- 1Department of Public Health Laboratory Sciences, School of Public Health, Hengyang Medical School, University of South China, Hengyang, Hunan, China
- 2The Affiliated Nanhua Hospital, Department of Clinical Laboratory, Hengyang Medical School, University of South China, Hengyang, China
Mycoplasma genitalium is a newly emerged sexually transmitted disease pathogen and an independent risk factor for female cervicitis and pelvic inflammatory disease. The clinical symptoms caused by M. genitalium infection are mild and easily ignored. If left untreated, M. genitalium can grow along the reproductive tract and cause salpingitis, leading to infertility and ectopic pregnancy. Additionally, M. genitalium infection in late pregnancy can increase the incidence of preterm birth. M. genitalium infections are often accompanied by co-infection with other sexually transmitted pathogens (Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis) and viral infections (Human Papilloma Virus and Human Immunodeficiency Virus). A recent study suggested that M. genitalium plays a role in tumor development in the female reproductive system. However, few studies endorsed this finding. In recent years, M. genitalium has evolved into a new “superbug” due to the emergence of macrolide-and fluoroquinolone-resistant strains leading to frequent therapy failures. This review summarizes the pathogenic characteristics of M. genitalium and the female reproductive diseases caused by M. genitalium (cervicitis, pelvic inflammatory disease, ectopic pregnancy, infertility, premature birth, co-infection, reproductive tumors, etc.), as well as its potential relationship with reproductive tumors and clinical treatment.
1. Introduction
In 1981, Tully et al. (1981) first successfully isolated two strains of Mycoplasma genitalium (G-37 and M-30) from 13 male patients with non-gonococcal urethritis. With the development of culture technology and detection methods, M. genitalium, a newly emerged sexually transmitted disease pathogen, has received increased attention. According to the 2021 guidelines for the Sexually Transmitted Infections Treatment issued by the United States Centers for Disease Control and Prevention, M. genitalium infection can cause cervicitis and pelvic inflammatory disease (PID) and more evidence is needed to determine whether M. genitalium infection is associated with adverse pregnancy outcomes (Workowski et al., 2021). A meta-analysis showed that M. genitalium infection was significantly associated with an increased risk of cervicitis (pooled odds ratio [OR] 1.66), pelvic inflammatory disease (pooled OR 2.14), female infertility (pooled OR 2.43), preterm birth (pooled OR 1.89), and spontaneous abortion (pooled OR 1.82; Lis et al., 2015). A recent meta-analysis from China showed that the infection rate of M. genitalium infection in pregnant women was 4.86%, and the M. genitalium infection rates in women with ectopic pregnancy, spontaneous abortion, induced abortion, and premature rupture of membranes were 13.01, 11.81, 6.11, and 12.63%, respectively (Yan et al., 2022). This suggests that the incidence of M. genitalium infection in pregnant women with ectopic pregnancy, spontaneous abortion, and premature rupture of membranes is higher than that in other pregnant women. Therefore, it is necessary to pay attention to the related diseases and treatment of M. genitalium.
Routine screening for sexually transmitted infections (STIs) usually targets only Chlamydia trachomatis and Neisseria gonorrhoeae, the two most common bacterial STIs globally, whereas M. genitalium infection is an emerging STI with symptoms similar to these two infections. Studies on the prevalence of M. genitalium infection in women indicated that M. genitalium infection is often co-infected with other sexually transmitted pathogens, including C. trachomatis, Trichomonas vaginalis, and N. gonorrhoeae. Co-infection of M. genitalium with C. trachomatis was reported at 29.9% and co-infection of M. genitalium with N. gonorrhoeae at 23.6% in young women at high risk in the United States (Sena et al., 2018), while in a study of testing centers in Belgium, Germany, Spain, and the United Kingdom, the rate of co-infection of M. genitalium with C. trachomatis/N. gonorrhoeae was only 0.1 to 0.6% (Perry et al., 2022). It is unclear whether this difference is related to the underrepresentation of the collected samples and further investigation is needed. C. trachomatis is the most common pathogen co-infected with M. genitalium. Most importantly, the detection rate of M. genitalium in high-risk populations is higher than that of C. trachomatis, T. vaginalis, and N. gonorrhoeae in some studies (Sena et al., 2018; Trent et al., 2018; Nolskog et al., 2019). The 2015 French guidelines for diagnosing and treating PID have included M. genitalium, N. gonorrhoeae, and C. trachomatis as routine targets of microbial examination for PID (Brun et al., 2016).
In addition to causing inflammation, many studies showed that persistent exposure to other Mycoplasma species is associated with oncogenic transformation and leads to proliferation, invasiveness, and metastasis of cancer cells (Zarei et al., 2013; Gedye et al., 2016; Zella and Gallo, 2021). Recent studies suggested that M. genitalium is associated with ovarian cancer (Fortner et al., 2019) and high-risk Human Papilloma Virus (HPV) infection (Ye et al., 2018). These findings indicate that M. genitalium adversely affects the health of the female reproductive system. Hence, this article reviews the diseases of the female reproductive system caused by M. genitalium and its treatment.
2. Molecular pathogenic characteristics of Mycoplasma genitalium
Mycoplasma genitalium harbors the smallest genome among self-replicating prokaryotic cells and lacks a cell wall. It is estimated that the genome contains about 500 genes, including guanine - cytosine average content is shallow, about 31% (McGowin and Totten, 2017). Lacking almost all enzymes required for amino acid biosynthesis, de novo nucleic acid synthesis, and fatty acid biosynthesis, M. genitalium shows marked metabolic limitations and can metabolize glucose but not arginine or urea. M. genitalium, is thus dependent on the host’s nutrients and grows slowly in an SP-4 medium rich in amino acids, nucleotides, glucose, vitamins, and cholesterol. In the absence of a cell wall, M. genitalium encodes a large number of lipid-associated membrane protein (LAMPs) genes (Fraser et al., 1995), which may be in prolonged contact with the epithelial surface during acute and chronic infection. These LAMPs play a role in innate immune activation of epithelial and resident immune cells by binding to highly expressed pattern recognition receptors, ultimately leading to activation of cell-host defense pathways, secretion of pro-inflammatory cytokines, and continuation of local inflammatory responses (Dehon and McGowin, 2017).
MgpB and MgpC are major genes involved in the genetic diversity of M. genitalium. MgpB (also known as MG_191) encodes the MgPa protein (also known as P140), which mediates adhesion to cell types such as human oviduct hairy epithelial cells. MgpC (also known as MG_192) encodes the P110 protein (also known as P114). In vivo, MgpC heterogeneity is extensive and evolves throughout for persistent infection, with sequence variation within MgpC supporting recombination with MgPar regions. MgPar is a general term for the nine repetitive DNA sequences that compose the chromosome of M. genitalium, and contains 79–90% of MgpB and MgpC gene homology sequences (Fraser et al., 1995). Since MgPar sequences have only partial and incomplete copies of MgpB and/or MgpC, which are homologous to different regions of these two genes, such homologous but not identical MgPar sequences are recombined into MgpB or MgpC, resulting in the expression of variant MgPa and P110 proteins, causing genome heterogeneity of M. genitalium (Iverson-Cabral et al., 2007; Hakim et al., 2021). The antigenic variation of these proteins can not only optimize adhesion, but also help to avoid host immune response and promote persistence, which plays a key role in the pathogenicity of M. genitalium and escape from the host immune system, and is essential for the survival of M. genitalium (Taylor-Robinson and Jensen, 2011). Genotyping revealed that the variety of MgpC repeat regions occurred in a single infected strain, suggesting that the antigenic variation of M. genitalium could be achieved by recombining between different sites of the MgPar sequence and recombining of MgpB, MgpC, and MgPar (Iverson-Cabral et al., 2007). In addition, MgpB genotyping has significant genetic diversity in different populations of patients, so it is necessary to carry out molecular typing of M. genitalium. Currently, M. genitalium strain typing is mainly based on analyzing different short tandem repeats of MgpB alleles MG_191 and MG_309 (Pineiro et al., 2019; Laumen et al., 2021; Dumke, 2022).
Mycoplasma genitalium morphologically presents as a bottle or flask shape, with a rod-like structure at the end, and a tiny protrusion (7–8 nm) similar to the protrusion of myxoviruses, which can promote adhesion and make it have adhesion force. The terminal structure is composed of various proteins, among which P140 and P110 are the major adhesion proteins of M. genitalium (Hu et al., 1987), which need to cooperate with accessory proteins. The adhesion of P140 protein is an essential step in producing pathogenic changes (Taylor-Robinson and Jensen, 2011). It is not only an important functional component of organelles but also the strongest immunogen according to human and experimental animal models (McGowin et al., 2013). P110 is an immunodominant protein of M. genitalium, and its binding to sialic acid oligosaccharides activates the adhesion of M. genitalium to human cells (Aparicio et al., 2018). P140 and P110 interact to form a transmembrane complex called “Nap,” which accumulates at polar terminal structures and plays an important role in cell adhesion and motility (Aparicio et al., 2020). In addition, the biofilms generated by M. genitalium in vitro are rich in polysaccharides composed of poly-N-acetylglucosamine (PNAG), which can reduce contact with antibiotics and increase the resistance of M. genitalium (Daubenspeck et al., 2020).
The above characteristics of M. genitalium justify its pathogenicity at the molecular biological level. It is not known whether the dependence of M. genitalium on its environment is related to its selectivity and persistence in the infected host. For patients with multidrug-resistant M. genitalium infection, it is necessary to determine whether microecological regulation of infection facilitates pathogen clearance.
3. Mycoplasma genitalium and the female reproductive system
The incidence of M. genitalium infection varies greatly among different ages, regions, and populations. Studies demonstrated that the infection rate of females aged 15–21 was higher than that of females aged 22–25 (22.6 and 17.7%, respectively; Lillis et al., 2019). In a retrospective study from 2009 to 2019, the detection rate of M. genitalium was 3.4% among 17,573 women who presented a pregnancy termination and contraception clinic, with the highest prevalence of 5.4% among 20–24 years. The prevalence fluctuated over time, from 4.4% in 2009 to 2.1% in 2013 and 4.8% in 2019 (Shilling et al., 2022). A meta-analysis revealed that the prevalence of M. genitalium in the general population of developed countries was 1.3%, lower than 3.9% in underdeveloped countries, and the prevalence of sex workers was as high as 15.9% (Baumann et al., 2018). A sample of female sex workers from Burkina Faso showed an infection rate of 11.54% of M. genitalium (Tovo et al., 2021). The frequencies of M. genitalium in the New Orleans sexually transmitted disease clinics (Lillis et al., 2019), a multicenter clinical study in the United States (Getman et al., 2016), Adolescent/Young Adult Medicine and Obstetrics and Gynecology clinics (Trent et al., 2018), 10 different clinical sites (i.e., sexually transmitted disease, family planning, obstetrics-gynecology, and clinical research clinics) in the United States (Sena et al., 2018) were 17.5, 16.3, 16, and 20.5%, respectively. The frequencies of M. genitalium in the obstetrics and gynecology clinics in Jalisco, Mexico (Casillas-Vega et al., 2016) and sexually transmitted infection clinics in the Netherlands (de Jong et al., 2016) were only 2.4 and 4.5%, respectively. In addition, a London cohort study discovered that high-risk sexual behavior (more than two sexual partners within 12 months) was an independent risk factor for M. genitalium positivity. As the number of sexual partners increased, so did the infection rate. Among women with 0, 1, 2, and 12 partners, the positive rates were 1.2, 2.0, 3.8, and 6.5%, respectively (Oakeshott et al., 2010).
Previous studies and prevalence characteristics indicate that the high-risk factors for M. genitalium infection are as follows: black population (Taylor et al., 2018), younger age [age ≤ 30 years (Stafford et al., 2021), age ≤ 25 years (Lillis et al., 2019), age ≤ 20 years (Sena et al., 2018)], symptom status, low socioeconomic status, a series of risky sexual behaviors [increase in a total partner, new partner, and condomless sex, etc. (Taylor et al., 2018). ≥2 sexual partners in the past 12 months (Lillis et al., 2019)]. Due to the low prevalence of M. genitalium, universal screening in the general population and pregnant women is not currently supported, but screening cervical secretions from young women with high-risk factors could enhance the diagnosis and treatment of M. genitalium infection in women.
Mycoplasma genitalium infection can cause inflammation of the reproductive system, such as pelvic inflammatory disease, salpingitis, cervicitis, and vaginitis in the female reproductive tract, which may lead to infertility, ectopic pregnancy, and premature delivery, and other reproductive diseases. At the same time, M. genitalium may be related to the occurrence and co-infection of ovarian tumors and HPV (Figure 1).
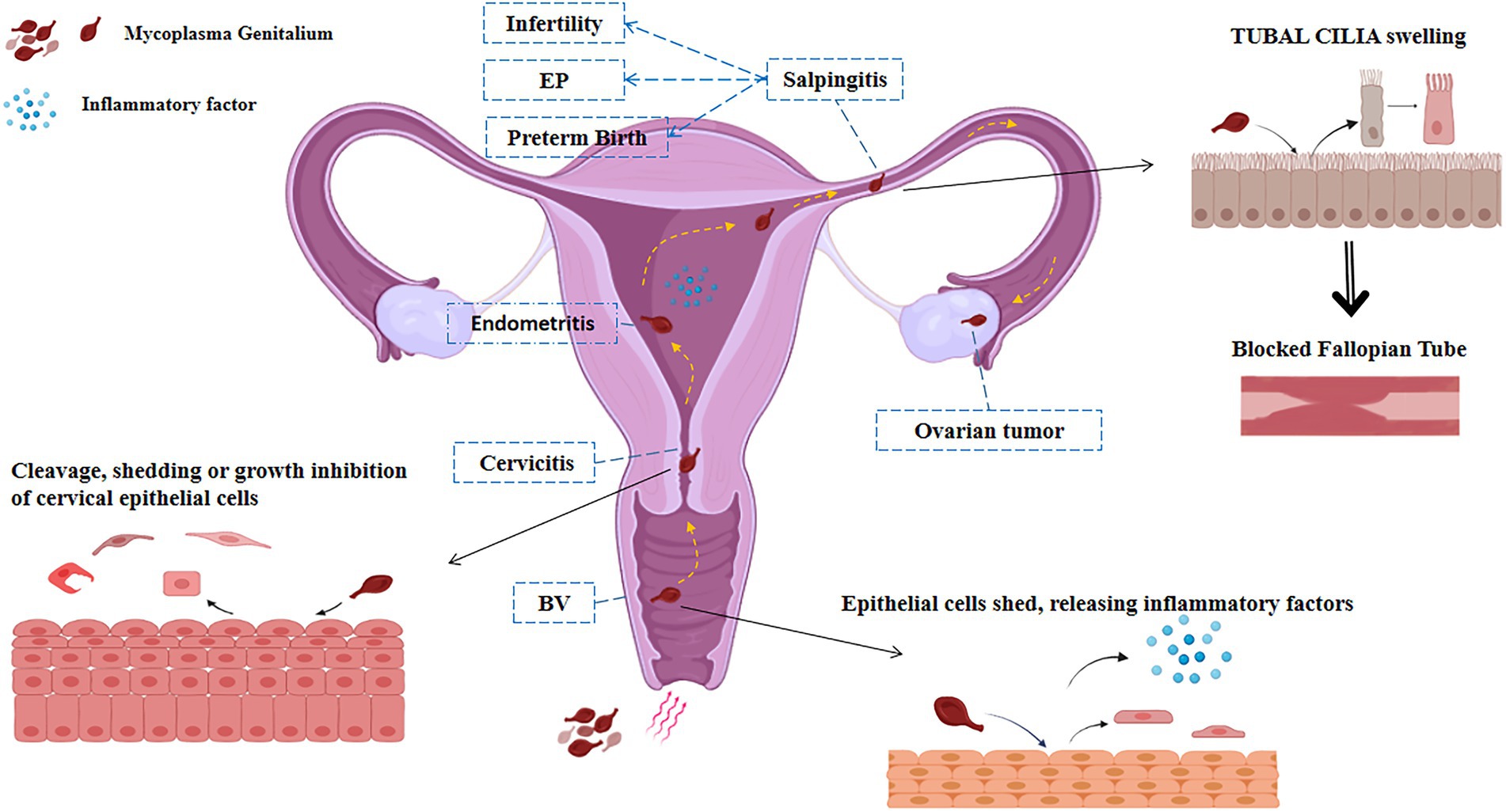
Figure 1. Pathways of infection of M. genitalium in the female reproductive system. M. genitalium is carried by sperm and other risk factors into the female vagina and colonizes there to cause vaginitis. M. genitalium travels up the reproductive tract to the cervix, endometrium, and fallopian tube epithelium, causing cell swelling and shedding, releasing inflammatory factors, and triggering acute inflammatory reactions such as pelvic inflammatory disease and endometritis, inducing adverse pregnancy outcomes such as infertility, preterm delivery, and ectopic pregnancy. Long-term infection with M. genitalium transforms into a chronic inflammatory response that may be associated with tumor outcomes such as ovarian cancer.
3.1. Reproductive tract diseases caused by Mycoplasma genitalium infection
3.1.1. Mycoplasma genitalium brings about inflammation in the lower genital tract
Inflammation of the lower genital tract includes cervicitis and vaginitis. There is no consensus on cervicitis’s definition and diagnostic criteria. The United States 2021 guidelines for the diagnosis and treatment of sexually transmitted diseases identified two major diagnostic signs that characterize cervicitis: (1) a purulent or mucopurulent endocervical exudate visible in the endocervical canal or on an endocervical swab specimen (commonly referred to as mucopurulent cervicitis) and (2) sustained endocervical bleeding easily induced by gentle passage of a cotton swab through the cervical os (Workowski et al., 2021). C. trachomatis and N. gonorrhoeae are the two most frequent sexually transmitted pathogens that cause cervicitis, and studies have indicated M. genitalium infection as an important new causal pathogen (Roy et al., 2021).
The pathogenic effect of M. genitalium on the cervix has been confirmed in the cell (McGowin et al., 2013) and animal experiments (McGowin et al., 2012). Clinical studies have reported that M. genitalium infection can cause mucopurulent cervicitis in women (Manhart et al., 2003) and is significantly related to cervical contact bleeding (Oliphant and Azariah, 2013). In vitro studies have discovered that 4–14 days after inoculation with high levels of M. genitalium (200 MOI), cervical epithelial cells undergo significant cleavage, shedding, or growth inhibition (McGowin et al., 2012), suggesting that M. genitalium infection can cause acute toxic reactions in cervical and oviduct epithelial cells, which is directly related to the number of M. genitalium. After M. genitalium inoculation of cervical epithelial cells for 48 h, interleukin (IL-6, IL-7, IL-8), monocyte chemoattractant protein 1, and granulocyte-macrophage-colony-stimulating factor levels were significantly increased (McGowin et al., 2012, 2013), suggesting that M. genitalium infection produces an acute immune response to cervical epithelial cells, leading to increased secretion of inflammatory cytokines. In addition, in the mouse model (McGowin et al., 2010), it was found that M. genitalium was detected in the upper genital tract only 3 days after injection of M. genitalium into the vagina, suggesting that M. genitalium could travel up from the lower end of the genital tract to the upper end. At the same time, the adhesion of M. genitalium to cultured cervical epithelial cells in vitro can trigger the release of inflammatory signals (Toll-like receptors 2 and 6), which leads to the activation of NF-κB and the induction of host defense-related genes, and ultimately leads to the aggregation of leukocytes at the infection site and the induction of genital tract inflammation (McGowin and Totten, 2017). In contrast, the localization of M. genitalium to vaginal and cervical cells provides a survival site and protection from immune responses, as phagocytosis by macrophages can be avoided, thereby facilitating the establishment and maintenance of reproductive tract infections (McGowin et al., 2009). During persistent infection, M. genitalium can continuously replicate and provoke the continuous secretion of inflammatory cytokines. In vitro studies of cervical epithelial cells have shown that the average number of M. genitalium increased 500 to 5,000 times after 85 days of vaccination with a low concentration (10 MOI) of M. genitalium. During that time, inflammatory cytokines (IL-6, IL-7, IL-8, monocyte chemoattractant protein 1, and granulocyte-macrophage-colony-stimulating factor) were also detected (McGowin et al., 2012). IL-6 is involved in acute and chronic inflammatory processes and plays a key role in the production of acute phase proteins, the transition from acute to chronic inflammation, and the maintenance of chronic inflammatory response, while weakening the ciliary activity of oviduct epithelium (Papathanasiou et al., 2008).
Likewise, the incidence of cervicitis is significantly higher in M. genitalium-positive women than in M. genitalium-negative women (Olson et al., 2021), and multiple studies reported M. genitalium infection as an independent predictor of cervicitis that increases the risk of cervicitis in women by 2.5–3.3-fold (Lillis et al., 2019). Additionally, M. genitalium can cause inflammatory damage to the cervix and endometritis through the cervical canal. The outflow of abnormal cervix secretion also represents a manifestation of inflammation of the upper genital tract.
The current evidence for a link between M. genitalium and vaginitis is limited and requires further confirmation. Although M. genitalium has been found in vaginal specimens, it is interesting to note whether M. genitalium is associated with vaginitis is contentious. Some studies showed a significant correlation between M. genitalium infection and bacterial vaginitis (OR, 1.7; Nye et al., 2020; Moore et al., 2021), some showed a negative correlation between M. genitalium infection and bacterial vaginitis (OR, 0.4; Manhart et al., 2003), and some showed no correlation between them (p > 0.05; Wang et al., 2022). A recent study showed that M. genitalium susceptibility might be enhanced in the presence of bacterial vaginitis in a manner similar to N. gonorrhoeae and C. trachomatis (Shipitsyna et al., 2020). At the same time, there is a strong correlation between M. genitalium infection and bacterial vaginosis and trichomonas vaginitis (Masha et al., 2018). Consequently, for women at high risk of M. genitalium infection, abnormal vaginal discharge, or cervical contact bleeding, it is necessary to research the detection of M. genitalium, timely diagnosis, and effective treatment.
3.1.2. Mycoplasma genitalium gives rise to inflammation in the upper genital tract (pelvic inflammatory disease)
Pelvic inflammatory disease describes the inflammation of the upper genital tract, including endometritis, tubal inflammation, tubo-ovarian abscess, pelvic peritonitis, etc., which can present with different clinical symptoms, and some patients present with mild or even unconscious symptoms. In recent years, numerous studies have confirmed that M. genitalium is an important new pathogen that causes PID.
Mycoplasma genitalium can provoke endometritis and salpingitis in non-human primates (McGowin et al., 2010; Taylor-Robinson and Jensen, 2011), and induce oviduct edema in rats (De Carvalho et al., 2020). Tubal experiments further show that M. genitalium can adhere to the surface of cilia, causing abnormal swelling and shedding (Baczynska et al., 2007). Animal studies have revealed that cervical inoculation of M. genitalium can infect the uterus and Fallopian tubes (McGowin et al., 2010). Clinical data show that the positive rate of M. genitalium infection in endometrial specimens of women with endometritis is 8–12% (Haggerty et al., 2006). Nonetheless, in endometrial and Fallopian tube specimens from patients with acute salpingitis, this proportion is only 4% (Cohen et al., 2005). The detection rate was lower than that of C. trachomatis and N. gonorrhoeae, which may be related to the mild clinical symptoms of PID caused by M. genitalium infection. In addition, the study found that M. genitalium infection increased the incidence of PID. Another study reported that the histopathological diagnosis of endometritis incidence was 68.3% in patients with PID and M. genitalium positive cervical or endometritis results, but only 44.9% in patients with M. genitalium negative results (Haggerty et al., 2008). In addition, a prospective study showed that the incidence of PID in patients with M. genitalium infection was 4.9%. In comparison, the incidence of PID in patients with M. genitalium negative infection was only 0.6%, suggesting an independent correlation between M. genitalium infection and PID (Bjartling et al., 2012). These studies confirm that M. genitalium infection can increase the incidence of PID. Previous studies showed that 6 out of 49 women who had M. genitalium infection and terminated pregnancy before surgery were infected with PID (incidence: 12.2%), while the infection rate was only 2.4% in 168 women who had no M. genitalium infection or C. trachomatis infection and terminated pregnancy before surgery (Bjartling et al., 2010). It is suggested that there is a certain relationship between M. genitalium infection and PID after the termination of pregnancy. Screening for M. genitalium before pregnancy termination and routine treatment of M. genitalium-positive patients may prevent PID after pregnancy termination.
Pelvic tenderness is the clinical diagnosis basis for PID, while pelvic inflammation caused by Mycoplasma genitalium infection is mild or has no specific discomfort symptoms (Sena et al., 2018), and the changes in inflammatory indicators are not obvious (Ah-Kit et al., 2019). This also leads to infections that are easily overlooked, delayed, or left untreated, leading to long-term reproductive complications, including infertility or ectopic pregnancy (EP) (Ma et al., 2021). Therefore, accurate identification and detection of PID caused by M. genitalium infection are crucial, suggesting that PID diagnosis and detection methods need to be further studied.
3.2. Reproductive system diseases generated by Mycoplasma genitalium infection
3.2.1. Mycoplasma genitalium infections may contribute to higher infertility rates
Infertility is defined as the inability to conceive after 12 months of regular unprotected sexual intercourse. Currently, 9% of women of reproductive age worldwide are infertile, among which tubal factors are one of the most common causes of infertility (Carson and Kallen, 2021). In vitro and animal experiments have shown that M. genitalium infection can cause tubal cilia swelling, shedding (Baczynska et al., 2007), and hydrosalpinx (McGowin et al., 2010). Another study reported 16.1 and 2.2% positivity for M. genitalium in cervical swabs from infertile and normal women, respectively (Ajani et al., 2017). This is in agreement with the results of a Polish study on M. genitalium (19.9 and 4.4%, respectively; Grzesko et al., 2009), which found that the detection rate of M. genitalium in abdominal fluid samples collected by laparoscopy was 5.88 and 0% in infertile and control women, respectively (Grzesko et al., 2009), similar to the results of an Indian study (6.1 and 0.6%; Rekha et al., 2019). The above results suggest that the detection rate of M. genitalium in infertile women is significantly higher than that in normal women, which suggests that M. genitalium is an independent risk factor for infertility. In 2001, serum levels of antibodies to M. genitalium were measured by western blotting in infertile women. The serological positivity rate for MgPa protein of M. genitalium was 6.3% in patients with normal Fallopian tubes, and 22.0% in patients with tubal factor infertility (Clausen et al., 2001), which is similar to data from a 2005 study (respectively, 13.2 and 22.0%; Baczynska et al., 2005), were similar. Unfortunately, the experiment only compared rates between groups with infertility factors and not women with childbearing potential.
These findings suggest that the rate of M. genitalium infection is higher in cervical and peritoneal fluid and serum from infertile women. This supports previous findings of M. genitalium in cervical, endometrial, and Fallopian tube tissue samples, suggesting that M. genitalium translocates through the endometrium to the Fallopian tubes, where it attaches to the human Fallopian tube epithelium, impairing tubal function and causing infertility. In addition, M. genitalium has been shown to bind to human sperm, with less cell binding at 5 min of incubation; significant binding at 30 min of incubation (Svenstrup et al., 2003). Thus M. genitalium may be carried by active sperm to the female reproductive tract and thus be transferred to the uterus and Fallopian tubes, colonizing and destroying the ciliated epithelium in vivo, leading to female infertility. These results suggest that routine screening of infertile women for M. genitalium and prompt treatment of positive patients may improve infertility outcomes.
3.2.2. Mycoplasma genitalium infections and ectopic pregnancy formation
Ectopic pregnancy is a pregnancy implanted outside the intrauterine cavity and accounts for 1–2% of natural pregnancies, whereas more than 98% of pregnancies occur in the Fallopian tube (Ashshi et al., 2015). Salpingitis caused by upper reproductive-tract infection is an important cause of EP (Zhang et al., 2018). In vitro experiments involving the oviduct confirmed that M. genitalium infection can cause tubal cilia swelling and shedding (Baczynska et al., 2007) and reduce the ciliary activity of the tubal epithelium by increasing IL-6 secretion (Papathanasiou et al., 2008). This suggests that M. genitalium promotes EP by changing the structure and function of the Fallopian tubes. Two studies conducted in Saudi Arabia from 2015 to 2016 showed that the rate of M. genitalium infection in Fallopian-tube tissues from patients with EP ranged from 19.8 to 20.2%, whereas that in normal Fallopian-tube tissues was only 1.6–3.9% (Ashshi et al., 2015; Refaat et al., 2016). Moreover, they found that serum IL-6 levels in M. genitalium infected patients with EP were significantly elevated, suggesting that M. genitalium infection was significantly correlated with EP (Ashshi et al., 2015; Refaat et al., 2016).
In 2007, a Swedish study compared serum M. genitalium antibodies of pregnant female patients with EP and found an antibody positive rate of 18% in EP patients compared with 15% in controls, indicating no significant correlation between M. genitalium antibodies and EP (Jurstrand et al., 2007). Interestingly, another Swedish study in 2015 used the M. genitalium serological test to assess differences in M. genitalium antibodies between infertile and pregnant women, with the results showing that the rate of M. genitalium-positive serum IgG levels in infertile women was 5.4% relative to 1.6% in the control group (Idahl et al., 2015). Furthermore, two other studies reported that patients with a history of PID showed a 7.5-fold increased risk of EP (Ashshi et al., 2015; Refaat et al., 2016). These findings suggest that timely and proper treatment of PID can reduce the incidence of EP.
3.2.3. Mycoplasma genitalium infection and preterm birth
Preterm birth is defined as delivery at 28 weeks of gestation but less than 37 weeks, accounting for 5.5–12% of all deliveries, and infectious factors account for 50% of spontaneous preterm births (Kayem et al., 2018). Intrauterine infection is a common and important cause of preterm birth, and epidemiological studies have shown that 25–40% of spontaneous preterm births are associated with intrauterine infections caused by bacteria. M. genitalium establishes long-term infection in the upper female genital tract. At the same time, pathogen ascension causes an inflammatory cascade and disrupts the chorionic metaphase gap, which is thought to be the mechanism of genital tract infection leading to preterm labor (Averbach et al., 2013), where the inflammatory factor IL-6 is the main marker of preterm labor. A prospective study on the detection of M. genitalium in the vaginal secretions of pregnant women at 23–32 weeks of gestation demonstrated a 20.2% vaginal colonization rate of M. genitalium in pregnant women with spontaneous preterm delivery. This suggests that M. genitalium colonization is a significant independent risk factor for spontaneous preterm birth (Edwards et al., 2006). Another study of M. genitalium testing in spontaneously preterm and term pregnant women within 48 h after delivery showed a 4% (29/661) rate of M. genitalium infection in the preterm group and 2% (12/667) in the control group (p = 0.007), suggesting that M. genitalium positivity is significantly associated with spontaneous preterm delivery (Hitti et al., 2010). An Australian study tested vaginal secretions for M. genitalium in the same group of pregnant women at three gestational ages (median gestational age 21, 29, and 36 weeks) and showed a 15 and 2% prevalence of M. genitalium infection in women who delivered prematurely and at term, respectively (Payne et al., 2016). However, two prospective studies (Averbach et al., 2013) in pregnant women <11 weeks’ gestation (Kataoka et al., 2006) and < 16 weeks’ gestation reported that vaginal discharge and cervical swab testing for M. genitalium showed that the incidence of preterm delivery was not associated with colonization by M. genitalium. These data suggest that the median time to clearance of lower genital tract M. genitalium infection ranges from 1.5 months to 85.5 days (Lokken et al., 2017; Balkus et al., 2018). Thus, early gestation with M. genitalium infection is not the same as late gestation with M. genitalium colonization, which is associated with spontaneous preterm delivery in the lower genital tract. This suggests that M. genitalium testing should be used as a routine test for microbiology in the lower genital tract in late pregnancy.
In the United States (Kayem et al., 2018) and Australia (Rowlands et al., 2017), the positive rate of amniotic fluid testing for M. genitalium in mid-pregnancy was 1.3 and 0%, respectively. In addition, in Italy (Contini et al., 2018) and the United Kingdom (Cox et al., 2016), M. genitalium was not detected in chorionic villi samples from spontaneous abortion and placenta after preterm delivery, respectively. These results suggest that M. genitalium infections rarely occur in the amniotic cavity, chorionic villi, and placenta.
3.3. Co-infection with Mycoplasma genitalium
In many countries, M. genitalium is generally not recommended for routine sexually transmitted infection (STI) screening due to the lack of information on the prevalence of M. genitalium infections and their co-infection with other sexually transmitted bacteria. A study in California showed that the co-infection rate with M. genitalium and C. trachomatis was 3.1% in women and 9.7% in men (Getman et al., 2016). A study of the prevalence of C. trachomatis and M. genitalium among women attending termination of pregnancy and contraception clinics during 2009–2019 showed a co-infection rate of 10.1% for C. trachomatis and M. genitalium (Shilling et al., 2022). In a recent HIV pre-exposure prophylaxis (PrEP) demonstration trial involving 200 men, molecular testing for N. gonorrhoeae, C. trachomatis, and M. genitalium found significantly higher gonococcal bacterial loads in the presence of M. genitalium, suggesting that M. genitalium may be a cofactor for N. gonorrhoeae transmission. If this finding is confirmed in other studies, M. genitalium may be required as a cofactor for N. gonorrhoeae transmission in specific populations such as MSM (Van Dijck et al., 2022). Therefore, future studies should investigate co-infections with M. genitalium and other sexually transmitted bacteria (e.g., C. trachomatis or N. gonorrhoeae; Desdorf et al., 2021). It is suggested that testing for M. genitalium should be included in routine screening for sexually transmitted infections.
Results of a study on the prevalence of M. genitalium conducted at the National Sexual Health Clinic in Singapore showed that M. genitalium was strongly associated with C. trachomatis infection (8.1% of cases) but only 2.4% of C. trachomatis-negative cases, suggesting that targeted screening for M. genitalium in C. trachomatis positive patients could reduce the incidence of M. genitalium (Hart et al., 2020). An assessment of the prevalence of M. genitalium co-infection in 302 women with C. trachomatis attending a clinic for sexually transmitted diseases in Birmingham found only 22 (7.3%) M. genitalium co-infections, concluding that M. genitalium co-infection is uncommon in women with C. trachomatis infection (Harrison et al., 2019). A multicenter clinical study cohort in the United States tested 946 subjects from seven geographically diverse clinical sites for M. genitalium, C. trachomatis, N. gonorrhoeae, and T. vaginalis, and found that the prevalence of M. genitalium was 16.1% in women and 17.2% in men. The single infection rate of M. genitalium in females was significantly higher than the co-infection rate of C. trachomatis and N. gonorrhoeae. The single infection rate of M. genitalium in males was significantly higher than the co-infection rate of C. trachomatis and M. genitalium, indicating that M. genitalium single infection was high. The co-infection rate of M. genitalium with other sexually transmitted pathogens was low (Getman et al., 2016). As mentioned above, infection with C. trachomatis or N. gonorrhoeae is highly likely to be complicated by infection with M. genitalium, so the inclusion of M. genitalium testing in routine STI screening will likely reduce morbidity.
A 2009 meta-analysis of the association between M. genitalium and HIV infection showed a statistically significant twofold increase in the odds of HIV infection (OR 2.1) in the M. genitalium-infected population (Napierala Mavedzenge and Weiss, 2009). Since both M. genitalium and HIV are sexually transmitted, HIV-positive patients may be more vulnerable to M. genitalium infection. A cross-sectional analysis of men who have sex with men (MSM) population in Shenyang, China, found that M. genitalium infection (aOR = 3.2) was associated with an increased risk of HIV infection among MSM (Zhao et al., 2019). This suggests the need for more widespread screening for sexually transmitted infections, particularly for M. genitalium infections among MSM. However, an African cohort study that examined M. genitalium infection in HIV-positive pregnant women by transcription-mediated amplification assay found a higher detection rate of M. genitalium in this cohort (21.4%) as well as higher HIV plasma levels in M. genitalium-infected women (p = 0.02), suggesting that M. genitalium-infected women may be more likely to transmit HIV to male sexual partners (Roxby et al., 2019). However, the sample selected for this study was more limited and should be evaluated in a larger study.
The vaginal microbiota plays a role in the acquisition and persistence of HPV in the human vagina and the subsequent development and progression of cervical intraepithelial neoplasia. Noma et al. (2021) found no significant association between M. genitalium and high-risk HPV infection. However, this was a statistical investigation of a small sample. A survey of 802 female sex workers showed that M. genitalium was significantly associated with certain types of high-risk HPV (HPV-16 and HPV-56) infections (Yin et al., 2013). The results of previous studies on the relationship between M. genitalium and high-risk HPV are controversial. In 2018, a meta-analysis showed that M. genitalium was associated with a significantly increased risk of high-risk HPV infection (OR 1.50; Ye et al., 2018). Since HPV exposure to cervical intraepithelial neoplasia and cancer progression takes several years, cofactors may play a key role in different stages of the natural history of HPV-induced tumorigenesis. As mentioned above, M. genitalium infection can reduce microvilli in cervical epithelial cells, releasing inflammatory factors and disrupting the physical and local immune barriers of the cervix (McGowin et al., 2013). This creates favorable conditions for HPV infection of cervical epithelial basal cells and leads to persistent infection. However, the role of M. genitalium as a risk factor for the progression of cervical lesions is unclear and requires further study.
3.4. Mycoplasma genitalium and reproductive tumor
As early as 1965, Paton et al. (1965) found that Mycoplasma orale infection can cause chromosomal abnormalities in cells, which are aggravated with the extension of infection time. In 1966, Macpherson and Russell (1966) demonstrated that some Mycoplasmas act as a “co-carcinogen” and mediate inheritable cell changes in vitro. Subsequently, many experiments confirmed that Mycoplasma chronic infection promoted malignant transformation resulting from the dysregulation of genes such as Ras, Myc, or P53 and nuclear factor-κB activation in host cells (Borchsenius et al., 2018). Moreover, Mycoplasma infection promotes tumor progression by increasing the expression of epithelial cell adhesion molecule (Kim et al., 2019), inducing epithelial-mesenchymal transition (Duan et al., 2014b), and activating the β-catenin signaling pathway (Liu et al., 2019) and epidermal growth factor receptor-phosphoinositide 3-kinase-AKT signaling axis (Duan et al., 2014a). Although there is no evidence that Mycoplasma can be directly tumorigenic in vivo, analysis of the association between Mycoplasma infection and cell transformation, apoptosis, genomic instability, tumor invasion and metastasis, and drug resistance suggests that Mycoplasma infection is involved in the development of tumors (Li et al., 2022).
The studies on the correlation between mycoplasma and tumor mainly focused on Mycoplasma hyorhinis, Mycoplasma penetrans, Mycoplasma fermentans, and Mycoplasma hominis, but very few studies investigated the association between M. genitalium and tumors. In 2009, Namiki et al. (2009) first reported the capacity of M. genitalium infection to lead to the malignant transformation of benign human prostate cells. A updated meta-analysis showed that a history of PID was associated with an increased risk of ovarian cancer (HR 1.18, 95% CI 1.13 to 1.22; Piao et al., 2020). M. genitalium was also linked to ovarian cancer because of its role in PID. A study by Idahl et al. (2011) verified that M. genitalium IgG antibodies were associated with borderline ovarian tumors. However, two other studies by Idahl et al. (2020) showed that M genitalium is not detectable in ovarian tissues either from women with benign conditions and borderline tumors or from women with ovarian cancer, and serum antibodies to M. genitalium were not associated with epithelial ovarian cancers. Fortner et al. (2019) revealed that M. genitalium was positively associated with ovarian cancer (response rate: 1.92). However, Trabert et al. (2019) observed no association.
Recent studies indicated that serous ovarian cancer originates in the Fallopian tube and that serous tubal intraepithelial carcinoma represents a precursor lesion in high-grade serous ovarian carcinoma (Wang et al., 2019). As described above, M. genitalium typically causes chronic and asymptomatic infection and is associated with PID, tubal pregnancy, and tubal infertility, suggesting that this organism can traverse from the lower to the upper genital tract to cause infection and cause persistent inflammation in the Fallopian tubes and ovaries. Inflammation is characterized by the production of free radicals, cytokines, and prostaglandins. These mediators of inflammation can induce genetic and epigenetic changes, including point mutations in tumor suppressor genes, DNA methylation, and post-translational modifications, causing alterations in critical pathways responsible for maintaining normal cellular homeostasis and leading to the development and progression of cancer (Hussain and Harris, 2007). Accordingly, an association between M. genitalium and the risk of ovarian tumors is biologically plausible and can be explained by the inflammation hypothesis.
Currently, epidemiological evidence explaining the potential of M. genitalium to cause cancer development is lacking and the possible mechanisms are also unclear. The role of M. genitalium in cancer remains conjectural. To improve the understanding of this microorganism’s initial or co-factorial probable roles in cancer, additional in vitro and in vivo studies on its mechanisms for malignant transformation are needed.
3.5. Diagnosis of Mycoplasma genitalium infection
The clinical manifestations of M. genitalium infection lack specificity, and its diagnosis mainly depends on the etiological diagnosis. Due to the lack of a cell wall, M. genitalium is not visible by gram staining microscopy. In addition, in vitro culture of M. genitalium is difficult and time-consuming, so isolation culture is usually not carried out clinically. And the specificity of the M. genitalium serological test is poor, and there is a lack of standard diagnostic reagents. The first M. genitalium diagnostic kit was approved by the US Food and Drug Administration (FDA) in January 2019 (Shipitsyna and Unemo, 2020). Due to the widespread presence of macrolide resistance in Europe, the 2021 European Guidelines recommend testing for macrolide resistance mutations in all positive specimens of M. genitalium (Jensen et al., 2022). On the types of samples suitable for detection of M. genitalium: (1) For men, first void urine (FVU) is usually recommended, which has the advantages of the non-invasive and convenient collection (Gaydos et al., 2019; Van Der Pol et al., 2020); (2) In women, the sensitivity of M. genitalium in vaginal swab samples detected by NAAT is higher than that of cervical swabs and urine samples (Gaydos et al., 2019). The United Kingdom guidelines recommend women collect vaginal swab samples (Soni et al., 2019); (3) Rectal samples are recommended in men who have sex with men (MSM) or in patients with proctitis (Jensen et al., 2022).
4. Treatment of Mycoplasma genitalium-related diseases
Typical therapeutic agents for M. genitalium infections are divided into three main groups: macrolides, fluoroquinolones, and tetracyclines. In the past 10 years, the resistance of M. genitalium to macrolide antibiotics (azithromycin) as the first-line treatment and to fluoroquinolones (moxifloxacin) as the second-line treatment has increased globally, partly due to the frequent use of similar antibiotics to treat other bacterial pathogens such as C. trachomatis, leading to the selection of drug-resistant M. genitalium (van der Schalk et al., 2020). The data showed that macrolide resistance for M. genitalium increased: from 10% in studies before 2010 to around 50% in studies published in 2016 and 2017, whereas resistance to fluoroquinolones is 7.7% (CI 4.5–11.4; Machalek et al., 2020). And the resistance levels in M. genitalium vary by region, population, and gender (Sweeney et al., 2019; Fernandez-Huerta et al., 2020; Iwuji et al., 2022). The increase in drug resistance led to frequent treatment failures, so we need to explore more treatment options and research the resistance mechanisms in M. genitalium.
Resistance-guided therapy is necessary to improve the effectiveness of the treatment for M. genitalium infections and reduce macrolide resistance options. Both the 2021 European Guidelines and the 2021 STI Treatment Guidelines recommend that give doxycycline as initial empiric therapy to decrease organism load and the risk of macrolide resistance selection, followed by resistance testing. After the initial 7 days of doxycycline, patients sensitive to macrolides should be given azithromycin, and patients resistant to macrolides should be given moxifloxacin, according to the results of the resistance test (Workowski et al., 2021; Jensen et al., 2022). Sweeney et al. (2019, 2022) highlight the need for ongoing surveillance of resistance in M. genitalium and suggest that introducing fluoroquinolone resistance testing would greatly improve the management of M. genitalium infection. According to the resistance testing results, the Melbourne Sexual Health Centre gave azithromycin to non-macrolide-resistant patients and sitafloxacin to macrolide-resistant patients, which reached 94.8 and 92.2% of the cure rates, respectively (Read et al., 2019). In 2021 STI Treatment Guidelines, it is recommended that patients with macrolide sensitivity should be given doxycycline followed by azithromycin after screening for macrolide resistance; Patients with macrolide resistance should be given doxycycline followed by moxifloxacin; When resistance testing is not available, patients should be given an initial week of doxycycline followed by a week of moxifloxacin (Workowski et al., 2021). For patients resistant to macrolides and fluoroquinolones, a previous study suggested that pristinamycin is the only clinically available drug to treat M. genitalium infections (Martin et al., 2017). This conclusion was supported in an Australian study, which identified bacterial load as an important factor influencing the failure of pristinamycin treatment (Read et al., 2018) and pristinamycin is listed as a third-line treatment in the 2021 European guidelines for the management of M. genitalium infections (Jensen et al., 2022). The latest recommendations for treatment options following resistance testing are shown in Figure 2, panel A.
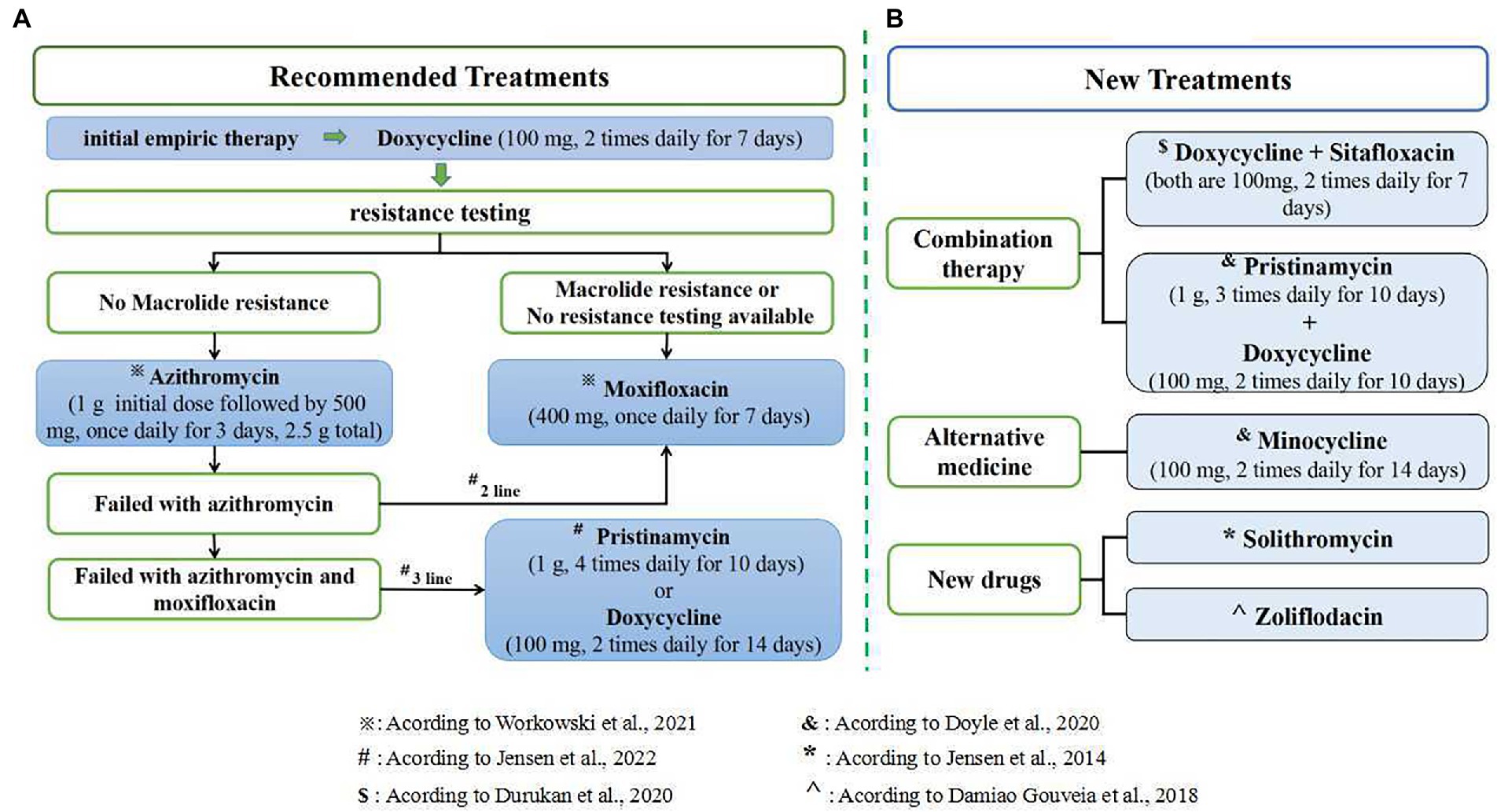
Figure 2. Treatment options for M. genitalium. (A) The latest recommendations for treatment options following resistance testing; (B) several new treatments and drugs related to M. genitalium infection.
A prospective evaluation (Read et al., 2019) showed that treatment of M. genitalium with only a single antibiotic has a high failure rate due to the rapid emergence of resistance. In contrast, combination therapy reduces bacterial escape from antibiotic treatment (Read et al., 2019; van der Schalk et al., 2020). Durukan et al. (2020) found that combination therapy with doxycycline and sitafloxacin was well tolerated and effective in treatment-resistant M. genitalium. Meanwhile, Doyle et al. (2020) reported a 75% (55/73) cure rate for macrolide-resistant M. genitalium infections treated with a combination of pristinamycin and doxycycline and a 71% (25/35) cure rate with a 14-day course of minocycline. It is suggested that minocycline may be used as an alternative agent for treating M. genitalium infections resistant to macrolides and quinolones. However, neither sitafloxacin nor pristinamycin are available in the US at present. In addition, an evaluation of the in vitro activity found that fluoroketolide solithromycin has a clinical cure rate of 65 to 85% for the treatment of macrolide-resistant bacteria, suggesting that solithromycin may be a new drug for the treatment of M. genitalium infections (Jensen et al., 2014). Damiao Gouveia et al. (2018) found that Zoliflodacin was more effective than moxifloxacin against M. genitalium (p = 0. 009) through sensitivity evaluation in vitro, and there was no cross-reactivity between the two. So Zoliflodacin may also be used as a new drug for the treatment of M. genitalium infections. It is suggested that combination therapy can be used, or new drugs and alternative drugs can be developed to reduce drug resistance, shorten the course of treatment, and improve the cure rate. However, a recent prospective review from the Melbourne Sexual Health Centre showed that doxycycline combined with moxifloxacin did not improve treatment in the context of rising quinolone resistance (Vodstrcil et al., 2022). Therefore, the applicability of combination therapy for M. genitalium infection needs to be further verified. Several new treatments and drugs for M. genitalium infection are shown in Figure 2, panel B.
5. Conclusion
Mycoplasma genitalium infection is an independent risk factor for female cervicitis and PID. M. genitalium can be carried by sperm and other risk factors to the female vagina and colonize there, thus moving up the reproductive tract and causing swelling of the cervix, endometrium, and Fallopian tube epithelium, causing acute inflammatory reactions such as pelvic inflammatory disease and endometritis. Because of the lack of typical clinical manifestations, it is easy to be ignored. Untreated or delayed treatment leads to further transformation of inflammation into chronic long-term infection, resulting in impaired tubal function or blockage, which could cause infertility, ectopic pregnancy, premature birth, and other reproductive diseases. For pregnant women, M. genitalium infection can increase the incidence of PID after the termination of pregnancy, and late intravaginal colonization with M. genitalium can increase the incidence of preterm delivery. Based on limited studies in recent years, M. genitalium may to play a role in the development of ovarian cancer and in the acquisition and persistence of high-risk HPV, but the current findings are inconsistent.
Mycoplasma genitalium plays a vital role in inflammatory diseases of the female reproductive tract. However, the question of whether high-risk groups should be screened, how to identify M. genitalium infections early, whether timely treatment of M. genitalium infections can reduce the incidence of PID, EP, and infertility, and how to improve pregnancy outcomes requires further analysis. Additional epidemiological studies, prospective studies, and in vitro and animal studies are needed to confirm whether M. genitalium infection can cause tubal inflammation and reduce oncogene activity, leading to ovarian cancer. Whether M. genitalium infection increases the risk of high-risk HPV infection and affects the natural history of HPV infection by initiating cellular abnormalities remains to be further explored.
Author contributions
JY, YZ, and JH prepared and wrote the original draft. HL, XS, TG, and JW provided critical revisions for this review. ZY and ZD contributed to the interpretation and editing of the manuscript. JH is responsible for the concept and final revision of the manuscript. All authors contributed to the review and approved the submitted manuscript.
Funding
This work was supported by the Scientific Research Project of Hunan Provincial Health Committee (Grant No. 20201915), the Clinical Medical Technology Innovation Guidance Project of Hunan Province (Grant No. 2020SK51901), the Emergency special project of epidemic prevention and control of COVID-19 pneumonia in the University of South China (Grant No. 12), the Hengyang Science and Technology Planning Project (Grant No. 202010021604 and 202250045307), and the Natural Science Foundation of Hunan Province (No. 2022JJ40406).
Acknowledgments
We are grateful to Zeng Yi-Hua, Institute of Pathogenic Biology, University of South China, for valuable comments and amendments to this review and the figure is created with BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ah-Kit, X., Hoarau, L., Graesslin, O., and Brun, J. L. (2019). Follow-up and counselling after pelvic inflammatory disease: CNGOF and SPILF pelvic inflammatory diseases guidelines. Gynecol. Obstet. Fertil. Senol. 47, 458–464. doi: 10.1016/j.gofs.2019.03.009
Ajani, T. A., Oluwasola, T. A. O., Ajani Bakare, R., and A Ajani, M., Department of Medical Microbiology, University College Hospital, Ibadan, Nigeria, Department of Histopathology, Babcock University, Ilishan-Remo, Ogun State, Nigeria, Department of Medical Microbiology, College of Medicine, University of Ibadan, Ibadan, Nigeria (2017). The prevalence of, and risk factors for, Mycoplasma genitalium infection among infertile women in Ibadan: A cross-sectional study. Int. J. Reprod. Biomed. 15, 613–618. doi: 10.29252/ijrm.15.10.3
Aparicio, D., Scheffer, M. P., Marcos-Silva, M., Vizarraga, D., Sprankel, L., Ratera, M., et al. (2020). Structure and mechanism of the nap adhesion complex from the human pathogen Mycoplasma genitalium. Nat. Commun. 11:2877. doi: 10.1038/s41467-020-16511-2
Aparicio, D., Torres-Puig, S., Ratera, M., Querol, E., Pinol, J., Pich, O. Q., et al. (2018). Mycoplasma genitalium adhesin P110 binds sialic-acid human receptors. Nat. Commun. 9:4471. doi: 10.1038/s41467-018-06963-y
Ashshi, A. M., Batwa, S. A., Kutbi, S. Y., Malibary, F. A., Batwa, M., and Refaat, B. (2015). Prevalence of 7 sexually transmitted organisms by multiplex real-time PCR in fallopian tube specimens collected from Saudi women with and without ectopic pregnancy. BMC Infect. Dis. 15:569. doi: 10.1186/s12879-015-1313-1
Averbach, S. H., Hacker, M. R., Yiu, T., Modest, A. M., Dimitrakoff, J., and Ricciotti, H. A. (2013). Mycoplasma genitalium and preterm delivery at an urban community health center. Int. J. Gynaecol. Obstet. 123, 54–57. doi: 10.1016/j.ijgo.2013.06.005
Baczynska, A., Friis Svenstrup, H., Fedder, J., Birkelund, S., and Christiansen, G. (2005). The use of enzyme-linked immunosorbent assay for detection of mycoplasma hominis antibodies in infertile women serum samples. Hum. Reprod. 20, 1277–1285. doi: 10.1093/humrep/deh780
Baczynska, A., Funch, P., Fedder, J., Knudsen, H. J., Birkelund, S., and Christiansen, G. (2007). Morphology of human fallopian tubes after infection with Mycoplasma genitalium and mycoplasma hominis--in vitro organ culture study. Hum. Reprod. 22, 968–979. doi: 10.1093/humrep/del455
Balkus, J. E., Manhart, L. E., Jensen, J. S., Anzala, O., Kimani, J., Schwebke, J., et al. (2018). Mycoplasma genitalium infection in Kenyan and US women. Sex. Transm. Dis. 45, 514–521. doi: 10.1097/OLQ.0000000000000799
Baumann, L., Cina, M., Egli-Gany, D., Goutaki, M., Halbeisen, F. S., Lohrer, G. R., et al. (2018). Prevalence of Mycoplasma genitalium in different population groups: systematic review and meta-analysis. Sex. Transm. Infect. 94, 255–262. doi: 10.1136/sextrans-2017-053384
Bjartling, C., Osser, S., and Persson, K. (2010). The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG 117, 361–364. doi: 10.1111/j.1471-0528.2009.02455.x
Bjartling, C., Osser, S., and Persson, K. (2012). Mycoplasma genitalium in cervicitis and pelvic inflammatory disease among women at a gynecologic outpatient service. Am. J. Obstet. Gynecol. 206:476.e1. doi: 10.1016/j.ajog.2012.02.036
Borchsenius, S. N., Daks, A., Fedorova, O., Chernova, O., and Barlev, N. A. (2018). Effects of mycoplasma infection on the host organism response via p53/NF-kappaB signaling. J. Cell. Physiol. 234, 171–180. doi: 10.1002/jcp.26781
Brun, J. L., Graesslin, O., Fauconnier, A., Verdon, R., Agostini, A., Bourret, A., et al. (2016). Updated French guidelines for diagnosis and management of pelvic inflammatory disease. Int. J. Gynaecol. Obstet. 134, 121–125. doi: 10.1016/j.ijgo.2015.11.028
Carson, S. A., and Kallen, A. N. (2021). Diagnosis and management of infertility: a review. JAMA 326, 65–76. doi: 10.1001/jama.2021.4788
Casillas-Vega, N., Morfin-Otero, R., Garcia, S., Llaca-Diaz, J., Rodriguez-Noriega, E., Camacho-Ortiz, A., et al. (2016). Sexually transmitted pathogens, coinfections and risk factors in patients attending obstetrics and gynecology clinics in Jalisco, Mexico. Salud Publica Mex 58, 437–445. doi: 10.21149/spm.v58i4.8024
Clausen, H. F., Fedder, J., Drasbek, M., Nielsen, P. K., Toft, B., Ingerslev, H. J., et al. (2001). Serological investigation of Mycoplasma genitalium in infertile women. Hum. Reprod. 16, 1866–1874. doi: 10.1093/humrep/16.9.1866
Cohen, C. R., Mugo, N. R., Astete, S. G., Odondo, R., Manhart, L. E., Kiehlbauch, J. A., et al. (2005). Detection of Mycoplasma genitalium in women with laparoscopically diagnosed acute salpingitis. Sex. Transm. Infect. 81, 463–466. doi: 10.1136/sti.2005.015701
Contini, C., Rotondo, J. C., Magagnoli, F., Maritati, M., Seraceni, S., Graziano, A., et al. (2018). Investigation on silent bacterial infections in specimens from pregnant women affected by spontaneous miscarriage. J. Cell. Physiol. 234, 100–107. doi: 10.1002/jcp.26952
Cox, C., Saxena, N., Watt, A. P., Gannon, C., McKenna, J. P., Fairley, D. J., et al. (2016). The common vaginal commensal bacterium Ureaplasma parvum is associated with chorioamnionitis in extreme preterm labor. J. Matern. Fetal Neonatal Med. 29, 3646–3651. doi: 10.3109/14767058.2016.1140734
Damiao Gouveia, A. C., Unemo, M., and Jensen, J. S. (2018). In vitro activity of zoliflodacin (ETX0914) against macrolide-resistant, fluoroquinolone-resistant and antimicrobial-susceptible Mycoplasma genitalium strains. J. Antimicrob. Chemother. 73, 1291–1294. doi: 10.1093/jac/dky022
Daubenspeck, J. M., Totten, A. H., Needham, J., Feng, M., Balish, M. F., Atkinson, T. P., et al. (2020). Mycoplasma genitalium biofilms contain poly-GlcNAc and contribute to antibiotic resistance. Front. Microbiol. 11:585524. doi: 10.3389/fmicb.2020.585524
De Carvalho, N. S., Palu, G., and Witkin, S. S. (2020). Mycoplasma genitalium, a stealth female reproductive tract. Eur. J. Clin. Microbiol. Infect. Dis. 39, 229–234. doi: 10.1007/s10096-019-03707-8
de Jong, A. S., Rahamat-Langendoen, J. C., van Alphen, P., Hilt, N., van Herk, C., Pont, S., et al. (2016). Large two-Centre study into the prevalence of Mycoplasma genitalium and trichomonas vaginalis in the Netherlands. Int. J. STD AIDS 27, 856–860. doi: 10.1177/0956462415596496
Dehon, P. M., and McGowin, C. L. (2017). The Immunopathogenesis of Mycoplasma genitalium infections in women: A narrative review. Sex. Transm. Dis. 44, 428–432. doi: 10.1097/OLQ.0000000000000621
Desdorf, R., Andersen, N. M., and Chen, M. (2021). Mycoplasma genitalium prevalence and macrolide resistance-associated mutations and coinfection with chlamydia trachomatis in southern Jutland, Denmark. APMIS 129, 706–710. doi: 10.1111/apm.13174
Doyle, M., Vodstrcil, L. A., Plummer, E. L., Aguirre, I., Fairley, C. K., and Bradshaw, C. S. (2020). Nonquinolone options for the treatment of Mycoplasma genitalium in the era of increased resistance. Open Forum Infect. Dis. 7:ofaa291. doi: 10.1093/ofid/ofaa291
Duan, H., Qu, L., and Shou, C. (2014a). Activation of EGFR-PI3K-AKT signaling is required for mycoplasma hyorhinis-promoted gastric cancer cell migration. Cancer Cell Int. 14:135. doi: 10.1186/s12935-014-0135-3
Duan, H., Qu, L., and Shou, C. (2014b). Mycoplasma hyorhinis induces epithelial-mesenchymal transition in gastric cancer cell MGC803 via TLR4-NF-kappaB signaling. Cancer Lett. 354, 447–454. doi: 10.1016/j.canlet.2014.08.018
Dumke, R. (2022). Molecular tools for typing Mycoplasma pneumoniae and Mycoplasma genitalium. Front. Microbiol. 13:904494. doi: 10.3389/fmicb.2022.904494
Durukan, D., Doyle, M., Murray, G., Bodiyabadu, K., Vodstrcil, L., Chow, E. P. F., et al. (2020). Doxycycline and Sitafloxacin combination therapy for treating highly resistant Mycoplasma genitalium. Emerg. Infect. Dis. 26, 1870–1874. doi: 10.3201/eid2608.191806
Edwards, R. K., Ferguson, R. J., Reyes, L., Brown, M., Theriaque, D. W., and Duff, P. (2006). Assessing the relationship between preterm delivery and various microorganisms recovered from the lower genital tract. J. Matern. Fetal Neonatal Med. 19, 357–363. doi: 10.1080/00207170600712071
Fernandez-Huerta, M., Barbera, M. J., Serra-Pladevall, J., Esperalba, J., Martinez-Gomez, X., Centeno, C., et al. (2020). Mycoplasma genitalium and antimicrobial resistance in Europe: a comprehensive review. Int. J. STD AIDS 31, 190–197. doi: 10.1177/0956462419890737
Fortner, R. T., Terry, K. L., Bender, N., Brenner, N., Hufnagel, K., Butt, J., et al. (2019). Sexually transmitted infections and risk of epithelial ovarian cancer: results from the Nurses' health studies. Br. J. Cancer 120, 855–860. doi: 10.1038/s41416-019-0422-9
Fraser, C. M., Gocayne, J. D., White, O., Adams, M. D., Clayton, R. A., Fleischmann, R. D., et al. (1995). The minimal gene complement of Mycoplasma genitalium. Science 270, 397–404. doi: 10.1126/science.270.5235.397
Gaydos, C. A., Manhart, L. E., Taylor, S. N., Lillis, R. A., Hook, E. W. 3rd, Klausner, J. D., et al. (2019). Molecular testing for Mycoplasma genitalium in the United States: results from the AMES prospective multicenter clinical study. J. Clin. Microbiol. 57:e01125. doi: 10.1128/JCM.01125-19
Gedye, C., Cardwell, T., Dimopoulos, N., Tan, B. S., Jackson, H., Svobodova, S., et al. (2016). Mycoplasma infection alters cancer stem cell properties in vitro. Stem Cell Rev. Rep. 12, 156–161. doi: 10.1007/s12015-015-9630-8
Getman, D., Jiang, A., O'Donnell, M., and Cohen, S. (2016). Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J. Clin. Microbiol. 54, 2278–2283. doi: 10.1128/JCM.01053-16
Grzesko, J., Elias, M., Maczynska, B., Kasprzykowska, U., Tlaczala, M., and Goluda, M. (2009). Occurrence of Mycoplasma genitalium in fertile and infertile women. Fertil. Steril. 91, 2376–2380. doi: 10.1016/j.fertnstert.2008.03.060
Haggerty, C. L., Totten, P. A., Astete, S. G., Lee, S., Hoferka, S. L., Kelsey, S. F., et al. (2008). Failure of cefoxitin and doxycycline to eradicate endometrial Mycoplasma genitalium and the consequence for clinical cure of pelvic inflammatory disease. Sex. Transm. Infect. 84, 338–342. doi: 10.1136/sti.2008.030486
Haggerty, C. L., Totten, P. A., Astete, S. G., and Ness, R. B. (2006). Mycoplasma genitalium among women with nongonococcal, nonchlamydial pelvic inflammatory disease. Infect. Dis. Obstet. Gynecol. 2006:30184. doi: 10.1155/IDOG/2006/30184
Hakim, M. S., Annisa, L., Jariah, R. O. A., and Vink, C. (2021). The mechanisms underlying antigenic variation and maintenance of genomic integrity in Mycoplasma pneumoniae and Mycoplasma genitalium. Arch. Microbiol. 203, 413–429. doi: 10.1007/s00203-020-02041-4
Harrison, S. A., Olson, K. M., Ratliff, A. E., Xiao, L., Van Der Pol, B., Waites, K. B., et al. (2019). Mycoplasma genitalium coinfection in women with chlamydia trachomatis infection. Sex. Transm. Dis. 46, e101–e104. doi: 10.1097/OLQ.0000000000001028
Hart, T., Tang, W. Y., Mansoor, S. A. B., Chio, M. T. W., and Barkham, T. (2020). Mycoplasma genitalium in Singapore is associated with chlamydia trachomatis infection and displays high macrolide and fluoroquinolone resistance rates. BMC Infect. Dis. 20:314. doi: 10.1186/s12879-020-05019-1
Hitti, J., Garcia, P., Totten, P., Paul, K., Astete, S., and Holmes, K. K. (2010). Correlates of cervical Mycoplasma genitalium and risk of preterm birth among Peruvian women. Sex. Transm. Dis. 37, 81–85. doi: 10.1097/OLQ.0b013e3181bf5441
Hu, P. C., Schaper, U., Collier, A. M., Clyde, W. A. Jr., Horikawa, M., Huang, Y. S., et al. (1987). A Mycoplasma genitalium protein resembling the mycoplasma pneumoniae attachment protein. Infect. Immun. 55, 1126–1131. doi: 10.1128/iai.55.5.1126-1131.1987
Hussain, S. P., and Harris, C. C. (2007). Inflammation and cancer: an ancient link with novel potentials. Int. J. Cancer 121, 2373–2380. doi: 10.1002/ijc.23173
Idahl, A., Jurstrand, M., Olofsson, J. I., and Fredlund, H. (2015). Mycoplasma genitalium serum antibodies in infertile couples and fertile women. Sex. Transm. Infect. 91, 589–591. doi: 10.1136/sextrans-2015-052011
Idahl, A., Le Cornet, C., Gonzalez Maldonado, S., Waterboer, T., Bender, N., Tjonneland, A., et al. (2020). Serologic markers of Chlamydia trachomatis and other sexually transmitted infections and subsequent ovarian cancer risk: results from the EPIC cohort. Int. J. Cancer 147, 2042–2052. doi: 10.1002/ijc.32999
Idahl, A., Lundin, E., Jurstrand, M., Kumlin, U., Elgh, F., Ohlson, N., et al. (2011). Chlamydia trachomatis and Mycoplasma genitalium plasma antibodies in relation to epithelial ovarian tumors. Infect. Dis. Obstet. Gynecol. 2011:824627. doi: 10.1155/2011/824627
Iverson-Cabral, S. L., Astete, S. G., Cohen, C. R., and Totten, P. A. (2007). mgpB and mgpC sequence diversity in Mycoplasma genitalium is generated by segmental reciprocal recombination with repetitive chromosomal sequences. Mol. Microbiol. 66, 55–73. doi: 10.1111/j.1365-2958.2007.05898.x
Iwuji, C., Pillay, D., Shamu, P., Murire, M., Nzenze, S., Cox, L. A., et al. (2022). A systematic review of antimicrobial resistance in Neisseria gonorrhoeae and Mycoplasma genitalium in sub-Saharan Africa. J. Antimicrob. Chemother. 77, 2074–2093. doi: 10.1093/jac/dkac159
Jensen, J. S., Cusini, M., Gomberg, M., Moi, H., Wilson, J., and Unemo, M. (2022). 2021 European guideline on the management of Mycoplasma genitalium infections. J. Eur. Acad. Dermatol. Venereol. 36, 641–650. doi: 10.1111/jdv.17972
Jensen, J. S., Fernandes, P., and Unemo, M. (2014). In vitro activity of the new Fluoroketolide solithromycin (CEM-101) against macrolide-resistant and-susceptible Mycoplasma genitalium strains. Antimicrob. Agents Chemother. 58, 3151–3156. doi: 10.1128/AAC.02411-14
Jurstrand, M., Jensen, J. S., Magnuson, A., Kamwendo, F., and Fredlund, H. (2007). A serological study of the role of Mycoplasma genitalium in pelvic inflammatory disease and ectopic pregnancy. Sex. Transm. Infect. 83, 319–323. doi: 10.1136/sti.2007.024752
Kataoka, S., Yamada, T., Chou, K., Nishida, R., Morikawa, M., Minami, M., et al. (2006). Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J. Clin. Microbiol. 44, 51–55. doi: 10.1128/JCM.44.1.51-55.2006
Kayem, G., Doloy, A., Schmitz, T., Chitrit, Y., Bouhanna, P., Carbonne, B., et al. (2018). Antibiotics for amniotic-fluid colonization by Ureaplasma and/or mycoplasma spp. to prevent preterm birth: a randomized trial. PLoS One 13:e0206290. doi: 10.1371/journal.pone.0206290
Kim, M. K., Shin, S. J., Lee, H. M., Choi, H. S., Jeong, J., Kim, H., et al. (2019). Mycoplasma infection promotes tumor progression via interaction of the mycoplasmal protein p37 and epithelial cell adhesion molecule in hepatocellular carcinoma. Cancer Lett. 454, 44–52. doi: 10.1016/j.canlet.2019.04.007
Laumen, J. G. E., van Alphen, L. B., Maduna, L. D., Hoffman, C. M., Klausner, J. D., Medina-Marino, A., et al. (2021). Molecular epidemiological analysis of Mycoplasma genitalium shows low prevalence of azithromycin resistance and a well-established epidemic in South Africa. Sex. Transm. Infect. 97, 152–156. doi: 10.1136/sextrans-2019-054371
Li, C., Xl, S., Ke-ying, L., and Jun, H. (2022). Research progress of mycoplasma in tumor genesis and development. J. Pathogen Biol. 17, 356–360. doi: 10.13350/j.cjpb.220324
Lillis, R. A., Martin, D. H., and Nsuami, M. J. (2019). Mycoplasma genitalium infections in women attending a sexually transmitted disease clinic in New Orleans. Clin. Infect. Dis. 69, 459–465. doi: 10.1093/cid/ciy922
Lis, R., Rowhani-Rahbar, A., and Manhart, L. E. (2015). Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin. Infect. Dis. 61, 418–426. doi: 10.1093/cid/civ312
Liu, X., Rong, Z., and Shou, C. (2019). Mycoplasma hyorhinis infection promotes gastric cancer cell motility via beta-catenin signaling. Cancer Med. 8, 5301–5312. doi: 10.1002/cam4.2357
Lokken, E. M., Balkus, J. E., Kiarie, J., Hughes, J. P., Jaoko, W., Totten, P. A., et al. (2017). Association of recent bacterial vaginosis with acquisition of Mycoplasma genitalium. Am. J. Epidemiol. 186, 194–201. doi: 10.1093/aje/kwx043
Ma, C., Du, J., Dou, Y., Chen, R., Li, Y., Zhao, L., et al. (2021). The associations of genital mycoplasmas with female infertility and adverse pregnancy outcomes: a systematic review and meta-analysis. Reprod. Sci. 28, 3013–3031. doi: 10.1007/s43032-020-00399-w
Machalek, D. A., Tao, Y., Shilling, H., Jensen, J. S., Unemo, M., Murray, G., et al. (2020). Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect. Dis. 20, 1302–1314. doi: 10.1016/S1473-3099(20)30154-7
Macpherson, I., and Russell, W. (1966). Transformations in hamster cells mediated by mycoplasmas. Nature 210, 1343–1345. doi: 10.1038/2101343a0
Manhart, L. E., Critchlow, C. W., Holmes, K. K., Dutro, S. M., Eschenbach, D. A., Stevens, C. E., et al. (2003). Mucopurulent cervicitis and Mycoplasma genitalium. J. Infect. Dis. 187, 650–657. doi: 10.1086/367992
Martin, D. H., Manhart, L. E., and Workowski, K. A. (2017). Mycoplasma genitalium from basic science to public health: summary of the results from a National Institute of allergy and infectious Disesases technical consultation and consensus recommendations for future research priorities. J. Infect. Dis. 216, S427–S430. doi: 10.1093/infdis/jix147
Masha, S. C., Cools, P., Descheemaeker, P., Reynders, M., Sanders, E. J., and Vaneechoutte, M. (2018). Urogenital pathogens, associated with trichomonas vaginalis, among pregnant women in Kilifi, Kenya: a nested case-control study. BMC Infect. Dis. 18:549. doi: 10.1186/s12879-018-3455-4
McGowin, C. L., Annan, R. S., Quayle, A. J., Greene, S. J., Ma, L., Mancuso, M. M., et al. (2012). Persistent Mycoplasma genitalium infection of human endocervical epithelial cells elicits chronic inflammatory cytokine secretion. Infect. Immun. 80, 3842–3849. doi: 10.1128/IAI.00819-12
McGowin, C. L., Popov, V. L., and Pyles, R. B. (2009). Intracellular Mycoplasma genitalium infection of human vaginal and cervical epithelial cells elicits distinct patterns of inflammatory cytokine secretion and provides a possible survival niche against macrophage-mediated killing. BMC Microbiol. 9:139. doi: 10.1186/1471-2180-9-139
McGowin, C. L., Radtke, A. L., Abraham, K., Martin, D. H., and Herbst-Kralovetz, M. (2013). Mycoplasma genitalium infection activates cellular host defense and inflammation pathways in a 3-dimensional human endocervical epithelial cell model. J. Infect. Dis. 207, 1857–1868. doi: 10.1093/infdis/jit101
McGowin, C. L., Spagnuolo, R. A., and Pyles, R. B. (2010). Mycoplasma genitalium rapidly disseminates to the upper reproductive tracts and knees of female mice following vaginal inoculation. Infect. Immun. 78, 726–736. doi: 10.1128/IAI.00840-09
McGowin, C. L., and Totten, P. A. (2017). The unique microbiology and molecular pathogenesis of Mycoplasma genitalium. J. Infect. Dis. 216, S382–S388. doi: 10.1093/infdis/jix172
Moore, K. R., Tomar, M., Taylor, B. D., Gygax, S. E., Hilbert, D. W., and Baird, D. D. (2021). Mycoplasma genitalium and bacterial vaginosis-associated bacteria in a non-clinic-based sample of African American women. Sex. Transm. Dis. 48, 118–122. doi: 10.1097/OLQ.0000000000001275
Namiki, K., Goodison, S., Porvasnik, S., Allan, R. W., Iczkowski, K. A., Urbanek, C., et al. (2009). Persistent exposure to mycoplasma induces malignant transformation of human prostate cells. PLoS One 4:e6872. doi: 10.1371/journal.pone.0006872
Napierala Mavedzenge, S., and Weiss, H. A. (2009). Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS 23, 611–620. doi: 10.1097/QAD.0b013e328323da3e
Nolskog, P., Backhaus, E., Nasic, S., and Enroth, H. (2019). STI with Mycoplasma genitalium-more common than chlamydia trachomatis in patients attending youth clinics in Sweden. Eur. J. Clin. Microbiol. Infect. Dis. 38, 81–86. doi: 10.1007/s10096-018-3395-3
Noma, I. H. Y., Shinobu-Mesquita, C. S., Suehiro, T. T., Morelli, F., De Souza, M. V. F., Damke, E., et al. (2021). Association of Righ-Risk Human Papillomavirus and Ureaplasma parvum co-infections with increased risk of low-grade squamous intraepithelial cervical lesions. Asian Pac. J. Cancer Prev. 22, 1239–1246. doi: 10.31557/APJCP.2021.22.4.1239
Nye, M. B., Harris, A. B., Pherson, A. J., and Cartwright, C. P. (2020). Prevalence of Mycoplasma genitalium infection in women with bacterial vaginosis. BMC Womens Health 20:62. doi: 10.1186/s12905-020-00926-6
Oakeshott, P., Aghaizu, A., Hay, P., Reid, F., Kerry, S., Atherton, H., et al. (2010). Is Mycoplasma genitalium in women the "new chlamydia?" A community-based prospective cohort study. Clin. Infect. Dis. 51, 1160–1166. doi: 10.1086/656739
Oliphant, J., and Azariah, S. (2013). Cervicitis: limited clinical utility for the detection of Mycoplasma genitalium in a cross-sectional study of women attending a New Zealand sexual health clinic. Sex. Health 10, 263–267. doi: 10.1071/SH12168
Olson, E., Gupta, K., Van Der Pol, B., Galbraith, J. W., and Geisler, W. M. (2021). Mycoplasma genitalium infection in women reporting dysuria: A pilot study and review of the literature. Int. J. STD AIDS 32, 1196–1203. doi: 10.1177/09564624211030040
Papathanasiou, A., Djahanbakhch, O., Saridogan, E., and Lyons, R. A. (2008). The effect of interleukin-6 on ciliary beat frequency in the human fallopian tube. Fertil. Steril. 90, 391–394. doi: 10.1016/j.fertnstert.2007.07.1379
Paton, G. R., Jacobs, J. P., and Perkins, F. T. (1965). Chromosome changes in human diploid-cell cultures infected with mycoplasma. Nature 207, 43–45. doi: 10.1038/207043a0
Payne, M. S., Ireland, D. J., Watts, R., Nathan, E. A., Furfaro, L. L., Kemp, M. W., et al. (2016). Ureaplasma parvum genotype, combined vaginal colonisation with Candida albicans, and spontaneous preterm birth in an Australian cohort of pregnant women. BMC Pregnancy Childbirth 16:312. doi: 10.1186/s12884-016-1110-x
Perry, M. D., Jones, S., Bertram, A., de Salazar, A., Barrientos-Duran, A., Schiettekatte, G., et al. (2022). The prevalence of Mycoplasma genitalium (MG) and Trichomonas vaginalis (TV) at testing centers in Belgium, Germany, Spain, and the UK using the cobas TV/MG molecular assay. Eur. J. Clin. Microbiol. Infect. Dis. 42, 43–52. doi: 10.1007/s10096-022-04521-5
Piao, J., Lee, E. J., and Lee, M. (2020). Association between pelvic inflammatory disease and risk of ovarian cancer: an updated meta-analysis. Gynecol. Oncol. 157, 542–548. doi: 10.1016/j.ygyno.2020.02.002
Pineiro, L., Idigoras, P., and Cilla, G. (2019). Molecular typing of Mycoplasma genitalium-positive specimens discriminates between persistent and recurrent infections in cases of treatment failure and supports contact tracing. Microorganisms 7:609. doi: 10.3390/microorganisms7120609
Read, T. R. H., Fairley, C. K., Murray, G. L., Jensen, J. S., Danielewski, J., Worthington, K., et al. (2019). Outcomes of resistance-guided sequential treatment of Mycoplasma genitalium infections: A prospective evaluation. Clin. Infect. Dis. 68, 554–560. doi: 10.1093/cid/ciy477
Read, T. R. H., Jensen, J. S., Fairley, C. K., Grant, M., Danielewski, J. A., Su, J., et al. (2018). Use of Pristinamycin for macrolide-resistant Mycoplasma genitalium infection. Emerg. Infect. Dis. 24, 328–335. doi: 10.3201/eid2402.170902
Refaat, B., Ashshi, A. M., Batwa, S. A., Ahmad, J., Idris, S., Kutbi, S. Y., et al. (2016). The prevalence of chlamydia trachomatis and Mycoplasma genitalium tubal infections and their effects on the expression of IL-6 and leukaemia inhibitory factor in fallopian tubes with and without an ectopic pregnancy. Innate Immun. 22, 534–545. doi: 10.1177/1753425916662326
Rekha, S., Nooren, M., Kalyan, S., Mohan, M., Bharti, M., Monika, R., et al. (2019). Occurrence of Mycoplasma genitalium in the peritoneal fluid of fertile and infertile women with detailed analysis among infertile women. Microb. Pathog. 129, 183–186. doi: 10.1016/j.micpath.2019.02.006
Rowlands, S., Danielewski, J. A., Tabrizi, S. N., Walker, S. P., and Garland, S. M. (2017). Microbial invasion of the amniotic cavity in midtrimester pregnancies using molecular microbiology. Am. J. Obstet. Gynecol. 217, 71 e71–71 e75. doi: 10.1016/j.ajog.2017.02.051
Roxby, A. C., Yuhas, K., Farquhar, C., Bosire, R., Mbori-Ngacha, D., Richardson, B. A., et al. (2019). Mycoplasma genitalium infection among HIV-infected pregnant African women and implications for mother-to-child transmission of HIV. AIDS 33, 2211–2217. doi: 10.1097/QAD.0000000000002335
Roy, A., Dadwal, R., Yadav, R., Singh, P., Krishnamoorthi, S., Dasgupta, A., et al. (2021). Association of Chlamydia trachomatis, Neisseria gonorrhoeae, mycoplasma genitalium and Ureaplasma species infection and organism load with cervicitis in north Indian population. Lett. Appl. Microbiol. 73, 506–514. doi: 10.1111/lam.13520
Sena, A. C., Lee, J. Y., Schwebke, J., Philip, S. S., Wiesenfeld, H. C., Rompalo, A. M., et al. (2018). A silent epidemic: the prevalence, incidence and persistence of Mycoplasma genitalium among young, asymptomatic high-risk women in the United States. Clin. Infect. Dis. 67, 73–79. doi: 10.1093/cid/ciy025
Shilling, H. S., Garland, S. M., Costa, A. M., Marceglia, A., Fethers, K., Danielewski, J., et al. (2022). Chlamydia trachomatis and Mycoplasma genitalium prevalence and associated factors among women presenting to a pregnancy termination and contraception clinic, 2009-2019. Sex. Transm. Infect. 98, 115–120. doi: 10.1136/sextrans-2020-054695
Shipitsyna, E., Khusnutdinova, T., Budilovskaya, O., Krysanova, A., Shalepo, K., Savicheva, A., et al. (2020). Bacterial vaginosis-associated vaginal microbiota is an age-independent risk factor for chlamydia trachomatis, Mycoplasma genitalium and Trichomonas vaginalis infections in low-risk women, St. Petersburg, Russia. Eur. J. Clin. Microbiol. Infect. Dis. 39, 1221–1230. doi: 10.1007/s10096-020-03831-w
Shipitsyna, E., and Unemo, M. (2020). A profile of the FDA-approved and CE/IVD-marked Aptima Mycoplasma genitalium assay (Hologic) and key priorities in the management of M. genitalium infections. Expert. Rev. Mol. Diagn. 20, 1063–1074. doi: 10.1080/14737159.2020.1842198
Soni, S., Horner, P., Rayment, M., Pinto-Sander, N., Naous, N., Parkhouse, A., et al. (2019). British Association for Sexual Health and HIV national guideline for the management of infection with Mycoplasma genitalium (2018). Int. J. STD AIDS 30, 938–950. doi: 10.1177/0956462419825948
Stafford, I. A., Hummel, K., Dunn, J. J., Muldrew, K., Berra, A., Kravitz, E. S., et al. (2021). Retrospective analysis of infection and antimicrobial resistance patterns of Mycoplasma genitalium among pregnant women in the southwestern USA. BMJ Open 11:e050475. doi: 10.1136/bmjopen-2021-050475
Svenstrup, H. F., Fedder, J., Abraham-Peskir, J., Birkelund, S., and Christiansen, G. (2003). Mycoplasma genitalium attaches to human spermatozoa. Hum. Reprod. 18, 2103–2109. doi: 10.1093/humrep/deg392
Sweeney, E. L., Bradshaw, C. S., Murray, G. L., and Whiley, D. M. (2022). Individualised treatment of Mycoplasma genitalium infection-incorporation of fluoroquinolone resistance testing into clinical care. Lancet Infect. Dis. 22, e267–e270. doi: 10.1016/S1473-3099(21)00629-0
Sweeney, E. L., Trembizki, E., Bletchly, C., Bradshaw, C. S., Menon, A., Francis, F., et al. (2019). Levels of Mycoplasma genitalium antimicrobial resistance differ by both region and gender in the State of Queensland, Australia: implications for treatment guidelines. J. Clin. Microbiol. 57:555. doi: 10.1128/JCM.01555-18
Taylor, B. D., Zheng, X., O'Connell, C. M., Wiesenfeld, H. C., Hillier, S. L., and Darville, T. (2018). Risk factors for Mycoplasma genitalium endometritis and incident infection: a secondary data analysis of the T cell response against chlamydia (TRAC) study. Sex. Transm. Infect. 94, 414–420. doi: 10.1136/sextrans-2017-053376
Taylor-Robinson, D., and Jensen, J. S. (2011). Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin. Microbiol. Rev. 24, 498–514. doi: 10.1128/CMR.00006-11
Tovo, S. F., Zohoncon, T. M., Dabire, A. M., Ilboudo, R., Tiemtore, R. Y., Obiri-Yeboah, D., et al. (2021). Molecular epidemiology of human papillomaviruses, Neisseria gonorrhoeae, chlamydia trachomatis and Mycoplasma genitalium among female sex Workers in Burkina Faso: prevalence, coinfections and drug resistance genes. Trop Med. Infect. Dis. 6:90. doi: 10.3390/tropicalmed6020090
Trabert, B., Waterboer, T., Idahl, A., Brenner, N., Brinton, L. A., Butt, J., et al. (2019). Antibodies against Chlamydia trachomatis and ovarian cancer risk in two independent populations. J. Natl. Cancer Inst. 111, 129–136. doi: 10.1093/jnci/djy084
Trent, M., Coleman, J. S., Hardick, J., Perin, J., Tabacco, L., Huettner, S., et al. (2018). Clinical and sexual risk correlates of Mycoplasma genitalium in urban pregnant and non-pregnant young women: cross-sectional outcomes using the baseline data from the Women's BioHealth study. Sex. Transm. Infect. 94, 411–413. doi: 10.1136/sextrans-2017-053367
Tully, J. G., Taylor-Robinson, D., Cole, R. M., and Rose, D. L. (1981). A newly discovered mycoplasma in the human urogenital tract. Lancet 1, 1288–1291. doi: 10.1016/s0140-6736(81)92461-2
Van Der Pol, B., Waites, K. B., Xiao, L., Taylor, S. N., Rao, A., Nye, M., et al. (2020). Mycoplasma genitalium detection in urogenital specimens from symptomatic and asymptomatic men and women by use of the cobas TV/MG test. J. Clin. Microbiol. 58:2124. doi: 10.1128/JCM.02124-19
van der Schalk, T. E., Braam, J. F., and Kusters, J. G. (2020). Molecular basis of antimicrobial resistance in Mycoplasma genitalium. Int. J. Antimicrob. Agents 55:105911. doi: 10.1016/j.ijantimicag.2020.105911
Van Dijck, C., De Baetselier, I., Cuylaerts, V., Buyze, J., Laumen, J., Vuylsteke, B., et al. (2022). Gonococcal bacterial load in PrEP users with Mycoplasma genitalium coinfection. Int. J. STD AIDS 33, 129–135. doi: 10.1177/09564624211048678
Vodstrcil, L. A., Plummer, E. L., Doyle, M., Murray, G. L., Bodiyabadu, K., Jensen, J. S., et al. (2022). Combination therapy for Mycoplasma genitalium, and new insights into the utility of parC mutant detection to improve cure. Clin. Infect. Dis. 75, 813–823. doi: 10.1093/cid/ciab1058
Wang, Y., Hong, S., Mu, J., Wang, Y., Lea, J., Kong, B., et al. (2019). Tubal origin of "ovarian" low-grade serous carcinoma: A gene expression profile study. J. Oncol. 2019:8659754. doi: 10.1155/2019/8659754
Wang, R., Trent, M. E., Bream, J. H., Nilles, T. L., Gaydos, C. A., Carson, K. A., et al. (2022). Mycoplasma genitalium infection is not associated with genital tract inflammation among adolescent and young adult women in Baltimore, Maryland. Sex Transm. Dis. 49, 139–144. doi: 10.1097/OLQ.0000000000001524
Workowski, K. A., Bachmann, L. H., Chan, P. A., Johnston, C. M., Muzny, C. A., Park, I., et al. (2021). Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm. Rep. 70, 1–187. doi: 10.15585/mmwr.rr7004a1
Yan, X., Xiang, H., Tao, Y., Xu, Z., Xuelong, S., Jinyan, Q., et al. (2022). Mycoplasma genitalium infection rate among pregnancy females in China: a meta-analysis. Chin. J. Evid. Med. 22, 90–95. doi: 10.7507/1672-2531.202111133
Ye, H., Song, T., Zeng, X., Li, L., Hou, M., and Xi, M. (2018). Association between genital mycoplasmas infection and human papillomavirus infection, abnormal cervical cytopathology, and cervical cancer: a systematic review and meta-analysis. Arch. Gynecol. Obstet. 297, 1377–1387. doi: 10.1007/s00404-018-4733-5
Yin, Y. P., Li, H. M., Xiang, Z., Liang, G. J., Shi, M. Q., Zhou, Y. J., et al. (2013). Association of sexually transmitted infections with high-risk human papillomavirus types: a survey with 802 female sex workers in China. Sex. Transm. Dis. 40, 493–495. doi: 10.1097/OLQ.0b013e31828b32b8
Zarei, O., Rezania, S., and Mousavi, A. (2013). Mycoplasma genitalium and cancer: a brief review. Asian Pac. J. Cancer Prev. 14, 3425–3428. doi: 10.7314/apjcp.2013.14.6.3425
Zella, D., and Gallo, R. C. (2021). Viruses and bacteria associated with cancer: an overview. Viruses 13:1039. doi: 10.3390/v13061039
Zhang, S., Sun, Q., Jiang, X., and Gao, F. (2018). Clinical significance of expression of hsa-mir-1247 and hsa-mir-1269a in ectopic pregnancy due to salpingitis. Exp. Ther. Med. 15, 4901–4905. doi: 10.3892/etm.2018.5998
Keywords: Mycoplasma genitalium, female reproductive diseases, inflammation, tumor, treatment
Citation: Yu J, Zhou Y, Luo H, Su X, Gan T, Wang J, Ye Z, Deng Z and He J (2023) Mycoplasma genitalium infection in the female reproductive system: Diseases and treatment. Front. Microbiol. 14:1098276. doi: 10.3389/fmicb.2023.1098276
Edited by:
Nan Shen, Shanghai Jiao Tong University, ChinaReviewed by:
Luis Piñeiro, Donostia University Hospital, SpainRebecca Ann Lillis, Louisiana State University, United States
Copyright © 2023 Yu, Zhou, Luo, Su, Gan, Wang, Ye, Deng and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun He, ✉ anVuaGUyMDA4QDE2My5jb20=; Zhongliang Deng, ✉ ZHpsMDIxMDEyQDE2My5jb20=
 Jianwei Yu1
Jianwei Yu1 Yan Zhou
Yan Zhou Jun He
Jun He