
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 17 February 2023
Sec. Extreme Microbiology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1096826
This article is part of the Research TopicAdaptation of Halophilic/Halotolerant Microorganisms and Their ApplicationsView all 12 articles
 Yalpi Karthik1
Yalpi Karthik1 Manjula Ishwara Kalyani1*
Manjula Ishwara Kalyani1* Srinivasa Krishnappa2
Srinivasa Krishnappa2 Ramakrishna Devappa3
Ramakrishna Devappa3 Chengeshpur Anjali Goud4
Chengeshpur Anjali Goud4 Krishnaveni Ramakrishna5
Krishnaveni Ramakrishna5 Muneeb Ahmad Wani6
Muneeb Ahmad Wani6 Mohamed Alkafafy7
Mohamed Alkafafy7 Maram Hussen Abduljabbar8
Maram Hussen Abduljabbar8 Amal S. Alswat9
Amal S. Alswat9 Samy M. Sayed10
Samy M. Sayed10 Muntazir Mushtaq11,12*
Muntazir Mushtaq11,12*The Glutamicibacter group of microbes is known for antibiotic and enzyme production. Antibiotics and enzymes produced by them are important in the control, protection, and treatment of chronic human diseases. In this study, the Glutamicibacter mysorens (G. mysorens) strain MW647910.1 was isolated from mangrove soil in the Mangalore region of India. After optimization of growth conditions for G. mysorens on starch casein agar media, the micromorphology of G. mysorens was found to be spirally coiled spore chain, each spore visualized as an elongated cylindrical hairy appearance with curved edges visualized through Field Emission Scanning Electron Microscopy (FESEM) analysis. The culture phenotype with filamentous mycelia, brown pigmentation, and ash–colored spore production was observed. The intracellular extract of G. mysorens characterized through GCMS analysis detected bioactive compounds reported for pharmacological applications. The majority of bioactive compounds identified in intracellular extract when compared to the NIST library revealed molecular weight ranging below 1kgmole−1. The Sephadex G-10 could result in 10.66 fold purification and eluted peak protein fraction showed significant anticancer activity on the prostate cancer cell line. Liquid Chromatography–Mass Spectrometry (LC–MS) analysis revealed Kinetin-9-ribose and Embinin with a molecular weight below 1 kDa. This study showed small molecular weight bioactive compounds produced from microbial origin possess dual roles, acting as antimicrobial peptides (AMPs) and anticancer peptides (ACPs). Hence, the bioactive compounds produced from microbial origin are a promising source of future therapeutics.
The environmental conditions in a particular ecosystem play an important role in determining biodiversity composition. High tides, hypersaline water, significant temperature fluctuations, and optimal flora and fauna diversity are just a few of the distinctive environmental characteristics of the mangrove ecosystem (Karthik et al., 2020). Microbes can better adapt to any extreme environment in these vulnerable situations. The isolation of bioactive chemicals will be greatly aided by this habitat (Alongi, 2015).
Actinomyces word derived from the words “atkis” which means “a ray” and “mykes” which means “fungi” are filamentous, Gram–positive bacteria distinguished by different coloration and spore production at maturity (Chater, 2006). Actinomyces share the characteristics of bacteria and fungi. The Actinomyces group’s genetic and environmental flexibility facilitates the development of worthwhile bioactive substances. Actinomyces contribute more in enzyme production to pharmacological industries for the treatment, and prevention of various ailments (Chater, 2013).
The pharmaceutical industry is constantly looking for drugs with innovative structures and new modes of action as a result of the rise in antibiotic resistance. There are still many environmental niches to investigate as potential sources of antibiotics (Karthik and Kalyani, 2021, 2022). One such Actinomyces group Glutamicibacter genus is broadly utilized in the control, treatment, and prevention of diseases through the production of bioactive compounds, widely used as antibiotics (Phuong and Diep, 2020), anti-tumor, anti-tubercular (Khusro et al., 2020), anti-helminthic, anti-diabetic, anti-oxidant from an exo-polysaccharide (Xiong et al., 2020; Fukuda and Kono, 2021; Hidri et al., 2022), anti-angiogenic, growth hormones (Qin et al., 2018; Hidri et al., 2022), immuno-suppressors, neuritogenic (Tang et al., 2021), anti-inflammatory (Hui et al., 2021), anti-algal (Agamennone et al., 2018), anti-fungal with enzymatic source (Mihooliya et al., 2017; Asif et al., 2020), anti-proliferative (Baig et al., 2021), anti-parasitic, anti-malarial, anti-viral, anti-bacterial and many more biological applications (Nishioka and Katayama, 1978; Renner et al., 1999; Fernebro, 2011; Janardhan et al., 2014; Desouky et al., 2015; Abd-Elnaby et al., 2016).
The various species of genus Glutamicibacter shown huge biological importance as detailed above. Whereas G. creatinolyticus shown resistance to antibiotics as well as heavy metals (copper, arsenic, cadmium, cobalt, zinc, and chromium; Santos et al., 2020). The G. arilaitensis produced pink colored pigment and coprophorphyrin binds zinc and regulates in cheese rinds (Cleary et al., 2018). Another Gluamicibacter sps. Possessing genes that regulates the growth of plant under saline conditions, cold adaptation, efficient degradation and chitinase enzyme producing genes which help in control the growth of pathogenic bacteria (Borker et al., 2021; Fu et al., 2021). While G. nicotianae involved in heavy metals degradation (Wang et al., 2021). The G. mishrai and halophytocola isolated from Andaman sea sample. Genes involved in cell wall biogenesis, replication, recombination, repair mechanism and amino acid metabolism along possess important role in physiology and behavior of insects (Qin et al., 2018; Das et al., 2020; Wang W, et al., 2022).
Antimicrobial peptides (AMPs) are peptides with antimicrobial properties. In multicellular organisms, these positively charged host defense molecules, or AMPs, serve as the initial line of protection. Many AMP’s from both prokaryotes and eukaryotes have been categorized (Brandenburg et al., 2012; Desriac et al., 2013). Several genera of AMP’s -producing microorganisms have been discovered, including bacteriocins produced by Leuconostoc gelidum, Enterococcus faecium, and other species (Juturu and Wu, 2018; Khodaei and Sh, 2018). Microcins A and B, antimicrobial bacteriocins derived from Streptomyces pluripotens, have been shown to be effective against Escherichia coli, Salmonella typhimurium, Staphylococcus aureus, and Listeria monocytogenes (Collin and Maxwell, 2019; Kurnianto et al., 2021).These AMPs have been found to be effective in the treatment of a broad range of ailments (Sugrue et al., 2019; Karthik et al., 2020; Khadayat et al., 2020; Zhang et al., 2020). AMP’s are peptides derived from microbes that exhibit antimicrobial activity. AMPs have been shown to target cell walls or cell membranes, permitting them to penetrate cells and affect vital components while inhibiting growth (Desriac et al., 2013; Wang et al., 2020). As a result of their target-specific activity against resistant microbial species, AMPs are thought to be anti–microbial compounds.
Peptides with selective action and non-selective activity, i.e., those that have activity against bacteria, cancer cells, and healthy cells, can be categorized as having antitumor activity in Hoskin and Ramamoorthy’s investigations (Hoskin and Ramamoorthy, 2008).The peptides have antibacterial and anticancer properties, but not against normal cells. Cecropins, buforins, and magainins, among other peptides, have demonstrated anticancer effects without harming normal eukaryotic cells (Cruciani et al., 1991; Cho et al., 2009). These studies go into great detail and provide a compelling case for the fact that many peptides have biological activity in a variety of dimensions and properties and can possess dual activity as AMPs and ACPs. Therefore, we are searching for mangrove soil Actinomyces in the Mangalore region to isolate and characterize bioactive peptides that can function as both AMPs and ACPs.
In our previous study, we reported the detailed procedures for isolation, microscopic and macroscopic characters, identified as Glutamicibacter mysorens with GenBank accession number MW647910.1, the intracellular protein; extraction, estimation, along with their potential antimicrobial activity was observed against test pathogens Salmonella typhimurium (ATCC23564), Staphylococcus aureus (ATCC6538P),Bacillus cereus (ATCC10876), Proteus vulgaris (ATCC13315), and Pseudomonas aeruginosa (ATCC9027) cultures. The protein was characterized through LCMS and SDS PAGE techniques and small peptides were detected (Karthik and Kalyani, 2021).
In this study, the optimization of suitable growth media for G. mysorens and its micromorphology were analyzed using FESEM. The isolation of intracellular extract of G. mysorens was characterized through GCMS and LCMS. These GCMS studies revealed a large number of small bioactive compounds that possess significant biological activities are discussed. Whereas the LCMS studies resulted in the detection of low molecular weight Kinetin-9-ribose and Embinin showed significant anti-tumor potential against PC3 cell line in comparison to standard cisplatin drug.
Soil samples were collected from Mangroves soil in Mangalore, Dakshina Kannada. Jeppinamogaru (JPMU) is located at 12°50′31.4”N 74°51′36.4″E. At the collecting site, the soil was brown with a powdery texture, and environmental parameters; the temperature of 21°C and pH of 7.2 was recorded. The collected samples were shifted to the Molecular Research Laboratory (MRL), Department of Microbiology, Jnana Kaveri, Mangalore University, India, in aseptic containers. To prevent fungal and bacterial growth, the soil sample was pre-heated for 2 h at 60°C prior to serial dilution and isolation (Mohan et al., 2013; Sridevi et al., 2015; Sapkota et al., 2020).
The isolated G. mysorens strain was subjected to FESEM analysis at different objectives distances; spore structure (1 and 2 μm) mycelial structure (10 and 20 μm) to visualize the complete micromorphological structures. The sequencing and identification of G. mysorens are reported by Karthik and Kalyani (2021).
The G. mysorens strain was grown in SCN broth for 7 days at 30 ± 2°C with continuous shaking at 100 rpm. Centrifugation at 7000 rpm for 8 min separated the cultured biomass cells, which were then washed twice using phosphate-buffered saline devoid of Mg2+ and Ca2+ and centrifuged again. The cells were then re-suspended in 10 ml of chilled acetone for 5 min before being centrifuged at 7,000 rpm for 8 min. The intracellular extract was incubated for 2 min with 1.0 ml of 1% SDS after the traces of acetone was removed with a nitrogen stream (Bhaduri and Demchick, 1983). This intracellular extract was characterized using spectrometric (LCMS, GCMS) tools along with a comparison to the NIST library.
The following equipment was assessed for the GC–MS studies of G. mysorens intracellular extract: a PerkinElmer Clarus 680 Gas Chromatograph and a PerkinElmer Clarus SQ 8C Mass Spectrometer. A PerkinElmer Elite-5MS standard column with dimensions of 30 m long x 0.250 mm inner diameter x 1 micron (60–350°C) is utilized in the equipment. With an equivalence ratio of 10:1, the injected volume of 2 μl was completely run for 26.6 min. Helium is used as the carrier gas, with a flow rate of 2 ml/min. The source temperature was set to 230 degrees Celsius, and the inlet temperature was set to 250 degrees Celsius. The oven temperature was initially set to 80°C with a hold time of 2.0 min; ramp1 was set to 10.0 /min to 150°C with a hold time of 1.0 min; and ramp2 was set to 15.0 /min to 250°C with a hold time of 10.0 min. The components were identified by comparing them to those contained in the NIST computer library, which was linked to the GC–MS apparatus, and the results were published.
The microbial proteins were purified using SephadexG-10. For 5 h, the Sephadex G-10 was allowed to swell in excess of dH2O in a boiling water bath. After decanting the gel to remove fines, it was equilibrated with 0.05 M sodium phosphate buffer, pH 7.0. Under gravity, the gel was packed into a 1.0 cm × 110.0 cm column. At a flow rate of 10 ml/h, the column was standardized with two-bed volumes of phosphate buffer of concentration 0.05 M, pH 7.0. The 20 mg of isolate protein sample was loaded onto the gel, eluted with 0.05 M sodium phosphate buffer, pH 7.0, and 2.0 ml fractions were collected and further analyzed (Bharadwaj et al., 2018).
The Sephadex G-10 peak fraction was analyzed using LC–MS, model Synapt G2, an analytical chemistry technique that combines the physical separation capabilities of liquid chromatography with mobile phases A: 0.1% Formic acid in Water and mobile phase B: 0.1% Formic acid in Acetonitrile with the mass analysis capabilities of mass spectrometry (MS) an Agilent 1100 LC system with a vacuum degasser, A BEH C18, 50 mm × 1.0 mm, 1.7 μm C18 column (Waters, United States) was used to achieve chromatographic separation in comparison to the NIST computer library.
Prostate cancer cells (PC3) procured from NCCS Pune; were harvested in T-25 flasks for the in vitro studies. PC3 cells were trypsinized and aspirated into a 5 ml centrifuge tube. After centrifugation at 300 rpm for 10 min, the cell pellet was separated. The cell count was adjusted using DMEM HG medium so that 200 μl of suspension contained approximately 10,000 cells. In an ESCO model CLM170B-8-UV CO2 incubator, a 200 μl cell suspension was added to each well of the 96-well microtiter plate, and the plate was incubated for 24 h at 37°C and 5% CO2 atmosphere. After 24 h, the spent medium was aspirated. In each well, 200 μl of various test drug concentrations and the standard drug cisplatin were added. After that, the plates were incubated for 24 h at 37°C and 5% CO2. The drug-containing media was aspirated after the plate was removed from the incubator. The plate was then incubated for 3 h at 37\u00B0C and 5% CO2 atmosphere with 200 μl of medium containing 10% MTT reagent in each well to achieve a final concentration of 0.5 mg/ml. The culture medium was completely removed without disturbing the formed crystals. To solubilize the formed formazan, the plate was gently shaken in a gyrator shaker with 100 μl of solubilization solution (DMSO). The absorbance was read at 570 and 630 nm using the microplate reader of a Multiskan sky ELISA spectrophotometer.
The Mangrove region in Jeppinamogaru located at Mangalore, India, served as a suitable source for isolating G. mysorens strain. The G. mysorens strain received a GenBank accession number MW647910.1 and was isolated and their biological activities were reported by Karthik and Kalyani (2021). In continuation to previous work; initially, the G. mysorens strain was observed for morphological characteristics after performing FESEM analysis. Also, biologically important chemical components present in the intracellular extract of the G. mysorens strain were characterized using GCMS and a partially purified protein sample was characterized using LCMS and have a shown significant number of bioactive compounds.
The cultural characteristics of mangrove adapted G. mysorens strain upon growth on starch casein nitrate agar medium exhibited as white colored filamentous mycelia and at maturity showed ash-colored spores. Production of brown pigmentation on SCNA media was observed. Further microscopic analysis showed Gram staining positive. The isolate when further subjected to FESEM microscopic studies revealed mycelial morphological characteristics of the genus Glutamicibacter. Further, the culture showed filamentous mycelia possessing spirally coiled spore chains. Each spore is visualized as an elongated cylindrical hairy appearance with curved edges as shown in Figure 1. The G. mysorens when grown on different Actinomyces-specific media have shown distinctive phenotypic characteristics as listed in Table 1. Excellent growth was achieved on starch casein nitrate agar, whereas good growth was seen on, glucose leucine agar, yeast extract agar, and nutrient agar media. Moderate growth was seen on sucrose peptone agar, and malt extract agar.Whereas in another study, lysogeny agar was chosen as the best growth media for G. mysorens according to Wang Y. et al. (2022) and Deb et al. (2020).
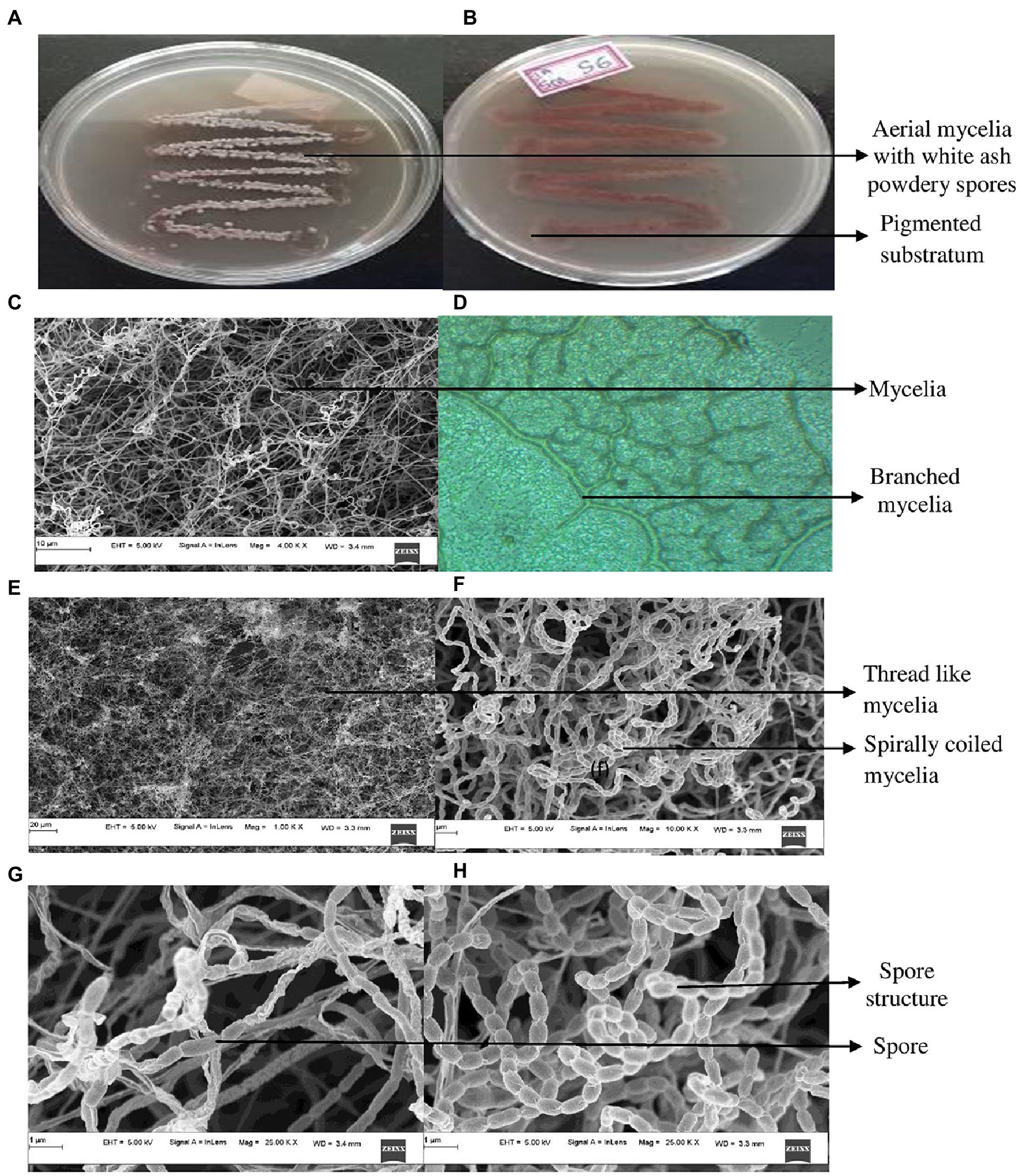
Figure 1. Cultural characteristics of Glutamicibacter mysorens. (A) Front view of isolate. (B) Rear view of isolate. (C) Mycelia observations under FESEM. (D) Phase contrast microscopic analysis. (E,F) Mycelia along with spore analysis under FESEM. (G,H) Spore structure analysis using FESEM.
In our previous report, the G. mysorens strain when subjected to simple and rapid disruption followed according to the method of Bhaduri yielded significant intracellular extraction in buffer (Bhaduri and Demchick, 1983). A 20 mg of protein was loaded on top of the column and 2 ml fractions were collected and about 2.5 times (216 ml) bed volumes of protein elutions were collected. The absorbance of protein fractions was checked at 280 nm and graphs were plotted. The X-axis indicates fraction numbers and absorbance plotted on Y-axis for each fraction collected from Sephadex G-10 column chromatography as showed in Figure 2. This column separation chromatography purifies 10.66 folds as detailed in Table 2. The GCMS studies depicted the presence of 155 bioactive molecules present in the intracellular extract of G. mysorens and the obtained elution profile is as shown in Figure 3. Whereas GCMS analysis depicted the highest probable compounds such as Cyclopentane undecanoic acid, methyl ester 22.7% and Glutaric acid, 2,2-dichloroethyl 3-fluorophenyl ester 34% probability as shown in Figure 4. All the compounds detected through GCMS showed low molecular weight below 1Kgmol−1with various pharmacological applications. The majority of bioactive compounds have shown antimicrobial, enzyme inhibitors, activators, antioxidants, anti-inflammatory, anticancer, agrochemical, insecticide, anti-obese, and many other applications as listed in Table 3. The intracellular extract of G. mysorens had shown potent antimicrobial activity to a broad spectrum of test pathogens such as Salmonella typhimurium (ATCC23564), Staphylococcus aureus (ATCC6538P), Pseudomonas aeruginosa (ATCC9027), Proteus vulgaris (ATCC13315), and Bacillus cereus (ATCC10876) cultures. In order to focus further on prominent bioactive compounds the intracellular extract was partially purified using a Sephadex G-10 column. The eluted peak fraction upon spectrophotometry and electrophoretic analysis revealed the presence of peptide and is reported in our previous article (Karthik and Kalyani, 2021). A similar study was illustrated on 41 different Actinomyces species and majority isolates shown antagonist activity against Staphylococcus aureus, Escherichia coli and Klebsiella pneumoniae (Sapkota et al., 2020).
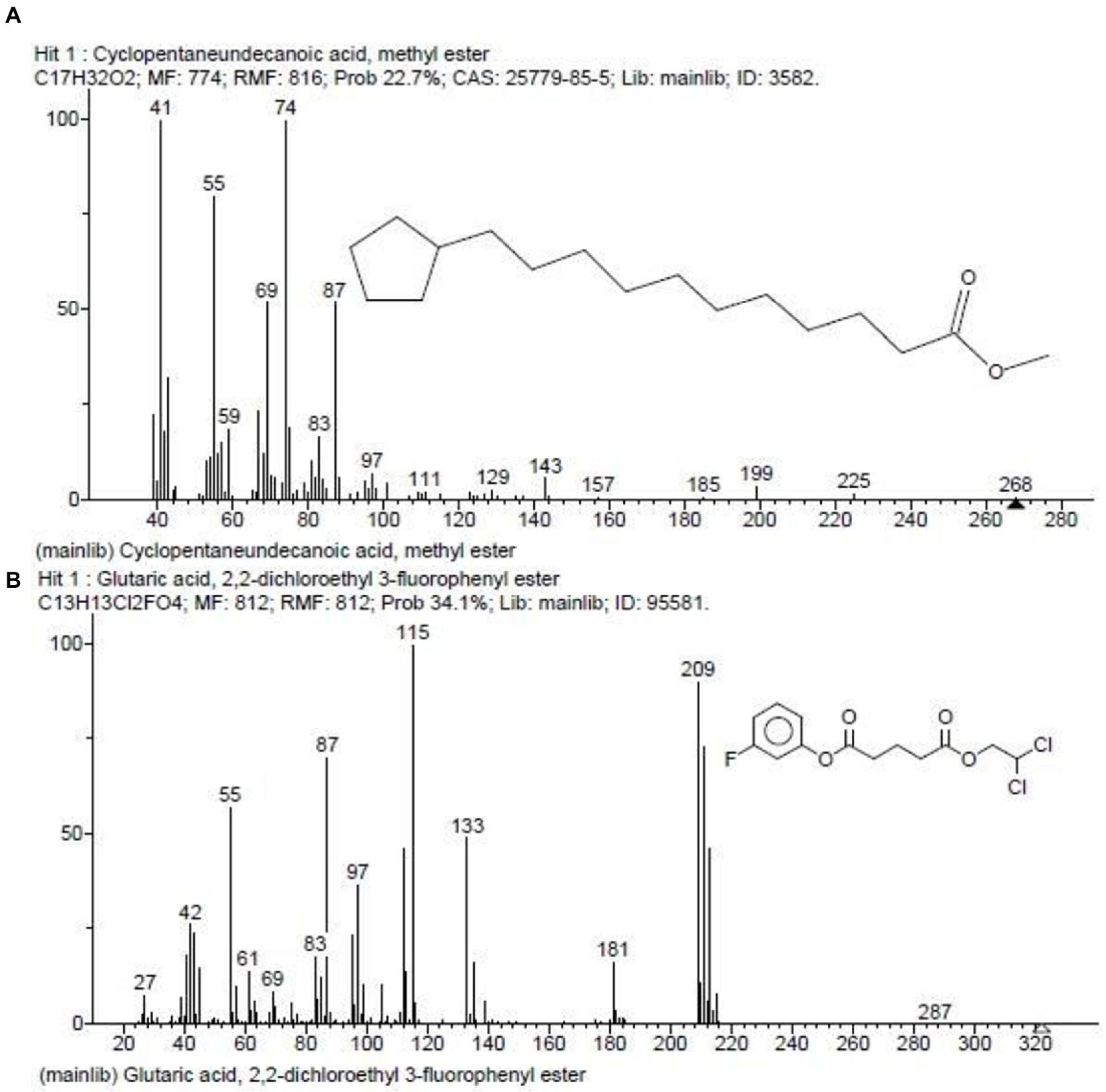
Figure 4. GCMS depicted highest probable compounds. (A) Cyclopentaneundecanoic acid, methyl ester 22.7%. (B) Glutaric acid, 2,2-dichloroethyl 3-fluorophenyl ester 34% probability.
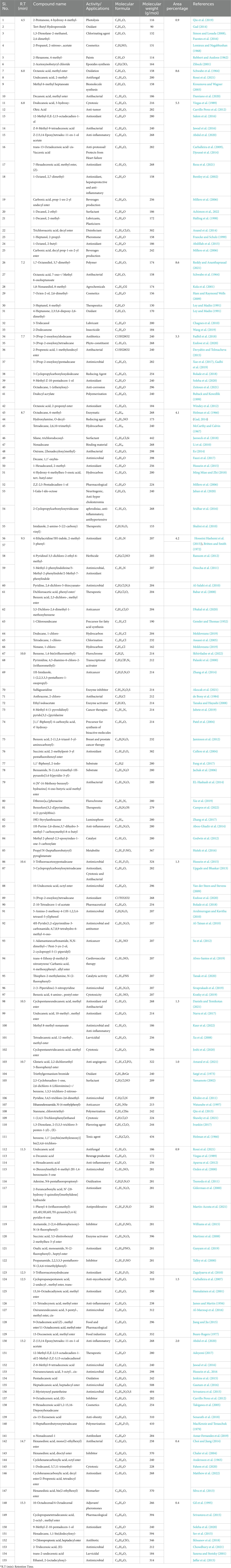
Table 3. List of GC–MS analysis of bioactive compounds from Glutamicibacter mysorens intracellular extract.
One of the previous study; extracellular protein of Actinomyces are actively producers for enzyme ligno cellulase (Clark Mason et al., 1988). The eluted peak fraction for proteins of G. mysorens has shown significant activity for different concentrations 50 μg of protein fraction showed 24% antiproliferative activity against prostate cancer PC3 cell line, for 100 μg 35% antiproliferative activity was observed, for 150 μg 47% antiproliferative activity was observed and for 200 μg 56% antiproliferative activity was observed in comparison with standard drug cisplatin at 5 μg showed 47% antiproliferative activity as showed in Figure 5.
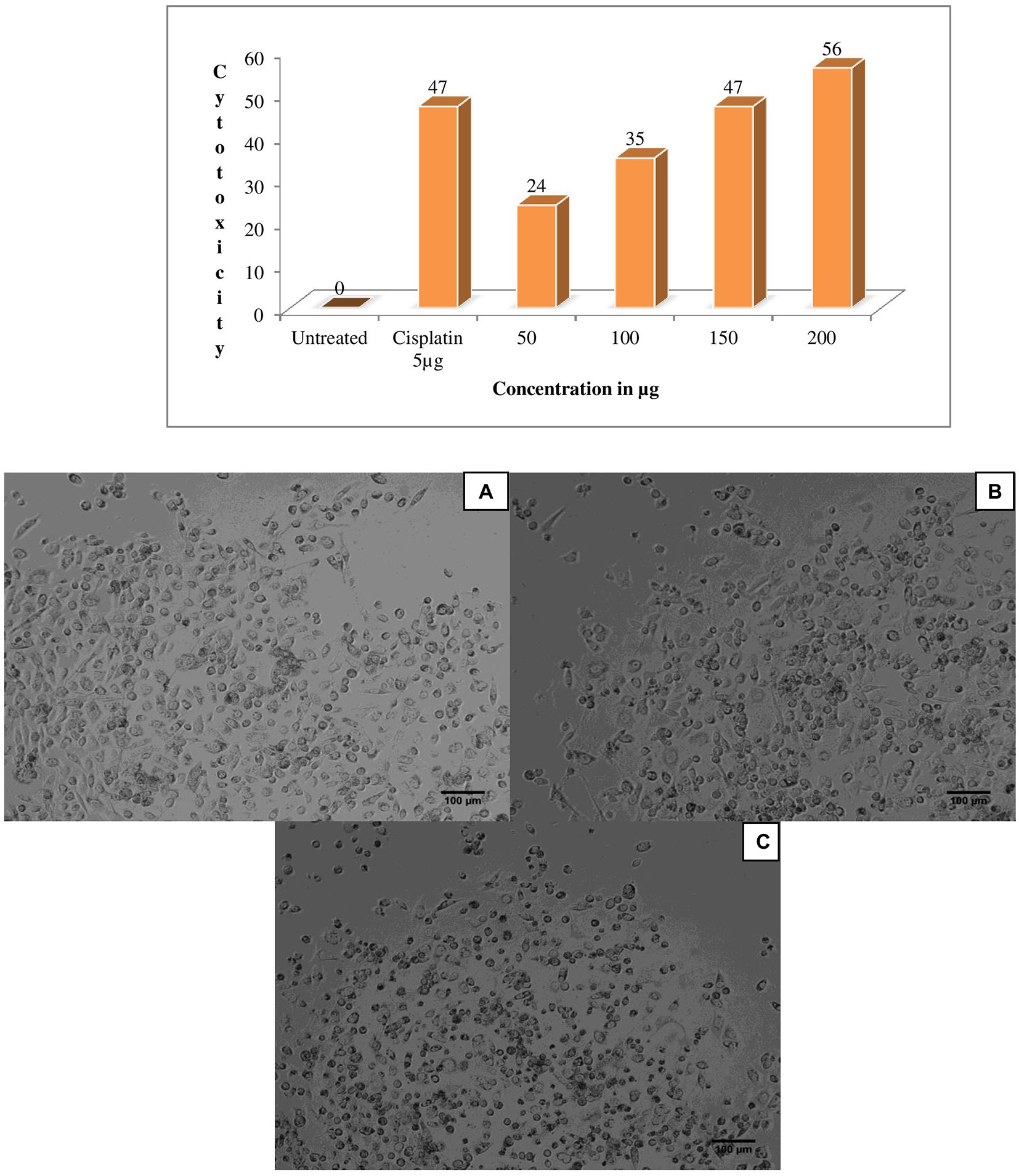
Figure 5. Anticancer activity of Glutamicibacter mysorens strain protein. MTT assay performed by using prostate cancer PC3 cell line. (A) Untreated cells of PC3 cell line, (B) Standard cisplatin at 5 μg/ml, (C) 56% Anticancer activity of Glutamicibacter mysorens protein at 200 μg/ml.
Similar studies reported that other bioactive compounds isolated from the genus Glutamicibacter have been characterized for antimicrobial activity (Phuong and Diep, 2020; Xiong et al., 2020). In another study reported that plant-growth promoting bioactive compounds was produced by Glutamicibacter halophytocola coastal region of China (Qin et al., 2018). Whereas another study describes the anti-fungal efficiency of the Glutamicibacter genus with chitin hydrolyzing activity (Asif et al., 2020). The intracellular protein extraction already reported in our previous studies characterized for an antimicrobial activity that can be considered as antimicrobial peptides (AMPs) from the microbial origin (Karthik and Kalyani, 2021). In the present work the G. mysorens protein fraction is also exhibiting antiproliferative activity against cancerous cells acting also as anticancer peptides (ACP’s) and the protein molecules detected and characterized by LC–MS analysis. We are also reporting GCMS analysis and detected bioactive compounds from G. mysorens.
As discussed above the Sephadex G-10 eluted peak protein fraction was further subjected to LCMS analysis. The LCMS analysis and elution profile as shown in Figure 6, revealed the detection of pharmacologically applicable bioactive peptide compounds. With respect to elution peak from LCMS analysis and detection through the NIST, the computer library resulted in the identification of Kinetin-9-riboside and Embinin. The detected Kinetin-9-riboside with 347 Da molecular weight structure and mass confirmation are shown in Figure 7. The mass confirmation and structure of Embinin with a molecular weight of 606 Da showed in Figure 8. These bioactive molecules are well-known for their effective activity in various biological applications.
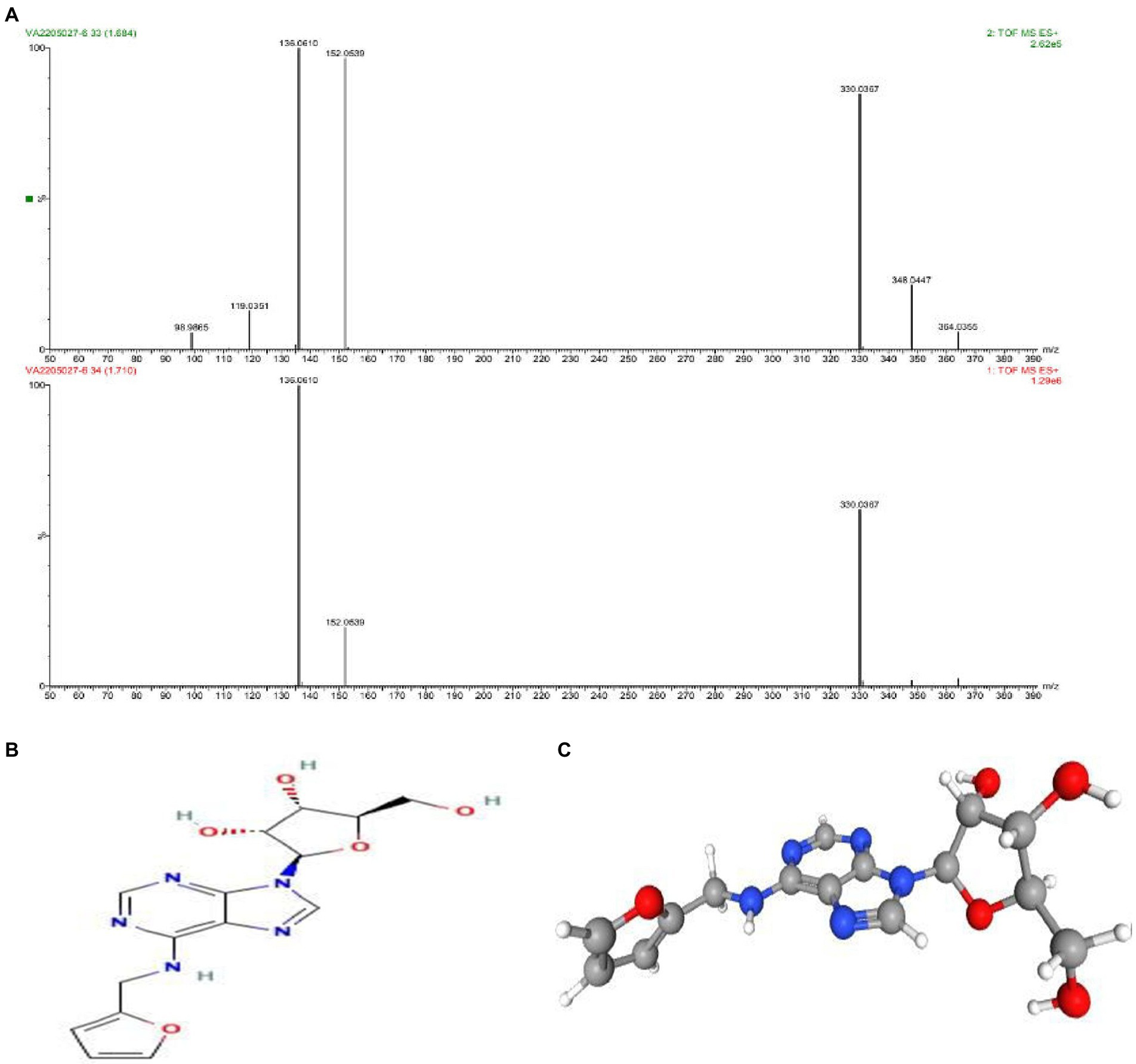
Figure 7. (A) Mass confirmation and analysis record. (B) 2D structure of kinetin-9-ribose, (C) 3D structure of kinetin-9-ribose molecule.
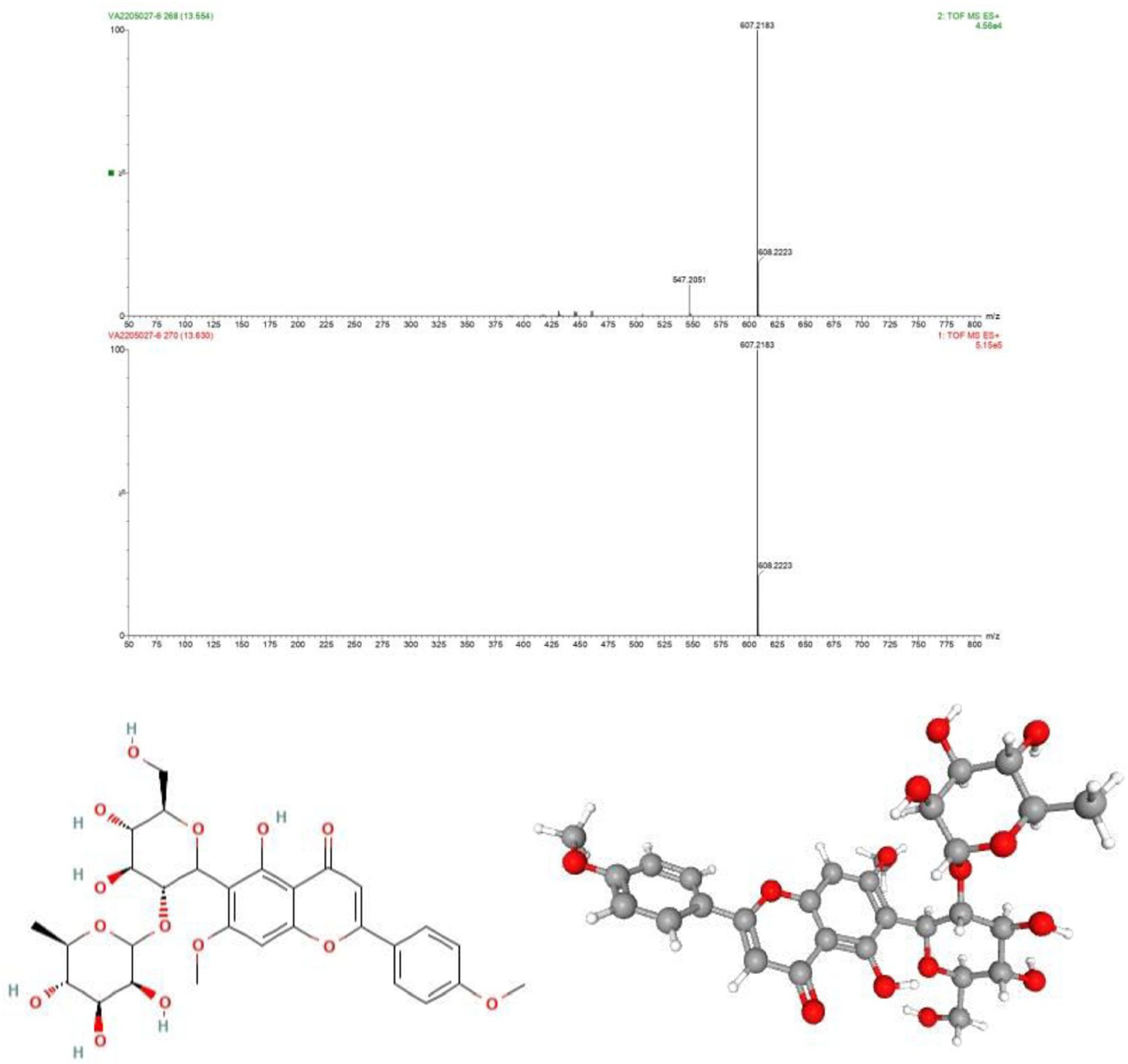
Figure 8. (A) Mass confirmation of Embinin, (B) 2D structure of Embinin, (C) 3D structure of Embinin.
In a previous study, the therapeutic and biological studies of Kinetin-9-riboside as an immuno-stimulant; immuno-stimulatory activities, and their uses as an adjuvant were reported. Because mutations in induced putative kinase 1 (PINK1) induce severe Parkinson’s disease, there’s a lot of interest in finding small molecules that boost PINK1’s kinase activity. Several studies on the design, synthesis, serum stability and hydrolysis of four kinetin riboside ProTides have been published. These ProTides, in combination with kinetin riboside, activated PINK1 in cells that had not been depolarized by mitochondria. This demonstrates the therapeutic potential of modified nucleosides and their phosphate prodrugs for Parkinson’s disease, the second most common neurodegenerative disease (Osgerby et al., 2017).
Another study found that the epithelial-mesenchymal transition (EMT) is a molecular phenomenon associated with increased vimentin expression and raised activity of transcriptional factors (Snail, Twist) that inhibit E-cadherin. EMT has been linked to prostate cancer metastatic potential, therapy resistance, and poor outcomes. Kinetin riboside (KR) is a naturally occurring cytokinin with effective anticancer activity against several human cancer cell lines. mRNA and protein levels of AR, E-, N-cadherins, Vimentin, Snail, Twist, and MMPs were measured using Western Blot and RT-PCR or RQ-PCR techniques to determine the effect of KR on human prostate cell lines.KR inhibited the growth of human prostate cancer cells and, to a lesser extent, normal cells. The cell type and androgen sensitivity determined this effect. KR also decreased the level of p-Akt, which is involved in androgen signaling modulation. When cancer cell lines are exposed to KR, the anti-apoptotic Bcl-2 protein is down-regulated, whereas the Bax protein is up-regulated. KR was involved in E-cadherin re-expression as well as pivotal changes in cell migration. Taken together, the findings suggest that, for the first time, KR can be anticipated as a factor for signaling pathway regulation that involves the inhibition of the development of aggressive forms of prostate cancer, potentially leading to future therapeutic interventions. As a result, research indicates that KR is an effective inhibitor of EMT in human prostate cells (Thakor et al., 2016; Dulińska-Litewka et al., 2020).
Whereas Embinin is a C-Glycosyl flavone and has a wide therapeutic applications in cardiovascular diseases (Ivkin et al., 2018). Another study reports the production of Embinin frompetals of Iris germanica Linnaeus and Iris lactea Leaves (Kawase and Yagishita, 1968; Chen et al., 2018). Our study elucidates the cytotoxicity activity of G. mysorens bioactive peptide as characterized by LCMS/MS revealed the presence of Kinetin-9-Riboside and Embininin the peptide fraction showing its antiproliferative effect on the prostate cancer cell line. Thus microbial-originated intracellular peptides have potential antimicrobial (AMPs) and anticancer (ACPs) have been significantly substantiated in our studies.
The present study is illustrative for exploring untapped mangrove habitat in the Mangalore region of Karnataka. In our study, we could demonstrate that mangrove G. mysorens is an efficient microbe to produce bioactive compounds and enzymes responsible for both antimicrobial and anticancer activity. The antimicrobial potentiality was detailed in our previous article. In this present study; anticancer activity on prostate cancer cell lines and to treat various other related ailments. Peptides from reliable sources such as Actinomyces could be demonstrated as having dual roles as AMPs as well as ACPs. Hence, this study supports and proves that the genus Glutamicibacter is an effective microbial group for the isolation of peptides to treat multidrug-resistant pathogens.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: NCBI - MW647910.
YK conducted the research and wrote the manuscript. MI, SK, RD, and CA performed the data analyses and reviewed the manuscript. SK, YK, KR, MAh, MAl, MH, AA, SS, and MM edited and reviewed the manuscript. MI provided experimental support, planned, supervised, and organized the experiment, and wrote and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This work is supported by Science & Engineering Research Board, DST, Govt. of India and Vision Group of Science and Technology Govt. of Karnataka by providing financial and equipment grants.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abd-Elnaby, H., Abo-Elala, G., Abdel-Raouf, U., Abd-elwahab, A., and Hamed, M. (2016). Antibacterial and anticancer activity of marine Streptomyces parvus: optimization and application. Biotechnol. Biotechnol. Equip. 30, 180–191. doi: 10.1080/13102818.2015.1086280
Abdillah, S., Tambunan, R. M., Farida, Y., Sandhiutami, N. M. D., and Dewi, R. M. (2015). Phytochemical screening and antimalarial activity of some plants traditionally used in Indonesia. Asian Pac. J. Trop. Dis. 5, 454–457. doi: 10.1016/S2222-1808(15)60814-3
Abdul, K., Shaheed, A., Imtair, N., Awi, A. N., Kariem, A., Abbas, Z., et al. (2020). Analysis of bioactive phytochemical compound of (Cyperus iria L.) by using gas chromatography -mass spectrometry.
Abou-Ghadir, O., Alaa, M. H., Abdel-Moty, S., and Hussein, M. (2014). Design and synthesis of some new purine-dione derivatives of potential anti-inflammatory activity. Der Pharma Chem. 6, 199–211.
Achimon, F., Brito, V. D., Pizzolitto, R. P., and Zygadlo, J. A. (2022). Effect of carbon sources on the production of volatile organic compounds by Fusarium verticillioides. J. Fungi 8:158. doi: 10.3390/jof8020158
Adeyemi, M. (2017). Phytochemical analysis and GC-MS determination of Lagenaria breviflora R. Fruit. Int. J. Pharmacogn. Phytochem. Res. 9, 1045–1050. doi: 10.25258/phyto.v9i07.11178
Agamennone, V., Roelofs, D., van Straalen, N., and Janssens, T. K. S. (2018). Antimicrobial activity in culturable gut microbial communities of springtails. J. Appl. Microbiol. 125, 740–752. doi: 10.1111/jam.13899
Akocak, S., Taslimi, P., Lolak, N., Işık, M., Durgun, M., Budak, Y., et al. (2021). Synthesis, characterization, and inhibition study of novel substituted Phenylureido Sulfaguanidine derivatives as α-glycosidase and cholinesterase inhibitors. Chem. Biodivers. 18:e2000958. doi: 10.1002/cbdv.202000958
Al-Marzoqi, A., Hadi, M., and Hameed, I. (2016). Determination of metabolites products by Cassia angustifolia and evaluate antimicobial activity. J. Pharmacogn. Phytother. 8, 25–48. doi: 10.5897/JPP2015.0367
Alongi, D. M. (2015). The impact of climate change on mangrove forests. Curr. Clim. Change Rep. 1, 30–39. doi: 10.1007/s40641-015-0002-x
Al-Salahi, R. A., Al-Omar, M. A., and Amr, A. E.-G. E. (2010). Synthesis of chiral macrocyclic or linear pyridine carboxamides from pyridine-2,6-dicarbonyl dichloride as antimicrobial agents. Molecules 15, 6588–6597. doi: 10.3390/molecules15096588
Al-Taisan, K. M., Al-Hazimi, H. M. A., and Al-Shihry, S. S. (2010). Synthesis, characterization and biological studies of some novel thieno[2,3-d]pyrimidines. Molecules 15, 3932–3957. doi: 10.3390/molecules15063932
Alves-Santos, T. R., Silva, O. A., Moreira, H. S., Borges, R. S., Duarte, G. P., Magalhães, P. J. C., et al. (2019). Cardiovascular effects of trans-4-Methoxy-β-nitrostyrene in spontaneously hypertensive rats: comparison with its parent drug β-nitrostyrene. Front. Pharmacol. 10:1407. doi: 10.3389/fphar.2019.01407
Amaral, A. U., Ferreira, G. C., Seminotti, B., Leipnitz, G., and Wajner, M. (2021). “Glutaric acid neurotoxicity: mechanisms and actions” in Handbook of neurotoxicity. ed. R. M. Kostrzewa (Cham: Springer International Publishing), 1–35.
Anand, S. S., Philip, B. K., and Mehendale, H. M. (2014). “Chlorination byproducts” in Encyclopedia of toxicology. ed. P. Wexler. Third ed (Oxford: Academic Press), 855–859.
Andersson, L., Nilsson, I. M., Niléhn, J.-E., Hedner, U., Granstrand, B., and Melander, B. (1965). Experimental and clinical studies on AMCA, the antifibrinolytically active isomer of p-Aminomethyl cyclohexane carboxylic acid. Scand. J. Haematol. 2, 230–247. doi: 10.1111/j.1600-0609.1965.tb01300.x
Aparna, V., Dileep, K. V., Mandal, P. K., Karthe, P., Sadasivan, C., and Haridas, M. (2012). Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem. Biol. Drug Des. 80, 434–439. doi: 10.1111/j.1747-0285.2012.01418.x
Arulmurugan, S., and Kavitha, H. (2010). 2-Methyl-3-{4-[2-(1H-tetrazol-5-yl)ethylamino]phenyl}-3H-quinazolin-4-one. Mol. Ther. 2010, 1–5. doi: 10.3390/M695
Asif, T., Javed, U., Zafar, S. B., Ansari, A., Ul Qader, S. A., and Aman, A. (2020). Bioconversion of colloidal chitin using novel Chitinase from Glutamicibacter uratoxydans exhibiting anti-fungal potential by hydrolyzing chitin within fungal Cell Wall. Waste Biomass Valor 11, 4129–4143. doi: 10.1007/s12649-019-00746-2
Assassi, N., Tazerouti, A., and Canselier, J. (2005). Analysis of chlorinated, sulfochlorinated and sulfonamide derivatives of n-tetradecane by gas chromatography/mass spectrometry. J. Chromatogr. A 1071, 71–80. doi: 10.1016/j.chroma.2005.01.102
Aznar-Fernandez, T., Cimmino, A., Masi, M., Rubiales, D., and Evidente, A. (2019). Antifeedant activity of long-chain alcohols, and fungal and plant metabolites against pea aphid (Acyrthosiphon pisum) as potential biocontrol strategy. Nat. Prod. Res. 33, 2471–2479. doi: 10.1080/14786419.2018.1452013
Babar, T., Qadeer, G., Rama, N., Ruzicka, A., and Padelkova, Z. (2008). Methyl 2,5-dichlorobenzoate. Acta Crystallogr. Sect. E: Struct. Rep. Online 64:o1970. doi: 10.1107/S1600536808029541
Baig, U., Dahanukar, N., Shintre, N., Holkar, K., Pund, A., Lele, U., et al. (2021). Phylogenetic diversity and activity screening of cultivable actinobacteria isolated from marine sponges and associated environments from the western coast of India. Access Microbiol. 3:000242. doi: 10.1099/acmi.0.000242
Beare-Rogers, J. L. (1977). Docosenoic acids in dietary fats. Prog. Chem. Fats Other Lipids 15, 29–56. doi: 10.1016/0079-6832(77)90006-4
Bentley, S. D., Chater, K. F., Cerdeño-Tárraga, A.-M., Challis, G. L., Thomson, N. R., James, K. D., et al. (2002). Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417, 141–147. doi: 10.1038/417141a
Bhaduri, S., and Demchick, P. H. (1983). Simple and rapid method for disruption of bacteria for protein studies. Appl. Environ. Microbiol. 46, 941–943. doi: 10.1128/aem.46.4.941-943.1983
Bharadwaj, R. P., Raju, N. G., and Chandrashekharaiah, K. S. (2018). Purification and characterization of alpha-amylase inhibitor from the seeds of underutilized legume, Mucuna pruriens. J. Food Biochem. 42:e12686. doi: 10.1111/jfbc.12686
Bolade, O. P., Akinsiku, A. A., Adeyemi, A. O., Williams, A. B., and Benson, N. U. (2018). Dataset on phytochemical screening, FTIR and GC–MS characterisation of Azadirachta indica and Cymbopogon citratus as reducing and stabilising agents for nanoparticles synthesis. Data Brief 20, 917–926. doi: 10.1016/j.dib.2018.08.133
Borker, S. S., Thakur, A., Kumar, S., Kumari, S., Kumar, R., and Kumar, S. (2021). Comparative genomics and physiological investigation supported safety, cold adaptation, efficient hydrolytic and plant growth-promoting potential of psychrotrophic Glutamicibacter arilaitensis LJH19, isolated from night-soil compost. BMC Genomics 22:307. doi: 10.1186/s12864-021-07632-z
Brandenburg, L.-O., Merres, J., Albrecht, L.-J., Varoga, D., and Pufe, T. (2012). Antimicrobial peptides: multifunctional drugs for different applications. Polymers 4, 539–560. doi: 10.3390/polym4010539
Britten, A. Z., and Smith, G. F. (1972). Autoxidation of 3,3′-dimethyl-2,2′-bi-indolyl. J. Chem. Soc. Perkin Trans. 1, 418–420. doi: 10.1039/P19720000418
Buback, M., and Kowollik, C. (1999). Termination kinetics in free-radical bulk copolymerization: the systems dodecyl acrylate−dodecyl methacrylate and dodecyl acrylate−methyl acrylate. Macromolecules 32, 1445–1452. doi: 10.1021/ma9814806
Campos, J. F., Besson, T., and Berteina-Raboin, S. (2022). Review on the synthesis and therapeutic potential of pyrido[2,3-d], [3,2-d], [3,4-d] and [4,3-d]pyrimidine derivatives. Pharmaceuticals 15:352. doi: 10.3390/ph15030352
Carballeira, N. M., Montano, N., Balaña-Fouce, R., and Prada, C. F. (2009). First total synthesis and antiprotozoal activity of (Z)-17-methyl-13-octadecenoic acid, a new marine fatty acid from the sponge Polymastia penicillus. Chem. Phys. Lipids 161, 38–43. doi: 10.1016/j.chemphyslip.2009.06.140
Carballeira, N. M., Montano, N., Vicente, J., and Rodriguez, A. D. (2007). Novel cyclopropane fatty acids from the phospholipids of the Caribbean sponge Pseudospongosorites suberitoides. Lipids 42, 519–524. doi: 10.1007/s11745-007-3047-3
Carrillo Perez, C., Del, C. C. M., and Alonso De La Torre, S. (2012). Antitumor effect of oleic acid; mechanisms of action. A review. Nutr. Hosp. 27, 1860–1865. doi: 10.3305/nh.2012.27.6.6010
Chagnes, A., Rager, M.-N., Courtaud, B., Thiry, J., and Cote, G. (2010). Speciation of vanadium (V) extracted from acidic sulfate media by trioctylamine in n-dodecane modified with 1-tridecanol. Hydrometallurgy 104, 20–24. doi: 10.1016/j.hydromet.2010.04.004
Chaler, R., Canton, L., Vaquero, M., and Grimalt, J. O. (2004). Identification and quantification of n-octyl esters of alkanoic and hexanedioic acids and phthalates as urban wastewater markers in biota and sediments from estuarine areas. J. Chromatogr. A 1046, 203–210.
Chater, K. F. (2006). Streptomyces inside-out: a new perspective on the bacteria that provide us with antibiotics. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 361, 761–768. doi: 10.1098/rstb.2005.1758
Chater, K. F. (2013). “Streptomyces,” in Brenner’s encyclopedia of genetics. eds. S. Maloy and K. Hughes (Cambridge, Massachusetts, United States: Elsevier), 565–567.
Chen, D., Meng, Y., Zhu, Y., Wu, G., Yuan, J., Qin, M., et al. (2018). Qualitative and quantitative analysis of C-glycosyl-flavones of Iris lactea leaves by liquid chromatography/tandem mass spectrometry. Molecules 23:3359. doi: 10.3390/molecules23123359
Cho, J. H., Sung, B. H., and Kim, S. C. (2009). Buforins: histone H2A-derived antimicrobial peptides from toad stomach. Biochim. Biophys. Acta Biomembr. 1788, 1564–1569. doi: 10.1016/j.bbamem.2008.10.025
Choi, W. H., and Jiang, M. (2014). Evaluation of antibacterial activity of hexanedioic acid isolated from Hermetia illucens larvae. J. Appl. Biomed. 12, 179–189. doi: 10.1016/j.jab.2014.01.003
Chowdhury, S. K., Dutta, T., Chattopadhyay, A. P., Ghosh, N. N., Chowdhury, S., and Mandal, V. (2021). Isolation of antimicrobial tridecanoic acid from Bacillus sp. LBF-01 and its potentialization through silver nanoparticles synthesis: a combined experimental and theoretical studies. J. Nanostruct. Chem. 11, 573–587. doi: 10.1007/s40097-020-00385-3
Clark Mason, J., Richards, M., Zimmermann, W., and Broda, P. (1988). Identification of extracellular proteins from actinomycetes responsible for the solubilisation of lignocellulose. Appl. Microbiol. Biotechnol. 28, 276–280. doi: 10.1007/BF00250455
Cleary, J. L., Kolachina, S., Wolfe, B. E., and Sanchez, L. M. (2018). Coproporphyrin III produced by the bacterium Glutamicibacter arilaitensis binds zinc and is upregulated by fungi in cheese rinds. mSystems 3, e00036–e00018. doi: 10.1128/mSystems.00036-18
Collin, F., and Maxwell, A. (2019). The microbial toxin microcin B17: prospects for the development of new antibacterial agents. J. Mol. Biol. 431, 3400–3426. doi: 10.1016/j.jmb.2019.05.050
Cruciani, R. A., Barker, J. L., Zasloff, M., Chen, H. C., and Colamonici, O. (1991). Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc. Natl. Acad. Sci. U. S. A. 88, 3792–3796. doi: 10.1073/pnas.88.9.3792
Cullere, L., Cacho, J., and Ferreira, V. (2004). Analysis for wine C5–C8 aldehydes through the determination of their O-(2,3,4,5,6-pentafluorobenzyl)oximes formed directly in the solid phase extraction cartridge. Anal. Chim. Acta 524, 201–206. doi: 10.1016/j.aca.2004.03.025
Damiano, F., De Benedetto, G. E., Longo, S., Giannotti, L., Fico, D., Siculella, L., et al. (2020). Decanoic acid and not octanoic acid stimulates fatty acid synthesis in U87MG glioblastoma cells: a metabolomics study. Front. Neurosci. 14:783. doi: 10.3389/fnins.2020.00783
Daniels, A., and Temikotan, T. (2021). Fatty acid profile, antioxidant and antibacterial effect of the ethyl acatate extract of cleistopholis patens. Bull. Sci. Res. 3, 21–31. doi: 10.34256/bsr2113
Das, L., Deb, S., and Das, S. (2020). Glutamicibacter mishrai sp. nov., isolated from the coral Favia veroni from Andaman Sea. Arch. Microbiol. 202, 1–13. doi: 10.1007/s00203-019-01783-0
de Bony, J., Martin, G., Welby, M., and Tocanne, J. F. (1984). Evidence for a homogeneous lateral distribution of lipids in a bacterial membrane: a photo cross-linking approach using anthracene as a photoactivable group. FEBS Lett. 174, 1–6. doi: 10.1016/0014-5793(84)81065-0
Deb, S., Das, L., and Das, S. K. (2020). Phylogenomic analysis reveals that Arthrobacter mysorens Nand and Rao 1972 (approved lists 1980) and Glutamicibacter mysorens Busse 2016 are later heterotypic synonyms of Arthrobacter nicotianae Giovannozzi-Sermanni 1959 (approved lists 1980) and Glutamicibacter nicotianae Busse 2016. Curr. Microbiol. 77, 3793–3798. doi: 10.1007/s00284-020-02176-z
Deryabin, D. G., and Tolmacheva, A. A. (2015). Antibacterial and anti-quorum sensing molecular composition derived from Quercus cortex (oak bark) extract. Molecules 20, 17093–17108. doi: 10.3390/molecules200917093
Desouky, S. E., Shojima, A., Singh, R. P., Matsufuji, T., Igarashi, Y., Suzuki, T., et al. (2015). Cyclodepsipeptides produced by actinomycetes inhibit cyclic-peptide-mediated quorum sensing in Gram-positive bacteria. FEMS Microbiol. Lett. 362:fnv109. doi: 10.1093/femsle/fnv109
Desriac, F., Jegou, C., Balnois, E., Brillet, B., Chevalier, P. L., and Fleury, Y. (2013). Antimicrobial peptides from marine proteobacteria. Mar. Drugs 11, 3632–3660. doi: 10.3390/md11103632
Dhakal, R., Li, X., Parkin, S. R., and Lehmler, H.-J. (2020). Synthesis of mono- and dimethoxylated polychlorinated biphenyl derivatives starting from fluoroarene derivatives. Environ. Sci. Pollut. Res. Int. 27, 8905–8925. doi: 10.1007/s11356-019-07133-3
Djoussé, L., Matsumoto, C., Hanson, N. Q., Weir, N. L., Tsai, M. Y., and Gaziano, J. M. (2014). Plasma cis-vaccenic acid and risk of heart failure with antecedent coronary heart disease in male physicians. Clin. Nutr. 33, 478–482. doi: 10.1016/j.clnu.2013.07.001
Dulińska-Litewka, J., Gąsiorkiewicz, B., Litewka, A., Gil, D., Gołąbek, T., and Okoń, K. (2020). Could the kinetin riboside be used to inhibit human prostate cell epithelial–mesenchymal transition? Med. Oncol. 37:17. doi: 10.1007/s12032-020-1338-1
EL-Hashash, M. A., Essawy, A., and Sobhy Fawzy, A. (2014). Synthesis and antimicrobial activity of some novel heterocyclic candidates via Michael addition involving 4-(4-Acetamidophenyl)-4-oxobut-2-enoic acid. Adv. Chem. 2014:e619749, 1–10. doi: 10.1155/2014/619749
Es, B. (2014). Antibacterial potential of Luprops tristis - the nuisance rubber plantation Pest from Western Ghats of India. IJAIR 3
Ezekwe, S., Rizwan, A., Rabiu, K., and Ogbonnaya, E. (2020). Qualitative phytochemical and GC-MS analysis of some commonly consumed vegetables. GSC Biol. Pharm. Sci. 12, 208–214. doi: 10.30574/gscbps.2020.12.3.0299
Fadhil, L., Kadhim, S., and Hameed, I. (2018). Detection of bioactive secondary metabolites produced by Bacillus subtilis using gas chromatography-mass spectrometry technique. Indian J. Public Health Res. Dev. 9:1097. doi: 10.5958/0976-5506.2018.00877.X
Fahem, N., Djellouli, A. S., and Bahri, S. (2020). Cytotoxic activity assessment and GC-MS screening of two codium species extracts. Pharm. Chem. J. 54, 755–760. doi: 10.1007/s11094-020-02266-z
Fang, B.-Z., Salam, N., Han, M.-X., Jiao, J.-Y., Cheng, J., Wei, D.-Q., et al. (2017). Insights on the effects of heat pretreatment, pH, and calcium salts on isolation of rare actinobacteria from Karstic caves. Front. Microbiol. 8:1535. doi: 10.3389/fmicb.2017.01535
Fauzi, A., Jawad, M., and Hameed, I. (2017). Phytochemical profiles of methanolic seeds extract of Cuminum cyminum using GC-MS technique. Int. J. Curr. Pharm. Rev. Res. 8, 1–9. doi: 10.25258/ijcprr.v8i02.9194
Fernebro, J. (2011). Fighting bacterial infections-future treatment options. Drug Resist. Updat. 14, 125–139. doi: 10.1016/j.drup.2011.02.001
Francke, W., and Schulz, S. (1999). “8.04 - pheromones” in Comprehensive natural products chemistry. eds. S. D. Barton, K. Nakanishi, and O. Meth-Cohn (Oxford: Pergamon), 197–261.
Fu, B., Olawole, O., and Beattie, G. A. (2021). Biological control and microbial ecology draft genome sequence data of Glutamicibacter sp. FBE-19, a bacterium antagonistic to the plant pathogen Erwinia tracheiphila. Phytopathology 111, 765–768. doi: 10.1094/PHYTO-09-20-0380-A
Fuentes, A. S., del Pozo Losada, C., and Vora, H. U. (2016). “(−)-(4R,5R)-4,5-Bis[hydroxy(diphenyl)methyl]-2,2-dimethyl-1,3-dioxolane” in Encyclopedia of reagents for organic synthesis. eds. A. Charette, J. Bode, T, Rovis, and R. Shenvi (Hoboken, New Jersey, United States: John Wiley & Sons, Ltd), 1–9.
Fukuda, K., and Kono, H. (2021). “Cost-benefit analysis and industrial potential of exopolysaccharides,” in Microbial exopolysaccharides as novel and significant biomaterials. eds. A. K. Nadda, K. V. Sajna and S. Sharma (Cham: Springer), 303–339.
Gad, S. E. (2014). “Hydroperoxide, tert-Butyl” in Encyclopedia of toxicology. ed. P. Wexler. Third ed (Oxford: Academic Press), 977–978.
Gadhi, A., El-Sherbiny, M., Al-Sofynai, A., Baakdah, M., and Sathianeson, S. (2019). Antimicrofouling activities of marine macroalga Dictyota dichotoma from the Red Sea. J. Agric. Mar. Sci. 23:58. doi: 10.24200/jams.vol23iss0pp58-67
Ganyam, M., Anaduaka, E., Gabriel, F., Sani, S., and Fedilis, I. (2019). Effects of methanol extract of toasted african yam bean seeds (Sphenostylis stenocarpa) on anti-inflammatory properties. Pharmacol. Online 3, 100–113.
Gautam, V., Sharma, A., Arora, S., and Bhardwaj, R. (2016). Bioactive compounds in the different extracts of flowers of Rhododendron arboreum Sm. J. Chem. Pharm. Res. 2016, 439–444.
Gensler, W. J., and Thomas, G. R. (1952). Synthesis of unsaturated fatty acids: vaccenic acid. J. Am. Chem. Soc. 74, 3942–3943. doi: 10.1021/ja01135a511
Gil, S., Lázaro, M. A., Mestres, R., Millan, F., and Parra, M. (1995). Components of the sex pheromone of chilo supressalis: efficient syntheses of (Z)-11-hexadecenal and (Z)-13-Octadecenal. Synth. Commun. 25, 351–361. doi: 10.1080/00397919508011366
Godwin, J., Chukwu, U. J., and Gad, T. (2012). Distribution of iron (ii) between buffered aqueous solutions and chloroform solution of N, N’-ethylenebis(4-butanoyl-2,4-dihydro-5-methyl-2-phenyl-3h-pyrazol-3-oneimine). J. Adv. Chem. 8, 1581–1589. doi: 10.24297/jac.v8i2.4039
Gülerman, N., Oruç-Emre, E., Kartal, F., and Rollas, S. (2000). In vivo metabolism of 4-fluorobenzoic acid [(5-nitro-2-furanyl)methylene] hydrazide in rats. Eur. J. Drug Metab. Pharmacokinet. 25, 103–108. doi: 10.1007/BF03190075
Halling, P. J., Ross, A. C., and Bell, G. (1998). “Inactivation of enzymes at the aqueous-organic interface” in Progress in biotechnology stability and stabilization of biocatalysts. eds. A. Ballesteros, F. J. Plou, J. L. Iborra, and P. J. Halling (Amsterdam, Netherlands: Elsevier), 365–372.
Ham, J. E., and Raymond Wells, J. (2009). Surface chemistry of dihydromyrcenol (2,6-dimethyl-7-octen-2-ol) with ozone on silanized glass, glass, and vinyl flooring tiles. Atmos. Environ. 43, 4023–4032. doi: 10.1016/j.atmosenv.2009.05.007
Hamalainen, T. I., Sundberg, S., Makinen, M., Kaltia, S., Hase, T., and Hopia, A. (2001). Hydroperoxide formation during autoxidation of conjugated linoleic acid methyl ester. Eur. J. Lipid Sci. Technol. 103, 588–593. doi: 10.1002/1438-9312(200109)103:9<588::AID-EJLT5880>3.0.CO;2-L
Hidri, R., Mahmoud, O. M.-B., Zorrig, W., Mahmoudi, H., Smaoui, A., Abdelly, C., et al. (2022). Plant growth-promoting rhizobacteria alleviate high salinity impact on the halophyte Suaeda fruticosa by modulating antioxidant defense and soil biological activity. Front. Plant Sci. 13:821475. doi: 10.3389/fpls.2022.821475
Holman, R. T., Deubig, M., and Hayes, H. (1966). Pyrolysis chromatography of lipids. I. Mass spectrometric identification of pyrolysis products of hydrocarbons. Lipids 1, 247–253. doi: 10.1007/BF02531610
Hoskin, D. W., and Ramamoorthy, A. (2008). Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta - Biomembr. 1778, 357–375. doi: 10.1016/j.bbamem.2007.11.008
Hosseini Hashemi, S. K., Anooshei, H., Aghajani, H., and Salem, M. (2015). Chemical composition and antioxidant activity of extracts from the inner bark of Berberis vulgaris stem. Bioresources 10, 7958–7969. doi: 10.15376/biores.10.4.7958-7969
Hui, M. L.-Y., Tan, L. T.-H., Letchumanan, V., He, Y.-W., Fang, C.-M., Chan, K.-G., et al. (2021). The extremophilic actinobacteria: from microbes to medicine. Antibiotics 10:682. doi: 10.3390/antibiotics10060682
Hušek, P., Švagera, Z., Hanzlíková, D., Řimnáčová, L., Zahradníčková, H., Opekarová, I., et al. (2016). Profiling of urinary amino-carboxylic metabolites by in-situ heptafluorobutyl chloroformate mediated sample preparation and gas chromatography–mass spectrometry. J. Chromatogr. A 1443, 211–232. doi: 10.1016/j.chroma.2016.03.019
Hussein, A. O., Hameed, I. H., Jasim, H., and Kareem, M. A. (2015). Determination of alkaloid compounds of Ricinus communis by using gas chromatography- mass spectroscopy (GC-MS). JMPR 9, 349–359. doi: 10.5897/JMPR2015.5750
Hussein, J., Mohammed, Y. H., and Imad, H. H. (2016). Study of chemical composition of Foeniculum vulgare using Fourier transform infrared spectrophotometer and gas chromatography -mass spectrometry. J. Pharmacogn. Phytother. 8, 60–89. doi: 10.5897/JPP2015.0372
Ikhsanov, Y. S., Abilov Zh, A., Choudhary, M. I., and Sultanova, N. A. (2018). Study of the chemical composition of dichloromethane extract Tamarix hispida. Bulletin of the Karaganda University. Chemistry Series.
Ivankin, A. (2017). Biotechnology for formation of aromatic properties of National- Foodstuffs on the basis of meat raw material under influence of bacterial crops and Chromato-mass-spectrometric analysis of the flavoring components. J. Appl. Biotechnol. Bioeng. 3, 366–372. doi: 10.15406/jabb.2017.03.00072
Ivkin, D. Y., Luzhanin, V. G., Karpov, A. A., Minasyan, S. M., Poleshchenko, Y. I., Mamedov, A. E., et al. (2018). Embinin is a perspective cardiotonic mean for natural origin. Drug Dev. Regist. 0, 166–170.
Jachak, M., Bhusnar, A., Medhane, V., and Toche, R. (2006). A convenient route for the synthesis of pyrazolo[3,4-d]pyrimidine, pyrazolo[3,4-b][1,6]naphthyridine and pyrazolo[3,4-b]quinoline derivatives. J. Heterocyclic Chem. 43, 1169–1175. doi: 10.1002/jhet.5570430506
Jaffar, A., Somanath, B., and Karthi, S. (2015). Efficacy of methanolic extract of a marine ascidian, Lissoclinum bistratum for antimicrobial activity. J. Chem. Biol. Phys. Sci. 5:4119.
Jahan, I., Tona, M. R., Sharmin, S., Sayeed, M. A., Tania, F. Z., Paul, A., et al. (2020). GC-MS phytochemical profiling, pharmacological properties, and in Silico studies of Chukrasia velutina leaves: a novel source for bioactive agents. Molecules 25:3536. doi: 10.3390/molecules25153536
James, A. T., and Martin, A. J. P. (1956). Gas–liquid chromatography: the separation and identification of the methyl esters of saturated and unsaturated acids from formic acid to n-octadecanoic acid. Biochem. J. 63, 144–152. doi: 10.1042/bj0630144
Jamieson, S. M. F., Brooke, D. G., Heinrich, D., Atwell, G. J., Silva, S., Hamilton, E. J., et al. (2012). 3-(3,4-Dihydroisoquinolin-2(1H)-ylsulfonyl)benzoic acids: highly potent and selective inhibitors of the type 5 17-β-hydroxysteroid dehydrogenase AKR1C3. J. Med. Chem. 55, 7746–7758. doi: 10.1021/jm3007867
Janardhan, A., Kumar, A. P., Viswanath, B., Saigopal, D. V. R., and Narasimha, G. (2014). Production of bioactive compounds by actinomycetes and their antioxidant properties. Biotechnol. Res. Int. 2014, 1–9. doi: 10.1155/2014/217030
Janneck, R., Heremans, P., Genoe, J., and Rolin, C. (2018). Influence of the surface treatment on the solution coating of single-crystalline organic thin-films. Adv. Mater. Interfaces 5:1800147. doi: 10.1002/admi.201800147
Jawad, M., Mohammad, G., and Hussein, H. (2016). Analysis of bioactive metabolites from Candida albicans using (GC-MS) and evaluation of antibacterial activity 8, 655–670.
Jenkins, B., West, J. A., and Koulman, A. (2015). A review of odd-chain fatty acid metabolism and the role of pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0) in health and disease. Molecules 20, 2425–2444. doi: 10.3390/molecules20022425
Jiang, J., and Jia, X. (2015). Profiling of fatty acids composition in suet oil based on GC–EI-qMS and chemometrics analysis. Int. J. Mol. Sci. 16, 2864–2878. doi: 10.3390/ijms16022864
Joshi, P. R., Paudel, M. R., Chand, M. B., Pradhan, S., Pant, K. K., Joshi, G. P., et al. (2020). Cytotoxic effect of selected wild orchids on two different human cancer cell lines. Heliyon 6:e03991. doi: 10.1016/j.heliyon.2020.e03991
Jubete, G., Puig de la Bellacasa, R., Estrada-Tejedor, R., Teixidó, J., and Borrell, J. I. (2019). Pyrido[2,3-d]pyrimidin-7(8H)-ones: synthesis and biomedical applications. Molecules 24:4161. doi: 10.3390/molecules24224161
Juturu, V., and Wu, J. C. (2018). Microbial production of bacteriocins: latest research development and applications. Biotechnol. Adv. 36, 2187–2200. doi: 10.1016/j.biotechadv.2018.10.007
Karthik, Y., and Kalyani, M. I. (2021). Molecular profiling of Glutamicibacter Mysorens strain YKIKM.MU and bioactive peptides characterization for antibacterial activity. Int. J. Pharm. Clin. Res. 13:15.
Karthik, Y., and Kalyani, M. I. (2022). Occurrence of Streptomyces tauricus in mangrove soil of Mangalore region in Dakshina Kannada as a source for antimicrobial peptide. J. Basic Microbiol. 62, 1–15. doi: 10.1002/jobm.202200108
Karthik, Y., Kalyani, M. I., Sheetal, K., Rakshitha, D., and Bineesha, B. K. (2020). Cytotoxic and antimicrobial activities of microbial proteins from mangrove soil actinomycetes of Mangalore, Dakshina Kannada. Biomedicine 40, 59–67. doi: 10.51248/.v40i1.104
Kaur, S., Sharma, P., Bains, A., Chawla, P., Sridhar, K., Sharma, M., et al. (2022). Antimicrobial and anti-inflammatory activity of low-energy assisted nanohydrogel of Azadirachta indica oil. Gels 8:434. doi: 10.3390/gels8070434
Kawase, A., and Yagishita, K. (1968). On the structure of a new C-glycosyl flavone, embinin, isolated from the petals of Iris germanica Linnaeous*1. Agric. Biol. Chem. 32, 537–538. doi: 10.1080/00021369.1968.10859095
Khadayat, K., Sherpa, D. D., Malla, K. P., Shrestha, S., Rana, N., Marasini, B. P., et al. (2020). Molecular identification and antimicrobial potential of Streptomyces species from nepalese soil. Int. J. Microbiol. 2020:e8817467. doi: 10.1155/2020/8817467
Khidre, R., Abuhashem, A., and El-Shazly, M. (2011). Synthesis and anti-microbial activity of some 1-substituted amino-4, 6-dimethyl-2-oxo-pyridine-3-carbonitrile derivatives. Eur. J. Med. Chem. 46, 5057–5064. doi: 10.1016/j.ejmech.2011.08.018
Khodaei, M., and Sh, S. N. (2018). Isolation and molecular identification of Bacteriocin-producing enterococci with broad antibacterial activity from traditional dairy products in Kerman Province of Iran. Korean J. Food Sci. Anim. Resour. 38, 172–179. doi: 10.5851/kosfa.2018.38.1.172
Khusro, A., Aarti, C., and Agastian, P. (2020). Microwave irradiation-based synthesis of anisotropic gold nanoplates using Staphylococcus hominis as reductant and its optimization for therapeutic applications. J. Environ. Chem. Eng. 8:104526. doi: 10.1016/j.jece.2020.104526
Kratky, M., Konečná, K., Janoušek, J., Brablíková, M., Janďourek, O., Trejtnar, F., et al. (2019). 4-Aminobenzoic acid derivatives: converting folate precursor to antimicrobial and cytotoxic agents. Biomol. Ther. 10:9. doi: 10.3390/biom10010009
Kroumova, A. B., and Wagner, G. J. (2003). Different elongation pathways in the biosynthesis of acyl groups of trichome exudate sugar esters from various solanaceous plants. Planta 216, 1013–1021. doi: 10.1007/s00425-002-0954-7
Kula, J., Quang, T. B., and Smigielski, K. (2001). Convenient synthesis of (r)-1,3-nonanediol. Synth. Commun. 31, 463–467. doi: 10.1081/SCC-100000540
Kurnianto, M. A., Kusumaningrum, H. D., Lioe, H. N., and Chasanah, E. (2021). Partial purification and characterization of bacteriocin-like inhibitory substances produced by Streptomyces sp. isolated from the gut of Chanos chanos. Biomed. Res. Int. 2021:7190152. doi: 10.1155/2021/7190152
Lemieux, R. U., and Nagabhushan, T. L. (1968). The synthesis of 2-amino-2-deoxyhexoses: D-glucosamine, D-mannosamine, D-galactosamine, and D-talosamine. Can. J. Chem. 46, 401–403. doi: 10.1139/v68-064
Ley, S. V., and Madin, A. (1991). “2.7 - oxidation adjacent to oxygen of alcohols by chromium reagents” in Comprehensive organic synthesis. eds. B. M. Trost and I. Fleming (Oxford: Pergamon), 251–289.
Li, H., Liu, X., and Fang, G. (2010). Preparation and characteristics of n-nonadecane/cement composites as thermal energy storage materials in buildings. Energ. Buildings 42, 1661–1665. doi: 10.1016/j.enbuild.2010.04.009
MacKenzie, S. L., and Tenaschuk, D. (1979). Quantitative fromation of N(O,S)-heptafluorobutyryl isobutyl amino acids for gas chromatographic analysis: I. Esterification. J. Chromatogr. A 171, 195–208. doi: 10.1016/S0021-9673(01)95299-9
Martín-Acosta, P., Amesty, A., Guerra-Rodríguez, M., Guerra, B., Fernández-Pérez, L., and Estévez-Braun, A. (2021). Modular synthesis and antiproliferative activity of new dihydro-1H-pyrazolo[1,3-b]pyridine embelin derivatives. Pharmaceuticals (Basel) 14:1026. doi: 10.3390/ph14101026
Martinez, C., Hu, S., Dumond, Y., Tao, J., Kelleher, P., and Tully, L. (2008). Development of a Chemoenzymatic manufacturing process for pregabalin. Org. Process Res. Dev. 12, 392–398. doi: 10.1021/op7002248
Matthew, O., James, A., Akogwu, I., Fabunmi, T., Godwin, I., Ebun, B., et al. (2022). The reference colored blue in the reference section should be cited in the body of the work evaluation of in vitro antioxidant, phytochemical and Gc- Ms analysis of aqueous extract of Solanum Dasyphyllum fruits.
McCarthy, E. D., and Calvin, M. (1967). The isolation and identification of the C17 saturated isoprenoid hydrocarbon 2,6,10-trimethyltetradecane from a Devonian shale: the role of squalane as a possible precursor. Tetrahedron 23, 2609–2619. doi: 10.1016/0040-4020(67)85125-1
Mihooliya, K. N., Nandal, J., Swami, L., Verma, H., Chopra, L., and Sahoo, D. K. (2017). A new pH indicator dye-based method for rapid and efficient screening of l-asparaginase producing microorganisms. Enzym. Microb. Technol. 107, 72–81. doi: 10.1016/j.enzmictec.2017.08.004
Millero, F. J., Graham, T. B., Huang, F., Bustos-Serrano, H., and Pierrot, D. (2006). Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Mar. Chem. 100, 80–94. doi: 10.1016/j.marchem.2005.12.001
Ming Miao, Y., and Zhi, Y. (2018). Mass spectrum of acids, alcohols, esters and amines of coffee hull (Typical.Arabica).
Mohan, Y., Sirisha, B., Haritha, R., and Ramana, T. (2013). Selective screening, isolation and characterization of antimicrobial agents from marine actinomycetes. Int J Pharm Pharm Sci 5, 443–449.
Moldoveanu, S. C. (2019). “Chapter 2 - pyrolysis of hydrocarbons,” in Pyrolysis of organic molecules. ed. S. C. Moldoveanu. Second ed (Amsterdam, Netherlands: Elsevier), 35–161.
Narra, N., Kaki, S. S., Badari, R., Prasad, R. B. N., Misra, S., Koude, D., et al. (2017). Synthesis and evaluation of anti-oxidant and cytotoxic activities of novel 10-undecenoic acid methyl ester based lipoconjugates of phenolic acids. Beilstein J. Org. Chem. 2017, 26–32. doi: 10.3762/bjoc.13.4
Nishioka, K., and Katayama, I. (1978). Angiogenic activity in culture supernatant of antigen-stimulated lymph node cells. J. Pathol. 126, 63–69. doi: 10.1002/path.1711260202
Onocha, A., Abimbade, S., and Oloyede, G. (2011). Chemical composition, toxicity, antimicrobial and antioxidant activities of leaf and stem essential oils of Dieffenbachia picta (Araceae). Eur. J. Sci. Res. 49, 567–580.
Osgerby, L., Lai, Y.-C., Thornton, P. J., Amalfitano, J., Le Duff, C. S., Jabeen, I., et al. (2017). Kinetin riboside and its ProTides activate the Parkinson’s disease associated PTEN-induced putative kinase 1 (PINK1) independent of mitochondrial depolarization. J. Med. Chem. 60, 3518–3524. doi: 10.1021/acs.jmedchem.6b01897
Ozden, S., Oztürk, A., Goker, H., and Altanlar, N. (2000). Synthesis and antimicrobial activity of some new 4-hydroxy-2H-1,4-benzoxazin-3(4H)-ones. Farmaco 55, 715–718. doi: 10.1016/S0014-827X(00)00098-7
Palanki, M., Erdman, P. E., Goldman, M. E., Suto, C., and Suto, M. J. (2000). Synthesis and structure-activity relationship studies of conformationally restricted, analogs of 2-chloro-4-trifluoromethylpyrimidine- 5-[N-(3′,5′-bis(trifluoromethyl)phenyl)]carboxamide. Med. Chem. Res. 10, 19–29.
Patel, K., Piagentini, M., Rascher, A., Tian, Z.-Q., Buchanan, G. O., Regentin, R., et al. (2004). Engineered biosynthesis of geldanamycin analogs for Hsp90 inhibition. Chem. Biol. 11, 1625–1633. doi: 10.1016/j.chembiol.2004.09.012
Phuong, T. V., and Diep, C. N. (2020). Isolation and selection of actinobacteria against pathogenic bacteria from shrimp pond water on Duyen Hai District, Tra Vinh Province, Vietnam. Int. J. Environ. Agric. Res. 6:8.
Qin, S., Feng, W.-W., Zhang, Y.-J., Wang, T.-T., Xiong, Y.-W., and Xing, K. (2018). Diversity of bacterial microbiota of coastal halophyte Limonium sinense and amelioration of salinity stress damage by symbiotic plant growth-promoting actinobacterium Glutamicibacter halophytocola KLBMP 5180. Appl. Environ. Microbiol. 84, e01533–e01518. doi: 10.1128/AEM.01533-18
Qiu, H., Liu, R., and Long, L. (2019). Analysis of chemical composition of extractives by acetone and the chromatic aberration of teak (Tectona Grandis L.F.) from China. Molecules 24, 1–10. doi: 10.3390/molecules24101989
Qiu, D., Meng, H., Jin, L., Wang, S., Tang, S., Wang, X., et al. (2013). ChemInform abstract: synthesis of aryl trimethylstannanes from aryl amines: a sandmeyer-type stannylation reaction. Angew. Chem. Int. Ed. Eng. 125, 11581–11584. doi: 10.1002/anie.201304579
Ransom, C., Grey, T., Howatt, K., Johnson, E., Keese, R., Nurse, R., et al. (2012). Common and chemical names of herbicides approved by the weed science Society of America. Weed Sci. 60, 650–657.
Rebbert, R. E., and Ausloos, P. (1962). Intramolecular rearrangements in the solid phase photolysis of 4-methyl-2-hexanone and sec-butyl acetate. J. Chem. Phys. 37, 1158–1159. doi: 10.1063/1.1733239
Reddy, N., and Ananthaprasad, M. G. (2021). “Chapter 11 - polymeric materials for three-dimensional printing” in Additive manufacturing Woodhead publishing reviews: Mechanical engineering series. eds. M. Manjaiah, K. Raghavendra, N. Balashanmugam, and J. P. Davim (Darya Ganj, Delhi, India: Woodhead Publishing), 233–274.
Renner, M. K., Shen, Y.-C., Cheng, X.-C., Jensen, P. R., Frankmoelle, W., Kauffman, C. A., et al. (1999). Cyclomarins a−C, new antiinflammatory cyclic peptides produced by a marine bacterium (Streptomyces sp.). J. Am. Chem. Soc. 121, 11273–11276. doi: 10.1021/ja992482o
Reza, A. S. M. A., Haque, M. A., Sarker, J., Nasrin Mst, S., Rahman, M. M., Tareq, A. M., et al. (2021). Antiproliferative and antioxidant potentials of bioactive edible vegetable fraction of Achyranthes ferruginea Roxb. In cancer cell line. Food Sci. Nutr. 9, 3777–3805. doi: 10.1002/fsn3.2343
Rossi, A., Martins, M. P., Bitencourt, T. A., Peres, N. T. A., Rocha, C. H. L., Rocha, F. M. G., et al. (2021). Reassessing the use of undecanoic acid as a therapeutic strategy for treating fungal infections. Mycopathologia 186, 327–340. doi: 10.1007/s11046-021-00550-4
Salem, M. Z. M., Zayed, M. Z., Ali, H. M., and Abd El-Kareem, M. S. M. (2016). Chemical composition, antioxidant and antibacterial activities of extracts from Schinus molle wood branch growing in Egypt. J. Wood Sci. 62, 548–561. doi: 10.1007/s10086-016-1583-2
Santos, R. G., Hurtado, R., Gomes, L. G. R., Profeta, R., Rifici, C., Attili, A. R., et al. (2020). Complete genome analysis of Glutamicibacter creatinolyticus from mare abscess and comparative genomics provide insight of diversity and adaptation for Glutamicibacter. Gene 741:144566. doi: 10.1016/j.gene.2020.144566
Sapkota, A., Thapa, A., Budhathoki, A., Sainju, M., Shrestha, P., and Aryal, S. (2020). Isolation, characterization, and screening of antimicrobial-producing actinomycetes from soil samples. Int. J. Microbiol. 2020, 1–7. doi: 10.1155/2020/2716584
Satgé, J., Massol, M., and Rivière, P. (1973). Divalent germanium species as starting materials and intermediates in organo germanium chemistry. J. Organomet. Chem. 56, 1–39. doi: 10.1016/S0022-328X(00)89951-9
Saxena, D., and Stotzky, G. (2001). Bacillus thuringiensis (Bt) toxin released from root exudates and biomass of Bt corn has no apparent effect on earthworms, nematodes, protozoa, bacteria, and fungi in soil. Soil Biol. Biochem. 33, 1225–1230. doi: 10.1016/S0038-0717(01)00027-X
Schwabe, A. D., Bennett, L. R., and Bowman, L. P. (1964). Octanoic acid absorption and oxidation in humans. J. Appl. Physiol. 19, 335–337. doi: 10.1152/jappl.1964.19.2.335
Senarath, S., Yoshinaga, K., Nagai, T., Yoshida, A., Beppu, F., and Gotoh, N. (2018). Differential effect of cis-eicosenoic acid positional isomers on adipogenesis and lipid accumulation in 3T3-L1 cells. Eur. J. Lipid Sci. Technol. 120:1700512. doi: 10.1002/ejlt.201700512
Ser, H. L., Palanisamy, U., Yin, W.-F., Abd Malek, N., Chan, K.-G., Goh, B. H., et al. (2015). Presence of antioxidative agent, pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- in newly isolated Streptomyces mangrovisoli sp. nov. Front. Microbiol. 6:854. doi: 10.3389/fmicb.2015.00854
Shalini, K., Sharma, P. K., and Kumar, N. (2010). Imidazole and its biological activities: a review 13, 36–47.
Shawky, A. M., Ibrahim, N. A., Abdalla, A. N., Abourehab, M. A. S., and Gouda, A. M. (2021). Novel pyrrolizines bearing 3,4,5-trimethoxyphenyl moiety: design, synthesis, molecular docking, and biological evaluation as potential multi-target cytotoxic agents. J. Enzyme Inhib. Med. Chem. 36, 1312–1332. doi: 10.1080/14756366.2021.1937618
Silva, M. J., Samandar, E., Ye, X., and Calafat, A. M. (2013). In vitro metabolites of Di-2-ethylhexyl adipate (DEHA) as biomarkers of exposure in human biomonitoring applications. Chem. Res. Toxicol. 26, 1498–1502. doi: 10.1021/tx400215z
Simon, A., and Losada, C. (2008). (−)-(4 R,5 R )-4,5-Bis[hydroxy(diphenyl)methyl]-2,2-dimethyl-1,3-dioxolane.
Sivaprakash, S., Prakash, S., Mohan, S., and Jose, S. P. (2019). Quantum chemical studies and spectroscopic investigations on 2-amino-3-methyl-5-nitropyridine by density functional theory. Heliyon 5:e02149. doi: 10.1016/j.heliyon.2019.e02149
Skhirtladze, L., Leitonas, K., Bucinskas, A., Volyniuk, D., Mahmoudi, M., Mukbaniani, O., et al. (2022). 1,4-Bis(trifluoromethyl)benzene as a new acceptor for the design and synthesis of emitters exhibiting efficient thermally activated delayed fluorescence and electroluminescence: experimental and computational guiding. J. Mater. Chem. C 10, 4929–4940. doi: 10.1039/D1TC05420A
Soleha, M., Pratiwi, D., Sari, I., Hermiyanti, E., Yunarto, N., and Setyorini, H. (2020). Antioxidant activity of methanol extract Tetracera scanden L Merr predicted active compound of methanol extract with GCMS NIST library. J. Phys. Conf. Ser. 1665:012028. doi: 10.1088/1742-6596/1665/1/012028
Sridevi, V., Priyadarshini, P. S., and Gautam, Y. A. (2015). Selection of marine actinomycetes with bioactive potential isolated from sediments of Bay of Bengal and characterization of promising isolate, ABT-103. Microbiol. Res. J. Int. 10, 1–9. doi: 10.9734/BMRJ/2015/20132
Sridhar, K., Bhargavan, R., and Kasinathan, S. (2016). Phytochemical screening and gc-ms analysis of ethanolic extract of Tribulus terrestris. Int. J. Pharmacol. Res. 6, 44–50.
Srivastava, R., Mukerjee, A., and Verma, A. (2015). GC-MS analysis of Phytocomponents in, pet ether fraction of Wrightia tinctoria seed. Pharm. J. 7, 249–253. doi: 10.5530/pj.2015.4.7
Su, X., Halem, H. A., Thomas, M. P., Moutrille, C., Culler, M. D., Vicker, N., et al. (2012). Adamantyl carboxamides and acetamides as potent human 11β-hydroxysteroid dehydrogenase type 1 inhibitors. Bioorg. Med. Chem. 20, 6394–6402. doi: 10.1016/j.bmc.2012.08.056
Sugrue, I., O’Connor, P. M., Hill, C., Stanton, C., and Ross, R. P. (2019). Actinomyces produces defensin-like bacteriocins (Actifensins) with a highly degenerate structure and broad antimicrobial activity. J. Bacteriol. 202, 1–15. doi: 10.1128/JB.00529-19
Takigawa, H., Nakagawa, H., Kuzukawa, M., Mori, H., and Imokawa, G. (2005). Deficient production of hexadecenoic acid in the skin is associated in part with the vulnerability of atopic dermatitis patients to colonization by Staphylococcus aureus. DRM 211, 240–248. doi: 10.1159/000087018
Talley, J. J., Bertenshaw, S. R., Brown, D. L., Carter, J. S., Graneto, M. J., Kellogg, M. S., et al. (2000). N-[[(5-methyl-3-phenylisoxazol-4-yl)- phenyl]sulfonyl]propanamide, sodium salt, parecoxib sodium: a potent and selective inhibitor of COX-2 for parenteral administration. J. Med. Chem. 43, 1661–1663. doi: 10.1021/jm000069h
Tanak, H., Karataş, Ş., Meral, S., and Agar, A. A. (2020). Synthesis, molecular structure and quantum chemical studies of N-(2-fluorophenyl)-1-(5-nitrothiophen-2-yl)methanimine. Crystallogr. Rep. 65, 1212–1216. doi: 10.1134/S106377452007024X
Tanaka, T., and Hayashi, M. (2008). Catalytic enantioselective reformatsky reaction of alkyl iodoacetate with aldehydes catalyzed by chiral Schiff Base. Chem. Lett. 37, 1298–1299. doi: 10.1246/cl.2008.1298
Tang, W., Meng, Z., Li, N., Liu, Y., Li, L., Chen, D., et al. (2021). Roles of gut microbiota in the regulation of hippocampal plasticity, inflammation, and hippocampus-dependent behaviors. Front. Cell. Infect. Microbiol. 10, 1–15. doi: 10.3389/fcimb.2020.611014
Thakor, P., Mehta, J. B., Patel, R. R., Patel, D. D., Subramanian, R. B., and Thakkar, V. R. (2016). Extraction and purification of phytol from Abutilon indicum: cytotoxic and apoptotic activity. RSC Adv. 6, 48336–48345. doi: 10.1039/C5RA24464A
Tsunoda, H., Kudo, T., Masaki, Y., Ohkubo, A., Seio, K., and Sekine, M. (2011). Biochemical behavior of N-oxidized cytosine and adenine bases in DNA polymerase-mediated primer extension reactions. Nucleic Acids Res. 39, 2995–3004. doi: 10.1093/nar/gkq914
Upgade, A., and Bhaskar, A. (2013). Characterization and medicinal importance of phytoconstituents of C. Papaya from down south Indian region using gas chromatography and mass spectroscopy. Asian J. Pharm. Clin. Res. 6, 101–106.
Van der Steen, M., and Stevens, C. V. (2009). Undecylenic acid: a valuable and physiologically active renewable building block from castor oil. ChemSusChem 2, 692–713. doi: 10.1002/cssc.200900075
Viegas, C. A., Rosa, M. F., Sa-Correia, I., and Novais, J. M. (1989). Inhibition of yeast growth by octanoic and decanoic acids produced during ETHANOLIC fermentation. Appl. Environ. Microbiol. 55, 21–28. doi: 10.1128/aem.55.1.21-28.1989
Wang, C., Lu, Y., and Cao, S. (2020). Antimicrobial compounds from marine actinomycetes. Arch. Pharm. Res. 43, 677–704. doi: 10.1007/s12272-020-01251-0
Wang, X., Shen, S., Wu, H., Wang, H., Wang, L., and Lu, Z. (2021). Acinetobacter tandoii ZM06 assists Glutamicibacter nicotianae ZM05 in resisting cadmium pressure to preserve dipropyl phthalate biodegradation. Microorganisms 9:1417. doi: 10.3390/microorganisms9071417
Wang, Y., Su, X., Liu, J., Fang, W., and Zhu, L. (2022). Complete genome sequence of Glutamicibacter mysorens NBNZ-009, isolated from Jin Lake sediment. Microbiol. Resour. Announc. 0, e00762–e00722. doi: 10.1128/mra.00762-22
Wang, W., Xiao, G., Du, G., Chang, L., Yang, Y., Ye, J., et al. (2022). Glutamicibacter halophytocola-mediated host fitness of potato tuber moth on Solanaceae crops. Pest Manag. Sci. 78, 3920–3930. doi: 10.1002/ps.6955
Wang, Y., Zhang, L.-T., Feng, Y.-X., Guo, S.-S., Pang, X., Zhang, D., et al. (2019). Insecticidal and repellent efficacy against stored-product insects of oxygenated monoterpenes and 2-dodecanone of the essential oil from Zanthoxylum planispinum var. dintanensis. Environ. Sci. Pollut. Res. 26, 24988–24997. doi: 10.1007/s11356-019-05765-z
Watanabe, M., Koike, H., Ishiba, T., Okada, T., Seo, S., and Hirai, K. (1997). Synthesis and biological activity of methanesulfonamide pyrimidine- and N-methanesulfonyl pyrrole-substituted 3,5-dihydroxy-6-heptenoates, a novel series of HMG-CoA reductase inhibitors. Bioorg. Med. Chem. 5, 437–444. doi: 10.1016/S0968-0896(96)00248-9
Williams, J. D., Torhan, M. C., Neelagiri, V., Brown, C., Bowlin, N. O., Di, M., et al. (2015). Synthesis and structure-activity relationships of novel phenoxyacetamide inhibitors of the Pseudomonas aeruginosa type III secretion system (T3SS). Bioorg. Med. Chem. 23, 1027–1043. doi: 10.1016/j.bmc.2015.01.011
Windey, K., De Preter, V., Louat, T., Schuit, F., Herman, J., Vansant, G., et al. (2012). Modulation of protein fermentation does not affect fecal water toxicity: a randomized cross-over study in healthy subjects. PLoS One 7:e52387. doi: 10.1371/journal.pone.0052387
Xie, F.-M., Li, H.-Z., Dai, G.-L., Li, Y.-Q., Cheng, T., Xie, M., et al. (2019). Rational molecular design of dibenzo[a,c]phenazine-based thermally activated delayed fluorescence emitters for orange-red OLEDs with EQE up to 22.0%. ACS Appl. Mater. Interfaces 11, 26144–26151. doi: 10.1021/acsami.9b06401
Xiong, Y.-W., Ju, X.-Y., Li, X.-W., Gong, Y., Xu, M.-J., Zhang, C.-M., et al. (2020). Fermentation conditions optimization, purification, and antioxidant activity of exopolysaccharides obtained from the plant growth-promoting endophytic actinobacterium Glutamicibacter halophytocola KLBMP 5180. Int. J. Biol. Macromol. 153, 1176–1185. doi: 10.1016/j.ijbiomac.2019.10.247
Xu, Y., Li, H., Li, X., Xiao, X., and Qian, P.-Y. (2008). Inhibitory effects of a branched-chain fatty acid on larval settlement of the polychaete hydroides elegans. Mar. Biotechnol. 11, 495–504. doi: 10.1007/s10126-008-9161-2
Xue, J., Zhuo, J., Liu, M., Chi, Y., Zhang, D., and Yao, Q. (2017). Synergetic effect of co-pyrolysis of cellulose and polypropylene over an all-silica mesoporous catalyst MCM-41 using Thermogravimetry–Fourier transform infrared spectroscopy and pyrolysis–gas chromatography–mass spectrometry. Energy Fuel 31, 9576–9584. doi: 10.1021/acs.energyfuels.7b01651
Yamamoto, T. (2002). Chlorination of bisphenol a in aqueous media: formation of chlorinated bisphenol a congeners and degradation to chlorinated phenolic compounds. Chemosphere 46, 1215–1223. doi: 10.1016/S0045-6535(01)00198-9
Zagulyaeva, A. A., Yusubov, M. S., and Zhdankin, V. V. (2010). A general and convenient preparation of [bis(trifluoroacetoxy)iodo]perfluoroalkanes and [bis(trifluoroacetoxy)iodo]arenes by oxidation of organic iodides using oxone and trifluoroacetic acid. J. Organomet. Chem. 75, 2119–2122. doi: 10.1021/jo902733f
Zeitoun, M., Adel, M., Abulfotouh, F., and Ebrahim, S. (2021). Thermophysical properties enhancement of octadecane using reduced graphene oxide and graphene oxide nanoplatelets. J. Energy Storage 38:102512. doi: 10.1016/j.est.2021.102512
Zhang, D., Lu, Y., Chen, H., Wu, C., Zhang, H., Chen, L., et al. (2020). Antifungal peptides produced by actinomycetes and their biological activities against plant diseases. J. Antibiot. 73, 265–282. doi: 10.1038/s41429-020-0287-4
Zhang, L., Peng, X.-M., Damu, G. L. V., Geng, R.-X., and Zhou, C.-H. (2014). Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 34, 340–437. doi: 10.1002/med.21290
Zhang, X., Wang, Y.-X., Zhao, J., Duan, P., Chen, Y., and Chen, L. (2017). Structural insights into 9-Styrylanthracene-based luminophores: geometry control versus mechanofluorochromism and sensing properties. Chem. Asian J. 12, 830–834. doi: 10.1002/asia.201700183
Keywords: anticancer, chromatography, FESEM, Glutamicibacter mysorens, mangrove soil, microbial peptides
Citation: Karthik Y, Ishwara Kalyani M, Krishnappa S, Devappa R, Anjali Goud C, Ramakrishna K, Wani MA, Alkafafy M, Hussen Abduljabbar M, Alswat AS, Sayed SM and Mushtaq M (2023) Antiproliferative activity of antimicrobial peptides and bioactive compounds from the mangrove Glutamicibacter mysorens. Front. Microbiol. 14:1096826. doi: 10.3389/fmicb.2023.1096826
Received: 12 November 2022; Accepted: 26 January 2023;
Published: 17 February 2023.
Edited by:
Sumit Kumar, Amity University, IndiaReviewed by:
Amr Shehabeldine, Al-Azhar University, EgyptCopyright © 2023 Karthik, Ishwara Kalyani, Krishnappa, Devappa, Anjali Goud, Ramakrishna, Wani, Alkafafy, Hussen Abduljabbar, Alswat, Sayed and Mushtaq. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manjula Ishwara Kalyani, ✉ bWFuanVnYW5lc2g3MTc2QGdtYWlsLmNvbQ==; Muntazir Mushtaq, ✉ bXVudGF6aXJodWRhQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.