- 1School of Ecology and Nature Conservation, Beijing Forestry University, Beijing, China
- 2Department of Biology, National Museum of Natural Science, Taichung, Taiwan
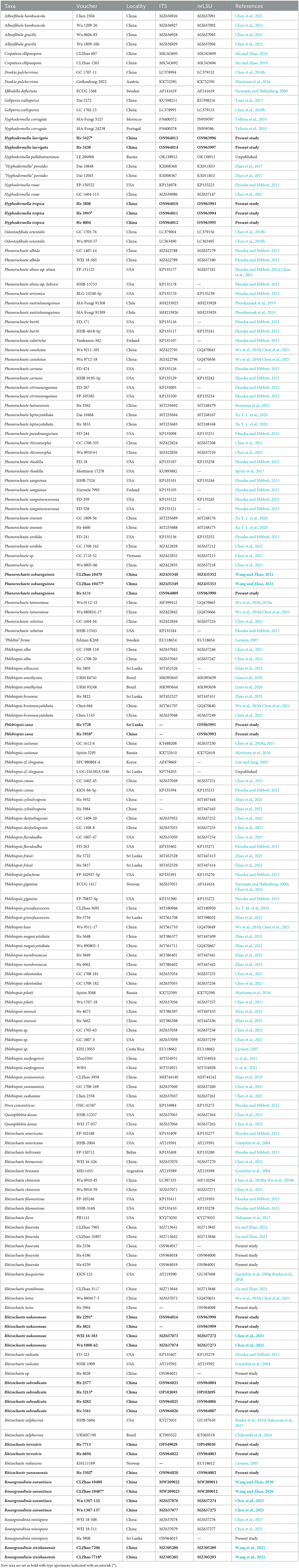
The species diversity, taxonomy, and phylogeny of five corticioid genera of Phanerochaetaceae, namely, Hyphodermella, Roseograndinia, Phlebiopsis, Rhizochaete, and Phanerochaete, in East Asia are studied by using the morphological and molecular methods. Phylogenetic analyses were performed separately for the Donkia, Phlebiopsis, Rhizochaete, and Phanerochaete clades based on ITS1-5.8S-ITS2 and nrLSU sequence data. In total, seven new species were found, two new combinations are suggested, and a new name is proposed. In the Donkia clade, Hyphodermella sensu stricto was strongly supported with two new lineages, namely H. laevigata and H. tropica, which were recovered. Hyphodermella aurantiaca and H. zixishanensis are members of Roseograndinia, while R. jilinensis is proved to be a later synonym of H. aurantiaca. In the Phlebiopsis clade, P. cana sp. nov. was found on the bamboo from tropical Asia. In the Rhizochaete clade, four new species, R. nakasoneae, R. subradicata, R. terrestris, and R. yunnanensis were recovered based mainly on molecular analyses. In the Phanerochaete clade, P. subsanguinea nom. nov. is proposed to replace Phanerochaete rhizomorpha C.L. Zhao & D.Q. Wang, which is an invalid name because it was published after Phanerochaete rhizomorpha C.C. Chen, Sheng H. Wu & S.H. He, representing another species. Descriptions and illustrations are provided for the new species, and discussions are given for new taxa and names. Identification keys to Hyphodermella species worldwide and Rhizochaete species in China are given separately.
Introduction
The species diversity, taxonomy, and phylogeny of the large phlebioid clade, which includes three families, namely, Phanerochaetaceae, Irpicaceae and Meruliaceae, have been intensively studied in recent years, and the number of taxa has been dramatically increased (Floudas and Hibbett, 2015; Miettinen et al., 2016; Justo et al., 2017; Nakasone et al., 2017, 2021; Chen et al., 2018b, 2020, 2021, 2022; Ma and Zhao, 2019; Huang and Zhao, 2020; Xu Y. L. et al., 2020; Gu and Zhao, 2021; Zhao et al., 2021; Li et al., 2022). To date, 60 genera of poroid, corticioid, and hydnoid fungi are recognized in the three families, which mostly cause white rot on both angiosperms and gymnosperms (Chen et al., 2021; Nakasone et al., 2021; Lira et al., 2022). Among corticioid fungi, Phanerochaete s.l. and Phlebia s.l. are the two largest groups in the clade, which have been divided into many small genera with the aid of molecular systematics.
East Asia, especially the tropic areas, has been shown to be rich in the species diversity of the corticioid fungi of the phlebioid clade (Ma and Zhao, 2019; Huang and Zhao, 2020; Xu Y. L. et al., 2020; Chen et al., 2021; Gu and Zhao, 2021; Li et al., 2021, 2022; Zhao et al., 2021). Although many new taxa were described from this region, the species diversity, especially for the cryptic species, has not been completely understood, and the generic positions of some species need to be further studied (Chen et al., 2021). In this study, we carried out complete taxonomic and phylogenetic studies on the newly collected specimens and some newly described taxa of corticioid fungi in Phanerochaetaceae from East Asia. Seven new species in Hyphodermella, Phlebiopsis, and Rhizochaete were found, and two new combinations of Roseograndinia and a new name for Phanerochaete are proposed. These results are continuations and supplements to the research of phlebioid fungi in China.
Materials and methods
Specimen collection
Field trips for specimen collection in several nature reserves and forest parks in China and other countries were carried out by the authors. In situ photos of the fungi were taken with a Canon EOS 70D camera (Canon Corporation, Japan). Fresh specimens were dried at 40°C with a portable drier (manufactured in Finland). Dried specimens were labeled and then stored in a refrigerator at −40°C for 2 weeks to kill the insects and their eggs before they were ready for morphological and molecular studies. Voucher specimens are deposited at the herbaria of Beijing Forestry University, Beijing, China (BJFC), the National Museum of Natural Science, Taichung, Taiwan (TNM), and the Center for Forest Mycology Research, Madison, Wisconsin, U.S.A. (CFMR). Herbarium code designations follow the Index Herbariorum.1
Morphological studies
Thin, freehand sections were made from dried basidiomes and mounted in 2% (w/v) aqueous potassium hydroxide (KOH) and 1% (w/v) aqueous phloxine. Amyloidity and dextrinoidity of basidiospores were checked in Melzer's reagent (IKI). Cyanophily of hyphal and basidiospore walls was observed in 1% (weight/volume) cotton blue in 60% (w/v) lactic acid (CB). Microscopic examinations were carried out with a Nikon Eclipse 80i microscope (Nikon Corporation, Japan) at magnifications up to 1,000×. Drawings were made with the aid of a drawing tube. The following abbreviations are used: IKI– = neither amyloid nor dextrinoid, CB– = acyanophilous, L = mean spore length, W = mean spore width, Q = L/W ratio, and n (a/b) = the number of spores (a) measured from the number of specimens (b). Color codes and names were followed as suggested by Kornerup and Wanscher (1978).
DNA extraction and sequencing
A CTAB plant genomic DNA extraction kit, DN14 (Aidlab Biotechnologies Co., Ltd, Beijing, China), was used to extract total genomic DNA from dried specimens, which was then amplified by the polymerase chain reaction (PCR), according to the manufacturer's instructions. The ITS1-5.8S-ITS2 region (ITS) was amplified with the primer pair ITS5/ITS4 (White et al., 1990) using the following protocol: initial denaturation at 95°C for 4 min, followed by 34 cycles at 94°C for 40 s, 58°C for 45 s, 72°C for 1 min, and the final extension at 72°C for 10 min. The nrLSU D1-D2 region (nrLSU) was amplified with the primer pair LR0R/LR72 using the following procedure: initial denaturation at 94°C for 1 min, followed by 34 cycles at 94°C for 30 s, 50°C for 1 min, 72°C for 1.5 min, and the final extension at 72°C for 10 min. DNA sequencing was performed at the Beijing Genomics Institute, and the sequences were deposited in GenBank3 (Table 1). BioEdit v.7.0.5.3 (Hall, 1999) and Geneious Basic v.11.1.15 (Kearse et al., 2012) were used to review the chromatograms and for contig assembly.
Phylogenetic analyses
Four separate datasets of concatenated ITS-nrLSU sequences of the Donkia, Phlebiopsis, Rhizochaete, and Phanerochaete clades in the Phanerochaeteaceae were analyzed. The clades recognition and ingroup and outgroup taxa selections were mainly discussed in the study by Chen et al. (2021). The Phanerochaete clade included only taxa in the core group of the genus. Rhizochaete radicata (Henn.) Gresl., Nakasone & Rajchenb. was selected as the outgroup for the Donkia and Phlebiopsis clades, while Phlebiopsis gigantea (Fr.) Jülich was used as the outgroup for the Rhizochaete and Phanerochaete clades. The ITS and nrLSU sequences were aligned separately using MAFFT v.74 (Katoh et al., 2017) with the G-INS-I iterative refinement algorithm and optimized manually in BioEdit v.7.0.5.3. Then, the separate alignments were concatenated using Mesquite v.3.5.1 (Maddison and Maddison, 2018).
Maximum parsimony (MP), maximum likelihood (ML) analyses, and Bayesian inference (BI) were carried out using PAUP* v.4.0b10 (Swofford, 2002), RAxML v.8.2.10 (Stamatakis, 2014), and MrBayes 3.2.6 (Ronquist et al., 2012), respectively. In MP analysis, trees were generated using 100 replicates of random stepwise addition of sequence and the tree-bisection reconnection (TBR) branch-swapping algorithm with all characters given equal weight. Branch supports for all parsimony analyses were estimated by performing 1,000 bootstrap replicates with a heuristic search of 10 random-addition replicates for each bootstrap replicate. In ML analysis, statistical support values were obtained using rapid bootstrapping with 1,000 replicates, with default settings for other parameters. For BI, the best-fit substitution model was estimated with jModeltest v.2.17 (Darriba et al., 2012). Four Markov chains were run for 1,000,000 for the three datasets of the Donkia, Phanerochaete, and Phlebiopsis clades and the 2,000,000 for the dataset of the Rhizochaete clade until the split deviation frequency value was lower than 0.01. Trees were sampled every 100th generation. The first quarter of the trees, which represented the burn-in phase of the analyses, were discarded, and the remaining trees were used to calculate posterior probabilities (BPP) in the majority rule consensus tree.
Results
Phylogenetic analyses
The dataset of the Donkia clade contained 39 ITS and 38 nrLSU sequences from 39 samples, representing 19 ingroup taxa and the outgroup (Table 1), and had an aligned length of 2,009 characters, of which 251 were parsimony-informative. MP analysis yielded two equally parsimonious trees (TL = 899, CI = 0.617, RI = 0.793, RC = 0.489, HI = 0.383). The dataset of Phlebiopsis clade contained 45 ITS and 46 nrLSU sequences from 49 samples, representing 28 ingroup taxa and the outgroup, and had an aligned length of 2,006 characters, of which 224 were parsimony-informative. MP analysis yielded 17 equally parsimonious trees (TL = 862, CI = 0.542, RI = 0.770, RC = 0.417, HI = 0.458). The dataset of the Rhizochaete clade contained 34 ITS and 35 nrLSU sequences from 37 samples, representing 20 ingroup taxa and the outgroup, and had an aligned length of 2,164 characters, of which 295 were parsimony-informative. MP analysis yielded nine equally parsimonious trees (TL = 1,150, CI = 0.572, RI = 0.703, RC = 0.402, HI = 0.428). The dataset of the Phanerochaete clade contained 42 ITS and 33 nrLSU sequences from 42 samples, representing 24 ingroup taxa and the outgroup, and had an aligned length of 2,065 characters, of which 275 were parsimony-informative. MP analysis yielded 19 equally parsimonious trees (TL = 885, CI = 0.591, RI = 0.745, RC = 0.440, HI = 0.409).
jModelTest suggested GTR+I+G as the best-fit models of nucleotide evolution for the four datasets. The average standard deviations of the split frequencies of BI were 0.007436, 0.007608, 0.007786, and 0.006954 for the Donkia, Phlebiopsis, Rhizochaete, and Phanerochaete clades, respectively, at the end of the runs. The MP and BI analyses resulted in almost identical tree topologies with the ML analysis. Only the ML trees of the four clades are shown in Figures 1–4 with the parsimony bootstrap values (≥50%, first), the likelihood bootstrap values (≥50%, second), and Bayesian posterior probabilities (≥0.95, third) labeled along the branches.
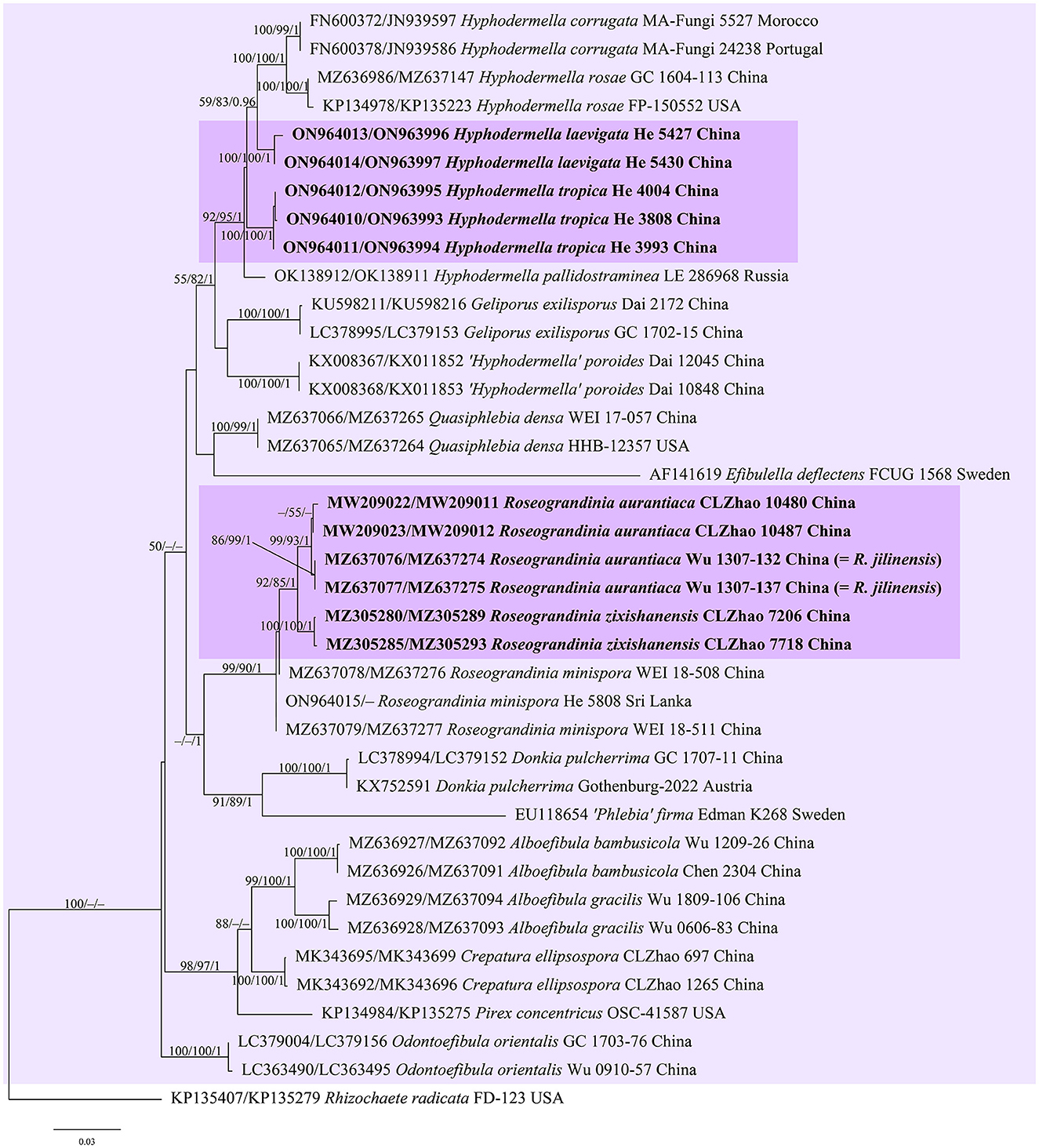
Figure 1. Phylogenetic tree obtained from ML analysis of the ITS-nrLSU sequences of the Donkia clade. Branches are labeled with parsimony bootstrap values (≥50%, first), likelihood bootstrap values (≥50%, second), and Bayesian posterior probabilities (≥0.95, third). New taxa are set in bold and highlighted.
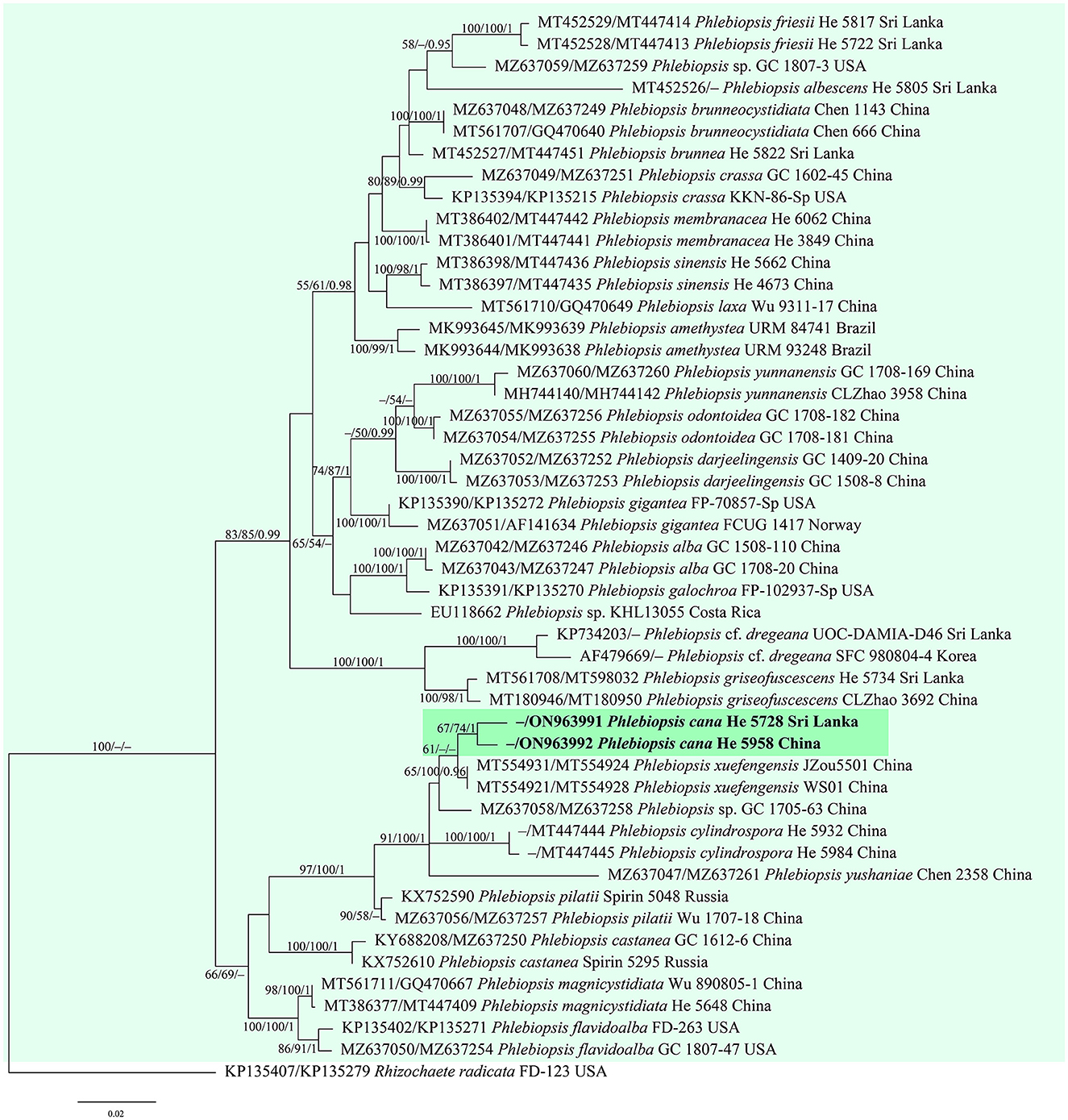
Figure 2. Phylogenetic tree obtained from ML analysis of the ITS-nrLSU sequences of Phlebiopsis. Branches are labeled with parsimony bootstrap values (≥50%, first), likelihood bootstrap values (≥50%, second), and Bayesian posterior probabilities (≥0.95, third). The new species are set in bold and highlighted.
Figure 3. Phylogenetic tree obtained from ML analysis of the ITS-nrLSU sequences of Rhizochaete. Branches are labeled with parsimony bootstrap values (≥50%, first), likelihood bootstrap values (≥50%, second), and Bayesian posterior probabilities (≥0.95, third). New species are set in bold and highlighted.
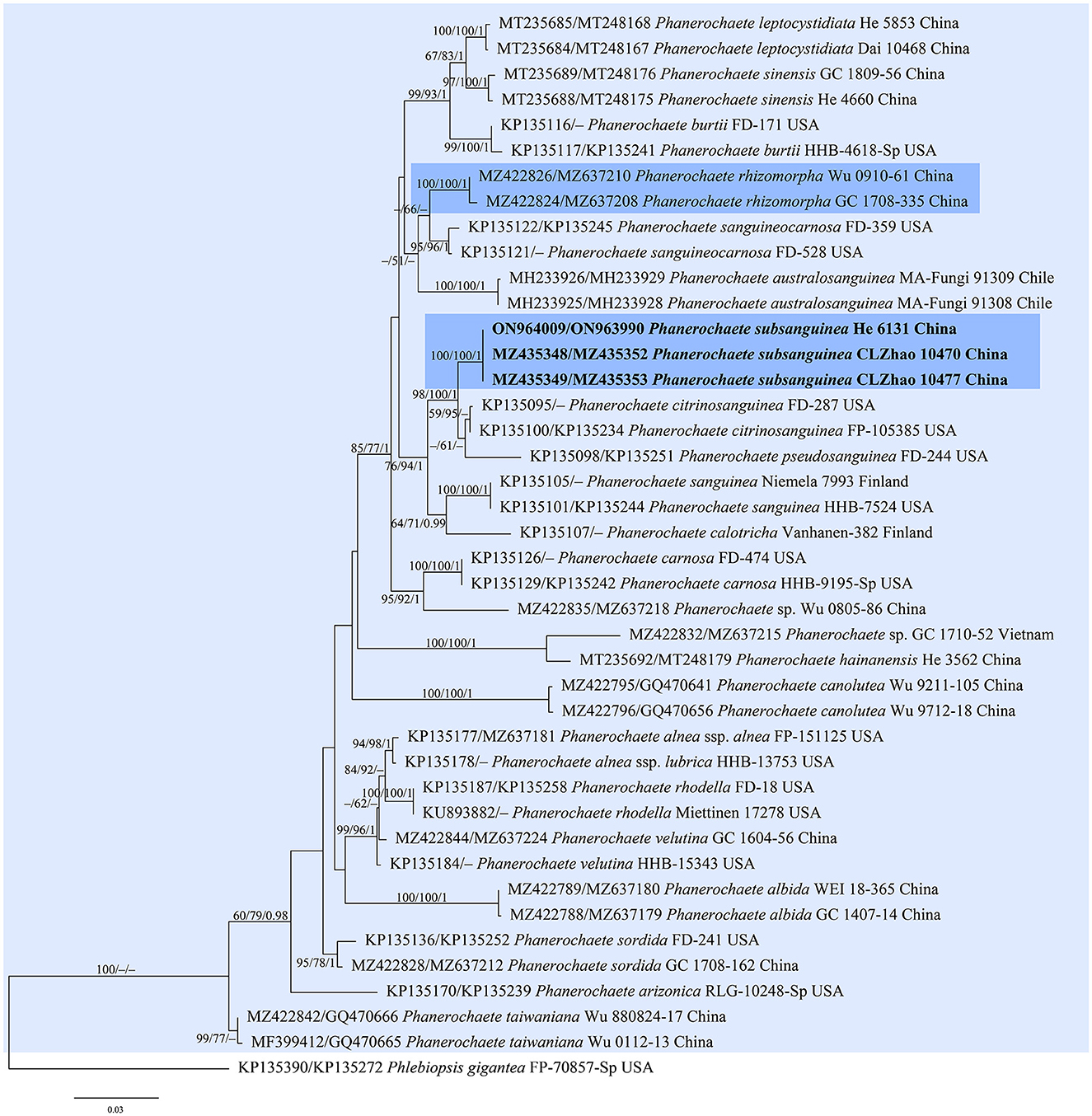
Figure 4. Phylogenetic tree obtained from ML analysis of the ITS-nrLSU sequences of Phanerochaete. Branches are labeled with parsimony bootstrap values (≥50%, first), likelihood bootstrap values (≥50%, second), and Bayesian posterior probabilities (≥0.95, third). The new replacement names are set in bold and highlighted.
In the trees (Figures 1–4), seven new species, Hyphodermella laevigata, H. tropica, Phlebiopsis cana, Rhizochaete nakasoneae, R. subradicata, R. terrestris, and R. yunnanensis, formed distinct lineages. In our tree of the Donkia clade (Figure 1), Hyphodermella s.s. and Roseograndinia were strongly supported as separate genera (92/95/1 and 99/90/1). Meanwhile, H. zixishanensis C.L. Zhao and H. aurantiaca C.L. Zhao that is conspecific with R. jilinensis C.C. Chen & Sheng H. Wu were nested within Roseograndinia. In the Phanerochaete clade (Figure 4), the new names P. subsanguinea (= P. rhizomorpha C.L. Zhao & D.Q. Wang) and P. rhizomorpha (C.C. Chen, Sheng H. Wu & S.H. He) formed two distinct lineages.
Taxonomy
Hyphodermella laevigata Yue Li & S.H. He, sp. nov.
MycoBank: MB846336
Diagnosis—The species is recognized by a smooth hymenophore, the absence of cystidia and cystidioid hyphal ends, and small ellipsoid basidiospores.
Type—China, Guizhou Province, Chishui County, Suoluo Nature Reserve, on a dead angiosperm branch, 7 July 2018, He 5427 (BJFC 026488, holotype).
Etymology—Refer to the smooth hymenophore.
Fruiting body—Basidiomes annual, resupinate, widely effused, closely adnate, inseparable from substrate, and membranaceous, first as small patches, later confluent up to 10 cm long, 2 cm wide, and up to 80 μm thick in section. Hymenophore smooth, pale yellow (4A3) to grayish yellow (4B3), slightly darkening in KOH, and not cracked; margin thinning out, determinate, adnate, fimbriate, and concolorous with hymenophore surface; and context is pale yellow.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate. Subiculum distinct; hyphae colorless, thick-walled, smooth, frequently branched, moderately septate, loosely interwoven, and 2.5–6.5 μm in diameter. Subhymenium thin; hyphae colorless, thin-walled, smooth, infrequently branched, moderately septate, more or less vertical, and 1.5–2.5 μm in diameter. Cystidia absent. Basidia clavate to subcylindrical, colorless, thin-walled, smooth, with a basal simple septum and four sterigmata, and 10–30 × 5–7 μm; basidioles similar to basidia, but smaller. Basidiospores ellipsoid, with an apiculus, usually with one or two oil drops, colorless, thin-walled, smooth, (4.6–) 4.8–6 (−6.3) × 3–3.8 (−4.1) μm, L = 5.3 μm, W = 3.5 μm, and Q = 1.46–1.56 (n = 60/2).
Additional specimens examined—China, Guizhou Province, Chishui County, Suoluo Nature Reserve, on a dead angiosperm branch, 7 July 2018, He 5430 (BJFC 026491).
Notes—Hyphodermella laevigata (Figure 5) is characterized by membranaceous basidiomes with smooth hymenophores, the absence of cystidia and cystidioid hyphal ends, and small ellipsoid basidiospores. In the phylogenetic tree (Figure 1), H. laevigata formed a distinct lineage sister to H. corrugata (Fr.) J. Erikss. & Ryvarden and H. rosae (Bres.) Nakasone, which have grandinioid or odontioid hymenophores, encrusted hyphal ends projecting beyond the hymenium and obviously larger basidiospores (8–10 × 5–7 μm of H. corrugata, 7–8.5 × 4.5–5.5 μm of H. rosae, Bernicchia and Gorjón, 2010). Hyphodermella maunakeaensis Gilb. & Hemmes from Hawaii is similar to H. laevigata by sharing a loose texture and relatively small basidiospores but differs in having a finely hydnaceous hymenophore with projecting fascicles of encrusted hyphoid cystidia and slightly longer basidiospores (6.5–7.5 μm, Gilbertson, 2001).
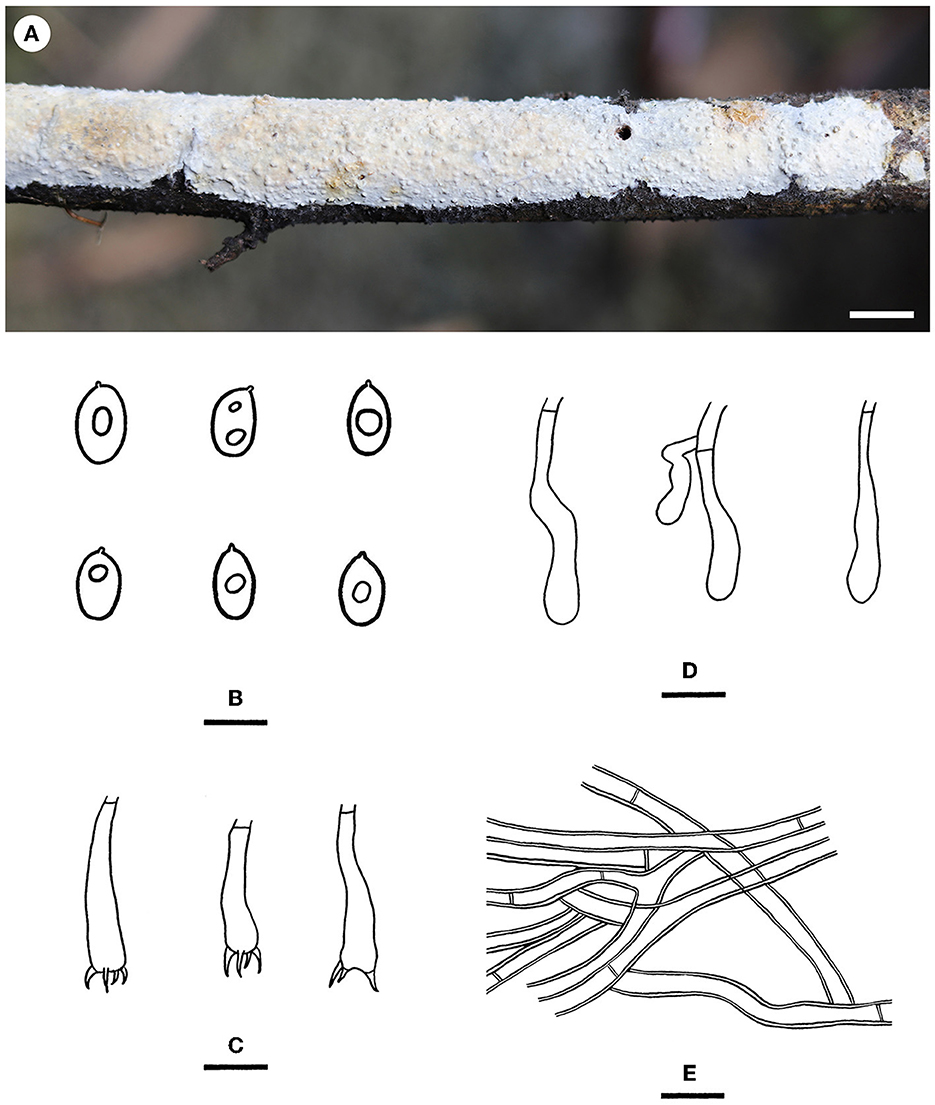
Figure 5. Hyphodermella laevigata [from the holotype He 5427; scale bars: (A) = 1 cm; (B–E) = 10 μm]. (A) Basidiomes; (B) Basidiospores; (C) Basidia; (D) Basidioles; (E) Hyphae from subiculum.
Hyphodermella tropica Yue Li & S.H. He, sp. nov.
MycoBank: MB846337
Diagnosis—The species is recognized by a grandinioid hymenophore, the presence of encrusted cystidioid hyphal ends, and broad ellipsoid to ovoid basidiospores.
Type—China, Hainan Province, Baoting County, Qixianling Forest Park, on a dead angiosperm branch, 11 June 2016, He 3993 (BJFC 022495, holotype, CFMR, isotype).
Etymology—Refer to the known distribution in tropic China.
Fruiting body—Basidiomes annual, resupinate, widely effused, closely adnate, inseparable from substrate, and coriaceous, first as small patches and later confluent up to 11 cm long, 3 cm wide, and up to 80 μm thick in section. Hymenophore grandinioid, grayish orange (5B4) to brownish orange [5C (4–5)], darkening in KOH, and not cracked upon drying; the margin thinning out, adnate, indistinct, and concolorous with hymenophore surface; and context is grayish orange.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate. Subiculum distinct; hyphae colorless, thick-walled, smooth, frequently branched, moderately septate, more or less parallel to the substrate, and 2–4 μm in diameter. Subhymenium distinct, thickening, composed of encrusted cystidioid hyphal ends, and hyphae with masses of crystals; hyphae colorless, slightly thick-walled, smooth, moderately branched and septate, loosely interwoven, and 1.5–3 μm in diameter. Cystidioid hyphal ends present, subcylindrical, colorless, thick-walled, heavily encrusted, mostly embedded, and 20–50 × 6–10 μm (crystals included). Basidia subcylindrical, colorless, thin-walled, smooth, usually with oil drops, with a basal simple septum and four sterigmata, and 15–27 × 4–8 μm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores which are broadly ellipsoid to ovoid, with an apiculus, usually with oil drops, colorless, thin-walled, smooth, IKI–, CB–, 5–6 (−6.5) × (3–) 4–5 (−5.5) μm, L = 5.7 μm, W = 4.4 μm, and Q = 1.23–1.34 (n = 60/2).
Additional specimens examined—China, Guizhou Province, Libo County, Maolan Nature Reserve, on a dead angiosperm branch, 16 June 2016, He 3808 (BJFC 022307, CFMR); Hainan Province, Baoting County, Qixianling Forest Park, on a dead angiosperm branch, 11 June 2016, He 4004 (BJFC 022506, CFMR).
Notes—Hyphodermella tropica (Figure 6) is characterized by coriaceous basidiomes with a grandinioid hymenophore, the presence of encrusted cystidioid hyphal ends, and broadly ellipsoid to ovoid basidiospores. In the phylogenetic tree (Figure 1), H. tropica formed a distinct lineage sister to H. pallidostraminea Bukharova & Volobuev and H. laevigata. Hyphodermella pallidostraminea from Russia differs from H. tropica by having a smooth to slightly tuberculate hymenophore, not encrusted cystidioid hyphal ends in hymenium, and narrower ellipsoid basidiospores (3–3.5 μm, Crous et al., 2021). Hyphodermella laevigata can be easily distinguished from H. tropica by the smooth hymenophore, the loose texture of the subiculum, and the lack of encrusted cystidioid hyphal ends. Hyphodermella brunneocontexta Duhem & Buyck from France is similar to H. tropica by sharing an odontioid hymenophore, encrusted cystidioid hyphal ends, and ellipsoid to ovoid basidiospores but differs in having a brown context with densely interwoven hyphae (Duhem and Buyck, 2011).
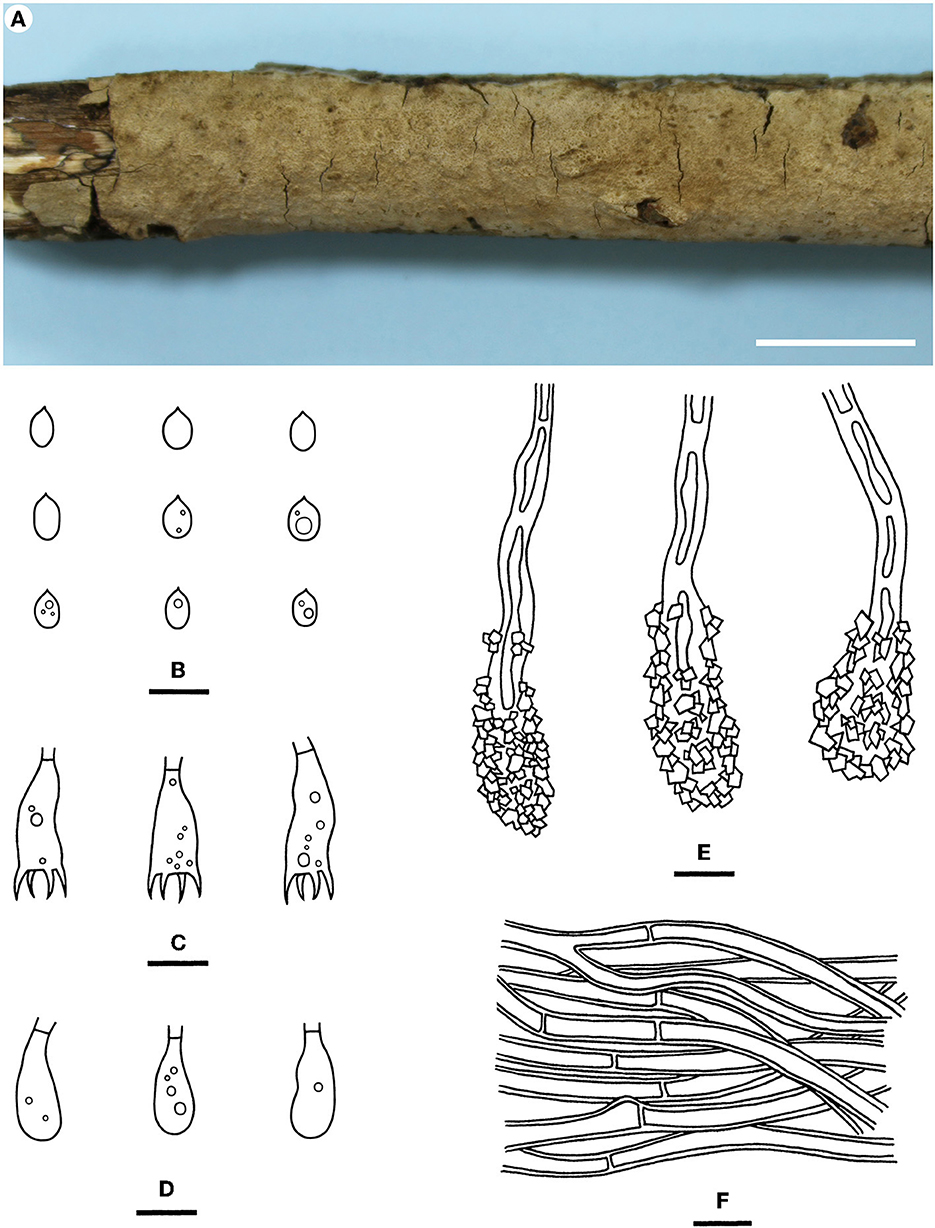
Figure 6. Hyphodermella tropica [from the holotype He 3933; scale bars: (A) = 1 cm; (B–F) = 10 μm]. (A) Basidiomes; (B) Basidiospores; (C) Basidia; (D) Basidioles; (E) Encrusted cystidioid hyphal ends; (F) Hyphae from subiculum.
Roseograndinia aurantiaca (C.L. Zhao) Yue Li & S.H. He, comb. nov.
MycoBank: MB846338
= Hyphodermella aurantiaca C.L. Zhao, Annales Botanici Fennici 58: 65, 2020. [MB#837949]
= Roseograndinia jilinensis C.C. Chen & Sheng H. Wu, Fungal Diversity 111: 396, 2021. [MB#840765]
Notes—Both H. aurantiaca and R. jilinensis were recently described from China, and are similar to each other by sharing a smooth to tuberculate, reddish hymenophore, a compact context with a thickened subhymenium, the absence of cystidia, and relatively small ellipsoid basidiospores but differ in the size of the basidiospores according to the descriptions (3–4 × 2–2.8 μm of H. aurantiaca vs. 4.6–5.5 × 2.6–3.1 μm of R. jilinensis, Wang and Zhao, 2020; Chen et al., 2021). However, in our phylogenetic tree (Figure 1), the types of the two species clustered in a strongly supported lineage in the Roseograndinia clade (99/93/1) with the ITS sequence similarity reaching 99.5% (3 differences of 600 base pairs). Thus, based on the morphological and molecular evidence, the new combination R. aurantiaca is proposed herein, since the epithet “aurantiaca” has the priority.
Roseograndinia zixishanensis (C.L. Zhao) Yue Li & S.H. He, comb. nov.
MycoBank: MB846339
= Hyphodermella zixishanensis C.L. Zhao, Nordic Journal of Botany 39 (8): e03329, 4, 2021. [MB#839869]
Notes—The species H. zixishanensis was recently described from Yunnan Province, southwestern China, based on morphological and molecular evidence (Wang et al., 2021). In our phylogenetic tree based on a more complete sampling, including many newly described taxa (Figure 1), the species was nested within the Roseograndinia clade, which was strongly supported as a monophyletic clade with three species and independent of Hyphodermella. Morphologically, Roseograndinia zixishanensis has the typical characteristics of the genus by possessing ceraceous basidiomes with a smooth to tuberculate hymenophore, a monomitic hyphal system with simple-septate generative hyphae, and a lack of cystidia (Chen et al., 2021; Wang et al., 2021).
Phlebiopsis cana Yue Li & S.H. He, sp. nov.
MycoBank: MB846340
Diagnosis—The species is recognized by a smooth hymenophore with a gray to brownish gray hymenial surface, the presence of short lamprocystidia and short cylindrical basidiospores, and its growth on bamboo.
Type—China, Hainan Province, Lingshui County, Diaoluoshan Nature Reserve, on a culm of dead bamboo, 2 July 2019, He 5958 (BJFC 030834, holotype).
Etymology—Refer to the gray color of basidiomes.
Fruiting body—Basidiomes annual, resupinate, widely effused, closely adnate, inseparable from the substrate, and coriaceous, first as small patches and later confluent up to 11 cm long, 2 cm wide, and up to 40 μm thick in section. Hymenophore smooth, gray (4C1) to brownish gray (4D2), slightly darkening in KOH, and not cracked upon drying; the margin thinning out, adnate, indistinct, and concolorous with hymenophore surface when juvenile, and darkening with age; and context is gray.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate. Subiculum distinct, a compact texture, pale yellowish-brown, and up to 20 μm thick; hyphae colorless to pale yellow, thick-walled, smooth, rarely branched, moderately septate, more or less parallel to the substrate, slightly agglutinated, and 3–4 μm in diameter. Subhymenium indistinct; hyphae colorless, slightly thick-walled, rarely branched, moderately septate, densely interwoven, and 2–3 μm in diameter. Lamprocystidia scattered, subcylindrical to subfusiform, colorless, thick-walled, apically encrusted with small crystals, projecting beyond the hymenium up to 10 μm, and 15–30 × 5–7 μm (crystals included). Basidia clavate, colorless, thin-walled, smooth, with a basal simple septum and four sterigmata, and 12–18 × 3.5–5 μm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores short cylindrical, with an apiculus, colorless, thin-walled, smooth, IKI–, CB–, (4.6–) 4.8–5.6 (−5.8) × 1.8–2 (−2.2) μm, L = 5.4 μm, W = 2 μm, and Q = 2.66–2.77 (n = 60/2).
Additional specimen examined—Sri Lanka, Western Province, Ingiriya, Dombagaskanda Forest Reserve, on a culm dead bamboo, 27 February 2019, He 5728 (BJFC 030595).
Notes—Phlebiopsis cana (Figure 7) is characterized by having thin basidiomes with a gray, smooth hymenophore surface, short lamprocystidia, and cylindrical basidiospores. In the phylogenetic tree (Figure 2), P. cana formed a lineage sister to P. xuefengensis J. Zou, which is an endophyte of Gastrodia elata from China. The descriptions of P. xuefengensis were based on cultures and not standard or comparable with P. cana (Li et al., 2021). Phlebiopsis cylindrospora Y.N. Zhao & S.H. He that was also found on bamboos in China is similar to P. cana by sharing short lamprocystidia and cylindrical basidiospores but differs in having hymenophores turning purple in KOH, generative hyphae encrusted with yellow, resinous granules, and slightly larger basidiospores (5.5–7.5 × 1.8–2.8 μm, Zhao et al., 2021). Another Phlebiopsis species on bamboo from Taiwan, P. yushaniae C.C. Chen & Sheng H. Wu, differs from P. cana by possessing white to cream basidiomes, possessing broadly ellipsoid to subglobose basidiospores (6.9–7.9 × 5.2–6.2 μm), and possessing a lack of cystidia (Chen et al., 2021). Phylogenetically, P. cana, P. cylindrospora, and P. yushaniae are grouped together with strong support values but formed distinct lineages (Figure 2).
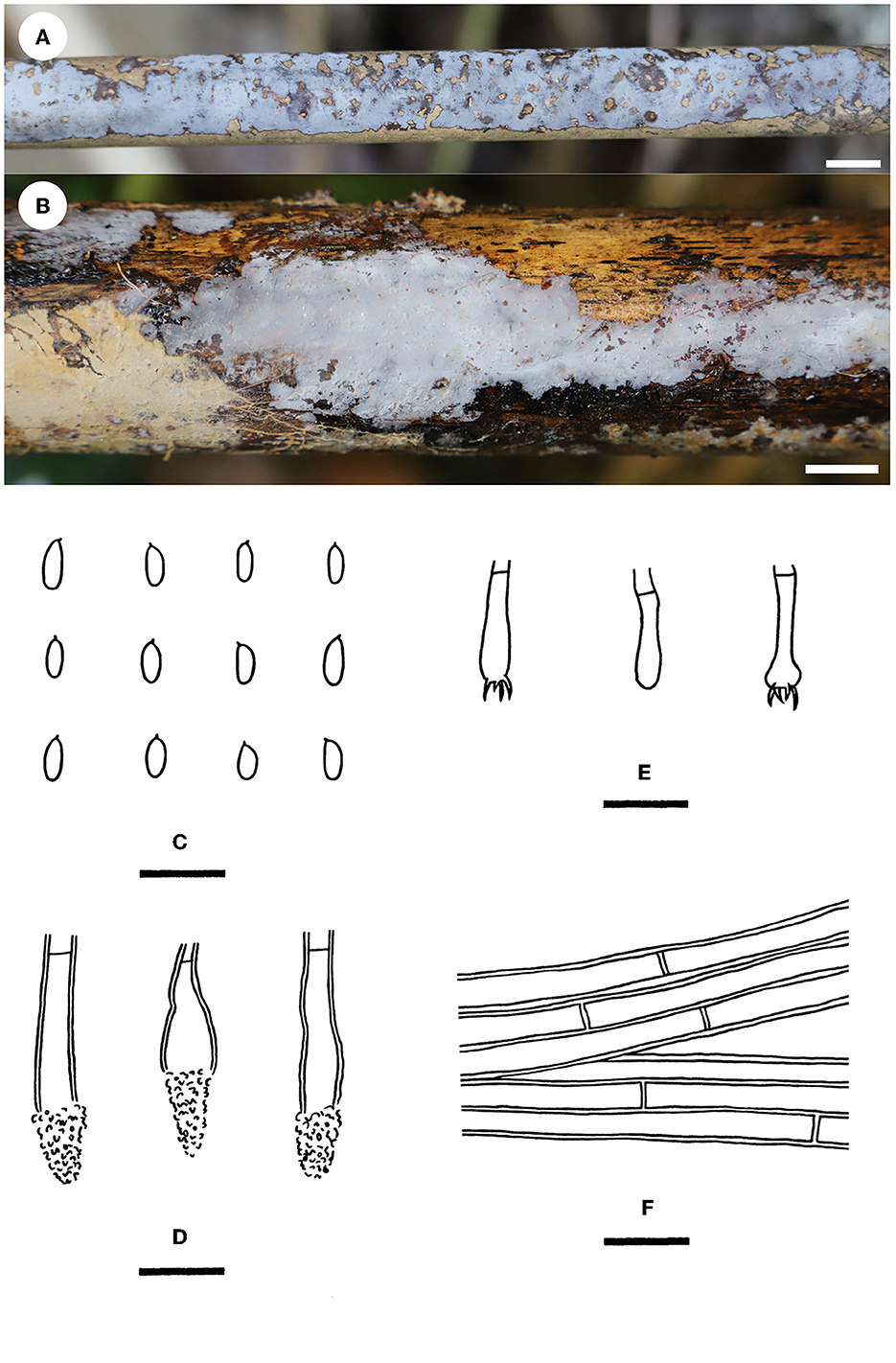
Figure 7. Phlebiopsis cana [(A, C–F) from the holotype He 5958; (B) from He 5728; scale bars: (A, B) = 1 cm; (C–F) = 10 μm]. (A, B) Basidiomes; (C) Basidiospores; (D) Basidia and a basidiole; (E) Lamprocystidia; (F) Hyphae from subiculum.
Rhizochaete nakasoneae Yue Li, C.C. Chen & S.H. He, sp. nov.
MycoBank: MB846341
Diagnosis—The species is recognized by a smooth hymenophore turning pinkish buff in KOH, the presence of thick-walled lamprocystidia, and broadly ellipsoid to ovoid basidiospores.
Type—China, Hunan Province, Zhangjiajie, Zhangjiajie Forest Park, on a fallen angiosperm trunk, 7 July 2015, He 2291 (BJFC 020746, holotype).
Etymology—Named to honor Dr. Karen K. Nakasone (CFMR, USA), who contributed much to the taxonomy and phylogeny of Rhizochaete.
Fruiting body—Basidiomes annual, resupinate, widely effused, loosely adnate, easily separated from substrate, and membranaceous to pellicular, first as small patches, later confluent up to 4 cm long, 2 cm wide, and up to 120 μm thick in section. Hymenophore smooth, light orange (6A5) to grayish orange (5B5), turning pinkish buff in KOH, not cracked upon drying; the margin thinning out, adnate, fimbriate, sterile, white when fresh, and becoming pale yellow upon drying; hyphal cords are present, brownish yellow, and turning pinkish buff in KOH; and context is pale yellow.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate. Subiculum distinct; hyphae colorless, thin- to slightly thick-walled, usually encrusted with crystals, frequently branched and septate, loosely interwoven, and 3–5 μm in diameter. Subhymenium thickening, composed of lamprocystidia and hyphae; hyphae colorless, thin- to slightly thick-walled, smooth, vertically arranged, moderately branched, rarely septate, and 2–3 μm in diameter. Lamprocystidia numerous, metuloid, subfusiform, colorless, thick-walled, heavily encrusted with crystals, embedded or projecting beyond the hymenium up to 25 μm, and 34–50 × 8–14 μm (crystals included), with walls up to 1.5–2 μm. Basidia clavate, colorless, thin-walled, smooth, with a basal simple septum and four sterigmata, and 18–24 × 3.5–5 μm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores broadly ellipsoid to ovoid, with an apiculus, colorless, thin-walled, smooth, usually with one or two oil drops, IKI–, CB–, 2.5–4 × (1.8–) 2–2.5 μm, L = 3.1 μm, W = 2.1 μm, and Q = 1.34–1.59 (n = 60/2).
Additional specimens examined—China, Guangxi Zhuang Autonomous Region, Xing'an County, Mao'ershan Nature Reserve, on an angiosperm stump, 13 July 2017, He 4821 (BJFC 024340, CFMR); Hunan Province, Zhangjiajie, Zhangjiajie Forest Park, on a rotten angiosperm trunk, 17 August 2010, Wu 1008-62 (TNM F0025327); Taiwan, Nantou County, Jenai Township, Aowanda National Forest Recreation Area, on a fallen angiosperm branch, 3 October 2016, WEI 16-383 (TNM F0031055).
Notes—Rhizochaete nakasoneae (Figures 8A, 9) is characterized by the thin pellicular basidiomes, simple-septate generative hyphae, thick-walled subfusiform cystidia, and small, broadly ellipsoid to ovoid basidiospores. Morphologically and phylogenetically, R. belizensis Nakasone, K. Draeger & B. Ortiz is closely related to R. nakasoneae but differs by having thicker basidiomes, encrusted hyphae, and distribution in Belize (Nakasone et al., 2017). Rhizochaete fissurata C.L. Zhao, recently described from China, formed a sister lineage to R. nakasoneae and R. belizensis but differs from R. nakasoneae in having thicker basidiomes with a cracked hymenophore, thinner cystidia walls (<1 μm), and slightly larger basidiospores (3–4.5 × 2.5–3 μm, Gu and Zhao, 2021). Rhizochaete radicata differs from R. nakasoneae by longer cystidia (60–100 μm) and slightly larger basidiospores (4–5 × 2.5–3 μm, Greslebin et al., 2004).
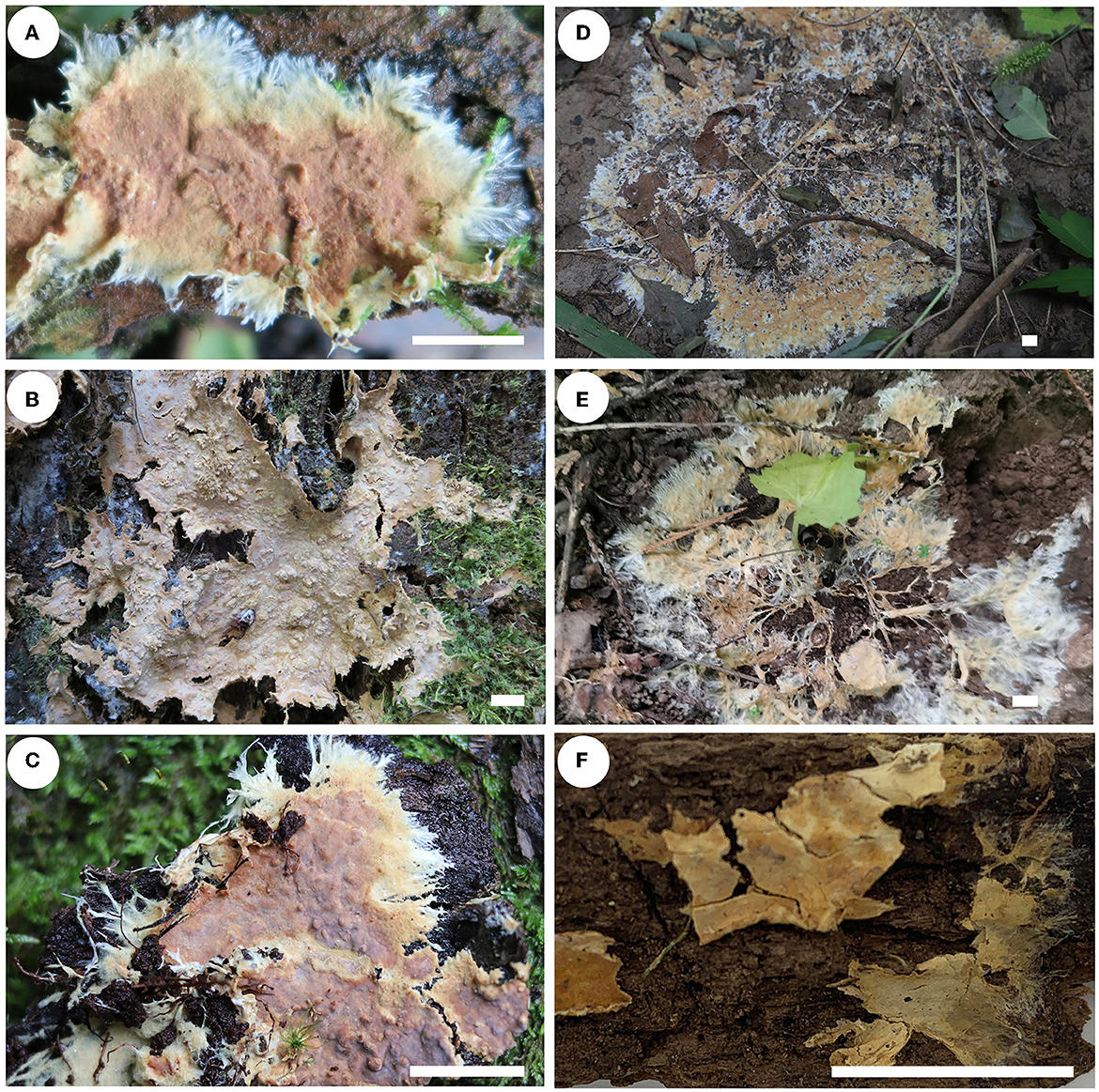
Figure 8. Basidiomes of Rhizochaete [scale bars: (A–F) = 1 cm]. (A) R. nakasoneae (He 4821, paratype); (B, C) R. subradicata [(B) He 3213, holotype; (C) He 5561, paratype]; (D, E) R. terrestris [(D) He 7713, holotype; (E) He 6694, paratype]; (F) R. yunnanensis (He 3302, holotype).
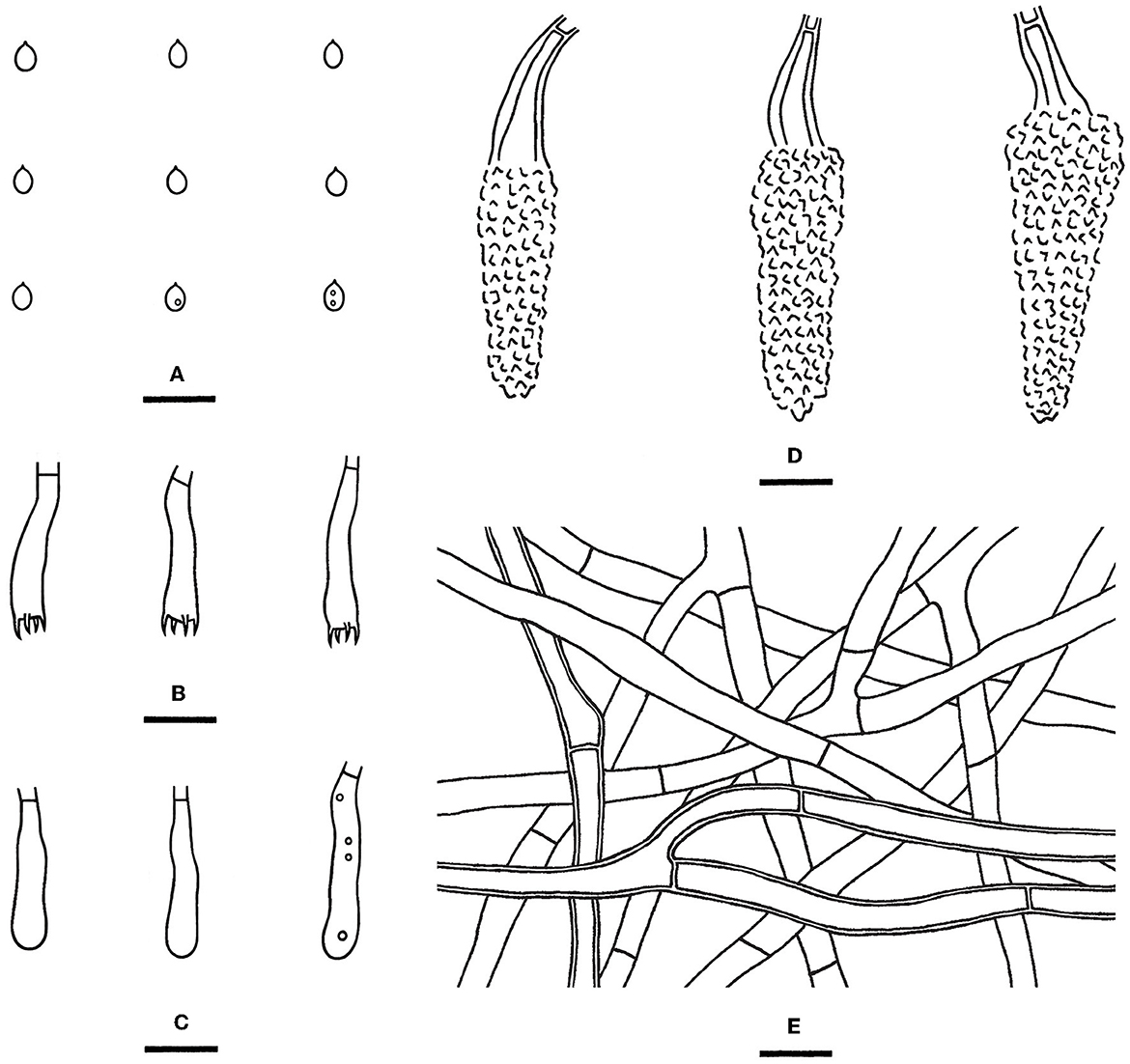
Figure 9. Rhizochaete nakasoneae [from the holotype He 2291; scale bars: (A–E) = 10 μm]. (A) Basidiospores; (B) Basidia; (C) Basidioles; (D) Lamprocystidia; (E) Hyphae from subiculum.
Rhizochaete subradicata Yue Li & S.H. He, sp. nov.
MycoBank: MB846342
Diagnosis—The species is recognized by a smooth hymenophore turning reddish brown in KOH, the presence of long lamprocystidia, and small ellipsoid basidiospores.
Type—China, Yunnan Province, Yongping City, Baotaishan Forest Park, on an angiosperm stump, 27 November 2015, He 3213 (BJFC 021608, holotype).
Etymology—Refer to the morphological similarity and close phylogenetic relationship of R. radicata.
Fruiting body—Basidiomes annual, resupinate, widely effused, loosely adnate, easily separated from substrate, and membranaceous to pellicular, first as small patches, later confluent up to 6 cm long, 3 cm wide, and up to 200 μm thick in section. Hymenophore smooth, light orange (5A5) to grayish orange (5B5), turning reddish brown in KOH, and not cracked upon drying; the margin thinning out, adnate, fimbriate, sterile, white or pale orange when juvenile, and slightly darkening with age; hyphal cords present, brownish yellow, and turning reddish brown in KOH; and context is pale yellow.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate. Subiculum distinct, a loose texture; hyphae colorless, slightly thick-walled, smooth, frequently branched and septate, more or less parallel to substrate, and 3–6 μm in diameter. Subhymenium thickening, composed of lamprocystidia and hyphae; hyphae colorless, slightly thick-walled, smooth, more or less vertically arranged, moderately branched and septate, and 2.5–4 μm in diameter. Lamprocystidia numerous, subcylindrical to subfusiform, slightly thick-walled, encrusted with crystals in the upper part, mostly embedded or slightly projecting beyond the hymenium, and 40–75 (−90) × 7–15 μm (crystals included), with walls up to 1.5–2 μm. Basidia clavate, colorless, thin-walled, smooth, with a basal simple septum and four sterigmata, and 25–40 × 4–6 μm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores ellipsoid, with an apiculus, colorless, thin-walled, smooth, usually with one or two oil drops, IKI–, CB–, (3.5–) 3.8–4.5 (−4.8) × 2.2–2.8 μm, L = 4.2 μm, W = 2.4 μm, and Q = 1.64–1.82 (n = 90/3).
Additional specimens examined—China, Guizhou Province, Jiangkou County, Fanjingshan Nature Reserve, on a rotten angiosperm trunk, 11 July 2018, He 5561 (BJFC 026622); Hubei Province, Wufeng County, Houhe Nature Reserve, on a fallen angiosperm trunk, 16 August 2017, He 5086 (BJFC 024604, CFMR); Hunan Province, Dong'an County, Shunhuangshan Nature Reserve, on a rotten angiosperm stump, 13 July 2015, He 2377 (BJFC 020831, CFMR); Jilin Province, Jiaohe County, forestry experimental area, on a fallen angiosperm trunk, 3 September 2017, He 5152 (BJFC 024670, CFMR); Jiangxi Province, Ji'an County, Jinggangshan Forest Park, on a fallen Rhododendron trunk, 11 August 2016, He 4282 (BJFC 023724, CFMR); Liaoning Province, Qingyuan County, Qingyuan forest ecological test station, on a dead angiosperm bark, 26 August 2015, He 2958 (BJFC 022025); Yunnan Province, Lushui County, Gaoligongshan Nature Reserve, on a fallen angiosperm trunk, 30 November 2015, He 3424 (BJFC 021820, CFMR); Yongde County, Daxueshan Nature Reserve, Dabaoshan, on a dead Quercus stump, 27 August 2015, He 2672 (BJFC 021111, CFMR); Yongping County, Baotaishan Forest Park, on a dead angiosperm tree, 27 November 2015, He 3246 (BJFC 021641).
Notes—Rhizochaete subradicata (Figures 8B, C, 10) is characterized by the thin pellicular basidiomes turning reddish brown in KOH, relatively long cystidia with thickened walls, and ellipsoid basidiospores. Morphologically and phylogenetically, R. subradicata is closely related to R. radicata, which differs by having thicker basidiomes and longer cystidia (60–100 μm, Nakasone et al., 1994). Rhizochaete radicata has been reported worldwide (Nakasone et al., 1994, 2017), but here we show that the widely distributed species in China is R. subradicata. Also, the occurrence of R. radicata in India, Japan, and Vietnam needs further study. Rhizochaete grandinosa C.L. Zhao & Z.R. Gu, recently described from Yunnan Province, southwestern China, differs from R. subradicata by having smaller cystidia (24–50 × 4–9 μm) and shorter basidia (14.5–21 μm, Gu and Zhao, 2021).
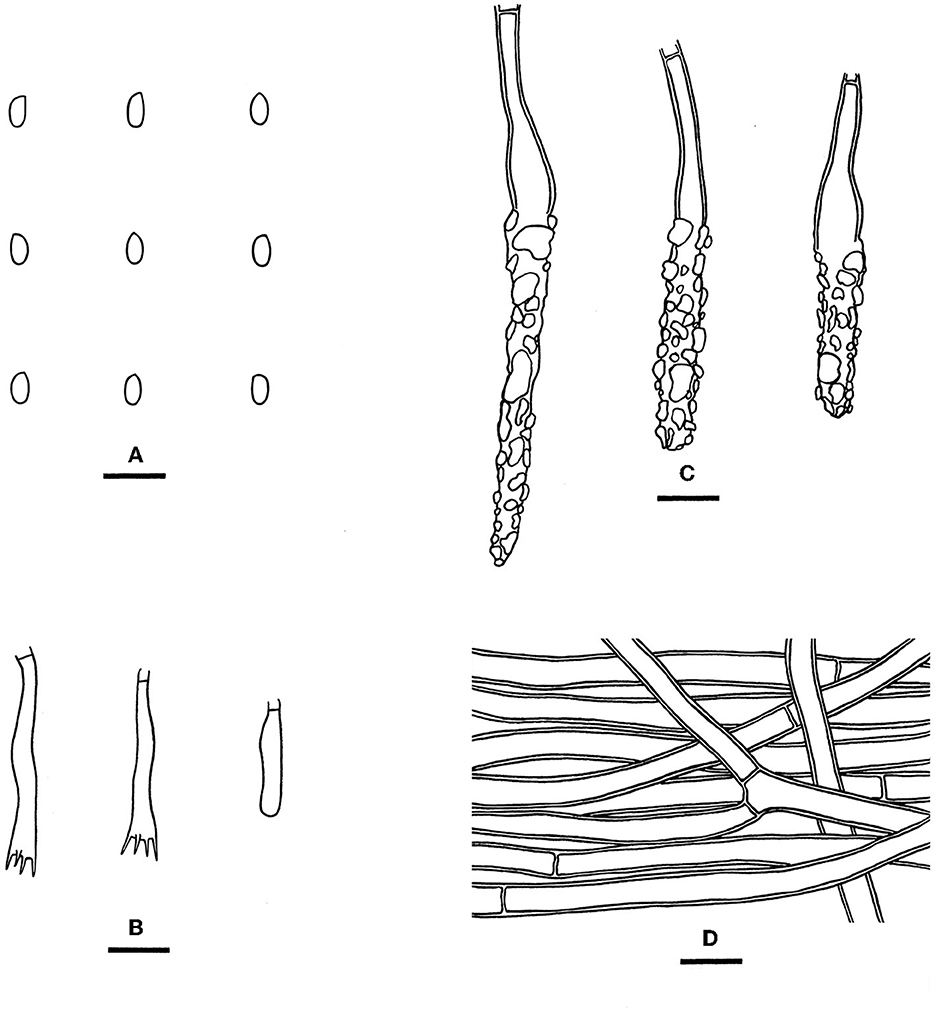
Figure 10. Rhizochaete subradicata [from the holotype He 3213; scale bars: (A–D) = 10 μm]. (A) Basidiospores; (B) Basidia and a basidiole; (C) Lamprocystidia; (D) Hyphae from subiculum.
Rhizochaete terrestris Yue Li & S.H. He, sp. nov.
MycoBank: MB846343
Diagnosis—The species is recognized by a discontinuous hymenophore with well-developed hyphal cords, the absence of cystidia, and growth on the ground.
Type—China, Beijing, Haidian District, Yuyuantan Park, on the ground, 12 August 2022, He 7713 (BJFC 038849, holotype).
Etymology—Refer to the habitat on the ground.
Fruiting body—Basidiomes annual, resupinate, widely effused, adnate, on soil or rotten twigs and leaves, membranaceous or pellicular, fragile, the colonies up to 30 × 20 cm, up to 240 μm thick in section. Hymenophore discontinuous, smooth in the developed part, light yellow (4B5) to yellowish orange (4B7), turning pale purple in KOH, not cracked upon drying; the margin thinning out, adnate, fimbriate, and white; hyphal cords well developed, white to pale orange, and turning pale purple in KOH; and context is white.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate with single clamps occasionally present. Subiculum distinct; hyphae colorless, thin- to slightly thick-walled, slightly encrusted with fine crystals, frequently branched and septate, more or less parallel to the substrate, and 2.5–7 μm in diameter; Subhymenium indistinct. Cystidia not observed. Basidia subclavate, colorless, thin-walled, smooth, with a basal simple septum and four sterigmata, and 28–38 × 4.5–6.5 μm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores ellipsoid to broadly ellipsoid, with an apiculus, colorless, thin-walled, smooth, usually with two oil drops, IKI–, CB–, (3.8–) 4–5 (−5.2) × 2.5–3.2 (−3.5) μm, L = 4.4 μm, W = 2.9 μm, and Q = 1.47–1.56 (n = 60/2).
Additional specimens examined—China, Beijing, Haidian District, Jiufeng Forest Park, on the ground, 5 August 2020, He 6694 (BJFC 033642, holotype).
Notes—Rhizochaete terrestris (Figures 8D, E, 11) is characterized by the discontinuous basidiomes growing on the ground, well-developed hyphal cords, and the absence of cystidia. Rhizochaete lutea (Sheng H. Wu) C.C. Chen & Sheng H. Wu also lacks cystidia but differs from R. terrestris by the well-developed basidiomes unchanging in KOH, smaller basidia (19–23.5 × 3.7–4.2 μm), slightly narrower basidiospores (2–2.5 μm), and growth on bamboo in tropical areas (Wu, 1990). In the phylogenetic tree, R. terrestris formed a distinct lineage among the strongly supported group (93/83/1, Figure 3) that includes R. radicata and several other newly described species.
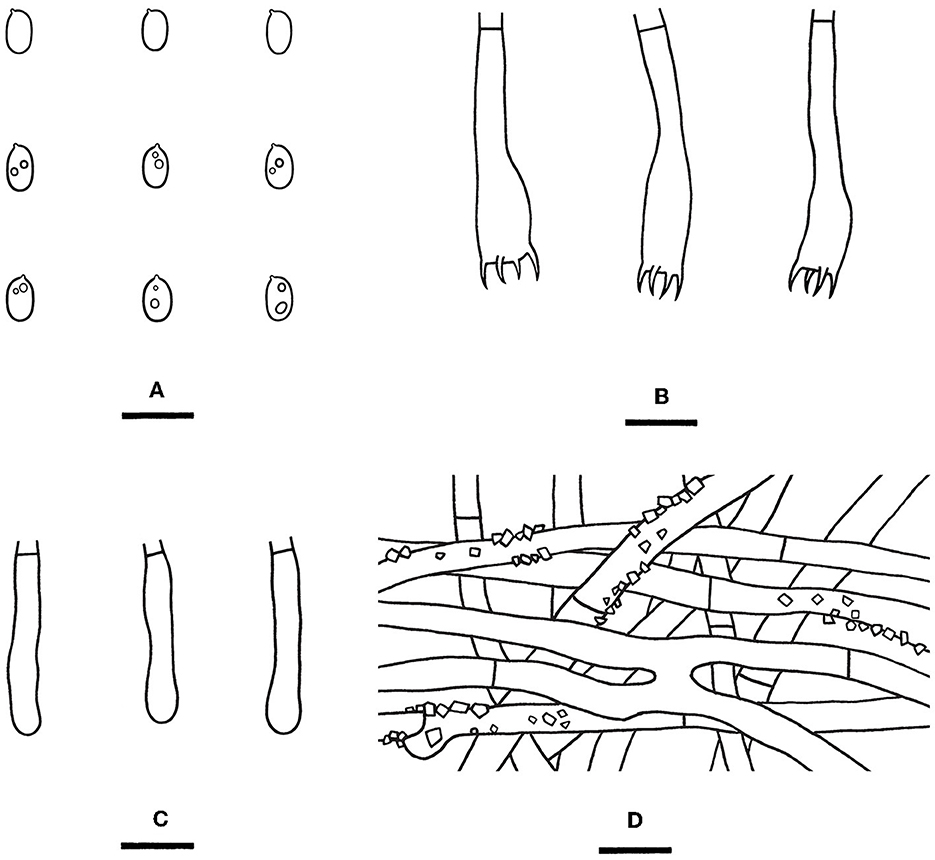
Figure 11. Rhizochaete terrestris [from the holotype He 7713; scale bars: (A–D) = 10 μm]. (A) Basidiospores; (B) Basidia; (C) Basidioles; (D) Hyphae from subiculum.
Rhizochaete yunnanensis Yue Li & S.H. He, sp. nov.
MycoBank: MB846344
Diagnosis—The species is recognized by a smooth hymenophore turning reddish brown in KOH and the presence of apically encrusted lamprocystidia, long basidia, and ellipsoid basidiospores.
Type—China, Yunnan Province, Lushui County, Gaoligongshan Nature Reserve, on a fallen angiosperm trunk, 28 November 2015, He 3302 (BJFC 021697, holotype; CFMR, isotype).
Etymology—Refer to the type locality in Yunnan Province, southwestern China.
Fruiting body—Basidiomes annual, resupinate, widely effused, loosely adnate, easily separated from the substrate, and membranaceous to pellicular, first as small patches, later confluent up to 5 cm long, 2 cm wide, and up to 160 μm thick in section. Hymenophore smooth, grayish yellow (4B4) to brownish orange (5B6), turning reddish brown in KOH, and not cracked upon drying; the margin thinning out, adnate, fimbriate, paler than hymenophore surface, and cream; hyphal cords yellow and turning reddish brown in KOH; and context is yellow.
Microscopic structures—Hyphal system monomitic; generative hyphae simple-septate with rare single clamps. Subiculum distinct; hyphae colorless, thin- to slightly thick-walled, smooth, frequently branched, sometimes with H- or Y-connections, frequently septate, loosely interwoven, and 3.5–6 μm in diameter. Subhymenium thickening, composed of lamprocystidia and hyphae, hyphae colorless, thin- to slightly thick-walled, smooth, loosely interwoven, moderately branched, rarely septate, and 1.5–3 μm in diameter. Lamprocystidia is numerous, subfusiform with an obtuse apex, apically encrusted, mostly embedded, and 40–52 × 6–10 μm (crystals included), with walls up to 1–1.5 μm. Basidia clavate, colorless, thin-walled, smooth, with a basal simple septum and four sterigmata, and 33–45 × 4.5–7 μm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores ellipsoid, with an apiculus, colorless, thin-walled, smooth, usually with oil drops, IKI–, CB–, 4.2–5.2 × 2.8–3.2 μm, L = 4.8 μm, W = 3 μm, and Q = 1.6 (n = 30/1).
Notes—Rhizochaete yunnanensis (Figures 8F, 12) is characterized by thin basidiomes turning reddish brown in KOH, apically encrusted thick-walled cystidia, and long basidia. In the phylogenetic tree (Figure 3), R. yunnanensis is the sister to R. violascens (Fr.) K.H. Larss., which differs in having a violaceous hymenophore and clamped generative hyphae and lacking cystidia (Eriksson and Ryvarden, 1973). Rhizochaete subradicata is similar to R. yunnanensis by sharing similar-sized basidia and basidiospores but differs in having larger cystidia (40–75 × 7–15 μm) with more proportions of the length encrusted.
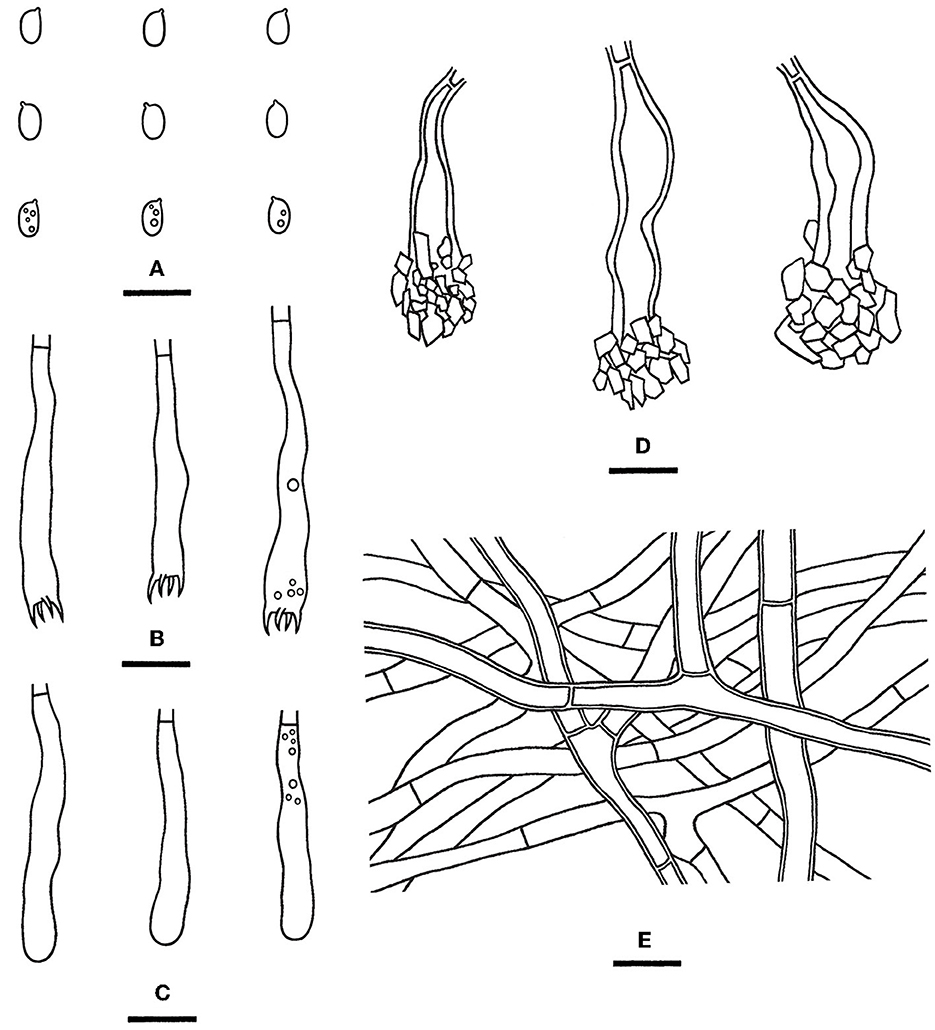
Figure 12. Rhizochaete yunnanensis [from the holotype He 3302; scale bars: (A–E) = 10 μm]. (A) Basidiospores; (B) Basidia; (C) Basidioles; (D) Lamprocystidia; (E) Hyphae from subiculum.
Phanerochaete subsanguinea Yue Li & S.H. He, nom. nov.
MycoBank: MB846345
Replaced synonym—Phanerochaete rhizomorpha C.L. Zhao & D.Q. Wang, Journal of Fungi 7: 10. 2021. [MB#841272]
Etymology—Refer to the morphological similarity and close phylogenetic relationship of P. sanguinea (Fr.) Pouzar.
Description—See Wang and Zhao (2021).
Notes—According to Art. 53.1, the name P. rhizomorpha C.L. Zhao & D.Q. Wang published on 11 December 2021 is an invalid name since it is a homonym of P. rhizomorpha C.C. Chen, Sheng H. Wu & S.H. He published on 15 November 2021 (Chen et al., 2021; Wang and Zhao, 2021). Our morphological and phylogenetic studies on the representative specimens showed that the two names represented two distinct species (Figure 4). Therefore, we propose the new name P. subsanguinea (Figure 13) to replace P. rhizomorpha C.L. Zhao & D.Q. Wang.
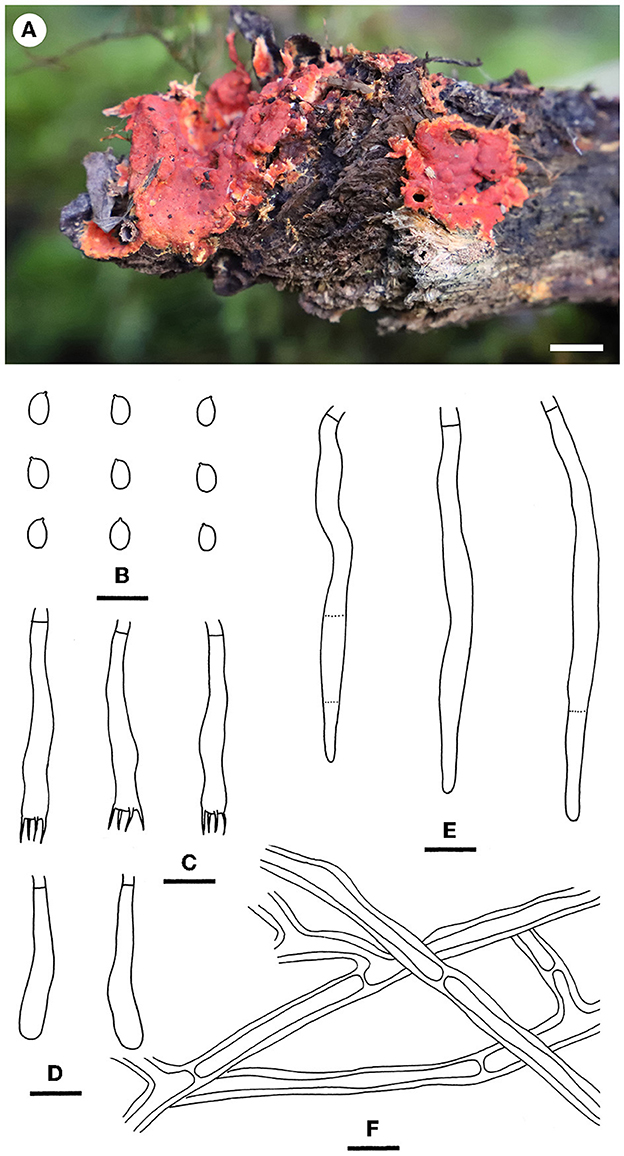
Figure 13. Phanerochaete subsanguinea [from He 6131; scale bars: (A) = 1 cm; (B–F) = 10 μm]. (A) Basidiomes; (B) Basidiospores; (C) Basidia; (D) Basidioles; (E) Cystidia; (F) Hyphae from subiculum.
Discussion
The phylogeny of the Phanerochaetaceae at the generic level is becoming much clearer with some new genera introduced for the independent lineages (Floudas and Hibbett, 2015; Miettinen et al., 2016; Yuan et al., 2017; Chen et al., 2018b, 2021; Ma and Zhao, 2019). At present, the family includes 23 genera, most of which are corticioid fungi (Chen et al., 2021). Although most of the newly described taxa in the family originated from East Asia, some groups in this area still need more systematic studies based on more complete samplings.
Chen et al. (2021) used Roseograndinia for a distinct lineage of two new species, which are morphologically similar to the type, R. rosea (Henn.) Hjortstam & Ryvarden. Morphologically, the genus is similar to Hyphodermella, which differs by having encrusted cystidioid hyphal ends. Our phylogenetic analyses based on more samples of the two genera showed that two recently described species without cystidioid hyphal ends, Hyphodermella aurantica and H. zixishanensis, nested within the Roseograndinia. Meanwhile, two new species, H. laevigata and H. tropica, in the core clade of Hyphodermella were found, though the former species also lacks cystidioid hyphal ends. The poroid species, Hyphodermella poroides Y.C. Dai & C.L. Zhao, did not nest within the core group of the genus but clustered with Geliporus exilisporus (Y.C. Dai & Niemelä) Yuan, Jia J. Chen & S.H. He. However, their relationship was not well supported, and there are clear morphological differences between the two species (Yuan et al., 2017; Zhao et al., 2017). At present, we accept a broad concept of Hyphodermella to include nine species as follows:
A key to accepted species of Hyphodermella s.l.
1. Hymenophore poroid ………………………..H. poroides
1. Hymenophore non-poroid ……………………………2
2. Hymenophore smooth to tuberculate ……………………3
2. Hymenophore grandinioid, odontoid to hydnaceous ……….4
3. Cystidioid hyphal ends absent, reported in southern China …………………………………………...H. laevigata
3. Cystidioid hyphal ends present, reported in Far East of Russia ……………………………………..H. pallidostraminea
4. Context distinctly brown ………………H. brunneocontexta
4. Context cream, pale yellow ……………………………5
5. Basidiospores <6 μm long …………………….H. tropica
5. Basidiospores > 6 μm long ……………………………6
6. Basidiospores narrowly ellipsoid, <4 μm wide ………………………………………H. maunakeaensis
6. Basidiospores ellipsoid, > 4 μm wide ……………………7
7. Basidiomes up to 500 μm thick, basidia up to 60 μm long …………………………………………..H. corrugata
7. Basidiomes up to 110 μm thick, basidia up to 40 μm long ……………………………………………………8
8. Subicular hyphae agglutinated, widely distributed in Mediterranean area and China on many hosts ………………………………………………H. rosae
8. Subicular hyphae separable, found in Italy on ………………………………….Ampelopsis H. ochracea
Rhizochaete is morphologically well-circumscribed and phylogenetically well-supported in the Phanerochaetaceae. It contains 17 species worldwide, including four new species recently described from China (Nakasone et al., 2017; Chen et al., 2021; Gu and Zhao, 2021). Our analyses herein demonstrated that the species diversity of the genus in China is rich, and four additional new species were found based mainly on molecular evidence. Meanwhile, according to our investigation records, some species are abundantly distributed locally, for example, R. chinensis C.C. Chen, Sheng H. Wu & S.H. He is one of the most common corticioid species in the Beijing area. The species, R. sulphurina (P. Karst.) K.H. Larss., was recorded in northeastern China earlier [as Ceraceomyces sulphurinus (P. Karst.) J. Erikss. & Ryvarden, Dai, 2011], but we did not recover this species based on our investigations and analyses. Thus, we accepted eight species of Rhizochaete in China as follows:
A key to species of Rhizochaete in China
1. Cystidia absent ……………………………………..2
1. Cystidia present …………………………………….3
2. Basidiomes on wood or bamboo, color unchanged in KOH, found in tropical areas ……………………………R. lutea
2. Basidiomes on the ground, turning purple in KOH, found in temperate area ……………………………….R. terrestris
3. Basidiospores broadly ellipsoid to ovoid, usually <2.5 μm wide ………………………………………....R. nakasoneae
3. Basidiospores ellipsoid, usually > 2.5 μm wide …………...4
4. Basidiomes turning purple in KOH …………………….5
4. Basidiomes turning reddish brown in KOH ………………6
5. Cystidia 18–60.5 × 6–11 μm, basidia 11–33 × 3–5 μm ……………………………………………R. fissurata
5. Cystidia 20–50 × 4–9 μm, basidia 14.5–21 × 4.3–5.2 μm ………………………………………….R. grandinosa
6. Basidiomes up to 500 μm thick, basidia <27 μm long ……………………………………………R. chinensis
6. Basidiomes up to 200 μm thick, basidia mostly > 27 μm long ……………………………………………………7
7. Cystidia 40–75 × 7–15 μm, basidiospores 3.8–4.5 × 2.2–2.8 μm …………………………………………R. subradicata
7. Cystidia 40–52 × 6–10 μm, basidiospores 4.2–5.2 × 2.8–3.2 μm …………………………………………R. yunnanensis
Data availability statement
The data presented in the study are deposited in the NCBI and MycoBank repositories, accession numbers ON963990–ON964026, OP549029, OP549030, OP102693, OP102695 for NCBI repository, and accession numbers MB846336–MB846345 for MycoBank repository.
Author contributions
YL performed the phylogenetic analyses and did most of the measurements, descriptions, and illustrations. C-CC provided some specimens and sequences and revised the manuscript. S-HH designed the research, collected most of the specimens, and wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Financial support was provided by the National Natural Science Foundation of China (Nos. 31870011 and 31750001).
Acknowledgments
The authors would like to express their deep appreciation to Dr. Karen K. Nakasone (Center for Forest Mycology Research, Northern Research Station, U.S. Forest Service, USA) for improving the manuscript and Dr. Shi-Liang Liu (Institute of Microbiology, Chinese Academy of Sciences, Beijing, China) for assisting with the drawing of the microscopic structures of Phanerochaete subsanguinea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ITS, internal transcribed spacer; nrLSU, nuclear ribosomal large subunit; BJFC, the herbarium of Beijing Forestry University, Beijing, China; TNM, National Museum of Natural Science, Taichung, Taiwan, China; KOH, 2% (w/v) potassium hydroxide; IKI, Melzer's reagent; CB, cotton blue; IKI–, neither amyloid nor dextrinoid; CB–, acyanophilous; L, mean spore length; W, mean spore width; Q, L/W ratio, n (a/b), number of spores (a) measured from the number of specimens (b); CTAB, cetyltrimethylammonium bromide; DNA, deoxyribonucleic acid; PCR, polymerase chain reaction; MP, maximum parsimony; ML, maximum likelihood; BI, Bayesian inference; BPP, Bayesian posterior probability.
Footnotes
1. ^http://sweetgum.nybg.org/science/ih/
2. ^https://sites.duke.edu/vilgalyslab/rdna_primers_for_fungi/
References
Bernicchia, A., and Gorjón, S. P. (2010). Corticiaceae s.l. Fungi Europaei 12. Edizioni Candusso. Alassio, Italia
Binder, M., Larsson, K. H., Matheny, P. B., and Hibbett, D. S. (2010). Amylocorticiales ord. nov. and Jaapiales ord. nov.: early diverging clades of Agaricomycetidae dominated by corticioid forms. Mycologia 102, 865–880. doi: 10.3852/09-288
Boonmee, S., Wanasinghe, D. N., Calabon, M. S., Huanraluek, N., Chandrasiri, S. K. U., Jones, G. E. B., et al. (2021). Fungal diversity notes 1387–1511: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Div. 111, 1–335. doi: 10.1007/s13225-021-00489-3
Chen, C. C., Chen, C. Y., Lim, Y. W., and Wu, S. H. (2020). Phylogeny and taxonomy of Ceriporia and other related taxa and description of three new species. Mycologia 112, 64–82. doi: 10.1080/00275514.2019.1664097
Chen, C. C., Chen, C. Y., and Wu, S. H. (2021). Species diversity, taxonomy and multi-gene phylogeny of phlebioid clade (Phanerochaetaceae, Irpicaceae, Meruliaceae) of Polyporales. Fungal Div. 111, 337–442. doi: 10.1007/s13225-021-00490-w
Chen, C. C., Wu, S. H., and Chen, C. Y. (2018a). Four species of polyporoid fungi newly recorded from Taiwan. Mycotaxon 133, 45–54. doi: 10.5248/133.45
Chen, C. C., Wu, S. H., and Chen, C. Y. (2018b). Hydnophanerochaete and Odontoefibula, two new genera of phanerochaetoid fungi (Polyporales, Basidiomycota) from East Asia. MycoKeys 39, 75–96. doi: 10.3897/mycokeys.39.28010
Chen, J. J., Wang, Y. R., Wang, C. G., and Dai, Y. C. (2022). Two new species of Ceriporia (Irpicaceae, Basidiomycota) from the Asia Pacific area. Mycological Progress 21, 39–48. doi: 10.1007/s11557-021-01731-7
Chikowski, R. S., Larsson, K. H., and Gibertoni, T. B. (2016). Three new combinations in Rhizochaete (Agaricomycetes, Fungi) and a new record to the Brazilian Amazonia. Nova Hedwigia 102, 185–196. doi: 10.1127/nova_hedwigia/2015/0298
Crous, P. W., Osieck, E. R., Jurjevic, Ž., Boers, J., van Iperen, A. L., Starink-Willemse, M., et al. (2021). Fungal Planet description sheets: 1284–1382. Persoonia 47, 178–374. doi: 10.3767/persoonia.2021.47.06
Dai, Y. C. (2011). A revised checklist of corticioid and hydnoid fungi in China for 2010. Mycoscience 52, 69–79. doi: 10.1007/S10267-010-0068-1
Darriba, D., Taboada, G. L., Doallo, R., and Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772. doi: 10.1038/nmeth.2109
Duhem, B., and Buyck, B. (2011). Hyphodermella brunneocontexta sp. nov. de l'île de Mayotte (France) (Basidiomycota, Polyporales). Cryptogamie Mycologie 32, 413–420. doi: 10.7872/crym.v32.iss4.2011.413
Eriksson, J., and Ryvarden, L. (1973). The Corticiaceae of North Europe. Cryptogamie Mycologie 2, 60–287.
Floudas, D., and Hibbett, D. S. (2015). Revisiting the taxonomy of Phanerochaete (Polyporales, Basidiomycota) using a four gene dataset and extensive ITS sampling. Fungal Biology 119, 679–719. doi: 10.1016/j.funbio.2015.04.003
Gilbertson, R. L. (2001). Fungi from the mamane-naio vergetation zone of Hawaii. Fungal Div. 6, 35–68.
Greslebin, A., Nakasone, K. K., and Rajchenberg, M. (2004). Rhizochaete, a new genus of phanerochaetoid fungi. Mycologia 96, 260–271. doi: 10.1080/15572536.2005.11832976
Gu, Z. R., and Zhao, C. L. (2021). The hidden wood-decaying fungal diversity: Rhizochaete from East Asia. J. Fungi 13, 503. doi: 10.3390/d13100503
Hall, T. A. (1999). Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Sympos. Series 41, 95–98.
Huang, R. X., and Zhao, C. L. (2020). Three new species of Phlebia (Polyporales, Basidiomycota) based on the evidence from morphology and DNA sequence data. Mycologic. Progr. 19, 753–767. doi: 10.1007/s11557-020-01591-7
Justo, A., Miettinen, O., Floudas, D., Ortiz-Santana, B., Sjökvist, E., Lindner, D., et al. (2017). A revised family-level classification of the Polyporales (Basidiomycota). Fung. Biol. 121, 798–824. doi: 10.1016/j.funbio.2017.05.010
Katoh, K., Rozewicki, J., and Yamada, K. D. (2017). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20, 1160–1166. doi: 10.1093/bib/bbx108
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., et al. (2012). Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649. doi: 10.1093/bioinformatics/bts199
Kornerup, A., and Wanscher, J. H. (1978). Methuen handbook of colour. London: E. Methuen and Co., Ltd.
Larsson, K. H. (2007). Re-thinking the classifcation of corticioid fungi. Mycolog. Res. 111, 1040–1063. doi: 10.1016/j.mycres.2007.08.001
Li, T., Gao, J. L., Huang, J. H., Gu, L., Zou, J., and Wu, X. J. (2021). Phlebiopsis xuefengensis sp. nov. from Gastrodia elata (Orchidaceae) in Hunan Province, Southern China. South Afric. J. Bot. 142, 299–304. doi: 10.1016/j.sajb.2021.06.034
Li, Y., He, S. H., Chen, C. C., Nakasone, K. K., and Ma, H. X. (2022). Global taxonomy and phylogeny of Irpicaceae (Polyporales, Basidiomycota) with descriptions of seven new species and proposals of two new combinations. Front. Microbiol. 13, 1–26. doi: 10.3389/fmicb.2022.911978
Lim, Y. W., and Jung, H. S. (2003). Irpex hydnoides, sp. nov. is new to science, based on morphological, cultural and molecular characters. Mycologia 95, 694–699. doi: 10.1080/15572536.2004.11833073
Lima, V. X., Sousa, L. C., dos Santos, C. R., Santos, C., Lima, N., and Gibertoni, T. B. (2020). Additions to neotropical stereoid fungi (Polyporales, Basidiomycota): one new species of Lopharia and one new combination in Phlebiopsis. Mycologic. Progr. 19, 31–40. doi: 10.1007/s11557-019-01538-7
Lira, C. R. S., Chikowski, R. S., de Lima, V. X., Gibertoni, T. B., and Larsson, K. H. (2022). Allophlebia, a new genus to accomodate Phlebia ludoviciana (Agaricomycetes, Polyporales). Mycologic. Progr. 21, 1–11. doi: 10.1007/s11557-022-01781-5
Ma, X., and Zhao, C. L. (2019). Crepatura ellipsospora gen. et sp. nov. in Phanerochaetaceae (Polyporales, Basidiomycota) bearing a tuberculate hymenial surface. Mycologic. Progr. 18, 785–793. doi: 10.1007/s11557-019-01488-0
Maddison, W. P., and Maddison, D. R. (2018). Mesquite: a modular system for evolutionary analysis. Version 3.5.1. Available online at: http://www.mesquiteproject.org (accessed October 01, 2022).
Miettinen, O., Spirin, V., Vlasák, J., Rivoire, B., Stenroos, S., and Hibbett, D. (2016). Polypores and genus concepts in Phanerochaetaceae (Polyporales, Basidiomycota). MycoKeys 17, 1–46. doi: 10.3897/mycokeys.17.10153
Nakasone, K. K., Bergman, C. R., and Burdsall, H. H. (1994). Phanerochaete filamentosa—Corticium radicatum species complex in North America. Sydowia 46, 44–62.
Nakasone, K. K., Draeger, K. R., and Ortiz-Santana, B. (2017). A contribution to the taxonomy of Rhizochaete (Polyporales, Basidiomycota). Cryptogam Mycologie 38, 81–99. doi: 10.7872/crym/v38.iss1.2017.81
Nakasone, K. K., Ortiz-Santana, B., and He, S. H. (2021). Taxonomic studies of crust fungi with spines in Radulomyces, Sarcodontia, and the new genus Noblesia. Mycologic. Progr. 20, 1479–1501. doi: 10.1007/s11557-021-01746-0
Parmasto, E., and Hallenberg, N. (2000). A taxonomic study of phlebioid fungi (Basidiomycota). Nordic J. Bot. 20, 105–118. doi: 10.1111/j.1756-1051.2000.tb00740.x
Phookamsak, R., Hyde, K. D., Jeewon, R., Bhat, D. J., Gareth Jones, E. B., Maharachchikumbura, S. S. N., et al. (2019). Fungal diversity notes 929–1035: taxonomic and phylogenetic contributions on genera and species of fungi. Fung. Diver. 95, 1–273. doi: 10.1007/s13225-019-00421-w
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Hohna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Spirin, V., Volobuev, S., Okun, M., Miettinen, O., and Larsson, K. H. (2017). What is the type species of Phanerochaete (Polyporales, Basidiomycota). Mycologic. Progr. 16, 171–183. doi: 10.1007/s11557-016-1267-8
Stamatakis, A. (2014). RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Swofford, D. L. (2002). PAUP*: Phylogenetic analysis using parsimony (*and other methods). Version 4.0b10. Sinauer Associates, Sunderland, Massachusetts.
Telleria, M. T., Dueñas, M., Melo, I., and Martín, M. P. (2010). Morphological and molecular studies of Hyphodermella in the Western Mediterranean area. Mycologic. Progr. 9, 585–596. doi: 10.1007/s11557-010-0666-5
Wang, D. Q., and Zhao, C. L. (2021). Morphological and phylogenetic evidence for recognition of two new species of Phanerochaete from East Asia. J. Fungi 7, 1063. doi: 10.3390/jof7121063
Wang, H., Gu, Z., and Zhao, C. L. (2021). Hyphodermella zixishanensis (Polyporales, Basidiomycota), a new species with reddish hymenial surface. Nordic J. Bot. 39, e03329. doi: 10.1111/njb.03329
Wang, H., and Zhao, C. L. (2020). Hyphodermella aurantiaca sp. nova (Polyporales, Basidiomycota) as evidenced by morphological characters and phylogenetic analyses. Annales Botanici Fennici 58, 61–68. doi: 10.5735/085.058.0110
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR Protocols: A Guide to Methods and Applications, eds Innis, M.A., Gelfand, D.H., Sninsky, J.J., and White, T.J. (San Diego: Academic Press), 315–322.
Wu, S. H. (1990). The Corticiaceae (Basidiomycetes) subfamilies Phlebioideae, Phanerochaetoideae and Hyphodermoideae in Taiwan. Acta Botanica Fennica 142, 1–123.
Wu, S. H., Chen, Y. P., Wei, C. L., Floudas, D., and Dai, Y. C. (2018a). Two new species of Phanerochaete (Basidiomycota) and redescription of P. robusta. Mycologic. Progr. 17, 425–435. doi: 10.1007/s11557-017-1368-z
Wu, S. H., Nilsson, H. R., Chen, C. T., Yu, S. Y., and Hallenberg, N. (2010). The white-rotting genus Phanerochaete is polyphyletic and distributed throughout the phleboid clade of the Polyporales (Basidiomycota). Fungal Diver. 42, 107–118. doi: 10.1007/s13225-010-0031-7
Wu, S. H., Wang, D. M., and Chen, Y. P. (2018b). Purpureocorticium microsporum (Basidiomycota) gen. et sp. nov. from East Asia. Mycologic. Progr. 17, 357–364. doi: 10.1007/s11557-017-1362-5
Xu, T. M., Zeng, Y. F., Cheng, Y. H., and Zhao, C. L. (2020). Phlebiopsis lacerata sp. nov. (Polyporales, Basidiomycota) from southern China. Phytotaxa 440, 268–280. doi: 10.11646/phytotaxa.440.4.2
Xu, Y. L., Cao, Y. F., Nakasone, K. K., Chen, C. C., and He, S. H. (2020). Taxonomy and phylogeny of Phanerochaete sensu stricto (Polyporales, Basidiomycota) with emphasis on Chinese collections and descriptions of nine new species. Mycosphere 11, 1527–1552. doi: 10.5943/mycosphere/11/1/12
Yuan, Y., Chen, J. J., and He, S. H. (2017). Geliporus exilisporus gen. et comb. nov., a xanthochroic polypore in Phanerochaetaceae from China. Mycoscience 58, 197–203. doi: 10.1016/j.myc.2017.01.006
Zhao, C. L., Liu, X. F., and Ma, X. (2019). Phlebiopsis yunnanensis sp. nov. (Polyporales, Basidiomycota) evidenced by morphological characters and phylogenetic analysis. Nova Hedwigia 108, 265–279. doi: 10.1127/nova_hedwigia/2018/0508
Zhao, C. L., Ren, G. J., and Wu, F. (2017). A new species of Hyphodermella (Polyporales, Basidiomycota) with a poroid hymenophore. Mycoscience 58, 452–456. doi: 10.1016/j.myc.2017.06.007
Keywords: phlebioid clade, phylogeny, taxonomy, white rot, wood-decaying fungi
Citation: Li Y, Chen C-C and He S-H (2023) New corticioid taxa in Phanerochaetaceae (Polyporales, Basidiomycota) from East Asia. Front. Microbiol. 14:1093096. doi: 10.3389/fmicb.2023.1093096
Received: 22 November 2022; Accepted: 06 February 2023;
Published: 09 March 2023.
Edited by:
Jie Chen, Universidad Veracruzana, MexicoReviewed by:
Samantha Chandranath Karunarathna, Qujing Normal University, ChinaYusufjon Gafforov, Academy of Science of the Republic of Uzbekistan, Uzbekistan
Copyright © 2023 Li, Chen and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuang-Hui He, aGVzaHVhbmdodWlAYmpmdS5lZHUuY24=
 Yue Li
Yue Li Che-Chih Chen
Che-Chih Chen Shuang-Hui He
Shuang-Hui He