- 1Collage of Animal Science, Guizhou University, Guiyang, Guizhou, China
- 2Key Laboratory of Animal Genetics, Breeding and Reproduction in the Plateau Mountainous Region, Ministry of Education, Guizhou University, Guiyang, Guizhou, China
To develop a new high-yielding and polysaccharide-containing forage resource for livestock, the effects of different cutting methods and additives on Saccharum arundinaceum silage were evaluated. The wilted S. arundinaceum were chopped and knead-wired. The silages from each cutting method were treated with Lactobacillus plantarum (LP), cellulase (CE) and the combination of LP and CE (LP + CE) for 3, 7, 15, 30, and 60 days. Compared with the CK treatment, CE treatment exhibited better effects in the degradation of neutral detergent fiber (NDF), LP exhibited a better performance in preserving the content of dry matter (DM), and adding LP + CE significantly enhanced (P < 0.05) the contents of lactic acid (LA), crude protein (CP) and DM and significantly reduced (P < 0.05) the pH and NDF content during ensiling. In addition, both additives exerted a remarkable effect on the silage bacterial community (P < 0.05), with a dramatic increase in the Lactobacillus abundance and a decrease in the abundance of Enterobacter. Lactic acid bacteria (LAB) became the most dominant bacteria that affected the fermentation quality of LP and LP + CE silages. Meanwhile, chopped silages showed better fermentation quality and nutrient preservation and a higher abundance of LAB. Our research indicated that the chopped S. arundinaceum ensiling with LP + CE could exert a positive effect on LA fermentation and preservation of nutrient substances by shifting the bacterial community. In conclusion, S. arundinaceum can serve as a new silage resource for feed utilization by the ensiling method of LP + CE-chopped.
Introduction
Saccharum arundinaceum is a tall-growing, caespitose perennial herbaceous grass native to southern China, parts of South and Southeast Asia and other countries (Wang W. G. et al., 2019). S. arundinaceum is a C4 plant and produces large amounts of biomass, for which the dry matter yield is approximately 40–60 t⋅ha–1⋅year–1; lignocellulosic biomass includes a high content of cellulose and polysaccharides (Heaton et al., 2008; Hattori and Morita, 2010). Therefore, S. arundinaceum has received increasing attention as a potential biofuel feedstock (Kirupa et al., 2018; Muthuvelu et al., 2018; Zhang et al., 2020). The high biomass yield and high polysaccharide content indicate that S. arundinaceum can be a potential feed source (Muthuvelu et al., 2018). In addition, the feeding value of S. arundinaceum can be increased through certain processing methods (such as ensiling). However, to our knowledge, research on S. arundinaceum has been mainly focused on its ecological restoration and biomass energy (Muthuvelu et al., 2018; Zhao et al., 2020). Few studies have paid attention to its feeding value and improving its quality through ensiling.
Ensiling is a process of rapid anaerobic fermentation of plant material by either epiphytic or exogenously applied lactic acid bacteria (Cai et al., 1998; Ren et al., 2020). Silage is one of the most important ruminant feedstocks in China, particularly southern China, where local climactic conditions make traditional hay production impractical (Dunière et al., 2013; Du et al., 2021). However, low amounts of epiphytic LAB and water-soluble carbohydrate (WSC) limit the applicability of some forages for ensiling (Xu et al., 2020; Du et al., 2021). Therefore, biological additives, including Lactobacillus plantarum and cellulase, can be crucial for improving the fermentation and nutrients of silage (Xu et al., 2018; Cai et al., 2020; Guo et al., 2020). Previous studies showed that adding L. plantarum could decrease the DM loss, pH, ammonia-N (NH3-N) content and butyric acid (BA) concentration, increase the LA concentration and change the bacterial community composition of silage (Ren et al., 2020; Xu et al., 2020; Zhang et al., 2021). Moreover, ensiling with cellulase could degrade part of the cellulose, thus providing a large amount of WSCs for LAB utilization of S. arundinaceum silage (Feng et al., 2020; Xu et al., 2020). Therefore, it is inferred that S. arundinaceum could potentially be used as a feed source for animals if L. plantarum and cellulase were applied during ensiling.
Cutting is used to obtain the appropriate particle size of the raw material, which is a necessary step in silage processing technology and an important basis to ensure the quality of silage (Savoie et al., 1992; Thomson et al., 2017). Different raw materials may have different optimal particle sizes by different cutting methods, such as chopping and knead wiring, due to the physical characteristics of the crop stems (Savoie et al., 1992; Sun et al., 2020). In general, raw materials of conventional silage are chopped into 1∼3 cm lengths by a straw crusher (Dong et al., 2020; Ren et al., 2020; Wang et al., 2021). Knead wire uses a kneading machine to flatten, shred, beat, tear and crush the forage grass, which is then kneaded and cut into soft silks. However, to the best of our knowledge, the effect of chopping and knead-wiring on silage fermentation with a high content of lignocellulosic biomass is unclear. Moreover, we hypothesized that the two cutting methods would have different effects on S. arundinaceum silage fermentation and the microbial community.
Therefore, chopped and knead-wired S. arundinaceum were ensiled with different additives in our study. The silage quality and microbial community were explored to evaluate the optimal cutting methods and additives. This study lays a research foundation for the development of new forage resources for animal production.
Materials and methods
Silage material production
Both the tender stems and leaves of S. arundinaceum were harvested from Sichuan Province, China. The raw materials were allowed to wilt until they reached approximately 70% moisture and then cut using two different methods: (1) a straw crusher was used to chop material into lengths of 1∼2 cm; or (2) a kneading machine was used to knead-wire the material. Lactobacillus plantarum was previously isolated by our research team. Cellulase was obtained from Yakult (Yakult Pharmaceutical Industry Co., Tokyo, Japan). All additives were added to fresh S. arundinaceum material after cutting.
Our 2 × 4 × 5 experiment was performed with a completely randomized design: 2 cutting methods (chopping, knead-wiring) × 4 additive treatments [1.0 × 106 colony forming units (cfu)/g FM (Fresh matter, FM) LP, 50 U/g FM CE, 1 × 106 cfu/g FM LP + 50 U/g FM CE (LP + CE), and the control (CK)] × 5 fermentation periods (3, 7, 15, 30, and 60 day). Three polyethylene bags (300 mm × 230 mm) were loaded with 500 g of silage and vacuum sealed per treatment, for a total of 120 experimental bags. All bags were stored at room temperature (approximately 25°C). Three bags for each treatment were opened to examine fermentation quality and microbial population at days 3, 7, 15, 30, and 60 during ensiling. The chemical components were evaluated after 60 day of fermentation. The bacterial community was analyzed after 60 day of ensiling.
Chemical analyses
To measure fermentation indices, 10 g of each silage sample was blended with 90 mL of sterilized water and then filtered through qualitative filter paper. The silage pH and contents of NH3-N and organic acids were then determined using the resulting filtrate. The pH was immediately measured with a PHS-3C pH meter equipped with a glass electrode (INESA Scientific Instrument Co., Shanghai, China). Then, the filtrate was frozen in −20°C storage prior to determining the concentration of NH3-N and organic acids. The NH3-N concentration was quantified using the phenol-hypochlorite method proposed by Broderick and Kang (1980). The concentrations of various organic acids, including acetic acid (AA), BA, propionic acid (PA), and LA, were determined through high-performance liquid chromatography (HPLC) (LC-20A, Shimadzu, Tokyo, Japan) (Zhang et al., 2016).
To quantify the silage DM content, the samples were dried at 65°C in an air circulation oven for 48 h. Then, the samples were pulverized and passed through a 1 mm screen. The Van Soest et al. (1991) method was employed to determine both acid detergent fiber (ADF) and NDF. The Association of Official Agricultural Chemists [AOAC] (1995) method was employed to determine the content of CP. Murphy (1958) anthrone method was used to quantify the WSC content.
Investigation of microbial populations
The plate count method (Li P. et al., 2019) was employed to quantify the size of silage microbial populations. Briefly, each 10 g sample was mixed 1:9 (w/v) with sterilized water, followed by serial dilution from 10–1 to 10–6 using sterilized water. Yeast population densities were enumerated by plating on Rose Bengal agar (YM01435, Shyuanmu Biomart Biotech. Co., Shanghai, China) containing 1.5 mg/L tetracycline, and LAB population densities were enumerated by De Man, Rogosa and Sharpe (MRS) agar (GCM188, Beijing Land Bridge Technology Co., Beijing, China). Yeasts and LAB were incubated for 72 h at 30°C and for 48 h at 37°C, respectively. Each plate count was transformed into log10 cfu/g FM.
Analysis of bacterial community structure
Total bacterial DNA was extracted from 60-day silage samples according to the sodium dodecyl sulfate (SDS)/cetyltrimethylammonium bromide (CTAB) method. We used 1% agarose gels to assess DNA concentration and purity and extracted DNA diluted with sterilized water to a standardized concentration of 1 ng/μL. We amplified the bacterial 16S rRNA genes with the broad-range primers 27F (5-GAGAGTTTGATC CT GGCTCAG-3) and 1541R (5-AAGGAGGTG ATCC AGCCGCA-3) (Yan et al., 2019). TransStart® FastPfu DNA Polymerase (TransGen Biotech, Beijing, China) was used to carry out polymerase chain reactions (PCRs). The same volume of 1X loading buffer was mixed with PCR products, and electrophoresis was performed on a 2% agarose gel for detection. The QIAquick@ Gel Extraction Kit (Qiagen, Hilden, Germany) was used for PCR product purification. The SMRTbell™ Template Prep Kit (Pacific Biosciences, Menlo Park, CA, USA) was used to generate the SMRTbell library following the manufacturer’s standard instructions. The Qubit@ 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA) and a FEMTO Pulse system were used to ensure library quality.
Raw sequences were processed, and a sequencing library was created by Novogene Bio Technology Co. (Beijing, China) using the PacBio Sequel sequencer (Pacific Biosciences, Menlo Park, CA, USA). Taxonomic information was annotated in the Silva SSUrRNA Database.1 After determining the phylogenetic relationships, the Magic platform was employed to calculate alpha diversity (Chao 1, Shannon, and Ace) and coverage values and perform bacterial community functional prediction (Li et al., 2021).
Data processing and statistical analyses
SPSS ver. 23.0 for Windows (SPSS Inc. Chicago, IL, USA) was employed to carry out all statistical analyses. One- and two-way analyses of variance (ANOVA) were carried out using the fixed effects of additives, cutting methods, and ensiling days. To assess the separation of means under different fermentation additives, cutting methods, and fermentation times, Duncan’s multiple range test was performed. P < 0.05 was set as the threshold of statistical significance. Heatmaps based on Spearman correlation coefficients between the bacterial population and fermentation quality and function prediction were calculated with SPSS software. All data are reported as the mean with statistical significance indicated with letters. All figures were generated using OriginPro 2017.2
Results and discussion
Characteristics of fresh Saccharum arundinaceum before ensiling
The characteristics of the fresh material before ensiling are presented in Table 1. The content of DM in fresh S. arundinaceum was 284.2 g/kg. The CP content of S. arundinaceum was 104.13 g/kg on a DM basis, which was similar to that of oats (Zhao et al., 2018). However, the relatively high contents of NDF (672.51 g/kg DM) and ADF (302.32 g/kg DM) and the low content of WSCs (g/kg DM) are not conducive to the fermentation of LAB and animal utilization (Xu et al., 2020; Wan et al., 2021). The epiphytic yeasts (3.72 log cfu/g FM) on S. arundinaceum were higher than LAB (1.29 log cfu/g FM).
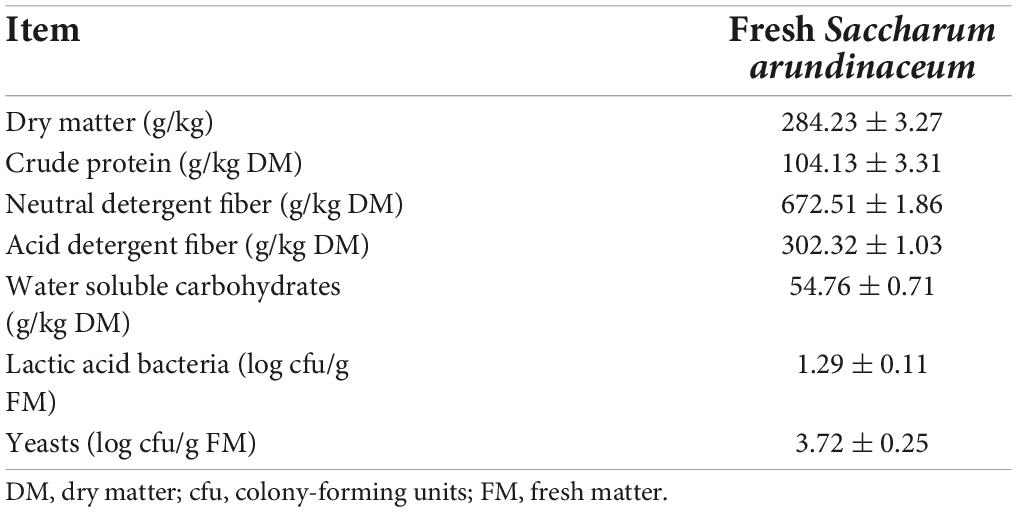
Table 1. Microbial and chemical characterization of unfermented Saccharum arundinaceum (mean ± SEM, n = 3).
Chemical characteristics of Saccharum arundinaceum after ensiling
The effects of cutting methods and additives on the chemical characteristics (DM, CP, NDF, ADF, and WSC contents) of S. arundinaceum silage on day 60 are shown in Figure 1. As shown in Figure 1A, the contents of DM in the silages with two additives were well preserved and were significantly higher (P < 0.05) than those of the CK treatments (except for the CE-chopped silage). This result was consistent with previous studies showing that adding LP and CE could suppress the growth and/or activity of undesirable microorganisms by rapidly decreasing the pH (Li F. et al., 2019; Wang Y. et al., 2019). The DM content of the chopped treatments was higher than that of the knead-wired silages (P < 0.001), which could be attributed to the lower pH of the chopped treatments (Table 2), and more nutrient substances were preserved. Another reason may be that the knead wire could provide high levels of fermentable carbohydrates by its more complex processing method, and epiphytic microorganisms consume much more biomass during ensiling, especially undesirable bacteria (Kõiv et al., 2019). The variation in CP content in S. arundinaceum silages (Figure 1B) was similar to that of the DM content, and adding LP + CE also led to higher CP contents than that of the CK-treated silages with chopping method (P < 0.05). LP and CE inoculation provided abundant LAB and increased the fermentation material, respectively, which induced the accumulation of organic acids and a quick decrease in pH. The relatively low pH inhibited undesirable microorganism growth and activity and/or proteolytic enzyme activity (Ni et al., 2017; Wang C. et al., 2019); hence, more crude protein in the silages was preserved. The above reason also contributing to the higher CP contents in chopped silages compared to knead wired silages. Moreover, the interaction between additives and cutting methods had a statistically significant effect on the preservation of CP (P < 0.05). Therefore, LP + CE-chopped silages had a better effect on preserving the CP.
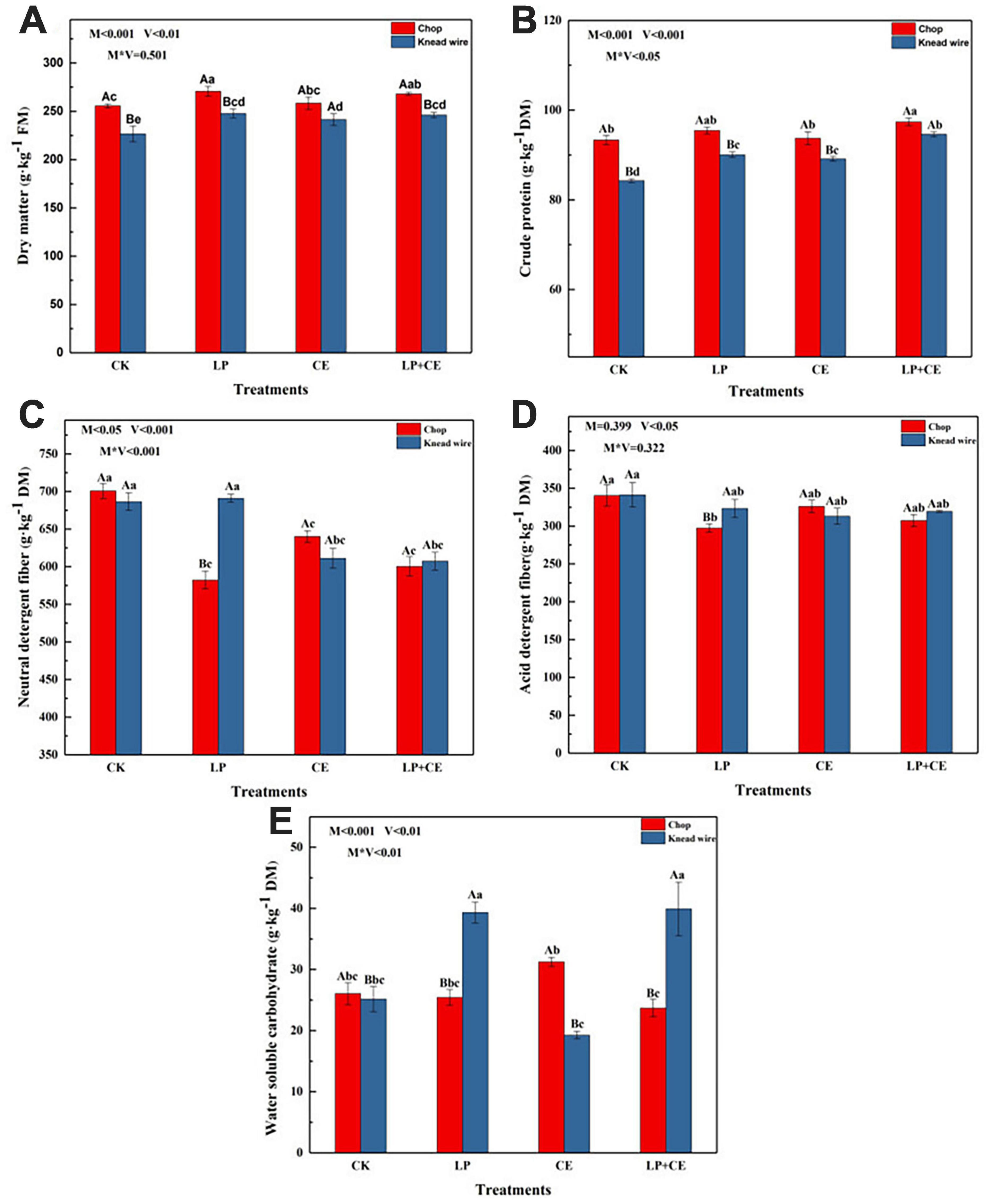
Figure 1. Effects of cutting methods and fermentation additives on (A) DM, (B) CP, (C) NDF, (D) ADF, and (E) WSC of Saccharum arundinaceum after 60 day of ensiling. CK, control; LP, Lactobacillus plantarum; CE, cellulase; LP + CE, L. plantarum plus cellulase. Significant differences (P < 0.05) between the same additive treatments are indicated by capital letters, and significant differences (P < 0.05) among different treatments are indicated by lowercase letters. DM, dry matter; FM, fresh matter; M, cutting methods; V, additive; V*M, the interaction between additive and cutting methods.
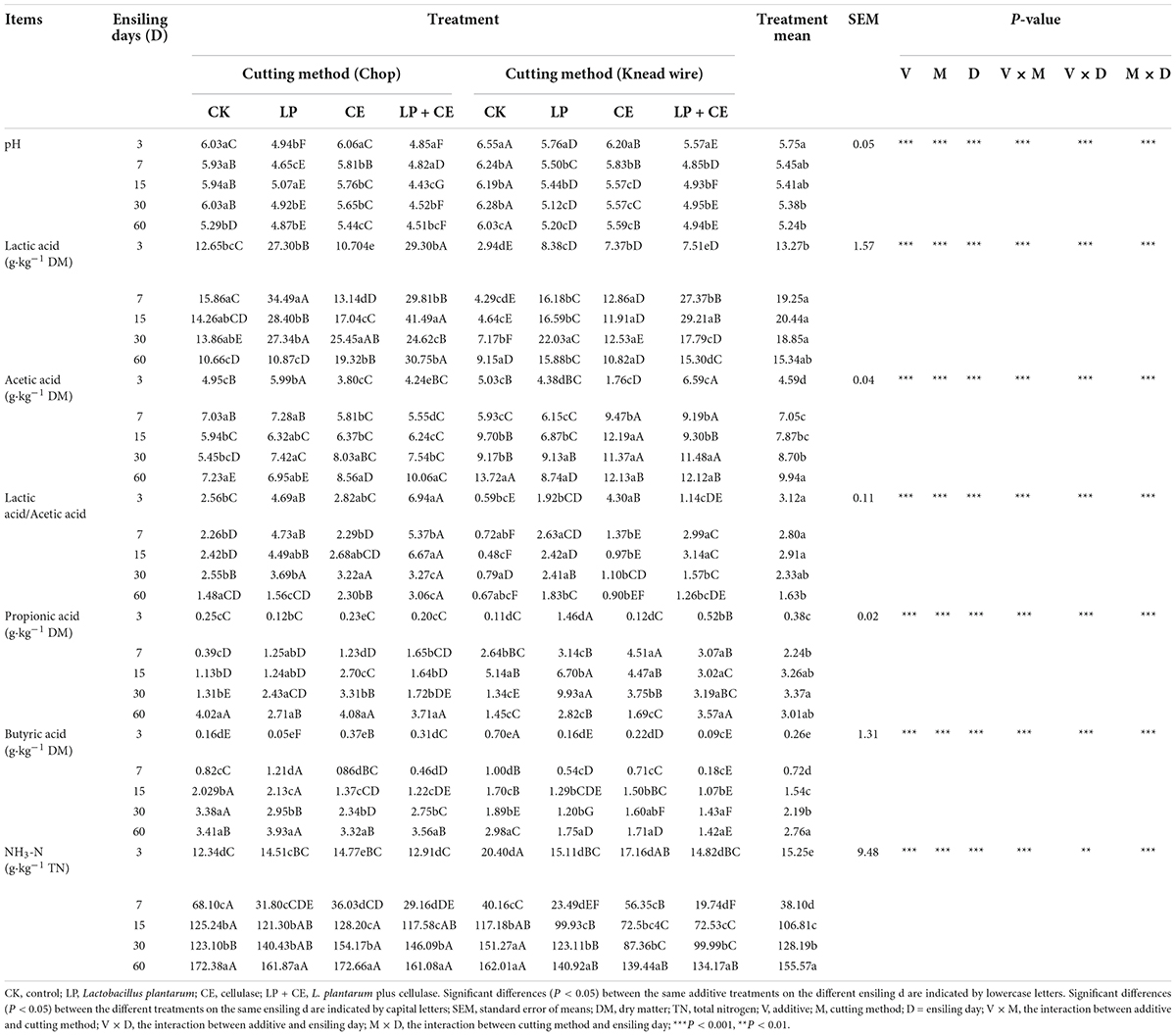
Table 2. Fermentative profile of Saccharum arundinaceum silage subjected to different cutting methods and additives during fermentation.
As shown in Figures 1C,D, M (cutting methods) × V (additives) significantly influenced the content of NDF (P < 0.001) and V (additives) significantly influenced the ADF content (P < 0.05). The NDF contents in the CE- and LP + CE-treated S. arundinaceum silages were lower than those of the CK (P < 0.05), while there was no significant difference (P > 0.05) between CE and LP + CE. Cellulase can catalyze the hydrolysis of cellulose; therefore, the CE- and LP + CE-treated silages showed a large reduction in NDF content (Xu et al., 2020; Du et al., 2021). Moreover, the NDF contents of LP-chopped silages were also significantly decreased compared with those of CK-chopped silages, but LP-kneaded silages showed no difference from those of the CK. The above result was probably due to the pH lower than 5.0 of LP-chopped silages in most of the fermentation time (Table 2), while LP-kneaded silages had a higher pH than LP-chopped treatments. The relatively low pH led to acid hydrolysis, which reduced the lignocellulosic structure (Yang et al., 2019). For ADF contents, all the treatments had no significant difference from CK, except for the LP-chopped silages. Lignin and hemicellulose constitute lignin-carbohydrate complexes that act as a protective layer to prevent cellulose degradation (Yu et al., 2005), which may result in high levels of NDF degradation and low levels of ADF degradation in S. arundinaceum silages. Another reason for this observation may be that ADL (included in the ADF) is difficult to degrade, and S. arundinaceum has high levels of lignin; moreover, lignin is closely associated with hemicellulose by ester and hydrogen bonds (Li et al., 2018).
Water-soluble carbohydrates are the major fermentation substrates for LAB during ensiling and can be converted into organic acids and rapidly decrease the pH of silages by LAB (Ahmadi et al., 2019; Xu et al., 2020). The influence of different cutting methods and additives on the WSC contents of S. arundinaceum silage is shown in Figure 1E. The WSC content of CE-chopped silages following 60 day of ensiling was higher than that of other silages, but only significantly higher than LP + CE-chopped silages. These results indicate that the addition of CE improved lignocellulosic degradation and preserved more WSCs (Xu et al., 2020). The lower WSC contents of LP + CE silages than CE silages were probably because the addition of LP enhanced the abundance of LAB to consume more WSCs, corresponding to the result of bacterial community abundance, in which the relative abundance of Lactobacillus in CE and LP + CE silages was 0.27 and 80.38%, respectively (Figure 3). In general, the interaction of additives × cutting methods existed for the WSC contents (P < 0.01). Moreover, for LP and LP + CE, the knead-wired silages had higher WSC contents, had higher pH values and poorer fermentation quality than chopped silages (Table 2), which needs further study.
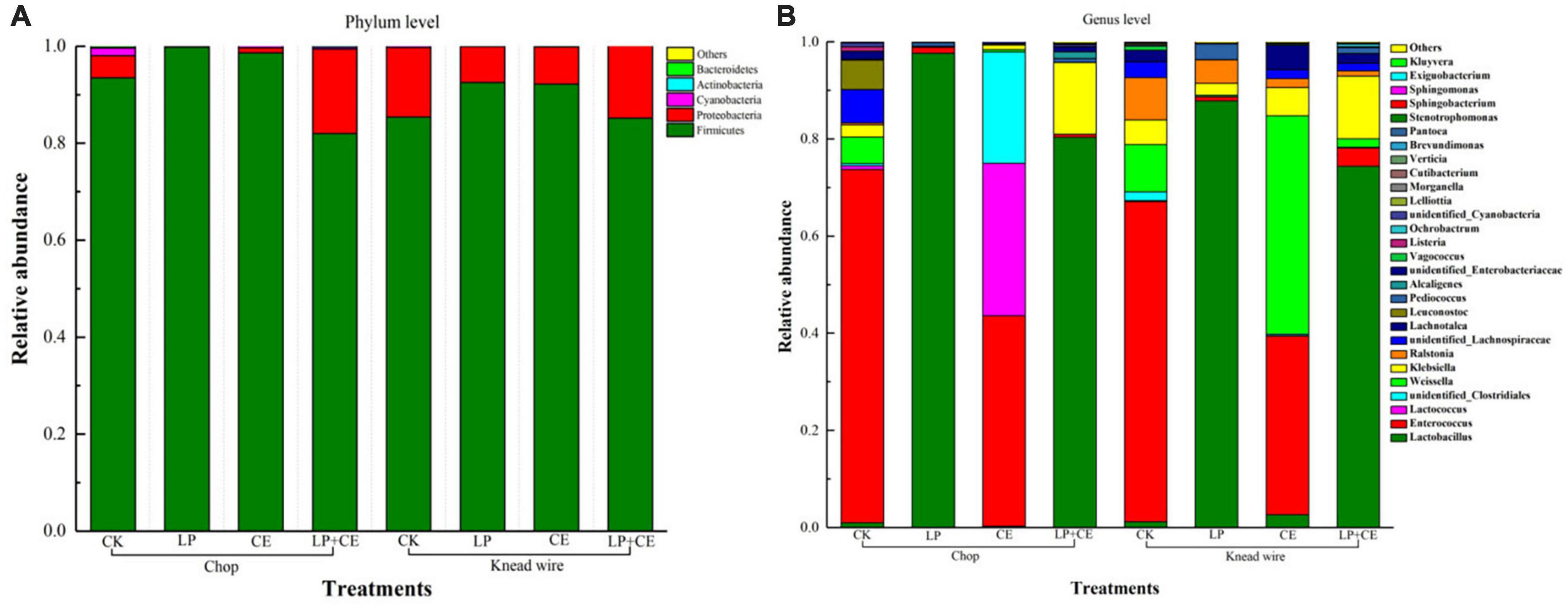
Figure 2. Taxonomic classification and relative abundances of the bacterial community of 60-day Saccharum arundinaceum silage. Bacteria were identified to the (A) phylum and (B) genus levels. CK, control; LP, Lactobacillus plantarum; CE, cellulase; LP + CE, L. plantarum plus cellulase.
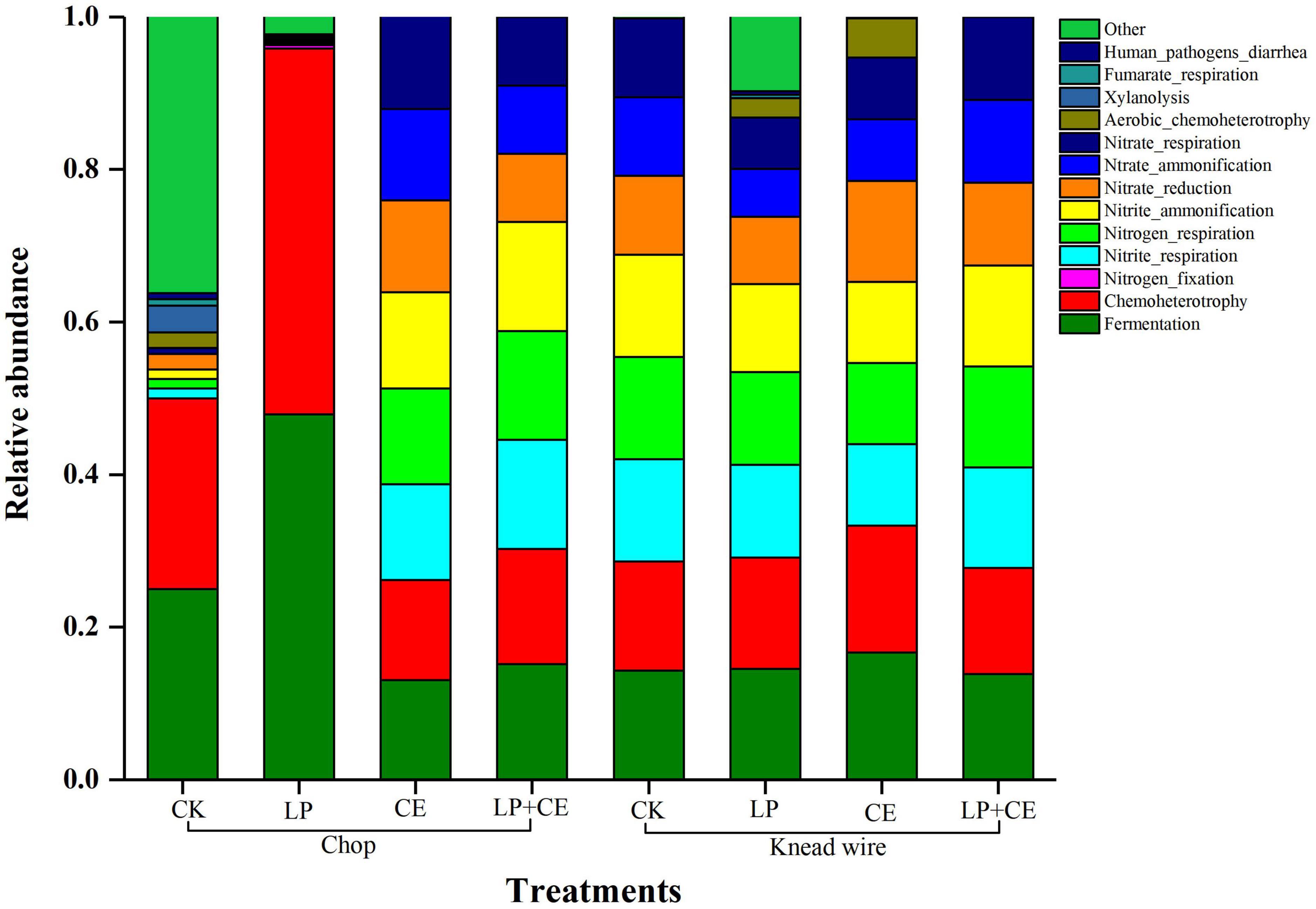
Figure 3. Functional prediction of the bacterial community of 60-day Saccharum arundinaceum silage. CK, control; CE, cellulase; LP, Lactobacillus plantarum; LP + CE, combination of L. plantarum and cellulase. CK, control; LP, L. plantarum; CE, cellulase; LP + CE, L. plantarum plus cellulase.
Fermentation profile and microbial population of Saccharum arundinaceum silages
The effects of cutting methods and additives on the silage fermentation profile of S. arundinaceum are listed in Table 2. In our study, the interaction of V × M existed for pH (P < 0.001), concentrations of LA (P < 0.001), AA (P < 0.001), PA (P < 0.001), BA (P < 0.001), and NH3-N (P < 0.001), and LA/AA (P < 0.001).
Silage pH is an important indicator for ensiling (Larsen et al., 2019; He et al., 2020). During fermentation, the pH value of LP and LP + CE-chopped silages decreased to 4.94 and 4.85 in the first 3 day and continuously decreased to 4.87 and 4.51 on day 60, respectively, which were significantly lower (P < 0.05) than those of the other treatments on each ensiling day. Therefore, LP addition accelerated the pH decline. As expected, adding LP (LP and LP + CE treatments) to the chopped and knead-wired silages led to a lower pH than that of the CK groups (P < 0.05), as LP addition could improve fermentation by producing abundant lactic acid (Li F. et al., 2019; Guo et al., 2021). Moreover, compared with LP treatments, the pH value of LP + CE silages significantly decreased (except for the chopped silages on day 3), which might be because the addition of CE improved the efficiency of cellulose degradation, thereby increasing the substrate available for LAB fermentation (Xu et al., 2020). For the same additive treatment, kneaded wired silages had higher pH values than chopped silages, which was probably due to the lower concentration of lactic acid and the higher abundance of Weissella and lower abundance of Lactobacillus (Mu et al., 2020; Ren et al., 2020; Du et al., 2021).
In Table 2, the dynamics of the organic acid concentration of S. arundinaceum silages are shown. Inoculation with LP significantly (P < 0.05) increased the silage contents of LA subjected to both cutting methods (except for the chopped silages on day 60). L. plantarum is known to potentially improve fermentation quality and reduce silage pH (Ni et al., 2017), which could contribute to the lower pH values of both the LP and LP + CE treatments. In contrast to the silage pH value, knead-wired silages have a lower concentration of LA than chopped silages, especially at the initial fermentation. The AA concentration was higher in S. arundinaceum silages with knead wire (especially in the CE groups) vs. chopped silages (P < 0.001). The major reason for the higher AA concentration in knead wire-treated S. arundinaceum silages could be attributed to the enhancement of heterolactic acid fermentation (Li F. et al., 2019). The reason for this phenomenon may be that heterofermentative microorganisms are dominant in tropical forages of spontaneous fermentation form, as previously reported (Shao et al., 2003). The addition of CE to knead wire-treated silages may lead to a higher relative abundance of Weissella, which are obligatory heterofermentative LAB (Figure 2; Graf et al., 2016; Ren et al., 2020). As expected, inoculation with LP increased the ratios of LA/AA of the silages compared to those of the CK- and CE-treated silages (P < 0.001), which could be illustrated by the addition of homofermentative LAB (L. plantarum), increasing the homolactic fermentation and LA production of the silage (Lin et al., 2020).
For the concentration of PA and BA, adding LP resulted in lower concentrations of these two organic acids (except for PA in knead-wired silages) (P < 0.05). Generally, excess moisture can impair silage fermentation and secondary fermentation by Clostridia and Enterobacteria, which can convert LA into PA (Kung, 2008). Adding LP (LP and LP + CE) significantly reduced the NH3-N content compared with the CK and CE-treated silages (P < 0.05). The above phenomenon showed that LP silage inhibited the proteolytic action of plant enzymes and the growth and/or activity of Clostridia and Enterobacteria (Figure 3; Guan et al., 2018; Cai et al., 2020; Dong et al., 2020), which can be found in Figure 3. In contrast, a lower content of NH3-N was detected in knead-wired silages than in chopped silages (P < 0.001); further studies are required to explore this phenomenon.
The dynamics of the LAB and yeast populations of S. arundinaceum silages are listed in Table 3. The number of LAB increased at the initial stage and then decreased from day 7 (Treatment mean), as many LAB strains are relatively intolerant to lower pH values (Ohmomo et al., 2002). Although no significant difference (P > 0.05) was observed in LAB populations between chopped and knead-wired silages, additives had a strikingly significant (P < 0.001) impact on the LAB population in S. arundinaceum silage. Before 15 days of fermentation, adding LP resulted in lower numbers of yeast because of the decrease in pH. Yeast was not detected in any of the samples after 30 days, which was due to the anaerobic environment during the late period of silage fermentation (Zhang et al., 2021), indicating better fermentation performance.
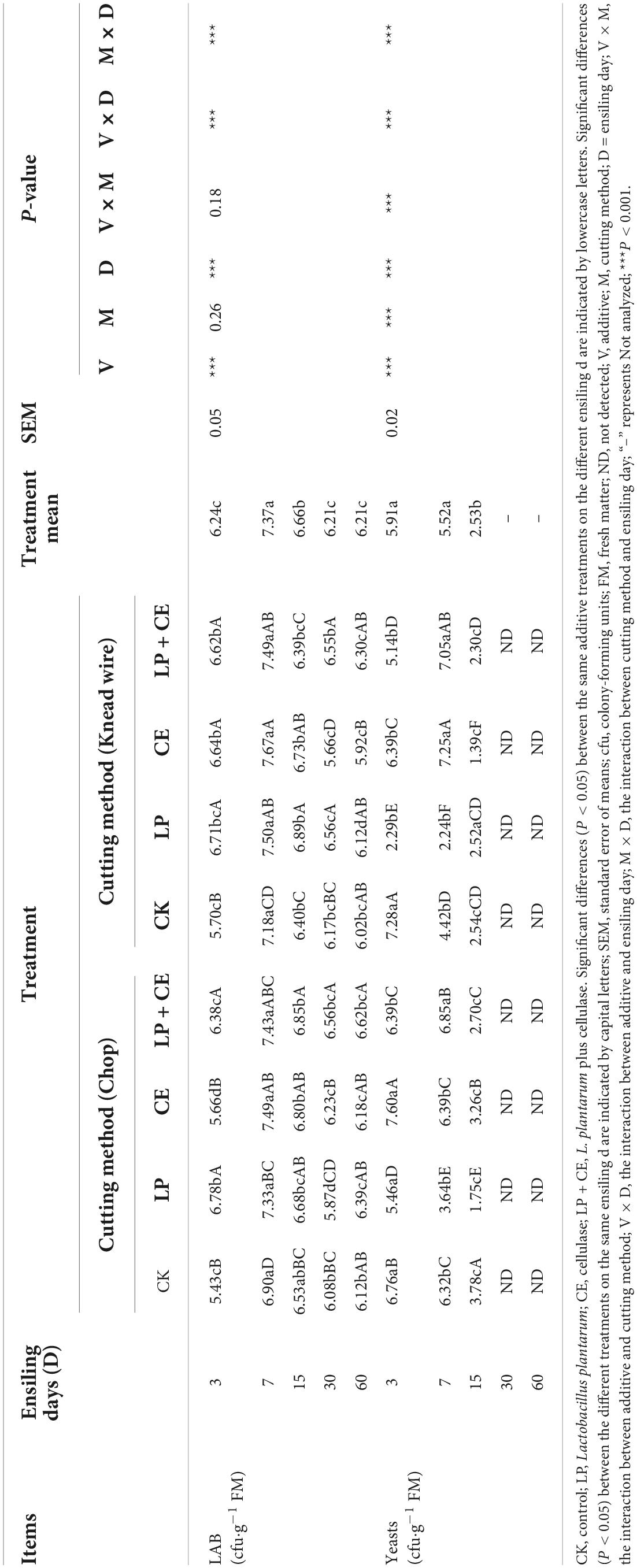
Table 3. Microbial population of Saccharum arundinaceum subjected to different cutting methods and additives during fermentation.
Bacterial community diversity of Saccharum arundinaceum silage
The observed species, Good’s coverage, alpha diversity including ACE, Chao1 estimation, and Shannon index of bacteria are summarized in Table 4. Alpha diversity reflects the bacterial abundance (ACE and Chao1) and species diversity (Shannon index) in a sample (Du et al., 2021). After ensiling, the LP- and CE-inoculated chopped silage produced lower observed species, ACE, Chao1 estimation, and Shannon index than the CK silages, possibly due to the lower pH in the additive treatments, which could inhibit the growth of some microorganisms (Méndez-García et al., 2015). In contrast, CE-treated knead-wired silages had greater species richness, abundance (ACE and Chao1), and diversity (Shannon index) than CE-chopped silages. Knead-wired silages tended to have an increased pH, likely conducive to the survival of many microbes, including heterofermentative LAB (Hu et al., 2009; Yang et al., 2019). Good’s coverage of all samples was above 0.99, the depth of sequencing results which can represent the real situation of the silage samples well and makes it feasible to analyze the microbial community (Yang et al., 2019).
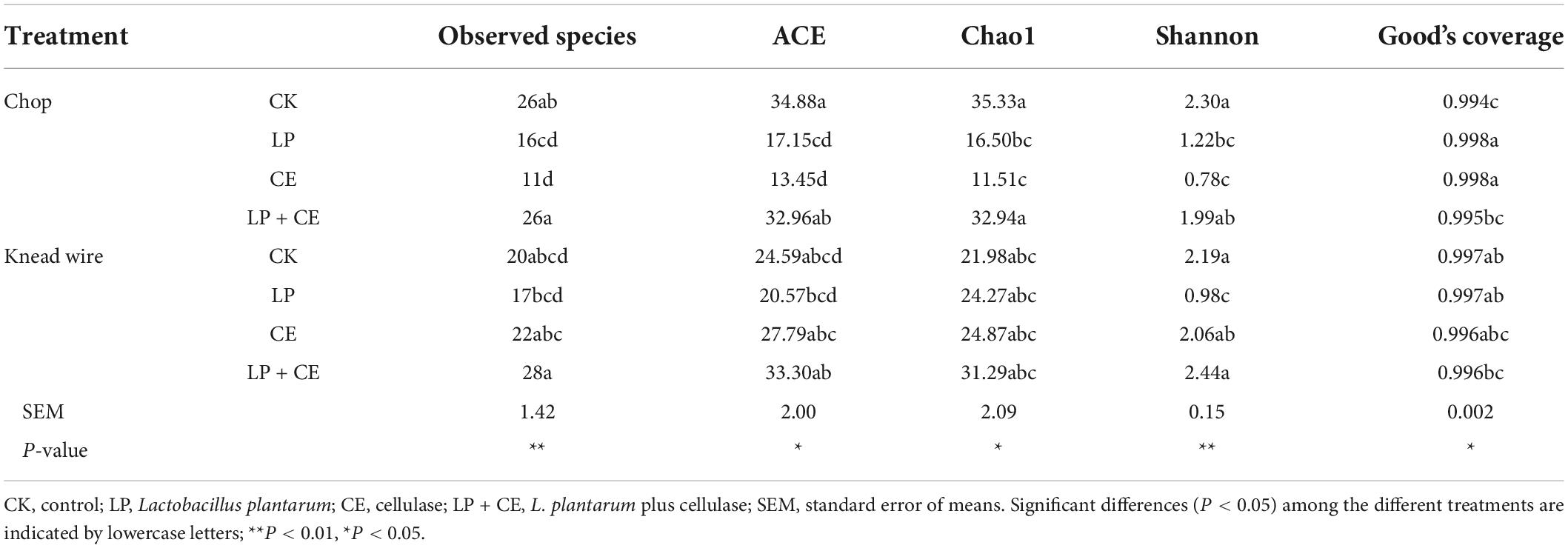
Table 4. Bacterial species richness and diversity of Saccharum arundinaceum silage subjected to different cutting methods and fermentation additives.
The bacterial community structure in the S. arundinaceum silage on day 60 was explored by high-throughput Illumina MiSeq sequencing. As shown in Figure 2, the phylum and genus levels of bacterial community compositions are visualized as stacked columns. Figure 2A shows that the silages were dominated by two main phyla (Firmicutes and Proteobacteria) at the end of ensiling, which can be commonly found in previous studies (Mu et al., 2020; Ren et al., 2020). Firmicutes, which is crucial to producing organic acids such as LA and AA at the later stage of fermentation (Zhao et al., 2017), became the most dominant bacterial phylum in all the samples, with a relatively high abundance of 82%∼99.8%, especially in the LP- and CE-chopped silages (99.8 and 98.62%, respectively). For knead-wired silages, the higher relative abundance of Proteobacteria, which can digest organic matter, was probably due to a variety of factors, including climate and silage material composition (Guan et al., 2018; Mu et al., 2020; Yuan et al., 2020). In our study, the reason may be the cutting methods.
In Figure 2B, the most predominant genera in the CK treatments of the chopped and knead-wired silages were Enterococcus (72.74 and 65.93%, 0.31%, respectively), Weissella (5.46 and 9.74%, respectively) and Klebsiella (2.46 and 5.06%, respectively), and in the CE treatments were Enterococcus (43.3 and 36.77%, respectively), Lactococcus (31.43 and 0.09%, respectively), unidentified_ Clostridiales (22.93 and 0.31%, respectively), Weissella (0.51 and 45.01%, respectively). The dominant genera in the LP and LP + CE treatments of the two cutting methods were Lactobacillus (74.37∼97.65%) and Klebsiella (0∼12.85%), while chop-treated silages had a relatively higher abundance of Lactobacillus and a relatively lower abundance of Klebsiella. The LAB genera Lactobacillus, Weissella, and Lactococcus are functional bacteria that can be used to improve silage quality (Yang et al., 2016). Moreover, Lactobacillus played a vital role in silage fermentation, as they could decrease the pH of silage rapidly to preserve the nutrient substance and could remain active and viable at low pH values (Dunière et al., 2013; Zhang et al., 2021). Therefore, adding LP significantly enhanced Lactobacillus abundance, which resulted in the lower silage pH of the LP and LP + CE treatments of the chopped and knead-wired silages compared with that of the CK and CE groups, which is in line with the findings of previous studies (Ren et al., 2020; Zhang et al., 2021). Relatively speaking, Enterobacter are considered undesirable bacteria in silage, as they may compete with LAB to utilize fermentation substrates and produce NH3-N. As a result, the high relative abundance of Enterobacter in the CK and CE treatments led to a slower decline in pH and an increase in protein degradation (Wang Y. et al., 2019), which is shown in Table 2 and Figure 1. Therefore, for LP and LP + CE, especially in chopped silages, undesirable growth of microorganisms of Enterobacter was inhibited, and the dominant Lactobacillus growth was increased, which resulted in a low pH value in the silage (Dunière et al., 2013). A higher Weissella abundance was found in the CK- and CE-knead wire-treated silages compared with that of other knead wired silages, which belong to the heterofermentative LAB and consume WSCs to produce both LA and AA, leading to an increasing concentration of AA (Table 2; Graf et al., 2016). Lactococcus were identified at the early stage of silage fermentation and exhibited a declining trend as fermentation proceeded, which decreased gradually due to the rapid drop in pH value and severe acidic conditions (Liu B. Y. et al., 2019). Bacteria from the genus Klebsiella can convert amino acids into ammonia and biogenic amines (Zhang et al., 2021), and the higher abundance of Klebsiella probably resulted in the lower CP content of knead wire-treated silages (Figure 1).
Function prediction and correlation analysis of the microbial community based on some silage parameters
Functional prediction of the bacterial community of S. arundinaceum silage fermentation on day 60 was visualized as a stacked column (Figure 3). Fermentation was the main function of the microorganism community, followed by chemoheterotrophy, nitrite respiration, nitrogen respiration and nitrite ammonification. The bacterial communities of chopped silages had a higher proportion of fermentation and chemoheterotrophy functions compared with knead-wired silages, indicating that carbon fixation was inhibited and energy was obtained through organic oxidation in these silages (Zhang et al., 2018). Therefore, chopped treatment could enhance ensiling fermentation, resulting in lower pH values (Table 2), which were favorable for silage preservation. The LP-treated chopped silages promoted the fermentation of the bacterial community, whereas LP + CE-treated chopped silages had no significant effect vs. the CK-chopped silages. The deceased rate and extent of fermentation of the treatment with LP + CE need further study. Moreover, the high levels of nitrite respiration, nitrogen respiration and nitrite ammonification of the bacterial community in knead-wired silages could lead to lower CP contents (Figure 1; Li et al., 2021).
As shown in Figure 4, the interactions between some silage parameters and bacterial community/function prediction of the bacterial community in the S. arundinaceum silages were presented by establishing their Spearman correlations and then visualized in heatmaps. Figure 4A shows the correlations between the metabolites and microbial diversity. In general, silage fermentation metabolites can be improved by the diversity of microorganisms, and the produced metabolites can affect the bacterial community and thus silage quality (Dong et al., 2020; Li et al., 2021). Therefore, many previous studies have investigated the correlations between microorganisms and silage fermentation parameters (Xu et al., 2018; Ren et al., 2020; Li et al., 2021). For example, Li et al. (2021) found that the bacteria in the genus Pediococcus were negatively correlated with pH value and positively correlated with the LA concentration in silage, which was consistent with our findings. In our study, the dominant genus Lactobacillus was positively correlated with the contents of CP (r = 0.494, P < 0.05) and LA (r = 0.494, P < 0.05) and negatively correlated with pH (r = −0.765, P < 0.001) in the S. arundinaceum silage. However, the relative abundance of Enterococcus was positively correlated with pH (r = 0.656, P < 0.001) and negatively correlated with CP content (r = −0.458, P < 0.05). These results indicated that inoculation with L. plantarum enhanced the concentration of LA, which decreased the pH and inhibited undesirable microorganisms during ensiling to preserve the silage nutrient substance (e.g., CP and DM) (Dong et al., 2020; Ren et al., 2020). Weissella can produce both LA and AA (Yan et al., 2019); therefore, pH (r = 0.453, P < 0.05) and AA concentration (r = 0.425, P < 0.05) were significantly positively correlated with Weissella abundance, probably slowing the pH decline and thus resulting in unfavorable silage fermentation. Therefore, the contents of CP (r = –0.431, P < 0.05), WSC (r = –0.503, P < 0.05), PA (r = –0.571, P < 0.05) and BA (r = –0.407, P < 0.05) were significantly negatively correlated with Weissella abundance. The relationships between the ensiling characteristics and functional prediction in the bacterial community are shown in Figure 4B. Silage pH was significantly positively correlated with fermentation and chemoheterotrophy (P < 0.01). The CP contents were highly positively correlated with nitrite respiration (r = 0.537, P < 0.01), nitrogen respiration (r = 0.537, P < 0.01) and nitrite ammonification (r = 0.532, P < 0.01) of the bacterial community, which could result in the lower CP content. This phenomenon can be found in Figure 1.
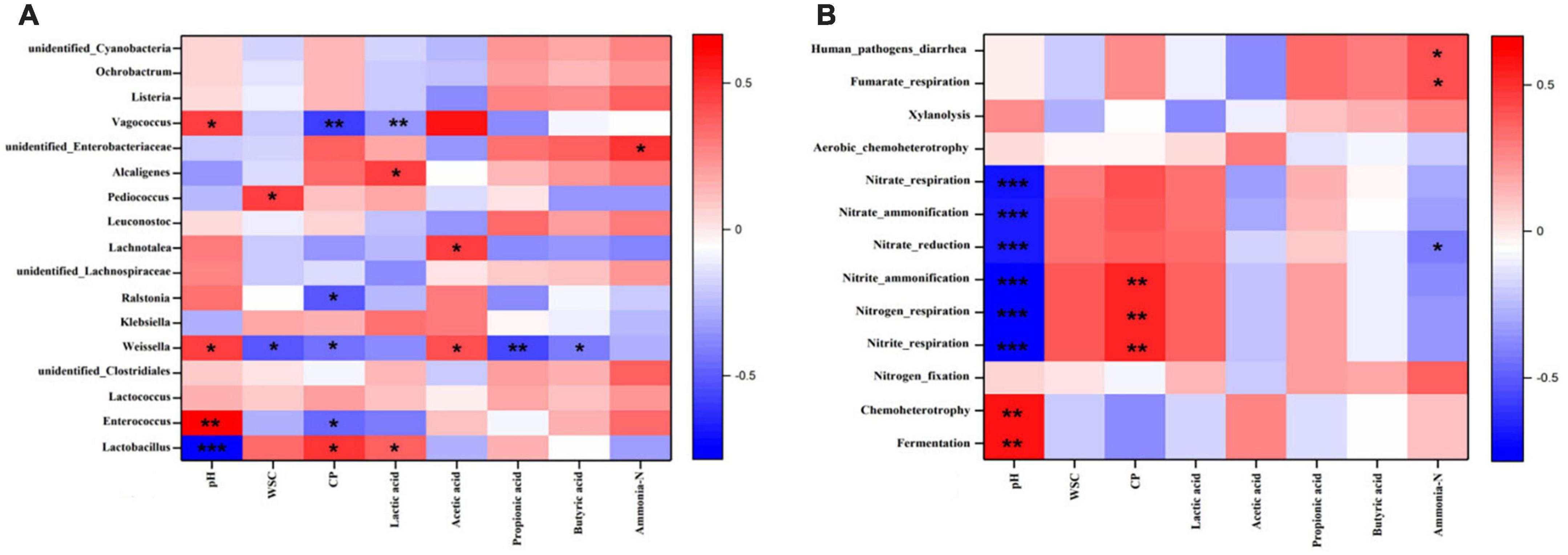
Figure 4. Heatmap of Spearman correlations between bacterial community (A) predicted functions (B) and silage characteristics of 60-day Saccharum arundinaceum silage. Heatmap colors denote the Spearman correlation coefficient “r” (–1 to 1). r > 0 represents a positive correlation, and r < 0 represents a negative correlation. The asterisks *, **, and *** represent statistical significance levels of P < 0.05, < 0.01 and < 0.001, respectively.
Conclusion
The results showed that adding LP and CE efficiently increased the content of DM, CP, and LA and decreased the content of NDF and pH of S. arundinaceum silage, especially chop-treated silage. Moreover, the bacterial community structure underwent great changes with different cutting methods and additives, with increased abundance of Lactobacillus in the LP and CE-chopped treatment. In summary, the chopped S. arundinaceum silages treated with the LP + CE inoculant achieved better fermentation quality and preservation of nutrient substances. Therefore, S. arundinaceum can serve as a new silage resource for feed utilization by the ensiling method of LP + CE-chopped.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YZ, PL, and CC designed the study, wrote the manuscript, and were involved in the revision of the manuscript. YZ, PL, ML, JX, and HS performed the experiments. QC, YX, CW, and PL conducted the statistical analysis. All authors reviewed and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (Grant Number: 2021YFD1300300) and Guizhou Provincial Science and Technology Projects (Grant Number: QKHJC -ZK[2022)General 156].
Acknowledgments
We thank Dr. Wenlong Gou, Dr. Jiajun Yan, and Dr. Minghong You from Sichuan Academy of Grassland Sciences for their help during sampling preparation and collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Ahmadi, F., Lee, Y. H., Lee, W. H., Oh, Y. K., Park, K., and Kwak, W. S. (2019). Long-term anaerobic conservation of fruit and vegetable discards without or with moisture adjustment after aerobic preservation with sodium metabisulfite. Waste. Manage. 87, 258–267. doi: 10.1016/j.wasman.2019.02.010
Association of Official Agricultural Chemists [AOAC] (1995). Official methods of analysis of the AOAC International, 16th Edn. Washington, MD: AOAC International.
Broderick, G. A., and Kang, J. H. (1980). Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 63, 64–75. doi: 10.3168/jds.S0022-0302(80)82888-8
Cai, Y. M., Benno, Y., Ogawa, M., Ohamomo, S., Kumai, S., and Nakase, T. (1998). Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microbiol. 64, 2982–2987. doi: 10.1128/AEM.64.8.2982-2987.1998
Cai, Y. M., Du, Z. M., Yamasaki, S., Nguluve, D., Tinga, B., Macome, F., et al. (2020). Community of natural lactic acid bacteria and silage fermentation of corn stover and sugarcane tops in Africa. Asian. Austral. J. Anim. 33, 1252–1264.
Dong, L. F., Zhang, H. S., Gao, Y. H., and Diao, Q. Y. (2020). Dynamic profiles of fermentation characteristics and bacterial community composition of Broussonetia papyrifera ensiled with perennial ryegrass. Bioresource. Technol. 310:123396. doi: 10.1016/j.biortech.2020.123396
Du, Z., Sun, L., Chen, C., Lin, J., Yang, F., and Cai, Y. (2021). Exploring microbial community structure and metabolic gene clusters during silage fermentation of paper mulberry, a high-protein woody plant. Anim. Feed Sci. Tech. 275:114766. doi: 10.1016/j.anifeedsci.2020.114766
Dunière, L., Sindou, J., Chaucheyras-Durand, F., Chevallier, I., and Thévenot-Sergentet, D. (2013). Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed. Sci. Tech 182, 1–15. 2013. 04.006 doi: 10.1016/j.anifeedsci
Feng, L., Perschkea, Y. M. L., Fontaineb, D., Nikolauszc, M., Warda, A. J., da Rochac, U. N., et al. (2020). Anaerobic digestion of co-ensiled cover crop and barley straw: Effect of coensiling ratios, manure addition and impact on microbial community structure. Ind. Crop. Prod. 144:112025.
Graf, K., Ulrich, A., Idler, C., and Klocke, M. (2016). Bacterial community dynamics during ensiling of perennial ryegrass at two compaction levels monitored by terminal restriction fragment length polymorphism. J. Appl. Microbiol. 120, 1479–1491. doi: 10.1111/jam.13114
Guan, H., Yan, Y. H., Li, X. L., Li, X. M., Shuai, Y., Feng, G. Y., et al. (2018). Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour. Technol. 265, 282–290. /j.biortech.2018.06.018 doi: 10.1016
Guo, L. N., Lu, Y. X., Li, P., Chen, L. Y., Gou, W. L., and Zhang, C. B. (2021). Effects of delayed harvest and additives on fermentation quality and bacterial community of corn stalk silage. Front. Microbiol. 10:687481. doi: 10.3389/fmicb.2021.687481
Guo, L., Yao, D., Li, D., Lin, Y., Bureenok, S., Ni, K., et al. (2020). Effects of lactic acid bacteria isolated from rumen fluid and feces of dairy cows on fermentation quality, microbial community, and in vitro digestibility of alfalfa silage. Front. Microbiol. 10:2998. doi: 10.3389/fmicb.2019.02998
Hattori, T., and Morita, S. (2010). Energy crops for sustainable bioethanol production; which, where and how? Plant. Prod. Sci. 13, 221–234. doi: 10.1626/pps.13.221
He, L. W., Lv, H. J., Xing, Y. Q., Wang, C., You, X. W., Chen, X. Y., et al. (2020). The nutrients in Moringa oleifera leaf contribute to the improvement of stylo and alfalfa silage: Fermentation, nutrition and bacterial community. Bioresour. Technol. 231:122733. doi: 10.1016/j.biortech.2020.122733
Heaton, E. A., Dohleman, F. G., and Long, S. P. (2008). Meeting US biofuel goals with less land: The potential of Miscanthus. Global. Change. Biol. 14, 2000–2014. j.1365-2486.2008.01662.x
Hu, W., Schmidt, R. J., McDonell, E. E., Klingerman, C. M., and Kung, L. (2009). The effect of Lactobacillus buchneri 40788 or Lactobacillus plantarum MTD-1 on the fermentation and aerobic stability of corn silages ensiled at two dry matter contents. J. Dairy Sci. 92:390703914. doi: 10.3168/jds.2008-1788
Kirupa, S. M., Ravikumar, R., Kumar, N. M., and Sivakumar, U. (2018). Development of co-immobilized tri-enzyme biocatalytic system for one-pot pretreatment of four different perennial lignocellulosic biomass and evaluation of their bioethanol production potential. Bioresour. Technol. 269, 227–236. j. biortech.2018.08.091 doi: 10.1016/
Kõiv, V., Arbo, K., Maiväli, Ü, Kisand, V., Roosaare, M., Remm, M., et al. (2019). Endophytic bacterial communities in peels and pulp of five root vegetables. PLoS. One 14:e0210542. doi: 10.1371/journal.pone.0210542
Kung, L. Jr. (2008). “Silage fermentation end products and microbial populations: Their relationships to silage quality and animal productivity,” in Annual conference of the american association of bovine practitioners, (Charlotte, NC), 25–27. doi: 10.3389/fvets.2022.969321
Larsen, S. U., Arnbye-Jensen, M., Jorgensen, H., and Jorgensen, U. (2019). Ensiling of the pulp fraction after biorefining of grass into pulp and protein juice. Ind. Crop. Prod. 139:111576. doi: 10.1016/j.indcrop.2019.111576
Li, F., Ding, Z., Ke, W., Xu, D., Zhang, P., and Bai, J. (2019). Ferulic acid esterase- producing lactic acid bacteria and cellulase pretreatments of corn stalk silage at two different temperatures: Ensiling characteristics, carbohydrates composition and enzymatic saccharification. Bioresour. Technol. 282, 211–221. biortech.2019.03.022 doi: 10.1016/j
Li, J., Yuan, X., Dong, Z., Mugabe, W., and Shao, T. (2018). The effects of fibrolytic enzymes, cellulolytic fungi and bacteria on the fermentation characteristics, structural carbohydrates degradation, and enzymatic conversion yields of Pennisetum sinese silage. Bioresour. Technol. 264, 123–130. doi: 10.1016/j.biortech.2018.05.059
Li, P., Zhao, W. J., Yan, L. J., Chen, L. Y., Chen, Y. L., and Gou, W. L. (2021). Inclusion of abandoned rhubarb stalk enhanced anaerobic fermentation of alfalfa on the Qinghai Tibetan Plateau. Bioresour. Technol. 347:126347. 2021.126347
Li, P., Zhao, W. J., Yan, L. J., Chen, L. Y., Chen, Y. L., Gou, W. L., et al. (2019). Silage fermentation and bacterial community of bur clover, annual ryegrass and their mixtures prepared with microbial inoculant and chemical additive. Anim. Feed Sci. Technol. 247, 285–293. doi: 10.1016/j.anifeedsci.2018.11.009
Liu, B. Y., Huan, H. L., Gu, H. R., Xu, N. X., Shen, Q., and Ding, C. L. (2019). Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 273, 212–219. doi: 10.1016/j.biortech.2018.10.041
Méndez-García, C., Peláez, A. I., Mesa, V., Sánchez, J., Golyshina, O. V., and Ferrer, M. (2015). Microbial diversity and metabolic networks in acid mine drainage habitats. Front. Microbiol. 6:475. doi: 10.3389/fmicb.2015.00475
Mu, L., Xie, Z., Hu, L. X., Chen, G. H., and Zhang, Z. F. (2020). Cellulase interacts with Lactobacillus plantarum to affect chemical composition, bacterial communities, and aerobic stability in mixed silage of high-moisture amaranth and rice straw. Bioresour. Technol. 315:123772. doi: 10.1016/j.biortech.2020.123772
Murphy, R. P. (1958). A method for the extraction of plant samples and the determination of total soluble carbohydrates. J. Sci. Food. Agr. 9, 714–717. 27400 91104
Muthuvelu, K. S., Rajarathinam, R., Manickam, N. K., and Uthandi, S. (2018). Development of co-immobilized tri-enzyme biocatalytic system for one-pot pretreatment of four different perennial lignocellulosic biomass andevaluation of their bioethanol production potential. Bioresour. Technol. 269, 227–236. doi: 10.1016/j.biortech.2018.08.091
Ni, K., Wang, F., Zhu, B., Yang, J., Zhou, G., Pan, Y., et al. (2017). Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 238, 706–715. 2017.04.055
Ohmomo, S., Tanaka, O., Kitamoto, H. K., and Cai, Y. M. (2002). Silage and microbial performance, old story but new problems. JARQ Jpn. Agr. Res. Q. 36, 59–71. doi: 10.6090/jarq.36.59
Ren, H. W., Feng, Y. P., Pei, J. W., Li, J. P., Wang, Z. Y., Fu, S. F., et al. (2020). Effects of Lactobacillus plantarum additive and temperature on the ensiling quality and microbial community dynamics of cauliflower leaf silages. Bioresour. Technol. 307:123238. doi: 10.1016/j.biortech.2020.123238
Savoie, P., Tremblay, D., Tremblay, G. F., Wauthy, J. M., Flipot, P. M., and Theriault, R. (1992). Effect of length of cut on quality of stack silage and milk-production. Can. J. Anim. Sci. 72, 253–263. doi: 10.4141/cjas92-032
Shao, T., Ohba, N., Shimojo, M., and Masuda, Y. (2003). Changes in mono-and disaccharides compositions of guineagrass (Panicum maximum Jacq.) silage during early stages of ensiling. J. Fac. Agr. Kyushu U. 47, 333–339.
Sun, Z. Q., Jia, T. T., Gao, R., Xu, S. Y., Wu, Z., and Wang, B. (2020). Effects of chopping length and additive on the fermentation quality and aerobic stability in silage of Leymus chinensis. Processes 8:1283. doi: 10.3390/pr8101283
Thomson, A. L., Humphries, D. J., Kliem, K. E., Dittmann, M. T., and Reynolds, C. K. (2017). Effects of replacing maize silage with lucerne silage and lucerne silage chop length on rumen function and milk fatty acid composition. J. Dairy Sci. 100, 7127–7138. doi: 10.3168/jds.2017-12914
Van Soest, P. J., Robertsom, J. B., and Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy. Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Wan, J. C., Xie, K. Y., Wang, Y. X., Liu, L., Yu, Z., and Wang, B. (2021). Effects of wilting and additives on the ensiling quality and in vitro rumen fermentation characteristics of sudangrass silage. Anim. Biosci. 34, 56–65. doi: 10.5713/ajas.20.0079
Wang, C., He, L., Xing, Y., Zhou, W., Yang, F., Chen, X., et al. (2019). Fermentation quality and microbial community of alfalfa and stylo silage mixed with Moringa oleifera leaves. Bioresour. Technol. 284, 240–247. doi: 10.1016/j.biortech.2019.03.129
Wang, C., Pian, R. Q., Chen, X. Y., and Zhang, Q. (2021). Effects of polyphenol oxidases on proteolysis and lipolysis during ensiling of Moringa oleifera leaves with or without pyrocatechol. Anim. Feed. Sci. Tech. 275:114870. doi: 10.1016/j.anifeedsci.2021.114870
Wang, W. G., Li, R., Wang, H., Qi, B. F., Jiang, X. M., Zhu, Q. L., et al. (2019). Sweetcane (Erianthus arundinaceums) as a native bioenergy crop with environmental remediation potential in southern China: A review. GCB Bioenergy 11, 1012–1025. doi: 10.1111/gcbb.12600
Wang, Y., He, L. W., Xing, Y. Q., Zhou, W., Pian, R. Q., Yang, F. Y., et al. (2019). Bacterial diversity and fermentation quality of Moringa oleifera leaves silage preparedwith lactic acid bacteria inoculants and stored at different temperatures. Bioresour. Technol. 284, 349–358. doi: 10.1016/j.biortech.2019.03.139
Xu, D. M., Ding, Z. T., Bai, J., Ke, W. C., Zhang, Y. X., Li, F. H., et al. (2020). Evaluation of the effect of feruloyl esterase-producing Lactobacillus plantarum and cellulase pretreatments on lignocellulosic degradation and cellulose conversion of co-ensiled corn stalk and potato pulp. Bioresour. Technol. 310:123476. /j.biortech.2020.123476 doi: 10.1016
Xu, D., Ding, W., Ke, W., Li, F., Zhang, P., and Guo, X. (2018). Modulation of metabolome and bacterial community in whole crop corn silage by inoculating homofermentative Lactobacillus plantarum and heterofermentative Lactobacillus buchneri. Front. Microbiol. 9:3299. doi: 10.3389/fmicb.2018.0329
Yan, Y. H., Li, X. M., Guan, H., Huang, L. K., Ma, X., and Peng, Y. (2019). Microbial community and fermentation characteristic of Italian ryegrass silage prepared with corn stover and lactic acid bacteria. Bioresour. Technol. 279, 166–173. doi: 10.1016/j.biortech.2019.01.107
Yang, J. S., Tan, H. S., and Cai, Y. M. (2016). Characteristics of lactic acid bacteria isolates and their effect on silage fermentation of fruit residues. J. Dairy. Sci. 99, 5325–5334. doi: 10.3168/jds.2016-10952
Yang, L. L., Yuan, X. J., Li, J. F., Dong, Z. H., and Shao, T. (2019). Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour. Technol. 275, 280–287. doi: 10.1016/j.biortech.2018.12.067
Yu, P., Mckinnon, J. J., and Christensen, D. A. (2005). Hydroxycinnamic acids and ferulic acid esterase in relation to biodegradation of complex plant cell walls. Can. J. Anim. Sci. 85, 255–267. doi: 10.4141/A04-010
Yuan, X. J., Dong, Z., Li, J., and Shao, T. (2020). Microbial community dynamics and their contributions to organic acid production during the early stage of the ensiling of Napier grass (Pennisetum purpureum). Grass. Forage. Sci. 75, 37–44. 12455 doi: 10.1111/gfs
Zhang, Q., Guo, X., Zheng, M. Y., Chen, D. K., and Chen, X. Y. (2021). Altering microbial communities: A possible way of lactic acid bacteria inoculants changing smell of silage. Anim. Feed. Sci. Tech. 279:114998. doi: 10.1016/j.anifeedsci.2021.114998
Zhang, Q., Yu, Z., Yang, H., and Na, R. S. (2016). The effects of stage of growth and additives with or without cellulase on fermentation and in vitro degradation characteristics of Leymus chinensis silage. Grass. Forage. Sci. 71, 595–606. doi: 10.1111/gfs.12210
Zhang, X., Hu, B. X., Ren, H., and Zhang, J. (2018). Composition and functional diversity of microbial community across a mangrove-inhabited mudflat as revealed by 16S rDNA gene sequences. Sci. Total. Environ. 633, 518–528. 2018.03.158 doi: 10.1016/j.scitotenv
Zhang, Y., Zhang, H., Lee, D. J., Zhang, T., Jiang, D. P., Zhang, Z. P., et al. (2020). Effect of enzymolysis time on biohydrogen production from photofermentation by using various energy grasses as substrates. Bioresour. Technol. 305:123062. doi: 10.1016/j.biortech.2020.123062
Zhao, G. Q., Ju, Z. L., Chai, J. K., Jiao, T., Jia, Z. F., Casper, D. P., et al. (2018). Effects of silage additives and varieties on fermentation quality, aerobic stability, and nutritive value of oat silage. J. Anim. Sci. 96, 3151–3160. doi: 10.1093/jas/sky207
Zhao, X. L., Liu, J. H., Liu, J. J., Yang, F. Y., Zhu, W. B., Yuan, X. F., et al. (2017). Effect of ensiling and silage additives on biogas production and microbial community dynamics during anaerobic digestion of switchgrass. Bioresour. Technol. 241, 349–359. doi: 10.1016/j.biortech.2017.03.183
Keywords: silage, Saccharum arundinaceum, Lactobacillus plantarum, cellulase, cutting methods, bacterial community
Citation: Zheng Y, Li M, Xu J, Sun H, Cheng Q, Xie Y, Wang C, Chen C and Li P (2022) Effects of different cutting methods and additives on the fermentation quality and microbial community of Saccharum arundinaceum silage. Front. Microbiol. 13:999881. doi: 10.3389/fmicb.2022.999881
Received: 21 July 2022; Accepted: 30 August 2022;
Published: 23 September 2022.
Edited by:
Qing Zhang, South China Agricultural University, ChinaReviewed by:
Yimin Cai, Japan International Research Center for Agricultural Sciences (JIRCAS), JapanLinna Guo, Henan University of Technology, China
Copyright © 2022 Zheng, Li, Xu, Sun, Cheng, Xie, Wang, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Li, bHB5em1Ac2luYS5jbg==
 Yulong Zheng
Yulong Zheng Mengxin Li
Mengxin Li Jinyi Xu1
Jinyi Xu1 Qiming Cheng
Qiming Cheng Ping Li
Ping Li