- 1Department of Clinical Laboratory, The First Affiliated Hospital of Wenzhou Medical University and Key Laboratory of Clinical Laboratory Diagnosis and Translational Research of Zhejiang Province, Wenzhou, Zhejiang, China
- 2Department of Microbiology, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, China
- 3Department of Medical Lab Science, School of Laboratory Medicine and Life Science, Wenzhou Medical University, Wenzhou, Zhejiang, China
The rise in infections caused by the hypervirulent carbapenem-resistant Klebsiella pneumoniae (hv-CRKP) is an emergent threat to public health. We assessed the effects of chlorogenic acid (CA), a natural phenolic compound, on antibacterial, antivirulence, and anti-quorum sensing (QS) of hv-CRKP. Five hv-CRKP were selected for antimicrobial susceptibility test and confirmed to carry virulence genes and carbapenem-resistant genes by polymerase chain reaction (PCR). Subsequently, a series of time-kill assay, determinations of protease activity and capsule content, biofilm-related experiment, scanning electron microscopy (SEM) and transmission electron microscope (TEM) observation, G. mellonella infection model, quantitative real-time PCR (qRT-PCR) of QS-related genes and biofilm formation genes, as well as AI-2 binding test were conduct to verify the effect of CA on hv-CRKP. Five CRKP strains showed varying degrees of resistance to antibacterial agents. All strains carried the blaKPC–2 gene, primarily carrying rmpA2, iucA, and peg-344. CA showed no effect on CRKP growth at the 1/2 minimum inhibitory concentration (MIC), 1/4 MIC, and 1/8 MIC, CA could reduce the production of extracellular protease and capsular polysaccharides, and improve the survival rate of larvae in Galleria mellonella (G. mellonella) infection model. By means of crystal violet staining and scanning electron microscopy experiments, we observed that CA can inhibit the formation of CRKP biofilm. On the quantitative real-time PCR analysis, the expression of the luxS, mrkA and wbbm genes in most CRKP strains appeared downregulated because of the CA treatment. Besides, CA significantly inhibited the effect of AI-2 activity of BB170. Our study suggests that CA can be an effective antimicrobial, antivirulent compound that can target QS in hv-CRKP infections, thus providing a new therapeutic direction for treating bacterial infections.
1 Introduction
Klebsiella pneumoniae (K. pneumoniae), an important opportunistic Gram-negative bacteria and one of the most common pathogenic bacteria in hospitals (Ballen et al., 2021), is responsible for the frequent incidence of a serious hospital- and community-acquired infections, including urinary tract infections, lung infections, and bloodstream infections (M Campos et al., 2020; Hu et al., 2021). Studies have shown that the clinical detection rate of K. pneumoniae is second only to Escherichia coli, exhibiting a year-on-year increase (Yang F. et al., 2019). In recent years, the resistance of K. pneumoniae to antibacterial drugs has been increasing, therefore, carbapenems, because of their broad antibacterial spectrum and strong antibacterial activity, are being increasingly used to treat serious bacterial infections (Doi, 2019). However, the wide use and abuse of carbapenems in clinical practice is giving rise to carbapenem-resistant K. pneumoniae (CRKP), even causing nosocomial infection outbreaks, posing considerable challenges to clinical treatment (Candan and Aksoz, 2015; Xu et al., 2017; Tang et al., 2020; Zhao et al., 2020).
According to virulence characteristics, K. pneumoniae can be classified as classic K. pneumoniae (cKp) and hypervirulent K. pneumoniae (hvKp) (Bialek-Davenet et al., 2014). The hvKp strain carries plasmids pLVPK-encoding virulence genes such as rmpA and rmpA2, and the highly transmissible hvKp infections often occur in multiple (Russo and Marr, 2019), responsible for a variety of life-threatening infections, such as liver abscess, endomyelitis, and meningitis (Cubero et al., 2016; Wang et al., 2020). To date, because of selective pressure of antibiotics, multidrug resistance isolates and hvKp have been detected in clinical practice, in particular, the emergence of hypervirulent CRKP (hv-CRKP), which is highly challenging to anti-infection treatment protocols (Tang et al., 2020). Therefore, new treatment strategies to effectively inhibit hv-CRKP is emergently required.
Quorum sensing (QS) is a mechanism of communication between bacteria that depends on the bacterial population density; QS aids regulation of bacterial biological functions, such as, secretion of pathogenic extracellular protease and toxins, biofilm formation, and resistance development to various antibacterial drugs (Pena et al., 2019; Wu et al., 2020). K. pneumoniae primarily use LuxS/AI-2 as the self-induction factor of its QS system, and the AI-2 QS system of K. pneumoniae has a regulatory effect on biofilm formation and lipopolysaccharide synthesis (De Araujo et al., 2010; Papenfort and Bassler, 2016). Thus, inhibiting QS will potentially help in fighting the pathogenic bacteria.
Quorum sensing inhibitors (QSIs) by interfering with the QS system can reduce virulence factors and pathogenic effects of the bacteria without affecting its growth of bacteria, thus effectively inhibiting infections caused by the pathogen (Kalia et al., 2019). In addition, QSIs can act as synergists in delaying antimicrobial resistance development. The combination of QSIs and antibacterial drugs can restore bacterial sensitivity to previously tolerated drugs, thus helping reduce the effective dose and improve the efficiency of antibacterial drugs (Ham et al., 2021). Natural QSIs mainly exist in bacteria, fungi, animals, and plants, and marine organisms (Kalia, 2013). Chlorogenic acid (CA), a polyphenolic compound richly found in fruits and vegetables, has antitumor, anti-inflammatory, and antiviral activities (Santana-Galvez et al., 2017). CA can inhibit the biofilm formation, virulence factor, and QS system of Pseudomonas aeruginosa (Xu et al., 2022). However, relatively few studies on the antibacterial and antivirulence sensing of CRKP. The emergence of hv-CRKP has created an urgency to develop a new strategy to deal with related infections.
Therefore, we aimed to explore the effects of CA on hv-CRKP and its potential antivirulence mechanism.
2 Materials and methods
2.1 Bacterial strains and antimicrobial susceptibility testing
A total of 5 strains were selected from 20 CRKP strains isolated from the Wenzhou Medical University from January to May 2020. These isolates were identified by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS; bioMérieux, Lyons, France). In addition, duplicate strains isolated from the same part of the same patient were removed during strains collection. The minimum inhibitory concentrations (MICs) of meropenem, imipenem, ertapenem, ampicillin, ceftriaxone, ceftazidime, cefepime, ciprofloxacin, levofloxacin, gentamycin, tobramycin, colistin, and CA were determined by the microdilution broth method with cation-adjusted Mueller–Hinton broth as per the guidelines of the Clinical and Laboratory Standards Institute (CLSI, 2021). In addition, CA (purity ≥ 98%, Beijing Soleibao Co., Ltd., Beijing, China) was dissolved in dimethyl sulphoxide (DMSO). ATCC 27853 and ATCC 25922 were used for quality control.
2.2 Detection of virulence and resistance genes of CRKP
The DNA of CRKP isolates were extracted using the BioFlux Bacterial DNA Extraction Kit (Bioflux, Tokyo, Japan). The virulence genes (rmpA, rmpA2, iucA, iroB, and peg-344) and carbapenem-resistant genes (blaKPC–2, blaNDM, blaVIM, and blaOXA–232) were amplified by the polymerase chain reaction (PCR) with specific primers (Supplementary Table 1). The amplified PCR products were sequenced by Shanghai Majorbio Bio-Pharm Technology Co. (Shanghai, China). Further sequence alignment was performed to analyze any possible gene mutations by BLAST on the National Center for Biotechnology Information (NCBI).1
2.3 Time-kill assay
The time-kill assay was performed to evaluate the effect of CA on the growth of CRKP, as described previously with some minor modifications (Lu et al., 2013). Briefly, the five CRKP strains were inoculated into 20 mL of cation-adjusted Mueller-Hinton broth (CAMHB) containing different concentrations of CA (1/2, 1/4, and 1/8 MIC). Tubes containing the LB medium alone served as the negative control. The bacterial suspensions were incubated at 37°C with moderate shaking for 2, 4, 6, 12, and 24 h. According to the growth rate of bacteria, appropriate dilution concentration was prepared. At the corresponding time points, 100 μL of the bacterial suspension was spotted on the Mueller-Hinton (MH) agar plates, and the colony forming unit (CFU) was counted after incubating the plate overnight at 37°C. All studies were conducted in duplicate.
2.4 Protease activity assay
We measured the diameter of the precipitation ring on Luria-Bertani (LB) skim-milk agar plate at 37°C for 18–36 h to test the protease activity semi-quantitatively. The diameter of the precipitation ring reflected the protease activity (Phrommao et al., 2011).
We also quantitatively measured the protease activity of bacterial supernatants treated with different concentrations of CA (1/2 and 1/4 MIC) by a modified azocasein assay (Nicodeme et al., 2005). Briefly, 1 mL of culture supernatant was combined with 1 ml of 10 mM Tris–Cl buffer (pH 7.5), 3 mg/mL of azocasein, and 0.5 mM CaCl2 for the protease test. With mild shaking, the reaction mixture was incubated at 37°C for 1 h. The reaction was terminated with the addition of 0.4 mL of 10% trichloroacetic acid. Then, the mixture was centrifuged at 10,000 × g for 10 min, and the absorbance of the supernatant was measured at 420 nm. By dividing the A420 values by the A600 (cell density) of the various bacterial cultures, the specific proteolytic activity unit (U) was computed.
2.5 Quantification of capsule assay
Capsules were quantified as described previously, albeit with some modifications (Yang X. et al., 2019). Briefly, 500 μL of the cultured bacterial suspensions were resuspended and adjusted to 106 CFU/mL, and 1.2 mL sodium tetraborate in sulfuric acid was added to the resuspensions placed on an ice bath and incubated for 5 min at 100°C, and then left on the ice for 10 min. A 20-μL volume of 1.5 mg/mL m-hydroxydiphenyl was then added to its mixture and mixed well. After a 5-min incubation at room temperature, the absorbance at 590 nm was measured. The glucuronic acid content was determined with reference to a standard curve of glucuronic acid and expressed as μg/108 CFU. The results were presented as the mean of data of three independent experiments.
2.6 Transmission electron microscopy (TEM)
In order to better understand the changes in the capsule of CRKP when treated with different concentrations of CA, a transmission electron microscopy (stocktickerTEM; Hitachi HT7700, Germany) examination was performed, using the experimental method as previously described with some modifications (Wang et al., 2022). In short, fresh bacterial cells of K. pneumoniae FK 8123 with different treatments of CA (none, 1/2 MIC, and 1/4 MIC) were tested with stocktickerTEM. 10 μL of bacterial solution was applied to copper mesh and allowed to connect for around 10 min after fresh bacterial cells had been rinsed with phosphate-buffered saline (PBS) once. Filter paper was used to absorb any extra bacterial fluid before samples were dyed for 1 min with 1% uranyl acetate dihydrate applied to copper mesh.
2.7 Evaluation of the antivirulence of CA in G. mellonella infection model in vivo
We detected the efficacy of CA in Galleria mellonella infection in vivo through a survival assay, as described previously with some modifications (Brackman et al., 2011). FK 8036 was selected for the experiment. Healthy larvae weighing at least 250 mg and free of any gray markings were selected at random for the experiments. Then, 10 G. mellonellas were randomly selected from each group. Overnight cultures of CRKP strain were washed with PBS and further adjusted with PBS to a concentration of 1 × 104 CFU/mL. The insects infected with PBS were used as control. We considered an average haemolymph volume of 50 μL and the increase in the volume contributed to bacterial and antibiotic injections (each 10 μL). For example, 10 μL of an antibiotic solution with 7-time greater concentration was injected into the larvae. Then, 10 μL of the bacterial solution was injected into the rear left proleg of G. mellonella by using a microinjector, followed by the test of the CA (1/2 and 1/4 MIC) of 7-time within 2 h of infection. Then G. mellonellas at 37°C and recorded their survival rate after 24, 48, 72, 96, 120, 144, and 168 h. All experiments were conducted in triplicate. Death was considered when the larvae repeatedly failed to respond to a physical stimuli. Kaplan–Meier analysis and log-rank test were performed to analyze the survival rate of G. mellonella larvae.
2.8 Biofilm-formation inhibition assay
Biofilm-formation assays were performed in 96-well polystyrene microtiter plates, as previously described (Yang X. et al., 2019) with some modifications. A single colony on the blood plate was shaken overnight in 3 mL of fresh LB broth medium at 37°C. Then, the culture was adjusted to 0.5 McFarland with sterile normal saline and further diluted by 1:100 in LB broth and dispensed in a 96-well microtiter plate with 1/2, 1/4, and 1/8 MIC CA. The 96-well plates were incubated at 37°C for 24 h. Then, the cell suspension was removed and the plates were washed twice with 1 × PBS (Sigma-Aldrich, Milan, Italy) and inverted to dry at the room temperature. Next, 200 μL of 1% crystal violet (CV) solution (Beijing Solarbio Biotechnology Co., Ltd., China) was added to the wells for 15 min. After staining, CV was removed and the wells were washed thrice with 1 × PBS. After the plate dried naturally at room temperature, the bound CV was solubilized by adding 200 μL of ethanol–acetone (95:5 v/v) solution. The absorbance of CV was read at 595 nm on a microplate reader (Multiskan FC). All tests were performed in triplicate, with at least three independent experiments.
2.9 Mature biofilm eradication assay
We further detected the eradication effect of CA on mature biofilms, as suggested elsewhere (Yu et al., 2020), albeit with some modifications. Briefly, the 5 CRKP overnight cultures in 3 mL of fresh LB broth medium were adjusted to 0.5 McFarland with sterile normal saline, followed by further dilution to 1:100 in LB broth medium and dispensing in a 96-well microtiter plate. After 24 h of static incubation at 37°C (the formation of mature biofilms), the supernatant was discarded and the plates were washed thrice with 0.9% saline to remove the unattached cells. Next, fresh LB broth containing 2, 4, and 8 MIC CA was, respectively, added to each well (200 μL/well). The LB broth medium without antimicrobials was served as control, and the 96-well plates were incubated for 24 h at 37°C. Subsequent staining and treatment were performed as described previously. Each assay was performed in triplicate.
2.10 Scanning electron microscopy (SEM)
To evaluate the morphological changes of the bacterial biofilm after different treatments (Hemmati et al., 2020), FK 8036 was randomly selected for SEM. The overnight culture in LB medium incubated at 37°C was adjusted to 0.5 McFarland with sterile normal saline, and sterile coverslips were placed in each well of a 6-well plate to which 100 μL of diluted culture plus 1,900 μL CA (1/2 and 1/4 MIC) were added. Blank LB broth served as the negative control group. Biofilms were grown on coverslips at 37°C for 18–24 h. After which, the bacterial culture was removed and washed with 1 × PBS. The biofilm samples were fixed with 2.5% glutaraldehyde fixation solution in a fresh 6-well plate and incubated at 4°C for 4 h, after which the samples were subjected to a gradient series of ethanol (30, 50, 70, 90, and 100% v/v) for 10 min. All samples were then dried for 2 h and observed by SEM (S-3000N, Japan).
2.11 Quantitative real-time PCR (qRT-PCR)
The quantification of the expression of rmpA2, iucA, luxS, mrkA, wzm, wbbm, and treC in 5 CRKP was performed by quantitative real-time PCR (qRT-PCR), while 16SrRNA served as the standardized reference gene for CRKP. Briefly, the strain was cultured in the LB broth for 16–18 h, to which CA (1/2 MIC) was added and the mixture was incubated for 6 h. The total RNA from the cultured strain was extracted by using a commercial RNA extraction kit (Tiangen Biotech, Beijing, China). The gene expression was quantified by the 2–ΔΔCt method. The experiments for each gene were performed in triplicate. The primer sequences were listed in the Supplementary Table 2.
2.12 AI-2 binding assays in vitro
The steps for detecting the activity of culture supernatant can refer to reference and be modified as appropriate (Panayi et al., 2022). The steps are as follows: Vibrio harveyi (V. harveyi) BB170 is inoculated in autoinducer bioassay (AB) culture medium and cultured overnight at 30°C. Before detection, the overnight culture solution of V. harveyi BB170 was diluted with fresh AB medium at a ratio of 1:5,000, and shaken well. A total of 900 μL diluted V. harveyi BB170 cell were mixed with 100 μL bacterial supernatant treated with different concentrations of CA (1/2, 1/4, and 1/8 MIC). At the same time, V. harveyi and the supernatant of AB culture medium were used as positive and negative controls. Continue shaking culture at 30°C, then take 200 μL/well to the black 96-well flat base plate every 1 h for 5 h, and use the multifunctional microplate reader (BioTek) to detect its bioluminescence intensity. When the luminous intensity of the negative control reaches the minimum, the luminous value of the positive control is set to 100%. The data result is expressed by relative activity.
2.13 Statistical analysis
The statistical significance was evaluated by using one-way analysis of variance (ANOVA) with post hoc analysis using Tukey’s test to evaluate significant differences between groups. Student’s t-test and log-rank test were used to determine the statistical significance of gene expression and survival rate, respectively. For all analyses: *P < 0.05, **P < 0.01, ***P < 0.001, and ns P > 0.05.
3 Results
3.1 Microbiological characteristics of CRKP isolates
We identified the biochemical characteristics of 5 CRKP strains (FK 7887, FK 7917, FK 8002, FK 8036, and FK 81230) and found that their oxidase, indole, methyl red, hydrogen sulfide (H2S) production, and motility test were negative, while Voges–Proskauer and Simmons citric acid test were positive, which accorded with the biochemical characteristics of K. pneumoniae. Five CRKP strains in our study did not have hypermucoviscous phenotype.
3.2 Antimicrobial susceptibility testing
We used CA and common clinical antibiotics to determine the MICs of five CRKP strains. For all the five CRKP strains, the MIC of CA was 10,240 μg/mL (Table 1); for all five CRKP strains exhibited different degrees of resistance to clinical antibiotics.
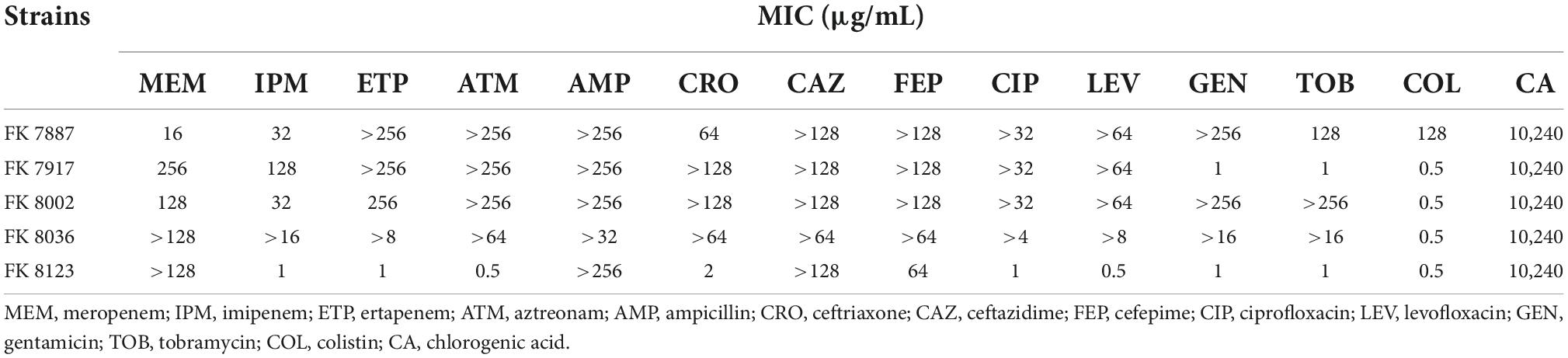
Table 1. Minimum inhibitory concentration (MIC) value of carbapenem-resistant Klebsiella pneumoniae against clinical antibiotics and chlorogenic acid (CA).
3.3 Virulence and drug resistance genes
The strains that carried their respective virulence genes are as follows: FK 7887 carried rmpA, iucA, iroB, and peg-344; FK 7917, FK 8002, and FK 8036 strains carried rmpA, rmpA2, iucA, and peg-344; and FK 8123 strain carried virulence genes rmpA2, iucA, iroB, and Peg-344; and all five CRKP strains only carried carbapenem-resistant gene blaKPC–2 (Figure 1).
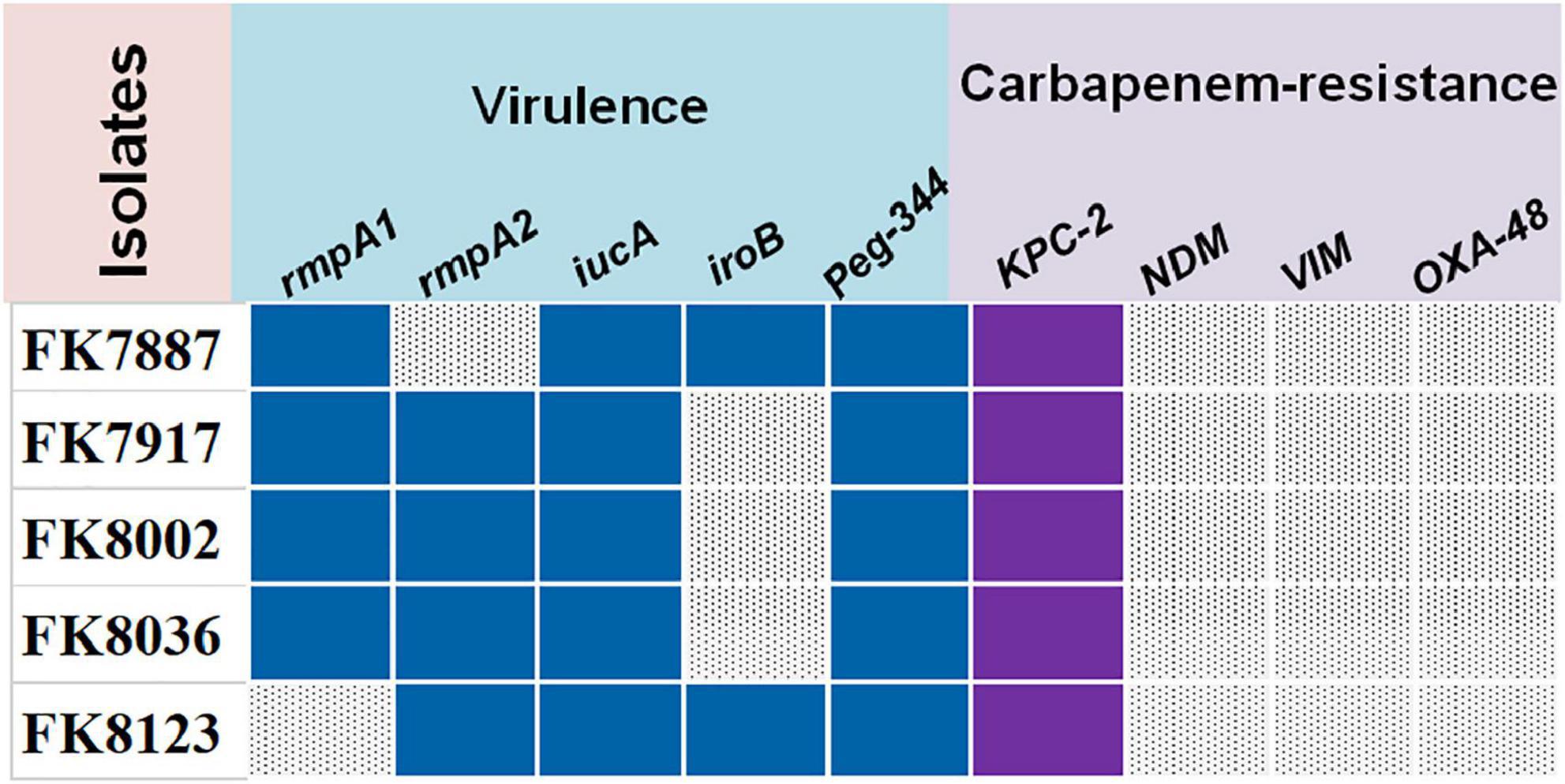
Figure 1. The virulence and carbapenem-resistant genes of five carbapenem-resistant Klebsiella pneumoniae (CRKP) strains. Blue color represents virulence genes, purple color represents carbapenem-resistant genes, and gray color represents don’t carry the corresponding gene.
3.4 Time-kill analysis
1/2, 1/4, and 1/8 MIC CA did not affect bacterial growth (Figure 2), suggesting that CA has no effect on the growth of CRKP at sub-MICs (P > 0.05).
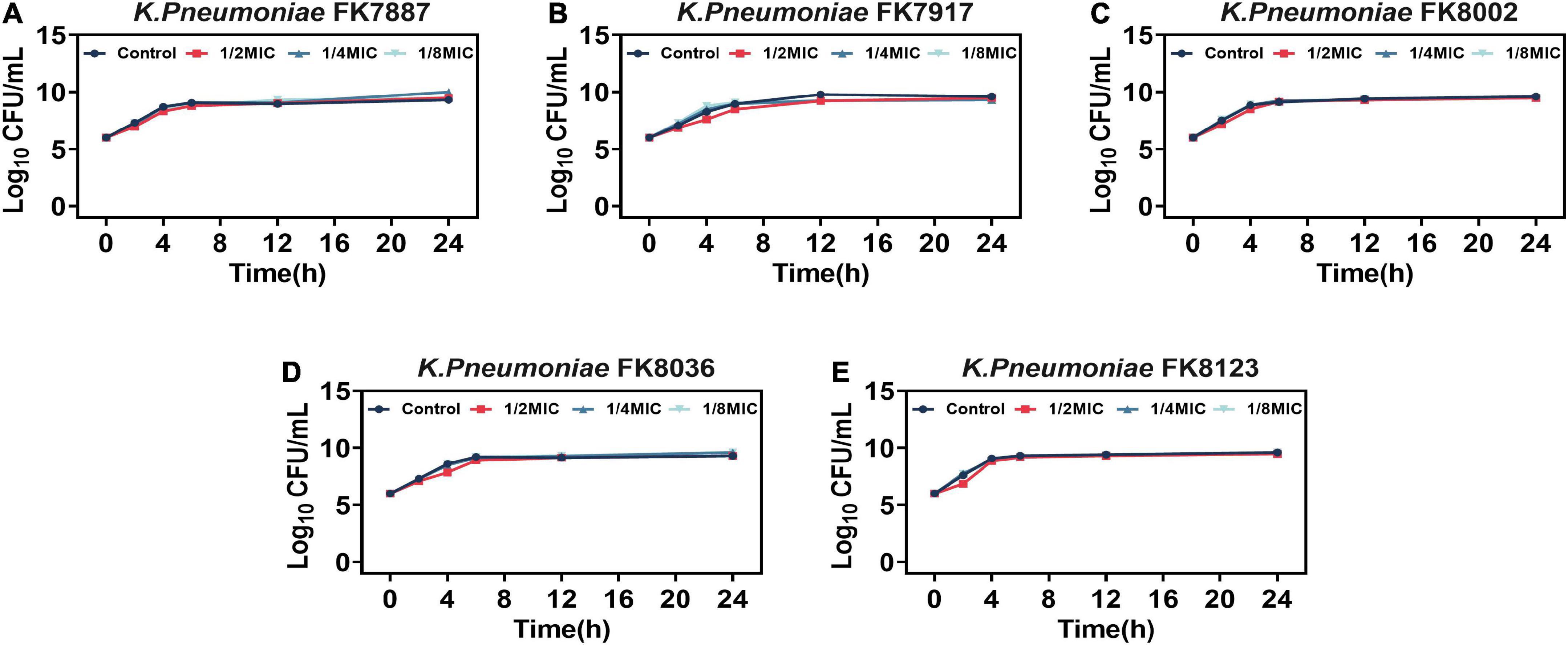
Figure 2. (A-E) Effects of different concentrations of chlorogenic acid (CA) on the growth of five carbapenem-resistant Klebsiella pneumoniae (CRKP) strains. Different color represents different concentrations of CA. The dark blue represents the control group, the red represents 1/2 MIC, the light blue represents 1/4 MIC, and the green represents 1/8 MIC.
3.5 Inhibition of protease production
Klebsiella pneumoniae–secreted protease has the ability to destroy host tissue proteins, and is an important virulence factor for the pathogenicity of this organism. The protease secretion detection of the five CRKP strain showed that only the FK 8123 strain produced protease. Therefore, the FK 8123 strain was used to assess the effect of CA on the strain’s protease production capacity, results are shown in Figure 3. When CA was not used, the diameter of the colony growth was larger and the hydrolysis ring was obvious, indicating the ability of the FK 8123 strain to produce protease. After the addition of 1/2 and 1/4 MIC CA, the hydrolysis ring of the strain became significantly smaller (P < 0.05), indicating that CA had a certain inhibitory effect on protease production. Besides, we assessed the ability of the FK 8123 supernatant to degrade casein with control group, 1/2 and 1/4 MIC CA treatment. With an increase in CA concentration under test conditions, the ability of the FK 8123 supernatant to degrade casein decreased (P < 0.05), further indicating that CA had a certain inhibitory effect on protease production.
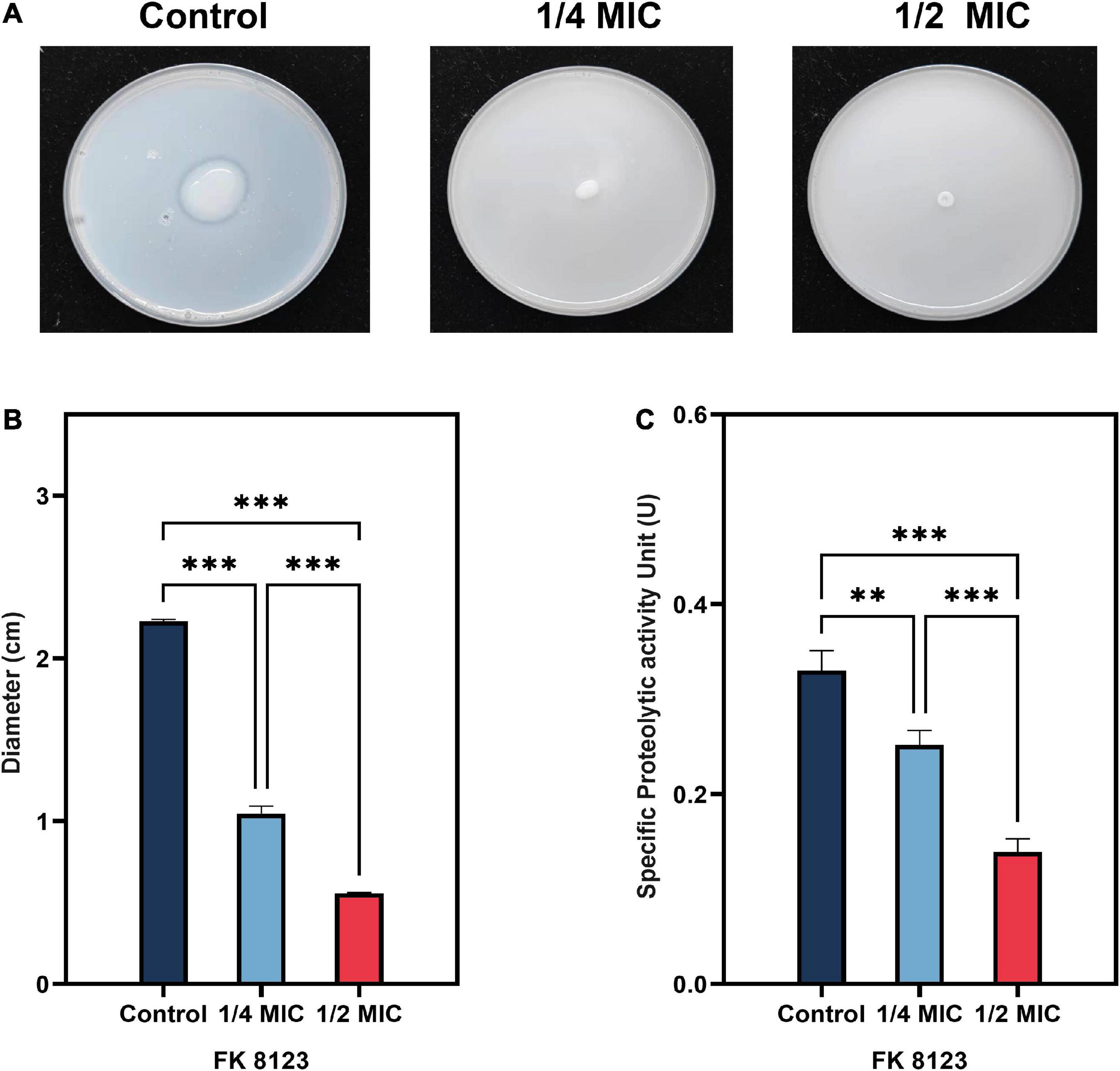
Figure 3. Protease inhibition assay. (A) Milk agar containing a series of concentrations of chlorogenic acid (CA) [1/2 and 1/4 minimum inhibitory concentration (MIC)] supplemented with carbapenem-resistant Klebsiella pneumoniae (CRKP); (B) Average diameter of five CRKP isolates; (C) Specific protease activity unit. The “**” means P < 0.01, “***” means P < 0.001.
3.6 Inhibition of capsular polysaccharide
The presence of capsular polysaccharide in K. pneumoniae contributes to its high-viscosity and high-virulence phenotype. We assessed the effect of CA on the capsular polysaccharide production of the five CRKP strains. CA had a significant inhibitory effect on the capsular polysaccharide production of the FK 7917, FK 8036, and FK 8123 strains (P < 0.05) (Figure 4). We conducted the TEM experiment to better comprehend the changes in the capsule of CRKP treated with CA. Under the treatment of CA (1/2 or 1/4 MIC), the capsule became thinner, further demonstrating the inhibitory effect of CA on the capsular (Figure 5).
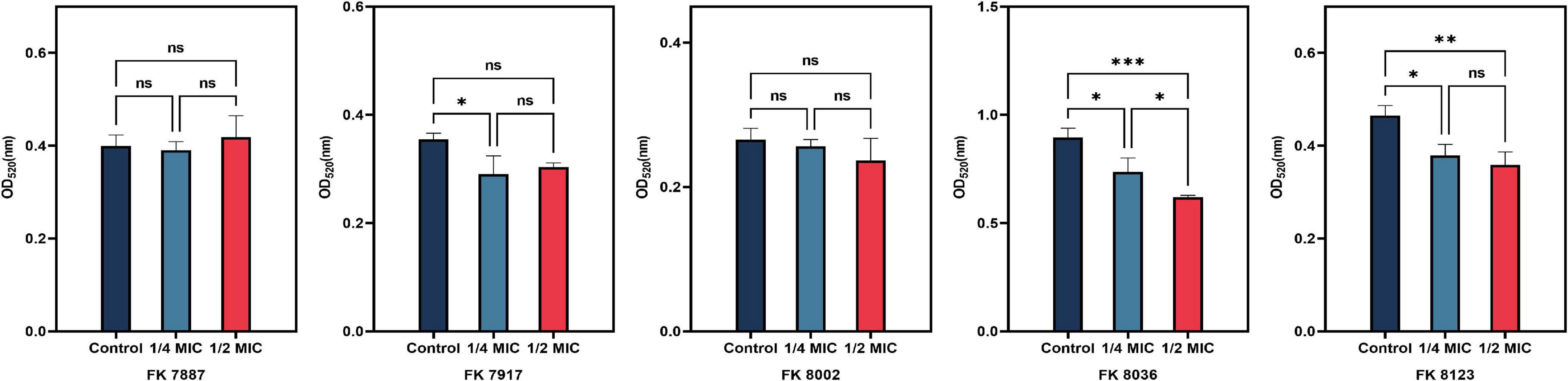
Figure 4. Capsular polysaccharide inhibition assay. Different color represents different concentrations of chlorogenic acid (CA) [1/2 and 1/4 minimum inhibitory concentration (MIC)] supplemented with carbapenem-resistant Klebsiella pneumoniae (CRKP). “*” means P < 0.05, “**” means P < 0.01, “***” means P < 0.001, and “ns” means P > 0.05.
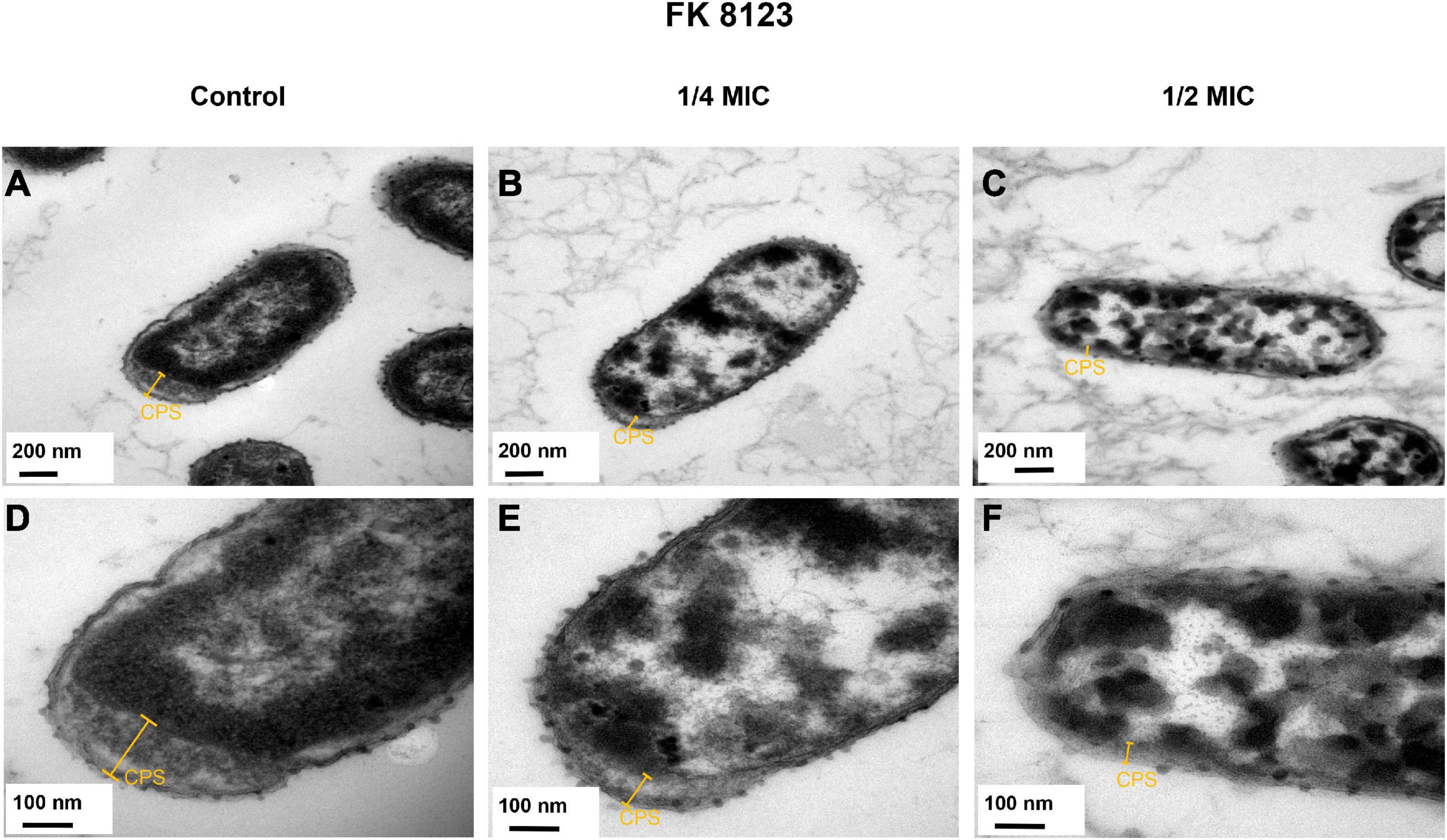
Figure 5. Transmission electron microscopy (TEM) images of the effects of chlorogenic acid (CA) treatment on the capsular of FK 8123. (A) LB broth control group, 30,000×; (B) 1/4 minimum inhibitory concentration (MIC) CA monotherapy, 30,000×; (C) 1/2 MIC CA monotherapy, 30,000×; (D) LB broth control group, 80,000×; (E) 1/4 MIC CA monotherapy, 80,000×; (F) 1/2 MIC CA monotherapy, 80,000×.
3.7 Virulence assays in vivo by G. mellonella larvae infection model
The number of surviving G. mellonella larvae in the FK 8036 + PBS control group gradually decreased within 7 days after infection with K. pneumoniae (Figure 6). Compared with the survival rate of the control group, the survival rate of G. mellonella larvae treated with 1/2 MIC CA was significantly improved (P < 0.05).
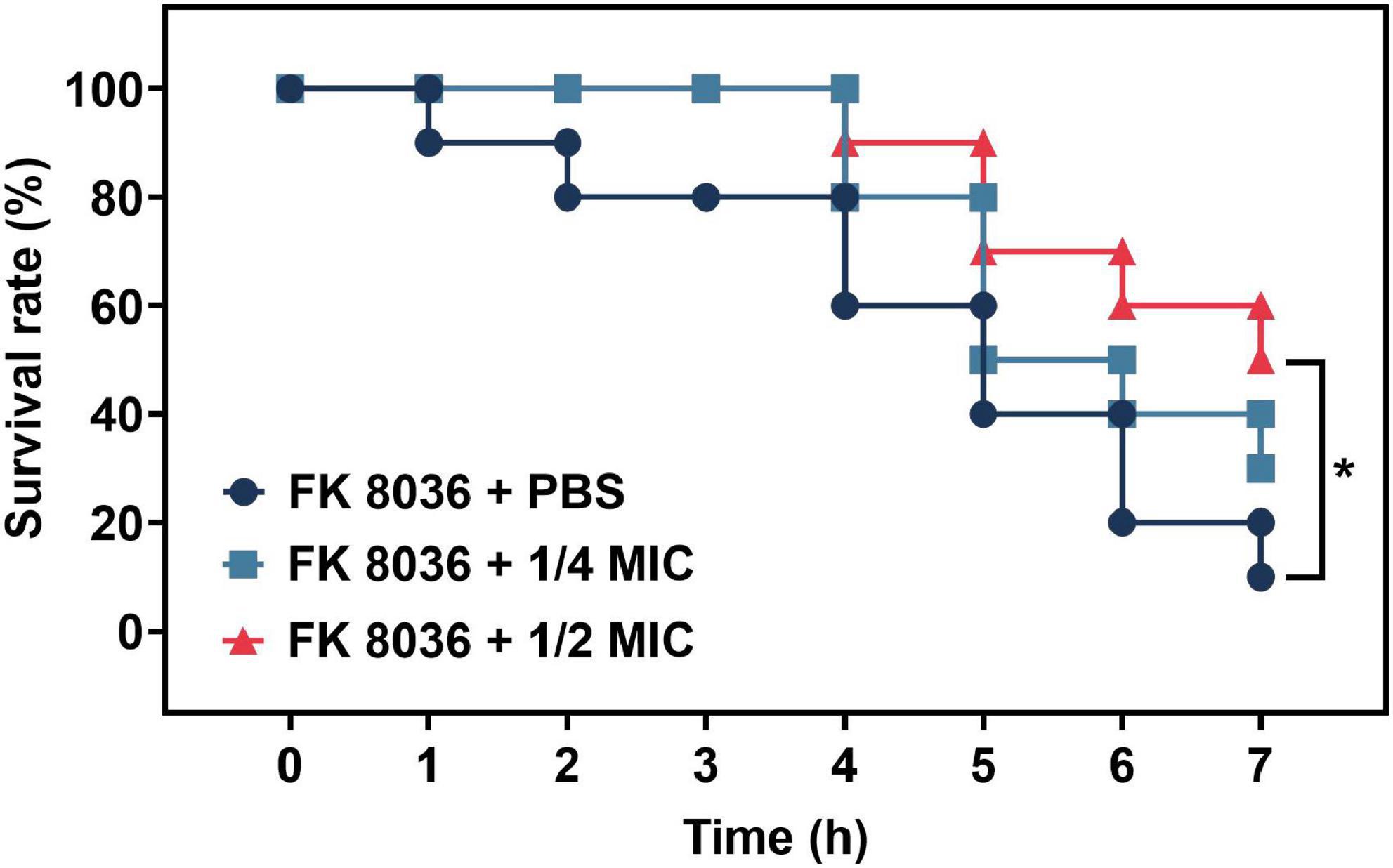
Figure 6. Survival rate of Galleria mellonella after 7 days of treatment with different concentrations of chlorogenic acid (CA) [1/2 and 1/4 minimum inhibitory concentration (MIC)] against FK 8036. Kaplan–Meier analysis and log-rank test were performed to analyze the survival rate of G. mellonella larvae.
3.8 Inhibition of biofilm formation
All the five CRKP strains had the weakest biofilm formation ability in the presence of 1/2 MIC CA (Figure 7). The inhibitory ability of CRKP strains was significantly different (P < 0.05), except that of the FK 8002 strain. This insignificant effect on the FK 8002 strain after treatment could be because of this strain’s weak biofilm forming ability.
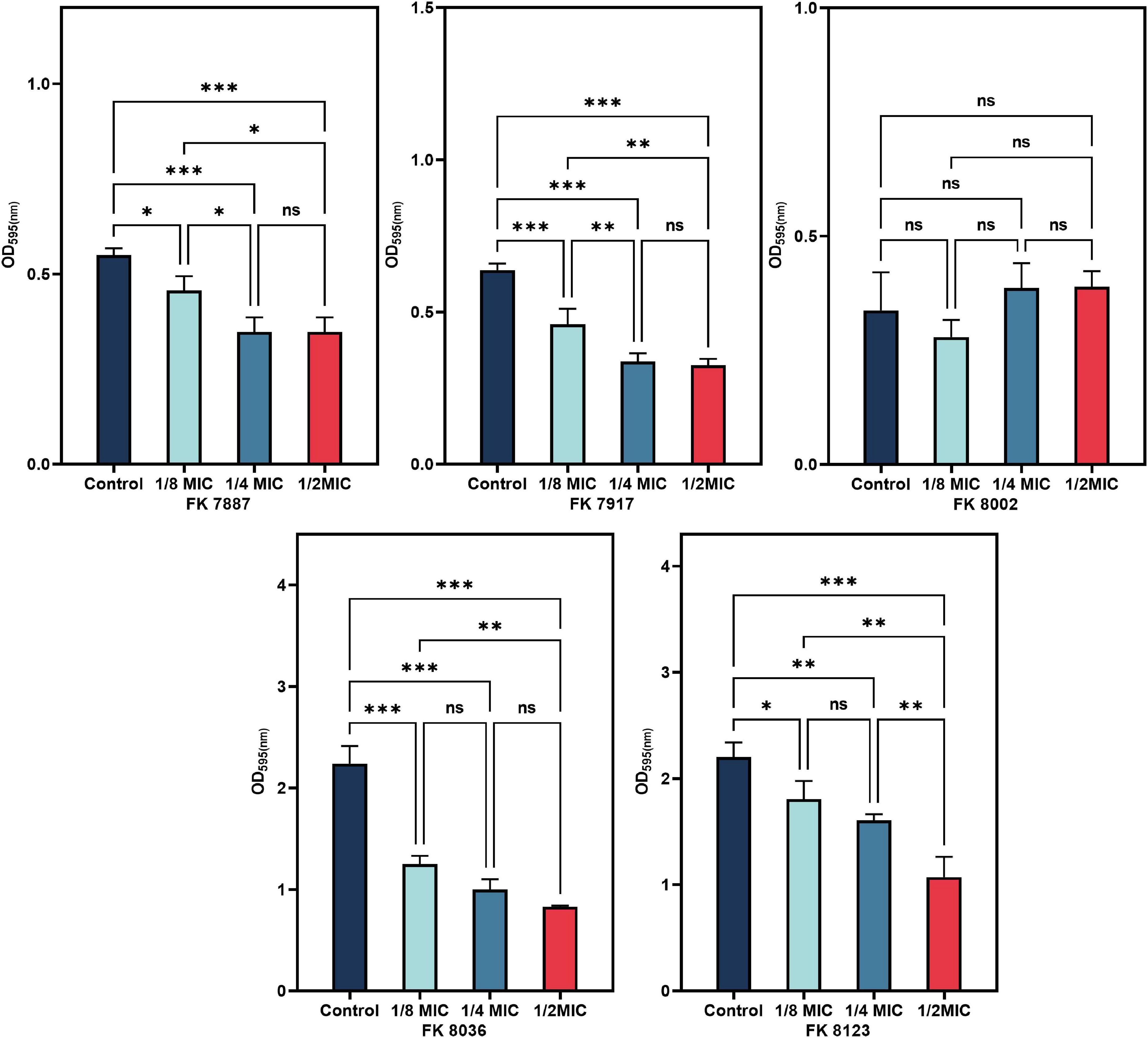
Figure 7. The inhibitory effect of biofilm formation of five carbapenem-resistant Klebsiella pneumoniae (CRKP) treated with chlorogenic acid (CA) at 1/2, 1/4, and 1/8 minimum inhibitory concentration (MIC). “*” Means P < 0.05, “**” means P < 0.01, “***” means P < 0.001, and “ns” means P > 0.05.
3.9 Mature biofilm eradication assays
A bacterial biofilm is difficult to eradicate because of the persistent presence of bacteria. Therefore, to assessed the ability of CA to remove the biofilm formed by K. pneumoniae, we selected different concentrations of CA for experiments; and the results were shown in Figure 8. The ability of CA to scavenge the biofilms formed by K. pneumoniae was not significant, except that of FK 7917 at 4 and 8 MIC and that of FK 8002 at 8 MIC (P < 0.05). The ability of each CA concentration measured to remove biofilms of other strains was not significant.
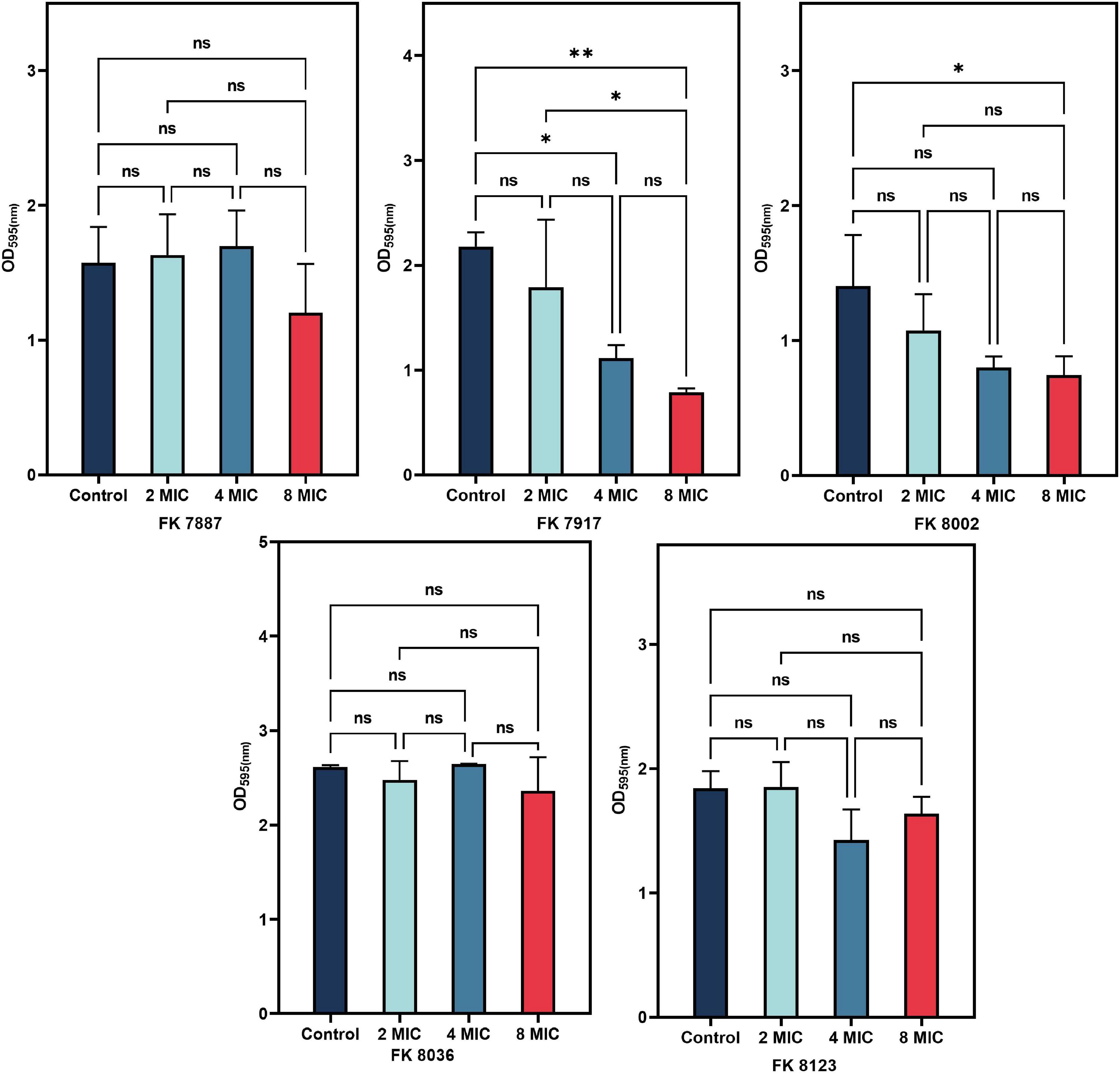
Figure 8. Radiation effect of chlorogenic acid (CA) on five carbapenem-resistant Klebsiella pneumoniae (CRKP) mature biofilm at 1/2, 1/4, and 1/8 minimum inhibitory concentration (MIC). “*” Means P < 0.05, “**” means P < 0.01, and “ns” means P > 0.05.
3.10 Biofilm morphology
We used SEM to assess the biofilm morphology and distribution of the FK 8036 strain after CA treatment; the results were shown in Figure 9. The form and quantity of the FK 8036 strain-formed biofilm were different before and after CA treatment. In the strain treated with the LB broth, the number of colonies, and their network structure were large and the bacterial morphology appeared complete. Whereas, in the strains treated with different concentrations of CA, the formed biofilm was weak, the network structure of colony growth appeared damaged, the strains were scattered, and the bacteria were aggregated in small parts.
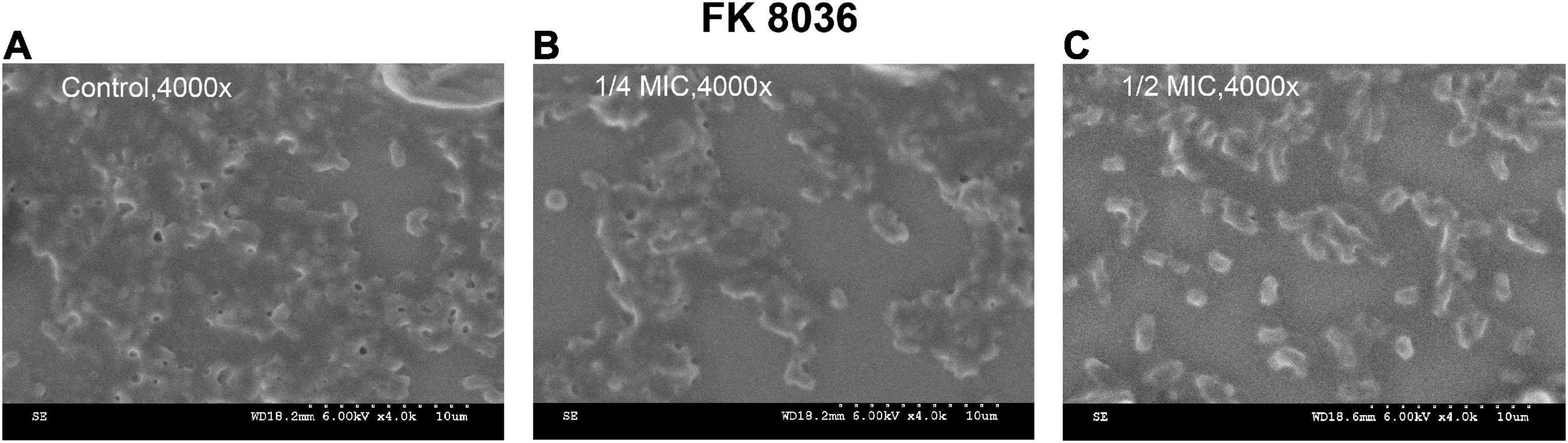
Figure 9. Scanning electron microscopy (SEM) images of the effects of chlorogenic acid (CA) treatment on the bacterial number and biofilm formation of FK 8036. (A) LB broth control group, 4,000×; (B) 1/4 minimum inhibitory concentration (MIC) CA monotherapy, 4,000×; (C) 1/2 MIC CA monotherapy, 4,000×.
3.11 CA regulated the expression of QS-related genes and biofilm formation genes
We used qRT-PCR to further verify the inhibitory of CA on the virulence genes, QS-related genes and biofilm formation genes of CRKP isolated from clinics. The effects of CA on gene expression helped elucidate its regulatory effect on strain virulence, biofilm formation, and QS at the transcriptional level. As shown in Figures 10, 11, CA had different inhibitory effects on different genes in the five CRKP strains, with 1/2 MIC CA treatment, the expression levels of the luxS genes and biofilm formation regulators mrkA and wbbm were downregulated in five CRKP strains (P < 0.05).
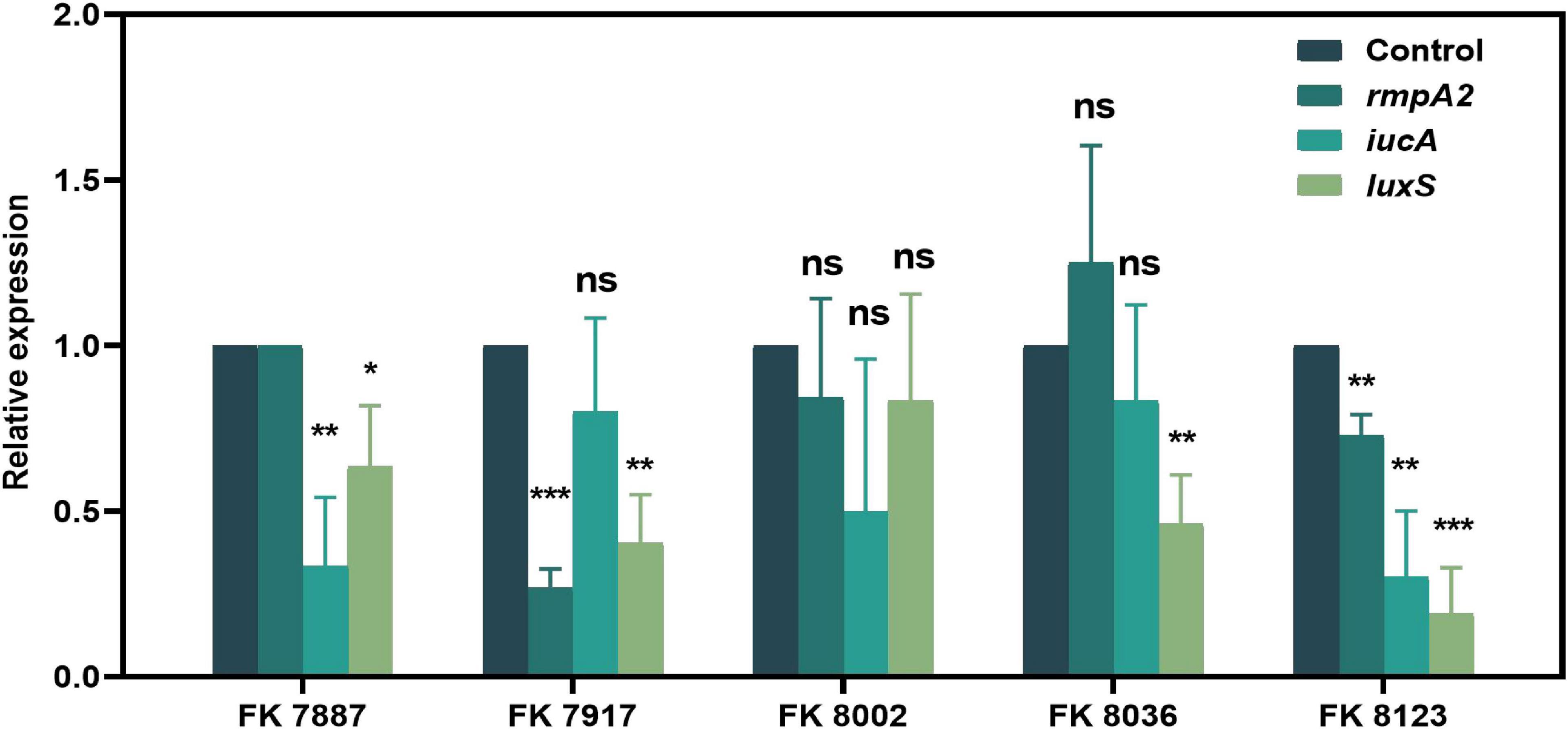
Figure 10. The expression of quorum sensing (QS)-related genes of five carbapenem-resistant Klebsiella pneumoniae (CRKP) strains in different treatment groups. The strain FK 7887 did not carry the rmpA2 gene, hence this result will not be discussed. “*” Means P < 0.05, “**” means P < 0.01, “***” means P < 0.001, and “ns” means P > 0.05.
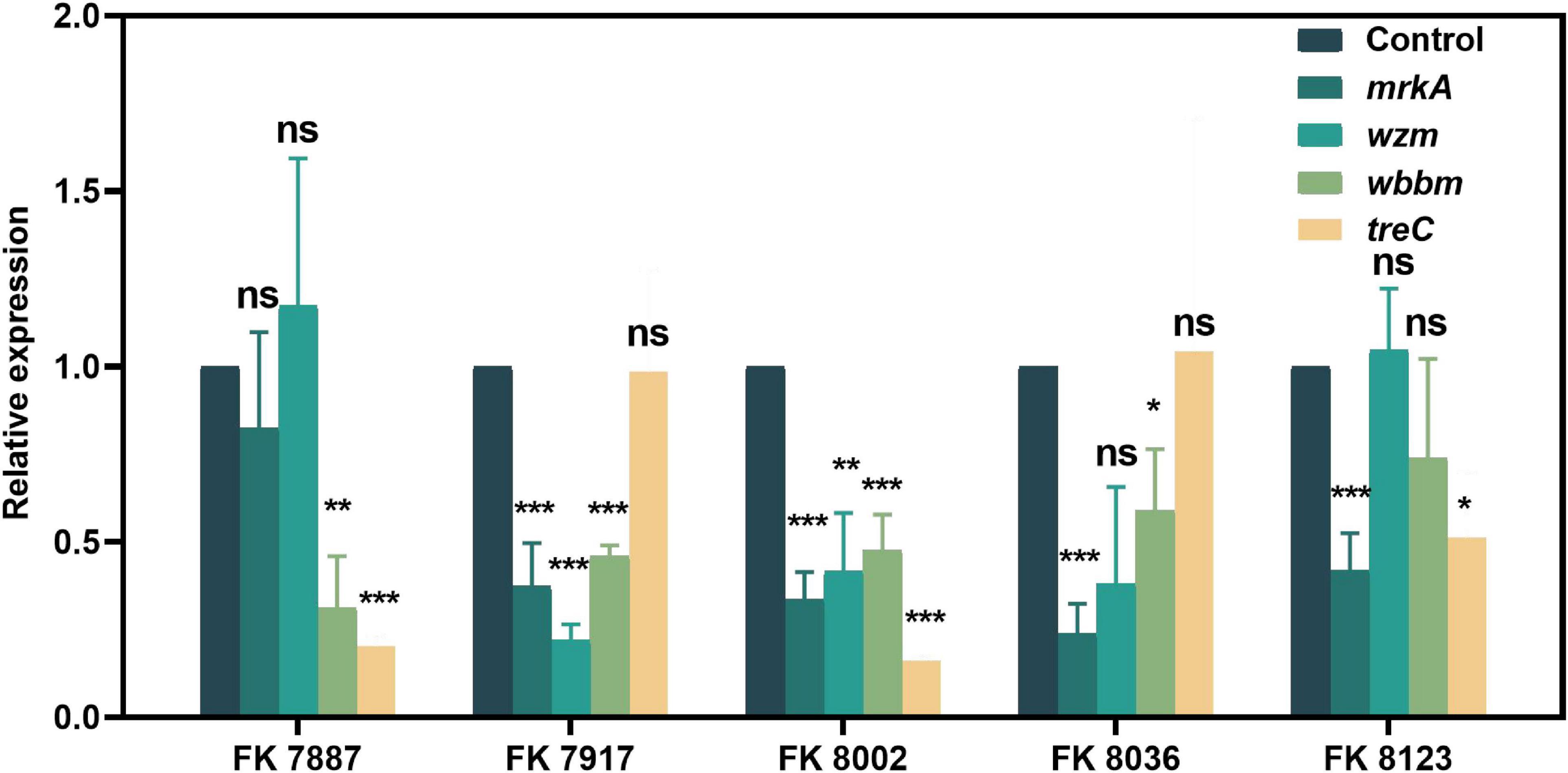
Figure 11. The expression of biofilm formation genes of five carbapenem-resistant Klebsiella pneumoniae (CRKP) strains deal with different treatment groups. “*” Means P < 0.05, “**” means P < 0.01, “***” means P < 0.001, and “ns” means P > 0.05.
3.12 Inhibition of AI-2 activity
We used V. harveyi BB170 to investigated the inhibition of CA on AI-2 activity. As shown in Figure 12, CA significantly inhibited the effect of AI-2 activity of BB170 (P < 0.05).
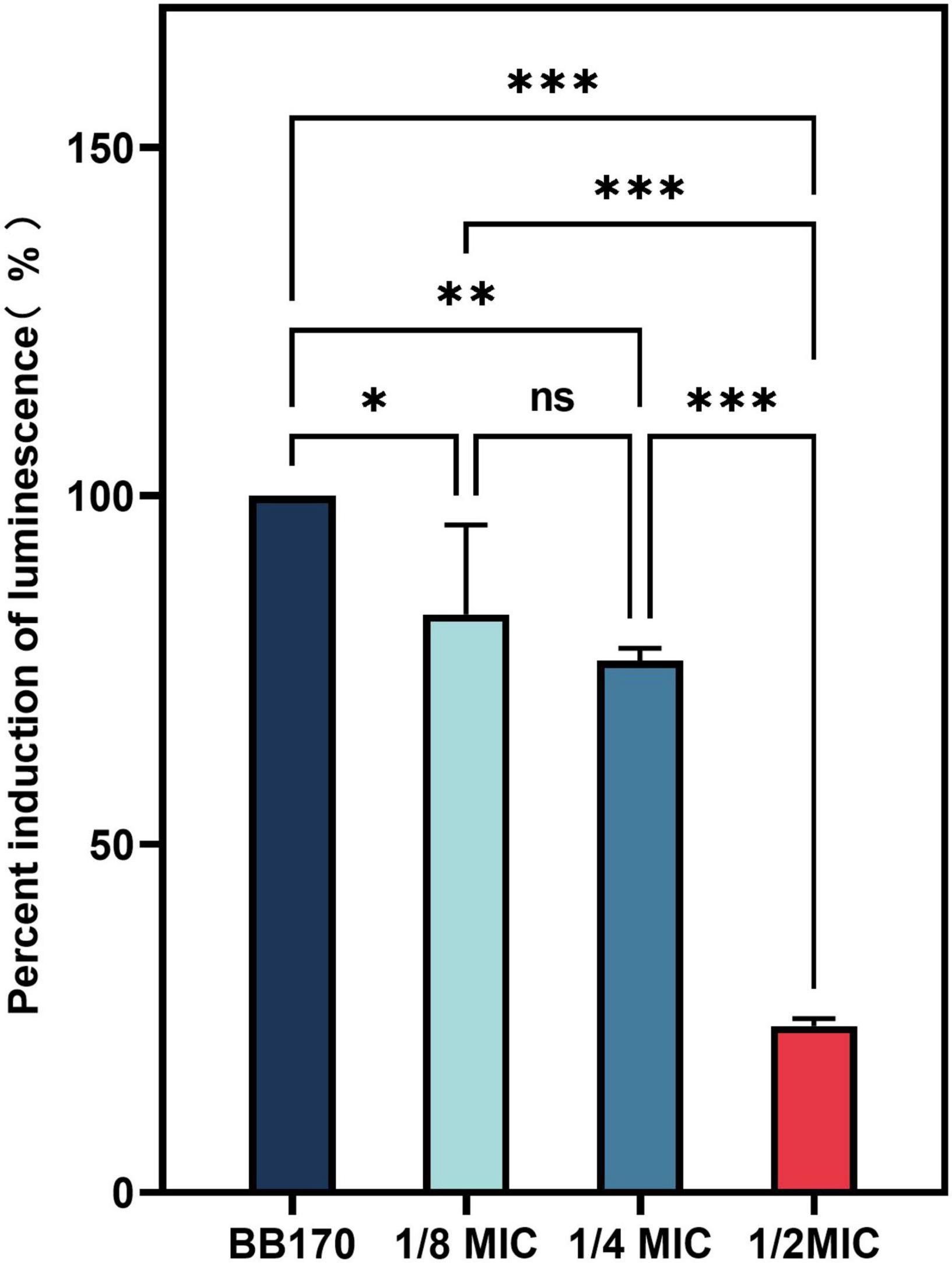
Figure 12. The effect of chlorogenic acid (CA) on the percent induction of luminescence of Vibrio harveyi. “*” Means P < 0.05, “**” means P < 0.01, “***” means P < 0.001, and “ns” means P > 0.05.
4 Discussion
Klebsiella pneumoniae, an important Gram-negative opportunistic pathogen, widely exists in environment and on mucosal surfaces of animals and humans; it is responsible for urinary tract infection, bacteremia, lung infection, and liver abscess (Bengoechea and Sa Pessoa, 2019). According to its virulence, K. pneumoniae can be classified as cKp and hvKp, with hvKp being stronger than cKp in virulence and pathogenicity, hvKp infection occurs usually appears in multiple parts of the body and is highly transferable and transmission, therefore, intervention and control strategies are urgently required (Shon et al., 2013). In general, highly virulent isolates have high adhesion, and protease and capsular polysaccharide levels (Russo and Marr, 2019). The QS system is a communication system between microbial cells, and it regulates the virulence, ability for biofilm formation, and other pathogenic factors of bacterial strains. The QS allows the pathogenic bacteria to achieve high cell density before the virulence determinant can be expressed, helping it carry out a coordinated attack against host defense (Kai, 2018). Therefore, the inhibition of virulence factors and virulence-related QS is vital to control the pathogenicity of bacterial strains.
Because of the antibiotic selective pressure caused by its long-term use, clinical drug-resistant K. pneumoniae strains have emerged, with CRKP appearing more frequently and poses considerable challenges to clinical treatment. Many scholars attempted to develop and use non-traditional antibacterial drugs, such as antimicrobial peptides, bacteriophages, and plant extracts that had certain antibacterial and virulence and biofilm inhibition effects, in place of traditional antibacterial drugs (Khan et al., 2021; Witherell et al., 2021). The QSIs can decrease the strain virulence factors, biofilm formation ability of the strain; therefore, they proved to be ideal for treatment purposes (Ham et al., 2021). Substances such as quercetin, resveratrol, and 6-gingerol (Quecan et al., 2019; Ham et al., 2021). However, some QSI retain some form of toxicity, hindering their optimal clinical application. Therefore, further investigation to find suitable QSIs is required.
Chlorogenic acid, also known as 5-Caffeoylquinic acid, is found in many plants and hence part of the human diet (Singh et al., 2022). A natural phenolic compound, it is frequently used in food- and conventional medicine–related fields because of its low toxicity level. CA has been approved by China Food and Drug Administration (CFDA) for phase I and phase II clinical trials (Nwafor et al., 2022). In our previous studies (Xu et al., 2022), CA had a good inhibitory effect on the motility and virulence phenotypes of multidrug resistant P. aeruginosa. The growth and virulence phenotypes of K. pneumoniae, especially CRKP, needed to be further studied.
The five CRKP strains exhibited different antimicrobial resistance to different antibacterial drugs, better sensitivity to gentamicin, tobramycin, and colistin and weak sensitivity to non-antibacterial CA whose MIC value was > 10,240 μg/mL. Based on this study, the detection results of peg-344, iroB, iucA, rmpA, and rmpA2 virulence genes of the five CRKP strains, most strains carry virulence genes of varying degrees, suggesting that the incidence of K. pneumoniae with high virulence and multidrug resistance characteristics is increasing in clinical practice. Efforts need to be made toward timely detection of infections caused by the multidrug-resistant K. pneumoniae, because of its high pathogenicity mediated by its high virulence phenotype. In contrast, detection results of carbapenem-resistant genes blaKPC–2, blaNDM, blaVIM, and blaOXA–48 showed that all the five strains carried blaKPC–2, a result consistent with that of previous reports. The prevalence of carbapenem-resistant genes expressed by K. pneumoniae in different countries and regions is diverse and not similar, but in K. pneumoniae isolated from China, the blaKPC–2 strain remains the most important prevalent epidemic type.
Because the growth of the strain influences the virulence phenotype of the strain, we used different CA concentrations to assess its effect on the growth of the five CRKP strains to test its effect, on the virulence phenotype of the strains. We found that CA at concentrations of 1/2, 1/4, and 1/8 MIC had no effect on the growth of CRKP. The extracellular products of K. pneumoniae, including various extracellular enzymes play a vital role in the infection of the bacterial strain in the host (Mayville et al., 1999). In addition, the high capsule production of K. pneumoniae can convert the colony into a high-viscosity phenotype that has a protective effect on phagocytes and human defensin-mediated bactericidal activity (Hoh et al., 2019). Our results indicated that CA reduced the generation of extracellular protease of the FK 8123 strain, the only strain producing protease among the five CRKP strains. We will include more strains in future studies to verify their ability for protease inhibition of CA. Moreover, CA had a significant inhibitory effect on the capsular polysaccharide production of the FK 7917, FK 8036, and FK 8123 strains, but its effect on that of the FK 7887 and FK 8002 strains was not obvious, which we speculated could be related to the capsular polysaccharide content secreted by the strain or to the characteristics of the strain itself. TEM showed that under the treatment of CA (1/2 or 1/4 MIC), the capsule became thinner. G. mellonella infection model was used to evaluate the effect of many non-antibacterial drugs on the virulence of bacteria, therefore, we used the CRKP infection model of G. mellonella to test the effect of CA on the virulence of the strain. Our results showed that 1/2 MIC CA could inhibit the virulence of the strain to a certain extent and improve the survival rate of G. mellonella. In our previous studies, we have shown that 1/4 MIC (2,560 μg/mL) CA had no cytotoxicity on RAW 264.7 macrophages (Xu et al., 2022), and the pre-experiment conducted in the G. mellonella infection assay showed that the CA used in this study was not toxic to the survival rate of G. mellonella, compared with the control group (data not shown), indicating CA has no cytotoxicity and could be further developed for clinical treatment use. Because of the complex mechanism of in vivo infection model, the inhibitory effect of CA on an in vivo strain virulence model needs to be further verified using other animal models and infection models.
The increase in pathogenic invasiveness and drug resistance to infection caused by K. pneumoniae has a significant correlation with biofilm. A biofilm, is a complex microbial community that can be formed on surfaces and can form dynamic structures (Karatan and Watnick, 2009). Many persistent, recurrent and refractory chronic infectious diseases, such as endocarditis, urinary tract infection, and chronic otitis media, are closely related to the formation of biofilms (Davey and O’Toole, 2000). K. pneumoniae has the ability to form biofilms on the inner surface of catheters and other medical devices, making these conducive to the development of invasive infection (Singhai et al., 2012). The biofilm formation process of K. pneumoniae is closely related to the factors mediating virulence, such as pili, and capsular polysaccharide and lipopolysaccharide, which are essential for maintaining the stability of biofilm structure and for inter-cell communication. The QS system coordinates and controls the signal and response of gene expression in bacterial population, thus playing an important role in bacterial adhesion and regulation of the formation of bacterial biofilm. However, deletion of QS genes may not reduce biofilm formation or virulence, therefore, the research on the interference with the signal molecules of the QS system this has certain significance.
Many plant extracts, such as resveratrol and quercetin, have good anti-toxicity, anti-biofilm, and anti-QS activities (Vasavi et al., 2017). The results of our study showed that CA can inhibit the biofilm formation ability of most the CRKP except the FK 8002 strain. This could be attributed to the fact that the biofilm formation ability of FK 8002 itself is weak; therefore, the inhibition effect on its ability is not significant under different concentrations of CA. SEM images showed that the network structure of the biofilm was destroyed after CA treatment and the number of colonies reduced and appeared dispersed, suggesting that CA does have a certain inhibitory effect on the process of biofilm formation, this result can help the potential use of CA in treatment of medical device–related diseases by resisting biofilm formation. In addition, our results showed that CA (8, 4, and 2 MIC) had poor clearance effect on the formed biofilm, which may be related to the existence of resistant persistent bacteria in the biofilm. Due to the biofilm were affected by many factors, it is worth further to explore the mechanism behind their inhibitory effect.
In the QS system, bacteria communicate with each other by secreting chemical signaling molecules. Through QS, many bacteria regulate the expression of various physiological functions, such as strain locomotion, virulence, biofilm formation, and bacteriocin production. The QS system of K. pneumoniae mainly uses type-2 QS, namely, AI-2, which is typically involved in the expression regulation of virulence-related factors, secretion system, regulatory proteins, and peptides, as the media molecule for communication with other bacterial population, and it is primarily regulated by the luxS gene (Zhu and Mekalanos, 2003). In addition, the virulence genes of K. pneumoniae are also correlated with their virulence phenotypes. rmpA2 can regulate the synthesis of polysaccharide in the outer capsular membrane to make the strain have high stickiness; the siderophore-related gene iucA is also closely related to the high stickiness of K. pneumoniae. Our results showed that CA downregulated the expression levels of luxS genes in most CRKP, but showed weak effect on the expression levels of rmpA2 and iucA, suggesting that CA may affect the virulence phenotype or biofilm formation of K. pneumoniae by downregulating the luxS gene. However, although CA downregulated the expression level of the luxS in the FK 8002 strain, there was no significant difference, which may be the reason why CA could not effectively inhibit the biofilm formation of the FK 8002. Furthermore, Balestrino et al. (2005) revealed that the luxS mutant could form a mature biofilm but one that had reduced capacity to develop microcolonies, mostly in the early steps of the biofilm formation, indicting the deletion of QS genes may not influence biofilm formation. Pan et al. (2021) found that no major differences were observed between the wild-type and the luxS mutant in regard to outer membrane protein profiles, biofilm formation, exopoly saccharides (EPS) production, or intracellular survival, which indicating that the deletion of QS genes may not reduce biofilm formation. The genes of mrkA, wzm, wbbm, and treC are all involved in the biofilm formation of K. pneumoniae (Vuotto et al., 2017), wzm and wbbM are lipopolysaccharide (LPS) synthesis-related genes and participate in LPS production. MrkA controls the production of type 3 fimbriae (Gual-de-Torrella et al., 2022), and treC participates in the production of capsular polysaccharide (Devanga Ragupathi et al., 2020). We further investigated the expression of regulators of biofilm formation mrkA, wzm, wbbm, and treC, the results showed that CA downregulated the expression level of mrkA and wbbm in five CRKP strains, suggesting that CA may regulate biofilm formation by regulating these factors. Besides, CA significantly inhibited the percent induction of luminescence of V. harveyi, but the AI-2 QS system network of K. pneumoniae is complex, the effect of CA on other factors of the QS system of K. pneumoniae type II and its mutual regulation with virulence factors need to be further explored in a future study. In view of the rapid increase of bacterial resistance, combining non-traditional antibiotic compounds and antibiotics as a new treatment regimen has thus garnered considerable scientific attention. We performed the checkerboard assays to evaluate the synergistic or additive effects between CA and known antimicrobials (meropenem, imipenem, ertapenem, ceftazidime, ciprofloxacin, levofloxacin, gentamicin, tobramycin, and ampicillin) on five CRKP strains in our study. The results showed that the CA/antimicrobial combinations exhibited a irrelevant effect on all five CRKP strains (data not shown). We believe that the development of molecular modifications in the future will help CA play a better role in clinical treatment, and we will pay more attention to this point in future research.
5 Conclusion
The present study found that CA can inhibit the virulence phenotype of CRKP and downregulate the expression level of the QS system and virulence-related genes. Because of the increase in the incidence of hv-CRKP in clinical settings, timely and accurate diagnosis of the infection and effective treatment are vital. As a new therapeutic drug for bacterial diseases, QSIs that can reduce the virulence of bacterial strains need to be further discussed in future research.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
LW conducted the experiments, analyzed the data, and wrote the manuscript. YZ participated in experiments. YL and MX took part in analysis of results. ZY and XZ participated in analysis of results. MS and TZ helped to design the study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Planned Science and Technology Project of Traditional Chinese Medicine of Zhejiang Province of China (No. 2023ZL352).
Acknowledgments
We thank all the medical workers of the Department of Clinical Laboratory, The First Affiliated Hospital of Wenzhou Medical University for co-operating this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.997310/full#supplementary-material
Footnotes
References
Balestrino, D., Haagensen, J. A., Rich, C., and Forestier, C. (2005). Characterization of type 2 quorum sensing in Klebsiella pneumoniae and relationship with biofilm formation. J. Bacteriol. 187, 2870–2880. doi: 10.1128/JB.187.8.2870-2880.2005
Ballen, V., Gabasa, Y., Ratia, C., Ortega, R., Tejero, M., and Soto, S. (2021). Antibiotic resistance and virulence profiles of Klebsiella pneumoniae strains isolated from different clinical sources. Front. Cell. Infect. Microbiol. 11:738223. doi: 10.3389/fcimb.2021.738223
Bengoechea, J. A., and Sa Pessoa, J. (2019). Klebsiella pneumoniae infection biology: Living to counteract host defences. FEMS Microbiol. Rev. 43, 123–144. doi: 10.1093/femsre/fuy043
Bialek-Davenet, S., Criscuolo, A., Ailloud, F., Passet, V., Jones, L., Delannoy-Vieillard, A. S., et al. (2014). Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg. Infect. Dis. 20, 1812–1820. doi: 10.3201/eid2011.140206
Brackman, G., Cos, P., Maes, L., Nelis, H. J., and Coenye, T. (2011). Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 55, 2655–2661. doi: 10.1128/AAC.00045-11
Candan, E. D., and Aksoz, N. (2015). Klebsiella pneumoniae: Characteristics of carbapenem resistance and virulence factors. Acta Biochim. Pol. 62, 867–874. doi: 10.18388/abp.2015_1148
CLSI (2021). Performance standards for antimicrobial susceptibility testing, 31th Edn. Wayne, PA: Clinical and Laboratory Standards Institute.
Cubero, M., Grau, I., Tubau, F., Pallares, R., Dominguez, M. A., Linares, J., et al. (2016). Hypervirulent Klebsiella pneumoniae clones causing bacteraemia in adults in a teaching hospital in Barcelona, Spain (2007-2013). Clin. Microbiol. Infect. 22, 154–160. doi: 10.1016/j.cmi.2015.09.025
Davey, M. E., and O’Toole, G. A. (2000). Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64, 847–867. doi: 10.1128/MMBR.64.4.847-867.2000
De Araujo, C., Balestrino, D., Roth, L., Charbonnel, N., and Forestier, C. (2010). Quorum sensing affects biofilm formation through lipopolysaccharide synthesis in Klebsiella pneumoniae. Res. Microbiol. 161, 595–603. doi: 10.1016/j.resmic.2010.05.014
Devanga Ragupathi, N. K., Muthuirulandi Sethuvel, D. P., Triplicane Dwarakanathan, H., Murugan, D., Umashankar, Y., Monk, P. N., et al. (2020). The influence of biofilms on carbapenem susceptibility and patient outcome in device associated K. pneumoniae infections: Insights into phenotype vs genome-wide analysis and correlation. Front. Microbiol. 11:591679. doi: 10.3389/fmicb.2020.591679
Doi, Y. (2019). Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin. Infect. Dis. 69(Suppl. 7) S565–S575. doi: 10.1093/cid/ciz830
Gual-de-Torrella, A., Delgado-Valverde, M., Perez-Palacios, P., Oteo-Iglesias, J., Rojo-Molinero, E., Macia, M. D., et al. (2022). Prevalence of the fimbrial operon mrkABCD, mrkA expression, biofilm formation and effect of biocides on biofilm formation in carbapenemase-producing Klebsiella pneumoniae isolates belonging or not belonging to high-risk clones. Int. J. Antimicrob. Agents 60:106663. doi: 10.1016/j.ijantimicag.2022.106663
Ham, S. Y., Kim, H. S., Jo, M. J., Lee, J. H., Byun, Y., Ko, G. J., et al. (2021). Combined treatment of 6-gingerol analog and tobramycin for inhibiting Pseudomonas aeruginosa infections. Microbiol. Spectr. 9, e00192-21. doi: 10.1128/Spectrum.00192-21
Hemmati, F., Salehi, R., Ghotaslou, R., Kafil, H. S., Hasani, A., Gholizadeh, P., et al. (2020). The assessment of antibiofilm activity of chitosan-zinc oxide-gentamicin nanocomposite on Pseudomonas aeruginosa and Staphylococcus aureus. Int. J. Biol. Macromol. 163, 2248–2258. doi: 10.1016/j.ijbiomac.2020.09.037
Hoh, C. H., Tan, Y. H., and Gan, Y. H. (2019). Protective role of kupffer cells and macrophages in Klebsiella pneumoniae-induced liver abscess disease. Infect. Immun. 87:e00369-19. doi: 10.1128/IAI.00369-19
Hu, Y., Anes, J., Devineau, S., and Fanning, S. (2021). Klebsiella pneumoniae: Prevalence, reservoirs, antimicrobial resistance, pathogenicity, and infection: A hitherto unrecognized zoonotic bacterium. Foodborne Pathog. Dis. 18, 63–84. doi: 10.1089/fpd.2020.2847
Kai, K. (2018). Bacterial quorum sensing in symbiotic and pathogenic relationships with hosts. Biosci. Biotechnol. Biochem. 82, 363–371. doi: 10.1080/09168451.2018.1433992
Kalia, V. C. (2013). Quorum sensing inhibitors: An overview. Biotechnol. Adv. 31, 224–245. doi: 10.1016/j.biotechadv.2012.10.004
Kalia, V. C., Patel, S. K. S., Kang, Y. C., and Lee, J. K. (2019). Quorum sensing inhibitors as antipathogens: Biotechnological applications. Biotechnol. Adv. 37, 68–90. doi: 10.1016/j.biotechadv.2018.11.006
Karatan, E., and Watnick, P. (2009). Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol. Mol. Biol. Rev. 73, 310–347. doi: 10.1128/MMBR.00041-08
Khan, F. M., Gondil, V. S., Li, C., Jiang, M., Li, J., Yu, J., et al. (2021). A novel Acinetobacter baumannii bacteriophage endolysin LysAB54 with high antibacterial activity against multiple Gram-negative microbes. Front. Cell. Infect. Microbiol. 11:637313. doi: 10.3389/fcimb.2021.637313
Lu, X., Yang, X., Li, X., Lu, Y., Ren, Z., Zhao, L., et al. (2013). In vitro activity of sodium new houttuyfonate alone and in combination with oxacillin or netilmicin against methicillin-resistant Staphylococcus aureus. PLoS One 8:e68053. doi: 10.1371/journal.pone.0068053
M Campos, J. C., Antunes, L. C., and Ferreira, R. B. (2020). Global priority pathogens: Virulence, antimicrobial resistance and prospective treatment options. Future Microbiol. 15, 649–677. doi: 10.2217/fmb-2019-0333
Mayville, P., Ji, G., Beavis, R., Yang, H., Goger, M., Novick, R. P., et al. (1999). Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc. Natl. Acad. Sci. U.S.A. 96, 1218–1223. doi: 10.1073/pnas.96.4.1218
Nicodeme, M., Grill, J. P., Humbert, G., and Gaillard, J. L. (2005). Extracellular protease activity of different Pseudomonas strains: Dependence of proteolytic activity on culture conditions. J. Appl. Microbiol. 99, 641–648. doi: 10.1111/j.1365-2672.2005.02634.x
Nwafor, E. O., Lu, P., Zhang, Y., Liu, R., Peng, H., Xing, B., et al. (2022). Chlorogenic acid: Potential source of natural drugs for the therapeutics of fibrosis and cancer. Transl. Oncol. 15:101294. doi: 10.1016/j.tranon.2021.101294
Pan, Y., Siddaramappa, S., Sandal, I., Dickerman, A., Bandara, A. B., and Inzana, T. J. (2021). The role of luxS in Histophilus somni virulence and biofilm formation. Infect. Immun. 89:e00567-20. doi: 10.1128/IAI.00567-20
Panayi, T., Sarigiannis, Y., Mourelatou, E., Hapeshis, E., and Papaneophytou, C. (2022). Anti-quorum-sensing potential of ethanolic extracts of aromatic plants from the flora of cyprus. Plants 11:2632. doi: 10.3390/plants11192632
Papenfort, K., and Bassler, B. L. (2016). Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 14, 576–588. doi: 10.1038/nrmicro.2016.89
Pena, R. T., Blasco, L., Ambroa, A., Gonzalez-Pedrajo, B., Fernandez-Garcia, L., Lopez, M., et al. (2019). Relationship between quorum sensing and secretion systems. Front. Microbiol. 10:1100. doi: 10.3389/fmicb.2019.01100
Phrommao, E., Yongsawatdigul, J., Rodtong, S., and Yamabhai, M. (2011). A novel subtilase with NaCl-activated and oxidant-stable activity from Virgibacillus sp. SK37. BMC Biotechnol. 11:65. doi: 10.1186/1472-6750-11-65
Quecan, B. X. V., Santos, J. T. C., Rivera, M. L. C., Hassimotto, N. M. A., Almeida, F. A., and Pinto, U. M. (2019). Effect of quercetin rich onion extracts on bacterial quorum sensing. Front. Microbiol. 10:867. doi: 10.3389/fmicb.2019.00867
Russo, T. A., and Marr, C. M. (2019). Hypervirulent Klebsiella pneumoniae. Clin. Microbiol. Rev. 32, e00001–e19. doi: 10.1128/CMR.00001-19
Santana-Galvez, J., Cisneros-Zevallos, L., and Jacobo-Velazquez, D. A. (2017). Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 22:358. doi: 10.3390/molecules22030358
Shon, A. S., Bajwa, R. P., and Russo, T. A. (2013). Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence 4, 107–118. doi: 10.4161/viru.22718
Singh, A. K., Singla, R. K., and Pandey, A. K. (2022). Chlorogenic acid: A dietary phenolic acid with promising pharmacotherapeutic potential. Curr. Med. Chem. doi: 10.2174/0929867329666220816154634
Singhai, M., Malik, A., Shahid, M., Malik, M. A., and Goyal, R. (2012). A study on device-related infections with special reference to biofilm production and antibiotic resistance. J. Glob. Infect. Dis. 4, 193–198. doi: 10.4103/0974-777X.103896
Tang, M., Kong, X., Hao, J., and Liu, J. (2020). Epidemiological characteristics and formation mechanisms of multidrug-resistant hypervirulent Klebsiella pneumoniae. Front. Microbiol. 11:581543. doi: 10.3389/fmicb.2020.581543
Vasavi, H. S., Sudeep, H. V., Lingaraju, H. B., and Shyam Prasad, K. (2017). Bioavailability-enhanced Resveramax modulates quorum sensing and inhibits biofilm formation in Pseudomonas aeruginosa PAO1. Microb. Pathog. 104, 64–71. doi: 10.1016/j.micpath.2017.01.015
Vuotto, C., Longo, F., Pascolini, C., Donelli, G., Balice, M. P., Libori, M. F., et al. (2017). Biofilm formation and antibiotic resistance in Klebsiella pneumoniae urinary strains. J. Appl. Microbiol. 123, 1003–1018. doi: 10.1111/jam.13533
Wang, G., Zhao, G., Chao, X., Xie, L., and Wang, H. (2020). The characteristic of virulence, biofilm and antibiotic resistance of Klebsiella pneumoniae. Int. J. Environ. Res. Public Health 17:6258. doi: 10.3390/ijerph17176278
Wang, S., Ding, Q., Zhang, Y., Zhang, A., Wang, Q., Wang, R., et al. (2022). Evolution of virulence, fitness, and carbapenem resistance transmission in ST23 hypervirulent Klebsiella pneumoniae with the capsular polysaccharide synthesis gene wcaJ inserted via insertion sequence elements. Microbiol. Spectr. e0240022. doi: 10.1128/spectrum.02400-22
Witherell, K. S., Price, J., Bandaranayake, A. D., Olson, J., and Call, D. R. (2021). In vitro activity of antimicrobial peptide CDP-B11 alone and in combination with colistin against colistin-resistant and multidrug-resistant Escherichia coli. Sci. Rep. 11:2151. doi: 10.1038/s41598-021-81140-8
Wu, S., Liu, J., Liu, C., Yang, A., and Qiao, J. (2020). Quorum sensing for population-level control of bacteria and potential therapeutic applications. Cell. Mol. Life Sci. 77, 1319–1343. doi: 10.1007/s00018-019-03326-8
Xu, L., Sun, X., and Ma, X. (2017). Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann. Clin. Microbiol. Antimicrob. 16:18. doi: 10.1186/s12941-017-0191-3
Xu, W., Zhang, X., Wang, L., Zeng, W., Sun, Y., Zhou, C., et al. (2022). Effect of chlorogenic acid on the quorum-sensing system of clinically isolated multidrug-resistant Pseudomonas aeruginosa. J. Appl. Microbiol. 132, 1008–1017. doi: 10.1111/jam.15275
Yang, F., Deng, B., Liao, W., Wang, P., Chen, P., and Wei, J. (2019). High rate of multiresistant Klebsiella pneumoniae from human and animal origin. Infect. Drug Resist. 12, 2729–2737. doi: 10.2147/IDR.S219155
Yang, X., Wai-Chi Chan, E., Zhang, R., and Chen, S. (2019). A conjugative plasmid that augments virulence in Klebsiella pneumoniae. Nat. Microbiol. 4, 2039–2043. doi: 10.1038/s41564-019-0566-7
Yu, W., Zhang, J., Tong, J., Zhang, L., Zhan, Y., Huang, Y., et al. (2020). In vitro antimicrobial activity of fosfomycin, vancomycin and daptomycin alone, and in combination, against linezolid-resistant Enterococcus faecalis. Infect. Dis. Ther. 9, 927–934. doi: 10.1007/s40121-020-00342-1
Zhao, Y., Zhang, S., Fang, R., Wu, Q., Li, J., Zhang, Y., et al. (2020). Dynamic epidemiology and virulence characteristics of carbapenem-resistant Klebsiella pneumoniae in Wenzhou, China from 2003 to 2016. Infect. Drug Resist. 13, 931–940. doi: 10.2147/IDR.S243032
Keywords: chlorogenic acid, carbapenem-resistant, hypervirulent, Klebsiella pneumoniae, quorum sensing
Citation: Wang L, Zhang Y, Liu Y, Xu M, Yao Z, Zhang X, Sun Y, Zhou T and Shen M (2022) Effects of chlorogenic acid on antimicrobial, antivirulence, and anti-quorum sensing of carbapenem-resistant Klebsiella pneumoniae. Front. Microbiol. 13:997310. doi: 10.3389/fmicb.2022.997310
Received: 18 July 2022; Accepted: 29 November 2022;
Published: 13 December 2022.
Edited by:
Manuela Mandrone, University of Bologna, ItalyCopyright © 2022 Wang, Zhang, Liu, Xu, Yao, Zhang, Sun, Zhou and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tieli Zhou, d3l6dGxpQDE2My5jb20=; Mo Shen, c2hlbm1vNjAxQDE2My5jb20=
†These authors have contributed equally to this work
 Lingbo Wang
Lingbo Wang Yi Zhang
Yi Zhang Yan Liu
Yan Liu Mengxin Xu
Mengxin Xu Zhuocheng Yao
Zhuocheng Yao Xiaodong Zhang
Xiaodong Zhang Yao Sun
Yao Sun Tieli Zhou
Tieli Zhou Mo Shen
Mo Shen