
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 11 October 2022
Sec. Infectious Agents and Disease
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.989879
China experienced another widespread Coronavirus disease 2019 (COVID-19) outbreak recently caused by the Omicron variant, which is less severe but far more contagious than the other COVID-19 variants, leading local governments to focus efforts on eliminating the spread of the disease. Previous studies showed that after “recovering” from the virus, some patients could re-test positive for COVID-19 with nucleic acid tests, challenging the control of disease spread. In this study, we aimed to analyze the clinical and laboratory characteristics of re-positive COVID-19 patients in Northeast China. We retrospectively analyzed data from confirmed reverse transcription polymerase chain reaction (RT-PCR) re-positive COVID-19 patients who were admitted to the First Hospital of Jilin University, Jilin Province, China, from March to June 2022. Detailed clinical symptoms, medical history, anti-Corona Virus (CoV) IgG and IgM levels, and CoV nucleic acid cycle threshold (Ct) values during the re-positive period were collected and analyzed. A total of 180 patients were included in this study, including 62 asymptomatic cases and 118 mild cases. The cohort included 113 men and 67 women, with an average age of 45.73 years. The median time between recovery from the virus and re-positivity was 13 days. Our results showed that the proportion of re-positive patients with symptoms was lower, and the nucleic acid test-positive duration was shorter during the re-positive period. Furthermore, in patients with underlying disease, the proportion of patients with symptoms was higher, anti-CoV IgG levels were lower, and the total disease duration was longer. In conclusion, during the re-positive period, the symptoms were milder, and the CoV nucleic acid test-positive course was shorter. The concomitant underlying disease is an important factor associated with clinical symptoms, and the overall course of COVID-19 re-positive patients may be associated with lower anti-CoV IgG levels. Large-scale and multicenter studies are recommended to better understand the pathophysiology of recurrence in patients with COVID-19.
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has had a worldwide impact since its first identified case in 2019. The Omicron variant of SARS-CoV-2, identified in November 2021, has contributed to a recent increase in the number of cases worldwide. This variant is heavily mutated, with a very high risk of infection, making the pandemic more complex and difficult to control (Araf et al., 2022). The pandemic in Changchun, Jilin Province, from early March 2022, was caused by the new highly contagious Omicron mutant BA.2 strain. However, infected individuals have atypical symptoms or are asymptomatic, resulting in the spread of the disease within the community.
Previous studies have shown that re-positive tests for SARS-CoV-2 using nucleic acid reverse transcription polymerase chain reaction (RT-PCR) in recovered COVID-19 patients are common (Simon et al., 2020; Li et al., 2021; Wong et al., 2021). The proportion of re-positive tests in recovered COVID-19 patients varied from 2.4 to 69.2% and ranged from 1 to 111 days after recovery, depending on region, sample size, age of patients, and type of specimen (Chen et al., 2020; Mattiuzzi et al., 2020; Vancsa et al., 2021). However, the mechanisms underlying these positive test results remain unclear. Currently, several causes of re-positive tests for SARS-CoV-2 in recovered COVID-19 patients are proposed, including false-negative RT-PCR tests, prolonged or recurrent viral shedding, reactivation, or reinfection with coronavirus (Miyamae et al., 2020). The false-negative RT-PCR results may be due to the sources of samples. Oropharyngeal and nasopharyngeal (OP/NP) swab samples are often used for testing; however, SARS-CoV-2 has been found in fecal specimens of patients with clinically suspected COVID-19 but multiple negative RT-PCR test results for SARS-CoV-2 using OP/NP specimens (Brogna B. et al., 2021). Sensitivity/specificity problems of the nucleic acid test kit and the sampling procedure itself have also been reported to contribute to the false-negative results. Reinfection needs to be confirmed by viral gene sequencing results or clear epidemiological data; knowledge about reinfected patients remains limited (Farrukh et al., 2021).
Theoretically, re-positive patients can be potential transmission sources. The transmission capacity of recurrent SARS-CoV-2 depends on viral replication. The viral load in re-positive cases is very low, and the viral culture of the OP/NP samples from re-positive patients showed no cytopathic effect (Liang et al., 2021). However, there is evidence showing spontaneous replication of SARS-CoV-2 in the bacterial cultures of patients’ feces for up to 30 days and beyond, indicating that the gut microbiota could affect the severity of COVID-19 (Yeoh et al., 2021). In addition, toxin-like peptides, which were almost identical to the toxic components of animal venoms, such as conotox, phospholipases, phosphodiesterases, zinc-metal proteinases, and bradykinins, were reported in the blood, feces, and urine obtained from patients with COVID-19 (Brogna et al., 2022). The existence of toxin-like peptides has been suggested to be associated with a large set of heterogeneous extra-pulmonary COVID-19 clinical manifestations (Brogna B. et al., 2021). Owing to the potential risk of virus transmission in recovered patients with re-positive virus detection, after discharge, recovered patients were transferred to quarantine locations for another 7 days, and a coronavirus nucleic acid test (COVNAT) was performed during the quarantine period.
In this study, we aimed to analyze the clinical and laboratory characteristics of re-positive COVID-19 patients from Changchun, Jilin Province, and to discuss the factors influencing disease severity in these patients. Our findings will aid in the management of patients with COVID-19 caused by the Omicron variant of SARS-CoV-2.
Clinical and laboratory data from patients admitted to the First Hospital of Jilin University between March and June 2022 were retrospectively collected. COVID-19 was diagnosed according to the Diagnosis and Treatment Protocol for COVID-19 (Trial Version 9) criteria (National Health Commission & National Administration of Traditional Chinese Medicine, 2022) issued by the National Health Commission of China.
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Hospital of Jilin University. Written informed consent for participation was not required for this study in accordance with national legislation and institutional requirements.
Coronavirus disease 2019 was diagnosed by measuring SARS-CoV-2 RNA in oropharyngeal swabs. RNA was measured using RT-PCR according to the standard protocol by specialized laboratory personnel at the Changchun Infectious Hospital.
The severity of COVID-19 was defined according to the Diagnosis and Treatment Protocol for COVID-19 (Trial Version 9) criteria: (1) asymptomatic type: COVNAT positive without any clinical symptoms; (2) mild type: mild clinical symptoms without any radiological findings; (3) general type: limited clinical symptoms (i.e., fever, cough, and other common pneumonia-related symptoms with radiological abnormality); (4) severe type: patients with any of the following: (a) respiratory distress, respiratory rate ≥ 30 per min; (b) oxygen saturation on room air at rest ≤ 93%; and (c) partial pressure of oxygen in arterial blood/fraction of inspired oxygen ≤ 300 mmHg; and (5) critical type: patients with any of the following: (a) respiratory failure with mechanical ventilation required; (b) shock; and (c) other organ dysfunction requiring intensive care unit monitoring treatment.
The COVID-19 patients were considered to be discharged when they met all the criteria from the Treatment Protocol for COVID-19 (Trial Version 9): briefly, (1) normal body temperature for more than 3 days; (2) significant recovery of respiratory symptoms; (3) lung imaging showing obvious absorption and recovery of acute exudative lesions; and (4) negative results of the nucleic acid tests of respiratory pathogens for two consecutive samples (sampling interval of at least 24 h).
The term “re-positive” is used to refer to the phenomenon of a confirmed COVID-19 patient with detected SARS-CoV-2 RNA-positive post-discharge (with random test timing).
The RNA was measured by the RT-PCR method against open reading frame 1ab (ORF1ab) and nucleocapsid protein N, separately. The sequence of primers for the ORF1ab gene are as follows: Forward (5′–>3′): CCCTGTGGGTTTTACACTTAA; Reverse (5′–>3′): ACGATTGTGCATCAGCTGA. The sequence of primers for nucleocapsid protein N gene are: Forward (5′–>3′): GGGGAACTTCTCCTGCTAGAAT; Reverse (5′–>3′): CAGACATTTTGCTCTCAAGCTG. IgG or IgM antibodies against SARS-CoV-2 were detected using magnetic particle chemiluminescence, which is a highly sensitive immunoassay that can be used to determine all antigenic substances.
Continuous variables are presented as the mean ± standard deviation for normally distributed data or median with interquartile range (IQR) for non-normally distributed data. Categorical variables are expressed as n (%). Differences in clinical characteristics and laboratory findings between groups were compared using the independent sample T-test or Mann–Whitney U test (continuous variables) and the chi-square test or Fisher’s exact test (categorical variables). A two-sided significance level of 0.05 was used to evaluate the statistical significance.
The data from 180 patients were collected in this study, of whom 118 were mild cases and 62 were asymptomatic. The patients were between 4 and 93 years of age, with an average age of 45.73 years, and 113 patients were men (62.7%). The median initial onset course was 12 days, the median course between the first COVNAT-negative and re-positive time point was 13 days, the median re-positive course was 6 days, and the median total disease course was 36 days. Fifty-three of the patients had underlying diseases (29.4%), including 18 hypertension patients, 11 diabetes patients, six cerebral infarction patients, five coronary heart disease patients, two hypotension patients, two asthma patients, two tracheitis patients, two depression patients, two pancreatitis patients, one chronic hepatitis patient, one brucellosis patient, and one arthritis patient.
The comparison of clinical symptoms and laboratory results between the initial onset and re-positive period is summarized in Table 1. Our results showed that the proportion of patients with symptoms during the re-positive period was significantly lower than that during the initial onset period. Moreover, we compared specific symptoms including fever, headache, nasal congestion, cough, dry throat, decreased sense of smell, fatigue, soreness of the body, diarrhea, nausea, vomiting, and poor appetite between the initial onset and the re-positive period. All proportions of the above symptoms were lower in the re-positive period than those in the initial onset period. Furthermore, the disease course was longer in the initial onset period than that in the re-positive period. Moreover, 37 patients had persistent symptoms, including cough (50%), dry or sore throat (44.7%), fatigue (13.5%), headache (13.5%), decreased sense of smell (5.2%), and fever (2.6%).
To compare the clinical characteristics between patients with or without underlying diseases at the initial onset and re-positive period, the patients were grouped as with (n = 53) and without (n = 127) underlying diseases, respectively, and the clinical characteristics and laboratory results were compared between the two groups. Our results showed no significant differences between the two groups in terms of sex, clinical symptoms at the initial onset period, COVNAT-negative to re-positive duration, initial onset to COVNAT-negative duration, re-positive to COVNAT-negative duration, CoV O gene and N gene levels at the re-positive time, lowest CoV O gene and N gene levels during the re-positive period, anti-CoV IgM levels, and proportion of patients with elevated anti-CoV IgG and anti-CoV IgM levels. However, the patients were older, the proportion of patients with symptoms was significantly higher (at the re-positive period only), the proportion of patients who were vaccinated was lower, the total disease course was longer, and the levels of anti-CoV IgG were lower in patients with underlying diseases than those in patients without underlying diseases (Table 2).
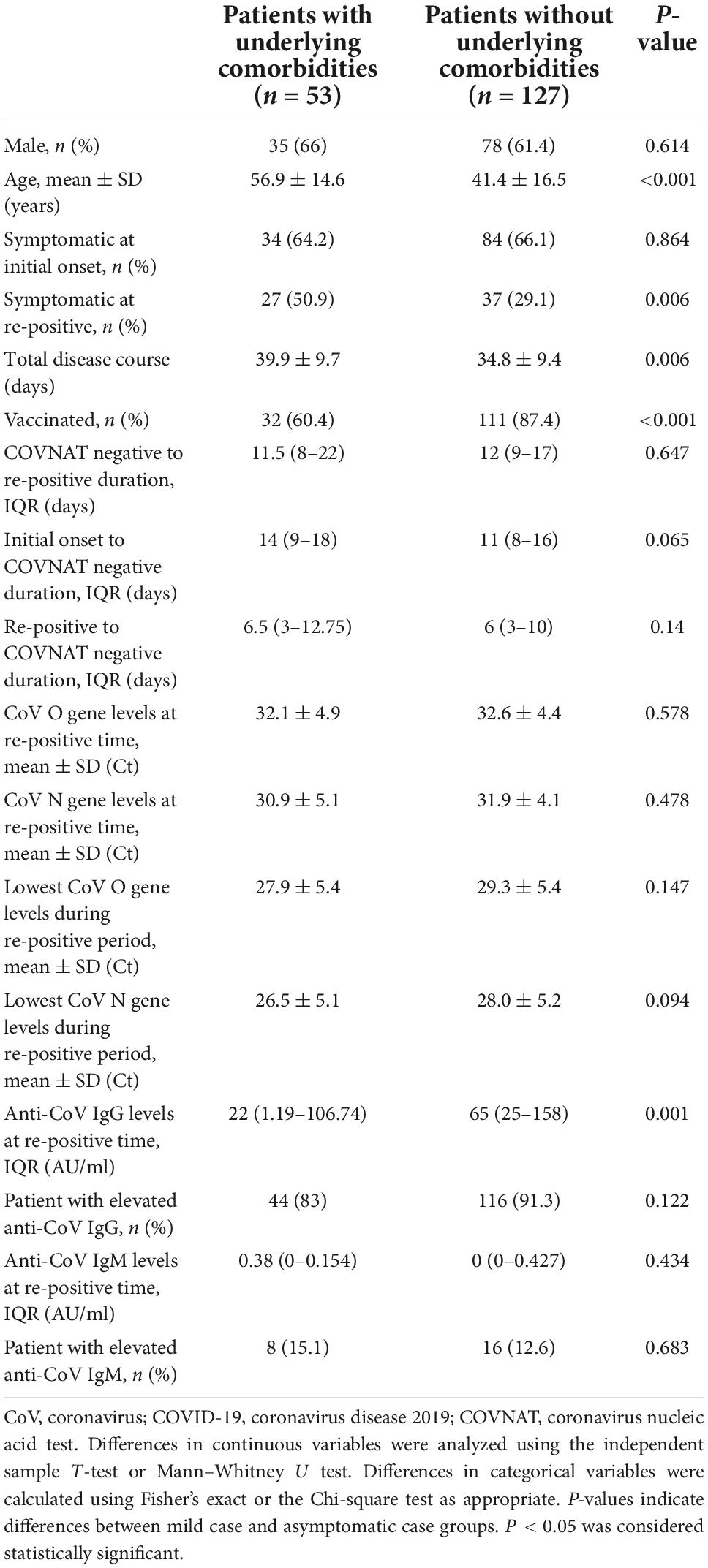
Table 2. Comparison of clinical characteristics and laboratory results between patients with or without underlying diseases at first positive and re-positive period.
To compare the clinical characteristics by age at the initial onset and re-positive period, the patients were grouped by age >50 years (n = 71) and ≤50 years (n = 109), and the clinical characteristics and laboratory results were compared between the two groups. Our results showed no significant differences between the two groups regarding sex, clinical symptoms at the initial onset period and at the re-positive period, COVNAT-negative to re-positive duration, initial onset to COVNAT-negative duration, re-positive to COVNAT-negative duration, CoV O and N gene levels at re-positive time, anti-CoV IgM levels, and proportion of patients with elevated anti-CoV IgM levels. However, the proportion of patients with underlying diseases and the proportion of non-vaccinated patients were higher, and the lowest CoV O gene Ct values, N gene Ct values, levels of anti-CoV IgG, and proportion of patients with elevated anti-CoV IgG levels were lower in patients aged >50 years (Table 3).
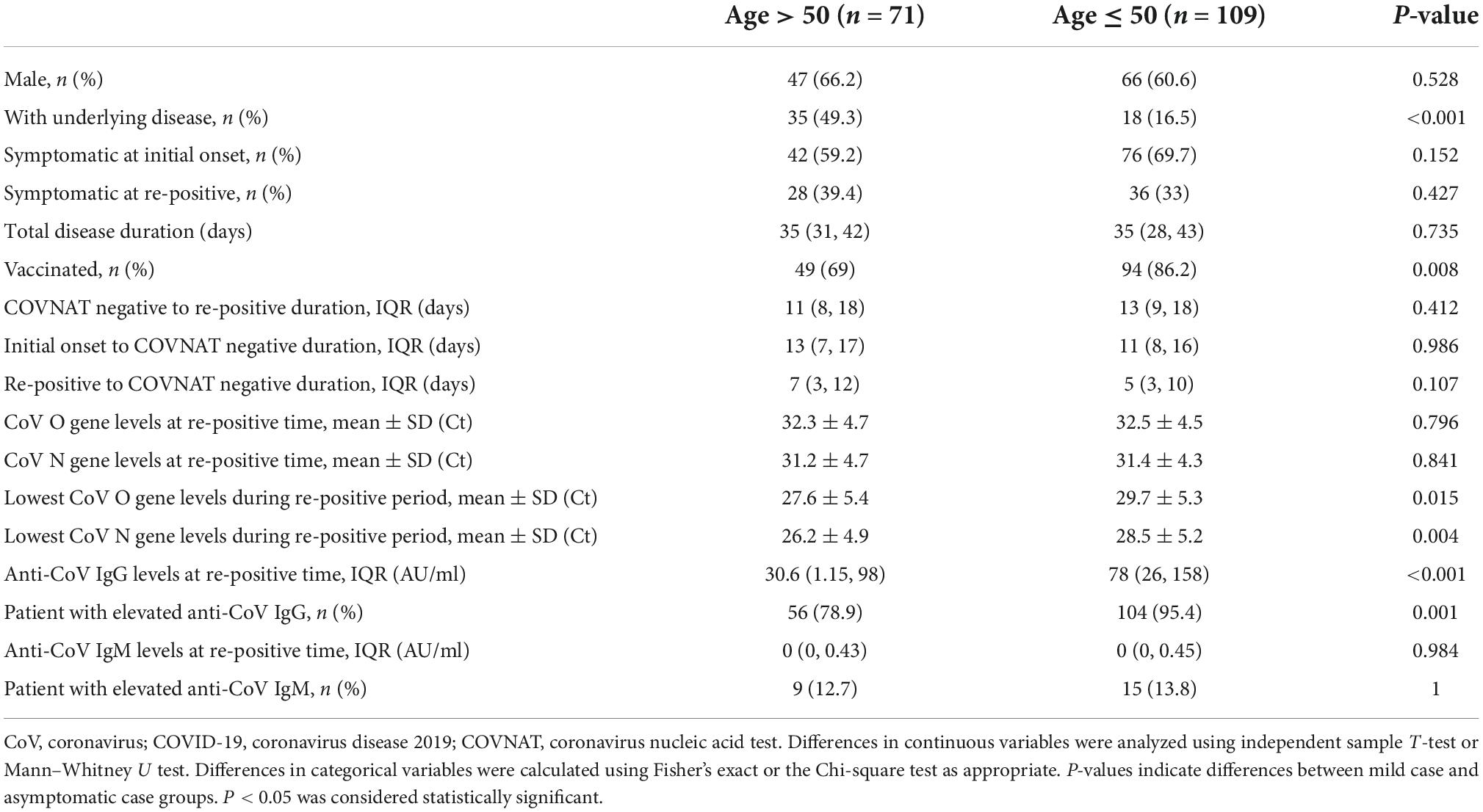
Table 3. Comparison of clinical characteristics and laboratory results between patients >50 and ≤50 years old.
To compare the clinical characteristics between patients with or without vaccination at initial onset and in the re-positive period, the patients were grouped by vaccinated (n = 143) and non-vaccinated (n = 37) individuals, and the clinical characteristics were compared between the two groups at the first positive and re-positive periods, separately. Our results showed no significant differences between the two groups regarding sex, clinical symptoms at the initial onset period and at the re-positive period, COVNAT-negative to re-positive duration, initial onset to COVNAT-negative duration, re-positive to COVNAT-negative duration, CoV O and N gene levels at the re-positive time, lowest CoV N gene levels during the re-positive time, anti-CoV IgM levels, and proportion of patients with elevated anti-CoV IgM levels. However, the patients were older, the proportion of patients with underlying diseases was higher, and the lowest CoV O gene Ct values and levels of anti-CoV IgG, and proportion of patients with elevated anti-CoV IgG levels were lower in patients who were not vaccinated (Table 4).
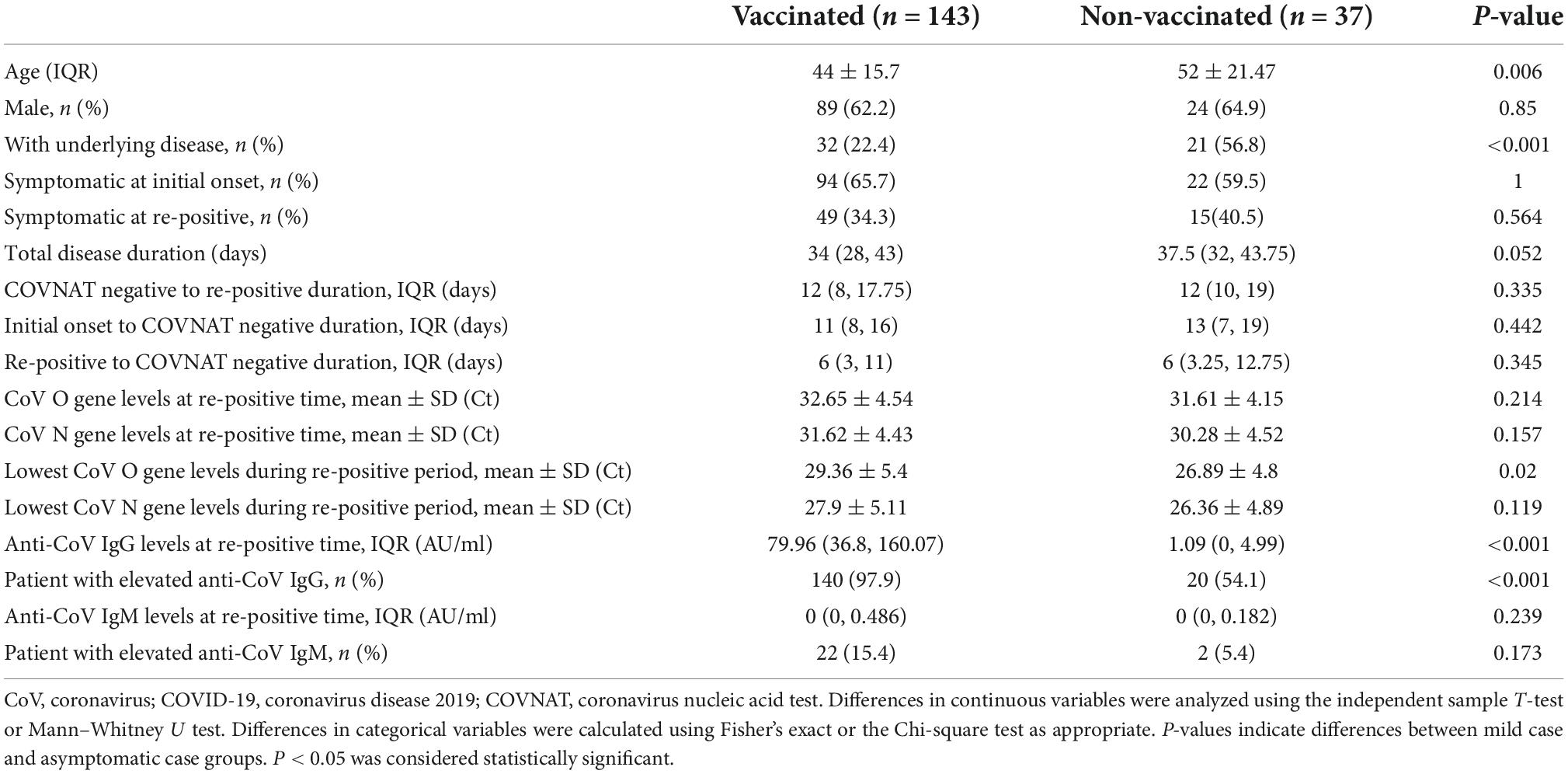
Table 4. Comparison of clinical characteristics and laboratory results between vaccinated and non-vaccinated patients.
Re-positive tests for COVNAT in recovered COVID-19 patients are common, challenging the elimination of the spread of the disease. In the present study, we analyzed the data from 180 re-positive patients, including 62 asymptomatic cases and 118 mild cases, who were admitted at the First Hospital of Jilin University. Our results showed that the proportion of re-positive patients with symptoms was lower and the COVNAT-positive duration was shorter during the re-positive period. Furthermore, the proportion of patients with symptoms was higher, anti-CoV IgG levels were lower, and the total disease course was longer in patients with underlying diseases.
The results from previous studies have shown that the symptoms were generally milder in the re-positive period than those in the initial infection period (Wu et al., 2020; Li et al., 2022), most of the re-positive patients turned negative in the next few tests, and patients were found positive for antibodies against SARS-CoV-2 (Yuan et al., 2020). Accordingly, our results showed that the proportion of re-positive patients with symptoms was lower and the COVNAT-positive course was shorter during the re-positive period. The reason why symptoms were milder in the re-positive period may be due to the low viral load of re-positive patients; indeed, viral culture of samples from patients in the re-positive period showed no cytopathic effect, and nucleic acid testing of the culture medium from viral cultures showed negative results (Lu et al., 2020; Liang et al., 2021), indicating that a re-positive test is likely due to inactivated viral RNA rather than reactivation or reinfection.
Many risk factors have been reported to be associated with the severity of COVID-19, including old age; male sex; underlying comorbidities such as hypertension; diabetes; chronic lung diseases; heart, liver, and kidney diseases; obesity; tumors; clinically apparent immunodeficiencies; local immunodeficiencies; and pregnancy (Gao et al., 2021). In our cohort, 53 patients had underlying diseases (29.4%), and the proportion of patients with symptoms was higher in those with underlying diseases. In line with our findings, previous reports showed that among re-positive cases, increased underlying comorbidity was observed in re-positive patients compared to that in non-re-positive patients with COVID-19 (Guan et al., 2020; Hoang, 2021). Another study using multivariate Cox regression analysis showed that the risk factors associated with re-positivity were the presence of comorbidities, particularly hypertension or chronic diseases in the respiratory system (Zhou et al., 2020). Moreover, we found that the levels of anti-CoV IgG were lower and the total disease duration was longer in patients with underlying diseases. Interestingly, in the group with underlying diseases, the patients were older and had a lower proportion of vaccinated patients when compared with the group of patients without underlying diseases. Anti-CoV IgG is a protective IgG that is capable of adhering to the virus and preventing it from entering the host cell (Dinc et al., 2022). Vaccination is the most effective way to boost the levels of anti-CoV IgG, and our results showed that patients with underlying disease are older and less vaccinated, indicating that age and underlying disease are factors influencing the symptomatic patients and long total disease course in re-positive patients.
The patients in our cohort were between 4 and 93 years of age, with an average age of 45.73 years, and 113 patients were men (62.7%). The initial onset duration was 12 days, the time between the first COVNAT and re-positive time was 13 days, and the median total disease course was 36 days. It has been reported that re-positivity can occur in any person, but juveniles, women, and patients with mild/moderate existing symptoms have higher rates of re-positivity (Liu et al., 2021). Another study showed that patients younger than 18 years had higher re-positive rates, and no significant differences were observed regarding sex between re-positives and non-re-positives (Yuan et al., 2020). These differences may be explained by the different regions and populations included in the study. Even though pollution is mild in the northeast of China, it has recently been reported that chronic exposure to PM2.5 causes overexpression of the alveolar ACE2 receptor, the entry route of SARS-CoV-2 into the organism, shared by the lungs and testis where expression is highest in the body (Montano et al., 2021). Hence, the influence of air pollution cannot be ignored.
No significant difference in terms of clinical symptoms was found between patients aged >50 years and those aged ≤50 years. However, the proportion of patients with underlying disease were higher, the proportion of patients who were vaccinated, the lowest CoV O gene and N gene Ct values, and the levels of anti-CoV IgG were lower in patients who were older than 50 years, indicating that age, underlying disease, and vaccination are factors that influence the capacity of patients to produce protective antibodies.
Our results showed no significant difference in clinical symptoms between the vaccinated and non-vaccinated groups at either the initial onset period or the re-positive period. However, the lowest CoV O gene Ct values and the levels of anti-CoV IgG were lower in patients who were not vaccinated. The full vaccination rate was 81.1%, and the booster immunization rate was 32.1% in Jilin Province, according to the National Health Commission of China. In the present study, 81.67% of patients had the first vaccination dose, 60.5% of patients were fully vaccinated, and 18.9% of patients had a booster vaccination. Whether current vaccines are effective against the new Omicron variant of COVID-19 has been investigated: effectiveness of the vaccine against SARS-CoV-2 was lower for omicron than that for pre-omicron variants 1 month after vaccine completion, and little protection remained by 6 months after the primary vaccine series (Higdon et al., 2022). Both re-positives and non-re-positives have been reported to have similar patterns of IgG and IgM (Huang et al., 2021). Therefore, large-scale and multicenter studies are recommended to better understand the pathophysiology of the potential recurrence of SARS-CoV-2 in patients with COVID-19.
Our study had several limitations. First, it only included patients who tested re-positive for SARS-CoV-2, and no control group of non-re-positive patients was enrolled. Second, we could not differentiate between re-positive tests that were due to prolonged viral shedding of non-viable viral fragments and those that were true new infections. We should isolate samples of the live virus instead of performing RT-PCR assays to determine the true infectivity of patients for the purpose of continuous disease management.
In conclusion, recurrence of SARS-CoV-2 in patients who have recovered from COVID-19 after discharge from the hospital is common. However, the cause of this re-positivity remains unclear. Symptoms were milder, and the nucleic acid test-positive course was shorter during the re-positive period. The concomitant underlying disease is an important factor associated with clinical symptoms and the overall course of COVID-19 re-positive patients may be associated with lower anti-CoV IgG levels. Large-scale and multicenter studies are recommended to better understand the pathophysiology of the potential recurrence of SARS-CoV-2 in patients with COVID-19.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of the First Hospital of Jilin University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JC and ZW: designed the study. PZ, XY, and JN: interpreted the data. MZ: drafted the manuscript. All authors revised the manuscript critically for important intellectual content and gave final approval of the version to be published.
The authors thank all medical professionals who worked on the frontlines during the COVID-19 pandemic.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.989879/full#supplementary-material
Araf, Y., Akter, F., Tang, Y. D., Fatemi, R., Parvez, M. S. A., Zheng, C., et al. (2022). Omicron variant of sars-cov-2: Genomics, transmissibility, and responses to current covid-19 vaccines. J. Med. Virol. 94, 1825–1832. doi: 10.1002/jmv.27588
Brogna, B., Brogna, C., Petrillo, M., Conte, A. M., Benincasa, G., Montano, L., et al. (2021). Sars-cov-2 detection in fecal sample from a patient with typical findings of covid-19 pneumonia on ct but negative to multiple sars-cov-2 rt-pcr tests on oropharyngeal and nasopharyngeal swab samples. Medicina 57:290. doi: 10.3390/medicina57030290
Brogna, C., Brogna, B., Bisaccia, D. R., Lauritano, F., Marino, G., Montano, L., et al. (2022). Could sars-cov-2 have bacteriophage behavior or induce the activity of other bacteriophages? Vaccines 10:708. doi: 10.3390/vaccines10050708
Brogna, C., Cristoni, S., Petrillo, M., Querci, M., Piazza, O., and Van den Eede, G. (2021). Toxin-like peptides in plasma, urine and faecal samples from covid-19 patients. F1000Res 10:550. doi: 10.12688/f1000research.54306.2
Chen, S. L., Xu, H., Feng, H. Y., Sun, J. F., Li, X., Zhou, L., et al. (2020). Epidemiological and clinical findings of short-term recurrence of severe acute respiratory syndrome coronavirus 2 ribonucleic acid polymerase chain reaction positivity in 1282 discharged coronavirus disease 2019 cases: A multicenter, retrospective, observational study. Open Forum Infect. Dis. 7:ofaa432. doi: 10.1093/ofid/ofaa432
Dinc, H. O., Demirci, M., Ozdemir, Y. E., Sirekbasan, S., Aktas, A. N., Karaali, R., et al. (2022). Anti-sars-cov-2 igg and neutralizing antibody levels in patients with past covid-19 infection: A longitudinal study. Balkan. Med. J. 39, 172–177. doi: 10.4274/balkanmedj.galenos.2022.2021-8-131
Farrukh, L., Mumtaz, A., and Sana, M. K. (2021). How strong is the evidence that it is possible to get sars-cov-2 twice? a systematic review. Rev. Med. Virol. 31, 1–12. doi: 10.1002/rmv.2203
Gao, Y. D., Ding, M., Dong, X., Zhang, J. J., Kursat Azkur, A., Azkur, D., et al. (2021). Risk factors for severe and critically ill covid-19 patients: A review. Allergy 76, 428–455. doi: 10.1111/all.14657
Guan, W. J., Ni, Z. Y., Hu, Y., Liang, W. H., Ou, C. Q., He, J. X., et al. (2020). Clinical characteristics of coronavirus disease 2019 in china. N. Engl. J. Med. 382, 1708–1720. doi: 10.1056/NEJMoa2002032
Higdon, M. M., Baidya, A., Walter, K. K., Patel, M. K., Issa, H., Espie, E., et al. (2022). Duration of effectiveness of vaccination against covid-19 caused by the omicron variant. Lancet Infect. Dis. 22, 1114–1116. doi: 10.1016/S1473-3099(22)00409-1
Hoang, T. (2021). Characteristics of covid-19 recurrence: A systematic review and meta-analysis. Ann. Glob. Health 87:28. doi: 10.5334/aogh.3163
Huang, K., Liu, W., Zhou, J., Wang, Y., Zhang, Y., Tang, X., et al. (2021). Repositive rt-pcr test in discharged covid-19 patients during medical isolation observation. Int. J. Med. Sci. 18, 2545–2550. doi: 10.7150/ijms.58766
Li, S., Wang, X., Li, L., Pan, Y., Yang, S., Tan, D., et al. (2022). Factors associated with sars-cov-2 repeat positivity - beijing, china, june-september 2020. China CDC Wkly 4, 88–95. doi: 10.46234/ccdcw2022.017
Li, Y., Ji, D., Cai, W., Hu, Y., Bai, Y., Wu, J., et al. (2021). Clinical characteristics, cause analysis and infectivity of covid-19 nucleic acid repositive patients: A literature review. J. Med. Virol. 93, 1288–1295. doi: 10.1002/jmv.26491
Liang, L., Guo, Q., Zhang, H., Lin, S., Zheng, H., Li, B., et al. (2021). Low infectious risk of re-positive covid-19 patients: A single-center study. Int. J. Infect. Dis. 111, 5–9. doi: 10.1016/j.ijid.2021.08.019
Liu, H. Q., Yuan, B., An, Y. W., Chen, K. J., Hu, Q., Hu, X. P., et al. (2021). Clinical characteristics and follow-up analysis of 324 discharged covid-19 patients in shenzhen during the recovery period. Int. J. Med. Sci. 18, 347–355. doi: 10.7150/ijms.50873
Lu, J., Peng, J., Xiong, Q., Liu, Z., Lin, H., Tan, X., et al. (2020). Clinical, immunological and virological characterization of covid-19 patients that test re-positive for sars-cov-2 by rt-pcr. EBio Med. 59:102960. doi: 10.1016/j.ebiom.2020.102960
Mattiuzzi, C., Henry, B. M., Sanchis-Gomar, F., and Lippi, G. S. A. R. S. - (2020). Cov-2 recurrent rna positivity after recovering from coronavirus disease 2019 (covid-19): A meta-analysis. Acta Biomed. 91:e2020014. doi: 10.23750/abm.v91i3.10303
Miyamae, Y., Hayashi, T., Yonezawa, H., Fujihara, J., Matsumoto, Y., Ito, T., et al. (2020). Duration of viral shedding in asymptomatic or mild cases of novel coronavirus disease 2019 (covid-19) from a cruise ship: A single-hospital experience in tokyo, japan. Int. J. Infect. Dis. 97, 293–295. doi: 10.1016/j.ijid.2020.06.020
Montano, L., Donato, F., Bianco, P. M., Lettieri, G., Guglielmino, A., Motta, O., et al. (2021). Air pollution and covid-19: A possible dangerous synergy for male fertility. Int. J. Environ. Res. Public Health 18:6846. doi: 10.3390/ijerph18136846
National Health Commission & National Administration of Traditional Chinese Medicine (2022). Diagnosis and treatment protocol for COVID-19 patients (Trial Version 9). Health care sci. doi: 10.1002/hcs2.1
Simon, V., van Bakel, H., and Sordillo, E. M. (2020). Positive, again! what to make of “re-positive” sars-cov-2 molecular test results. EBio Med. 60:103011. doi: 10.1016/j.ebiom.2020.103011
Vancsa, S., Dembrovszky, F., Farkas, N., Szako, L., Teutsch, B., Bunduc, S., et al. (2021). Repeated sars-cov-2 positivity: Analysis of 123 cases. Viruses 13:512. doi: 10.3390/v13030512
Wong, C. L., Lei, S. K., Lei, C. I., Lo, I. L., Lam, C., and Leong, I. H. (2021). Re-positive of sars-cov-2 test is common in covid-19 patients after hospital discharge. data from high standard post-discharge quarantined patients in macao sar, china. PeerJ. 9:e11170. doi: 10.7717/peerj.11170
Wu, J., Xia, X. Y., Liu, H. L., Xia, H., Huang, W. X., Jia, B., et al. (2020). Clinical characteristics and outcomes of discharged covid-19 patients with reoccurrence of sars-cov-2 rna. Future Virol. 15, 663–671. doi: 10.2217/fvl-2020-0142
Yeoh, Y. K., Zuo, T., Lui, G. C., Zhang, F., Liu, Q., Li, A. Y., et al. (2021). Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with covid-19. Gut 70, 698–706. doi: 10.1136/gutjnl-2020-323020
Yuan, B., Liu, H. Q., Yang, Z. R., Chen, Y. X., Liu, Z. Y., Zhang, K., et al. (2020). Recurrence of positive sars-cov-2 viral rna in recovered covid-19 patients during medical isolation observation. Sci. Rep. 10:11887. doi: 10.1038/s41598-020-68782-w
Keywords: COVID-19, re-positive, Northeast China, anti-CoV IgG, underlying disease
Citation: Li H, Zhu M, Zhang P, Yan X, Niu J, Wang Z and Cao J (2022) Milder symptoms and shorter course in patients with re-positive COVID-19: A cohort of 180 patients from Northeast China. Front. Microbiol. 13:989879. doi: 10.3389/fmicb.2022.989879
Received: 09 July 2022; Accepted: 07 September 2022;
Published: 11 October 2022.
Edited by:
Leland Shapiro, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Guerrino Macori, University College Dublin, IrelandCopyright © 2022 Li, Zhu, Zhang, Yan, Niu, Wang and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenyu Wang, emhlbnl1QGpsdS5lZHUuY24=; Jie Cao, Y2ppZUBqbHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.