- 1Key Laboratory of Integrated Oncology and Intelligent Medicine of Zhejiang Province, Department of Hepatobiliary and Pancreatic Surgery, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Zhejiang University School of Medicine, Hangzhou, China
- 3Westlake Laboratory of Life Sciences and Biomedicine, Hangzhou, China
- 4NHC Key Laboratory of Combined Multi-Organ Transplantation, Hangzhou, China
- 5Institute of Organ Transplantation, Zhejiang University, Hangzhou, China
Viral hepatitis is a major global public health problem that affects hundreds of millions of people and is associated with significant morbidity and mortality. Five biologically unrelated hepatotropic viruses account for the majority of the global burden of viral hepatitis, including hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV), and hepatitis E virus (HEV). Omics is defined as the comprehensive study of the functions, relationships and roles of various types of molecules in biological cells. The multi-omics analysis has been proposed and considered key to advancing clinical precision medicine, mainly including genomics, transcriptomics and proteomics, metabolomics. Overall, the applications of multi-omics can show the origin of hepatitis viruses, explore the diagnostic and prognostics biomarkers and screen out the therapeutic targets for viral hepatitis and related diseases. To better understand the pathogenesis of viral hepatitis and related diseases, comprehensive multi-omics analysis has been widely carried out. This review mainly summarizes the applications of multi-omics in different types of viral hepatitis and related diseases, aiming to provide new insight into these diseases.
Introduction
Viral hepatitis is a major global public health problem that affects hundreds of millions of people and is associated with significant morbidity and mortality. Five biologically unrelated hepatotropic viruses account for the majority of the global burden of viral hepatitis, including hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV), and hepatitis E virus (HEV). Despite that HAV does not develop into a chronic infection, HBV, HCV, HDV and occasionally HEV may cause chronic infections, of which HBV and HCV have a significant association with chronic incidence. Most deaths from viral hepatitis are due to HBV and HCV infections. According to the statistics, it was estimated that 296 million people were infected with hepatitis B, 58 million people were infected with hepatitis C and 1.1 million people died as a consequence of viral hepatitis infections in 2019 (Tanaka et al., 2022). In 2015, the United Nations adopted a resolution to combat viral hepatitis as part of the agenda to achieve the 2030 sustainable development goals. Subsequently, the first global strategy was developed in 2016 for the elimination of viral hepatitis (Vo Quang et al., 2021).
The advances in technology have created a variety of new fields of study, commonly referred to as omics. Omics is defined as the comprehensive study of the functions, relationships and roles of various types of molecules in biological cells. The multi-omics analysis has been proposed and considered key to advancing clinical precision medicine, including genomics, transcriptomics and proteomics, metabolomics and so on (Olivier et al., 2019). Genomics focuses on genomic DNA, the genome is usually divided into small fragments and then iteratively assembled by bioinformatics algorithms, along with gene annotation and other data analysis (Low et al., 2019). Transcriptomics can be performed to study the sum of mRNAs at a certain time point, and can also use known gene probe for specific genes (Kalisky et al., 2018). Besides, proteomics is oriented to the whole protein based on 2D-Gel and mass spectrometry. It is divided into top–down and bottom–up analysis methods. Similar to the genomics, the protein is decomposed into small peptide segments, and the known and unknown protein sequences are thus identified (Aslam et al., 2017). Through the liquid phase and mass spectrometry, metabolomics can analyze a mixture of metabolites, such as macromolecules and small molecules (Li B. et al., 2017). Technological innovation has promoted the awareness of several diseases (Wu et al., 2015, 2017a,b; Crooke et al., 2021). In general, the applications of multi-omics have provided new insights into the diagnosis, prognosis and treatment of these diseases.
To better understand the pathogenesis of viral hepatitis and related diseases, comprehensive multi-omics analysis has been widely carried out. Hence, this review summarizes the applications of multi-omics in different types of viral hepatitis and related diseases, aiming to throw light on the development of these diseases.
Multi-omics in hepatitis A and related diseases
Hepatitis A virus is a positive-strand RNA virus, which is transmitted through the fecal oral route. HAV outbreaks are often associated with poor sanitation, overcrowding or contamination of food and water (Abutaleb and Kottilil, 2020). HAV infections in children are usually asymptomatic, but adults will present symptoms with jaundice, abdominal pain and hyperbilirubinemia.
Using genomics, Wassenaar et al. (2020) compared the intraspecific genome diversity of the single-stranded RNA(+) viruses of HAV, HCV, and HEV, and they found that these viruses all can cause hepatitis, but have no genetic similarity. Heydari et al. (2021) performed whole-genome sequencing of two patients with acute hepatitis A and plotted an HAV genome-wide phylogenetic tree. Whole-genome sequencing can clearly reveal HAV sequence. After sequence alignment, the researchers can explore its origin and spread history.
To better understand the biogenesis of quasi-enveloped HAV (eHAV) virions, McKnight et al. (2017) used proteomics quantitative analysis to successfully identify surface markers for eHAV vesicles and supported exosome-like mechanisms of eHAV outflow. In the study of duck HAV genotype 3 (DHAV-3), Liang et al. (2020) collected DHAV-3-infected duck livers for proteomic analysis, and they found that type I interferon plays an extremely important role in the pathogenic mechanism of DHAV-3. Similarly, by proteomics, DHAV-1 infection was considered to cause endoplasmic reticulum stress-induced duck embryo fibroblast cell autophagy, and proteins involved in the DHAV-1 infection process or endoplasmic reticulum stress-induced autophagy process were successfully identified (Lan et al., 2019). Infection process of HAV in the hosts associated with specific proteins will be shown using proteomics.
Kanda et al. (2015) demonstrated that epigenetic control is involved in HAV internal ribosomal entry site-dependent translation and HAV replication. It was suggested that in the clinical application of epigenetic therapy for malignant tumors, special attention should also be paid to the underlying viral disease (Kanda et al., 2015).
Multi-omics in hepatitis B and related diseases
Hepatitis B virus is the most common cause of acute and chronic liver diseases worldwide, and approximately 4 million people are infected with HBV every year, especially in Asia and Africa (Khan A. et al., 2021). About 10% of patients infected with HBV will develop chronic infections, including liver fibrosis and cirrhosis. Each year, about one million people die from hepatitis B-related chronic liver diseases (Lu et al., 2018; Asrani et al., 2019). Most chronic hepatitis B (CHB) patients show no obvious symptoms, but as the disease progresses, they eventually develop liver cirrhosis and hepatocellular carcinoma (HCC) (Khan A. A. et al., 2021; Su et al., 2022). The applications of multi-omics can show the molecular and functional maps of HBV and related diseases.
Genomics
By comparing the genomes of ancient African strains and HBV, Guzmán-Solís et al. (2021) found a high degree of similarity between the two viruses, which suggested that HBV may originate on the African continent and was transported to America during the transatlantic slave trade and subsequently introduced to New Spain. Next-generation sequencing methods are used to sequence concurrently, enabling us to detect any pre-existing mutations before antiviral therapy. Hence, drug resistance mutations were detected in CHB patients receiving nucleos(t)ide analog therapy using genomics (Widasari et al., 2014). Genome comparisons can enrich the discussion of HBV origin and transmission. Besides, genomics also allows us to understand HBV genotypes, quasi-species, splicing, defective HBV, virus evolution within a single host and so on.
As the DNA virus, HBV is different from other RNA hepatitis viruses since its viral genome can be integrated into the host liver cell genome. HBV integration is considered to lead to the occurrence of HCC, and the study of its structure is of great significance to the occurrence and development of HBV-related HCC. Ramirez et al. (2021) successfully fabricated a panel of HBV-targeting biotinylated oligonucleotide probes, and they described the structure and transcriptional signatures of integrated HBV in different HCC cell lines. At the junctions between chromosomes, five chromosomal translocations integrating HBV DNA were found, and many integrations and translocations were transcriptionally silent, which further revealed the possible mediating mechanism of HBV-related HCC (Ramirez et al., 2021). To clearly describe the structure of HBV integration, Péneau et al. (2022) found that clonal selection for HBV integration may be associated with two mechanisms that lead to HCC through long-read sequencing or Bionano whole genome mapping. The first possible mechanism is that the integration of viral enhancers near the cancer driver gene may lead to overexpression of the oncogene, and the second possible mechanism is that frequent chromosomal rearrangements at the HBV integration site can cause changes in the distance of the cancer driver gene. Therefore, HBV integration is thought to have the ability to predict HBV-associated patients with HCC and has a certain clinical value (Péneau et al., 2022). In addition, the structure of HBV isolated from HCC patients was also determined, and it was found that HBV immune escape mutants may be an important factor in the occurrence and development of HCC (Lin et al., 2002). The applications of genomics in HBV-related diseases mainly focus on HBV-induced HCC. Gene expression profiling can facilitate the discovery of diagnostic and prognostic markers for HBV-related HCC.
Advances in genomics have deepened the understanding of the diagnosis, prognosis of HBV-related HCC. The detailed genetic analysis of liver tissue provides important information for tumorigenesis and progression (Dhanasekaran et al., 2019; Wu Y. et al., 2020; Wei et al., 2022). The findings of genomics research may promote the progress of individualized management of HCC, thereby innovating therapeutic methods.
Proteomics
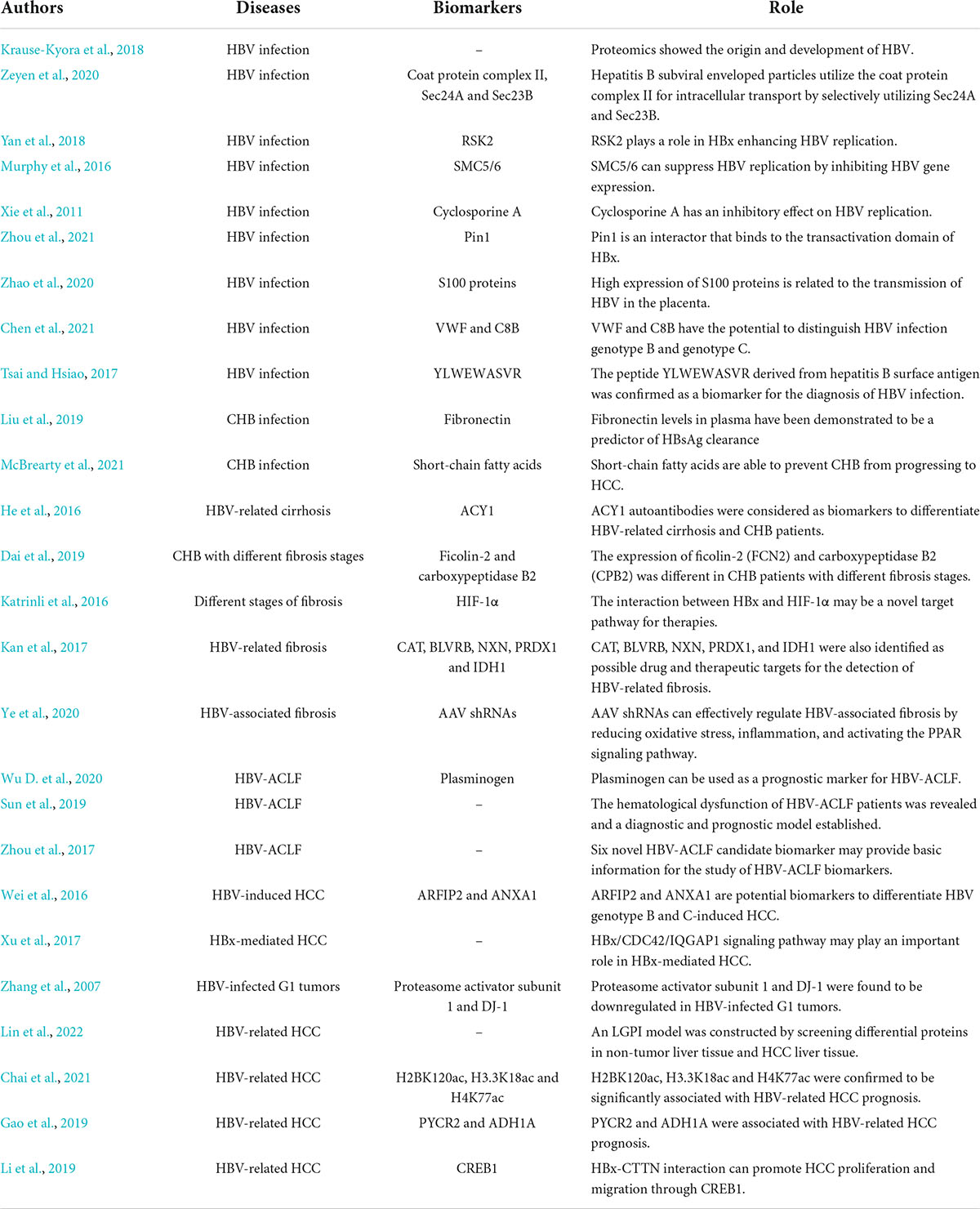
The applications of proteomics are of great significance in hepatitis B and related diseases (Table 1). Proteomics can help reveal the origin and development of HBV. Krause-Kyora et al. (2018) showed that HBV has been circulating in European populations for over 7,000 years through proteomics.
The applications of proteomics provide new strategies for the occurrence, progression and replication of HBV. Based on yeast proteomics, Zeyen et al. (2020) found that hepatitis B subviral enveloped particles utilize the coat protein complex II component for intracellular transport by selectively utilizing Sec24A and Sec23B. Based on isobaric tags for relative and absolute quantitation (iTRAQ) quantitative comparative proteomics, RSK2 was identified as a novel host protein that plays a role in HBx enhancing HBV replication (Yan et al., 2018). Using the substrate capture proteomics, Murphy et al. (2016) showed that the main function of HBx is to degrade SMC5/6, which can suppress HBV replication by inhibiting HBV gene expression. Xie et al. (2011) found that HBx has a promoting effect on HBV replication, while they confirmed that cyclosporine A has an inhibitory effect on HBV replication. Pin1 is considered to be an interactor that binds to the transactivation domain of HBx, suggesting the potential relationship between Pin1 and the function of HBx in HBV replication (Zhou et al., 2021). In addition, through iTRAQ proteomic analysis, Zhao et al. (2020) found that the high expression of S100 proteins is related to the transmission of HBV in the placenta, which provides new insight into the mother-to-infant transmission of HBV.
In addition to the traditional HBV markers HBsAg and anti-HBs, the applications of proteomics have also expanded the development of diagnostic markers. Two differential proteins, VWF and C8B were considered to have the potential to distinguish HBV infection genotypes B and C and could provide precise guidance for HBV genotyping (Chen et al., 2021). The peptide YLWEWASVR derived from the hepatitis B surface antigen was confirmed as a biomarker for the diagnosis of hepatitis B virus infection (Tsai and Hsiao, 2017). Moreover, the use of proteomics/genomics databased in the identification of the HBV receptor in 2012, which is considered as one of the most important discoveries related to HBV in the last decade (Yan et al., 2012).
In CHB patients, fibronectin levels in plasma have been demonstrated to be a predictor of HBsAg clearance (Liu et al., 2019). Long-term HBV infection has been shown to lead to cellular proteome remodeling, which can mediate the pathological effect (Zai et al., 2022). Through the mass spectrometry-based proteomic analysis, McBrearty et al. (2021) found that short-chain fatty acids can prevent CHB from progressing to HCC.
In recent years, the applications of proteomics in HBV-related cirrhosis have also been reported. Autoantibodies recognized aminoacylase-1 (ACY1) were considered biomarkers to differentiate HBV-related cirrhosis and CHB patients by serum proteomic detection (He et al., 2016). Proteomics also provides new ideas for the diagnosis, prognosis and treatment of HBV-induced fibrosis. Through serum proteomics analysis, Dai et al. found that the expression of ficolin-2 (FCN2) and carboxypeptidase B2 (CPB2) was different in CHB patients with different fibrosis stages, indicating the diagnostic value of FCN2 and CPB2 (Dai et al., 2019). At different stages of fibrosis, Katrinli et al. (2016) observed changes in the glycolytic pathway caused by the presence of HBx, so the interaction between it and HIF-1α may be a novel target pathway for therapies. Kan et al. (2017) screened 28 HBV-specific proteins by comprehensive proteomics and transcriptomics, and they emphasized the critical role of oxidative stress in HBV-related liver fibrosis. Catalase (CAT), Biliverdin Reductase B (BLVRB), Nucleoredoxin (NXN), Peroxiredoxin 1 (PRDX1), and Isocitrate Dehydrogenase [NADP(+)] 1 (IDH1) were also identified as possible drug and therapeutic targets for the detection of HBV-related fibrosis. AAV shRNAs were found to effectively regulate HBV-associated fibrosis by reducing oxidative stress, inflammation, and activating the PPAR signaling pathway (Ye et al., 2020).
Proteomics also plays an important role in HBV-related liver failure. Wu D. et al. (2020) used TMT-labeled quantitative proteomics to find that plasminogen can be used as a prognostic marker for HBV-ACLF. Sun et al. (2019) revealed the hematological dysfunction of HBV-ACLF patients through targeted proteomics and established a diagnostic and prognostic model. Quantitative proteomics analyses have identified six novel HBV-ACLF candidate biomarkers, which may provide basic information for the study of HBV-ACLF biomarkers (Zhou et al., 2017).
As HBV is a risk factor for the development of HCC, the applications of HBV-related HCC proteomics have also been paid more and more attention. Wei et al. (2016) concluded that ARFIP2 and ANXA1 are potential biomarkers to differentiate HBV genotype B and C-induced HCC through quantitative proteomic analysis. With the altered protein expression during the progression of HBV-related HCC, some proteins can be considered potential biomarkers for diagnosis and therapy (Jiang et al., 2019). Through the quantitative proteomics, Xu et al. (2017) found that the HBx/CDC42/IQGAP1 signaling pathway may play an important role in HBx-mediated HCC. Similarly, proteasome activator subunit 1 and DJ-1 were found to be downregulated in HBV-infected G1 tumors, revealing their possible mediating mechanisms (Zhang et al., 2007). Lin et al. (2022) constructed an LGPI model by screening differential proteins in non-tumor liver tissue and HCC liver tissue, predicting the overall survival and prognosis of patients with HBV-related HCC. By quantitative proteomics, H2BK120ac, H3.3K18ac and H4K77ac were confirmed to be significantly associated with HBV-related HCC prognosis (Chai et al., 2021). Gao et al. (2019) screened out two HCC metabolic reprogram prognostic markers associated with HBV-related HCC, PYCR2, and ADH1A. HBx-CTTN interaction can promote HCC proliferation and migration through CREB1, and the HBx/CTTN/CREB1 axis was considered a potential new therapeutic target for HCC (Li et al., 2019).
Metabolomics
Through the differential metabolomic analysis, Luteolin-7-O-glucoside was confirmed to inhibit HBsAg and HBV replication through mechanisms involving mitochondria (Cui et al., 2017). Yu et al. (2022) focused on metabolic changes during HBV replication and infection, and they found that the high levels of amino acids depletion and phosphatidylcholines and lysophosphatidylcholines biosynthesis play important roles in the pathogenesis of HBV infection. Through the metabolomic analysis, Hu et al. (2021) found that HBP-mediated O-GlcNAcylation can positively regulate the host’s antiviral response to HBV.
Combined with serum-targeted metabolomics, the metabolic signature of CHB infection progression was further revealed (Schoeman et al., 2016). By analyzing metabolomics data at different stages in patients with CHB, Nguyen et al. (2021) found that ammonia detoxification, glutamine and glutamate metabolism, methionine metabolism, branched-chain amino acid imbalance, and disorders of the tricarboxylic acid cycle are influencing factors in the progression of patients with CHB. Combined with the gut microbiome and metabolome, Sun et al. (2021) provided new insights into bile acid metabolic pathways in patients with CHB.
Through serum metabolomics, Nie et al. (2014) found that 17 metabolites were associated with the prognosis of HBV-related acute-on-chronic liver failure (HBV-ACLF), providing information for markers for the diagnosis and prognosis of HBV-ACLF. Lian et al. (2016) analyzed serum samples from HBV-ACLF, HBV-related chronic liver failure (HBV-CLF) and healthy populations, and they found that phosphatidylcholines, lysophosphatidylcholines and conjugated bile acids (GCDCA, GUDCA) metabolites may act as markers for ACLF and CLF diagnosis and provide new insights into the pathogenesis of ACLF and CLF.
Through metabolomics, amino acid imbalance metabolism was thought to play an important role in the development and progression of HBV-related HCC (Huang et al., 2020). Compared with HBV-infected patients, HBV-related HCC patients have lower levels of metabolite lysophosphatidylcholines in their blood, which may serve as a clinical diagnostic marker for HCC (Li et al., 2021). Through serum metabolomics, Cai et al. (2020) discovered enzymes associated with HBV-related HCC diagnosis and prognosis. Through the genetic screening, combined with RNA-seq and metabolomic analysis, Chen et al. (2022) found the joint effect of PSTK as a resistance medium for targeted therapy of HCC cells, suggesting an ideal treatment method for HBV-related HCC.
Transcriptomics
Transcriptomics functions in the study of HBV and related diseases. Using RNA-seq transcriptomics, König et al. (2019) demonstrated that HBV does not induce significant gene expression changes in HepG2-NTCPsec+ and that HepG2-NTCPsec + cells support a net amplification of the HBV genome, leading to the development of a new model of HBV infection. Hou et al. (2017) conducted an in-depth transfer group analysis of formalin-fixed paraffin-embedding liver biopsy in the clinical stage. They found that viral load and liver injury are associated with the fluctuations that coincided with those of the liver transcriptome (Hou et al., 2017). Using transcriptomic and proteomic methods, the RIG-I-like receptor signaling pathway was confirmed to be the main signaling pathway for changes in HBV-related fibrosis (Kan et al., 2017). In addition, Li et al. (2022) found that HBV exacerbation-induced immune dysregulation disorder is the underlying mechanism identified in HBV-ACLF through mRNA sequencing of peripheral blood mononuclear cells in patients.
Multi-omics in hepatitis C and related diseases
Hepatitis C virus virions are spherical and are single-stranded positive-stranded RNA viruses. HCV can often cause hepatitis C infection. According to estimates, approximately 71 million people worldwide suffer from chronic hepatitis C virus (CHC) infection (Rabaan et al., 2020). CHC infection is associated with advanced liver disease and can induce hepatocellular carcinoma, which will cause many extrahepatic manifestations. The applications of multi-omics also help researchers understand HCV and related diseases deeply.
Genomics
Through functional genomics, Li et al. (2016) found that E-cadherin is a mediator of HCV entry into host cells and is closely related to HCV-induced epithelial-mesenchymal transition. Takagi et al. (2021) revealed the sequence of HCV-G4-KM long clones by sequencing the HCV-G4-KM long clones in mouse serum, and they proved that the sequence of HCV-G4-KM long NAs plays an important role in infectious cloning. By combining proteomics and genomics, Ramage et al. (2015) demonstrated the role of HCV between the infectious process and the host, and they explored the mechanism by which HCV affects the function of the infected host. In addition, functional genomics has been used to explore the interaction between HCV and miRNA and also demonstrated the HCV-mediated pathogenesis (Li Q. et al., 2017). Genomics can reveal the process of HCV entering and infecting host cells.
Proteomics
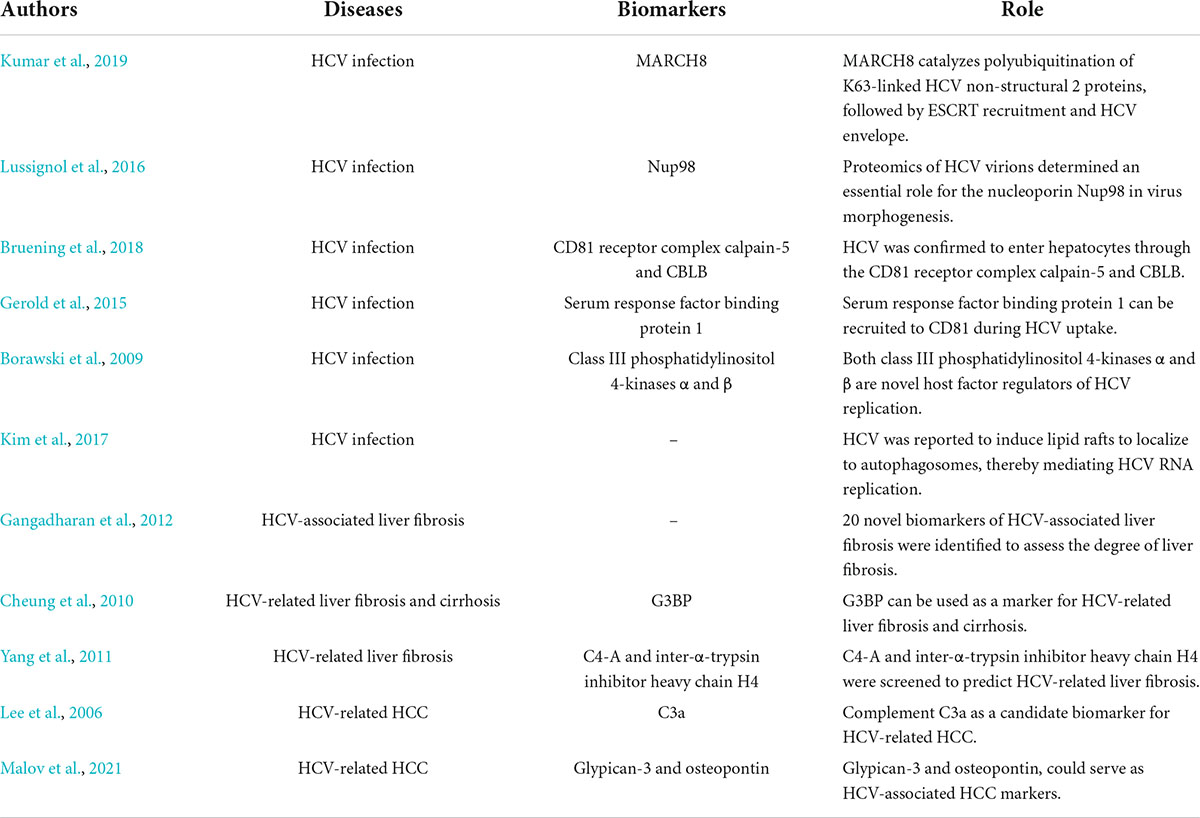
Proteomics matters in hepatitis C and related diseases (Table 2). Proteomics can show the process of HCV assembly, host cell entry and replication. Kumar et al. (2019) reported that MARCH8 catalyzes polyubiquitination of K63-linked HCV non-structural 2 proteins, followed by ESCRT recruitment and HCV envelope. Proteomics of HCV virions determined an essential role for the nucleoporin Nup98 in virus morphogenesis (Lussignol et al., 2016). HCV was confirmed to enter hepatocytes through the CD81 receptor complex calpain-5 and CBLB (Bruening et al., 2018). Gerold et al. (2015) identified serum response factor binding protein 1 by quantitative proteomics, which can be recruited to CD81 during HCV uptake and support HCV infection in HCC cells and primary human hepatocytes. Borawski et al. (2009) demonstrated that both class III phosphatidylinositol 4-kinases α and β are novel host factor regulators of HCV replication. In addition, HCV was reported to induce lipid rafts to localize to autophagosomes, thereby mediating HCV RNA replication (Kim et al., 2017).
Gangadharan et al. (2012) identified 20 novel biomarkers of HCV-associated liver fibrosis to assess the degree of liver fibrosis. Cheung et al. (2010) found that G3BP can be used as a marker for HCV-related liver fibrosis and cirrhosis. Through the serum proteomics, C4-A and inter-α-trypsin inhibitor heavy chain H4 were screened to predict HCV-related liver fibrosis (Yang et al., 2011). The development of biomarkers for HCV-associated fibrosis and cirrhosis were also explored using genomics.
Diagnostic and prognostic biomarkers of HCV-related HCC can be discovered by proteomics. Lee et al. (2006) identified complement C3a as a candidate biomarker for HCV-related HCC by proteomics. Malov et al. (2021) confirmed that heparin binds to growth factors, glypican-3 and osteopontin could serve as HCV-associated HCC markers. The screening of specific proteins is conducive to accurate diagnosis and prognosis in patients with HCV infection and related diseases.
Metabolomics
Through the comprehensive metabolomics analysis, Fitian et al. (2014) identified overall metabolic disorders in patients with HCV-associated HCC and cirrhosis, and they hypothesized that abnormal dicarboxylic acid metabolism, enhanced bile acid metabolism, and elevated fibrinogen-cleaved peptides might be the signs of liver cirrhosis. Shanmuganathan et al. (2021) compared the ability of multisegment injection-capillary electrophoresis-mass spectrometry and nuclear magnetic resonance to characterize the serum metabolome, and they found that both instrumental techniques can quickly and reliably quantify serum metabolites in large-scale metabolomics research with the good overlap of biomarker replication. Therefore, metabolomics can show the metabolic process of HCV infection and its associated diseases.
Transcriptomics
Through the genome-wide miRNA functional screening and transcriptomic analysis, Sodroski et al. (2019) generated a comprehensive map of HCV-miRNA interactions. They found that inhibition of key host restriction factors mediates the proviral effects of miR-135a on HCV transmission (Sodroski et al., 2019). Through the comprehensive functional genomics analysis, miR-25, let-7, and miR-130 families were also proved to inhibit the necessary HCV cofactors, thus limiting HCV infection in multiple stages (Li Q. et al., 2017). Hence, cellular microRNAs have been shown to regulate HCV infection by acting directly on the viral genome or indirectly on virus-associated host factors.
Multi-omics in hepatitis D and related diseases
Hepatitis D virus is only found in humans currently. It is a satellite virus, which is assembled, released and entered by the envelope protein of HBV. It is the smallest known RNA virus, encoding a single protein.
Using meta-transcriptomics, Wille et al. (2018) identified the genome of a novel HDV in duck. Sequence analysis showed that HDVs share a common secondary structure. The predicted viral protein shares a 32% amino acid similarity with the small delta antigen of HDV, which contains a distinct phylogenetic lineage. The discovery of avian influenza virus-like pathogens helps us better understand the origin of HDV and subviral pathogens (Wille et al., 2018). Taking meta-transcriptomic data, Chang et al. (2019) found that highly differentiated HDV-like viruses also exist in fish, amphibians and invertebrates. None of these novel HDV-like infections is associated with other hepatitis virus infections, supporting the idea that the HDV-HBV association may be unique to humans (Chang et al., 2019). To summary, transcriptomics can reveal the diversity and host range of HDV, and also indicate the origin and evolutionary history of HDV.
Multi-omics in hepatitis E and related diseases
Hepatitis E virus (HEV) is an important zoonotic virus that can infect various hosts. It has 7 main genotypes. Patients with HEV infection are mostly asymptomatic, some patients will present jaundice and symptoms of acute hepatitis (Desai, 2020). Besides, HEV infection can also cause many extrahepatic manifestations (European Association for the Study of the Liver, 2018; Wu et al., 2021).
The study by Shen et al. (2014) employed a comparative gel proteomics approach to investigate the changes in A549 cell proteins following in vitro HEV exposure, which was beneficial for the study of the interaction between HEV and host cells. Three different strains of porcine HEV were identified by Rogée et al. (2015). They revealed the process by which HEV damages cells, providing important evidence for the replication factors and related pathogenesis of HEV (Rogée et al., 2015). Through serum metabolomics, it was demonstrated that dynamic changes in serum metabolites were associated with AHE infection and severity (Wu et al., 2022b). Through the meta-transcriptomic, Zhang et al. (2019) determined the HEV virus subtypes in broilers and further proved by the phylogenetic analysis that the avian HEV identified in the study is a novel subtype of genotype 3 avian HEV. Thus, transcriptomics provides complete genomic data on the evolutionary relationships of avian HEV, which helps us further understand the evolution of HEV. Besides, Wu et al. (2022a) performed the 16S ribosomal ribonucleic acid gene sequencing, and they found that gut microbiota dysbiosis is associated with plasma levels of Interferon-γ and viral load in patients with acute hepatitis E infection. Overall, the investigation on HEV infection and related diseases using multi-omics are less, which requires more efforts.
Conclusion and perspectives
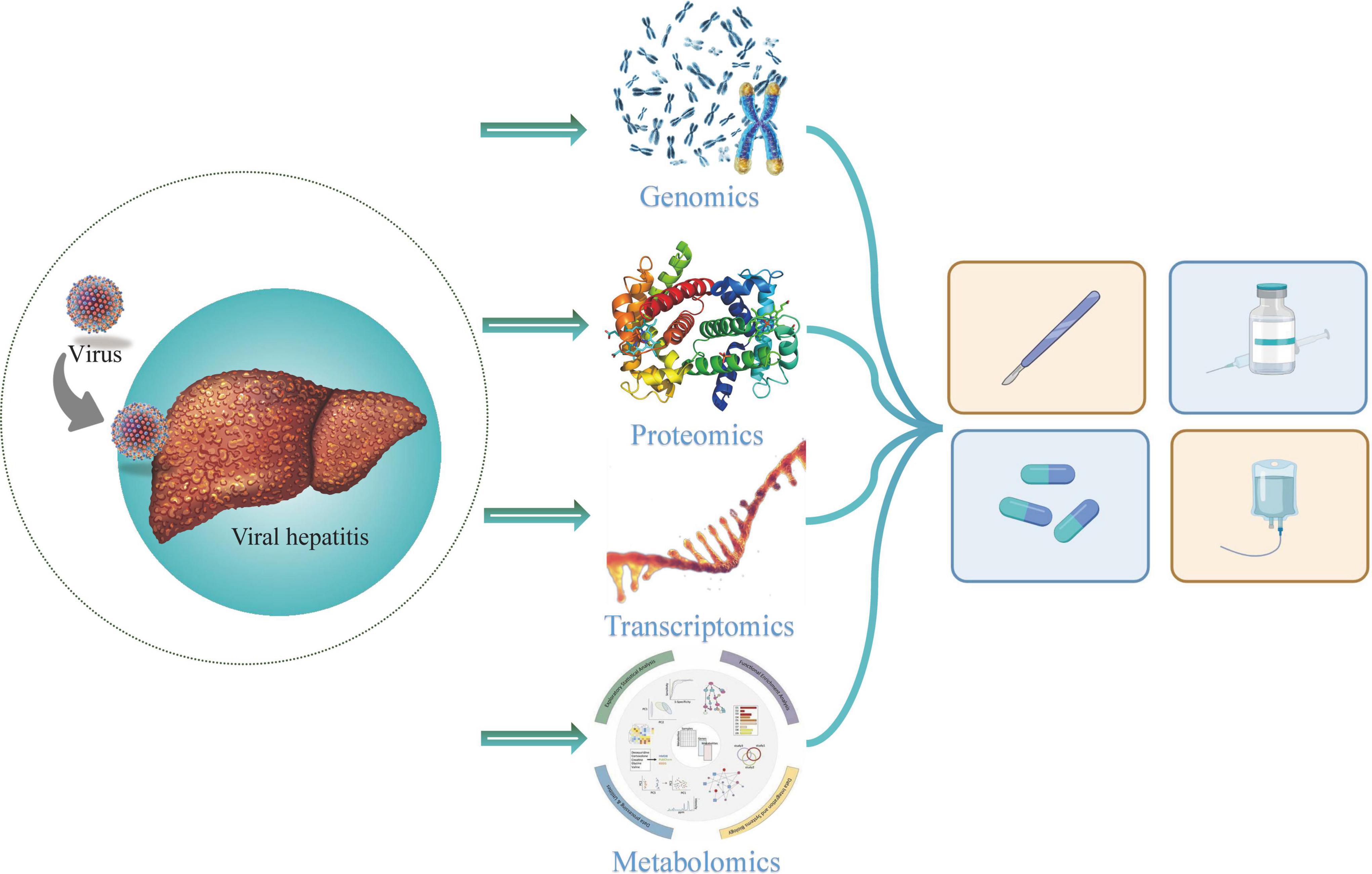
In conclusion, the applications of multi-omics have shown the origin and development of the hepatitis virus and provided new strategies for the diagnosis, prognosis and treatment of viral hepatitis and related diseases (Figure 1). There are many multi-omics studies on HBV infection, HCV infection and related diseases, several biomarkers were found and more correlations were revealed. Nevertheless, the accuracy of these screened biomarkers for the diagnosis and prognosis in patients still needs to be discussed. Moreover, multi-omics studies on other hepatitis are inadequate, and more efforts should be made.
Besides, multi-omics applications are not limited to genomics, proteomics, metabolomics and transcriptomics, and other omics are also developing, including radiomics, viromics, and so on. The joint application of these omics is believed to provide new insight into viral hepatitis and related diseases.
Author contributions
ZX and JL had the idea for the manuscript. ZX and DL performed the literature search and data analysis. XX and XW drafted and critically revised the work. All authors contributed to the article and approved the submitted version.
Funding
This study was support from the National Key Research and Development Program of China (No. 2021YFA1100500), the Major Research Plan of the National Natural Science Foundation of China (No. 92159202), the National Natural Science Foundation of China (No. 81930016), the Key Research & Development Plan of Zhejiang Province (No. 2019C03050), the Construction Fund of Key Medical Disciplines of Hangzhou (OO20200093), and the Young Program of National Natural Science Funds (No. 82000617).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abutaleb, A., and Kottilil, S. (2020). Hepatitis A: Epidemiology, natural history, unusual clinical manifestations, and prevention. Gastroenterol. Clin. 49, 191–199. doi: 10.1016/j.gtc.2020.01.002
Aslam, B., Basit, M., Nisar, M. A., Khurshid, M., and Rasool, M. H. (2017). Proteomics: Technologies and their applications. J. Chromatographic Sci. 55, 182–196. doi: 10.1093/chromsci/bmw167
Asrani, S. K., Devarbhavi, H., Eaton, J., and Kamath, P. S. (2019). Burden of liver diseases in the world. J. Hepatol. 70, 151–171. doi: 10.1016/j.jhep.2018.09.014
Borawski, J., Troke, P., Puyang, X., Gibaja, V., Zhao, S., Mickanin, C., et al. (2009). Class III phosphatidylinositol 4-kinase alpha and beta are novel host factor regulators of hepatitis C virus replication. J. Virol. 83, 10058–10074. doi: 10.1128/JVI.02418-08
Bruening, J., Lasswitz, L., Banse, P., Kahl, S., Marinach, C., Vondran, F. W., et al. (2018). Hepatitis C virus enters liver cells using the CD81 receptor complex proteins calpain-5 and CBLB. PLoS Pathogens 14:e1007111. doi: 10.1371/journal.ppat.1007111
Cai, F.-F., Song, Y.-N., Lu, Y.-Y., Zhang, Y., Hu, Y.-Y., and Su, S.-B. (2020). Analysis of plasma metabolic profile, characteristics and enzymes in the progression from chronic hepatitis B to hepatocellular carcinoma. Aging 12:14949. doi: 10.18632/aging.103554
Chai, X., Guo, J., Dong, R., Yang, X., Deng, C., Wei, C., et al. (2021). Quantitative acetylome analysis reveals histone modifications that may predict prognosis in hepatitis B-related hepatocellular carcinoma. Clin. Trans. Med. 11:e313. doi: 10.1002/ctm2.313
Chang, W.-S., Pettersson, J. H., Le Lay, C., Shi, M., Lo, N., Wille, M., et al. (2019). Novel hepatitis D-like agents in vertebrates and invertebrates. Virus Evol. 5:vez021. doi: 10.1093/ve/vez021
Chen, Y., Li, L., Lan, J., Cui, Y., Rao, X., Zhao, J., et al. (2022). CRISPR screens uncover protective effect of PSTK as a regulator of chemotherapy-induced ferroptosis in hepatocellular carcinoma. Mol. Cancer 21:11. doi: 10.1186/s12943-021-01466-9
Chen, Y., Wei, D., and Deng, M. (2021). Comparative Analysis of Serum Proteins Between Hepatitis B Virus Genotypes B and C Infection by DIA-Based Quantitative Proteomics. Infect. Drug Resist. 14:4701. doi: 10.2147/IDR.S335666
Cheung, K. J., Libbrecht, L., Tilleman, K., Deforce, D., Colle, I., and Van Vlierberghe, H. (2010). Galectin-3-binding protein: A serological and histological assessment in accordance with hepatitis C-related liver fibrosis. Eur. J. Gastroenterol. Hepatol. 22, 1066–1073. doi: 10.1097/MEG.0b013e328337d602
Crooke, S. T., Baker, B. F., Crooke, R. M., and Liang, X.-H. (2021). Antisense technology: An overview and prospectus. Nat. Rev. Drug Discov. 20, 427–453. doi: 10.1038/s41573-021-00162-z
Cui, X.-X., Yang, X., Wang, H.-J., Rong, X.-Y., Jing, S., Xie, Y.-H., et al. (2017). Luteolin-7-O-glucoside present in lettuce extracts inhibits hepatitis B surface antigen production and viral replication by human hepatoma cells in vitro. Front. Microbiol. 8:2425. doi: 10.3389/fmicb.2017.02425
Dai, Y.-N., Tu, Y.-X., Meng, D., Chen, M.-J., Zhang, J.-J., Gong, Y.-H., et al. (2019). Serum proteomic changes as candidate biomarkers of intermediate liver fibrosis in chronic Hepatitis B infection. OMICS 23, 167–179. doi: 10.1089/omi.2018.0179
Dhanasekaran, R., Nault, J.-C., Roberts, L. R., and Zucman-Rossi, J. (2019). Genomic medicine and implications for hepatocellular carcinoma prevention and therapy. Gastroenterology 156, 492–509. doi: 10.1053/j.gastro.2018.11.001
European Association for the Study of the Liver (2018). EASL Clinical Practice Guidelines on hepatitis E virus infection. J. Hepatol. 68, 1256–1271. doi: 10.1016/j.jhep.2018.03.005
Fitian, A. I., Nelson, D. R., Liu, C., Xu, Y., Ararat, M., and Cabrera, R. (2014). Integrated metabolomic profiling of hepatocellular carcinoma in hepatitis C cirrhosis through GC/MS and UPLC/MS-MS. Liver Int. 34, 1428–1444. doi: 10.1111/liv.12541
Gangadharan, B., Bapat, M., Rossa, J., Antrobus, R., Chittenden, D., Kampa, B., et al. (2012). Discovery of novel biomarker candidates for liver fibrosis in hepatitis C patients: A preliminary study. PloS One 7:e39603. doi: 10.1371/journal.pone.0039603
Gao, Q., Zhu, H., Dong, L., Shi, W., Chen, R., Song, Z., et al. (2019). Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma. Cell 179, 561–577.e22. doi: 10.1016/j.cell.2019.08.052
Gerold, G., Meissner, F., Bruening, J., Welsch, K., Perin, P. M., Baumert, T. F., et al. (2015). Quantitative proteomics identifies serum response factor binding protein 1 as a host factor for hepatitis C virus entry. Cell Rep. 12, 864–878. doi: 10.1016/j.celrep.2015.06.063
Guzmán-Solís, A. A., Villa-Islas, V., Bravo-López, M. J., Sandoval-Velasco, M., Wesp, J. K., Gómez-Valdés, J. A., et al. (2021). Ancient viral genomes reveal introduction of human pathogenic viruses into Mexico during the transatlantic slave trade. eLife 10:e68612. doi: 10.7554/eLife.68612
He, X., Hong, Y., Wang, X., Zhang, X., Long, J., Li, H., et al. (2016). Identification and clinical significance of an elevated level of serum aminoacylase-1 autoantibody in patients with hepatitis B virus-related liver cirrhosis. Mol. Med. Rep. 14, 4255–4262. doi: 10.3892/mmr.2016.5740
Heydari, H., Majd, A., Hamidi-Fard, M., Bahramali, G., and Aghasadeghi, M. R. (2021). Full-length Sequencing and Genotyping of Hepatitis A Virus among Acute Hepatic Patients in Iran. Clin. Lab. 67. doi: 10.7754/Clin.Lab.2020.200919
Hou, J., Brouwer, W. P., Kreefft, K., Gama, L., Price, S. L., Janssen, H. L., et al. (2017). Unique intrahepatic transcriptomics profiles discriminate the clinical phases of a chronic HBV infection. PLoS One 12:e0179920. doi: 10.1371/journal.pone.0179920
Hu, J., Gao, Q., Yang, Y., Xia, J., Zhang, W., Chen, Y., et al. (2021). Hexosamine biosynthetic pathway promotes the antiviral activity of SAMHD1 by enhancing O-GlcNAc transferase-mediated protein O-GlcNAcylation. Theranostics 11:805. doi: 10.7150/thno.50230
Huang, B. Y., Tsai, M. R., Hsu, J. K., Lin, C. Y., Lin, C. L., Hu, J. T., et al. (2020). Longitudinal change of metabolite profile and its relation to multiple risk factors for the risk of developing hepatitis B-related hepatocellular carcinoma. Mol. Carcinogenesis 59, 1269–1279. doi: 10.1002/mc.23255
Jiang, Y., Sun, A., Zhao, Y., Ying, W., Sun, H., Yang, X., et al. (2019). Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature 567, 257–261. doi: 10.1038/s41586-019-0987-8
Kalisky, T., Oriel, S., Bar-Lev, T. H., Ben-Haim, N., Trink, A., Wineberg, Y., et al. (2018). A brief review of single-cell transcriptomic technologies. Brief. Funct. Genomics 17, 64–76. doi: 10.1093/bfgp/elx019
Kan, F., Ye, L., Yan, T., Cao, J., Zheng, J., and Li, W. (2017). Proteomic and transcriptomic studies of HBV-associated liver fibrosis of an AAV-HBV-infected mouse model. BMC Genomics 18:641. doi: 10.1186/s12864-017-3984-z
Kanda, T., Sasaki, R., Nakamoto, S., Haga, Y., Nakamura, M., Shirasawa, H., et al. (2015). The sirtuin inhibitor sirtinol inhibits hepatitis A virus (HAV) replication by inhibiting HAV internal ribosomal entry site activity. Biochem. Biophys. Res. Commun. 466, 567–571. doi: 10.1016/j.bbrc.2015.09.083
Katrinli, S., Ozdil, K., Sahin, A., Ozturk, O., Kir, G., Baykal, A. T., et al. (2016). Proteomic profiling of HBV infected liver biopsies with different fibrotic stages. Proteome Sci. 15:7. doi: 10.1186/s12953-017-0114-4
Khan, A., Ahsan, O., Wei, D.-Q., Ansari, J. K., Najmi, M. H., Muhammad, K., et al. (2021). Computational evaluation of abrogation of hbx-bcl-xl complex with high-affinity carbon nanotubes (Fullerene) to halt the hepatitis b virus replication. Molecules 26:6433. doi: 10.3390/molecules26216433
Khan, A. A., Liu, Z.-K., and Xu, X. (2021). Recent advances in immunotherapy for hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. 20, 511–520. doi: 10.1016/j.hbpd.2021.06.010
Kim, J. Y., Wang, L., Lee, J., and Ou, J.-H. J. (2017). Hepatitis C virus induces the localization of lipid rafts to autophagosomes for its RNA replication. J. Virol. 91:e541–e517. doi: 10.1128/JVI.00541-17
König, A., Yang, J., Jo, E., Park, K. H. P., Kim, H., Than, T. T., et al. (2019). Efficient long-term amplification of hepatitis B virus isolates after infection of slow proliferating HepG2-NTCP cells. J. Hepatol. 71, 289–300. doi: 10.1016/j.jhep.2019.04.010
Krause-Kyora, B., Susat, J., Key, F. M., Kühnert, D., Bosse, E., Immel, A., et al. (2018). Neolithic and medieval virus genomes reveal complex evolution of hepatitis B. eLife 7:e36666. doi: 10.7554/eLife.36666
Kumar, S., Barouch-Bentov, R., Xiao, F., Schor, S., Pu, S., Biquand, E., et al. (2019). MARCH8 ubiquitinates the hepatitis C virus nonstructural 2 protein and mediates viral envelopment. Cell Rep. 26, 1800–1814.e5. doi: 10.1016/j.celrep.2019.01.075
Lan, J., Zhang, R., Yu, H., Wang, J., Xue, W., Chen, J., et al. (2019). Quantitative proteomic analysis uncovers the mediation of endoplasmic reticulum stress-induced autophagy in DHAV-1-infected DEF cells. Int. J. Mol. Sci. 20:6160. doi: 10.3390/ijms20246160
Lee, I. N., Chen, C. H., Sheu, J. C., Lee, H. S., Huang, G. T., Chen, D. S., et al. (2006). Identification of complement C3a as a candidate biomarker in human chronic hepatitis C and HCV-related hepatocellular carcinoma using a proteomics approach. Proteomics 6, 2865–2873. doi: 10.1002/pmic.200500488
Li, B., He, X., Jia, W., and Li, H. (2017). Novel applications of metabolomics in personalized medicine: A mini-review. Molecules 22:1173. doi: 10.3390/molecules22071173
Li, Q., Lowey, B., Sodroski, C., Krishnamurthy, S., Alao, H., Cha, H., et al. (2017). Cellular microRNA networks regulate host dependency of hepatitis C virus infection. Nat. Commun. 8:1789. doi: 10.1038/s41467-017-01954-x
Li, H., Wang, Y., Ma, S., Zhang, C., Liu, H., and Sun, D. (2021). Clinical significance of small molecule metabolites in the blood of patients with different types of liver injury. Sci. Rep. 11:11642. doi: 10.1038/s41598-021-91164-9
Li, J., Liang, X., Jiang, J., Yang, L., Xin, J., Shi, D., et al. (2022). PBMC transcriptomics identifies immune-metabolism disorder during the development of HBV-ACLF. Gut 71, 163–175. doi: 10.1136/gutjnl-2020-323395
Li, Q., Sodroski, C., Lowey, B., Schweitzer, C. J., Cha, H., Zhang, F., et al. (2016). Hepatitis C virus depends on E-cadherin as an entry factor and regulates its expression in epithelial-to-mesenchymal transition. Proc. Natl. Acad. Sci.U.S.A. 113, 7620–7625. doi: 10.1073/pnas.1602701113
Li, Y., Fu, Y., Hu, X., Sun, L., Tang, D., Li, N., et al. (2019). The HBx–CTTN interaction promotes cell proliferation and migration of hepatocellular carcinoma via CREB1. Cell Death Dis. 10:405. doi: 10.1038/s41419-019-1650-x
Lian, J., Li, X., Wang, Y., Yang, J., Liu, W., Ma, J., et al. (2016). Metabolite variations between acute-on-chronic liver failure and chronic liver failure caused by hepatitis B virus based on ultra-performance liquid chromatography mass spectrometry. Biomed. Pharmacotherapy 84, 994–1000. doi: 10.1016/j.biopha.2016.09.079
Liang, S., Xie, M., Tang, J., Wang, M., Zhang, D., and Hou, S. (2020). Proteomics reveals the effect of type I interferon on the pathogenicity of duck hepatitis A virus genotype 3 in Pekin ducks. Vet. Microbiol. 248:108813. doi: 10.1016/j.vetmic.2020.108813
Lin, P., Wen, D. Y., Pang, J. S., Liao, W., Chen, Y. J., He, Y., et al. (2022). Proteomics profiling of nontumor liver tissues identifies prognostic biomarkers in hepatitis B-related hepatocellular carcinoma. J. Med. Virol. Epub ahead of print. doi: 10.1002/jmv.27732
Lin, X., Ma, Z. M., Yao, X., Zhang, Y. P., and Wen, Y. M. (2002). Replication efficiency and sequence analysis of full-length hepatitis B virus isolates from hepatocellular carcinoma tissues. Int. J. Cancer 102, 487–491. doi: 10.1002/ijc.10733
Liu, F., Seto, W.-K., Wong, D. K.-H., Huang, F.-Y., Cheung, K.-S., Mak, L.-Y., et al. (2019). Plasma fibronectin levels identified via quantitative proteomics profiling predicts hepatitis B surface antigen seroclearance in chronic hepatitis B. J. Infect. Dis. 220, 940–950. doi: 10.1093/infdis/jiz223
Low, W. Y., Tearle, R., Bickhart, D. M., Rosen, B. D., Kingan, S. B., Swale, T., et al. (2019). Chromosome-level assembly of the water buffalo genome surpasses human and goat genomes in sequence contiguity. Nat. Commun. 10:260. doi: 10.1038/s41467-018-08260-0
Lu, D., Zhuo, J., Yang, M., Wang, C., Pan, L., Xie, H., et al. (2018). The association between donor genetic variations in one-carbon metabolism pathway genes and hepatitis B recurrence after liver transplantation. Gene 663, 121–125. doi: 10.1016/j.gene.2018.03.071
Lussignol, M., Kopp, M., Molloy, K., Vizcay-Barrena, G., Fleck, R. A., Dorner, M., et al. (2016). Proteomics of HCV virions reveals an essential role for the nucleoporin Nup98 in virus morphogenesis. Proc. Natl. Acad. Sci.U.S.A. 113, 2484–2489. doi: 10.1073/pnas.1518934113
Malov, S., Malov, I., Kuvshinov, A., Marche, P., Decaens, T., Macek-Jilkova, Z., et al. (2021). Search for effective serum tumor markers for early diagnosis of hepatocellular carcinoma associated with hepatitis C. Sovrem. Tekhnologii. Med. 13, 27–33. doi: 10.17691/stm2021.13.1.03
McBrearty, N., Arzumanyan, A., Bichenkov, E., Merali, S., Merali, C., and Feitelson, M. (2021). Short chain fatty acids delay the development of hepatocellular carcinoma in HBx transgenic mice. Neoplasia 23, 529–538. doi: 10.1016/j.neo.2021.04.004
McKnight, K. L., Xie, L., González-López, O., Rivera-Serrano, E. E., Chen, X., and Lemon, S. M. (2017). Protein composition of the hepatitis A virus quasi-envelope. Proc.Natl. Acad. Sci. 114, 6587–6592. doi: 10.1073/pnas.1619519114
Murphy, C. M., Xu, Y., Li, F., Nio, K., Reszka-Blanco, N., Li, X., et al. (2016). Hepatitis B virus X protein promotes degradation of SMC5/6 to enhance HBV replication. Cell Rep. 16, 2846–2854. doi: 10.1016/j.celrep.2016.08.026
Nguyen, H. T. T., Wimmer, R., Le, V. Q., and Krarup, H. B. (2021). Metabolic fingerprint of progression of chronic hepatitis B: Changes in the metabolome and novel diagnostic possibilities. Metabolomics 17:16. doi: 10.1007/s11306-020-01767-y
Nie, C., Han, T., Zhang, L., Li, Y., Liu, H., Xiao, S., et al. (2014). Cross-sectional and dynamic change of serum metabolite profiling for H epatitis B-related acute-on-chronic liver failure by UPLC/MS. J. Viral Hepatitis 21, 53–63. doi: 10.1111/jvh.12122
Olivier, M., Asmis, R., Hawkins, G. A., Howard, T. D., and Cox, L. A. (2019). The need for multi-omics biomarker signatures in precision medicine. Int. J. Mol. Sci. 20:4781. doi: 10.3390/ijms20194781
Péneau, C., Imbeaud, S., La Bella, T., Hirsch, T. Z., Caruso, S., Calderaro, J., et al. (2022). Hepatitis B virus integrations promote local and distant oncogenic driver alterations in hepatocellular carcinoma. Gut 71, 616–626. doi: 10.1136/gutjnl-2020-323153
Rabaan, A. A., Al-Ahmed, S. H., Bazzi, A. M., Alfouzan, W. A., Alsuliman, S. A., Aldrazi, F. A., et al. (2020). Overview of hepatitis C infection, molecular biology, and new treatment. J. Infect. public health 13, 773–783. doi: 10.1016/j.jiph.2019.11.015
Ramage, H. R., Kumar, G. R., Verschueren, E., Johnson, J. R., Von Dollen, J., Johnson, T., et al. (2015). A combined proteomics/genomics approach links hepatitis C virus infection with nonsense-mediated mRNA decay. Mol. Cell 57, 329–340. doi: 10.1016/j.molcel.2014.12.028
Ramirez, R., van Buuren, N., Gamelin, L., Soulette, C., May, L., Han, D., et al. (2021). Targeted Long-Read Sequencing Reveals Comprehensive Architecture, Burden, and Transcriptional Signatures from Hepatitis B Virus-Associated Integrations and Translocations in Hepatocellular Carcinoma Cell Lines. J. Virol. 95:e299–e221. doi: 10.1128/JVI.00299-21
Rogée, S., Le Gall, M., Chafey, P., Bouquet, J., Cordonnier, N., Frederici, C., et al. (2015). Quantitative proteomics identifies host factors modulated during acute hepatitis E virus infection in the swine model. J. Virol. 89, 129–143. doi: 10.1128/JVI.02208-14
Schoeman, J. C., Hou, J., Harms, A. C., Vreeken, R. J., Berger, R., Hankemeier, T., et al. (2016). Metabolic characterization of the natural progression of chronic hepatitis B. Genome Med. 8:64. doi: 10.1186/s13073-016-0318-8
Shanmuganathan, M., Sarfaraz, M. O., Kroezen, Z., Philbrick, H., Poon, R., Don-Wauchope, A., et al. (2021). A Cross-Platform Metabolomics Comparison Identifies Serum Metabolite Signatures of Liver Fibrosis Progression in Chronic Hepatitis C Patients. Front. Mol. Biosci. 8:676349. doi: 10.3389/fmolb.2021.676349
Shen, Q., Pu, Y., Fu, X., Xie, Y., Bian, X., Yang, S., et al. (2014). Changes in the cellular proteins of A549 infected with Hepatitis E virus by proteomics analysis. BMC Vet. Res. 10:188. doi: 10.1186/s12917-014-0188-5
Sodroski, C., Lowey, B., Hertz, L., Jake Liang, T., and Li, Q. (2019). MicroRNA-135a modulates hepatitis C virus genome replication through downregulation of host antiviral factors. Virol. Sinica 34, 197–210. doi: 10.1007/s12250-018-0055-9
Su, R.-Y., Ling, S.-B., Shan, Q.-N., Wei, X.-Y., Wang, R., Jia, C.-K., et al. (2022). Efficacy and safety of sirolimus early conversion protocol in liver transplant patients with hepatocellular carcinoma: A single-arm, multicenter, prospective study. Hepatobiliary Pancreat. Dis. Int. 21, 106–112. doi: 10.1016/j.hbpd.2021.09.001
Sun, Z., Huang, C., Shi, Y., Wang, R., Fan, J., Yu, Y., et al. (2021). Distinct Bile Acid Profiles in Patients With Chronic Hepatitis B Virus Infection Reveal Metabolic Interplay Between Host, Virus and Gut Microbiome. Front. Med. 8:708495. doi: 10.3389/fmed.2021.708495
Sun, Z., Liu, X., Wu, D., Gao, H., Jiang, J., Yang, Y., et al. (2019). Circulating proteomic panels for diagnosis and risk stratification of acute-on-chronic liver failure in patients with viral hepatitis B. Theranostics 9:1200. doi: 10.7150/thno.31991
Takagi, A., Amako, Y., Yamane, D., Kitab, B., Tokunaga, Y., El-Gohary, A., et al. (2021). Longer Poly (U) Stretches in the 3’ UTR Are Essential for Replication of the Hepatitis C Virus Genotype 4a Clone in in vitro and in vivo. Front. Microbiol. 12:764816. doi: 10.3389/fmicb.2021.764816
Tanaka, J., Kurisu, A., Ohara, M., Ouoba, S., Ohisa, M., Sugiyama, A., et al. (2022). Burden of chronic hepatitis B and C infections in 2015 and future trends in Japan: A simulation study. Lancet Reg. Health Western Pacific 22:100428. doi: 10.1016/j.lanwpc.2022.100428
Tsai, H.-F., and Hsiao, H.-H. (2017). Synthesis of stable isotopically labeled peptides with filter-assisted enzymatic labeling for the diagnosis of hepatitis B virus infection utilizing mass spectrometry-based proteomics strategy. Analytica Chimica acta 956, 32–39. doi: 10.1016/j.aca.2016.12.015
Vo Quang, E., Shimakawa, Y., and Nahon, P. (2021). Epidemiological projections of viral-induced hepatocellular carcinoma in the perspective of WHO global hepatitis elimination. Liver Int. 41, 915–927. doi: 10.1111/liv.14843
Wassenaar, T. M., Jun, S. R., Robeson, M., and Ussery, D. W. (2020). Comparative genomics of hepatitis A virus, hepatitis C virus, and hepatitis E virus provides insights into the evolutionary history of Hepatovirus species. Microbiol. Open 9:e973. doi: 10.1002/mbo3.973
Wei, D., Zeng, Y., Xing, X., Liu, H., Lin, M., Han, X., et al. (2016). Proteome differences between hepatitis B virus genotype-B-and genotype-C-induced hepatocellular carcinoma revealed by iTRAQ-based quantitative proteomics. J. Proteome Res. 15, 487–498. doi: 10.1021/acs.jproteome.5b00838
Wei, R.-L., Fan, G.-H., Zhang, C.-Z., Chen, K.-C., Zhang, W.-H., Li, C.-B., et al. (2022). Prognostic implication of early posttransplant hypercholesterolemia in liver transplantation for patients with hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. [Epub ahead of print]. doi: 10.1016/j.hbpd.2022.05.005
Widasari, D. I., Yano, Y., Heriyanto, D. S., Utsumi, T., Yamani, L. N., Rinonce, H. T., et al. (2014). A deep-sequencing method detects drug-resistant mutations in the hepatitis B virus in Indonesians. Intervirology 57, 384–392. doi: 10.1159/000366420
Wille, M., Netter, H. J., Littlejohn, M., Yuen, L., Shi, M., Eden, J.-S., et al. (2018). A divergent hepatitis D-like agent in birds. Viruses 10:720. doi: 10.3390/v10120720
Wu, Y., Liu, Z., and Xu, X. (2020). Molecular subtyping of hepatocellular carcinoma: A step toward precision medicine. Cancer Commun. 40, 681–693. doi: 10.1002/cac2.12115
Wu, D., Zhang, S., Xie, Z., Chen, E., Rao, Q., Liu, X., et al. (2020). Plasminogen as a prognostic biomarker for HBV-related acute-on-chronic liver failure. J. Clin. Investig. 130, 2069–2080. doi: 10.1172/JCI130197
Wu, J., Bortolanza, M., Zhai, G., Shang, A., Ling, Z., Jiang, B., et al. (2022a). Gut microbiota dysbiosis associated with plasma levels of Interferon-γ and viral load in patients with acute hepatitis E infection. J. Med. Virol. 94, 692–702. doi: 10.1002/jmv.27356
Wu, J., Xu, Y., Cui, Y., Bortolanza, M., Wang, M., Jiang, B., et al. (2022b). Dynamic changes of serum metabolites associated with infection and severity of patients with acute hepatitis E infection. J. Med. Virol. 94, 2714–2726. doi: 10.1002/jmv.27669
Wu, J., Chen, Z.-P., Shang, A.-Q., Wang, W.-W., Chen, Z.-N., Tao, Y.-J., et al. (2017a). Systemic bioinformatics analysis of recurrent aphthous stomatitis gene expression profiles. Oncotarget 8:111064. doi: 10.18632/oncotarget.22347
Wu, J., Cui, L. L., Yuan, J., Wang, Y., and Song, S. (2017b). Clinical significance of the phosphorylation of MAPK and protein expression of cyclin D1 in human osteosarcoma tissues. Mol. Med. Rep. 15, 2303–2307. doi: 10.3892/mmr.2017.6224
Wu, J., Lu, W. Y., and Cui, L. L. (2015). Clinical significance of STAT3 and MAPK phosphorylation, and the protein expression of cyclin D1 in skin squamous cell carcinoma tissues. Mol. Med. Rep. 12, 8129–8134. doi: 10.3892/mmr.2015.4460
Wu, J., Xiang, Z., Zhu, C., Yao, Y., Bortolanza, M., Cao, H., et al. (2021). Extrahepatic manifestations related to hepatitis E virus infection and their triggering mechanisms. J. Infect. 83, 298–305. doi: 10.1016/j.jinf.2021.07.021
Xie, H.-Y., Cheng, J., Xing, C.-Y., Wang, J.-J., Su, R., Wei, X.-Y., et al. (2011). Evaluation of hepatitis B viral replication and proteomic analysis of HepG2. 2.15 cell line after knockdown of HBx. Hepatobiliary Pancreat. Dis. Int. 10, 295–302. doi: 10.1016/s1499-3872(11)60049-0
Xu, Y., Qi, Y., Luo, J., Yang, J., Xie, Q., Deng, C., et al. (2017). Hepatitis B virus X protein stimulates proliferation, wound closure and inhibits apoptosis of HuH-7 cells via CDC42. Int. J. Mol. Sci. 18:586. doi: 10.3390/ijms18030586
Yan, H., Zhong, G., Xu, G., He, W., Jing, Z., Gao, Z., et al. (2012). Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 1:e00049. doi: 10.7554/eLife.00049
Yan, L. B., Yu, Y. J., Zhang, Q. B., Tang, X. Q., Bai, L., Huang, F., et al. (2018). Identification of p90 Ribosomal S6 Kinase 2 as a Novel Host Protein in HBx Augmenting HBV Replication by iTRAQ-Based Quantitative Comparative Proteomics. Proteomics Clin. Appl. 12:1700090. doi: 10.1002/prca.201700090
Yang, L., Rudser, K. D., Higgins, L., Rosen, H. R., Zaman, A., Corless, C. L., et al. (2011). Novel biomarker candidates to predict hepatic fibrosis in hepatitis C identified by serum proteomics. Digest. Dis. Sci. 56, 3305–3315. doi: 10.1007/s10620-011-1745-4
Ye, L., Chen, T., Cao, J., Sun, L., Li, W., and Zhang, C. (2020). Short hairpin RNA attenuates liver fibrosis by regulating the PPAR-γ and NF-κB pathways in HBV-induced liver fibrosis in mice. Int. J. Oncol. 57, 1116–1128. doi: 10.3892/ijo.2020.5125
Yu, L., Zeng, Z., Tan, H., Feng, Q., Zhou, Q., Hu, J., et al. (2022). Significant metabolic alterations in patients with hepatitis B virus replication observed via serum untargeted metabolomics shed new light on hepatitis B virus infection. J. Drug Target. 30, 442–449. doi: 10.1080/1061186X.2021.2009841
Zai, W., Hu, K., Ye, J., Ding, J., Huang, C., Li, Y., et al. (2022). Long-Term Hepatitis B Virus Infection Induces Cytopathic Effects in Primary Human Hepatocytes, and Can Be Partially Reversed by Antiviral Therapy. Microbiol. Spectrum 10:e1328–e1321. doi: 10.1128/spectrum.01328-21
Zeyen, L., Döring, T., Stieler, J. T., and Prange, R. (2020). Hepatitis B subviral envelope particles use the COPII machinery for intracellular transport via selective exploitation of Sec24A and Sec23B. Cell. Microbiol. 22:e13181.
Zhang, D., Lim, S. G., and Koay, E. S. (2007). Proteomic identification of down-regulation of oncoprotein DJ-1 and proteasome activator subunit 1 in hepatitis B virus-infected well-differentiated hepatocellular carcinoma. Int. J. Oncol. 31, 577–584. doi: 10.3892/ijo.31.3.577
Zhang, X.-L., Li, W.-F., Yuan, S., Guo, J.-Y., Li, Z.-L., Chi, S.-H., et al. (2019). Meta-transcriptomic analysis reveals a new subtype of genotype 3 avian hepatitis E virus in chicken flocks with high mortality in Guangdong. China. BMC Vet. Res. 15:131. doi: 10.1186/s12917-019-1884-y
Zhao, P., Wen, J., Qian, L., Zhu, X., Wang, H., and Bai, X. (2020). Expression of S100 proteins is associated with HBV intrauterine transmission. Arch. Gynecol. Obstetrics 302, 1389–1399. doi: 10.1007/s00404-020-05753-6
Zhou, N., Wang, K., Fang, S., Zhao, X., Huang, T., Chen, H., et al. (2017). Discovery of a potential plasma protein biomarker panel for acute-on-chronic liver failure induced by hepatitis B virus. Front. Physiol. 8:1009. doi: 10.3389/fphys.2017.01009
Keywords: viral hepatitis, genomics, proteomics, transcriptomics, metabolomics
Citation: Xiang Z, Li J, Lu D, Wei X and Xu X (2022) Advances in multi-omics research on viral hepatitis. Front. Microbiol. 13:987324. doi: 10.3389/fmicb.2022.987324
Received: 06 July 2022; Accepted: 11 August 2022;
Published: 02 September 2022.
Edited by:
Zhipeng Xu, Nanjing Medical University, ChinaReviewed by:
Yuzhu Dai, The 903th Hospital of the People’s Liberation Army, ChinaMin Tang, Tongji Hospital Affiliated to Tongji University, China
Copyright © 2022 Xiang, Li, Lu, Wei and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Xu, emp4dUB6anUuZWR1LmNu; Xuyong Wei, MTMxNTAwOUB6anUuZWR1LmNu
†These authors have contributed equally to this work
 Ze Xiang
Ze Xiang Jiayuan Li
Jiayuan Li Di Lu
Di Lu Xuyong Wei1,3,4,5*
Xuyong Wei1,3,4,5* Xiao Xu
Xiao Xu