
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 15 September 2022
Sec. Microbe and Virus Interactions with Plants
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.984200
This article is part of the Research Topic Community Series in Plants and Microbial Communities: Diversity, Pathogens and Biological Control, volume II View all 25 articles
 Yajun Wang1†
Yajun Wang1† Lan Ma2†
Lan Ma2† Ziyang Liu2
Ziyang Liu2 Jingwei Chen2
Jingwei Chen2 Hongxian Song1
Hongxian Song1 Jiajia Wang1
Jiajia Wang1 Hanwen Cui2
Hanwen Cui2 Zi Yang1
Zi Yang1 Sa Xiao2
Sa Xiao2 Kun Liu2
Kun Liu2 Lizhe An1
Lizhe An1 Shuyan Chen1*
Shuyan Chen1*Plant species and microbial interactions have significant impacts on the diversity of bacterial communities. However, few studies have explored interactions among these factors, such the role of microbial interactions in regulating the effects of plant species on soil bacterial diversity. We assumed that plant species not only affect bacterial community diversity directly, but also influence bacterial community diversity indirectly through changing microbial interactions. Specifically, we collected soil samples associated with three different plant species, one evergreen shrub (Rhododendron simsii) and the other two deciduous shrubs (Dasiphora fruticosa and Salix oritrepha). Soil bacterial community composition and diversity were examined by high-throughput sequencing. Moreover, soil bacterial antagonistic interactions and soil edaphic characteristics were evaluated. We used structural equation modeling (SEM) to disentangle and compare the direct effect of different plant species on soil bacterial community diversity, and their indirect effects through influence on soil edaphic characteristics and microbial antagonistic interactions. The results showed that (1) Plant species effects on soil bacterial diversity were significant; (2) Plant species effects on soil microbial antagonistic interactions were significant; and (3) there was not only a significant direct plant species effect on bacterial diversity, but also a significant indirect effect on bacterial diversity through influence on microbial antagonistic interactions. Our study reveals the difference among plant species in their effects on soil microbial antagonistic interactions and highlights the vital role of microbial interactions on shaping soil microbial community diversity.
The linkages between above-ground and below-ground communities have been widely studied, and much attention has been paid to the effects of plants on soil microbial communities (Garbeva et al., 2004; Badri and Vivanco, 2009; Bakker et al., 2013a; Schlatter et al., 2015a). Plants strongly influence microbial communities both directly and indirectly. For example, plants provide a wide range of compounds, such as allelopathic constituents (e.g., catechin) and secondary metabolites through root exudation to affect soil microbial communities (Pennanen et al., 1999; Fierer et al., 2012). Moreover, plants may induce competition for nutrients, such as carbon compounds through affecting the amount and quality of litter to influence soil microbial communities (Rousk et al., 2010). Microbe-microbe interactions, such as antagonism and competition, play a significant role in microbial community structure and function (Schlatter et al., 2015b). However, a clearer model is required to allow us to better understand the important role of microbial interactions in regulating the effects of plant species on soil microbial structure.
There are many evidences that plants can significantly influence microbial interactions, which are revealed for instance through assessment of antagonistic activity (Bergsma-Vlami et al., 2005; Bakker et al., 2013b). The plant was suggested to provide environmental context for microbial interactions (Schlatter et al., 2015b). Specifically, plant species significantly impact the availability of soil resources, which may have a direct bearing on resource competition and microbial interactions among soil bacteria (Schlatter et al., 2015b). For instance, plant productivity is suggested to influence microbial competition phenotypes (Wiggins and Kinkel, 2005a). Furthermore, the quality of resources is suggested to mediate microbial interactions (Kinkel et al., 2011, 2012). For example, a higher concentration of phenolic compounds in plant humus reduces microbial activities (Wardle et al., 1998; Inderjit and van der Putten, 2010).
Microbial interactions are broadly perceived to affect the composition and diversity of soil bacterial community (Kinkel et al., 2011; Schlatter et al., 2015b), and are essential for the development and maintenance of microbial communities (Ryan and Dow, 2008). Specifically, soil microbial interactions are expected to change the density and dynamics of microbial populations. To be specific, competing microbial groups can coexist steadily under a certain nutrient concentration ratio, but in the case of nutritional restrictions, certain microbial groups may be defeated (Hibbing et al., 2010). Interspecies signaling molecules, such as sub-inhibitory concentrations of antibiotics, are predicted to mediate the steady state of microbial communities (Davies et al., 2006; Seshasayee et al., 2006). Furthermore, competitive bacteria have been shown to increase bacterial diversity (Domin et al., 2018). Similarly, it has been found that the proportion of soil microbes which have antagonistic activity against other microbes is significantly positively correlated with soil bacterial diversity (Bakker et al., 2013b).
Antagonism is a common phenomenon within soil bacterial communities (Kinkel et al., 2012; Schlatter and Kinkel, 2014; Essarioui et al., 2017), in which one organism causes inhibition of the development or growth of other microorganisms. Such antagonism plays an important role in the formation and maintenance of microbial communities, such as suppressing diverse plant diseases (Chou et al., 2009). Streptomyces, a genus of gram-positive bacteria, is ubiquitous in nature (Doumbou et al., 2001) and is well known owing to various metabolic abilities (Bakker et al., 2013b) and producing diverse antibiotics (Kinkel et al., 2012). Streptomyces possess diverse antagonistic activities against a variety of plant pathogens among soil bacteria (Jauri and Kinkel, 2014; Takano et al., 2016). Because of their ubiquitous presence and ability to produce antagonistic compounds (Kinkel et al., 2012; Bakker et al., 2013a), Streptomyces spp. have been considered as a target for evaluating antagonists (Schlatter et al., 2015b). Studies have shown that microbial antagonism driven by Streptomyces is affected by plant species and plant productivity (Wiggins and Kinkel, 2005a; Kinkel et al., 2012; Bakker et al., 2013a). More plant productivity is hypothesized to favor antagonism among soil microbes (Sun et al., 2015).
Although plant species and microbial interactions both have significant impacts on bacterial communities, few studies have explored the role of microbial interactions in regulating the effect of plant species on soil bacterial diversity. In this study, we compared the effects of three dominant shrub species (Rhododendron simsii, Dasiphora fruticosa, and Salix oritrepha) on soil bacterial diversity and microbial interactions in an alpine meadow of north-west China. Shrubs have been shown to positively influence the activity of soil microbial communities (Xu and Coventry, 2003). It has been shown that shrubs support spatial heterogeneity (Grime, 1984), and provide opportunities for a wide variety of interactions, including microbial and plant-microbe interactions (Lopez-Pintor et al., 2006). Rhododendron simsii, a kind of evergreen shrub (Kudo et al., 2001; Myers-Smith et al., 2011), produces recalcitrant and uneasily decomposed plant litter (Cornelissen, 1996; Cornelissen et al., 1999), resulting in lower ecosystem productivity (Pastor et al., 1993). Meanwhile, the higher concentrations of phenolic allelochemicals have been found in Rhododendron plants (Wardle et al., 1998) than under other ground-cover taxa (Ward et al., 2009; Adamczyk et al., 2016). Dasiphora fruticosa and Salix oritrepha are deciduous shrubs (Kudo et al., 2001; Myers-Smith et al., 2011), producing higher quality and more easily decomposed plant litter (DeMarco et al., 2014). Research has shown that dominant plant can modify the diversity of soil microbial communities by impacting soil physicochemical properties through litter inputs (Zhu et al., 2022). The aims of this study were to (1) analyze the bacterial community diversity, microbial antagonistic interaction, and soil properties under different shrub types (i.e., evergreen shrub and deciduous shrubs); and (2) analyze the pathway of deciduous shrubs’ effects on bacterial community diversity compared to evergreen shrub.
The experiment was conducted at the Gansu Qilianshan National Reserve in Tianzhu (103°11′E, 37°13′N), Gansu Province, China. The site is located on the northeastern edge of the Tibetan plateau with an average elevation at 2,892 m above sea level. The climate belongs to the alpine arid and semi-arid desert climate. The mean annual temperature ranges from −0.6°C to 2°C, with approximately 240–270 frost days per year, and the annual precipitation ranges from 300 to 500 mm, falling mainly during the cool summer. The experimental site vegetation is mainly dominated by the shrubs R. simsii, D. fruticosa, and S. oritrepha.
In early August 2018, we chose a representative area with more than 100 individuals of each of the shrubs R. simsii, D. fruticosa, and S. oritrepha. Then, we randomly allocated 30 plots (30 cm ∗ 30 cm) that each included one of the target shrub species. Distance between neighboring plots was less than 5 m, to ensure that the same climatic conditions were present across plots. Thus, a total of 10 individuals were selected for each of the three species of shrub.
Around each selected individual host plant, we randomly collected three soil cores, which were well mixed to form a composite sample. Then all the composite soil samples were divided into three equal-sized aliquots. One aliquot was stored at 4°C and used for evaluating the soil microbial antagonistic potential. One aliquot was stored at −80°C for DNA extraction, and the remaining aliquot was used to measure the soil edaphic properties.
To measure soil water content, 10 g fresh soil was left at 105°C for 72 h and the dry weight was determined. The remaining air-dried soil samples were sieved through a 0.15 mm mesh and analyzed for total phosphorus, nitrogen and organic carbon, ammonium, nitrate, and pH. Soil total phosphorus and nitrogen were measured following the semi micro-Kjeldahl protocol and wet oxidation method (Wang et al., 2018). Soil organic carbon was determined based on the wet oxidation method. Both ammonium and nitrate were determined after extracting with 2 M KCl. Soil pH was determined by using a pH meter (PHSJ-3F, Shanghai INESA Scientific Instrument Co., Ltd., China) in a slurry of 1:2.5 (w/v) soil: deionized water (Wang et al., 2018, 2019).
DNA was extracted using the DNeasy® PowerSoil® Kit (Qiagen, Hilden, Germany) from 0.25 g of each soil samples following the manufacturer’s protocol. For bacterial community composition, the polymerase chain reaction (PCR) was used to amplify the 16S rRNA gene within the V4 hypervariable region with the primers: 515F (GTGCCAGCMGCCGCGGTAA) and 806R (GGACTACHVGGGTWTCTAAT) (Caporaso et al., 2011). The PCR products were visualized using a 2% agarose gel and purified using the GeneJET Gel Extraction Kit (Thermo Scientific), according to the manufacturer’s protocol. The purified 16S rRNA amplicons were sequenced with a lon S5™ XL instrument (Thermo Fisher Scientific). Sequencing data were quality-filtered using Cutadapt v1.9.1 (Jiao et al., 2016), and vsearchGITHUB (Qin et al., 2012) was used to conduct chimera detection. Finally, the high-quality sequences were binned and classified into different operational taxonomic units (OTUs; 97% similarity) by Uparse v7.01 (Rognes et al., 2016). After this process, we obtained 31,212 bacterial OTUs, and bacterial diversity was calculated at the OTU level.
Soil samples were stored at 4°C and were processed in random order over 6 months. Each soil sample was evaluated for total Streptomyces density, antagonistic Streptomyces density, the intensity of inhibition, and frequency of inhibition as described in Bakker et al. (2013b). Due to the ubiquitous presence of Streptomyces and the ability to produce antagonistic compounds (Davelos et al., 2004a), Streptomyces has been used as a target for evaluating microbial antagonistic interactions (Schlatter et al., 2015b). Briefly, 5 g fresh soil from each sample were dried overnight under sterile cheesecloth, and dispersed in 50 ml H2O on a shaker (175 rpm, 60 min, 4°C). Soil dilutions were spread on 15 ml agar plates and overlaid with 5 ml of cooled starch-casein agar (SCA) (Wiggins and Kinkel, 2005b). The plates were incubated for 3 days at 28°C. After counting the total Streptomyces densities, each plate was overlaid with a second thin layer (10 ml) of SCA. Then plates were spread with spore suspensions of each of three indicator Streptomyces strains (Streptomyces olivochromogenes, Streptomyces mirabilis, and Streptomyces colombiensis) having different antibiotic resistance profiles, similar to the approach of Davelos et al. (2004b), and were incubated for an additional 3 days at 28°C. Zones of inhibition were measured for each plate, and antagonistic Streptomyces densities were counted of each plate. Proportions of antagonistic Streptomyces were averaged across indicator strains for each sample (Bakker et al., 2013b).
The Shapiro-Wilk test and Levene’s Test were used to check the assumptions of normal distribution and variance homogeneity among treatments. All indices used in this article met the assumption of normality and equal variance, except the total Streptomyces density, antagonistic Streptomyces density, soil pH, soil total nitrogen, and soil ammonia nitrogen; for these variables, we assessed impacts of shrub species via one-way permutation test. One-way analysis of variance (ANOVA) was used to test the effects of shrub species on the remaining indices, including bacterial Shannon diversity, size of inhibition zone, proportions of antagonistic Streptomyces, soil water content, organic carbon, total phosphorus, and nitrate nitrogen. Non-metric multidimensional scaling (NMDS) was used to analyze the structural variation of bacterial communities across samples.
We established theoretical structural equation modeling (SEM) to explore, compared to the evergreen shrub (R. simsii), how bacterial diversity and bacterial community composition were impacted by the direct effects of deciduous shrubs (D. fruticosa and S. oritrepha), and by indirect effects of deciduous shrubs (D. fruticosa and S. oritrepha) via changes in soil microbial antagonism interactions and soil physical-chemical properties (Supplementary Figure 3). The evergreen shrub (R. simsii) was used as a control group to explore the ways in which deciduous shrubs (D. fruticosa and S. oritrepha) affect soil bacterial diversity and bacterial community composition. We used the method as described by Veen et al. (2010) and used PC1 scores (i.e., the first axis of principal components analysis) of total Streptomyces density, antagonist density, the intensity of inhibition, and frequency of inhibition to represent microbial antagonistic interactions. Variables were chosen with lower P-value according to ANOVA results.
We applied SEM analyses according to the following premises: we hypothesized that (1) There was a significant difference of plant species on microbial antagonistic interaction of associated with Streptomyces communities (Bakker et al., 2013b); (2) Plant species will trigger changes in soil physicochemical properties (Bakker et al., 2013a; Wang et al., 2018); (3) Microbial antagonistic interactions are related to the soil microbial diversity (Schlatter et al., 2015b); (4) Soil bacterial diversity is related to the soil physicochemical properties (Herold et al., 2014); and (5) Soil bacterial diversity can be shaped by plant species (Bakker et al., 2013a).
There are many indicators to examine the goodness of fit of SEM, yet there is no single index that is generally accepted. Hence, we used both the χ2-test (P > 0.05) and the root mean square error of approximation (RMSEA) test to assess the goodness of fit of SEM (Wang et al., 2019). A smaller RMSEA and χ2 represents a better model fit. When the RMSEA > 0.06, models should be rejected. If two models have similarly RMSEA and χ2, the more parsimonious model is accepted (Danner et al., 2015).
All data analyses were performed in R software, version 4.0.1. The variance homogeneity was tested with the “car” package (Fox et al., 2013). SEMs were conducted using the “lavaan” package (Rosseel, 2012).
Targeting the v4 region of the 16S rRNA gene, we obtained 2,054,980 quality-screened sequences from the 30 soil samples using lon S5™ XL sequencing, ranging from 53,142 to 76,845 reads per sample. At the 97% sequence similarity level, 31,212 bacterial OTUs were obtained in total. Shannon index was used to estimate bacterial richness and evenness. Especially, the average values of Shannon index were 7.62 ± 0.04, 7.52 ± 0.03, and 7.60 ± 0.04 (mean ± SE) for soil bacterial communities associated with R. simsii, D. fruticosa, and S. oritrepha, respectively. The richness of soil bacterial community differed significantly among shrub species (Figure 1A). Soil bacterial Shannon diversity did not vary significantly among shrub species (Figure 1B). However, shrub species had significant effects on the composition of bacterial community (Supplementary Figure 1).
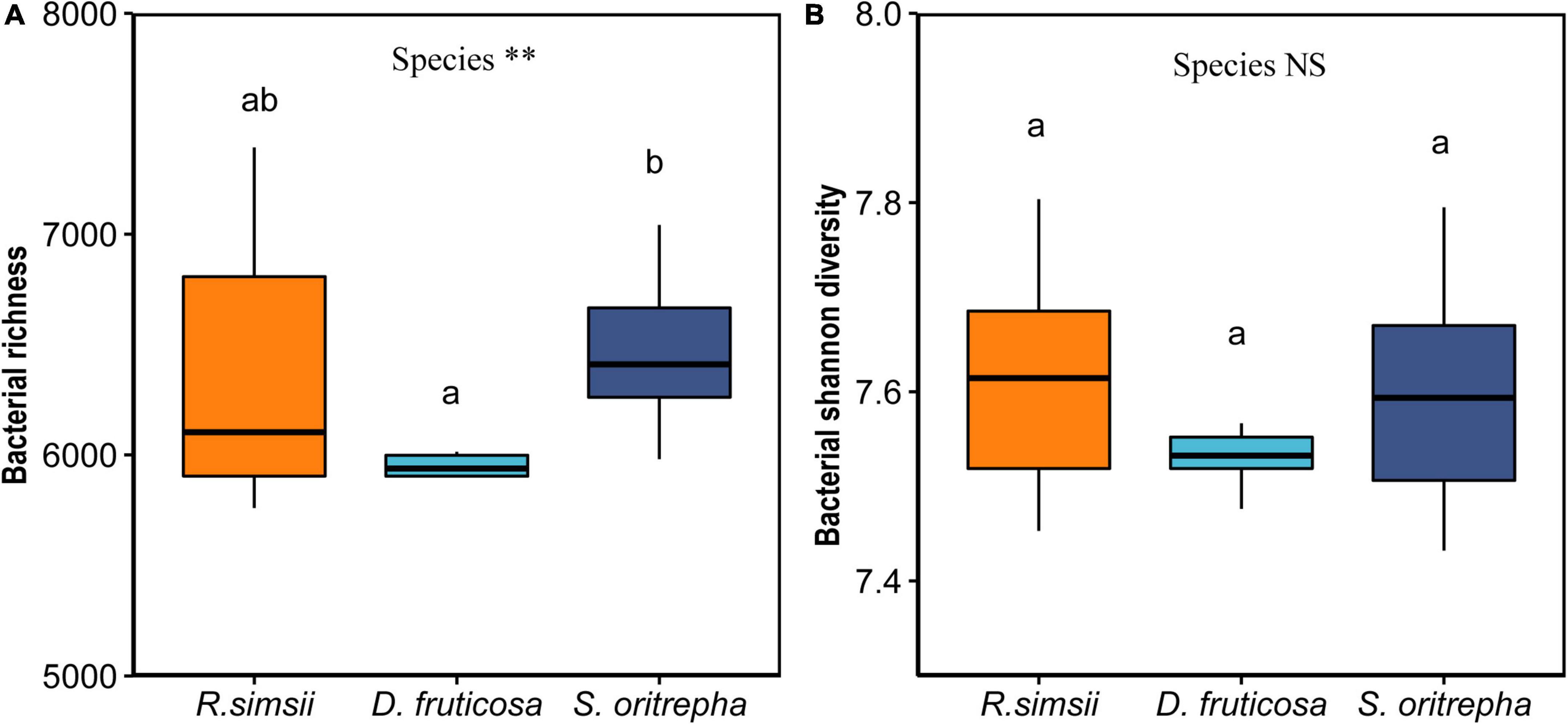
Figure 1. Overview of effects of shrub species on soil bacterial Shannon diversity (mean ± standard error). (A) Bacterial richness. (B) Bacterial Shannon diversity. Symbol: NSP > 0.05; ∗∗P < 0.001 (one-way ANOVA). The same letter means no significant difference (P > 0.1).
Several measures of soil edaphic properties were significantly impacted by the shrub species (Figure 2). Soil ammonium differed marginally significantly among shrub species (Figure 2B). S. oritrepha supported higher soil ammonium than R. simsii, and D. fruticosa supported lower soil ammonium than R. simsii. The soil nitrate also differed among shrub species (Figure 2C), with S. oritrepha harboring lower soil nitrate than R. simsii, and D. fruticosa harboring higher soil nitrate than R. simsii. The soil total nitrogen differed among shrub species (Figure 2D), with D. fruticosa and S. oritrepha supporting lower total nitrogen than R. simsii. The soil carbon nitrogen ratio differed significantly among shrub species (Figure 2H), with S. oritrepha supporting higher carbon nitrogen ratio than R. simsii.
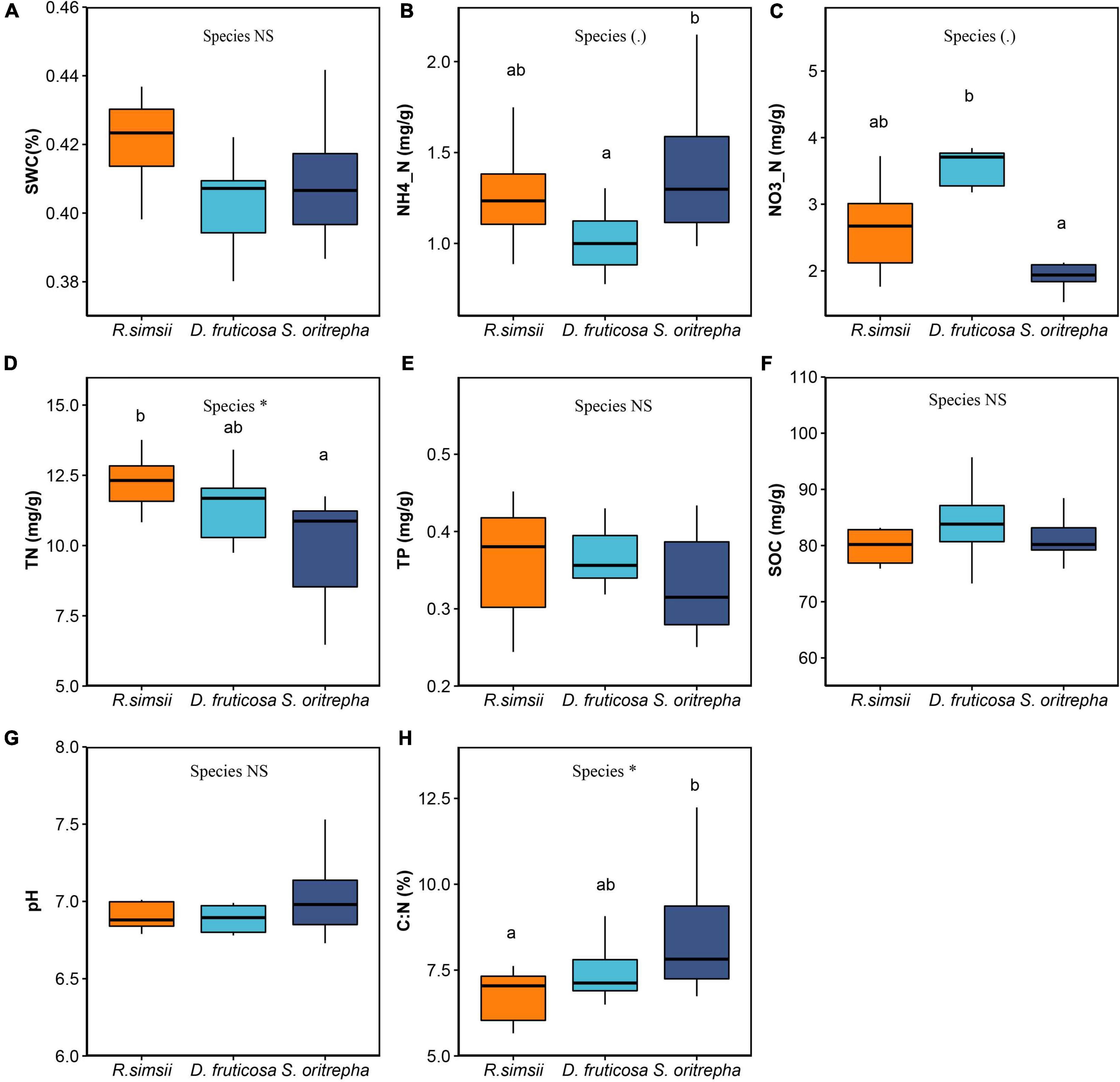
Figure 2. Overview of effects of shrub species on soil edaphic properties. (A) Soil water content, (B) ammonium, (C) nitrate, (D) total nitrogen, (E) total phosphorus, (F) soil organic carbon, (G) soil pH, and (H) soil carbon-nitrogen ratio. Symbol: ∗P < 0.05; NSP > 0.1 (one-way ANOVA). The same letter means no significant difference (P > 0.1).
Soil total Streptomyces density, soil antagonistic Streptomyces density, and Streptomyces antagonism intensity varied among shrub species. Across all experimental treatments, soil cultivable total Streptomyces densities varied nearly twofold, ranging from 2.3 × 106 to 5.7 × 106 colony forming units (CFU) per gram of soil (mean 4.1 × 106 CFU/g). Antagonistic Streptomyces densities ranged from 0.9 × 105 to 2.4 × 105 CFU per gram of soil, with a mean of 1.6 × 105 CFU/g. The intensity of inhibition, measured as the average diameter of inhibition zones against indicator strains, varied from 0.47 to 0.78 cm, with a mean value of 0.64 cm. The frequency of inhibition did not change much, from 0.038 to 0.044.
Three out of four indices of microbial antagonistic interaction were significantly influenced by shrub species. Specifically, there was a significant difference in soil cultivable total Streptomyces density associated with shrub species, the total Streptomyces density associated with R. simsii was significantly lower than those associated with S. oritrepha (Figure 3A, P < 0.001, ANOVAP with Kruskal-Wallis Test). Moreover, the soil cultivable antagonistic Streptomyces density also differed significantly among shrub species, and antagonistic Streptomyces density associated with R. simsii was significantly lower than those associated with S. oritrepha (Figure 3B, P < 0.01, ANOVA with Tukey HSD test). Similarly, there was a significant difference in the diameter of the inhibition zone (Figure 3C, P < 0.05, ANOVAP with Kruskal-Wallis Test), and intensity of inhibition associated with these three species of R. simsii, D. fruticosa, and S. oritrepha increased in turn. Generally, the soil total Streptomyces density, soil antagonistic Streptomyces density as well as the intensity of inhibition consistently showed the same trend of variation among different shrub species, which is the lowest associated with R. simsii and the highest associated with S. oritrepha. However, there was no significant difference in the proportion of inhibitory Streptomyces among shrub species (Figure 3D).
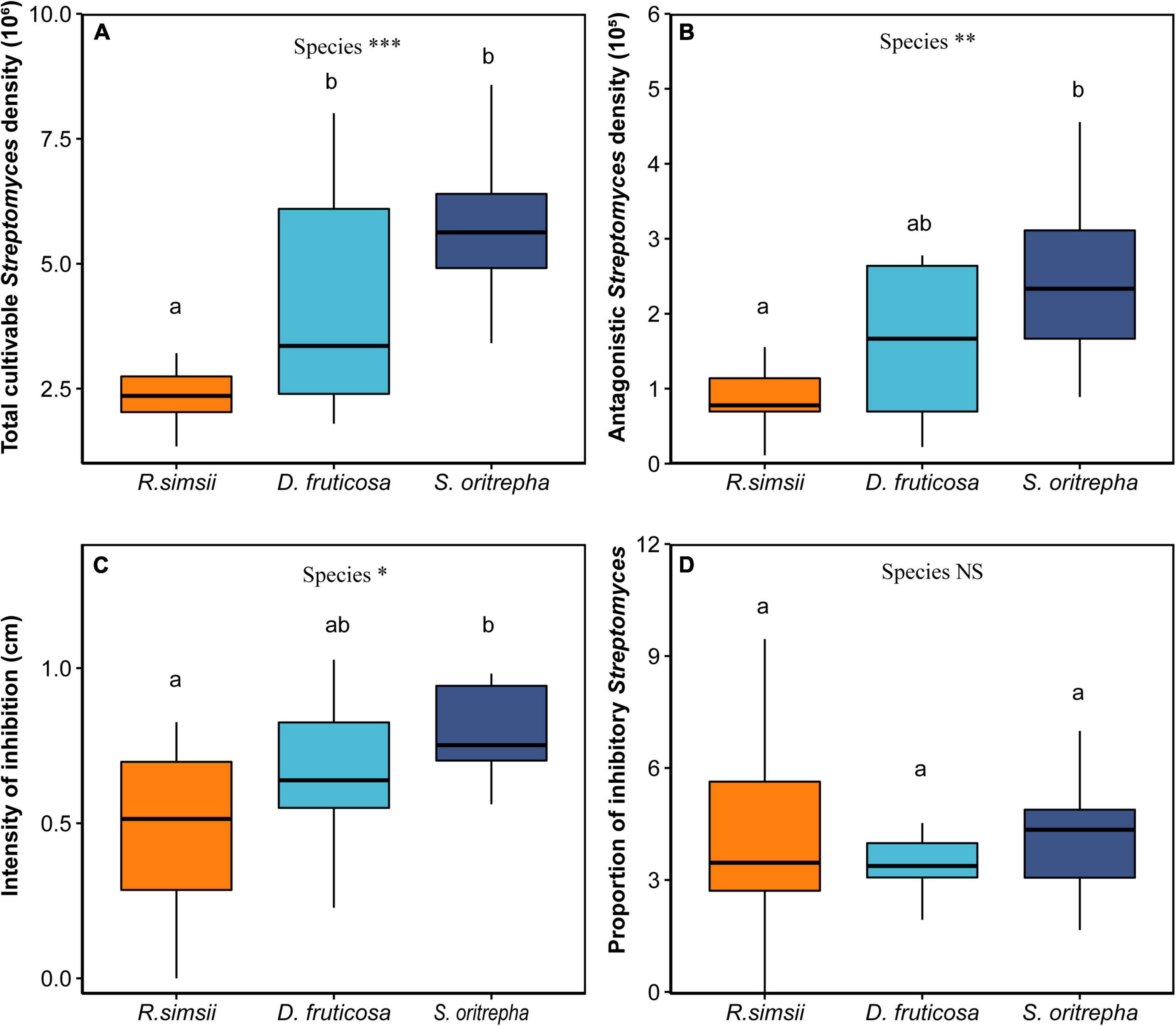
Figure 3. Overview of the effects of shrub species on soil Streptomyces antagonism potential (mean ± SE), Effects of plant species on (A) the soil total cultivable Streptomyces density, (B) the soil cultivable antagonistic Streptomyces density, (C) the diameter of the inhibition zone, (D) the proportion of inhibitory Streptomyces. Symbol: ***P < 0.001; **P < 0.01; *P < 0.05; NSP > 0.1 (one-way ANOVA). The same letter means no significant difference (P > 0.1).
Our SEM model explained 27% of the variation of bacterial diversity among samples (Figure 4). The SEM showed that compared with R. simsii, both D. fruticosa and S. oritrepha had not only significantly negative direct correlations with bacterial diversity, but also positive indirect correlations with bacterial diversity through Streptomyces’s antagonism potential. Specifically, there were significant relationships between D. fruticosa and S. oritrepha compared to R. simsii and Streptomyces’s antagonistic interactions, and Streptomyces’s antagonism potential were marginally significantly positively related to bacterial diversity. Although D. fruticosa and S. oritrepha compared to R. simsii were significantly negatively related to soil water content, and S. oritrepha compared to R. simsii was significantly negatively related to soil total nitrogen, there were no significant relationships of soil water content or soil total nitrogen directly on bacterial diversity.
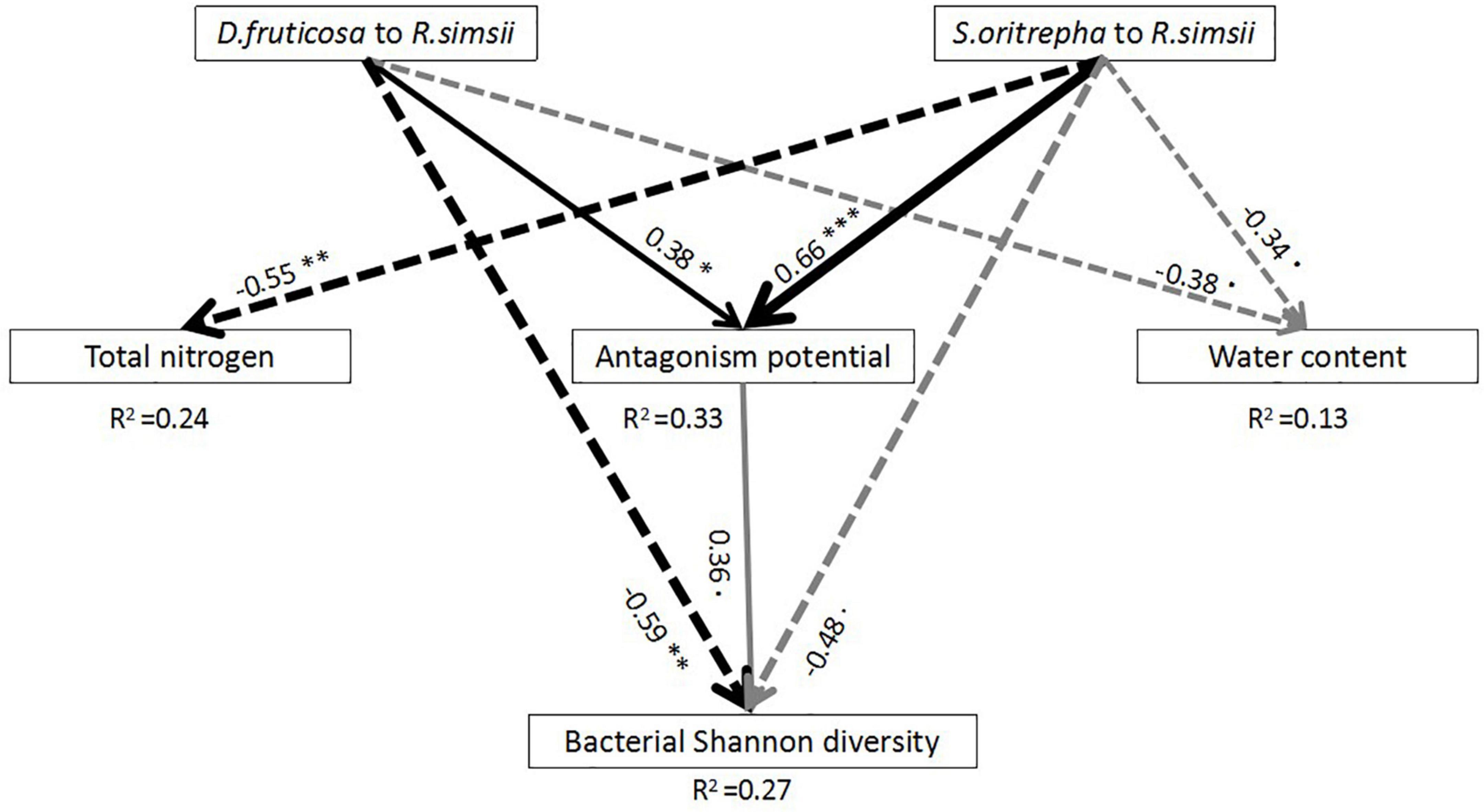
Figure 4. Results of the SEM analyses indicating direct and indirect effects of shrub species on soil bacterial diversity [P (Chi-square) = 0.84, df = 3, RMSEA = 0]. Square boxes displayed variables included in the model: effect of D. fruticosa compared to R. simsii, effect of S. oritrepha compared to R. simsii, total nitrogen, water content, microbial antagonism potential (PC1 scores of total Streptomyces density, antagonistic Streptomyces density, intensity of inhibition, and frequency of inhibition), and bacterial diversity. Black arrows indicated significant effects (at the level P < 0.05), and gray arrows indicated marginally significant effects (at the level P < 0.1). Solid arrows indicated positive effects, while dashed arrows indicated negative effects. R2 values associated with response variables indicated the proportion of explained variation by relationships with other variables. Values associated with solid arrows represented standardized path coefficients. Symbol: ∗∗∗P < 0.001; ∗∗P < 0.01; ∗P < 0.05.
Numerous research results have shown that plant species have significant impacts on the composition and diversity of bacterial communities (Marschner et al., 2004; Costa et al., 2006; De Deyn et al., 2011). Our results showed that there were significant differences in soil bacterial community composition among shrub species (Supplementary Figure 1), and it may be that soil physicochemical properties regulated the effects of shrub species on bacterial community composition. Specifically, compared to R. simsii, S. oritrepha affected the composition of associated bacterial communities through changing soil total nitrogen (Supplementary Figure 2). However, we did not find that plant species had a significant effect on the soil bacterial Shannon community in our studies (Figure 1). The mechanisms that plant impact soil microbial communities are complex (Bever et al., 2012). Sometimes, although the total effect of plant species on microbial diversity is not significant, the direct or indirect effect are significant (Schlatter et al., 2015a; Wang et al., 2018). Similarly, our result showed that the indirect effects of plants species mediated by Streptomyces’s antagonism potential may counteract the direct effects on bacterial diversity (Figure 4).
Compared to the evergreen shrub species (R. simsii), deciduous shrubs (D. fruticosa and S. oritrepha) had directly negative effects on bacterial diversity (Figure 4). Many studies showed that the impacts of plant species on soil bacterial diversity depend on the resources provided by the plant species (Sanaei et al., 2022). Kinkel et al. (2011) predicted that high nutrient input would decrease the soil microbial community diversity by decreasing the competition between the microbes in a high density initial soil microbial community. The leaf litter produced by deciduous shrubs is generally easier to decompose than that of evergreen shrubs (DeMarco et al., 2014; Vankoughnett and Grogan, 2016), while evergreen shrubs produce more recalcitrant litter (Cornelissen, 1996; Cornelissen et al., 1999). Further, more easily decomposed plant litter produced by deciduous shrubs is thought to increase soil nutrient storage (Cornelissen et al., 2007). Some studies showed that deciduous shrubs maintain higher rates of primary production compared to evergreen shrubs (van Wijk et al., 2004). Our results showed that the soil organic carbon under deciduous shrubs was higher than under evergreen shrubs, although the difference was not significant (Figure 4). Thus, we inferred that deciduous shrubs (D. fruticosa and S. oritrepha) induce a higher-resource environment, which directly reduces soil bacterial diversity, compared to the evergreen shrub (R. simsii).
Shrub species had indirect effects on bacterial diversity through influence on the antagonism potential of Streptomyces (Figure 4). Compared to the evergreen shrub species (R. simsii), deciduous shrubs (D. fruticosa and S. oritrepha) significantly increased the soil microbial antagonism potential driven by Streptomyces. It has been demonstrated that resources are an important factor affecting microbial interactions (Kinkel et al., 2012). The majority of the resources obtained by saprotrophic Streptomyces are ultimately from plants (Wiggins and Kinkel, 2005a). More importantly, plant root exudation and litter are suggested to modify the effect of plants on microbial interaction (Bakker et al., 2013b). Thus, we inferred that plant characteristics are very likely to affect the potential of microbial antagonism driven by Streptomyces (Wiggins and Kinkel, 2005a).
High phenolic-allelopathy (Moral and Cates, 1971) and other secondary compounds (Ward et al., 2009) have been found in evergreen shrubs. It is reported that Rhododendron plants possessed various constituents, and presented significant allelopathic potential (Chou et al., 2009). Rhododendron plants release allelopathic constituents (e.g., catechin) to affect surrounding plants and microorganisms through root exudation and litter (An et al., 2003). Allelopathic constituents are concentrated in plant senescent leaves (Wang et al., 2013), and plant litter decomposition is accompanied by the release of nutrients and allelochemicals. Researchers have proposed that catechin acts as the major allelopathic compound in leaves of Rhododendron plants (Wang et al., 2013); it seems to transform rapidly after being injected into the soil, and subsequently reduces the total cultivable numbers of bacterial community and inhibits the growth of some soil bacterial populations (Inderjit and van der Putten, 2010; Wang et al., 2013). There are also some studies showing that catechin can suppress the growth and activity of microorganisms groups that are sensitive to it (Wang et al., 2013). Thus, we inferred that the allelopathy of the evergreen shrub R. simsii may be the key factor to inhibit the growth of the Streptomyces population, as well as inhibiting microbial antagonistic interactions driven by Streptomyces. However, more controlled experiments are needed to explore the mechanistic effect of the allelopathy of R. simsii on the soil antagonism potential of Streptomyces. In addition, compared to the evergreen shrub (R. simsii), deciduous shrubs (D. fruticosa and S. oritrepha) contain lower concentrations of allelopathic constituents (e.g., tannins) and other secondary compounds (Cornelissen et al., 1999; Ward et al., 2009). Therefore, the results showed that D. fruticosa and S. oritrepha promote the amicrobial antagonistic interaction driven by Streptomyces.
Moreover, we found that the diversity of the soil bacterial community was mediated by the antagonism potential of Streptomyces. Microbial antagonism has been shown to significantly increase soil bacterial diversity. Several studies have found a close relationship existed in microbial interactions and the composition and diversity of soil bacterial communities (Czaran et al., 2002; Ryan and Dow, 2008; Hibbing et al., 2010; Becker et al., 2012). Research showed that specific genotypes of Escherichia coli produce antibiotic colicins, and its toxic effect promotes antagonistic genotype diversity. Likewise, based on a spatially theoretical model, antibiotic interactions within microbial communities have been shown to play an important role in maintaining microbial diversity (Czaran et al., 2002). Additionally, ubiquitous antagonism among the bacterial community (Validov et al., 2005) is hypothesized to favor higher community diversity (Kerr et al., 2002). At the same time, research has shown that a higher proportion of antagonism can significantly increase the diversity of soil bacterial communities (Bakker et al., 2013a). Similarly, a higher frequency of antagonistic bacteria is hypothesized to support the more diverse bacterial communities (Czaran et al., 2002). Therefore, our results are consistent with these findings, and more antagonism activities are expected to increase bacterial diversity.
Our research provides a better understanding of how plant species directly and indirectly affect bacterial diversity and emphasizes the importance of Streptomyces’s antagonism potential in regulating the effects of plant species on soil bacterial diversity. Overall, compared with an evergreen shrub (R. simsii), deciduous shrubs (D. fruticosa and S. oritrepha) decreased bacterial community diversity directly, but they increased bacterial community diversity indirectly through enhancing Streptomyces’s antagonistic interactions. Thus, Streptomyces’s antagonism potential can offset the directly negative effects of plant species on microbial diversity. This study provides a theoretical basis for studying the microbial interactions that regulate the effects of plant species on soil microbial community diversity.
The data presented in this study can be found at the link below: https://datadryad.org/stash/share/tSSena_Xd20RBrgJktKKA7uR0yYxJlxSeIxBinxPGSw.
SC: conception and design of study. YW, LM, ZL, JC, HS, JW, HC, ZY, SX, and KL: acquisition of data. YW, LM, and SX: analysis and interpretation of data. YW, LM, and SC: drafting the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Research Station of Alpine Meadow and Wetland Ecosystems of Lanzhou University for permission to use their site. This research was supported by the Project of the National Natural Science Foundation of China (Nos. 41830321, 31870412, and 32071532).
We thank the Gannan Grassland Ecosystem National Field Scientific Observation and Research Station of Lanzhou University. We thank Aifeng Guo, Limin Zhang, and Lihua Meng for their assistance on field experiment. We also thank Dr. Matthew G. Bakker for his help in revising the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.984200/full#supplementary-material
Adamczyk, B., Ahvenainen, A., Sietio, O. M., Kanerva, S., Kieloaho, A. J., Smolander, A., et al. (2016). The contribution of ericoid plants to soil nitrogen chemistry and organic matter decomposition in boreal forest soil. Soil Biol. Biochem. 103, 394–404. doi: 10.1016/j.soilbio.2016.09.016
An, M., Liu, D. L., Johnson, I. R., and Lovett, J. V. (2003). Mathematical modelling of allelopathy: II. The dynamics of allelochemicals from living plants in the environment. Ecol. Model. 161, 53–66. doi: 10.1016/S0304-3800(02)00289-2
Badri, D. V., and Vivanco, J. M. (2009). Regulation and function of root exudates. Plant Cell Environ. 32, 666–681. doi: 10.1111/j.1365-3040.2009.01926.x
Bakker, M. G., Bradeen, J. M., and Kinkel, L. L. (2013a). Effects of plant host species and plant community richness on streptomycete community structure. FEMS Microbiol. Ecol. 83, 596–606. doi: 10.1111/1574-6941.12017
Bakker, M. G., Otto-Hanson, L., Lange, A. J., Bradeen, J. M., and Kinkel, L. L. (2013b). Plant monocultures produce more antagonistic soil Streptomyces communities than high-diversity plant communities. Soil Biol. Biochem. 65, 304–312. doi: 10.1016/j.soilbio.2013.06.007
Becker, J., Eisenhauer, N., Scheu, S., and Jousset, A. (2012). Increasing antagonistic interactions cause bacterial communities to collapse at high diversity. Ecol. Lett. 15, 468–474. doi: 10.1111/j.1461-0248.2012.01759.x
Bergsma-Vlami, M., Prins, M. E., and Raaijmakers, J. M. (2005). Influence of plant species on population dynamics, genotypic diversity and antibiotic production in the rhizosphere by indigenous Pseudomonas spp. FEMS Microbiol. Ecol. 52, 59–69. doi: 10.1016/j.femsec.2004.10.007
Bever, J. D., Platt, T. G., and Morton, E. R. (2012). Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu. Rev. Microbiol. 66, 265–283. doi: 10.1146/annurev-micro-092611-150107
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Lozupone, C. A., Turnbaugh, P. J., et al. (2011). Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. U.S.A. 108, 4516–4522. doi: 10.1073/pnas.1000080107
Chou, S. C., Krishna, V., and Chou, C. H. (2009). Hydrophobic Metabolites from Rhododendron formosanum and their Allelopathic Activities. Nat. Product Commun. 4, 1189–1192. doi: 10.1177/1934578x0900400906
Cornelissen, J. H. C. (1996). An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. J. Ecol. 84, 573–582. doi: 10.2307/2261479
Cornelissen, J. H. C., Perez-Harguindeguy, N., Diaz, S., Grime, J. P., Marzano, B., Cabido, M., et al. (1999). Leaf structure and defence control litter decomposition rate across species and life forms in regional floras on two continents. New Phytol. 143, 191–200. doi: 10.1046/j.1469-8137.1999.00430.x
Cornelissen, J. H. C., van Bodegom, P. M., Aerts, R., Callaghan, T. V., van Logtestijn, R. S. P., Alatalo, J., et al. (2007). Global negative vegetation feedback to climate warming responses of leaf litter decomposition rates in cold biomes. Ecol. Lett. 10, 619–627. doi: 10.1111/j.1461-0248.2007.01051.x
Costa, R., Gotz, M., Mrotzek, N., Lottmann, J., Berg, G., and Smalla, K. (2006). Effects of site and plant species on rhizosphere community structure as revealed by molecular analysis of microbial guilds. FEMS Microbiol. Ecol. 56, 236–249. doi: 10.1111/j.1574-6941.2005.00026.x
Czaran, T. L., Hoekstra, R. F., and Pagie, L. (2002). Chemical warfare between microbes promotes biodiversity. Proc. Natl. Acad. Sci. U.S.A. 99, 786–790. doi: 10.1073/pnas.012399899
Danner, D., Hagemann, D., and Fiedler, K. (2015). Mediation analysis with structural equation models: Combining theory, design, and statistics. Eur. J. Soc. Psychol. 45, 460–481. doi: 10.1002/ejsp.2106
Davelos, A. L., Kinkel, L. L., and Samac, D. A. (2004a). Spatial variation in frequency and intensity of antibiotic interactions among streptomycetes from prairie soil. Appl. Environ. Microbiol. 70, 1051–1058. doi: 10.1128/Aem.70.2.1051-1058.2004
Davelos, A. L., Xiao, K., Flor, J. M., and Kinkel, L. L. (2004b). Genetic and phenotypic traits of streptomycetes used to characterize antibiotic activities of field-collected microbes. Can. J. Microbiol. 50, 79–89. doi: 10.1139/W03-107
Davies, J., Spiegelman, G. B., and Yim, G. (2006). The world of subinhibitory antibiotic concentrations. Curr. Opin. Microbiol. 9, 445–453. doi: 10.1016/j.mib.2006.08.006
De Deyn, G. B., Quirk, H., and Bardgett, R. D. (2011). Plant species richness, identity and productivity differentially influence key groups of microbes in grassland soils of contrasting fertility. Biol. Lett. 7, 75–78. doi: 10.1098/rsbl.2010.0575
DeMarco, J., Mack, M. C., and Bret-Harte, M. S. (2014). Effects of arctic shrub expansion on biophysical vs. biogeochemical drivers of litter decomposition. Ecology 95, 1861–1875. doi: 10.1890/13-2221.1
Domin, H., Zurita-Gutierrez, Y. H., Scotti, M., Buttlar, J., Humeida, U. H., and Fraune, S. (2018). Predicted bacterial interactions affect in vivo microbial colonization dynamics in nematostella. Front. Microbiol. 9:728. doi: 10.3389/Fmicb.2018.00728
Doumbou, C. L., Salove, M. K. H., Crawford, D. L., and Beaulieu, C. (2001). Actinomycetes, promising tools to control plant diseases and to promote plant growth. Phytoprotection 82, 85–102. doi: 10.7202/706219ar
Essarioui, A., LeBlanc, N., Kistler, H. C., and Kinkel, L. L. (2017). Plant community richness mediates inhibitory interactions and resource competition between Streptomyces and fusarium populations in the rhizosphere. Microb. Ecol. 74, 157–167. doi: 10.1007/s00248-016-0907-5
Fierer, N., Lauber, C. L., Ramirez, K. S., Zaneveld, J., Bradford, M. A., and Knight, R. (2012). Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 6, 1007–1017. doi: 10.1038/ismej.2011.159
Fox, J., Friendly, M., and Weisberg, S. (2013). Hypothesis Tests for Multivariate Linear Models Using the car Package. R J. 5, 39–52.
Garbeva, P., van Veen, J. A., and van Elsas, J. D. (2004). Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu. Rev. Phytopathol. 42, 243–270. doi: 10.1146/annurev.phyto.42.012604.135455
Grime, J. P. (1984). The ecology of species, families and communities of the contemporary british flora. New Phytol. 98, 15–33. doi: 10.1111/j.1469-8137.1984.tb06096.x
Herold, N., Schoning, I., Gutknecht, J., Alt, F., Boch, S., Muller, J., et al. (2014). Soil property and management effects on grassland microbial communities across a latitudinal gradient in Germany. Appl. Soil Ecol. 73, 41–50. doi: 10.1016/j.apsoil.2013.07.009
Hibbing, M. E., Fuqua, C., Parsek, M. R., and Peterson, S. B. (2010). Bacterial competition: surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 8, 15–25. doi: 10.1038/nrmicro2259
Inderjit van der Putten, W. H. (2010). Impacts of soil microbial communities on exotic plant invasions. Trends Ecol. Evol. 25, 512–519. doi: 10.1016/j.tree.2010.06.006
Jauri, P. V., and Kinkel, L. L. (2014). Nutrient overlap, genetic relatedness and spatial origin influence interaction-mediated shifts in inhibitory phenotype among Streptomyces spp. FEMS Microbiol. Ecol. 90, 264–275. doi: 10.1111/1574-6941.12389
Jiao, S., Liu, Z. S., Lin, Y. B., Yang, J., Chen, W. M., and Wei, G. H. (2016). Bacterial communities in oil contaminated soils: biogeography and co-occurrence patterns. Soil Biol. Biochem. 98, 64–73. doi: 10.1016/j.soilbio.2016.04.005
Kerr, B., Riley, M. A., Feldman, M. W., and Bohannan, B. J. M. (2002). Local dispersal promotes biodiversity in a real-life game of rock-paper-scissors. Nature 418, 171–174. doi: 10.1038/nature00823
Kinkel, L. L., Bakker, M. G., and Schlatter, D. C. (2011). A Coevolutionary Framework for Managing Disease-Suppressive Soils. Annu. Rev. Phytopathol. 49, 47–67. doi: 10.1146/annurev-phyto-072910-095232
Kinkel, L. L., Schlatter, D. C., Bakker, M. G., and Arenz, B. E. (2012). Streptomyces competition and co-evolution in relation to plant disease suppression. Res. Microbiol. 163, 490–499. doi: 10.1016/j.resmic.2012.07.005
Kudo, G., Molau, U., and Wada, N. (2001). Leaf-trait variation of tundra plants along a climatic gradient: an integration of responses in evergreen and deciduous species. Arctic Antarct. Alpine Res. 33, 181–190. doi: 10.2307/1552219
Lopez-Pintor, A., Sal, A. G., and Benayas, J. M. R. (2006). Shrubs as a source of spatial heterogeneity - the case of Retama sphaerocarpa in Mediterranean pastures of central Spain. Acta Oecol. Int. J. Ecol. 29, 247–255. doi: 10.1016/j.actao.2005.11.001
Marschner, P., Crowley, D., and Yang, C. H. (2004). Development of specific rhizosphere bacterial communities in relation to plant species, nutrition and soil type. Plant Soil 261, 199–208. doi: 10.1023/B:Plso.0000035569.80747.C5
Moral, R. D., and Cates, R. G. (1971). Allelopathic potential of the dominant vegetation of Western Washington. Ecology 52, 1030–1037. doi: 10.2307/1933809
Myers-Smith, I. H., Forbes, B. C., Wilmking, M., Hallinger, M., Lantz, T., Blok, D., et al. (2011). Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ. Res. Lett. 6:045509. doi: 10.1088/1748-9326/6/4/045509
Pastor, J., Dewey, B., Naiman, R. J., Mcinnes, P. F., and Cohen, Y. (1993). Moose browsing and soil fertility in the boreal forests of isle-royale-national-park. Ecology 74, 467–480. doi: 10.2307/1939308
Pennanen, T., Liski, J., Baath, E., Kitunen, V., Uotila, J., Westman, C. J., et al. (1999). Structure of the microbial communities in coniferous forest soils in relation to site fertility and stand development stage. Microb. Ecol. 38, 168–179. doi: 10.1007/s002489900161
Qin, J. J., Li, Y. R., Cai, Z. M., Li, S. H., Zhu, J. F., Zhang, F., et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60. doi: 10.1038/nature11450
Rognes, T., Flouri, T., Nichols, B., Quince, C., and Mahe, F. (2016). VSEARCH: a versatile open source tool for metagenomics. Peerj 4:e2584. doi: 10.7717/Peerj.2584
Rosseel, Y. (2012). lavaan: An R package for structural equation modeling. J. Stat. Softw. 48, 1–36. doi: 10.18637/Jss.V048.I02
Rousk, J., Baath, E., Brookes, P. C., Lauber, C. L., Lozupone, C., Caporaso, J. G., et al. (2010). Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 4, 1340–1351. doi: 10.1038/ismej.2010.58
Ryan, R. P., and Dow, J. M. (2008). Diffusible signals and interspecies communication in bacteria. Microbiol. SGM 154, 1845–1858. doi: 10.1099/mic.0.2008/017871-0
Sanaei, A., Sayer, E. J., Yuan, Z., Lin, F., Fang, S., Ye, J., et al. (2022). Soil stoichiometry mediates links between tree functional diversity and soil microbial diversity in a temperate forest. Ecosystems 25, 291–307. doi: 10.1007/s10021-021-00655-3
Schlatter, D. C., Bakker, M. G., Bradeen, J. M., and Kinkel, L. L. (2015a). Plant community richness and microbial interactions structure bacterial communities in soil. Ecology 96, 134–142.
Schlatter, D. C., Bakker, M. G., Bradeen, J. M., and Kinkel, L. L. (2015b). Plant community richness and microbial interactions structure bacterial communities in soil. Ecology 96, 134–142. doi: 10.1890/13-1648.1
Schlatter, D. C., and Kinkel, L. L. (2014). Global biogeography of Streptomyces antibiotic inhibition, resistance, and resource use. FEMS Microbiol. Ecol. 88, 386–397. doi: 10.1111/1574-6941.12307
Seshasayee, A. S. N., Bertone, P., Fraser, G. M., and Luscombe, N. M. (2006). Transcriptional regulatory networks in bacteria: from input signals to output responses. Curr. Opin. Microbiol. 9, 511–519. doi: 10.1016/j.mib.2006.08.007
Sun, P. P., Otto-Hanson, L. K., Arenz, B. E., Ma, Q., and Kinkel, L. L. (2015). Molecular and functional characteristics of streptomycete communities in relation to soil factors and potato common scab. Eur. J. Soil Biol. 70, 58–66. doi: 10.1016/j.ejsobi.2015.07.004
Takano, H., Nishiyama, T., Amano, S., Beppu, T., Kobayashi, M., and Ueda, K. (2016). Streptomyces metabolites in divergent microbial interactions. J. Ind. Microbiol. Biotechnol. 43, 143–148. doi: 10.1007/s10295-015-1680-z
Validov, S., Mavrodi, O., De La Fuente, L., Boronin, A., Weller, D., Thomashow, L., et al. (2005). Antagonistic activity among 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. FEMS Microbiol. Lett. 242, 249–256. doi: 10.1016/j.femsle.2004.11.013
van Wijk, M. T., Clemmensen, K. E., Shaver, G. R., Williams, M., Callaghan, T. V., Chapin, F. S., et al. (2004). Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: generalizations and differences in ecosystem and plant type responses to global change. Global Change Biol. 10, 105–123. doi: 10.1111/j.1365-2486.2003.00719.x
Vankoughnett, M. R., and Grogan, P. (2016). Plant production and nitrogen accumulation above- and belowground in low and tall birch tundra communities: the influence of snow and litter. Plant Soil 408, 195–210. doi: 10.1007/s11104-016-2921-2
Veen, G. F., Olff, H., Duyts, H., and van der Putten, W. H. (2010). Vertebrate herbivores influence soil nematodes by modifying plant communities. Ecology 91, 828–835. doi: 10.1890/09-0134.1
Wang, C. M., Li, T. C., Jhan, Y. L., Weng, J. H., and Chou, C. H. (2013). The impact of microbial biotransformation of catechin in enhancing the allelopathic effects of rhododendron formosanum. PLoS One 8:85162. doi: 10.1371/journal.pone.0085162
Wang, X. T., Nielsen, U. N., Yang, X. L., Zhang, L. M., Zhou, X. H., Du, G. Z., et al. (2018). Grazing induces direct and indirect shrub effects on soil nematode communities. Soil Biol. Biochem. 121, 193–201. doi: 10.1016/j.soilbio.2018.03.007
Wang, X. T., Xiao, S., Yang, X. L., Liu, Z. Y., Zhou, X. H., Du, G. Z., et al. (2019). Dominant plant species influence nematode richness by moderating understory diversity and microbial assemblages. Soil Biol. Biochem. 137:107566. doi: 10.1016/j.soilbio.2019.107566
Ward, S. E., Bardgett, R. D., McNamara, N. P., and Ostle, N. J. (2009). Plant functional group identity influences short-term peatland ecosystem carbon flux: evidence from a plant removal experiment. Funct. Ecol. 23, 454–462. doi: 10.1111/j.1365-2435.2008.01521.x
Wardle, D. A., Nilsson, M. C., Gallet, C., and Zackrisson, O. (1998). An ecosystem-level perspective of allelopathy. Biol. Rev. 73, 305–319. doi: 10.1017/S0006323198005192
Wiggins, B. E., and Kinkel, L. L. (2005a). Green manures and crop sequences influence potato diseases and pathogen inhibitory activity of indigenous streptomycetes. Phytopathology 95, 178–185. doi: 10.1094/Phyto-95-0178
Wiggins, E., and Kinkel, L. L. (2005b). Green manures and crop sequences influence alfalfa root rot and pathogen inhibitory activity among soil-borne streptomycetes. Plant Soil 268, 271–283. doi: 10.1007/s11104-004-0300-x
Xu, R. K., and Coventry, D. R. (2003). Soil pH changes associated with lupin and wheat plant materials incorporated in a red-brown earth soil. Plant Soil 250, 113–119. doi: 10.1023/A:1022882408133
Keywords: bacterial diversity, structural equation model, antagonism, microbial interactions, plant species
Citation: Wang Y, Ma L, Liu Z, Chen J, Song H, Wang J, Cui H, Yang Z, Xiao S, Liu K, An L and Chen S (2022) Microbial interactions play an important role in regulating the effects of plant species on soil bacterial diversity. Front. Microbiol. 13:984200. doi: 10.3389/fmicb.2022.984200
Received: 01 July 2022; Accepted: 12 August 2022;
Published: 15 September 2022.
Edited by:
Yong Wang, Guizhou University, ChinaReviewed by:
Christopher Blackwood, Kent State University, United StatesCopyright © 2022 Wang, Ma, Liu, Chen, Song, Wang, Cui, Yang, Xiao, Liu, An and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuyan Chen, Y2hlbnNoeUBsenUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.