- 1Department of Clinical Laboratory, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Key Laboratory of Precision Medicine in Diagnosis and Monitoring Research of Zhejiang Province, Hangzhou, China
Ureaplasma spp. and Mycoplasma hominis, frequent colonizers in the lower urogenital tract, have been implicated in various infections, with antibiotic resistance growing and varying regionally. This study aims to investigate the prevalence and antibiotic resistance profiles of Ureaplasma spp. and M. hominis in outpatients in Hangzhou, China, from 2013 to 2019. A total of 135,263 outpatients were examined to determine the prevalence of Ureaplasma spp. and M. hominis, including 48,638 males and 86,625 females. Furthermore, trends in antibiotic susceptibility of Ureaplasma spp. and M. hominis during 1999–2019 were analyzed. The cultivation, identification, and antibiotic susceptibility of the bacteria (ofloxacin, ciprofloxacin, erythromycin, clarithromycin, azithromycin, josamycin, tetracycline, doxycycline, and pristinamycin) were determined using the Mycoplasma IST2 kit. Our study indicated that the overall prevalence of total Ureaplasma spp./M. hominis was 38.1% from 2013 to 2019. Ureaplasma spp. were the most frequently isolated species (overall prevalence, 31.3%), followed by Ureaplasma spp./M. hominis coinfection (6.0%) and single M. hominis infection (0.8%). The prevalence of Ureaplasma spp. and M. hominis was significantly higher in females than in males, and the highest positive rates of total Ureaplasma spp./M. hominis were observed in both female and male outpatients aged 14–20 years. During 2013–2019, josamycin, tetracycline, doxycycline, and pristinamycin maintained exceptionally high activity (overall resistance rates, <5%) against both Ureaplasma spp. and M. hominis, but ofloxacin and ciprofloxacin showed limited activity (overall resistance rates, >70%). During 1999–2019, the rates of resistance to ofloxacin and ciprofloxacin increased against both Ureaplasma spp. and M. hominis but decreased to erythromycin, clarithromycin, azithromycin, tetracycline, and doxycycline against Ureaplasma spp. In conclusion, our study demonstrates a high prevalence of Ureaplasma spp. compared to M. hominis and Ureaplasma spp./M. hominis, and their distribution was associated with sex and age. Josamycin, doxycycline, and tetracycline are promising antibiotics that have remarkable activity against Ureaplasma species and M. hominis.
Introduction
Ureaplasma species and Mycoplasma hominis, members of the class Mollicutes, are the smallest self-replicating and free-living organisms known, and are routinely identified as common commensal bacteria in the lower urogenital tract of healthy individuals. They are, however, sometimes implicated in various types of infections, such as chorioamnionitis, infertility, adverse pregnancy outcomes, and neonatal diseases (Waites et al., 2005; Taylor-Robinson and Lamont, 2011; Huang et al., 2015; Kletzel et al., 2018). Genital mycoplasmas can be identified in cervicovaginal and urethral specimens of 40–80% healthy humans. But they are relatively common in the urogenital tracts of sexually active adults with clinical manifestations, where Ureaplasma spp. and M. hominis can be found, with Ureaplasma spp. being the most prevalent (Song et al., 2014; Kasprzykowska et al., 2018; Beeton and Jones, 2019; Piscopo et al., 2020; Doroftei et al., 2021).
Both Ureaplasma spp. and M. hominis lack cell wall; thus, antibiotic therapies are restricted to those that prevent DNA replication (e.g., fluoroquinolones) and protein synthesis (e.g., macrolides and tetracyclines). The prevalence and antibiotic susceptibility profiles vary geographically, depending on antibiotic use and history of previous antibiotic exposure. Antibiotic resistance has been increasing in recent years probably due to the inappropriate use of antibiotics, which is most likely acquired through gene mutation or the acquisition of resistance determinants (Yang et al., 2020; Chalker et al., 2021). Therefore, it is critical to monitor the change of antibiotic susceptibility regularly to provide guidelines for the treatment of Ureaplasma spp. and M. hominis infections. The objective of this study was to determine the prevalence and antibiotic susceptibility of Ureaplasma spp. and M. hominis in outpatients in Hangzhou, China, from 2013 to 2019.
Materials and methods
Study participants
During the period of January 2013 to December 2019, a total of 135,263 outpatients were examined in the clinical laboratory at Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, China. Of these, 48,638 were males aged 14–89 years, and 86,625 were females aged 14–94 years. Furthermore, 804 Ureaplasma spp. isolates were collected from outpatients between March and June of 1999–2004, and 1,278 isolates were obtained from outpatients with genital manifestations, such as vaginal or cervical discharge, painful or burning urination, dysuria, frequent urination, and other symptoms, between January 2005 and December 2012, to determine the trend in the antibiotic susceptibility of Ureaplasma spp. (Xie and Zhang, 2006; Song et al., 2014). Additionally, 267 M. hominis isolates recovered from outpatients between January 2005 and December 2012 were included to determine the trend in the antibiotic susceptibility of M. hominis (Kong et al., 2016).
Sample collection, culture, and antibiotic susceptibility testing
Urethral specimens of male patients were obtained by inserting Dacron swabs 2–3 cm into the urethra and spinning for 5 s, and cervicovaginal specimens of female patients were obtained from the cervical area after exocervical mucus was cleansed with a swab. A commercial Mycoplasma IST2 assay (bioMe’rieux, Marcy-l’E’ toile, France) was used for the identification, semi-quantification of the concentration, and antibiotic susceptibility testing of Ureaplasma spp. and M. hominis. The specimens were inoculated and incubated according to the manufacturer’s instructions. Briefly, urethral and cervical swabs were inoculated in R1 medium, and the mixture was added to R2 medium and vortexed until the pellet dissolved. Then, the rehydrated R2 growth medium was distributed into wells on the Mycoplasma IST2 strip and protected from drying with mineral oil. The strip and the remaining broth were incubated at 37°C for 48 h and color changes were recorded at 24 h for Ureaplasma spp. and 48 h for M. hominis. Positive results were noticed when the color of the broth changed from yellow to red with an estimated density of each organism ≥104 CFU.
Antibiotic susceptibility testing was performed for the following antibiotics: ofloxacin, ciprofloxacin, erythromycin, clarithromycin, azithromycin, josamycin, tetracycline, doxycycline, and pristinamycin. The antibiotic resistance breakpoints for the above nine antibiotics (mg/L) were as follows: ofloxacin, resistant (R) ≥ 4; ciprofloxacin, R ≥ 2; erythromycin, R ≥ 4; clarithromycin, R ≥ 4; azithromycin, R ≥ 4; josamycin, R ≥ 8; tetracycline, R ≥ 8; doxycycline, R ≥ 8; and pristinamycin, R ≥ 2 (Kenny and Cartwright, 2001).
Statistical analysis
The SPSS Statistics for Windows v.21.0 was used to analyze the prevalence and occurrence of resistance to the nine antibiotics tested based on the Chi-square test and Fisher’s exact test. p-values of <0.05 were considered significant statistically.
Results
Prevalence of Ureaplasma spp. and Mycoplasma hominis from 2013 to 2019
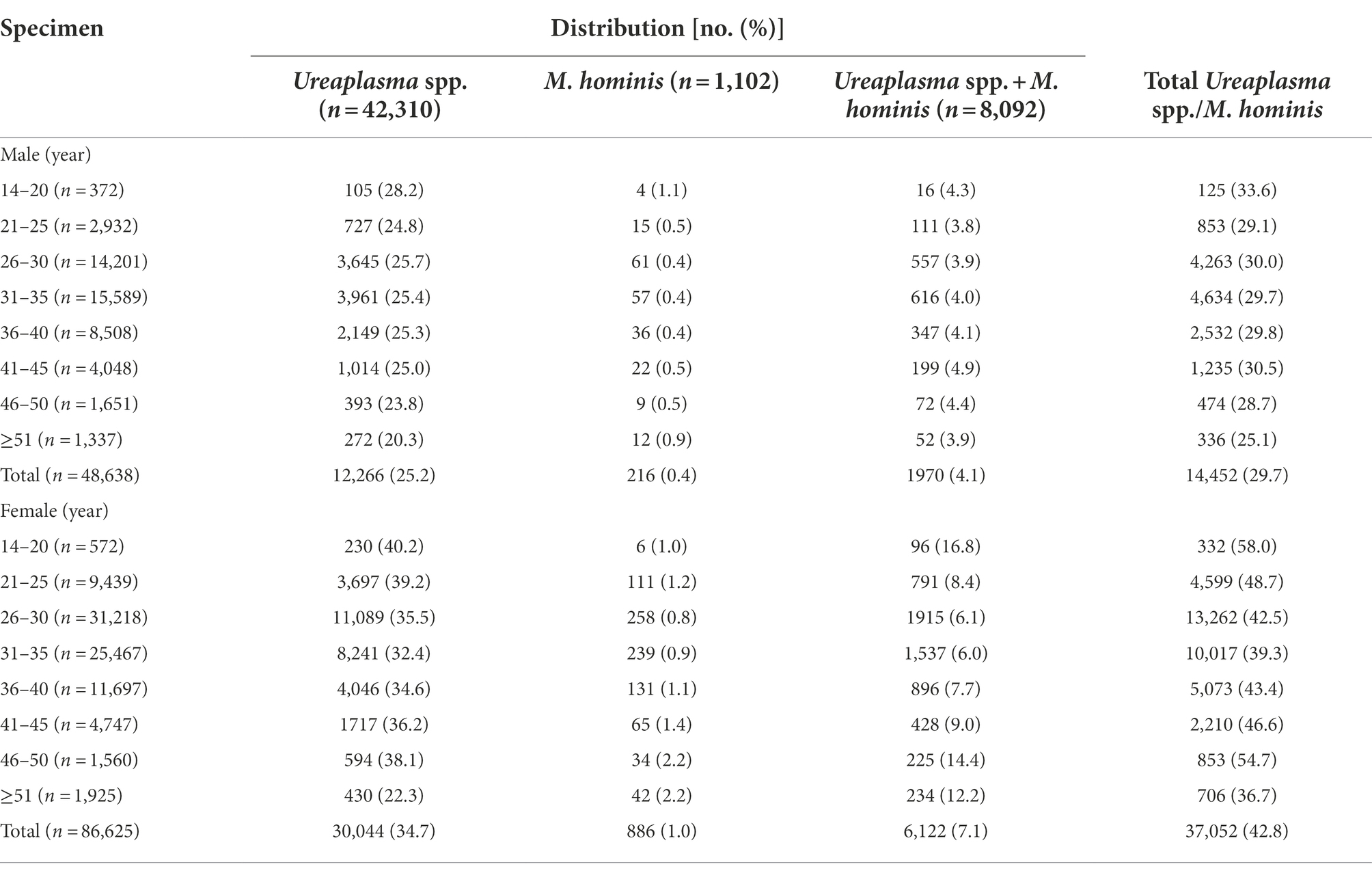
Among the 135,263 specimens tested, the overall positive rate of total Ureaplasma spp./M. hominis was 38.1% (51,504 out of 135,263). Ureaplasma spp. infection was more common than Ureaplasma spp./M. hominis coinfection (31.3% vs. 6.0%, p < 0.001) and M. hominis infection (31.3% vs. 0.8%, p < 0.001). Of the 48,638 specimens obtained from male outpatients, 12,266 (25.2%) were positive for Ureaplasma spp., 216 (0.4%) for M. hominis, and 1970 (4.1%) for both Ureaplasma spp. and M. hominis. Females had a significantly higher prevalence of Ureaplasma spp. and M. hominis than males (p < 0.001). Of the 86,625 specimens obtained from female outpatients, 30,044 (34.7%) were positive for Ureaplasma spp., 886 (1.0%) for M. hominis, and 6,122 (7.1%) for both Ureaplasma spp. and M. hominis.
Trends in the prevalence of Ureaplasma spp. and M. hominis during the test period are shown in Figure 1. Ureaplasma spp. infection rates were ranged from 23.1 to 27.1% in males and from 32.7 to 39.9% in females, which were higher than those of M. hominis infection and Ureaplasma spp./M. hominis coinfection (M. hominis, 0.3–0.6% for males and 0.8–1.4% for females; coinfection, 3.1–5.1% for males and 5.9–8.2% for females).

Figure 1. Trends in the prevalence of Ureaplasma spp. and Mycoplasma hominis in males (A) and females (B) from 2013 to 2019.
Distribution of Ureaplasma spp. and Mycoplasma hominis in different age groups from 2013 to 2019
The distribution of Ureaplasma spp. and M. hominis according to the age group from 2013 to 2019 is presented in Table 1. The overall positive rate of total Ureaplasma spp./M. hominis was highest in male patients aged 14–20 years (33.6%) and lowest in male patients aged ≥51 years (25.1%), with a declining trend as age increased. Similarly, the overall positive rate of total Ureaplasma spp./M. hominis was highest in female patients aged 14–20 years (58.0%), followed by 46–50 years (54.7%), and lowest in female patients aged 50–94 years (36.7%). For Ureaplasma spp. infection, the highest positive rates were found in both male and female patients aged 14–20 years, with 28.2% for males and 40.2% for females. For M. hominis infection, the detection rate was highest in males aged 14–20 years (1.1%) and ≥ 51 years (0.9%), but it occurred most commonly in females aged 46–50 years (2.2%) and ≥ 51 years (2.2%). Notably, among the patients with Ureaplasma spp./M. hominis coinfection, the highest detection rate was found in females aged 14–20 years (16.8%) and 46–50 years (14.4%); however, close detection rates were found in males of different age groups, ranging from 3.8 to 4.9%.
Antibiotics effectiveness from 2013 to 2019
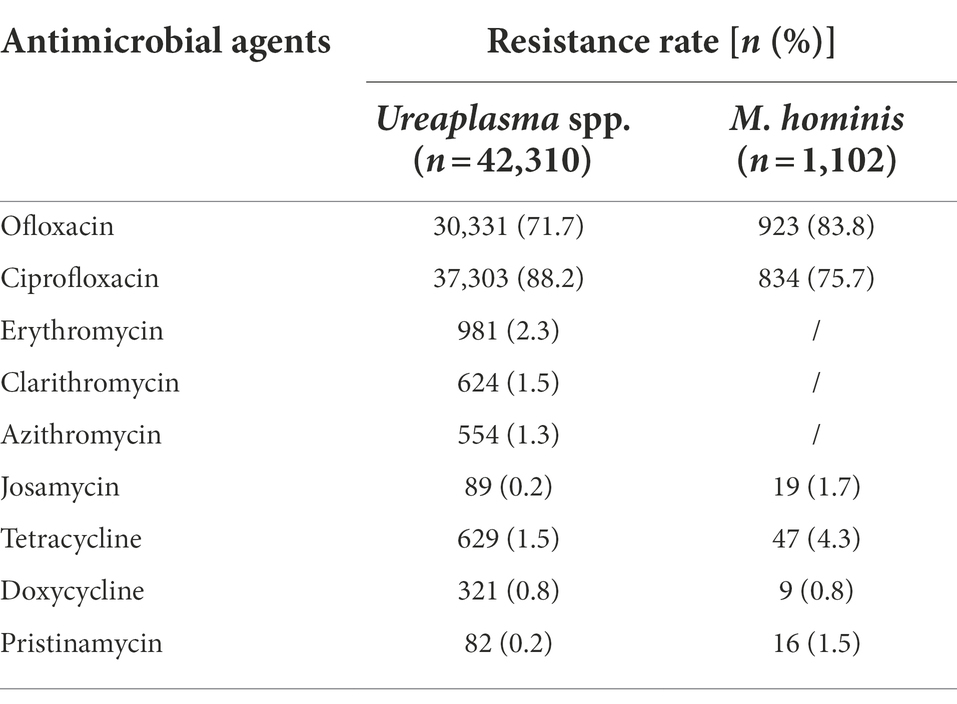
The overall resistance rates of Ureaplasma spp. and M. hominis from 2013 to 2019 are shown in Table 2. Josamycin, tetracycline, doxycycline, and pristinamycin maintained high activity against Ureaplasma spp. and M. hominis, with resistance rates all <5%. Erythromycin, clarithromycin, and azithromycin were effective against the majority of Ureaplasma spp. isolates (resistant rates, <3%). In comparison, ofloxacin and ciprofloxacin displayed limited effectiveness against both Ureaplasma spp. and M. hominis (resistant rates, >70%).
Antibiotic susceptibility patterns of Ureaplasma spp. and Mycoplasma hominis over 20 years
The antibiotic susceptibility of Ureaplasma spp. isolates collected during 2013–2019 was compared to those collected during 1999–2004 and during 2005–2012 (Figure 2). Ofloxacin resistance of Ureaplasma spp. increased from 1999 (resistance rate, 24.1%) to 2019 (resistance rate, 71.9%), whereas ciprofloxacin resistance maintained high from 2001 to 2019, with resistance rates ranging from 64.2 to 93.2% (p < 0.001). Resistance to erythromycin, clarithromycin, and azithromycin decreased, with the exception of josamycin, which maintained extremely low (resistance rates, 0–2.8%) during the test period. Resistance to tetracycline and doxycycline increased from 1999 (tetracycline, 4.6%; doxycycline, 3.7%) to 2001 (tetracycline, 12%) or 2002 (doxycycline, 11.3%), then decreased to 2019 (tetracycline, 1.3%, p < 0.001; doxycycline, 0.6%, p < 0.001). Additionally, resistance rates to pristinamycin were low, ranging from 0 to 4.6%.
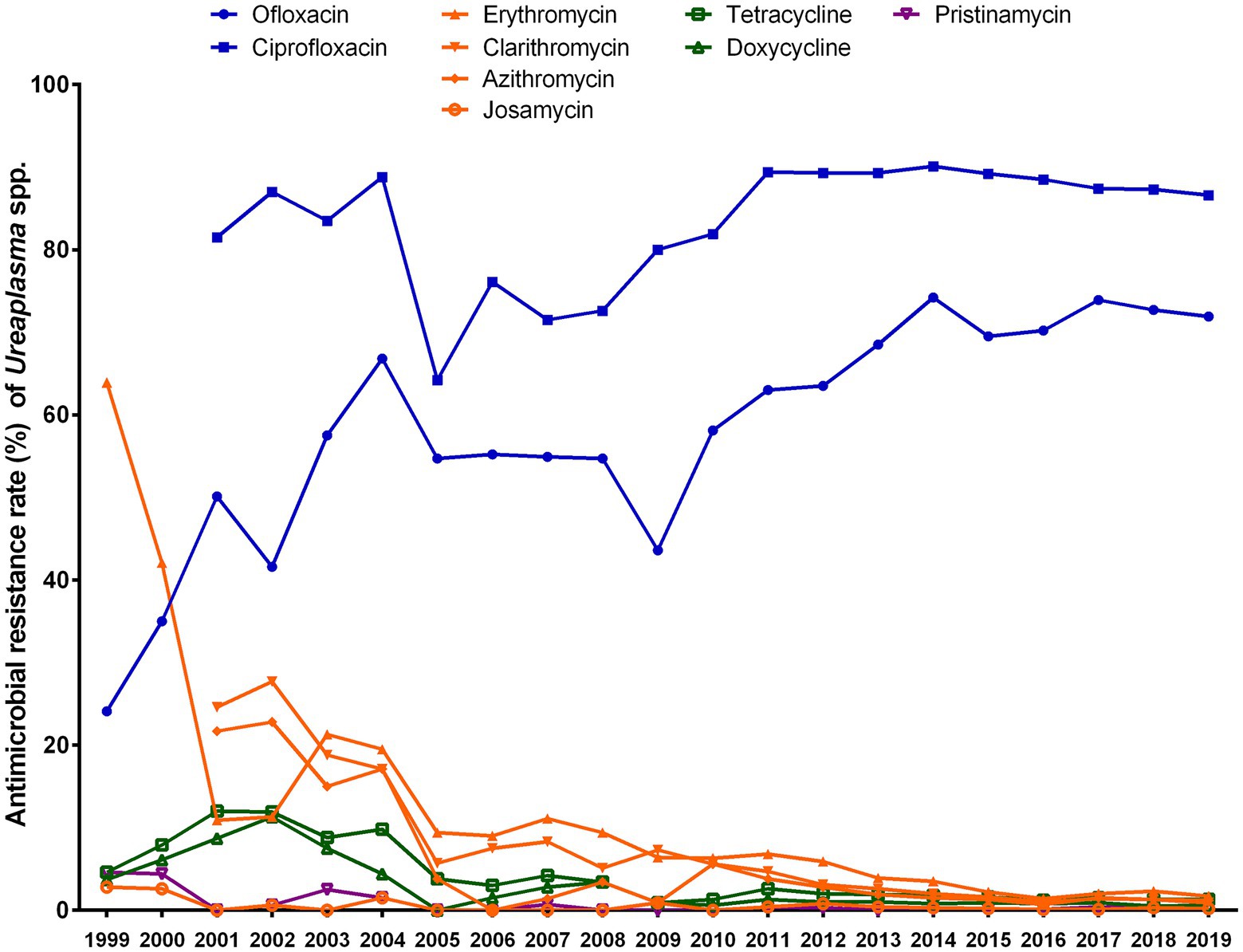
Figure 2. Trends in the antibiotic resistance rates of Ureaplasma spp. to nine antibiotics from 1999 to 2019.
The trend in the antibiotic susceptibility of M. hominis isolates during 2005–2019 is shown in Figure 3. Resistance to ofloxacin and ciprofloxacin rose from 2005 (ofloxacin, 47.1%; ciprofloxacin, 41.2%) to 2019 (ofloxacin, 81.3%; ciprofloxacin, 65.4%), with peaks in 2017 for ofloxacin (87.1%, p < 0.001) and 2014 for ciprofloxacin (83.6%; p < 0.001). Resistance rates to josamycin, tetracycline, doxycycline, and pristinamycin remained low, ranging from 0 to 8.7%.
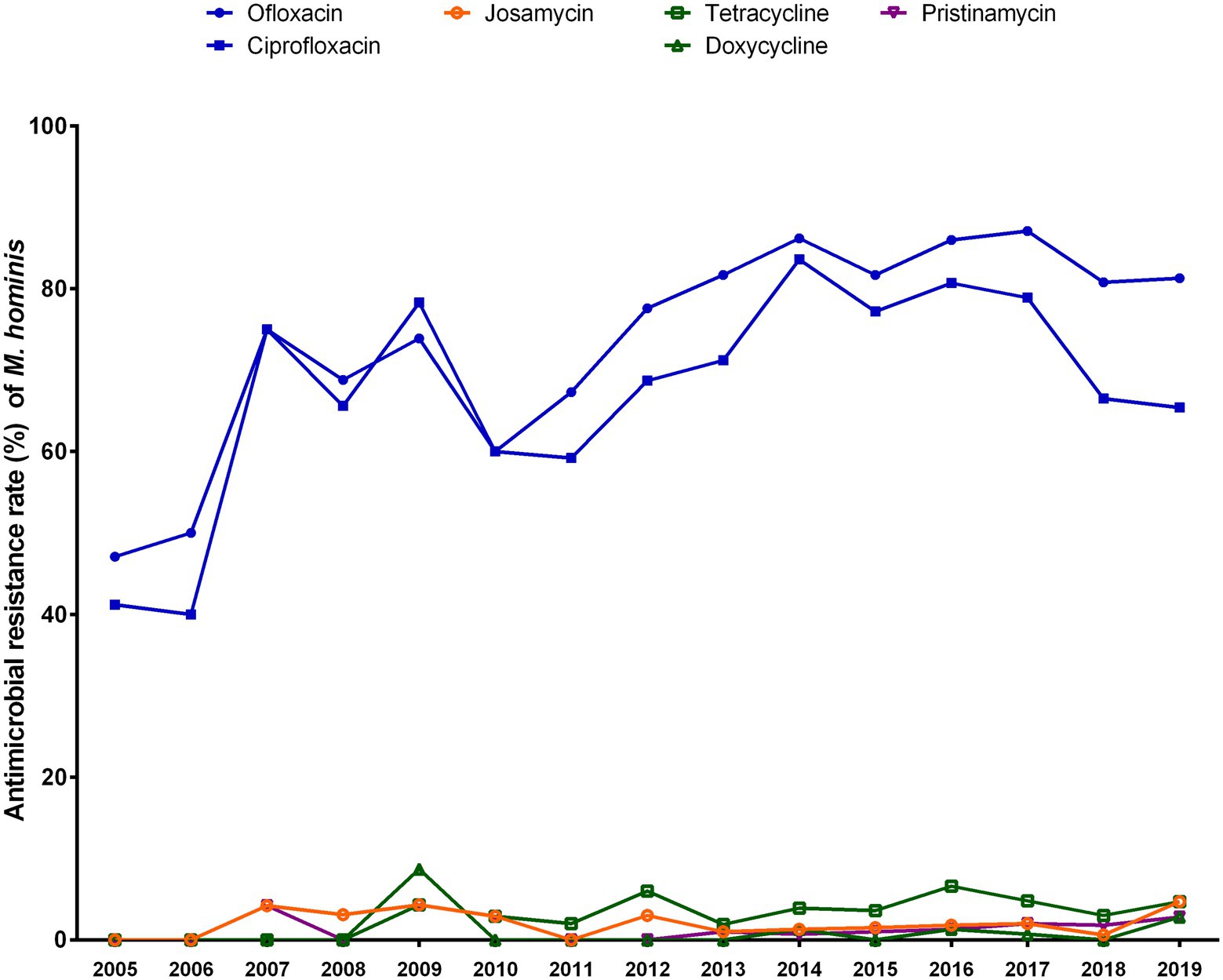
Figure 3. Trends in the antibiotic resistance rates of Mycoplasma hominis to six antibiotics from 2005 to 2019.
Discussion
Ureaplasma species and M. hominis are frequent colonizers in the urogenital tract of adults but are sometimes associated with a variety of diseases. This study aimed to evaluate the prevalence and antibiotic susceptibility of these species between 2013 and 2019. Our findings identified a high prevalence of Ureaplasma species and M. hominis (overall prevalence, 38.1%). Ureaplasma spp. infection was the most common (31.3%), followed by Ureaplasma spp./M. hominis coinfection (6.0%) and single M. hominis infection (0.8%).
Compared to our previous study analyzing the period of 2005 to 2013, the positive rates of both Ureaplasma spp. and M. hominis were decreased in females but increased in males in this study (Song et al., 2014). High prevalence of genital mycoplasmas was also observed in other provinces of China. The positive rates of genital mycoplasmas were detected in 33.9% of female outpatients in Beijing, 38.7% of infertile men in Shanghai, and 47.11% of outpatients for gynecologic healthcare screening or the presence of urogenital infection symptoms in Xi’an (Wang et al., 2016; Zeng et al., 2016; Zhou et al., 2018). Similar results were reported in South Korea, Russia, and Romania (Rumyantseva et al., 2019; Lee and Yang, 2020; Doroftei et al., 2021), but relatively lower positive rates were reported in Poland, Italy, and Brazil (Ponyai et al., 2013; Foschi et al., 2018; Piscopo et al., 2020), which could be explained by the discrepancy in socioeconomic conditions, living standards, and the experimental methods used. Notably, the identification of Ureaplasma spp. and M. hominis in clinical specimens depends on a variety of commercial Mycoplasma testing kits based on molecular or culture methods, the sensitivity and specificity of which are mostly unknown. Moreover, an unequal prevalence between sexes was observed, in which the detection rates of Ureaplasma spp. and M. hominis were higher in the female population than in the male population. The higher occurrence of Ureaplasma spp. and M. hominis in females appears to be a general trend, as evidenced by an increasing number of studies (Ponyai et al., 2013; Zeng et al., 2016; Foschi et al., 2018; Kasprzykowska et al., 2018).
Our study also indicated that the prevalence of Ureaplasma spp. was higher in younger individuals and declined with age, but we cannot ignore the fact that the number of both male and female patients aged 14–20 years was considerably lower than that of any other age group. However, M. hominis was more frequently isolated from the older individuals, with an increasing trend as age increased, especially in female patients. This result is consistent with our previous study, which showed that M. hominis was more prevalent in male patients aged 56–60 years, and in female patients aged 61–65 years and 46–50 years (Kong et al., 2016). Lee et al. reported that Ureaplasma spp. were most commonly found in female patients aged 18–29 years, but M. hominis was more common in females aged 60–89 years, followed by 30–39 years, in Seoul, South Korea (Lee and Yang, 2020). However, Zhou et al. showed that Ureaplasma spp. and M. hominis occurred mostly in infertile men aged 26–30 years and 21–25 years, respectively, in Shanghai, China (Zhou et al., 2018). These findings suggest that Ureaplasma spp. are more likely to be detected in younger patients, but further studies are required to determine the association between M. hominis prevalence and age.
Antibiotic resistance is the leading cause of treatment failure in genital mycoplasmas infections, and the increasing antibiotic resistance has prompted researchers to conduct ongoing monitoring investigations. In this study, a significant variation in levels of sensitivity to various antibiotics was discovered. The majority of clinical Ureaplasma spp. isolates were susceptible to macrolides (erythromycin, clarithromycin, azithromycin, and josamycin), tetracyclines (tetracycline and doxycycline), and streptogramins (pristinamycin), suggesting that macrolides, tetracyclines, and streptogramins are effective antibiotics against Ureaplasma spp. However, the current findings revealed that Ureaplasma spp. were extremely resistant to fluoroquinolones, which is consistent with our and other recent studies on fluoroquinolone resistance in Ureaplasma spp. in China (Xie and Zhang, 2006; Song et al., 2014; Wang et al., 2016; Yang et al., 2020; Ma et al., 2021). In our recent study, the resistance rates of levofloxacin were 84.69% for U. parvum and 82.43% for U. urealyticum, and those of moxifloxacin were 51.44% for U. parvum and 62.16% for U. urealyticum (Yang et al., 2020). Notably, fluoroquinolone resistance levels differed significantly between countries. In Italy, 77.1% of Ureaplasma spp. were ciprofloxacin-resistant, and 26.3% of isolates were ofloxacin-resistant (Foschi et al., 2018). In the United States, however, the resistance rates of levofloxacin in Ureaplasma spp. were extremely low, with only 1.6% for U. parvum and 0% for U. urealyticum (Valentine-King and Brown, 2017). The primary variation is perhaps related to the strategy or inclination for using antibiotics in different regions.
M. hominis is intrinsically resistant to C14- and C15-membered macrolides (erythromycin, clarithromycin, and azithromycin), but susceptible to C16-membered macrolides (josamycin). Our results showed that the majority of clinical M. hominis isolates were susceptible to C16-membered macrolides (josamycin), tetracyclines (tetracycline and doxycycline), and streptogramins (pristinamycin), but most of them were resistant to fluoroquinolones (ofloxacin and ciprofloxacin). These findings were consistent with several previous studies (Wang et al., 2016; Zeng et al., 2016; Foschi et al., 2018) but differed from others (Valentine-King and Brown, 2017; Foschi et al., 2018). During the test period in China, fluoroquinolone resistance increased and reached an extraordinarily high level against both Ureaplasma spp. and M. hominis, perhaps due to the inappropriate use of fluoroquinolone agents in both poultry industry and clinical settings (Chen Z. et al., 2021; Chen H. et al., 2021).
Overall, our results showed that josamycin, tetracycline, doxycycline, and pristinamycin maintained outstanding activity against Ureaplasma spp. and M. hominis. Due to its toxicity, pristinamycin was no longer a viable alternative, so it has been unavailable for therapeutic prescription in several countries. Additionally, erythromycin, clarithromycin, and azithromycin are all candidates for Ureaplasma spp. infection therapy.
This study has some important limitations. First, it was unable to discriminate between actual genital mycoplasma infection and common commensal colonization due to the lack of clinical data on the participants. Second, the Mycoplasma IST2 kit failed to separate between Ureaplasma spp. (U. parvum and U. urealyticum), as well as produce distinct findings for mixed cultures of Ureaplasma spp. and M. hominis, which might result in inaccurate reporting of antibiotic resistance. Third, since all clinical isolates of Ureaplasma spp. and M. hominis were generated as part of routine clinical laboratory procedures and were disposed of after being tested, the identification and antibiotic susceptibility results produced by the Mycoplasma IST2 kit cannot be compared with some other molecular-based methods or the standardized guidelines of the Clinical and Laboratory Standards Institute (CLSI) on Antimicrobial Susceptibility Testing. Regrettably, the antibiotics and breakpoints used in the Mycoplasma IST2 kit conflict with CLSI recommendations. Fourth, we were unable to perform further studies to determine the mechanisms of resistance to fluoroquinolones, macrolides, and tetracyclines in Ureaplasma spp. and M. hominis.
In conclusion, our study retrospectively analyzed the prevalence and antibiotic susceptibility of Ureaplasma spp. and M. hominis in Hangzhou, China, from 2013 to 2019. Ureaplasma spp. infection was relatively common, but M. hominis infection and Ureaplasma spp./M. hominis coinfection were exceedingly rare. Furthermore, both Ureaplasma spp. and M. hominis were more prevalent in females than in males, and their distribution was associated with age. Josamycin, doxycycline, and tetracycline are promising antibiotics with outstanding activity against Ureaplasma spp. and M. hominis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
This study was approved by the local Research Ethics Committee of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, China. All isolates were generated as part of routine clinical laboratory procedures, and no identifiable patient information was collected.
Author contributions
JS, XX, and JZ designed experiments. JS and XW carried out experiments and analyzed the results. YK and HJ checked data. JS, TY, XX, and JZ wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (82072342 and 82102429).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Beeton, M. L. P. M., and Jones, L. (2019). The role of Ureaplasma spp. in the development of nongonococcal urethritis and infertility among men. Clin. Microbiol. Rev. 32:e00137-18. doi: 10.1128/CMR.00137-18
Chalker, V. J., Sharratt, M. G., Rees, C. L., Bell, O. H., Portal, E., Sands, K., et al. (2021). Tetracycline resistance mediated by tet(M) Has variable integrative conjugative element composition in mycoplasma hominis strains isolated in the United Kingdom from 2005 to 2015. Antimicrob Agents Chemother 65:e02513-20. doi: 10.1128/AAC.02513-20
Chen, Z., Bai, J., Zhang, X., Wang, S., Chen, K., Lin, Q., et al. (2021). Highly prevalent multidrug resistance and QRDR mutations in salmonella isolated from chicken, pork and duck meat in southern China, 2018-2019. Int. J. Food Microbiol. 340:109055. doi: 10.1016/j.ijfoodmicro.2021.109055
Chen, H., Song, J., Zeng, X., Chen, D., Chen, R., Qiu, C., et al. (2021). National Prevalence of salmonella enterica serotype Kentucky ST198 with high-level resistance to ciprofloxacin and extended-Spectrum Cephalosporins in China, 2013 to 2017. mSystems 6:e00935-20. doi: 10.1128/mSystems.00935-20
Doroftei, B, Ilie, OD, Armeanu, T, Anton, E, Scripcariu, I, and Maftei, R. (2021). The prevalence of Ureaplasma Urealyticum and Mycoplasma Hominis infections in infertile patients in the northeast region of Romania. Kaunas Medicina 57:57030211. doi: 10.3390/medicina57030211
Foschi, C., Salvo, M., Galli, S., Moroni, A., Cevenini, R., and Marangoni, A. (2018). Prevalence and antimicrobial resistance of genital Mollicutes in Italy over a two-year period. New Microbiol. 41, 153–158.
Huang, C., Zhu, H. L., Xu, K. R., Wang, S. Y., Fan, L. Q., and Zhu, W. B. (2015). Mycoplasma and ureaplasma infection and male infertility: a systematic review and meta-analysis. Andrology 3, 809–816. doi: 10.1111/andr.12078
Kasprzykowska, U., Sobieszczanska, B., Duda-Madej, A., Secewicz, A., Nowicka, J., and Gosciniak, G. (2018). A twelve-year retrospective analysis of prevalence and antimicrobial susceptibility patterns of Ureaplasma spp. and Mycoplasma hominis in the province of lower Silesia in Poland. Eur. J. Obstet. Gynecol. Reprod. Biol. 220, 44–49. doi: 10.1016/j.ejogrb.2017.11.010
Kenny, G. E., and Cartwright, F. D. (2001). Susceptibilities of mycoplasma hominis, M. pneumoniae, and Ureaplasma urealyticum to GAR-936, dalfopristin, dirithromycin, evernimicin, gatifloxacin, linezolid, moxifloxacin, quinupristin-dalfopristin, and telithromycin compared to their susceptibilities to reference macrolides, tetracyclines, and quinolones. Antimicrob. Agents Chemother. 45, 2604–2608. doi: 10.1128/AAC.45.9.2604-2608.2001
Kletzel, H. H., Rotem, R., Barg, M., Michaeli, J., and Reichman, O. (2018). Ureaplasma urealyticum: the role as a pathogen in Women’s health, a systematic review. Curr. Infect. Dis. Rep. 20:33. doi: 10.1007/s11908-018-0640-y
Kong, Y. Q. Y., Song, J., Ruan, Z., Fei, C., Huang, J., Song, T., et al. (2016). Comparative analysis of male and female populations on prevalence and antibiotic resistance of mycoplasma hominis in China, 2005-2014. J Glob Antimicrob Resist 6, 69–72. doi: 10.1016/j.jgar.2016.03.004
Lee, J. Y., and Yang, J. S. (2020). Prevalence and antimicrobial susceptibility of mycoplasma hominis and Ureaplasma species in nonpregnant female patients in South Korea indicate an increasing trend of Pristinamycin-resistant isolates. Antimicrob. Agents Chemother. 64:e01065. doi: 10.1128/AAC.01065-20
Ma, H., Zhang, X., Shi, X., Zhang, J., and Zhou, Y. (2021). Phenotypic antimicrobial susceptibility and genotypic characterization of clinical Ureaplasma isolates circulating in Shanghai, China. Front. Microbiol. 12:724935. doi: 10.3389/fmicb.2021.724935
Piscopo, R. C., Guimaraes, R. V., Ueno, J., Ikeda, F., Bella, Z. I. J., Girao, M. J., et al. (2020). Increased prevalence of endocervical mycoplasma and Ureaplasma colonization in infertile women with tubal factor. JBRA Assist Reprod 24, 152–157. doi: 10.5935/1518-0557.20190078
Ponyai, K., Mihalik, N., Ostorhazi, E., Farkas, B., Parducz, L., Marschalko, M., et al. (2013). Incidence and antibiotic susceptibility of genital mycoplasmas in sexually active individuals in Hungary. Eur. J. Clin. Microbiol. Infect. Dis. 32, 1423–1426. doi: 10.1007/s10096-013-1892-y
Rumyantseva, T., Khayrullina, G., Guschin, A., and Donders, G. (2019). Prevalence of Ureaplasma spp. and mycoplasma hominis in healthy women and patients with flora alterations. Diagn. Microbiol. Infect. Dis. 93, 227–231. doi: 10.1016/j.diagmicrobio.2018.10.001
Song, T., Ye, A., Xie, X., Huang, J., Ruan, Z., Kong, Y., et al. (2014). Epidemiological investigation and antimicrobial susceptibility analysis of ureaplasma species and mycoplasma hominis in outpatients with genital manifestations. J. Clin. Pathol. 67, 817–820. doi: 10.1136/jclinpath-2014-202248
Taylor-Robinson, D., and Lamont, R. F. (2011). Mycoplasmas in pregnancy. Bjog 118, 164–174. doi: 10.1111/j.1471-0528.2010.02766.x
Valentine-King, M. A., and Brown, M. B. (2017). Antibacterial resistance in Ureaplasma species and mycoplasma hominis isolates from urine cultures in college-aged females. Antimicrob. Agents Chemother. 61:e01104. doi: 10.1128/AAC.01104-17
Waites, K. B., Katz, B., and Schelonka, R. L. (2005). Mycoplasmas and ureaplasmas as neonatal pathogens. Clin. Microbiol. Rev. 18, 757–789. doi: 10.1128/CMR.18.4.757-789.2005
Wang, Q. Y., Li, R. H., Zheng, L. Q., and Shang, X. H. (2016). Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and mycoplasma hominis in female outpatients, 2009-2013. J. Microbiol. Immunol. Infect. 49, 359–362. doi: 10.1016/j.jmii.2014.06.007
Xie, X., and Zhang, J. (2006). Trends in the rates of resistance of Ureaplasma urealyticum to antibiotics and identification of the mutation site in the quinolone resistance-determining region in Chinese patients. FEMS Microbiol. Lett. 259, 181–186. doi: 10.1111/j.1574-6968.2006.00239.x
Yang, T., Pan, L., Wu, N., Wang, L., Liu, Z., Kong, Y., et al. (2020). Antimicrobial resistance in clinical Ureaplasma spp. and mycoplasma hominis and structural mechanisms underlying quinolone resistance, Antimicrob Agents Chemother 64, e02560-19. doi: 10.1128/AAC.02560-19
Zeng, X. Y., Xin, N., Tong, X. N., Wang, J. Y., and Liu, Z. W. (2016). Prevalence and antibiotic susceptibility of Ureaplasma urealyticum and mycoplasma hominis in Xi'an, China. Eur. J. Clin. Microbiol. Infect. Dis. 35, 1941–1947. doi: 10.1007/s10096-016-2745-2
Zhou, Y. H., Ma, H. X., Yang, Y., and Gu, W. M. (2018). Prevalence and antimicrobial resistance of Ureaplasma spp. and mycoplasma hominis isolated from semen samples of infertile men in Shanghai, China from 2011 to 2016. Eur. J. Clin. Microbiol. Infect. Dis. 37, 729–734. doi: 10.1007/s10096-017-3167-5
Keywords: Ureaplasma spp., Mycoplasma hominis, prevalence, antibiotic resistance, activity
Citation: Song J, Wu X, Kong Y, Jin H, Yang T, Xie X and Zhang J (2022) Prevalence and antibiotics resistance of Ureaplasma species and Mycoplasma hominis in Hangzhou, China, from 2013 to 2019. Front. Microbiol. 13:982429. doi: 10.3389/fmicb.2022.982429
Edited by:
Fang He, Zhejiang Provincial People's Hospital, ChinaReviewed by:
Yun Heng Zhou, Zhabei Central Hospital of Jing’an District, ChinaXiaoyan Li, Southern Medical University, China
Arthur H. Totten, Stony Brook Medicine, United States
Copyright © 2022 Song, Wu, Kong, Jin, Yang, Xie and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinyou Xie, c2NvdHR4aWVAemp1LmVkdS5jbg==; Jun Zhang, amFtZXN6aGFuZzIwMDBAemp1LmVkdS5jbg==
†These authors share first authorship
 Jingjuan Song1,2†
Jingjuan Song1,2† Yingying Kong
Yingying Kong Xinyou Xie
Xinyou Xie Jun Zhang
Jun Zhang