- The State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST), Key Laboratory of Oral Biomedicine Ministry of Education, School and Hospital of Stomatology, Wuhan University, Wuhan, China
Bacteria residing within biofilms are more resistant to drugs than planktonic bacteria. They can thus play a significant role in the onset of chronic infections. Dispersion of biofilms is a promising avenue for the treatment of biofilm-associated diseases, such as dental caries. In this review, we summarize strategies for dispersion of cariogenic biofilms, including biofilm environment, signaling pathways, biological therapies, and nanovehicle-based adjuvant strategies. The mechanisms behind these strategies have been discussed from the components of oral biofilm. In the future, these strategies may provide great opportunities for the clinical treatment of dental diseases.
Introduction
Dental caries is a common oral disease that is mainly caused by cariogenic biofilm. Cariogenic biofilms constantly form in the oral cavity. Besides mechanical cleaning, auxiliary chemical methods are necessary to control their spread (Pratten et al., 1998).
For better physical settlement, microorganisms produce, and wrap themselves in a matrix that acts like a “protective scaffold” (Jamal et al., 2018). As an architectural colony, the microbial ecosystem of caries is an ordered and spatial community (Kim and Koo, 2020), which offers opportunities for close relationships and high mutation frequency to virulence genes. In addition, as active and complex organizations, cariogenic biofilms colonize competitive niches and are resistant to stressful environments. Cariogenic biofilms restrict and sequestrate the penetration of chemicals through the matrix (Sims et al., 2020). Therefore, it is not surprising that bacteria in the biofilm state are more tolerant to various antibiotics, and thus, are more difficult to control than bacteria in the planktonic state (Davies, 2003). With long-term applications, antibiofilm drugs may not only induce resistance of cariogenic bacteria but also disrupt healthy microbiota, resulting in the limitation of existing antimicrobial therapies (Perez-Diaz et al., 2015).
Several studies have focused on the inhibition of biofilms and most present agents can inhibit biofilm-forming bacteria without eradicating the mature biofilm. Due to the short effect time of inhibitors, they cannot control the biofilm well. Therefore, to some extent, dispersion and eradication of mature biofilms are very important for biofilm control.
Most strategies for cariogenic biofilm dispersion are restricted to a particular approach; however, each approach has advantages and disadvantages. It is important to reinforce the concept of co-administration of different strategies. In this review, we summarize the applications and mechanisms of the strategies for dispersion of cariogenic biofilms, including changing the micro-environment, modulating signaling molecules, and so on. In addition, we explore a few novel biological and nanovehicle-based strategies, which have the potential to be combined with traditional approaches or strengthen the effects of cariogenic biofilm dispersion.
The biofilm lifestyle and dispersion
The life cycle of cariogenic biofilms has already been studied thoroughly. The development of biofilms is generally considered to be a different stage of a cyclic process. During the infection, biofilm formation is initiated by the aggregation of planktonic cells. In the biofilm, single bacterial cells are protected against the immune system and antimicrobial agents (Serra and Hengge, 2014). The concentration gradient of oxygen, nutrient resources and waste products become steepening. These stress factors of the different micro-environment may activate the starvation mechanisms and accumulation of molecules to induce dispersion (Nguyen et al., 2011). The life cycle of a biofilm is finalized with the cells escaping via dispersion to new sites for colonization. The biofilm releases the bacterial cells and allows them to recolonize at other sites (Koo et al., 2017). Evacuation of bacteria leave behind voids in the center of mature biofilm (Rumbaugh and Sauer, 2020). Although self-disassembly can result in infection and bacteremia (Fleming and Rumbaugh, 2018), the dispersed bacteria and biofilm with center voids become much more sensitive to antimicrobial agents.
Therefore, in the final stage of biofilm development, dispersion provides a great opportunity for us to remove biofilm unaffectedly (Lin et al., 2022). It is possible to create an environment that is experienced by bacteria in biofilms during the terminal stages (such as by mediating extracellular signaling molecules, nutrient resources, and oxygen) to induce biofilm degradation and diffusion. Taking advantage of the metabolites or enzymes of microorganisms would be a gentle and specific approach that would not affect the development of dysbiosis or the balance of the beneficial oral microbiome (Pleszczynska et al., 2015). Therefore, biofilm life cycles can be exploited in effective biofilm dispersion strategies.
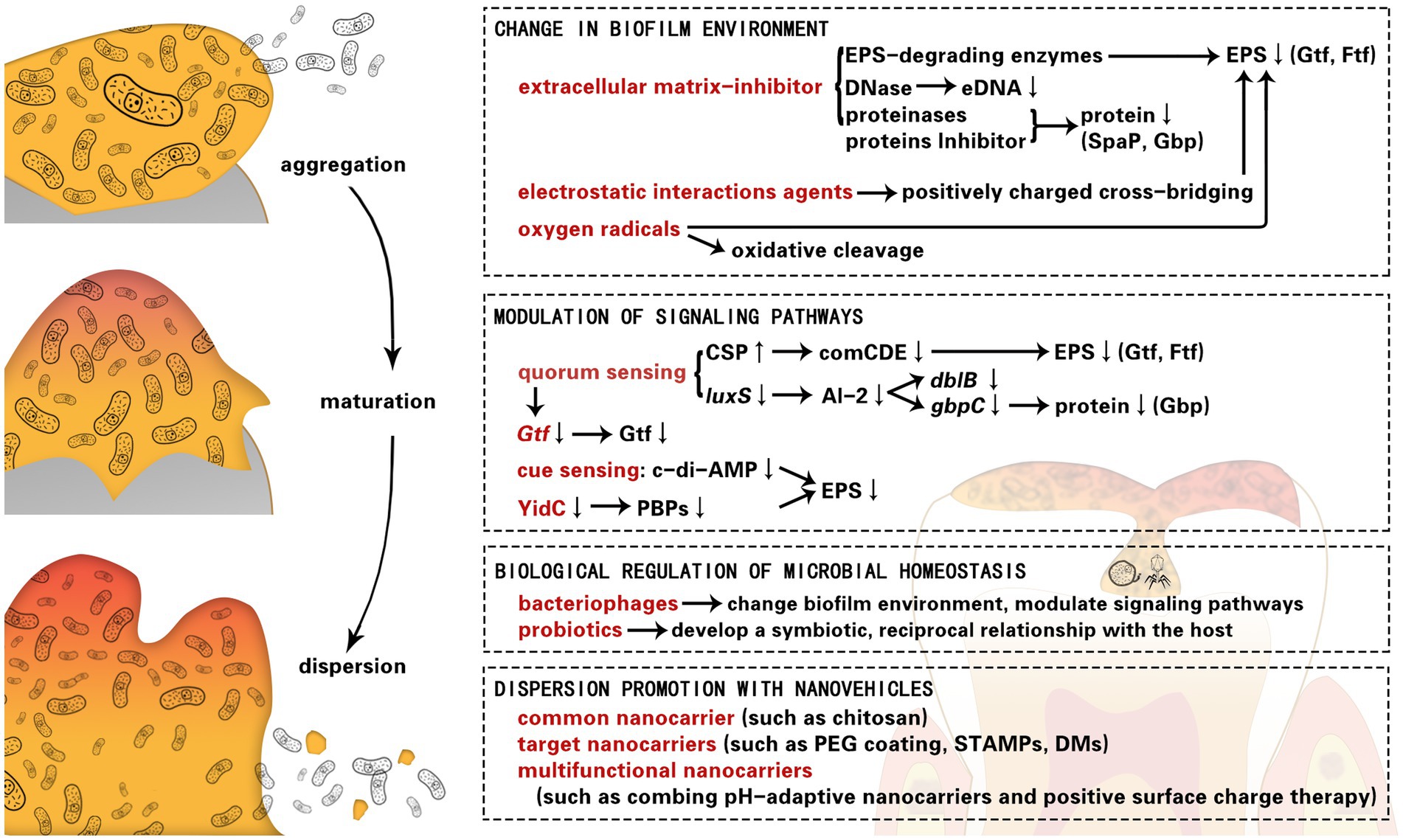
Change in biofilm environment
Extracellular matrix-inhibitor
Degradation of the matrix is an effective strategy for the “physical collapse” of the biofilms (Rainey et al., 2019). As mentioned before, the extracellular matrix is a shield for the biofilm residents, which not only provides structural protection to encase the community but also gain nutrients for metabolic utilization. Generally, these matrices comprise extracellular polymeric substances (EPS), extracellular DNA (eDNA), proteins, and lipids (Petersen et al., 2005; Jakubovics and Burgess, 2015).
Extracellular polymeric substance-degrading enzymes and inhibitor
Extracellular polymeric substances is one of the most important components in cariogenic biofilm matrices and includes glucans, and fructans. The polysaccharides in EPS promote bacterial aggregation and mediate biofilm adhesion (Lynch et al., 2007), which aids in avoiding a collapse of the biofilm architecture (Liljemark and Bloomquist, 1996). EPS are synthesized by extracellular enzymes of oral bacteria (Townsend-Lawman and Bleiweis, 1991; Vacca Smith and Bowen, 2000), such as glucosyltransferase (Gtf) and fructosyltransferase (Ftf). Gtf and Ftf transform glucose and fructose to glucan and fructans, respectively (Munro et al., 1991). Some antiplaque agents inhibit the activity of dental plaques by reducing the production of extracellular glucans (Koo et al., 2000) and fructans (Steinberg et al., 2002).
Glucans and fructans comprise primarily a mixture of different linkages, including α-(1→3), α-(1→6), and β-(1→6) glucans (Bowen and Koo, 2011), as well as β-(2→6)-linked fructan (Willcox and Drucker, 1987). As the key fractions of the matrix, EPS provides sites for the formation of metabolizable polysaccharides, cell aggregation microbial colonization (Koo et al., 2010; Xiao and Koo, 2010), and adhesion among different species (Gregoire et al., 2011). The α-(1→3)-linked glucan is presented in insoluble glucans with high concentrations and α-(1→6)-linked glucan is abundant in soluble glucans (Bowen and Koo, 2011). Glucanohydrolases contain mutanase for insoluble glucans and dextranase for soluble glucan (Hayacibara et al., 2004). Mutanases catalyze the hydrolysis of glucosidic linkages and effectively help fight against Streptococcus mutans (S. mutans) (Thallinger et al., 2013). These abilities of mutanases mainly manifest in the degree of saccharification and dissolution of water-insoluble EPS in S. mutans. Dextranase hydrolyzes dextran, which is an acceptor molecule to synthesize soluble glucans (Xiao et al., 2012). Similar effects have been observed for dispersin B, which hydrolyzes β-(1→6)-glucans (Kaplan et al., 2004). However, breaking one of the linkages in EPS monomers is not sufficient to degrade the biofilm completely. Ren et al. (2019) found that a combination of dextranase and mutanase can synergistically degrade different glycosyl linkages in a biofilm more efficiently.
Moreover, several phenols (including eugenol, catechins, quercetins, and sylvestris) showed similar functions as mutanase or dextranase. Eugenol inhibits both insoluble and soluble glucan activities of Streptococcus sobrinus (S. sobrinus) considerably (Li et al., 2012). Burt et al., reported that eugenol exhibits significant activity against the biofilm of Candida albicans (C. albicans) (Burt, 2004). C. albicans is one of the major etiological agents in early childhood caries (ECC), which may enhance the virulence of S. sobrinus and S. mutans as well (Wan et al., 2021). Meanwhile, eugenol presents low cytotoxicity and hemolytic activity. Catechins and quercetins interfere with both insoluble and soluble glucan activities (Zeng et al., 2019) by interacting with Gtf in S. mutans (Nakahara et al., 1993). This anti-Gtf action is also associated with sylvestris, which affects the quality of glucans formed by inhibiting GtfB activity. Ribeiro et al. (2019) reported that FLO/SC, PAC/CE, and PRE/SP extracts remove a significant amount of S. mutans biofilms, probably because of a decrease in the biomass of glucans produced by GtfB.
Although enzymes that degrade EPS can be used as moderate anti-biofilms agents (Otsuka et al., 2015), their applications alone have not been tested clinically due to their limited antimicrobial activity (Balakrishnan et al., 2000). Promising antibacterial activity in plant species has been noted. Piceatannol could be acted as an inhibitor of gtfC, which shares the same space as acarbose (Ito et al., 2011). Due to its specificity for the S. mutans Gtf, piceatannol interacted specifically with the adhesion of S. mutans biofilms and did not influence cell viability (Nijampatnam et al., 2018). Similarly, osteopontin exhibited an apparent selectivity toward Streptococcus mitis SK24 biofilms instead of the planktonic cells by changing the hydrophobicity of the biofilm surface (Schlafer et al., 2012).
Extracellular enzymes can successfully weaken the structure of the biofilms by targeting glucans, fructans, and their different linkages in EPS. Furthermore, a synergistic approach that combines antimicrobial agents with EPS matrix-degrading enzymes can potentially increase the effect of biofilm disruption and prevent dental caries.
Deoxyribonuclease
In recent years, environmental DNA (eDNA) has attracted much attention as a component of the matrix of cariogenic biofilms (Pedraza et al., 2017; Tawakoli et al., 2017). There are multiple functions of eDNA in biofilm formation, such as establishing the basis for initial bacterial adhesion and mediating subsequent attachment (Das et al., 2011). In addition, eDNA facilitates the transmission of genetic information among oral biofilms (Roberts and Kreth, 2014). It can also be a source of nutrients, including phosphate, carbon, and fixed nitrogen for oral bacteria (Liu et al., 2018b).
Zhang et al. (2020) designed a type of helical peptide, which could interact with eDNA to induce dispersion of the S. mutans biofilm. Several studies have used DNase to cleave eDNA. Endogenous DNase encoded by deoC can significantly decrease the biofilm biomass and regulate the dispersion of the S. mutans biofilm (Liu et al., 2017). Although DNase barely decreases the viability of planktonic C. albicans and S. mutans, the human recombinant DNase I can significantly enhance the eradication of dual-species biofilm during its initial stages (Guo et al., 2021). DNase can enhance the susceptibility of antimicrobial agents and their antibiofilm activities by cleaving eDNA.
Proteinases and proteins inhibitor
Besides EPS and eDNA, proteins also act as a scaffold to protect the community. Bacteria produce multiple proteins to enhance bacterial adhesion. Surface adhesion proteins, including glucan-binding proteins (GbpA, GbpB, GbpC, and GbpD) and streptococcal protein antigen P (SpaP), can facilitate sucrose-dependent attachment of matrix glucans, salivary agglutinin, and bacteria (Biswas and Biswas, 2005). For instance, Gbp in S. mutans can mediate the adhesion of S. mutans and promote the functions of the viscoelastic structure (Matsumoto-Nakano, 2018). Proteinase acting on the Gbp proteins exhibits an anti-Gtf effect, which leads to a reduction in the volume of EPS or even bacterial biomass. Proteinase K could affect the biofilm infrastructure of S. mutans and Streptococcus oralis (S. oralis) by removing most of the extracellular proteins (Karygianni et al., 2020). Flavonoids have a similar function as proteinase. They not only interact with extracellular and soluble proteins on the bacterial surface but also inhibit the activity of Gtfs (Koo et al., 2003).
In sum, endogenous and exogenous nucleases for eDNA and proteinase for surface adhesion proteins may effectively promote the dispersion of cariogenic biofilm.
Electrostatic interactions agents
The electrostatic interactions involved in the bonding of the biofilm matrix could be affected by hydrophilic agents (Venault et al., 2014), surfactants (Wang et al., 2021), and metal chelators (Roman et al., 2014). Such electrostatic interaction agents have been found to destabilize the biofilm matrix and facilitate biofilm separation (Xavier et al., 2005). However, there are a relatively small amount of studies about electrostatic interactions involved in the bonding of the cariogenic biofilm matrix.
Furthermore, electrostatic interactions exist among anionic metabolites and anionic components on the bacterial surface. Postollec et al. (2003) reported that static electricity affects bacterial adhesion and aggregation via isothermal reaction calorimetry. For instance, the static electricity of polypyrrole affects the positively charged cross-bridging. Most surfaces of the biofilm are negatively charged, and polypyrrole also binds to negatively charged amino acids. Enhancing electrostatic interactions may promote the physical removal of bacteria from the tooth surface by facilitating the biofilm to remain intact and by inhibiting cell separation from long chains. It has been shown that aspartic acid451 is a part of the active site that controls the catalytic activity in Gtfs in response to sucrose binding, i.e., the DSIRVDAVD (residues 446–454) (Mooser et al., 1991). High concentrations of polypyrrole can absorb Gtf-I and Gtf-SI and block the action of Gtfs (Kato et al., 1992). Through the electrostatic interactions with S. mutans, the polypyrrole structure physically inhibits the formation and colonization of the biofilm. It can also promote the physical removal of the biofilm from the tooth surface by enhancing electrostatic adsorption aggregation (Senpuku et al., 2019).
Either synchronous modification of antimicrobial polyethylene glycol (PEG) or pH-activated charge conversion with cationic peptides has recently emerged as effective approaches to target negatively charged sites (Tian et al., 2020). In this manner, the micelle structures enhanced penetration and self-regulation by anchoring to the targeted biofilm.
Such specific electrostatic interaction agents can facilitate cariogenic biofilm removal by promoting concentrations of effective constituent and affecting the Gtfs.
Oxygen radicals
During metabolism, endogenous H2O2 is produced by natural bacteria. The neighboring streptococci in oral micro-ecology, such as Streptococcus gordonii (S. gordonii), Streptococcus sanguis, and Streptococcus oligofermentans, can impact the pathogenesis of S. mutans via self-produced H2O2 (Kreth et al., 2009). H2O2 generates free radicals, which not only degrade EPS but also promote the physical removal of biofilms by oxidative cleavage (Noyori et al., 2003). Although S. mutans is sensitive to oxidative stress (Liu et al., 2018b), the inhibitory effect of S. gordonii through H2O2 is far from adequate (Tanzer et al., 2012).
Exogenous application of H2O2 is common in household and clinical disinfection. It has little toxicity even at concentrations as high as 10% of effective concentration. The high peroxidase-like catalytic activity of metals or metal oxides under acidic pH has led to an increased interest in their biomedical application. Silver (Metin-Gursoy et al., 2017) and zinc oxide nanoparticles (Hernandez-Sierra et al., 2008), as well as iron oxide nanozymes (Cormode et al., 2018), have been reported to have potent antibiofilm nature. For instance, iron oxide nanozymes in acidic environments have the similar activity as peroxidase. They disrupts the constituents of the biofilm matrix and kill S. mutans (Liu et al., 2018a). Dextran-coated iron oxide nanoparticles (Dex-NZM) can degrade EPS at an acidic pH (Naha et al., 2019). Furthermore, the combination of iron oxide nanozymes and H2O2-generating bacteria improves the overall cleansing effect (Wang et al., 2020). Gao et al. (2016) synthesized catalytic nanoparticles (CAT-NP) to degrade insoluble glucans by the generation of free radicals from H2O2 in pathogenic acidic biofilms.
Photosensitizer (PS) can also activate molecular oxygen radicals and produce reactive oxygen species (ROS) (Cieplik et al., 2018). Through an oxidative burst, the PS compounds cause bacterial death and biofilm dispersion (de Souza et al., 2020; Martins Antunes de Melo et al., 2021). This method provides a robust direct ablation without drug resistance (Zhao et al., 2019a). Methylene blue (MB) caused a significant reduction in S. mutans biofilms, allowing the prospect of eliminating bacterial infections in deep carious lesions (Legenova et al., 2020). Fotoenticine (FTC) is a new derivative of chlorin e-6, which showed significant photodynamic effects against cariogenic bacteria, including S. mutans that was isolated from patients with dental caries (Terra Garcia et al., 2018). Due to the high carbohydrate content, the S. mutans biofilms exhibited greater absorption to PS than fungal cells, which might be the reason for the susceptibility of S. mutans (Sharma et al., 2011). Even in a complex polymicrobial biofilm, S. mutans are more susceptible to FTC-mediated photodynamic therapy (Garcia et al., 2021).
H2O2 and nanoparticles with the peroxidase-like activity present an ideal antibiofilm strategy by generating free radicals for the elimination of oral biofilms.
Modulation of signaling pathways
Instead of targeting the biofilm matrix, small molecules have been used to influence signaling systems by disaggregating bacteria (Ren et al., 2016; Fleming and Rumbaugh, 2017; Snarr et al., 2017). Due to its unique patterns of gene expression and protein production in each developmental stage of biofilms, bacterial signaling systems can minimize the impact on normal bacterial flora and prevent dental plaque infectious diseases (Lamont et al., 2018).
Quorum sensing
Quorum sensing (QS) is a microbial communication response in the entire cell population and has a significant impact on the biofilm life cycle (Li and Tian, 2012). QS is a typical microbial communication mode that enables bacteria to display cooperative group mechanistic behavior, which controls the expression of genes to virulence factors, biofilm dispersion, biofilm activity, and secondary metabolism (Li et al., 2001). Therefore, inhibition of the QS pathway would be a potential strategy for attenuating bacterial virulence.
The comCDE system
The comCDE system and the agglutinin-like sequence (Als) family are important in QS. The comCDE system responds to environmental signals, such as acid, and mediates pheromone competence stimulating peptide (CSP) activity (Lemos and Burne, 2008). High concentrations of CSP, which is a QS molecule in streptococci, may reduce biofilms and elongate the cells (Qi et al., 2005). Cvitkovitch et al., synthesized an analog of CSP (KBI-3221), which specifically targeted the QS pathway and decreased biofilms in various streptococcus biofilm dispersal (LoVetri and Madhyastha, 2010). Carolacton triggered the death of S. mutans by interfering with the comCDE system, and ComX in a growth-dependent way (Kunze et al., 2010).
Curcumin could downregulate the expression of the comCDE system (comC, comD, and comE) (Li et al., 2019) to inhibit QS (Li et al., 2018) and alter the EPS production (Falsetta et al., 2014). Hoyer et al., indicated that the expression of the Als family in C. albicans, which controls adhesion and aggregation, is suppressed by curcumin (Hoyer and Cota, 2016).
The LuxS system
In S. oralis, S. gordonii, and S. mutans, sulfated vizantin (Viz-S) reduces the expression of luxS and the downstream pathway of AI-2. With the deletion of the luxS gene, gtfB and gtfC genes are upregulated, which markedly reduces biofilm formation (Yoshida et al., 2005). Activation of the luxS gene downregulates the expression of gtfG in S. gordoni (Mcnab et al., 2003). AI-2 was also found in the inner cellular matrix of S. mutans and S. sobrinus. AI-2 inhibits the expression of gbpC and dblB, and induces the production of dextran-dependent aggregation (DDAG) (Lee et al., 2015).
Downregulation of the luxS gene alters biofilm structure in S. oralis and S. gordonii resulting in dispersion (Cuadra-Saenz et al., 2012).
Others
There has been increased interest in the QS system for the development of Chinese traditional medicine in recent years. Zingiber officinale reduces the expression of the entire set of S. mutans virulent genes and genes related to the biofilm life cycle, including comDE (for part of the QS cascade), relA (for oxidative stress and acid tolerance mechanisms) (Liu et al., 2011), brpA (for biofilm development and maturity), and gtfC (for the synthesis of glucans). The repression of these genes, especially their inhibition through the QS system, would attenuate their internal communication systems (Hasan et al., 2015). Cannabigerol also exerted an anti-bacterial effect against S. mutans (Karas et al., 2020; Aqawi et al., 2021). Cannabigerol suppressed the expressions of gbpB (for growth essential), vicR (for cell wall derivation and biofilm formation) (Lei et al., 2018), brpA (Wen et al., 2018), and wapA (for cell aggregation and biofilm architecture) (Zhu et al., 2006), with a concomitant increase in spaA (for binding S. mutans to tooth surfaces) expression and activity (Yang et al., 2019). Taken together, the above findings show that affecting the QS pathway can alter various gene expressions and attenuate the internal communication system, which may lead to biofilm disruption.
The Gtf gene family
All the QS pathways mentioned above involving the Gtf gene family. As we mentioned before, Gtfs maintain the integrity of the biofilm (Klein et al., 2015). The Gtf gene family, which encodes all Gtfs in S. mutans, directly responds to glucan matrix formation (Lei et al., 2015) and is regulated by the rnc gene. Increased expression of the rnc gene down-regulates vicRKX by posttranscriptional repression, followed by the promotion of the expression of gtfB and gtfC genes (Stipp et al., 2013; Mao et al., 2016). Therefore, the rnc gene could be responsible for decreasing the EPS (Mao et al., 2018).
Mao et al. (2021) reported that graphene oxide with Cu nanocomposites (GOCuNPs) can the antibacterial effects by decreasing the expression of the rnc gene. The regulatory role of graphene oxide with Ag nanocomposites has been reported to be the same as GOCuNPs. They can alter the QS gene expressions of S. mutans and the biological process of adherence (Kulshrestha et al., 2017). GOCuNPs can also regulate the expression of the Cop family, including CopA (for P1-ATPase copper export), CopY (for negative DNA-binding repression), and CopZ (for copper chaperone) (Garcia et al., 2016). Cu is consistent with the effect of GOCuNPs in transcriptional repression of Gtfs by inhibiting the expression of the Cop family (Singh et al., 2015).
Besides being regulated by the QS system (Viszwapriya et al., 2017), WIG-synthesizing Gtf genes promoted caries in Streptococcus species (Xu et al., 2018). Therefore, Gtf genes family plays an important role in biofilm dispersion.
Cue sensing
In addition to QS, cue sensing also plays a key role in bacterial communication. Cue sensing and its signal transmission eventually lead to the downregulation of the cyclic di-guanosine monophosphate (c-di-GMP). c-di-GMP is an intracellular secondary messenger for signal transduction. c-di-GMP-based regulatory systems are involved in diverse aspects of each stage of biofilm development, including biofilm dispersion (Rumbaugh and Sauer, 2020).
Peng demonstrated that S. mutans modulates the production of EPS and biofilm formation by regulating c-di-AMP levels (Peng et al., 2016). The gcp gene in S. mutans encodes AAN59731, which is a conserved hypothetical protein, which acts as a diadenylate cyclase (Yan et al., 2010). It was reported that downregulation of cdaA decreases the production of diadenylate cyclase and the levels of c-di-AMP, resulting in reduced EPS content and increased sensitivity to H2O2 (Cheng et al., 2016). Due to a reduction in c-di-GMP levels, the expression of matrix-degrading enzymes increases, resulting in matrix dispersal (Romling et al., 2013; Srivastava et al., 2013). Therefore, a decrease in the levels of c-di-GMP induces biofilm dispersion to planktonic mode, while an increase in intracellular c-di-GMP levels fosters it to a sessile mode (Hengge, 2009).
YidC family
The deletion of YidC in S. aureus inhibited biofilm formation and attenuated virulence. In Escherichia coli, YidC mutations were lethal (Samuelson et al., 2000). Although with phenotypic differences, mutants of either YidC1 or YidC2 still reduce virulence in S. mutans (Palmer et al., 2012; Crowley and Brady, 2016). Particularly, YidC2 has recently been identified to have the capability of folding plasminogen-binding protein (PBPs) and secreting enzymatic activities. Therefore, deletion of YidC2 causes significant alterations not only in cell physiology properties and division, but also in the EPS matrix assembly and mechanical stability associated with dental caries (Palmer et al., 2019).
Biological regulation of microbial homeostasis
Bacteriophages
Bacteriophages are viruses that invade bacteria with high strain specificity and low toxicity (Chan and Abedon, 2015). When bacteriophages infect bacteria, they induce EPS depolymerization and lysis, which degrades the biofilm matrix and impairs cell wall integrity (Azeredo and Sutherland, 2008). After accessing the biofilm, bacteriophages disrupt key metabolic processes, such as the QS system, and even affect the regulation of the eDNA release, which induce bacterial lysis (Rehman et al., 2019). Bacteriophages are good candidates for genetic engineering. They can co-evolve with the bacterial host to resist the antibiotic (Khalifa et al., 2016). Dalmasso et al. (2015) isolated phage, ɸAPCM01, successfully. ɸAPCM01 is a S. mutans bacteriophage that inhibits the growth of S. mutans and efficiently destroys its biofilms. SMHBZ8 is also a S. mutans bacteriophage that is isolated from salivary samples and it has similar antimicrobial properties as ɸAPCM01 (Ben-Zaken et al., 2021). Overall, by invading bacteria, bacteriophages offer a broad prospect to be used as a novel biotherapy.
Probiotics
Probiotics treat oral infections by developing a symbiotic or reciprocal relationship with the host (Roberts and Darveau, 2015). They can prolong the therapeutic efficacy by niche occupation and prevent recolonization of the pathogenic bacteria. An ecological approach to caries treatment is to modulate and maintain the beneficial properties of the indigenous oral microflora.
There are already various commercial mouthwash and lozenges that are supplemented with probiotic bacteria, such as PerioBalance®, KForce Breath Guard®, and ProBiora3® (Yao and Fine, 2014). Streptococcus salivarius (S. salivarius) K12 and Lactobacillus rhamnosus GG are probiotic formulations for oral health (Caglar et al., 2005). Aggregation of S. mutans can cover up their surficial sites, rendering them unavailable for drug binding (di Cologna et al., 2021). Co-aggregation of Lactobacillus paracasei DSMZ16671 and S. mutans exposes these sites and removes S. mutans without disruption of other oral commensal species (Lang et al., 2010). Besides, lactococcus such as Lactococcus lactis produces nisin and disrupts oral pathogenic biofilms (Radaic et al., 2020). Therefore, by maintaining a healthy balance, probiotic bacteria and their metabolite can inhibit the process of biofilm development and preserve the beneficial properties of the oral microflora.
Dispersion promotion with nanovehicles
Due to the particularity of tooth anatomical structure, improper treatment for biofilm removal may expose pulp tissue or adjacent soft tissue (Schwendicke et al., 2018). Potent antibiotics, such as CHX, are significantly cytotoxic with side effects, including discoloration or nerve damage due to pulp exposure (Nemezio et al., 2017). Furthermore, bacteria that may survive in the inner layer or the unintentional removal of tissues can weaken the tooth structure and even cause toothaches (Orhan et al., 2010). However, we can still take advantage of biofilm infiltration and intramembrane transport of drug delivery nanotechnology (Zhou et al., 2016). Polymer micelles (Zhao et al., 2019b), vesicles (Xi et al., 2019), and liposomes (Benoit et al., 2019) have been proven to have great potential for drug delivery. These nanocarriers are ideal materials with high surface area and specific catalytic and magnetic properties for use in nanomedicine (Ramos et al., 2017). Nanocarriers loaded with antimicrobials have displayed unique characteristics, including targeted bacterial enzyme decomposition of micellar carriers (Li et al., 2016) and enhanced infiltration or accumulation (Landis et al., 2017).
Common nanocarrier
Chitosan is a common nano-carrier, which can interact with both biofilm bacteria and enamel (Li et al., 2013). Chitosan, as a bio-adhesive polymer, can improve the adherence of its contents and interfere with the adhesion of biofilm bacteria (Aliasghari et al., 2016). Covarrubias et al. (2018) demonstrated that Cu coating inside chitosan (CuChNP) improves the adherence of Cu to S. mutans and the tooth surface. CuO-chitosan hybrid structure, silver nanoparticles containing lactose-modified chitosan (Chitlac-nAg) (Ionescu et al., 2015), poloxamer 407 formulations, capped lysozyme, and lactoferrin nanoparticles are known to reduce S. mutans biofilm burden (Tonguc-Altin et al., 2015). Nanocarriers, such as chitosan, can increase adherence or aggregation of the active ingredient to improve biofilm dispersion.
Target nanocarriers
One of the most important features of cariogenic biofilm microenvironments is their acidic nature. Once inside a biofilm, pH-responsive nanocarriers would expedite the release of antimicrobials through degradation of their biodegradable linkages. Zhao et al. (2019b) designed a pH-responsive detachable PEG shell that infiltrated the oral biofilms and embedded itself in the interlayer of the nanoplatforms through dynamic borate linkages. In the weakly acidic micro-ecological environments (pH 6.5), the linkages shed their PEG coating. The pH-responsive nanoparticles are capable of readily binding to EPS and reinforcing its penetration, which leads to enhanced drug anchorage followed by “on-site” drug release. Collectively, it can be a feasible strategy for the treatment of dental caries.
Specifically-targeted antimicrobial peptides (STAMPs) ensure targeted delivery to specific species in a mixed-species environment. Eckert et al. (2006) designed a STAMP molecule by combining a species-specific targeting peptide and a non-specific killing peptide. This STAMP bound specifically to S. mutans and eliminated it effectively while maintaining a healthy biofilm. It also showed considerable protective effects with the competitiveness of healthy normal flora against S. mutans colonization (Li et al., 2010).
Dextranomer (DMs) has a similar targeted delivery function as STAMPs with different principles. DMs exhibit a specific affinity for pathogenic oral streptococci, while causing limited disturbance to healthy biofilms. The affinity between DMs and oral streptococci may increase depending on the presence of sucrose. DMs with antimicrobial cargo not only protect healthy bacteria, but also improve bacterial aggregation of selectively adhered bacteria (Mashburn-Warren et al., 2017). Targeting a particular microbial species or a specific kind of pathogen can help maintain microbial homeostasis, and thus, and better eliminate pathogens significantly.
Multifunctional nanocarriers
Nanotechnology-based therapeutic modalities provide many versatile strategies to coordinate biofilm infiltration and bacterial anchoring functions.
To combine pH-adaptive nanocarriers and positive surface charge therapy, Benoit et al., developed p(DMAEMA)-b-p(DMAMEA-co-BMA-co-PAA) nanocarriers, which offer outstanding adhesion effect and can target negatively charged tooth matrix or biofilm components for drug accumulation in cariogenic biofilms (Horev et al., 2015). Furthermore, most of the cariogenic S. mutans are characterized by esterase activity, which degrades the ester-linkage of PAE (Hansel et al., 1998). Under acidic conditions, PAE is exposed, and can penetrate and accumulate in the biofilm. It also targets negatively charged bacterial cell surfaces with its positive charge (Liu et al., 2016). Combining the function of stealthy penetration with low pH and electrostatic attraction allows accumulation in biofilms. Therefore, PEG-PAE micelles significantly increase the efficacy of Triclosan (Wang et al., 2016). Such properties thwart dental caries by the enrichment of local drugs. The high drug bioavailability impacts overall biofilm dispersion, allowing bacterial retention at the infection site, which is a highly promising strategy for efficient bacteria killing.
Conclusion
In this review, we summarized the applications and mechanisms of the strategies for dispersion of cariogenic biofilms. Most of the studies that we have discussed focus on mono species. However, the real cariogenic biofilms comprise various acidogenic and aciduric microorganisms, including S. mutans, S. sobrinus, Lactobacillus reuteri, and even fungi (Pires et al., 2019). In addition, the interaction between pathogenic species and salivary components can help bacterial species adapt to environmental stress, while aiding in the bacterial evolution of cariogenic biofilms. This phenomenon is referred to as horizontal gene transfer (HGT) (Kim et al., 2017). HGT is the main means for species to exchange metabolites and generate resistance (Lobo et al., 2019). Therefore, it is necessary to expand research on dual-species biofilms and biofilms with mixed pathogens.
Although much research has addressed bacterial biofilms, experimental conditions vary from one study to another. The oral hygiene of patients is also dependent on individual cleaning habits and orthodontic appliances used. There are novel research models that mimic the oral environment. To close the knowledge gap between ideal experimental conditions and the actual oral environment, more suitable experimental models and in vivo, mechanistic models are needed. Such research will play an important role in facilitating practical clinical applications. Furthermore, such therapeutic strategies can potentially be extended to other pathological conditions, such as periodontitis (Natan and Banin, 2017; Sun et al., 2021), and microbial communities. Useful strategies are by no means limited to one condition. Further research that aims to improve available strategies can shift their time, the proportion of medication applied, and dependence on auxiliary medical equipment, such as irradiation.
Many Chinese medicine ingredients comprise natural products that can contribute to overcoming the problem of chemical agents, including narrow specificity, slow action, expensive manufacturing, and drug purification for biomedical applications (Hannig et al., 2010). Focusing on strategies that can achieve biofilm dispersion to a certain degree can help preserve a balanced oral microbiome, and thus, can aid in preventing drug-resistant bacteria. It is worth noting that some of the strategies should be used together with antimicrobials to maximize biofilm dispersion. Based on the review of numerous relevant studies, we can improve therapeutic approaches by combining strategies instead of monotherapies (Xiao et al., 2018).
Author contributions
RC wrote the manuscript. CL designed this project and wrote the manuscript. MD designed this project. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by grants from the National Natural Science Foundation of China (grant nos. 81201260 and 81771084).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aliasghari, A., Rabbani Khorasgani, M., Vaezifar, S., Rahimi, F., Younesi, H., and Khoroushi, M. (2016). Evaluation of antibacterial efficiency of chitosan and chitosan nanoparticles on cariogenic streptococci: an in vitro study. Iran J. Microbiol. 8, 93–100. doi: none.
Aqawi, M., Sionov, R. V., Gallily, R., Friedman, M., and Steinberg, D. (2021). Anti-bacterial properties of cannabigerol toward Streptococcus mutans. Front. Microbiol. 12:656471. doi: 10.3389/fmicb.2021.656471
Azeredo, J., and Sutherland, I. W. (2008). The use of phages for the removal of infectious biofilms. Curr. Pharm. Biotechnol. 9, 261–266. doi: 10.2174/138920108785161604
Balakrishnan, M., Simmonds, R. S., and Tagg, J. R. (2000). Dental caries is a preventable infectious disease. Aust. Dent. J. 45, 235–245. doi: 10.1111/j.1834-7819.2000.tb00257.x
Benoit, D. S. W., Sims, K. R. Jr., and Fraser, D. (2019). Nanoparticles for oral biofilm treatments. ACS Nano. 13, 4869–4875. doi: 10.1021/acsnano.9b02816
Ben-Zaken, H., Kraitman, R., Coppenhagen-Glazer, S., Khalifa, L., Alkalay-Oren, S., Gelman, D., et al. (2021). Isolation and characterization of Streptococcus mutans phage as a possible treatment agent for caries. Viruses 13. doi: 10.3390/v13050825
Biswas, S., and Biswas, I. (2005). Role of Htra in surface protein expression and biofilm formation by Streptococcus mutans. Infect. Immun. 73, 6923–6934. doi: 10.1128/IAI.73.10.6923-6934.2005
Bowen, W. H., and Koo, H. (2011). Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 45, 69–86. doi: 10.1159/000324598
Burt, S. (2004). Essential oils: their antibacterial properties and potential applications in foods-a review. Int. J. Food Microbiol. 94, 223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022
Caglar, E., Kargul, B., and Tanboga, I. (2005). Bacteriotherapy and probiotics' role on oral Health. Oral Dis. 11, 131–137. doi: 10.1111/j.1601-0825.2005.01109.x
Chan, B. K., and Abedon, S. T. (2015). Bacteriophages and their enzymes in biofilm control. Curr. Pharm. Des. 21, 85–99. doi: 10.2174/1381612820666140905112311
Cheng, X., Zheng, X., Zhou, X., Zeng, J., Ren, Z., Xu, X., et al. (2016). Regulation of oxidative response and extracellular polysaccharide synthesis by a diadenylate cyclase in Streptococcus mutans. Environ. Microbiol. 18, 904–922. doi: 10.1111/1462-2920.13123
Cieplik, F., Deng, D., Crielaard, W., Buchalla, W., Hellwig, E., Al-Ahmad, A., et al. (2018). Antimicrobial photodynamic therapy-what we know and what we don't. Crit. Rev. Microbiol. 44, 571–589. doi: 10.1080/1040841X.2018.1467876
Cormode, D. P., Gao, L., and Koo, H. (2018). Emerging biomedical applications of enzyme-like catalytic nanomaterials. Trends Biotechnol. 36, 15–29. doi: 10.1016/j.tibtech.2017.09.006
Covarrubias, C., Trepiana, D., and Corral, C. (2018). Synthesis of hybrid copper-chitosan nanoparticles with antibacterial activity against cariogenic Streptococcus mutans. Dent. Mater. J. 37, 379–384. doi: 10.4012/dmj.2017-195
Crowley, P. J., and Brady, L. J. (2016). Evaluation of the effects of Streptococcus mutans chaperones and protein secretion machinery components on cell surface protein biogenesis, competence, and mutacin production. Mol. Oral. Microbiol. 31, 59–77. doi: 10.1111/omi.12130
Cuadra-Saenz, G., Rao, D. L., Underwood, A. J., Belapure, S. A., Campagna, S. R., Sun, Z., et al. (2012). Autoinducer-2 influences interactions amongst pioneer colonizing streptococci in oral biofilms. Microbiology 158, 1783–1795. doi: 10.1099/mic.0.057182-0
Dalmasso, M., De Haas, E., Neve, H., Strain, R., Cousin, F. J., Stockdale, S. R., et al. (2015). Isolation of a novel phage with activity against Streptococcus mutans biofilms. PLoS One 10:e0138651. doi: 10.1371/journal.pone.0138651
Das, T., Sharma, P. K., Krom, B. P., Van Der Mei, H. C., and Busscher, H. J. (2011). Role of eDNA on the adhesion forces between Streptococcus mutans and substratum surfaces: influence of ionic strength and substratum hydrophobicity. Langmuir 27, 10113–10118. doi: 10.1021/la202013m
Davies, D. (2003). Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2, 114–122. doi: 10.1038/nrd1008
De Souza, C. M., Garcia, M. T., De Barros, P. P., Pedroso, L. L. C., Ward, R., Strixino, J. F., et al. (2020). Chitosan enhances the antimicrobial photodynamic inactivation mediated by Photoditazine (R) against Streptococcus mutans. Photodiagnosis Photodyn. Ther. 32:102001. doi: 10.1016/j.pdpdt.2020.102001
Di Cologna, N. M., Samaddar, S., Valle, C. A., Vargas, J., Aviles-Reyes, A., Morales, J., et al. (2021). Amyloid aggregation of Streptococcus mutans Cnm influences its collagen-binding activity. Appl. Environ. Microbiol. 87:e0114921. doi: 10.1128/AEM.01149-21
Eckert, R., He, J., Yarbrough, D. K., Qi, F., Anderson, M. H., and Shi, W. (2006). Targeted Killing of Streptococcus mutans by a pheromone-guided "smart" antimicrobial peptide. Antimicrob. Agents Chemother. 50, 3651–3657. doi: 10.1128/AAC.00622-06
Falsetta, M. L., Klein, M. I., Colonne, P. M., Scott-Anne, K., Gregoire, S., Pai, C. H., et al. (2014). Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 82, 1968–1981. doi: 10.1128/IAI.00087-14
Fleming, D., and Rumbaugh, K. (2018). The consequences of biofilm dispersal on the host. Sci. Rep. 8:10738. doi: 10.1038/s41598-018-29121-2
Fleming, D., and Rumbaugh, K. P. (2017). Approaches to dispersing medical biofilms. Microorganisms 5. doi: 10.3390/microorganisms5020015
Gao, L., Liu, Y., Kim, D., Li, Y., Hwang, G., Naha, P. C., et al. (2016). Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 101, 272–284. doi: 10.1016/j.biomaterials.2016.05.051
Garcia, M. T., Ward, R., Goncalves, N. M. F., Pedroso, L. L. C., Neto, J., Strixino, J. F., et al. (2021). Susceptibility of dental caries microcosm biofilms to photodynamic therapy mediated by fotoenticine. Pharmaceutics 13. doi: 10.3390/pharmaceutics13111907
Garcia, S. S., Du, Q., and Wu, H. (2016). Streptococcus mutans copper chaperone, Copz, is critical for biofilm formation and competitiveness. Mol. Oral. Microbiol. 31, 515–525. doi: 10.1111/omi.12150
Gregoire, S., Xiao, J., Silva, B. B., Gonzalez, I., Agidi, P. S., Klein, M. I., et al. (2011). Role of glucosyltransferase b in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Appl. Environ. Microbiol. 77, 6357–6367. doi: 10.1128/AEM.05203-11
Guo, H., Chen, Y., Guo, W., and Chen, J. (2021). Effects of extracellular DNA on dual-species biofilm formed by Streptococcus mutans and Candida albicans. Microb. Pathog. 154:104838. doi: 10.1016/j.micpath.2021.104838
Hannig, C., Spies, B., Spitzmuller, B., and Hannig, M. (2010). Efficacy of enzymatic mouth rinses for immobilisation of protective enzymes in the in situ pellicle. Arch. Oral. Biol. 55, 1–6. doi: 10.1016/j.archoralbio.2009.10.004
Hansel, C., Leyhausen, G., Mai, U. E., and Geurtsen, W. (1998). Effects of various resin composite (co) monomers and extracts on two caries-associated micro-organisms in vitro. J. Dent. Res. 77, 60–67. doi: 10.1177/00220345980770010601
Hasan, S., Danishuddin, M., and Khan, A. U. (2015). Inhibitory effect of zingiber officinale towards Streptococcus mutans virulence and caries development: in vitro and in vivo studies. BMC Microbiol. 15:1. doi: 10.1186/s12866-014-0320-5
Hayacibara, M. F., Koo, H., Vacca-Smith, A. M., Kopec, L. K., Scott-Anne, K., Cury, J. A., et al. (2004). The influence of mutanase and dextranase on the production and structure of glucans synthesized by streptococcal glucosyltransferases. Carbohydr. Res. 339, 2127–2137. doi: 10.1016/j.carres.2004.05.031
Hengge, R. (2009). Principles of C-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 7, 263–273. doi: 10.1038/nrmicro2109
Hernandez-Sierra, J. F., Ruiz, F., Pena, D. C., Martinez-Gutierrez, F., Martinez, A. E., Guillen Ade, J., et al. (2008). The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine 4, 237–240. doi: 10.1016/j.nano.2008.04.005
Horev, B., Klein, M. I., Hwang, G., Li, Y., Kim, D., Koo, H., et al. (2015). Ph-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano. 9, 2390–2404. doi: 10.1021/nn507170s
Hoyer, L. L., and Cota, E. (2016). Candida albicans agglutinin-like sequence (Als) family vignettes: a review of Als protein structure and function. Front. Microbiol. 7:280. doi: 10.3389/fmicb.2016.00280
Ionescu, A. C., Brambilla, E., Travan, A., Marsich, E., Donati, I., Gobbi, P., et al. (2015). Silver-polysaccharide antimicrobial nanocomposite coating for methacrylic surfaces reduces streptococcus mutans biofilm formation in vitro. J. Dent. 43, 1483–1490. doi: 10.1016/j.jdent.2015.10.006
Ito, K., Ito, S., Shimamura, T., Weyand, S., Kawarasaki, Y., Misaka, T., et al. (2011). Crystal structure of glucansucrase from the dental caries pathogen Streptococcus mutans. J. Mol. Biol. 408, 177–186. doi: 10.1016/j.jmb.2011.02.028
Jakubovics, N. S., and Burgess, J. G. (2015). Extracellular DNA in oral microbial biofilms. Microbes Infect. 17, 531–537. doi: 10.1016/j.micinf.2015.03.015
Jamal, M., Ahmad, W., Andleeb, S., Jalil, F., Imran, M., Nawaz, M. A., et al. (2018). Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 81, 7–11. doi: 10.1016/j.jcma.2017.07.012
Kaplan, J. B., Ragunath, C., Velliyagounder, K., Fine, D. H., and Ramasubbu, N. (2004). Enzymatic detachment of staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 48, 2633–2636. doi: 10.1128/AAC.48.7.2633-2636.2004
Karas, J. A., Wong, L. J. M., Paulin, O. K. A., Mazeh, A. C., Hussein, M. H., Li, J., et al. (2020). The antimicrobial activity of cannabinoids. Antibiotics (Basel) 9. doi: 10.3390/antibiotics9070406
Karygianni, L., Attin, T., and Thurnheer, T. (2020). Combined DNase and proteinase treatment interferes with composition and structural integrity of multispecies oral biofilms. J. Clin. Med. 9. doi: 10.3390/jcm9040983
Kato, C., Nakano, Y., Lis, M., and Kuramitsu, H. K. (1992). Molecular genetic analysis of the catalytic site of Streptococcus mutans glucosyltransferases. Biochem. Biophys. Res. Commun. 189, 1184–1188. doi: 10.1016/0006-291x(92)92329-v
Khalifa, L., Shlezinger, M., Beyth, S., Houri-Haddad, Y., Coppenhagen-Glazer, S., Beyth, N., et al. (2016). Phage therapy against enterococcus faecalis in dental root canals. J. Oral. Microbiol. 8:32157. doi: 10.3402/jom.v8.32157
Kim, D., and Koo, H. (2020). Spatial design of polymicrobial oral biofilm in its native disease state. J. Dent. Res. 99, 597–603. doi: 10.1177/0022034520909313
Kim, D., Sengupta, A., Niepa, T. H., Lee, B. H., Weljie, A., Freitas-Blanco, V. S., et al. (2017). Candida albicans stimulates streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci. Rep. 7:41332. doi: 10.1038/srep41332
Klein, M. I., Hwang, G., Santos, P. H., Campanella, O. H., and Koo, H. (2015). Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front. Cell. Infect. Microbiol. 5:10. doi: 10.3389/fcimb.2015.00010
Koo, H., Allan, R. N., Howlin, R. P., Stoodley, P., and Hall-Stoodley, L. (2017). Targeting microbial biofilms: current and prospective therapeutic strategies. Nat. Rev. Microbiol. 15, 740–755. doi: 10.1038/nrmicro.2017.99
Koo, H., Hayacibara, M. F., Schobel, B. D., Cury, J. A., Rosalen, P. L., Park, Y. K., et al. (2003). Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and Tt-farnesol. J. Antimicrob. Chemother. 52, 782–789. doi: 10.1093/jac/dkg449
Koo, H., Vacca Smith, A. M., Bowen, W. H., Rosalen, P. L., Cury, J. A., and Park, Y. K. (2000). Effects of Apis mellifera propolis on the activities of streptococcal glucosyltransferases in solution and adsorbed onto saliva-coated hydroxyapatite. Caries Res. 34, 418–426. doi: 10.1159/000016617
Koo, H., Xiao, J., Klein, M. I., and Jeon, J. G. (2010). Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J. Bacteriol. 192, 3024–3032. doi: 10.1128/JB.01649-09
Kreth, J., Merritt, J., and Qi, F. (2009). Bacterial and host interactions of oral streptococci. DNA Cell. Biol. 28, 397–403. doi: 10.1089/dna.2009.0868
Kulshrestha, S., Qayyum, S., and Khan, A. U. (2017). Antibiofilm efficacy of green synthesized graphene oxide-silver nanocomposite using lagerstroemia speciosa floral Extract: a comparative study on inhibition of gram-positive and gram-negative biofilms. Microb. Pathog. 103, 167–177. doi: 10.1016/j.micpath.2016.12.022
Kunze, B., Reck, M., Dotsch, A., Lemme, A., Schummer, D., Irschik, H., et al. (2010). Damage of Streptococcus mutans biofilms by carolacton, a secondary metabolite from the Myxobacterium sorangium Cellulosum. BMC Microbiol. 10, 199. doi: 10.1186/1471-2180-10-199
Lamont, R. J., Koo, H., and Hajishengallis, G. (2018). The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 16, 745–759. doi: 10.1038/s41579-018-0089-x
Landis, R. F., Gupta, A., Lee, Y. W., Wang, L. S., Golba, B., Couillaud, B., et al. (2017). Cross-linked polymer-stabilized nanocomposites for the treatment of bacterial biofilms. ACS Nano. 11, 946–952. doi: 10.1021/acsnano.6b07537
Lang, C., Bottner, M., Holz, C., Veen, M., Ryser, M., Reindl, A., et al. (2010). Specific lactobacillus/mutans streptococcus co-aggregation. J. Dent. Res. 89, 175–179. doi: 10.1177/0022034509356246
Lee, H. J., Kim, S. C., Kim, J., Do, A., Han, S. Y., Lee, B. D., et al. (2015). Synergistic inhibition of streptococcal biofilm by ribose and xylitol. Arch. Oral Biol. 60, 304–312. doi: 10.1016/j.archoralbio.2014.11.004
Legenova, K., Kovalcikova, M., Cernakova, L., and Bujdakova, H. (2020). The contribution of photodynamic inactivation vs. corsodyl mouthwash to the control of streptococcus mutans biofilms. Curr. Microbiol. 77, 988–996. doi: 10.1007/s00284-020-01901-y
Lei, L., Stipp, R. N., Chen, T., Wu, S. Z., Hu, T., and Duncan, M. J. (2018). Activity of Streptococcus mutans vicR is modulated by antisense RNA. J. Dent. Res. 97, 1477–1484. doi: 10.1177/0022034518781765
Lei, L., Yang, Y., Mao, M., Li, H., Li, M., Yang, Y., et al. (2015). Modulation of biofilm exopolysaccharides by the Streptococcus mutans Vicx gene. Front. Microbiol. 6:1432. doi: 10.3389/fmicb.2015.01432
Lemos, J. A., and Burne, R. A. (2008). A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology 154, 3247–3255. doi: 10.1099/mic.0.2008/023770-0
Li, B., Li, X., Lin, H., and Zhou, Y. (2018). Curcumin as a promising antibacterial agent: effects on metabolism and biofilm formation in S. Mutans. Biomed Res. Int. 2018, 4508709. doi: 10.1155/2018/4508709
Li, F., Weir, M. D., Chen, J., and Xu, H. H. (2013). Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dent. Mater. 29, 450–461. doi: 10.1016/j.dental.2013.01.012
Li, L. N., Guo, L. H., Lux, R., Eckert, R., Yarbrough, D., He, J., et al. (2010). Targeted antimicrobial therapy against Streptococcus mutans establishes protective non-cariogenic oral biofilms and reduces subsequent infection. Int. J. Oral. Sci. 2, 66–73. doi: 10.4248/IJOS10024
Li, M. Y., Lai, G. Y., Wang, J., and Ye, D. X. (2012). The inhibition of eugenol on glucan is essential for the biofilm eradication effect on caries-related biofilm in an artificial mouth model. Nat. Prod. Res. 26, 1152–1155. doi: 10.1080/14786419.2011.561799
Li, X., Yin, L., Ramage, G., Li, B., Tao, Y., Zhi, Q., et al. (2019). Assessing the impact of curcumin on dual-species biofilms formed by Streptococcus mutans and Candida albicans. Microbiologyopen 8:e937. doi: 10.1002/mbo3.937
Li, Y., Liu, G., Wang, X., Hu, J., and Liu, S. (2016). Enzyme-responsive polymeric vesicles for bacterial-strain-selective delivery of antimicrobial agents. Angew. Chem. Int. Ed. Engl. 55, 1760–1764. doi: 10.1002/anie.201509401
Li, Y. H., Lau, P. C., Lee, J. H., Ellen, R. P., and Cvitkovitch, D. G. (2001). Natural genetic transformation of Streptococcus mutans growing in biofilms. J. Bacteriol. 183, 897–908. doi: 10.1128/JB.183.3.897-908.2001
Li, Y. H., and Tian, X. (2012). Quorum sensing and bacterial social interactions in biofilms. Sensors 12, 2519–2538. doi: 10.3390/s120302519
Liljemark, W. F., and Bloomquist, C. (1996). Human oral microbial ecology and dental caries and periodontal diseases. Crit. Rev. Oral Biol. Med. 7, 180–198. doi: 10.1177/10454411960070020601
Lin, Y., Zhou, X., and Li, Y. (2022). Strategies for Streptococcus mutans biofilm dispersal through extracellular polymeric substances disruption. Mol. Oral Microbiol. 37, 1–8. doi: 10.1111/omi.12355
Liu, C., Worthington, R. J., Melander, C., and Wu, H. (2011). A new small molecule specifically inhibits the cariogenic bacterium Streptococcus mutans in multispecies biofilms. Antimicrob. Agents Chemother. 55, 2679–2687. doi: 10.1128/AAC.01496-10
Liu, J., Sun, L., Liu, W., Guo, L., Liu, Z., Wei, X., et al. (2017). A nuclease from Streptococcus mutans facilitates biofilm dispersal and escape from killing by neutrophil extracellular traps. Front. Cell. Infect. Microbiol. 7:97. doi: 10.3389/fcimb.2017.00097
Liu, Y., Busscher, H. J., Zhao, B., Li, Y., Zhang, Z., Van Der Mei, H. C., et al. (2016). Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms. ACS Nano 10, 4779–4789. doi: 10.1021/acsnano.6b01370
Liu, Y., Naha, P. C., Hwang, G., Kim, D., Huang, Y., Simon-Soro, A., et al. (2018a). Topical ferumoxytol nanoparticles disrupt biofilms and prevent tooth decay in vivo via intrinsic catalytic activity. Nat. Commun. 9:2920. doi: 10.1038/s41467-018-05342-x
Liu, Y., Palmer, S. R., Chang, H., Combs, A. N., Burne, R. A., and Koo, H. (2018b). Differential oxidative stress tolerance of Streptococcus mutans isolates affects competition in an ecological mixed-species biofilm model. Environ. Microbiol. Rep. 10, 12–22. doi: 10.1111/1758-2229.12600
Lobo, C. I. V., Rinaldi, T. B., Christiano, C. M. S., De Sales Leite, L., Barbugli, P. A., and Klein, M. I. (2019). Dual-species biofilms of Streptococcus mutans and Candida albicans exhibit more biomass and are mutually beneficial compared with single-species biofilms. J. Oral Microbiol. 11:1581520. doi: 10.1080/20002297.2019.1581520
Lovetri, K., and Madhyastha, S. (2010). Antimicrobial and antibiofilm activity of quorum sensing peptides and peptide analogues against oral biofilm bacteria. Methods Mol. Biol. 618, 383–392. doi: 10.1007/978-1-60761-594-1_24
Lynch, D. J., Fountain, T. L., Mazurkiewicz, J. E., and Banas, J. A. (2007). Glucan-binding proteins are essential for shaping Streptococcus mutans biofilm architecture. FEMS Microbiol. Lett. 268, 158–165. doi: 10.1111/j.1574-6968.2006.00576.x
Mao, M., Zhang, W., Huang, Z., Huang, J., Wang, J., Li, W., et al. (2021). Graphene oxide-copper nanocomposites suppress cariogenic Streptococcus mutans biofilm formation. Int. J. Nanomed. 16, 7727–7739. doi: 10.2147/IJN.S303521
Mao, M. Y., Li, M., Lei, L., Yin, J. X., Yang, Y. M., and Hu, T. (2018). The regulator gene rnc is closely involved in biofilm formation in Streptococcus mutans. Caries Res. 52, 347–358. doi: 10.1159/000486431
Mao, M. Y., Yang, Y. M., Li, K. Z., Lei, L., Li, M., Yang, Y., et al. (2016). The rnc gene promotes exopolysaccharide synthesis and represses the vicRkx gene expressions via microrna-size small rnas in Streptococcus mutans. Front. Microbiol. 7:687. doi: 10.3389/fmicb.2016.00687
Martins Antunes de Melo, W. C., Celiesiute-Germaniene, R., Simonis, P., and Stirke, A. (2021). Antimicrobial photodynamic therapy (Apdt) for biofilm treatments. Possible synergy between Apdt and pulsed electric fields. Virulence 12, 2247–2272. doi: 10.1080/21505594.2021.1960105
Mashburn-Warren, L., Downey, J. S., and Goodman, S. D. (2017). Novel method for the depletion of cariogenic bacteria using dextranomer microspheres. Mol. Oral Microbiol. 32, 475–489. doi: 10.1111/omi.12186
Matsumoto-Nakano, M. (2018). Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 54, 22–29. doi: 10.1016/j.jdsr.2017.08.002
Mcnab, R., Ford, S. K., El-Sabaeny, A., Barbieri, B., Cook, G. S., and Lamont, R. J. (2003). Luxs-based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J. Bacteriol. 185, 274–284. doi: 10.1128/JB.185.1.274-284.2003
Metin-Gursoy, G., Taner, L., and Akca, G. (2017). Nanosilver coated orthodontic brackets: in vivo antibacterial properties and ion release. Eur. J. Orthod. 39, 9–16. doi: 10.1093/ejo/cjv097
Mooser, G., Hefta, S. A., Paxton, R. J., Shively, J. E., and Lee, T. D. (1991). Isolation and sequence of an active-site peptide containing a catalytic aspartic acid from two Streptococcus sobrinus alpha-glucosyltransferases. J. Biol. Chem. 266, 8916–8922. doi: 10.1016/S0021-9258(18)31531-X
Munro, C., Michalek, S. M., and Macrina, F. L. (1991). Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect. Immun. 59, 2316–2323. doi: 10.1128/iai.59.7.2316-2323.1991
Naha, P. C., Liu, Y., Hwang, G., Huang, Y., Gubara, S., Jonnakuti, V., et al. (2019). Dextran-coated iron oxide nanoparticles as biomimetic catalysts for localized and Ph-activated biofilm disruption. ACS Nano 13, 4960–4971. doi: 10.1021/acsnano.8b08702
Nakahara, K., Kawabata, S., Ono, H., Ogura, K., Tanaka, T., Ooshima, T., et al. (1993). Inhibitory effect of oolong tea polyphenols on glycosyltransferases of mutans streptococci. Appl. Environ. Microbiol. 59, 968–973. doi: 10.1128/aem.59.4.968-973.1993
Natan, M., and Banin, E. (2017). From nano to micro: using nanotechnology to combat microorganisms and their multidrug resistance. FEMS Microbiol. Rev. 41, 302–322. doi: 10.1093/femsre/fux0003
Nemezio, M. A., De Souza Farias, S. S., Borsatto, M. C., Aires, C. P., and Corona, S. A. M. (2017). Effect of methylene blue-induced photodynamic therapy on a Streptococcus mutans biofilm model. Photodiagnosis Photodyn. Ther. 20, 234–237. doi: 10.1016/j.pdpdt.2017.10.025
Nguyen, D., Joshi-Datar, A., Lepine, F., Bauerle, E., Olakanmi, O., Beer, K., et al. (2011). Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 334, 982–986. doi: 10.1126/science.1211037
Nijampatnam, B., Zhang, H., Cai, X., Michalek, S. M., Wu, H., and Velu, S. E. (2018). Inhibition of Streptococcus mutans biofilms by the natural stilbene piceatannol through the inhibition of glucosyltransferases. ACS Omega 3, 8378–8385. doi: 10.1021/acsomega.8b00367
Noyori, R., Aoki, M., and Sato, K. (2003). Green oxidation with aqueous hydrogen peroxide. Chem. Commun. 1977-86:1977. doi: 10.1039/b303160h
Orhan, A. I., Oz, F. T., and Orhan, K. (2010). Pulp exposure occurrence and outcomes after 1- or 2-visit indirect pulp therapy vs. complete caries removal in primary and permanent molars. Pediatr. Dent. 32, 347–355. doi: none.
Otsuka, R., Imai, S., Murata, T., Nomura, Y., Okamoto, M., Tsumori, H., et al. (2015). Application of chimeric glucanase comprising mutanase and dextranase for prevention of dental biofilm formation. Microbiol. Immunol. 59, 28–36. doi: 10.1111/1348-0421.12214
Palmer, S. R., Crowley, P. J., Oli, M. W., Ruelf, M. A., Michalek, S. M., and Brady, L. J. (2012). Yidc 1 and Yidc 2 are functionally distinct proteins involved in protein secretion, biofilm formation and cariogenicity of Streptococcus mutans. Microbiology 158, 1702–1712. doi: 10.1099/mic.0.059139-0
Palmer, S. R., Ren, Z., Hwang, G., Liu, Y., Combs, A., Soderstrom, B., et al. (2019). Streptococcus mutans Yidc 1 and Yidc 2 impact cell envelope biogenesis, the biofilm matrix, and biofilm biophysical properties. J. Bacteriol. 201. doi: 10.1128/JB.00396-18
Pedraza, M. C. C., Novais, T. F., Faustoferri, R. C., Quivey, R. G., Terekhov, A., Hamaker, B. R., et al. (2017). Extracellular DNA and lipoteichoic acids interact with exopolysaccharides in the extracellular matrix of Streptococcus mutans biofilms. Biofouling 33, 722–740. doi: 10.1080/08927014.2017.1361412
Peng, X., Zhang, Y., Bai, G., Zhou, X., and Wu, H. (2016). Cyclic di-amp mediates biofilm formation. Mol. Microbiol. 99, 945–959. doi: 10.1111/mmi.13277
Perez-Diaz, M. A., Boegli, L., James, G., Velasquillo, C., Sanchez-Sanchez, R., Martinez-Martinez, R. E., et al. (2015). Silver nanoparticles with antimicrobial activities against Streptococcus mutans and their cytotoxic effect. Mater. Sci. Eng. C Mater. Biol. Appl. 55, 360–366. doi: 10.1016/j.msec.2015.05.036
Petersen, F. C., Tao, L., and Scheie, A. A. (2005). DNA binding-uptake system: a link between cell-to-cell communication and biofilm formation. J. Bacteriol. 187, 4392–4400. doi: 10.1128/JB.187.13.4392-4400.2005
Pires, J. G., Braga, A. S., Andrade, F. B., Saldanha, L. L., Dokkedal, A. L., Oliveira, R. C., et al. (2019). Effect of hydroalcoholic extract of Myracrodruon urundeuva all and Qualea grandiflora mart leaves on the viability and activity of microcosm biofilm and on enamel demineralization. J. Appl. Oral Sci. 27:e20180514. doi: 10.1590/1678-7757-2018-0514
Pleszczynska, M., Wiater, A., Janczarek, M., and Szczodrak, J. (2015). (1-->3)-alpha-D-glucan hydrolases in dental biofilm prevention and control: a review. Int. J. Biol. Macromol. 79, 761–778. doi: 10.1016/j.ijbiomac.2015.05.052
Postollec, F., Norde, W., Van Der Mei, H. C., and Busscher, H. J. (2003). Enthalpy of interaction between coaggregating and non-coaggregating oral bacterial pairs--A microcalorimetric study. J. Microbiol. Methods 55, 241–247. doi: 10.1016/s0167-7012(03)00145-3
Pratten, J., Wills, K., Barnett, P., and Wilson, M. (1998). In vitro studies of the effect of antiseptic-containing mouthwashes on the formation and viability of Streptococcus sanguis biofilms. J. Appl. Microbiol. 84, 1149–1155. doi: 10.1046/j.1365-2672.1998.00462.x
Qi, F., Kreth, J., Levesque, C. M., Kay, O., Mair, R. W., Shi, W., et al. (2005). Peptide pheromone induced cell death of Streptococcus mutans. FEMS Microbiol. Lett. 251, 321–326. doi: 10.1016/j.femsle.2005.08.018
Radaic, A., Ye, C., Parks, B., Gao, L., Kuraji, R., Malone, E., et al. (2020). Modulation of pathogenic oral biofilms towards health with nisin probiotic. J. Oral Microbiol. 12:1809302. doi: 10.1080/20002297.2020.1809302
Rainey, K., Michalek, S. M., Wen, Z. T., and Wu, H. (2019). Glycosyltransferase-mediated biofilm matrix dynamics and virulence of Streptococcus mutans. Appl. Environ. Microbiol. 85. doi: 10.1128/AEM.02247-18
Ramos, A. P., Cruz, M. A. E., Tovani, C. B., and Ciancaglini, P. (2017). Biomedical applications of nanotechnology. Biophys. Rev. 9, 79–89. doi: 10.1007/s12551-016-0246-2
Rehman, S., Ali, Z., Khan, M., Bostan, N., and Naseem, S. (2019). The dawn of phage therapy. Rev. Med. Virol. 29:e2041. doi: 10.1002/rmv.2041
Ren, Z., Cui, T., Zeng, J., Chen, L., Zhang, W., Xu, X., et al. (2016). Molecule targeting glucosyltransferase inhibits Streptococcus mutans biofilm formation and virulence. Antimicrob. Agents Chemother. 60, 126–135. doi: 10.1128/AAC.00919-15
Ren, Z., Kim, D., Paula, A. J., Hwang, G., Liu, Y., Li, J., et al. (2019). Dual-targeting approach degrades biofilm matrix and enhances bacterial killing. J. Dent. Res. 98, 322–330. doi: 10.1177/0022034518818480
Ribeiro, S. M., Fratucelli, E. D. O., Bueno, P. C. P., De Castro, M. K. V., Francisco, A. A., Cavalheiro, A. J., et al. (2019). Antimicrobial and antibiofilm activities of Casearia sylvestris extracts from distinct Brazilian biomes against Streptococcus mutans and Candida albicans. BMC Complement Altern. Med. 19:308. doi: 10.1186/s12906-019-2717-z
Roberts, A. P., and Kreth, J. (2014). The Impact of horizontal gene transfer on the adaptive ability of the human oral microbiome. Front. Cell. Infect. Microbiol. 4:124. doi: 10.3389/fcimb.2014.00124
Roberts, F. A., and Darveau, R. P. (2015). Microbial protection and virulence in periodontal tissue as a function of polymicrobial communities: symbiosis and dysbiosis. Periodontol 69, 18–27. doi: 10.1111/prd.12087
Roman, M. J., Decker, E. A., and Goddard, J. M. (2014). Metal-chelating active packaging film enhances lysozyme inhibition of listeria monocytogenes. J. Food. Prot. 77, 1153–1160. doi: 10.4315/0362-028X.JFP-13-545
Romling, U., Galperin, M. Y., and Gomelsky, M. (2013). Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. R. 77, 1–52. doi: 10.1128/MMBR.00043-12
Rumbaugh, K. P., and Sauer, K. (2020). Biofilm dispersion. Nat. Rev. Microbiol. 18, 571–586. doi: 10.1038/s41579-020-0385-0
Samuelson, J. C., Chen, M., Jiang, F., Moller, I., Wiedmann, M., Kuhn, A., et al. (2000). Yidc mediates membrane protein insertion in bacteria. Nature 406, 637–641. doi: 10.1038/35020586
Schlafer, S., Meyer, R. L., Sutherland, D. S., and Stadler, B. (2012). Effect of osteopontin on the initial adhesion of dental bacteria. J. Nat. Prod. 75, 2108–2112. doi: 10.1021/np300514z
Schwendicke, F., Leal, S., Schlattmann, P., Paris, S., Dias Ribeiro, A. P., Gomes Marques, M., et al. (2018). Selective carious tissue removal using subjective criteria or polymer bur: study protocol for a randomised controlled trial (select). BMJ Open 8:e022952. doi: 10.1136/bmjopen-2018-022952
Senpuku, H., Tuna, E. B., Nagasawa, R., Nakao, R., and Ohnishi, M. (2019). The inhibitory effects of polypyrrole on the biofilm formation of Streptococcus mutans. PLoS One 14:e0225584. doi: 10.1371/journal.pone.0225584
Serra, D. O., and Hengge, R. (2014). Stress responses go three dimensional - the spatial order of physiological differentiation in bacterial macrocolony biofilms. Environ. Microbiol. 16, 1455–1471. doi: 10.1111/1462-2920.12483
Sharma, S. K., Dai, T., Kharkwal, G. B., Huang, Y. Y., Huang, L., De Arce, V. J., et al. (2011). Drug discovery of antimicrobial photosensitizers using animal models. Curr. Pharm. Des. 17, 1303–1319. doi: 10.2174/138161211795703735
Sims, K. R. Jr., Maceren, J. P., Liu, Y., Rocha, G. R., Koo, H., and Benoit, D. S. W. (2020). Dual antibacterial drug-loaded nanoparticles synergistically improve treatment of streptococcus mutans biofilms. Acta Biomater. 115, 418–431. doi: 10.1016/j.actbio.2020.08.032
Singh, K., Senadheera, D. B., Levesque, C. M., and Cvitkovitch, D. G. (2015). The copYAZ operon functions in copper efflux, biofilm formation, genetic transformation, and stress tolerance in Streptococcus mutans. J. Bacteriol. 197, 2545–2557. doi: 10.1128/JB.02433-14
Snarr, B. D., Baker, P., Bamford, N. C., Sato, Y., Liu, H., Lehoux, M., et al. (2017). Microbial glycoside hydrolases as antibiofilm agents with cross-kingdom activity. Proc. Natl. Acad. Sci. U.S.A. 114, 7124–7129. doi: 10.1073/pnas.1702798114
Srivastava, D., Hsieh, M. L., Khataokar, A., Neiditch, M. B., and Waters, C. M. (2013). Cyclic di-GMP Inhibits vibrio cholerae motility by repressing induction of transcription and inducing extracellular polysaccharide production. Mol. Microbiol. 90, 1262–1276. doi: 10.1111/mmi.12432
Steinberg, D., Bachrach, G., Gedalia, I., Abu-Ata, S., and Rozen, R. (2002). Effects of various antiplaque agents on fructosyltransferase activity in solution and immobilized onto hydroxyapatite. Eur. J. Oral Sci. 110, 374–379. doi: 10.1034/j.1600-0722.2002.21303.x
Stipp, R. N., Boisvert, H., Smith, D. J., Hofling, J. F., Duncan, M. J., and Mattos-Graner, R. O. (2013). Covr and vicRk regulate cell surface biogenesis genes required for biofilm formation in Streptococcus mutans. PLoS One 8:e58271. doi: 10.1371/journal.pone.0058271
Sun, Y., Sun, X., Li, X., Li, W., Li, C., Zhou, Y., et al. (2021). A versatile nanocomposite based on nanoceria for antibacterial enhancement and protection from aPDT-aggravated inflammation via modulation of macrophage polarization. Biomaterials 268:120614. doi: 10.1016/j.biomaterials.2020.120614
Tanzer, J. M., Thompson, A., Sharma, K., Vickerman, M. M., Haase, E. M., and Scannapieco, F. A. (2012). Streptococcus mutans out-competes Streptococcus gordonii in vivo. J. Dent. Res. 91, 513–519. doi: 10.1177/0022034512442894
Tawakoli, P. N., Neu, T. R., Busck, M. M., Kuhlicke, U., Schramm, A., Attin, T., et al. (2017). Visualizing the dental biofilm matrix by means of fluorescence lectin-binding analysis. J. Oral Microbiol. 9. doi: 10.1080/20002297.2017.1345581
Terra Garcia, M., Correia Pereira, A. H., Figueiredo-Godoi, L. M. A., Jorge, A. O. C., Strixino, J. F., and Junqueira, J. C. (2018). Photodynamic therapy mediated by chlorin-type photosensitizers against Streptococcus mutans biofilms. Photodiagnosis Photodyn. Ther. 24, 256–261. doi: 10.1016/j.pdpdt.2018.08.012
Thallinger, B., Prasetyo, E. N., Nyanhongo, G. S., and Guebitz, G. M. (2013). Antimicrobial enzymes: an emerging strategy to fight microbes and microbial biofilms. Biotechnol. J. 8, 97–109. doi: 10.1002/biot.201200313
Tian, S., Su, L., Liu, Y., Cao, J., Yang, G., Ren, Y., et al. (2020). Self-targeting, zwitterionic micellar dispersants enhance antibiotic killing of infectious biofilms—an intravital imaging study in mice. Sci. Adv. 6:eabb1112. doi: 10.1126/sciadv.abb1112
Tonguc-Altin, K., Sandalli, N., Duman, G., Selvi-Kuvvetli, S., Topcuoglu, N., and Kulekci, G. (2015). Development of novel formulations containing lysozyme and lactoferrin and evaluation of antibacterial effects on mutans streptococci and lactobacilli. Arch. Oral Biol. 60, 706–714. doi: 10.1016/j.archoralbio.2015.02.004
Townsend-Lawman, P., and Bleiweis, A. S. (1991). Multilevel control of extracellular sucrose metabolism in Streptococcus salivarius by sucrose. J. Gen. Microbiol. 137, 5–13. doi: 10.1099/00221287-137-1-5
Vacca Smith, A. M., and Bowen, W. H. (2000). In situ studies of pellicle formation on Hydroxyapatite discs. Arch. Oral Biol. 45, 277–291. doi: 10.1016/s0003-9969(99)00141-7
Venault, A., Yang, H. S., Chiang, Y. C., Lee, B. S., Ruaan, R. C., and Chang, Y. (2014). Bacterial resistance control on mineral surfaces of hydroxyapatite and human teeth via surface charge-driven antifouling coatings. ACS Appl. Mater. Interfaces 6, 3201–3210. doi: 10.1021/am404780w
Viszwapriya, D., Subramenium, G. A., Radhika, S., and Pandian, S. K. (2017). Betulin inhibits cariogenic properties of Streptococcus mutans by targeting vicRk and Gtf genes. Antonie Van Leeuwenhoek 110, 153–165. doi: 10.1007/s10482-016-0785-3
Wan, S. X., Tian, J., Liu, Y., Dhall, A., Koo, H., and Hwang, G. (2021). Cross-kingdom cell-to-cell interactions in cariogenic biofilm initiation. J. Dent. Res. 100, 74–81. doi: 10.1177/0022034520950286
Wang, B., Liu, T., Chen, H., Yin, B., Zhang, Z., Russell, T. P., et al. (2021). Molecular brush surfactants: versatile emulsifiers for stabilizing and structuring liquids. Angew. Chem. Int. Ed. Engl. 60, 19626–19630. doi: 10.1002/anie.202104653
Wang, L. S., Gupta, A., and Rotello, V. M. (2016). Nanomaterials for the treatment of bacterial biofilms. ACS Infect. Dis. 2, 3–4. doi: 10.1021/acsinfecdis.5b00116
Wang, Y., Shen, X., Ma, S., Guo, Q., Zhang, W., Cheng, L., et al. (2020). Oral biofilm elimination by combining iron-based nanozymes and hydrogen peroxide-producing bacteria. Biomater. Sci. 8, 2447–2458. doi: 10.1039/c9bm01889a
Wen, Z. Z. T., Scott-Anne, K., Liao, S. M., De, A. P., Luo, M., Kovacs, C., et al. (2018). Deficiency of brpA in Streptococcus mutans reduces virulence in rat caries model. Mol. Oral Microbiol. 33, 353–363. doi: 10.1111/omi.12230
Willcox, D. P., and Drucker, D. B. (1987). A simple method for determining extracellular polysaccharide-producing ability of oral streptococci. Microbios 51, 175–181. doi: none.
Xavier, J. B., Picioreanu, C., Rani, S. A., Van Loosdrecht, M. C. M., and Stewart, P. S. (2005). Biofilm-control strategies based on enzymic disruption of the extracellular polymeric substance matrix--a modelling study. Microbiology 151, 3817–3832. doi: 10.1099/mic.0.28165-0
Xi, Y., Wang, Y., Gao, J., Xiao, Y., and Du, J. (2019). Dual corona vesicles with intrinsic antibacterial and enhanced antibiotic delivery capabilities for effective treatment of biofilm-induced periodontitis. ACS Nano 13, 13645–13657. doi: 10.1021/acsnano.9b03237
Xiao, J., Grier, A., Faustoferri, R. C., Alzoubi, S., Gill, A. L., Feng, C., et al. (2018). Association between oral candida and bacteriome in children with severe ECC. J. Dent. Res. 97, 1468–1476. doi: 10.1177/0022034518790941
Xiao, J., Klein, M. I., Falsetta, M. L., Lu, B. W., Delahunty, C. M., Yates, J. R., et al. (2012). The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. Plos Pathogens 8:e1002623. doi: 10.1371/journal.ppat.1002623
Xiao, J., and Koo, H. (2010). Structural organization and dynamics of exopolysaccharide matrix and microcolonies formation by Streptococcus mutans in biofilms. J. Appl. Microbiol. 108, 2103–2113. doi: 10.1111/j.1365-2672.2009.04616.x
Xu, R. R., Yang, W. D., Niu, K. X., Wang, B., and Wang, W. M. (2018). An update on the evolution of glucosyltransferase (Gtf) genes in streptococcus. Front. Microbiol. 9:2979. doi: 10.3389/fmicb.2018.02979
Yan, W., Qu, T., Zhao, H., Su, L., Yu, Q., Gao, J., et al. (2010). The effect of C-di-GMP (3'-5'-cyclic diguanylic Acid) on the biofilm formation and adherence of Streptococcus mutans. Microbiol. Res. 165, 87–96. doi: 10.1016/j.micres.2008.10.001
Yang, J. M., Deng, D. M., Brandt, B. W., Nazmi, K. R., Wu, Y. F., Crielaard, W., et al. (2019). Diversity of SpaP in genetic and salivary agglutinin mediated adherence among Streptococcus mutans strains. Sci. Rep. 9:19943. doi: 10.1038/s41598-019-56486-9
Yao, S. G., and Fine, J. B. (2014). Probiotics for bacterial disease treatment in the oral environment. Compend. Contin. Educ. Dent. 35, 658–663. quiz 664. doi: none
Yoshida, A., Ansai, T., Takehara, T., and Kuramitsu, H. K. (2005). Luxs-based signaling affects Streptococcus mutans biofilm formation. Appl. Environ. Microbiol. 71, 2372–2380. doi: 10.1128/AEM.71.5.2372-2380.2005
Zeng, Y., Nikitkova, A., Abdelsalam, H., Li, J., and Xiao, J. (2019). Activity of quercetin and kaemferol against Streptococcus mutans biofilm. Arch. Oral Biol. 98, 9–16. doi: 10.1016/j.archoralbio.2018.11.005
Zhang, J., Chen, C., Chen, J., Zhou, S., Zhao, Y., Xu, M., et al. (2020). Dual mode of anti-biofilm action of G3 against Streptococcus mutans. ACS Appl. Mater. Interfaces 12, 27866–27875. doi: 10.1021/acsami.0c00771
Zhao, Y., Guo, Q., Dai, X., Wei, X., Yu, Y., Chen, X., et al. (2019a). A biomimetic non-antibiotic approach to eradicate drug-resistant infections. Adv. Mater. 31:e1806024. doi: 10.1002/adma.201806024
Zhao, Z., Ding, C., Wang, Y., Tan, H., and Li, J. (2019b). Ph-responsive polymeric nanocarriers for efficient killing of cariogenic bacteria in biofilms. Biomater. Sci. 7, 1643–1651. doi: 10.1039/c8bm01640b
Zhou, J., Horev, B., Hwang, G., Klein, M. I., Koo, H., and Benoit, D. S. (2016). Characterization and optimization of PH-responsive polymer nanoparticles for drug delivery to oral biofilms. J. Mater. Chem. B. 4, 3075–3085. doi: 10.1039/C5TB02054A
Keywords: dispersion, eradication, disruption, cariogenic biofilms, dental plaque, Streptococcus mutans
Citation: Chen R, Du M and Liu C (2022) Strategies for dispersion of cariogenic biofilms: applications and mechanisms. Front. Microbiol. 13:981203. doi: 10.3389/fmicb.2022.981203
Edited by:
Huancai Lin, Sun Yat-sen University, ChinaReviewed by:
Dongyeop Kim, Jeonbuk National University, South KoreaWei Hu, Shandong University, China
Copyright © 2022 Chen, Du and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chang Liu, bGl1YzA3MjhAd2h1LmVkdS5jbg==
 Rourong Chen
Rourong Chen Minquan Du
Minquan Du Chang Liu
Chang Liu