
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 14 September 2022
Sec. Terrestrial Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.979383
This article is part of the Research Topic Recent advancements in microbe-pesticide interaction: A smart-soil bioremediation approach View all 18 articles
Synthetic pesticides are extensively and injudiciously applied to control agriculture and household pests worldwide. Due to their high use, their toxic residues have enormously increased in the agroecosystem in the past several years. They have caused many severe threats to non-target organisms, including humans. Therefore, the complete removal of toxic compounds is gaining wide attention to protect the ecosystem and the diversity of living organisms. Several methods, such as physical, chemical and biological, are applied to degrade compounds, but as compared to other methods, biological methods are considered more efficient, fast, eco-friendly and less expensive. In particular, employing microbial species and their purified enzymes makes the degradation of toxic pollutants more accessible and converts them into non-toxic products by several metabolic pathways. The digestive tract of insects is usually known as a superior organ that provides a nutrient-rich environment to hundreds of microbial species that perform a pivotal role in various physiological and ecological functions. There is a direct relationship between pesticides and insect pests: pesticides reduce the growth of insect species and alter the phyla located in the gut microbiome. In comparison, the insect gut microbiota tries to degrade toxic compounds by changing their toxicity, increasing the production and regulation of a diverse range of enzymes. These enzymes breakdown into their derivatives, and microbial species utilize them as a sole source of carbon, sulfur and energy. The resistance of pesticides (carbamates, pyrethroids, organophosphates, organochlorines, and neonicotinoids) in insect species is developed by metabolic mechanisms, regulation of enzymes and the expression of various microbial detoxifying genes in insect guts. This review summarizes the toxic effects of agrochemicals on humans, animals, birds and beneficial arthropods. It explores the preferential role of insect gut microbial species in the degradation process and the resistance mechanism of several pesticides in insect species. Additionally, various metabolic pathways have been systematically discussed to better understand the degradation of xenobiotics by insect gut microbial species.
In modern agriculture, for the management of various kinds of pests and the production of high-yield crops to meet the food availability for human beings, pesticides are extensively applied all over the world (Giambò et al., 2021). Pesticides are chemicals that control different pests such as rodents, arthropods, weeds and microbial pathogens (Huang and Chen, 2022). Pest management strategy is a vigorous arms race: on the one hand, farmers, pesticide inventors, agribusiness men, and researchers throughout the world struggle for the protection of crops and their higher production (Damalas and Koutroubas, 2018). While on the other hand, insects and other microbial pathogens follow their biological metabolism and drive to live and reproduce their generations (Pietri and Liang, 2018). Due to the repetitive application of pesticides with higher concentrations, insects and other pathogens fail to control them and develop cross-resistance (Daisley et al., 2018; Gressel, 2018). However, insect resistance against insecticides produces severely threaten non-target living organisms and contaminates the ecosystem (Khalid et al., 2021). Various studies have reported that pesticides’ toxic residues are abundantly present in soil, sediments, and water bodies (Mulla, Ameen et al., 2020).
These hazardous compounds and their toxic metabolite residues significantly affect the climate and living organisms such as soil biota, fish, birds, mammals, plants and human beings (Lee et al., 2021; Pujar et al., 2022). In addition, their toxic residues ruin organisms’ behavior, reproduction cycles and metabolism mechanisms, which can permanently alter the interrelated ecosystem (Zhao et al., 2019). These toxic compounds are degraded into simpler or less toxic substances using various methods such as chemical reactions, physical methods, photodegradation and biodegradation. Compared to other techniques, biological methods are less expensive, environment-friendly, more effective and easier to adapt to remove emerging pollutants (Hao et al., 2018).
Microbial species have been extensively applied for the biodegradation of environmental pollutants, including agrochemicals (Chen et al., 2015). To date, researchers throughout the world have screened millions of microbial species (bacteria, fungi, yeasts, algae, etc.) from the soil, sewage sludge, wastewater and other contaminated sites (Yang L. et al., 2021). Investigation of pure cultures of microbial species has revealed that toxic molecules are transformed into various metabolites (Ahlawat et al., 2020). Nevertheless, due to considering prominent features of insect gut microbial species like high resistance to pesticides and purification of novel suitable enzymes, various researchers have isolated a diverse number of microbial species for the biological treatment of wastewater and clean-up of the contaminated environment (Skidmore and Hansen, 2017).
Gut microbial species play a pivotal role in the detoxification, mineralization, and catabolism of organic molecules employed in pest control as determined by degradation or histochemical mechanisms (Berasategui et al., 2016). They are also considered superior organs for producing pheromones, synthesizing vitamins, and different enzymes to prevent pathogens (Ozdal et al., 2016). The gut of insects and other arthropods provides a rich nutrient medium for developing microbial species that can produce some essential enzymes and contribute significantly to insect physiology (Ramakrishnan et al., 2019; Figure 1). Insect gut microflora provides a prominent environment for transforming genes, mutant traits, and conjugative plasmids, which can adapt to harsh environmental conditions and perform smoothly in biodegradation processes (Xia et al., 2018).
More importantly, microbial species isolated from this source are rarely indigenous to the polluted environment. Hence, their use in bioaugmentation and biodegradation enhances their efficiency to remove environmental pollutants (Kadri et al., 2018). To keep in mind these critical points, insect associated-microbial species, especially bacteria, are more vigorous and beneficial because they are interrelated with the application of active ingredients (Blanton and Peterson, 2020). The coordination between symbiotic microbial species and resistance to pesticides in arthropods would provide new opportunities for managing pests and isolating efficient microbial species to protect the agroecosystem (Li et al., 2018).
This review investigates the resistance mechanisms of different pesticides in insect pathogens and the transforming mechanisms of their parent toxic compounds into less toxic intermediates by the isolation of gut microflora. Additionally, microbial species interlinked with insects and involved in the detoxification of pesticides will be essential in the designing of future novel ingredients to ensure their long-term efficiency. Therefore, investigating the linkages between environmental contaminants and gut microflora is of great significance. This literature review will be beneficial and guider to reveal the possible impacts of gut microflora on the fate of organic pollutants and provide a more comprehensive insight into the mineralization, transformation, and biodegradation of pesticides and other emerging pollutants from the environment.
Throughout the world, an extensive range of pesticides like insecticides, herbicides, rodenticides, fungicides, nematicides, molluscicides, rodenticides, bactericides, repellents, insect growth regulators and disinfectants have been generated for the management of specific target pests in agriculture, aquaculture, horticulture and households (van de Merwe et al., 2018). Currently, more than 3.5 million tons of pesticides are used throughout the world, out of which 47.5% are herbicides, 29.5% are insecticides, 17.5% are fungicides, and 5.5% are other types of pesticides (Sharma et al., 2019). Since its inception 50 years ago, China has grown to be the world’s largest producer and consumer of pesticides. The other major pesticide-consuming countries are the United States, Argentina, Thailand, Brazil, Italy, France, Canada, Japan, and India (Olisah et al., 2019). These agrochemicals are extensively introduced into modern agriculture and urban ecosystems during their production, transportation, improper storage and unwise applications, which cause severe environmental threats (Ahmad et al., 2021). Local governments and environmental protection agencies regulate the production of pesticides and their applications. But the ecological management and risk assessment rules and regulations are generally restricted to formulating agrochemicals and their active ingredients and additives (Pokhrel et al., 2018). According to a combined statement of WHO and UNEP, approximately 200,000 people worldwide die, and roughly three million are affected yearly by pesticide residues (Meftaul et al., 2020). Another study revealed that the majority of cases, nearly 95% of them are reported from developing countries (Yadav et al., 2015). Agrochemicals severely effect the ecosystem through toxic residues at the application sites, such as agricultural farms, lawns and parks (Wu et al., 2007). However, these compounds pose severe threats to aquatic organisms by leaching down into the groundwater and through surface runoff into lakes, rivers, and other water bodies (Ahmad et al., 2022a). Furthermore, when pesticides are applied to crops, horticulture areas, home lawns and school parks, many people including, children and women, animals, beneficial arthropods, birds and wildlife creatures, are seriously affected (Masud et al., 2018).
Due to unwise applications of pesticides with higher concentrations, their toxic residues have been frequently revealed in the urban air, dust, soil and water bodies than in those of rural areas, predominantly due to primary, secondary and re-emissions of the parent compound and their toxic derivatives (Ren et al., 2018). Agronomic crops and other ornamental plants can easily absorb these chemicals from contaminated sites and transfer them to their vegetative and reproductive parts (Kim et al., 2017). When farmers apply higher concentrations of pesticides to protect their crops from pests and diseases, their residues are entered into food commodities (Dai et al., 2010). However, various researchers are working to investigate toxic pesticide residues in fruits and vegetables growing in agricultural, rural and urban areas in developing and developed countries (Pietrzak et al., 2020; Barbieri et al., 2021; Zamule et al., 2021). Incorporating pesticide residues into daily food consumption is a foremost safety issue for consumers worldwide (Hasan et al., 2017). The excessive use of pesticides deliberately affects flora, fauna and the ecosystem (Arunkumar et al., 2017). We briefly discuss the risk of pesticides to humans’ health and other non-target living organisms in the following sections.
The labors working in pesticide formulation industries, agriculture areas, and assassinators for managing household pests are generally affected by direct or indirect pesticide exposure. There are higher chances of risk for people working in the pesticide manufacturing industries at the time of formulation, packaging and production because they handle crude materials and other hazardous solvents (Gangemi et al., 2016; Nicolopoulou-Stamati et al., 2016). Various kinds of health disorders such as cancer, diabetes problems, respiratory issues, neurological disorders, reproductive syndromes and oxidative stress are produced due to direct or indirect exposure and handling of pesticides or their toxic active ingredients in foodstuffs (Carles et al., 2017; Grewal, 2017; Rani et al., 2021). Some studies have revealed that due to continuous risk assessment of highly toxic compounds, including pesticides such as lung cancer, breast cancer, leukemia and multiple myeloma have occurred in human beings (Han et al., 2018; Ruiz et al., 2018; Huang et al., 2019; Jaacks et al., 2019). Meng et al. (2016) carried out a study to investigate agrochemical exposure in indoor dust and blood samples. Results of this study revealed asthma is positively interlinked with exposure to alpha-hexachlorocyclohexane in humans.
In another study to evaluate pesticide exposure and its effects on human health, Tanner et al. (2009) carried out a study. They discovered that Parkinson’s disease and 2-4 D herbicides are closely associated with their cause. Yan et al. (2016) reported that pesticides and Alzheimer’s disease are closely interlinked, and a meta-analysis proved that pesticide exposure is hazardous for the brain and eyes. Recently, Shah et al. (2020) carried out a study investigating the effect of organochlorine pesticides such as β-hexachlorocyclohexane, dichlorodiphenyldichloroethylene and dieldrin on human epithelial ovary cells for the risk prediction of ovarian cancer. The findings of this study revealed that organochlorine pesticides highly affect human health and stimulate the measurement of reactive oxygen species (ROS), pro-inflammatory response and DNA damage in human epithelial ovary cells. Besides this, DDT organochlorine pesticide caused DNA damage, genetic instability, micronucleus formation and the sister chromatid exchange in humans (Yáñez et al., 2004; Savant et al., 2018). Cassidy et al. (2005) studied the relationship between women’s breast cancer and heptachlor pesticide. The results of this study revealed that women’s breast cancer risk was positively correlated with the level of heptachlor epoxide. Richardson et al. (2014) investigated the effect of dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) on human health and found that these both pesticides were responsible for causing Alzheimer’s disease. In another study, due to high exposure to organochlorine pesticides their toxic effects on human health were examined, and it was found that these pesticides cause Parkinson’s disease (Freire and Koifman, 2012).
Imprudent application of pesticides in farming could pollute surface water via draining, runoff, leaching and drift. Polluted surface water harms non-target organisms, including humans and animals (Chrustek et al., 2018; Lee and Choi, 2020). Surface water is considered a major drinking water source in developing nations such as Pakistan, India, Bangladesh, Nepal, and Sri Lanka (Mojiri et al., 2020; Teklu et al., 2022). The residues of various pesticides from the groups of organochlorines, organophosphate, carbamates, neonicotinoids, and pyrethroids are found in the rivers of California (Anderson et al., 2018). Besides this, several other European countries also investigated pesticide residues and noticed that 76 types of pesticide residues are present in European soil. Furthermore, it was revealed that 83% of soil contained one type of residue, and 58% of soil contained two, three or more types of residues. The highest concentrations of glyphosate and its derivatives were detected frequently. The presence of pesticide residues in the surface water and rivers all over the world causes critical threats to aquatic organisms (Mitchell et al., 2017; Tian et al., 2018; Dias et al., 2020).
A study was conducted to evaluate the exposure of organochlorine and pyrethroid pesticide residues in surface water, fish, sediments and aquatic weeds in the southern region. Results of this study revealed that residues of organochlorine pesticides were identified in surface water, sediments, fish muscle, gills, liver, and aquatic weeds at a concentration of 0.001–34.44 μg/L, 0.01–16.72 μg/Kg, 0.01–26.05 μg/Kg, 0.01–40.56 μg/Kg, 0.01–65.14 μg/Kg, 0.01–5.53 μg/Kg, respectively. This study further explained that organochlorine pesticides such as eldrin, dieldrin, endosulfan, endrin, and heptachlor were the prominent pesticides identified with the above level of maximum residue limit set by the World Health Organization in surface water, sediments and fish (Arisekar et al., 2019). In another study, residues of pesticides in the Guayas River at 181 places were investigated using the solid phase extraction method. Results of this study explained that 26 types of pesticide residues in fresh water at 108 sampling sites (60%) were detected with higher concentrations. The major types of pesticides found in river water are cadusafos, butachlor, and pendimethalin at 62, 21, and 21, with concentrations of 0.081, 2.006, and 0.557 μg/L, respectively. Finally, this study also demonstrated that all detected pesticides in river water are frequently found in agriculture and horticulture crops such as rice and banana, with higher concentrations due to irregular application methods like aerial spraying. Finally, their residues are transferred to the rice field and river water. This study also suggested that precaution measures such as legal regulations and awareness campaigns for farmers and local industries are highly recommended to control environmental contamination and prevent the accumulation of pesticide residues in aquatic and terrestrial systems (Deknock et al., 2019).
Recently, for the investigation of highly used herbicide residues such as atrazine, acetochlor, alachlor, hexazinone, metolachlor, simazine, terbuthylazine, trifluralin, and phenoxy acids (MCPA and 2,4-D) in two type of fishes (Clarias gariepinus, Oreochromis mossambicus) an experimental study was conducted. This study showed that all herbicides’ residues were found in analyzed samples with a total concentration ranging from 42.3 to 238 ng/g in Clarias gariepinus and 72.2–291 ng/g in Oreochromis mossambicus. The most dominant herbicides which are found in fish tissues, gills and liver are phenoxy acid herbicides, acetochlor, atrazine and terbuthylazine with the ranges of 17.6 ± 12 ng/g, 28.9 ± 16 ng/g, 15.4 ± 5.8 ng/g, 12.7 ± 7.1 ng/g, 12.4 ± 12 ng/g, respectively (Tyohemba et al., 2021).
Insect pollinators and predators play a pivotal role in developing many crops, as insect pollinators increase the yield and predators protect crops from pest infestation (Ihara et al., 2017). But due to excessive use of agrochemicals and unselective treatment in the modern agriculture system, their diversity and abundance are severely affected (Joshi et al., 2020). It has been reported that only 1% of pesticides reach the target site, whereas the remaining amount accumulates in the environment and contaminates it (Goergen et al., 2016; Fine et al., 2017; Stein et al., 2017). Beneficial arthropods are directly linked with pesticide exposure at the time of application or immediately after applying pesticides. The droplets of toxic residues could inlet on their cuticle by ingestion and influence their growth and mating behavior (Sgolastra et al., 2017).
Abraham et al. (2018) investigated the effects of glyphosate herbicides on beneficial insects (Apis mellifera, Hypotrigona ruspolii) under laboratory conditions. The bees were treated with the recommended concentration, a two-fold higher recommended concentration, and distilled water for control. The impact of glyphosate herbicide was compared with the lambda cyhalothrin. The herbicide was sprayed on plants as well as the bees were treated with herbicide-sprayed filter paper. Results of this study revealed that a more significant number of bees died after contact with the herbicide in both ways. This study concluded that spraying glyphosate herbicide was very dangerous for beneficial insects by contacting or spraying on fresh plants with more than the recommended dose. Another study investigated the effects of widely used neonicotinoids (acetamiprid, imidacloprid, thiamethoxam, and thiacloprid) on spiders. All the neonicotinoids with recommended doses were applied in field conditions, and short-term exposure was evaluated on spiders. Results of this study revealed that after 1 h, imidacloprid showed more critical effects and revealed partial acute lethality (15–32%). Acetamiprid showed strong sublethal effects, particularly when employed dorsally on Philodromus cespitum. After 1 day of application of thiacloprid and acetamiprid, Linyphiidae species were paralyzed or finally caused death, especially in males (Øezáè et al., 2019).
A study was conducted to reveal the toxicity of imidacloprid, thiamethoxam and sulfoxaflor on the aphid (Aphis gossypii). The impact of these pesticides on natural enemies of aphids, especially parasitoids (Aphidius colemani), was investigated with low lethal, median lethal and sublethal concentrations. This study showed that the median lethal concentration caused maximum mortality of parasitoids compared to sulfoxaflor, while imidacloprid had the least negligible impact on the diversity of parasitoids (Ricupero et al., 2020). To study the effects of more neonicotinoid pesticides such as imidacloprid, thiamethoxam, clothianidin and dinotefuran on the parasitoid larvae (Coccinella septempunctata) by the application of lethal and median lethal doses using the direct contact method. This study indicated that neonicotinoid pesticides are hazardous for the survival of larvae. The median lethal dose highly impacts the emergence of larval instars, pupal emergence and weight. Finally, this study concluded that pesticide application is hazardous for the survival of beneficial insects, primarily involved in integrated pest management services (Wu et al., 2021).
Every day, increasing environmental pollution affects many living organisms and has always been considered a critical challenge in the scientific community (Niroumand et al., 2016). The high accumulation of pesticides in agricultural soils and their cumulative behavior and toxicity pose severe threats to beneficial plants (Ferrando and Matamoros, 2020). It is well known that the accumulation of pesticides affects the behavior of soil microbial species and enzymes and is absorbed by plants, which further transfers it to non-target organisms through the food chain process (Sayed et al., 2020). The adaptation of medicinal plants to cure various diseases has been practiced for several centuries and even today plays a pivotal role in primary health care as a therapeutic agent in several developing nations (Reinholds et al., 2017; Kumar et al., 2018).
The application of herbal medicines to treat various illnesses has increased significantly in the past few decades due to their prominent features such as minimum side effects compared to synthetic drugs, inexpensive and excellent viability (Ammar et al., 2020). Besides their numerous benefits, toxic pesticide residues could be more dangerous and cause many diseases in humans and other living organisms (Righi et al., 2018). Recently, Luo et al. (2021) have studied the accumulation of pesticide residuals in various medicinal plants, which are frequently used throughout the world for the welfare of humanity. Results of this study explained that in 1771 samples, 88% of pesticide residues were detected. Terrifyingly, 59% of pesticide residues are beyond the European Pharmacopoeia (EP) limit and 43% are confined to 35 types of banned pesticides worldwide. Additionally, this study demonstrated that eight pesticide residues were five hundred times higher than the default maximum residue limit set by the environmental protection agency.
In another study, Li R. -X. et al. (2020) investigated the presence of various pesticide residues in herbal plants using the Quick Easy Cheap Effective Rugged Safe (QuEChERS) extraction method. All residues were detected through Ultra-Performance Liquid Chromatography and Gas Chromatography-Mass spectrometry analysis. This method was applied to 39 real samples of Ophiopogon japonicus, Polygonatum odoratum, and Paeonia suffruticosa obtained from different locations, and the results of this study revealed that in 92.3% of samples, residues of pesticides were detected. This study showed that 26% pesticide residues are frequently detected in three traditional Chinese medicine plants. In addition, tebuconazole and paclobutrazol residue levels were considerably higher in nine samples compared to the maximum residue limit.
The primary way of transformation of pesticides in the general population is the consumption of food commodities that might be polluted with toxic residues of pesticides (Nagy et al., 2020). However, their residues can ultimately be inserted into animals’ digestive tracts via various pathways and affect their physical conditions (Altun et al., 2017; Yuan et al., 2019). Recently, Nerozzi et al. (2020) investigated the effects of glyphosate and its most famous formulation Roundup, on animal health and reproductive functions. In this study, the pig was chosen as a model animal. The commercial semen of pigs was treated with glyphosate and Roundup formulation at 0–360 μg/mL concentrations and incubated at 38°C for 3 h. The consequences of this study indicated that the application of high concentrations of glyphosate significantly reduced sperm viability, motility, mitochondrial activity and acrosome integrity. While on the other side, by treating lower concentrations (5–100 μg/mL) of Roundup formulation, all the disorders were observed after 1 h of incubation. Finally, this study concluded that pesticides active ingredients and inert materials negatively affect animals and the human reproductive system. Jarrell et al. (2020) also reported that glyphosate-based herbicides are more dangerous for animals’ health and cause severe diseases such as the reproductive system, altering the regulation of enzymes, disrupting serum levels and activity, and loss of fertility.
An investigation was carried out to evaluate deltamethrin and ivermectin residues on local sheep milk and meat. A total of eighty samples (40 each for milk and meat) were obtained from different places, and detection of pesticide residues was observed by performing High-Performance Liquid Chromatography. Results of this study indicated that 92.5 of milk samples and 90% of meat samples were polluted with toxic deltamethrin residues. More alarmingly, this study highlighted that all samples were contaminated with ivermectin residues above the maximum residue limit set by the World Health Organization (WHO) and Food and Agriculture Organization (FAO) (Mani and Al Araji, 2022).
However, to remove various environmental pollutants, protect the diversity of living organisms, and save crops from pests, effective, eco-friendly, less expensive, and more applicable methods are urgently required.
In order to gain a better knowledge of plants and microbes, researchers are using system biology technologies (Bhatt et al., 2016). Numerous details on the interactions between microbes, plants, humans and other non-target organisms by pesticides in nature have been fabricated because of advances in the fields of genomics and proteomics (Bhandari et al., 2021). Recently, a biological system-based approach was carried out to remove atrazine residues from the contaminated environment and to understand the complex biological network with different cellular systems modeling and simulation of atrazine were performed. The findings of this study revealed that two functional enzymes from bacteria (chlorohydrolase and monooxygenase) actively performed and completely degraded atrazine from the environment. To learn more about the biochemistry and physiology of atrazine in various cellular networks, additional analysis and simulations of the utilized model were performed. Atrazine degradation’s 289 nodes and 300 edges were verified by topological analysis (Bhatt et al., 2020). Insecticides containing pyrethroids are frequently used to control pests in homes and agricultural crops (Bhatt et al., 2022b). The complete removal of various pyrethroid pesticides was achieved using a system biological based approach and a simulated model. Results of this study explain that the toxic metabolites of pyrethroids severely affect non-target organisms, especially beneficial arthropods, microorganisms and human health. In addition, this investigation actively contributed to analyzing the toxicity and removal of other emerging pollutants from the agroecosystem (Bhatt et al., 2021a). In another study, a potential bacterial strain, Bacillus subtilis 1D, was isolated from a polluted agriculture field and investigated their degradation efficiency to degrade cypermethrin from the environment. The findings of this study showed that bacterial stain efficiently degraded 95% of cypermethrin within 15 days and converted it into various metabolites. In addition, laccase and esterase enzymes were identified from bacterial strain and observed that both enzymes more rapidly degraded cypermethrin as compared to free cells (Gangola et al., 2018).
A biological molecular model and a purified methyl transferase enzyme were adopted to degrade residues of methyl halide from a polluted environment. This study explained that the enzymes played a crucial role in the remediation of methyl halide. In addition, this model demonstrates that a volatile poisonous substance impacts the earth’s environmental layers and life systems (Bhatt et al., 2019). A potential Bacillus sp. FA3 was isolated from the contaminated environment and examined their degradation efficiency using the Box-Behnken design to degrade fipronil from the soil and water systems. Results of this study revealed that at optimum conditions (temperature of 32°C, pH 7, and rotational speed of 110 rpm), the bacterial strain performed efficiently and degraded 76% of fipronil within 15 days. Finally, this study concluded that Bacillus sp. FA3 was a superior candidate for removing fipronil from the wastewater and soil system and could be helpful for large-scale treatment (Bhatt et al., 2021b).
A diverse range of symbiotic microbial species have been produced within the insect gut and have contributed a very significant role in the regulation of insect metabolism, enhanced food digestion, increased excretion of waste fluids, protecting the host from enemies, developing resistance against toxins and degrading them into their intermediates (Smith et al., 2017; Heys et al., 2018; Tokuda et al., 2018; Hauffe and Barelli, 2019; Figure 1). The identification and characterization of insect gut microbial species are investigated mainly by culture-dependent or culture-independent techniques (Bourguignon et al., 2018; Bruno et al., 2019). However, the culture-dependent method usually produces biased results. It relies on various parameters and techniques, while in the culture-independent method, a lot of omics and molecular approaches are applied, such as 16S rRNA and BLAST analysis, which provide a better and more comprehensive picture of the microbial communities located in insect guts (Eski et al., 2018; Erlandson et al., 2019; Erb and Kliebenstein, 2020). The application of high throughput and next-generation sequencing provides new insights into obtaining microbial ecology (Harishankar et al., 2013). It reveals that the diversity of microbial species by using independent culture methods identified a higher number of microbial communities than traditional culture-based and conventional molecular methods (Bhatt et al., 2021c,a, 2022a; Mishra et al., 2021; Ahmad et al., 2022a). Therefore, a comprehensive evaluation of microbial communities within a host species plays a vital role in understanding insect physiology and their interactions with insect hosts (Armitage et al., 2022).
A comprehensive investigation was carried out to evaluate insect symbiotic microbial species and their significant roles in 305 insect samples belonging to 218 insect species in 21 taxonomic orders. Using an independent culture method and adopting 16S rRNA analysis, 454 pyrosequencing were performed, and 174,374 sequence reads were gained. This study’s results indicated a total of 9301 bacterial operational taxonomic units (OTUs) at a distance level of 3% from all samples, with an average of 84.3% (± 97.7) OTUs per sample. In addition, this study suggested that gut microbial species were dominated by Proteobacteria, Wolbachia, and Firmicutes with a ratio of 62.1, 14.1, and 20.7%, respectively. Finally, this study concluded that these bacterial communities could help in food digestion, the development of larval stages and enhanced the insect immune system (Yun et al., 2014). The findings of another study showed that the hindgut of subterranean termites contained a 90% population of bacteria and archaea (Hongoh, 2010). The diversity of bacterial species in the digestive tract of fruit flies (Drosophila melanogaster) was studied using 454 pyrosequencing of 16S rRNA gene amplicons. Results of this investigation explained that 5 OTUs enriched the sequence reads, and ≤97% of that sequence identity could be related to Acetobacter pomorum, Acetobacter tropicalis, Lactobacillus brevis, Lactobacillus fructivorans, and Lactobacillus plantarum (Wong et al., 2011).
In another study, using an independent culture technique and adopting molecular approaches such as denaturing gradient gel electrophoreses and 16S rRNA analysis, a high diversity of genus Gammaproteobacteria were identified in the gut of the locust Schistocerca gregaria. The results of this study suggested that this diversity of bacterial species engaged with a defensive mechanism and enhanced it against external pathogens and toxic chemicals (Dillon et al., 2010). Recently, Xue et al. (2021) investigated the diversity of gut microbial species in various life stages of Adelphocoris suturalis by adopting the independent culture technique. Results of this study explained that the gut of the first and second instar was highly accomplished with the diversity of bacterial species. This study demonstrated that in the phylum, Proteobacteria and Firmicutes were dominant with a ratio of 87.06 and 9.43%, respectively, while at the genus level, Erwinia (28.98%), Staphylococcus (5.69%), and Acinetobacter (4.54%) were dominant bacteria. Finally, this study concluded that the diversity of bacterial species could be applied for biological control.
The insect gut is divided into three primary regions: the anterior midgut or foregut, the posterior midgut, and the hindgut (Wang G. -H. et al., 2020). The anterior midgut and hindgut arise from the embryonic epithelium. They are sheltered from pathogens by an exoskeleton of chitin and integument glycoproteins, while the posterior midgut is mainly used for absorption and digestion (He et al., 2018). Additionally, the hindgut of insects serves as an extension of the body cavity and is used to collect dietary waste (Siddiqui et al., 2022). However, it offers an appropriate environment that stimulates the proliferation and diversification of insect gut microbiomes (Bruno et al., 2019). Many studies have reported that insect gut microbiota plays a significant role in developing symbiotic insect interactions facilitated by secondary metabolites (Shang et al., 2021a). Besides this, they also play an essential role in the detoxification of pesticides, providing a natural defense system, nutrient availability, development of resistance against toxins and pathogens, breakdown of food, and suitable for proper growth of insects (Jang and Kikuchi, 2020; Jing et al., 2020; Mogren and Shikano, 2021; Tilottama et al., 2021).
High benefits and more prominent features of insect gut microbial species provide new insights into the development of beneficial arthropods, which are often used as biocontrol agents to solve environmental problems and further applications for the welfare of humans (Samoilova et al., 2016; Borrelli et al., 2017; Liao et al., 2017). However, considering the superior features of insect gut microbiota, this review mainly focuses on their potential applications for the detoxification of pesticides and their toxic metabolites for the cleanup of the environment (Wang S. et al., 2020).
Pesticides have been applied to manage pests and diseases since the start of agriculture for the production and protection of crops. However, the unwise use of pesticides accumulates in the ecosystem and contaminates plants, air, water and soil (Lewis et al., 2016). The storage of pesticides in plants can develop resistance or tolerance against various pests (Ramakrishnan et al., 2019). A lot of studies have demonstrated that resistance is also developed due to reduction of toxicity of a compound, the introduction of a new pesticide group, target site mutation or over expression, pre-date or wrong selection of pesticide, repetition of the same chemical, environmental changes, and degradation of parent compounds into their metabolites by insect gut microbiota and their detoxifying enzymes (Naik et al., 2018; Hawkins et al., 2019; Matsuda et al., 2020; Table 1).
Insect digestive systems have a robust defensive system mainly equipped with various microbial species such as bacteria, fungi, archaea, and protozoa (Chen et al., 2021). In a recent study, the isolation of various microbial species in the digestive tract of worker honeybees (Apis mellifera). Results of this study demonstrated that nine species of bacteria from various genera were isolated; five belonged to Snodgrassella alvi, Gilliamella apicola, two species were from Lactobacillus, and one from Bifidobacterium (Douglas, 2018). Various microbial communities allow insects to tolerate or reject toxic compounds through various metabolic processes and develop a peritrophic medium composed of chitin microfibrils and a protein-carbohydrate medium (Kamalakkannan et al., 2017; Rumbos et al., 2018). This peritrophic medium plays a pivotal role in the development of resistance against chemicals due to some prominent features such as releasing digestive enzymes, availability of nutrients and providing protection to epithelial cells from external microbes and toxins through a semipermeable membrane (Puri et al., 2022; Siddiqui et al., 2022). These physiological obstacles between the lumen and epithelium actively contribute to the defense mechanism and minimize the activation of pesticides on the host rather than reducing microbial load in the gut (Chen et al., 2022; Figure 2).
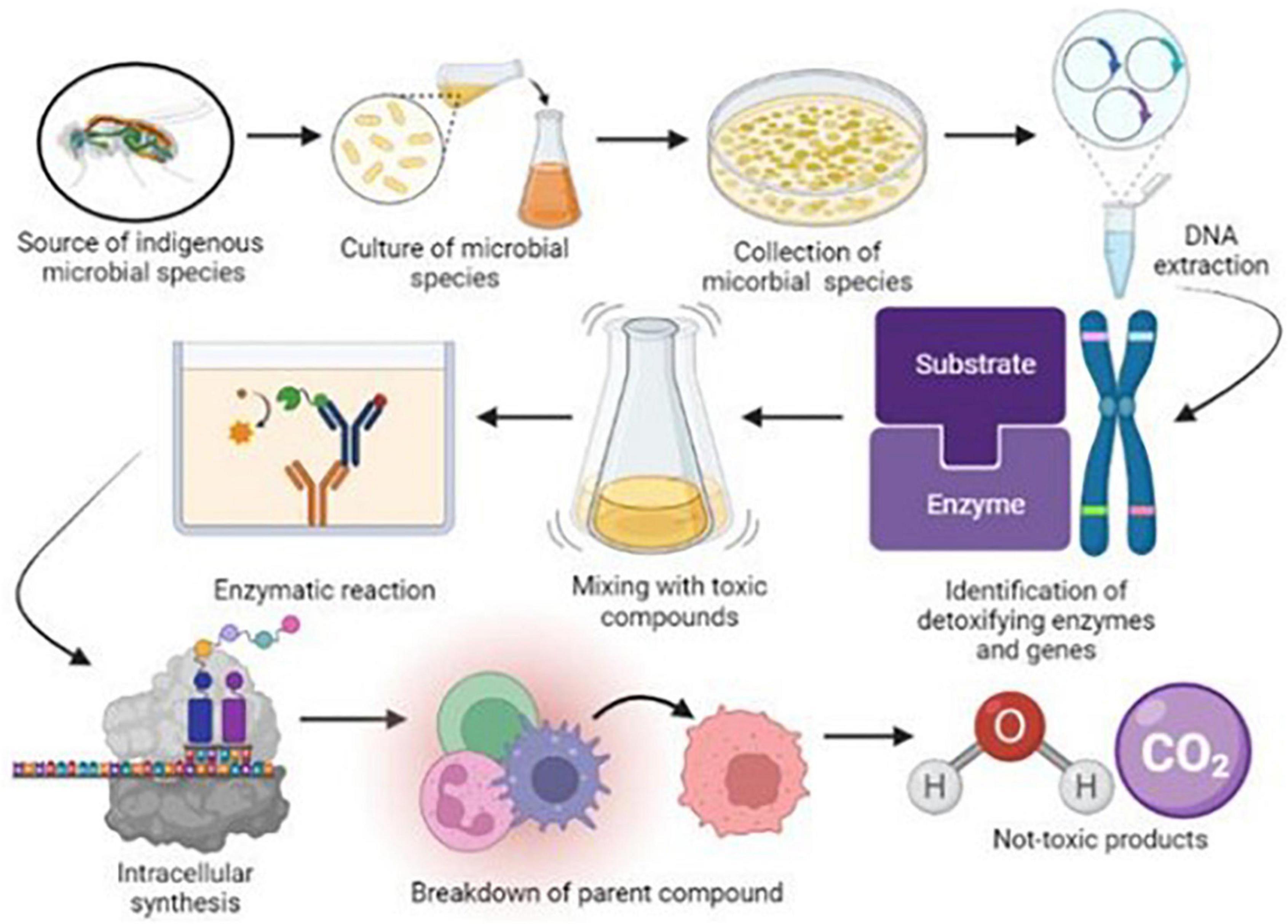
Figure 2. Schematic diagram of isolation of insect gut microbial species and their functions in biodegradation of environmental pollutants.
Recently, a laboratory experiment was conducted to investigate organophosphate pesticide resistance in a serious rice pest, Cletus punctiger by the gut symbiont. Results of this study demonstrated that a rice bug effectively degraded organophosphate fenitrothion by Burkholderia bacterial specie via oral infection and stayed it in the midgut part of the rice bug. The degradation of fenitrothion by the isolating bacterial species from the midgut revealed that gut microbiomes are highly capable of degrading pesticides in insects, and insect gut symbiosis plays a significant role in the development of resistance against fenitrothion in the host rice bug (Ishigami et al., 2021). In another study, the resistance of stored grain products against phosphine fumigation was studied. Four major stored grain pests (Rhyzopertha dominica, Sitophilus granaries, Tribolium castaneum, and Trogoderma granarium) were reared under laboratory conditions for up to seven generations. Results of this study indicated that insect gut symbiosis develops resistance in all pests. Regarding their level of resistance, Rhyzopertha dominica was highly resistant followed by Tribolium castaneum, Trogoderma granarium, and Sitophilus granaries. Although this study concluded that phosphine tablets are excessively applied to manage stored products and are considered very efficient against various stored grain pests, the development of resistance may lead to a serious failure of their applications (Wakil et al., 2021).
An investigation was carried out to study the role of gut microbiota in developing resistance against various insecticides in the laboratory and open field conditions in the larvae of Spodoptera frugiperda. The insect pests were collected from various corn fields in five Brazilian states. In a metagenomic experiment and 16S rRNA analysis, the isolation of bacterial species from insect gut in the selective medium was achieved. The maximum growth of microbial species in insecticides was observed, and it was found that all microbes utilized it as a sole source of carbon and energy. This study indicated that bacteria isolated from field larvae grew better and degraded insecticides more efficiently than those collected from laboratory-selected strains. However, this study concluded that due to the high efficiency and diversity of insect gut microbes in the field, larval insects are more capable of degrading pesticides and showed high resistance (Gomes et al., 2020).
A study was conducted to evaluate the resistance of chlorpyriphos in diamondback moths (Plutella xylostella) by insect gut microbiota. In this investigation, three bacterial species from insect guts such as Enterococcus sp., Enterobacter sp., and Serratia sp. were isolated and examined for their role in detoxifying chlorpyriphos and developing resistance in diamondback moths. Results of this study indicated that Enterococcus sp. increased resistance against the most widely used insecticide, chlorpyriphos. At the same time, Serratia sp. reduced resistance in the diamondback moth and for Enterobacter sp. no effect was observed. In addition, this study explained that Enterococcus sp., vitamin C and acetylsalicylic acid increased the regulation of antimicrobial peptides, which played a crucial role in the development of insecticide resistance (Xia et al., 2018). In another study, Wang et al. (2021) explained that insect gut microbial species play a significant role in insecticide deltamethrin resistance in Aedes albopictus. Additionally, experimental results indicated that by full-length 16S rRNA analysis, two bacterial species were collected from insect guts such as Serratia oryzae and Acinetobacter junii and investigated their growth in six kinds of growth media in biotic and abiotic conditions. Further, they observed that both symbiotic bacteria are mainly facultative in an anaerobic environment. Moreover, this study explained that insect symbiotic bacterial species actively promoted insect resistance against insect pesticides.
To identify the complete profile and total biodiversity of microbial communities in insect gut microbiota and polluted sites, modern molecular biological approaches including clone libraries, probes, reverse sample genome probing, fluorescence in situ hybridization, community profiling or DNA fingerprinting, next-generation sequencing and pyrosequencing provide a more significant explanation as compared to the conventional biological tools (Ahmad et al., 2022b). Various functional parts of an insect’s gut microbes, such as enzymes and genes, are responsible for developing pesticide resistance in insects (Bhatt et al., 2022b). Metagenomic analysis was performed to identify major microbial species in the gut of a honeybee (Apis mellifera) and their functional roles in developing resistance. This study’s results revealed that insect gut microbe gene contents (Gilliamella apicola) are related to various host-dependent symbiotic functions. Moreover, as evidenced by the case of pectin breakdown by G. apicola, genetic variations are related to functional variations. The glycoside hydrolase and polysaccharide lyase enzyme families discovered in the honeybee metagenome are depicted with their respective cleavage sites on the schematic of the pectin molecule (Engel and Moran, 2013). In another study, an investigation of gut microbial species from three diamondback moth larvae was carried out to study prothiofos resistance. Findings from 16S rRNA showed that the bacterial community from the prothiofos-resistant larval gut was more diversified. In addition, the secretion of chitinase enzymes from the population of insect gut bacteria significantly contributed to host antagonism against entomopathogens and nutrition (Indiragandhi et al., 2007). Pesticide-resistance cockroach species such as German cockroaches, American cockroaches, and Oriental cockroaches are rich sources of insect gut microbiota and play a crucial role in insect physiology (Zhang X. C. et al., 2022). For example, the effect of beta-cypermethrin resistance development in cockroaches (Blattella germanica) by the gut microbial population and their genetic association with host growth was investigated. Results of 16S rRNA gene sequencing and metagenomics indicated that Lactobacillus spp. were abundantly present in the foregut and midgut of cockroaches. In addition, carbohydrate-active enzymes actively contribute to developing resistance, insect growth, and fitness (Zhang and Yang, 2019). However, modern molecular biological tools efficiently describe the microbial interaction with the host and external pathogens. Moreover, in the future, these approaches could be applied to using and managing environmental bioprocesses through knowledge-based control.
In many insect species, resistance to pesticides has been confirmed. It has been found that they are very beneficial for degrading toxic compounds due to their digesting abilities (Schmidt and Engel, 2021). The degradation of pesticides depends on various factors such as microbial remediation and the chemical hydrolysis process, which are additionally correlated with many physiological properties such as pH, temperature, organic matter, and moisture content (Bhatt et al., 2022b). However, the insect gut provides a favorable environment for developing diverse microbial communities. Hence, they efficiently deliver many promising facilities to their host (Shan et al., 2021). Symbiotic microbial species isolated from insect gut can perform in extreme environmental conditions to degrade pesticides and other emerging pollutants (Francis and Aneesh, 2022; Figure 3).
Recently, Wang et al. (2022) investigated the degradation of various pesticides by isolating microbial species from stored grain pests and studied the resistance mechanism. In this experiment, from multiple locations, adults of different stored grain pests (Sitophilus oryzae, Cryptolestes ferrugineus, and Rhyzopertha dominica) were collected and isolated as five bacterial species. Results of this study indicated that all screened bacterial species could degrade deltamethrin, malathion, and pirimiphos-methyl efficiently and use their residues as a source of carbon and energy, which are favorable for their growth. Additionally, this study revealed that when bacterial species are treated with 0.5–10 mg/kg of malathion, pirimiphos-methyl, and 0.3–0.75 mg/kg of deltamethrin, gnotobiotic reinoculation and their survival rates in the host are significantly increased, which implies that development of insecticide resistance highly depends on concentration rate. Moreover, this study also explained that the in vitro biodegradation of pesticides through gut bacteria was not entirely consistent with their in vivo operation in host pesticide resistance, which suggested that instead of direct degradation of pesticides, other physiological and morphological processes are also responsible for pesticide tolerance or resistance.
In another study, insects of Orthoptera and Dermaptera were collected from various sites. Fourteen bacterial species were isolated for the biodegradation of deltamethrin from a polluted environment. All the bacterial species were analyzed by 16SRNA and identified as Poecilimon tauricola, Locusta migratoria, Gryllus bimaculatus, Forficula Auricularia, Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Bacillus atrophaeus, Acinetobacter lwoffii, Rhodococcus coprophilus, Brevundimonas vesicularis, Pseudomonas syringae, Yersinia frederiksenii, Bacillus licheniformis, Enterobacter intermedius, and Serratia marcescen. In addition, this study explained that eleven of them were gram-negative bacteria, and three were gram-positive bacteria, potentially deleting deltamethrin up to 100 mg/L. This study concluded that insecticide-tolerated gut microbiota is enriched with nutrients and is considered a powerful tool for the remediation of various kinds of pollutants from the ecosystem (Özdal and Algur, 2020). An indigenous rod-shaped gram-negative bacterium was isolated from the digestive tract of a grasshopper (Poecilimon tauricola) for the potential biodegradation of α-endosulfan and α-cypermethrin. By morphological, physiological, and 16S rRNA sequence analysis, the bacterial strain was characterized as Acinetobacter schindler and named B7. This study demonstrated that when the bacterial strain was treated with 100 mg/L of both pesticides in a glucose-mediated non-sulfur medium, significant growth of the bacterial strain was observed. Additionally, this study showed that within 10 days, the bacterial strain was capable of degrading 67.31 and 68.4% of α-endosulfan and α-cypermethrin, respectively (Ozdal and Algur, 2022).
Organophosphates are an influential group of pesticides that are excessively applied to control insect pest infestations across several agricultural and horticultural crops. The residues of this group are very toxic to the environment and spread many diseases to non-target organisms. Therefore, it is essential to remove their residues from the ecosystem by a potential degradation method. Recently, a study was conducted on the effective biodegradation of organophosphate pesticides, chlorpyriphos and polyethylene, by isolating insect gut microbial species. This study isolated four potential bacterial species: Bacillus licheniformis, Pseudomonas cereus, Pseudomonas putida, and Bacillus subtilis, from the gut of the citrus mealybug (Planococcus citri). Results of this study revealed that all symbiotic bacterial species utilized chlorpyriphos and polyethylene as a sole source of carbon and energy and enhanced their growth and enzymatic activity. Findings of the degradation experiment showed that after the treatment of 45 days, a satisfactory reduction of polyethylene weight was noticed, and scanning electron microscope analysis suggested that a biofilm formation around the polyethylene sheet by bacterial isolates was also observed. While in the case of chlorpyriphos, results indicated that after 21 days, significant degradation was observed in soil and water. In addition, this study revealed that Pseudomonas cereus and Pseudomonas putida have more potential to degrade both pollutants in diverse environmental conditions (Ibrahim et al., 2021).
To degrade clothianidin residues in an open environment, seven bacterial species such as Edwardsiella sp., two Serratia sp., Rahnella sp., Pantoea sp., Hafnia sp., and Enterobacter sp. were isolated from the digestive tract of the honeybee. To examine the growth of all bacterial strains, they were treated with various concentrations of clothianidin as a sole source of carbon and energy. They found that all bacterial strains provide satisfactory growth up to 10 ppb of clothianidin. Results of the degradation experiment showed that within 3 days, all endogenous bacterial strains noticed complete degradation of clothianidin (El Khoury et al., 2022).
For the biodegradation of multi-pesticides such as chlorpyriphos, cypermethrin, malathion, quinalphos, and triazophos, 13 indigenous microbial species were isolated from the gut of the cotton bollworm (Helicoverpa armigera) and tested for their degradation efficiency. After physiochemical, morphological and 16S rRNA sequence analysis, all bacterial strains were identified as Bacillus pumillis CL1, Enterococcus casseliflavus CL2, Bacillus subtilis CL3, Rhodococcus sp. CL4, Pseudomonas sp. CL5, Staphylococcus sp. CL6, Pseudomonas aeruginosa CL7, Proteus vulgaris HL1, Cellulosimicrobium cellulans HL2, Klebsiella oxytoca HL3, Bacillus subtilis HL4, Stenotrophomonas maltophilla HL5, and Pseudomonas sp. HL6. Results of this study indicated that strains CL2 and CL4 provided more rapid growth in the presence of malathion and chlorpyriphos in a mineral salt medium. Gas chromatography and mass spectrometry analysis revealed that strain CL4 has the potential to degrade 44% of chlorpyriphos and strain CL2 was capable to degrade 26% of chlorpyriphos and 57.1% of malathion in mineral salt medium (Madhusudhan et al., 2021). In another study, a symbiotic gut bacterium, Citrobacter sp. (CF-BD), was isolated from the digestive tract of tephritid fruit flies (Bactrocera dorsalis) and investigated their degradation efficiency against trichlorphon insecticide. Results of this study showed that insect gut microbiota plays a vital role in developing resistance against toxin chemicals and efficiently degrade trichlorphon into metabolites. This study also explains that various hydrolase genes were identified in the bacterial isolate CF-BD. In the presence of trichlorphon, the maximum gene expression was observed, and it was found that these critical genes play a crucial role in developing resistance in tephritid fruit flies (Cheng et al., 2017).
In the insect body system, various potential natural enzymes are linked with different biological processes and play a vital role in toxic detoxifying substances in target sites (Lin et al., 2015; Naik et al., 2018; Bhandari et al., 2021). Based on some previous reports, it is found that resistance or tolerance of pesticides is interlinked with these biochemical processes which are held in the insect body and sensitivity of pesticides which are further degraded by metabolic enzymes such as hydrolase, esterase, laccase, acetylcholinesterase, carboxylesterase, glutathione S-transferase, cytochrome P450 and many more (Ismail, 2020; Clark et al., 2021; Yang Y. -X. et al., 2021; Ahmad et al., 2022b; Siddiqui et al., 2022). Insect pests’ resistance against different pesticides by insect gut microbial enzymes was reported (Table 2). In the midgut of the tobacco budworm (Heliothis virescens), many essential enzymes were purified, such as 58 proteinases, four cadherins, 13 aminopeptidases, and five alkaline phosphatases. Other putative detoxification enzymes include 20 cytochrome P450 oxidases, 11 glutathione S-transferases, nine esterase’s, and 15 cytochrome oxidases. These enzymes contributed to insect physiology and reduced the toxicity of pesticides (Zhu et al., 2011).
An investigation was conducted to understand the enzymatic molecular mechanism for biodegradation of chlorpyriphos, glyphosate, phoxim, and esfenvalerate. In this study, 263 bacterial colonies were isolated from the gut of a cricket (Teleogryllus occipitalis), cultured individually, and examined for their degradation efficiency. Based on morphological, physiological, and 16S rRNA analysis and found that 55 bacteria species showed a high resemblance to 28 genera. Among these 55 bacterial species, 18 have the potential to degrade 50%, and six were able to degrade 70% of chlorpyriphos at an initial concentration of 400 mg/L within 1 day of incubation in a mineral salt medium. In addition, purification of extracellular hydrolase enzymes was studied in these isolates and found that free cells and hydrolase enzymes play a crucial role in the degradation of chlorpyriphos, glyphosate, phoxim, and esfenvalerate. A carboxyl esterase enzyme was purified from the mosquito gut bacteria (Escherichia coli) and studied for its effectiveness in degrading malathion. The results of the degradation experiment revealed that these carboxylesterase enzymes could efficiently degrade more than 80% of malathion. This study concluded that due to their rapid degradation ability, superior stability, and high activity, these enzymes could further degrade other organophosphate pesticides that contaminate the environment (Zhang et al., 2004).
In another study, a study was carried out on the biodegradation of organophosphate and pyrethroid pesticides from the contaminated environment to evaluate the resistance mechanism in insect pests (Helicoverpa armigera). In this study, a yeast (Pichia pastoris HaGST-8) was isolated from the insect gut and purified glutathione-S-transferase enzymes to detoxify chlorpyriphos dichlorvos and cypermethrin up to a concentration of 2–15 mg/L. Results of this study revealed that these enzymes have the potential to degrade all organophosphate pesticides completely and cypermethrin partially (53%) in an aqueous solution. Moreover, this study suggested that isolated yeast provides satisfactory growth in all pesticides at higher concentrations (200–400 mg/L) and concluded that these purified enzymes could be further utilized to degrade other pesticide groups such as organophosphates, carbamates, pyrethroids, organochlorines, and organophosphates from food, soil, and water resources (Labade et al., 2018). Two potential enzymes, such as cytochrome P450 monooxygenase and esterase, were purified from an insect gut (Aedes aegypti) bacterium and characterized for their efficiency in degrading propoxur and naled insecticides. Results of this study indicated that both types of enzymes play a crucial role in the degradation of pesticides and are further metabolized into non-toxic substances. This study concluded that insect gut symbiotic bacteria and their associated enzymes reduced the toxicity of pesticides, enhanced resistance, and played an essential role in the digestion of food (Scates et al., 2019).
The potential and purification of three enzymes such as hydrolases, transferases, and oxygenase’s, were purified from the insect (Plutella xylostella) gut bacterium (Bacillus thuringiensis var. kurstaki HD-1) and investigated their role in the development of resistance and degradation of insecticides such as fenvalerate, fipronil, and flufenoxuron. This study demonstrated that all detoxifying enzymes were responsible for developing resistance against the diamondback moth insecticides and metabolizing them into less toxic substances (Mohan and Gujar, 2003). However, it has been proven that a diverse number of prokaryotic and eukaryotic microbial species in the insect gut and their associated enzymes actively contribute to the degradation of various pesticides and metabolize them into less toxic substances. These less toxic substances were further utilized by microbiota as a sole source of carbon, sulfur, and energy and play a key role in insect physiology (Mohammadi et al., 2021).
The high application of pesticides to control various pests has produced long-term hazardous residual pollution in the ecosystem (Shahid et al., 2021). A lot of insect pest species depend on insect gut microbial species to attain nutrients, in defense mechanisms, exploit novel food resources and undergo metabolization of parent compounds into their intermediates (Chen et al., 2020). Various microbial species break down parent compounds into their metabolites, which pose the same or higher toxicity than the parent compound and disturb the environmental equilibrium.
An acetamiprid-degrading bacterium that provides the highest growth on a mineral salt medium was isolated and, based on morphological, physiological, 16S rRNA, and BLAST analysis, identified as Rhodococcus sp. BCH2. This study showed that the acetamiprid was rapidly metabolized into its three metabolites, such as N-amidoamide derivative, 1-(6-chloropyridin-3yl)-N-methylmethanamine, and 6-chloronicotinic acid. Additionally, toxicological effects of the parent compound and their metabolites on silkworm (Bombax mori) concerning genotoxicity, antioxidant enzymes, lipid peroxidation, and protein oxidation were also investigated. This study suggests that the parental molecule has more hazardous effects on insect physiology than its derivatives (Phugare and Jadhav, 2015; Figure 4). In another study, five intestinal bacterial species were screened to understand the chlorpyriphos biodegradation mechanism. After characterization of physiological and morphological properties, bacterial species were identified as Lactobacillus lactis, Lactobacillus fermentum, Lactobacillus plantarum, Escherichia coli, and Enterococcus faecalis. Plate assay findings indicated that three of them (Lactobacillus fermentum, Escherichia coli, and Lactobacillus lactis) could provide maximum growth even using higher dosages (>1400 μg/L), while the other two bacterial species (Lactobacillus plantarum and Enterococcus faecalis) were able to grow using less concentrations (100 and 400 μg/L), respectively. Based on growth parameters, the best three bacterial species were investigated to degrade chlorpyriphos and found that L. fermentum was able to degrade 70% of chlorpyriphos and generate one metabolite named 3,5,6-trichloro-2-pyridinol, L. lactis degraded 61% of chlorpyriphos into chlorpyrifos oxon, and E. coli provided less chlorpyriphos degradation (16%) and breakdown in chlorpyrifos oxon and diethyl phosphate (Figure 5).
Resistance to pesticides in insect pest species is increased via the metabolism pathway and regulation of various genes and enzymes and is considered a significant problem throughout the globe (Kalsi and Palli, 2017). A study was carried out to examine the resistance of imidacloprid through the regulation of genes in vinegar flies (Drosophila melanogaster) and evaluate how imidacloprid metabolites are generated and affect vinegar flies. These questions have been addressed by coupling the genetic tools of gene overexpression and CRISPR gene knock-out with the mass spectrometric technique, the Twin-Ion Method. In this study, the Cyp6g1 gene, responsible for developing resistance against different insecticides, including imidacloprid, was identified. It found that gut microbes living in vinegar flies were responsible for generating oxidative and nitro-reduced metabolites, which were further interconnected with overexpression of the gene Cyp6g1. Additionally, this study revealed that imidacloprid was metabolized into toxic metabolites that were not further degraded into less harmful products and were excreted relatively hardly (Fusetto et al., 2017). In another study, the biodegradation of imidacloprid by the strain Klebsiella pneumoniae BCH1 and the effect of its toxic metabolites on silkworm (Bombyx mori) were studied. The strain was able to degrade 78% of imidacloprid within a week and produced three metabolites: nitrosoguanidine, imidacloprid guanidine, and 6-chloronicotinic acid by using gas chromatography and mass spectrometry. The toxicity of imidacloprid and its metabolites revealed that they enhanced oxidative stress, lipid peroxidation, protein oxidation, DNA damage, and changed the activity of antioxidant enzymatic status (Phugare et al., 2013; Figure 6).
The applications of synthetic pesticides are heavily applied throughout the globe to develop insect pests’ infestation strategies. Their toxic residues are accumulated in the agroecosystem, which causes severe threats to animals, humans, birds, and other non-target organisms. Investigating microbial species and their associated enzymes in insect pests’ digestive tracts is essential in agricultural research. Recent advances in independent culture methods such as next-generation sequencing, BLAST analysis, and 16S rRNA analysis have provided new insights into understanding the extensive range of symbiotic microbial communities and their functions with insects. Symbiotic microbial species are very beneficial for the regulation of insect physiology and contribute to a very significant role, such as in insect fitness, by providing amino acids, vitamins, lactic acids, and sterols, enhanced immunity system, food digestion, excretion of waste fluids, host fertility, increased resistance to toxins and external pathogens; and degradation of pesticides and allelochemicals into less toxic products by the production of different hydrolytic enzymes. The biodegradation of pesticides by isolating indigenous insect symbiotic microbial species and their associated catabolic enzymes, genes, and proteins has become an excellent option to clean up the contaminated environment.
Additionally, insects can swiftly obtain novel metabolic activities and colonize new ecological niches through symbiotic interactions with microbiota that have previously fully developed well-tuned metabolic pathways and converted toxic compounds into derivatives. However, the various characteristics of symbiotic microbial species and their associated enzymes that work mutually with insect guts as a superior biocontrol agent are yet to be ascertained. Several omic approaches to predict hidden microbial communities, database approaches for their identification, and systematic biomolecular tools are urgently required to discover unknown features of insect gut microbial species. In the coming days, the relevant discoveries will immensely provide new myriads to explore the proliferation and diversification of insect gut microbial communities and, on the other hand, develop several industrial applications and environmentally friendly technologies for generating wealth, such as the production of biofuels.
YL conceived the project and contributed to revise the manuscript. SJ prepared the original draft. SA prepared the tables and figures and revised all the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Common Key Technology R&D Innovation Team Project in Modern Agricultural Industry of Guangdong (2022kj134).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdallah, I. S., Abou-Yousef, H. M., Fouad, E. A., and Kandil, E.-H. (2016). The role of detoxifying enzymes in the resistance of the cowpea aphid (Aphis craccivora Koch) to thiamethoxam. J. Plant Prot. Res. 56:1. doi: 10.1515/jppr-2016-0010
Abraham, J., Benhotons, G. S., Krampah, I., Tagba, J., Amissah, C., and Abraham, J. D. (2018). Commercially formulated glyphosate can kill non-target pollinator bees under laboratory conditions. Entomol. Exp. Appl. 166, 695–702. doi: 10.1111/eea.12694
Ahlawat, S., Singh, D., Yadav, A., Singh, A. K., Virdi, J. S., and Sharma, K. K. (2020). Proteomic analysis reveals the damaging role of low redox laccase from Yersinia enterocolitica strain 8081 in the midgut of Helicoverpa armigera. Biotechnol. Lett. 42, 2189–2210. doi: 10.1007/s10529-020-02925-x
Ahmad, S., Ahmad, H. W., and Bhatt, P. (2022a). Microbial adaptation and impact into the pesticide’s degradation. Arch. Microbiol. 204, 1–25. doi: 10.1007/s00203-022-02899-6
Ahmad, S., Bhatt, P., Ahmad, H. W., Cui, D., Guo, J., Zhong, G., et al. (2022b). “Enzymes involved in the bioremediation of pesticides,” in Industrial applications of microbial enzymes, ed. P. Bhatt (Boca Raton, FL: CRC Press), 133–168. doi: 10.1201/9781003202998-7
Ahmad, S., Dongming, C., Zhong, G., and Liu, J. (2021). Microbial technologies employed for biodegradation of neonicotinoids in the agroecosystem. Front. Microbiol. 12:759439. doi: 10.3389/fmicb.2021.759439
Alberoni, D., Favaro, R., Baffoni, L., Angeli, S., and Di Gioia, D. (2021). Neonicotinoids in the agroecosystem: In-field long-term assessment on honeybee colony strength and microbiome. Sci. Total Environ. 762:144116. doi: 10.1016/j.scitotenv.2020.144116
Altun, S., Özdemir, S., and Arslan, H. (2017). Histopathological effects, responses of oxidative stress, inflammation, apoptosis biomarkers and alteration of gene expressions related to apoptosis, oxidative stress, and reproductive system in chlorpyrifos-exposed common carp (Cyprinus carpio L.). Environ. Pollut. 230, 432–443. doi: 10.1016/j.envpol.2017.06.085
Ammar, S., Noui, H., Djamel, S., Madani, S., Maggi, F., Bruno, M., et al. (2020). Essential oils from three Algerian medicinal plants (Artemisia campestris, Pulicaria arabica, and Saccocalyx satureioides) as new botanical insecticides? Environ. Sci. Pollut. Res. 27, 26594–26604. doi: 10.1007/s11356-020-09064-w
Anderson, B. S., Phillips, B. M., Voorhees, J. P., Deng, X., Geraci, J., Worcester, K., et al. (2018). Changing patterns in water toxicity associated with current use pesticides in three California agriculture regions. Integr. Environ. Assess. Manag. 14, 270–281. doi: 10.1002/ieam.2005
Arisekar, U., Shakila, R. J., Jeyasekaran, G., Shalini, R., Kumar, P., Malani, A. H., et al. (2019). Accumulation of organochlorine and pyrethroid pesticide residues in fish, water, and sediments in the Thamirabarani river system of southern peninsular India. Environ. Nanotechnol. Monit. Manag. 11:100194. doi: 10.1016/j.enmm.2018.11.003
Armitage, S. A., Genersch, E., Mcmahon, D. P., Rafaluk-Mohr, C., and Rolff, J. (2022). Tripartite interactions: How immunity, microbiota and pathogens interact and affect pathogen virulence evolution. Curr. Opin. Insect Sci. 50:100871. doi: 10.1016/j.cois.2021.12.011
Arunkumar, N., Banu, J. G., Gopalakrishnan, N., and Prakash, A. H. (2017). Isolation, screening and characterization of microbial surfactants producing wax degrading bacteria from cotton mealybugs, Phenacoccus solenopsis Tinsley and Ferrisia virgata cockerell (Homoptera: Pseudococcidae). Zool. Stud. 5, 1191–1195. doi: 10.1016/jezs.2017.2.1191.1195
Aslanturk, A., Kalender, S., Uzunhisarcikli, M., and Kalender, Y. (2011). Effects of methidathion on antioxidant enzyme activities and malondialdehyde level in midgut tissues of Lymantria dispar (Lepidoptera) larvae. J. Entomol. Res. Soc. 13, 27–38. doi: 10.1016/1302.0250
Barbieri, M. V., Peris, A., Postigo, C., Moya-Garcés, A., Monllor-Alcaraz, L. S., Rambla-Alegre, M., et al. (2021). Evaluation of the occurrence and fate of pesticides in a typical Mediterranean delta ecosystem (Ebro River Delta) and risk assessment for aquatic organisms. Environ. Pollut. 274:115813. doi: 10.1016/j.envpol.2020.115813
Barman, M., Samanta, S., Thakur, H., Chakraborty, S., Samanta, A., Ghosh, A., et al. (2021). Effect of neonicotinoids on bacterial symbionts and insecticide-resistant gene in whitefly, Bemisia tabaci. Insects 12:742. doi: 10.3390/insects12080742
Berasategui, A., Shukla, S., Salem, H., and Kaltenpoth, M. (2016). Potential applications of insect symbionts in biotechnology. Appl. Microbiol. Biotechnol. 100, 1567–1577. doi: 10.1007/s00253-015-7186-9
Bhandari, G., Sharma, M., Negi, S., Gangola, S., Bhatt, P., and Chen, S. (2021). System biology analysis of endosulfan biodegradation in bacteria and its effect in other living systems: Modeling and simulation studies. J. Biomol. Struct. Dyn. 8, 1–13. doi: 10.1080/07391102.2021.1982773
Bhatt, P., Ahmad, S., Joshi, S., and Bhatt, K. (2022b). “Recent advancement in microbial enzymes and their industrial applications,” in Industrial applications of microbial enzymes, ed. P. Bhatt (Boca Raton, FL: CRC Press), 1–17. doi: 10.1201/9781003202998-1
Bhatt, P., Sethi, K., Gangola, S., Bhandari, G., Verma, A., Adnan, M., et al. (2022a). Modeling and simulation of atrazine biodegradation in bacteria and its effect in other living systems. J. Biomol. Struct. Dyn. 40, 3285–3295. doi: 10.1080/07391102.2020.1846623
Bhatt, P., Bhatt, K., Huang, Y., Lin, Z., and Chen, S. (2020). Esterase is a powerful tool for the biodegradation of pyrethroid insecticides. Chemosphere 244:125507. doi: 10.1016/j.chemosphere.2019.125507
Bhatt, P., Negi, G., Gangola, S., Khati, P., Kumar, G., Srivastava, A., et al. (2016). Differential expression and characterization of cypermethrin-degrading potential proteins in Bacillus thuringiensis strain, SG4. 3 Biotech 6:225. doi: 10.1007/s13205-016-0541-4
Bhatt, P., Pal, K., Bhandari, G., and Barh, A. (2019). Modelling of the methyl halide biodegradation in bacteria and its effect on environmental systems. Pestic. Biochem. Physiol. 158, 88–100. doi: 10.1016/j.pestbp.2019.04.015
Bhatt, P., Rene, E. R., Huang, Y., Lin, Z., Pang, S., Zhang, W., et al. (2021a). Systems biology analysis of pyrethroid biodegradation in bacteria and its effect on the cellular environment of pests and humans. J. Environ. Chem. Eng. 9:106582. doi: 10.1016/j.jece.2021.106582
Bhatt, P., Sharma, A., Rene, E. R., Kumar, A. J., Zhang, W., and Chen, S. (2021b). Bioremediation of fipronil using Bacillus sp. FA3: Mechanism, kinetics and resource recovery potential from contaminated environments. J. Water Process. Eng. 39:101712. doi: 10.1016/j.jwpe.2020.101712
Bhatt, P., Zhou, X., Huang, Y., Zhang, W., and Chen, S. (2021c). Characterization of the role of esterases in the biodegradation of organophosphate, carbamate, and pyrethroid pesticides. J. Hazard. Mater. 411:125026. doi: 10.1016/j.jhazmat.2020.125026
Blanton, A. G., and Peterson, B. F. (2020). Symbiont-mediated insecticide detoxification as an emerging problem in insect pests. Front. Microbiol. 11:547108. doi: 10.3389/fmicb.2020.547108
Borrelli, L., Coretti, L., Dipineto, L., Bovera, F., Menna, F., Chiariotti, L., et al. (2017). Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 7, 1–11. doi: 10.1038/s41598-017-16560-6
Bourguignon, T., Lo, N., Dietrich, C., Šobotník, J., Sidek, S., Roisin, Y., et al. (2018). Rampant host switching shaped the termite gut microbiome. Curr. Biol. 28, 649–654.e2. doi: 10.1016/j.cub.2018.01.035
Bruno, D., Bonelli, M., Cadamuro, A. G., Reguzzoni, M., Grimaldi, A., Casartelli, M., et al. (2019). The digestive system of the adult Hermetia illucens (Diptera: Stratiomyidae): Morphological features and functional properties. Cell Tissue Res. 378, 221–238. doi: 10.1007/s00441-019-03025-7
Cai, H., Yang, L., Zuo, Z., Liao, W., and Yang, Z. (2021). Resistance status of Myzus persicae to pesticide and its relationship with enzymes. Agron. J. 113, 806–819. doi: 10.1002/agj2.20490
Cariño, F., Koener, J., Plapp, F. Jr., and Feyereisen, R. (1994). Constitutive overexpression of the cytochrome P450 gene CYP6A1 in a house fly strain with metabolic resistance to insecticides. Insect Biochem. Mol. Biol. 24, 411–418. doi: 10.1016/0965-1748(94)90034-5
Carles, C., Bouvier, G., Lebailly, P., and Baldi, I. (2017). Use of job-exposure matrices to estimate occupational exposure to pesticides: A review. J. Expo. Sci. Environ. Epidemiol. 27, 125–140. doi: 10.1038/jes.2016.25
Cassidy, R. A., Natarajan, S., and Vaughan, G. M. (2005). The link between the insecticide heptachlor epoxide, estradiol, and breast cancer. Breast Cancer Res. Treat. 90, 55–64. doi: 10.1007/s10549-004-2755-0
Chen, B., Xie, S., Zhang, X., Zhang, N., Feng, H., Sun, C., et al. (2020). Gut microbiota metabolic potential correlates with body size between mulberry-feeding lepidopteran pest species. Pest Manag. Sci. 76, 1313–1323. doi: 10.1002/ps.5642
Chen, H., Singh, H., Bhardwaj, N., Bhardwaj, S. K., Khatri, M., Kim, K.-H., et al. (2022). An exploration on the toxicity mechanisms of phytotoxins and their potential utilities. Crit. Rev. Environ. Sci. Technol. 52, 395–435. doi: 10.1080/10643389.2020.1823172
Chen, M., Xu, P., Zeng, G., Yang, C., Huang, D., and Zhang, J. (2015). Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 33, 745–755. doi: 10.1016/j.biotechadv.2015.05.003
Chen, X. D., Neupane, S., Gossett, H., Pelz-Stelinski, K. S., and Stelinski, L. L. (2021). Insecticide rotation scheme restores insecticide susceptibility in thiamethoxam-resistant field populations of Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae), in Florida. Pest Manag. Sci. 77, 464–473. doi: 10.1002/ps.6039
Cheng, D., Guo, Z., Riegler, M., Xi, Z., Liang, G., and Xu, Y. (2017). Gut symbiont enhances insecticide resistance in a significant pest, the oriental fruit fly Bactrocera dorsalis (Hendel). Microbiome 5, 1–12. doi: 10.1186/s40168-017-0236-z
Chmiel, J. A., Daisley, B. A., Burton, J. P., and Reid, G. (2019). Deleterious effects of neonicotinoid pesticides on Drosophila melanogaster immune pathways. mBio 10:e01395-19. doi: 10.1128/mBio.01395-19
Chrustek, A., Hołyńska-Iwan, I., Dziembowska, I., Bogusiewicz, J., Wróblewski, M., Cwynar, A., et al. (2018). Current research on the safety of pyrethroids used as insecticides. Medicina 54:61. doi: 10.3390/medicina54040061
Clark, M., Tepper, K., Petroll, K., Kumar, S., Sunna, A., and Maselko, M. (2021). Bioremediation of industrial pollutants by insects expressing a fungal laccase. ACS Synth. Biol. 11, 308–316. doi: 10.1021/acssynbio.1c00427
Cuesta-Maté, A., Renelies-Hamilton, J., Kryger, P., Jensen, A. B., Sinotte, V. M., and Poulsen, M. (2021). Resistance and vulnerability of honeybee (Apis mellifera) gut bacteria to commonly used pesticides. Front. Microbiol. 12:717990. doi: 10.3389/fmicb.2021.717990
Dada, N., Lol, J. C., Benedict, A. C., López, F., Sheth, M., Dzuris, N., et al. (2019). Pyrethroid exposure alters internal and cuticle surface bacterial communities in Anopheles albimanus. ISME J. 13, 2447–2464. doi: 10.1038/s41396-019-0445-5
Dai, Y.-J., Ji, W.-W., Chen, T., Zhang, W.-J., Liu, Z.-H., Ge, F., et al. (2010). Metabolism of the neonicotinoid insecticides acetamiprid and thiacloprid by the yeast Rhodotorula mucilaginosa strain IM-2. J. Agric. Food Chem. 58, 2419–2425. doi: 10.1021/jf903787s
Daisley, B. A., Trinder, M., Mcdowell, T. W., Collins, S. L., Sumarah, M. W., and Reid, G. (2018). Microbiota-mediated modulation of organophosphate insecticide toxicity by species-dependent interactions with Lactobacilli in a Drosophila melanogaster insect model. Appl. Environ. Microbiol. 84:e02820-17. doi: 10.1128/AEM.02820-17
Damalas, C. A., and Koutroubas, S. D. (2018). Farmers’ behaviour in pesticide use: A key concept for improving environmental safety. Curr. Opin. Environ. Sci. Health. 4, 27–30. doi: 10.1016/j.coesh.2018.07.001
David, J. P., Faucon, F. Chandor-Proust, A., Poupardin, R., Riaz, M. A., and Bonin, A., et al., (2014). Comparative analysis of response to selection with three insecticides in the dengue mosquito Aedes aegypti using mRNA sequencing. BMC Genom. 15:174. doi: 10.1186/1471-2164-15-174
DeGrandi-Hoffman, G., Corby-Harris, V., Dejong, E. W., Chambers, M., and Hidalgo, G. (2017). Honey bee gut microbial communities are robust to the fungicide Pristine® consumed in pollen. Apidologie 48, 340–352. doi: 10.1007/s13592-016-0478-y
Deknock, A., De Troyer, N., Houbraken, M., Dominguez-Granda, L., Nolivos, I., Van Echelpoel, W., et al. (2019). Distribution of agricultural pesticides in the freshwater environment of the Guayas river basin (Ecuador). Sci. Total Environ. 646, 996–1008. doi: 10.1016/j.scitotenv.2018.07.185
Dias, L. D. A., Gebler, L., Niemeyer, J. C., and Itako, A. T. (2020). Destination of pesticide residues on biobeds: State of the art and future perspectives in Latin America. Chemosphere 248:126038. doi: 10.1016/j.chemosphere.2020.126038
Dickel, F., Münch, D., Amdam, G. V., Mappes, J., and Freitak, D. (2018). Increased survival of honeybees in the laboratory after simultaneous exposure to low doses of pesticides and bacteria. PLoS One 13:e0191256. doi: 10.1371/journal.pone.0191256
Dillon, R. J., Webster, G., Weightman, A. J., and Keith Charnley, A. (2010). Diversity of gut microbiota increases with aging and starvation in the desert locust. Antonie Van Leeuwenhoek 97, 69–77. doi: 10.1007/s10482-009-9389-5
Douglas, A. E. (2018). Omics and the metabolic function of insect–microbial symbioses. Curr. Opin. Insect. Sci. 29, 1–6. doi: 10.1016/j.cois.2018.05.012
Edi, C. V., Djogbenou, L., Jenkins, A. M., Regna, K., Muskavitch, M. A., Poupardin, R., et al. (2014). CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 10:e1004236. doi: 10.1371/journal.pgen.1004236
El Khoury, S., Giovenazzo, P., and Derome, N. (2022). Endogenous honeybee gut microbiota metabolize the pesticide clothianidin. Microorganisms 10:493. doi: 10.3390/microorganisms10030493
Engel, P., and Moran, N. A. (2013). Functional and evolutionary insights into the simple yet specific gut microbiota of the honeybee from metagenomic analysis. Gut Microbes 4, 60–65. doi: 10.4161/gmic.22517
Erb, M., and Kliebenstein, D. J. (2020). Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 184, 39–52. doi: 10.1104/pp.20.00433
Erlandson, M. A., Toprak, U., and Hegedus, D. D. (2019). Role of the peritrophic matrix in insect-pathogen interactions. J. Insect Physiol. 117:103894. doi: 10.1016/j.jinsphys.2019.103894
Eski, A., Demir, I., Güllü, M., and Demirbağ, Z. (2018). Biodiversity and pathogenicity of bacteria associated with the gut microbiota of beet armyworm, Spodoptera exigua Hübner (Lepidoptera: Noctuidae). Microb. Pathog. 121, 350–358. doi: 10.1016/j.micpath.2018.05.012
Faucon, F., Dusfour, I., Gaude, T., Navratil, V., Boyer, F., Chandre, F., et al. (2015). Identifying genomic changes associated with insecticide resistance in the dengue mosquito Aedes aegypti by deep targeted sequencing. Genome Res. 25, 1347–1359. doi: 10.1101/gr.189225.115
Fernández, M. D. M., Meeus, I., Billiet, A., Van Nieuwerburgh, F., Deforce, D., Vandamme, P., et al. (2019). Influence of microbiota in the susceptibility of parasitic wasps to abamectin insecticide: Deep sequencing, esterase and toxicity tests. Pest Manag. Sci. 75, 79–86. doi: 10.1002/ps.5195
Ferrando, L., and Matamoros, V. (2020). Attenuation of nitrates, antibiotics and pesticides from groundwater using immobilised microalgaebased systems. Sci. Total Environ. 703:134740. doi: 10.1016/j.scitotenv.2019.134740
Fine, J. D., Cox-Foster, D. L., and Mullin, C. A. (2017). An inert pesticide adjuvant synergizes viral pathogenicity and mortality in honey bee larvae. Sci. Rep. 7, 1–9. doi: 10.1038/srep40499
Flores, A. E., Grajales, J. S., Salas, I. F., Garcia, G. P., Becerra, M. H. L., Lozano, S., et al. (2006). Mechanisms of insecticide resistance in field populations of Aedes aegypti (L.) from Quintana Roo, Southern Mexico. J. Am. Mosq. Control Assoc. 22, 672–677. doi: 10.2987/8756-971X(2006)22[672:MOIRIF]2.0.CO;2
Francis, C., and Aneesh, E. M. (2022). Gut bacterium induced pesticide resistance in insects with special emphasis to mosquitoes. Int. J. Trop. Insect Sci. 42, 2051–2064. doi: 10.1007/s42690-022-00761-2
Freire, C., and Koifman, S. (2012). Pesticide exposure and Parkinson’s disease: Epidemiological evidence of association. Neurotoxicology 33, 947–971. doi: 10.1016/j.neuro.2012.05.011
Fusetto, R., Denecke, S., Perry, T., O’hair, R. A., and Batterham, P. (2017). Partitioning the roles of CYP6G1 and gut microbes in the metabolism of the insecticide imidacloprid in Drosophila melanogaster. Sci. Rep. 7, 1–12. doi: 10.1038/s41598-017-09800-2
Fuxa, J., and Richter, A. (1990). Response of nuclear polyhedrosis virus-resistant Spodoptera frugiperda larvae to other pathogens and to chemical insecticides. J. Invertebr. Pathol. 55, 272–277. doi: 10.1016/0022-2011(90)90063-C
Gangemi, S., Miozzi, E., Teodoro, M., Briguglio, G., De Luca, A., Alibrando, C., et al. (2016). Occupational exposure to pesticides as a possible risk factor for the development of chronic diseases in humans. Mol. Med. Rep. 14, 4475–4488. doi: 10.3892/mmr.2016.5817
Gangola, S., Sharma, A., Bhatt, P., Khati, P., and Chaudhary, P. (2018). Presence of esterase and laccase in Bacillus subtilis facilitates biodegradation and detoxification of cypermethrin. Sci. Rep. 8, 1–11. doi: 10.1038/s41598-018-31082-5
Giambò, F., Teodoro, M., Costa, C., and Fenga, C. (2021). Toxicology and microbiota: How do pesticides influence gut microbiota? A review. Int. J. Environ. Res. Public Health 18:5510. doi: 10.3390/ijerph18115510
Giglio, A., Vommaro, M. L., Gionechetti, F., and Pallavicini, A. (2021). Gut microbial community response to herbicide exposure in a ground beetle. J. Appl. Entomol. 145, 986–1000. doi: 10.1111/jen.12919
Goergen, G., Kumar, P. L., Sankung, S. B., Togola, A., and Tamò, M. (2016). First report of outbreaks of the fall armyworm Spodoptera frugiperda (JE Smith)(Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS One 11:e0165632. doi: 10.1371/journal.pone.0165632
Gomes, A. F. F., Omoto, C., and Cônsoli, F. L. (2020). Gut bacteria of field-collected larvae of Spodoptera frugiperda undergo selection and are more diverse and active in metabolizing multiple insecticides than laboratory-selected resistant strains. J. Pest Sci. 93, 833–851. doi: 10.1007/s10340-020-01202-0
Gómez-Gallego, C., Rainio, M. J., Collado, M. C., Mantziari, A., Salminen, S., Saikkonen, K., et al. (2020). Glyphosate-based herbicide affects the composition of microbes associated with Colorado potato beetle (Leptinotarsa decemlineata). FEMS Microbiol. Lett. 367:fnaa050. doi: 10.1093/femsle/fnaa050
Gonzalez-Morales, M. A., and Romero, A. (2019). Effect of synergists on deltamethrin resistance in the common bed bug (Hemiptera: Cimicidae). J. Econ. Entomol. 112, 786–791. doi: 10.1093/jee/toy376
Gowda, G. B., Patil, N., Sahu, M., Prabhukarthikeyan, S., Raghu, S., Pandi, G., et al. (2021). Differential gut bacteria in phosphine resistant and susceptible population of Tribolium castaneum (Herbst) and their biochemical and molecular characterization. Pak. J. Zool. 54:3. doi: 10.17582/JOURNAL.PJZ/20201204111217
Granada, Y., Mejía-Jaramillo, A. M., Zuluaga, S., and Triana-Chávez, O. (2021). Molecular surveillance of resistance to pyrethroids insecticides in Colombian Aedes aegypti populations. PLoS Negl. Trop. Dis. 15:e0010001. doi: 10.1371/journal.pntd.0010001
Gressel, J. (2018). Microbiome facilitated pest resistance: Potential problems and uses. Pest Manag. Sci. 74, 511–515. doi: 10.1002/ps.4777
Grewal, A. (2017). Pesticide res-idues in food grains, vegetables and fruits: A hazard to human health. J. Med. Chem. Toxicol. 2, 1–7. doi: 10.15436/2575-808X.17.1355
Guo, Y., Zhang, J., Yu, R., Zhu, K. Y., Guo, Y., and Ma, E. (2012). Identification of two new cytochrome P450 genes and RNA interference to evaluate their roles in detoxification of commonly used insecticides in Locusta migratoria. Chemosphere 87, 709–717. doi: 10.1016/j.chemosphere.2011.12.061
Guo, Z., Lu, Y., Yang, F., Zeng, L., Liang, G., and Xu, Y. (2017). Transmission modes of a pesticide-degrading symbiont of the oriental fruit fly Bactrocera dorsalis (Hendel). Appl. Microbiol. Biotechnol. 101, 8543–8556. doi: 10.1007/s00253-017-8551-7
Ozdal, O. G., and Algur, O. F. (2022). Biodegradation α-endosulfan and α-cypermethrin by Acinetobacter schindleri B7 isolated from the microflora of grasshopper (Poecilimon tauricola). Arch. Microbiol. 204, 1–8. doi: 10.1007/s00203-022-02765-5
Gurr, G. M., Sun, B., Xia, X., Vasseur, L., Xue, M., and You, M. (2018). Gut microbiota mediate insecticide resistance in the diamondback moth, Plutella xylostella (L.). Front. Microbiol. 9:25. doi: 10.3389/fmicb.2018.00025
Han, W., Tian, Y., and Shen, X. (2018). Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere 192, 59–65. doi: 10.1016/j.chemosphere.2017.10.149
Hao, X., Zhang, X., Duan, B., Huo, S., Lin, W., Xia, X., et al. (2018). Screening and genome sequencing of deltamethrin-degrading bacterium ZJ6. Curr. Microbiol. 75, 1468–1476. doi: 10.1007/s00284-018-1546-5
Harishankar, M., Sasikala, C., and Ramya, M. (2013). Efficiency of the intestinal bacteria in the degradation of the toxic pesticide, chlorpyrifos. 3 Biotech 3, 137–142. doi: 10.1007/s13205-012-0078-0
Hasan, R., Prodhan, M., Rahman, S., Khanom, R., and Ullah, A. (2017). Determination of organophosphorus insecticide residues in country bean collected from different markets of Dhaka. J. Environ. Anal. Toxicol. 7, 2161–2525. doi: 10.4172/2161-0525.1000489
Hauffe, H. C., and Barelli, C. (2019). Conserve the germs: The gut microbiota and adaptive potential. Conserv. Genet. 20, 19–27. doi: 10.1007/s10592-019-01150-y
Hawkins, N. J., Bass, C., Dixon, A., and Neve, P. (2019). The evolutionary origins of pesticide resistance. Biol. Rev. 94, 135–155. doi: 10.1111/brv.12440
He, L., Liu, B., Tian, J., Lu, F., Li, X., and Tian, Y. (2018). Culturable epiphytic bacteria isolated from Teleogryllus occipitalus crickets metabolize insecticides. Arch. Insect Biochem. Physiol. 99:e21501. doi: 10.1002/arch.21501
Heys, C., Lizé, A., Blow, F., White, L., Darby, A., and Lewis, Z. J. (2018). The effect of gut microbiota elimination in Drosophila melanogaster: A how-to guide for host–microbiota studies. Ecol. Evol. 8, 4150–4161. doi: 10.1002/ece3.3991
Hongoh, Y. (2010). Diversity and genomes of uncultured microbial symbionts in the termite gut. Biosci. Biotechnol. Biochem. 74, 1145–1151. doi: 10.1271/bbb.100094
Hou, J., Yu, J., Qin, Z., Liu, X., Zhao, X., Hu, X., et al. (2021). Guadipyr, a new insecticide, induces microbiota dysbiosis and immune disorders in the midgut of silkworms (Bombyx mori). Environ. Pollut. 286:117531. doi: 10.1016/j.envpol.2021.117531
Hsu, J.-C., Haymer, D. S., Wu, W.-J., and Feng, H.-T. (2006). Mutations in the acetylcholinesterase gene of Bactrocera dorsalis associated with resistance to organophosphorus insecticides. Insect Biochem. Mol. Biol. 36, 396–402. doi: 10.1016/j.ibmb.2006.02.002
Huang, W., He, Y., Xiao, J., Huang, Y., Li, A., He, M., et al. (2019). Risk of breast cancer and adipose tissue concentrations of polychlorinated biphenyls and organochlorine pesticides: A hospital-based case-control study in Chinese women. Environ. Sci. Pollut. Res. 26, 32128–32136. doi: 10.1007/s11356-019-06404-3
Huang, Y., and Chen, S. (2022). “Pyrethroid-degrading microorganisms and their potential application for the bioremediation of contaminated environments,” in Enzymes for pollutant degradation, microorganisms for sustainability, Vol. 30, eds S. I. Mulla and R. N. Bharagava (Singapore: Springer), 119–137. doi: 10.1007/978-981-16-4574-7_6
Ibrahim, S., Gupta, R. K., War, A. R., Hussain, B., Kumar, A., Sofi, T., et al. (2021). Degradation of chlorpyriphos and polyethylene by endosymbiotic bacteria from citrus mealybug. Saudi J. Biol. Sci. 28, 3214–3224. doi: 10.1016/j.sjbs.2021.03.058
Ihara, M., Buckingham, S. D., Matsuda, K., and Sattelle, D. B. (2017). Modes of action, resistance and toxicity of insecticides targeting nicotinic acetylcholine receptors. Curr. Med. Chem. 24, 2925–2934. doi: 10.2174/0929867324666170206142019
Indiragandhi, P., Anandham, R., Madhaiyan, M., Poonguzhali, S., Kim, G., Saravanan, V., et al. (2007). Cultivable bacteria associated with larval gut of prothiofos-resistant, prothiofos-susceptible and field-caught populations of diamondback moth, Plutella xylostella and their potential for, antagonism towards entomopathogenic fungi and host insect nutrition. J. Appl. Microbiol. 103, 2664–2675. doi: 10.1111/j.1365-2672.2007.03506.x
Ishigami, K., Jang, S., Itoh, H., and Kikuchi, Y. (2021). Insecticide resistance governed by gut symbiosis in a rice pest, Cletus punctiger, under laboratory conditions. Biol. Lett. 17:20200780. doi: 10.1098/rsbl.2020.0780
Ismail, S. M. (2020). Effect of sublethal doses of some insecticides and their role on detoxication enzymes and protein-content of Spodoptera littoralis (Boisd.)(Lepidoptera: Noctuidae). Bull. Natl. Res. Cent. 44, 1–6. doi: 10.1186/s42269-020-00294-z
Itoh, H., Hori, T., Sato, Y., Nagayama, A., Tago, K., Hayatsu, M., et al. (2018). Infection dynamics of insecticide-degrading symbionts from soil to insects in response to insecticide spraying. ISME J. 12, 909–920. doi: 10.1038/s41396-017-0021-9
Jaacks, L. M., Yadav, S., Panuwet, P., Kumar, S., Rajacharya, G. H., Johnson, C., et al. (2019). Metabolite of the pesticide DDT and incident type 2 diabetes in urban India. Environ. Int. 133:105089. doi: 10.1016/j.envint.2019.105089
Jackson, C. J., Liu, J.-W., Carr, P. D., Younus, F., Coppin, C., Meirelles, T., et al. (2013). Structure and function of an insect α-carboxylesterase (αEsterase7) associated with insecticide resistance. Proc. Natl. Acad. Sci. U.S.A. 110, 10177–10182. doi: 10.1073/pnas.1304097110
Jang, S., and Kikuchi, Y. (2020). Impact of the insect gut microbiota on ecology, evolution, and industry. Curr. Opin. Insect. Sci. 41, 33–39. doi: 10.1016/j.cois.2020.06.004
Jarrell, Z. R., Ahammad, M. U., and Benson, A. P. (2020). Glyphosate-based herbicide formulations and reproductive toxicity in animals. Vet. Anim. Sci. 10:100126. doi: 10.1016/j.vas.2020.100126
Jing, T.-Z., Qi, F.-H., and Wang, Z.-Y. (2020). Most dominant roles of insect gut bacteria: Digestion, detoxification, or essential nutrient provision? Microbiome 8, 1–20. doi: 10.1186/s40168-020-00823-y
Joshi, N. K., Leslie, T., Rajotte, E. G., and Biddinger, D. J. (2020). Environmental impacts of reduced-risk and conventional pesticide programs differ in commercial apple orchards, but similarly influence pollinator community. Chemosphere 240:124926. doi: 10.1016/j.chemosphere.2019.124926
Joußen, N., Agnolet, S., Lorenz, S., Schöne, S. E., Ellinger, R., Schneider, B., et al. (2012). Resistance of Australian Helicoverpa armigera to fenvalerate is due to the chimeric P450 enzyme CYP337B3. Proc. Natl. Acad. Sci. U.S.A. 109, 15206–15211. doi: 10.1073/pnas.1202047109
Kadri, T., Magdouli, S., Rouissi, T., and Brar, S. K. (2018). Ex-situ biodegradation of petroleum hydrocarbons using Alcanivorax borkumensis enzymes. Biochem. Eng. J. 132, 279–287. doi: 10.1016/j.bej.2018.01.014
Kalsi, M., and Palli, S. R. (2017). Cap n collar transcription factor regulates multiple genes coding for proteins involved in insecticide detoxification in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 90, 43–52. doi: 10.1016/j.ibmb.2017.09.009
Kamalakkannan, V., Salim, A. A., and Capon, R. J. (2017). Microbiome-mediated biotransformation of cane toad bufagenins. J. Nat. Prod. 80, 2012–2017. doi: 10.1021/acs.jnatprod.7b00134
Karthi, S., Panneerselvam, B., Senthil-Nathan, S., Vasantha-Srinivasan, P., Shivakumar, M. S., and Krutmuang, P. (2020). Functional identification and characterization of midgut microbial flora derived from lepidopteran larvae Spodoptera litura Fab. Biocatal. Agric. Biotechnol. 28:101758. doi: 10.1016/j.bcab.2020.101758
Khalid, M. Z., Ahmad, S., Ngegba, P. M., and Zhong, G. (2021). Role of Endocrine system in the regulation of female insect reproduction. Biology 10:614. doi: 10.3390/biology10070614
Kim, K.-H., Kabir, E., and Jahan, S. A. (2017). Exposure to pesticides and the associated human health effects. Sci. Total Environ. 575, 525–535. doi: 10.1016/j.scitotenv.2016.09.009
Kontsedalov, S., Zchori-Fein, E., Chiel, E., Gottlieb, Y., Inbar, M., and Ghanim, M. (2008). The presence of Rickettsia is associated with increased susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides. Pest Manag. Sci. 64, 789–792. doi: 10.1002/ps.1595
Kumar, N., Kulsoom, M., Shukla, V., Kumar, D., Kumar, S., Tiwari, J., et al. (2018). Profiling of heavy metal and pesticide residues in medicinal plants. Environ. Sci. Pollut. Res. 25, 29505–29510. doi: 10.1007/s11356-018-2993-z
Labade, C. P., Jadhav, A. R., Ahire, M., Zinjarde, S. S., and Tamhane, V. A. (2018). Role of induced glutathione-S-transferase from Helicoverpa armigera (Lepidoptera: Noctuidae) HaGST-8 in detoxification of pesticides. Ecotoxicol. Environ. Saf. 147, 612–621. doi: 10.1016/j.ecoenv.2017.09.028
Lee, G.-H., and Choi, K.-C. (2020). Adverse effects of pesticides on the functions of immune system. Comp. Biochem. Physiol. Toxicol. Pharmacol. 235:108789. doi: 10.1016/j.cbpc.2020.108789
Lee, J. H., Lee, H. Y., Cho, D. Y., Kim, M. J., Jung, J. G., Jeong, E. H., et al. (2021). Biodegradable properties of organophosphorus insecticides by the potential probiotic Lactobacillus plantarum WCP931 with a degrading gene (opdC). Appl. Biol. Chem. 64, 1–12. doi: 10.1186/s13765-021-00632-3
Lewis, K. A., Tzilivakis, J., Warner, D. J., and Green, A. (2016). An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Inter. J. 22, 1050–1064. doi: 10.1080/10807039.2015.1133242
Li, F., Li, M., Mao, T., Wang, H., Chen, J., Lu, Z., et al. (2020). Effects of phoxim exposure on gut microbial composition in the silkworm, Bombyx mori. Ecotoxicol. Environ. Saf. 189:110011. doi: 10.1016/j.ecoenv.2019.110011
Li, R.-X., Li, M.-M., Wang, T., Wang, T.-L., Chen, J.-Y., Francis, F., et al. (2020). Screening of pesticide residues in traditional Chinese medicines using modified QuEChERS sample preparation procedure and LC-MS/MS analysis. J. Chromatogr. B. 1152:122224. doi: 10.1016/j.jchromb.2020.122224
Li, W., Jin, D., Shi, C., and Li, F. (2017). Midgut bacteria in deltamethrin-resistant, deltamethrin-susceptible, and field-caught populations of Plutella xylostella, and phenomics of the predominant midgut bacterium Enterococcus mundtii. Sci. Rep. 7, 1–13. doi: 10.1038/s41598-017-02138-9
Li, Y., Liu, X., and Guo, H. (2018). Variations in endosymbiont infection between buprofezin-resistant and susceptible strains of Laodelphax striatellus (Fallén). Curr. Microbiol. 75, 709–715. doi: 10.1007/s00284-018-1436-x
Liao, X., Mao, K., Ali, E., Zhang, X., Wan, H., and Li, J. (2017). Temporal variability and resistance correlation of sulfoxaflor susceptibility among Chinese populations of the brown planthopper Nilaparvata lugens (Stål). Crop Prot. 102, 141–146. doi: 10.1016/j.cropro.2017.08.024
Lin, H., Chuan-Hua, X., Jin-Jun, W., Ming, L., Wen-Cai, L., and Zhi-Mo, Z. (2009). Resistance selection and biochemical mechanism of resistance to two Acaricides in Tetranychus cinnabarinus (Boiduval). Pestic. Biochem. Physiol. 93, 47–52. doi: 10.1016/j.pestbp.2008.11.001
Lin, T., Cai, Z., and Wu, H. (2015). Transcriptome analysis of the Japanese pine sawyer beetle, Monochamus alternatus (Coleoptera: Cerambycidae) by high-throughput Illumina sequencing. J. Asia Pac. Entomol. 18, 439–445. doi: 10.1016/j.aspen.2015.04.011
Lu, Q., Li, G., Lan, H., Yu, D., Yin, X., Yang, W., et al. (2022). Effects of exposure to trace pyriproxyfen on the intestinal bacterial diversity and immune signal pathways of silkworm (Bombyx mori) larvae. J. Asia Pac. Entomol. 25:101895. doi: 10.1016/j.aspen.2022.101895
Lumjuan, N., Rajatileka, S., Changsom, D., Wicheer, J., Leelapat, P., Prapanthadara, L.-A., et al. (2011). The role of the Aedes aegypti Epsilon glutathione transferases in conferring resistance to DDT and pyrethroid insecticides. Insect Biochem. Mol. Biol. 41, 203–209. doi: 10.1016/j.ibmb.2010.12.005
Luo, L., Dong, L., Huang, Q., Ma, S., Fantke, P., Li, J., et al. (2021). Detection and risk assessments of multi-pesticides in 1771 cultivated herbal medicines by LC/MS-MS and GC/MS-MS. Chemosphere 262:127477. doi: 10.1016/j.chemosphere.2020.127477
Madhusudhan, S., Jalali, S., and Sibi, G. (2021). Molecular identification of insecticide degradation by gut bacteria isolated from Helicoverpa armigera of Cotton plants. J. Appl. Nat. Sci. 13, 641–653. doi: 10.31018/jans.v13i2.2678
Malathi, V. M., Jalali, S. K., Gowda, D. K. S., Mohan, M., and Venkatesan, T. (2017). Establishing the role of detoxifying enzymes in field-evolved resistance to various insecticides in the brown planthopper (Nilaparvata lugens) in South India. Insect Sci. 24, 35–46. doi: 10.1111/1744-7917.12254
Mani, A. M., and Al Araji, R. R. (2022). Determination of pyrothyroid (Deltamethrin) pesticides and ivermectine residues in local sheep milk and mutton by high performance liquid chromatography (HPLC). J. Vet. Med. Anim. Sci. 5:1106.
Masud, M., Mourshed, M., Arefin, A. M. E., Islam, M. T., and Akhter, M. S. (2018). Inhalation risk of indoor aerosol spray products commercially available in Bangladesh. Int. J. Environ. Stud. 75, 732–739. doi: 10.1080/00207233.2018.1440104
Matsuda, K., Ihara, M., and Sattelle, D. B. (2020). Neonicotinoid insecticides: Molecular targets, resistance, and toxicity. Annu. Rev. Pharmacol. Toxicol. 60, 241–255. doi: 10.1146/annurev-pharmtox-010818-021747
Meftaul, I. M., Venkateswarlu, K., Dharmarajan, R., Annamalai, P., and Megharaj, M. (2020). Pesticides in the urban environment: A potential threat that knocks at the door. Sci. Total Environ. 711:134612. doi: 10.1016/j.scitotenv.2019.134612
Meng, G., Nie, Z., Feng, Y., Wu, X., Yin, Y., and Wang, Y. (2016). Typical halogenated persistent organic pollutants in indoor dust and the associations with childhood asthma in Shanghai, China. Environ. Pollut. 211, 389–398. doi: 10.1016/j.envpol.2015.12.006
Mishra, S., Pang, S., Zhang, W., Lin, Z., Bhatt, P., and Chen, S. (2021). Insights into the microbial degradation and biochemical mechanisms of carbamates. Chemosphere 279:130500. doi: 10.1016/j.chemosphere.2021.130500
Mitchell, E. A., Mulhauser, B., Mulot, M., Mutabazi, A., Glauser, G., and Aebi, A. (2017). A worldwide survey of neonicotinoids in honey. Science 358, 109–111. doi: 10.1126/science.aan3684
Mogren, C. L., and Shikano, I. (2021). Microbiota, pathogens, and parasites as mediators of tritrophic interactions between insect herbivores, plants, and pollinators. J. Invertebr. Pathol. 186:107589. doi: 10.1016/j.jip.2021.107589
Mohammadi, M., Shadnoush, M., Sohrabvandi, S., Yousefi, M., Khorshidian, N., and Mortazavian, A. M. (2021). Probiotics as potential detoxification tools for mitigation of pesticides: A mini review. Int. J. food Sci. Technol. 56, 2078–2087. doi: 10.1111/ijfs.14880
Mohan, M., and Gujar, G. (2003). Local variation in susceptibility of the diamondback moth, Plutella xylostella (Linnaeus) to insecticides and role of detoxification enzymes. Crop Prot. 22, 495–504. doi: 10.1016/S0261-2194(02)00201-6
Mojiri, A., Zhou, J. L., Robinson, B., Ohashi, A., Ozaki, N., Kindaichi, T., et al. (2020). Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 253:126646. doi: 10.1016/j.chemosphere.2020.126646
Mulla, S. I., Ameen, F., Talwar, M. P., Eqani, S. A. M. A. S., Bharagava, R. N., Saxena, G., et al. (2020). “Organophosphate pesticides: Impact on environment, toxicity, and their degradation,” in Bioremediation of industrial waste for environmental safety, eds G. Saxena and R. Bharagava (Singapore: Springer), 265–290. doi: 10.1007/978-981-13-1891-7_13
Nagy, K., Duca, R. C., Lovas, S., Creta, M., Scheepers, P. T., Godderis, L., et al. (2020). Systematic review of comparative studies assessing the toxicity of pesticide active ingredients and their product formulations. Environ. Res. 181:108926. doi: 10.1016/j.envres.2019.108926
Naik, V. C., Kumbhare, S., Kranthi, S., Satija, U., and Kranthi, K. R. (2018). Field-evolved resistance of pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), to transgenic Bacillus thuringiensis (Bt) cotton expressing crystal 1Ac (Cry1Ac) and Cry2Ab in India. Pest Manag. Sci. 74, 2544–2554. doi: 10.1002/ps.5038
Nerozzi, C., Recuero, S., Galeati, G., Bucci, D., Spinaci, M., and Yeste, M. (2020). Effects of Roundup and its main component, glyphosate, upon mammalian sperm function and survival. Sci. Rep. 10, 1–9. doi: 10.1038/s41598-020-67538-w
Nicolopoulou-Stamati, P., Maipas, S., Kotampasi, C., Stamatis, P., and Hens, L. (2016). Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 4:148. doi: 10.3389/fpubh.2016.00148
Niroumand, M. C., Farzaei, M. H., Razkenari, E. K., Amin, G., Khanavi, M., Akbarzadeh, T., et al. (2016). An evidence-based review on medicinal plants used as insecticide and insect repellent in traditional Iranian medicine. Iran. Red Crescent Med. J. 18:e22361. doi: 10.5812/ircmj.22361
Olisah, C., Okoh, O. O., and Okoh, A. I (2019). Global evolution of organochlorine pesticides research in biological and environmental matrices from 1992 to 2018: A bibliometric approach. Emerg. Contam. 5, 157–167. doi: 10.1016/j.emcon.2019.05.001
Omoke, D., Kipsum, M., Otieno, S., Esalimba, E., Sheth, M., Lenhart, A., et al. (2021). Western kenyan Anopheles gambiae showing intense permethrin resistance harbour distinct microbiota. Malar. J. 20, 1–14. doi: 10.1186/s12936-021-03606-4
Ozdal, M., Ozdal, O. G., and Algur, O. F. (2016). Isolation and characterization of α-endosulfan degrading bacteria from the microflora of cockroaches. Pol. J. Microbiol. 65, 63–68. doi: 10.5604/17331331.1197325
Özdal, ÖG., and Algur, ÖF. (2020). Isolation and identification of the pyrethroid insecticide deltamethrin degrading bacteria from insects. Avrupa Bilim ve Teknoloji Dergisi 18, 905–910. doi: 10.31590/ejosat.677008
Paris, L., Peghaire, E., Mone, A., Diogon, M., Debroas, D., Delbac, F., et al. (2020). Honeybee gut microbiota dysbiosis in pesticide/parasite co-exposures is mainly induced by Nosema ceranae. J. Invertebr. Pathol. 172:107348. doi: 10.1016/j.jip.2020.107348
Phugare, S. S., and Jadhav, J. P. (2015). Biodegradation of acetamiprid by isolated bacterial strain Rhodococcus sp. BCH2 and toxicological analysis of its metabolites in silkworm (Bombax mori). Clean Soil Air Water 43, 296–304. doi: 10.1002/clen.201200563
Phugare, S. S., Kalyani, D. C., Gaikwad, Y. B., and Jadhav, J. P. (2013). Microbial degradation of imidacloprid and toxicological analysis of its biodegradation metabolites in silkworm (Bombyx mori). Chem. Eng. J. 230, 27–35. doi: 10.1016/j.cej.2013.06.042
Pietri, J. E., and Liang, D. (2018). The links between insect symbionts and insecticide resistance: Causal relationships and physiological tradeoffs. Ann. Entomol. Soc. Am. 111, 92–97. doi: 10.1093/aesa/say009
Pietrzak, D., Kania, J., Kmiecik, E., Malina, G., and Wator, K. (2020). Fate of selected neonicotinoid insecticides in soil–water systems: Current state of the art and knowledge gaps. Chemosphere 255:126981. doi: 10.1016/j.chemosphere.2020.126981
Pokhrel, B., Gong, P., Wang, X., Chen, M., Wang, C., and Gao, S. (2018). Distribution, sources, and air–soil exchange of OCPs, PCBs and PAHs in urban soils of Nepal. Chemosphere 200, 532–541. doi: 10.1016/j.chemosphere.2018.01.119
Poupardin, R., Reynaud, S., Strode, C., Ranson, H., Vontas, J., and David, J.-P. (2008). Cross-induction of detoxification genes by environmental xenobiotics and insecticides in the mosquito Aedes aegypti: Impact on larval tolerance to chemical insecticides. Insect Biochem. Mol. Biol. 38, 540–551. doi: 10.1016/j.ibmb.2008.01.004
Prabhakar, C. S., Sood, P., and Mehta, P. K. (2008). Protein hydrolyzation and pesticide tolerance by gut bacteria of Bactrocera tau (Walker). Pest Manag. Econ. Zool. 16, 123–129. doi: 10.1007/s12600-012-0278-5
Puinean, A. M., Foster, S. P., Oliphant, L., Denholm, I., Field, L. M., Millar, N. S., et al. (2010). Amplification of a cytochrome P450 gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. PLoS Genet. 6:e1000999. doi: 10.1371/journal.pgen.1000999
Pujar, N. K., Premakshi, H., Ganeshkar, M. P., and Kamanavalli, C. M. (2022). “Biodegradation of pesticides used in agriculture by soil microorganisms,” in Enzymes for pollutant degradation microorganisms for sustainability, Vol. 30, eds S. I. Mulla and R. N. Bharagava (Singapore: Springer), 213–235. doi: 10.1007/978-981-16-4574-7_11
Puri, S., Singh, S., and Sohal, S. K. (2022). Oviposition behaviour and biochemical response of an insect pest, Zeugodacus cucurbitae (Coquillett)(Diptera: Tephritidae) to plant phenolic compound phloroglucinol. Comp. Biochem. Physiol. Toxicol. Pharmacol. 255:109291. doi: 10.1016/j.cbpc.2022.109291
Ramakrishnan, B., Venkateswarlu, K., Sethunathan, N., and Megharaj, M. (2019). Local applications but global implications: Can pesticides drive microorganisms to develop antimicrobial resistance? Sci. Total Environ. 654, 177–189. doi: 10.1016/j.scitotenv.2018.11.041
Ramoutar, D., Cowles, R. S., and Alm, S. R. (2009). Pyrethroid resistance mediated by enzyme detoxification in Listronotus maculicollis (Coleoptera: Curculionidae) from connecticut. J. Econ. Entomol. 102, 1203–1208. doi: 10.1603/029.102.0345
Rani, L., Thapa, K., Kanojia, N., Sharma, N., Singh, S., Grewal, A. S., et al. (2021). An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 283:124657. doi: 10.1016/j.jclepro.2020.124657
Reinholds, I., Pugajeva, I., Bavrins, K., Kuckovska, G., and Bartkevics, V. (2017). Mycotoxins, pesticides and toxic metals in commercial spices and herbs. Food Addit. Contam. Part B 10, 5–14. doi: 10.1080/19393210.2016.1210244
Ren, X., Zeng, G., Tang, L., Wang, J., Wan, J., Liu, Y., et al. (2018). Sorption, transport and biodegradation–an insight into bioavailability of persistent organic pollutants in soil. Sci. Total Environ. 610, 1154–1163. doi: 10.1016/j.scitotenv.2017.08.089
Øezáè, M., Øezáèová, V., and Heneberg, P. (2019). Contact application of neonicotinoids suppresses the predation rate in different densities of prey and induces paralysis of common farmland spiders. Sci. Rep. 9, 1–9. doi: 10.1038/s41598-019-42258-y
Riaz, M. A., Poupardin, R., Reynaud, S., Strode, C., Ranson, H., and David, J.-P. (2009). Impact of glyphosate and benzo [a] pyrene on the tolerance of mosquito larvae to chemical insecticides. Role of detoxification genes in response to xenobiotics. Aquat. Toxicol. 93, 61–69. doi: 10.1016/j.aquatox.2009.03.005
Richardson, J. R., Roy, A., Shalat, S. L., Von Stein, R. T., Hossain, M. M., Buckley, B., et al. (2014). Elevated serum pesticide levels and risk for Alzheimer disease. JAMA Neurol. 71, 284–290. doi: 10.1001/jamaneurol.2013.6030
Ricupero, M., Desneux, N., Zappalà, L., and Biondi, A. (2020). Target and non-target impact of systemic insecticides on a polyphagous aphid pest and its parasitoid. Chemosphere 247:125728. doi: 10.1016/j.chemosphere.2019.125728
Riga, M., Tsakireli, D., Ilias, A., Morou, E., Myridakis, A., Stephanou, E., et al. (2014). Abamectin is metabolized by CYP392A16, a cytochrome P450 associated with high levels of acaricide resistance in Tetranychus urticae. Insect Biochem. Mol. Biol. 46, 43–53. doi: 10.1016/j.ibmb.2014.01.006
Righi, K., Righi, F. A., Boubkeur, A., Boungab, K., Elouissi, A., and Djendara, A. C. (2018). Toxicity and repellency of three Algerian medicinal plants against pests of stored product: Ryzopertha dominica (Fabricius)(Coleoptera: Bostrichidae). Banats J. Biotechnol. 9, 50–59.
Rouzé, R., Moné, A., Delbac, F., Belzunces, L., and Blot, N. (2019). The honeybee gut microbiota is altered after chronic exposure to different families of insecticides and infection by Nosema ceranae. Microbes Environ. 34, 226–233. doi: 10.1264/jsme2.ME18169
Ruiz, D., Becerra, M., Jagai, J. S., Ard, K., and Sargis, R. M. (2018). Disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care 41, 193–205. doi: 10.2337/dc16-2765
Rumbos, C. I., Dutton, A. C., and Athanassiou, C. G. (2018). Insecticidal effect of spinetoram and thiamethoxam applied alone or in combination for the control of major stored-product beetle species. J. Stored Prod. Res. 75, 56–63. doi: 10.1016/j.jspr.2017.10.004
Safi, N. H. Z., Ahmadi, A. A., Nahzat, S., Ziapour, S. P., Nikookar, S. H., Fazeli-Dinan, M., et al. (2017). Evidence of metabolic mechanisms playing a role in multiple insecticides resistance in Anopheles stephensi populations from Afghanistan. Malar. J. 16, 1–10. doi: 10.1186/s12936-017-1744-9
Samoilova, E., Kostina, N., and Striganova, B. (2016). Microbial population of the digestive tract of click beetle larvae (Elateridae, Coleoptera). Biol. Bull. 43, 457–467. doi: 10.1134/S1062359016050083
Sarfraz, M., and Keddie, B. (2005). Conserving the efficacy of insecticides against Plutella xylostella (L.)(Lep., Plutellidae). J. Appl. Entomol. 129, 149–157. doi: 10.1111/j.1439-0418.2005.00930.x
Savant, S. S., Sriramkumar, S., and O’hagan, H. M. (2018). The role of inflammation and inflammatory mediators in the development, progression, metastasis, and chemoresistance of epithelial ovarian cancer. Cancers 10:251. doi: 10.3390/cancers10080251
Sayed, S. M., Alotaibi, S. S., Gaber, N., and Elarrnaouty, S.-A. (2020). Evaluation of five medicinal plant extracts on Aphis craccivora (Hemiptera: Aphididae) and its predator, Chrysoperla carnea (Neuroptera: Chrysopidae) under laboratory conditions. Insects 11:398. doi: 10.3390/insects11060398
Scates, S. S., O’neal, S. T., and Anderson, T. D. (2019). Bacteria-mediated modification of insecticide toxicity in the yellow fever mosquito, Aedes aegypti. Pestic Biochem. Physiol. 161, 77–85. doi: 10.1016/j.pestbp.2019.07.016
Schmidt, K., and Engel, P. (2021). Mechanisms underlying gut microbiota–host interactions in insects. J. Exp. Biol. 224:jeb207696. doi: 10.1242/jeb.207696
Serebrov, V., Gerber, O., Malyarchuk, A., Martemyanov, V., Alekseev, A., and Glupov, V. (2006). Effect of entomopathogenic fungi on detoxification enzyme activity in greater wax moth Galleria mellonella L.(Lepidoptera, Pyralidae) and role of detoxification enzymes in development of insect resistance to entomopathogenic fungi. Biol. Bull. 33, 581–586. doi: 10.1134/S1062359006060082
Sgolastra, F., Porrini, C., Maini, S., Bortolotti, L., Medrzycki, P., Mutinelli, F., et al. (2017). Healthy honey bees and sustainable maize production: Why not. Bull. Insectol. 70, 156–160. doi: 10.1016/j.biocon.2019.108356
Shah, H. K., Sharma, T., and Banerjee, B. D. (2020). Organochlorine pesticides induce inflammation, ROS production, and DNA damage in human epithelial ovary cells: An in vitro study. Chemosphere 246:125691. doi: 10.1016/j.chemosphere.2019.125691
Shahid, M., Khan, M. S., Ahmed, B., Syed, A., and Bahkali, A. H. (2021). Physiological disruption, structural deformation and low grain yield induced by neonicotinoid insecticides in chickpea: A long term phytotoxicity investigation. Chemosphere 262:128388. doi: 10.1016/J.CHEMOSPHERE.2020.128388
Shan, H., Wu, W., Sun, Z., Chen, J., and Li, H. (2021). The gut microbiota of the insect infraorder Pentatomomorpha (Hemiptera: Heteroptera) for the light of ecology and evolution. Microorganisms 9:464. doi: 10.3390/microorganisms9020464
Shang, J., Yao, Y. S., Zhu, X. Z., Wang, L., Li, D. Y., Zhang, K. X., et al. (2021b). Evaluation of sublethal and transgenerational effects of sulfoxaflor on Aphis gossypii via life table parameters and 16S rRNA sequencing. Pest Manag. Sci. 77, 3406–3418. doi: 10.1002/mbo3.652
Shang, J., Yao, Y.-S., Chen, L.-L., Zhu, X.-Z., Niu, L., Gao, X.-K., et al. (2021a). Sublethal exposure to deltamethrin stimulates reproduction and alters symbiotic bacteria in Aphis gossypii. J. Agric. Food Chem. 69, 15097–15107. doi: 10.1021/acs.jafc.1c05070
Sharma, A., Kumar, V., and Shahzad, B. (2019). Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 1:1446. doi: 10.1007/s42452-019-1485-1
Siddiqui, J. A., Khan, M. M., Bamisile, B. S., Hafeez, M., Qasim, M., Rasheed, M. T., et al. (2022). Role of insect gut microbiota in pesticide degradation: A review. Front. Microbiol. 13:870462. doi: 10.3389/fmicb.2022.870462
Skidmore, I. H., and Hansen, A. K. (2017). The evolutionary development of plant-feeding insects and their nutritional endosymbionts. Insect Sci. 24, 910–928. doi: 10.1111/1744-7917.12463
Smith, C. C., Srygley, R. B., Healy, F., Swaminath, K., and Mueller, U. G. (2017). Spatial structure of the mormon cricket gut microbiome and its predicted contribution to nutrition and immune function. Front. Microbiol. 8:801. doi: 10.3389/fmicb.2017.00801
Soh, L.-S., and Veera Singham, G. (2022). Bacterial symbionts influence host susceptibility to fenitrothion and imidacloprid in the obligate hematophagous bed bug, Cimex hemipterus. Sci. Rep. 12, 1–16. doi: 10.1038/s41598-022-09015-0
Soltani, A., Vatandoost, H., Oshaghi, M. A., Enayati, A. A., and Chavshin, A. R. (2017). The role of midgut symbiotic bacteria in resistance of Anopheles stephensi (Diptera: Culicidae) to organophosphate insecticides. Pathog. Glob. Health 111, 289–296. doi: 10.1080/20477724.2017.1356052
Somwang, P., Yanola, J., Suwan, W., Walton, C., Lumjuan, N., Prapanthadara, L.-A., et al. (2011). Enzymes-based resistant mechanism in pyrethroid resistant and susceptible Aedes aegypti strains from northern Thailand. Parasitol. Res. 109, 531–537. doi: 10.1007/s00436-011-2280-0
Song, Y., Shi, J., Xiong, Z., Shentu, X., and Yu, X. (2021). Three antimicrobials alter gut microbial communities and causing different mortality of brown planthopper, Nilaparvata lugens Stål. Pestic Biochem. Physiol. 174:104806. doi: 10.1016/j.pestbp.2021.104806
Stein, K., Coulibaly, D., Stenchly, K., Goetze, D., Porembski, S., Lindner, A., et al. (2017). Bee pollination increases yield quantity and quality of cash crops in Burkina Faso, West Africa. Sci. Rep. 7, 1–10. doi: 10.1038/s41598-017-17970-2
Tanner, C. M., Ross, G. W., Jewell, S. A., Hauser, R. A., Jankovic, J., Factor, S. A., et al. (2009). Occupation and risk of Parkinsonism: A multicenter case-control study. Arch. Neurol. 66, 1106–1113. doi: 10.1001/archneurol.2009.195
Teklu, B. M., Haileslassie, A., and Mekuria, W. (2022). Pesticides as water pollutants and level of risks to environment and people: An example from central rift valley of ethiopia. Environ. Dev. Sustain. 24, 5275–5294. doi: 10.1007/s10668-021-01658-9
Tian, J., Long, X., Zhang, S., Qin, Q., Gan, L., and Tian, Y. (2018). Screening cyhalothrin degradation strains from locust epiphytic bacteria and studying Paracoccus acridae SCU-M53 cyhalothrin degradation process. Environ. Sci. Pollut. Res. 25, 11505–11515. doi: 10.1007/s11356-018-1410-y
Tilottama, M., Teh, B. S., Aishwarya, M., Schmidt-Heck, W., Schlenker, Y., Vogel, H., et al. (2021). Transcriptomics reveal the survival strategies of Enterococcus mundtii in the gut of Spodoptera littoralis. J. Chem. Ecol. 47, 227–241. doi: 10.1007/s10886-021-01246-1
Tokuda, G., Mikaelyan, A., Fukui, C., Matsuura, Y., Watanabe, H., Fujishima, M., et al. (2018). Fiber-associated spirochetes are major agents of hemicellulose degradation in the hindgut of wood-feeding higher termites. Proc. Natl. Acad. Sci. U.S.A. 115, E11996–E12004. doi: 10.1073/pnas.1810550115
Tyohemba, R. L., Pillay, L., and Humphries, M. S. (2021). Bioaccumulation of current-use herbicides in fish from a global biodiversity hotspot: Lake St Lucia, South Africa. Chemosphere 284:131407. doi: 10.1016/j.chemosphere.2021.131407
van de Merwe, J. P., Neale, P. A., Melvin, S. D., and Leusch, F. D. (2018). In vitro bioassays reveal that additives are significant contributors to the toxicity of commercial household pesticides. Aquat. Toxicol. 199, 263–268. doi: 10.1016/j.aquatox.2018.03.033
Wakil, W., Kavallieratos, N. G., Usman, M., Gulzar, S., and El-Shafie, H. A. (2021). Detection of phosphine resistance in field populations of four key stored-grain insect pests in Pakistan. Insects 12:288. doi: 10.3390/insects12040288
Wang, C., Henderson, G., and Gautam, B. K. (2013). Lufenuron suppresses the resistance of Formosan subterranean termites (Isoptera: Rhinotermitidae) to entomopathogenic bacteria. J. Econ. Entomol. 106, 1812–1818. doi: 10.1603/ec13068
Wang, G.-H., Berdy, B. M., Velasquez, O., Jovanovic, N., Alkhalifa, S., Minbiole, K. P., et al. (2020). Changes in microbiome confer multigenerational host resistance after sub-toxic pesticide exposure. Cell Host Microbe 27, 213–224.e7. doi: 10.1016/j.chom.2020.01.009
Wang, H., Shi, Y., Wang, L., Liu, S., Wu, S., Yang, Y., et al. (2018). CYP6AE gene cluster knockout in Helicoverpa armigera reveals role in detoxification of phytochemicals and insecticides. Nat. Commun. 9, 1–8. doi: 10.1038/s41467-018-07226-6
Wang, H., Zhang, C., Cheng, P., Wang, Y., Liu, H., Wang, H., et al. (2021). Differences in the intestinal microbiota between insecticide-resistant and-sensitive Aedes albopictus based on full-length 16S rRNA sequencing. Microbiol. Open 10:e1177. doi: 10.1002/mbo3.1177
Wang, L., Zhang, Y., Han, Z., Liu, Y., and Fang, J. (2010). Cross-resistance and possible mechanisms of chlorpyrifos resistance in Laodelphax striatellus (Fallén). Pest Manag. Sci. 66, 1096–1100. doi: 10.1002/ps.1984
Wang, S., Wang, L., Fan, X., Yu, C., Feng, L., and Yi, L. (2020). An insight into diversity and functionalities of gut microbiota in insects. Curr. Microbiol. 77, 1976–1986. doi: 10.1007/s00284-020-02084-2
Wang, Z., Wang, W., and Lu, Y. (2022). Biodegradation of insecticides by gut bacteria isolated from stored grain beetles and its implication in host insecticide resistance. J. Stored Prod. Res. 96:101943. doi: 10.1016/j.jspr.2022.101943
Wong, C. N. A., Ng, P., and Douglas, A. E. (2011). Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ. Microbiol. 13, 1889–1900. doi: 10.1111/j.1462-2920.2011.02511.x
Wu, C., Zhang, H., He, M., Dong, F., Xu, J., Wu, X., et al. (2021). Toxicity of neonicotinoid insecticides on key non-target natural predator the larvae of Coccinella septempunctata in environmental. Environ. Technol. Innov. 23:101523. doi: 10.1016/j.eti.2021.101523
Wu, G., Miyata, T., Kang, C. Y., and Xie, L. H. (2007). Insecticide toxicity and synergism by enzyme inhibitors in 18 species of pest insect and natural enemies in crucifer vegetable crops. Pest Manag. Sci. 63, 500–510. doi: 10.1002/ps.1361
Xia, X., Sun, B., Gurr, G. M., Vasseur, L., Xue, M., and You, M. (2018). Gut microbiota mediate insecticide resistance in the diamondback moth, Plutella xylostella (L.). Front. Microbiol. 9:25. doi: 10.3389/fmicb.2017.00663
Xia, X., Zheng, D., Zhong, H., Qin, B., Gurr, G. M., Vasseur, L., et al. (2013). DNA sequencing reveals the midgut microbiota of diamondback moth, Plutella xylostella (L.) and a possible relationship with insecticide resistance. PLoS One 8:e68852. doi: 10.1371/journal.pone.0068852
Xie, W., Meng, Q.-S., Wu, Q.-J., Wang, S.-L., Yang, X., Yang, N.-N., et al. (2012). Pyrosequencing the Bemisia tabaci transcriptome reveals a highly diverse bacterial community and a robust system for insecticide resistance. PLoS One 7:e35181. doi: 10.1371/journal.pone.0035181
Xue, H., Zhu, X., Wang, L., Zhang, K., Li, D., Ji, J., et al. (2021). Gut bacterial diversity in different life cycle stages of Adelphocoris suturalis (Hemiptera: Miridae). Front. Microbiol. 12:1258. doi: 10.3389/fmicb.2021.670383
Yadav, I. C., Devi, N. L., Syed, J. H., Cheng, Z., Li, J., Zhang, G., et al. (2015). Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Sci. Total Environ. 511, 123–137. doi: 10.1016/j.scitotenv.2014.12.041
Yahouédo, G. A., Chandre, F., Rossignol, M., Ginibre, C., Balabanidou, V., Mendez, N. G. A., et al. (2017). Contributions of cuticle permeability and enzyme detoxification to pyrethroid resistance in the major malaria vector Anopheles gambiae. Sci. Rep. 7, 1–10. doi: 10.1038/s41598-017-11357-z
Yan, D., Zhang, Y., Liu, L., and Yan, H. (2016). Pesticide exposure and risk of Alzheimer’s disease: A systematic review and meta-analysis. Sci. Rep. 6, 1–9. doi: 10.1038/srep32222
Yáñez, L., Borja-Aburto, V. C. H., Rojas, E., De La Fuente, H., González-Amaro, R., Gómez, H., et al. (2004). DDT induces DNA damage in blood cells. Studies in vitro and in women chronically exposed to this insecticide. Environ. Res. 94, 18–24. doi: 10.1016/S0013-9351(03)00047-1
Yang, L., Gao, J., Liu, Y., Zhuang, G., Peng, X., Wu, W.-M., et al. (2021). Biodegradation of expanded polystyrene and low-density polyethylene foams in larvae of Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae): Broad versus limited extent depolymerization and microbe-dependence versus independence. Chemosphere 262:127818. doi: 10.1016/j.chemosphere.2020.127818
Yang, Y.-X., Lin, R.-H., Li, Z., Wang, A.-Y., Xue, C., Duan, A.-L., et al. (2021). Function analysis of P450 and GST genes to imidacloprid in Aphis craccivora (Koch). Front. Physiol. 11:624287. doi: 10.3389/fphys.2020.624287
Yu, L., Yang, H., Cheng, F., Wu, Z., Huang, Q., He, X., et al. (2021). Honey bee Apis mellifera larvae gut microbial and immune, detoxication responses towards flumethrin stress. Environ. Pollut. 290:118107. doi: 10.1016/j.envpol.2021.118107
Yuan, X., Pan, Z., Jin, C., Ni, Y., Fu, Z., and Jin, Y. (2019). Gut microbiota: An underestimated and unintended recipient for pesticide-induced toxicity. Chemosphere 227, 425–434. doi: 10.1016/j.chemosphere.2019.04.088
Yun, J.-H., Roh, S. W., Whon, T. W., Jung, M.-J., Kim, M.-S., Park, D.-S., et al. (2014). Insect gut bacterial diversity determined by environmental habitat, diet, developmental stage, and phylogeny of host. Appl. Environ. Microbiol. 80, 5254–5264. doi: 10.1128/AEM.01226-14
Zamule, S. M., Dupre, C. E., Mendola, M. L., Widmer, J., Shebert, J. A., Roote, C. E., et al. (2021). Bioremediation potential of select bacterial species for the neonicotinoid insecticides, thiamethoxam and imidacloprid. Ecotoxicol. Environ. Saf. 209:111814. doi: 10.1016/j.ecoenv.2020.111814
Zeng, J.-Y., Shi, J.-H., Shi, Z.-B., Guo, J.-X., Zhang, G.-C., and Bi, B. (2020). Avermectin stress varied structure and function of gut microbial community in Lymantria dispar asiatica (Lepidoptera: Lymantriidae) larvae. Pestic. Biochem. Physiol. 164, 196–202. doi: 10.1016/j.pestbp.2020.01.013
Zhang, F., and Yang, R. (2019). Life history and functional capacity of the microbiome are altered in beta-cypermethrin-resistant cockroaches. Int. J. Parasitol. 49, 715–723. doi: 10.1016/j.ijpara.2019.04.006
Zhang, J., Ai, Z., Liu, H., Tang, D., Yang, X., Wang, G., et al. (2022). Short-term N addition in a Pinus tabuliformis plantation: Microbial community composition and interactions show different linkages with ecological stoichiometry. Appl. Soil Ecol. 174:104422. doi: 10.1016/j.apsoil.2022.104422
Zhang, J., Lan, W., Qiao, C., and Jiang, H. (2004). Bioremediation of organophosphorus pesticides by surface-expressed carboxylesterase from mosquito on Escherichia coli. Biotechnol. Prog. 20, 1567–1571. doi: 10.1021/bp049903c
Zhang, X. C., Jiang, M., Zang, Y. N., Zhao, H. Z., Liu, C. X., Liu, B. R., et al. (2022). Metarhizium anisopliae is a valuable grist for biocontrol in beta-cypermethrin-resistant Blattella germanica (L.). Pest Manag. Sci. 78, 1508–1518. doi: 10.1002/ps.6769
Zhao, J., Jia, D., Du, J., Chi, Y., and Yao, K. (2019). Substrate regulation on co-metabolic degradation of β-cypermethrin by Bacillus licheniformis B-1. AMB Express 9, 1–11. doi: 10.1186/s13568-019-0808-3
Zhou, C., Yang, H., Wang, Z., Long, G.-Y., and Jin, D.-C. (2018). Comparative transcriptome analysis of Sogatella furcifera (Horváth) exposed to different insecticides. Sci. Rep. 8, 1–12. doi: 10.1038/s41598-018-27062-4
Zhu, Y. C., Blanco, C. A., Portilla, M., Adamczyk, J., Luttrell, R., and Huang, F. (2015). Evidence of multiple/cross resistance to Bt and organophosphate insecticides in Puerto Rico population of the fall armyworm, Spodoptera frugiperda. Pestic. Biochem. Physiol. 122, 15–21. doi: 10.1016/j.pestbp.2015.01.007
Zhu, Y. C., Guo, Z., Chen, M.-S., Zhu, K. Y., Liu, X. F., and Scheffler, B. (2011). Major putative pesticide receptors, detoxification enzymes, and transcriptional profile of the midgut of the tobacco budworm, Heliothis virescens (Lepidoptera: Noctuidae). J. Invertebr. Pathol. 106, 296–307. doi: 10.1016/j.jip.2010.10.007
Keywords: symbiotic microbes, enzymes, pesticides, non-target organisms, metabolic pathways
Citation: Jaffar S, Ahmad S and Lu Y (2022) Contribution of insect gut microbiota and their associated enzymes in insect physiology and biodegradation of pesticides. Front. Microbiol. 13:979383. doi: 10.3389/fmicb.2022.979383
Received: 27 June 2022; Accepted: 19 August 2022;
Published: 14 September 2022.
Edited by:
Pankaj Bhatt, Purdue University, United StatesReviewed by:
Sikandar I. Mulla, REVA University, IndiaCopyright © 2022 Jaffar, Ahmad and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongyue Lu, bHV5b25neXVlQHNjYXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.