
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 08 September 2022
Sec. Evolutionary and Genomic Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.975344
This article is part of the Research Topic Microbiome and Microbial Informatics View all 18 articles
Paenibacillus peoriae is a plant growth-promoting rhizobacteria (PGPR) widely distributed in various environments. P. peoriae ZBFS16 was isolated from the wheat rhizosphere and significantly suppressed grape white rot disease caused by Coniella vitis. Here, we present the complete genome sequence of P. peoriae ZBFS16, which consists of a 5.83 Mb circular chromosome with an average G + C content of 45.62%. Phylogenetic analyses showed that ZBFS16 belongs to the genus P. peoriae and was similar to P. peoriae ZF390, P. peoriae HS311 and P. peoriae HJ-2. Comparative analysis with three closely related sequenced strains of P. peoriae identified the conservation of genes involved in indole-3-acetic acid production, phosphate solubilization, nitrogen fixation, biofilm formation, flagella and chemotaxis, quorum-sensing systems, two-component systems, antimicrobial substances and resistance inducers. Meanwhile, in vitro experiments were also performed to confirm these functions. In addition, the strong colonization ability of P. peoriae ZBFS16 was observed in soil, which provides it with great potential for use in agriculture as a PGPR. This study will be helpful for further studies of P. peoriae on the mechanisms of plant growth promotion and biocontrol.
Paenibacillus peoriae (previously Bacillus peoriae) is a Gram-positive, facultatively anaerobic, rod-shaped bacterium with flagella and belongs to the genus Paenibacillus and the family Paenibacillaceae. Species in the genus Paenibacillus are either Gram-positive or variable, facultatively anaerobic or strictly aerobic, produce ellipsoidal endospores, and are nonpigmented, rod-shaped and motile (Ash et al., 1993; Siddiqi et al., 2015). Currently, the genus Paenibacillus contains 240 species, including the plant-beneficial species of P. polymyxa (Zhang et al., 2018; Timmusk et al., 2019), P. ehimensis (Naing et al., 2015), P. alvei (Emmanouil et al., 2016), P. macerans (Liang et al., 2014), P. lentimorbus (DasGupta et al., 2006) and P. peoriae (Von der Weid et al., 2003; Jiang et al., 2022). Previously, P. peoriae was reported to act as a plant growth-promoting rhizobacteria (PGPR), which can produce biofilms, stably colonize the rhizosphere of plants and compete with other microbiota (Von der Weid et al., 2003; Vejan et al., 2016; Jiang et al., 2022). Meanwhile, P. peoriae has the ability to act as a biological control agent against many plant pathogens, including Fusarium spp., Diplodia macrospora, D. maydis, Verticillium dahlia, Rhizoctonia solani, Colletotrichum gloeosporioides, and C. graminicola (Von der Weid et al., 2003; Yadav D. et al., 2021; Jiang et al., 2022), and even the antimicrobial peptide purified from P. peoriae could protect against Staphylococcus aureus, Escherichia coli, and Candida albicans (Ngashangva et al., 2021).
PGPR has been considered environmentally friendly alternatives to fertilizers or agrochemicals for improving crop yield and quality (Vejan et al., 2016; Hashem et al., 2019). Many microorganisms, such as Bacillus, Pseudomonas, Burkholderia, Caulobacter, and Paenibacillus spp., are PGPRs, and some have or will be successfully applied in practical applications (Ahemad, 2015; Garcia-Seco et al., 2015; Hashem et al., 2019). Production of indole-3-acetic acid (IAA), the capability of fixation of nitrogen, dissolution of phosphorus, secretion of ferriphagin and plant hormones, and antibiotic biosynthesis are important mechanisms of PGPR (Li et al., 2020; Yin et al., 2022). IAA is an important phytohormone that controls cell enlargement and tissue differentiation of plants. Nitrogen (N) and phosphorus (P) are important nutrients for plant growth and productivity. PGPRs are called diazotrophs because of their ability to fix N2 in nonleguminous plants and form a nonobligate interaction with host plants (Ahemad, 2015). Additionally, by providing P to plants, PGPRs solubilize inorganic P in soil to low molecular weight organic acids (Zaidi et al., 2009; Yuan et al., 2020). Siderophores can form stable complexes with Fe and other heavy metals (Al, Cd, Cu, Ga, In, Pb and Zn), and most plant growth promotion occurs via siderophore-mediated Fe uptake (Rajkumar et al., 2010). P. polymyxa., which is closest to P. peoriae, was identified as having key genes or gene clusters related to IAA, phosphate solubilization and nitrogen fixation for plant growth promotion (Li et al., 2020; Zhou et al., 2020).
The predominant genera of PGPRs are Pseudomonas and Bacillus, which have the feature of biocontrol, as well as most species in Paenibacillus (Naing et al., 2015; Grady et al., 2016; Hashem et al., 2019). Paenibacillus helps to control phytopathogens (bacteria, fungi, nematodes and viruses) by triggering induced systemic resistance (ISR) by producing secondary metabolites (Grady et al., 2016). Antimicrobial substances produced by Paenibacillus, including peptides, enzymes, and volatile organic compounds, could be used to control soil-borne fungal pathogens and food-borne bacteria (Zhai et al., 2021). Paenicidin A and penisin are antimicrobial peptides produced by P. polymyxa NRRL B-30509 and Paenibacillus sp. strain A3, respectively (Baindara et al., 2015; Van Belkum et al., 2015). Paenibacillin exhibits excellent tolerance to pH and heat, with activity against a broad range of fungi and bacteria (Abriouel et al., 2011; Li Y. et al., 2019; Li L. et al., 2019). Nonribosomal peptide synthetases are large multimodular biocatalysts that utilize complex regiospecific and stereospecific reactions to assemble structurally and functionally diverse peptides that have important medicinal applications (Strieker et al., 2010).
The role of P. peoriae in plant growth promotion and biological control remained unexplored until very recently, and few reports revealed the mechanisms regarding the plant growth promotion and biological control of P. peoriae. P. peoriae ZBSF16 exhibit significant broad inhibitory spectra against various pathogenic fungi and bacteria on grape and possess perfect characteristics and potential for the biocontrol of grape diseases. In this study, we demonstrated the sequence and annotation of P. peoriae strain ZBSF16 and compared its genome with the three major representative P. peoriae strains (P. peoriae ZF390, P. peoriae HS311 and P. peoriae HJ-2) that are beneficial to plant growth. Our aim was to provide important insights into the functions of the biocontrol strains and analyze the mechanisms of plant growth promotion and biological control at the gene level, which will benefit improved application of P. peoriae to plants in the field.
P. peoriae ZBSF16 was isolated from the wheat rhizosphere in Shandong Province, China on May 7, 2020 and was deposited as a reference strain (strain no. 24769) in the China General Microbiological Culture Collection Center. Strain ZBSF16 was cultivated in LB (Luria broth) medium at 28°C with shaking at 180 rpm for 24 h. The growth curve and the dynamic change in pH were measured every 4 h by spectrophotometer (Persee, TU-1900) and pH meter (Sartorius, PB-10) and the biochemical tests were performed as described by Yin et al. (2022). The morphology of the strains was observed scanning electron microscope (TESCAN VEGA3 SBU). Strain ZBSF16 was evaluated for its antagonistic activities to Coniella vitis, Gloeosporium fructigrum, Pestalotiopsis clavispora, Alternaria viticola, Diaporthe eres, F. oxysporum, Botrytis cinerea, Botryosphaeria dothidea, Aspergillus niger, F. graminearum, F. pseudograminearum and Allorhizobium vitis by plate bioassays inoculated with 2 μl of bacterial suspension (Li Y. et al., 2019). The inoculation concentration of strain ZBSF16 was determined by the optical density at 600 nm (OD600 = 0.8). Genomic DNA was extracted from cultured ZBSF16 cells (OD600 = 0.8) using a QIAamp® DNA Mini Kit (Qiagen, Valencia, CA, United States) according to the manufacturer’s instructions.
The genomic DNA of P. peoriae ZBSF16 was sequenced at Biomarker Technologies with the Pacific Biosciences (PacBio) RSII Single Molecule Real Time (SMRT) sequencing platform (Li Y. et al., 2019). For genome assembly, the filtered subreads were assembled by Canu v1.5 software, and then, circlator v1.5.5 was used to cyclize the assembled genome. A 10-kb insert size template library was prepared according to the PacBio Sequel gDNA protocol and sequenced using the PacBio Sequel instrument. Circular genome views of the alignments were generated by CGView (Yuan et al., 2020).
Genes and components of the genome were predicted by using Prodigal v2.6.3, and functional annotation was performed by comparisons against multiple databases, including NR (nonredundant) protein databases, SwissProt and the enhanced COG database, KEGG database, TrEMBL, and the Eggnog database. Transfer RNA (tRNA) genes were predicted with tRNAscan-SE v2.0, and ribosome RNA (rRNA) genes were predicted with Infernal v1.1.3. antiSMASH v5.0.0 was used to predict secondary metabolic gene clusters, and CRT v1.2 was used for CRISPR identification. Furthermore, pathogenicity and drug resistance can be researched by BLAST against the CAZy, TCDB, CARD, PHI, and VFDB databases.
The evolutionary position of P. peoriae ZBSF16 was determined by 16S rDNA gene sequence analysis, multilocus sequence analysis (MLSA) and PhyloPhlAn method (Segata et al., 2013; Asnicar et al., 2020; Yin et al., 2022). 22 strains belonging to Paenibacillus were selected for constructing phylogenetic trees to investigate the evolution of strain ZBSF16 (Supplementary Table 1). Five housekeeping genes (16S rRNA, gyrB, rpoD, rho, and pgk) were selected for MLSA, sequence alignments of ZBSF16 with other Paenibacillus strains were carried out using the maximum likelihood clustering method, which was performed in MEGA6 with a bootstrapping test of 1,000 replications to generate phylogenetic trees.
For the comparative genomic analysis, the genome sequences of P. peoriae ZBSF16 were compared to P. peoriae ZF390, P. peoriae HS311 and P. peoriae HJ-2 by MAUVE comparison software (Darling et al., 2004). Additionally, a circular chromosomal map of all the genomes used in the pan-genome analysis was prepared by using BLAST Ring Image Generator (BRIG) v 0.95, taking strain ZBSF16 as a reference genome (Alikhan et al., 2011; Mukhia et al., 2022). Furthermore, average nucleotide identity (ANI) was conducted by using the orthologous average nucleotide identity (OrthoANI) tool, and in silico DNA–DNA hybridization (DDH) was calculated by using the Genome-to-Genome Distance Calculator (GGDC) (Goris et al., 2007). Functional genes involved in plant growth promotion, such as genes responsible for IAA production, phosphate solubilization, nitrogen fixation, biofilm formation and synthesis resistance inducers, were searched in the NCBI databases as described by Kumar et al. (2019). The blast search was performed against the locally constructed database of the publically available genomes of P. peoriae, with the genome of P. peoriae ZBSF16 as a query. The identities of different functional genes at the amino acid level were compared among the strains by using BLAST, with an E-value cut off of 1e-15 was used for the BLAST search (Kumar et al., 2019). Secondary metabolite gene clusters were predicted by antiSMASH 4.0.2 (Jiang et al., 2022).
To determine the production of IAA, strain ZBSF16 was cultured in DF (peptone, 5.0 g; yeast extract, 1.5 g; beef extract, 1.5 g/l; NaCl, 5.0 g/l; tryptophan, 0.5 g/l) salt minimal medium, with a concentration of L-tryptophan of 1.02 g/l. After incubation for 24 h at 28°C, the IAA concentration was estimated as the method described by Yuan et al., (2020). The capability of strain ZBSF16 to solubilize phosphate was estimated via National Botanical Research Institute Phosphate (NBRIP) solid medium as described by Yin et al., (2022), and the clear zone around the colony was measured after 7 days at 28°C. A CAS agar plate was used for qualitative analysis of siderophores, and yellow circles that appeared around the colonies were measured after 7 days at 28°C. The capability of strain ZBSF16 to produce ammonia was detected by the method described by Przemienieck, and Nessler’s reagent was used to determine its ability to produce ammonium (Przemieniecki et al., 2019; Elhaissoufi et al., 2020).
The characteristics of antibiotic resistance of strain ZBSF16 were tested on nine antibiotics, including ampicillin (200 μg/ml), kanamycin (50 μg/ml), rifampicin (50 μg/ml), vancomycin (50 μg/ml), streptomycin (10 μg/ml), spectinomycin (50 μg/ml), gentamycin (10 μg/ml), tetracycline (5 μg/ml), and chloramphenicol (20 μg/ml). The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of spectinomycin for strain ZBSF16 were determined as previously described. P. peoriae ZBSF16 was grown in LB broth at 28°C for 24 h, Wagstsuma Blood Agar Base (Hopebio, China) was used to determine hemolysis as described previously (Brillard et al., 2001; Yuan et al., 2020).
To determine the plant growth promotion capability of ZBSF16, ten Vitis vinifera seedlings (cv. Red globe) were treated with 50 ml of ZBSF16 culture (108 CFU/ml) by irrigation every 15 days for 2 months. Another ten V. vinifera seedlings used as controls were treated with sterile water. All treated grape plants were placed in a greenhouse maintained at temperature 28°C and 90% relative humidity (RH). At 60 days after inoculation, the root length, shoot length, fresh weight, and dry weight of the seedlings were measured. Meanwhile, the infection rate and disease index of grape white rot on Vitis vinifera seedlings (cv. Red globe) were calculated after inoculating C. vitis conidial suspension (106 conidial/ml) two month later at 28°C and 70–80% RH (Chethana et al., 2017; Ji et al., 2021).
To observe the population dynamics of the ZBSF16 strain in the rhizosphere soil, Vitis vinifera seedlings (cv. Red globe) were transplanted into nursery pots containing sterile soil, and each seedling was irrigated with 50 ml of P. peoriae ZBSF16 bacterial suspension at a concentration of 108 CFU ml−1. Rhizosphere soil was collected at different time points (0, 7, 14, 21, 28, 35, 42, 49 and 56 days after inoculation), and the number of ZBSF16 in the rhizosphere soil was determined by the plating counting method with LB medium containing spectinomycin and streptomycin.
Grape white rot caused by Coniella vitis was used as the pathosystem to determine the biocontrol potential of ZBSF16. Leaves and fruit of V. vinifera (cv. Red globe) were used to assess the preventive effect and control effect of strain ZBSF16 as described by Yin et al. (2022). Ten biological replicates were performed for each treatment, and the experiments were independently repeated three times. All the leaves and fruit were maintained at 28°C and 90% RH.
All experimental data were analyzed by SPSS 22.0 software, and all the values are presented as the mean ± standard error of at least three replications. Significant differences (p < 0.05) were determined by one-way analysis (ANOVA) of variance and Duncan’s multiple range test (Yuan et al., 2020; Yin et al., 2022).
As a gram-positive, anaerobic, rod-shaped bacterium with a length of 3–5 μm and a diameter of 0.8–1.2 μm, ZBSF16 can utilize diverse carbon sources and belongs to the Paenibacillus genus (Supplementary Figures 1A,B; Supplementary Table 2). The growth curve showed that the strain was in the exponential growth phase between 4 and 20 h after inoculation, with the pH value increasing to 7.77 (Supplementary Figure 1C). Additionally, the strain grew best when the pH value was between 6 and 8 and could endure 2% NaCl (Supplementary Figures 1G,H).
P. peoria ZBSF16 was isolated as a biocontrol agent for use against Coniella vitis, which exhibited the highest inhibitory rate of 64.44% (Supplementary Figure 2A). Antagonistic spectrum assays showed that strain ZBSF16 presented broad, strong antipathogenic activities against various fungi on grape, including Gloeosporium fructigrum, Botrytis cinerea, Diaporthe eres, Alternaria viticola, F. oxysporum, Aspergillus niger, Pestalotiopsis clavispora, and Allorhizobium vitis (Figure 1A). In addition, ZBSF16 is considered a biocontrol agent for its extracellular enzyme activity, and it can produce protease, cellulase and lipoidase, which is an important mechanism for inhibiting pathogens (Supplementary Figures 1D–F).
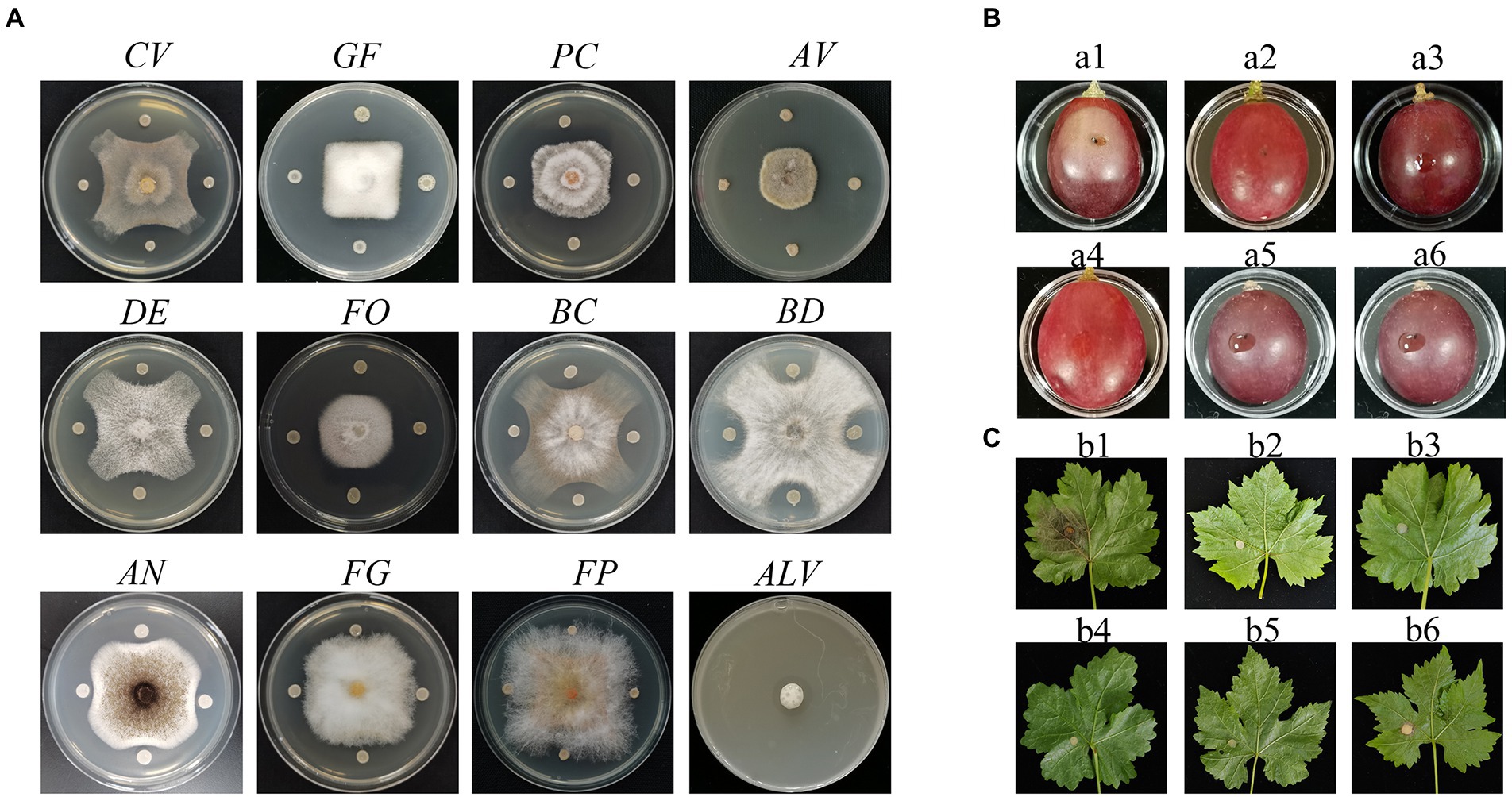
Figure 1. Antagonistic assay of Paenibacillus peoriae ZBSF16 against eleven pathogenic fungi and one pathogenic bacterium. (A) Antagonistic assay of P. peoriae ZBSF16. Coniella vitis (CV). Gloeosporium fructigrum (GF). Pestalotiopsis clavispora (Pc). Alternaria viticola (Av). Diaporthe eres (DE). Fusarium oxysporum (FO). Botrytis cinerea (BC). Botryosphaeria dothidea (BD). Aspergillus niger (AN). Fusarium graminearum (FG). Fusarium pseudograminearum (FP). Allorhizobium vitis (ALV). (B,C) Biocontrol efficiency of P. peoriae ZBSF16 on grape white rot caused by Coniella vitis. (a1, b1) Inoculated with C. vitis; (a2, b2) LB broth; (a3, b3) sterile water; (a4, b4) culture of ZBSF16; (a5, b5) inoculated with C. vitis 24 h after inoculation with the culture of ZBSF16; (a6, b6) inoculated culture of ZBSF16 24 h after inoculation with C. vitis.
The ability of ZBSF16 to promote growth was verified by inoculating the rhizosphere of plants of V. vinifera (cv. Red globe) with the suspension in the greenhouse. P. peoriae ZBSF16 produced siderophores and was considered an excellent PGRP (Supplementary Figure 3E). The rate of growth promotion for the length (weight) of the aboveground parts and the root length (fresh weight, dry weight) were 46.56% (60.20, 183.75%) and 60.78% (137.25, 454.54%), respectively (Figure 2C). In addition, the bacterial counts of ZBSF16 on the root surface were maintained at 105 CFU/g after 1 month of inoculation (Supplementary Figure 3F). Further study showed that the infection rate and disease index of grape white rot on V. vinifera caused by C. vitis were decreased 70% and 62.97, inoculating with strain ZBSF16 compared to the control plants (Supplementary Figures 2D,E).
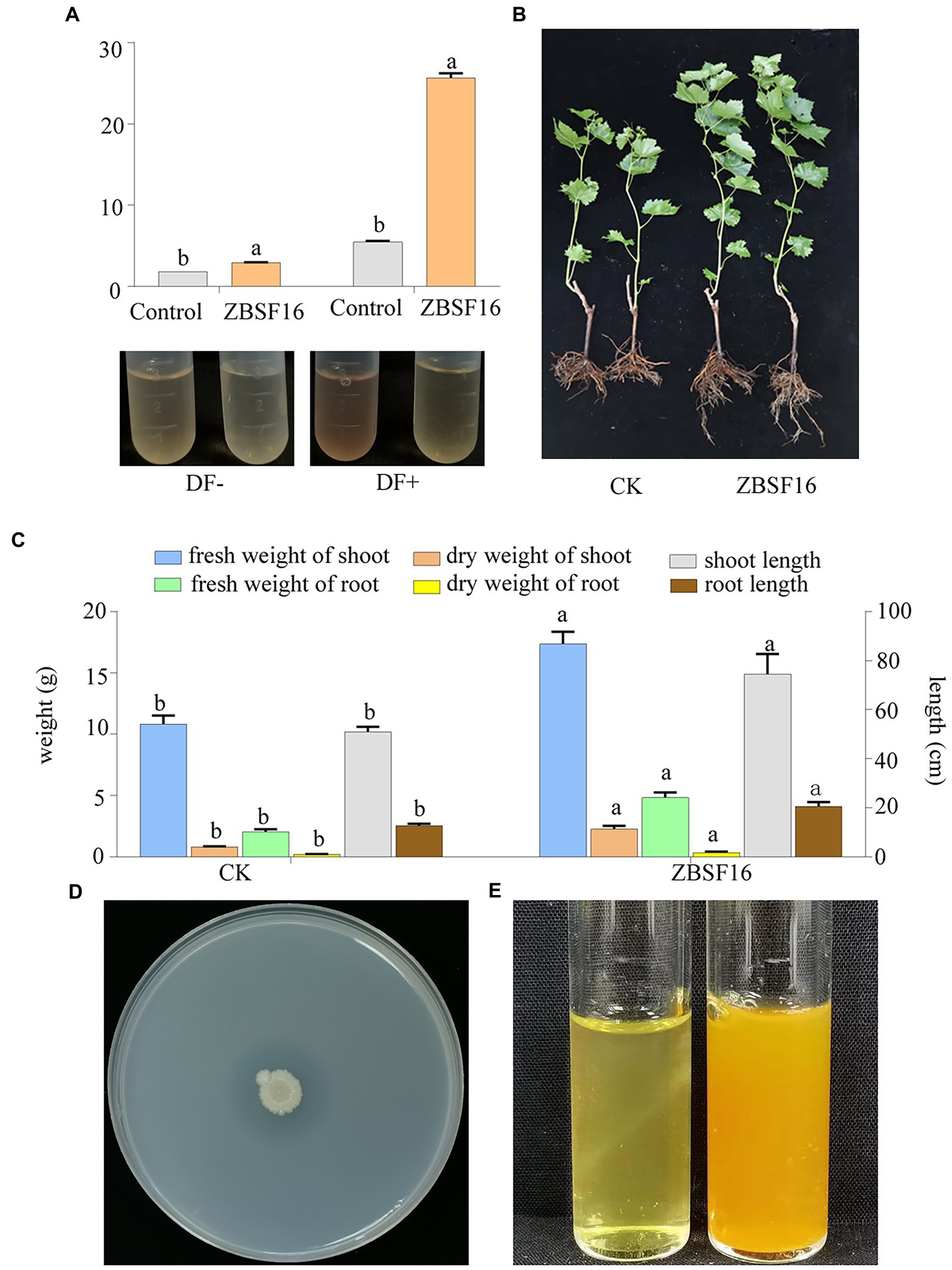
Figure 2. Determination of the plant growth-promoting properties of Paenibacillus peoriae ZBSF16. (A) IAA production of P. peoriae ZBSF16. DF−, DF medium without L-tryptophan; DF+, DF medium containing L-tryptophan. (B,C) The growth-promoting effect of Paenibacillus peoriae ZBSF16 on grape; (D) mineral phosphate solubilization of P. peoriae ZBSF16; (E) ammonia production of P. peoriae ZBSF16.
Two treatments were performed to determine the preventive effect and control effect of strain ZBSF16. The results demonstrated that strain ZBSF16 displayed excellent biocontrol traits for grape white rot disease (Figures 1B,C), with the preventive effects for detached leaf and detached fruit being 90.59 and 94.52%, respectively. The control effects for detached leaves and detached fruit were 94.52 and 84.70%, respectively (Supplementary Figures 2B,C).
The strain ZBSF16 exhibited resistance to ampicillin, chloramphenicol, tetracycline, gentamycin, rifampicin, kanamycin and vancomycin but not to streptomycin or spectinomycin. In addition, strain ZBSF16 showed an MIC of spectinomycin of 216 μg/ml and an MBC of 1,024 μg/ml (Supplementary Figures 3A–C). Meanwhile, the strain was unable to produce hemolysin activity on plates according to the blood agar hemolysis assay (Supplementary Figure 3D).
The completed genome of the rod-shaped bacterium P. peoria ZBSF161 has been shown to be composed of one circular chromosome of 5,839,239 bp in size, with an average G + C content of 45.62% (Figure 3). The details of the assembly information and genomic features are summarized in Supplementary Tables 3, 4. A total of 5,188 predicted genes were identified in the genome, including 4,944 protein-coding sequences, 39 ribosomal RNA operons, 109 tRNAs, and 4 other RNAs. Genes associated with carbohydrate transport and metabolism (7.98%) were the highest density, followed by transcription (7.51%), amino acid transport and metabolism (5.59%), inorganic ion transport and metabolism (4.81%), signal transduction mechanisms (3.82%), replication, cell wall/membrane/envelope biogenesis (3.92%), replication, recombination, and repair (3.61%) and energy production and conversion (3.31%) (Figure 3). In addition, four crisprs were involved in ZBSF16, and the length of the repeated sequences ranged from 19 to 30 bp (Supplementary Table 4).
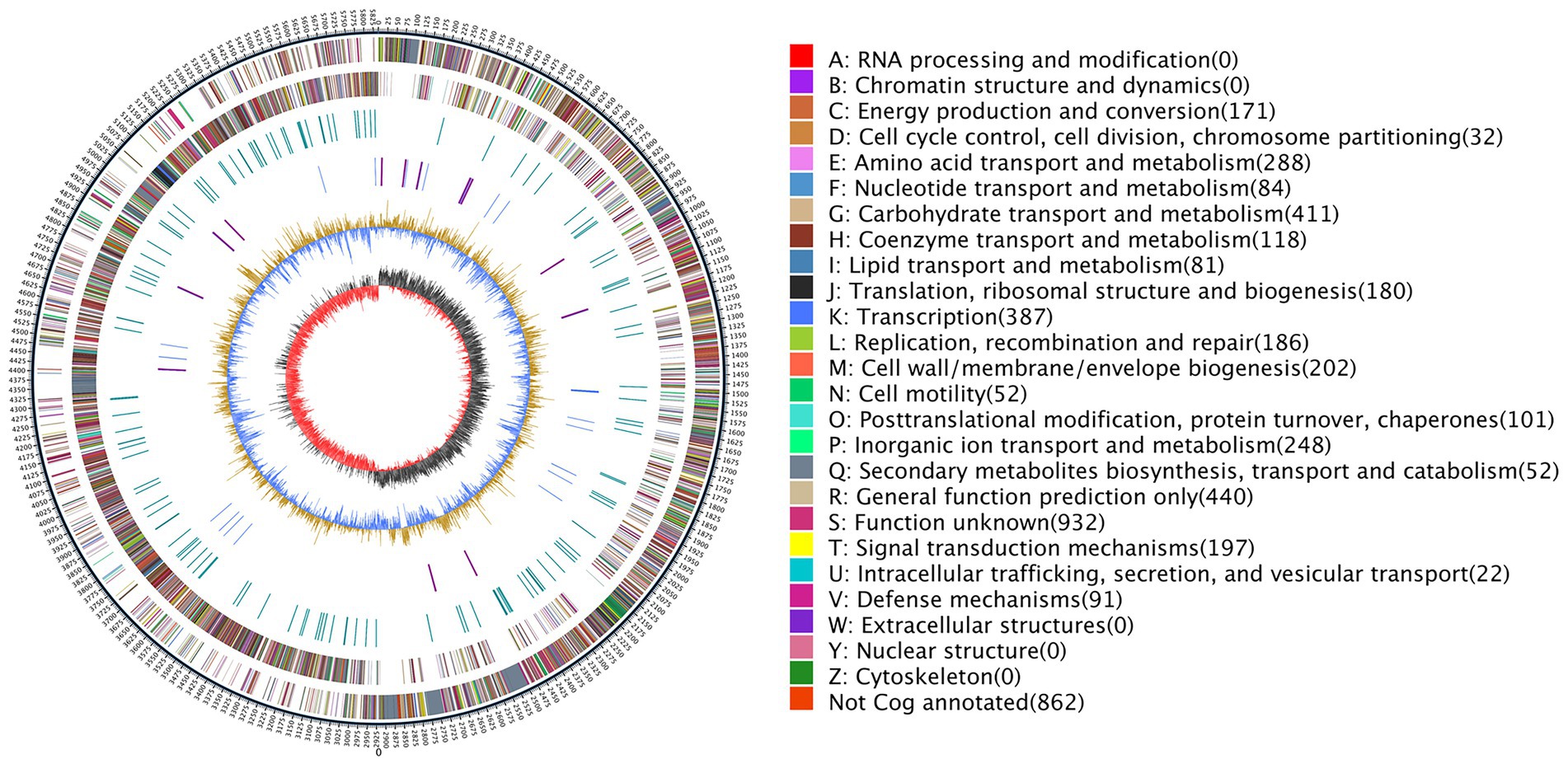
Figure 3. Genome map of Paenibacillus peoriae ZBSF16. The distribution of the circle from the outside indicates the genome size, forward CDS, reverse CDS, repeat sequence, tRNA (blue), rRNA (purple), GC ratio (yellow and blue indicate regions where the GC ratio is higher than average and lower than average, respectively), and CG skew positive (dark) and negative (red).
To determine the relationships of P. peoria ZBSF16 with Paenibacillus spp. strains, phylogenetic trees based on the 16S rRNA gene sequences were built. The result indicated that ZBSF16 was close to the strain P. peoria ZF390; however, P. kribbensis AM49 and P. peoria ZF390 were in a clade (Supplementary Figure 4A). Additionally, strain ZBSF16 was clearly classified as P. peoria in the phylogenetic tree based on the MLSA, and P. peoria ZBSF16 was most closely related to strains P. peoria ZF390, P. peoria HS311 and P. peoria HJ-2 (Supplementary Figure 4B). PhyloPhlAn method was performed to verify the evolutionary position. As expected, P. peoria ZBSF16 was most closely related to strains P. peoria ZF390, P. peoria HS311 (Figure 4).
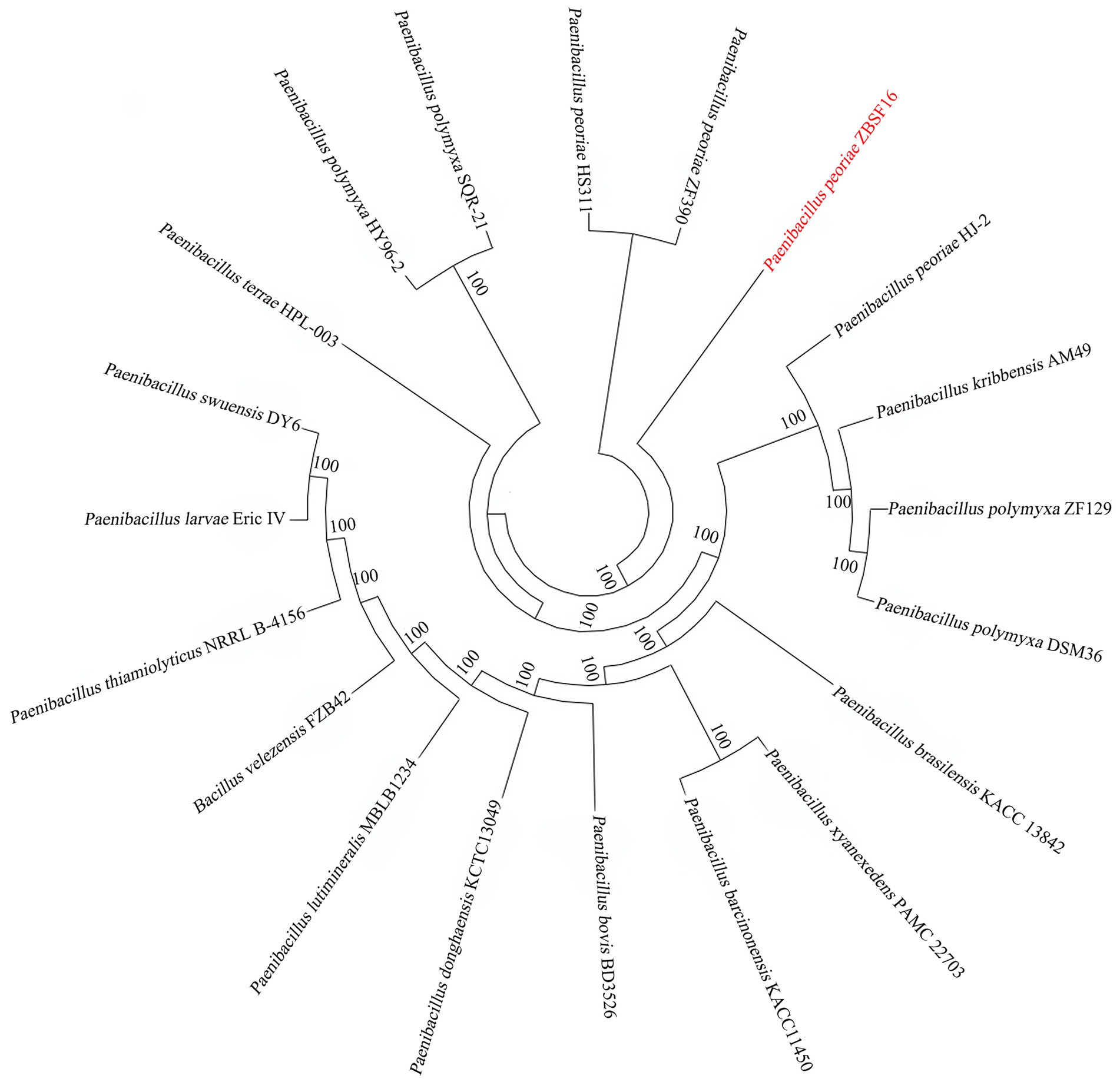
Figure 4. Phylogenetic analysis of Paenibacillus peoriae ZBSF16 against six other Paenibacillus from genomes using PhyloPhlAn 3.0.2.
Average nucleotide identity (ANI) and DNA–DNA hybridization (DDH) are powerful approaches for evolutionary distance assessment between bacteria at the genomic level, and compared strains usually with ANI values > 96% and DDH values ≥ 70% are regarded as the same species (Richter and Rosselló-Móra, 2009; Jiang et al., 2022). ANI values showed that ZBSF16 between P. peoria ZF390, HS311 and HJ-2 were 95.22, 95.23 and 95.24%, respectively. However, the DDH value between ZBSF16 and P. peoria HS311 was > 70% (Supplementary Figure 4). Obviously, ZBSF16 did not belong to P. polymyxa and P. kribbensis, according to the lower ANI values (< 91%) and DDH values (< 50%; Supplementary Figure 5).
In comparison, the entire genome size of the four P. peoriae strains ranged from 5.84 to 6.19 Mb, the G + C content ranged from 44.99 to 45.62%, and the predicted coding genes ranged from 5,188 to 5,894. Furthermore, the genomes of strains ZF390 and HS311 contained three and one plasmids, respectively. ZBSF16 and HJ-2 both contained one circular chromosome, and the additional genomic features of the six strains are described in Table 1.
To evaluate the evolutionary distance among these sequenced strains in relation to several Paenibacillus strains, the genome sequence of ZBSF16 was compared to three sequenced P. peoriae strains (ZF390, HS311 and HJ-2), two P. polymyxa strains (HY96-2 and SQR-21) and one P. kribbensis (AM49) by mauve. The alignments among Paenibacillus strains are presented in Figure 5A. Horizontal gene transfer was obviously observed among Paenibacillus strains, and the ZBSF16 genome is much more similar to HS311 than to ZF390 within P. peoriae strains based on comparative analysis. There were 3,479 conserved genes shared by the seven sequenced strains of the Paenibacillus strains, and 3,960 genes were shared within the four sequenced P. peoriae strains, including ZBSF16, ZF390, HS311 and HJ-2. In detail, ZBSF16 shared 4,152, 4,143 and 4,135 genes with ZF390, HS311 and HJ-2, respectively. Furthermore, 357 unique genes were present in the genome of P. peoriae ZBSF16, genomes with their unique regions are presented in circular images (Figure 5B), and the functions of most unique genes are still unknown. Notably, only 3,772 genes were shared by ZBSF16 and P. kribbensis AM49, which is less than those in The P. polymyxa strains (Figures 5B,C; Supplementary Figure 6).
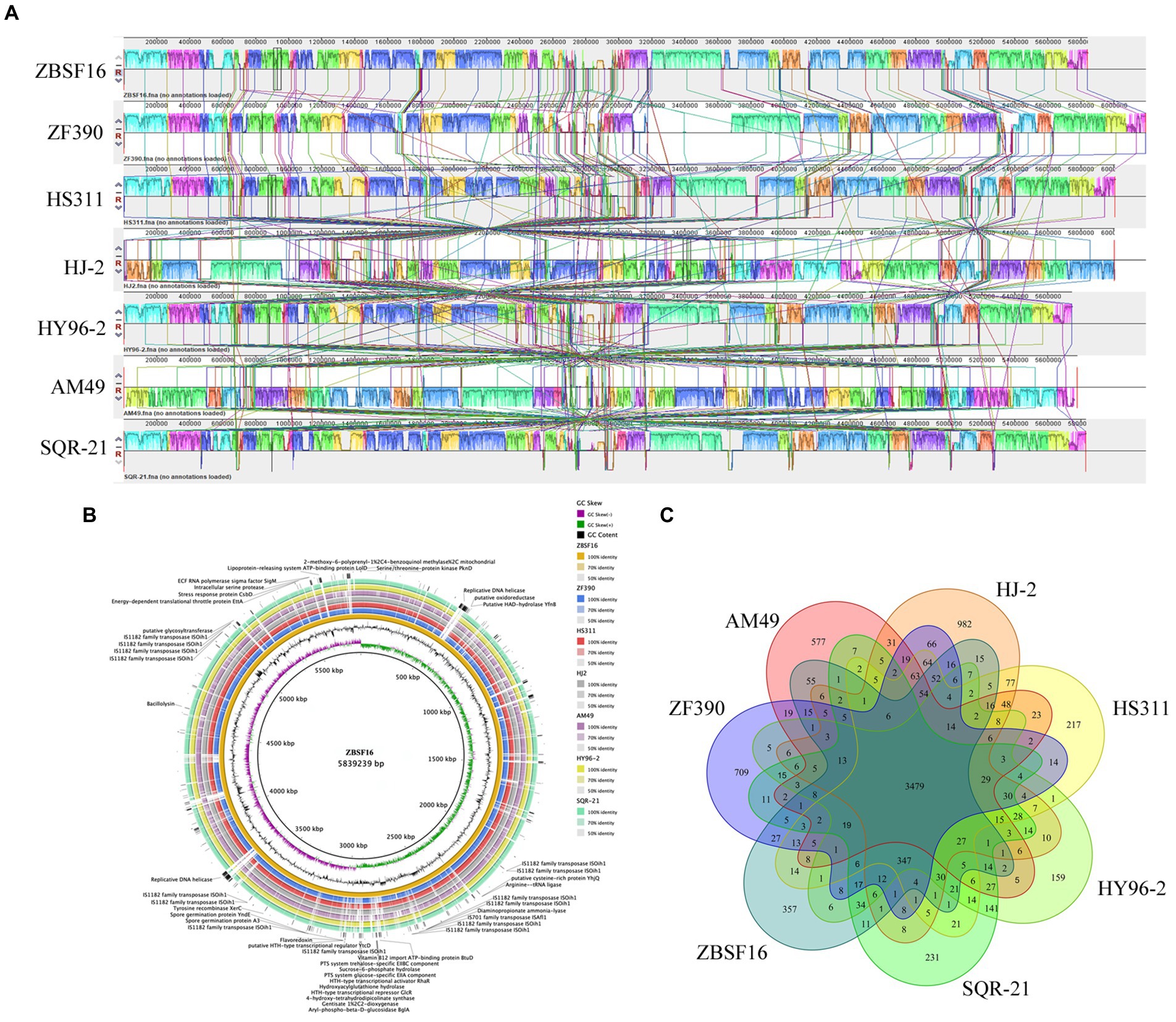
Figure 5. Comparison of Paenibacillus peoriae ZBSF16 genome sequences against six other Paenibacillus genome sequences. (A) Synteny analysis of P. peoriae ZBSF16 with the P. peoriae ZF390, P. peoriae HS311, P. peoriae HJ-2, P. polymyxa HY96-2 P. polymyxa SQR-21 and P. kribbensis AM49 genomes. Pairwise alignments of the genomes were generated using MAUVE. The genome of strain ZBSF16 was used as the reference genome. Boxes with the same color indicate syntenic regions. Boxes below the horizontal strain line indicate inverted regions. Rearrangements are shown by colored lines. Scale is in nucleotides. (B) Pangenome analysis with closely related strains identified the unique genes present in the query genomes that are highlighted in the outermost circle, the strain ZBSF16 as the query genome is placed in the innermost circle. (C) Venn diagram showing the number of clusters of orthologous genes shared and unique genes.
IAA is an important phytohormone that controls cell enlargement and tissue differentiation in plants. In this study, ZBSF16 showed a higher IAA biosynthetic capacity (28.67 μg/ml; Figure 2A), and 12 genes related to IAA biosynthesis were identified in strain ZBSF16. Nine genes in the IAA biosynthesis pathway were shared among the four P. peoriae strains with homology higher than 90%, except for three genes (trpE, trpG and trpCF) that were not found in strain HJ-2 (Table 2). As a major essential nutrient, phosphorus and nitrogen are necessary for the growth and development of plants, and ZBSF16 exhibits the capability of phosphate solubilization and nitrogen fixation (Figure 2D). Additionally, comparative genome analysis showed 14 genes related to phosphate solubilization in ZBSF16, which was highly similar to ZF390, HS311 and HJ-2, and the gene iap, which is shared by strain ZF390 and HS311 (Table 2). Furthermore, 15 genes responsible for nitrogen fixation were all found in the genomes of ZBSF16, HS311 and HJ-2, most of which were highly conserved, with sequence identities ranging from 93 to 100%. However, nifH, nifN, nifB, nifD, nifE, nifK, nifX and hesA were absent in strain ZF390 (Table 2). Meanwhile, 30 genes involved in flagella and 12 genes related to biofilm formation were discovered in strain ZBSF16, and 40 genes involved in flagella (except for fliD and fliS) and biofilm formation exhibited high conservation (> 88%) in ZF390, HS311, HJ-2 and ZBSF16 (Supplementary Tables 5, 6). Quorum sensing (QS) relegated many traits of bacteria, including biofilm formation and colonization. QS is conserved across hundreds of species belonging to the Paenibacillaceae family, and seven genes related to QS were identified in P. peoriae strains in this study (Supplementary Table 7). Additionally, 11 genes associated with the chemtaxis and two-component systems (TCS), except CitG and DcuS, were conservative in different strains of P. peoriae (Supplementary Table 8).
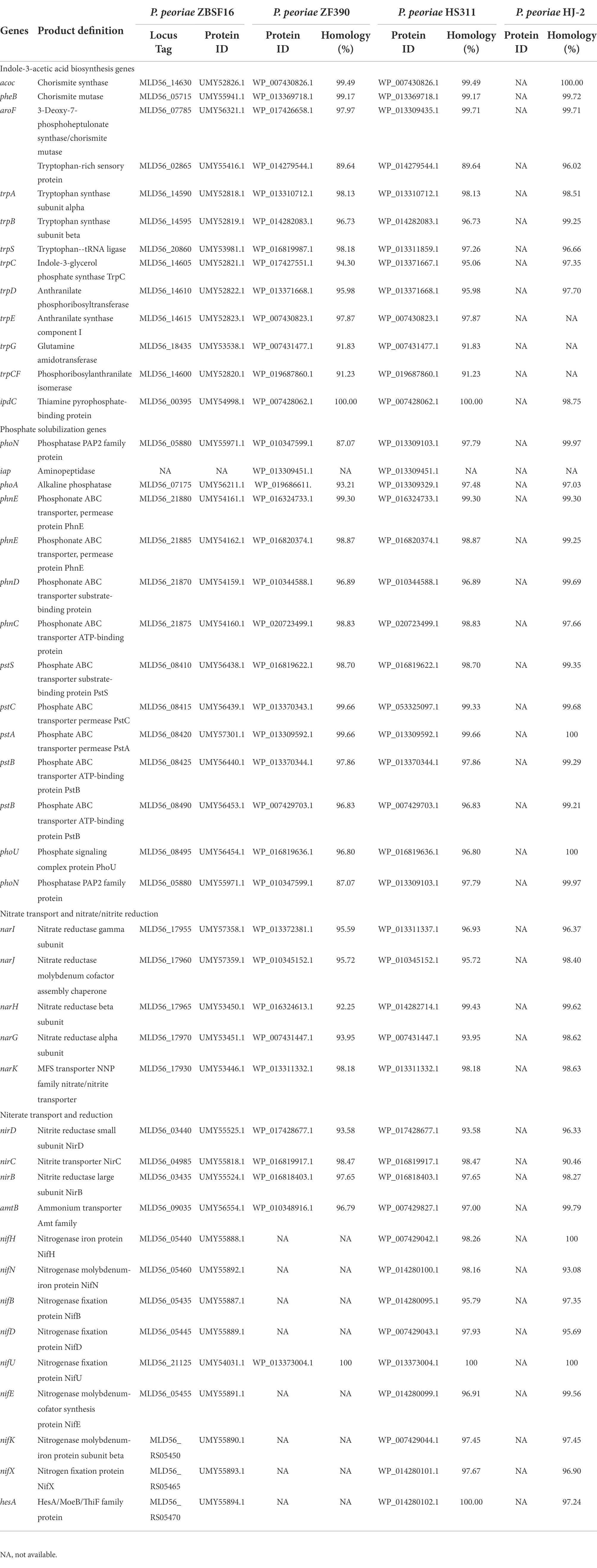
Table 2. Homolog analysis of genes involved in plant growth promotion in Paenibacillus peoriae ZBSF16 and other P. peoriae strains.
P. peoriae ZBSF16 showed potent broad-spectrum antifungal activities. Based on the antiSMASH database, 14 clusters related to secondary metabolite synthesis were identified in ZBSF16. Among these gene clusters, three clusters (Cluster 1 related to fusarcidinB, Cluster 8 related to cyclic-lactone-autoinducer, and Cluster 9 related to tridecaptin) were shared among the four P. peoriae strains, the two P. polymyxa and P. kribbensis; the functions of fusarcidin B and tridecaptin were antifungal and antibacterial, respectively. Cluster 3 related to paenibacillin was specific and only found in strain ZBSF16, which was a kind of lantibiotic. In addition, polymyxin and paenilan did not appear in P. kribbensis, paeninodin could not be detected in P. polymyxa, and genes related to Cluster 17 encoding the biosynthesis of paenilan, pelgipeptin, aurantinin and so on were not found in ZBSF16 (Figure 6; Supplementary Table 9).
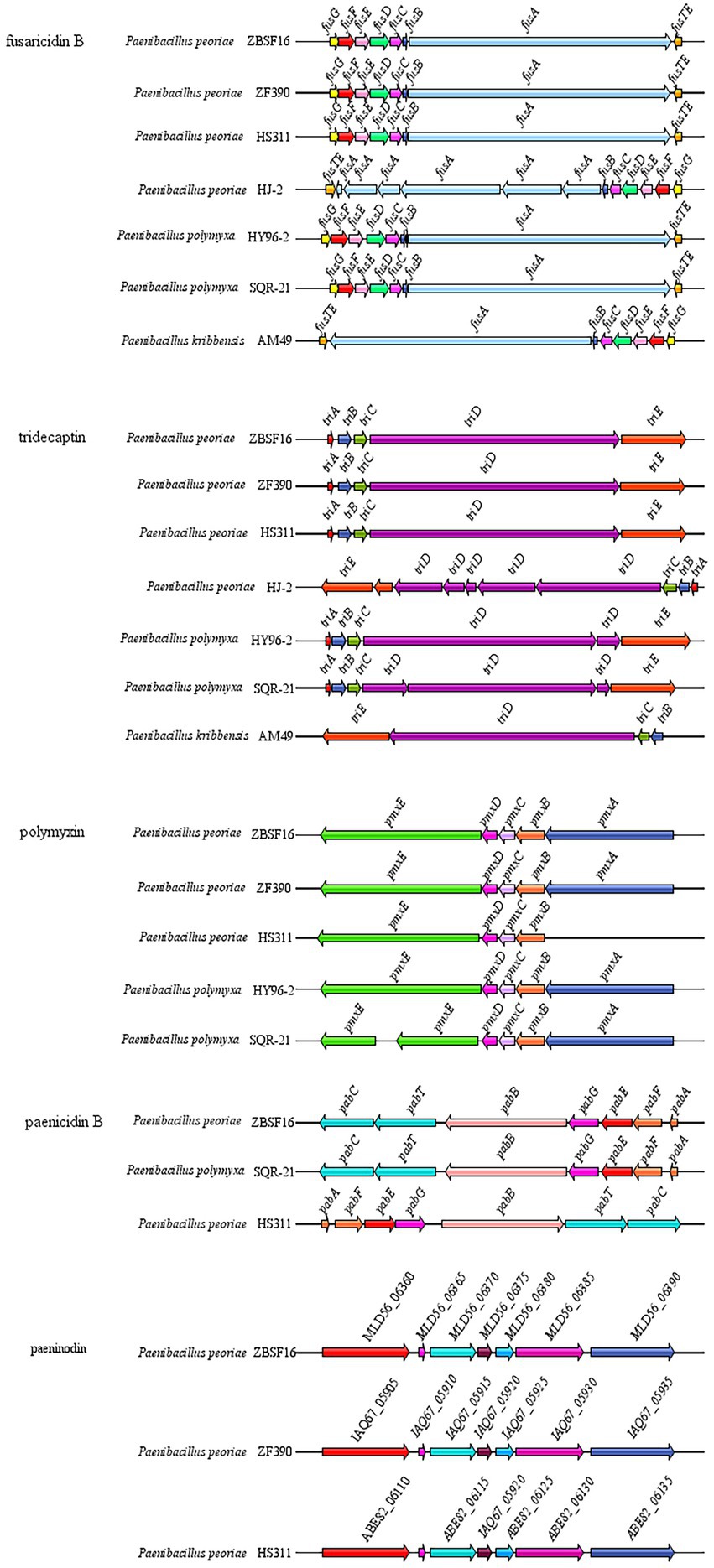
Figure 6. Comparison of antibiotic synthesis clusters of Paenibacillus strains. Antibiotic synthesis clusters were identified using antiSMASH, and gene cluster intraspecific genes were compared.
The resistance inducer biosynthesis gene cluster, including 11 genes related to ISR and 3 genes involved in PAMP-triggered immunity (PTI), was analyzed in strain ZBSF16, which is highly conserved in the selected P. peoriae strains (> 79% identity). The genes alsS and budA were identified in strain ZBSF16, which showed a lower similarity to ZF390. The gene flgL involved in PTI of plants showed higher similarity to ZF390, and it could not be identified in strains HS311 and HJ-2 (Table 3).
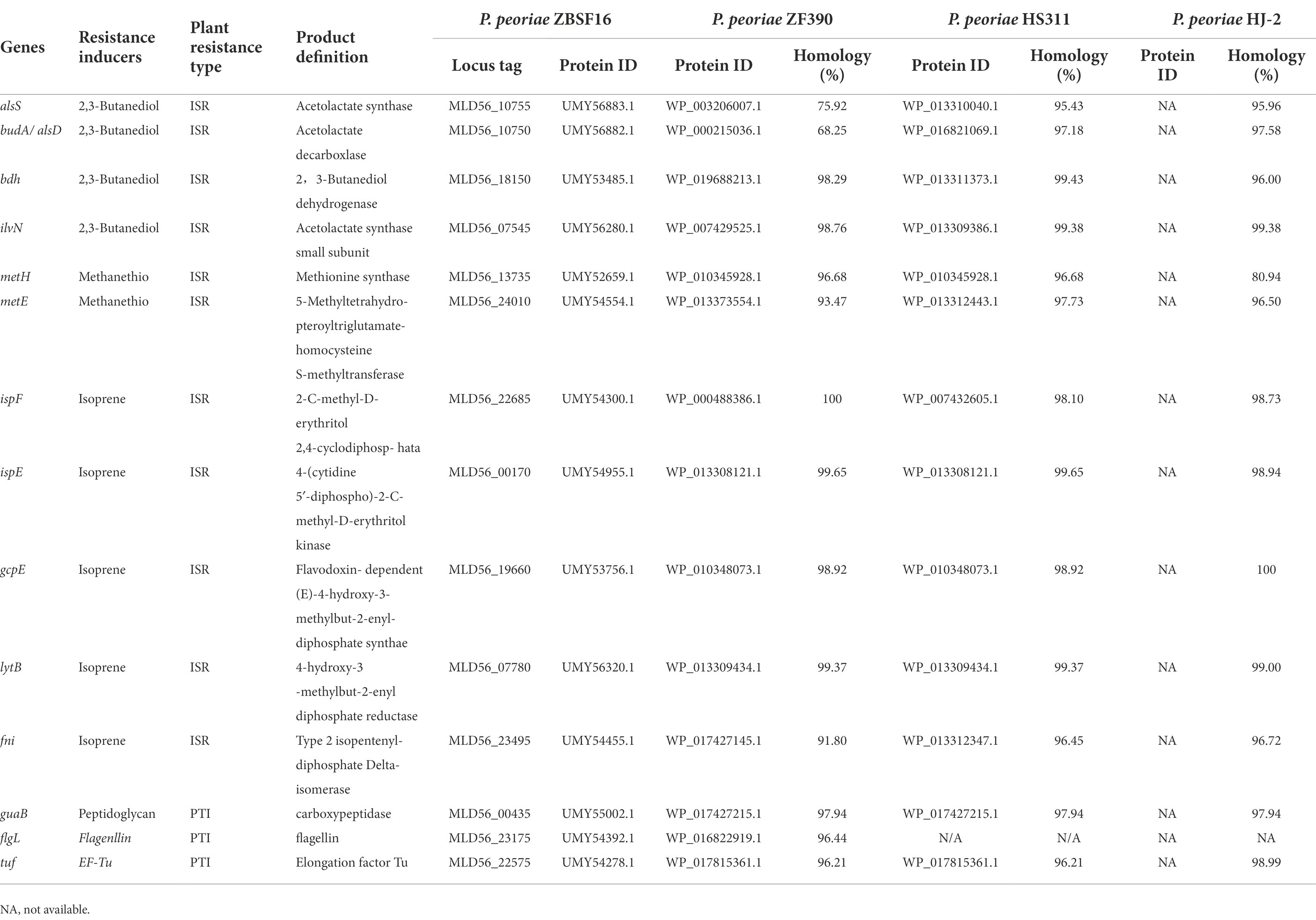
Table 3. Genes related to synthesis resistance inducer in Paenibacillus peoriae ZBSF16 and other P. peoriae strains.
Paenibacillus is widely distributed in a variety of environments, including wetlands, meadow soil, desert sand, oceans, wheat soil rhizospheres, cucumber greenhouses and infected honeybees (Jeon et al., 2009; Wang et al., 2013; Ahn et al., 2014). The genus Paenibacillus is reported to have the ability to promote the growth of many plants, such as maize, wheat, tomato, and pumpkin (Hao and Chen, 2017; Dixit et al., 2018). The genome size of Paenibacillus species ranges from 3.02 Mbp to 8.82 Mbp. As a member of 200 species in Paenibacillus, P. peoriae was described to play a role in promoting the growth of plants by some studies in the past and was confirmed in this study (Figure 2), with a genome size of 5.74–6.19 Mbp and GC content of 44.99–45.62% (Table 1). P. peoriae was close to P. polymyxa and P. kribbensis in terms of evolutionary status, and ZBSF16 was identified and confirmed to belong to P. peoriae by ANI and DDH. Compared to P. peoriae HJ-2, which presented antagonistic activity against Fusarium spp., ZBSF16 had a broad antifungal and antibacterial spectrum, which could protect against 10 species of fungi and 2 species of bacteria.
Many PGPRs, including Bacillus, Rahnella, Pseudomonas, Klebsiella, Agrobacterium and Paenibacillus sp. can produce IAA to stimulate the growth of plants, and Paenibacillus nonsymbiotic bacteria yielded high concentrations of IAA (in the range of 4.90–0.19 IAA/mg biomass; Shokri and Emtiazi, 2010; Trinh et al., 2018). P. polymyxa, P. borealis, and P. terrae showed the secretion of a significant amount of IAA, but no P. graminis had the ability to produce IAA (Navarro-Noya et al., 2012; Kim et al., 2017). P. peoriae HJ-2 isolated from soil significantly promoted the growth of P. polyphylla, and P. peoriae ZBSF16 for the first time was used to describe the ability to synthesize IAA and promote the growth of grape, with IAA production of 28.67 μg ml−1. The various pathways for IAA biosynthesis include tryptophan (Trp), tryptamine (Tam), indole-3-pyruvic acid (IPyA) and indole-3-acetamide (IAAm) pathways, and the IPyA pathway was suggested in Paenibacillus because of the absence of tryptophan monooxygenase or indole-3-acetamide hydrolase (Mano and Nemoto, 2012; Xie et al., 2016). In addition, the ipdC gene, encoding a key enzyme in the IPyA pathway, is shared in all Paenibacillus (Xie et al., 2016). In this study, ipdC homologies were present in all sequenced P. peoriae, which demonstrated that P. peoriae may rely on the IPyA pathway for IAA synthesis.
P. polymyxa strains have long been known to solubilize phosphate, which carries the phn genes (phnABCDEWXM) responsible for solubilizing organic phosphate (Zhou et al., 2020; Soni et al., 2021). The phnB gene was absent in some species of Paenibacillus, including P. beijingensis 1–18, P. peoriae KCTC 3763 and P. terrae HPL-003 (Jeong et al., 2012; Shin et al., 2012; Li L. et al., 2019). In this study, phnA and phnB were not found in the genomes of P. peoriae. The Pst (phosphate-specific transport) system is a major transport system for Pi. The pst operon of Paenibacillu is composed of pstS, pstC, pstA and pstB (Li et al., 2020), and the four pst genes were all present in P. peoriae ZBSF16, which contribute to the solubilization of phosphate. It has been reported that Rahnella aquatilis ZF7 can produce acid, which may have high activity for solubilizing organic phosphate (Yuan et al., 2020). A higher phosphate solubilization ability of P. peoriae ZBSF16 was observed, although the pH value of ZBSF16 remained alkaline when cultured.
Nitrogen fixation is one characteristic of the genus Paenibacillus, and more than 20 species of the genus Paenibacillus can fix nitrogen (Grady et al., 2016; He et al., 2021). Nitrogen fixation is mainly catalyzed by Mo-nitrogenase, and the nif gene cluster (nifB, nifH, nifD, nifK, nifE, nifN, nifX, hesA and nifV) encoding Mo-nitrogenase is shared in N2-fixing Paenibacillus strains (Xie et al., 2014). When the nif gene cluster is lost, non-N2-fixing strains are produced, such as P. peoriae KTCT 3763, P. polymyxa SC2 and P. polymyxa E681 (Kim et al., 2010; Ma et al., 2011). When acquiring the vnf and anf genes, strains of vnfHDGKEN encoding V-nitrogenase and anfHDGK encoding Fe-nitrogenase appeared, such as P. azotofixans ATCC 35681 and P. forsythia T98 (Xie et al., 2014, 2016). Most likely due to gene loss, the nifV gene was absent in the gene cluster in P. peoriae ZBSF16, but ZBSF16 retained its nitrogen-fixing capacity.
The genus Paenibacillus is known for its ability to produce antibacterial metabolites, including fusaricidins, pelgipeptin, surfactins and polymyxins (Grady et al., 2016). The antibacterial metabolites of P. polymyxa ZF129 and P. polymyxa ZF197 were significantly different, but paeninodin, fusaricidin, paenibacterin and tridecaptin were shared by the two strains (Li et al., 2020). In our study, fusaricidin B, tridecaptin, polymyxin and paenicidin B were found in P. peoriae ZBSF16, which contribute to its strong antipathogenic activities. In addition, fusaricidin B, tridecaptin and polymyxin were conserved in P. peoriae, P. polymyxa and P. kibbensis, which were also shared in P. polymyxa ZF129 and P. polymyxa ZF197. The antifungal mechanism of fusaricidin is permeabilization and disruption of cell membranes (Jiang et al., 2022), which may be one of the reasons why P. peoriae ZBSF16 showed a broad antifungal spectrum.
ISR is the form of induced resistance wherein plant defenses are preconditioned by prior treatment that results in resistance against subsequent challenge by a pathogen or parasite (Choudhary et al., 2007). ISR can increase systemic levels of the plant hormone salicylic acid (SA) and trigger the jasmonic acid/ethylene pathway. Paenibacillus-mediated ISR has been demonstrated against fungi (e.g., C. truncatum, C. orbiculare and F. oxysporum) and bacteria (e.g., Xanthomonas axonopodis pv. vesicatoria, Erwinia carotovora subsp. carotovora) in pepper, cucumber, banana, and Arabidopsis thaliana (Sang et al., 2014; Nakkeeran et al., 2021; Yadav M. et al., 2021). Nine genes involved in ISR were explored in P. polymyxa, with higher sequence identity (> 95%) in different strains, while key genes associated with volatile organic compounds (2,3-butanediol, methanethiol and isoprene) were contained (Li et al., 2020). A total of 12 genes related to ISR were found in P. peoriae ZBSF16, which were highly similar to those in P. polymyxa (homology > 99%). The results demonstrated that P. peoriae and P. polymyxa could induce similar systemic resistance in plants.
P. peoriae ZBSF16 showed broad-spectrum antagonistic activities against 12 plant pathogens and exhibited obvious biocontrol effects against grape white rot disease. The aim of this study was to reveal the plant growth-promoting and biocontrol mechanisms of P. peoriae. Whole-genome analysis and phylogenetic analysis revealed that ZBSF16 belongs to P. peoriae and is closely related to P. peoriae ZF390. Comparative analysis of the genome of P. peoriae ZBSF16 with other Paenibacillus spp. indicated that ZBSF16 harbored many genes related to IAA production, nitrogen fixation, phosphate solubilization, biofilms and flagella, which have been proven to be beneficial to plant growth. In addition, genes associated with antibiotic synthesis and induction of resistance were identified. Overall, the features of P. peoriae ZBSF16 make it a high-probability biocontrol agent and biofertilizer, and these results will contribute to in-depth research on the mechanisms of plant growth promotion and biocontrol.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: NCBI GenBank - CP092831.1.
LY, YW, and XY conceived and designed the experiments. XY, HJ, TL, PL, and XJ performed the experiments and analyzed the data. LY and YW wrote the manuscript. TL, XJ, PL, and HJ revised the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by Shandong Provincial Natural Science Foundation (ZR2021QC131), Innovation Project of Shandong Academy of Agricultural Sciences (CXGC2022E15), and Shandong Academy of Grape Guide Fund (SDAG2021B06, SDAG2021B10, and SDAG2021B02).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.975344/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | General characteristics of Paenibacillus peoriae ZBSF16. (A) Image of ZBSF16 colony morphology. (B) Image of ZBSF16 cells via scanning electron microscopy. (C) Growth dynamics and pH change of P. peoriae ZBSF16. Bars plot the means ± standard deviation of three replicate experiments. P (D) Production of protease. (E) Cellulose degradation. (F) Production of lipase. Determination of NaCl (G) and pH (H) tolerance capabilities of P. peoriae ZBSF16.
SUPPLEMENTARY FIGURE 2 | Antagonistic assay and biocontrol effect of Paenibacillus peoriae ZBSF16. (A) Colony radius and inhibition rate of each microorganism. Bars plot the means ± standard deviation of three replicate experiments. Coniella vitis (CV). Gloeosporium fructigrum (GF). Pestalotiopsis clavispora (Pc). Alternaria viticola (Av). Diaporthe eres (DE). Fusarium oxysporum (Fo). Botrytis cinerea (BC). Botryosphaeria dothidea (BD). Aspergillus niger (AN). Fusarium graminearum (FG). Fusarium pseudograminearum (FP). Allorhizobium vitis (ALV). (B,C) Incidence, disease index and control efficiency of P. peoriae ZBSF16. (a1, b1) Inoculated with C. vitis; (a2, b2) LB broth; (a3, b3) sterile water; (a4, b4) culture of ZBSF16; (a5, b5) inoculated with C. vitis 24 h after inoculation with the culture of ZBSF16; (a6, b6) inoculated culture of ZBSF16 24 h after inoculation with C. vitis. (D) Disease symptoms and growth state of Vitis vinifera (cv. Red globe) inoculated with strain ZBSF16. (E) The infection rate and disease index of grape white rot on Vitis vinifera (cv. Red globe) inoculated with strain ZBSF16. CK plants were treated with sterile water. Different letters above the bars denote a significant difference at p < 0.05 according to Duncan’s multi-range test.
SUPPLEMENTARY FIGURE 3 | Determination of antibiotic resistance of Paenibacillus peoriae ZBSF16. (A) Survival of P. peoriae ZBSF16 treated with different antibiotics. Spectinomycin (Spe), streptomycin (Str), ampicillin (Amp), vancomycin (Van), kanamycin (Kan), gentamycin (Gen), chloramphenicol (Chl), tetracycline (Tet) and rifampicin (Rif). (B) Minimum inhibitory concentration (MIC) of spectinomycin for strain ZBSF16. (C) Minimum bactericidal concentration (MBC) of spectinomycin for strain ZBSF16. (D) Hemolysis assay of ZBSF16. (E) Siderophores production of P. peoriae ZBSF16. (F) Population dynamics of P. peoriae ZBSF16 in the rhizosphere soil of grape.
SUPPLEMENTARY FIGURE 4 | (A) Phylogenetic tree for P. peoriae ZBSF16 and the genus Paenibacillus based on 16S rRNA (Bacillus velezensis FZB42 was used as an outgroup). (B) Phylogenetic tree of Paenibacillus peoriae ZBSF16 among other Paenibacillus species. The phylogenetic tree was constructed based on five housekeeping genes (16S rRNA, gyrB, rpoD, rho, and pgk) according to the aligned gene sequences using the maximum likelihood method in MEGA 6.0. Bootstrap values (1,000 replicates) are shown at the branch points. The scale bar indicates 0.05 nucleotide substitutions per nucleotide position. GenBank accession numbers associated with the housekeeping loci of all strains can be found in Supplementary Table 1.
SUPPLEMENTARY FIGURE 5 | ANI (A) and DDH (B) value matrix heatmap between Paenibacillus peoriae ZBSF16 and six other Paenibacillus genome sequences.
SUPPLEMENTARY FIGURE 6 | Venn diagram showing the number of clusters of orthologous genes shared and unique genes.
Abriouel, H., Franz, C. M., Ben Omar, N., and Gálvez, A. (2011). Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 35, 201–232. doi: 10.1111/j.1574-6976.2010.00244.x
Ahemad, M. (2015). Phosphate-solubilizing bacteria-assisted phytoremediation of metalliferous soils: a review. 3 Biotech 5, 111–121. doi: 10.1007/s13205-014-0206-0
Ahn, J. H., Kim, B. C., Kim, B. Y., Kim, S. J., Song, J., Kwon, S. W., et al. (2014). Paenibacillus cucumis sp. nov. isolated from greenhouse soil. J. Microbiol. 52, 460–464. doi: 10.1007/s12275-014-4071-7
Alikhan, N. F., Petty, N. K., Zakour, N. L., and Ben Beatson, S. A. (2011). BLAST ring image generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12, 1–10. doi: 10.1186/1471-2164-12-402
Ash, C., Priest, F. G., and Collins, M. D. (1993). Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Proposal for the creation of a new genus Paenibacillus. Antonie Van Leeuwenhoek 64, 253–260. doi: 10.1007/BF00873085
Asnicar, F., Thomas, A. M., Beghini, F., Mengoni, C., Manara, S., Manghi, P., et al. (2020). Precise phylogenetic analysis of microbial isolates and genomes from metagenomes using Phylo PhlAn 3.0. Nat. Commun. 11, 2500. doi: 10.1038/s41467-020-16366-7
Baindara, P., Chaudhry, V., Mittal, G., Liao, L. M., Matos, C. O., Khatri, N., et al. (2015). Characterization of the antimicrobial peptide penisin, a class Ia novel lantibiotic from Paenibacillus sp. strain A3. Antimicrob. Agents Chemother. 60, 580–591. doi: 10.1128/AAC.01813-15
Brillard, J., Ribeiro, C., Boemare, N., Brehelin, M., and Givaudan, A. (2001). Two distinct hemolytic activities in Xenorhabdus nematophila are active against immunocompetent insect cells. Appl. Environ. Microb. 67, 2515–2525. doi: 10.1128/AEM.67.6.2515-2525.2001
Chethana, K. W. T., Zhou, Y., Zhang, W., Liu, M., Xing, Q. K., Li, X. H., et al. (2017). Coniella vitis sp. nov. is the common pathogen of white rot in Chinese vineyards. Plant Dis. 101, 2123–2136. doi: 10.1094/pdis-12-16-1741-re
Choudhary, D. K., Prakash, A., and Johri, B. N. (2007). Induced systemic resistance (ISR) in plants: mechanism of action. Indian J. Microbiol. 47, 289–297. doi: 10.1007/s12088-007-0054-2
Darling, A. C., Mau, B., Blattner, F. R., and Perna, N. T. (2004). Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 14, 1394–1403. doi: 10.1101/gr.2289704
DasGupta, S. M., Khan, N., and Nautiyal, C. S. (2006). Biologic control ability of plant growth-promoting Paenibacillus lentimorbus NRRL B-30488 isolated from milk. Curr. Microbiol. 53, 502–505. doi: 10.1007/s00284-006-0261-9
Dixit, R., Agrawal, L., Singh, S. P., Prateeksha,, Singh, P. C., Prasad, V., et al. (2018). Paenibacillus lentimorbus induces autophagy for protecting tomato from Sclerotium rolfsii infection. Microbiol. Res. 215, 164–174. doi: 10.1016/j.micres.2018.07.008
Elhaissoufi, W., Khourchi, S., Ibnyasser, A., Ghoulam, C., Rchiad, Z., Zeroual, Y., et al. (2020). Phosphate solubilizing rhizobacteria could have a stronger influence on wheat root traits and aboveground physiology than rhizosphere P. solubilization. Front. Plant Sci. 11, 979. doi: 10.3389/fpls.2020.00979
Emmanouil, A., Markakis Sotirios, E., Tjamos Polymnia, P., Antoniou Epameinondas, J., and Paplomatas Eleftherios, C. T. (2016). Biological control of Verticillium wilt of olive by Paenibacillus alvei, strain K165. Biol. Control 61, 293–303. doi: 10.1007/s10526-015-9669-0
Garcia-Seco, D., Zhang, Y., Gutierrez-Mañero, F. J., Martin, C., and Ramos-Solano, B. (2015). Application of Pseudomonas fluorescens to blackberry under field conditions improves fruit quality by modifying flavonoid metabolism. PLoS One 10:e0142639. doi: 10.1371/journal.pone.0142639
Goris, J., Konstantinidis, K. T., Klappenbach, J. A., Coenye, T., Vandamme, P., and Tiedje, J. M. (2007). DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 57, 81–91. doi: 10.1099/ijs.0.64483-0
Grady, E. N., MacDonald, J., Liu, L., Richman, A., and Yuan, Z. C. (2016). Current knowledge and perspectives of Paenibacillus: a review. Microb. Cell Factories 15, 203. doi: 10.1186/s12934-016-0603-7
Hao, T., and Chen, S. (2017). Colonization of wheat, maize and cucumber by Paenibacillus polymyxa WLY78. PLoS One 12:e0169980. doi: 10.1371/journal.pone.0169980
Hashem, A., Tabassum, B., and Fathi Abd Allah, E. (2019). Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi. J. Biol. Sci. 26, 1291–1297. doi: 10.1016/j.sjbs.2019.05.004
He, X., Li, Q., Wang, N., and Chen, S. (2021). Effects of an EPS biosynthesis gene cluster of Paenibacillus polymyxa WLY78 on biofilm formation and nitrogen fixation under aerobic conditions. Microorganisms. 9, 289. doi: 10.3390/microorganisms9020289
Jeon, C. O., Lim, J. M., Lee, S. S., Chung, B. S., Park, D. J., Xu, L. H., et al. (2009). Paenibacillus harenae sp. nov., isolated from desert sand in China. Int. J. Syst. Evol. Microbiol. 59, 13–17. doi: 10.1099/ijs.0.65664-0
Jeong, H., Choi, S. K., Park, S. Y., Kim, S. H., and Park, S. H. (2012). Draft genome sequence of Paenibacillus peoriae strain KCTC 3763t. J. Bacterio. 194, 1237–1238. doi: 10.1128/JB.06577-11
Ji, T., Languasco, L., Li, M., and Rossi, V. (2021). Effects of temperature and wetness duration on infection by Coniella diplodiella, the fungus causing white rot of grape berries. Plan. Theory 10, 1696. doi: 10.3390/plants10081696
Jiang, A., Zou, C., Xu, X., Ke, Z., Hou, J., Jiang, G., et al. (2022). Complete genome sequence of biocontrol strain Paenibacillus peoriae HJ-2 and further analysis of its biocontrol mechanism. BMC Genomics 23, 161. doi: 10.1186/s12864-022-08330-0
Kim, J. F., Jeong, H., Park, S. Y., Kim, S. B., Park, Y. K., Choi, S. K., et al. (2010). Genome sequence of the polymyxin-producing plant-probiotic rhizobacterium Paenibacillus polymyxa E681. J. Bacteriol. 192, 6103–6104. doi: 10.1128/JB.00983-10
Kim, A. Y., Shahzad, R., Kang, S. M., Khan, A. L., Lee, S. M., Park, Y. G., et al. (2017). Paenibacillus terrae AY-38 resistance against Botrytis cinerea in Solanum lycopersicum L. plants through defence hormones regulation. J. Plant Interact. 12, 244–253. doi: 10.1080/17429145.2017.1319502
Kumar, R., Acharya, V., Mukhia, S., Singh, D., and Kumar, S. (2019). Complete genome sequence of Pseudomonas frederiksbergensis ERDD5: 01 revealed genetic bases for survivability at high altitude ecosystem and bioprospection potential. Genomics 111, 492–499. doi: 10.1016/j.ygeno.2018.03.008
Li, J. Y., Gao, T. T., and Wang, Q. (2020). Comparative and functional analyses of two sequenced Paenibacillus polymyxa genomes provides insights into their potential genes related to plant growth-promoting features and biocontrol mechanisms. Front. Genet. 11:564939. doi: 10.3389/fgene.2020.564939
Li, Y., Li, Y., Zhang, H., Wang, M., and Chen, S. (2019). Diazotrophic Paenibacillus beijingensis BJ-18 provides nitrogen for plant and promotes plant growth, nitrogen uptake and metabolism. Front. Microbiol. 10, 1119. doi: 10.3389/fmicb.2019.01119
Li, L., Yuan, L., Shi, Y., Xie, X., Chai, A., Wang, Q., et al. (2019). Comparative genomic analysis of pseudomonas amygdali pv. Lachrymans NM002: insights into its potential virulence genes and putative invasion determinants. Genomics 111, 1493–1503. doi: 10.1016/j.ygeno.2018.10.004
Liang, T. W., Wu, C. C., Cheng, W. T., Chen, Y. C., Wang, C. L., Wang, I. L., et al. (2014). Exopolysaccharides and antimicrobial biosurfactants produced by Paenibacillus macerans TKU029. Appl. Biochem. Biotechnol. 172, 933–950. doi: 10.1007/s12010-013-0568-5
Ma, M., Wang, C., Ding, Y., Li, L., Shen, D., Jiang, X., et al. (2011). Complete genome sequence of Paenibacillus polymyxa SC2, a strain of plant growth-promoting rhizobacterium with broad-spectrum antimicrobial activity. J. Bacteriol. 193, 311–312. doi: 10.1128/JB.01234-10
Mano, Y., and Nemoto, K. (2012). The pathway of auxin biosynthesis in plants. J. Exp. Bot. 63, 2853–2872. doi: 10.1093/jxb/ers091
Mukhia, S., Kumar, A., Kumari, P., and Kumar, R. (2022). Psychrotrophic plant beneficial bacteria from the glacial ecosystem of Sikkim Himalaya: genomic evidence for the cold adaptation and plant growth promotion. Microbiol. Res. 260:127049. doi: 10.1016/j.micres.2022.127049
Naing, K. W., Nguyen, X. H., Anees, M., Lee, Y. S., Kim, Y. C., Kim, S. J., et al. (2015). Biocontrol of Fusarium wilt disease in tomato by Paenibacillus ehimensis KWN38. World J. Microbiol. Biotechnol. 31, 165–174. doi: 10.1007/s11274-014-1771-4
Nakkeeran, S., Rajamanickam, S., Saravanan, R., Vanthana, M., and Soorianathasundaram, K. (2021). Bacterial endophytome-mediated resistance in banana for the management of Fusarium wilt. 3 Biotech 11, 267. doi: 10.1007/s13205-021-02833-5
Navarro-Noya, Y. E., Hernández-Mendoza, E., Morales-Jiménez, J. M., Jan-Roblero, J., Martínez-Romero, E., and Hernández-Rodríguez, C. (2012). Isolation and characterization of nitrogen fixing heterotrophic bacteria from the rhizosphere of pioneer plants growing on mine tailings. Appl. Soil Ecol. 62, 52–60. doi: 10.1016/j.apsoil.2012.07.011
Ngashangva, N., Mukherjee, P., Sharma, K. C., Kalita, M. C., and Indira, S. (2021). Analysis of antimicrobial peptide metabolome of bacterial endophyte isolated from traditionally used medicinal plant millettia pachycarpa benth. Front. Microbiol. 12:656896. doi: 10.3389/fmicb.2021.656896
Przemieniecki, S. W., Kurowski, T. P., Kotlarz, K., Krawczyk, K., Damszel, M., Pszczółkowska, A., et al. (2019). Bacteria isolated from treated wastewater for biofertilization and crop protection against Fusarium spp. pathogens. J. Soil Sci Plant Nut. 19, 1–11. doi: 10.1007/s42729-018-0001-9
Rajkumar, M., Ae, N., Prasad, M. N. V., and Freitas, H. (2010). Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 28, 142–149. doi: 10.1016/j.tibtech.2009.12.002
Richter, M., and Rosselló-Móra, R. (2009). Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. U. S. A. 106, 19126–19131. doi: 10.1073/pnas.0906412106
Sang, M. K., Kim, E. N., Han, G. D., Kwack, M. S., Jeun, Y. C., and Kim, K. D. (2014). Priming-mediated systemic resistance in cucumber induced by pseudomonas azotoformans GC-B19 and Paenibacillus elgii MM-B22 against Colletotrichum orbiculare. Phytopathology 104, 834–842. doi: 10.1094/PHYTO-11-13-0305-R
Segata, N., Börnigen, D., Morgan, X. C., and Huttenhower, C. (2013). Phylo PhlAn is a new method for improved phylogenetic and taxonomic placement of microbes. Nat. Commun. 4, 2304. doi: 10.1038/ncomms3304
Shin, S. H., Kim, S., Kim, J. Y., Song, H. Y., Cho, S. J., Kim, D. R., et al. (2012). Genome sequence of Paenibacillus terrae HPL-003, a xylanase-producing bacterium isolated from soil found in forest residue. J. Bacteriol. 194, 1266. doi: 10.1128/JB.06668-11
Shokri, D., and Emtiazi, G. (2010). Indole-3-acetic acid (IAA) production in symbiotic and non-symbiotic nitrogen-fixing bacteria and its optimization by Taguchi design. Curr. Microbiol. 61, 217–225. doi: 10.1007/s00284-010-9600-y
Siddiqi, M. Z., Siddiqi, M. H., Im, W. T., Kim, Y. J., and Yang, D. C. (2015). Paenibacillus kyungheensis sp. nov., isolated from flowers of magnolia. Int. J. Syst. Evol. Microbiol. 65, 3959–3964. doi: 10.1099/ijsem.0.000521
Soni, R., Rawal, K., and Keharia, H. (2021). Genomics assisted functional characterization of Paenibacillus polymyxa HK4 as a biocontrol and plant growth promoting bacterium. Microbiol. Res. 248:126734. doi: 10.1016/j.micres.2021
Strieker, M., Tanović, A., and Marahiel, M. A. (2010). Nonribosomal peptide synthetases: structures and dynamics. Curr. Opin. Struct. Biol. 20, 234–240. doi: 10.1016/j.sbi.2010.01.009
Timmusk, S., Copolovici, D., Copolovici, L., Teder, T., Nevo, E., and Behers, L. (2019). Paenibacillus polymyxa biofilm polysaccharides antagonise Fusarium graminearum. Sci. Rep. 9, 662. doi: 10.1038/s41598-018-37718-w
Trinh, C. S., Jeong, C. Y., Lee, W. J., Truong, H. A., Chung, N., Han, J., et al. (2018). Paenibacillus pabuli strain P7S promotes plant growth and induces anthocyanin accumulation in Arabidopsis thaliana. Plant Physiol. Biochem. 129, 264–272. doi: 10.1016/j.plaphy.2018.06.001
Van Belkum, M. J., Lohans, C. T., and Vederas, J. C. (2015). Draft genome sequences of Paenibacillus polymyxa NRRL B-30509 and Paenibacillus terrae NRRL B-30644, strains from a poultry environment that produce tridecaptin A and paenicidins. Genome Announc. 3, e00372-15. doi: 10.1128/genomeA.00372-15
Vejan, P., Abdullah, R., Khadiran, T., Ismail, S., and Nasrulhaq Boyce, A. (2016). Role of plant growth promoting rhizobacteria in agricultural sustainability-a review. Molecules 21, 573. doi: 10.3390/molecules21050573
Von der Weid, I., Alviano, D. S., Santos, A. L. S., Soares, R. M. A., Alviano, C. S., and Seldin, L. (2003). Antimicrobial activity of Paenibacillus peoriae strain NRRL BD-62 against a broad spectrum of phytopathogenic bacteria and fungi. J. Appl. Microbiol. 95, 1143–1151. doi: 10.1046/j.1365-2672.2003.02097.x
Wang, L. Y., Li, J., Li, Q. X., and Chen, S. F. (2013). Paenibacillus beijingensis sp. nov., a nitrogen-fixing species isolated from wheat rhizosphere soil. Antonie Van Leeuwenhoek 104, 675–683. doi: 10.1007/s10482-013-9974-5
Xie, J. B., Du, Z., Bai, L., Tian, C., Zhang, Y., and Xie, J. Y. (2014). Comparative genomic analysis of N2-fixing and non-N2-fixing Paenibacillus spp.: organization, evolution and expression of the nitrogen fixation genes. PLoS Genet. 10:e1004231. doi: 10.1371/journal.pgen.1004231
Xie, J., Shi, H., Du, Z., Wang, T., Liu, X., and Chen, S. (2016). Comparative genomic and functional analysis reveal conservation of plant growth promoting traits in Paenibacillus polymyxa and its closely related species. Sci. Rep. 6, 21329. doi: 10.1038/srep21329
Yadav, D. R., Adhikari, M., Kim, S. W., Kim, H. S., and Lee, Y. S. (2021). Suppression of Fusarium wilt caused by Fusarium oxysporum f. sp. lactucae and growth promotion on lettuce using bacterial isolates. J. Microbiol. Biotechnol. 31, 1241–1255. doi: 10.4014/jmb.2104.04026
Yadav, M., Dubey, M. K., and Upadhyay, R. S. (2021). Systemic resistance in chilli pepper against anthracnose (caused by Colletotrichum truncatum) induced by Trichoderma harzianum, Trichoderma asperellum and Paenibacillus dendritiformis. J. Fungi (Basel). 7, 307. doi: 10.3390/jof7040307
Yin, X. T., Li, T. G., Jiang, X. L., Tang, X. N., Zhang, J. K., Yuan, L. F., et al. (2022). Suppression of grape white rot caused by Coniella vitis using the potential biocontrol agent Bacillus velezensis GSBZ09. Pathogens. 11, 248. doi: 10.3390/pathogens11020248
Yuan, L., Li, L., Zheng, F., Shi, Y., Xie, X., Chai, A., et al. (2020). The complete genome sequence of Rahnella aquatilis ZF7 reveals potential beneficial properties and stress tolerance capabilities. Arch. Microbiol. 202, 483–499. doi: 10.1007/s00203-019-01758-1
Zaidi, A., Khan, M. S., Ahemad, M., and Oves, M. (2009). Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol. Immunol. Hung. 56, 263–284. doi: 10.1556/AMicr.56.2009.3.6
Zhai, Y., Zhu, J. X., Tan, T. M., Xu, J. P., Shen, A. R., Yang, X. B., et al. (2021). Isolation and characterization of antagonistic Paenibacillus polymyxa HX-140 and its biocontrol potential against Fusarium wilt of cucumber seedlings. BMC Microbiol. 21, 75. doi: 10.1186/s12866-021-02131-3
Zhang, F., Li, X. L., Zhu, S. J., Ojaghian, M. R., and Zhang, J. Z. (2018). Data on the ultrastructural characteristics of Paenibacillus polymyxa isolates and biocontrol efficacy of P. polymyxa ShX301. Data Brief 21, 259–262. doi: 10.1016/j.dib.2018.09.058
Keywords: Paenibacillus peoriae, comparative genome analysis, plant growth-promoting, biocontrol, antimicrobial substances
Citation: Yuan L, Jiang H, Jiang X, Li T, Lu P, Yin X and Wei Y (2022) Comparative genomic and functional analyses of Paenibacillus peoriae ZBSF16 with biocontrol potential against grapevine diseases, provide insights into its genes related to plant growth-promoting and biocontrol mechanisms. Front. Microbiol. 13:975344. doi: 10.3389/fmicb.2022.975344
Received: 22 June 2022; Accepted: 10 August 2022;
Published: 08 September 2022.
Edited by:
Zheng Zhang, Shandong University, ChinaReviewed by:
Guotian Liu, Northwest A&F University Hospital, ChinaCopyright © 2022 Yuan, Jiang, Jiang, Li, Lu, Yin and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanfeng Wei, d2VpeWFuZmVuZzIwMjJAMTYzLmNvbQ==; Xiangtian Yin, eXh0MTk4NUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.