- 1College of Animal Science, Guizhou University, Guiyang, China
- 2Sichuan Academy of Grassland Sciences, Chengdu, China
- 3Key Laboratory of Animal Genetics, Breeding and Reproduction in the Plateau Mountainous Region, Ministry of Education, Guizhou University, Guiyang, China
This study aimed to isolate, characterize, and identify lactic acid bacteria (LAB) strains from various sources and evaluate their effects on the nutritional quality, fermentation characteristics, and microbial compositions of paper mulberry (PM) after 60 days of ensiling. Forty-nine LAB strains were isolated from Phalaris arundinacea silage, pickle, and fresh PM leaves; three of these strains (Lactiplantibacillus plantarum, YC1; Levilactobacillus brevis, PC3; and Lactiplantibacillus plantarum, BP17) and one commercial inoculant Gaofuji (GFJ) were subsequently used. Compared with other treatments, PC3 and BP17 increased (P < 0.05) the LAB count and crude protein content and decreased (P < 0.05) the molds and coliform bacteria counts, pH, and ammonia-N content of PM silages. BP17 and PC3 increased the relative Lactiplantibacillus abundance and decreased that of Lelliottia and Cladosporium, improving PM silage quality. Therefore, PC3 and BP17 can improve the fermentation quality of PM silage and could be used as silage starter cultures.
Introduction
With the rapid development of animal husbandry in China, traditional feeds such as fodder crops, grasses, and grain are insufficient to meet the demand for livestock. The development and utilization of new feed resources has proven to be a viable solution to the feed crisis. Recently, woody forage processing and feeding technology have been studied in China (Si et al., 2018; Zhang et al., 2019a). Paper mulberry (PM; Broussonetia papyrifera L.), a typical woody forage, is fast growing; rich in crude protein (211.50–245.92 g/kg of DM), amino acids and flavonoids; and widely distributed in Asia (Pang et al., 2014; Cheng et al., 2021a; Wang et al., 2021a). The PM is widelyx distributed, with annual production of about 2.25 × 108 t in temperate and tropical zones of China (Dong et al., 2020). As one of the country’s top 10 targeted poverty alleviation initiatives, China has planted more than 300,000 hectares of PM for use as an unconventional animal feed (Hao et al., 2021). There have been many studies on the nutritional value of PM and the effects of feeding PM on livestock. For example, some studies found that adding an appropriate amount of PM silage to the diets of cattle and goats could improve feed efficiency, growth performance, meat quality, and immune and antioxidant function (Hao et al., 2020; Hua et al., 2020; Tao et al., 2020; Tian et al., 2020). Another study revealed the use of PM as a new type of animal feed that could be a candidate protein feed resource in response to the feed crisis (Guo et al., 2021).
PM is harvested during the rainy season, resulting in a high moisture content, and ensiling has been indicated to be the best way to preserve PM (Zhang et al., 2019a). In the process of ensiling, epiphytic lactic acid bacteria (LAB) ferment soluble carbohydrates in fresh forage into organic acids, mainly lactic acid, thereby reducing pH and inhibiting harmful microorganisms (Dong et al., 2019). As a result, ensiling is a microbial-driven process, in which LAB play a key role. Our previous studies have shown that without treatment, PM is difficult to ensile well due to the considerably high buffering capacity and the low epiphytic LAB count [< 105 cfu/g of fresh matter (FM)] of fresh forage (Cheng et al., 2021a). Exogenous LABs are frequently used to speed up the process of ensiling, prevent the growth of harmful microorganisms, and improve the silage quality of PM (Du et al., 2021; Wang et al., 2021b). Because the adaptability, establishment, and development of LAB in forages during ensiling is unknown, the conditions used do not always result in successful regulation of silage fermentation with LAB inoculants (Kobayashi et al., 2010; Tohno et al., 2012). Wang et al. (2021b) found that adding LAB [Lactiplantibacillus plantarum (L. plantarum) or Lacticaseibacillus casei] isolated from Leymus chinensis silage did not significantly improve the fermentation quality of PM silage, which was mainly reflected in the high pH (> 6) value and ammonia nitrogen (NH3–N) content (>16% TN), indicating that these LAB strains were not the best choice for PM ensiling. Previous studies have shown that adding LAB (isolated from fresh PM leaves) can improve the fermentation quality of PM silage (Cheng et al., 2021a). According to previous research, the best isolates for boosting fermentation quality may originate from that specific forage (Zhang et al., 2015; Wang et al., 2018a). Therefore, it is necessary to explore LAB strains that are adaptable and can play a role in PM silage. However, to our knowledge, few studies have focused on the effects of epiphytic LAB isolated from various forage sources on the fermentation quality of PM silage.
It is widely established that bacterial community structure and abundance are key factors that affect the fermentation quality of silage. Previously published studies indicated that a high abundance of harmful bacteria (such as Enterobacter or Clostridium) and a low abundance of beneficial bacteria (such as Lactobacillus) in silage were the main challenges for proper PM ensiling (Cheng et al., 2021a; Du et al., 2021). However, the structure and abundance of fungal community members (such as Saccharomyces, Cladosporium or Issatchenkia) during the ensiling process also affected the fermentation quality of silage (Li et al., 2021). Therefore, the composition and shift in bacteria and fungi may affect the fermentation quality of PM silage. In recent years, 16S rRNA and ITS sequencing has changed our understanding of bacterial and fungal communities in fresh and ensiled forages, including alfalfa (Bai et al., 2020), sugarcane (Wang et al., 2020), and timothy (Li et al., 2021). To the best of our knowledge, there have been no reports on the diversity of the fungal community in fresh and ensiled PM. Hence, the objective of the present study was to compare the effects of specific LAB, including epiphytic, exogenous, and commercial LAB inoculants, on the nutritional quality, fermentation characteristics, and bacterial and fungal community compositions of PM silage. Our hypothesis was that LAB strains isolated from different sources could functionally improve the silage quality of PM.
Materials and methods
Isolation, screening, characterization, and identification of lactic acid bacteria isolated from different sources
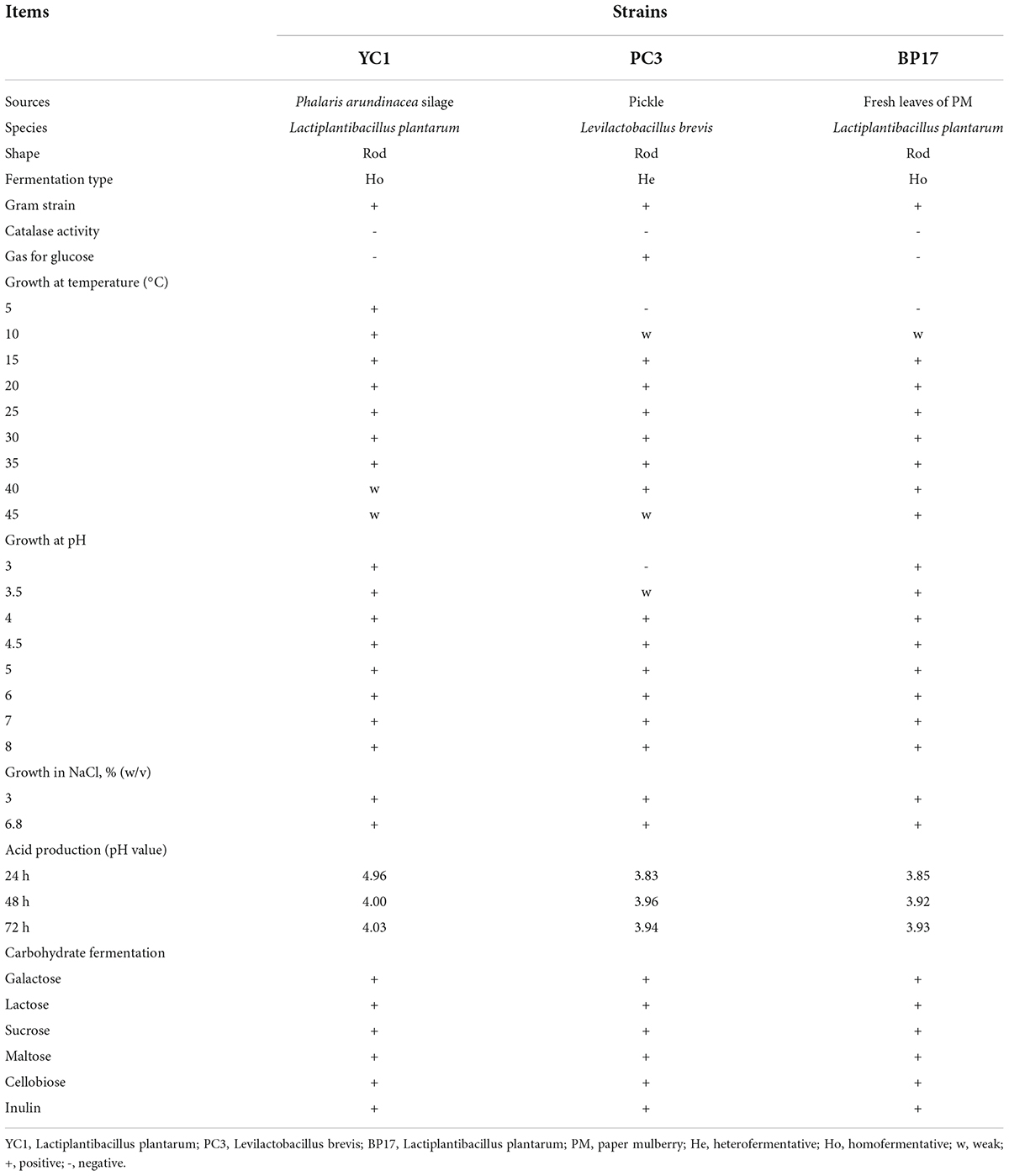
A total of 49 LAB strains were isolated from Phalaris arundinacea (P. arundinacea) silage, pickle, and fresh PM leaves according to the method of Cai et al. (1998). Ten grams of sample from each material was put into a sterile glass bottle and blended with 90 mL of sterile water. Serial dilutions were used for the isolation of LAB using de Man, Rugose, Sharpe (MRS) agar (GCM188, Land Bridge Technology Co., Ltd., Beijing, China). The morphological characteristics, growth ability, and acid production capacity of all identified strains were measured on MRS agar medium. The physiological and biochemical features of three LAB strains (L. plantarum isolated from P. arundinacea silage, YC1; L. plantarum isolated from pickle, PC3; and Levilactobacillus brevis isolated from fresh PM leaves, BP17) with rapid growth ability and high acid production ability were investigated. In addition, the tested strains were genetically identified by 16S rRNA gene sequencing (Sangon Biotech (Shanghai) Co., Ltd., Shanghai, China) and preserved in China General Microbiological Culture Collection Center under accession numbers CGMCC NO. 17813 (YC1), CGMCC No. 17726 (PC3) and CGMCC No. 17814 (BP17), respectively.
Silage preparation
This study was conducted at the experimental base (Chengdu) of Sichuan Academy of Grassland Science (103°22′E, 33°33′N). Whole PM leaves were harvested as ensiling material on July 1, 2020 and chopped to a length of 2–3 cm. Silages were prepared on a small-scale system by packing 500 g of chopped PM leaves without or with LAB inoculants into polyethylene bags (25 cm × 30 cm) and then vacuum packed using a vacuum packing machine (SJ-400, Shanghai Precision Machinery Manufacturing Co., Ltd.). The treatments were as follows: (1) CK, control without additives, treated with 5 mL kg–1 FM 0.9% physiological saline; (2) YC1, applied at 1.0 × 106 cfu g–1 of FM; (3) PC3, applied at 1.0 × 106 cfu g–1 of FM; (4) BP17, applied at 1.0 × 106 cfu g–1 of FM (Cheng et al., 2021a); (5) Gaofuji (GFJ), a combination of L. plantarum and Lentilactobacillus buchneri, produced by Sichuan Gaofuji Biotechnology Co., Ltd., applied at 1.0 × 106 cfu g–1 of FM. Three polyethylene bags of silage with the same treatment were sampled for analysis after 60 d of ensiling in a dark room at room temperature (20–25°C). Samples from fresh and ensiled PM leaves were subjected to analyses of chemical composition, fermentation characteristics, microbial population, and bacterial and fungal community compositions.
Analysis of microbial population, chemical composition, and fermentation quality
The microbial populations on fresh material and silage samples were determined according to the method of Cai et al. (1999). Ten grams of each sample was suspended in 90 mL of sterilized water and serially diluted from 10–1 to 10–5. The number of LAB was measured by plate counting on MRS agar (GCM188, Land Bridge Technology Co., Ltd., Beijing, China) and kept in an anaerobic incubator for 48 h at 37°C. Molds and yeasts were incubated in a general incubator on malt extract agar (CM173, Land Bridge Technology Co., Ltd., Beijing, China) for 48 h at 30°C, and yeasts were distinguished from molds by colony appearance and cell morphology. Aerobic bacteria were counted on nutrient agar (Nissui) and incubated for 48 h at 30°C under aerobic conditions. Coliform bacteria were incubated on blue light broth agar (Nissur Ltd., Tokyo, Japan) for 48 h at 30°C. The microbial counts were expressed as log cfu/g of FM.
Oven drying at 65°C to constant weight was used to determine the dry matter (DM) content of fresh material and silage samples. All dried samples were milled to pass through a 0.20 mm screen for determination of the chemical compositions. The total nitrogen (TN) content was determined using a Kjeldahl nitrogen analyzer (Kjeltec 8400, FOSS, Sweden), and crude protein (CP) was calculated by multiplying TN by 6.25 (AOAC, 1990). The neutral detergent fiber (NDF) and acid detergent fiber (ADF) levels were analyzed with a modified procedure using an ANKOM 2000 Fiber Analyzer (ANKOM Technology Corp., Fairport, NY, United States) (Van Soest et al., 1991). The water-soluble carbohydrate (WSC) was determined using the method of Mcdonald et al. (1991). The in vitro ruminal DM digestibility (IVDMD) of all silage samples was determined according to the method of Goto and Minson (1977) using the two-stage fermentation technique.
A silage sample of 10 g was mixed with 90 mL ultrapure water for 3 min in a stomacher blender. The pH value of the silage extract was determined by a pH meter (3-Star 310P-02, Thermo Electron, Boston, United States). The ammonia nitrogen (NH3–N) content was determined in the silages by the phenol-hypochlorite procedure (Kleinschmit et al., 2005). A filtrate of approximately 10 mL was subjected to centrifugation (12,000 × g, 10 min, 4°C), and the lactic acid (LA), acetic acid (AA), propionic acid (PA), and butyric acid (BA) contents in the supernatant were analyzed using high-performance liquid chromatography (HPLC) (LC-20A; Shimadzu, Tokyo, Japan) with a UV detector (210 nm) and a column (e2695, Waters Co., Ltd.) (Cheng et al., 2021b).
Sequencing-based microbial analyses
Microbial DNA was extracted from the fresh PM and silage according to the method described in Li et al. (2021). In brief, a Power Soil DNA Isolation Kit (MO BIO Laboratories) was used to extract microbial DNA following the manufacturer’s instructions. All microbial DNA samples were immediately sent to Novogene Company (Beijing, China) for PCR amplification and bioinformatic analysis. The primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′) were chosen to amplify the V3-V4 region of the 16S rRNA gene. The primers ITS1F (5′– CTTGGTCATTTAGAGGAAGTAA–3′) and ITS2-2043R (5′-GCTGCGTTCTTCATCGATGC-3′) were used to amplify the ITS gene (Li et al., 2021). Novogene (Beijing, China) completed library construction and Illumina S5 sequencing according to the manufacturer’s instructions. The data were analyzed using the Novogene Magic Cloud Platform.1
Statistical analyses
Data on the microbial population, chemical composition and fermentation quality of fresh and ensiled paper mulberry were analyzed using one-way analysis of variance (ANOVA) to evaluate the effects of LAB inoculants. The differences between means were assessed using Duncan’s multiple range method. The effect was considered significant when P < 0.05. The analyses were conducted using IBM SPSS Statistics 26.0 (SPSS, Inc., Chicago, IL).
Results and discussion
Characteristics and identification of selected lactic acid bacteria strains
More than 200 strains were isolated from different sources, and Gram staining, colony morphology and catalase activity tests identified 49 of them as LAB strains. Three strains, YC1, PC3, and BP17, were selected for further study according to their growth ability and acid production ability. The morphological, physiological, and biochemical properties of the three LAB strains used in our study are shown in Table 1. Previous studies reported that 16S rDNA sequencing was a good method for identifying microorganisms by genus and species (Cai et al., 1998; Wang et al., 2019a). In our study, 16S rDNA sequence analysis was used to identify these three strains. Among them, YC1 isolated from P. arundinacea silage and BP17 isolated from fresh PM leaves were L. plantarum, and PC3 isolated from pickle was Levilactobacillus brevis. The YC1 and BP17 strains were rod shaped, homofermentative, Gram positive, catalase negative, and glucose negative. This result is consistent with the study of Guo et al. (2020), who reported that F1 and F50 isolated from feces were L. plantarum and could be used to improve the silage quality of alfalfa. The PC3 strain was rod shaped, heterofermentative, Gram positive, catalase negative, and glucose positive. Previous studies revealed that Levilactobacillus brevis isolated from pickle had antifungal, antioxidant, and probiotic properties, and it has been successfully used in silage (Grant et al., 1994; Arasu et al., 2015). The YC1 strain could grow normally at temperatures from 5 to 35°C, while the PC3 and BP17 strains could grow normally at 15–40°C. This indicates that the YC1 strain is more resistant to low temperature than the PC3 and BP17 strains. The raw material from which YC1 was isolated (P. arundinacea silage) came from the Qinghai-Tibetan Plateau, while the raw materials from which PC3 (pickle) and BP17 (fresh leaves of PM) were isolated came from Chengdu; therefore, this difference in optimal growth temperature could have arisen due to long-term evolution and natural selection on the different environmental temperatures (You et al., 2021). This unique characteristic of the YC1 strain suggests that it may be used as a low-temperature-tolerant LAB inoculant, consistent with the results of Chen et al. (2020a), who reported that L. plantarum isolated from naturally fermented silage on the Qinghai Tibetan Plateau could grow normally at 5–30°C. The YC1 and BP17 strains could grow normally at pH values ranging from 3.0 to 8.0, while the PC3 strain could grow normally at pH 4–8, and all isolated strains could grow normally with NaCl concentrations of 3% (w/v) and 6.8% (w/v). These findings indicated that the tolerance of PC3 to an acidic environment was weaker than that of YC1 and BP17. This is because the PC3 strain is Levilactobacillus brevis, a heterofermentative LAB that plays a role in the early period of ensiling and is less resistant to the acidic environment than L. plantarum (Zheng et al., 2017; Amaral et al., 2020). This finding is consistent with the results of Wang et al. (2018a), who reported that L. plantarum was highly tolerant to low pH. The ability to reduce the pH of the medium has long been one of the main principles for selecting LAB as inoculants (Liu et al., 2014). In this study, the strains were inoculated in MRS medium and cultured at 30°C for 24–72 h. The pH ranges of the MRS medium from YC1, PC3, and BP17 were 4.00–4.96, 3.83–3.96, and 3.85–3.93, respectively. All of the strains could produce acid from galactose, lactose, sucrose, maltose, cellobiose, and inulin, which indicated that the strains isolated in our study had a wide variety of fermentation substrates. The selected strains, YC1, PC3, and BP17, demonstrated a wide range of temperature, pH, and salt tolerances, as well as a high acid production and a wide variety of fermentation substrates. These unique properties of these three selected strains offer the potential for practical applications as inoculants.
Microbial counts and chemical compositions of fresh and ensiled paper mulberry
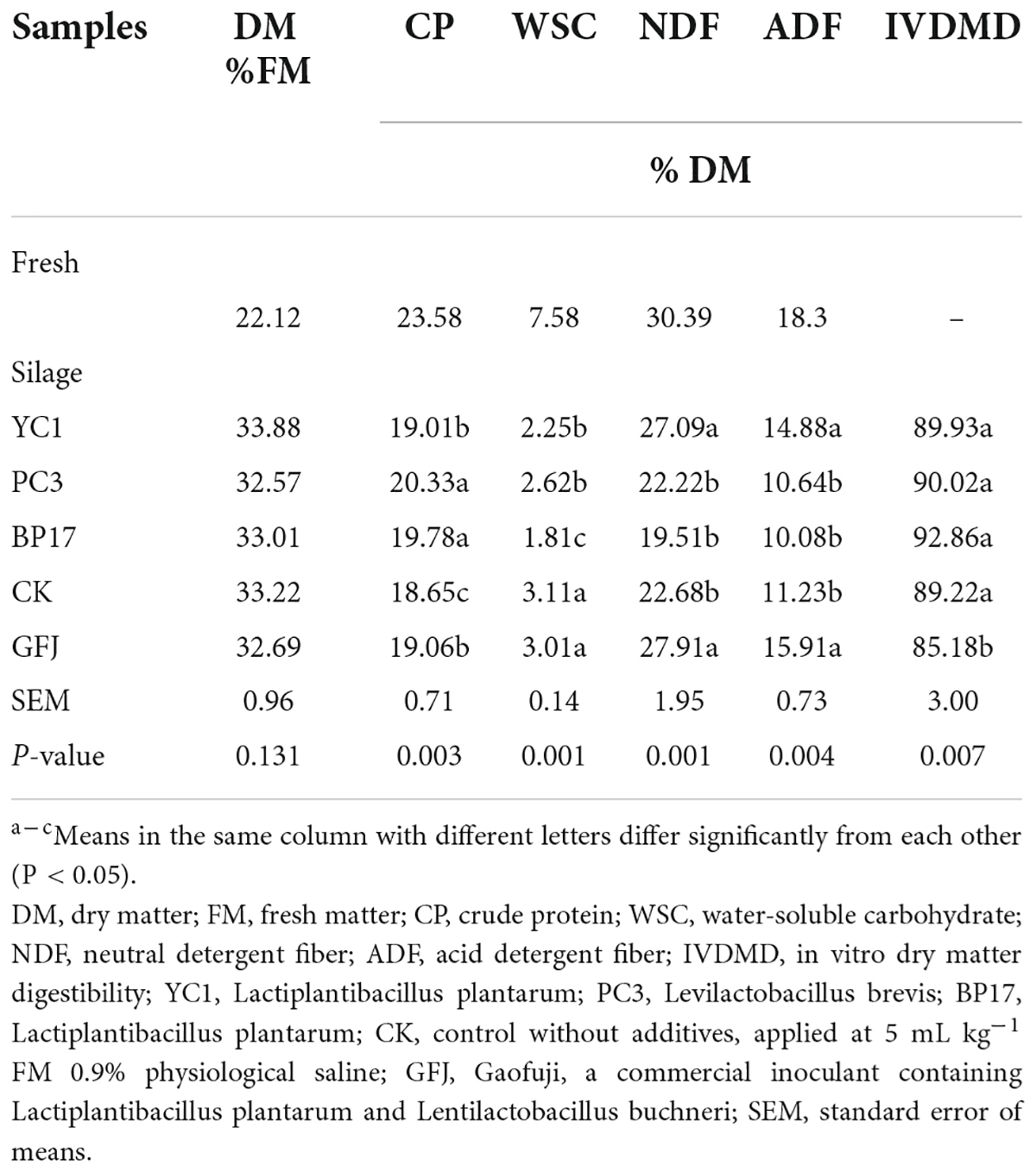
The microbial populations of fresh and ensiled PM are presented in Table 2. A low count of LAB (102.75 cfu/g of FM) was detected in the fresh PM, and high counts of undesirable microorganisms, including molds, yeast, aerobic bacteria, and coliform bacteria, were detected (103.89–106.19 cfu/g FM). The number of epiphytic LAB on the fresh PM (102.48 cfu/g of FM) in our study was similar to the result reported by Cheng et al. (2021a). In our study, the low count of epiphytic LAB (< 105 cfu/g of FM) and high count of harmful microorganisms on fresh PM made it difficult to ensile well, and additional LAB must be added to improve silage quality. As expected, as fermentation progressed, the counts of LAB increased (> 105 cfu/g of FM), whereas the counts of undesirable microorganisms decreased (P < 0.05). Similarly, Cheng et al. (2021b) reported that the number of helpful microorganisms increased, and the number of harmful microorganisms decreased after ensiling. Moreover, compared with other treatments, the PC3 and BP17 treatments had a higher (P < 0.05) number of LAB and lower (P < 0.05) numbers of molds and coliform bacteria.
The chemical compositions of fresh and ensiled PM are displayed in Table 3. Studies have found that the presence of a sufficient WSC content (6–8% DM) in the fresh material is essential to ensure the fermentation quality of silage (Wang et al., 2018a). In our study, the WSC concentration (7.58% DM) of fresh PM was enough to promote LAB fermentation. After 60 d of ensiling, the YC1, PC3, and BP17 treatment silages consistently showed lower (P < 0.05) WSC contents than the CK and GFJ treatment silages. This may be due to the conversion of WSC content into acid, ethanol, and carbon dioxide by LAB during fermentation (Muck et al., 2018). All LAB treatments showed a greater (P < 0.05) CP concentration than the CK treatment, which could be due to LAB limiting clostridia growth and proteolysis (Arriola et al., 2011). Among the LAB treatments, PC3 and BP17 showed the best inhibitory effect, mainly due to their significantly higher CP content. The NDF and ADF levels of fresh PM were 30.39% DM and 18.30% DM, respectively, which were lower than the results (50.28% DM and 34.89% DM, respectively) reported in Hao et al. (2021). This is because previous studies focused on the whole PM plant, while this study focused on the PM leaves. The PC3 and BP17 treatments resulted in significantly lower (P < 0.05) NDF and ADF levels than the GFJ and YCI treatments, which indicated that PC3 and BP17 were more capable of degrading fiber than other LAB additives. Feeding well-fermented silage helps to improve animal performance because of the high level of LA and the low level of AA and ammonia nitrogen in the silage (Wang et al., 2019b). LAB strains could improve rumen DM digestibility by interacting with rumen microorganisms (Wang et al., 2019b). Compared with the GFJ treatment, inoculation with YC1, PC3, and BP17 enhanced (P < 0.05) the IVDMD of PM silage. This is because PM silage inoculated with YC1, PC3, and BP17 had a lower pH, which inhibited the growth of other microorganisms, thus reducing dry matter loss during silage fermentation. However, the in vitro DM digestibility of PM silage with GFJ was lower than that of PM silage without LAB. Similar result from Weinberg et al. (2007), who reported that the in vitro DM digestibility of wheat silage with LAB was lower than that of wheat silage without LAB. Based on these results, LAB isolated from different sources could maintain the nutritional value of PM silage.
Fermentation quality of ensiled paper mulberry
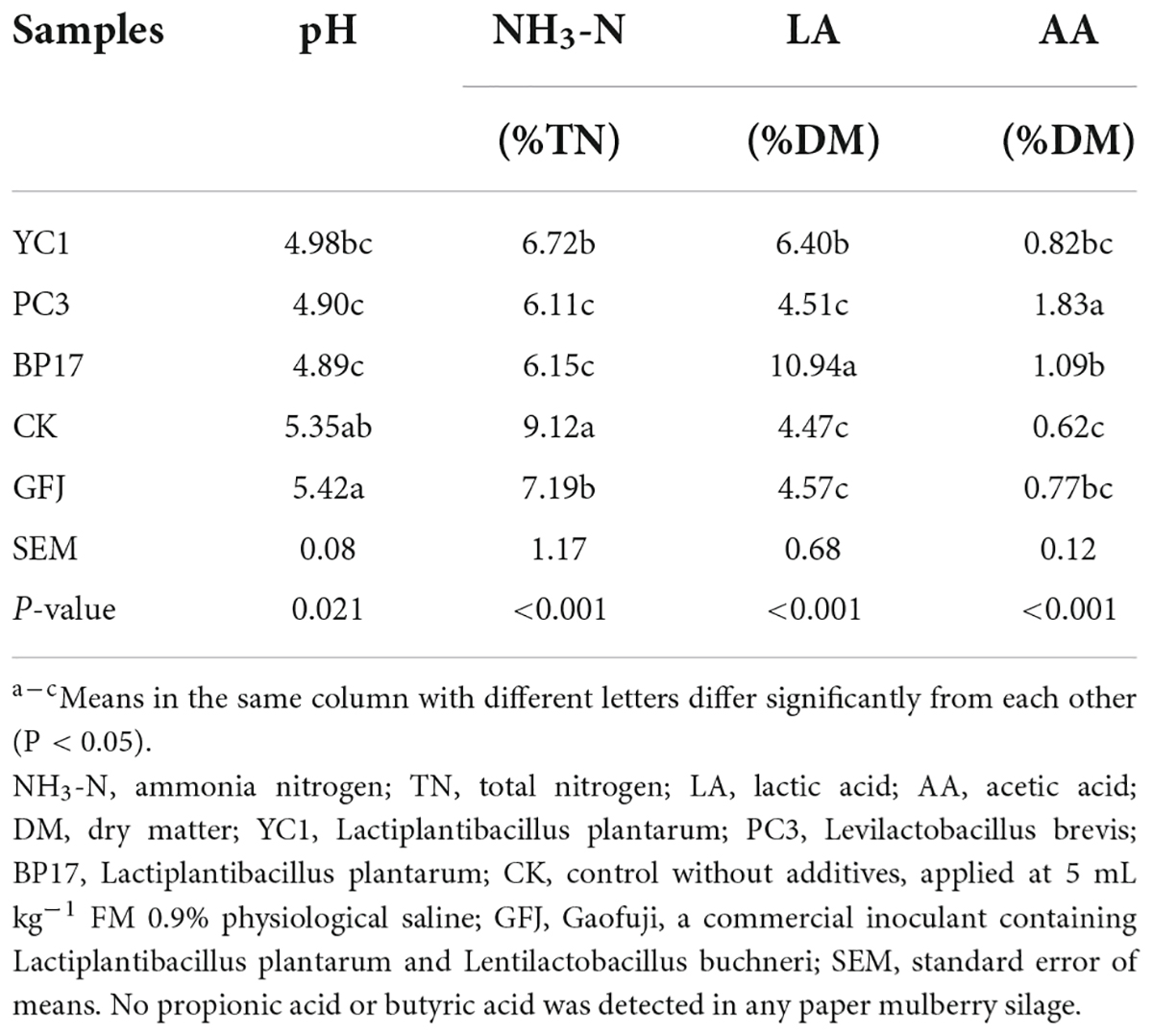
The fermentation quality of PM silage is shown in Table 4. The LAB treatments had significant effects (P < 0.05) on the pH value and NH3-N, LA, and AA levels of PM silage. Compared with the CK and GFJ treatments, the BP17 and PC3 treatments had lower (P < 0.05) pH values and NH3-N contents. This is likely because the LAB could convert WSC into LA, resulting in a decrease in pH and an increase in the LA content (You et al., 2021). However, since LAB activity plays an important role in LA accumulation and pH reduction in the early stages of ensiling (Davies et al., 1998), LAB from different sources have different effects on the silage quality of PM. In addition, the BP17 treatment had the lowest pH (4.89) and the highest LA content (10.94% DM). This result was attributed to the high count (108.63 cfu/g FM) of LAB contained in the BP17 treatment. However, although the PC3 treatment contained a large number of LAB (108.92 cfu/g FM), the LA level of silage was lower than that of the BP17 and YC1 treatments. This is because PC3 is a heterofermentative LAB (Levilactobacillus brevis) that can convert LA to AA (Danner et al., 2003), so the PC3-treated silage had the highest (P < 0.05) AA content (1.83% DM) (Table 4). The NH3-N content of silage is an indication of the degree of proteolysis (Wang et al., 2018b). Previous studies have suggested that well-preserved silage should contain NH3-N < 10% TN (Umaña et al., 1991). However, the NH3-N content (6.11% TN-9.12% TN) of all silages in our study was below the recommended levels. In addition, the NH3-N content in all LAB-treated silages was lower (P < 0.05) than that in the CK silage. This result confirmed that LAB reduced the pH value of the silage environment, thereby inhibiting the development and proteolytic activity of other microorganisms (such as clostridia) (Heinritz et al., 2012). PA and BA are undesirable because they waste metabolic energy in their creation (Dong et al., 2020). No PA or BA was detected in any PM silage in our study, which is consistent with the results of Cheng et al. (2021a).
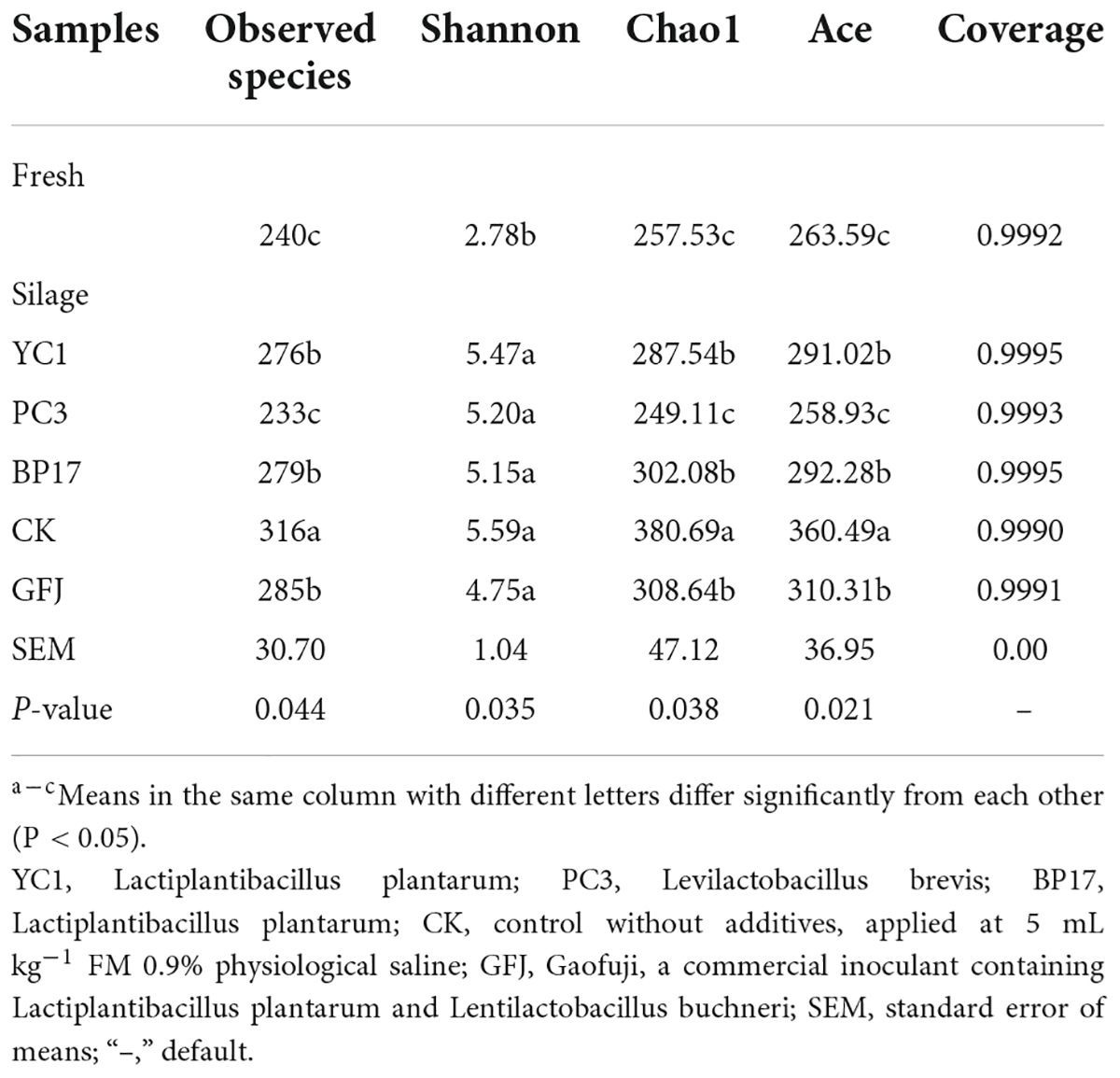
Alpha diversities of bacteria and fungi in fresh and ensiled paper mulberry
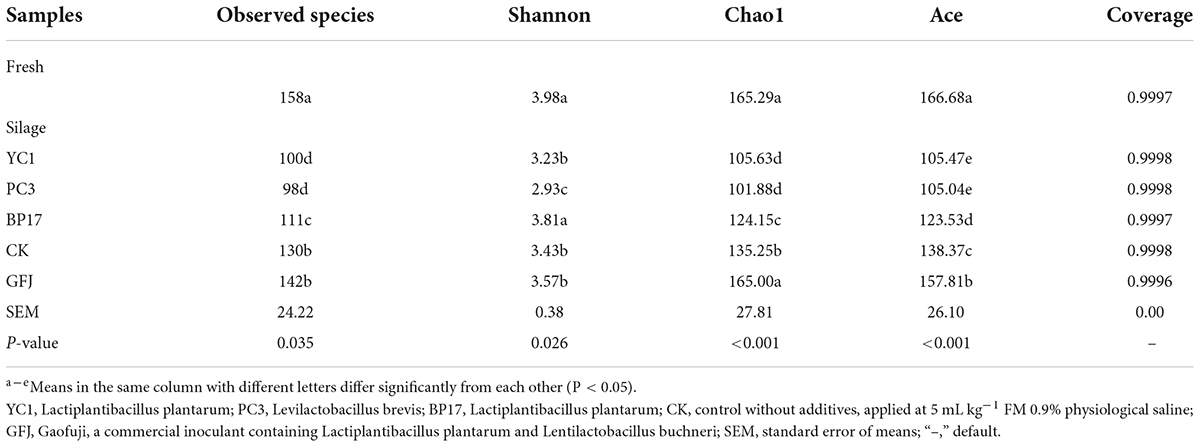
The sequencing information and bacterial diversity analysis of ensiled PM are shown in Table 5. The Good’s coverage of all samples was more than 0.99, suggesting that the sequencing results could reveal the bacterial diversity of PM silage. Inoculation with LAB had a significant (P < 0.05) effect on the number of OTUs and on the Shannon, Ace and Chao1 indexes of PM silage. During ensiling, a decrease in observed species was found in YC1-, PC3-, and BP17-treated silages compared with the CK and GFJ-treated silages (P < 0.05). This is likely because many microorganisms are replaced by anaerobic LAB during ensiling, and the LA produced by LAB also inhibits harmful microorganisms. The Shannon index of the PC3-treated silage was decreased compared with that of the CK and GFJ-treated silages (P < 0.05). Similar results were found by Wang et al. (2021a), who reported that the Shannon index decreased after inoculating PM silage with Lentilactobacillus buchneri in PM silage. However, the Shannon index of the BP17-treated silage was higher (P < 0.05) than that of the CK and GFJ-treated silages. This was in accordance with the results from Wang et al. (2021a), who reported that the Shannon index increased after inoculating PM silage with L. plantarum. The Ace and Chao1 indexes of the YC1-, PC3-, and BP17-treated silages were lower than those of the CK and GFJ-treated silages (P < 0.05). These results agree with the results of Du et al. (2021), who found that the Chao and Ace indexes of PM silage increased after inoculation with LAB compared with the CK. These results suggest that our screened LAB can quickly reduce the pH of PM silage and thus inhibit harmful microorganisms and reduce the alpha diversity of bacteria (Li et al., 2021).
The sequencing information and fungal diversity of PM silage are shown in Table 6. The majority of research on fungi has focused on toxin-producing fungi (Duniere et al., 2017), but few studies have investigated the epiphytic fungal community in silage. In our study, the Good’s coverage of all samples was more than 0.99, indicating that the sequencing depth was sufficient for revealing the complete fungal diversity. During ensiling, a decrease in the OTUs, Ace and Chao1 indexes was found in LAB silages compared with the CK silage (P < 0.05). A consistent result was reported by Keshri et al. (2018), who found that the alpha index of fungi in LAB-treated corn silage was lower than that in CK silage. Therefore, LAB reduces the diversity and richness of the fungal community in PM silage.
Bacterial and fungal communities of fresh and ensiled paper mulberry
The relative abundance of bacterial communities in the PM silages is shown in Figure 1. The main phyla detected in fresh PM were Firmicutes, Cyanobacteria, and Proteobacteria (Figure 1A). This is in accordance with the results from He et al. (2021), who reported that these bacteria were present in fresh and ensiled PM. Firmicutes and Proteobacteria were the main phyla detected in the PM silages. Compared to the fresh PM, the PM silages showed decreased relative abundances of Cyanobacteria and increased relative abundances of Firmicutes. Similar results were obtained by He et al. (2021), who reported that the relative abundance of Cyanobacteria in PM silages was lower and the relative abundance of Firmicutes was higher than those in fresh PM. Compared with the CK silage, the YC1-, PC3-, and BP17-treated silages had lower relative abundances of Cyanobacteria and Proteobacteria and a higher relative abundance of Firmicutes. The results of our study agree with those of Liu et al. (2019), who found that LAB-treated silage exhibited greater Firmicutes abundance and lower Proteobacteria and Cyanobacteria abundances than the CK silage. However, the relative abundance of Cyanobacteria was decreased and the relative abundance of Proteobacteria was increased in the GFJ-treated silage compared with other silages. This result indicated that GFJ did not improve the fermentation quality of PM silage. Similar results were obtained by Wang et al. (2021a), who reported that inoculation with LAB derived from Leymus chinensis silage had no discernible effect on the fermentation quality of PM silage. As illustrated in Figure 1B, Cyanobacteria and Bacillus were the predominant genera in fresh PM; these genera are not conducive to silage fermentation, and LAB treatments need to be added to improve the fermentation quality of PM silage. In the present study, Lactiplantibacillus and Enterococcus were the predominant genera in the PM silages. This was consistent with the findings of Cheng et al. (2021b), who showed that the predominant bacteria in the PM silage inoculated with LAB were Enterococcus and Lactiplantibacillus. A variety of beneficial LAB, including Lactiplantibacillus, Weissella, Lactococcus, Leuconostoc, and Streptococcus, are essential for enhancing LA production and reducing pH (Dong et al., 2020). Compared with the CK and GFJ-treated silages, the PC3- and BP17-treated silages had increased relative abundances of Lactiplantibacillus and decreased relative abundances of Enterococcus. Since Lactiplantibacillus is strongly acid tolerant, it would remain unaffected at low pH values, while less acid-tolerant microorganisms such as Enterococcus would be inhibited (Li et al., 2021). In the present study, the relative abundance of Kosakonia in the GFJ-treated silage was higher than that in the other silages. Studies have found that Kosakonia, a bacterium similar to Enterobacter, can produce NH3-N, leading to silage spoilage (Kosako et al., 1996; Duniere et al., 2013). Compared with the CK and GFJ-treated silages, the PC3- and BP17-treated silages showed decreased relative abundances of Lelliottia. Lelliottia has been rarely reported to be distributed in silage. Lelliottia, an Enterobacteriaceae member (Yuk et al., 2018), can create NH3-N, resulting in poor silage quality.
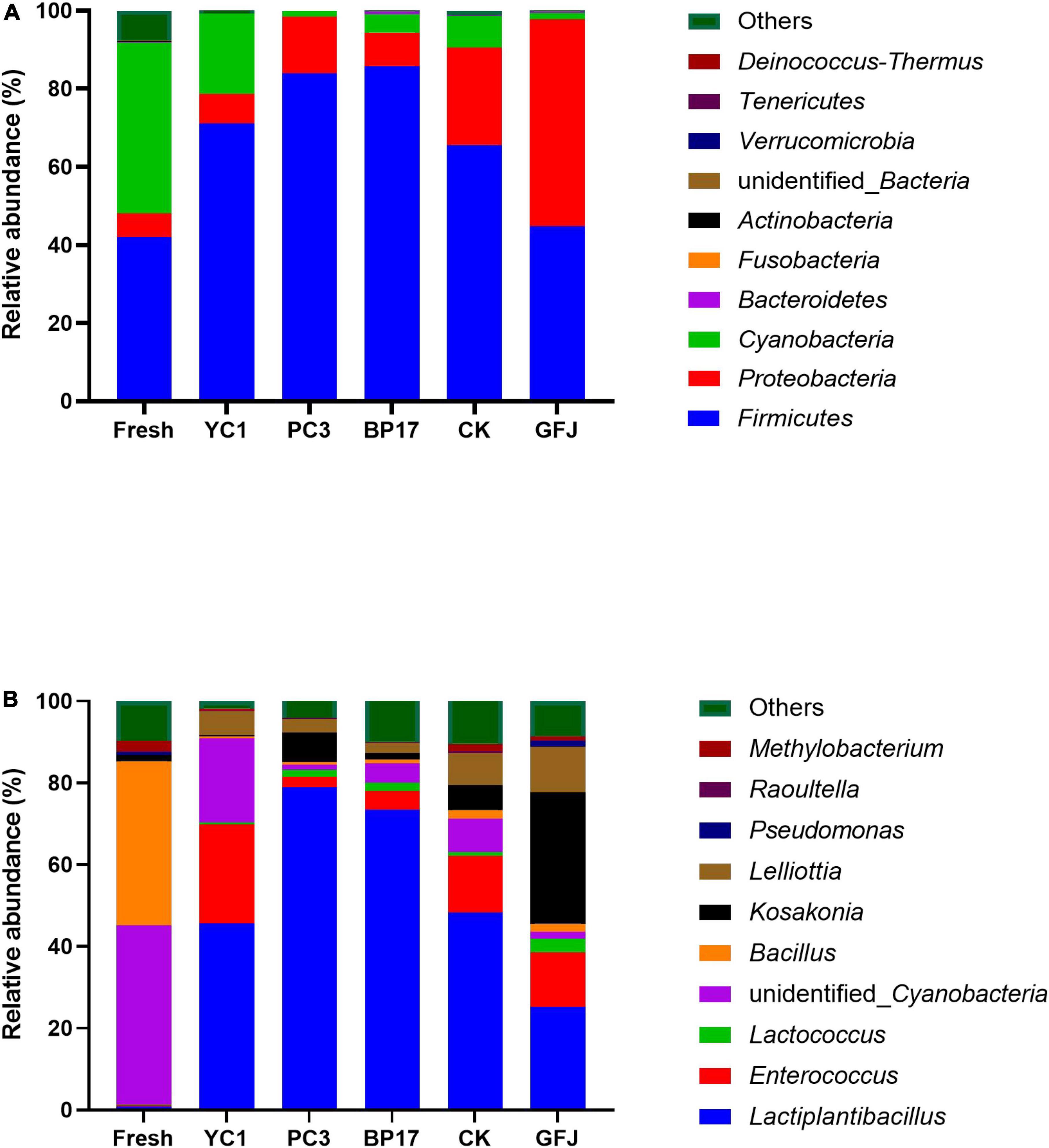
Figure 1. The relative abundance of bacteria at the phylum (A) and genus (B) levels in fresh paper mulberry and silages inoculated with saline (CK), with a commercial inoculant (GFJ, a combination of Lactiplantibacillus plantarum and Lentilactobacillus buchneri, 1.0 × 106 cfu/g of FM) or with one of the three selected strains (YC1, Lactiplantibacillus plantarum; PC3, Levilactobacillus brevis; BP17, Lactiplantibacillus plantarum) at 1.0 × 106 cfu/g of FM.
The fungal composition of PM silages is shown in Figure 2. Since well-fermented silages do not contain toxin-producing fungi, researchers rarely consider the fungal community in silage (Duniere et al., 2017). The main phyla detected in the fresh PM were Ascomycota and Basidiomycota (Figure 2A). A similar result was found in Zhang et al. (2019b), which reported that Ascomycota were highly abundant, followed by Basidiomycota, in silages. Compared to the PM silage, the fresh PM had a higher relative abundance of Ascomycota and a lower relative abundance of Basidiomycota. However, an opposite result was obtained by Bai et al. (2020), who found that Ascomycota abundance increased and Basidiomycota abundance decreased in alfalfa silage compared with fresh material. Hence, we attribute that LAB have different effects on the fungal communities of different forage species. The dominant fungal genus in fresh PM was Aureobasidium (Figure 2B), which has been reported to be the predominant genus on the skin of grapes (Gao et al., 2019). However, inconsistent results were reported by Chen et al. (2020b), who reported that the dominant fungal genera in fresh PM were Mortierella and Hannaella. Numerous factors, such as forage types and environmental factors, result in differences in the fungal community composition (Li et al., 2021). The dominant fungal genus in the PM silages was unclassified fungi (Figure 2B), which was similar to the results of Li et al. (2021), who found that unclassified fungi were the most abundant microorganisms in additive-treated timothy silage. Compared with the CK and GFJ-treated silages, the PC3- and YC1-treated silages had increased relative abundances of Strelitziana. However, this is the first study to report that Strelitziana is distributed in PM silage. Further study is needed to investigate the effect of Strelitziana on silage quality. Compared with the CK silage, the BP17- and PC3-treated silages had decreased relative abundances of Cladosporium. Cladosporium, which produces mycotoxins, is a mold commonly found in plants (Tabuc et al., 2011). The reduction in Cladosporium indicated that inoculation with BP17 and PC3 improved the quality of silage. The relative abundance of Gibberella was decreased in the BP17-treated silage compared with the CK silage, while it was decreased in the PC3-treated silage. Gibberella can produce mycotoxins such as zearalenone, a fungus commonly found in corn (Anderson et al., 2017). Thus, inoculation of PM silage with BP17 could inhibit mycotoxin. However, inoculation with PC3 showed a limited effect on the activity of Gibberella. In addition, the highest relative abundance of Isaria was observed in the PC3-treated silage. Several studies have shown that Isaria could be used for the biotransformation of flavonoids, glycosides, steroids, and other compounds (Dymarska et al., 2018; Kozłowska et al., 2018). There are few reports on Isaria in silage; therefore, additional research is needed to investigate the role of Isaria in silage.
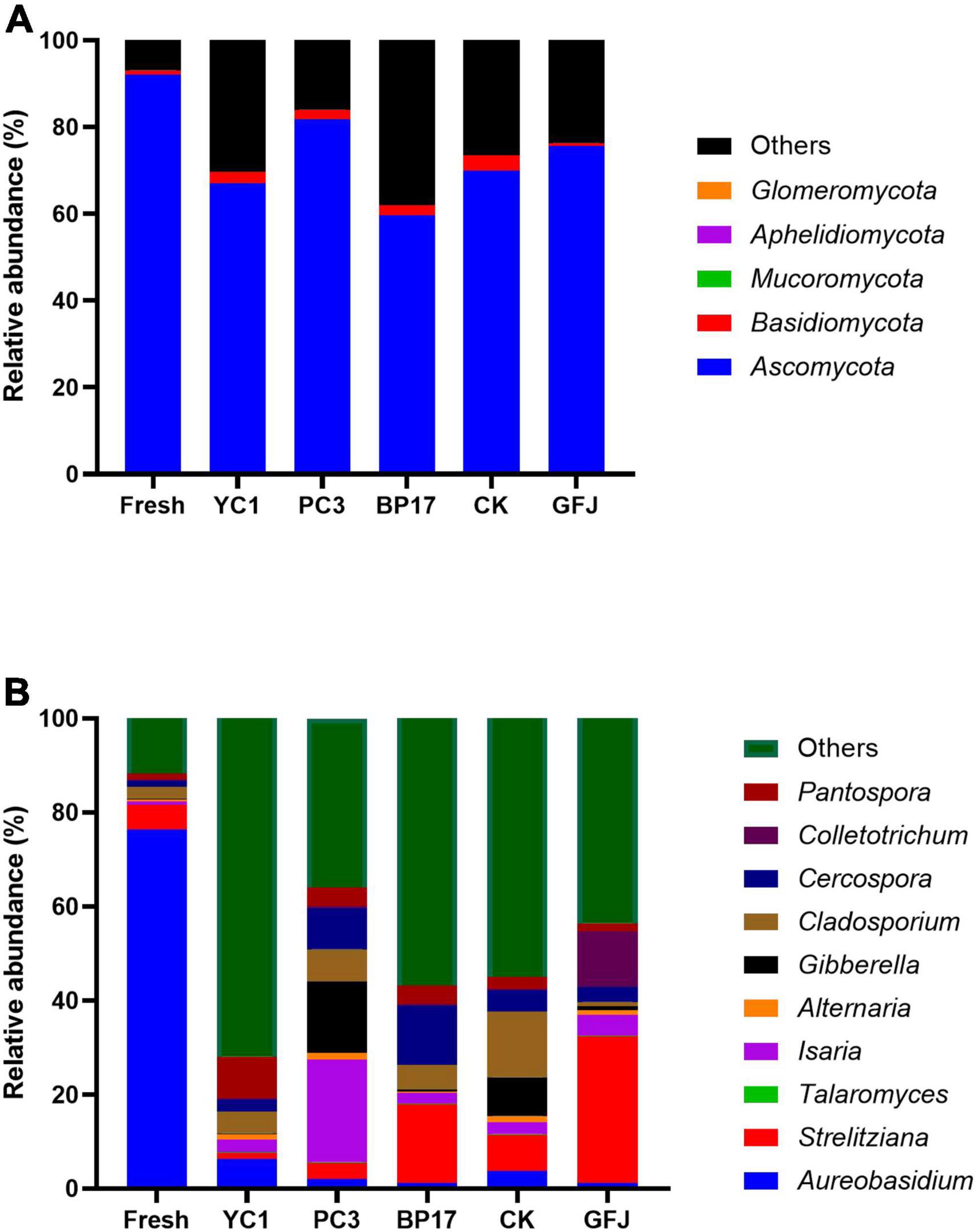
Figure 2. The relative abundance of fungi at the phylum (A) and genus (B) levels in fresh paper mulberry and silages treated inoculated with saline (CK), with a commercial inoculant (GFJ, a combination of Lactiplantibacillus plantarum and Lentilactobacillus buchneri, 1.0 × 106 cfu/g of FM) or with one of the three selected strains (YC1, Lactiplantibacillus plantarum; PC3, Levilactobacillus brevis; BP17, Lactiplantibacillus plantarum) at 1.0 × 106 cfu/g of FM.
These results suggested that inoculation with BP17 and PC3 increased the relative abundance of Lactiplantibacillus and decreased the relative abundance of Lelliottia and Cladosporium, resulting in improved PM silage quality.
Conclusion
This study evaluated the effect of LAB strains from various sources on the silage quality and the PM microbial community. Among the evaluated LAB strains, BP17 isolated from the fresh PM leaves and PC3 isolated from pickle could significantly improve the silage quality of PM, mainly in terms of high CP and LA contents and low pH and NH3-N contents. Inoculation with BP17 and PC3 increased the relative abundance of Lactiplantibacillus and decreased the relative abundances of Lelliottia and Cladosporium, thereby improving the silage quality of PM. This study showed that BP17 and PC3 could be used as silage additives.
Data availability statement
The original contributions presented in this study are included in the article, further inquiries can be directed to the corresponding author.
Authors contributions
PL and CC designed the experiments and revised the manuscript. ML, XF, YC, HS, YX, and YZ performed the experiments. QC and ML wrote the manuscript. QC carried out the data analysis. All authors reviewed and considered the manuscript.
Funding
This work was supported by the National Key Research and Development Subject (2021YFD1300302), the Guizhou Talent Base of Grassland Ecological Animal Husbandry (RCJD2018-13), the Guizhou University Introduced Talents Scientific Research Project (Guida Renji Hezi (2020)71), and the Guizhou University Cultivation Project (Guida Renji Hezi [2020]9).
Acknowledgments
We thank the Key Laboratory of Grassland Resources of the Ministry of Education and the Key Laboratory of Forage Cultivation, Processing and High Efficient Utilization of the Ministry of Agriculture and Rural Affairs and Novogene Company (Beijing, China) for providing technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Amaral, R. C., Carvalho, B. F., Costa, D. M., Morenz, M. J. F., Schwan, R. F., and Ávila, C. L. D. S. (2020). Novel lactic acid bacteria strains enhance the conservation of elephant grass silage cv. BRS Capiaçu. Anim. Feed Sci. Tech. 264:114472. doi: 10.1016/j.anifeedsci.2020.114472
Anderson, N. R., Luna, M. P., Ravellette, J. D., and Wise, K. A. (2017). Impact of foliar fungicides on gibberella ear rot and deoxynivalenol levels in Indiana Corn. Plant. Health Prog. 18, 186–191. doi: 10.1094/PHP-01-17-0010-RS
AOAC (1990). Official methods of analysis. Arlington, TX: Association of Official Analytical Chemists.
Arasu, M. V., Al-Dhabi, N. A., Rejiniemon, T. S., Lee, K. D., Huxley, V. A. J., Kim, D. H., et al. (2015). Identification and characterization of lactobacillus brevis p68 with antifungal, antioxidant and probiotic functional properties. Indian J. Microbiol. 55, 19–28. doi: 10.1007/s12088-014-0495-3
Arriola, K. G., Kim, S. C., and Adesogan, A. T. (2011). Effect of applying inoculants with heterolactic or homolactic and heterolactic bacteria on the fermentation and quality of corn silage. J. Dairy Sci. 94, 1511–1516. doi: 10.3168/jds.2010-3807
Bai, J., Xu, D., Xie, D., Wang, M., Li, Z., and Guo, X. (2020). Effects of antibacterial peptide-producing Bacillus subtilis and Lactobacillus buchneri on fermentation, aerobic stability, and microbial community of alfalfa silage. Bio. Technol. 315:123881. doi: 10.1016/j.biortech.2020.123881
Cai, Y., Benno, Y., Ogawa, M., and Kumai, S. (1999). Effect of applying lactic acid bacteria isolated from forage crops on fermentation characteristics and aerobic deterioration of silage. J. Dairy Sci. 82, 520–526. doi: 10.3168/jds.S0022-0302(99)75263-X
Cai, Y., Benno, Y., Ogawa, M., Ohmomo, S., Kumai, S., and Nakase, T. (1998). Influence of lactobacillus spp. from an inoculant and of weissella and leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microb. 64, 2982–2987. doi: 10.1128/AEM.64.8.2982-2987.1998
Chen, L., Bai, S., You, M., Xiao, B., Li, P., and Cai, Y. (2020a). Effect of a low temperature tolerant lactic acid bacteria inoculant on the fermentation quality and bacterial community of oat round bale silage. Anim. Feed Sci. Tech. 269:114669. doi: 10.1016/j.anifeedsci.2020.114669
Chen, P., Hu, Y., Tang, F., Zhao, M., Peng, X., and Shen, S. (2020b). Cooperation between Broussonetia papyrifera and its symbiotic fungal community to improve local adaptation of the host. Appl. Environ. Microb. 86, e420–e464. doi: 10.1128/AEM.00464-20
Cheng, Q., Chen, Y., Bai, S., Chen, L., You, M., Zhang, K., et al. (2021a). Study on the bacterial community structure and fermentation characteristics of fresh and ensiled paper mulberry. Anim. Sci. J. 92:e13656. doi: 10.1111/asj.13656
Cheng, Q., Li, P., Xiao, B., Yang, F., Li, D., Ge, G., et al. (2021b). Effects of LAB inoculant and cellulase on the fermentation quality and chemical composition of forage soybean silage prepared with corn stover. Grassl. Sci. 67, 83–90. doi: 10.1111/grs.12289
Danner, H., Holzer, M., Mayrhuber, E., and Braun, R. (2003). Acetic acid increases stability of silage under aerobic conditions. Appl. Environ. Microb. 69, 562–567. doi: 10.1128/AEM.69.1.562-567.2003
Davies, D. R., Merry, R. J., Williams, A. P., Bakewell, E. L., Leemans, D. K., and Tweed, J. K. S. (1998). Proteolysis during ensilage of forages varying in soluble sugar content. J. Dairy Sci. 81, 444–453. doi: 10.3168/jds.S0022-0302(98)75596-1
Dong, L., Liu, J., Zhong, Z., Wang, S., Wang, H., Huo, Y., et al. (2019). Dietary tea tree oil supplementation improves the intestinal mucosal immunity of weanling piglets. Anim. Feed Sci. Tech. 255:114209. doi: 10.1016/j.anifeedsci.2019.114209
Dong, L., Zhang, H., Gao, Y., and Diao, Q. (2020). Dynamic profiles of fermentation characteristics and bacterial community composition of Broussonetia papyrifera ensiled with perennial ryegrass. Bio. Technol. 310:123396. doi: 10.1016/j.biortech.2020.123396
Du, Z., Sun, L., Chen, C., Lin, J., Yang, F., and Cai, Y. (2021). Exploring microbial community structure and metabolic gene clusters during silage fermentation of paper mulberry, a high-protein woody plant. Anim. Feed Sci. Tech. 275:114766. doi: 10.1016/j.anifeedsci.2020.114766
Duniere, L., Sindou, J., Chaucheyras-Durand, F., Chevallier, I., and Thevenot-Sergentet, D. (2013). Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed Sci. Tech. 182, 1–15. doi: 10.1016/j.anifeedsci.2013.04.006
Duniere, L., Xu, S., Long, J., Elekwachi, C., Wang, Y., Turkington, K., et al. (2017). Bacterial and fungal core microbiomes associated with small grain silages during ensiling and aerobic spoilage. BMC Microbiol. 17:50. doi: 10.1186/s12866-017-0947-0
Dymarska, M., Janeczko, T., and Kostrzewa-Susłow, E. (2018). Glycosylation of 3-hydroxyflavone, 3-methoxyflavone, quercetin and baicalein in fungal cultures of the genus isaria. Molecules 23:2477. doi: 10.3390/molecules23102477
Gao, F., Chen, J., Xiao, J., Cheng, W., Zheng, X., Wang, B., et al. (2019). Microbial community composition on grape surface controlled by geographical factors of different wine regions in Xinjiang. China. Food Res. Int. 122, 348–360. doi: 10.1016/j.foodres.2019.04.029
Goto, I., and Minson, D. J. (1977). Prediction of the day matter digestibility of tropical grasses using a pepsin-cellulase assay. Anim. Feed Sci. Tech. 2, 247–253. doi: 10.1016/0377-8401(77)90028-1
Grant, M. A., Harrison, J. H., Rink, S., and Loney, K. A. (1994). Novel use of bacteria from pickle fermentation as a silage additive. 1. PH and Microbial Analysis1. J. Dairy Sci. 77, 3388–3400. doi: 10.3168/jds.S0022-0302(94)77281-7
Guo, L., Wang, X., Lin, Y., Yang, X., Ni, K., and Yang, F. (2021). Microorganisms that are critical for the fermentation quality of paper mulberry silage. Food Energy Secur. 10:e304. doi: 10.1002/fes3.304
Guo, L., Yao, D., Li, D., Lin, Y., Bureenok, S., Ni, K., et al. (2020). Effects of lactic acid bacteria isolated from rumen fluid and feces of dairy cows on fermentation quality, microbial community, and in vitro digestibility of alfalfa silage. Front. Microbiol. 10:2998. doi: 10.3389/fmicb.2019.02998
Hao, Y., Huang, S., Liu, G., Zhang, J., Liu, G., Cao, Z., et al. (2021). Effects of different parts on the chemical composition, silage fermentation profile, in vitro and in situ digestibility of paper mulberry. Animals 11:413. doi: 10.3390/ani11020413
Hao, Y., Huang, S., Si, J., Zhang, J., Gaowa, N., Sun, X., et al. (2020). Effects of paper mulberry silage on the milk production, apparent digestibility, antioxidant capacity, and fecal bacteria composition in holstein dairy cows. Animals 10:1152. doi: 10.3390/ani10071152
He, Q., Zhou, W., Chen, X., and Zhang, Q. (2021). Chemical and bacterial composition of Broussonetia papyrifera leaves ensiled at two ensiling densities with or without Lactobacillus plantarum. J. Clean. Prod. 329:129792. doi: 10.1016/j.jclepro.2021.129792
Heinritz, S. N., Martens, S. D., Avila, P., and Hoedtke, S. (2012). The effect of inoculant and sucrose addition on the silage quality of tropical forage legumes with varying ensilability. Anim. Feed Sci. Tech. 174, 201–210. doi: 10.1016/j.anifeedsci.2012.03.017
Hua, J., Xu, T., Shen, Q., Liu, Y., Huang, G., Rao, D., et al. (2020). Productive and metabolic increments of the inclusion of Broussonetia papyrifera to replace maize silage in growing goats. Czech J. Anim. Sci. 65, 303–310. doi: 10.17221/10/2020-CJAS
Keshri, J., Chen, Y., Pinto, R., Kroupitski, Y., Weinberg, Z. G., and Sela Saldinger, S. (2018). Microbiome dynamics during ensiling of corn with and without Lactobacillus plantarum inoculant. Appl. Microbiol. Biot. 102, 4025–4037. doi: 10.1007/s00253-018-8903-y
Kleinschmit, D. H., Schmidt, R. J., and Kung, L. (2005). The effects of various antifungal additives on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 88, 2130–2139. doi: 10.3168/jds.S0022-0302(05)72889-7
Kobayashi, H., Cai, Y., Uegaki, R., Shimizu, M., Nakajima, M., Kanaya, C., et al. (2010). Microorganism composition of high moisture italian ryegrass (Lolium multiflorum lam.) and its fermentation characteristics of silage inoculated with lactic acid bacteria. JPN J. Grassl. Sci. 56, 39–46. doi: 10.14941/grass.56.39
Kosako, Y., Tamura, K., Sakazaki, R., and Miki, K. (1996). Enterobacter kobei sp. nov., a new species of the family Enterobacteriaceae resembling Enterobacter cloacae. Curr. Microbiol. 33, 261–265. doi: 10.1007/s002849900110
Kozłowska, E., Hoc, N., Sycz, J., Urbaniak, M., Dymarska, M., Grzeszczuk, J., et al. (2018). Biotransformation of steroids by entomopathogenic strains of Isaria farinosa. Microb. Cell Fact. 17:71. doi: 10.1186/s12934-018-0920-0
Li, P., Lu, Y., Zhao, M., Chen, L., Zhang, C., Cheng, Q., et al. (2021). Effects of phenyllactic acid, lactic acid bacteria, and their mixture on fermentation characteristics and microbial community composition of timothy silage. Front. Microbiol. 12:743433. doi: 10.3389/fmicb.2021.743433
Liu, B., Huan, H., Gu, H., Xu, N., Shen, Q., and Ding, C. (2019). Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bio. Technol. 273, 212–219. doi: 10.1016/j.biortech.2018.10.041
Liu, Q. H., Yang, F. Y., Zhang, J. G., and Shao, T. (2014). Characteristics of Lactobacillus parafarraginis ZH1 and its role in improving the aerobic stability of silages. J. Appl. Microbiol. 117, 405–416. doi: 10.1111/jam.12530
Mcdonald, P., Henderson, A. R., and Heron, S. (1991). The biochemistry of silage. J. Wiley. 28, 125–125. doi: 10.1017/S0014479700023115
Muck, R. E., Nadeau, E. M. G., McAllister, T. A., Contreras-Govea, F. E., Santos, M. C., and Kung, L. (2018). Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 101, 3980–4000. doi: 10.3168/jds.2017-13839
Pang, X., Teng, L., Wang, X., Wang, Y., and Shen, S. (2014). De novo assembly of expressed transcripts and global transcriptomic analysis from seedlings of the paper mulberry (Broussonetia kazinoki x Broussonetia papyifera). PLoS One 9:e97487. doi: 10.1371/journal.pone.0097487
Si, B., Tao, H., Zhang, X., Guo, J., Cui, K., Tu, Y., et al. (2018). Effect of Broussonetia papyrifera L. (paper mulberry) silage on dry matter intake, milk composition, antioxidant capacity and milk fatty acid profile in dairy cows. Asian Austral. J. Anim. 31, 1259–1266. doi: 10.5713/ajas.17.0847
Tabuc, C., Taranu, I., and Calin, L. (2011). Survey of mould and mycotoxin contamination of cereals in south-eastern romania in 2008–2010. Archiva Zootechnica. 14, 25–38.
Tao, H., Si, B., Xu, W., Tu, Y., and Diao, Q. (2020). Effect of Broussonetia papyrifera L. silage on blood biochemical parameters, growth performance, meat amino acids and fatty acids compositions in beef cattle. Asian Austral. J. Anim. 33, 732–741. doi: 10.5713/ajas.19.0150
Tian, H., Chen, Y., Zhu, N., Guo, Y., Deng, M., Liu, G., et al. (2020). Effect of Broussonetia papyrifera silage on the serum indicators, hindgut parameters and fecal bacterial community of holstein heifers. AMB Exp. 10:197. doi: 10.1186/s13568-020-01135-y
Tohno, M., Kobayashi, H., Nomura, M., Kitahara, M., Ohkuma, M., Uegaki, R., et al. (2012). Genotypic and phenotypic characterization of lactic acid bacteria isolated from Italian ryegrass silage. Anim. Sci. J. 83, 111–120. doi: 10.1111/j.1740-0929.2011.00923.x
Umaña, R., Staples, C. R., Bates, D. B., Wilcox, C. J., and Mahanna, W. C. (1991). Effects of a microbial inoculant and(or) sugarcane molasses on the fermentation, aerobic stability, and digestibility of bermudagrass ensiled at two moisture contents. J. Anim. Sci. 69, 4588–4601. doi: 10.2527/1991.69114588x
Van Soest, P. J., Robertson, J. B., and Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Wang, S., Li, J., Dong, Z., Chen, L., Yuan, X., and Shao, T. (2018a). The effects of lactic acid bacteria strains isolated from various substrates on the fermentation quality of common vetch (Vicia sativa L.) in tibet. Grass Forage Sci. 73, 639–647. doi: 10.1111/gfs.12363
Wang, S., Dong, Z., Li, J., Chen, L., and Shao, T. (2018b). Pediococcus acidilactici strains as silage inoculants for improving the fermentation quality, nutritive value and in vitro ruminal digestibility in different forages. J. Appl. Microbiol. 126, 424–434. doi: 10.1111/jam.14146
Wang, Y., He, L., Xing, Y., Zhou, W., Pian, R., Yang, F., et al. (2019a). Bacterial diversity and fermentation quality of moringa oleifera leaves silage prepared with lactic acid bacteria inoculants and stored at different temperatures. Bio. Technol. 284, 349–358. doi: 10.1016/j.biortech.2019.03.139
Wang, S., Li, J., Dong, Z., Chen, L., and Shao, T. (2019b). Effect of microbial inoculants on the fermentation characteristics, nutritive value, and in vitro digestibility of various forages. Anim. Sci. J. 90, 178–188. doi: 10.1111/asj.13134
Wang, T., Teng, K., Cao, Y., Shi, W., Xuan, Z., Zhou, J., et al. (2020). Effects of Lactobacillus hilgardii 60TS-2, with or without homofermentative Lactobacillus plantarum B90, on the aerobic stability, fermentation quality and microbial community dynamics in sugarcane top silage. Bio. Technol. 312:123600. doi: 10.1016/j.biortech.2020.123600
Wang, X., Cao, X., Liu, H., Guo, L., Lin, Y., Liu, X., et al. (2021a). Effects of lactic acid bacteria on microbial metabolic functions of paper mulberry silage: A BIOLOG ECO microplates approach. Front. Microbiol. 12:689174. doi: 10.3389/fmicb.2021.689174
Wang, X., Liu, H., Xie, Y., Zhang, Y., Lin, Y., Zheng, Y., et al. (2021b). Effect of sucrose and lactic acid bacteria additives on fermentation quality, chemical composition and protein fractions of two typical woody forage silages. Agriculture 11:256. doi: 10.3390/agriculture11030256
Weinberg, Z. G., Shatz, O., Chen, Y., Yosef, E., Nikbahat, M., Ben-Ghedalia, D., et al. (2007). Effect of lactic acid bacteria inoculants on in vitro digestibility of wheat and corn silages. J. Dairy Sci. 90, 4754–4762. doi: 10.3168/jds.2007-0176
You, S., Du, S., Ge, G., Wan, T., and Jia, Y. (2021). Selection of lactic acid bacteria from native grass silage and its effects as inoculant on silage fermentation. Agron. J. 113, 3169–3177. doi: 10.1002/agj2.20720
Yuk, K. J., Kim, Y. T., Huh, C. S., and Lee, J. H. (2018). Lelliottia jeotgali sp nov., isolated from a traditional Korean fermented clam. Int. J. Syst. Evol. Micrcb. 68, 1725–1731. doi: 10.1099/ijsem.0.002737
Zhang, Y. C., Li, D. X., Wang, X. K., Lin, Y. L., Zhang, Q., Chen, X. Y., et al. (2019a). Fermentation dynamics and diversity of bacterial community in four typical woody forages. Ann. Microbiol. 69, 233–240. doi: 10.1007/s13213-018-1398-z
Zhang, L., Zhou, X., Gu, Q., Liang, M., Mu, S., Zhou, B., et al. (2019b). Analysis of the correlation between bacteria and fungi in sugarcane tops silage prior to and after aerobic exposure. Bio. Technol. 291:121835. doi: 10.1016/j.biortech.2019.121835
Zhang, Q., Yu, Z., and Wang, X. (2015). Isolating and evaluating lactic acid bacteria strains with or without sucrose for effectiveness of silage fermentation. Grassl. Sci. 61, 167–176. doi: 10.1111/grs.12097
Keywords: lactic acid bacteria, paper mulberry, silage, fermentation quality, microbial community
Citation: Cheng Q, Li M, Fan X, Chen Y, Sun H, Xie Y, Zheng Y, Chen C and Li P (2022) Effects of epiphytic and exogenous lactic acid bacteria on fermentation quality and microbial community compositions of paper mulberry silage. Front. Microbiol. 13:973500. doi: 10.3389/fmicb.2022.973500
Received: 20 June 2022; Accepted: 28 July 2022;
Published: 25 August 2022.
Edited by:
Qing Zhang, South China Agricultural University, ChinaReviewed by:
Smerjai Bureenok, Rajamangala University of Technology Isan, ThailandWang Tianwei, Chinese Academy of Sciences (CAS), China
Copyright © 2022 Cheng, Li, Fan, Chen, Sun, Xie, Zheng, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Li, bHB5em1Ac2luYS5jbg==
†These authors have contributed equally to this work
 Qiming Cheng
Qiming Cheng Maoya Li
Maoya Li Xueying Fan1,3
Xueying Fan1,3 Ping Li
Ping Li