
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 29 September 2022
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.969769
This article is part of the Research Topic Horizontal Gene Transfer Mediated Bacterial Antibiotic Resistance, Vol II View all 11 articles
 Shaqiu Zhang1,2,3*†
Shaqiu Zhang1,2,3*† Jinfeng Wen1†
Jinfeng Wen1† Yuwei Wang4†
Yuwei Wang4† Mingshu Wang1,2,3
Mingshu Wang1,2,3 Renyong Jia1,2,3
Renyong Jia1,2,3 Shun Chen1,2,3
Shun Chen1,2,3 Mafeng Liu1,2,3
Mafeng Liu1,2,3 Dekang Zhu1,3
Dekang Zhu1,3 Xinxin Zhao1,2,3
Xinxin Zhao1,2,3 Ying Wu1,2,3
Ying Wu1,2,3 Qiao Yang1,2,3
Qiao Yang1,2,3 Juan Huang1,2,3
Juan Huang1,2,3 Xumin Ou1,2,3
Xumin Ou1,2,3 Sai Mao1,2,3
Sai Mao1,2,3 Qun Gao1,2,3
Qun Gao1,2,3 Di Sun1,2,3
Di Sun1,2,3 Bin Tian1,2,3
Bin Tian1,2,3 Anchun Cheng1,2,3*
Anchun Cheng1,2,3*With the large-scale use of antibiotics, antibiotic resistant bacteria (ARB) continue to rise, and antibiotic resistance genes (ARGs) are regarded as emerging environmental pollutants. The new tetracycline-class antibiotic, tigecycline is the last resort for treating multidrug-resistant (MDR) bacteria. Plasmid-mediated horizontal transfer enables the sharing of genetic information among different bacteria. The tigecycline resistance gene tet(X) threatens the efficacy of tigecycline, and the adjacent ISCR2 or IS26 are often detected upstream and downstream of the tet(X) gene, which may play a crucial driving role in the transmission of the tet(X) gene. Since the first discovery of the plasmid-mediated high-level tigecycline resistance gene tet(X4) in China in 2019, the tet(X) genes, especially tet(X4), have been reported within various reservoirs worldwide, such as ducks, geese, migratory birds, chickens, pigs, cattle, aquatic animals, agricultural field, meat, and humans. Further, our current researches also mentioned viruses as novel environmental reservoirs of antibiotic resistance, which will probably become a focus of studying the transmission of ARGs. Overall, this article mainly aims to discuss the current status of plasmid-mediated transmission of different tet(X) genes, in particular tet(X4), as environmental pollutants, which will risk to public health for the “One Health” concept.
The discovery of antibiotics is a milestone event in human medicine. With the large-scale use of antibiotics, while reducing the morbidity and mortality of bacterial infections, strains carrying different antibiotic resistance genes (ARGs) appeared and spread rapidly (Davies and Davies, 2010; Ahmad and Khan, 2019). The global sales of antimicrobials are estimated to reach 104,079 tons in 2030, an increase of 11.5% since 2017 (Tiseo et al., 2020). Antimicrobial resistance (AMR) is one of the public health issues of widely concern around the world, and ARGs are regarded as new environmental pollutants (Plantinga et al., 2015; Zhang et al., 2020c).Tetracycline have many desirable properties of antibiotics, such as their excellent anti-bacterial activity and oral benefits. They have been widely used in the treatment of human and animal infections or as animal growth-promoting feed additives (Roberts, 2003). However, only a small part of tetracycline can be absorbed after entering the body, and more than 75% of tetracycline will be excreted in the form of a prototype or metabolite (Liao et al., 2021).
Tigecycline belonged to tetracycline-class drugs, is a new class of glycylcycline antibiotics, approved by the FDA in 2005 (Wenzel et al., 2005; Stein and Babinchak, 2013; Hirabayashi et al., 2021). It has broad-spectrum anti-bacterial activity, especially against multidrug-resistant (MDR) gram-negative bacteria (Zha et al., 2020). Tigecycline is also considered as a drug of last resort to combat bacterial infections, and which is mainly used for the treatment of infections within skin tissue, anti-tumor, bacterial pneumonia, and complex intra-abdominal (Olson et al., 2006; Kaewpoowat and Ostrosky-Zeichner, 2015; Zhao et al., 2021). Furthermore, it is a third-generation tetracycline-class antibiotic, which was improved by adding a 9-tert-butyl-glycylamido side-chain modification structure to the central framework of minocycline, and thereby forming a steric hindrance, overcoming normal mechanisms of resistance to tetracyclines, such as parts of the efflux pump mechanism[tet(A-E), tet(K)] and ribosome protection mechanism[tet(M)] (Chopra, 2002; Livermore, 2005; Linkevicius et al., 2016). Tigecycline can act on bacterial ribosomes and inhibit bacterial protein synthesis by interfering with aminoacyl-tRNA binding to ribosomes (Chopra and Roberts, 2001). We have gathered, appraised, and reviewed the accessible relevant literature from online sources, including Science Direct, PubMed, and Google Scholar. The keywords were included but not limited to tet(X) genes, Escherichia coli (E. coli), ISCR2, IS26, antibiotic resistant bacteria (ARB), AMR, ARGs, MDR, plasmids, environmental pollutants, public health, resistance contact, clinical and veterinary settings. Moreover, the cited references were also explored for further referencing. This article summarized the mechanisms of tigecycline resistance and the prevalence of the plasmid-mediated high-level tigecycline resistance gene tet(X4) among the environment, animals, and humans. In addition, the origin of the tet(X) and the importance of mobile genetic elements (MGEs) during the dissemination of the tet(X) are discussed. The purpose of this article is to collect and organize the information available so far in one platform, and to provide a bridge for readers to understand that the prevalence of plasmid-mediated high-level tigecycline resistance genes, which can contaminate the natural environment, and further risking to public health. Moreover, we also made a positive outlook for the transmission of ARGs by viruses.
At present, the main mechanisms of bacterial resistance to tigecycline are efflux pump mechanism, cell membrane pore channel protein variation, ribosome protection mechanism, and drug-degrading enzyme mechanism (Figure 1).
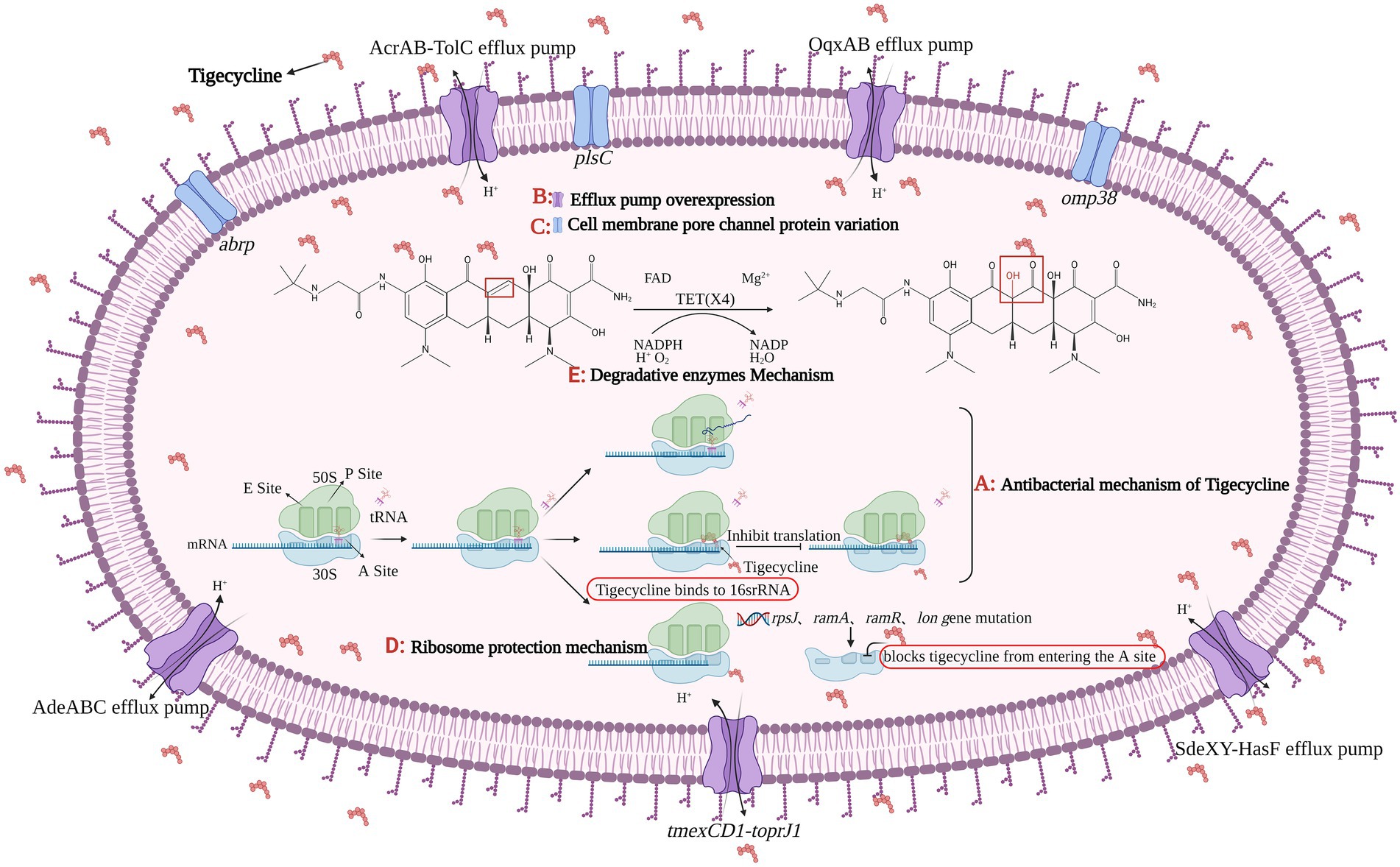
Figure 1. The figure was created with “BioRender.com” showing antibacterial mechanism and drug resistance mechanism of Tigecycline. (A) Tigecycline can act on bacterial ribosomes. After entering bacteria, tigecycline reversibly binds to the 16S rRNA in the 30S subunit of the ribosome, preventing tRNA from entering the A site, which eventually inhibits the process of transcription and translation in protein synthesis. There are four main tigecycline resistance mechanisms: (B) efflux pump overexpression; (C) cell membrane porin mutation; (D) ribosomal protection and (E) degrading enzyme mechanism. Among them, the expression product of plasmid-mediated tet(X4) gene belongs to the core member of degradative enzyme mechanism, and the Tet(X4) can catalyze the selective hydroxylation of tigecycline in the presence of FAD, Mg2+, O2 and NADPH, thus making tigecycline ineffective.
An active efflux pump is a protein transport system of bacteria, it can excrete antibiotics entering the bacteria from itself, reducing antibiotic concentration in bacteria, so as to promote the growth of ARB (Venter et al., 2015; Bankan et al., 2021). There are five main efflux pump families involved in the active efflux of antibiotics, one is the ATP binding cassette (ABC) superfamily, which is the “primary active” transporter that directly uses ATP binding and hydrolysis to drive the free efflux of drugs (Rempel et al., 2019). The other four families are secondary active transport proteins, which are energy-acquiring transporters with proton pumps, including the major facilitator super (MFS) family, multidrug and toxic compound extrusion (MATE) family, small multidrug resistance (SMR) family, and resistance modulation division (RND) superfamily (Kumar et al., 2016; Lamut et al., 2019). In Gram-negative bacteria, overexpression of MFS family and RND family efflux pumps plays a significant role in tigecycline resistance, such as Tet(A), AcrAB-TolC, OqxAB, and AdeABC (Ruzin et al., 2007; Zhong et al., 2014; Chen et al., 2017), Tet(A) and AcrAB-TolC efflux pumps have been studied relatively comprehensively (Munita and Arias, 2016), their coding genes can be located on chromosomes or plasmids and can be transmitted via plasmids or transposons (Sheykhsaran et al., 2019). As a tetracycline efflux pump gene, tet(A) has no effect on tigecycline sensitivity (Fluit et al., 2005), but studies showed the double frameshift mutation of tet(A) can make strains resistant to tigecycline at a low level (Hentschke et al., 2010; Akiyama et al., 2013). A new RND type efflux pump gene cluster, named tmexCD1-toprJ1, was first identified in Klebsiella pneumoniae (K. pneumonia) in 2020. TmexCD1-toprJ1 is widely present in K. pneumoniae, leading to a 4–32 fold increase in the minimal inhibitory concentration (MIC) of K. pneumoniae to tigecycline and eravacycline (Lv L. et al., 2020).
The 1-acyl-3-glycerol phosphatidyl transferase encoded by the plsC gene is located on the cell membrane of E. coli, and its primary function is to catalyze the synthesis of phospholipids, and then participate in the biosynthesis of bacterial cell membranes (Lu et al., 2005). By inducing Acinetobacter baumannii (A. baumannii) to be resistant to tigecycline, the researchers performed whole-genome sequencing analysis of the strains before and after induction, and found three factors that could reduce the sensitivity of tigecycline, which were the frameshift mutation of plsC and omp38 as well as SNP synonymous mutation (Li et al., 2015). A new abrp gene was found in A. baumannii, which encodes the C13 family of peptidases and makes the bacteria less sensitive to tigecycline (Li et al., 2016).
The rpsJ gene can encode the production of the ribosomal structural protein S10. When there is a 12 bp deletion in rpsJ, the amino acid Rath at positions 53–56 of the S10 protein will be removed, resulting in a change in the binding site of tigecycline and bacteria, making bacteria resistant to tigecycline (Beabout et al., 2015; Bender et al., 2020). In addition to the S10 protein, mutations in the S3 and S13 proteins can also make bacteria resistant to tigecycline (Lupien et al., 2015). In K. pneumoniae, mutations in the ramR operon, ramA, lon, and rpsJ genes result in decreasing bacterial sensitivity to tigecycline (Fang et al., 2016). Mutation of rpsJ in Enterococci also leads to resistance to tigecycline (Cattoir et al., 2015). Mutations in the rff, ropB and adeS genes in A. baumannii can affect the normal function of the ribosome and thus confer tigecycline resistance to the strain (Hua et al., 2021).
Tet(X) is a FAD-dependent monooxygenase that regioselectively hydroxylates tetracycline substrates, leading to the non-enzymatic breakdown of an unstable compound (Ghosh et al., 2015). Tet(X) can only produce effect in the presence of FAD, NADPH, Mg2+, and O2 at the same time (Moore et al., 2005). Researchers proved that tigecycline was a substrate of Tet(X) by X-ray crystallography (Volkers et al., 2011), and in fact, Tet(X) can effectively degrade almost all tetracycline antibiotics, making bacteria resistant to tetracycline (Ghosh et al., 2015; Xu et al., 2022). Tet(X) gene was originally isolated from the anaerobic bacteria Bacteroides fragilis (Speer et al., 1991), however, according to recent reports, tet(X) appeared in Riemerella anatipestifer (R. anatipestifer) as early as the 1860s (Zhang et al., 2021a). In 2004, the tet(X) gene and its variant tet(X2) were discovered in anaerobic Bacteroides, then pointing out Tet(X) can degrade tigecycline, although it showed low levels of resistance to tigecycline, this phenomenon would still exist when tet(X) was transferred into E. coli (Guiney et al., 1984; Yang et al., 2004). Various tet(X) gene variants mediate different levels of tigecycline resistance. Compared with the Tet(X-X7), the enzymatic activity of the Tet(X4) has increased significantly. Researchers found five key residues (H231, M372, E43, R114, D308) could affect Tet(X4) enzyme activity in the tetracycline and FAD binding regions of the Tet(X4) (Xu et al., 2019). Subsequently, a new study has identified five mutants (L282S, A339T, D340N, V350I and K351E) in the structural domain of Tet(X2) when compared to Tet(X4), and demonstrated that the MIC of tigecycline increased 2–8 folds, when these five amino acid residues were mutated in the Tet(X2)-producing strain (Cui et al., 2021).
The plasmid-mediated tigecycline resistance genes tet(X3) and tet(X4) were first isolated from animal samples in 2019, which mediate high levels of antibiotic resistance to tigecycline, the MIC value can reach 32–64 mg/l (He et al., 2019). Tet(X4) is most commonly found in mobile plasmids and occasionally in chromosomes (Sun J. et al., 2019, 2020; Li et al., 2020b). Since the report of tet(X3/4), the degradative enzyme mechanism has gained more and more attention (He et al., 2019; Xu et al., 2022). At present, bismuth drugs and plumbagin can be used as Tet(X) inhibitors to improve the sensitivity of strains to tigecycline, which provides a new therapeutic strategy for the treatment of tigecycline-resistant bacterial infections (Deng et al., 2022; Xu et al., 2022).
Although, the tet(X) gene was first isolated from the anaerobic Bacteroidetes, the current study points the origin of the tet(X) to R. anatipestifer, the tet(X) and its variants share the same ancestry with the monooxygenase gene carried in the chromosomes of Flavobacteriaceae bacteria. In Zhu’s study, 170 of 212 strains of R. anatipestifer carried the tet(X) gene (Zhu et al., 2018). Among 6,692 strains isolated from 13 different hospitals, almost all of the tet(X)-positive strains belonged to the Flavobacteriaceae. They then performed a phylogenetic analysis of the different evolutionary patterns of tet(X), in which one of the pathways involving the Flavobacteriaceae produced a major evolutionary branch, suggesting that it can be considered as the potential ancestral source of tet(X) (Zhang et al., 2020a). Umar et al. collect 57 non-repetitive sequences of R. anatipestifer in GenBank, of which tet(X) gene was detected in 47 genomes, and they have high similarity when compared with tet(X4) gene (Umar et al., 2021). The same finding was also reported in other study (Cui et al., 2021). When analyzing the evolutionary trajectory of the tet(X) gene, they found that most of the tet(X)-positive strains belonged to the Flavobacteriaceae, it has a higher detection rate than other species and is widely distributed in different clades of tet(X). Their latest study also inferred that the tet(X) gene originated in Flavobacteriaceae and can be transmitted to environmental and clinical strains such as E. coli and Acinetobacter with the help of the mobilization of ISCR2 element (Chen et al., 2020).
The MGE such as ISCR2 and IS26 are essential for the spread of tet(X) gene. A 4608 bp element consisting of an ISCR2, a tet(X4) and a partner gene catD forms a canonical RC transposable unit (RC-TU) mediated by ISCR2, of which the 2,760 bp element of catD-tet(X4) is highly conserved. When transposition occurs, the ISCR2-catD-tet(X4)-ISCR2 composite transposon structure is often generated, and the upstream or downstream of ISCR2 element may be inserted and truncated by other IS elements, such as IS26 (Chen et al., 2021; Liu et al., 2022). In addition, only single-copy ISCR2 elements was sufficient to transpose adjacent DNA sequences through the process of rolling circle transposition (Poirel et al., 2009; Partridge et al., 2018). IS26 was also often found in plasmids resistant to antibiotics, and it can participate in the progress of plasmid fusion and gene recombination (He et al., 2015; Du et al., 2020; Li et al., 2020b), and IS26 can also be inserted into both ends of RC-TU, allowing ISCR2 residues-tet(X4) to spread through a novel transmission mechanism (Liu et al., 2022). It has been found that the ISCR2 element is frequent adjacent to tet(X4) or other tet(X) variants, which suggests ISCR2 is more likely to participate in spread of tet(X) variants (Wang L. et al., 2019; Liu et al., 2020; Fu et al., 2021). In a conserved genetic environment and uncertain transferability among different bacteria, the co-action of ISCR2 and IS26 may be the main driving forces for the widespread of tet(X4; Dai et al., 2022; Zhang et al., 2022).
Tetracycline resistance genes speculated to be of environmental origin but are now widely distributed in commensal and pathogenic bacteria (Thaker et al., 2010). The extensive use of first or second-generation tetracycline-class drugs played a major role in the emergance of tetracycline resistance genes, especially oxytetracycline, chlortetracycline, and doxycycline (Aminov, 2021). Since the discovery of the plasmid-mediated high-level tigecycline resistance genes tet(X3/X4) in 2019, reports of tet(X) have gradually increased around the world (Table 1). Tet(X4)-positive strains have spread globally and have been detected in animals, humans and the environment, which largely limited the use of tigecycline (Xu et al., 2022). The tet(X) gene and its variants were present in 23 countries on six continents (Pan et al., 2020; Wang J. et al., 2021), which are also widely present in various bacterial species, including R. anatipestifer, E. coli, Acinetobacter, K. pneumoniae, Salmonella, Proteus, La Providencia bacteria, Bacteroides bacteria, Pseudomonas bacteria, and Aeromonas caviae (Chen et al., 2019a, 2020). Moreover, most of the tet(X4) genes are located on different types of plasmids such as IncQ1, IncX1, IncFIB, IncHI1, F-:A18:B-, ColE2-like, IncN, p0111 and hybrid plasmids (Fang et al., 2020), among which the IncX1 type is the most common (Cai et al., 2021; Cui et al., 2022). The Nomenclature Center1 recommends that only tet(X) will be used in the future, because the tet(X) gene variant DNA similarity is in the range of 83–100% among tet(X2)-tet(X14), corresponding amino acid similarity is between 82 and 100%, which is greater than the standard of 79% amino acid similarity. In this article, for the convenience of description, the previous classification method is still used. This article also summarizes the prevalence of tet(X) gene and its variants in China in recent years as shown in Figure 2.
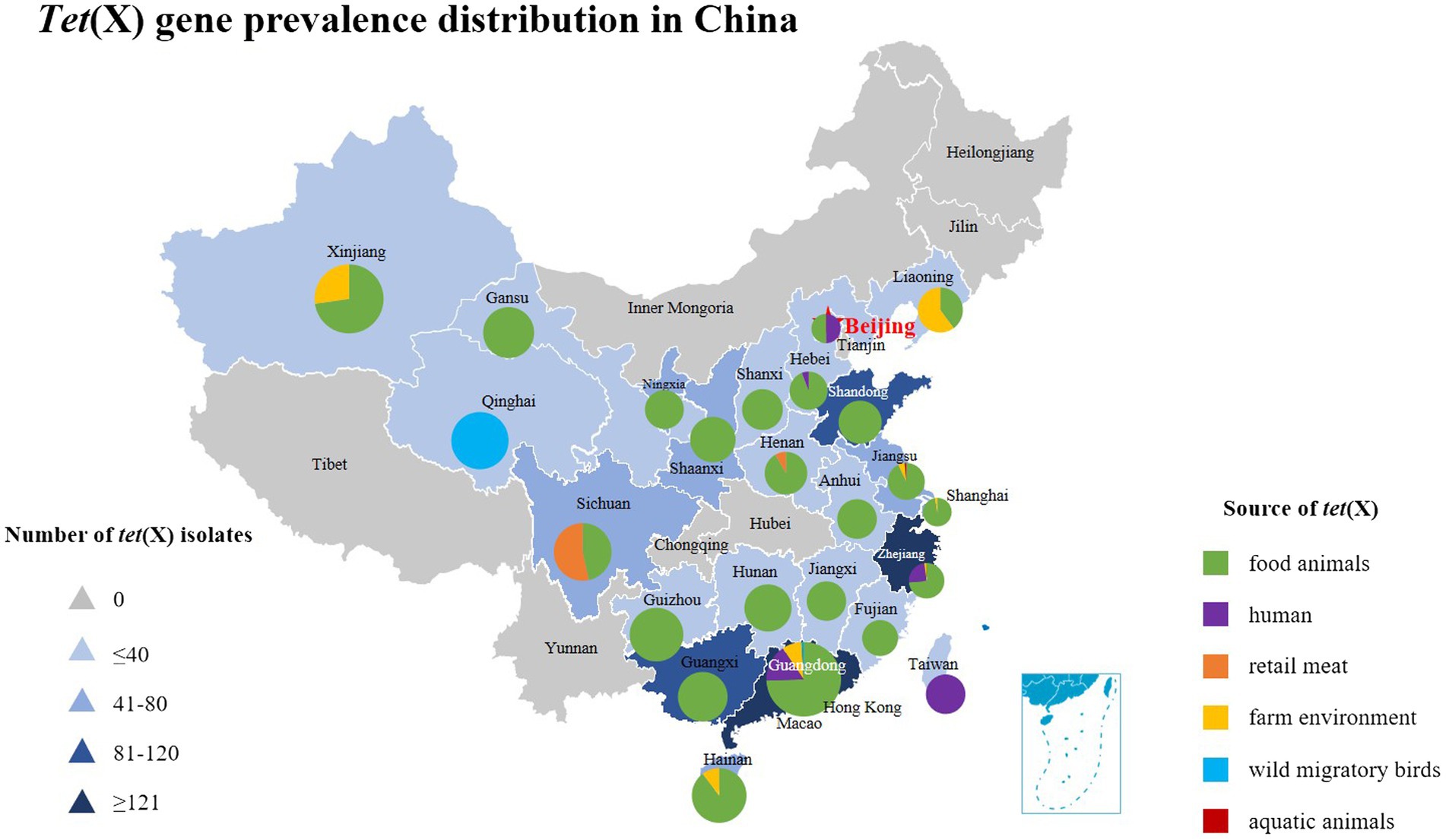
Figure 2. The distribution of tet(X) genes in different parts of China showing these genes have been found in 24 provinces from 2015 to 2022. The triangle in the figure indicates the number of tet(X)-positive strains isolated in each region of China, and the square indicates different sources of tet(X) genes, which corresponds to the pie chart in the figure. Furthermore, the different sources of tet(X) genes in the listed provinces can also be found, using color indication.
Antibiotics are commonly used in livestock production to maintain animal health and productivity. However, the absorption of antibiotics in the body is low, and most of them are excreted in the form of metabolites with feces and urine (Qiu et al., 2016). The antibiotic residues and ARGs carried in animal feces can be transmitted to the environment or humans, showing a potential source of ARGs (Ji et al., 2012; Van Boeckel et al., 2015). Tigecycline is currently approved for Human clinical use only, but the tet(X4) gene has been detected in food animals, retail meat, aquatic animals, and wild animals (Figure 3). Moreover, tet(X4) is currently detected in isolates from various animal origin samples, including pigs, ducks, geese, chickens, cattle, freshwater fish and shrimp, and migratory birds, with pig sources in particular predominating (Table 1). In a study based on a metagenomics approach, it was shown that among the abundant of ARGs in pig manure and its receiving environment (sewage, crops, soil, etc.), the tetracycline resistance genes were prevalent in pig farms (Tong et al., 2022). The same is true for pig slaughterhouses, suggesting that tet(X4)-carrying plasmids play an essential role in the spread of this drug related ARGs (Li et al., 2020b). Worth noting that the first isolation of plasmid mediated-tet(X4) was also obtained from the pig-derived sample (He et al., 2019). So far, 24 provinces in China have reported the emergence of tet(X), with Guangdong, Zhejiang, and Shandong having the largest number of positive strains (Figure 2). Li et al. (2021c) isolated 32 tet(X4)-positive strains from feces and anal swabs of pigs in Shanxi. At the same time, tet(X4)-positive E. coli were also detected in the sewage and soil of the pig farm environment. These isolates have different ST types, but their tet(X4)-carrying plasmids have the same replicon type, indicating that these plasmids are transferred horizontally among different reservoirs, and horizontal transfer maybe the main way for tet(X4) to spread in the surrounding environment (Sun J. et al., 2019). During 2016–2018, researchers isolated the tet(X)-positive Acinetobacter from pig, chicken, duck and goose feces in multi-regional farms of seven provinces, China (Guangdong, Hainan, Guangxi, Fujian, Shandong, Xinjiang, and Liaoning; Cui et al., 2020). Zhang et al. have detected 51 (17%) tet(X)-positive strains from 296 rectal swabs of healthy dairy cows, including the strains of tet(X3)-positive Acinetobacter and tet(X4)-positive E. coli (Zhang et al., 2020b). The prevalent range of tet(X) continues to expand, tet(X) and its variant genes have been detected in different reservoirs, and tet(X)-carrying plasmids have high mobility, which can be transmitted horizontally among different species.
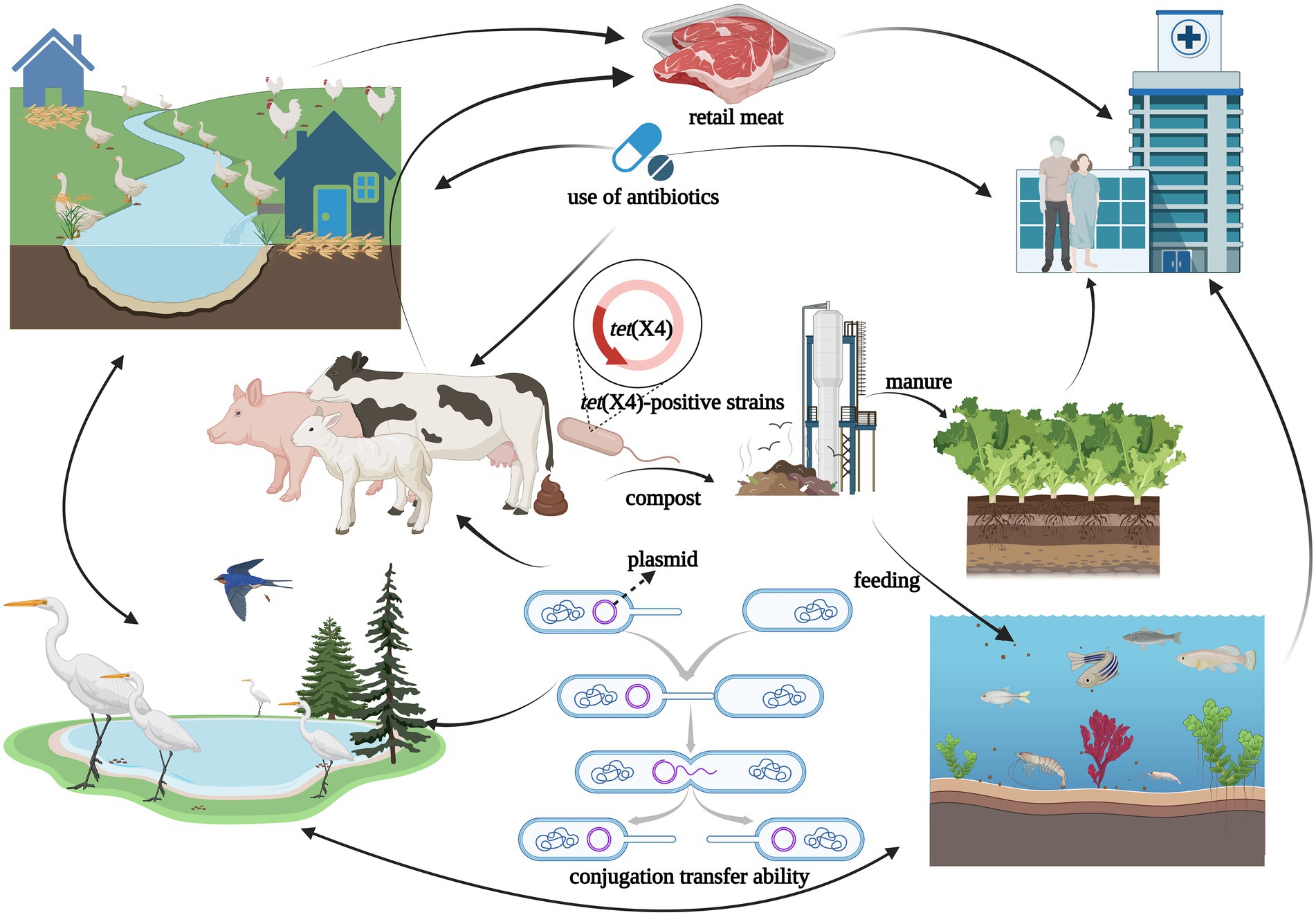
Figure 3. The figure was created with “BioRender.com” showing transmission routes illustration of tet(X4)-positive strains in natural environment. Possible dissemination routes of tet(X4)-positive strains showed by arrows among different reservoirs such as ducks, geese, migratory birds, chickens, pigs, cattle, aquatic animal, agricultural field, meat, and humans. The horizontal transmission of tet(X4) among reservoirs risked to public health for the “One Health” concept.
The co-existence of tet(X4) with other important ARGs is noteworthy. Specifically, the tet(X) gene co-existed with the flor gene in most cases, the latter encoding chloramphenicol efflux pumps, which can be also co-transferred (Du et al., 2004; Fu et al., 2021). Further, ESBL genes and colistin resistance genes often co-existed with tet(X4) in Enterobacteriaceae (Table 1). In a retrospective study, five pig-derived tet(X4)-positive strains were detected in Sichuan, Henan, and Guangdong of China, and two of these tet(X4)-positive E. coli also carried the mcr-1 gene (Sun C. et al., 2019). Tang et al. (2021) found eight tet(X4)-positive strains in two commercial pig farms in Sichuan, and three of them co-existed with the cfr gene in E. coli, and both ARGs were located on a novel hybrid plasmid, which could be transferred to the recipient bacteria. Li et al. (2020c) screened one strain of tet(X4)-positive E. coli and two strains of tet(X6)-positive aspergillus in different chicken farms, while the tet(X6) gene co-existed with the carbapenem resistance gene blaNDM-1. The same situation also existed in other country, where the tet(X4) gene was detected to co-exist with the colistin resistance gene in Pakistan (Mohsin et al., 2021; Li et al., 2022). Specifically, Li et al. (2022) detected 36 tet(X4)-positive strains, of which 24 tet(X4)-positive strains co-carried the mcr-1 gene. Mohsin et al. (2021) detected four tet(X4)-positive E. coli from farm animals and slaughterhouse effluents, and three E. coli contained the mcr-1.1 gene. It should be noted that the resistance to tigecycline or colistin can be transferred by the transmission of plasmids, which posed an enormous threat to the clinical treatment of MDR bacterial infections (Ruan et al., 2020; Xu et al., 2021; Zhang et al., 2021b).
Food animals such as pigs and poultry are the primary source of high-quality protein for humans (Henchion et al., 2014), they have been slaughtered in slaughterhouses before entering the market, and tet(X) has also been detected in retail meat, which indicated that the slaughterhouse might be a potential reservoir for tet(X) (Homeier-Bachmann et al., 2021; Mohsin et al., 2021). There are also some reports on tet(X) from retail meat sources in Sichuan and Henan. In 2019, Sun et al. collected 311 retail meat samples from Sichuan province and detected 25 tet(X4)-positive E. coli strains, most of which were isolated from the raw pork (52%), chicken (40%), duck (4%), and beef (4%; Sun et al., 2021a). In addition, five tet(X4)-positive E. coli strains were isolated from retail chicken during routine monitoring of ARGs in the Sichuan market in 2020. Interestingly, one of the tet(X4)-carrying plasmids from retail chicken was 99% identity to the pig-derived tet(X4)-carrying plasmid, and others had the tet(X4) gene localized on hybrid plasmids (Lv H. et al., 2020). This phenomenon suggests that tet(X4)-carrying plasmids can spread among different animals, which lead to the dissemination of tet(X4) in the ecological environment.
In addition to food animals, tigecycline resistance genes have also been detected in wild animals. In 2018, Chen et al. (2019b) isolated three strains of tet(X4)-positive E. coli from the feces of migratory birds in Guangdong, two of which were located on the plasmid, and the remaining one was located on the chromosome. The tet(X4)-carrying plasmid isolated from the migratory birds had a high degree of similarity with one plasmid isolated from human samples. In addition, five tet(X4)-positive Acinetobacter were also isolated from the bar-headed goose samples in Qinghai (Chen et al., 2020). In the latest report, researchers also detected the tet(X) variant genes in wild fish and shrimp (Li et al., 2019; Concha et al., 2021). Wild animals were not directly exposed to clinical antibiotics, but more and more ARGs were detected in them, indicating wild animals including migratory birds, were likely to be involved in the large-scale exchange of ARGs, especially long-distance transmission of cross species (Allen et al., 2010; Wang et al., 2017; Zeballos-Gross et al., 2021; Luo et al., 2022).
Tigecycline was approved for clinical use in 2005, and which was introduced in China in 2012. Tet(X) was detected in human clinical samples in 2013, with 11 tet(X) positive strains isolated from 52 samples, including stool, semen, blood, and urine in a Sierra Leonean hospital (Leski et al., 2013). Ding et al. (2020) conducted a retrospective screening study on 109 fecal samples, and detected tet(X4)-positive strains in the intestinal microflora of healthy human, with an isolation rate of 10.1%. Subsequently, tet(X4)-positive E. coli were also reported in clinical isolates from Guangdong, Hebei, Zhejiang, Beijing, Sichuan, and other places in China (Table 1). It can be seen that the tet(X) gene is not uncommon in hospital clinical isolates, and tet(X4) may be widely distributed in the human gut microflora, with great risk of transmission. In 2019, Cui et al. (2022) collected 1,001 stool samples from hospital inpatients in Guangdong Province of China, isolated 48 (4.8%) tet(X4)-positive E. coli. Notably, the hybrid plasmid was found to be prevalent in tet(X4)-positive strains of animal origin, with the characteristics of stable existence and horizontal transfer (Sun C. et al., 2019), which predicted this tet(X4)-carrying plasmid can be transmitted among humans, animals and the environment, thus facilitating the wide spread of tet(X4) in the ecosystem. The co-existence of tet(X4) with mcr and ESBL genes in the clinical setting is a great concern. Ruan et al. (2020) found one E. coli strain co-harboring tet(X4) with mcr-1 on the same conjugative plasmid from the urine sample of a clinical patient in Zhejiang Province, China. Further, two E. coli strains carrying both mcr-1 and tet(X4) were isolated in Singapore (Ding et al., 2020). Meanwhile, blaCTX, blaOXA, blaNDM, and blaSHV genes were also detected to be co-existence with tet(X) in one strain (Table 1). Tigecycline and colistin are the last resort for treating MDR bacteria, and the co-existence of tet(X) with mcr and ESBL genes limited the choice of clinical antibiotics, which subsequently poses a significant threat to public health.
ARB are persistent pollutants in the environment in which humans are in close contact (Kim and Aga, 2007). ARB can be transmitted to other hosts through human activities when conditions are favorable (Allen et al., 2010). Except for the hospital clinical environment, the live poultry market (LPM) is also a vast reservoir of ARGs (Wang Y. et al., 2019; Wang et al., 2021b). The tet(X3) and tet(X4) genes have been detected in the intestinal flora of LPM workers and the surrounding environment (Wang Y. et al., 2020), which indicated that the plasmid-mediated tigecycline resistance gene might exist in LPM for a long period. The ARGs are likely to be transmitted from live poultry to LPM staff, ecological environments or other animals.
Antibiotics and ARGs were detected in various environments (Qiao et al., 2018). The humans, animals, and ecological environments are components of the “One health” concept, and they have important connections and can influence each other. Therefore, they can acquire ARGs through different pathways and achieve the flow of ARGs among different reservoirs (Anyanwu et al., 2021), including tet(X4) (Figure 3). In recent years, the environment has played an increasing role on the spread of antibiotic resistance (Finley et al., 2013; Bengtsson-Palme et al., 2014; Bondarczuk et al., 2016; Lerminiaux and Cameron, 2019). The ARGs and ARB existed in large numbers within the environment and can be transmit to reservoirs (Lin et al., 2021), such as rivers contaminated by animal manure, the soil around livestock farms, manure-irrigated agricultural fields, and sewage treatment plants. The abuse use of antibiotics and the spread of antibiotic resistance caused by animal husbandry is one of the main concerns of sustainable agriculture(Manyi-Loh et al., 2018), where the use of first or second-generation tetracycline-class drugs was high, with subtherapeutic dosing in the forage (Yezli and Li, 2012). In animal husbandry, a wider range of antibiotic options lead to the spread of ARGs in agriculture to the human microbiota (Aminov, 2011). Animal manure as the valuable renewable fertilizer was often applied to the cropland (Zhou X. et al., 2019; Lima et al., 2020), which was found to contain different ARB and ARGs. Moreover, water as a good transport route for nutrients and contaminants was also a major reservoir for ARGs (Vaz-Moreira et al., 2014; Manaia et al., 2016; Miłobedzka et al., 2022). Specifically, macrogenomic analysis of wetland effluents and sediments in the Yangtze Delta region revealed a high abundance of the tet(X) gene (Du et al., 2022). Tet(X) and their variants were detected in farm soil, manure, and lettuce samples near chicken farms in Jiangsu, Jiangxi, and Sichuan provinces of China, and even in soil samples far from these farms (He et al., 2021). Cui et al. (2020) collected samples from some poultry farms in seven provinces across China, where tet(X)-positive strains from sewage and soil were isolated at 7.5% and 6.7%, respectively, and tet(X) was detected to be localized on the same plasmid with blaNDM-1. These reports on identification and analysis of tet(X4) in the farm environment suggest that animal manure, sewage, and soil can influence with each other in this ecology. Moreover, tet(X4) can be transmitted among them, and the farm environment may be a massive reservoir of ARGs.
The phenomenon of MDR of bacteria is a significant concern worldwide. Colistin and tigecycline are considered as the last resort drugs against carbapenem-resistant bacteria (Cunha et al., 2017; Zhou Y. et al., 2019). Either the global distribution of colistin-resistant E. coli or the rapid spread of the carbapenem-resistant Enterobacteriaceae have created enormous challenges for public health security. It is a more and more headache to solve the infection caused by MDR pathogens in human clinical treatment and animal husbandry (Gao et al., 2016; Potter et al., 2016; Rehman et al., 2020; Zhang et al., 2021b, 2021c). As a result, tigecycline has been recognized as the important antibiotic of last resort for the clinical treatment of certain bacterial infections. Through this article, we found that the tet(X) is prevalent on six continents around the world, with China having the highest prevalence, and most of tet(X4)-carrying plasmids can spread tigecycline resistance among different bacteria by means of horizontal transfer.
The mechanisms that cause antibiotic resistance to tigecycline are mainly overexpression of active efflux pump and ribosomal protection mechanisms. However, more and more tet(X4) has been detected in plasmids, and many different types of tet(X4)-carrying plasmids have strong ability of horizontal transfer, which means plasmids mediated transmission of tigecycline resistance genes may gradually increase, risking to public health (Pereira et al., 2021). The widespread use of antimicrobial drugs in domestic animals is an important reason for the rapid increase of AMR. The researchers reported the AMR monitoring results of E. coli in China’s pig farms from 2018 to 2019, showing that multidrug resistance was detected in 91% of isolates (1871 in total), and resistance to last resort drugs including tigecycline, colistin and carbapenem was found (Peng et al., 2022). Recent studies have also found the antibiotic resistance of livestock has increased from 1970 to 2019, indicating that if the use of antibiotics is not restricted, it may not be able to effectively protect the livestock. By testing the sensitivity of several recent strains of E. coli to various antibiotics, researchers found their resistance was far higher than that of the strains in the 1970s. In addition, the researchers also pointed out although the specific antibiotics used to treat bacterial infections may be different, the types are often the same, so the rapid rise in drug resistance will eventually affect human beings (Yang et al., 2022). Surprisingly, the potential spread of virus-mediated ARGs is likely to exacerbate AMR, including tetracycline resistance and harm to public health (Calero-Cáceres et al., 2019; Debroas and Siguret, 2019; Shi et al., 2022), which needs our wider attention. Moreover, viruses might be linked to Enterobacteriaceae or Vibrionaceae and were considered as gene shuttles in ARGs transfer, like plasmids. This indicates that viruses and bacteria may have a synergistic effect on the transmission of ARGs. Therefore,we should look at AMR from a holistic perspective that includes humans, animals as well as the environment, and develop a plan for rational use of antibiotics to reduce the long-term and single use of tigecycline in the clinical environment, avoiding reduced clinical efficacy and increased mortality (Yahav et al., 2011). Controlling the “spillover effect” of ARGs is also important from “One Health” concept (Collignon, 2015; Tyrrell et al., 2019; Olesen et al., 2020; Aslam et al., 2021). In-depth studies of tigecycline resistance or transmission mechanisms, and continuous monitoring of tet(X) prevalence are urgent needed to determine the precise transmission route of ARB and ARGs, so as to provide reference for designing more effective public health intervention strategies. However, due to the limitation of the length of the article, we did not summarize the current methods and strategies of various countries or regions to limit the transmission of tet(X4)-positive strains, and what beneficial substances (like probiotics, prebiotics and antimicrobial peptide) can replace use of specific antibiotics in the post-antibiotic era to avoid the spread of tigecycline resistance.
SZ and JW wrote this manuscript. JW, YuW, and SZ contributed to the design of this manuscript. MW, XO, QY, YiW, RJ, ML, DZ, SC, and QG provided ideas for the conception of this manuscript. BT, DS, XZ, SM, and JH helped to create figures and tables. SZ and AC modified this manuscript, and acquired funding. All authors contributed to the article and approved the submitted version.
This work was supported by the NSFC (31902267), the earmarked fund for China Agriculture Research System (CARS-42-17), the funds of Key Laboratory of Livestock and Poultry Provenance Disease Research in Mianyang, Sichuan Veterinary Medicine and Drug Innovation Group of China Agricultural Research System (SCCXTD-2022-18), and the Applied Basic Research Programs of Science and Technology Department of Sichuan Province (2019YJ0410).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmad, M., and Khan, A. (2019). Global economic impact of antibiotic resistance: a review. J. Glob. Antimicrob. Resist. 19, 313–316. doi: 10.1016/j.jgar.2019.05.024
Akiyama, T., Presedo, J., and Khan, A. (2013). The tetA gene decreases tigecycline sensitivity of Salmonella enterica isolates. Int. J. Antimicrob. Agents 42, 133–140. doi: 10.1016/j.ijantimicag.2013.04.017
Allen, H., Donato, J., Wang, H., Cloud-Hansen, K., Davies, J., and Handelsman, J. (2010). Call of the wild: antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 8, 251–259. doi: 10.1038/nrmicro2312
Aminov, R. (2011). Horizontal gene exchange in environmental microbiota. Front. Microbiol. 2:158. doi: 10.3389/fmicb.2011.00158
Aminov, R. (2021). Acquisition and spread of antimicrobial resistance: a tet(X) case study. Int. J. Mol. Sci. 22:3905. doi: 10.3390/ijms22083905
Anyanwu, M., Okpala, C., Chah, K., and Shoyinka, V. (2021). Prevalence and traits of mobile colistin resistance gene harbouring isolates from different ecosystems in Africa. Biomed. Res. Int. 2021, 1–20. doi: 10.1155/2021/6630379
Aslam, B., Khurshid, M., Arshad, M., Muzammil, S., Rasool, M., Yasmeen, N., et al. (2021). Antibiotic resistance: one health one world outlook. Front. Cell. Infect. Microbiol. 11:771510. doi: 10.3389/fcimb.2021.771510
Bai, L., Du, P., Du, Y., Sun, H., Zhang, P., Wan, Y., et al. (2019). Detection of plasmid-mediated tigecycline-resistant gene tet(X4) in Escherichia coli from pork, Sichuan and Shandong provinces, China, February 2019. Euro Surveill. 24. doi: 10.2807/1560-7917.ES.2019.24.25.1900340
Bankan, N., Koka, F., Vijayaraghavan, R., Basireddy, S., and Jayaraman, S. (2021). Overexpression of the ade B efflux pump gene in Tigecycline-resistant Acinetobacter baumannii clinical isolates and its inhibition by (+)Usnic acid as an adjuvant. Antibiotics 10:1900340. doi: 10.3390/antibiotics10091037
Beabout, K., Hammerstrom, T., Perez, A., Magalhães, B., Prater, A., Clements, T., et al. (2015). The ribosomal S10 protein is a general target for decreased tigecycline susceptibility. Antimicrob. Agents Chemother. 59, 5561–5566. doi: 10.1128/AAC.00547-15
Bender, J., Klare, I., Fleige, C., and Werner, G. (2020). A nosocomial cluster of Tigecycline-and vancomycin-resistant enterococcus faecium isolates and the impact of rps J and tet (M) mutations on tigecycline resistance. Microb. Drug Resist. 26, 576–582. doi: 10.1089/mdr.2019.0346
Bengtsson-Palme, J., Boulund, F., Fick, J., Kristiansson, E., and Larsson, D. (2014). Shotgun metagenomics reveals a wide array of antibiotic resistance genes and mobile elements in a polluted lake in India. Front. Microbiol. 5:648. doi: 10.3389/fmicb.2014.00648
Bondarczuk, K., Markowicz, A., and Piotrowska-Seget, Z. (2016). The urgent need for risk assessment on the antibiotic resistance spread via sewage sludge land application. Environ. Int. 87, 49–55. doi: 10.1016/j.envint.2015.11.011
Cai, W., Tang, F., Jiang, L., Li, R., Wang, Z., and Liu, Y. (2021). Histone-like nucleoid structuring protein modulates the fitness of tet(X4)-bearing Inc X1 plasmids in gram-negative bacteria. Front. Microbiol. 12:763288. doi: 10.3389/fmicb.2021.763288
Calero-Cáceres, W., Ye, M., and Balcázar, J. L. (2019). Bacteriophages as environmental reservoirs of antibiotic resistance. Trends Microbiol. 27, 570–577. doi: 10.1016/j.tim.2019.02.008
Cattoir, V., Isnard, C., Cosquer, T., Odhiambo, A., Bucquet, F., Guérin, F., et al. (2015). Genomic analysis of reduced susceptibility to tigecycline in Enterococcus faecium. Antimicrob. Agents Chemother. 59, 239–244. doi: 10.1128/AAC.04174-14
Chen, C., Chen, L., Zhang, Y., Cui, C., Wu, X., He, Q., et al. (2019a). Detection of chromosome-mediated tet(X4)-carrying Aeromonas caviae in a sewage sample from a chicken farm. J. Antimicrob. Chemother. 74, 3628–3630. doi: 10.1093/jac/dkz387
Chen, C., Cui, C., Wu, X., Fang, L., He, Q., He, B., et al. (2021). Spread of tet (X5) and tet(X6) genes in multidrug-resistant Acinetobacter baumannii strains of animal origin. Vet. Microbiol. 253:108954. doi: 10.1016/j.vetmic.2020.108954
Chen, C., Cui, C., Yu, J., He, Q., Wu, X., He, Y., et al. (2020). Genetic diversity and characteristics of high-level tigecycline resistance Tet(X) in Acinetobacter species. Genome Med. 12:111. doi: 10.1186/s13073-020-00807-5
Chen, C., Cui, C., Zhang, Y., He, Q., Wu, X., Li, G., et al. (2019b). Emergence of mobile tigecycline resistance mechanism in Escherichia coli strains from migratory birds in China. Emerg. Microbes Infect. 8, 1219–1222. doi: 10.1080/22221751.2019.1653795
Chen, Y., Hu, D., Zhang, Q., Liao, X., Liu, Y., and Sun, J. (2017). Efflux pump overexpression contributes to tigecycline heteroresistance in Salmonella enterica serovar Typhimurium. Front. Cell. Infect. Microbiol. 7:37. doi: 10.3389/fcimb.2017.00037
Cheng, Y., Chen, Y., Liu, Y., Guo, Y., Zhou, Y., Xiao, T., et al. (2020). Identification of novel tetracycline resistance gene tet(X14) and its co-occurrence with tet(X2) in a tigecycline-resistant and colistin-resistant Empedobacter stercoris. Emerg. Microbes Infect. 9, 1843–1852. doi: 10.1080/22221751.2020.1803769
Cheng, Y., Chen, Y., Liu, Y., Song, J., Chen, Y., Shan, T., et al. (2021a). Detection of a new tet(X6)-encoding plasmid in Acinetobacter towneri. J. Glob. Antimicrob. Resist. 25, 132–136. doi: 10.1016/j.jgar.2021.03.004
Cheng, Y., Liu, Y., Chen, Y., Huang, F., Chen, R., Xiao, Y., et al. (2021b). Sporadic dissemination of tet(X3) and tet(X6) mediated by highly diverse plasmidomes among livestock-associated Acinetobacter. Microbiol. Spectr. 9:e0114121. doi: 10.1128/Spectrum.01141-21
Chopra, I. (2002). New developments in tetracycline antibiotics: glycylcyclines and tetracycline efflux pump inhibitors. Drug Resist. Updat. 5, 119–125. doi: 10.1016/S1368-7646(02)00051-1
Chopra, I., and Roberts, M. (2001). Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65, 232–260; second page, table of contents. doi: 10.1128/MMBR.65.2.232-260.2001
Collignon, P. (2015). Antibiotic resistance: are we all doomed? Intern. Med. J. 45, 1109–1115. doi: 10.1111/imj.12902
Concha, C., Miranda, C., Santander, J., and Roberts, M. (2021). Genetic characterization of the tetracycline-resistance gene tet(X) carried by two Epilithonimonas strains isolated from farmed diseased rainbow trout, Oncorhynchus mykiss in Chile. Antibiotics 10:1051. doi: 10.3390/antibiotics10091051
Cui, C., Chen, C., Liu, B., He, Q., Wu, X., Sun, R., et al. (2020). Co-occurrence of plasmid-mediated tigecycline and carbapenem resistance in Acinetobacter spp. from waterfowls and their neighboring environment. Antimicrob. Agents Chemother. 64:e02502-19. doi: 10.1128/AAC.02502-19
Cui, C., He, Q., Jia, Q., Li, C., Chen, C., Wu, X., et al. (2021). Evolutionary trajectory of the Tet(X) family: critical residue changes towards high-level Tigecycline resistance. mSystems 6:e00050-21. doi: 10.1128/mSystems.00050-21
Cui, C., Li, X., Chen, C., Wu, X., He, Q., Jia, Q., et al. (2022). Comprehensive analysis of plasmid-mediated tet(X4)-positive Escherichia coli isolates from clinical settings revealed a high correlation with animals and environments-derived strains. Sci. Total Environ. 806:150687. doi: 10.1016/j.scitotenv.2021.150687
Cunha, B., Baron, J., and Cunha, C. (2017). Once daily high dose tigecycline – pharmacokinetic/pharmacodynamic based dosing for optimal clinical effectiveness: dosing matters, revisited. Expert Rev. Anti-Infect. Ther. 15, 257–267. doi: 10.1080/14787210.2017.1268529
Dai, S., Liu, D., Han, Z., Wang, Y., Lu, X., Yang, M., et al. (2022). Mobile tigecycline resistance gene tet(X4) persists with different animal manure composting treatments and fertilizer receiving soils. Chemosphere 307:135866. doi: 10.1016/j.chemosphere.2022.135866
Dao, T., Kasuga, I., Hirabayashi, A., Nguyen, D., Tran, H., Vu, H., et al. (2022). Emergence of mobile tigecycline resistance gene tet(X4)-harbouring Shewanella xiamenensis in a water environment. J. Glob. Antimicrob. Resist. 28, 140–142. doi: 10.1016/j.jgar.2021.12.022
Davies, J., and Davies, D. (2010). Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 74, 417–433. doi: 10.1128/MMBR.00016-10
Debroas, D., and Siguret, C. (2019). Viruses as key reservoirs of antibiotic resistance genes in the environment. ISME J. 13, 2856–2867. doi: 10.1038/s41396-019-0478-9
Deng, T., Jia, Y., Tong, Z., Shi, J., Wang, Z., and Liu, Y. (2022). Bismuth drugs reverse Tet(X)-conferred tigecycline resistance in gram-negative bacteria. Microbiol. Spectr. 10:e0157821. doi: 10.1128/spectrum.01578-21
Ding, Y., Saw, W., Tan, L., Moong, D., Nagarajan, N., Teo, Y., et al. (2020). Emergence of tigecycline-and eravacycline-resistant Tet(X4)-producing Enterobacteriaceae in the gut microbiota of healthy Singaporeans. J. Antimicrob. Chemother. 75, 3480–3484. doi: 10.1093/jac/dkaa372
Du, J., Xu, T., Guo, X., and Yin, D. (2022). Characteristics and removal of antibiotics and antibiotic resistance genes in a constructed wetland from a drinking water source in the Yangtze River Delta. Sci. Total Environ. 813:152540. doi: 10.1016/j.scitotenv.2021.152540
Du, P., Liu, D., Song, H., Zhang, P., Li, R., Fu, Y., et al. (2020). Novel IS26-mediated hybrid plasmid harbouring tet(X4) in Escherichia coli. J. Glob. Antimicrob. Resist. 21, 162–168. doi: 10.1016/j.jgar.2020.03.018
Du, X., Xia, C., Shen, J., Wu, B., and Shen, Z. (2004). Characterization of florfenicol resistance among calf pathogenic Escherichia coli. FEMS Microbiol. Lett. 236, 183–189. doi: 10.1111/j.1574-6968.2004.tb09645.x
Fang, L., Chen, Q., Shi, K., Li, X., Shi, Q., He, F., et al. (2016). Step-wise increase in tigecycline resistance in Klebsiella pneumoniae associated with mutations in ram R, lon and rpsJ. PLoS One 11:e0165019. doi: 10.1371/journal.pone.0165019
Fang, L., Chen, C., Cui, C., Li, X., Zhang, Y., Liao, X., et al. (2020). Emerging high-level tigecycline resistance: novel tetracycline destructases spread via the Mobile Tet(X). BioEssays 42:e2000014. doi: 10.1002/bies.202000014
Feng, J., Su, M., Li, K., Ma, J., Li, R., Bai, L., et al. (2022). Extensive spread of tet(X4) in multidrug-resistant Escherichia coli of animal origin in western China. Vet. Microbiol. 269:109420. doi: 10.1016/j.vetmic.2022.109420
Finley, R., Collignon, P., Larsson, D., Mcewen, S., Li, X., Gaze, W., et al. (2013). The scourge of antibiotic resistance: the important role of the environment. Clin. Infect. Dis. 57, 704–710. doi: 10.1093/cid/cit355
Fluit, A., Florijn, A., Verhoef, J., and Milatovic, D. (2005). Presence of tetracycline resistance determinants and susceptibility to tigecycline and minocycline. Antimicrob. Agents Chemother. 49, 1636–1638. doi: 10.1128/AAC.49.4.1636-1638.2005
Fu, Y., Chen, Y., Liu, D., Yang, D., Liu, Z., Wang, Y., et al. (2021). Abundance of tigecycline resistance genes and association with antibiotic residues in Chinese livestock farms. J. Hazard. Mater. 409:124921. doi: 10.1016/j.jhazmat.2020.124921
Gao, R., Hu, Y., Li, Z., Sun, J., Wang, Q., Lin, J., et al. (2016). Dissemination and mechanism for the MCR-1 Colistin resistance. PLoS Pathog. 12:e1005957. doi: 10.1371/journal.ppat.1005957
Gao, X., He, X., Lv, L., Cai, Z., Liu, Y., and Liu, J. (2022). Detection of Tet(X4)-producing Klebsiella pneumoniae from the environment and wide spread of Inc FIA-Inc HI1A-Inc HI1B plasmid carrying tet(X4) in China. J. Glob. Antimicrob. Resist. 30, 130–132. doi: 10.1016/j.jgar.2022.05.028
Ghosh, S., Lapara, T., and Sadowsky, M. (2015). Transformation of tetracycline by Tet X and its subsequent degradation in a heterologous host. FEMS Microbiol. Ecol. 91:fiv059. doi: 10.1093/femsec/fiv059
Guiney, D. Jr., Hasegawa, P., and Davis, C. (1984). Expression in Escherichia coli of cryptic tetracycline resistance genes from bacteroides R plasmids. Plasmid 11, 248–252. doi: 10.1016/0147-619X(84)90031-3
He, D., Wang, L., Zhao, S., Liu, L., Liu, J., Hu, G., et al. (2020). A novel tigecycline resistance gene, tet(X6), on an SXT/R391 integrative and conjugative element in a Proteus genomospecies 6 isolate of retail meat origin. J. Antimicrob. Chemother. 75, 1159–1164. doi: 10.1093/jac/dkaa012
He, S., Hickman, A., Varani, A., Siguier, P., Chandler, M., Dekker, J., et al. (2015). Insertion sequence IS26 reorganizes plasmids in clinically isolated multidrug-resistant bacteria by replicative transposition. MBio 6:e00762. doi: 10.1128/mBio.00762-15
He, T., Li, R., Wei, R., Liu, D., Bai, L., Zhang, L., et al. (2020). Characterization of Acinetobacter indicus co-harbouring tet(X3) and blaNDM-1 of dairy cow origin. J. Antimicrob. Chemother. 75, 2693–2696. doi: 10.1093/jac/dkaa182
He, T., Wang, R., Liu, D., Walsh, T., Zhang, R., Lv, Y., et al. (2019). Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 4, 1450–1456. doi: 10.1038/s41564-019-0445-2
He, T., Wei, R., Zhang, L., Gong, L., Zhu, L., Gu, J., et al. (2021). Dissemination of the tet(X)-variant genes from layer farms to manure-receiving soil and corresponding lettuce. Environ. Sci. Technol. 55, 1604–1614. doi: 10.1021/acs.est.0c05042
Henchion, M., Mccarthy, M., Resconi, V., and Troy, D. (2014). Meat consumption: trends and quality matters. Meat Sci. 98, 561–568. doi: 10.1016/j.meatsci.2014.06.007
Hentschke, M., Christner, M., Sobottka, I., Aepfelbacher, M., and Rohde, H. (2010). Combined ram R mutation and presence of a Tn1721-associated tet(A) variant in a clinical isolate of Salmonella enterica serovar Hadar resistant to tigecycline. Antimicrob. Agents Chemother. 54, 1319–1322. doi: 10.1128/AAC.00993-09
Hirabayashi, A., Dao, T., Takemura, T., Hasebe, F., Trang, L., Thanh, N., et al. (2021). A transferable Inc C-Inc X3 hybrid plasmid cocarrying blaNDM-4, tet(X), and tmex CD3-topr J3 confers resistance to carbapenem and tigecycline. mSphere 6:e0059221. doi: 10.1128/mSphere.00592-21
Homeier-Bachmann, T., Heiden, S., Lübcke, P., Bachmann, L., Bohnert, J., Zimmermann, D., et al. (2021). Antibiotic-resistant Enterobacteriaceae in wastewater of abattoirs. Antibiotics 10:568. doi: 10.3390/antibiotics10050568
Hsieh, Y., Wu, J., Chen, Y., Quyen, T., Liao, W., Li, S., et al. (2021). An outbreak of tet(X6)-carrying tigecycline-resistant Acinetobacter baumannii isolates with a new capsular type at a hospital in Taiwan. Antibiotics 10:1239. doi: 10.3390/antibiotics10101239
Hua, X., He, J., Wang, J., Zhang, L., Zhang, L., Xu, Q., et al. (2021). Novel tigecycline resistance mechanisms in Acinetobacter baumannii mediated by mutations in ade S, rpo B and rrf. Emerg. Microbes Infect. 10, 1404–1417. doi: 10.1080/22221751.2021.1948804
Ji, X., Shen, Q., Liu, F., Ma, J., Xu, G., Wang, Y., et al. (2012). Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai; China. J. Hazard. Mater. 235–236, 178–185. doi: 10.1016/j.jhazmat.2012.07.040
Kaewpoowat, Q., and Ostrosky-Zeichner, L. (2015). Tigecycline: a critical safety review. Expert Opin. Drug Saf. 14, 335–342. doi: 10.1517/14740338.2015.997206
Kim, S., and Aga, D. (2007). Potential ecological and human health impacts of antibiotics and antibiotic-resistant bacteria from wastewater treatment plants. J. Toxicol. Environ. Health B Crit. Rev. 10, 559–573. doi: 10.1080/15287390600975137
Kumar, S., He, G., Kakarla, P., Shrestha, U., Ranjana, K., Ranaweera, I., et al. (2016). Bacterial multidrug efflux pumps of the major facilitator superfamily as targets for modulation. Infect. Disord. Drug Targets 16, 28–43. doi: 10.2174/1871526516666160407113848
Kürekci, C., Lu, X., Celil, B., Disli, H., Mohsin, M., Wang, Z., et al. (2022). Emergence and characterization of tigecycline resistance gene tet(X4) in ST609 Escherichia coli isolates from wastewater in Turkey. Microbiology Spectrum 10, e00732–e00722. doi: 10.1128/spectrum.00732-22
Lamut, A., Peterlin Mašič, L., Kikelj, D., and Tomašič, T. (2019). Efflux pump inhibitors of clinically relevant multidrug resistant bacteria. Med. Res. Rev. 39, 2460–2504. doi: 10.1002/med.21591
Lerminiaux, N., and Cameron, A. (2019). Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 65, 34–44. doi: 10.1139/cjm-2018-0275
Leski, T., Bangura, U., Jimmy, D., Ansumana, R., Lizewski, S., Stenger, D., et al. (2013). Multidrug-resistant tet(X)-containing hospital isolates in Sierra Leone. Int. J. Antimicrob. Agents 42, 83–86. doi: 10.1016/j.ijantimicag.2013.04.014
Li, B. (2020a). Investigation of tigecycline resistant Escherichia coli from raw meat reveals potential transmission among food-producing animals. Food Control 121:107633. doi: 10.1016/j.foodcont.2020.107633
Li, R., Li, Y., Peng, K., Yin, Y., Liu, Y., He, T., et al. (2021a). Comprehensive genomic investigation of tigecycline resistance gene tet(X4)-bearing strains expanding among different settings. Microbiol. Spectr. 9:e0163321. doi: 10.1128/spectrum.01633-21
Li, R., Liu, Z., Peng, K., Liu, Y., Xiao, X., and Wang, Z. (2019). Co-occurrence of two tet(X) variants in an Empedobacter brevis of shrimp origin. Antimicrob. Agents Chemother. 63:e01636-19. doi: 10.1128/AAC.01636-19
Li, R., Lu, X., Munir, A., Abdullah, S., Liu, Y., Xiao, X., et al. (2022). Widespread prevalence and molecular epidemiology of tet(X4) and mcr-1 harboring Escherichia coli isolated from chickens in Pakistan. Sci. Total Environ. 806:150689. doi: 10.1016/j.scitotenv.2021.150689
Li, R., Lu, X., Peng, K., Liu, Z., Li, Y., Liu, Y., et al. (2020b). Deciphering the structural diversity and classification of the mobile tigecycline resistance gene tet(X)-bearing plasmidome among bacteria. mSystems 5:e00134-20. doi: 10.1128/mSystems.00134-20
Li, R., Peng, K., Li, Y., Liu, Y., and Wang, Z. (2020c). Exploring tet(X)-bearing tigecycline-resistant bacteria of swine farming environments. Sci. Total Environ. 733:139306. doi: 10.1016/j.scitotenv.2020.139306
Li, X., Liu, L., Ji, J., Chen, Q., Hua, X., Jiang, Y., et al. (2015). Tigecycline resistance in Acinetobacter baumannii mediated by frameshift mutation in pls C, encoding 1-acyl-sn-glycerol-3-phosphate acyltransferase. Eur. J. Clin. Microbiol. Infect. Dis. 34, 625–631. doi: 10.1007/s10096-014-2272-y
Li, X., Quan, J., Yang, Y., Ji, J., Liu, L., Fu, Y., et al. (2016). Abrp, a new gene, confers reduced susceptibility to tetracycline, glycylcine, chloramphenicol and fosfomycin classes in Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 35, 1371–1375. doi: 10.1007/s10096-016-2674-0
Li, Y., Peng, K., Yin, Y., Sun, X., Zhang, W., Li, R., et al. (2021b). Occurrence and molecular characterization of abundant tet(X) variants among diverse bacterial species of chicken origin in Jiangsu, China. Front. Microbiol. 12:751006. doi: 10.3389/fmicb.2021.751006
Li, Y., Wang, Q., Peng, K., Liu, Y., Li, R., and Wang, Z. (2020d). Emergence of carbapenem- and tigecycline-resistant Proteus cibarius of animal origin. Front. Microbiol. 11:1940. doi: 10.3389/fmicb.2020.01940
Li, Y., Wang, Q., Peng, K., Liu, Y., Xiao, X., Mohsin, M., et al. (2021c). Distribution and genomic characterization of tigecycline-resistant tet(X4)-positive Escherichia coli of swine farm origin. Microb. Genom. 7:000667. doi: 10.1099/mgen.0.000667
Liao, Q., Rong, H., Zhao, M., Luo, H., Chu, Z., and Wang, R. (2021). Interaction between tetracycline and microorganisms during wastewater treatment: a review. Sci. Total Environ. 757:143981. doi: 10.1016/j.scitotenv.2020.143981
Lima, T., Domingues, S., and Da Silva, G. (2020). Manure as a potential hotspot for antibiotic resistance dissemination by horizontal gene transfer events. Vet. Sci. 7:110. doi: 10.3390/vetsci7030110
Lin, Z., Yuan, T., Zhou, L., Cheng, S., Qu, X., Lu, P., et al. (2021). Impact factors of the accumulation, migration and spread of antibiotic resistance in the environment. Environ. Geochem. Health 43, 1741–1758. doi: 10.1007/s10653-020-00759-0
Linkevicius, M., Sandegren, L., and Andersson, D. (2016). Potential of tetracycline resistance proteins to evolve tigecycline resistance. Antimicrob. Agents Chemother. 60, 789–796. doi: 10.1128/AAC.02465-15
Liu, D., Wang, T., Shao, D., Song, H., Zhai, W., Sun, C., et al. (2022). Structural diversity of the ISCR2-mediated rolling-cycle transferable unit carrying tet(X4). Sci. Total Environ. 826:154010. doi: 10.1016/j.scitotenv.2022.154010
Liu, D., Zhai, W., Song, H., Fu, Y., Schwarz, S., He, T., et al. (2020). Identification of the novel tigecycline resistance gene tet(X6) and its variants in Myroides, Acinetobacter and Proteus of food animal origin. J. Antimicrob. Chemother. 75, 1428–1431. doi: 10.1093/jac/dkaa037
Livermore, D. (2005). Tigecycline: what is it, and where should it be used? J. Antimicrob. Chemother. 56, 611–614. doi: 10.1093/jac/dki291
Lu, B., Jiang, Y., Man, M., Brown, B., Elias, P., and Feingold, K. (2005). Expression and regulation of 1-acyl-sn-glycerol-3-phosphate acyltransferases in the epidermis. J. Lipid Res. 46, 2448–2457. doi: 10.1194/jlr.M500258-JLR200
Luo, Y., Tan, L., Zhang, H., Bi, W., Zhao, L., Wang, X., et al. (2022). Characteristics of wild bird resistomes and dissemination of antibiotic resistance genes in interconnected bird-habitat systems revealed by similarity of blaTEM polymorphic sequences. Environ. Sci. Technol. doi: 10.1021/acs.est.2c01633
Lupien, A., Gingras, H., Leprohon, P., and Ouellette, M. (2015). Induced tigecycline resistance in Streptococcus pneumoniae mutants reveals mutations in ribosomal proteins and rRNA. J. Antimicrob. Chemother. 70, 2973–2980. doi: 10.1093/jac/dkv211
Lv, H., Huang, W., Lei, G., Liu, L., Zhang, L., and Yang, X. (2020). Identification of novel plasmids containing the tigecycline resistance gene tet(X4) in Escherichia coli isolated from retail chicken meat. Foodborne Pathog. Dis. 17, 792–794. doi: 10.1089/fpd.2020.2822
Lv, L., Wan, M., Wang, C., Gao, X., Yang, Q., Partridge, S., et al. (2020). Emergence of a plasmid-encoded resistance-nodulation-division efflux pump conferring resistance to multiple drugs, including tigecycline, in Klebsiella pneumoniae. MBio 11:e02930-19. doi: 10.1128/mBio.02930-19
Manaia, C., Macedo, G., Fatta-Kassinos, D., and Nunes, O. (2016). Antibiotic resistance in urban aquatic environments: can it be controlled? Appl. Microbiol. Biotechnol. 100, 1543–1557. doi: 10.1007/s00253-015-7202-0
Manyi-Loh, C., Mamphweli, S., Meyer, E., and Okoh, A. (2018). Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules 23:150689. doi: 10.3390/molecules23040795
Marathe, N., Svanevik, C., Ghavidel, F., and Grevskott, D. (2021). First report of mobile tigecycline resistance gene tet(X4)-harbouring multidrug-resistant Escherichia coli from wastewater in Norway. J. Glob. Antimicrob. Resist. 27, 37–40. doi: 10.1016/j.jgar.2021.07.019
Martelli, F., Abuoun, M., Cawthraw, S., Storey, N., Turner, O., Ellington, M., et al. (2022). Detection of the transferable tigecycline resistance gene tet(X4) in Escherichia coli from pigs in the United Kingdom. J. Antimicrob. Chemother. 77, 846–848. doi: 10.1093/jac/dkab439
Miłobedzka, A., Ferreira, C., Vaz-Moreira, I., Calderón-Franco, D., Gorecki, A., Purkrtova, S., et al. (2022). Monitoring antibiotic resistance genes in wastewater environments: the challenges of filling a gap in the one-health cycle. J. Hazard. Mater. 424:127407. doi: 10.1016/j.jhazmat.2021.127407
Mohsin, M., Hassan, B., Martins, W., Li, R., Abdullah, S., Sands, K., et al. (2021). Emergence of plasmid-mediated tigecycline resistance tet(X4) gene in Escherichia coli isolated from poultry, food and the environment in South Asia. Sci. Total Environ. 787:147613. doi: 10.1016/j.scitotenv.2021.147613
Moore, I., Hughes, D., and Wright, G. (2005). Tigecycline is modified by the flavin-dependent monooxygenase Tet X. Biochemistry 44, 11829–11835. doi: 10.1021/bi0506066
Munita, J., and Arias, C. (2016). Mechanisms of antibiotic resistance. Microbiol. Spectr. 4:VMBF-0016-2015. doi: 10.1128/microbiolspec.VMBF-0016-2015
Olesen, S., Lipsitch, M., and Grad, Y. (2020). The role of “spillover” in antibiotic resistance. Proc. Natl. Acad. Sci. U. S. A. 117, 29063–29068. doi: 10.1073/pnas.2013694117
Olson, M., Ruzin, A., Feyfant, E., Rush, T. 3rd, O'connell, J., and Bradford, P. (2006). Functional, biophysical, and structural bases for antibacterial activity of tigecycline. Antimicrob. Agents Chemother. 50, 2156–2166. doi: 10.1128/AAC.01499-05
Pan, Y., Awan, F., Zhenbao, M., Zhang, X., Zeng, J., Zeng, Z., et al. (2020). Preliminary view of the global distribution and spread of the tet(X) family of tigecycline resistance genes. J. Antimicrob. Chemother. 75, 2797–2803. doi: 10.1093/jac/dkaa284
Partridge, S., Kwong, S., Firth, N., and Jensen, S. (2018). Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 31:e00088-17. doi: 10.1128/CMR.00088-17
Peng, K., Li, R., He, T., Liu, Y., and Wang, Z. (2020). Characterization of a porcine Proteus cibarius strain co-harbouring tet(X6) and cfr. J. Antimicrob. Chemother. 75, 1652–1654. doi: 10.1093/jac/dkaa047
Peng, Z., Hu, Z., Li, Z., Zhang, X., Jia, C., Li, T., et al. (2022). Antimicrobial resistance and population genomics of multidrug-resistant Escherichia coli in pig farms in mainland China. Nat. Commun. 13:1116. doi: 10.1038/s41467-022-28750-6
Pereira, A., Paranhos, A., De Aquino, S., and Silva, S. (2021). Distribution of genetic elements associated with antibiotic resistance in treated and untreated animal husbandry waste and wastewater. Environ. Sci. Pollut. Res. Int. 28, 26380–26403. doi: 10.1007/s11356-021-13784-y
Plantinga, N., Wittekamp, B., Van Duijn, P., and Bonten, M. (2015). Fighting antibiotic resistance in the intensive care unit using antibiotics. Future Microbiol. 10, 391–406. doi: 10.2217/fmb.14.146
Poirel, L., Mugnier, P., Toleman, M., Walsh, T., Rapoport, M., Petroni, A., et al. (2009). ISCR2, another vehicle for blaVEB gene acquisition. Antimicrob. Agents Chemother. 53, 4940–4943. doi: 10.1128/AAC.00414-09
Potter, R., D'souza, A., and Dantas, G. (2016). The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist. Updat. 29, 30–46. doi: 10.1016/j.drup.2016.09.002
Qiao, M., Ying, G., Singer, A., and Zhu, Y. (2018). Review of antibiotic resistance in China and its environment. Environ. Int. 110, 160–172. doi: 10.1016/j.envint.2017.10.016
Qiu, J., Zhao, T., Liu, Q., He, J., He, D., Wu, G., et al. (2016). Residual veterinary antibiotics in pig excreta after oral administration of sulfonamides. Environ. Geochem. Health 38, 549–556. doi: 10.1007/s10653-015-9740-x
Rehman, M., Yang, H., Zhang, S., Huang, Y., Zhou, R., Gong, S., et al. (2020). Emergence of Escherichia coli isolates producing NDM-1 carbapenemase from waterfowls in Hainan island, China. Acta Trop. 207:105485. doi: 10.1016/j.actatropica.2020.105485
Rempel, S., Stanek, W., and Slotboom, D. (2019). ECF-type ATP-binding cassette transporters. Annu. Rev. Biochem. 88, 551–576. doi: 10.1146/annurev-biochem-013118-111705
Roberts, M. (2003). Tetracycline therapy: update. Clin. Infect. Dis. 36, 462–467. doi: 10.1086/367622
Ruan, Z., Jia, H., Chen, H., Wu, J., He, F., and Feng, Y. (2020). Co-existence of plasmid-mediated tigecycline and colistin resistance genes tet(X4) and mcr-1 in a community-acquired Escherichia coli isolate in China. J. Antimicrob. Chemother. 75, 3400–3402. doi: 10.1093/jac/dkaa317
Ruzin, A., Keeney, D., and Bradford, P. (2007). Ade ABC multidrug efflux pump is associated with decreased susceptibility to tigecycline in Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J. Antimicrob. Chemother. 59, 1001–1004. doi: 10.1093/jac/dkm058
Sheykhsaran, E., Baghi, H., Soroush, M., and Ghotaslou, R. (2019). An overview of tetracyclines and related resistance mechanisms. Rev. Med. Microbiol. 30, 69–75. doi: 10.1097/MRM.0000000000000154
Shi, L. D., Dong, X., Liu, Z., Yang, Y., Lin, J. G., Li, M., et al. (2022). A mixed blessing of viruses in wastewater treatment plants. Water Res. 215:118237. doi: 10.1016/j.watres.2022.118237
Speer, B., Bedzyk, L., and Salyers, A. (1991). Evidence that a novel tetracycline resistance gene found on two Bacteroides transposons encodes an NADP-requiring oxidoreductase. J. Bacteriol. 173, 176–183. doi: 10.1128/jb.173.1.176-183.1991
Stein, G., and Babinchak, T. (2013). Tigecycline: an update. Diagn. Microbiol. Infect. Dis. 75, 331–336. doi: 10.1016/j.diagmicrobio.2012.12.004
Sun, C., Cui, M., Zhang, S., Liu, D., Fu, B., Li, Z., et al. (2020). Genomic epidemiology of animal-derived tigecycline-resistant Escherichia coli across China reveals recent endemic plasmid-encoded tet(X4) gene. Commun. Biol. 3:412. doi: 10.1038/s42003-020-01148-0
Sun, C., Cui, M., Zhang, S., Wang, H., Song, L., Zhang, C., et al. (2019). Plasmid-mediated tigecycline-resistant gene tet(X4) in Escherichia coli from food-producing animals, China, 2008–2018. Emerg. Microbes Infect. 8, 1524–1527. doi: 10.1080/22221751.2019.1678367
Sun, H., Wan, Y., Du, P., Liu, D., Li, R., Zhang, P., et al. (2021a). Investigation of tigecycline resistant Escherichia coli from raw meat reveals potential transmission among food-producing animals. Food Control 121:107633. doi: 10.1016/j.foodcont.2020.107633
Sun, H., Zhai, W., Fu, Y., Li, R., Du, P., and Bai, L. (2021b). Co-occurrence of plasmid-mediated resistance genes tet(X4) and blaNDM-5 in a multidrug-resistant Escherichia coli isolate recovered from chicken in China. J. Glob. Antimicrob. Resist. 24, 415–417. doi: 10.1016/j.jgar.2021.02.010
Sun, J., Chen, C., Cui, C., Zhang, Y., Liu, X., Cui, Z., et al. (2019). Plasmid-encoded tet(X) genes that confer high-level tigecycline resistance in Escherichia coli. Nat. Microbiol. 4, 1457–1464. doi: 10.1038/s41564-019-0496-4
Tang, Y., Lai, Y., Kong, L., Wang, X., Li, C., Wang, Y., et al. (2021). Characterization of three porcine Escherichia coli isolates co-harbouring tet(X4) and cfr. J. Antimicrob. Chemother. 76, 263–264. doi: 10.1093/jac/dkaa384
Thaker, M., Spanogiannopoulos, P., and Wright, G. (2010). The tetracycline resistome. Cell. Mol. Life Sci. 67, 419–431. doi: 10.1007/s00018-009-0172-6
Tiseo, K., Huber, L., Gilbert, M., Robinson, T., and Van Boeckel, T. (2020). Global trends in antimicrobial use in food animals from 2017 to 2030. Antibiotics 9:918. doi: 10.3390/antibiotics9120918
Tong, C., Xiao, D., Xie, L., Yang, J., Zhao, R., Hao, J., et al. (2022). Swine manure facilitates the spread of antibiotic resistome including tigecycline-resistant tet(X) variants to farm workers and receiving environment. Sci. Total Environ. 808:152157. doi: 10.1016/j.scitotenv.2021.152157
Tyrrell, C., Burgess, C. M., Brennan, F., and Walsh, F. (2019). Antibiotic resistance in grass and soil. Biochem. Soc. Trans. 47, 477–486. doi: 10.1042/BST20180552
Umar, Z., Chen, Q., Tang, B., Xu, Y., Wang, J., Zhang, H., et al. (2021). The poultry pathogen Riemerella anatipestifer appears as a reservoir for Tet(X) tigecycline resistance. Environ. Microbiol. 23, 7465–7482. doi: 10.1111/1462-2920.15632
Usui, M., Fukuda, A., Suzuki, Y., Nakajima, C., and Tamura, Y. (2021). Broad-host-range Inc W plasmid harbouring tet(X) in Escherichia coli isolated from pigs in Japan. J. Glob. Antimicrob. Resist. 28, 97–101. doi: 10.1016/j.jgar.2021.12.012
Van Boeckel, T., Brower, C., Gilbert, M., Grenfell, B., Levin, S., Robinson, T., et al. (2015). Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. U. S. A. 112, 5649–5654. doi: 10.1073/pnas.1503141112
Vaz-Moreira, I., Nunes, O., and Manaia, C. (2014). Bacterial diversity and antibiotic resistance in water habitats: searching the links with the human microbiome. FEMS Microbiol. Rev. 38, 761–778. doi: 10.1111/1574-6976.12062
Venter, H., Mowla, R., Ohene-Agyei, T., and Ma, S. (2015). RND-type drug efflux pumps from gram-negative bacteria: molecular mechanism and inhibition. Front. Microbiol. 6:377. doi: 10.3389/fmicb.2015.00377
Volkers, G., Palm, G., Weiss, M., Wright, G., and Hinrichs, W. (2011). Structural basis for a new tetracycline resistance mechanism relying on the Tet X monooxygenase. FEBS Lett. 585, 1061–1066. doi: 10.1016/j.febslet.2011.03.012
Wang, J., Ma, Z., Zeng, Z., Yang, X., Huang, Y., and Liu, J. (2017). The role of wildlife (wild birds) in the global transmission of antimicrobial resistance genes. Zool. Res. 38, 55–80. doi: 10.24272/j.issn.2095-8137.2017.003
Wang, J., Wang, Y., Wu, H., Wang, Z., Shen, P., Tian, Y., et al. (2020). Coexistence of blaOXA-58 and tet(X) on a novel plasmid in Acinetobacter sp. from pig in Shanghai, China. Front. Microbiol. 11:578020. doi: 10.3389/fmicb.2020.578020
Wang, J., Wu, H., Mei, C., Wang, Y., Wang, Z., Lu, M., et al. (2021). Multiple mechanisms of tigecycline resistance in Enterobacteriaceae from a pig farm, China. Microbiol. Spectr. 9:e0041621. doi: 10.1128/Spectrum.00416-21
Wang, L., Liu, D., Lv, Y., Cui, L., Li, Y., Li, T., et al. (2019). Novel plasmid-mediated tet (X5) gene conferring resistance to tigecycline, eravacycline, and omadacycline in a clinical Acinetobacter baumannii isolate. Antimicrob. Agents Chemother. 64:e01326-19. doi: 10.1128/AAC.01326-19
Wang, Y., Hu, Y., Cao, J., Bi, Y., Lv, N., Liu, F., et al. (2019). Antibiotic resistance gene reservoir in live poultry markets. J. Infect. 78, 445–453. doi: 10.1016/j.jinf.2019.03.012
Wang, Y., Liu, F., Xu, X., Huang, H., Lyu, N., Ma, S., et al. (2021a). Detection of plasmid-mediated tigecycline resistance gene tet(X4) in a Salmonella enterica serovar Llandoff Isolate. Infect. Microb. Dis. 3, 198–204. doi: 10.1097/IM9.0000000000000077
Wang, Y., Liu, F., Zhu, B., and Gao, G. (2020). Discovery of tigecycline resistance genes tet(X3) and tet(X4) in live poultry market worker gut microbiomes and the surrounded environment. Sci. Bull. 65, 340–342. doi: 10.1016/j.scib.2019.12.027
Wang, Y., Lyu, N., Liu, F., Liu, W., Bi, Y., Zhang, Z., et al. (2021b). More diversified antibiotic resistance genes in chickens and workers of the live poultry markets. Environ. Int. 153:106534. doi: 10.1016/j.envint.2021.106534
Wenzel, R., Bate, G., and Kirkpatrick, P. (2005). Tigecycline. Nat. Rev. Drug Discov. 4, 809–810. doi: 10.1038/nrd1857
Wu, Y., He, R., Qin, M., Yang, Y., Chen, J., Feng, Y., et al. (2022). Identification of plasmid-mediated tigecycline-resistant gene tet(X4) in Enterobacter cloacae from pigs in China. Microbiol. Spectr. 10:e0206421. doi: 10.1128/spectrum.02064-21
Xu, L., Zhou, Y., Niu, S., Liu, Z., Zou, Y., Yang, Y., et al. (2022). A novel inhibitor of monooxygenase reversed the activity of tetracyclines against tet(X3)/tet(X4)-positive bacteria. EBioMedicine 78:103943. doi: 10.1016/j.ebiom.2022.103943
Xu, Y., Liu, L., Zhang, H., and Feng, Y. (2021). Co-production of Tet(X) and MCR-1, two resistance enzymes by a single plasmid. Environ. Microbiol. 23, 7445–7464. doi: 10.1111/1462-2920.15425
Xu, Y., Liu, L., Sun, J., and Feng, Y. (2019). Limited distribution and mechanism of the Tet X4 tetracycline resistance enzyme. Sci. Bull. 64, 14–17. doi: 10.1016/j.scib.2019.08.024
Yahav, D., Lador, A., Paul, M., and Leibovici, L. (2011). Efficacy and safety of tigecycline: a systematic review and meta-analysis. J. Antimicrob. Chemother. 66, 1963–1971. doi: 10.1093/jac/dkr242
Yang, L., Shen, Y., Jiang, J., Wang, X., Shao, D., Lam, M., et al. (2022). Distinct increase in antimicrobial resistance genes among Escherichia coli during 50 years of antimicrobial use in livestock production in China. Nat. Food 3, 197–205. doi: 10.1038/s43016-022-00470-6
Yang, W., Moore, I., Koteva, K., Bareich, D., Hughes, D., and Wright, G. (2004). Tet X is a flavin-dependent monooxygenase conferring resistance to tetracycline antibiotics. J. Biol. Chem. 279, 52346–52352. doi: 10.1074/jbc.M409573200
Yezli, S., and Li, H. (2012). Antibiotic resistance amongst healthcare-associated pathogens in China. Int. J. Antimicrob. Agents 40, 389–397. doi: 10.1016/j.ijantimicag.2012.07.009
Yu, Y., Cui, C., Kuang, X., Chen, C., Wang, M., Liao, X., et al. (2021). Prevalence of tet(X4) in Escherichia coli from duck farms in Southeast China. Front. Microbiol. 12:716393. doi: 10.3389/fmicb.2021.716393
Zeballos-Gross, D., Rojas-Sereno, Z., Salgado-Caxito, M., Poeta, P., Torres, C., and Benavides, J. (2021). The role of gulls as reservoirs of antibiotic resistance in aquatic environments: a scoping review. Front. Microbiol. 12:703886. doi: 10.3389/fmicb.2021.703886
Zeng, Y., Lu, J., Liu, C., Ling, Z., Sun, Q., Wang, H., et al. (2021). A method for screening tigecycline-resistant gene tet(X) from human gut. J. Glob. Antimicrob. Resist. 24, 29–31. doi: 10.1016/j.jgar.2020.11.010
Zha, L., Pan, L., Guo, J., French, N., Villanueva, E., and Tefsen, B. (2020). Effectiveness and safety of high dose tigecycline for the treatment of severe infections: a systematic review and meta-analysis. Adv. Ther. 37, 1049–1064. doi: 10.1007/s12325-020-01235-y
Zhai, W., Tian, Y., Lu, M., Zhang, M., Song, H., Fu, Y., et al. (2022). Presence of mobile tigecycline resistance gene tet(X4) in clinical Klebsiella pneumoniae. Microbiol. Spectr. 10:e0108121. doi: 10.1128/spectrum.01081-21
Zhang, R., Dong, N., Shen, Z., Zeng, Y., Lu, J., Liu, C., et al. (2020a). Epidemiological and phylogenetic analysis reveals Flavobacteriaceae as potential ancestral source of tigecycline resistance gene tet(X). Nat. Commun. 11:4648. doi: 10.1038/s41467-020-18475-9
Zhang, R., Dong, N., Zeng, Y., Shen, Z., Lu, J., Liu, C., et al. (2020b). Chromosomal and plasmid-borne tigecycline resistance genes tet(X3) and tet(X4) in dairy cows on a Chinese farm. Antimicrob. Agents Chemother. 64:e00674-20. doi: 10.1128/AAC.00674-20
Zhang, R., Sun, J., Sun, R. Y., Wang, M., Cui, C., Fang, L., et al. (2021a). Source tracking and global distribution of the Tigecycline non-susceptible tet(X). Microbiol. Spectr. 9:e0116421. doi: 10.1128/Spectrum.01164-21
Zhang, S., Abbas, M., Rehman, M. U., Huang, Y., Zhou, R., Gong, S., et al. (2020c). Dissemination of antibiotic resistance genes (ARGs) via integrons in Escherichia coli: a risk to human health. Environ. Pollut. 266:115260. doi: 10.1016/j.envpol.2020.115260
Zhang, S., Abbas, M., Rehman, M. U., Wang, M., Jia, R., Chen, S., et al. (2021b). Updates on the global dissemination of colistin-resistant Escherichia coli: an emerging threat to public health. Sci. Total Environ. 799:149280. doi: 10.1016/j.scitotenv.2021.149280
Zhang, S., Chen, S., Rehman, M. U., Yang, H., Yang, Z., Wang, M., et al. (2021c). Distribution and association of antimicrobial resistance and virulence traits in Escherichia coli isolates from healthy waterfowls in Hainan, China. Ecotoxicol. Environ. Saf. 220:112317. doi: 10.1016/j.ecoenv.2021.112317
Zhang, Z., Zhan, Z., and Shi, C. (2022). International spread of Tet(X4)-producing Escherichia coli isolates. Foods 11:2010. doi: 10.3390/foods11142010
Zhao, E., Wang, X., Ji, J., Wang, Z., Wang, Y., and Cui, H. (2021). Anti-tumor activity of tigecycline: a review. Sheng Wu Gong Cheng Xue Bao 37, 3031–3041. doi: 10.13345/j.cjb.200630
Zheng, X., Ma, J., Lu, Y., Sun, D., Yang, H., Xia, F., et al. (2022). Detection of tet(X6) variant-producing Proteus terrae subsp. cibarius from animal cecum in Zhejiang, China. J. Glob. Antimicrob. Resist. 29, 124–130. doi: 10.1016/j.jgar.2022.02.011
Zheng, X., Zhu, J., Zhang, J., Cai, P., Sun, Y., Chang, M., et al. (2020). A novel plasmid-borne tet(X6) variant co-existing with blaNDM-1 and blaOXA-58 in a chicken Acinetobacter baumannii isolate. J. Antimicrob. Chemother. 75, 3397–3399. doi: 10.1093/jac/dkaa342
Zhong, X., Xu, H., Chen, D., Zhou, H., Hu, X., and Cheng, G. (2014). First emergence of acr AB and oqx AB mediated tigecycline resistance in clinical isolates of Klebsiella pneumoniae pre-dating the use of tigecycline in a Chinese hospital. PLoS One 9:e115185. doi: 10.1371/journal.pone.0115185
Zhou, X., Qiao, M., Su, J. Q., Wang, Y., Cao, Z., Cheng, W., et al. (2019). Turning pig manure into biochar can effectively mitigate antibiotic resistance genes as organic fertilizer. Sci. Total Environ. 649, 902–908. doi: 10.3389/fmicb.2019.02957
Zhou, Y., Liu, P., Zhang, C., Liao, X., Sun, J., and Liu, Y. (2019). Colistin combined with tigecycline: a promising alternative strategy to combat Escherichia coli harboring blaNDM-5 and mcr-1. Front. Microbiol. 10:2957. doi: 10.3389/fmicb.2019.02957
Keywords: antibiotic resistant bacteria, tigecycline resistance gene, plasmid-mediated, tet(X4), transmission, one health
Citation: Zhang S, Wen J, Wang Y, Wang M, Jia R, Chen S, Liu M, Zhu D, Zhao X, Wu Y, Yang Q, Huang J, Ou X, Mao S, Gao Q, Sun D, Tian B and Cheng A (2022) Dissemination and prevalence of plasmid-mediated high-level tigecycline resistance gene tet (X4). Front. Microbiol. 13:969769. doi: 10.3389/fmicb.2022.969769
Received: 15 June 2022; Accepted: 05 September 2022;
Published: 29 September 2022.
Edited by:
Xingmin Sun, University of South Florida, United StatesReviewed by:
Fangkun Wang, Shandong Agricultural University, ChinaCopyright © 2022 Zhang, Wen, Wang, Wang, Jia, Chen, Liu, Zhu, Zhao, Wu, Yang, Huang, Ou, Mao, Gao, Sun, Tian and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaqiu Zhang, c2hhcWl1ODZAaG90bWFpbC5jb20=; Anchun Cheng, Y2hlbmdhbmNodW5AdmlwLjE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.