
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Microbiol., 04 August 2022
Sec. Food Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.957167
This article is part of the Research TopicAcetic Acid BacteriaView all 14 articles
Beer is the result of a multistep brewing process, including a fermentation step using in general one specific yeast strain. Bacterial presence during beer production (or presence in the beer itself) is considered as bad, since bacteria cause spoilage, produce off-flavors, and/or turbidity. Although most problems in the past related to lack of hygiene and/or cleaning, bacteria do still cause problems nowadays. Despite this negative imago, certain bacteria play an irreplaceable role during fermentation and/or maturation of more unique, funky, and especially refreshing sour beers. The term sour beers or sours is not restricted to one definition but covers a wide variety of beers produced via different techniques. This review proposes an uncluttered sour beer classification scheme, which includes all sour beer production techniques and pays special attention to the functional role of acetic acid bacteria. Whereas their oxidation of ethanol and lactate into acetic acid and acetoin usually spoils beer, including sour beers, organoleptically, a controlled growth leads to a desirable acidic flavor in sour beers, such as lambic-style, lambic-based, and red-brown acidic ales.
Food fermentations have been done by humans for thousands of years as means of preservation of raw materials from agricultural and husbandry origin (Hutkins, 2019). Other desirable attributes of fermented food products, such as unique flavors, textures, appearances, or other functionalities were recognized rapidly as well (Leroy and De Vuyst, 2004). With the development of other preservation techniques, a lot of fermentation processes have been replaced, and the main goal of the production of fermented foods has shifted from preservation to flavor production and health promotion (Marco et al., 2021). Also, food fermentations are associated with cultural connotations, gastronomic qualities, artisan characteristics, and natural appeal. In several food fermentation processes, not only yeasts and lactic acid bacteria (LAB) but also acetic acid bacteria (AAB) are involved, such as in the production of cocoa, kombucha, lambic beer, vinegar, and water kefir (Gullo et al., 2016; Pothakos et al., 2016; Cousin et al., 2017; De Roos and De Vuyst, 2018; Gomes et al., 2018; Villareal-Soto et al., 2018; De Vuyst and Leroy, 2020; Bongaerts et al., 2021; Lynch et al., 2021).
Fermented foods and drinks play a major role in the human diet and human nutrition worldwide (Marco et al., 2017, 2021). Beer, the end-product of the brewing process, including a fermentation and maturation step, is the most consumed fermented beverage by humans worldwide (Neves et al., 2011; Colen and Swinnen, 2015). Whereas, originally, beer was fermented in a spontaneous way, due to lack of knowledge and starter cultures, all beers were at least slightly acidic (Hornsey, 2003). This acidity contributed to a safe water supply for beer drinkers, as hop and spice antimicrobial compounds do for non-sour beers. Nowadays, for almost all beers produced the fermentation relies on specific yeast strains and the presence of bacteria is completely undesirable due to their spoilage potential (Briggs et al., 2004; Vriesekoop et al., 2012). However, although their spoilage capacity, some specific beers do require LAB and/or AAB to introduce characteristic beer flavors (Van Oevelen et al., 1977; De Keersmaecker, 1996; Tonsmeire, 2014; De Roos and De Vuyst, 2019; Bongaerts et al., 2021; Kubizniaková et al., 2021). Sour beers, with their typical refreshing and (slightly) acidic flavor because of high organic acid concentrations are an example of such beers, during the production process of some LAB or even both LAB and AAB are part of the core microbiota and hence contribute to their flavor formation (Van Oevelen et al., 1976; Snauwaert et al., 2016; De Roos and De Vuyst, 2019; Bongaerts et al., 2021). Today, the production of sour beers or sours knows an increasing trend.
AAB are traditionally well known for their beer-spoiling capacity. Beer spoilage has been a problem since multiple decades (Sakamoto and Konings, 2003). The combination of multiple inhibitory factors or hurdles, such as the presence of ethanol (up to 10%, v/v), hop antimicrobial compounds, a low pH, relatively high carbon dioxide concentrations, low oxygen concentrations, and the lack of nutritive compounds makes beer hard to spoil (Kourtis and Arvanitoyannis, 2001; Sakamoto and Konings, 2003; Briggs et al., 2004; Menz et al., 2009; Dysvik et al., 2020c). Despite this harsh environment, some Gram-positive bacteria, Gram-negative bacteria, and so-called wild yeasts are capable of spoiling beer (Van Vuuren et al., 1979; Vriesekoop et al., 2012; Schneiderbanger et al., 2020; Suiker and Wösten, 2021). The Gram-negative bacteria capable of beer spoilage include enterobacteria (such as Citrobacter, Klebsiella, Rahnella, and Obesumbacterium), Zymomonas spp., Pectinatus spp., Megasphaera spp., Selenomonas spp., Zymophilus spp., and AAB, whereof Acetobacter and Gluconobacter have been mainly reported (Sakamoto and Konings, 2003; Van Vuuren and Priest, 2003). Even though AAB species are strict aerobic, some strains have been detected and identified from beer and have been reported as micro-aerotolerant (Harper, 1980; Briggs et al., 2004; Jeon et al., 2014). Today, aerobic AAB species do not form a big problem of beer spoilage anymore thanks to the development of improved brewing technology and beer storage, capable of lowering oxygen levels drastically (Sakamoto and Konings, 2003).
As AAB are in particular acid- and ethanol-tolerant and not inhibited by hop compounds, they may grow in beer. Beer spoilage by AAB species is characterized by a sour taste and vinegary aroma, caused by ethanol oxidation into acetic acid (Ingledew, 1979; Magnus et al., 1986). Besides off-flavor formation, AAB species, such as Acetobacter aceti, Acetobacter liquefaciens, Acetobacter pasteurianus, Acetobacter hansenii and Gluconobacter oxydans, can cause haziness and ropiness in the beer or form pellicles on the beer surface (Van Vuuren, 1999; Van Vuuren and Priest, 2003; Briggs et al., 2004; Hill, 2015; Paradh, 2015).
Since most spoilage incidents with AAB are related to oxygen, the key to prevent spoilage by AAB is to limit oxygen ingress as much as possible and apply good hygiene regimes. Additional care should be applied during bottling in the brewery and cleaning of beer lines, taps, and dispense systems in pubs (Briggs et al., 2004; Vriesekoop et al., 2012). Alternatively, when AAB belong to the desired fermentation microbiota, key will be to have their growth under control.
Beers are generally classified within four different beer production types, being bottom-fermented beers, top-fermented beers, spontaneously fermented beers, and mixed-fermented beers (Briggs et al., 2004; De Roos and De Vuyst, 2019; Bongaerts et al., 2021). This classification is made according to the fermentation step and which microorganisms are involved, in particular related to their origin. The use of specific strain(s) of the yeast species Saccharomyces bayanus or Saccharomyces pastorianus for bottom fermentation and Saccharomyces cerevisiae for top fermentation characterizes these yeast-based fermentation processes, which are carried out in stainless-steel vessels. In stark contrast to these very controlled fermentation processes stands a spontaneous one, for which a diverse multistage fermentation and maturation process in horizontal wooden barrels results in a unique sour beer, thanks to the successive activities of different microbial groups, in particular yeasts, LAB, and AAB (Bokulich et al., 2012; Spitaels et al., 2014a, 2015b; De Roos et al., 2018a,b, 2020; De Roos and De Vuyst, 2019; Bongaerts et al., 2021). Mixed fermentation is a combination of top and spontaneous fermentation techniques, for which process an in-house starter culture, a yeast slurry also containing LAB from previous fermentations, is added, and maturation in vertical wooden barrels (potentially involving AAB) or stainless-steel vessels (Tonsmeire, 2014; Snauwaert et al., 2016; Spitaels et al., 2017; De Roos and De Vuyst, 2019).
The traditional classification into four different beer production types does not cover all beers on the market nowadays, especially more experimental beers, craft beers, or beers produced by microbreweries, which are known to experiment more in the search for new flavor profiles. The craft beer industry, growing since the 1970s, is characterized not only by reusing traditional techniques and brewing with traditional ingredients but also by their diverging application regarding ingredients used, yeasts applied, alcohol content, aging, isotonic claims, and/or packaging (Donadini and Porretta, 2017; Li et al., 2017; Baiano, 2020; Lattici et al., 2020).
The traditional four-type classification system hence only includes three added Saccharomyces species, namely S. cerevisiae for top fermentation and S. bayanus or S. pastorianus for bottom fermentation. Therefore, beers produced using other, non-Saccharomyces yeast species, such as Brettanomyces spp., Torulaspora spp., Pichia spp., etc., cannot be classified unambiguously. Consequently, an extended classification system is suggested in this review, taking into account the fermentation type (distinguishing five production processes), the microorganisms involved (not only yeasts but also LAB and AAB), and the acidification principle (Figure 1). A separate beer production type, indicated as non-conventional fermented beers, covers all beers fermented solely using yeasts, except for S. cerevisiae, S. pastorianus and S. bayanus (Type C in Figure 1). This beer production type likely makes the transition from yeast-based fermentation processes to sour beer production types.
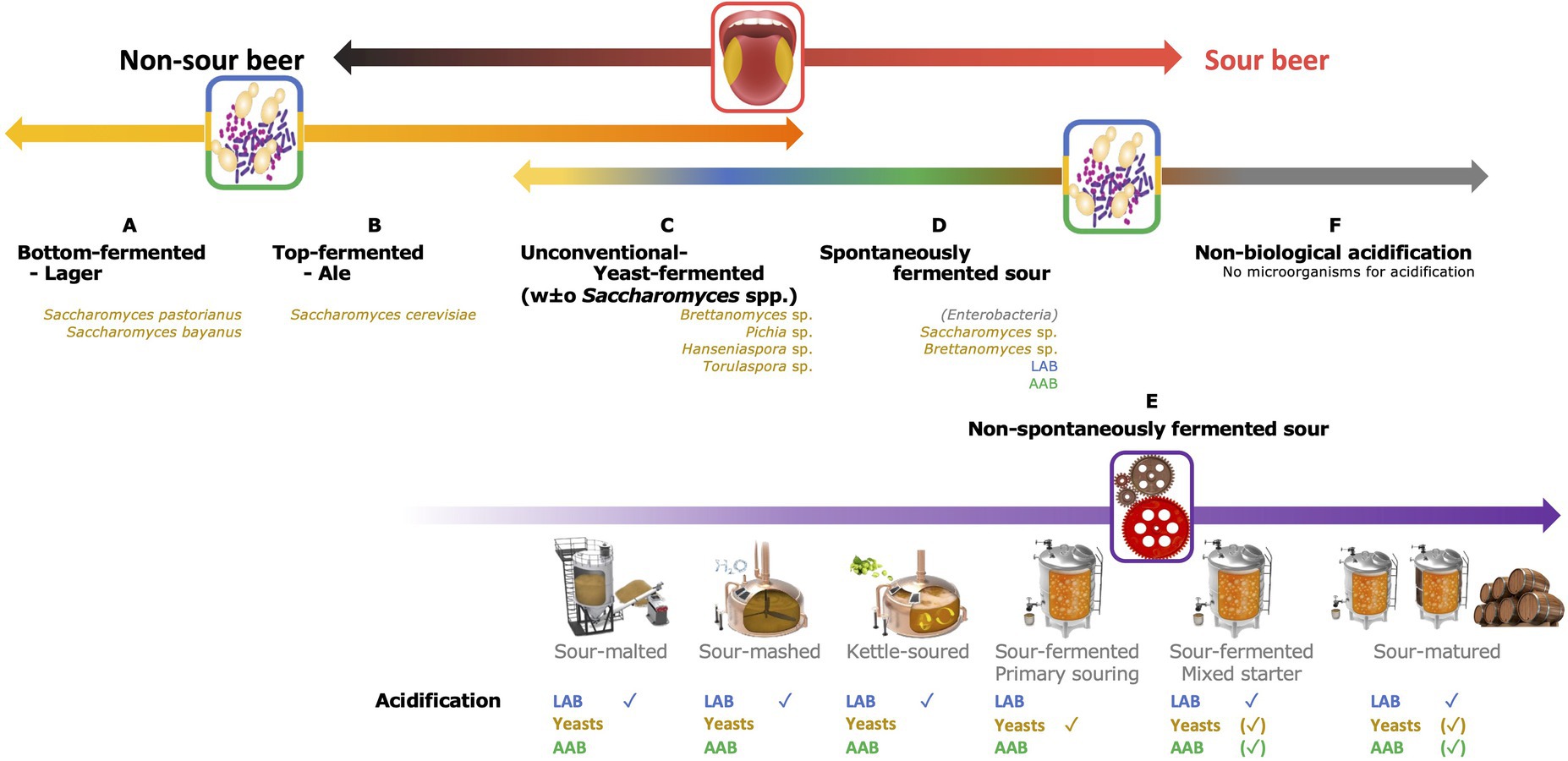
Figure 1. Schematic representation of an extended beer classification. On top, a first subdivision is made based on taste [from left to right non-sour (black) to sour (red)]; in the middle, distinction is made based on the fermentation technology applied and the microorganisms involved [yeasts (yellow-orange); lactic acid bacteria (LAB, blue); acetic acid bacteria (AAB, green); and no microorganisms (gray)]. Category E is further subdivided according to the acidification technique applied and the microorganisms responsible for the acidification are indicated.
Whereas non-sour beers rely on fermentation and/or maturation steps done using an axenic Saccharomyces yeast strain, the story differs for sour beers (Bokulich and Bamforth, 2013; Tonsmeire, 2014; Bossaert et al., 2019; Dysvik et al., 2020c).
One of the, if not the, most traditional sour beer production processes is based on spontaneous inoculation, thus without initiation of the fermentation of the wort by addition of a starter culture (Type D in Figure 1). It mainly concerns Belgian lambic beer and American coolship ale (ACA) productions, two of the most popular spontaneously fermented beers worldwide. Generally, the boiled and sterile wort is inoculated via multiple ways with a so-called wild microbiota, consisting of both wanted and unwanted yeasts and bacteria (De Keersmaecker, 1996; Spitaels et al., 2014a, 2015b; De Roos and De Vuyst, 2019; Bongaerts et al., 2021). Inoculation takes place when the environmental air gets into contact with the wort during an overnight cooling step using a metallic open vessel or coolship, when the wort gets into contact with the surfaces of the brewery equipment, and especially by contact of the fermenting wort and maturing beer with the interior surfaces of the horizontal, wooden barrels during the long-lasting fermentation and maturation steps (De Keersmaecker, 1996; De Roos and De Vuyst, 2019; Bongaerts et al., 2021). Therefore, spontaneously fermented beers are produced during the winter period to cool down the boiled wort fast enough, and so limit enterobacterial growth, in particular when the wort is not acidified manually, and in temperature-controlled cellars to achieve an optimal microbial succession (Bokulich et al., 2012; De Roos et al., 2018a; De Roos and De Vuyst, 2019; Bongaerts et al., 2021).
Specifically for bacteria, a broad range of species, including enterobacteria, LAB, and AAB have been isolated and identified from spontaneously fermenting lambic beer and ACA worts (Bokulich et al., 2012; De Roos and De Vuyst, 2019; Bongaerts et al., 2021; De Roos et al., unpublished results). Bacteria are typically present from the start of the lambic beer wort fermentation till the end of the extended barrel maturation process, which can last up to 3 years (Van Oevelen et al., 1977; Verachtert and Iserentant, 1995; De Roos et al., 2018b; Bongaerts et al., 2021). They are also present in lambic-based beers, such as gueuze, a bottle-refermented blend of young and old lambic beers (Spitaels et al., 2015a; De Roos et al., 2018a,b, 2020; Bongaerts et al., 2021; Piraine et al., 2021). More specifically, AAB are encountered during nearly the whole fermentation and maturation process of lambic beers but are mainly active during the first weeks, called the wild or enterobacterial and thus initial fermentation phase, during the acidification phase (two up to 12 months), and during maturation (up to 3 years; Bokulich et al., 2012; Spitaels et al., 2014a; De Roos and De Vuyst, 2019; Bongaerts et al., 2021). Next to gueuze, different types of lambic-based sour beers are produced in a traditional way, in particular fruit beers, such as Oude Kriek [additional fermentation in barrels of young (around 1 year old) lambic beer blended with fresh sour cherries], Framboise (young lambic beer blended with raspberries), and Pecheresse (young lambic beer blended with peaches), as well as Faro (young lambic beer blended with rock sugar; De Keersmaecker, 1996; Briggs et al., 2004; Tonsmeire, 2014; Verachtert and Derdelinckx, 2014; Pothakos et al., 2016; Dysvik et al., 2020c).
Culture-dependently, AAB species belonging to only two genera (Acetobacter and Gluconobacter) have been isolated and identified from spontaneously fermented sour beers (Bokulich et al., 2012; Spitaels et al., 2014a,b,c, 2015a,b; Snauwaert et al., 2016; De Roos et al., 2018a,b; Table 1). Culture-independent methods, such as metagenetics (targeting a part of or the whole 16S rRNA marker gene) and shotgun metagenomics, have led to the identification of not only Acetobacter spp. and Gluconobacter spp. but also Gluconacetobacter spp. and Komagataeibacter spp. (Bokulich et al., 2012; Snauwaert et al., 2016; De Roos et al., 2018a, 2020; Piraine et al., 2021; Tyakht et al., 2021; De Roos et al., unpublished results; Table 2).

Table 2. Overview of acetic acid bacterial species identified culture-independently from sour beers.
Multiple techniques of sour beer production fall in this category, differing according to the acidification method and especially where and when the acidification takes place (Bossaert et al., 2019; Dysvik et al., 2020a,b,c; Type E in Figure 1).
Malt, barley grains processed during the malting process, is used for almost all beers produced worldwide (Briggs et al., 2004). In the case of sour malting and sour mashing, acidification takes place during malting or mashing, respectively (Bossaert et al., 2019; Dysvik et al., 2019, 2020b,c). The acidification is achieved by the growth and metabolic activity of LAB, such as Lactiplantibacillus plantarum or Pediococcus pentosaceus (Laitila et al., 2006; Vriesekoop et al., 2012; Peyer et al., 2017). Carbohydrates present on the surfaces of the malted grains or in the mash are converted into lactic acid and in the case of sour malting, this lactic acid is retained on the grain surfaces (Lowe and Arendt, 2004; Vriesekoop et al., 2012). Lactic acid typically lowers the pH value around 0.15 to 0.25 units and therefore only 3–10% of the total grist will be acidified malt (Lowe et al., 2004; Lowe and Arendt, 2004). The resulting wort using acidified malts has a pH value around 5.2, in contrast with a pH of around 5.5 for non-acidified malt-based wort (Back, 2005; Vriesekoop et al., 2012). Whereas the influence of LAB starter cultures seems marginally, it results in an improved malting process, by suppressing rootlet growth of the germinating grain kernels, which has been shown to outperform chemical rootlet inhibitors (Vriesekoop et al., 2012). LAB starter culture application during the malting process results in an increased malt yield, an improved filterability, and lower wort viscosity (Vriesekoop et al., 2012). Additionally, LAB show certain antifungal and antibacterial activities, lowering the growth of fungi, such as Fusarium, which are involved in gushing and potential mycotoxin production (Batish et al., 1997; Lowe and Arendt, 2004; Shetty and Jespersen, 2006; Rouse et al., 2008; Vriesekoop et al., 2012).
During kettle souring, LAB acidify the wort in the brewing kettle. Sometimes, wort is boiled (without hops) prior to LAB pitching or the LAB inoculation can happen straight after mashing, without boiling (Cantwell and Bouckaert, 2016; Bossaert et al., 2019; Dysvik et al., 2020c). Kettle souring, also called quick souring, is a modern technique with the biggest advantage that the desired acidification typically takes place after one to 3 days (Bossaert et al., 2019). Acidification can be stopped either by boiling or by the addition of (heavily) hopped wort. LAB are then inactivated due to their sensitivity to hop-related compounds, such as 𝛼-acids (humulones), 𝛽-acids (lupulones), or their isomerized forms (iso-𝛼-acids or iso-humulones and iso-𝛽-acids or iso-lupulones, respectively; Almaguer et al., 2014). Further, addition of heavily hopped wort limits the loss of flavor compounds, which can happen during boiling (Tonsmeire, 2014; Bossaert et al., 2019; Dysvik et al., 2020c). Yet, when a less complex sour beer is wanted, boiling after acidification can be desired (Tonsmeire, 2014; Admassie, 2018).
Sour-fermented beers comprise all techniques for which ethanol production and acidification take place at the same moment. Sour-fermented beers can be divided into two large categories, based on the fact if there are bacteria present or not. For both, wort is prepared as usual, and the fermentation takes place in stainless-steel vessels (Tonsmeire, 2014).
Sour-fermented beers produced in the total absence of any bacteria must be produced through fermentation with yeast species capable of degradation of carbohydrates into lactic acid, ethanol, and carbon dioxide (Osburn et al., 2016, 2018). Hanseniaspora vineae, Lachancea fermentati, Lachancea thermotolerans, Schizosaccharomyces japonicus, and Wickerhamomyces anomalus species have been tested before and most strains examined are able to completely ferment the wort within 4 weeks (Domizio et al., 2016; Osburn et al., 2018; Bossaert et al., 2019). Whereas these yeast species have mainly been reported as members of mixed-starter cultures for beer wort and wine must fermentations, generally combined with Saccharomyces yeast species, they are able to attenuate beer wort sufficiently when used as the sole yeast starter, and they are capable of producing L-lactic acid in sufficient concentrations to produce sour beers (Banilas et al., 2016; Domizio et al., 2016; Benito, 2018; Osburn et al., 2018; Larroque et al., 2021; Vicente et al., 2022). Strain selection is of major importance, since large within-species and -strain variations occur, which greatly influences the final products (Osburn et al., 2018; Gatto et al., 2020). A high variability has been reported regarding lactic acid and ester production, flavor formation, sourness perception, and final pH (Domizio et al., 2016; Gamero et al., 2016; Osburn et al., 2018; Gatto et al., 2020; Vicente et al., 2021). Brewing primary-soured beers has the additional advantage of being produced without blocking the brewing kettle for days. It results in better sensory profiles compared to kettle-soured beers, without a long barrel aging process as is the case for spontaneously fermented sour beers (Osburn et al., 2018). The absence of acidifying bacteria in the brewing apparatus and brewery eliminates the risk of contaminating non-sour beers, especially when both sour and non-sour beers are brewed on the same site and/or using the same brewing equipment (Osburn et al., 2018; Bossaert et al., 2019). Additionally, yeast growth is very limited or completely not impacted by the hop dosing and release of iso-𝛼-acids into the wort. Consequently, higher hop dosages can be applied during primary souring brewing, since acidification does not rely on bacteria, which are generally more sensitive to iso-𝛼-acids, in particular Gram-positive LAB (Hazelwood et al., 2010; Almaguer et al., 2014; Domizio et al., 2016; Osburn et al., 2018).
The term mixed culture is used when more than one specific microbial strain and/or species is present during fermentation. The application of a mixed-starter culture differs from spontaneously fermented beers, such as lambic beer, in that in the latter case all yeasts and bacteria originate from the environment and/or brewing tools (De Keersmaecker, 1996; De Roos et al., 2018b; Bongaerts et al., 2021). Both traditional, red/brown (Flanders red ales) and old-brown (Flanders brown ales) acidic (Flemish) ales, and modern mixed-culture sour beers (Flanders-style sour ales) exist on industrial scale nowadays (Tonsmeire, 2014; Snauwaert et al., 2016; Bossaert et al., 2019; Dysvik et al., 2020c). During red/brown acidic ale production, a yeast slurry from previous fermentation processes is added to the cooled wort to perform the fermentation. Although the yeast slurry typically undergoes an acid wash, it still contains LAB (Martens et al., 1997). Initially, AAB have not been detected in the slurry or during primary fermentation (Martens et al., 1997). However, making use of appropriate selective agar media and incubation conditions, AAB have been isolated from beers at the end of the maturation phase, in particular A. pasteurianus (Snauwaert et al., 2016). Notice that the final beers representing Flanders red ales are also blends of two-year barrel-matured beers and young, non-matured beers, whereby different blend ratios give different red-brown sours (such as Rodenbach Classic, Grand Cru, and Vintage). During modern mixed fermentation processes, a mixture of yeasts (Saccharomyces and/or non-Saccharomyces spp.) and bacteria (LAB and/or AAB) is added as starter culture after wort production, either all at the same time, or spread over time (Peyer et al., 2017; Ciosek et al., 2019; Dysvik et al., 2019, 2020a,b,c).
When acidification happens during the maturation phase, either solely during maturation or as further souring during maturation, the beers can be classified as sour-matured. Sour maturation can take place in both stainless-steel vessels or wooden barrels, but maturation differs according to the container used (Tonsmeire, 2014; Snauwaert et al., 2016).
Wooden barrels were historically by far the most applied beer transport and storage tool, but have later disappeared due to practical reasons and the unpredictability of the quality of the resulting beers (Twede, 2005; Tonsmeire, 2014; Bossaert et al., 2019, 2020, 2022b). Yet, wooden barrels are nowadays gaining interest again, either for production or maturation, to introduce additional flavors and hence achieve more complex flavor profiles and/or sour beers (Garcia et al., 2012; Tonsmeire, 2014; Cantwell and Bouckaert, 2016; Bossaert et al., 2019, 2020; Shayevitz et al., 2020). Although introducing wood-associated flavors seems to be the most obvious reason, wooden barrel maturation does more. Flavors from previous uses of the barrels, such as for the production of port wine, wine or spirits, or for storage, can be introduced in the maturing beer (De Rosso et al., 2009; Fernandez de Simon et al., 2014; Cantwell and Bouckaert, 2016; Shayevitz et al., 2020). Also, microbial activity during barrel maturation can lead to new flavors, including a sour taste and acidic notes (Tonsmeire, 2014; Cantwell and Bouckaert, 2016; Bossaert et al., 2019, 2022a). Wooden barrels are generally made of oak, chestnut, cherry, and/or acacia wood (Cantwell and Bouckaert, 2016; Bossaert et al., 2019, 2020, 2022c). Due to the porous nature of the wood, wooden barrels are slightly permeable for oxygen gas and thus create a microaerobic environment, which may allow the growth of AAB (Cantwell and Bouckaert, 2016; De Roos et al., 2019; Bongaerts et al., 2021). Also linked with their porosity, wooden barrels harbor microorganisms up to 1.2 cm deep and act so as inoculation source for the fermenting wort and/or maturing beer (De Roos et al., 2019; Shayevitz et al., 2020; Bongaerts et al., 2021). The typical wooden barrel-associated microbiota consists of Brettanomyces yeasts, LAB and AAB, which have been isolated numerously from barrel-aged beers, including barrel-aged ales (Bokulich et al., 2012; Spitaels et al., 2014a; De Roos et al., 2018b, 2019; Shayevitz et al., 2020). In combination with a microaerobic environment, ethanol can be oxidized to acetic acid easily, which in turn impacts the beer flavor significantly and causes acidification of the beer (Cantwell and Bouckaert, 2016; De Roos et al., 2019; Shayevitz et al., 2020). Despite widespread use of wooden barrels, barrel maturation is generally a long-lasting process, and it remains trial and error concerning the flavor of the final beer, since the outcome relies on many factors including barrel characteristics, such as barrel history, barrel cleaning methods applied, barrel condition, and barrel wood, intrinsic beer characteristics such as alcohol level and pH, duration of the maturation, temperature, and humidity (Garcia et al., 2012; Sterckx et al., 2012; Cantwell and Bouckaert, 2016; Bossaert et al., 2022a,c).
In contrast with wooden barrels, stainless-steel vessels are not permeable for oxygen gas and do not harbor microorganisms. Consequently, acidification in metallic vessels can take place if the beer itself contains acidifying microorganisms, such as LAB, or acidifying microorganisms can be added (Tonsmeire, 2014). One of the most known beers produced through acidification in metallic vessels are old-brown acidic ales (Flanders brown ales; Tonsmeire, 2014; De Roos and De Vuyst, 2019). Old-brown acidic ales are produced as red/red-brown acidic ales but differ in the usage of metallic vessels for old-brown acidic ales and wooden barrels for red/red-brown acidic ales, and have been described as more malty and less acidic (Verachtert and Iserentant, 1995; Martens et al., 1997; Preedy, 2008; Tonsmeire, 2014; Snauwaert et al., 2016; De Roos and De Vuyst, 2019). The sour taste and acidic notes of old-brown acidic ales mainly comes from LAB activity during fermentation, prior to the metallic vessel maturation, but these beers do not acidify by acetic acid formation during maturation (Tonsmeire, 2014; Snauwaert et al., 2016). This must be ascribed to the lack of inoculation of barrel-associated AAB and the anaerobic environment inside stainless-steel vessels.
Whereas all beers of Types D and E are acidified by bacteria and/or yeasts, sour beers can also be acidified without microbial interference (Tonsmeire, 2014; Dysvik et al., 2019; Tan et al., 2021; Type F in Figure 1). Non-biologically acidified beers, also called chemically acidified beers, are produced by adding food-grade organic acids, such as lactic acid, fresh fruits, fruit juices, or lemonades (Franz et al., 2009; Tonsmeire, 2014; Dysvik et al., 2019; Tan et al., 2021). The main advantage linked with this production technique is the ability to experiment extensively with juice/beer ratios or the kinds of fruits used (Tonsmeire, 2014). In general, the most used fruits are berries, such as blueberries and raspberries, and cherries, but many more have been used, such as citrus fruits, peaches, mangoes, etc. (Tonsmeire, 2014; Tan et al., 2021). Beers produced by blending fruit juices with finished (eventually pasteurized) beers should not be confused with traditional fruit lambic beers, as the latter still evolve over time by the presence of metabolically active microorganisms, whereas non-biological acidified beers do not evolve anymore.
AAB, and bacteria in general, are completely unwanted during non-sour beer production, mainly due to their acetic acid production turning the beer into vinegar (Bokulich and Bamforth, 2013). Despite their bad reputation, AAB do contribute unambiguously to desired flavor formation during the fermentation and/or maturation of certain sour beer types (Vriesekoop et al., 2012; De Roos and De Vuyst, 2019; Bongaerts et al., 2021; Figure 1). As mentioned above, the best studied example of AAB in beers is their appearance and functionality during fermentation and maturation of spontaneously fermented beers, such as lambic beer, and to a lesser extent the ACA analogue (Martens et al., 1997; Bokulich et al., 2012; Spitaels et al., 2014a, 2015b, 2017; Tonsmeire, 2014; Snauwaert et al., 2016; De Roos and De Vuyst, 2019; Bongaerts et al., 2021). Historically, the functional role of AAB in spontaneously fermented beers was considered limited and solely restricted to the oxidation of ethanol to acetic acid. Although this is their most impacting and characterizing feature, more functionalities and contributions during fermentation and maturation have been described in the last decade. Limited literature is available about AAB presence in sour beers, except for red-brown acidic ales, ACAs and especially lambic beer, and so is the following writing mainly based on findings during spontaneously fermented beers, and to a lesser extent on findings originating from research applied on red-brown acidic ales.
Acetic acid bacteria occurrence in beers is mainly linked with their most characterizing feature, being the aerobic, incomplete oxidation of ethanol, carbohydrates, or sugar alcohols by dehydrogenase activities into the corresponding organic acids, sometimes referred to as oxidative fermentation (Cleenwerck et al., 2002; Ashtavinayak and Elizabeth, 2016; De Roos and De Vuyst, 2018; De Roos et al., 2018a). The two-step catalytic oxidation of ethanol comprises first oxidation of ethanol by membrane-bound pyrroloquinoline quinone (PQQ)-dependent alcohol dehydrogenase (ADH) activity to acetaldehyde and the further oxidation of the latter compound by a membrane-bound aldehyde dehydrogenase (ALDH) to acetic acid (Yakushi and Matsushita, 2010; Gomes et al., 2018). Since both enzymes, ADH and ALDH, form a multienzyme complex, acetaldehyde is not released (Gomes et al., 2018). Acetic acid influences the beer flavor in a drastic way, since it is described as harsh, and it thus contributes a sharp sourness and vinegary notes if present above the threshold concentration of around 200 mg/l (Van Oevelen et al., 1976; De Roos et al., 2019). Although acetic acid possibly causes problems in non-sour beers or when it is present in excessively high concentrations in sour beers, it is crucial to get the unique flavor profile and refreshing character of most sour beers (Van Oevelen et al., 1976; De Roos et al., 2019; Bongaerts et al., 2021).
Maltooligosaccharides (MOS) is the overarching term of linearly 𝛼-1-4 glycosidically bound glucopyranosyl units, covering chain lengths of two up to 10 glucose molecules. MOS are formed from starch due to a breakdown by heat and endogenous amylase activity, mainly during the mashing process (Fangel et al., 2018). During beer production with pitching of an axenic yeast culture of S. cerevisiae, S. pastorianus or S. bayanus, MOS remain untouched, due to the lack of the expression of the degrading enzymes (Sheih et al., 1979). Other yeast species, such as Saccharomyces kudriavzevii but especially Brettanomyces/Dekkera spp., are known to express 𝛼-glucosidases, which allows the metabolism of MOS (Kumara et al., 1993; De Roos et al., 2020). Especially Brettanomyces/Dekkera spp. can metabolize this additional substrate when all mono- and disaccharides are depleted and hence explaining their growth and activity during the maturation phase of lambic beer production (De Roos and De Vuyst, 2019; Bongaerts et al., 2021). Besides MOS breakdown by yeasts, shotgun metagenomic research of fermenting lambic beer wort has shown that two genes for MOS breakdown, encoding maltooligosyl trehalose synthase and maltooligosyl trehalose, are associated mainly with A. pasteurianus as well as with Acetobacter pomorum and an unknown Acetobacter species, most likely A. lambici (De Roos et al., 2020). Shotgun metagenomic research of fermenting lambic beer wort has additionally demonstrated that A. cerevisiae and Acetobacter malorum contain these two genes, encoding maltooligosyl trehalose synthase and maltooligosyl trehalose, as well (De Roos et al., unpublished results). These two enzymes allow the degradation of MOS by their conversion into maltooligosyl trehalose, followed by the release of trehalose (disaccharide of 𝛼-1-1 glycosidically bound glucopyranosyl molecules) by maltooligosyl trehalose activity, to protect the cells against osmotic stress (Zhang et al., 2015; De Roos et al., 2020). Although these two genes are present, it still has to be confirmed if MOS degradation indeed takes place by the latter two species, since high acetic acid concentrations inhibit the biosynthesis of trehalose (Yang et al., 2019). Surprisingly, examining the whole genome of one of the most encountered AAB species, A. lambici, it is not one of the species possessing the latter two enzymes for MOS degradation via trehalose, although it is very well adapted to the harsh late stages of the lambic beer production process when all mono- and disaccharides are depleted (De Roos et al., 2020; De Roos et al., unpublished results).
Esters are extremely important for flavor formation of fermented beverages, including beer, among which ethyl acetate, isoamyl acetate, ethyl hexanoate and ethyl octanoate are the most desired ones produced by yeasts (Engan, 1974; Verstrepen et al., 2003; He et al., 2014). The ester profile of sour beers covers two main types, namely ethyl esters (the condensation products of ethanol and fatty acids) and acetate esters (the condensation products of acetic acid and higher alcohols; Pires et al., 2014; Bongaerts et al., 2021). The ester profile of sour beers differs from top-fermented and bottom-fermented beers, as AAB species contribute to not only acetic acid formation but also ester formation by their expression of esterases (Kashima et al., 2000; De Roos et al., 2020). AAB, more specifically Acetobacter spp., possess the intracellular esterases EST1 and EST2 and are thus able to catalyze the condensation of ethanol and acetic acid into ethyl acetate, the most abundant ester in lambic beers (Van Oevelen et al., 1976; Kashima et al., 1998, 2000; Tonsmeire, 2014; Witrick et al., 2017; De Roos and De Vuyst, 2019; De Roos et al., 2020; Bongaerts et al., 2021). Ethyl acetate is of indisputable importance for the lambic beer flavor, and by extension sour beer flavor, due to its high odor activity value and high concentrations. Its formation is ascribed to not only the activities of Brettanomyces yeasts and AAB but also chemical esterification of ethanol and acetate (Van Oevelen et al., 1976; Verstrepen et al., 2003; De Roos et al., 2018a). Besides with the formation of ethyl acetate, esterase EST1 is also linked with the formation of isoamyl acetate, an abundantly formed ester by S. cerevisiae (Kashima et al., 2000).
Acetoin or 3-hydroxy-2-butanone is a flavor-active compound associated with yoghurt flavor, cream odor, and buttery taste (Xiao and Lu, 2014; De Roos and De Vuyst, 2018, 2019; Bongaerts et al., 2021). Acetoin can be produced by yeasts, LAB (such as P. damnosus) and AAB, since they all possess the necessary genes. Acetobacter spp. are most likely the main producers of acetoin in sour beers, as has been shown during lambic beer productions and cocoa fermentation processes (Adler et al., 2014; Moens et al., 2014; De Roos et al., 2018a,b, 2020). The acetoin concentrations increase when AAB appear and acetoin is produced more at the air/liquid interface of fermenting lambic beer wort in wooden barrels, where typically AAB are present in higher numbers (De Roos et al., 2018a,b, 2020). Indeed, lactic acid can be used as carbon source and oxidized into pyruvate, further converted by acetolactate synthase and/or acetolactate decarboxylase into 𝛼-acetolactate and finally by diacetyl reductase into acetoin (Adler et al., 2014; Moens et al., 2014; De Roos et al., 2020; Pelicaen et al., 2020). These features have been supported by metagenomic identification of the appropriate genes and phenotypically in both lambic beer productions and cocoa fermentation processes (Moens et al., 2014; De Roos et al., 2020). Acetoin, in combination with acetic acid, contributes to sour beer flavors. However, excessive concentrations should be avoided, since high acetoin levels can cause undesirable buttery notes (De Roos et al., 2018a; Bongaerts et al., 2021). AAB growth should always be controlled by using well-sealed wooden barrels, which do allow microaerobic conditions through the pores of the wood and the formation of a yeast pellicle at the surface of the liquor to limit oxygen inlet from the headspace into the beer. Volume adjustments over time compensate volume losses by evaporation, decrease headspace volumes containing oxygen, and decrease the contact surface between the maturing beer and the barrel headspace, all limiting oxygen entering the beer, and so preventing AAB to grow too extensively (Tonsmeire, 2014; De Roos et al., 2018b, 2019). Finally, the temperature should always be controlled and kept stable, typically below 20°C, again to prevent excessive AAB growth (Van den Steen, 2012; Tonsmeire, 2014).
Sour beers, especially the ones produced by a spontaneous fermentation process, can be linked with health benefits (De Roos and De Vuyst, 2022). First, sour beers containing living LAB and/or AAB cells may contribute to a good microbial balance inside the human gut (Marco et al., 2017, 2021; De Roos and De Vuyst, 2022). Regarding this feature, sour beers have been produced using probiotic LAB strains, for instance with Lacticaseibacillus paracasei L26 and DTA-81, and sour beers have been evaluated regarding their suitability as probiotic delivery matrix, which has been demonstrated successfully if produced using a semi-separated co-culture system (Chan et al., 2019; Silva et al., 2020). Further, the occurrence of high-molecular-mass pentosans and ß-glucans, produced by LAB, in sour beer can provide this beer with a natural source of prebiotics (Peyer et al., 2017; Silva et al., 2020).
Regarding caloric values, sour beers can be a helping hand. Whereas non-sour beers are rich in calories, mainly caused by the ethanol content in combination with the presence of residual unfermentable carbohydrates, most sour beers are almost carbohydrate-free, thanks to their complete MOS degradation by interference of non-conventional yeasts, LAB, and/or AAB (De Cort et al., 1994; Briggs et al., 2004; Tonsmeire, 2014; Bossaert et al., 2019; De Roos et al., 2020; De Roos et al., unpublished results). Indeed, if sour beers are produced using Brettanomyces yeast species, intentionally added or not, the fraction of unfermentable carbohydrates decreases because of extracellular and intracellular 𝛼-glucosidase activities, causing the breakdown of MOS with chain lengths of at least up to eight glucose molecules (Kumara et al., 1993; De Roos and De Vuyst, 2019; De Roos et al., 2020; Bongaerts et al., 2021).
Finally, regarding antioxidant properties, especially those provided by polyphenolic compounds, sour beers produced by the addition of fresh fruits contain significantly high concentrations of these compounds (Cho et al., 2018; Kawa-Rygielska et al., 2019; Zapata et al., 2019; Nardini and Garaguso, 2020). Fruit addition acts as additional source of antioxidant compounds (e.g., carotenoids, tocopherols, and/or ascorbic acid), besides those provided by barley and hops (Nardini and Garaguso, 2020). Beer antioxidant activities and polyphenolic contents are influenced by the raw materials used, the quantity and quality of the fruits added, and the brewing process applied (Kawa-Rygielska et al., 2019; Nardini and Garaguso, 2020). Dietary intake of antioxidants counters negative effects of oxidative stress, which is caused by the overproduction of reactive oxygen species or reactive nitrogen species (Martinez-Gomes et al., 2020).
Historically, the role of AAB in beers was underestimated and limited to solely oxidation of ethanol into acetic acid, causing a sharp sour taste and pungent smell, impacting the flavor of most beers negatively. Despite their negative imago, research has extended the knowledge about AAB, exposing new features of AAB in sour beers. Sour beer production involving AAB can possibly result in more complex and funky beers. To achieve a positive contribution of AAB to the beer flavor, controlled growth should always be aimed for. Despite an increased understanding of AAB and their functional role during sour beer production, controlled growth of AAB with sufficient but not excessive production of flavor compounds, such as acetic acid and acetoin, requires skills only experienced brewers do master.
AB drafted the manuscript. AB and LDV revised and edited the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Research Council of the Vrije Universiteit Brussel (SRP7 and IOF3017 projects).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adler, P., Frey, L. J., Berger, A., Bolten, C. J., Hansen, C. E., and Wittmann, C. (2014). The key to acetate: metabolic fluxes of acetic acid bacteria under cocoa pulp fermentation-simulating conditions. Appl. Environ. Microbiol. 80, 4702–4716. doi: 10.1128/AEM.01048-14
Admassie, M. (2018). A review on food fermentation and the biotechnology of lactic acid bacteria. World J. Food Sci. Technol. 2, 19–24. doi: 10.11648/j.wjfst.20180201.13
Almaguer, C., Schönenberger, C., Gastl, M., Arendt, E. K., and Becker, T. (2014). Humulus lupulus - a story that begs to be told. A review. J. Inst. Brew. 120, 289–314. doi: 10.1002/jib.160
Ashtavinayak, P., and Elizabeth, H. A. (2016). Review: gram negative bacteria in brewing. Adv. Microbiol. 6, 195–209. doi: 10.4236/aim.2016.63020
Baiano, A. (2020). Craft beer: an overview. Compr. Rev. Food Sci. Food Saf. 20, 1829–1856. doi: 10.1111/1541-4337.12693
Banilas, G., Sgouros, G., and Nisiotou, A. (2016). Development of microsatellite markers for Lachancea thermotolerans typing and population structure of wine-associated isolates. Microbiol. Res. 193, 1–10. doi: 10.1016/j.micres.2016.08.010
Batish, V. K., Roy, U., Lal, R., and Grower, S. (1997). Antifungal attributes of lactic acid bacteria - A review. Crit. Rev. Biotechnol. 17, 209–225. doi: 10.3109/07388559709146614
Benito, S. (2018). The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 102, 6775–6790. doi: 10.1007/s00253-018-9117-z
Bokulich, N. A., and Bamforth, C. W. (2013). The microbiology of malting and brewing. Microbiol. Mol. Biol. Rev. 77, 157–172. doi: 10.1128/MMBR.00060-12
Bokulich, N. A., Bamforth, C. W., and Mills, D. A. (2012). Brewhouse-resident microbiota are responsible for multi-stage fermentation of American coolship ale. PLoS One 7:e35507. doi: 10.1371/journal.pone.0035507
Bongaerts, D., De Roos, J., and De Vuyst, L. (2021). Technological and environmental features determine the uniqueness of the lambic beer microbiota and production process. Appl. Environ. Microbiol. 87, e00612–e00621. doi: 10.1128/AEM.00612-21
Bossaert, S., Crauwels, S., De Rouck, G., and Lievens, B. (2019). The power of sour - a review: old traditions, new opportunities. BrewSci. 72, 78–88. doi: 10.23763/BrSc19-10bossaert
Bossaert, S., Kocijan, T., Winne, V., Schlich, J., Herrera-Malaver, B., Verstrepen, K. J., et al. (2022a). Beer ethanol and iso-α-acid level affect microbial community establishment and beer chemistry throughout wood maturation of beer. BioRxiv [Preprint]. Available at: https://www.biorxiv.org/content/10.1101/2022.03.07.483260v1.full.pdf (Accessed May 03, 2022).
Bossaert, S., Kocijan, T., Winne, V., Van Opstaele, F., Schlich, J., Herrera-Malaver, B., et al. (2022b). Development of a tractable model system to mimic wood-ageing of beer on a lab scale. BioRxiv [Preprint]. Available at: https://www.biorxiv.org/content/10.1101/2022.03.11.483928v1 (Accessed May 26, 2022).
Bossaert, S., Winne, V., Van Opstaele, F., Buyse, J., Verreth, C., Herrera-Malaver, B., et al. (2020). Description of the temporal dynamics in microbial community composition and beer chemistry in sour beer production via barrel ageing of finished beers. Int. J. Food Microbiol. 339:109030. doi: 10.1016/j.ijfoodmicro.2020.109030
Bossaert, S., Winne, V., Van Opstaele, F., Buyse, J., Verreth, C., Herrera-Malaver, B., et al. (2022c). Impact of wood species on microbial community composition, beer chemistry and sensory characteristics during barrel-ageing of beer. Int. J. Food Sci. Technol. 57, 1122–1136. doi: 10.1111/ijfs.15479
Briggs, D. E., Boulton, C., Brookes, P., and Stevens, R. (2004). Brewing Science and Practice. Cambridge: Woodhead Publishing Limited.
Cantwell, D., and Bouckaert, P. (2016). Wood & Beer: A Brewer's Guide. Boulder: Brewers Publications.
Chan, M. Z. A., Chua, J. Y., Toh, M., and Liu, S.-Q. (2019). Survival of probiotic strain Lactobacillus paracasei L26 during co-fermentation with S. cerevisiae for the development of a novel beer beverage. Food Microbiol. 82, 541–550. doi: 10.1016/j.fm.2019.04.001
Cho, J.-H., Kim, I.-D., Dhungana, S. K., Do, H.-M., and Shin, D.-H. (2018). Persimmon fruit enhanced quality characteristics and antioxidant potential of beer. Food Sci. Biotechnol. 27, 1067–1073. doi: 10.1007/s10068-018-0340-2
Ciosek, A., Rusiecka, I., and Poreda, A. (2019). Sour beer production: impact of pitching sequence of yeast and lactic acid bacteria. J. Inst. Brew. 126, 53–58. doi: 10.1002/jib.590
Cleenwerck, I., Vandemeulebroecke, K., Janssens, D., and Swings, J. (2002). Re-examination of the genus Acetobacter, with descriptions of Acetobacter cerevisiae sp. nov. and Acetobacter malorum sp. nov. Int. J. Syst. Evol. Microbiol. 52, 1551–1558. doi: 10.1099/00207713-52-5-1551
Colen, L., and Swinnen, J. (2015). Economic growth, globalisation and beer consumption. J. Agric. Econ. 67, 186–207. doi: 10.1111/1477-9552.12128
Cousin, F. J., Le Guellec, R., Schlusselhuber, M., Dalmasso, M., Laplace, J.-M., and Cretenet, M. (2017). Microorganisms in fermented apple beverages: current knowledge and future directions. Microorg. 5:39. doi: 10.3390/microorganisms5030039
De Cort, S., Kumara, H. S., and Verachtert, H. (1994). Localization and characterization of α-glucosidase activity in Lactobacillus brevis. Appl. Environ. Microbiol. 60, 3074–3078. doi: 10.1128/aem.60.9.3074-3078.1994
De Keersmaecker, J. (1996). The mystery of lambic beer. SciAm 275, 74–80. doi: 10.1038/scientificamerican0896-74
De Roos, J., and De Vuyst, L. (2018). Acetic acid bacteria in fermented foods and beverages. Curr. Opin. Biotechnol. 49, 115–119. doi: 10.1016/j.copbio.2017.08.007
De Roos, J., and De Vuyst, L. (2019). Microbial acidification, alcoholization, and aroma production during spontaneous lambic beer production. J. Sci. Food Agric. 99, 25–38. doi: 10.1002/jsfa.9291
De Roos, J., and De Vuyst, L. (2022). “Lambic beer, a unique blend of tradition and good microorganisms,” in Good Microbes in Medicine, Food Production, Biotechnology, Bioremediation, and Agriculture, eds. F. J. deBruijn, H. Smidt, L. Cocolin, M. Sauer, D. Dowling, and L. Thomashow (Hoboken: John Wiley & Sons), 219–229. doi: 10.1128/AEM.02226-18
De Roos, J., Vandamme, P., and De Vuyst, L. (2018a). Wort substrate consumption and metabolite production during lambic beer fermentation and maturation explain the successive growth of specific bacterial and yeast species. Front. Microbiol. 9:2763. doi: 10.3389/fmicb.2018.02763
De Roos, J., Van der Veken, D., and De Vuyst, L. (2019). The interior surfaces of wooden barrels are an additional microbial inoculation source for lambic beer production. Appl. Environ. Microbiol. 85, e02226–e02218.
De Roos, J., Verce, M., Aerts, M., Vandamme, P., and De Vuyst, L. (2018b). Temporal and spatial distribution of the acetic acid bacterium communities throughout the wooden casks used for the fermentation and maturation of lambic beer underlines their functional role. Appl. Environ. Microbiol. 84, e02846–e02817. doi: 10.1128/AEM.02846-17
De Roos, J., Verce, M., Weckx, S., and De Vuyst, L. (2020). Temporal shotgun metagenomics revealed the potential metabolic capabilities of specific microorganisms during lambic beer production. Front. Microbiol. 11:1692. doi: 10.3389/fmicb.2020.01692
De Rosso, M., Panighel, A., Dalla Vedova, A., Stella, L., and Flamini, R. (2009). Changes in chemical composition of a red wine aged in acacia, cherry, chestnut, mulberry, and oak wood barrels. J. Agric. Food Chem. 57, 1915–1920. doi: 10.1021/jf803161r
De Vuyst, L., and Leroy, F. (2020). Functional role of yeasts, lactic acid bacteria and acetic acid bacteria in cocoa fermentation processes. FEMS Microbiol. Rev. 44, 432–453. doi: 10.1093/femsre/fuaa014
Domizio, P., House, J. F., Joseph, C. M. L., Bisson, L. F., and Bamforth, C. W. (2016). Lachancea thermotolerans as an alternative yeast for the production of beer. J. Inst. Brew. 122, 599–604. doi: 10.1002/jib.362
Donadini, G., and Porretta, S. (2017). Uncovering patterns of consumers’ interest for beer: a case study with craft beers. Food Res. Int. 91, 183–198. doi: 10.1016/j.foodres.2016.11.043
Dysvik, A., La Rosa, S. L., Buffetto, F., Liland, K. H., Myhrer, K. S., Rukke, E. O., et al. (2020a). Secondary lactic acid bacteria fermentation with wood-derived xylooligosaccharides as a tool to expedite sour beer production. J. Agric. Food Chem. 68, 301–314. doi: 10.1021/acs.jafc.9b05459
Dysvik, A., La Rosa, S. L., De Rouck, G., Rukke, E.-O., Westereng, B., and Wicklund, T. (2020c). Microbial dynamics in traditional and modern sour beer production. Appl. Environ. Microbiol. 86, e00566–e00520. doi: 10.1128/AEM.00566-20
Dysvik, A., La Rosa, S. L., Liland, K. H., Myhrer, K. S., Østlie, H. M., De Rouck, G., et al. (2020b). Co-fermentation involving Saccharomyces cerevisiae and Lactobacillus species tolerant to brewing-related stress factors for controlled and rapid production of sour beer. Front. Microbiol. 11:279. doi: 10.3389/fmicb.2020.00279
Dysvik, A., Liland, K. H., Myhrer, K. S., Westereng, B., Rukke, E.-O., De Rouck, G., et al. (2019). Pre-fermentation with lactic acid bacteria in sour beer production. J. Inst. Brew. 125, 342–356. doi: 10.1002/jib.569
Fangel, J. U., Eiken, J., Sierksma, A., Schols, H. A., Willats, W. G. T., and Harholt, J. (2018). Tracking polysaccharides through the brewing process. Carbohydr. Polym. 196, 465–473. doi: 10.1016/j.carbpol.2018.05.053
Fernandez de Simon, B., Martinez, J., Sanz, M., Cadahaia, E., Esteruelas, E., and Muñoz, A. M. (2014). Volatile compounds and sensorial characterisation of red wine aged in cherry, chestnut, false acacia, ash and oak wood barrels. Food Chem. 147, 346–356. doi: 10.1016/j.foodchem.2013.09.158
Franz, O., Gastl, M., and Back, W. (2009). “Beer-based mixed drinks,” in Handbook of Brewing: Processes, Technology, Markets. ed. H. M. Esslinger (Wiley-VCH Verlag GmbH, Weinheim, Germany), 257–273.
Gamero, A., Quintilla, R., Groenewald, M., Alkema, W., Boekhout, T., and Hazelwood, L. (2016). High-throughput screening of a large collection of non-conventional yeasts reveals their potential for aroma formation in food fermentation. Food Microbiol. 60, 147–159. doi: 10.1016/j.fm.2016.07.006
Garcia, R., Soares, B., Dias, C. B., Freitas, A. M. C., and Cabrita, M. J. (2012). Phenolic and furanic compounds of Portuguese chestnut and French, American and Portuguese oak wood chips. Eur. Food Res. Technol. 235, 457–467. doi: 10.1007/s00217-012-1771-2
Gatto, V., Binati, R. L., Lemos Junior, W. J. F., Basile, A., Treu, L., de Almeida, O. G. G., et al. (2020). New insights into the variability of lactic acid production in Lachancea thermotolerans at the phenotypic and genomic level. Microbiol. Res. 238:126525. doi: 10.1016/j.micres.2020.126525
Gomes, R. J., Borges, M. D. F., Rosa, M. F., Castro-Gómez, R. J. H., and Spinosa, W. M. (2018). Acetic acid bacteria in the food industry: systematics, characteristics and applications. Food Technol. Biotechnol. 56, 139–151. doi: 10.17113/ftb.56.02.18.5593
Gullo, M., Verzelloni, E., and Canonico, M. (2016). Aerobic submerged fermentation by acetic acid bacteria for vinegar production: process and biotechnological aspects. Process Biochem. 49, 1571–1579. doi: 10.1016/j.procbio.2014.07.003
Harper, D. R. (1980). Microbial contamination of draught beer in public houses. Process Biochem. 16, 2–7.
Hazelwood, L. A., Walsh, M. C., Pronk, J. T., and Daran, J.-M. (2010). Involvement of vacuolar sequestration and active transport in tolerance of Saccharomyces cerevisiae to hop iso-a-acids. Appl. Environ. Microbiol. 76, 318–328. doi: 10.1128/AEM.01457-09
He, Y., Dong, J., Yin, H., Zhao, Y., Chen, R., Wan, X., et al. (2014). Wort composition and its impact on the flavour-active higher alcohol and ester formation of beer – a review. J. Inst. Brew. 120, 157–163. doi: 10.1002/jib.145
Hill, A. (2015). “Gram-negative spoilage bacteria in brewing,” in Brewing Microbiology: Managing Microbes, Ensuring Quality and Valorising Waste. ed. A. D. Paradh (Amsterdam: Elsevier Science & Technology), 175–194.
Hutkins, R. W. (2019). Microbiology and Technology of Fermented Foods. Ames: Wiley-Blackwell Publishing.
Ingledew, M. W. (1979). Effect of bacterial contaminants on beer. A review. J. Am. Soc. Brew. Chem. 37, 145–150.
Jeon, S. H., Kim, N. H., Shim, M. B., Jeon, Y. W., Ahn, J. H., Lee, S. H., et al. (2014). Microbiological diversity and prevalence of spoilage and pathogenic bacteria in commercial fermented alcoholic beverages (beer, fruit wine, refined rice wine, and yakju). J. Food Prot. 78, 812–818. doi: 10.4315/0362-028X.JFP-14-431
Kashima, Y., Iijima, M., Nakano, T., Tayama, K. A., Koizumi, Y., Udaka, S., et al. (2000). Role of intracellular esterases in the production of esters by Acetobacter pasteurianus. J. Biosci. Bioeng. 89, 81–83. doi: 10.1016/S1389-1723(00)88055-X
Kashima, Y., Iijima, M., Okamoto, A., Koizumi, Y., Udaka, S., and Yanagida, F. (1998). Purification and characterization of intracellular esterases related to ethyl acetate formation in Acetobacter pasteurianus. J. Ferm. Bioeng. 85, 584–588. doi: 10.1016/S0922-338X(98)80009-3
Kawa-Rygielska, J., Adamenko, K., Kucharska, A. Z., Prorok, P., and Piórecki, N. (2019). Physicochemical and antioxidative properties of cornelian cherry beer. Food Chem. 281, 147–153. doi: 10.1016/j.foodchem.2018.12.093
Kourtis, L., and Arvanitoyannis, I. (2001). Implementation of hazard analysis critical control point (HACCP) system to the alcoholic beverages industry. Food Rev. Int. 17, 1–44. doi: 10.1081/FRI-100000514
Kubizniaková, P., Kyselová, L., Brožová, M., Hanzalíková, K., and Matoulková, D. (2021). The role of acetic acid bacteria in brewing and their detection in operation. Kvasny Prumysl 67, 511–522. doi: 10.18832/kp2021.67.511
Kumara, H. M., De Cort, S., and Verachtert, H. (1993). Localization and characterization of 𝛼-glucosidase activity in Brettanomyces lambicus. Appl. Environ. Microbiol. 59, 2352–2358. doi: 10.1128/aem.59.8.2352-2358.1993
Laitila, A., Sweins, H., Vilpola, A., Kotaviita, E., Olkku, J., Home, S., et al. (2006). Lactobacillus plantarum and Pediococcus pentosaceus starter cultures as a tool for microflora management in malting and for enhancement of malt processability. J. Agric. Food Chem. 54, 3840–3851. doi: 10.1021/jf052979j
Larroque, M. N., Carrau, F., Fariña, L., Boido, E., Dellacassa, E., and Medina, K. (2021). Effect of Saccharomyces and non-Saccharomyces native yeasts on beer aroma compounds. Int. J. Food Microbiol. 337:108953. doi: 10.1016/j.ijfoodmicro.2020.108953
Lattici, F., Catallo, M., and Solieri, L. (2020). Designing new yeasts for craft brewing: when natural biodiversity meets biotechnology. Beverages 6:3. doi: 10.3390/beverages6010003
Leroy, F., and De Vuyst, L. (2004). Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 15, 67–78. doi: 10.1016/j.tifs.2003.09.004
Li, F., Shi, Y., Boswell, M., and Rozelle, C. (2017). “Craft beer in China,” in Economic Perspectives on Craft Beer: A Revolution in the Global Beer Industry. eds. C. Garavaglia and J. Swinnen (London: Palgrave Macmillan), 457–484.
Lowe, D. P., and Arendt, E. K. (2004). The use and effects of lactic acid bacteria in malting and brewing with their relationships to antifungal activity, mycotoxins and gushing: a review. J. Inst. Brew. 110, 163–180. doi: 10.1002/j.2050-0416.2004.tb00199.x
Lowe, D. P., Ulmer, H. M., van Sinderen, D., and Arendt, E. K. (2004). Application of biological acidification to improve the quality and processability of wort produced from 50% raw barley. J. Inst. Brew. 110, 133–140. doi: 10.1002/j.2050-0416.2004.tb00192.x
Lynch, K. M., Wilkinson, S., Daenen, L., and Arendt, A. K. (2021). An update on water kefir: microbiology, composition and production. Int. J. Food Microbiol. 345:109128. doi: 10.1016/j.ijfoodmicro.2021.109128
Magnus, C. A., Ingledew, M. W., and Casey, G. (1986). High-gravity brewing: influence of high-ethanol beer on the viability of contaminating brewery bacteria. J. Am. Soc. Brew. Chem. 44, 158–161. doi: 10.1094/ASBCJ-44-0158
Marco, M. L., Heeney, D., Binda, S., Cifelli, C. J., Cotter, P. D., Foligné, B., et al. (2017). Health benefits of fermented foods: microbiota and beyond. Curr. Opin. Biotechnol. 44, 94–102. doi: 10.1016/j.copbio.2016.11.010
Marco, M. L., Sanders, M. E., Gänzle, M., Arrieta, M. C., Cotter, P. D., De Vuyst, L., et al. (2021). The international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 18, 196–208. doi: 10.1038/s41575-020-00390-5
Martens, H., Iserentant, D., and Verachtert, H. (1997). Microbiological aspects of a mixed yeast-bacterial fermentation in the production of a special Belgian acidic ale. J. Inst. Brew. 103, 85–91. doi: 10.1002/j.2050-0416.1997.tb00939.x
Martinez-Gomes, A., Caballero, I., and Blanco, C. A. (2020). Phenols and Melanoidins as Natural Antioxidants in Beer. Structure, Reactivity and Antioxidant Activity. Biomolecules 10:400. doi: 10.3390/biom10030400
Menz, G., Aldred, P., and Vriesekoop, F. (2009). “Pathogens in beer,” in Beer in Health and Disease Prevention. ed. V. R. Preedy (Amsterdam: Academic Press), 403–413.
Moens, F., Lefeber, T., and De Vuyst, L. (2014). Oxidation of metabolites highlights the microbial interactions and role of Acetobacter pasteurianus during cocoa bean fermentation. Appl. Environ. Microbiol. 80, 1848–1857. doi: 10.1128/AEM.03344-13
Nardini, M., and Garaguso, I. (2020). Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 305:125437. doi: 10.1016/j.foodchem.2019.125437
Neves, M. F., Trombin, V. G., Lopes, F. F., Kalaki, R., and Milan, P. (2011). The Orange Juice Business. (Wageningen: Wageningen Academic Publishers).
Osburn, K., Ahmadd, N. N., and Bochman, M. L. (2016). Bio-prospecting, selection, and analysis of wild yeasts for ethanol fermentation. Zymurgy 39, 81–88. doi: 10.13140/RG.2.2.16952.14080
Osburn, K., Amaral, J., Metcalf, S. R., Nickens, D. M., Rogers, C. M., Sausen, C., et al. (2018). Primary souring: a novel bacteria-free method for sour beer production. Food Microbiol. 70, 76–84. doi: 10.1016/j.fm.2017.09.007
Paradh, A. D. (2015). “Gram-negative spoilage bacteria in brewing,” in Brewing Microbiology Managing Microbes, Ensuring Quality and Valorising Waste. ed. A. E. Hill (Sawston: Woodhead Publishing), 175–194.
Pelicaen, R., Gonze, D., De Vuyst, L., and Weckx, S. (2020). Genome-scale metabolic modeling of Acetobacter pasteurianus 386B reveals its metabolic adaptation to cocoa fermentation conditions. Food Microbiol. 92:103597. doi: 10.1016/j.fm.2020.103597
Peyer, L. C., Zarnkow, M., Jacob, F., De Schutter, D. P., and Arendt, E. K. (2017). Sour brewing: impact of Lactobacillus amylovorus FST2.11 on technological and quality attributes of acid beers. J. Am. Soc. Brew. Chem. 75, 207–216. doi: 10.1094/ASBCJ-2017-3861-01
Piraine, R. E. A., Leite, F. P. L., and Bochman, M. L. (2021). Mixed-culture metagenomics of the microbes making sour beer. Fermentation 7:174. doi: 10.3390/fermentation7030174
Pires, E. J., Teixeira, J. A., Brányik, T., and Vicente, A. A. (2014). Yeast: the soul of beer’s aroma - A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 98, 1937–1949. doi: 10.1007/s00253-013-5470-0
Pothakos, V., Illeghems, K., Laureys, D., Spitaels, F., Vandamme, P., and De Vuyst, L. (2016). “Acetic acid bacteria in fermented food and beverage ecosystems,” in Acetic Acid Bacteria: Ecology and Physiology. eds. K. Matsushita, H. Toyama, N. Tonouchi, and A. Okamoto-Kainuma (Tokyo: Springer), 73–100.
Rouse, S., Harnett, D., Vaughan, A., and van Sinderen, D. (2008). Lactic acid bacteria with potential to eliminate fungal spoilage in foods. J. Appl. Microbiol. 104, 915–923. doi: 10.1111/j.1365-2672.2007.03619.x
Sakamoto, K., and Konings, W. N. (2003). Beer spoilage bacteria and hop resistance. Int. J. Food Microbiol. 89, 105–124. doi: 10.1016/S0168-1605(03)00153-3
Schneiderbanger, J., Jacob, F., and Hutzler, M. (2020). Mini-review: The current role of lactic acid bacteria in beer spoilage. BrewSci. 73, 19–28. doi: 10.23763/BrSc19-28schneiderbanger
Shayevitz, A., Harrison, K., and Curtin, C. D. (2020). Barrel-induced variation in the microbiome and mycobiome of aged sour ale and imperial porter beer. J. Am. Soc. Brew. Chem. 79, 33–40. doi: 10.1080/03610470.2020.1795607
Sheih, K. K., Donnely, B. J., and Scallet, B. L. (1979). Reactions of oligosaccharides. IV. Fermentability by yeasts. Cereal Chem 50, 169–175.
Shetty, P. H., and Jespersen, L. (2006). Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Technol. 17, 48–55. doi: 10.1016/j.tifs.2005.10.004
Silva, L. C., Schmidt, G. B., Alves, L. G. O., Oliveira, V. S., Laureano-Melo, R., Stutz, E., et al. (2020). Use of probiotic strains to produce beers by axenic or semi-separated co-culture system. Food Bioprod. Process. 124, 408–418. doi: 10.1016/j.fbp.2020.10.001
Snauwaert, I., Roels, S. P., Van Nieuwerburg, F., Van Landschoot, A., De Vuyst, L., and Vandamme, P. (2016). Microbial diversity and metabolite composition of Belgian red-brown acidic ales. Int. J. Food Microbiol. 221, 1–11. doi: 10.1016/j.ijfoodmicro.2015.12.009
Spitaels, F., Li, L., Wieme, A., Balzarini, T., Cleenwerck, I., Van Landschoot, A., et al. (2014c). Acetobacter lambici sp. nov., isolated from fermenting lambic beer. Int. J. Syst. Evol. Microbiol. 64, 1083–1089. doi: 10.1099/ijs.0.057315-0
Spitaels, F., Van Kerrebroeck, S., Wieme, A. D., Snauwaert, I., Aerts, M., Van Landschoot, A., et al. (2015a). Microbiota and metabolites of aged bottled gueuze beers converge to the same composition. Food Microbiol. 47, 1–11. doi: 10.1016/j.fm.2014.10.004
Spitaels, F., Wieme, A. D., Balzarini, T., Cleenwerck, I., Van Landschoot, A., De Vuyst, L., et al. (2014b). Gluconobacter cerevisiae sp. nov., isolated from the brewery environment. Int. J. Syst. Evol. Microbiol. 64, 1134–1141. doi: 10.1099/ijs.0.059311-0
Spitaels, F., Wieme, A. D., Janssens, M., Aerts, M., Daniel, H.-M., Van Landschoot, A., et al. (2014a). The microbial diversity of traditional spontaneously fermented lambic beer. PLoS One 9:e95384. doi: 10.1371/journal.pone.0095384
Spitaels, F., Wieme, A. D., Janssens, M., Aerts, M., Van Landschoot, A., De Vuyst, L., et al. (2015b). The microbial diversity of an industrially produced lambic beer shares members of a traditionally produced one and reveals a core microbiota for lambic beer fermentation. Food Microbiol. 49, 23–32. doi: 10.1016/j.fm.2015.01.008
Spitaels, F., Wieme, A. D., Snauwaert, I., De Vuyst, L., and Vandamme, P. (2017). “Microbial ecology of traditional beer fermentations,” in Brewing Microbiology: Current Research, Omics and Microbial Ecology. eds. N. Bokulich and C. Bamforth (Poole: Caister Academic Press), 179–196.
Sterckx, F. L., Saison, D., and Delvaux, F. R. (2012). Wood aging of beer. Part II: influence of wood aging parameters on monophenol concentrations. J. Am. Soc. Brew. Chem. 70, 62–69. doi: 10.1094/ASBCJ-2011-1201-02
Suiker, I. M., and Wösten, H. A. B. (2021). Spoilage yeasts in beer and beer products. Curr. Opin. Food Sci. 44:100815. doi: 10.1016/j.cofs.2022.100815
Tan, Z., Hou, C., Jiang, X., Li, Y., Song, Y., and Dong, X. (2021). “The Brewing process of the Schisandra sour beer,” in 3rd World Congress on Chemistry, Biotechnology and Medicine (WCCBM 2021), (Cambridge: Francis Academic Press); July 2-4, 2021; 52–58.
Tonsmeire, M. (2014). American Sour Beers: Innovative Techniques For Mixed Fermentations, Colorado: Brewers Publications.
Twede, D. (2005). The cask age: the technology and history of wooden barrels. Pack. Technol. Sci. 18, 253–264. doi: 10.1002/pts.696
Tyakht, A., Kopeliovich, A., Klimenko, N., Efimova, D., Dovidchenko, N., Odintsova, V., et al. (2021). Characteristics of bacterial and yeast microbiomes in spontaneous and mixed-fermentation beer and cider. Food Microbiol. 94:103658. doi: 10.1016/j.fm.2020.103658
Van Oevelen, D., Delescaille, F., and Verachtert, H. (1976). Synthesis of aroma components during spontaneous fermentation of lambic and gueuze. J. Inst. Brew. 82, 322–326. doi: 10.1002/j.2050-0416.1975.tb06953.x
Van Oevelen, D., Spaepen, M., Timmermans, P., and Verachtert, H. (1977). Microbiological aspects of spontaneous wort fermentation in the production of lambic and gueuze. J. Inst. Brew. 83, 356–360. doi: 10.1002/j.2050-0416.1977.tb03825.x
Van Vuuren, H. J. J. (1999). “Gram negative spoilage bacteria,” in Brewing Microbiology. eds. F. Priest and I. Campbell (Gaithersburg: Aspen Publishers Inc.), 163–191.
Van Vuuren, H. J. J., Loos, M. A., Louw, H. A., and Meisel, R. (1979). Distribution of bacterial contaminants in a south African lager brewery. J. Appl. Bacteriol. 47, 421–424. doi: 10.1111/j.1365-2672.1979.tb01202.x
Van Vuuren, H. J. J., and Priest, F. G. (2003). “Gram-negative brewery bacteria,” in Brewing Microbiology. eds. F. G. Priest and I. Campbell (New York: Kluwer Academic/Plenum Publishers), 219–245.
Verachtert, H., and Derdelinckx, G. (2014). Belgian acidic beers daily reminiscences of the past. Cerevisia 38, 121–128. doi: 10.1016/j.cervis.2014.04.002
Verachtert, H., and Iserentant, D. (1995). Properties of Belgian acidic beers and their microflora. I. The production of gueuze and related refreshing acid beers. Cerevisia 20, 37–41.
Verstrepen, K. J., Derdelinckx, G., Dufour, J.-P., Winderickx, J., Thevelein, J. M., Pretorius, I. S., et al. (2003). Flavor-active esters: adding fruitiness to beer. J. Biosci. Bioeng. 96, 110–118. doi: 10.1016/S1389-1723(03)90112-5
Vicente, J., Baran, Y., Navascués, E., Santos, A., Caldéron, F., Marquina, D., et al. (2022). Biological management of acidity in wine industry: a review. Int. J. Food Microbiol. 375:109726. doi: 10.1016/j.ijfoodmicro.2022.109726
Vicente, J., Navascués, E., Calderón, F., Santos, A., Marquina, D., and Benito, S. (2021). An integrative view of the role of Lachancea thermotolerans in wine technology. Foods 10:2878. doi: 10.3390/foods10112878
Villareal-Soto, S. A., Beaufort, S., Bouajila, J., Souchard, J.-P., and Taillandier, P. (2018). Understanding kombucha tea fermentation: a review. J. Food Sci. 83, 580–588. doi: 10.1111/1750-3841.14068
Vriesekoop, F., Krahl, M., Hucker, B., and Menz, G. (2012). 125th anniversary review: Bacteria in brewing: the good, the bad and the ugly. J. Inst. Brew. 118, 335–345. doi: 10.1002/jib.49
Witrick, K. T., Duncan, S. E., Hurley, K. E., and O’Keefe, S. F. (2017). Acid and volatiles of commercially available lambic beers. Beverages 3:51. doi: 10.3390/beverages3040051
Xiao, Z., and Lu, J. R. (2014). Generation of acetoin and its derivatives in foods. J. Agric. Food Chem. 62, 6487–6497. doi: 10.1021/jf5013902
Yakushi, T., and Matsushita, K. (2010). Alcohol dehydrogenase of acetic acid bacteria: structure, mode of action, and applications in biotechnology. Appl. Microbiol. Biotechnol. 86, 1257–1265. doi: 10.1007/s00253-010-2529-z
Yang, H., Yu, Y., Fu, C., and Chen, F. (2019). Bacterial acid resistance toward organic weak acid revealed by RNA-seq transcriptomic analysis in Acetobacter pasteurianus. Front. Microbiol. 10:16–16. doi: 10.3389/fmicb.2019.01616
Zapata, P. J., Martínez-Esplá, A., Gironés-Vilaplana, A., Santos-Lax, D., Noguera-Artiaga, L., and Carbonell-Barrachina, Á. A. (2019). Phenolic, volatile, and sensory profiles of beer enriched by macerating quince fruits. Food Sci. Technol. 103, 139–146. doi: 10.1016/j.lwt.2019.01.002
Keywords: sour beer, beer classification, lambic beer, Acetobacter, Brettanomyces, acetic acid bacteria
Citation: Bouchez A and De Vuyst L (2022) Acetic Acid Bacteria in Sour Beer Production: Friend or Foe? Front. Microbiol. 13:957167. doi: 10.3389/fmicb.2022.957167
Received: 30 May 2022; Accepted: 15 June 2022;
Published: 04 August 2022.
Edited by:
Isidoro García-García, University of Cordoba, SpainReviewed by:
Shao Quan Liu, National University of Singapore, SingaporeCopyright © 2022 Bouchez and De Vuyst. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luc De Vuyst, bHVjLmRlLnZ1eXN0QHZ1Yi5iZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.