- 1Ministry of Education Key Laboratory of Molecular Microbiology and Technology, Department of Microbiology, College of Life Sciences, Nankai University, Tianjin, China
- 2Institute of Cultural Relics and Archaeology, Jinan, China
- 3Department of Cultural Relics and Museums, College of History and Culture, Qufu Normal University, Qufu, China
- 4Institute of Cultural Heritage and History of Science and Technology, University of Science and Technology Beijing, Beijing, China
In April 2020, 232 tombs of the Western Han Dynasty were found in Sundayuan, Heze City. In total, 141 pottery figurines of significant historical, cultural, and artistic value were unearthed from the tombs. Some of the figurines are currently being stored in warehouses, and the surface of some of the figurines show fungal deterioration. To thoroughly analyze the fungal deterioration on the surface of the pottery figurines and find appropriate control methods, we used high-through sequencing, scanning electron microscopy observation, pure cultures of culturable fungi, and optical microscopy observation and molecular identification of culturable fungi. We conducted fungistatic and simulation experiments in the laboratory to find appropriate control methods. We found that the fungi on the surface of the figurines were mainly of the phylum Ascomycota, and a few fungi were of the phyla Basidiomycota and Mucoromycota. We isolated seven culturable fungal strains and observed their colony morphology. The seven fungal strains were Lecanicillium aphanocladii, Penicillium aurantiogriseum, Clonostachys rosea, Mortierella sp., Mortierella alpina, Aspergillus flavus, and Cladosporium halotolerans. Through the fungistatic and simulation experiments conducted in the laboratory, we found that 50 mg/ml cinnamaldehyde and 0.5% K100 (2-methyl-4-isothiazolin-3-one) have a good fungistatic effect. They can not only inhibit the growth of fungi on medium, but also inhibit the growth of fungi on the surface of pottery figurines. This study has good reference significance for the analysis and control of fungal deterioration of unearthed pottery figurines.
Introduction
An increasing number of studies have been focusing on the biological weathering and degradation of cultural relics, including monuments, murals, art exhibits, historical manuscripts, archaeological sites, and tombs. These studies show that cultural relics can provide ideal habitats for microbial colonization and growth (Pruvost et al., 2011; Sterflinger and Pinzari, 2012; Sterflinger and Piñar, 2013; Piñar et al., 2015). Fungi are ubiquitous colonists, and according to some researchers, they are the most important factor in the deterioration of cultural heritage materials in the outdoor environment (Masaphy et al., 2014; Liu et al., 2022). Over time, cultural relics are often contaminated and colonized by fungi (Milanesi et al., 2006; Pepe et al., 2010). Therefore, biological degradation caused by fungi has been considered an important aspect of cultural relics protection in the past 40 years (Sampo and Mosca, 1989). Many cultural relics contain organic substances that support fungal growth. It is well known that fungi can accelerate the degradation of cultural relics physically and chemically through mycelium growth and metabolites, respectively (Imperi et al., 2007; Sterflinger and Pinzari, 2012). Studies have reported that fungal hyphae can penetrate and expand cracks in rocks and building materials and produce a wide range of enzymes and other compounds, including pigments, leading to erosion, discoloration, and peeling of stones and murals (Sterflinger, 2010; Unković et al., 2016; Li et al., 2019).
From April 2020 to July 2021, the Shandong Institute of Cultural Relics and Archaeology and the local cultural relics department of Heze City explored and excavated the Sundayuan site. It was discovered that the whole site contains 232 ancient tombs. An analysis of the shape and objects of the tombs showed that these brick coffin tombs are most likely from between the middle and late periods of the Western Han Dynasty (from 202 B.C. to 8 A.D.). During the excavation, 141 exquisitely painted pottery figurines were found buried in brick coffin tombs. A significant number of the pottery figurines unearthed from the Sundayuan site are stored in warehouses. Owing to the high temperature and humidity of the storage environment, fungal deterioration has appeared on the surface of some of the pottery figurines.
The methods of controlling cultural relic diseases are mainly divided into the following categories: physical, chemical, and biological methods. Physical methods involve using physical technologies to inhibit the microorganisms causing cultural relic diseases. For example, ultraviolet rays can be used to damage microorganisms, destroy their nucleic acid function, and thus inactivate them to inhibit microorganisms on cultural relics (Scheerer et al., 2009). Titanium dioxide is a kind of nanoparticle that can produce peroxides and other components that can inhibit the microorganisms causing cultural relic diseases. This method has fewer side effects. Currently, this technology is being used in the protection of a few stone cultural relics (Russa et al., 2012; Coutinho et al., 2016). Although this method has been applied to the protection of cultural relics, further investigation is needed into whether it has the potential to cause harm to cultural relic workers. Certain mechanical methods are also included among physical methods. Mechanical methods refer to the method of manually removing organisms from the surface of cultural relics by directly using scalpels, tweezers, vacuum cleaners, and other tools. This method is simple to implement and has a significant inhibitory effect on the diseases of cultural relics caused by algae, lichens, and mosses. However, the disadvantage of using this method is that it cannot remove fungi that have invaded the interior of cultural relics and cannot completely inhibit the growth of organisms (Municchia et al., 2018). Moreover, since sharp tools are used, operating them carelessly may cause damage to cultural relics. Chemical methods mainly refer to the use of various liquid antibacterial agents or the use of gas for fumigation. Although many kinds of chemical bacteriostatic agents are available on the market, the types of bacteriostatic agents that can be applied to cultural relics protection are very few. Currently, antibacterial agents that can be used in cultural relics protection mainly include formaldehyde polymers, quaternary amines, isothiazolinone, etc. (Polo et al., 2010). In addition to these liquid bacteriostatic agents mentioned above, some gases, such as formaldehyde and ethylene oxide, are often used for fumigation in the process of cultural relics protection, but these gases are very toxic and cause harm to cultural relic workers. Biological methods mainly refer to the inhibition of microorganisms on artifacts using metabolic substances produced by organisms. In recent years, scientists have been paying attention to the antibacterial and insecticidal properties of natural substances. Scholars collected the essential oils of different plants such as anise, clove, and laurel and tested them for their inhibitory effects on various microorganisms related to the degradation of heritage artifacts, such as Aspergillus, Penicillium, and Fusarium as well as Bacillus, and found that the inhibitory effect was good and targeted, and that the constituents of these essential oils included terpenes, aromatic aldehydes, terpene aldehydes, and phenolic compounds (Borrego et al., 2012; Ashraf et al., 2014). Marjanlo reported the high inhibitory activity of cumin oil against Botrytis cinerea and attributed this effect to the cumin aldehyde component in the oil (Marjanlo et al., 2009). Besides plant essential oils, it has been found that some secondary metabolites of microorganisms are also potential natural bacteriostatic agents, which are also easily synthesized in the laboratory because of their simple chemical structure. These secondary metabolites mainly include aliphatic, cyclic aliphatic, aromatic, and terpene components (Gazzano et al., 2013). For example, Silva showed that lipopeptide secondary metabolites produced by Bacillus have antifungal activity (Silva et al., 2017). Overall, these natural products with bacteriostatic activity have great application prospects in artifacts protection, as they are naturally non-contaminated, cause little harm to the environment and human body, and thus can be used directly as biocides, as well as serve as a basic model for the development of natural biocides.
Pottery was not only a tool in daily life, but also an object of cultural identification for ancient communities. Technological choices and manufacturing techniques demonstrate the level of understanding and evolution of specific cultural and social backgrounds (Tite, 2008; Peña and McCallum, 2009; De Bonis et al., 2013; Dal Sasso et al., 2014; De Vito et al., 2014; Giannossa et al., 2014). The research and protection of underground historical relics is of great significance. Underground historical relics and their burial places (such as caves, necropolises, monuments, catacombs, tombs, and crypts) are valuable heritage of human civilization (Koh and Birney, 2019; Botticelli et al., 2020; Luo et al., 2021). To analyze and study the fungal deterioration on the surface of the pottery figurines and determine the appropriate control measures for them, we visited the warehouse the pottery figurines were stored in for on-site observation in July 2021 and took some samples for analysis. This study took the unearthed pottery figurines as the research object, comprehensively analyzed the fungal deterioration on the surface of the figurines using various biological technologies, determined the suitable fungistatic products among the fungistatic agents currently used in the field of cultural relics protection, and actively tried a few environmentally friendly biological metabolic products as fungistatic agents. This study is of great significance for the storage and protection of the pottery figurines unearthed in the Sundayuan site, and it has reference significance for the protection of pottery figurines unearthed in other areas as well.
Materials and methods
Study site and sampled artifacts
The Sundayuan site is located in the southwest of Weilou reservoir in Heze City, Shandong Province, about 200 meters southwest of the original Sundayuan village (115°18′0.51″ E, 35°17′41.58″ N; Supplementary Figure 1). The site has a north–south orientation and a shuttle shape. It is approximately 220 meters long from north to south and 80 meters wide from east to west, with a total area of about 12,000 square meters. Four unearthed pottery figurines were selected for this study. Their cultural relic numbers are TN61E65M47: 2, TN61E65M47: 3, TN61E65M47: 5, and TN61E65M169: 1 (Supplementary Figure 2).
SEM and optical microscope observation
Three pottery figurines (cultural relic numbers TN61E65M47: 3, TN61E65M47: 2, and TN61E65M47: 5) were selected. Their surface had six spots suspected to be microbial colonization (Figure 1). The samples obtained using double-sided carbon conductive adhesive were glued to the sample cups and sprayed with gold. The gold-plated samples were observed with SEM (FEI Quanta 200, United States), and images were obtained at a magnification of 1,000× to 2,500×. An optical microscope (Nikon E200, Japan) was used to observe the morphology of mycelia and spores of dominant fungi. The microbial morphology was recorded under 40 times microscope.
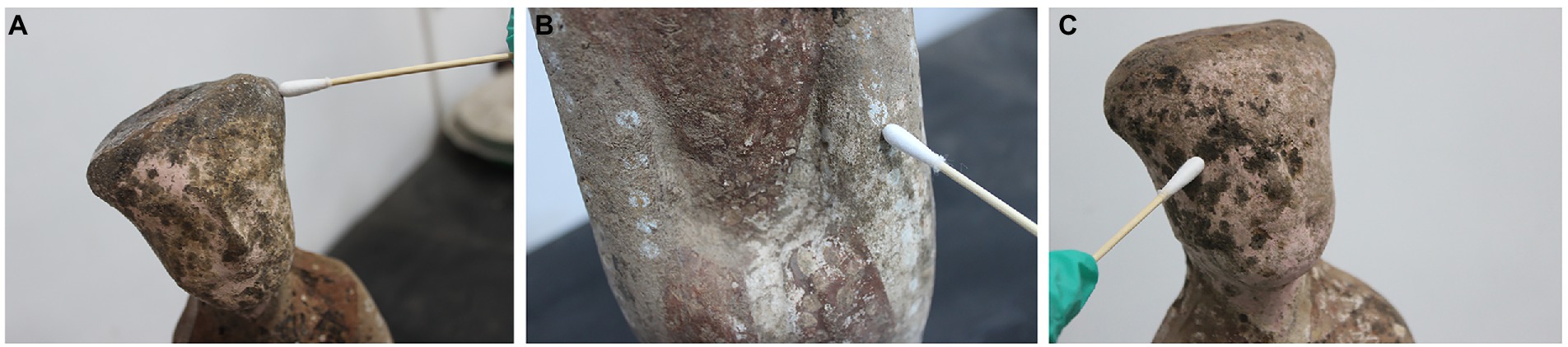
Figure 1. Spots on the surface of pottery figurines. (A) Ear of pottery figurine TN61E65M47:3. (B) Left arm of pottery figurine TN61E65M47:3. (C) Face of pottery figurine TN61E65M47: 2.
Total DNA extractions and high-throughput sequencing
Five pottery figurine samples (PF1–PF5) were selected for high-throughput sequencing. The five samples were taken from pottery figurines TN61E65M47: 5, TN61E65M47: 3, TN61E65M169: 1, and TN61E65M47: 2. DNeasy PowerSoil Kit (QIAGEN, Germany) was used to extract the genome DNA of all the samples. The total DNA was sent to Majorbio Genome Sequencing Company. The 18S rRNA gene region was sequenced using HiSeq platform. The primers used are SSU0817F’ (5′-TTAGCATGGAATAATRRAATAGG’-3′) and 1196R’ (5′-TCTGGACCTGGTGAGTTTC’-3′). The amplification regions are V5–V7 and the length of amplified fragment is 379 bp. The microbial community in the samples was analyzed using the sequencing results.
Alpha diversity analysis
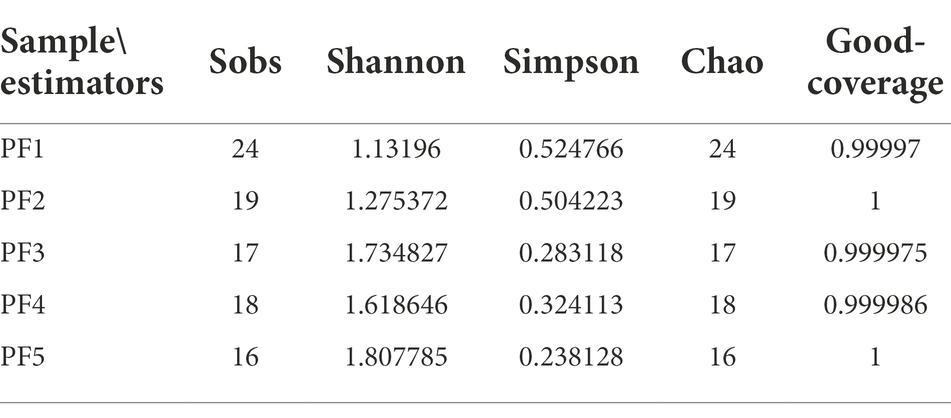
Alpha diversity was applied in analyzing the complexity of species diversity for a sample through five indices, including Sobs, Chao, Shannon, Simpson, and Good’s coverage. Sobs and Chao can reflect community richness, Shannon and Simpson can reflect community diversity, and Good’s coverage can reflect community coverage. All these indices in our samples were calculated using QIIME (Version 1.7.0) and displayed with R software (Version 2.15.3).
Isolation and identification of culturable fungi
PDA medium was used to culture and isolate fungi. The PDA mediums were cultured at 28°C for 3–7 days. Fungi with significantly different morphology in each medium were isolated and purified. The T5 Direct PCR Mix kit (TSINGKE, China) was used for DNA extraction and PCR amplification of the dominant fungi according to the manufacturer’s protocol. The ITS1-5.8srRNA-ITS2 gene of the fungus was amplified with ITS1/ITS4 primers. The PCR system and conditions are detailed in the manual of the kit. The purified PCR products were sent to GENEWIZ (Beijing, China) for sequencing and then the sequence homology of the amplified fragments was analyzed by NCBI.
Fungistatic experiment
The fungistatic efficacy of six biocides against the fungi isolated from the surface of the pottery figurines was examined. The biocides selected in our experiment can be divided into two categories: the biocides currently used for the protection of the cultural relics (K100, boric acid/borax solution, and miconazole nitrate) and the metabolites of plants and microorganisms (cinnamaldehyde, Glucosinolate crude extracts, and 5,5-Dimethyl-1, 3-cyclohexanedione). The concentration of various biocides was determined according to the literature and some previous experimental results. The fungistatic effects of 0.5% K100, 5% boric acid/borax solution (1: 1), and 0.5% miconazole nitrate were tested with ultrapure water as negative control, and the fungistatic effects of 50 mg/ml cinnamaldehyde, 2.7 mg/ml Glucosinolate crude extracts, and 0.5 g/ml 5,5-Dimethyl-1,3-cyclohexanedione were tested with methanol as negative control. The details of the biocides are provided in Supplementary Table 1. The experimental strains were fungi isolated from the surface of the pottery figurines. First, the strain was cultured by scribing on the PDA medium with a cotton swab, and the filter papers (each with a diameter of 7 mm) were placed on the medium at four places. One of the filter papers had the negative control, while the remaining three were impregnated with the different biocides (15 μl each). They were then placed in a 28°C incubator for culture. The fungistatic results were observed on the third day of culture.
Laboratory simulation experiment
The fungus used in the experiment is Lecanicillium aphanocladii (NK-PF1). We selected 12 unearthed fragments of pottery figurines (Supplementary Table 2) as experimental materials, and added 1.5 ml well-distributed fungal suspension to them. Each fragment was then placed in a plastic box (27.5 cm × 20 cm × 16.5 cm) covered with gauze. The original condition of the pottery figurine fragments can be seen in Supplementary Figure 3. Subsequently, they were randomly divided into four groups. Each group was gently sprayed with 5 ml solvent of cinnamaldehyde (mixture of 1% dimethyl sulfate and 0.1% Tween-80), 50 mg/ml cinnamaldehyde, ultrapure water, and 0.5% K100 every day. They were cultured at room temperature (Average temperature 23°C) for 6 days. The growth of microorganisms on the pottery figurines was observed.
Results
SEM results of surface samples of pottery figurines
SEM observation showed a large number of microorganisms on the ear and face of TN61E65M47:3. The mycelium and spores of fungi could be clearly seen at a magnification of 2000× (Figures 2A,B). There were no obvious microorganisms in the other four places (Figures 2C–F); these spots may have been caused by the peeling of the pigments on the surface of the pottery figurines or some chemical reactions.

Figure 2. SEM results of spots. (A) Ear of pottery figurine TN61E65M47:3. (B) Face of pottery figurine TN61E65M47:3. (C) Left arm of pottery figurine TN61E65M47:3. (D) Face of pottery figurine TN61E65M47: 2. (E) Leg of pottery figurine TN61E65M47: 2. (F) Leg of pottery figurine TN61E65M47: 5.
Fungal community analysis by high-throughput sequencing
It was found that most of the fungi on the surface of the pottery figurines were of the phylum Ascomycota, and a few were of the phyla Basidiomycota and Mucoromycota. The average proportion of Ascomycota reached 92.86%, while Mucoromyceta only existed in PF1, PF3, and PF4 (Figure 3A). In these 5 samples, Thysanophora, Hypocreales, Fusarium, and Orbiliaceae were present at higher levels and Puccinia, Pichia, Knufia, Sordariomycetes, and Colpodea were present at lower levels (Figure 3B). We simultaneously calculated the Alpha diversity index of these five samples. It can be seen that PF5 has the highest community richness and diversity, while PF1 has the lowest community richness and diversity (Table 1).
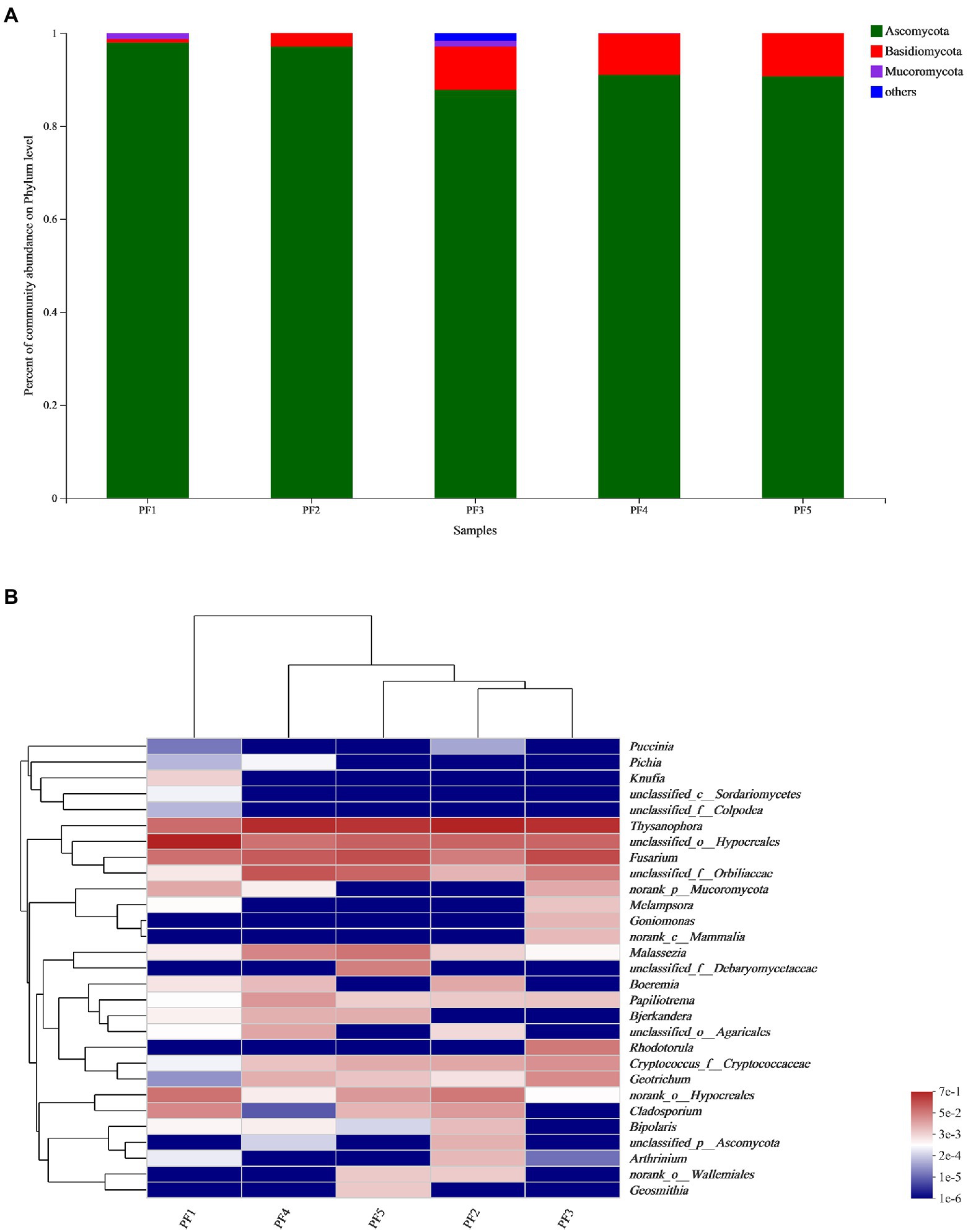
Figure 3. Fungal community analysis. (A) Histogram of species relative abundance of the pottery figurines at the phylum level; (B) Heatmap at the genus level.
Molecular identification and observation of purified fungi on the surface of the pottery figurines
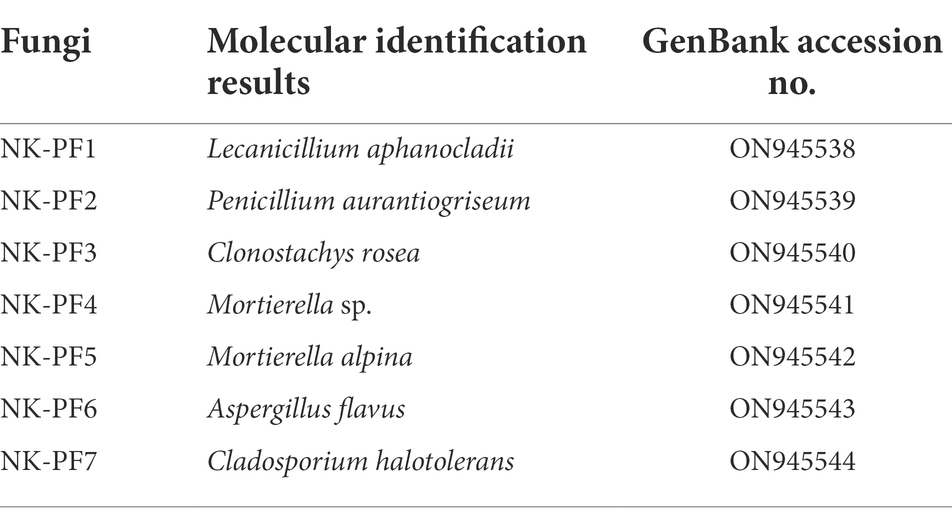
We cultured seven fungal strains on PDA medium, namely, L. aphanocladii, Penicillium aurantiogriseum, Clonostachys rosea, Mortierella sp., Mortierella alpina, Aspergillus flavus, and Cladosporium halotolerans (Table 2). We observed their single colony state and observed their hyphae and spores using chamber culture and microscopy (Figures 4, 5).
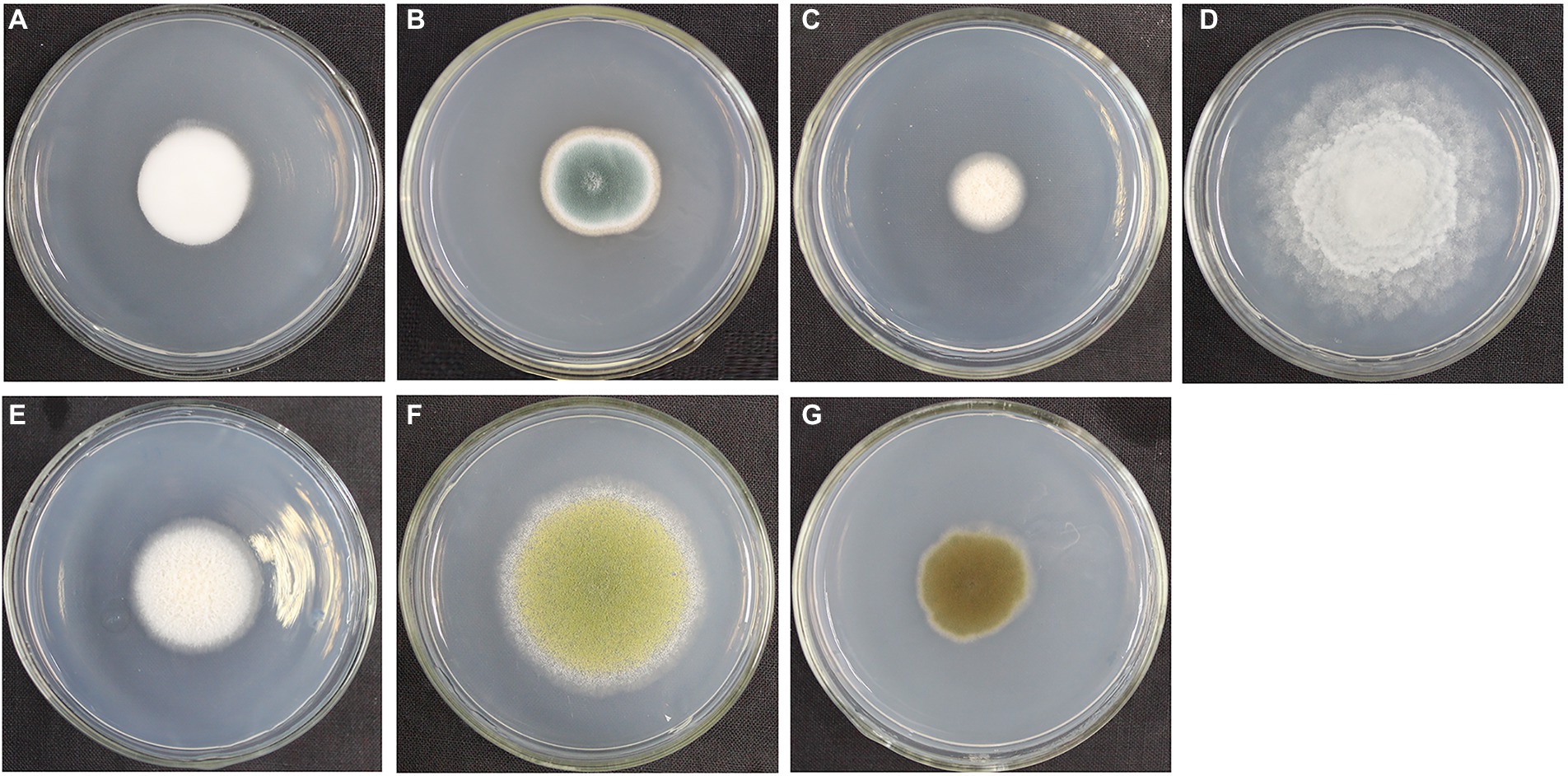
Figure 4. Single colony morphology of fungi isolated from the surface of pottery figurines. The fungi were inoculated onto the PDA medium and incubated at 28°C for 3 days. (A) Lecanicillium aphanocladii (NK-PF1). (B) Penicillium aurantiogriseum (NK-PF2). (C) Clonostachys rosea (NK-PF3). (D) Mortierella sp. (NK-I). (E) Mortierella alpina (NK-PF5). (F) Aspergillus flavus (NK-PF6). (G) Cladosporium halotolerans (NK-PF7).
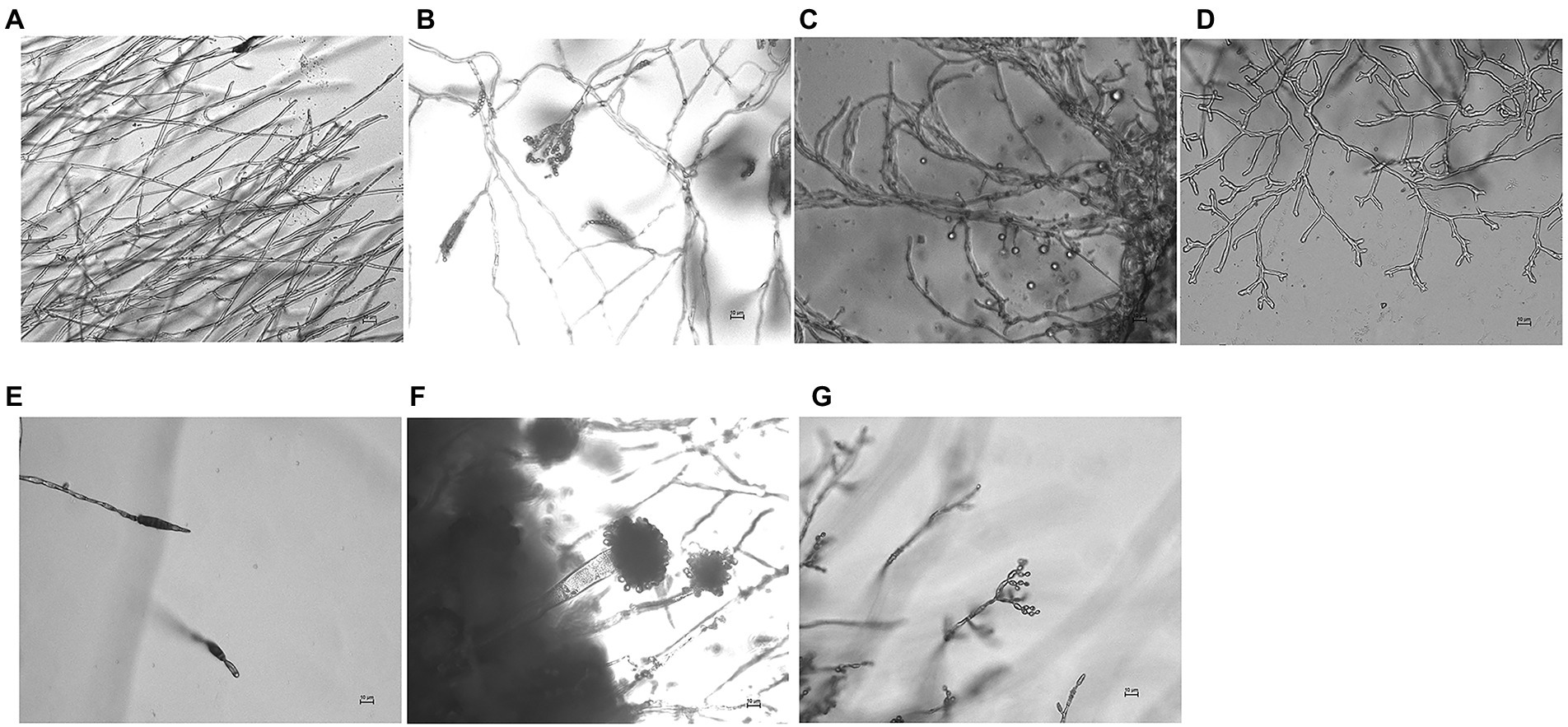
Figure 5. Micromorphology of fungi isolated from the surface of pottery figurines. The fungi were inoculated onto the PDA medium and incubated at 28°C for 3 days. The scale is 10 μm. (A) L. aphanocladii (NK-PF1). (B) P. aurantiogriseum (NK-PF2). (C) C. rosea (NK-PF3). (D) Mortierella sp. (IPF4). (E) M. alpina (NK-PF5). (F) A. flavus (NK-PF6). (G) C. halotolerans (NK-PF7).
Susceptibility of fungi to biocides
As shown in Figure 6, 0.5% miconazole nitrate showed no inhibitory effect on these 7 fungal strains and 5% boric acid/borax solution (1:1) showed only a slight inhibitory effect on L. aphanocladii (NK-PF1), P. aurantiogriseum (NK-PF2), and C. rosea (NK-PF3). 0.5% K100 showed a good inhibitory effect on all the fungi. As shown in Figure 7, 50 mg/ml cinnamaldehyde and 2.7 mg/ml glucosinolate crude extracts have certain inhibitory effects on these 7 fungal strains, and 50 mg/ml cinnamaldehyde has a better inhibitory effect than 2.7 mg/ml glucosinolate crude extracts. 0.5 g/ml 5,5-Dimethyl-1,3-cyclohexanedione also had a certain inhibitory effect on Mortierella sp. (NK-PF4).
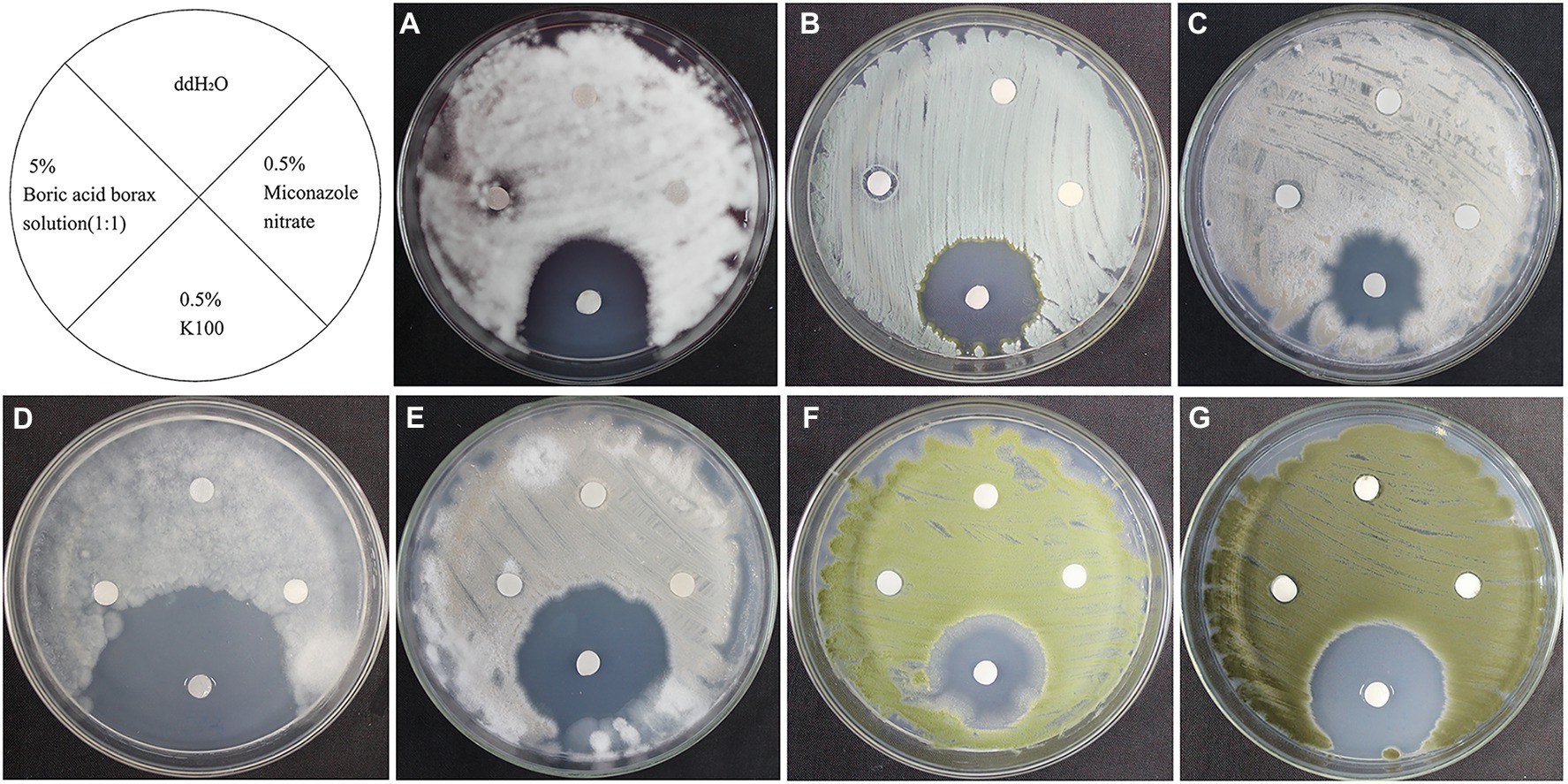
Figure 6. The fungistatic effects of 0.5% K100, 5% boric acid/borax solution (1:1), and 0.5% miconazole nitrate. The fungi were inoculated onto the PDA medium and incubated at 28°C for 3 days. (A) L. aphanocladii (NK-PF1). (B) P. aurantiogriseum (NK-PF2). (C) C. rosea (NK-PF3). (D) Mortierella sp. (NK-PF4). (E) M. alpina (NK-PF5). (F) A. flavus (NK-PF6). (G) C. halotolerans (NK-PF7).
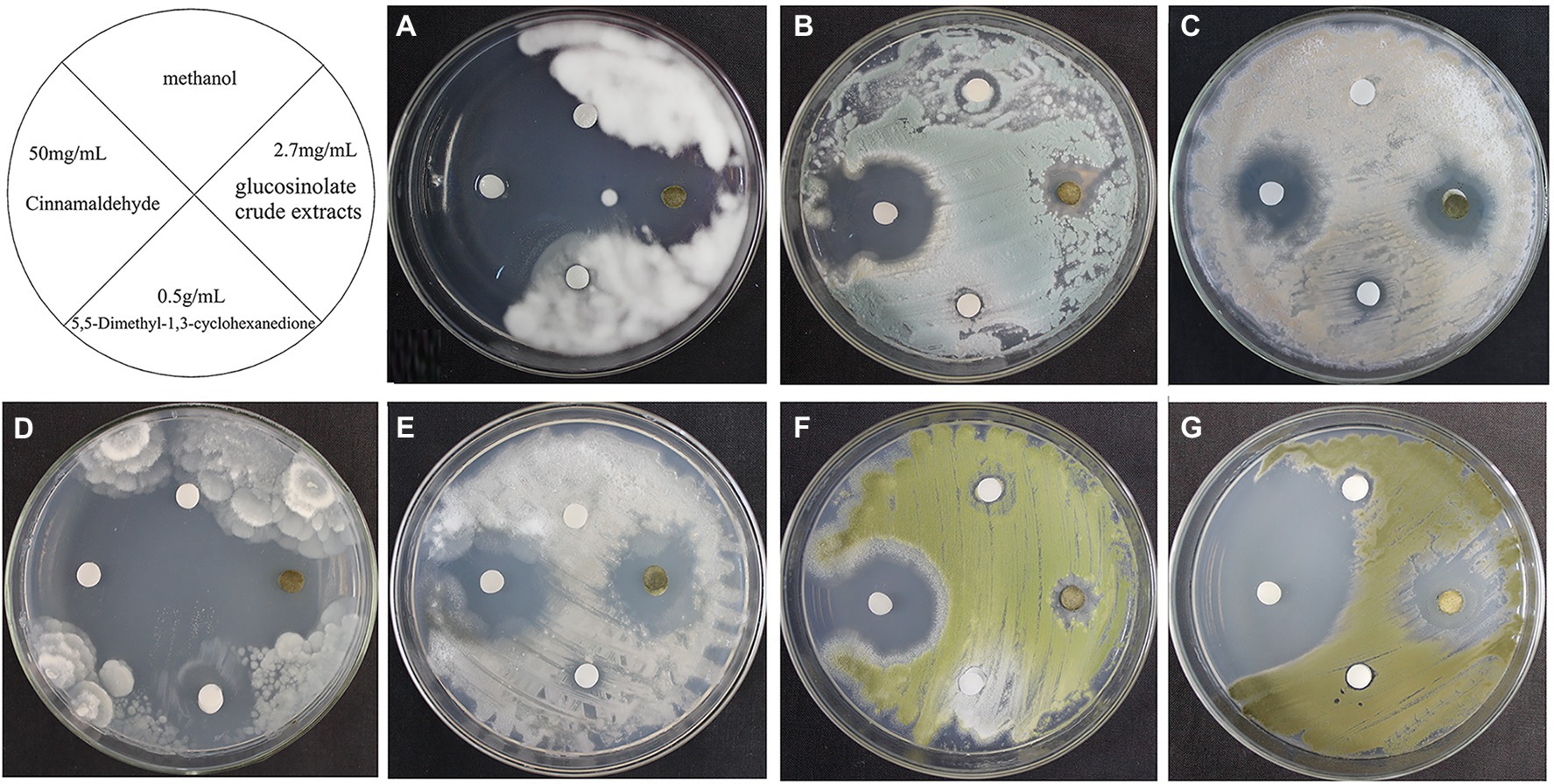
Figure 7. The fungistatic effects of 50 mg/ml Cinnamaldehyde, 2.7 mg/ml Glucosinolate crude extracts, and 0.5 g/ml 5,5-Dimethyl-1,3-cyclohexanedione. The fungi were inoculated onto the PDA medium and incubated at 28°C for 3 days. (A) L. aphanocladii (NK-PF1). (B) P. aurantiogriseum (NK-PF2). (C) C. rosea (NK-PF3). (D) Mortierella sp. (NK-PF4). (E) M. alpina (NK-PF5). (F) A. flavus (NK-PF6). (G) C. halotolerans (NK-PF7).
Simulation experiment results
Through the fungistatic experiment, we found that 0.5% K100 and 50 mg/ml cinnamaldehyde had better fungistatic effect. Therefore, we conducted the simulation experiment on controlling the growth of fungus on the surface of the pottery figurines in the laboratory. As shown in Figure 8, the fungi inoculated on the surface of pottery figurine fragments sprayed with ddH2O and a solvent of cinnamaldehyde could colonize and grow in large quantities, while the fungi on the surface of pottery figurine fragments sprayed with 0.5% K100 and 50 mg/ml cinnamaldehyde did not increase, indicating that spraying 0.5% K100 or 50 mg/ml cinnamaldehyde can effectively inhibit the growth and reproduction of disease fungi on the surface of pottery figurines.

Figure 8. Pictures of pottery figurine fragments on day 1 (−1d) and day 6 (−6d) of the simulation experiment. Each group has three samples, and the same amount of NK-PF1 was added to each pottery figurine fragment. (A) Sprayed with 5 ml solvent of cinnamaldehyde (mixture of 1% dimethyl sulfate and 0.1% Tween-80) every day. (B) Sprayed with 5 ml 50 mg/ml cinnamaldehyde every day. (C) Sprayed with 5 ml ddH2O every day. (D) Sprayed with 5 ml 0.5% K100 every day.
Discussion
In this study, fungal deteriorations on the surface of pottery figurines were thoroughly analyzed and studied. Using a scanning electron microscope, we found that not all the spots on the surface of pottery figurines were caused by fungal colonization. Some spots may have been formed by the chipping of the surface materials of the pottery figurines. As we can see in Figure 1, the surface of the pottery figurines were not smooth, the shedding of the material was visible, and the locations where the black spots were exactly concave were confirmed. These phenomena, together with the SEM observations, confirm our conjecture. Similar studies have been previously reported in the literature. After thousands of years of burial, the unearthed cultural relics have adapted to their burial environment. Even after centuries of burial, many historical relics are still well preserved (Cacace et al., 2003; Saiz-Jimenez et al., 2011). It is generally believed that the outstanding preservation of unearthed historical relics is due to the abnormal stability of internal climate parameters in the underground environment (Elez et al., 2013; Bourges et al., 2014). The thermal and humidity conditions in the burial environment are considered the basic factors for the integrity of historical relics. If cultural relics are excavated suddenly, violent fluctuations in temperature and relative humidity lead to irreversible deformation, such as curls and cracks. At the beginning of excavation, the Terra Cotta Warriors were intact and painted in vivid colors. Unfortunately, the raw lacquer underneath suffered severe warping, curling, and peeling, and the gray layer was exposed just a few hours after excavation (Zhang et al., 2007). Studies have also shown that some spots are evidence of microorganisms colonizing the surface. When studying the brown spots on the walls of the tomb of King Tutankhamun, Vasanthakumar et al. (2013) found through a scanning electron microscope that the primitive organisms that caused these spots no longer existed. Malic acid was detected only in the brown spots, not in the gypsum, indicating that microorganisms were involved in the formation of the spots. Many microorganisms, including fungi such as Aspergillus spp. (Peleg et al., 1988) and bacteria such as Arthrobacter and Pseudomonas (Vyas and Gulati, 2009) can produce malic acid in the environment.
The fungi on the surface of the pottery figurines may have come from the environment in which they were stored. The culturable fungi that colonize on the surface of cultural relics are closely related to their environment, and these fungi are potentially harmful to cultural relics (Samson et al., 2014). We isolated Penicillium, Aspergillus, and Cladosporium from the pottery figurines, and they are very common fungi, which are often isolated from the surface of cultural relics. For example, the predominant fungi in the air of Tianjin Museum are Penicillium and Cladosporium (Zhang et al., 2019a). Cladosporium was found in the air of an art museum in Tokyo (Abe, 2010). Cladosporium and Penicillium were also found in the ambient air test conducted for the storage of the Nanhai No. 1 shipwreck in 2019 and 2020 (Han et al., 2021), in the ambient air test for the Mogao Grottoes in Dunhuang from 2008 to 2009 (Wang et al., 2010a,b), and in the ambient air test for the canoe of the Tang Dynasty preserved in the National Marine Museum of China in 2019 (Zhang et al., 2019a). Aspergillus is one of the most abundant and widely distributed organisms on earth. It can produce a large number of conidia to spread widely (Pangallo et al., 2012). In addition, Aspergillus has the ability to produce a variety of enzymes and can colonize on any substrate. Penicillium and Aspergillus caused black spots in two 1700-year-old tombs (Ma et al., 2020). Mortierella has strong acid production capacity, and the organic acids produced by metabolism also have a deteriorative impact on cultural relics (Zhang et al., 2019b; IvančićŠantek et al., 2021). The hypha of L. aphanocladii is white. When growing on PDA medium, a red pigment is produced, which is a threat to cultural relics. This phenomenon has been previously reported (Dong et al., 2016). Therefore, controlling the storage environment is very important for the prevention and control of fungal deterioration. Controlling temperature and relative humidity is a key factor in cultural relics protection (Brimblecombe, 2013; Smedemark et al., 2020). In an indoor environment, fungi can grow with relatively low water content. Water accumulation promotes the growth of microorganisms on cultural relics, and humidity is positively correlated with fungal richness (Li et al., 2020; Trovão et al., 2020). When the temperature and humidity conditions are appropriate, fungal spores have the ability of long-term survival and growth (Vukojević and Grbić, 2010; Kakakhel et al., 2019). When we took samples, the temperature of the warehouse where the pottery figurines were stored was 26°C and the relative humidity was 70%. Such temperature and humidity conditions are conducive to the growth of fungi. Moreover, studies have reported that unsuitable temperature and relative humidity lead to mechanical, biological, and chemical degradation of cultural relics (Pinzari et al., 2020; Sadlowska-Salega and Radon, 2020). Currently, heating, ventilation, and air conditioning systems are the main methods used to meet the requirements of heat and humidity (Ferdyn-Grygierek and Grygierek, 2019; Schito et al., 2020). However, even if the excavated cultural relics can be stored in an environment with relatively stable temperature and humidity, secondary damage is still inevitable. Therefore, it is of great significance to study the environment before the excavation of cultural relics. It can serve as an important basis for preventive protection to avoid and minimize decay (Lucchi, 2018). We want to inhibit the fungi that grow on the surface of the pottery figurines using some targeted biocides. K100 and cinnamaldehyde were experimentally found to have good effects. The main component of fungistatic agent K100, which showed good fungistatic effect in this experiment, is a type of isothiazolinone. It can not only inhibit the growth of a variety of fungi on the culture medium, but also effectively control the growth and reproduction of the fungus L. aphanocladiiz on the surface of pottery figurines. Currently, K100 is also used as an effective fungistatic agent in the protection of the Nanhai No. 1 shipwreck (Han et al., 2021). However, as mentioned before, K100 may also have a slight impact on operators and the environment. Therefore, we are also looking for safer and environmentally friendly fungistatic substances. We also selected cinnamaldehyde, a plant essential oil, and crude glucosinolate extract from plants as fungistatic agents. Cinnamaldehyde is an aldehyde organic compound, which is a yellow, sticky liquid and abundantly found in plants such as cinnamon. It has a bacteriostatic effect and it can be added to aquatic products, meat products, etc., as a natural food preservative, so cinnamaldehyde is less harmful to humans and is promising for the protection of artifacts. In this experiment, fungistatic agents with a good inhibitory effect were selected. In the future, the abovementioned fungistatic agents can be considered for use on the pottery figurines to inhibit the fungal deteriorations on their surface.
Conclusion
In this paper, the fungal deteriorations on the surface of pottery figurines unearthed from the Sundayuan site and their control were studied comprehensively. The results show that not all the spots on the surface of the pottery figurines were caused by fungal colonization. Moreover, the fungi that colonize on the surface of cultural relics are closely related to their environment. Two fungistatic agents, namely, K100 and cinnamaldehyde, can effectively inhibit the growth and reproduction of microorganisms on the surface of the pottery figurines.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA827628.
Author contributions
JP conceived and designed the study, reviewed and edited the manuscript. YW, CW, and XY performed the experiments. KM and PG provided assistance during the experiments. QS and SJ provided samples. YW analyzed the data and wrote the manuscript. All authors contributed to the article and approved the final version of this manuscript.
Funding
This work was continuously supported by the Natural Science Foundation of Tianjin (19JCZDJC33700).
Acknowledgments
We gratefully acknowledge the assistance of Xingzhong Liu, Dongsheng Wei, and Haikun Ma of Nankai University, Ling Wang of the Protection Research Center of the Mausoleum of the Dingtao King, and Chenghao Li of the Shandong Institute of Cultural Relics and Archaeology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.956774/full#supplementary-material
References
Abe, K. (2010). Assessment of the environmental conditions in a museum storehouse by use of a fungal index. Int. Biodeterior. Biodegradation 64, 32–40. doi: 10.1016/j.ibiod.2009.10.004
Ashraf, M. A., Ullah, S., Ahmad, I., Qureshi, A. K., Balkhair, K. S., and Abdur Rehman, M. (2014). Green biocides, a promising technology: current and future applications to industry and industrial processes. J. Sci. Food Agric. 94, 388–403. doi: 10.1002/jsfa.6371
Borrego, S., Valdes, O., Vivar, I., Lavin, P., Guiamet, P., Battistoni, P., et al. (2012). Essential oils of plants as biocides against microorganisms isolated from Cuban and argentine documentary heritage. ISRN Microbiol. 2012:826786, 1–7. doi: 10.5402/2012/826786
Botticelli, M., Mignardi, S., De Vito, C., Liao, Y., Montanari, D., Shakarna, M., et al. (2020). Variability in pottery production at Khalet al-Jam’anecropolis, Bethlehem (West Bank): from the early-middle bronze to the iron age. Ceram. Int. 46, 16405–16415. doi: 10.1016/j.ceramint.2020.03.200
Bourges, F., Genthon, P., Genty, D., Lorblanchet, M., Mauduit, E., and D’Hulst, D. (2014). Conservation of prehistoric caves and stability of their inner climate: lessons from Chauvet and other French caves. Sci. Total Environ. 493, 79–91. doi: 10.1016/j.scitotenv.2014.05.137
Brimblecombe, P. (2013). Temporal humidity variations in the heritage climate of South East England. Heritage Sci. 1:3. doi: 10.1186/2050-7445-1-3
Cacace, C., Caneva, G., Gallo, F., Georgiadis, T., Maggi, O., and Valenti, P. (2003). Cultural Heritage and Aerobiology. Dordrecht: Springer.
Coutinho, M., Miller, A., Martin-Sanchez, P., Mirão, J., Gomez-Bolea, A., Machado-Moreira, B., et al. (2016). A multiproxy approach to evaluate biocidal treatments on biodeteriorated majolica glazed tiles. Environ. Microbiol. 18, 4794–4816. doi: 10.1111/1462-2920.13380
Dal Sasso, G., Maritan, L., Salvatori, S., Mazzoli, C., and Artioli, G. (2014). Discriminating pottery production by image analysis: a case study of Mesolithic and Neolithic pottery from Al Khiday (Khartoum, Sudan). J. Archaeol. Sci. 46, 125–143. doi: 10.1016/j.jas.2014.03.004
De Bonis, A., Grifa, C., Cultrone, G., De Vita, P., Langella, A., and Morra, V. (2013). Raw materials for archaeological pottery from the Campania region of Italy: a petrophysical characterization. Geoarchaeology 28, 478–503. doi: 10.1002/gea.21450
De Vito, C., Medeghini, L., Mignardi, S., Orlandi, D., Nigro, L., Spagnoli, F., et al. (2014). Technological fingerprints of black-gloss ware from Motya (Western Sicily, Italy). Appl. Clay Sci. 88–89, 202–213. doi: 10.1016/j.clay.2013.12.026
Dong, X., Zeng, G., Chen, W., Han, Y., and Liang, Z. (2016). A new fungus strain (producing red pigment) of Lecanicillium aphanocladii. J. Mount. Agric. Biol. 35, 91–94. doi: 10.15958/j.cnki.sdnyswxb.2016.03.018
Elez, J., Cuezva, S., Fernandez-Cortes, A., Garcia-Anton, E., Benavente, D., Cañaveras, J. C., et al. (2013). A GIS-based methodology to quantitatively define an Adjacent Protected Area in a shallow karst cavity: the case of Altamira cave. J. Environ. Manag. 118, 122–134. doi: 10.1016/j.jenvman.2013.01.020
Ferdyn-Grygierek, J., and Grygierek, K. (2019). Proposed strategies for improving poor hygrothermal conditions in museum exhibition rooms and their impact on energy demand. Energies 12:620. doi: 10.3390/en12040620
Gazzano, C., Favero-Longo, S., Iacomussi, P., and Piervittori, R. (2013). Biocidal effect of lichen secondary metabolites against rock-dwelling microcolonial fungi, cyanobacteria and green algae. Int. Biodeterior. Biodegr. 84, 300–306. doi: 10.1016/j.ibiod.2012.05.033
Giannossa, L. C., Acquaviva, M., De Benedetto, G. E., Acquafredda, P., Laviano, R., and Mangone, A. (2014). Methodology of a combined approach: analytical techniques to identify the technology and raw materials used in thin-walled pottery from Herculaneum and Pompeii. Anal. Methods 6, 3490–3499. doi: 10.1039/C3AY42195C
Han, Y., Huang, X., Wang, Y., Du, J., Ma, K., Chen, Y., et al. (2021). Fungal community and biodeterioration analysis of hull wood and its storage environment of the Nanhai No. 1 shipwreck. Front. Microbiol. 11:609475. doi: 10.3389/fmicb.2020.609475
Imperi, F., Caneva, G., Cancellieri, L., Ricci, M. A., Sodo, A., and Visca, P. (2007). The bacterial aetiology of rosy discoloration of ancient wall paintings. Environ. Microbiol. 9, 2894–2902. doi: 10.1111/j.1462-2920.2007.01393.x
IvančićŠantek, M., Grubišić, M., Galić Perečinec, M., Beluhan, S., and Šantek, B. (2021). Lipid production by Mortierella isabellina from pretreated corn cobs and effect of lignocellulose derived inhibitors on growth and lipid synthesis. Process Biochem. 109, 46–58. doi: 10.1016/j.procbio.2021.06.021
Kakakhel, M., Wu, F., Gu, J., Feng, H., Shah, K., and Wang, W. (2019). Controlling biodeterioration of cultural heritage objects with biocides: a review. Int. Biodeterior. Biodegradation 143:104721. doi: 10.1016/j.ibiod.2019.104721
Koh, A., and Birney, K. (2019). Ancient organic residues as cultural and environmental proxies: the value of legacy objects. Sustainability 11:656. doi: 10.3390/su11030656
Li, T., Hu, Y., and Zhang, B. (2019). Evaluation of efficiency of six biocides against microorganisms commonly found on Feilaifeng Limestone, China. J. Cult. Herit. 43, 45–50. doi: 10.1016/j.culher.2019.11.006
Li, Y., Huang, Z., Petropoulos, E., Ma, Y., and Shen, Y. (2020). Humidity governs the wall-inhabiting fungal community composition in a 1600-year tomb of Emperor Yang. Sci. Rep. 10:8421. doi: 10.1038/s41598-020-65478-z
Liu, Z., Zhu, H., Wu, M., Li, Y., Cao, H., and Rong, R. (2022). Seasonal dynamics of airborne culturable fungi and its year-round diversity monitoring in Dahuting Han Dynasty Tomb of China. Sci. Total Environ. 838:155990. doi: 10.1016/j.scitotenv.2022.155990Get
Lucchi, E. (2018). Review of preventive conservation in museum buildings. J. Cult. Heritage 29, 180–193. doi: 10.1016/j.culher.2017.09.003
Luo, X., Dang, Y., Yu, C. W., and Gu, Z. (2021). The practice of local environment control for the funerary pits of Emperor Qin’s Mausoleum Site Museum. Indoor Built Environ. 30, 293–297. doi: 10.1177/1420326X20985620
Ma, W., Wu, F., Tian, T., He, D., Zhang, Q., Gu, J., et al. (2020). Fungal diversity and its contribution to the biodeterioration of mural paintings in two 1700-year-old tombs of China. Int. Biodeterior. Biodegrad. 152:104972. doi: 10.1016/j.ibiod.2020.104972
Marjanlo,, Mostofi, Y., Shoeibi, S., and Fattahi, M. (2009). Effect of cumin essential oil on postharvest decay and some quality factors of strawberry. J. Med. Plants 8, 25–43.
Masaphy, S., Lavi, I., Sultz, S., and Zabari, L. (2014). Laboratory study of fungal bioreceptivity of different fractions of composite flooring tiles showing efflorescence. Appl. Microbiol. Biotechnol. 98, 5251–5260. doi: 10.1007/s00253-014-5628-4
Milanesi, C., Baldi, F., Borin, S., Vignani, R., Ciampolini, F., Faleri, C., et al. (2006). Biodeterioration of a fresco by biofilm forming bacteria. Int. Biodeterior. Biodegrad. 57, 168–173. doi: 10.1016/j.ibiod.2006.02.005
Municchia, A., Bartoli, F., Taniguchi, Y., Giordani, P., and Caneva, G. (2018). Evaluation of the biodeterioration activity of lichens in the cave Church of Üzümlü (Cappadocia, Turkey). Int. Biodeterior. Biodegradation 127, 160–169. doi: 10.1016/j.ibiod.2017.11.023
Pangallo, D., Kraková, L., Chovanová, K., Simonovicová, A., De Leo, F., and Urzì, C. (2012). Analysis and comparison of the microflora isolated from fresco surface and from surrounding air environment through molecular and biodegradative assays. World J. Microbiol. Biotechnol. 28, 2015–2027. doi: 10.1007/s11274-012-1004-7
Peleg, Y., Stieglitz, B., and Goldberg, I. (1988). Malic acid accumulation by Aspergillus flavus. Appl. Microbiol. Biotechnol. 28, 69–75. doi: 10.1007/BF00250501
Peña, J. T., and McCallum, M. (2009). The production and distribution of pottery at Pompeii: a review of the evidence; part 2, the material basis for production and distribution. Am. J. Archaeol. 113, 165–201. doi: 10.3764/aja.113.2.165
Pepe, O., Sannino, L., Palomba, S., Anastasio, M., Blaiotta, G., Villani, F., et al. (2010). Heterotrophic microorganisms in deteriorated medieval wall paintings in southern Italian churches. Microbiol. Res. 165, 21–32. doi: 10.1016/j.micres.2008.03.005
Piñar, G., Sterflinger, K., and Pinzari, F. (2015). Unmasking the measles-like parchment discoloration: molecular and microanalytical approach. Environ. Microbiol. 17, 427–443. doi: 10.1111/1462-2920.12471
Pinzari, F., Cornish, L., and Jungblut, A. D. (2020). Skeleton bones in museum indoor environments offer niches for fungi and are affected by weathering and deposition of secondary minerals. Environ. Microbiol. 22, 59–75. doi: 10.1111/1462-2920.14818
Polo, A., Cappitelli, F., Brusetti, L., Principi, P., Villa, F., Giacomucci, L., et al. (2010). Feasibility of removing surface deposits on stone using biological and chemical remediation methods. Microb. Ecol. 60, 1–14. doi: 10.1007/s00248-009-9633-6
Pruvost, M., Bellone, R., Benecke, N., Sandoval-Castellanos, E., Cieslak, M., and Kuznetsova, T. (2011). Genotypes of predomestic horses match phenotypes painted in Paleolithic works of cave art. Proc. Natl. Acad. Sci. U. S. A. 108, 18626–18630. doi: 10.2307/23058525
Russa, M., Ruffolo, S., Rovella, N., Belfiore, C., Palermo, A., Guzzi, M., et al. (2012). Multifunctional TiO2 coatings for cultural. Heritage 74, 186–191. doi: 10.1016/j.porgcoat.2011.12.008
Sadlowska-Salega, A., and Radon, J. (2020). Feasibility and limitation of calculative determination of hygrothermal conditions in historical buildings: case study of st. Martin church in Wisniowa. Build. Environ. 186:107361. doi: 10.1016/j.buildenv.2020.107361
Saiz-Jimenez, C., Cuezva, S., Jurado, V., Fernandez-Cortes, A., Porca, E., Benavente, D., et al. (2011). Paleolithic art in peril: policy and science collide at Altamira Cave. Science 334, 42–43. doi: 10.1126/science.1206788
Sampo, S., and Mosca, A. M. L. (1989). A study of the fungi occurring on 15th-century frescoes in Florence, Italy. Int. Biodeterior. 25, 343–353. doi: 10.1016/0265-3036(89)90014-6
Samson, R., Visagie, C., Houbraken, J., Hong, S., Hubka, V., Klaassen, C., et al. (2014). Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 78, 141–173. doi: 10.1016/j.simyco.2014.07.004
Scheerer, S., Ortega-Morales, O., and Gaylarde, C. (2009). Microbial deterioration of stone monuments—an updated overview. Adv. Appl. Microbiol. 66, 97–139. doi: 10.1016/S0065-2164(08)00805-8
Schito, E., Conti, P., Urbanucci, L., and Testi, D. (2020). Multi-objective optimization of HVAC control in museum environment for artwork preservation, visitors’ thermal comfort and energy efficiency. Build. Environ. 180:107018. doi: 10.1016/j.buildenv.2020.107018
Silva, M., Rosado, T., Teixeira, D., Candeias, A., and Caldeira, A. (2017). Green mitigation strategy for cultural heritage: bacterial potential for biocide production. Environ. Sci. Pollut. Res. Int. 24, 4871–4881. doi: 10.1007/s11356-016-8175-y
Smedemark, S. H., Ryhl-Svendsen, M., and Toftum, J. (2020). Distribution of temperature, moisture and organic acids in storage facilities with heritage collections. Build. Environ. 175:106782. doi: 10.1016/j.buildenv.2020.106782
Sterflinger, K. (2010). Fungi: their role in deterioration of cultural heritage. Fungal Biol. Rev. 24, 47–55. doi: 10.1016/j.fbr.2010.03.003
Sterflinger, K., and Piñar, G. (2013). Microbial deterioration of cultural heritage and works of art — tilting at windmills? Appl. Microbiol. Biotechnol. 97, 9637–9646. doi: 10.1007/s00253-013-5283-1
Sterflinger, K., and Pinzari, F. (2012). The revenge of time: fungal deterioration of cultural heritage with particular reference to books, paper and parchment. Environ. Microbiol. 14, 559–566. doi: 10.1111/j.1462-2920.2011.02584.x
Tite, M. S. (2008). Ceramic production, provenance and use - A review. Archaeometry 50, 216–231. doi: 10.1111/j.1475-4754.2008.00391
Trovão, J., Gil, F., Catarino, L., Soares, F., Tiago, I., and Portugal, A. (2020). Analysis of fungal deterioration phenomena in the first Portuguese King tomb using a multianalytical approach. Int. Biodeterior. Biodegrad. 149:104933. doi: 10.1016/j.ibiod.2020.104933
Unković, N., Grbić, M. L., Stupar, M., Savković, Ž., Jelikić, A., Stanojević, D., et al. (2016). Fungal-induced deterioration of mural paintings: in situ and mock-model microscopy analyses. Microsc. Microanal. 22, 410–421. doi: 10.1017/S1431927616000544
Vasanthakumar, A., Dearaujo, A., Mazurek, J., Schilling, M., and Mitchell, R. (2013). Microbiological survey for analysis of the brown spots on the walls of the tomb of King Tutankhamun. Int. Biodeterior. Biodegrad. 79, 56–63. doi: 10.1016/j.ibiod.2013.01.014
Vukojević, J., and Grbić, M. (2010). Moulds on paintings in Serbian fine art museums. Afr. J. Microbiol. Res. 4, 1453–1456. doi: 10.1186/1475-2859-9-53
Vyas, P., and Gulati, A. (2009). Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent pseudomonas. BMC Microbiol. 9:174. doi: 10.1186/1471-2180-9-174
Wang, W., Ma, X., Ma, Y., Mao, L., Wu, F., Ma, X., et al. (2010a). Seasonal dynamics of airborne fungi in different caves of the Mogao Grottoes, Dunhuang, China. Int. Biodeterior. Biodegr. 64, 461–466. doi: 10.1016/j.ibiod.2010.05.005
Wang, W., Ma, Y., Ma, X., Wu, F., Ma, X., An, L., et al. (2010b). Seasonal variations of airborne bacteria in the Mogao Grottoes, Dunhuang, China. Int. Biodeterior. Biodegr. 64, 309–315. doi: 10.1016/j.ibiod.2010.03.004
Zhang, J., Cai, L., Gao, X., and Liu, M. (2007). Thought and suggestion on protection techniques of paint layers of Qin Terracotta Army. Sci. Conserv. Archaeol. 1, 51–56.
Zhang, F., Li, L., Sun, M., Hu, C., Zhang, Z., Shao, H., et al. (2019a). Fungal community analyses of a pirogue from the tang dynasty in the national maritime museum of China. Appl. Sci. 9:4129. doi: 10.3390/app9194129
Keywords: tombs of the Western Han Dynasty, pottery figurines, fungal deterioration, fungistatic experiment, cultural heritage protection
Citation: Wang Y, Wang C, Yang X, Ma K, Guo P, Sun Q, Jia S and Pan J (2022) Analysis and control of fungal deterioration on the surface of pottery figurines unearthed from the tombs of the Western Han Dynasty. Front. Microbiol. 13:956774. doi: 10.3389/fmicb.2022.956774
Edited by:
Mukesh Kumar Awasthi, Northwest AandF University, ChinaReviewed by:
Yimin Yang, University of Chinese Academy of Sciences, ChinaFasi Wu, Dunhuang Research Academy, China
Copyright © 2022 Wang, Wang, Yang, Ma, Guo, Sun, Jia and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiao Pan, cGFuamlhb25rQG5hbmthaS5lZHUuY24=; cGFuamlhb0B1c3RiLmVkdS5jbg==
†These authors have equally contributed to this work
 Yu Wang
Yu Wang Cen Wang1†
Cen Wang1† Kaixuan Ma
Kaixuan Ma Jiao Pan
Jiao Pan