- 1College of Animal Science, Guizhou University, Guiyang, China
- 2Key Laboratory of Animal Genetics, Breeding and Reproduction in the Plateau Mountainous Region, Ministry of Education, Guizhou University, Guiyang, China
Paper mulberry (Broussonetia papyrifera L., PM) is being used as a new type of animal protein feed to address the feed crisis. To investigate the effect of additives on the chemical composition, fermentation quality, and bacterial community of PM silage (at room temperature, 25°), paper mulberry was fermented with formic acid (FA), Amomum villosum essential oil (AVEO) and lactic acid bacteria (LAB) inoculant treatments. The results showed that fresh PM had a low water-soluble carbohydrate (WSC) content and large amounts of unclassified bacteria. Compared with the CK and LAB treatments, the FA and AVEO treatments significantly (P < 0.05) decreased the pH and increased the lactic acid content of PM silage after 60 days of ensiling. In the AVEO-treated silages the abundance of Lactococcus in the early stage of ensiling increased by 14.09%, the abundances of Levilactobacillus and Lentilactobacillus in the late stage of ensiling increased by 58.34 and 91.12%, respectively, and the abundance of Stenotrophomonas decreased by 94.71%, resulting in improved PM silage quality. These results confirmed that AVEO could potentially be developed as a new additive for improving the fermentation quality of silage.
Introduction
With the rapid development of China’s livestock industry, traditional feed is no longer sufficient to fulfill the needs of livestock (Dong et al., 2020). The exploitation of new feed resources to alleviate the feed crisis has been demonstrated (Guo et al., 2021). Paper mulberry (Broussonetia papyifera L., PM) is a perennial tree or shrub that is highly adaptable, fast growing, affordable to produce, and widely distributed (Peñailillo et al., 2016). China has cultivated approximately 300,000 hectares of PM as animal feed because of its high crude protein (CP) content and its high levels of vitamins and amino acids (Liu et al., 2019). Moreover, some studies have indicated that feeding with PM silage could enhance the immunity and antioxidant functions of beef cattle and dairy cows (Hao et al., 2020; Tao et al., 2020).
PM is harvested in the high-temperature and high-precipitation season, causing PM to become moist, and ensiling has been indicated to be the best way to preserve PM (Zhang et al., 2019). However, Cheng et al. (2021) observed that untreated PM was difficult to ensile due to its high buffering capacity and due to the low lactic acid bacteria (LAB) concentration (< 105 cfu/g FM) in fresh forage. LAB additives are widely used to accelerate the ensiling process, limit the growth of harmful microorganisms, and improve the quality of PM silage (Cheng et al., 2021; Du et al., 2021; Wang et al., 2021). Since the adaptability and developmental processes of LAB in forage during ensiling are unknown, LAB additives do not always improve the fermentation quality of silage. Kobayashi et al. (2010) discovered that adding LAB additives derived from Leymus chinensis silage had no significant effect on the fermentation quality of PM silage, as indicated by the high pH value (> 6) and ammonia nitrogen (NH3–N) content (> 16% TN). Therefore, it is imperative to find LAB additives or other additives that can significantly improve the silage quality of PM.
Formic acid (FA) is commonly used as a chemical additive to rapidly reduce the pH of silage at an early stage of ensiling, suppress the growth of harmful microorganisms, and enhance silage quality (Wei et al., 2021). According to Andrade and Melotti (2004), adding 0.5% FA can improve the fermentation quality of elephant grass and increase animal digestibility. To date, there has been no report on the effect of FA on the quality of PM silage. Plant essential oils are naturally occurring secondary metabolites derived from aromatic plants that have a wide range of antimicrobial properties (Soycan-Önenç et al., 2015). Numerous studies have demonstrated that plant essential oils affect the growth and metabolism of a variety of microorganisms (rumen bacteria, gram-positive bacteria, and fungi) (Wallace, 2004). Amomum villosum, an herbaceous plant in the ginger family, is a famous traditional Chinese medicine (Chen et al., 2018). A. villosum essential oil (AVEO) is an edible spice oil that exhibits broad-spectrum antibacterial activity (Suo et al., 2018). It can be utilized as a food additive, animal feed supplement, or medicine (Hou and Jiang, 2013). Suo et al. (2018) found that AVEO significantly inhibited the growth of a range of harmful bacteria, including Escherichia coli, Pseudomonas aeruginosa and Staphylococcus sp. Moreover, Chen et al. (2018) showed that AVEO significantly inhibited lipopolysaccharide from entering blood circulation. This inhibitory effect may be associated with the beneficial effects on the equilibrium of the animal intestinal microflora (Chen et al., 2018). Although A. villosum has been widely used in the treatment of gastrointestinal diseases in animals, whether AVEO can be applied to improve PM silage quality and the underlying mechanisms of action are still unknown. The objective of our present study was to evaluate the effects of LAB, FA, and AVEO on the chemical composition, fermentation quality, and bacterial community of PM silage. Our hypothesis was that AVEO can improve the silage quality of PM.
Materials and methods
Silage preparation
Whole PM plants (Broussonetia papyifera L. Zhongke No.1) were harvested and chopped to a length of 1–2 cm on May 1st, 2021, in Changshun County, Guiyang city, Guizhou Province. To produce silage, approximately 300 g of chopped PM was mixed homogenously with each additive, packed manually into polyethylene bags (25 cm × 30 cm), and then vacuum packed using a vacuum packing machine (SJ-400, Shanghai Precision Machinery Manufacturing Co., Ltd.). The treatments were as follows: (1) CK (without additives), distilled water applied at 5 mL kg–1 fresh weight (FW); (2) FA, applied at 5 mL kg–1 FW (88%) (Zhongke Jiayi Biological Engineering Co., Ltd., Shandong, China); (3) LAB, combined application of Lactiplantibacillus plantarum and Lactiplantibacillus buchneri (Zhongke Jiayi Biological Engineering Co., Ltd., Shandong, China) at 2 × 107 cfu/g FW; (4) AVEO, applied at 1 ml kg–1 FW (Baishengyuan Industrial Co., Ltd., Yang Jiang, China). Silages were stored at room temperature (22–25°C) and were opened in triplicate for each treatment after 3, 7, 15, 30, and 60 days of ensiling to analyze fermentation quality, after 3 and 60 days of ensiling to analyze microbial diversity and after 60 days of ensiling to analyze chemical composition.
Analysis of chemical components and fermentation characteristics
Each sample was dried at 65°C, crushed, and sieved through a 0.5 mm sieve to estimate the DM content. Both the neutral detergent fiber (NDF) and acid detergent fiber (ADF) levels were analyzed using the methods of Van Soest et al. (1991). The water-soluble carbohydrate (WSC) content was determined by the anthrone colorimetric method as described by Zhang et al. (2017). The crude protein (CP) content was determined by the method of AOAC (1990).
Each 20 g silage sample was mixed homogeneously with 180 mL of sterile water for 3 min and then filtered through four layers of cheesecloth. The filtrate was used to measure the pH, organic acid content, and ammonia nitrogen (NH3-N) content. The concentrations of organic acids [lactic acid (LA), acetic acid (AA), propionic acid (PA), and butyric acid (BA)] were measured using high-performance liquid chromatography as described by Mu et al. (2021). The levels of organic acids were determined using an Agilent 1260 HPLC system with a 210 nm ultraviolet detector and an Acclaim TM Organic Acid column (Dionex Co., Ltd., Sunnyvale, CA, United States). The NH3-N content was measured as described by Broderick and Kang (1980) using the phenol–hypochlorite reaction.
DNA extraction, amplification, and sequencing
To measure the bacterial community compositions of fresh and ensiled PM, each sample was immediately transported to Biomarker Technologies (Beijing, China). The bacterial community in the samples was analyzed by second-generation sequencing technology as described by Chen et al. (2020). The total DNA from each sample was extracted by a Power Soil DNA Isolation Kit (MO BIO Laboratories) according to the manufacturer’s protocol. After purification, the DNA was diluted to 1 ng/mL using sterile water. The 16S rDNA V3–V4 regions were amplified using a forward primer (50-ACTCCTACGGGAGGCAGCA-30) and reverse primer (50GGACTACHVGGGTWTCTAAT-30) combined with specific barcode sequences. The total PCR amplification volume was 50 μl, which included 10 μl of buffer, 0.2 μl of Q5 High-Fidelity DNA Polymerase, 10 μl of High GC Enhancer, 1 μl of dNTPs, 10 μM each primer, and 60 ng of genomic DNA. The polymerase chain reaction (PCR) was performed under the following conditions: initial activation of the hot-start polymerase at 95°C for 4 min, followed by 15 cycles of 95°C for 60 s, 60°C for 40 s, and 72°C for 60 s and a final extension at 72°C for 10 min. The final PCR products were pooled and quantified using the Quant-iT™ dsDNA HS Reagent. High-throughput sequencing was conducted on an Illumina HiSeq 2500 platform (2 × 250 paired ends) by Biomarker Technologies Corporation (Beijing, China) according to protocols described by Caporaso et al. (2012) and Kozich et al. (2013). Sequences with ≥ 97% comparability were assigned to the same operational classification unit (OTU) using UPARSE software. After standardizing the OTU abundance information, alpha diversity indexes (Chao, Shannon, Simpson, and Ace) and coverage values were computed using QIIME software (version 2.15.3).
Statistical analyses
The chemical composition of PM mixed with additives was evaluated using one-way analysis of variance in SPSS Statistics (version 22.0) software (IBM Crop., Armonk, NY, United States). Data for fermentation quality and the bacterial community were analyzed via two-way analysis of variance to evaluate the effects of additives (T), ensiling period (D), and their interaction (T × D). The means were then compared to determine significance using Duncan’s multiple range method. All statistical analyses were performed using the general linear model procedure with SPSS 26 software (IBM Crop., Armonk, NY, United States). Significance was declared at P < 0.05 unless otherwise noted.
Results and discussion
Chemical compositions of fresh and ensiled paper mulberry
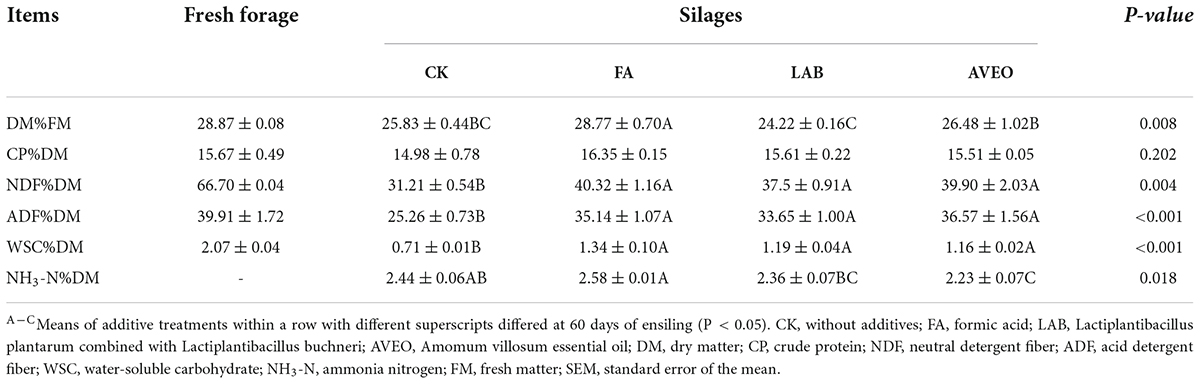
The chemical compositions of fresh and ensiled PM are shown in Table 1. The DM content of fresh PM was 28.87% FM, which was similar to the result of Hao et al. (2021), who reported that the DM content of whole PM plants was 26.6% FM. The NDF and ADF levels in PM were 66.70% DM and 39.92% DM, respectively, which were much higher than those observed by Du et al. (2021) (45.67% DM and 16.68% DM). The NDF and ADF levels observed in our study being higher because the previous study focused on PM leaves, while this study focused on the whole PM plant. However, the CP content of fresh PM was 15.67% DM, which was similar to that observed by Sun et al. (2022) (16.47% DM). The presence of sufficient WSC content (6–7% DM) in the fresh material is essential to ensure the quality of silage fermentation (Smith, 1962). However, the WSC content of fresh PM (2.07% DM) was < 6–7% DM in our study. As a result, silage additives were required to improve the fermentation quality of PM during ensiling (Dong et al., 2020).
The additive significantly (P < 0.05) affected the DM, NDF, ADF, NH3-N, and WSC levels in the silage. The DM content is an important indicator in silage, as LAB require moisture for growth and reproduction (Xu et al., 2020). The DM content in all the PM silages was lower than that in fresh forage. According to Ogunade et al. (2016), this decrease could be due to the metabolism of soluble substrates by microbial fermentation. The DM content of the FA-treated silage was significantly higher than that of the other treated silages (P < 0.05). The addition of FA can effectively restrict the growth of harmful microorganisms, thereby minimizing DM loss (Wei et al., 2021). Moreover, the FA-, LAB-, and AVEO-treated silages had greater NDF and ADF levels than the CK silage (P < 0.05). However, consistent results were reported by Sun et al. (2022), who found that silage additives can increase the NDF and ADF levels in PM silage. This result might be attributed to the reduction in DM content (Li et al., 2021a). The WSC content in forage plays an essential role in the production of LA during ensiling. Compared with the CK, the additive treatment significantly (P < 0.05) increased the WSC content. Similar results were obtained by Li et al. (2021a), who reported that inoculation with additives increased the WSC content of mulberry silage. AVEO treatment significantly (P < 0.05) decreased the NH3-N content compared with that in the CK silage. Similar to the result of our study, cinnamon essential oil also reduced the NH3-N content in pea silage (Cantoia Júnior et al., 2020). This may have occurred because the essential oil could inhibit the growth of NH3-N-producing bacteria, thereby reducing the NH3-N content of silage (Tang et al., 2021).
Fermentation characteristics of paper mulberry silages
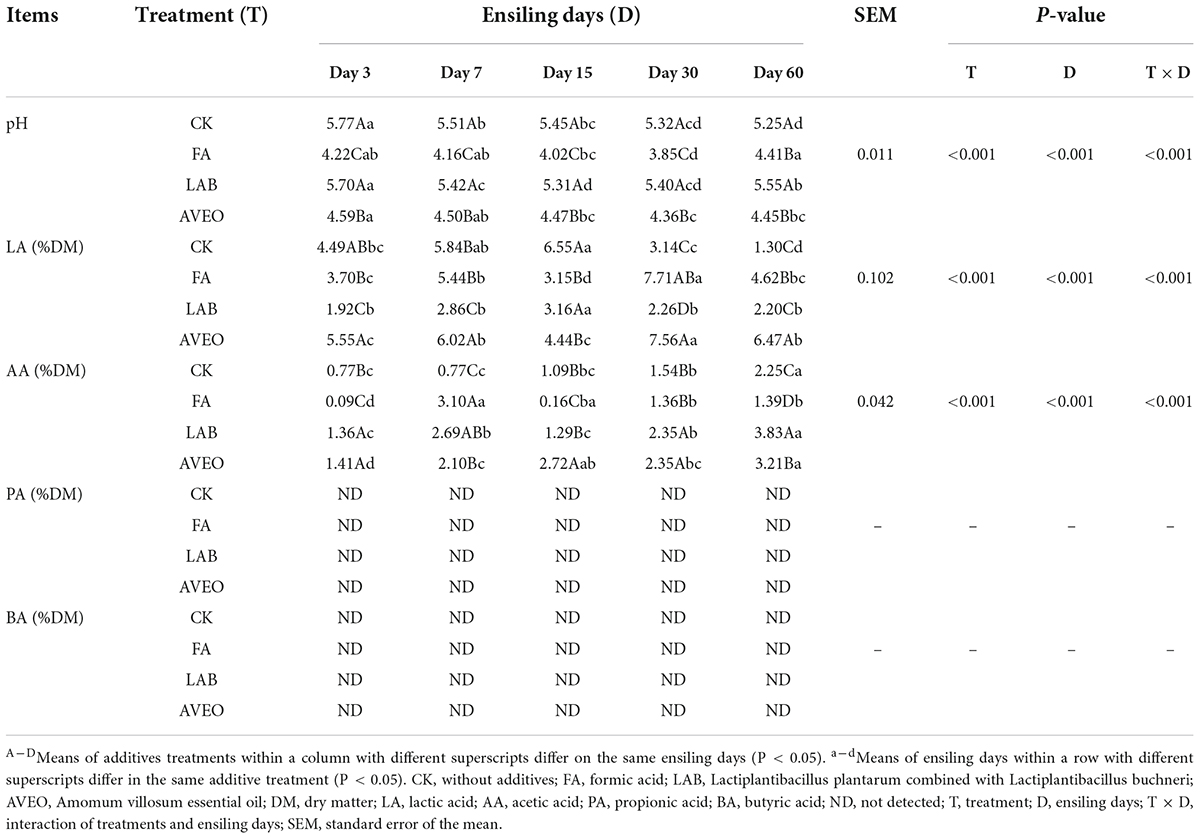
The fermentation quality of the PM silages is shown in Table 2. The FA- and AVEO-treated silages exhibited lower (P < 0.05) pH values than the CK silage during the ensiling process. This may be attributable to the acidification function of FA (Wei et al., 2021). Administration of AVEO significantly increased the abundance of Lactiplantibacillus and decreased the abundance of Proteobacteria, such as Desulfovibrio and Helicobacter, which could be an explanation for the observed effects in this study (Chen et al., 2018). However, in our study, there was no difference in pH between the LAB- and CK-treated silages. A similar situation was reported by Cheng et al. (2021), who found that PM silage treated with a commercial inoculant (Lactiplantibacillus) did not exhibit improved fermentation quality. At later stages of fermentation, the FA-treated silage exhibited higher LA content and lower AA content than the CK silage (P < 0.05). However, the opposite results were obtained by Tyrolová et al. (2017) and Wei et al. (2021), who reported that FA treatment significantly (P < 0.05) decreased the LA content and increased the AA content of silage. This may be due to the differences in forage characteristics and in the FA application rate, which can lead to inconsistent results in the fermentation quality of silage (Huhtanen et al., 2013). However, after 60 days of ensiling, the LAB-treated silage exhibited lower LA content than the FA- and AVEO-treated silages but had the highest AA content (3.83% DM) (P < 0.05). This was similar to the results of Queiroz et al. (2013), who shown that LAB treatment increased the AA content but not the LA content in corn silage. A study from Driehuis and van Wikselaar (2000) demonstrated that changes in the LA and AA levels of silage were related to the pH and LAB types. The reason for the higher AA in the LAB-treated silage could be due to the ability of the added L. buchneri to convert LA to AA (Danner et al., 2003). After 60 days of ensiling, the AVEO-treated silage exhibited the highest (P < 0.05) LA content (6.47% DM) compared with the other silages. According to Vousough et al. (2009), mint essential oil has a stimulatory effect on the growth of several strains of Lactiplantibacillus in vitro. Therefore, the increase in LA content was attributed to AVEO significantly increasing the abundance of Lactiplantibacillus and decreasing the abundance of harmful microorganisms to metabolize LA (Chen et al., 2018). These results showed that AVEO could improve the fermentation quality of PM silage. Interestingly, PA and BA were not detected in any of the silages, which was similar to the results of Cheng et al. (2021), who found that neither PA nor BA was detected in PM silage treated with additives.
Bacterial community composition of paper mulberry silages
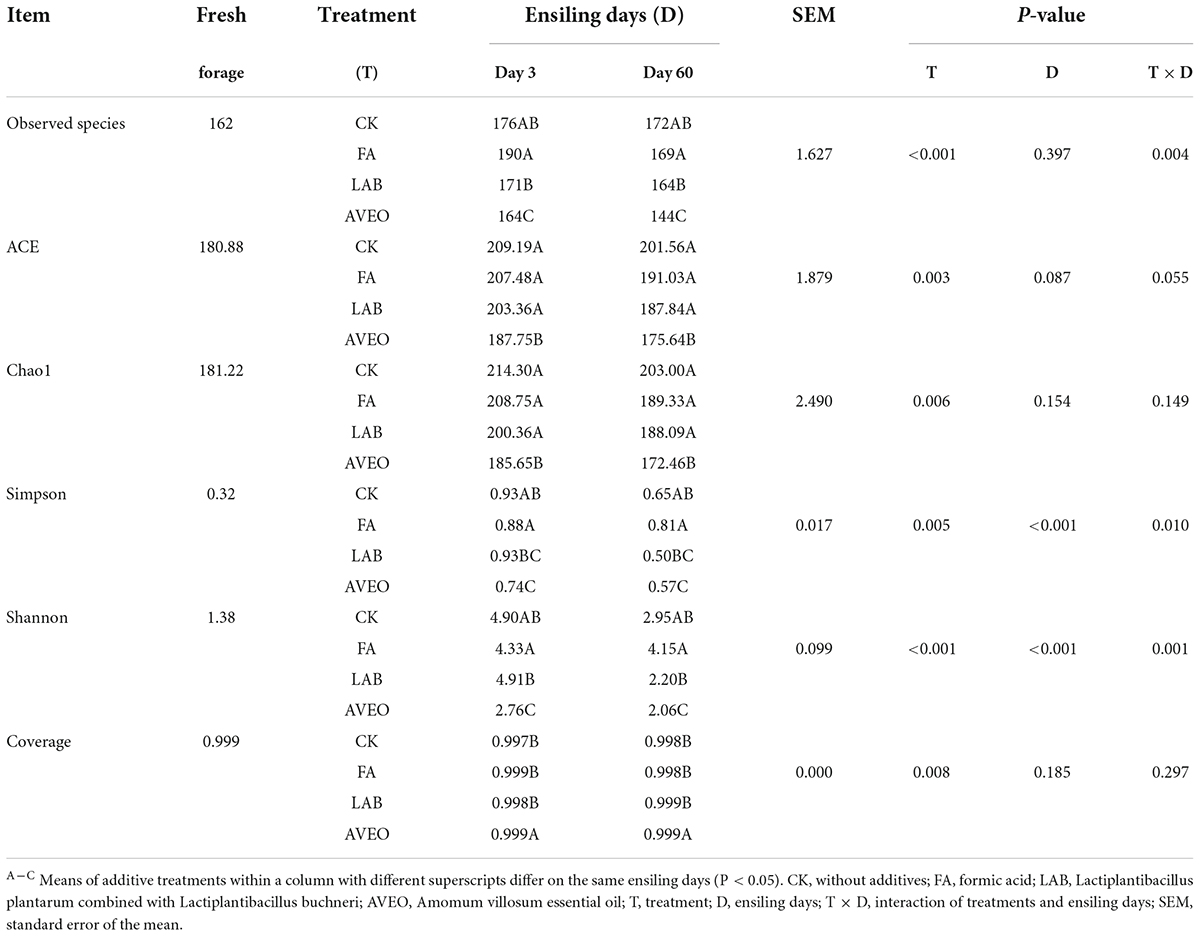
The bacterial diversity of the PM silages is shown in Table 3. The coverage value of all samples was more than 0.99, indicating that the sequencing depth was sufficient for determining the microbial composition. During the ensiling process, numerous microorganisms were replaced by an anaerobic LAB population, and the observed species index decreased, leading to the production of well-fermented silage. During ensiling, a decrease in the observed species index was observed in the AVEO-treated silage compared with the CK silage (P < 0.05). Similar results were found in the study of Dong et al. (2020), who reported that the observed species index in PM silage decreased after ensiling. The addition of AVEO during ensiling significantly decreased (P < 0.05) the bacterial alpha-diversity (ACE, Chao1, Shannon, and Simpson indexes) indexes of the PM silage compared to the CK silage. The results of our study are consistent with those of Dong et al. (2020), who showed that the Shannon, Simpson, and Chao1 indexes decreased after ensiling, suggesting that the diversity of the microbial community decreased when the growth of some microorganisms was inhibited by AVEO. However, in this study, there was no significant difference in bacterial alpha diversity among the silages treated with LAB, FA, and CK.
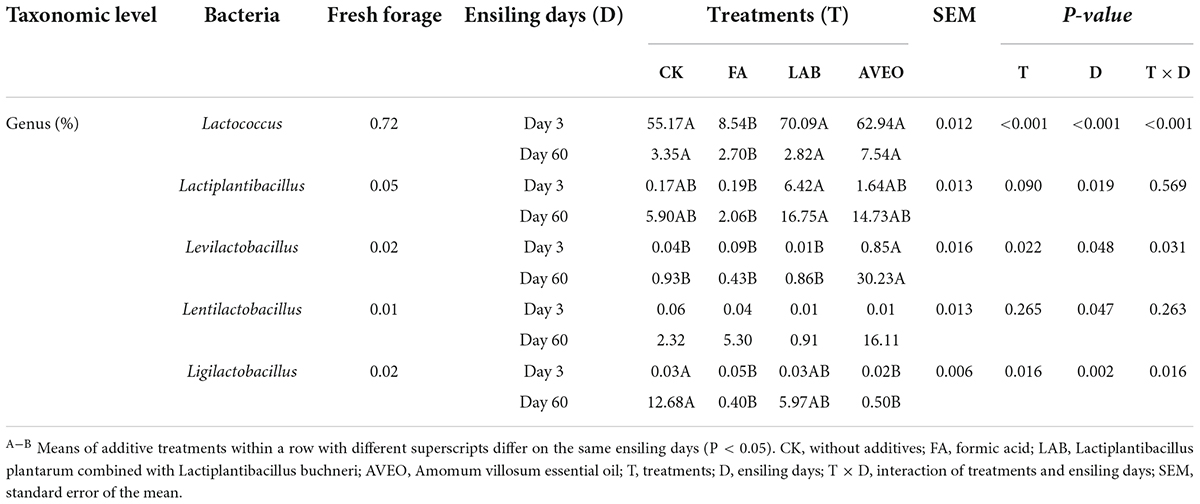
Changes in the bacterial community composition during the fermentation process in PM silages at the phylum level are shown in Figure 1A. Firmicutes and Proteobacteria were mainly detected during the ensiling process. The results of our study are consistent with those of He et al. (2021), who reported that Firmicutes and Proteobacteria were the main phyla detected during the ensiling process in PM silage. Moreover, the addition of LAB and AVEO reduced the relative abundance of Proteobacteria while increasing the relative abundance of Firmicutes. The addition of exogenous LAB (which are members of Firmicutes) increased the relative abundance of Firmicutes (Liu et al., 2019) and the acidic environment produced by LAB suppressed the growth of many undesirable microorganisms (Yan et al., 2019), resulting in a reduction in the relative abundance of Proteobacteria. Moreover, the main components of AVEO are alkenes, esters, and alcohols, and these chemicals have marked inhibitory effects on Proteobacteria (Knobloch et al., 1989; Zhang et al., 2011). Changes in the bacterial community composition during the fermentation process in PM silage at the genus level are shown in Figure 1B and Table 4. The main microorganisms in fresh PM were unculturable bacteria, which is consistent with the results of Cheng et al. (2021). Overall, both unclassified bacteria and Lactococcus were dominant in the PM silages at 3 days of ensiling. This result is consistent with those of Zhang et al. (2019), who found that the dominant genus in PM silage was Lactococcus. However, Lactococcus is considered an LA-producing microorganism in silage during the initiation of fermentation (Liu et al., 2019). Moreover, the abundance of Lactococcus in the LAB- and AVEO-treated silages increased (P < 0.05) compared to that in the CK treatment at 3 days of ensiling. This confirms our results for fermentation quality, with higher LA content observed in the LAB and AVEO treatments (Table 2) because the addition of LAB and AVEO inhibits the growth of Proteobacteria, such as Desulfovibrio, Sutterella, and Helicobacter (Chen et al., 2018; Dong et al., 2020), thus providing sufficient nutrients for the growth of Lactococcus. However, at 3 days of ensiling, the relative abundance of Lactococcus in FA-treated silage was lower (P < 0.05) than that in the CK treatment. This may be because FA rapidly reduces the pH and inhibits the growth of acid-intolerant microorganisms, such as Lactococcus (Dong et al., 2020). Acinetobacter was not detected in the AVEO-treated silage but could be detected in the other treated silages. We speculated that the level of Acinetobacter could not be determined due to the ability of AVEO to suppress bacterial growth. It has been found that Acinetobacter can utilize AA to survive in an anaerobic environment, and its abundance may increase with increasing AA content (Fuhs and Chen, 1975; Ogunade et al., 2017). Therefore, in our study, the abundance of Acinetobacter in the CK, LAB and FA treatments might have been related to the AA content. The relative abundance of Lactococcus decreased in all the silages treated for 60 days compared to 3 days due to a decrease in pH as the ensiling duration increased, thereby inhibiting the growth of acid-intolerant microorganisms (Dong et al., 2020). In addition, at 60 days of ensiling, the relative abundance of Lactiplantibacillus in the LAB- and AVEO-treated silages was higher than that in the CK silage. This is because the main component of the LAB additive was Lactiplantibacillus. The results of our study are consistent those of Chen et al. (2018), who found that administration of AVEO significantly increased the relative abundance of Lactiplantibacillus. Bornyl acetate is considered the main active ingredient of AVEO and exerts its antibacterial effect via modulation of p38 MAPK kinase and Caspase 3 expression (Chen et al., 2018). We found that Levilactobacillus, Lentilactobacillus and Ligilactobacillus were present in the PM silage after 60 days of ensiling. Levilactobacillus and Lentilactobacillus are heterofermentative LAB (Zheng et al., 2020). Species of Ligilactobacillus are homofermentative and express urease (Krumbeck et al., 2016). At 60 days of ensiling, the relative abundance of Levilactobacillus and Lentilactobacillus in the AVEO-treated silage was higher than that in the other silages. This may have been due to the inhibitory effects of AVEO on harmful bacteria making the relative abundance of LAB with strong acid tolerance higher than that in other silages. In summary, AVEO increased the abundance of Lactococcus in the early stage of ensiling and Levilactobacillus and Lentilactobacillus in the late stage of ensiling, resulting in improved PM silage quality.
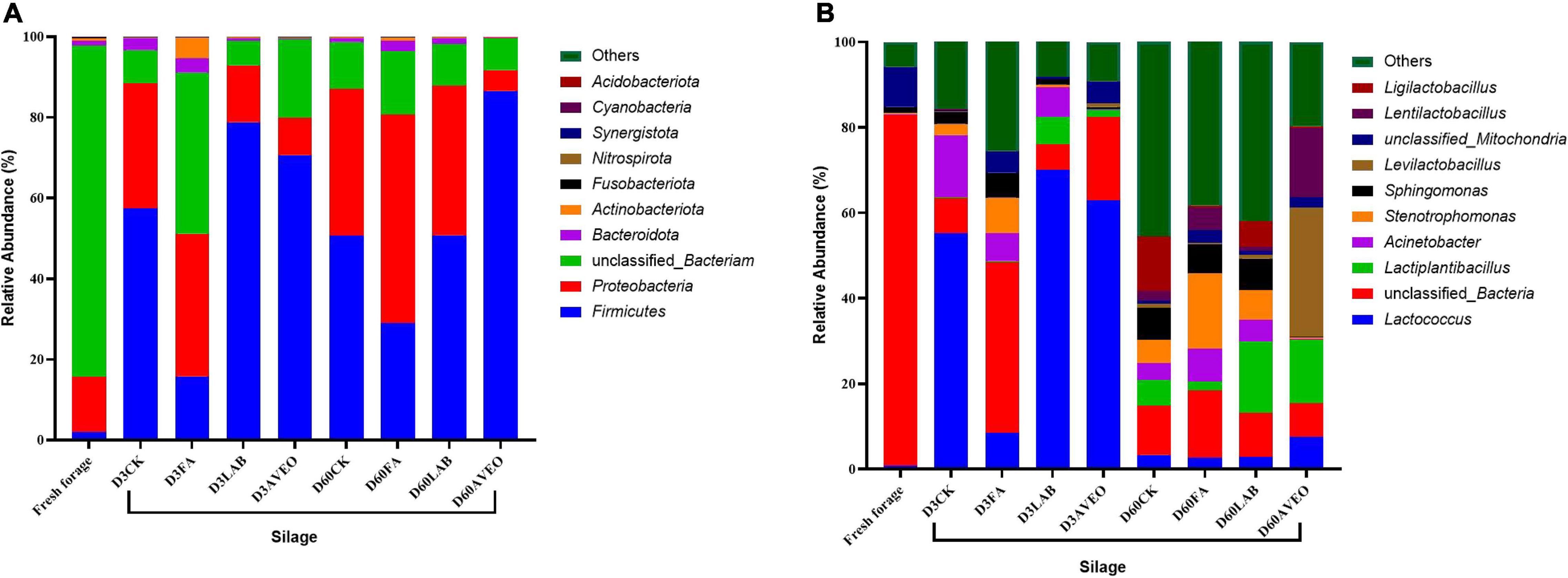
Figure 1. Relative abundance of the bacterial community at the phylum (A) and genus (B) levels in fresh paper mulberry and the control treatment without additives (CK) or with formic acid (FA); Lactiplantibacillus plantarum combined with Lactiplantibacillus buchneri (LAB); Amomum villosum essential oil (AVEO); D3, 3 days of ensiling; D60, 60 days of ensiling.
Functional prediction for the bacterial community
The results of functional prediction for the bacterial community during silage fermentation are shown in Figure 2. Chemoheterotrophs were the primary functional components of the bacterial population in the silage, followed by fermenters, aerobic chemoheterotrophs, and animal parasites or symbionts. At 3 days of ensiling, treatments with AVEO and LAB promoted silage fermentation, whereas treatment with FA reduced the rate and extent of fermentation. The degree of fermentation was greater in the LAB and AVEO treatments due to the higher abundance of Lactococcus, which could be an explanation for the effects observed in this study. At 60 days of ensiling, treatment with AVEO promoted silage fermentation. Similar results were found in the study of Li et al. (2021b) who reported that treatment with an additive promoted the silage fermentation. Since the AVEO treatment showed increased abundances of LAB, such as Levilactobacillus and Lentilactobacillus, the degree of fermentation in the PM silage increased under AVEO treatment. Treatment with AVEO reduced the abundances of aerobic chemoheterotrophs and animal parasites or symbionts compared to the control. Furthermore, Tang et al. (2021) reported that an essential oil induced bacterial death by inhibiting the TCA cycle, decreasing ATP and ROS generation, and enhancing SOD activity, which resulted in a reduction in the abundance of aerobic bacteria and animal parasites.
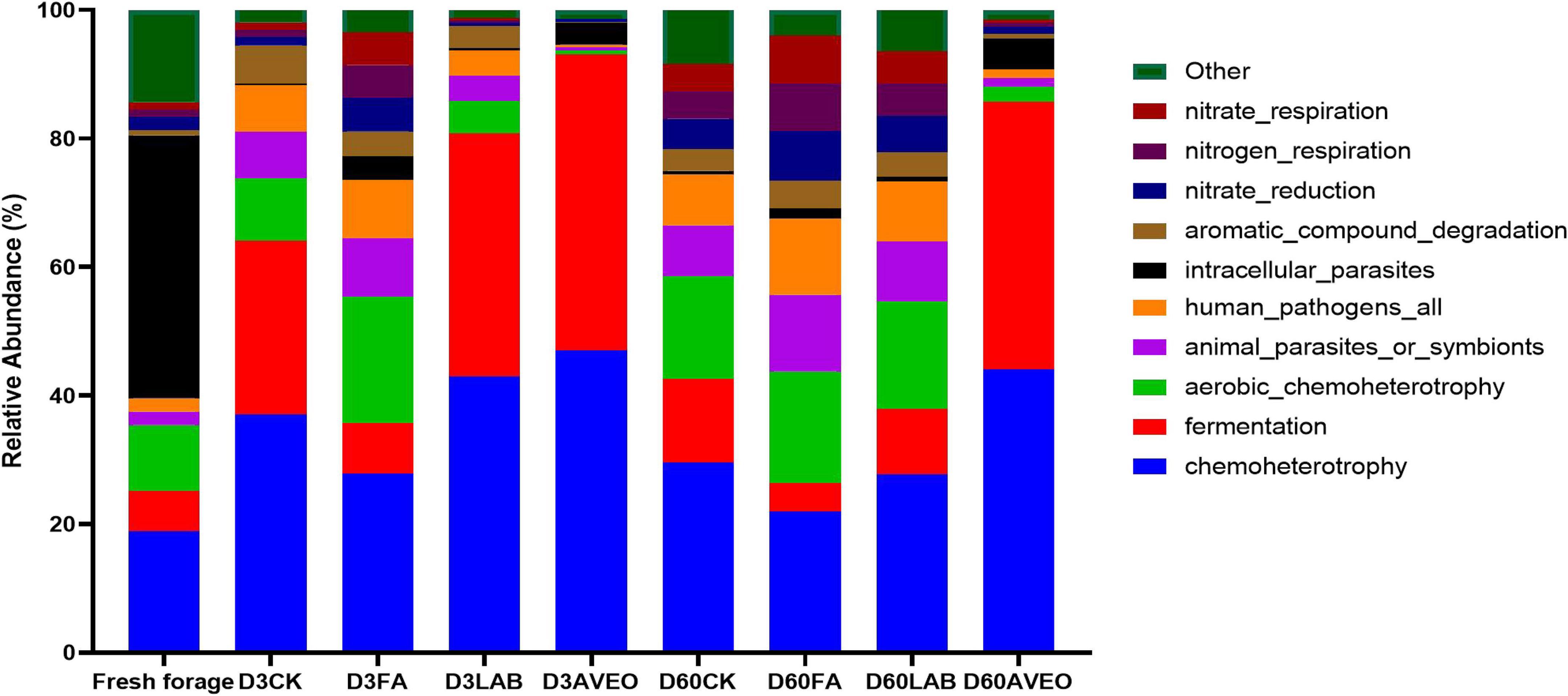
Figure 2. Functional prediction for the bacterial community in fresh paper mulberry and the control without additives (CK) or with formic acid (FA); Lactiplantibacillus plantarum combined with Lactiplantibacillus buchneri (LAB); Amomum villosum essential oil (AVEO); D3, 3 days of ensiling; D60, 60 days of ensiling.
Conclusion
Additives can affect the fermentation quality of PM silage by altering the bacterial community structure during ensiling, with the AVEO additive providing the best effect. Mainly, AVEO increased the abundance of Lactococcus in the early stage of ensiling and Levilactobacillus and Lentilactobacillus in the late stage of ensiling, resulting in improved PM silage quality. Therefore, AVEO could potentially be developed as a new additive for improving the fermentation quality of silage.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by the National Key Research and Development Subject (2021YFD1300302), Guizhou Talent Base of Grassland Ecological Animal Husbandry (RCJD2018-13), the Guizhou University Introduced Talents Scientific Research Project [Guida Renji Hezi (2020)71], and the Guizhou University Cultivation Project [Guida Renji Hezi [2020]9].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Biomarker Technologies Company (Beijing, China) for providing technical support.
Abbreviations
PM, paper mulberry; FA, formic acid; AVEO, Amomum villosum essential oil; LAB, lactic acid bacteria; OTU, operational taxonomic unit; PCR, polymerase chain reaction; DM, dry matter; CP, rude protein; NDF, neutral detergent fiber; ADF, acid detergent fiber; WSC, water-soluble carbohydrate; NH3-N, ammonia nitrogen; FM, fresh matter; LA, lactic acid; AA, acetic acid; PA, propionic acid; BA, butyric acid.
References
Andrade, S. J. T. D., and Melotti, L. (2004). Efeito de alguns tratamentos sobre a qualidade da silagem de capim-elefante cultivar Napier (Pennisetum purpureum. Schum). Braz. J. Vet. Res. Anim. Sci. 41, 409–415. doi: 10.1590/S1413-95962004000600009
Broderick, G. A., and Kang, J. H. (1980). Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media1. J. Dairy Sci. 63, 64–75. doi: 10.3168/jds.S0022-0302(80)82888-8
Cantoia Júnior, R., Capucho, E., Garcia, T. M., Del Valle, T. A., Campana, M., Zilio, E. M. C., et al. (2020). Lemongrass essential oil in sugarcane silage: fermentative profile, losses, chemical composition, and aerobic stability. Anim. Feed Sci. Tech. 260:114371. doi: 10.1016/j.anifeedsci.2019.114371
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Huntey, J., Fierer, N., et al. (2012). Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624. doi: 10.1038/ismej.2012.8
Chen, L., Qu, H., Bai, S., Yan, L., You, M., Gou, W., et al. (2020). Effect of wet sea buckthorn pomace utilized as an additive on silage fermentation profile and bacterial community composition of alfalfa. Bioresour. Technol. 314:123773. doi: 10.1016/j.biortech.2020.123773
Chen, Z., Ni, W., Yang, C., Zhang, T., Lu, S., Zhao, R., et al. (2018). Therapeutic Effect of Amomum villosum on Inflammatory Bowel Disease in Rats. Front. Pharmacol. 9:639. doi: 10.3389/fphar.2018.00639
Cheng, Q., Chen, Y., Bai, S., Chen, L., You, M., Zhang, K., et al. (2021). Study on the bacterial community structure and fermentation characteristics of fresh and ensiled paper mulberry. Anim. Sci. J. 92:e13656. doi: 10.1111/asj.13656
Danner, H., Holzer, M., Mayrhuber, E., and Braun, R. (2003). Acetic acid increases stability of silage under aerobic conditions. Appl. Environ. Microbiol. 69, 562–567. doi: 10.1128/AEM.69.1.562-567.2003
Dong, L., Zhang, H., Gao, Y., and Diao, Q. (2020). Dynamic profiles of fermentation characteristics and bacterial community composition of Broussonetia papyrifera ensiled with Perennial ryegrass. Bioresour. Technol. 310:123396. doi: 10.1016/j.biortech.2020.123396
Driehuis, F., and van Wikselaar, P. G. (2000). The occurrence and prevention of ethanol fermentation in high-dry-matter grass silage. J. Sci. Food Agric. 80, 711–718. doi: 10.1002/(SICI)1097-0010(20000501)80:6<711::AID-JSFA593<3.0.CO;2-6
Du, Z., Sun, L., Chen, C., Lin, J., Yang, F., and Cai, Y. (2021). Exploring microbial community structure and metabolic gene clusters during silage fermentation of paper mulberry, a high-protein woody plant. Anim. Feed Sci. Tech. 275:114766. doi: 10.1016/j.anifeedsci.2020.114766
Fuhs, G. W., and Chen, M. (1975). Microbiological basis of phosphate removal in the activated sludge process for the treatment of wastewater. Microbiol. Ecol. 2, 119–138. doi: 10.1007/BF02010434
Guo, L., Wang, X., Lin, Y., Yang, X., Ni, K., and Yang, F. (2021). Microorganisms that are critical for the fermentation quality of paper mulberry silage. Food Energy Secur. 10:e304. doi: 10.1002/fes3.304
Hao, Y., Huang, S., Liu, G., Zhang, J., Liu, G., Cao, Z., et al. (2021). Effects of different parts on the chemical composition, silage fermentation profile, in vitro and in situ digestibility of paper mulberry. Animals 11:413. doi: 10.3390/ani11020413
Hao, Y., Huang, S., Si, J., Zhang, J., Gaowa, N., Sun, X., et al. (2020). Effects of paper mulberry silage on the milk production, apparent digestibility, antioxidant capacity, and fecal bacteria composition in holstein dairy cows. Animals 10:1152. doi: 10.3390/ani10071152
He, Q., Zhou, W., Chen, X., and Zhang, Q. (2021). Chemical and bacterial composition of Broussonetia papyrifera leaves ensiled at two ensiling densities with or without Lactobacillus plantarum. J. Clean. Prod. 329:129792. doi: 10.1016/j.jclepro.2021.129792
Hou, Y., and Jiang, J. G. (2013). Origin and concept of medicine food homology and its application in modern functional foods. Food Funct. 4, 1727–1741. doi: 10.1039/c3fo60295h
Huhtanen, P., Jaakkola, S., and Nousiainen, J. (2013). An overview of silage research in Finland: from ensiling innovation to advances in dairy cow feeding. Agric. Food Sci. 22, 35–56. doi: 10.23986/afsci.6632
Knobloch, K., Pauli, A., Iberl, B., Weigand, H., and Weis, N. (1989). Antibacterial and antifungal properties of essential oil components. J. Essent. Oil Res. 1, 119–128. doi: 10.1080/10412905.1989.9697767
Kobayashi, H., Cai, Y., Uegaki, R., Shimizu, M., Nakajima, M., Kanaya, C., et al. (2010). Microorganism composition of high moisture italian ryegrass (Lolium multiflorum lam.) and its fermentation characteristics of silage inoculated with lactic acid bacteria. JPN J. Grassl. Sci. 56, 39–46. doi: 10.14941/grass.56.39
Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K., and Schloss, P. D. (2013). Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120. doi: 10.1128/AEM.01043-13
Krumbeck, J. A., Marsteller, N. L., Frese, S. A., Peterson, D. A., Ramer-Tait, A. E., Hutkins, R. W., et al. (2016). Characterization of the ecological role of genes mediating acid resistance in Lactobacillus reuteri during colonization of the gastrointestinal tract. Environ. Microbiol. 18, 2172–2184. doi: 10.1111/1462-2920.13108
Li, P., You, M., Du, Z., Lu, Y., Zuo, C., Zhao, M., et al. (2021a). Effects of fertilization during cultivation and lactobacillus plantarum inoculation at ensiling on chemical composition and bacterial community of mulberry silage. Front. Microbiol. 12:735767. doi: 10.3389/fmicb.2021.735767
Li, P., Zhao, W., Yan, L., Chen, L., Chen, Y., Gou, W., et al. (2021b). Inclusion of abandoned rhubarb stalk enhanced anaerobic fermentation of alfalfa on the Qinghai Tibetan Plateau. Bioresour. Technol. 347:126347. doi: 10.1016/j.biortech.2021.126347
Liu, B., Huan, H., Gu, H., Xu, N., Shen, Q., and Ding, C. (2019). Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 273, 212–219. doi: 10.1016/j.biortech.2018.10.041
Mu, L., Xie, Z., Hu, L., Chen, G., and Zhang, Z. (2021). Lactobacillus plantarum and molasses alter dynamic chemical composition, microbial community, and aerobic stability of mixed (amaranth and rice straw) silage. J. Sci. Food Agric. 101, 5225–5235. doi: 10.1002/jsfa.11171
Ogunade, I. M., Jiang, Y., Kim, D. H., Cervantes, A. A. P., Arriola, K. G., Vyas, D., et al. (2017). Fate of Escherichia coli O157:H7 and bacterial diversity in corn silage contaminated with the pathogen and treated with chemical or microbial additives. J. Dairy Sci. 100, 1780–1794. doi: 10.3168/jds.2016-11745
Ogunade, I. M., Kim, D. H., Jiang, Y., Weinberg, Z. G., Jeong, K. C., and Adesogan, A. T. (2016). Control of Escherichia coli O157:H7 in contaminated alfalfa silage: effects of silage additives. J. Dairy Sci. 99, 4427–4436. doi: 10.3168/jds.2015-10766
Peñailillo, J., Olivares, G., Moncada, X., Payacán, C., Chang, C., Chung, K., et al. (2016). Sex Distribution of Paper Mulberry (Broussonetia papyrifera) in the Pacific. PLoS One 11:e161148. doi: 10.1371/journal.pone.0161148
Queiroz, O. C. M., Arriola, K. G., Daniel, J. L. P., and Adesogan, A. T. (2013). Effects of 8 chemical and bacterial additives on the quality of corn silage. J. Dairy Sci. 96, 5836–5843. doi: 10.3168/jds.2013-6691
Smith, L. H. (1962). Theoretical carbohydrates requirement for alfalfa silage production1. Agron. J. 54, 291–293. doi: 10.2134/agronj1962.00021962005400040003x
Soycan-Önenç, S., Koc, F., Coşkuntuna, L., Özdüven, M. L., and Gümüş, T. (2015). The effect of oregano and cinnamon essential oils on fermentation quality and aerobic stability of field pea silages. Asian Austral. J. Anim. 28, 1281–1287. doi: 10.5713/ajas.15.0122
Sun, W., Huang, Y., Wu, C., Peng, C., Zheng, Y., Chen, C., et al. (2022). Addition of lactic acid bacteria can promote the quality and feeding value of Broussonetia papyrifera (Paper mulberry) silage. Fermentation 8:25. doi: 10.3390/fermentation8010025
Suo, S., Lai, Y., Li, M., Song, Q., Cai, J., Zhao, J., et al. (2018). Phytochemicals, pharmacology, clinical application, patents, and products of Amomi fructus. Food Chem. Toxicol. 119, 31–36. doi: 10.1016/j.fct.2018.05.051
Tang, C., Chen, J., Zhou, Y., Ding, P., He, G., Zhang, L., et al. (2021). Exploring antimicrobial mechanism of essential oil of Amomum villosum Lour through metabolomics based on gas chromatography-mass spectrometry in methicillin-resistant Staphylococcus aureus. Microbiol. Res. 242:126608. doi: 10.1016/j.micres.2020.126608
Tao, H., Si, B., Xu, W., Tu, Y., and Diao, Q. (2020). Effect of Broussonetia papyrifera L. Silage on blood biochemical parameters, growth performance, meat amino acids and fatty acids compositions in beef cattle. Asian Austral. J. Anim. 33, 732–741. doi: 10.5713/ajas.19.0150
Tyrolová, Y., Bartoò, L., and Louèka, R. (2017). Effects of biological and chemical additives on fermentation progress in maize silage. Czech J. Anim. Sci. 62, 306–312. doi: 10.17221/67/2016-CJAS
Van Soest, P. J., Robertson, J. B., and Lewis, B. A. (1991). Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Vousough, A. S., Khomeyri, M., Kashaninezhad, M., and Mahdi, J. S. (2009). Effects of mint extract on the viability of probiotic bacteria in a native Iranian dairy drink (doogh). J. Agric. Sci. Cambridge 16, 156–164.
Wallace, R. J. (2004). Antimicrobial properties of plant secondary metabolites. Proc. Nutr. Soc. 63, 621–629. doi: 10.1079/PNS2004393
Wang, X., Liu, H., Xie, Y., Zhang, Y., Lin, Y., Zheng, Y., et al. (2021). Effect of sucrose and lactic acid bacteria additives on fermentation quality, chemical composition and protein fractions of two typical woody forage silages. Agriculture 11:256. doi: 10.3390/agriculture11030256
Wei, S. N., Li, Y. F., Jeong, E. C., Kim, H. J., and Kim, J. G. (2021). Effects of formic acid and lactic acid bacteria inoculant on main summer crop silages in Korea. J. Anim. Sci. Biotechnol. 63, 91–103. doi: 10.5187/jast.2021.e7
Xu, D., Ding, Z., Wang, M., Bai, J., Ke, W., Zhang, Y., et al. (2020). Characterization of the microbial community, metabolome and biotransformation of phenolic compounds of sainfoin (Onobrychis viciifolia) silage ensiled with or without inoculation of Lactobacillus plantarum. Bioresour. Technol. 316:123910. doi: 10.1016/j.biortech.2020.123910
Yan, Y., Li, X., Guan, H., Huang, L., Ma, X., Peng, Y., et al. (2019). Microbial community and fermentation characteristic of Italian ryegrass silage prepared with corn stover and lactic acid bacteria. Bioresour. Technol. 279, 166–173. doi: 10.1016/j.biortech.2019.01.107
Zhang, Q., Zhao, M., Wang, X. G., Yu, Z., and Na, R. (2017). Ensiling alfalfa with whole crop corn improves the silage quality and in vitro digestibility of the silage mixtures. Grassl. Sci. 63, 211–217. doi: 10.1111/grs.12168
Zhang, S. T., Wang, Z. Y., Wang, T. S., Miao-Xia, L. I., and Kitahara, M. (2011). Composition and antimicrobial activities of essential oil of fructus amomi. Nat. Prod. Res. Dev. 23, 464–472. doi: 10.16333/j.1001-6880.2011.03.017
Zhang, Y. C., Li, D. X., Wang, X. K., Lin, Y. L., Zhang, Q., Chen, X. Y., et al. (2019). Fermentation dynamics and diversity of bacterial community in four typical woody forages. Ann. Microbiol. 69, 233–240. doi: 10.1007/s13213-018-1398-z
Zheng, J., Wittouck, S., Salvetti, E., Franz, C., Harris, H., Mattarelli, P., et al. (2020). A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 70, 2782–2858. doi: 10.1099/ijsem.0.004107
Keywords: Amomum villosum essential oil, paper mulberry, silage, additives, fermentation quality, bacterial community
Citation: Li M, Fan X, Cheng Q, Chen Y, Long J, Lei Y, Li P and Chen C (2022) Effect of Amomum villosum essential oil as an additive on the chemical composition, fermentation quality, and bacterial community of paper mulberry silage. Front. Microbiol. 13:951958. doi: 10.3389/fmicb.2022.951958
Received: 24 May 2022; Accepted: 29 June 2022;
Published: 22 July 2022.
Edited by:
Gang Fan, Huazhong Agricultural University, ChinaReviewed by:
Yimin Cai, Japan International Research Center for Agricultural Sciences (JIRCAS), JapanWei Zhou, South China Agricultural University, China
Copyright © 2022 Li, Fan, Cheng, Chen, Long, Lei, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiming Cheng, NDI5ODQ1ODAxQHFxLmNvbQ==; Chao Chen, Z3pneXhnYzM4NTUyMThAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Maoya Li
Maoya Li Xueying Fan
Xueying Fan Qiming Cheng
Qiming Cheng Yulian Chen1,2
Yulian Chen1,2 Ping Li
Ping Li