
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 29 June 2022
Sec. Microbiotechnology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.932940
This article is part of the Research Topic Biological Nitrogen Removal from Low Carbon Wastewater View all 11 articles
 Xing-Hui Feng1,2
Xing-Hui Feng1,2 Xiao-Jun Wang3
Xiao-Jun Wang3 Hai-Xiang Li1,2
Hai-Xiang Li1,2 Hai-Ya Zhang4
Hai-Ya Zhang4 Zong-Qiang Zhu1,2
Zong-Qiang Zhu1,2 Yan-Peng Liang1,2
Yan-Peng Liang1,2 Kun Dong1
Kun Dong1 Hong-Hu Zeng1*
Hong-Hu Zeng1*Acquisition of stable nitritation and efficient anammox play a crucial role in partial nitritation (PN) combined with anammox for nitrogen removal from ammonium-rich wastewater. Due to the limitation of ammonia-oxidizing bacteria (AOB) enrichment and nitrite-oxidizing bacteria (NOB) control in traditional membrane biological reactor (MBR), it can result in a lower nitrite production rate (NPR) and unstable PN, eventually reducing the nitrogen removal rate (NRR) via PN-anammox. In this study, we developed a zeolite membrane biological reactor (ZMBR) to enhance the PN of iron oxide red wastewater (IORW), in which the biofilm derived from the zeolite surface can provide free ammonia (FA)-containing microenvironment for AOB enrichment and NOB inhibition. The results showed that ZMBR can tolerate a higher influent nitrogen loading rate (NLR) of 2.78 kg/(m3⋅day) in comparison to the traditional MBR [2.02 kg/(m3⋅day)] and the NPR in ZMBR and traditional MBR were 1.39 and 0.96 kg/(m3⋅day), respectively. The mass concentration ratio of -N/-N ranged from 1.05 to 1.33 in ZMBR, suggesting a suitable condition for nitrogen removal via anammox. Subsequently, the domesticated granular sludge obtained from a paper-making wastewater treatment was used as the carrier of anammox bacteria to remove nitrogen. After 93 days of operation, the NRR was observed to be 2.33 kg/(m3⋅day) and high-throughput sequencing indicated that the relatively higher abundance (45.0%) of Candidatus Kuenenia stuttgartiensis was detected in the granular sludge of the bottom part of the reactor, which can produce more proteins and lipids, suggesting a good settleability. Overall, this study provides a high-efficient method to control PN and domesticate anammox for nitrogen removal from IORW.
About 15 tons of iron oxide red wastewater (IORW) occurred per ton of finished product, which contains lots of ammonium (-N), Fe2+, and . The previous study has proved that aeration oxidation and alkaline neutralization are effective solutions for removing Fe2+ from which iron mud can be recycled and used as the inoculating crystal (Feng et al., 2019). However, the remaining -N is at high concentrations (700–1,000 mg/L) in the supernatant. The improper management or direct discharge of IORW could cause serious water eutrophication; accordingly, a low-carbon and low-cost technology for nitrogen removal is a significant requirement. Due to the presence of , the traditional nitrification-denitrification technology might not be an acceptable process for nitrogen removal because sulfate-reducing bacteria (SRB) convert sulfate to H2S, which would lead to serious secondary pollution and inhibition of nitrification.
Recently, the partial nitritation (PN) combined with anaerobic ammonium oxidation (PN-anammox) has been widely used in nitrogen removal system via anammox by using -N as the electron donor and -N as the electron acceptor, and its engineering application, is drawing increasing attention from researchers. The PN-anammox reduces the need for organic carbon and aeration and decreases sludge production compared with traditional nitrification-denitrification (Lackner et al., 2014). Currently, a limiting step in PN-anammox is to obtain stable PN. Generally, ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB) play a crucial role in the nitritation and nitratation process, respectively (Sinha and Annachhatre, 2007; Laureni et al., 2016). Nitrite accumulation depends on nitritation (Anthonisen et al., 1976) and AOB abundance increased, while NOB number decreased at high ammonium loading conditions (Shao et al., 2018). The literature reports numerous indirect methods to maintain effective AOB and inhibit NOB in the same reactor, for instance, controlling free nitrous acid (FNA) (Pedrouso et al., 2017), maintaining a low concentration of dissolved oxygen (DO) in a sequencing batch reactor (SBR) (Li et al., 2011) and regulating free ammonia (FA) in zeolite biological aeration filter (ZBAF). A previous study investigated the PN in two-stage ZBAF by controlling the alkalinity dosage resulting that stable PN being obtained in this combined system (Feng et al., 2019). In addition, high FA induced by Na2CO3 was avoided resulting that Na2CO3 as an alkalinity donor could save about 40% dosage than common NaHCO3. Although ZBAF can be stable and low-cost in the PN from IORW, the low treatment efficiency and frequent backwashing requirement result that it might not be suitable for large-scale implementation. Moreover, the detached biofilm from zeolite would outflow along with the affluent, affecting the substance concentration (nitrite and ammonium). Besides, numerous theoretical and practical applications claim that membrane biological reactors (MBRs) have advantages in higher volumetric loading, better effluent quality, and less sludge loss (Barreto et al., 2017). These advantages from MBRs coupled with zeolite to maintain high-efficiency PN and used Na2CO3 as the alkalinity donor, which should have a wide market foreground and important engineering significance for IORW treatment. However, the premise is to achieve stable PN in a zeolite membrane biological reactor (ZMBR).
Anammox bacteria cultivated by feeding with real wastewater is another key step in nitrogen removal from IORW treatment because it has a high sensitivity to changing environmental conditions, which makes their cultivation extremely difficult (Ni and Meng, 2011). Besides, the growth rate of anammox bacteria is regarded as restrictive to engineering applications (Jin et al., 2012). Nitrogen removal efficiency is the common parameter to reflect anammox activity. The literature reports the different doubling times of anammox bacteria as 10–14 (Marc et al., 2006), 4.8 (Tang et al., 2011), and 1.8 days (Isaka et al., 2006). Different sludge morphologies (e.g., floc and granule) have different adhesive surfaces, which have differences in anammox bacteria enrichment. The characteristics of granular sludge mainly include well-defined shape, high settleability, enhanced microbial activities, and its application in domestic or industrial wastewater treatment (Sarma et al., 2017). Although flocculent sludge has a larger specific surface area that could offer more attachment sites for anammox bacteria than granular sludge, sludge loss would be a challenge in practical application. Moreover, extracellular polymeric substances (EPSs) are usually found in the metabolic activities of microorganisms and look like a gel-like network on the surface of granules and play a crucial role in maintaining their structural stability (Cai et al., 2017). Especially the proteins, lipids, and β-D-mannose are also beneficial to maintaining sludge structure and promoting sludge aggregation (Jia et al., 2017). In other words, EPS might be beneficial for the attachment of microorganisms to the sludge. Granular sludge is often considered a typical solid waste that needs reasonable disposal in papermaking plants. Because of this, using granular sludge as the carrier of anammox bacteria is also a potential resource utilization method, which still needs experimental verification.
In this study, the PN of IORW was conducted in ZMBR, followed by using granular sludge-based anammox for further nitrogen removal. The objective of this study was to: (1) obtain controllable PN of IORW in ZMBR and evaluate the biofilm formation on the surface of zeolite; (2) recycle granular sludge from the papermaking wastewater treatment process for the carrier of anammox bacteria; (3) investigate the living environment of anammox bacteria through EPS (proteins, β-D-mannose, and lipid) detection by using confocal laser scanning microscopy (CLSM); and (4) use ZMBR combined with granular sludge-based anammox for the nitrogen removal from IORW. The obtained result might provide a high-efficiency and cost-effective strategy for IORW treatment.
The ZMBR was designed as a rectangular vessel made of stalinite with a total volume of 10 L (working volume was 8 L) and the surface area of microfiltration polymeric membranes was 2 m2 (the ratio of the addition of membrane to the working volume of the reactor was 0.25 m2/L). Natural zeolite was filled as the -N adsorbents that the average particle size was 0.5–1 mm (diameter) and the filling rate was ranged from 2 to 5% of the reactor volume. Based on upflow anaerobic sludge blanket (UASB) and anammox reactors, they were made of transparent acrylic plates of 1,000 mm in height and 80 mm in diameter. The total volume was 5 L and the sludge volume was about 1.5 L. The schematic diagram of the PN-anammox process is shown in Figure 1.
Test wastewater was taken from the pretreatment process of an iron oxide red plant (Jiangmen, China) which has completed Fe2+ removal under the condition of aeration and added NaOH then static settlement. The concentration of -N was between 700 and 1,000 mg/L and was in the range of 8,000–10,000 mg/L in the supernatant.
The seed sludge of nitritation was obtained from a refuse landfill (Guangzhou, China) and using clean water washed several times then cultivated under the laboratory condition (feed with alkalinity and -N). The granular sludge came from the sewage treatment process of a papermaking plant (Liuzhou, China). The seed sludge of anammox was taken from an anammox reactor that was fed with synthetic water in a laboratory and the dominant anammox bacteria were Candidatus Brocadia sinica and Candidatus Kuenenia stuttgartiensis.
The ZMBR startup was divided into three phases: Phase I, -N was enriched in the zeolite via adsorption; phase II, added 2 L seed sludge to activate PN [the mixed liquid suspended solids (MLSSs) was about 3,000 mg/L]; and phase III, gradually increase the influent -N concentration or shortening the hydraulic retention time (HRT) to increase influent nitrogen loading rate (NLR). Using an air pump to maintain the DO concentration in the range of 4–6 mg/L and the operating temperature was 28 ± 2°C in the ZMBR.
Using MgSO4 (0.1 mg/L), KH2PO4 (0.05 mg/L) to provide essential microelement and Na2CO3 as alkalinity donor. According to the previous study, alkalinity/-N = 4.33 (mass concentration ratio) was an optimum ratio to obtain -N/-N = 1.0–1.4 that is suitable for further treatment via anammox (Feng et al., 2019). The concentrations of -N, -N, and -N were detected in every 12 h to assess the nitritation efficiency and FA (Anthonisen et al., 1976), nitrite production rate (NPR), NLR, and nitrogen removal rate (NRR) were calculated by the following equations:
Anammox reactor was started up by inoculating with anammox sludge, increasing the influent substrate concentration (-N and -N), and put on a heating jacket to maintain the temperature between 28 and 32°C.
Absolute quantification-PCR (AQ-PCR) technology was based on the known copy number variation (CNV) from the standard sample to obtain a standard curve. According to the cycle threshold (Ct) value, the absolute quantification of CNV from messenger RNA (mRNA) or DNA could be calculated according to the standard curve (Walker, 2001). Because of the relatively low abundance in NOB, it results that it was usually not detected by the common high-throughput sequencing technology. Therefore, PCR was employed to detect the relative ratio of AOB and NOB, which coexistence on the biofilm of zeolite.
Ct refers to the cycle index when the fluorescence signal of the amplified product reaches the set threshold value and K was the slope and b as the y-intercept of standard curve.
This detection was supported by the Shanghai Sunny Biotechnology Corporation Ltd. The related primer information of AOB and NOB is given in Table 1. The real-time PCR samples were carried out by using 20 μl reaction volumes that contained 10 μl mixture A, which consist of 10 μl 2 × SYBR real-time PCR premixture and 0.4 μl from primer-F and -R (10 μM, the primer information from AOB and NOB was found in Table 1), 10 μl DNA diluent as template (using DNA extraction kit obtained 1.61 μg DNA in 50 μl solution, cat: DP305, TianGen Biotechnology Corporation Ltd., Beijing, China). The thermal cycling was performed under the following conditions: 95°C for 5 min, followed by 40 cycles of 95°C for 15 s, and a final extension at 60°C for 30 s. All the operations, primers, and analysis results were provided by Sunny Biotechnology Corporation Ltd. (Shanghai, China).
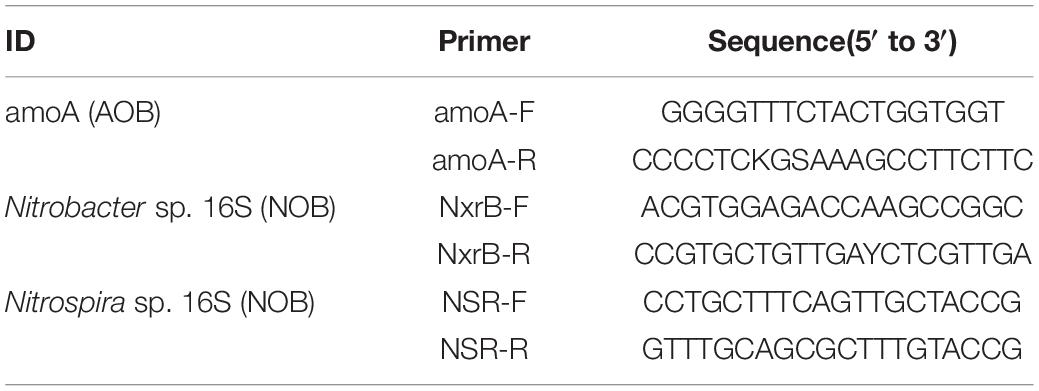
Table 1. The related primer information of ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB).
The samples from the anammox reactor were pretreated by staining and then observed by CLSM to detect the EPS (proteins, β-D-mannose, and lipid). The procedures of staining were adopted from a reference (Wu et al., 2018) and with a little modification that is described as follows: (1) Fluorescein isothiocyanate (FITC) (CAS No. 27072-45-3, Macklin, Shanghai, China) was applied to stain proteins; (2) Calcofluor white (CalW) (CAS No. 4404-43-47, Sigma-Aldrich Corporation, California, CA, United States) for β-D-mannose detection; and (3) Nile Red (CAS No. 7385-67-3, Macklin, Shanghai, China) was used to stain lipids.
The detailed staining steps were followed by adding a drop of FITC (10 mg/L, dimethyl sulfoxide as solvent) to the samples standing for 30 min and washing for three times by using phosphate-buffered saline (PBS) (0.01 M, pH = 7.4). Then, using CalW solution combined with 10% KOH (1:1) stained 1 min and washed with PBS several times. Next, put the samples in Nile red solution (5 mg/L, methanol as solvent) to stain for 5 min. Finally, a drop of the antifade mounting medium was added to the test samples. After staining, using PBS washing then put on the glass slide and observed by CLSM (Leica TCS SP8, Germany) and amplified about 4–10 times. The measuring parameter and observable color from different staining conditions are given in Table 2.
Based on the standard examination of water and wastewater (Walter, 1961), the concentrations of ammonium, nitrite, and nitrate were periodically detected to evaluate the nitrogen removal performance and an automatic potentiometric titration was applied for the alkalinity measurement (based on CaCO3 calculation, mg/L). A multifunctional portable instrument (HQ30d, HACH) was employed for the detection of temperature and DO. A pH meter (PHS-3C) was used to obtain the pH value. Using high-throughput sequencing technology, it investigates the bacterial community structure in sludge samples [this part was supported by The Beijing Genomics Institute (BGI) Corporation Ltd.].
Partial nitritation was maintained in ZMBR by gradually increasing the influent ammonium concentration and shortening HRT. As shown in Figure 2, ZMBR exhibited excellent nitritation efficiency and obtained 2.78 kg/(m3⋅day) in NLR and 1.40 kg/(m3⋅day) in NPR by influent -N of 803 mg/L, while the MBR without zeolite achieved 2.02 kg/(m3⋅day) (NLR) and NPR was 0.96 kg/(m3⋅day). As compared to a previous study, it showed that the maximum influent NLR from one-stage ZBAF was 1.20 kg/(m3⋅day) and obtained 0.76 kg/(m3⋅day) in NPR by influent 550 mg/L -N from synthetic wastewater (Feng et al., 2019). The PN performed in two-stage ZBAF achieved the NPR up to 0.67 kg/(m3⋅day) by influent 1.46 kg/(m3⋅day) NLR at 752 mg/L -N from IORW (Feng et al., 2019). Dramatically, the PN in non-zeolite MBR would be seriously inhibited, if NLR was up to 2.14 kg/(m3⋅day) then nitritation was gradually inhibited and FA increase to 109.0 mg/L. However, it would not be inhibited until the NLR is high to 3.01 kg/(m3⋅day) in ZMBR and the corresponding FA was 88.5 mg/L. Due to the higher NLR maintained in ZMR, more DO was consumed to support high-efficient nitritation, which resulted that DO concentration in ZMBR (4–6 mg/L) being higher than ZBAF (3–6 mg/L). Moreover, PN in ZMBR can obtain the range of -N/-N ratio was 1.07–1.33, which would provide the necessary influent substrate ratio for nitrogen removal via anammox. Stable PN is maintained in ZMBR, which can support nitrogen removal from high ammonium wastewater and profit by the low carbon and energy-saving of anammox.
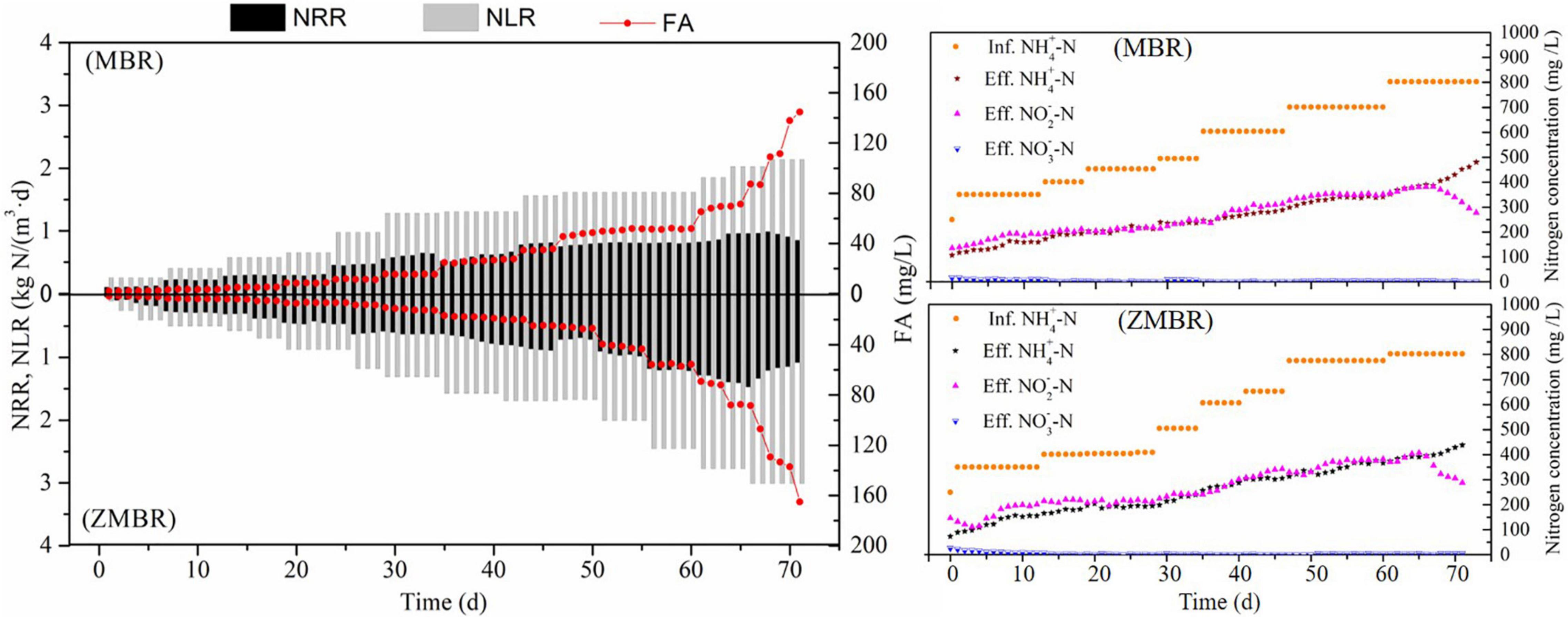
Figure 2. Evaluation of the PN, free ammonia (FA), and the nitrogen concentrations in MBR and zeolite membrane biological reactor (ZMBR).
In the PN phase, zeolite and biofilm played the role of -N adsorption-desorption and N biochemical conversion, respectively, which can be a key factor in maintaining high-efficiency PN. Due to zeolite, a special adsorptivity to ammonium and the FA concentration in liquid were lower than that on the surface of zeolite; therefore, AOB would easily grow on the biofilm than in suspended sludge. The biofilm on the surface of zeolite formed by different microorganisms and the dominant bacterial community (AOB) might be valid evidence to hold the efficient nitritation. According to the amplification and copy numbers in the biofilm from the surface of zeolite (Table 3), NOB can coexist with AOB on the biofilm and more copy numbers were obtained from AOB than from NOB. Since the FA inhibited NOB and AOB kept working, the ZMBR profited from the special existence of zeolite that supported a higher influent loading than the MBR.
Anammox bacteria are belonging to branching from Planctomycetes (Kuenen, 2008). At present, Candidatus Brocadia sinica, Ca. K. stuttgartiensis, Candidatus Jettenia asiatica, Candidatus Scalindua wagneri, and Candidatus Anammoxoglobus propionicus had been identified as the effective bacteria in anammox process (Hu et al., 2011; Mamoru et al., 2011). Microbial analysis at different taxonomy levels provides a further understanding of bacterial community function. Ca. K. stuttgartiensis can rapidly enrich in the IORW treatment process and dominate in granular sludge. The literature reports that Ca. K. stuttgartiensis has advantages in adapting to the environment of the nitrite-limiting environment (Park et al., 2015), while the other four prefer to grow in the substrate-rich anammox-based process (Gonzalezgil et al., 2015). Besides, sludge structure, inoculum, and organic matter might be other potential factors that affect bacterial community structure (Date et al., 2009). IORW is typical industrial wastewater and the possible affecting factors to anammox still need deep analysis. Generally, anammox was started by using seed sludge to shorten the startup time and rapidly increased treatment efficiency. In this study, granular sludge was obtained from a papermaking wastewater treatment process, which was applied as the microbial carrier and seeded with anammox sludge to further remove ammonium and nitrite. In the first 47 days, influent TN concentration was 226–450 mg/L (the -N/-N ratio was in the range of 1.07–1.33) and effective nitrogen removal was achieved. As shown in Figure 3, the granular sludge-based anammox achieved stable nitrogen removal efficiency after 93 days’ operation by influent IORW and the corresponding NRR was 2.33 kg/(m3⋅day). The literature reports that overloads lead to system instability and the anammox process would be seriously inhibited when the nitrite concentration is higher than 280 mg/L (Trigo et al., 2006; Isaka et al., 2007).
The granular sludge was used as a carrier of anammox bacteria in the further nitrogen removal process, which was obtained from a papermaking wastewater treatment process. According to the high-throughput sequencing detection, 632 operational taxonomic units (OTUs) and 1,093 OTUs were obtained from seed sludge samples and cultivating sludge in 90-day samples, respectively. Based on the classification at different taxonomy levels, the bacterial community structure from floc particle (settle at the bottom of the reactor), float sludge (floating on the surface of the liquid), and bottom sludge (accumulate at the bottom of the reactor) were similar, while the relative abundance exhibited significant difference when achieved stable anammox. As shown in Figure 4, the four most abundant bacteria at the phylum level were Bacteroidetes (28.5%), Proteobacteria (12.6%), Chloroflexi (8.9%), and Chlorobi (7.2%) in seed sludge sample, while it changed to Planctomycetes, Proteobacteria, and Bacteroidetes in the samples from floc particle, float sludge, and bottom sludge after 90 days of operation. Compared to the seed sludge, an obvious increase can be seen in Planctomycetes and Proteobacteria, which proved that anammox bacteria gradually increase on the granular sludge.
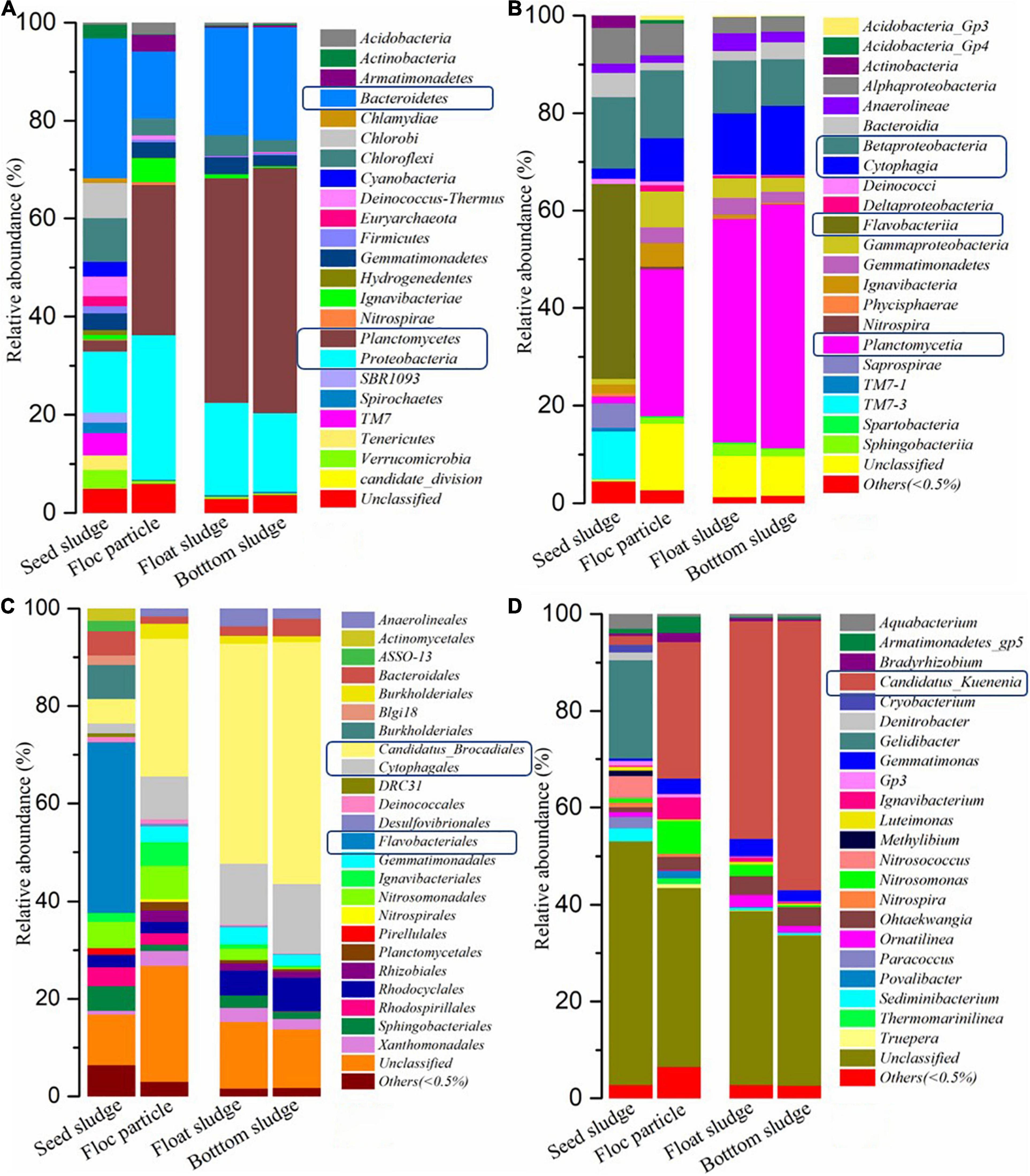
Figure 4. Microbial community structure and relative abundance in different sludge samples. Taxonomy in phylum (A), class (B), order (C), and genus (D).
The granular sludge from the bottom has the most abundant Ca. K. stuttgartiensis (56.6%), followed by fine particle granular sludge (45.6%) and the last was float sludge (45.1%). The influent substances were from the bottom to the top (UASB) and the bottom sludge preferential using the substances than the floating sludge, which might be the reason for the different content of anammox bacteria. The reference reported that Ca. B. sinica and Ca. K. stuttgartiensis are usually dominant in large granules (greater than 400 μm in diameter) and Ca. J. asiatica was enriched in fine particles (less than 200 μm in diameter) that were cultivated with synthetic water (Liu et al., 2017). However, this study showed obvious differences compared to the reference because of different wastewater treatment and inoculation. In addition, the possible hydraulic and pneumatic shock cause channeling phenomenon with the result that substances maldistribution. This might easily lead to the different microenvironments with a high concentration of substances or severe shortage of substances concurrence. The other literature reports that the anammox activity would be completely lost when the anammox biomass aggregates exposure to a -N solution of 100 mg/L (Van De Graaf et al., 1995) and the other results show that the -N concentration of 40 mg/L over several days would lead to an irreversible inactivation (Hulle et al., 2010). This study believed that the tolerance to -N concentration depends on the relative abundance of anammox bacteria. Moreover, substance distribution was another important factor in anammox bacteria enrichment.
Zeolite has a special characteristic of absorption-desorption to -N, which should be the reason for the better performance than the process without zeolite. Besides, this kind of particularity can avoid -N substantial increase or decrease. Therefore, the ZMBR exhibited higher nitritation efficiency than the MBR by the same influent NLR, which might benefit from the stable concentration of -N and FA. As shown in Figure 5, zeolite was an efficient carrier that could maintain large areas of biofilm and lots of microorganisms attached to the biofilm and the biofilm formation on the zeolite might reduce a large amount of biofilm attached to the membranes. Moreover, the friction effect of the interfacial concrete from zeolite and membrane under aeration conditions might be another factor affecting a large amount of biofilm formation on the membrane. According to the above, the ZMBR obtained higher treatment efficiency than the MBR in the PN of IORW. Compared to the PN in a one-stage or two-stage ZBAF and SBR, the ZMBR has higher treatment efficiency and effluent quality. In addition, a previous study reported that using Na2CO3 as the alkalinity donor for PN could save about 40% dosage than common NaHCO3, while it would bring higher FA and cause inhibition to PN (Feng et al., 2019). Thus, cost efficiency and high efficiency were achieved in the practical application. In this study, the ZMBR could maintain higher efficiency for PN than the MBR or ZBAF by using Na2CO3 as the alkalinity donor. Besides, the most practical merit of the ZMBR is its rapid startup, stable operation, and tolerance to high influent NLR, which provides a controllable and highly efficient method for the PN of IORW.
The granular sludge profited from better sludge settleability than flocculent sludge, which could achieve greater treatment efficiency. Moreover, the relative abundance of ammonium bacteria was directly affected by the nitrogen removal and the living environment of sludge might also be an important factor. Meanwhile, the different microbial structures would bring different excreta that might have positive or negative effects on the surface of sludge. This still needs further verification. Since granular sludge has different spatial distribution in the reactor resulting that a part of granular sludge float on the liquid and another part and floc sludge at the bottom of the reactor. This study disagrees with the opinion of dosing Fe2+ can suppress the flotation problem (Wang et al., 2018) because the residual concentration of Fe2+ in IORW is still at 15 ± 5 mg/L and believed that the continuous minute bubbles from anammox might be the main reason for the phenomenon of sludge floating. In addition, another study found that using vibration technology is an effective and quick method to form settleable granules (Zhang et al., 2019). The floc particle sludge should be formed from granular collision or decomposition. The efficient anammox consisted of these three different spatial distribution granular sludge and microorganism communities presenting different abundances.
Using CLSM observation technology can simultaneously exhibit the distribution and the relative abundance of different EPSs on the granular sludge. As shown in Figure 6, the fluorescence intensity indicated that proteins, lipids, and β-D-mannose were erratically distributed on the granular sludge and the proteins were patchily distributed on the granular sludge (the green color). Weaker fluorescence was found in lipid and β-D-mannose detection compared to the proteins. The EPS distribution revealed that the higher relative abundance of Ca. K. stuttgartiensis on granular sludge the better sludge settleability was maintained. Besides, EPSs might play a crucial role during fast anammox bacteria enrichment, which was different from the granular sludge with a low abundance of anammox bacteria (Ni et al., 2015). In addition, the aggregation property from EPS was usually applied to support the anammox bacteria preferably attached to the surface of sludge (Jia et al., 2017). Recent studies reveal that EPSs can enhance the NRR (Liu et al., 2018). EPS might contribute to the anammox bacteria attached to the surface of sludge. As a common carrier, granular sludge provides a suitable environment for anammox bacteria and is beneficial to the nitrogen removal of IORW. According to the microscopic image, a small cavity was common in float sludge in the cultivation phase, while the granular sludge at the bottom of the reactor was a solid sphere. The potential explanation was that ammonium and nitrite were simultaneously removed by anammox bacteria, which would produce a lot of gas on the surface of granular sludge, which can destroy the surface structure. Therefore, floc particles were released from the inside of the granular sludge, which might possible formatted new granular sludge because of the adhesion from abundant EPSs.
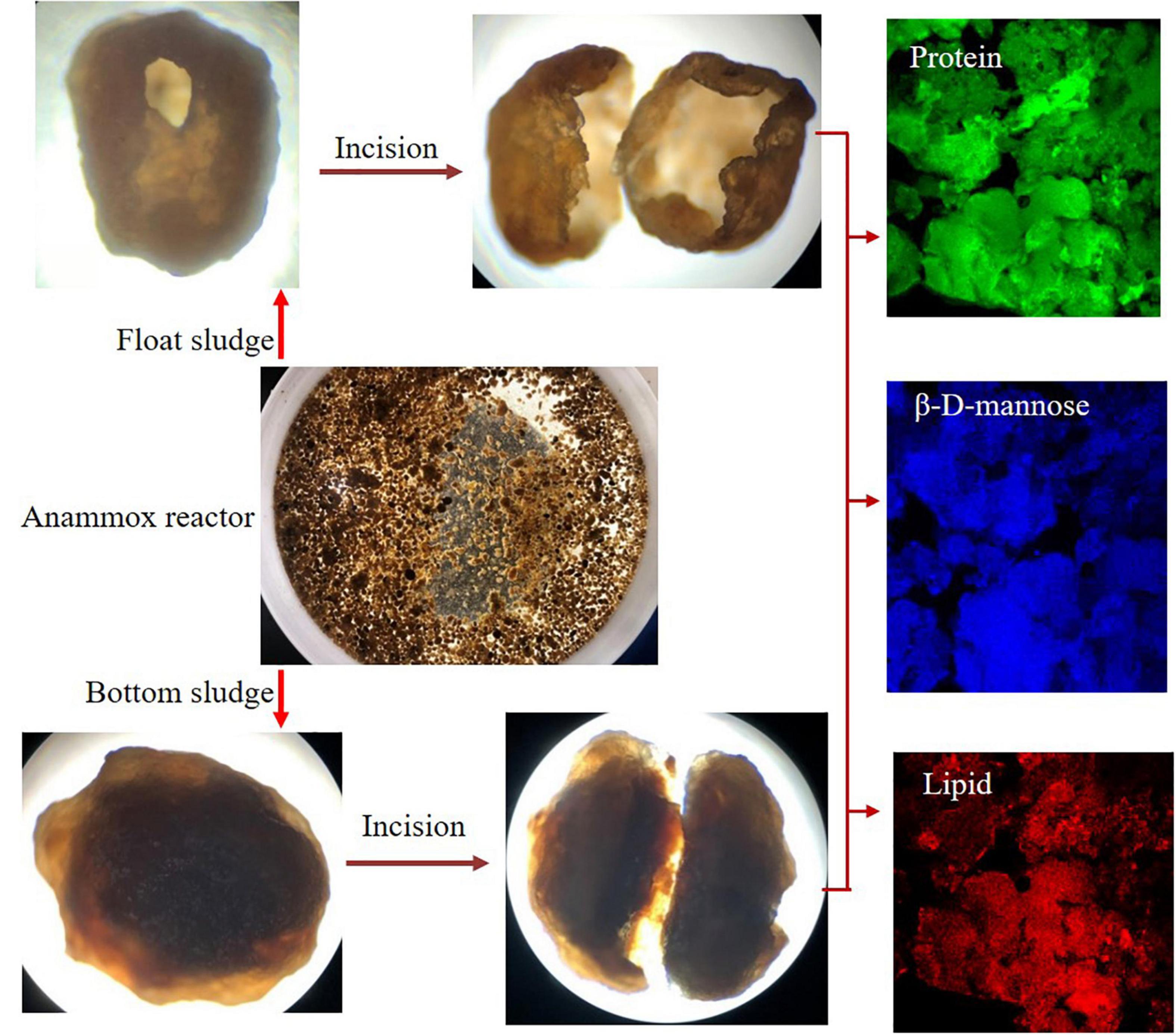
Figure 6. Morphology structure and extracellular polymeric substance (EPS) detection of granular sludges.
The stable PN was maintained in a ZMBR and obtained 2.78 kg/(m3⋅day) in NLR and 1.40 kg/(m3⋅day) in NPR by influent IORW with -N concentration at 803 mg/L treatment. Granular sludge was taken from a papermaking wastewater treatment process can be used as a bacterial carrier and Ca. K. stuttgartiensis gradually dominant on the sludge. Moreover, the morphology structure and EPSs distribution on granular sludge were investigated resulting that anammox bacteria activities and floc particles released from the inside of the granular sludge being an important reason for sludge floating. Based on the recycle utilization of granular sludge in anammox combined with ZMBR (PN-anammox) can obtain NRR was 2.33 kg/(m3⋅day). Because of low-cost and high-efficient, this combined process would have great potential for IORW treatment and larger-scale engineering implementation.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
X-HF, X-JW, and H-HZ: conceptualization and methodology. X-HF, KD, and Z-QZ: project administration and data curation. X-HF and KD: original draft. X-HF and H-YZ: review and editing. X-HF, Y-PL, and H-XL: supervision. All authors have contributed to the article and approved the submitted version of the manuscript.
This study was supported by the following research funds: the Guangxi Science and Technology Project (AD21220117), the Guangxi Natural Science Foundation (2022GXNSFFA035033), and the Guangxi Key Laboratory of Theory and Technology for Environmental Pollution Control (2001K008).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anthonisen, A. C., Loehr, R. C., Prakasam, T. B., and Srinath, E. G. (1976). Inhibition of nitrification by ammonia and nitrous acid. J. Water Pollut. Control Federat. 48, 835–852.
Barreto, C., Garcia, H. A., Hooijmans, C. M., Herrera, A., and Brdjanovic, D. (2017). Assessing the performance of an MBR operated at high biomass concentrations. Int. Biodeterior. Biodegrad. 119, 528–537.
Cai, X., Zhang, M., Yang, L., Lin, H., Wu, X., He, Y., et al. (2017). Quantification of interfacial interactions between a rough sludge floc and membrane surface in a membrane bioreactor. J. Colloid. Interf. Sci. 490, 710–718. doi: 10.1016/j.jcis.2016.12.005
Date, Y., Isaka, K., Ikuta, H., Sumino, T., Kaneko, N., Yoshie, S., et al. (2009). Microbial diversity of anammox bacteria enriched from different types of seed sludge in an anaerobic continuous-feeding cultivation reactor. J. Biosci. Bioeng. 107, 281–286. doi: 10.1016/j.jbiosc.2008.11.015
Feng, X., Wang, X., Chen, Z., and Chen, J. (2019). Nitrogen removal from iron oxide red wastewater via partial nitritation-Anammox based on two-stage zeolite biological aerated filter. Bioresour. Technol. 279, 17–24. doi: 10.1016/j.biortech.2019.01.113
Gonzalezgil, G., Sougrat, R., Behzad, A. R., Lens, P. N. L., and Saikaly, P. E. (2015). Microbial community composition and ultrastructure of granules from a full-scale anammox reactor. Microb. Ecol. 70, 118–131. doi: 10.1007/s00248-014-0546-7
Hu, B. L., Shen, L. D., Xu, X. Y., and Zheng, P. (2011). Anaerobic ammonium oxidation (anammox) in different natural ecosystems. Biochem. Soc. T. 39, 1811–1816. doi: 10.1042/BST20110711
Hulle, S. W. H. V., Vandeweyer, H. J. P., Meesschaert, B. D., Vanrolleghem, P. A., Dejans, P., and Dumoulin, A. (2010). Engineering aspects and practical application of autotrophic nitrogen removal from nitrogen rich streams. Chem. Eng. J. 162, 1–20.
Isaka, K., Date, Y., Sumino, T., Yoshie, S., and Tsuneda, S. (2006). Growth characteristic of anaerobic ammonium-oxidizing bacteria in an anaerobic biological filtrated reactor. Appl. Microbiol. Biotechnol. 70, 47–52. doi: 10.1007/s00253-005-0046-2
Isaka, K., Sumino, T., and Tsuneda, S. (2007). High nitrogen removal performance at moderately low temperature utilizing anaerobic ammonium oxidation reactions. J. Biosci. Bioeng. 103, 486–490. doi: 10.1263/jbb.103.486
Jia, F., Yang, Q., Liu, X., Li, X., Li, B., Zhang, L., et al. (2017). Stratification of Extracellular Polymeric Substances (EPS) for Aggregated Anammox Microorganisms. Environ. Sci. Technol. 51, 3260–3268. doi: 10.1021/acs.est.6b05761
Jin, R. C., Yang, G. F., Yu, J. J., and Zheng, P. (2012). The inhibition of the Anammox process: a review. Chem. Eng. J. 197, 67–79.
Kuenen, J. G. (2008). Anammox bacteria: from discovery to application. Nat. Rev. Microbiol. 6, 320–326. doi: 10.1038/nrmicro1857
Lackner, S., Gilbert, E. M., Vlaeminck, S. E., Joss, A., Horn, H., and Loosdrecht, M. C. M. V. (2014). Full-scale partial nitritation/anammox experiences – An application survey. Water Res. 55, 292–303. doi: 10.1016/j.watres.2014.02.032
Laureni, M., Falas, P., Robin, O., Wick, A., Weissbrodt, D. G., Nielsen, J. L., et al. (2016). Mainstream partial nitritation and anammox: long-term process stability and effluent quality at low temperatures. Water Res. 101, 628–639. doi: 10.1016/j.watres.2016.05.005
Li, J., Elliott, D., Nielsen, M., Healy, M. G., and Zhan, X. (2011). Long-term partial nitrification in an intermittently aerated sequencing batch reactor (SBR) treating ammonium-rich wastewater under controlled oxygen-limited conditions. Biochem. Eng. J. 55, 215–222.
Liu, W., Yang, D., Chen, W., and Xiao, G. (2017). High-throughput sequencing-based microbial characterization of size fractionated biomass in an anoxic anammox reactor for low-strength wastewater at low temperatures. Bioresour. Technol. 231, 45–52. doi: 10.1016/j.biortech.2017.01.050
Liu, Y., Guo, J., Lian, J., Chen, Z., Li, Y., Xing, Y., et al. (2018). Effects of extracellular polymeric substances (EPS) and N-acyl-L-homoserine lactones (AHLs) on the activity of anammox biomass. Int. Biodeterior. Biodegrad. 129, 141–147.
Mamoru, O., Masaki, S., Naoki, F., Hisashi, S., and Satoshi, O. (2011). Physiological characteristics of the anaerobic ammonium-oxidizing bacterium ‘Candidatus Brocadia sinica’. Microbiology 157, 1706–1713. doi: 10.1099/mic.0.048595-0
Marc, S., Eric, P., Sophie, M., Thomas, R., Angelika, L., Taylor, M. W., et al. (2006). Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440, 790–794. doi: 10.1038/nature04647
Ni, S., and Meng, J. (2011). Performance and inhibition recovery of anammox reactors seeded with different types of sludge. Water Sci. Technol. 63, 710–718. doi: 10.2166/wst.2011.293
Ni, S., Sun, N., Yang, H., Zhang, J., and Ngo, H. H. (2015). Distribution of extracellular polymeric substances in anammox granules and their important roles during anammox granulation. Biochem. Eng. J. 101, 126–133.
Park, H., Sundar, S., Ma, Y., and Chandran, K. (2015). Differentiation in the microbial ecology and activity of suspended and attached bacteria in a nitritation-anammox process. Biotechnol. Bioeng. 112, 272–279. doi: 10.1002/bit.25354
Pedrouso, A., Río, Á. V. D., Morales, N., Vázquez-Padín, J. R., Campos, J. L., Méndez, R., et al. (2017). Nitrite oxidizing bacteria suppression based on in-situ free nitrous acid production at mainstream conditions. Sep. Purif. Technol. 186, 55–62.
Sarma, S. J., Tay, J., and Chu, A. (2017). Finding Knowledge Gaps in Aerobic Granulation Technology. Trends Biotechnol. 35, 66–78. doi: 10.1016/j.tibtech.2016.07.003
Shao, Y., Yang, S., Mohammed, A., and Liu, Y. (2018). Impacts of ammonium loading on nitritation stability and microbial community dynamics in the integrated fixed-film activated sludge sequencing batch reactor (IFAS-SBR). Int. Biodeterior. Biodegrad. 133, 63–69.
Sinha, B., and Annachhatre, A. P. (2007). Partial nitrification—operational parameters and microorganisms involved. Rev. Environ. Sci. Biotechnol. 6, 285–313.
Tang, C. J., Zheng, P., Wang, C. H., Mahmood, Q., Zhang, J. Q., Chen, X. G., et al. (2011). Performance of high-loaded ANAMMOX UASB reactors containing granular sludge. Water Res. 45, 135–144. doi: 10.1016/j.watres.2010.08.018
Trigo, C., Campos, J. L., Garrido, J. M., and Mendez, R. (2006). Start-up of the Anammox process in a membrane bioreactor. J. Biotechnol. 126, 475–487.
Van De Graaf, A. A., Mulder, A., Bruijn, P. D., Jetten, M. S., Robertson, L. A., and Kuenen, J. G. (1995). Anaerobic oxidation of ammonium is a biologically mediated process. Appl. Environ. Microbiol. 61, 1246–1251. doi: 10.1128/aem.61.4.1246-1251.1995
Walker, N. J. (2001). Real-time and quantitative PCR: applications to mechanism-based toxicology. J. Biochem. Mol. Toxic. 15, 121–127. doi: 10.1002/jbt.8
Walter, W. G. (1961). Apha standard methods for the examination of water and wastewater. Am. J. Public Health 51:940. doi: 10.2105/AJPH.51.6.940-a
Wang, B., Wu, D., Zhang, X., Mackey, H. R., and Chen, G. (2018). Sludge flotation, its causes and control in granular sludge upflow reactors. Appl. Microbiol. Biotechnol. 102, 6383–6392. doi: 10.1007/s00253-018-9131-1
Wu, L., Tang, B., Bin, L., Chen, G., Huang, S., Li, P., et al. (2018). Heterogeneity of the diverse aerobic sludge granules self-cultivated in a membrane bioreactor with enhanced internal circulation. Bioresour. Technol. 263, 297–305. doi: 10.1016/j.biortech.2018.05.004
Keywords: nitrogen removal, PN-anammox, granular sludge, IORW, alkalinity
Citation: Feng X-H, Wang X-J, Li H-X, Zhang H-Y, Zhu Z-Q, Liang Y-P, Dong K and Zeng H-H (2022) Integration of Zeolite Membrane Bioreactor With Granular Sludge-Based Anammox in High-Efficiency Nitrogen Removal From Iron Oxide Red Wastewater. Front. Microbiol. 13:932940. doi: 10.3389/fmicb.2022.932940
Received: 30 April 2022; Accepted: 23 May 2022;
Published: 29 June 2022.
Edited by:
Chongjun Chen, Suzhou University of Science and Technology, ChinaReviewed by:
Da Kang, Beijing University of Technology, ChinaCopyright © 2022 Feng, Wang, Li, Zhang, Zhu, Liang, Dong and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Hu Zeng, emVuZ2hvbmdodUBnbHV0LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.