
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 13 October 2022
Sec. Microorganisms in Vertebrate Digestive Systems
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.932294
This article is part of the Research TopicRadiotherapy and Commensal MicrobiomeView all 7 articles
 Jing-Yu Yang1†
Jing-Yu Yang1† Meng-Jie Liu1†
Meng-Jie Liu1† Lin Lv2†
Lin Lv2† Jin-Rong Guo1
Jin-Rong Guo1 Kai-Yue He1
Kai-Yue He1 Hong Zhang1
Hong Zhang1 Ke-Ke Wang3
Ke-Ke Wang3 Cui-Yun Cui4
Cui-Yun Cui4 Bei-Zhan Yan4
Bei-Zhan Yan4 Dan-Dan Du5
Dan-Dan Du5 Jin-Hua Wang3
Jin-Hua Wang3 Qiang Ding6
Qiang Ding6 Guo-Long Liu2*
Guo-Long Liu2* Zhi-Xiang Xu1,6*
Zhi-Xiang Xu1,6* Yong-Ping Jian1*
Yong-Ping Jian1*Abdominal irradiation (IR) destroys the intestinal mucosal barrier, leading to severe intestinal infection. There is an urgent need to find safe and effective treatments to reduce IR-induced intestinal injury. In this study, we reported that metformin protected mice from abdominal IR-induced intestinal injury by improving the composition and diversity of intestinal flora. The elimination of intestinal microbiota (Abx) abrogated the protective effects of metformin on irradiated mice. We further characterized that treatment of metformin increased the murine intestinal abundance of Lactobacillus, which mediated the radioprotective effect. The administration of Lactobacillus or fecal microbiota transplantation (FMT) into Abx mice considerably lessened IR-induced intestinal damage and restored the radioprotective function of metformin in Abx mice. In addition, applying the murine intestinal organoid model, we demonstrated that IR inhibited the formation of intestinal organoids, and metformin alone bore no protective effect on organoids after IR. However, a combination of metformin and Lactobacillus or Lactobacillus alone displayed a strong radioprotection on the organoid formation. We demonstrated that metformin/Lactobacillus activated the farnesoid X receptor (FXR) signaling in intestinal epithelial cells and hence upregulated tight junction proteins and mucins in intestinal epithelia, increased the number of goblet cells, and augmented the mucus layer thickness to maintain the integrity of intestinal epithelial barrier, which eventually contributed to reduced radiation intestinal injury. In addition, we found that Lactobacillus abundance was significantly increased in the intestine of patients receiving metformin while undergoing abdominal radiotherapy and the abundance was negatively correlated with the diarrhea duration of patients. In conclusion, our results demonstrate that metformin possesses a protective effect on IR-induced intestinal injury by upregulating the abundance of Lactobacillus in the intestine.
Radiotherapy is an important adjuvant treatment for abdominal tumors (Miller et al., 2019). Intestinal radiotoxicity is a major limitation to the application of radiotherapy in abdominal tumors although strategies for the therapy have been greatly improved (Fransson and Widmark, 2007; De Ruysscher et al., 2019). Irradiation (IR)-induced intestinal injury destroys the intestinal mucosal barrier (Andreyev, 2005; Hauer-Jensen et al., 2014), leading to severe intestinal infection (Chaves-Pérez et al., 2019; Jian et al., 2021). Therefore, there is an urgent need to find safe and effective treatments to reduce IR-induced intestinal injury (Hauer-Jensen et al., 2014).
Human gastrointestinal microbiota, also known as gut microbiota, comprises microorganisms that live in the human digestive tract. It plays a critical role in the maintenance of nutrient absorption, metabolism, and immune function (McKenzie et al., 2017). It was reported that intestinal flora protects germ-free mice from radiation-induced intestinal damage and death (Guo et al., 2020), suggesting that intestinal dysbiosis plays a crucial role in the pathogenesis of IR-induced intestinal injury. Gerassy-Vainberg et al. (2018) reported rectal radiation-induced dysbiosis, hence increasing intestinal inflammation susceptibility. However, it remains unclear whether alterations in gut microbiota affect the occurrence and outcome of radiation-induced intestinal damage. Therefore, elucidating the mechanism of interaction between the microbiome and the host may provide potential therapeutic benefits for patients with radiation-induced intestinal injury.
We previously found that Lactobacillus activates FXR–FGF15 signaling in intestinal epithelial cells and hence promotes DNA damage repair (Jian et al., 2022). FXR is a bile acid-responsive transcriptional regulator for liver bile metabolism. The expression and function of FXR in the intestine (especially the ileum), immune cells, and endothelial tissues have recently been characterized (Gadaleta et al., 2011; Verbeke et al., 2014). FXR knockout mice not only show obvious liver inflammation and fibrosis but also develop an inflammatory bowel disease-like phenotype, with increased intestinal inflammation and permeability (Wang et al., 2008). FXR activation promotes the expression of tight junction proteins to improve the ileal barrier function (Verbeke et al., 2015), demonstrating a crucially protective role of FXR in the intestine.
Metformin, a first-line agent for the treatment of type 2 diabetes, improves upper small intestine microbiota composition and restores the sodium-glucose cotransporter-1 (SGLT1)-dependent glucose sensing to regulate glucose homeostasis (Wu et al., 2017; Bauer et al., 2018). Metformin was previously reported to enhance radiation sensitivity (Gulati et al., 2020) and improve radiation-induced lung injury, alleviate pulmonary fibrosis and inflammatory infiltration, and hence bear a potential for application in radiation protection (Wang et al., 2017). Chen et al. reported metformin alleviated radiation-induced intestinal injury by optimizing mitophagy, an AMPK-dependent process (Chen et al., 2020). The effect of metformin on the gut microbiota in radiation-induced intestinal injury is unclear. In this study, we established an animal model of abdominal IR-induced intestinal injury and characterized the role and underlying signaling of a specific microorganism, Lactobacillus, and its activator, metformin, in the prevention and treatment of radiation-induced intestinal injury in mice. Our results suggest that corrections of abnormal gut microbiota, such as administration of Lactobacillus or elevation of the probiotic by metformin, protected IR mice and abdominal IR patients from intestinal injury. Our findings reveal a novel target for preventing radiation-induced intestinal damage.
Female BALB/c mice were maintained in a specific pathogen-free (SPF) animal facility at Henan University. Mice were kept on a 12-h light-dark cycle with food and water available ad libitum. Metformin (250 mg/kg/day) was administered intragastrically for 7 days before and 3 days after abdominal IR (Higurashi et al., 2016). In the model, 8 Gy one-time abdominal IR with a dose rate of 1.0 Gy/min by X-ray radiation source was used (Ottewell et al., 2003). After radiation, mice were housed in an animal facility for routine observation and treatment. Ethical regulations for animal testing and research at Henan University were strictly implemented. Protocols for animal usage were approved by the institutional animal care and use committee (IACUC) at Henan University, China.
Feces of mice were collected after IR. For 16S rRNA sequencing, 30 mg of feces were kept in a sterilized Eppendorf tube.
After the mouse was euthanized, the ileocecal junction of the intestine was identified and located. The ileum was connected to one end of the ileocecal junction. Within 5 cm of the distal ileum, 60 mg of ileal content was collected and stored in sterilized Eppendorf tubes at −80°C.
Both mucosa and the epithelial tissue in the ileum of mice were scraped for collecting ileal contents. DNA from the ileal contents of mice was extracted for PCR amplification. The amplified sample was confirmed by 2% agarose gel electrophoresis. The high-throughput sequencing involved was completed by Shanghai Meiji Biology Company (Shanghai, China) (Jian et al., 2022).
The paraffin sections were dewaxed and hydrated for thermal antigen retrieval, endogenous peroxidase blocking, antibody action, and development as we reported previously (Jian et al., 2022). The immunoreaction was quantified using the immunohistochemistry (IHC) plugin of the Image J software (Jian et al., 2022).
The preparation of paraffin sections and hematoxylin-eosin (H&E) staining of the intestine were performed as we reported previously (Jian et al., 2022). Intestinal damage was scored and quantitated in the H&E staining. The intestinal mucosal injury was classified using Chiu's method (Chiu et al., 1970) as follows: 0, intestinal mucosal villus without abnormality; 1, cystic gaps and capillary hyperemia under the epithelium in villus apex; 2, cystic gaps enlarged under the epithelium, edema expanded to lamina propria, and central cheliferous vessels dilated; 3, severe edema in lamina propria, deterioration, and necrosis of intestinal epithelial cells (IECs), and abscission of a small number of villus apex; 4, open of lamina propria, dilation of capillary, and hyperemia; degeneration, necrosis, and exfoliation of IECs, abscission of a number of villi; and 5, fragmentation of lamina propria, and bleeding or ulceration, abscission of the complete villus (Mori et al., 2011).
The ilea of mice were isolated, rinsed with 0.9% saline, and prefixed with 4% glutaraldehyde. Samples were then processed to complete TEM biological sample preparation and electron microscopy observation (Ding et al., 2020).
Fresh fecal pellets were collected in PBS with a concentration of 50 mg feces/ml. Pooled samples were centrifuged and the supernatant was used for fecal microbiota transplantation (FMT) (van den Berg et al., 2021). A total of 200 μl of FMT or PBS alone were intragastrically administered into microbiota-eliminated mice each day for 4 weeks continuously before and 1 week after IR to reconstitute the intestinal microbiome of mice (Jian et al., 2022).
Mice were euthanized with carbon dioxide, and intestinal crypts were isolated. Villi of the intestine were scraped off with a coverslip and incubated in PBS and 2 mM EDTA at 4°C for 20 min, and then incubated at 37°C for 8 min. The digested crypts were then centrifuged at 300 g for 3 min, embedded in Matrigel, polymerized at 37°C for 30 min, and supplemented with a complete culture medium (Sato et al., 2011; Sato and Clevers, 2013).
Intestinal organoids were seeded in a 96-well plate with 10% Matrigel and exposed to Lactobacillus plantarum (LP) with a multiplicity of infection (MOI) of 100 for 4 h. Wells containing organoids were then washed with the medium containing the bacteria and then removed. Culture wells were washed 3 times with a medium containing 1% penicillin-streptomycin and 40 μg/ml gentamicin. Organoids were then placed in the medium with the antibiotics aforementioned for up to 3 days and measured to determine the organoid size (Yang et al., 2022).
Intestinal organoids were seeded and cultured in a 96-well plate with 10% Matrigel. The growth of intestinal organoids was assessed with a 10× objective light microscope (day 1). Intestinal organoids were then exposed to treatments for another 48 h. Images of intestinal organoids were captured using the EVOS FL cell imaging system at the end of the 3rd day (Thermo Fisher Scientific) (Park et al., 2021). Intestinal organoid sizes were determined using the Image J software.
Intestinal organoids were prepared and cultured as aforementioned. MTS assay buffer (10 μl) (CellTiter 96 AQueous One Solution, Promega, Madison, WI, USA) was added to each well containing 100 μl of basal medium 3 h before the end of the experiment. After incubation, the optical density at 490 nm was measured using a BioTek microplate reader (BioTek Instruments Inc., Winooski, VT, USA) (Park et al., 2021).
Specific pathogen-free mice were treated with a mixture of 4 antibiotics including 200 mg/L ampicillin, 200 mg/L metronidazole, 200 mg/L neomycin, and 100 mg/L vancomycin in drinking water for 4 weeks (Castellanos et al., 2018; Scott et al., 2018; Zarrinpar et al., 2018; Yang et al., 2019). All antibiotics were purchased from Meilunbio, China. At the end of the treatment, the intestinal content of mice was collected for 16S rRNA sequencing to validate the elimination of intestinal microbiota.
Lactobacillus plantarum (LP) was obtained from the American Type Culture Collection (202195) and cultured in Lactobacillus MRS broth (Panigrahi et al., 2017). To reconstitute the intestinal microbiome of mice before IR, 100 million CFU of LP in 0.2 ml of PBS or PBS alone was intragastrically administered into microbiota-eliminated mice each day for 4 weeks.
Paraffin sections were dewaxed and hydrated as described previously. The slide was stained with 1% periodic acid for 10 min and washed 3 times with distilled water for 5 min each time. The slide was then stained with Schiff reagent for 30 min. Differentiation, development, nucleus staining, dehydration, transparency, and sealing were carried out following the protocol reported previously (Hänninen et al., 2018).
We collected feces from cervical cancer patients registered in the department of gynecology of Jiangsu Cancer Hospital and undergoing abdominal radiotherapy. The irradiation dose was 2 Gy/time, 5 times/week for 5 consecutive weeks. The inclusion criteria were as follows: (1) 30–60 years of age; (2) receiving abdominal radiation therapy; (3) with or without clinical manifestations of radiation-induced intestine injury (tenesmus, diarrhea, rectal bleeding, mucus and fecal incontinence, etc.); (4) with or without metformin administration recently. The exclusion criteria were as follows: radiotherapy was interrupted, inflammatory bowel disease, and tumor involving the compressing bowel. Screened according to the inclusion criteria, fecal samples from 45 female patients were collected for 16S rRNA sequencing. In addition, 15 healthy female subjects, aged between 30 and 60, were recruited locally, and their stool samples were collected to serve as normal controls.
Experimental data were analyzed using SPSS 17.0. Student's t-test and one-way ANOVA were used for the significant test. A P < 0.05 (* P < 0.05, ** P < 0.01, *** P < 0.001) was set to be statistically significant, whereas when the P > 0.05, the experimental results were defined as statistically insignificant.
To establish a model of irradiation (IR)-induced intestinal injury, we administered abdominal IR of 8 Gy to BALB/c mice (Cui et al., 2017). To determine the radioprotective effects of metformin on IR mice, we analyzed the survival, body weight, and intestinal injury in abdominal IR mice treated with metformin. As shown in Figures 1A,B, treatment with metformin led to a marked improvement in the survival and body weight of IR mice (Figures 1A,B). Metformin did not affect the blood glucose, drinking water, and diet intake of mice in our experimental settings (Supplementary Figures S1A–C). In contrast to the striking reduction of feces and markedly decreased ileal length in IR mice, administration of metformin substantially improved fecal output and prevented the mice from IR-induced ileum shortening (Figures 1C,D). The application of metformin reduced histological injury score and abrogated IR-induced intestinal damages, such as reduction in intestinal villus height and cell death (Figure 1E; Supplementary Figure S1D). Under the electron microscope, the epithelial microvilli of control and metformin-treated mice were arranged in order, and no abnormalities in the endoplasmic reticulum, mitochondria, intercellular gap, and nuclear were displayed. In IR mice, however, epithelial microvilli were rare, short, sloughing, and irregularly arranged (Figure 1F; Supplementary Figure S1E). Increased cytosolic lysosomes, vacuolar degeneration, nuclear condensation, fragmentation, dissolution, chromatin margination, increased intercellular space, and cell necrosis were observed in intestinal epithelia of mice treated with IR (Figure 1F). Treatment of metformin led to a striking improvement in IECs of abdominal IR mice under the electron microscopy analysis, with only marginal damages observed in epithelial microvilli and chromatin margination (Figure 1F). These results demonstrate that radiation-induced intestinal tissue damages were relieved with the application of metformin in mice.
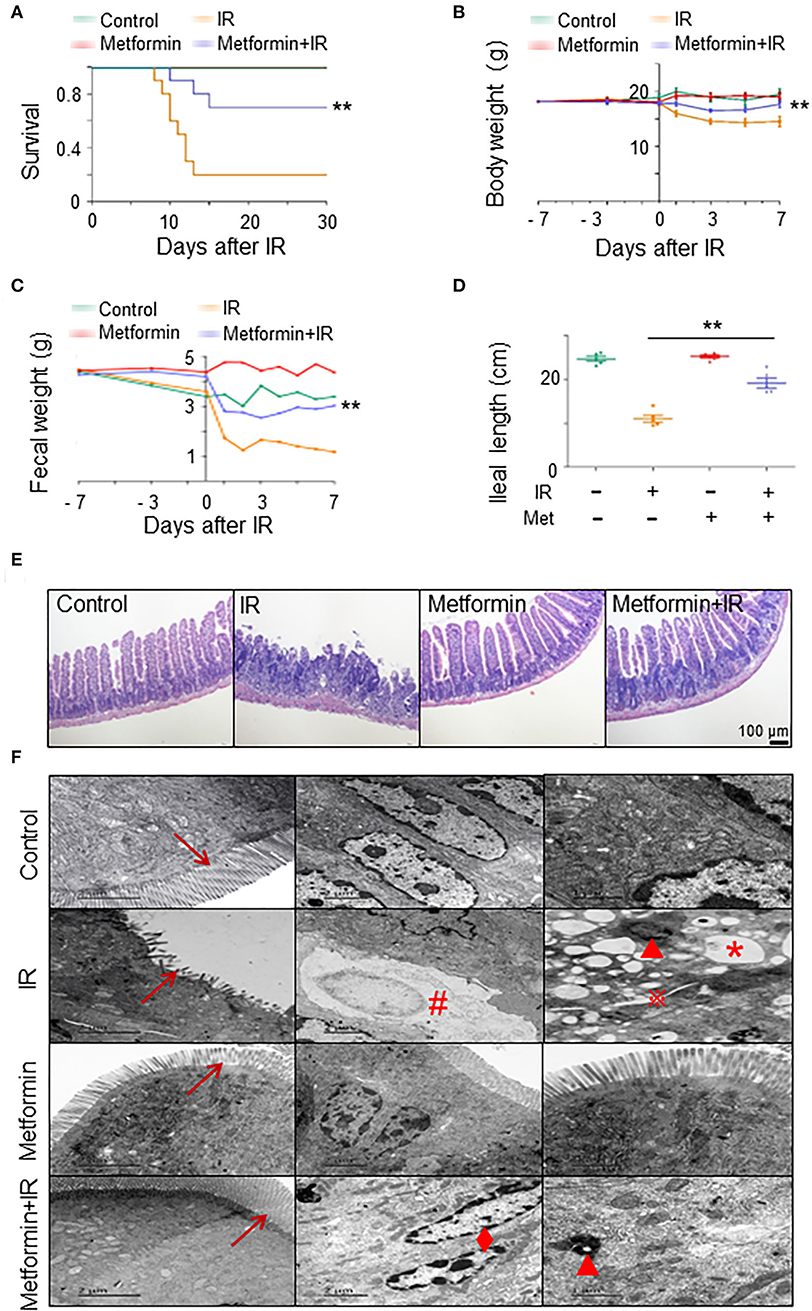
Figure 1. Metformin mitigates intestinal damage in IR mice. Metformin was intragastrically administered to mice at a dose of 250 mg/kg/day. Each mouse was administered continuously for 7 days before and 3 days after radiation for a total of 10 days. Mice were given 8 Gy one-time abdominal IR with a dose rate of 1.0 Gy/min in an X-ray radiation source or a mock treatment. Ilea were collected for light and electron microscopy 3 days after the IR. Mice were analyzed for (A) survival, (B) body weight, (C) fecal weight, (D) ileal length, (E) H&E staining of ilea, and (F) electron microscopic detection of ilea. → , microvillus of intestinal epithelial cells;  , increased intercellular space; *, vacuolar degeneration; ▴, increased cytosolic lysosomes; ♦, chromatin margination; #, cell necrosis. Data represent mean ± SEM. ** P < 0.01, n = 5, compared with IR. Also see Supplementary Figure S1.
, increased intercellular space; *, vacuolar degeneration; ▴, increased cytosolic lysosomes; ♦, chromatin margination; #, cell necrosis. Data represent mean ± SEM. ** P < 0.01, n = 5, compared with IR. Also see Supplementary Figure S1.
It was reported that metformin, a first-line agent for the treatment of type 2 diabetes, improves upper small intestine microbiota composition and restores the SGLT1-dependent glucose sensing to regulate glucose homeostasis (Wu et al., 2017; Bauer et al., 2018). Thus, we asked whether metformin could improve dysbiosis in IR mice. We performed a 16S rRNA sequencing with ileal contents from IR mice treated with metformin. Evaluation of the credibility of the sequencing by Sobs curves and Shannon curves indicated that the number of sequencing samples was acceptable (Supplementary Figure S2). Treatment of metformin markedly reversed the reduction in gut microbiota diversity in IR mice in the Shannon index analysis (Figure 2A). The abundance of intestinal flora was also improved in IR mice treated with metformin in the Chao index analysis (Figure 2B). Collectively, our results suggest that metformin improved the diversity and abundance of intestinal flora in abdominal IR mice.

Figure 2. Metformin improves the composition and abundance of intestinal flora in abdominal IR mice. Female BALB/c mice were treated with abdominal IR and/or metformin as described in Figure 1. Total DNAs were isolated from intestinal contents as described in the Materials and methods section and subjected to 16S rRNA sequencing. Intestinal flora is shown as follows: (A) diversity (Shannon index), (B) abundance (Chao index), (C) Venn in genus level, and (D) bacterial composition in the intestine of mice treated with or without metformin. Data represent mean ± SEM. * P < 0.05, ** P < 0.01, n = 3, metformin+IR vs. IR. Also, see Supplementary Figures S2, S3.
We further analyzed the influence of metformin on the composition of intestinal flora in mice. The Venn plot analysis, which is used to display the distribution of the number of elements between various sets, showed that there were 11 phyla and 75 genera in common between the IR and control mice, whereas metformin+IR and control mice shared 14 phyla and 119 genera (Supplementary Figure S3A; Figure 2C), indicating that treatment of metformin renders IR mice more similarities in intestinal flora composition as in non-irradiated healthy mice. The community composition analysis chart (bar chart) (Jain et al., 2021) showed that the dominant phylum in all mice was Firmicutes. The flora composition was similar. However, the relative abundance was different at the phylum and genus levels among control-, metformin-, and metformin + IR-treated mice. The composition of the IR group was quite different from that of the aforementioned three groups (Supplementary Figure S3B; Figure 2D). Based on the bacterial classification, the microbiota was divided into three distinct taxa in the sample hierarchical cluster map. The distance between metformin+IR and control mice was relatively close, indicating that the composition of the flora in the two groups was similar, whereas a far distance between IR and control group was observed, indicating less similarity in the composition of microbiota in the two groups (Supplementary Figure S3C). Similar results for the beneficial effects of metformin in the maintenance of microbiota composition in mice with or without IR were observed in the PCA analysis (Supplementary Figure S3D). Collectively, these results demonstrate that IR led to the disorder of intestinal flora composition in mice, which could be corrected by metformin.
To determine whether the role of metformin in radioprotection of the intestine is mediated by gut microbiota, we treated normal and microbiota-eliminated (Abx) mice with metformin followed by IR. Interestingly, metformin no longer bore a radioprotective effect on the intestine in the Abx mice. The survival and body weight were similar in Abx+IR mice with or without metformin treatment, which was significantly lower than those in control mice treated with metformin and IR (metformin+IR) (Figures 3A,B). The amount of feces was reduced, and ileal length was decreased in Abx mice with IR regardless of the application of metformin (Figures 3C,D). Histologically, the protective effects of metformin on intestinal epithelial cells in IR mice were abrogated in Abx mice (Figures 3E,F). These results indicate that intestinal flora is required for metformin-mediated alleviation of radioactive intestinal injury. To verify this hypothesis, we reconstituted the gut microbiome with feces from control or metformin-treated non-IR mice into gut microbiota-eliminated mice. Fecal microbiota transplant (FMT) from control or metformin-treated mice conferred microbiota-eliminated mice resistance to abdominal IR, and FMT from metformin-treated mice was more effective (Figures 3G,H). In addition, we also reconstituted the gut microbiome with feces from IR or metformin+IR mice into mice with intestinal flora elimination. Microbiota-eliminated mice transplanted with feces from IR mice are sensitive to abdominal IR. Intestinal epithelial damage and the histological score of the damaged intestine were severe (Figures 3G,H). However, feces from metformin+IR mice markedly reduced radiation-induced intestinal damage in the recipient mice (Figures 3G,H). Collectively, our data suggest that metformin reduces IR-induced intestinal damage in the presence of microbiota in mice.
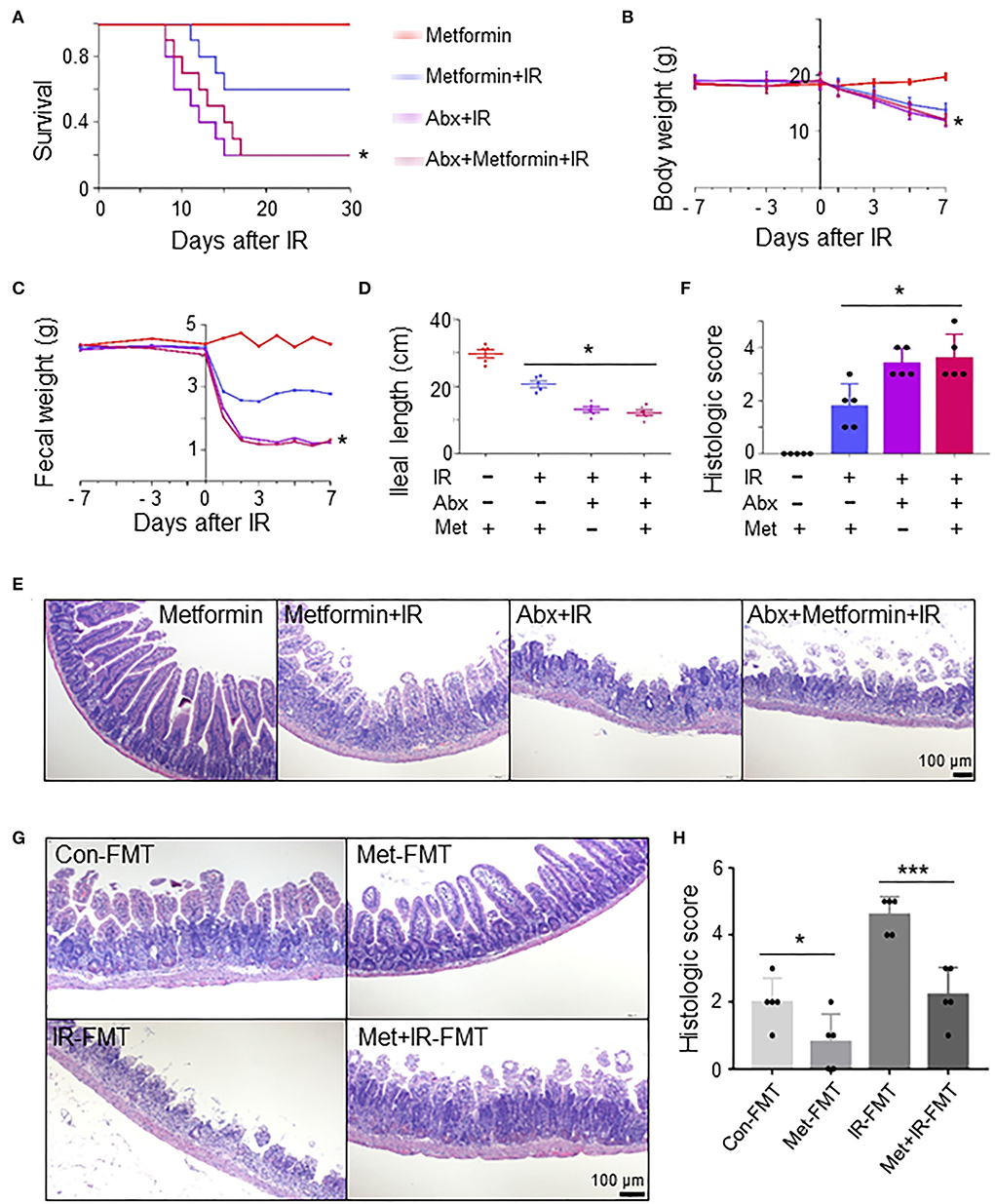
Figure 3. Metformin reduces IR-induced intestinal damage in mice by improving the composition and abundance of microbiota. Mice were pretreated with an antibiotic mixture for 4 weeks before metformin treatment. Mice were irradiated as described in Figure 1. Ilea were collected for light microscopy 3 days after IR. The mice were monitored for (A) survival, (B) body weight, (C) fecal output, (D) ileal length, (E) H&E staining of ileum, and (F) damage score of ileal tissue. * P < 0.05, Abx+metformin+IR vs. metformin+IR. (G,H) A total of 200 μl FMT using feces from control-, metformin-, IR-, or metformin+IR-treated mice were intragastrically administered into microbiota-eliminated mice (Abx) for 4 weeks continuously before and 1 week after IR to reorganize the intestinal microenvironment. (G) H&E staining of ileal tissues from IR mice received FMT with feces from control (Con-FMT), metformin-treated (Met-FMT), IR mice (IR-FMT), or metformin-treated IR mice (Met+IR-FMT). (H) Damage score of ileal tissues of mice in (G). Data represent mean ± SEM. * P < 0.05, *** P < 0.001, n = 5.
We analyzed the species of intestinal flora in IR mice treated with metformin based on the Pie and heat maps. They showed that the dominant genus in control- and metformin-treated mice was Lactobacillus (Figure 4). The abundance of Lactobacillus and Akkermansia in metformin-treated mice was increased, whereas that of Escherichia-Shigella was decreased, as compared with those in control mice (Figure 4). In addition to the enrichment of Lactobacillus and Akkermansia, metformin-treated IR mice also displayed a striking increase in Lactococcus, Streptococcus, Clostridium, and Burkholderia-Paraburkholderia and a marked decrease in Escherichia-Shigella, Candidatus-Arthromitus, and Enterococcus (Figure 4A; Supplementary Figure S3E). Among them, Lactobacillus was the top genus identified in the increase due to exposure to metformin (Figure 4B).
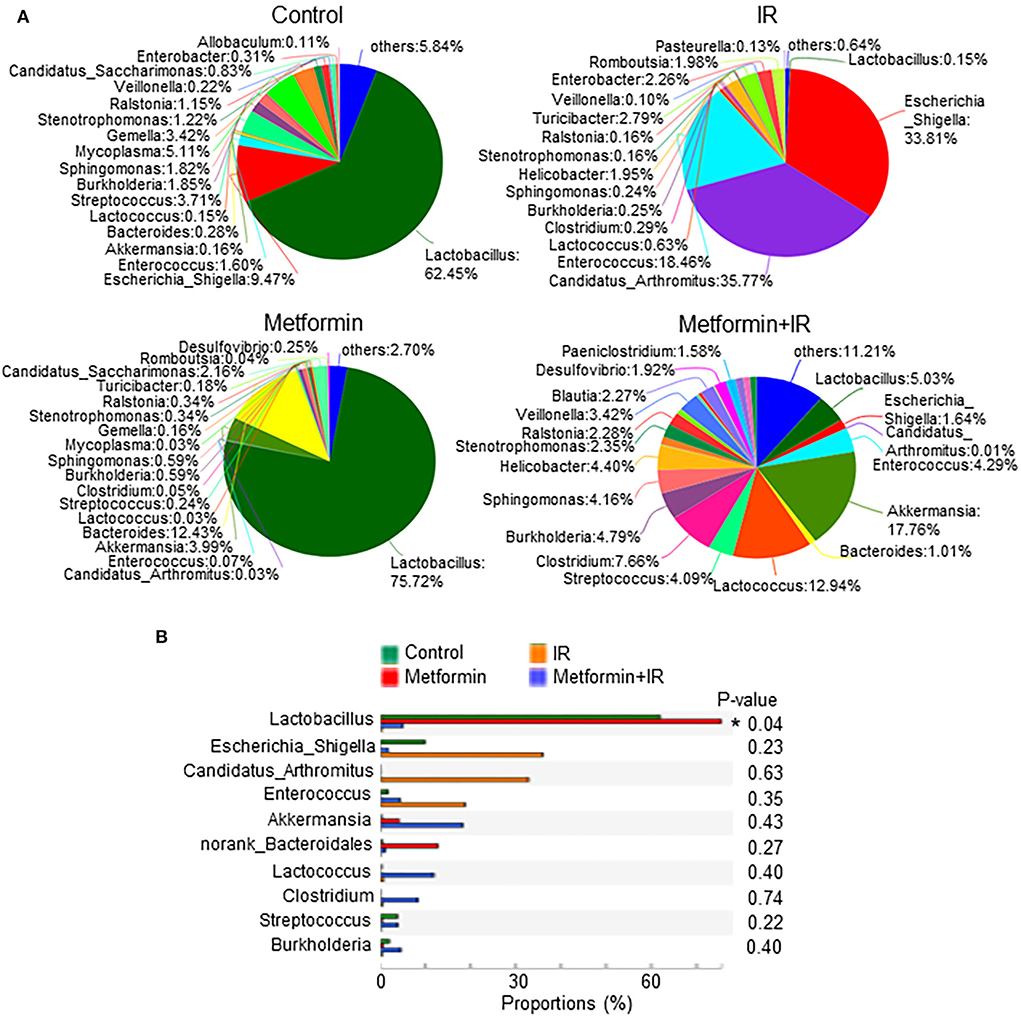
Figure 4. Metformin increases the abundance of Lactobacillus. Female BALB/c mice were treated with abdominal IR and/or metformin as described in Figure 1. Total DNAs were isolated from intestinal contents as described in the “Materials and methods” section and subjected to 16S rRNA sequencing. (A) Pie analysis and (B) comparison of bacteria in intestinal flora in mice treated with or without metformin and identification of Lactobacillus as the most changed bacteria in abundance after metformin treatment. Data represent mean ± SEM. * P < 0.05, n = 3, metformin+IR vs. IR.
To characterize the role of Lactobacillus in metformin-induced radioprotection in abdominal IR mice, we administrated metformin into Abx mice by gavage. The administration of metformin did not protect against IR-induced intestinal damage in Abx mice (Figures 5A,B). Then we reconstituted Abx mice with Lactobacillus before metformin and IR treatment. Surprisingly, administration of Lactobacillus substantially lessened IR-induced intestinal damage in Abx mice (Figures 5A,B). More importantly, administration of Lactobacillus restored the radioprotective effect of metformin on the Abx mice (Figures 5A,B), further supporting the notion that metformin protects mice from IR-induced gut injury by improving the abundance of Lactobacillus in the intestine.
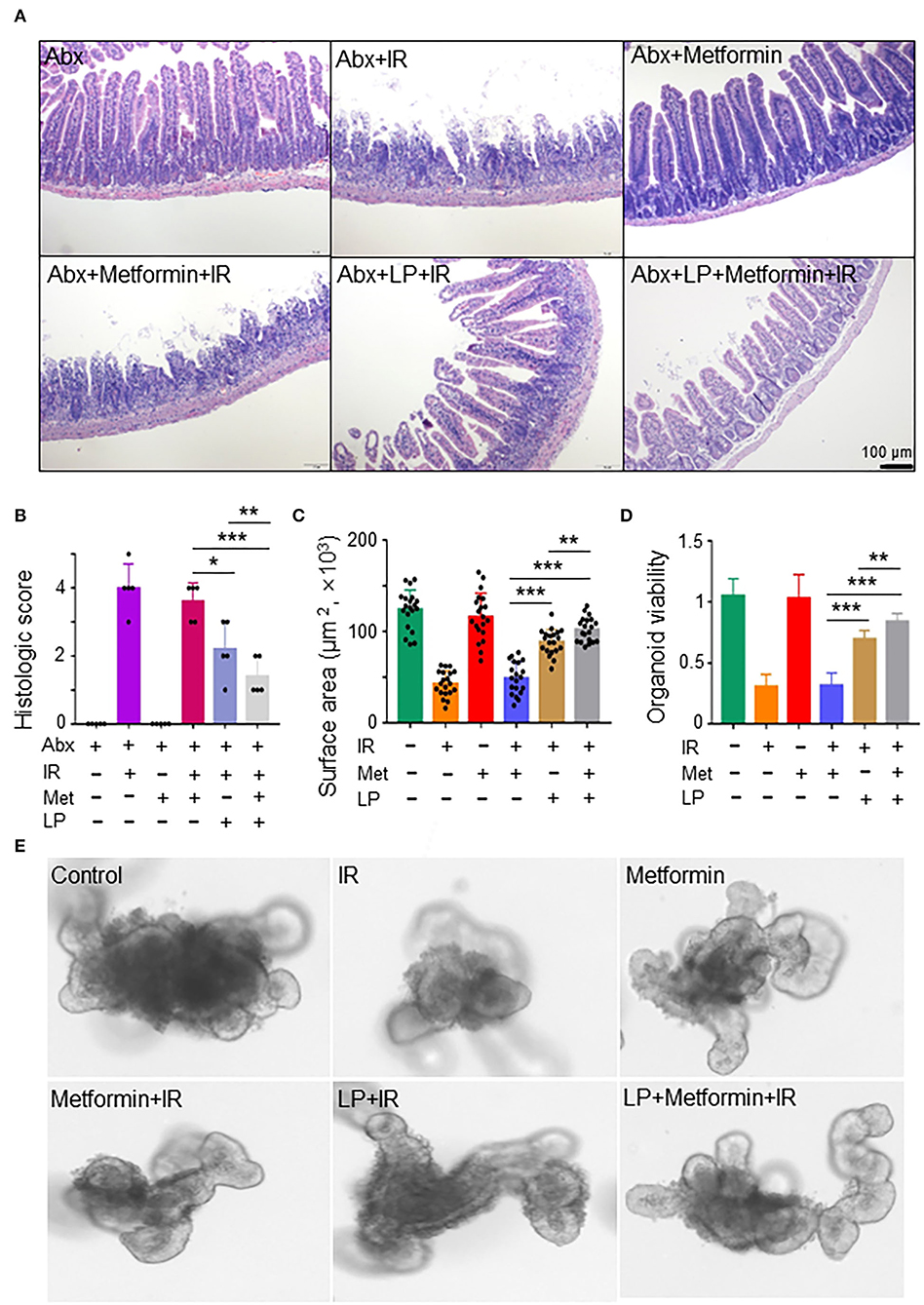
Figure 5. Metformin protects the murine intestine against IR in the presence of Lactobacillus. (A,B) Mice were pretreated with a mixture of antibiotics for 4 weeks before metformin and/or Lactobacillus treatment. Mice were irradiated as described in Figure 1. Ilea were collected for light microscopy 3 days after IR. (A) H&E staining of ilea. (B) Damage score of ileal tissues. Data represent mean ± SEM. * P < 0.05, *** P < 0.001, n = 5, compared with Abx+metformin+IR. (C–E) Ileal tissues of mice were collected for the intestinal organoid culture. Intestinal organoids were treated with metformin and/or Lactobacillus as described in the Materials and methods section. (C) Intestinal organoid surface area. (D) Intestinal organoid viability (OD490nm). (E) Representative intestinal organoid image. Scale bar = 20 μm. Data represent mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001, n = 5, compared with metformin+IR.
To further validate our finding that metformin reduces IR-induced intestinal damage by increasing the abundance of Lactobacillus, we analyzed the response of murine intestinal organoids to IR in the presence or absence of metformin and/or Lactobacillus. We found that IR inhibited intestinal organoid formation, and metformin alone had no protective effect on intestinal organoids treated with radiation (Figures 5C–E). However, combined treatment of metformin and Lactobacillus or Lactobacillus alone had an obviously protective effect on intestinal organoid formation following IR, leading to significantly larger organoids as compared with those treated with IR or metformin+IR (Figures 5C,E). In addition, intestinal organoids treated with metformin and Lactobacillus or Lactobacillus alone survived much better than those treated with PBS (control) or metformin alone under IR (Figures 5D,E). Together, these results support our notion that metformin exerts a radioprotective effect on murine intestines through Lactobacillus.
Studies have shown that gut microbiota decreased hepatic bile acid by activating farnesoid X receptor (FXR) in intestinal epithelial cells, leading to the alleviation of excessive hepatic bile-induced liver injury and fibrosis in mice (Liu et al., 2020). FXR activation promotes the expression of tight junction proteins to improve ileal barrier function (Verbeke et al., 2015), demonstrating a crucially protective role of FXR in the intestine. To determine the role of FXR in metformin-induced radioprotection, we analyzed the expression of FXR in irradiated intestinal organoids treated with metformin and/or Lactobacillus. We found that metformin substantially upregulated the level of FXR mRNA in irradiated mouse organoids in the presence of LP. Metformin-induced elevation of FXR was abrogated in irradiated murine intestinal organoids without the cotreatment of LP (Figure 6A). In addition, the mRNA levels of occludin and ZO-1, two important components of tight junctions between intestinal epithelial cells and MUC2, a mucus layer protein secreted by intestinal goblet cells, were also increased in irradiated mouse intestinal organoids pretreated with metformin and LP, indicating that cotreatment of metformin with LP increased the intestinal barrier function (Figures 6B–D). Consistently, knocking down of FXR markedly reduced the expression of occludin, ZO-1, and MUC2 induced by metformin and LP in mouse intestinal organoids (Figure 6E). Together, our data indicate that the activation of FXR signaling likely explains the radioprotective effect of metformin in mouse intestinal organoids.
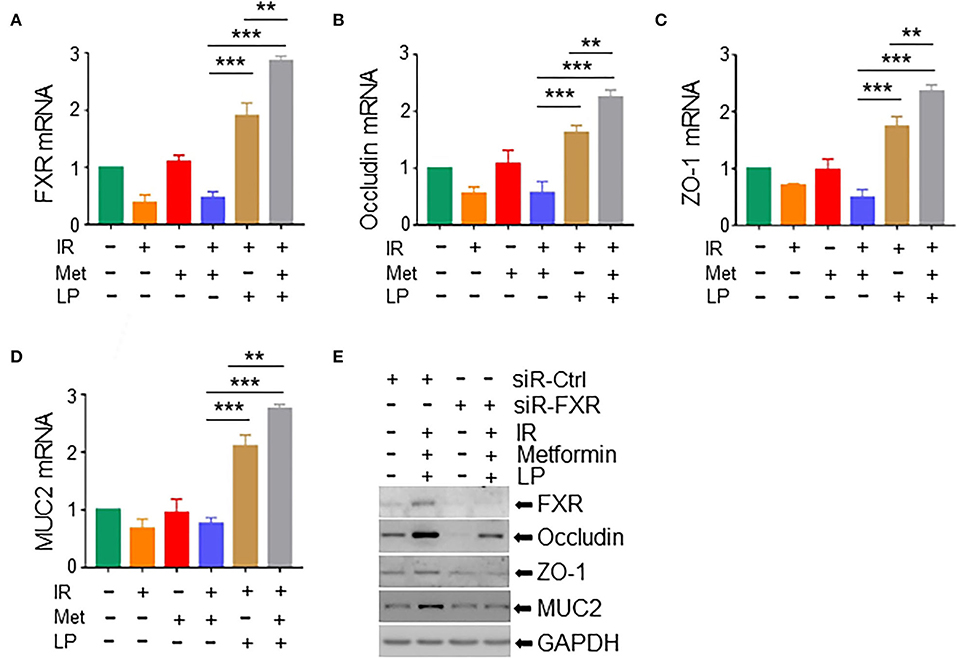
Figure 6. Metformin/Lactobacillus exerts a radioprotective effect via activating the FXR signaling. Intestinal organoids were treated with metformin and/or Lactobacillus as described in the Materials and methods section and Figure 5. Organoids were irradiated with 8-Gy X-ray. Total RNAs or whole cell lysates were extracted from the intestinal organoids 16 h after the irradiation. mRNA levels of FXR (A), occludin (B), ZO-1 (C), and MUC2 (D) in intestinal organoids. qPCR was used for the quantitation. (E) Expressions of FXR, occludin, ZO-1, and MUC2 in intestinal organoids with FXR knockdown were detected with Western blotting. Data represent mean ± SEM. ** P < 0.01, *** P < 0.001, n = 5, compared with metformin+IR.
To validate the necessity of FXR signaling activation in metformin/Lactobacillus-mediated radioprotective effects on intestinal epithelia, we pretreated abdominal IR mice with GW 4064, an FXR activator (Seok et al., 2014). The application of FXR activator GW 4064 markedly alleviated intestinal tissue damage in microbiota-eliminated IR mice (Figures 7A,B). To determine how activation of FXR signaling reduces intestinal damage by IR, we detected intestinal epithelial barrier integrity in abdominal IR mice treated with or without GW 4064. Expressions of FXR, occludin, ZO-1, and MUC2 in the intestine of abdominal IR mice treated with GW 4064 were increased as compared with those in IR mice without treatment with GW 4064 (Figures 7C,D). More importantly, the number of goblet cells in the small intestine of IR mice was upregulated after GW 4064 treatment (Figure 7E, upper panels, Figure 7F). The mucus layer areas on the surface of the small intestinal villi were also increased (Figure 7E, lower panels, Figure 7F). Collectively, our data suggest that activation of FXR maintains the integrity of intestinal epithelial barriers and promotes the reduction of IR-induced intestinal injury.
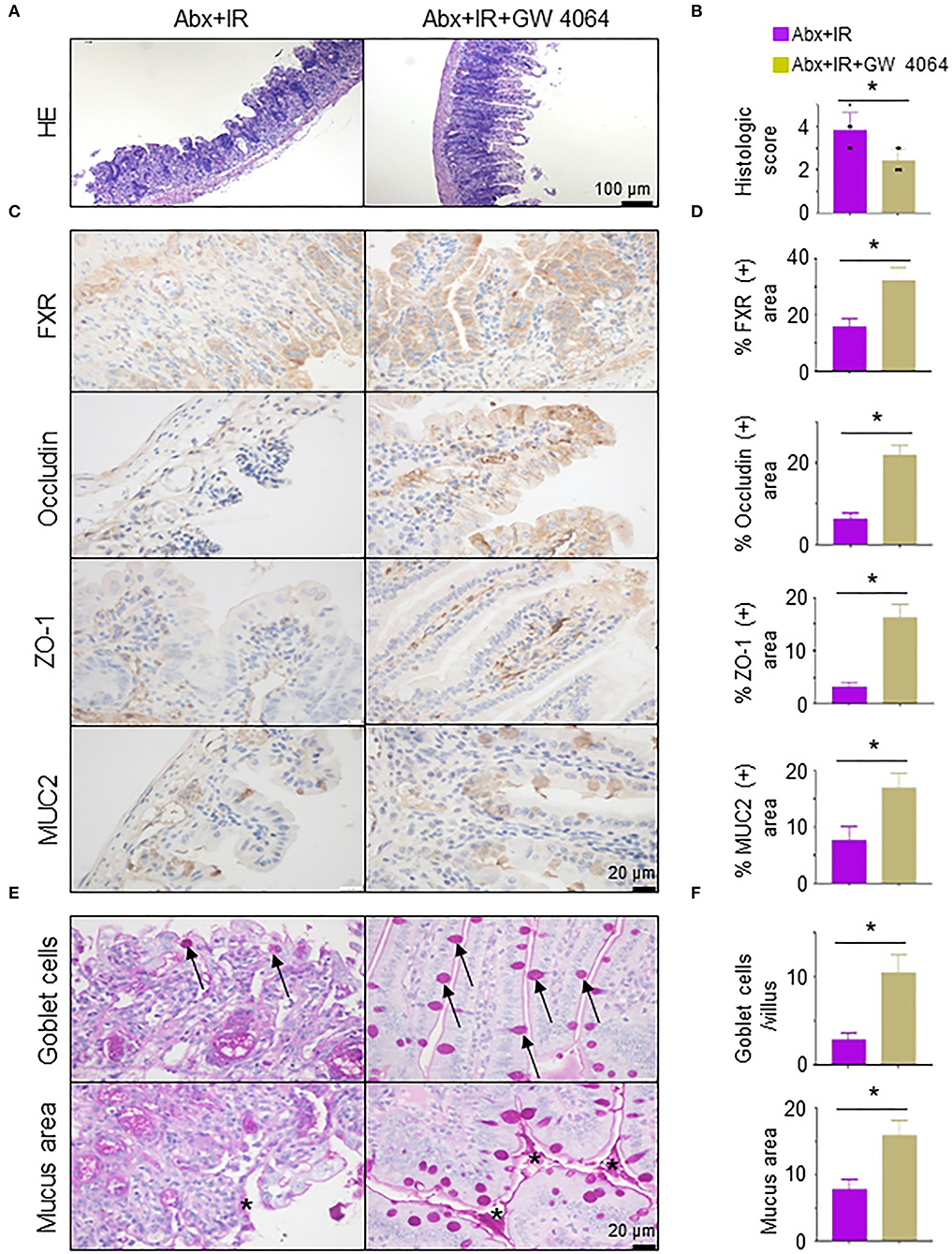
Figure 7. Activation of FXR reduces radiation-induced intestinal injury in microbiota-eliminated mice. Microbiota-eliminated mice (Abx) were injected intraperitoneally with an FXR activator (GW 4064, 30 mg/kg/day, MedChemExpress, China) 3 days before IR. Irradiation of the mice was performed as described in Figure 1. The mice were subjected to the following analyses. (A) H&E staining of ilea in IR mice treated with FXR activator GW 4064. (B) Damage score of ilea in (A). (C,D) IHC staining was performed in ilea from IR mice treated with or without FXR activator GW 4064 (C). Percentage of FXR, occludin, ZO-1, and MUC2 positive area in IHC staining of ilea in IR mice treated with FXR activator GW 4064 (D). (E,F) PAS staining of goblet cells and mucus in the intestine of mice treated with FXR activator GW 4064. Arrows, goblet cells; Stars, mucus. (F) Goblet cells per villus and quantitation of the mucus area. Data represent mean ± SEM. * P < 0.05, n = 5, Abx+GW 4064+IR vs. Abx+IR.
To further validate the role of FXR activation in the radioprotection of metformin/Lactobacillus against IR-induced intestinal injury, we pretreated the mice with Gly-β-MCA, an FXR inhibitor (Fu et al., 2019), before abdominal IR. Surprisingly, exposure to the FXR inhibitor Gly-β-MCA abrogated Lactobacillus-mediated protection in the ileum in IR mice (Supplementary Figures S4A,B). Expression of FXR, occludin, ZO-1, and MUC2 was substantially reduced in the intestine of abdominal IR mice treated with Gly-β-MCA as compared with those in IR mice treated without Gly-β-MCA (Supplementary Figures S4C,D). In addition, the number of goblet cells and the area of the mucus layer were dramatically decreased in abdominal IR mice treated with Gly-β-MCA in spite of the existence of LP (Supplementary Figures S4E,F). Taken all together, our results suggest that metformin/Lactobacillus-activated FXR signaling upregulates the levels of tight junction proteins and mucins in intestinal epithelia, increases the number of goblet cells, and augments the mucus layer thickness to maintain the integrity of intestinal epithelial barrier (Supplementary Figure S5), which eventually contributes to reduced radiation intestinal injury.
To investigate the effect of metformin on gut microbiota composition and the prevention of diarrhea in patients with abdominal radiotherapy, we collected feces from abdominal IR patients with or without metformin treatment (500 mg, 3 times per day, oral administration for more than 3 months before radiotherapy) and age-matched healthy subjects (HC) for 16S rRNA sequencing. The sequencing results showed that the intestinal flora of IR patients was different from that of HC. Metformin treatment improved the composition of gut microbiota of IR patients (Figure 8A). To clarify the effect of metformin on gut microbiota composition, we performed a statistical analysis of specific microbiota. We found that the abundance of Lactobacillus, Akkermansia, Bifidobacterium, Romboutsia, Subdoligranulum, Faecalibacterium, and Bacteroides was increased, and Escherichia-Shigella, Prevotella, and Agathobacter decreased in the feces of IR patients treated with metformin as compared with those without metformin (Figures 8A–C), among which Lactobacillus raised the most in abundance (Figure 8B). The amount of Lactobacillus was negatively correlated with the diarrhea duration of patients (Figure 8D). Collectively, our data support the observation that abdominal irradiation leads to gut dysbiosis in patients. Metformin promotes the abundance of Lactobacillus in the intestine of abdominal IR patients.
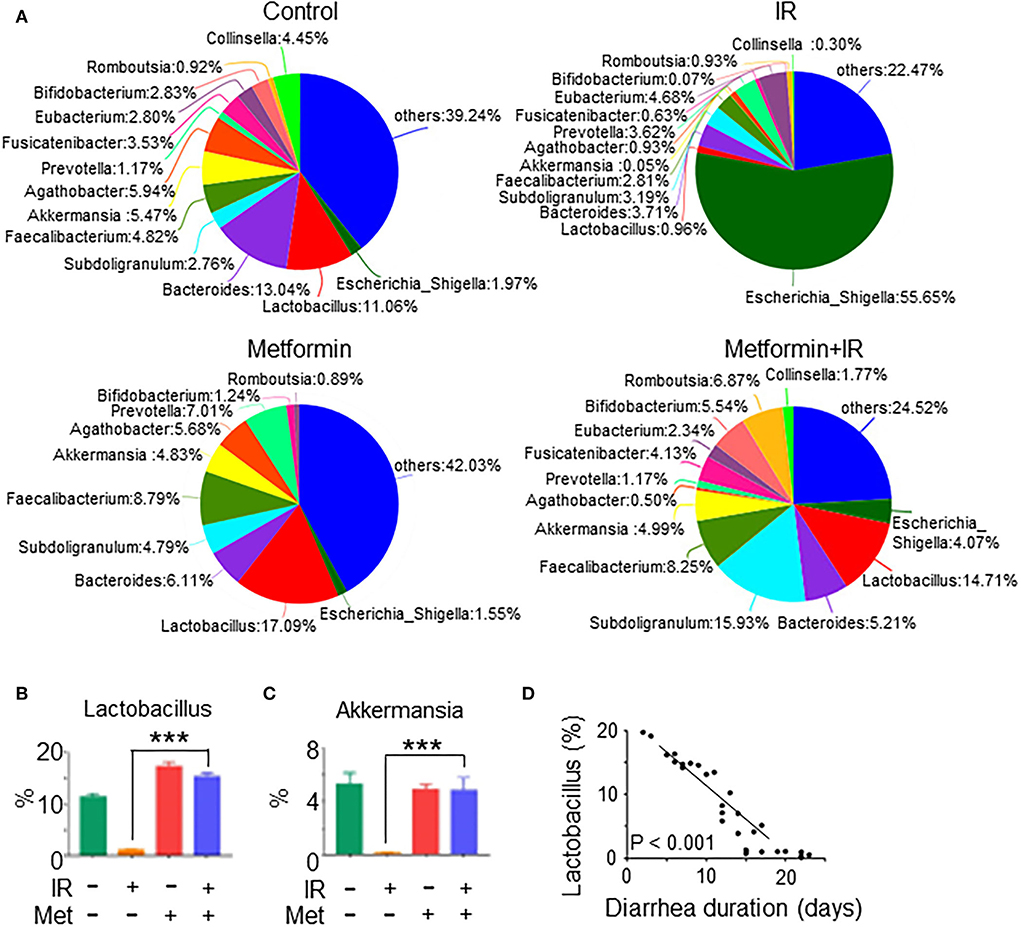
Figure 8. Metformin improves the composition and abundance of intestinal flora in abdominal IR patients. Feces samples from cervical cancer patients receiving abdominal IR with or without metformin treatment and healthy subjects were collected. Total DNAs were isolated from the feces as described in the Materials and methods section and subjected to 16S rRNA sequencing. (A) Pie analysis of intestinal flora in abdominal IR patients with or without metformin treatment and healthy subjects. (B,C) Percentage of Lactobacillus (B) and Akkermansia (C) in the intestinal flora. (D) Correlation of Lactobacillus abundance and diarrhea duration in abdominal IR patients. Data represent mean ± SEM. *** P < 0.001, compared with abdominal IR patients, n = 15 in each group.
Irradiation is one of the most widely used strategies in abdominal tumor treatment, but the toxicity of IR in normal tissues is usually dose-limiting, as it often leads to IR-induced intestinal injury. As a result, there is an increasing interest to identify agents that manage both short- and long-term radiation damage (Fransson and Widmark, 2007; De Ruysscher et al., 2019).
Metformin is the most widely used drug for the treatment of type 2 diabetes (García-Calzón et al., 2020; Koh et al., 2020). Metformin exerts its antihyperglycemic action mainly by reducing hepatic glucose production (Hunter et al., 2018), hence reducing the level of glucose. It was found that metformin also alleviates type 2 diabetes by maintaining the homeostasis of intestinal flora (Bauer et al., 2018). The role of metformin in IR-induced intestinal dysbiosis was not characterized previously. In this study, we identified that metformin improved the microbiota abundance and diversity in the intestine of IR mice (Figures 2A,B). In the follow-up analysis, we identified that Lactobacillus in intestinal flora was markedly elevated by metformin (Figure 4). Elimination of intestinal microbiota abrogated the radioprotective effect of metformin on the intestine of mice. In addition, we found that Lactobacillus abundance was significantly increased in the intestine of abdominal radiotherapy patients treated with metformin and its abundance was negatively correlated with the diarrhea duration (Figure 8). Thus, our data endorse a robust role of metformin in the radioprotection of the intestine by maintaining the integrity of intestinal microbiota.
Metformin was previously reported to enhance radiation sensitivity (Gulati et al., 2020) and improve radiation-induced lung injury, alleviate pulmonary fibrosis and inflammatory infiltration, and hence bear a potential for application in radiation protection (Wang et al., 2017). Chen et al. reported that metformin alleviates radiation-induced intestinal injury by optimizing mitophagy, an AMPK-dependent process (Chen et al., 2020). However, the impact of metformin on gut microbiota in radiation-induced intestinal injury is unclear. In view of the capability of metformin in the improvement of intestinal flora in type 2 diabetes, in this study, we asked whether metformin was able to enhance the composition and abundance of intestinal flora in IR mice, and hence alleviate the intestinal injury and improve the survival of the mice. We found that metformin improved the composition and abundance of intestinal microbiota, in particular the enrichment of Lactobacillus in the intestine of IR mice. Fecal microbiota transplant (FMT) from metformin-treated mice or administration of Lactobacillus conferred microbiota-eliminated mice resistance to abdominal IR and promoted the survival of mice. Furthermore, we identified that metformin enhanced the intestinal barrier integrity via Lactobacillus-mediated activation of the FXR signaling in intestinal epithelial cells. More importantly, in the microbiota-eliminated mice, metformin lacked the protective effect against abdominal IR, further supporting the notion that intestinal flora, especially Lactobacillus, played a crucial role in the radioprotective effect of metformin. These findings provide robust evidence warranting that metformin is a novel agent for the treatment of IR-induced intestinal injury. Metformin is also reported to be able to ameliorate IR-induced bone marrow injury (Xu et al., 2015). It is an interesting question to be explored in the future to determine whether metformin exerts a radioprotective effect on the bone marrow through the intestinal flora.
The pivotal contributor to IR-induced intestinal injury is increased intestinal permeability. Therefore, we focused on FXR, a bile acid-responsive nuclear transcription factor, which is essential for the regulation of hepatic bile acid and lipid and carbohydrate metabolism (Vanwijngaerden et al., 2011; Mudaliar et al., 2013; Verbeke et al., 2014). Interestingly, FXR is also highly expressed in the intestine (Figure 6; Supplementary Figure S4), indicating that the effect of FXR may extend beyond the liver. The crucial role of FXR in maintaining intestinal homeostasis and its involvement in the gut-liver axis are increasingly recognized. In cholestasis, activation of FXR improves ileal barrier function (Modica et al., 2012). Lack of FXR is associated with increased intestinal permeability of the gastrointestinal tract (Wang et al., 2008; Gadaleta et al., 2011). In this study, we observed that knockdown or suppression of FXR led to the reduction in occludin, ZO-1, and MUC2 in murine intestinal organoids (Figure 6) and depletion of radioprotective effect of metformin and LP on IR mice (Supplementary Figure S4). Collectively, our data suggest that the activation of FXR signaling is important for the radioprotective effect of metformin on mouse intestines.
In addition, we applied GW 4064, a synthetic FXR agonist, to mice and found that activation of FXR alleviated IR-induced intestinal damage and strengthened the intestinal barrier in microbiota-eliminated mice. In stark contrast, an opposite outcome was obtained in IR mice treated with FXR inhibitor Gly-β-MCA, bile acid, and potent, stable, intestine-selective, and high-affinity FXR inhibitor (Jiang et al., 2015). These data support the conclusion that activation of FXR signaling is essential for the alleviation of IR-induced intestinal damage.
Taken together, in this study, using the abdominal irradiation mouse model, we made a series of novel findings. We demonstrated that metformin improved the composition and abundance of intestinal microbiota, in particular the enrichment of Lactobacillus in the intestine of IR mice. FMT from metformin-treated mice or administration of Lactobacillus conferred microbiota-eliminated mice resistant to abdominal IR and promoted the survival of mice. Furthermore, we identified that metformin enhanced the intestinal barrier integrity via Lactobacillus-induced activation of the FXR signaling in intestinal epithelial cells. These findings provide robust evidence warranting that metformin is a novel agent for the treatment of IR-induced intestinal injury. Our findings not only provide new insights into the role of gut microbiota in radiation-induced intestinal injury but also shed new light on the application of probiotics for the protection of radiation-damaged individuals.
Metformin/Lactobacillus activated the FXR signaling in intestinal epithelial cells and hence strengthened the intestinal barrier. Corrections of abnormal gut microbiota, such as administration of Lactobacillus or elevation of the probiotic by metformin, could be a novel therapeutic strategy for IR-induced intestinal damage.
The data presented in the study are deposited in the figshare.com repository, accession number 21164911.V1.
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Henan University, China.
Y-PJ designed the study and wrote the manuscript. J-YY, M-JL, LL, J-RG, K-YH, K-KW, C-YC, B-ZY, and D-DD performed the experiments. HZ, QD, G-LL, and J-HW explained and discussed the data. Z-XX contributed to the conception and writing. All authors read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (Nos. 82020108024, 32161143021, and 81772924).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.932294/full#supplementary-material
Supplementary Figure S1. Metformin treatment does not affect blood glucose, drinking water, and food intake in IR mice. Healthy BALB/c mice raised in an SPF environment were irradiated with 8 Gy abdominal IR (X-ray, dosage rate 1 Gy/min) or mock treatment. Metformin was intragastrically administered to the mice at a dose of 250 mg/kg/day for 7 days before and 3 days after IR for a total of 10 days. The mice were subjected to the following analyses. (A) Blood glucose, (B) drinking water intake, (C) food intake, (D) damage score of ileal tissue, and (E) intestinal microvillus length (μm) of ilea. Data represent mean ± SEM. *** P < 0.001, n = 5, NS represents no statistical difference, compared with IR.
Supplementary Figure S2. 16S rRNA sequencing of murine ileal contents after radiation. Healthy BALB/c mice raised in an SPF environment were irradiated with 8 Gy abdominal IR (X-ray, dosage rate 1 Gy/min) or mock treatment. Intestinal contents around the ileum of the mice were collected 3 days after the IR. Total DNAs were isolated from the contents as described in the Materials and methods section and subjected to 16S rRNA sequencing. (A) Sobs index dilution curves of intestinal flora. (B) Shannon index dilution curves of intestinal flora.
Supplementary Figure S3. Metformin treatment improves gut microbiota composition. Mice were treated and irradiated as described in Supplementary Figure S1. Intestinal contents around the ileum of mice were collected 3 days after IR. Total DNAs were isolated from the contents as described in the “Materials and methods” section and subjected to 16S rRNA sequencing. Intestinal flora is shown as follows. (A) Venn on phylum level, (B) composition on phylum level, (C) hierarchical clustering maps, (D) PCA (principal component analysis), and (E) heat map. Data represent mean ± SEM. n = 3, metformin+IR vs. IR.
Supplementary Figure S4. Inhibition of FXR abolishes the radioprotective effect of Lactobacillus. Microbiota-eliminated (Abx) mice reconstituted with Lactobacillus were injected intraperitoneally with FXR inhibitor Gly-β-MCA (30 mg/kg/day, MedChemExpress, China). Irradiation of mice was performed 3 days after the treatment, as described in Figure 1. Mice were subjected to the following analyses. (A) H&E staining of ilea in IR mice treated with FXR inhibitor Gly-β-MCA. (B) Damage score of ilea in IR mice treated with or without FXR inhibitor Gly-β-MCA in (A). (C,D) IHC staining (C) and its quantitation (D) were performed in ilea from IR mice treated with FXR inhibitor Gly-β-MCA. (E,F) PAS staining of goblet cells and mucus in the intestine of IR mice treated with FXR inhibitor Gly-β-MCA. Arrows, goblet cells; Stars, mucus. (F) Goblet cells per villus and quantitation of the mucus area. Data represent mean ± SEM. * P < 0.05, n = 5, Abx + LP + MCA + IR vs. Abx + LP + IR.
Supplementary Figure S5. The role of metformin/Lactobacillus-FXR signaling in the reduction of radiation-induced intestinal injury. Metformin activates FXR signaling by increasing the abundance of Lactobacillus to upregulate levels of tight junction proteins and mucins in intestinal epithelia, increase the number of goblet cells, and augment the mucus layer thickness to maintain the integrity of the intestinal epithelial barrier in abdominal IR subjects.
IR, irradiation; Abx, elimination of intestinal microbiota through the antibiotic mixture; FMT, fecal microbiota transplantation; FXR, farnesoid X receptor; SGLT1, sodium-glucose cotransporter-1; IHC, immunohistochemistry; HE, hematoxylin-eosin; IECs, intestinal epithelial cells; MOI, multiplicity of infection; LP, Lactobacillus plantarum; PAS, periodic acid-Schiff.
Andreyev, J. (2005). Gastrointestinal complications of pelvic radiotherapy: are they of any importance? Gut 54, 1051–1054. doi: 10.1136/gut.2004.062596
Bauer, P. V., Duca, F. A., Waise, T. M. Z., Rasmussen, B. A., Abraham, M. A., Dranse, H. J., et al. (2018). Metformin alters upper small intestinal microbiota that impact a glucose-SGLT1-sensing glucoregulatory pathway. Cell Metab.27, 101–117.e105. doi: 10.1016/j.cmet.2017.09.019
Castellanos, J. G., Woo, V., Viladomiu, M., Putzel, G., Lima, S., Diehl, G. E., et al. (2018). Microbiota-induced TNF-like ligand 1A drives group 3 innate lymphoid cell-mediated barrier protection and intestinal T cell activation during colitis. Immunity 49, 1077–1089.e1075. doi: 10.1016/j.immuni.2018.10.014
Chaves-Pérez, A., Yilmaz, M., Perna, C., De La Rosa, S., and Djouder, N. (2019). URI is required to maintain intestinal architecture during ionizing radiation. Science 364, eaaq1165. doi: 10.1126/science.aaq1165
Chen, L., Liao, F., Jiang, Z., Zhang, C., Wang, Z., Luo, P., et al. (2020). Metformin mitigates gastrointestinal radiotoxicity and radiosensitises P53 mutation colorectal tumours via optimising autophagy. Br. J. Pharmacol. 177, 3991–4006. doi: 10.1111/bph.15149
Chiu, C. J., Mcardle, A. H., Brown, R., Scott, H. J., and Gurd, F. N. (1970). Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch. Surg. 101, 478–483. doi: 10.1001/archsurg.1970.01340280030009
Cui, M., Xiao, H., Li, Y., Zhou, L., Zhao, S., Luo, D., et al. (2017). Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol. Med. 9, 448–461. doi: 10.15252/emmm.201606932
De Ruysscher, D., Niedermann, G., Burnet, N. G., Siva, S., Lee, A. W. M., and Hegi-Johnson, F. (2019). Radiotherapy toxicity. Nat. Rev. Dis. Primers 5, 13. doi: 10.1038/s41572-019-0064-5
Ding, S., Song, Y., Brulois, K. F., Pan, J., Co, J. Y., Ren, L., et al. (2020). Retinoic acid and lymphotoxin signaling promote differentiation of human intestinal M cells. Gastroenterology159, 214–226.e211. doi: 10.1053/j.gastro.2020.03.053
Fransson, P., and Widmark, A. (2007). 15-year prospective follow-up of patient-reported outcomes of late bowel toxicity after external beam radiotherapy for localized prostate cancer. A comparison with age-matched controls. Acta Oncol. 46, 517–524. doi: 10.1080/02841860601113596
Fu, T., Coulter, S., Yoshihara, E., Oh, T. G., Fang, S., Cayabyab, F., et al. (2019). FXR regulates intestinal cancer stem cell proliferation. Cell 176, 1098–1112.e1018. doi: 10.1016/j.cell.2019.01.036
Gadaleta, R. M., Van Erpecum, K. J., Oldenburg, B., Willemsen, E. C., Renooij, W., Murzilli, S., et al. (2011). Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60, 463–472. doi: 10.1136/gut.2010.212159
García-Calzón, S., Perfilyev, A., Martinell, M., Ustinova, M., Kalamajski, S., Franks, P. W., et al. (2020). Epigenetic markers associated with metformin response and intolerance in drug-naïve patients with type 2 diabetes. Sci. Transl. Med. 12, eaaz1803. doi: 10.1126/scitranslmed.aaz1803
Gerassy-Vainberg, S., Blatt, A., Danin-Poleg, Y., Gershovich, K., Sabo, E., Nevelsky, A., et al. (2018). Radiation induces proinflammatory dysbiosis: transmission of inflammatory susceptibility by host cytokine induction. Gut 67, 97–107. doi: 10.1136/gutjnl-2017-313789
Gulati, S., Desai, J., Palackdharry, S. M., Morris, J. C., Zhu, Z., Jandarov, R., et al. (2020). Phase 1 dose-finding study of metformin in combination with concurrent cisplatin and radiotherapy in patients with locally advanced head and neck squamous cell cancer. Cancer 126, 354–362. doi: 10.1002/cncr.32539
Guo, H., Chou, W. C., Lai, Y., Liang, K., Tam, J. W., Brickey, W. J., et al. (2020). Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 370, eaay9097. doi: 10.1126/science.aay9097
Hänninen, A., Toivonen, R., Pöysti, S., Belzer, C., Plovier, H., Ouwerkerk, J. P., et al. (2018). Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 67, 1445–1453. doi: 10.1136/gutjnl-2017-314508
Hauer-Jensen, M., Denham, J. W., and Andreyev, H. J. (2014). Radiation enteropathy–pathogenesis, treatment and prevention. Nat. Rev. Gastroenterol. Hepatol. 11, 470–479. doi: 10.1038/nrgastro.2014.46
Higurashi, T., Hosono, K., Takahashi, H., Komiya, Y., Umezawa, S., Sakai, E., et al. (2016). Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 17, 475–483. doi: 10.1016/S1470-2045(15)00565-3
Hunter, R. W., Hughey, C. C., Lantier, L., Sundelin, E. I., Peggie, M., Zeqiraj, E., et al. (2018). Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat. Med. 24, 1395–1406. doi: 10.1038/s41591-018-0159-7
Jain, U., Ver Heul, A. M., Xiong, S., Gregory, M. H., Demers, E. G., Kern, J. T., et al. (2021). Debaryomyces is enriched in Crohn's disease intestinal tissue and impairs healing in mice. Science 371, 1154–1159. doi: 10.1126/science.abd0919
Jian, Y., Zhang, D., Liu, M., Wang, Y., and Xu, Z. X. (2021). The impact of gut microbiota on radiation-induced enteritis. Front. Cell. Infect. Microbiol. 11, 586392. doi: 10.3389/fcimb.2021.586392
Jian, Y. P., Yang, G., Zhang, L. H., Liang, J. Y., Zhou, H. L., Wang, Y. S., et al. (2022). Lactobacillus plantarum alleviates irradiation-induced intestinal injury by activation of FXR-FGF15 signaling in intestinal epithelia. J. Cell. Physiol. 237, 1845–1856. doi: 10.1002/jcp.30651
Jiang, C., Xie, C., Lv, Y., Li, J., Krausz, K. W., Shi, J., et al. (2015). Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun. 6, 10166. doi: 10.1038/ncomms10166
Koh, A., Mannerås-Holm, L., Yunn, N. O., Nilsson, P. M., Ryu, S. H., Molinaro, A., et al. (2020). Microbial imidazole propionate affects responses to metformin through p38γ-dependent inhibitory AMPK phosphorylation. Cell Metab. 32, 643-653.e644. doi: 10.1016/j.cmet.2020.07.012
Liu, Y., Chen, K., Li, F., Gu, Z., Liu, Q., He, L., et al. (2020). Probiotic Lactobacillus rhamnosus GG prevents liver fibrosis through inhibiting hepatic bile acid synthesis and enhancing bile acid excretion in mice. Hepatology 71, 2050–2066. doi: 10.1002/hep.30975
McKenzie, C., Tan, J., Macia, L., and Mackay, C. R. (2017). The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol. Rev. 278, 277–295. doi: 10.1111/imr.12556
Miller, K. D., Nogueira, L., Mariotto, A. B., Rowland, J. H., Yabroff, K. R., Alfano, C. M., et al. (2019). Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 69, 363–385. doi: 10.3322/caac.21565
Modica, S., Petruzzelli, M., Bellafante, E., Murzilli, S., Salvatore, L., Celli, N., et al. (2012). Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology 142, 355–365.e351–354. doi: 10.1053/j.gastro.2011.10.028
Mori, K., Morisaki, H., Yajima, S., Suzuki, T., Ishikawa, A., Nakamura, N., et al. (2011). Beta-1 blocker improves survival of septic rats through preservation of gut barrier function. Intensive Care Med. 37, 1849–1856. doi: 10.1007/s00134-011-2326-x
Mudaliar, S., Henry, R. R., Sanyal, A. J., Morrow, L., Marschall, H. U., Kipnes, M., et al. (2013). Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 145, 574–582.e571. doi: 10.1053/j.gastro.2013.05.042
Ottewell, P. D., Watson, A. J., Wang, T. C., Varro, A., Dockray, G. J., and Pritchard, D. M. (2003). Progastrin stimulates murine colonic epithelial mitosis after DNA damage. Gastroenterology 124, 1348–1357. doi: 10.1016/S0016-5085(03)00288-9
Panigrahi, P., Parida, S., Nanda, N. C., Satpathy, R., Pradhan, L., Chandel, D. S., et al. (2017). A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 548, 407–412. doi: 10.1038/nature23480
Park, M., Kwon, J., Youk, H., Shin, U. S., Han, Y. H., and Kim, Y. (2021). Valproic acid protects intestinal organoids against radiation via NOTCH signaling. Cell Biol. Int. 45, 1523–1532. doi: 10.1002/cbin.11591
Sato, T., and Clevers, H. (2013). Primary mouse small intestinal epithelial cell cultures. Methods Mol. Biol. 945, 319–328. doi: 10.1007/978-1-62703-125-7_19
Sato, T., Stange, D. E., Ferrante, M., Vries, R. G., Van Es, J. H., Van Den Brink, S., et al. (2011). Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762–1772. doi: 10.1053/j.gastro.2011.07.050
Scott, N. A., Andrusaite, A., Andersen, P., Lawson, M., Alcon-Giner, C., Leclaire, C., et al. (2018). Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Sci. Transl. Med. 10, eaao4755. doi: 10.1126/scitranslmed.aao4755
Seok, S., Fu, T., Choi, S. E., Li, Y., Zhu, R., Kumar, S., et al. (2014). Transcriptional regulation of autophagy by an FXR-CREB axis. Nature 516, 108–111. doi: 10.1038/nature13949
van den Berg, F. F., Van Dalen, D., Hyoju, S. K., Van Santvoort, H. C., Besselink, M. G., Wiersinga, W. J., et al. (2021). Western-type diet influences mortality from necrotising pancreatitis and demonstrates a central role for butyrate. Gut 70, 915–927. doi: 10.1136/gutjnl-2019-320430
Vanwijngaerden, Y. M., Wauters, J., Langouche, L., Vander Perre, S., Liddle, C., Coulter, S., et al. (2011). Critical illness evokes elevated circulating bile acids related to altered hepatic transporter and nuclear receptor expression. Hepatology 54, 1741–1752. doi: 10.1002/hep.24582
Verbeke, L., Farre, R., Trebicka, J., Komuta, M., Roskams, T., Klein, S., et al. (2014). Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology 59, 2286–2298. doi: 10.1002/hep.26939
Verbeke, L., Farre, R., Verbinnen, B., Covens, K., Vanuytsel, T., Verhaegen, J., et al. (2015). The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am. J. Pathol. 185, 409–419. doi: 10.1016/j.ajpath.2014.10.009
Wang, J., Wang, Y., Han, J., Mei, H., Yu, D., Ding, Q., et al. (2017). Metformin attenuates radiation-induced pulmonary fibrosis in a murine model. Radiat. Res. 188, 105–113. doi: 10.1667/RR14708.1
Wang, Y. D., Chen, W. D., Wang, M., Yu, D., Forman, B. M., and Huang, W. (2008). Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology 48, 1632–1643. doi: 10.1002/hep.22519
Wu, H., Esteve, E., Tremaroli, V., Khan, M. T., Caesar, R., Manneras-Holm, L., et al. (2017). Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 23, 850–858. doi: 10.1038/nm.4345
Xu, G., Wu, H., Zhang, J., Li, D., Wang, Y., Wang, Y., et al. (2015). Metformin ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic. Biol. Med. 87, 15–25. doi: 10.1016/j.freeradbiomed.2015.05.045
Yang, D., Chen, X., Wang, J., Lou, Q., Lou, Y., Li, L., et al. (2019). Dysregulated lung commensal bacteria drive interleukin-17B production to promote pulmonary fibrosis through their outer membrane vesicles. Immunity50, 692–706.e697. doi: 10.1016/j.immuni.2019.02.001
Yang, J., Wei, H., Zhou, Y., Szeto, C. H., Li, C., Lin, Y., et al. (2022). High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology162, 135–149.e2. doi: 10.1053/j.gastro.2021.08.041
Keywords: irradiation-induced intestinal injury, microbiota, Lactobacillus, metformin, intestinal barrier, farnesoid X receptor (FXR)
Citation: Yang J-Y, Liu M-J, Lv L, Guo J-R, He K-Y, Zhang H, Wang K-K, Cui C-Y, Yan B-Z, Du D-D, Wang J-H, Ding Q, Liu G-L, Xu Z-X and Jian Y-P (2022) Metformin alleviates irradiation-induced intestinal injury by activation of FXR in intestinal epithelia. Front. Microbiol. 13:932294. doi: 10.3389/fmicb.2022.932294
Received: 29 April 2022; Accepted: 21 September 2022;
Published: 13 October 2022.
Edited by:
Maria De Lourdes Pereira, University of Aveiro, PortugalReviewed by:
Yaoxiang Li, Georgetown University Medical Center, United StatesCopyright © 2022 Yang, Liu, Lv, Guo, He, Zhang, Wang, Cui, Yan, Du, Wang, Ding, Liu, Xu and Jian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-Long Liu, ZXlnbGxpdUBzY3V0LmVkdS5jbg==; Zhi-Xiang Xu, emhpeGlhbmd4dTA4QGdtYWlsLmNvbQ==; Yong-Ping Jian, eW9uZ3BpbmdqaWFuMTIzQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.