- 1Department of Molecular Biology and Genetics, Faculty of Health Sciences, Democritus University of Thrace, Alexandroupolis, Greece
- 2Department of Agricultural Development, Democritus University of Thrace, Orestiada, Greece
The Lacticaseibacillus paracasei species is comprised by nomadic bacteria inhabiting a wide variety of ecological niches, from fermented foodstuffs to host-associated microenvironments. Lc. paracasei SP5 is a novel strain, originally isolated from kefir grains that presents desirable probiotic and biotechnological attributes. In this study, we applied genomic tools to further characterize the probiotic and biotechnological potential of the strain. Firstly, whole genome sequencing and assembly, were performed to construct the chromosome map of the strain and determine its genomic stability. Lc. paracasei SP5 carriers several insertion sequences, however, no plasmids or mobile elements were detected. Furthermore, phylogenomic and comparative genomic analyses were utilized to study the nomadic attributes of the strain, and more specifically, its metabolic capacity and ability to withstand environmental stresses imposed during food processing and passage through the gastrointestinal (GI) tract. More specifically, Kyoto Encyclopedia of Genes and Genomes (KEGG) and Carbohydrate-active enzyme (CAZymes) analyses provided evidence for the ability of the stain to utilize an array of carbohydrates as growth substrates. Consequently, genes for heat, cold, osmotic shock, acidic pH, and bile salt tolerance were annotated. Importantly bioinformatic analysis showed that the novel strain does not harbor acquired antimicrobial resistance genes nor virulence factors, in agreement with previous experimental data. Putative bacteriocin biosynthesis clusters were identified using BAGEL4, suggesting its potential antimicrobial activity. Concerning microbe-host interactions, adhesins, moonlighting proteins, exopolysaccharide (EPS) biosynthesis genes and pilins mediating the adhesive phenotype were, also, pinpointed in the genome of Lc. paracasei SP5. Validation of this phenotype was performed by employing a microbiological method and confocal microscopy. Conclusively, Lc. paracasei SP5 harbors genes necessary for the manifestation of the probiotic character and application in the food industry. Upcoming studies will focus on the mechanisms of action of the novel strain at multiple levels.
Introduction
The amended Lactobacillus genus is comprised by more than 200 species and subspecies, organized in clades based on metabolic and phenotypic attributes (Zheng et al., 2015). Full genome sequencing has provided evidence for the vast heterogeneity of this taxonomic group, leading to its reclassification into 26 genera (Zheng et al., 2020). The Lacticaseibacillus paracasei (formerly Lactobacillus paracasei) species includes non-motile, non-spore-forming, rod shaped, facultative anaerobic lactic acid bacteria (LAB) that populate several niches, from fermented foodstuffs to host-associated microenvironments (Zheng et al., 2020). The versatility is imprinted in their genomes, as they code for genes mediating adaptation to both anaerobic and aerobic environments, as well as in plant, insect, and animal hosts (Hatti-Kaul et al., 2018). Their employment as starter cultures for novel dairy and non-dairy fermented products, such as white brined cheese (Terpou et al., 2018), rice-flour (D’Auria et al., 2021), cranberry juice (Mantzourani et al., 2019a) and pomegranate beverage (Mantzourani et al., 2020) and therefore their biotechnological importance has been well documented. Moreover, their role in the prevention or management of several intestinal and extraintestinal diseases, and their potential application as probiotics, have been described in several studies (Hill et al., 2018). For example, consumption of milk fermented with Lc. paracasei strain Shirota showed to alleviate constipation in depressed individuals (Otaka et al., 2021), whereas Lc. paracasei Lpc-37® administration could modulate stress and anxiety, in healthy adults (Patterson et al., 2020). In parallel, mechanistic studies have shown that Lc. paracasei isolates may present antiproliferative (Saxami et al., 2017), immunomodulatory (Chondrou et al., 2020), antibiofilm and antimicrobial activities (Acurcio et al., 2020), in a strain-specific manner.
The development and application of genomic methodologies have contributed significantly to the knowledge and understating of the probiotic and biotechnological potential of novel LAB strains (Kiousi et al., 2021). Indeed, phylogenomics paired with comparative genomics facilitate the taxonomic classification of isolates, pinpoint putative probiotic markers, and provide information about their fermentation profile and niche preference (Pan et al., 2021; Surve et al., 2022). Furthermore, genome mining helps toward the identification of virulence phenotypes and resistance to common antibiotics, offering valuable insights into potential health hazards associated with their consumption (Wang et al., 2021a).
Potential probiotic microorganisms can be found as autochthonous or allochthonous in the mammalian GI tract. Indeed, genomic data showed that they have co-evolved with the animal host, undergoing selective pressure and extensive genome modifications to adapt to the gut mucosa and epithelium (Duar et al., 2017). Specifically, host-associated LAB strains have traded the ability to produce vitamins and amino acids, that can be readily provided by the host, for the capacity to code for proteins associated with stress survival and host interactions (Salvetti and O’Toole, 2017). In this context, genes encoding for bile acid hydrolases, proton pumps and proteolytic enzymes, are abundant in their genome (Arnold et al., 2018).
In order to be efficient, probiotic bacteria must adhere to epithelial cells of the host, at least transiently (Monteagudo-Mera et al., 2019). Adhesion is a complex process that is mediated by protein-protein or protein-polysaccharide interactions. More specifically, host-associated lactobacilli code for proteins that can participate in specific carbohydrate-binding interactions, or non-specific electrostatic and hydrophobic attachment to the gut niche (Monteagudo-Mera et al., 2019). Importantly, the cell wall of numerous LAB strains is decorated with pili, housekeeping proteins and glycolytic enzymes with moonlighting functions, major contributors to mucin attachment (Reunanen et al., 2012; Waśko et al., 2014). Several in silico studies have identified these genetic clusters in the genome of Lc. paracasei strains, strengthening the notion that they can interact with the gut niche, when consumed (Bäuerl et al., 2010; Koryszewska-Bagińska et al., 2019).
Lc. paracasei SP5 is a LAB strain, originally isolated from kefir grains (Mantzourani et al., 2019b). Preliminary evaluation, based on a series of established in vitro tests, including tolerance to bile salts, resistance to digestion enzymes and acidic pH, as well as susceptibility to common antibiotics, demonstrated that, Lc. paracasei SP5 presents probiotic potential (Mantzourani et al., 2019b). Recent studies from our lab have also shown that the novel strain possesses desirable biotechnological properties. Indeed, Lc. paracasei SP5 was successfully utilized for the production of fermented chokeberry juice (Bontsidis et al., 2021) and white brined cheese (Plessas et al., 2021). In the present study, genomic approaches were applied to further characterize the probiotic and biotechnological attributes of Lc. paracasei SP5. Firstly, whole genome sequencing and assembly, were performed to construct the chromosome map of the strain. The genomic stability of the strain was estimated based on the presence of plasmids and mobile elements. Average nucleotide identity (ANI) was used as a metric to confirm that the novel strain is unique, while comprehensive phylogenomic analysis was employed to validate its classification to the Lc. paracasei species. Moreover, comparative genomic analysis was performed to detect genetic loci related to resistance to extreme conditions, bile acid, low pH, osmotic and oxidative stress, and KEGG and CAZymes analyses to determine the metabolic profile of the novel strain. Annotation algorithms were employed to detect genetic clusters for bacteriocin production and biofilm formation, as well as genes implicated in the adhesive phenotype and the production of pili and exopolysaccharides. The adhesion properties of the novel strain were also studied and evaluated in vitro, employing a quantitative microbiological method and confocal microscopy.
Materials and Methods
Bacterial Strains, Culture Conditions and DNA Isolation
Lc. paracasei SP5 was previously isolated from kefir grains (Mantzourani et al., 2019b). Lc. rhamnosus GG ATCC 53103 (LGG) was acquired from DSMZ (Braunschweig, Germany). The bacterial strains were grown O/N in de Man, Rogosa and Sharp (MRS) broth (Condalab, Madrid, Spain) at 37°C, under anaerobic conditions. For DNA isolation, overnight cultures of Lc. paracasei SP5 were pelleted by centrifugation at 8,000 × g for 4 min. The pellet was lysed, and DNA was extracted using the NucleoSpin® Tissue kit (Macherey-Nagel, Düren, Germany), according to manufacturer’s instructions. DNA fragmentation was determined in 1% (w/v) agarose gel, and the quantity and quality of the isolated nucleic acids were also determined spectrophotometrically at 260 nm using NanoDrop® ND-1000 UV-Vis Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States).
Whole Genome Sequencing and de novo Assembly
The isolated genomic DNA was sequenced using the Illumina NovaSeq6000 (2 × 151 paired ends) platform. A total of 10,389,922 paired-end reads were obtained. The quality of the reads was determined using FASTQC (v0.11.9; Andrews, 2010), and low-quality reads were removed using Trimmomatic (version 0.39; Bolger et al., 2014). De novo assembly was executed with SPAdes (version 3.15.1), with default parameters, selecting the –careful parameter to minimize mismatches (Bankevich et al., 2012). Assembly metrics were calculated with the QUality Assessment Tool (QUAST; version 5.2.0; Gurevich et al., 2013).
Genome Annotation
Genome annotation was performed using Prokka (version 1.14.5; Seemann, 2014), and the local version of Prokaryotic Genome Annotation Pipeline (PGAP; Tatusova et al., 2016), with default parameters. The presence of plasmids in the assembled sequence was determined using PlasmidFinder (Carattoli et al., 2014) and of mobile genetic elements with MobileElementFinder (Johansson et al., 2021). Prophage regions were detected using PHAge Search Tool Enhanced Release (PHASTER; Arndt et al., 2016) and insertion sequence elements with ISFinder (Siguier et al., 2006). The assembly was searched for the presence of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) arrays using CRISPRDetect (version 2.4; Biswas et al., 2016) and PILER-CR (Edgar, 2007). The EggNOGmapper (version 2.0) tool of the online EggNOG database (version 5.0; Huerta-Cepas et al., 2019) was used for the classification of predicted proteins into Clusters of Orthologous Groups (COGs). BlastKOALA (version 2.2) was utilized for the assignment of proteins into KEGG Orthology (KO) groups and the production of KEGG maps (Kanehisa et al., 2016). Additionally, CAZyme annotation was performed using the dbCAN2 meta server (Zhang et al., 2018) and Traitar was used for phenotypic characteristics prediction (Weimann et al., 2016). The visualization of the genome assembly was completed using Artemis (version 18.1.0; Carver et al., 2012).
Comparative Genomics
The genome sequences of all available Lc. paracasei strains (January 2022) were obtained using a python script and were categorized based on isolation source. ANI was calculated for all strains using Pyani, a python module (version 0.2.10; Pritchard et al., 2015) to verify that Lc. paracasei SP5 is a unique strain. Pangenome analysis of Lc. paracasei strains was performed with Roary (version 3.13.0; Page et al., 2015), and core genome sequences were used to construct a phylogenomic tree with FastTree 2.1 (Price et al., 2010). Whole genome sequences of 14 Lc. paracasei strains originating from dairy and non-dairy sources, LGG, Lactiplantibacillus pentosus BGM48, Lactiplantibacillus plantarum DF and Staphylococcus aureus NCTC 8325, were aligned with progressiveMauve (Darling et al., 2010). The publicly available online EMBL tool “Interactive Tree of Life” (iTol; version 6.1.1; Letunic and Bork, 2016) was used for the visualization of the trees.
Detection of Genetic Elements Associated With Probiotic Characteristics
Genes involved in antimicrobial resistance were investigated using Resistance Gene Identifier (RGI; version 5.2.0) and ResFinder 4.1 (Zankari et al., 2012; Bortolaia et al., 2020). Genes coding for virulence factors were determined with VirulenceFinder 2.0 (Joensen et al., 2014; Tetzschner et al., 2020), while putative pathogenic sequences were determined with PathogenFinder 1.1 (Cosentino et al., 2013). Putative bacteriocin clusters were identified using BAGEL4 (van Heel et al., 2018). The presence of proteins involved in the adhesion phenotype was investigated using BLAST+ (Basic Local Alignment Search Tool; Camacho et al., 2009) and alignment of genes of the spaCBA and spaFED clusters was performed with ClustalW (Thompson et al., 1994). Visualization of the alignment of spaF gene sequences was performed with Jalview (Waterhouse et al., 2009) and of the phylogenetic tree with the publicly available tool, iTol. Classification of protein families involved in the adhesion phenotype was performed by InterPro (Blum et al., 2021) and conserved domains were identified with Pfam (Mistry et al., 2021) and ScanProsite (de Castro et al., 2006). Characterization of the physicochemical properties of the putative spaF pilin was performed using ProtParam (Gasteiger et al., 2005). SignalP 6.0 was utilized to predict N-terminal signals (Teufel et al., 2022).
Human Cancer Cell Line
The human colon adenocarcinoma cell line HT-29 was purchased from the American-Type Culture Collection (ATCC, LGC Standards, Middlesex, United Kingdom). Cells were maintained at 37°C, 5% CO2, in a humidified atmosphere under sterile conditions in RPMI-1640 medium, supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Thermo Fisher Scientific, Waltham, MA United States).
Preparation of Viable Bacteria for Adhesion Assays
Strains Lc. paracasei SP5 and LGG were incubated O/N in MRS broth, at 37°C, under anaerobic conditions. The next day, the bacterial cells were centrifuged at 4,000 × g for 10 min. The cell pellet was resuspended in RPMI-1640 medium supplemented with 10% heat-inactivated FBS and 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; all from Thermo Fisher Scientific) at a final concentration of 108 CFU/mL.
Assessment of Bacterial Adhesion by Quantitative Analysis
The quantitative adhesion assay was performed as described previously, with minor modifications (Plessas et al., 2020). HT-29 cells were seeded in 24-well plates at a density of 40 × 104 cells per well and incubated for 14 days to form a monolayer. A day prior to the treatments, the cell monolayer was washed with phosphate buffered saline (PBS; Thermo Fisher Scientific) and fresh, antibiotic-free medium was added. The next day, viable L. paracasei SP5 or LGG cells, at a final concentration of 108 CFU/mL were added to each well, with each strain being tested in duplicate. After 4 h of coincubation at 37°C, cells were washed twice with PBS and lysed with 1% Triton X-100 (Sigma-Aldrich, Saint Louis, MO, United States). The lysates were serially diluted in Ringer’s solution (Lab M, Lancashire, United Kingdom), plated on MRS agar, and incubated at 37°C, until the formation of visible colonies. Colony forming units per milliliter (CFU/mL) were determined with the formula: CFU/mL = (No. of colonies × dilution factor)/volume of culture plate and was used as viable count measure. The experimental procedure was repeated three independent times and the results are represented as mean ± standard deviation.
Assessment of Bacterial Adhesion via Confocal Microscopy
Confocal microscopy was used for the visualization of bacterial attachment onto HT-29 cells. To this end, cells were seeded in a 6-well plate at a density of 5 × 105 cells per well on No. 1.5 coverslips and were incubated overnight at 37°C with 5% CO2 in a humidified incubator. T he next day, cells were washed with PBS and were stained with Hoechst 33342 (Biotium, Hayward, CA, United States) for 20 min, according to manufacturer’s instructions. Simultaneously, lactobacilli were stained with 10 μM carboxyfluorescein succinimidyl ester (CFSE; ThermoFisher Scientific) in PBS to a final concentration of 108 CFU/mL, for 20 min at 37°C. After staining, the lactobacilli suspension was co-cultured with HT-29 cells for three and a half hours at 37°C with 5% CO2 in a humidified incubator. Subsequently, cells were washed three times with fresh medium and were stained with CellBrite Red Cytoplasmic Membrane Dye (Biotium), according to manufacturer’s instructions. Then, cells were washed with fresh PBS and fixed in 4% paraformaldehyde (PFA; AppliChem, Darmstadt, Germany) in PHEM solution consisted of 25 mM HEPES, 10 mM EGTA (Merck Millipore, Burlington, MA, United States), 60 mM PIPES, 2 mM MgCl2 (Applichem) pH 6.9, for 12 min at room temperature, followed by three washes with PBS. Finally, coverslips were mounted in homemade mowiol 4-88 (AppliChem) medium.
Image acquisition was performed on a customized Andor Revolution Spinning Disk Confocal system (Yokogawa CSU-X1; Yokogawa, Tokyo, Japan), built around an Olympus IX81 (Olympus Shinjuku, Tokyo, Japan), with 40 × 0.95NA air lens (UPlanSApo; Olympus Shinjuku, Tokyo, Japan) and a digital camera (Andor Ixon+885; Andor Technology Ltd., Belfast, Northern Ireland). The system was controlled by Andor IQ3 software (Andor Technology). Images were acquired as z-stacks with a z-step of 1 μm, through the entire volume of the cells. For each image, maximum projection of z-stacks was generated, and background was subtracted using a custom script in ImageJ (National Institute of Health, United States). The number of HT-29 cells with adhered lactobacilli was counted manually with the Cell Counter plugin on ImageJ (version 1.53f51). For each condition, more than 1000 cells were counted in two independent experiments and the ratio of the HT-29 cells with adhered bacteria to the total number of eukaryotic cells were calculated.
Results and Discussion
Genome Features and Stability
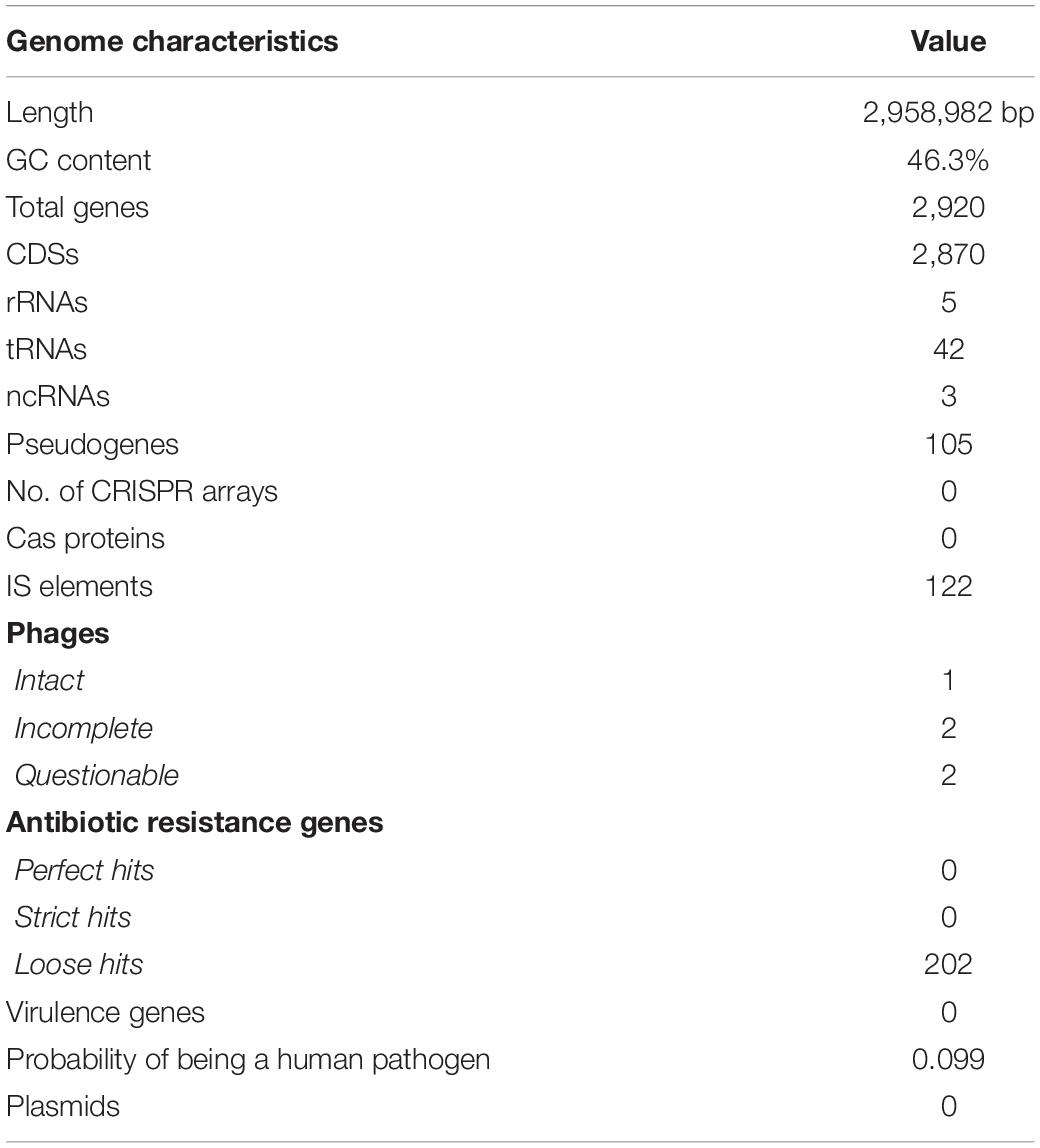
Whole-genome sequencing, de novo assembly and genome annotation were performed to investigate the genomic features of Lc. paracasei SP5 (Figure 1). The genome of Lc. paracasei SP5 has a total length of 2,958,982 bp and a GC content of 46.3%. The strain harbors 2,920 predicted genes; 2,870 coding DNA sequences (CDSs), 105 pseudogenes, 5 rRNA, 42 tRNA and 3 ncRNAs (Table 1). Genome metrics can be a crude indicator of the lifestyle of LAB strains. Indeed, studies have shown that genome size of lactobacilli ranges from 1.28 to 4 Mb, depending on their preferred environmental niche (Duar et al., 2017). Over the course of evolution, members of the Lactobacillus sensu lato have underwent a process of genome reduction, during the transition from free living to nomadic and matrix-associated bacteria (Sachs et al., 2011). Free-living and nomadic strains carry larger genomes (3–4 Mb) to support their survival in heterogenous matrixes. Lc. paracasei is a member of the former Lc. casei group alongside the nomadic Lc. casei and Lc. rhamnosus species (Hill et al., 2018). These species harbor genomes with median length and GC content of 2.9 Mb and 46–47%, respectively. They inhabit similar niches, predominantly dairy products, while they can also be found in association with the host (Hill et al., 2018). In this context, Lc. paracasei SP5 genome metrics may support its classification in nomadic lactobacilli. On the other hand, strictly host-associated strains, possess smaller genomes (1.28–3 Mb), resulting from extensive gene loss (Zheng et al., 2015; Duar et al., 2017). This phenomenon can be attributed to the fact that specializing to a nutrient-dense environment leads to the loss of redundant functions, such as amino acid biosynthesis, while strains become more selective in their energy sources (Fontana et al., 2018). In this context, Lactobacillus iners with a median genome length of 1.28 Mb is the most prominent example of extreme niche specialization, as it has acquired clusters for survival in the human vagina (Macklaim et al., 2011). The reverse process (gene accumulation) has been recorded in the Limosilactobacillus fermentum species, that is undergoing transition from host-associated to free living (Duar et al., 2017).
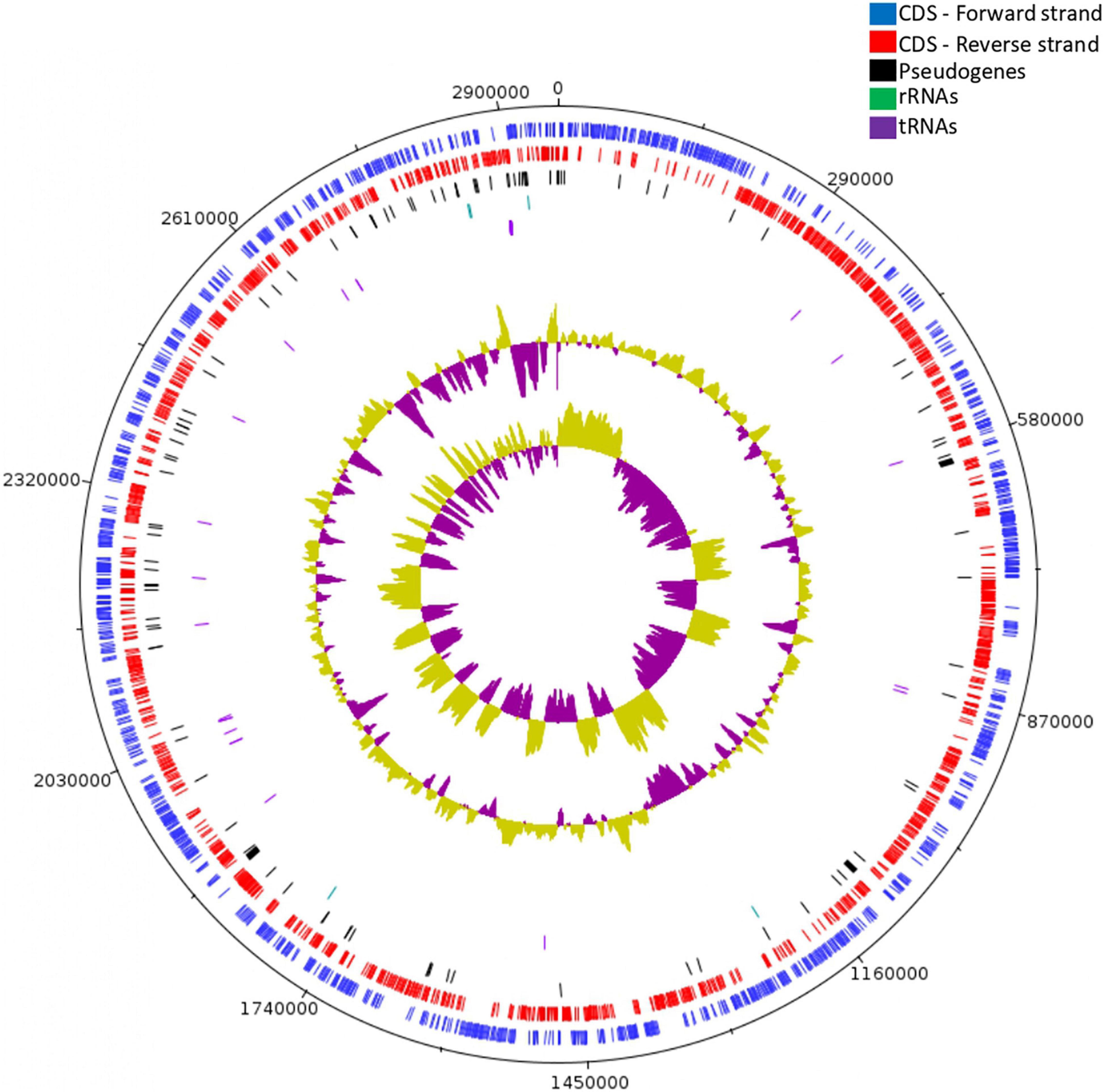
Figure 1. Circular genome map of Lc. paracasei SP5, constructed using Artemis. From outer circle to inner, genomic features are presented as: forward strand CDS (blue), reverse strand CDS (red), pseudogenes (black), rRNA genes (green) tRNA genes (purple), GC content and GC skew.
Lc. paracasei SP5 carries 122 insertion elements, 5 prophage regions (1 intact, 2 incomplete and 2 questionable; Supplementary Table 1) and no mobile genetic elements or plasmids (Table 1). Importantly, the strain lacks functional CRISPR arrays and does not code for Cas proteins, being therefore susceptible to bacteriophage assaults and accumulation of insertion sequences (Rath et al., 2015). Genome analysis with ISFinder showed that the majority of the insertion sequences originate from Lc. casei and Lc. rhamnosus, while several sequences were also derived from Leuconostoc and Pediococcus species. These bacteria are commonly found in the microbiome of dairy products, alluding to events of genetic transfer in these matrixes (Bonham et al., 2017). Insertion elements could play an important role in the expression of bacterial genes and genome evolution, and the availability of a large number of whole genome sequences have paved the way for the characterization of novel elements (Siguier et al., 2014). However, they can compromise genome stability, an important safety indicator for probiotics (Hill et al., 2014). Lactobacilli are known to possess a large number of transposons and mobile elements scattered in their genomes, providing evidence for transfer of genetic material in food and/or animal microbiota (Nicolas et al., 2007; Liu et al., 2009). Indeed, the intestinal cavity promotes these events in high frequencies (Lerner et al., 2017), and thus, it is of outmost importance to determine if they carry virulence factors and resistance genes that they can share with malignant species. On that note, bioinformatic analysis with RGI and VirulenceFinder showed that the novel strain does not harbor mobile antibiotic resistance genes or other virulence factors that could transport to bacteria of the gut microbiome. In this context, the probability of Lc. paracasei SP5 being a human pathogen is estimated to be below 0.1% (Table 1). Notably, Lc. paracasei strains have a long history of safe consumption, and previous studies have shown that administration in supplements or in fermented foodstuffs is well tolerated (Costa et al., 2014; Ren et al., 2022).
Phylogenetic Analysis
An approximately-maximum-likelihood phylogenetic tree based on orthologous gene clusters was built with 1,000 bootstrap replications to reveal the position of the novel strain within the Lc. paracasei species (Figure 2). Furthermore, a phylogenetic tree based on whole genome sequence of Lc. paracasei SP5 was, also, constructed, with Staphylococcus aureus NCTC8325 as an outgroup (Supplementary Figure 1). These findings agree with the preliminary classification of the strain to the Lc. paracasei species, based on 16S rRNA sequencing and species-specific multiplex PCR, with primers targeting the conserved tuf gene (Mantzourani et al., 2019b). Importantly, this is the first published work that presents a phylogenetic tree based on the core genome sequences of all available Lc. paracasei strains (as of January 2022), including their isolation source. As shown in Figure 2, Lc. paracasei SP5 clusters with other dairy isolates, as well as with one strain isolated from fermented soybeans, and several human-associated members of the species. The fact that strains do not form phylogenetic clusters based on their origin, reflects the nomadic character that is hardwired in their genomes (Duar et al., 2017). On a larger scale, the members of the former Lactobacillus genus, form clades based on their metabolic and fermentation capabilities, rather than their origin (Zheng et al., 2015). It is suggested that fermented foods are hardly the primary habitat of lactobacilli, however, tracing their origins is a challenging task (Duar et al., 2017).
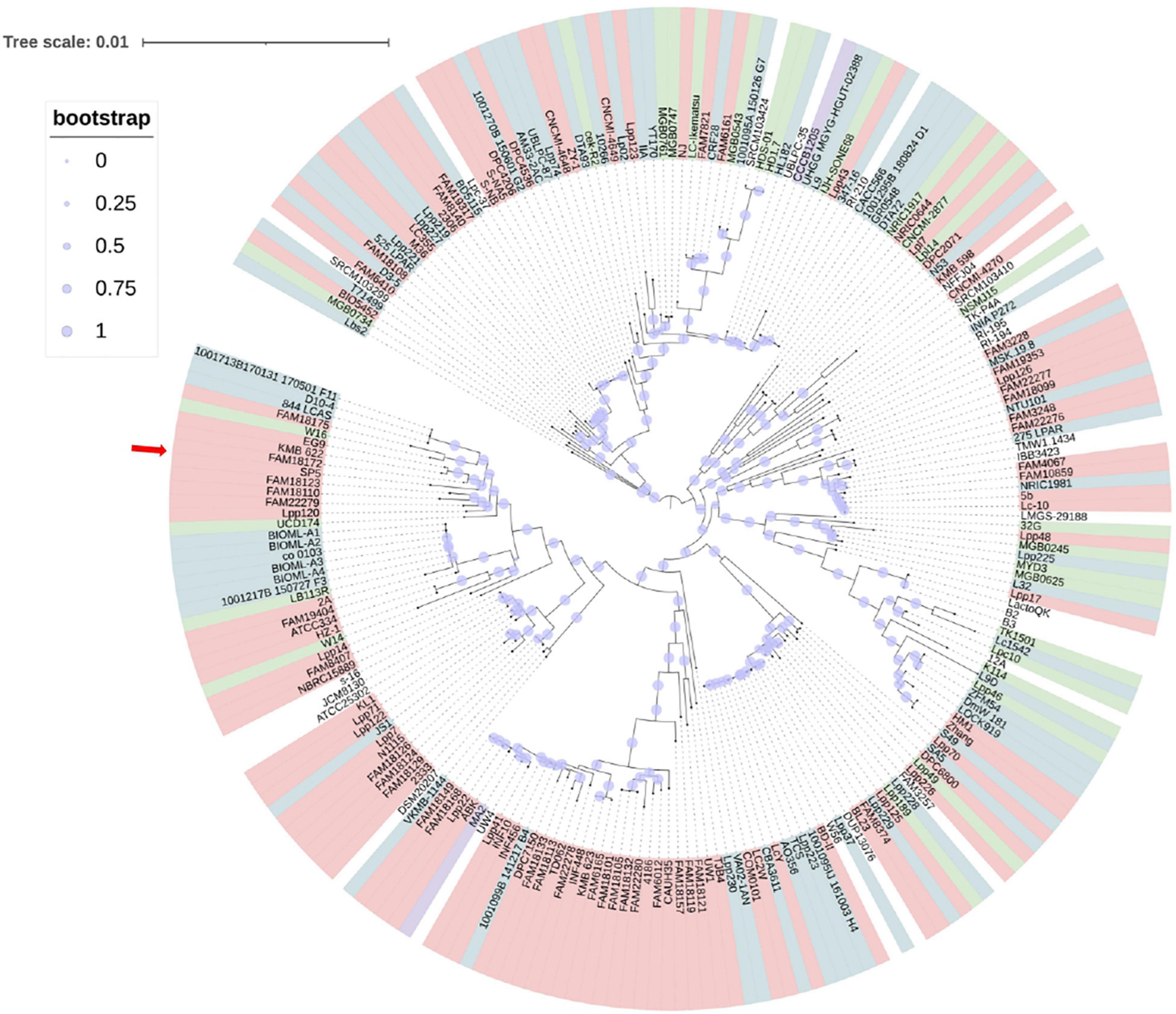
Figure 2. Approximately-maximum-likelihood phylogenetic tree of all available (239 as of January 2022) Lc. paracasei isolated from various sources (light pink – dairy products, blue – host-associated strains, green – vegeTables–associated strains, lilac – strains isolated from non-dairy beverages) based on orthologous genes calculated by Roary (version 3.13.0) and built with 1,000 bootstrap replications. The red arrow indicates the position of Lc. paracasei SP5 in the phylogenetic tree.
ANI was selected as a metric for the nucleotide-level genomic similarity of Lc. paracasei SP5 with other strains of the species (Figure 3 and Supplementary Table 2), as well as for taxonomic classification with the cutoff for species boundaries set at 96% (Ciufo et al., 2018). In this study, ANI analysis was performed for all available strains showing that the novel strain is unique and presents high similarity with both dairy and non-dairy Lc. paracasei strains. More specifically, Lc. paracasei SP5 presents high genome similarity to Lc. paracasei Lpp120, Lc. paracasei KMB_622, Lc. paracasei FAM18172, all isolated from fermented milk products (Smokvina et al., 2013; Wüthrich et al., 2018; Figure 3B). Interestingly, Lc. paracasei SP5, also, presents high-level ANI (> 99%) to human-derived isolates, such as Lc. paracasei 844_LCAS (Roach et al., 2015), Lc. paracasei D10-4 and Lc. paracasei co_0103 (Jiang et al., 2019), as well as to Lc. paracasei W14 and W16, originally isolated from fermented soybeans (National Center for Biotechnology Information, 2017a,b) (Supplementary Table 2).
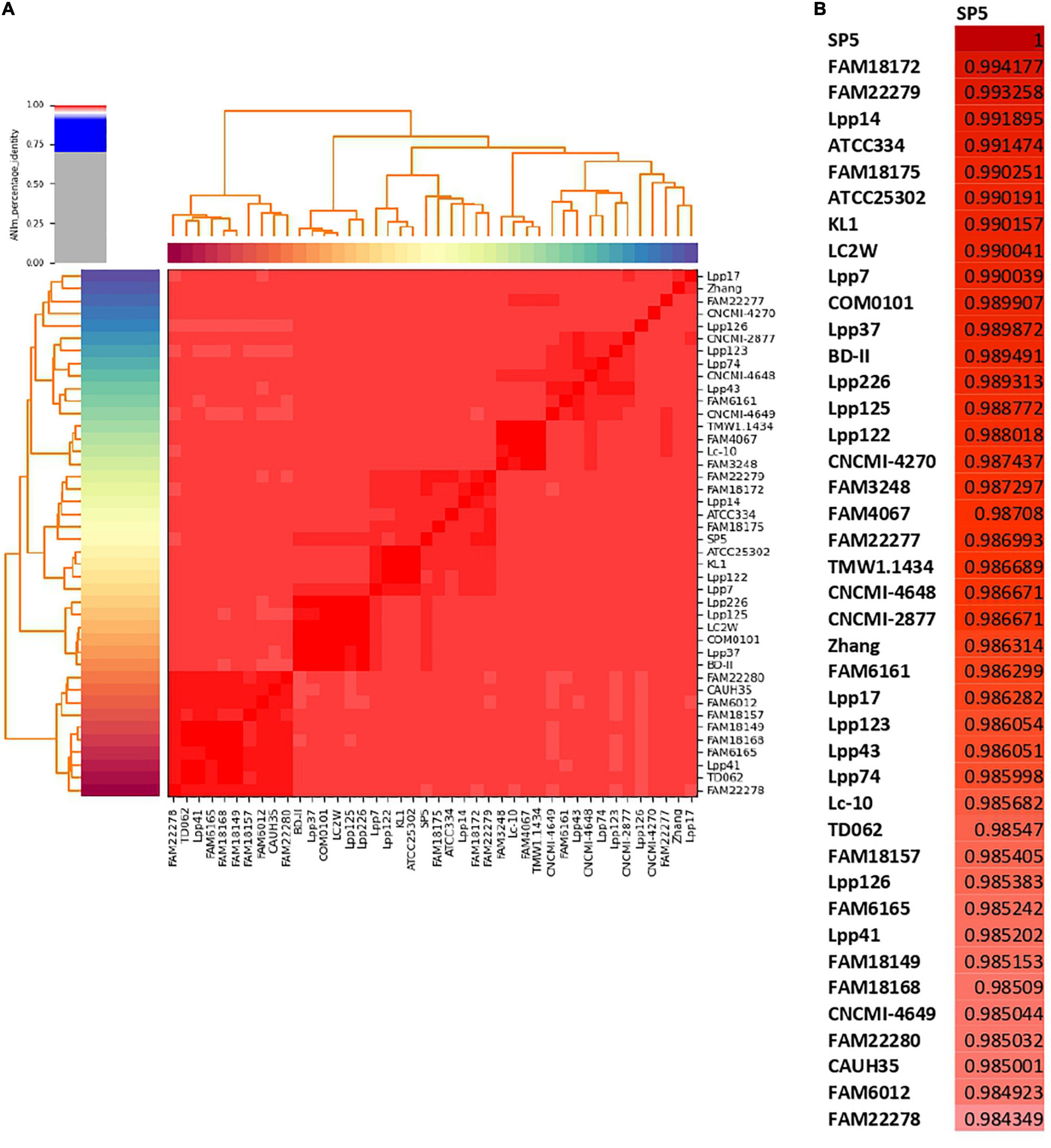
Figure 3. ANI of Lc. paracasei strains derived from fermented milk products estimated using Pyani (version 0.2.10). (A) ANI heatmap of all dairy isolates, (B) ANI of Lc. paracasei SP5 with dairy isolates.
Pangenome Analysis and Comparative Genomics
Pangenome analysis was performed in a subset of 42 strains derived from fermented milk products. The pangenome of these strains consists of a total of 12,423 orthologous groups (Figure 4). Amongst these, 489 genes belong to the conserved core genome, 741 to the soft-core genome, 2,596 to the shell genome and 8,596 genes to the cloud genome. The pangenome of these strains was further classified into COG categories (Table 2). The most represented category is “Function unknown” (17.11%), followed by “Replication and repair” (16.11%). Lc. paracasei SP5 codes for 97 unique, strain-specific gene groups that can be categorized into 15 COG categories. The majority of these strain-specific proteins possess unknown functions (19.05%) or are implicated in “Carbohydrate metabolism and transport” (16.67%). Further annotation revealed the presence of unique genes coding for transposases and for proteins involved in polysaccharide biosynthesis and transport (Supplementary Table 3). A previous study on a subset of 34 Lc. paracasei strains derived from a variety of sources, revealed that genes involved in microbe-host interactions can be found at the core genome of the species (Smokvina et al., 2013). Interestingly, genes mediating adhesion on the GI mucosa were also identified in the core genome of the dairy isolates included in this study. More specifically, genes coding for the moonlighting proteins with adhesive properties enolase (eno) and phosphoglycerate mutase (pgm6) were annotated in the core genome of the strains (Supplementary Table 4). It should be noted that the classification of pangenome proteins and proteins encoded by Lc. paracasei SP5 to COG categories followed a similar pattern (Table 2). However, the pangenome of the strains contains more proteins in the “Replication and Repair” (16.11%) category than Lc. paracasei SP5. This finding could be attributed to the fact that the vast array of transposable and insertion elements encoded by Lc. paracasei strains are classified into this category. In a previous pangenome comparative study of probiotic genera that included the emended Lactobacillus genus, it was found that the majority of proteins in the pangenome possess unknown functions, or cluster in the L category (Lukjancenko et al., 2012), in agreement with the pattern observed in the present study.
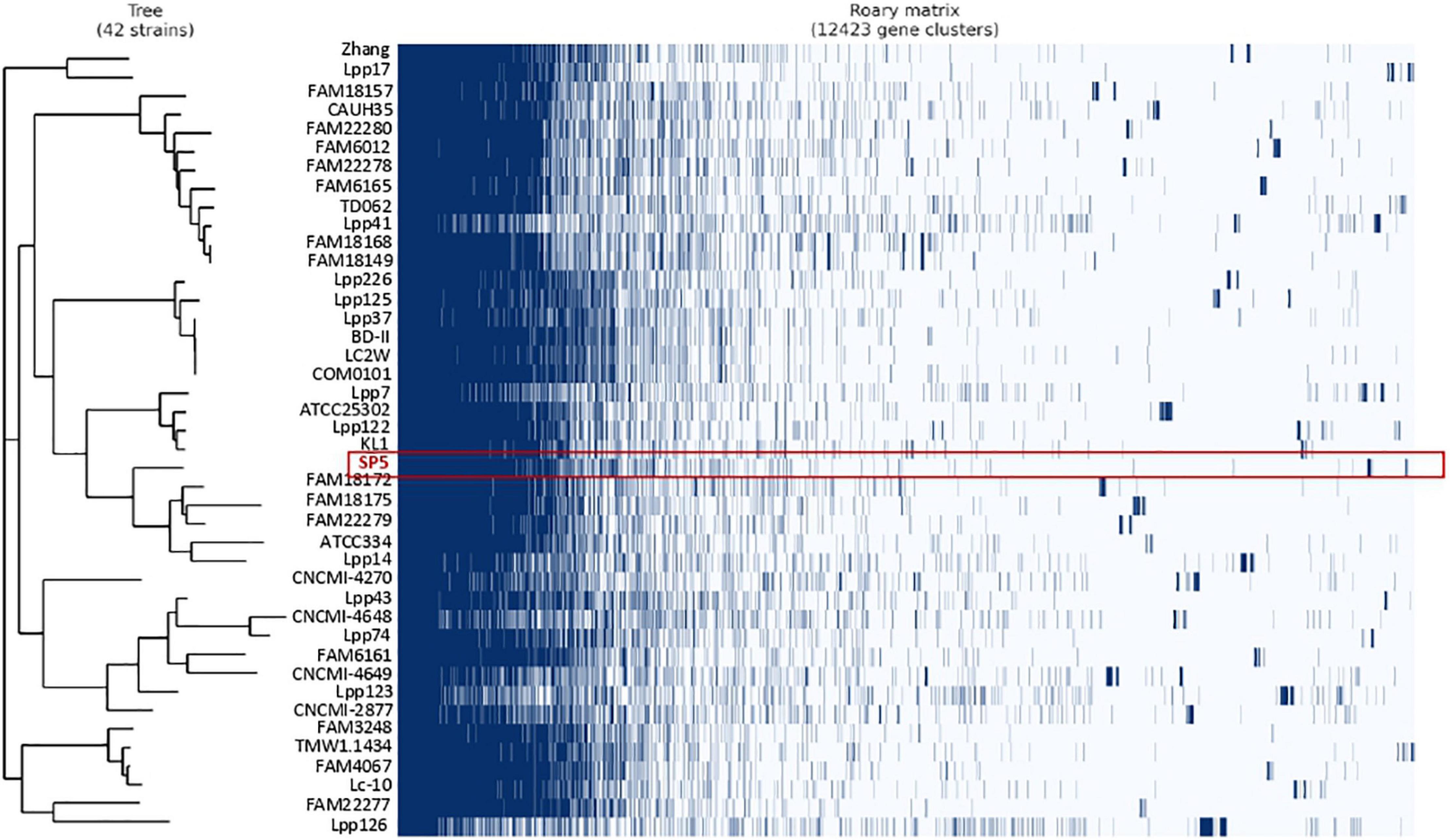
Figure 4. Pangenome analysis of Lc. paracasei strains isolated from fermented milk products, performed using Roary (version 3.13.0). The absence (white) or presence (dark blue) of core and accessory genes are depicted in the matrix. Highlighted in red are clusters assigned to Lc. paracasei SP5.
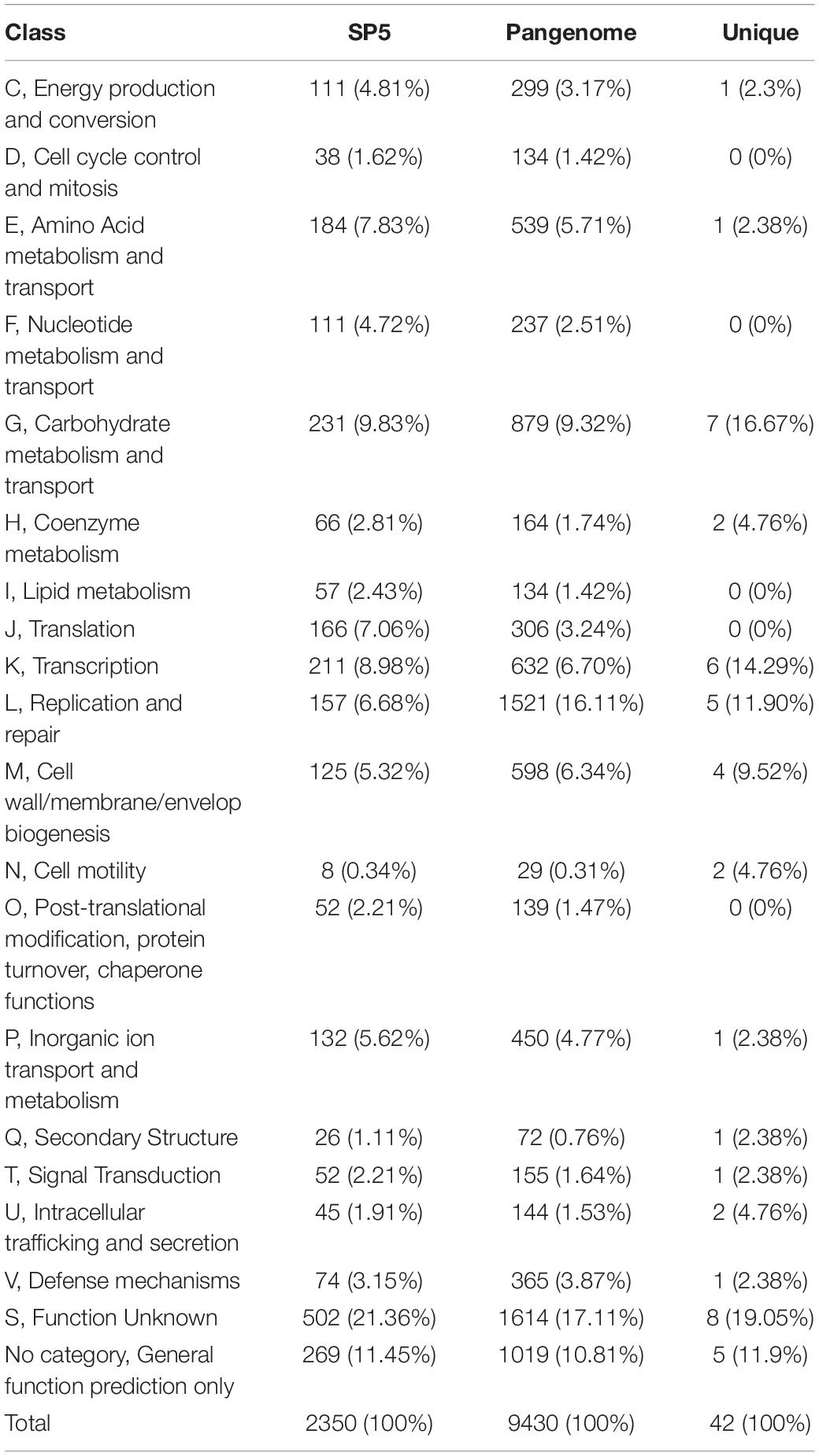
Table 2. Categorization of genes (SP5, Pangenome, SP5 unique genes) to clusters of orthologous genes (COGs).
Functional Classification of Genes and Prediction of the Metabolic Potential of Lc. paracasei SP5
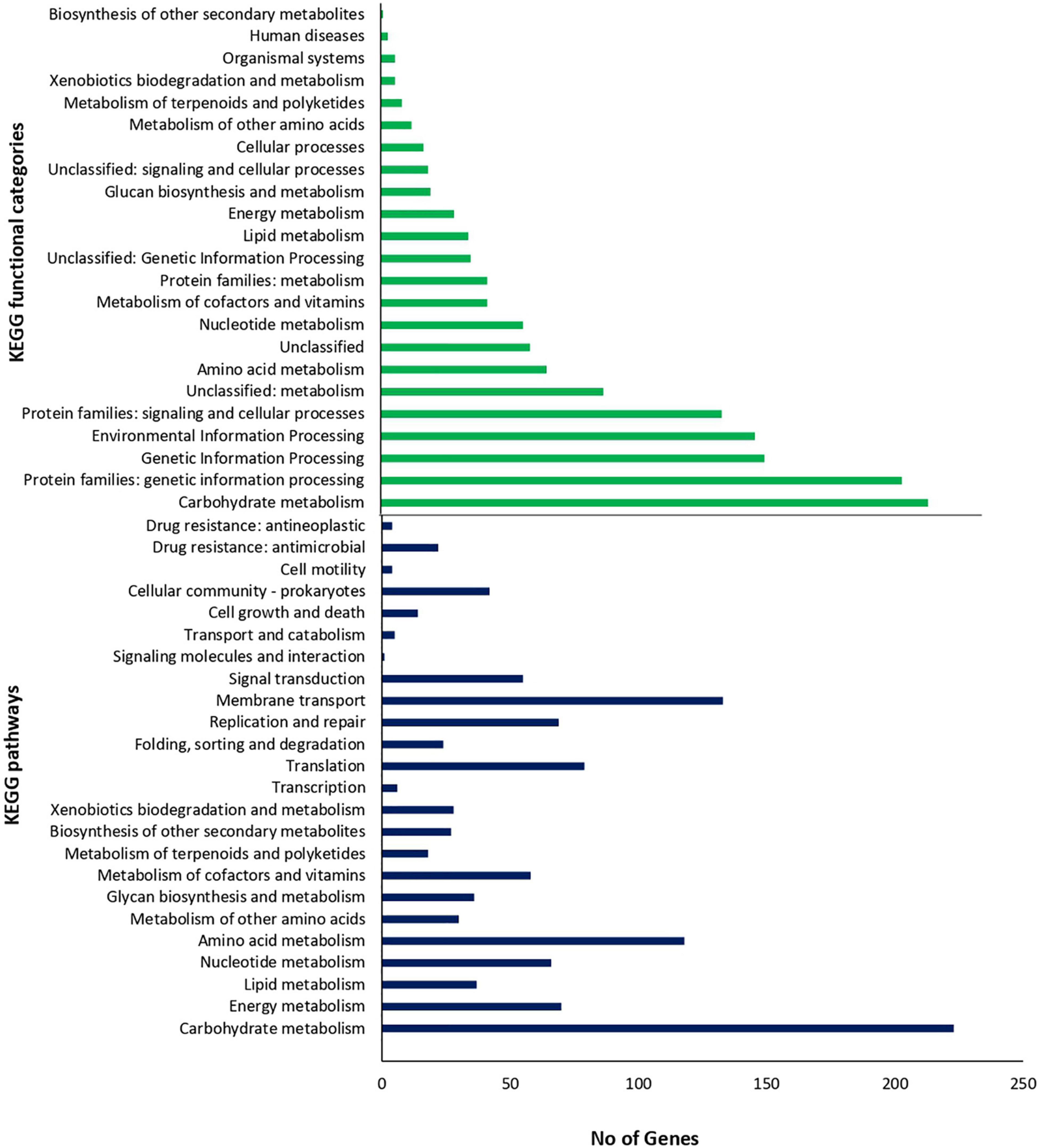
To gain a better insight into the functional characteristics of the genome of Lc. paracasei SP5, its protein sequences were allocated into the 20 COG categories using EggNog. As shown in Table 2, the most represented category is “Function Unknown” (21.36%), followed by “Carbohydrate metabolism and transport” (9.83%), “Transcription” (8.98%), “Translation” (7.06%) and “Replication and repair” (6.68%). KEGG analysis of the assembled genome resulted in protein assignment into 191 pathways, organized into 24 broader groups, and 23 functional categories (Figure 5). Similarly, to the COG profile of Lc. paracasei SP5 genes, most proteins were assigned to the “Carbohydrate metabolism” category (223 proteins), followed by the “Membrane transport” (133 proteins) and “Amino acid metabolism” (118 proteins) categories (Figure 5). Concerning KEGG pathway assignment, the majority of annotated proteins cluster in the “Biosynthesis of secondary metabolites” (162 proteins, KO 01110) and the “Microbial metabolism in diverse environments” (95 proteins, KO 01120) pathways (Figure 5). Complete biosynthetic pathways for the production of 3 (threonine, lysine and proline) out of 20 amino acids (Supplementary Figures 2–4), and incomplete clusters with one block missing for the production of cysteine, methionine, arginine, histidine, tryptophan and glutathione were, also, found in the genome of Lc. paracasei SP5. This finding could be indicative of the gene decay that the strain underwent during its evolutionary history. More specifically, loss of amino acid biosynthetic capability is a shared characteristic of strains transitioning from a free-living to a matrix-associated lifestyle (Duar et al., 2017). Importantly, Lc. paracasei SP5 codes for numerous proteolytic enzymes, peptidases (e.g., membrane dipeptidase, pepD2), oligopeptidases (e.g., oligopeptidase F, pepF2) and amino acid transporters, including the Di-/tripeptide transporter (dtpT), compensating for its limited biosynthetic capability. Recently, we have reached similar conclusions for the nomadic strains Lp. pentosus L33 and Lp. plantarum L125 (Stergiou et al., 2021; Tegopoulos et al., 2021). The proteolytic capability of strains to be incorporated in fermented foodstuffs could be an asset in the functional food industry, due to degradation of allergens or the production of bioactive metabolites after amino acid catabolism (Wang et al., 2021b). However, casein degradation could negatively affect the quality of fermented dairy products (Lesme et al., 2020). In this context, Traitar analysis showed that Lc. paracasei SP5 cannot break down this protein (Supplementary Figure 5), a finding supporting its application in the production of dairy foodstuffs. Additionally, amino acid catabolism could lead to the accumulation of biogenic amines in the food matrix (Wang et al., 2021b). Biogenic amines are commonly found in fermented products in low quantities, without necessarily affecting their quality and organoleptic characteristics (Li et al., 2018), however, their concentration should be monitored. The enzymes implicated in their production are decarboxylases and deiminases. PGAP annotation showed that Lc. paracasei SP5 codes for ornithine decarboxylase, an enzyme that catalyzes the production of putrescine from ornithine.
CAZymes analysis combined with KO assignment was used to decipher the ability of the strain to encode glycolytic and carbohydrate-binding modules and enzymes (Supplementary Table 5). More specifically, Lc. paracasei SP5 codes for 69 genes that can be further classified into four classes: 31 glycoside hydrolase (GH) genes, 32 glycosyltransferase (GT) genes, 3 carbohydrate-binding modules (CBMs), 3 carbohydrate esterase (CE) genes, supporting the catabolism of a broad range of carbohydrates, including glucose, mannose, glycogen, chitin. Traitar analysis also showed that it can utilize sucrose, maltose, D-mannose, malonate, citrate and possibly lactose, as growth substrates (Supplementary Figure 5). Furthermore, biosynthetic clusters for the production of chitin, cellulose, and lipopolysaccharides were also identified (Supplementary Table 5). KEGG, CAZymes and Traitar analysis showed that it heavily relies on carbohydrate metabolism, possessing full pathways for glycolysis through the Embden-Meyerhof pathway, gluconeogenesis, pyruvate oxidation and the pentose phosphate pathway. These metabolic attributes are characteristic of homofermentative strains, that produce lactic acid as the major byproduct of glycolysis (Duar et al., 2017). In this context, COG annotation showed that Lc. paracasei SP5 codes for D-lactate dehydrogenase that catalyzes the formation of D-lactic acid from pyruvate. Interestingly, it can also degrade galactose, and possibly starch, alluding to the fact that this strain could be used in both dairy and vegetable fermentation. These findings support the lack of niche specialization of the strain, revealing its ability to grow in a great variety of nutrient-dense environments, in agreement with previous data from our lab, where Lc. paracasei SP5 was successfully incorporated in cheese and fermented juice products (Bontsidis et al., 2021; Plessas et al., 2021). The promiscuity of the strain is a very useful tool for functional food industry, as it enables novel application in dairy and non-dairy food products. Accordingly, another presumed nomadic strain, Lc. paracasei K5, originally isolated from dairy products, was utilized to produce a pomegranate beverage (Mantzourani et al., 2020) and sourdough bread (Mantzourani et al., 2019c). Strain specific differences in the metabolic capacity of other members of the Lc. paracasei species also supports their use as starter cultures for green table olives (Sisto and Lavermicocca, 2012) or short ripened Caciotta-type cheese (Bancalari et al., 2020).
Lc. paracasei SP5 also contains genes that participate in the production of a plethora of vitamins and co-factors, however, most of these modules are incomplete (Supplementary Figures 6–9). The strain carries a full biosynthetic cluster for the production of C10-C20 isoprenoids, through the mevalonate pathway and can produce intermediates for diterpenoid, carotenoid and indole diterpene alkaloid biosynthesis. This pathway is scattered amongst eukaryotes and prokaryotes, playing an important role in the production of isoprenoids, the largest family of organic compounds that is comprised by quinones, hormones and other signaling molecules (Hoshino and Gaucher, 2018). Previously, a gene cluster for the mevalonate pathway was identified in Lactobacillus helveticus (Smeds et al., 2009). The ability of the Lc. paracasei SP5 to produce isoprenoids and other secondary metabolites of biological importance should be further characterized in vitro and in situ.
Genome-Wide Analysis of Loci Conferring Probiotic and Biotechnological Potential
Genome annotation and comparative bioinformatical analysis were utilized for the detection of sequences that could be implicated in resistance to stress conditions, prevalent during industrial processing and digestion. Specifically, genes conferring tolerance to heat and cold shock, such as the cold shock proteins (Csp)A, B, C, and members of the HSP20 family, were pinpointed in the genome of the novel isolate (Table 3). Accordingly, Lc. paracasei SP5 codes for the GroEL/GroES chaperonin system assisting protein folding in extreme conditions. Based on the genetic attributes of the strain, it could also be resistant to osmotic shock, as it codes for response elements and osmoprotectant proteins. In greater detail, Lc. paracasei SP5 harbors genes for the production of glycine betaine binding factors (opuAC, opuCC, choS) and transporters (gbuA, B), an osmolyte that is accumulated in bacterial cells under hypertonic shock (Boch et al., 1996). No biosynthetic clusters for the production of glycine betaine were identified, suggesting that the strain may depend on extracellular supply. Additionally, the synergistic activity of GrpE with DnaK and DnaJ, during hyperosmotic shock could remedy possible damages in the macromolecular machinery of the cell (Schroder et al., 1993). It should be noted that in a transcriptomic study, Lc. paracasei SMN-LBK exhibited resistance to ethanol, that was accompanied by the induction of phosphofructokinase (PFK), GAPDH, and glycerol kinase (GK; Guo et al., 2020), also annotated in the genome of the novel strain. This finding could suggest that, Lc. paracasei SP5 could survive in beverages with low alcoholic content, however, this should be further investigated in situ. Furthermore, survival in saline media (6.5% NaCl) was predicted by the Traitar tool (Supplementary Figure 5). Physicochemical stress, including heat, high pressure, and osmotic shock, is a common strategy employed by the food industry to minimize the proliferation of human pathogens (Burgess et al., 2016). Strains of the former Lc. casei group generally present high tolerance to these conditions, supporting their application as starter or non-starter cultures in the food industry (Reale et al., 2015). Furthermore, Lc. paracasei SP5 harbors an effective oxidative stress response system (Table 3) that can support survival and damage repair in aerobic conditions during production. More specifically, the strain codes for peroxidases and NADH oxidases, as well as for redox-regulated molecular chaperones. Lc. paracasei strains, additionally, present another mechanism for oxidative stress resistance, manifested by the intracellular accumulation of manganese (Nierop Groot et al., 2005). The genetic cluster (mtsCBA) involved in this phenotype is also encoded by Lc. paracasei SP5, regulating the production of ABC-type manganese transporters. This cluster was shown to play an important role in the survival of Lc. paracasei strain Shirota in aerobic conditions (Serata et al., 2018). Apart from the innate ability of probiotic strains to cope with these conditions, additional strategies to guarantee survival of starter cultures have been adapted in the food industry. These commonly include the addition of oxygen-consuming enzymes or antioxidants, encapsulation or the modification of the food matrix to include prebiotics (Feng and Wang, 2020).
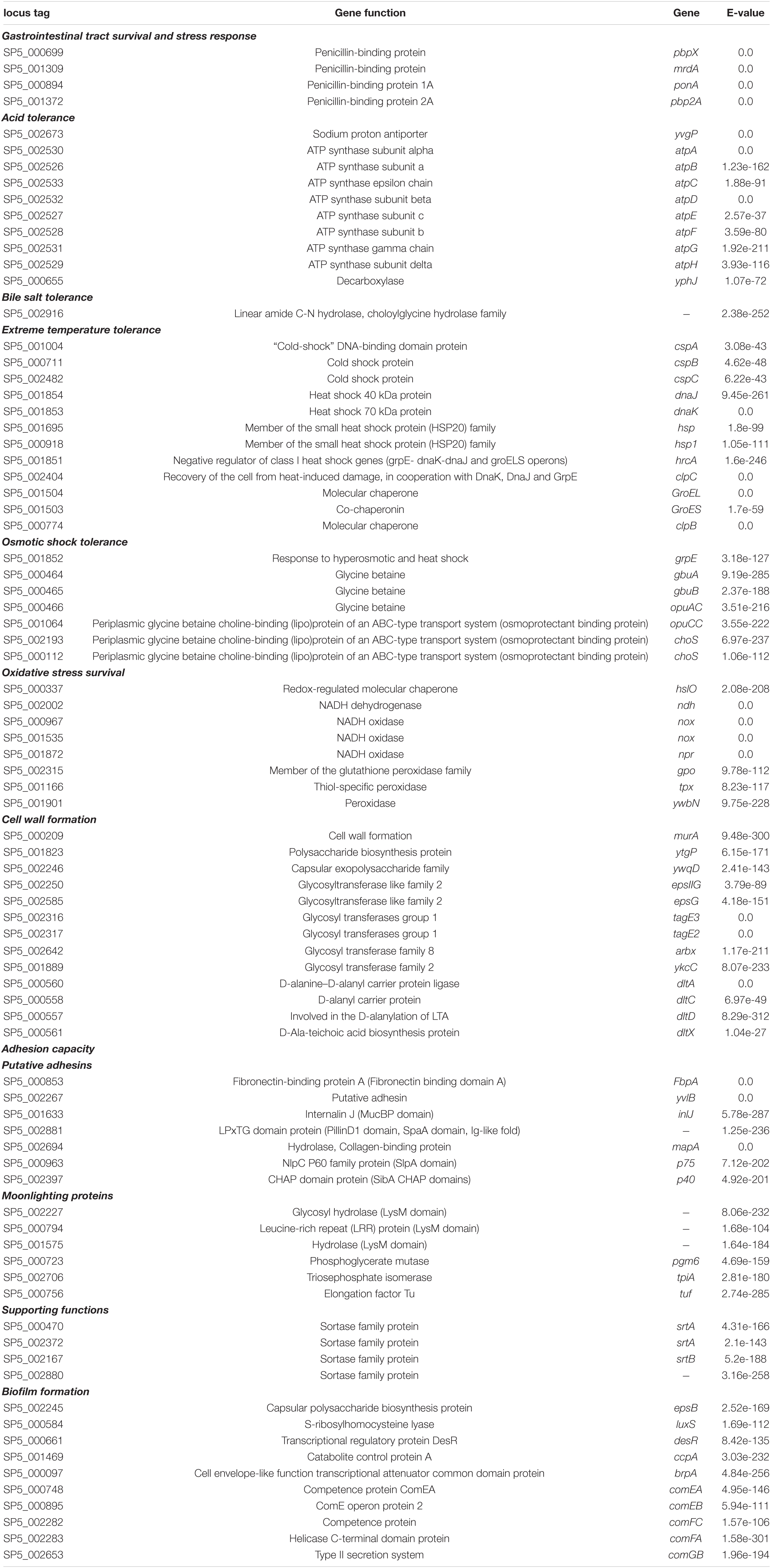
Table 3. Annotation of genes coded by Lc. paracasei SP5 that are implicated in stress response and host-microbe interactions.
Genes conferring resistance to the acidic pH of the stomach, bile salts and digestion enzymes were, also, annotated in the genome of Lc. paracasei SP5, using interconnected approaches. Specifically, 11 loci coding for acid tolerance proteins were identified (Table 3). These include a complete cluster for F0-F1 ATPase proton pump, that regulates cytoplasmic pH by pumping out H+ after ATP hydrolysis (Deckers-Hebestreit and Altendorf, 2003), as well as a sodium: proton antiporter for sodium and pH homeostasis (Huang et al., 2016). I t should be noted that acid tolerance can be manifested in a plethora of ways, including cell wall modifications and biofilm formation (Liu et al., 2021). In this context, biofilm formation supports survival and proliferation in hostile environments, as viable bacteria are protected in a polysaccharidic capsule (Liu et al., 2021). Genes implicated in biofilm formation, such as luxS and comC, comD and comE, were also annotated. Molecular chaperones and co-chaperons, as well as repair mechanisms, including ultraviolet (UV) excinuclease gene (uvrA) and RecA-assisted DNA repair, also found in the genome of Lc. paracasei SP5, could support survival in acidic conditions (Table 3; Thompson and Blaser, 1995; Hanna et al., 2001). Concerning resistance to bile acids, Lc. paracasei SP5 codes for a linear amide C-N hydrolase belonging to the choloylglycine hydrolase family. Proteins of this family were previously shown to neutralize bile acids via deconjugation (Begley et al., 2006). No other bile salt hydrolases were identified, however, phenotypic predictions and previous experimental data show that the strain is resistant to bile (Supplementary Figure 5; Mantzourani et al., 2019a).
The probiotic character also includes sensitivity to common antibiotics and lack of virulence genes. In this context, the strain does not possess acquired genes conferring antimicrobial resistance. However, it harbors a genetic cluster involved in vancomycin resistance. Vancomycin specifically binds to the D-alanine/D-alanine terminus of the muramyl pentapeptide of peptidoglycan precursors, inhibiting its polymerization and, thus, cell wall formation. Genes conferring resistance to this antibiotic usually regulate the production of different peptidoglycan precursors. The genome of Lc. paracasei SP5 carries the resistance genes vanR and vanZ. The mechanism of action of VanZ remains unknown, however, there are indications that it can exclude vancomycin and other lipoglycopeptide antibiotics from the cell wall, increasing the minimum inhibitory concentration required (Vimberg et al., 2020). VanR is a response element that activates transcription of the vanHAX cluster after vancomycin exposure. These genes are chromosomally encoded, and thus, they cannot participate in events of horizontal gene transfer. Vancomycin resistance is a shared characteristic of lactobacilli that does not raise safety concerns (Gueimonde et al., 2013). It is important to note, however, that vancomycin resistance is not intrinsic in all LAB strains. The most characteristic example is that of Enterococcus strains that, although present a wide spectrum antimicrobial activity and potential probiotic attributes, do not possess the Generally Recognized as Safe (GRAS) or Qualified Presumption of Safety (GPS) status (Ricci et al., 2017) due to the fact that they harbor transferable vancomycin resistance genes and virulence factors (Hanchi et al., 2018). Whole genome analysis of Lc. paracasei SP5 with PathogenFinder and VirulenceFinder algorithms did not reveal any homologous genes to virulence factors of common, clinically relevant pathogens. Additionally, the Traitar tool predicted that the strain does not possess beta hemolytic or coagulase activity (Supplementary Figure 5). Antimicrobial capacity is one of the most well-studied attributes of potentially probiotic strains (Silva et al., 2020). To this aim, BAGEL4 analysis revealed the presence of five regions of interest for bacteriocin production in the genome Lc. paracasei SP5 (Supplementary Figure 10). Specifically, these genetic clusters encode the production of Enterolysin A (Class III bacteriocin), carnocine CP (putative class II bacteriocin) and Enterocin X beta chain (Class IIc bacteriocin, circular peptide), suggesting the potential antimicrobial capacity of the strain. Furthermore, the lantibiotic transporter LanT (Singh and Sareen, 2014) was identified in the genome of the strain, indicating the possible presence of novel clusters for lantibiotic production. In this context, other Lc. paracasei strains capable of producing functional bacteriocins in a plethora of matrixes, where found active against gram negative strains, such as Escherichia coli (Belguesmia et al., 2020; Madi-Moussa et al., 2022), gram positive bacteria, including Staphylococcus aureus (Jiang et al., 2022), as well as food spoilage microbiota (Zhu et al., 2021). Interestingly, previous findings indicate that, Lc. paracasei SP5 can present antibacterial and antifungal activity in situ (Plessas et al., 2021). In that sense, comprehensive characterization of the antimicrobial potential of the strain is currently being undertaken.
Genome-Wide Analysis and in vitro Study of the Adhesive Phenotype of Lc. paracasei SP5
Adhesins carrying motifs for exposure on the bacterial surface (LPxTG) and for specific interactions with host receptors, glycosylated proteins and polysaccharides of the gut niche and extracellular matrix (WxL) were annotated using PGAP and studied further using InterPro, Pfam and ScanProsite. Amongst the predicted proteins, genes coding for fibronectin-binding protein A (fbpA), two mucin binding proteins; Internalin J (inlJ), and the mucus adhesion promoting protein (mapA) were annotated in the genome sequence (Table 3). Interestingly, proteomic studies have revealed that bile acids and heat stress resulted in their overexpression in Lc. paracasei species, leading to increased adhesion ability (Bengoa et al., 2018; Adu et al., 2020). Several sortase genes (srtA, strB, srtC1, srtC2) were found in the genome of Lc. paracasei SP5. Sortases play an important role in the maturation and exposure of LPxTG motif-carrying proteins on the cell wall, supporting the adhesive ability of strains. Furthermore, the presence of spaCBA or spaFED pili was estimated using BLASTp locally, using as template sequences derived from LGG, one of the first lactobacilli shown to produce these pili (Reunanen et al., 2012). Lc. paracasei SP5 codes for spaA, spaB and srtC1, however, the adhesin spaC is missing (pseudogene), suggesting that the pilus may not be functional (Figure 6A). Furthermore, its genome contains a full cluster for the production of SpaFED pili (Figures 6A,B), that presents more than 77 % similarity to that of LGG, according to BLASTp analysis. Further bioinformatic analysis on the sequence of the SpaFED pilus adhesin, spaF revealed that it presents high similarity (> 99 %) to cell wall proteins encoded by other lactobacilli, showing a close phylogenetic relationship with an LPXTG cell wall anchor domain-containing protein, derived from Lc. paracasei subsp. paracasei isolate AS01afH2WH_17 (Figure 6C). It has a length of 915 aa, and a molecular weight of approximately 10 kDa. Furthermore, SpaF has an acidic isoelectric point (pI) of 5.18, and ProtParam categorizes it as stable. Domain and motif analysis revealed that it contains two Cna-B domains that enclose a collagen adhesive domain, and a C-terminal LPxTG (LPKTG) motif (Figure 6D). It is important to note that the sequence is lacking a N-terminal signal peptide and thus, questions about its successful incorporation on the bacterial surface are being raised. However, THMM 2.0 analysis showed that the first 887 aa may be exposed on the cellular surface, while a transmembrane region including the LPxTG domain, and an intercellular domain were identified in the remaining amino acids. The spaCBA cluster plays a significant role in the adhesive capacity of the Lacticaseibacillus genus and was previously identified in LGG (Reunanen et al., 2012), Lc. casei LOCK 0919 (Aleksandrzak-Piekarczyk et al., 2016) and Lc. paracasei LP10266 (Tang et al., 2021), among others. The spaFED cluster is not as widespread as the spaCBA genetic locus; however, it is detected in most Lc. rhamnosus strains, as well as in several Lc. paracasei strains (Broadbent et al., 2012). It is important to note, that the regulatory factors triggering its expression remain elusive (Rintahaka et al., 2014), while the crystal structures of the pilins have only recently been solved (Megta et al., 2019). Therefore, visualization of these structures on Lc. paracasei SP5 and the comprehensive characterization of their physicochemical properties are required to better understand their contribution to the adhesion capacity of the strain.
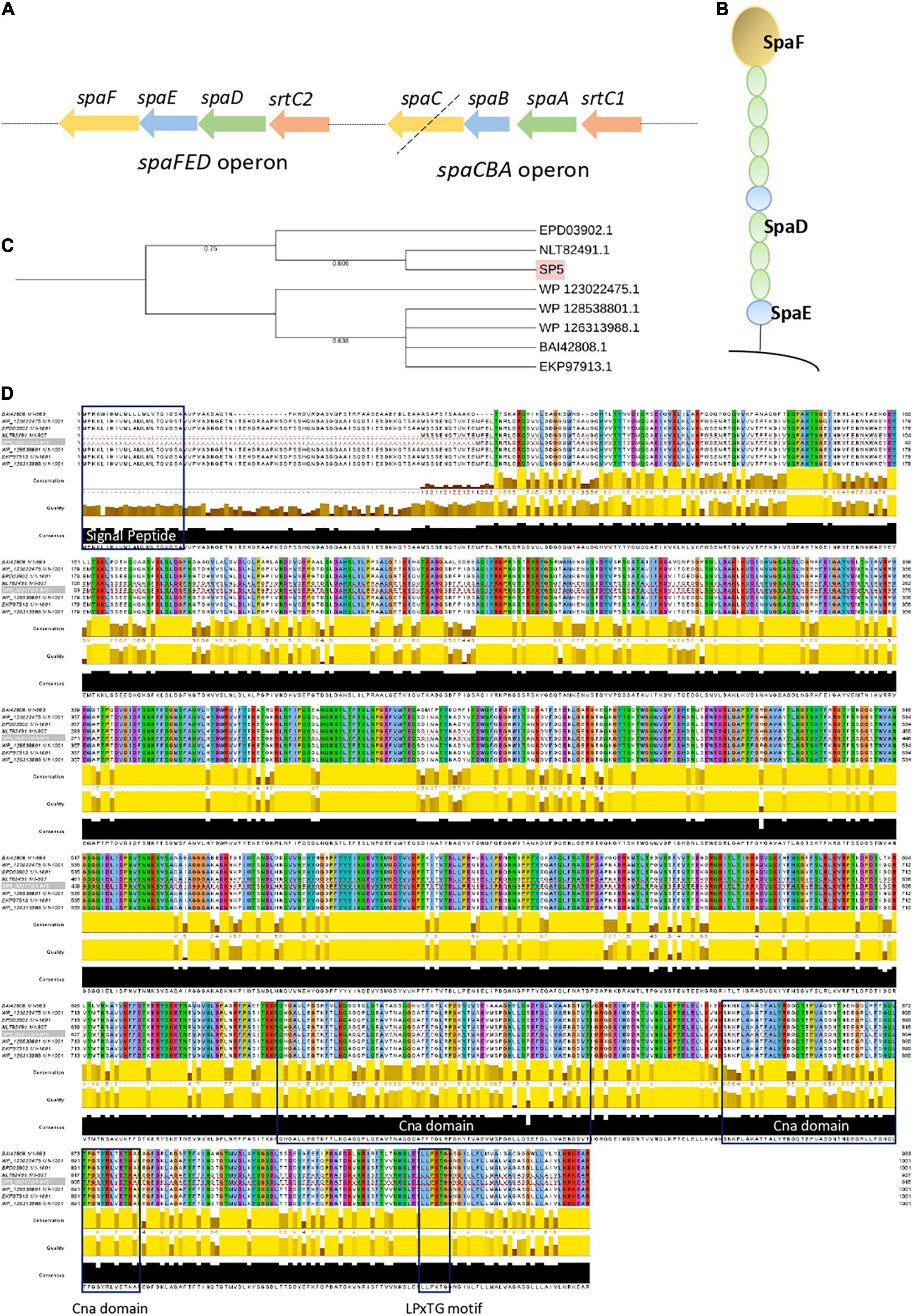
Figure 6. Analysis of the spaCBA and spaFED cluster encoded in the genome of Lc. paracasei SP5. (A) Graphical depiction of the spaCBA and spaFED and pili clusters annotated by PGAP in the genome of Lc. paracasei SP5. The black dotted line signifies that spaC is a pseudogene. (B) Graphical depiction of the spaFED pilus. (C) Neighbor-Joining phylogenetic tree of Lc. paracasei (EPD03902.1, NLT82491.1, WP_123022475.1, WP_128538801.1, WP_126313988.1) and Lc. rhamnosus GG (BAI42808.1) putative spaF gene sequences. Gene alignment was performed using ClustalW and the tree was constructed on the iTol server. Highlighted in pink is the spaF gene sequence of Lc. paracasei SP5 (D) Visualization of spaF alignment was performed using Jalview. Blue boxes indicate the N-terminal signal peptide, two Cna domains (669–735 aa, 764–818 aa), and a C-terminal LPxTG motif.
Moonlighting proteins with putative adhesive properties were also identified in the genome (Table 3). These include the chaperone GroEL and the co-chaperonin GroES, as well as glycolytic enzymes, such as triosephosphate isomerase (tpiA) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), that under appropriate conditions can be exposed in the cellular surface and participate in the adhesion phenotype of strains (Jeffery, 2019). Studies have shown that availability of carbon sources, plant polyphenols and prebiotic fiber could influence their exposure on the cell surface, and subsequently the adhesion capacity of strains (Jeffery, 2019). Concerning the host factors that moonlighting proteins bind to, GAPDH was shown to interact with host mucin (Patel et al., 2016), TpiA with laminin (Pereira et al., 2007) and GroEL with plasminogen (Hagemann et al., 2017) and mucin (Bergonzelli et al., 2006). Interestingly, pathogens utilize moonlighting proteins to colonize the host’s epithelia, and thus probiotics can compete with them for binding sites (Jeffery, 2018). More specifically, probiotics that code for homologous surface proteins with pathogenic species can effectively block their adhesion in the gut niche (van Zyl et al., 2020). In this context, the exclusion of Listeria monocytogenes EGDe by Lp. plantarum 423 in vivo, was attributed to the presence of the mapA gene (van Zyl et al., 2019), also annotated in the genome of Lc. paracasei SP5. Similar observations were made, for other lactobacilli proteins, such as S-layer proteins and sortase-dependent cell surface proteins (SDPs; van Zyl et al., 2020). Furthermore, non-protein macromolecules, such as exopolysaccharides can also mediate the adhesive phenotype (Alp and Kuleaşan, 2019). In this light, genes for exopolysaccharide biosynthesis and transport were pinpointed in the genome of Lc. paracasei SP5 (Table 3). Concerning, microbe-microbe adhesive interactions Lc. paracasei SP5 harbors genes participating in autoaggregation, biofilm production and LuxS signaling (Table 3). Proteins with moonlighting functions, pilins and the capsular polysaccharides of the cellular surface may also contribute to these phenotypes, as previously described (Nwoko and Okeke, 2021).
The adhesive properties of the strain were further investigated in vitro using a microbiological method and confocal microscopy. More specifically, it was shown that Lc. paracasei SP5 can bind to HT-29 monolayers with comparable efficiency to LGG (Figure 7). LGG was used as a reference strain, due its well-characterized adhesive properties (Rasinkangas et al., 2020). Confocal microscopy provided visual evidence of these interactions and was used to determine the percentage of cells carrying adhered bacteria (Figures 7A,B). More specifically, Lc. paracasei SP5 adhered to 231 out of total 1575 cells (14.7%), while LGG adhered to 464 out of total 1195 cells (38.3%). Further comparative bioinformatic analysis revealed that both strains present a very similar profile in terms of the production of adhesins and adhesion-related proteins (Figure 7C). Many LAB strains present adhesion capacity in vitro, however, when in the gut niche, probiotics can attach to the mucus and epithelium with varying degrees of success, only leading to transient gut colonization (Monteagudo-Mera et al., 2019). Thus, studies on more sophisticated models of the gut or in animals could provide better insights into the behaviour of Lc. paracasei SP5 in physiological conditions.
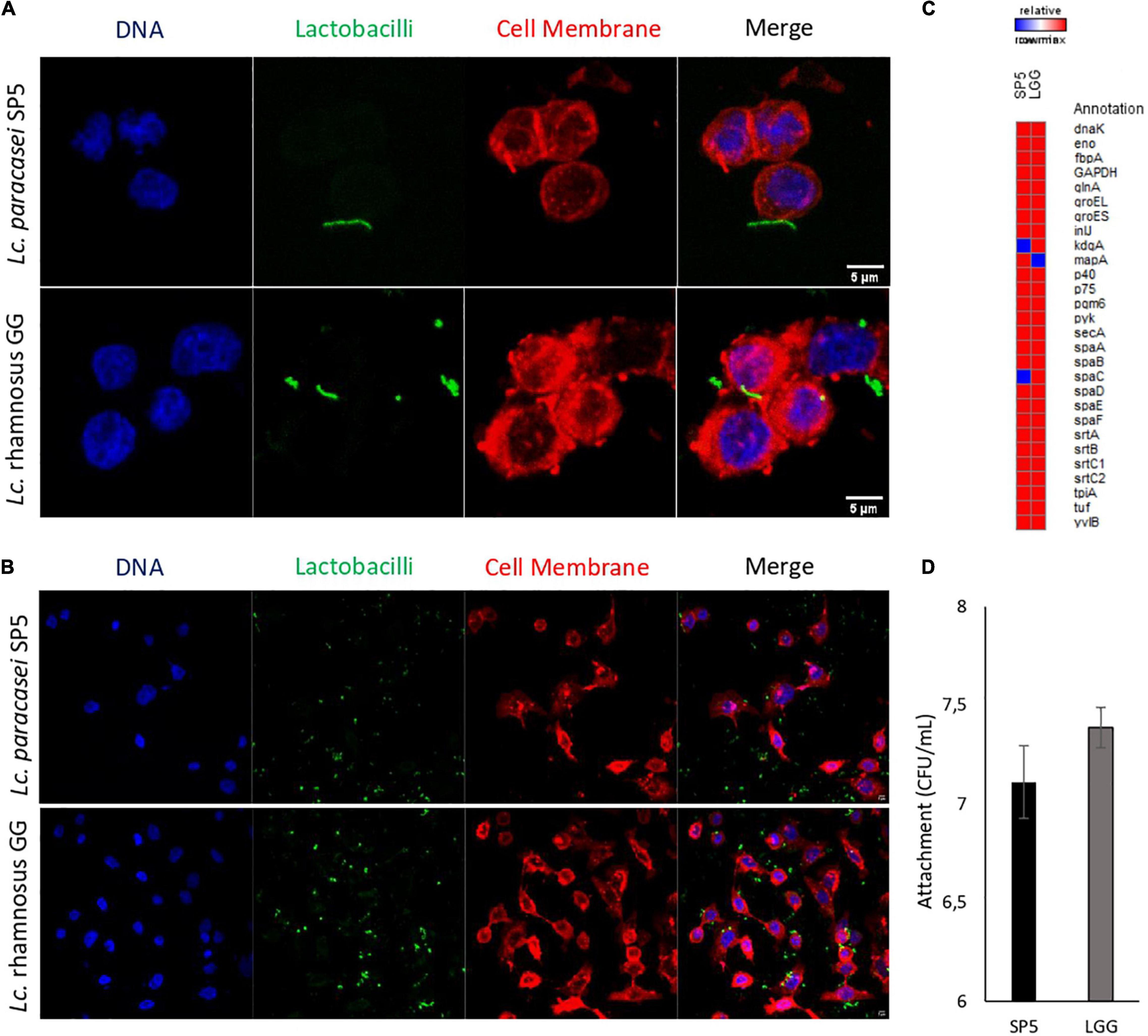
Figure 7. Evaluation of the adhesion capacity of Lc. paracasei SP5 onto the human colon cancer adenocarcinoma cell line, HT-29. (A,B) Representative photos from confocal fluorescent microscopy showing the adhesion capacity of Lc. paracasei SP5 or Lc. rhamnosus GG. Panel A represents a zoomed in image of a 40x photo, edited by ImageJ (version 1.53f51), while panel B represents a photo of the original magnification (40×). Bacteria are stained with CFSE (green), eukaryotic nuclei (blue) and cell membranes (red) are stained with Hoescht and CellBrite Red Cytoplasmic Membrane Dye, respectively (scale bar, 5 μm). (C) Gene matrix depicting the presence or absence of adhesion-related proteins in the genomes of Lc. paracasei SP5 and Lc. rhamnosus GG, annotated by PGAP. (D) Determination of attached bacterial counts (CFU/mL) of Lc. paracasei SP5 (black bar) or Lc. rhamnosus GG (grey bar) after 4 h co-incubation with HT-29 monolayers. Results are presented as mean ± standard deviation.
Conclusion
In this work, whole genome sequencing, annotation and comprehensive bioinformatic analyses were utilized to further characterize the probiotic and biotechnological potential of Lc. paracasei SP5, a strain originally isolated from kefir grains. Comprehensive phylogenomic analysis confirmed the classification of the novel strain to the Lc. paracasei species. Concerning genome stability and safety, Lc. paracasei SP5 does not harbor mobile elements, acquired antimicrobial resistance genes or virulence factors. Genome annotation and functional characterization revealed that the strain can utilize a plethora of carbohydrates as energy sources, supporting its nomadic character. Furthermore, it was found that Lc. paracasei SP5 codes for several factors mediating survival during food processing and GI passage, as well as for microbe-host interactions. Indeed, it possesses genes implicated in the production of proteins and polysaccharides that participate in mucosa and epithelium attachment, and subsequently the adhesion capacity of the novel strain was validated in vitro. It was shown that, Lc. paracasei SP5 adheres to cell monolayers with comparable efficiency to LGG. These findings suggest that, Lc. paracasei SP5 is a good probiotic candidate, with capacity to be incorporated in novel fermented food products, providing fertile ground for biotechnological innovation. Further studies, including metabolomic and proteomic approaches, to characterize the strain at multiple levels, will provide a more complete view of its mechanisms of action and health-promoting properties.
Data Availability Statement
The data presented in this study are deposited in the DDBJ/ENA/GenBank repository, accession number JAKJPP000000000. The version described in this manuscript is JAKJPP010000000.
Author Contributions
PK, MK, and AG designed the study. DK, CE, KT, and IM carried out the experiments. DK, KT, AA, PK, and MK analysed the data. DK, CE, and AG participated in the writing of the manuscript. AA, SP, PK, MK, and AG contributed to editing and critical reviewing of the manuscript. AA, SP, MK, and AG took charge of the resources. All authors had read and approved the final manuscript.
Funding
This research has been financed by the project “InTechThrace: Integrated Technologies in biomedical research: multilevel biomarker analysis in Thrace” (MIS Code 5047285), under the Operational Program “Competitiveness, Entrepreneurship & Innovation” (EPAnEK), co-funded by the European Regional Development Fund (ERDF), and national resources (Partnership Agreement 2014-2020).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge the support of the Bioimaging Facility and the Biomedical Data Science and Bioinformatics Facility of the Department of Molecular Biology and Genetics, Democritus University of Thrace.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.922689/full#supplementary-material
References
Acurcio, L. B., Wuyts, S., de Cicco Sandes, S. H., Sant’anna, F. M., Pedroso, S. H. S. P., Bastos, R. W., et al. (2020). Milk fermented by Lactobacillus paracasei NCC 2461 (ST11) modulates the immune response and microbiota to exert its protective effects against Salmonella typhimurium infection in mice. Probiotics Antimicrob. Proteins 12, 1398–1408. doi: 10.1007/S12602-020-09634-X
Adu, K. T., Wilson, R., Baker, A. L., Bowman, J., and Britz, M. L. (2020). Prolonged heat stress of Lactobacillus paracasei GCRL163 improves binding to human colorectal adenocarcinoma HT-29 cells and modulates the relative abundance of secreted and cell surface-located proteins. J. Proteome Res. 19, 1824–1846. doi: 10.1021/ACS.JPROTEOME.0C00107
Aleksandrzak-Piekarczyk, T., Koryszewska-Bagińska, A., Grynberg, M., Nowak, A., Cukrowska, B., Kozakova, H., et al. (2016). Genomic and functional characterization of the unusual pLOCK 0919 plasmid harboring the SPACBA pili cluster in Lactobacillus casei LOCK 0919. Genome Biol. Evol. 8, 202–217. doi: 10.1093/GBE/EVV247
Alp, D., and Kuleaşan, H. (2019). Adhesion mechanisms of lactic acid bacteria: conventional and novel approaches for testing. World J. Microbiol. Biotechnol. 35:156. doi: 10.1007/s11274-019-2730-x
Andrews, S. (2010). FastQC: a Quality Control Tool for High Throughput Sequence Data. Available online at: https://www.bioinformatics.babraham.ac.uk/projects/fastqc [Accessed January 19, 2022].
Arndt, D., Grant, J. R., Marcu, A., Sajed, T., Pon, A., Liang, Y., et al. (2016). PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 44, W16–W21. doi: 10.1093/NAR/GKW387
Arnold, J. W., Simpson, J. B., Roach, J., Kwintkiewicz, J., and Azcarate-Peril, M. A. (2018). Intra-species genomic and physiological variability impact stress resistance in strains of probiotic potential. Front. Microbiol. 9:242. doi: 10.3389/FMICB.2018.00242/BIBTEX
Bancalari, E., Montanari, C., Levante, A., Alinovi, M., Neviani, E., Gardini, F., et al. (2020). Lactobacillus paracasei 4341 as adjunct culture to enhance flavor in short ripened Caciotta-type cheese. Food Res. Int. 135:109284. doi: 10.1016/J.FOODRES.2020.109284
Bankevich, A., Nurk, S., Antipov, D., Gurevich, A. A., Dvorkin, M., Kulikov, A. S., et al. (2012). Original articles SPADES: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comp. Biol. 19, 455–477. doi: 10.1089/cmb.2012.0021
Bäuerl, C., Pérez-Martínez, G., Yan, F., Polk, D. B., and Monedero, V. (2010). Functional Analysis of the p40 and p75 proteins from Lactobacillus casei BL23. J. Mol. Microbiol. Biotechnol. 19, 231–241. doi: 10.1159/000322233
Begley, M., Hill, C., and Gahan, C. G. M. (2006). Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 72:1729. doi: 10.1128/AEM.72.3.1729-1738.2006
Belguesmia, Y., Hazime, N., Kempf, I., Boukherroub, R., and Drider, D. (2020). New bacteriocins from Lacticaseibacillus paracasei CNCM I-5369 adsorbed on alginate nanoparticles are very active against Escherichia coli. Int. J. Mol. Sci. 21, 1–15. doi: 10.3390/IJMS21228654
Bengoa, A. A., Zavala, L., Carasi, P., Trejo, S. A., Bronsoms, S., Serradell, M., et al. (2018). Simulated gastrointestinal conditions increase adhesion ability of Lactobacillus paracasei strains isolated from kefir to Caco-2 cells and mucin. Food Res. Int. 103, 462–467. doi: 10.1016/J.FOODRES.2017.09.093
Bergonzelli, G. E., Granato, D., Pridmore, R. D., Marvin-Guy, L. F., Donnicola, D., and Corthésy-Theulaz, I. E. (2006). GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect. Immun. 74:425. doi: 10.1128/IAI.74.1.425-434.2006
Biswas, A., Staals, R. H. J., Morales, S. E., Fineran, P. C., and Brown, C. M. (2016). CRISPRDetect: a flexible algorithm to define CRISPR arrays. BMC Genomics 17:356. doi: 10.1186/S12864-016-2627-0/FIGURES/6
Blum, M., Chang, H. Y., Chuguransky, S., Grego, T., Kandasaamy, S., Mitchell, A., et al. (2021). The InterPro protein families and domains database: 20 years on. Nucleic Acids Res. 49, D344–D354. doi: 10.1093/NAR/GKAA977
Boch, J., Kempf, B., Schmid, R., and Bremer, E. (1996). Synthesis of the osmoprotectant glycine betaine in Bacillus subtilis: characterization of the gbsAB Genes. J. Bacteriol. 178, 5121–5129. doi: 10.1128/jb.178.17.5121-5129.1996
Bolger, A. M., Lohse, M., and Usadel, B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/BIOINFORMATICS/BTU170
Bonham, K. S., Wolfe, B. E., and Dutton, R. J. (2017). Extensive horizontal gene transfer in cheese-associated bacteria. Elife 6:e22144. doi: 10.7554/ELIFE.22144
Bontsidis, C., Mallouchos, A., Terpou, A., Nikolaou, A., Batra, G., Mantzourani, I., et al. (2021). Microbiological and chemical properties of chokeberry juice fermented by novel lactic acid bacteria with potential probiotic properties during fermentation at 4 °c for 4 weeks. Foods 10:768. doi: 10.3390/FOODS10040768
Bortolaia, V., Kaas, R. S., Ruppe, E., Roberts, M. C., Schwarz, S., Cattoir, V., et al. (2020). ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 75, 3491–3500. doi: 10.1093/JAC/DKAA345
Broadbent, J. R., Neeno-Eckwall, E. C., Stahl, B., Tandee, K., Cai, H., Morovic, W., et al. (2012). Analysis of the Lactobacillus casei supragenome and its influence in species evolution and lifestyle adaptation. BMC Genomics 13:533. doi: 10.1186/1471-2164-13-533/FIGURES/7
Burgess, C. M., Gianotti, A., Gruzdev, N., Holah, J., Knøchel, S., Lehner, A., et al. (2016). The response of foodborne pathogens to osmotic and desiccation stresses in the food chain. Int. J. Food Microbiol. 221, 37–53. doi: 10.1016/J.IJFOODMICRO.2015.12.014
Camacho, C., Coulouris, G., Avagyan, V., Ma, N., Papadopoulos, J., Bealer, K., et al. (2009). BLAST+: architecture and applications. BMC Bioinformatics 10:421. doi: 10.1186/1471-2105-10-421/FIGURES/4
Carattoli, A., Zankari, E., Garciá-Fernández, A., Larsen, M. V., Lund, O., Villa, L., et al. (2014). In Silico detection and typing of plasmids using plasmidfinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 58:3895. doi: 10.1128/AAC.02412-14
Carver, T., Harris, S. R., Berriman, M., Parkhill, J., and McQuillan, J. A. (2012). Artemis: an integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 28, 464–469. doi: 10.1093/bioinformatics/btr703
Chondrou, P., Karapetsas, A., Kiousi, D. E., Vasileiadis, S., Ypsilantis, P., Botaitis, S., et al. (2020). Assessment of the immunomodulatory properties of the probiotic strain Lactobacillus paracasei K5 in vitro and in vivo. Microorganisms 8:709. doi: 10.3390/MICROORGANISMS8050709
Ciufo, S., Kannan, S., Sharma, S., Badretdin, A., Clark, K., Turner, S., et al. (2018). Using average nucleotide identity to improve taxonomic assignments in prokaryotic genomes at the NCBI. Int. J. Syst. Evol. 68:2386. doi: 10.1099/IJSEM.0.002809
Cosentino, S., Voldby Larsen, M., Møller Aarestrup, F., and Lund, O. (2013). Pathogen finder–distinguishing friend from foe using bacterial whole genome sequence data. PLoS One 8:e77302. doi: 10.1371/JOURNAL.PONE.0077302
Costa, D. J., Marteau, P., Amouyal, M., Poulsen, L. K., Hamelmann, E., Cazaubiel, M., et al. (2014). Efficacy and safety of the probiotic Lactobacillus paracasei LP-33 in allergic rhinitis: a double-blind, randomized, placebo-controlled trial (GA2LEN Study). Eur. J. Clin. Nutr. 68, 602–607. doi: 10.1038/EJCN.2014.13
D’Auria, E., Panelli, S., Lunardon, L., Pajoro, M., Paradiso, L., Beretta, S., et al. (2021). Rice flour fermented with Lactobacillus paracasei CBA L74 in the treatment of atopic dermatitis in infants: a randomized, double- blind, placebo- controlled trial. Pharmacol. Res. 163:105284. doi: 10.1016/J.PHRS.2020.105284
Darling, A. E., Mau, B., and Perna, N. T. (2010). Progressive mauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5:e11147. doi: 10.1371/JOURNAL.PONE.0011147
de Castro, E., Sigrist, C. J. A., Gattiker, A., Bulliard, V., Langendijk-Genevaux, P. S., Gasteiger, E., et al. (2006). ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 34:W362. doi: 10.1093/NAR/GKL124
Deckers-Hebestreit, G., and Altendorf, K. (2003). The F0F1-type ATP synthases of bacteria: structure and function of the F0 complex. Annu. Rev. Microbiol. 50, 791–824. doi: 10.1146/annurev.micro.50.1.791
Duar, R. M., Lin, X. B., Zheng, J., Martino, M. E., Grenier, T., Pérez-Muñoz, M. E., et al. (2017). Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS Microbiol. Rev. 41, S27–S48. doi: 10.1093/FEMSRE/FUX030
Edgar, R. C. (2007). PILER-CR: fast and accurate identification of CRISPR repeats. BMC Bioinformatics 8:18. doi: 10.1186/1471-2105-8-18/TABLES/2
Feng, T., and Wang, J. (2020). Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review. Gut Microb. 12:1801944. doi: 10.1080/19490976.2020.1801944
Fontana, A., Zacconi, C., and Morelli, L. (2018). Genetic signatures of dairy lactobacillus casei group. Front. Microbiol. 9:2611. doi: 10.3389/FMICB.2018.02611
Gasteiger, E., Hoogland, C., Gattiker, A., Duvaud, S., Wilkins, M. R., Appel, R. D., et al. (2005). ‘Protein Identification and Analysis Tools on the ExPASy Server.’ in The Proteomics Protocols Handbook, ed John M. Walker (Humana Press), pp. 571-607. Available online at: https://web.expasy.org/protparam/protpar-ref.html [Accessed January 19, 2022].
Gueimonde, M., Sánchez, B., de los Reyes-Gavilán, C. G., and Margolles, A. (2013). Antibiotic resistance in probiotic bacteria. Front. Microbiol. 4:202. doi: 10.3389/FMICB.2013.00202/BIBTEX
Guo, J., Li, X., Li, B., Yang, J., Jin, D., and Li, K. (2020). Transcriptome analysis of Lactobacillus paracasei SMN-LBK under ethanol stress. Int. J. Dairy Sci. 103, 7813–7825. doi: 10.3168/JDS.2019-16955
Gurevich, A., Saveliev, V., Vyahhi, N., and Tesler, G. (2013). QUAST: quality assessment tool for genome assemblies. Bioinformatics 29:1072. doi: 10.1093/BIOINFORMATICS/BTT086
Hagemann, L., Gründel, A., Jacobs, E., and Dumke, R. (2017). The surface-displayed chaperones GroEL and DnaK of Mycoplasma pneumoniae interact with human plasminogen and components of the extracellular matrix. Pathog. Dis. 75:17. doi: 10.1093/FEMSPD/FTX017
Hanchi, H., Mottawea, W., Sebei, K., and Hammami, R. (2018). The genus enterococcus: between probiotic potential and safety concerns-an update. Front. Microbiol. 9:1791. doi: 10.3389/FMICB.2018.01791
Hanna, M. N., Ferguson, R. J., Li, Y. H., and Cvitkovitch, D. G. (2001). uvrA is an acid-inducible gene involved in the adaptive response to low pH in Streptococcus mutans. J. Bacteriol. 183, 5964–5973. doi: 10.1128/JB.183.20.5964-5973.2001
Hatti-Kaul, R., Chen, L., Dishisha, T., and El Enshasy, H. (2018). Lactic acid bacteria: from starter cultures to producers of chemicals. FEMS Microbiol. Lett. 365:fny213. doi: 10.1093/femsle/fny213
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
Hill, D., Sugrue, I., Tobin, C., Hill, C., Stanton, C., and Ross, R. P. (2018). The Lactobacillus casei group: history and health related applications. Front. Microbiol. 9:2107. doi: 10.3389/FMICB.2018.02107
Hoshino, Y., and Gaucher, E. A. (2018). On the origin of isoprenoid biosynthesis. Mol. Biol. Evol. 35, 2185–2197. doi: 10.1093/MOLBEV/MSY120
Huang, Y., Chen, W., Dotson, D. L., Beckstein, O., and Shen, J. (2016). Mechanism of pH-dependent activation of the sodium-proton antiporter NhaA. Nat. Commun. 7, 1–10. doi: 10.1038/ncomms12940
Huerta-Cepas, J., Szklarczyk, D., Heller, D., Hernández-Plaza, A., Forslund, S. K., Cook, H., et al. (2019). eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 47, D309–D314. doi: 10.1093/NAR/GKY1085
Jeffery, C. J. (2018). Protein moonlighting: what is it, and why is it important? Philos. Trans. R. Soc. Lond. B Biol. Sci. 373:20160523. doi: 10.1098/RSTB.2016.0523
Jeffery, C. J. (2019). Mini-review intracellular/surface moonlighting proteins that aid in the attachment of gut microbiota to the host. AIMS Microbiol. 5, 77–86. doi: 10.3934/microbiol.2019.1.77
Jiang, X., Brantley Hall, A., Arthur, T. D., Plichta, D. R., Covington, C. T., Poyet, M., et al. (2019). Invertible promoters mediate bacterial phase variation, antibiotic resistance, and host adaptation in the gut. Science 363:181. doi: 10.1126/SCIENCE.AAU5238
Jiang, Y. H., Xin, W. G., Yang, L. Y., Ying, J. P., Zhao, Z. S., Lin, L. B., et al. (2022). A novel bacteriocin against Staphylococcus aureus from Lactobacillus paracasei isolated from Yunnan traditional fermented yogurt: purification, antibacterial characterization, and antibiofilm activity. J. Dairy Sci. 105, 2094–2107. doi: 10.3168/JDS.2021-21126
Joensen, K. G., Scheutz, F., Lund, O., Hasman, H., Kaas, R. S., Nielsen, E. M., et al. (2014). Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 52, 1501–1510. doi: 10.1128/JCM.03617-13
Johansson, M. H. K., Bortolaia, V., Tansirichaiya, S., Aarestrup, F. M., Roberts, A. P., and Petersen, T. N. (2021). Detection of mobile genetic elements associated with antibiotic resistance in Salmonella enterica using a newly developed web tool: mobile element finder. J. Antimicrob. Chemother. 76, 101–109. doi: 10.1093/jac/dkaa390
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M., and Tanabe, M. (2016). KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44:D457. doi: 10.1093/NAR/GKV1070
Kiousi, D. E., Rathosi, M., Tsifintaris, M., Chondrou, P., and Galanis, A. (2021). Pro-biomics: omics technologies to unravel the role of probiotics in health and disease. Adv. Nutr. 12, 1802–1820. doi: 10.1093/ADVANCES/NMAB014
Koryszewska-Bagińska, A., Gawor, J., Nowak, A., Grynberg, M., and Aleksandrzak-Piekarczyk, T. (2019). Comparative genomics and functional analysis of a highly adhesive dairy Lactobacillus paracasei subsp. paracasei IBB3423 strain. Appl. Microbiol. Biotechnol. 103, 7617–7634. doi: 10.1007/s00253-019-10010-1
Lerner, A., Matthias, T., and Aminov, R. (2017). Potential effects of horizontal gene exchange in the human gut. Front. Immunol. 8:1630. doi: 10.3389/FIMMU.2017.01630
Lesme, H., Rannou, C., Famelart, M. H., Bouhallab, S., and Prost, C. (2020). Yogurts enriched with milk proteins: texture properties, aroma release and sensory perception. Trends Food Sci. Technol. 98, 140–149. doi: 10.1016/J.TIFS.2020.02.006
Letunic, I., and Bork, P. (2016). Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 44, W242–W245. doi: 10.1093/NAR/GKW290
Li, L., Wen, X., Wen, Z., Chen, S., Wang, L., and Wei, X. (2018). Evaluation of the biogenic amines formation and degradation abilities of Lactobacillus curvatus from Chinese bacon. Front. Microbiol. 9:1015. doi: 10.3389/FMICB.2018.01015
Liu, M., Siezen, R. J., and Nauta, A. (2009). In silico prediction of horizontal gene transfer events in Lactobacillus bulgaricus and Streptococcus thermophilus reveals protocooperation in yogurt manufacturing. Appl. Environ. Microbiol. 75, 4120–4129. doi: 10.1128/AEM.02898-08
Liu, N., Qin, L., and Miao, S. (2021). Regulatory mechanisms of l-lactic acid and taste substances in chinese acid rice soup (Rice-Acid) fermented with a Lacticaseibacillus paracasei and Kluyveromyces marxianus. Front. Microbiol. 12:1078. doi: 10.3389/FMICB.2021.594631
Lukjancenko, O., Ussery, D. W., and Wassenaar, T. M. (2012). Comparative genomics of Bifidobacterium, Lactobacillus and related probiotic genera. Microb. Ecol. 63, 651–673. doi: 10.1007/s00248-011-9948-y
Macklaim, J. M., Gloor, G. B., Anukam, K. C., Cribby, S., and Reid, G. (2011). At the crossroads of vaginal health and disease, the genome sequence of Lactobacillus iners AB-1. Proc. Natl. Acad. Sci. U.S.A. 108, 4688–4695. doi: 10.1073/PNAS.1000086107
Madi-Moussa, D., Belguesmia, Y., Charlet, A., Drider, D., and Coucheney, F. (2022). Lacticaseicin 30 and colistin as a promising antibiotic formulation against gram-negative β-lactamase-producing strains and colistin-resistant strains. Antibiotics 11:20. doi: 10.3390/ANTIBIOTICS11010020/S1
Mantzourani, I., Bontsidis, C. A., Plessas, S., Alexopoulos, A., Theodoridou, E., Tsigalou, C., et al. (2019a). Comparative susceptibility study against pathogens using fermented cranberry juice and antibiotics. Front. Microbiol. 10:1294. doi: 10.3389/FMICB.2019.01294/BIBTEX
Mantzourani, I., Chondrou, P., Bontsidis, C., Karolidou, K., Terpou, A., Alexopoulos, A., et al. (2019b). Assessment of the probiotic potential of lactic acid bacteria isolated from kefir grains: evaluation of adhesion and antiproliferative properties in in vitro experimental systems. Ann. Microbiol. 69, 751–763. doi: 10.1007/S13213-019-01467-6
Mantzourani, I., Terpou, A., Alexopoulos, A., Bezirtzoglou, E., and Plessas, S. (2019c). Assessment of ready-to-use freeze-dried immobilized biocatalysts as innovative starter cultures in sourdough bread making. Foods 8:40. doi: 10.3390/FOODS8010040
Mantzourani, I., Terpou, A., Bekatorou, A., Mallouchos, A., Alexopoulos, A., Kimbaris, A., et al. (2020). Functional pomegranate beverage production by fermentation with a novel synbiotic L. paracasei biocatalyst. Food Chem. 308:125658. doi: 10.1016/J.FOODCHEM.2019.125658
Megta, A. K., Mishra, A. K., Palva, A., von Ossowski, I., and Krishnan, V. (2019). Crystal structure of basal pilin SpaE reveals the molecular basis of its incorporation in the lactobacillar SpaFED pilus. J. Struct. Biol. 207, 74–84. doi: 10.1016/J.JSB.2019.04.016
Mistry, J., Chuguransky, S., Williams, L., Qureshi, M., Salazar, G. A., Sonnhammer, E. L. L., et al. (2021). Pfam: the protein families database in 2021. Nucleic Acids Res. 49, D412–D419. doi: 10.1093/NAR/GKAA913
Monteagudo-Mera, A., Rastall, R. A., Gibson, G. R., Charalampopoulos, D., and Chatzifragkou, A. (2019). Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 103, 6463–6472. doi: 10.1007/S00253-019-09978-7
National Center for Biotechnology Information (2017a). Available online at: https://www.ncbi.nlm.nih.gov/biosample?LinkName=bioproject_biosample_all&from_uid=378123 [Accessed February 15, 2022].
National Center for Biotechnology Information (2017b). Available online at: https://www.ncbi.nlm.nih.gov/biosample?LinkName=bioproject_biosample_all&from_uid=378122 [Accessed February 15, 2022].
Nicolas, P., Bessières, P., Ehrlich, S. D., Maguin, E., and van de Guchte, M. (2007). Extensive horizontal transfer of core genome genes between two Lactobacillus species found in the gastrointestinal tract. BMC Evol. Biol. 7:141. doi: 10.1186/1471-2148-7-141
Nierop Groot, M. N., Klaassens, E., de Vos, W. M., Delcour, J., Hols, P., and Kleerebezem, M. (2005). Genome-based in silico detection of putative manganese transport systems in Lactobacillus plantarum and their genetic analysis. Microbiology (Reading) 151, 1229–1238. doi: 10.1099/MIC.0.27375-0
Nwoko, E. S. Q. A., and Okeke, I. N. (2021). Bacteria autoaggregation: how and why bacteria stick together. Biochem. Soc. Trans. 49, 1147–1157. doi: 10.1042/BST20200718
Otaka, M., Kikuchi-Hayakawa, H., Ogura, J., Ishikawa, H., Yomogida, Y., Ota, M., et al. (2021). Effect of Lacticaseibacillus paracasei strain shirota on improvement in depressive symptoms, and its association with abundance of actinobacteria in gut microbiota. Microorganisms 9:1026. doi: 10.3390/MICROORGANISMS9051026
Page, A. J., Cummins, C. A., Hunt, M., Wong, V. K., Reuter, S., Holden, M. T. G., et al. (2015). Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31, 3691–3693. doi: 10.1093/BIOINFORMATICS/BTV421
Pan, Q., Cen, S., Yu, L., Tian, F., Zhao, J., Zhang, H., et al. (2021). Niche-specific adaptive evolution of Lactobacillus plantarum strains isolated from human feces and paocai. Front. Cell. Infect. Microbiol. 10:615876. doi: 10.3389/FCIMB.2020.615876/BIBTEX
Patel, D. K., Shah, K. R., Pappachan, A., Gupta, S., and Singh, D. D. (2016). Cloning, expression and characterization of a mucin-binding GAPDH from Lactobacillus acidophilus. Int J Biol Macromol 91, 338–346. doi: 10.1016/J.IJBIOMAC.2016.04.041
Patterson, E., Griffin, S. M., Ibarra, A., Ellsiepen, E., and Hellhammer, J. (2020). Lacticaseibacillus paracasei Lpc-37® improves psychological and physiological markers of stress and anxiety in healthy adults: a randomized, double-blind, placebo-controlled and parallel clinical trial (the Sisu study). Neurobiol. Stress 13:100277. doi: 10.1016/J.YNSTR.2020.100277
Pereira, L. A., Báo, S. N., Barbosa, M. S., da Silva, J. L. M., Felipe, M. S. S., de Santana, J. M., et al. (2007). Analysis of the Paracoccidioides brasiliensis triosephosphate isomerase suggests the potential for adhesin function. FEMS Yeast Res. 7, 1381–1388. doi: 10.1111/J.1567-1364.2007.00292.X
Plessas, S., Ganatsios, V., Mantzourani, I., and Bosnea, L. (2021). White brined cheese production by incorporation of a traditional milk-cereal prebiotic matrix with a candidate probiotic bacterial strain. Appl. Sci. 11:6182. doi: 10.3390/APP11136182
Plessas, S., Kiousi, D. E., Rathosi, M., Alexopoulos, A., Kourkoutas, Y., Mantzourani, I., et al. (2020). Isolation of a Lactobacillus paracasei strain with probiotic attributes from kefir grains. Biomedicines 8, 1–15. doi: 10.3390/BIOMEDICINES8120594
Price, M. N., Dehal, P. S., and Arkin, A. P. (2010). FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/JOURNAL.PONE.0009490
Pritchard, L., Glover, R. H., Humphris, S., Elphinstone, J. G., and Toth, I. K. (2015). Genomics and taxonomy in diagnostics for food security: soft-rotting enterobacterial plant pathogens. Anal. Methods 8, 12–24. doi: 10.1039/C5AY02550H
Rasinkangas, P., Tytgat, H. L. P., Ritari, J., Reunanen, J., Salminen, S., Palva, A., et al. (2020). Characterization of highly mucus-adherent Non-GMO derivatives of Lacticaseibacillus rhamnosus GG. Front. Bioeng. Biotechnol. 8:1024. doi: 10.3389/FBIOE.2020.01024
Rath, D., Amlinger, L., Rath, A., and Lundgren, M. (2015). The CRISPR-Cas immune system: biology, mechanisms and applications. Biochimie 117, 119–128. doi: 10.1016/J.BIOCHI.2015.03.025
Reale, A., di Renzo, T., Rossi, F., Zotta, T., Iacumin, L., Preziuso, M., et al. (2015). Tolerance of Lactobacillus casei, Lactobacillus paracasei and Lactobacillus rhamnosus strains to stress factors encountered in food processing and in the gastro-intestinal tract. LWT 60, 721–728. doi: 10.1016/J.LWT.2014.10.022
Ren, Z., Zhao, A., Zhang, J., Yang, C., Zhong, W., Mao, S., et al. (2022). Safety and tolerance of Lacticaseibacillus paracasei N1115 in caesarean-born young children: a randomised, placebo-controlled trial. Benef. Microbes 1–16. doi: 10.3920/BM2021.0132
Reunanen, J., von Ossowski, I., Hendrickx, A. P. A., Palva, A., and de Vosa, W. M. (2012). Characterization of the SpaCBA pilus fibers in the probiotic Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 78, 2337–2344. doi: 10.1128/AEM.07047-11
Ricci, A., Allende, A., Bolton, D., Chemaly, M., Davies, R., Girones, R., et al. (2017). Scientific opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA†. EFSA J. 15:4664. doi: 10.2903/J.EFSA.2017.4664
Rintahaka, J., Yu, X., Kant, R., Palva, A., and von Ossowski, I. (2014). Phenotypical analysis of the lactobacillus rhamnosus GG fimbrial spaFED operon: surface expression and functional characterization of recombinant SpaFED pili in Lactococcus lactis. PLoS One 9:e113922. doi: 10.1371/JOURNAL.PONE.0113922
Roach, D. J., Burton, J. N., Lee, C., Stackhouse, B., Butler-Wu, S. M., Cookson, B. T., et al. (2015). A year of infection in the intensive care unit: prospective whole genome sequencing of bacterial clinical isolates reveals cryptic transmissions and novel microbiota. PLoS Genet. 11:e1005413. doi: 10.1371/JOURNAL.PGEN.1005413
Sachs, J. L., Skophammer, R. G., and Regus, J. U. (2011). Evolutionary transitions in bacterial symbiosis. Proc. Natl. Acad. Sci. U.S.A. 108, 10800–10807. doi: 10.1073/PNAS.1100304108
Salvetti, E., and O’Toole, P. W. (2017). The genomic basis of lactobacilli as health-promoting organisms. Microbiol. Spectr. 5:BAD-0011-2016. doi: 10.1128/microbiolspec.BAD-0011-2016
Saxami, G., Karapetsas, A., Chondrou, P., Vasiliadis, S., Lamprianidou, E., Kotsianidis, I., et al. (2017). Potentially probiotic Lactobacillus strains with anti-proliferative activity induce cytokine/chemokine production and neutrophil recruitment in mice. Benef. Microbes 8, 615–623. doi: 10.3920/BM2016.0202
Schroder, H., Langer, T., Hartl, F. U., and Bukau, B. (1993). DnaK, DnaJ and GrpE form a cellular chaperone machinery capable of repairing heat-induced protein damage. EMBO J. 12, 4137–4144. doi: 10.1002/j.1460-2075.1993.tb06097.x
Seemann, T. (2014). Genome analysis prokka: rapid prokaryotic genome annotation. Bioinformatics (Oxford, England) 30, 2068–2069. doi: 10.1093/bioinformatics/btu153
Serata, M., Yasuda, E., and Sako, T. (2018). Effect of superoxide dismutase and manganese on superoxide tolerance in Lactobacillus casei strain Shirota and analysis of multiple manganese transporters. Biosci. Microbiota Food Health 37:31. doi: 10.12938/BMFH.17-018
Siguier, P., Gourbeyre, E., and Chandler, M. (2014). Bacterial insertion sequences: their genomic impact and diversity. FEMS Microbiol. Rev. 38, 865–891. doi: 10.1111/1574-6976.12067
Siguier, P., Perochon, J., Lestrade, L., Mahillon, J., and Chandler, M. (2006). ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 34, D32–D36. doi: 10.1093/NAR/GKJ014
Silva, D. R., Sardi, J. C. O., Pitangui, N. S., Roque, S. M., da Silva, A. C. B., and Rosalen, P. L. (2020). Probiotics as an alternative antimicrobial therapy: current reality and future directions. J. Funct. Foods 73:104080. doi: 10.1016/j.jff.2020.104080
Singh, M., and Sareen, D. (2014). Novel LanT associated Lantibiotic Clusters identified by genome database mining. PLoS One 9:e91352. doi: 10.1371/journal.pone.0091352
Sisto, A., and Lavermicocca, P. (2012). Suitability of a probiotic Lactobacillus paracasei strain as a starter culture in olive fermentation and development of the innovative patented product “probiotic table olives.”. Front. Microbiol. 3:174. doi: 10.3389/FMICB.2012.00174
Smeds, A., Joutsjoki, T., and Palva, A. (2009). Identification of a gene cluster for the mevalonate pathway in Lactobacillus helveticus. DNA Seq. 12, 187–190. doi: 10.3109/10425170109080773
Smokvina, T., Wels, M., Polka, J., Chervaux, C., Brisse, S., Boekhorst, J., et al. (2013). Lactobacillus paracasei comparative genomics: towards species pan-genome definition and exploitation of diversity. PLoS One 8:e68731. doi: 10.1371/JOURNAL.PONE.0068731
Stergiou, O. S., Tegopoulos, K., Kiousi, D. E., Tsifintaris, M., Papageorgiou, A. C., Tassou, C. C., et al. (2021). Whole-genome sequencing, phylogenetic and genomic analysis of Lactiplantibacillus pentosus L33, a potential probiotic strain isolated from fermented sausages. Front. Microbiol. 12:746659. doi: 10.3389/FMICB.2021.746659
Surve, S., Shinde, D. B., and Kulkarni, R. (2022). Isolation, characterization and comparative genomics of potentially probiotic Lactiplantibacillus plantarum strains from Indian foods. Sci. Rep. 12:1940. doi: 10.1038/s41598-022-05850-3
Tang, Q., Hao, Y., Wang, L., Lu, C., Li, M., Si, Z., et al. (2021). Characterization of a bacterial strain Lactobacillus paracasei LP10266 recovered from an endocarditis patient in Shandong, China. BMC Microbiol. 21:183. doi: 10.1186/S12866-021-02253-8
Tatusova, T., Dicuccio, M., Badretdin, A., Chetvernin, V., Nawrocki, E. P., Zaslavsky, L., et al. (2016). NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 44, 6614–6624. doi: 10.1093/NAR/GKW569
Tegopoulos, K., Stergiou, O. S., Kiousi, D. E., Tsifintaris, M., Koletsou, E., Papageorgiou, A. C., et al. (2021). Genomic and phylogenetic analysis of Lactiplantibacillus plantarum L125, and evaluation of its anti-proliferative and cytotoxic activity in cancer cells. Biomedicines 9:1718. doi: 10.3390/BIOMEDICINES9111718
Terpou, A., Mantzourani, I., Galanis, A., Kanellaki, M., Bezirtzoglou, E., Bekatorou, A., et al. (2018). Employment of L. paracasei K5 as a novel potentially probiotic freeze-dried starter for feta-type cheese production. Microorganisms 7:3. doi: 10.3390/MICROORGANISMS7010003
Tetzschner, A. M. M., Johnson, J. R., Johnston, B. D., Lund, O., and Scheutz, F. (2020). In Silico genotyping of Escherichia coli isolates for extraintestinal virulence genes by use of whole-genome sequencing data. J. Clin. Microbiol. 58:e01269–20. doi: 10.1128/JCM.01269-20
Teufel, F., Almagro Armenteros, J. J., Johansen, A. R., Gíslason, M. H., Pihl, S. I., Tsirigos, K. D., et al. (2022). SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 1–3. doi: 10.1038/s41587-021-01156-3
Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673. doi: 10.1093/NAR/22.22.4673
Thompson, S. A., and Blaser, M. J. (1995). Isolation of the Helicobacter pylori recA gene and involvement of the recA region in resistance to low pH. Infect. Immun. 63, 2185–2193. doi: 10.1128/iai.63.6.2185-2193.1995
van Heel, A. J., de Jong, A., Song, C., Viel, J. H., Kok, J., and Kuipers, O. P. (2018). BAGEL4: a user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic Acids Res. 46, W278–W281. doi: 10.1093/NAR/GKY383
van Zyl, W. F., Deane, S. M., and Dicks, L. M. T. (2019). Bacteriocin production and adhesion properties as mechanisms for the anti-listerial activity of Lactobacillus plantarum 423 and Enterococcus mundtii ST4SA. Benef. Microbes 10, 329–349. doi: 10.3920/BM2018.0141
van Zyl, W. F., Deane, S. M., and Dicks, L. M. T. (2020). Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microb. 9:1831339. doi: 10.1080/19490976.2020.1831339
Vimberg, V., Zieglerová, L., Buriánková, K., Branny, P., and Balíková Novotná, G. (2020). VanZ reduces the binding of lipoglycopeptide antibiotics to Staphylococcus aureus and Streptococcus pneumoniae cells. Front. Microbiol. 11:566. doi: 10.3389/FMICB.2020.00566
Wang, Y., Liang, Q., Lu, B., Shen, H., Liu, S., Shi, Y., et al. (2021a). Whole-genome analysis of probiotic product isolates reveals the presence of genes related to antimicrobial resistance, virulence factors, and toxic metabolites, posing potential health risks. BMC Genomics 22:210. doi: 10.1186/S12864-021-07539-9
Wang, Y., Wu, J., Lv, M., Shao, Z., Hungwe, M., Wang, J., et al. (2021b). Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 9:378. doi: 10.3389/FBIOE.2021.612285
Waśko, A., Polak-Berecka, M., Paduch, R., and Jóźwiak, K. (2014). The effect of moonlighting proteins on the adhesion and aggregation ability of Lactobacillus helveticus. Anaerobe 30, 161–168. doi: 10.1016/J.ANAEROBE.2014.10.002
Waterhouse, A. M., Procter, J. B., Martin, D. M. A., Clamp, M., and Barton, G. J. (2009). Jalview Version 2-A multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191. doi: 10.1093/BIOINFORMATICS/BTP033
Weimann, A., Mooren, K., Frank, J., Pope, P. B., Bremges, A., and McHardy, A. C. (2016). From genomes to phenotypes: traitar, the microbial trait analyzer. mSystems 1:e00101–16. doi: 10.1128/mSystems.00101-16
Wüthrich, C. D., Irmler, S., Berthoud, H., Guggenbühl, B., Eugster, E., and Bruggmann, R. (2018). Conversion of methionine to cysteine in Lactobacillus paracasei depends on the highly mobile cysK-ctl-cysE gene cluster. Front. Microbiol. 9:2415. doi: 10.3389/FMICB.2018.02415
Zankari, E., Hasman, H., Cosentino, S., Vestergaard, M., Rasmussen, S., Lund, O., et al. (2012). Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 67, 2640–2644. doi: 10.1093/JAC/DKS261
Zhang, H., Yohe, T., Huang, L., Entwistle, S., Wu, P., Yang, Z., et al. (2018). dbCAN2: a meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 46, W95–W101. doi: 10.1093/nar/gky418
Zheng, J., Ruan, L., Sun, M., and Gänzle, M. (2015). A genomic view of Lactobacilli and pediococci demonstrates that phylogeny matches ecology and physiology. Appl. Environ. Microbiol. 81, 7233. doi: 10.1128/AEM.02116-15
Zheng, J., Wittouck, S., Salvetti, E., Franz, C. M. A. P., Harris, H. M. B., Mattarelli, P., et al. (2020). A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol 70, 2782–2858. doi: 10.1099/IJSEM.0.004107
Keywords: Lacticaseibacillus paracasei, probiotics, whole-genome sequence, comparative genomics, adhesion capacity, confocal microscopy, biotechnological potential
Citation: Kiousi DE, Efstathiou C, Tegopoulos K, Mantzourani I, Alexopoulos A, Plessas S, Kolovos P, Koffa M and Galanis A (2022) Genomic Insight Into Lacticaseibacillus paracasei SP5, Reveals Genes and Gene Clusters of Probiotic Interest and Biotechnological Potential. Front. Microbiol. 13:922689. doi: 10.3389/fmicb.2022.922689
Received: 18 April 2022; Accepted: 16 May 2022;
Published: 16 June 2022.
Edited by:
Agapi Doulgeraki, Institute of Technology of Agricultural Products, GreeceReviewed by:
Baokun Li, Shihezi University, ChinaVictor Sebastian Blancato, CONICET Instituto de Biología Molecular y Celular de Rosario (IBR), Argentina
Copyright © 2022 Kiousi, Efstathiou, Tegopoulos, Mantzourani, Alexopoulos, Plessas, Kolovos, Koffa and Galanis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stavros Plessas, c3BsZXNzYXNAYWdyby5kdXRoLmdy; Alex Galanis, YWdhbGFuaXNAbWJnLmR1dGguZ3I=
 Despoina Eugenia Kiousi
Despoina Eugenia Kiousi Christos Efstathiou
Christos Efstathiou Konstantinos Tegopoulos
Konstantinos Tegopoulos Ioanna Mantzourani
Ioanna Mantzourani Athanasios Alexopoulos
Athanasios Alexopoulos Stavros Plessas
Stavros Plessas Petros Kolovos
Petros Kolovos Maria Koffa
Maria Koffa Alex Galanis
Alex Galanis