- 1Gastroenterology Unit, Instituto de Investigación Sanitaria Princesa (IIS-Princesa), Hospital Universitario de La Princesa, Madrid, Spain
- 2Universidad Autónoma de Madrid (UAM), Madrid, Spain
- 3Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD), Madrid, Spain
Background: Treating Helicobacter pylori infection according to antibiotic resistance has been frequently recommended. However, information on its real effectiveness is scarce.
Aim: The aim of this study is to perform a meta-analysis comparing empirical vs. susceptibility-guided treatment of H. pylori.
Methods: Selection of studies: Studies comparing empirical versus susceptibility-guided treatment were selected. Search strategy: electronic and manual up to August 2021. Data synthesis: by intention-to-treat (random-effects model).
Results: Overall, 54 studies were included (6,705 patients in the susceptibility-guided group and 7,895 in the empirical group). H. pylori eradication rate was 86 vs. 76%, respectively (RR: 1.12; 95% CI: 1.08–1.17; I2: 83%). Similar results were found when only RCTs were evaluated (24 studies; RR: 1.16; 95% CI: 1.11–1.22; I2: 71%) and when susceptibility testing was assessed by culture (RR: 1.12; 95% CI: 1.06–1.18) or PCR (RR: 1.14; 95% CI: 1.05–1.23). For first-line treatments (naïve patients; 30 studies), better efficacy results were obtained with the susceptibility-guided strategy (RR: 1.15; 95% CI: 1.11–1.20; I2: 79%). However, for empirical first-line quadruple regimens, in particular (both with and without bismuth, excluding the suboptimal triple therapies), not based on CYP2C19 gene polymorphism, no differences in efficacy were found compared with the susceptibility-guided group (RR: 1.04; 95% CI: 0.99–1.09); this lack of difference was confirmed in RCTs (RR: 1.05; 95% CI: 0.99–1.12). For rescue therapies (13 studies, most 2nd-line), similar results were demonstrated for both strategies, including all studies (RR: 1.09; 95% CI: 0.97–1.22; I2: 82%) and when only RCTs were considered (RR: 1.15; 95% CI: 0.97–1.36).
Conclusion: The benefit of susceptibility-guided treatment over empirical treatment of H. pylori infection could not be demonstrated, either in first-line (if the most updated quadruple regimens are prescribed) or in rescue therapies.
Introduction
Helicobacter pylori (H. pylori) infection affects billions of people worldwide, which is the main cause of gastritis, peptic ulcer disease, and gastric cancer (Hooi et al., 2017). However, after more than 30 years of experience in the management of this infection, the ideal treatment regimen remains undefined.
Antibiotic resistance has been identified as the major factor affecting our ability to cure H. pylori infection, and the rate of resistance to several antibiotics—mainly clarithromycin—is steadily increasing in many geographic areas (Dore et al., 2000; Megraud et al., 2013; Camargo et al., 2014; Thung et al., 2016). A recent systematic review and meta-analysis assessed the distribution of H. pylori resistance to commonly used antibiotics in 65 countries and found that primary resistance rates to clarithromycin, metronidazole, and levofloxacin were ≥15% in most regions. Furthermore, increasing antibiotic resistance was observed in most countries (Savoldi et al., 2018). Accordingly, the World Health Organization (WHO) has designated clarithromycin-resistant H. pylori a high priority for antibiotic research and development.
Since antibiotic resistance is an evolving process, it seems mandatory to carry out point prevalence surveys on a regular basis to guide clinicians in their therapeutic choice (Megraud et al., 2013). A strategy that has been suggested to increase the eradication rate is individualized treatment according to antibiotic susceptibility testing (personalized treatment). However, the true utility of culture—with consequent antibiotic susceptibility testing—and the moment when it must be performed (before the first treatment or only after eradication failure) are both controversial. Of note, H. pylori culture is time-consuming, not always available on a routine basis, offers quite low sensitivity, and implies the performance of an endoscopic exploration (Zullo et al., 2003; Gisbert, 2011). Furthermore, culture is relatively expensive, not because of the cost of the procedure per se, but mainly because of the costs of the associated endoscopy required to obtain biopsy specimens.
Although susceptibility-guided therapy is recommended by many H. pylori consensus reports, the number of studies evaluating this strategy is, however, quite limited, and the evidence available to date regarding when and in whom culture should be performed is surprisingly scant. Currently, most physicians treat H. pylori infection without relying on antimicrobial susceptibility testing to choose the best regimen (Gisbert, 2020).
Therefore, the present study aimed to perform a meta-analysis comparing empirical vs. susceptibility-guided treatment of H. pylori including both first-line and rescue regimens.
Methods
General Criteria for Considering Studies for This Review
Randomized, quasi-randomized, and non-randomized controlled trials were eligible for inclusion in this review, whereas case reports, letters, editorials, comments, and reviews were excluded. Full-text forms and abstracts of the articles selected (in each of the searches) were reviewed, and those dealing with the susceptibility-guided treatment of H. pylori infection were recorded and were eligible for inclusion. No restrictions by date of publication or by language were considered.
The studied population included adults or children diagnosed as positive for H. pylori. Patients could be treated with any of the available eradication treatments for H. pylori infection in any line of treatment. Trials had to compare the efficacy of an H. pylori eradication treatment based on a previous susceptibility-guided diagnostic test with that of empirical treatment. Pre-treatment diagnostic methods for H. pylori detection should comprise one or more of the most commonly validated tests: urea breath test, histology, rapid urease test, and stool antigen test; for susceptibility-guided treatments, studies should include methods to test antimicrobial susceptibility on gastric biopsies such as PCR or culture.
Eligible studies should include accessible data on successful eradication rates in both tailored and empirical groups.
Outcome Measures
The primary endpoint was intention-to-treat efficacy (H. pylori eradication rate). Reported efficacy was considered as the rate (proportion) of patients cured among the total of treated patients. Trials were included if they reported the number of patients with H. pylori eradication in each treatment arm; otherwise, the numerator was calculated from the percentage of eradication reported and the intention-to-treat (ITT) sample size.
Trials were eligible if H. pylori eradication was confirmed using a rapid urease test, histology or culture of an endoscopic biopsy sample, or by a urea breath test or a monoclonal stool antigen test, at least 4 weeks after completion of treatment. Trials, in which only serology test was performed, were excluded.
Search Methods for Identification of Studies
Search Strategy
Bibliographical searches were performed in the MEDLINE, EMBASE, and the Cochrane Library electronic databases up to August 2021 based on the following words (all fields): pylori AND [(culture OR culture-based OR culture-guided OR tailored OR susceptibility OR susceptibility-guided OR “antimicrobial susceptibility” OR “susceptibility testing”) OR (empiric OR empirical)].
Reference lists of the articles selected by electronic searching were examined in detail to further identify relevant studies. In addition, references of articles retrieved, significant reviews, and the personal databases of the authors were also checked for eligible publications.
Data Collection and Analysis
Selection of Studies
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) approach (www.prismastatement.org) was used to develop a diagram to schematize the different steps of study selection (Liberati et al., 2009; Page et al., 2021).
Before the selection of studies, duplicates were removed in the citation manager. The selection of studies was conducted in two phases: a first screening of titles and abstracts to identify potentially relevant citations; and a second phase, where full texts of the previously selected studies were retrieved. Selection criteria were applied to full texts for definite inclusion. Two reviewers (OPN and ME) performed the screenings independently; disagreements were resolved by consensus with a third reviewer (JPG). The reason for the exclusion of a given study was reported in the second phase only as appropriate.
Data Extraction
A pre-tested data extraction form was used in a pilot test before the final collection of data to test its reliability. The following information was extracted from each study: first author; year of publication of the study; country; population (adult or children); study design (RCT or non-RCT); treatment line; susceptibility test; clarithromycin resistance rate (%); metronidazole resistance rate (%); levofloxacin resistance rate (%); type of empirical regimen; eradication rate with the empirical regimen; and eradication rate with the susceptibility-guided regimen. Two reviewers (OPN and ME) performed the data extraction independently; disagreements were resolved by consensus with a third reviewer (JPG).
Assessment of the Risk of Bias in Included Studies
The risk of bias was assessed independently by two reviewers (OPN and ME); disagreements were resolved by consensus with a third reviewer (JPG) in accordance with the Cochrane Collaboration's current recommendations (Higgins et al., 2009).
For RCTs, the Cochrane Risk of Bias (RoB) tool was used and the six quality items were evaluated: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias). A study was considered to be an RCT if it was explicitly described as “randomized.” This should include the use of words such as “random,” “randomly,” or “randomization.” We then rated the potential randomized trial as truly random, pseudo-random (randomization was mentioned but the method used was not reported), or non-random, based on the definitions by the Cochrane Handbook (Higgins et al., 2009).
For quasi-randomized trials (that is, non-random but controlled studies) and non-RCTs, the RoB criteria for EPOC Reviews (Guide for review authors on assessing study quality) advocated by the Cochrane was used. The same quality domains (as for RCTs) were assessed, but that related to the evaluation of randomization was reported as “high risk of bias” as no allocation of the sequence was generated as per the study design.
Assessment of Heterogeneity
The possible sources of diversity in the trial's characteristics were evaluated. We performed the Chi2 test for heterogeneity for each combined analysis, where P < 0.10 indicated significant heterogeneity between studies (Higgins and Thompson, 2002). Graphical methods (forest plots) were also used to complete the Chi2 test assessment.
The I2 statistic was used to assess the heterogeneity of the studies, following the recommendation of the Cochrane Collaboration's Handbook for Systematic Reviews of Interventions (Higgins et al., 2009), as follows: 0 to 40%, unimportant heterogeneity; 40 to 75%, moderate heterogeneity; 75 to 100% considerable heterogeneity.
Assessment of Reporting Biases
To assess publication bias, funnel plot asymmetry was inspected visually by examining the relationship between the treatment effects and the standard error of the estimate.
Data Synthesis
To collate, combine, and summarize the information obtained, a quantitative approach was undertaken. The evidence collected in the included studies was synthesized by summarizing the information related to the effect size of all studies, for each comparison and for each subgroup analysis. A meta-analysis was therefore performed combining the calculated risk ratios (RRs) of the individual studies with their corresponding 95% confidence intervals (CIs), using a random effects model (Mantel–Haenszel). Additional sensitivity analyses were performed to check the robustness of the results (DerSimonian and Laird, 1986; Egger et al., 1997).
The subgroup analyses were pre-planned to explore the possible sources of heterogeneity according to the study design (RCT vs. non-RCT), treatment line (naïve vs. rescue), susceptibility testing (culture vs. PCR), RCT by treatment line, and RCT by susceptibility test. The last group evaluating the empirical first-line quadruple treatments only was also included, to perform the most equitable comparison according to the most updated recommended first empirical quadruple treatments (i.e., non-bismuth and bismuth quadruple therapy) (Malfertheiner et al., 2017).
Analyses were performed using the freeware program Review Manager (RevMan) version 5.4.1 (2020).
Results
Description of Studies
In total, 17,383 citations were retrieved from the following electronic databases: PubMed and EMBASE, up to August 2021. After removing duplicates, a total of 13,941 citations were screened. After reviewing the abstracts and full texts, 47 studies (Romano et al., 2000, 2003; Toracchio et al., 2000; Avidan et al., 2001; Street et al., 2001; Lamouliatte et al., 2003; Miwa et al., 2003; Neri et al., 2003; Marzio et al., 2006; Yahav et al., 2006; Furuta et al., 2007; Kawai et al., 2008; Wang et al., 2008; Zhou et al., 2010, 2016; Bontems et al., 2011; Molina-Infante et al., 2012; Cosme et al., 2013, 2016; Lee et al., 2013; Martos et al., 2014; Park et al., 2014; Dong et al., 2015; Zhuo et al., 2015; Kwon et al., 2016; Miyaki et al., 2016; Ferenc et al., 2017; Gweon et al., 2018; Huang et al., 2018; Liou et al., 2018; Mascellino et al., 2018; Tanabe et al., 2018; Byambajav et al., 2019; Chen et al., 2019; Cho et al., 2019; Choi et al., 2019, 2021; Delchier et al., 2019; Ong et al., 2019; Pan et al., 2020; Saracino et al., 2020; Zhang et al., 2020; Bonoso Criado et al., 2021; Cha et al., 2021; Chang et al., 2021; Choe et al., 2021) were finally included in the present systematic review. A total of 54 different treatment comparisons were evaluated both in the quantitative and qualitative synthesis since some studies assessed more than one treatment comparison.
The different steps in the selection of studies and the reasons for the exclusion of studies [non-comparative (Gasbarrini et al., 2000; Gomollon et al., 2000; Chi et al., 2003; Cammarota et al., 2004; Fiorini et al., 2013; Liou et al., 2013; Choi et al., 2014; Sugimoto et al., 2014; Draeger et al., 2015; Stamboliyska et al., 2015; Han et al., 2016; Cosme et al., 2017, 2019; Costa et al., 2017; Králícek et al., 2017; Blümel et al., 2018; Kwon et al., 2019; Lee et al., 2019; Yu et al., 2019; Zhu and Wu, 2019) and cost-effectiveness (Breuer and Graham, 1999; Qasim et al., 2004; Faber et al., 2005; Cammarota et al., 2014)] are reported in the PRISMA flow chart (Figure 1).
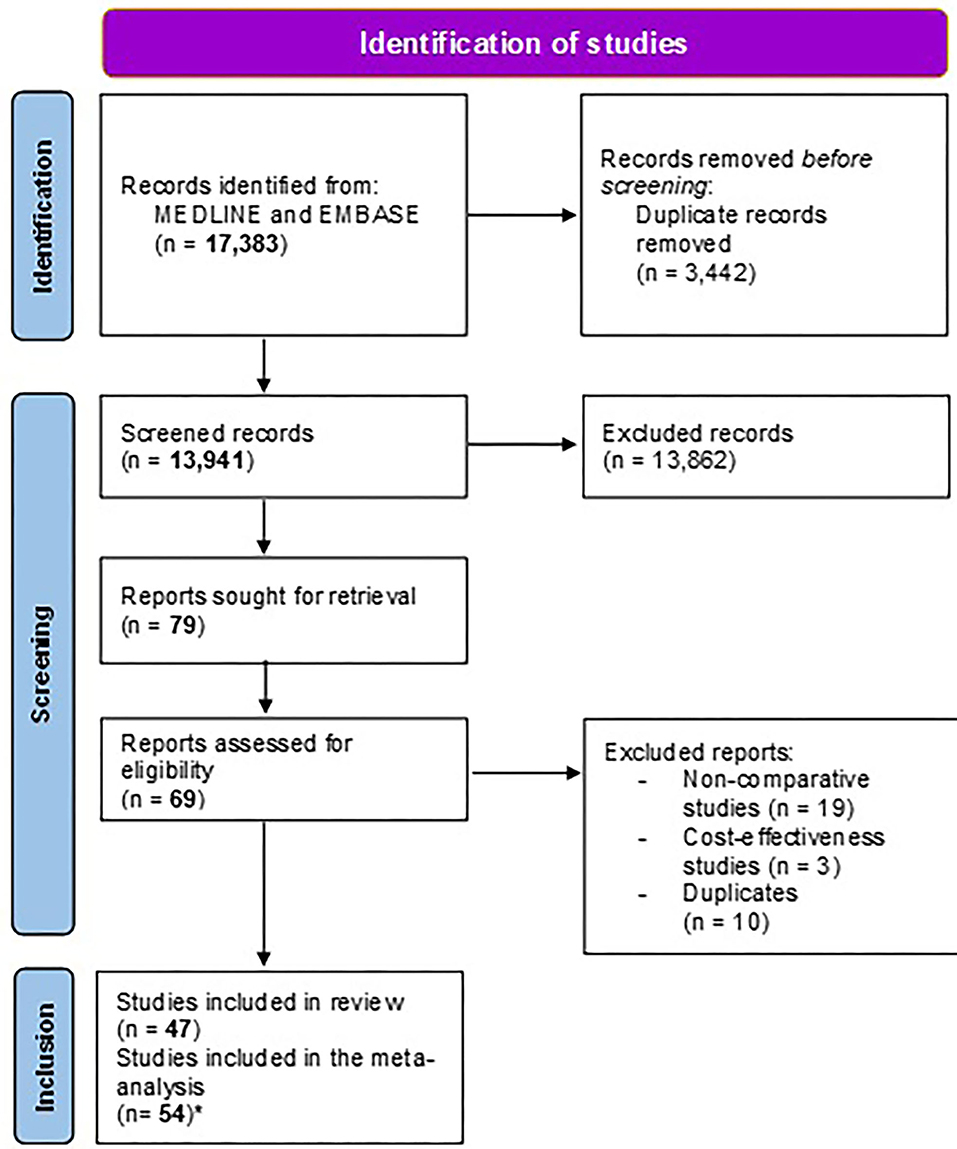
Figure 1. Study flow chart. *The number of studies included in the meta-analysis is higher than the number of studies included in the systematic review because certain studies included different treatment groups that were meta-analyzed separately (i.e., we considered as many study groups/comparisons as treatment arms).
The search time span was from the year 2000 to 2021, and published studies were mainly from Asian and European countries. All studies were on adults except for two, which evaluated children. One of them was an RCT (Bontems et al., 2011) and the other one was a prospective observational cohort (Zhang et al., 2020), and both used culture for the diagnosis of the infection and tailoring of the treatment.
We found 31 RCTs and 23 non-RCTs. In 30 studies, patients were naïve to treatment and the majority assessed patients treated with a second-line rescue therapy, except for three studies (Bontems et al., 2011; Huang et al., 2018; Liou et al., 2018), in which patients were treated with a third-line therapy. Baseline characteristics, diagnostic methods, and prescribed treatments are reported in Table 1. Most studies are tested for clarithromycin resistance. In four studies, (Miwa et al., 2003; Furuta et al., 2007; Zhou et al., 2016; Zhang et al., 2020) patients received a tailored therapy based on CYP2C19 polymorphism.
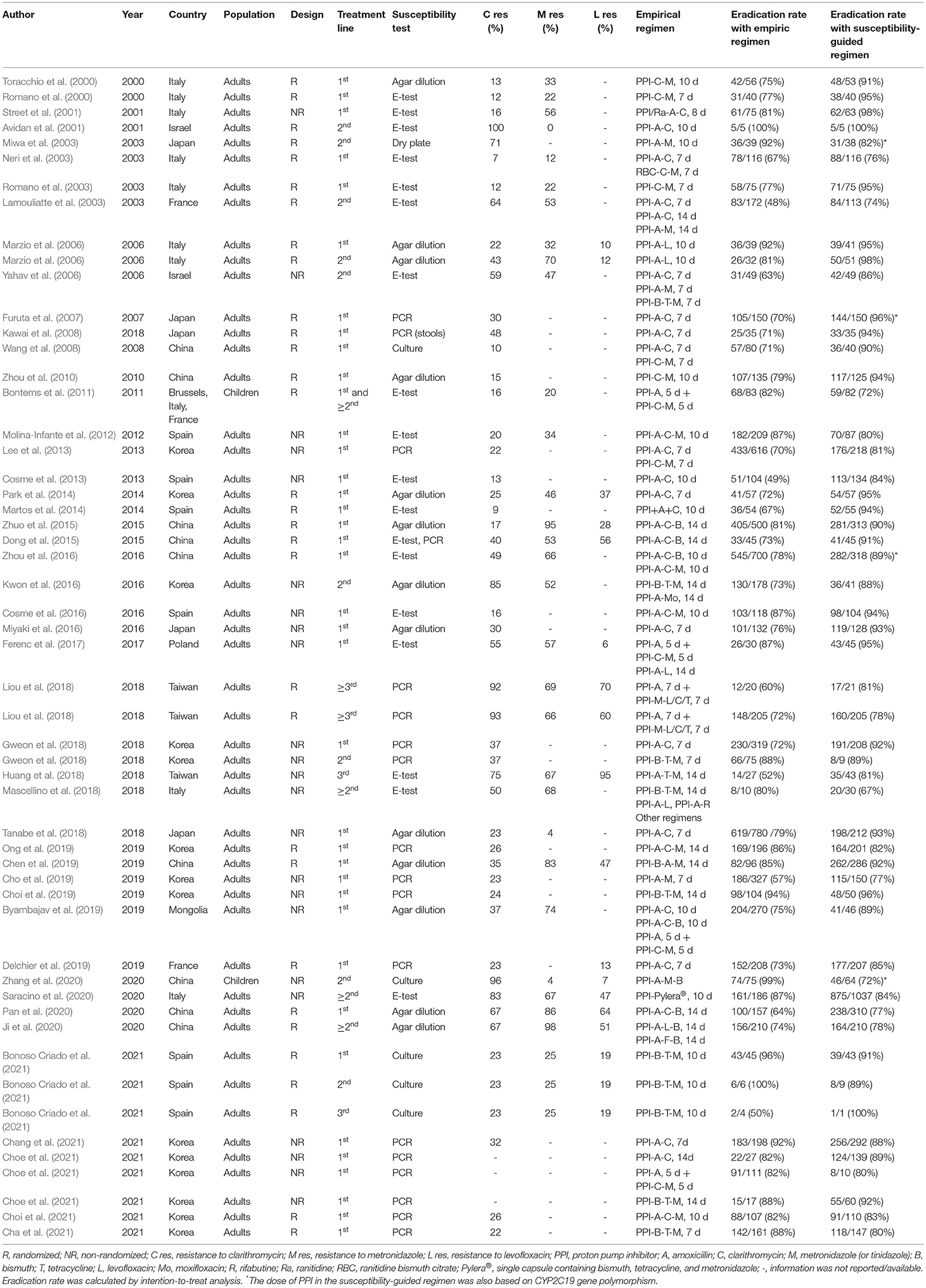
Table 1. Characteristics of studies comparing empirical vs. susceptibility-guided treatment for H. pylori infection.
Overall Results
From all studies, 14,600 patients were analyzed (6,705 patients in the susceptibility-guided group and 7,895 in the empirical group). Overall, H. pylori eradication was significantly better for the susceptibility-guided treatment than for the empirical treatment, 86 vs. 76%, respectively (RR: 1.12; 95% CI: 1.08–1.17; I2: 83%; Supplementary Figure 1).
Results of meta-analyses comparing the effectiveness of the empirical and the susceptibility-guided treatments between different groups (by treatment line, study design, tailored therapy, or recommended empirical quadruple therapy) are detailed below.
Treatment Line
First-Line Therapy
A total of 35 studies were included in this analysis, with 10,894 patients treated with first-line treatment. Statistically significant differences were found in cure rates in favor of susceptibility-guided therapy (87%) vs. empirical treatment (78%); however, results were highly heterogeneous (RR: 1.13; 95%CI: 1.08, 1.17; I2: 83%; Supplementary Figure 2).
Sensitivity analyses confirmed that susceptibility-guided therapy was also superior to first-line clarithromycin-based triple therapy, in areas with high (i.e., over 20%) clarithromycin resistance (RR: 1.13; 95% CI: 1.03, 1.25; I2: 90%) and also in those with low clarithromycin resistance (RR: 1.24; 95% CI: 1.15, 1.32; I2: 45%).
Rescue Therapy
A total of 16 studies were on rescue (more than one treatment failure) therapy. When patients receiving a second- (1,131) or third-line (152) treatment were evaluated separately, no differences were found between groups. Likewise, when all rescue lines were grouped (from 2nd to 3rd) and analyzed together (1,356 participants), no differences were reported (RR: 1.07; 95% CI: 0.97–1.18; I2: 78%, Supplementary Figure 2).
Susceptibility Test
Similar results were reported when culture (36 studies; RR: 1.11; 95% CI: 1.05–1.16; I2: 83%) or PCR (16 studies; RR: 1.08; 95% CI: 1,01–1,16; I2: 84%; Supplementary Figure 3) was used as a method to test antibiotic susceptibility; in both cases, the efficacy of the susceptibility-guided treatment was higher than that of the empirical treatment (85 vs. 77% and 86 vs. 78%, respectively). Heterogeneity between groups was high (I2: 83%); however, no significant variation in the mean effects was found between the different subgroups (P = 0.64).
Randomized Controlled Trials vs. Non-randomized Controlled Trials
In total, 27 RCTs (encompassing 31 comparisons) were included in the meta-analysis; that is, 7,325 patients (3,502 in the susceptibility-guided and 3,823 in the empirical treatment group) were evaluated. H. pylori eradication was achieved in 85% of patients in the susceptibility-guided group vs. 76% in the empirical group (RR: 1.13; 95% CI: 1.07–1.18; I2: 74%; Supplementary Figure 4). In this subgroup, one study (Bontems et al., 2011) was on children, nevertheless, excluding this study from the group did not vary the result of the sensitivity analysis. Heterogeneity was considerable in the RCT group, but lower than that of the overall assessment including all study designs (74 vs. 82%; respectively).
In non-RCTs, 8,000 patients (3,698 in the susceptibility-guided group and 4,302 in the empirical treatment group) were analyzed. In this sub-group, eradication was also higher in the susceptibility-guided group than in the empirical group (RR: 1.07; 95% CI: 1.01–1.14; I2: 86%). Likewise, the exclusion of one study (Zhang et al., 2020) on children did not vary the overall result of the sensitivity analysis. In addition, heterogeneity was significantly higher in the non-RCT group than when only RCTs were evaluated (86 vs. 74%, respectively; p < 0.001).
Randomized-Controlled Trials by Treatment Line
All the RCTs included could be meta-analyzed by treatment line except for the one by Bontems et al. (2011), in which eradication data could not be extracted separately for the first- and second-line treatment arms.
In total, 21 comparisons were evaluated, where 5,819 naïve patients had been randomized to receive either a first-line empirical therapy or a susceptibility-guided treatment. Statistically significant differences were reported in eradication rates between groups (78 vs. 87%, respectively), with moderate heterogeneity between arms (RR: 1.14; 95% CI: 1.08–1.20; I2: 75%; Figure 2).
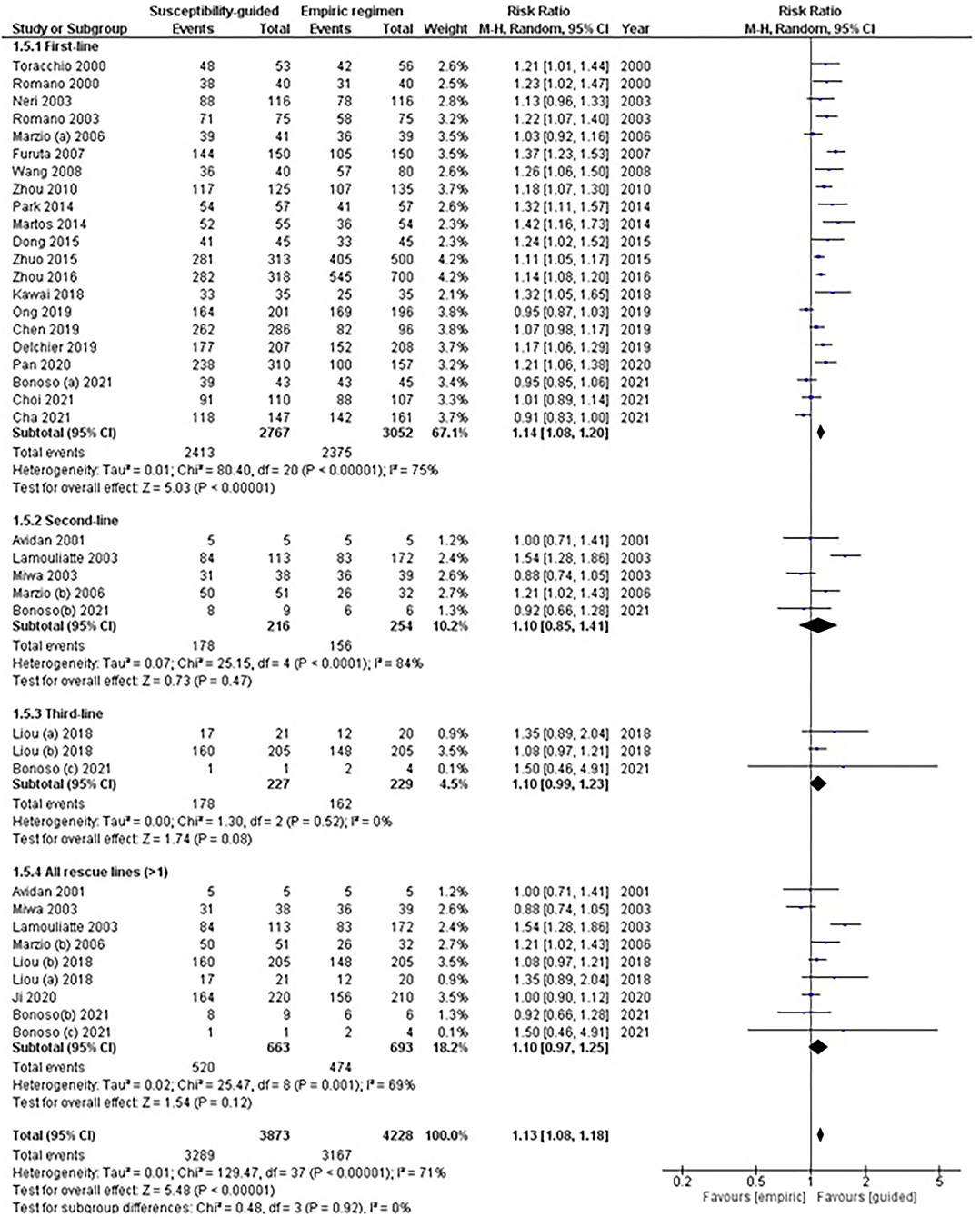
Figure 2. Forest plot of randomized controlled studies comparing the effectiveness of susceptibility-guided and empirical treatment according to treatment line. M-H, Mantel–Haenszel; CI, confidence interval.
No statistical differences were observed in second- (RR: 1.10; 95% CI: 0.85–1.42; I2: 84%) or subsequent rescue treatment lines; that is, when participants received more than one eradication treatment (RR: 1.10; 95% CI: 0.97–1.25; I2: 69%). Two RCTs (Liou et al., 2018; Bonoso Criado et al., 2021) reported data on patients treated with a third-line treatment, with no differences between treatment arms.
Randomized-Controlled Trials by Susceptibility Test
A total of 24 RCTs used culture and 8 PCR-based methods to determine (on gastric biopsies only) any bacterial antibiotic resistance (Table 1). Overall data were reported with moderate to high heterogeneity for each of the subgroup analyses and the test for subgroup differences was reported not significant.
Among studies with culture testing, results were moderately heterogeneous (RR: 1.13, 95% CI: 1.08–1.19, I2: 65%; Figure 3), and eradication rates were statistically higher in the guided-treatment arm than in empirically treated participants (86 vs. 76%; respectively). In this same subgroup, sensitivity analyses performed among naïve patients showed similar results (RR: 1.13, 95% CI: 1.07–1.20, I2: 81%). However, when rescue treatments (more than one treatment failure) were considered, no statistically significant differences were found between treatment arms (RR: 1.05, 95% CI: 0.95–1.17, I2: 85%).
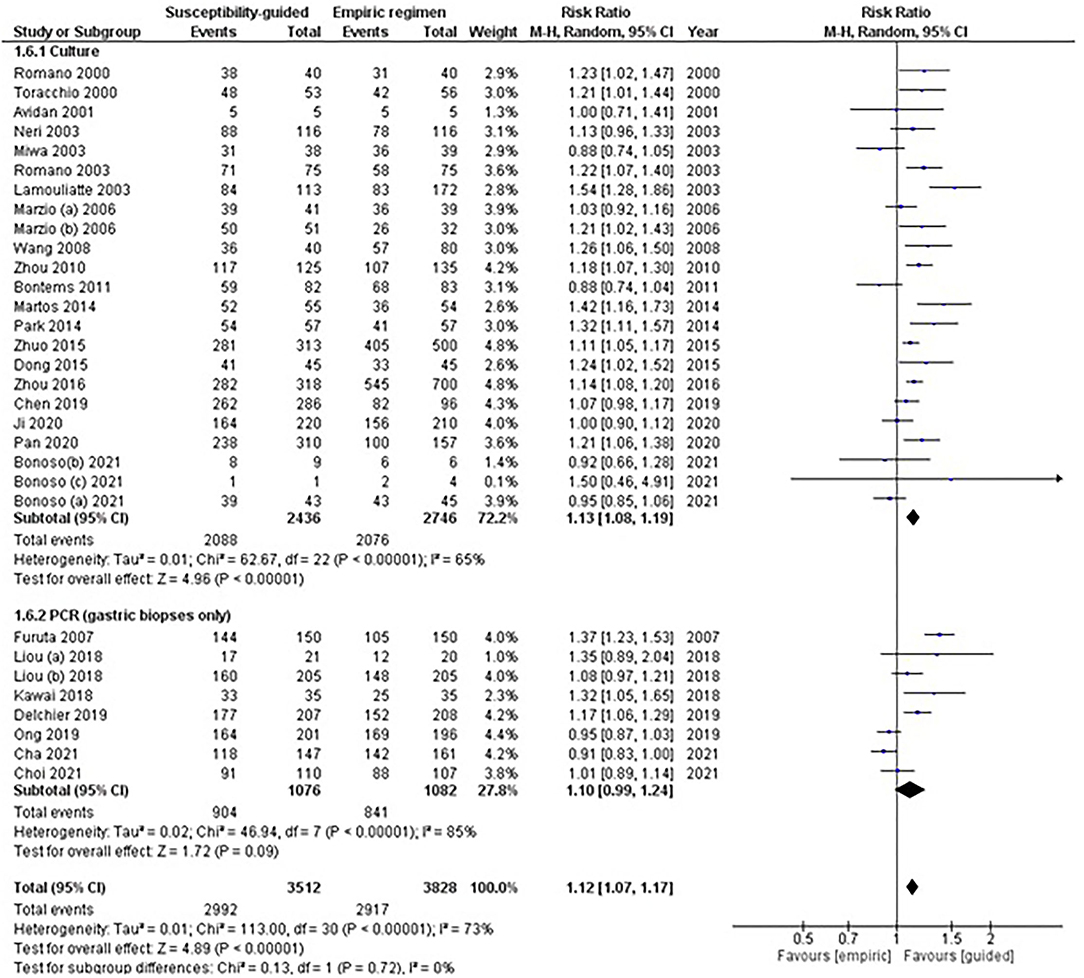
Figure 3. Forest plot of randomized controlled studies comparing the effectiveness of susceptibility-guided and empirical treatment according to susceptibility method (culture vs. polymerase chain reaction). M-H, Mantel–Haenszel; CI, confidence interval; PCR, polymerase chain reaction.
Among studies using PCR, no statistically significant differences were found between treatment groups (RR: 1.10, 95% CI: 0.99–1.24, I2: 85%). In this subgroup, sensitivity analyses performed among naïve patients showed similar results (RR: 1.10, 95% CI: 0.95–1.26, I2: 89%). One study (Liou et al., 2018) assessing third-line treatment, where two comparisons were available, showed no significant differences between treatments when data from both comparisons were pooled (RR: 1.10, 95% CI: 0.98–1.24, I2: 3%).
Two other studies (Kawai et al., 2008; Dong et al., 2015) were not included in this subgroup meta-analysis as they used PCR on stool, and both PCR and E-test, respectively, and results were not reported separately.
Empirical First-Line Quadruple Treatment
The recommendations of the Consensus guidelines on H. pylori first-line therapy were used to select studies for this subgroup meta-analysis. Only those studies evaluating naïve patients treated with an empirical first-line quadruple therapy—either with or without bismuth—and excluding unaccepted and suboptimal triple therapies and tailored treatment based on the CYP2C19 polymorphism were included.
In total, 12 studies (RCTs and non-RCTs) including 2,762 naïve patients (1,455 in the susceptibility-guided and 1,307 in the empirical group) were evaluated. No statistically significant differences were found in cure rates between the guided therapy (87%) and the empirical treatment (78%), with low to moderate heterogeneity between treatment arms (RR: 1.04; 95% CI: 0.99–1.09; I2: 72%; Figure 4). A post hoc sensitivity analysis including only RCTs (eight studies) in this same subgroup meta-analysis also confirmed the lack of difference in effectiveness between both groups (RR: 1.05; 95% CI: 0.99–1.12; I2: 77%). Moreover, excluding the RCT by Zhou et al. (2016), because the susceptibility-guided treatment was based on the CYP2C19 polymorphism, the effectiveness results remained similar in both treatment arms, with no significant differences (RR: 1.04; 95% CI: 0.97–1.12; I2: 75%).
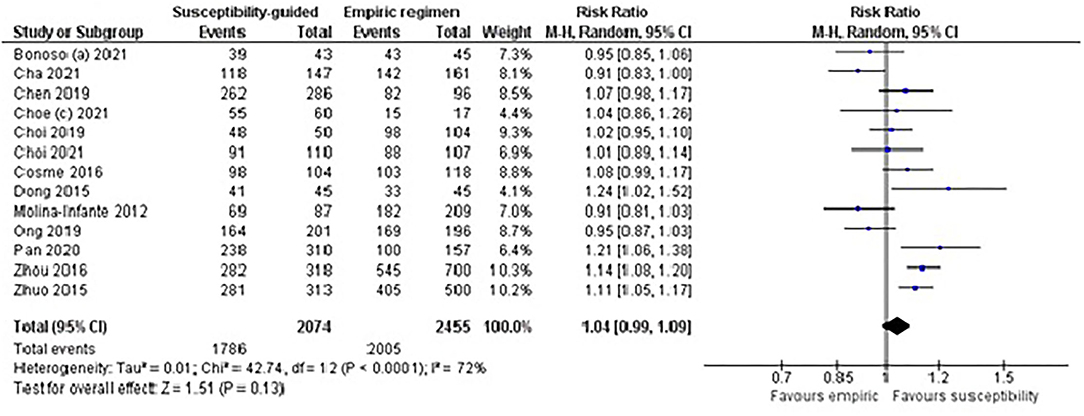
Figure 4. Forest plot of studies comparing the effectiveness of susceptibility-guided and empirical treatment in naïve patients treated with a (bismuth or non-bismuth) quadruple therapy. M-H, Mantel–Haenszel; CI, confidence interval.
Quality Assessment
A summary of the quality of included studies is shown in Supplementary Figures 5, 6.
Quality assessment of all studies included in the meta-analysis is presented in the summary table, following Cochrane instructions for evaluation of comparative studies (both RCTs and non-RCTs) with the RoB tool.
The RoB for both the randomization and allocation items was either unclear or high-risk in 50 to 75% of the studies (Supplementary Figure 5). The quality items related to the blinding of participants and personnel and to the outcome assessment are unlikely to affect the eradication outcome because H. pylori is an objective measurable endpoint. Therefore, these items were considered as introducing a low risk of bias, even for open-labeled studies.
All studies reported complete outcome data with no imbalance between arms in the patient's participation flow; therefore, no attrition bias was identified. Likewise, no selective reporting bias was detected [except for one study (Bontems et al., 2011)], as results of the primary endpoint were always correctly reported and data could be extracted (Supplementary Figure 6).
The funnel plot comparing the susceptibility-guided vs. empirical regimen groups of all included studies are shown in Supplementary Figure 7. This plot shows asymmetry suggesting a possible publication bias.
Discussion
Susceptibility testing has been proposed for antibiotic stewardship, aiming to reduce unnecessary antibiotic prescriptions; theoretically, treatment of H. pylori infections should not be an exception (Dang and Graham, 2017). Furthermore, through the application of susceptibility testing before treatment, the development of antimicrobial resistance could be minimized (Arslan et al., 2017), as antibiotic resistance in the outpatient community is positively correlated with antibiotic use (Megraud et al., 2013). However, in the present study (meta-analysis), the benefit of susceptibility-guided treatment over empirical treatment of H. pylori infection could not be demonstrated.
Several meta-analyses have previously compared cure rates of susceptibility-guided vs. empirical therapy for H. pylori first-line treatment, but all of them had limitations. The first meta-analysis was published by Wenzhen et al. (2010) and was focused specifically on first-line treatment. It only included five RCTs and concluded that culture-guided triple therapy was more effective than standard triple therapy (which was the regimen prescribed in most studies at that time) for first-line treatment. The second meta-analysis was published by Lopez-Gongora et al. (2015) and concluded that, in first-line treatment (nine studies only), susceptibility-guided therapy was more efficacious than empirical 7- to 10-day triple therapy (which, again, was the generally prescribed treatment at that time). The third meta-analysis was published by Chen et al. (2016) (including also nine studies only), and, again, showed that first-line tailored therapy achieved higher eradication rates than empirical regimens. The fourth meta-analysis was published by Gingold-Belfer et al. (2021) (including 16 studies only), also focusing mainly on first-line treatment (as only three RCTs were on rescue treatment), and concluded that susceptibility-guided therapy was superior to first-line clarithromycin-based triple therapy only when clarithromycin resistance exceeded 20%.
In our meta-analysis, the most updated in the literature, we have included 47 comparative studies (involving 6,705 patients in the susceptibility-guided group and 7,895 in the empirical group and including both RCTs and non-RCTs). Therefore, this study presents the highest number of studies in each subgroup published so far. Furthermore, the subgroup analyses performed in the present meta-analysis were more comprehensive than those of previous systematic reviews, and our protocol established no language restrictions.
Overall better efficacy results were obtained with the susceptibility-guided strategy for first-line treatments (naïve patients, 35 studies), although the results were borderline statistically significant (RR: 1.13; 95% CI: 1.08, 1.17). However, when prescribing only empirical quadruple regimens—that is, excluding the suboptimal triple therapies—no differences in efficacy were found vs. the susceptibility-guided group; this lack of difference was confirmed when only RCTs were considered. Therefore, we may conclude that susceptibility-guided treatment of H. pylori infection is not better than empirical treatment in first-line if the most updated bismuth or non-bismuth quadruple regimens are empirically prescribed, in agreement with a previous study (Gingold-Belfer et al., 2021).
The results of our meta-analysis are in agreement with the well-known high effectiveness of bismuth quadruple therapy, even in patients with clarithromycin or metronidazole resistance. In particular, when a bismuth quadruple therapy [either with tetracycline (Choi et al., 2019) or with amoxicillin (Chen et al., 2019)] was empirically prescribed, the efficacy was similar to that obtained with the susceptibility-based strategy. As an example, in the study by Choi et al. (2019), the eradication rate with the empirical bismuth quadruple and the susceptibility-based therapy was 94 and 96%, respectively. An advantage of prescribing a bismuth-based quadruple therapy is that we do not need to worry about previous antibiotic use or antimicrobial resistance as the risk of having a tetracycline or amoxicillin-resistant strain is extremely low and metronidazole resistance has limited impact on the effectiveness of this regimen (Gisbert and Pajares, 2002; Gisbert, 2020). In addition, the results of our meta-analysis are also in agreement with the encouraging results that are generally obtained with the empirical use of non-bismuth quadruple concomitant therapy, even when single clarithromycin or metronidazole resistance is present (only dual clarithromycin and metronidazole resistance seems to jeopardize effectiveness with this regimen) (Gisbert and Calvet, 2011).
Some meta-analyses have compared H. pylori cure rates of susceptibility-guided therapies with those of empirical therapy specifically for second-line treatment. In the meta-analysis by Lopez-Gongora et al., only four RCTs assessing H. pylori second-line rescue therapies were included (Lopez-Gongora et al., 2015). Results were highly heterogeneous and no significant differences were found between susceptibility-guided and empirical strategies in terms of efficacy. The other meta-analysis, performed by Chen et al., also found no differences between tailored and empirical rescue regimens, although only three studies were included (Chen et al., 2016). Finally, in our updated meta-analysis, for rescue therapies (16 studies, mostly as second-line), similar efficacy results were demonstrated with the two strategies—tailored and empirical—both when all the comparative studies were included and when only RCTs were considered.
It has been frequently recommended that performing culture at first-line treatment or after a first eradication failure may not be necessary and therefore assessing H. pylori sensitivity to antibiotics in clinical practice may be suggested only after failure of the second treatment (O'Connor et al., 2000). However, previous meta-analyses could not find any RCT comparing cure rates of susceptibility-guided therapies vs. those of empirical third-line therapy (Lopez-Gongora et al., 2015). Another systematic review aimed to evaluate the effectiveness of susceptibility-guided therapy as third-line therapy (without comparing it with empirical treatment) (Puig et al., 2016): four observational studies were included (no comparative studies were found), and the pooled mean eradication rate with susceptibility-guided therapy was only 72%. Therefore, the authors concluded that cure rates with susceptibility-guided therapy were, at best, moderate (Puig et al., 2016). Similarly, a more recent meta-analysis identified up to three studies and one sub-study showing a third-line therapy success of 79.9% in the susceptibility-guided therapy group vs. 65.2% in the empirical one (Gingold-Belfer et al., 2021). In our meta-analysis, four studies (of which two were RCTs) evaluated this comparison in the scenario of third-line treatment (Huang et al., 2018; Liou et al., 2018; Bonoso Criado et al., 2021; Choe et al., 2021), reporting no differences between the empirical and the susceptibility-guided arms. Therefore, the evidence is in favor of susceptibility-guided therapy as rescue therapy is currently insufficient to recommend its use.
In routine clinical laboratories, the detection of H. pylori antimicrobial resistance is mainly based on phenotypic methods performed after culture, including gradient diffusion susceptibility testing (E-test) and the agar dilution method (Arslan et al., 2017). In the last years, different PCR-based approaches have been developed as alternative tools to bacterium culture (Ierardi et al., 2017). In our meta-analysis, similar results were observed when susceptibility testing was assessed by culture or by PCR. Molecular tests are accurate in finding even minimal genotypic traces of certain resistant strains and are faster than conventional culture-based assays. Furthermore, PCR is technically feasible for clinical application in small- and medium-sized hospitals in developing countries (Xuan et al., 2016). However, the correlation between both methods is not perfect, probably due to the relatively low sensitivity of phenotypic assessment, the possibility that the E-test may identify resistant strains with point mutations different from those tested by PCR, or its inability to detect hetero-resistance (Ierardi et al., 2017; Jung et al., 2018).
Finally, some limitations of the strategy of performing culture systematically in all patients should be recognized: (1) culture implies the performance of endoscopic exploration, which is uncomfortable, expensive, and not free of risk. In addition, as endoscopy centers have been facing increasing demands, the technique frequently involves prolonged waiting times. As a consequence of the aforementioned problems, several diagnostic policies have been proposed for selecting patients with symptoms of dyspepsia, the most outstanding being the so-called “test-and-treat” strategy. Several prospective studies and decision analyses support the use of the test-and-treat strategy for dyspeptic patients (Gisbert and Calvet, 2013; Beresniak et al., 2020). Accordingly, this strategy has been recommended by all international consensus conferences (Fallone et al., 2016; Chey et al., 2017; Malfertheiner et al., 2017). (2) Culture is not always available on a routine basis. (3) The sensitivity of bacterial culture is not 100% (Megraud et al., 1997); indeed, even in the optimal conditions usually encountered in therapeutic trials, culture sensitivity is <90% (Zullo et al., 2003; Cammarota et al., 2014; Baylina et al., 2019). (4) Antibiotic susceptibility testing in clinical practice yields useful information only for a few antibiotics: clarithromycin, quinolones, and, less clearly, metronidazole (the relevance of in vitro metronidazole resistance for the in vivo treatment is quite limited) (Gisbert and Pajares, 2002); on the other hand, resistance to amoxicillin and tetracycline is extremely low. (5) Even knowing the susceptibility of H. pylori, eradication rates do not achieve 100%, as the results observed in vivo by following in vitro susceptibility to antibiotics are often disappointing (Guslandi, 2001; Gisbert and Pajares, 2002; Zullo et al., 2003; Baylina et al., 2019). The reverse situation is also possible, as H. pylori eradication may, nonetheless, be achieved in the presence of H. pylori metronidazole- or clarithromycin-resistant strains even with a drug combination including these antibiotics (Zullo et al., 2003; Bujanda et al., 2020, 2021). Furthermore, probably due to the synergistic effect of bismuth, the addition of this drug to triple therapy with clarithromycin may allow achieving a cure rate of approximately 90% even in patients with resistance against this antibiotic (Gisbert and McNicholl, 2017; Gisbert and Nyssen, 2021). (6) As previously mentioned, high eradication rates (≥90%) have been obtained with current up-to-date empirical first-line treatments, such as the bismuth or non-bismuth quadruple regimens. (7) Some studies have evaluated different empirical regimens after the failure of one, two, or more eradication treatments and have achieved a final (overall) eradication rate of almost 100% (Bock et al., 2000; Chan et al., 2000; Gasbarrini et al., 2000; Gomollon et al., 2000; Perri et al., 2000; Seppala et al., 2000; Beales, 2001; Canducci et al., 2001; Zullo et al., 2001, 2003; Treiber et al., 2002; Dore et al., 2003; Gisbert et al., 2003, 2004, 2008; Rokkas et al., 2009; Burgos-Santamaria et al., 2019). Thus, the empirical strategy should be based on the avoidance of repeating similar eradicating schemes, mainly clarithromycin- and quinolone-containing regimens, in the same patients during different eradicating regimens (Gisbert and Pajares, 2002; Roccarina et al., 2012; Calvet, 2018; Baylina et al., 2019; Nyssen et al., 2022). (8) Finally, different cost-effectiveness studies of the susceptibility-guided treatment of H. pylori infection have achieved contradictory results (Breuer and Graham, 1999; Romano et al., 2003; Qasim et al., 2004; Faber et al., 2005; Furuta et al., 2007; Cosme et al., 2013; Cammarota et al., 2014; Gweon et al., 2018; Liou et al., 2018; Cho et al., 2019).
Some relevant limitations affect studies comparing empirical vs. susceptibility-guided strategies, and consequently also the reliability of our meta-analysis. A major limitation of the current evidence regarding susceptibility-guided therapy is that comparative studies of susceptibility-guided therapy randomized patients after diagnostic endoscopy or even after successful culture (Lopez-Gongora et al., 2015). Therefore, the comparative effectiveness of susceptibility-guided therapy vs. the current non-invasive diagnosis and empirical treatment policy in patients with suspected H. pylori infection has not been evaluated in RCTs (Lopez-Gongora et al., 2015). Thus, a study adequately evaluating the effectiveness of susceptibility-guided therapy as a first-line treatment should randomize patients with non-investigated dyspepsia into non-invasive testing and endoscopy plus culture groups. In this same line, most of the studies evaluate the effectiveness of susceptibility-guided therapy as rescue therapy included the patients when the culture had been already obtained. Therefore, the effectiveness of susceptibility-guided therapy and empirical rescue therapy has never been properly compared (Puig et al., 2016). On the other hand, most studies using susceptibility-guided therapy only include patients with a positive culture. Therefore, the number of susceptibility-guided therapy failures due to patients' refusal of endoscopy has not been estimated or included (Baylina et al., 2019). When the applicability and effectiveness of this strategy were reviewed (Baylina et al., 2019), the rate of acceptance of endoscopy for biopsy and culture was described only in one article with only 60 patients and was reported to be as low as 60% (Matsumoto et al., 2005). In addition, given the diversity of studies included, our meta-analysis showed considerable heterogeneity (with asymmetric funnel plots) of the different a priori subgroup analyses performed comparing both therapeutic strategies; although such variability was investigated, it only could be partially explained. However, it is important to highlight that overall methodological quality was frequently high, and most studies were likely to avoid performance or detection biases (as per the therapeutic context) as well as attrition or reporting biases (as per the robustness of the outcome).
In summary, we think that susceptibility tests (culture or PCR) should be routinely performed, even before prescribing first-line treatment, in specialized centers with an interest in H. pylori management, to evaluate the prevalence of antibiotic resistance in the treatment of naïve patients and the influence of such resistances on the efficacy of up-to-date first-line eradication treatments. However, the present meta-analysis shows that the evidence is too limited to support the generalized use of susceptibility-guided therapy for H. pylori treatment in routine clinical practice, either as first-line or as rescue treatment. Undoubtedly, the most effective first-line H. pylori eradication treatment—that is, those regimens that have demonstrated to achieve cure rates ≥90% in our setting—must always be prescribed and the rescue treatment should be carefully chosen depending on which treatment was used initially. The results (H. pylori cure rates) of our clinical practice should be continuously audited to confirm that we always maintain a high success rate.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
OPN and JPG interpreted the data. OPN performed the analysis and prepared the first draft of the manuscript. ME, JPG, and OPN contributed to the literature search and data extraction. ME reviewed and approved the final draft of the manuscript. JPG analyzed and reviewed the draft manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
JPG has served as a speaker, consultant, and advisory member for or has received research funding from Mayoly, Allergan, Diasorin, Gebro Pharma, and Richen. OPN has received research funding from Mayoly and Allergan.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.913436/full#supplementary-material
Abbreviations
H. pylori, Helicobacter pylori; MIC, minimal inhibitory concentration; PPI, proton pump inhibitor; RCT, randomized controlled trial.
References
Arslan, N., Yilmaz, O., and Demiray-Gurbuz, E. (2017). Importance of antimicrobial susceptibility testing for the management of eradication in Helicobacter pylori infection. World J. Gastroenterol. 23, 2854–2869. doi: 10.3748/wjg.v23.i16.2854
Avidan, B., Melzer, E., Keller, N., and Bar-Meir, S. (2001). The effect of culture results for Helicobacter pylori on the choice of treatment following failure of initial eradication. Isr. Med. Assoc. J. 3, 163–165.
Baylina, M., Munoz, N., Sanchez-Delgado, J., Lopez-Gongora, S., Calvet, X., and Puig, I. (2019). Systematic review: Would susceptibility-guided treatment achieve acceptable cure rates for second-line Helicobacter pylori therapy as currently practiced? Helicobacter. 24, e12584. doi: 10.1111/hel.12584
Beales, I. L. (2001). Efficacy of Helicobacter pylori eradication therapies: a single centre observational study. BMC Gastroenterol. 1, 7. doi: 10.1186/1471-230X-1-7
Beresniak, A., Malfertheiner, P., Franceschi, F., Liebaert, F., Salhi, H., and Gisbert, J. P. (2020). Helicobacter pylori “Test-and-Treat” strategy with urea breath test: A cost-effective strategy for the management of dyspepsia and the prevention of ulcer and gastric cancer in Spain-Results of the Hp-Breath initiative. Helicobacter. 13, e12693. doi: 10.1111/hel.12693
Blümel, B., Goelz, H., Kist, M., and Glocker, E. O. (2018). Retrospective study on outcome of salvage Helicobacter pylori eradication therapies based on molecular genetic susceptibility testing. Helicobacter. 23, e12494. doi: 10.1111/hel.12494
Bock, H., Koop, H., Lehn, N., and Heep, M. (2000). Rifabutin-based triple therapy after failure of Helicobacter pylori eradication treatment: preliminary experience. J. Clin. Gastroenterol. 31, 222–225. doi: 10.1097/00004836-200010000-00007
Bonoso Criado, R., Pérez Citores, L., Pérez Millán, G., Montero Moretón, A., González de Castro, E., Cabezudo Molleda, L., et al. (2021). Estudio comparativo prospectivo del tratamiento de Helicobacter pylori con triple terapia guiada por antibiograma frente a cuádruple terapia con subcitrato de bismuto-metronidazol-tetraciclina. Rev. Esp. Enferm. Dig. 113, 597–601.
Bontems, P., Kalach, N., Oderda, G., Salame, A., Muyshont, L., and Miendje, D. Y. (2011). Sequential therapy versus tailored triple therapies for Helicobacter pylori infection in children. J. Pediatr. Gastroenterol. Nutr. 53, 646–650. doi: 10.1097/MPG.0b013e318229c769
Breuer, T., and Graham, D. Y. (1999). Costs of diagnosis and treatment of Helicobacter pylori infection: when does choosing the treatment regimen based on susceptibility testing become cost effective? Am. J. Gastroenterol. 94, 725–729. doi: 10.1111/j.1572-0241.1999.00943.x
Bujanda, L., Nyssen, O. P., Cosme, A., Bordin, D. S., Tepes, B., and Perez-Aisa, A. (2020). Impact of Helicobacter pylori clarithromycin resistance on the treatment effectiveness: data of the European Registry on H. pylori Management (Hp-EuReg). United Eur. Gastroenterol. J. 8, 256–257.
Bujanda, L., Nyssen, O. P., Vaira, D., Saracino, I. M., Fiorini, G., and Lerang, F. (2021). Antibiotic Resistance Prevalence and Trends in Patients Infected with Helicobacter pylori in the Period 2013-2020: Results of the European Registry on H. pylori Management (Hp-EuReg). Antibiotics. 10, 1058. doi: 10.3390/antibiotics10091058
Burgos-Santamaria, D., McNicholl, A. G., and Gisbert, J. P. (2019). Empirical Helicobacter pylori rescue therapy: an 18-year singlecentre study of 1200 patients. GastroHep. 1, 311–324. doi: 10.1002/ygh2.372
Byambajav, T. O., Bira, N., Choijamts, G., Davaadorj, D., Gantuya, B., and Sarantuya, T. (2019). Initial trials with susceptibility-based and empiric anti-h. pylori therapies in mongolia. Front. Pharmacol. 10, 394. doi: 10.3389/fphar.2019.00394
Calvet, X. (2018). Dealing with uncertainty in the treatment of Helicobacter pylori. Ther. Adv. Chronic. Dis. 9, 93–102. doi: 10.1177/2040622318758240
Camargo, M. C., Garcia, A., Riquelme, A., Otero, W., Camargo, C. A., and Hernandez-Garcia, T. (2014). The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am. J. Gastroenterol. 109, 485–495. doi: 10.1038/ajg.2014.24
Cammarota, G., Ianiro, G., Bibb,ò, S., Di Rienzo, T. A., Masucci, L., Sanguinetti, M., et al. (2014). Culture-guided treatment approach for Helicobacter pylori infection: review of the literature. World J. Gastroenterol. 20, 5205–5211. doi: 10.3748/wjg.v20.i18.5205
Cammarota, G., Martino, A., Pirozzi, G., Cianci, R., Branca, G., and Nista, E. C. (2004). High efficacy of 1-week doxycycline- and amoxicillin-based quadruple regimen in a culture-guided, third-line treatment approach for Helicobacter pylori infection. Aliment. Pharmacol. Therap. 19, 789–795. doi: 10.1111/j.1365-2036.2004.01910.x
Canducci, F., Ojetti, V., Pola, P., Gasbarrini, G., and Gasbarrini, A. (2001). Rifabutin-based Helicobacter pylori eradication 'rescue therapy'. Aliment. Pharmacol. Therap. 15, 143. doi: 10.1046/j.1365-2036.2001.00909.x
Cha, B., Bang, B. W., Shin, J. B., Ko, E. J., Ko, W., and Kwon, K. S. (2021). Bismuth containing quadruple therapy versus tailored therapy as first-line treatments for Helicobacter pylori infection in a high clarithromycin resistance area. Scandin. J. Gastroenterol. 56, 1017–1022. doi: 10.1080/00365521.2021.1948606
Chan, F. K., Sung, J. J., Suen, R., Wu, J. C., Ling, T. K., and Chung, S. C. (2000). Salvage therapies after failure of Helicobacter pylori eradication with ranitidine bismuth citrate-based therapies. Aliment. Pharmacol. Therap. 14, 91–95. doi: 10.1046/j.1365-2036.2000.00674.x
Chang, Y. W., Shin, G. Y., Kim, J. W., Moon, J. C., Chang, E. J., and Oh, C. H. (2021). Cost-effectiveness of empirical bismuth-based quadruple therapy and tailored therapy after clarithromycin resistance tests for helicobacter pylori eradication. Dig. Dis. Sci. 23, 1–9. doi: 10.1007/s10620-021-06938-y
Chen, H., Dang, Y., Zhou, X., Liu, B., Liu, S., and Zhang, G. (2016). Tailored therapy versus empiric chosen treatment for helicobacter pylori eradication: a meta-analysis. Medicine. 95, e2750. doi: 10.1097/MD.0000000000002750
Chen, Q., Long, X., Ji, Y., Liang, X., Li, D., Gao, H., et al. (2019). Randomised controlled trial: susceptibility-guided therapy versus empiric bismuth quadruple therapy for first-line Helicobacter pylori treatment. Aliment. Pharmacol. Therap. 49, 1385–1394. doi: 10.1111/apt.15273
Chey, W. D., Leontiadis, G. I., Howden, C. W., and Moss, S. F. A. C. G. C. (2017). Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 112, 212–239. doi: 10.1038/ajg.2016.563
Chi, C. H., Lin, C. Y., Sheu, B. S., Yang, H. B., Huang, A. H., and Wu, J. J. (2003). Quadruple therapy containing amoxicillin and tetracycline is an effective regimen to rescue failed triple therapy by overcoming the antimicrobial resistance of Helicobacter pylori. Aliment. Pharmacol. Therap. 18:347–353. doi: 10.1046/j.1365-2036.2003.01653.x
Cho, J. H., Jeon, S. R., Kim, H. G., Jin, S. Y., and Park, S. (2019). Cost-effectiveness of a tailored Helicobacter pylori eradication strategy based on the presence of a 23S ribosomal RNA point mutation that causes clarithromycin resistance in Korean patients. J. Gastroenterol. Hepatol. 34, 700–706. doi: 10.1111/jgh.14383
Choe, A. R., Shim, K. N., Park, Y., Song, E. M., Tae, C. H., and Jung, S. A. (2021). Cost-effectiveness, efficacy, and safety analysis of tailored therapy in patients with helicobacter pylori infection. J. Clin. Med. 10, 2619. doi: 10.3390/jcm10122619
Choi, M. G., Lee, J. Y., Lim, C. H., Kim, J. S., Park, J. M., and Lee, I. S. (2014). Helicobacter pylori eradication according to23s ribosomal RNA point mutations. Gastroenterology. 146, S-557. doi: 10.1016/S0016-5085(14)62017-5
Choi, Y. I., Chung, J. W., Kim, K. O., Kwon, K. A., Kim, Y. J., and Kim, J. H. (2021). Tailored eradication strategy vs concomitant therapy for Helicobacter pylori eradication treatment in Korean patients. World J. Gastroenterol. 27, 5247–5258. doi: 10.3748/wjg.v27.i31.5247
Choi, Y. I., Chung, J. W., Park, D. K., Kim, K. O., Kwon, K. A., and Kim, Y. J. (2019). Tailored eradication vs empirical bismuth-containing quadruple therapy for first-line Helicobacter pylori eradication: A comparative, open trial. World J. Gastroenterol. 25, 6743–6751. doi: 10.3748/wjg.v25.i46.6743
Cosme, A., Lizasoan, J., Montes, M., Tamayo, E., Alonso, H., and Mendarte, U. (2016). Antimicrobial susceptibility-guided therapy versus empirical concomitant therapy for eradication of helicobacter pylori in a region with high rate of clarithromycin resistance. Helicobacter. 21, 29–34. doi: 10.1111/hel.12231
Cosme, A., Montes, M., Ibarra, B., Tamayo, E., Alonso, H., and Mendarte, U. (2017). Antimicrobial susceptibility testing before first-line treatment for Helicobacter pylori infection in patients with dual or triple antibiotic resistance. World J. Gastroenterol. 23, 3367–3373. doi: 10.3748/wjg.v23.i18.3367
Cosme, A., Montes, M., Martos, M., Gil, I., Mendarte, U., and Salicio, Y. (2013). Usefulness of antimicrobial susceptibility in the eradication of Helicobacter pylori. Clin. Microbiol. Infec. 19, 379–383. doi: 10.1111/j.1469-0691.2012.03844.x
Cosme, A., Torrente Iranzo, S., Montes Ros, M., Fernandez-Reyes Silvestre, M., Alonso Galan, H., and Lizasoain, J. (2019). Helicobacter pylori antimicrobial resistance during a 5-year period (2013-2017) in northern Spain and its relationship with the eradication therapies. Helicobacter. 24, e12557. doi: 10.1111/hel.12557
Costa, S., Soares, J. B., and Goncalves, R. (2017). Efficacy and tolerability of culture-guided treatment for Helicobacter pylori infection. Eur. J. Gastroenterol. Hepatol. 29, 1258–1263. doi: 10.1097/MEG.0000000000000960
Dang, B. N., and Graham, D. Y. (2017). Helicobacter pylori infection and antibiotic resistance: a WHO high priority? Nat. Rev. Gastroenterol. Hepatol. 14, 383–384. doi: 10.1038/nrgastro.2017.57
Delchier, J. C., Bastuji-Garin, S., Raymond, J., Megraud, F., Amiot, A., and Cambau, E. (2019). Efficacy of a tailored PCR-guided triple therapy in the treatment of Helicobacter pylori infection. Med. Malad. Infect. 50, 492–9. doi: 10.1016/j.medmal.2019.06.001
DerSimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Control Clin. Trials. 7, 177–188. doi: 10.1016/0197-2456(86)90046-2
Dong, F., Ji, D., Huang, R., Zhang, F., Huang, Y., Xiang, P., et al. (2015). Multiple genetic analysis system-based antibiotic susceptibility testing in helicobacter pylori and high eradication rate with phenotypic resistance-guided quadruple therapy. Medicine. 94, e2056. doi: 10.1097/MD.0000000000002056
Dore, M. P., Leandro, G., Realdi, G., Sepulveda, A. R., and Graham, D. Y. (2000). Effect of pretreatment antibiotic resistance to metronidazole and clarithromycin on outcome of Helicobacter pylori therapy: a meta-analytical approach. Dig. Dis. Sci. 45, 68–76. doi: 10.1023/A:1005457226341
Dore, M. P., Marras, L., Maragkoudakis, E., Nieddu, S., Manca, A., and Graham, D. Y. (2003). Salvage therapy after two or more prior Helicobacter pylori treatment failures: the super salvage regimen. Helicobacter. 8, 307–309. doi: 10.1046/j.1523-5378.2003.00150.x
Draeger, S., Wuppenhorst, N., Kist, M., and Glocker, E. O. (2015). Outcome of second- and third-line Helicobacter pylori eradication therapies based on antimicrobial susceptibility testing. J. Antimicrob. Chemother. 70, 3141–3145. doi: 10.1093/jac/dkv223
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ. 315, 629–634. doi: 10.1136/bmj.315.7109.629
Faber, J., Bar-Meir, M., Rudensky, B., Schlesinger, Y., Rachman, E., and Benenson, S. (2005). Treatment regimens for Helicobacter pylori infection in children: is in vitro susceptibility testing helpful? J. Pediatr. Gastroenterol. Nutr. 40, 571–574. doi: 10.1097/01.MPG.0000155567.71902.75
Fallone, C. A., Chiba, N., van Zanten, S. V., Fischbach, L., Gisbert, J. P., and Hunt, R. H. (2016). The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 151, 51–69. e14. doi: 10.1053/j.gastro.2016.04.006
Ferenc, S., Gnus, J., Koscielna, M., Kinda, M., Yarka, A., and Stewart, L. (2017). High antibiotic resistance of Helicobacter pylori and its effect on tailored and empiric eradication of the organism in Lower Silesia, Poland. Helicobacter. 22, e12365. doi: 10.1111/hel.12365
Fiorini, G., Vakil, N., Zullo, A., Saracino, I. M., Castelli, V., and Ricci, C. (2013). Culture-based selection therapy for patients who did not respond to previous treatment for Helicobacter pylori infection. Clin. Gastroenterol. Hepatol. 11, 507–510. doi: 10.1016/j.cgh.2012.12.007
Furuta, T., Shirai, N., Kodaira, M., Sugimoto, M., Nogaki, A., and Kuriyama, S. (2007). Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin. Pharmacol. Ther. 81, 521–528. doi: 10.1038/sj.clpt.6100043
Gasbarrini, A., Ojetti, V., Armuzzi, A., Branca, G., Canducci, F., and Torre, E. S. (2000). Efficacy of a multistep strategy for Helicobacter pylori eradication. Aliment. Pharmacol. Therap. 14, 79–83. doi: 10.1046/j.1365-2036.2000.00685.x
Gingold-Belfer, R., Niv, Y., Schmilovitz-Weiss, H., Levi, Z., and Boltin, D. (2021). Susceptibility-guided versus empirical treatment for Helicobacter pylori infection: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 36, 2649–2658. doi: 10.1111/jgh.15575
Gisbert, J. P. (2011). Is culture necessary before first-line treatment for Helicobacter pylori infection? Intern Med. 50, 2717. doi: 10.2169/internalmedicine.50.5135
Gisbert, J. P. (2020). Empirical or susceptibility-guided treatment for Helicobacter pylori infection? A comprehensive review. Therap. Adv. Gastroenterol. 13, 1756284820968736. doi: 10.1177/1756284820968736
Gisbert, J. P., and Calvet, X. (2011). Review article: non-bismuth quadruple (concomitant) therapy for eradication of Helicobater pylori. Aliment. Pharmacol. Therap. 34, 604–617. doi: 10.1111/j.1365-2036.2011.04770.x
Gisbert, J. P., and Calvet, X. (2013). Helicobacter Pylori “Test-and-Treat” strategy for management of dyspepsia: a comprehensive review. Clin. Transl. Gastroenterol. 4, e32. doi: 10.1038/ctg.2013.3
Gisbert, J. P., Calvet, X., Bujanda, L., Marcos, S., Gisbert, J. L., and Pajares, J. M. (2003). 'Rescue' therapy with rifabutin after multiple Helicobacter pylori treatment failures. Helicobacter. 8, 90–94. doi: 10.1046/j.1523-5378.2003.00128.x
Gisbert, J. P., Gisbert, J. L., Marcos, S., Jimenez-Alonso, I., Moreno-Otero, R., and Pajares, J. M. (2008). Empirical rescue therapy after Helicobacter pylori treatment failure: a 10-year single-centre study of 500 patients. Aliment. Pharmacol. Therap. 27, 346–354. doi: 10.1111/j.1365-2036.2007.03573.x
Gisbert, J. P., Gisbert, J. L., Marcos, S., and Pajares, J. M. (2004). Empirical Helicobacter pylori “rescue” therapy after failure of two eradication treatments. Dig. Liver Dis. 36, 7–12. doi: 10.1016/j.dld.2003.09.018
Gisbert, J. P., and McNicholl, A. G. (2017). Optimization strategies aimed to increase the efficacy of H. pylori eradication therapies. Helicobacter. 22, e12392. doi: 10.1111/hel.12392
Gisbert, J. P., and Nyssen, O. P. (2021). “Ten common errors in the treatment of Helicobacter pylori infection”, in Recent Advances in Gastroenterology. New Delhi: C Probert Jaypee Brothers Medical Publishers Ltd. p. 106–150. ISBN13 9781909836242.
Gisbert, J. P., and Pajares, J. M. (2002). Review article: Helicobacter pylori “rescue” regimen when proton pump inhibitor-based triple therapies fail. Aliment. Pharmacol. Therap. 16, 1047–1057. doi: 10.1046/j.1365-2036.2002.01276.x
Gomollon, F., Sicilia, B., Ducons, J. A., Sierra, E., Revillo, M. J., and Ferrero, M. (2000). Third line treatment for Helicobacter pylori: a prospective, culture-guided study in peptic ulcer patients. Aliment. Pharmacol. Therap. 14, 1335–1338. doi: 10.1046/j.1365-2036.2000.00833.x
Guslandi, M. (2001). Review article: alternative antibacterial agents for Helicobacter pylori eradication. Aliment. Pharmacol. Therap. 15, 1543–1547. doi: 10.1046/j.1365-2036.2001.01064.x
Gweon, T. G., Kim, J. S., and Kim, B. W. (2018). An economic modeling study of helicobacter pylori eradication: comparison of dual priming oligonucleotide-based multiplex polymerase chain reaction and empirical treatment. Gut Liver. 12, 648–654. doi: 10.5009/gnl18079
Han, F., Ji, Z. Z., Jin, X., Wan, L., Cai, C. X., and Chen, Y. P., et al. (2016). Antimicrobial susceptibility testing for helicobacterf pylori treatment: A follow-up study on clinical effects. World Chin. J. Digestol. 24, 808–814. doi: 10.11569/wcjd.v24.i5.808
Higgins, J. P., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558. doi: 10.1002/sim.1186
Higgins, JPT., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, MJ., and Welcg VA, (editors) (2009). Cochrane Handbook for Systematic Reviews of Interventions version 6, 2. (updated February 2022). Cochrane, 2022. Available online at: www.training.cochrane.org/handbook
Hooi, J. K. Y., Lai, W. Y., Ng, W. K., Suen, M. M. Y., Underwood, F. E., and Tanyingoh, D. (2017). Global prevalence of helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 153, 420–429. doi: 10.1053/j.gastro.2017.04.022
Huang, H. T., Wang, H. M., Yang, S. C., Tai, W. C., Liang, C. M., and Wu, K. L. (2018). Efficacy of a 14-day quadruple-therapy regimen for third-line Helicobacter pylori eradication. Infect. Drug Resist. 11, 2073–2080. doi: 10.2147/IDR.S185511
Ierardi, E., Giorgio, F., Iannone, A., Losurdo, G., Principi, M., and Barone, M. (2017). Noninvasive molecular analysis of Helicobacter pylori: Is it time for tailored first-line therapy? World J. Gastroenterol. 23, 2453–2458. doi: 10.3748/wjg.v23.i14.2453
Ji, C. R., Liu, J., Li, Y. Y., Qiao, C., Qu, J. Y., Hu, J. N., et al. (2020). Susceptibility-guided quadruple therapy is not superior to medication history-guided therapy for the rescue treatment of Helicobacter pylori infection: a randomized controlled trial. J. Dig. Dis. 21, 549–557. doi: 10.1111/1751-2980.12934
Jung, D. H., Kim, J. H., Jeong, S. J., Park, S. Y., Kang, I. M., and Lee, K. H. (2018). Peptide Nucleic Acid Probe-Based Analysis as a New Detection Method for Clarithromycin Resistance in Helicobacter pylori. Gut. liver. 12, 641–647. doi: 10.5009/gnl18111
Kawai, T., Yamagishi, T., Yagi, K., Kataoka, M., Kawakami, K., and Sofuni, A. (2008). Tailored eradication therapy based on fecal Helicobacter pylori clarithromycin sensitivities. J. Gastroenterol. Hepatol. 23, S171–S174. doi: 10.1111/j.1440-1746.2008.05408.x
Králícek, L., Kroupa, R., Ševcíková, A., Otrubová, M., and Kulhánek, V. (2017). Outcome of treatment of Helicobacter pylori infection based on microbiological susceptibility test ing fol low ing the unsucces sful second-line eradication treatment. Gastroenterol. Hepatol. 71, 304–309. doi: 10.14735/amgh2017csgh.info02
Kwon, Y. H., Jeon, S. W., Nam, S. Y., Lee, H. S., and Park, J. H. (2019). Efficacy of tailored therapy for Helicobacter pylori eradication based on clarithromycin resistance and survey of previous antibiotic exposure: A single-center prospective pilot study. Helicobacter. 24, e12585. doi: 10.1111/hel.12585
Kwon, Y. H., Kim, N., Lee, J. Y., Choi, Y. J., Yoon, K., and Nam, R. H. (2016). Comparison of the efficacy of culture-based tailored therapy for Helicobacter pylori eradication with that of the traditional second-line rescue therapy in Korean patients: a prospective single tertiary center study. Scandin. J. Gastroenterol. 51, 270–276. doi: 10.3109/00365521.2015.1095352
Lamouliatte, H., Megraud, F., Delchier, J. C., Bretagne, J. F., Courillon-Mallet, A., and De Korwin, J. D. (2003). Second-line treatment for failure to eradicate Helicobacter pylori: a randomized trial comparing four treatment strategies. Aliment. Pharmacol. Therap. 18, 791–797. doi: 10.1046/j.1365-2036.2003.01759.x
Lee, H. J., Kim, J. I., Cheung, D. Y., Kim, T. H., Jun, E. J., and Oh, J. H. (2013). Eradication of Helicobacter pylori according to 23S ribosomal RNA point mutations associated with clarithromycin resistance. J. Infect. Dis. 208, 1123–1130. doi: 10.1093/infdis/jit287
Lee, J. W., Kim, N., Nam, R. H., Lee, S. M., Kwon, Y. H., and Sohn, S. D. (2019). Favorable outcomes of culture-based Helicobacter pylori eradication therapy in a region with high antimicrobial resistance. Helicobacter. 24, e12561. doi: 10.1111/hel.12561
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gotzsche, P. C., and Ioannidis, J. P. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 339, b2700. doi: 10.1136/bmj.b2700
Liou, J. M., Chen, C. C., Chang, C. Y., Chen, M. J., Fang, Y. J., and Lee, J. Y. (2013). Efficacy of genotypic resistance-guided sequential therapy in the third-line treatment of refractory Helicobacter pylori infection: a multicentre clinical trial. J. Antimicrob. Chemother. 68, 450–456. doi: 10.1093/jac/dks407
Liou, J. M., Chen, P. Y., Luo, J. C., Lee, J. Y., Chen, C. C., and Fang, Y. J. (2018). Efficacies of genotypic resistance-guided vs empirical therapy for refractory helicobacter pylori infection. Gastroenterology. 155, 1109–1119. doi: 10.1053/j.gastro.2018.06.047
Lopez-Gongora, S., Puig, I., Calvet, X., Villoria, A., Baylina, M., and Munoz, N. (2015). Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J. Antimicrob. Chemother. 70, 2447–2455. doi: 10.1093/jac/dkv155
Malfertheiner, P., Megraud, F., O'Morain, C. A., Gisbert, J. P., Kuipers, E. J., and Axon, A. T. (2017). Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 66, 6–30. doi: 10.1136/gutjnl-2016-312288
Martos, M., Bujanda, L., Salicio, Y., Sarasqueta, C., Ibarra, B., and Mendarte, U. (2014). Clarithromycin for first-line treatment of Helicobacter pylori infection after culture in high-resistance regions. Eur. J. Gastroenterol. Hepatol. 26, 1380–1384. doi: 10.1097/MEG.0000000000000197
Marzio, L., Coraggio, D., Capodicasa, S., Grossi, L., and Cappello, G. (2006). Role of the preliminary susceptibility testing for initial and after failed therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and esomeprazole. Helicobacter. 11, 237–242. doi: 10.1111/j.1523-5378.2006.00407.x
Mascellino, M. T., Oliva, A., De Angelis, M., Pontone, S., and Porowska, B. (2018). Helicobacter pylori infection: antibiotic resistance and eradication rate in patients with gastritis showing previous treatment failures. New Microbiol. 41, 306–309.
Matsumoto, Y., Miki, I., Aoyama, N., Shirasaka, D., Watanabe, Y., and Morita, Y. (2005). Levofloxacin- versus metronidazole-based rescue therapy for H. pylori infection in Japan. Dig. Liver. Dis. 37, 821–825. doi: 10.1016/j.dld.2005.06.002
Megraud, F., Coenen, S., Versporten, A., Kist, M., Lopez-Brea, M., and Hirschl, A. M. (2013). Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 62, 34–42. doi: 10.1136/gutjnl-2012-302254
Megraud, F., O'MORAIN, C., and Malfertheiner, P. (1997). Technical annex: tests used to assess Helicobacter pylori infection. Working Party of the European Helicobacter pylori Study Group. Gut. 41, S10–S18.
Miwa, H., Nagahara, A., Kurosawa, A., Ohkusa, T., Ohkura, R., and Hojo, M. (2003). Is antimicrobial susceptibility testing necessary before second-line treatment for Helicobacter pylori infection? Aliment. Pharmacol. Therap. 17, 1545–1551. doi: 10.1046/j.1365-2036.2003.01541.x
Miyaki, A., Yamaguchi, K., Ida, A., and Miyauchi, T. (2016). An assessment of the efficacy of first-line Helicobacter pylori-eradication therapy based on clarithromycin susceptibility. Minerva Gastroenterol. Dietol. 62, 234–239.
Molina-Infante, J., Pazos-Pacheco, C., Vinagre-Rodriguez, G., Perez-Gallardo, B., Duenas-Sadornil, C., and Hernandez-Alonso, M. (2012). Nonbismuth quadruple (concomitant) therapy: empirical and tailored efficacy versus standard triple therapy for clarithromycin-susceptible Helicobacter pylori and versus sequential therapy for clarithromycin-resistant strains. Helicobacter. 17, 269–276. doi: 10.1111/j.1523-5378.2012.00947.x
Neri, M., Milano, A., Laterza, F., Di Bonaventura, G., Piccolomini, R., Caldarella, M. P., et al. (2003). Role of antibiotic sensitivity testing before first-line Helicobacter pylori eradication treatments. Aliment. Pharmacol. Therap. 18, 821–827. doi: 10.1046/j.1365-2036.2003.01757.x
Nyssen, O. P., Vaira, D., Tepes, B., Kupcinskas, L., Bordin, D., and Perez-Aisa, A. (2022). Room for Improvement in the Treatment of Helicobacter pylori Infection: Lessons from the European Registry on H. pylori Management (Hp-EuReg). J. Clin. Gastroenterol. 56, e98–e108. doi: 10.1097/MCG.0000000000001482
O'Connor, A., Furuta, T., Gisbert, J. P., and O'Morain, C. (2000). Regular review: treatment of Helicobacter pylori infection. BMJ. 320, 31–34. doi: 10.1136/bmj.320.7226.31
Ong, S., Kim, S. E., Kim, J. H., Yi, N. H., Kim, T. Y., Jung, K., et al. (2019). Helicobacter pylori eradication rates with concomitant and tailored therapy based on 23S rRNA point mutation: A multicenter randomized controlled trial. Helicobacter. 24, e12654. doi: 10.1111/hel.12654
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372, n71. doi: 10.1136/bmj.n71
Pan, J., Shi, Z., Lin, D., Yang, N., Meng, F., and Lin, L. (2020). Is tailored therapy based on antibiotic susceptibility effective? a multicenter, open-label, randomized trial. Front. Med. 14, 43–50. doi: 10.1007/s11684-019-0706-8
Park, C. S., Lee, S. M., Park, C. H., Koh, H. R., Jun, C. H., and Park, S. Y. (2014). Pretreatment antimicrobial susceptibility-guided vs. clarithromycin-based triple therapy for Helicobacter pylori eradication in a region with high rates of multiple drug resistance. Am. J. Gastroenterol. 109, 1595–1602. doi: 10.1038/ajg.2014.222
Perri, F., Festa, V., Clemente, R., Quitadamo, M., and Andriulli, A. (2000). Rifabutin-based 'rescue therapy' for Helicobacter pylori infected patients after failure of standard regimens. Aliment. Pharmacol. Therap. 14, 311–316. doi: 10.1046/j.1365-2036.2000.00719.x
Puig, I., Lopez-Gongora, S., Calvet, X., Villoria, A., Baylina, M., and Sanchez-Delgado, J. (2016). Systematic review: third-line susceptibility-guided treatment for Helicobacter pylori infection. Therap. Adv. Gastroenterol. 9, 437–448. doi: 10.1177/1756283X15621229
Qasim, A., Sebastian, S., Buckley, M., O'Connor, H., and O'Morain, C. (2004). Pretreatment antimicrobial susceptibility testing is not cost saving in the standard eradication of Helicobacter pylori. Clin. Gastroenterol. Hepatol. 2, 85. discussion doi: 10.1016/S1542-3565(03)00296-9
Roccarina, D., Franceschi, F., Zocco, M. A., Garcovich, M., Gasbarrini, G., and Gasbarrini, A. (2012). Different Antibiotic No Culture Eradicating (DANCE) strategy: an easy way to manage H. pylori eradication. Dig. Liver Dis. 44, 889–892. doi: 10.1016/j.dld.2012.05.010
Rokkas, T., Sechopoulos, P., Robotis, I., Margantinis, G., and Pistiolas, D. C. (2009). Pylori eradication rates in clinical practice by adopting first and second-line regimens proposed by the Maastricht III consensus and a third-line empirical regimen. Am. J. Gastroenterol. 104, 21–25. doi: 10.1038/ajg.2008.87
Romano, M., Iovene, M. R., Montella, F., Vitale, L. M., De Simone, T., and Blanco, C. D. V. (2000). Pretreatment antimicrobial-susceptibility testing in the eradication of H. pylori infection. Am. J. Gastroenterol. 95, 3317–3318. doi: 10.1111/j.1572-0241.2000.03317.x
Romano, M., Marmo, R., Cuomo, A., De Simone, T., Mucherino, C., and Iovene, M. R. (2003). Pretreatment antimicrobial susceptibility testing is cost saving in the eradication of Helicobacter pylori. Clin Gastroenterol Hepatol. 1, 273–278. doi: 10.1016/S1542-3565(03)00131-9
Saracino, I. M., Pavoni, M., Zullo, A., Fiorini, G., Saccomanno, L., and Lazzarotto, T. (2020). Antibiotic resistance and therapy outcome in h. pylori eradication failure patients. Antibiotics. 9, 121. doi: 10.3390/jcm9051299
Savoldi, A., Carrara, E., Graham, D. Y., Conti, M., and Tacconelli, E. (2018). Prevalence of antibiotic resistance in helicobacter pylori: a systematic review and meta-analysis in world health organization regions. Gastroenterology. 155, 1372–82.e17. doi: 10.1053/j.gastro.2018.07.007
Seppala, K., Kosunen, T. U., Nuutinen, H., Sipponen, P., Rautelin, H., and Sarna, S. (2000). Cure of Helicobacter pylori infection after failed primary treatment: one-center results from 120 patients. Scandin. J. Gastroenterol. 35, 929–934. doi: 10.1080/003655200750022977
Stamboliyska, M., Kaludova, V., Mirchev, M., Kotzev, I., Madjov, R., and Metodiev, K. (2015). Role and importance of Microbiology testing in the Diagnosis and Treatment of Helicobacter pylori infection. J. IMAB – Ann. Proc. 21, 969–973. doi: 10.5272/jimab.2015214.969
Street, M. E., Caruana, P., Caffarelli, C., Magliani, W., Manfredi, M., and Fornaroli, F. (2001). Antibiotic resistance and antibiotic sensitivity based treatment in Helicobacter pylori infection: advantages and outcome. Arch. Dis. Child. 84, 419–422. doi: 10.1136/adc.84.5.419
Sugimoto, M., Uotani, T., Sahara, S., Ichikawa, H., Yamade, M., and Sugimoto, K. (2014). Efficacy of tailored Helicobacter pylori eradication treatment based on clarithromycin susceptibility and maintenance of acid secretion. Helicobacter. 19, 312–318. doi: 10.1111/hel.12128
Tanabe, H., Yoshino, K., Ando, K., Nomura, Y., Ohta, K., and Satoh, K. (2018). Vonoprazan-based triple therapy is non-inferior to susceptibility-guided proton pump inhibitor-based triple therapy for Helicobacter pylori eradication. Ann. Clin. Microbiol. Antimicrob. 17, 29. doi: 10.1186/s12941-018-0281-x
Thung, I., Aramin, H., Vavinskaya, V., Gupta, S., Park, J. Y., and Crowe, S. E. (2016). Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol Therap. 43, 514–533. doi: 10.1111/apt.13497
Toracchio, S., Cellini, L., Di Campli, E., Cappello, G., Malatesta, M. G., Ferri, A., et al. (2000). Role of antimicrobial susceptibility testing on efficacy of triple therapy in Helicobacter pylori eradication. Aliment. Pharmacol Therap. 14, 1639–1643. doi: 10.1046/j.1365-2036.2000.00870.x
Treiber, G., Ammon, S., Malfertheiner, P., and Klotz, U. (2002). Impact of furazolidone-based quadruple therapy for eradication of Helicobacter pylori after previous treatment failures. Helicobacter. 7, 225–231. doi: 10.1046/j.1523-5378.2002.00087.x
Wang, G., Zhao, Q., and Li, S. (2008). Study of drug sensitivity test in Helicobacter pylori eradication therapy. J. Clin. Intern. Med. 25, 474–477.
Wenzhen, Y., Yumin, L., Quanlin, G., Kehu, Y., Lei, J., and Donghai, W. (2010). Is antimicrobial susceptibility testing necessary before first-line treatment for Helicobacter pylori infection? Meta-analysis of randomized controlled trials. Intern. Med. 49, 1103–1109. doi: 10.2169/internalmedicine.49.3031
Xuan, S. H., Wu, L. P., Zhou, Y. G., and Xiao, M. B. (2016). Detection of clarithromycin-resistant Helicobacter pylori in clinical specimens by molecular methods: A review. J. Glob. Antimicrob. Resist. 4, 35–41. doi: 10.1016/j.jgar.2016.01.002
Yahav, J., Samra, Z., Niv, Y., Evans, C. T., Passaro, D. J., and Dinari, G. (2006). Susceptibility-guided vs. empiric retreatment of Helicobacter pylori infection after treatment failure. Dig. Dis. Sci. 51, 2316–2321. doi: 10.1007/s10620-006-9302-2
Yu, L., Luo, L., Long, X., Liang, X., Ji, Y., Chen, Q., et al. (2019). Susceptibility-guided therapy for Helicobacter pylori infection treatment failures. Therap. Adv. Gastroenterol. 12, 1756284819874922. doi: 10.1177/1756284819874922
Zhang, Y. D., Dong, Q. W., Zhang, S. H., Gu, F., Zhang, Y., and Song, H. B. (2020). Effectiveness of eradication regimen based on the bacterial susceptibility and CYP2C19 genotype in children with refractory Helicobacter pylori infection. Zhonghua er ke za zhi = Chinese J. Pediat. 58, 41–45. doi: 10.3760/cma.j.issn.0578-1310.2020.01.010
Zhou, J., Wu, M., and Jiang, X. (2010). Role of drug sensitivity test in the triple therapy for eradication of Helicobacter pylori. Chin. J. Gastroenterol. 15, 358–360.
Zhou, L., Zhang, J., Song, Z., He, L., and Qian, Li, Y. (2016). Tailored versus triple plus bismuth or concomitant therapy as initial helicobacter pylori treatment: a randomized trial. Helicobacter. 21, 91–99. doi: 10.1111/hel.12242
Zhu, D. B., and Wu, J. M. (2019). Risk factors for treatment ineffectiveness in patients with H. Pylori infection undergoing eradication treatment according to drug susceptibility testing results. World Chin. J. Digestol. 27, 509–514. doi: 10.11569/wcjd.v27.i8.509
Zhuo, R. P., Chen, X. P., Wu, S. Z., Xie, J. L., and Hu, S. K. (2015). Clinical effects of quadruple therapy based on antimicrobial susceptibility testing in treatment of Helicobacter pylori associated upper digestive tract diseases. World Chin. J. Digestol. 23, 196–201. doi: 10.11569/wcjd.v23.i2.196
Zullo, A., Hassan, C., Campo, S. M., Lorenzetti, R., Febbraro, I., Matthaeis, D., et al. (2001). A triple therapy regimen after failed Helicobacter pylori treatments. Aliment. Pharmacol. Therap. 15, 1193–1197. doi: 10.1046/j.1365-2036.2001.01028.x
Keywords: Helicobacter pylori, culture, tailored, susceptibility, empirical
Citation: Nyssen OP, Espada M and Gisbert JP (2022) Empirical vs. Susceptibility-Guided Treatment of Helicobacter pylori Infection: A Systematic Review and Meta-Analysis. Front. Microbiol. 13:913436. doi: 10.3389/fmicb.2022.913436
Received: 06 April 2022; Accepted: 17 May 2022;
Published: 14 June 2022.
Edited by:
Maria Teresa Mascellino, Sapienza University of Rome, ItalyReviewed by:
Matteo Pavoni, Università di Bologna, ItalyKathryn Haley, Grand Valley State University, United States
Carola Severi, Sapienza University of Rome, Italy
Copyright © 2022 Nyssen, Espada and Gisbert. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Javier P. Gisbert, javier.p.gisbert@gmail.com
 Olga P. Nyssen1,2,3
Olga P. Nyssen1,2,3