- 1College of Science, Hainan University, Haikou, China
- 2Key Laboratory of Tropical Translational Medicine of Ministry of Education, School of Pharmacy, Hainan Medical University, Haikou, China
- 3College of Life Science, Hunan Normal University, Changsha, China
- 4Institut Systématique, Evolution, Biodiversité (ISYEB), UMR 7205, Muséum National d’ Histoire Naturelle, CNRS, Sorbonne Université, Paris, France
- 5School of Pharmaceutical Sciences and Yunnan Key Laboratory of Pharmacology for Natural Products, Kunming Medical University, Kunming, China
- 6Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Ganzhou, China
- 7School of Horticulture, Anhui Agricultural University, Hefei, China
Species of Craterellus (Hydnaceae, Cantharellales) in China are investigated on the basis of morphological and molecular phylogenetic analyses of DNA sequences from nuc 28S rDNA D1-D2 domains (28S) and nuc rDNA internal transcribed spacer ITS1-5.8S-ITS2 region. Five species are recognized in China, of which three of them are described as new, viz. C. fulviceps, C. minor, and C. parvopullus, while two of them are previously described taxa, viz. C. aureus, and C. lutescens. A key to the known Chinese taxa of the genus is also provided.
Introduction
Craterellus Pers. (Hydnaceae, Cantharellales), typified by C. cornucopioides (L.) Pers., is characterized by a small, funnel-shaped basidioma with a hollow stipe (Petersen, 1979a). Recent molecular phylogenetic data have confirmed the monophyly of the genus (Hibbett et al., 2014). To date, many taxa of Craterellus have been discovered in Africa, America, and Asia (Dahlman et al., 2000; Matheny et al., 2010; Beluhan and Ranogajec, 2011; Kumari et al., 2012; Wilson et al., 2012; Das et al., 2017; Hembrom et al., 2017; Bijeesh et al., 2018; Zhong et al., 2018; Zhang et al., 2020; Cao et al., 2021a,b). They have received much attention for their edibility and medicinal value; for example, C. cornucopioides is considered a highly nutritious edible fungus and has antihyperglycemic, antioxidative, and antitumor activities (Beluhan and Ranogajec, 2011; Liu et al., 2012; Fan et al., 2014), and C. tubaeformis (Fr.) Quél. has antioxidant, antimicrobial, and anti-inflammatory activities (Li, 1996; O’Callaghan et al., 2014).
A total of thirteen taxa of Craterellus have been described/reported from China in previous studies, viz. C. albidus Chun Y. Deng, M. Zhang & Jing Zhang, C. atrobrunneolus T. Cao & H.S. Yuan, C. aureus Berk. & M.A. Curtis., C. badiogriseus T. Cao & H.S. Yuan, C. croceialbus T. Cao & H.S. Yuan, C. cornucopioides, C. cornucopioides var. parvisporus Heinem., C. lutescens (Fr.) Fr., C. luteus T.H. Li & X.R. Zhong, C. odoratus (Schwein.) Fr., C. macrosporus T. Cao & H.S. Yuan, C. squamatus T. Cao & H.S. Yuan, and C. tubaeformis (Li, 1996, 2005; Beluhan and Ranogajec, 2011; Xiao et al., 2012; Zhang et al., 2020; Cao et al., 2021a,b). Most of them are well known in the country, for mushrooms identified as C. aureus, C. cornucopioides, C. cornucopioides var. parvisporus, C. lutescens, or C. tubaeformis are sold as edibles in the market of Yunnan Province, southwestern China (Wang et al., 2004; Zhang et al., 2021; Figure 1). In addition, interesting compounds such as merosesquiterpenids, acetylenic acids, and derivatives have been isolated from collections identified as C. lutescens and C. odoratus in the country (Zhang et al., 2010; Huang et al., 2016, 2017).

Figure 1. Collections of Craterellus lutescens sold as edibles in the market of Yunnan Province, southwestern China. Photos: H.-Y. Huang.
Recently, lots of collections of Craterellus in China have been made, which were studied using morphological and molecular phylogenetic analyses. The aim was to (i) describe new taxa and (ii) reevaluate some reports of previously described taxa.
Materials and Methods
Morphological Studies
Field notes and digital photographs were made from fresh specimens which were dried and deposited in the Fungal Herbarium of Hainan Medical University (FHMU) (Index Herbariorum), Haikou City, Hainan Province of China. Color codes follow Kornerup and Wanscher (1981). An optical light microscope (CX23, Olympus, Tokyo, Japan) was used to observe and measure the microstructures of basidiomata; the samples were hand-sectioned and mounted in a 5% KOH solution. The notation [n/m/p] indicates “n” basidiospores measured from “m” basidiomata of “p” collections. Dimensions of basidiospores are presented as (a–)b–e–c(–d), where the range “b–c” represents a minimum of 90% of the measured values (5th to 95th percentile), and extreme values (a and d), whenever present (a <5th percentile, d >95th percentile), are in parentheses, “e” refers to the average length/width of basidiospores. “Q” refers to the length/width ratio of basidiospores; “Qm” refers to the average “Q” of basidiospores and is presented with standard deviation. The terms referring to the size of basidioma are based on Bas (1969).
Molecular Procedures
Total genomic DNA was extracted from dried basidiomata (10–20 mg) using the Plant Genomic DNA Kit (CWBIO, Beijing, China) according to the manufacturer’s instructions. Protocols for polymerase chain reaction (PCR) amplification and sequencing followed An et al. (2017). The universal primer pairs ITS5/ITS4 (White et al., 1990) and LR0R/LR5 (Vilgalys and Hester, 1990; James et al., 2006) were used for PCR amplification of nuclear ribosomal internal transcribed spacer (ITS) and large subunit ribosomal DNA (28S), respectively. PCR conditions followed Zhang et al. (2021). PCR products were checked using 1% (w/v) agarose gel electrophoresis. The amplified PCR products were sequenced using an ABI 3730 DNA Analyzer (BGI, Guangzhou, China) with the PCR primers. Forward or reverse sequences were assembled with BioEdit (Hall, 1999). All newly obtained sequences were deposited in GenBank1.
Dataset Assembly
A total of thirty DNA sequences (16 of 28S, 14 of ITS) from 17 collections were newly generated for this study (Table 1). For the concatenated dataset, the 28S and ITS sequences generated in the study were aligned with selected sequences from previous studies and GenBank (Table 1). Hydnum minus FHMU2461 and Hydnum cremeoalbum FHMU2153 were chosen as outgroups as described by An et al. (2017). Sequences of 28S and ITS were aligned separately to test for phylogenetic conflict. The topologies of the phylogenetic trees based on a single gene were identical, indicating that the phylogenetic signals present in the different gene fragments were not in conflict. Then, the sequences of the different genes were aligned using MUSCLE (Edgar, 2004), and alignments were purged from unreliably aligned positions and gaps using Gblocks (Castresana, 2000). The sequences of the different genes were concatenated using Phyutility v2.2 for further analyses (Smith and Dunn, 2008).
Phylogenetic Analyses
The combined nuclear dataset (28S + ITS) was analyzed using maximum likelihood (ML) and Bayesian inference (BI) methods. ML tree generation and bootstrap (BS) analyses were performed using RAxML v7.2.6 (Stamatakis, 2006), running 1,000 replicates combined with the ML search. BI was conducted in MrBayes v3.1 (Huelsenbeck and Ronquist, 2005) on the CIPRES Science Gateway portal (Miller et al., 2011). The best-fit likelihood models of 28S (GTR + I + G) and ITS (HKY + I + G) were estimated in MrModeltest v2.3 (Nylander, 2004) based on the Akaike information criterion. Bayesian analysis was repeated for 30 million generations and sampled every 1,000 generations. Trees sampled from the first 25% generations were discarded as burn-in, and Bayesian posterior probabilities (PP) were then calculated for a majority-rule consensus tree of the retained sampled trees.
Results
Molecular Data
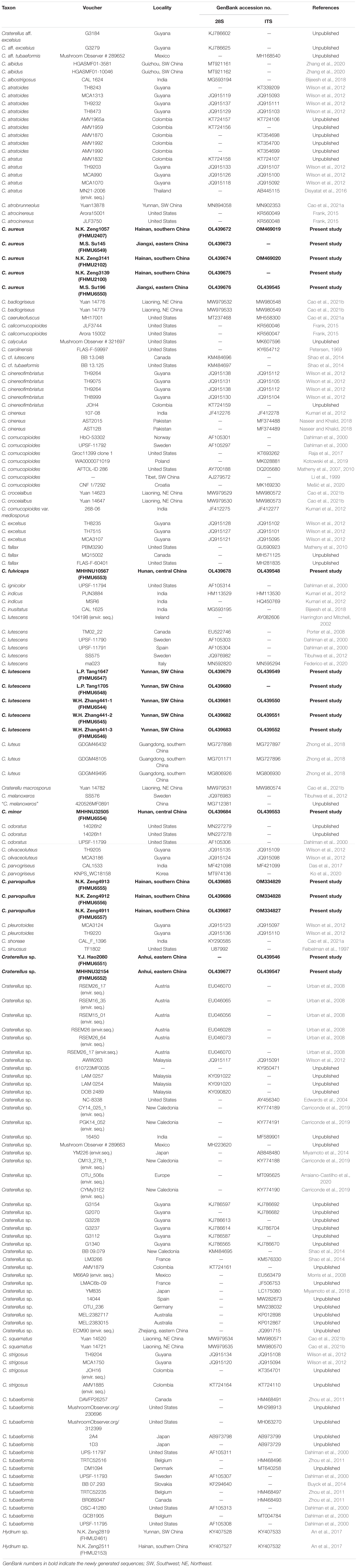
The combined dataset (28S + ITS) of Craterellus consisted of 161 taxa and 2,173 nucleotide sites (Figure 2), and the alignment was submitted to TreeBase (S28981). The topologies of the phylogenetic trees based on the combined dataset generated from ML and BI analyses were identical, but statistical support showed slight differences. In this study, we focused on lineages 1–14 from China (Figure 2). Lineage 1, with strong statistical support (BS = 85%, PP = 0.99), comprised of three collections (GDGM46432, GDGM48105, and GDGM49495) of C. luteus, and three collections (FHMU2100, FHMU2102, and FHMU2407) from southern China, and two collections (FHMU6549, FHMU6550) from eastern China. Lineage 2, with strong statistical support (BS = 82%, PP = 1.0), comprised of two collections (FHMU6551, and FHMU6552) from eastern China. Lineage 3, three collections (FHMU6555, FHMU6556, and FHMU6557) from southern China grouped together with high statistical support (BS = 100%, PP = 0.99). Lineage 4 comprised of the holotype of C. atrobrunneolus. Lineage 5, with strong statistical support (BS = 94%, PP = 1.0), comprised of two collections (Yuan 14,520, and Yuan 14,721) of C. squamatus from northeastern China. Lineage 6 comprised of the holotype of C. macrosporus. Lineage 7, with strong statistical support (BS = 100%, PP = 1.0), comprised of two collections (Yuan 14,623, and Yuan 14,647) of C. croceialbus from northeastern China. Lineage 8 comprised of one collection named C. cornucopioides from western China. Lineage 9, with strong statistical support (BS = 100%, PP = 1.0), comprised of two collections (Yuan 14,776 and Yuan 14,779) of C. badiogriseus from northeastern China. Lineage 10, with strong statistical support (BS = 96%, PP = 1.0), comprised of two collections (HGASMF01-10046, and HGASMF01-3581) of C. albidus from southwestern China. Lineage 11, with strong statistical support (BS = 95%, PP = 1.0), comprised of one collection (FHMU6553) from central China, and one collection labeled as C. tubaeformis from Japan. Lineage 12, with strong statistical support (BS = 99%, PP = 1.0), comprised of one collection (FHMU6554) from central China, and one collection labeled as C. melanoxeros also from China. Lineage 13 comprised of one collection (ECM90) from eastern China. Lineage 14, with strong statistical support (BS = 90%, PP = 1.0), comprised of seven collections of C. lutescens (UPSF-11789, UPSF-11790, UPSF-11791, 104198, SS575, ma023, and TM02_22), five collections labeled as Craterellus sp. (RSEM15_01, RSEM16_35, RSEM26, RSEM26_17, and RSEM26_64), and five collections (FHMU6544–FHMU6548) from southwestern China.
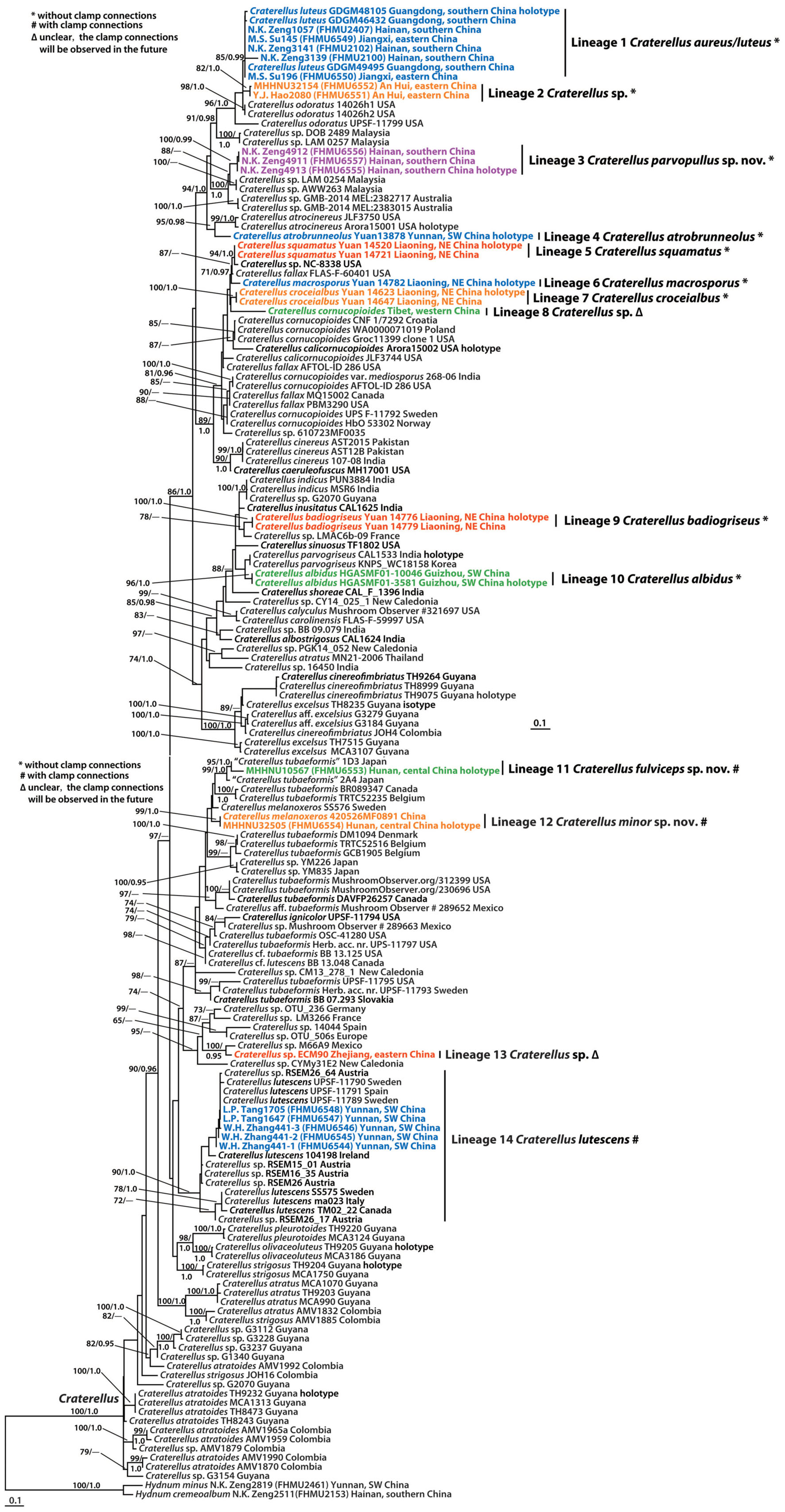
Figure 2. Phylogram inferred from a combined dataset (28S + ITS) of Craterellus using RAxML. RAxML bootstrap percentages (BS ≥ 70%) and Bayesian posterior probabilities (PP ≥ 0.95) are indicated above or below the branches as BS/PP.
Taxonomy
Craterellus aureus Berk. & M.A. Curtis, Proc. Amer. Acad. Arts & Sci. 4: 123, 1860 Figures 3A–E, 4.

Figure 3. Basidiomata of Craterellus species. (A–E) C. aureus (A) FHMU2100; (B) FHMU6549; (C) FHMU2102; (D) FHMU2407; (E) FHMU6550; (F) C. fulviceps (FHMU6553, holotype). Photos: (A,C,D) N.-K. Zeng; (B,E) M.-S. Su; (F) P. Zhang.
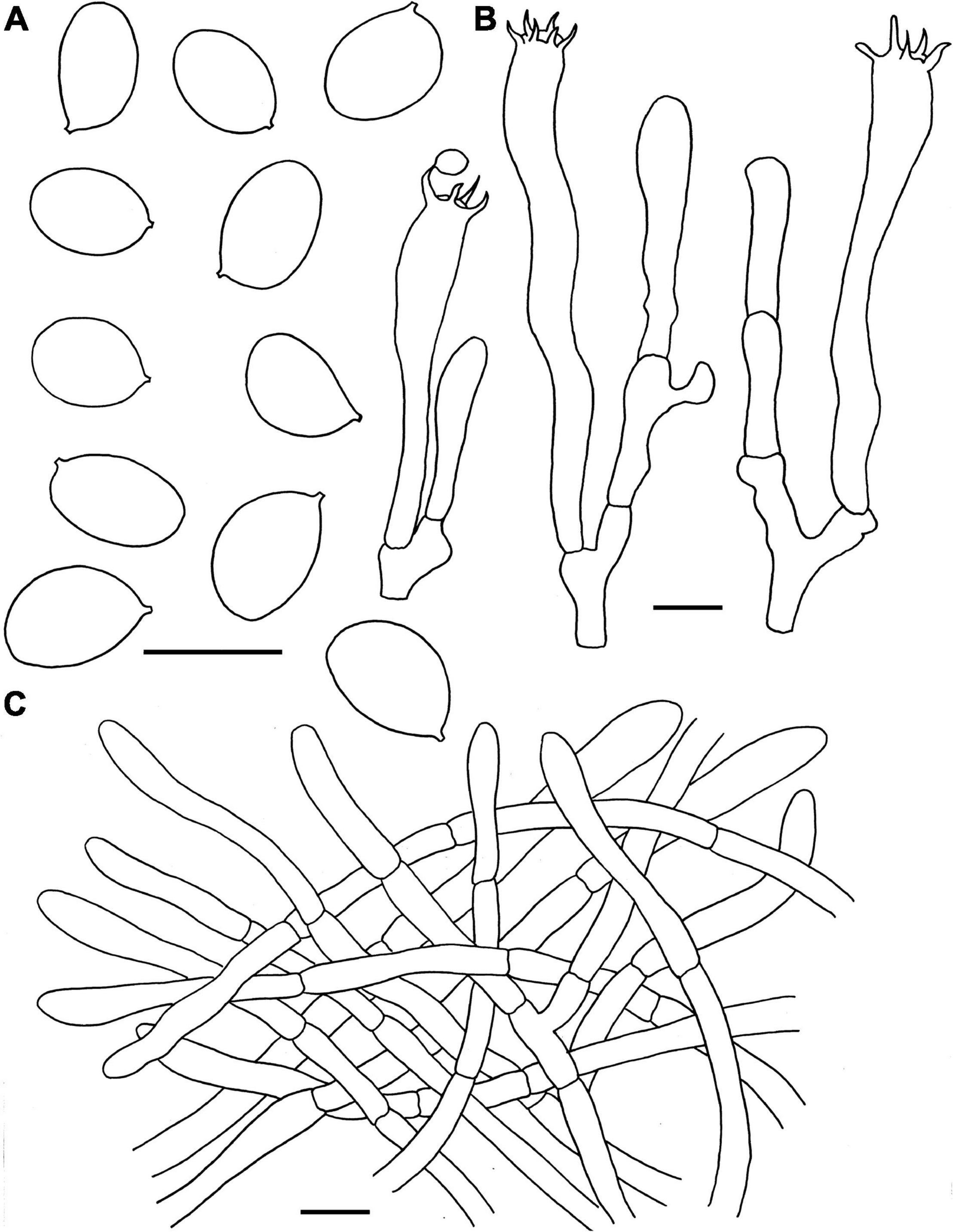
Figure 4. Microscopic features of Craterellus aureus (FHMU2407). (A) Basidiospores. (B) Basidia. (C) Pileipellis. Scale bars = 10 μm. Drawings by Y.-Z. Zhang.
Basidiomata medium-sized. Pileus 1.5–5 cm diam, infundibuliform, broadly infundibuliform with age; surface dry, vivid yellow (1A5) to orange (3A7); margin straight when young, wavy or lobed at maturity. Hymenophore nearly smooth, dirty white (1B2), yellow (4A7) to pale orange (1A2); context 0.1–0.15 cm in thickness, whitish (3A1) to pale yellow (3A2). Stipe 1.2–2.7 × 0.35–0.45 cm, central, hollow, usually curved, without any obvious demarcation between pileus and stipe; surface dry, yellowish-white (3A2), yellow (4A4) to pale orange (1A2). Basal mycelium white. Odor mild. Spore print not obtained.
Basidiospores [60/9/5] (7–)7.5–8.21–9(–9.5) × 5.5–5.97–6.5(–7) μm, Q = (1.17–)1.23–1.55(–1.64), Qm = 1.38 ± 0.1, ellipsoid to broadly ellipsoid, smooth, slightly thick-walled (up to 0.5 μm), hyaline or yellowish in KOH. Basidia 50–83 × 6.5–8.5 μm, cylindro-clavate, with irregular flexuous, slightly thick-walled (up to 0.5 μm), 4–6-spored, pale yellowish in KOH; sterigmata 5–6 μm in length. Cystidia absent. Pileipellis intricate trichoderm composed of cylindrical, 4–9 μm wide, slightly thick-walled (0.5–0.7 μm) hyphae, faintly pale yellow in KOH; terminal cells 27–59 × 4–8 μm, subcylindrical to subclavate with obtuse apex. Clamp connections absent in all tissues.
Habitat: Gregarious, caespitose, or rarely solitary on the ground of forests dominated by Castanea spp. and Quercus spp. (Zhong et al., 2018).
Known distribution: Eastern China (Jiangxi Province), and southern China (Guangdong and Hainan Provinces, Hong Kong) (Berkeley and Curtis, 1860).
Specimens examined: CHINA. Hainan Province: Jianfengling of Hainan Tropical Rainforest National Park, elev. 850 m, 4 July 2012, N.K. Zeng1057 (FHMU2407); Limushan of Hainan Tropical Rainforest National Park, elev. 750 m, 27 July 2017, N.K. Zeng3139, 3141 (FHMU2100, 2102). Jiangxi Province: Ganzhou City, Shangyou Town, Youshixiangmeiling Village, elev. 180 m, 9 June 2016, M.S. Su145 (FHMU6549); Nanchang City, Wanli District, Zhaoxian Town, Dongyuan Village, elev. 180 m, 24 June 2018, M.S. Su196 (FHMU6550).
Notes: Our recent collections and the holotype of C. luteus, a species originally described from Guangdong Province, southern China (Zhong et al., 2018), phylogenetically group together with high statistical support (Figure 2), which suggests that these new specimens belong to C. luteus. Morphologically, these newly collected materials easily remind us of C. aureus, a species first described in Hong Kong, southern China. When C. luteus was first described (Zhong et al., 2018), the species looked different from the original diagnosis of C. aureus (Berkeley and Curtis, 1860; Corner, 1966): the bright yellow cap, large size, and robust aspect of the basidiomata and the white hymenophore made it impossible to associate C. luteus with Berkeley and Curtis’ original description. Our new collections, which share near-identical (BS = 83%, PP = 1.0) sequences with the holotype of C. luteus, indicate that this species might be more variable in overall aspect and color, thereby, significantly reducing the morphological differences with the orange C. aureus. Our collections also have a near-identical basidiospore size compared with those reported for C. aureus, whereas basidiospores of C. luteus are longer [(8.5–)9–11(–12.5) μm]. The fact that both species were described from southern China, sharing the same climate and vegetation, suggests C. luteus is a synonym of C. aureus, but it does not exclude the presence of a larger species complex in southern China within this clade.
The phylogenetic analyses also showed that C. aureus is closely related to C. odoratus (Schwein.) Fr. (Figure 2), a species originally described in North America (Petersen, 1979b; Knopf, 1981). However, C. odoratus has a more fragile basidioma, narrower basidiospores measuring 8.9–11.8 × 4.4–6.3 μm, and a strong pleasant odor (Petersen, 1979b; Knopf, 1981).
Craterellus fulviceps N.K. Zeng, Y.Z. Zhang, P. Zhang & Zhi Q. Liang, sp. nov. Figures 3F, 5 MycoBank: MB841969.
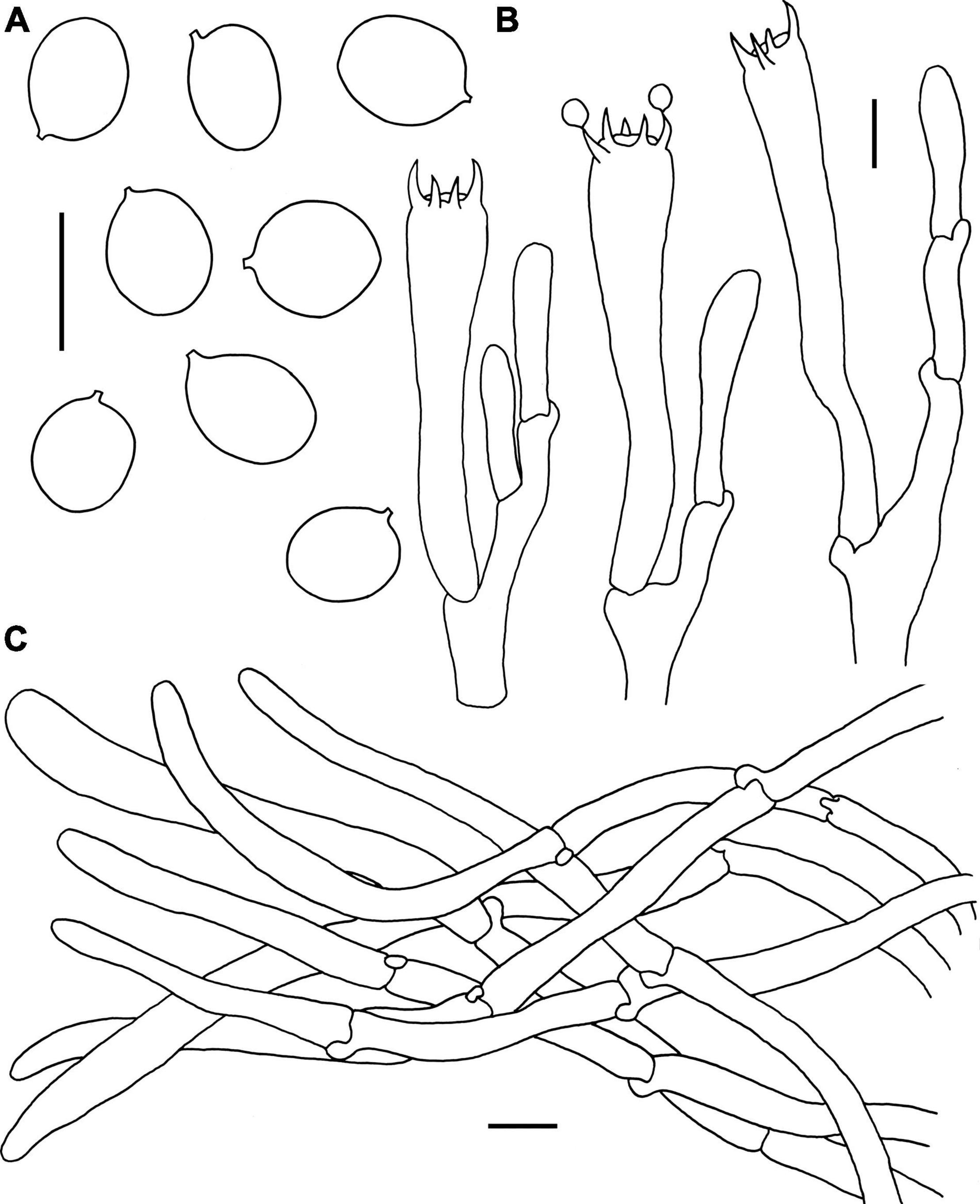
Figure 5. Microscopic features of Craterellus fulviceps (FHMU6553, holotype). (A) Basidiospores. (B) Basidia. (C) Pileipellis. Scale bars = 10 μm. Drawings by Y.-Z. Zhang.
Diagnosis: This species is distinguished from others in Craterellus by its very small-sized basidioma, a fulvous pileus, a veined hymenophore, an egg-yolk yellow stipe, and a presence of clamp connections in all parts of the basidioma.
Etymology: Latin “fulvi-,” meaning fulvous, and “ceps,” meaning pileus, refer to the fulvous pileus of our new species.
Holotype: CHINA. Hunan Province: Rucheng County, Jiulongjiang Nature Reserve, elev. 600 m, 2 October 2020, P. Zhang MHHNU10567 (FHMU6553). GenBank accession number: 28S = OL439678, ITS = OL439548.
Basidiomata very small-sized. Pileus 1–3 cm diam, convex to applanate, center slightly depressed; surface nearly smooth, fulvous (2A3); margin decurved; context very thin. Hymenophore veined, decurrent; folds about 0.1 cm broad, distant, relatively spaced, yellowish (1A2). Stipe 2–4 × 0.3–0.8 cm, central, slightly concave and curved in the middle; surface dry, egg-yolk yellow (2A4). Basal mycelium white. Odor not distinctive. Spore print not obtained.
Basidiospores [40/2/1] 8–9–10 × 6.5–7.6–8.5 μm, Q = 1.06–1.36(–1.38), Qm = 1.19 ± 0.09, ellipsoid, rarely subglobose, smooth, slightly thick-walled (up to 0.5 μm), yellowish in KOH. Basidia 58–82 × 9–15.5 μm, long, narrow, subcylindrical, slightly thick-walled (up to 0.5 μm), 2–5-spored, yellowish in KOH; sterigmata 3–7 μm in length. Cystidia absent. Pileipellis a cutis composed of mostly cylindrical, 4–10.5 μm wide, slightly thick-walled (0.5–0.7 μm) hyphae, faintly pale yellow in KOH; terminal cells 45–75 × 5–10 μm, subcylindrical to subclavate with obtuse apex. Clamp connections abundant in all parts of the basidioma.
Habitat: Solitary, scattered, or gregarious on the ground of forests dominated by fagaceous trees.
Known distribution: Central China (Hunan Province).
Notes: The collection from central China phylogenetically clustered with one specimen (1D3) identified as C. tubaeformis from Japan with strong statistical support (Lineage 11 of Figure 2). Our molecular phylogenetic data also show that specimens identified as C. tubaeformis were present in several different parts of the tree (Figure 2). Although the true position of C. tubaeformis in the molecular tree should be defined in the future, now we are sure that the Chinese collection in Lineage 11 (Figure 2) is not true C. tubaeformis, for the European species has a fuscous or fuacous umber pileus, larger basidiospores measuring 8–11 × 5.5–8 μm, and narrower basidia 60–90 × 8–11 μm (Corner, 1966), which is morphologically different from the Chinese specimen. And thus, the Chinese collection was proposed as a new species.
Craterellus lutescens (Fr.) Fr., Epic. Syst. Mycol. (Upsaliae): 532, 1838 Figures 6A–D, 7.

Figure 6. Basidiomata of Craterellus species. (A–D) C. lutescens (A) FHMU6547; (B) FHMU6548; (C,D) FHMU6544; (E,F) C. minor (FHMU6554, holotype); (G–I) C. parvopullus (G) FHMU6557; (H) FHMU6555, holotype; (I) FHMU6556. Photos: (A,B) L.-P. Tang; (C,D) W.-H. Zhang; (E,F) P. Zhang; (G–I) N.-K. Zeng.
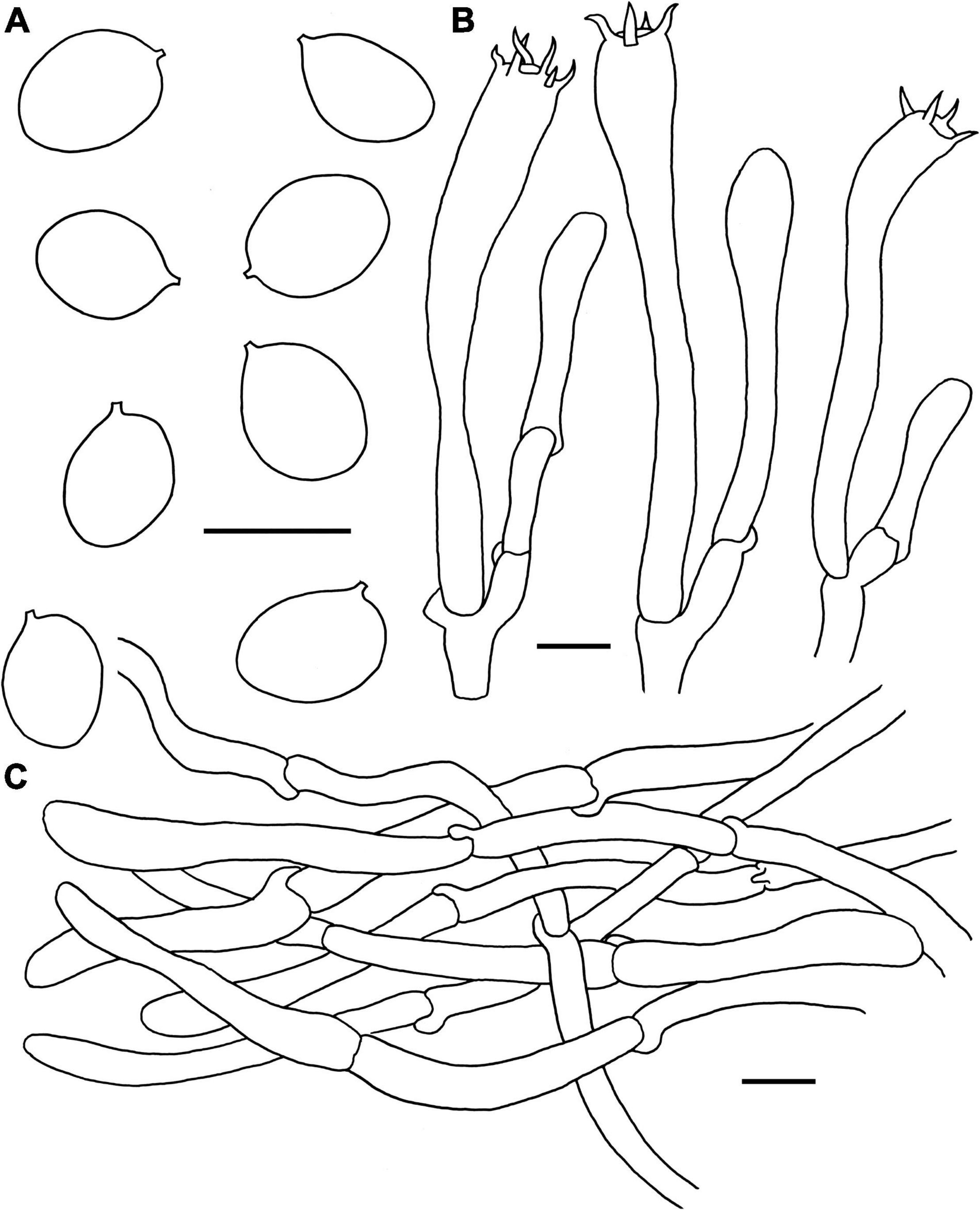
Figure 7. Microscopic features of Craterellus lutescens (FHMU6544). (A) Basidiospores. (B) Basidia. (C) Pileipellis. Scale bars = 10 μm. Drawings by Y.-Z. Zhang.
Basidiomata very small-sized. Pileus about 3 cm diam, nearly convex to applanate, center slightly depressed; margin inrolled; surface nearly smooth, brown (6D5); context about 0.2 cm in thickness, yellowish (2A3). Hymenophore veined, sometimes smooth, decurrent; folds very thin, light orange-yellow (4A4) to orange-yellow (4A6). Stipe 4–6 × 0.5–0.8 cm, central, cylindrical, hollow; surface dry, sunflower yellow (3A8) to dark yellow (4B8); context yellowish-white (4A2). Odor pleasant, milky. Spore print not obtained.
Basidiospores [240/12/5] (8–)8.5–9.7–11(–11.5) × (6.5–)7–7.8–9(–9.5) μm, Q = 1.13–1.36(–1.46), Qm = 1.23 ± 0.16, ellipsoid, smooth, slightly thick-walled (up to 0.5 μm), pale yellowish in KOH. Basidia 61–84 × 7.5–10 μm, long, narrow, subcylindrical, thin to slightly thick-walled (up to 0.5 μm), 4–6-spored, yellowish in KOH; sterigmata 5.5–7 μm in length. Cystidia absent. Pileipellis a cutis composed of 5.5–10.5 μm wide, slightly thick-walled (0.5–0.7 μm) hyphae, yellowish in KOH; terminal cells 30–58 × 4–8.5 μm, subcylindrical to subclavate with obtuse apex. Clamp connections abundant in all parts of the basidioma.
Habitat: Solitary, scattered, or gregarious on the ground of forests dominated by Pinus yunnanensis Franch. and Quercus L.
Known distribution: Southwestern China (Yunnan Province); Europe (Dahlman et al., 2000).
Specimens examined: CHINA. Yunnan Province: Jianchuan County, Shibaoshan Nature Reserve, near the grotto parking lot, elev. 2,499 m, 16 August 2014, L.P. Tang1647 (FHMU6547); same location, elev. 2,542 m, 19 August 2014, L.P. Tang1705 (FHMU6548); Lijiang City, bought from a market, 19 August 2020, W.H. Zhang441-1, 441-2, 441-3 (FHMU6544, FHMU6546, and FHMU6545).
Notes: Our collections and three Swedish specimens (UPSF-11789, UPSF-11790, and SS575) of C. lutescens phylogenetically group together with strong statistical support (Figure 2). Morphologically, the Chinese specimens match well with those of C. lutescens provided by Petersen (1969). Therefore, the specimen from China is recognized as C. lutescens.
Craterellus minor N.K. Zeng, Y.Z. Zhang, P. Zhang & Zhi Q. Liang, sp. nov. Figures 6E,F, 8 MycoBank: MB841974.
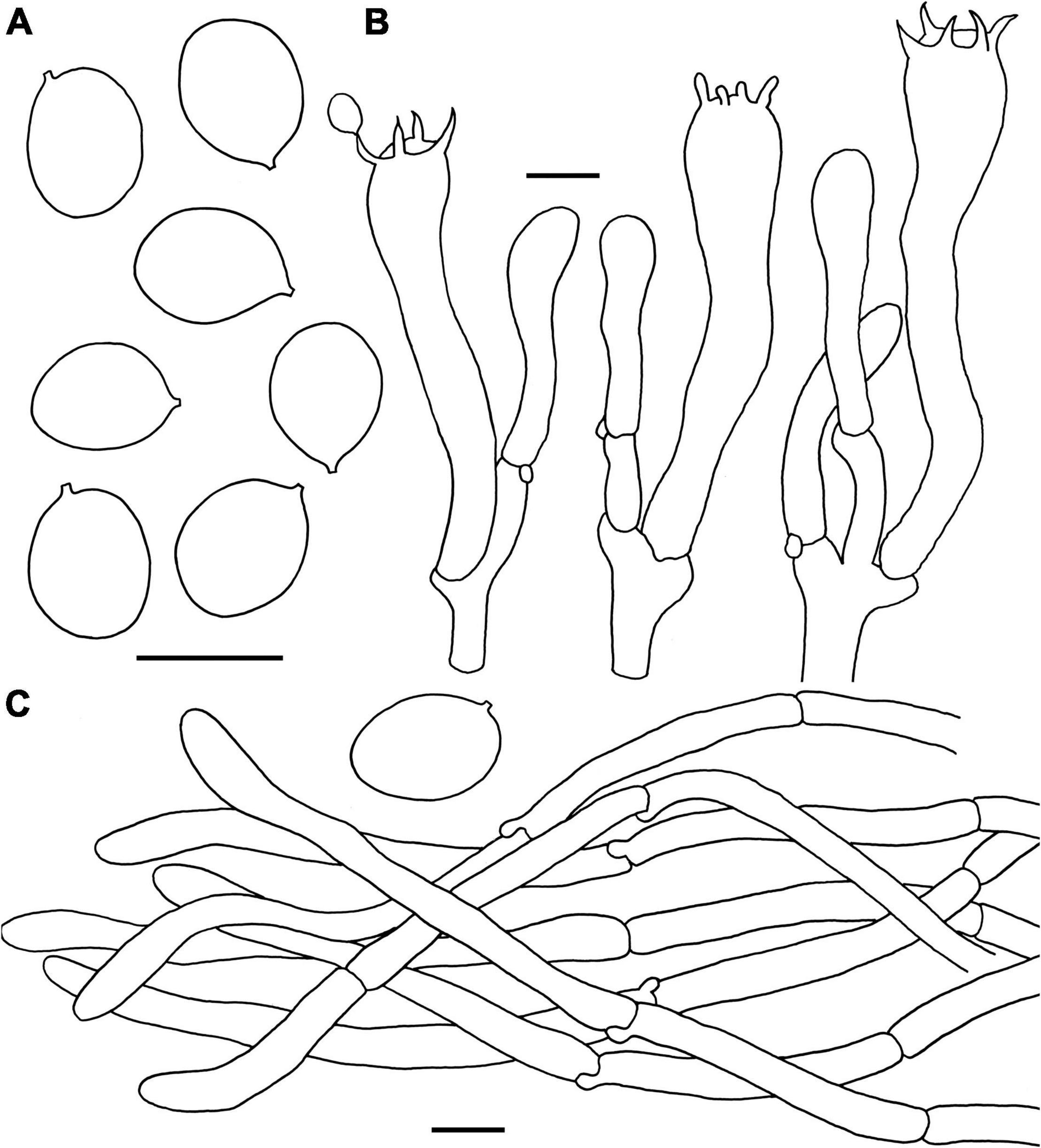
Figure 8. Microscopic features of Craterellus minor (FHMU6554, holotype). (A) Basidiospores. (B) Basidia. (C) Pileipellis. Scale bars = 10 μm. Drawings by Y.-Z. Zhang.
Diagnosis: This species is distinguished from others in Craterellus by its very small-sized basidioma, a grayish yellow pileus without dark pigments, a veined hymenophore, a lemon-yellow stipe, and the presence of clamp connections in all parts of the basidioma.
Etymology: Latin “minor”, refers to very small-sized basidioma of the new species.
Holotype: CHINA. Hunan Province: Sangzhi County, Badagong Mountain, Tianping Mountain, elev. 750 m, 15 September 2020, P. Zhang MHHNU32505 (FHMU6554). GenBank accession number: 28S = OL439684, ITS = OL439553.
Basidiomata very small-sized. Pileus about 1.7 cm in diam, center strongly depressed; margin inrolled, with irregular small crenulate; surface dry, grayish-yellow (1B2); context very thin, white or whitish (2A1). Hymenophore veined, decurrent; folds about 0.1 cm broad, forking gill-folds, white to pale (5A1). Stipe 2.6 × 0.3 cm, central, hollow, cylindrical, slightly concave and curved in the middle; surface dry, pale lemon yellow (1A4) with white base (3A1). Odor indistinct. Spore print not obtained.
Basidiospores [40/1/1] (8–)8.5–9.4–10.5 × 7–7.7–8.5 μm, Q = (1.07–)1.12–1.4, Qm = 1.23 ± 0.08, ellipsoid to broadly ellipsoid, smooth, inamyloid, slightly thick-walled (up to 0.5 μm), yellowish in KOH. Basidia 56–75 × 8–13 μm, long, narrow, subcylindrical, slightly thick-walled (up to 0.5 μm), 2–5-spored, yellowish in KOH; sterigmata 4.5–8 μm in length. Cystidia absent. Pileipellis a cutis composed of mostly cylindrical, 5–10 μm wide, slightly thick-walled (up to 0.5 μm) hyphae, faintly pale yellow in KOH; terminal cells 35–85 × 5–7 μm, subcylindrical to subclavate with obtuse apex. Clamp connections present in all parts of the basidioma.
Habitat: Solitary to scattered on the ground of forests dominated by fagaceous trees.
Known distribution: Central China (Hunan Province).
Notes: The new collection from central China phylogenetically clustered with one specimen labeled as C. melanoxeros (Desm.) Pérez-De-Greg (420526MF0891) also from China with strong statistical support (Figure 2). The Chinese species is morphologically related to European C. melanoxeros (SS576). However, C. melanoxeros has a large basidioma, a presence of dark pigments, and narrower basidiospores (Dahlman et al., 2000; Akata and Kumbasli, 2014).
Craterellus parvopullus N.K. Zeng, Y.Z. Zhang & Zhi Q. Liang, sp. nov. Figures 6G–I, 9 MycoBank: MB841977.
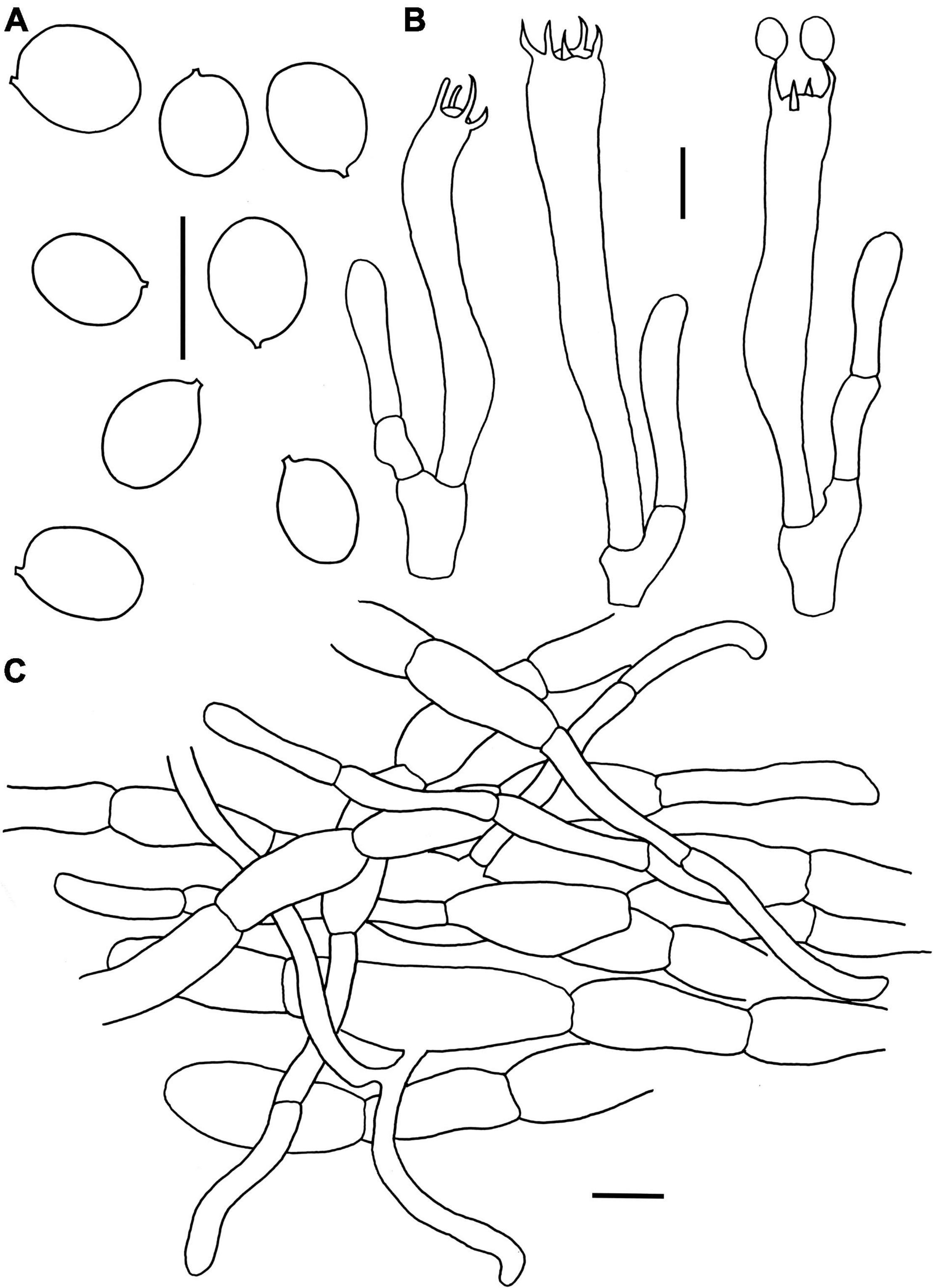
Figure 9. Microscopic features of Craterellus parvopullus (FHMU6555, holotype). (A) Basidiospores. (B) Basidia. (C) Pileipellis. Scale bars = 10 μm. Drawings by Y.Z. Zhang.
Diagnosis: This species is distinguished from others in Craterellus by its basidioma without any obvious demarcation between pileus and stipe, a blackish brown to blackish pileus, a smooth grayish hymenophore, subglobose to ellipsoid or broadly ellipsoid basidiospores, hyphae in pileipellis more or less inflated, but obviously slender in terminations, an absence of clamp connections in all parts of the basidioma, and it is associated with the trees of Dipterocarpaceae.
Etymology: Latin “parvo,” meaning small, and “pullus,” meaning blackish, refer to the small and blackish pileus of our new species.
Holotype: CHINA. Hainan Province: Wanning County, Bofangling, elev. 80 m, 29 August 2020, N.K. Zeng4913 (FHMU6555). GenBank accession number: 28S = OL439685, ITS = OM334829.
Basidiomata very small to small-sized. Pileus 1.8–4.6 cm diam, infundibuliform; margin slightly incurved, wavy, irregularly folded; surface dry, blackish brown (6F7) to black (5F1); context very thin, grayish (1E1). Hymenophore smooth to slightly folded, ashen gray (4B1). Stipe 1.2–2.6 × 0.15–0.4 cm, confluent with pileus, hollow; surface dry, ashen gray (4B1); context very thin, grayish (1E1). Odor not distinctive. Spore print not obtained.
Basidiospores [80/16/3] (6.5–)7–7.7–8.5(–9) × (5–)5.5–6.2–7(–7.5) μm, Q = (1.07–)1.14–1.42(–1.45), Qm = 1.25 ± 0.09, subglobose to ellipsoid or broadly ellipsoid, smooth, slightly thick-walled (up to 0.5 μm), yellowish in KOH. Basidia 53–73 × 7–10 μm, subcylindrical to subclavate, slightly thick-walled (up to 0.5 μm), 3–5-spored, hyaline or yellowish in KOH; sterigmata 4–6.5 μm in length. Cystidia absent. Pileipellis a cutis composed of mostly cylindrical, occasionally branched hyphae, hyphae 8–14 μm wide, but slender in terminations (3–6 μm wide), thin- to thick-walled (up to 1.5 μm), yellowish in KOH; terminal cells 21–46 × 3–9 μm, clavate or subcylindrical with obtuse apex. Clamp connections absent in all tissues.
Habitat: Gregarious on the ground in forests of Vatica mangachapoi Blanco.
Known distribution: Southern China (Hainan Province).
Additional specimens examined: CHINA. Hainan Province: Wanning County, Bofangling, elev. 80 m, 29 August 2020, N.K. Zeng4911, 4912 (FHMU6557, FHMU6556).
Notes: The Chinese C. atrobrunneolus T. Cao & H.S. Yuan, C. badiogriseus T. Cao & H.S. Yuan, C. croceialbus T. Cao & H.S. Yuan, C. macrosporus T. Cao & H.S. Yuan, and C. squamatus T. Cao & H.S. Yuan are morphologically similar to C. parvopullus. However, C. atrobrunneolus is distributed in subtropical areas (Cao et al., 2021a), while C. badiogriseus, C. croceialbus, C. macrosporus, and C. squamatus grow in temperate regions (Cao et al., 2021b); all of them are not associated with trees of Dipterocarpaceae (Cao et al., 2021a,b). Moreover, C. atrobrunneolus has smaller basidiospores measuring (6.2–)6.5–7.8(–8) × (4.2–)4.5–6(–6.2) μm (Cao et al., 2021a); C. badiogriseus has larger basidiospores measuring (7.5–)8–10.5(–11) × (6.5–)6.8–7.5(–8) μm, and a pileipellis composed of thick-walled hyphae without slender terminations (Cao et al., 2021b); C. croceialbus has a brown pileus with an orange-white margin, larger basidiospores measuring (9–)10–12(–12.5) × (6.5–)6.8–8(–8.2) μm, and a pileipellis composed of hyphae without slender terminations (Cao et al., 2021b); C. macrosporus has a brown pileus, larger basidiospores measuring (12.5–)12.8–14.5(–15) × (8.8–)9–11(–11.5) μm, and a pileipellis composed of thin-walled hyphae without slender terminations (Cao et al., 2021b); C. squamatus has a squamulose pileus, larger basidiospores measuring (11.5–)12–13.8(–14) × (8.2–)8.5–9.5(–10) μm, and a pileipellis composed of thick-walled hyphae without slender terminations (Cao et al., 2021b).
Besides the five species found in China, Malaysian C. cornucopioides var. mediosporus Corner and C. verrucosus Massee, European C. cornucopioides, North American C. atrocinereus D. Arora & J.L. Frank, C. calicornucopioides D. Arora & J.L. Frank and C. fallax A.H. Sm are also morphologically similar to C. parvopullus. However, C. verrucosus has a rugulose hymenophore, larger basidiospores measuring 8–10 × 6.5–8 μm, and wider hyphae (up to 20 μm) more or less vertically arranged in the pileipellis (Corner, 1966); C. cornucopioides var. mediosporus has larger basidiospores measuring 8–10 × 6.5–7.5 μm, and a pileipellis composed of uninflated hyphae (Corner, 1966); C. cornucopioides s.s. has larger basidiospores measuring (7–)11–15(–20) × (5–)7(–11) μm, and its distribution in temperate areas (Pilz et al., 2003); C. atrocinereus has larger basidiospores measuring 8–10 × 4.5–6 μm, a prominently folded, distinctly thick hymenium, and groups on the ground under hardwoods, especially Quercus and Neolithocarpus (Frank, 2015); C. calicornucopioides has larger basidiospores measuring 11–14 × 8–10 μm, a presence of abundant clamp connections, and is mainly distributed with Quercus, Arctostaphylos, Vaccinium and Arbutus (Frank, 2015); C. fallax has larger basidiospores measuring 10–13 × 7–9 μm, and is mainly distributed in a broad host range, including Pinaceae (Pinus and Tsuga) and Fagaceae (Quercus and Castanea) (Matheny et al., 2010). Phylogenetically, C. parvopullus is not closely related to C. atrobrunneolus, C. atrocinereus, C. calicornucopioides, C. cornucopioides, and C. fallax (Figure 2).
Key to Known Craterellus Species in China
1. Without any obvious demarcation between pileus and stipe……………………………………………………………………………….. 2
1. Obvious demarcation between pileus and stipe……………….8
2. Pileus vivid yellow to orange…………………………………C. aureus
2. Pileus brown, gray brown, dark brown to almost black……3
3. Pileal surface scabrous…………………………………….C. squamatus
3. Pileal surface subglabrous to glabrous……………………………..4
4. Pileal surface blackish brown, blackish to almost black……5
4. Pileal surface brown, gray-brown to dark brown, without black tinge………………………………………………………………………7
5. Hyphal width in pileipellis usually uneven, obviously slender in terminations, and distributed in tropical areas………………………………………………………………C. parvopullus
5. Hyphal width in pileipellis usually even, and distributed in subtropical or temperate areas………………………………………..6
6. Basidiospores larger [(7.5–)8–10.5(–11) × (6.5–)6.8–7.5(–8) μm]………………………………………………………….C. badiogriseus
6. Basidiospores smaller [(6.2–)6.5–7.8(–8) × (4.2–)4.5–6(–6.2) μm]…………………………………………………..C. atrobrunneolus
7. Pileal margin orange-white, basidiospores smaller [(9–)10–12(–12.5) × (6.5–)6.8–8(–8.2) μm]……………..C. croceialbus
7. Pileal margin dark brown, basidiospores larger [(12.5–)12.8–14.5(–15) × (8.8–)9–11.0(–11.5) μm].C. macrosporus
8. Basidomata very pale, whitish, hyphal clamp connections absent, grow on dead wood…………………………………..C. albidus
8. Basidiomata brown, yellow, hyphal clamp connections abundant, grow on ground……………………………………………..9
9. Pileus brown, hymenophore veined, sometimes smooth……………………………………………………………….C. lutescens
9. Pileus fulvous, grayish-yellow, hymenophore veined, never smoot……………………………………………………………………………10
10. Stipe egg-yolk yellow………………………………………….C. fulviceps
10. Stipe pale lemon yellow………………………………………….C. minor
Discussion
Craterellus cornucopioides and Craterellus tubaeformis Complexes
Craterellus cornucopioides, originally described in Europe, was previously considered a widely distributed species (Akata and Kumbasli, 2014). However, recent studies have indicated that C. cornucopioides represents a species complex rather than a single widespread species (Dahlman et al., 2000). Our molecular phylogenetic data also show that specimens identified as C. cornucopioides were present in several different parts of the tree (Figure 2). Interestingly, collections of C. cornucopioides from Europe were present in more than one part of the tree (Figure 2). The species concept of C. cornucopioides should be confirmed by obtaining collections and DNA sequences from the holotype locality. Craterellus cornucopioides s. str. likely occurs in fewer areas of Europe; one specimen identified as C. cornucopioides from Tibet, western China (Lineage 8 in Figure 2), might represent another species. Craterellus tubaeformis was also present in several parts of the tree (Figure 2), which indicates that C. tubaeformis represents a species complex rather than a single widespread species; the collections identified as C. tubaeformis in China from previous studies should be re-evaluated.
Species Diversity of Craterellus in China
High species diversity of Craterellus in China was revealed in this study, with fourteen species-level lineages identified (Figure 2). Three lineages (3, 11, and 12) were described as new species, viz. C. minor, C. parvopullus, and C. fulviceps. Eight lineages (1, 4–7, 9, 10, and 14) represent previously described species, viz. C. albidus, C. atrobrunneolus, C. aureus, C. badiogriseus, C. croceialbus, C. lutescens, C. macrosporus, and C. squamatus. Three lineages (2, 8, and 13) remain undescribed because of insufficient materials. Five additional species have been reported from China, viz. C. cornucopioides, C. cornucopioides var. parvisporus, C. luteus, C. odoratus, and C. tubaeformis. Craterellus luteus is a synonym of C. aureus, and the occurrence of C. cornucopioides, C. cornucopioides var. parvisporus, C. odoratus, and C. tubaeformis has not yet been confirmed in China.
Phylogenetic Relationships and Geographic Divergence of Craterellus
Our molecular phylogenetic data based on two-locus DNA sequences (28S + ITS) with a large number of collections from China have uncovered useful information regarding the phylogeny and geography of Craterellus. Our data indicate that the affinities of Craterellus species between China and Europe, North America, and Australia are evident (Figure 2); for example, C. lutescens (Lineage 14 in Figure 2) is found in China, Europe, and North America; C. badiogriseus (Lineage 9 in Figure 2) is associated with one specimen (LMAC6b-09) from Europe; C. aureus (Lineage 1 in Figure 2), and two Chinese specimens (FHMU6551 and FHMU6552) (Lineage 2 in Figure 2) of Craterellus are closely related to North American C. odoratus; C. parvopullus (Lineage 3 in Figure 2) is closely related to two specimens (GMB-2014 MEL:2382717 and GMB-2014 MEL:2383015) from Australia; C. macrosporus (Lineage 6 in Figure 2), C. squamatus (Lineage 5 in Figure 2), and two North American specimens (NC-8338 and FLAS-F-60401) labeled as C. sp. and C. fallax, respectively, are in the same clade; a Chinese specimen (ECM90) labeled as C. sp. (Lineage 13 in Figure 2) is closely related to one collection (M66A9) from Mexico. Moreover, C. fulviceps (lineage 11 in Figure 2) is found in China and Japan; C. parvopullus (lineage 3 in Figure 2) is associated with two specimens (LAM 0254 and AWW263) from Malaysia.
We also noted that there is little or no statistical support in some deeper nodes of the phylogeny, although the molecular data provided new insights into the phylogeny and geography of Craterellus with a large number of collections from China included. In the future, with more genes investigated and more Craterellus species discovered, a molecular phylogenetic tree of Craterellus should be constructed on the basis of the present data, which will provide more interesting information.
Disclosure
All the experiments undertaken in this study comply with the current laws of the People’s Republic of China.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: National Center for Biotechnology Information (NCBI) GenBank, https://www.ncbi.nlm.nih.gov/genbank/, OL439672–OL439687, OM334827–OM334829, OL439545–OL439553, OM469019–OM469020 and MycoBank, https://www.mycobank.org/, MB841969, MB841974, MB841977.
Author Contributions
Z-QL and N-KZ: conceptualization and writing—original draft preparation. Y-ZZ: methodology, performing the experiment, and formal analysis. N-KZ, PZ, L-PT, Z-HC, M-SS, Y-JH, H-YH, and W-HZ: resources. N-KZ, BB, Z-QL, PZ, H-YH, and W-HZ: writing—review and editing. N-KZ and Z-QL: supervision. N-KZ: project administration and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 32160001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor BD declared a past co-authorship with the author BB.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are very grateful to Professor Z. L. Yang, Kunming Institute of Botany, Chinese Academy of Sciences, and H. S. Yuan, Institute of Applied Ecology, Chinese Academy of Sciences, for providing valuable literature; G. Lu, L. X. Yuan, and L. Li, Haikou Duotan Wetlands Institute, the forest rangers of Liji Qingpilin Nature Reserve of Hainan and Hainan Tropical Rainforest National Park, for their help during the field investigations. We would like to thank TopEdit (www.topeditsci.com) for linguistic assistance during the preparation of this manuscript.
Footnotes
References
Akata, I., and Kumbasli, M. (2014). A new and rare record for turkish Cantharellus. Biodivers. Conserv. 7, 143–145.
An, D. Y., Liang, Z. Q., Jiang, S., Su, M. S., and Zeng, N. K. (2017). Cantharellus hainanensis, a new species with a smooth hymenophore from tropical China. Mycoscience 58, 438–444. doi: 10.1016/j.myc.2017.06.004
Arraiano-Castilho, R., Bidartondo, M. I., Niskanen, T., Clarkson, J. J., Brunner, I., Zimmermann, S., et al. (2020). Habitat specialization controls ectomycorrhizal fungi above the treeline in the European Alps. New Phytol. 229, 2901–2916. doi: 10.1111/nph.17033
Bas, C. (1969). Morphology and subdivision of Amanita and a monograph of its section Lepidella. Persoonia 5, 285–579.
Beluhan, S., and Ranogajec, A. (2011). Chemical composition and non-volatile components of Croatian wild edible mushrooms. Food Chem. 124, 1076–1082. doi: 10.1016/j.foodchem.2010.07.081
Berkeley, M. J., and Curtis, M. A. (1860). Characters of new fungi, collected in the north pacific exploring expedition by Charles Wright. Proc. Am. Acad. Arts. Sci. 4, 111–130.
Bijeesh, C., Kumar, A. M., Vrinda, K. B., and Pradeep, C. K. (2018). Two new species of Craterellus (Cantharellaceae) from tropical India. Phytotaxa 346, 157–168. doi: 10.11646/phytotaxa.372.1.5
Buyck, B., Kauff, F., Eyssartier, G., Couloux, A., and Hofstetter, V. (2014). A multilocus phylogeny for worldwide Cantharellus (Cantharellales, Agaricomycetidae). Fungal Divers. 64, 101–121. doi: 10.1007/s13225-013-0272-3
Cao, T., Yu, J. R., Hu, Y. P., and Yuan, H. S. (2021a). Craterellus atrobrunneolus sp. nov. from southwestern China. Mycotaxon 136, 59–71. doi: 10.5248/136.59
Cao, T., Hu, Y. P., Yu, J. R., Wei, T. Z., and Yuan, H. S. (2021b). A phylogenetic overview of the Hydnaceae (Cantharellales, Basidiomycota) with new taxa from China. Stud. Mycol. 99, 100–121. doi: 10.1016/j.simyco.2021.100121
Carriconde, F., Gardes, M., Bellanger, J. M., Letellier, K., Gigante, S., Gourmelon, V., et al. (2019). Host effects in high ectomycorrhizal diversity tropical rainforests on ultramafic soils in New Caledonia. Fungal Ecol. 39, 201–212. doi: 10.1016/j.funeco.2019.02.006
Castresana, J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17, 540–552. doi: 10.1093/oxfordjournals.molbev.a026334
Dahlman, M., Danell, E., and Spatafora, J. W. (2000). Molecular systematics of Craterellus: cladistic analysis of nuclear LSU rDNA sequence data. Mycol. Res. 104, 388–394. doi: 10.1017/S0953756299001380
Das, K., Ghosh, A., Chakraborty, D., Li, J., Qiu, L., Baghela, A., et al. (2017). Fungal biodiversity profiles 31–40. Mycologia 38, 353–406. doi: 10.7872/crym/v38.iss3.2017.353
Disyatat, N. R., Yomyart, S., Sihanonth, P., and Piapukiew, J. (2016). Community structure and dynamics of ectomycorrhizal fungi in a dipterocarp forest fragment and plantation in Thailand. Plant Ecol. Divers. 9, 577–588. doi: 10.1080/17550874.2016.1264018
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Edwards, I. P., Cripliver, J. L., Gillespie, A. R., Johnsen, K. H., Scholler, M., and Turco, R. F. (2004). Nitrogen availability alters macrofungal basidiomycete community structure in optimally fertilized loblolly pine forests. New Phytol. 162, 755–770. doi: 10.1111/j.1469-8137.2004.01074.x
Fan, X. D., Chen, Y. H., Chang, J., and Chen, J. (2014). Structure and antitumor activity of water-soluble polysaccharide from Craterellus cornucopioides. Mod. Food Sci. Technol. 30, 50–54+79. doi: 10.1016/j.carbpol.2018.02.077
Federico, D. R., Manuel, A., and Francesco, T. (2020). The history of conifers in central Italy supports long-term persistence and adaptation of mesophilous conifer fungi in arbutus-dominated shrublands. Rev. Palaeobot. Palynol. 282, 104300–104314. doi: 10.1016/j.revpalbo.2020.104300
Feibelman, T. P., Doudrick, R. L., Cibula, W. G., and Bennett, J. W. (1997). Phylogenetic relationships within the Cantharellaceae inferred from sequence analysis of the nuclear large subunit rDNA. Mycol. Res. 101, 1423–1430. doi: 10.1017/S0953756297004115
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analyses program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98.
Harrington, T. J., and Mitchell, D. T. (2002). Characterization of Dryas octopetala ectomycorrhizas from limestone karst vegetation, western Ireland. Can. J. Bot. 80, 970–982. doi: 10.1139/b02-082
Hembrom, M. E., Das, K., Adhikari, S., Parihar, A., and Buyck, B. (2017). First report of Pterygellus from Rajmahal hills of Jharkhand (India) and its relation to Craterellus (Hydnaceae, Cantharellales). Phytotaxa 306, 201–210. doi: 10.11646/phytotaxa.306.3.2
Hibbett, D. S., Bauer, R., Binder, M., Giachini, A. J., Hosaka, K., Justo, A., et al. (2014). “Agaricomycetes,” in The Mycota vol.VII, Systematics and Evolution part A, 2nd Edn, eds D. J. McLaughlin and J. W. Spatafora (Berlin, DE: SpringerVerlag), 373–429. doi: 10.1007/978-3-642-55318-9_14
Huang, Y., Zhang, S. B., Chen, H. P., Zhao, Z. Z., Li, Z. H., Feng, T., et al. (2016). New acetylenic acids and derivatives from the Basidiomycete Craterellus lutescens (Cantharellaceae). Fitoterapia 115, 177–181. doi: 10.1016/j.fitote.2016.10.006
Huang, Y., Zhang, S. B., Chen, H. P., Zhao, Z. Z., Zhou, Z. Y., Li, Z. H., et al. (2017). New acetylenic acids and derivatives from the edible mushroom Craterellus lutescens (Cantharellaceae). J. Agric. Food Chem. 65, 3835–3841. doi: 10.1021/acs.jafc.7b00899
Huelsenbeck, J. P., and Ronquist, F. (2005). “Bayesian analysis of molecular evolution using MrBayes,” in Statistical Methods in Molecular Evolution, ed. R. Nielsen (New York, NY: Springer), 183–226. doi: 10.1007/0-387-27733-1_7
James, T. Y., Kauff, F., Schoch, C., Matheny, P. B., Hofstetter, V., Cox, C., et al. (2006). Reconstructing the early evolution of the fungi using a six gene phylogeny. Nature 443, 818–822. doi: 10.1038/nature05110
Knopf, A. A. (1981). The Audubon Society field guide to North American Mushrooms. New York, NY: Lincoff.
Ko, P. Y., Lee, S. H., Kim, T. H., Hong, K. S., Choe, S. Y., and Jeun, Y. C. (2020). Distribution of spontaneously growing mushrooms in the Wolchulsan National Park. J. Mushrooms 18, 201–207. doi: 10.14480/JM.2020.18.3.201
Kornerup, A., and Wanscher, J. H. (1981). Taschenlexikon der Farben 3. Northeim, DE: Muster-Schmidt Verlagsgesellschaft.
Kotowski, M. A., Pietras, M., and Łuczaj, Ł (2019). Extreme levels of mycophilia documented in Mazovia, a region of Poland. J. Ethnobiol. Ethnomed. 15, 12–31. doi: 10.1186/s13002-019-0291-6
Kumari, D., Upadhyay, R. C., and Reddy, M. S. (2012). Craterellus indicus sp. nov., a new species associated with Cedrus deodara from the western Himalayas, India. Mycol. Prog. 11, 769–774. doi: 10.1007/s11557-011-0788-4
Li, T. H., Chen, Y. Q., Qu, L. H., Lu, Y. J., and Song, B. (1999). Partial 25S rDNA sequence of Cantharellus and ITS phylogenetic implications. Mycosystema 18, 12–19.
Liu, Y. T., Sun, J., Luo, Z. Y., Rao, S. Q., Su, Y. J., Xu, R. R., et al. (2012). Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food Chem. Toxicol. 50, 1238–1244. doi: 10.1016/j.fct.2012.01.023
Matheny, P. B., Austin, E. A., Birkebak, J. M., and Wolfenbarger, A. D. (2010). Craterellus fallax, a black trumpet mushroom from eastern North America with a broad host range. Mycorrhiza 20, 569–575. doi: 10.1007/s00572-010-0326-2
Matheny, P. B., Wang, Z., Binder, M., Curtis, J. M., Lim, Y. W., Nilsson, R. H., et al. (2007). Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Mol. Phylogenet. Evol. 43, 430–51. doi: 10.1016/j.ympev.2006.08.024
Mešić, A., Šamec, D., Jadan, M., Bahun, V., and Tkalčec, Z. (2020). Integrated morphological with molecular identification and bioactive compounds of 23 Croatian wild mushrooms samples. Food Biosci. 37, 100720–100730. doi: 10.1016/j.fbio.2020.100720
Miller, M. A., Pfeiffer, W., and Schwartz, T. (2011). “The CIPRES science gateway: a community resource for phylogenetic analyses,” in Proceedings of the 2011 TeraGrid Conference: extreme Digital Discovery, (New York, NY: ACM), 41.
Miyamoto, Y., Nakano, T., Hattori, M., and Nara, K. (2014). The mid-domain effect in ectomycorrhizal fungi: range overlap along an elevation gradient on Mount Fuji. Japan. ISME J. 8, 1739–1746. doi: 10.1038/ismej.2014.34
Miyamoto, Y., Narimatsu, M., and Nara, K. (2018). Effects of climate, distance, and a geographic barrier on ectomycorrhizal fungal communities in Japan: a comparison across Blakiston’s Line. Fungal Ecol. 33, 125–133. doi: 10.1016/j.funeco.2018.01.007
Morris, M. H., Perez-Perez, M. A., Smith, M. E., and Bledsoe, C. S. (2008). Multiple species of ectomycorrhizal fungi are frequently detected on individual oak root tips in a tropical cloud forest. Mycorrhiza 18, 375–383. doi: 10.1007/s00572-008-0186-1
Naseer, A., and Khalid, A. N. (2018). A new record of genus Craterellus, edible basidiomycotous fungus from Pakistan. Saudi J. Med. Pharm. Sci. 4, 656–659. doi: 10.21276/sjmps.2018.4.6.2
Nylander, J. A. A. (2004). MrModeltest 2.3. Program Distributed by the Author. Uppsala: Uppsala University.
O’Callaghan, Y. C., O’Brien, N. M., Kenny, O., Harrington, T., Brunton, N., and Smyth, T. J. (2014). Anti-inflammatory effects of wild Irish mushroom extracts in RAW264.7 mouse macrophage cells. J. Med. Food 18, 202–7. doi: 10.1089/jmf.2014.0012
Petersen, R. H. (1969). Notes on cantharelloid fungi—II. Some new taxa, and notes on Pseudocraterellus. Persoonia 5, 211–223.
Petersen, R. H. (1979a). Notes on cantharelloid fungi. IX. Illustrations of new or poorly understood taxa. Nova Hedwigia 31, 1–23.
Petersen, R. H. (1979b). Notes on cantharelloid fungi. X. Cantharellus confluens and C. lateritius, Craterellus odoratus and C. aureus. Sydowia 32, 198–208.
Pilz, D., Norvell, L., Danell, E., and Molina, R. (2003). Ecology and management of commercially harvested chanterelle mushrooms. Washington, DC: US Department of Agriculture, Forest Service.
Porter, T. M., Skillman, J. E., and Moncalvo, J. M. (2008). Fruiting body and soil rDNA sampling detects complementary assemblage of Agaricomycotina (Basidiomycota, Fungi) in a hemlock-dominated forest plot in southern Ontario. Mol. Ecol. 17, 3037–3050. doi: 10.1111/j.1365-294X.2008.03813.x
Raja, H. A., Baker, T. R., Little, J. G., and Oberlies, N. H. (2017). DNA barcoding for identification of consumer-relevant mushrooms: a partial solution for product certification? Food Chem. 214, 383–392. doi: 10.1016/j.foodchem.2016.07.052
Shao, S. C., Buyck, B., Hofstetter, V., Tian, Y. H., Yu, F. Q., and Liu, P. G. (2014). Cantharellus hygrophorus, a new species in subgenus Afrocantharellus from tropical southwestern China. Cryptogamie Mycol. 35, 283–291. doi: 10.7872/crym.v35.iss3.2014.283
Smith, S. A., and Dunn, C. W. (2008). Phyutility: a phyloinformatics tool for trees, alignments andmolecular data. Bioinformatics 24, 715–716. doi: 10.1093/bioinformatics/btm619
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihoodbased phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. doi: 10.1093/bioinformatics/btl446
Tibuhwa, D. D., Savić, S., Tibell, L., and Kivaisi, A. K. (2012). Afrocantharellus gen. stat. nov. is part of a rich diversity of African Cantharellaceae. IMA Fungus 3, 25–38. doi: 10.5598/imafungus.2012.03.01.04
Urban, A., Puschenreiter, M., Strauss, J., and Gorfer, M. (2008). Diversity and structure of ectomycorrhizal and co-associated fungal communities in a serpentine soil. Mycorrhiza 18, 339–354. doi: 10.1007/s00572-008-0189-y
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Wang, X. H., Liu, P. G., and Yu, F. Q. (2004). Color atlas of wild commercial mushrooms in Yunnan. Kunming: Yunnan Science and Technology Press.
White, T. J., Bruns, T., Lee, S., and Taylor, J. W. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenies,” in PCR Protocols: A Guide to Methods and Applications, eds M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (New York, NY: Academic Press), 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wilson, A. W., Aime, M. C., Dierks, J., Mueller, G. M., and Henkel, T. W. (2012). Cantharellaceae of Guyana I: new species, combinations and distribution records of Craterellus and a synopsis of known taxa. Mycologia 104, 1466–1477. doi: 10.3852/11-412
Xiao, Z. D., Song, B., Li, T. H., Deng, C. Y., and Huang, H. (2012). Resource of Cantharellaceae from Chebaling Nature Reserve, Guangdong Province. Edible Fungi China 31, 12–13.
Zhang, J., Wu, D. I., Deng, C. Y., Zhang, M., Dauner, L., and Wijayawardene, N. N. (2020). A new species of Craterellus (Cantharellales, Hydnaceae) from Guizhou Province, China. Phytotaxa 472, 259–268. doi: 10.11646/phytotaxa.472.3.4
Zhang, L., Shen, Y., Wang, F., Leng, Y., and Liu, J. K. (2010). Rare merosesquiterpenoids from basidiomycete Craterellus odoratus and their inhibition of 11β-hydroxysteroid dehydrogenases. Phytochemistry 71, 100–103. doi: 10.1016/j.phytochem.2009.09.020
Zhang, Y., Mo, M. Z., Yang, L., Mi, F., Cao, Y., Liu, C. L., et al. (2021). Exploring the species diversity of edible mushrooms in Yunnan, southwestern China, by DNA barcoding. J. Fungi 7, 310–333. doi: 10.3390/jof7040310
Zhong, X. R., Li, T. H., Jiang, Z. D., Deng, W. Q., and Huang, H. (2018). A new yellow species of Craterellus (Cantharellales, Hydnaceae) from China. Phytotaxa 360, 35–44. doi: 10.11646/phytotaxa.360.1.3
Keywords: East Asia, molecular phylogeny, morphology, new taxa, taxonomy
Citation: Zhang Y-Z, Zhang P, Buyck B, Tang L-P, Liang Z-Q, Su M-S, Hao Y-J, Huang H-Y, Zhang W-H, Chen Z-H and Zeng N-K (2022) A Contribution to Knowledge of Craterellus (Hydnaceae, Cantharellales) in China: Three New Taxa and Amended Descriptions of Two Previous Species. Front. Microbiol. 13:906296. doi: 10.3389/fmicb.2022.906296
Received: 28 March 2022; Accepted: 20 June 2022;
Published: 13 July 2022.
Edited by:
Bálint Dima, Eötvös Loránd University, HungaryReviewed by:
Samantha Chandranath Karunarathna, Qujing Normal University, ChinaSaowaluck Tibpromma, Qujing Normal University, China
Copyright © 2022 Zhang, Zhang, Buyck, Tang, Liang, Su, Hao, Huang, Zhang, Chen and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Qun Liang, bGl6aHF1MTk4MEAxMjYuY29t; Nian-Kai Zeng, bmlhbmthaXpAMTYzLmNvbQ==
 Yu-Zhuo Zhang
Yu-Zhuo Zhang Ping Zhang3
Ping Zhang3 Nian-Kai Zeng
Nian-Kai Zeng