- 1Key Laboratory of Tropical Translational Medicine of Ministry of Education, School of Pharmacy, Hainan Medical University, Haikou, China
- 2College of Science, Hainan University, Haikou, China
- 3Institute of Edible and Medicinal Fungi, College of Life Sciences, Zhejiang University, Hangzhou, China
- 4UMR 7205, Institut Systématique, Evolution, Biodiversité, Muséum National d’Histoire Naturelle, Sorbonne Université, CNRS, Paris, France
- 5Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases of Ministry of Education, Gannan Medical University, Ganzhou, China
- 6College of Life Science, Hunan Normal University, Changsha, China
- 7School of Pharmaceutical Sciences and Yunnan Key Laboratory of Pharmacology for Natural Products, Kunming Medical University, Kunming, China
- 8Yinggeling Substation, Hainan Tropical Rainforest National Park, Baisha, China
Species of Cantharellus subgenus Cantharellus are interesting and important for their mycorrhizal properties, medicinal values, and edibility. In China, there are many undescribed species of the subgenus. In this study, four new species of subg. Cantharellus, viz. Cantharellus albopileatus, Cantharellus chuiweifanii, Cantharellus pinetorus, and Cantharellus ravus from Hainan and Hunan Provinces, respectively, were described based on morphological and phylogenetic evidence as a contribution to the knowledge of the species diversity in China. Detailed descriptions, color photographs of fresh basidiomata, and line drawings of microstructures of these four new species are presented as well as comparisons with related species.
Introduction
Species diversity, taxonomy, and phylogeny of macrofungi have been investigated in the recent years, and many new species have been discovered (Zeng et al., 2013; Han et al., 2016; Chai et al., 2019; Cui et al., 2019; Shen et al., 2019; Sun et al., 2020; Liu et al., 2021a,b, 2022; Wu et al., 2021; Ji et al., 2022; Xie et al., 2022). Species of Cantharellus Adans. ex Fr. (Hydnaceae, Cantharellales), interesting and important fungi, have also received a lot of attention by mycologists for their mycorrhizal properties, medicinal values, and edibility (Pilz et al., 2003; Yun and Hall, 2004; Shao et al., 2012). Molecular phylogeny has delimited abundant species within the genus and revealed unexpected species diversity (Buyck et al., 2011, 2013, 2014, 2016a,b; Foltz et al., 2013; Leacock et al., 2016). Until now, a large number of Cantharellus taxa have been described in Europe, Africa, and North America (Corner, 1966; Buyck et al., 2011, 2013, 2014, 2016a,b, 2018; Kumari et al., 2011, 2013; Suhara and Kurogi, 2015; De Kesel et al., 2016; Leacock et al., 2016).
In China, many species of Cantharellus have been uncovered (Chiu, 1973; Zang, 1980; Wei et al., 2008; Tian et al., 2009, 2012; Shao et al., 2011, 2012, 2014, 2016a,2021; Buyck et al., 2018; An et al., 2017; Jian et al., 2020; Cao et al., 2021; Zhang M. et al., 2021). They are well known to the public in the country because most of them have activities of anticancer, antimicrobial, immune regulation, and antioxidant (Dulger et al., 2004; Daniewskia et al., 2012; Nowacka-Jechalkea et al., 2018; Zhao et al., 2018). Interestingly, basidiocarps of Cantharellus such as Cantharellus yunnanensis W. F. Chiu are sold as edibles in markets in Yunnan Province, southwestern China (Figure 1), which generate good economic value (Watling, 1997; Wu and Lu, 2006).

Figure 1. Collections of chanterelle sold as edibles in the market of Yunnan Province, southwestern China. Photographs were taken by N. K. Zeng.
Recently, the genus Cantharellus has been divided into six subgenera including subg. Afrocantharellus Eyssart. and Buyck, subg. Cantharellus Adans. ex Fr., subg. Cinnabarinus Buyck and V. Hofst., subg. Parvocantharellus Eyssart. and Buyck, subg. Pseudocantharellus Eyssart. and Buyck, and subg. Rubrinus Eyssart. and Buyck (Buyck et al., 2014). Among them, subg. Cantharellus has received considerable attention, which is characterized by basidiomata that is medium- to large-sized; cap and stipe are usually smooth, sometimes with appressed squama; hymenophore is veined with blunt, mostly strongly forking-anastomosing ridges, rarely smooth or nearly so; hyphal endings are mostly thick-walled; clamp connections are abundant everywhere (Buyck et al., 2014). In China, although several taxa of subg. Cantharellus have been described or reported in previous studies (Chiu, 1973; Shao et al., 2011, 2016b,2021; An et al., 2017; Cao et al., 2021; Zhang Y. Z. et al., 2021), many more undescribed novel species probably exist in the country. Recently, many collections of subg. Cantharellus in China have been collected, and they were studied using morphological and molecular phylogenetic analyses, aiming to (i) describe new taxa and (ii) elucidate the species diversity of subg. Cantharellus in China.
Materials and Methods
Morphological Studies
Field notes and digital photographs were made from fresh specimens. Specimens examined in this study were deposited in the Fungal Herbarium of Hainan Medical University (FHMU), Haikou City, Hainan Province of China. Macroscopic descriptions are based on the detailed notes and photographs taken from fresh basidiomata. Color codes follow Kornerup and Wanscher (1981). Samples were hand-sectioned and mounted in 5% KOH solution and 1% congo red. Sections of the pileipellis were cut radial-perpendicularly and halfway between the center and the margin of the pileus. The following notations [n/m/p] indicate that n basidiospores measured m basidiomata of p collections. Dimensions of basidiospores were presented in the form (a–)b–e–c(–d), where the range b–c contains at least 90% of the measured values, “a” and “d” were the extreme values, and “e” refers to the average length/width of basidiospores. Q refers to the length/width ratio of basidiospores; Qm refers to the average Q of basidiospores and is given with standard deviation. For basidiospore shape, Qm = 1.15–1.3 describes “broadly ellipsoid,” Qm = 1.3–1.6 “ellipsoid,” and Qm = 1.6–2.0 “elongate” (Yang, 2005). The terms referring to the size of basidioma are based on Bas (1969).
Molecular Procedures
Total genomic DNA was obtained with the Plant Genomic DNA Kit (CWBIO, Beijing, China) according to the manufacturer’s instructions from collections dried with silica gel. Primer pairs used for amplification were as follows: nuc 28S rDNA D1-D2 domains (28S) with LR0R/LR5 (Vilgalys and Hester, 1990; James et al., 2006) and the translation elongation factor 1-α gene (TEF1) with EF1-α-F/EF1-α-R (Mikheyev et al., 2006). Polymerase chain reaction (PCR) conditions followed the program of Zhang Y. Z. et al. (2021). PCR products were checked in 1% (w/v) agarose gels. Amplified PCR products were sequenced using an ABI 3730 DNA Analyzer (Guangzhou Branch of BGI, China) with the same primers. Forward or reverse sequences were compiled with BioEdit (Hall, 1999). All sequences newly obtained in this study were deposited to GenBank1.
Dataset Assembly
A total of sixty-nine DNA sequences (34 of 28S, and 35 of TEF1) from 36 collections were newly generated. Edited sequences were deposited in GenBank; the GenBank accession numbers are listed in Table 1. For the concatenated dataset, the sequences of 28S and TEF1 from new collections were aligned with selected sequences of subg. Cantharellus from previous studies and GenBank (Table 1); Cantharellus ibityensis Buyck, Randrianj. and V. Hofst. was chosen as an outgroup inferred from Buyck et al. (2014). To test for phylogenetic conflict among 28S and TEF1, single-gene phylogenetic trees based on each of these two fragments were analyzed. The results of analyses showed that 28S and TEF1 were not in conflict. Thus, two datasets (28S and TEF1) were aligned with MUSCLE v3.6 (Edgar, 2004) and manually optimized on BioEdit v7.0.9 (Hall, 1999); then, the two datasets were concatenated using Phyutility v2.2 for further analyses (Smith and Dunn, 2008).
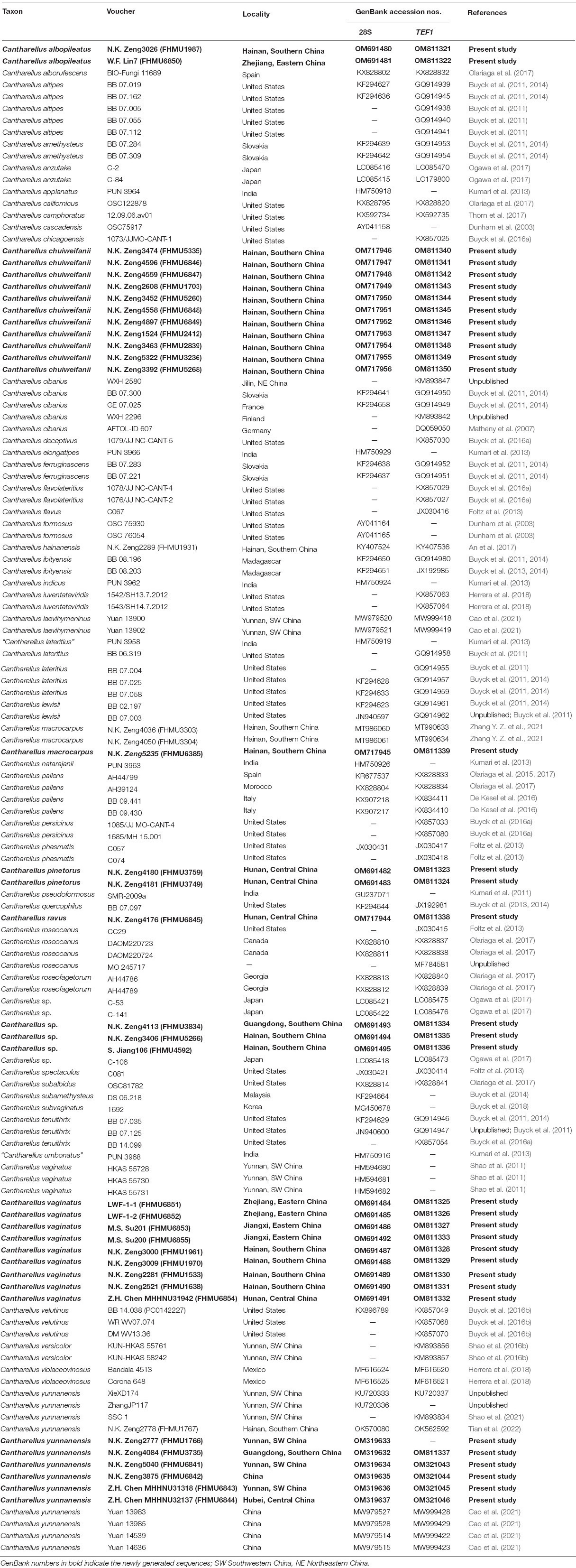
Table 1. Taxa, vouchers, locations, and GenBank accession numbers of DNA sequences used in this study.
Phylogenetic Analyses
The combined nuclear dataset (28S + TEF1) was analyzed using maximum likelihood (ML) and Bayesian inference (BI). Maximum likelihood tree generation and bootstrap analyses were performed with the program RAxML7.2.6 (Stamatakis, 2006) running 1,000 replicates combined with an ML search. Bayesian analysis with MrBayes 3.1 (Huelsenbeck and Ronquist, 2005) implementing the Markov chain Monto Carlo (MCMC) technique and parameters predetermined with MrModeltes 2.3 (Nylander, 2004) was performed. The best-fit likelihood model of 28S and TEF1 was GTR + I + G and SYM + I + G, respectively. Bayesian analysis of the combined nuclear dataset (28S + TEF1) was repeated for 20 million generations and sampled every 100 generations. Trees sampled from the first 25% of the generations were discarded as burn-in, and Bayesian posterior probabilities (PP) were then calculated for a majority consensus tree of the retained Bayesian trees. Runs were terminated once the average standard deviation of split frequencies went below 0.01.
Results
Molecular Data
The combined dataset (28S + TEF1) of subg. Cantharellus consisted of 129 taxa and 1,707 nucleotide sites, and the alignment was submitted to TreeBase (S29413). The phylogram with branch lengths generated from RAxML and support values is shown in Figure 2. The topologies of the phylogenetic trees based on the combined dataset generated from ML and BI analyses were almost identical, but there was a slight variation in statistical support.
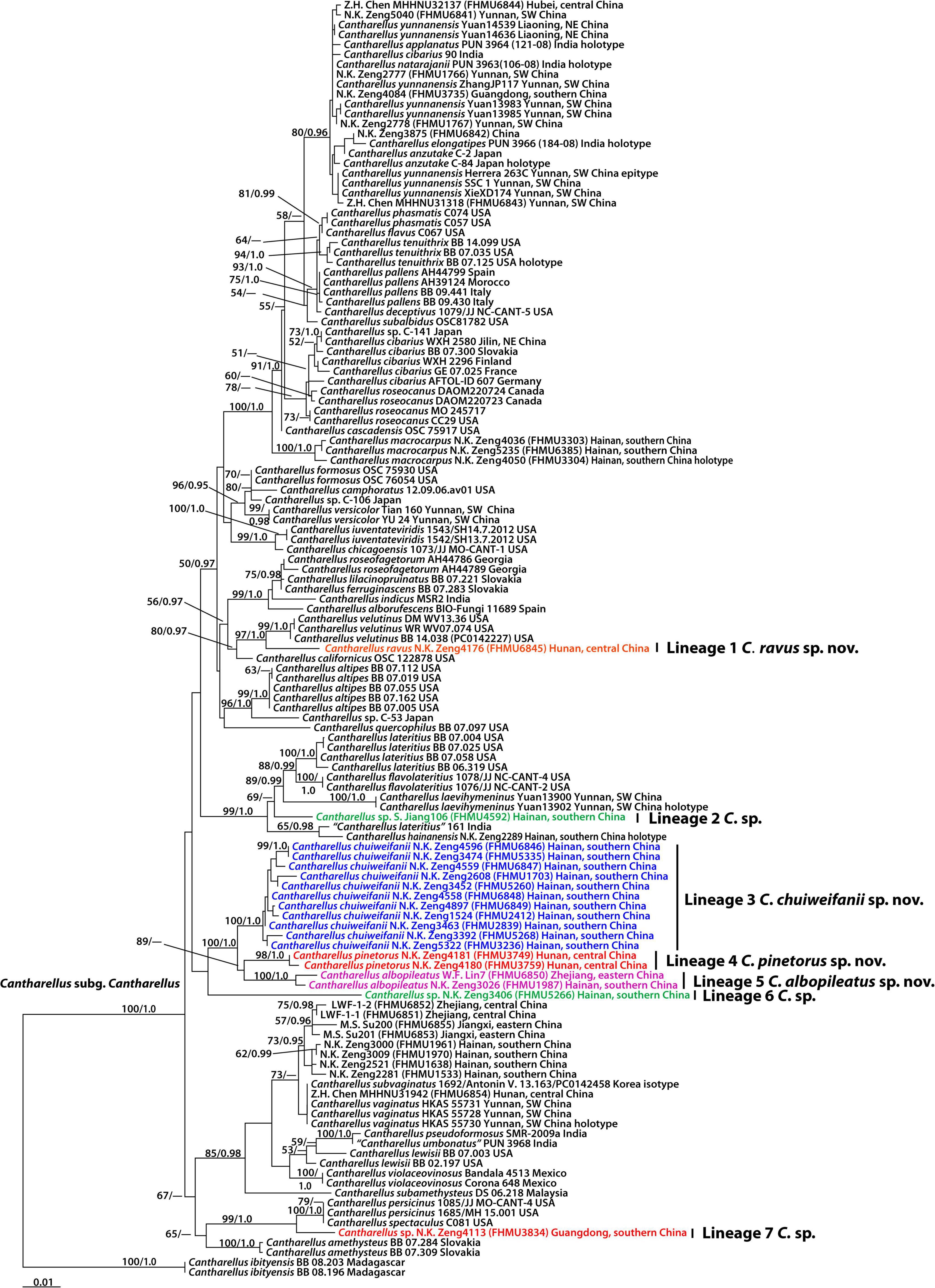
Figure 2. Phylogram inferred from a combined dataset (28S and TEF1) of the Cantharellus subg. Cantharellus using RAxML. RAxML likelihood bootstrap (BS ≥ 50%) and Bayesian posterior probabilities (PP ≥ 0.95) are indicated above or below the branches as RAxML BS/PP.
The present molecular data indicate that the Chinese species of subg. Cantharellus were grouped into fourteen independent lineages (Figure 2). A total of seven new lineages were identified in this study (Lineages 1–7 of Figure 2). Lineage 1 was comprised of one collection (FHMU6845) from central China; lineage 2 was comprised of one material (FHMU4592) from southern China; lineage 3, with strong statistical support (BS = 100%, PP = 1.0), was comprised of eleven collections (FHMU5335, FHMU6846, FHMU6847, FHMU1703, FHMU5260, FHMU6848, FHMU6849, FHMU2412, FHMU2839, FHM3236, and FHMU5268) from southern China; lineage 4, with high statistical support (BS = 98%, PP = 1.0), was comprised of two specimens (FHMU3749 and FHMU3759) both from central China; lineage 5, with strong statistical support (BS = 100%, PP = 1.0), was comprised of two materials (FHMU1987 and FHMU6850) from southern China and eastern China, respectively; lineage 6 was comprised of one collection (FHMU5266) from southern China; and lineage 7 was comprised of one specimen (FHMU3834) also from southern China (Figure 2).
Taxonomy
Cantharellus albopileatus N.K. Zeng, Y.Z. Zhang, and W.F. Lin, sp. nov.

Figure 3. Basidiomata of Cantharellus subg. Cantharellus species. (A,B) Cantharellus albopileatus (A) FHMU1987, holotype; (B) FHMU6850; (C–E) Cantharellus chuiweifanii (C,D) FHMU5335; (E) FHMU2839; (F,G) Cantharellus pinetorus (F) FHMU3759, holotype; (G) FHMU3749; (H) Cantharellus ravus (FHMU6845, holotype). Photographs: (A,C–H) N. K. Zeng; (B) W. F. Lin.
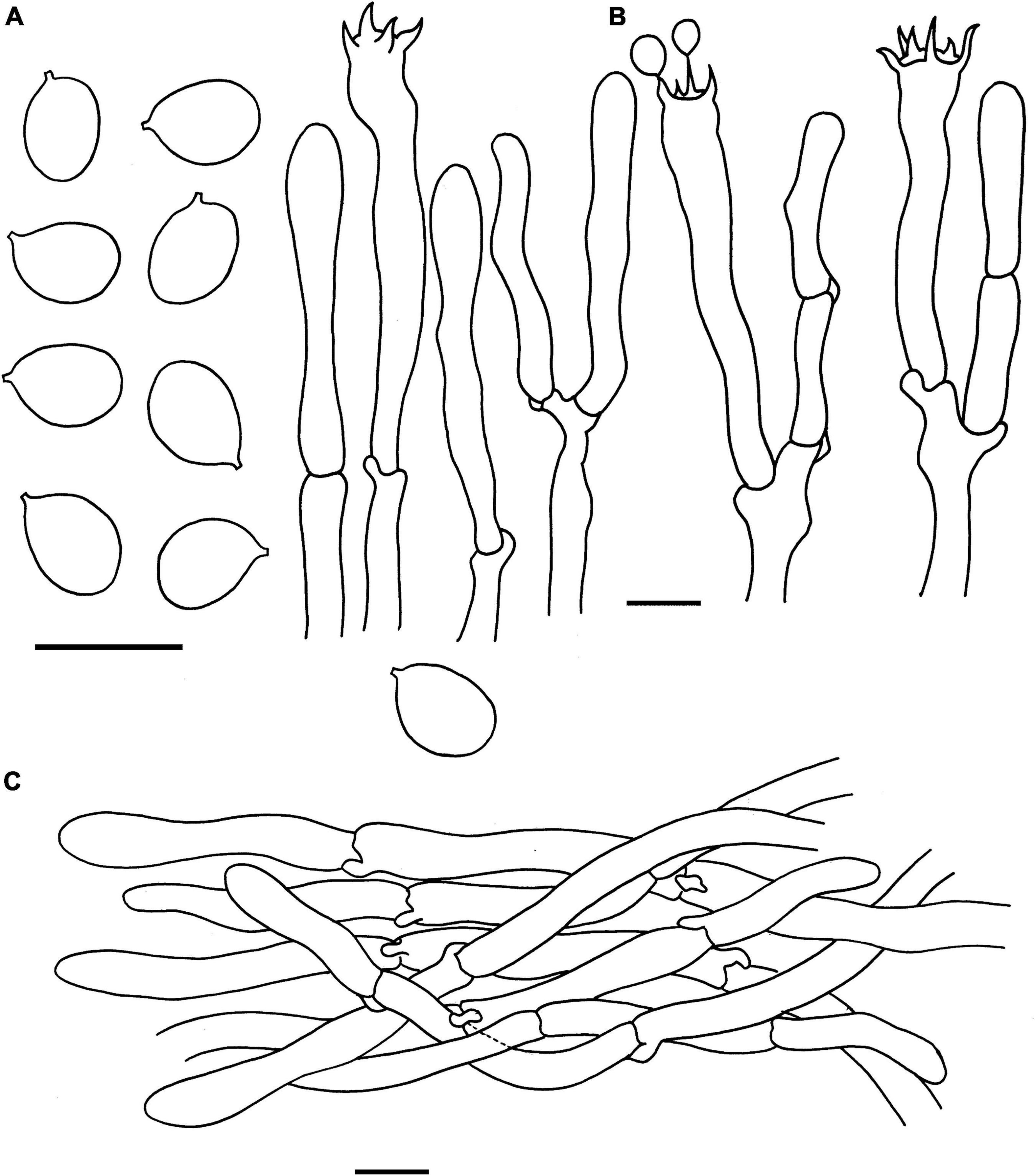
Figure 4. Microscopic features of Cantharellus albopileatus (FHMU1987, holotype). (A) Basidiospores. (B) Basidia. (C) Pileipellis. Scale bars = 10 μm. Drawings by Y. Z. Zhang.
MycoBank: MB843063
Diagnosis: It differs from other species of subg. Cantharellus by a cream to off-white basidioma, a well-developed hymenophore, and ellipsoid basidiospores.
Etymology: Latin “albo-” means white, “pileatus” means pileus, referring to the off-white pileus of our new species.
Holotype: China. Hainan Province: Yinggeling of Hainan Tropical Rainforest National Park, elev. 750 m, 28 May 2017, N. K. Zeng3026 (FHMU1987). GenBank accession number: 28S = OM691480, ITS = OM835804, TEF1 = OM811321.
Basidiomata are very small to small-sized. Pileus is 2.5–5 cm in diameter and convex with a depressed center; the margin was strongly incurved, irregular, often wavy, and lobed; the surface is smooth, slightly greasy, and cream (3A1) to off-white (4A1) in color; the context above stipe was 0.3 cm in thickness, whitish (4A1), unchanging in color when injured. Hymenophore is composed of relatively well-developed, decurrent gill folds, branched to furcate, which becomes strongly intervened with age; these folds are about 0.1 cm broad and are yellowish (2A2) to white (4A1) in color. Stipe is 2–3 × 0.4–0.6 cm, central, subcylindrical, young solid, hollowing with age, and curved at the base; the surface was dry, whitish (4A1) to very pale cream (3A1), and nearly concolorous with hymenophore; the context is fleshy, firm, and whitish (3A1). Taste and odor is not distinctive. Spore print is not obtained.
Basidiospores [40/2/2] 6–7.18–8 × 5–5.46–6(–6.5) μm, Q = (1.09–)1.15–1.50(–1.6), Qm = 1.32 ± 0.12, are ellipsoid, smooth, slightly thick-walled (0.5 μm), yellowish in KOH. Basidia is 43–65 × 5–10 μm, narrowly clavate, slightly thick-walled (up to 0.5 μm), 4-5-6-spored, and yellowish in KOH; sterigmata is 4–6 μm in length. Cystidia is absent. Pileipellis has a cutis that is 30–70 μm thick, is composed of mostly interwoven, cylindrical hyphae, is 6–12 μm wide, is thick-walled (up to 1 μm), and is faintly pale yellow in KOH; terminal cells are 21–108 × 5–10 μm in length, thin to slightly thick-walled (up to 0.5 μm), subcylindrical to subclavate, with obtuse apex. Clamp connections are present in all parts of basidioma.
Habitat: Solitary, scattered, or gregarious on the ground in forests dominated by fagaceous trees such as Castanopsis fissa (Champion ex Bentham) Rehder et E. H. Wilson.
Known distribution: southern and eastern China.
Other specimens were examined: China. Zhejiang Province: Hangzhou City, Tianmushan Nature Reserve, elev. 1100 m, 22 July 2020, W. F. Lin7 (FHMU6850).
Notes: Cantharellus albus S. P. Jian and B. Feng, originally described from Yunnan, southwestern China, has a white basidioma. However, by presenting the features such as a stipe turning yellow when bruised and a lower value of Qm, it is a member of subg. Parvocantharellus Eyssart. and Buyck (Jian et al., 2020). The white basidioma makes Cantharellus albopileatus somewhat similar to the American Cantharellus subalbidus A. H. Sm. and Morse; however, this latter species in addition to being associated with trees of Pinaceae (Smith and Morse, 1947) possesses larger basidioma [pileus 5–10 (–14) cm], and it is also distinctly differentiated at the molecular level (Figure 2).
In the phylogenetic analyses, Cantharellus albopileatus forms a well-supported (BS = 100%, PP = 1.0) monophyletic clade, together with the newly described Cantharellus chuiweifanii, equally a tropical species, and Cantharellus pinetorus from central China (Figure 2), which is quite different from any of the other northern hemisphere species in subg. Cantharellus. However, Cantharellus pinetorus is associated with trees of Pinaceae (refer to the descriptions under Cantharellus pinetorus); both Cantharellus pinetorus and Cantharellus chuiweifanii have a yellow pileus (refer to the descriptions under Cantharellus chuiweifanii).
Cantharellus chuiweifanii N.K. Zeng, Y.Z. Zhang, and Zhi Q. Liang, sp. nov.
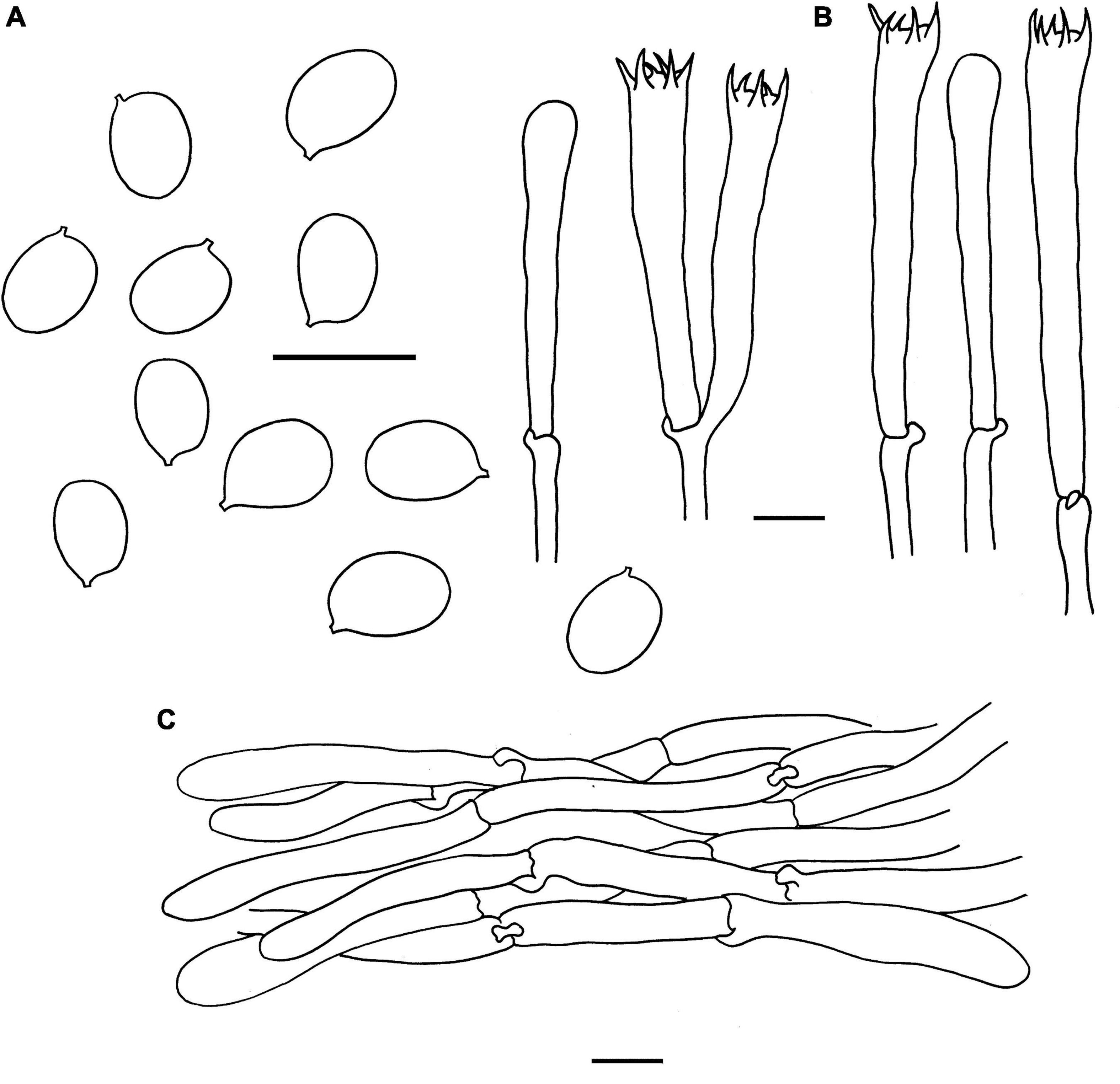
Figure 5. Microscopic features of Cantharellus chuiweifanii (FHMU2412, holotype). (A) Basidiospores. (B) Basidia. (C) Pileipellis. Scale bars = 10 μm. Drawings by Y. Z. Zhang and D. Y. An.
MycoBank: MB843062
Diagnosis: It differs from other species of subg. Cantharellus by an egg-yolk yellow to bright yellow, shiny pileus, a whitish stipe, a well-developed hymenophore, ellipsoid basidiospores, and a distribution in tropical Asia.
Etymology: Latin “chuiweifanii” is named after Chinese mycologist W. F. Chiu; for that, he published the first new species of Cantharellus in China, i.e., Cantharellus yunnanensis.
Holotype: China. Hainan Province: Limushan of Hainan Tropical Rainforest National Park, elev. 650 m, 11 May 2014, N. K. Zeng1524 (FHMU2412). GenBank accession number: 28S = OM717953, TEF1 = OM811347.
Basidiomata are very small to medium-sized. Pileus is 2–5 cm in diameter and plano-convex at first but soon depressed in the center; the margin was first very regular and strongly incurved and then became more wavy, the surface is smooth, pale yellow, egg-yolk yellow (1A5) to bright yellow (1A7), sometimes tinged with brownish, and shiny in color; the context above stipe was 0.05–0.15 cm in thickness, yellowish (3A3) to orange yellow (3A8), but unchanging in color when injured. Hymenophore is composed of well-developed, decurrent, well-spaced, and unequal gill folds, especially near the extreme cap margin with many very short lamellulae or also often forked; these folds are 0.1–0.2 cm broad and are pale yellow, egg-yolk yellow (1A4) to orange yellow in color (2A7). Stipe is 1.2–4 × 0.4–1.0 cm, central, cylindrical; surface dry, whitish (5A1), or yellowish-brown (1A2) in color; the context is fleshy, firm, and yellowish (2A2). Taste and odor is not distinctive. Spore print is not obtained.
Basidiospores [173/18/11] 6–7.05–8 × 4.5–5.03–5.5(–6) μm, Q = (1.18–)1.27–1.60(–1.67), Qm = 1.41 ± 0.10, are ellipsoid, smooth, slightly thick-walled (0.5 μm), yellowish in KOH. Basidia is –57-67 × 8-10 long, narrow, subcylindric, slightly thick-walled (0.5–0.7 μm), 4-5-6-spored, yellowish in KOH; sterigmata is 5–7 μm in length. Cystidia is absent. Pileipellis has a cutis that is 70–180 μm thick, is composed of mostly interwoven, cylindrical hyphae, is 5–8 μm wide, is slightly thick-walled (0.5–0.8 μm), and is faintly pale yellow in KOH; terminal cells are 52–148 × 4–7 μm in length, slightly thick-walled (0.5–0.8 μm), subcylindrical to subclavate, with obtuse apex. Clamp connections are present in all parts of basidioma.
Habitat: Solitary, scattered, or gregarious on the ground in forests dominated by fagaceous trees such as Lithocarpus spp.
Known distribution: southern China.
Other specimens were examined: China. Hainan Province: Yinggeling of Hainan Tropical Rainforest National Park, elev. 750 m, 5 August 2015, N. K. Zeng2608 (FHMU1703); Jianfengling of Hainan Tropical Rainforest National Park, elev. 850 m, 27 June 2018, N. K. Zeng3392 (FHMU5268); same location, 28 June 2018, N. K. Zeng3452, 3463, 3474 (FHMU5260, FHMU2839, FHMU5335); same location, 10 August 2020, N. K. Zeng4558, 4559 (FHMU6848, FHMU6847); same location, 11 August 2020, N. K. Zeng4596 (FHMU6846); same location, 29 July 2021, N. K. Zeng5322 (FHMU3236); Wanning County, Bofangling, elev. 70 m, 29 August 2020, N. K. Zeng4897 (FHMU6849).
Notes: Cantharellus chuiweifanii looks like Cantharellus subcibarius Corner and Cantharellus yunnanensis. However, Cantharellus subcibarius has a yellow stipe, a context turning yellow to orange-brown when bruised, and larger basidiospores [(7.3–)7.5–7.89–8.3(–8.5) × (5.2–)5.6–6.10–6.5(–6.9) μm, Q = (1.12–)1.23–1.30–1.36(–1.40)] (Corner, 1966; Buyck et al., 2021); Cantharellus yunnanensis has a yellow stipe, wider basidiospores measuring (6.5–)7–8(–8.5) × 5–6(–6.5) μm (Shao et al., 2021), and it is also distinctly differentiated at the molecular level (Figure 2). Cantharellus chuiweifanii is phylogenetically associated with Cantharellus albopileatus and Cantharellus pinetorus (Figure 2), and the differences in the three taxa have been discussed under Cantharellus albopileatus.
Cantharellus pinetorus N.K. Zeng, Y.Z. Zhang and Zhi Q. Liang, sp. nov.
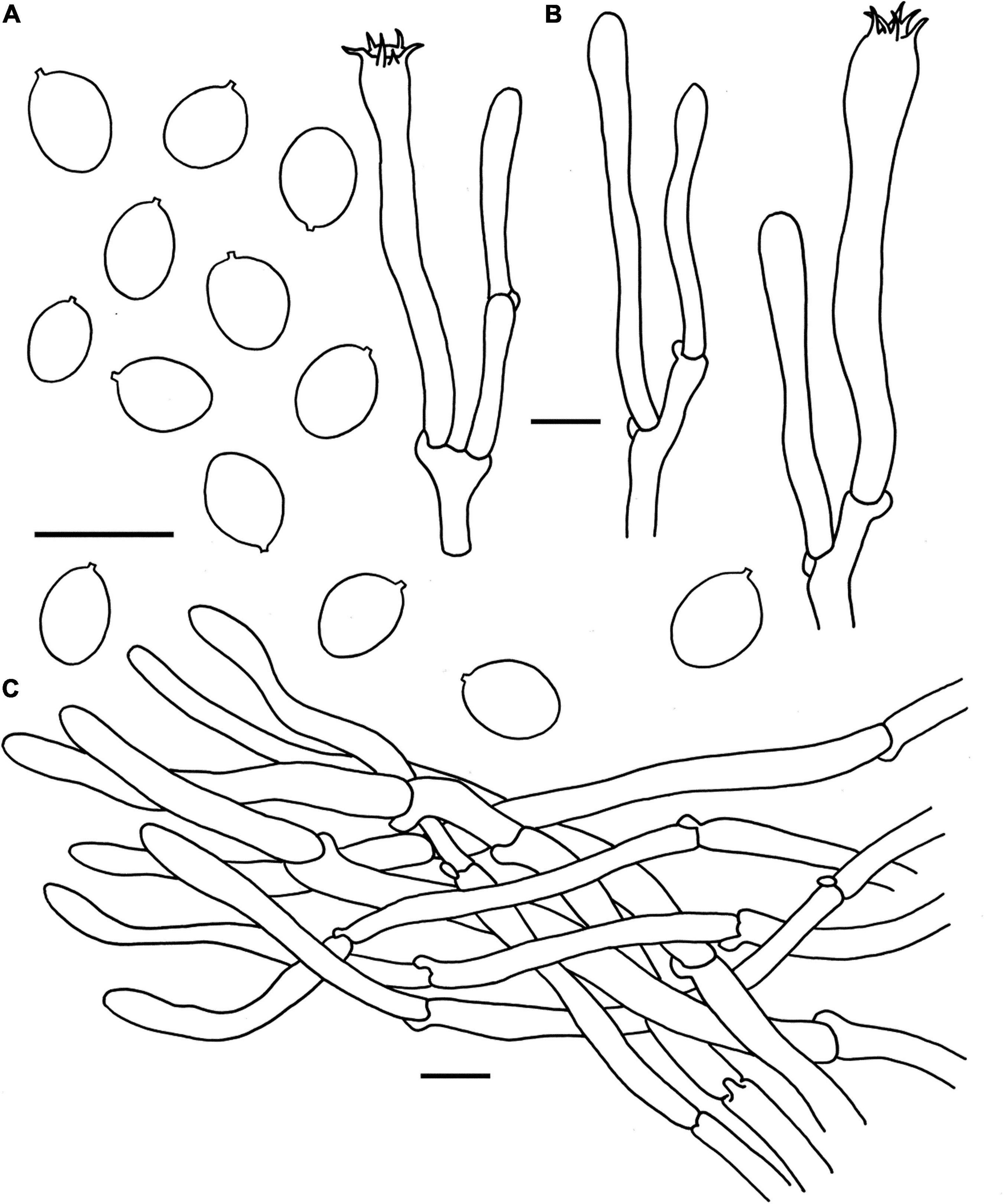
Figure 6. Microscopic features of Cantharellus pinetorus (FHMU3759, holotype). (A) Basidiospores. (B) Basidia. (C) Pileipellis. Scale bars = 10 μm. Drawings by Y. Z. Zhang.
MycoBank: MB843064
Diagnosis: It differs from other species of subg. Cantharellus by a bright yellow to orange-yellow pileus, a cream to grayish yellow stipe, a well-developed hymenophore, broadly ellipsoid to ellipsoid basidiospores, and it is associated with pine trees.
Etymology: Latin “pinetorus” refers to the association of the new species with pine forests.
Holotype: China. Hunan Province: Yizhang County, Mangshan National Nature Reserve, elev. 750 m, 30 July 2019, N. K. Zeng4180 (FHMU3759). GenBank accession number: 28S = OM691482, TEF1 = OM811323.
Basidiomata are small to medium-sized. Pileus is 3.5–5 cm in diameter and convex when young and then applanate with depressed center; the surface is smooth, bright yellow (3A5) to orange-yellow in color (3A6); the margin is incurved and irregularly wavy; the context above stipe is 0.35–0.55 cm in thickness, yellow (3A7), but unchanging in color when injured. Hymenophore is veined and decurrent; these folds are 0.05–0.2 cm broad and are lemon yellow (1A6) to pale yellow in color (4A2). Stipe is 2.5–3 × 0.5–0.6 cm, cylindrical, central, and hollow; the surface is dry and cream (4A1) to grayish yellow (4A2) in color; the context is white (3A2). Taste and odor is not distinctive. Spore print is not obtained.
Basidiospores [40/2/2] 6–6.98–7.5(–8) × 5–5.36–6 μm, Q = (1.09–)1.17–1.5, Qm = 1.30 ± 0.09, are broadly ellipsoid to ellipsoid, smooth, slightly thick-walled (0.5 μm), yellowish in KOH. Basidia is 57–68 × 4–10 μm, long, narrow, subcylindric, slightly thick-walled (up to 0.5 μm), 3-4-5-spored, and yellowish in KOH; sterigmata is 2–4 μm in length. Cystidia is absent. Pileipellis has a cutis that is 100–150 μm thick, is composed of mostly interwoven, cylindrical hyphae, is 6–9 μm wide, is slightly thick-walled (up to 0.5 μm), and is faintly pale yellow in KOH; terminal cells are 40–61 × 4–6 μm in length, slightly thick-walled (0.5–0.7 μm), subcylindrical to subclavate, with obtuse apex. Clamp connections are present in all parts of basidioma.
Habitat: Solitary, scattered, or gregarious on the ground, in forests dominated by Pinus massoniana Lamb.
Known distribution: central China.
Other specimens were examined: China. Hunan Province: Yizhang County, Mangshan National Nature Reserve, elev. 750 m, 30 July 2019, N. K. Zeng4181 (FHMU3749).
Notes: Malaysian Cantharellus ianthinus Corner and Cantharellus subcibarius are morphologically similar to Cantharellus pinetorus. However, Cantharellus ianthinus has purple fibrils on the surfaces of the pileus and stipe, and larger basidiospores measuring 8–10.5 × 5.5–7 μm (Corner, 1966); Cantharellus subcibarius has a context turning yellow to orange-brown when bruised, and it is not associated with trees of Pinaceae (Corner, 1966; Buyck et al., 2021). In the phylogenetic analyses, Cantharellus pinetorus is allied with Cantharellus albopileatus and Cantharellus chuiweifanii (Figure 2), the differences in the three taxa have been discussed under Cantharellus albopileatus.
Cantharellus ravus N.K. Zeng, Y.Z. Zhang, and Zhi Q. Liang, sp. nov.
Figures 3H, 7.
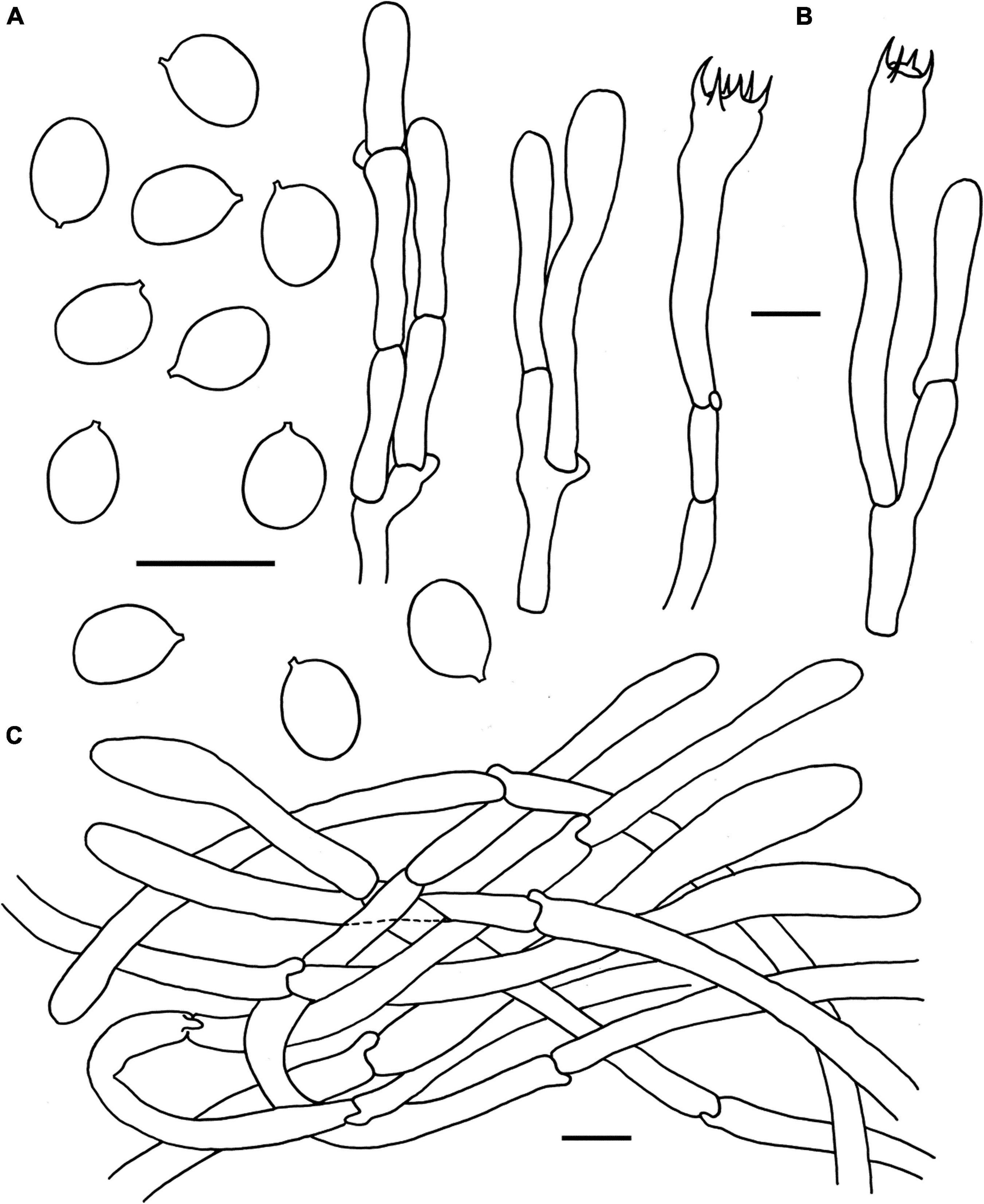
Figure 7. Microscopic features of Cantharellus ravus (FHMU6845, holotype). (A) Basidiospores. (B) Basidia. (C) Pileipellis. Scale bars = 10 μm. Drawings by Y. Z. Zhang.
MycoBank: MB843065
Diagnosis: It differs from other species of subg. Cantharellus by a yellowish to grayish yellow, dull pileus, a well-developed hymenophore, and ellipsoid basidiospores.
Etymology: Latin “ravus” refers to the grayish yellow basidioma of our new species.
Holotype: China. Hunan Province: Yizhang County, Mangshan National Nature Reserve, elev. 550 m, 29 July 2019, N. K. Zeng4176 (FHMU6845). GenBank accession number: 28S = OM717944, TEF1 = OM811338.
Basidiomata are small to medium-sized. Pileus is 3.5–8 cm in diameter and plano-convex to infundibuliform; the surface is smooth, yellowish (1A2) to grayish yellow (2A4) in color, and dull; the margin is incurved or downward; the context above stipe is about 0.3 cm in thickness, yellowish (3A3), unchanging in color when injured. Hymenophore is veined and decurrent; these folds are about 0.1 cm high, forking, creamy yellow (1A4) to yellowish (3A2) in color. Stipe is 3–4 × 0.6–0.9 cm, central, cylindrical; surface dry, grayish yellow to fulvous (4A4), but whitish (5A1) at base. Taste and odor is not distinctive. Spore print is not obtained.
Basidiospores [37/2/1] (6–)6.5–7.04–7.5 × 4.5–5.24–5.5 μm, Q = (1.18–)1.2–1.5(–1.56), Qm = 1.35 ± 0.08, are ellipsoid, smooth, slightly thick-walled (0.5 μm), yellowish in KOH. Basidia is 45–63 × 8–9 μm, long, narrow, subcylindric, slightly thick-walled (up to 0.5 μm), 4-5-6 spored, yellowish in KOH; sterigmata is 6–8 μm in length. Cystidia is absent. Pileipellis has a cutis composed of mostly cylindrical, that is 5–12 μm wide, is slightly thick-walled (up to 1 μm) hyphae, and is faintly pale yellow in KOH; terminal cells are 45–82 × 6–10 μm, slightly thick-walled (0.5–0.7 μm), subcylindrical to subclavate, with obtuse apex. Clamp connections are present in all parts of basidioma.
Habitat: Gregarious on the ground in forests dominated by fagaceous trees such as Lithocarpus spp.
Known distribution: central China.
Notes: Cantharellus subcibarius and Cantharellus yunnanensis are also morphologically similar to our new species. However, both Cantharellus subcibarius and Cantharellus yunnanensis have shiny pileal surfaces. Moreover, Cantharellus subcibarius has a context turning yellow to orange-brown when bruised, and larger basidiospores [(7.3–)7.5–7.89–8.3(–8.5) × (5.2–)5.6–6.10–6.5(–6.9) μm, Q = (1.12–)1.23–1.30–1.36(–1.40)] (Corner, 1966; Buyck et al., 2021); Cantharellus yunnanensis also has larger basidiospores measuring (6.5–) 7–8 (–8.5) × 5–6 (–6.5) μm (Shao et al., 2021), and it is distinctly differentiated at the molecular level (Figure 2). Our molecular data also indicated that Cantharellus ravus is closely related to the North American Cantharellus californicus D. Arora and Dunham and Cantharellus velutinus Buyck and V. Hofst (Figure 2). However, Cantharellus californicus has much larger basidiospores measuring 7–9.30–12 μm × 5–6.45–8 μm (Arora and Dunham, 2008); Cantharellus velutinus, a very variable species with yellow to pink fruiting bodies and pubescent pileus surface, has longer but narrower basidiospores measuring (6.7–)7.3–7.84–8.4(–9.2) × (3.7–)4.2–4.61–5.0(–5.2) μm and hyphal extremities of the pileipellis with conspicuously thickened cell walls (Buyck et al., 2016b).
Discussion
Morphological Features and Hosts of Cantharellus Subgenus Cantharellus
In agreement with the previous hypotheses (Buyck et al., 2014), most species in China have nearly glabrous pileus, with the exception of Cantharellus vaginatus S. C. Shao, X. F. Tian, and P. G. Liu and Cantharellus versicolor S. C. Shao and P. G. Liu having a squamulose cap (Shao et al., 2011, 2016b). As noted by Buyck et al. (2014), most taxa in subg. Cantharellus have yellow pileus. In China, Cantharellus chuiweifanii, Cantharellus cibarius Fr, Cantharellus hainanensis N. K. Zeng, Zhi Q. Liang, and S. Jiang, Cantharellus laevihymeninus T. Cao and H. S. Yuan, Cantharellus macrocarpus N. K. Zeng, Y. Z. Zhang, and Zhi Q. Liang, Cantharellus pinetorus, Cantharellus ravus, Cantharellus vaginatus, and Cantharellus yunnanensis also have pilei colored with yellow; Cantharellus albopileatus is characterized with white pileus; squamules on pileal surface of Cantharellus vaginatus are fulvous to brown (Shao et al., 2011), and sandy brown to dark brown in Cantharellus versicolor was observed (Shao et al., 2016b).
Besides macro-morphology, some micro-morphological features can also be used to discriminate subg. Cantharellus species. For example, species of the subgenus usually have abundant clamps, and hyphal endings in pileipellis are mostly thick-walled (Buyck et al., 2014). Our four new species and previous taxa of subg. Cantharellus in China also possess clamp connections and thick-walled hyphal endings in pileipellis (Shao et al., 2011, 2016b,2021; An et al., 2017; Cao et al., 2021; Zhang Y. Z. et al., 2021).
In regard to ecological preference, many species such as Cantharellus altipes Buyck and V. Hofst, Cantharellus macrocarpus, and Cantharellus tenuithrix Buyck and V. Hofst were reported to grow in pine-oak woods (Buyck and Hofstetter, 2011; Shao et al., 2021; Zhang Y. Z. et al., 2021). In China, Cantharellus cibarius, Cantharellus vaginatus, and Cantharellus yunnanensis were also reported to grow in pine-oak forests (Shao et al., 2011, 2021); Cantharellus albopileatus, Cantharellus chuiweifanii, Cantharellus hainanensis, and Cantharellus ravus grow in forests dominated by fagaceous trees, whereas Cantharellus pinetorus and Cantharellus versicolor are associated with trees of Pinaceae (Shao et al., 2011, 2016b; An et al., 2017).
Most species of subg. Cantharellus sections Cantharellus and Amethystini Buyck and V. Hofstetter have well-developed hymenophore, section Sublaeves Buyck and V. Hofstetter harbors taxa with smooth hymenophore, and section Amethystini Buyck and V. Hofstetter usually has a pileus with appressed squama. Judging from the positions of our new species and Chinese previous taxa in the molecular phylogenetic tree (Figure 2), plus the morphological features, Cantharellus cibarius, Cantharellus macrocarpus, Cantharellus ravus, Cantharellus versicolor, and Cantharellus yunnanensis are the members of section Cantharellus, Cantharellus hainanensis, and Cantharellus laevihymeninus, also have ill-developed hymenophore (An et al., 2017; Cao et al., 2021), and belong to the section Sublaeves. Cantharellus vaginatus is a member of section Amethystini, and Cantharellus albopileatus, Cantharellus chuiweifanii, and Cantharellus pinetorus probably represent a new section, which will be further studied in the future.
Species Diversity of Cantharellus Subgenus Cantharellus
High species diversity of subg. Cantharellus in China was revealed in this study, and fourteen lineages were identified (Figure 2). A total of four (lineages 1, 3–5 of Figure 2) were here described as new species: Cantharellus albopileatus, Cantharellus chuiweifanii, Cantharellus pinetorus, and Cantharellus ravus; others represent previously described taxa: Cantharellus cibarius, Cantharellus hainanensis, Cantharellus laevihymeninus, Cantharellus macrocarpus, Cantharellus vaginatus, Cantharellus versicolor, and Cantharellus yunnanensis, whereas three lineages (lineages 2, 6–7 of Figure 2) remain still undescribed.
Up to now, taxa in subg. Cantharellus are all from northern hemisphere (Buyck et al., 2014). Most species of the subgenus included in the present dataset are from temperature areas of northern hemisphere including North America and Europe (Figure 2). In China, with the exception of Cantharellus cibarius and Cantharellus versicolor in temperate areas, most species, viz. Cantharellus albopileatus, Cantharellus chuiweifanii, Cantharellus hainanensis, Cantharellus macrocarpus, Cantharellus pinetorus, Cantharellus ravus, Cantharellus vaginatus, and Cantharellus yunnanensis, occur in subtropical or tropical China. With more field investigations, more taxa of the subgenus will be uncovered in the subtropical and tropical regions.
Phylogenetic Relationships and Geographic Divergence of Chinese Cantharellus Subgenus Cantharellus
Our molecular data based on two-locus DNA sequences (28S + TEF1) with many new specimens from China have contributed to our knowledge of subg. Cantharellus.
It is clear that there are several clades having taxa from both sides of the Pacific, and allied species from East Asia and North America are obviously inferred from this molecular phylogenetic tree (Figure 2). For example, Chinese Cantharellus versicolor is related to North American Cantharellus camphoratus R. H. Petersen and Cantharellus formosus Corner; our new species Cantharellus ravus is affiliated with Cantharellus californicus and Cantharellus velutinus, two species both described from United States; one collection tentatively named C. sp. (FHMU3834) appears closely related to North American-type collection of Cantharellus spectaculus Foltz and T. J. Volk (Figure 2), which is an earlier synonym of Cantharellus persicinus Petersen (Buyck et al., 2016c). Our study did not identify disjunct populations of the same purported taxon in the two areas (Figure 2). Similar scenarios have been documented for many other fungi (Zhang et al., 2004; Zeng et al., 2013, 2016).
Biogeographic connections between East Asia and Europe have also been discussed in other fungi such as Amanita Pers., Phylloporus Quél. and Rhodotus Maire (Zhang et al., 2004; Zeng et al., 2013; Tang et al., 2014). In this study, we found that Cantharellus cibarius occurs in northeastern China and Europe (Figure 2).
In addition, we also noted that Cantharellus hainanensis is associated with one specimen labeled as Cantharellus lateritius from India (Figure 2). Besides northeastern China and Europe, the geographical distribution range of Cantharellus cibarius also extends to Japan (Figure 2).
Data Availability Statement
The data presented in the study are deposited in the https://www.ncbi.nlm.nih.gov/GenBank and https://www.mycobank.org/page/Home/MycoBank repository, accession number of GenBank (28S: OM319632-OM319637, OM691480-OM691495, OM717944-OM717956; TEF1: OM321043-OM321046, OM811321-OM811350; and ITS: OM835791-OM835808) and MycoBank (MB843062-MB843065).
Author Contributions
Z-QL and N-KZ contributed to the conceptualization. Y-ZZ performed the methodology, wrote the original draft preparation, and carried out the formal analysis. Y-ZZ and D-YA performed the experiment. N-KZ, W-FL, M-SS, Z-HC, PZ, and SJ carried out the resources. N-KZ, BB, and Z-QL wrote, reviewed, and edited the manuscript. N-KZ and Z-QL supervised the data. N-KZ carried out the project administration and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 32160001), which includes funds for open access publication fees.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
An, D. Y., Liang, Z. Q., Jiang, S., Su, M. S., and Zeng, N. K. (2017). Cantharellus hainanensis, a new species with a smooth hymenophore from tropical China. Mycoscience 58, 438–444. doi: 10.1016/j.myc.2017.06.004
Arora, D., and Dunham, S. M. (2008). A new, commercially valuable chanterelle species, Cantharellus californicus sp. nov., associated with live oak in California, USA. Econ. Bot. 62, 376–391. doi: 10.1007/s12231-008-9042-7
Bas, C. (1969). Morphology and subdivision of Amanita and a monograph of its section Lepidella. Persoonia 5, 285–579.
Buyck, B., Antonín, V., Chakraborty, D., Baghela, A., Das, K., and Hofstetter, V. (2018). Cantharellus sect. Amethystini in Asia. Mycol. Prog. 17, 917–924. doi: 10.1007/s11557-018-1403-8
Buyck, B., Cruaud, C., Couloux, A., and Hofstetter, V. (2011). Cantharellus texensis sp. nov. from Texas, a southern lookalike of C. cinnabarinus revealed by tef-1 sequence data. Mycologia 103, 1037–1046. doi: 10.3852/10-261
Buyck, B., Eyssartier, G., Dima, B., Consiglio, G., Noordeloos, M. E., Papp, V., et al. (2021). Fungal biodiversity profiles 101–110. Cryptogam. Mycol. 42, 63–89. doi: 10.5252/cryptogamie-mycologie2021v42a5
Buyck, B., and Hofstetter, V. (2011). The contribution of tef-1 sequences to species delimitation in the Cantharellus cibarius complex in the southeastern USA. Fungal Divers. 49, 35–46. doi: 10.1007/s13225-011-0095-z
Buyck, B., Hofstetter, V., and Olariaga, I. (2016a). Setting the record straight on North American Cantharellus. Cryptogam. Mycol. 37, 405–417. doi: 10.7872/crym/v37.iss3.2016.405
Buyck, B., Kauff, F., Cruaud, C., and Hofstetter, V. (2013). Molecular evidence for novel Cantharellus (Cantharellales, Basidiomycota) from tropical African miombo woodland and a key to all tropical African chanterelles. Fungal Divers. 58, 281–298. doi: 10.1007/s13225-012-0215-4
Buyck, B., Kauff, F., Eyssartier, G., Couloux, A., and Hofstetter, V. (2014). A multilocus phylogeny for worldwide Cantharellus (Cantharellales, Agaricomycetidae). Fungal Divers. 64, 101–121. doi: 10.1007/s13225-013-0272-3
Buyck, B., Olariaga, I., Justice, J., Lewis, D., Roody, W., and Hofstetter, V. (2016b). The dilemma of species recognition in the field when sequence data are not in phase with phenotypic variability. Cryptogam. Mycol. 37, 367–389. doi: 10.7872/crym/v37.iss3.2016.367
Buyck, B., Olariaga, I., Looney, B., Justice, J., and Hofstetter, V. (2016c). Wisconsin chanterelles revisited and first indications for very wide distributions of Cantharellus species in the United States East of the Rocky Mountains. Cryptogam. Mycol. 37, 345–366. doi: 10.7872/crym/v37.iss3.2016.345
Cao, T., Hu, Y. P., Yu, J. R., Wei, T. Z., and Yuan, H. S. (2021). A phylogenetic overview of the Hydnaceae (Cantharellales, Basidiomycota) with new taxa from China. Stud. Mycol. 99, 100–121. doi: 10.1016/j.simyco.2021.100121
Chai, H., Liang, Z. Q., Xue, R., Jiang, S., Luo, S. H., Wang, Y., et al. (2019). New and noteworthy boletes from subtropical and tropical China. Mycokeys 46, 55–96. doi: 10.3897/mycokeys.46.31740
Chiu, W. F. (1973). Ten new species of Agaricales from Yunnan. China. Acta Microbiol. Sin. 13, 129–135. doi: 10.1016/j.toxicon.2016.07.018
Cui, B. K., Li, H. J., Ji, X., Zhou, J. L., Song, J., Si, J., et al. (2019). Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Divers. 97, 137–392. doi: 10.1007/s13225-019-00427-4
Daniewskia, W. M., Danikiewiczb, W., Gołêbiewskic, W. M., Gucma, M., Łysik, A., Grodner, J., et al. (2012). Search for bioactive compounds from Cantharellus cibarius. Nat. Prod. Commun. 7, 917–918. doi: 10.1177/1934578X1200700729
De Kesel, A., Amalfi, M., Ngoy, B. K. W., Yorou, N. S., Raspé, O., Degreef, J., et al. (2016). New and interesting Cantharellus from tropical Africa. Cryptogam. Mycol. 37, 283–327. doi: 10.7872/crym/v37.iss3.2016.283
Dulger, B., Gonuz, A., and Gucin, F. (2004). Antimicrobial activity of the macrofungus Cantharellus cibarius. P. J. B. S. 7, 1535–1539. doi: 10.3923/pjbs.2004.1535.1539
Dunham, S. M., O’Dell, T. E., and Molina, R. (2003). Analysis of nrDNA sequences and microsatellite allele frequencies reveals a cryptic chanterelle species Cantharellus cascadensis sp. nov. from the American Pacific Northwest. Mycol. Res. 107, 1163–1177. doi: 10.1017/S0953756203008475
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Foltz, M. J., Perez, K. E., and Volk, T. J. (2013). Molecular phylogeny and morphology reveal three new species of Cantharellus within 20 m of one another in western Wisconsin, USA. Mycologia 105, 447–461. doi: 10.3852/12-181
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analyses program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98. doi: 10.1016/S1468-1641(10)60416-1
Han, M. L., Chen, Y. Y., Shen, L. L., Song, J., Vlasák, J., Dai, Y. C., et al. (2016). Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Divers. 80, 343–373. doi: 10.1007/s13225-016-0364-y
Herrera, M., Bandala, V. M., and Montoya, L. (2018). Cantharellus violaceovinosus, a new species from tropical Quercus forests in eastern Mexico. MycoKeys 32, 91–109. doi: 10.3897/mycokeys.32.22838
Huelsenbeck, J. P., and Ronquist, F. (2005). “Bayesian analysis of molecular evolution using MrBayes,” in Statistical Methods in Molecular Evolution, ed. R. Nielsen (New York, NY: Springer), 183–226. doi: 10.1007/0-387-27733-1_7
James, T. Y., Kauff, F., Schoch, C., Matheny, P. B., Hofstetter, V., Cox, C., et al. (2006). Reconstructing the early evolution of the fungi using a six gene phylogeny. Nature 443, 818–822. doi: 10.1038/nature05110
Ji, X., Zhou, J. L., Song, C. G., Xu, T. M., Wu, D. M., and Cui, B. K. (2022). Taxonomy, phylogeny and divergence times of Polyporus (Basidiomycota) and related genera. Mycosphere 13, 1–52. doi: 10.5943/mycosphere/13/1/1
Jian, S. P., Dai, R., Gao, J., and Feng, B. (2020). Cantharellus albus, a striking new species from Southwest China. Phytotaxa 470, 133–144. doi: 10.11646/phytotaxa.470.2.2
Kornerup, A., and Wanscher, J. H. (1981). Taschenlexikon der Farben, 3. Göttingen: Muster-Schmidt Verlag.
Kumari, D., Reddy, M. S., and Upadhyay, R. C. (2013). New records of Cantharellus species from the northwestern Himalayas of India. Mycology 4, 205–220. doi: 10.1080/21501203.2013.872205
Kumari, D., Upadhyay, R. C., and Reddy, M. S. (2011). Cantharellus pseudoformosus, a new species associated with Cedrus deodara from India. Mycoscience 52, 147–151. doi: 10.1007/s10267-010-0080-5
Leacock, P. R., Riddell, J., Wilson, A. W., Zhang, R., Ning, C., and Mueller, G. M. (2016). Cantharellus chicagoensis sp. nov. is supported by molecular and morphological analysis as a new yellow chanterelle in midwestern United States. Mycologia 108, 765–772. doi: 10.3852/15-230
Liu, S., Han, M. L., Xu, T. M., Wang, Y., Wu, D. M., and Cui, B. K. (2021a). Taxonomy and phylogeny of the Fomitopsis pinicola complex with descriptions of six new species from east Asia. Front. Microbiol. 12:644979. doi: 10.3389/fmicb.2021.644979
Liu, S., Shen, L. L., Wang, Y., Xu, T. M., Gates, G., and Cui, B. K. (2021b). Species diversity and molecular phylogeny of Cyanosporus (Polyporales. Basidiomycota). Front. Microbiol. 12:631166. doi: 10.3389/fmicb.2021.631166
Liu, S., Song, C. G., Xu, T. M., Ji, X., Wu, D. M., and Cui, B. K. (2022). Species diversity, molecular phylogeny and ecological habits of Fomitopsis (Polyporales. Basidiomycota). Front. Microbiol. 13:859411. doi: 10.3389/fmicb.2022.859411
Matheny, P. B., Wang, Z., Binder, M., Curtis, J. M., Lim, Y. W., Henrik Nilsson, R., et al. (2007). Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Mol. Phylogenet. Evol. 43, 430–451. doi: 10.1016/j.ympev.2006.08.024
Mikheyev, A. S., Mueller, U. G., and Abbot, P. (2006). Cryptic sex and many-to-one coevolution in the fungus-growing ant symbiosis. Proc. Natl. Acad. Sci. U.S.A. 103, 10702–10706. doi: 10.1073/pnas.0601441103
Nowacka-Jechalkea, N., Nowak, R., Juda, M., Malm, A., Lemieszek, M., Rzeski, W., et al. (2018). New biological activity of the polysaccharide fraction from Cantharellus cibarius and its structural characterization. Food Chem. 268, 355–361. doi: 10.1016/j.foodchem.2018.06.106
Nylander, J. A. A. (2004). MrModeltest 2.3. Program Distributed by the Author. Uppsala: University: Evolutionary Biology Center.
Ogawa, W., Endo, N., Fukuda, M., and Yamada, A. (2017). Phylogenetic analyses of Japanese golden chanterelles and a new species description, Cantharellus anzutake sp. nov. Mycoscience 59, 153–165. doi: 10.1016/j.myc.2017.08.014
Olariaga, I., Buyck, B., Esteve-Raventós, F., Hofstetter, V., Manjón, J. L., Moreno, G., et al. (2015). Assessing the taxonomic identity of white and orange specimens of Cantharellus: occasional colour variants or independent species? Cryptogam. Mycol. 36, 287–300. doi: 10.7872/crym/v36.iss3.2015.287
Olariaga, I., Moreno, G., Manjón, J. L., Salcedo, I., Hofstetter, V., Rodríguez, D., et al. (2017). Cantharellus (Cantharellales, Basidiomycota) revisited in Europe through a multigene phylogeny. Fungal Divers. 83, 263–292. doi: 10.1007/s13225-016-0376-7
Pilz, D., Norvell, L., Danell, E., and Molina, R. (2003). Ecology and Management of Commercially Harvested Chanterelle Mushrooms. Washington: US Department of Agriculture Pacific Northwest Research Station.
Shao, S. C., Buyck, B., Hofstetter, V., Tian, X. F., Geng, Y. H., Yu, F. Q., et al. (2014). Cantharellus hygrophorus, a new species in subgenus Afrocantharellus from tropical southwestern China. Cryptogam. Mycol. 35, 283–291. doi: 10.7872/crym.v35.iss3.2014.283
Shao, S. C., Buyck, B., Tian, X. F., Liu, P. G., and Geng, Y. H. (2016a). Cantharellus phloginus, a new pink-colored species from southwestern China. Mycoscience 57, 144–149. doi: 10.1016/j.myc.2015.12.004
Shao, S. C., Liu, P. G., Tian, X. F., Buyck, B., and Geng, Y. H. (2016b). A new species of Cantharellus (Cantharellales, Basidiomycota, Fungi) from subalpine forest in Yunnan, China. Phytotaxa 252, 273–279. doi: 10.11646/phytotaxa.252.4.3
Shao, S. C., Liu, P. G., Wei, T. Z., and Herrera, M. (2021). New insights into the taxonomy of the genus Cantharellus in China: epityfication of C. yunnanensis W.F. Chiu and the first record of C. cibarius Fr. Cryptogam. Mycol. 42, 25–37. doi: 10.5252/cryptogamie-mycologie2021v42a3
Shao, S. C., Tian, X. F., and Liu, P. G. (2011). Cantharellus in southwestern China: a new species and a new record. Mycotaxon 116, 437–446. doi: 10.5248/116.437
Shao, S. C., Tian, X. F., and Liu, P. G. (2012). Two species with intercontinental disjunct distribution of the genus Cantharellus. J. Yunnan Agri. Univ. 27, 150–155.
Shen, L. L., Wang, M., Zhou, J. L., Xing, J. H., Cui, B. K., and Dai, Y. C. (2019). Taxonomy and phylogeny of Postia. Multi-gene phylogeny and taxonomy of the brown-rot fungi: Postia (Polyporales, Basidiomycota) and related genera. Persoonia 42, 101–126. doi: 10.3767/persoonia.2019.42.05
Smith, A. H., and Morse, E. E. (1947). The genus Cantharellus in the western United States. Mycologia 39, 497–534. doi: 10.1080/00275514.1947.12017631
Smith, S. A., and Dunn, C. W. (2008). Phyutility: a phyloinformatics tool for trees, alignments andmolecular data. Bioinformation 24, 715–716. doi: 10.1093/bioinformatics/btm619
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihoodbased phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. doi: 10.1093/bioinformatics/btl446
Suhara, H., and Kurogi, S. (2015). Cantharellus cyphelloides (Cantharellales), a new and unusual species from a Japanese evergreen broadleaved forest. Mycol. Prog. 14:55. doi: 10.1007/s11557-015-1079-2
Sun, Y. F., Costa-Rezende, D. H., Xing, J. H., Zhou, J. L., Zhang, B., Gibertoni, T. B., et al. (2020). Multi-gene phylogeny and taxonomy of Amauroderma s. lat. (Ganodermataceae). Persoonia 44, 206–239. doi: 10.3767/persoonia.2020.44.08
Tang, L. P., Hao, Y. J., Cai, Q., Tolgor, B., and Yang, Z. L. (2014). Morphological and molecular evidence for a new species of Rhodotus from tropical and subtropical Yunnan, China. Mycol. Prog. 13, 45–53. doi: 10.1007/s11557-013-0890-x
Thorn, R. G., Kim, J. I., Lebeuf, R., and Voitk, A. (2017). The golden chanterelles of Newfoundland and Labrador: a new species, a new record for North America, and a lost species rediscovered. Botany 95, 547–560. doi: 10.1139/cjb-2016-0213
Tian, R., Liang, Z. Q., Wang, Y., and Zeng, N. K. (2022). Analysis of aromatic components of two edible mushrooms, Phlebopus portentosus and Cantharellus yunnanensis using HS-SPME/GC-MS. Results Chem. 4:100282. doi: 10.1016/j.rechem.2022.100282
Tian, X. F., Buyck, B., Shao, S. C., Liu, P. G., and Fang, Y. (2012). Cantharellus zangii, a new subalpine basidiomycete from southwestern China. Mycotaxon 120, 99–103. doi: 10.5248/120.99
Tian, X. F., Shao, S. C., and Liu, P. G. (2009). Two notable species of the genus Cantharellus Adans (Cantharellales, Basidiomycota) new to China. Edible Fungi China 28, 10–11. doi: 10.13629/j.cnki.53-1054.2009.04.006
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Wei, T. Z., Zhang, X. Q., and Guo, L. D. (2008). Cantharellus cibarius var. squamosus, a new record of China. Mycosystema 27, 627–629. doi: 10.13346/j.mycosystema.2008.04.004
Wu, J. C., and Lu, H. (2006). Development prospect and related measures of wild edible fungi industry in Yunnan. West. For. Sci. 35, 154–158.
Wu, L. L., Liang, Z. Q., Su, M. S., Fan, Y. G., Zhang, P., Jiang, S., et al. (2021). Updated taxonomy of Chinese Phylloporus (Boletaceae, Boletales): six new taxa and four redescribed species. Mycol. Prog. 20, 1243–1273. doi: 10.1007/s11557-021-01722-8
Xie, H. J., Tang, L. P., Mu, M., Fan, Y. G., Jiang, S., Su, M. S., et al. (2022). A contribution to knowledge of Gyroporus (Gyroporaceae, Boletales) in China: three new taxa, two previous species, and one ambiguous taxon. Mycol. Prog. 21, 71–92. doi: 10.1007/s11557-021-01754-0
Yun, W., and Hall, I. R. (2004). Edible ectomycorrhizal mushrooms: challenges and achievements. Can. J. Bot. 82, 1063–1073. doi: 10.1139/b04-051
Zang, M. (1980). Some new species of Basidiomycetes from the Xizang autonomous region of China. Acta. Microbiol. Sin. 20, 29–34.
Zeng, N. K., Liang, Z. Q., Wu, G., Li, Y. C., Yang, Z. L., and Liang, Z. Q. (2016). The genus Retiboletus in China. Mycologia 108, 363–380. doi: 10.3852/15-072
Zeng, N. K., Tang, L. P., Li, Y. C., Tolgor, B., Zhu, X. T., Zhao, Q., et al. (2013). The genus Phylloporus (Boletaceae, Boletales) from China: morphological and multilocus DNA sequence analyses. Fungal Divers. 58, 73–101. doi: 10.1007/s13225-012-0184-7
Zhang, L. F., Yang, J. B., and Yang, Z. L. (2004). Molecular phylogeny of eastern Asian species of Amanita (Agaricales, Basdiomycota): taxonomic and biogeographic implications. Fungal Divers. 17, 219–238.
Zhang, M., Wang, C. Q., Buyck, B., Deng, W. Q., and Li, T. H. (2021). Multigene phylogeny and morphology reveal unexpectedly high number of new species of Cantharellus Subgenus Parvocantharellus (Hydnaceae, Cantharellales) in China. J. Fungi 7:919. doi: 10.3390/jof7110919
Zhang, Y. Z., Liang, Z. Q., Xie, H. J., Wu, L. L., Xue, R., and Zeng, N. K. (2021). Cantharellus macrocarpus (Cantharellaceae, Cantharellales), a new species from tropical China. Phytotaxa 484, 170–180. doi: 10.11646/phytotaxa.484.2.2
Keywords: chanterelle, molecular phylogeny, morphology, new taxa, taxonomy
Citation: Zhang Y-Z, Lin W-F, Buyck B, Liang Z-Q, Su M-S, Chen Z-H, Zhang P, Jiang S, An D-Y and Zeng N-K (2022) Morphological and Phylogenetic Evidences Reveal Four New Species of Cantharellus Subgenus Cantharellus (Hydnaceae, Cantharellales) From China. Front. Microbiol. 13:900329. doi: 10.3389/fmicb.2022.900329
Received: 20 March 2022; Accepted: 28 April 2022;
Published: 27 June 2022.
Edited by:
Baokai Cui, Beijing Forestry University, ChinaReviewed by:
Victor Manuel Bandala, Instituto de Ecología (INECOL), MexicoTolgor Bau, Jilin Agricultural University, China
Junfeng Liang, Chinese Academy of Forestry, China
Copyright © 2022 Zhang, Lin, Buyck, Liang, Su, Chen, Zhang, Jiang, An and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Qun Liang, bGl6aHF1MTk4MEAxMjYuY29t; Nian-Kai Zeng, bmlhbmthaXpAMTYzLmNvbQ==
 Yu-Zhuo Zhang
Yu-Zhuo Zhang Wen-Fei Lin3
Wen-Fei Lin3 Nian-Kai Zeng
Nian-Kai Zeng