
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 12 July 2022
Sec. Evolutionary and Genomic Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.894641
This article is part of the Research Topic Basidiomycete Fungi: From Biosystematics and Biodiversity to Biotechnology View all 21 articles
Auriculariales is a fungal order with highly diverse morphological traits of basidiomes, which partially leads to a poor understanding of its taxonomic system at the generic level. To identify our recently collected specimens of Auriculariales to a species level, we perform a comprehensive phylogenetic analysis of the generic relationships in Auriculariales. In association with morphological characteristics, a new genus Alloexidiopsis belonging to Auriculariaceae is erected with two new species, namely, A. australiensis and A. schistacea. Moreover, Exidiopsis calcea separated from the generic type E. effusa and Heteroradulum niveum and H. yunnanense recently inaccurately described as members of Heteroradulum are recovered in the clade of Alloexidiopsis. These three species are thus transferred to this new genus. One collection of Exidiopsis grisea also falls in the clade of Alloexidiopsis, whereas another collection of this species is separated far from Alloexidiopsis and E. effusa. Since we have no collection to confirm the species identity of E. grisea, its generic position is uncertain. The main taxonomic morphological differences among Alloexidiopsis and related corticioid genera in Auriculariales are summarized. A key to all the five accepted species of Alloexidiopsis is provided. As two unnamed lineages exist in Alloexidiopsis besides the abovementioned five species, it is assumed that more new species will be revealed from this genus under its current circumscription.
Auriculariales is a fungal order being mainly composed of wood-inhabiting macrofungi in Agaricomycetes and Basidiomycota (Hibbett et al., 2007). The type genus of this order is Auricularia, which together with several other gelatinous genera, namely, Exidia, Guepinia, and Pseudohydnum, comprise important edible and medicinal fungi (Wu et al., 2019). Therefore, interest in species diversity in these gelatinous genera has grown significantly in recent years (Bandara et al., 2015; Chen et al., 2020; Shen and Fan, 2020; Ye et al., 2020; Wang and Thorn, 2021; Wu et al., 2021).
Contrary to the gelatinous genera, most species in Auriculariales bear tough, resupinate, and effused to reflexed basidiomes as corticioid and polyporoid fungi (Miettinen et al., 2012; Zhou and Dai, 2013; Malysheva and Spirin, 2017). With the aid of molecular phylogeny, the corticioid species traditionally placed in Eichleriella, Exidiopsis, and Heterochaete according to morphological characters have been rearranged to make genera monophyletic. After the erection of some new genera, e.g., Adustochaete, Amphistereum, Crystallodon, Proterochaete, and Sclerotrema and reinstatement of several previously known genera, e.g., Hirneolina, Heteroradulum, and Tremellochaete (Malysheva and Spirin, 2017), Eichleriella is accepted to be a monophyletic genus, while Exidiopsis and Heterochaete seem to be synonymous with a priority of the latter genus (Malysheva and Spirin, 2017; Alvarenga et al., 2019; Alvarenga and Gibertoni, 2021). However, certain species of Exidiopsis, even sequenced ones such as E. calcea and E. grisea, still have no appropriate placement at the generic level (Malysheva and Spirin, 2017; Li et al., 2022b). In addition, the generic placement of certain recently described species of Heteroradulum is questionable as indicated in a study by Li et al. (2022b) and our understanding of the phylogenies in Guan et al. (2020) and Li et al. (2022a). This phenomenon indicates the generic delimitation in Auriculariales that should be further clarified.
When revisiting specimens collected in the last few years, some of them are identified to be previously known and new species in Auriculariales, but cannot be placed in any known genus. Therefore, a new genus is erected for these species and also for other related species.
Sixteen studied specimens were sampled in northwestern and southwestern China, Vietnam, and Australia from May to November 2017–2020. These specimens were dried using a portable drying instrument at 35°C on the day of sampling and are preserved at the Fungarium, Institute of Microbiology, Chinese Academy of Sciences (HMAS), Beijing, China and the National Herbarium of Victoria (MEL), Melbourne, Australia. Macromorphological characters of basidiomes were examined with the aid of a Leica M125 stereomicroscope (Wetzlar, Germany) at magnifications up to 100 × . Color terms follow Petersen (1996). Microscopic examination was carried out with an Olympus BX43 light microscope (Tokyo, Japan) at magnifications up to 1,000 × following a study by Liu et al. (2021). All the measurements were taken from the sections mounted in cotton blue. The following abbreviations are used: L = mean basidiospore length (arithmetic average of all the basidiospores), W = mean basidiospore width (arithmetic average of all the basidiospores), Q = variation in the L/W ratios between the specimens studied, and n = number of basidiospores measured from a given number of specimens.
The cetyltrimethylammonium bromide (CTAB) plant genome rapid extraction kit (Beijing Demeter Biotech Co., Ltd., Beijing, China) was employed for DNA extraction from dried specimens. The internal transcribed spacer (ITS) and nuclear large subunit (nLSU) gene regions were amplified with the primer pairs ITS5/ITS4 (White et al., 1990) and LR0R/LR7 (Vilgalys and Hester, 1990), respectively. The PCR procedure for ITS was initial denaturation at 95°C for 3 min, followed by 35 cycles at 94°C for 40 s, 54°C for 45 s, 72°C for 1 min, and a final extension of 72°C for 10 min, while that for nLSU was initial denaturation at 94°C for 1 min, followed by 34 cycles at 94°C for 30 s, 50°C for 1 min, 72°C for 1.5 min, and a final extension of 72°C for 10 min. The PCR products were purified and sequenced at the Beijing Genomics Institute (BGI), China. All the newly generated sequences were submitted to GenBank (https://www.ncbi.nlm.nih.gov/genbank/).
The current dataset for phylogenetic analysis included all the main lineages in Auriculariales as ingroup taxa, while Sistotrema brinkmannii was selected as an outgroup taxon following a study by Li et al. (2022b). The ITS and nLSU regions were separately aligned using MAFFT version 7.110 (Katoh and Standley, 2013) with the G-INS-i strategy (Katoh et al., 2005), and then the two resulting alignments were concatenated as a single alignment. The concatenated alignment was submitted to TreeBASE (http://www.treebase.org; accession number S29452). jModelTest 2.1.10 (Guindon and Gascuel, 2003; Darriba et al., 2012) was used to determine the best-fit evolutionary model of the concatenated alignment based on the Akaike information criterion (AIC). Following the resulting model, maximum likelihood (ML) and Bayesian inference (BI) analyses were performed. For the ML analysis, raxmlGUI 2.0 (Stamatakis, 2014; Edler et al., 2021) was used with the calculation of bootstrap (BS) replicates under the auto fiber channel (FC) option (Pattengale et al., 2009). For the BI analysis, MrBayes 3.2 (Ronquist et al., 2012) was used with two independent runs of four chains, and trees were sampled every 1,000th generation. The first 25% of the resulting trees were discarded as burn-in, while the remaining 75% of the resulting trees were used for constructing a 50% majority consensus tree and calculating Bayesian posterior probabilities (BPPs). Chain convergence was determined using Tracer 1.7 (Rambaut et al., 2018). The trees were visualized in FigTree 1.4.4 (Rambaut, 2018) and edited in Adobe Illustrator cc 2020.
A total of 15 ITS and 15 nLSU sequences were newly generated from all the 16 studied specimens (Table 1). The concatenated alignment of ITS and nLSU regions has 117 collections and 1,675 characters. GTR + I + G was estimated as the best-fit evolutionary model for this alignment. The ML analysis ended after 200 BS replicates. The BI analysis converged after 20 million generations, which was indicated by the effective sample sizes of all the parameters above 5,000 and the potential scale reduction factors close to 1.000. The topology resulting from the ML analysis is shown along with BS values of more than 50% and BPPs of more than 0.8 at the nodes (Figure 1).
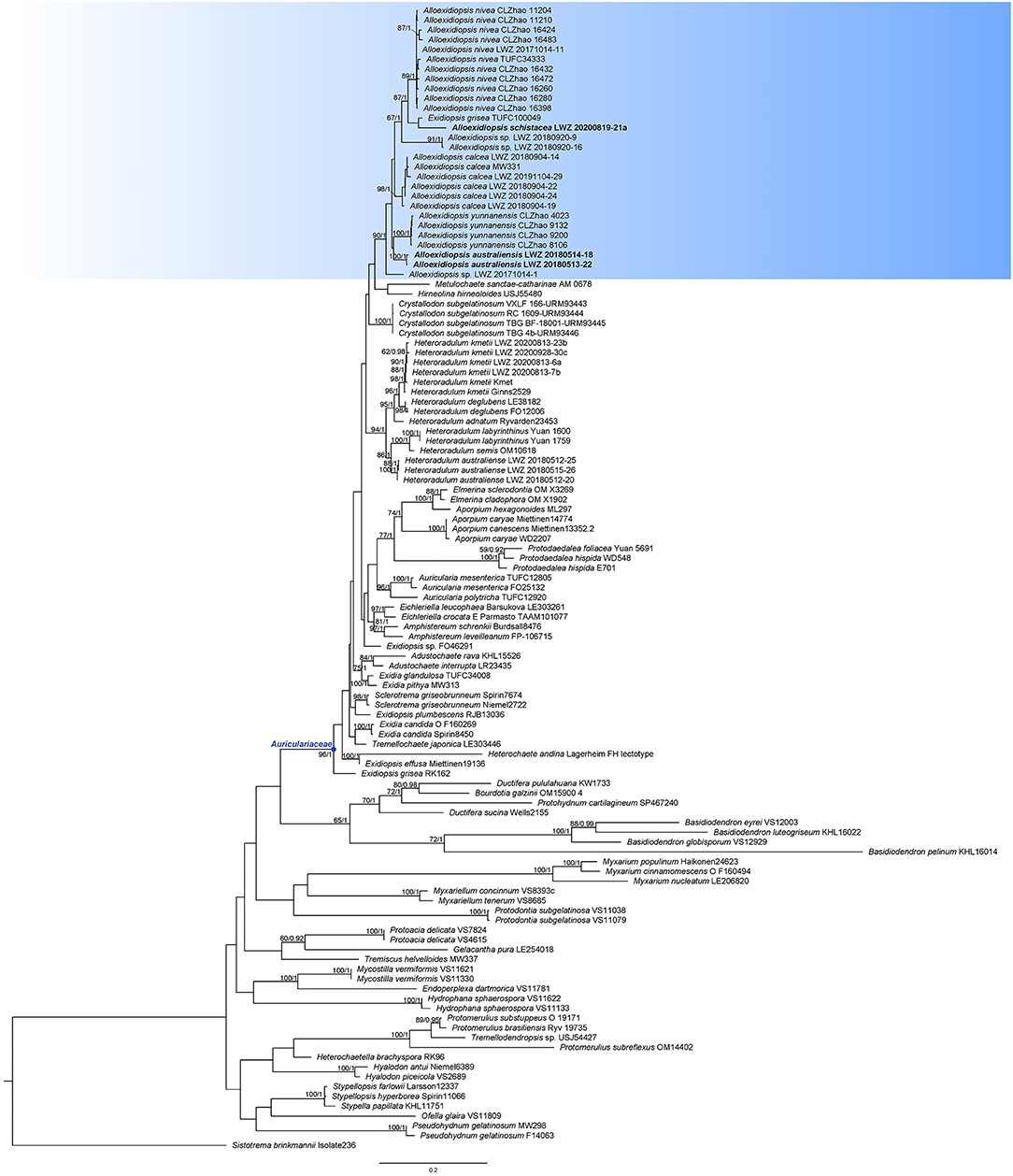
Figure 1. Phylogenetic position of Alloexidiopsis in Auriculariales inferred from the concatenated dataset of internal transcribed spacer (ITS) and nuclear large subunit (nLSU) regions. The topology generated from the maximum likelihood analysis is shown along with bootstrap values and Bayesian posterior probabilities of more than 50% and 0.8, respectively, at the nodes. The new genus Alloexidiopsis is highlighted with the bluish background color, while the specimens of the newly described species are in boldface.
The Auriculariaceae is well recovered (BS = 96%, BPP = 1) by the current phylogeny (Figure 1). In Auriculariaceae, besides four sequences representing Heteroradulum kmetii (LWZ 20200813-6a, LWZ 20200813-7b, LWZ 20200813-23b, and LWZ 20200928-30c), additional newly sequenced specimens (Table 1) grouped with Exidiopsis calcea, one of the collections of “E. grisea” (TUFC 100049), Heteroradulum niveum, and H. yunnanense as a strongly supported clade (BS = 94%, BPP = 1) that is separated from the generic types of Exidiopsis (E. effusa) and Heteroradulum (H. kmetii). This clade is described as a new genus below. In this clade, five of our new sequences turned out to represent E. calcea (LWZ 20180904-14, LWZ 20180904-19, LWZ 20180904-22, LWZ 20180904-24, and LWZ 20191104-29), one belongs to H. niveum (LWZ 20171014-11). The remaining sequences formed four new lineages. The specimens such as LWZ 20171014-1, LWZ 20180920-9, and LWZ 20180920-16 are sterile and thus, the two lineages represented by them are not included in the subsequent taxonomic treatment. The other two lineages, represented by the specimens LWZ 20180513-22 and LWZ 20180514-18 and LWZ 20200819-21a are, respectively, described as two new species in association with morphological examinations. Exidiopsis calcea, H. niveum, and H. yunnanense are transferred to the new genus, while the species identity of “E. grisea” cannot be confirmed and thus, a taxonomic change for this species at the generic level is not proposed.
Alloexidiopsis L.W. Zhou & S.L. Liu, gen. nov.
MycoBank: MB 844125.
Etymology: Alloexidiopsis (Latin), refers to the segregation from Exidiopsis.
Diagnosis: It differs from Exidiopsis in the combination of resupinate, leathery basidiomes and the presence of cystidia and hyphidia.
Type species: Alloexidiopsis schistacea S.L. Liu, Z.Q. Shen & L.W. Zhou (described below).
Type specimen: China: Sichuan, Pingshan County, Laojunshan National Nature Reserve, on the fallen angiosperm trunk, 19 August 2020, LW Zhou, LWZ 20200819-21a (holotype in HMAS).
Description: Basidiomes annual, resupinate, effused, thin, leathery, closely adnate. Hymenophore smooth or with sterile spines, greyish white to ochraceous, cracked or not. Hyphal system monomitic, generative hyphae with clamp connections, hyaline, thin-walled. Cystidia cylindrical to clavate, thin-walled. Hyphidia abundant, covering hymenium, branched, thin-walled. Basidia ellipsoid to ovoid, longitudinally septate, two- to four-celled, hyaline. Basidiospores cylindrical to broadly cylindrical, slightly curved (allantoid), hyaline, thin-walled, smooth, inamyloid, indextrinoid, acyanophilous. On wood.
Notes: Alloexidiopsis is characterized by grayish-white to ochraceous, corticioid basidiomes, a monomitic hyphal system, and the presence of cystidia and hyphidia. Besides Exidiopsis as indicated in diagnosis, this new genus is also close to Crystallodon and Heteroradulum in morphology. However, Crystallodon differs in the presence of hyphal pegs surrounded by crystals (Alvarenga and Gibertoni, 2021), while Heteroradulum has brightly colored (pinkish or reddish) basidiomes and a mono- or dimitic hyphal system with thick-walled generative hyphae (Malysheva and Spirin, 2017; Li et al., 2022b). The main taxonomic morphological differences among Alloexidiopsis and related corticioid genera in Auriculariales are summarized in Table 2.
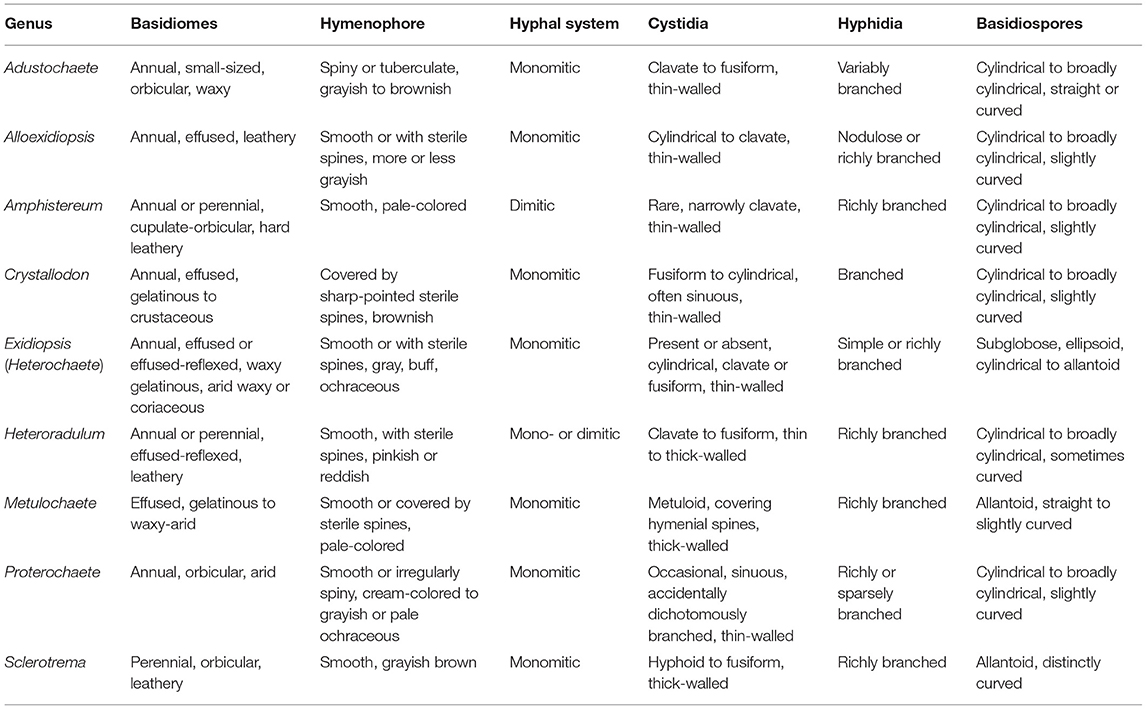
Table 2. Morphological comparison among Alloexidiopsis and related corticioid genera in Auriculariales.
Alloexidiopsis australiensis S.L. Liu, Z.Q. Shen & L.W. Zhou, sp. nov. (Figures 2A,B, 3).
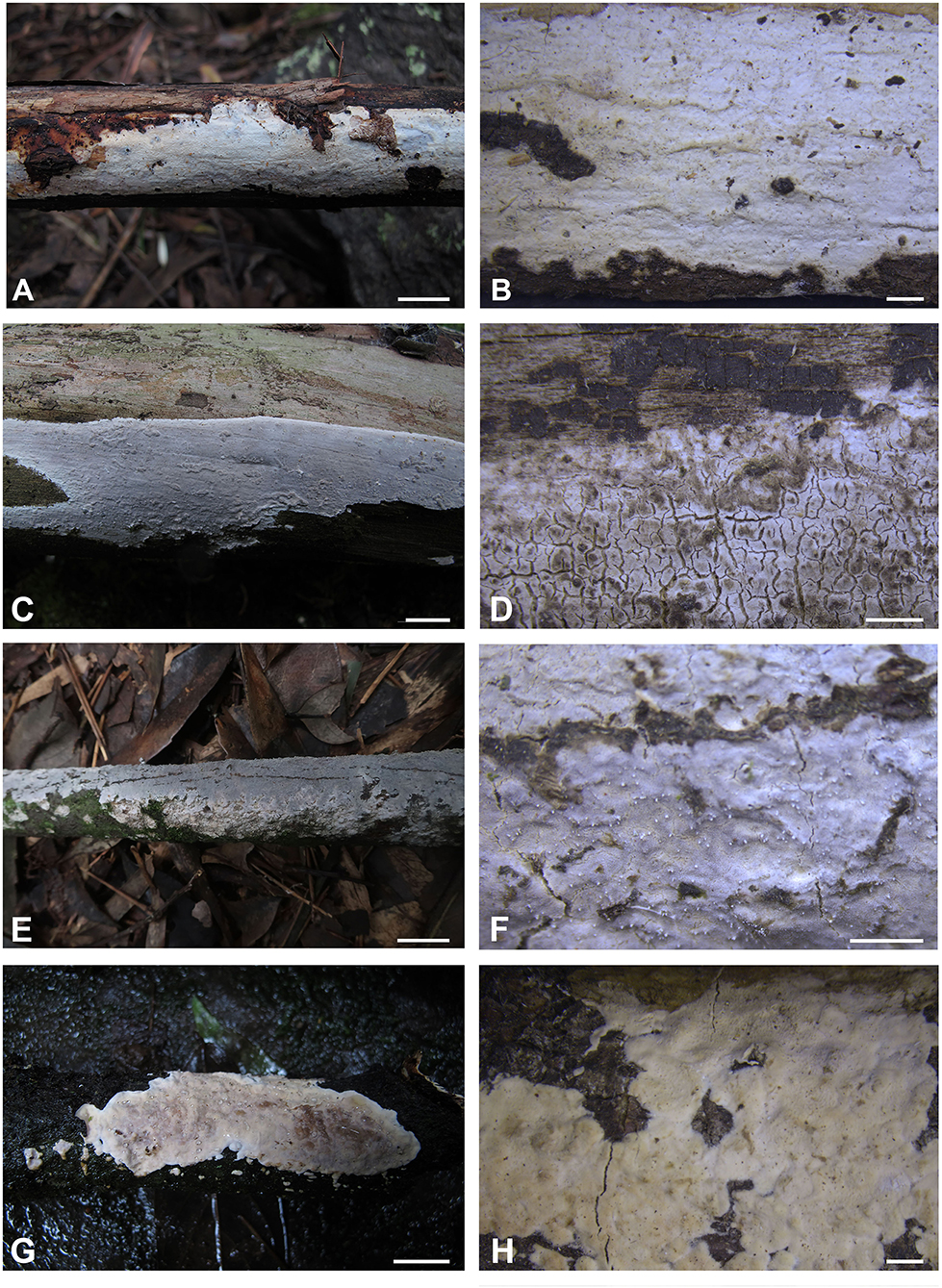
Figure 2. Basidiomes of Alloexidiopsis. (A,B) A. australiensis (LWZ 20180513-22, holotype). (C,D) A. calcea (LWZ 20180904-24). (E,F) A. schistacea (LWZ 20200819-21a, holotype). (G,H) A. sp. (LWZ 20180920-16). Scale bars: (A,C,E,G) = 1 cm, (B,D,F,H) = 2 mm.
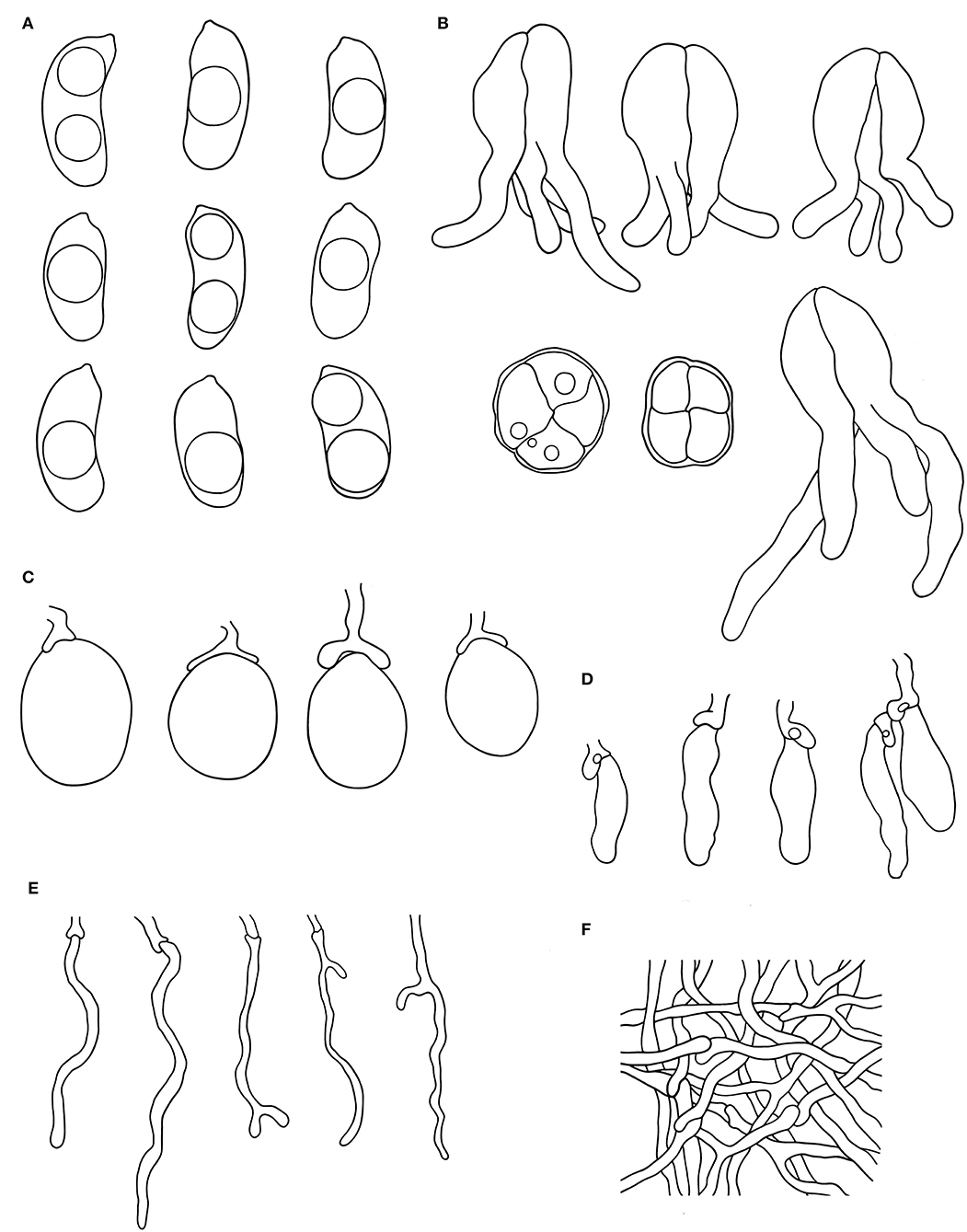
Figure 3. Microscopic structures of Alloexidiopsis australiensis (drawn from the holotype). (A) Basidiospores. (B) Basidia. (C) Basidioles. (D) Cystidia. (E) Hyphidia. (F) Hyphae from subiculum. Scale bars = 10 μm.
MycoBank: MB 844126.
Etymology: australiensis (Latin), refers to Australia.
Diagnosis: It is characterized by smooth, cream hymenophore.
Type: Australia: Tasmania, Hobart, and Mount Wellington, on the fallen angiosperm branch, 13 May 2018, LW Zhou, LWZ 20180513-22 (holotype in MEL, isotype in HMAS).
Description: Basidiomes annual, resupinate, membranaceous, becoming leathery upon drying, closely adnate, widely effused, up to 12 cm long, 2 cm wide, 100–200 μm thick. Hymenophore smooth, cream to pale orange when fresh, becoming white upon drying. Margin gradually thinning out, thin, concolorous with or slightly darker than subiculum.
Hyphal system monomitic; generative hyphae with clamp connections. Subiculum composed of crystal clusters and agglutinated hyphae; subicular hyphae hyaline, thin-walled, frequently branched, closely interwoven, 1–2 μm in diam. Cystidia cylindrical with an obtuse apex, ventricose, 21.5–24.5 × 9.5–12 μm, with a clamp connection at base. Hyphidia arising from hyphae, nodulose or richly branched, hyaline, thin-walled, 22–33 × 1–2 μm. Basidia ellipsoid to ovoid, longitudinally septate, four-celled, embedded, 18–21 μ 13–18 μm, occasionally with a short base stalk, with a clamp connection at base. Basidiospores cylindrical to broadly cylindrical, slightly curved (allantoid), hyaline, thin-walled, smooth, acyanophilous, inamyloid, indextrinoid, with oily inclusions, (12−)13–25(−25.5) × (6.5−)7–11(−12) μm, L = 20.0 μm, W = 9.0 μm, Q = 2.3 (n = 60/2).
Other specimens (paratype) are also examined: Australia: Timbs Track, on dead standing angiosperm, 14 May 2018, LW Zhou, LWZ 20180514-18 (HMAS).
Notes: Alloexidiopsis australiensis resembles A. calcea and A. nivea (both transferred below) by smooth hymenophore in Alloexidiopsis. However, A. calcea differs in grayish-white to ochraceous hymenophore when fresh and has a distribution in the Northern Hemisphere (Wells, 1961), while A. nivea differs in smaller basidiospores (6.5–13.5 × 2.7–5.5 μm; Li et al., 2022a). Exidiopsis macrospora is similar to A. australiensis by the leathery basidiomes and the presence of cystidia and hyphidia; however, it differs in the reflexed basidiomes when dry and smaller basidiospores (10–15 μm × 5–7.5 μm; Wells, 1961).
Alloexidiopsis calcea (Pers.) L.W. Zhou & S.L. Liu, comb. nov. (Figures 2C,D).
MycoBank: MB 844128.
Basionym: Thelephora calcea Pers., Syn. meth. fung. (Göttingen) 2:581 (1801).
≡ Auricularia calcea (Pers.) Mérat, Nouv. Fl. Environs Paris, Edn 2 1:35 (1821).
≡ Corticium calceum (Pers.) Fr., Epicr. syst. mycol. (Upsaliae): 562 (1838) (1836–1838).
≡ Terana calcea (Pers.) Kuntze, Revis. gen. pl. (Leipzig) 2:872 (1891).
≡ Sebacina calcea (Pers.) Bres., Fung. trident. 2(11–13):64 (1892).
≡ Exidiopsis calcea (Pers.) K. Wells, Mycologia 53(4):348 (1962) (1961).
Notes: Alloexidiopsis calcea has been successively placed in several genera. Before the current study, its latest generic placement was Exidiopsis, which is accepted by the first and also the only comprehensively phylogenetic analyses of Auriculariales (Weiß and Oberwinkler, 2001). The phylogeny in Malysheva and Spirin (2017) recognized that Exidiopsis calcea was separated from the generic type E. effusa, but no taxonomic change was proposed may be due to a lack of specimens for careful morphological examinations. Here, five additional specimens were collected from Northwest and Southwest China grouped with E. calcea represented by the German collection of molecular weight (MW) 331 (BS = 94%, BPP = 1; Figure 1). Moreover, the morphological characters of these Chinese specimens are consistent with the description of E. calcea (Wells, 1961). Taking E. calcea falling within the clade of the newly erected genus into consideration together, this species is transferred to Alloexidiopsis.
Alloexidiopsis nivea (J.J. Li & C.L. Zhao) L.W. Zhou & S.L. Liu, comb. nov.
MycoBank: MB 844129.
Basionym: Heteroradulum niveum J.J. Li & C.L. Zhao, in Li, Zhao, and Liu, Diversity 14 (1, no. 40):5 (2022).
Notes: Alloexidiopsis nivea was recently described as a member of Heteroradulum (Li et al., 2022a). When the independence of this species was phylogenetically supported, its relationship with additional species of Heteroradulum, however, failed to receive reliable statistical support in the original phylogeny with a sampling on Auriculariaceae (Figure 1 in Li et al., 2022a). Although the original phylogeny with a narrower sampling focusing mainly on Heteroradulum did not reject the close relationship of H. niveum with other species of Heteroradulum, the practice for this phylogenetic analysis (lack of additional in-group taxa for reference) cannot accurately determine the monophyly of Heteroradulum and, thus, the phylogenetic position of H. niveum (Figure 2 in Li et al., 2022a). Including a broader sampling of reference sequences, the current phylogeny unambiguously recovers this species in the newly erected genus Alloexidiopsis (Figure 1), so we formally propose the transfer here.
Alloexidiopsis schistacea L.W. Zhou & S.L. Liu, sp. nov. (Figures 2E,F, 4).
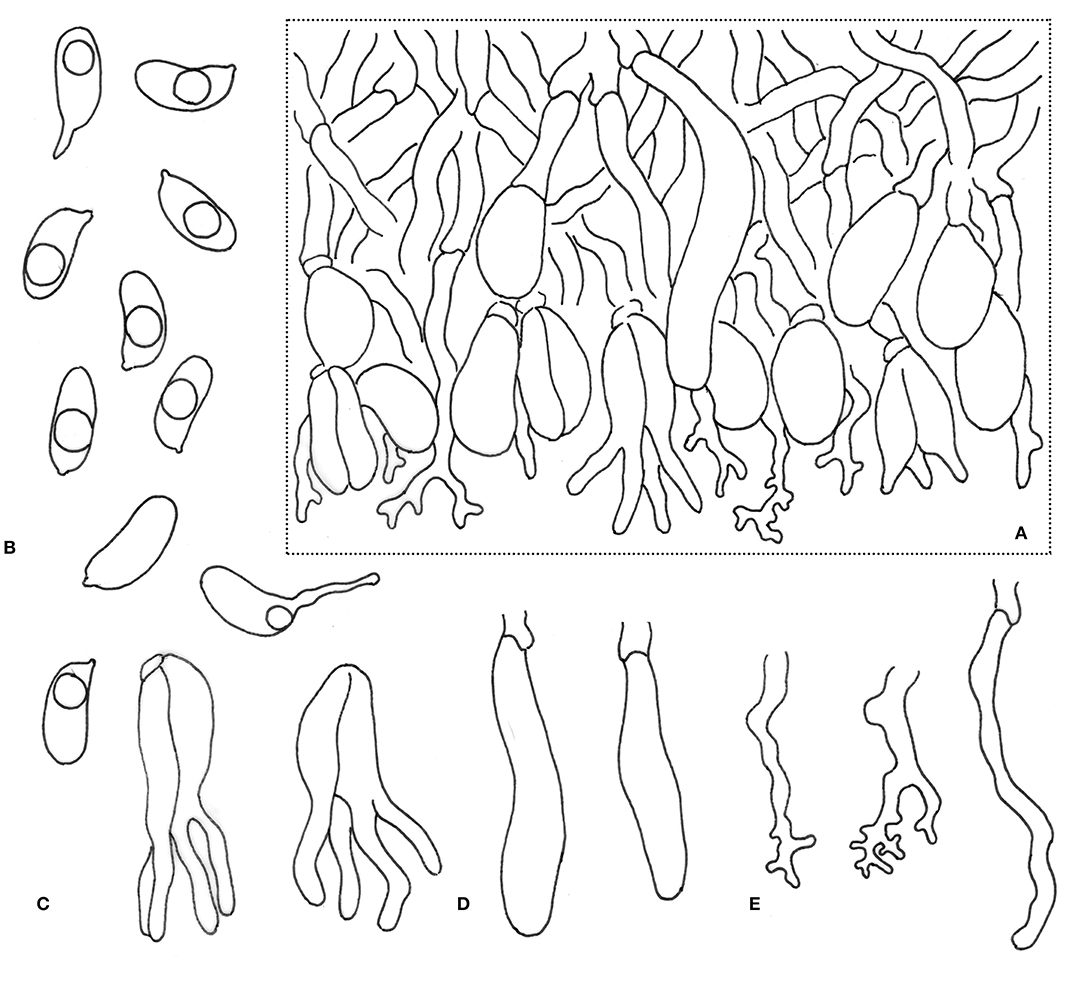
Figure 4. Microscopic structures of Alloexidiopsis schistacea (drawn from the holotype). (A) A section of hymenium. (B) Basidiospores. (C) Basidia. (D) Cystidia. (E) Hyphidia. Scale bars = 10 μm.
MycoBank: MB 844127.
Etymology: schistacea (Latin), refers to the slate-like color (grayish) of hymenophore.
Diagnosis: Characterized by grayish hymenophore with small tubercles.
Type: China: Sichuan, Pingshan County, Laojunshan National Nature Reserve, on the fallen angiosperm trunk, 19 Aug 2020, LW Zhou, LWZ 20200819-21a (holotype in HMAS).
Description: Basidiomes annual, resupinate, membranaceous, becoming leathery upon drying, closely adnate, widely effused, up to 15 cm long, 2.5 cm wide, about 200 μm thick. Hymenophore smooth, covered by regularly arranged sterile spines, greyish when fresh. Margin gradually thinning out, thin, concolorous with or slightly darker than subiculum.
Hyphal system monomitic; generative hyphae with clamp connections. Subiculum composed of crystal clusters and agglutinated hyphae; subicular hyphae hyaline, thin-walled, frequently branched, closely interwoven, 2–3 μm in diam. Cystidia cylindrical with an obtuse apex, 25–50 × 4–6 μm, with a clamp connection at base. Hyphidia arising from hyphae, nodulose or branched, hyaline, thin-walled, 20–40 × 1.5–3 μm. Basidia ellipsoid to ovoid, longitudinally septate, four-celled, embedded, 15–20 × 7–10 μm. Basidiospores cylindrical to broadly cylindrical, slightly curved (allantoid), hyaline, thin-walled, smooth, acyanophilous, inamyloid, indextrinoid, with oily inclusions, (8.5−)9.5–11(−12.5) × (4.3−)4.5–5.5 μm, L = 10.4 μm, W = 5.0 μm, Q = 2.1 (n = 30/1).
Notes: Alloexidiopsis schistacea resembles Alloexidiopsis yunnanensis (transferred below) by grayish, grandinioid to odontioid hymenophore; however, the latter species differs in two- to three-celled basidia and larger basidiospores (17–24 μm × 5–8 μm; Guan et al., 2020). Micromorphologically, Exidiopsis badia and E. umbrina resemble A. schistacea by the presence of cystidia and hyphidia; however, these two species produce gelatinous, but not leathery basidiomes (Roberts, 2003). Moreover, E. badia has larger basidiospores than A. schistacea (13–15 μm × 5.5–6 μm; Roberts, 2003). Although only one collection is available for A. schistacea, its distinct morphological characters and phylogenetic position make the large enough basidiomes suitable to be described as a new species.
Alloexidiopsis yunnanensis (C.L. Zhao) L.W. Zhou & S.L. Liu, comb. nov.
MycoBank: MB 844130.
Basionym: Heteroradulum yunnanense C.L. Zhao (as “yunnanensis”), in Guan, Liu, Zhao and Zhao, Phytotaxa 437(2):57 (2020).
Notes: Alloexidiopsis yunnanensis was originally described in Yunnan, China as a member of Heteroradulum (Guan et al., 2020). However, the generic placement of this species is inaccurate as indicated in a study by Li et al. (2022b), who, thus, excluded it from Heteroradulum and left its generic position open. The current phylogeny recovers this species in the newly erected genus Alloexidiopsis (Figure 1), so we formally propose the taxonomic transfer here.
1. Hymenophore smooth…………………………………2
2. Hymenophore grandinioid to odontioid…………………4
3. Basidiospores less than 7 μm wide…………………A. nivea
4. Basidiospores more than 7 μm wide……………………3
5. Hymenophore greyish white to ochraceous when fresh; in the Northern Hemisphere……………………………A. calcea
6. Hymenophore cream to pale orange when fresh; in the Southern Hemisphere………………………A. australiensis
7. Basidiospores more than 14 μm long………A. yunnanensis
8. Basidiospores less than 14 μm long……………A. schistacea
In this study, we further revise the generic delimitation of corticioid fungi in Auriculariales based on previous studies (Malysheva and Spirin, 2017; Li et al., 2022b). A new genus Alloexidiopsis is erected for two new species, namely, A. australiensis and A. schistacea, a new combination from Exidiopsis as A. calcea and two new combinations from Heteroradulum as A. nivea and A. yunnanensis. A key to all the five species currently accepted in Alloexidiopsis is provided.
Besides the five accepted species, two unnamed distinct lineages are recovered in Alloexidiopsis (Figures 1, 2G,H). The poor growth stage of these specimens restricts accurate morphological examinations, so no taxonomic treatment is proposed for them. However, this phylogeny indicates that the species diversity in Alloexidiopsis could be higher. Systematic field trips for collections of Alloexidiopsis and comprehensive taxonomic studies will result in more new members of Alloexidiopsis.
After the transfer of Exidiopsis calcea to Alloexidiopsis, Exidiopsis is closer to being a monophyletic genus. A sample “E. grisea” (TUFC100049) also falls in the clade of Alloexidiopsis, whereas another collection of this species (RK 162) is separated far from Alloexidiopsis as a basal lineage of Auriculariaceae (Figure 1). We have neither collection for morphological examinations and, thus, cannot challenge the taxonomic determinations given. Moreover, the texture of E. grisea is waxy gelatinous (Wells, 1961), which makes this species distinguished from all the members of Alloexidiopsis. Consequently, it is premature to change the taxonomic position of E. grisea at this stage.
It is noteworthy that the same research group separately described two new species of Heteroradulum, viz., H. niveum and H. yunnanensis quite recently (Guan et al., 2020; Li et al., 2022a). However, the generic placement of these two species is inaccurate and thus, they are transferred to the new genus Alloexidiopsis. Even if the inaccurate placement has mainly resulted from the practice of phylogenetic analyses, this phenomenon also indicates that the taxonomic system of Auriculariales is poorly established. It has not been tried to do so since the publication of Weiß and Oberwinkler (2001) 20 years ago, which even leaves the monophyly of Auriculariales unconfirmed. A multilocus-based phylogeny with a wider sampling of various morphological groups in Auriculariales is urgently needed to achieve a more natural classification of this order, as in other orders within Agaricomycetes (Wang et al., 2021).
The data presented in the study can be found in the GenBank (https://www.ncbi.nlm.nih.gov/GenBank; accession numbers: OM801918-OM801947) and TreeBASE (http://www.treebase.org; accession number: S29452) repositories.
S-LL, Z-QS, X-YL, and L-WZ made morphological examinations. S-LL and Q-ZL performed phylogenetic analyses. L-WZ conceived and supervised the study. S-LL, Z-QS, and L-WZ wrote the manuscript. All authors have approved the final version of the manuscript.
This study was financed by the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment, China (Project No. 2019HJ2096001006) and the National Natural Science Foundation of China (Project Nos. 31970012 and 32100004).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Drs. Tom W. May (MEL, Australia) and Genevieve Gates (Tasmanian Institute of Agriculture, Australia) are thanked for kindly arranging the L-WZ's field trip to Australia.
Alvarenga, R. L. M., and Gibertoni, T. B. (2021). Crystallodon Alvarenga gen. nov., a new genus of the Auriculariales from the Neotropics. Cryptogamie Mycol. 42, 17–24. doi: 10.5252/cryptogamie-mycologie2021v42a2
Alvarenga, R. L. M., Spirin, V., Malysheva, V., Gibertoni, T. B., and Larsson, K. H. (2019). Two new genera and six other novelties in Heterochaete sensu lato (Auriculariales, Basidiomycota). Botany 97, 439–451. doi: 10.1139/cjb-2019-0046
Bandara, A. R., Chen, J., Karunarathna, S., Hyde, K. D., and Kakumyan, P. (2015). Auricularia thailandica sp. nov. (Auriculariaceae, Auriculariales) a widely distributed species from Southeastern Asia. Phytotaxa 208, 147–156. doi: 10.11646/phytotaxa.208.2.3
Chen, Y. L., Su, M. S., Zhang, L. P., Zou, Q., Wu, F., Zeng, N. K., et al. (2020). Pseudohydnum brunneiceps (Auriculariales, Basidiomycota), a new species from Central China. Phytotaxa 441, 87–94. doi: 10.11646/phytotaxa.441.1.8
Darriba, D., Taboada, G. L., Doallo, R., and Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772. doi: 10.1038/nmeth.2109
Edler, D., Klein, J., Antonelli, A., and Silvestro, D. (2021). raxmlGUI 2.0 beta: a graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 12, 373–377. doi: 10.1111/2041-210X.13512
Guan, Q. X., Liu, C. M., Zhao, T. J., and Zhao, C. L. (2020). Heteroradulum yunnanensis sp. nov. (Auriculariales, Basidiomycota) evidenced by morphological characters and phylogenetic analyses in China. Phytotaxa 437, 51–59. doi: 10.11646/phytotaxa.437.2.1
Guindon, S., and Gascuel, O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. System. Biol. 52, 696–704. doi: 10.1080/10635150390235520
Hibbett, D. S., Binder, M., Bischoff, J. F., Blackwell, M., Cannon, P. F., Eriksson, O. E., et al. (2007). A higher-level phylogenetic classification of the Fungi. Mycol. Res. 111, 509–547. doi: 10.1016/j.mycres.2007.03.004
Katoh, K., Kuma, K., Toh, H., and Miyata, T. (2005). MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucl. Acids Res. 33, 511–518. doi: 10.1093/nar/gki198
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Li, J. J., Zhao, C. L., and Liu, C. M. (2022a). qThe morphological characteristics and phylogenetic analyses revealed an additional taxon in Heteroradulum (Auriculariales). Diversity 14. doi: 10.3390/d14010040
Li, Q. Z., Liu, S. L., Wang, X. W., May, T. W., and Zhou, L. W. (2022b). Redelimitation of Heteroradulum (Auriculariales, Basidiomycota) with H. australiense sp. nov. MycoKeys 87–101. doi: 10.3897/mycokeys.86.76425
Liu, S. L., He, S. H., Liu, D. M., and Zhou, L. W. (2021). Two new species of Fibrodontia (Trechisporales, Basidiomycota) with a key to worldwide species. J. Fungi 7. doi: 10.3390/jof7110982
Malysheva, V., and Spirin, V. (2017). Taxonomy and phylogeny of the Auriculariales (Agaricomycetes, Basidiomycota) with stereoid basidiocarps. Fungal Biology 121, 689–715. doi: 10.1016/j.funbio.2017.05.001
Miettinen, O., Spirin, V., and Niemel,ä, T. (2012). Notes on the genus Aporpium (Auriculariales, Basidiomycota), with a new species from temperate Europe. Ann. Botanici Fennici. 49, 359–368. doi: 10.5735/085.049.0607
Pattengale, N. D., Alipour, M., Bininda-Emonds, O. R. P., Moret, B. M. E., and Stamatakis, A. (2009). How many bootstrap replicates are necessary? J. Comput. Biol. 17:337–354. doi: 10.1089/cmb.2009.0179
Petersen, J. H. (1996). Farvekort. The Danish Mycological Society's colour-chart. Greve: Foreningen til Svampekundskabens Fremme.
Rambaut, A. (2018). FigTree, a graphical viewer of phylogenetic trees. Available online at: http://tree.bio.ed.ac.uk/software/figtree/ (accessed November 11, 2018).
Rambaut, A., Drummond, A. J., Xie, D, Baele, G, and Suchard, M.A. (2018). Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 67, 901–904. doi: 10.1093/sysbio/syy032
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Hohna, S., et al. (2012). MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. System. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Shen, Y., and Fan, L. (2020). Morphological and molecular analyses reveal two new species of Guepinia from China. Phytotaxa 475, 91–101. doi: 10.11646/phytotaxa.475.2.3
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Wang, S., and Thorn, R. G. (2021). Exidia qinghaiensis, a new species from China. Mycoscience 62, 212–216. doi: 10.47371/mycosci.2021.03.002
Wang, X. W., May, T. W., Liu, S. L., and Zhou, L. W. (2021). Towards a natural classification of Hyphodontia sensu lato and the trait evolution of basidiocarps within Hymenochaetales (Basidiomycota). J. Fungi 7, 478. doi: 10.3390/jof7060478
Weiß, M., and Oberwinkler, F. (2001). Phylogenetic relationships in Auriculariales and related groups–hypotheses derived from nuclear ribosomal DNA sequences. Mycol. Res. 105, 403–415. doi: 10.1017/S095375620100363X
Wells, K. (1961). Studies of some Tremellaceae. IV. Exidiopsis. Mycologia 53, 317–370. doi: 10.1080/00275514.1961.12017967
White, T. J., Bruns, T. D., Lee, S., and Taylor, J. (1990). Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics in PCR Protocols, a Guide to Methods and Applications. Cambridge, MA: Academic Press. p. 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wu, F., Tohtirjap, A., Fan, L. F., Zhou, L. W., Alvarenga, R. L. M., Gibertoni, T. B., et al. (2021). Global diversity and updated phylogeny of Auricularia (Auriculariales, Basidiomycota). J. Fungi 7, 933. doi: 10.3390/jof7110933
Wu, F., Zhou, L. W., Yang, Z. L., Bau, T., Li, T. H., and Dai, Y. C. (2019). Resource diversity of Chinese macrofungi: edible, medicinal and poisonous species. Fungal Diversity 98, 1–76. doi: 10.1007/s13225-019-00432-7
Ye, S. Y., Zhang, Y. B., Wu, F., and Liu, H. X. (2020). Multi-locus phylogeny reveals two new species of Exidia (Auriculariales, Basidiomycota) from China. Mycol. Progress 19, 859–868. doi: 10.1007/s11557-020-01601-8
Keywords: Agaricomycetes, Auriculariaceae, Exidiopsis, Heteroradulum, wood-inhabiting fungi, six new taxa
Citation: Liu S-L, Shen Z-Q, Li Q-Z, Liu X-Y and Zhou L-W (2022) Alloexidiopsis gen. nov., A Revision of Generic Delimitation in Auriculariales (Basidiomycota). Front. Microbiol. 13:894641. doi: 10.3389/fmicb.2022.894641
Received: 12 March 2022; Accepted: 09 June 2022;
Published: 12 July 2022.
Edited by:
Masoomeh Ghobad-Nejhad, Iranian Research Organization for Science and Technology, IranReviewed by:
Samantha Chandranath Karunarathna, Qujing Normal University, ChinaCopyright © 2022 Liu, Shen, Li, Liu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Wei Zhou, bGl3ZWlfemhvdTE5ODJAaW0uYWMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.