- 1Department of Mycology, Pasteur Institute of Iran, Tehran, Iran
- 2Department of Biotechnology, Faculty of Life Sciences and Biotechnology, Shahid Beheshti University, Tehran, Iran
- 3Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Fungal co-infections are frequent in patients with coronavirus disease 2019 (COVID-19) and can affect patient outcomes and hamper therapeutic efforts. Nonetheless, few studies have investigated fungal co-infections in this population. This study was performed to assess the rate of fungal co-infection in patients with COVID-19 as a systematic review. EMBASE, MEDLINE, and Web of Science were searched considering broad-based search criteria associated with COVID-19 and fungal co-infection. We included case reports and case series studies, published in the English language from January 1, 2020 to November 30, 2021, that reported clinical features, diagnosis, and outcomes of fungal co-infection in patients with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Totally, 54 case reports and 17 case series were identified, and 181 patients (132 men, 47 women, and 2 not mentioned) co-infected with COVID-19 and fungal infection enrolled. The frequency of fungal co-infection among patients with COVID-19 was 49.7, 23.2, 19.8, 6.6, and 0.5% in Asia, America, Europe, Africa, and Australia, respectively. Diabetes (59.6%) and hypertension (35.9%) were found as the most considered comorbidities in COVID-19 patients with fungal infections. These patients mainly suffered from fever (40.8%), cough (30.3%), and dyspnea (23.7%). The most frequent findings in the laboratory results of patients and increase in C-reactive protein (CRP) (33.1%) and ferritin (18.2%), and lymphopenia (16%) were reported. The most common etiological agents of fungal infections were Aspergillus spp., Mucor spp., Rhizopus spp., and Candida spp. reported in study patients. The mortality rate was 54.6%, and the rate of discharged patients was 45.3%. Remdesivir and voriconazole were the most commonly used antiviral and antifungal agents for the treatment of patients. The global prevalence of COVID-19-related deaths is 6.6%. Our results showed that 54.6% of COVID-19 patients with fungal co-infections died. Thus, this study indicated that fungal co-infection and COVID-19 could increase mortality. Targeted policies should be considered to address this raised risk in the current pandemic. In addition, fungal infections are sometimes diagnosed late in patients with COVID-19, and the severity of the disease worsens, especially in patients with underlying conditions. Therefore, patients with fungal infections should be screened regularly during the COVID-19 pandemic to prevent the spread of the COVID-19 patients with fungal co-infection.
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19) that started as a local epidemic but evolved within a few months into a worldwide pandemic with high morbidity and mortality rates, and the World Health Organization declared it as a global epidemic on January 30, 2020 (Dos Santos et al., 2020; Gorbalenya et al., 2020). The prognosis of this disease is severe in patients with underlying conditions. Diabetes, hypertension, cancer, chronic kidney disease, heart failure, and mental disorders increased mortality. However, success in developing specific therapeutic against COVID-19 infection is still needed (Robinson et al., 2022). Therefore, the most effective way to deal with an epidemic is to prevent further infection. The elevated prevalence of mortality and infection in patients with COVID-19 can be due to natural immunity and replication of the virus in the lower respiratory tract, and also due to superinfections and secondary infections, resulting in severe lung damage as well as acute respiratory distress syndrome (ARDS) (Zheng et al., 2003; Farhan et al., 2021). Patients with COVID-19 are found with co-infections with respiratory viruses, bacteria, fungi, and secondary infections that have been identified as a fatal predictor. From the outbreak of COVID-19, we found that fungal co-infection of patients with COVID-19 could significantly increase mortality rates (Yang S. et al., 2021). The significance of fungal co-infection in patients with COVID-19, however, especially in patients with severe and critical conditions, is still poorly understood (Yang et al., 2020). Invasive fungal infections, including aspergillosis and candidiasis, are frequent in hospitalized patients (Sadeghi et al., 2018; Jamzivar et al., 2019; Hughes et al., 2020; Nasir et al., 2020). Acute respiratory diseases, such as invasive pulmonary aspergillosis (IPA), are common in intensive care units (ICUs) and immunocompromised patients (Prattes et al., 2021). Fungal infections, before or after COVID-19, are capable of complicating COVID-19 diagnosis, treatment, and progression (Talento and Hoenigl, 2020). According to data obtained from other COVID-19 outbreaks [severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS)], invasive aspergillosis and also other systemic fungal infections play a role in severe outcomes of patients in ICUs (Song et al., 2020). In addition, patients with COVID-19 with predisposing factors (mechanical ventilation, diabetes, and cytokine storm) were found with a dramatic increase in the incidence of opportunistic fungal infections (Silva et al., 2020; Song et al., 2020). In contrast, because of the complicated medical situations of the patients with COVID-19 and the improper collection of the clinical species, many fungal infections in these patients are misidentified (Silva et al., 2020). Researchers are facing several challenges in the diagnosis and identification of fungal infections. In this systematic review, we reviewed the case reports and case series with patients with COVID-19 presenting fungal co-infections to evaluate the various aspects such as symptoms, diagnosis, and the most frequent etiological agents of patients with fungal co-infecting COVID-19, treatment, and outcome.
Materials and Methods
Search Strategy
A comprehensive systematic literature search was conducted by reviewing original research papers published in Medline, Web of Science, and Embase databases. The following keywords were used for the search: “coronavirus,” “coronavirus infections,” “HCoV,” “nCoV,” “Covid,” “SARS,” “COVID-19,” “nCoV19,” “SARS-CoV-2,” “SARS coronavirus 2,” “2019 novel corona virus,” “Human,” “pneumonia,” “SARS,” “co-infection,” “Superinfection,” “fungus,” “mycosis,” “co-infect,” “secondary infection,” “mixed infection,” “Fungal infection,” “aspergillosis,” “CAPA,” and “upper respiratory” alone or in combination with “OR” and/or “AND.” The search included English language studies from January 1, 2020 to November 30, 2021. Then, articles were kept if the title and abstract contained discussion about bacterial, fungal, and/or respiratory viral co-infection in patients with SARS-CoV-2. The systematic review was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) instructions (Moher et al., 2010).
Ethical Statement
As this study was a systematic review, it did not require any ethics committee approval.
Inclusion and Exclusion Criteria
All case reports/case series that were about the fungal infection among patients with COVID-19 in English were evaluated. They included adequate data for analysis, namely, country of origin, the number of patients with COVID-19, and the number of cases with fungal infections, fungal species/group, clinical signs, laboratory results, diagnostic techniques, outcomes, and treatment.
The following exclusion criteria were used: (1) only animal studies, (2) research on fungal infections only, (3) research on patients with COVID-19 only, (4) review articles, (5) meeting or congress abstracts, (6) editorials, (7) letters, (8) languages other than English, (9) meta-analyses or systematic reviews, (10) articles available only in abstract, and (11) duplicate studies.
Study Selection and Data Extraction
The obtained studies were merged, followed by removing the duplicates using EndNote X7 (Thomson Reuters, United States). Two authors (PB and MG) separately screened the studies according to their titles and abstracts, considering the exclusion and inclusion criteria of this study. The full texts were analyzed by a third author (SS). Data extracted included the first author’s last name, research type, publication year, country, number of patients with COVID-19, number of cases with fungal confection, co-infecting fungi, clinical symptoms, laboratory findings and outcomes, diagnostic methods, and treatment. The data were obtained by two independent individuals and validated by another investigator.
Quality Assessment
Quality assessment was performed by a checklist provided by the Joanna Briggs Institute (JBI).
Results
Characteristics of the Selected Studies
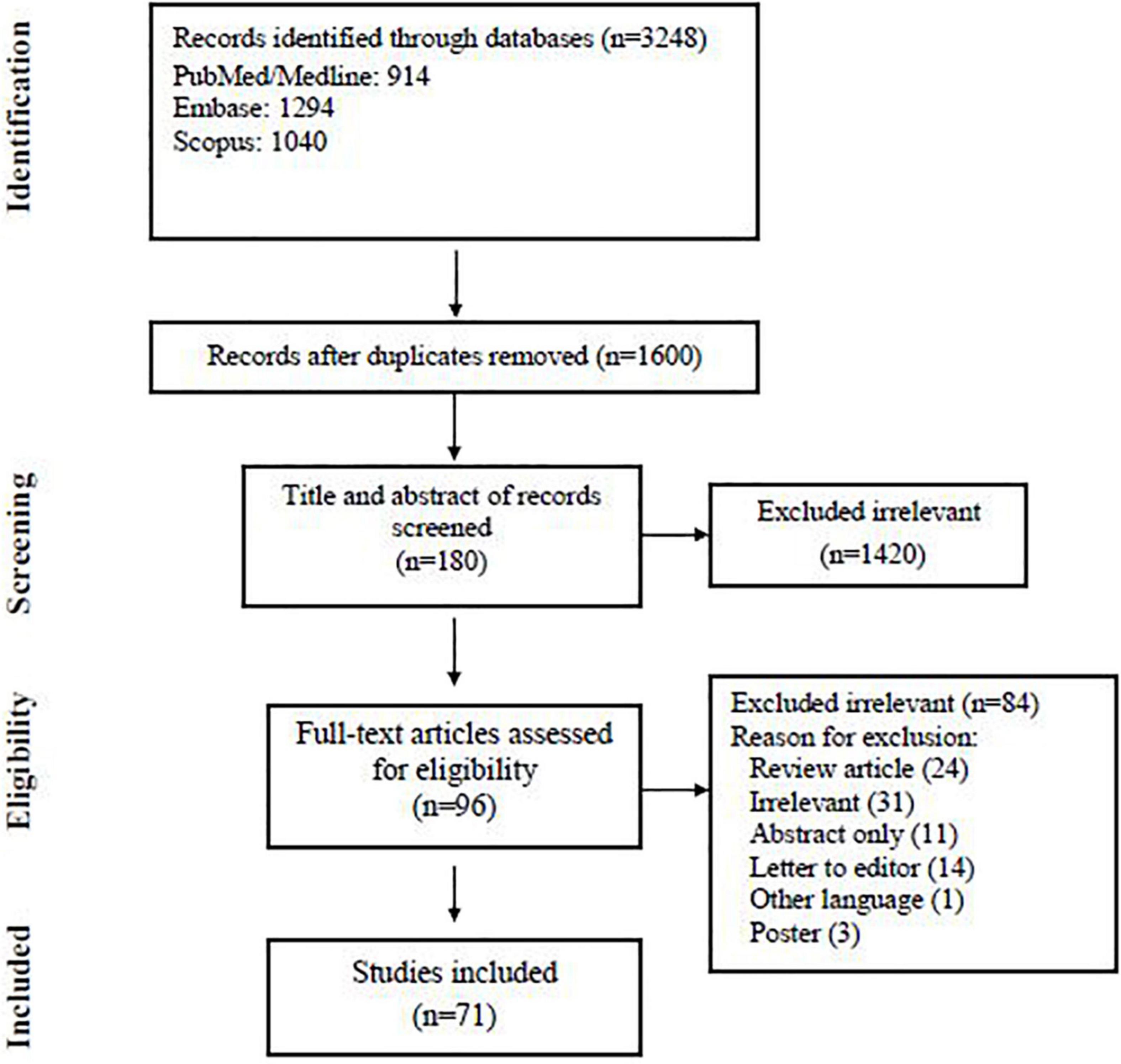
Our search yielded 3,248 records from three databases; we excluded 1,648 duplicates and screened 1,600 articles. At the abstract and title review stage, we excluded 1,420 articles, leaving 180 articles for full-text review. After reviewing the full text of 180 studies, eventually, 71 articles met the inclusion criteria and were subjected to the final assessment (Figure 1). Table 1 summarizes the characteristics of published data related to fungal co-infection in patients with COVID-19.
The Frequency of Fungal Infections Among Patients With COVID-19
The characteristics of the 71 included articles are shown in Table 2. Fifty-four case reports and seventeen case series highlighted fungal co-infection in 60 and 121 patients with COVID-19, respectively. Conforming to the results of these studies, 181 patients with fungal infections had been declared among 188 patients with COVID-19 from 23 countries (Table 2). Based on the data in this table, most of the patients in this study were reported from India (68 patients), United States (19 patients), Brazil (18 patients), and Spain/Egypt (12 patients for each), respectively. Among the cases with defined gender, 47 cases with fungal infections were women and 132 were men. The rate of co-infection in the age group of less than 50 years and more than 50 years was 23.7 and 66.2%, respectively. Table 3 shows more details of the subgroup analysis of the studies.
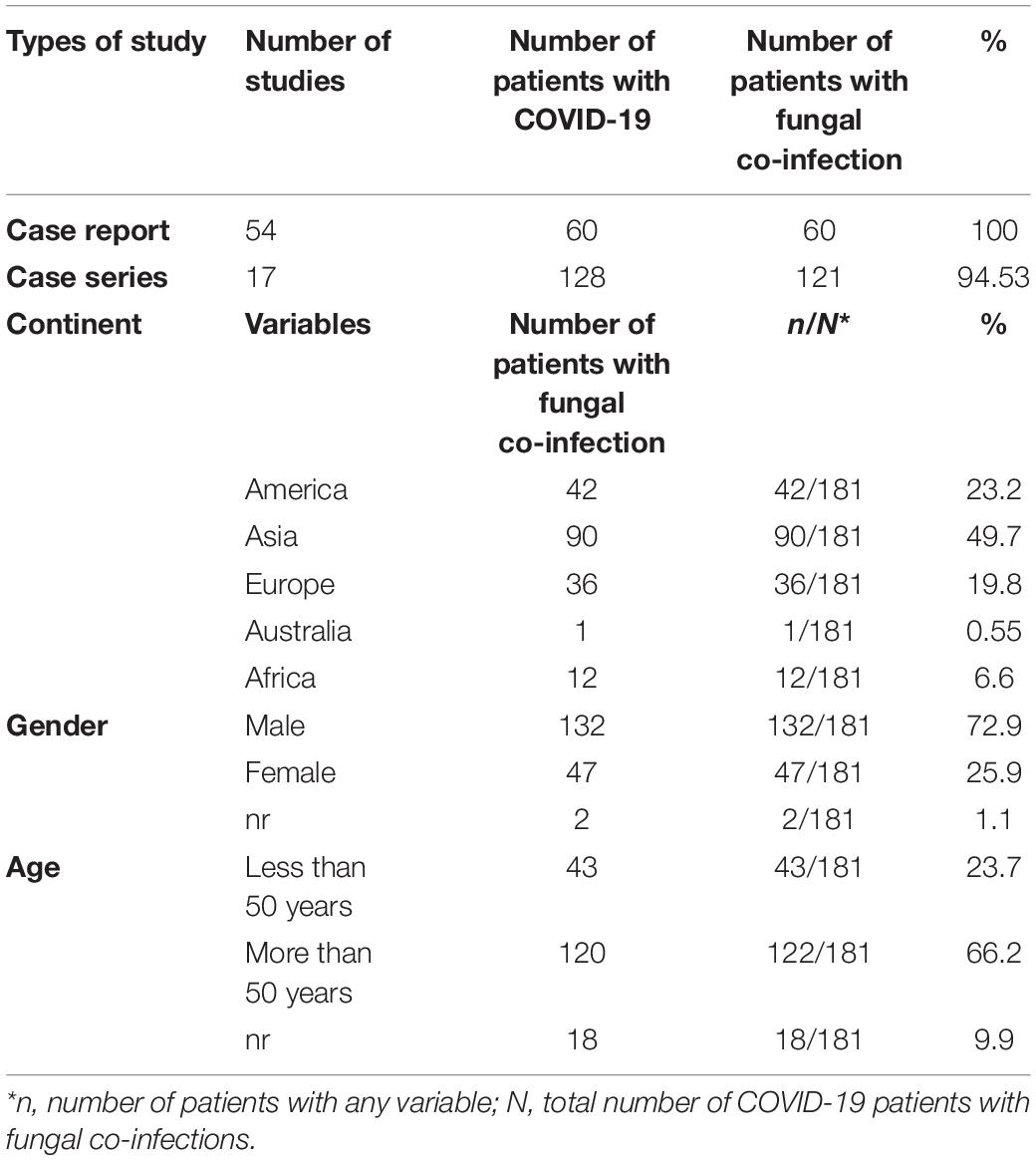
Table 2. Frequency of fungal co-infection among patients with COVID-19 based on different subgroups.
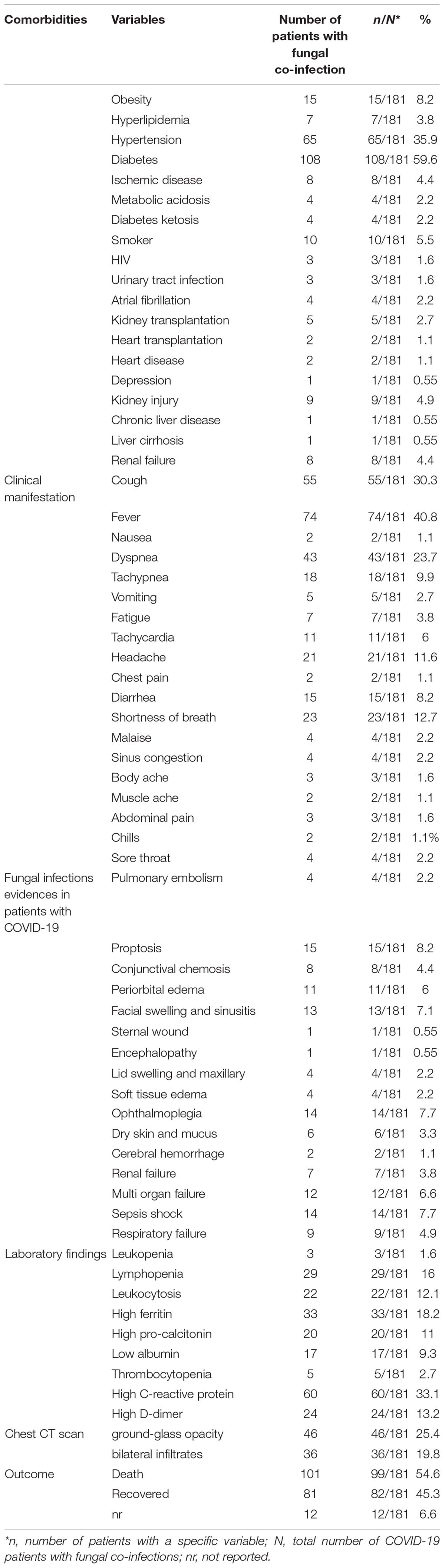
Among 19 types of comorbidities, diabetes (59.6%), hypertension (35.9%), and obesity (8.2%) were the commonest comorbidities. Fever (40.8%), cough (30.3%), dyspnea (23.7%), and shortness of breath (12.7%) were the commonest clinical symptoms in COVID-19 patients with fungal infections. Laboratory assessment of patients indicated that elevated C-reactive protein (CRP) (>100 mg/L) (33.1%), high ferritin (>500 ng/mL) (18.2%), lymphopenia (<800 cells/μl) (16%), leukocytosis, and increased D-dimer (>1,000 ng/ml) (13.2%) were the most common findings (Table 3).
Computerized tomography (CT) scan has been reported in studies as a diagnostic method employed for COVID-19, and its findings are as follows: ground-glass opacification (25.4%) and bilateral infiltrates (19.8%). The CT results in the majority of the assessed patients were ground-glass opacification. We also considered the patients’ outcomes, and of 181 patients (mentioned in Table 2), 81 improved, 101 died, and in 12 patients, the outcome was unknown (Table 3).
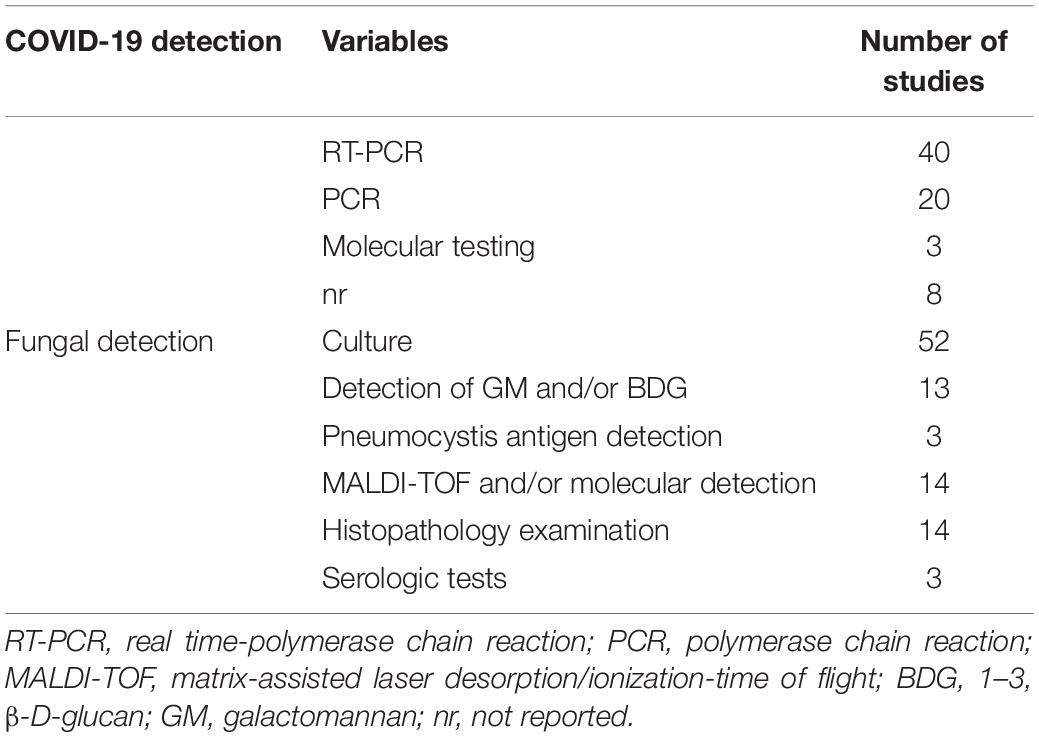
According to the results of this study (Table 4), RT-PCR was the most common laboratory technique for the detection of SARS-CoV-2 in the study patients (43 articles). The most frequently used laboratory techniques for co-fungal detection within studies included 52 that used culture, 13 that used galactomannan (GM) and/or 1,3 β-D-glucan (BDG) detection test, 14 that used histopathology examination, and 14 that used matrix-assisted laser desorption ionization time of flight (MALDI-TOF) and/or molecular detection.
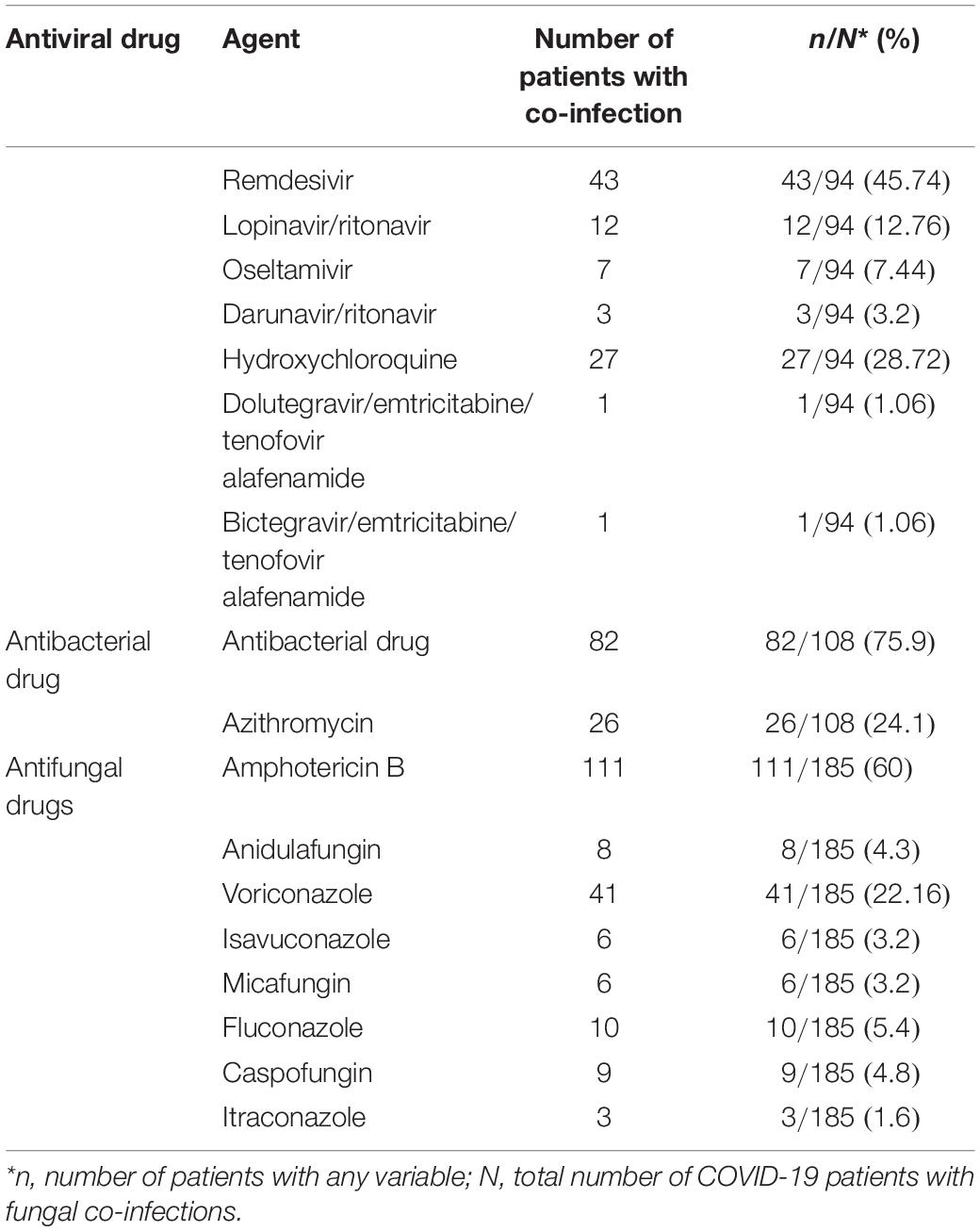
From the fungal co-infections registered, the most common etiological agents were as follows: Aspergillus spp. (82 isolates), Mucor spp. (69 isolates), Rhizopus spp. (24 isolates), Candida spp. (21 isolates), Pneumocystis jirovecii (four isolates), Saccharomyces cerevisiae (three isolates), Coccidioides spp. and Cryptococcus neoformans (two for each), Trichosporon asahii (six isolates), and Histoplasma capsulatum and Lichteimia (Absidia) (one for each) were infections in patients with fungal-COVID-19 (Table 5). In the study articles, the drugs applied to treat COVID-19 patients with fungal infections were characterized into three categories, namely, antibacterial, antiviral, and antifungal drugs (Table 6). Remdesivir (45.74%) and lopinavir/ritonavir (12%) were the most common antiviral drugs used. Among the antifungal drugs reported in Table 6, amphotericin B (50%) and voriconazole (22.16%) were widely used as an antifungal agent. Among the antifungal drugs reported in Table 6, amphotericin B (50%) and voriconazole (22.16%) were the most widely used antifungal agents for treating patients.
Discussion
This systematic review is a detailed description of fungal co-infections in patients with COVID-19. There is a special concern for fungal infections, before or after COVID-19 exposure, which leads to treatment failure and deterioration of disease and imposes high healthcare costs on patients and hospitals. Overall, it is well established that all genders and ages are at risk for COVID-19 infection (Kalantari et al., 2020; Song et al., 2020; Talento and Hoenigl, 2020).
In this systematic review, we analyzed 181 fungal patients with COVID-19 from 23 countries, and co-infection in the age group of over 50 years was higher than under 50 years (66.2 vs. 23.7%) which is in agreement with studies that exhibited elderly patients have a higher risk of COVID-19 infection and mortality. Our data are in concordance with a study conducted in the United Kingdom on co-infection patients with COVID-19 symptoms, which reported that the highest prevalence of co-infection patients was in the age group of 55–81 years (Hughes et al., 2020). In this connection, Senok et al. (2021) found that the mean age of patients with co-infections was 49.3 ± 12.5 years in the United Arab Emirates. These observations indicated that declined immune system ability and increasing comorbid conditions with age could be a rational justification for the observed increased infection in older patients. The patients’ gender was assessed in 71 studies that indicated COVID-19 infection in men (72.9%) was higher than that of women (25.9%). A research performed by Senok et al. (2021) on patients hospitalized with COVID-19 in the United Arab Emirates notified that the most cases (84.2%) were men. Garcia-Vidal et al. (2021) found that the majority of patients hospitalized with COVID-19 in Spain were in the age of 62 years and also the most cases (55.8%) were men. In a single-center experiment performed by Jin et al. (2020), in China, out of 43 patients with COVID-19, 51.2% were found to be men. As a finding, it can be inferred that sex hormones and X chromosomes as factors involved in innate and adaptive immunity may have an important role in less susceptibility to COVID-19 infection among women. Overall, the high occurrence of many diseases in men compared to women could likely indicate a shorter life expectancy in this sex. Consequently, gender would be considered a risk factor for higher morbidity and severity in patients with COVID-19.
The disease pattern of COVID-19 can range from mild to life-threatening pneumonia associated with bacterial and fungal co-infections (Mehta and Pandey, 2020). Due to the associated comorbidities [e.g., diabetes mellitus, hypertension, and chronic obstructive pulmonary disease (COPD)] and immunocompromised conditions, these patients are prone to develop severe opportunistic infections. The findings of this study indicated that diabetes, hypertension, and obesity were the most common comorbidities reported in patients with fungal co-infections and COVID-19. This result is in line with the results of Abdalla et al. (2020) which indicated that diabetes, hepatitis B, and hypertension are the common comorbidities in patients with COVID-19-associated pulmonary aspergillosis. Other reports showed that in a patient with diabetes and leukemia, Aspergillus fumigatus was isolated from BAL (Dallalzadeh et al., 2021). Published data have indicated that obesity is a risk factor for infection with COVID-19 (Albashir, 2020; Yang J. et al., 2021). Based on the evidence, the relationship between inflammation and hypertension is well documented. Patients with inflammatory responses increase the disease’s severity and complications, which make the infection worse. In line with our report, Mirzaei et al. (2021) in their review reported diabetes, obesity, and COPD as the most common underlying diseases in patients with COVID-19. Underlying factors could lead to the deterioration of the disease and make the scenario worse. However, the impact of comorbidities on COVID-19 must be carefully considered.
In this analysis, patients had various symptoms but fever, cough, dyspnea, diarrhea, and shortness of breath were the most common clinical symptoms among patients with fungal co-infections and COVID-19. So far, similar results have been reported in this context (Singhal, 2020; Team, 2020). One study of 53 cases of HIV co-infection with COVID-19 indicated that fever, cough, and respiratory and gastrointestinal problems were the most common clinical symptoms reported in patients with SARS-CoV-2-HIV co-infection (Patel et al., 2021). In another study performed by Galván Casas et al. (2020) in Spain, the most common clinical symptoms among patients with COVID-19 were found to be fever, cough, pneumonia, vomiting, diarrhea, headache, nausea, and dyspnea.
As stated in the literature, concurrent involvement of various microorganisms in patients with COVID-19 is a serious threat, especially in patients with underlying diseases, which can lead to exacerbation of complications and subsequently increase the mortality rate. Infection with this virus is related to immune dysregulation, overexpression of pro-inflammatory cytokines, impaired cell-mediated immunity, and decreased CD4 and CD8+ T-cells that can increase the risk of invasive fungal infections (Hughes et al., 2020; Rawson et al., 2020; Farhan et al., 2021). However, there is scarce information regarding fungal co-infections and COVID-19. Therefore, adequate information is required on the simultaneous infection in patients with COVID-19 in adopting more appropriate treatment regimens for these patients. As it is well documented, patients with COVID-19 are at a greater risk of developing fungal infections because of its effect on the immune system and because treatments for COVID-19 can weaken the body’s defenses against fungi (Pemán et al., 2020; Rawson et al., 2020). According to the evidence, the number of reports of fungal co-infections in patients with COVID-19 was steadily growing worldwide. Awareness of the possibility of fungal co-infection with COVID-19 is essential to reduce delays in diagnosis and treatment in order to help prevent severe illness and death from these infections. In this analysis, infection with Aspergillus spp., Mucor spp., Rhizopus spp., Candida spp., and P. jirovecii was the most recorded fungal co-infections in patients with COVID-19. Similar findings of the main fungal co-infections in patients with COVID-19, such as Aspergillus, were also reported by studies conducted in China and Spain (Pemán et al., 2020; Song et al., 2020). In other study performed by Hoenigl (2020) and Garcia-Vidal et al. (2021), the most fungal infections in patients with COVID-19 include aspergillosis, invasive candidiasis, and mucormycosis. A study conducted by Chen et al. (2020) indicated a high prevalence of opportunistic fungal pathogens, such as Aspergillus spp., Candida glabrata, and Candida albicans, in patients with COVID-19. In this connection, Peng et al. (2021) in their systematic review and meta-analysis noted a 0.12 pooled proportion of fungal co-infection in patients with COVID-19. In a recent meta-analysis of eighteen studies, Rawson et al. (2020) reported that 8% of patients with COVID-19 had bacterial/fungal co-infection. The findings of this study indicated that the most COVID-19-associated mucormycosis is found in India. A study conducted in 2021 found that more than 47,000 cases of COVID-19-associated mucormycosis were reported in just 3 months in India (Muthu et al., 2021). Uncontrolled diabetes and overuse of steroids for COVID-19 treatment are important risk factors.
Geological differences have influenced the occurrences of fungal co-infection. Based on this meta-analysis, the frequency of fungal co-infection in patients with COVID-19 was higher in Asia than in other continents. Peng et al. (2021) reported that the fungal co-infection rate was significantly higher in patients from Asia than non-Asian patients.
The use of proper diagnostic techniques is an important issue in the management of COVID-19 diseases. CT scan is considered a relatively high sensitive method for diagnosing cases of COVID-19. This diagnostic method can be a useful factor for diagnosis and assessment of the infection progression in patients with COVID-19. However, the aforementioned technique may not find the involvement of the lung in the first stages of the disease and may not reliably confirm COVID-19 in the patients. According to the CT scan findings obtained from case reports and case series research, ground-glass opacification and bilateral infiltrates were reported as the predominant features in patients with fungal co-infections and COVID-19. This finding was similar to the findings of Radpour et al. (2020) and Omidi et al. (2021).
Diagnosing fungal co-infections in patients with COVID-19 is a serious challenge for clinicians, and it requires detection by a comprehensive diagnostic test for the achievement of an effective treatment. According to the analysis performed in this study, culture was the most common diagnostic method for the presence of fungal infections. As presented in the current analysis, the frequency of fungi in research using non-molecular assays is higher than in studies using molecular assays. As specified by Song et al. (2020), laboratory tests, including direct microscopic, culture, histopathology, BDG, real-time PCR, PCR, and MALDI-TOF techniques, can be used for the detection of fungal co-infections in patients with COVID-19. Since this laboratory evidence can alert us related to the severity of the disease, therefore, it is important to use these methods in combination for the diagnosis of fungal co-infections in these patients.
This study has some limitations. Since only case reports and case series studies have been selected for this review, they are more likely to be biased than other studies. Case studies and case series are descriptive and describe the patient’s signs and symptoms. The prevalence and percentage of co-infection in them have not been studied. For this reason, it was not possible to perform meta-analysis calculations in this review. Therefore, the prevalence of fungal infections among patients with COVID-19 has not been calculated.
Conclusion
There have been many reported cases of viral, fungal, and bacterial infections associated with COVID-19. In this study, we studied the association between fungal infections and COVID-19. We discussed the clinical characteristics, diagnosis, treatment, and mortality rate of patients with COVID-19 co-infected with fungal infections. Sometimes the diagnosis of fungal infections occurs later in patients with COVID-19, which causes the progression and severity of the disease. Both diseases have similar risk factors, such as old age, diabetes, immunodeficiency, HIV, and COPD. Finally, a regular program is recommended to detect fungal infections during the outbreak of COVID-19 and follow it up continuously to prevent the occurrence of these two diseases simultaneously.
Author Contributions
SS, MR-A, and MG designed the study. PB, MG, and MN performed the search, study selection, and data synthesis. SS and MG wrote the first draft of the manuscript. MN, MR-A, and SS revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by a research grant from the Research Deputy of Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant No. 30923). The funding agency had no role in the design of the project, work execution, analyses, interpretation of the data, and manuscript writing and submission as well.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdalla, S., Almaslamani, M. A., Hashim, S. M., Ibrahim, A. S., and Omrani, A. S. (2020). Fatal coronavirus disease 2019-associated pulmonary aspergillosis; a report of two cases and review of the literature. IDCases 22:e00935. doi: 10.1016/j.idcr.2020.e00935
Al Osta, S., Atwi, G., El Ahmar, N., Bejjani, N., Abillama, F., and Matli, K. (2021). Coronavirus disease (COVID-19) associated rhinocerebral mucormycosis and complications: a case report. Int. J. Clin. Res. 2, 93–99. doi: 10.38179/ijcr.v2i1.102
Albashir, A. A. D. (2020). The potential impacts of obesity on COVID-19. Clin. Med. 20:e109. doi: 10.7861/clinmed.2020-0239
Aldaas, M. B., Goldsmith, D., and Arnold, F. W. (2021). COVID-19-associated pulmonary aspergillosis: a case report from the COVID-19 surveillance program. Univ. Louisville J. Respir. Infect. 5:31. doi: 10.18297/jri/vol5/iss1/31
Alekseyev, K., Didenko, L., and Chaudhry, B. (2021). Rhinocerebral mucormycosis and COVID-19 pneumonia. J. Med. Case Rep. 12, 85–89. doi: 10.14740/jmc3637
Ali, G. A., Husain, A., Salah, H., and Goravey, W. (2021). Trichosporon asahii fungemia and COVID-19 co-infection: an emerging fungal pathogen; case report and review of the literature. IDCases 25:e01244. doi: 10.1016/j.idcr.2021.e01244
Almeida, J. N. D., Francisco, E. C., Hagen, F., Brandão, I. B., Pereira, F. M., Presta Dias, P. H., et al. (2021). Emergence of Candida auris in Brazil in a COVID-19 intensive care unit. J. Fungi 7:220. doi: 10.3390/jof7030220
Alobaid, K., Yousuf, B., Al-Qattan, E., Muqeem, Z., and Al-Subaie, N. (2021). Pulmonary aspergillosis in two COVID-19 patients from Kuwait. Access. Microbiol. 3:000201. doi: 10.1099/acmi.0.000201
Antinori, S., Rech, R., Galimberti, L., Castelli, A., Angeli, E., Fossali, T., et al. (2020). Invasive pulmonary aspergillosis complicating SARS-CoV-2 pneumonia: a diagnostic challenge. Travel. Med. Infect. Dis. 38:101752. doi: 10.1016/j.tmaid.2020.101752
Arana, C., Cuevas Ramírez, R. E., Xipell, M., Casals, J., Moreno, A., Herrera, S., et al. (2021). Mucormycosis associated with covid19 in two kidney transplant patients. Transpl. Infect. Dis. 23:e13652. doi: 10.1111/tid.13652
Ashour, M. M., Abdelaziz, T. T., Ashour, D. M., Askoura, A., Saleh, M. I., and Mahmoud, M. S. (2021). Imaging spectrum of acute invasive fungal rhino-orbital-cerebral sinusitis in COVID-19 patients: a case series and a review of literature. J. Neuroradiol. 48, 319–324. doi: 10.1016/j.neurad.2021.05.007
Basso, R. P., Poester, V. R., Benelli, J. L., Stevens, D. A., Zogbi, H. E., Vasconcellos, I. C. D. S., et al. (2021). COVID-19-associated histoplasmosis in an AIDS patient. Mycopathologia 186, 109–112. doi: 10.1007/s11046-020-00505-1
Benedetti, M. F., Alava, K. H., Sagardia, J., Cadena, R. C., Laplume, D., Capece, P., et al. (2021). COVID-19 associated pulmonary aspergillosis in ICU patients: report of five cases from Argentina. Med. Mycol. Case Rep. 31, 24–28. doi: 10.1016/j.mmcr.2020.11.003
Bowalekar, C. S., Yadav, R. R., and Sharma, N. (2021). Fungal sinusitis post COVID 19 – a case series from a diagnostic microbiology laboratory. Indian J. Med. Microbiol. 8, 249–255. doi: 10.18231/j.ijmr.2021.051
Chang, C. C., Senining, R., Kim, J., and Goyal, R. (2020). An acute pulmonary coccidioidomycosis coinfection in a patient presenting with multifocal pneumonia with COVID-19. J. Investig. Med. High Impact Case Rep. 8:2324709620972244. doi: 10.1177/2324709620972244
Chaudhary, D., Behera, A., and Sharma, N. (2022). Renal mucormycosis following COVID-19 treatment with immune modulators: a case report. Front. Emerg. Med. 6:e12. doi: 10.18502/fem.v6i1.7684
Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y., et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395, 507–513. doi: 10.1016/S0140-6736(20)30211-7
Costache, C., Botan, A., Mihaila, R. M., Colosi, I. A., Buksa, S. B., and Chiorescu, R. M. (2021). Mixed etiology COVID-19 associated pulmonary aspergillosis (CAPA)—a case report and brief review of the literature. J. Fungi 7:877. doi: 10.3390/jof7100877
Dallalzadeh, L. O., Ozzello, D. J., Liu, C. Y., Kikkawa, D. O., and Korn, B. S. (2021). Secondary infection with rhino-orbital cerebral mucormycosis associated with COVID-19. Orbit [Epub ahead of print]. doi: 10.1080/01676830.2021.1903044
De Francesco, M. A., Alberici, F., Bossini, N., Scolari, F., Pascucci, F., Tomasoni, G., et al. (2020). Pneumocystis jirevocii and SARS-CoV-2 co-infection: a common feature in transplant recipients? Vaccines 8:544. doi: 10.3390/vaccines8030544
Do Monte Junior, E. S., Dos Santos, M. E. L., Ribeiro, I. B., de Oliveira Luz, G., Baba, E. R., Hirsch, B. S., et al. (2020). Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: a case report. Clin. Endosc. 53, 746–749. doi: 10.5946/ce.2020.180
Dos Santos, J. A., Normando, A. G. C., da Silva, R. L. C., De Paula, R. M., Cembranel, A. C., Santos-Silva, A. R., et al. (2020). Oral mucosal lesions in a COVID-19 patient: new signs or secondary manifestations? Int. J. Infect. Dis. 97, 326–328. doi: 10.1016/j.ijid.2020.06.012
Falces-Romero, I., Ruiz-Bastián, M., Díaz-Pollán, B., Maseda, E., García-Rodríguez, J., and Sars-CoV-2 Working Group. (2020). Isolation of Aspergillus spp. in respiratory samples of patients with COVID-19 in a Spanish tertiary care hospital. Mycoses 63, 1144–1148. doi: 10.1111/myc.13155
Farhan, C., Sohail, M. U., Abdelhafez, I., Salman, S., Attique, Z., Kamareddine, L., et al. (2021). SARS-CoV-2 and immune-microbiome interactions: lessons from respiratory viral infections. Int. J. Infect. Dis. 105, 540–550. doi: 10.1016/j.ijid.2021.02.071
Fernandez, N. B., Caceres, D. H., Beer, K. D., Irrazabal, C., Delgado, G., Farias, L., et al. (2021). Ventilator-associated pneumonia involving Aspergillus flavus in a patient with coronavirus disease 2019 (COVID-19) from Argentina. Med. Mycol. Case Rep. 31, 19–23. doi: 10.1016/j.mmcr.2020.07.001
Flikweert, A. W., Grootenboers, M. J., Yick, D. C., du Mée, A. W., van der Meer, N. J., Rettig, T. C., et al. (2020). Late histopathologic characteristics of critically ill COVID-19 patients: different phenotypes without evidence of invasive aspergillosis, a case series. J. Crit. Care 59, 149–155. doi: 10.1016/j.jcrc.2020.07.002
Galván Casas, C., Catala, A., Carretero Hernández, G., Rodríguez−Jiménez, P., Fernández−Nieto, D., Rodríguez−Villa Lario, A., et al. (2020). Classification of the cutaneous manifestations of COVID−19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 183, 71–77. doi: 10.1111/bjd.19163
Garcia-Vidal, C., Sanjuan, G., Moreno-García, E., Puerta-Alcalde, P., Garcia-Pouton, N., Chumbita, M., et al. (2021). Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin. Microbiol. Infect. 27, 83–88. doi: 10.1016/j.cmi.2020.07.041
Ghelfenstein-Ferreira, T., Saade, A., Alanio, A., Bretagne, S., de Castro, R. A., Hamane, S., et al. (2021). Recovery of a triazole-resistant Aspergillus fumigatus in respiratory specimen of COVID-19 patient in ICU–A case report. Med. Mycol. Case Rep. 31, 15–18. doi: 10.1016/j.mmcr.2020.06.006
Gorbalenya, A. E., Baker, S. C., Baric, R., Groot, R. J. D., Drosten, C., Gulyaeva, A. A., et al. (2020). Severe acute respiratory syndrome-related coronavirus: the species and its viruses–a statement of the Coronavirus Study Group. bioRxiv Preprint doi: 10.1101/2020.02.07.937862
Haglund, A., Christensen, S., Kristensen, L., Gertsen, J. B., Buus, L., and Lausch, K. R. (2021). Invasive pulmonary aspergillosis and hyperthermia in an immunocompetent patient with COVID-19. Med. Mycol. Case Rep. 31, 29–31. doi: 10.1016/j.mmcr.2020.11.004
Hoenigl, M. (2020). Invasive fungal disease complicating Coronavirus Disease 2019: when it rains, it spores. Clin. Infect. Dis. 73, e1645–e1648. doi: 10.1093/cid/ciaa1342
Hughes, S., Troise, O., Donaldson, H., Mughal, N., and Moore, L. S. (2020). Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 26, 1395–1399. doi: 10.1016/j.cmi.2020.06.025
Imoto, W., Himura, H., Matsuo, K., Kawata, S., Kiritoshi, A., Deguchi, R., et al. (2021). COVID-19-associated pulmonary aspergillosis in a Japanese man: a case report. J. Infect. Chemother. 27, 911–914. doi: 10.1016/j.jiac.2021.02.026
Jamzivar, F., Shams-Ghahfarokhi, M., Khoramizadeh, M., Yousefi, N., Gholami-Shabani, M., and Razzaghi-Abyaneh, M. (2019). Unraveling the importance of molecules of natural origin in antifungal drug development through targeting ergosterol biosynthesis pathway. Iran. J. Microbiol. 11, 448–459. doi: 10.18502/ijm.v11i6.2216
Jin, J.-M., Bai, P., He, W., Wu, F., Liu, X.-F., Han, D.-M., et al. (2020). Gender differences in patients with COVID-19: focus on severity and mortality. Front. Public Health 8:152. doi: 10.3389/fpubh.2020.00152
Johnson, A. K., Ghazarian, Z., Cendrowski, K. D., and Persichino, J. G. (2021). Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med. Mycol. Case Rep. 32, 64–67. doi: 10.1016/j.mmcr.2021.03.006
Kalantari, H., Tabrizi, A. H. H., and Foroohi, F. (2020). Determination of COVID-19 prevalence with regards to age range of patients referring to the hospitals located in western Tehran, Iran. Gene Rep. 21:100910. doi: 10.1016/j.genrep.2020.100910
Kalpana, G., Patil, A. A., Shaan, M., Verma, M., and Harshe, G. (2021). A case series on post-COVID deadly fungal infection: mucormycosis. Int. J. Otorhinolaryngol. Head Neck Surg. 42, 1503–1508. doi: 10.18203/issn.2454-5929.ijohns20213287
Kanwar, A., Jordan, A., Olewiler, S., Wehberg, K., Cortes, M., and Jackson, B. R. (2021). A fatal case of Rhizopus azygosporus pneumonia following COVID-19. J. Fungi 7:174. doi: 10.3390/jof7030174
Khan, N., Gutierrez, C. G., Martinez, D. V., and Proud, K. C. (2020). A case report of COVID-19 associated pulmonary mucormycosis. Arch. Clin. Cases 7, 46–51. doi: 10.22551/2020.28.0703.10172
Khatri, A., Chang, K.-M., Berlinrut, I., and Wallach, F. (2021). Mucormycosis after Coronavirus disease 2019 infection in a heart transplant recipient–case report and review of literature. J. Mycol. Med. 31:101125. doi: 10.1016/j.mycmed.2021.101125
Khodavaisy, S., Khajavirad, N., Hashemi, S. J., Izadi, A., Manshadi, S. A. D., Abdollahi, A., et al. (2021). Proven pulmonary aspergillosis in a COVID-19 patient: a case report. Curr. Med. Mycol. 7, 39–42. doi: 10.18502/cmm.7.2.7031
Legnani, C., and Dusi, E. (2021). Novel pathogens, same old habits. A call for evidence-based research in the fight against COVID-19. Infect. Dis. Now 51, 632–633. doi: 10.1016/j.idnow.2021.01.004
Lescure, F.-X., Bouadma, L., Nguyen, D., Parisey, M., Wicky, P.-H., Behillil, S., et al. (2020). Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 20, 697–706. doi: 10.1016/S1473-3099(20)30200-0
Maini, A., Tomar, G., Khanna, D., Kini, Y., Mehta, H., and Bhagyasree, V. (2021). Sino-orbital mucormycosis in a COVID-19 patient: a case report. Int. J. Surg. Case Rep. 82:105957. doi: 10.1016/j.ijscr.2021.105957
Martins, A. C., Psaltikidis, E. M., de Lima, T. C., Fagnani, R., Schreiber, A. Z., de Oliveira Conterno, L., et al. (2021). COVID-19 and invasive fungal coinfections: a case series at a Brazilian referral hospital. J. Mycol. Med. 31:101175. doi: 10.1016/j.mycmed.2021.101175
Mehrabi, Z., Salimi, M., Niknam, K., Mohammadi, F., Mamaghani, H. J., Sasani, M. R., et al. (2021). Sinoorbital mucormycosis associated with corticosteroid therapy in COVID-19 infection. Case Rep. Ophthalmol. Med. 2021:9745701. doi: 10.1155/2021/9745701
Mehta, S., and Pandey, A. (2020). Rhino-orbital mucormycosis associated with COVID-19. Cureus 12:e10726. doi: 10.7759/cureus.10726
Meijer, E. F., Dofferhoff, A. S., Hoiting, O., Buil, J. B., and Meis, J. F. (2020). Azole-resistant COVID-19-associated pulmonary aspergillosis in an immunocompetent host: a case report. J. Fungi 6:79. doi: 10.3390/jof6020079
Mekonnen, Z. K., Ashraf, D. C., Jankowski, T., Grob, S. R., Vagefi, M. R., Kersten, R. C., et al. (2021). Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plast. Reconstr. Surg. 37, e40–e80. doi: 10.1097/IOP.0000000000001889
Merchant, E. A., Flint, K., Barouch, D. H., and Blair, B. M. (2021). Co-infection with coronavirus disease 2019, previously undiagnosed human immunodeficiency virus, Pneumocystis jirovecii pneumonia and cytomegalovirus pneumonitis, with possible immune reconstitution inflammatory syndrome. IDCases 24:e01153. doi: 10.1016/j.idcr.2021.e01153
Mirzaei, H., McFarland, W., Karamouzian, M., and Sharifi, H. (2021). COVID-19 among people living with HIV: a systematic review. AIDS Behav. 25, 85–92. doi: 10.1007/s10461-020-02983-
Mishra, N., Mutya, V. S. S., Thomas, A., Rai, G., Reddy, B., Mohanan, A., et al. (2021). A case series of invasive mucormycosis in patients with COVID-19 infection. Int. J. Otorhinolaryngol. Head Neck Surg. 7, 867–870. doi: 10.18203/issn.2454-5929.ijohns20211583
Mitaka, H., Perlman, D. C., Javaid, W., and Salomon, N. (2020). Putative invasive pulmonary aspergillosis in critically ill patients with COVID−19: an observational study from New York City. Mycoses 63, 1368–1372. doi: 10.1111/myc.13185
Mohamed, A., Hassan, T., Trzos-Grzybowska, M., Thomas, J., Quinn, A., O’Sullivan, M., et al. (2021). Multi-triazole-resistant Aspergillus fumigatus and SARS-CoV-2 co-infection: a lethal combination. Med. Mycol. Case Rep. 31, 11–14. doi: 10.1016/j.mmcr.2020.06.005
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2010). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–341. doi: 10.1016/j.ijsu.2010.02.007
Muthu, V., Rudramurthy, S. M., Chakrabarti, A., and Agarwal, R. (2021). Epidemiology and pathophysiology of COVID-19-associated mucormycosis: India versus the rest of the world. Mycopathologia 186, 739–754. doi: 10.1007/s11046-021-00584-8
Nasir, N., Farooqi, J., Mahmood, S. F., and Jabeen, K. (2020). COVID−19−associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID−19 pneumonia: an observational study from Pakistan. Mycoses 63, 766–770. doi: 10.1111/myc.13135
Nasri, E., Shoaei, P., Vakili, B., Mirhendi, H., Sadeghi, S., Hajiahmadi, S., et al. (2020). Fatal invasive pulmonary Aspergillosis in COVID-19 patient with acute myeloid leukemia in Iran. Mycoses 185, 1077–1084. doi: 10.1007/s11046-020-00493-2
Nehara, H. R., Puri, I., Singhal, V., Sunil, I., Bishnoi, B. R., and Sirohi, P. (2021). Rhinocerebral mucormycosis in COVID-19 patient with diabetes a deadly trio: case series from the north-western part of India. Indian J. Med. Microbiol. 39, 380–383. doi: 10.1016/j.ijmmb.2021.05.009
Ohashi, N., Ideta, Y., Takeda, A., Iwai, T., Kioi, M., Miyazaki, A., et al. (2021). Oral candidiasis caused by ciclesonide in a patient with COVID-19 pneumonia: a case report and literature review. SAGE Open Med. Case Rep. 9:2050313X211048279. doi: 10.1177/2050313X211048279
Omidi, F., Hajikhani, B., Kazemi, S. N., Tajbakhsh, A., Riazi, S., Mirsaeidi, M., et al. (2021). COVID-19 and cardiomyopathy: a systematic review. Front. Cardiovasc. Med. 8:695206. doi: 10.3389/fcvm.2021.695206
Pasero, D., Sanna, S., Liperi, C., Piredda, D., Branca, G. P., Casadio, L., et al. (2021). A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection 49, 1055–1060. doi: 10.1007/s15010-020-01561-x
Passarelli, V. C., Perosa, A. H., de Souza Luna, L. K., Conte, D. D., Nascimento, O. A., Ota-Arakaki, J., et al. (2020). Detected SARS-CoV-2 in ascitic fluid followed by cryptococcemia: a case report. SN Compr. Clin. Med. 2, 2414–2418. doi: 10.1007/s42399-020-00574-9
Patel, R. H., Acharya, A., Chand, H. S., Mohan, M., and Byrareddy, S. N. (2021). Human immunodeficiency virus and severe acute respiratory syndrome coronavirus 2 coinfection: a systematic review of the literature and challenges. AIDS Res. Hum. Retrovir. 37, 266–282. doi: 10.1089/aid.2020.0284
Pemán, J., Ruiz-Gaitán, A., García-Vidal, C., Salavert, M., Ramírez, P., Puchades, F., et al. (2020). Fungal co-infection in COVID-19 patients: should we be concerned? Rev. Iberoam. Micol. 37, 41–46. doi: 10.1016/j.riam.2020.07.001
Peng, J., Wang, Q., Mei, H., Zheng, H., Liang, G., She, X., et al. (2021). Fungal co-infection in COVID-19 patients: evidence from a systematic review and meta-analysis. Aging 13, 7745–7757. doi: 10.18632/aging.202742
Placik, D. A., Taylor, W. L., and Wnuk, N. M. (2020). Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol. Case Rep. 15, 2378–2381. doi: 10.1016/j.radcr.2020.09.026
Posteraro, B., Torelli, R., Vella, A., Leone, P. M., De Angelis, G., De Carolis, E., et al. (2020). Pan-echinocandin-resistant Candida glabrata bloodstream infection complicating COVID-19: a fatal case report. J. Fungi 6:163. doi: 10.3390/jof6030163
Prattes, J., Valentin, T., Hoenigl, M., Talakic, E., Reisinger, A. C., and Eller, P. (2021). Invasive pulmonary aspergillosis complicating COVID-19 in the ICU-A case report. Med. Mycol. Case Rep. 31, 2–5. doi: 10.1016/j.mmcr.2020.05.001
Radpour, A., Bahrami-Motlagh, H., Taaghi, M. T., Sedaghat, A., Karimi, M. A., Hekmatnia, A., et al. (2020). COVID-19 evaluation by low-dose high resolution CT scans protocol. Acad. Radiol. 27:901. doi: 10.1016/j.acra.2020.04.016
Rawson, T. M., Moore, L. S., Zhu, N., Ranganathan, N., Skolimowska, K., Gilchrist, M., et al. (2020). Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 71, 2459–2468. doi: 10.1093/cid/ciaa530
Revannavar, S. M., Supriya, P., Samaga, L., and Vineeth, V. (2021). COVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world? BMJ Case Rep. 14:e241663. doi: 10.1136/bcr-2021-241663
Robinson, P. C., Liew, D. F., Tanner, H. L., Grainger, J. R., Dwek, R. A., Reisler, R. B., et al. (2022). COVID-19 therapeutics: challenges and directions for the future. Proc. Natl. Acad. Sci. U.S.A. 119:e2119893119. doi: 10.1073/pnas.2119893119
Roushdy, T., and Hamid, E. (2021). A case series of post COVID-19 mucormycosis—a neurological prospective. Egypt. J. Neurol. Psychiatry Neurosurg. 57:100. doi: 10.1186/s41983-021-00355-8
Rubiano, C., Tompkins, K., Sellers, S. A., Bramson, B., Eron, J., Parr, J. B., et al. (2021). Pneumocystis and severe acute respiratory syndrome coronavirus 2 coinfection: a case report and review of an emerging diagnostic dilemma. Open Forum Infect. Dis. 8:ofaa633. doi: 10.1093/ofid/ofaa633
Sadeghi, G., Ebrahimi-Rad, M., Mousavi, S., Shams-Ghahfarokhi, M., and Razzaghi-Abyaneh, M. (2018). Emergence of non-Candida albicans species: epidemiology, phylogeny and fluconazole susceptibility profile. J. Mycol. Med. 28, 51–58. doi: 10.1016/j.mycmed.2017.12.008
Saldanha, M., Reddy, R., and Vincent, M. J. (2021). Title of the article: paranasal mucormycosis in COVID-19 patient. Indian J. Otolaryngol. Head Neck Surg. [Epub ahead of print]. doi: 10.1007/s12070-021-02574-0
Sari, A. P., Darnindro, N., Yohanes, A., and Mokoagow, M. I. (2021). Role of tocilizumab for concomitant systemic fungal infection in severe COVID-19 patient: case report. Medicine 100:e25173. doi: 10.1097/MD.0000000000025173
Schein, F., Munoz-Pons, H., Mahinc, C., Grange, R., Cathébras, P., and Flori, P. (2020). Fatal aspergillosis complicating severe SARS-CoV-2 infection: a case report. J. Mycol. Med. 30:101039. doi: 10.1016/j.mycmed.2020.101039
Seitz, T., Hoepler, W., Weseslindtner, L., Aberle, J., Aberle, S., Puchhammer-Stoeckl, E., et al. (2020). Successful management of the first reported case in Austria of COVID-19 with ARDS. Infection 48, 647–651. doi: 10.1007/s15010-020-01458-9
Senok, A., Alfaresi, M., Khansaheb, H., Nassar, R., Hachim, M., Al Suwaidi, H., et al. (2021). Coinfections in patients hospitalized with COVID-19: a descriptive study from the United Arab Emirates. Infect. Drug Resist. 14, 2289–2296. doi: 10.2147/IDR.S314029
Shah, A. S., Heidari, A., Civelli, V. F., Sharma, R., Clark, C. S., Munoz, A. D., et al. (2020). The coincidence of 2 epidemics, coccidioidomycosis and SARS-CoV-2: a case report. J. Investig. Med. High Impact Case Rep. 8:2324709620930540. doi: 10.1177/2324709620930540
Sharma, A., Hofmeyr, A., Bansal, A., Thakkar, D., Lam, L., Harrington, Z., et al. (2021). COVID-19 associated pulmonary aspergillosis (CAPA): an Australian case report. Med. Mycol. Case Rep. 31, 6–10. doi: 10.1016/j.mmcr.2020.06.002
Silva, L. N., de Mello, T. P., de Souza Ramos, L., Branquinha, M. H., Roudbary, M., and Dos Santos, A. L. S. (2020). Fungal infections in COVID-19-positive patients: a lack of optimal treatment options. Curr. Top. Med. Chem. 20, 1951–1957. doi: 10.2174/156802662022200917110102
Singh, V., Prasad, A., Panda, P. K., Totaganti, M., Tyagi, A., Thaduri, A., et al. (2021). Mixed invasive molds among COVID-19 patients. medRxiv Preprint doi: 10.1101/2021.08.09.21261555
Singh, Y., Ganesh, V., Kumar, S., Patel, N., Aggarwala, R., Soni, K. D., et al. (2021). Coronavirus disease-associated mucormycosis from a tertiary care hospital in India: a case series. Cureus 13:e16152. doi: 10.7759/cureus.16152
Singhal, T. (2020). A review of coronavirus disease-2019 (COVID-19). Indian J. Pediatr. 87, 281–286. doi: 10.1007/s12098-020-03263-6
Song, G., Liang, G., and Liu, W. (2020). Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia 185, 599–606. doi: 10.1007/s11046-020-00462-9
Talento, A. F., and Hoenigl, M. (2020). Fungal infections complicating COVID-19: with the rain comes the spores. J. Fungi 6:279. doi: 10.3390/jof6040279
Team, E. (2020). The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Wkly. 2, 113–122. doi: 10.46234/ccdcw2020.032
Teixeira, I. S., Leal, F. S., Tateno, R. Y., Palma, L. F., and Campos, L. (2021). Photobiomodulation therapy and antimicrobial photodynamic therapy for orofacial lesions in patients with COVID-19: a case series. Photodiagnosis. Photodyn. Ther. 34:102281. doi: 10.1016/j.pdpdt.2021.102281
Trovato, L., Calvo, M., Migliorisi, G., Astuto, M., Oliveri, F., and Oliveri, S. (2021). Fatal VAP-related pulmonary aspergillosis by Aspergillus niger in a positive COVID-19 patient. Respir. Med. Case Rep. 32:101367. doi: 10.1016/j.rmcr.2021.101367
Veisi, A., Bagheri, A., Eshaghi, M., Rikhtehgar, M. H., Rezaei Kanavi, M., and Farjad, R. (2021). Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: a case report. Eur. J. Ophthalmol. [Epub ahead of print]. doi: 10.1177/11206721211009450
Ventoulis, I., Sarmourli, T., Amoiridou, P., Mantzana, P., Exindari, M., Gioula, G., et al. (2020). Bloodstream infection by Saccharomyces cerevisiae in two COVID-19 patients after receiving supplementation of Saccharomyces in the ICU. J. Fungi 6:98. doi: 10.3390/jof6030098
Viceconte, G., Buonomo, A. R., Lanzardo, A., Pinchera, B., Zappulo, E., Scotto, R., et al. (2021). Pneumocystis jirovecii pneumonia in an immunocompetent patient recovered from COVID-19. Infect. Dis. 53, 382–385. doi: 10.1080/23744235.2021.1890331
Waizel-Haiat, S., Guerrero-Paz, J. A., Sanchez-Hurtado, L., Calleja-Alarcon, S., and Romero-Gutierrez, L. (2021). A case of fatal rhino-orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID-19. Cureus 13:e13163. doi: 10.7759/cureus.13163
Wang, J., Yang, Q., Zhang, P., Sheng, J., Zhou, J., and Qu, T. (2020). Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID-19 in Zhejiang, China: a retrospective case series. Crit. Care 24:299. doi: 10.1186/s13054-020-03046-7
Werthman-Ehrenreich, A. (2021). Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am. J. Emerg. Med. 42, e264.e5–e264.e8. doi: 10.1016/j.ajem.2020.09.032
Yang, J., Hu, J., and Zhu, C. (2021). Obesity aggravates COVID−19: a systematic review and meta−analysis. J. Med. Virol. 93, 257–261. doi: 10.1002/jmv.26237
Yang, S., Hua, M., Liu, X., Du, C., Pu, L., Xiang, P., et al. (2021). Bacterial and fungal co-infections among COVID-19 patients in intensive care unit. Microbes Infect. 23:104806. doi: 10.1016/j.micinf.2021.104806
Yang, X., Yu, Y., Xu, J., Shu, H., Liu, H., Wu, Y., et al. (2020). Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 8, 475–481. doi: 10.1016/S2213-2600(20)30079-5
Keywords: COVID-19, co-infection, fungal infection, systematic review, Aspergillus
Citation: Seyedjavadi SS, Bagheri P, Nasiri MJ, Razzaghi-Abyaneh M and Goudarzi M (2022) Fungal Infection in Co-infected Patients With COVID-19: An Overview of Case Reports/Case Series and Systematic Review. Front. Microbiol. 13:888452. doi: 10.3389/fmicb.2022.888452
Received: 02 March 2022; Accepted: 03 June 2022;
Published: 06 July 2022.
Edited by:
Matthaios Papadimitriou-Olivgeris, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Raquel Sabino, National Institute of Health Dr. Ricardo Jorge, PortugalTong-Bao Liu, Southwest University, China
Copyright © 2022 Seyedjavadi, Bagheri, Nasiri, Razzaghi-Abyaneh and Goudarzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mehdi Goudarzi, Z3VkYXJ6aW1AeWFob28uY29t; Mehdi Razzaghi-Abyaneh, bXJhYjQyQHlhaG9vLmNvbQ==, bXJhYjQyQHBhc3RldXIuYWMuaXI=
 Sima Sadat Seyedjavadi1
Sima Sadat Seyedjavadi1 Parmida Bagheri
Parmida Bagheri Mohammad Javad Nasiri
Mohammad Javad Nasiri Mehdi Goudarzi
Mehdi Goudarzi