
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 25 April 2022
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.874354
This article is part of the Research Topic Advances in the discovery of Natural Molecules and their Analogues Against Microbial Infection-Related Biofilms View all 14 articles
 Huaqiao Tang1†
Huaqiao Tang1† Suqi Hao1†
Suqi Hao1† Muhammad Faraz Khan2
Muhammad Faraz Khan2 Ling Zhao1
Ling Zhao1 Fei Shi1
Fei Shi1 Yinglun Li1
Yinglun Li1 Hongrui Guo1
Hongrui Guo1 Yuanfeng Zou1
Yuanfeng Zou1 Cheng Lv1
Cheng Lv1 Jie Luo3,4
Jie Luo3,4 Ze Zeng3
Ze Zeng3 Qiang Wu5
Qiang Wu5 Gang Ye1*
Gang Ye1*The superbug Pseudomonas aeruginosa is among the most formidable antibiotic-resistant pathogens. With declining options for antibiotic-resistant infections, new medicines are of utmost importance to combat with P. aeruginosa. In our previous study, we demonstrated that Epigallocatechin-3-gallate (EGCG) can inhibit the production of quorum sensing (QS)-regulated virulence factors in vitro. Accordingly, the protective effect and molecular mechanisms of EGCG against P. aeruginosa-induced pneumonia were studied in a mouse model. The results indicated that EGCG significantly lessened histopathological changes and increased the survival rates of mice infected with P. aeruginosa. EGCG effectively alleviated lung injury by reducing the expression of virulence factors and bacterial burden. In addition, EGCG downregulated the production of pro-inflammatory cytokines, such as TNF-α, IL-1, IL-6, and IL-17, and increased the expression of anti-inflammatory cytokines IL-4 and IL-10. Thus, the experimental results supported for the first time that EGCG improved lung damage in P. aeruginosa infection by inhibiting the production of QS-related virulence factors in vivo.
Globally, pneumonia is a severe public health problem with a large disease burden and a major cause of mortality and morbidity. The disease burden caused by pneumonia affects more children worldwide than other diseases (O’Brien et al., 2019). Older patients have a high risk of death afflicted by pneumonia (Quinton and Mizgerd, 2015). Some of the bacterial and viral infections causing acute pneumonia or sepsis result in serious inflammatory damage to the lungs, thereby leading to the progression of acute lung injury (ALI) or acute respiratory distress syndrome (ARDS), especially in critically ill patients (Dai et al., 2018). Multidrug-resistant microbes (“superbugs”), such as Streptococcus pneumoniae, group A Streptococcus, Klebsiella pneumoniae, Staphylococcus aureus, Mycoplasma pneumoniae, and Pseudomonas aeruginosa can cause severe pneumonia and were the main inducers of acute pathogenic factors (Ravi Kumar et al., 2018).
P. aeruginosa, a common opportunistic pathogen, can cause life-threatening respiratory infections and is responsible for hospital-acquired infections and ventilator-associated pneumonia (VAP). The high adaptability and an increasing number of multidrug-resistant P. aeruginosa constitute a threat for those who suffer from Chronic Obstructive Pulmonary Disease (COPD) or Cystic Fibrosis (CF; Aloush et al., 2006). Unfortunately, because of its intrinsic and extrinsic drug resistance and the capacity of P. aeruginosa to form biofilms, a P. aeruginosa infection is notoriously difficult to treat with antibiotics (Moore and Flaws, 2011). Multidrug-resistant pathogen-induced death is estimated to reach 10 million by 2050, and thereby exceeding deaths caused by cancer and diabetes combined worldwide. Thus, the development of alternative therapeutic methods is critical (O’Neill, 2016). The QS system is a cell-to-cell system that enables microbe populations to change their behavior based on population density. The QS system of P. aeruginosa plays a key role in coordinating various activities, including biofilm formation and the release of virulence factors. The QS system of P. aeruginosa is mainly regulated by four QS network subsystems, including lasI/lasR, rhlI/rhlR, PQS, and IQS systems (Lee and Zhang, 2015).
P. aeruginosa-induced pneumonia expresses a myriad of virulence factors, including flagella, pili, lipopolysaccharides (LPS), elastase, alkaline phosphatase, exotoxin A, as well as components of the type III secretion system (T3SS; Sadikot et al., 2005). In addition, the ability of P. aeruginosa to form biofilms is conducive to establishing infections in VAP and CF patients and is difficult to eradicate (Costerton et al., 1999). P. aeruginosa-induced pneumoniae is a complex process. Several surface-associated elements, including flagella, fimbriae, and LPS, contact host respiratory epithelia through a number of aggregated pilis (Gellatly and Hancock, 2013). Once contact with host epithelia has occurred, T3SS can be activated. P. aeruginosa T3SS is a major determinant of virulence, and its expression is frequently associated with acute invasive infections. Moreover, P. aeruginosa T3SS has been linked to increased mortality in infected patients (Hauser, 2009). Elastase (lasB lasA) has been shown to directly injure lung tissue through disruption of epithelial tight junctions and basal membranes. Elastase may increase the recruitment of neutrophils into the airways, which can result in serious inflammation (Kipnis et al., 2006). Pyocyanin induces direct damage to the respiratory tract as epithelial necrosis, and slowing tracheal mucociliary transport results from damaged ciliary movement (Lau et al., 2004). Virulence factors, such as type III secretory proteins, QS systems, and LPS, activate the host immune response. Therefore, the activation of macrophages, neutrophilic granulocytes, and T cells induces the secretion of cytokines, chemotactic factors, and another inflammatory mediator, thus leading to lung injury and mortality (Driscoll et al., 2007).
For thousands of years, tea originating from China has gained the world’s taste. It has become the daily health drink for many individuals. In general, these health benefits result from the phenolic compounds that are present in green tea, particularly catechins. Numerous studies have demonstrated the diverse activities of Epigallocatechin-3-gallate (EGCG), including antioxidant, antibacterial, antiviral, antitumor and anti-inflammatory activities. In our previous study, we demonstrated that EGCG significantly acted against the expression of P. aeruginosa QS-regulated virulence in vitro, and significantly increased the survival rate of Caenorhabditis elegans infected with P. aeruginosa (Hao et al., 2021). QS quenching, called anti-virulence therapy, has been considered a suitable strategy to settle the multidrug resistance problem (Clatworthy et al., 2007). Taken together, our study provides additional support for drinking tea as an effective method against bacterial infections. These findings can pave the way for the use of EGCG as a therapeutic medicine to treat bacterial infections in the lungs.
Eight-week-old male ICR mice (25–30 g) were purchased from Sibeifu Biotechnology Co. Ltd. (Beijing, China). Mice were housed at 22–25°C with a 12 h day-night cycle, were fed standard rodent chow, and sterile water ad libitum for 1 week of acclimation. All protocols involving animal studies were reviewed and approved by the Animal Ethical Committee of Sichuan Agricultural University (#20210020). P. aeruginosa (PAO1) were cultured in Luria Bertani (LB) medium (Sangon Biotech, Shanghai, China). EGCG was obtained from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China); CIP was obtained from Sichuan Chuanlong Dongke Pharmaceutical Co., Ltd.
A total of 90 mice were randomly divided into six groups (n = 15 mice per group): a control group, PAO 1 group, PAO 1 + EGCG (20 mg/kg) group, PAO 1 + EGCG (40 mg/kg) group, PAO 1 + EGCG (80 mg/kg) group, and a Ciprofloxacin (CIP; 20 mg/kg) group. CIP was used as positive control. Mice were administered the same volume of saline or drugs by intragastric administration for 3 days. Mice were infected by using a previously described method with modifications (Traber et al., 2019). Briefly, mice were weighed and anesthetized, and received intratracheal instillation of PAO 1 (2.5 × 108 CFU) in 20 μl phosphate buffered saline (PBS) to model an acute infection with PAO 1. Mice were anesthetized and sacrificed at 24 h after infection. Samples were harvested rapidly for subsequent analysis. Per group, 10 mice were randomly selected to collect blood and lung tissue; another five mice were selected for the collection of bronchial-alveolar lavage fluid (BALF).
To assess bacterial burden in the lung, fresh lung tissues were extracted aseptically, and homogenized in sterile NaCl 0.9%. As previously described (Pylaeva et al., 2019), colony forming units (CFU) were enumerated by plating serial dilutions of lung homogenates on Pseudomonas Agar Medium for the detection of Pyocyanin (PDP, Haibo Biotech, Shandong, China). After incubation at 37°C for 24 h, CFU were counted and recorded.
Lung tissue was fixed with 4% paraformaldehyde overnight, and embedded in paraffin. Subsequently, lung tissue was cut into 5-μm-thick sections. Sections were stained with hematoxylin and eosin (H&E) and the degree of damage to lung tissue was validated (Sen-Kilic et al., 2019).
BALF was collected and centrifuged at 1500 g for 5 min, then the supernatant was removed and transferred to a clean tube. The expression of TNF-α, IL-10, and IL-17 in the supernatant was determined by ELISA according to the manufacturer’s instructions [Multisciences (Lianke) Biotech Co., Ltd., Hangzhou, China]. The absorbance was measured using a microplate reader at 450 nm.
Total RNA was extracted from lung tissue homogenate using TRIzol reagent (Songon Biotech, Shanghai, China) according to the manufacturer’s instructions. Then, cDNA synthesis was conducted using a RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, K1622, Waltham, MA, United States) in a 20 μl volume of reaction mixture. Real-time PCR was performed in a total reaction volume of 10 μl, containing 5 μl PerfectStartTM Green qPCR SuperMix (TransGen Biotech, Beijing, China), 2 μl template cDNA, 1 μl of primers (Shenzhen Huada Gene Research Institute, Shenzhen, China), and 2 μl DNase/RNase-free water (Tiangen Biotech, Beijing, China). Finally, pvdQ of P. aeruginosa was chosen as the reference gene. The qRT-PCR reaction was conducted on a LightCycler® 480II Master Mix (Roche, Germany). Data from qRT-PCR experiments were analyzed using the 2®-ΔΔCT method (Yang et al., 2021). Primer sequences are listed in Table 1.
Data are expressed as the mean ± standard deviation (SD), and all experimental groups were compared with the control group. Data were analyzed using SPSS 20.0 by ANOVA (IBM SPSS Statistics, CA, United States), and p < 0.05 or p < 0.01 was considered significantly different.
As shown in Figure 1, the survival of mice with a P. aeruginosa-induced acute lung infection was evaluated to test the protective effect of EGCG. Of the mice in the P. aeruginosa group, only 53.4% survived. The survival rates after EGCG early intervening treatment with 80 mg/kg, 40 mg/kg, 20 mg/kg, respectively, were 71.8, 66.7, 66.7%, whereas the survival rate of the positive CIP group was 60%, thus the protective effect was less compared to that of EGCG. Next, the alleviation of P. aeruginosa-induced pulmonary edema by EGCG was assessed. The results showed that the wet weight of mice in the P. aeruginosa group increased more than 2-fold compared with mice in the control group. EGCG significantly reduced the lung edema, and no significant differences in lung weight were observed between CIP and P. aeruginosa. The bacterial burden in the lungs was determined by plating serial dilutions and counting viable bacteria at 24 h post the challenge. As expected, the bacterial load in the lungs of EGCG-treated mice was significantly lower compared to that of mice in the P. aeruginosa group. These findings demonstrated the preventive effect of EGCG in decreasing acute lung infection of P. aeruginosa. The control group did not show any histopathological changes in the lungs under the light microscope. The results indicated that lung sections after P. aeruginosa infection showed significant changes, including PMN infiltration, alveolar interstitial edema, interstitial hemorrhage, and alveolar wall thickening. Importantly, our results showed that EGCG treatment reduced lung damage and maintained alveolar integrity due to structural disruptions and hemorrhage in a concentration-dependent manner compared with the control. Taken together, these results demonstrated that EGCG alleviated histopathological damage in lungs of mice challenged with P. aeruginosa.
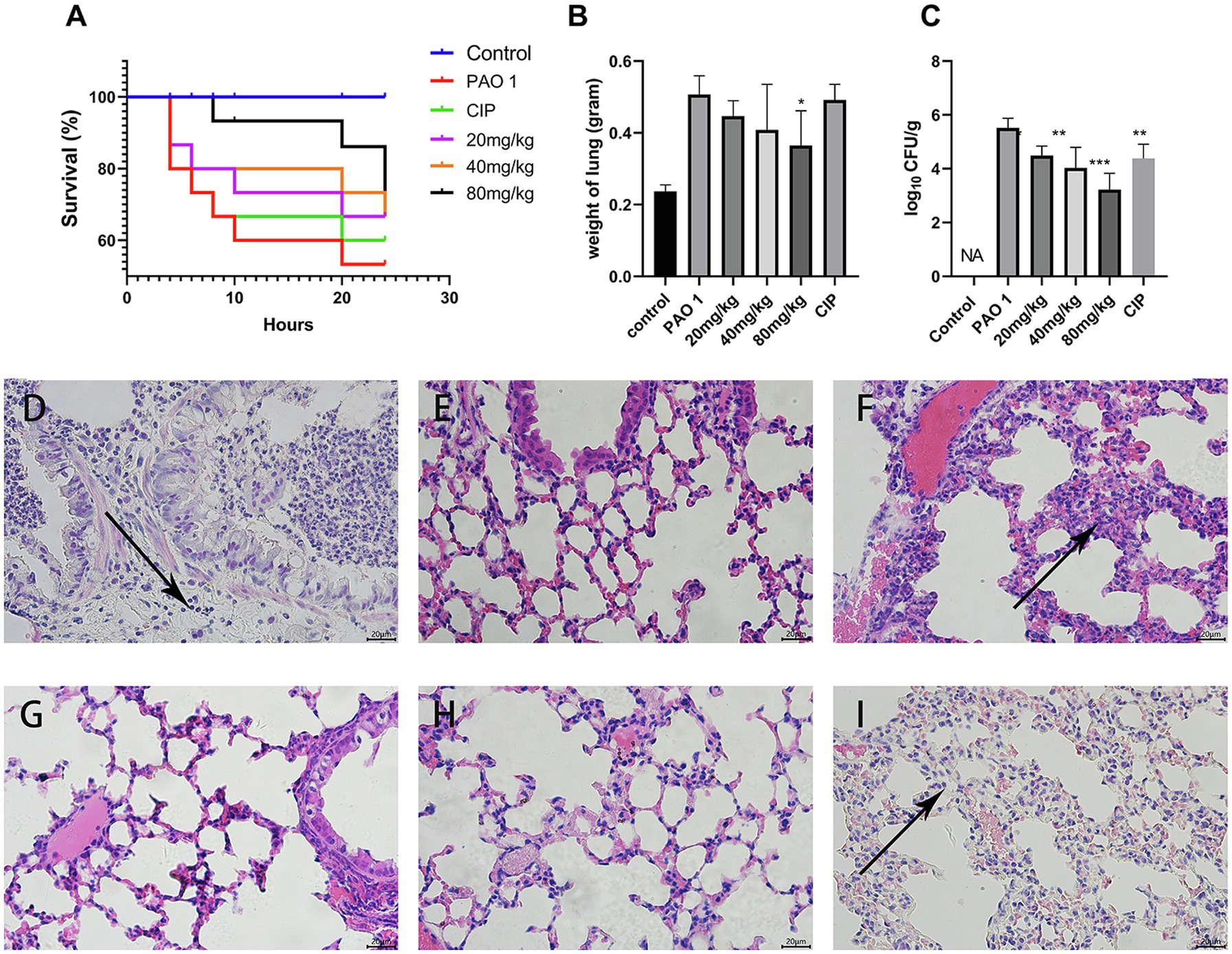
Figure 1. Protective efficacy of EGCG against acute lung infection. (A) The survival rate was measured at different time points post P. aeruginosa challenged. (B) The weight of the lungs after P. aeruginosa challenged. (C) Bacterial load in the lungs were evaluated at 24 h post infection. (D–I) Pathological changes in (D) P. aeruginosa group, (E) control group, (F) EGCG (20 mg/kg) group, (G) EGCG (40 mg/kg) group, (H) EGCG (80 mg/kg) group, (I) CIP was used as positive control. Data are presented as mean ± SD and analyzed with one-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.005 vs. PAO 1 group.
In this study, the expression of QS system genes was examined in acute lung injury. As shown in Figure 2, EGCG and CIP significantly downregulated the expression of QS-related virulence factors in vivo. First, EGCG inhibited the master QS regulatory system lasI/lasR in a dose-dependent manner. The expression of lasR was significantly inhibited at 80 mg/kg. At a dose of 80 mg/kg, the expression of lasA and lasB, which are regulated via the lasI/lasR pathway, was also significantly inhibited in a dose-dependent manner. Moreover, rhlI/rhlR and pqsA/pqsR systems were downregulated as well. Our data showed that the expression level of the pqsA/pqsR system was lower than that of lasI/lasR and rhlI/rhlR systems. Therefore, we hypothesized that lasI/lasR and rhLI/rhlR systems played a dominant role in acute lung infection (Figure 3). The expression of virulence factors was significantly downregulated by EGCG. The expression of phzA, phzS, phzM, and phzH was also inhibited by EGCG compared with mice in the P. aeruginosa group. Furthermore, the rhlA gene, which controls the production of rhamnolipid, was significantly inhibited.
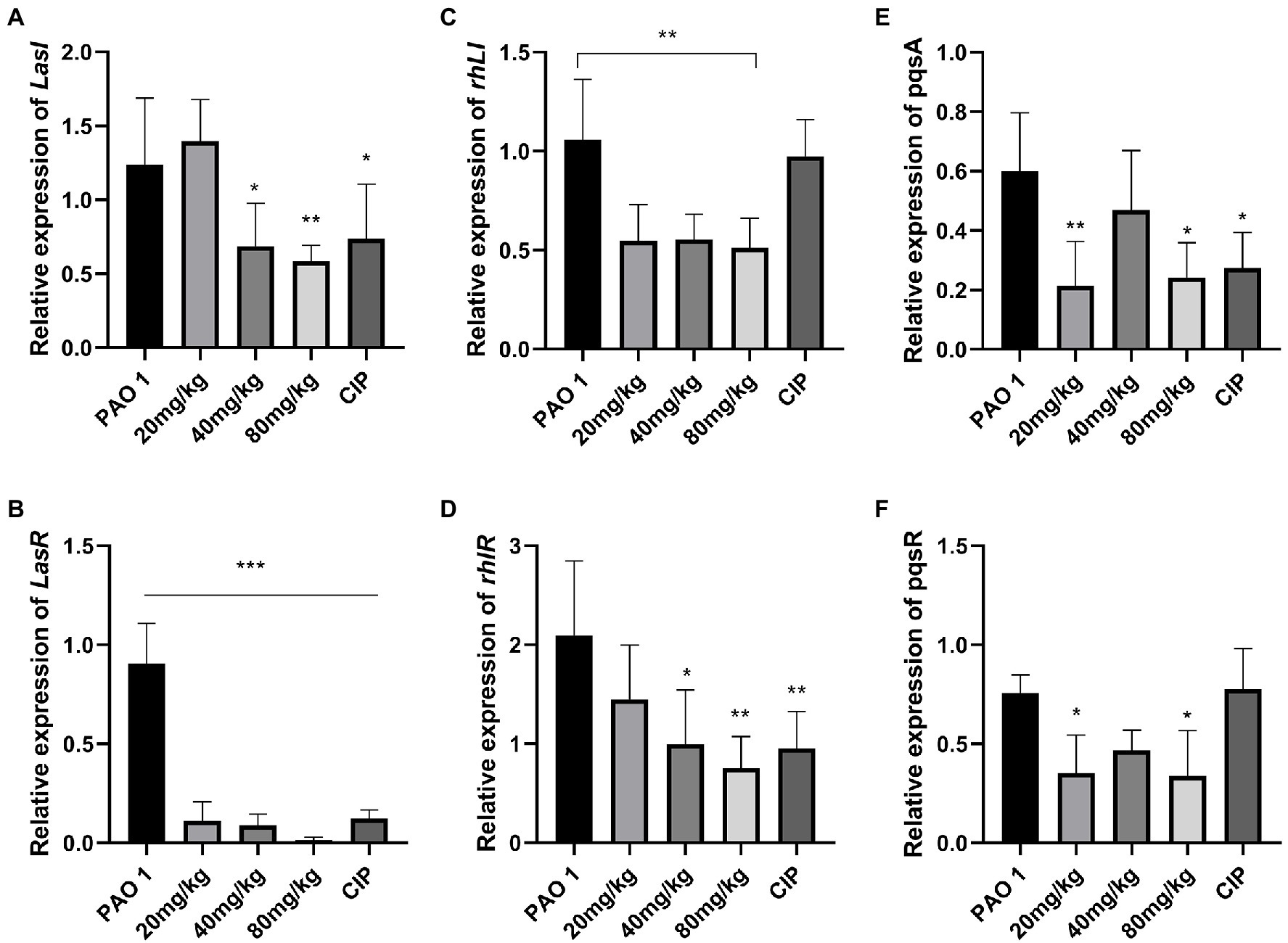
Figure 2. The effect of EGCG on the expression of QS system in vivo. (A) lasI, (B) lasR, (C) rhlI, (D) rhlR, (E) pqsA, and (F) pqsR. Data are presented as mean ± SD and analyzed with one-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.005 vs. PAO 1 group.
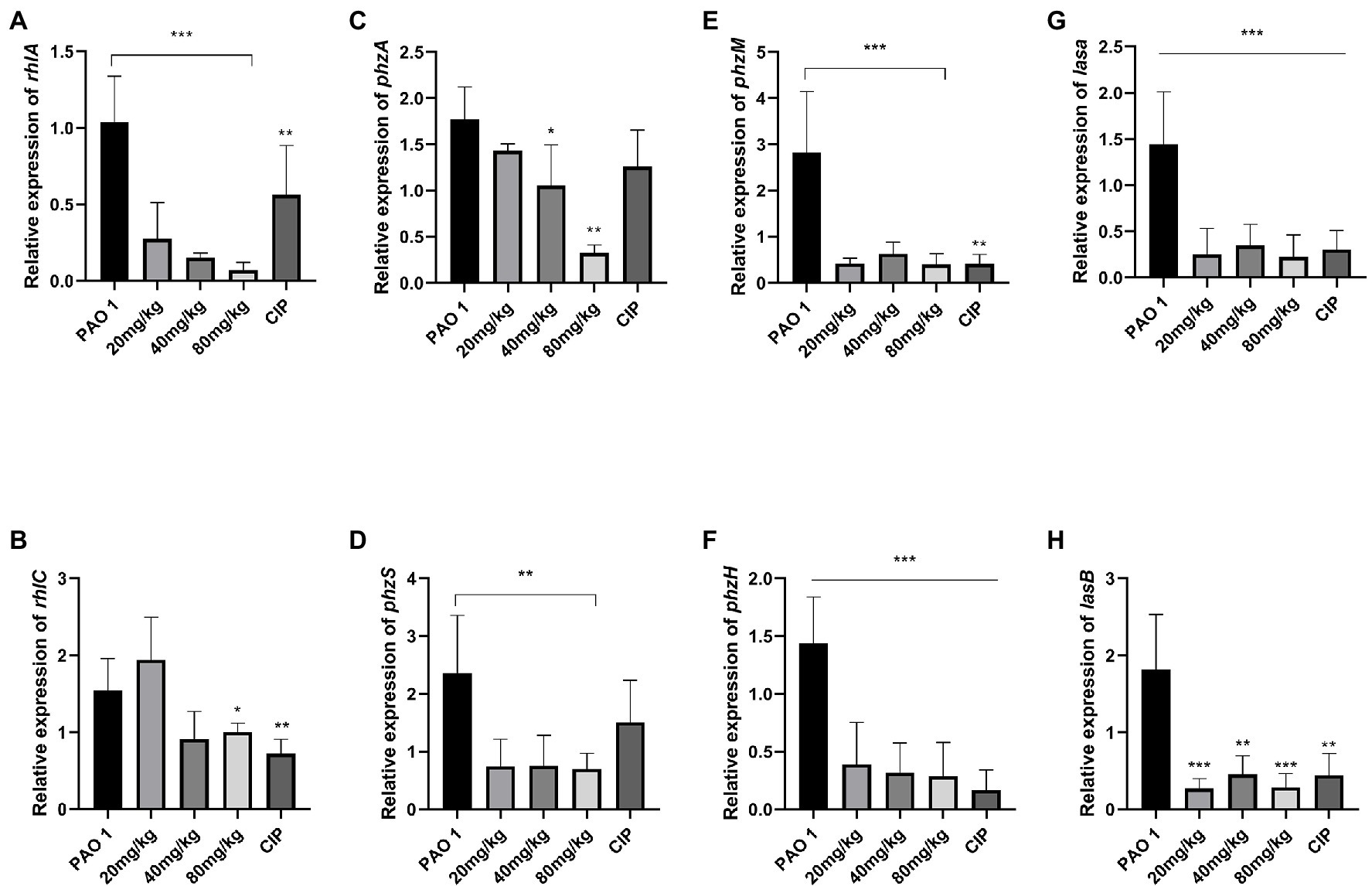
Figure 3. The inhibition of EGCG on the expression of QS-regulated virulence factors in the lung challenged by P. aeruginosa. (A) rhlA, (B) rhlC (C) phzA, (D) phzS, (E) phzM (F) phzH, (G) lasA, (H) lasB. Data are presented as mean ± SD and analyzed with one-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.005 vs. PAO 1 group.
Pseudomonas aeruginosa biofilm formation is regulated by the QS system (Sauer et al., 2002). The expression of biofilm matrix genes and subsequent development of biofilm structure are also adjusted by the QS system (Skariyachan et al., 2018). The expression of biofilm-regulated factors (fila, pela, pila, pslb) was evaluated in vivo. The results indicated that EGCG significantly inhibited biofilm maturation at 18 h in vitro, and a better inhibitory effect was found in vivo. The expression of pela, pila, and pslb was significantly inhibited by EGCG in comparison to mice in the P. aeruginosa infection group. Thus, these results suggested that EGCG can effectively inhibit biofilm formation and prevent the activation of virulence factors of P. aeruginosa in vivo. The data are presented in Figure 4.
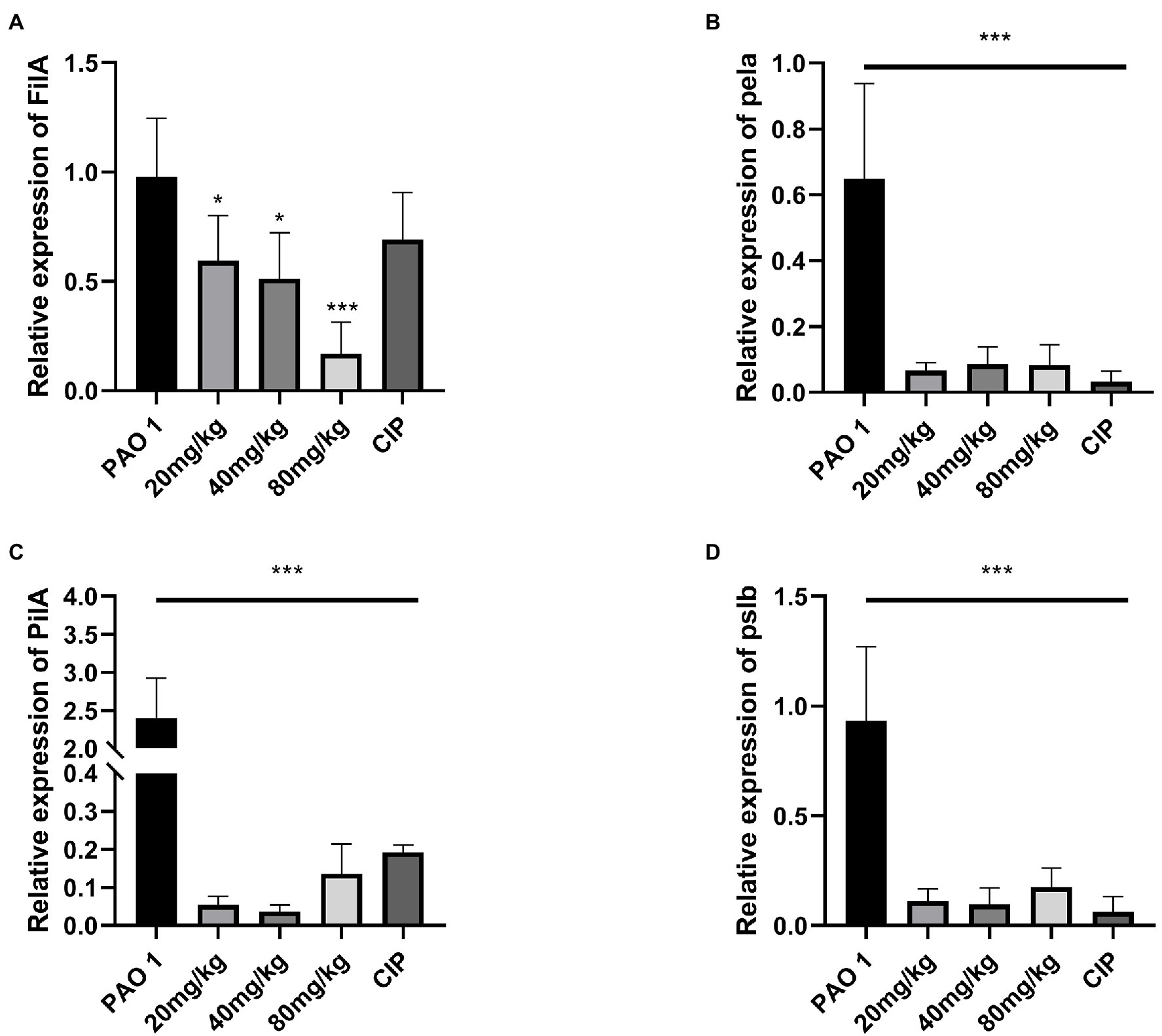
Figure 4. EGCG significantly affected the expression of the biofilm formation relevant genes in vivo. (A) FilA, (B) pela (C) PilA, (D) pslb. Data are presented as mean ± SD and analyzed with one-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.005 vs. PAO 1 group.
To assess the inflammatory state of infected mice, the expression of inflammatory cytokines was determined by real-time PCR and ELISA. Compared to the control, EGCG significantly decreased the expression of proinflammatory cytokines TNF-α, IL-1, IL-6, and IL-17. Moreover, the expression of proinflammatory cytokines in the CIP group was lower than that in the EGCG group. The EGCG group had a higher expression of anti-inflammatory cytokines IL-4 and IL-10 (Figure 5).
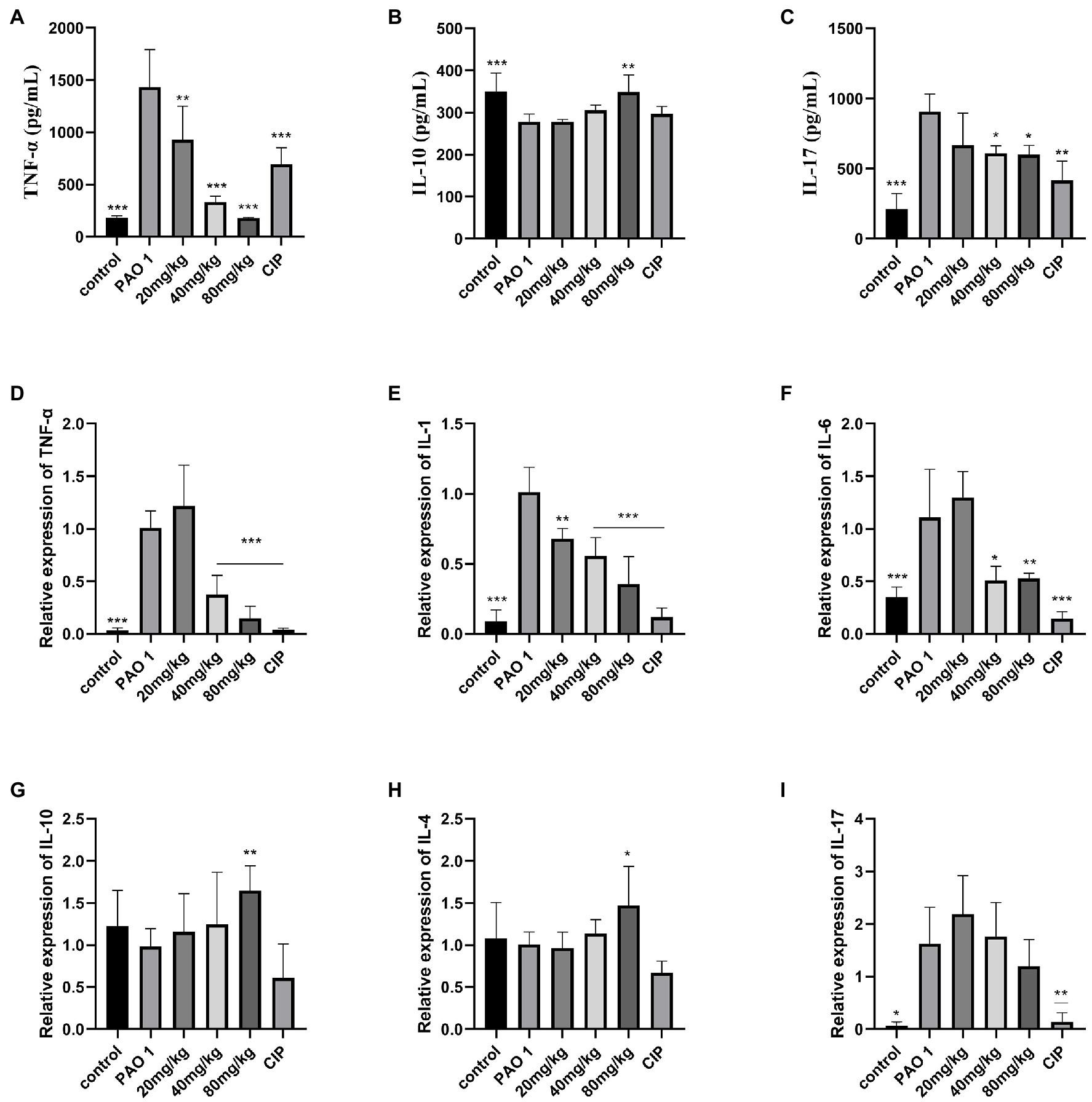
Figure 5. Assay of inflammatory cytokines by ELISA and QRT-PCR. Secreted TNF-α, IL-10, and IL-17 from the bronchial-alveolar lavage fluid was assessed by ELISA assay kit (A–C). EGCG affected the expression of cytokines with a dosage dependent manner in the lung (D–I). Data are presented as mean ± SD and analyzed with one-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.005 vs. PAO 1 group.
In this study, it was demonstrated that EGCG protects mice against P. aeruginosa-induced lung damage by inhibiting the virulence controlled by QS systems. P. aeruginosa is a major cause of acute nosocomial infections and pneumonia (Kizny Gordon et al., 2017). Nosocomial bacteremia pneumonia is associated with significant mortality, morbidity, length of hospital stays and cost. In addition, despite important medical advances, there have been no improvements in the treatment of P. aeruginosa infections over the past two decades (Melsen et al., 2013; Jain et al., 2015). The mechanisms by which P. aeruginosa resists antibiotics include intrinsic, acquired and adaptive resistance, such as efflux pumps, antibiotic-inactivating enzymes, impermeable outer membrane proteins, horizontal transfer of resistance genes or mutational changes, and adaptive resistance refers to the formation of biofilms (Livermore, 2002; Breidenstein et al., 2011). P. aeruginosa is resistant to diverse types of antibiotics, including quinolones, aminoglycosides, and β-lactams (Hancock and Speert, 2000). Due to the increasing frequency of antibiotic resistance of P. aeruginosa, novel therapeutic approaches to treat P. aeruginosa infections are of utmost importance. Furthermore, novel treatments may be used alone or in combination with conventional therapies, such as the inhibition of QS and bacterial lectins, the use of iron chelation, phage therapy, vaccine strategies, as well as the use of nanoparticles, antimicrobial peptides and electrochemical scaffolds (Chatterjee et al., 2016). In our previous study, the minimal inhibitory concentration (MIC) of EGCG against P. aeruginosa was confirmed to be 512 μg/ml (Hao et al., 2021). However, EGCG reduced virulence phenotypes, such as biofilm, protease, elastase activity, swimming, and swarming motility at concentrations where no growth inhibition was observed. In the present study, we aimed to explore the anti-infection ability of EGCG based on the QS mechanism in a mouse model of P. aeruginosa infection. Our results indicated that EGCG significantly alleviated P. aeruginosa-induced pulmonary edema, decreased the bacterial load and the level of proinflammatory cytokines in the lung, and enhanced the survival rates of mice. Moreover, EGCG significantly reduced the expression of QS-regulated virulence factors in vivo. Taken together, these findings showed that EGCG has the ability to mitigate the release of virulence and may alleviate inflammation-caused damage in vivo.
EGCG is one of the richest ingredients in green tea-derived polyphenols, and its biological activities have been extensively studied (Jigisha et al., 2012; Steinmann et al., 2013). EGCG can inhibit infections by reducing biofilm formation and toxin release in bacteria (Zhao et al., 2021). However, few studies have evaluated the clinical effect of EGCG in animal models. In several previous studies, the anti-inflammation effect of EGCG was demonstrated. Lee et al. demonstrated that EGCG protected against TNF-α-mediated lung inflammation by down-regulation of oxidative stress and expression of intercellular adhesion molecule (ICAM)-1 in A549 cells or human pulmonary alveolar epithelial cells (HPAEpiCs) as well as in mouse lungs (Lee et al., 2013). In a recent study, the effect of green tea (GTE) and EGCG on macrophage polarization was evaluated in vitro and it was determined whether the treatment could ameliorate inflammatory responses in vivo. Results showed that GTE and EGCG decreased M1-macrophages and increased Treg cells in bone marrow to inhibit inflammation. EGCG and GTE prevent LPS-induced inflammatory damage contributing to restore the immune system homeostasis through increasing M2-macrophages, N2-neutrophils and Tregs in the spleen and blood (Azambuja et al., 2022). The activation of M2 macrophages, which polarized by Th2 cytokines such as IL-4 and IL-13, can enhance the effect anti-inflammatory and immunoregulatory. Consequently, increasing the release of anti-inflammatory cytokines IL-10 and TGF-β (Shapouri-Moghaddam et al., 2018). Wang et al. (2019) demonstrated that pretreatment with EGCG attenuated LPS-induced ALI as manifested by fewer pathological changes in pulmonary edema and the expression of proinflammatory cytokines TNF-α, IL-1β, and IL-6 in the lung, serum, and BALF. The protective mechanism was associated with suppressing the TLR-4/NF-kB-p65 pathway that mediates inflammation.
These results were similar to the data obtained in our study; we demonstrated that EGCG alleviates lung damage, including pathological injury and pulmonary edema in P. aeruginosa-induced pulmonary infection. Moreover, EGCG decreased the P. aeruginosa load and infection mortality in the lung. EGCG significantly inhibited the expression of TNF-α, IL-1β, IL-6, and IL-17 in the lung but increased the expression of anti-inflammatory cytokines IL-4 and IL-10. We speculate that EGCG may improve the immunity ability through promoting the polarization of M2 macrophages. Hence EGCG increased the level of anti-inflammatory cytokines IL-4 and IL-10. Further work needs to be performed to identify the underlying mechanisms involved. The ELISA results showed that the protein expression levels of TNF-α and IL-17 decreased in a dose-dependent manner, compared with PAO 1 group. Therefore EGCG can reduce P. aeruginosa-induced inflammation in the lung. Liu et al. demonstrated that tea polyphenols increased the survival rate of Caenorhabditis elegans against K. pneumonia infection to 73.3 and 82.2% (Liu et al., 2020). Previous results showed that microencapsulation of EGCG exhibited therapeutic outcomes by resolution of inflammation in tuberculosis bacteria-infected lungs and a significant reduction in bacterial burden (Sharma et al., 2020). Many studies have suggested that EGCG and other polyphenol compounds could be potential antivirulence agents for pulmonary infection (Sriram et al., 2009; Ling et al., 2012). The potent anti-inflammatory activities add further appeal to the medicinal use of EGCG or other green tea polyphenol-rich products.
P. aeruginosa can express a plethora of virulence factors that facilitate invasion and damage host tissues (Crousilles et al., 2015), and are controlled by complex, intersecting regulatory circuits and multiple signaling systems (Nadal Jimenez et al., 2012; Balasubramanian et al., 2013). Among these, QS regulates virulence factors (proteases, elastase, rhamnolipid, exotoxins, and pyocyanin) and plays an important role in acute P. aeruginosa infections (Hauser, 2009; Lee and Zhang, 2015). QS participates in biofilm formation, which plays an important role during chronic infections and antibiotic resistance. In recent years, antimicrobial agents with great potential have been studied and are now known as antivirulence therapies (Hauser, 2009). This intervention targets virulence factors or virulence regulatory pathways that will not result in inhibition of bacterial growth or bacterial cell death but block their pathogenicity (Hauser, 2009). Consequently, the emergence of drug-resistant strains is decreasing. Interference with QS-mediated signaling and alleviation of virulence contribute to clearance of infecting bacteria by host defenses (Saeki et al., 2020). Our results confirmed the anti-QS ability of EGCG against P. aeruginosa in vivo. EGCG significantly inhibited the expression of QS-relevant genes, including lasI, lasR, rhlI, rhlR, pqsA, pqsR, phzA, phzH, phzM, phzS, lasA, lasB, rhlA, and rhlC. Moreover, EGCG had a better capacity to inhibit virulence than the positive CIP group. According to the gene expression results, EGCG displayed an excellent ability to inhibit proinflammatory cytokines. At 80 mg/kg, EGCG significantly increased anti-inflammatory cytokines IL-10 and IL-4, which was not observed in the CIP group. We found that the expression of lasR/lasI and rhlR/rhlI systems, the leading virulence factor during P. aeruginosa infection, was significantly inhibited at 80 mg/kg. Pyocyanin, a green color product of P. aeruginosa, can interfere with host oxidative stress responses by increasing intracellular levels of reactive oxygen species (Lau et al., 2004). Our results show that the inhibition ability of EGCG on the expression of QS-regulated virulence factors was better than that of CIP, especially in the QS systems lasR/lasI, rhlR/rhlI and pqsR/pqsA. Thus, these results suggested that EGCG not only alleviates inflammation but also reduces the damage of P. aeruginosa infection by targeting QS system-regulated virulence, thereby resulting in a higher protective effect than CIP. EGCG is a great potential QS inhibitor against infection that not only reduces the release of virulence factors in vitro but also effectively inhibits the expression of virulence factors in vivo.
In conclusion, our results provide proof that EGCG alone can ameliorate acute P. aeruginosa infection in the lungs by QS and inflammation inhibition. The in vivo results indicate that the QS inhibitor, EGCG,s may serve as a potential approach in treating bacterial infections. EGCG, a rich ingredient of tea, is indispensable to human life and may play an important role in preventing bacterial infections.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Animal Ethical Committee of Sichuan Agricultural University.
HT and SH contributed to conceptualization, methodology, validation, and investigation. LZ and FS contributed to formal analysis and investigation. YL, QW, HG, CL, JL, and ZZ contributed to writing—original draft and resources. GY contributed to writing—review and editing, supervision, resources, and funding acquisition. All authors contributed to the article and approved the submitted version.
This work was supported by International Science and Technology Cooperation Program of Sichuan: 2020YFH0143; Traditional Chinese Veterinary Medicine Laboratory of the National Ethnic Affairs Commission [2020] 03.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aloush, V., Navon-Venezia, S., Seigman-Igra, Y., Cabili, S., and Carmeli, Y. (2006). Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob. Agents Chemother. 50, 43–48. doi: 10.1128/AAC.50.1.43-48.2006
Azambuja, J. H., Mancuso, R. I., Della Via, F. I., Torello, C. O., and Saad, S. T. O. (2022). Protective effect of green tea and epigallocatechin-3-gallate in a LPS-induced systemic inflammation model. J. Nutr. Biochem. 101:108920. doi: 10.1016/j.jnutbio.2021.108920
Balasubramanian, D., Schneper, L., Kumari, H., and Mathee, K. (2013). A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 41, 1–20. doi: 10.1093/nar/gks1039
Breidenstein, E. B., de la Fuente-Núñez, C., and Hancock, R. E. (2011). Pseudomonas aeruginosa: all roads lead to resistance. Trends Microbiol. 19, 419–426. doi: 10.1016/j.tim.2011.04.005
Chatterjee, M., Anju, C., Biswas, L., Kumar, V. A., Mohan, C. G., and Biswas, R. (2016). Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int. J. Med. Microbiol. 306, 48–58. doi: 10.1016/j.ijmm.2015.11.004
Clatworthy, A. E., Pierson, E., and Hung, D. T. (2007). Targeting virulence: a new paradigm for antimicrobial therapy. Nat. Chem. Biol. 3, 541–548. doi: 10.1038/nchembio.2007.24
Costerton, J. W., Stewart, P. S., and Greenberg, E. P. (1999). Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322. doi: 10.1126/science.284.5418.1318
Crousilles, A., Maunders, E., Bartlett, S., Fan, C., Ukor, E.-F., Abdelhamid, Y., et al. (2015). Which microbial factors really are important in Pseudomonas aeruginosa infections? Future Microbiol. 10, 1825–1836. doi: 10.2217/fmb.15.100
Dai, R.-X., Kong, Q.-H., Mao, B., Xu, W., Tao, R.-J., Wang, X.-R., et al. (2018). The mortality risk factor of community acquired pneumonia patients with chronic obstructive pulmonary disease: a retrospective cohort study. BMC Pulm. Med. 18, 12–10. doi: 10.1186/s12890-018-0587-7
Driscoll, J. A., Brody, S. L., and Kollef, M. H. (2007). The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 67, 351–368. doi: 10.2165/00003495-200767030-00003
Gellatly, S. L., and Hancock, R. E. (2013). Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Path. Dis. 67, 159–173. doi: 10.1111/2049-632X.12033
Hancock, R. E., and Speert, D. P. (2000). Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist. Updat. 3, 247–255. doi: 10.1054/drup.2000.0152
Hao, S., Yang, D., Zhao, L., Shi, F., Ye, G., Fu, H., et al. (2021). EGCG-mediated potential inhibition of biofilm development and quorum sensing in Pseudomonas aeruginosa. Int. J. Mol. Sci. 22:4946. doi: 10.3390/ijms22094946
Hauser, A. R. (2009). The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat. Rev. Microbiol. 7, 654–665. doi: 10.1038/nrmicro2199
Jain, S., Self, W. H., Wunderink, R. G., Fakhran, S., Balk, R., Bramley, A. M., et al. (2015). Community-acquired pneumonia requiring hospitalization among US adults. N. Engl. J. Med. 373, 415–427. doi: 10.1056/NEJMoa1500245
Jigisha, A., Nishant, R., Navin, K., and Pankaj, G. (2012). Green tea: a magical herb with miraculous outcomes. Int. Res. J. Pharm 3, 139–148.
Kipnis, E., Sawa, T., and Wiener-Kronish, J. (2006). Targeting mechanisms of Pseudomonas aeruginosa pathogenesis. Med. et Mal. Infect. 36, 78–91. doi: 10.1016/j.medmal.2005.10.007
Kizny Gordon, A. E., Mathers, A. J., Cheong, E. Y., Gottlieb, T., Kotay, S., Walker, A. S., et al. (2017). The hospital water environment as a reservoir for carbapenem-resistant organisms causing hospital-acquired infections—a systematic review of the literature. Clin. Infect. Dis. 64, 1435–1444. doi: 10.1093/cid/cix132
Lau, G. W., Hassett, D. J., Ran, H., and Kong, F. (2004). The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 10, 599–606. doi: 10.1016/j.molmed.2004.10.002
Lee, I.-T., Lin, C.-C., Lee, C.-Y., Hsieh, P.-W., and Yang, C.-M. (2013). Protective effects of (−)-epigallocatechin-3-gallate against TNF-α-induced lung inflammation via ROS-dependent ICAM-1 inhibition. J. Nutr. Biochem. 24, 124–136. doi: 10.1016/j.jnutbio.2012.03.009
Lee, J., and Zhang, L. (2015). The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 6, 26–41. doi: 10.1007/s13238-014-0100-x
Ling, J.-X., Wei, F., Li, N., Li, J.-L., Chen, L.-J., Liu, Y.-Y., et al. (2012). Amelioration of influenza virus-induced reactive oxygen species formation by epigallocatechin gallate derived from green tea. Acta Pharmacol. Sin. 33, 1533–1541. doi: 10.1038/aps.2012.80
Liu, W., Lu, H., Chu, X., Lou, T., Zhang, N., Zhang, B., et al. (2020). Tea polyphenols inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances resistance to Klebsiella pneumoniae infection in Caenorhabditis elegans model. Microb. Pathog. 147:104266. doi: 10.1016/j.micpath.2020.104266
Livermore, D. M. (2002). Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34, 634–640. doi: 10.1086/338782
Melsen, W. G., Rovers, M. M., Groenwold, R. H., Bergmans, D. C., Camus, C., Bauer, T. T., et al. (2013). Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 13, 665–671. doi: 10.1016/S1473-3099(13)70081-1
Moore, N. M., and Flaws, M. L. (2011). Antimicrobial resistance mechanisms in Pseudomonas aeruginosa. Clin. Lab. Sci. 24, 47–51. doi: 10.29074/ascls.24.1.47
Nadal Jimenez, P., Koch, G., Thompson, J. A., Xavier, K. B., Cool, R. H., and Quax, W. J. (2012). The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 76, 46–65. doi: 10.1128/MMBR.05007-11
O’Brien, K. L., Baggett, H. C., Brooks, W. A., Feikin, D. R., Hammitt, L. L., Higdon, M. M., et al. (2019). Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet 394, 757–779. doi: 10.1016/S0140-6736(19)30721-4
Pylaeva, E., Bordbari, S., Spyra, I., Decker, A. S., Häussler, S., Vybornov, V., et al. (2019). Detrimental effect of type I IFNs During acute lung infection With Pseudomonas aeruginosa is mediated Through the stimulation of neutrophil NETosis. Front. Immun. 10:2190, 2190. doi: 10.3389/fimmu.2019.02190
Quinton, L. J., and Mizgerd, J. P. (2015). Dynamics of lung defense in pneumonia: resistance, resilience, and remodeling. Annu. Rev. Physiol. 77, 407–430. doi: 10.1146/annurev-physiol-021014-071937
Ravi Kumar, S., Paudel, S., Ghimire, L., Bergeron, S., Cai, S., Zemans, R. L., et al. (2018). Emerging roles of inflammasomes in acute pneumonia. Am. J. Respir. Crit. Care Med. 197, 160–171. doi: 10.1164/rccm.201707-1391PP
Sadikot, R. T., Blackwell, T. S., Christman, J. W., and Prince, A. S. (2005). Pathogen–host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 171, 1209–1223. doi: 10.1164/rccm.200408-1044SO
Saeki, E. K., Kobayashi, R. K. T., and Nakazato, G. (2020). Quorum sensing system: target to control the spread of bacterial infections. Microb. Pathog. 142:104068. doi: 10.1016/j.micpath.2020.104068
Sauer, K., Camper, A. K., Ehrlich, G. D., Costerton, J. W., and Davies, D. G. (2002). Pseudomonas aeruginosa displays Multiple phenotypes during development as a biofilm. SAM J. 184, 1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002
Sen-Kilic, E., Blackwood, C. B., Boehm, D. T., Witt, W. T., Malkowski, A. C., Bevere, J. R., et al. (2019). Intranasal peptide-based FpvA-KLH conjugate vaccine protects mice from Pseudomonas aeruginosa acute murine pneumonia. Front. Immunol. 10:2497. doi: 10.3389/fimmu.2019.02497
Shapouri-Moghaddam, A., Mohammadian, S., Vazini, H., Taghadosi, M., Esmaeili, S. A., Mardani, F., et al. (2018). Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 233, 6425–6440. doi: 10.1002/jcp.26429
Sharma, A., Vaghasiya, K., Ray, E., Gupta, P., Gupta, U. D., Singh, A. K., et al. (2020). Targeted pulmonary delivery of the green tea polyphenol Epigallocatechin Gallate controls the growth of mycobacterium tuberculosis by enhancing the autophagy and suppressing bacterial burden. ACS Biomater Sci. Eng. 6, 4126–4140. doi: 10.1021/acsbiomaterials.0c00823
Skariyachan, S., Sridhar, V. S., Packirisamy, S., Kumargowda, S. T., and Challapilli, S. B. (2018). Recent perspectives on the molecular basis of biofilm formation by Pseudomonas aeruginosa and approaches for treatment and biofilm dispersal. Folia Microbiol. 63, 413–432. doi: 10.1007/s12223-018-0585-4
Sriram, N., Kalayarasan, S., and Sudhandiran, G. (2009). Epigallocatechin-3-gallate exhibits anti-fibrotic effect by attenuating bleomycin-induced glycoconjugates, lysosomal hydrolases and ultrastructural changes in rat model pulmonary fibrosis. Chem. Biol. Interact. 180, 271–280. doi: 10.1016/j.cbi.2009.02.017
Steinmann, J., Buer, J., Pietschmann, T., and Steinmann, E. (2013). Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 168, 1059–1073. doi: 10.1111/bph.12009
Traber, K. E., Dimbo, E. L., Symer, E. M., Korkmaz, F. T., Jones, M. R., Mizgerd, J. P., et al. (2019). Roles of interleukin-11 during acute bacterial pneumonia. PLoS One 14:e0221029. doi: 10.1371/journal.pone.0221029
Wang, J., Fan, S. M., and Zhang, J. (2019). Epigallocatechin-3-gallate ameliorates lipopolysaccharide-induced acute lung injury by suppression of TLR4/NF-κB signaling activation. Braz. J. Med. Biol. Res. 52:e8092. doi: 10.1590/1414-431x20198092
Yang, D., Hao, S., Zhao, L., Shi, F., Ye, G., Zou, Y., et al. (2021). Paeonol attenuates quorum-sensing regulated virulence and biofilm formation in Pseudomonas aeruginosa. Front. microbiol. 12:692474. doi: 10.3389/fmicb.2021.692474
Keywords: anti-virulence, quorum sensing, EGCG, P. aeruginosa, acute lung infection
Citation: Tang H, Hao S, Khan MF, Zhao L, Shi F, Li Y, Guo H, Zou Y, Lv C, Luo J, Zeng Z, Wu Q and Ye G (2022) Epigallocatechin-3-Gallate Ameliorates Acute Lung Damage by Inhibiting Quorum-Sensing-Related Virulence Factors of Pseudomonas aeruginosa. Front. Microbiol. 13:874354. doi: 10.3389/fmicb.2022.874354
Received: 12 February 2022; Accepted: 04 April 2022;
Published: 25 April 2022.
Edited by:
Laura Quintieri, Italian National Research Council, ItalyReviewed by:
Mohammad Minnatul Karim, Islamic University, BangladeshCopyright © 2022 Tang, Hao, Khan, Zhao, Shi, Li, Guo, Zou, Lv, Luo, Zeng, Wu and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Ye, eWVnYW5nX3NpY2F1QDE2My5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.