- 1College of Horticulture and Landscape Architecture, Tianjin Agricultural University, Tianjin, China
- 2College of Engineering and Technology, Tianjin Agricultural University, Tianjin, China
Subtilisin, a serine protease, can trigger defense responses in a wide variety of plants, both locally and systemically, to protect against pathogens. However, key residues of subtilisin to improve resistance to plant diseases remain unknown. In this study, Nicotiana benthamiana (N. benthamiana) leaves expressing subtilisin from Bacillus velezensis LJ02 were shown to improve protection against Botrytis cinerea (B. cinerea). Furthermore, the underlying mechanism that LJ02 subtilisin improved the protective effect was explored, and the direct inhibitory effect of subtilisin on B. cinerea was excluded in vitro. Subsequently, reactive oxygen species (ROS) burst and upregulation of resistance-related genes in systemic leaves of N. benthamiana further verified that subtilisin could induce systemic protection against B. cinerea. G307A/T308A and S213A/L214A/G215A subtilisin significantly reduced the ability to resist B. cinerea infection in N. benthamiana. Furthermore, the ROS content and expression levels of resistance-related genes of both mutants were significantly decreased compared with that of wild-type subtilisin. This work identified key residues essential for the activation function of subtilisin plant immunity and was crucial in inducing plant defense responses against B. cinerea.
Introduction
Botrytis cinerea (B. cinerea), a necrotrophic fungal pathogen, is the causal agent of blight, rot and gray mold in more than 1000 plant species (Fillinger and Elad, 2016), including almost all economically important vegetables, fruits and crops, and annually causes huge economic losses worldwide (Weiberg et al., 2013). Gray mold is prone to occur prior to harvest, or even at the seedling stage in some plants. In severe cases, the incidence of gray mold in plants can reach 100% (Wang et al., 2019), resulting in massive losses of plants seedlings. Bacillus velezensis (B.velezensis) such as CE100 (Choi et al., 2020), XT1 (Torres et al., 2020), and LJ02 (Li et al., 2015), as potential and efficient agents demonstrated robust biocontrol activity against B. cinerea. B. velezensis can encode immune proteins that can be recognized like the perception of a pathogen by plant to induce disease resistance, which has attracted wide attention, and thus has the advantage of triggering sophisticated and effective defense responses. It was found that flagellin from B. velezensis LJ02 can stimulate defense responses and increase resistance to Nicotiana tabacum var. Xanthi to Tobacco mosaic virus (TMV) (Wei et al., 2021). Further studies showed that PeBA1 of B. amyloliquefaciens NC6 induced resistance in Nicotiana tabacum against TMV (Wang et al., 2016). However, there are limited reports that immune proteins secreted by B. velezensis can enhance plants disease resistance to B. cinerea.
Plant recognition of pathogen-associated molecular pattern (PAMP) triggers a defense response known as PAMP-triggered immunity (PTI), which activates a cascade of signaling events that culminate in immune responses, including ion fluxes, activation of mitogen-activated protein kinases (MAPK), and the production of reactive oxygen species (ROS; Vatsa et al., 2011; Ngou et al., 2021). Bacterial PAMPs not only play an important role in basal resistance to pathogens but also contribute to the induction of systemic-acquired resistance (SAR; Mishina and Zeier, 2007). SAR, which often develops in uninfected areas of plants, is a systemic protection against other infections that gradually spread throughout the plant, and usually develops a long-lasting improved resistance to further attacks by pathogens (Gao et al., 2015). Recognition of PAMP recognition initiates the MAPK signaling cascade, one of the earliest signaling events consisting of the first step in PTI (Nie et al., 2017), and activates WRKY transcription factors (e.g., WRKY7/WRKY8), resulting in the upregulation expression of resistance-related genes (Zhong et al., 2018). PTI is accompanied by a set of induced responses that usually repel pathogen attacks, such as activation of PTI marker genes ACRE31 (Ma et al., 2021) and FRK (Wen et al., 2021). The ROS burst is believed to act downstream of PAMP/pathogen-responsive MAPKs (Yoshioka et al., 2003). Pti1 serine-threonine kinase acts early in PTI by inducing ROS production in response to perception of PAMP (Schwizer et al., 2017). ROS contributes to the restriction of further infection by pathogens, direct attack on pathogens, and triggering SAR (Alvarez et al., 1998; Torres et al., 2006). The rapidly generated Avr9/cf-9 genes (Acre) are candidates for components of signaling pathways involved in the activation of later defense responses in PTI. ACRE 31, a putative calcium-binding protein, can be rapidly induced by the elicitor and involved in the plant defense signaling cascade (Durrant et al., 2000).
Endogenous plant proteases are essential in many aspects of plant immunity (Rawlings et al., 2014), among which serine proteases are the most abundant in plants (Clemente et al., 2019). Subtilisins of the S8 family are one of the main serine proteases and have shown multiple roles in defence responses, ranging from immune priming to the activation of resistance-related genes, the generation of antimicrobial peptides, and the recognition or processing of pathogen effectors (Balakireva and Zamyatnin, 2018). Due to the large number of duplications, losses, and functional diversifications in the evolution of subtilisin superfamily, there are large differences in subtilisin phylogeny (Li et al., 2017). Subtilisin has a certain degree of conservation across different classes of microbes to general microbial fitness (Caro et al., 2020), while conserved functional residues are critically important for protein function. For example, specific residues of AtZAR1, a canonical CC-type NLR protein from Arabidopsis, are required for its immune function against Pseudomonas syringae pv. tomato DC3000 (Baudin et al., 2017). In search of whether the proteolytic activity of AsES, a subtilisin from Acremonium strictum, is required to trigger the defence response, its mutants at the active site (S226A) have confirmed that AsES induced the plant defense, and that enzymatic and eliciting activities were not associated (Caro et al., 2020). However, key residues of subtilisin triggering defence responses to pathogens remain largely unknown.
In this study, we focused on subtilisin secreted by Bacillus velezensis LJ02. Previous studies have shown that subtilisin can induce SAR and confer resistance against Botrytis cinerea in plants (Hael-Conrad et al., 2018). However, key residues of subtilisin essential for activation of plant defense responses were not yet identified. In this study, we verified the protective effect of subtilisin from LJ02 against B. cinerea by transient expression in Nicotiana benthamiana. ROS burst and upregulated expression of resistance-related genes showed that subtilisin can induce plant defense responses. Multiple alignment analysis of subtilisin revealed the conserved amino acids of subtilisin in different strains. We performed comparative analysis and mutation of conserved residues and expressed subtilisin and its mutants in N. benthamiana. Moreover, we measured ROS accumulation and resistance-related gene expression levels of systemic leaves to identify residues with key roles in subtilisin-activated plant defense responses.
Materials and Methods
Pathogen and Plant Culture
Nicotiana benthamiana was grown in an INE800 Memmert incubator at a temperature of 25°C in a 16 h light/8 h dark cycle for 4 weeks after sowing. B. velezensis LJ02 and B. cinerea were provided and preserved by the Plant Immunity and Biological Control Laboratory, Tianjin Agricultural College.
Subtilisin Protein Prokaryotic Expression and Purification
Genomic DNA from B. velezensis LJ02 was isolated using the Bacterial Genomic DNA Extraction kit (Solarbio). The subtilisin coding sequence (Supplementary Data 1) was cloned using genomic DNA from B. velezensis LJ02. Both amplified subtilisin fragments and prokaryotic expression vector pET-28a were ligated by Rapid DNA Ligation kit (Roche). Constructed pET-28a-subtilisin plasmid were transformed into the Escherichia coli BL21(DE3). The competent cells with OD600 of 0.6 were induced with isopropyl β-D-1-thiogalactopyranoside (IPTG) at 16°C for 14 h. Cells were harvested by centrifugation for 30 min at 4°C. Supernatants were removed and analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Cells were suspended in 10 ml of lysis buffer (50 mM Tris-HCl (pH 8.0), 300 mM NaCl, and 20% glycerol (w/v) prior to disruption at 4°C by ultrasonication (3 min × 1 min). Cell debris was pelleted by centrifugation for 1 h at 4°C. Supernatants were applied onto Ni Sepharose 6 Fast Flow (Cytiva) for purification. Recombinant subtilisin proteins were analyzed in 12% SDS-PAGE stained with Coomassie Brilliant Blue (Solarbio).
Western Blot
Proteins were extracted from plants as described by Han et al. (2022). Western blots were performed by SDS-PAGE with the Mini-PROTEA§ Tetra System (BIO-RAD). Proteins were transferred to the polyvinylidene fluoride (PVDF) membrane with the TransBlot turboTM system (Bio-rad), and the membrane was blocked for 2 h with 1 × Tris-buffered saline (TBS) containing skim milk (0.5%) followed by 2 h incubation with primary antibody. After washing with TBS, the membrane was incubated with the secondary antibody for 1 h, followed by wash with TBS. The photographic developer was sprayed (A solution + B solution mix at 1:1) onto the membrane. The primary antibody was Anti-DYKDDDDK Monoclonal Antibody (TRANS), Actin mouse anti-antibody (Bioss), and the secondary antibody was Goat Anti-Mouse IgG, HRP (TRANS). The blot was recorded using Tanon 5200 Multi.
Subtilisin Protease Assay
As described previously by Kobayashi et al. (1995) and Jaouadi et al. (2012), subtilisin protease activity was measured with N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide (Suc-AAPF-pNA; 2 mg/ml in 50% dimethyl formamide) as substrates. One hundred microliters of enzyme prepared in 50 mM Tris–HCl buffer (pH 8.0), containing 5 mM CaCl2 at a concentration of 8.25 mg/ml, were added to 20 ml of substrate. After incubation at 37°C for 2 h, the amount of p-nitroaniline released was measured by monitoring the absorbance at 405 nm. A sample blank without substrate was routinely included. One unit (U) of enzymatic activity was defined as the amount of enzyme that liberated 1 μmol of p-nitroaniline per minute under the conditions of the assay.
The activity of the subtilisin protease was measured at pH values ranging from 3 to 11 using Nsuc-AAPF-pNA as the substrate. The following 50 mM buffers containing 5 mM CaCl2 were used: (pH 3.0–5.0), Tris–HCl (pH 6.0–8.0), and carbonate (pH 9.0–11.0). The effect of incubation temperature (25, 30, 35, 40, and 45°C) and storage time (0, 1, 2, 3, and 4 days) on the degradation of Nsuc-AAPF-pNA by the subtilisin protease was evaluated.
Effects of Subtilisin on Botrytis cinerea
All plates and materials were sterilized in an autoclave oven before experiments, and experimental operations were conducted in a sterile bench. The oxford cup method was conducted according to previously described method (Li et al., 2013). Antifungal activity was evaluated by the diameter of transparent circles around oxford cups.
Botrytis cinerea was induced to sporulate on potato dextrose agar (PDA) medium. Spores were scraped from the agar plates and then made into a spore suspension (30–100 spores per field of view under low power). Spores Germination Assays were performed according to the method of Maribel (Plascencia-Jatomea et al., 2003). The number of spore germination was checked under the microscope (the length of the germ tube was longer than the short radius of the spore was regarded as germination) after 12 h. The inhibition rate of spore germination was calculated according to the method described previously (Zhao et al., 2014).
Subtilisin was infiltrated into the lower leaves of N. benthamiana by needleless syringe. Upper systemic leaves of N. benthamiana were collected and inoculated with mycelial discs of B. cinerea at 3 days after infiltrated (DAI; Wang et al., 2016). The inoculated leaves are stored in a humid chamber. The lesions’ diameter was measured using the criss-cross method (Ji et al., 2014) and then photographed.
Sequence Alignment, Classification, and Phylogenetic Tree Analysis
Totally, 20 top organisms of subtilisin amino acid sequences by taxon were downloaded from NCBI (Supplementary Data 2) and analyzed using DNAMAN for multiple alignments to identify and present conserved residues. The evolutionary history was inferred using the UPGMA method (Sneath and Sokal, 1973). The optimal tree is shown. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site. Poorly aligned positions and divergent regions of MSA were eliminated using Gblocks Version 0.91b with a less stringent setting to make it more suitable for phylogenetic analysis. Then, a neighbor-joining (NJ) tree was constructed using MEGA 11 with 1,000 of bootstrap replications, pairwise deletion, and Poisson model.
Transient Expression of Subtilisin and Its Mutants in Nicotiana benthamiana
The subtilisin gene was amplified and homologously recombined with the pK7WG2D (a transient expression vector, expressed by 35S promoter) by using the ClonExpressII One Step Cloning Kit (Vazyme Biotech). The point mutation was introduced into the subtilisin DNA fragments by fusion PCR method with primers bearing desired point mutation. In total, 13 coding sequences of subtilisin mutants were amplified and cloned into vector pK7WG2D. The primer sequences and vectors used for the plasmid constructs are shown in Supplementary Table 4. The constructed vectors were transformed into Agrobacterium GV3101. Agrobacterium-mediated transient expression was performed as described (Li Z. et al., 2019). The upper systemic leaves of N. benthamiana were collected and inoculated with B. cinerea mycelial discs at 3 DAI as previously described. The inoculated leaves were stored in a humid chamber. The lesions’ diameter were measured using the criss-cross-method (Ji et al., 2014) at 3 days after inoculation with B. cinerea and then photographed.
ROS Measurement
The generation of ROS during subtilisin infiltration was determined using a commercial plant ROS enzyme-linked immnunosorbent assay (ELISA) kit (Chundubio). Three or four upper leaves without infiltration of N. benthamiana were harvested at various times after infiltration with subtilisin, 0.1 g was fully ground in liquid nitrogen, and phosphate-buffered saline (pH 7.4) was added. The plant tissue was homogenized with a low-temperature homogenizer, centrifuged twice for about 20 min, and then the precipitate was discarded for use in ELISA. The standard curve was used to determine the amount in each unknown sample. OD450 values were measured with an ELISA reader (Promega). Meanwhile, ROS generation was detected using 3,3′-diaminobenzidine (DAB) solution (Solarbio) as described previously (Yang et al., 2018). Leaves were then decolorized in boiling ethanol (90%) for 30 min and were photographed by camera (Zhao et al., 2018).
RNA Extraction and Quantitative RT-PCR
WRKY7, WRKY8, ACRE31, Pti1, CYP71D20, and FRK primers in N. benthamiana genome database1 were designed using the SnapGene software. Actin (Tao et al., 2011) was used as the internal reference gene (Supplementary Table 5). The extraction of total RNA from N. benthamiana leave samples was carried out according to the instructions of TIANGEN RNAsimple Total RNA Extraction Kit. Using total RNA as a template, Takara Prime Script TM RT regent Kit with gDNA Eraser (Perfect Real-Time) was used for reverse transcription. The primers and reaction system were added according to the instructions (Takara). The 2–ΔΔCt calculation method was used for analysis (Livak and Schmittgen, 2001).
Results
Subtilisin Improves the Protective Effect of Nicotiana benthamiana to Botrytis cinerea
The systemic leaves of N. benthamiana expressing pK7WG2D (CK) and N. benthamiana expressing pK7WG2D-subtilisin (NES) were inoculated with B. cinerea. A severe symptom was observed on the systemic leaves of CK compared with NES at 3 days after inoculation (Figure 1A), and the lesion diameter on the systemic leaves of NES was significantly smaller than that of CK (Figure 1B). Western blot analysis of NES leaves showed that the subtilisin protein was stably accumulated at 3 DAI (Figure 1C). In addition, the lesion diameter of the systemic leaves in N. benthamiana infiltrated with purified subtilisin protein was significantly smaller than that in N. benthamiana infiltrated with buffer, similar to the transient expression results (Supplementary Figure 1).
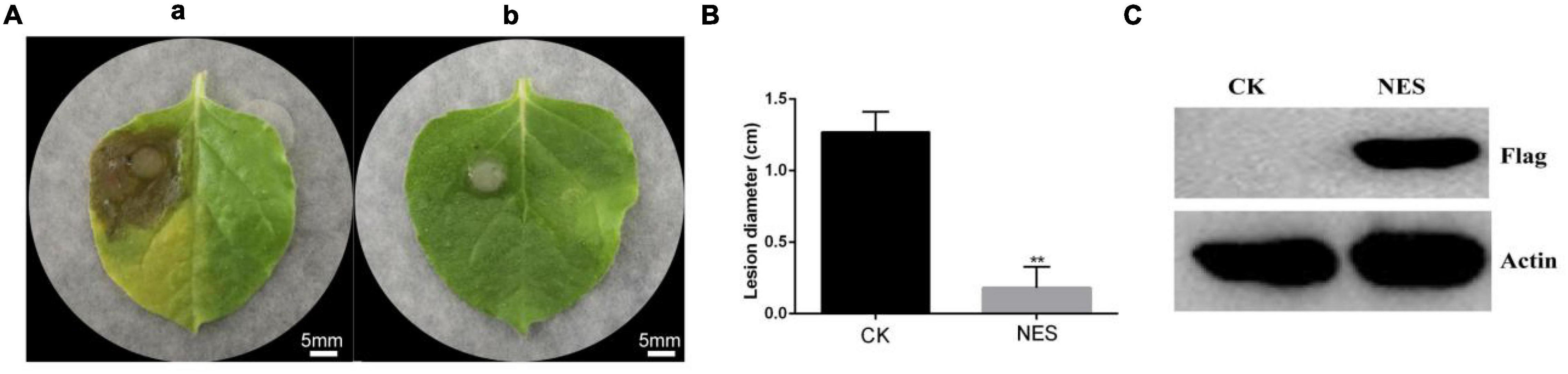
Figure 1. Subtilisin transient expression improves the control effect in N. benthamiana against B. cinerea. (A) Representative phenotypes of the disease caused by B. cinerea in N. benthamiana leaves expressing pK7WG2D (a) and pK7WG2D-subtilisin (b) at 3 DAI. Photographs were taken at 3 days after inoculation with B. cinerea. Experiments were carried out with five leaves per treatment. (B) Lesion diameter of disease caused by B. cinerea in N. benthamiana leaves expressing pK7WG2D (CK) and pK7WG2D subtilisin (NES). Data presented in (B) are the means ± SD of lesion diameter of five leaves. The statistical analyses were performed using the Student’s t-test; and asterisks indicate significant differences between pK7WG2D and pK7WG2D-subtilisin treatment (** p < 0.01). (C) Protein accumulation detected by western blot using anti-flag and anti-Actin antibody in N. benthamiana leaves expressing pK7WG2D (CK) and pK7WG2D subtilisin (NES).
Subtilisin Has No Direct Inhibitory Effect on Botrytis cinerea
Subtilisin has been shown to provide protection in N. benthamiana against B. cinerea. To further determine whether subtilisin itself has a direct antifungal effect against B. cinerea, subtilisin protein activity was measured, the purified subtilisin protein was used for bacteriostatic experiments (Figure 2A), and the experimental group with protein buffer was used as CK. The activity of subtilisin protein was relatively stable in PDA medium when incubated at 28°C for 3 days (Supplementary Figure 2), and there is no inhibition zone around the oxford cup with purified subtilisin (Figure 2B), indicating that subtilisin had no clear inhibitory effect on hyphal growth in vitro. Furthermore, the germination rate of spores inoculated with purified subtilisin did not show significant differences compared with that of CK (Figure 2C), suggesting that subtilisin does not have an effect on the germination of spores. Together, the hypothesis that subtilisin directly inhibits the growth of B. cinerea was ruled out.
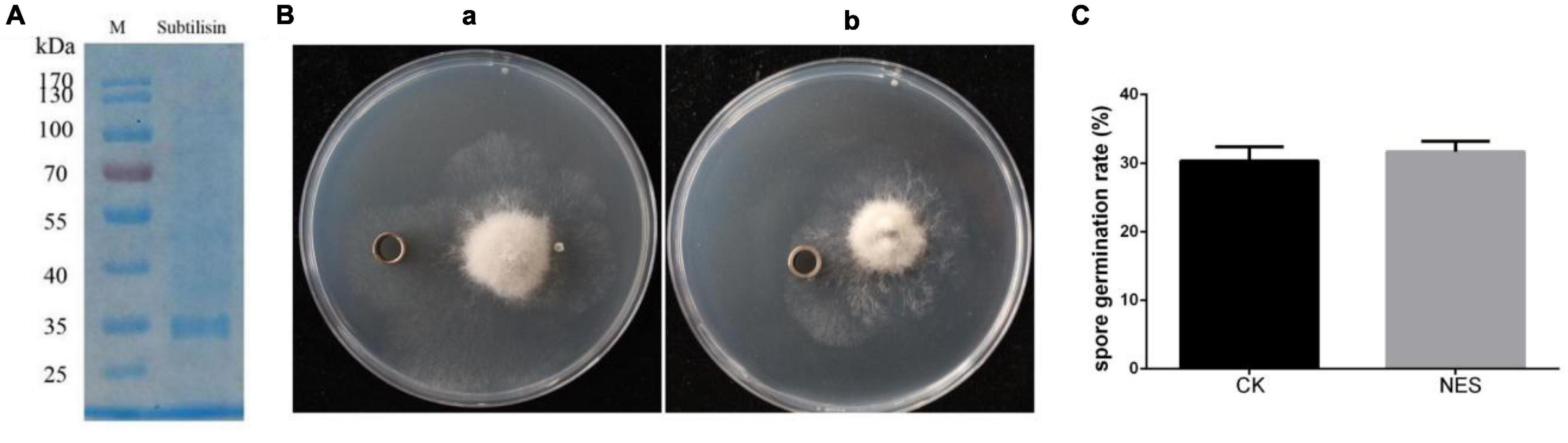
Figure 2. Antifungal effect of subtilisin in vitro. (A) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) image of purified subtilisin protein, Molecular mass markers (M) indicated on the left from top to bottom is 170, 130, 100, 70, 55, 40, 35, and 25 kDa. (B) Antifungal experiment of buffer (a) and purified subtilisin (b) in vitro. After B. cinerea was cultured on the potato dextrose agar (PDA) medium for 3 days, 100 μl of 100 μg⋅ml–1 subtilisin protein was added to the Oxford cup and placed in a 28°C incubator for 3 days, and then, the diameter of transparent circles around oxford cups was observed. (C) The effect of subtilisin on the B. cinerea germination. Data presented in (C) are the means ± SD from three independent experiments. The statistical analyses were performed using the Student’s t-test, and asterisks indicate significant differences.
Subtilisin Stimulates the Immune Resistance of Nicotiana benthamiana
Since subtilisin cannot inhibit the growth of hyphals and germination of B. cinerea, it implied that subtilisin may act by an effect on plant defense responses. To further verify this hypothesis, we characterized the defense responses induced by subtilisin in N. benthamiana. The standard curve was generated by plotting the average OD450 of ROS standard concentrations (Figure 3A) and the ROS accumulation and concentration in N. benthamiana expressing PK7WG2D (CK) and pK7WG2D-subtilisin (NES) at various times were analyzed. Compared with CK, ROS concentration (Figure 3B) and brown DAB-stained precipitates (Figure 3C) in NES were increased at 2–4 DAI, demonstrating that subtilisin infiltration of N. benthamiana can cause ROS accumulation, a typical phenotype of plant defense response.
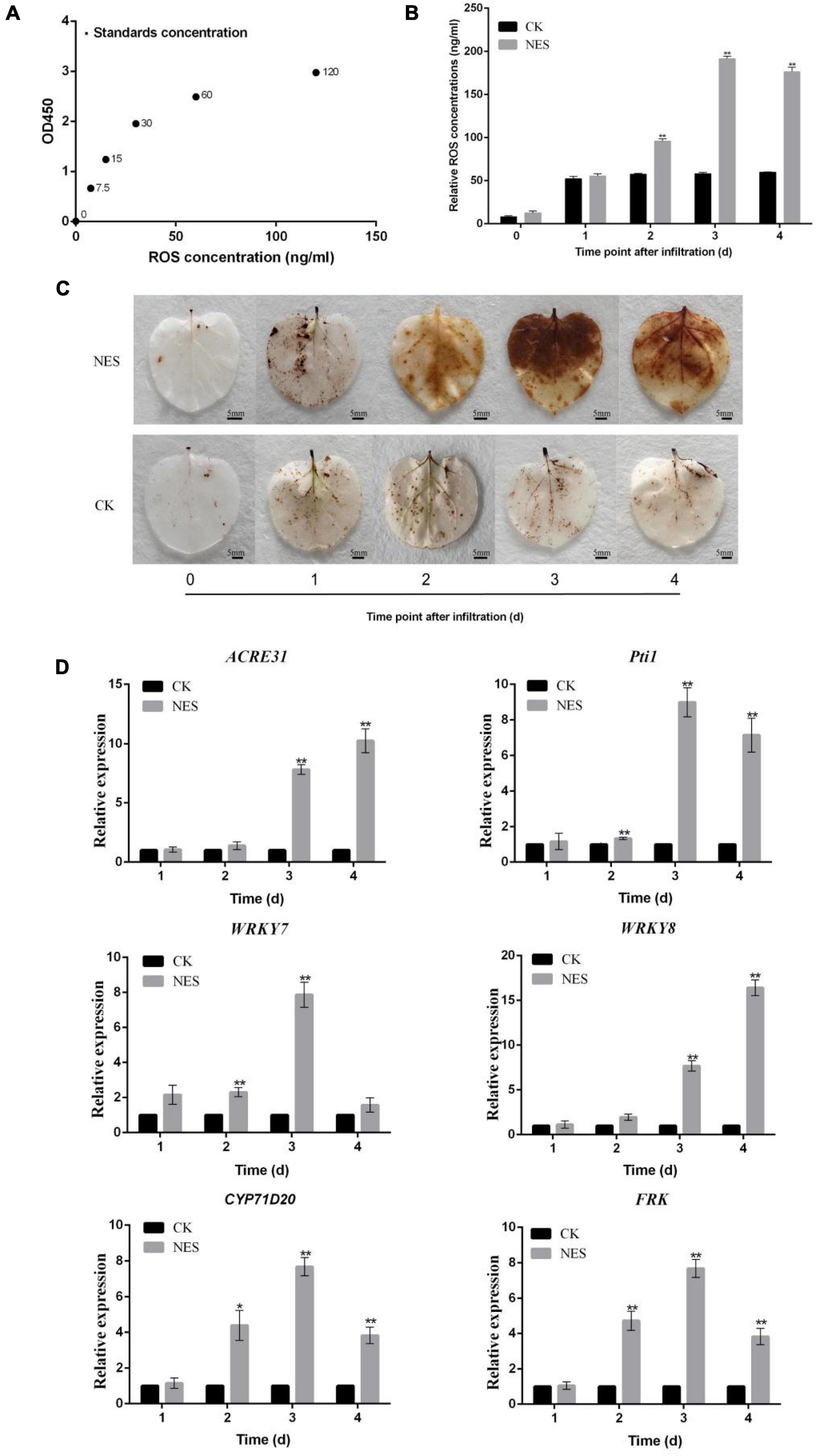
Figure 3. Changes in reactive oxygen species (ROS) and gene expression in response to subtilisin. (A) Standard curve for the determination of ROS concentration. The standard curve was generated by plotting the average OD450 of the six plant ROS standard concentrations (0, 7.5, 15, 30, 60, and 120 ng/ml) provided in the kit on the vertical axis (X) vs. the corresponding concentration on the horizontal axis (Y). (B) ROS concentration in N. benthamiana leaves expressing pK7WG2D (CK) and pK7WG2D-subtilisin (NES) measured by enzyme-linked immunosorbent assay (ELISA) at 1–4 DAI. (C) Accumulation of ROS was analyzed in N. benthamiana leaves expressing pK7WG2D (CK) and pK7WG2D-subtilisin (NES) at 1–4 DAI. ROS was visualized by 3,3′ diaminobenzidine (DAB) staining methods. Brownish deposits were indicative of ROS. (D) Expression analysis of resistance-related genes WRKY7/8, ACRE31, Pti1, CYP71D20, and FRK in N. benthamiana expressing pK7WG2D (CK) and pK7WG2D-subtilisin (NES) at 1–4 DAI. The samples were normalized against Actin and expression levels are represented as fold changes relative to the control. Data presented in (D) are the means ± SD of three independent experiments. The statistical analyses were performed using the Student’s t-test, and asterisks indicate significant differences (*p < 0.05; **p < 0.01).
Changes in expression pattern of resistance-related genes are important plant responses to PAMPs and pathogen (Meng and Zhang, 2013). Therefore, the expression levels of resistance-related genes WRKY7, WRKY8, ACRE31, Pti1, CYP71D20, and FRK were systematically investigated in CK and NES at 1–4 DAI. Compared with CK, the expression levels of WRKY7 of NES all showed a significant increase at 2 DAI and 3 DAI. ACRE31 and WRKY8 expression levels of NES all showed significantly increased at 3 DAI and 4 DAI. Pti1, CYP71D20, and FRK expression levels of NES were significantly higher than those of CK at 2–4 DAI (Figure 3D). The expression of the resistance-related gene indicated that subtilisin is involved in the activation of plant defense responses in N. benthamiana.
Identification of Conserved Amino Acids in Subtilisin
Considering the domain conservation of subtilisin may be the result of evolution, multiple sequence alignments were performed to identify the conserved residues in subtilisin, which may be helpful to elucidate the mechanism for its immune functions. We conducted phylogenetic tree analysis (Figure 4A) and similarity analysis (Supplementary Figure 2) of 20 amino acid sequences of subtilisin. After comparing sequences with identities greater than 75%, a total of 13 conserved sites were selected. To determine which amino acids play a key role in subtilisin-induced immunity, we generated subtilisin mutants in which these conserved sites were replaced with alanine. In total, 13 subtilisin mutants were constructed (Figure 4B).

Figure 4. Multiple sequence alignment of subtilisins. (A) Phylogenetic analysis of 20 subtilisin amino acid sequences after grouping. (B) Identification of conserved residues in subtilisin for point mutation analysis. List of point mutation sites for subtilisin in each subtilisin construct. All selected conserved sites of amino acids were mutated to alanine. Taking M1 as an example, 116V, 120D, and 122G were mutated to A and are thus noted as V116A, D120A, and G122A.
Conserved Amino Acids of Subtilisin Were Crucial for Its Induced Resistance Activity
To determine the induced resistance activity of the subtilisin mutants, systemic leaves of N. benthamiana expressing subtilisin and its mutants were inoculated with B. cinerea. The resistance of N. benthamiana expressing subtilisin-p7 mutants (M7, S213A/L214A/G215A) and subtilisin-p13 mutants (M13, G307A/T308A) to B. cinerea showed small differences compared with that of N. benthamiana expressing pK7WG2D control (EV) but lower than that in N. benthamiana expressing wild-type subtilisin (WT). The resistance of N. benthamiana expressing other 11 mutants except M7 and M13 to B. cinerea showed small differences compared with WT but higher than EV (Figure 5A). The lesion diameter of M7 and M13 showed significantly larger than WT but formed small differences compared with EV, and the lesion diameter of other 11 mutants except M7 and M13 showed significantly smaller than EV but formed small differences compared with WT (Figure 5B), indicating M7 and M13 reduced disease resistance to B. cinerea in N. benthamiana. Furthermore, the lesion diameter of M7 was smaller than that of M13.
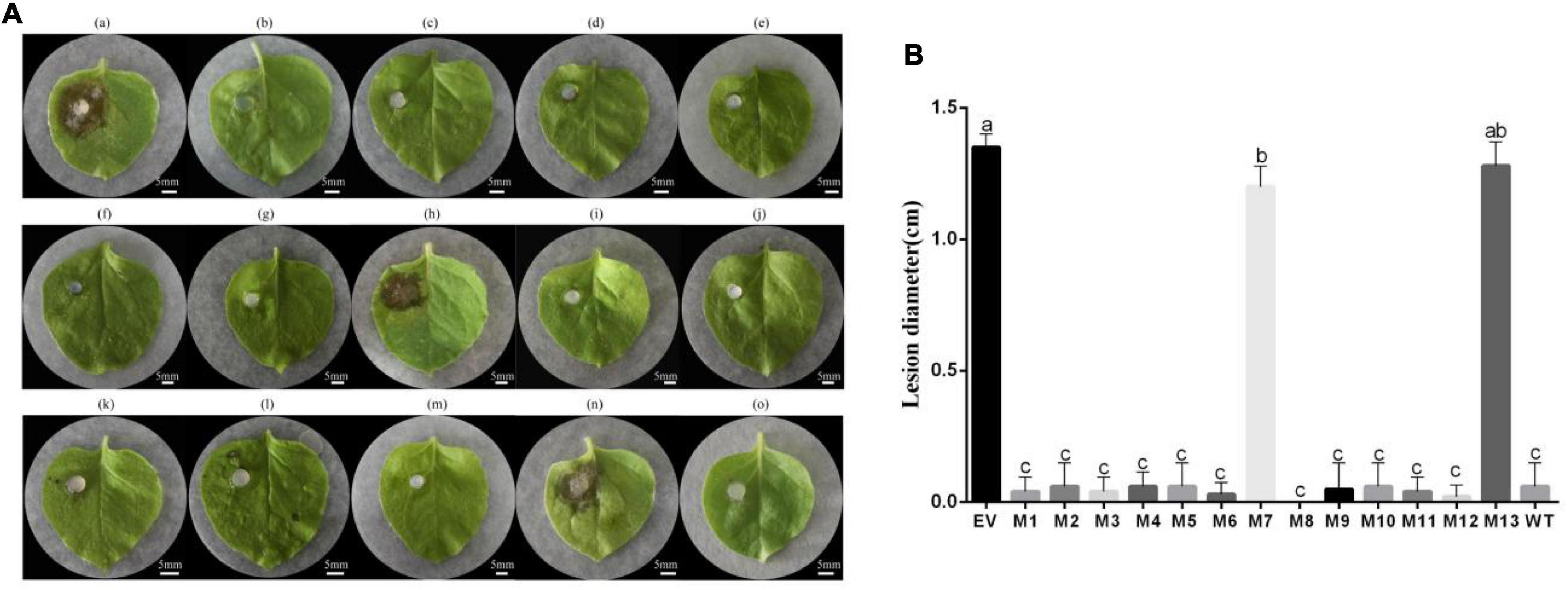
Figure 5. The transient expression vector of subtilisin mutants induced resistance in N. benthamiana against B. cinerea. (A) (a)–(o), respectively, are representative phenotypes of disease caused by B. cinerea in leaves of N. benthamiana expressing pK7WG2D control (EV), subtilisin-p1 mutants (M1), subtilisin-p2 mutants (M2), subtilisin-p3 mutants (M3), subtilisin-p4 mutants (M4), subtilisin-p5 mutants (M5), subtilisin-p6 mutants (M6), subtilisin-p7 mutants (M7), subtilisin-p8 mutants (M8), subtilisin-p9 mutants (M9), subtilisin-p10 mutants (M10), subtilisin-p11 mutants (M11), subtilisin-p12 mutants (M12), subtilisin-p13 mutants (M13), and wild-type subtilisin (WT). Photographs were taken at 3 days after inoculation with B. cinerea. Experiments were carried out with five leaves per treatment. (B) Lesion diameter caused by B. cinerea was measured in N. benthamiana leaves expressing EV, M1, M2, M3, M4, M5, M6, M7, M8, M9, M10, M11, M12, M13, and WT. Data presented in (B) are the means ± SD of lesion diameter of five leaves. Columns with different letters indicate significant differences according to Duncan’s multiple tests (p < 0.05).
Reactive oxygen species plays a vital role in pathogen–plant interactions as signaling modules (Zhang et al., 2018) and has a crucial role in plants systemic resistance (Bóka and Orbán, 2007). Therefore, we generated the standard curve by plotting the average OD450 of ROS standard concentrations (Figure 6A) and detected the ROS accumulation and concentration in EV, M7, M13, and WT at different time points. Compared with WT, the ROS accumulation and concentration of M7 and M13 decreased at 2–4 DAI. The ROS accumulation and concentration of M7 and M13 were still higher than that of EV at 2–4 DAI. Furthermore, the concentration (Figure 6B) and ROS accumulation (Figure 6C) of M7 were higher than that of M13 at 2–4 DAI. These results established that the mutation of G307A/T308A and S213A/L214A/G215A impaired subtilisin-mediated ROS production in N. benthamiana, and the weakening effect of G307A/T308A was even greater.
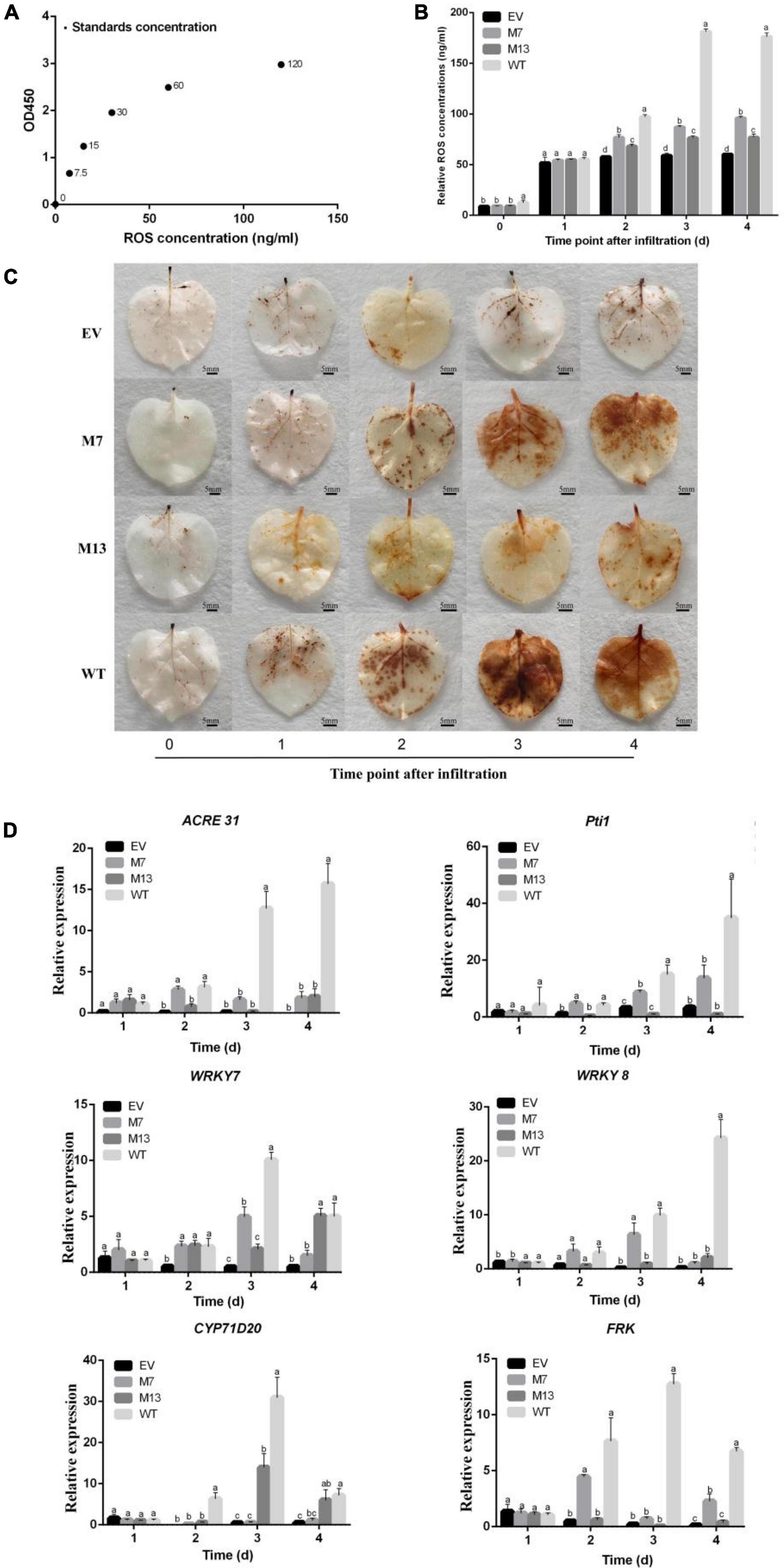
Figure 6. Changes in ROS and gene expression in response to subtilisin mutants. (A) The standard curve was generated from the average optical density OD450 obtained for each of the six plant ROS standard concentrations (0, 7.5, 15, 30, 60, and 220 ng/ml). (B) The ROS concentration of N. benthamiana expressing pK7WG2D control (EV), subtilisin-p7 mutants (M7), subtilisin-p13 mutants (M13), and wild-type subtilisin (WT) were measured by ELISA at various times (0, 1, 2, 3, and 4 days). (C) Accumulation of ROS was analyzed in N. benthamiana leaves expressing pK7WG2D control (EV), subtilisin-p7 mutants (M7), subtilisin-p13 mutants (M13), and wild-type subtilisin (WT) at 0–4 DAI. ROS was visualized by 3,3′ diaminobenzidine (DAB) staining methods. Brownish deposits were indicative of ROS. (D) Expression analysis of resistance-related genes WRKY7/8, ACRE31, Pti1, CYP71D20, and FRK in EV, M7, M13, and WT at 1–4 DAI. The samples were normalized against Actin and expression levels are represented as fold changes relative to the control. Data are means ± SD of three independent experiments. Columns with different letters indicate significant differences according to Duncan’s multiple tests (p < 0.05).
To further illustrate the roles of G307/T308 and S213/L214/G215 in subtilisin-induced disease resistance, we analyzed the expression levels of the WRKY7, WRKY8, ACRE31, Pti1, CYP71D20, and FRK gene in EV, M7, M13, and WT. RT-qPCR analysis showed that Pti1, ACRE31, FRK, and WRKY7 expression levels in M7 decreased significantly compared with those of WT at 3–4 DAI, among which ACRE31 and FRK expression levels of M7 did not differ significantly from those of EV at 3 DAI, and ACRE31, Pti1, and WRKY7 expression levels in M7 did not differ significantly from those of EV at 4 DAI. The expression levels of CYP71D20 in M7 decreased significantly compared with those of WT at 2–4 DAI. WRKY8 expression levels of M7 were significantly decreased compared with those of WT at 4 DAI. ACRE31, FRK, and Pti1 expression levels of M13 were significantly decreased compared with WT at 2–4 DAI. CYP71D20 expression levels of M13 were significantly decreased compared with WT at 2 DAI and 3 DAI. WRKY8 expression levels in M13 decreased significantly compared with those of WT, while not significantly different from EV in 3 DAI and 4 DAI (Figure 6D). ACRE31, FRK, and Pti1 of M7 were significantly higher than those of M13 at 2 DAI. The expression levels of pti1, WRKY7, and WRKY8 in M7 were significantly higher than those of M13 at 3 DAI.
Discussion
In recent years, proteases related to plant immunity have gained increasing attention (Thomas and Van Der Hoorn, 2018). Subtilisin, a serine protease, is involved in plant–pathogen resistance and plays an important role in pathogen recognition and initiation of resistance-related signal pathways (Xu et al., 2020). Most of the subtilisin reported so far are directly or indirectly generated by plants in order to recognize one or more components secreted by the attackers and as a consequence activate defense responses to prevent pathogens invasion (Figueiredo et al., 2014). In this study, we observed a systemic effect of subtilisin in N. bethamiana against B. cinerea. The lesion diameter of the systemic leaf in NES was significantly smaller than that of CK at 3 DAI (Figure 1). Furthermore, subtilisin itself did not cause the inhibition of B. cinerea in vitro (Figure 2). On the basis of these results, we assumed that the induced protection is due to plant systemic resistance rather than directly bacteriostatic effect to the fungus. The result was in line with previous studies reporting that the protection exerted by AsES against B. cinerea (Hael-Conrad et al., 2015) was systemically induced. Establishment of systemic resistance was usually accompanied with potentiated activation of various cellular defense responses against other pathogens infection (Conrath et al., 2006). Thus, in an attempt to characterize the systemic resistance, the production and concentration of ROS were analyzed in a systemic leaves. NES showed a significantly higher ROS concentration (Figure 3B) and more brown DAB-stained precipitates (Figure 3C) than that of CK at 2–4 DAI. This implied that subtilisin could prime plants for an increased and faster capacity to activate ROS accumulation in systemic tissue, which was consistent with the results reported by Li on the PeFOC1 protein elicitor that can activate ROS burst and trigger systemic resistance in N. tabacum cv. samsun NN (Li S. et al., 2019). The same ROS burst was also found in Zhang’s research on SsCut protein elicitor (Zhang et al., 2014).
Pathogen-associated molecular pattern–triggered immunity (PTI) plays an important role in plant disease resistance against B. cinerea and was a potent elicitor to induce SAR. Therefore, we examined some typical PTI elements during the subtilisin-induced SAR to further investigate the relationship between SAR and PTI. WRKYs participated in mediating disease resistance and expression of resistance-related genes in SAR (Birkenbihl et al., 2012). WRKY7/WRKY8 functioned downstream of MAPK during PTI. MAPKs were also reported to act upstream of ROS accumulation (Meng and Zhang, 2013). Pti1 can induce ROS production in response to PAMP perception. ACRE31 was rapidly induced by the elicitor and was involved in a later plant defense signaling cascade. Systemic signals were produced in the primary infected leaves and signal movement into distant organs (leaves). Tissues exhibiting SAR in the distant, pathogen-free parts of plants, displayed a “prepared” state associated with faster and stronger defence mechanisms (Ádám et al., 2018). The expression level of WRKY7 of untreated distal leaves in NES was significantly higher than that of CK at 2 DAI and 3 DAI. The Pti1 expression level of NES was significantly increased compared with CK at 2–4 DAI (Figure 3D), suggesting that MAPK activation by subtilisin may be related to the ROS burst against pathogen infection. We also examined PTI marker genes, such as FRK and CYP71D20 (Dagvadorj et al., 2017). qRT-PCR analysis showed that the expression level of FRK and CYP71D20 was significantly increased in NES at 2–4 DAI (Figure 3D), implying that the generation of subtilisin-induced SAR may be associated with the ROS burst and the activation response of PTI. In line with previous results, it was shown that AsES induces a systemic protection against Colletotrichum acutatum in strawberry (Chalfoun et al., 2013). These results confirmed that there is a close association between subtilisin-induced SAR and activation of PTI.
Pathogen-associated molecular patterns have a certain degree of conservation across different classes of microbes to general microbial fitness, whereas effectors that either facilitate infection or trigger defense responses often exhibit species-, race- or strain-specific as a result of natural selection (Yang et al., 2017), and the common distinction between PAMP and effectors cannot always be strictly maintained (Thomma et al., 2011). Subtilisins are especially abundant in plants and have undergone evolution, and the subtilisin encoding gene sequence is highly specific. Subtilisin from B. velezensis LJ02 was evolutionarily closely related to Sarocladium strictum and Bacillus anthracis because it showed the highest similarity (Figure 4A). Conserved functional residues played critically important role in protein function. For example, Li demonstrated that conservative sites of the Arabidopsis thaliana natural resistance-associated macrophage protein, D72 and N75, were essential for transport activity (Li et al., 2018). Pawel Z suggested that Thr807, Thr812, Tyr815, and Tyr820 of receptor-like kinase ERECTA in the activation segment of the kinase domain were functionally important (Kosentka et al., 2017). Since several subtilisin homologs can all stimulate the defense responses of plants to B. cinerea, such as the fungal subtilase AsES (Ádám et al., 2018) and the subtilisin-like protease Bcser2 (Liu et al., 2020), we speculated that one or more conserved amino acids from the active site or adjacent residues may be essential for subtilisin to induced defense response in different species. Sites with more than 75% similarity of 20 homologous subtilisin sequences were selected, and a total of 13 conserved sites were obtained (Figure 4B). To identify conserved residues that may be important for the defense response induced by subtilisin, we generated 13 point mutants with alanine substitutions for those conserved sites and found that the lesion diameter of M1, M2, M3, M4, M5, M6, M8, M9, M10, M11, and M12 formed small differences compared with WT, but all M7 and M13 resulted in a significantly decreased resistance of N. benthamiana resistance to B. cinerea compared with that of WT by the Agrobacterium infiltration assay (Figure 5), indicating that G307/T308 and S213/L214/G215 were indispensable for the resistance induced by subtilisin to B. cinerea. The ROS concentration (Figure 6B) and accumulation (Figure 6C) of M7 and M13 showed decreased compared with WT at 2–4 DAI, and M13 exhibited lower ROS accumulation and concentration than M7. The expression levels of Pti1, ACRE31, FRK, and WRKY7 in M7 were significantly decreased compared with those of WT at 3 DAI and 4 DAI. The expression levels of CYP71D20 in M7 formed significantly decreased compared with WT at 2–4 DAI. WRKY8 expression levels of M7 were decreased significantly compared to WT at 4 DAI. Pti1, ACRE31, and FRK expression levels of M13 formed significantly decreased compared with WT at 2–4 DAI, and showed no significantly different from that in EV. CYP71D20 expression levels of M13 were significantly decreased compared with WT at 2 DAI and 3 DAI. Furthermore, WRKY8 expression levels decreased significantly compared with those of WT and were not significantly different from those of EV at 3 DAI and 4 DAI (Figure 6D). These combined results suggested that mutations of G307/T308 or S213/L214/G215 directly affect the activation of plant defense responses. These amino acids may be the key sites for subtilisin to improve disease resistance. The reason why these amino acid sites activate plant defense responses, such as active oxygen bursts, is not known. This study provided theoretical guidance for further analysis of the key functional sites at which subtilisin can activate defense responses and contribute to the development of disease biocontrol strategies in plants.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
YW and ZL designed the study. JH performed the research, analyzed most of the data, and wrote the first draft of the manuscript. YY and RC contributed to refining the ideas and finalizing this manuscript. YW and ZL wrote the final draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was funded by the National Key R&D Program (SQ2017ZY060083) and Scientific Project of Tianjin Municipal Education Commission (2021KJ105).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Qing Wei for advice on this manuscript. We also thank the National Key R&D Program (SQ2017ZY060083) and Scientific Project of Tianjin Municipal Education Commission (2021KJ105) for supporting this work.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.869596/full#supplementary-material
Footnotes
References
Ádám, A. L., Nagy, Z. Á, Kátay, G., Mergenthaler, E., and Viczián, O. (2018). Signals of systemic immunity in plants: progress and open questions. Int. J. Mol. Sci. 19:1146. doi: 10.3390/ijms19041146
Alvarez, M. E., Pennell, R. I., Meijer, P. J., Ishikawa, A., Dixon, R. A., and Lamb, C. (1998). Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92, 773–784. doi: 10.1016/1369-5266(88)80032-3
Balakireva, A. V., and Zamyatnin, A. A. (2018). Indispensable role of proteases in plant innate immunity. Int. J. Mol. Sci. 19:629. doi: 10.3390/ijms19020629
Baudin, M., Hassan, J. A., Schreiber, K. J., and Lewis, J. D. (2017). Analysis of the ZAR1 immune complex reveals determinants for immunity and molecular interactions. Plant Physiol. 174, 2038–2053. doi: 10.1104/pp.17.00441
Birkenbihl, R. P., Diezel, C., and Somssich, I. E. (2012). Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiol. 159, 266–285. doi: 10.1104/pp.111.192641
Bóka, K., and Orbán, N. (2007). New aspect of H2O2 signaling. Plant Signal. Behav. 2, 498–500. doi: 10.4161/psb.2.6.4582
Caro, M. D. P., Holton, N., Conti, G., Venturuzzi, A. L., Martínez-Zamora, M. G., Zipfel, C., et al. (2020). The fungal subtilase AsES elicits a PTI-like defence response in Arabidopsis thaliana plants independently of its enzymatic activity. Mol. Plant Pathol. 21, 147–159. doi: 10.1111/mpp.12881
Chalfoun, N. R., Grellet-Bournonville, C. F., Martínez-Zamora, M. G., Díaz-Perales, A., Castagnaro, A. P., and Díaz-Ricci, J. C. (2013). Purification and characterization of AsES protein: a subtilisin secreted by Acremonium strictum is a novel plant defense elicitor. J. Biol. Chem. 288, 14098–14113. doi: 10.1074/jbc.m112.429423
Choi, T. G., Maung, C. E. H., Lee, D. R., Henry, A. B., Lee, Y. S., and Kim, K. Y. (2020). Role of bacterial antagonists of fungal pathogens, Bacillus thuringiensis KYC and Bacillus velezensis CE 100 in control of root-knot nematode, Meloidogyne incognita and subsequent growth promotion of tomato. Biocontrol Sci. Technol. 30, 685–700. doi: 10.1080/09583157.2020.1765980
Clemente, M., Corigliano, M. G., Pariani, S. A., Sánchez-López, E. F., Sander, V. A., and Ramos-Duarte, V. A. (2019). Plant serine protease inhibitors: biotechnology application in agriculture and molecular farming. Int. J. Mol. Sci. 20:1345. doi: 10.3390/ijms20061345
Conrath, U., Beckers, G. J., Flors, V., García-Agustín, P., Jakab, G., Mauch, F., et al. (2006). Priming: getting ready for battle. Mol. Plant Microbe Interact. 19, 1062–1071. doi: 10.1094/mpmi-19-1062
Dagvadorj, B., Ozketen, A. C., Andac, A., Duggan, C., Bozkurt, T. O., and Akkaya, M. S. (2017). A Puccinia striiformis f. sp. tritici secreted protein activates plant immunity at the cell surface. Sci. Rep. 7, 1–10. doi: 10.1038/s41598-017-01100-z
Durrant, W. E., Rowland, O., Piedras, P., Hammond-Kosack, K. E., and Jones, J. D. (2000). cDNA-AFLP reveals a striking overlap in race-specific resistance and wound response gene expression profiles. Plant Cell 12, 963–977. doi: 10.1105/tpc.12.6.963
Figueiredo, A., Monteiro, F., and Sebastiana, M. (2014). Subtilisin-like proteases in plant–pathogen recognition and immune priming: a perspective. Front. Plant Sci. 5:739. doi: 10.3389/fpls.2014.00739
Fillinger, S., and Elad, Y. (2016). Botrytis-the Fungus, the Pathogen and its Management in Agricultural Systems. Cham: Springer International Publishing.
Gao, Q. M., Zhu, S., Kachroo, P., and Kachroo, A. (2015). Signal regulators of systemic acquired resistance. Front. Plant Sci. 6:228. doi: 10.3389/fpls.2015.00228
Hael-Conrad, V., Abou-Mansour, E., Díaz-Ricci, J.-C., Métraux, J.-P., and Serrano, M. (2015). The novel elicitor AsES triggers a defense response against Botrytis cinerea in Arabidopsis thaliana. Plant Sci. 241, 120–127. doi: 10.1016/j.plantsci.2015.09.025
Hael-Conrad, V., Perato, S. M., Arias, M. E., Martínez-Zamora, M. G., Di Peto, P. l. Á, Martos, G. G., et al. (2018). The elicitor protein AsES induces a systemic acquired resistance response accompanied by systemic microbursts and micro–hypersensitive responses in Fragaria ananassa. Mol. Plant Microbe Interact. 31, 46–60. doi: 10.1094/MPMI-05-17-0121-FI
Han, H., Wang, Y., Zheng, T., Peng, Q., Qiu, L., Hu, X., et al. (2022). NtAGO1 positively regulates the generation and viral resistance of dark green islands in Nicotiana tabacum. Plant Physiol. Bioch. 174, 1–10. doi: 10.1016/j.plaphy.2022.01.028
Jaouadi, N. Z., Jaouadi, B., Aghajari, N., and Bejar, S. (2012). The overexpression of the SAPB of Bacillus pumilus CBS and mutated sapB-L31I/T33S/N99Y alkaline proteases in Bacillus subtilis DB430: new attractive properties for the mutant enzyme. Bioresour. Technol. 105, 142–151. doi: 10.1016/j.biortech.2011.11.115
Ji, M., Wu, X., Yao, K., Chen, H., Yang, J., Wang, L., et al. (2014). Identification of strawberry anthracnose pathogens and screening of germicides. Agric. Sci. Technol. 15:94. doi: 10.16175/j.cnki.1009-4229.2014.01.014
Kobayashi, T., Hakamada, Y., Adachi, S., Hitomi, J., Yoshimatsu, T., Koike, K., et al. (1995). Purification and properties of an alkaline protease from alkalophilic Bacillus sp. KSM-K16. Appl. Microbiol. Biotechnol. 43, 473–481. doi: 10.1007/BF00218452
Kosentka, P. Z., Zhang, L., Simon, Y. A., Satpathy, B., Maradiaga, R., Mitoubsi, O., et al. (2017). Identification of critical functional residues of receptor-like kinase ERECTA. J. Exp. Bot. 68, 1507–1518. doi: 10.1093/jxb/erx022
Li, B., Li, Y., Zhao, Y., and Sun, L. (2013). Shape-controlled synthesis of Cu2O nano/microcrystals and their antibacterial activity. J. Phys. Chem. Solids 74, 1842–1847. doi: 10.1016/j.jpcs.2013.07.017
Li, J., Gu, F., Wu, R., Yang, J., and Zhang, K. (2017). Phylogenomic evolutionary surveys of subtilase superfamily genes in fungi. Sci. Rep. 7, 1–15. doi: 10.1038/srep45456
Li, J., Wang, L., Zheng, L., Wang, Y., Chen, X., and Zhang, W. (2018). A functional study identifying critical residues involving metal transport activity and selectivity in natural resistance-associated macrophage protein 3 in Arabidopsis thaliana. Int. J. Mol. Sci. 19:1430. doi: 10.3390/ijms19051430
Li, S., Nie, H., Qiu, D., Shi, M., and Yuan, Q. (2019). A novel protein elicitor PeFOC1 from Fusarium oxysporum triggers defense response and systemic resistance in tobacco. Biochem. Biophys. Res. Commun. 514, 1074–1080. doi: 10.1016/j.bbrc.2019.05.018
Li, Y., Gu, Y., Li, J., Xu, M., Wei, Q., and Wang, Y. (2015). Biocontrol agent Bacillus amyloliquefaciens LJ02 induces systemic resistance against cucurbits powdery mildew. Front. Microbiol. 6:883. doi: 10.3389/fmicb.2015.00883
Li, Z., He, Y., Luo, T., Zhang, X., Wan, H., Ur Rehman, A., et al. (2019). Identification of key residues required for RNA silencing suppressor activity of p23 protein from a mild strain of citrus tristeza virus. Viruses 11:782. doi: 10.3390/v11090782
Liu, X., Xie, J., Fu, Y., Jiang, D., Chen, T., and Cheng, J. (2020). The subtilisin-like protease Bcser2 affects the sclerotial formation, conidiation and virulence of Botrytis cinerea. Int. J. Mol. Sci. 21:603. doi: 10.3390/ijms21020603
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Ma, T., Chen, S., Liu, J., Fu, P., Wu, W., Song, S., et al. (2021). Plasmopara viticola effector PvRXLR111 stabilizes VvWRKY40 to promote virulence. Mol. Plant Pathol. 22, 231–242. doi: 10.1111/mpp.13020
Meng, X., and Zhang, S. (2013). MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 51, 245–266. doi: 10.1146/annurev-phyto-082712-102314
Mishina, T. E., and Zeier, J. (2007). Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J. 50, 500–513. doi: 10.1111/j.1365-313x.2007.03067.x
Ngou, B. P. M., Ahn, H. K., Ding, P., and Jones, J. D. (2021). Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 592, 110–115. doi: 10.1038/s41586-021-03315-7
Nie, P., Li, X., Wang, S., Guo, J., Zhao, H., and Niu, D. (2017). Induced systemic resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET-and NPR1-dependent signaling pathway and activates PAMP-triggered immunity in Arabidopsis. Front. Plant Sci. 8:238. doi: 10.3389/fpls.2017.00238
Plascencia-Jatomea, M., Viniegra, G., Olayo, R., Castillo-Ortega, M. M., and Shirai, K. (2003). Effect of chitosan and temperature on spore germination of Aspergillus niger. Macromol. Biosci. 3, 582–586. doi: 10.1002/mabi.200350024
Rawlings, N. D., Waller, M., Barrett, A. J., and Bateman, A. (2014). MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 42, 503–509. doi: 10.1093/nar/gkr987
Schwizer, S., Kraus, C. M., Dunham, D. M., Zheng, Y., Fernandez-Pozo, N., Pombo, M. A., et al. (2017). The tomato kinase Pti1 contributes to production of reactive oxygen species in response to two flagellin-derived peptides and promotes resistance to Pseudomonas syringae infection. Mol. Plant Microbe Interact. 30, 725–738. doi: 10.1094/mpmi-03-17-0056-r
Sneath, P., and Sokal, R. (1973). Numerical Taxonomy: The Principles and Practice of Numerical Classification. San Francisco: WF Freeman & Co.
Tao, Y., Zeng, F., Ho, H., Wei, J., Wu, Y., Yang, L., et al. (2011). Pythium vexans causing stem rot of Dendrobium in Yunnan Province, China. J. Phytopathol. 159, 255–259. doi: 10.1111/j.1439-0434.2010.01756.x
Thomas, E. L., and Van Der Hoorn, R. A. (2018). Ten prominent host proteases in plant-pathogen interactions. Int. J. Mol. Sci. 19:639. doi: 10.3390/ijms19020639
Thomma, B. P., Nürnberger, T., and Joosten, M. H. (2011). Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell 23, 4–15. doi: 10.1105/tpc.110.082602
Torres, M. A., Jones, J. D., and Dangl, J. L. (2006). Reactive oxygen species signaling in response to pathogens. Plant Physiol. 141, 373–378. doi: 10.1104/pp.106.079467
Torres, M., Llamas, I., Torres, B., Toral, L., Sampedro, I., and Bejar, V. (2020). Growth promotion on horticultural crops and antifungal activity of Bacillus velezensis XT1. Appl. Soil Ecol. 150:103453. doi: 10.1016/j.apsoil.2019.103453
Vatsa, P., Chiltz, A., Bourque, S., Wendehenne, D., Garcia-Brugger, A., and Pugin, A. (2011). Involvement of putative glutamate receptors in plant defence signaling and NO production. Biochimie 93, 2095–2101. doi: 10.1016/j.biochi.2011.04.006
Wang, H., Li, L., Zhang, Z., Zhou, H., Cai, L., and Yu, Z. (2019). Toxicity of four fungicides against fungus Botrytis cinerea in tobacco and their inhibition effects against tobacco gray mold. J. Plant Prot. 46, 377–384. doi: 10.13802/j.cnki.zwbhxb.2019.2017206
Wang, N., Liu, M., Guo, L., Yang, X., and Qiu, D. (2016). A novel protein elicitor (PeBA1) from Bacillus amyloliquefaciens NC6 induces systemic resistance in tobacco. Int. J. Biol. Sci. 12:757. doi: 10.7150/ijbs.14333
Wei, Y., Li, Z., Yuan, Y., Zhang, B., Wang, Y., and Chang, R. (2021). Screening and Function of Plant Immune Proteins from Bacillus velezensis LJ02. J. Plant Prot. 54, 3451–3460.
Weiberg, A., Wang, M., Lin, F., Zhao, H., Zhang, Z., Kaloshian, I., et al. (2013). Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 342, 118–123. doi: 10.1126/science.1239705
Wen, Q., Sun, M., Kong, X., Yang, Y., Zhang, Q., Huang, G., et al. (2021). The novel peptide NbPPI1 identified from Nicotiana benthamiana triggers immune responses and enhances resistance against Phytophthora pathogens. J. Integr. Plant Biol. 63, 961–976. doi: 10.1111/jipb.13033
Xu, L., Wang, H., Zhang, C., Wang, J., Chen, A., Chen, Y., et al. (2020). System-wide characterization of subtilases reveals that subtilisin-like protease FgPrb1 of Fusarium graminearum regulates fungal development and virulence. Fungal Genet. Biol. 144:103449. doi: 10.1016/j.fgb.2020.103449
Yang, B., Wang, Q., Jing, M., Guo, B., Wu, J., Wang, H., et al. (2017). Distinct regions of the Phytophthora essential effector Avh238 determine its function in cell death activation and plant immunity suppression. New Phytol. 214, 361–375. doi: 10.1111/nph.14430
Yang, T., Zhu, L.-S., Meng, Y., Lv, R., Zhou, Z., Zhu, L., et al. (2018). Alpha-momorcharin enhances Tobacco mosaic virus resistance in tobaccoNN by manipulating jasmonic acid-salicylic acid crosstalk. J. Plant Physiol. 223, 116–126. doi: 10.1016/j.jplph.2017.04.011
Yoshioka, H., Numata, N., Nakajima, K., Katou, S., Kawakita, K., Rowland, O., et al. (2003). Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15, 706–718. doi: 10.1105/tpc.008680
Zhang, H., Wu, Q., Cao, S., Zhao, T., Chen, L., Zhuang, P., et al. (2014). A novel protein elicitor (SsCut) from Sclerotinia sclerotiorum induces multiple defense responses in plants. Plant Mol. Biol. 86, 495–511. doi: 10.1007/s11103-014-0244-3
Zhang, S., Wang, L., Zhao, R., Yu, W., Li, R., Li, Y., et al. (2018). Knockout of SlMAPK3 reduced disease resistance to Botrytis cinerea in tomato plants. J. Agric. Food Chem. 66, 8949–8956. doi: 10.1021/acs.jafc.8b02191
Zhao, Q., Xiang, X., Liu, D., Yang, A., and Wang, Y. (2018). Tobacco transcription factor NtbHLH123 confers tolerance to cold stress by regulating the NtCBF pathway and reactive oxygen species homeostasis. Front. Plant Sci. 9:381. doi: 10.3389/fpls.2018.00381
Zhao, W., Wisniewski, M., Wang, W., Liu, J., and Liu, Y. (2014). Heat-induced oxidative injury contributes to inhibition of Botrytis cinerea spore germination and growth. World J. Microbiol. Biotechnol. 30, 951–957. doi: 10.1007/s11274-013-1513-z
Keywords: Bacillus velezensis LJ02, subtilisin point mutant, systemic acquired resistance, PAMP-triggered immunity, Botrytis cinerea
Citation: Hu J, Chang R, Yuan Y, Li Z and Wang Y (2022) Identification of Key Residues Essential for the Activation of Plant Immunity by Subtilisin From Bacillus velezensis LJ02. Front. Microbiol. 13:869596. doi: 10.3389/fmicb.2022.869596
Received: 04 February 2022; Accepted: 17 June 2022;
Published: 15 August 2022.
Edited by:
Lei Huang, Purdue University, United StatesReviewed by:
Long Yang, Huazhong Agricultural University, ChinaMaofeng Jing, Nanjing Agricultural University, China
Copyright © 2022 Hu, Chang, Yuan, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanhong Wang, d2FuZ3loQHRqYXUuZWR1LmNu; Zhuoran Li, enJsaUB0amF1LmVkdS5jbg==
 Jianan Hu1
Jianan Hu1 Yuanhong Wang
Yuanhong Wang