- 1Department of Food Science, College of Agriculture and Veterinary Medicine, United Arab Emirates University (UAEU), Al Ain, United Arab Emirates
- 2Department of Clinical Nutrition and Dietetics, College of Health Sciences, University of Sharjah, Sharjah, United Arab Emirates
- 3Sharjah Institute for Medical Research, University of Sharjah, Sharjah, United Arab Emirates
- 4Department of Nutrition and Food Technology, Faculty of Agriculture, Jordan University of Science and Technology, Irbid, Jordan
- 5Campus Director at Higher Colleges of Technology, Dubai, United Arab Emirates
The study aimed to determine the prevalence of different species of Vibrio spp. in fish and shellfish sold in subtropical-arid countries (United Arab Emirates). It also examined the antimicrobial resistance of the isolated species and their growth behavior upon in vitro environmental changes concerning temperature, pH, and salinity. The prevalence of Vibrio spp. in fish and shellfish samples, was 64.5 and 92%, respectively. However, Vibrio parahemolyticus were detected in a mere 7.5 and 13.0% of the samples, respectively. On the other hand, Vibrio mimicus was detected in 1.5 and 8.5% of the samples, respectively. None of the six antibiotics studied except for Sulfamethoxazole-trimethoprim were effective against fish Vibrio spp. isolates. On a similar note, three antibiotics, namely Penicillin, Daptomycin, and Vancomycin, were ineffective against the shellfish isolates. The growth of the microorganisms did not show any significant trend with changes in pH and salinity. The optimum temperature for Vibrio spp. growth was observed to be 37°C.
Introduction
Vibrio spp. are Gram-negative halophile and mesophile bacteria (Broberg et al., 2011). Different species of Vibrio exist, such as V. parahaemolyticus and V. vulnificus, to name a few (Chowdhury et al., 2004; Bonnin-Jusserand et al., 2019). Vibrio cholerae causes cholera which could be fatal (CDC, 2018). The symptoms associated with a Vibrio spp. infection are usually gastrointestinal, commonly expressed as watery diarrhea, abdominal cramping, nausea, vomiting, and fever (CDC, 2019d). The symptoms may show within 24 h of ingestion and may last up to 3 days (CDC, 2019c). Immunocompromised patients or those with a pre-existing condition are at a greater fatality risk (CDC, 2019c). The bacteria could also be resistant to multiple antibiotics making their treatment in a human infection difficult (Alsalem et al., 2018).
Vibrio spp. are good swimmers and can attach to other organisms living in the water (McCarter, 1999). Thus, a contaminated water body could potentially infect all its fish inhabitants (Kim and Lee, 2017). Multiple deadly outbreaks associated with Vibrio spp. have been observed worldwide (CDC, 2018; WHO, 2021). In a developed country like the United States, Vibriosis causes 80,000 illnesses and about 100 deaths every year (CDC, 2019b). There is a very high possibility that the actual number of outbreaks is higher than the reported figures (WHO, 2021).
Oysters feed by filtering water; hence, there is a chance that microorganisms concentrate in their bodies; thereby, they are at a higher risk of Vibrio spp. infection as compared to fish (CDC, 2019a). The prevalence of V. parahaemolyticus (from all the Vibrio spp. analyzed) in shellfish in Egypt ranged from 9.3 to 16.7% (Abd-Elghany and Sallam, 2013; Youssef et al., 2018). The contamination was even higher in Kuwait, with 78% of the seafood being contaminated with Vibrio spp. (Al-Mouqati et al., 2012). Raw contaminated seafood may contaminate other foods or surfaces in contact, like chopping boards, knives, etc. This cross-contamination puts other food items being prepared at the facility at risk, which may result in outbreaks, especially where the food is served to the masses, for instance, restaurants, and food catering organizations (Kramer et al., 2006). Moreover, slightly cooked or uncooked seafood delicacies may increase the risk of infection (CDC, 2019b).
The global fish and seafood trade value was estimated to be around USD 152 billion in 2017 (UNSTAD, 2019). The average seafood consumption per capita in the United Arab Emirates (UAE) is 29 kg/year (FAO, 2021). A total of 2,598 tonnes of fish worth 15 million USD were caught in UAE waters as of 2019 (SCAD, 2020). The fish production in the country is of substantial value, which could pose a major risk if contamination levels are high.
To the best of our knowledge, this is the first study to evaluate the microbiological safety of fish and shellfish in the UAE concerning Vibrio spp. Therefore, this study aimed to determine the prevalence of different species of Vibrio spp. in fish and shellfish sold in the local markets of the UAE. It also examined the antimicrobial resistance of the isolated species and their growth behavior upon in vitro environmental changes.
Materials and Methods
Sample Collection
Fresh most sold local 200 fish samples (spangled emperor, prang spotted, pearly goatfish, greater amberjack, and yellowstripe scad) and 200 shellfish (shrimp, oysters, crab, clam, and lobster) were purchased from local markets in four different emirates in the UAE (Al-Ain, Dubai, Fujairah, and Abu Dhabi). These emirates are housing the larger fish markets in the whole UAE. From each emirate, 50 fish and 50 shellfish samples were collected (10 samples per each above-mentioned type). The samples were purchased from June to September 2017 and transferred into sterile, sealable, labeled plastic bags. The samples were transported in dry ice to the food microbiology laboratory at the United Arab Emirates University (UAEU) for analysis.
Vibrio spp. Isolation
Twenty-five grams of the flesh from fish and shellfish samples were homogenized in 225 ml alkaline peptone saline water (APSW, Hi Media®, Bombay, India) using a stomacher circular Unit 400 (Seward Ltd.®, London, United Kingdom) for 2 min at 260 rpm followed by incubation at 42°C for 8 h. Then, 10 ml of the incubated homogenate were streaked in duplicate on Thiosulfate-Citrate-Bile Salts-Sucrose Agar (TCBS Agar; Oxoid, Thermo Fischer Scientific) and Modified Cellobiose-Polymyxin B-Colistin Agar (mCPC Agar; APSW, Hi Media®, Bombay, India) followed by incubation at 37°C for 24 h. The suspected colonies were re-streaked on Tryptone Soy Agar (Oxoid, Thermo Fischer Scientific) supplemented with 3% Sodium Chloride (TSA + 3% NaCl) and incubated at 37°C for 24 h to obtain a pure isolate (Sujeewa et al., 2009).
DNA Extraction
The isolated bacteria were grown individually in Tryptone Soy Broth (Oxoid, Thermo Fischer Scientific) supplemented with 3% NaCl (TSB + 3% NaCl) and incubated at 37°C for 24 h. The isolates’ DNA was extracted using a QIAGEN DNA extraction kit as per the manufacturer’s instructions.
Confirmation of Vibrio spp. by Polymerase Chain Reaction
PCR assay was performed separately for general (Vibrio spp.) and specific (16S rRNA) genes of the suspected Vibrio spp. isolates. The process conditions were 35 cycles of amplification, denaturation at 94°C for 1 min, annealing at 58°C for 1 min, extension at 72°C for 1 min, and final extension at 72°C for 7 min. The reaction mixtures were resolved by electrophoresis in 2% agarose gel and visualized under UV light. Gel bands were compared with reference strains (V. parahaemolyticus DSM2172 and DSM19130, V. vulnificus DSM10143, and V. mimicus DSM19130). The reference strains were purchased from Leibniz-Institut DSMZ—Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany). The listed primers in Table 1 were employed to identify the Vibrio spp. and strains. A 100 bp marker was employed (Sujeewa et al., 2009).
Antibiotic Sensitivity of Vibrio spp. Isolates
Twenty-eight Vibrio isolates including 15 V. parahaemolyticus, 10 V. vulnificus, and 3 V. mimicus were used for antibiotic test. The isolates were inoculated into sterile TSB + 3% NaCl, which was then incubated at 37°C until turbidity (~16 h). Using a sterile cotton swab, the bacterium was inoculated on Muller Hinton Agar plates (Oxoid, Thermo Fischer Scientific). Antimicrobial susceptibility test disks (Oxoid, Thermo Fischer Scientific) of Penicillin G (10 iu), Vancomycin (2 mcg), Daptomycin (30 mcg), Ampicillin (10 mcg), Erythromycin (15 mcg), and Sulphamethoxazole/Trimethoprim (SXT; 25 mcg) were gently placed on the agar plates post which they were incubated at 37°C for 24 h. The inhibition zone was measured in millimeters (Yaashikaa et al., 2016).
Analysis of Factors Affecting Growth and Survival of Vibrio spp.
Effect of Temperature on the Growth of Vibrio spp. Isolates
Quantities of 0.1 ml of the isolated Vibrio spp. cultures were inoculated in sterilized nutrient broth (Oxoid, Thermofischer scientific) and incubated for 20–24 h at different temperatures (25, 37, and 45°C). Appropriate serial dilutions using Peptone water were then prepared, and the tubes were re-incubated for 20–24 h at 37°C. The viable counts were determined using a spectrophotometer adjusted to 620 nm at regular time intervals (Yaashikaa et al., 2016).
The growth rate was calculated in comparisons with Optical Density (OD) at time 0 h (t0) and time the specimen was analyzed (t):
Effect of pH on the Growth of Vibrio spp. Isolates
Quantities of 0.1 ml of the isolated Vibrio spp. cultures were inoculated in nutrient broth adjusted to a pH of 3, 5, and 7 using 0.1 N HCl and incubated for 20–24 h at 37°C. After that, appropriate serial dilutions using Peptone water were made, and the tubes were re-incubated at 37°C for 24 h. The viable count of bacteria was determined with the help of a spectrophotometer adjusted to 620 nm at regular time intervals (Yaashikaa et al., 2016). The growth rate was calculated in comparison with OD at time 0 h.
Effect of Salinity on the Growth of Vibrio spp. Isolates
NaCl was added at various concentrations (0.5, 1.0, and 2.0%; Yang et al., 2010) to nutrient broth, after which the pH was adjusted to 8.5 using Sodium Hydroxide (NaOH, 0.1 N). The test tubes containing the mixture were then autoclaved. The tubes were then inoculated with 0.1 ml of fresh Vibrio spp. isolates, and incubated for 20–24 h at 37°C. Appropriate serial dilutions were performed using Peptone water for each concentration. Growth of isolates was observed by measuring the absorbance using a spectrophotometer adjusted to 620 nm at regular time intervals (Yaashikaa et al., 2016). The growth rate was calculated compared to OD at time 0 h as described in the section “Effect of temperature on the growth of Vibrio spp. isolates.”
Statistical Analysis
Growth profile data in triplicate were subjected to the ANOVA using a general linear model (GLM). Mean comparisons were performed using Duncan’s multiple range test to compare significant differences between means for all analyses. Statistical analysis was carried out using Statistical Package for the Social Sciences (SPSS®, Version 21). Values are expressed as mean ± SD. The contour plots were performed using Minitab v21 (Pennsylvania, United States). The regression equations used were:
For fish isolates, OD = −1.203 + 0.4149 Salt + 0.1402 pH + 0.00745 Temp.
For shellfish isolates, OD = −0.341 + 0.2859 Salt + 0.1119 pH − 0.00590 Temp.
Results and Discussion
Isolation of Vibrio spp. in Fish and Shellfish Samples
Fish can harbor various microorganisms like Listeria monocytogenes, Yersinia spp., Salmonella spp., and Clostridium botulinum (Novoslavskij et al., 2016). The microorganisms in fish can use it as a nutrient medium and hydrolyze proteins to form biogenic amines (Lou et al., 2021). However, one notorious pathogen of great public health concern that fish can harbor is the Vibrio spp. (Novoslavskij et al., 2016). The cases of Vibriosis are on the rise even in developed countries like the United States (Baker-Austin et al., 2018).
In this study, eighth respect to fish and shellfish samples, the results showed that a total of 129 (64.5%), 184 (92%) were Vibrio spp., positive, respectively. The high prevalence of Vibrio spp. could be attributed to ecological contamination like that of the feed or to the water salinity and temperatures (Blanch et al., 2009; Baker-Austin et al., 2018). The UAE follows strict practices to prevent marine pollution; thereby contamination through this means is highly unlikely. High seawater rising temperatures attributable to the worldwide phenomenon of global warming could be a possible explanation (Baker-Austin et al., 2018). However, the current prevalence rates observed in this study may not necessarily be a cause of concern as the country has not witnessed any major outbreak of Cholera or Vibriosis (to the best of our knowledge; SCAD, 2020). Vibrio spp. is indigenous to marine waters (Hsiao and Zhu, 2020). Furthermore, not all variants of Vibrio spp., are considered as pathogenic (Lopez-Joven et al., 2015; Song et al., 2017).
The comparative higher prevalence in shellfish than fish is understandable because actively growing clear shellfish particles from water at rates ranging from 1 to 4 L/h (Rice, 2001; CDC, 2019a). It is thereby possible that during this filtration step, the Vibrio spp., present in the water body, is retained within the shellfish body (Rice, 2001; CDC, 2019a). A meta-analysis did indicate a general trend of higher Vibrio spp., contamination in oysters and clams compared to fish (Odeyemi, 2016).
Moreover, Fish is usually consumed after applying a heat treatment, which is expected to destroy any pathogenic Vibrio spp. (USDA, 2020). Further analysis regarding the prevalence of virulent strains is needed for better comprehension.
The prevalence of Vibrio spp., isolates in shellfish from Egypt, was observed to be 33% (Abd-Elghany and Sallam, 2013). A study in Iran reported a 17.1% prevalence of Vibrio spp., in shrimp samples (Asgarpoor et al., 2018). A higher population of Vibrio spp., in shrimp samples purchased from wet markets (5.04–6.34 log CFU/ml) compared to supermarkets (4.21–4.43 log CFU/ml) was observed in Malaysia (Letchumanan et al., 2015). Another study conducted in Iran revealed 26.8% of the examined fish samples were Vibrio spp., positive (Raissy et al., 2014). The prevalence of Vibrio spp., varies based on water temperature, level of salinity, season, water depth, and total suspended solids (Akoachere and Mbuntcha, 2014; Kokashvili et al., 2015; Lopez-Joven et al., 2015; Williams et al., 2017).
Molecular Identification of Vibrio spp. Isolates in Fish and Shellfish Samples
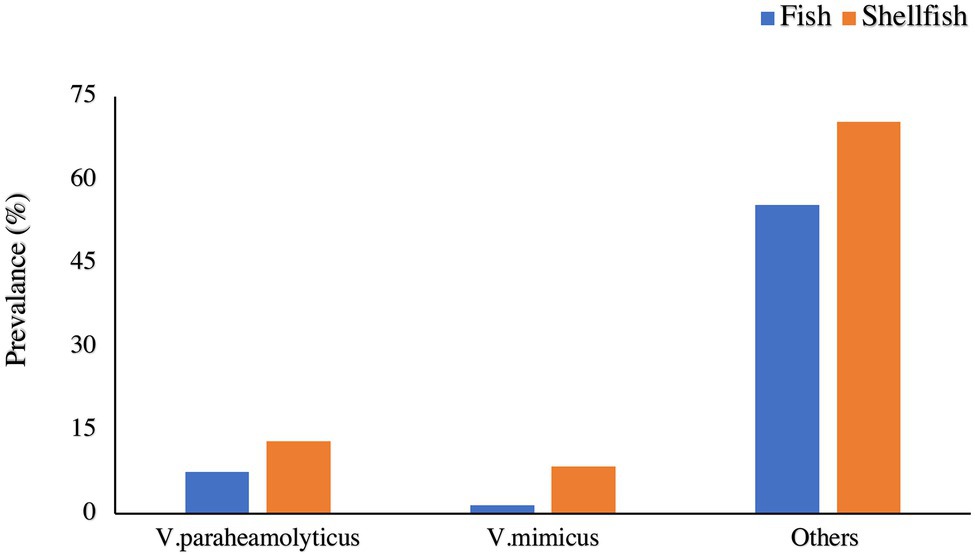
In fish, the majority species prevalence was Vibrio parahemolyticus and V. mimicus (7.5 and 1.5%, respectively; Figure 1). A similar result was observed in shellfish samples, with 13 and 8.5% prevalence, respectively (Figure 1). Vibrio vulnificus was found in fish (5%), but it was not present in the shellfish samples. A study conducted in the Suez Canal area (Egypt) reported an overall V. parahaemolyticus prevalence of 9.3% in shellfish (Youssef et al., 2018). The entire prevalence of the above-mentioned species does not need to be pathogenic. The virulence genes present in the bacteria are a cause of concern in terms of public health (Hasan et al., 2010; Gennari et al., 2012; Lopez-Joven et al., 2015). This study is limited to the typification of the species, and further research is needed to analyze the pathogenicity of these strains. The strain pathogenicity depends on factors like the presence of enzymes, such as urease, lipase, gelatinase, and hemolysin or the adhesiveness potential (Baffone et al., 2001).
The percentage of V. parahaemolyticus in shellfish harvested from Turkey was observed to be 0.8% (Colakoglu et al., 2006). Amongst all the Vibrio spp., studied, some studies indicate that V. alginolyticus was present in the greatest amount of samples (Gopal et al., 2005; Colakoglu et al., 2006; Yücel and Balci, 2010), while others were indicating a higher prevalence of other strains (Vibrio cholerae, Vibrio communis; Adebayo-Tayo et al., 2011; Amalina et al., 2019).
PCR was used for the molecular identification of the Vibrio spp., positive isolates. The presence of Vibrio spp. was confirmed by using both general and Vibrio spp., specific sequences. In this study, the presence of Vibrio spp., in shellfish samples were atypical in different locations. 16S rRNA is present in all Vibrio spp., isolates and could be used as a marker gene for specific detection of this bacterium (Kim et al., 1999).
Antibiotic Resistance of Vibrio spp. Isolates
Various antibiotic-resistant Vibrio spp., isolates have been reported worldwide (Baker-Austin et al., 2009; Kitaoka et al., 2011; Jun et al., 2012; Kang et al., 2017; Kurdi Al-Dulaimi and Ariffin, 2019). These bacteria develop the resistance through various mechanisms, including the development of multidrug efflux pumps, horizontal gene transfer, plasmid conjugation, or just simple random mutations (Kitaoka et al., 2011). If the antibiotic-resistant bacteria get transferred to humans via the food chain, it will create difficulties in disease treatment (Igbinosa, 2016).
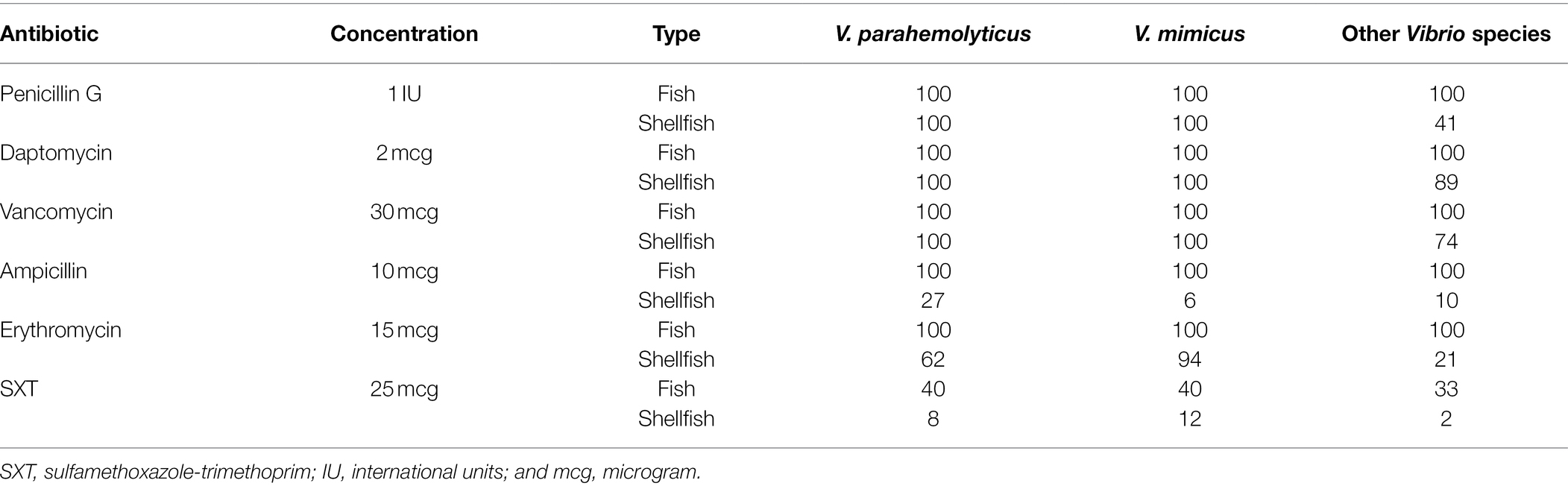
With respect to V. parahemolyticus and V. mimicus isolated from fish in this study, none of the studied antibiotics (penicillin g, daptomycin, vancomycin, ampicillin, and erythromycin) were effective except for SXT (40% resistance). On a similar note, in shellfish isolates, treatment with penicillin, daptomycin, and vancomycin proved to be futile (Table 2). Erythromycin and SXT were more effective against V. parahemolyticus isolates (62.0 and 8.0% were resistant, respectively) compared to V. mimicus (94 and 12.0% were resistant, respectively) in shellfish (Table 2). The difference in resistance to antibiotics in fish and shellfish isolates despite belonging to the same strain could be because the immune response varies based on fish type—those strains isolated from fish with higher immune responses may have developed mechanisms for enhanced survival compared to fish with lower immunity (Smith et al., 2019). Further studies are needed to confirm this hypothesis. Similarly, any previous exposure to antibiotics may also encourage survival (Smith et al., 2019). Other modes of treatment could decrease the prevalence of the Vibrio spp., such as ultrasound, low-temperature treatment, and the use of ozone and saline (Zhou et al., 2002). Moreover, the fish/shellfish are expected to be marinated prior to consumption. The use of sugar, vinegar, lemon juice, or citric acid has been associated with decreasing the contamination of Vibrio spp., in fish and shellfish, respectively (Borazjani et al., 2003; Ibrahim et al., 2018).
In accordance with our observation, a previous study on Vibrio spp., isolates from cockles and clams showed resistance toward Penicillin (93%), Ampicillin (70%), Cephalothin (65%), Clindamycin (66%), Vancomycin (64%), and Erythromycin (51%; Kurdi Al-Dulaimi and Ariffin, 2019). A study conducted on 44 V. parahaemolyticus isolates from oysters in Korea revealed 90.9, 86.4, and 75.0% of the isolates being resistant to Vancomycin, Ampicillin, and Streptomycin treatment, respectively (Kang et al., 2017). Another study in Korea reported all 19 isolates of V. parahaemolyticus obtained from seafood to be resistant to more than four commercial antibiotics (Jun et al., 2012). Other studies also report similar observations (Baker-Austin et al., 2009; Lopatek et al., 2015). On the other hand, an assessment of the antimicrobial susceptibility profile of V. parahaemolyticus isolated from short mackerels (Rastrelliger brachysoma) in Malaysia revealed majority of the isolates were highly susceptible to Ampicillin Sulbactam, Meropenem, Ceftazidime, and Imipenem (Tan et al., 2017). Treatment with Ampicillin/Sulbactam or Chloramphenicol was effective against around 95% of the V. parahaemolyticus strains isolated from shrimps in Malaysia (Letchumanan et al., 2015).
In contrast to antibiotic therapy, acid electrolyzed ice water decreased Vibrio spp., populations by about 3% (He et al., 2022). Meanwhile, a synergistic antimicrobial effect on the microbiota of fish was observed when it was vacuum packaged with a coating of gelatin composed of grape seed extract. This strategy may also be useful on pathogenic species like Vibrio spp. (Zhao et al., 2021).
Factors Affecting the Growth Rate of Vibrio spp. Isolates of Shellfish
Effect of Salt Concentration on the Growth Rate of Vibrio spp. Isolates
Salt in the form of NaCl has been observed to enhance the formation of wrinkle colonies and pellicle in Vibrio spp. (Marsden et al., 2017). The formation of wrinkled colonies happens at the earlier stages of biofilm formation (Ray et al., 2012). Even at high salt concentrations, the rapid growth of bacteria is a cause of concern as it is a very common/cost-effective preservative method. The Vibrio spp., isolates from fish and shellfish in this study were thereby tested for their ability to grow in the presence of varying salt concentrations. After 16 h of storage, at a salt concentration of 0.5%, the growth rates of the Vibrio isolates (n = 28) ranged from 58.9 to 92.5% and 51.7 to 83.3% in fish and shellfish, respectively. Increasing the salt concentration to 1% resulted in growth rates of 44.8–82.3 and 34.8–72.3%, respectively. Furthermore, a salt concentration of 2% resulted in growth rates ranging from 56.6 to 87.1% and 38.6 to 81.0%, respectively. Great variation among strains was observed with no specific trend (Supplementary Figure S1).
The literature regarding the impact of NaCl on Vibrio spp., growth is bifurcated. A study conducted on V. parahaemolyticus and V. vulnificus showed that the bacteria reached a viable-but-nonculturable (VBNC) state when the concentration of NaCl was elevated up to a level of 30% (Yoon et al., 2017). Decreased water salinity levels have also been associated with increased concentration of V. parahaemolyticus in shrimp aquaculture systems (Bauer et al., 2021). The toxicity of Vibrio spp., grown in 1% NaCl conditions, was greater than a 3% concentration (Whitaker et al., 2010). On the other hand, the growth of V. parahaemolyticus in 1% NaCl being significantly less when compared to growth in 3% NaCl has also been reported (Whitaker et al., 2010). About 80% of the Vibrio spp., extracted from fish and prawns, could grow in salt concentrations ranging from 1.5 to 3.5% (Surendran et al., 1983). The variance in observation could be explained. Vibrio spp. can be classified as moderate halophiles (Surendran et al., 1983). They do need salt for their survival/growth (Fujiwara-Nagata and Eguchi, 2004); however, increasing the concentration beyond a certain limit seems detrimental (Yoon et al., 2017; Bauer et al., 2021). A study conducted on V. alginolyticus indicated that the highest growth of the microbe was observed at 3% NaCl solution, increasing the concentration to 6% or decreasing it to 0.5% lowered growth (Farid and Larsen, 1981). Sodium is essential for Vibrio spp., for growth and starvation survival mechanisms (Fujiwara-Nagata and Eguchi, 2004). To overcome salt stress, the microbe remodels its outer membrane proteins to keep an intact cell membrane (Yang et al., 2010). As can be seen from the previous studies, the ability of each strain to use sodium for its growth or to protect itself from the harsh environment is expected to vary, and this does explain the no consistent trend seen in our results.
Effect of pH on the Growth Rate of Vibrio spp. Isolates
The acidity or alkalinity of the surrounding environment exerts antimicrobial action on the bacterial cell by affecting the proteins involved in cell membrane maintenance and motility function (Hommais et al., 2002). Furthermore, the pH affects the ability of the Vibrio spp., cell in terms of its ability to resist drugs and interferes with its biofilm formation (Hommais et al., 2002). The Vibrio spp., isolates from fish and shellfish of this study were tested for their ability to withstand varying pH conditions. The growth rate (depending on the isolate) at pH = 3.0 ranged from 1.5 to 60.1 and 1.8 to 65.0% in fish and shellfish, respectively. At a pH of 5.0, the growth rate range was 44.5–87.1 and 8.7–67.4%, respectively. At neutral pH = 7.0, the growth rates for fish and shellfish were from 25.6 to 81.6 and 38.6 to 76.2%, respectively (Supplementary Figure S2).
The V. cholerae and V. parahaemolyticus isolated from prawn (Penaeus monodon) were reported to grow well at pH 5.0 and 7.0. Increasing the pH to 9.0 or reducing it to 3.0 decreased the growth of V. cholerae and V. parahaemolyticus isolates (Yaashikaa et al., 2016). Another study indicated the ability of Vibrio spp., to survive broad spectra of pH ranging from 5.2 to 9.2 (Beuchat, 1973). Our study results are compatible with the above literature, as the minimal growth rate at pH 3.0 is lower by a good degree when compared to a pH of 5.0 or 7.0 (Supplementary Figure S2).
Effect of Temperature on the Growth Rate of Vibrio spp. Isolates
The temperature of the environment alters the cell membrane fluidity and thereby impacts pathogen survival (Kandušer et al., 2008). At a temperature of 25°C, the growth rate of Vibrio spp., in fish and shellfish ranged from 47.5 to 82.8 and 8.4 to 80.5%, respectively. Increasing the temperature to 37°C resulted in growth rates ranging from 54.5 to 84.7 and 34.0 to 80.9%, respectively. At 45°C, the growth rates were 19.2–75.3 and 27.7–76.1%, respectively. The results indicate that the optimum temperature for the Vibrio spp., growth was at 37°C (Supplementary Figure S3).
In a previous study, V. parahemolyticus populations in fresh seafood were 33.4%, while the prevalence in frozen and iced samples was observed to be 14.9% (Yang et al., 2008). In another study, V. parahaemolyticus grew well at 15, 25, and 35°C; however, a lower growth rate was observed at 5°C (Wang et al., 2018). Vibrio parahaemolyticus in live clams held at 28°C multiplied rapidly while no significant growth was observed and 4 and 15°C (Lopez-Joven et al., 2018). In Oyster meat stored at 16°C, V. vulnificus populations showed a maximum increase by 1.5 log CFU/g, while a storage at 36°C, resulted in a maximum increase by 2.8 log CFU/g, respectively (Kim et al., 2012). In Oyster slurry, V. parahaemolyticus growth was not observed at 10 and 15°C. Vibrio parahemolyticus was observed to have an optimum growth rate at temperatures ranging from 37 to 39°C, increasing or decreasing the temperature beyond 8.3 and 45.3°C resulting in hampering their growth (Miles et al., 1997). Our results are in accordance with the above-mentioned studies.
Regressions Between the Growth Factors
Figure 2 displays the contour plots of the effect of the three factors pH, salt, and temperature on the growth (OD) of Vibrio spp., from fish (A–C) and shellfish (D–F) after 16 h of incubation. One factor was fixed when the other two factors were changed. As can be seen from the figure, the variation in the contour ranges was pronounced when pH was changed. This implies that pH had a greater influence on the Vibrio spp., growth than salt and temperature. This suggests that using acidulant agents would be an efficient approach to preserve fish and shellfish products in addition to temperature.
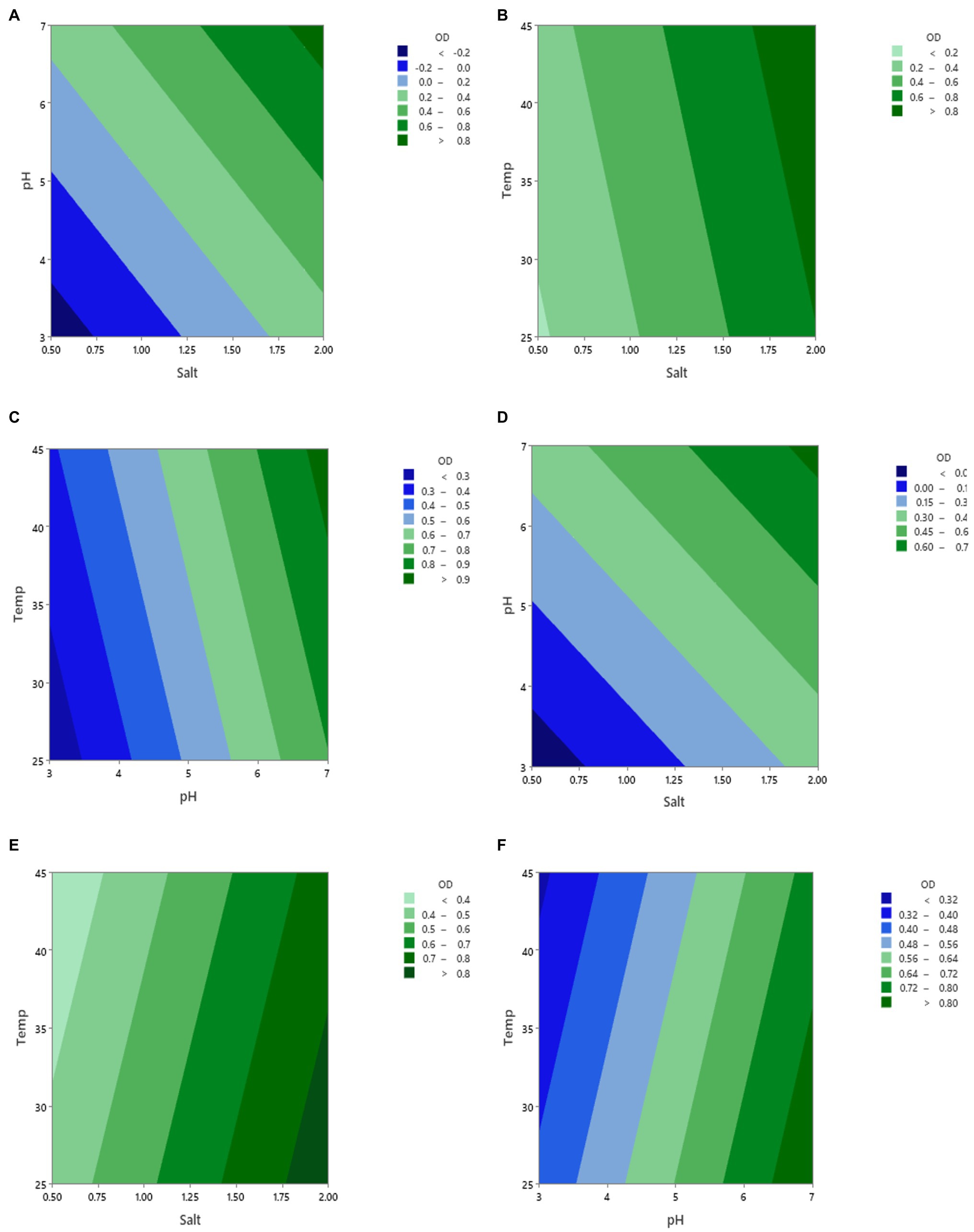
Figure 2. Contour plots of the growth (OD) of vibrio spp. isolated from fish (A–C) and shellfish (D–F) during changing three factors (pH, salt, and temperature) and incubation for 16 h. The fixed values of each factor were 2.0 for salt, 7.0 for pH, and 37°C for temperature.
Conclusion
To the best of our knowledge, our study is the first comprehensive report regarding the prevalence, growth characteristics, and antibiotic susceptibility of Vibrio spp., isolates from fish and shellfish samples in the UAE. The prevalence of V. parahemolyticus and V. mimicus was low. V. vulnificus was found only in a minor portion of fish samples. A definitive conclusion cannot be made about the risk they pose. This is because the presence of virulence genes present in the microorganism defines its pathogenicity. The analysis at the gene level was not conducted in this study. However, fish and shellfish are usually given a heat treatment prior to consumption in our region, thereby posing less risk. The impact of environmental growth conditions was observed to vary greatly based on the strain. SXT was determined to be the most effective antibiotic in the treatment of V. parahemolyticus and V. vulnificus isolates from fish, while both Ampicillin and SXT were effective in shellfish.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author Contributions
MA, TO, and AA: conceptualization. TA and HA-R: methodology, investigation, and data curation. MA and TO: validation. TA, HA-R, and MA: formal analysis. MA: resources, supervision, project administration, and funding acquisition. TA, HA-R, TO, and FH: writing—original draft preparation. MA, TO, and RO: writing—review and editing. TA, HA-R, TO, and MA: visualization. All authors contributed to the article and approved the submitted version.
Funding
The authors are thankful to the United Arab Emirates University for funding this project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.861547/full#supplementary-material
References
Abd-Elghany, S. M., and Sallam, K. I. (2013). Occurrence and molecular identification of Vibrio parahaemolyticus in retail shellfish in Mansoura, Egypt. Food Control 33, 399–405. doi: 10.1016/j.foodcont.2013.03.024
Adebayo-Tayo, B. C., Okonko, I. O., Esen, C. U., Odu, N. N., Onoh, C. C., and Igwiloh, N. J. P. (2011). Incidence of potentially pathogenic Vibrio spp., in fresh seafood from Itu Creek in Uyo, Akwa Ibom state, Nigeria. World Appl. Sci. J. 15, 985–991.
Akoachere, J.-F. T. K., and Mbuntcha, C. K. P. (2014). Water sources as reservoirs of vibrio cholerae O1 and non-O1 strains in Bepanda, Douala (Cameroon): relationship between isolation and physico-chemical factors. BMC Infect. Dis. 14:421. doi: 10.1186/1471-2334-14-421
Al-Mouqati, S., Azad, I. S., Al-Baijan, D., and Benhaji, A. (2012). Vibrio detection in market seafood samples of Kuwait by biochemical (API 20E) strips and its evaluation against 16s rDNA-based molecular methods. Res. J. Biotechnol. 7, 63–69.
Alsalem, Z., Elhadi, N., Aljeldah, M., Alzahrani, F., and Nishibuchi, M. (2018). Characterization of Vibrio vulnificus isolated from the coastal areas in the Eastern Province of Saudi Arabia. J. Pure Appl. Microbiol. 12, 1355–1364. doi: 10.22207/JPAM.12.3.38
Amalina, N. Z., Santha, S., Zulperi, D., Amal, M. N. A., Yusof, M. T., Zamri-Saad, M., et al. (2019). Prevalence, antimicrobial susceptibility and plasmid profiling of Vibrio spp., isolated from cultured groupers in Peninsular Malaysia. BMC Microbiol. 19:251. doi: 10.1186/s12866-019-1624-2
Asgarpoor, D., Haghi, F., and Zeighami, H. (2018). Detection and molecular characterization of Vibrio parahaemolyticus in shrimp samples. Open Biotechnol. J. 12, 46–50. doi: 10.2174/1874070701812010046
Baffone, W., Citterio, B., Vittoria, E., Casaroli, A., Pianetti, A., Campana, R., et al. (2001). Determination of several potential virulence factors in vibrio spp., Isolated from Sea Water. Food Microbiol. 18, 479–488. doi: 10.1006/fmic.2001.0441
Baker-Austin, C., McArthur, J. V., Lindell, A. H., Wright, M. S., Tuckfield, R. C., Gooch, J., et al. (2009). Multi-site analysis reveals widespread antibiotic resistance in the marine pathogen Vibrio vulnificus. Microb. Ecol. 57, 151–159. doi: 10.1007/s00248-008-9413-8
Baker-Austin, C., Oliver, J. D., Alam, M., Ali, A., Waldor, M. K., Qadri, F., et al. (2018). Vibrio spp. Infections. Nat. Rev. Dis. Prim. 4, 1–19. doi: 10.1038/s41572-018-0005-8
Bauer, J., Teitge, F., Neffe, L., Adamek, M., Jung, A., Peppler, C., et al. (2021). Impact of a reduced water salinity on the composition of Vibrio spp., in recirculating aquaculture systems for Pacific white shrimp (Litopenaeus vannamei) and its possible risks for shrimp health and food safety. J. Fish Dis. 44, 89–105. doi: 10.1111/jfd.13270
Beuchat, L. R. (1973). Interacting effects of pH, temperature, and salt concentration on growth and survival of Vibrio parahaemolyticus. Appl. Microbiol. 25, 844–846. doi: 10.1128/am.25.5.844-846.1973
Blanch, A. R., Hispano, C., Bultó, P., Ballesté, E., González-López, J. J., and Vilanova, X. (2009). Comparison of Vibrio spp., populations found in seawater, in exhibition aquaria, in fish intestine and in fish feed. J. Appl. Microbiol. 106, 57–65. doi: 10.1111/j.1365-2672.2008.03974.x
Bonnin-Jusserand, M., Copin, S., Le Bris, C., Brauge, T., Gay, M., Brisabois, A., et al. (2019). Vibrio species involved in seafood-borne outbreaks (Vibrio cholerae, V. parahaemolyticus and V. vulnificus): review of microbiological versus recent molecular detection methods in seafood products. Crit. Rev. Food Sci. Nutr. 59, 597–610. doi: 10.1080/10408398.2017.1384715
Borazjani, A., Andrews, L., and Veal, C. (2003). Novel nonthermal methods to reduce Vibrio vulnificus in raw oysters. J. Food Saf. 23, 179–187. doi: 10.1111/j.1745-4565.2003.tb00361.x
Broberg, C. A., Calder, T. J., and Orth, K. (2011). Vibrio parahaemolyticus cell biology and pathogenicity determinants. Microbes Infect. 13, 992–1001. doi: 10.1016/j.micinf.2011.06.013
CDC (2018). Selected outbreaks. Available at: https://www.cdc.gov/vibrio/outbreaks.html (Accessed September 27, 2018).
CDC (2019a). Vibrio and Food. Available at: https://www.cdc.gov/vibrio/food.html (Accessed September 11, 2021).
CDC (2019b). Vibrio species causing Vibriosis. Available at: https://www.cdc.gov/vibrio/index.html (Accessed October 15, 2021).
CDC (2019c). Vibrio species causing Vibriosis—People at Risk. Available at: https://www.cdc.gov/vibrio/people-at-risk.html (Accessed October 15, 2021).
CDC (2019d). Vibrio species causing Vibriosis—Symptoms. Available at: https://www.cdc.gov/vibrio/symptoms.html (Accessed October 15, 2021).
Chowdhury, A., Ishibashi, M., Thiem, V. D., Tuyet, D. T. N., Van Tung, T., Chien, B. T., et al. (2004). Emergence and serovar transition of Vibrio parahaemolyticus pandemic strains isolated during a diarrhea outbreak in Vietnam between 1997 and 1999. Microbiol. Immunol. 48, 319–327. doi: 10.1111/j.1348-0421.2004.tb03513.x
Colakoglu, F. A., Sarmasik, A., and Koseoglu, B. (2006). Occurrence of Vibrio spp., and Aeromonas spp., in shellfish harvested off Dardanelles cost of Turkey. Food Control 17, 648–652. doi: 10.1016/j.foodcont.2005.04.014
FAO (2021). GLOBEFISH—Information and Analysis on World Fish Trade. Available at: http://www.fao.org/in-action/globefish/fishery-information/resource-detail/en/c/338614/ (Accessed September 11, 2021).
Farid, A. F., and Larsen, J. L. (1981). Growth of vibrio alginolyticus: interacting effects on pH, temperature, salt concentration, and incubation time. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene 2, 68–75. doi: 10.1016/S0721-9571(81)80019-1
Fujiwara-Nagata, E., and Eguchi, M. (2004). Significance of Na+ in the fish pathogen, Vibrio anguillarum, under energy depleted condition. FEMS Microbiol. Lett. 234, 163–167. doi: 10.1111/j.1574-6968.2004.tb09528.x
Gennari, M., Ghidini, V., Caburlotto, G., and Lleo, M. M. (2012). Virulence genes and pathogenicity islands in environmental Vibrio strains nonpathogenic to humans. FEMS Microbiol. Ecol. 82, 563–573. doi: 10.1111/j.1574-6941.2012.01427.x
Gopal, S., Otta, S. K., Kumar, S., Karunasagar, I., Nishibuchi, M., and Karunasagar, I. (2005). The occurrence of Vibrio species in tropical shrimp culture environments; implications for food safety. Int. J. Food Microbiol. 102, 151–159. doi: 10.1016/j.ijfoodmicro.2004.12.011
Hasan, N. A., Grim, C. J., Haley, B. J., Chun, J., Alam, M., Taviani, E., et al. (2010). Comparative genomics of clinical and environmental Vibrio mimicus. Proc. Natl. Acad. Sci. 107, 21134–21139. doi: 10.1073/pnas.1013825107
He, Y., Xie, Z., Xu, Y., Zhao, X., Zhao, L., and Yang, H. (2022). Preservative effect of slightly acid electrolysed water ice generated by the developed sanitising unit on shrimp (Penaeus vannamei). Food Control 136:108876. doi: 10.1016/j.foodcont.2022.108876
Hommais, F., Laurent-Winter, C., Labas, V., Krin, E., Tendeng, C., Soutourina, O., et al. (2002). Effect of mild acid pH on the functioning of bacterial membranes in vibrio cholerae. Proteomics 2, 571–579. doi: 10.1002/1615-9861(200205)2:5<571::AID-PROT571>3.0.CO;2-G
Hsiao, A., and Zhu, J. (2020). Pathogenicity and virulence regulation of Vibrio cholerae at the interface of host-gut microbiome interactions. Virulence 11, 1582–1599. doi: 10.1080/21505594.2020.1845039
Ibrahim, H. M., Amin, R. A., and Ghanaym, H. R. M. (2018). Effect of marination on Vibrio parahaemolyticus in tilapia fillets. Benha Vet. Med. J. 34, 234–245. doi: 10.21608/bvmj.2018.29434
Igbinosa, E. O. (2016). Detection and antimicrobial resistance of Vibrio isolates in aquaculture environments: implications for public health. Microb. Drug Resist. 22, 238–245. doi: 10.1089/mdr.2015.0169
Jun, J. W., Kim, J. H., Choresca, C. H. Jr., Shin, S. P., Han, J. E., Han, S. Y., et al. (2012). Isolation, molecular characterization, and antibiotic susceptibility of Vibrio parahaemolyticus in Korean seafood. Foodborne Pathog. Dis. 9, 224–231. doi: 10.1089/fpd.2011.1018
Kandušer, M., Šentjurc, M., and Miklavčič, D. (2008). The temperature effect during pulse application on cell membrane fluidity and permeabilization. Bioelectrochemistry 74, 52–57. doi: 10.1016/j.bioelechem.2008.04.012
Kang, C.-H., Shin, Y., Jang, S., Yu, H., Kim, S., An, S., et al. (2017). Characterization of Vibrio parahaemolyticus isolated from oysters in Korea: resistance to various antibiotics and prevalence of virulence genes. Mar. Pollut. Bull. 118, 261–266. doi: 10.1016/j.marpolbul.2017.02.070
Kim, J. Y., and Lee, J.-L. (2017). Correlation of total bacterial and vibrio spp., populations between fish and water in the aquaculture system. Front. Mar. Sci. 4:147. doi: 10.3389/fmars.2017.00147
Kim, Y. W., Lee, S. H., Hwang, I. G., and Yoon, K. S. (2012). Effect of temperature on growth of Vibrio paraphemolyticus and Vibrio vulnificus in flounder, salmon sashimi and oyster meat. Int. J. Environ. Res. Public Health 9, 4662–4675. doi: 10.3390/ijerph9124662
Kim, Y. B., Okuda, J. U. N., Matsumoto, C., Takahashi, N., Hashimoto, S., and Nishibuchi, M. (1999). Identification of Vibrio parahaemolyticus strains at the species level by PCR targeted to the toxR gene. J. Clin. Microbiol. 37, 1173–1177. doi: 10.1128/JCM.37.4.1173-1177.1999
Kitaoka, M., Miyata, S. T., Unterweger, D., and Pukatzki, S. (2011). Antibiotic resistance mechanisms of Vibrio cholerae. J. Med. Microbiol. 60, 397–407. doi: 10.1099/jmm.0.023051-0
Kokashvili, T., Whitehouse, C. A., Tskhvediani, A., Grim, C. J., Elbakidze, T., Mitaishvili, N., et al. (2015). Occurrence and diversity of clinically important Vibrio species in the aquatic environment of Georgia. Front. Public Health 3:232. doi: 10.3389/fpubh.2015.00232
Kramer, A., Schwebke, I., and Kampf, G. (2006). How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 6:130. doi: 10.1186/1471-2334-6-130
Kurdi Al-Dulaimi, M. M., and Ariffin, A. A. (2019). Multiple antibiotic resistance (MAR), plasmid profiles, and DNA polymorphisms among Vibrio vulnificus isolates. Antibiotics 8:68. doi: 10.3390/antibiotics8020068
Letchumanan, V., Yin, W.-F., Lee, L.-H., and Chan, K.-G. (2015). Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shrimps in Malaysia. Front. Microbiol. 6:33. doi: 10.3389/fmicb.2015.00033
Lopatek, M., Wieczorek, K., and Osek, J. (2015). Prevalence and antimicrobial resistance of Vibrio parahaemolyticus isolated from raw shellfish in Poland. J. Food Prot. 78, 1029–1033. doi: 10.4315/0362-028X.JFP-14-437
Lopez-Joven, C., de Blas, I., Furones, M. D., and Roque, A. (2015). Prevalences of pathogenic and non-pathogenic Vibrio parahaemolyticus in mollusks from the Spanish Mediterranean coast. Front. Microbiol. 6:736. doi: 10.3389/fmicb.2015.00736
Lopez-Joven, C., de Blas, I., and Roque, A. (2018). Temperature affects the growth and survival of tdh positive Vibrio parahaemolyticus in tissues of postharvest Manila clam (Ruditapes philippinarum). Food Microbiol. 75, 61–64. doi: 10.1016/j.fm.2017.10.016
Lou, X., Zhai, D., and Yang, H. (2021). Changes of metabolite profiles of fish models inoculated with Shewanella baltica during spoilage. Food Control 123:107697. doi: 10.1016/j.foodcont.2020.107697
Marsden, A. E., Grudzinski, K., Ondrey, J. M., DeLoney-Marino, C. R., and Visick, K. L. (2017). Impact of salt and nutrient content on biofilm formation by Vibrio fischeri. PLoS One 12:e0169521. doi: 10.1371/journal.pone.0169521
McCarter, L. (1999). The multiple identities of Vibrio parahaemolyticus. J. Mol. Microbiol. Biotechnol. 1, 51–57.
Miles, D. W., Ross, T., Olley, J., and McMeekin, T. A. (1997). Development and evaluation of a predictive model for the effect of temperature and water activity on the growth rate of Vibrio parahaemolyticus. Int. J. Food Microbiol. 38, 133–142. doi: 10.1016/S0168-1605(97)00100-1
Novoslavskij, A., Terentjeva, M., Eizenberga, I., Valciņa, O., Bartkevičs, V., and Bērziņš, A. (2016). Major foodborne pathogens in fish and fish products: a review. Ann. Microbiol. 66, 1–15. doi: 10.1007/s13213-015-1102-5
Odeyemi, O. A. (2016). Incidence and prevalence of Vibrio parahaemolyticus in seafood: a systematic review and meta-analysis. Springerplus 5:464. doi: 10.1186/s40064-016-2115-7
Raissy, M., Khamesipour, F., Rahimi, E., and Khodadoostan, A. (2014). Occurrence of Vibrio spp., Aeromonas hydrophila, Escherichia coli and Campylobacter spp., in crayfish (Astacus leptodactylus) from Iran. Iran. J. Fish. Sci. 13, 944–954.
Ray, V. A., Morris, A. R., and Visick, K. L. (2012). A semi-quantitative approach to assess biofilm formation using wrinkled colony development. J. Vis. Exp. 64:e4035. doi: 10.3791/4035
Rice, M. A. (2001). “Environmental impacts of shellfish aquaculture: filter feeding to control eutrophication,” in Marine Aquaculture and the Environment: A Meeting for Stakeholders in the Northeast. ed. M. F. Tlusty (Falmouth, MA, USA: Cape Cod Press), 77–86.
SCAD (2020). Livestock statistics and Fisheries statistics. Available at: https://www.scad.gov.ae/Release%20Documents/Livestock%20Statistics_2020_Annual_Yearly_en.pdf?msclkid=6ae69486aff611ec86e190c8af90b7b8 (Accessed September 11, 2021).
Smith, N. C., Rise, M. L., and Christian, S. L. (2019). A comparison of the innate and adaptive immune systems in cartilaginous fish, ray-finned fish, and lobe-finned fish. Front. Immunol. 10:2292. doi: 10.3389/fimmu.2019.02292
Song, X., Ma, Y., Fu, J., Zhao, A., Guo, Z., Malakar, P. K., et al. (2017). Effect of temperature on pathogenic and non-pathogenic Vibrio parahaemolyticus biofilm formation. Food Control 73, 485–491. doi: 10.1016/j.foodcont.2016.08.041
Sujeewa, A. K. W., Norrakiah, A. S., and Laina, M. (2009). Prevalence of toxic genes of Vibrio parahaemolyticus in shrimps (Penaeus monodon) and culture environment. Int. Food Res. J. 16, 89–95. doi: 10.20431/2349-0365.0406003
Surendran, P. K., Mahadeva Iyer, K., and Gopakumar, K. (1983). Salt tolerance of bacteria isolated from tropical marine fish and prawn. Fish. Technol. 20, 105–110.
Tan, C. W., Malcolm, T. T. H., Kuan, C. H., Thung, T. Y., Chang, W. S., Loo, Y. Y., et al. (2017). Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from short mackerels (Rastrelliger brachysoma) in Malaysia. Front. Microbiol. 8:1087. doi: 10.3389/fmicb.2017.01087
UNSTAD (2019). Advancing Sustainable Development Goal 14: Sustainable fish, seafood value chains, trade and climate. Available at: https://unctad.org/system/files/official-document/ditcted2019d3_en.pdf (Accessed September 11, 2021).
USDA (2020). Safe Minimum Internal Temperature Chart. Available at: https://www.fsis.usda.gov/food-safety/safe-food-handling-and-preparation/food-safety-basics/safe-temperature-chart (Accessed September 19, 2021).
Wang, Y., Zhang, H., Fodjo, E. K., Kong, C., Gu, R.-R., Han, F., et al. (2018). Temperature effect study on growth and survival of pathogenic Vibrio parahaemolyticus in Jinjiang oyster (Crassostrea rivularis) with rapid count method. J. Food Qual. 2018, 1–10. doi: 10.1155/2018/2910481
Whitaker, W. B., Parent, M. A., Naughton, L. M., Richards, G. P., Blumerman, S. L., and Boyd, E. F. (2010). Modulation of responses of Vibrio parahaemolyticus O3: K6 to pH and temperature stresses by growth at different salt concentrations. Appl. Environ. Microbiol. 76, 4720–4729. doi: 10.1128/AEM.00474-10
WHO (2021). Cholera. Available at: https://www.who.int/news-room/fact-sheets/detail/cholera (Accessed September 11, 2021).
Williams, T. C., Froelich, B. A., Phippen, B., Fowler, P., Noble, R. T., and Oliver, J. D. (2017). Different abundance and correlational patterns exist between total and presumed pathogenic Vibrio vulnificus and V. parahaemolyticus in shellfish and waters along the North Carolina coast. FEMS Microbiol. Ecol. 93, 1–11. doi: 10.1093/femsec/fix071
Yaashikaa, P. R., Saravanan, A., and Kumar, P. S. (2016). Isolation and identification of Vibrio cholerae and Vibrio parahaemolyticus from prawn (Penaeus monodon) seafood: preservation strategies. Microb. Pathog. 99, 5–13. doi: 10.1016/j.micpath.2016.07.014
Yang, Z., Jiao, X., Zhou, X., Cao, G., Fang, W., and Gu, R. (2008). Isolation and molecular characterization of Vibrio parahaemolyticus from fresh, low-temperature preserved, dried, and salted seafood products in two coastal areas of eastern China. Int. J. Food Microbiol. 125, 279–285. doi: 10.1016/j.ijfoodmicro.2008.04.007
Yang, L., Zhan, L., Han, H., Gao, H., Guo, Z., Qin, C., et al. (2010). The low-salt stimulon in Vibrio parahaemolyticus. Int. J. Food Microbiol. 137, 49–54. doi: 10.1016/j.ijfoodmicro.2009.11.006
Yoon, J.-H., Bae, Y.-M., and Lee, S.-Y. (2017). Effects of varying concentrations of sodium chloride and acidic conditions on the behavior of Vibrio parahaemolyticus and Vibrio vulnificus cold-starved in artificial sea water microcosms. Food Sci. Biotechnol. 26, 829–839. doi: 10.1007/s10068-017-0105-3
Youssef, A. I., Farag, A. L., and Helal, I. M. (2018). Molecular characterization of Vibrio parahaemolyticus isolated from shellfish and their harvesting water from Suez Canal area, Egypt. Int. Food Res. J. 25, 2375–2381. doi: 10.21608/avmj.2018.168732
Yücel, N., and Balci, Ş. (2010). Prevalence of Listeria, Aeromonas, and Vibrio species in fish used for human consumption in Turkey. J. Food Prot. 73, 380–384. doi: 10.4315/0362-028X-73.2.380
Zhao, X., Chen, L., Wongmaneepratip, W., He, Y., Zhao, L., and Yang, H. (2021). Effect of vacuum impregnated fish gelatin and grape seed extract on moisture state, microbiota composition, and quality of chilled seabass fillets. Food Chem. 354:129581. doi: 10.1016/j.foodchem.2021.129581
Keywords: Vibrio spp., antibiotic resistance, pH, salinity, temperature, prevalence
Citation: Abdalla T, Al-Rumaithi H, Osaili TM, Hasan F, Obaid RS, Abushelaibi A and Ayyash MM (2022) Prevalence, Antibiotic-Resistance, and Growth Profile of Vibrio spp. Isolated From Fish and Shellfish in Subtropical-Arid Area. Front. Microbiol. 13:861547. doi: 10.3389/fmicb.2022.861547
Edited by:
Hongshun Yang, National University of Singapore, SingaporeReviewed by:
Shaojuan Lai, Guizhou University of Traditional Chinese Medicine, ChinaLigia Virginia Da Silva, University of Maryland Eastern Shore, United States
Copyright © 2022 Abdalla, Al-Rumaithi, Osaili, Hasan, Obaid, Abushelaibi and Ayyash. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mutamed M. Ayyash, bXV0YW1lZC5heXlhc2hAdWFldS5hYy5hZQ==; Tareq M. Osaili, dG9zYWlsaUBzaGFyamFoLmFjLmFl
†These authors have contributed equally to this work
 Tarfa Abdalla1†
Tarfa Abdalla1† Reyad S. Obaid
Reyad S. Obaid Mutamed M. Ayyash
Mutamed M. Ayyash