- 1State Key Laboratory of Virology, School of Public Health, Wuhan University, Wuhan, China
- 2Jingmen Municipal Health Commission, Jingmen, China
- 3Jingmen Center for Disease Control and Prevention, Jingmen, China
- 4Jingzhou Center for Disease Control and Prevention, Jingzhou, China
- 5Department of Clinical Laboratory, The Second Hospital of Shandong University, Jinan, China
Background: The delta variant (B.1.617.2) of SARS-CoV-2 was the dominant viral strain causing COVID-19 in China, 2021. We reported a SARS-CoV-2 delta variant outbreak in Jingmen City, Hubei Province, China.
Methods: The data of epidemiological, clinical, laboratorial, and vaccination of COVID-19 cases were collected through field investigation and analyzed.
Results: During the outbreak from 4 to 20 August 2021, 58 cases of the SARS-CoV-2 delta variant (B.1.617.2) were identified with 15 (25.9%) asymptomatic and 43 (74.1%) symptomatic (mild and moderate) patients. The mean serial interval was 2.6 days (standard deviation: 2.0, 95% CI: 1.9–3.6). The median age of the patients was 39 years (ranging from 1 to 60 years) with the high proportion in children (19.0%). The secondary attack rate was 9.8% higher from parents to their children (<18 years) (46.2%, 95% CI: 14.8–77.5%) than that between spouses (36.4%, 95% CI: 14.5–58.2%), but no significant difference was observed (p > 0.05). Approximately half (27; 46.6%) of cases were vaccine breakthrough infections. In vaccine breakthrough cases (fully vaccinated), viral loads decreased 1.9–3.4-folds (p < 0.05), duration of viral shedding shortened 5 days (p < 0.05), and the risk of becoming symptomatic from asymptomatic decreased 33% (95% CI: 5–53%) (aged ≥12 years) than those in unvaccinated infections.
Conclusions: Children are highly susceptible to the SARS-CoV-2 delta variant in the COVID-19 outbreak in Jingmen City in 2021. Inactivated vaccine derived from wide-type strain can effectively reduce the viral load, duration of viral shedding, and clinical severity in vaccine breakthrough cases. Our study indicates that protective measures that include full vaccination against COVID-19, especially in children, should be strengthened.
Introduction
Since the pandemic of coronavirus disease 2019 (COVID-19) was reported in Wuhan in December 2019 (Li Q. et al., 2020), 416 million COVID-19 confirmed cases and over 5 million deaths were reported globally as of 17 February 2022 [WHO Coronavirus (COVID-19) Dashboard, 2022]. Currently, pediatric cases are on the rise composing 18.9% of all COVID-19 cases with nearly 7 million additional cases since the first week of September 2021 in the US (Children and COVID-19: State-Level Data Report, 2022). According to the definition of the World Health Organization (WHO), SARS-CoV-2 variants of concern have signature substitutions in key amino acids of the immunodominant spike protein which can affect virus characteristics with one or more of the following changes at a degree of global public health significance: increased transmissibility or detrimental change in COVID-19 epidemiology; increase in virulence or change in clinical disease presentation; and decrease in the effectiveness of public health and social measures or available diagnostics, vaccines, and therapeutics (Tracking SARS-CoV-2 Variants, 2022). Several SARS-CoV-2 variants of concern have emerged in the past 2 years including the alpha variant (B.1.1.7), beta variant (B.1.351), gamma variant (P.1), delta variant (B.617.2), lambda variant (C.37), and omicron variant (B.1.1.529) (Faria et al., 2021; Tegally et al., 2021; Vaidyanathan, 2021; Volz et al., 2021; Tracking SARS-CoV-2 Variants, 2022). The delta variant was first identified in October 2020 in India and rapidly spread to more than 200 countries, areas, or territories worldwide (Vaidyanathan, 2021; World Health Organization, 2021; Tracking of Variants, 2022; Tracking SARS-CoV-2 Variants, 2022). Based on the data from India and the UK, the delta variant was about 50–60% more transmissible than the alpha variant (Dhar et al., 2021; Public Health England, 2021).
In China, the first local transmission caused by the SARS-CoV-2 delta variant emerged in Guangzhou City in May 2021 (Zhang et al., 2021). Since then, the SARS-CoV-2 delta variant outbreaks have been reported in several cities and most of them infected <100 persons. However, there have been few publications related to SARS-CoV-2 delta variant outbreak in China, especially Hubei Province which was the epicenter of the first wave of the COVID-19 pandemic. Jingmen City in the middle of Hubei Province, a prefectural-level municipality with a population of 2.9 million, experienced the first wave of the COVID-19 pandemic with 928 confirmed cases between January and March 2020. The second COVID-19 outbreak caused by the delta variant occurred in August 2021. This outbreak gave us an opportunity to systematically analyze the characteristics of epidemiology, transmission dynamic, household transmission, and vaccination in the outbreak caused by the SARS-CoV-2 delta variant in Jingmen City, China.
Methods
Study Design and Data Sources
A retrospective observational study was conducted to explore the epidemiological, clinical, laboratorial, and effectiveness of vaccination in the SARS-CoV-2 delta variant outbreak in Jingmen City, Hubei Province, China, in August 2021. The relevant data were obtained by filed investigation and from official information system.
Case Definition
The definitions of confirmed case, asymptomatic infection, close contact, and disease severity (mild, moderate, and severe) were all defined according to the China's national guidelines for the prevention and control of COVID-19 (the eighth version) and the guidelines for the diagnosis and treatment of COVID-19 (the eighth revised version) as described in Table 1 (National Health Commission of the People's Republic of China, 2021a,b). A COVID-19 vaccine breakthrough case was defined as a fully vaccinated case who received two doses of vaccine ≥14 days before the last exposure to a person with COVID-19.

Table 1. Case definition in the SARS-CoV-2 delta variant outbreak in Jingmen City, Hubei Province, China in August 2021.
Transmission Dynamic
The serial interval is the time between the onset of illness in the primary case and secondary case. The basic reproductive number (R0) is defined as the expected number of additional cases that one case will generate without intervention and control measures. The effective reproduction number (Rt) is an indicator of real-time transmissibility of disease, which is often used to evaluate intervention and control measures. The incubation period is defined as the period of time between infection and illness onset.
Household Transmission
A household was defined as two or more individuals living in a family in this study. Household contacts were defined as the individuals living in the same family for at least 1 day or night with the household index starting from 4 days before the onset or sampling of the index case (Li et al., 2021). For asymptomatic infections, onset was defined as the date of specimen collection for the first positive SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR). A household secondary attack rate (SAR) was defined as the number of secondary symptomatic cases and asymptomatic cases in the household within 14 days after the index case was identified divided by the total number of contacts in the household.
Effect of Vaccination
The COVID-19 vaccination history of each patient was obtained from the electronic health information system and field investigation. The vaccines used in Jingmen City were inactivated virions of SARS-CoV-2 wide-type strain and produced by Beijing Institute of Biological Products Co., Ltd, Wuhan Institute of Biological Products Co., Ltd, and Sinovac Life Science Co., Ltd.
The effect of inactivated SARS-CoV-2 vaccines against the delta variant was evaluated by comparing the clinical severity, RT-PCR cycle threshold (Ct) value, and duration of viral shedding of vaccinated patients with breakthrough infection or partially vaccinated to the unvaccinated. Asymptomatic case was considered as effective vaccination when evaluating the impact of the vaccine on clinical symptoms. Vaccine status was categorized as the unvaccinated, partly vaccinated, and fully vaccinated based on the fact that it takes 14 days to develop an effective protection against SARS-CoV-2 after vaccination (National Health Commission of the People's Republic of China, 2021b). The unvaccinated individuals were either not vaccinated before the last exposure to a person with COVD-19 or received one dose of vaccine <14 days before the last exposure. Partially vaccinated referred to individuals who received one dose of vaccine ≥14 days or received two doses of vaccine <14 days before the last exposure. Fully vaccinated referred to individuals who received two doses of vaccine ≥14 days before the last exposure.
Laboratory Testing
The SARS-CoV-2 testing was performed according to the national guideline (National Health Commission of the People's Republic of China, 2021a). Nasopharyngeal swab samples were obtained from individuals, and RNA was extracted and tested by real-time RT-PCR with SARS-CoV-2-specific primers and probes aimed at the gene of the open reading frame (ORF) and nucleocapsid (N) protein. Cycle threshold (Ct) value <37 was defined as positive, and persons with the Ct value between 37 and 40 were resampled and tested to confirm. Viral load was estimated by the Ct value of real-time RT-PCR. A difference of 1 Ct unit was approximately equal to a factor of 2 in the number of virus per sample (Levine-Tiefenbrun et al., 2021). High-throughput sequencing was performed for SARS-CoV-2-positive specimens using MiniSeq high-output kit and Misq v2 Reagent Kit (Illumina, US) by the Hubei Provincial Center for Disease Control and Prevention. The whole genome sequence was compared with the nucleotide sequences in the GenBank and GISAID database.
Statistical Analysis
The analysis of the serial interval, R0, Rt, and incubation period, was performed with R software 4.0.5. The package of “R0” and “EpiEstim” was used for the estimation of R0 and Rt, respectively. Categorical variables were compared with the chi-square test or the Fisher's exact test, and continuous variables were compared with the Mann–Whitney U-test using SPSS 25.0 as appropriate. Two-sided p < 0.05 and 95% confidence intervals (CIs) not including 1 value were considered as statistically significant.
Ethics Approval and Consent
Institutional review board approval was waived as a part of a public health outbreak investigation in China. The data for analysis in our study were anonymous.
Results
Outbreak Description
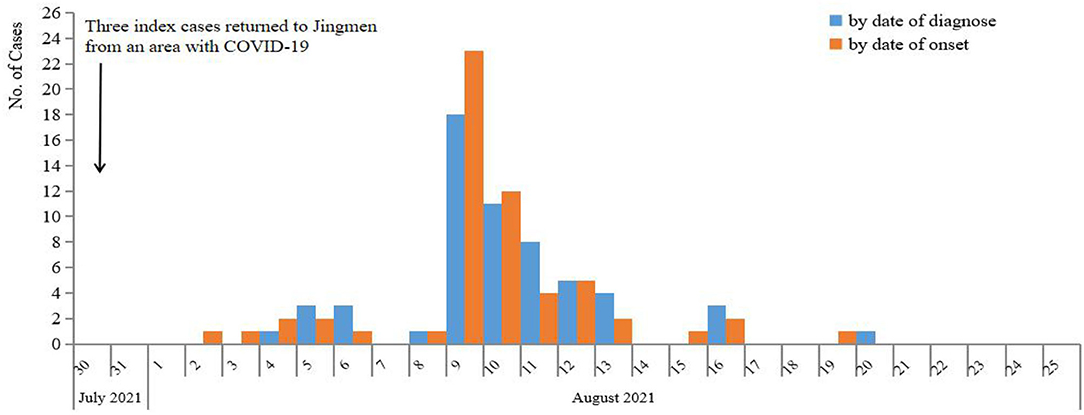
On 4 August 2021, a 33-year-old male who had illness onset 2 days ago was diagnosed as COVID-19 in Jingmen City, Hubei Province, China. The patient was a migrant worker and had worked in a construction filed in an area with COVID-19 before return to Jingmen City 5 days ago. After he was confirmed to be infected with SARS-CoV-2, another three co-workers who returned to Jinmen City together with the index patient were also tested for SARS-CoV-2 and two of his co-workers were also SARS-CoV-2-positive. After discovering the three patients, totally 4,436 close contacts were identified in Jingmen City, and all of them were tested for SARS-CoV-2 with RT-PCR from 4 to 20 August 2021. The PCR results showed that another 55 individuals to be SARS-CoV-2-positive. In total, 58 cases were identified in this COVID-19 epidemic in Jingmen City (Figure 1). DNA sequencing showed that the SARS-CoV-2 strains from the cases were delta variant. The median age of the cases was 39 years old ranging from 1 to 60 with 11 (19.0%) children (≤18 years), and the male and female ratio was 1.9:1 (38 men and 20 women). According to the clinical severity, 25.9% (15/58) of patients were asymptomatic, and 74.1% (43/58) of patients had clinical symptoms with 41.3% (24/58) mild and 32.8% (19/58) moderate. The asymptomatic proportion was 27.3% (3/11) for children and 25.5% (12/47) for adults (Fisher's exact: p > 0.05). The mean interval time between illness onset and diagnosis was 2 days (95% CI: 1.7–2.4). The median Ct value of the cases for the first real-time RT-PCR result was 26.3 (inter quartile range, IQR: 22.7–31.0) for the N gene and 27.35 (IQR: 22.0–32.23) for the ORF gene (Table 2).
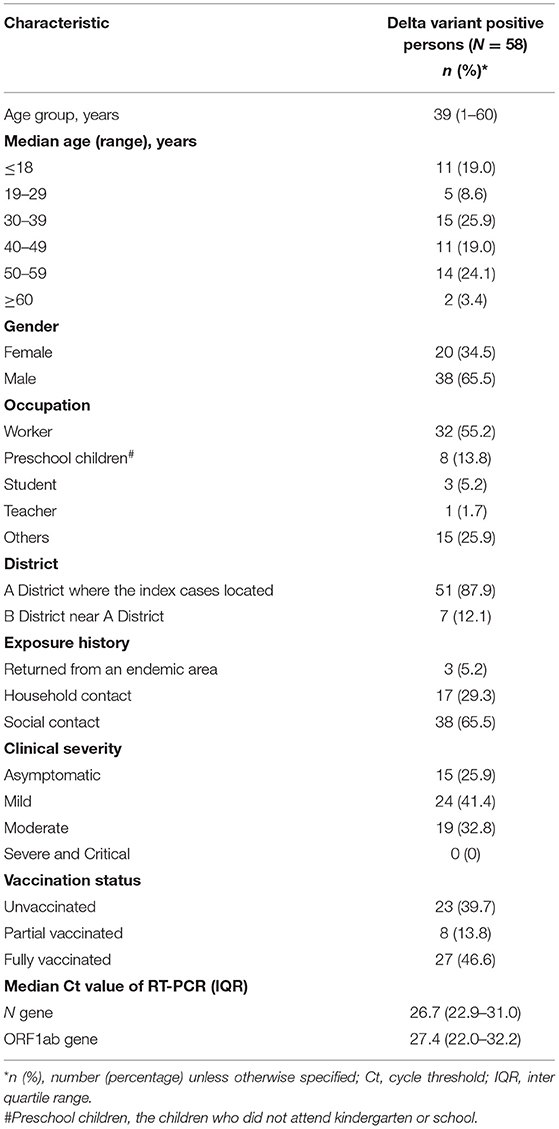
Table 2. Epidemiological characteristics of the SARS-CoV-2 delta variant outbreak in Jingmen City, Hubei Province, China in August 2021.
Transmission Dynamics
Based on the epidemiological data of 58 infector–infectee pairs, the transmissibility parameters were calculated. The mean serial interval was 2.6 days (standard deviation: 2.0, 95% CI: 1.9–3.6) and mean incubation period was 4.0 days (95% CI: 2.0–4.8). The evaluated R0was 2.8 (95% CI: 1.6–4.8) using the maximum likelihood method (the coefficient of determination R2 = 0.96). Then, 9 days after the initial case was reported, the effective reproduction number (Rt) decreased to 1 on 13 August, and fluctuated close to 1 from 13 to 20 August. No new case was reported since 20 August (Figure 2).
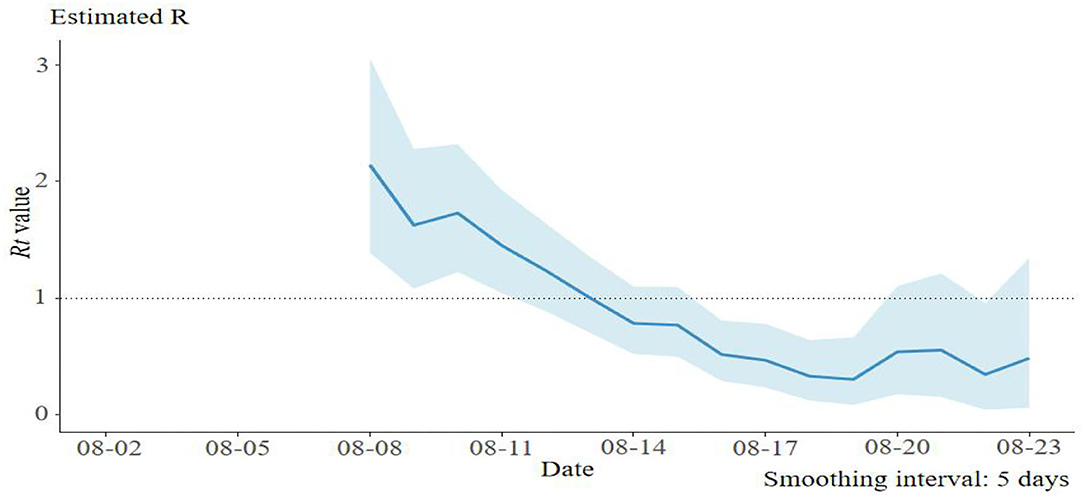
Figure 2. The effective reproduction number (Rt) of the SARS-CoV-2 delta variant outbreak in Jingmen City, Hubei Province, China in August 2021.
Close Contacts Tracing
In total, 4,436 close contacts were traced and quarantined, and 55 (1.2%, 95% CI: 0.9–1.6%) were found to be secondarily infected. The highest SAR occurred in the household setting (17/68, 25%, 95% CI: 14.4–35.6%), followed by 2.2% (23/1,025, 95% CI: 1.3–3.2%) in the workplace, and 0.5% (15/3,343, 95% CI: 0.2–0.7%) in other places, with significant differences (p < 0.05) (Figure 3).
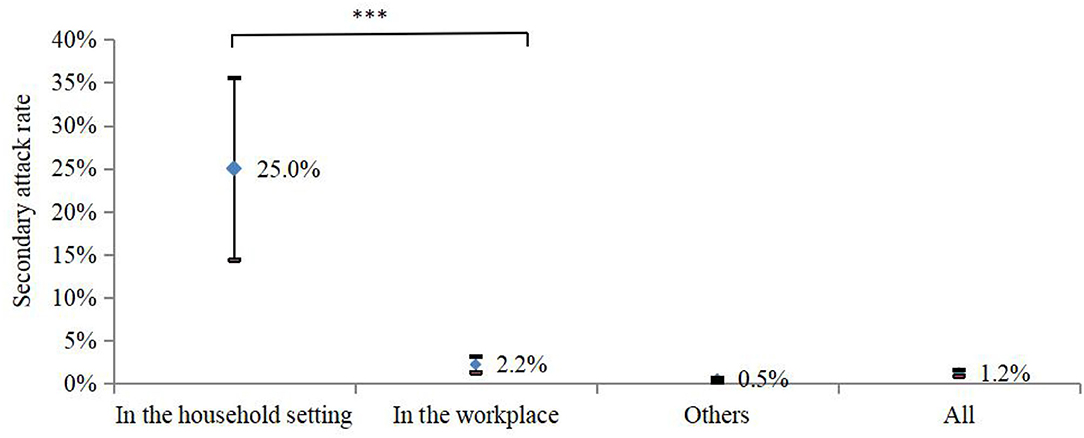
Figure 3. SAR occurred in the different settings in Jingmen City, Hubei Province, China in August 2021. ***p < 0.001.
Household Transmission
Among all 58 COVID-19 cases, 44 cases belonged to 27 households and 14 cases lived alone or in working dormitories. In 27 households with COVID-19 cases, there were 68 household contacts with the median number of persons per household of three, which ranged from 2 to 7. For the 27 households with COVID-19, 11 households (39.3%) had 17 secondary cases. The SAR was 25.0% (17/68) (95% CI: 14.4–35.6%) among the household contacts. Although the SAR was 9.8% higher between parents and children (<18 years) (46.2%, 95% CI: 14.8–77.5%) than that between spouses (36.4%, 95% CI: 14.5–58.2%), no statistical difference was observed (p > 0.05) (Figure 4). Among the 11 households with secondary transmission, the SAR was 60.7% (17/28, 95% CI: 41.4–80.0%), and 3 (27.3%) out of 11 households had a 100% SAR. The average number of secondarily infected individuals was 2, which ranged from 2 to 6, and the median serial interval was 2.0 days (standard deviation: 2.2, 95% CI: 1.5–4.0).
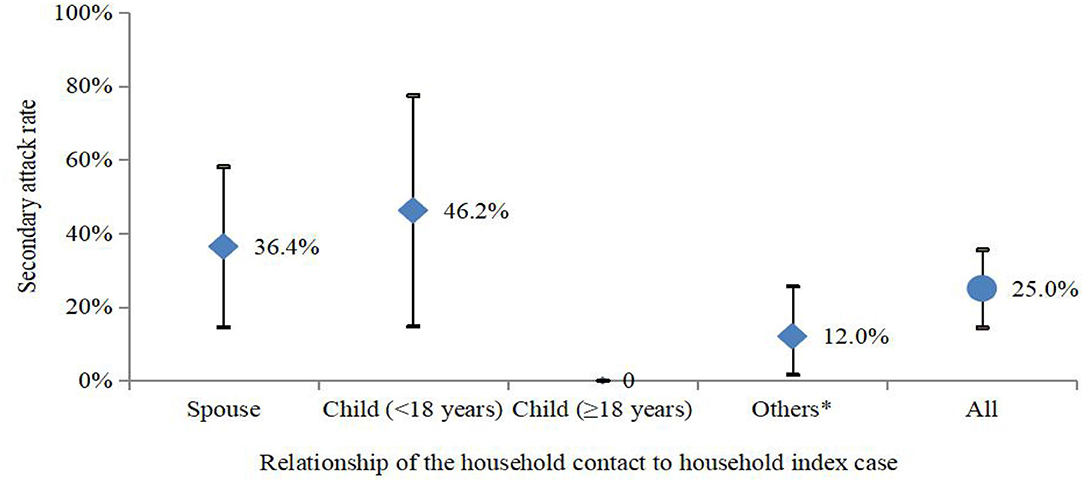
Figure 4. SAR (95% CI) in households in the SARS-CoV-2 delta variant outbreak in Jingmen City, Hubei Province, China in August 2021. *Others included parents, siblings, and grandparents.
The median Ct values for the N gene and ORF1ab gene were 23.1 (21.7–26.4) and 23.2 (21.1–27.9) in households with secondary transmission and 27.1 (22.4–29.1) and 29.1 (23.0–31.2) in household without secondary transmission, which represented an increase of 4.0–5.9-folds in viral load in household indexes with secondary transmission compared to those without secondary transmission (p < 0.05), which indicated a high viral load might contribute to secondary transmission. There was no statistically difference in the age, gender, and clinical severity between the household indexes with secondary transmission and those without secondary transmission (p > 0.05) (Table 3).
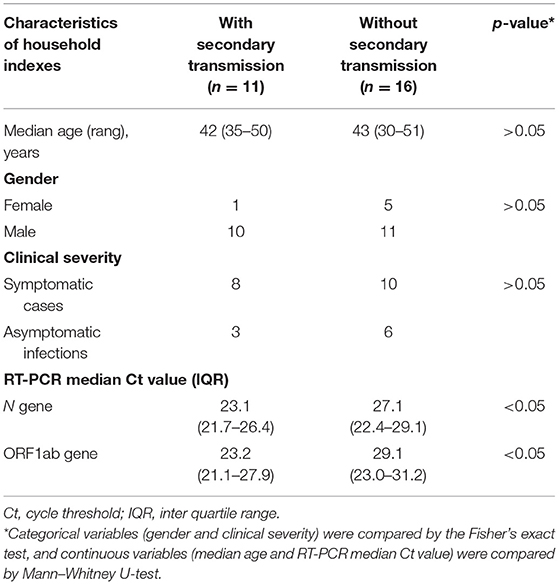
Table 3. Characteristics of household indexes in the SARS-CoV-2 delta variant outbreak in Jingmen City, Hubei Province, China in August 2021.
The Effect of Vaccination
Among the 58 COVID-19 cases, 27 (46.6%) were vaccine breakthrough cases (fully vaccinated with inactivated virion vaccine of wide-type strain). The first-time PCR results showed that the median Ct values of both N gene and ORF1ab gene in vaccine breakthrough cases were higher than that in unvaccinated cases (Table 4), which represents a 1.9–3.4-fold decrease of viral load in vaccine breakthrough cases compared to the unvaccinated cases (p < 0.05), while no statistical difference was observed in partially vaccinated cases compared to the unvaccinated cases (p > 0.05). The duration of viral shedding was 5 days shorter in vaccine breakthrough cases (median: 19 days; IQR: 15–23 days) than that in unvaccinated cases (median: 24 days; IQR: 18–30 days) (p < 0.05) (Table 4). The risk of progressing from asymptomatic to symptomatic decreased 33% (95% CI: 5–53%) in fully vaccinated patients than that in unvaccinated patients aged ≥12 years (Table 5).
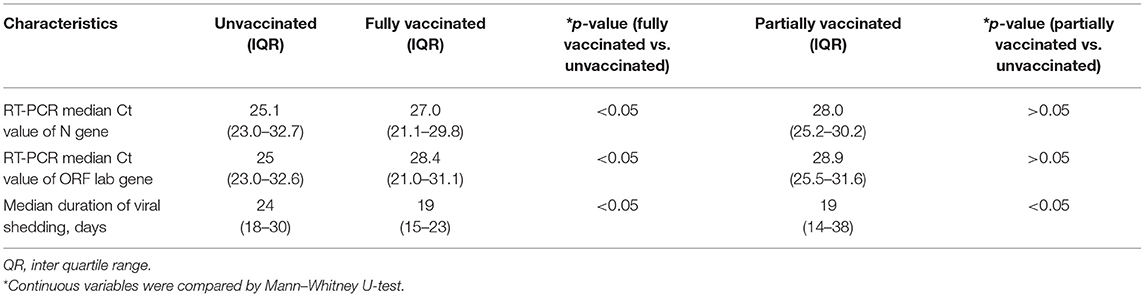
Table 4. The viral load of the SARS-CoV-2 delta variant infected patients in different vaccination status in Jingmen City, Hubei Province, China in August 2021.
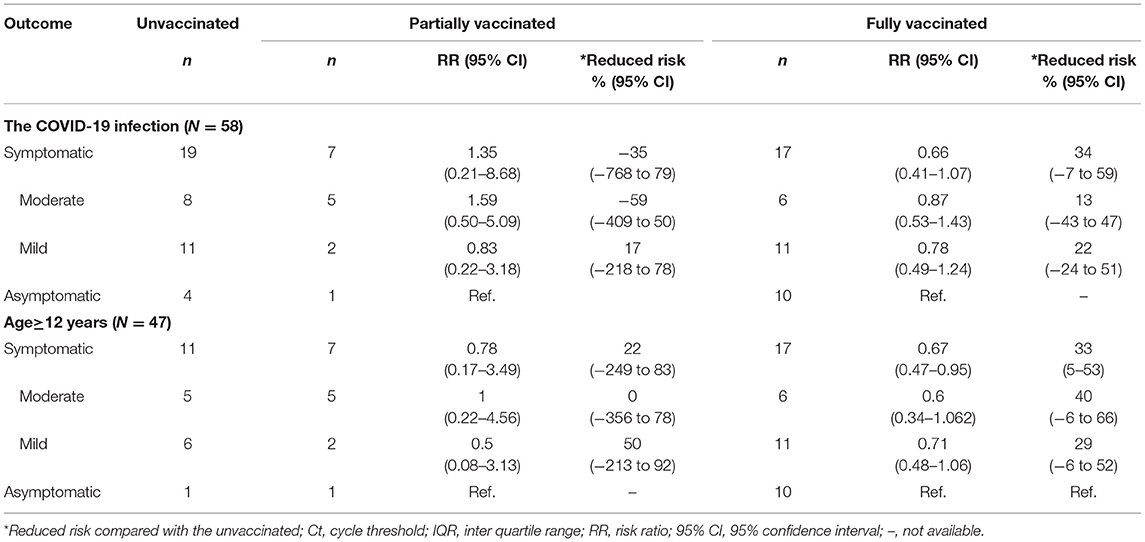
Table 5. Clinical severity of delta variant infected patients in different vaccination status in Jingmen City, Hubei Province in August 2021.
Discussion
The SARS-CoV-2 delta variant outbreak in Jingmen City in August 2021 was eliminated in 17 days with 58 cases and the proportion of infected children was relatively high (19%) in this outbreak. In the first wave of COVID-19 pandemic caused by wide-type SARS-CoV-2 in Jingmen City in 2020, the proportion of infected children was only 0.5% (5/928). The previous study showed that children appeared to be less infected by the wide-type strain of SARS-CoV-2 (infection rate varied from 0.9–7.9%) than adults based on a large scale COVID-19-related epidemiological studies (Mehta et al., 2020). A meta-analysis based on 213 published literatures of household transmission showed that the SAR of other strains of SARS-CoV-2 in pediatric household contacts was lower than in adult household contacts (RR, 0.62; 95% CI, 0.42–0.91) (Zhu et al., 2021). We found that all pediatric cases were between ages 1 and 10 years in this outbreak and did not get vaccinated. Vaccination might be a confounding factor to affect the susceptibility in children under 12 years to delta variant. However, there was no vaccine available during wide-type strain epidemic in China. Children under 12 years were not vaccinated both in the previous wide-type strain epidemic and this delta variant outbreak in Jingmen City. Therefore, it could be ruled out that the vaccine affects the susceptibility of children to the wide-type strain outbreaks and this outbreak. Children are more susceptible to SARS-CoV-2 delta variant in Jingmen City in 2021 than wide-type strain in 2019–2020, which could be caused by different exposure opportunity and/or viral type.
The SAR between parents and children (<18 years) was much higher (9.8%) than that between spouses in our study. However, no statistical difference was observed (p > 0.05), which was most likely caused by the small sample size. The higher SAR in children than parents in the household could be influenced by the mode of interactions between the adults and children (as in preparation of food, for instance), or more greatly associated with a particular type of interaction, or vaccination status and not an inherent increased susceptibility of children. Whether children are more susceptible than adults to SARS-CoV-2 delta variant needs to be further investigated.
As of 4 February 2022, a total of 8.6 billion doses of COVID-19 vaccine have been administered globally including 3.0 billion in China (WHO, 2022). COVID-19 vaccine breakthrough infections with varied proportion have been observed (Abu-Raddad et al., 2021; Bergwerk et al., 2021). In this study, we systematically evaluated the effect of vaccination derived from the wide-type strain in viral loads, duration of viral shedding, and clinical severity in the outbreak of SARS-CoV-2 delta variant in Jingmen City in 2021. Our findings showed that full vaccination with inactivated COVID-19 vaccines derived from the wide-type strain had partial cross-protection against the SARS-CoV-2 delta variant (B.1.617.2). In vaccine breakthrough cases, the SARS-CoV-2 delta variant viral load in the first positive PCR test and duration of viral shedding decreased 1.9–3.4-folds and shortened 5 days, respectively, compared with those in unvaccinated patients. The previous study in Israel (n = 3,776) showed a decrease of 2.8–4.5-folds in SARS-CoV-2 viral load in BNT162b2 messenger RNA vaccine breakthrough patients compared with those of the unvaccinated (Levine-Tiefenbrun et al., 2021). Our study showed that inactivated SARS-CoV-2 vaccine derived from the wide-type strain also had the similar function in reducing the viral load in the vaccine breakthrough infections of SARS-CoV-2 delta variant. A study in the US between January and August 2021 showed that symptomatic delta variant breakthrough cases had longer duration of viral shedding compared with non-delta variants (Siedner et al., 2022). However, to our knowledge, few published studies explored the difference of the viral shedding duration between the vaccine breakthrough infections and the unvaccinated for the individuals who both infected with SARS-CoV-2 delta variant. This study added the knowledge to this aspect. Vaccine breakthrough patients aged ≥12 years had a 33% reduced risk in progressing from asymptomatic to symptomatic compared with that in unvaccinated cases in this outbreak. A SARS-CoV-2 delta variant outbreak that occurred in Guangzhou City, China, in May and June 2021 showed that the function of inactive full vaccination was 59.0% vaccination effectiveness against infection, 70.2% against moderate COVID-19, 100% against severe by case-control study, 51.8% against infection, 60.4% against symptomatic infection, 78.4% against pneumonia, and 100% against severe or critical illness by retrospective cohort study (Li et al., 2021; Kang et al., 2022). A previous study in the UK showed that the BNT162b2 and ChAdOx1 nCoV-19 vaccines were 88.0 and 67.0% effectiveness against the SARS-COV-2 delta variant (Lopez Bernal et al., 2021).
In our study, the mean serial interval was 2.6 days, which was similar to what was observed in the SARS-CoV-2 delta variant outbreak in Guangdong Province (2.3 days), but significantly shorter than what was observed from the wide-type strain of SARS-CoV-2 outbreak in Wuhan City (early estimation: 7.5 days) (Li Q. et al., 2020), Jingzhou City (5.8 days) (Liu et al., 2020), Hubei Province (4.0 days) (Du et al., 2020), and Hunan Province (5.5 days) in 2020 (Hu et al., 2021). The shorter serial interval of SARS-CoV-2 delta variant implies control measures such as contact tracing and quarantining which should be conducted as soon as possible to stop further transmission. The effective reproduction number (Rt) was an indicator of real-time transmissibility of disease and the effect of interventions. In this epidemic, Rtdecreased to about 1, 9 days after the first case was reported, which was similar to the delta variant outbreak in Guangzhou City (Zhang et al., 2021). A series of rigorous intervention measures were used to contain this outbreak, which includes active case finding, rapid contacts tracing and quarantining, three times of nucleic acid test for the whole population in the city, lockdown of affected area, community management, and travel restrictions. The quick decline of Rtindicated that the control measures worked well.
High transmissibility in households was another feature in this outbreak. The study of household SARs caused by the SARS-CoV-2 delta variant (B.1.617.2) was limited (Dougherty et al., 2021). In the previous studies of epidemics caused by the wide-type of SARS-CoV-2 or other related variants, household SARs varied from 3 to 32% in China and the US (Jing et al., 2020; Lewis et al., 2020; Li W. et al., 2020; Wu et al., 2020; Cerami et al., 2021). Compared with outbreaks caused by wide-type strain of SARS-CoV-2, the overall household SAR in this outbreak (25%) was higher than that in Guangzhou City, China (12.4–17.1%) and lower than that in the US households (29–32%) and Zhuhai City, China (32%) (Jing et al., 2020; Lewis et al., 2020; Wu et al., 2020; Cerami et al., 2021). However, high SAR (60.7%) was observed among households with secondary transmission including 100% SAR in 3 households in this outbreak, which was similar to SARS-CoV-2 delta variant (B.1.617.2) outbreak in Oklahoma, US (SARs among households with secondary transmission ranged from 80 to 100%) (Dougherty et al., 2021). The viral load in index patients with household secondary transmission was much higher (4.0–5.9-folds) than those index patients without household secondary transmission. Factors such as household contacts without protective measures cohabitation with the index case and household crowding may increase the risk of secondary infection (Lewis et al., 2020; Wu et al., 2020; Cerami et al., 2021). In our study, although the primary infections were sent to the designated hospitals as soon as possible, precautionary practices such as mask use and increased social distance basically were not observed in households, which probably increased the odds of household transmission (Wu et al., 2020).
Our study has several limitations. First, recall bias could not be fully avoided in the investigation of cases and their contacts despite the in-depth investigation. Second, the sample size in this outbreak is small, which may affect the statistical test. Third, the valuation of vaccination effectiveness by respective cohort study, case–control, or subgroup analyses was limited by insufficient data such as few cases, no severe and critical case, and unavailable data for the vaccination status of all close contacts in this study. Fourth, although the hospitalization is an important index to evaluate the effectiveness of vaccination, we could not use it because all individuals infected with SARS-CoV-2 were hospitalized even if asymptomatic in China.
In conclusion, our findings showed that children are highly susceptible to the SARS-CoV-2 delta variant (B.1.617.2), and the transmission could easily happen from parents to their children. Inactivated COVID-19 vaccine derived from wide-type strain of SARS-CoV-2 showed protection against the SARS-CoV-2 delta variant in reducing viral load, duration of viral shedding, and disease severity in vaccine breakthrough cases compared with those in unvaccinated cases. Protective measures against COVID-19 among children should be strengthened, and increased full vaccination efforts should be taken not only in adults, but also in children to reduce the disease severity in individuals and prevent the spread of the virus in the community.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
DL contributed to field investigation, analysis, and manuscript drafting. A-eL contributed to study design and supervision. Z-qL and YB contributed to field investigation. TL contributed to field investigation and revised the manuscript. X-RQ contributed to supervision. X-jY contributed to study design, revised the manuscript, and supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Funds of China (grant number: 81971939), the Natural Science Foundation of Shandong Province of China (ZR202102210339), and Jingmen Science and Technology Plan Project (grant number: 2020YFYB114).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Local and Provincial Health Sectors and Local Public Security Bureau for their supports in this investigation.
References
Abu-Raddad, L. J., Chemaitelly, H., Ayoub, H. H., Yassine, H. M., Benslimane, F. M., Al Khatib, H. A., et al. (2021). Association of prior SARS-CoV-2 infection with risk of Breakthrough infection following mRNA vaccination in Qatar. JAMA 326, 1930–1939. doi: 10.1001/jama.2021.19623
Bergwerk, M., Gonen, T., Lustig, Y., Amit, S., Lipsitch, M., Cohen, C., et al. (2021). Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 385, 1474–1484. doi: 10.1056/NEJMoa2109072
Cerami, C., Popkin-Hall, Z. R., Rapp, T., Tompkins, K., Zhang, H., Muller, M. S., et al. (2021). Household transmission of SARS-CoV-2 in the United States: living density, viral load, and disproportionate impact on communities of color. Clin. Infect. Dis. 12, ciab701. doi: 10.1093/cid/ciab701
Children COVID-19: State-Level Data Report. (2022) Available online at: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/ (accessed February 18 2022).
Dhar, M. R., Radhakrishnan, V., Ponnusamy, K., and Rakshit, P. (2021). Genomic characterization and epidemiology of an emerging SARS-CoV-2 variant in Delhi, India. Science. 374, 995–999. doi: 10.1126/science.abj9932
Dougherty, K., Mannell, M., Naqvi, O., Matson, D., and Stone, J. (2021). SARS-CoV-2 B.1.617.2 (Delta) Variant COVID-19 outbreak associated with a gymnastics facility - Oklahoma. Morb. Mortal. Wkly. Rep. 70, 1004–1007. doi: 10.15585/mmwr.mm7028e2
Du, Z. W., Xu, X. K., Wu, Y., Wang, L., Cowling, B. J., and Meyers, L. A. (2020). Serial interval of COVID-19 among publicly reported confirmed cases. Emerg. Infect. Dis. 26, 1341–1343. doi: 10.3201/eid2606.200357
Faria, N. R., Mellan, T. A., Whittaker, C., Claro, I. M., Candido, D. D. S., Mishra, S., et al. (2021). Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science 372, 815–821. doi: 10.1126/science.abh2644
Hu, S., Wang, W., Wang, Y., Litvinova, M., Luo, K., Ren, L., et al. (2021). Infectivity, susceptibility, and risk factors associated with SARS-CoV-2 transmission under intensive contact tracing in Hunan, China. Nat. Commun. 12, 1533. doi: 10.1038/s41467-021-21710-6
Jing, Q. L., Liu, M. J., Zhang, Z. B., Fang, L. Q., Yuan, J., Zhang, A. R., et al. (2020). Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect. Dis. 20, 1141–1150. doi: 10.1016/S1473-3099(20)30471-0
Kang, M., Yi, Y., Li, Y., Sun, L. M., Deng, A. P., Hu, T., et al. (2022). Effectiveness of inactivated COVID-19 vaccines against illness caused by the b.1.617.2 (Delta) variant during an Outbreak in Guangdong, China. Ann. Intern. Med. M21–3509. doi: 10.7326/M21-3509
Levine-Tiefenbrun, M., Yelin, I., Katz, R., Herzel, E., Golan, Z., Schreiber, L., et al. (2021). Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat. Med. 27, 790–792. doi: 10.1038/s41591-021-01316-7
Lewis, N. M., Chu, V. T., Ye, D., Conners, E. E., Gharpure, R., Laws, R. L., et al. (2020). Household transmission of SARS-CoV-2 in the United States. Clin. Infect. Dis. 73, 1805–1813. doi: 10.1093/cid/ciaa1166
Li, Q., Guan, X., Wu, P., Wang, X., Zhou, L., Tong, Y., et al. (2020a). Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 382, 1199–1207. doi: 10.1056/NEJMoa2001316
Li, W., Zhang, B., Lu, J., Liu, S., Chang, Z., Peng, C., et al. (2020b). Characteristics of household transmission of COVID-19. Clin. Infect. Dis. 71, 1943–1946. doi: 10.1093/cid/ciaa450
Li, X. N., Huang, Y., Wang, W., Jing, Q. L., Zhang, C. H., Qin, P. Z., et al. (2021). Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case-control real-world study. Emerg. Microbes Infect. 10, 1751–1759. doi: 10.1080/22221751.2021.1969291
Liu, T., Qi, L., Yao, M., Tian, K., Lin, M., and Jiang, H. (2020). Serial interval and reproductive number of COVID-19 among 116 infector-infectee Pairs — Jingzhou City, Hubei Province, China, 2020. China CDC Wkly. 2, 491–495. doi: 10.46234/ccdcw2020.118
Lopez Bernal, J., Andrews, N., Gower, C., Gallagher, E., Simmons, R., Thelwall, S., et al. (2021). Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 385, 585–594. doi: 10.1056/NEJMoa2108891
Mehta, N. S., Mytton, O. T., Mullins, E. W. S., Fowler, T. A., Falconer, C. L., Murphy, O. B., et al. (2020). SARS-CoV-2 (COVID-19): what do we know about children? A systematic review. Clin. Infect. Dis. 71, 2469–2479. doi: 10.1093/cid/ciaa556
National Health Commission of the People's Republic of China. (2021a). Guidelines for the Prevention and Control of COVID-19 (the eighth version). Available online at: http://www.nhc.gov.cn/jkj/s3577/202105/6f1e8ec6c4a540d99fafef52fc86d0f8.shtml (accessed September 1, 2021).
National Health Commission of the People's Republic of China. (2021b). Guidelines for the Diagnosis and Treatment of COVID-19 (the eighth version). Available online at: http://www.nhc.gov.cn/jkj/s3577/202105/6f1e8ec6c4a540d99fafef52fc86d0f8.shtml (accessed September 1, 2021).
Public Health England. (2021) SARS-CoV-2 Variants of Concern and Variants Under Investigation in England: Technical Briefing 13. Available online at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/990339/Variants_of_Concern_VOC_Technical_Briefing_13_England.pdf (accessed September 18 2021).
Siedner, M. J., Boucau, J., Gilbert, R. F., Uddin, R., Luu, J., Haneuse, S., et al. (2022). Duration of viral shedding and culture positivity with postvaccination SARS-CoV-2 delta variant infections. JCI Insight 7, e155483. doi: 10.1172/jci.insight.155483
Tegally, H., Wilkinson, E., Giovanetti, M., Iranzadeh, A., Fonseca, V., Giandhari, J., et al. (2021). Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 592, 438–443. doi: 10.1038/s41586-021-03402-9
Tracking of Variants. (2022) Available online at: https://www.gisaid.org/hcov19-variants/ (accessed February 18 2022).
Tracking SARS-CoV-2 Variants. (2022) Available online at: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed February 18, 2022).
Vaidyanathan, G. (2021). Coronavirus variants are spreading in India - what scientists know so far. Nature 593, 321–322. doi: 10.1038/d41586-021-01274-7
Volz, E., Mishra, S., Chand, M., Barrett, J. C., Johnson, R., Geidelberg, L., et al. (2021). Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature 593, 266–269. doi: 10.1038/s41586-021-03470-x
WHO Coronavirus (COVID-19) Dashboard. (2022) Available online at: https://covid19.who.int/ (accessed February 18, 2022).
World Health Organization. (2021) CORONAVIRUS disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. Available online at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-8-june-2021 (accessed September 18 2021).
Wu, J., Huang, Y., Tu, C., Bi, C., Chen, Z., Luo, L., et al. (2020). Household transmission of SARS-CoV-2, Zhuhai, China, 2020. Clin. Infect. Dis. 71, 2099–2108. doi: 10.1093/cid/ciaa557
Zhang, M., Xiao, J., Deng, A., Zhang, Y., Zhuang, Y., Hu, T., et al. (2021). Transmission dynamics of an outbreak of the COVID-19 delta variant B.1.617.2 — Guangdong Province, China. China CDC Weekly 3, 584–586. doi: 10.46234/ccdcw2021.151
Keywords: SARS-CoV-2, delta variant (B.1.617.2), children, breakthrough infection, household transmission
Citation: Li D, Li A-e, Li Z-q, Bao Y, Liu T, Qin X-R and Yu X-j (2022) SARS-CoV-2 Delta Variant in Jingmen City, Hubei Province, China, 2021: Children Susceptible and Vaccination Breakthrough Infection. Front. Microbiol. 13:856757. doi: 10.3389/fmicb.2022.856757
Received: 17 January 2022; Accepted: 14 March 2022;
Published: 13 April 2022.
Edited by:
Axel Cloeckaert, Institut National de recherche pour l'agriculture, l'alimentation et l'environnement (INRAE), FranceReviewed by:
Antoni Soriano-Arandes, Vall d'Hebron University Hospital, SpainMark Parcells, University of Delaware, United States
Silvia Spoto, Policlinico Universitario Campus Bio-Medico, Italy
Zhenhai Zhang, Southern Medical University, China
Copyright © 2022 Li, Li, Li, Bao, Liu, Qin and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ai-e Li, NTY1MDgyNDcxQHFxLmNvbQ==; Xiang-Rong Qin, NDkwMTYxMzM3QHFxLmNvbQ==; Xue-jie Yu, eXV4dWVqaWVAd2h1LmVkdS5jbg==
 Dan Li
Dan Li Ai-e Li2*
Ai-e Li2* Tian Liu
Tian Liu Xue-jie Yu
Xue-jie Yu