- 1Department of Infectious Diseases and Public Health, Jockey Club College of Veterinary Medicine and Life Sciences, City University of Hong Kong, Kowloon, Hong Kong SAR, China
- 2Department of Biology, College of Science, United Arab Emirates University, Al Ain, United Arab Emirates
- 3Department of Epidemiology and Public Health, University of Veterinary and Animal Sciences, Lahore, Pakistan
- 4School of Natural and Environmental Sciences, Newcastle University, Newcastle upon Tyne, United Kingdom
- 5Anses, INRAE, Ecole Nationale Vétérinaire d’Alfort, UMR BIPAR, Laboratoire de Santé Animale, Maisons-Alfort, France
Among blood-sucking arthropods, ticks are recognized as being of prime global importance because of their role as vectors of pathogens affecting human and animal health. Ticks carry a variety of pathogenic, commensal, and symbiotic microorganisms. For the latter, studies are available concerning the detection of endosymbionts, but their role in the physiology and ecology of ticks remains largely unexplored. This review paper focuses on tick endosymbionts of the genera Coxiella, Rickettsia, Francisella, Midichloria, and Wolbachia, and their impact on ticks and tick-pathogen interactions that drive disease risk. Tick endosymbionts can affect tick physiology by influencing nutritional adaptation, fitness, and immunity. Further, symbionts may influence disease ecology, as they interact with tick-borne pathogens and can facilitate or compete with pathogen development within the vector tissues. Rickettsial symbionts are frequently found in ticks of the genera of Ixodes, Amblyomma, and Dermacentor with relatively lower occurrence in Rhipicephalus, Haemaphysalis, and Hyalomma ticks, while Coxiella-like endosymbionts (CLEs) were reported infecting almost all tick species tested. Francisella-like endosymbionts (FLEs) have been identified in tick genera such as Dermacentor, Amblyomma, Ornithodoros, Ixodes, and Hyalomma, whereas Wolbachia sp. has been detected in Ixodes, Amblyomma, Hyalomma, and Rhipicephalus tick genera. Notably, CLEs and FLEs are obligate endosymbionts essential for tick survival and development through the life cycle. American dog ticks showed greater motility when infected with Rickettsia, indirectly influencing infection risk, providing evidence of a relationship between tick endosymbionts and tick-vectored pathogens. The widespread occurrence of endosymbionts across the tick phylogeny and evidence of their functional roles in ticks and interference with tick-borne pathogens suggests a significant contribution to tick evolution and/or vector competence. We currently understand relatively little on how these endosymbionts influence tick parasitism, vector capacity, pathogen transmission and colonization, and ultimately on how they influence tick-borne disease dynamics. Filling this knowledge gap represents a major challenge for future research.
Introduction
Ticks are hematophagous, obligate ectoparasites of terrestrial vertebrates such as amphibians, reptiles, birds, mammals, and humans (Black and Piesman, 1994; Estrada-Peña and Jongejan, 1999; Stafford et al., 2007; Anderson and Magnarelli, 2008). Ticks play a significant role in transmitting infectious diseases (Hussain et al., 2021b; Perveen et al., 2021b), and are competent vectors of a wide range of pathogens affecting animal and human health globally (de la Fuente et al., 2017; Hussain et al., 2021a,c). They are the most important vectors among those transmitting vector-borne pathogens to animals, and the second, after mosquitoes, for pathogens with impact for human health (de la Fuente et al., 2017; Hussain et al., 2021a,c). As a taxonomic group, ticks are well-adapted to a wide range of climatic conditions, thriving in tropical, temperate and even subarctic habitats (Anderson and Magnarelli, 2008). Ticks (Acari: Ixodida) are divided into three families: Ixodidae, Argasidae, and Nuttalliellidea (Anderson and Magnarelli, 2008; Perveen, 2021), and almost 28 tick species have been reported to transmit pathogens to humans (Anderson and Magnarelli, 2008). The prevalence of many tick-borne pathogens, such as Babesia, Theileria, and Borrelia has increased in recent years due to climate change and other anthropogenic factors such as land use change, deforestation, urbanization, global travel, and trade (Rochlin and Toledo, 2020). Ticks acquire these pathogens during blood feeding on an infected vertebrate host (Raffel et al., 2014). Some colonize the tick midgut (Raffel et al., 2014), and migrate to the salivary gland from where they are transmitted via tick feeding to a new host. Although less common, direct transmission of some pathogens (e.g., Borrelia afzelii) from tick midguts has also been reported (Pospisilova et al., 2019).
Some bacteria, mainly present in the Malpighian tubules or ovaries of ticks, act as endosymbionts and are harmless to vertebrate hosts (Rowley et al., 2004). Obligate symbionts are indispensable for tick survival and fitness, frequently transmitted from adult females to their offspring, while some facultative symbionts as Cardinium or Spiroplasma are reproductive parasites and have major impacts on reproduction of arthropods (Perlman et al., 2006). Bacterial symbionts that are vertically transmitted from mother to offspring increase the fitness of the tick by directly enhancing the reproductive capacity of the vector, which in turn facilitates and increases the symbiont survival and persistence across arthropod hosts generations (Brouqui et al., 1993). However, whilst our current understanding of the life cycle of these symbionts is typically framed within vertical transmission only, horizontal transmission has been observed in a number of cases. For example, evidence of horizontal transmission has been reported in tick symbionts such as Midichloria (Bazzocchi et al., 2013), Coxiella (Shivaprasad et al., 2008; Seo et al., 2016; Mioni et al., 2020; Kobayashi et al., 2021) and Arsenophonus (Edouard et al., 2013) strains (Bonnet et al., 2017). Horizontal transmission is also common in symbionts of other arthropods such as parasitoid wasps (Parratt et al., 2016). Exposure of vertebrate hosts to tick symbionts have been regarded as evidence of the pathogenic potential of symbionts (Shivaprasad et al., 2008). Tick-host-symbiont relationships have been thus described as a continuum of “mutualism,” “commensalism,” or “parasitism” (Bonnet et al., 2017). But the role of vertebrate hosts in the ecology and life cycle of tick symbionts remains poorly understood due to a paucity of research in this area.
Other bacteria, that are neither human pathogens nor strict symbionts, can be regarded more generally as tick microbiota (Wu-Chuang et al., 2021a). Within the text, “microbiota” only refers to the microbes themselves, whereas “microbiome” refers to the microorganisms and their genes. Cowdry (1925) first recognized the relationship between ticks and their microbiome at the beginning of the twentieth century. His work reported Rickettsia-like bacteria in the ovaries, eggs, Malpighian tubules, and intestinal epithelial cells of 16 different tick species (Cowdry, 1925). The first tick microbiome study to employ next-generation sequencing (NGS) was published in 2011 by Andreotti et al. (2011). Since then, an increasing number of NGS studies have been used to characterize the tick microbiome, allowing for a broader view of its taxonomic composition in several tick species (Wu-Chuang et al., 2021a). In addition to pathogens and obligate endosymbionts, ticks carry commensal non-pathogenic microorganisms that complement tick nutrition and interact with tick-borne pathogens, affecting tick fitness and vector competence (Wu-Chuang et al., 2021a). Due to the complex nature of colonization of tick microbiota, the interaction between endosymbionts and pathogens is sometimes hard to understand, with these associations potentially affecting tick physiology and ecology (Bonnet et al., 2017). Nevertheless, it is known that changes in microbial communities can modulate vector competence by decreasing, for example, Borrellia burgdorferi colonization in Ixodes scapularis larvae (Narasimhan et al., 2014). Similar examples have been previously reviewed (de la Fuente et al., 2017; Wu-Chuang et al., 2021a). Not only the microbiota modulates pathogen colonization, but pathogen entry within the tick midgut milieu trigger changes in the microbial communities (Abraham et al., 2017). For example, Anaplasma phagocytophilum infection in ticks disturbs the gut microbiota, increasing the presence of Pseudomonas and reducing the abundance of Rickettsia and Enterococcus (Abraham et al., 2017), which was associated with a reduction of bacterial biofilms in tick midguts (Heisig et al., 2014; Abraham et al., 2017) and also a decreased representation of biofilm synthesis pathways in the tick microbiome (Estrada-Peña et al., 2020b). B. burgdorferi infection increased the expression of the tick protein PIXR, which alter the gut microbiome, metabolome and immune responses and facilitates B. burgdorferi infection and molting of larvae (Narasimhan et al., 2017). Accordingly, it has been proposed that the relation between pathogens and microbiota is bidirectional (Cabezas-Cruz et al., 2018).
Developing a comprehensive understanding of the role of microbiota in governing the physiology and ecology of ticks, and its interaction with tick-borne pathogens could prove highly beneficial for devising new strategies to control and prevent tick-borne diseases. This review paper describes and discusses the most common symbiotic and endosymbiotic tick-microbiota relationships, highlighting their relevance for tick physiology and ecology. We focus primarily on published studies providing species-level resolutions for symbiont characterization, most often through PCR and whole genome sequencing. We also included studies using metagenomics approaches and NGS, a technique that has revolutionized microbiome research (Kulski, 2016), allowing for high throughput and high-resolution assessment of the assortment of circulating microorganisms in tick vectors. Thanks to these advances, it is now relatively straight-forward to characterize tick endosymbiont assemblages to species levels, with the resulting growing body of published work in this area prompting this review.
The Symbiotic Continuum in Ticks
Non-pathogenic microbes found in ticks can classified as commensals and endosymbionts. Within commensals, a highly variable group of bacterial taxa, referred to as microbiota, have been described (Narasimhan and Fikrig, 2015). The microbiota composition is under the influence of several factors including the tick species, physiological stress by environmental traits, blood-meal, host species, tick immunity, and developmental stage (Narasimhan and Fikrig, 2015). Despite the variability of bacterial taxa associated to ticks, bacterial communities in the tick microbiome are functionally redundant (Estrada-Peña et al., 2020a). For example, the microbiome of I. scapularis larvae and nymphs shared 80 taxa (24.6%, total 324), while out of 342 predicted metabolic pathways, 82.7%, were shared by all the tick samples (Estrada-Peña et al., 2020a). Furthermore, the I. scapularis microbiota exposed to pathogen infection, antimicrobial peptide or anti-tick host immunity shared a very reduced taxonomic core of 61 bacterial genera (7.4%, total 821), while the majority (i.e., 381) of the metabolic pathways (87.2%, total 437) were identified in all the samples (Estrada-Peña et al., 2020b), Also, despite high temperature altered the structure of the microbial community in I. scapularis (Thapa et al., 2019; Wu-Chuang et al., 2021b) four keystone taxa found across the temperature gradient, and their directly connected neighbors, contributed to more than 99% of the predicted pathways regardless the incubation temperature (Wu-Chuang et al., 2021b). This suggests that the tick microbiome is a stable source of metabolic functions with potential implications for tick physiology. A mechanism can then be proposed by which ticks are permissive to colonization by environmental bacteria fulfilling a specialized set of functions.
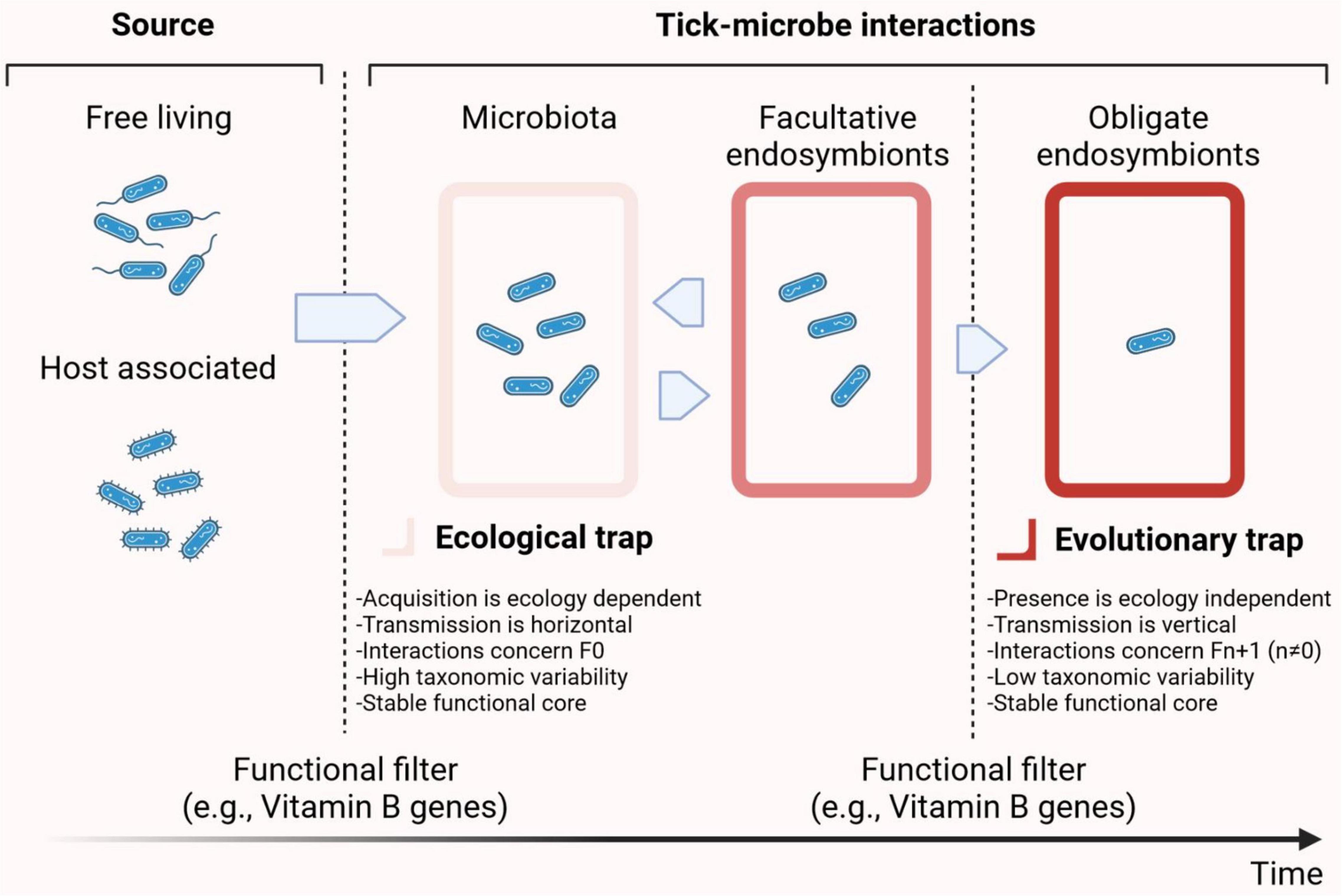
Intriguingly, metabolic pathways associated with nutritional complementation by symbionts are broadly distributed in the tick microbiome. For example, the presence of vitamin B synthesis genes in Francisella or Coxiella symbionts (see details below) compensate for the shortage of vitamin B in the blood meal (Duron et al., 2018). However, vitamin B synthesis genes are not restricted to symbiotic bacteria of the genera Francisella or Coxiella, but are widely distributed throughout several bacterial genera of tick microbiota (Obregón et al., 2019). In addition, considering the high diversity of genes and metabolic pathways encoded in the genomes of tick microbiota bacteria, we hypothesize that the contribution of bacteria to tick physiology and survival could extend well beyond vitamin B supplementation (Obregón et al., 2019; Estrada-Peña et al., 2020b). Indeed, the metabolic pathways associated with the tick microbiome include processes as diverse as amino acid, antibiotic, pyrimidine, lipid, and amino sugar metabolism (Obregón et al., 2019). Thus, an ecological-to-evolutionary continuum could be proposed in which environmental, free-living and/or host-associated, bacteria colonize tick tissues and later, under certain conditions, establish a symbiotic relationship with the tick host (Figure 1).
Impact of Symbionts on Tick Physiology
The recent emphasis on tick microbiome research has provided a new dimension of the microbes carried by ticks. Scientists are investigating symbiotic interactions and their impact on invertebrate host’s physiology (Guizzo et al., 2017). Ticks usually harbor two types of endosymbionts. The first group includes obligate mutualistic symbionts, transmitted from mother to offspring, which are essential for the development and growth of their host, supporting various functions such as nutrition (Wernegreen, 2012), and behavior (Zhong et al., 2021). The second group comprises facultative symbionts, which are not as important as their obligate counterparts in ensuring host survival (Ohtaka and Ishikawa, 1991). Nevertheless, facultative symbionts can also affect their host, where, for example, those found within reproductive organs can manipulate reproduction and physiology by inducing parthenogenesis and cytoplasmic incompatibility (Cordaux et al., 2011). Coxiella-like endosymbionts (CLEs) preferentially colonize the ovaries and Malpighian tubules of ticks (Cibichakravarthy et al., 2022). The colonization of CLEs in the ovaries of ticks promote reproduction and developmental processes and assists its maternal transmission to the offspring (Klyachko et al., 2007). The dense colonization of CLEs in Malpighian tubules assist in its nutritional role as Malpighian tubules are engaged in nitrogenous products excretion and osmoregulation (Dhooria, 2016; Cibichakravarthy et al., 2022). CLEs may recycle arthropods’ hemolymph metabolites to synthesize vitamin B (Cibichakravarthy et al., 2022). Further, vitamin B synthetic pathway enzymes have been detected significantly more abundant in Malpighian tubules as compared to ovaries (Cibichakravarthy et al., 2022) in Rh. sanguineus. FLEs are maternally inherited symbionts of ticks (Gerhart et al., 2016; Kumar et al., 2021). FLE was identified as an obligate nutritional mutualist in the life cycle of Ornithodoros moubata through experiments and synthesizes B vitamins (Duron et al., 2018) that are deficient in the ticks’ blood meal. The elimination of Francisella F-Om alters ticks’ life history traits (Duron et al., 2018). Comparison of the metabolic pathways present in FLE-Am to that of CLEAA showed that the metabolic capability of FLE-Am is extensive than CLEAA, for example, FLE-Am can produce heme in addition to cofactors (except thiamine) synthesized by CLEAA (Gerhart et al., 2016). Further, FLE provides cysteine, which found in fewer concentrations in blood meal and can synthesize glutamine from glutamic acid and ammonia, so recycling cellular ammonia waste to useful products. Therefore, FLE-Am has superior biosynthetic capability as compared to CLE (Gerhart et al., 2016). Wolbachia have been shown to influence the reproduction of infected insects in various ways, including parthenogenesis, male killing, cytoplasmic incompatibility, and feminization (Hurst et al., 1999; Stouthamer et al., 1999). Candidatus Midichloria mitochondrii is widespread in various ixodid ticks (Epis et al., 2008) was found abundant in unfed and semi-engorged Ixodes ricinus females that enhance the host fitness by supplying essential nutrients (Olivieri et al., 2019). In general, symbiotic partners enter into an evolutionary spiral that leads to an irreversible codependence with associated risks (Bennett and Moran, 2015), while the microbiota allows for more flexible tick-microbe interactions determined by and adapted to ecological conditions. Tick microbiota and its interactions with the tick and pathogens have been recently revised (Wu-Chuang et al., 2021a). In the sections below, we collate our current understanding about tick endosymbionts of the genera Coxiella, Rickettsia, Francisella, Midichloria, and Wolbachia, as summarized in Supplementary Table 1.
Coxiella
Coxiella-like endosymbionts is a obligate intracellular, maternally inherited bacterium, and found in high prevalence in tick populations (Duron, 2015). It usually engaged in mutualistic interactions with tick hosts (Brenner et al., 2021; Nardi et al., 2021). Several tick genera have been found to harbor CLE, including Amblyomma (Clay et al., 2008), Dermacentor (Jiao et al., 2021), Haemaphysalis (Lee et al., 2004), Rhipicephalus (Špitalská et al., 2018), Ixodes (Kurtti et al., 2002; Schabereiter-Gurtner et al., 2003; Špitalská et al., 2018), Ornithodoros (Duron, 2015), and Carios (Reeves et al., 2005). Douglas (2007) proposed that the benefits derived from the symbiotic relationship with CLE is limited in ticks, but it is considered that they play an important role in the synthesis of several nutrients that are required by their tick hosts (Moran et al., 2003; Douglas, 2007). As strict hematophagous parasites, ticks rely on the nutritional composition of host blood to support their metabolism (Heisig et al., 2014). Nutritional deficiencies in the blood, such as the lack of sufficient amounts of vitamin B, provide the basis for symbiotic partnerships with bacteria that synthesize vitamin B. This is a common challenge for arthropods feeding on blood, which frequently carry bacteria that synthesize vitamins and thus contribute to the fitness of their tick host (Beard et al., 2001; Akman et al., 2002; Wu et al., 2006). Further, Zhong et al. (2021) demonstrated that reduction in the abundance of the CLE in Haemaphysalis longicornis (CHI) decreases blood intake in ticks. It was found that reduced CLE abundance reduces serotonin biosynthesis that is essential in regulating tick-feeding activity. Researcher determined that providing tetracycline-treated ticks with the CHI-derived tryptophan precursor chorismate restores the feeding defect. Further, its increased level in the synganglion and midgut promotes tick feeding. Herbicide glyphosate treatment suppresses blood-feeding behavior in ticks by inhibition of CHI chorismate biosynthesis. Therefore, -CHI regulate tick feeding activity (Zhong et al., 2021).
The genome size of CLE is smaller than that of Coxiella burnetii, an obligate intracellular bacterium that causes Q fever in humans and animals (Stein and Raoult, 1992), and they are deficient in certain proteins including recN gene product that involved in DNA repair along with that Amblyomma bacterium may also lack the DNA repair function provided by recN, hence it impairs DNA replication (Jasinskas et al., 2007). This evidence indicated that this gene loss in this bacterium is the same as C. burnetii but also that it has a reduced genome, a common feature of obligate endosymbionts of invertebrates (Moran and Wernegreen, 2000; Jasinskas et al., 2007).
Coxiella-like endosymbionts have evolved as endosymbionts, being found at greater loads in the A. americanum tick salivary glands (Klyachko et al., 2007), where presence has been reported as necessary for ensuring the survival of this tick species. CLE also play a key role in the reproductive fitness of female A. americanum ticks, where clearance of this bacterium by antibiotic treatment reduces egg hatchability, and increases the time to oviposition (Zhong et al., 2007). CLEs have also been identified through field collection in Rh. turanicus, where these were higher in females than males, further supporting sex-specific benefits, driven by vertical transmission of these endosymbionts that occurs in Rh. turanicus after CLE proliferation and colonization in ovaries (Lalzar et al., 2014). Additional studies have shown that CLE are more abundant in ovaries and Malpighian tubules, but less abundant in other organs as salivary glands (Noda et al., 1997; Lalzar et al., 2014; Wang et al., 2017; Buysse et al., 2019). As Rh. turanicus females display higher blood consumption and increased metabolic rates compared with males, sex-specific benefits could be expected to extend beyond reproduction in female ticks of this species, with CLEs suppling essential nutrients in larger quantities in feeding females. Indeed, research has shown a direct positive correlation between the abundance of symbionts and female weight during feeding in this species (Lalzar et al., 2014). In contrast, symbiont levels appeared to be lower in males than in females, where weight increases were also lower during feeding.
In the Cayenne tick, Amblyomma cajennense, CLE was identified in salivary glands, ovaries and Malpighian tubules (Machado-Ferreira et al., 2011). Further, this bacterium found with high prevalence rate (100%) in all life stages and in eggs of ticks that confirm its transovarial and transstadial transmission (Machado-Ferreira et al., 2011). Similarly, CLEs proteomes detected from Malpighian tubule (75%) and the ovaries (80%) of the brown dog tick, Rh. sanguineus (Cibichakravarthy et al., 2022)possible roles in metabolism, fecundity and osmoregulation (Machado-Ferreira et al., 2011; Sonenshine, 2014; Cibichakravarthy et al., 2022). It is reported that CLEs use few substances from the hemolymph for the synthesis of B vitamins (Magnúsdóttir et al., 2015), potentially explaining why they are rarely found in other tick organs, such as midgut and salivary glands. Nevertheless, the importance of tissue tropism of CLEs has not been fully elucidated (Duron et al., 2017).
Coxiella-like endosymbionts have also been found in Haemaphysalis concinna collected in different geographical areas of Russia (Mediannikov et al., 2003), and in Rhipicephalus in Switzerland (albeit in only a small fraction of the ticks sampled; Bernasconi et al., 2002), Such findings indicate that this symbiont group plays a variable role across different tick species, being important in many of those researched to date. Further investigation of CLEs in ticks could therefore be recommended to explain their role and importance in tick population fitness (Supplementary Table 1).
Rickettsia
Rickettsia is obligate, intracellular gram-negative bacteria (Perlman et al., 2006). These microbes are widely distributed in accordance with their tick hosts across the globe, causing many diseases in humans and animals. Despite being regularly reported as tick symbionts with ticks, however, the symbiotic potential of some species remains poorly understood (Parola and Raoult, 2001; Goddard, 2009). In cases where our understanding is clearer, these symbionts have been shown to play a vital role in tick physiology, fitness and population dynamics and pathogen transmission (Childs and Paddock, 2002). Endosymbiotic Rickettsia might have nutritional importance in ticks, as many phylotypes of Rickettsia can produce folate (vitamin B), which are not present in the blood of vertebrates on which ticks usually fed (Kagemann and Clay, 2013).
Through various studies, it has been shown that Dermacentor variabilis harboring symbiotic Rickettsia sp. demonstrate higher motility than ticks without Rickettsia sp. (Kagemann and Clay, 2013). Rickettsia sp. symbionts are most common in ticks of the genera of Ixodes, Amblyomma, and Dermacentor, whereas it has been less frequently found to in Rhipicephalus, Haemaphysalis, and Hyalomma ticks (Burgdorfer et al., 1973; Nováková and Šmajs, 2018). In I. scapularis, Rickettsia endosymbionts has been reported in 100% of eggs, 81% in larva, 90.5% in nymph, and 98.9% in adult females tested (Rounds et al., 2012). Identification of endosymbionts in ticks was done by broad-range polymerase chain reaction and electrospray ionization mass spectrometry. The same study reports this bacterium to be present at high prevalence (100%) in I. pacificus (Rounds et al., 2012).
While Rickettsia symbionts may play roles in influencing the physiology of ticks, they often found as diverse species assemblages, interacting with one another. Dermacentor andersoni harbor symbionts such as Rickettsia montanensis (formerly R. montana) and Rickettsia peacockii (Burgdorfer et al., 1980; Macaluso et al., 2002), for example, and D. variabilis can be infected by Rickettsia rickettsia, Rickettsia bellii, and R. montanensis concurrently (Carmichael and Fuerst, 2006). It has been reported that R. montanensis is vertically transmitted in D. variabilis, but with this transmission inhibited by co-infection with a second Rickettsia sp. (Macaluso et al., 2002). Capillary feeding trials involving PCR diagnostics have also shown that ticks already harboring Rickettsia sp. are resistant to challenge with a secondary Rickettsia infection. In resisting co-infection, it has been proposed that Rickettsia present in tick ovaries change the molecular expression of oocytes, which prevents the occurrence of secondary residential infection (Carmichael and Fuerst, 2006). Soft ticks have also been reported to harbor Rickettsia. Particularly, two novel species named Candidatus Rickettsia Africa septentrionalis and Ca. Rickettsia mauretanica, were detected in Ornithodoros occidentalis from Morocco, Ornithodoros erraticus from Algeria and Ornithodoros normandi from Tunisia (Buysse and Duron, 2020).
Francisella
Francisella-like endosymbionts are gram-negative coccobacilli and facultative intracellular bacteria that are widespread in natural surroundings. In natural ecosystems, the survival of Francisella depends on temperature, direct sunlight exposure, and other physical factors (Kılıç, 2010; Celli and Zahrt, 2013). These bacteria are probably sustained in the environment through associations with various animals, including lagomorphs, rodents, insectivores, carnivores, ungulates, marsupials, birds, amphibians, and various species of invertebrates. Transmission to/between animals and humans may occur via bites of both ticks and mosquitoes (Momer, 1992; Eliasson et al., 2006). Ticks may serve as reservoirs, carrying the bacteria in their bodies throughout their lives, including in their salivary glands from where Francisella may be transmitted to new hosts at the site of tick feeding (Goethert and Telford, 2005; Gürcan, 2014; Yeni et al., 2021). Francisella have also been found in the reproductive tissues of female D. andersoni (Niebylski et al., 1997), with instances of unfed, infected larvae of A. americanum occurring in nature. Supporting the idea that transovarial transmission can occur (Calhoun and Alford, 1955). Transstadial transmission of Francisella tularensis has also been confirmed under laboratory conditions for multiple tick species, including D. andersoni, D. variabilis and A. americanum (Petersen et al., 2009).
FLEs are assumed to be non-pathogenic to humans, though they may cause limited pathogenicity in small animals (Keim et al., 2007) and are found in human-biting ticks, including those belonging to the genera Dermacentor, Amblyomma, Ixodes, and Hyalomma (Scoles, 2004; Machado-Ferreira et al., 2009; Ivanov et al., 2011; de Carvalho et al., 2016; Azagi et al., 2017). Presence of the genus Francisella has been reported in the camel tick, Hyalomma dromedarii, through 16s rRNA sequencing, with high relative abundance (99%) (Perveen et al., 2020b) and subsequent PCR confirming close relation to FLE (Perveen et al., 2021a). In the same geographic area, H. dromedarii ticks were reported throughout the year with high prevalence (94%) (Perveen et al., 2020a). It is possible that the high prevalence of camel ticks reported in this work may have been due to high abundance of these endosymbionts, though favorable microclimatic/environmental conditions and abundance of hosts for blood feeding (due to an increase in camel farming) may have also played a role. Previously, FLE was also detected with high prevalence in Hyalomma species (90.6%). In addition, maternal transmission rates of up to 91.8% were reported and FLE were localized in Malpighian tubules, ovaries, and salivary glands in H. marginatum (Azagi et al., 2017).
Francisella-like endosymbionts are closely related to pathogenic species of the genus Francisella (Noda et al., 1997; Scoles, 2004). Therefore, precise identification of this endosymbiont, its prevalence and interactions with other members of tick microbiota (co-existence) are crucial to understand and estimate its pathogenic potential, and investigate possible transmission to humans and animals. For example, the presence of closely related FLEs in tick species, including D. variabilis, D. andersoni, and D. occidentalis (Staples et al., 2006; Petersen et al., 2009) that can sustain and transmit pathogens causing tularemia poses a public health challenge.
Francisella-like endosymbiont is needed for tick growth and life cycle (Duron et al., 2018). In addition, FLEs have been found to be dominant symbionts in the Gulf Coast tick, Amblyomma maculatum microbiome (Binetruy et al., 2020) and reported in soft as well as hard ticks, especially in species belonging to the Ornithodoros genera (Gerhart et al., 2018). FLEs affect tick nutrition, and their capability to synthesize vitamin B make them especially suitable as mutualistic symbionts with Ornithodoros moubata (Duron et al., 2018). Further, elimination of FLE cause physical abnormalities and deficiencies in ticks that were restored by providing supplement of B vitamins (Duron et al., 2018). Gerhart et al. (2016) demonstrated the metabolic capability of FLE of A. americanum and found that FLE synthesizes cysteine, threonine, tyrosine, tryptophan, phenylalanine, and serine from pyruvate, and can break down glutamate, glutamine, and asparagine into ATP. This higher biosynthetic capability of FLE-Am could have led to replacing an ancestral symbiont, CLE in A. maculatum (Gerhart et al., 2016). Therefore, FLEs improve tick fitness by supplying vitamins and cofactors found in low concentrations in vertebrate blood (Gerhart et al., 2018).
Wolbachia
Wolbachia is an intracellular gram-negative bacterium that is the most commonly found microorganism infecting various arthropods. This bacterium is present in almost 60% of insect species and plays a role in multiple mechanisms, including altering host reproduction by inducing reproductive disorders, driving parthenogenesis, and acting as a defensive endosymbiont (Sazama et al., 2019). The presence of Wolbachia in ticks has been associated with parasitism by an Ixodiphagus parasitoid (Plantard et al., 2012; Luu et al., 2021). Ixodiphagus hookeri (Hymenoptera, Chalcidoidea, and Encyrtidae) that are endoparasitoids of I. ricinus, were found infected with Wolbachia pipientis (99.2% prevalence) (Plantard et al., 2012). Further, it was reported that natural populations of I. ricinus ticks harboring Wolbachia were parasitized by I. hookeri, and ticks that were not parasitized by I. hookeri were Wolbachia-free (Plantard et al., 2012). Therefore, the occurrence of W. pipientis in I. ricinus ticks is probably attributable to the presence of I. hookeri (Plantard et al., 2012). Excluding the transmission of Wolbachia from Ixodiphagus to ticks, evidence suggests that Wolbachia is rarely found in ticks and other maternally inherited endosymbionts as Spiroplasma, Midchloria, or Rickettsiella are more common (Tijsse-Klasen et al., 2011). In agreement with this, this bacterium has been reported with a prevalence rate of only 14% in I. ricinus (Subramanian et al., 2012). Likewise, in A. americanum it was found with a prevalence rate of 3.5–25% in females (Zhang et al., 2011).
In a study in Southern Maryland (United States), Wolbachia infection was present only at low frequency in nymphs of A. americanum, and observed primarily in females and only rarely in males. This significant difference in male and female ticks could be due to male-killing effects of infection or a sampling bias. The impact of Wolbachia species on the reproductive potential of A. americanum is still unknown and warrants further attention to investigate any relationship (Benson et al., 2004). Furthermore, a more extensive range of tick species should be examined for Wolbachia, evaluating sex-specific infection rates and the role of this bacterium in host reproduction processes. Indeed, this bacterium may have a potential role in the biological control of ticks, as it does for various disease vectors and other arthropod pests (Ahantarig and Kittayapong, 2011).
Candidatus Midichloria Mitochondrii
Candidatus Midichloria mitochondrii is an intracellular bacterium belonging to the order Rickettsiales (Stavru et al., 2020), mostly reported in I. ricinus and this bacterium has vertical transmission in this tick (Sassera et al., 2006; Mariconti et al., 2012a). This alphaproteobacterium colonize mitochondria and was found residing in the mitochondrial intermembrane space through electron microscopy (Sassera et al., 2006; Stavru et al., 2020). It has been noted as the most prevalent endosymbionts in I. ricinus, having 100% (Olivieri et al., 2019), and 44% (Sassera et al., 2006) prevalence rates in females and males, respectively. Candidatus Midichloria mitochondrii was found to be abundant in ovaries of female I. ricinus (Olivieri et al., 2019) and vertically transmitted symbiont reported with 100% prevalence in females and immatures. Further, Ca. Midichloria mitochondrii can be found in gut, rostrum, tracheae, Malpighian tubules, and salivary glands of I. ricinus females (Olivieri et al., 2019). Results suggest subpopulations of Ca. Midichloria mitochondrii with different specializations due to tissue tropism. Further, in silico metabolic reconstruction indicate that Ca. Midichloria mitochondrii could enhance the host fitness, help in the anti-oxidative defense, maintain of homeostasis, water balance, and stable its population through vertical and horizontal transmission in the tick host (Olivieri et al., 2019). In silico metabolic reconstruction from the Ca. Midichloria mitochondrii genome showed that several genes involved in interaction with I. ricinus and complete biosynthesis pathways for B vitamins, especially B9 (folate) and B7 (biotin) suggest its nutrient-provisioning role. However, more experiments are required to better understand the mechanisms underlying this symbiotic interaction.
Mariconti et al. (2012a) and Perlman et al. (2006) showed that 47 patients exposed to bites of Ixodes ricinus ticks were seropositive to antigens of Ca. Midichloria mitochondrii. This suggests that Ca. Midichloria mitochondrii antigens secreted together with tick saliva could trigger the human immune response, or that ticks could transmit this endosymbiont to humans. The second scenario raises the possibility that Ca. Midichloria mitochondrii can then be acquired by a second tick feeding on the same host. Making this an example of an endosymbiont completing a tick-to-host-to-tick transmission route similar to that reported of tick-borne pathogens. Whether, in addition to the vertical transmission, such host-mediated transmission route plays any role on Ca. Midichloria mitochondrii life cycle remains unknown. Coincidentally, patients found seropositive with Ca. Midichloria mitochondrii were among 80 tick-exposed patients hospitalized due to clinical signs of Lyme disease, and 31 of them were also found seropositive for B. burgdorferi sensu lato (Mariconti et al., 2012a). Deducing a direct relation between B. burgdorferi and Ca. Midichloria mitochondrii colonization in I. ricinus from co-occurrence data in ticks is not justified, as this endosymbiont is present in nearly 100% of tick females, while Borrelia prevalence in ticks is highly variable. However, whether patient exposure to Ca. Midichloria mitochondrii favors, or not, Borrelia infection and Lyme disease is an interesting, but unanswered, question. A previous report reported that the growth of a pathogenic R. parkeri in A. maculatum along with endosymbionts (Budachetri et al., 2018). Further, Budachetri et al. (2018) found that R. parkeri interaction with tick symbionts, FLE and Ca. Midichloria mitochondrii (CMM) modulate host’s defenses by up-regulating tick selenoproteins.
Mutualistic “Pathogens”
TBPs considered as having a “mutualist” relation with the tick, but not symbiotic. Some TBPs have vertical and horizontal transmission (e.g., Babesia) (Young et al., 2019) and others only horizontal (A. phagocytophilum and B. burgdorferi) (Jaarsma et al., 2019; Kurokawa et al., 2020). During blood feeding, various microbes are acquired that can trigger an immunological response in ticks. The immune system of ticks includes various enzymes and proteins, with endosymbionts also known to interact with tick immune function. For instance, B. burgdorferi can be phagocytosed by tick hemocytes (Zchori-Fein and Bourtzis, 2011; Hussain et al., 2021a). For instance, l. scapularis transmits the Lyme disease spirochete, B. burgdorferi whereas the D. variabilis is unable to transmit the bacterium to vertebrate host (Johns et al., 2001). It’s due to immune function of both tick species. In I. scapularis, some Borrelia spirochete were found connected with hemocytes, while several spiral-shaped intact bacteria were free in the hemolymph, however, in D. variabilis intact spirochetes were very less. Further, I. scapularis tissues contained culturable bacteria unlike D. variabilis tissues (Johns et al., 2001). In vitro, it was demonstrated that spirochetes motility and survival not reduced when they were incubated with I. scapularis hemolymph plasma, however, more than 50% spirochetes became non-motile after incubation of spirochetes with D. variabilis hemolymph plasma. Furthermore, I. scapularis showed immunotolerance against B. burgdorferi and slow phagocytic response whereas, D. variabilis showed high immunocompetence and rapid phagocytic activity to clear pathogens (Johns et al., 2001). A. phagocytophilum, an intracellular bacteria transmitted by I. scapularis. Its transmission is restricted by a member of the 5.3 kDa family of antimicrobial peptides expressed in the salivary glands of tick (Liu et al., 2012). Studies have been conducted on A. phagocytophilum how it manipulate gene expression and activate signaling pathways (Cabezas-Cruz et al., 2017). For instance, infection of A. phagocytophilum stimulates the expression of antimicrobial peptides that is mediated by the activation of the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway (Liu et al., 2012) to control bacterial load in tick salivary glands. Cabezas-Cruz et al. (2017) discussed about how tick-pathogen interactions increase the fitness in tick hosts. Pathogens manipulate tick protective responses to facilitate infection, however, preserve tick feeding and vector capacity to assure the survival of the pathogens as well as ticks (Cabezas-Cruz et al., 2017). A. phagocytophilum infections in I. scapularis showed higher tick fitness due to the induced expression of a tick antifreeze glycoprotein that increases their survival in the cold conditions (Neelakanta et al., 2010).
Symbiont-Pathogen Interactions
Ecological relationships among organisms are broad and diverse. The interactions that underpin them can range from beneficial to harmful along a continuum (Pérez-Brocal et al., 2011). Accurately defining symbiotic, mutualistic, or pathogenic interactions is challenging, especially where they may be influenced by various external factors such as climate change, land-use change, and invasion of parasites and hosts into new geographic ranges. Modern methodologies are helping researchers to better map this complex landscape of interactions, where molecular-based diagnostics have proven especially useful in confirming associations between microbes and various protozoa, other bacteria, fungi, and/or animals (Pérez-Brocal et al., 2011).
Multiple pathogens (Baneth, 2014) and symbiotic bacteria (Noda et al., 1997) may co-exist in tick midgut. But, many endosymbiotic microorganisms have established themselves in the ovaries of female ticks (Noda et al., 1997), and are often transmitted to eggs (and subsequently nymphs) from the mother. Examples can be found from Coxiella-, Francisella-, and Rickettsia-like endosymbionts, which all affect/improve the host fitness (Ahantarig et al., 2013), and defense against environmental stress (Bonnet et al., 2017). In some cases, these endosymbionts may infect humans (Ahantarig et al., 2013), with this potentially then affecting the tick-borne microbes occurrence and transmission (Bonnet et al., 2017; Bonnet and Pollet, 2021). Pre-existence of another symbiont can also influence the performance of a different species. For instance, the vertical transmission of a second Rickettsia species in the D. variabilis is inhibited by pre-existing Rickettsial infection in these ticks (De Sousa et al., 2001). For example, R. peacockii is found in Rocky Mountain wood ticks, D. andersoni and displays a close phylogenetic relationship to Rickettsia rickettsii, a bacteria of the spotted fever group (SFG), but many of the characteristics of rickettsial pathogens are missing (Burgdorfer et al., 1980). The major difference between pathogenic and non-pathogenic SFG Rickettsia is the ability to proliferate within macrophages distinguishable factor in pathogenic Rickettsia. In addition, pathogenic Rickettsia may be able to increase the endoplasmic reticulum protein folding capacity, while non-pathogenic Rickettsia do not show such capacity (Curto et al., 2019). Similarly, -FLEs- have been detected in a range of tick genera, such as Amblyomma, Hyalomma, Ixodes, and Ornithodoros (Sun et al., 2000; Scoles, 2004; Machado-Ferreira et al., 2009; Ivanov et al., 2011; de Carvalho et al., 2016; Azagi et al., 2017). The occurrence of closely related Francisella sp. in Dermacentor. species that can transmit tularemia (Elston and Apperson, 1977) suggests an important role for precise screening of F. tularensis in laboratories by PCR (Kugeler et al., 2005). Indeed, full assessment of the pathogenic potential of FLEs is crucial and has recently been undertaken for Rickettsia species (Felsheim et al., 2009). This work demonstrated that symbionts previously considered to be non-pathogenic (Raoult and Roux, 1997) may in fact be the opposite, as was true for Rickettsia slovaca and Rickettsia helvetica (Raoult and Roux, 1997). This supports that the presence, infection rate, and ecological/biological roles of bacterial endosymbionts need to be examined closely to reveal their contribution to tick-borne diseases epidemiology.
Impact of Symbionts on Disease Ecology
Vector-borne zoonotic diseases are a global health threat and involve humans, pathogens, vectors, and wildlife (Harrus and Baneth, 2005; Collinge and Ray, 2006). Novel disease emergence can be especially significant and may be facilitated through the anthropogenic spread of disease vectors and pathogens across the globe. Virulent lineages of pathogens can also be distributed globally through anthropogenic activities, such as the international trade of animals (Cunningham et al., 2017), deforestation, agricultural development, and the magnitude of human interaction in the disease ecosystem, with climate change and socio-economic and environmental factors also influencing the dynamics of pathogen populations (Paul et al., 2016). The regional pattern of risk of pathogen transmission and circulation in an area is very important to human health and influenced by several factors. Spatial distribution of tick-borne pathogens, microclimatic conditions, and host abundance may affect pathogen dynamics, as well as the survival of ticks as potential vectors. Host species presence and abundance may impact pathogen spread (Estrada-Peña and de la Fuente, 2014), as may host and tick vector competence, as influenced by vector density and longevity (de la Fuente et al., 2017). Logically, any habitat considered fit for the tick-borne pathogen’s circulation and spread must meet the prerequisite of housing ticks and their hosts for any risk to be realized (Pfäffle et al., 2013). High-throughput sequencing approaches have also emphasized the potential implications of the composition, diversity, and functional role of tick microbial fauna to public health (de la Fuente et al., 2017).
Symbionts may impact disease ecology in two ways. Firstly, through their impact on tick physiology they can contribute to increase fitness and vector abundance which indirectly increases pathogen circulation and disease risk. Secondly, symbionts compete or favor pathogen colonization and can directly impact disease ecology by decreasing or increasing the chance of host being bitten by tick able to transmit pathogens.
Symbionts may benefit ticks through impacts on their survival, growth, and defense systems (Ahantarig et al., 2013; Bonnet and Pollet, 2021), all of which can have knock-on effects to their significance as disease vectors for humans. The assessment of symbiont associations is very important because these impact tick reproduction and fitness (Ahantarig et al., 2013). For example, a decrease in progeny number and increase in oviposition time has been reported in A. americanum following the elimination of CLEs through antibiotics (Zhong et al., 2007). Other key processes in ticks, as supported through their symbionts, could potentially be targeted in a similar fashion. Candidatus Midichloria mitochondrii in I. ricinus affect tick molting process (Zchori-Fein and Bourtzis, 2011; de la Fuente et al., 2017). Further, recent sequencing and analysis of this bacterium genome suggest that the bacteria may serve as a source of ATP for the host cell during oogenesis (Epis et al., 2008; Gnainsky et al., 2021). Rickettsia-infected Dermacentor ticks showed more motility than uninfected ticks (Kagemann and Clay, 2013). Higher motility associated to host-seeking behavior in ticks indirectly influences infection risk by increasing the rates of tick bites and pathogen transmission. Therefore, for a given tick species, various symbionts and their population rate that varies across geographic locations (Lalzar et al., 2012; Duron et al., 2017) affect vector fitness, their abundance and impact disease risks.
Symbionts may interfere with pathogens replication and transmission by affecting pathogen diversity and abundance in various tick species and their transmission to humans and animals (Bonnet et al., 2017). Because symbionts compete with pathogens for nutrients or tissues and may excrete molecules directly inhibiting the growth of pathogens or facilitate pathogens development by immunosuppressing the invertebrate hosts (Bonnet et al., 2017). For example, Wolbachia may affect with the replication and transmission of pathogens such as bacteria, viruses, protozoa, etc. and protect arthropods from parasite-induced mortality, may be through up-regulating the immune system (Brownlie and Johnson, 2009). Further, Wolbachia can cause pathogen interference by reducing the chance of pathogen infection and decrease pathogen load, and cytoplasmic incompatibility by reducing hatchability of eggs (when infected males mate with uninfected females) in mosquitoes (Caragata et al., 2016). Several pathogens may affect vector competence (Hajdusek et al., 2013). Tick innate immunity involves several cellular and humoral response pathways that mediate defense to various infections caused by microorganisms, such as Borrellia, Flavivirus, and Babesia (Hajdusek et al., 2013; de la Fuente et al., 2017). Tick symbionts may influence tick immunity that in turn may influence pathogen infection. FLE in D. andersoni was found positively associated with pathogenic Francisella novicida infection (Gall et al., 2016). The presence of FLEs has positively influenced the establishment of F. novicida in D. andersoni that may suppress the tick immune system favoring the acquisition of F. novicida (Gall et al., 2016). Therefore, these microbial associations allow symbionts to facilitate or limit pathogen transmission and directly influence vector-borne infections.
Using Obligate Hematophagy and Endosymbionts as Weak Spots for the Control of Ticks and Tick-Borne Pathogens
As obligate hematophagous parasites, ticks ingest large amounts of blood from the vertebrate host during feeding. The tick midgut is the first organ in contact with host immune components present in the blood. After crossing the gut barrier (Ackerman et al., 1981; Ben-Yakir et al., 1987; Wang and Nuttall, 1994), host antibodies (Willadsen, 1997) and complement proteins (Rathinavelu et al., 2003) can reach the tick hemolymph (Ackerman et al., 1981; Ben-Yakir et al., 1987; Wang and Nuttall, 1994) and access the tick ovaries and eggs (Galay et al., 2018) as well as salivary glands and be secreted back to the host (Wang and Nuttall, 1994). For example, in D. variabilis and I. scapularis ticks, the crossing of host IgG from the midgut into the hemocoel occur during the later phases of engorgement (Vaughan et al., 2002). Notably, the immune functions of antibodies and complement are retained in the tick tissues (Ackerman et al., 1981; Ben-Yakir et al., 1987; Wang and Nuttall, 1994). Host antibodies interact not only with tissues and surface proteins (Willadsen, 1997), but can also be specifically transported inside the tick cells where they can interact with intracellular proteins (de la Fuente et al., 2011; Rodríguez-Mallon et al., 2012, 2015). Functional host antibodies have also been shown to interact with symbionts in Rodnius prolixus (Ben-Yakir, 1987), and Glossina morsitans (Nogge, 1978), as well as with bacterial microbiota in mosquitoes (Noden et al., 2011) and ticks (Mateos-Hernández et al., 2020, 2021).
Targeting vector microbiota with host antibodies is the rationale behind anti-microbiota vaccines for the control of vector arthropods such as ticks (Mateos-Hernández et al., 2020, 2021). Immunization with a tick microbiota Enterobacteriaceae, caused significant mortality of engorging ticks (Mateos-Hernández et al., 2020). Antibodies against the glycan α-Gal, present in tick bacterial microbiota, were associated with a mean mortality of approximately 45% in ticks fed on α1,3GT-deficient mice (Mateos-Hernández et al., 2020). Anti-microbiota vaccine directed at Enterobacteriaceae in the microbiota of I. scapularis disrupted both the makeup and functions of the microbiome and decreased pathways central to lysine degradation (Mateos-Hernandez et al., 2021). Anti-microbiota vaccines are a microbiome manipulation tool for the induction of infection-refractory states in the vector microbiome (Maitre et al., 2022).
The preliminary results with anti-microbiota vaccines justify their use to target tick endosymbionts for vector and/or tick-borne pathogen control. Rhodnius prolixus fed exclusively on blood from rabbits immunized against the symbiont Rhodoccocus rhodnii have developmental alterations such as prolonged molting times, incomplete development, and malformed limbs (Ben-Yakir, 1987). Feeding of Rhodnius prolixus larvae on hosts immunized against their symbiont produces retardation of the symbiont growth (Ben-Yakir, 1987). Developmental alterations observed in R. prolixus fed on Rhodococcus rhodnii-immunized animals were similar to those described in aposymbiotic triatomines (sterile raised and germ-free insects that lack R. rhodnii) (Salcedo-Porras et al., 2020). Similar results were obtained by Nogge (1978) who found that tsetse flies fed on rabbits immunized with symbionts became aposymbiotic and their fecundity decreased drastically while their longevity was not affected. Furthermore, Glossina morsitans morsitans flies maintained on rabbits immunized with gut bacteria had high mortality rates and permanently laterally extended wings, which in turn impairs flying and therefore trypanosomes transmission (Kaaya and Alemu, 1984). In addition to targeting tick endosymbionts with live-bacteria vaccines (Mateos-Hernández et al., 2020, 2021), selected enzymes encoded in the symbiont genome and transcriptionally active could also be targeted with host antibodies.
Conclusion and Perspectives
In this review, we have discussed five main tick-borne endosymbiont groups, comprising Coxiella-, Rickettsia-, Francisella-, Wolbachia, and Midichloria -like species, reporting key symbiont-pathogen interactions and exploring potential impacts of symbionts and their associations on tick physiology and tick-borne disease ecology. Examples are provided of various bacteria that can benefit their host’s health, with further examples of opportunistic or pathogenic bacteria that may stress the host in some way while exploiting it. It is clear from many of these examples that tick-symbiont relationships are complex and context-specific, and better defining the pathways that dictate how pathogenic microbes contribute to disease ecology will allow improved treatments that target the virulence responses associated with this microbiota.
Endosymbionts play a significant role in providing nutrients (Greay et al., 2018) that support host persistence, for example, rickettsial symbionts of D. andersoni and Amblyomma americanum, and the CLE of A. Americanum, and are essential for the fitness of their tick hosts (Greay et al., 2018). Experimentation using antibiotics have shown that cleansing ticks from its bacterial endosymbionts have major negative impact of vector fitness and survival as well as vector competence. For example, D. andersoni exposure to oxytetracycline caused a significant decline in tick feeding, molting competence, and survival (Wade et al., 2014). Similarly, injecting laboratory-reared A. americanum ticks with rifampin resulted in reduced endosymbionts (Coxiella sp. and Rickettsia sp.) and a reduction in weight of adult ticks post-molting (Zhong et al., 2007). However, antibiotic overuse is associated to several undesirable effects such as the emergence of microbial antibiotic resistance, agro-ecosystem contamination, and alteration on animal and human microbiota with potential negative effect on health. In addition, the effect of antibiotics on the microbiota is not specific, as several bacterial species can be depleted by antimicrobial treatment. All this, had led to the implementation of strong regulations to limit antibiotic use, particularly in livestock. Therefore, preventing the use of antibiotics to target tick endosymbionts. In this context, anti-microbiota vaccines emerge as an environmentally friendly alternative to target endosymbionts for the control of ticks and tick-borne pathogens.
The latest high throughput molecular diagnostic approaches have enabled researchers to explore microbiomes of different arthropods species rapidly and cost-effectively. However, the microbial composition of many tick species still needs to be explored, and additional studies are required to understand the functional role of these microorganisms in disease ecology and epidemiology (Greay et al., 2018). Tick-borne infections are a severe threat to public health and food security globally, and researchers, scientists, public health professionals and veterinarians must work both independently and collaboratively to reduce the risk they pose. Vector-borne infections thrive where infectious pathogens, competent vectors, and an infection-compatible microbiome exist, with disruption of any one of these drivers likely to reduce overall pathogen transmission by vectors (Maitre et al., 2022). Manipulation and targeting of tick endosymbionts have been under-explored to this end to date but may provide significant opportunities in the future.
Author Contributions
SH conceived the study and searched the literature. SH and OS planned and designed the review manuscript. SH, NP, AC-C, and OS screened and organized the data. SH, NP, AH, AC-C, and OS drafted the manuscript. AC-C provided intellectual inputs and share ideas. SH, NP, AH, AC-C, OS, MA, BS, and JZ revised the manuscript. OS, AC-C, DG, and JL critically revised the manuscript. All authors read and approved the final manuscript.
Funding
OS is a Principal Investigator of an Internal Research Fund of the Department of Infectious Diseases and Public Health of the City University of Hong Kong (Project number 9380108).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are thankful to all authors whose articles are included in this study. We also appreciate the Run Run Shaw library facility provided by the City University of Hong Kong.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.854803/full#supplementary-material
References
Abraham, N. M., Liu, L., Jutras, B. L., Yadav, A. K., Narasimhan, S., Gopalakrishnan, V., et al. (2017). Pathogen-mediated manipulation of arthropod microbiota to promote infection. Proc. Natl. Acad. Sci. U. S. A. 114, E781–E790. doi: 10.1073/pnas.1613422114
Ackerman, S., Clare, F. B., McGill, T., and Sonenshine, D. E. (1981). Passage of host serum components, including antibody, across the digestive tract of Dermacentor variabilis (Say). J. Parasitol. 67, 737–740. doi: 10.2307/3280459
Adegoke, A., Kumar, D., Bobo, C., Rashid, M. I., Durrani, A. Z., Sajid, M. S., et al. (2020). Tick-borne pathogens shape the native microbiome within tick vectors. Microorganisms 8:1299. doi: 10.3390/microorganisms8091299
Ahantarig, A., and Kittayapong, P. (2011). Endosymbiotic Wolbachia bacteria as biological control tools of disease vectors and pests. J. Appl. Entomol. 135, 479–486.
Ahantarig, A., Trinachartvanit, W., Baimai, V., and Grubhoffer, L. (2013). Hard ticks and their bacterial endosymbionts (or would be pathogens). Folia Microbiol. 58, 419–428. doi: 10.1007/s12223-013-0222-1
Akman, L., Yamashita, A., Watanabe, H., Oshima, K., Shiba, T., Hattori, M., et al. (2002). Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat. Genet. 32, 402–407. doi: 10.1038/ng986
Al-Deeb, M. A., Frangoulidis, D., Walter, M. C., Kömpf, D., Fischer, S. F., Petney, T., et al. (2016). Coxiella-like endosymbiont in argasid ticks (Ornithodoros muesebecki) from a Socotra Cormorant colony in Umm Al Quwain, United Arab Emirates. Ticks Tick Borne Dis. 7, 166–171. doi: 10.1016/j.ttbdis.2015.10.012
Al-Khafaji, A. M., Armstrong, S. D., Boccazzi, I. V., Gaiarsa, S., Sinha, A., Li, Z., et al. (2020). Rickettsia buchneri, symbiont of the deer tick Ixodes scapularis, can colonise the salivary glands of its host. Ticks Tick Borne Dis. 11:101299. doi: 10.1016/j.ttbdis.2019.101299
Al-Khafaji, A. M., Clegg, S. R., Pinder, A. C., Luu, L., Hansford, K. M., Seelig, F., et al. (2019). Multi-locus sequence typing of Ixodes ricinus and its symbiont Candidatus Midichloria mitochondrii across Europe reveals evidence of local co-cladogenesis in Scotland. Ticks Tick Borne Dis. 10, 52–62. doi: 10.1016/j.ttbdis.2018.08.016
Anderson, J. F., and Magnarelli, L. A. (2008). Biology of ticks. Infect. Dis. Clin. North Am. 22, 195–215.
Andreotti, R., de León, A. A. P., Dowd, S. E., Guerrero, F. D., Bendele, K. G., and Scoles, G. A. (2011). Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol. 11:6. doi: 10.1186/1471-2180-11-6
Arthan, W., Sumrandee, C., Hirunkanokpun, S., Kitthawee, S., Baimai, V., Trinachartvanit, W., et al. (2015). Detection of Coxiella-like endosymbiont in Haemaphysalis tick in Thailand. Ticks Tick Borne Dis. 6, 63–68. doi: 10.1016/j.ttbdis.2014.09.005
Azagi, T., Klement, E., Perlman, G., Lustig, Y., Mumcuoglu, K. Y., Apanaskevich, D. A., et al. (2017). Francisella-like endosymbionts and Rickettsia species in local and imported Hyalomma ticks. Appl. Environ. Microbiol. 83, e01302–17. doi: 10.1128/AEM.01302-17
Baneth, G. (2014). Tick-borne infections of animals and humans: a common ground. Int. J. Parasitol. 44, 591–596. doi: 10.1016/j.ijpara.2014.03.011
Bazzocchi, C., Mariconti, M., Sassera, D., Rinaldi, L., Martin, E., Cringoli, G., et al. (2013). Molecular and serological evidence for the circulation of the tick symbiont Midichloria (Rickettsiales: Midichloriaceae) in different mammalian species. Parasites Vectors 6:350.
Beard, C., Dotson, E., Pennington, P., Eichler, S., Cordon-Rosales, C., and Durvasula, R. (2001). Bacterial symbiosis and paratransgenic control of vector-borne Chagas disease. Int. J. Parasitol. 31, 621–627. doi: 10.1016/s0020-7519(01)00165-5
Beninati, T., Riegler, M., Vilcins, I.-M. E., Sacchi, L., McFadyen, R., Krockenberger, M., et al. (2009). Absence of the symbiont Candidatus Midichloria mitochondrii in the mitochondria of the tick Ixodes holocyclus. FEMS Microbiol. Lett. 299, 241–247. doi: 10.1111/j.1574-6968.2009.01757.x
Bennett, G. M., and Moran, N. A. (2015). Heritable symbiosis: the advantages and perils of an evolutionary rabbit hole. Proc. Natl. Acad. Sci. U. S. A. 112, 10169–10176. doi: 10.1073/pnas.1421388112
Benson, M. J., Gawronski, J. D., Eveleigh, D. E., and Benson, D. R. (2004). Intracellular symbionts and other bacteria associated with deer ticks (Ixodes scapularis) from Nantucket and Wellfleet, Cape Cod, Massachusetts. Appl. Environ. Microbiol. 70, 616–620. doi: 10.1128/AEM.70.1.616-620.2004
Ben-Yakir, D. (1987). Growth retardation of Rhodnius prolixus symbionts by immunizing host against Nocardia (Rhodococcus) rhodnii. J. Insect Physiol. 33, 379–383. doi: 10.1016/0022-1910(87)90015-1
Ben-Yakir, D., Fox, C., Homer, J., and Barker, R. (1987). Quantification of host immunoglobulin in the hemolymph of ticks. J. Parasitol. 73, 669–671. doi: 10.2307/3282157
Ben-Yosef, M., Rot, A., Mahagna, M., Kapri, E., Behar, A., and Gottlieb, Y. (2020). Coxiella-like endosymbiont of Rhipicephalus sanguineus is required for physiological processes during ontogeny. Front. Microbiol. 11:493. doi: 10.3389/fmicb.2020.00493
Bernasconi, M. V., Casati, S., Péter, O., and Piffaretti, J.-C. (2002). Rhipicephalus ticks infected with Rickettsia and Coxiella in southern Switzerland (Canton Ticino). Infect. Genet. Evol. 2, 111–120. doi: 10.1016/s1567-1348(02)00092-8
Binetruy, F., Buysse, M., Lejarre, Q., Barosi, R., Villa, M., Rahola, N., et al. (2020). Microbial community structure reveals instability of nutritional symbiosis during the evolutionary radiation of Amblyomma ticks. Mol. Ecol. 29, 1016–1029. doi: 10.1111/mec.15373
Black, W. C., and Piesman, J. (1994). Phylogeny of hard-and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. U. S. A. 91, 10034–10038. doi: 10.1073/pnas.91.21.10034
Bobo, C. G. (2020). Molecular Characterization of Wolbachia and Its Impact on the Microbiome of Exotic and United States Ticks. Ph.D. thesis. Hattiesburg: The University of Southern Mississippi.
Bonnet, S. I., and Pollet, T. (2021). Update on the intricate tango between tick microbiomes and tick-borne pathogens. Parasite Immunol. 43:e12813. doi: 10.1111/pim.12813
Bonnet, S. I., Binetruy, F., Hernández-Jarguín, A. M., and Duron, O. (2017). The tick microbiome: why non-pathogenic microorganisms matter in tick biology and pathogen transmission. Front. Cell. Infect. Microbiol. 7:236. doi: 10.3389/fcimb.2017.00236
Brenner, A. E., Muñoz-Leal, S., Sachan, M., Labruna, M. B., and Raghavan, R. (2021). Coxiella burnetii and related tick endosymbionts evolved from pathogenic ancestors. Genome Biol. Evol. 13:evab108. doi: 10.1093/gbe/evab108
Brouqui, P., Dupont, H. T., Drancourt, M., Berland, Y., Etienne, J., Leport, C., et al. (1993). Chronic Q fever: ninety-two cases from France, including 27 cases without endocarditis. Arch. Intern. Med. 153, 642–648. doi: 10.1001/archinte.153.5.642
Brownlie, J. C., and Johnson, K. N. (2009). Symbiont-mediated protection in insect hosts. Trends Microbiol. 17, 348–354. doi: 10.1016/j.tim.2009.05.005
Budachetri, K., Kumar, D., Crispell, G., Beck, C., Dasch, G., and Karim, S. (2018). The tick endosymbiont Candidatus Midichloria mitochondrii and selenoproteins are essential for the growth of Rickettsia parkeri in the Gulf Coast tick vector. Microbiome 6:141. doi: 10.1186/s40168-018-0524-2
Burgdorfer, W., Brinton, L., and Hughes, L. E. (1973). Isolation and characterization of symbiotes from the Rocky Mountain wood tick, Dermacentor andersoni. J. Invertebr. Pathol. 22, 424–434. doi: 10.1016/0022-2011(73)90173-0
Burgdorfer, W., Hayes, S., and Mavros, A. (1980). “Nonpathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distribution of Rickettsia rickettsii,” in Rickettsiae and Rickettsial Diseases, eds W. Burgdorfer and R. L. Anacker (New York: Academic Press), 585–594.
Buysse, M., and Duron, O. (2020). Two novel Rickettsia species of soft ticks in North Africa:‘Candidatus Rickettsia africaseptentrionalis’ and ‘Candidatus Rickettsia mauretanica’. Ticks Tick Borne Dis. 11:101376. doi: 10.1016/j.ttbdis.2020.101376
Buysse, M., Plantard, O., McCoy, K. D., Duron, O., and Menard, C. (2019). Tissue localization of Coxiella-like endosymbionts in three European tick species through fluorescence in situ hybridization. Ticks Tick Borne Dis. 10, 798–804. doi: 10.1016/j.ttbdis.2019.03.014
Cabezas-Cruz, A., Estrada-Peña, A., Rego, R. O., and De la Fuente, J. (2017). Tick-pathogen ensembles: do molecular interactions lead ecological innovation? Front. Cell. Infect. Microbiol. 7:74. doi: 10.3389/fcimb.2017.00074
Cabezas-Cruz, A., Vayssier-Taussat, M., and Greub, G. (2018). Tick-borne pathogen detection: what’s new? Microbes Infect. 20, 441–444. doi: 10.1016/j.micinf.2017.12.015
Calhoun, E. L., and Alford, H. I. Jr. (1955). Incidence of tularemia and Rocky Mountain spotted fever among common ticks of Arkansas. Am. J. Trop. Med. Hyg. 4, 310–317. doi: 10.4269/ajtmh.1955.4.310
Caragata, E. P., Dutra, H. L., and Moreira, L. A. (2016). Exploiting intimate relationships: controlling mosquito-transmitted disease with Wolbachia. Trends Parasitol. 32, 207–218. doi: 10.1016/j.pt.2015.10.011
Carmichael, J. R., and Fuerst, P. A. (2006). A rickettsial mixed infection in a Dermacentor variabilis tick from Ohio. Ann. N. Y. Acad. Sci. 1078, 334–337. doi: 10.1196/annals.1374.064
Celli, J., and Zahrt, T. C. (2013). Mechanisms of Francisella tularensis intracellular pathogenesis. Cold Spring Harb. Perspect. Med. 3:a010314. doi: 10.1101/cshperspect.a010314
Chao, L.-L., Castillo, C. T., and Shih, C.-M. (2021). Molecular detection and genetic identification of Wolbachia endosymbiont in Rhipicephalus sanguineus (Acari: Ixodidae) ticks of Taiwan. Exp. Appl. Acarol. 83, 115–130. doi: 10.1007/s10493-020-00574-3
Childs, J. E., and Paddock, C. D. (2002). Passive surveillance as an instrument to identify risk factors for fatal Rocky Mountain spotted fever: is there more to learn? Am. J. Trop. Med. Hyg. 66, 450–457. doi: 10.4269/ajtmh.2002.66.450
Chisu, V., Loi, F., Foxi, C., Chessa, G., Masu, G., Rolesu, S., et al. (2020). Coexistence of tick-borne pathogens in ticks collected from their hosts in Sardinia: an update. Acta Parasitol. 65, 999–1004. doi: 10.1007/s11686-020-00240-z
Chisu, V., Mura, L., Foxi, C., and Masala, G. (2021). Coxiellaceae in ticks from human, domestic and wild hosts from Sardinia, Italy: high diversity of coxiella-like endosymbionts. Acta Parasitol. 66, 654–663. doi: 10.1007/s11686-020-00324-w
Cibichakravarthy, B., Oses-Prieto, J. A., Ben-Yosef, M., Burlingame, A. L., Karr, T. L., and Gottlieb, Y. (2022). Comparative Proteomics of Coxiella like Endosymbionts (CLEs) in the symbiotic organs of Rhipicephalus sanguineus Ticks. Microbiol. Spectr. 10:e0167321. doi: 10.1128/spectrum.01673-21
Clay, K., Klyachko, O., Grindle, N., Civitello, D., Oleske, D., and Fuqua, C. (2008). Microbial communities and interactions in the lone star tick, Amblyomma americanum. Mol. Ecol. 17, 4371–4381. doi: 10.1111/j.1365-294x.2008.03914.x
Collinge, S. K., and Ray, C. (2006). Disease Ecology: Community Structure and Pathogen Dynamics. Oxford: Oxford University Press.
Cordaux, R., Bouchon, D., and Grève, P. (2011). The impact of endosymbionts on the evolution of host sex-determination mechanisms. Trends Genet. 27, 332–341. doi: 10.1016/j.tig.2011.05.002
Cowdry, E. (1925). A group of microorganisms transmitted hereditarily in ticks and apparently unassociated with disease. J. Exp. Med. 41, 817–830. doi: 10.1084/jem.41.6.817
Cunningham, A. A., Daszak, P., and Wood, J. L. (2017). One Health, emerging infectious diseases and wildlife: two decades of progress? Philos. Trans. R. Soc. B Biol. Sci. 372:20160167. doi: 10.1098/rstb.2016.0167
Curto, P., Santa, C., Allen, P., Manadas, B., Simões, I., and Martinez, J. J. (2019). A pathogen and a non-pathogen spotted fever group Rickettsia trigger differential proteome signatures in macrophages. Front. Cell. Infect. Microbiol. 9:43. doi: 10.3389/fcimb.2019.00043
Dasch, G. A., Ramaiah, A., Holmes, Z. C., Zambrano, M. L., and Shirey, T. B. (2019). “Use of the Ion Torrent PGM for determining the genomic sequences of francisella and coxiella-like endosymbionts and rickettsia directly from hard ticks,” in Contemporary Acarology, eds M. J. Skvarla, R. Ochoa, J. C. V. Rodrigues, and H. J. Hutcheson (Berlin: Springer), 1–35. doi: 10.1007/978-3-030-17265-7_1
de Carvalho, I. L., Toledo, A., Carvalho, C., Barandika, J., Respicio-Kingry, L., Garcia-Amil, C., et al. (2016). Francisella species in ticks and animals, Iberian Peninsula. Ticks Tick Borne Dis. 7, 159–165. doi: 10.1016/j.ttbdis.2015.10.009
de la Fuente, J., Antunes, S., Bonnet, S., Cabezas-Cruz, A., Domingos, A. G., Estrada-Peña, A., et al. (2017). Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front. Cell. Infect. Microbiol. 7:114. doi: 10.3389/fcimb.2017.00114
de la Fuente, J., Moreno-Cid, J. A., Canales, M., Villar, M., de la Lastra, J. M. P., Kocan, K. M., et al. (2011). Targeting arthropod subolesin/akirin for the development of a universal vaccine for control of vector infestations and pathogen transmission. Vet. Parasitol. 181, 17–22. doi: 10.1016/j.vetpar.2011.04.018
De Sousa, G. B., Blanco, A., and Gardenal, C. N. (2001). Genetic relationships among Aedes aegypti (Diptera: Culicidae) populations from Argentina using random amplified polymorphic DNA polymerase chain reaction markers. J. Med. Entomol. 38, 371–375. doi: 10.1603/0022-2585-38.3.371
Dergousoff, S. J., and Chilton, N. B. (2011). Novel genotypes of Anaplasma bovis,“Candidatus Midichloria” sp. and Ignatzschineria sp. in the Rocky Mountain wood tick, Dermacentor andersoni. Vet. Microbiol. 150, 100–106. doi: 10.1016/j.vetmic.2011.01.018
Douglas, A. E. (2007). Symbiotic microorganisms: untapped resources for insect pest control. Trends Biotechnol. 25, 338–342. doi: 10.1016/j.tibtech.2007.06.003
Duron, O. (2015). The IS1111 insertion sequence used for detection of Coxiella burnetii is widespread in Coxiella-like endosymbionts of ticks. FEMS Microbiol. Lett. 362:fnv132. doi: 10.1093/femsle/fnv132
Duron, O., Binetruy, F., Noël, V., Cremaschi, J., McCoy, K. D., Arnathau, C., et al. (2017). Evolutionary changes in symbiont community structure in ticks. Mol. Ecol. 26, 2905–2921. doi: 10.1111/mec.14094
Duron, O., Morel, O., Noël, V., Buysse, M., Binetruy, F., Lancelot, R., et al. (2018). Tick-bacteria mutualism depends on B vitamin synthesis pathways. Curr. Biol. 28, 1896–1902.85. doi: 10.1016/j.cub.2018.04.038
Edouard, S., Subramanian, G., Lefevre, B., Santos, A. Dos, Pouedras, P., Poinsignon, Y., et al. (2013). Co-infection with Arsenophonus nasoniae and Orientia tsutsugamushi in a traveler. Vector Borne Zoonotic Dis. 13, 565–571.
Ehounoud, C. B., Yao, K. P., Dahmani, M., Achi, Y. L., Amanzougaghene, N., Kacou N’Douba, A., et al. (2016). Multiple pathogens including potential new species in tick vectors in Côte d’Ivoire. PLoS Negl. Trop. Dis. 10:e0004367. doi: 10.1371/journal.pntd.0004367
Elbir, H., Almathen, F., and Elnahas, A. (2020). Low genetic diversity among Francisella-like endosymbionts within different genotypes of Hyalomma dromedarii ticks infesting camels in Saudi Arabia. Vet. World 13, 1462–1472. doi: 10.14202/vetworld.2020.1462-1472
Eliasson, H., Broman, T., Forsman, M., and Bäck, E. (2006). Tularemia: current epidemiology and disease management. Infect. Dis. Clin. 20, 289–311. doi: 10.1016/j.idc.2006.03.002
Elston, R., and Apperson, C. (1977). A light-activated on-off switch for the CDC light trap. J. Med. Entomol. 14, 254–255. doi: 10.1093/jmedent/14.2.254
Epis, S., Mandrioli, M., Genchi, M., Montagna, M., Sacchi, L., Pistone, D., et al. (2013). Localization of the bacterial symbiont Candidatus Midichloria mitochondrii within the hard tick Ixodes ricinus by whole-mount FISH staining. Ticks Tick Borne Dis. 4, 39–45. doi: 10.1016/j.ttbdis.2012.06.005
Epis, S., Sassera, D., Beninati, T., Lo, N., Beati, L., Piesman, J., et al. (2008). Midichloria mitochondrii is widespread in hard ticks (Ixodidae) and resides in the mitochondria of phylogenetically diverse species. Parasitology 135, 485–494. doi: 10.1017/S0031182007004052
Eremeeva, M. E., Oliveira, A., Robinson, J. B., Ribakova, N., Tokarevich, N. K., and Dasch, G. A. (2006). Prevalence of bacterial agents in Ixodes persulcatus ticks from the Vologda Province of Russia. Ann. N. Y. Acad. Sci. 1078, 291–298. doi: 10.1196/annals.1374.054
Estrada-Peña, A., and de la Fuente, J. (2014). The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Res. 108, 104–128. doi: 10.1016/j.antiviral.2014.05.016
Estrada-Peña, A., and Jongejan, F. (1999). Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp. Appl. Acarol. 23, 685–715. doi: 10.1023/a:1006241108739
Estrada-Peña, A., Cabezas-Cruz, A., and Obregón, D. (2020b). Resistance of tick gut microbiome to anti-tick vaccines, pathogen infection and antimicrobial peptides. Pathogens 9:309. doi: 10.3390/pathogens9040309
Estrada-Peña, A., Cabezas-Cruz, A., and Obregón, D. (2020a). Behind taxonomic variability: the functional redundancy in the tick microbiome. Microorganisms 8:1829. doi: 10.3390/microorganisms8111829
Felsheim, R. F., Kurtti, T. J., and Munderloh, U. G. (2009). Genome sequence of the endosymbiont Rickettsia peacockii and comparison with virulent Rickettsia rickettsii: identification of virulence factors. PLoS One 4:e8361. doi: 10.1371/journal.pone.0008361
Galay, R. L., Matsuo, T., Hernandez, E. P., Talactac, M. R., Kusakisako, K., Umemiya-Shirafuji, R., et al. (2018). Immunofluorescent detection in the ovary of host antibodies against a secretory ferritin injected into female Haemaphysalis longicornis ticks. Parasitol. Int. 67, 119–122. doi: 10.1016/j.parint.2017.10.006
Gall, C. A., Reif, K. E., Scoles, G. A., Mason, K. L., Mousel, M., Noh, S. M., et al. (2016). The bacterial microbiome of Dermacentor andersoni ticks influences pathogen susceptibility. ISME J. 10, 1846–1855. doi: 10.1038/ismej.2015.266
Gerhart, J. G., Auguste Dutcher, H., Brenner, A. E., Moses, A. S., Grubhoffer, L., and Raghavan, R. (2018). Multiple acquisitions of pathogen-derived Francisella endosymbionts in soft ticks. Genome Biol. Evol. 10, 607–615. doi: 10.1093/gbe/evy021
Gerhart, J. G., Moses, A. S., and Raghavan, R. (2016). A Francisella-like endosymbiont in the Gulf Coast tick evolved from a mammalian pathogen. Sci. Rep. 6:33670. doi: 10.1038/srep33670
Gnainsky, Y., Zfanya, N., Elgart, M., Omri, E., Brandis, A., Mehlman, T., et al. (2021). Systemic regulation of host energy and oogenesis by microbiome-derived mitochondrial coenzymes. Cell Rep. 34:108583. doi: 10.1016/j.celrep.2020.108583
Goddard, J. (2009). Historical and recent evidence for close relationships among Rickettsia parkeri, R. conorii, R. africae, and R. sibirica: implications for rickettsial taxonomy. J. Vector Ecol. 34, 238–242.
Goethert, H. K., and Telford, S. R. (2005). A new Francisella (Beggiatiales: Francisellaceae) inquiline within Dermacentor variabilis say (Acari: Ixodidae). J. Med. Entomol. 42, 502–505. doi: 10.1603/0022-2585(2005)042[0502:anfbfi]2.0.co;2
Gofton, A. W., Oskam, C. L., Lo, N., Beninati, T., Wei, H., McCarl, V., et al. (2015). Inhibition of the endosymbiont “Candidatus Midichloria mitochondrii” during 16S rRNA gene profiling reveals potential pathogens in Ixodes ticks from Australia. Parasites Vectors 8:345. doi: 10.1186/s13071-015-0958-3
Gottlieb, Y., Lalzar, I., and Klasson, L. (2015). Distinctive genome reduction rates revealed by genomic analyses of two Coxiella-like endosymbionts in ticks. Genome Biol. Evol. 7, 1779–1796. doi: 10.1093/gbe/evv108
Greay, T. L., Gofton, A. W., Paparini, A., Ryan, U. M., Oskam, C. L., and Irwin, P. J. (2018). Recent insights into the tick microbiome gained through next-generation sequencing. Parasites Vectors 11:12. doi: 10.1186/s13071-017-2550-5
Guizzo, M. G., Parizi, L. F., Nunes, R. D., Schama, R., Albano, R. M., Tirloni, L., et al. (2017). mutualist symbiont is essential to the development of Rhipicephalus microplus. Sci. Rep. 7:17554. doi: 10.1038/s41598-017-17309-x
Hajdusek, O., Sima, R., Ayllón, N., Jalovecká, M., Perner, J., De La Fuente, J., et al. (2013). Interaction of the tick immune system with transmitted pathogens. Front. Cell. Infect. Microbiol. 3:26. doi: 10.3389/fcimb.2013.00026
Harrus, S., and Baneth, G. (2005). Drivers for the emergence and re-emergence of vector-borne protozoal and bacterial diseases. Int. J. Parasitol. 35, 1309–1318. doi: 10.1016/j.ijpara.2005.06.005
Harrus, S., Perlman-Avrahami, A., Mumcuoglu, K., Morick, D., Eyal, O., and Baneth, G. (2011). Molecular detection of Ehrlichia canis, Anaplasma bovis, Anaplasma platys, Candidatus Midichloria mitochondrii and Babesia canis vogeli in ticks from Israel. Clin. Microbiol. Infect. 17, 459–463. doi: 10.1111/j.1469-0691.2010.03316.x
Hartelt, K., Oehme, R., Frank, H., Brockmann, S. O., Hassler, D., and Kimmig, P. (2004). Pathogens and symbionts in ticks: prevalence of Anaplasma phagocytophilum (Ehrlichia sp.), Wolbachia sp., Rickettsia sp., and Babesia sp. in Southern Germany. Int. J. Med. Microbiol. Suppl. 293, 86–92. doi: 10.1016/s1433-1128(04)80013-5
Heisig, M., Abraham, N. M., Liu, L., Neelakanta, G., Mattessich, S., Sultana, H., et al. (2014). Antivirulence properties of an antifreeze protein. Cell Rep. 9, 417–424. doi: 10.1016/j.celrep.2014.09.034
Hensley, J. R., Zambrano, M. L., Williams-Newkirk, A. J., and Dasch, G. A. (2021). Detection of Rickettsia Species, and Coxiella-Like and Francisella-Like Endosymbionts in Amblyomma americanum and Amblyomma maculatum from a Shared Field Site in Georgia, United States of America. Vector Borne Zoonotic Dis. 21, 509–516. doi: 10.1089/vbz.2020.2683
Hirunkanokpun, S., Ahantarig, A., Baimai, V., and Trinachartvanit, W. (2018). A new record of Wolbachia in the elephant ticks from Thailand. Sci. Asia 44, 44–47.
Hurst, G. D., Jiggins, F. M., von der Schulenburg, J. H. G., Bertrand, D., West, S. A., Goriacheva, I. I., et al. (1999). Male–killing Wolbachia in two species of insect. Proc. R. Soc. Lond. Ser. B Biol. Sci. 266, 735–740.
Hussain, S., Hussain, A., Ho, J., Li, J., George, D., Rehman, A., et al. (2021b). An epidemiological survey regarding ticks and tick-borne diseases among livestock owners in Punjab, Pakistan: a one health context. Pathogens 10:361. doi: 10.3390/pathogens10030361
Hussain, S., Hussain, A., Aziz, U., Song, B., Zeb, J., George, D., et al. (2021a). The role of ticks in the emergence of Borrelia burgdorferi as a zoonotic pathogen and its vector control: a global systemic review. Microorganisms 9:2412. doi: 10.3390/microorganisms9122412
Hussain, S., Hussain, A., Rehman, A., George, D., Li, J., Zeb, J., et al. (2021c). Spatio-temporal distribution of identified tick species from small and large ruminants of Pakistan. Biologia 76, 1–11.
Ivanov, I. N., Mitkova, N., Reye, A. L., Hübschen, J. M., Vatcheva-Dobrevska, R. S., Dobreva, E. G., et al. (2011). Detection of new Francisella-like tick endosymbionts in Hyalomma spp. and Rhipicephalus spp. (Acari: Ixodidae) from Bulgaria. Appl. Environ. Microbiol. 77, 5562–5565. doi: 10.1128/AEM.02934-10
Jaarsma, R. I., Sprong, H., Takumi, K., Kazimirova, M., Silaghi, C., Mysterud, A., et al. (2019). Anaplasma phagocytophilum evolves in geographical and biotic niches of vertebrates and ticks. Parasites Vectors 12:328. doi: 10.1186/s13071-019-3583-8
Jasinskas, A., Zhong, J., and Barbour, A. G. (2007). Highly prevalent Coxiella sp. bacterium in the tick vector Amblyomma americanum. Appl. Environ. Microbiol. 73, 334–336. doi: 10.1128/AEM.02009-06
Jiao, J., Lu, Z., Yu, Y., Ou, Y., Fu, M., Zhao, Y., et al. (2021). Identification of tick-borne pathogens by metagenomic next-generation sequencing in Dermacentor nuttalli and Ixodes persulcatus in Inner Mongolia, China. Parasites Vectors 14:287. doi: 10.1186/s13071-021-04740-3
Johns, R., Ohnishi, J., Broadwater, A., Sonenshine, D. E., De Silva, A. M., and Hynes, W. L. (2001). Contrasts in tick innate immune responses to Borrelia burgdorferi challenge: immunotolerance in Ixodes scapularis versus immunocompetence in Dermacentor variabilis (Acari: Ixodidae). J. Med. Entomol. 38, 99–107. doi: 10.1603/0022-2585-38.1.99
Jourdain, E., Duron, O., Barry, S., González-Acuña, D., and Sidi-Boumedine, K. (2015). Molecular methods routinely used to detect Coxiella burnetii in ticks cross-react with Coxiella-like bacteria. Infect. Ecol. Epidemiol. 5:29230. doi: 10.3402/iee.v5.29230
Kaaya, G. P., and Alemu, P. (1984). Further observations on survival and fertility of Glossina morsitans morsitans maintained on immunized rabbits. Int. J. Trop. Insect Sci. 5, 443–446.
Kagemann, J., and Clay, K. (2013). Effects of infection by Arsenophonus and Rickettsia bacteria on the locomotive ability of the ticks Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis. J. Med. Entomol. 50, 155–162. doi: 10.1603/me12086
Kazimírová, M., Hamšíková, Z., Špitalská, E., Minichová, L., Mahríková, L., Caban, R., et al. (2018). Diverse tick-borne microorganisms identified in free-living ungulates in Slovakia. Parasites Vectors 11:495. doi: 10.1186/s13071-018-3068-1
Keim, P., Johansson, A., and Wagner, D. M. (2007). Molecular epidemiology, evolution, and ecology of Francisella. Ann. N. Y. Acad. Sci. 1105, 30–66. doi: 10.1196/annals.1409.011
Khoo, J.-J., Lim, F.-S., Chen, F., Phoon, W.-H., Khor, C.-S., Pike, B. L., et al. (2016). Coxiella detection in ticks from wildlife and livestock in Malaysia. Vector Borne Zoonotic Dis. 16, 744–751. doi: 10.1089/vbz.2016.1959
Klyachko, O., Stein, B. D., Grindle, N., Clay, K., and Fuqua, C. (2007). Localization and visualization of a Coxiella-type symbiont within the lone star tick, Amblyomma americanum. Appl. Environ. Microbiol. 73, 6584–6594. doi: 10.1128/AEM.00537-07
Kobayashi, T., Chatanga, E., Qiu, Y., Simuunza, M., Kajihara, M., Hang’ombe, B. M., et al. (2021). Molecular detection and genotyping of Coxiella-Like Endosymbionts in ticks collected from animals and vegetation in Zambia. Pathogens 10:779. doi: 10.3390/pathogens10060779
Krawczyk, A. I. (2021). Questing Microbioticks: Interactions of Microbes, Ticks, Vertebrates, and the Environment. Wageningen: Wageningen University.
Kreizinger, Z., Hornok, S., Dán, Á, Hresko, S., Makrai, L., Magyar, T., et al. (2013). Prevalence of Francisella tularensis and Francisella-like endosymbionts in the tick population of Hungary and the genetic variability of Francisella-like agents. Vector Borne Zoonotic Dis. 13, 160–163. doi: 10.1089/vbz.2012.1065
Kugeler, K. J., Gurfield, N., Creek, J. G., Mahoney, K. S., Versage, J. L., and Petersen, J. M. (2005). Discrimination between Francisella tularensis and Francisella-like endosymbionts when screening ticks by PCR. Appl. Environ. Microbiol. 71, 7594–7597. doi: 10.1128/AEM.71.11.7594-7597.2005
Kulski, J. K. (2016). Next-generation sequencing—an overview of the history, tools, and “Omic” applications. Next Generat. Sequencing Adv. Appl. Challenges 3:60.
Kumar, D., Sharma, S. R., Adegoke, A., Kennedy, A., Tuten, H. C., Li, A. Y., et al. (2021). Recently evolved Francisella-like endosymbiont outcompetes an ancient and evolutionarily associated Coxiella-like endosymbiont in the lone star tick (Amblyomma americanum) linked to the Alpha-Gal Syndrome. Res. Sq. [Preprint]. doi: 10.21203/rs.3.rs-856007/v1
Kurokawa, C., Lynn, G. E., Pedra, J. H., Pal, U., Narasimhan, S., and Fikrig, E. (2020). Interactions between Borrelia burgdorferi and ticks. Nat. Rev. Microbiol. 18, 587–600. doi: 10.1038/s41579-020-0400-5
Kurtti, T. J., Palmer, A. T., and Oliver, J. H. (2002). Rickettsiella-like bacteria in Ixodes woodi (Acari: Ixodidae). J. Med. Entomol. 39, 534–540. doi: 10.1603/0022-2585-39.3.534
Lalzar, I., Friedmann, Y., and Gottlieb, Y. (2014). Tissue tropism and vertical transmission of C oxiella in R hipicephalus sanguineus and R hipicephalus turanicus ticks. Environ. Microbiol. 16, 3657–3668. doi: 10.1111/1462-2920.12455
Lalzar, I., Harrus, S., Mumcuoglu, K. Y., and Gottlieb, Y. (2012). Composition and seasonal variation of Rhipicephalus turanicus and Rhipicephalus sanguineus bacterial communities. Appl. Environ. Microbiol. 78, 4110–4116. doi: 10.1128/AEM.00323-12
Lee, J. H., Park, H. S., Jang, W. J., Koh, S. E., Park, T. K., Kang, S. S., et al. (2004). Identification of the Coxiella sp. detected from Haemaphysalis longicornis ticks in Korea. Microbiol. Immunol. 48, 125–130. doi: 10.1111/j.1348-0421.2004.tb03498.x
Li, N., Li, S., Wang, D., Yan, P., Wang, W., Li, M., et al. (2019). Characterization of the Rickettsia-like and Coxiella-like symbionts in the tick Dermacentor everestianus Hirst, 1926 (Acari: Ixodidae) from the Qinghai-Tibet Plateau. Syst. Appl. Acarol. 24, 106–117.
Liu, L., Dai, J., Zhao, Y. O., Narasimhan, S., Yang, Y., Zhang, L., et al. (2012). Ixodes scapularis JAK-STAT pathway regulates tick antimicrobial peptides, thereby controlling the agent of human granulocytic anaplasmosis. J. Infect. Dis. 206, 1233–1241. doi: 10.1093/infdis/jis484
Liu, L., Li, L., Liu, J., Hu, Y., Liu, Z., Guo, L., et al. (2013). Coinfection of Dermacentor silvarum Olenev (Acari: Ixodidae) by Coxiella-like, Arsenophonus-like, and Rickettsia-like symbionts. Appl. Environ. Microbiol. 79, 2450–2454. doi: 10.1128/AEM.03575-12
Liu, L., Li, L., Liu, J., Yu, Z., Yang, X., and Liu, J. (2016). Population dynamics of multiple symbionts in the hard tick, Dermacentor silvarum Olenev (Acari: Ixodidae). Ticks Tick Borne Dis. 7, 188–192. doi: 10.1016/j.ttbdis.2015.10.002
Luu, L., Palomar, A. M., Farrington, G., Schilling, A.-K., Premchand-Branker, S., McGarry, J., et al. (2021). Bacterial pathogens and symbionts harboured by Ixodes ricinus ticks parasitising red squirrels in the United Kingdom. Pathogens 10:458. doi: 10.3390/pathogens10040458
Macaluso, K. R., Sonenshine, D. E., Ceraul, S. M., and Azad, A. F. (2002). Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 39, 809–813. doi: 10.1603/0022-2585-39.6.809
Machado-Ferreira, E., Dietrich, G., Hojgaard, A., Levin, M., Piesman, J., Zeidner, N. S., et al. (2011). Coxiella symbionts in the Cayenne tick Amblyomma cajennense. Microbial Ecol. 62, 134–142. doi: 10.1007/s00248-011-9868-x
Machado-Ferreira, E., Piesman, J., Zeidner, N. S., and Soares, C. A. (2009). Francisella-like endosymbiont DNA and Francisella tularensis virulence-related genes in Brazilian ticks (Acari: Ixodidae). J. Med. Entomol. 46, 369–374.
Machado-Ferreira, E., Vizzoni, V. F., Balsemão-Pires, E., Moerbeck, L., Gazeta, G. S., Piesman, J., et al. (2016). Coxiella symbionts are widespread into hard ticks. Parasitol. Res. 115, 4691–4699. doi: 10.1007/s00436-016-5230-z
Magnúsdóttir, S., Ravcheev, D., de Crécy-Lagard, V., and Thiele, I. (2015). Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 6:148. doi: 10.3389/fgene.2015.00148
Maitre, A., Wu-Chuang, A., Aželytë, J., Palinauskas, V., Mateos-Hernández, L., Obregon, D., et al. (2022). Vector microbiota manipulation by host antibodies: the forgotten strategy to develop transmission-blocking vaccines. Parasites Vectors 15:4. doi: 10.1186/s13071-021-05122-5
Mariconti, M., Epis, S., Gaibani, P., Dalla Valle, C., Sassera, D., Tomao, P., et al. (2012a). Humans parasitized by the hard tick Ixodes ricinus are seropositive to Midichloria mitochondrii: is Midichloria a novel pathogen, or just a marker of tick bite? Pathog. Glob. Health 106, 391–396.
Mariconti, M., Epis, S., Sacchi, L., Biggiogera, M., Sassera, D., Genchi, M., et al. (2012b). A study on the presence of flagella in the order Rickettsiales: the case of ‘Candidatus Midichloria mitochondrii’. Microbiology 158, 1677–1683. doi: 10.1099/mic.0.057174-0
Mateos-Hernández, L., Obregón, D., Maye, J., Borneres, J., Versille, N., de la Fuente, J., et al. (2020). Anti-tick microbiota vaccine impacts Ixodes ricinus performance during feeding. Vaccines 8:702. doi: 10.3390/vaccines8040702
Mateos-Hernández, L. Obregón, D. Wu-Chuang, A., and Maye, J. Bornéres, J. Versillé, N., et al. (2021). Anti-microbiota vaccines modulate the tick microbiome in a taxon-specific manner. Front. immunol., 12:2780.
Mateos-Hernandez, L., Obregon, D., Wu-Chuang, A., Maye, J., Borneres, J., Versille, N., et al. (2021). Anti-microbiota vaccines modulate the tick microbiome in a taxon-specific manner. bioRxiv [Preprint]. doi: 10.1101/2021.05.12.443756
Mattila, J. T., Burkhardt, N. Y., Hutcheson, H. J., Munderloh, U. G., and Kurtti, T. J. (2007). Isolation of cell lines and a rickettsial endosymbiont from the soft tick Carios capensis (Acari: Argasidae: Ornithodorinae). J. Med. Entomol. 44, 1091–1101. doi: 10.1603/0022-2585(2007)44[1091:ioclaa]2.0.co;2
Mediannikov, O., Ivanov, L., Nishikawa, M., Saito, R., Sidelnikov, Y. N., Zdanovskaya, N. I., et al. (2003). Molecular evidence of Coxiella-like microorganism harbored by Haemaphysalis concinnae ticks in the Russian Far East. Ann. N. Y. Acad. Sci. 990, 226–228. doi: 10.1111/j.1749-6632.2003.tb07367.x
Mioni, M. S. R., Costa, F. B., Ribeiro, B. L. D., Teixeira, W. S. R., Pelicia, V. C., Labruna, M. B., et al. (2020). Coxiella burnetii in slaughterhouses in Brazil: a public health concern. PLoS One 15:e0241246. doi: 10.1371/journal.pone.0241246
Moran, N. A., and Wernegreen, J. J. (2000). Lifestyle evolution in symbiotic bacteria: insights from genomics. Trends Ecol. Evol. 15, 321–326. doi: 10.1016/s0169-5347(00)01902-9
Moran, N. A., Plague, G. R., Sandström, J. P., and Wilcox, J. L. (2003). A genomic perspective on nutrient provisioning by bacterial symbionts of insects. Proc. Natl. Acad. Sci. 100, 14543–14548. doi: 10.1073/pnas.2135345100
Najm, N.-A., Silaghi, C., Bell-Sakyi, L., Pfister, K., and Passos, L. M. F. (2012). Detection of bacteria related to Candidatus Midichloria mitochondrii in tick cell lines. Parasitol. Res. 110, 437–442. doi: 10.1007/s00436-011-2509-y
Narasimhan, S., and Fikrig, E. (2015). Tick microbiome: the force within. Trends Parasitol. 31, 315–323. doi: 10.1016/j.pt.2015.03.010
Narasimhan, S., Rajeevan, N., Liu, L., Zhao, Y. O., Heisig, J., Pan, J., et al. (2014). Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host Microbe 15, 58–71. doi: 10.1016/j.chom.2013.12.001
Narasimhan, S., Schuijt, T. J., Abraham, N. M., Rajeevan, N., Coumou, J., Graham, M., et al. (2017). Modulation of the tick gut milieu by a secreted tick protein favors Borrelia burgdorferi colonization. Nat. Commun. 8:184. doi: 10.1038/s41467-017-00208-0
Nardi, T., Olivieri, E., Kariuki, E., Sassera, D., and Castelli, M. (2021). Sequence of a Coxiella endosymbiont of the tick Amblyomma nuttalli suggests a pattern of convergent genome reduction in the Coxiella genus. Genome Biol. Evol. 13:evaa253. doi: 10.1093/gbe/evaa253
Neelakanta, G., Sultana, H., Fish, D., Anderson, J. F., and Fikrig, E. (2010). Anaplasma phagocytophilum induces Ixodes scapularis ticks to express an antifreeze glycoprotein gene that enhances their survival in the cold. J. Clin. Investig. 120, 3179–3190. doi: 10.1172/jci42868
Niebylski, M. L., Peacock, M. G., Fischer, E. R., Porcella, S. F., and Schwan, T. G. (1997). Characterization of an endosymbiont infecting wood ticks, Dermacentor andersoni, as a member of the genus Francisella. Appl. Environ. Microbiol. 63, 3933–3940. doi: 10.1128/aem.63.10.3933-3940.1997
Noda, H., Munderloh, U. G., and Kurtti, T. J. (1997). Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl. Environ. Microbiol. 63, 3926–3932. doi: 10.1128/aem.63.10.3926-3932.1997
Noden, B. H., Vaughan, J. A., Pumpuni, C. B., and Beier, J. C. (2011). Mosquito ingestion of antibodies against mosquito midgut microbiota improves conversion of ookinetes to oocysts for Plasmodium falciparum, but not P. yoelii. Parasitol. Int. 60, 440–446. doi: 10.1016/j.parint.2011.07.007
Nogge, G. (1978). Aposymbiotic tsetse flies, Glossina morsitans morsitans obtained by feeding on rabbits immunized specifically with symbionts. J. Insect Physiol. 24, 299–304. doi: 10.1016/0022-1910(78)90026-4
Nováková, M., and Šmajs, D. (2018). “Rickettsial endosymbionts of ticks,” in Ticks Tick Borne Pathogens, eds M. Abubakar and P. K. Perera (London: IntechOpen). doi: 10.1590/S1984-29612016045
Obregón, D., Bard, E., Abrial, D., Estrada-Peña, A., and Cabezas-Cruz, A. (2019). Sex-specific linkages between taxonomic and functional profiles of tick gut microbiomes. Front. Cell. Infect. Microbiol. 9:298. doi: 10.3389/fcimb.2019.00298
Ohtaka, C., and Ishikawa, H. (1991). Effects of heat treatment on the symbiotic system of an aphid mycetocyte. Symbiosis 11, 19–30.
Olivieri, E., Epis, S., Castelli, M., Boccazzi, I. V., Romeo, C., Desirò, A., et al. (2019). Tissue tropism and metabolic pathways of Midichloria mitochondrii suggest tissue-specific functions in the symbiosis with Ixodes ricinus. Ticks Tick Borne Dis. 10, 1070–1077. doi: 10.1016/j.ttbdis.2019.05.019
Papa, A., Tsioka, K., Kontana, A., Papadopoulos, C., and Giadinis, N. (2017). Bacterial pathogens and endosymbionts in ticks. Ticks Tick Borne Dis. 8, 31–35.
Parola, P., and Raoult, D. (2001). Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin. Infect. Dis. 32, 897–928. doi: 10.1086/319347
Parratt, S. R., Frost, C. L., Schenkel, M. A., Rice, A., Hurst, G. D., and King, K. C. (2016). Superparasitism drives heritable symbiont epidemiology and host sex ratio in a wasp. PLoS Pathog. 12:e1005629. doi: 10.1371/journal.ppat.1005629
Paul, R. E., Cote, M., Le Naour, E., and Bonnet, S. I. (2016). Environmental factors influencing tick densities over seven years in a French suburban forest. Parasites Vectors 9, 1–10. doi: 10.1186/s13071-016-1591-5
Pérez-Brocal, V., Latorre, A., and Moya, A. (2011). Symbionts and pathogens: what is the difference? Curr. Top. Microbiol. Immunol. 358, 215–243.
Perlman, S. J., Hunter, M. S., and Zchori-Fein, E. (2006). The emerging diversity of Rickettsia. Proc. R. Soc. B Biol. Sci. 273, 2097–2106. doi: 10.1098/rspb.2006.3541
Perveen, N. (2021). Livestock Ticks In The Uae: Prevalence, Distribution, Population Dynamics, And Associated Microorganisms. Ph.D. thesis. Al Ain: United Arab Emirates University.
Perveen, N., Muzaffar, S. B., Vijayan, R., and Al-Deeb, M. A. (2020b). Microbial communities associated with the camel tick, Hyalomma dromedarii: 16S rRNA gene-based analysis. Sci. Rep. 10:17035. doi: 10.1038/s41598-020-74116-7
Perveen, N., Bin Muzaffar, S., and Al-Deeb, M. A. (2020a). Population dynamics of Hyalomma dromedarii on camels in the United Arab Emirates. Insects 11:320. doi: 10.3390/insects11050320
Perveen, N., Muzaffar, S. B., and Al-Deeb, M. A. (2021b). Ticks and Tick-borne diseases of livestock in the Middle East and North Africa: a review. Insects 12:83. doi: 10.3390/insects12010083
Perveen, N., Muzaffar, S. B., and Al-Deeb, M. A. (2021a). Four tick-borne microorganisms and their prevalence in Hyalomma ticks collected from livestock in United Arab Emirates. Pathogens 10:1005. doi: 10.3390/pathogens10081005
Petersen, J. M., Mead, P. S., and Schriefer, M. E. (2009). Francisella tularensis: an arthropod-borne pathogen. Vet. Res. 40:07. doi: 10.1051/vetres:2008045
Pfäffle, M., Littwin, N., Muders, S. V., and Petney, T. N. (2013). The ecology of tick-borne diseases. Int. J. Parasitol. 43, 1059–1077.
Plantard, O., Bouju-Albert, A., Malard, M.-A., Hermouet, A., Capron, G., and Verheyden, H. (2012). Detection of Wolbachia in the tick Ixodes ricinus is due to the presence of the hymenoptera endoparasitoid Ixodiphagus hookeri. PLoS One 7:e30692. doi: 10.1371/journal.pone.0030692
Pospisilova, T., Urbanova, V., Hes, O., Kopacek, P., Hajdusek, O., and Sima, R. (2019). Tracking of Borrelia afzelii transmission from infected Ixodes ricinus nymphs to mice. Infect. Immun. 87, e00896–18. doi: 10.1128/IAI.00896-18
Raele, D. A., Galante, D., Pugliese, N., De Simone, E., and Cafiero, M. A. (2015). Coxiella-like endosymbiont associated to the “Anatolian brown tick” Rhipicephalus bursa in Southern Italy. Microbes Infect. 17, 799–805. doi: 10.1016/j.micinf.2015.09.011
Raffel, S. J., Battisti, J. M., Fischer, R. J., and Schwan, T. G. (2014). Inactivation of genes for antigenic variation in the relapsing fever spirochete Borrelia hermsii reduces infectivity in mice and transmission by ticks. PLoS Pathog. 10:e1004056. doi: 10.1371/journal.ppat.1004056
Rahal, M., Medkour, H., Diarra, A. Z., Bitam, I., Parola, P., and Mediannikov, O. (2020). Molecular identification and evaluation of Coxiella-like endosymbionts genetic diversity carried by cattle ticks in Algeria. Ticks Tick Borne Dis. 11:101493. doi: 10.1016/j.ttbdis.2020.101493
Ramaiah, A., and Dasch, G. A. (2018). Genome sequence of Coxiella-like endosymbiont strain CLE-RmD, a bacterial agent in the cattle tick (Rhipicephalus microplus) Deutsch strain. Genome Announc. 6, e00003–18. doi: 10.1128/genomeA.00003-18
Raoult, D., and Roux, V. (1997). Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 10, 694–719. doi: 10.1128/CMR.10.4.694
Rathinavelu, S., Broadwater, A., and De Silva, A. M. (2003). Does host complement kill Borrelia burgdorferi within ticks? Infect. Immun. 71, 822–829. doi: 10.1128/IAI.71.2.822-829.2003
Reeves, W. K., Loftis, A. D., Priestley, R. A., Wills, W., Sanders, F., and Dasch, G. A. (2005). Molecular and biological characterization of a novel Coxiella-like agent from Carios capensis. Ann. N. Y. Acad. Sci. 1063, 343–345. doi: 10.1196/annals.1355.055
Rochlin, I., and Toledo, A. (2020). Emerging tick-borne pathogens of public health importance: a mini-review. J. Med. Microbiol. 69, 781–791. doi: 10.1099/jmm.0.001206
Rodríguez-Mallon, A., Encinosa, P. E., éndez-Pérez, L. M., Bello, Y., Fernández, R. R., Garay, H., et al. (2015). High efficacy of a 20 amino acid peptide of the acidic ribosomal protein P0 against the cattle tick, Rhipicephalus microplus. Ticks Tick Borne Dis. 6, 530–537. doi: 10.1016/j.ttbdis.2015.04.007
Rodríguez-Mallon, A., Fernández, E., Encinosa, P. E., Bello, Y., éndez-Pérez, L. M., Ruiz, L. C., et al. (2012). A novel tick antigen shows high vaccine efficacy against the dog tick, Rhipicephalus sanguineus. Vaccine 30, 1782–1789. doi: 10.1016/j.vaccine.2012.01.011
Rounds, M. A., Crowder, C. D., Matthews, H. E., Philipson, C. A., Scoles, G. A., Ecker, D. J., et al. (2012). Identification of endosymbionts in ticks by broad-range polymerase chain reaction and electrospray ionization mass spectrometry. J. Med. Entomol. 49, 843–850. doi: 10.1603/me12038
Rowley, S. M., Raven, R. J., and McGraw, E. A. (2004). Wolbachia pipientis in Australian spiders. Curr. Microbiol. 49, 208–214. doi: 10.1007/s00284-004-4346-z
Salcedo-Porras, N., Umaña-Diaz, C., de Oliveira Barbosa Bitencourt, R., and Lowenberger, C. (2020). The role of bacterial symbionts in triatomines: an evolutionary perspective. Microorganisms 8:1438. doi: 10.3390/microorganisms8091438
Sassera, D., Beninati, T., Bandi, C., Bouman, E. A., Sacchi, L., Fabbi, M., et al. (2006). ‘Candidatus Midichloria mitochondrii’, an endosymbiont of the tick Ixodes ricinus with a unique intramitochondrial lifestyle. Int. J. Syst. Evol. Microbiol. 56, 2535–2540. doi: 10.1099/ijs.0.64386-0
Sassera, D., Lo, N., Bouman, E. A., Epis, S., Mortarino, M., and Bandi, C. (2008). “Candidatus Midichloria” endosymbionts bloom after the blood meal of the host, the hard tick Ixodes ricinus. Appl. Environ. Microbiol. 74, 6138–6140. doi: 10.1128/AEM.00248-08
Sazama, E. J., Ouellette, S. P., and Wesner, J. S. (2019). Bacterial endosymbionts are common among, but not necessarily within, insect species. Environ. Entomol. 48, 127–133. doi: 10.1093/ee/nvy188
Schabereiter-Gurtner, C., Lubitz, W., and Rölleke, S. (2003). Application of broad-range 16S rRNA PCR amplification and DGGE fingerprinting for detection of tick-infecting bacteria. J. Microbiol. Methods 52, 251–260. doi: 10.1016/s0167-7012(02)00186-0
Scoles, G. A. (2004). Phylogenetic analysis of the Francisella-like endosymbionts of Dermacentor ticks. J. Med. Entomol. 41, 277–286. doi: 10.1603/0022-2585-41.3.277
Seo, M.-G., Lee, S.-H., Ouh, I.-O., Lee, G. H., Goo, Y.-K., Kim, S., et al. (2016). Molecular detection and genotyping of Coxiella-like endosymbionts in ticks that infest horses in South Korea. PLoS One 11:e0165784. doi: 10.1371/journal.pone.0165784
Shivaprasad, H., Cadenas, M., Diab, S. S., Nordhausen, R., Bradway, D., Crespo, R., et al. (2008). Coxiella-like infection in psittacines and a toucan. Avian Dis. 52, 426–432. doi: 10.1637/8192-120707-Reg
Smith, T. A., Driscoll, T., Gillespie, J. J., Raghavan, R., and Coxiella-like, A. (2015). endosymbiont is a potential vitamin source for the Lone Star tick. Genome Biol. Evol. 7, 831–838. doi: 10.1093/gbe/evv016
Sonenshine, D. (2014). Excretion and water balance: hindgut, malpighian tubules, and coxal glands. Biol. Ticks 1, 206–219.
Špitalská, E., Sparagano, O., Stanko, M., Schwarzová, K., Špitalský, Z., and Škultéty, Ł, et al. (2018). Diversity of Coxiella-like and Francisella-like endosymbionts, and Rickettsia spp., Coxiella burnetii as pathogens in the tick populations of Slovakia, Central Europe. Ticks Tick Borne Dis. 9, 1207–1211. doi: 10.1016/j.ttbdis.2018.05.002
Stafford, G. P., Evans, L. D., Krumscheid, R., Dhillon, P., Fraser, G. M., and Hughes, C. (2007). Sorting of early and late flagellar subunits after docking at the membrane ATPase of the type III export pathway. J. Mol. Biol. 374, 877–882. doi: 10.1016/j.jmb.2007.09.080
Staples, J. E., Kubota, K. A., Chalcraft, L. G., Mead, P. S., and Petersen, J. M. (2006). Epidemiologic and molecular analysis of human tularemia, United States, 1964–2004. Emerg. Infect. Dis. 12, 1113–1118. doi: 10.3201/eid1207.051504
Stavru, F., Riemer, J., Jex, A., and Sassera, D. (2020). When bacteria meet mitochondria: the strange case of the tick symbiont Midichloria mitochondrii. Cell. Microbiol. 22:e13189. doi: 10.1111/cmi.13189
Stein, A., and Raoult, D. (1992). Detection of Coxiella burnetti by DNA amplification using polymerase chain reaction. J. Clin. Microbiol. 30, 2462–2466. doi: 10.1128/jcm.30.9.2462-2466.1992
Stouthamer, R., Breeuwer, J. A., and Hurst, G. D. (1999). Wolbachia pipientis: microbial manipulator of arthropod reproduction. Ann. Rev. Microbiol. 53, 71–102. doi: 10.1146/annurev.micro.53.1.71
Subramanian, G., Sekeyova, Z., Raoult, D., and Mediannikov, O. (2012). Multiple tick-associated bacteria in Ixodes ricinus from Slovakia. Ticks Tick Borne Dis. 3, 406–410. doi: 10.1016/j.ttbdis.2012.10.001
Sun, L. V., Scoles, G. A., Fish, D., and O’Neill, S. L. (2000). Francisella-like endosymbionts of ticks. J. Invertebr. Pathol. 76, 301–303. doi: 10.1006/jipa.2000.4983
Takhampunya, R., Sakolvaree, J., Chanarat, N., Youngdech, N., Phonjatturas, K., Promsathaporn, S., et al. (2021). The Bacterial community in questing ticks from Khao Yai National Park in Thailand. Front. Vet. Sci. 8:764763. doi: 10.3389/fvets.2021.764763
Thapa, S., Zhang, Y., and Allen, M. S. (2019). Effects of temperature on bacterial microbiome composition in Ixodes scapularis ticks. Microbiol. Open 8:e00719. doi: 10.1002/mbo3.719
Tijsse-Klasen, E., Braks, M., Scholte, E.-J., and Sprong, H. (2011). Parasites of vectors-Ixodiphagus hookeri and its Wolbachia symbionts in ticks in the Netherlands. Parasites Vectors 4:228. doi: 10.1186/1756-3305-4-228
Travanty, N. V., Ponnusamy, L., Kakumanu, M. L., Nicholson, W. L., and Apperson, C. S. (2019). Diversity and structure of the bacterial microbiome of the American dog tick, Dermacentor variabilis, is dominated by the endosymbiont Francisella. Symbiosis 79, 239–250. doi: 10.1007/s13199-019-00642-2
Trinachartvanit, W., Rakthong, P., Baimai, V., and Ahantarig, A. (2018b). Candidatus midichloria sp in a Rhipicephalus sanguineus sL nymphal tick collected from a cat in Thailand. Southeast Asian J. Trop. Med. Public Health 49, 251–255.
Trinachartvanit, W., Maneewong, S., Kaenkan, W., Usananan, P., Baimai, V., and Ahantarig, A. (2018a). Coxiella-like bacteria in fowl ticks from Thailand. Parasites Vectors 11:670. doi: 10.1186/s13071-018-3259-9
Trinachartvanit, W., Wutha, W., Kaenkan, W., Chelong, I.-A., Bahakheeree, M., Baimai, V., et al. (2019). Co-infection with Coxiella-like bacteria and Babesia in goat ticks from southern Thailand. Southeast Asian J. Trop. Med. Public Health 50, 643–650.
Vaughan, J. A., Sonenshine, D. E., and Azad, A. F. (2002). Kinetics of ingested host immunoglobulin G in hemolymph and whole body homogenates during nymphal development of Dermacentor variabilis and Ixodes scapularis ticks (Acari: Ixodidae). Exp. & Appl. Acarol. 27, 329–340. doi: 10.1023/a:1023347930746
Wade, D. M., Hankins, M., Smyth, D. A., Rhone, E. E., Mythen, M. G., Howell, D. C., et al. (2014). Detecting acute distress and risk of future psychological morbidity in critically ill patients: validation of the intensive care psychological assessment tool. Crit. Care 18:519. doi: 10.1186/s13054-014-0519-8
Wang, H., and Nuttall, P. (1994). Excretion of host immunoglobulin in tick saliva and detection of IgG-binding proteins in tick haemolymph and salivary glands. Parasitology 109, 525–530. doi: 10.1017/s0031182000080781
Wang, R., Li, N., Liu, J., Li, T., Liu, M., Yu, Z., et al. (2017). Symbiont dynamics of the Tibetan tick Haemaphysalis tibetensis (Acari: Ixodidae). Parasites Vectors 10:259. doi: 10.1186/s13071-017-2199-0
Willadsen, P. (1997). Novel vaccines for ectoparasites. Vet. Parasitol. 71, 209–222. doi: 10.1016/s0304-4017(97)00028-9
Williams-Newkirk, A. J., Rowe, L. A., Mixson-Hayden, T. R., and Dasch, G. A. (2012). Presence, genetic variability, and potential significance of “Candidatus Midichloria mitochondrii” in the lone star tick Amblyomma americanum. Exp. Appl. Acarol. 58, 291–300. doi: 10.1007/s10493-012-9582-5
Wu, D., Daugherty, S. C., Van Aken, S. E., Pai, G. H., Watkins, K. L., Khouri, H., et al. (2006). Metabolic complementarity and genomics of the dual bacterial symbiosis of sharpshooters. PLoS Biol. 4:e188. doi: 10.1371/journal.pbio.0040188
Wu-Chuang, A., Hodžić, A., Mateos-Hernández, L., Estrada-Peña, A., Obregon, D., and Cabezas-Cruz, A. (2021a). Current debates and advances in tick microbiome research. Curr. Res. Parasitol. Vector Borne Dis. 1:100036. doi: 10.1016/j.crpvbd.2021.100036
Wu-Chuang, A., Obregon, D., Estrada-Peña, A., and Cabezas-Cruz, A. (2021b). Thermostable keystone bacteria maintain the functional diversity of the Ixodes scapularis microbiome under heat stress. Microb. Ecol. [Epub Online ahead of print]. doi: 10.1007/s00248-021-01929-y
Yeni, D. K., Büyük, F., Ashraf, A., and Shah, M. (2021). Tularemia: a re-emerging tick-borne infectious disease. Folia Microbiol. 66, 1–14. doi: 10.1007/s12223-020-00827-z
Young, K. M., Corrin, T., Wilhelm, B., Uhland, C., Greig, J., Mascarenhas, M., et al. (2019). Zoonotic Babesia: a scoping review of the global evidence. PLoS One 14:e0226781. doi: 10.1371/journal.pone.0226781
Zchori-Fein, E., and Bourtzis, K. (2011). Manipulative Tenants: Bacteria Associated with Arthropods. Boca Raton: CRC press.
Zhang, X., Norris, D. E., and Rasgon, J. L. (2011). Distribution and molecular characterization of Wolbachia endosymbionts and filarial nematodes in Maryland populations of the lone star tick (Amblyomma americanum). FEMS Microbiol. Ecol. 77, 50–56. doi: 10.1111/j.1574-6941.2011.01089.x
Zhong, J., Jasinskas, A., and Barbour, A. G. (2007). Antibiotic treatment of the tick vector Amblyomma americanum reduced reproductive fitness. PLoS One 2:e405. doi: 10.1371/journal.pone.0000405
Keywords: tick microbiota, tick symbiont, symbiont-pathogen interactions, tick physiology, tick-borne diseases ecology, tick continuum
Citation: Hussain S, Perveen N, Hussain A, Song B, Aziz MU, Zeb J, Li J, George D, Cabezas-Cruz A and Sparagano O (2022) The Symbiotic Continuum Within Ticks: Opportunities for Disease Control. Front. Microbiol. 13:854803. doi: 10.3389/fmicb.2022.854803
Received: 14 January 2022; Accepted: 15 February 2022;
Published: 17 March 2022.
Edited by:
Olivier Duron, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Jianmin Zhong, Humboldt State University, United StatesJingwen Wang, Yale University, United States
Copyright © 2022 Hussain, Perveen, Hussain, Song, Aziz, Zeb, Li, George, Cabezas-Cruz and Sparagano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sabir Hussain, c2FodXNzYWluOC1jQG15LmNpdHl1LmVkdS5oaw==; Olivier Sparagano, b2xpdmllci5zcGFyYWdhbm9AY2l0eXUuZWR1Lmhr
 Sabir Hussain
Sabir Hussain Nighat Perveen
Nighat Perveen Abrar Hussain
Abrar Hussain Baolin Song
Baolin Song Muhammad Umair Aziz1
Muhammad Umair Aziz1 Jun Li
Jun Li Alejandro Cabezas-Cruz
Alejandro Cabezas-Cruz Olivier Sparagano
Olivier Sparagano