
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 22 March 2022
Sec. Infectious Agents and Disease
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.847313
High-touch environmental surfaces are acknowledged as potential sources of pathogen transmission, particularly in health care settings where infectious agents may be readily abundant. Methods of disinfecting these surfaces often include direct application of a chemical disinfectant or simply wiping the surface with a disinfectant pre-soaked wipe (DPW). In this study, we examine the ability of four disinfectants, ethanol (EtOH), sodium hypochlorite (NaOCl), chlorine dioxide (ClO2), and potassium monopersulfate (KMPS), to inactivate SARS-CoV-2 on a hard, non-porous surface, assessing the effects of concentration and contact time. The efficacy of DPWs to decontaminate carriers spiked with SARS-CoV-2, as well as the transferability of the virus from used DPWs to clean surfaces, is also assessed. Stainless steel carriers inoculated with approximately 6 logs of SARS-CoV-2 prepared in a soil load were disinfected within 5 min through exposure to 66.5% EtOH, 0.5% NaOCl, and 1% KMPS. The addition of mechanical wiping using DPWs impregnated with these biocides rendered the virus inactive almost immediately, with no viral transfer from the used DPW to adjacent surfaces. Carriers treated with 100 ppm of ClO2 showed a significant amount of viable virus remaining after 10 min of biocide exposure, while the virus was only completely inactivated after 10 min of treatment with 500 ppm of ClO2. Wiping SARS-CoV-2-spiked carriers with DPWs containing either concentration of ClO2 for 5 s left significant amounts of viable virus on the carriers. Furthermore, higher titers of infectious virus retained on the ClO2-infused DPWs were transferred to uninoculated carriers immediately after wiping. Overall, 66.5% EtOH, 0.5% NaOCl, and 1% KMPS appear to be highly effective biocidal agents against SARS-CoV-2, while ClO2 formulations are much less efficacious.
The coronavirus SARS-CoV-2, first discovered in December 2019 in Wuhan City, Hubei province, China, has caused millions of human deaths since its declaration as a pandemic by the World Health Organization in March 2020. Aerosols in close proximity are believed to be the primary mode of transmission (Anderson et al., 2020; Asadi et al., 2020; Morawska and Cao, 2020; World Health Organization [WHO], 2020, 2021; Yang et al., 2021); however, fomite transmission is often overlooked as a method of spread. Fomites are a concern for healthcare settings, where patients shed viral particles on surfaces that may remain viable from hours to weeks depending on the surface type and environmental conditions (i.e., ambient temperature and relative humidity) involved (Chin et al., 2020; Harbourt et al., 2020; Pastorino et al., 2020; Ren et al., 2020; Riddell et al., 2020; van Doremalen et al., 2020). A number of studies have focused on surfaces within health care facilities where patients infected with SARS-CoV-2 are being treated (Zeitler and Rapp, 2014; Chia et al., 2020; Cutts et al., 2020b; Guo et al., 2020; Ong et al., 2020; Wang et al., 2020; Wei et al., 2020; Zhang et al., 2020; Zhou et al., 2020; Zou et al., 2020; Kasloff et al., 2021; Wan et al., 2021) and on high-touch environmental surfaces (HITES; Bloise et al., 2020; Gholipour et al., 2020; Ijaz et al., 2020, 2021; Gavaldà-Mestre et al., 2021; Harvey et al., 2021; Hu et al., 2021; Kozer et al., 2021; Shah et al., 2021). HITES are often decontaminated by applying a suitable disinfectant and allowing it to dry or cleaning with a disinfectant pre-soaked wipe (DPW). Both methods rely on the biocidal nature of the applied chemical; however, the addition of mechanical action combines both inactivation and physical removal of the virus to render a surface “decontaminated” (Sattar and Maillard, 2013). Historically, such sanitation products were not typically tested in a manner recapitulating their use under real-life conditions. Gaps in testing included uncontrolled wiping action, the applied pressure during wiping, and inappropriate contact times (Williams et al., 2007; Sattar and Maillard, 2013; Edwards et al., 2017; Song et al., 2019). Such studies also failed to consider that during wiping, microorganisms could be transferred to adjacent surfaces instead of being removed, depending on the retaining ability of the wipe and the biocidal activity of the disinfectant (Edwards et al., 2017). In response to these shortcomings, ASTM International-E2967-15 (2015) was developed to measure the activity of antimicrobial wipes using a mechanical apparatus: the Wiperator specifically addresses the effect of pressure, cleaning strokes, and time against pathogens on hard non-porous surfaces (ASTM International-E2967-15, 2015).
In this study, we test the ability of DPWs impregnated with one of four microbicidal actives common in the marketplace (Abreu et al., 2013), namely, ethanol (EtOH), sodium hypochlorite (NaOCl), chlorine dioxide (ClO2), and potassium monopersulfate (KMPS), to effectively inactivate SARS-CoV-2 using ASTM International-E2967-15 (2015). We also compare the effect of the above disinfectants against dried SARS-CoV-2 in a sit-and-soak assay using ASTM International-E2197-17e1 (2017). This study will provide additional and novel information on the efficacy of these four biocidal agents, in both liquid and DPW forms, on the deadly pathogen, SARS-CoV-2.
African green monkey Vero E6 cells (ATCC CRL-1586) were propagated in cell culture medium (CCM) (DMEM; HyClone SH302243.01) supplemented with 10% fetal bovine serum [FBS; Gibco (catalog #) and 1% v/v penicillin–streptomycin (pen–strep; Gibco LS15140122)]. One day prior to testing, cells were trypsinized (Gibco LS25200056) and seeded into appropriate flasks or plates to reach ∼80% confluence the following day. On the day of testing, the CCM was removed and replaced with virus culture medium (VCM; DMEM + 2% FBS + 10 μl/ml pen/strep) for the duration of the experiment.
Virus stocks were prepared as previously described (Cook et al., 2016; Cutts et al., 2020a). Briefly, flasks containing 80% confluent Vero E6 cells were infected with passage 3 stocks of SARS-CoV-2 (hCoV-19/Canada/ON-VIDO-01/2020, GISAID accession# EPI_ISL_425177) at an MOI of 0.01. At 3–5 days post infection, the infected supernatant was removed and clarified using low-speed centrifugation at 4,500 × g for 10 min. Supernatant was overlaid onto a 20% w/v sucrose cushion in Tris-NaCl-EDTA buffer (prepared in-house), centrifuged at 28,000 RPM in a Beckman Coulter SW 32 TI rotor for 2 h, and the resulting viral pellet re-suspended in VCM overnight at 4°C. The following day, the pellets were pooled, aliquoted, and quantified as per Reed and Muench (1938). As SARS-CoV-2 is classified as a Risk Group 3 pathogen, all experimental procedures, from inoculum preparation to drying of carriers to disinfectant assays, took place within a Class II B2 BSC in a high-containment laboratory at the National Microbiology Laboratory in Winnipeg, Canada.
All four chemical biocides were prepared in accordance with ASTM International-E2197-17e1 (2017). Disinfectants and associated concentrations were chosen based on their potential use in health care facilities, within a laboratory setting, or being a common component in commercial disinfectant formulations.
The disinfectants 66.5% (v/v) EtOH (Commercial Alcohols P016EA95), 0.5% (v/v) NaOCl (Imperial Soap and Supplies IMP750-1), and 1% (w/v) KMPS (Osorno Enterprises Inc., KMPS-1KG-JAR) were prepared in sterile hard water (i.e., containing 0.04% w/v calcium carbonate). For assays using ClO2 (Osorno Enterprises Inc., Power Oxide POT-1L-SET), solutions were prepared 1 day prior to testing and placed at 4°C overnight as per the manufacturer’s instructions. Active chlorine was measured the following morning, and solutions were diluted to 100 ppm (i.e., 0.01%) and 500 ppm (i.e., 0.05%) in hard water for disinfectant assays. Fresh batches of disinfectant were used for each independent experiment.
A neutralization assay was performed as described previously (Cutts et al., 2019, 2020a) in order to evaluate any interactions between the neutralizers, disinfectants, host cells, and pathogen (Table 1). Combinations of neutralizers and disinfectants were evaluated and are described in the Supplementary Material. Wherever possible, culture medium (VCM) was used as neutralizer for the various experimental assays. However, in quantitative carrier test 2 (QCT-2) assays involving KMPS or ClO2, a 1% sodium thiosulfate solution was required to more effectively neutralize the biocides, leading to a more sensitive readout in the reporter assay with the lowest possible limit of detection to assure that any remaining viable virus was detected.
The QCT-2 disinfectant efficacy assay was conducted in accordance with ASTM International-E2197-17e1 (2017). Briefly, 170 μl of concentrated SARS-CoV-2 (8.5 logs/ml) was added to a tripartite soil load [12.5 μl of 5% BSA (Sigma A1933), 17.5 μl of 5% tryptone (Sigma T7293), and 50 μl of 4% mucin (Sigma M3895)], used to represent an organic matrix (ASTM International-E2197-17e1, 2017) and simulate the virus in its natural environment (Sattar et al., 2003). Using a positive displacement pipette, 10 μl of inoculum was deposited onto sterile stainless-steel carriers and dried for 1 h within a Class II B2 BSC. Fifty microliters of prepared disinfectant was added to carriers and incubated for 30 s, 1 min, 5 min, or 10 min, after which 950 μl of neutralizer (Table 1) was added and mixed via vigorous pipetting. The resulting neutralized solution is herein referred to as the “neat dilution.”
Neat dilutions were 10-fold serially diluted in VCM and, in replicates of five per dilution, added to 96-well plates containing 80% confluent Vero E6 cells for titration. The remaining neat material from each time point was added to 80% confluent Vero E6 cells in a six-well plate to ensure no viable virus remained. All plates were incubated at 37°C for 5 days and any cytopathic effect (CPE) was recorded (Reed and Muench, 1938). A total of three independent experiments with three biological replicates per time point were carried out for each disinfectant.
The effects of mechanical wiping were determined in accordance with the Wiperator methodology, ASTM International-E2967-15 (2015). Inoculum was prepared and deposited onto sterile stainless-steel carriers (“test carriers”) in 10-μl aliquots and dried for 1 h within a Class II B2 BSC. Carriers were placed onto the carrier plate adjacent to non-inoculated secondary carriers (“transfer carriers”) and secured with a magnet on the underside. Sterile J cloths (4 cm × 4 cm) were saturated with 320 μl of freshly prepared biocide and loaded onto the Wiperator Boss (Cutts et al., 2020a). Plates were lifted into place with test carriers subjected to 5 s of automated wiping action at 150 g of pressure. Plates were rotated and transfer carriers subjected to 5 s of wiping with the previously used J cloth. Test and transfer carriers were eluted with 1 ml of the predetermined neutralizer (see Table 1), and the presence of residual viable virus was determined both quantitatively and qualitatively as described for the QCT-2 assay. To determine the additive effect of drying on wiped surfaces, additional test carriers were wiped with each biocide as described; left to air dry for 30 s, 1 min, or 5 min; and subsequently neutralized. No transfer carriers were included for experiments with added drying times. Three independent experiments were conducted for all biocides, with the exception of 100 ppm ClO2, which was only assessed in two independent experiments.
When exposed to 66.5% EtOH, the titer of the dried inoculum (6.19 logs) decreased by 5.12 logs/carrier to 1.07 after 30 s of exposure. By 60 s, titers fell below the limit of quantitation (Figure 1), although one of nine wells showed CPE during safety testing (Table 2). Treatment with 0.5% NaOCl resulted in a log decrease of only 2.03 logs/carrier after 30 s and 3.45 logs after 1 min from an initial titer of 6.22 logs/carrier. It was only at the 5-min time point that the virus was completely inactivated by either EtOH or NaOCl in both the TCID50 assays (Figure 1) and safety tests (Table 2).
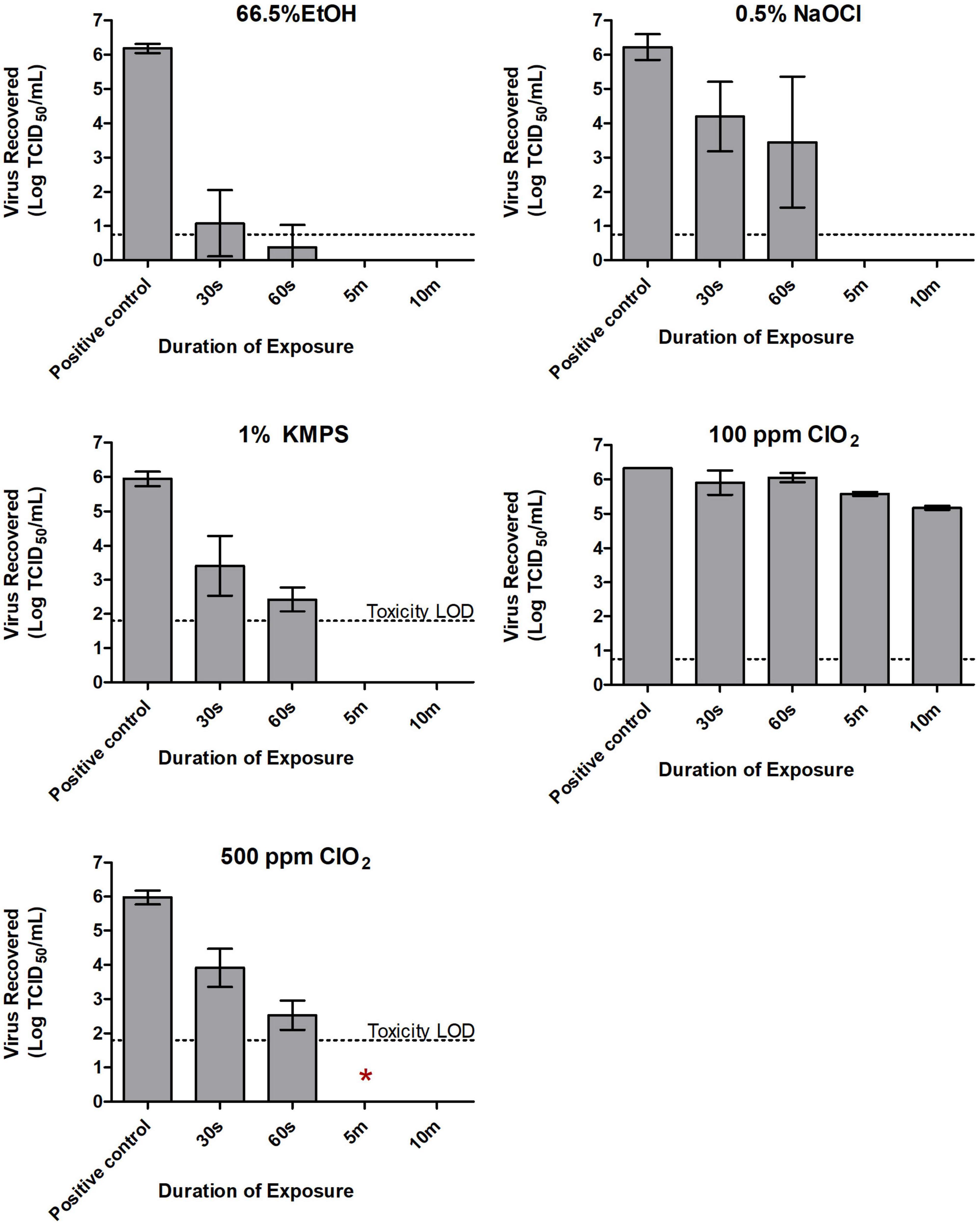
Figure 1. Inactivation of SARS-CoV-2 with 66.5% ethanol (EtOH), 0.5% sodium hypochlorite (NaOCl), 1% potassium monopersulfate (KMPS), 100 ppm chlorine dioxide (ClO2), and 500 ppm ClO2 during the quantitative carrier test 2 (QCT-2 assay). Inoculated carriers were subjected to various durations of exposure with each of the examined disinfectants. All carriers were neutralized in a pre-determined neutralizer following indicated exposure times, with subsequent viability testing in Vero E6 cells. Dashed lines indicate limits of quantification using the TCID50 assay. Toxicity LOD (i.e., limit of detection) reflects residual cytotoxicity in neat dilutions from neutralized carriers. Results represent the means of three independent experiments including three biological replicates each. Symbol *Indicates presence of viable virus in safety testing of a single biological replicate from the indicated treatment group.
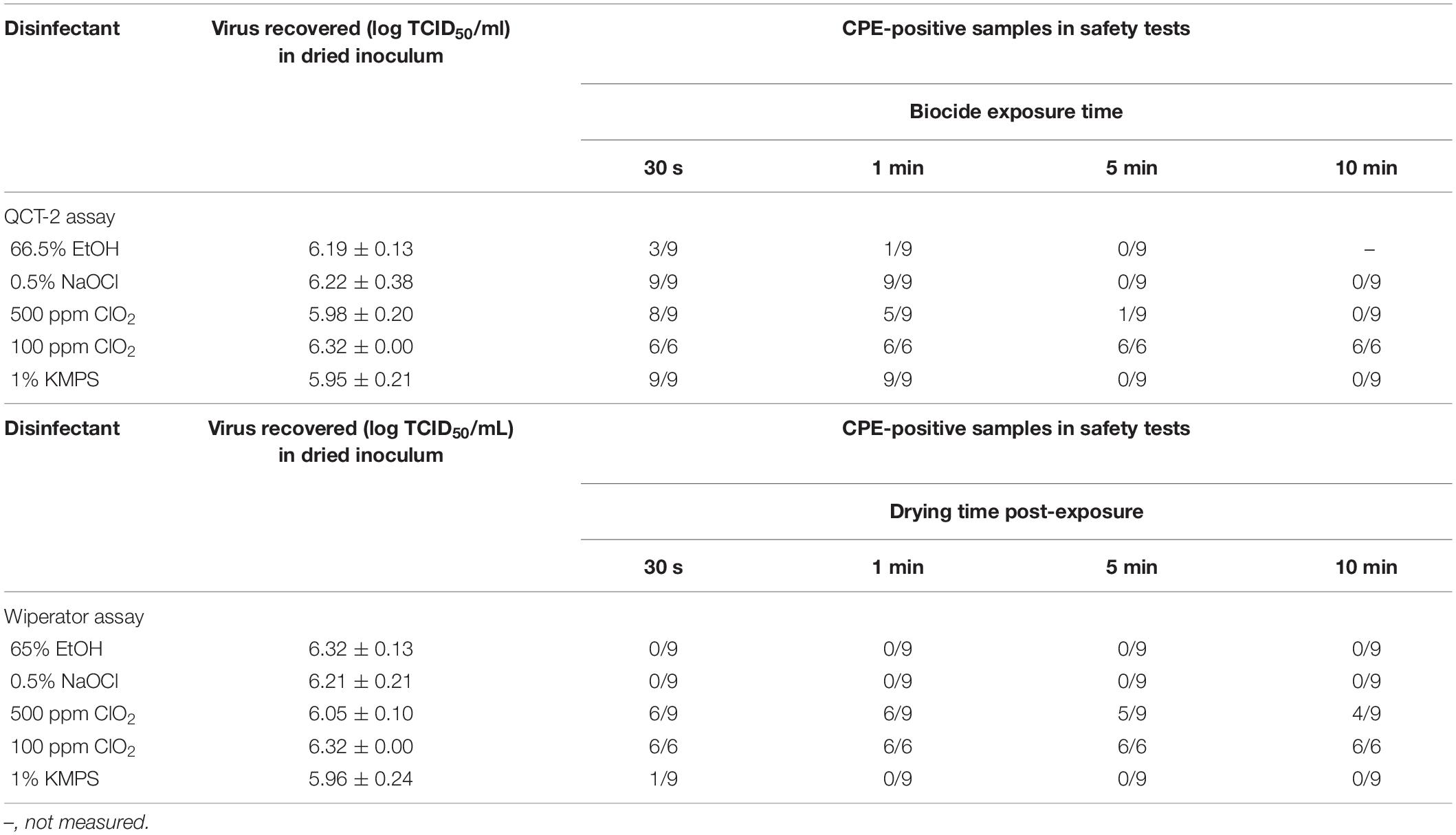
Table 2. Number of tissue culture-treated wells showing a cytopathic effect following treatment with disinfectants and disinfectant pre-soaked wipes during safety testing during the QCT-2 and Wiperator assays, respectively.
Exposing SARS-CoV-2 to ClO2 at a concentration of 100 ppm had a negligible effect on virus viability. A contact time of 30 s and 1 min with the disinfectant resulted in less than 0.5 log loss of viable virus (Figure 1). Even with 10 min of contact, only a 1.15 log decrease in viral titer was recorded (Figure 1), with all wells showing CPE in safety tests (Table 2). As such, only two independent experiments were carried out at this concentration. Increasing the concentration of ClO2 to 500 ppm produced more favorable results. An initial concentration of 5.98 logs of dried SARS-CoV-2 was reduced to 3.91 logs/carrier after a 30-s exposure (Figure 1). By 60 s, 3.45 logs of viable virus were recovered, and by 5 min, no detectable virus remained on the carrier surface in TCID50 assays (Figure 1). However, one of nine biological replicates (i.e., a single carrier from only one of three independent experiments) showed CPE in safety tests at the 5-min mark (Table 2). Increasing contact time to 10 min left no viable virus detectable in either the TCID50 assay (Figure 1) or safety test (Table 2).
Following exposure of 1% KMPS to the inoculated carriers, a 2.54 log decrease in viable virus from 5.95 to 3.41 logs/carrier occurred within 30 s and a 3.52 log decrease to 2.43 logs/carrier occurred by 1 min (Figure 1), with all nine safety test wells showing CPE (Table 2). After 5 min, no detectable virus remained in TCID50 assays (Figure 1) and safety tests (Table 2).
Incorporating the examined disinfectants into a mechanical wipe markedly reduced the time required to inactivate SARS-CoV-2. With 66.5% EtOH, 0.5% NaOCl, and 1% KMPS, no viable virus remained on inoculated test carriers after 5 s of wiping, nor did any remain on secondary transfer carrier after 5 s of wiping with the used DPW (Figure 2). Infectious virus was recovered, however, on both test and transfer carriers after treatment with the ClO2-impregnated DPWs. Not surprisingly, more virus remained on test carriers treated with the lower concentration of disinfectant, with 3.54 and 1.78 logs of SARS-CoV-2 recovered after treatment with DPWs containing 100 and 500 ppm ClO2, respectively (Figure 2). Notably, the amount of viable virus declined only slightly after the inoculated carriers were dried for increasingly longer durations post-wiping. Safety testing of the remaining neutralized viral solutions showed no viable virus for carriers treated with 66.5% EtOH or 0.5% NaOCl (Figure 2). However, a single biological replicate wiped with 1% KMPS resulted positive in safety testing, while the majority of wells were positive when using ClO2 (Table 2).
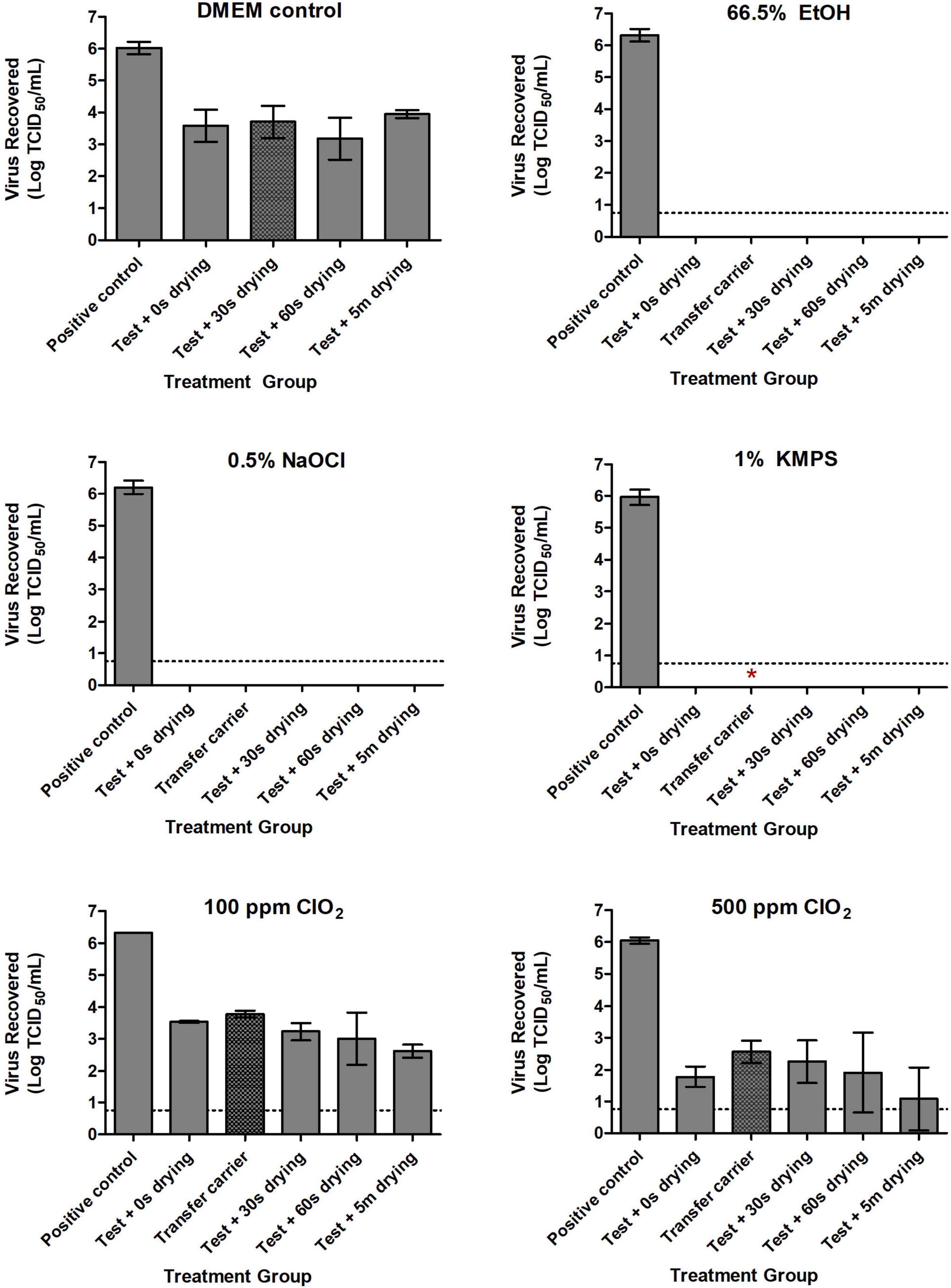
Figure 2. Inactivation of SARS-CoV-2 with Dulbecco’s Modified Eagle Medium (DMEM; positive control), 66.5% ethanol (EtOH), 0.5% sodium hypochlorite (NaOCl), 1% potassium monopersulfate (KMPS), 100 ppm chlorine dioxide (ClO2), and 500 ppm ClO2 during the Wiperator assay. Inoculated carriers were subjected to 5 s of wiping with disinfectant pre-soaked wipes infused with each of the examined disinfectants. Sterile uninoculated “transfer” carriers demonstrate transfer potential of viable virus to a clean surface through a used disinfectant presoaked wipe. All carriers were neutralized in 1 ml of a pre-determined neutralizer following indicated treatments, with subsequent viability testing in Vero E6 cells. Dashed lines indicate limits of quantification using the TCID50 assay. Results represent the means of three independent experiments including three biological replicates each. Symbol *Indicates presence of viable virus in safety testing of a single biological replicate from the indicated treatment group.
The ability of SARS-CoV-2 to remain viable on non-porous surfaces for days to weeks, depending on environmental conditions, has been well documented (Chin et al., 2020; Harbourt et al., 2020; Pastorino et al., 2020; Ren et al., 2020; Riddell et al., 2020; van Doremalen et al., 2020). This observation poses significant implications for HITES and healthcare settings where the pathogen may be abundant due to congregation and prolonged stay of infirm patients within a confined tempered space. As a generalization, enveloped viruses, including SARS-CoV-2, are more susceptible to environmental factors that non-enveloped viruses, as their phospholipid envelope is sensitive to ambient conditions such as heat, desiccation, detergents, and chemical degradation (Navaratnarajah et al., 2008; Lin et al., 2020). In this study, rather than exploiting this sensitivity, we attempt to inactivate SARS-CoV-2 as it would be found on environmental surfaces [i.e., within a protective organic matrix (Sattar et al., 2003; Terpstra et al., 2007)] using four chemical biocides, EtOH, NaOCl, KMPS, and ClO2, alone or with the addition of a mechanical wipe.
Several studies have examined the ability of DPWs, with or without incorporation of the Wiperator, to effectively decontaminate inoculated surfaces (Sattar et al., 2003; Williams et al., 2009; Sattar and Maillard, 2013; Lopez et al., 2014; ASTM International-E2967-15, 2015; Ramm et al., 2015; Becker et al., 2019; Song et al., 2019; Cutts et al., 2020a,2021). However, studies examining the effect of EtOH and NaOCl on SARS-CoV-2 have only examined their ability to inactivate the virus in liquid suspension for prolonged contact times (Chin et al., 2020). Additional inactivation studies involving these two biocides and human coronaviruses do exist; however, those involving EtOH have typically only examined various hand sanitizer formulations (Kratzel et al., 2020), while research involving NaOCl has been limited to coronaviruses existing prior to the emergence of SARS-CoV-2 (Kampf et al., 2020; Janik et al., 2021). Furthermore, the direct effects of KMPS and ClO2 on SARS-CoV-2 have yet to be reported in vitro, although both agents have displayed notable antimicrobial activity against a wide array of pathogens (Su and D’Souza, 2012; Hinenoya et al., 2015; Morin et al., 2015; Conlon-Bingham et al., 2016; Praeger et al., 2018; Sonthipet et al., 2018).
For the QCT-2 assay, the inactivation of SARS-CoV-2 upon exposure to the different disinfectants under analysis was variable. Viral titer showed the steepest decline once exposed to 66.5% EtOH, with a 5.12 log reduction after only 30 s and the majority of remaining virus below the limit of quantification after 60 s. Considering the disinfectant’s widespread incorporation into hand sanitizers targeting SARS-CoV-2 (Kratzel et al., 2020), its efficacy against other human coronaviruses (Kampf et al., 2020; Janik et al., 2021), and the widespread antimicrobial activity of alcohols in general (McDonnell and Russell, 1999; Guthery et al., 2005), these findings are not surprising. The virus displayed a more gradual inactivation profile while exposed to 0.5% NaOCl, with all viable virus inactivated by the 5-min mark. As with all of the disinfectants tested in this study, no additional time points were examined between 1 and 5 min of biocide exposure and, consequently, more precise times-to-inactivation within this range could not be determined. Interestingly, in a study of mouse hepatitis virus (MHV), a proposed potential surrogate for SARS-CoV-1, a 30-s exposure to 0.21% NaOCl inactivated >6 logs of challenge virus when dried on a non-porous surface to below the limit of detection (a 4.4-log demonstrable loss) (Dellanno et al., 2009). The use of an organic soil load in our study was likely a significant contributor to the extended exposure time required to inactivate SARS-CoV-2, despite the NaOCl concentration being 2× higher. Additionally, it is possible that differences between these coronaviruses are sufficient enough to alter their susceptibility to NaOCl. This has been seen in other closely related viruses, and it has been suggested that even minor changes in their viral composition can result in substantial differences in their inactivation kinetics (Sigstam et al., 2013; Ge et al., 2021).
Like with 66.5% EtOH and 0.5% NaOCl, complete inactivation of nearly 6 logs of dried SARS-CoV-2 was achieved after a 5-min exposure to 1% KMPS on stainless steel during the QCT-2 assay. Notably, as with 66.5% EtOH, the majority of virus (i.e., 4.25 logs) was degraded within the first minute of contact with the biocide, with full inactivation by the 5-min mark. KMPS is widely used as a chlorine-free oxidizing agent and is the main active ingredient in the multi-purpose laboratory disinfectant, Virkon (Cleanroom Technology, 2020). Virkon is known to have a wide range of antimicrobial activity against viruses, bacteria, and fungi (Gasparini et al., 1995; LANXESS, 2017) and has proven to be effective against SARS-CoV-2 (Syndel) Syndel (2019). It is noteworthy, however, that the KMPS ingredient by itself can inactivate the virus without the other reagents in Virkon (i.e., the cleaning agent sodium dodecylbenzenesulfonate and the detergent sulfamic acid), which are seemingly essential for its biocidal action.
The use of ClO2 has been shown to inactivate a variety of viruses, including SARS-CoV-2, in wastewater, albeit in conjunction with several other antimicrobial agents at low concentrations (Ge et al., 2021; Singh et al., 2021). Furthermore, ClO2 was shown to effectively inactivate SARS-CoV-1 after 30 min of exposure at a dosage of only 40 ppm (Wang et al., 2005). More recently, researchers inactivated 5 logs of SARS-CoV-2 using pure ClO2 at 80 ppm against SARS-CoV-2 in a suspension for as little as 10 s (Hatanaka et al., 2021). Our study followed the ASTM 2197-17 standard and showed that ClO2 at a lower concentration of 100 ppm did not fare as effectively against SARS-CoV-2 when dried on a hard non-porous surface, with only a 1.39-log reduction after a full 10 min of exposure. While others inactivated SARS-CoV-2 at lower concentrations of ClO2, those studies used a suspension test, had reduced protein content, used greater volumes of the ClO2, and in some cases had less virus. Our study format is a more challenging approach than a suspension test as it uses high titers of virus incorporating a higher protein content along with a mucin protectorate and uses lower amount of ClO2. These factors illustrate the importance of comparing efficiencies against biocides and their practical use under real-world conditions.
The effect of combining microbicidal actives and mechanical wiping action on the inactivation of viruses and bacteria has been evaluated in several studies (Tebbutt, 1988; Threlkeld et al., 1993; Williams et al., 2009; Abreu et al., 2013; Lopez et al., 2014; ASTM International-E2967-15, 2015; Ramm et al., 2015; Sattar et al., 2015; Edwards et al., 2017; Becker et al., 2019; Song et al., 2019; Cutts et al., 2020a). The benefits of mechanical action include its ability to remove the organic debris that could hinder the biocidal action of the disinfectant (Song et al., 2019), as well as dislodging the infectious material from its dried state to allow optimal penetration by the disinfectant. Here, we impregnated J Cloths with each of four disinfectants and employed the Wiperator to apply a continuous controlled wiping action on stainless steel carriers inoculated with SARS-CoV-2. Remarkably, after only 5 s of wiping with either 66.5% EtOH, 0.5% NaOCl, or 1% KMPS, no viable virus remained on any of the inoculated test carriers. The viral inactivation time for these three biocides during the QCT-2 assay was 5 min, signifying that the incorporated wiping action greatly increased the disinfectants’ efficacy. Furthermore, no quantifiable virus was recovered from transfer carriers after a 5-s wipe with used DPWs infused with these biocides. These data are similar to other studies assessing the wiping action of DPWs on viruses. Cutts et al. (2020a) demonstrated a complete inactivation and >6 log reduction of Ebola virus and vesicular stomatitis virus, respectively, on inoculated test carriers with no transfer of infectious virus to secondary carriers after 15 s of wiping with accelerated hydrogen peroxide (AHP)- or quaternary ammonium compound (QAC)-impregnated wipes. Moreover, Threlkeld et al. (1993) showed that wiping with DPWs infused with either isopropyl alcohol, hydrogen peroxide (H2O2), or iodophor for 5 s removed adenovirus 8 from Goldmann tonometer and pneumotonometer tips, while a 5-min submersion time was required for inactivation by the same compounds in the absence of wiping.
Although SARS-CoV-2 was resistant to degradation by 100 ppm ClO2, it was inactivated using 500 ppm (0.05%) ClO2 by the 5-min time point during the QCT-2 assay. Notably, the virus was far more susceptible to both concentrations of the biocide when wiping action was implemented. After 5 s of wiping with 100 ppm (0.01%) and 500 ppm ClO2-infused wipes, a 2.78 and 4.27 log reduction in viable virus was observed on inoculated test carriers, respectively. Considering that both concentrations of the disinfectant had negligible-to-minor effects on the virus during the QCT-2 assay (a 0.42 and 2.07 log reduction after 30 s of exposure to 100 and 500 ppm, respectively), these findings provide further support for the enhancement of viral inactivation when mechanical wiping is incorporated in the disinfection process.
Interestingly, in Wiperator tests involving ClO2, considerably high amounts of viable virus were recovered from both test and transfer carriers, indicating that transferring an infectious material from one surface to another via wiping is a legitimate concern. As such, it is imperative that any disinfection strategy involve the use of biocides proven effective against the infectious agent of concern rather than simply relying on commercial products due to ease or availability.
As seen in other studies, many factors contribute to the efficacy of antimicrobial disinfection, including the pathogen type (e.g., bacteria, viruses, spores, fungi, etc.), presence of an organic matrix, disinfectant used (e.g., type and concentration), and duration of exposure to the disinfectant (Sattar et al., 2003, 2015; Terpstra et al., 2007; Williams et al., 2009; Sattar and Maillard, 2013; Lopez et al., 2014; ASTM International-E2967-15, 2015; Ramm et al., 2015; Becker et al., 2019; Song et al., 2019; Cutts et al., 2020a; Ijaz et al., 2020). We have shown here that SARS-CoV-2 is most quickly inactivated by 66.5% EtOH, followed by 1% KMPS, 0.5% NaOCl, and then ClO2. Even when dried within a protective organic matrix, roughly 6 logs of virus can be inactivated using the three former biocides within 5 min of exposure, while 500 ppm ClO2 requires a longer duration (at least 10 min). We have also demonstrated that incorporating these biocides into a mechanical wipe greatly enhances their efficacy against the virus, and in agreement with other studies (Tebbutt, 1988; Ramm et al., 2015; Cutts et al., 2020a), infectious agents can be transferred to adjacent surfaces through wiping with a DPW if impregnated with an ineffective biocide. Nevertheless, wiping surfaces with DPWs infused with either 66.5% EtOH, 0.5% NaOCl, or 1% KMPS should be considered an effective means of inactivating viable SARS-CoV-2.
Although this study was on SARS-CoV-2 isolate Wuhan-Hu-1, we believe that the present findings would also apply to other circulating variants. Others have observed that there was no difference between SARS-CoV-1 and SARS-CoV-2 deposited on surfaces (van Doremalen et al., 2020) or in aerosol survival (Smither et al., 2020). Furthermore, other investigators have looked at the effect of biocides such as alcohol on variants and no difference was reported (Meister et al., 2021).
Here, we evaluated readily available disinfectants or components of formulated disinfectants that can be used within healthcare facilities or by the general public, either on their own or by incorporating wiping. Following simple application, ethanol was the most effective at reducing 6 logs of dried virus within a soil load in a short amount of time, while sodium hypochlorite (bleach) and KMPS required longer contact times. By incorporating a mechanical wipe, the virucidal effects of ethanol, sodium hypochlorite, and KMPS were immediate, with no detectable virus remaining after only 5 s of wiping the surface.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
SK and TC contributed to the conception and design of the study and performed the methods. AS drafted the manuscript. All authors analyzed the results, contributed to the article, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the Vaccine and Infectious Disease Organization (VIDO) in Saskatoon, SK, Canada, for kindly providing the original SARS-CoV-2 virus stock and Jay Krishnan for providing constructive comments upon review of the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.847313/full#supplementary-material
ClO2, chlorine dioxide; DPW, disinfectant pre-soaked wipe; EtOH, ethanol; HITES, high-touch environmental surfaces; KMPS, potassium monopersulfate; NaOCl, sodium hypochlorite.
Abreu, A. C., Tavares, R. R., Borges, A., Mergulhão, F., and Simões, M. (2013). Current and emergent strategies for disinfection of hospital environments. J. Antimicrob. Chemother. 68, 2718–2732. doi: 10.1093/jac/dkt281
Anderson, E. L., Turnham, P., Griffin, J. R., and Clarke, C. C. (2020). Consideration of the Aerosol Transmission for COVID-19 and Public Health. Risk Anal. 40, 902–907. doi: 10.1111/risa.13500
Asadi, S., Bouvier, N., Wexler, A. S., and Ristenpart, W. D. (2020). The coronavirus pandemic and aerosols: does COVID-19 transmit via expiratory particles? Aerosol Sci. Technol. 54, 635–638. doi: 10.1080/02786826.2020.1749229
ASTM International-E2197-17e1 (2017). Standard Quantitative Disk Carrier Test Method for Determining Bactericidal, Virucidal, Fungicidal, Mycobactericidal, and Sporicidal Activities of Chemicals. West Conshohocken: ASTM International.
ASTM International-E2967-15 (2015). Standard Test Method for Assessing the Ability of Pre-wetted Towelettes to Remove and Transfer Bacterial Contamination on Hard, Non-Porous Environmental Surfaces Using the Wiperator. West Conshohocken: ASTM International. doi: 10.1520/E2967-15
Becker, B., Henningsen, L., Paulmann, D., Bischoff, B., Todt, D., Steinmann, E., et al. (2019). Evaluation of the virucidal efficacy of disinfectant wipes with a test method simulating practical conditions. Antimicrob. Resist. Infect. Control 8:121. doi: 10.1186/s13756-019-0569-4
Bloise, I., Gómez-Arroyo, B., and García-Rodríguez, J. (2020). Detection of SARS-CoV-2 on high-touch surfaces in a clinical microbiology laboratory. J. Hosp. Infect. 105, 784–786. doi: 10.1016/j.jhin.2020.05.017
Chia, P. Y., Coleman, K. K., Tan, Y. K., Ong, S. W. X., Gum, M., Lau, S. K., et al. (2020). Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 11:2800. doi: 10.1038/s41467-020-16670-2
Chin, A. W. H., Chu, J. T. S., Perera, M. R. A., Hui, K. P. Y., Yen, H.-L., Chan, M. C. W., et al. (2020). Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 1:e10. doi: 10.1016/S2666-5247(20)30003-3
Cleanroom Technology. (2020). Lanxess plans to expand capacity for Oxone monopersulfate. Cleanroom Technol. Available online at: https://www.cleanroomtechnology.com/news/article_page/Lanxess_plans_to_expand_capacity_for_Oxone_monopersulfate/170170 (accessed June 27, 2021).
Conlon-Bingham, G., Aldeyab, M., Kearney, M. P., Scott, M. G., Baldwin, N., and McElnay, J. C. (2016). Reduction in the incidence of hospital-acquired MRSA following the introduction of a chlorine dioxide 275 ppm based disinfecting agent in a district general hospital. Eur. J. Hosp. Pharm. 23, 28–32. doi: 10.1136/ejhpharm-2014-000608
Cook, B. W. M., Cutts, T. A., Nikiforuk, A. M., Leung, A., Kobasa, D., and Theriault, S. S. (2016). The Disinfection Characteristics of Ebola Virus Outbreak Variants. Sci. Rep. 6:38293. doi: 10.1038/srep38293
Cutts, T. A., Ijaz, M. K., Nims, R. W., Rubino, J. R., and Theriault, S. S. (2019). Effectiveness of Dettol Antiseptic Liquid for Inactivation of Ebola Virus in Suspension. Sci. Rep. 9:6590. doi: 10.1038/s41598-019-42386-5
Cutts, T. A., Kasloff, S. B., Krishnan, J., Nims, R. W., Theriault, S. S., Rubino, J. R., et al. (2021). Comparison of the Efficacy of Disinfectant Pre-impregnated Wipes for Decontaminating Stainless Steel Carriers Experimentally Inoculated With Ebola Virus and Vesicular Stomatitis Virus. Front. Public Heal. 9:657443. doi: 10.3389/fpubh.2021.657443
Cutts, T. A., Robertson, C., Theriault, S. S., Nims, R. W., Kasloff, S. B., Rubino, J. R., et al. (2020b). Efficacy of microbicides for inactivation of Ebola–Makona virus on a non-porous surface: a targeted hygiene intervention for reducing virus spread. Sci. Rep. 10:15247. doi: 10.1038/s41598-020-71736-x
Cutts, T. A., Robertson, C., Theriault, S. S., Nims, R. W., Kasloff, S. B., Rubino, J. R., et al. (2020a). Assessing the Contributions of Inactivation, Removal, and Transfer of Ebola Virus and Vesicular Stomatitis Virus by Disinfectant Pre-soaked Wipes. Front. Public Heal. 8:183. doi: 10.3389/fpubh.2020.00183
Dellanno, C., Vega, Q., and Boesenberg, D. (2009). The antiviral action of common household disinfectants and antiseptics against murine hepatitis virus, a potential surrogate for SARS coronavirus. Am. J. Infect. Control 37, 649–652. doi: 10.1016/j.ajic.2009.03.012
Edwards, N. W. M., Best, E. L., Connell, S. D., Goswami, P., Carr, C. M., Wilcox, M. H., et al. (2017). Role of surface energy and nano-roughness in the removal efficiency of bacterial contamination by nonwoven wipes from frequently touched surfaces. Sci. Technol. Adv. Mater. 18, 197–209. doi: 10.1080/14686996.2017.1288543
Gasparini, R., Pozzi, T., Magnelli, R., Fatighenti, D., Giotti, E., Poliseno, G., et al. (1995). Evaluation of in vitro efficacy of the disinfectant Virkon. Eur. J. Epidemiol. 11, 193–197. doi: 10.1007/BF01719487
Gavaldà-Mestre, L., Ramírez-Tarruella, D., Gutiérrez-Milla, C., Guillamet-Roig, F., Orriols-Ramos, R., Tisner, S. R., et al. (2021). Nondetection of SARS-CoV-2 on high-touch surfaces of public areas next to COVID-19 hospitalization units. Am. J. Infect. Control 49, 840–842. doi: 10.1016/j.ajic.2021.01.007
Ge, Y., Zhang, X., Shu, L., and Yang, X. (2021). Kinetics and Mechanisms of Virus Inactivation by Chlorine Dioxide in Water Treatment: a Review. Bull. Environ. Contam. Toxicol. 106, 560–567. doi: 10.1007/s00128-021-03137-3
Gholipour, S., Nikaeen, M., Manesh, R. M., Aboutalebian, S., Shamsizadeh, Z., Nasri, E., et al. (2020). Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Contamination of High-touch Surfaces in Field Settings. Biomed. Environ. Sci. 33, 925–929. doi: 10.3967/bes2020.126
Guo, Z.-D., Wang, Z.-Y., Zhang, S.-F., Li, X., Li, L., Li, C., et al. (2020). Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerg. Infect. Dis. 26, 1583–1591. doi: 10.3201/eid2607.200885
Guthery, E., Seal, L. A., and Anderson, E. L. (2005). Zinc pyrithione in alcohol-based products for skin antisepsis: persistence of antimicrobial effects. Am. J. Infect. Control 33, 15–22. doi: 10.1016/j.ajic.2004.07.012
Harbourt, D. E., Haddow, A. D., Piper, A. E., Bloomfield, H., Kearney, B. J., Fetterer, D., et al. (2020). Modeling the stability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on skin, currency, and clothing. PLoS Negl. Trop. Dis. 14:e0008831. doi: 10.1371/journal.pntd.0008831
Harvey, A. P., Fuhrmeister, E. R., Cantrell, M. E., Pitol, A. K., Swarthout, J. M., Powers, J. E., et al. (2021). Longitudinal Monitoring of SARS-CoV-2 RNA on High-Touch Surfaces in a Community Setting. Environ. Sci. Technol. Lett. 8, 168–175. doi: 10.1021/acs.estlett.0c00875
Hatanaka, N., Xu, B., Yasugi, M., Morino, H., Tagishi, H., Miura, T., et al. (2021). Chlorine dioxide is a more potent antiviral agent against SARS-CoV-2 than sodium hypochlorite. J. Hosp. Infect. 118, 20–26. doi: 10.1016/j.jhin.2021.09.006
Hinenoya, A., Awasthi, S. P., Yasuda, N., Shima, A., Morino, H., Koizumi, T., et al. (2015). Chlorine dioxide is a better disinfectant than sodium hypochlorite against multi-drug resistant Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter baumannii. Jpn. J. Infect. Dis. 68, 276–279. doi: 10.7883/yoken.JJID.2014.294
Hu, X., Ni, W., Wang, Z., Ma, G., Pan, B., Dong, L., et al. (2021). The distribution of SARS-CoV-2 contamination on the environmental surfaces during incubation period of COVID-19 patients. Ecotoxicol. Environ. Saf. 208:111438. doi: 10.1016/j.ecoenv.2020.111438
Ijaz, M. K., Nims, R. W., Zhou, S. S., Whitehead, K., Srinivasan, V., Kapes, T., et al. (2021). Microbicidal actives with virucidal efficacy against SARS-CoV-2 and other beta- and alpha-coronaviruses and implications for future emerging coronaviruses and other enveloped viruses. Sci. Rep. 11:5626. doi: 10.1038/s41598-021-84842-1
Ijaz, M. K., Sattar, S. A., Rubino, J. R., Nims, R. W., and Gerba, C. P. (2020). Combating SARS-CoV-2: leveraging microbicidal experiences with other emerging/re-emerging viruses. PeerJ. 8:e9914. doi: 10.7717/peerj.9914
Janik, E., Bartos, M., Niemcewicz, M., Gorniak, L., and Bijak, M. (2021). SARS-CoV-2: outline, Prevention, and Decontamination. Pathogens 10:114. doi: 10.3390/pathogens10020114
Kampf, G., Todt, D., Pfaender, S., and Steinmann, E. (2020). Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 104, 246–251. doi: 10.1016/j.jhin.2020.01.022
Kasloff, S. B., Leung, A., Strong, J. E., Funk, D., and Cutts, T. (2021). Stability of SARS-CoV-2 on critical personal protective equipment. Sci. Rep. 11:984. doi: 10.1038/s41598-020-80098-3
Kozer, E., Rinott, E., Kozer, G., Bar-Haim, A., Benveniste-Levkovitz, P., Klainer, H., et al. (2021). Presence of SARS-CoV-2 RNA on playground surfaces and water fountains. Epidemiol. Infect. 149:e67. doi: 10.1017/S0950268821000546
Kratzel, A., Kratzel, A., Todt, D., V’kovski, P., Steiner, S., Steiner, S., et al. (2020). Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 by WHO-Recommended Hand Rub Formulations and Alcohols. Emerg. Infect. Dis. 26, 1592–1595. doi: 10.3201/eid2607.200915
LANXESS (2017). Virkon S. Available online at: https://virkon.us/wp-content/uploads/sites/15/2017/11/EPA-39967-137_Virkon-S_10lb_20170513. pdf (accessed March 4, 2022).
Lin, Q., Lim, J. Y. C., Xue, K., Yew, P. Y. M., Owh, C., Chee, P. L., et al. (2020). Sanitizing agents for virus inactivation and disinfection. View 1:e16. doi: 10.1002/viw2.16
Lopez, G. U., Kitajima, M., Havas, A., Gerba, C. P., and Reynolds, K. A. (2014). Evaluation of a disinfectant wipe intervention on fomite-to-finger microbial transfer. Appl. Environ. Microbiol. 80, 3113–3118. doi: 10.1128/AEM.04235-13
McDonnell, G., and Russell, A. D. (1999). Antiseptics and Disinfectants: activity, Action, and Resistance. Clin. Microbiol. Rev. 12, 147–179. doi: 10.1128/CMR.12.1.147
Meister, T. L., Fortmann, J., Todt, D., Heinen, N., Ludwig, A., Brüggemann, Y., et al. (2021). Comparable environmental stability and disinfection profiles of the currently circulating SARS-CoV-2 variants of concern B.1.1.7 and B.1.351. J. Infect. Dis. 224, 420–424. doi: 10.1093/infdis/jiab260
Morawska, L., and Cao, J. (2020). Airborne transmission of SARS-CoV-2: the world should face the reality. Elsevier 139, 1–3. doi: 10.1016/j.envint.2020.105730
Morin, T., Martin, H., Soumet, C., Fresnel, R., Lamaudière, S., Le Sauvage, A. L., et al. (2015). Comparison of the virucidal efficacy of peracetic acid, potassium monopersulphate and sodium hypochlorite on bacteriophages P001 and MS2. J. Appl. Microbiol. 119, 655–665. doi: 10.1111/jam.12870
Navaratnarajah, C. K., Warrier, R., and Kuhn, R. J. (2008). Assembly of Viruses: enveloped ParticlesB. W. J. Mahy. Encycl. Virol. 2008, 193–200. doi: 10.1016/B978-012374410-4.00667-1
Ong, S. W. X., Tan, Y. K., Chia, P. Y., Lee, T. H., Ng, O. T., Wong, M. S. Y., et al. (2020). Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) from a Symptomatic Patient. J. Am. Med. Assoc. 323, 1610–1612. doi: 10.1001/jama.2020.3227
Pastorino, B., Touret, F., Gilles, M., de Lamballerie, X., and Charrel, R. N. (2020). Prolonged Infectivity of SARS-CoV-2 in Fomites. Emerg. Infect. Dis. 26, 2256–2257. doi: 10.3201/eid2609.201788
Praeger, U., Herppich, W. B., and Hassenberg, K. (2018). Aqueous chlorine dioxide treatment of horticultural produce: effects on microbial safety and produce quality–A review. Crit. Rev. Food Sci. Nutr. 58, 318–333. doi: 10.1080/10408398.2016.1169157
Ramm, L., Siani, H., Wesgate, R., and Maillard, J. Y. (2015). Pathogen transfer and high variability in pathogen removal by detergent wipes. Am. J. Infect. Control 43, 724–728. doi: 10.1016/j.ajic.2015.03.024
Reed, L. J., and Muench, H. (1938). A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27, 493–497. doi: 10.7723/antiochreview.72.3.0546
Ren, S. Y., Wang, W. B., Hao, Y. G., Zhang, H. R., Wang, Z. C., Chen, Y. L., et al. (2020). Stability and infectivity of coronaviruses in inanimate environments. World J. Clin. Cases 8, 1391–1399. doi: 10.12998/WJCC.V8.I8.1391
Riddell, S., Goldie, S., Hill, A., Eagles, D., and Drew, T. W. (2020). The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 17, 1–7. doi: 10.1186/s12985-020-01418-7
Sattar, S. A., Bradley, C., Kibbee, R., Wesgate, R., Wilkinson, M. A. C., Sharpe, T., et al. (2015). Disinfectant wipes are appropriate to control microbial bioburden from surfaces: use of a new ASTM standard test protocol to demonstrate efficacy. J. Hosp. Infect. 91, 319–325. doi: 10.1016/j.jhin.2015.08.026
Sattar, S. A., and Maillard, J. Y. (2013). The crucial role of wiping in decontamination of high-touch environmental surfaces: review of current status and directions for the future. Am. J. Infect. Control 41, S97–S104. doi: 10.1016/j.ajic.2012.10.032
Sattar, S. A., Springthorpe, V. S., Adegbunrin, O., Zafer, A. A., and Busa, M. (2003). A disc-based quantitative carrier test method to assess the virucidal activity of chemical germicides. J. Virol. Methods 112, 3–12. doi: 10.1016/S0166-0934(03)00192-7
Shah, M. R., Jan, I., Johns, J., Singh, K., Kumar, P., Belarmino, N., et al. (2021). SARS-CoV-2 nosocomial infection: real-world results of environmental surface testing from a large tertiary cancer center. Cancer 127, 1926–1932. doi: 10.1002/cncr.33453
Sigstam, T., Gannon, G., Cascella, M., Pecson, B. M., Wigginton, K. R., and Kohn, T. (2013). Subtle differences in virus composition affect disinfection kinetics and mechanisms. Appl. Environ. Microbiol. 79, 3455–3467. doi: 10.1128/AEM.00663-13
Singh, S., Kumar, V., Kapoor, D., Dhanjal, D. S., Bhatia, D., Jan, S., et al. (2021). Detection and disinfection of COVID-19 virus in wastewater. Environ. Chem. Lett. 19, 1917–1933. doi: 10.1007/s10311-021-01202-1
Smither, S. J., Eastaugh, L. S., Findlay, J. S., and Lever, M. S. (2020). Experimental aerosol survival of SARS-CoV-2 in artificial saliva and tissue culture media at medium and high humidity. Emerg. Microbes Infect. 9, 1415–1417. doi: 10.1080/22221751.2020.1777906
Song, X., Vossebein, L., and Zille, A. (2019). Efficacy of disinfectant-impregnated wipes used for surface disinfection in hospitals: a review. Antimicrob. Resist. Infect. Control 8, 1–14. doi: 10.1186/s13756-019-0595-2
Sonthipet, S., Ruenphet, S., and Takehara, K. (2018). Bactericidal and virucidal efficacies of potassium monopersulfate and its application for inactivating avian influenza virus on virus-spiked clothes. J. Vet. Med. Sci. 80, 568–573. doi: 10.1292/jvms.17-0599
Su, X., and D’Souza, D. H. (2012). Inactivation of human norovirus surrogates by benzalkonium chloride, potassium peroxymonosulfate, tannic acid, and gallic acid. Foodborne Pathog. Dis. 9, 829–834. doi: 10.1089/fpd.2012.1155
Syndel (2019). Virkon-s. Virkon-s Added to Epa n List; Virkon now Meets Epa’s Criteria for Use Against Sars-cov-2, the Virus that Causes Covid-19. Available online at: https://syndel.com/product/virkon-s/ (accessed June 29, 2021).
Tebbutt, G. M. (1988). Laboratory evaluation of disposable and reusable disinfectant cloths for cleaning food contact surfaces. Epidemiol. Infect. 101, 367–375. doi: 10.1017/S0950268800054315
Terpstra, F. G., van den Blink, A. E., Bos, L. M., Boots, A. G. C., Brinkhuis, F. H. M., Gijsen, E., et al. (2007). Resistance of surface-dried virus to common disinfection procedures. J. Hosp. Infect. 66, 332–338. doi: 10.1016/j.jhin.2007.05.005
Threlkeld, A. B., Froggatt, J. W., Schein, O. D., and Forman, M. S. (1993). Efficacy of a Disinfectant Wipe Method for the Removal of Adenovirus 8 from Tonometer Tips. Ophthalmology 100, 1841–1845. doi: 10.1016/S0161-6420(93)31388-6
van Doremalen, N., Bushmaker, T., Morris, D. H., Holbrook, M. G., Gamble, A., Williamson, B. N., et al. (2020). Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 382, 1564–1567. doi: 10.1056/nejmc2004973
Wan, B., Zhang, X., Luo, D., Zhang, T., Chen, X., Yao, Y., et al. (2021). On-site analysis of COVID-19 on the surfaces in wards. Sci. Total Environ. 753:141758. doi: 10.1016/j.scitotenv.2020.141758
Wang, X. W., Li, J. S., Jin, M., Zhen, B., Kong, Q. X., Song, N., et al. (2005). Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods 126, 171–177. doi: 10.1016/j.jviromet.2005.02.005
Wang, Y., Qiao, F., Zhou, F., and Yuan, Y. (2020). Surface distribution of severe acute respiratory syndrome coronavirus 2 in Leishenshan Hospital in China. Indoor Built Environ. 26:1583. doi: 10.1177/1420326X20942938
Wei, L., Huang, W., Lu, X., Wang, Y., Cheng, L., Deng, R., et al. (2020). Contamination of SARS-CoV-2 in patient surroundings and on personal protective equipment in a non-ICU isolation ward for COVID-19 patients with prolonged PCR positive status. Antimicrob. Resist. Infect. Control 9, 1–5. doi: 10.1186/s13756-020-00839-x
World Health Organization [WHO] (2020). Coronavirus Disease (COVID-19): how is it Transmitted?. Switzerland: World Health Organization. www.who.int. Available online at: https://www.who.int/health-topics/coronavirus (accessed March 2, 2021).
World Health Organization [WHO] (2021). Roadmap to Improve and Ensure good Indoor Ventilation in the Context of COVID-19. Switzerland: World Health Organization.
Williams, G. J., Denyer, S. P., Hosein, I. K., Hill, D. W., and Maillard, J. Y. (2007). The development of a new three-step protocol to determine the efficacy of disinfectant wipes on surfaces contaminated with Staphylococcus aureus. J. Hosp. Infect. 67, 329–335. doi: 10.1016/j.jhin.2007.08.012
Williams, G. J., Denyer, S. P., Hosein, I. K., Hill, D. W., and Maillard, J.-Y. (2009). Limitations of the Efficacy of Surface Disinfection in the Healthcare Setting. Infect. Control Hosp. Epidemiol. 30, 570–573. doi: 10.1086/597382
Yang, Q., Saldi, T. K., Gonzales, P. K., Lasda, E., Decker, C. J., Tat, K. L., et al. (2021). Just 2% of SARS-CoV-2-positive individuals carry 90% of the virus circulating in communities. Proc. Natl. Acad. Sci. U. S. A. 118, 1–24. doi: 10.1073/pnas.2104547118
Zeitler, B., and Rapp, I. (2014). Surface-dried viruses can resist glucoprotamin-based disinfection. Appl. Environ. Microbiol. 80, 7169–7175. doi: 10.1128/AEM.02462-14
Zhang, S., Wang, C., Lin, M., Deng, Q., Ye, Y., Li, Z., et al. (2020). Analysis of the Virus Contamination and Disinfection Effect in Isolation Ward of Patients With COVID-19. Front. Public Heal. 8:486. doi: 10.3389/fpubh.2020.00486
Zhou, J., Otter, J. A., Price, J. R., Cimpeanu, C., Garcia, D. M., Kinross, J., et al. (2020). Investigating SARS-CoV-2 Surface and Air Contamination in an Acute Healthcare Setting During the Peak of the COVID-19 Pandemic in London. United States: Cold Spring Harbor Laboratory. doi: 10.1093/cid/ciaa905
Keywords: SARS-CoV-2, biocide, disinfection, fomites, QCT-2, wiping, wipe
Citation: Sloan A, Kasloff SB and Cutts T (2022) Mechanical Wiping Increases the Efficacy of Liquid Disinfectants on SARS-CoV-2. Front. Microbiol. 13:847313. doi: 10.3389/fmicb.2022.847313
Received: 02 January 2022; Accepted: 03 February 2022;
Published: 22 March 2022.
Edited by:
Matteo Guidotti, National Research Council (CNR), ItalyReviewed by:
Davide Mileto, Luigi Sacco Hospital, ItalyCopyright © 2022 Sloan, Kasloff and Cutts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Todd Cutts, dG9kZC5jdXR0c0BwaGFjLWFzcGMuZ2MuY2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.