
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 22 March 2022
Sec. Food Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.846653
This article is part of the Research Topic Insights in Food Microbiology: 2021 View all 14 articles
Staphylococcus aureus and staphylococcal enterotoxins are a serious public health concern associated with hospital and community-acquired illnesses. Dairy animals frequently shed S. aureus into the milk supply which can lead to food poisoning in humans. This study aims to investigate the prevalence and genetic diversity of S. aureus and staphylococcal enterotoxins in raw milk from the main dairy region of mainland Portugal. S. aureus was found in 53.0% (95% CI: 40.6–65.4%) of 100 raw cow’s milk samples collected from bulk cooling tanks. The highest contamination level was 3.4 log10 CFU.mL–1, and in some samples more than one S. aureus strain was identified. Staphylococcal enterotoxins (SEA-SEE) were detected in one sample. Spa typing revealed 62 distinct S. aureus isolates, being t529 (17.7%, 95% CI: 8.2–27.3%) and t1403 (16.1%, 95% CI: 7.0–25.3%) the predominant types, commonly associated with livestock infection or carriage. The antimicrobial susceptibility test showed that 35.5% of the S. aureus isolates were resistant to at least one antimicrobial agent, with resistance to penicillin being the highest (32.3%, 95% CI: 20.6–43.9%) followed by tetracycline (24.2%, 95% CI: 13.5–34.9%), ciprofloxacin (16.1%, 95% CI: 7.0–25.3%) and chloramphenicol (16.1%, 95% CI: 7.0–25.3%). Moreover, five isolates (8.1%, 95% CI: 1.3–14.8%) were identified as methicillin-resistant S. aureus (MRSA, cefoxitin resistant). Regarding virulence/resistance genes, 46,8% (95% CI: 34.4–59.2%) isolates harbored at least one enterotoxin-encoding gene, and the seg gene was the most frequently detected (41.9%, 95% CI: 29.7–54.2%) followed by the sei (40.3%, 95% CI: 28.1–52.5%), sec (6.5%, 95% CI: 0.3–12.6%), seh (4.8%, 95% CI: 0.0–10.2%), and sea (1.6%, 95% CI: 0.0–4.7%) genes. Five (8.1%, 95% CI: 1.3–14.8%) non-enterotoxigenic isolates carried the mecA gene (corresponding to isolates phenotypically classified as MRSA), and 4.8% (95% CI: 0.0–10.2%) enterotoxigenic strains also had the tsst-1 gene. Our study confirm that raw milk can be a zoonotic source of S. aureus, including enterotoxigenic and MRSA strains. Furthermore, the majority of enterotoxigenic isolates were found to contain genes encoding SEs (SEG, SEH and SEI) not routinely screened. This shows the need for a broader SE screening in food safety control, as well as the relevance of risk mitigation measures to control S. aureus transmission along the food chain in Portugal.
Food and water are well-known vectors for the dissemination of zoonotic microorganisms, some of them can be extremely harmful to human health (Gallo et al., 2020). Foodborne diseases are a serious public health concern, associated with losses in productivity and high medical expenses every year (Garcia et al., 2020). Milk, as a central food in the human diet is a critical vehicle of both beneficial and pathogenic microorganisms. Several pathogens including Brucella spp., Campylobacter spp., Shiga toxin-producing Escherichia coli, Listeria monocytogenes, Mycobacterium spp., Salmonella spp., and also bacterial toxins have been associated with milk-borne diseases (Dhanashekar et al., 2012). Furthermore, there has been a drastic increase in antimicrobial resistance among zoonotic pathogens. Thus, food surveillance is a major concern for the food industry and for public health (Pérez-Rodríguez and Mercanoglu Taban, 2019).
Staphylococcus aureus is ubiquitous in the environment and a major cause of bovine mastitis. Thus, milk is a common source of contamination for the dairy supply chain, the environment, as well as for final consumers (Rola et al., 2016). In the European Union, 43 foodborne outbreaks (FBO), 402 cases and 32 hospitalizations associated to this pathogen were reported in 2020, according to the recent European Food Safety Authority report (European Food Safety Authority and European Centre for Disease Prevention and Control, 2021b). Typically, staphylococcal food poisoning (SFP) are caused by the ingestion of food contaminated with preformed staphylococcal enterotoxins (SEs) produced by coagulase-positive staphylococci (CPS) (Argudín et al., 2010). There are 24 SEs and enterotoxin like (SEl-) toxins currently identified, including the classical (SEA-SEE) and the newer (SEG-SElY), which are encoded on different pathogenicity islands (Fisher et al., 2018). Most SFP outbreaks are reported in countries where consumption of unpasteurized milk cheeses is common, such as France and Italy. Additionally, raw milk vending machines and traditionally made food products, such as cheese manufactured at local dairy farms, are becoming more popular throughout Europe, increasing the risk of SFP (Rola et al., 2016).
Staphylococcus aureus can also produce several other extracellular virulent proteins such as toxic shock syndrome toxin 1 (TSST-1), Panton-Valentine Leukocidin (PVL), hemolysins, and coagulase. These proteins can contribute to a broad spectrum of pathologies beyond food poisoning that can range from toxin-mediated syndromes to fatal systemic diseases (Chambers and DeLeo, 2009). Moreover, this pathogen is a well-known example of acquired resistance to multiple antibiotics. Methicillin-resistant Staphylococcus aureus (MRSA) are of particular concern to human health because it is virtually resistant to all available β-lactam antibiotics and represent a significant cause of morbidity and mortality throughout the world. These have often been found outside the health environment, including in farm animals (van Duijkeren et al., 2014; Wu et al., 2018). Surveillance of raw milk is therefore essential for a better understanding of the risk factors along the milk food chain and to guarantee public health safety.
In Portugal, data on S. aureus circulating in raw milk are scarce. Pereira et al. (2009) characterized several S. aureus isolates from different foods in Portugal, including a limited number of raw milk samples, and detected the presence of enterotoxigenic S. aureus. Molecular typing can be a powerful tool for improve such epidemiological studies with data about clonal relatedness, genetic diversity, and tracking the spread of pathogens. S. aureus protein A (spa) typing is a rapid, affordable, and easy molecular typing method that assigns a classification to S. aureus strains from the number/sequence variation in repeats at a specific region of the spa gene. It offers excellent discriminatory results to the study of S. aureus diversity (Sabat et al., 2013). Molecular typing and characterization of virulence factors are thus an important tool in the control of zoonotic diseases.
The aim of this study was to determine the prevalence and diversity of S. aureus and staphylococcal enterotoxins in raw cow’s milk collected from bulk cooling tanks on dairy farms from the main dairy region of mainland Portugal. Genetic determinants associated with enterotoxinogenicity (i.e., SE-encoding genes), antimicrobial resistance (i.e., mecA and mecC genes) and severe human infections (i.e., pvl and tsst-1 genes) were investigated.
Between November 2020 and August 2021, 100 raw cow’s milk samples were collected from bulk cooling tanks of 100 dairy farms located in the “Bacia Leiteira Primária de Entre Douro e Minho.” Only dairy cow farms were included in this study. One-liter samples were collected in sterile labeled screwed top bottles, quickly stored at 4°C and analyzed within 24 h. Farms participation was voluntary and anonymous. Information on sampling and number of milking cows of each dairy farm can be found in Supplementary Table 1.
The presence of staphylococcal enterotoxins A, B, C, D, and E on raw milk samples was analyzed by a two-step method: extraction/concentration and toxin detection by enzyme linked fluorescent assay (ELFA) with the VIDAS SET2 test (bioMerieux, Marcy-I’Etoile, France) according to ISO 19020:2017 (International Organization for Standardization [ISO], 2017). Confidence Interval (CI), for proportions (Wald method), were determined considering a 95% CI (critical z value of 1.96), prevalence/frequency values and the sample size.
Bulk tank milk samples were analyzed according to ISO 6888–2 (International Organization for Standardization [ISO], 2021) method for the enumeration of coagulase-positive staphylococci (CPS). Briefly, 1 mL of serial dilutions of raw milk were plated on Baird-Parker agar with rabbit plasma fibrinogen (bioMerieux). After 48 h of incubation at 37 ± 1°C, the number of colonies displaying a phenotype characteristic for CPS were counted and morphologically different colonies were subcultured on tryptic soya agar (TSA) for further identification.
Then, colonies identified as CPS were confirmed as S. aureus by the presence of the nuc gene. For this, total DNA of each colony was extracted using the boiling method (95°C for 15 min). The suspensions were centrifuged at 12,000 g for 5 min and supernatants employed as DNA template. The primers used to amplify the nuc gene had the sequences forward: 5′-GCGATTGATGGTGATACGGTT-3 and reverse: 5′- AGCCAAGCCTTGACGAACTAAAGC-3, as described by Brakstad et al. (1992). The PCR amplification consisted of a reaction mixture containing 1X Supreme NZYTaq II 2 × Green Master Mix (NZYTech, Portugal), 0.25 μM of each primer and 2 μL of DNA. Thermal cycling reaction conditions used were: initial denaturation at 95°C for 5 min, 35 cycles of denaturation at 94°C for 30 s, annealing for 30 s at 56°C and extension at 72°C for 30 s, and a final extension at 72°C for 5 min. S. aureus ATCC 25923 and S. epidermidis CECT 231 were used as positive control and negative control, respectively. The PCR products were subjected to electrophoresis at 100 V for 1 h in a 1.5% (w/v) agarose gel, previous stained with GreenSafe Premium (NZYTech), and finally analyzed under UV light. CIs for prevalence of CPS were determined by the Wald method as mentioned in “Detection of Staphylococcal Enterotoxins” section.
Spa typing was performed as described by Shopsin et al. (1999) and Roussel et al. (2015). Briefly, amplification and sequencing of the spa gene were performed using the total DNA of each S. aureus isolate. The PCR amplification consisted of a reaction mixture containing 1X NZYProof Green Master Mix (NZYTech), 0.2 μM of primers spa-1113f (5′-TAA AGA CGA TCC TTC GGT GAG C-3′) and spa-1514r (5′-CAG CAG TAG TGC CGT TTG CTT-3′) and 2 μL of DNA template (Shopsin et al., 1999). Thermal cycling reaction conditions used were: 95°C for 5 min for the initial denaturation, followed by 35 cycles of denaturation at 94°C for 30 s, annealing for 30 s at 56°C and extension at 72°C for 30 s. A final extension was set at 72°C for 5 min. Amplification was confirmed by electrophoresis on a 2% (w/v) agarose gel at 100 V for 1 h, previous stained with GreenSafe Premium (NZYTech). Both strands were then purified and sequenced by I3S genomics platform (Porto, Portugal). The sequences were analyzed using automated workflow provided by BioNumerics software (bioMérieux) which analyze raw sequencer trace files and assign the repeat codes and spa types in connection to SeqNet/Ridom Spa Server1. CIs for prevalence of spa types were determined by the Wald method as mentioned in “Detection of Staphylococcal Enterotoxins” section.
Antimicrobial susceptibility profile for the S. aureus isolates was determined by the Kirby–Bauer disk diffusion method and interpreted according to the criteria of the Clinical and Laboratory Standards Institute guidelines (Clinical and Laboratory Standards Institute [CLSI], 2020, 2021). Briefly, a colony suspension of each S. aureus isolate was resuspended in saline solution at 0.5 McFarland standard. The suspension was streaked on Muller-Hinton Agar (Oxoid, Basingstoke, United Kingdom) and allowed to dry. Then, the antibiotic disks were placed on the medium and incubated at 37°C for 16–18h. The incubation time was extended to 24 h for cefoxitin, which was used as a surrogate test for methicillin resistance. After the appropriate incubation time, the zones of inhibition were measured and interpreted as sensitive (S), intermediate (I), and resistant (R). The following antimicrobials agents were used: penicillin G (PG, 10 IU), cefoxitin (FOX, 30 μg), ceftaroline (CPT, 5 μg), cefoperazone (CFP, 30 μg), ceftiofur (EFT, 30 μg), tetracycline (TE, 30 μg), chloramphenicol (C, 30 μg), gentamicin (CN, 10 μg), trimethoprim-sulfamethoxazole (SXT, 1.25/23.75 μg), trimethoprim (TM, 5 μg), sulfonamides (S, 300 μg), erythromycin (E, 15 μg), ciprofloxacin (CIP, 5 μg), clindamycin (DA, 2 μg), quinupristin-dalfopristin (QD, 15 μg), and linezolid (LZD, 30 μg). S. aureus strains ATCC 25923 was used as quality control. CIs for antimicrobial susceptibility ratios were determined by the Wald method as mentioned in “Detection of Staphylococcal Enterotoxins” section.
The isolates identified as S. aureus were screened for the presence of 11 main SE-encoding genes suspected to cause SFP outbreaks (sea, seb, sec, sed, see, ser, seg, seh, sei, sej, and sep) using two multiplex PCR assays according to the European Reference Laboratory (EURL) official method (Roussel et al., 2015). For sea, seb, sec, sed, see, and ser genes, the PCR amplification consisted of a reaction mixture containing 1X Supreme NZYTaq II 2 × Green Master Mix (NZYTech), 0.2 μM of each primer for sea, seb, sec, ser, 0.8 μM of each primer for sed, 0.6 μM of each primer for see and 2 μL of DNA template. Regarding the seg, seh, sei, sej and sep genes, the PCR amplification consisted of a reaction mixture containing 1X Supreme NZYTaq II 2 × Green Master Mix (NZYTech), 0.4 μM of each primer for seh, 0.6 μM of each primer for seg, 0.8 μM of each primer for sei and sep, 1.0 μM of each primer for sej and 2 μL of DNA template. Thermal cycling reaction conditions used were an initial denaturation at 95°C for 5 min, 35 cycles of denaturation at 94°C for 30 s, annealing for 30 s at 58°C and extension at 72°C for 30 s, and a final extension at 72°C for 5 min. Five reference S. aureus strains supplied by EURL CPS were used as positive controls (FRI137 for sec, seg, seh, sei; FRI361 for seg, sei, sej, sec, sed, ser; FRIS6 for sea, seb; FRI326 for see; and A900322 for sei, seg, sep). The PCR products were subjected to electrophoresis at 100 V for 1 h in a 2.5% (w/v) agarose gel, previous stained with GreenSafe Premium (NZYTech), and finally analyzed under UV light. CIs for prevalence of SE genes were determined by the Wald method as mentioned in “Detection of Staphylococcal Enterotoxins” section.
S. aureus isolates were, also, characterized regarding the presence of methicillin resistance genes (mecA and mecC), as well as pvl and tsst1 virulence genes. For the detection of mecA, mecC and pvl genes, a multiplex reaction was used as described by EURL protocol (Stegger et al., 2012). The PCR amplification consisted of a reaction mixture containing 1X Supreme NZYTaq II 2 × Green Master Mix (NZYTech), 0.2 μM of each primer for mecA, mecC and pvl and 2 μL of DNA template. The detection of the tsst1 gene was performed using the primers described by Johnson et al. (1991) and a reaction mixture containing 1X Supreme NZYTaq II 2 × Green Master Mix (NZYTech), 0.5 μM of each primer for tsst1 and 2 μL of DNA template. The thermal cycling reaction conditions used for both PCR were an initial denaturation at 95°C for 5 min, 35 cycles of denaturation at 94°C for 30 s, annealing for 30 s at 56°C for mecA, mecC and pvl genes or 54°C for tsst1 gene, extension at 72°C for 30 s, and a final extension 72°C for 5 min. The PCR products were subjected to electrophoresis at 100 V for 1 h in a 2.0% (w/v) agarose gel, previous stained with GreenSafe Premium (NZYTech), and finally analyzed under UV light. All PCR reactions were performed in 20 μL and using MJ Mini Personal Thermal Cycler (Bio-Rad). CIs for the prevalence of virulence/resistance genes were determined by the Wald method as mentioned in “Detection of Staphylococcal Enterotoxins” section.
CPS were identified in 54.0% (95% CI: 41.6–66.4%) of raw milk samples and the number of colonies forming units (CFU) ranged between 0 and 3.4 log10 CFU.mL–1 (Figure 1A). One hundred and ten morphologically different CPS colonies were isolated, which means that in some cases more than one colony per raw milk sample was CPS characteristic and catalase positive. All isolates were tested for the nuc gene, and from the 110 CPS isolated, 104 isolates were confirmed as S. aureus. The remaining six CPS isolates were identified as S. hyicus (API® Staph, bioMérieux). Of the 54 samples positive for CPS, S. aureus strains were identified in 53 (53.0%, 95% CI: 40.6–65.4%) of the samples.
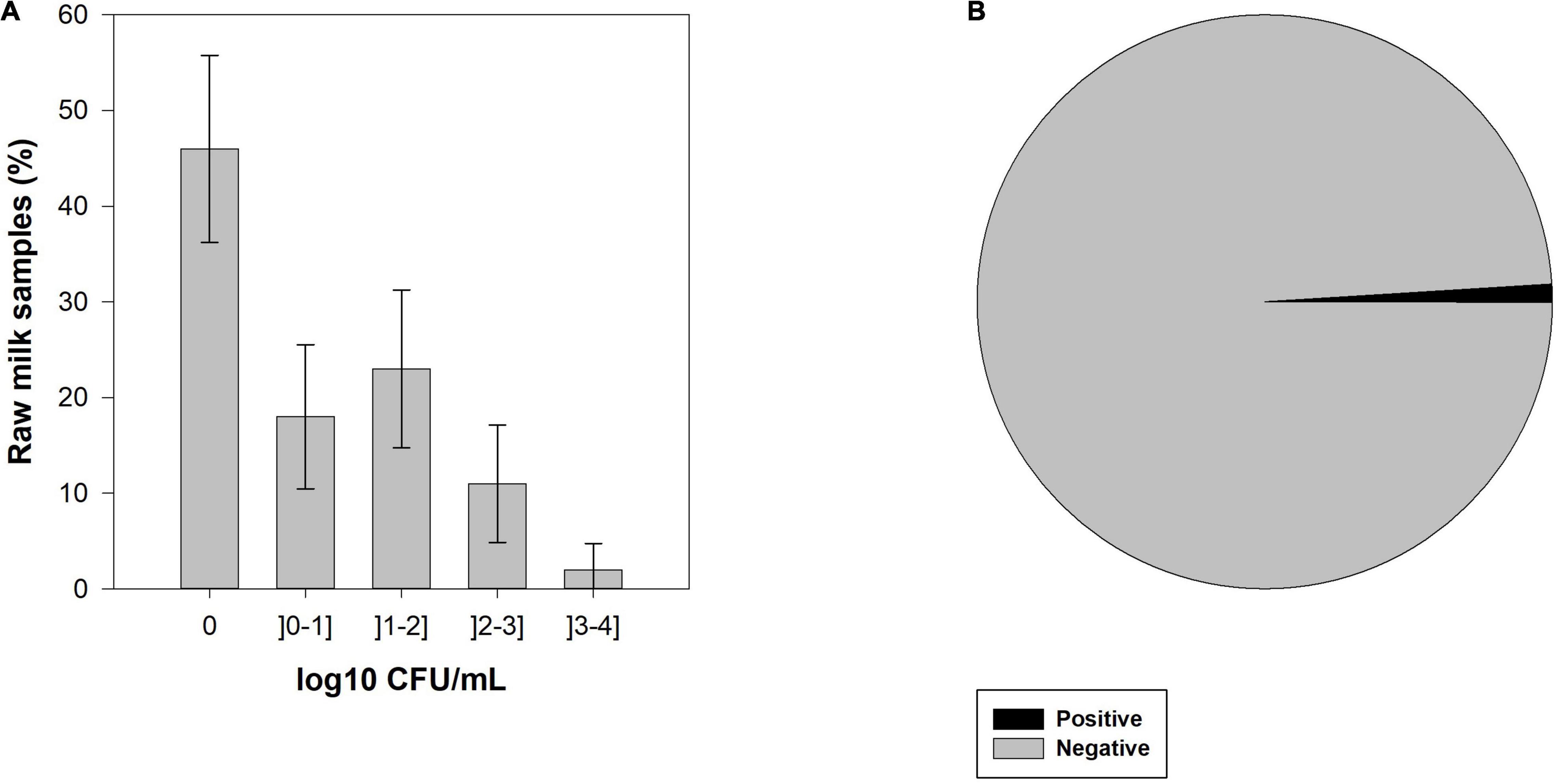
Figure 1. (A) Enumeration of bulk tank milk samples with coagulase-positive staphylococci (CPS) by colony forming units (CFU) categories and its 95% confidence interval (CI), and (B) prevalence of staphylococcal enterotoxins (SEs) in raw cow’s milk samples from bulk cooling tanks of 100 dairy farms located in the main dairy basin of mainland Portugal.
From 100 bulk tank milk samples, only one was positive for the presence of SEA-SEE (Figure 1B). Interestingly, the SEs-positive sample was negative for the presence of CPS and, consequently, no S. aureus was isolated from that sample.
Among the 104 S. aureus isolates, 25 different spa types were detected. As the same S. aureus type was identified in different colonies from the same raw milk sample, only 62 S. aureus strains from different raw milk samples were considered as distinct and used for further characterization. In terms of prevalence, the most common spa type was t529 (17.7%, 95% CI: 8.2–27.3%) followed by t1403 (16.1%, 95% CI: 7.0–25.3%), t337 (9.7%, 95% CI: 2.3–17.0%), t543 (9.7%, 95% CI: 2.3–17.0%), and t011 (8.1%, 95% CI: 1.3–14.8%). Other spa types, such as t528, t571, t2802, and t2873 were associated with two (3.2%, 95% CI: 0.0–7.6%) distinct isolates, while t002, t108, t117, t127, t189, t208, t267, t843, t899, t1200, t1207, t1334, t2383, t3585, t9216, and t19272, were associated to one (1.6%, 95% CI: 0.0–4.7%) S. aureus isolate (Figure 2).
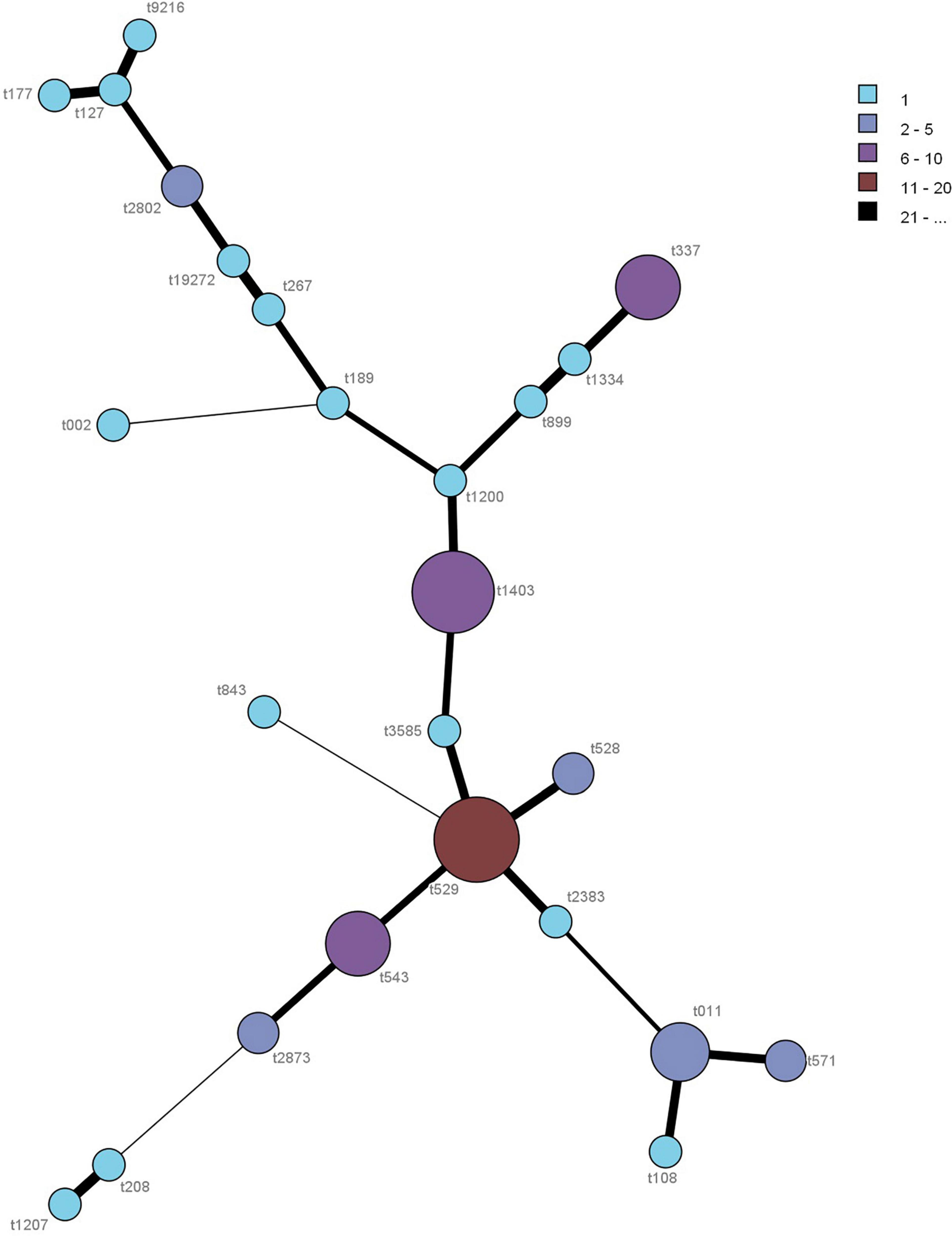
Figure 2. Minimum spanning tree of the spa typing for the 62 S. aureus isolates. Each spa type is depicted by a single node, with the size of the node representing the number of isolates associated with this spa type.
The antimicrobial susceptibility of the 62 S. aureus isolates to 16 antimicrobial agents are shown in Figure 3. The highest resistance was observed for penicillin G (32.3%, 95% CI: 20.6–43.9%) followed by tetracycline (24.2%, 95% CI: 13.5–34.9%), chloramphenicol (16.1%, 95% CI: 7.0–25.3%) and ciprofloxacin (16.1%, 95% CI: 7.0–25.3%), and to a lesser extent clindamycin (14.5%, 95% CI: 5.7–23.3%), erythromycin (12.9%, 95% CI: 4.6–21.2%), trimethoprim (12.9%, 95% CI: 4.6–21.2%), gentamicin (9.7%, 95% CI: 2.3–17.0%), cefoperazone (8.1%, 95% CI: 1.3–14.8%), ceftiofur (8.1%, 95% CI: 1.3–14.8%), sulfonamides (4.8%, 95% CI: 0–10.2%), and trimethoprim–sulfamethoxazole (1.6%, 95% CI: 0–4.7%). No resistance was observed for ceftaroline, quinupristin-dalfopristin and linezolid. In addition, five isolates (8.1%, 95% CI: 1.3–14.8%) were identified as methicillin-resistant strains (cefoxitin resistance) and showed complete resistance to all other β-lactams tested, except to ceftaroline. Overall, 64.5% (95% CI: 52.6–76.4%) of the S. aureus isolates were susceptible to all antimicrobial agents tested and 35.5% (95% CI: 23.6–47.4%) of the S. aureus isolates were resistant to at least one of the antimicrobial agents tested, being that 9.7% (95% CI: 2.3–17.0%) were resistant to only one, 3.2% (95% CI: 0–7.6%) to two, and 22.6% (95% CI: 12.2–33.0%) were multi-drug resistant (resistant to three or more antimicrobial agents of different classes). Individual resistance profiles can be found in the Supplementary Table 2.
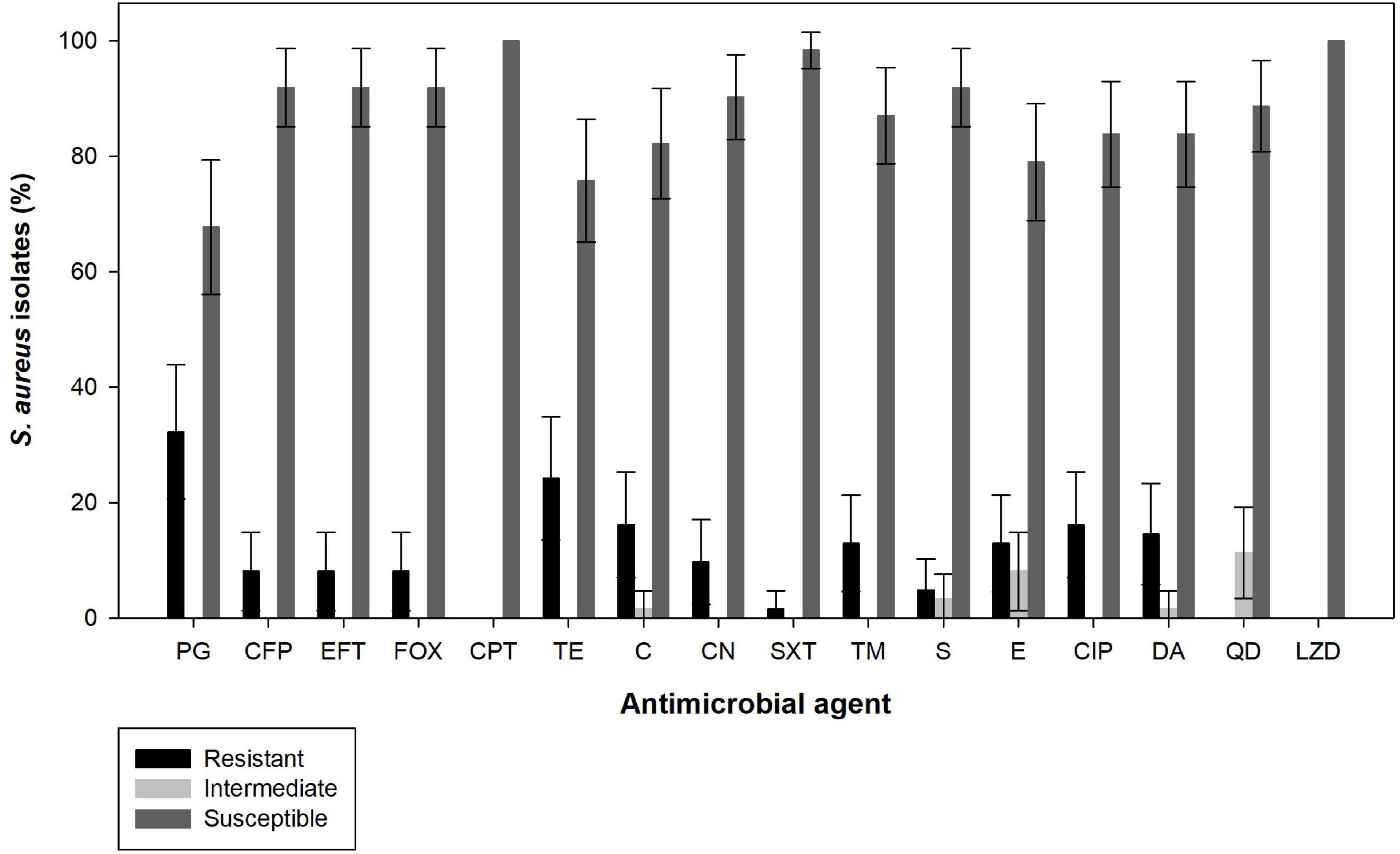
Figure 3. Antimicrobial susceptibility of S. aureus isolated from raw cow’s milk samples collected from bulk cooling tanks of 100 dairy farms located in the from main dairy basin in of mainland Portugal. PG, Penicillin G; FOX, cefoxitin; CPT, ceftaroline; CFP, cefoperazone; EFT, ceftiofur; TE, tetracycline; C, chloramphenicol; CN, gentamicin; SXT, trimethoprim-sulphamethoxazole; TM, trimethoprim; S, sulfonamides; E, erythromycin; CIP, ciprofloxacin; DA, clindamycin; QD, quinupristin-dalfopristin; LZD, linezolid.
Among the 62 S. aureus isolates, 46.8% (95% CI: 34.4–59.2%) harbored at least one of the SEs gene analyzed, 35.5% (95% CI: 23.6–47.4%) isolates had two SE genes and 4.8% (95% CI: 0.0–10.2%) isolates carried three SE genes (Figure 4A). From the 11 investigated SE genes, the seg was the most frequently detected (41.9%, 95% CI: 29.7–59.2%) followed by the sei (40.3%, 95% CI: 28.1–52.5%), sec (6.4%, 95% CI: 0.3–12.6%), seh (4.8%, 95% CI: 0–10.2%), and sea (1.6%, 95% CI: 0.0–4.7%). The genes seg and sei were always detected together (i.e., 40.3%, 95% CI: 28. 1–52.5%) of the S. aureus isolates, except one S. aureus isolate that was positive only for the seg gene. The gene sec was always detected in combination with seg and sei, and the isolate positive for sea was also positive for the seh gene. Moreover, seh gene was found alone in two isolates.
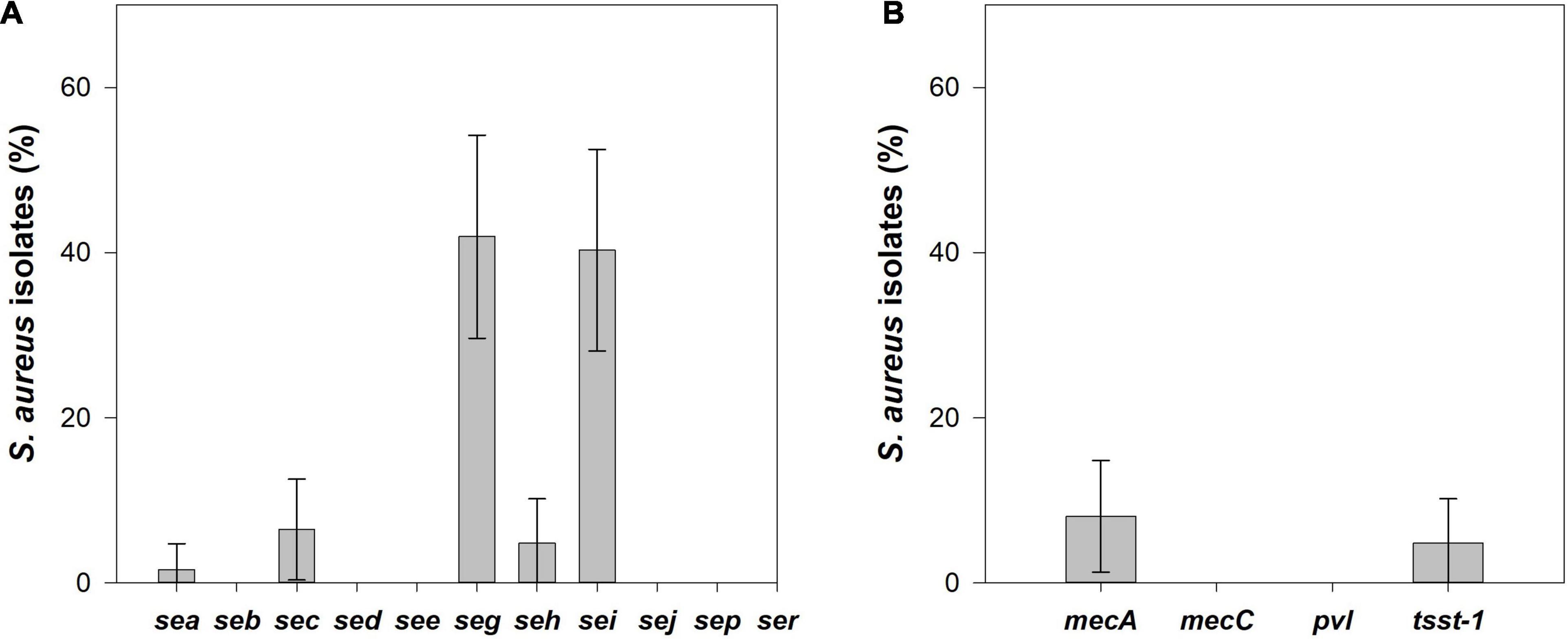
Figure 4. (A) Prevalence and its 95% confidence interval (CI) of enterotoxin coding genes, and (B) methicillin resistance genes, mecA and mecC, toxic shock syndrome toxin-1 (tsst-1) and Panton-Valentine leukocidin (pvl) genes in S. aureus isolated from raw cow’s milk samples collected from bulk cooling tanks of 100 dairy farms located in the main dairy basin of mainland Portugal.
From the 62 S. aureus isolates, 8.1% (95% CI: 1.3–14.8%), the same isolates that showed a cefoxitin resistance phenotype were confirmed as MRSA since they harbored the mecA gene. The tsst1 gene was identified in 4.8% (95% CI: 0.0–10.2%) of the S. aureus isolates. No pvl or mecC genes were detected among the S. aureus analyzed (Figure 4B). MRSA isolates did not contain any genes encoding SEs or TSST-1, but all tsst1-positive isolates were enterotoxigenic S. aureus with the same SE gene pattern, sec/seg/sei.
Based on the spa characterization and distribution of SEs-, resistance-, and other virulence-encoding genes, an analysis of the molecular patterns of S. aureus disseminated in raw cow’s milk was performed. Individual data for each isolate can be found in the Supplementary Table 3.
Seven genetic patterns can be identified: (1) seg; (2) seh; (3) sea/seh; (4) seg/sei; (5) sec/seg/sei; (6) tsst-1/sec/seg/sei; and (7) mecA. Furthermore, 45.2% (95% CI: 32.8–57.5%) of the S. aureus isolates did not present any of the virulence or resistance genes analyzed. Combining spa types and virulence/resistance determinants, 32 S. aureus patterns were detected among 62 isolates in raw milk samples from northern Portugal. Enterotoxigenic S. aureus are associated to spa types t002, t117, t127, t337, t528, t529, t543, t899, t2873, and t9216. Out of the five mecA-MRSA isolates, four were found to be t011 and one t2383. S. aureus t1403, t2802, t571, t108, t189. t208, t267, t843, t1200, t1207, t1334, and t19272 were exclusively associated with strains that did not contain any of the virulence/resistance genes evaluated. In total, S. aureus t1403-none (16.1%, 95% CI: 7.0–25.3%) is the predominant molecular pattern, followed by t529-seg/sei and t543-seg/sei (9.8%, 95% CI: 2.3–17.0%) (Figure 5).
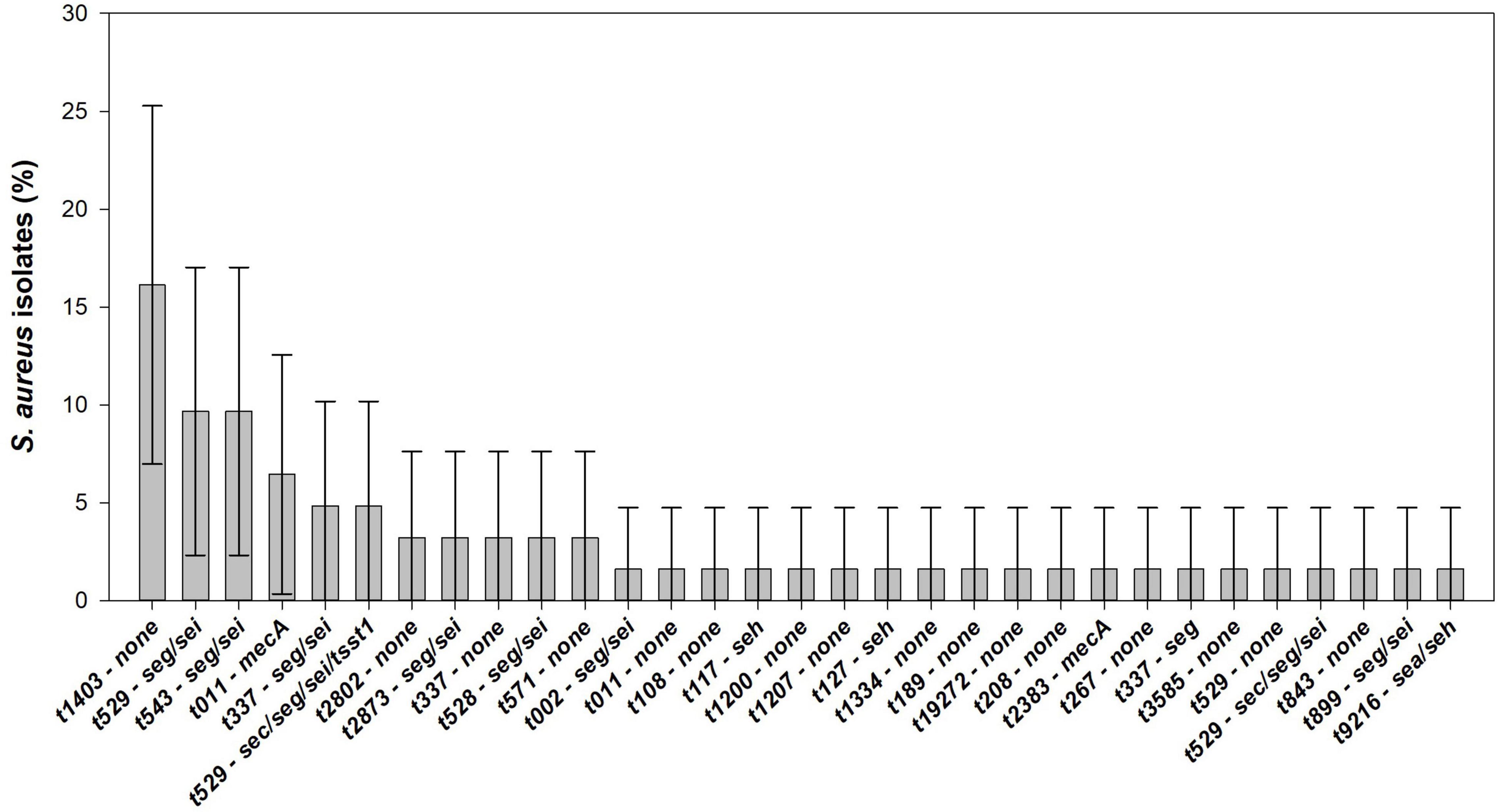
Figure 5. Distribution and its 95% confidence interval (CI) of virulence factors analyzed in S. aureus isolated from raw cow’s milk samples collected from bulk cooling tanks of 100 dairy farms located in the main dairy basin of mainland Portugal.
The presence of S. aureus and its enterotoxins in raw milk can be a serious threat to public health given the importance of dairy products in human diet. Therefore, an estimation of the prevalence and genetic determinants of S. aureus is always important to implement rational mitigation strategies and avoid the dissemination of this pathogen through the food chain (Kadariya et al., 2014).
In this study, a prevalence of 53.0% of S. aureus was established in raw cow’s milk collected from the bulk cooling tanks of 100 dairy farms located in the main dairy region of mainland Portugal. To date, this is the first significant study of S. aureus in raw milk carried out in Portugal. A similar level of contamination (approximately 40–70%) in raw cow’s milk has been found in other countries such as Poland, Italy, New Zealand, Norway, India and China (Jørgensen et al., 2005b; Jakobsen et al., 2011; Hill et al., 2012; Bianchi et al., 2014; Sudhanthiramani et al., 2015; Rola et al., 2016; Liu et al., 2017; Wang et al., 2018; Kou et al., 2021). In fact, the presence of S. aureus in bulk tank milk is not surprising given that this species is ubiquitous in nature and one of the main causes of bovine mastitis. Even so, the EU regulation established a criterion for CPS in cheeses made from raw milk (m = 104 CFU/g – M = 105 CFU/g) during the manufacturing process. If values are higher than 105 CFU/g cheese, the batch has to be tested for staphylococcal enterotoxin (EU Commission, 2007). In the present study, the CFU counts obtained on the CPS positive samples were all below the imposed limits. Low levels of CPS at the farms tanks are expected since milk storage is controlled at low temperatures (≈4°C) and for a short time after milking (12 – 24 h), as a way to prevent the growth of pathogens, including S. aureus (Owusu-Kwarteng et al., 2020).
Still, S. aureus presence in foods can represent a risk for human health since this pathogen produces an array of exoproteins with toxicological effects that are highly stable. Although the conditions used during the pasteurization process are suitable to destroy harmful microorganisms, they are not capable of eliminating some proteins, such as toxins. They will keep their activity in pasteurized milk, even at few micrograms, and be a serious threat to end consumers. From those toxins, SEs are the most relevant in the context of foodstuff. In fact, SEs are responsible for several SFP outbreaks reported every year worldwide (Argudín et al., 2010). Thus, their presence in foods is strictly prohibited in Europe (EU Commission, 2007; European Food Safety Authority and European Centre for Disease Prevention and Control, 2021a). In this study, one raw milk sample was positive for staphylococcal enterotoxins (SEA-SEE). Data on SEs in raw milk are scarce, as direct analysis on the raw material is out of scope of the European regulations. Although the presence of enterotoxins in raw milk has never been studied in Portugal, it has been found in cheeses during official controls, and also an outbreak affecting 13 persons due to an unknown food source (European Food Safety Authority [EFSA], 2019; European Food Safety Authority and European Centre for Disease Prevention and Control, 2021a). In Poland, the presence of SEs in raw milk was assessed without any positive result (Rola et al., 2016). Furthermore, SEs production usually requires the presence of S. aureus in high amounts, at least 5–6 log10 CFU.mL–1 (Argudín et al., 2010). None of the bulk tank milk samples analyzed had a level of contamination near or above this value, contributing to a lower probability of detection of SEs in the analyzed samples. Surprisingly, in the SE-positive sample found in this study no CPS was detected. Several hypotheses can be made to explain this result. Contamination of milk directly with enterotoxin from the udder of an animal is one of them. In fact, if S. aureus was in the udder of dairy cows with enough number of cells able to produce SEs, then enterotoxins could be transferred to the milk during the milking process, even after the bacteria have been eliminated (Rola et al., 2016). However, neither clinical nor subclinical mastitis were indicated in the animals from the dairy farm. Another possible explanation might be related with a dilution effect in the bulk milk, if only one or a few caws are infected. This dilution effect might affect more significantly culture techniques than SE determination, considering that the detection limit reported for VIDAS SET 2 is as low as 0.25 ng toxin per gram of food (Schultz et al., 2004). Enterotoxigenic coagulase-negative staphylococci (CNS) cannot be ruled out as several virulence determinants, usually associated with S. aureus, such as SE-encoding genes, have been detected in CNS genomes (Park et al., 2011; Podkowik et al., 2013) and different studies have identified several enterotoxigenic CNS species in dairy animals, capable of producing enterotoxins, predominantly SEC (Bergonier et al., 2003; Taponen et al., 2006; Ünal and Çinar, 2012). Since the methodology followed, in this study, for the isolation of S. aureus reject CNS strains, it is not possible to evaluate if CNS are responsible for the SE-positive sample. Finally, we should consider the possibility of a false positive result. Nonetheless, replicates were tested to confirm the sample result.
In recent years, the emergence of antimicrobial resistant strains, particularly MRSA, in livestock animals that is readily transferable to humans, has also become a growing public health concern (Sharma et al., 2018). In the present study, only 35.5% of the S. aureus isolates showed resistance to at least one antimicrobial agent. Similar results were reported on studies carry out in Italy (39.4%), Poland (23.0%) and China (38.5%) (Rola et al., 2015; Giacinti et al., 2017; Wang et al., 2018). Penicillin resistance was the most prevalent (32.3%) among the antimicrobial agents tested in accordance with previous studies in raw milk (Rola et al., 2015; Wang et al., 2018). Tetracycline, a broad-spectrum antimicrobial agent frequently used in the treatment of infections in cattle, was the second most prevalent antimicrobial resistance (24.2%), a level similar found to other studies (Liu et al., 2017). It was notable that ciprofloxacin- and chloramphenicol-resistant S. aureus were the third most frequently detected antimicrobial resistance. Ciprofloxacin is a third generation fluoroquinolone used at clinical level, while chloramphenicol is an antibiotic not authorized for use in food-producing animals in the European Union (Benford et al., 2014; Wang et al., 2018). Moreover, five isolates (8.1%) were identified as MRSA by antimicrobial susceptibility, revealing resistance to cefoxitin and all other β-lactams, except ceftaroline (Clinical and Laboratory Standards Institute [CLSI], 2020). Despite the low percentage of resistance observed, antibiotics should be used with caution in dairy animals because they may compromise the treatment of future infections. The presence of isolates with the MRSA phenotype is of major concern for human public health.
Although the presence of SEs was not detected in samples carrying S. aureus, enterotoxins’ production is still possible under appropriate environmental conditions (e.g., temperature) if the SE-encoding genes are part of the S. aureus genome. In our study, we have identified the presence of five different SE-encoding genes among 46.8% of S. aureus isolates, including two classical SE genes (sea and sec) and three non-classical SE genes (seh, seg and sei). Only five (8.1%) S. aureus isolates had classical SE genes (sea-see), although the literature suggested that about 95% of SFP outbreaks are caused by classical SEs (Argudín et al., 2010). Based on our results, the official method recommended for the detection of SEs in foodstuff does not reflect the complete diversity of enterotoxins found in nature. In fact, three of the SEs genes identified in this study, including the two most prevalent SEs (seg and sei), are not covered by ISO 19020:2017 methodology. The low prevalence of sea-see genes explains the low prevalence of SEA-SEE found in the bulk tank raw milk samples. Moreover, since the presence of non-classic SEs is not covered by the recommended methodologies, their prevalence may be underestimated. In Portugal, the characterization of S. aureus from a small number of raw milk samples had already verified the predominance of the seg and sei genes (Pereira et al., 2009). High prevalence of seg and sei was also observed in raw milk in Italy, and its co-existence in most isolates is also consistent with previous reports (Blaiotta et al., 2006; Bianchi et al., 2014; Johler et al., 2018). In fact, these two SE genes are typically located in tandem on the ecg locus and have already been implicated in scarlet fever, toxic shock and neonatal intractable diarrhea cases (Naik et al., 2008). However, these genes do not exist strictly together, as verified in one isolate of this study (only seg) and suggested by Jørgensen et al. (2005b). Other studies on raw milk have observed a higher prevalence of sea-see genes in Italy, Poland and China, yet most of them are in agreement with our study on the predominance of non-classic SE genes (Bianchi et al., 2014; Rola et al., 2016; Wu et al., 2018). Classical SE genes seems to be more prevalent in isolates of human and non-animal food origin than in animal food origin, such as S. aureus from raw milk (Chao et al., 2015). Furthermore, it should be mentioned that despite the prevalence of certain enterotoxin genes, they may have different genomic localization that may differentiate the virulence potential of the strains. Enterotoxins can be encoded in prophages, pathogenicity islands, genomic islands or plasmids, which can have different levels of regulation and expression of these virulence factors (Malachowa and Deleo, 2010).
Regarding the presence of resistance genes, the detection of mecA (8.1%) shows that raw cow’s milk can also be an antimicrobial resistance vehicle. The positive mecA-MRSA isolates correspond with the isolates classified as MRSA by the antimicrobial susceptibility test, demonstrating a complete correspondence between the genomic and phenotype results. The presence of MRSA strains have been reported in raw milk and dairy cattle in Europe (Tenhagen et al., 2014, 2018; Cortimiglia et al., 2016; Hansen et al., 2019; Schnitt et al., 2020; Lienen et al., 2021), but also in Algeria, Uganda, Brazil, and China (Asiimwe et al., 2017; Guimarães et al., 2017; Dai et al., 2019; Titouche et al., 2019). In Europe, MRSA prevalence in raw milk was 3–10%, which is in accordance with our results (Schnitt et al., 2020). In Portugal, mecA-MRSA strains have also been reported in bovine mastitis (Pereira et al., 2009), while mecA is also the predominant gene in most MRSA isolates worldwide. No association between MRSA strains and enterotoxigenic determinants was found in our analysis. In contrast, TSST-1 genetic determinant was found in enterotoxigenic isolates (sec/sec/sei-positive). Such relation may result in an increase in the toxigenic consequences of these strains. No pvl or mecC genes were detected, consistent with previous studies demonstrating the low prevalence of these genes among S. aureus isolated from dairy products (van Duijkeren et al., 2014; Johler et al., 2018). However, three isolates were identified as spa types (t843 and t528) commonly associated to CC130, the frequent genetic background of mecC (Bortolami et al., 2017). Given the epidemiological relevance of this genetic determinant of MRSA, the negative result for the presence of the mecC gene was confirmed. Furthermore, these isolates revealed a complete susceptibility to the tested antimicrobials, including cefoxitin (mecC-positive strains are typically cefoxitin resistant).
Regarding the diversity of S. aureus, we have identified 25 spa types among the 62 S. aureus isolates from the 100 bulk tank milk samples. Wang et al. (2018) found seven spa types of S. aureus in 96 isolates from raw milk collected on 2 dairy farms in China. The lower diversity obtained on that study might be due to the limited number of farms enrolled. Furthermore, the most predominant types observed in our study, t529 and t1403, differed from the t127 observed in China. A better correspondence is observed when compared to studies in European countries. Higher diversity and t529 and t1403 types were also predominant in raw milk and bovine isolates in Denmark and Switzerland (Johler et al., 2011; Ronco et al., 2018). Most of the other spa types detected in our study were also associated with bovine S. aureus from different European countries (Boss et al., 2016). Thus, the diversity of S. aureus found in raw milk in Portugal seems to be in line with that found in the rest of Europe, suggesting a geographic predominance of some spa types. In terms of human health, spa types t002 and t127, detected in one isolate each, are frequently observed in human invasive infections (Grundmann et al., 2010). Combining spa typing with virulence factors, 32 distinct S. aureus types can be identified in this study. Some spa types were exclusively associated to enterotoxigenic S. aureus, mainly t002, t117, t127, t528, t543, t899, t2873, and t9216. In contrast, S. aureus t1403 was also exclusively associated with the absence of virulence/resistance factors. The MRSA-t011 type have recently emerged in European countries, such as Germany and Denmark, associated with livestock-associated MRSA (LA-MRSA) strains including from raw milk isolates (van Duijkeren et al., 2014; Tenhagen et al., 2018; Hansen et al., 2019; Schnitt et al., 2020; Lienen et al., 2021). Accordingly, our results support that this trend is also present in Portugal. MRSA-t2383 type is a rare relative of t011 and was never reported in raw milk or dairy cattle, but in human and other animals as pigs and seabream (Fanoy et al., 2009; Salgueiro et al., 2020). Thus, spa type/virulence/resistance factors can be a good way to assess the variation in S. aureus diversity over time. In most instances, a spa types are highly associated with a specific multilocus sequence type (MLST), which is usually related with a specific clonal lineage (Tegegne et al., 2019). As an example, identified spa-types t002, t108, t117, t529, t1334, and t1207 are usually assigned to either sequence type (ST) ST151 or ST504, both belonging to clonal complex (CC) CC70, while t1403 is usually associated with ST97-CC97 or ST133-CC133. In the case of MRSA isolates, the spa-types here identified are usually assigned to ST398-CC398, which is the prevalent LA-MRSA in Europe, occasionally involved in human disease (Bortolami et al., 2017). Overall, most of the ST/CC associated with the spa-types identified in this study are commonly associated with livestock infection or carriage, especially of bovine origin (Smith et al., 2005; Hasman et al., 2010; Boss et al., 2016).
In conclusion, the high prevalence of enterotoxigenic S. aureus and the detection of MRSA strains in raw cow’s milk collected from bulk cooling tanks on dairy farms from the main dairy region of mainland Portugal is of particular concern. Furthermore, a high diversity of S. aureus was found in a relatively small geographical area, however, most with genomic lineages associated with livestock infection or carriage. This study also points out the predominance of SE-encoding genes that are not currently covered by the gold standard methodology (ISO 19020:2017) applied in the control of food samples. The higher prevalence of non-classical SEs, mainly SEG, SEH and SEI, should not be ignored, as these have been implicated in food poisoning outbreaks (Jørgensen et al., 2005a; Tang et al., 2011; Ciupescu et al., 2018; Hait et al., 2018). Commercial tests with proven effectiveness for the non-classic SEs are available on the market, which may facilitate their integration in food safety control standard for SEs analysis of foods (Hait et al., 2018). As SFP outbreaks linked to raw cow’s milk consumption and raw milk products have been increasing, the presented data on the characterization of S. aureus and its virulence determinants are important to improve risk assessment and develop solutions to limit the dissemination of this pathogen in Portugal.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
RO, EP, GA, NA, and CA designed the experiments. RO performed the experiments. RO, EP, and CA analyzed the data. All authors contributed to the writing of the manuscript.
This work was financially supported by LA/P/0045/2020 (ALiCE), UIDB/00511/2020 and UIDP/00511/2020 (LEPABE), funded by national funds through FCT/MCTES (PIDDAC); Project POCI-01-0145-FEDER-028659, funded by FEDER funds through COMPETE2020 – Programa Operacional Competitividade e Internacionalização (POCI); and by national funds (PIDDAC) through FCT/MCTES. The authors also thank FCT for the Ph.D. Fellowship SFRH/BD/138883/2018.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We sincerely thank all the dairy farmers who voluntarily participated in this study and the professionals in the field who assisted in collecting the samples. We would also like to thank the Reference Laboratory for Coagulase Positive Staphylococci (EURL CPS) for providing the control strains.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.846653/full#supplementary-material
Argudín, M. Á., Mendoza, M. C., and Rodicio, M. R. (2010). Food Poisoning and Staphylococcus aureus Enterotoxins. Toxins 2, 1751–1773. doi: 10.3390/toxins2071751
Asiimwe, B. B., Baldan, R., Trovato, A., and Cirillo, D. M. (2017). Prevalence and molecular characteristics of Staphylococcus aureus, including methicillin resistant strains, isolated from bulk can milk and raw milk products in pastoral communities of South-West Uganda. BMC Infect. Dis. 17:422. doi: 10.1186/s12879-017-2524-4
Benford, D., Ceccatelli, S., Cottrill, B., DiNovi, M., Dogliotti, E., Edler, L., et al. (2014). Scientific Opinion on Chloramphenicol in food and feed. EFSA J. 12:3907. doi: 10.2903/j.efsa.2014.3907
Bergonier, D., de Crémoux, R., Rupp, R., Lagriffoul, G., and Berthelot, X. (2003). Mastitis of dairy small ruminants. Vet. Res. 34, 689–716. doi: 10.1051/vetres:2003030
Bianchi, D. M., Gallina, S., Bellio, A., Chiesa, F., Civera, T., and Decastelli, L. (2014). Enterotoxin gene profiles of Staphylococcus aureus isolated from milk and dairy products in Italy. Lett. Appl. Microbiol. 58, 190–196. doi: 10.1111/lam.12182
Blaiotta, G., Fusco, V., Von Eiff, C., Villani, F., and Becker, K. (2006). Biotyping of enterotoxigenic Staphylococcus aureus by enterotoxin gene cluster (egc) polymorphism and spa typing analyses. Appl. Environ. Microbiol. 72, 6117–6123. doi: 10.1128/AEM.00773-06
Bortolami, A., Verin, R., Chantrey, J., Corrò, M., Ashpole, I., Lopez, J., et al. (2017). Characterization of Livestock-Associated Methicillin-Resistant Staphylococcus aureus CC398 and mecC-positive CC130 from Zoo Animals in the United Kingdom. Microb. Drug Resist. 23, 908–914. doi: 10.1089/mdr.2017.0161
Boss, R., Cosandey, A., Luini, M., Artursson, K., Bardiau, M., Breitenwieser, F., et al. (2016). Bovine Staphylococcus aureus: subtyping, evolution, and zoonotic transfer. J. Dairy Sci. 99, 515–528. doi: 10.3168/jds.2015-9589
Brakstad, O. G., Aasbakk, K., and Maeland, J. A. (1992). Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 30, 1654–1660. doi: 10.1128/jcm.30.7.1654-1660.1992
Chambers, H. F., and DeLeo, F. R. (2009). Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 7, 629–641. doi: 10.1038/nrmicro2200
Chao, G., Bao, G., Cao, Y., Yan, W., Wang, Y., Zhang, X., et al. (2015). Prevalence and diversity of enterotoxin genes with genetic background of Staphylococcus aureus isolates from different origins in China. Int. J. Food Microbiol. 211, 142–147. doi: 10.1016/j.ijfoodmicro.2015.07.018
Ciupescu, L. M., Auvray, F., Nicorescu, I. M., Meheut, T., Ciupescu, V., Lardeux, A. L., et al. (2018). Characterization of Staphylococcus aureus strains and evidence for the involvement of non-classical enterotoxin genes in food poisoning outbreaks. FEMS Microbiol. Lett. 365:139. doi: 10.1093/femsle/fny139
Clinical and Laboratory Standards Institute [CLSI] (2020). VET01 | Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, 5th Edn. Available online at: https://clsi.org/standards/products/veterinary-medicine/documents/vet01/ (Accessed February 4, 2022).
Clinical and Laboratory Standards Institute [CLSI] (2021). M100 | Performance Standards for Antimicrobial Susceptibility Testing, 31st Edn. Pennsylvania: Clinical & Laboratory Standards Institute.
EU Commission (2007). Commission regulation (EC) No 1441/2007 of 5 December 2007 amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 322, 2–29.
Cortimiglia, C., Luini, M., Bianchini, V., Marzagalli, L., Vezzoli, F., Avisani, D., et al. (2016). Prevalence of Staphylococcus aureus and of methicillin-resistant S. aureus clonal complexes in bulk tank milk from dairy cattle herds in Lombardy Region (Northern Italy). Epidemiol. Infect. 144, 3046–3051. doi: 10.1017/S0950268816001576
Dai, J., Wu, S., Huang, J., Wu, Q., Zhang, F., Zhang, J., et al. (2019). Prevalence and Characterization of Staphylococcus aureus Isolated from Pasteurized Milk in China. Front. Microbiol. 10:641. doi: 10.3389/FMICB.2019.00641/BIBTEX
Dhanashekar, R., Akkinepalli, S., and Nellutla, A. (2012). Milk-borne infections. an analysis of their potential effect on the milk industry. Germs 2, 101–109. doi: 10.11599/germs.2012.1020
European Food Safety Authority [EFSA] (2019). Trends and Sources of Zoonoses and in Humans, Foodstuffs, Animals and Feedingstuffs - Portugal. Parma: European Food Safety Authority. Available online at: http://www.efsa.europa.eu/sites/default/files/zoocountryreport18it.pdf
European Food Safety Authority, European Centre for Disease Prevention and Control (2021b). The European Union One Health 2020 Zoonoses report. EFSA J. 19:e06971. doi: 10.2903/j.efsa.2021.6971
European Food Safety Authority, European Centre for Disease Prevention and Control (2021a). The European Union One Health 2019 Zoonoses Report. EFSA J 19:e06406. doi: 10.2903/j.efsa.2021.6406
Fanoy, E., Helmhout, L. C., van der Vaart, W. L., Weijdema, K., van Santen-Verheuvel, M. G., Thijsen, S. F., et al. (2009). An outbreak of non-typeable MRSA within a residential care facility. Euro Surveill. 14:19080. doi: 10.2807/ese.14.01.19080-en
Fisher, E. L., Otto, M., and Cheung, G. Y. C. (2018). Basis of virulence in enterotoxin-mediated staphylococcal food poisoning. Front. Microbiol. 9:436. doi: 10.3389/fmicb.2018.00436
Gallo, M., Ferrara, L., Calogero, A., Montesano, D., and Naviglio, D. (2020). Relationships between food and diseases: what to know to ensure food safety. Food Res. Int. 137:109414. doi: 10.1016/j.foodres.2020.109414
Garcia, S. N., Osburn, B. I., and Jay-Russell, M. T. (2020). One Health for Food Safety, Food Security, and Sustainable Food Production. Front. Sustain. Food Syst. 4:1. doi: 10.3389/fsufs.2020.00001
Giacinti, G., Carfora, V., Caprioli, A., Sagrafoli, D., Marri, N., Giangolini, G., et al. (2017). Prevalence and characterization of methicillin-resistant Staphylococcus aureus carrying mecA or mecC and methicillin-susceptible Staphylococcus aureus in dairy sheep farms in central Italy. J. Dairy Sci. 100, 7857–7863. doi: 10.3168/JDS.2017-12940
Grundmann, H., Aanensen, D. M., Van Den Wijngaard, C. C., Spratt, B. G., Harmsen, D., Friedrich, A. W., et al. (2010). Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular-epidemiological analysis. PLoS Med. 7:e1000215. doi: 10.1371/journal.pmed.1000215
Guimarães, F. F., Manzi, M. P., Joaquim, S. F., Richini-Pereira, V. B., and Langoni, H. (2017). Short communication: outbreak of methicillin-resistant Staphylococcus aureus (MRSA)-associated mastitis in a closed dairy herd. J. Dairy Sci. 100, 726–730. doi: 10.3168/JDS.2016-11700
Hait, J. M., Nguyen, A. T., and Tallent, S. M. (2018). Analysis of the VIDAS® Staph Enterotoxin III (SET3) for detection of staphylococcal enterotoxins G. H, and I in foods. J. AOAC Int. 101, 1482–1489. doi: 10.5740/jaoacint.17-0501
Hansen, J. E., Ronco, T., Stegger, M., Sieber, R. N., Fertner, M. E., Martin, H. L., et al. (2019). LA-MRSA CC398 in Dairy Cattle and Veal Calf Farms Indicates Spillover From Pig Production. Front. Microbiol. 10:2733. doi: 10.3389/FMICB.2019.02733/BIBTEX
Hasman, H., Moodley, A., Guardabassi, L., Stegger, M., Skov, R. L., and Aarestrup, F. M. (2010). spa type distribution in Staphylococcus aureus originating from pigs, cattle and poultry. Vet. Microbiol. 141, 326–331. doi: 10.1016/j.vetmic.2009.09.025
Hill, B., Smythe, B., Lindsay, D., and Shepherd, J. (2012). Microbiology of raw milk in New Zealand. Int. J. Food Microbiol. 157, 305–308. doi: 10.1016/j.ijfoodmicro.2012.03.031
International Organization for Standardization [ISO] (2017). ISO 19020:2017 - Microbiology of the food chain — Horizontal method for the immunoenzymatic detection of enterotoxins in foodstuffs. 2017–06, undefined-undefined. Available online at: https://www.mendeley.com/catalogue/6735a125-303d-3f3c-aabf-e8d701b029bb/?utm_source=desktop&utm_medium=1.19.8&utm_campaign=open_catalog&userDocumentId=%7B823ad3bb-0c0f-3d1f-a3d4-30ba3a6d0fad%7D (Accessed December 23, 2021)
International Organization for Standardization [ISO] (2021). ISO 6888-2:2021 - Microbiology of the food chain — Horizontal method for the enumeration of coagulase-positive staphylococci (Staphylococcus aureus and other species) — Part 2: Method using rabbit plasma fibrinogen agar medium. Available online at: https://www.iso.org/standard/76673.html (Accessed December 23, 2021)
Jakobsen, R. A., Heggebø, R., Sunde, E. B., and Skjervheim, M. (2011). Staphylococcus aureus and Listeria monocytogenes in Norwegian raw milk cheese production. Food Microbiol. 28, 492–496. doi: 10.1016/j.fm.2010.10.017
Johler, S., Layer, F., and Stephan, R. (2011). Comparison of virulence and antibiotic resistance genes of food poisoning outbreak isolates of Staphylococcus aureus with isolates obtained from bovine mastitis milk and pig carcasses. J. Food Prot. 74, 1852–1859. doi: 10.4315/0362-028X.JFP-11-192
Johler, S., Macori, G., Bellio, A., Acutis, P. L., Gallina, S., and Decastelli, L. (2018). Short communication: characterization of Staphylococcus aureus isolated along the raw milk cheese production process in artisan dairies in Italy. J. Dairy Sci. 101, 2915–2920. doi: 10.3168/jds.2017-13815
Johnson, W. M., Tyler, S. D., Ewan, E. P., Ashton, F. E., Pollard, D. R., and Rozee, K. R. (1991). Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J. Clin. Microbiol. 29, 426–430. doi: 10.1128/jcm.29.3.426-430.1991
Jørgensen, H. J., Mathisen, T., Løvseth, A., Omoe, K., Qvale, K. S., and Loncarevic, S. (2005a). An outbreak of staphylococcal food poisoning caused by enterotoxin H in mashed potato made with raw milk. FEMS Microbiol. Lett. 252, 267–272. doi: 10.1016/j.femsle.2005.09.005
Jørgensen, H. J., Mork, T., Høgåsen, H. R., and Rørvik, L. M. (2005b). Enterotoxigenic Staphylococcus aureus in bulk milk in Norway. J. Appl. Microbiol. 99, 158–166. doi: 10.1111/j.1365-2672.2005.02569.x
Kadariya, J., Smith, T. C., and Thapaliya, D. (2014). Staphylococcus aureus and Staphylococcal Food-Borne Disease: an Ongoing Challenge in Public Health. Biomed Res. Int. 2014:827965. doi: 10.1155/2014/827965
Kou, X., Cai, H., Huang, S., Ni, Y., Luo, B., Qian, H., et al. (2021). Prevalence and Characteristics of Staphylococcus aureus Isolated From Retail Raw Milk in Northern Xinjiang, China. Front. Microbiol. 12:2187. doi: 10.3389/fmicb.2021.705947
Lienen, T., Schnitt, A., Hammerl, J. A., Maurischat, S., and Tenhagen, B. A. (2021). Genomic Distinctions of LA-MRSA ST398 on Dairy Farms From Different German Federal States With a Low Risk of Severe Human Infections. Front. Microbiol. 11:3390. doi: 10.3389/FMICB.2020.575321/BIBTEX
Liu, H., Li, S., Meng, L., Dong, L., Zhao, S., Lan, X., et al. (2017). Prevalence, antimicrobial susceptibility, and molecular characterization of Staphylococcus aureus isolated from dairy herds in northern China. J. Dairy Sci. 100, 8796–8803. doi: 10.3168/jds.2017-13370
Malachowa, N., and Deleo, F. R. (2010). Mobile genetic elements of Staphylococcus aureus. Cell. Mol. Life Sci. 67, 3057–3071. doi: 10.1007/s00018-010-0389-4
Naik, S., Smith, F., Ho, J., Croft, N. M., Domizio, P., Price, E., et al. (2008). Staphylococcal Enterotoxins G and I, a Cause of Severe but Reversible Neonatal Enteropathy. Clin. Gastroenterol. Hepatol. 6, 251–254. doi: 10.1016/j.cgh.2007.09.004
Owusu-Kwarteng, J., Akabanda, F., Agyei, D., and Jespersen, L. (2020). Microbial safety of milk production and fermented dairy products in africa. Microorganisms 8:752. doi: 10.3390/microorganisms8050752
Park, J. Y., Fox, L. K., Seo, K. S., McGuire, M. A., Park, Y. H., Rurangirwa, F. R., et al. (2011). Detection of classical and newly described staphylococcal superantigen genes in coagulase-negative staphylococci isolated from bovine intramammary infections. Vet. Microbiol. 147, 149–154. doi: 10.1016/j.vetmic.2010.06.021
Pereira, V., Lopes, C., Castro, A., Silva, J., Gibbs, P., and Teixeira, P. (2009). Characterization for enterotoxin production, virulence factors, and antibiotic susceptibility of Staphylococcus aureus isolates from various foods in Portugal. Food Microbiol. 26, 278–282. doi: 10.1016/j.fm.2008.12.008
Pérez-Rodríguez, F., and Mercanoglu Taban, B. (2019). A State-of-Art Review on Multi-Drug Resistant Pathogens in Foods of Animal Origin: risk Factors and Mitigation Strategies. Front. Microbiol. 10:2091. doi: 10.3389/fmicb.2019.02091
Podkowik, M., Park, J. Y., Seo, K. S., Bystroń, J., and Bania, J. (2013). Enterotoxigenic potential of coagulase-negative staphylococci. Int. J. Food Microbiol. 163, 34–40. doi: 10.1016/j.ijfoodmicro.2013.02.005
Rola, J. G., Czubkowska, A., Korpysa-Dzirba, W., and Osek, J. (2016). Occurrence of Staphylococcus aureus on farms with small scale production of raw milk cheeses in poland. Toxins 8:62. doi: 10.3390/toxins8030062
Rola, J. G., Korpysa-Dzirba, W., Czubkowska, A., and Osek, J. (2015). Prevalence of enterotoxin genes and antimicrobial resistance of coagulase-positive staphylococci recovered from raw cow milk. J. Dairy Sci. 98, 4273–4278. doi: 10.3168/jds.2014-9064
Ronco, T., Klaas, I. C., Stegger, M., Svennesen, L., Astrup, L. B., Farre, M., et al. (2018). Genomic investigation of Staphylococcus aureus isolates from bulk tank milk and dairy cows with clinical mastitis. Vet. Microbiol. 215, 35–42. doi: 10.1016/j.vetmic.2018.01.003
Roussel, S., Felix, B., Vingadassalon, N., Grout, J., Hennekinne, J. A., Guillier, L., et al. (2015). Staphylococcus aureus strains associated with food poisoning outbreaks in France: comparison of different molecular typing methods, including MLVA. Front. Microbiol. 6:882. doi: 10.3389/fmicb.2015.00882
Sabat, A. J., Budimir, A., Nashev, D., Sá-Leão, R., van Dijl, J. M., Laurent, F., et al. (2013). Overview of molecular typing methods for outbreak detection and epidemiological surveillance. Euro Surveill. 18:20380. doi: 10.2807/ese.18.04.20380-en
Salgueiro, V., Manageiro, V., Bandarra, N. M., Ferreira, E., Clemente, L., and Caniça, M. (2020). Genetic relatedness and diversity of staphylococcus aureus from different reservoirs: humans and animals of livestock, poultry, zoo, and aquaculture. Microorganisms 8:1345. doi: 10.3390/microorganisms8091345
Schnitt, A., Lienen, T., Wichmann-Schauer, H., Cuny, C., and Tenhagen, B. A. (2020). The occurrence and distribution of livestock-associated methicillin-resistant Staphylococcus aureus ST398 on German dairy farms. J. Dairy Sci. 103, 11806–11819. doi: 10.3168/jds.2020-18958
Schultz, A. M., Mcmahon, W. A., Aleo, V. A., and Johnson, R. L. (2004). VIDAS Staphylococcal Enterotoxin II (SET2) Pre-collaborative Study Report: AOAC Performance Tested Method. AOAC interational Anu. Meet., 2004. Available online at: http://www.biomerieux-usa.com/sites/subsidiary_us/files/doc/staph_poster-1.pdf (Accessed February 15, 2022)
Sharma, C., Rokana, N., Chandra, M., Singh, B. P., Gulhane, R. D., Gill, J. P. S., et al. (2018). Antimicrobial resistance: its surveillance, impact, and alternative management strategies in dairy animals. Front. Vet. Sci. 4:237. doi: 10.3389/fvets.2017.00237
Shopsin, B., Gomez, M., Montgomery, S. O., Smith, D. H., Waddington, M., Dodge, D. E., et al. (1999). Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbiol. 37, 3556–3563. doi: 10.1128/jcm.37.11.3556-3563.1999
Smith, E. M., Green, L. E., Medley, G. F., Bird, H. E., Fox, L. K., Schukken, Y. H., et al. (2005). Multilocus sequence typing of intercontinental bovine Staphylococcus aureus isolates. J. Clin. Microbiol. 43, 4737–4743. doi: 10.1128/JCM.43.9.4737-4743.2005
Stegger, M., Andersen, P. S., Kearns, A., Pichon, B., Holmes, M. A., Edwards, G., et al. (2012). Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecALGA251. Clin. Microbiol. Infect. 18, 395–400. doi: 10.1111/j.1469-0691.2011.03715.x
Sudhanthiramani, S., Swetha, C. S., and Bharathy, S. (2015). Prevalence of antibiotic resistant Staphylococcus aureus from raw milk samples collected from the local vendors in the region of Tirupathi, India. Vet. World 8, 478–481. doi: 10.14202/vetworld.2015.478-481
Tang, J., Tang, C., Chen, J., Du, Y., Yang, X. N., Wang, C., et al. (2011). Phenotypic characterization and prevalence of enterotoxin genes in Staphylococcus aureus isolates from outbreaks of illness in Chengdu city. Foodborne Pathog. Dis. 8, 1317–1320. doi: 10.1089/fpd.2011.0924
Taponen, S., Simojoki, H., Haveri, M., Larsen, H. D., and Pyörälä, S. (2006). Clinical characteristics and persistence of bovine mastitis caused by different species of coagulase-negative staphylococci identified with API or AFLP. Vet. Microbiol. 115, 199–207. doi: 10.1016/j.vetmic.2006.02.001
Tegegne, H. A., Koláčková, I., Florianová, M., Gelbíčová, T., Wattiau, P., Boland, C., et al. (2019). Genome Sequences of Livestock-Associated Methicillin-Resistant Staphylococcus aureus spa Type t899 Strains Belonging to Three Different Sequence Types (ST398, ST9, and ST4034). Microbiol. Resour. Announc. 8, e01351–18. doi: 10.1128/mra.01351-18
Tenhagen, B. A., Alt, K., Pfefferkorn, B., Wiehle, L., Käsbohrer, A., and Fetsch, A. (2018). Short communication: methicillin-resistant Staphylococcus aureus in conventional and organic dairy herds in Germany. J. Dairy Sci. 101, 3380–3386. doi: 10.3168/jds.2017-12939
Tenhagen, B. A., Vossenkuhl, B., Käsbohrer, A., Alt, K., Kraushaar, B., Guerra, B., et al. (2014). Methicillin-resistant Staphylococcus aureus in cattle food chains - Prevalence, diversity, and antimicrobial resistance in Germany. J. Anim. Sci. 92, 2741–2751. doi: 10.2527/jas.2014-7665
Titouche, Y., Hakem, A., Houali, K., Meheut, T., Vingadassalon, N., Ruiz-Ripa, L., et al. (2019). Emergence of methicillin-resistant Staphylococcus aureus (MRSA) ST8 in raw milk and traditional dairy products in the Tizi Ouzou area of Algeria. J. Dairy Sci. 102, 6876–6884. doi: 10.3168/jds.2018-16208
Ünal, N., and Çinar, O. D. (2012). Detection of stapylococcal enterotoxin, methicillin-resistant and Panton-Valentine leukocidin genes in coagulase-negative staphylococci isolated from cows and ewes with subclinical mastitis. Trop. Anim. Health Prod. 44, 369–375. doi: 10.1007/s11250-011-0032-x
van Duijkeren, E., Hengeveld, P. D., Albers, M., Pluister, G., Jacobs, P., Heres, L., et al. (2014). Prevalence of methicillin-resistant Staphylococcus aureus carrying mecA or mecC in dairy cattle. Vet. Microbiol. 171, 364–367. doi: 10.1016/j.vetmic.2013.12.024
Wang, W., Lin, X., Jiang, T., Peng, Z., Xu, J., Yi, L., et al. (2018). Prevalence and characterization of Staphylococcus aureus cultured from raw milk taken from dairy cows with mastitis in Beijing, China. Front. Microbiol. 9:1123. doi: 10.3389/fmicb.2018.01123
Keywords: Staphylococcus aureus, raw milk, staphylococcal enterotoxins, MRSA, spa typing
Citation: Oliveira R, Pinho E, Almeida G, Azevedo NF and Almeida C (2022) Prevalence and Diversity of Staphylococcus aureus and Staphylococcal Enterotoxins in Raw Milk From Northern Portugal. Front. Microbiol. 13:846653. doi: 10.3389/fmicb.2022.846653
Received: 31 December 2021; Accepted: 16 February 2022;
Published: 22 March 2022.
Edited by:
Laurent Dufossé, Université de la Réunion, FranceReviewed by:
Nathalia Silva, State University of Campinas, BrazilCopyright © 2022 Oliveira, Pinho, Almeida, Azevedo and Almeida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carina Almeida, Y2FyaW5hLmFsbWVpZGFAaW5pYXYucHQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.