
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Microbiol. , 03 March 2022
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.841905
Direct lytic agents (DLAs) are novel antimicrobial compounds with unique mechanisms of action based on rapid cell wall destabilization and bacteriolysis. DLAs include two classes of purified polypeptides—lysins (peptidoglycan hydrolase enzymes) and amurins (outer membrane targeting peptides). Their intended use is to kill bacteria in a manner that is complimentary to and synergistic with traditional antibiotics without selection for DLA resistance. Lysins were originally described as having activity against Gram-positive pathogens and of those, exebacase, is the first to have advanced into Phase 3 of clinical development. Recently, both engineered and native DLAs have now been described with potent bactericidal activity against a range of Gram-negative pathogens, including multidrug-resistant (MDR) and extensively drug-resistant (XDR) Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii. Importantly, novel DLAs targeting Gram-negatives, including the lysin CF-370 and the amurin peptides, are active in biological matrices (blood/serum) and, as such, offer promise for therapeutic use as systemically administered agents for the treatment of life-threatening invasive infections. In this review, DLAs are discussed as potential new classes of antimicrobial biologics that can be used to treat serious systemic infections.
Since the introduction of penicillin, new antibiotics have been primarily identified from natural sources, often bacteria and fungi, or as derivatives of pre-existing compounds (Silver, 2011). There have been no novel antibacterial drugs with distinct targets and/or mechanisms of action introduced over the last 30+ years (Figure 1), despite technological progress and extensive high-throughput screening of natural and synthetic product libraries (Singh et al., 2017; Travis et al., 2018; Serafim et al., 2020). Compounding this problem, many large pharmaceutical firms have now abandoned the anti-infective sector, leaving smaller companies and funding bodies to fill the gap (Cole, 2014). Emerging resistance to traditional antibiotics coupled with this lack of new medical modalities to treat infections caused by multidrug-resistant (MDR) and extensively drug-resistant (XDR) pathogens, pose increasingly concerning potential public health threats (WHO, 2019; Boucher, 2020).
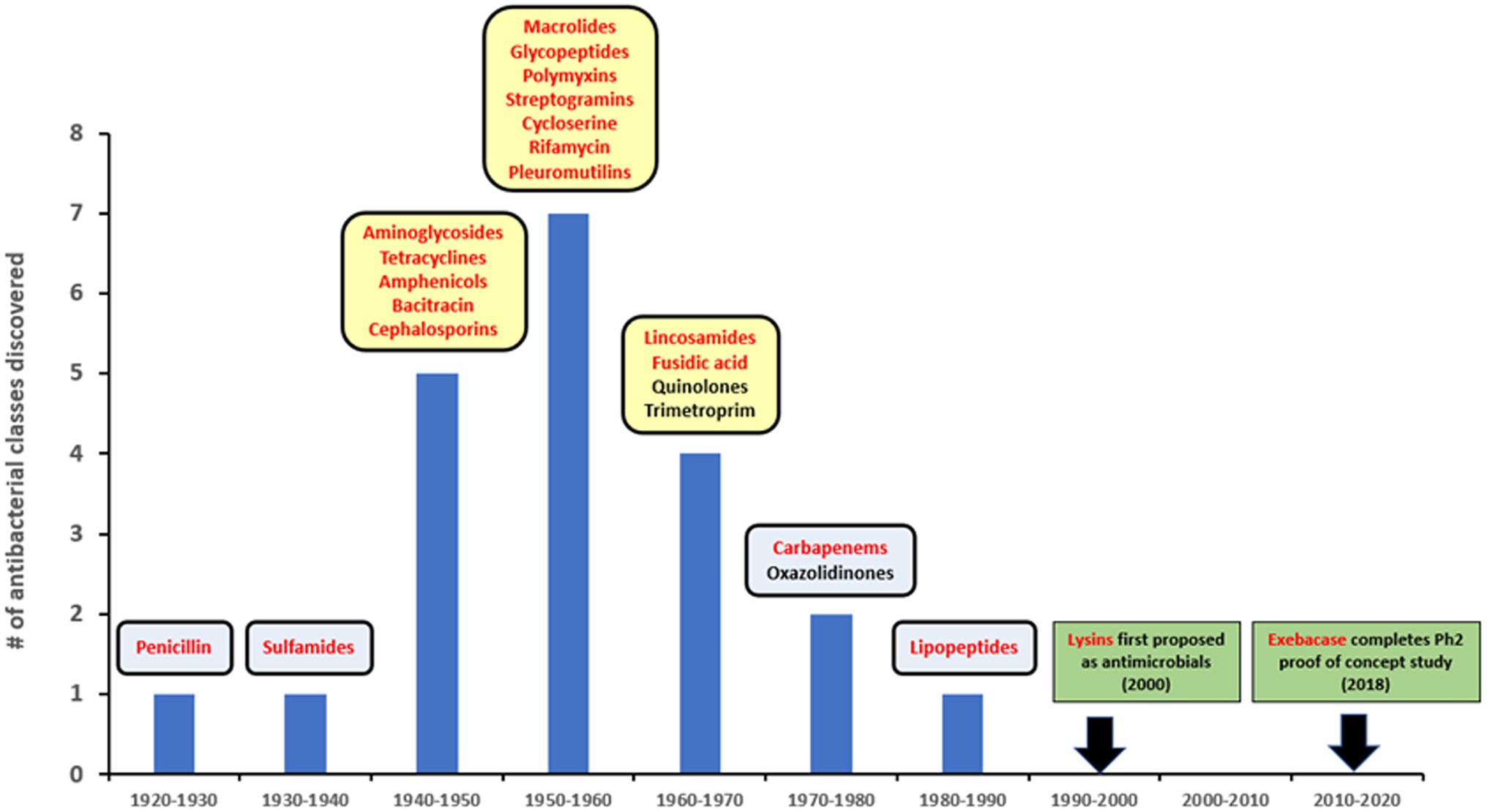
Figure 1. Lysins in the timeline of discovery for major antibiotic classes. Yellow squares represent golden age of antibiotics. Red font is used to show compounds with a natural origin.
While continued advances in genomics, bioinformatics, and structural biology still offer the promise of finding new small molecule antibiotics, altogether new chemotypes with innovative antimicrobial strategies are providing advanced leads that may eventually become the next generation of compounds in clinical use. Of particular note is a novel family of antimicrobials, recently referred to as “Direct Lytic Agents (DLAs)” by ContraFect Corporation (Sauve et al., 2021; Watson et al., 2021a,b). The DLA family consists of highly purified proteins and peptides, originally derived from the lytic systems of bacteriophage and which share a set of common microbiologic attributes and mode of action based on the direct lysis of target pathogens (Wittekind and Schuch, 2016). There are, thus far, two distinct classes of DLAs, including the lysins and amurin peptides, which are differentiated from each other and from antibiotics with respect to structure and activity.
Herein we review DLAs that are active in blood matrices and can be administered systemically, focusing primarily on the antistaphylococcal lysin exebacase, which is in Phase 3 clinical development, and then on recently described engineered lysins and amurin peptides that have demonstrated in vitro, ex vivo, or in vivo activity against Gram-negative pathogens. The identification of DLAs that can target Gram-negative bacteria which are amenable to systemic administration represents a major advance in the field, addressing unmet clinical needs by offering potential treatments against antibiotic-resistant pathogens that cause life-threatening infections for which there are no or limited therapeutic options.
The lysin class of DLAs encompass a large and structurally/functionally diverse family of cell wall hydrolytic enzymes (Fischetti et al., 2006; Nelson et al., 2012). There are five functional types of lysins: N-acetylmuramidases (lysozymes), endo-β-N-acetylglucosaminidases, and lytic transglycosylases, cleaving the amino sugar moieties of peptidoglycan; N-acetyl-muramoyl-L-alanine amidases, cleaving the amide bond between N-acetylmuramic acid and L-alanine in the stem peptide; and endopeptidases, cleaving within the peptide structure of peptidoglycan (Broendum et al., 2018; Vermassen et al., 2019). Lysins targeting Gram-positive bacteria (and to a lesser extent Gram-negative bacteria) typically also require cell wall-binding domains that direct catalytic activities to the cell wall via binding to either peptidoglycan or secondary cell wall components (Ganguly et al., 2013; Schuch et al., 2013; Bustamante et al., 2017; Santos et al., 2019; King et al., 2021). Binding domains direct rapid attachment with high equilibrium affinities (Schmelcher et al., 2010) and, depending on the binding epitope, may direct catalytic activities in either a species-specific (Schmelcher and Loessner, 2014) or more widespread (Schuch et al., 2019; Watson et al., 2019a) manner. Interestingly, the catalytic and cell wall-binding modules of lysins can be separable and swappable, providing ample opportunities for engineering (Gerstmans et al., 2018).
The use of lysins as non-antibiotic antimicrobials was first proposed in the early 2000s (Figure 1), based on studies showing that recombinantly produced and purified lysins can be applied exogenously to Gram-positive bacteria to elicit peptidoglycan hydrolysis and osmotic lysis (Loeffler et al., 2001; Nelson et al., 2001; Schuch et al., 2002; Fischetti et al., 2006). The ability of purified lysin to rapidly kill Gram-positive pathogens (as demonstrated in Figure 2) provided the foundation for their further development as powerful new antibacterial therapeutics.
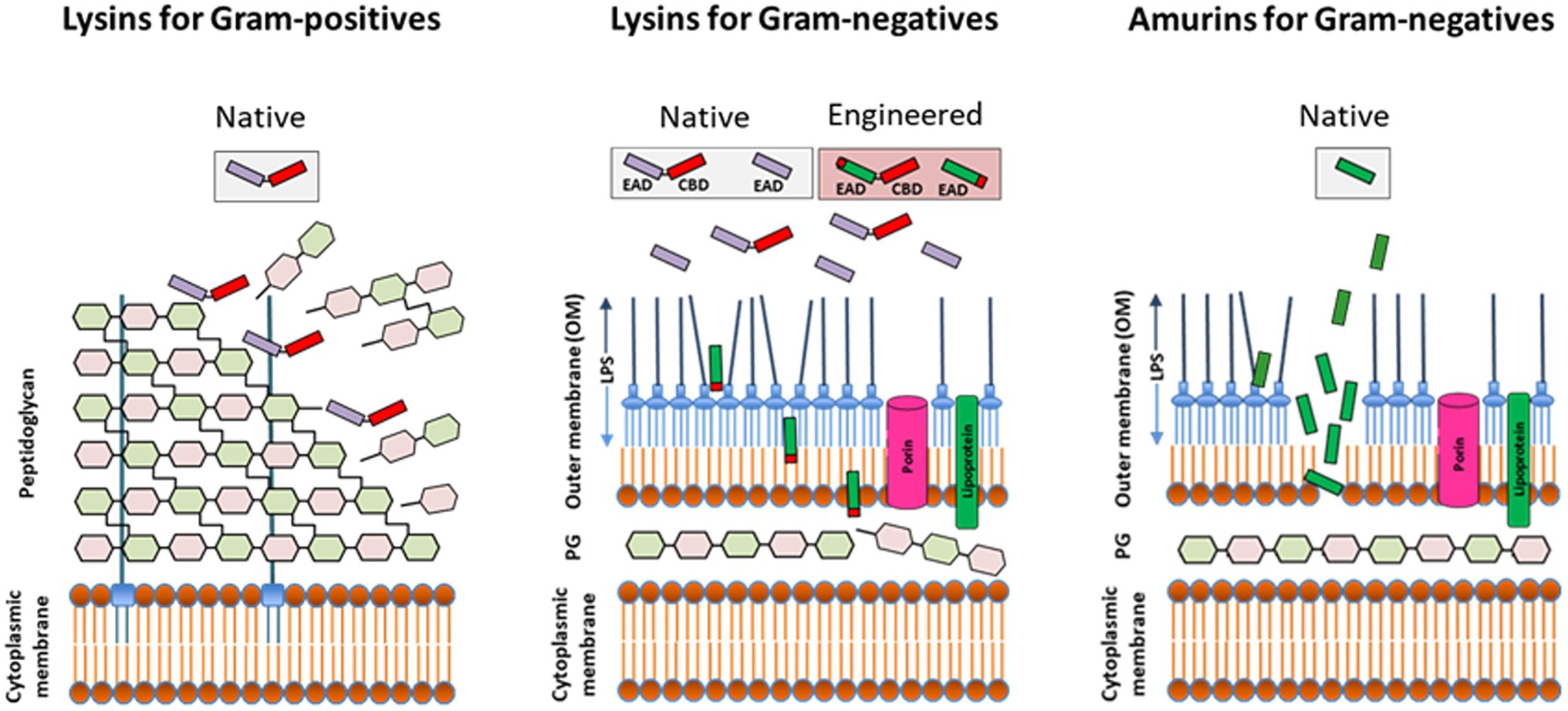
Figure 2. Direct lytic activity of purified (recombinant) lysins and synthesized amurin peptides applied exogenously to susceptible pathogens. The lysin class of Direct lytic agents (DLAs) consist of cell wall hydrolases targeting the surface exposed peptidoglycan of Gram-positives and the internal peptidoglycan layer of Gram-negatives. While native (unmodified) lysins are effective against Gram-positives, they require engineering to displace or disrupt the outer membrane (OM) and facilitate access to subjacent peptidoglycan in Gram-negatives. The amurin peptide class of DLAs has a non-murolytic MOA based on disruption of the OM. EAD, enzymatically active domain; CBD, cell wall-binding domain; PG, peptidoglycan; and LPS, lipopolysaccharide.
Lysins with in vitro bacteriolytic activities against an array of Gram-positive organisms, including staphylococci, streptococci, Clostridium spp., Paenibacillus larvae, Bacillus anthracis, Listeria monocytogenes and others, have been described and reviewed in the literature (Schuch et al., 2002; Nelson et al., 2012; Oliveira et al., 2015; Fischetti, 2017; Haddad Kashani et al., 2018; Murray et al., 2021; Sekiya et al., 2021). A limited number of these have advanced toward the clinic. Micreos Human Health (Bilthoven, Netherlands) recently reported results from open label, interventional, clinical study in South Africa, examining the safety and efficacy of a cream containing the antistaphylococcal lysin Endobioma™ (formerly Staphefekt™ SA.100) applied to 43 adults and children with mild to moderate atopic dermatitis, which produced a statistically and clinically significant reduction of disease severity and improved skin sensitivity and quality of life (Moreau et al., 2021). A second antistaphylococcal lysin, XZ.700, is also under development by Micreos as a topical treatment of atopic dermatitis and is currently in a phase I/IIa, single-center, randomized, double-blind, placebo-controlled, parallel treated dose-ranging study with 48 patients in The Netherlands (Murray et al., 2021). Roivant Sciences Inc. (New York, NY, United States) recently reported results from a Phase 1 study (ClinicalTrials.gov identifier NCT01855048) examining the safety, pharmacokinetics, and pharmacodynamics of single dose of LSVT-1701 (an antistaphylococcal lysin formerly called N-Rephasin® SAL200) in 32 healthy male subjects at a single center in South Korea (Wire et al., 2022). LSVT-1701 lysin was safe and well-tolerated; however, the Phase 2 trial was terminated for strategic reasons (ClinicalTrials.gov Identifier: NCT03089697).
Standing out as a potential therapeutic is the lysin exebacase (formerly CF-301), which is currently under development by ContraFect Corporation (Yonkers, NY, United States). Exebacase has been granted Breakthrough Therapy designation by the FDA and is the first and only lysin to advance into Phase 3 of clinical development as a systemically administered treatment to improve clinical outcomes for Staphylococcus aureus bacteremia, including right-sided endocarditis (ClinicalTrials.gov Identifier NCT04160468). Based on its position as a first-in-class and first-in-field compound, the discovery and development of exebacase is next described, with a focus on its hallmark in vitro microbiologic attributes, efficacy in animal models of infection, and results from a superiority-design, proof-of-concept Phase 2 clinical trial in the United States.
Exebacase was initially identified as a potent antistaphylococcal lysin with d-alanyl-l-glycyl endopeptidase activity (Schuch et al., 2014; Lood et al., 2017). It has a modular structure, 26 kilodaltons in size, with an N-terminal cysteine–histidine-dependent amidohydrolases/peptidases (CHAP) domain and a C-terminal SH3b cell wall-binding domain. A rapid bacteriolytic activity of exebacase against antibiotic-resistant S. aureus served as the initial basis for its further development (Schuch et al., 2014).
An accurate and reproducible method for antimicrobial susceptibility testing of S. aureus was developed for exebacase (CLSI, 2020; Oh et al., 2021a; Traczewski et al., 2021) and used in a surveillance study of clinical methicillin-susceptible and methicillin-resistant S. aureus isolates (MSSA and MRSA, respectively) collected from 2015 to 2017 in the United States, Europe, and South America. The clinical isolates were inhibited at ≤1 μg/ml of exebacase with 50% and 90% of organisms (MIC50 and MIC90, respectively) inhibited at 0.5 and 1 μg/ml, respectively (Traczewski et al., 2019). Exebacase was also uniformly active against a set of 666 contemporary S. aureus isolates responsible for bloodstream infections in the US in 2020, with an MIC range of 0.06–1 μg/ml and with MIC50, MIC90, and modal MIC values of 0.5 μg/ml (Mendes et al., 2021). No difference in activity was observed between the MSSA and MRSA isolates. In time-kill studies, exebacase used at 1x strain-specific MIC levels rapidly reduced S. aureus viability by 3-log10 within 30 min at the MIC (Schuch et al., 2014). Synergy was observed for interactions tested with each of 12 antibiotics, where checkerboard studies with S. aureus isolates showed fractional inhibitory concentration index values of ≤0.5, and this synergetic effect was further confirmed in time–kill assays (Watson et al., 2020). Exebacase is also highly active against S. aureus in human serum, plasma, and whole blood (Indiani et al., 2019), synovial fluid (Oh et al., 2019a), and pulmonary surfactant (Swift et al., 2021).
Exebacase is furthermore active against S. aureus persister cells and biofilm bacteria (Schuch et al., 2017). In biofilm studies, exebacase demonstrated a minimal biofilm eradicating concentration (MBEC) of ≤0.25 μg/ml for 90% of organisms tested, including 55 MRSA strains (Schuch et al., 2017). Biofilms formed on polystyrene, glass, surgical mesh, and catheter surfaces were all susceptible to exebacase, as were biofilms treated in the context of human serum, pulmonary surfactant (±daptomycin), and synovial fluid (Schuch et al., 2017; Swift et al., 2021). Bacteria in biofilms are highly recalcitrant to antibiotic therapy and novel strategies that target biofilms are highly desirable (de la Fuente-Nunez et al., 2013). Based on these findings, exebacase may have therapeutic potential as a new medical modality to treat biofilm-associated S. aureus infections.
Short and/or sub-MIC exposure of exebacase to S. aureus exerts suppressive effects on both growth and virulence traits (Oh et al., 2019b). Mean post-antibiotic effect (PAE), post-antibiotic sub-MIC effect (PA-SME), and sub-MIC effect (SME) values up to 4.8, 9.3, and 9.8 h, respectively, were observed in human serum, and growth inhibition was extended by up to 6 h in the additional presence of sub-MIC of daptomycin. Exposures to exebacase at sub-MIC levels as low as 0.001x MIC resulted in aberrant cell wall ultrastructure, increased membrane permeability, dissipation of membrane potential, and the inhibition of virulence phenotypes, including agglutination and biofilm formation. These findings suggest that the bactericidal activity of exebacase may be complemented by antivirulence functions at concentrations well below the MIC (Oh et al., 2019b).
Importantly, exebacase exhibits a low propensity for resistance development and the ability to suppress resistance development of other antibiotics when used in combination (Schuch et al., 2014; Oh and Schuch, 2017; Oh et al., 2018). Serial passage of exebacase alone resulted in only up to 2-fold increases in MIC, and when used at sub-MIC concentrations in addition to daptomycin, vancomycin, and oxacillin, exebacase suppressed the development of resistance for those antibiotics. Resistance development by spontaneous mutation seems to be a rare event for lysins, which may be attributed to the essential and conserved nature of peptidoglycan bonds that are the targets for these lysins (Fischetti et al., 2006). Resistance spread by horizontal transfer between intrinsically resistant and susceptible species, while unlikely, cannot be ruled out (Grishin et al., 2020).
The rapid bactericidal activity (Schuch et al., 2014), synergy with antibiotics (Schuch et al., 2014; Wittekind and Schuch, 2016; Watson et al., 2020), antibiofilm activity (Schuch et al., 2014, 2017), extended post-antibiotic effect (Oh et al., 2019b), and low propensity for resistance development (Schuch et al., 2014; Oh and Schuch, 2017; Oh et al., 2018) differentiate exebacase from traditional small molecule antibiotics and support further development of this DLA.
Results from in vitro synergy studies with exebacase and antibiotics, correlate with data from pre-clinical pharmacology studies showing potent in vivo activity for exebacase used in addition to conventional antistaphylococcal antibiotics including daptomycin, vancomycin, and oxacillin.
In a model of infective endocarditis infection with MRSA in rabbits, the addition of exebacase to either daptomycin or vancomycin resulted in significant reductions in colony-forming units (CFUs) in all target organs when compared to reductions observed with either antibiotic alone (Indiani et al., 2019). A single intravenous (i.v.) administration of exebacase as low as 0.09 mg/kg (in addition to a subhuman-equivalent dose of daptomycin) achieved a 6-log10-unit drop in CFUs per gram in heart valve vegetations compared to a 3-log10-unit drop in CFUs with daptomycin alone.
In a mouse model of MRSA bacteremia, exebacase administered i.v. in addition to daptomycin resulted in more than 70% survival, compared to less than 30% survival for daptomycin alone (Schuch et al., 2014). A single i.v. dose of exebacase in addition to daptomycin administered intraperitoneally every 12 h for 4 days also resulted in an additional decrease of more than 1.5 log10 CFU/gram of bone (compared to daptomycin alone) in a rat model of MRSA osteomyelitis (Karau et al., 2019). In the neutropenic murine thigh infection model, the addition of exebacase to daptomycin resulted in a 1.03 ± 0.72 log10 CFU/thigh reduction, whereas daptomycin alone resulted in growth of 0.39 ± 1.19 log10 CFU/thigh (Asempa et al., 2020). Finally, in a murine pneumonia model, exebacase used in addition to daptomycin resulted in 70% survival at 14 days, whereas treatment with daptomycin alone yielded no survivors (Swift et al., 2021).
As a first-in-class antimicrobial agent with the potential to improve clinical outcomes of S. aureus infections, exebacase completed a superiority-design, proof-of-concept Phase 2 clinical trial (Fowler et al., 2020). This randomized, double-blind, placebo-controlled study assessed the clinical response rates of patients with S. aureus bacteremia including endocarditis treated with a single i.v. infusion of exebacase in addition to standard-of-care antibiotics (SoCA) versus SoCA treatment.
Overall, 70.4% of patients in the exebacase and SoCA group and 60.0% of patients who received SoCA alone, were clinical responders at Day 14 (value of p = 0.3137). Importantly, in the pre-specified MRSA subgroup, the clinical responder rate at Day 14 was 42.8 percentage points higher in the exebacase-treated group compared with the SoCA alone group (responder rates of 74.1% vs. 31.3%, respectively; ad hoc value of p = 0.0101). Furthermore, a 21-percentage point lower 30-day all-cause mortality rate was observed among patients carrying MRSA that received exebacase with SoCA compared with those that received SoCA alone (3.7% vs. 25.0%, p = 0.0556). Among MRSA patients in the United States, the median length of stay was 6 days for patients treated with exebacase and SoCA, compared to 10 days for patients treated with antibiotics alone; a trend toward lower 30-day all-cause readmission rates (48% lower) was also observed in the exebacase-treated group compared with antibiotics alone. Exebacase was, furthermore, generally safe and well-tolerated, with adverse events consistent with those expected in critically ill, hospitalized patients with potentially life-threatening S. aureus bloodstream infection, including endocarditis and/or underlying comorbid conditions.
Based on the results of the Phase 2 study, exebacase has progressed into Phase 3 of clinical development for the treatment of S. aureus bacteremia including right-sided endocarditis and was granted Breakthrough Therapy designation for this indication by the FDA.
Unlike lysins targeting the surface exposed peptidoglycan of Gram-positive bacteria, exogenously applied lysins targeting Gram-negatives must first translocate across or permeabilize the outer membrane (OM) to reach their target peptidoglycan (Figure 2). The OM is a very effective barrier to bulky molecules with molecular masses of more than ∼600 Da, including lysins (Delcour, 2009; Silhavy et al., 2010). Thus, for most lysins to be active against Gram-negative bacteria, the use of either high hydrostatic pressures to transiently permeabilize the OM (Nakimbugwe et al., 2006; Briers et al., 2008) or the simultaneous addition of membrane destabilizing agents like poly-L-lysine, polymyxin B, ethylenediaminetetraacetic acid (EDTA), organic acids, or the essential oil carvacrol were originally required (Briers et al., 2011; Legotsky et al., 2014; Oliveira et al., 2014; Shavrina et al., 2016; Antonova et al., 2019; Ciepluch et al., 2019).
The long-held belief that the OM barrier precludes use of purified lysins against Gram-negative bacteria was first dispelled by the description of several lysins with “intrinsic” OM penetrating activity. Carboxy-terminal amphipathic α-helices within these lysins mediated translocation across the OM to reach the subjacent peptidoglycan with subsequent hydrolysis and cell killing (Lood et al., 2015; Larpin et al., 2018; Raz et al., 2019; Vasina et al., 2021; Vazquez et al., 2021). The intrinsic activity of these lysins is, however, restricted to low osmotic strength buffers and environments lacking physiological concentrations of NaCl and divalent cations, or human blood matrices, thus precluding, systemic administration and restricting their activity in vivo to topical use.
Further development of lysins targeting Gram-negative pathogens exploited the modular nature of such enzymes and the capability to append functional domains, in particular antimicrobial peptides and OM permeabilizing peptides (Briers et al., 2014a,b; Sauve et al., 2018, 2019). Engineered lysin CF-370 is distinguished here as it is the first Gram-negative lysin reported active in human serum and, thus potentially amenable for systemic administration. CF-370 is active with MIC90 values of 0.5–4 μg/ml against strain sets that include MDR and XDR P. aeruginosa, K. pneumoniae, Escherichia coli, A. baumannii, Enterobacter cloacae, and Stenotrophomonas maltophilia (Sauve et al., 2018, 2019; Watson et al., 2019b, 2021a,b; Oh et al., 2021b). CF-370 exhibits all of the microbiologic attributes associated with lysins targeting Gram-positive bacteria, including a rapid bactericidal effect against sensitive organisms, antibiofilm activity, synergy with a range of antibiotics, and the capacity to resensitize antibiotic-resistant bacteria (Watson et al., 2019b, 2021a,b).
Two pre-clinical pharmacology studies provided in vivo proof-of-concept efficacy data supporting the systemic administration of CF-370. In a model of acute P. aeruginosa pneumonia in rabbits, i.v. administration of CF-370 as a single dose (at 3 and 10 mg/kg) in addition to meropenem (20 mg/kg, q8h) achieved a 3-log10-CFU reduction per gram of lung compared to a 1-log10 reduction with either meropenem or CF-370 alone (Lehoux et al., 2021a). CF-370 also limited proliferation in secondary organs, as significant reduced bacterial counts were observed in kidney and spleen when used alone or in addition to meropenem. In a rabbit model of right-sided infective endocarditis caused by P. aeruginosa, 3 daily i.v. doses of CF-370 in addition to meropenem resulted in a 2-log10 CFU reduction per gram of heart vegetation compared to meropenem alone (Lehoux et al., 2021b). Up to 2.2-log10 were also observed in secondary organs, compared to meropenem alone. The in vivo efficacy observed for systemically administered CF-370 in these two models of invasive infection clearly distinguishes CF-370 from other engineered lysins which were not active in serum and for which efficacy data are limited to a skin infection model using human keratinocytes (Briers et al., 2014b), nematode and wax moth models (Briers et al., 2014b; Chen et al., 2021), an ex vivo pig and mouse burn wound model (Gerstmans et al., 2020; Li et al., 2021), or treatment of ear infections in dogs (Briers and Lavigne, 2015).
The in vitro and in vivo data for CF-370 is the first evidence that lysins can be engineered for i.v. delivery. This work spectrum antimicrobial provides a solid basis for further development of CF-370 (and, potentially, other engineered lysins) as a treatment for serious and life-threating infections with MDR and XDR Gram-negative pathogens.
An entirely new class of DLAs were recently identified, called amurin (for “amurolytic”) peptides, with bactericidal activity against a broad range of Gram-negative ESKAPE bacteria including carbapenem-resistant Enterobacterales (CRE), P. aeruginosa (CRP), and A. baumannii (CRA; Sauve et al., 2021). Derived from the lytic systems of phage, the amurins consist of a related family of small alpha-helical cationic peptides, typically 40–50 amino acids in length and distinct in sequence from previously described antimicrobial peptides (Sauve et al., 2021).
As synthesized peptides, amurins demonstrate a mechanism of action based on rapid OM-permeabilization, distinct from the cell wall hydrolytic activity of lysin enzymes (Figure 2; Sauve et al., 2021). For select amurins, MIC90 values of ≤1 μg/ml were reported against strain sets of P. aeruginosa, A. baumannii, K. pneumoniae, E. cloacae, and E. coli. The amurins are furthermore active in human serum and blood and exhibit many of the hallmark microbiological attributes associated with the lysins including in vitro and ex vivo antibiofilm activity (with MBEC values ranging from 0.125 to 2 μg/ml), synergy with up to 11 different conventional antibiotics tested and a low propensity for resistance development (Oh et al., 2019c; Sauve et al., 2021).
The discovery of an entirely new class of DLAs with potent activity against MDR and XDR Gram-negative pathogens and with a mechanism of action distinct from lysins brings the promise for further expanding the armamentarium of differentiated antimicrobial agents for treating invasive life-threatening infections.
In this era of diminished antibiotic susceptibility, DLAs provide new and highly differentiated antimicrobial modalities. The promise for DLAs lies in their diverse mechanisms of action and range of potent activity against both Gram-positive and Gram-negative bacteria. Key microbiological attributes shared among the DLAs are rapid bactericidal activity, antibiofilm activity, synergy with antibiotics, and a low propensity for resistance. Exebacase has provided proof-of-concept data in humans for the DLA platform, as it proceeds through clinical trials to treat life-threatening S. aureus bacteremia including right-sided endocarditis. CF-370 is the first DLA that can be administered systemically to treat infections caused by antibiotic-resistant Gram-negative bacteria. This enzyme was developed through protein engineering to allow new lysin specificities permeate through the OM barrier. Finally, the amurin peptides have been shown to be unique DLAs that provide differentiated mechanisms of antimicrobial action. Moving forward, still other lysins, amurin peptides, and perhaps altogether new DLAs are envisioned to reinvigorate the pipeline for new antimicrobial agents to satisfy the unmet medical need of treating infections caused by multidrug-resistant bacteria.
RS, CC, and XV-F conceptualized and wrote the manuscript. All authors contributed to the article and approved the submitted version.
ContraFect receives funding from CARB-X, which is sponsored by the Cooperative Agreement Number IDSEP160030 from ASPR/BARDA and by an award from Wellcome Trust.
RS, CC, and XV-F are employees of ContraFect Corporation.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Boudewijn DeJonge, Jane Ambler, Dario Lehoux, and Gary Woodnutt for reviewing this manuscript.
Antonova, N. P., Vasina, D. V., Lendel, A. M., Usachev, E. V., Makarov, V. V., Gintsburg, A. L., et al. (2019). Broad bactericidal activity of the myoviridae bacteriophage lysins LysAm24, LysECD7, and LysSi3 against gram-negative ESKAPE pathogens. Viruses 11:284. doi: 10.3390/v11030284
Asempa, T. E., Abdelraouf, K., Carabeo, T., Schuch, R., and Nicolau, D. P. (2020). Synergistic activity of exebacase (CF-301) in addition to daptomycin against Staphylococcus aureus in a neutropenic murine thigh infection model. Antimicrob. Agents Chemother. 64, e02176–19. doi: 10.1128/AAC.02176-19
Boucher, H. W. (2020). Bad bugs, no drugs 2002-2020: progress, challenges, and call to action. Trans. Am. Clin. Climatol. Assoc. 131, 65–71.
Briers, Y., Cornelissen, A., Aertsen, A., Hertveldt, K., Michiels, C. W., Volckaert, G., et al. (2008). Analysis of outer membrane permeability of Pseudomonas aeruginosa and bactericidal activity of endolysins KZ144 and EL188 under high hydrostatic pressure. FEMS Microbiol. Lett. 280, 113–119. doi: 10.1111/j.1574-6968.2007.01051.x
Briers, Y., and Lavigne, R. (2015). Breaking barriers: expansion of the use of endolysins as novel antibacterials against gram-negative bacteria. Future Microbiol. 10, 377–390. doi: 10.2217/fmb.15.8
Briers, Y., Walmagh, M., Grymonprez, B., Biebl, M., Pirnay, J. P., Defraine, V., et al. (2014a). Art-175 is a highly efficient antibacterial against multidrug-resistant strains and persisters of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 58, 3774–3784. doi: 10.1128/AAC.02668-14
Briers, Y., Walmagh, M., and Lavigne, R. (2011). Use of bacteriophage endolysin EL188 and outer membrane permeabilizers against Pseudomonas aeruginosa. J. Appl. Microbiol. 110, 778–785. doi: 10.1111/j.1365-2672.2010.04931.x
Briers, Y., Walmagh, M., Van Puyenbroeck, V., Cornelissen, A., Cenens, W., Aertsen, A., et al. (2014b). Engineered endolysin-based “Artilysins” to combat multidrug-resistant gram-negative pathogens. MBio 5, e01379–e01414. doi: 10.1128/mBio.01379-14
Broendum, S. S., Buckle, A. M., and Mcgowan, S. (2018). Catalytic diversity and cell wall binding repeats in the phage-encoded endolysins. Mol. Microbiol. 110, 879–896. doi: 10.1111/mmi.14134
Bustamante, N., Iglesias-Bexiga, M., Bernardo-Garcia, N., Silva-Martin, N., Garcia, G., Campanero-Rhodes, M. A., et al. (2017). Deciphering how Cpl-7 cell wall-binding repeats recognize the bacterial peptidoglycan. Sci. Rep. 7:16494. doi: 10.1038/s41598-017-16392-4
Chen, X., Liu, M., Zhang, P., Leung, S. S. Y., and Xia, J. (2021). Membrane-permeable antibacterial enzyme against multidrug-resistant Acinetobacter baumannii. ACS Infect. Dis. 7, 2192–2204. doi: 10.1021/acsinfecdis.1c00222
Ciepluch, K., Maciejewska, B., Galczynska, K., Kuc-Ciepluch, D., Bryszewska, M., Appelhans, D., et al. (2019). The influence of cationic dendrimers on antibacterial activity of phage endolysin against P. aeruginosa cells. Bioorg. Chem. 91:103121. doi: 10.1016/j.bioorg.2019.103121
CLSI (2020). “Performance Standards for Antimicrobial Susceptibility Testing, Document M100. ” 30th Edn. (Wayne, PA: Clinical and Laboratory Standards Institute).
Cole, S. T. (2014). Who will develop new antibacterial agents? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 369:20130430. doi: 10.1098/rstb.2013.0430
de la Fuente-Nunez, C., Reffuveille, F., Fernandez, L., and Hancock, R. E. (2013). Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 16, 580–589. doi: 10.1016/j.mib.2013.06.013
Delcour, A. H. (2009). Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta 1794, 808–816. doi: 10.1016/j.bbapap.2008.11.005
Fischetti, V. A. (2017). Lysin therapy for Staphylococcus aureus and other bacterial pathogens. Curr. Top. Microbiol. Immunol. 409, 529–540. doi: 10.1007/82_2015_5005
Fischetti, V. A., Nelson, D., and Schuch, R. (2006). Reinventing phage therapy: are the parts greater than the sum? Nat. Biotechnol. 24, 1508–1511. doi: 10.1038/nbt1206-1508
Fowler, V. G. Jr., Das, A. F., Lipka-Diamond, J., Schuch, R., Pomerantz, R., Jauregui-Peredo, L., et al. (2020). Exebacase for patients with Staphylococcus aureus bloodstream infection and endocarditis. J. Clin. Invest. 130, 3750–3760. doi: 10.1172/JCI136577
Ganguly, J., Low, L. Y., Kamal, N., Saile, E., Forsberg, L. S., Gutierrez-Sanchez, G., et al. (2013). The secondary cell wall polysaccharide of bacillus anthracis provides the specific binding ligand for the C-terminal cell wall-binding domain of two phage endolysins, PlyL and PlyG. Glycobiology 23, 820–832. doi: 10.1093/glycob/cwt019
Gerstmans, H., Criel, B., and Briers, Y. (2018). Synthetic biology of modular endolysins. Biotechnol. Adv. 36, 624–640. doi: 10.1016/j.biotechadv.2017.12.009
Gerstmans, H., Grimon, D., Gutierrez, D., Lood, C., Rodriguez, A., Van Noort, V., et al. (2020). A VersaTile-driven platform for rapid hit-to-lead development of engineered lysins. Sci. Adv. 6:eaaz1136. doi: 10.1126/sciadv.aaz1136
Grishin, A. V., Karyagina, A. S., Vasina, D. V., Vasina, I. V., Gushchin, V. A., and Lunin, V. G. (2020). Resistance to peptidoglycan-degrading enzymes. Crit. Rev. Microbiol. 46, 703–726. doi: 10.1080/1040841X.2020.1825333
Haddad Kashani, H., Schmelcher, M., Sabzalipoor, H., Seyed Hosseini, E., and Moniri, R. (2018). Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: current status of research and novel delivery strategies. Clin. Microbiol. Rev. 31, e00071–17. doi: 10.1128/CMR.00071-17
Indiani, C., Sauve, K., Raz, A., Abdelhady, W., Xiong, Y. Q., Cassino, C., et al. (2019). The antistaphylococcal lysin, CF-301, activates key host factors in human blood to potentiate methicillin-resistant Staphylococcus aureus bacteriolysis. Antimicrob. Agents Chemother. 63, e02291–e02318. doi: 10.1128/AAC.02291-18
Karau, M. J., Schmidt-Malan, S. M., Yan, Q., Greenwood-Quaintance, K. E., Mandrekar, J., Lehoux, D., et al. (2019). Exebacase in addition to daptomycin is more active than daptomycin or exebacase alone in methicillin-resistant Staphylococcus aureus osteomyelitis in rats. Antimicrob. Agents Chemother. 63, e01235–19. doi: 10.1128/AAC.01235-19
King, H., Ajay Castro, S., Pohane, A. A., Scholte, C. M., Fischetti, V. A., Korotkova, N., et al. (2021). Molecular basis for recognition of the group A carbohydrate backbone by the PlyC streptococcal bacteriophage endolysin. Biochem. J. 478, 2385–2397. doi: 10.1042/BCJ20210158
Larpin, Y., Oechslin, F., Moreillon, P., Resch, G., Entenza, J. M., and Mancini, S. (2018). In vitro characterization of PlyE146, a novel phage lysin that targets gram-negative bacteria. PLoS One 13:e0192507. doi: 10.1371/journal.pone.0192507
Legotsky, S. A., Vlasova, K. Y., Priyma, A. D., Shneider, M. M., Pugachev, V. G., Totmenina, O. D., et al. (2014). Peptidoglycan degrading activity of the broad-range Salmonella bacteriophage S-394 recombinant endolysin. Biochimie 107, 293–299. doi: 10.1016/j.biochi.2014.09.017
Lehoux, D., Abdelhady, W., Xiong, Y.Q., Swift, S., Cassino, C., Schuch, R., et al. (2021a). “CF-370, a systemically administered lysin with in vivo efficacy against a carbapenem-resistant Pseudomonas aeruginosa strain in a rabbit pulmonary infection model.” in European Congress of Clinical Microbiology and Infectious Diseases. April 13–16; Vienna, Austria.
Lehoux, D., Abdelhady, W., Xiong, Y.Q., Swift, S., Cassino, C., Schuch, R., et al. (2021b). “Lysin CF-370 in vivo efficacy against a carbapenem-resistant Pseudomonas aeruginosa in a rabbit infective endocarditis model.” in World Microbe Forum. June 20–24.
Li, C., Jiang, M., Khan, F. M., Zhao, X., Wang, G., Zhou, W., et al. (2021). Intrinsic antimicrobial peptide facilitates a new broad-spectrum lysin LysP53 to kill Acinetobacter baumannii in vitro and in a mouse burn infection model. ACS Infect. Dis. 7, 3336–3344. doi: 10.1021/acsinfecdis.1c00497
Loeffler, J. M., Nelson, D., and Fischetti, V. A. (2001). Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294, 2170–2172. doi: 10.1126/science.1066869
Lood, R., Molina, H., and Fischetti, V. A. (2017). Determining bacteriophage endopeptidase activity using either fluorophore-quencher labeled peptides combined with liquid chromatography-mass spectrometry (LC-MS) or forster resonance energy transfer (FRET) assays. PLoS One 12:e0173919. doi: 10.1371/journal.pone.0173919
Lood, R., Winer, B. Y., Pelzek, A. J., Diez-Martinez, R., Thandar, M., Euler, C. W., et al. (2015). Novel phage lysin capable of killing the multidrug-resistant gram-negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrob. Agents Chemother. 59, 1983–1991. doi: 10.1128/AAC.04641-14
Mendes, R. E., Lindley, J., Gurung, N., Castanheira, M., Schuch, R., and Ambler, J. E. (2021). In vitro activity of exebacase (CF-301) against Staphylococcus aureus causing bacteremia in the United States, including multidrug-resistant subsets. Open Forum Infect. Dis. 8(Supplement_1), S621–S622. doi: 10.1093/ofid/ofab466.1253
Moreau, M., Seite, S., Aguilar, L., Da Cruz, O., Puech, J., Frieling, J., et al. (2021). Topical S. aureus - targeting endolysin significantly improves symptoms and QoL in individuals With atopic dermatitis. J. Drugs Dermatol. 20, 1323–1328. doi: 10.36849/JDD.6363
Murray, E., Draper, L. A., Ross, R. P., and Hill, C. (2021). The advantages and challenges of using endolysins in a clinical setting. Viruses 13:680. doi: 10.3390/v13040680
Nakimbugwe, D., Masschalck, B., Atanassova, M., Zewdie-Bosuner, A., and Michiels, C. W. (2006). Comparison of bactericidal activity of six lysozymes at atmospheric pressure and under high hydrostatic pressure. Int. J. Food Microbiol. 108, 355–363. doi: 10.1016/j.ijfoodmicro.2005.11.021
Nelson, D., Loomis, L., and Fischetti, V. A. (2001). Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. U. S. A. 98, 4107–4112. doi: 10.1073/pnas.061038398
Nelson, D. C., Schmelcher, M., Rodriguez-Rubio, L., Klumpp, J., Pritchard, D. G., Dong, S., et al. (2012). Endolysins as antimicrobials. Adv. Virus Res. 83, 299–365. doi: 10.1016/B978-0-12-394438-2.00007-4
Oh, J. T., Ambler, J. E., Cassino, C., and Schuch, R. (2021a). Development of a broth microdilution method for exebacase susceptibility testing. Antimicrob. Agents Chemother. 65:e0258720. doi: 10.1128/AAC.02587-20
Oh, J., Cassino, C., and Schuch, R. (2019a). “Lysin exebacase (CF-301) exhibits potent bactericidal activity in human synovial fluid (HSF) against biofilm-forming Staphylococcus epidermidis (S. epidermidis) isolates.” in European Congress of Clinical Microbiology & Infectious Diseases (ECCMID). April 13–16; Amsterdam, Netherlands.
Oh, J. T., Cassino, C., and Schuch, R. (2019b). Postantibiotic and sub-MIC effects of exebacase (Lysin CF-301) enhance antimicrobial activity against Staphylococcus aureus. Antimicrob. Agents Chemother. 63, e02616–18. doi: 10.1128/AAC.02616-18
Oh, J., Noto, M.J., Borgmann, T., Sika, M., Cassino, C., Dwyer, J.P., et al. (2019c). “Amurin peptide App2-M1 eradicates Stenotrophomonas maltophilia biofilms formed on hemodialysis catheters in the setting of human infection.” in ASM-ESCMID. September 3–6; Boston, MA.
Oh, J., Sauve, K., Watson, A., Jandourek, A., Abdelhady, W., Xiong, Y.Q., et al. (2018). “Lysin CF-301 (exebacase) resensitizes methicillin-resistant Staphylococcus aureus (MRSA) to penicillin derivatives and first generation cephalosporins.” in ESCMID/ASM Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance. September 4–7; Lisbon, Portugal.
Oh, J., and Schuch, R. (2017). “Low propensity of resistance development in vitro in Staphylococcus aureus with lysin CF-301.” in ASM Microbe. June 1–5; New Orleans, LA.
Oh, J., Warner, M., Watson, A., Sauve, K., Swift, S., Ambler, J., et al. (2021b). “Direct lytic agents (DLAs), a novel family of antimicrobial agents, exert potent in vitro bactericidal activity against gram-negative (GN) pathogens which cause pulmonary infections in CF patients, including Pseudomonas aeruginosa, Stenotrophomonas maltophilia, and Achromobacter xylosoxidans.” in North American Cystic Fibrosis Conference. November 2–5.
Oliveira, A., Leite, M., Kluskens, L. D., Santos, S. B., Melo, L. D., and Azeredo, J. (2015). The first Paenibacillus larvae bacteriophage endolysin (PlyPl23) with high potential to control American foulbrood. PLoS One 10:e0132095. doi: 10.1371/journal.pone.0132095
Oliveira, H., Thiagarajan, V., Walmagh, M., Sillankorva, S., Lavigne, R., Neves-Petersen, M. T., et al. (2014). A thermostable salmonella phage endolysin, Lys68, with broad bactericidal properties against gram-negative pathogens in presence of weak acids. PLoS One 9:e108376. doi: 10.1371/journal.pone.0108376
Raz, A., Serrano, A., Hernandez, A., Euler, C. W., and Fischetti, V. A. (2019). Isolation of phage lysins that effectively kill Pseudomonas aeruginosa in mouse models of lung and skin infection. Antimicrob. Agents Chemother. 63, e00024–19. doi: 10.1128/AAC.00024-19
Santos, S. B., Oliveira, A., Melo, L. D. R., and Azeredo, J. (2019). Identification of the first endolysin cell binding domain (CBD) targeting Paenibacillus larvae. Sci. Rep. 9:2568. doi: 10.1038/s41598-019-39097-2
Sauve, K., Oh, J., Watson, A., Cassino, C., and Schuch, R. (2021). “Amurin peptides: new direct lytic agents (DLAs) with broad spectrum antimicrobial activity against Gram-negative (GN) ESKAPE pathogens.” in World Microbe Forum. June 20–24.
Sauve, K., Oh, J.T., Watson, A., Mozdierz, J., Indiani, C., Cassino, C., et al. (2019). “Lysins exhibit potent antimicrobial activity, synergy and antibiofilm effects against Pseudomonas aeruginosa in human serum and pulmonary surfactant.” in American Society For Microbiology (ASM) Microbe. June 20–24; San Francisco, California.
Sauve, K., Watson, A., Mozdierz, J., Indiani, C., Jandourek, A., Cassino, C., et al. (2018). “Bacteriophage-derived lysins can be engineered to exert a rapid and potent bactericidal effect against Pseudomonas aeruginosa in serum.” in American Society For Microbiology (ASM) Microbe. June 6–11; Atlanta, Georgia.
Schmelcher, M., and Loessner, M. J. (2014). Use of bacteriophage cell wall-binding proteins for rapid diagnostics of Listeria. Methods Mol. Biol. 1157, 141–156. doi: 10.1007/978-1-4939-0703-8_12
Schmelcher, M., Shabarova, T., Eugster, M. R., Eichenseher, F., Tchang, V. S., Banz, M., et al. (2010). Rapid multiplex detection and differentiation of Listeria cells by use of fluorescent phage endolysin cell wall binding domains. Appl. Environ. Microbiol. 76, 5745–5756. doi: 10.1128/AEM.00801-10
Schuch, R., Khan, B. K., Raz, A., Rotolo, J. A., and Wittekind, M. (2017). Bacteriophage lysin CF-301, a potent antistaphylococcal biofilm agent. Antimicrob. Agents Chemother. 61, e02666–e02716. doi: 10.1128/AAC.02666-16
Schuch, R., Lee, H. M., Schneider, B. C., Sauve, K. L., Law, C., Khan, B. K., et al. (2014). Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J. Infect. Dis. 209, 1469–1478. doi: 10.1093/infdis/jit637
Schuch, R., Nelson, D., and Fischetti, V. A. (2002). A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418, 884–889. doi: 10.1038/nature01026
Schuch, R., Pelzek, A. J., Nelson, D. C., and Fischetti, V. A. (2019). The PlyB endolysin of bacteriophage vB_BanS_Bcp1 exhibits broad-spectrum bactericidal activity against Bacillus cereus Sensu Lato isolates. Appl. Environ. Microbiol. 85, e00003–e00019. doi: 10.1128/AEM.00003-19
Schuch, R., Pelzek, A. J., Raz, A., Euler, C. W., Ryan, P. A., Winer, B. Y., et al. (2013). Use of a bacteriophage lysin to identify a novel target for antimicrobial development. PLoS One 8:e60754. doi: 10.1371/journal.pone.0060754
Sekiya, H., Okada, M., Tamai, E., Shimamoto, T., Shimamoto, T., and Nariya, H. (2021). A putative amidase endolysin encoded by Clostridium perfringens St13 exhibits specific lytic activity and synergizes with the muramidase endolysin psm. Antibiotics 10:245. doi: 10.3390/antibiotics10030245
Serafim, M. S. M., Kronenberger, T., Oliveira, P. R., Poso, A., Honorio, K. M., Mota, B. E. F., et al. (2020). The application of machine learning techniques to innovative antibacterial discovery and development. Expert Opin. Drug Discovery 15, 1165–1180. doi: 10.1080/17460441.2020.1776696
Shavrina, M. S., Zimin, A. A., Molochkov, N. V., Chernyshov, S. V., Machulin, A. V., and Mikoulinskaia, G. V. (2016). In vitro study of the antibacterial effect of the bacteriophage T5 thermostable endolysin on Escherichia coli cells. J. Appl. Microbiol. 121, 1282–1290. doi: 10.1111/jam.13251
Silhavy, T. J., Kahne, D., and Walker, S. (2010). The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2:a000414. doi: 10.1101/cshperspect.a000414
Silver, L. L. (2011). Challenges of antibacterial discovery. Clin. Microbiol. Rev. 24, 71–109. doi: 10.1128/CMR.00030-10
Singh, S. B., Young, K., and Silver, L. L. (2017). What is an “ideal” antibiotic? Discovery challenges and path forward. Biochem. Pharmacol. 133, 63–73. doi: 10.1016/j.bcp.2017.01.003
Swift, S. M., Sauve, K., Cassino, C., and Schuch, R. (2021). Exebacase is active In vitro in pulmonary surfactant and is efficacious alone and synergistic with daptomycin in a mouse model of lethal Staphylococcus aureus lung infection. Antimicrob. Agents Chemother. 65:e0272320. doi: 10.1128/AAC.02723-20
Traczewski, M. M., Ambler, J. E., and Schuch, R. (2021). Determination of MIC quality control parameters for exebacase, a novel lysin with antistaphylococcal activity. J. Clin. Microbiol. 59:e0311720. doi: 10.1128/JCM.03117-20
Traczewski, M., Oh, J., Cassino, C., and Schuch, R. (2019). In vitro activity of Exebacase (CF-301) against clinical Staphylococcus aureus surveillance isolates from the United States, Europe, and Latin America, 2015-2017. Diagn. Microbiol. Infect. Dis. 95:114879. doi: 10.1016/j.diagmicrobio.2019.114879
Travis, A., Chernova, O., Chernov, V., and Aminov, R. (2018). Antimicrobial drug discovery: lessons of history and future strategies. Expert Opin. Drug Discovery 13, 983–985. doi: 10.1080/17460441.2018.1515910
Vasina, D. V., Antonova, N. P., Grigoriev, I. V., Yakimakha, V. S., Lendel, A. M., Nikiforova, M. A., et al. (2021). Discovering the potentials of four phage endolysins to combat gram-negative infections. Front. Microbiol. 12:748718. doi: 10.3389/fmicb.2021.748718
Vazquez, R., Blanco-Ganan, S., Ruiz, S., and Garcia, P. (2021). Mining of gram-negative surface-active enzybiotic candidates by sequence-based calculation of physicochemical properties. Front. Microbiol. 12:660403. doi: 10.3389/fmicb.2021.660403
Vermassen, A., Leroy, S., Talon, R., Provot, C., Popowska, M., and Desvaux, M. (2019). Cell wall hydrolases in bacteria: insight on the diversity of cell wall amidases, glycosidases and peptidases toward peptidoglycan. Front. Microbiol. 10:331. doi: 10.3389/fmicb.2019.00331
Watson, A., Oh, J. T., Sauve, K., Bradford, P. A., Cassino, C., and Schuch, R. (2019a). Antimicrobial activity of exebacase (Lysin CF-301) against the most common causes of infective endocarditis. Antimicrob. Agents Chemother. 63, e01078–e01119. doi: 10.1128/AAC.01078-19
Watson, A., Sauve, K., Cassino, C., and Schuch, R. (2019b). “Lysin GN123 resensitizes carbapenem-resistant Pseudomonas aeruginosa to imipenem.” in European Congress of Clinical Microbiology & Infectious Diseases (ECCMID). April 13–16; Amsterdam, Netherlands.
Watson, A., Sauve, K., Cassino, C., and Schuch, R. (2020). Exebacase demonstrates in vitro synergy with a broad range of antibiotics against both methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 64, e01885–e01919. doi: 10.1128/AAC.01885-19
Watson, A., Sauve, K., Oh, J., Swift, S., Cassino, C., and Schuch, R. (2021a). “Lysin CF-370 exhibits potent bactericidal activity against clinical MDR and XDR Pseudomonas aeruginosa isolates including carbapenem and/or colistin resistant forms.” in European Congress of Clinical Microbiology & Infectious Diseases (ECCMID). April 13–16; Vienna, Austria.
Watson, A., Sauve, K., Oh, J., Warner, M., Swift, S., Cassino, C., et al. (2021b). “Lysin CF-370 exhibits broad spectrum antimicrobial activity against gram-negative (GN) ESKAPE pathogens.” in World Microbe Forum. June 20–24.
WHO (2019). “Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline ”. (Geneva, Switzerland: World Health Organization).
Wire, M. B., Jun, S. Y., Jang, I. J., Lee, S. H., Hwang, J. G., and Huang, D. B. (2022). A phase 1 study to evaluate the safety and pharmacokinetics following administration of single and multiple doses of the anti-staphylococcal lysin, LSVT-1701, in healthy adult subjects. Antimicrob. Agents Chemother. doi: 10.1128/AAC.01842-21, Epub ahead of print
Keywords: lysin, cell wall hydrolase, biologic, antimicrobial, antibiotic resistance, peptides
Citation: Schuch R, Cassino C and Vila-Farres X (2022) Direct Lytic Agents: Novel, Rapidly Acting Potential Antimicrobial Treatment Modalities for Systemic Use in the Era of Rising Antibiotic Resistance. Front. Microbiol. 13:841905. doi: 10.3389/fmicb.2022.841905
Received: 22 December 2021; Accepted: 28 January 2022;
Published: 03 March 2022.
Edited by:
Pilar García, Spanish National Research Council (CSIC), SpainReviewed by:
Pedro García, Spanish National Research Council (CSIC), SpainCopyright © 2022 Schuch, Cassino and Vila-Farres. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raymond Schuch, cnNjaHVjaEBjb250cmFmZWN0LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.