- Shandong Provincial Key Laboratory of Agricultural Microbiology, College of Plant Protection, Shandong Agricultural University, Tai’an, China
Glucose-inhibited division protein (GidA) plays a critical role in the growth, stress response, and virulence of bacteria. However, how gidA may affect plant growth-promoting bacteria (PGPB) is still not clear. Our study aimed to describe the regulatory function of the gidA gene in Lysobacter capsici, which produces a variety of lytic enzymes and novel antibiotics. Here, we generated an LC_GidA mutant, MT16, and an LC_GidA complemented strain, Com-16, by plasmid integration. The deletion of LC_GidA resulted in an attenuation of the bacterial growth rate, motility, and biofilm formation of L. capsici. Root colonization assays demonstrated that the LC_GidA mutant showed reduced colonization of wheat roots. In addition, disruption of LC_GidA showed a clear diminution of survival in the presence of high temperature, high salt, and different pH conditions. The downregulated expression of genes related to DNA replication, cell division, motility, and biofilm formation was further validated by real-time quantitative PCR (RT–qPCR). Together, understanding the regulatory function of GidA is helpful for improving the biocontrol of crop diseases and has strong potential for biological applications.
Introduction
Lysobacter spp. are bacteria natively present in the rhizosphere, water, and some extreme conditions (Park et al., 2008; Fang et al., 2020). In recent years, species, such as Lysobacter enzymogenes, Lysobacter antibioticus, and Lysobacter capsici, have attracted much interest for their antimicrobial activities, and they are regarded as effective biocontrol agents of plant diseases (Yu et al., 2018; Afoshin et al., 2020). For example, heat stable antifungal factor (HSAF), isolated from L. enzymogenes C3, has been exhibited to be inhibitory activities against a wide range of fungal species (Yu et al., 2007). Compared to L. enzymogenes, much less is known about the biological features of L. capsici. The L. capsici AZ78 genome has a gene pool that allows it to successfully interact with plant pathogenic microorganisms and environmental factors, providing a genetic framework for detailed analysis of potential biocontrol mechanisms of plant pathogens (Puopolo et al., 2016). In addition, the effective antifungal effect of L. capsici AZ78 and L. capsici PG4 has been shown (Puopolo et al., 2010; Brescia et al., 2020). Twenty-two volatile organic compounds to be produced by L. capsici AZ78, that contribute to biological control of soilborne plant pathogens (Vlassi et al., 2020). Overall, the species of L. capsici has considerable potential for biocontrol of plant pathogenic microorganisms.
tRNA modification ensures efficient and accurate protein synthesis and promotes cellular health and growth (Manickam et al., 2016). Glucose-inhibited division protein (GidA), which is highly conserved in prokaryotes, serves as a tRNA modification enzyme and catalyzes the addition of a carboxymethylaminomethyl (cmnm) group at the 5′ position of the wobble uridine (U34) of tRNAs (Yu et al., 2019; Gao et al., 2020). GidA modification is evolutionarily conserved in bacteria and Eukarya, which is essential for efficient and accurate protein translation (Fislage et al., 2014). The disruption of gidA causes pleiotropy and affects multiple phenotypic traits. Therefore, the GidA-mediated tRNA modification pathway is thought to be the main regulatory mechanism of pathogenicity (Shippy and Fadl, 2014). The gidA gene is recognized to function in the regulation of bacterial growth, stress response, and virulence (Shippy and Fadl, 2014). In Aeromonas hydrophila, disruption of gidA resulted in altered cell morphology, reduced growth, and decreased cytotoxic enterotoxin production (Sha et al., 2004). In other bacteria genera, such as Salmonella spp. and Streptococcus spp., gidA mutants had motility defects, reduced survival under stressful conditions, and decreased expression of virulence proteins (Rehl et al., 2013; Zhang et al., 2014; Gao et al., 2016). In Pseudomonas syringae, the causal agent of bean spot disease, the gidA mutant had altered cell morphology and could not produce toxin (Kinscherf and Willis, 2002). In reality, GidA can regulate the expression of a variety of proteins at the translational level through tRNA modification, and thus can regulate the survival of bacteria in response to environmental signals under stressful conditions (Gustilo et al., 2008). Taken together, these studies highlight the importance of this conserved tRNA modification pathway in cellular processes. However, little is known about GidA in L. capsici.
Lysobacter capsici X2-3 was isolated from the wheat rhizosphere and showed marked antimicrobial activity against plant pathogenic fungi, oomycetes, and Gram-positive bacteria. Genes in the X2-3 genome were annotated using a combined analysis of the KEGG, COG, and GO databases, and several genes were predicted to be associated with antibiotic production (Yi et al., 2015). Although GidA family proteins play important roles in the regulation of bacterial growth, pathogenicity, and human diseases in pathogenic species, there are few studies on plant growth-promoting bacteria (PGPB). In this study, the biological function of LC_GidA was characterized by constructing an LC_GidA mutant. We demonstrated that the inactivation of LC_GidA significantly reduced bacterial growth, twitching motility, biofilm formation, root colonization, and stress response in L. capsici X2-3.
Materials and Methods
Bacterial Strains, Growth Conditions, and Plasmids
The bacterial strains and plasmids used in this study are listed in Table 1. Unless otherwise stated, L. capsici X2-3 and its derivative strains were grown at 28°C in nutrient broth (NB) medium or on NA (NB with 1.5% agar) medium. Transformants from the first crossover for the LC_GidA knockout were cultured on NBN (NB without 1% sucrose) or NAN (NBN with 1.5% agar) medium. Transformants bearing the second crossover were plated on NAS (NAN plus 10% sucrose) medium (Zou et al., 2011). All bacterial strains were incubated at 28°C. Escherichia coli strains were cultured in Luria-Bertani (LB) or LB plus 1.5% agar plates at 37°C. When necessary, the media were supplemented with the antibiotic ampicillin (Amp, 50 μg/ml), kanamycin (Km, 50 μg/ml), or gentamicin (Gm, 50 μg/ml), depending on the strains used.
Construction of the LC_GidA Deletion Mutant and Its Complemented Strain
The LC_GidA mutant was generated from the wild-type X2-3 strain by allelic homologous recombination. Briefly, two LC_GidA flanking regions were amplified by PCR using the primer pairs up F/R and down F/R (Table 1). The upstream and downstream PCR products were digested with BamHI and HindIII, respectively. The digested fragments were ligated into the suicide vector pKMS1 (Table 1) to obtain the recombinant plasmid pKMS1-AB (Zou et al., 2011). The plasmid was transformed into X2-3 by electroporation. The LC_GidA mutant MT16 was obtained after two recombination events and confirmed by PCR and sequencing of the PCR products.
The fragment harboring the intact LC_GidA gene, which was amplified by PCR using the primers gidAF and gidAR (Table 1), was cloned into the expression vector pBBR1-MCS5 (Table 1) at the EcoRI and BamHI site, resulting in the recombinant plasmid pBBR1-gidA, and then pBBR1-gidA was transformed into the mutant MT16 by electroporation (1.8 KV, 200 Ω, and 25 μF). The complemented mutant strain Com-16 was selected on NA plates with gentamycin (Kovach et al., 1994).
Growth Curve Determination
The X2-3, MT16, and Com-16 strains were grown for 24 h at 28°C in NA medium and then inoculated into NB medium to OD600 = 1.0. The cultures were diluted 1:100 into NB medium. The strains were incubated at 28°C for 48 h with shaking at 180 rpm, and bacterial growth was examined every 4 h (Rehl et al., 2013).
Motility Assay
The motility assay was performed as previously described (Rashid and Kornberg, 2000; Tomada et al., 2016). To test twitching motility, bacteria were grown for 24 h in NA medium at 28°C, and 3 μl of the bacterial cultures at a normalized OD600 were added to NYGB medium (0.6% agar) plates. The diameters of the areas occupied by the bacterial cells were measured after 3 days.
Biofilm Formation Assay
The crystal violet technique was used to analyze the attachment of the different strains to an abiotic surface. The X2-3, MT16, and Com-16 strains were cultured in NB medium and adjusted to OD600 = 1. The cultures were diluted 1:100 into a glass tube containing 10 ml of NB medium supplemented with 1% sucrose or glucose. Then, the glass tubes were incubated at 28°C for 3 days with shaking at 180 rpm. The growth medium was removed, and the tubes were washed three times with sterile distilled water. Then, the glass tubes were stained with a 0.2% crystal violet solution for 10 min. The unbound crystal violet was removed, and the tubes were washed three times with sterile distilled water. Crystal violet was extracted with absolute ethanol, and the absorbance was measured at 575 nm (Zhang et al., 2018).
Pellicle Formation
All Lysobacter strains obtained throughout the study were tested for their ability to produce biofilms, which were visualized as floating pellicle at the air–broth interface that completely blocked the surface of the culture and could not be dispersed by shaking. The X2-3, MT16, and Com-16 strains were grown in glass test tubes containing NB medium (with 1% sucrose or 1% glucose) at 28°C for 5 days without shaking (Latasa et al., 2012).
Root Colonization Assay
Seven-day-old plants were collected, and the roots were cut into 1.5 cm segments. Fragments of uniform shape and size were placed into 96-well microtiter plate. Two hundred microliters of bacterial culture with an OD600 = 1.0 was added to the wells, and the plates were incubated at 28°C for 3 days. After the incubation period, the roots were removed from the cultures, washed with sterile water, and then added to 1 ml sterile water. The bacteria on the root surface were removed and dispersed in sterile water by shaking. One hundred microliters of the dispersed preparation was plated on NA agar and counted after 5 days (Tariq et al., 2014).
The plasmid pBBR1-gfp was transformed into the X2-3, MT16, and Com-16 strains by electroporation, and the transformants were selected on NA plates with gentamycin. The treatment was the same as above. To view the colonization of L. capsici X2-3-gfp, MT16-gfp, and Com-16-gfp on the root surfaces, the roots were observed using a confocal laser scanning microscope system (Zeiss LSM 800, Carl Zeiss AG, Jena, Germany) with an excitation wavelength of 488 nm. Images of at least 12 roots were obtained for each treatment (Liu et al., 2020).
Stress Tolerance Assays
The bacterial strains were diluted 1:100 into NB medium, and experiments were conducted to test the OD600 under five environmental stresses. Stress treatments were applied as follows: for UV radiation, the cells were exposed to shortwave UV radiation (254 nm in a biological safety cabinet) at a distance of 60 cm for 45 min. For salt stress, NaCl was added to the bacterial cultures at final concentrations of 0.15, 0.25, and 0.35 mol/L (Li et al., 2014). For temperature stress, the cultures were incubated at 37 and 42°C with shaking at 180 rpm. Resistance against H2O2 was determined as described previously with slight modifications (Liu et al., 2019). H2O2 at concentrations of 0.1, 0.01, and 0.001 mM was added to the bacterial cultures and, the samples were incubated at 28°C for 10 min with shaking. After serially diluting the bacteria five times (10−1–10−5), 3 μl of each cell sample was dropped onto NA plates and incubated at 28°C for 3 days. The pH stress test was similar to the H2O2 test. The bacterium was serially diluted five times (10−1–10−5), and then 3 μl of each cell sample was dropped onto NA plates with pH values ranging from 5.0 to 9.0.
RT–qPCR
The wild-type strain X2-3 and the mutant strain MT16 were cultivated until they reached an OD600 = 1. Total RNA was extracted using AG RNAex Pro Reagent [Accurate Biotechnology (Hunan) Co., Ltd.], and cDNA was synthesized by reverse transcription. Nineteen genes related to DNA replication, cell division, motility, and biofilm formation were chosen for RT–qPCR (Table 2). RT–qPCR experiments were carried out as instructed by the manufacturer [Accurate Biotechnology (Hunan) Co., Ltd.]. The 16S rRNA gene was used as an internal control (Qian et al., 2013). The relative transcription levels were calculated using the 2–ΔΔCT method (Livak and Schmittgen, 2001).
Statistical Analysis
All data were reported as mean standard at least triplicate experiments. The data were analyzed using the statistical SPSS software (version 18.0) by one-way ANOVA, and the mean was compared by Duncan’s multiple range test (DMRT) at the 5% probability level.
Results
General Analysis of GidA in X2-3
Glucose-inhibited division protein as a tRNA modification enzyme is highly conserved in bacteria and plays an important role in bacterial growth, stress response, and virulence (Shippy and Fadl, 2014). We conducted a search of the L. capsici X2-3 genome annotation (GenBank accession No. LBMI00000000.1) and observed that a potential ORF of approximately 1,890 bp in size was predicted to encode GidA (Supplementary Figure S1), which was named LC_GidA in L. capsici. BLAST analyses showed that the LC_GidA gene shares 62.43% identity with the E. coli gidA gene (GenBank accession No. NC_011750.1). The putative LC_GidA protein showed 63.81% identity with the E. coli GidA protein (GenBank accession No. YP_002410220.1; Supplementary Figure S1), which is a tRNA modification enzyme responsible for the proper biosynthesis of 5-methylaminomethyl-2-thiouridine (mnm5s2U) at position 5 of the wobble uridine (U34) of tRNAs.
Deletion of LC_GidA Attenuates the Growth and Motility of X2-3
To determine the function of the LC_GidA gene in L. capsici X2-3, a LC_GidA deletion mutant, termed MT16, was generated by integration of the pKMS1 plasmid (Supplementary Figure S2). The mutant was identified for the loss of 1,890 bp fragment coding region of the gidA gene by PCR with the primers gidAup-F and gidAdown-R (Supplementary Figure S3). Additionally, the complemented mutant Com-16 was generated by insertion of the full-length LC_GidA into pBBR1-MCS5 and transfer of the resultant plasmid into MT16. The growth of wild-type strain X2-3 and the LC_GidA gene deletion mutant MT16 was assayed by measuring OD600 values from 4 to 48 h at 4 h intervals. As shown in Figure 1A, the cell density of MT16 was lower than that of X2-3 and Com-16, and the MT16 colony size was obviously smaller than that of X2-3 at the same timepoints. These results suggest that the loss of LC_GidA resulted in the attenuation of bacterial growth.
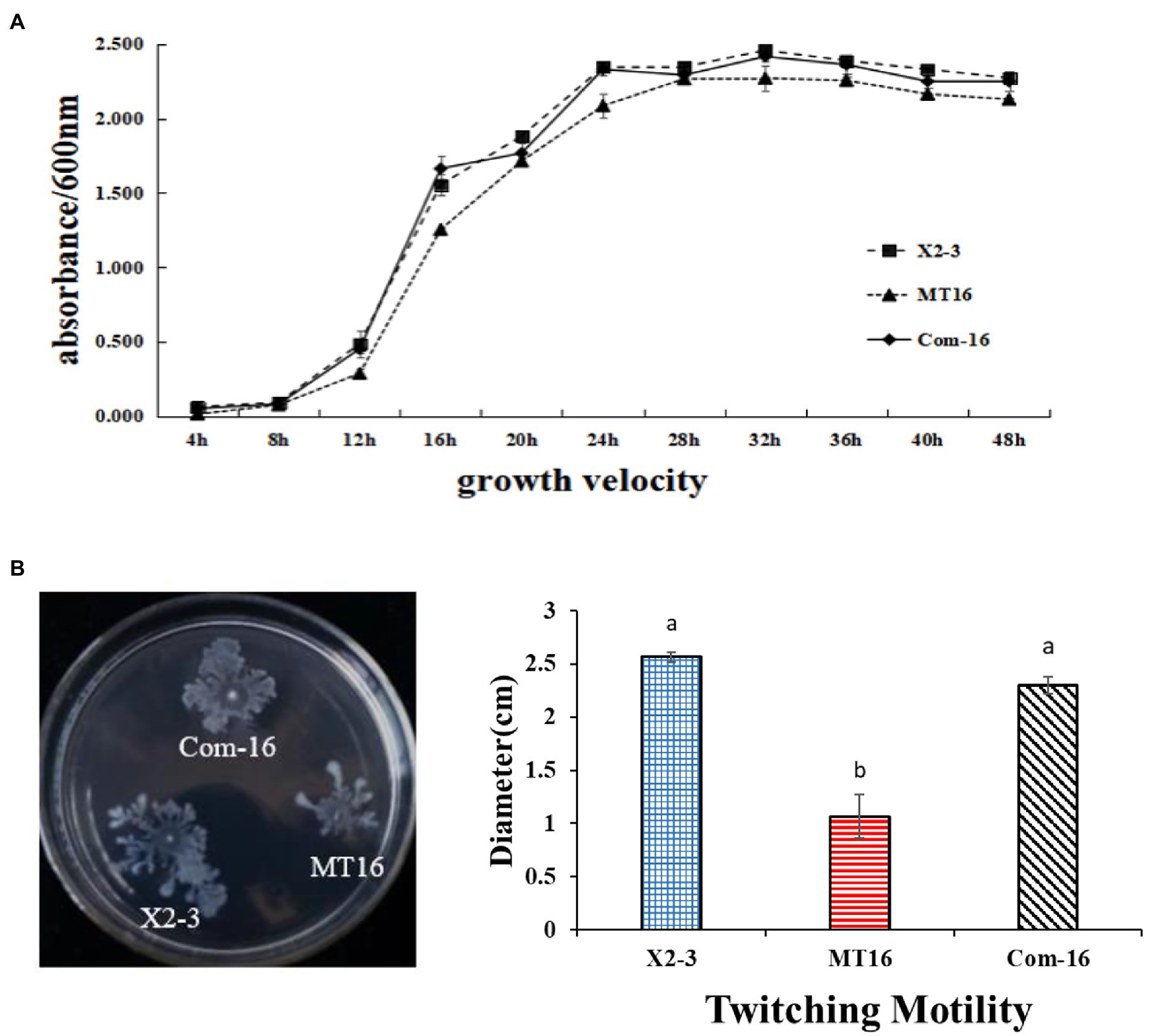
Figure 1. The growth and motility of X2-3, MT16, and Com-16. (A) X2-3, MT16, and Com-16 growth curves. The X2-3, MT16, and Com-16 strains were cultured in NB medium, adjusted to OD600 = 1.0, and then subcultured in fresh NB for 48 h. The OD600 values were tested every 4 h post-subculturing. All experiments were repeated at least three times. (B) Twitching motility of X2-3, MT16, and Com-16. The X2-3, MT16, and Com-16 strains were grown for 24 h in NB medium at 28°C and adjusted to OD600 = 1.0. Three microliters of each cell sample was dropped onto 0.6% agar plates for the motility tests. The diameters of each colony were measured after 3 days of incubation, and the resulting values were taken to indicate the bacterial motility. Each experiment was performed at least three times. a, not significant compared to X2-3. b, significant difference compared to X2-3.
The twitching motility of X2-3 and the mutant MT16 were tested on 0.6% agar plates. After 3 days of incubation at 28°C, the diameter of the Com-16 complemented strain was 2.30 cm in NYGB media, very similar to the X2-3 wild-type strain (2.57 cm). In contrast, the MT16 LC_GidA mutant had decreased twitching motility significantly (Figure 1B). These results indicated that the LC_GidA gene is required for the motility of L. capsici X2-3.
LC_GidA Is Involved in Biofilm and Pellicle Formation
To measure the difference in the biofilm biomass of the MT16 and X2-3 strains, they were cultured in NB medium supplemented with 1% sucrose or 1% glucose for 3 days. The samples were then stained with crystal violet, and the biofilm biomass was quantified by measuring their OD575. Staining of bacterial cells with CV-staining showed that X2-3 and Com-16 produced much more biofilms of cell mass adhered to the glass surface than those produced by MT16 strain (Figure 2A). The biofilm biomass of MT16 was 17 and 30% lower than that of X2-3 in 1% sucrose and 1% glucose media, respectively. By contrast, the biofilm biomass of Com-16 was similar to that of the wild-type strain (Figure 2B). Furthermore, the pellicle, robust biofilm formed at the air–liquid interface of the culture, could be observed in 1% sucrose or 1% glucose NB medium after static culture for 5 days. The MT16 pellicle was much thinner than that of X2-3, both in 1% sucrose and 1% glucose NB medium, while pellicle formation was partially or fully restored in the Com-16 strain (Figure 2C). From these results we also determined that the rate at which X2-3 utilized different C sources varied, for example, the utilization rate of sucrose was higher than that of glucose; the utilization rate of glucose by the LC_GidA deletion strain was relatively low. These results indicated that deletion of the LC_GidA gene in MT16 decreased the biofilm biomass, while the Com-16 complemented strain recovered biofilm formation ability.
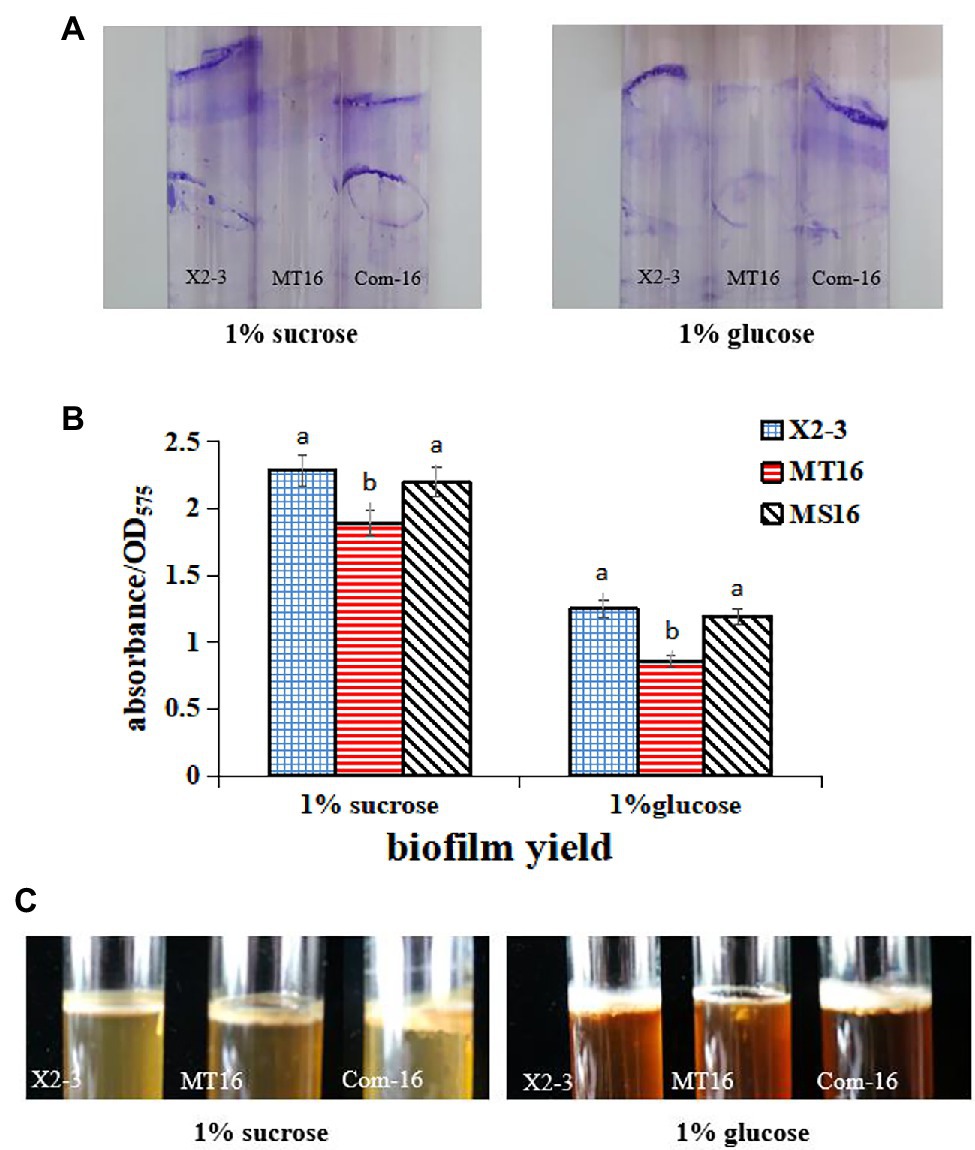
Figure 2. The ability to produce biofilms of X2-3, MT16, and Com-16. Biofilm formation of X2-3, MT16, and Com-16 on glass bottle surfaces after 3 days of incubation in NB medium supplemented with 1% sucrose or glucose. (A) Biofilm formation of X2-3, MT16, and Com-16 on glass bottle surfaces by crystal violet stain. (B) The results of the biofilm formation assays were quantified by measuring the absorbance of the crystal violet stain at 575 nm. Each experiment was performed at least three times. a, not significant compared to X2-3. b, significant difference compared to X2-3. (C) Pellicle formation by X2-3, MT16, and Com-16. All strains were analyzed after 5 days of incubation at 28°C, showing developed pellicles at the interface between the liquid and air in NB medium supplemented with 1% sucrose or glucose.
Inactivation of LC_GidA Decreased the Colonization of Lysobacter capsici X2-3 on Wheat Roots
Considering that the LC_GidA gene plays a role in biofilm formation, a quantitative measurement of root colonization was performed. Wheat roots were cultured in X2-3, MT16, or Com-16 for 3 days, and then 100 μl of the bacterial suspensions were plated on NA agar and cultured for 3 days. The results are shown in Figure 3B. The ability of the MT16 mutant to colonize wheat roots was significantly lower than that of the wild-type X2-3 strain; wheat root colonization was recovered in the Com-16 complemented strain. Green fluorescent protein-labeled X2-3, MT16, and Com-16 (X2-3-gfp, MT16-gfp, and Com-16-gfp) were used to detect the root colonization of L. capsici X2-3 under a confocal laser scanning microscope (Zeiss LSM 800, Carl Zeiss AG, Jena, Germany). GFP fluorescence shows successful colonization of X2-3 in root tip cells of wheat, the difference of colonization was determined by observing the GFP fluorescence area. As can be seen from Figure 3A, that the fluorescence area of the wild type is significantly larger than that of the mutant. The images showed that more X2-3-gfp cells were bound to the roots than MT16-gfp cells (Figure 3A). These results indicated that the inactivation of LC_GidA may affect the colonization of wheat roots.
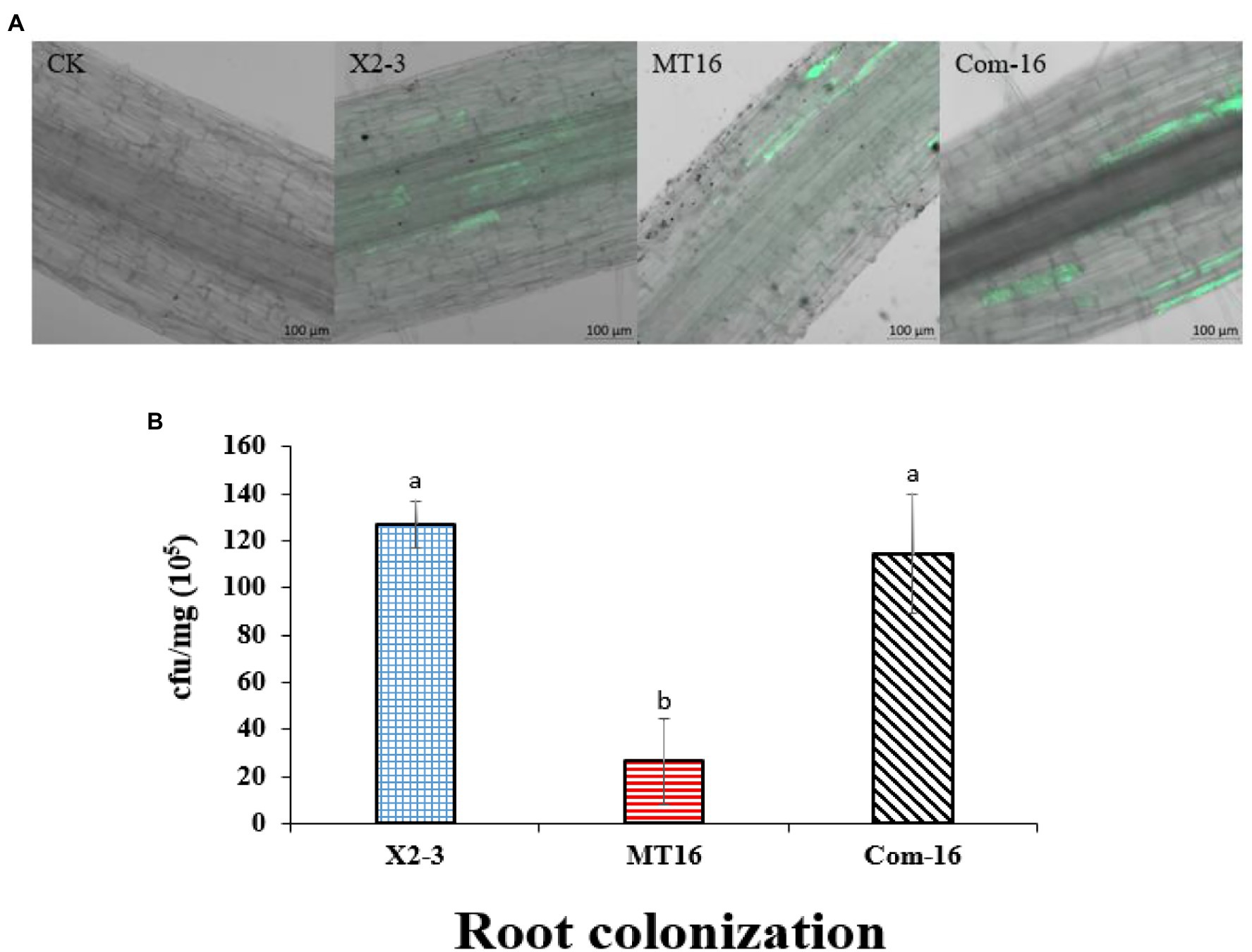
Figure 3. Qualitative and quantitative analysis of wheat root colonization by Lysobacter capsici X2-3 cells. The roots were cultured in X2-3, MT16, and Com-16 for 3 days. (A) Confocal scanning laser microscopy images of the roots colonized by L. capsici. Wheat roots without gfp inoculation as a control. Wheat roots colonized with X2-3-gfp, MT16-gfp, and Com-16-gfp for 3 days. Bar = 100 μm. (B) Quantitative analysis of root colonization by wild-type L. capsici, the LC_GidA deletion mutant and the complemented strain. a, not significant compared to X2-3. b, significant difference compared to X2-3.
The LC_GidA Mutation Impairs Bacterial Resistance to Temperature, Salt, pH, and H2O2 but Has No Significant Effect on UV Radiation
To assess the role of LC_GidA in stress tolerance, the growth yields of MT16, Com-16, and X2-3 were tested under different conditions, including temperature, salt, pH, and UV radiation. The growth of MT16 was significantly lower than that of X2-3 at 37 and 42°C, while Com-16 growth was basically restored to the level of the wild-type strain (Figure 4A). As shown in Figure 4A, the mutant had decreased survival at high osmotic pressure. When treated with UV radiation, there were no significant differences between the MT16 and X2-3 strains (Figure 4A). Compared with the wild-type strain, the growth of the mutant was inhibited at all concentrations of H2O2, and the growth of Com-16 was also slightly affected under the high and low H2O2 conditions (Figure 4B). The pH resistance of L. capsici was significantly affected by the deletion of LC_GidA (Figure 4C).
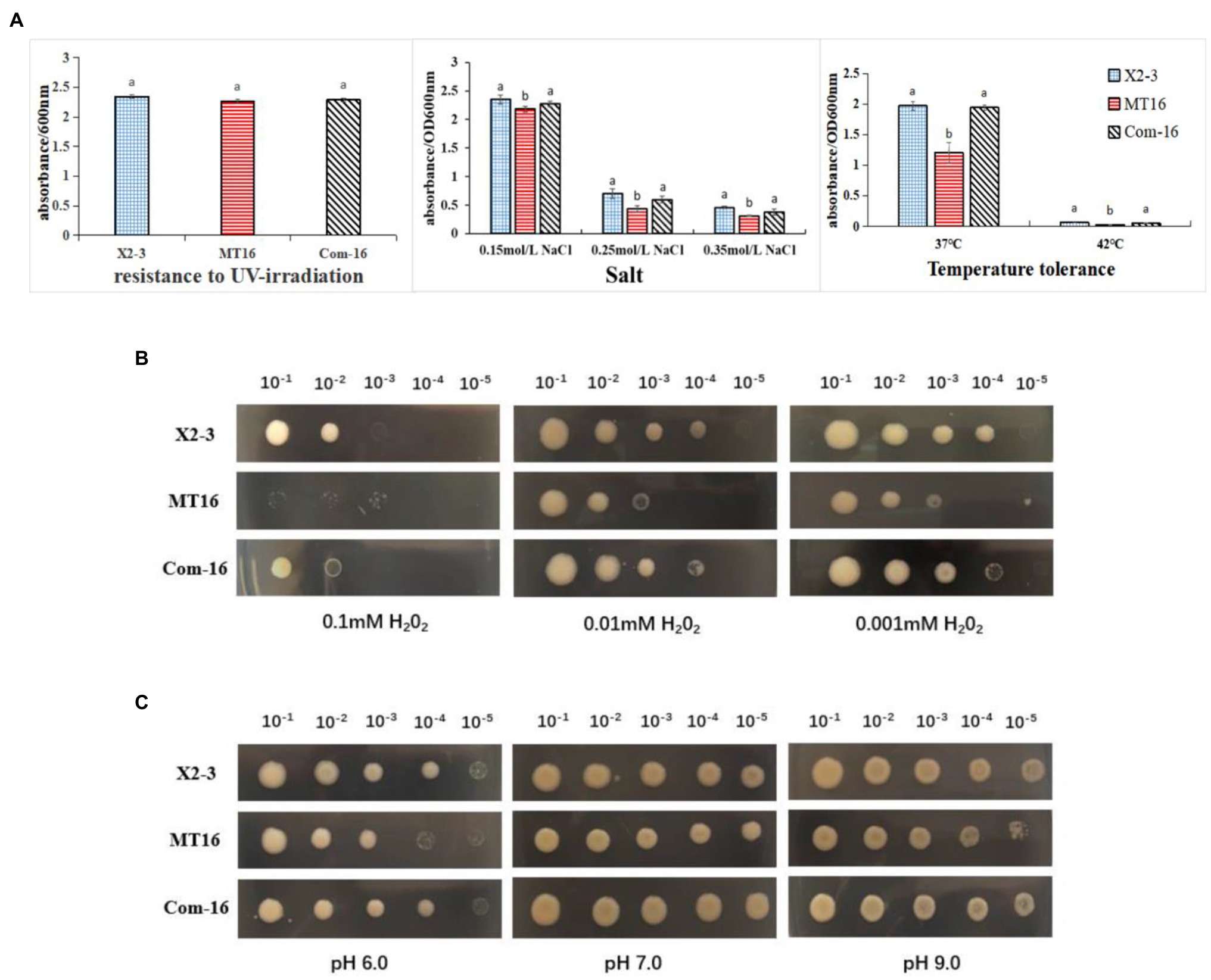
Figure 4. The survival under different stress conditions of X2-3, MT16, and Com-16. (A) Growth after 72 h of incubation of the wild-type X2-3, MT16 mutant, and Com-16 complemented strains after UV exposure for 45 min, at different concentrations of salt (0.15, 0.25, and 0.35 mol/L), and different temperatures (37 and 42°C). The results were quantified by measuring the absorbance at 600 nm. The data represent the means ± SDs of three independent experiments. a, not significant compared to X2-3. b, significant difference compared to X2-3. LC_GidA mutations impair resistance to (B) H2O2 and (C) pH in Lysobacter capsici. The wild-type X2-3, the mutant MT16, and the Com-16 complemented strains were grown on 0.1, 0.01, and 0.001 mM H2O2 (B) and at pH 6.0, pH 7.0, or pH 9.0 (C). The bacterium was serially diluted five times (10−1–10−5). Three replicates for each treatment were used, and the experiment was repeated three times.
The LC_GidA Gene Regulates the Expression of Different Genes
To assess the role of LC_GidA as a global regulatory factor and further show that the deletion of LC_GidA leads to a decrease in growth, motility, and biofilm formation, 19 genes related to DNA replication, repair, cell division, motility, and biofilm formation in X2-3 were chosen for RT–qPCR. The results showed that the expression of genes related to motility, replication, cell division, and biofilm formation was significantly downregulated. The genes radC, gyrA, recN, n6amt, dnaA, rmuC, ftsQ, ftsI, and ftsB, which are related to DNA replication, repair, and cell division, were markedly downregulated in the LC_GidA mutant (Figure 5A). Six genes related to motility, pilA, flgD, fliF, flhB, fliQ, and fliP, were significantly decreased in the mutant compared with wild-type X2-3 (Figure 5B). Among the biofilm formation genes, four genes, pgaA, pgaB, pgaC, and surA, were significantly repressed in the LC_GidA mutant (Figure 5C).
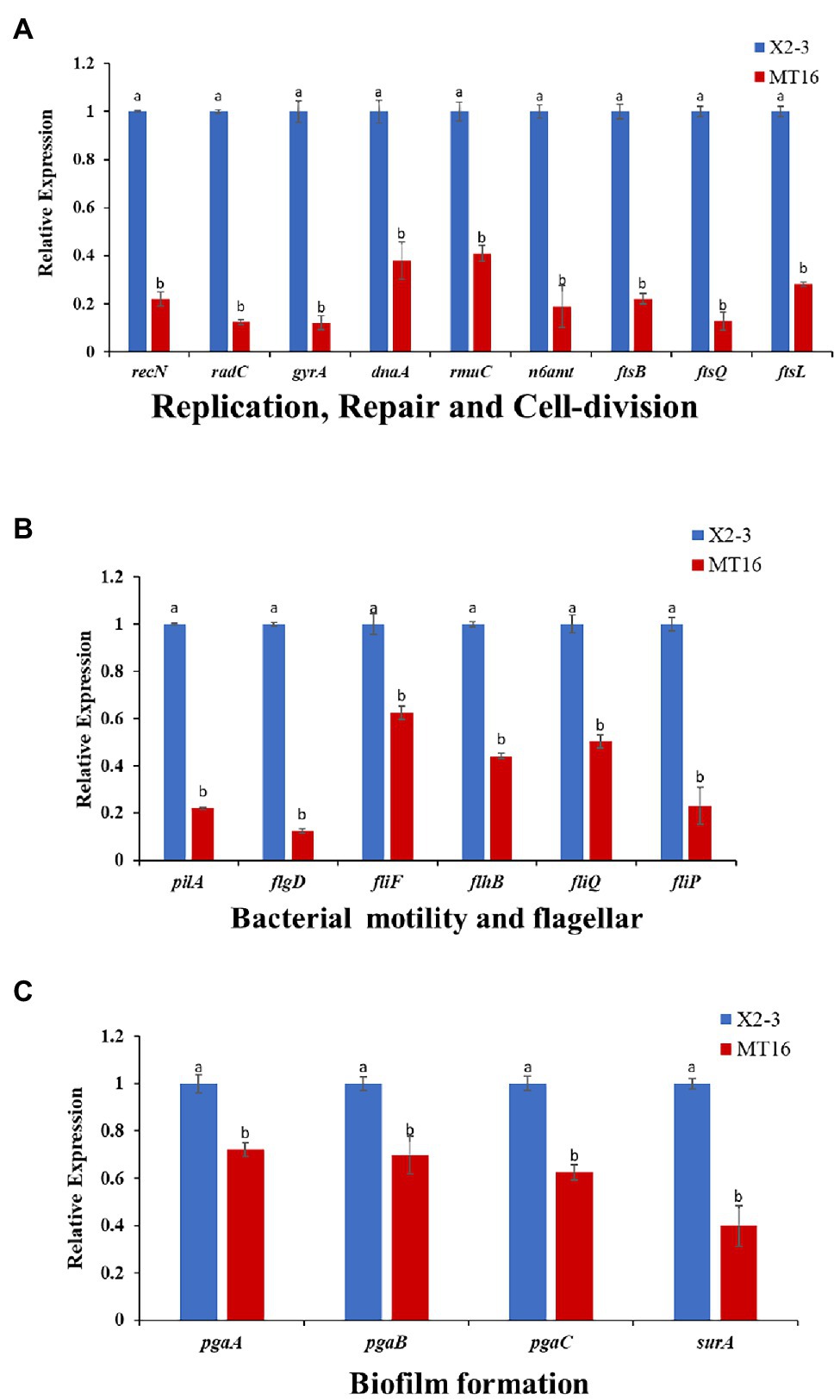
Figure 5. RT–qPCR of 19 selected differentially expressed genes. The X2-3 and MT16 mutant strains were cultivated to an OD600 = 1. RT–qPCR of 19 selected differentially expressed genes related to replication repair and cell division (A), bacterial motility and flagellar formation (B), and biofilm formation (C). Three replicates for each treatment were used, and the experiment was repeated three times. Vertical bars represent SEs. a, not significant compared to X2-3. b, significant difference compared to X2-3.
Discussion
Glucose-inhibited division protein, as an evolutionarily conserved tRNA modifying enzyme, catalyzes the addition of a cmnm group at the wobble uridine of tRNAs and is essential for proper and efficient protein translation (Fislage et al., 2014). GidA has exhibited important roles in regulating multiple biological processes, such as growth, cell division, and virulence in pathogenic bacteria (Shippy et al., 2011). However, the function in different bacterial species is not always the same. L. capsici is an effective biocontrol agents of plant diseases, and the role of GidA in L. capsici is unclear. In this study, we demonstrated that gidA affects cell growth, twitching motility, biofilm formation, root colonization, and stress response in L. capsici X2-3.
First, we obtained the gidA deletion mutant, we found that deletion of LC_GidA significantly reduced the growth and motility of L. capsici X2-3 (Figure 1), and this result is in agreement with previous reports on E. coli (Lies et al., 2015) and Salmonella enterica (Rehl et al., 2013). To further understand the regulatory effect of LC_GidA, nine genes related to growth, including six involved in DNA replication, recombination, and repair (radC, gyrA, recN, n6amt, dnaA, and rmuC), and three involved in cell division (ftsQ, ftsI, and ftsB), were analyzed in the LC_GidA mutant by RT–qPCR, and all of these genes were downregulated (Figure 5A). GyrA, n6amt, and dnaA are all related to DNA replication. GyrA is an essential gene that introduces negative supercoils into plasmid and chromosomal DNA (Rovinskiy et al., 2019); the n6amt gene encodes the main enzyme catalyzing the methylation of the adenine base (Zhang et al., 2016); and dnaA is the initiator of chromosomal DNA replication and has various activities in E. coli (Mizushima, 2000). RecN is a structural maintenance protein and is involved in RecA-mediated recombinational repair in Deinococcus radiodurans and E. coli (Uranga et al., 2017; Keyamura and Hishida, 2019). RmuC and radC function in recombination and repair via different mechanisms (Okaichi et al., 1995; Kosinski et al., 2005). Cell division is also essential in bacterial growth, and division regulated by the proteins FtsQ, FtsB, and FtsI is a key component in facilitating bacterial cell replication (Kureisaite-Ciziene et al., 2018). Taken together, these genes involved in DNA replication, recombination, repair, and cell division were all related to cell growth, and the downregulation of these genes in the LC_GidA mutant can explain the mechanism by which gidA disruption inhibits L. capsici X2-3 growth. Additionally, six genes related to motility, pilA, flgD, fliF, flhB, fliQ, and fliP, were downregulated in the LC_GidA mutant (Figure 5B). These RT–qPCR data related to replication, repair, cell division, and motility in the LC_GidA mutant strongly supported the biological results of attenuated cell growth and motility.
Deletion of gidA significantly reduced L. capsici biofilm formation and colonization of wheat roots. Biofilms attached to biological surfaces are indispensable for bacterial colonization and sessile growth (Kumara et al., 2017), and gidA is considered to play important roles in biofilm formation. In S. mutans, loss of gidA decreased the capacity for glucose-dependent biofilm formation by over 50% (Li et al., 2014). In our study, the deletion of LC_GidA attenuated biofilm formation in the LC_GidA mutant (Figure 2). This attenuation may be due to impaired growth of mutant MT16 or downregulation of genes associated with biofilm formation, or a dual function of impaired growth and downregulation of genes. Four genes, pgaA, pgaB, pgaC, and surA that were reported to be related to biofilm formation were tested by RT–qPCR. The results revealed that the genes pgaA, pgaB, pgaC, and surA were clearly downregulated in the LC_GidA mutant (Figure 5C). SurA is a major factor in the biogenesis of β-barrel outer membrane proteins, and the disruption of SurA in S. enterica serovar Typhi affects motility and biofilm formation (Lu et al., 2019). PgaA, pgaB, and pgaC have a profound role in the synthesis and secretion of poly-β-linked N-acetylglucosamine (PNAG), which has been characterized as a component of the bacterial surface responsible for biofilm formation in E. coli (Chen et al., 2014). Deletion of pgaC or pgaB dramatically reduced biofilms in Klebsiella pneumoniae and Aggregatibacter actinomycetemcomitans (Chen et al., 2014; Hathroubi et al., 2015; Shanmugam et al., 2017). Our results showed decreased biofilm formation and downregulated biofilm-related genes in the LC_GidA mutant, consistent with these studies. And the attenuation of biofilm formation in mutant can be explained by the downregulation of these genes. Biofilm formation is a determinant of the root colonization process in PGPBs, such as Bacillus (Chen et al., 2013; Xu et al., 2018). In our study, the LC_GidA mutant displayed an 80% reduction in bacterial colonization compared with X2-3 (Figure 3), suggesting that the LC_GidA gene is important for X2-3 colonization of wheat roots. Similar phenomena were found in a previous study with B. velezensis FZB42 (Al-Ali et al., 2018). In summary, the deletion of LC_GidA decreased X2-3 biofilm formation and colonization of the wheat rhizosphere.
In addition, biofilm formation is considered a generic mechanism for the survival of bacteria in stressful environments (Ansari and Ahmad, 2019; Gao et al., 2019; Masmoudia et al., 2019). As shown in Figure 4, the disruption of LC_GidA strongly reduced the growth of the mutant in high salt media, high temperature, different concentrations of H2O2, and different pH conditions. This result is in agreement with previous reports in S. mutans in which the gidA mutant showed a reduced ability to withstand stress conditions (Li et al., 2014). Moreover, in Xanthomonas oryzae, the PXO_RS20535 mutant produced significantly less biofilm and had a clear diminution of growth and survival under stress conditions (Antar et al., 2020). These results indicated that biofilm formation may be involved in the growth of X2-3 in various stressful environments. Previous study proved that as a global regulatory factor, deletion of gidA significantly reduced the growth in most bacteria (Shippy and Fadl, 2014). In our study, growth curves showed that the LC_GidA mutant resulted in an attenuation of the bacterial growth rate compared with the wild type and entered the stationary phase at a slightly lower density. While the LC_GidA mutant grew more slowly, this relatively small difference is not sufficient to explain the dramatic biofilm formation and stress respond observed. In addition, despite the modest growth defect, the LC_GidA mutant did not show any deficiency in UV stress compared with the wild type. And RT–qPCR assays also eliminate the effect due to the growth deficiency of LC_GidA in regulating biofilm formation and stress response. Taken together, our study indicated that the LC_GidA mutant decreased biofilm formation and stress respond of X2-3.
In conclusion, this study demonstrated that LC_GidA regulates the expression of a series of genes involved in cell growth, twitching motility, biofilm formation, rhizosphere colonization, and stress resistance in L. capsici X2-3. The antimicrobial activity of the LC_GidA mutant against Gram-positive bacteria was also markedly decreased (Supplementary Figure S7). However, no significant changes in the antimicrobial activity of the LC_GidA mutant against either fungi or oomycetes were observed (Supplementary Figure S6), although deletion of gidA in pathogenic bacteria resulted in reduced pathogenicity. The regulatory mechanisms of GidA in antibacterial activity remain to be investigated. These findings provide new insights to better understanding the regulatory function of gidA in PGPB. This is the first report on the regulation of LC_GidA in L. capsici, as well as in the genus Lysobacter.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
DZ and HW conceived this study. DZ performed the mainly experiments, and some experiments were performed with the assistance of ZL and SH. DZ analyzed the data. DZ, CH, and AL wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Key R&D Program of China (grant number 2017YFD0201100) and Outstanding Youth Foundation of Shandong Province (grant number ZR2021YQ20).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to Weiwen Kong in Yangzhou University for his pKMS1 plasmids.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.840792/full#supplementary-material
References
Afoshin, A. S., Kudryakova, I. V., Borovikova, A. O., Suzina, N. E., and Toropygin, I. Y. (2020). Lytic potential of Lysobacter capsici VKM B-2533t: bacteriolytic enzymes and outer membrane vesicles. Sci. Rep. 10:9944. doi: 10.1038/s41598-020-67122-2
Al-Ali, A., Deravel, J., Krier, F., Béchet, M., and Jacques, P. (2018). Biofilm formation is determinant in tomato rhizosphere colonization by Bacillus velezensis FZB42. Environ. Sci. Pollut. Res. 25, 29910–29920. doi: 10.1007/s11356-017-0469-1
Ansari, F. A., and Ahmad, I. (2019). Isolation, functional characterization and efficacy of biofilm-forming rhizobacteria under abiotic stress conditions. Antonie Van Leeuwenhoek 112, 1827–1839. doi: 10.1007/s10482-019-01306-3
Antar, A., Lee, M. A., Yoo, Y., Cho, M. H., and Lee, S. W. (2020). PXO_RS20535, encoding a novel response regulator, is required for chemotactic motility, biofilm formation, and tolerance to oxidative stress in Xanthomonas oryzae pv. Oryzae. Pathogens 9:956. doi: 10.3390/pathogens9110956
Brescia, F., Marchetti-Deschmann, M., Musetti, R., Perazzolli, M., Pertot, I., and Puopolo, G. (2020). The rhizosphere signature on the cell motility, biofilm formation and secondary metabolite production of a plant-associated Lysobacter strain. Microbiol. Res. 234:126424. doi: 10.1016/j.micres.2020.126424
Chen, K., Chiang, M., Wang, M., Ho, H., Lu, M., and Lai, Y. (2014). The role of pgaC in Klebsiella pneumoniae virulence and biofilm formation. Microb. Pathog. 77, 89–99. doi: 10.1016/j.micpath.2014.11.005
Chen, Y., Yan, F., Chai, Y. R., Liu, H. X., Kolter, R., Losick, R., et al. (2013). Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ. Microbiol. 15, 848–864. doi: 10.1111/j.1462-2920.2012.02860.x
Fang, B. Z., Xie, Y. G., Zhou, X. K., Zhang, X. T., Liu, L., Jiao, J. Y., et al. (2020). Lysobacter prati sp. nov., isolated from a plateau meadow sample. Antonie Van Leeuwenhoek 113, 763–772. doi: 10.1007/s10482-020-01386-6
Fislage, M., Brosens, E., Deyaert, E., Spilotros, A., Pardon, E., Loris, R., et al. (2014). SAXS analysis of the tRNA-modifying enzyme complex MnmE/MnmG reveals a novel interaction mode and GTP-induced oligomerization. Nucleic Acids Res. 42, 5978–5992. doi: 10.1093/nar/gku213
Gao, T., Ding, M., Yang, C., Fan, H., Chai, Y., and Li, Y. (2019). The phosphotransferase system gene ptsH plays an important role in MnSOD production, biofilm formation, swarming motility, and root colonization in Bacillus cereus 905. Res. Microbiol. 170, 86–96. doi: 10.1016/j.resmic.2018.10.002
Gao, T., Tan, M., Liu, W., Zhang, C., Zhang, T., Zheng, L., et al. (2016). GidA, a tRNA modification enzyme, contributes to the growth, and virulence of Streptococcus suis serotype 2. Front. Cell. Infect. Microbiol. 6:44. doi: 10.3389/fcimb.2016.00044
Gao, T., Yuan, F., Liu, Z., Liu, W., Zhou, D., Yang, K., et al. (2020). Proteomic and Metabolomic analyses provide insights into the mechanism on arginine metabolism regulated by tRNA modification enzymes GidA and MnmE of Streptococcus suis. Front. Cell. Infect. Microbiol. 10:597408. doi: 10.3389/fcimb.2020.597408
Gustilo, E. M., Vendeix, F. A., and Agris, P. F. (2008). tRNA’s modifications bring order to gene expression. Curr. Opin. Microbiol. 11, 134–140. doi: 10.1016/j.mib.2008.02.003
Hathroubi, S., Hancock, M. A., Bossé, J. T., Langford, P. R., Tremblay, Y. D. N., Labrie, J., et al. (2015). Surface polysaccharide mutants reveal that absence of O antigen reduces biofilm formation of Actinobacillus pleuropneumoniae. Infect. Immun. 84, 127–137. doi: 10.1128/IAI.00912-15
Keyamura, K., and Hishida, T. (2019). Topological DNA-binding of structural maintenance of chromosomes-like RecN promotes DNA double-strand break repair in Escherichia coli. Commun. Biol. 2:413. doi: 10.1038/s42003-019-0655-4
Kinscherf, T. G., and Willis, D. K. (2002). Global regulation by gidA in Pseudomonas syringae. J. Bacteriol. 184, 2281–2286. doi: 10.1128/JB.184.8.2281-2286.2002
Kosinski, J., Feder, M., and Bujnicki, J. M. (2005). The PD-(D/E)XK superfamily revisited: identification of new members among proteins involved in DNA metabolism and functional predictions for domains of (hitherto) unknown function. BMC Bioinformatics 6:172. doi: 10.1186/1471-2105-6-172
Kovach, M. E., Phillips, R. W., Elzer, P. H., Roop, R. M., and Peterson, K. M. (1994). pBBR1MCS: a broad-host-range cloning vector. BioTechniques 16, 800–802. doi: 10.1002/bip.360340510
Kumara, A., Alama, A., Ranib, M., Ehteshamc, N. Z., and Hasnaina, S. E. (2017). Biofilms: survival and defense strategy for pathogens. Int. J. Med. Microbiol. 307, 481–489. doi: 10.1016/j.ijmm.2017.09.016
Kureisaite-Ciziene, D., Varadajan, A., McLaughlin, S. H., Glas, M., Silva, A. M., Luirink, R., et al. (2018). Structural analysis of the interaction between the bacterial cell division proteins FtsQ and FtsB. MBio 9, e01346–e01358. doi: 10.1128/mBio.01346-18
Latasa, C., Garcia, B., Echeverz, M., Toledo-Arana, A., Valle, J., Campoy, S., et al. (2012). Salmonella biofilm development depends on the phosphorylation status of RcsB. J. Bacteriol. 194, 3708–3722. doi: 10.1128/JB.00361-12
Li, D., Shibata, Y., Takeshita, T., and Yamashita, Y. (2014). A novel gene involved in the survival of Streptococcus mutans under stress conditions. Appl. Environ. Microbiol. 80, 97–103. doi: 10.1128/AEM.02549-13
Lies, M., Visser, B. J., Joshi, M. C., Magnan, D., and Bates, D. (2015). MioC and GidA proteins promote cell division in E. coli. Front. Microbiol. 6:516. doi: 10.3389/fmicb.2015.00516
Liu, Y. P., Feng, H. C., Fu, R. X., Zhang, N., Du, W. B., Shen, Q. R., et al. (2020). Induced root-secreted D-galactose functions as a chemoattractant and enhances the biofilm formation of Bacillus velezensis SQR9 in an McpA-dependent manner. Appl. Microbiol. Biotechnol. 104, 785–797. doi: 10.1007/s00253-019-10265-8
Liu, X. L., Yan, Y. P., Wu, H., Zhou, C. Y., and Wang, X. F. (2019). Biological and transcriptomic studies reveal hfq is required for swimming, biofilm formation and stress response in Xanthomonas axonpodis pv. Citri. BMC Microbiol. 19:103. doi: 10.1186/s12866-019-1476-9
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCTmethods. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lu, R., Sun, J., Osei-Adjei, G., Zhang, Y., and Huang, X. (2019). The Periplasmic chaperone SurA affects motility and biofilm formation via the RcsCDB pathway in Salmonella enterica Serovar Typhi. J. Nanosci. Nanotechnol. 19, 5503–5509. doi: 10.1166/jnn.2019.16503
Manickam, N., Joshi, K., Bhatt, M. J., and Farabaugh, P. J. (2016). Effects of tRNA modification on translational accuracy depend on intrinsic codon-anticodon strength. Nucleic Acids Res. 44, 1871–1881. doi: 10.1093/nar/gkv1506
Masmoudia, F., Abdelmaleka, N., Tounsia, S., Dunlapb, C. A., and Trigui, M. (2019). Abiotic stress resistance, plant growth promotion and antifungal potential of halotolerant bacteria from a Tunisian solar saltern. Microbiol. Res. 229:126331. doi: 10.1016/j.micres.2019.126331
Mizushima, T. (2000). Site-directed mutational analysis of DnaA protein, the initiator of chromosomal DNA replication in E. coli. J. Biochem. 127, 1–7. doi: 10.1093/oxfordjournals.jbchem.a022569
Okaichi, K., Mori, T., Ihara, M., and Ohnishi, T. (1995). Unique DNA repair property of an ultraviolet-sensitive (radC) mutant of Dictyostelium discoideum. Photochem. Photobiol. 61, 281–284. doi: 10.1111/j.1751-1097.1995.tb03972.x
Park, J. H., Kim, R., Aslam, Z., Jeon, C. O., and Y.R., C. (2008). Lysobacter capsici sp. with antimicrobial activity isolated from the rhizosphere of pepper, and emended description of the genus Lysobacter. Int. J. Syst. Evol. Microbiol. 58, 387–392. doi: 10.1099/ijs.0.65290-0
Puopolo, G., Raio, A., and Zoina, A. (2010). Identification and characterization of Lysobacter capsici strain PG4: a new plant health-promoting rhizobacterium. J. Plant Pathol. 92, 157–164. doi: 10.4454/jpp.v92i1.25
Puopolo, G., Tomada, S., Sonego, P., Moretto, M., Engelen, K., Perazzolli, M., et al. (2016). The Lysobacter capsici AZ78 genome has a gene pool enabling it to interact successfully with phytopathogenic microorganisms and environmental factors. Front. Microbiol. 7:96. doi: 10.3389/fmicb.2016.00096
Qian, G. L., Wang, Y. L., Liu, Y. R., Xu, F. F., He, Y. W., and Du, L. C. (2013). Lysobacter enzymogenes uses two distinct cell-cell signaling systems for differential regulation of secondary-metabolite biosynthesis and colony morphology. Appl. Environ. Microbiol. 79, 6604–6616. doi: 10.1128/AEM.01841-13
Rashid, M. H., and Kornberg, A. (2000). Inorganic polyphosphate is needed for gliding, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. 97, 4885–4890. doi: 10.1073/pnas.060030097
Rehl, J. M., Shippy, D. C., Eakley, N. M., Brevik, M. D., Sand, J. M., Cook, M. E., et al. (2013). GidA expression in Salmonella is modulated under certain environmental conditions. Curr. Microbiol. 67, 279–285. doi: 10.1007/s00284-013-0361-2
Rovinskiy, N. S., Agbleke, A. A., Chesnokova, O. N., and Higgins, N. P. (2019). Supercoil levels in E. coli and Salmonella chromosomes are regulated by the C-terminal 35–38 amino acids of GyrA. Microorganisms 7:81. doi: 10.3390/microorganisms7030081
Sha, J., Kozlova, E. V., Fadl, A. A., Olano, J. P., Houston, C. W., Peterson, J. W., et al. (2004). Molecular characterization of a glucose-inhibited division gene, gidA, that regulates cytotoxic enterotoxin of Aeromonas hydrophila. Infect. Immun. 72, 1084–1095. doi: 10.1128/IAI.72.2.1084-1095.2004
Shanmugam, M., Oyeniyi, A. O., Parthiban, C., Gujjarlapudi, S. K., Pier, G. B., Ramasubbu, N., et al. (2017). Role of de-N-acetylase PgaB from Aggregatibacter actinomycetemcomitans in exopolysaccharide export in biofilm mode of growth. Mol Oral Microbiol 32, 500–510. doi: 10.1111/omi.12188
Shippy, D. C., Eakley, N. M., Bochsler, P. N., Chopra, A. K., and Fadl, A. A. (2011). Biological and virulence characteristics of Salmonella enterica serovar Typhimurium following deletion of glucose-inhibited division (gidA) gene. Microb. Pathog. 50, 303–313. doi: 10.1016/j.micpath.2011.02.004
Shippy, D. C., and Fadl, A. A. (2014). tRNA modification enzymes GidA and MnmE: potential role in virulence of bacterial pathogens. Int. J. Mol. Sci. 15, 18267–18280. doi: 10.3390/ijms151018267
Tariq, M., Hameed, S., Yasmeen, T., Zahid, M., and Zafar, M. (2014). Molecular characterization and identification of plant growth promoting endophytic bacteria isolated from the root nodules of pea (Pisum sativum L.). World J. Microbiol. Biotechnol. 30, 719–725. doi: 10.1007/s11274-013-1488-9
Tomada, S., Puopolo, G., Perazzolli, M., Musetti, R., Loi, N., and Pertot, I. (2016). Pea broth enhances the biocontrol efficacy of Lysobacter capsici AZ78 by triggering cell motility associated with biogenesis of type IV Pilus. Front. Microbiol. 7:1136. doi: 10.3389/fmicb.2016.01136
Uranga, L. A., Reyes, E. D., Patidar, P. L., Redman, L. N., and Lusetti, S. L. (2017). The cohesin-like RecN protein stimulates RecA-mediated recombinational repair of DNA double-strand breaks. Nat. Commun. 8:15282. doi: 10.1038/ncomms15282
Vlassi, A., Nesler, A., Perazzolli, M., Lazazzara, V., Büschl, C., Parich, A., et al. (2020). Volatile organic compounds from Lysobacter capsici AZ78 as potential candidates for biological control of soilborne plant pathogens. Front. Microbiol. 11:1748. doi: 10.3389/fmicb.2020.01748
Xu, S., Yang, N., Zheng, S. Y., Yan, F., Jiang, C. H., Yu, Y. Y., et al. (2018). The spo0A-sinI-sinR regulatory circuit plays an essential role in biofilm formation, nematicidal activities, and plant protection in Bacillus cereus AR156. Mol. Plant-Microbe Interact. 30, 603–619. doi: 10.1094/MPMI-02-17-0042-R
Yi, J. L., Wang, J., Li, Q., Liu, Z. X., Zhang, L., Liu, A. X., et al. (2015). Draft genome sequence of the bacterium Lysobacter capsici X2-3, with a broad spectrum of antimicrobial activity against multiple plant-pathogenic microbes. Genome Announc. 3, e00589–e00615. doi: 10.1128/genomeA.00589-15
Yu, N., Jora, M., Solivio, B., Thakur, P., Acevedo-Rocha, C. G., Randau, L., et al. (2019). tRNA modification profiles and codon-decoding strategies in Methanocaldococcus jannaschii. J. Bacteriol. 201, e00690–e00618. doi: 10.1128/JB.00690-18
Yu, L. J., Su, W., Fey, P. D., Liu, F. Q., and Du, L. C. (2018). Yield improvement of the anti-MRSA antibiotics WAP-8294A by CRISPR/dCas9 combined with refactoring self-protection genes in Lysobacter enzymogenes OH11. ACS Synth. Biol. 7, 258–266. doi: 10.1021/acssynbio.7b00293
Yu, F., Zaleta-Rivera, K., Zhu, X., Huffman, J., Millet, J. C., Harris, S. D., et al. (2007). Structure and biosynthesis of heat-stable antifungal factor (HSAF), abroad-spectrum antimycotic with a novel mode of action. Antimicrob. Agents Chemother. 51, 64–72. doi: 10.1128/AAC.00931-06
Zhang, Y., Gao, J., Wang, L., Liu, S., Bai, Z., Zhang, X., et al. (2018). Environmental adaptability and quorum sensing: iron uptake regulation during biofilm formation by Paracoccus Denitrificans. Appl. Environ. Microbiol. 84, e00865–e00818. doi: 10.1128/AEM.00865-18
Zhang, J., Xing, Y., Hou, B., Yuan, Z., Li, Y., Jie, W., et al. (2016). Amplification and function analysis of N6-adenine-specific DNA methyltransferase gene in Nilaparvata lugens. J. Integr. Agric. 15, 591–599. doi: 10.1016/S2095-3119(15)61180-7
Zhang, W., Zhao, Z., Zhang, B., Wu, X., Ren, Z., and Zhang, L. (2014). Posttranscriptional regulation of 2,4-Diacetylphloroglucinol production by GidA and TrmE in Pseudomonas fluorescens 2P24. Appl. Environ. Microbiol. 80, 3972–3981. doi: 10.1128/AEM.00455-14
Keywords: Lysobacter capsici, glucose-inhibited division protein, motility, biofilm formation, colonization, stress response
Citation: Zhao D, Wang H, Li Z, Han S, Han C and Liu A (2022) LC_Glucose-Inhibited Division Protein Is Required for Motility, Biofilm Formation, and Stress Response in Lysobacter capsici X2-3. Front. Microbiol. 13:840792. doi: 10.3389/fmicb.2022.840792
Edited by:
Milko Alberto Jorquera, University of La Frontera, ChileReviewed by:
Qian Guoliang, Nanjing Agricultural University, ChinaGang Zhou, Guangdong Academy of Science, China
Copyright © 2022 Zhao, Wang, Li, Han, Han and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aixin Liu, bGl1YXhAc2RhdS5lZHUuY24=; Chao Han, aGFuY2g4N0AxNjMuY29t
†These authors have contributed equally to this work
 Dan Zhao
Dan Zhao Aixin Liu
Aixin Liu
