
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 17 March 2022
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.800993
 Congcong Liu1†
Congcong Liu1† Yuchen Wu1†
Yuchen Wu1† Yinfei Fang1
Yinfei Fang1 Zi Sang2
Zi Sang2 Ling Huang1,3
Ling Huang1,3 Ning Dong4
Ning Dong4 Yu Zeng1
Yu Zeng1 Jiayue Lu1
Jiayue Lu1 Rong Zhang1
Rong Zhang1 Gongxiang Chen1*
Gongxiang Chen1*
CG258 is the dominant carbapenemase-producing Klebsiella pneumoniae clone worldwide and treatment of infections caused by this clone relies largely on the last-line antibiotics, colistin, and tigecycline. However, the emergence and global dissemination of mcr and tmexCD1-toprJ1 genes have significantly compromised their clinical applications. CG258 K. pneumoniae carrying both mcr and tmexCD1-toprJ1 have not been reported. A colistin-resistant strain T698-1 belonging to ST1326, a member of CG258, was isolated from the intestinal sample of a patient and characterized by the antimicrobial susceptibility testing, conjugation assay, WGS and bioinformatics analysis. It was resistant to colistin, tetracycline, aminoglycoside, fluoroqinolone, phenicols, sulfonamide, and some β-lactams, and positive for mcr-8.2, tmexCD1-toprJ1, and ESBL genes (blaDHA–1 and blaCTX–M–15). The tmexCD1-toprJ1 gene cluster was located in an multi-drug resistant (MDR) region flanked by TnAs1 elements on an IncHI1B/FIB plasmid. The genetic context of tmexCD1-toprJ1 was slightly distinct from previously reported Tn5393-like structures, with an IS26 element disrupting the upstream Tn5393 and its adjacent genetic elements. The mcr-8.2 gene was inserted into the backbone of an IncFII/FIA plasmid with the genetic context of ISEcl1-mcr-8.2-orf-ISKpn26. To our knowledge, this is the first report of co-occurrence of mcr-8.2 and tmexCD1-toprJ1 in a CG258 K. pneumoniae strain. Though this strain is tigecycline sensitive, the acquisition of colistin and tigecycline resistance determinants by the endemic CG258 K. pneumoniae clone still poses a serious public health concern. CG258, which became resistant to multiple last resort antibiotics, would be the next emerging superbug.
Klebsiella pneumoniae is an opportunistic pathogen causing both community- and hospital acquired severe infections such as bacteremia, respiratory tract infections, and liver abscess (Gu et al., 2018; David et al., 2019). The extensive use of antibiotics has led to the emergence and rapid dissemination of multidrug resistant K. pneumoniae, particularly those resistant to last-line antibiotics including carbapenems, colistin, and tigecycline (Navon-Venezia et al., 2017). The majority of carbapenem-resistant K. pneumoniae producing KPC worldwide belong to the notorious CG258 clonal group, with ST258 and ST11 being the dominant sequence types (Dong et al., 2018). Treatment regimens for carbapenem-resistant K. pneumoniae are mainly reliant on colistin and tigecycline, which are classified as critically important antimicrobials by the WHO (World Health Organization) (He et al., 2019). However, the clinical potential of both antibiotics has been significantly compromised by the global dissemination of plasmid-mediated colistin-resistance genes (mcr) and the mobile tigecycline-resistance genes (variants of the tet(X), tet(A), tet(K), and tet(M) genes), respectively (Linkevicius et al., 2016; Liu et al., 2016; He et al., 2019; Xu et al., 2021). Recently, a novel plasmid-mediated resistance-nodulation-division (RND) efflux pump, tmexCD1-toprJ1, conferring resistance to tigecycline, quinolones, cephalosporins, and aminoglycosides was identified in K. pneumoniae (Lv et al., 2020). These plasmid-mediated resistance determinants are highly transmissible, presenting a severe challenge for clinical management. In this study, we reported an ST1326 multidrug resistant K. pneumoniae isolate, belonging to CG258, harboring resistance determinants including ESBL genes, mcr-8.2, and tmexCD1-toprJ1 from a patient with critical illness. The emergence of colistin and tigecycline resistance determinants in the endemic K. pneumoniae clone constituted a true public threat.
An 84-year-old female patient with a previous history of pneumonia was admitted to Department of Respiratory Medicine in Dali Bai Autonomous Prefecture People’s Hospital on May 12, 2020 (Day 1) due to chronic cough. The patient experienced sudden loss of consciousness during hospitalization and received invasive mechanical ventilation via tracheal intubation. Anti-infective treatment with cefoperazone-sulbactam and caspofungin was also applied. She suffered from recurrent fungal and bacterial infections, fever, and gastro-intestinal bleeding. The drugs were switched to imipenem-cilastatin and ceftazidime 2 months later according to the patient’s condition. However, the patient responded poorly to the treatment and discharged on Day 96 with critical illness. On Day 104, a multidrug-resistant K. pneumoniae isolate (T698-1) was recovered from her intestinal samples for hospital infection surveillance. The species identity of T698-1 was confirmed by a matrix-assisted laser desorption ionization–time of flight mass spectrometer (MALDI-TOF MS) (Bruker, Germany). Antimicrobial susceptibility testing was conducted using the broth microdilution method and interpreted according to the CLSI guideline (CLSI, 2020).
ESBL genes (blaTEM, blaCTX–M, blaDHA, blaVEB, blaPER, blaGES), carbapenemase genes (blaVIM, blaNDM, blaIMP, blaOXA, and blaKPC), plasmid-mediated colistin resistance (mcr) genes, tigecycline resistance determinants [tet(X)], and the efflux pump gene cluster tmexCD1-toprJ1 were detected using previously reported primers (Dallenne et al., 2010; Poirel et al., 2011; Rebelo et al., 2018; Borowiak et al., 2020; Ji et al., 2020; Li et al., 2021). Conjugation experiments were performed by filter mating method using a rifampin resistant Escherichia coli EC600 (Rifr) strain as the recipient, to investigate the transferability of the mcr and tmexCD1-toprJ1 genes. Transconjugants were selected on MacConkey agar supplemented with 600 mg/L rifampin and 1 mg/L colistin or 0.5 mg/L ciprofloxacin for transconjugants, respectively.
The plasmid elimination experiment was performed using sodium dodecyl sulfate (SDS, 20%) as previously described with some modifications (Liu et al., 2017). Briefly, a single colony of strain T698-1 was picked and grown in 5 ml LB broth at 37°C overnight. Then 100 μl bacterial solution was transferred to fresh LB broth containing 20% SDS and incubated at 37°C. The overnight culture was spread on LB plates, the colonies on which were streaked onto drug-free LB plates and LB plates containing 2 μg ml–1 colistin or 2 μg ml–1 tigecycline simultaneously. The colonies losing plasmids were further confirmed by PCR.
Identification of mutations in mgrB gene was conducted using Kleborate version 2.0.4 (Lam et al., 2021). Mutations in amino acid sequences of PmrA/PmrB and PhoP/PhoQ were identified by aligning with a colistin-susceptible reference genome of the strain NJST258_1 (Genbank accession number: CP006923) reported previously (Deleo et al., 2014).
To decipher the genomic characterization, the genome of T698-1 was extracted from overnight cultures by using the PureLink Genomic DNA Mini Kit (Invitrogen, Carlsbad, CA, United States) and sequenced using both short-read sequencing (2 × 150 bp) by Illumina Hiseq 2500 platform and long-read sequencing by Oxford Nanopore Technologies MinION platform (Li et al., 2018). Hybrid assembly of both sequencing reads was constructed using Unicycler v 0.4.4 (Wick et al., 2017). Complete genome sequence was annotated by the RAST tool and modified manually (Overbeek et al., 2013). Genotyping including species identification, multi-locus sequence typing, serotyping, identification of antimicrobial resistance genes, and virulence genes was conducted using Kleborate v2.0.4 (Lam et al., 2021). Plasmid replicons were analyzed using PlasmidFinder v2.1 (Carattoli et al., 2014). Insertion sequences (ISs) were identified using ISfinder (Siguier et al., 2006). The genetic contexts of mcr and tmexCD1-toprJ1 genes were analyzed by comparing with similar items available in the NCBI nr (non-redundant) database. Plasmid comparisons and genetic context comparisons were visualized using BRIG and Easyfig (Alikhan et al., 2011; Sullivan et al., 2011).
Strain T698-1 isolated from the intestinal sample of the patient was identified as K. pneumoniae by both MALDI-TOF MS and whole genome sequencing. It was resistant to aminoglycoside (gentamycin), fluoroqinolone (ciprofloxacin), polymyxin (colistin), tetracycline, phenicols (florfenicol, chloramphenicol), sulfonamide (trimethoprime-sulfamethoxazole), and some β-lactams (ceftazidime, cefotaxime, cefoperazone/sulbactam, ampicillin, aztreonam), but remained susceptible to carbapenems (meropenem, imipenem) and tigecycline (Table 1). In line with the resistance phenotypes, no carbapenemase gene was detected in strain T698-1, while ESBL genes including blaDHA–1 and blaCTX–M–15 were detected. Meanwhile, strain T698-1 was negative for the tigecycline resistance determinant tet(X), but positive for the colistin resistance gene mcr-8.2 and the RND-type efflux pump gene cluster tmexCD1-toprJ1, which is associated with resistance to tetracyclines, quinolones, cephalosporins, and aminoglycosides. Despite carrying tmexCD1-toprJ1, strain T698-1 was susceptible to tigecycline, quite possibly due to the low expression level of this gene.
The genome of strain T698-1 (BioProject number: PRJNA747739) was assembled into four complete circularized contigs, including a 5,265,236 bp chromosome (CP079781) encoding 5207 predicted ORFs with a GC content of 57.6% and three multi-drug resistant (MDR) plasmids (pKPT698-tmexCD, pKPT698-mcr, pKPT698-tetA). Strain T698-1 belonged to ST1326 (gapA-infB-mdh-pgi-phoE-rpoB-tonB allele number 3-3-1-1-1-1-16), which is closely related to the endemic K. pneumoniae clones ST258 (3-3-1-1-1-1-79) and ST11 (3-3-1-1-1-1-4) in clonal group (CG) 258 with only one allele (tonB) variance among the three STs. According to the previous study, CGs were defined as groups for which MLST profiles showed only one allelic mismatch with at least one other member of the group, suggesting strain T698-1 belonged to the notorious CG258 clonal group (Bialek-Davenet et al., 2014). To date, 77 immunologically distinct Klebsiella capsule types (K1-K77) have been defined by serology, and comparative analysis of the full-length capsule synthesis loci (K-loci) extracted from whole genome sequences identified 134 distinct K-loci (KL1-KL134) (Wyres et al., 2016). Strain T698-1 belonged to KL102, which has not been verified with traditional immunoelectrophoresis techniques. Apart from the blaSHV–11 gene conferring intrinsic ampicillin resistance in K. pneumoniae, the chromosome of strain T698-1 carried acquired antimicrobial resistance genes sul1 and aadA2, which were bordered by mobile elements (IS26-IS6100-orf1-sul1-orf2-aadA2-IS26). Fluoroquinolone resistance-associated mutations including S80I in ParC and S83I in GyrA were detected in the chromosome of strain T698-1 (Wyres et al., 2019).
Plasmid pKPT698-tmexCD (CP079784) was 291,624 bp in length, with an average G + C content of 46.8%. It is an IncHI1B/FIB plasmid comprising 330 predicted ORFs. One plasmid, p18-29-MDR (MK262712), with similar backbone of 99.94% identity at 99% coverage to pKPT698-tmexCD was retrieved in the NCBI nr database by BLASTn analysis (Figure 1A). Plasmids pKPT698-tmexCD and p18-29-MDR, both from K. pneumoniae, shared the same backbone and plasmid replication genes, indicating that they might be originated from a common ancestor and have undergone evolution separately. A total of 18 different resistance genes containing tmexCD1-toprJ1, aadA1, aadA2, cmlA1, sul3, aac(3)-IVa, aph(4)-Ia, aph(3′)-Ic, strA (two copies), strB (two copies), mph(E), msr(E), armA, sul1, blaDHA–1 and qnrB4, and an truncated IS102-like gene, △tet(B), were identified on plasmid pKPT698-tmexCD. Antimicrobial resistance genes were located on a 65,340 bp MDR-encoding region bordered by the TnAs1 transposon. A further BLAST search of this region in the NCBI nr database returned seven plasmid hits (CP076031, MK262712, MN099026, MT647838, AP023338, CP075257, CP075287) with >99.7% identity and ≥95% coverage, indicating this MDR region was most likely acquired by horizontal gene transfer, and it was transmissible among diverse plasmid backbones. The MDR region harbored an array of mobile elements including TnAs1, Tn5393, ISEc28, ISEc29, ISEc59, IS903, ISCR1, IS1, 5 copies of IS26, and an IntI1 integrase, suggesting active genetic recombination could occur in this region (Figure 1B). The RND-type efflux pump gene cluster, tmexCD1-toprJ1, was first reported to be located on the transposon Tn5393 of plasmid pHNAH8I-1 (MK347425), which was considered to be associated with its translocation (Lv et al., 2020). On plasmid pKPT698-tmexCD, the upstream Tn5393 and its adjacent genetic elements were disrupted by an IS26, constituting the genetic context of IS26-tnfxB1-tmexC1-tmexD1-toprJ1-Tn5393:tnpA-Tn5393:tnpR-strA-strB, which was slightly distinct from that reported previously (Lv et al., 2020; Sun et al., 2020). Predicted to have originated from the chromosome of Pseudomonas sp., the RND efflux pump gene cluster tmexCD1-toprJ1, as well as its variants tmexCD2-toprJ2 and tmexCD3-toprJ3, has been reported from a wide diversity of species including Pseudomonas sp., K. pneumoniae, Klebsiella quasipneumoniae, Raoultella ornithinolytica, and Proteus mirabilis (Lv et al., 2020; Sun et al., 2020; Wan et al., 2020; Li et al., 2021; Wang C.-Z. et al., 2021; Wang Q. et al., 2021). Diverse mobile genetic elements including plasmids, insertion sequences, transposons, integrative, and conjugative elements (ICEs) were reported to have contributed to its wide dissemination (Lv et al., 2020; Wan et al., 2020; Hirabayashi et al., 2021; Wang Q. et al., 2021). Plasmid elimination was performed to demonstrate the influence of TMexCD1-TOprJ1 and MCR-9 to tigecycline and colistin resistance, respectively. Despite trying three times, we failed to get a single plasmid-eliminated derivative strain and only the strain T698-1-PC of which both pKPT698-tmexCD and pKPT698-mcr were cured of was obtained. The T698-1-PC showed the same or close MIC value to tigecycline (≤0.5 mg/L), tetracycline (32 mg/L), quinolones (>4 mg/L), gentamycin (>16 mg/L), and cephalosporins (>16 mg/L) as the original strain T698-1 and those antibacterial agents were the substrate spectrums of TMexCD1-TOprJ1 for K. pneumoniae (Lv et al., 2020), further proving the inefficacy of TMexCD1-TOprJ1 in T698-1.
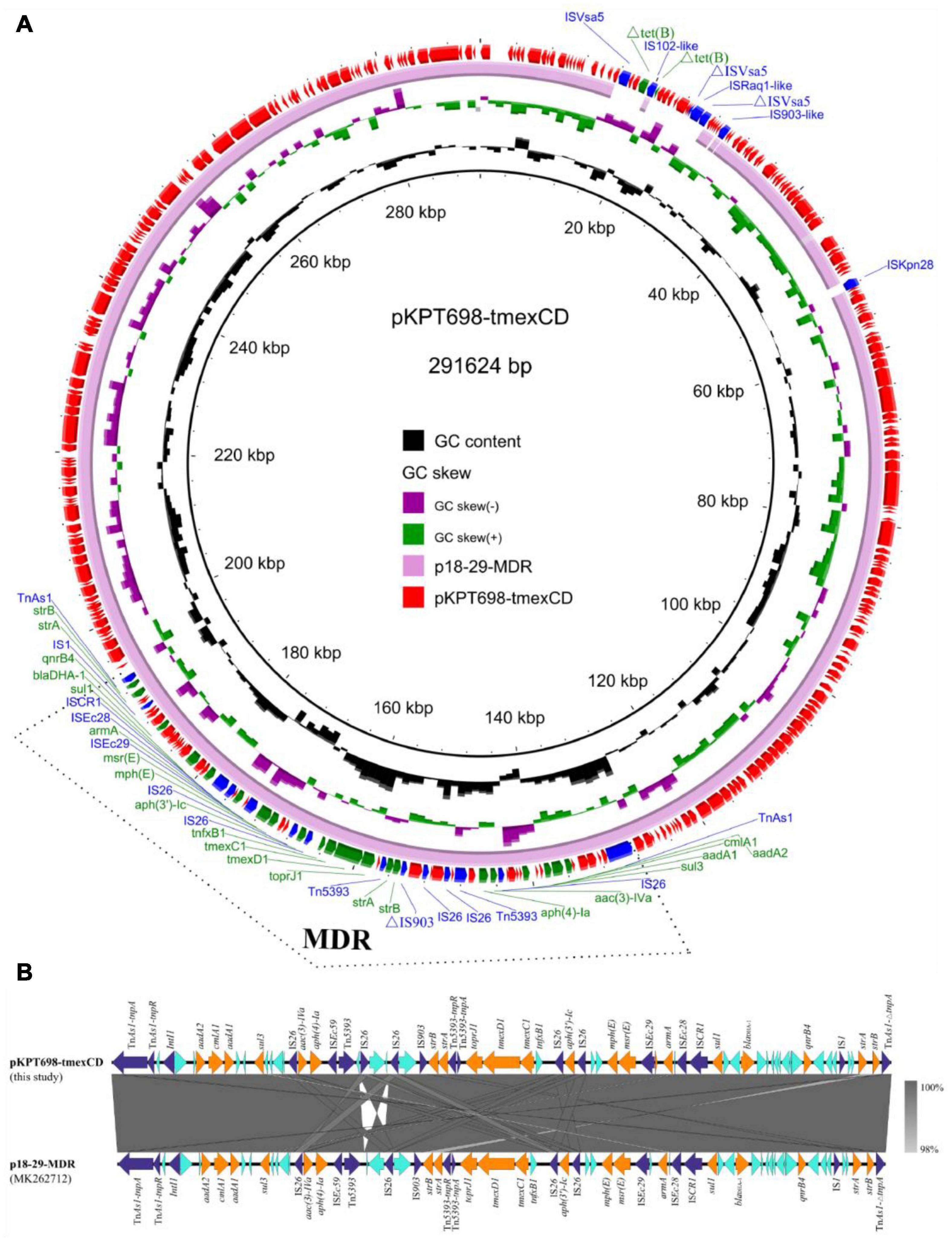
Figure 1. (A) Circular plasmid map of pKPT698-tmexCD. Green, blue, and red arrows indicate the antimicrobial resistance genes, mobile elements, and other predicted ORFs, respectively. The names of antimicrobial resistance genes, mobile elements are labeled alongside the corresponding arrows. The multi-drug resistant (MDR) region is indicated with dotted line frame. (B) Linear comparisons of the MDR region in plasmids pKPT698-tmexCD and p18-29-MDR. Cyan, blue, and yellow arrows indicated the antimicrobial resistance genes, mobile elements, and other predicted ORFs, respectively.
The second plasmid, pKPT698-tetA (CP079782), is a 138,834 bp, IncFIB plasmid which encodes 167 predicted ORFs with a G + C content of 52.3%. BLASTn analysis in the NCBI nr database returned no hits with similar backbones (>85% coverage), and the most closely related plasmid (pE196_IMP6, 250,392 bp, AP019405) exhibited 99.49% identity to pKPT698-tetA at 80% coverage. pKPT698-tetA harbored multiple antimicrobial resistance genes including aac(6′)Ib-cr, arr-3, dfrA27, aadA16, sul1, and catA2 located in a 23,779 bp MDR region bordered by the transposable element Tn2. The MDR region is >99.95% identical to its counterparts on plasmid pKp21774-135 (MG878868) from K. pneumoniae and plasmid pM206-NDM1 (AP018830) from Enterobacter xiangfangensis at 100% coverages, suggesting this region was acquired by horizontal gene transfer (Figure 2A).
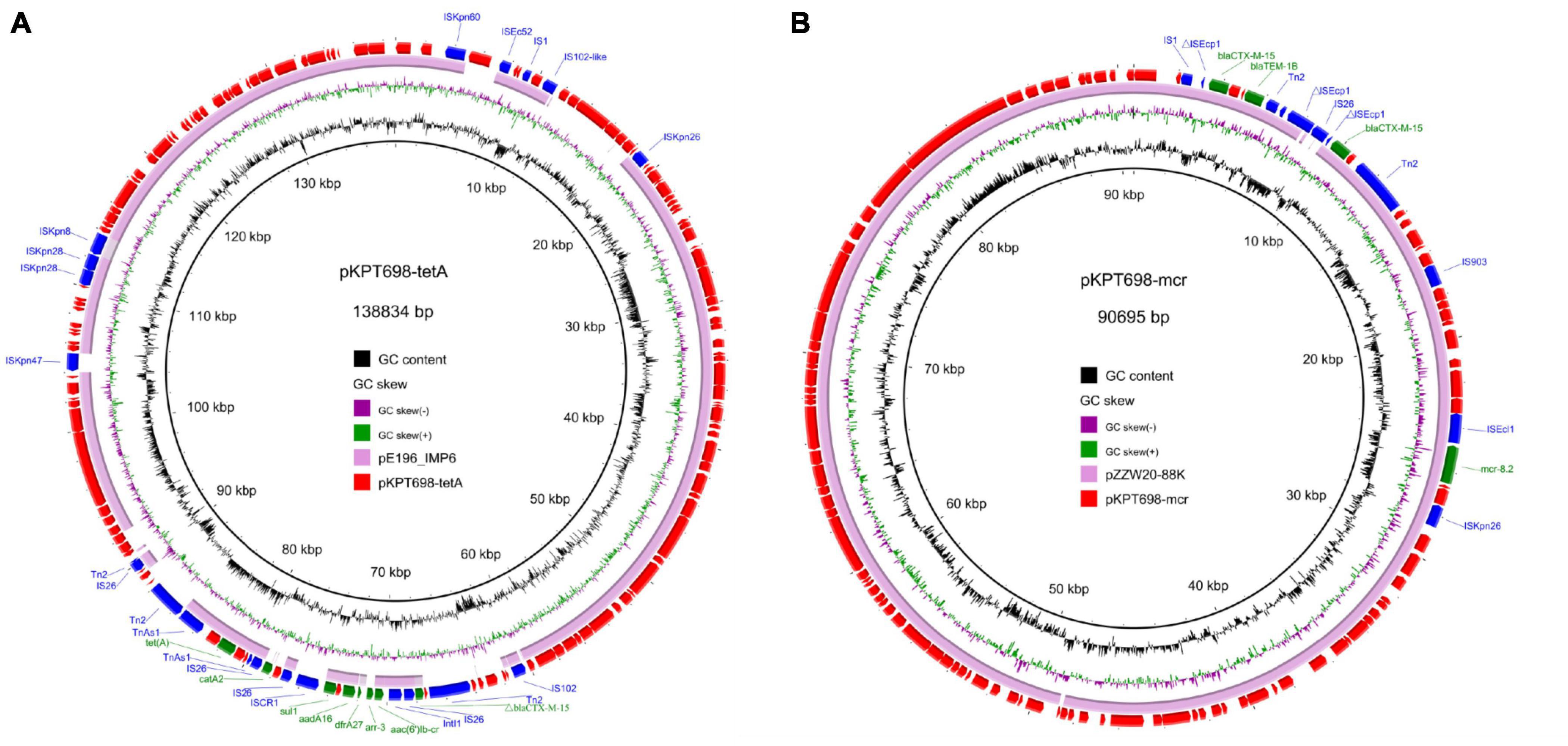
Figure 2. Circular plasmid maps of pKPT698-tetA (A) and pKPT698-mcr (B). Green, blue, and red arrows indicate the antimicrobial resistance genes, mobile elements, and other predicted ORFs, respectively. The names of antimicrobial resistance genes, mobile elements are labeled alongside the corresponding arrows. (A) The MDR region of pKPT698-tetA including aac(6′)Ib-cr, arr-3, dfrA27, aadA16, sul1, and catA2 was bordered by the transposable element Tn2. (B) pKPT698-mcr carried blaCTX–M–15 (two copies), blaTEM–1B, mcr-8.2 with mobile elements such as IS903B and ISEcl1 inserting into this segment.
The third plasmid, pKPT698-mcr (CP079783), is a 90,695 bp, IncFII/FIA plasmid which encodes predicted 112 ORFs with a G + C content of 50.1%. It was 99.97% identical to plasmid pZZW20-88K (accession: CP058962) at 98% coverage. Antimicrobial resistance genes carried by pKPT698-mcr include blaCTX–M–15 (two copies), blaTEM–1B, and mcr-8.2 (Figure 2B). Several mobile elements such as IS903B and ISEcl1 were reported to have played pivotal roles in the dissemination of mcr-8 among Enterobacteriaceae (Wang et al., 2018; Zhang et al., 2021). In line with the previous findings, the mcr-8.2 gene on pKPT698-mcr was associated with the genetic context of ISEcl1-mcr-8.2-orf-ISKpn26 (Zhang et al., 2021). Both pKPT698-tmexCD and pKPT698-mcr were shown to be non-conjugative under laboratory conditions. The elimination of pKPT698-mcr in strain T698-1-PC caused a marked decrease of MIC values to colistin from 64 mg/L in T698-1-PC to 1 mg/L in T698-1, indicating mcr-8.2 genes mediated colistin resistance in T698-1. Besides, we further screened common chromosomal mutations that caused colistin resistance. The result from Kleborate showed that no mutations associated with resistance to colistin were detected in mgrB gene. Compared with the colistin-susceptible genome of the strain NJST258_1, T698-1 did not possess any amino acid mutations of PmrA/PmrB, and PhoP/PhoQ. Therefore, these data suggested that the mcr-8.2-bearing plasmid in this strain is responsible for colistin resistance.
The plasmid-borne tigecycline-resistant RND efflux pump gene cluster tmexCD1-toprJ1 was first reported in K. pneumoniae in China in 2020 (Lv et al., 2020), with an extremely high prevalence of 52.4% in animals and 0.08∼2.5% in patients (Lv et al., 2020; Sun et al., 2020). This indicated that we should be alert to the further dissemination of TmexCD1-ToprJ1-positive K. pneumoniae in hospital despite that only one K. pneumoniae carrying TmexCD1-ToprJ1 was detected. Worse still, the K. pneumoniae strains even plasmids co-harboring tmexCD1-toprJ1 and mcr have been prevailing in China, and some strains belonged to the ST11 clone, the predominant and high risk clone of CRKP strains in China (Sun et al., 2020). The simultaneous presence of NDM carbapenemases further promoted the emergence of pan-drug resistant bacteria, especially in K. pneumoniae with high genome plasticity (Sun et al., 2020). The MDR strain T698-1 was susceptible to tigecycline and carbapenems but conferred resistance to colistin on account of mcr-8.2, a variant of the mcr-8 colistin resistance gene identified in 2020 (Ma et al., 2020), suggesting that future nosocomial infections surveillance pay more attention to K. pneumoniae.
In conclusion, we reported the emergence of a ST1326 MDR K. pneumoniae strain in a hospital in China, which belonged to the endemic CG258 clone -and carried mcr-8.2, ESBL genes, and the RND efflux pump gene cluster tmexCD1-toprJ1. K. pneumoniae of CG258 was commonly shown to be associated with carbapenem resistance. The acquisition of colistin and tigecycline resistance determinants by CG258 K. pneumoniae may pose a serious public health concern.
The complete genome sequence of strain T698-1 was deposited in the GenBank database under the BioProject number: PRJNA747739, with accession numbers CP079781-CP079784.
The study was approved by the Ethics Committee of Second Affiliated Hospital, Zhejiang University School of Medicine (2020-319). Written informed consent was obtained from the patients for the publication of any potentially identifiable data.
CL and YW performed the experiments and wrote the original draft. YF and ZS contributed to data analysis and manuscript writing. LH, ND, YZ, and JL helped with the experiments. RZ helped editing the manuscript and contributed to study design. GC put forward the conception, designed the study, and edited the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the National Natural Science Foundation of China (Nos. 81861138052, 82072341, and 81871705).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alikhan, N.-F., Petty, N. K., Zakour, N. L. B., and Beatson, S. A. (2011). BLAST ring image generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402. doi: 10.1186/1471-2164-12-402
Bialek-Davenet, S., Criscuolo, A., Ailloud, F., Passet, V., Jones, L., Delannoy-Vieillard, A.-S., et al. (2014). Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg. Infect. Dis. 20:1812. doi: 10.3201/eid2011.140206
Borowiak, M., Baumann, B., Fischer, J., Thomas, K., Deneke, C., Hammerl, J. A., et al. (2020). Development of a novel mcr-6 to mcr-9 multiplex PCR and assessment of mcr-1 to mcr-9 occurrence in colistin-resistant Salmonella enterica isolates from environment, feed, animals and food (2011–2018) in Germany. Front. Microbiol. 11:80. doi: 10.3389/fmicb.2020.00080
Carattoli, A., Zankari, E., García-Fernández, A., Larsen, M. V., Lund, O., Villa, L., et al. (2014). In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 58, 3895–3903. doi: 10.1128/AAC.02412-14
CLSI (2020). Performance Standards for Antimicrobial Susceptibility Testing. Approved Standard. CLSI Document M100. Wayne, PA: CLSI.
Dallenne, C., Da Costa, A., Decré, D., Favier, C., and Arlet, G. (2010). Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 65, 490–495. doi: 10.1093/jac/dkp498
David, S., Reuter, S., Harris, S. R., Glasner, C., Feltwell, T., Argimon, S., et al. (2019). Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat. Microbiol. 4, 1919–1929. doi: 10.1038/s41564-019-0492-8
Deleo, F., Chen, L., Porcella, S., Martens, C., Kobayashi, S., Porter, A., et al. (2014). Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae. Proc. Natl. Acad. Sci. U.S.A. 111, 4988–4993. doi: 10.1073/pnas.1321364111
Dong, N., Zhang, R., Liu, L., Li, R., Lin, D., Chan, E. W.-C., et al. (2018). Genome analysis of clinical multilocus sequence Type 11 Klebsiella pneumoniae from China. Microb. Genom. 4:e000149. doi: 10.1099/mgen.0.000149
Gu, D., Dong, N., Zheng, Z., Lin, D., Huang, M., Wang, L., et al. (2018). A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect. Dis. 18, 37–46. doi: 10.1016/S1473-3099(17)30489-9
He, T., Wang, R., Liu, D., Walsh, T. R., Zhang, R., Lv, Y., et al. (2019). Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat. Microbiol. 4, 1450–1456. doi: 10.1038/s41564-019-0445-2
Hirabayashi, A., Dao, T. D., Takemura, T., Hasebe, F., Thanh, N. H., Tran, H. H., et al. (2021). A transferable IncC-IncX3 hybrid plasmid co-carrying blaNDM–4, tet (X4), and tmexCD3-toprJ3 confers resistance to carbapenem and tigecycline. mSphere 6:e0059221. doi: 10.1128/mSphere.00592-21
Ji, K., Xu, Y., Sun, J., Huang, M., Jia, X., Jiang, C., et al. (2020). Harnessing efficient multiplex PCR methods to detect the expanding Tet(X) family of tigecycline resistance genes. Virulence 11, 49–56. doi: 10.1080/21505594.2019.1706913
Lam, M. M., Wick, R. R., Watts, S. C., Cerdeira, L. T., Wyres, K. L., and Holt, K. E. (2021). A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat. Commun. 12:4188. doi: 10.1038/s41467-021-24448-3
Li, R., Peng, K., Xiao, X., Liu, Y., Peng, D., and Wang, Z. (2021). Emergence of a multidrug resistance efflux pump with carbapenem resistance gene bla VIM–2 in a Pseudomonas putida megaplasmid of migratory bird origin. J. Antimicrob. Chemother. 76, 1455–1458. doi: 10.1093/jac/dkab044
Li, R., Xie, M., Dong, N., Lin, D., Yang, X., Wong, M. H. Y., et al. (2018). Efficient generation of complete sequences of MDR-encoding plasmids by rapid assembly of MinION barcoding sequencing data. Gigascience 7:gix132.
Linkevicius, M., Sandegren, L., and Andersson, D. I. (2016). Potential of tetracycline resistance proteins to evolve tigecycline resistance. Antimicrob. Agents Chemother. 60, 789–796. doi: 10.1128/AAC.02465-15
Liu, X., Tian, J., Liu, L., Zhu, T., Yu, X., Chu, X., et al. (2017). Identification of an operon involved in fluoride resistance in Enterobacter cloacae FRM. Sci. Rep. 7:6786. doi: 10.1038/s41598-017-06988-1
Liu, Y.-Y., Wang, Y., Walsh, T. R., Yi, L.-X., Zhang, R., Spencer, J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168. doi: 10.1016/S1473-3099(15)00424-7
Lv, L., Wan, M., Wang, C., Gao, X., Yang, Q., Partridge, S., et al. (2020). Emergence of a plasmid-encoded resistance-nodulation-division efflux pump conferring resistance to multiple drugs, including tigecycline, in Klebsiella pneumoniae. mBio 11:e02930-19. doi: 10.1128/mBio.02930-19
Ma, K., Feng, Y., Liu, L., Yao, Z., and Zong, Z. (2020). A cluster of colistin- and carbapenem-resistant Klebsiella pneumoniae carrying blaNDM–1 and mcr-8.2. J. Infect. Dis. 221, S237–S242.
Navon-Venezia, S., Kondratyeva, K., and Carattoli, A. (2017). Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 41, 252–275. doi: 10.1093/femsre/fux013
Overbeek, R., Olson, R., Pusch, G. D., Olsen, G. J., Davis, J. J., Disz, T., et al. (2013). The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 42, D206–D214. doi: 10.1093/nar/gkt1226
Poirel, L., Walsh, T. R., Cuvillier, V., and Nordmann, P. (2011). Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 70, 119–123. doi: 10.1016/j.diagmicrobio.2010.12.002
Rebelo, A. R., Bortolaia, V., Kjeldgaard, J. S., Pedersen, S. K., Leekitcharoenphon, P., Hansen, I. M., et al. (2018). Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Eurosurveillance 23:17-00672. doi: 10.2807/1560-7917.ES.2018.23.6.17-00672
Siguier, P., Pérochon, J., Lestrade, L., Mahillon, J., and Chandler, M. (2006). ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 34, D32–D36. doi: 10.1093/nar/gkj014
Sullivan, M. J., Petty, N. K., and Beatson, S. A. (2011). Easyfig: a genome comparison visualizer. Bioinformatics 27, 1009–1010. doi: 10.1093/bioinformatics/btr039
Sun, S., Gao, H., Liu, Y., Jin, L., Wang, R., Wang, X., et al. (2020). Co-existence of a novel plasmid-mediated efflux pump with colistin resistance gene mcr in one plasmid confers transferable multidrug resistance in Klebsiella pneumoniae. Emerg. Microbes Infect. 9, 1102–1113. doi: 10.1080/22221751.2020.1768805
Wan, M., Gao, X., Lv, L., Cai, Z., and Liu, J.-H. (2020). IS 26 mediate the acquisition of tigecycline resistance gene cluster tmexCD1-toprJ1 by IncHI1B-FIB plasmids in Klebsiella pneumoniae and Klebsie lla quasipneumoniae from food market sewage. Antimicrob. Agents Chemother. 65:e02178-20. doi: 10.1128/AAC.02178-20
Wang, C.-Z., Gao, X., Yang, Q.-W., Lv, L.-C., Wan, M., Yang, J., et al. (2021). A Novel transferable resistance-nodulation-division pump gene cluster, tmexCD2-toprJ2, confers tigecycline resistance in Raoultella ornithinolytica. Antimicrob. Agents Chemother. 65:e02229-20. doi: 10.1128/AAC.02229-20
Wang, Q., Peng, K., Liu, Y., Xiao, X., Wang, Z., and Li, R. (2021). Characterization of TMexCD3-TOprJ3, an RND-type efflux system conferring resistance to tigecycline in Proteus mirabilis, and its associated integrative conjugative element. Antimicrob. Agents Chemother. 65:e0271220. doi: 10.1128/AAC.02712-20
Wang, X., Wang, Y., Zhou, Y., Li, J., Yin, W., Wang, S., et al. (2018). Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg. Microb. Infect. 7:122. doi: 10.1038/s41426-018-0124-z
Wick, R. R., Judd, L. M., Gorrie, C. L., and Holt, K. E. (2017). Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Computat. Biol. 13:e1005595. doi: 10.1371/journal.pcbi.1005595
Wyres, K. L., Hawkey, J., Hetland, M. A., Fostervold, A., Wick, R. R., Judd, L. M., et al. (2019). Emergence and rapid global dissemination of CTX-M-15-associated Klebsiella pneumoniae strain ST307. J. Antimicrob. Chemother. 74, 577–581. doi: 10.1093/jac/dky492
Wyres, K. L., Wick, R. R., Gorrie, C., Jenney, A., Follador, R., Thomson, N. R., et al. (2016). Identification of Klebsiella capsule synthesis loci from whole genome data. Microb. Genom. 2:e000102. doi: 10.1099/mgen.0.000102
Xu, J., Zhu, Z., Chen, Y., Wang, W., and He, F. (2021). The Plasmid-borne tet (A) gene is an important factor causing tigecycline resistance in ST11 carbapenem-resistant Klebsiella pneumoniae under selective pressure. Front. Microbiol. 12:328. doi: 10.3389/fmicb.2021.644949
Keywords: Klebsiella pneumoniae, tmexCD1-toprJ1, ESBL genes, CG258, mcr-8.2
Citation: Liu C, Wu Y, Fang Y, Sang Z, Huang L, Dong N, Zeng Y, Lu J, Zhang R and Chen G (2022) Emergence of an ST1326 (CG258) Multi-Drug Resistant Klebsiella pneumoniae Co-harboring mcr-8.2, ESBL Genes, and the Resistance-Nodulation-Division Efflux Pump Gene Cluster tmexCD1-toprJ1 in China. Front. Microbiol. 13:800993. doi: 10.3389/fmicb.2022.800993
Received: 24 October 2021; Accepted: 15 February 2022;
Published: 17 March 2022.
Edited by:
Henrietta Venter, University of South Australia, AustraliaReviewed by:
Bela Kocsis, Semmelweis University, HungaryCopyright © 2022 Liu, Wu, Fang, Sang, Huang, Dong, Zeng, Lu, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gongxiang Chen, Y2hlbmdvbmd4aWFuZ0B6anUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.