
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 13 January 2023
Sec. Food Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1105888
 Yalei Liu1†
Yalei Liu1† Xinlin Jia1†
Xinlin Jia1† Junlei Chang1
Junlei Chang1 Xuemei Jiang1
Xuemei Jiang1 Lianqiang Che1
Lianqiang Che1 Yan Lin1
Yan Lin1 Yong Zhuo1
Yong Zhuo1 Bin Feng1
Bin Feng1 Zhengfeng Fang1
Zhengfeng Fang1 Jian Li1
Jian Li1 Lun Hua1
Lun Hua1 Jianping Wang1
Jianping Wang1 Zhihua Ren2
Zhihua Ren2 Mengmeng Sun3
Mengmeng Sun3 De Wu1
De Wu1 Shengyu Xu1*†
Shengyu Xu1*†Introduction: The effects of maternal addition of yeast cultures on offspring gut development and intestinal microorganisms are not yet known, so the aim of this study was to investigate the effects of maternal addition of yeast cultures to the diet of sows during late gestation and lactation on growth performance, antioxidant properties and intestinal microorganisms of offspring weaned piglets.
Methods: 40 Landrace × Yorkshire sows (3–7 of parity) with similar backfat were randomly divided into two treatment groups: control diet (CON) and control diet +2.0 g/kg yeast culture (XPC), and the trial started on day 90 of gestation and ended on day 21 of lactation.
Results: The results showed that maternal addition of yeast culture significantly increased weaned piglet weight and mean daily gain (p < 0.05), with a tendency to increase litter weight gain (p = 0.083) and liver weight (p = 0.076) compared to the control group. The content of thymus malondialdehyde (MDA) was significantly higher (p < 0.05) and the content of colon total antioxidant capacity (T-AOC) was significantly lower (p < 0.05) in the offspring weaned piglets of the XPC group compared to the control group. The expression of thymus SOD1 and SOD2, spleen SOD1, jejunum SOD2, and colon GPX1, SOD1, and SOD2 were significantly downregulated in the XPC group of offspring weaned piglets compared with the control group (p < 0.05). The intestinal morphology and the content of short-chain fatty acids in colonic chyme did not differ between the two groups (p > 0.05). Compared with the control group, the XPC group significantly increased the relative abundance of colonic chyme Bacteroidetes (p < 0.05), tended to decrease the relative abundance of Lactobacillus (p = 0.078), and tended to increase the relative abundance of Alloprevotella (p = 0.055). The XPC group significantly upregulated Blautia and Fournierella (p < 0.05) and significantly downregulated Candidatus_Competibacter, Nitrospira, Dechloromonas, Haliangium, and Oscillospira (p < 0.05).
Discussion: In conclusion, maternal addition of yeast cultures improved the growth performance of offspring weaned piglets and changed the intestinal microbial community, but did not improve their antioxidant performance.
In order to reduce the number of non-productive days and maximize the productivity of the sow, farms use the strategy of early weaning. Early weaned piglets have an underdeveloped immune system and are exposed to stress from various stressors such as physiological (separation from the sow, underdeveloped system), psychological (mixing with other litters, facing fighting and establishing a new social hierarchy) and nutritional (change from breast milk to solid feed), which leads to delayed growth and easy diarrhea after weaning (Modina et al., 2019; Xiong et al., 2019; Upadhaya and Kim, 2021). The main manifestations are a reduction in feed intake within 24–48 h, slowed or even negative growth, and increased susceptibility to pathogens (Lallès et al., 2007; Jayaraman and Nyachoti, 2017). Studies have shown that weaning older, more mature pigs helps prevent many of the adverse gastrointestinal effects associated with weaning stress (Craig et al., 2017; Wensley et al., 2021). In addition, reduced piglet organ weights, restricted digestion of colostrum and reduced serum immunoglobulin concentrations can lead to delayed development, resulting in differences in birth weight and subsequent growth of sow offspring (Craig et al., 2019). Therefore, nutritional and management strategies can be implemented prior to weaning to provide piglets with a good stress tolerance and thus better cope with weaning stress. For nutritional strategies, in-depth research can be conducted in sow nutrition to have a long-term beneficial effect on offspring growth performance by improving maternal uterine condition and lactational breast milk condition. It has been shown that maternal nutritional interventions can affect the intestinal health and growth performance of offspring (Cao et al., 2014; Liu et al., 2016; Chen et al., 2017), so it is important to investigate the effects of maternal nutritional interventions on offspring.
Yeast cultures are a class of biological products consisting of metabolites produced during the anaerobic fermentation of Saccharomyces cerevisiae and some live yeast (Jin et al., 2017). Yeast cultures have various functions, such as maintaining the intestinal health of animals, improving production performance, improving feed nutrition, and promoting the metabolism of the body (Fan et al., 2012; Diao, 2016; Burdick Sanchez et al., 2021). Yeast cultures have been widely used in swine production in recent years, and studies have found that the addition of yeast cultures to sow diets from late gestation to lactation improves feed intake, immune status, milk production, milk quality, and fecal microbiota, thereby improving the growth performance of piglets (Zhao et al., 2022). However, the effect of maternal addition of yeast cultures on offspring gut development and gut microbiota is unknown and needs further study. We hypothesized that maternal addition of yeast cultures would improve the growth and development of offspring weaned piglets. To test this hypothesis, we investigated the effects of adding yeast cultures to sow diets during late gestation and lactation on growth performance, antioxidant properties, intestinal morphology, short-chain fatty acids, and intestinal microorganisms of offspring weaned piglets.
The yeast cultures (Diamond V, United States) used in the test contained a variety of nutrients, including oligosaccharides, proteins, peptides, amino acids, yeast-derived enzymes, nucleic acids, and etc. The trial was conducted at Yile breeding pig farm (Dekang group Co., Ltd., China). All animal procedures were approved by the Animal Care and Use Committee of Sichuan Agricultural University (Ethical Approval Code: SICAU20220119).
40 Landrace × Yorkshire sows (3–7 of parity) with similar backfat (16.80 ± 0.42 mm) were randomly divided into two treatment groups: control diet (CON) and control diet +2.0 g/kg yeast culture (XPC), with 20 replicates per treatment and 1 sow per replicate. The trial started on day 90 of gestation and ended on day 21 of lactation. At the day 20 of lactation, 6 litters of piglets with similar average weight were selected from each group, weighed one by one after fasting overnight, and one piglet close to the average weight of the litter was selected from each weaned litter for slaughter, totaling 12 piglets (half of each male and half of each female).
The nutritional needs of basal diet for sows during gestation and lactation are formulated according to National Research Council (2012) (NRC, United States). The sows in the control group were fed the basal diet and the sows in the yeast culture group were supplemented with an additional 2.0 g/kg XPC on top of the basal diet. The composition and nutritional levels of the basal diet are shown in Supplementary Table S1. Gestating sows were fed twice a day (8:30; 14:30) with a total of 3 kg of basal diet. Sows are not fed on the day of farrowing, 2 kg on the following day, and then gradually increase 1 kg per day. Sows started to feed freely on day 7 of lactation, three times a day (8:30; 11:30; 17:30). During the whole test period, sows were free to drink water, pens were cleaned daily, disinfected and dewormed regularly, and kept the air circulation and temperature constant in the house.
On the day of weaning (21 days old), 10 ml of blood was collected from the anterior vena cava of piglets, blood was loaded into sodium heparin anticoagulation tubes, left at room temperature for 30 min, then centrifuged at 3500 r/min at 4°C for 15 min, and the supernatant plasma was divided and stored at −20°C for measurement. After the piglets were slaughtered, the intestinal tissues were removed, the duodenum, jejunum and ileum were separated, and about 2 cm of the middle section of each intestine was cut and fixed in 4% paraformaldehyde solution for the determination of intestinal morphology. In addition, the middle segments of duodenum, jejunum, ileum and colon were collected, and the thymus, liver, spleen and mesenteric lymph nodes were rinsed with saline and blotted dry with absorbent paper, then divided into lyophilization tubes, snap-frozen in liquid nitrogen and stored at −80°C for measurement (Mou et al., 2018). The colonic chyme was divided into lyophilization tubes and then snap frozen in liquid nitrogen and stored at −80°C for testing (Wan et al., 2020).
After sow farrowing, record the average daily feed intake (ADFI) of sow, born alive, litter weight of born alive and average alive piglet birth weigh. After weaning, record the number of offspring weaned piglets, weaned piglet weight, weaned litter weight, calculate the average daily weight gain and litter weight gain.
Morphological samples of duodenum, jejunum and ileum were sent to Yibaidao Technology Co., Ltd. (Chengdu, China) for determination. The samples were processed for paraffin preparation for HE staining, and Image Pro Plus 6.0 (media cybernetics, Inc., Rockville, MD, United States) software was applied to measure 5 villus height (VH) as well as corresponding crypt depth (CD) per field, making a total of 10 villi and their corresponding crypts. The V/C ratio is equal to the VH value divided by the CD value.
Sample pretreatment: All tissues were ground into powder using a mortar containing liquid nitrogen, and then weighed about 0.1 g of tissue powder and added saline in the ratio of powder weight: saline volume = 1:9, homogenized in an ice bath using a homogenizer, and then centrifuged at 4,000 r/min for 10 min at 4°C (Mou et al., 2020). The supernatant was divided and then used in a BCA protein concentration determination kit (Beyotime Biotechnology, Shanghai, China) to determine the protein concentration of the homogenate.
Catalase (CAT), glutathione peroxidase (GSH-Px), malondialdehyde (MDA), superoxide dismutase (SOD), and total antioxidant capacity (T-AOC) were measured in plasma and tissues using the corresponding kits (Grace Biotechnology Co., Ltd., Suzhou, China), and all indexes were measured according to the instructions (Bradford, 1976).
The expression of antioxidant-related genes GPX1 (glutathione peroxidase1), SOD1 (superoxide dismutase1), SOD2 (superoxide dismutase2), and CAT (catalase) in weaned piglet tissues was determined by quantitative real-time fluorescence PCR (RT-PCR). Tissue RNA was extracted using RNAiso Plus (TaKaRa, Japan), the concentration and quality of RNA was determined using a UV spectrophotometer (NanoDrop 2000, Thermo, United States), and the integrity of RNA was detected using gel electrophoresis. Using HiScript ® III RT SuperMix for qPCR (+gDNA wiper) kit (Vazyme, R323, Nanjing, China) removes genomic DNA from the sample under the reaction condition of 42°C for 2 min. Then a reverse transcription reaction system was prepared for reverse transcription to obtain cDNA at 37°C for 15 min; 85°C, 5 s. Fluorescent quantitative PCR was performed using the ChamQ Universal SYBR qPCR Master Mix quantification kit (Vazyme, Q711, Nanjing, China) according to the instructions. Statistical results were obtained using β-actin as an internal reference gene and the 2−∆∆Ct method was used to calculate the relative expression of target genes (Livak and Schmittgen, 2001). The target gene primer sequences (primers were synthesized by Tsingke Biotechnology Co., Ltd., Beijing, China) are shown in Supplementary Table S2.
The colonic chyme was mixed with ultrapure water and the supernatant was added with metaphosphoric acid and crotonic acid, mixed and the supernatant was added with chromatographic methanol, mixed and the supernatant was filtered through a 0.22 μm filter membrane, and then the content of short-chain fatty acids (SCFAs) was determined by gas chromatography (Varian, GC CP3800). In addition, the colonic chyme was sent to Novogene Technology Co., Ltd. (Beijing, China) for 16 s rRNA sequencing. The general steps are as follows: DNA extraction and detection of samples, PCR amplification, product purification, library preparation and library inspection, NovaSeq up-sequencing, splicing and filtering of the raw data obtained from sequencing, and noise reduction by DADA2 to obtain the final Amplicon Sequence Variants (ASVs) and feature tables. Subsequently, the obtained ASVs were compared with the database using the classify-sklearn module in the QIIME2 software to obtain species information for each ASV. For the obtained ASVs, abundance, Alpha diversity calculation, and Venn diagram were analyzed, and in addition, the differences between different samples (groups) were found from them by Beta diversity index inter-group variance analysis, and Principal Co-ordinates Analysis (PCoA; Xu et al., 2020; Li et al., 2021). T-test statistical analysis was selected to test the significance of differences in species composition and community structure of the samples.
IBM SPSS Statistics 27 software was used for independent sample T test. Before statistical analysis, Shapiro Wilk and Levene’s tests were used to test the normality and homogeneity of variance of all data. If the data did not conform to the normal distribution, nonparametric tests were used. Results are expressed as “mean ± standard error,” with p < 0.05 considered as significant difference and 0.05 ≤ p < 0.1 considered as significant trend.
Compared with the control group, maternal addition of XPC significantly increased offspring weaning piglet weight (p < 0.05) and significantly increased average daily weight gain of offspring weaned piglets (p < 0.05), with a tendency to increase sow ADFI (p = 0.081) and piglet litter gain (p = 0.083). However, there was no significant effect on the number of live births, number of offspring weaned piglets, litter weight of born alive, average alive piglet birth weigh, and weaned litter weight (p > 0.05; Table 1).
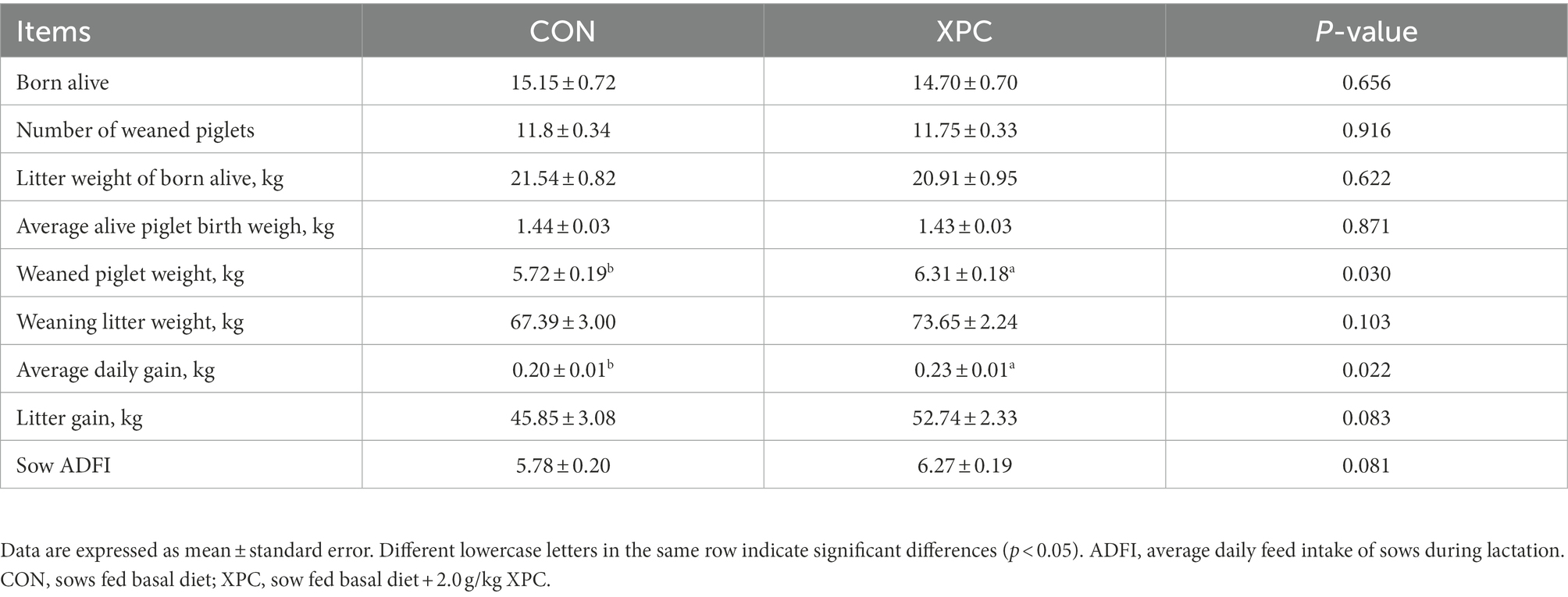
Table 1. Effects of maternal supplementation with XPC on growth performance of offspring weaned piglets.
Compared with the control group, maternal addition of XPC significantly increased slaughter weight of offspring weaned piglets (p < 0.05) and tended to increase liver weight (p = 0.076), but had no significant effect on spleen weight and organ index of offspring weaned piglets (p > 0.05; Table 2).
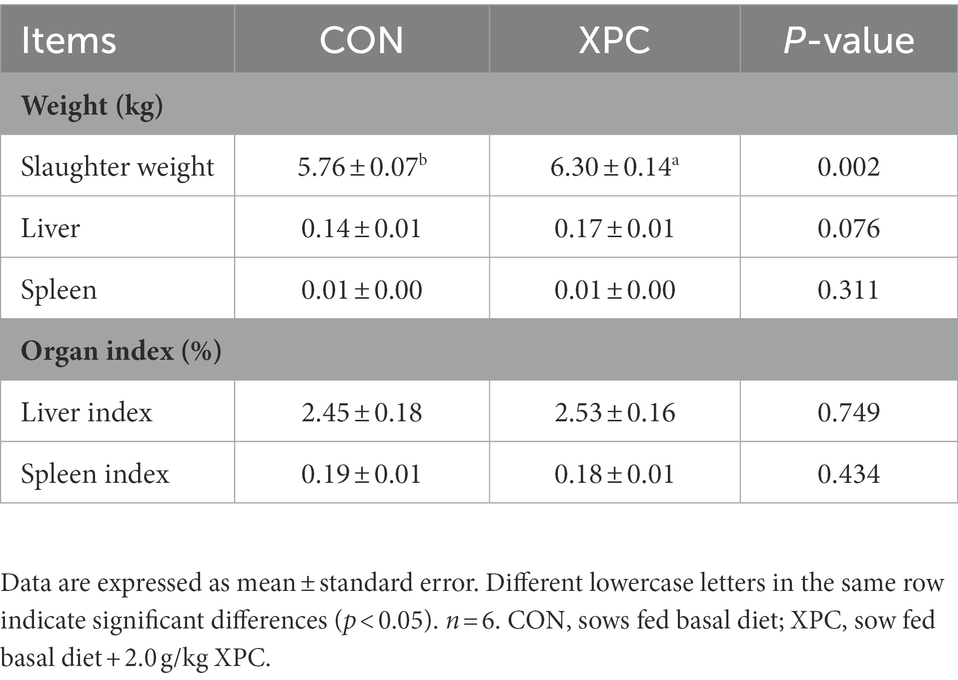
Table 2. The effect of maternal supplementation with XPC on the organ index of offspring weaned piglets.
The results showed that there was no significant difference in the VH, CD, V/C ratio and number of goblet cells in the duodenum, jejunum and ileum in the XPC group compared with the control group (p > 0.05; Supplementary Table S3).
The content of thymic MDA was significantly higher and the content of colonic T-AOC was significantly lower in the offspring weaned piglets of the XPC group compared to the control group (p < 0.05), while there was no significant difference in the antioxidant indexes of other tissues between the two groups (p > 0.05; Tables 3, 4).
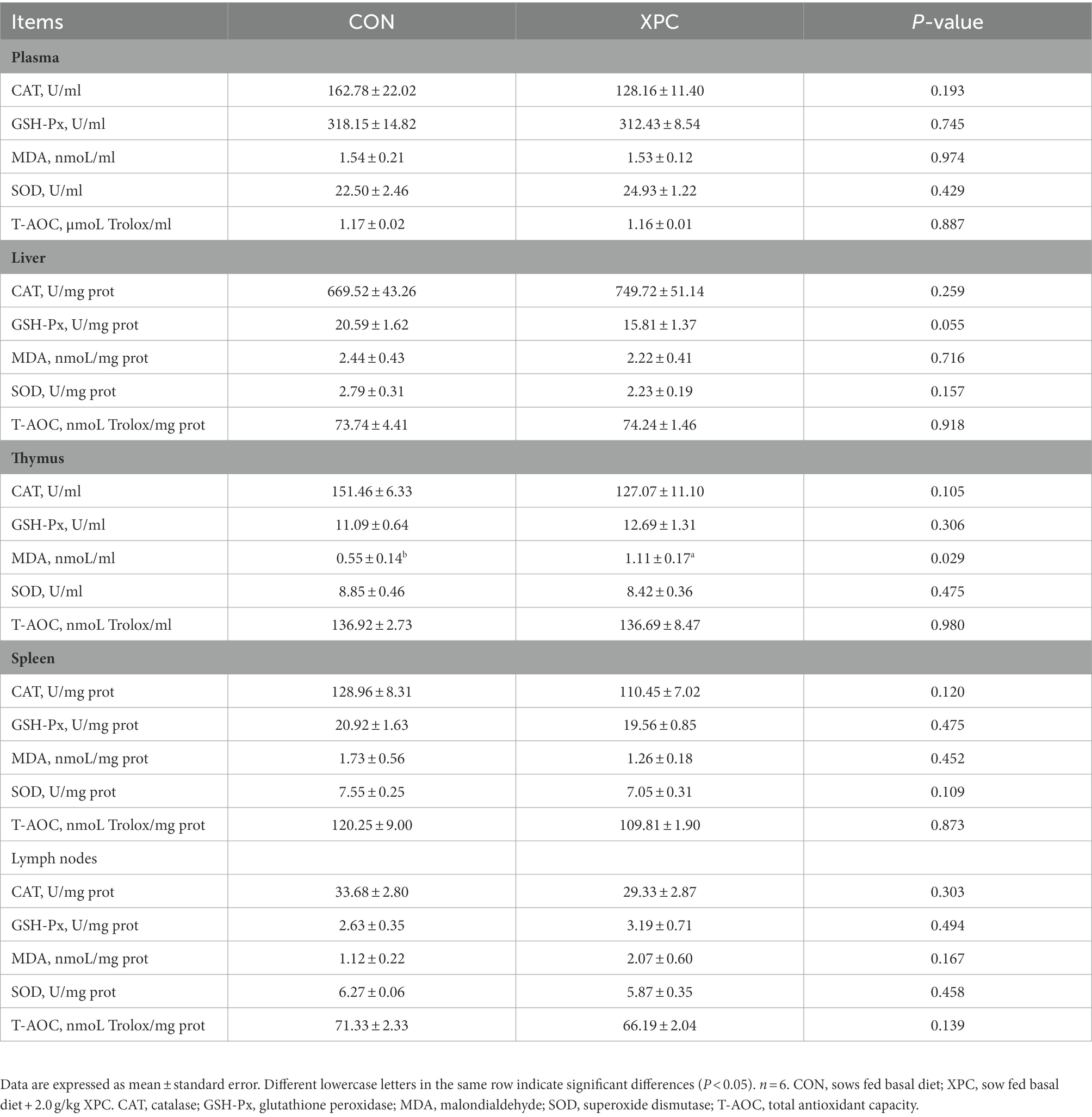
Table 3. Effects of maternal supplementation with XPC on plasma and tissue antioxidant properties of offspring weaned piglets.
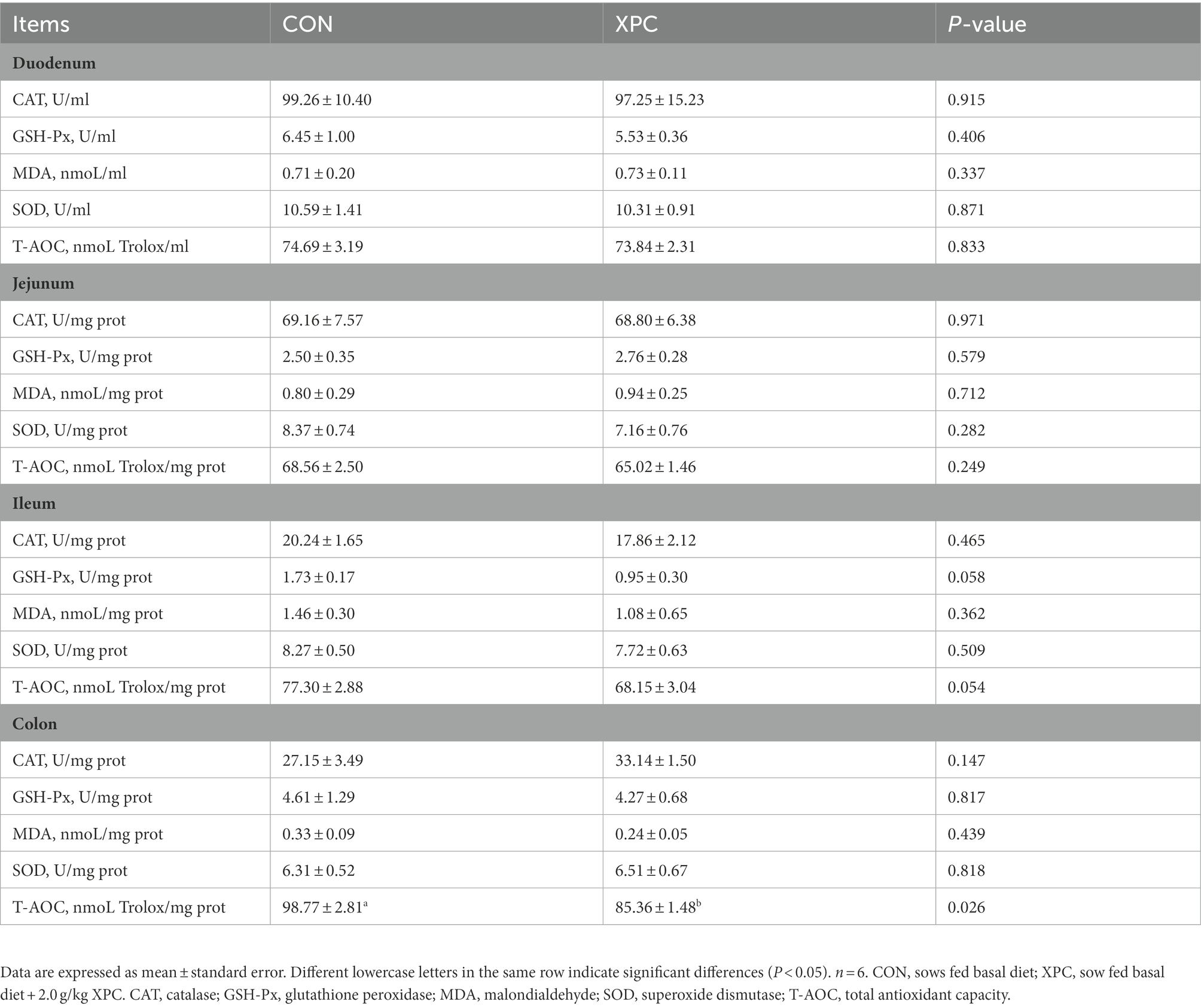
Table 4. Effect of maternal supplementation with XPC on intestinal antioxidant properties of offspring weaned piglets.
Compared with the control group, the expression of thymus SOD1 and SOD2 was significantly downregulated in the XPC group of offspring weaned piglets (p < 0.05, Figure 1A), the expression of liver GPX1 tended to be downregulated (p = 0.051, Figure 1B), and the expression of spleen SOD1 was significantly downregulated (p < 0.05, Figure 1C). There was no significant difference in the expression of lymph nodes and duodenum between the two groups (p < 0.05, Figures 1D,E). The expression of jejunal SOD2 was significantly downregulated (p < 0.05), the expression of SOD1 tended to be downregulated (p = 0.081, Figure 1F). There was no significant difference in the expression of ileum between the two groups (p < 0.05, Figure 1G). The expression of colonic GPX1, SOD1, and SOD2 was significantly downregulated (p < 0.05, Figure 1H).

Figure 1. Effect of maternal supplementation with XPC on the expression of antioxidant-related genes in different tissues (A) thymus, (B) liver, (C) spleen, (D) lymph nodes, (E) duodenum, (F) jejunum, (G) ileum, (H) colon, of offspring weaned piglets. Data are expressed as mean ± standard error, n = 6. *p < 0.05, **p < 0.01. CON, sows fed basal diet; XPC, sow fed basal diet +2.0 g/kg XPC. GPX, glutathione peroxidase; SOD, superoxide dismutase; CAT, catalase.
Maternal addition of XPC did not have a significant effect on colonic chyme SCFAs in offspring weaned piglets (p > 0.05; Supplementary Table S4).
Maternal addition of XPC had no significant effect on the colonic microbial α-diversity index in offspring weaned piglets compared to the control group (p > 0.05; Supplementary Table S5). Both groups enjoyed 546 ASVs together, 604 and 599 ASVs specific to the control and XPC groups, respectively (Figure 2A). The analysis of the principal coordinates revealed that the colonic microbial communities of the two groups were separately aggregated and had significantly different community structures (Figure 2B).
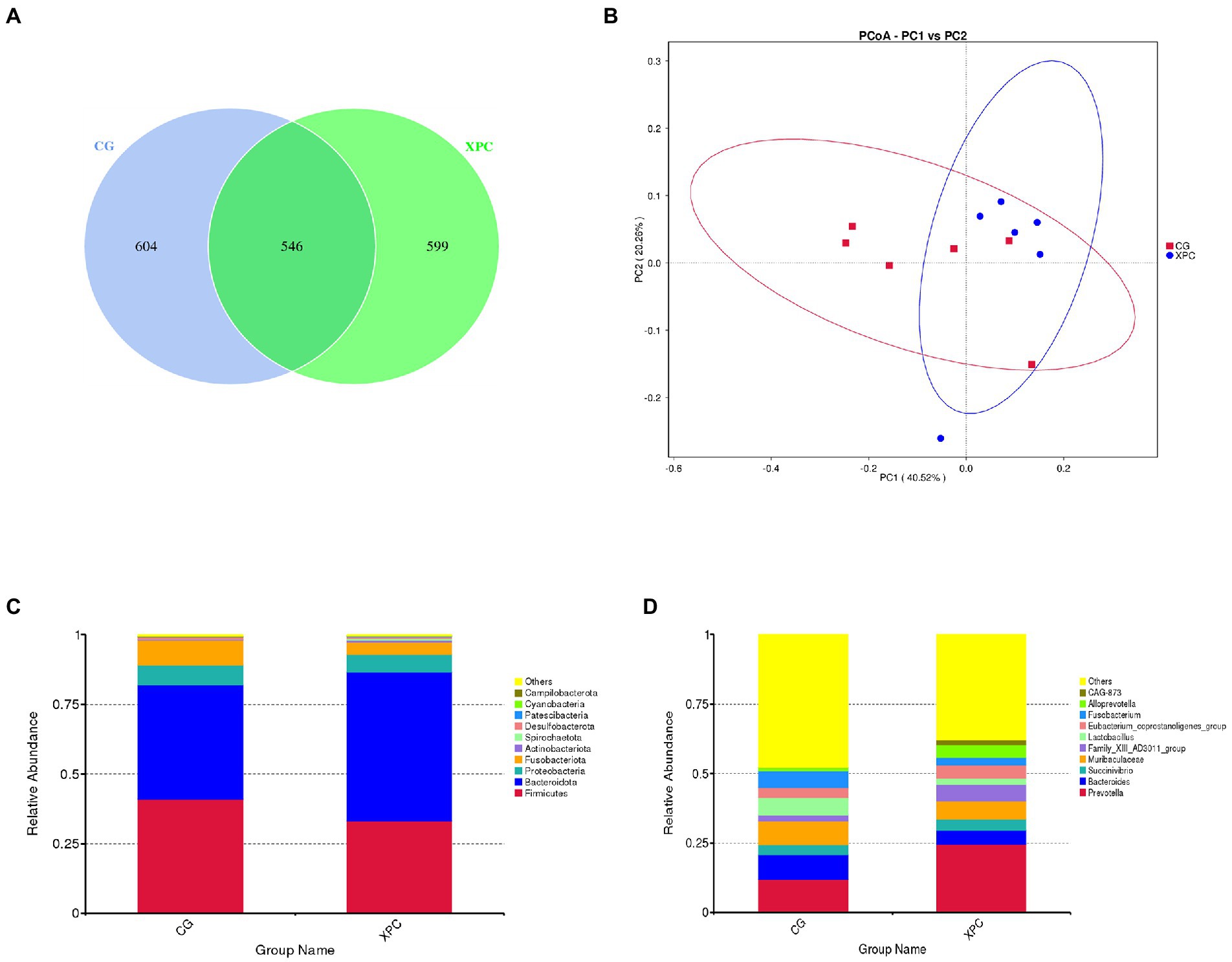
Figure 2. Effects of maternal supplementation with XPC on colonic microbial composition and relative abundance in offspring weaned piglets. (A) Amplicon Sequence Variants (ASVs) analysis. (B) Principal Co-ordinates Analysis (PCoA). (C) Relative abundance of phylum level. (D) Relative abundance of genus level. CG: control group (CON, sows fed basal diet); XPC, yeast culture group (sow fed basal diet +2.0 g/kg XPC). n = 6.
Firmicutes and Bacteroidetes were the dominant phylum among colonic microorganisms in offspring weaned piglets. Compared to the control group, the XPC group significantly increased the relative abundance of Bacteroidetes (p < 0.05; Table 5; Figure 2C), tended to decrease the relative abundance of Lactobacillus (p = 0.078), and tended to increase the relative abundance of Alloprevotella (p = 0.055; Table 6; Figure 2D).
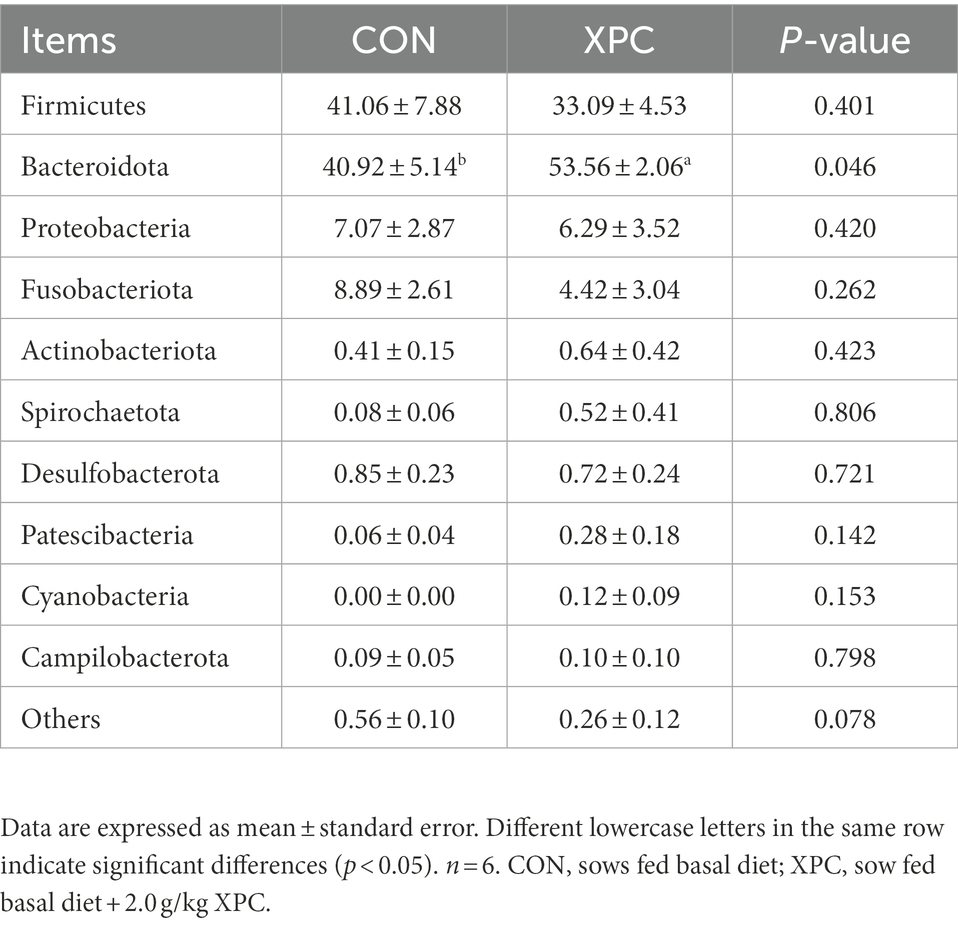
Table 5. Effect of maternal supplementation with XPC on the relative abundance of colonic microbial phylum level of offspring weaned piglets (%).
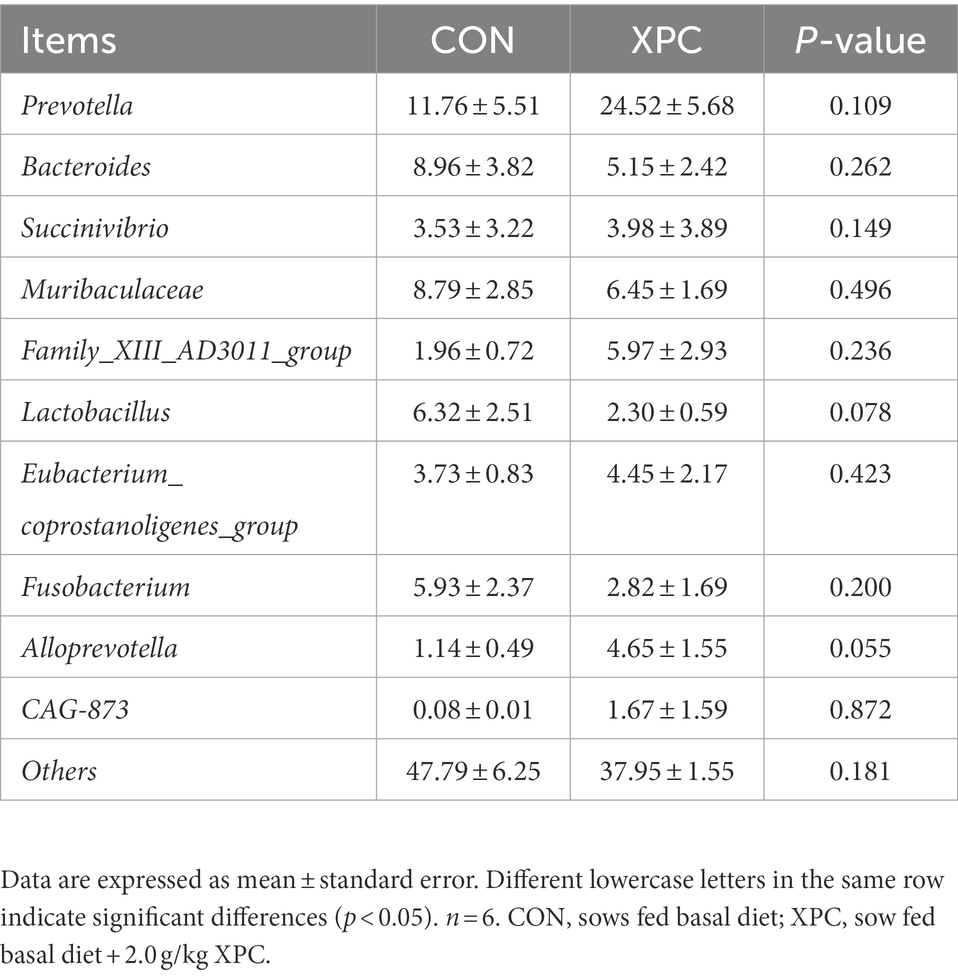
Table 6. Effect of maternal supplementation with XPC on the relative abundance of colonic microbial genus level of offspring weaned piglets (%).
Compared with the control group, the XPC group significantly upregulated Blautia and Fournierella (p < 0.05), and significantly downregulated Candidatus_Competibacter, Nitrospira, Dechloromonas, Haliangium, and Oscillospira (p < 0.05, Figure 3).
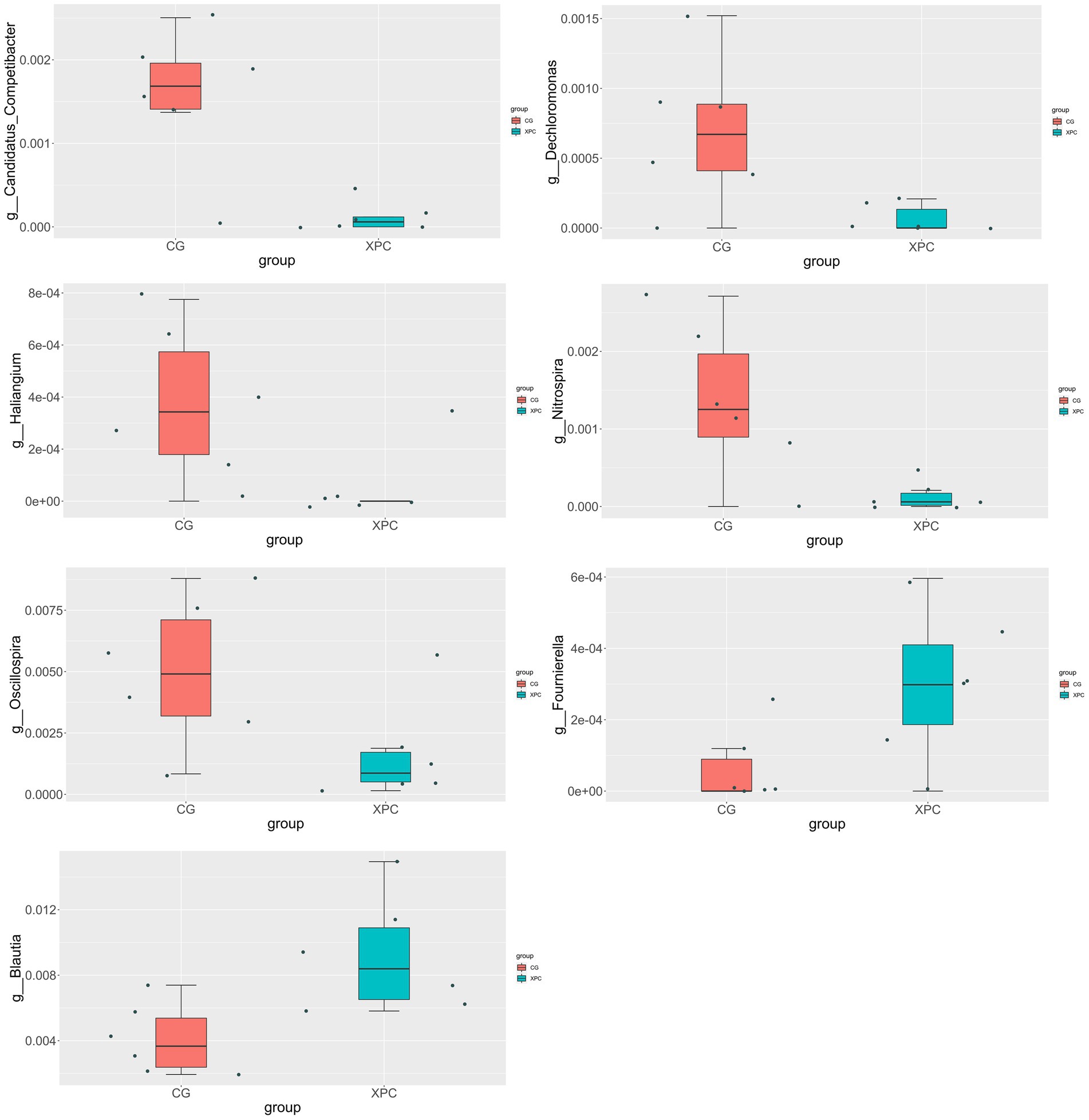
Figure 3. T-test analysis of species differences between groups. CG, control group (CON, sows fed basal diet); XPC, yeast culture group (sow fed basal diet +2.0 g/kg XPC). n = 6.
The development of piglets during the embryonic period and the source of nutrition such as breast milk after birth are crucial to the growth and development of piglets, so the nutritional and physiological status of the sow during the fetal and lactation stages of piglets will greatly affect the growth and development of piglets. Higher feed intake of sows during lactation increased milk production and thus contributed to higher weaning weight of piglets (Jiang et al., 2020). The results of this experiment showed that maternal addition of XPC significantly increased weaning piglet weight, average daily weight gain, and tended to increase sow ADFI and piglet litter weight gain, so we speculate that XPC is beneficial to increase the milk production of sows by increasing sow feed intake, so that piglets can get more nutrition and thus increase weaning piglet weight. It was found that the addition of brewer’s yeast fermentation products to sow diets had no significant effect on the number of live births and weaned piglets, but increased weaning litter weight and litter gain, probably due to a reduction in sow plasma urea nitrogen to improve sow protein utilization, which increased milk production without affecting colostrum and milk (Shen et al., 2011). The addition of 4% brewer’s yeast to the sow’s diet during late gestation and lactation significantly increased piglet weaning survival rate and weaning weight, significantly reduced piglet stillbirth rate, and improved sow milk production and quality (Song et al., 2017). It has also been found that the addition of yeast cultures to sows during late gestation and lactation significantly increased the average daily weight gain of piglets and improved the milk yield of sows (Zhao et al., 2022), which is consistent with the results of present experiment. Studies have shown that the nutritional status of the sow affects milk production, especially the availability of energy and protein during lactation, and that increased nutritional availability promotes mammary gland development as well as metabolism, which leads to increased milk production (Kim et al., 2000; Bass et al., 2019; Lu et al., 2019). It suggests that maternal addition of yeast cultures may increase the weaning weight of piglets as well as the average daily weight gain by increasing the milk production of the sow.
The intestine is the main digestive and absorption site of the body, in which intestinal epithelial cells play an important role. Villi cells absorb nutrients, water and electrolytes, while crypt cells secrete water and electrolytes (Xiong et al., 2019), and villi height and crypt depth are related to the number of villi and crypt cells (Hampson, 1986), and if villi become shorter and crypt deeper, which indicates a decrease in intestinal absorptive cells and an increase in secretory cells, it leads to poorer intestinal absorption and increased secretion, which may be related to piglet diarrhea (Nabuurs et al., 1993; Yang and Yu, 2021). Undigested dietary fiber is fermented by intestinal microorganisms to produce SCFAs. SCFAs play an important role in the body’s glycolipid metabolism, inflammatory response, maintenance of intestinal integrity, and immune homeostasis (Tan et al., 2014; Xu, 2017). The results of this experiment showed that maternal addition of XPC had no significant effect on intestinal morphology and colonic chyme SCFAs of offspring weaned piglets. This indicates that the intestinal digestion, absorption and secretion function of offspring could not be affected by maternal addition of XPC, and in addition, it could not affect the intestinal microbial dietary fiber fermentation of offspring. Antioxidant enzymes can scavenge reactive oxygen species (ROS) in the body and play an important role in maintaining the balance of the redox state of the body (Irshad and Chaudhuri, 2002). The results of this experiment showed that the content of thymic MDA was higher in the XPC group of offspring weaned piglets, while the content of colonic T-AOC was lower, and in addition, the expression of antioxidant-related genes in different tissues was downregulated to different degrees. It was found that the addition of selenium-enriched yeast to the diet improved the antioxidant capacity of weaned piglets (Liu, L. et al., 2021a). Another study found that the addition of yeast products to the diet increased the rate of diarrhea in weaned piglets, adversely affected both intestinal morphology and intestinal barrier, and to some extent improved the antioxidant capacity (Yang et al., 2016). Piglet diarrhea is usually associated with oxidative stress (Granot and Kohen, 2004), and the negative impact of yeast products on the piglet intestine leads to oxidative stress, which activates the antioxidant system and increases the activity of antioxidant enzymes in the body (Yang et al., 2016). Under the present conditions, XPC did not affect the intestinal morphology of the offspring weaned piglets, so it is possible that the piglets in the XPC group had lower levels of oxidants resulting in a lower redox status, or that the piglets in the XPC group had a lower antioxidant capacity themselves. It is noteworthy that piglets in the XPC group showed better growth performance, which may suggest better resistance to oxidative stress, thus facilitating growth.
Intestinal microorganisms play an important role in animal health and play an important role in nutrient metabolism, growth and development, maintenance of the intestinal barrier, immune regulation, and resistance to pathogen invasion (Yu et al., 2022). Piglets form intestinal microorganisms through contact with the sow’s birth canal, skin, feces, environmental microorganisms and by suckling milk (He et al., 2020; Zhang et al., 2022). It was found that the breast milk microbiota constitutes the initial microbiota of the newborn piglets gut and plays a crucial role in regulating newborn piglets health (Wu et al., 2006; Chen et al., 2018; Li et al., 2022). Thus, maternal addition of yeast cultures may affect the gut microbiology of piglets by influencing the composition of breast milk and sow feces microbes. This pilot study found that the relative abundance of Alloprevotella was higher in the XPC group and the relative abundance of Lactobacillus was lower compared to the control group. Lactobacillus as a common probiotic has good probiotic properties and significantly inhibits the multiplication of pathogenic bacteria (He et al., 2019). Alloprevotella is also a probiotic that mainly produces succinate and acetate, both of which have improved intestinal barrier and anti-inflammatory effects (Downes et al., 2013). Thus, while maternal addition of XPC decreased the relative abundance of the Lactobacillus in the intestine of offspring weaned piglets, it increased the relative abundance of another beneficial bacterium, Alloprevotella. Similarly, another study found that the addition of live yeast to sow diets significantly reduced the number of fecal Lactobacillus in piglets (Van et al., 2003), which may be related to differences in maternal transmission of XPC and the ability of XPC to better influence anti-inflammatory function in piglets.
Species difference analysis showed that Blautia and Fournierella were significantly up-regulated in the XPC group, and Candidatus_Competibacter, Nitrospira, Dechloromonas, Haliangium, and Oscillospira were significantly down-regulated. Blautia is a group of anaerobic bacteria with probiotic properties, widely present in the intestine and feces of mammals, that ferment different types of carbohydrates to produce acetic acid, lactic acid and ethanol and improve glucose metabolism in animals (Kiros et al., 2019; Liu, X. et al., 2021). Fournierella favors the production of short-chain fatty acids (Liu et al., 2020). Therefore, genera related to intestinal barrier, anti-inflammation and glucose metabolism were more abundant in the intestine of offspring weaned piglets in the XPC group, and these genera may have promoted the absorption of nutrients, better utilization of energy and resistance to pathogenic bacteria, ultimately promoting piglet growth and higher weaning weight. Both Candidatus_Competibacter and Dechloromonas belong to the Proteobacteria, while the Proteobacteria is associated with intestinal diseases and is mostly pathogenic and can cause inflammation (He et al., 2019). Nitrospira and Haliangium are mostly found in water bodies and soil and have a more specific function, while Oscillospira can produce butyric acid (Konikoff and Gophna, 2016). Therefore, XPC can increase the content of beneficial bacteria and reduce the content of harmful bacteria in the intestine of weaned piglets to some extent. It was found that the addition of yeast probiotics to the diet had no significant effect on the fecal α-diversity index of weaned piglets, but the microbiota was significantly different from the control group (Xu et al., 2018). In contrast, supplementation of live brewer’s yeast during lactation in piglets increased cecum microbial α-diversity and increased Blautia, Collinsella and Eubacterium (Kiros et al., 2019). The reason for the difference in test results may be the difference in yeast product, the object of addition, and the amount of additives. Another possible explanation is the difference in diet composition. The sow diet in this trial was not a corn-soybean meal diet, but a mixed diet, so the difference in sow diet largely affected the intestinal microorganisms. The addition of yeast cultures to sows may have a direct effect on breast milk as well as fecal microorganisms, and piglets’ intestinal microorganisms may also be affected through exposure to breast milk and sow feces. Therefore, a variety of factors caused the complexity of intestinal microbes in offspring weaned piglets.
Maternal addition of yeast cultures increased offspring piglet weaning weight, average daily weight gain and litter weight gain, and increased the content of beneficial intestinal bacteria, but had no effect on intestinal morphology and the content of SCFAs in colonic chyme, and reduced antioxidant capacity. Therefore, maternal addition of yeast cultures can improve the growth and development of the offspring to some extent, especially in terms of growth performance. Yeast cultures can be used as a potential growth promoter to promote the growth of offspring through maternal transmission.
The data presented in the study are deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA), accession number: PRJNA914903).
The animal study was reviewed and approved by Animal Care and Use Committee of Sichuan Agricultural University.
SX, YLiu, and DW designed the study. YLiu and XJia carried out the animal experiments and performed the laboratory work. YLiu, XJia, JC, XJian, LC, YLin, YZ, BF, ZF, JL, LH, JW, ZR, and MS performed the statistical analysis. YLiu wrote the paper. SX and DW revised the manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by Major Scientific and Technological Special Project of Sichuan Province (no. 2021ZDZX0009), Natural Science Foundation of Sichuan Province (2022NSFSC1628), and Sichuan Province “145” Breeding Tackle Project (2021YFYZ0008).
We would like to thank Diamond V, United States for kindly providing yeast culture (XPC) and Higher Education Discipline Innovation Project of China (D17015).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1105888/full#supplementary-material
Bass, B. E., Tsai, T. C., Yang, H., Perez, V., Holzgraefe, D., Chewning, J., et al. (2019). Influence of a whole yeast product (Pichia guilliermondii) fed throughout gestation and lactation on performance and immune parameters of the sow and litter. J. Anim. Sci. 97, 1671–1678. doi: 10.1093/jas/skz060
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Burdick Sanchez, N. C., Broadway, P. R., and Carroll, J. A. (2021). Influence of yeast products on modulating metabolism and immunity in cattle and swine. Animals 11:371. doi: 10.3390/ani11020371
Cao, M., Che, L., Wang, J., Yang, M., Su, G., Fang, Z., et al. (2014). Effects of maternal over-and undernutrition on intestinal morphology, enzyme activity, and gene expression of nutrient transporters in newborn and weaned pigs. Nutrition 30, 1442–1447. doi: 10.1016/j.nut.2014.04.016
Chen, W., Mi, J., Lv, N., Gao, J., Cheng, J., Wu, R., et al. (2018). Lactation stage-dependency of the sow Milk microbiota. Front. Microbiol. 9:945. doi: 10.3389/fmicb.2018.00945
Chen, Y., Mou, D., Hu, L., Zhen, J., Che, L., Fang, Z., et al. (2017). Effects of maternal low-energy diet during gestation on intestinal morphology, disaccharidase activity, and immune response to lipopolysaccharide challenge in pig offspring. Nutrients 9:1115. doi: 10.3390/nu9101115
Craig, J. R., Collins, C. L., Bunter, K. L., Cottrell, J. J., Dunshea, F. R., and Pluske, J. R. (2017). Poorer lifetime growth performance of gilt progeny compared with sow progeny is largely due to weight differences at birth and reduced growth in the preweaning period, and is not improved by progeny segregation after weaning. J. Anim. Sci. 95, 4904–4916. doi: 10.2527/jas2017.1868
Craig, J. R., Dunshea, F. R., Cottrell, J. J., Furness, J. B., Wijesiriwardana, U. A., and Pluske, J. R. (2019). A comparison of the anatomical and gastrointestinal functional development between gilt and sow progeny around birth and weaning. J. Anim. Sci. 97, 3809–3822. doi: 10.1093/jas/skz217
Diao, C. (2016). Application of yeast cultures in pig production. Swine Sci. 33, 80–82. doi: 10.3969/j.issn.1673-5358.2016.06.031
Downes, J., Dewhirst, F. E., Tanner, A., and Wade, W. G. (2013). Description of Alloprevotella rava gen. Nov., sp. nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae Moore et al. 1994 as Alloprevotella tannerae gen. Nov., comb. nov. Int. J. Syst. Evol. Microbiol. 63, 1214–1218. doi: 10.1099/ijs.0.041376-0
Fan, X., Chen, C., Zhnag, H., and Wang, D. (2012). Application of yeast cultures in piglet production. Feed Expo. 10, 33–35. doi: 10.3969/j.issn.1001-0084.2012.10.014
Granot, E., and Kohen, R. (2004). Oxidative stress in childhood--in health and disease states. Clin. Nutr. 23, 3–11. doi: 10.1016/s0261-5614(03)00097-9
Hampson, D. J. (1986). Alterations in piglet small intestinal structure at weaning. Res. Vet. Sci. 40, 32–40. doi: 10.1016/S0034-5288(18)30482-X
He, M., Dang, W., Liang, X., Yang, J., Zhang, J., Liang, J., et al. (2019). Effect of adding fermented soybean meal to the diet on the diversity of fecal flora in weaned piglets. Chin. J. Anim. Nutr. 31, 2560–2571. doi: 10.3969/j.issn.1006-267x.2019.06.015
He, J., Zhang, P., Shen, L., Niu, L., Tan, Y., Chen, L., et al. (2020). Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 21:6356. doi: 10.3390/ijms21176356
Irshad, M., and Chaudhuri, P. S. (2002). Oxidant-antioxidant system: role and significance in human body. Indian J. Exp. Biol. 40, 1233–1239.
Jayaraman, B., and Nyachoti, C. M. (2017). Husbandry practices and gut health outcomes in weaned piglets: a review. Anim. Nutr. 3, 205–211. doi: 10.1016/j.aninu.2017.06.002
Jiang, X., Lin, S., Lin, Y., Fang, Z., Xu, S., Feng, B., et al. (2020). Effects of silymarin supplementation during transition and lactation on reproductive performance, milk composition and haematological parameters in sows. J. Anim. Physiol. Anim. Nutr. 104, 1896–1903. doi: 10.1111/jpn.13425
Jin, X., Li, K., Huang, L., and Feng, J. (2017). Research and application of yeast cultures in animal production. Feed Res. 16, 7–10. doi: 10.13557/j.cnki.issn1002-2813.2017.16.002
Kim, S. W., Hurley, W. L., Hant, I. K., and Easter, R. A. (2000). Growth of nursing pigs related to the characteristics of nursed mammary glands. J. Anim. Sci. 78, 1313–1318. doi: 10.2527/2000.7851313x
Kiros, T. G., Luise, D., Derakhshani, H., Petri, R., Trevisi, P., D'Inca, R., et al. (2019). Effect of live yeast Saccharomyces cerevisiae supplementation on the performance and cecum microbial profile of suckling piglets. PLoS One 14:e0219557. doi: 10.1371/journal.pone.0219557
Konikoff, T., and Gophna, U. (2016). Oscillospira: a central, enigmatic component of the human gut microbiota. Trends Microbiol. 24, 523–524. doi: 10.1016/j.tim.2016.02.015
Lallès, J. P., Bosi, P., Smidt, H., and Stokes, C. R. (2007). Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 66, 260–268. doi: 10.1017/S0029665107005484
Li, Z., Dong, Y., Chen, S., Jia, X., Jiang, X., Che, L., et al. (2021). Organic selenium increased gilts antioxidant capacity, immune function, and changed intestinal microbiota. Front. Microbiol. 12:723190. doi: 10.3389/fmicb.2021.723190
Li, Q., Yang, S., Zhang, X., Liu, X., Wu, Z., Qi, Y., et al. (2022). Maternal nutrition during late gestation and lactation: association with immunity and the inflammatory response in the offspring. Front. Immunol. 12:758525. doi: 10.3389/fimmu.2021.758525
Liu, P., Che, L., Yang, Z., Feng, B., Che, L., Xu, S., et al. (2016). A maternal high-energy diet promotes intestinal development and intrauterine growth of offspring. Nutrients 8:258. doi: 10.3390/nu8050258
Liu, L., Chen, D., Yu, B., Luo, Y., Huang, Z., Zheng, P., et al. (2021). Influences of selenium-enriched yeast on growth performance, immune function, and antioxidant capacity in weaned pigs exposure to oxidative stress. Biomed. Res. Int. 2021:5533210. doi: 10.1155/2021/5533210
Liu, X., Mao, B., Gu, J., Wu, J., Cui, S., Wang, G., et al. (2021). Blautia-a new functional genus with potential probiotic properties? Gut Microbes 13, 1–21. doi: 10.1080/19490976.2021.1875796
Liu, Y. J., Tang, B., Wang, F. C., Tang, L., Lei, Y. Y., Luo, Y., et al. (2020). Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics 10, 5225–5241. doi: 10.7150/thno.43716
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2 ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lu, H., Wilcock, P., Adeola, O., and Ajuwon, K. M. (2019). Effect of live yeast supplementation to gestating sows and nursery piglets on postweaning growth performance and nutrient digestibility. J. Anim. Sci. 97, 2534–2540. doi: 10.1093/jas/skz150
Modina, S. C., Polito, U., Rossi, R., Corino, C., and Di Giancamillo, A. (2019). Nutritional regulation of gut barrier integrity in weaning piglets. Animals 9:1045. doi: 10.3390/ani9121045
Mou, D., Ding, D., Yan, H., Qin, B., Dong, Y., Li, Z., et al. (2020). Maternal supplementation of organic selenium during gestation improves sows and offspring antioxidant capacity and inflammatory status and promotes embryo survival. Food Funct. 11, 7748–7761. doi: 10.1039/d0fo00832j
Mou, D., Wang, J., Liu, H., Chen, Y., Che, L., Fang, Z., et al. (2018). Maternal methyl donor supplementation during gestation counteracts bisphenol A-induced oxidative stress in sows and offspring. Nutrients 45, 76–84. doi: 10.1016/j.nut.2017.03.012
Nabuurs, M. J., Hoogendoorn, A., van der Molen, E. J., and van Osta, A. L. (1993). Villus height and crypt depth in weaned and unweaned pigs, reared under various circumstances in the Netherlands. Res. Vet. Sci. 55, 78–84. doi: 10.1016/0034-5288(93)90038-h
National Research Council. (2012). Nutrient requirements of swine: Eleventh Revised Edition. Washington, DC: The National Academies Press.
Shen, Y. B., Carroll, J. A., Yoon, I., Mateo, R. D., and Kim, S. W. (2011). Effects of supplementing Saccharomyces cerevisiae fermentation product in sow diets on performance of sows and nursing piglets. J. Anim. Sci. 89, 2462–2471. doi: 10.2527/jas.2010-3642
Song, D., Li, X., Cheng, Y., Wu, G., Xiao, X., Wang, F., et al. (2017). Effects of supplementing sow diets with Saccharomyces cerevisiae refermented sorghum dried distiller's grains with solubles from late gestation to weaning on the performance of sows and progeny. J. Anim. Sci. 95, 2025–2031. doi: 10.2527/jas.2017.1438
Tan, J., McKenzie, C., Potamitis, M., Thorburn, A. N., Mackay, C. R., and Macia, L. (2014). The role of short-chain fatty acids in health and disease. Adv. Immunol. 121, 91–119. doi: 10.1016/B978-0-12-800100-4.00003-9
Upadhaya, S. D., and Kim, I. H. (2021). The impact of weaning stress on gut health and the mechanistic aspects of several feed additives contributing to improved gut health function in weanling piglets-a review. Animal 11:2418. doi: 10.3390/ani11082418
Van, H. E., Funderburke, D. W., and Dorton, K. L. (2003). Growth performance, nutrient digestibility, and fecal microflora in weanling pigs fed live yeast. J. Anim. Sci. 81, 1004–1012. doi: 10.2527/2003.8141004x
Wan, K., Li, Y., Sun, W., An, R., Tang, Z., Wu, L., et al. (2020). Effects of dietary calcium pyruvate on gastrointestinal tract development, intestinal health and growth performance of newly weaned piglets fed low-protein diets. J. Appl. Microbiol. 128, 355–365. doi: 10.1111/jam.14494
Wensley, M. R., Tokach, M. D., Woodworth, J. C., Goodband, R. D., Gebhardt, J. T., and DeRouchey, J. M. (2021). Maintaining continuity of nutrient intake after weaning. II. Review of post-weaning strategies. Transl. Anim. Sci. 5:txab022. doi: 10.1093/tas/txab022
Wu, G., Bazer, F. W., Wallace, J. M., and Spencer, T. E. (2006). Board-invited review: intrauterine growth retardation: implications for the animal sciences. J. Anim. Sci. 84, 2316–2337. doi: 10.2527/jas.2006-156
Xiong, X., Tan, B., Song, M., Ji, P., Kim, K., Yin, Y., et al. (2019). Nutritional intervention for the intestinal development and health of weaned pigs. Front. Vet. Sci. 6:46. doi: 10.3389/fvets.2019.00046
Xu, Q. (2017). Effect of active yeast supplementation in rations on growth performance and intestinal and immune function of weaned piglets after ETEC attack. Master's thesis, Sichuan Agricultural University. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201901&filename=1018070318.nh
Xu, J., Li, Y., Yang, Z., Li, C., Liang, H., Wu, Z., et al. (2018). Yeast probiotics shape the gut microbiome and improve the health of early-weaned piglets. Front. Microbiol. 9:2011. doi: 10.3389/fmicb.2018.02011
Xu, S., Shi, J., Dong, Y., Li, Z., and Shen, Y. (2020). Fecal bacteria and metabolite responses to dietary lysozyme in a sow model from late gestation until lactation. Sci. Rep. 10:3210. doi: 10.1038/s41598-020-60131-1
Yang, H. S., Wu, F., Long, L. N., Li, T. J., Xiong, X., Liao, P., et al. (2016). Effects of yeast products on the intestinal morphology, barrier function, cytokine expression, and antioxidant system of weaned piglets. J. Zhejiang Univ. Sci. B 17, 752–762. doi: 10.1631/jzus.B1500192
Yang, S., and Yu, M. (2021). Role of goblet cells in intestinal barrier and mucosal immunity. J. Inflamm. Res. 14, 3171–3183. doi: 10.2147/JIR.S318327
Yu, T., Li, X., Song, Z., Wang, X., Liu, B., and Liu, Z. (2022). Progress of research on the succession pattern of intestinal flora in piglets and its influencing factors. Chin. J. Vet. Sci. 42, 1281–1286. doi: 10.16303/j.cnki.1005-4545.2022.06.26
Zhang, D., Huang, J., Li, J., Zheng, H., Zheng, H., Wang, S., et al. (2022). Advances in the study of porcine intestinal microorganisms, their metabolites and intestinal barrier. Chin. J. Anim. Vet. Sci. 53, 1334–1344. doi: 10.11843/j.issn.0366-6964.2022.05.002
Keywords: yeast cultures, sows, weaned piglets, growth performance, antioxidant properties, intestinal microorganisms
Citation: Liu Y, Jia X, Chang J, Jiang X, Che L, Lin Y, Zhuo Y, Feng B, Fang Z, Li J, Hua L, Wang J, Ren Z, Sun M, Wu D and Xu S (2023) Effect of yeast culture supplementation in sows during late gestation and lactation on growth performance, antioxidant properties, and intestinal microorganisms of offspring weaned piglets. Front. Microbiol. 13:1105888. doi: 10.3389/fmicb.2022.1105888
Received: 23 November 2022; Accepted: 21 December 2022;
Published: 13 January 2023.
Edited by:
Xiangfeng Kong, Institute of Subtropical Agriculture (CAS), ChinaReviewed by:
Hua Yang, Zhejiang Academy of Agricultural Sciences, ChinaCopyright © 2023 Liu, Jia, Chang, Jiang, Che, Lin, Zhuo, Feng, Fang, Li, Hua, Wang, Ren, Sun, Wu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengyu Xu, ✉ shengyuxu@sicau.edu.cn
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.