
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 13 December 2022
Sec. Microbiological Chemistry and Geomicrobiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1093487
This article is part of the Research TopicMicrobial Ecological and Biogeochemical Processes in the Soil-Vadose Zone-Groundwater HabitatsView all 12 articles
 Yanyu Song1
Yanyu Song1 Xiaofeng Cheng1
Xiaofeng Cheng1 Changchun Song1,2*
Changchun Song1,2* Mengting Li1,3
Mengting Li1,3 Siqi Gao1,4
Siqi Gao1,4 Zhendi Liu1,4
Zhendi Liu1,4 Jinli Gao1
Jinli Gao1 Xianwei Wang1
Xianwei Wang1Changes in soil CO2 and N2O emissions due to climate change and nitrogen input will result in increased levels of atmospheric CO2 and N2O, thereby feeding back into Earth’s climate. Understanding the responses of soil carbon and nitrogen emissions mediated by microbe from permafrost peatland to temperature rising is important for modeling the regional carbon and nitrogen balance. This study conducted a laboratory incubation experiment at 15 and 20°C to observe the impact of increasing temperature on soil CO2 and N2O emissions and soil microbial abundances in permafrost peatland. An NH4NO3 solution was added to soil at a concentration of 50 mg N kg−1 to investigate the effect of nitrogen addition. The results indicated that elevated temperature, available nitrogen, and their combined effects significantly increased CO2 and N2O emissions in permafrost peatland. However, the temperature sensitivities of soil CO2 and N2O emissions were not affected by nitrogen addition. Warming significantly increased the abundances of methanogens, methanotrophs, and nirK-type denitrifiers, and the contents of soil dissolved organic carbon (DOC) and ammonia nitrogen, whereas nirS-type denitrifiers, β-1,4-glucosidase (βG), cellobiohydrolase (CBH), and acid phosphatase (AP) activities significantly decreased. Nitrogen addition significantly increased soil nirS-type denitrifiers abundances, β-1,4-N- acetylglucosaminidase (NAG) activities, and ammonia nitrogen and nitrate nitrogen contents, but significantly reduced bacterial, methanogen abundances, CBH, and AP activities. A rising temperature and nitrogen addition had synergistic effects on soil fungal and methanotroph abundances, NAG activities, and DOC and DON contents. Soil CO2 emissions showed a significantly positive correlation with soil fungal abundances, NAG activities, and ammonia nitrogen and nitrate nitrogen contents. Soil N2O emissions showed positive correlations with soil fungal, methanotroph, and nirK-type denitrifiers abundances, and DOC, ammonia nitrogen, and nitrate contents. These results demonstrate the importance of soil microbes, labile carbon, and nitrogen for regulating soil carbon and nitrogen emissions. The results of this study can assist simulating the effects of global climate change on carbon and nitrogen cycling in permafrost peatlands.
Soil carbon dioxide (CO2) emissions represent the second largest carbon (C) flux in terrestrial ecosystems, accounting for 70–90% of total ecosystem respiration (Schlesinger and Andrews, 2000; Cascio et al., 2017). Losses of soil C to the atmosphere through soil heterotrophic respiration play an important role in regulating atmospheric CO2. These losses are predicted to increase due to climate change, resulting in a positive C-climate feedback loop (Yuan et al., 2019; Dacal et al., 2022). The availability of nitrogen (N) changes the source-sink dynamics of ecosystem C by changing the soil CO2 flux (Cascio et al., 2017). Soils also act as an important source-sink for nitrous oxide (N2O; Wu et al., 2013, 2015). Climate warming and the input of N could change mineralization of soil N and N2O emissions (Ma et al., 2011). The increases in N2O emissions can cause changes in global warming potential, thus affecting the C sinks and CO2 emissions (Muhammad et al., 2022). However, little is known about how increases in temperature and N inputs interact to regulate soil emissions of CO2 and N2O and their temperature sensitivity. An increased comprehension of the microbial mechanisms under warming and N addition impact emissions of CO2 and N2O is vital for accurately simulating the consequences of a changing global climate on the C and N balance.
Low temperatures and nutrient concentration limited soil microbial activities and soil organic matter (SOM) decomposition (Koyama et al., 2014). An increase in temperature results in enhanced microbial growth and in the activation of the functional genes involved in C and N cycling (Xue et al., 2016; Wang et al., 2019). These result in increased soil C decomposition and respiration (Han et al., 2013). However, a previous study noted a reduction in N2O production with increasing temperature, especially due to denitrification (Duan et al., 2019), whereas the abundances of amoA, nifH, and nirK increased (Jung et al., 2011; Han et al., 2013). Warming could increase N limitation of microorganisms, which, in turn, could limit the impact of increased temperature on SOM mineralization. Previous studies found that N addition increased the abundances of C decomposition and N cycling genes (Jung et al., 2011; Wang et al., 2019), leading to a stronger positive correlation between soil available N and microbial properties exposed to elevated temperature (Huang et al., 2022). Greater insight into the impacts of warming and the addition of N on soil microorganisms can assist in improving understanding of the reactions of soil C and N emissions to a global changing climate.
Soil enzymes catalyze breakdown of high molecular weight compounds, and play important functions in SOM degradation (Yao et al., 2015), measuring their activities can provide useful indicators of soil emissions of CO2 and N2O (Chen et al., 2017). Soil enzyme activities can be used to investigate microbial nutrient cycling due to their connections with active microbial biomass, including microbial responses to environmental changes, transformation rates, and the location of the most active biomass (Wang et al., 2015). Warming can result in changes in enzyme activities, leading to functional changes in soil ecosystem processes (Xu et al., 2015). An improved understanding of decomposition and mechanisms of microbial enzyme production can assist in constraining long-term responses to warming (Sihi et al., 2016). Moreover, enzyme activities were applied as indicators of the impacts of N input within many recent experiments since they reflect the metabolic needs of soil microbial communities relative to available nutrients (Ochoa-Hueso et al., 2013). Nitrogen addition significantly stimulated activities of N- and phosphorus-acquiring hydrolytic enzymes and depressed the activities of oxidative enzymes (Tu et al., 2014). Maslov and Maslova (2021) investigated the effect of increased N availability on changes in soil enzyme activities to better understand the internal mechanisms of soil C and N cycling processes. Improved comprehension of soil enzymes and their regulatory mechanisms is needed to enhance comprehension of the impacts of temperature and N availability on soil CO2 and N2O emissions.
Peatlands represent an important C pool on Earth, storing 1,055 Gt of soil C, even though they only cover 3% of the land surface of the Earth (Nichols and Peteet, 2019). In particular, permafrost peatlands experience increased storage and emissions of C, and can act as key contributors to global warming. Permafrost thaw in northern peatlands results in alterations to ground thermal conditions, moisture, and chemistry, which, in turn, regulate microbial activities responsible for generating greenhouse gases (GHGs) from decomposing organic matter (Kirkwood et al., 2021). Newly thawed permafrost in Western Canada is predicted to release 0.2 to 25% of stored C by 2,100 (Jin and Ma, 2021). An increase in annual temperature by 1°C was predicted to increase respiration by up to 60% in an experiment conducted in Arctic blanket peatland (Dorrepaal et al., 2009). Moreover, increases in N input affected N2O emissions in northern peatlands due to increased N availability and/or changing vegetation composition (Le et al., 2020). Nitrogen addition could mitigate the positive effect of warming on methane fluxes in a coastal bog (Gong et al., 2021). However, the synergistic environmental parameters regulating GHGs emissions in northern permafrost peatlands remain largely unknown (AminiTabrizi et al., 2020). Clarifying the synergistic effects of both climate warming and a rising nitrogen availability on permafrost emissions of CO2 and N2O can provide a reference for future studies on potential responses of C and N sequestration of high latitude peatlands to climate change.
Northeastern China contains the second largest expanse of permafrost in China, primarily known as Xing’an-Baikal permafrost. This permafrost area lies on the southeastern edges of the Eurasian cryolithozone and is thermally unstable and sensitive to external changes (Wei et al., 2011). By the 2010s, the area of Xing’an-Baikal permafrost in Northeast China had declined by 40.6% compared with that in the 1960s (Li et al., 2021). The present study aimed to understand the synergistic effects of both climate warming and rising N availability on soil emissions of CO2 and N2O and its regulation mechanism in permafrost peatlands. An incubation experiment with temperature increase of 5°C and nitrogen addition of 50 mg N kg−1 was conducted in the Great Xing’an mountain peatland, Northeast China. The objectives of this research were to explore the response of CO2 and N2O emissions from permafrost peatland soil to warming and nitrogen addition, and clarify their driving mechanisms, which can help improve future predictions of responses of soil C and N cycling to climate warming.
The study site of the present study is a typical permafrost peatland nearby the Tuqiang Forestry Bureau, Great Xing’an Mountain (52°44′N, 122°39′E), Heilongjiang Province, China. Average yearly temperature and average yearly precipitation are −3.9°C and 452 mm, respectively. The dominant species of plants are Vaccinium uliginosum L., Moench, Sphagnum spp., Ledum palustre L., Eriophorum vaginatum L., and Chamaedaphne calyculata L. The soil type of the study area according to the United States Department of Agriculture (USDA) classification system is Glacic Histoturbels (Soil Survey Staff, 2010). A soil sample of the active layer (0–20 cm) was obtained using a hand auger soil core sampler, which was filtered through a 2-mm sieve. The total C (TC) and total N (TN) of the soil sample before incubation experiments were 408.74 and 15.34 g kg−1, respectively, whereas soil moisture and pH were 77.18% and 5.49, respectively.
Fresh soil samples (15 g according to completely dry soil) were placed in 500-ml glass flasks and preincubated at 15°C for 7 days. NH4NO3 solution (2 ml) was uniformly added to soil at a concentration of 50 mg N kg−1, with four replicates prepared. Deionized water (2 ml) was added to the control treatment. The flask lids were sealed with rubber septa to allow the analysis of rates of emissions of CO2 and N2O at 15 and 20°C (maximum monthly mean temperature in July of 18.4°C). These soils were incubated continuously for 18 days. Trapped air in the jars was removed for CO2 and N2O determination at intervals of 2 h, 1, 2, 3, 5, 7, 9, 12, 15, and 18 days. Headspace gas in the jars was extracted using a 50-ml syringe with a three-way valve. The concentrations of CO2 and N2O were measured utilizing a gas chromatograph (Agilent 7890B, United States). Deionized water corresponding to the reduction in weight after each collection of gas was added. Soil samples were collected to determine soil microbial abundances, enzyme activities, and labile C and N contents at the end of incubation.
Soil DNA was extracted from a 300-mg subsample using a FastDNA spin Kit (MPbio, Santa Ana, CA, United States) in accordance with the manufacturer’s instructions. Bacterial 16S rRNA, fungal IST, and functional genes encoding mcrA, pmoA, nirS, and nirK were quantitatively evaluated via qPCR using an ABI StepOne instrument (Applied Biosystems, San Francisco, CA, United States). Supplementary Table S1 lists the primers and amplification details used in the present study. The PCR mixture contained 10 ng soil DNA, 0.4 μl primers (10 μM), and 12.5-μl of SYBR Buffer (TaKaRa, Beijing, China) in a final volume of 25 μl. qPCR standard curves were created by purifying amplicon products of functional and phylogenetic markers using a cyclic purification kit (Omega Bio-Tek, United States), ligated to the pMD18-T (TaKaRa) vector, and transforming into Escherichia coli. A plasmid mini kit (Omega Bio-Tek, United States) was utilized to remove the plasmids, with a standard local alignment searching tool used to identify specificity of plasmids. Standard curves were produced by plasmid serial dilution (Song et al., 2021).
The potential activities of acid phosphatase (AP), β-1,4-glucosidase (βG), cellobiohydrolase (CBH), and NAG were measured for absorbance using a microplate spectrophotometer. Aliquots (200 μl) of slurry (1 g fresh soil sample homogenized in 125-ml 50-mM acetate buffer, pH 8) and 50-μl of substrate solution (200 μM) were placed into 96-well microplates. Every microplate had eight replicate wells per assay, as well as negative and positive controls for quench correction. The microplates were incubated in darkness at 20°C for 4 h. Excitation and emission fluorescence were identified at 365 and 450 nm, respectively using Cell Imaging Multi-Mode Reader (BioTek Cytation 5, United States).
Soil ammonia nitrogen (NH4+-N), nitrate (NO3−-N), and dissolved organic N (DON) were extracted through the addition of 2 M KCl at a 1:15 ratio, followed by 1 h of shaking at 150 rpm at a temperature of 20°C. DON concentrations of soil were calculated as the difference between total dissolved N and inorganic N. Soil dissolved organic C (DOC) contents were analyzed using a Multi N/C 2100 analyzer (Analytik Jena AG, Germany) after extracting fresh soil with a 2 M KCl solution. Soil TN contents were analyzed after digestion with sulfuric acid (H2SO4) and potassium sulfate (K2SO4), with cupric sulfate (CuSO4) used as a catalyst. The products of digestion were subsequently analyzed using an AA3 continuous flow chemical analyzer (Seal Analytical, Germany). Quantification of soil moisture was by oven drying of fresh soil at 105°C to a constant weight. The pH of soil was measured using a 1:10 soil-deionized water slurry.
Statistical analyses were performed in the SPSS 24.0 package. Results are shown as the average ± standard error. A two-way analysis of variance (ANOVA) was performed to evaluate the interactions between increasing temperature and addition of N on soil emissions of CO2 and N2O, microbial abundances, enzyme activities, and contents of soil C and N. Linear regression analysis was conducted to explore relationships between the soil CO2 and N2O emissions and soil microbial abundances, enzyme activities, and soil C and N contents.
The temperature sensitivities (Q10) of soil CO2 and N2O emission rates per 10°C were calculated as follows:
where T1 and T2 is the incubation temperatures for 15 and 20°C, respectively. K1 and K2 is the CO2 (mg CO2-C kg−1 d−1) and N2O (μg N2O-N kg−1 d−1) emission rates at 15 and 20°C, respectively.
An increase in temperature significantly stimulated soil emissions of CO2 and N2O in the permafrost peatlands (Figures 1A,B). Soil CO2 and N2O emissions in the control increased by 53.57 and 45.50% at 20°C compared to that at 15°C, respectively. The addition of N resulted in increases in CO2 and N2O emissions by 52.34 and 54.53% at 20°C compared to that at 15°C, respectively. The cumulative CO2 and N2O emissions were significantly higher under N addition than that in the control at 15°C and 20°C (Figures 2A,B). The increase in cumulative N2O emissions after the addition of N was significantly higher than the increase in CO2. There were significant interactions between rising temperature and addition of N on both CO2 and N2O emissions (p < 0.05; Table 1). The sensitivities of soil CO2 and N2O emissions to temperature in the control were 2.37 and 2.36, respectively. The addition of N did not impact the Q10 values of CO2 and N2O emissions of 2.50 and 2.44, respectively (Figure 2C).
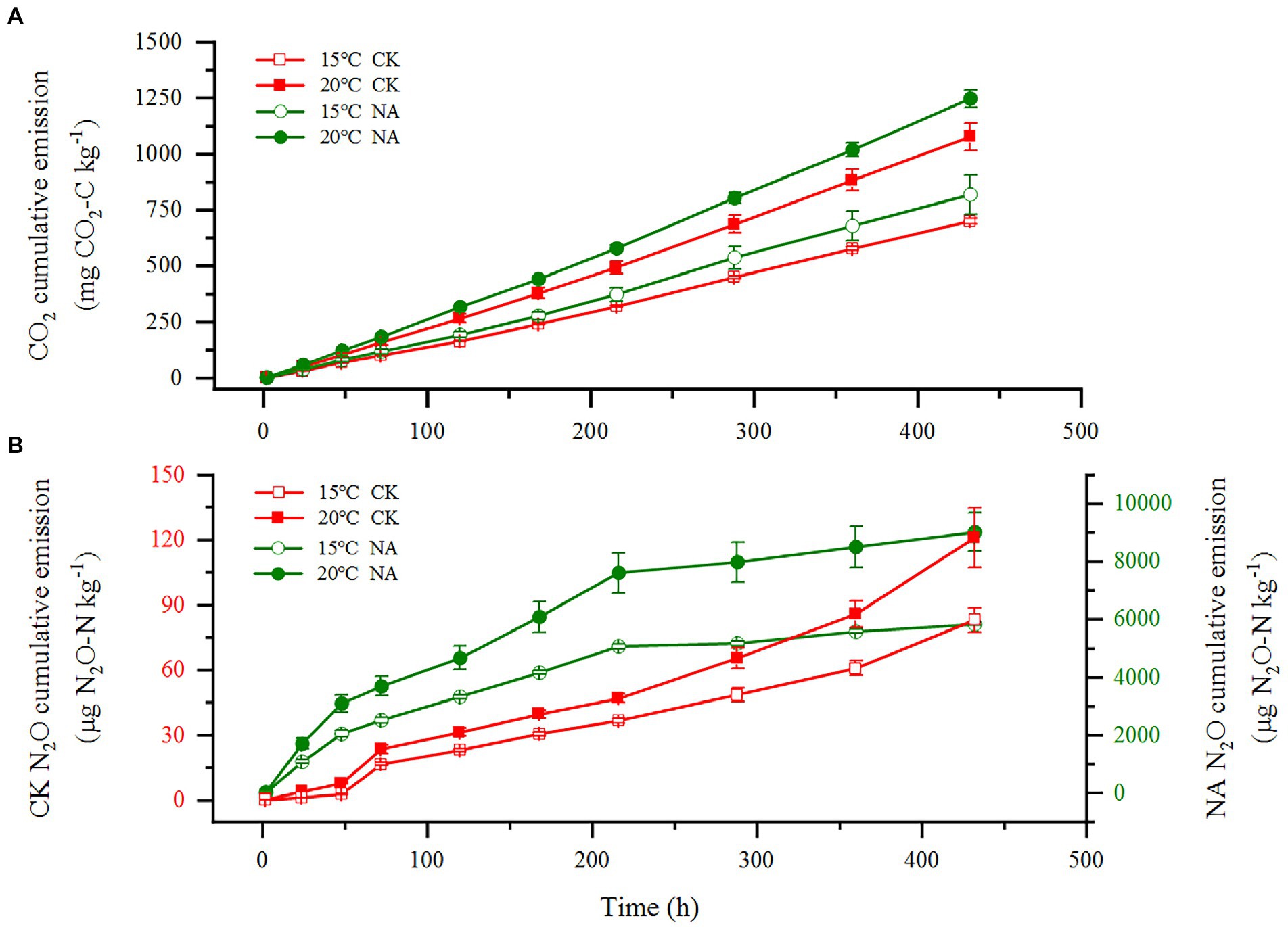
Figure 1. Effects of temperature rising and nitrogen addition on soil CO2 (A) and N2O (B) emissions in permafrost peatland. CK, control; NA, add 50 mg N kg−1 soil.
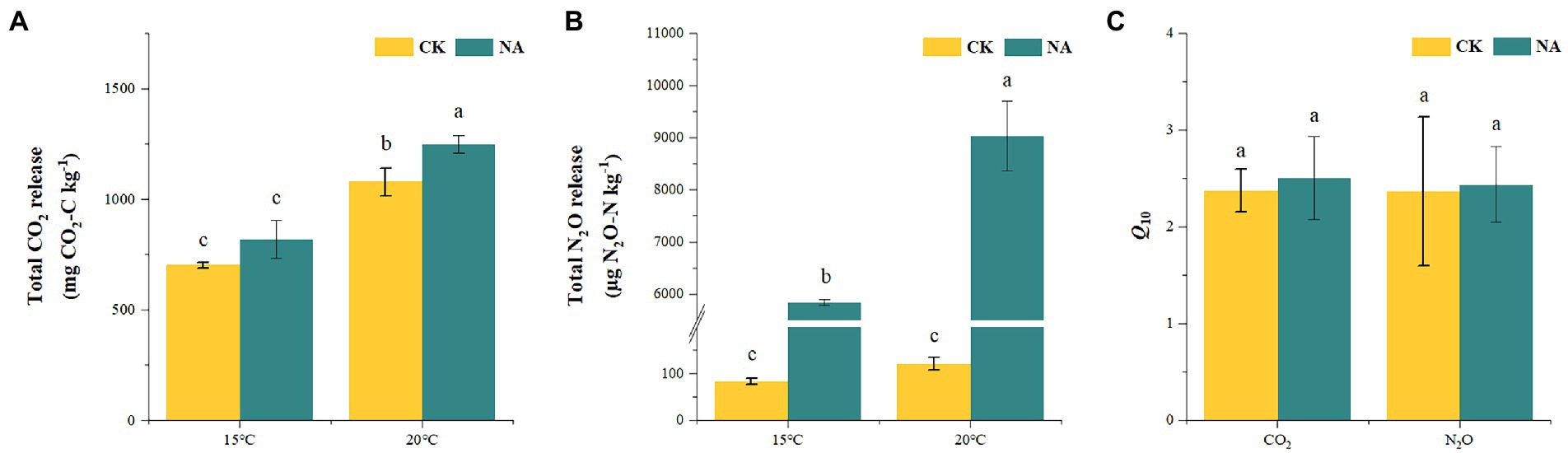
Figure 2. Effects of temperature rising and nitrogen addition on soil total CO2 (A) and N2O (B) release and their temperature sensitivity (Q10) (C) in permafrost peatland. CK, control; NA, add 50 mg N kg−1 soil. Different lowercase letters in the figure indicate significant differences in the means between different treatments.
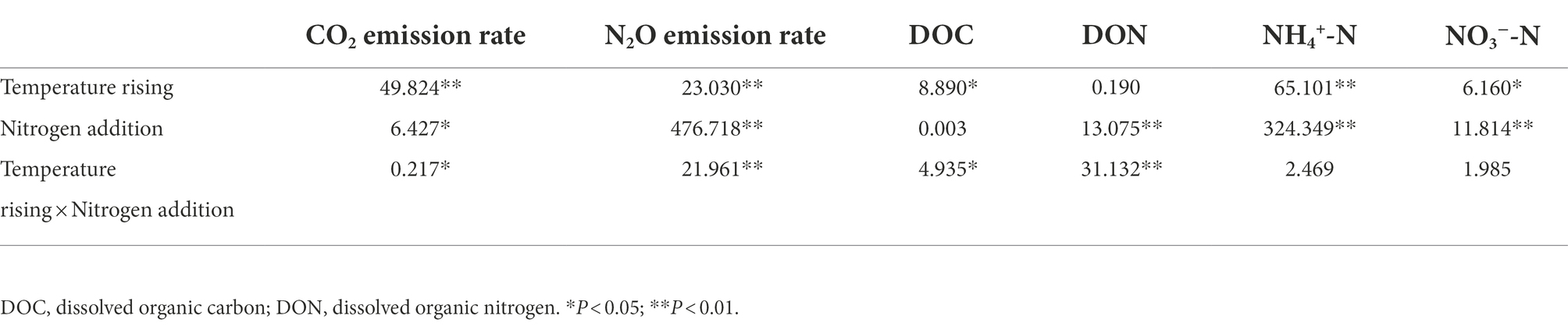
Table 1. Two-way ANOVA of effects of temperature rising and nitrogen addition on soil CO2, N2O release, and soil carbon and nitrogen contents.
Among the microbial community, bacteria were the most abundant (6.08–14.52 × 1012 copies g−1 dry soil). At 20°C, bacterial abundances in the control and N addition treatment decreased to 36.89 and 50.54% of that at 15°C (Figure 3A), respectively, indicating the preference of bacteria for lower temperature. At 20°C, fungal abundances increased significantly by 60.73% in the N addition treatment (Figure 3B). N addition appeared to reduce the abundances of bacteria under both temperatures, whereas fungal abundances were significantly stimulated at 20°C. Increased temperature resulted in the proliferation of methanogen (mcrA) by 28.04 and 31.46% in the control and N addition treatments, respectively (Figure 3C). However, N addition reduced methanogen abundances by 19.30 and 17.14% at 15 and 20°C, respectively. The abundances of methanotrophs (pmoA) significantly increased by 28.49-, 14.31-, and 18.16-fold under a rising temperature, N addition, and both increased temperature and N addition, respectively (Figure 3D). Adding N at 15°C significantly increased the abundances of nirK-type denitrifiers by 21.89% (Figure 3E). An increase in temperature resulted in decreases in the abundances of the nirS-type denitrifiers by 25.59 and 22.75% in the control and N addition treatments, respectively (Figure 3F). The addition of N resulted in increases in the abundances of nirS-type denitrifiers by 19.48 and 24.04% at 15 and 20°C, respectively. The increase in temperature and N addition had an interactive impact on the abundances of fungi and methanotrophs; however, there was no synergistic effect on bacterial, methanogen, and denitrifier abundances (p < 0.01; Table 2). There were significant relationships between the abundances of fungi and the contents of NH4+-N, NO3-_N, as well as emissions of CO2. This result indicated that fungi contributed to CO2 emissions and were affected by N concentrations. The significant correlations between N2O emissions and the abundances of fungi, methanotrophs, and nirK-type denitrifiers indicated the significant contribution of the microbial community to N2O emissions (p < 0.05; Figure 4).
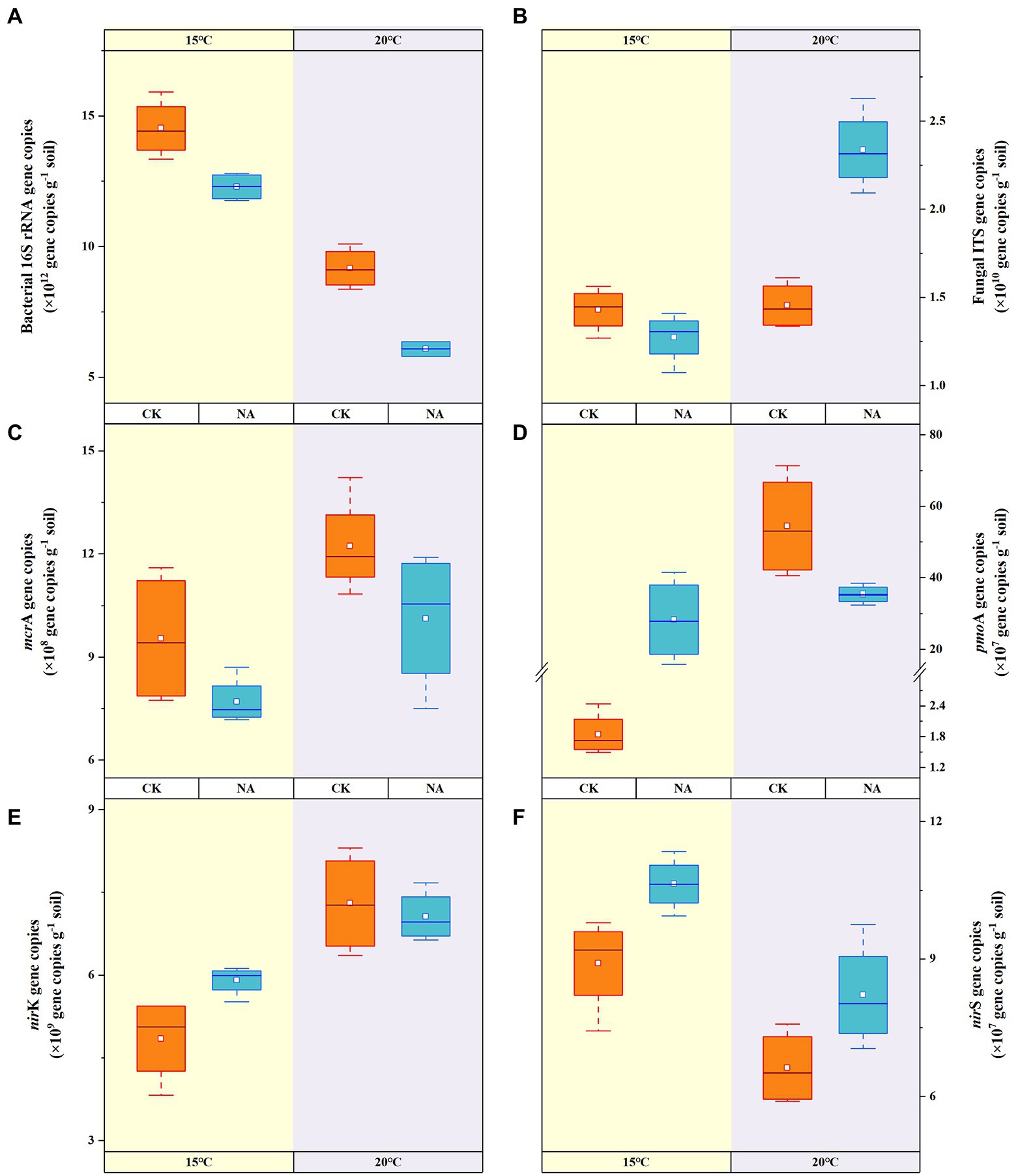
Figure 3. Effects of temperature rising and nitrogen addition on soil bacterial (A), fungal (B), mcrA (C), pmoA (D), nirK (E), and nirS (F) abundances in permafrost peatland. CK, control; NA, add 50 mg N kg−1 soil.
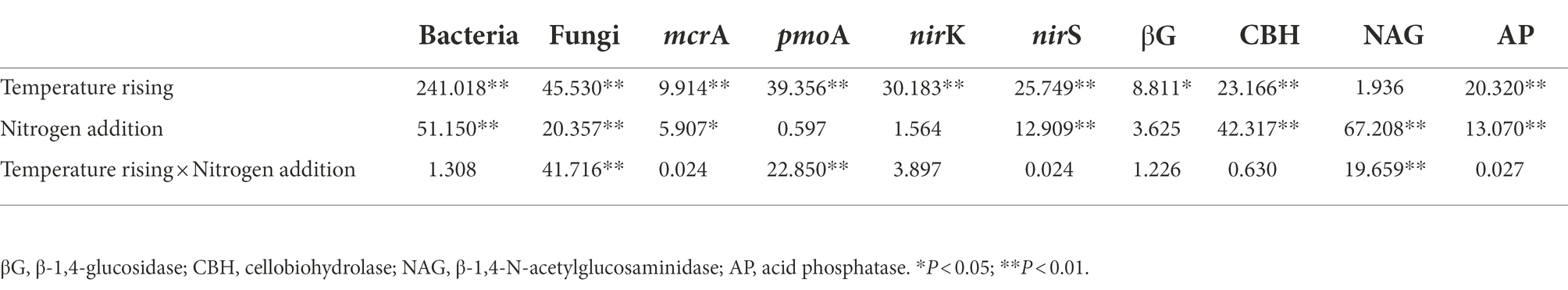
Table 2. Two-way ANOVA of the effects of nitrogen addition and temperature rising on soil microbial abundances and enzyme activities.
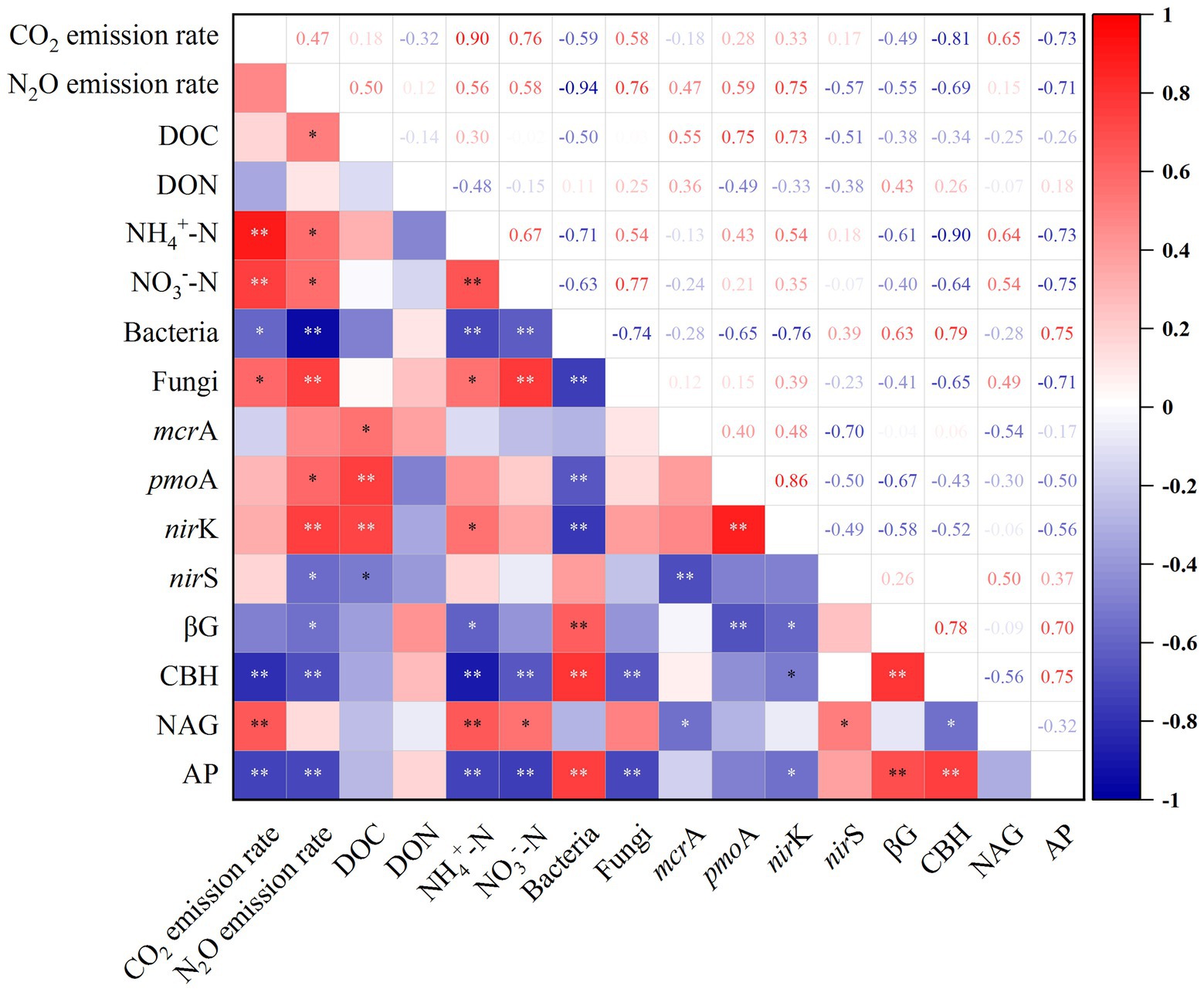
Figure 4. Pearson’s correlation analysis of soil CO2 and N2O emissions, carbon and nitrogen contents, microbial abundances, and enzyme activities. βG, β-1,4-glucosidase; CBH, cellobiohydrolase; NAG, β-1,4-N-acetylglucosaminidase; AP, acid phosphatase; DOC, dissolved organic carbon; DON, dissolved organic nitrogen; NH4+-N, ammonium nitrogen; NO3—N, nitrate nitrogen. * indicates significant p < 0.05; ** indicates significant p < 0.01.
The activities of the four soil enzymes responded significantly to a rising temperature and the addition of N (Figure 5). The C-cycling-related activities of βG and CBH decreased by 22.63 and 22.46% with a rising in temperature in the control, whereas they decreased by 12.40 and 46.03% in the N addition treatment, respectively (Figures 5A,B). The rise in temperature resulted in an increase in soil NAG activities by 11.83 and 48.57% in the control and N addition treatments, respectively (Figure 5C). Significant interactive effects were observed between the rising temperature and addition of N on soil NAG activities (p < 0.01; Table 2). NAG activities showed significant positive correlations with soil emissions of CO2 and contents of NO3−-N and NH4+-N (p < 0.05; Figure 4). Soil AP activities decreased with a rising temperature and the addition of N, with the highest and lowest activities of 2,089.23 and 1,730.22 nmol g−1 h−1 obtained at 15°C without N addition and 20°C with N addition, respectively (Figure 5D). There were no synergistic effects of the rising temperature and addition of N on soil βG, CBH, and AP activities (p > 0.05; Table 2).
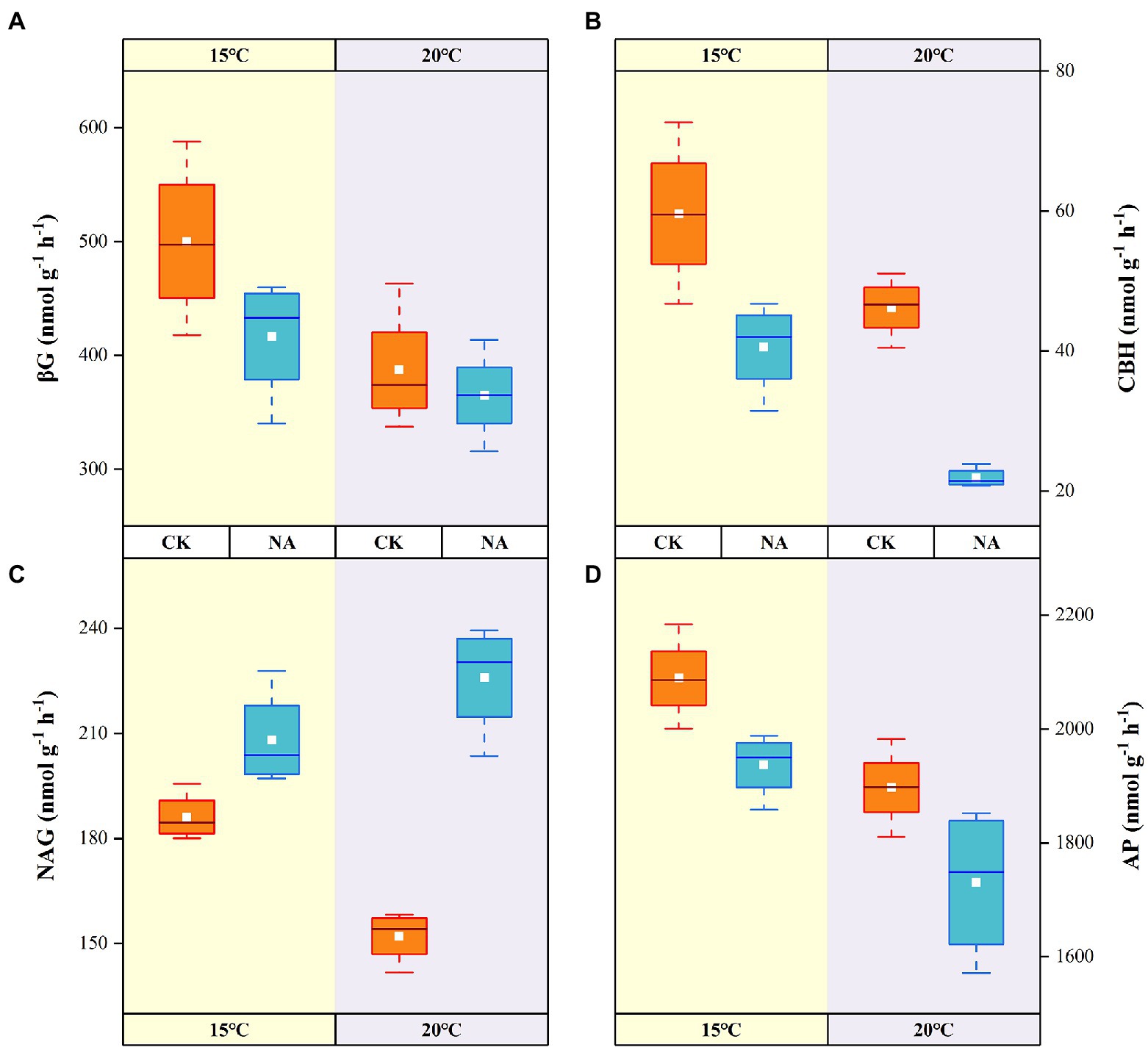
Figure 5. Effects of temperature rising and nitrogen addition on soil enzyme activities in permafrost peatland. CK, control; NA, add 50 mg N kg−1 soil. βG, β-1,4-glucosidase (A); CBH, cellobiohydrolase (B); NAG, β-1,4-N-acetylglucosaminidase (C); AP, acid phosphatase (D).
An increase in temperature increased DOC contents in the permafrost peatlands from 531.05 to 628.25 mg kg−1 in the control treatment (Figure 6A). However, the increase in temperature did not result in a significant change in soil DOC contents under the N addition treatment. N addition had a significantly negative impact on DON contents at 15°C, with DON decreasing from 169.80 to 116.80 mg kg−1, whereas soil DON was not significantly affected at 20°C (Figure 6B). NH4+-N in soil ranged from 35.70 to 62.25 mg kg−1. Both a rise in temperature and the addition of N resulted in increased contents of soil NH4+-N (Figure 6C). The contents of soil NO3−-N under N addition (19.73 mg kg−1) were significantly higher than that in the control (14.96 mg kg−1) at 20°C (Figure 6D). The increase in temperature and N addition had significant interactive impacts on soil DOC and DON contents (p < 0.05; Table 1), whereas the effects on NO3−-N and NH4+-N were not significant. The soil emissions of CO2 and N2O showed significant positive correlations with contents of soil NH4+-N and NO3−-N, whereas N2O emissions were positively correlated with DOC contents (p < 0.05; Figure 4).
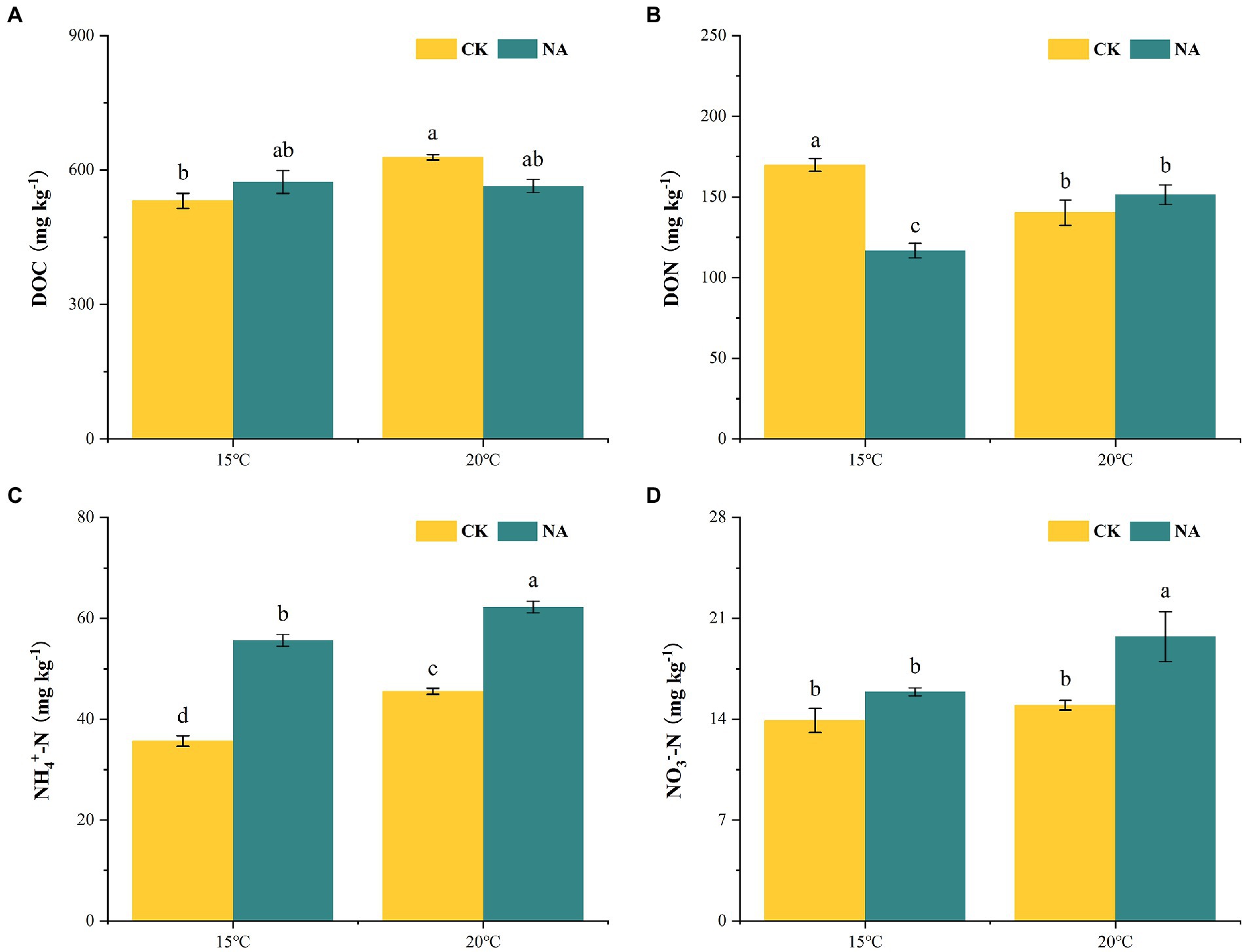
Figure 6. Effects of temperature rising and nitrogen addition on soil dissolved organic carbon (A), dissolved organic nitrogen (B), NH4+-N (C), and NO3−-N (D) contents in peatland. CK, control; NA, add 50 mg N kg−1 soil; DOC, dissolved organic carbon; DON, dissolved organic nitrogen; NH4+-N, ammonium nitrogen; NO3—N, nitrate nitrogen. Different lowercase letters in the figure indicate significant differences in the means between different treatments.
The rise in temperature and addition of N stimulated emissions of soil CO2 and N2O. Moreover, the rise in temperature and addition of N interacted within their effect on soil emissions of CO2 and N2O. However, the results of the current study demonstrated a strong negative effect of rise in temperature and the addition of N on the abundances of bacteria. This result indicated that bacteria in permafrost peatlands were adapted to a low temperature and N-limited environment. In line with our results, warming reduced 37% of bacterial abundance and microbial metabolic capacity in the deep organic layer of an Alaska tundra (Wu et al., 2022). Our results showed that the combined effects of temperature rising and N addition significantly increased fungal abundances and there were significantly positively correlations between fungal abundances and the emissions of CO2 and N2O, suggesting that there were differences in sensitivity of different microbial communities to environmental changes and fungi communities played a vital part in the variations of CO2 and N2O emissions at higher temperature and under the addition of N. Consistent with the outcomes of the current study, Xu et al. (2017) determined that fungal tolerance to high temperatures played a significant part in N2O emissions.
The results of the present study showed that methanotrophs were more sensitive to a changing temperature and the addition of N compared to other microbial communities. The higher abundances of nirK-type denitrifiers at 20°C compared to at 15°C observed in the present study were consistent with results of previous studies in which the abundances of nirK genes were promoted by higher temperatures (Jung et al., 2011; Cui et al., 2016). Declines the abundances of nirS-type denitrifiers were observed at 20°C compared to those at 15°C. This result demonstrated that nirS-type denitrifiers were better adapted to low temperature conditions. The significant positive correlations between the abundances of nirK-type denitrifiers and NH4+-N contents and N2O emissions observed in the present study indicated that the increase in emissions of N2O could be primarily attributed to the denitrification pathway mediated by nirK denitrifiers. Jung et al. (2011) similarly observed an increase in nirK genes abundances under both warming and the addition of N. The nirK denitrifiers mentioned above are bacterial nirK, fungal nirK also have clear relevance for N2O-producing, future understanding the abundance and distribution of denitrifying fungi may provide new insight into soil N2O emissions under various environmental settings (Chen et al., 2016).
Soil enzymes play an important role in the mineralization of soil C and N. Therefore, an improved comprehension of the reaction of soil enzyme activities to increasing temperature and the availability of N is crucial for understanding the mechanisms under which emissions of soil CO2 and N2O occur. An increased temperature can alter the nutrient acquisition strategies of microbial communities. This is achieved by changing extracellular enzyme activities through the priming of decomposition of SOM, which leads to increased emissions of CO2 from peatlands (AminiTabrizi et al., 2022). NAG participates in N conversion and plays a significant part in the decomposition of nitrogenous substances in soil as it facilitates the degradation of chitin (Liu et al., 2019). Chitin is a major source of soil organic N. The addition of N may affect the decomposition of chitin and peptidoglycan, which, in turn, accelerates the activities of NAG (Liu et al., 2019). Consistent with the outcomes of the present study, Chen et al. (2018) and Liu et al. (2019) determined that N addition significantly increased the activities of NAG by 5.5% and 56.40–204.78%, respectively. The increase in the activities of NAG can be attributed to soil acidification induced by the addition of N. A decrease in pH was shown to positively affect soil NAG activities (Chen et al., 2018). pH is a key driver for the turnover of organic matter in cold soil, regulatory role of pH needs consideration in the future studies (Leifeld et al., 2013). Although the rise in temperature decreased NAG activities in the control treatment, the increase in NAG activities in the N addition treatment indicated that within the combined effect of an elevated temperature and addition of N, the latter had the dominant effect on soil enzyme activities.
The rise in temperature inhibited the activities of soil βG, CBH, and AP. This result could be attributed to the decrease in enzyme activities possibly being related to a decrease in substrate (e.g., microbial biomass) availability at elevated temperatures. Wang J. Y. et al. (2020) determined that enzyme activities reduced with increasing incubation time, suggesting that the responses of enzymes reflected changes in the availability of substrate due to warming. The rate of enzyme production has been shown to decrease as substrate is exhausted. The outcomes of the current study illustrated that the warming stimulation of soil respiration readily depleted hydrolysable substrates during incubation without inputs of C sources. Therefore, decreases in the active pool due to warming can result in microbial C starvation (Metcalfe, 2017; Wang J. Y. et al., 2020). In addition, bacterial conversion of NH4+-N to NO2−-N in the first step of nitrification can further acidify soils through the release of H+ into soil solution. Accelerated acidification, in turn, is an important factor inhibiting soil microbial enzyme activities to acute nutrient amendment (Fatemi et al., 2016). Previous studies have also suggested that a decline in soil enzyme activities was attributable to their more rapid inactivation due to warming can help explain attenuation of the warming impact on mineralization of soil C (Alvarez et al., 2018). Changes in redox conditions driven by temperature can result in abiotic destabilization of Fe-organic matter (phenol) complexes. This is a peatland decomposition pathway that was previously underestimated and can result in increased production of CO2 and the accumulation of polyphenol-like compounds that could further inhibit the activities of extracellular enzymes (AminiTabrizi et al., 2022).
The emissions of soil CO2 and N2O were related to the concentrations of NO3−-N and NH4+-N. Also, soil emissions of N2O were related to the concentrations of DOC. Similarly, correlations between the soil CO2 release and NO3−-N and NH4+-N concentrations were revealed by Zhang et al. (2018) in mountain forest and meadow ecosystems. These results indicated that higher substrate availability enhanced the activities of soil microbes, which, in turn, resulted in increased emissions of CO2 and N2O. Soil DOC is composed of low molecular weight organic compounds and drives the growth and activity of microbes by acting as an energy source and a substrate (Wang C. M. et al., 2020). The results of the present study showed an increase in DOC with increasing incubation temperature in the control. An elevated temperature accelerated microbial processes and increased C availability in the control, resulting in higher heterotrophic respiration rates and increased release of CO2. However, soil DOC tended to decrease with the addition of N at a higher incubation temperature, indicating that N addition may limit available C. Warming significantly increased inorganic N (NH4+-N and NO3—N; Table 1) due to higher mineralization and nitrification of TN. The above results are consistent with the earlier study of Yuan et al. (2018), and suggest that warming increases soil N mineralization. Increase in N mineralization resulted in an increase in soil available N contents with increasing incubation temperature. Higher temperatures have been shown to accelerate the denitrification and nitrification processes (Inclan et al., 2012; Zhang et al., 2016). These processes are major pathways of soil emissions or production of N2O (Zhang et al., 2018; Li et al., 2019). N2O emissions due to nitrification accounted for 60–80% of total emissions (Zhang et al., 2020). Therefore, the increased availability of C and N in the soil substrate stimulated N2O emissions by accelerating N transformation under warming.
In addition to soil temperature, the addition of N had profound influences on the emissions of CO2 and N2O. N addition significantly elevated NH4+-N and NO3−-N, alleviated microbial N limitation, and promoted soil CO2 and N2O emissions, thereby accelerating soil C and N cycling. Menyailoa et al. (2014) similarly found an increase in heterotrophic activity by 20–30% after the addition of N. Increase in the availability of N often accelerates soil denitrification and nitrification processes and results in increased emissions of N-oxide (Davidson et al., 2000; Benanti et al., 2014). Especially, when C are available for microbial activity, N availability will have pronounced impacts on nitrification and denitrification (Lu et al., 2015). Consistent with the result of Guo et al. (2020), the results of the present study showed a positive correlation between DOC and N2O emissions. This result indicated that both labile C and available N concentrations were the dominant factors influencing the emissions of N2O. DOC is an important factor regulating denitrification and autotrophic and heterotrophic nitrification (Ferrarini et al., 2017). DOC concentrations influence the emissions of greenhouse gasses by regulating microbial metabolism, whereas soil ammonium and nitrate do not have the same regulatory function (Chen et al., 2020). Increased C availability enhances microbial activity, and, in turn, O2 consumption, which may lead to sub-aerobic microsites facilitating N2O emissions by denitrification and nitrifier denitrification (Ma et al., 2022). Consistent with the outcomes of the current study, Zhu et al. (2016) concluded that the sensitivity of soil respiration to temperature was not influenced by the addition of N, indicating that the availability of C substrate may be more important than that of N substrate.
This study showed that a rise in temperature and the addition of N promoted soil CO2 and N2O emissions. This result implies that future increases in temperature and availability of N will stimulate C and N cycling in the permafrost peatlands. The abundances of fungi were positively correlated with emissions of soil CO2 and N2O, suggesting that fungal communities may play a significant part in driving the exchange of C and N at the soil-atmosphere interface in permafrost peatlands. The abundances of the nirK-type denitrifiers were positively correlated with DOC and NH4+-N contents, and emissions of N2O, suggesting that the denitrification process mediated by nirK-type denitrifiers and available substrate may play a significant part in emissions of N2O. The activities of soil NAG increased with the addition of N and a rise in temperature, and were positively correlated with soil CO2 emissions. This result indicated that the activities of soil NAG are more important than those of other enzymes for regulating CO2 emissions. The results of the current study improve understanding of how temperature and N availability regulate soil emissions of greenhouse gasses in permafrost peatlands. However, a laboratory study cannot completely reflect the actual response of greenhouse gasses to global warming, and future research should focus on how plants and their interactions with soil microbes regulate greenhouse gas emissions under field conditions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YS: conceptualization, writing – review and editing, and funding acquisition. XC: methodology, data curation, and writing – review and editing. CS: supervision and funding acquisition. ML, ZL, JG, and XW: writing – review and editing. SG: methodology. All authors contributed to the article and approved the submitted version.
This research was funded by the National Natural Science Foundation of China (Nos. 41871090, 41730643, and 42271109), Professional Association of the Alliance of International Science Organizations (No. ANSO-PA-2020-14), and the Innovation Team Project of Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences (No. 2022CXTD02).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1093487/full#supplementary-material
Alvarez, G., Shahzad, T., Andanson, L., Bahn, M., Wallenstein, M. D., and Fontaine, S. (2018). Catalytic power of enzymes decreases with temperature: new insights for understanding soil C cycling and microbial ecology under warming. Glob. Change Biol. 24, 4238–4250. doi: 10.1111/gcb.14281
AminiTabrizi, R., Dontsova, K., Grachet, N. G., and Tfaily, M. M. (2022). Elevated temperatures drive abiotic and biotic degradation of organic matter in a peat bog under oxic conditions. Sci. Total Environ. 804:150045. doi: 10.1016/j.scitotenv.2021.150045
AminiTabrizi, R., Wilson, R. M., Fudyma, J. D., Hodgkins, S. B., Heyman, H. M., Rich, V. I., et al. (2020). Controls on soil organic matter degradation and subsequent greenhouse gas emissions across a permafrost thaw gradient in northern Sweden. Front. Earth Sci. 8:557961. doi: 10.3389/feart.2020.557961
Benanti, G., Saunders, M., Tobin, B., and Osborne, B. (2014). Contrasting impacts of afforestation on nitrous oxide and methane emissions. Agric. For. Meteorol. 198-199, 82–93. doi: 10.1016/j.agrformet.2014.07.014
Cascio, M. L., Morillas, L., Ochoa-Hueso, R., Munzi, S., Roales, J., Hasselquist, N. J., et al. (2017). Contrasting effects of nitrogen addition on soil respiration in two Mediterranean ecosystems. Environ. Sci. Pollut. Res. 24, 26160–26171. doi: 10.1007/s11356-017-8852-5
Chen, M. L., Chang, L., Zhang, J. M., Guo, F. C., Vymazal, J., He, Q., et al. (2020). Global nitrogen input on wetland ecosystem: the driving mechanism of soil labile carbon and nitrogen on greenhouse gas emissions. ESE 4:100063. doi: 10.1016/j.ese.2020.100063
Chen, H., Li, D. J., Zhao, J., Xiao, K. C., and Wang, K. L. (2018). Effects of nitrogen addition on activities of soil nitrogen acquisition enzymes: a meta-analysis. Agric. Ecosyst. Environ. 252, 126–131. doi: 10.1016/j.agee.2017.09.032
Chen, J., Luo, Y. Q., Li, J. W., Zhou, X. H., Cao, J. J., Wang, R. W., et al. (2017). Costimulation of soil glycosidase activity and soil respiration by nitrogen addition. Glob. Chang. Biol. 23, 1328–1337. doi: 10.1111/gcb.13402
Chen, H. H., Yu, F. B., and Shi, W. (2016). Detection of N2O-producing fungi in environment using nitrite reductase gene (nirK)-targeting primers. Fungal Biol. 120, 1479–1492. doi: 10.1016/j.funbio.2016.07.012
Cui, P. Y., Fan, F. L., Yin, C., Song, A. L., Huang, P. R., Tang, Y. J., et al. (2016). Long-term organic and inorganic fertilization alters temperature sensitivity of potential N2O emissions and associated microbes. Soil Biol. Biochem. 93, 131–141. doi: 10.1016/j.soilbio.2015.11.005
Dacal, M., Delgado-Baquerizo, M., Barquero, J., Berhe, A. A., Gallardo, A., Maestre, F. T., et al. (2022). Temperature increases soil respiration across ecosystem types and soil development, but soil properties determine the magnitude of this effect. Ecosystems 25, 184–198. doi: 10.1007/s10021-021-00648-2
Davidson, E. A., Keller, M., Erickson, H. E., Verchot, L. V., and Veldkamp, E. (2000). Testing a conceptual model of soil emissions of nitrous and nitric oxides. Bioscience 50, 667–680. doi: 10.1641/0006-3568(2000)050[0667:TACMOS]2.0.CO;2
Dorrepaal, E., Toet, S., van Logtestijn, R. S. P., Swart, E., van de Weg, M. J., Callaghan, T. V., et al. (2009). Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature 460, 616–619. doi: 10.1038/nature08216
Duan, P. P., Song, Y. F., Li, S. S., and Xiong, Z. Q. (2019). Responses of N2O production pathways and related functional microbes to temperature across greenhouse vegetable field soils. Geoderma 355:113904. doi: 10.1016/j.geoderma.2019.113904
Fatemi, F. R., Fernandez, I. J., Simon, K. S., and Dail, D. B. (2016). Nitrogen and phosphorus regulation of soil enzyme activities in acid forest soils. Soil Biol. Biochem. 98, 171–179. doi: 10.1016/j.soilbio.2016.02.017
Ferrarini, A., Fornasier, F., Serra, P., Ferrari, F., Trevisan, M., and Amaducci, S. (2017). Impacts of willow and miscanthus bioenergy buffers on biogeochemical N removal processes along the soil-groundwater continuum. GCB Bioenergy 9, 246–261. doi: 10.1111/gcbb.12340
Gong, Y., Wu, J. H., Sey, A. A., and Le, T. B. (2021). Nitrogen addition (NH4NO3) mitigates the positive effect of warming on methane fluxes in a coastal bog. Catena 203:105356. doi: 10.1016/j.catena.2021.105356
Guo, B. L., Zheng, X. Z., Yu, J. H., Ding, H., Pan, B. B., Luo, S. Z., et al. (2020). Dissolved organic carbon enhances both soil N2O production and uptake. Glob. Ecol. Conserv. 24:e01264. doi: 10.1016/j.gecco.2020.e01264
Han, J., Jung, J., Park, M., Hyun, S., and Park, W. (2013). Short-term effect of elevated temperature on the abundance and diversity of bacterial and archaeal amoA genes in Antarctic soils. J. Microbiol. Biotechnol. 23, 1187–1196. doi: 10.4014/jmb.1305.0501
Huang, S. P., Cui, X. C., Xu, Z. H., Zhang, Z. S., and Wang, X. M. (2022). Nitrogen addition exerts a stronger effect than elevated temperature on soil available nitrogen and relation to soil microbial properties in the rhizosphere of Camellia sinensis L. seedlings. Environ. Sci. Pollut. R. 29, 35179–35192. doi: 10.1007/s11356-022-18748-4
Inclan, R., Uribe, C., Sanchez, L., Sanchez, D. M., Clavero, A., Fernandez, A. M., et al. (2012). N2O and CH4 fluxes in undisturbed and burned holm oak, scots pine and Pyrenean oak forests in Central Spain. Biogeochemistry 115, 419–420. doi: 10.1007/s10533-013-9866-9
Jin, H., and Ma, Q. (2021). Impacts of permafrost degradation on carbon stocks and emissions under a warming climate: a review. Atmos 12:1425. doi: 10.3390/atmos12111425
Jung, J., Yeom, J., Kim, J., Han, J., Lim, H. S., Park, H., et al. (2011). Change in gene abundance in the nitrogen biogeochemical cycle with temperature and nitrogen addition in Antarctic soils. Res. Microbiol. 162, 1018–1026. doi: 10.1016/j.resmic.2011.07.007
Kirkwood, J. A. H., Roy-Léveillée, P., Mykytczuk, N., Packalen, M., McLaughlin, J., Laframboise, A., et al. (2021). Soil microbial community response to permafrost degradation in Palsa fields of the Hudson Bay lowlands: implications for greenhouse gas production in a warming climate. Glob. Biogeochem. Cy. 35:GB006954. doi: 10.1029/2021GB00695
Koyama, A., Wallenstein, M. D., Simpson, R. T., and Moore, J. C. (2014). Soil bacterial community composition altered by increased nutrient availability in Arctic tundra soils. Front. Microbiol. 5:516. doi: 10.3389/fmicb.2014.00516
Le, T. B., Wu, J. H., Gong, Y., and Vogt, J. (2020). Graminoid removal reduces the increase in N2O fluxes due to nitrogen fertilization in a boreal peatland. Ecosystems 24, 261–271. doi: 10.1007/s10021-020-00516-5
Leifeld, J., Bassin, S., Conen, F., Hajdas, I., Egli, M., and Fuhrer, J. (2013). Control of soil pH on turnover of belowground organic matter in subalpine grassland. Biogeochemistry 112, 59–69. doi: 10.1007/s10533-011-9689-5
Li, X. Y., Jin, H. J., Sun, L., Wang, H. W., He, R. X., Huang, Y. D., et al. (2021). Climate warming over 1961–2019 and impacts on permafrost zonation in Northeast China. J. For. Res. 33, 767–788. doi: 10.1007/s11676-021-01403-y
Li, J. Q., Nie, M., and Pendall, E. (2019). An incubation study of temperature sensitivity of greenhouse gas fluxes in three land-cover types near Sydney, Australia. Sci. Total Environ. 688, 324–332. doi: 10.1016/j.scitotenv.2019.06.206
Liu, Y., Chen, Q. M., Wang, Z. X., Zheng, H. F., Chen, Y. M., Chen, X., et al. (2019). Nitrogen addition alleviates microbial nitrogen limitations and promotes soil respiration in a subalpine coniferous forest. Forests 10:1038. doi: 10.3390/f10111038
Lu, C. Y., Bowman, D., Rufty, T., and Shi, W. (2015). Reactive nitrogen in Turfgrass systems: relations to soil physical, chemical, and biological properties. J. Environ. Qual. 44, 210–218. doi: 10.2134/jeq2014.06.0247
Ma, F., Li, M., Wei, N., Dong, L. B., Zhang, X. Y., Han, X., et al. (2022). Impacts of elevated atmospheric CO2 and N fertilization on N2O emissions and dynamics of associated soil labile C components and mineral N in a maize field in the North China plain. Agronomy 12:432. doi: 10.3390/agronomy12020432
Ma, L. N., Lu, X. T., Liu, Y., Guo, J. X., Zhang, N. Y., Yang, J. Q., et al. (2011). The effects of warming and nitrogen addition on soil nitrogen cycling in a temperate grassland, Northeastern China. PLoS One 6:e27645. doi: 10.1371/journal.pone.0027645
Maslov, M. N., and Maslova, O. A. (2021). Nitrogen limitation of microbial activity in alpine tundra soils along an environmental gradient: intra-seasonal variations and effect of rising temperature. Soil Biol. Biochem. 156:108234. doi: 10.1016/j.soilbio.2021.108234
Menyailoa, V., Matvienkoa, A. I., Makarov, M. I., and Cheng, C. H. (2014). Positive response of carbon mineralization to nitrogen addition in forest soils of Siberia. Dokl. Biol. Sci. 456, 173–176. doi: 10.1134/S0012496614030028
Metcalfe, D. B. (2017). Microbial change in warming soils. Science 358, 41–42. doi: 10.1126/science.aap7325
Muhammad, I., Lv, J. Z., Wang, J., Ahmad, S., Farooq, S., Ali, S., et al. (2022). Regulation of soil microbial community structure and biomass to mitigate soil greenhouse gas emission. Front. Microbiol. 13:868862. doi: 10.3389/fmicb.2022.868862
Nichols, J. E., and Peteet, D. M. (2019). Rapid expansion of northern peatlands and doubled estimate of carbon storage. Nat. Geosci. 12, 917–921. doi: 10.1038/s41561-019-0454-z
Ochoa-Hueso, R., Maestre, F. T., Ríos, A. D. I., Vale, S., Theobald, M. R., Vivanco, M. G., et al. (2013). Nitrogen deposition alters nitrogen cycling and reduces soil carbon content in low-productivity semiarid Mediterranean ecosystems. Environ. Pollut. 179, 185–193. doi: 10.1016/j.envpol.2013.03.060
Schlesinger, W. H., and Andrews, J. A. (2000). Soil respiration and the global carbon cycle. Biogeochemistry 48, 7–20. doi: 10.1023/A:1006247623877
Sihi, D., Gerber, S., Inglett, P. W., and Inglett, K. S. (2016). Comparing models of microbial–substrate interactions and their response to warming. Biogeosciences 13, 1733–1752. doi: 10.5194/bg-13-1733-2016
Soil Survey Staff (2010). Keys to Soil Taxonomy, 11th Edn. USDA-Natural Resources Conservation Service, Washington, D.C.
Song, Y. Y., Liu, C., Song, C. C., Wang, X. W., Ma, X. Y., Gao, J. L., et al. (2021). Linking soil organic carbon mineralization with soil microbial and substrate properties under warming in permafrost peatlands of northeastern China. Catena 203:105348. doi: 10.1016/j.catena.2021.105348
Tu, L. H., Chen, G., Peng, Y., Hu, H. L., Hu, T. X., Zhang, J., et al. (2014). Soil biochemical responses to nitrogen addition in a bamboo forest. PLoS One 9:e102315. doi: 10.1371/journal.pone.0102315
Wang, R. Z., Dorodnikov, M., Yang, S., Zhang, Y. Y., Filley, T. R., Turco, R. F., et al. (2015). Responses of enzymatic activities within soil aggregates to 9-year nitrogen and water addition in a semi-arid grassland. Soil Biol. Biochem. 81, 159–167. doi: 10.1016/j.soilbio.2014.11.015
Wang, Z. J., Lu, G. X., Yuan, M. T., Yu, H., Wang, S., Li, X., et al. (2019). Elevated temperature overrides the effects of N amendment in Tibetan grassland on soil microbiome. Soil Biol. Biochem. 136:107532. doi: 10.1016/j.soilbio.2019.107532
Wang, J. Y., Ren, C. J., Feng, X. X., Zhang, L., Doughty, R., and Zhao, F. Z. (2020). Temperature sensitivity of soil carbon decomposition due to shifts in soil extracellular enzymes after afforestation. Geoderma 374:114426. doi: 10.1016/j.geoderma.2020.114426
Wang, C. M., Zhang, Y. Y., and Li, Y. (2020). Soil type and a labile C addition regime control the temperature sensitivity of soil C and N mineralization more than N addition in wetland soils in China. Atmos 11:1043. doi: 10.3390/atmos11101043
Wei, Z., Jin, H. J., Zhang, J. M., Yu, S. P., Han, X. J., Ji, Y. J., et al. (2011). Prediction of permafrost changes in northeastern China under a changing climate. Sci. China Earth Sci. 54, 924–935. doi: 10.1007/s11430-010-4109-6
Wu, H. T., Lu, M. Z., Lu, X. G., Guan, Q., and He, X. H. (2015). Interactions between earthworms and mesofauna has no significant effect on emissions of CO2 and N2O from soil. Soil Biol. Biochem. 88, 294–297. doi: 10.1016/j.soilbio.2015.06.005
Wu, H. T., Lu, X. G., Wu, D. H., Song, L. H., Yan, X. M., and Liu, J. (2013). Ant mounds alter spatial and temporal patterns of CO2, CH4 and N2O emissions from a marsh soil. Soil Biol. Biochem. 57, 884–891. doi: 10.1016/j.soilbio.2012.10.034
Wu, L. W., Yang, F. L., Feng, J. J., Tao, X. Y., Qi, Q., Wang, C. E., et al. (2022). Permafrost thaw with warming reduces microbial metabolic capacities in subsurface soils. Mol. Ecol. 31, 1403–1415. doi: 10.1111/mec.16319
Xu, G., Chen, J., Berninger, F., Pumpanen, J., Bai, J. W., Yu, L., et al. (2015). Labile, recalcitrant, microbial carbon and nitrogen and the microbial community composition at two Abies faxoniana forest elevations under elevated temperatures. Soil Biol. Biochem. 91, 1–13. doi: 10.1016/j.soilbio.2015.08.016
Xu, X. Y., Liu, X. R., Li, Y., Ran, Y., Liu, Y. P., Zhang, Q. C., et al. (2017). High temperatures inhibited the growth of soil bacteria and archaea but not that of fungi and altered nitrous oxide production mechanisms from different nitrogen sources in an acidic soil. Soil Biol. Biochem. 107, 168–179. doi: 10.1016/j.soilbio.2017.01.003
Xue, K., Yuan, M. M., Zhou, J. S., Qin, Y., Deng, Y., Cheng, L., et al. (2016). Tundra soil carbon is vulnerable to rapid microbial decomposition under climate warming. Nat. Clim. Chang. 6, 595–600. doi: 10.1038/nclimate2940
Yao, H. Y., Bowman, D., Rufty, T., and Shi, W. (2015). Interactions between N fertilization, grass clipping addition and pH in turf ecosystems: implications for soil enzyme activities and organic matter decomposition. J. Environ. Qual. 44, 210–218. doi: 10.2134/jeq2014.06.0247
Yuan, X. C., Si, Y. T., Lin, W. S., Yang, J. Q., Wang, Z., Zhang, Q. F., et al. (2018). Effects of short-term warming and nitrogen addition on the quantity and quality of dissolved organic matter in a subtropical Cunninghamia lanceolata plantation. PLoS One 13:e0191403. doi: 10.1371/journal.pone.0191403
Yuan, C., Zhu, G., Yang, S., Xu, G., Li, Y., Gong, H., et al. (2019). Soil warming increases soil temperature sensitivity in subtropical forests of SW China. PeerJ 7:e7721. doi: 10.7717/peerj.7721
Zhang, J. J., Peng, C. H., Xue, W., Yang, Z. N., Yang, B., Li, P., et al. (2018). Soil CH4 and CO2 dynamics and nitrogen transformations with incubation in mountain forest and meadow ecosystems. Catena 163, 24–32. doi: 10.1016/j.catena.2017.12.005
Zhang, J. J., Peng, C. H., Zhu, Q., Xue, W., Shen, Y., Yang, Y. Z., et al. (2016). Temperature sensitivity of soil carbon dioxide and nitrous oxide emissions in mountain forest and meadow ecosystems in China. Atmos. Environ. 142, 340–350. doi: 10.1016/j.atmosenv.2016.08.011
Zhang, Y., Zhang, N., Yin, J. J., Yang, F., Zhao, Y. X., Jiang, Z. Q., et al. (2020). Combination of warming and N inputs increases the temperature sensitivity of soil N2O emission in a Tibetan alpine meadow. Sci. Total Environ. 704:135450. doi: 10.1016/j.scitotenv.2019.135450
Keywords: climate warming, nitrogen availability, soil microbial abundance, enzyme activity, boreal peatland
Citation: Song Y, Cheng X, Song C, Li M, Gao S, Liu Z, Gao J and Wang X (2022) Soil CO2 and N2O emissions and microbial abundances altered by temperature rise and nitrogen addition in active-layer soils of permafrost peatland. Front. Microbiol. 13:1093487. doi: 10.3389/fmicb.2022.1093487
Received: 09 November 2022; Accepted: 23 November 2022;
Published: 13 December 2022.
Edited by:
Zifang Chi, Jilin University, ChinaReviewed by:
Bin Chen, Beijing Normal University, ChinaCopyright © 2022 Song, Cheng, Song, Li, Gao, Liu, Gao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changchun Song, c29uZ2NjQGlnYS5hYy5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.