
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol., 10 January 2023
Sec. Food Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1085891
This article is part of the Research TopicAspergillus-Derived Mycotoxins In The Feed And Food Chain, Volume IIView all 9 articles
Aflatoxins are toxic secondary metabolites produced by Aspergillus spp. found in staple food and feed commodities worldwide. Aflatoxins are carcinogenic, teratogenic, and mutagenic, and pose a serious threat to the health of both humans and animals. The global economy and trade are significantly affected as well. Various models and datasets related to aflatoxins in maize have been developed and used but have not yet been linked. The prevention of crop loss due to aflatoxin contamination is complex and challenging. Hence, the set-up of advanced decontamination is crucial to cope with the challenge of climate change, growing population, unstable political scenarios, and food security problems also in European countries. After harvest, decontamination methods can be applied during transport, storage, or processing, but their application for aflatoxin reduction is still limited. Therefore, this review aims to investigate the effects of environmental factors on aflatoxin production because of climate change and to critically discuss the present-day and novel decontamination techniques to unravel gaps and limitations to propose them as a tool to tackle an increased aflatoxin risk in Europe.
Mycotoxins are toxic secondary metabolites produced by filamentous fungi which contaminate food and feed products worldwide. More than 25% of food is contaminated by at least one mycotoxin, with important implications for the health of humans and animals and the global trade and economy (Eskola et al., 2020; Ráduly et al., 2020).
Aflatoxins (AFs) are the most critical mycotoxins due to their toxic potential and occurrence. Indeed, they have been listed as Class 1 carcinogens by the International Agency on Research on Cancer, known to cause cancer, namely liver cancer, in humans (International Agency for Research on Cancer, 2002). Besides, they may cause liver inflammation and necrosis, immune depression, stunting, growth and development impairment, reproductive dysfunction, and even death when consumed at high dosages (Rushing and Selim, 2019; Ráduly et al., 2020).
AFs include more than 20 different furanocoumarin derivatives, which can be found on a wide variety of food commodities in the field during harvest, transport, and storage (Kumar et al., 2017). The most relevant ones are the AFs of the B-series (AFB1 and AFB2) and G-series (AFG1 and AFG2), which can be found in cereals, peanuts, nuts, and spices, and AFs of the M-series (AFM1 and AFM2), which are in vivo hydroxylated metabolites secreted in milk.
Being secondary metabolites, the production of AFs is sophistically regulated and depends upon a wide spectrum of biotic and abiotic factors (Reverberi et al., 2010; Bayram and Braus, 2012; Fountain et al., 2014; Keller, 2015; Pfliegler et al., 2020; Khan et al., 2021), thus making it difficult to prevent entirely. Pre-harvest technologies mainly rely on good agricultural practices and the field application of non-aflatoxigenic fungal strains as biological control agents displacing aflatoxigenic strains on crops (Abbas et al., 2017; Agbetiameh et al., 2019; Sarrocco et al., 2019; Dövényi-Nagy et al., 2020; Peles et al., 2021; Ola et al., 2022).
Once synthesized, AFs persist in food and feed because they are highly stable to heat and most common food and feed processing techniques. To reduce AFs contamination, various decontamination methods can be applied after harvest, either during transport, storage, or processing (Peles et al., 2021; Sipos et al., 2021). Considering that commodities can be imported from distant geographical areas and stored for long periods before being processed, all post-harvest handling stages are important to reduce aflatoxin contamination.
Cereals like maize, sorghum, wheat, and barley are amongst the most susceptible commodities to be contaminated by AFs. They represent the staple food of human and animal diet worldwide and will be increasingly required to feed the growing population. Nonetheless, cereal trade globalization is threatened not only by fungal infection and mycotoxin contamination but also by climate change and insecure political scenarios (King et al., 2017; Sarmast et al., 2021; Hellegers, 2022).
Cereal production is expected to decrease slightly in 2022/2023, making it an even more precious commodity in the following years (FAO, 2022). In Europe, the production in 2021 has been estimated to be around 270 million tons (FAOstat, 2022) and it will be 4% lower due to hot and dry weather conditions, prolonged periods of rainfall deficits, and reduced maize output caused by the war in Ukraine (FAO, 2022). Besides tighter supplies and market uncertainty, rising energy and input prices will also contribute to keeping world cereal prices elevated throughout 2023 (FAO, 2022). To further complicate this scenario, in the near future, climate change will significantly modify fungal and mycotoxin contamination patterns, and aflatoxin contamination of maize will be the main mycotoxin issue in Europe (Moretti et al., 2019). Aside from the increased contamination in the fields, increased aflatoxin M1 levels (also above the current European regulation limit of 50 ng/kg) are also expected in milk and dairy (Van der Fels-Klerx et al., 2019).
Nuts and nut products and oilseed are other two important AFs susceptible commodities. Nontheless, European production of these two commodities is far behind that of cereals, namely 2 and 27 million tons, respectively (FAOstat, 2022). While nuts are widely imported from abroad (e.g., Turkey, United States, Iran, Vietnam, China), they remain the most notified product category contaminated by AFs in 2021 by the Rapid Alert System for Food and Feed (2022). Regarding oilseed production, The EU cultivates three major types of oilseed crops; the main three are rape, sunflower, and soya. From a safety point of view, they do not represent the major oilseed crops to be contaminated by AFs (Einolghozati et al., 2021).Therefore, the set-up and application of advanced decontamination are crucial to cope with the challenge of climate change, growing population, unstable political scenarios, and food security problems, as summarized in Figure 1.
Due to the aflatoxin worldwide concern, over the past 20 years a wide variety of decontamination methods have been investigated, including physical, chemical, and biological ones. Therefore, this review aims to investigate the effects of environmental factors on aflatoxin production in view of the climate change to better understand how to model and prevent contamination in the field, as well as the present-day and novel decontamination techniques which can be applied to the in the field and at post-harvest stage. Gaps and perspectives of these methods were also critically discussed to propose them as a tool to tackle an increased aflatoxin risk in Europe.
Molecular genetic tools are absolutely needed (i) to decipher the function of aflatoxin biosynthetic genes (Amaike and Keller, 2011; Amare and Keller, 2014; Caceres et al., 2020; Gil-Serna et al., 2020), (ii) to shed light on the regulatory motifs and networks governing this high-complexity process (Yu, 2012; Eom et al., 2018; Pfannenstiel et al., 2018; Cary et al., 2019; Gil-Serna et al., 2020; Zhao et al., 2022), (iii) to track back genetic changes resulting in non-aflatoxigenic biological control fungal strains (Moore et al., 2009; Chang et al., 2012a; Adhikari et al., 2016; Pennerman et al., 2018; Chang, 2022), (iv) to study population structure and dynamics of aflatoxigenic molds in agriculture (Chang et al. 2019; Lewis et al., 2019; Weaver et al., 2019; Drott et al., 2020), (v) to map the mechanism of action of various agents interfering with the aflatoxin production by aflatoxigenic molds (Hua et al., 2014; Wang et al., 2015; Ren Y. et al., 2020; Safari et al., 2020), and (vi) to develop novel, RNA interference-based, host-induced AF control technologies (Majumdar et al., 2017; Wu, 2022). To make genetics and biotechnology-based approaches and innovations more effective, we need to intensify basic research aiming at the elucidation of the elements of the regulatory network that fine-tunes aflatoxin production adequately to the environmental stimuli perceived by aflatoxin producer molds.
The organization of the aflatoxin biosynthetic gene cluster and the functions of its genes have recently been reviewed in several publications (Caceres et al., 2020; Gil-Serna et al., 2020; Khan et al., 2021; Ferrara et al., 2022), and, therefore, only a short recapitulation of our knowledge on the genetic regulation of AF biosynthesis is presented here.
The biosynthesis of AFs is a complex, energy-consuming process that needs, as a minimum, 27 enzymatic reactions to build up these highly complex molecules. Studies on the genetic background of AF biosynthesis in A. flavus and A. parasiticus, as well as sterigmatocystin production in A. nidulans, led to the identification of the AF gene cluster (Yu et al., 2000, 2004; Georgianna and Payne, 2009; Amare and Keller, 2014).
The 75 kbp long AF gene cluster comprises 30 genes and is located near these fungi’s telomere of chromosome III. Of the enzyme encoding structural genes of the cluster, four genes, aflA, aflB, aflC, and hypC, encoding two fatty acid synthase subunits, a polyketide synthase, and an oxidase, respectively, are involved in building up the precursor, norsoloric acid (NOR) from hexanoate units. NOR is converted to versicolorin B (VERB) by a series of enzymes encoded by aflD, aflG, aflH, aflI, aflJ, aflK, aflV, and aflW genes. Transformation of VERB into versicolorin A (VERA) is catalyzed by a cytochrome P-450 monooxygenase, encoded by aflL. Five genes (aflM, aflN, aflY, aflX, and aflO) are responsible for converting VERA to sterigmatocystin (STER). Finally, STER is transformed into AFB1 in several Aspergillus species belonging to section Flavi with the aid of enzymes encoded by aflP, aflQ, hypB, hypE, and aflE. An additional gene of the cluster (aflT), encoding a putative MFS transporter, was found to have no significant role in AF secretion (Chang et al., 2004), despite the presence of its gene product (AflT) in the aflatoxisomes (Chanda et al., 2010).
The central positive regulator of the AF biosynthesis genes is aflR (Yu et al., 1996), encoding a Zn(II)2Cys6 transcription factor (Shimizu et al., 2003). The AflR protein, acting as a pathway-specific transcription factor, has binding sites on promoters of as many as 18 afl genes, including its own promoter, indicating autoregulation of aflR (Chang et al., 1995). Within the cluster, next to aflR, there is another regulatory gene, aflS acting as an enhancer. The product of this gene interacts with AflR, and the AflR-AflS complex formed in this manner allows a stringent binding to promoters of the target genes (Kong et al., 2014).
Not surprisingly, a wide spectrum of RNA interference based technologies has been developed and tested in planta to control AF production by aflatoxigenic fungi (McDonald et al., 2005;Majumdar et al., 2017; Wu, 2022). A frequently targeted gene is aflR in both peanut (Arias et al., 2015; Faustinelli et al., 2018; Power et al., 2020) and maize (Masanga et al., 2015). Other approaches targeted either the aflS regulatory gene (Arias et al., 2015; Power et al., 2020) or AF biosynthetic genes including aflC (encoding polyketide synthase; Arias et al., 2015; Thakare et al., 2017; Power et al., 2020; Niño-Sánchez et al., 2021) and aflM (coding for versicolorin dehydrogenase; Raruang et al., 2020). It is noteworthy that a RNAi-based AF control system simultaneously silencing aflR, aflR, aflC, aflep (coding for AF efflux pump) and pes1 (encoding a non-ribosomal peptide synthase with hypothesized function in cyclopiazonic acid biosynthesis) was also constructed and tested in peanut using both transgenic and non-transgenic delivery tools (Arias et al., 2015; Power et al., 2020). In addition to host induced gene silencing strategies (Majumdar et al., 2017; Wu, 2022), exogenous RNAi delivery-based, non-transgenic approaches are gaining ground including the application of RNAi-triggering dsDNA and dsRNA by gene gun (Power et al., 2020), DsiRNA (Dicer-substrate siRNA) after wounding (Faustinelli et al., 2018) and dsRNA by genetically engineered bacteria (RNAseIII-null mutant Escherichia coli, both living cells and crude whole-cell autolysates; Niño-Sánchez et al., 2021). Further AF biosynthetic structural genes like aflD (encoding an enzyme converting norsolorinic acid to averantin; Abdel-Hadi et al., 2011) are likely to be targeted in future RNA interference technologies.
In addition to pathway-specific transcription factors, secondary metabolite biosynthesis genes are regulated by global (or general) transcription factors, too; these transcription factors mediate (i) nutritional, (ii) environmental, and (iii) developmental signals (Reverberi et al., 2010; Roze et al., 2011; Yin et al., 2012, 2013; Montibus et al., 2013; Hong et al., 2013a,b; Ferrara et al., 2022). Such a complex regulatory network helps fungi to react to stressors by producing secondary metabolites. For example, AF biosynthesis has long been recognized as an essential oxidative stress response mechanism in Aspergilli (Roze et al., 2011), and AFs are natural scavengers of reactive oxygen species (Finotti et al., 2021).
As far as the nutrition-related factors are concerned, CreA and AreA are worth mentioning first. CreA, encoded by creA, a Cys2His2 zinc finger transcription factor, is the critical player in carbon catabolite repression in filamentous fungi. Deletion of creA in A. flavus resulted in the loss of AF production (Fasoyin et al., 2018), indicating a crucial role of this TF in regulating the AF gene cluster. Another transcription factor, AreA, a member of the GATA transcription factor family and acting as the primary regulator of N-utilization, also affects AF biosynthesis. A ΔareA strain of A. flavus increased or decreased AF production compared to the wild type depending on the N-source of the culture medium (Fasoyin et al., 2019). Among nitrogen forms, nitrate inhibits AF formation, increases the expression of aflS gene, and reduces the expression of other genes involved in AF synthesis (Price et al., 2005). However, actual AF synthesis is inhibited by a pathway other than AF synthesis, which is hypothesized that is associated with a change in the redox potential caused by nitrate, which affects the formation of the precursor of polyketide synthesis by increasing the activity of mannitol dehydrogenase (Niehaus and Jiang, 1989).
Simple sugars and acetate stimulate (Shantha and Murthy, 1981), and intermediates of the tricarboxylic acid cycle inhibit the formation of AF (Buchanan and Ayres, 1977; Shantha and Murthy, 1981). Although, at the same time, carbon sources do not regulate specifically the genes in the AF biosynthesis pathway. They affect the synthesis of AFs either through the precursors or the cAMP signaling pathway (Georgianna and Payne, 2009).
Further studies on the regulatory network fine-tuning the carbon and nitrogen metabolisms of aflatoxigenic molds are definitely needed because host-induced gene silencing of either A. flavus amy1 (encoding alpha-amylase) or alk (coding for alkaline protease) significantly reduced growth and AF production (Gilbert et al., 2018; Omolehin et al., 2021). Shedding light on the elements of this network and their target genes may help us to find novel targets for the construction of crops with enhanced AF resistance. Importantly, direct targeting of A. flavus by RNAi technologies may be more effective than the suppression of the AF biosynthetic gene cluster especially in late maturing grain under humid conditions (Gressel and Polturak, 2018). Hence, RNA interference-based targeting of other important genes maintaining fungal growth like spds (coding for spermidine synthase; Majumdar et al., 2018) is foreseeable.
Transcription factors involved in responses to environmental variables, like pH, oxidative stress, and light, influence AF/STER production and transcription of AF genes. PacC, a Cys2His2 zinc finger regulator, is the critical player in reacting to pH. An A. nidulans mutant with constitutive pacC activity produced 10-fold less STER than its wild-type parental strain (Keller et al., 1997). Acidic pH favors AF synthesis through the transcription factor PacC, whose binding point is in the promoter of aflR, while the inhibitory effect of alkaline pH on AF synthesis is realized through pkA (Tilburn et al., 1995; Ehrlich et al., 1999; Shimizu and Keller, 2001; Shimizu et al., 2003).
AP-1 and AtfB, bZIP transcription factors, MsnA, a Cys2His2 zinc finger, and SrrA, a winged helix-turn-helix transcription factor, were shown to form a regulatory network to mediate oxidative stress response and induce AF biosynthesis in A. parasiticus (Hong et al., 2013b). In this fungus, AtfB, by forming a heterodimer with AP-1, binds to seven genes of the AF gene clusters at the CRE binding sites and induces AF biosynthesis (Roze et al., 2011). Therefore, AtfB silencing brought about a remarkable reduction in AF production (Wee et al., 2017). AtfB binding and induction of AF production can be initiated by the elevation of extra- or intracellular ROS (Roze et al., 2011).
A comprehensive study by Zhao et al. (2022) revealed several basic region/leucine zipper motif (bZIPs) transcription factors involved in AF production in A. flavus. Ten bZIPs seem to regulate AF biosynthesis since gene deletion of the bZIP1, bZIP2, bZIP4, bZIP5, atfA, atfB, meaB, and metR reduced AF levels remarkably. It is worth noting that the deletion of A. nidulans atfA and Fusarium verticillioides FvatfA also resulted in drastically decreased mycotoxin, namely sterigmatocystin and fumonisin productions in these fungi (Szabó et al., 2020; Kocsis et al., 2022). In A. flavus, the elimination of the bZIP transcription factors hapX and jlbA decreased AF biosynthesis slightly but significantly (Zhao et al., 2022). Furthermore, all 10 bZIPs, except bZIP5, coupled with AF production reacted to oxidative stress (Zhao et al., 2022).
The bZIP transcription factor, AflRsmA (restorer of secondary metabolism A) is also associated with the AF production in A. flavus. The overexpression of AflrsmA increased the AFB1 production. At the same time, oxidative stress (menadione sodium bisulfite and tert-butyl-hydroperoxide) exposed ΔAflRsmA mutant showed reduced AF levels compared to the wild type and AflRsmAOE strains (Wang et al., 2020). Furthermore, this research group identified conserved motifs in promoters of both AF biosynthesis genes and stress-response genes, where these transcription factors can bind. Interestingly, deletion of Afap1, a Saccharomyces cerevisiae Yap1 ortholog bZIP type transcription factor, led to down-regulation of aflM and aflP and up-regulation of aflB and aflR, and this contrasting action resulted in a ~ 75% decrease of AF production in A. flavus (Guan et al., 2019).
Similar to the regulatory network orchestrating the carbon and nitrogen metabolisms of aflatoxigenic fungi, further elements of the environmental stress response system and their regulation should be revealed and considered as future targets to control the growth and AF production of these molds. Actually, there is a plethora of literature data demonstrating the efficiency of various antioxidants to hinder the AF production by aflatoxigenic fungi (Reverberi et al., 2005, 2006; Zjalic et al., 2006; Caceres et al., 2017; Zhao et al., 2018; Xu et al., 2021). Obviously, a deeper understanding of the regulatory elements interweaving the pathways responsible for the proper adjustment and co-regulation of mycotoxin production and environmental stress response may also help us to develop novel technologies for mycotoxin contamination control.
The light-responsive Velvet complex regulates secondary metabolite genes in a temperature-dependent manner (Lind et al., 2016). Accordingly, components of this complex (VeA, VelB, LaeA) affect AF production. In A. flavus, deletion of laeA resulted in the downregulation of aflR, aflS, and aflD, accompanied by loss of AF production (Chang et al., 2012b). Whereas, in the ΔveA mutants of A. parasiticus, no aflR, aflS, aflC and aflM transcripts were observed, and the mutant did not produce the AF precursor, versicolorin A (Calvo et al., 2004). VeA of A. flavus regulates not only the formation of AFs but also the biosynthesis of cyclopiazonic acid and aflatrem (Duran et al., 2007). Concomitantly, VeA is also a key player in the regulation of H2O2 stress response in A. flavus (Baidya et al., 2014). Interestingly, mycovirus-dependent suppression of AF production by A. flavus may also be a result of the interference of mycovirus (PcV) degradation products with veA expression (Schmidt, 2009). Mycovirus-based technologies to control aflatoxigenic molds can be promising but further molecular-level studies are needed in this field (Kotta-Loizou and Coutts, 2017).
Lipid synthesis correlates with AF production since the first step produces acetyl coenzyme A, which is necessary for forming STER and thus AFs (Dutton, 1988). Oxylipins are signal molecules derived from fatty acids and play an essential role in regulating development, pathogenic processes, and the production of secondary metabolites (Tsitsigiannis and Keller, 2006, 2007). Genes that encode fatty acid oxygenases (ppoA, ppoB, ppoC) affect the expression of brlA and veA genes; the veA gene also regulates the brlA gene that affects asexual reproduction, which may have an effect that may be exerted through oxylipins (Tsitsigiannis et al., 2005; Calvo, 2008). The effects of ppo genes on aflR can be exerted through the PKA or the pathway mediated by the G protein (Tsitsigiannis and Keller, 2006).
Finally, development-related transcription factors can also regulate AF biosynthesis genes. Home-box proteins (Hbx) control basic developmental processes, like conidiogenesis and fruiting body development in fungi. Disruption of hbx1 in A. flavus resulted in the loss of AF production. Transcriptome analysis revealed that Hbx1 is a master regulator, as its deletion affected the expression of more than 5,000 genes in the A. flavus genome, including down-regulation of aflO, aflP, and aflR (Cary et al., 2019). NsdC, a Cys2His2 zinc finger and NsdD, a GATA type TF, are required for sexual and asexual development in Aspergillus spp. and influence AF biosynthesis. In a ΔnsdC mutant of A. flavus, transcript levels of aflR increased, but that of aflM and aflP decreased, resulting in a loss of AF production (Cary et al., 2012).
Besides their roles in nucleosome positioning and RNA polymerase recruiting, the global transcription factors we summed up above assist, thus, the AF-producing Aspergilli by interconnecting the perception of environmental/developmental signals and the fine regulation of the AF biosynthesis genes. Future studies will hopefully point at novel targets for the development of effective RNA interference-based, host-induced plant biotechnological methods to control AF production in important crops sensitive to aflatoxigenic molds, possibly even in Europe.
A proper mapping of environmental factors affecting AF production are of paramount importance when good agricultural practices and effective storage protocols are adapted, developed, and evaluated (Dövényi-Nagy et al., 2020; Sipos et al., 2021). Monitoring these environmental factors may help experts to control AFs in feed and food production chains (Farkas et al., 2022).
Not surprisingly, several environmental factors affect the biosynthesis of AFs, such as temperature (O’Brian et al., 2007), light, pH, carbon sources, and nitrogen sources (Calvo et al., 2002; Price et al., 2005).
Water activity (aw) and temperature are crucial environmental aspects affecting A. flavus growth and AF production. The effect of temperature on AF synthesis is temporary; above the optimal temperature required for synthesis, the genes of the AF gene cluster are repressed, except for aflR and aflS, the level of which remains at a constant value (O’Brian et al., 2007). However, the level of the AflR protein is much lower at a higher temperature, which can be explained by transcription inhibition of the genes of the AF gene cluster and the inactivation of proteins (Liu and Chu, 1998).
The aw × temperature connections are related to the ratio of the two essential regulatory genes (aflS/aflR). The higher ratio of aflS/aflR relate to the higher level of AFs production (Schmidt-Heydt et al., 2009, 2010; Abdel-Hadi et al., 2010, 2012; Medina et al., 2014). For example, AFB1 production on polished rice occurs over a broader range of temperature × aw levels. For fungal growth on polished rice, the optimal conditions were a w 0.92–0.96 and 28–37°C. The maximum amounts of AFB1 were observed at 33°C and a w 0.96 on polished rice (Lv et al., 2019). Two regulatory genes (aflR and aflS) were up-regulated at a w 0.90 (Lv et al., 2019). In addition, the expression of 11 development-related genes amplified under 0.99 a w treatment (Zhang et al., 2014).
Moreover, the effect of relating conditions of a w × temperature × elevated CO2 had little consequence on fungal growth; they had a significant influence on structural aflD and regulatory aflR genes and can significantly stimulate the production of AFB1 (Medina et al., 2014).
For an A. nomius strain, isolated from Brazil nuts, the ideal temperature for growth was 30°C and the best state for expressing the aflR, aflD, and aflQ genes. However, maximum production of AF B and G occurred at 25°C (Yunes et al., 2020). On the other hand, in A. flavus, compared with 37°C, the transcript abundance of 30 AFs biosynthesis genes was much higher at 30°C, and most genes were up-regulated at both protein and transcription levels at 28°C (Bai et al., 2015).
Drought stress has been revealed to stimulate the production of reactive oxygen species (ROS) in plant tissues (Cruz de Carvalho, 2008). Cells use their antioxidant system to protect themselves from reactive oxygen radicals, which can react with DNA, proteins and lipids and damage their functions. When the balance between antioxidants and reactive oxygen radicals is lost, oxidative stress occurs (Apel and Hirt, 2004). Oxidative stress stimulates AF synthesis, while antioxidants such as gallic acid adversely affect the formation of AF by inhibiting aflM and aflD genes (Jayashree and Subramanyam, 2000; Mahoney and Molyneux, 2004; Reverberi et al., 2008). Superoxide dismutase plays an essential role in the effect of oxidative stress on AF formation, which catalyzes the conversion of superoxide into hydrogen peroxide and water and inhibits the formation of AF (Furukawa and Sakuda, 2019). Enzymes involved in oxidative stress presented significantly down-regulated in liquid media and up-regulated at 28°C (p ≤ 0.05) in A. flavus (Wang et al., 2019).
A clear understanding of how abiotic factors impact aflatoxin contamination is crucial to understand their real impact in new geographical locations in the climate change scenario. This information can be integrated in new models which can be exploited for early prediction (as described in section “Climatic effects and climatic models in Europe”) and pro-active intervention. Today, precision agriculture, nanotechnology, machine learning, and artificial intelligence can be used to set-up an innovative crop management/intervention system with real-time monitoring and responsiveness, especially in controlled-environment agriculture and during storage.
Naturally, A. flavus is hosted by a wide range of plants, while AF production is most common in plant types with higher oil content (e.g., maize, hazelnut, and other nuts). The distribution and growth of fungi in the soil are altered by geographical areas, soil type, soil water retention, climatic conditions (temperature, humidity, and rainfall), altitude, landform, type of crop grown, rotation, crop, and insect presence (Zhang et al., 2017). In addition, larger population densities were associated with soils with a significant organic matter content, abundant nitrate, phosphate, potassium, increased pH, and more significant electrical conductivity. Fungal spores can be spread by direct contact with the soil, dust-carrying soil particles, or insect vectors (Abbas et al., 2017; Zhang et al., 2017). Soil (specifically agricultural soils), where mould-infected plant residues are often present, serves as the main pool of mycotoxigenic fungi (Zhang et al., 2017). Therefore, considering mycotoxin concentrations, geographical location significantly affects the distribution of AFs. Therefore, the risk of a shift in traditional occurrence areas for AFs is expected in the World, while the incidence of AF is unpredictable.
Interestingly, AF notifications for the RASFF of the European Union are very low in Europe (Figure 2), especially for maize, compared to the other food sources and other continents, thanks to the regulations. The European Union has one of the most inclusive and sternest regulations on AF levels, set by the commission regulation 1881/2006 (European Commission, 2006) and later by its amending supplement 165/2010 (European Commission, 2010), that are binding upon the 27 member states of the EU. In the current climatic situation, European countries in which maize cultivation is expected, i.e., in France, Romania, North-East Italy and Hungary (in total reporting for 60% of the total production for the 28 EU Member States, FAOStat, 2013), show a low chance of AF occurrence (European Commission, 2007). The European nations trade most of their maize amongst each other; from the outside, several European nations also import maize from Brazil and Argentina and much less from the USA (Wu and Guclu, 2012).
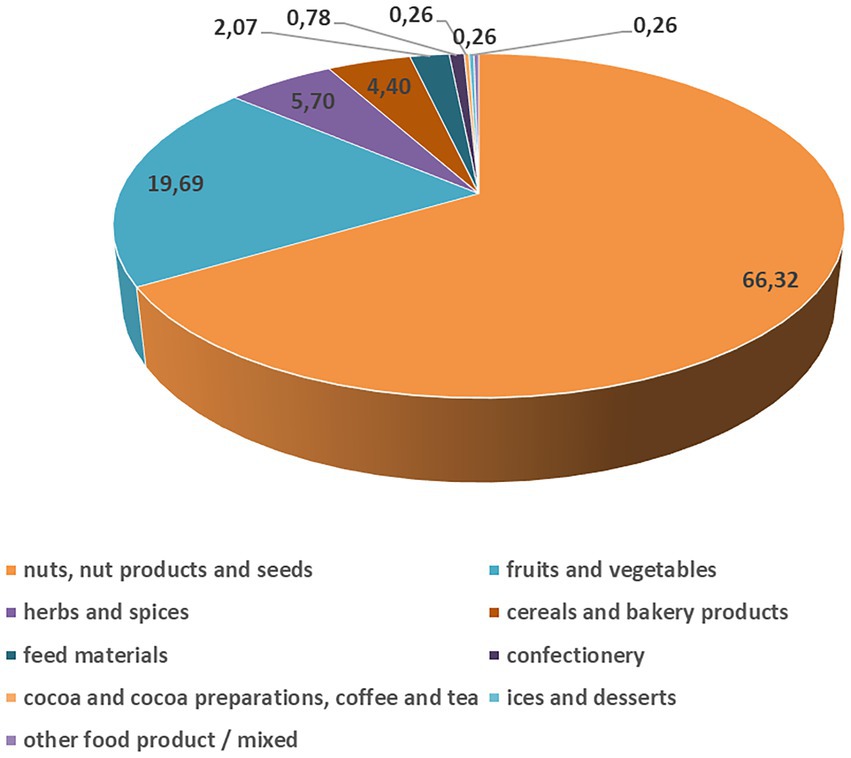
Figure 2. Severe aflatoxin B1 contamination for the different food and feed sources (%) reported in the European Union in 2021 (Rapid Alert System for Food and Feed, 2021). The data distribution from the 2021 and 2020 years was not statistically significantly different, but in 2021, the reported cases were twice as much (385) as in 2020 (165). However, the origin of the aflatoxin contaminated products was derived from all over the World. Serious aflatoxin contamination was reported only in some percent every year in the European Union.
Prediction models are an important tool to predict fungal occurrence and mycotoxin contamination. Nonetheless, there are still open issues, like: (i) keeping prediction accurate in climate change scenario, (ii) remodel the impact of cropping systems, (iii) consider co-occurring fungi and their ecology changing during the growing season, and (iv) multimycotoxin occurrence (Camardo Leggieri et al., 2020).
Various models and datasets related to AFs in maize have been developed and used, but they have not yet been linked. The EU green paper suggested that climate change effects will be regional and be either damaging or beneficial depending on geographical area (European Commission, 2007).
A model reflected a low to a medium probability of AF contamination under the +2°C increase. The climatic changes in Southern Europe are predicted to reach an increase of 4–5°C with more extended drought periods, causing increasing desertification and decreasing crop yields (Solomon et al., 2007). Serious contamination with AF was predicted in some southern European countries like Italy or Bulgaria (Battilani et al., 2016). However, maize production is marginal in these areas. Changes of +2.5–3.5°C with dryer and hotter summers are predicted for Western and Atlantic European areas. For the Central European countries, an increase of 3–4°C, higher rainfall and floods were forecasted, and longer growing periods would benefit crop yields. Northern Europe expects a mean temperature increase of 3–4.5°C and a significant (30–40%) increase in precipitation. It may lead to increases in crop yields and perhaps new crop cultivation techniques and changes in crop successions (European Commission, 2007; Solomon et al., 2007). The +5°C increase was predicted to lead to more expansive contaminated areas in the European domain. However, in southern Europe, AF increase was predicted to be limited since conditions would be less suitable for A. flavus growth (Battilani et al., 2016). In 2022, the Intergovernmental Panel on Climate Change reported that the global warming can reach 1.5°C in the near-term (2040) that would cause unavoidable increases in multiple climate hazards and present multiple risks to ecosystems and humans (very high confidence. The frequency of extreme agricultural droughts is projected to be 150 to 200% more likely at 2°C for the Mediterraneum (IPCC, 2022).
Biological control is regarded as one of the most promising solutions to counteract Aspergillus spp. growth in the field. Biological control is achieved by multiple means: (i) parasitism (deriving nutrients from the host); competition (for space and nutrients), and antibiosis (production of inhibitory metabolites and enzymes). The antagonistic behavior may derive from more than one mechanism, depending on the species involved and on the environmental conditions (Calistru et al., 1997). So far many species with Aspergillus inhibitory activity have been studied; atoxigenic strains and Trichoderma spp. are the most used and effective microorganisms, although bacteria and yeasts showed antagonistic and AFs reducing activities.
Atoxigenic Aspergillus strains are able to inhibit toxigenic fungi growth by competitive exclusion and to reduce mycotoxin production thanks to the production of organic volatile compounds (Moore et al., 2021).Atoxigenic Aspergilli are not able to produce AFs due to the partial or total deletion in AFs biosynthetic gene cluster (Moore, 2022) and when applied in the field, they can displace toxigenic strains and significantly lower both infection rate and aflatoxin production by native Aspergilli (Mauro et al., 2018; Moral et al., 2020). Different commercial products based on single or a combination of atoxigenic strains exist. In the US, Aspergillus flavus AF36, developed by USDA-ARS, and Afla-Guard® (A.flavus NRRL21882), developed by Syngenta, are commercialized. Another success story is Aflasafe® (developed by USDA-ARS, IITA, M&B Gates Foundations et al.), composed by four atoxigenic isolates belonging to distinct vegetative compatibility groups, native to the target nation (Bandyopadhyay et al., 2016). Aflasafe® was authorized in Nigeria, The Gambia, Senegal, Kenia, Burkina Faso, Ghana, Tanzania, and approved in Malawi, Zambia, and Mozambique.
Since 2003, several non-compliant, AF contaminated maize has been produced in Europe due to extreme temperature and dry weather (Mauro et al., 2013; Kos et al., 2018). This prompted the search for atoxigenic strains to be used as biocontrol agents, leading to the development of AF-X1™ (commercialized by Corteva Agriscience), a devitalised sorghum, coated with spores of A. flavus MUCL54911 (Mauro et al., 2018). To date, in Italy AF-X1™ has been granted a temporary authorization of 120 days that has to be renewed every year until full authorization as plant protection product from the European commission is granted.
Indeed, despite the high level of efficacy proved in several field trials and the outstanding success in low-income countries (>90% of AFB1 reduction), there are still concerns regarding the application of these biocontrol agents in Europe. Data is missing on the viability and population dynamics in water/sediment, its persistence and multiplication in natural environments. Several studies indicate that atoxigenic strains can persist year after year and to reduce aflatoxin contamination even if they are not re-applied (Moore, 2022). The genetic stability of these strains, i.e., if they still are atoxigenic generation after generation, still must be assessed. Additionally, the dietary and non-dietary risk assessments, and the ecotoxicological risk assessment (the impact in terms of pathogenicity and infectivity on non-target organisms, humans included) could not be finalized either due to lack of data (EFSA, 2022).
A concerning issue regards the potential promotion of fumonisin-producing strains and the possibility that non aflatoxigenic strains produce other mycotoxins in the field. Nonetheless, this hypothesis has not been yet verified (Ortega-Beltran et al., 2021).
Trichoderma spp. are among the most diffused biocontrol agents worldwide. They are commonly found in soil and root ecosystems, and elicit their biocontrol activities on a wide variety of plant pathogens through mycoparasitism, competition, and antibiosis. In fact, it is a fast growing, metabolically flexible species, able to parasitize other fungi and to produce a wide range of antibiotic substances (Ren et al., 2022).
The active substances produced by Trichoderma have shown to reduce Aspergillus growth and subsequently AFs production, or possibly to degrade AFs rather than to inhibit their synthesis (Gamal et al., 2022; Ren et al., 2022).
Among the extracellular cell wall degrading enzymes produced by Trichoderma spp., peroxidase has been shown to degrade AFs (Dini et al., 2022) and inhibit hyphal growth, while protease P6281 showed inhibitory activity on conidial germination and mycelial growth (Deng et al., 2018).
Trichoderma is presently marketed as active ingredients of more than 250 products worldwide, including bio-pesticides, biofertilizers, growth enhancers and stimulants of natural resistance (Woo et al., 2014).
Among bacteria, Bacillus, Pseudomonas, Lactobacillus, Streptomyces are the main genera with inhibitory activity towards Aspergillus spp. mycelial growth, conidial germination, and AFs production by competition and antibiosis. A wide range of inhibitory compounds have been identified among enzymes (chitinases, proteases, and glucanases), peptides, organic acids (lactiv and fatty acids) and miscellaneous alicyclic and aromatic compounds (Ren X. et al., 2020).
Different Saccharomyces and non- Saccharomyces yeasts (e.g., a Saccharomyces, Aureobasidium, Pichia, Metschnikowia, Dekkera (van der Walt), and Rhodotorula genera) have been studied in biological control, especially for Aspergillus infection in grapes (Di Canito et al., 2021). Also yeasts act via competition and antibiosis, the latter via production of lytic enzymes, peptides, toxic compounds, and volatile organic compounds (Ren et al., 2019).
Bacteria and yeast-based commercial biocontrol products are marketed in Europe after approval at EU level and authorization by Member States. Although they have been proven in several papers to be active against Aspergillus, they may be specifically registered to be used against other plant pathogens, like Botrytis cinerea, depending on the country.
The growing threat of aflatoxicosis requests novel decontamination measures to ensure food safety and food security. Decontamination techniques can be divided into three categories: (i) chemical, (ii) physical, and (iii) biological, according to the mechanism involved in the reduction or degradation.
Despite the enormous amount of literature published in the last years, there is currently a limited number of valid methods that have been authorized in Europe for aflatoxin reduction. Table 1 summarizes the main characteristics, advantages, and disadvantages of aflatoxin advanced decontamination techniques, which will be further described in the following sections.
Mycotoxin detoxifying agents (MDAs) were defined by the EFSA (European Food Safety Authority) et al. (2022) as “substances that can suppress or reduce the absorption, promote the excretion of mycotoxins or modify their mode of action” (Boudergue et al., 2019). Therefore, two main categories were identified, namely adsorbing and biotransforming agents. Adsorbing agents reduce mycotoxin bioavailability and uptake in living organisms by physically binding to the toxin, while biotransforming agents degrade mycotoxins into non-toxic metabolites (Boudergue et al., 2019).
From a regulatory point of view, Commission Regulation (EC 1831/2003; European Commission, 2003) introduced the category of technical additives intended to “suppress or reduce the absorption, promote the excretion of mycotoxins or modify their mode of action.” Nonetheless, detoxification processes can be applied in Europe to products intended for animal feed (Commission Regulation 786/2015; European Commission, 2015) if it is effective, characterized, does not result in harmful residues of both the substance/microorganism/enzyme used to detoxify and the toxin, and does not adversely affect the characteristics and the nature of the feed. This implies that a safety and efficacy assessment must be carried out before a method can be proposed and commercialized. Aside from these requirements, the cost of such a method must be considered, especially when treating a low-cost commodity, like, for example, maize for feed.
Adsorbents may be silica-based inorganic compounds or carbon-based organic polymers and can be used to sequester mycotoxins in feed or the gut of feedstock. Commercially available inorganic adsorbents are composed of aluminosilicates, such as bentonite, montmorillonite, zeolite, and hydrated sodium calcium aluminosilicates (HSCAS), and they can bind the β-keto-lactone or bilactone system of AFs through the uncoordinated metal ions in the mineral (Čolović et al., 2019). Carbon-based organic polymers include yeast cell wall and glucomannan, composed of lipids, proteins, polysaccharides, glucans and mannans. Being chemically and physically diverse compounds, they bind AFB1 through different types of non-covalent interactions, such as hydrogen bonds, and ionic or hydrophobic interactions (Kolawole et al., 2019).
The adsorption capability depends on the charge distribution, surface area, and pore size of the material. It may also occur within the layers of the adsorbent, thus increasing its binding capacity (Zhu et al., 2016). Adsorbents are widely commercialized as technical additives and proven to reduce the detrimental effects of AFs ingestion.
Despite being incredibly effective in binding AFB1, adsorbents display several disadvantages, like the unspecific binding to micro and macronutrients, veterinary medicinal products and additives, and the narrow spectrum of action towards multiple mycotoxins. To overcome this issue, research focuses on developing innovative hybrid adsorbents which combine the physical properties and binding capacity of different materials (Ma, 2019). Nano-adsorbents are a hot research topic because they have improved selectivity, sensitivity, and binding area. Graphene derivatives and clay minerals are the most promising materials because of their efficacy and lower cost. Nonetheless, large-scale production and commercialization are limited due to scale-up difficulties, high cost, and lack of long-term toxicity studies (Song and Qin, 2022).
Several recent papers investigated the potential of probiotics in AFs binding and, to a less extent, degradation, with regards to Lactobacillus spp., Lactocaseibacillus spp., Streptococcus spp., and Saccharomyces cerevisiae. The peptidoglycans and other cell wall polysaccharides are responsible of binding AFs. They show great potential even for a possible food application, considering their Qualified Presumption of Safety (QPS) status (Abdolmaleki et al., 2022).
Microbial detoxification of mycotoxins has been known since a very long time. The evidence that mycotoxins do not accumulate in agricultural soil and that can be reduced in some fermented foods prompted very productive research on microorganisms able to degrade mycotoxins and, consequently, on enzymes able to perform such degradation (Zhu et al., 2017). The isolation of microorganisms is often performed from contaminated environments, and followed by an enrichment step, in which the microorganism is grown in specific selective media which contain restricted carbon sources and a high level of mycotoxin contamination.
Microorganisms can reduce mycotoxin contamination by two different mechanisms, namely adsorption to cell wall, as discussed in Section “Atoxigenic Aspergillus strains,” and biotransformation. Biotransformation of AFs can be performed by different genera of filamentous fungi (Pleurotus, Armillariella, Armoracia, Trametes, Rhizopus, Trichoderma, Clonostachys, Cladosporium, Aspergillus), yeasts (Saccharomyces, Pichia, Candida, Kluyveromyces, Yarrowia, Rhodotorula, Rhodosporidium), and bacteria (Bacillus, Metschnikowia, Komagataella, Streptomyces, Rhodococcus, Pseudomonas, Pediococcus, Lactiplantibacillus, Lactiplantibacillus, Enterobacter, Cupriavidus, Brevibacterium) (Piotrowska, 2021; Nahle et al., 2022).
Currently, two commercial products based on living microorganisms have been released onto the market. A novel genus, formerly known as Eubacterium, belonging to the Coriobacteriaceae family (Biomin® BBSH® 797, DSM 11798) produces de-epoxidases able to detoxify trichothecenes, and Trichosporon mycotoxinivorans, a non-pathogenic yeast, produces peptidases able to detoxify ochratoxin A. However, so far, there is not any valid, commercially available microorganism-based solution to decontaminate AFs in feed.
Using whole microbial cells instead of the isolated enzyme has the advantage of being low-cost and potentially more effective. A single organism can produce enzymes able to degrade different toxins, or to perform multi step reactions. Indeed, as described by Heinl et al. (2010), fumonisin degradation can be performed by the consecutive action of a carboxylesterase and an aminotransferase. On the other hand, the production of degrading enzymes by microorganism may be difficult to standardize and to adjust to the level of contamination. Other enzymes and metabolites can be produced and their effect on the properties of the matrix might be difficult to predict. The only exception is represented by matrices specifically intended to be fermented, like silage, or by-products which can be fermented and used as ingredients in feeds, like spent grains or distilled dried grains with solubles (DGGS). For these reasons, the use of isolated enzymes represents a more convenient, though expensive option.
A wide variety of enzymes have been identified and characterized for their mycotoxin degrading capabilities in the last years (Loi et al., 2017; Wang et al., 2022).
So far only two enzyme-based commercial products have been released onto the European market. Fumzyme® by DSM is a fumonisin esterase capable of degrading fumonisin B1 to a non-toxic compound, which has been recently approved for use in all animal species (EFSA Panel on Additives, and Products or Substances used in Animal Feed (FEEDAP) et al., 2020; Regulation EU, 2021/363). ZENzyme® by DSM is a hydrolase able to detoxify zearalenone, recently approved for all terrestrial animal species (EFSA Panel on Additives, and Products or Substances used in Animal Feed (FEEDAP) et al., 2022).
There are no other successful examples, especially for AFs. Despite the many publications on AFs degradation by enzymes (Loi et al., 2016, 2020a; Yang et al., 2021), currently there are no commercial options onto the market. Most of the literature focuses on in vitro AFs degradation, lacks data on degradation products, and toxicity assessments.
Most AFs degrading enzymes reported in literature fall into the category of oxidoreductases, like laccases, peroxidase, or the so-called “AF oxidases” (Loi et al., 2017; Guan et al., 2021; Kumar et al., 2022). AF’s structure is highly stable and requires a strong oxidation to be degraded. These enzymes are incredibly versatile, yet unspecific. This complicates to study the mechanism, kinetics, degradation products, and toxicity of the compounds resulting from the reaction. AFs are metabolized in in vivo by endogenous oxidases, which activate AFB1 to the mutagenic 8,9-epoxyAFB1. This is performed by specific liver cytochromes P450 isoforms (CYP1A2, 2A6, and 2A13). Nonetheless, unspecific oxidation might also result in the generation of AFQ1, a less toxic compound, with other specific cytochrome isoforms (CYP3A4, 1A2, 3A7). Interestingly, it has been demonstrated that peroxidases and laccase-like enzymes can detoxify AFB1 to AFQ1 and do not activate the toxin to epoxide (Loi et al., 2020b; Qin et al., 2021). Other degradation mechanisms have been hypothesized, including the nucleophilic attack on the lactone and the furan rings, leading to their opening (Kumar et al., 2022). Although the enzymatic reduction has always been referred to be specific, this is not true for these enzymes. Their capability of degrading AFs relies on their high oxidative capacity, which is not restricted to AFs as substrates. Laccases and peroxidases also work in combination with redox mediators, which broaden even more the substrate spectrum of these enzymes. Indeed, when studied in protein and carbohydrate-rich foods, these enzymes have been shown to catalyze the formation of crosslinks and deeply modify the nutritional, technological, and rheological properties of foods (Isaschar-Ovdat and Fishman, 2018). In some cases, the modification resulted in an improvement of the nutritional (Loi et al., 2018) and technological properties (Loi et al., 2020a,b) of the food, therefore a careful case by case evaluation is necessary to effectively apply these enzymes.
Stability and activity in real matrices are challenges for the enzymes, and structure-based engineering has been shown to dramatically improve enzyme performance (Wang et al., 2022). The cost of enzyme production is another important hustle to overcome. Recombinant production is an effective strategy to increase production yield and lower costs.
The use of ultraviolet radiation (UV) proved to be an effective physical method to reduce contaminants and microorganisms through photochemical degradation and DNA damage, respectively (Sun et al., 2019). Ultraviolet radiation (UV) is a non-ionized radiation, with wavelength ranges between 100 and 400 nm. UV light spectrum is divided UV-A (315–400 nm), UV-B (280–315 nm), UV-C (200–280 nm), and UV-V (100–200 nm) (Rifna et al., 2019) and may be generated by solar radiation, UV lamps, or even Light Emitting Diodes (LEDs). LEDs show unique advantages, especially regarding the lack of radiant heat and can be easily applied in refrigerated storage (Loi et al., 2021). Due to its antibacterial properties, it finds application in the food industry to reduce the microbial load in air and water or on the surface of fresh products like fish, egg, chicken, liquid food, milk, fruit juices, or cider (Akhila et al., 2021). Two different mechanisms have been described in UV irradiation mediated degradation of contaminants: photolysis and photocatalysis. In the first case, degradation is due to the absorption of electromagnetic radiation, while in the latter, a photocatalyst (metal oxide, metal chalcogenide, or carbon-based material) is used to enhance degradation: TiO2 and ZnO are among the most used catalysts.
Photolysis occurs when reactant molecules absorb photons; electrons are excited to a high energy state, and when energy is released the chemical structure of the molecule is altered. The wavelength of UV radiation is inversely proportional to photon energy. Therefore, UV-C radiation has shown the most promising effects for microbial and mycotoxins decontamination (Shen and Singh, 2021).
UV light can also be given in a concentrated form, so that intense short bursts (pulses) have more penetration capacity. This technology is therefore called “pulsed UV.”
Aflatoxins are photosensitive and may be degraded by either photolysis or photocatalysis. AFB1 photocatalytic degradation is supposed to occur by direct oxidization of hydroxyl free radicals (•OH), H+, and other oxidative species generated by UV/photocatalyst. UV treatment leads to profound modifications in the chemical structure of AFB1, which preferentially start from the double bond on the bifuran moiety. Other degradation pathways involve the cycloaddition reaction at the furan ring and lactone ring, followed by further structure rearrangements (Sun et al., 2019; Murugesan et al., 2021; Song et al., 2022).
UV treatment has been employed for mycotoxins degradation in food commodities, such as oils, milk, wheat, and peanut. UV photocatalysis showed several limitations when applied to food, such as the oxidation of valuable nutritional components and low penetration in solids and turbid liquids (Shen and Singh, 2022). UV photolysis/photocatalysis is considered as an advanced oxidation method with significant advantages: no secondary pollution, easy and mild operational conditions, low cost, operability, and applicability in the food industry (Shen and Singh, 2022). For these reasons, it has been employed with success for the degradation of organic pollutants in the wastewater and air environment. Nonetheless, due to its oxidative nature, a careful evaluation of the detrimental effect on food in terms of sensory and nutritional profiles and toxin residues must be performed to achieve a satisfactory application. Scale-up of the technology for bulk-level applications, sustainability, and reusability of photocatalysts are important features that deserve further study (Magzoub et al., 2019).
Magnetic nanoparticles are small particles (less than 100 nm) made up of pure metals, metal alloys and metal oxides which have emerged as excellent adsorbents, due to their unique structural advantages, large surface area, tunable surface functionalities, and easy recovery with external magnetic fields (Horky et al., 2018).
Iron and zinc oxides, silver, copper, or selenium nanoparticles are gaining massive attention in mycotoxin research because of their effective binding capacity in agricultural feedstuff and foods. Nanoparticles can be functionalized to enhance mycotoxin binding capacity, to provide binding affinity towards various types of mycotoxins, or even to immobilize enzymes, cells (Duishemambet Kyzy et al., 2022) or build magnetic-propelled yeast cell robots (Lu et al., 2021) able to reduce mycotoxin contamination. AFB1 degradation has been studied using iron oxide nanoparticles in vitro and in edible oils magnetic graphene composite. The main issue for the practical use such material is the gap in the toxicity evaluation and in data collection to set safety limits (Malhotra et al., 2020).
Plasma is an ionized gas that generates several reactive charged and neutral species, including photons, positive and negative ions, and oxygen and nitrogen reactive species (Mandal et al., 2018). It can be divided into thermal and non-thermal (cold) plasma, depending on the type of gas generation methods, and working temperature. Cold plasma works at around room temperature (30–60°C), and for this reason, it finds multiple applications in sterilization, inactivation, decontamination, and disinfection in the food industry. The reactive species generated by the cold plasma are highly active oxidants.
The capability of cold plasma to inactivate fungal growth and mycotoxin production has been well documented. Nonetheless, recently some studies have also investigated the capability of degrading mycotoxins (Hojnik et al., 2021; Wu et al., 2021). The degradation mechanism has been recently unraveled: oxidative degradation of AFB1 occurred via the electrophilic addition of water on C8 and formation of AFB2a or H-atom abstraction from the bonds C–H at the 8 and 9 positions. These reactions lead to the opening of the lactone and terminal furan ring; these unstable intermediates undergo further degradation (Hojnik et al., 2021; Li et al., 2022).
Plasma technology is at its very first beginning, and there are substantial limitations to its concrete application. Suitable plasma equipment is still at the laboratory stage and mainly designed for other applications besides food. The process still needs standardization and improvement to overcome the low penetration capacity (Wu et al., 2021). Finally, the effects on food matrices have been poorly studied, and further research is needed to propose this technology as an AF decontamination method in food and feed.
Nanozymes are inorganic nanoparticles with enzyme-like properties in redox reactions. They combine the properties of nanomaterials and oxidases in a more stable and efficient system. Nanozymes with laccase and peroxidase-like activities were developed for contaminant removal, including AFB1 (Zhang et al., 2020; Guo et al., 2021; Wei et al., 2022). Nanozyme show also adsorptive characteristics due to the hierarchical porous structure (Wu et al., 2020; Ma et al., 2021; Pérez-Gómez et al., 2022) and can combine filtration, adsorption, and catalysis in a multifunctional removal process (Ren et al., 2019). These studies show that these innovative materials possess high efficiency (up to 96%), stability, and reusability (up to 5 cycles). Few authors investigated the effectiveness on real matrices (Ma et al., 2021; Wei et al., 2022) and the toxicity of both nanozyme and degradation products (Wei et al., 2022). These data are promising, as high removal rates were obtained in vegetable oils, with little impact on their quality. In the study conducted by Wei et al. (2022), the stability of the metal part was assessed to verify that no metal component leaked into the food.
Even though preliminary LC–MS/MS data suggest that less toxic compounds could be generated by nanozyme catalysis, additional research is needed to further characterize these products and confirm that they are less toxic. On the other hand, there is no data on their use in solid, low-water content materials like grains and nuts, which would represent the most important and useful application for AFs removal.
Despite the technical obstacles, there are several steps which must be considered for a successful development of a new post-harvest reduction method.
A mandatory prerequisite to research for a new product and invest in its commercial application is that there is a sufficient need, an end-user, and an economic benefit for both the producer and the final customer. Even if aflatoxin threat is per se a sufficient reason to motivate the research and development of new post-harvest reduction methods, the low perceived risk of incurring aflatoxicosis, the low cost of the raw material and the possibility to downgrade or divert the material to countries with less stringent regulation, might slow the process of developing new postharvest methods. A close partnership between research and companies is mandatory to overcome technical and economical gaps between research and practical application.
So far, the cost-effectiveness and technology readiness level (TRL) of innovative postharvest methods are weak points (Marshall et al., 2020). Some of the innovative technologies discussed in this review might be already in use and commercially available for different applications; nonetheless, they have low TRLs (3–5) if we consider mycotoxin reduction application (Marshall et al., 2020).
Large-scale production and the impact of such methods in actual application must be assessed (Ortega-Beltran and Bandyopadhyay, 2021). Another critical point is that regulatory approval through a dossier submission to EFSA must be obtained. Nonetheless, the biggest obstacle is still represented by the regulatory gap that hinders the application of such methods to food matrices.
AFs contamination is becoming an emerging risk in European countries and requires the implementation of novel post-harvest methods. Due to climate change and growing populations, environmental factors affecting mycotoxins production are also altering. This leads to a change in the distribution and growth of fungi in the soil which is difficult to predict and prevent.
AFs are among the most difficult toxin to degrade, and effective degradation only occurs when strong oxidants are used, irrespectively of their nature (physical or biological methods) and origin. Adsorption, on the other hand, can be used for AFs removal and commercial solutions exist for feed use. The toxicological impact and detrimental effects on food matrices still need to be deeply investigated for new technologies.
Adopting an integrated pre- and post-harvest approach, possibly using different reduction techniques remains the most effective way to counteract AF risk. None of the strategies can completely prevent or reduce AFs contamination or can be used as a general all-purpose decontamination method. Future research is needed to overcome gaps and limitations, possibly in close connection with industries and other stakeholders, to finally apply these novel methods at the industrial level.
AL and IP contributed to the conception and design of the paper. All authors wrote the first draft and contributed to the manuscript revision, read, and approved the submitted version.
This work was supported by Project no. 2018-1.2.1-NKP-2018-00002 which has been implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the 2018-1.2.1-NKP funding scheme, by Project no. TKP2021-EGA-20 810 (Biotechnology), which has been implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the TKP2021-EGA funding scheme, by the National Research, Development and Innovation Office of Hungary with the grant K142801. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 952337.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbas, H. K., Accinelli, C., and Shier, W. T. (2017). Biological control of aflatoxin contamination in US crops and the use of bioplastic formulations of Aspergillus flavus biocontrol strains to optimize application strategies. J. Agric. Food Chem. 65, 7081–7087. doi: 10.1021/acs.jafc.7b01452
Abdel-Hadi, A. M., Caley, D. P., Carter, D. R. F., and Magan, N. (2011). Control of aflatoxin production of Aspergillus flavus and Aspergillus parasiticus using RNA silencing technology by targeting aflD (nor-1) gene. Toxins 3, 647–659. doi: 10.3390/toxins3060647
Abdel-Hadi, A., Carter, D., and Magan, N. (2010). Temporal monitoring of the nor-1 (aflD) gene of Aspergillus flavus in relation to aflatoxin B1 production during storage of peanuts under different water activity levels. J. Appl. Microbiol. 109, 1914–1922. doi: 10.1111/j.1365-2672.2010.04820.x
Abdel-Hadi, A., Schmidt-Heydt, M., Parra, R., Geisen, R., and Magan, N. (2012). A systems approach to model the relationship between aflatoxin gene cluster expression, environmental factors, growth and toxin production by Aspergillus flavus. J. R. Soc. Interface 9, 757–767. doi: 10.1098/rsif.2011.0482
Abdolmaleki, K., Javanmardi, F., Gavahian, M., Phimolsiripol, Y., Ruksiriwanich, W., Mir, S. A., et al. (2022). Emerging technologies in combination with probiotics for aflatoxins removal: an updated review. Int. J. Food Sci. 57, 5712–5721. doi: 10.1111/ijfs.15926
Adhikari, B. N., Bandyopadhyay, R., and Cotty, P. J. (2016). Degeneration of aflatoxin gene clusters in Aspergillus flavus from Africa and North America. AMB Express 6, 62–16. doi: 10.1186/s13568-016-0228-6
Agbetiameh, D., Ortega-Beltran, A., Awuah, R. T., Atehnkeng, J., Islam, M. S., Callicott, K. A., et al. (2019). Potential of atoxigenic Aspergillus flavus vegetative compatibility groups associated with maize and groundnut in Ghana as biocontrol agents for aflatoxin management. Front. Microbiol. 10:2069. doi: 10.3389/fmicb.2019.02069
Akhila, P. P., Sunooj, K. V., Aaliya, B., Navaf, M., Sudheesh, C., Sabu, S., et al. (2021). Application of electromagnetic radiations for decontamination of fungi and mycotoxins in food products: A comprehensive review. Trends in Food Science & Technology 114, 399–409. doi: 10.1016/j.tifs.2021.06.013
Amaike, S., and Keller, N. P. (2011). Aspergillus flavus. Annu. Rev. Phytopathol. 49, 107–133. doi: 10.1146/annurev-phyto-072910-095221
Amare, M. G., and Keller, N. P. (2014). Molecular mechanisms of Aspergillus flavus secondary metabolism and development. Fungal Genet. Biol. 66, 11–18. doi: 10.1016/j.fgb.2014.02.008
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Arias, R. S., Dang, P. M., and Sobolev, V. S. (2015). RNAi-mediated control of aflatoxins in peanut: method to analyze mycotoxin production and transgene expression in the peanut/Aspergillus pathosystem. JoVE 106:e53398. doi: 10.3791/53398
Bai, Y., Wang, S., Zhong, H., Yang, Q., Zhang, F., Zhuang, Z., et al. (2015). Integrative analyses reveal transcriptome-proteome correlation in biological pathways and secondary metabolism clusters in A. flavus in response to temperature. Sci. Rep. 5:14582.
Baidya, S., Duran, R. M., Lohmar, J. M., Harris-Coward, P. Y., Cary, J. W., Hong, S. Y., et al. (2014). VeA is associated with the response to oxidative stress in the aflatoxin producer Aspergillus flavus. Eukaryot. Cell 13, 1095–1103. doi: 10.1128/EC.00099-14
Bandyopadhyay, R., Ortega-Beltran, A., Akane, A., Mutegi, C., Atehnkeng, J., Kaptoge, L., et al. (2016). Biological control of aflatoxins in Africa: current status and potential challenges in the face of climate change. World Mycotoxin J. 9, 771–789. doi: 10.3920/WMJ2016.2130
Battilani, P., Toscano, P., der Fels-Klerx, V., Moretti, A., Camardo Leggieri, M., Brera, C., et al. (2016). Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 6, 1–7. doi: 10.1038/srep24328(2016)
Bayram, Ö., and Braus, G. H. (2012). Coordination of secondary metabolism and development in fungi: the velvet family of regulatory proteins. FEMS Microbiol. Rev. 36, 1–24. doi: 10.1111/j.1574-6976.2011.00285.x
Boudergue, C., Burel, C., Dragacci, S., Favrot, M. C., Fremy, J. M., Massimi, C., et al. (2019). Review of mycotoxin-detoxifying agents used as feed additives: mode of action, efficacy and feed/food safety. EFSA Support. Publ. 6:22E. doi: 10.2903/sp.efsa.2009.EN-22
Buchanan, R. L., and Ayres, J. C. (1977). Effect of various glycolytic and TCA intermediates on aflatoxin production. J. Food Saf. 1, 19–28. doi: 10.1111/j.1745-4565.1977.tb00256.x
Caceres, I., El Khoury, R., Bailly, S., Oswald, I. P., Puel, O., and Bailly, J. D. (2017). Piperine inhibits aflatoxin B1 production in Aspergillus flavus by modulating fungal oxidative stress response. Fungal Genet. Biol. 107, 77–85. doi: 10.1016/j.fgb.2017.08.005
Caceres, I., Khoury, A. A., Khoury, R. E., Lorber, S., Oswald, I. P., Khoury, A. E., et al. (2020). Aflatoxin biosynthesis and genetic regulation: a review. Toxins 12:150. doi: 10.3390/toxins12030150
Calistru, C., McLean, M., and Berjak, P. (1997). In vitro studies on the potential for biological control of Aspergillus flavus and Fusarium moniliforme by Trichoderma species. Mycopathologia 137, 115–124. doi: 10.1023/A:1006802423729
Calvo, A. M. (2008). The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet. Biol. 45, 1053–1061.
Calvo, A. M., Bok, J. W., Brooks, W., and Keller, N. P. (2004). Vea is required for toxin and sclerotial production in Aspergillus parasiticus. Appl. Environ. Microbiol. 70, 4733–4739. doi: 10.1128/AEM.70.8.4733-4739.2004
Calvo, A. M., Wilson, R. A., Bok, J. W., and Keller, N. P. (2002). Relationship between secondary metabolism and fungal development. MMBR 66, 447–459. doi: 10.1128/MMBR.66.3.447-459.2002
Camardo Leggieri, M., Lanubile, A., DallAsta, C., Pietri, A., and Battilani, P. (2020). The impact of seasonal weather variation on mycotoxins: maize crop in 2014 in northern Italy as a case study. World Mycotoxin J. 13, 25–36. doi: 10.3920/WMJ2019.2475
Cary, J. W., Entwistle, S., Satterlee, T., Mack, B. M., Gilbert, M. K., Chang, P. K., et al. (2019). The transcriptional regulator Hbx1 affects the expression of thousands of genes in the aflatoxin-producing fungus Aspergillus flavus. G3: Genes Genom. Genet. 9, 167–178. doi: 10.1534/g3.118.200870.Doi:10.1534/g3.118.200870
Cary, J. W., Harris-Coward, P. Y., Ehrlich, K. C., Mack, B. M., Kale, S. P., Larey, C., et al. (2012). NsdC and NsdD affect Aspergillus flavus morphogenesis and aflatoxin production. Eukaryot. Cell 11, 1104–1111. doi: 10.1128/EC.00069-12
Chanda, A., Roze, L. V., and Linz, J. E. (2010). A possible role for exocytosis in aflatoxin export in Aspergillus parasiticus. Eukaryot. Cell 9:1727. doi: 10.1128/EC.00118-10
Chang, P. K. (2022). Aspergillus flavus La3279, a component strain of the Aflasafe™ biocontrol product, contains a partial aflatoxin biosynthesis gene cluster followed by a genomic region highly variable among A. flavus isolates. Int. J. Food Microbiol. 366:109559. doi: 10.1016/j.ijfoodmicro.2022.109559
Chang, P. K., Abbas, H. K., Weaver, M. A., Ehrlich, K. C., Scharfenstein, L. L., and Cotty, P. J. (2012a). Identification of genetic defects in the atoxigenic biocontrol strain Aspergillus flavus K49 reveals the presence of a competitive recombinant group in field populations. Int. J. Food Microbiol. 154, 192–196. doi: 10.1016/j.ijfoodmicro.2012.01.005
Chang, P.-K., Ehrlich, K. C., Yu, J., Bhatnagar, D., and Cleveland, T. E. (1995). Increased expression of Aspergillus parasiticus aflR, encoding a sequence- specific DNA-binding protein, relieves nitrate inhibition of aflatoxin biosynthesis. Appl. Environ. Microbiol. 61, 2372–2377. doi: 10.1128/aem.61.6.2372-2377.1995
Chang, P. K., Scharfenstein, L. L., Ehrlich, K. C., Wei, Q., Bhatnagar, D., and Ingber, B. F. (2012b). Effects of laeA deletion on Aspergillus flavus conidial development and hydrophobicity may contribute to loss of aflatoxin production. Fungal Biol. 116, 298–307. doi: 10.1016/j.funbio.2011.12.003
Chang, P. K., Yu, J., and Yu, J. H. (2004). aflT, a MFS transporter-encoding gene located in the aflatoxin gene cluster, does not have a significant role in aflatoxin secretion. Fungal Genet. Biol. 41, 911–920. doi: 10.1016/j.fgb.2004.06.007
Čolović, R., Puvača, N., Cheli, F., Avantaggiato, G., Greco, D., Đuragić, O., et al. (2019). Decontamination of mycotoxin-contaminated feedstuffs and compound feed. Toxins 11:617. doi: 10.3390/toxins11110617
Cruz de Carvalho, M. H. (2008). Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal. Behav. 3, 156–165. doi: 10.4161/psb.3.3.5536
Deng, J. J., Huang, W. Q., Li, Z. W., Lu, D. L., Zhang, Y., and Luo, X. C. (2018). Biocontrol activity of recombinant aspartic protease from Trichoderma harzianum against pathogenic fungi. Enzyme and microbial technology 112, 35–42. doi: 10.1016/j.enzmictec.2018.02.002
Di Canito, A., Mateo-Vargas, M. A., Mazzieri, M., Cantoral, J., Foschino, R., Cordero-Bueso, G., et al. (2021). The role of yeasts as biocontrol agents for pathogenic fungi on postharvest grapes: a review. Foods 10:1650. doi: 10.3390/foods10071650
Dini, I., Alborino, V., Lanzuise, S., Lombardi, N., Marra, R., Balestrieri, A., et al. (2022). Trichoderma enzymes for degradation of aflatoxin B1 and ochratoxin A. Molecules 27:3959. doi: 10.3390/molecules27123959
Dövényi-Nagy, T., Rácz, C., Molnár, K., Bakó, K., Szláma, Z., Jóźwiak, Á., et al. (2020). Pre-harvest modelling and mitigation of aflatoxins in maize in a changing climatic environment—a review. Toxins 12:768. doi: 10.3390/toxins12120768
Drott, M. T., Satterlee, T. R., Skerker, J. M., Pfannenstiel, B. T., Glass, N. L., Keller, N. P., et al. (2020). The frequency of sex: population genomics reveals differences in recombination and population structure of the aflatoxin-producing fungus Aspergillus flavus. MBio 11, e00963–e00920. doi: 10.1128/mBio.00963-20
Duishemambet Kyzy, A., Kocyigit, Y., and Ardag, A. H. (2022). Aflatoxin B1 bioremoval by fungal cells immobilised on magnetic nanoparticles. J. Environ. Anal. Chem., 1–14. doi: 10.1080/03067319.2022.2115897
Duran, R. M., Cary, J. W., and Calvo, A. M. (2007). Production of cyclopiazonic acid, aflatrem, and aflatoxin by Aspergillus flavus is regulated by veA, a gene necessary for sclerotial formation. Appl. Microbiol. Biotechnol. 73, 1158–1168. doi: 10.1007/s00253-006-0581-5
Dutton, M. F. (1988). Enzymes and aflatoxin biosynthesis. Microbiol. Rev. 52, 274–295. doi: 10.1128/mr.52.2.274-295.1988
EFSA (European Food Safety Authority)Alvarez, F., Arena, M., Auteri, D., Binaglia, M. C. A., Chiusolo, A., et al. (2022). Conclusion on the peer review of the pesticide risk assessment of the active substance Aspergillus flavus strain MUCL54911. EFSA J. 20:7202:21. doi: 10.2903/j.efsa.2022.7202
EFSA Panel on Additives, and Products or Substances used in Animal Feed (FEEDAP)Bampidis, V., Azimonti, G., Bastos, M. D. L., Christensen, H., Dusemund, B., et al. (2020). Safety and efficacy of fumonisin esterase from Komagataella phaffii DSM 32159 as a feed additive for all animal species. EFSA J. 18:e06207. doi: 10.2903/j.efsa.2018.5269
EFSA Panel on Additives, Products or Substances used in Animal Feed (FEEDAP)Bampidis, V., Azimonti, G., MDL, B., Christensen, H., Dusemund, B., et al. (2022). Safety and efficacy of a feed additive consisting of zearalenone hydrolase produced by Escherichia coli DSM 32731 for all terrestrial animal species (Biomin GmbH). EFSA J. 20:e07157. doi: 10.2903/j.efsa.2022.7157
Ehrlich, K. C., Cary, J. W., and Montalbano, B. G. (1999). Characterization of the promoter for the gene encoding the aflatoxin biosynthetic pathway regulatory protein AflR. Biochim. Biophys. Acta Gene Struct. Expr. 1444, 412–417. doi: 10.1016/s0167-4781(99)00022-6
Einolghozati, M., Talebi-Ghane, E., Ranjbar, A., and Mehri, F. (2021). Concentration of aflatoxins in edible vegetable oils: a systematic meta-analysis review. Eur. Food Res. Technol. 247, 2887–2897. doi: 10.1007/s00217-021-03844-5
Eom, T. J., Moon, H., Yu, J. H., and Park, H. S. (2018). Characterization of the velvet regulators in Aspergillus flavus. J. Microbiol. 56, 893–901. doi: 10.1007/s12275-018-8417-4
Eskola, M., Kos, G., Elliott, C. T., Hajšlová, J., Mayar, S., and Krska, R. (2020). Worldwide contamination of food-crops with mycotoxins: validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 60, 2773–2789. doi: 10.1080/10408398.2019.1658570
European Commission (2003). Regulation (EC) No 1831/2003 of the European Parliament and of the council of 22 September 2003 on additives for use in animal nutrition.
European Commission (2006). Commission Regulation (EC) No. 1881/2006 of 19 December 2006 Setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24.
European Commission (2007). Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52007DC0354&from=EN
European Commission (2010). Commission Regulation (EU) No. 165/2010 of 26 February 2010 Amending regulation (EC) no 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off. J. Eur. Union 2010, 50, 8–12.
European Commission (2015). Commission regulation (EU) 2015/786 of 19 May 2015 defining acceptability criteria for detoxification processes applied to products intended for animal feed as provided for in directive 2002/32/EC of the European Parliament and of the council.
FAO (2022). Crop prospects and food situation. Available at: https://www.fao.org/3/cc0868en/cc0868en.pdf [Accessed September 19, 2022].
FAOstat (2022). Available at: https://www.fao.org/faostat/en/ [Accessed November 26, 2022].
FAOstat (2013). Available at: https://www.fao.org/3/i3107e/i3107e.PDF
Farkas, Z., Országh, E., Engelhardt, T., Csorba, S., Kerekes, K., Zentai, A., et al. (2022). A systematic review of the efficacy of interventions to control aflatoxins in the dairy production chain—feed production and animal feeding interventions. Toxins 14:115. doi: 10.3390/toxins14020115
Fasoyin, O. E., Wang, B., Qiu, M., Han, X., Chung, K. R., and Wang, S. (2018). Carbon catabolite repression gene creA regulates morphology, aflatoxin biosynthesis and virulence in Aspergillus flavus. Fungal Genet. Biol. 115, 41–51. doi: 10.1016/j.fgb.2018.04.008
Fasoyin, O. E., Yang, K., Qiu, M., Wang, B., Wang, S., and Wang, S. (2019). Regulation of morphology, aflatoxin production, and virulence of Aspergillus flavus by the major nitrogen regulatory gene areA. Toxins 11:718. doi: 10.3390/toxins11120718
Faustinelli, P. C., Power, I. L., and Arias, R. S. (2018). Detection of exogenous double-stranded RNA movement in in vitro peanut plants. Plant Biol. 20, 444–449. doi: 10.1111/plb.12703
Ferrara, M., Perrone, G., and Gallo, A. (2022). Recent advances in biosynthesis and regulatory mechanisms of principal mycotoxins. Curr. Opin. Food Sci. doi: 10.1016/j.cofs.2022.100923
Finotti, E., Parroni, A., Zaccaria, M., Domin, M., Momeni, B., Fanelli, C., et al. (2021). Aflatoxins are natural scavengers of reactive oxygen species. Sci. Rep. 11, 1–9. doi: 10.1038/s41598-021-95325-8
Fountain, J. C., Scully, B. T., Ni, X., Kemerait, R. C., Lee, R. D., Chen, Z. Y., et al. (2014). Environmental influences on maize-Aspergillus flavus interactions and aflatoxin production. Front. Microbiol. 5:40. doi: 10.3389/fmicb.2014.00040
Furukawa, T., and Sakuda, S. (2019). Inhibition of aflatoxin production by paraquat and external superoxide dismutase in Aspergillus flavus. Toxins 11:107. doi: 10.3390/toxins11020107
Gamal, M., Abou Zaid, M., Abou Mourad, I. K., Abd El Kareem, H., and Gomaa, O. M. (2022). Trichoderma viride bioactive peptaibol induces apoptosis in Aspergillus niger infecting tilapia in fish farms. Aquaculture 547:737474. doi: 10.1016/j.aquaculture.2021.737474
Georgianna, D. R., and Payne, G. A. (2009). Genetic regulation of aflatoxin biosynthesis: from gene to genome. Fungal Genet. Biol. 46, 113–125. doi: 10.1016/j.fgb.2008.10.011
Gilbert, M. K., Majumdar, R., Rajasekaran, K., Chen, Z. Y., Wei, Q., Sickler, C. M., et al. (2018). RNA interference-based silencing of the alpha-amylase (amy1) gene in Aspergillus flavus decreases fungal growth and aflatoxin production in maize kernels. Planta 247, 1465–1473. doi: 10.1007/s00425-018-2875-0
Gil-Serna, J., Vázquez, C., and Patiño, B. (2020). Genetic regulation of aflatoxin, ochratoxin a, trichothecene, and fumonisin biosynthesis: a review. Int. Microbiol. 23, 89–96. doi: 10.1007/s10123-019-00084-2
Gressel, J., and Polturak, G. (2018). Suppressing aflatoxin biosynthesis is not a breakthrough if not useful. Pest Manag. Sci. 74, 17–21. doi: 10.1002/ps.4694
Guan, Y., Chen, J., Nepovimova, E., Long, M., Wu, W., and Kuca, K. (2021). Aflatoxin detoxification using microorganisms and enzymes. Toxins 13:46. doi: 10.1080/10408398.2021.2010647
Guan, X., Zhao, Y., Liu, X., Shang, B., Xing, F., Zhou, L., et al. (2019). The bZIP transcription factor Afap1 mediates the oxidative stress response and aflatoxin biosynthesis in Aspergillus flavus. Rev. Argent. Microbiol. 51, 292–301. doi: 10.1016/j.ram.2018.07.003
Guo, Y., Zhao, L., Ma, Q., and Ji, C. (2021). Novel strategies for degradation of aflatoxins in food and feed: a review. Food Res. Int. 140:109878. doi: 10.1016/j.foodres.2020.109878
Heinl, S., Hartinger, D., Thamhesl, M., Vekiru, E., Krska, R., Schatzmayr, G., et al. (2010). Degradation of fumonisin B1 by the consecutive action of two bacterial enzymes. J. Biotechnol. 145, 120–129. doi: 10.1016/j.jbiotec.2009.11.004
Hellegers, P. (2022). Food security vulnerability due to trade dependencies on Russia and Ukraine. Food Secur., 1–8. doi: 10.1007/s12571-022-01306-8
Hojnik, N., Modic, M., Walsh, J. L., Žigon, D., Javornik, U., Plavec, J., et al. (2021). Unravelling the pathways of air plasma induced aflatoxin B1 degradation and detoxification. J. Hazard. Mater. 403:123593. doi: 10.1016/j.jhazmat.2020.123593
Hong, S. Y., Roze, L. V., and Linz, J. E. (2013a). Oxidative stress-related transcription factors in the regulation of secondary metabolism. Toxins 5, 683–702. doi: 10.3390/toxins5040683
Hong, S. Y., Roze, L. V., Wee, J., and Linz, J. E. (2013b). Evidence that a transcription factor regulatory network coordinates oxidative stress response and secondary metabolism in Aspergilli. Microbiol. Open 2, 144–160. doi: 10.1002/mbo3.63
Horky, P., Skalickova, S., Baholet, D., and Skladanka, J. (2018). Nanoparticles as a solution for eliminating the risk of mycotoxins. Nano 8:727. doi: 10.3390/nano8090727
Hua, S. S. T., Beck, J. J., Sarreal, S. B. L., and Gee, W. (2014). The major volatile compound 2-phenylethanol from the biocontrol yeast, Pichia anomala, inhibits growth and expression of aflatoxin biosynthetic genes of Aspergillus flavus. Mycotoxin Res. 30, 71–78. doi: 10.1007/s12550-014-0189-z
International Agency for Research on Cancer (2002). Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. Available at: http://monographs.iarc.fr/ENG/Monographs/vol82/volume82.pdf
IPCC (2022). Climate change 2022: impacts, adaptation and vulnerability. Contribution of working group II to the sixth assessment report of the intergovernmental panel on climate change. eds. H.-O. Pörtner, D. C. Roberts, M. Tignor, E. S. Poloczanska, K. Mintenbeck, and A. Alegría, et al. (Cambridge, UK and New York, NY, United States: Cambridge University Press), 3056.
Isaschar-Ovdat, S., and Fishman, A. (2018). Crosslinking of food proteins mediated by oxidative enzymes–a review. Trends Food Sci. Technol. 72, 134–143. doi: 10.1016/j.tifs.2017.12.011
Jayashree, T., and Subramanyam, C. (2000). Oxidative stress as a prerequisite for aflatoxin production by Aspergillus parasiticus. Free Radic. Biol. Med. 29, 981–985. doi: 10.1016/s0891-5849(00)00398-1
Keller, N. P. (2015). Translating biosynthetic gene clusters into fungal armor and weaponry. Nat. Chem. Biol. 11, 671–677. doi: 10.1038/nchembio.1897.
Keller, N. P., Nesbitt, C., Sarr, B., Phillips, T. D., and Burow, G. B. (1997). pH regulation of sterigmatocystin and aflatoxin biosynthesis in Aspergillus spp. Phytopathology 87, 643–648. doi: 10.1094/PHYTO.1997.87.6.643
Khan, R., Ghazali, F. M., Mahyudin, N. A., and Samsudin, N. I. P. (2021). Aflatoxin biosynthesis, genetic regulation, toxicity, and control strategies: a review. J. Fungi 7:606. doi: 10.3390/jof7080606
King, T., Cole, M., Farber, J. M., Eisenbrand, G., Zabaras, D., Fox, E. M., et al. (2017). Food safety for food security: relationship between global megatrends and developments in food safety. Trends Food Sci. Technol. 68, 160–175. doi: 10.1016/j.tifs.2017.08.014
Kocsis, B., Lee, M. K., Yu, J. H., Nagy, T., Daróczi, L., Batta, G., et al. (2022). Functional analysis of the bZIP-type transcription factors AtfA and AtfB in Aspergillus nidulans. Front. Microbiol. 13:1003709. doi: 10.3389/fmicb.2022.1003709
Kolawole, O., Meneely, J., Greer, B., Chevallier, O., Jones, D. S., Connolly, L., et al. (2019). Comparative in vitro assessment of a range of commercial feed additives with multiple mycotoxin binding claims. Toxins 11:659. doi: 10.3390/toxins11110659
Kong, Q., Chi, C., Yu, J., Shan, S., Li, Q., Guan, B., et al. (2014). The inhibitory effect of Bacillus megaterium on aflatoxin and cyclopiazonic acid biosynthetic pathway gene expression in Aspergillus flavus. Appl. Environ. Microbiol. 98, 5161–5172. doi: 10.1007/s00253-014-5632-8
Kos, J., Janić Hajnal, E., Šarić, B., Jovanov, P., Mandić, A., Đuragić, O., et al. (2018). Aflatoxins in maize harvested in the Republic of Serbia over the period 2012–2016. Food Addit. Contam. Part B 11, 246–255. doi: 10.1080/19393210.2018.1499675
Kotta-Loizou, I., and Coutts, R. H. A. (2017). Mycoviruses in aspergilli: a comprehensive review. Front. Microbiol. 8:1699. doi: 10.3389/fmicb.2017.01699
Kumar, V., Bahuguna, A., Ramalingam, S., Dhakal, G., Shim, J. J., and Kim, M. (2022). Recent technological advances in mechanism, toxicity, and food perspectives of enzyme-mediated aflatoxin degradation. Crit. Rev. Food Sci. Nutr. 62, 5395–5412. doi: 10.1080/10408398.2021.2010647
Kumar, P., Mahato, D. K., Kamle, M., Mohanta, T. K., and Kang, S. G. (2017). Aflatoxins: a global concern for food safety, human health and their management. Front. Microbiol. 7:2170. doi: 10.3389/fmicb.2016.02170
Lewis, M. H., Carbone, I., Luis, J. M., Payne, G. A., Bowen, K. L., Hagan, A. K., et al. (2019). Biocontrol strains differentially shift the genetic structure of indigenous soil populations of Aspergillus flavus. Front. Microbiol. 10:1738. doi: 10.3389/fmicb.2019.01738
Li, S., Yao, X., Wang, X., Tian, S., and Zhang, Y. (2022). Reactive molecular dynamics simulation on degradation of aflatoxin B1 by cold atmospheric plasmas. IFSET 80:103101. doi: 10.1016/j.ifset.2022.103101
Lind, A. L., Smith, T. D., Saterlee, T., Calvo, A. M., and Rokas, A. (2016). Regulation of secondary metabolism by the velvet complex is temperature-responsive in Aspergillus. G3 Genes Genom. Genet. 6, 4023–4033. doi: 10.1128/aem.64.10.3718-3723.1998
Liu, B. H., and Chu, F. S. (1998). Regulation of aflR and its product, AflR, associated with aflatoxin biosynthesis. Appl. Environ. Microbiol. 64, 3718–3723. doi: 10.1128/aem.64.10.3718-3723.1998
Loi, M., Fanelli, F., Liuzzi, V. C., Logrieco, A. F., and Mulè, G. (2017). Mycotoxin biotransformation by native and commercial enzymes: present and future perspectives. Toxins 9:111. doi: 10.3390/toxins9040111
Loi, M., Fanelli, F., Zucca, P., Liuzzi, V. C., Quintieri, L., Cimmarusti, M. T., et al. (2016). Aflatoxin B1 and M1 degradation by Lac2 from Pleurotus pulmonarius and redox mediators. Toxins 8:245. doi: 10.3390/toxins8090245
Loi, M., Quintieri, L., De Angelis, E., Monaci, L., Logrieco, A. F., Caputo, L., et al. (2020a). Yield improvement of the Italian fresh Giuncata cheese by laccase–induced protein crosslink. Int. Dairy J. doi: 10.1016/j.idairyj.2019.104555
Loi, M., Quintieri, L., Fanelli, F., Caputo, L., and Mulè, G. (2018). Application of a recombinant laccase-chlorogenic acid system in protein crosslink and antioxidant properties of the curd. Food Res. Int. 106, 763–770. doi: 10.1016/j.foodres.2018.01.050
Loi, M., Renaud, J. B., Rosini, E., Pollegioni, L., Vignali, E., Haidukowski, M., et al. (2020b). Enzymatic transformation of aflatoxin B1 by Rh_DypB peroxidase and characterization of the reaction products. Chemosphere 250:126296. doi: 10.1016/j.chemosphere.2020.126296
Loi, M., Villani, A., Paciolla, F., Mulè, G., and Paciolla, C. (2021). Challenges and Opportunities of Light-Emitting Diode (LED) as Key to Modulate Antioxidant Compounds in Plants. A Review. Antioxidants 10:42. doi: 10.3390/antiox10010042
Lu, D., Tang, S., Li, Y., Cong, Z., Zhang, X., and Wu, S. (2021). Magnetic-propelled Janus yeast cell robots functionalized with metal-organic frameworks for mycotoxin decontamination. Micromachines 12:797. doi: 10.3390/mi12070797
Lv, C., Jin, J., Wang, P., Dai, X., Liu, Y., Zheng, M., et al. (2019). Interaction of water activity and temperature on the growth, gene expression and aflatoxin production by Aspergillus flavus on paddy and polished rice. Food Chem. 293, 472–478. doi: 10.1016/j.foodchem.2019.05.009
Ma, D. (2019). “Hybrid nanoparticles: an introduction,” in Noble metal-metal oxide hybrid nanoparticles (Duxford CB22 4QH, United Kindom: Woodhead Publishing), 3–6.
Ma, F., Cai, X., Mao, J., Yu, L., and Li, P. (2021). Adsorptive removal of aflatoxin B1 from vegetable oils via novel adsorbents derived from a metal-organic framework. J. Hazard. Mater. 412:125170. doi: 10.1016/j.jhazmat.2021.125170
Magzoub, R. A. M., Yassin, A. A. A., Abdel-Rahim, A. M., Gubartallah, E. A., Miskam, M., Saad, B., et al. (2019). Photocatalytic detoxification of aflatoxins in Sudanese peanut oil using immobilized titanium dioxide. Food Control 95, 206–214. doi: 10.1016/j.foodcont.2018.08.009
Mahoney, N., and Molyneux, R. J. (2004). Phytochemical inhibition of aflatoxigenicity in Aspergillus flavus by constituents of walnut (Juglans regia). J. Agric. Food Chem. 52, 1882–1889. doi: 10.1021/jf030812p
Majumdar, R., Lebar, M., Mack, B., Minocha, R., Minocha, S., Carter-Wientjes, C., et al. (2018). The Aspergillus flavus spermidine synthase (spds) gene, is required for normal development, aflatoxin production, and pathogenesis during infection of maize kernels. Front. Plant Sci. 9:317. doi: 10.3389/fpls.2018.00317
Majumdar, R., Rajasekaran, K., and Cary, J. W. (2017). RNA interference (RNAi) as a potential tool for control of mycotoxin contamination in crop plants: concepts and considerations. Front. Plant Sci. 8:200. doi: 10.3389/fpls.2017.00200
Malhotra, N., Lee, J. S., Liman, R. A. D., Ruallo, J. M. S., Villaflores, O. B., Ger, T. R., et al. (2020). Potential toxicity of iron oxide magnetic nanoparticles: a review. Molecules 25:3159. doi: 10.3390/molecules25143159
Mandal, R., Singh, A., and Pratap, S. A. (2018). Recent developments in cold plasma decontamination technology in the food industry. Trends Food Sci. Technol. 80, 93–103. doi: 10.1016/j.tifs.2018.07.014
Marshall, H., Meneely, J. P., Quinn, B., Zhao, Y., Bourke, P, Gilmore, B. F., and Elliott, C. T. (2020). Novel decontamination approaches and their potential application for post-harvest aflatoxin control. Trends Food Sci. Technol., 106, 489–496. doi: 10.1016/j.tifs.2020.11.001
Masanga, J. O., Matheka, J. M., Omer, R. A., Ommeh, S. C., Monda, E. O., and Alakonya, A. E. (2015). Downregulation of transcription factor aflR in Aspergillus flavus confers reduction to aflatoxin accumulation in transgenic maize with alteration of host plant architecture. Plant Cell Rep. 34, 1379–1387. doi: 10.1007/s00299-015-1794-9
Mauro, A., Battilani, P., Callicott, K. A., Giorni, P., Pietri, A., and Cotty, P. J. (2013). Structure of an Aspergillus flavus population from maize kernels in northern Italy. Int. J. Food Microbiol. 162, 1–7. doi: 10.1016/j.ijfoodmicro.2012.12.021
Mauro, A., Garcia-Cela, E., Pietri, A., Cotty, P. J., and Battilani, P. (2018). Biological control products for aflatoxin prevention in Italy: commercial field evaluation of atoxigenic Aspergillus flavus active ingredients. Toxins 10:30. doi: 10.3390/toxins10010030
McDonald, T., Brown, D., Keller, N. P., and Hammond, T. M. (2005). RNA silencing of mycotoxin production in Aspergillus and Fusarium species. Mol. Plant-Microbe Interact. 18, 539–545. doi: 10.1094/MPMI-18-0539
Medina, A., Rodriguez, A., and Magan, N. (2014). Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front. Microbiol. 5:348. doi: 10.3389/fmicb.2014.00348
Montibus, M., Pinson-Gadais, L., Richard-Forget, F., Barreau, C., and Ponts, N. (2013). Coupling of transcriptional response to oxidative stress and secondary metabolism regulation in filamentous fungi. Crit. Rev. Microbiol. 41, 295–308. doi: 10.3109/1040841X.2013.829416
Moore, G. G. (2022). Practical considerations will ensure the continued success of pre-harvest biocontrol using non-aflatoxigenic Aspergillus flavus strains. Crit. Rev. Food Sci. Nutr. 62, 4208–4225. doi: 10.1080/10408398.2021.1873731
Moore, G. G., Lebar, M. D., Carter-Wientjes, C. H., and Gilbert, M. K. (2021). The potential role of fungal volatile organic compounds in Aspergillus flavus biocontrol efficacy. Biol. Control 160:104686. doi: 10.1016/j.biocontrol.2021.104686
Moore, G. G., Singh, R., Horn, B. W., and Carbone, I. (2009). Recombination and lineage-specific gene loss in the aflatoxin gene cluster of Aspergillus flavus. Mol. Ecol. 18, 4870–4887. doi: 10.1111/j.1365-294X.2009.04414.x
Moral, J., Garcia-Lopez, M. T., Camiletti, B. X., Jaime, R., Michailides, T. J., Bandyopadhyay, R., et al. (2020). Present status and perspective on the future use of aflatoxin biocontrol products. Agronomy 10:491. doi: 10.3390/agronomy10040491
Moretti, A., Pascale, M., and Logrieco, A. F. (2019). Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Technol. 84, 38–40. doi: 10.1016/j.tifs.2018.03.008
Murugesan, P., Brunda, D. K., Moses, J. A., and Anandharamakrishnan, C. (2021). Photolytic and photocatalytic detoxification of mycotoxins in foods. Food Control 123:107748. doi: 10.1016/j.foodcont.2020.107748
Nahle, S., El Khoury, A., Savvaidis, I., Chokr, A., Louka, N., and Atoui, A. (2022). Detoxification approaches of mycotoxins: by microorganisms, biofilms and enzymes. Int. J. Food Contam. 9, 1–14. doi: 10.1186/s40550-022-00089-2
Niehaus, W. G., and Jiang, W. P. (1989). Nitrate induces enzymes of the mannitol cycle and suppresses versicolorin synthesis in Aspergillus parasiticus. Mycopathologia 107, 131–137.
Niño-Sánchez, J., Chen, L. H., De Souza, J. T., Mosquera, S., and Stergiopoulos, I. (2021). Targeted delivery of gene silencing in fungi using genetically engineered bacteria. J. Fungi 7:125. doi: 10.3390/jof7020125
O’Brian, G. R., Georgianna, D. R., Wilkinson, J. R., Yu, J., Abbas, H. K., Bhatnagar, D., et al. (2007). The effect of elevated temperature on gene transcription and aflatoxin biosynthesis. Mycologia 99, 232–239. doi: 10.3852/mycologia.99.2.232
Ola, O. T., Ogedengbe, O. O., Raji, T. M., Eze, B., Chama, M., Ilori, O. N., et al. (2022). Aflatoxin biocontrol effectiveness in the real world—private sector-led efforts to manage aflatoxins in Nigeria through biocontrol-centered strategies. Front. Microbiol. 13:977789. doi: 10.3389/fmicb.2022.977789
Omolehin, O., Raruang, Y., Hu, D., Han, Z. Q., Wei, Q., Wang, K., et al. (2021). Resistance to aflatoxin accumulation in maize mediated by host-induced silencing of the Aspergillus flavus alkaline protease (alk) gene. J. Fungi 7:904. doi: 10.3390/jof7110904
Ortega-Beltran, A., Agbetiameh, D., Atehnkeng, J., Falade, T. D., and Bandyopadhyay, R. (2021). Does use of Atoxigenic biocontrol products to mitigate aflatoxin in maize increase fumonisin content in grains? Plant Dis. 105, 2196–2201. doi: 10.1094/PDIS-07-20-1447-RE
Ortega-Beltran, A., and Bandyopadhyay, R. (2021). Contributions of integrated aflatoxin management strategies to achieve the sustainable development goals in various African countries. Glob. Food Sec. 30:100559. doi: 10.1016/j.gfs.2021.100559
Peles, F., Sipos, P., Kovács, S., Győri, Z., Pócsi, I., and Pusztahelyi, T. (2021). Biological control and mitigation of aflatoxin contamination in commodities. Toxins 13:104. doi: 10.3390/toxins13020104
Pennerman, K. K., Gonzalez, J., Chenoweth, L. R., Bennett, J. W., Yin, G., and Hua, S. S. T. (2018). Biocontrol strain Aspergillus flavus WRRL 1519 has differences in chromosomal organization and an increased number of transposon-like elements compared to other strains. MGG 293, 1507–1522. doi: 10.1007/s00438-018-1474-x
Pérez-Gómez, E. O., García-Rosales, G., Longoria-Gándara, L. C., and Gómez-Vilchis, J. C. (2022). Obtention of biochar-Ca nanoparticles using citrus tangerina: a morphological, surface and study remotion of aflatoxin AFB1. J. Hazard. Mater. 424:127339. doi: 10.1016/j.jhazmat.2021.127339
Pfannenstiel, B. T., Greco, C., Sukowaty, A. T., and Keller, N. P. (2018). The epigenetic reader SntB regulates secondary metabolism, development and global histone modifications in Aspergillus flavus. Fungal Genet. Biol. 120, 9–18. doi: 10.1016/j.fgb.2018.08.004
Pfliegler, W. P., Pócsi, I., Győri, Z., and Pusztahelyi, T. (2020). The aspergilli and their mycotoxins: metabolic interactions with plants and the soil biota. Front. Microbiol. 10:2921. doi: 10.3389/fmicb.2019.02921
Piotrowska, M. (2021). Microbiological decontamination of mycotoxins: opportunities and limitations. Toxins 13:819. doi: 10.3390/toxins13110819
Power, I. L., Faustinelli, P. C., Orner, V. A., Sobolev, V. S., and Arias, R. S. (2020). Analysis of small RNA populations generated in peanut leaves after exogenous application of dsRNA and dsDNA targeting aflatoxin synthesis genes. Sci. Rep. 10, 1–12. doi: 10.1038/s41598-020-70618-6
Price, M. S., Conners, S. B., Tachdjian, S., Kelly, R. M., and Payne, G. A. (2005). Aflatoxin conducive and non-conducive growth conditions reveal new gene associations with aflatoxin production. Fungal Genet. Biol. 42, 506–518. doi: 10.1016/j.fgb.2005.03.009
Qin, X., Xin, Y., Zou, J., Su, X., Wang, X., Wang, Y., et al. (2021). Efficient degradation of aflatoxin B1 and zearalenone by laccase-like multicopper oxidase from Streptomyces thermocarboxydus in the presence of mediators. Toxins 13:754. doi: 10.3390/toxins13110754
Ráduly, Z., Szabó, L., Madar, A., Pócsi, I., and Csernoch, L. (2020). Toxicological and medical aspects of Aspergillus-derived mycotoxins entering the feed and food chain. Front. Microbiol. 10:2908. doi: 10.3389/fmicb.2019.02908
Rapid Alert System for Food and Feed (2021). Annual Report. Available at: https://food.ec.europa.eu/system/files/2022-07/acn_annual-report_2021-final.pdf [Accessed November 26, 2022].
Rapid Alert System for Food and Feed (2022). Portal Database. Available at: https://webgate.ec.europa.eu/rasff-window/portal/ [Accessed August 3, 2022].
Raruang, Y., Omolehin, O., Hu, D., Wei, Q., Han, Z. Q., Rajasekaran, K., et al. (2020). Host induced gene silencing targeting Aspergillus flavus aflM reduced aflatoxin contamination in transgenic maize under field conditions. Front. Microbiol. 11:754. doi: 10.3389/fmicb.2020.00754
Regulation (EU) 2021/363. Commission Implementing Regulation (EU) 2021/363 of 26 February 2021. Concerning the authorisation of a preparation of fumonisin esterase produced by Komagataella phaffii DSM 32159 as a feed additive for all animal species (text with EEA relevance) C/2021/1140.
Ren, X., Branà, M. T., Haidukowski, M., Gallo, A., Zhang, Q., Logrieco, A. F., et al. (2022). Potential of trichoderma spp. for biocontrol of aflatoxin-producing Aspergillus flavus. Toxins 14:86. doi: 10.3390/toxins14020086
Ren, Y., Jin, J., Zheng, M., Yang, Q., and Xing, F. (2020). Ethanol inhibits aflatoxin B1 biosynthesis in Aspergillus flavus by up-regulating oxidative stress-related genes. Front. Microbiol. 10:2946. doi: 10.3389/fmicb.2019.02946
Ren, Z., Luo, J., and Wan, Y. (2019). Enzyme-like metal–organic frameworks in polymeric membranes for efficient removal of aflatoxin B1. ACS Appl. Mater. Interfaces 11, 30542–30550. doi: 10.1021/acsami.9b08011
Ren, X., Zhang, Q., Zhang, W., Mao, J., and Li, P. (2020). Control of aflatoxigenic molds by antagonistic microorganisms: inhibitory behaviors, bioactive compounds, related mechanisms, and influencing factors. Toxins 12:24. doi: 10.3390/toxins12010024
Reverberi, M., Fabbri, A. A., Zjalic, S., Ricelli, A., Punelli, F., and Fanelli, C. (2005). Antioxidant enzymes stimulation in Aspergillus parasiticus by Lentinula edodes inhibits aflatoxin production. Appl. Microbiol. Biotechnol. 69, 207–215. doi: 10.1007/s00253-005-1979-1
Reverberi, M., Ricelli, A., Zjalic, S., Fabbri, A. A., and Fanelli, C. (2010). Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl. Microbiol. Biotechnol. 87, 899–911. doi: 10.1007/s00253-010-2657-5
Reverberi, M., Zjalic, S., Ricelli, A., Fabbri, A. A., and Fanelli, C. (2006). Oxidant/antioxidant balance in Aspergillus parasiticus affects aflatoxin biosynthesis. Mycotoxin Res. 22, 39–47. doi: 10.1007/BF02954556
Reverberi, M., Zjalic, S., Ricelli, A., Punelli, F., Camera, E., Fabbri, C., et al. (2008). Modulation of antioxidant defence in Aspergillus parasiticus is involved in aflatoxin biosynthesis: a role for ApyapA gene. Eukaryot. Cell 7, 988–1000. doi: 10.1128/EC.00228-07
Rifna, E. J., Ramanan, K. R., and Mahendran, R. (2019). Emerging technology applications for improving seed germination. Trends in Food Science & Technology 86, 95–108. doi: 10.1016/j.tifs.2019.02.029
Roze, L. V., Chanda, A., Wee, J., Awad, D., and Linz, J. E. (2011). Stress-related transcription factor AtfB integrates secondary metabolism with oxidative stress response in Aspergilli. J. Biol. Chem. 286, 35137–35148. doi: 10.1074/jbc.M111.253468
Rushing, B. R., and Selim, M. I. (2019). Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 124, 81–100. doi: 10.1016/j.fct.2018.11.047
Safari, N., Mirabzadeh Ardakani, M., Hemmati, R., Parroni, A., Beccaccioli, M., and Reverberi, M. (2020). The potential of plant-based bioactive compounds on inhibition of aflatoxin B1 biosynthesis and down-regulation of aflR, aflM and aflP genes. Antibiotics 9:728. doi: 10.3390/antibiotics9110728
Sarmast, E., Fallah, A. A., Jafari, T., and Khaneghah, A. M. (2021). Occurrence and fate of mycotoxins in cereals and cereal-based products: a narrative review of systematic reviews and meta-analyses studies. Curr. Opin. Food Sci. 39, 68–75. doi: 10.1016/j.cofs.2020.12.013
Sarrocco, S., Mauro, A., and Battilani, P. (2019). Use of competitive filamentous fungi as an alternative approach for mycotoxin risk reduction in staple cereals: state of art and future perspectives. Toxins 11:701. doi: 10.3390/toxins11120701
Schmidt, F. R. (2009). The RNA interference-virus interplay: tools of nature for gene modulation, morphogenesis, evolution and a possible mean for aflatoxin control. Appl. Microbiol. Biotechnol. 83, 611–615. doi: 10.1007/s00253-009-2007-7
Schmidt-Heydt, M., Abdel-Hadi, A., Magan, N., and Geisen, R. (2009). Complex regulation of the aflatoxin biosynthesis gene cluster of Aspergillus flavus in relation to various combinations of water activity and temperature. Int. J. Food Microbiol. 135, 231–237. doi: 10.1016/j.ijfoodmicro.2009.07.026
Schmidt-Heydt, M., Rüfer, C. E., Abdel-Hadi, A., Magan, N., and Geisen, R. (2010). The production of aflatoxin B1 or G1 by Aspergillus parasiticus at various combinations of temperature and water activity is related to the ratio of aflS to aflR expression. Mycotox. Res. 26, 241–246. doi: 10.1007/s12550-010-0062-7
Shantha, T., and Murthy, V. S. (1981). Influence of tricarboxylic acid cycle intermediates and related metabolites on the biosynthesis of aflatoxin by resting cells of Aspergillus flavus. Appl. Environ. Microbiol. 42, 758–761. doi: 10.1128/aem.42.5.758-761.1981
Shen, M. H., and Singh, R. K. (2021). Effect of rotating peanuts on aflatoxin detoxification by ultraviolet C light and irradiation uniformity evaluated by AgCl-based dosimeter. Food Control 120:107533. doi: 10.1016/j.foodcont.2020.107533
Shen, M. H., and Singh, R. K. (2022). Effective UV wavelength range for increasing aflatoxins reduction and decreasing oil deterioration in contaminated peanuts. Food Res. Int. 154:111016. doi: 10.1016/j.foodres.2022.111016
Shimizu, K., Hicks, J. K., Huang, T. P., and Keller, N. P. (2003). Pka, Ras and RGS protein interactions regulate activity of AflR, a Zn(II)2Cys6 transcription factor in Aspergillus nidulans. Genetics 165, 1095–1104. doi: 10.1093/genetics/165.3.1095
Shimizu, K., and Keller, N. P. (2001). Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157, 591–600. doi: 10.1093/genetics/157.2.591
Sipos, P., Peles, F., Brassó, D. L., Béri, B., Pusztahelyi, T., Pócsi, I., et al. (2021). Physical and chemical methods for reduction in aflatoxin content of feed and food. Toxins 13:204. doi: 10.3390/toxins13030204
Solomon, S., Quin, D., Manning, M., Alley, R. B., Berstein, T., Bindoff, N. I., et al. (2007). “Technical summary,” in Climate change 2007: The physical science basis. Contribution of working group 1 to the fourth assessment report of the intergovernmental panel on climate change. eds. S. Solomon, D. Quin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor, and H. L. Miller (Cambridge, NY: Cambridge University Press), 996.
Song, C., and Qin, J. (2022). High-performance fabricated nano-adsorbents as emerging approach for removal of mycotoxins: a review. Int. J. Food Sci. 57, 5781–5789. doi: 10.1111/ijfs.15953
Song, C., Yang, J., Wang, Y., Ding, G., Guo, L., and Qin, J. (2022). Mechanisms and transformed products of aflatoxin B1 degradation under multiple treatments: a review. Crit. Rev. Food Sci. Nutr. 1–13. doi: 10.1080/10408398.2022.2121910
Sun, S., Zhao, R., Xie, Y., and Liu, Y. (2019). Photocatalytic degradation of aflatoxin B1 by activated carbon supported TiO2 catalyst. Food Control 100, 183–188. doi: 10.1016/j.foodcont.2019.01.014
Szabó, Z., Pákozdi, K., Murvai, K., Pusztahelyi, T., Kecskeméti, Á., Gáspár, A., et al. (2020). FvatfA regulates growth, stress tolerance as well as mycotoxin and pigment productions in Fusarium verticillioides. Appl. Microbiol. Biotechnol. 104, 7879–7899. doi: 10.1007/s00253-020-10717-6
Thakare, D., Zhang, J., Wing, R. A., Cotty, P. J., and Schmidt, M. A. (2017). Aflatoxin-free transgenic maize using host-induced gene silencing. Sci. Adv. 3:e1602382. doi: 10.1126/sciadv.1602382
Tilburn, J., Sarkar, S., Widdick, D. A., Espeso, E. A., Orejas, M., Mungroo, J., et al. (1995). The Aspergillus PacC zinc-finger transcription factor mediates regulation of both acid-expressed and alkaline-expressed genes by ambient pH. EMBO J. 14, 779–790. doi: 10.1002/j.1460-2075.1995.tb07056.x
Tsitsigiannis, D. I., and Keller, N. P. (2006). Oxylipins act as determinants of natural product biosynthesis and seed colonization in Aspergillus nidulans. Mol. Microbiol. 59, 882–892. doi: 10.1111/j.1365-2958.2005.05000.x
Tsitsigiannis, D. I., and Keller, N. P. (2007). Oxylipins as developmental and host–fungal communication signals. Trends Microbiol. 15, 109–118. doi: 10.1016/j.tim.2007.01.005
Tsitsigiannis, D. I., Kunze, S., Willis, D. K., Feussner, I., and Keller, N. P. (2005). Aspergillus infection inhibits the expression of peanut 13 S -HPODE-forming seed lipoxygenases. MPMI 18, 1081–1089. doi: 10.1094/MPMI-18-1081
Van der Fels-Klerx, H. J., Vermeulen, L. C., Gavai, A. K., and Liu, C. (2019). Climate change impacts on aflatoxin B1 in maize and aflatoxin M1 in milk: a case study of maize grown in Eastern Europe and imported to the Netherlands. PLoS One 14:e0218956. doi: 10.1371/journal.pone.0218956
Wang, P., Chang, P.-K., Kong, Q., Shan, S., and Wei, Q. (2019). Comparison of aflatoxin production of Aspergillus flavus at different temperatures and media: Proteome analysis based on TMT. Int J Food Microbiol. 16:108313. doi: 10.1016/j.ijfoodmicro.2019.108313
Wang, Y., Chen, Y., Jiang, L., and Huang, H. (2022). Improvement of the enzymatic detoxification activity towards mycotoxins through structure-based engineering. Biotechnol. Adv. 107927. doi: 10.1016/j.biotechadv.2022.107927
Wang, H., Lei, Y., Yan, L., Cheng, K., Dai, X., Wan, L., et al. (2015). Deep sequencing analysis of transcriptomes in Aspergillus flavus in response to resveratrol. BMC Microbiol. 15, 1–14. doi: 10.1186/s12866-015-0513-6
Wang, X., Zha, W., Liang, L., Fasoyin, O. E., Wu, L., and Wang, S. (2020). The bZIP transcription factor AflRsmA regulates aflatoxin B1 biosynthesis, oxidative stress response and sclerotium formation in Aspergillus flavus. Toxins 12:271. doi: 10.3390/toxins12040271
Weaver, M. A., Mack, B. M., and Gilbert, M. K. (2019). Genome sequences of 20 georeferenced Aspergillus flavus isolates. Microbiol. Resour. Announc. 8:e01718. doi: 10.1128/MRA.01718-18
Wee, J., Hong, S.-Y., Roze, L. V., Day, D. M., Chanda, A., and Linz, J. E. (2017). The fungal bZIP transcription factor AtfB controls virulence-associated processes in Aspergillus parasiticus. Toxins 9:287. doi: 10.3390/toxins9090287
Wei, J., Wu, X., Wu, C., Hou, F., Wu, L., and Huang, H. (2022). Metal-organic frameworks with peroxidase-like activity for efficient removal of aflatoxin B1. Food Chem. 378:132037. doi: 10.1016/j.foodchem.2021.132037
Woo, S. L., Ruocco, M., Vinale, F., Nigro, M., Marra, R., Lombardi, N., et al. (2014). Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 8, 71–126. doi: 10.2174/1874437001408010071
Wu, F. (2022). Mycotoxin risks are lower in biotech corn. Curr. Opin. Biotechnol. 78:102792. doi: 10.1016/j.copbio.2022.102792
Wu, Y., Cheng, J. H., and Sun, D. W. (2021). Blocking and degradation of aflatoxins by cold plasma treatments: applications and mechanisms. Trends Food Sci. Technol. 109, 647–661. doi: 10.1016/j.tifs.2021.01.053
Wu, Q., Fan, J., Chen, X., Zhu, Z., Luo, J., and Wan, Y. (2020). Sandwich structured membrane adsorber with metal organic frameworks for aflatoxin B1 removal. Sep. Purif. Technol. 246:116907. doi: 10.1016/j.seppur.2020.116907
Wu, F., and Guclu, H. (2012). Aflatoxin regulations in a network of global maize trade. PLoS One 7:e45151. doi: 10.1371/journal.pone.0045151
Xu, D., Peng, S., Guo, R., Yao, L., Mo, H., Li, H., et al. (2021). EGCG alleviates oxidative stress and inhibits aflatoxin B1 biosynthesis via MAPK signaling pathway. Toxins 13:693. doi: 10.3390/toxins13100693
Yang, P., Xiao, W., Lu, S., Jiang, S., Zheng, Z., Zhang, D., et al. (2021). Recombinant expression of Trametes versicolor aflatoxin B1-degrading enzyme (TV-AFB1D) in engineering Pichia pastoris GS115 and application in AFB1 degradation in AFB1-contaminated peanuts. Toxins 13:349. doi: 10.3390/toxins13050349
Yin, W. B., Amaike, S., Wohlbach, D. J., Gasch, A. P., Chiang, Y. M., Wang, C. C., et al. (2012). An Aspergillus nidulans bZIP response pathway hardwired for defensive secondary metabolism operates through aflR. Mol. Microbiol. 83, 1024–1034. doi: 10.1111/j.1365-2958.2012.07986.x
Yin, W. B., Reinke, A. W., Szilágyi, M., Emri, T., Chiang, Y. M., Keating, A. E., et al. (2013). bZIP transcription factors affecting secondary metabolism, sexual development and stress responses in Aspergillus nidulans. Microbiology 159, 77–88. doi: 10.1099/mic.0.063370-0
Yu, J. (2012). Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins 4, 1024–1057. doi: 10.3390/toxins4111024
Yu, J.-H., Butchko, R. E., Fernandes, M., Keller, N., Leonard, T., and Adams, T. (1996). Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus. Curr. Genet. 29, 549–555. doi: 10.1007/BF02426959
Yu, J., Chang, P., Ehrlich, K. C., Cary, J. W., Bhatnagar, D., Cleveland, T. E., et al. (2004). Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 70, 1253–1262. doi: 10.1128/AEM.70.3.1253-1262.2004
Yu, J., Woloshuk, C. P., Bhatnagar, D., and Cleveland, T. E. (2000). Cloning and characterization of avfA and omtB genes involved in aflatoxin biosynthesis in three Aspergillus species. Gene 248, 157–167. doi: 10.1016/s0378-1119(00)00126-8
Yunes, N., Oliveira, R. C., Reis, T. A., Baquião, A. C., Rocha, L. O., and Correa, B. (2020). Effect of temperature on growth, gene expression, and aflatoxin production by Aspergillus nomius isolated from Brazil nuts. Mycotoxin Res. 36, 173–180. doi: 10.1007/s12550-019-00380-w
Zhang, F., Guo, Z., Zhong, H., Wang, S., Yang, W., Liu, Y., et al. (2014). RNA-Seq-based transcriptome analysis of aflatoxigenic Aspergillus flavus in response to water activity. Toxins 6, 3187–3207. doi: 10.3390/toxins6113187
Zhang, X., Li, G., Wu, D., Liu, J., and Wu, Y. (2020). Recent advances on emerging nanomaterials for controlling the mycotoxin contamination: from detection to elimination. Food Front. 1, 360–381. doi: 10.1002/fft2.42
Zhang, C., Selvaraj, J. N., Yang, Q., and Liu, Y. (2017). A survey of aflatoxin-producing Aspergillus sp. from peanut field soils in four agroecological zones of China. Toxins 9:E40. doi: 10.3390/toxins9010040
Zhao, Q., Pei, H., Zhou, X., Zhao, K., Yu, M., Han, G., et al. (2022). Systematic characterization of bZIP transcription factors required for development and aflatoxin generation by high-throughput gene knockout in Aspergillus flavus. J Fungi 8:356. doi: 10.3390/jof8040356
Zhao, X., Zhi, Q. Q., Li, J. Y., Keller, N. P., and He, Z. M. (2018). The antioxidant gallic acid inhibits aflatoxin formation in Aspergillus flavus by modulating transcription factors FarB and CreA. Toxins 10:270. doi: 10.3390/toxins10070270
Zhu, Y., Hassan, Y. I., Lepp, D., Shao, S., and Zhou, T. (2017). Strategies and methodologies for developing microbial detoxification systems to mitigate mycotoxins. Toxins 9:130. doi: 10.3390/toxins9040130
Zhu, Y., Hassan, Y. I., Watts, C., and Zhou, T. (2016). Innovative technologies for the mitigation of mycotoxins in animal feed and ingredients—A review of recent patents. Anim. Feed Sci. Technol. 216, 19–29. doi: 10.1016/j.anifeedsci.2016.03.030
Keywords: aflatoxin, climate change, models, mycotoxin control, decontamination techniques
Citation: Loi M, Logrieco AF, Pusztahelyi T, Leiter É, Hornok L and Pócsi I (2023) Advanced mycotoxin control and decontamination techniques in view of an increased aflatoxin risk in Europe due to climate change. Front. Microbiol. 13:1085891. doi: 10.3389/fmicb.2022.1085891
Received: 31 October 2022; Accepted: 15 December 2022;
Published: 10 January 2023.
Edited by:
Antonio Bevilacqua, University of Foggia, ItalyReviewed by:
Massimo Reverberi, Sapienza University of Rome, ItalyCopyright © 2023 Loi, Logrieco, Pusztahelyi, Leiter, Hornok and Pócsi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martina Loi, ✉ bWFydGluYS5sb2lAaXNwYS5jbnIuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.