- 1Hubei Province Key Laboratory of Occupational Hazard Identification and Control, Department of Pharmacy, Institute of Infection, Immunology and Tumor Microenvironments, Institute of Pharmaceutical Process, Medical College, Wuhan University of Science and Technology, Wuhan, China
- 2Department of Pharmacy, Tongji Hospital Affiliated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Fungi are considered to be one of the wealthiest sources of bio-metabolites that can be employed for yielding novel biomedical agents. Alternaria, including parasitic, saprophytic, and endophytic species, is a kind of dark fungi that can produce a broad array of secondary metabolites (SMs) widely distributed in many ecosystems. These are categorized into polyketides, nitrogen-containing compounds, quinones, terpenes, and others based on the unique structural features of the metabolites. New natural products derived from Alternaria exhibit excellent bioactivities characterized by antibacterial, antitumor, antioxidative, phytotoxic, and enzyme inhibitory properties. Thus, the bio-metabolites of Alternaria species are significantly meaningful for pharmaceutical, industrial, biotechnological, and medicinal applications. To update the catalog of secondary metabolites synthesized by Alternaria fungi, 216 newly described metabolites isolated from Alternaria fungi were summarized with their diverse chemical structures, pharmacological activity, and possible biosynthetic pathway. In addition, possible insights, avenues, and challenges for future research and development of Alternaria are discussed.
1. Introduction
Fungi are vital microorganisms that reside in various environments where they play a significant role in protecting eco-balance and diversity (Keller, 2019; Noor et al., 2020; Ibrahim et al., 2021). Fungi have attracted considerable attention in the fields of natural product chemistry, medicine, and agriculture (Al-Obaidi et al., 2021; Ibrahim et al., 2022). Alternaria fungus is a widespread dark fungus, belonging to classes Ascomycota, Dothideomycetes, Pleosporales, and Pleosporaceae (Feng and Sun, 2020). The fungal genus Alternaria is a ubiquitous group growing in diverse ecosystems worldwide as a parasitic, saprophytic, or endophytic species (Wang et al., 2022). Of these, Alternaria alternata, Alternaria brassicicola, Alternaria penicillata, Alternaria cetera, Alternaria alternantherae, and another 28 groups are ubiquitous (Feng and Sun, 2020; Li et al., 2021; Wang et al., 2022). Alternaria species can produce a variety of secondary metabolites. These metabolites mainly include polyketides, nitrogen-containing compounds, quinones, terpenes, and other compounds (Yamada et al., 2019; Li et al., 2020a; Tian et al., 2021). A large number of potentially bioactive molecules have been found, with intriguing structural skeletons and remarkable activities (Lou et al., 2013; Wang et al., 2022). Bioactive metabolites secreted by Alternaria fungi often exhibit excellent pharmacological potential, such as anticancer, antibacterial, antioxidant, and enzyme inhibitory effects (Wang J. et al., 2015; Dalinova et al., 2020; Mahmoud et al., 2021; Tian et al., 2021). For example, the world's first plant immune protein biological insecticide, ATailing, has been successfully developed by enhancing the broad-spectrum resistance of plants (Sheng et al., 2017). In addition, bio-metabolites of Alternaria fungi also have the efficacy of weeding and insecticide, and enhance the role of plant immunity in agricultural and food applications (Shi et al., 2017, 2018b; Tan et al., 2019; Li et al., 2021).
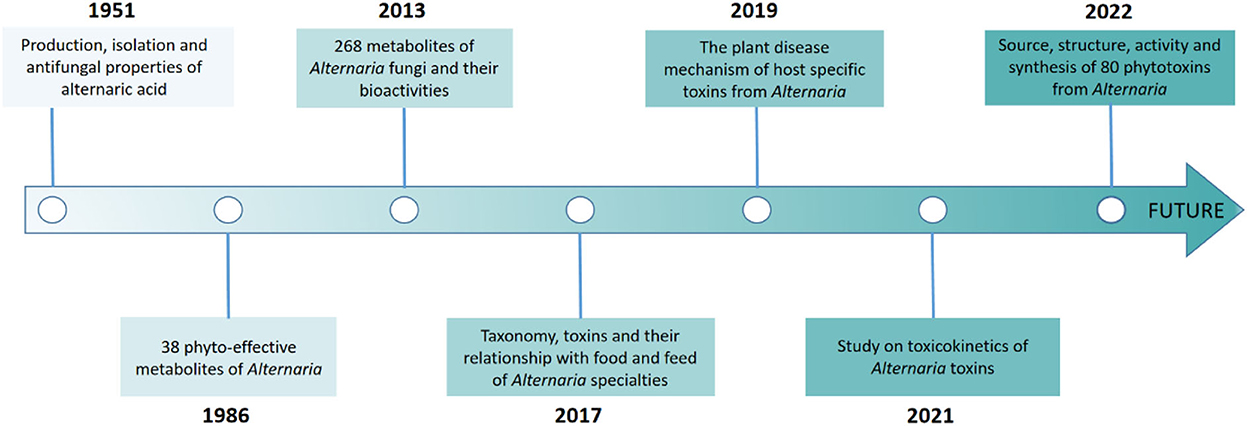
Furthermore, continuous studies on Alternaria metabolites have been carried out on the production, isolation, chemical complexity, culture conditions, plant disease mechanisms and toxicokinetics of toxin metabolomics (Figure 1) (Brian et al., 1951; Bemmann, 1986; Pinto and Patriarca, 2017; Sheng et al., 2017; Meena and Samal, 2019; Chen et al., 2021). A recent review focused on the 80 Alternaria phytotoxins with their classification, chemical structure, occurrence, bioactivity, and biosynthesis (Wang et al., 2022). These metabolites have an important but less-explored application value in the microorganism, where the chemical industry and fields of medicine, biological control, etc. have endeavored to discover structurally novel natural products. In this study, we summarize the new Alternaria-derived metabolites and give a general overview of the occurrence, chemical structure, and pharmacological properties of secondary metabolites as seen in research from 2014 to 2022. In addition, biosynthetic pathways with some biologically important metabolites are also discussed, which provide new research opportunities for the discovery of drug compounds and practical production technology in the future. Related literature can be found on various databases, including Science Direct, PubMed, Elsevier, Google Scholar, Baidu Scholar, CNKI, and Springer.
2. Secondary metabolites of Alternaria fungi
2.1. Polyketides
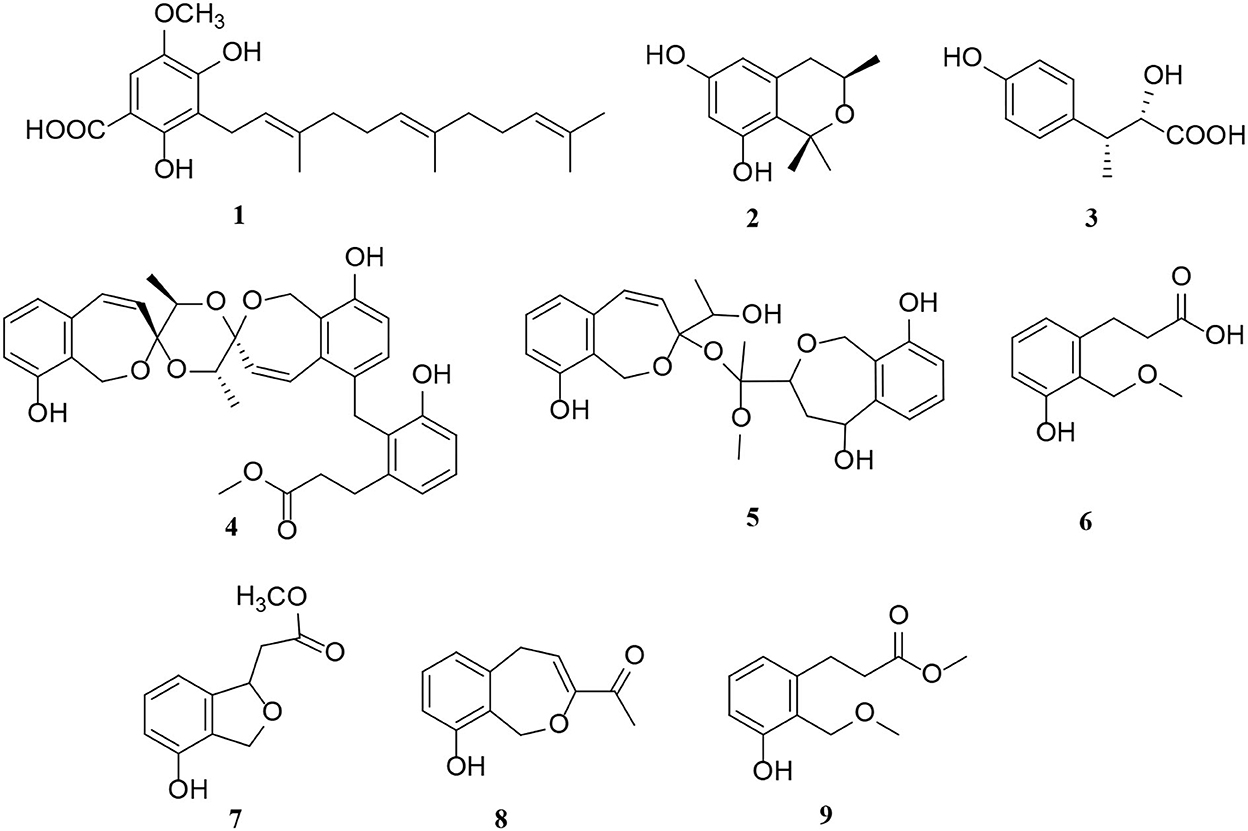
Polyketides are potential virulence factors and immunosuppressants. Pathogenic fungi, which can be synthesized from simple acyl building blocks, exhibit a high degree of structural diversity (Miyanaga, 2017). Polyketides are important natural metabolites that have attracted considerable attention. Simple phenylpropanoids and pyranones are the major groups of the polyketide family secreted by Alternaria sp. A total of 96 polyketides, 9 simple phenylpropanoids (1–9) (Figure 2), 76 pyranones (10–85) (Figures 3, 4), and 11 other polyketides (86–96) (Figure 5) are summarized. Most pyranones have intriguing stereoisomeric frameworks, which are described in detail in this article.
Simple phenylpropanoids are also common in Alternaria endophytes (Figure 2). A total of nine novel phenylpropanoid derivatives, namely alternaritins B–C (1–2), (2S, 3R)-2-hydroxy-3-(4-hydroxyphenyl) butanoic acid (3), and alternarias A–F (4–9), were isolated from the Alternaria species (Lu et al., 2021; Tian et al., 2021). Notably, alternaritin C (2), composed of hydrogenated pyran and tetrasubstituted benzene, is a rare carbon skeleton with double-ring units (Tian et al., 2021). In addition, compound 3 was a new natural product consisting of a p-substituted phenol moiety and a 2-hydroxybutyric acid fragment (Tian et al., 2021).
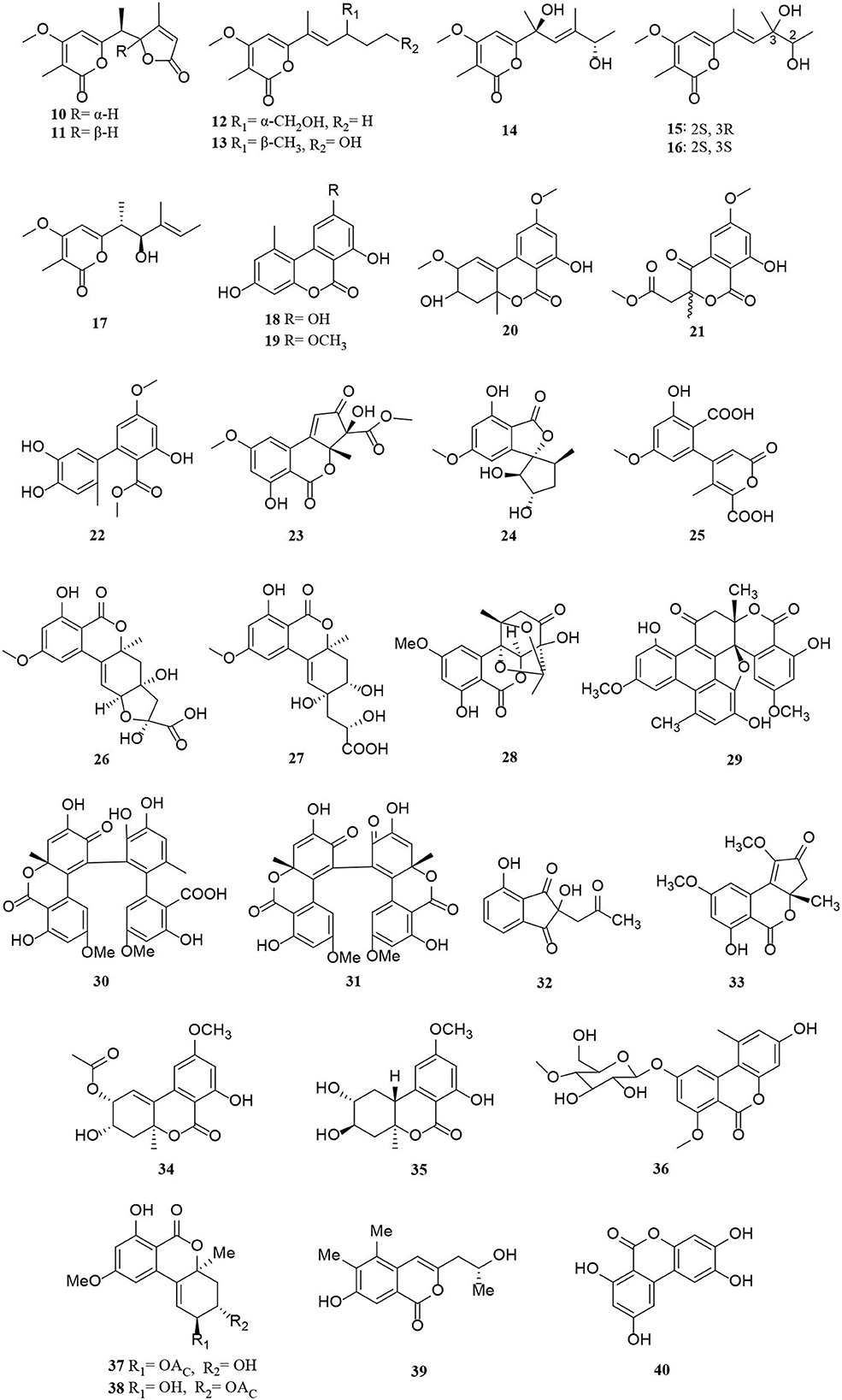
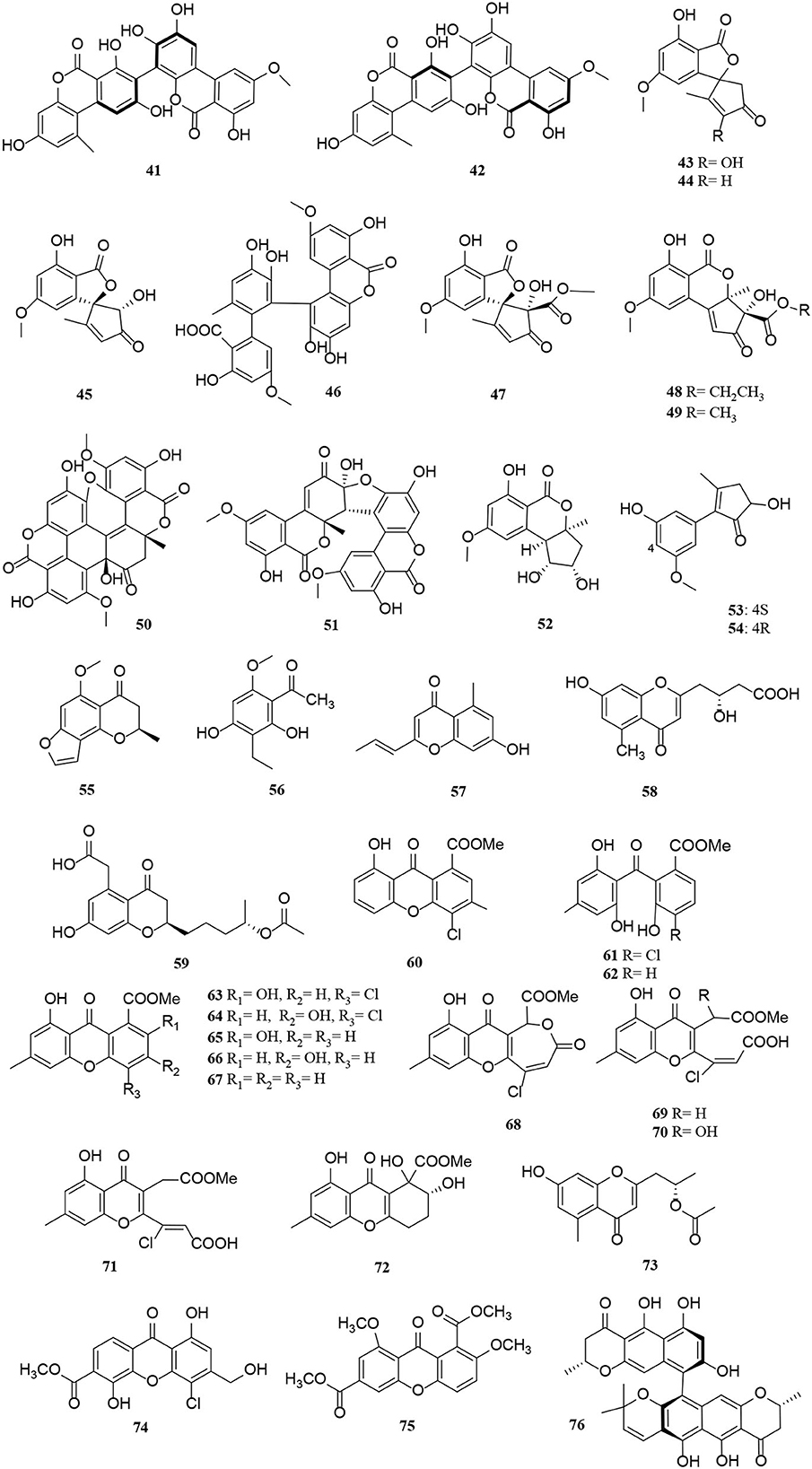
Pyranones, also known as pyrones, include α-, β-, and γ-pyranones. Most pyranones isolated from Alternaria fungi belong to α-pyranones, and most of these have enantiomeric structures, including dibenzo-α-pyranone derivatives (Tang et al., 2019), aromatic polyketone dimers (Yang C. L. et al., 2019), cyclopentane isochromone derivatives (Lu et al., 2018), and biphenyl structure derivatives (Kong et al., 2020). Of these, three pairs of unprecedented α-enantiomers of pyrone derivatives (10–12) were derived from Alternaria brassicicola, along with five diastereomeric structures, alterpyrones D-H (13–17) (Li et al., 2021). Structurally, two pyranone derivatives, alternariol (18) and alternariol-9-methyl ether (19), isolated from the marine endophytic Alternaria, have the same tricyclic skeleton as the alternates A–C (20–22) (Mahmoud et al., 2021; Wang et al., 2021). In addition, alternatiol (23) was reported as a new altenusin metabolite separated from Vitex rotundifolia Alternaria alternata JS0515 (Lee et al., 2019). Alternatains A–D (24–27) were obtained from the solid substrate cultures of Alternaria alternata MT-47 (Yang H. et al., 2019). It can be inferred that it is mainly composed of acetyl coenzyme A and polyketone synthase according to structural characteristics (Yang H. et al., 2019). The enantiomer (+)- and (–)- alternarilactone A (28) was identified as a dibenzo-α-pyranone derivative, possessing a diepoxy-cage-like moiety isolated from Alternaria sp. Hh930. (Tang et al., 2019). Interestingly, (+)- and (–)-alternamgin (29) is also an enantiomeric pyranone derivative with an unprecedented 6/6/6/6/5/6/6 seven-ring framework from Vitis quinquangularis (Wu J. C. et al., 2019). A new example of aromatic polyketone dimer metabolite, bialternacins E-F (30–31), was produced by Alternaria sp. NF2128 from the stem of Maianthemum bifolium fungus (Yang C. L. et al., 2019). Notably, indandione B (32), featuring an extremely rare indole ketone moiety, was found in the Morinda officinalis fungus Alternaria sp. A744 (Wang et al., 2017). The absolute configuration of compound 33 was determined as a pair of new cyclopentane isochromone enantiomers by 2D-nuclear magnetic resonance (2D-NMR) and high-resolution electrospray ionization mass spectroscopy (HRESIMS) (Lu et al., 2018). Compounds 34–42 possess a similar three-ring system, formed an ester bond between a six-membered ring and phenol. Interestingly, the third ring of 39 is open, and both 41 and 42 are dimers (Wang et al., 2014; Xu et al., 2015; Tian et al., 2017; Kong et al., 2020). The Alternaria alternata ZHJG5 produced a series of compounds (43–49), including five novel polyketide derivatives (43–46) and three pairs of dibenzo-α-pyrone derivatives (47–49) (Zhao et al., 2020, 2021). In this study, (±) alternarlactones A (50) and B (51) were two new isolated dimers, which were formed by the C-O- and C-C-bond between dehydroaltenusin and alternariol from Halophyte Salicornia sp. fungus Alternaria alternata P1210 (Shi et al., 2019). In addition, the isolation of the same marine fungi Alternaria sp. SCSIO41014 yielded three new α-pyranone derivatives (52–54) (Pang et al., 2018). Compounds 53 and 54 were proved to be two stereoisomeric configurations isolated from marine sponge (Pang et al., 2018).
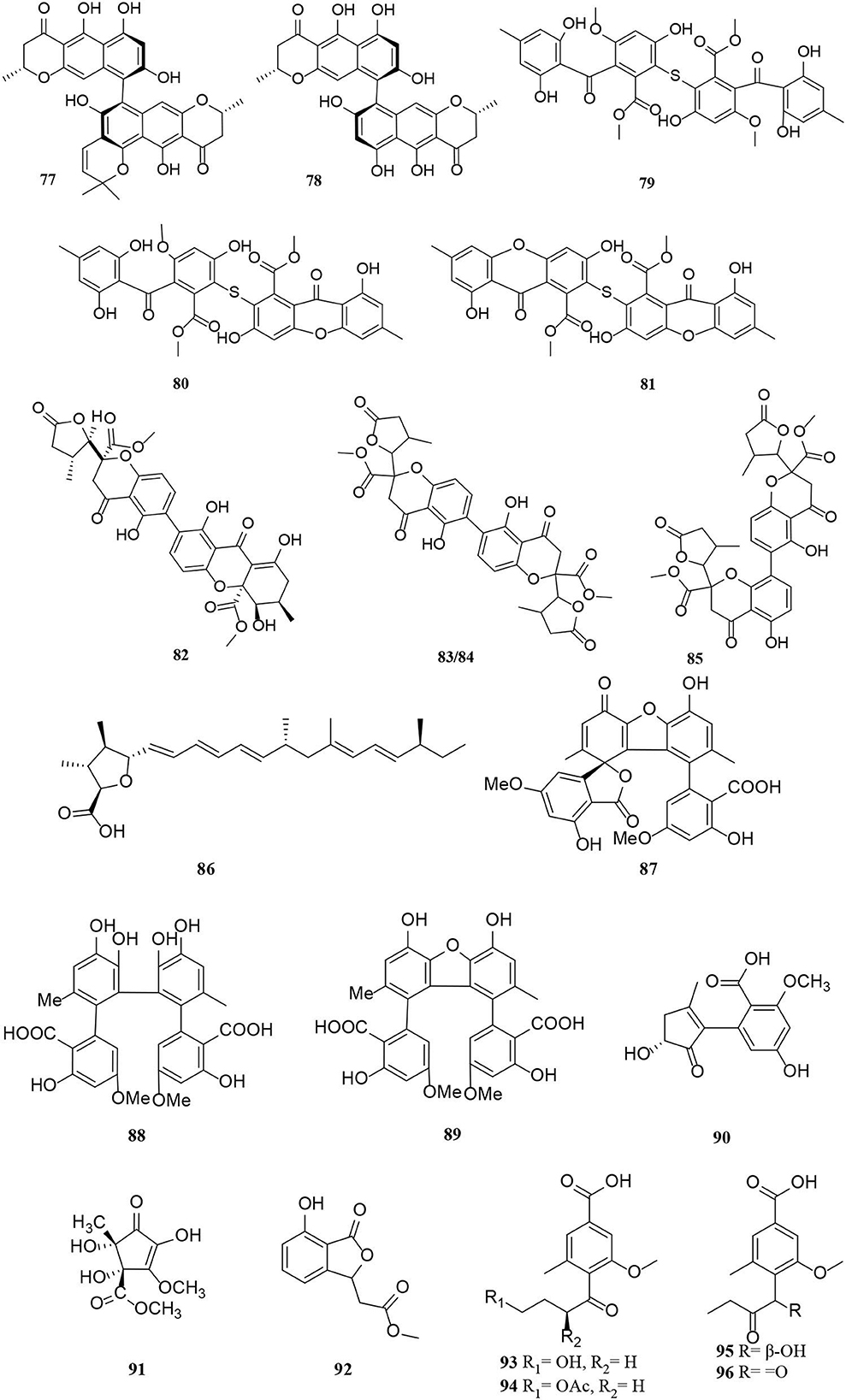
Two new phomalone derivatives, phomalichenones E-F (55–56), were isolated from a deep-sea-derived fungus, Alternaria sp. MCCC 3A00467 (Zhong et al., 2022). 56 is an open γ-pyranone ring with an acetyl group at C-1 compared with 55. Alterchromanone A (59) is a new chromanone derivative, also isolated from marine Alternaria longipes (Liu et al., 2021). Structurally, alternate D (57) and alternaritin D (58) have similar benzo-γ-pyranone moiety (Tian et al., 2021; Wang et al., 2021). A total of 13 compounds (60–72) were isolated from Alternaria sonchi, including chromones, xanthones, and benzophenones (Dalinova et al., 2020). Among them, 60 and 61 represent two new derivatives of chlorinated anthrone and benzophenone, respectively, which were determined by spectroscopy (mainly through 2D-NMR and MS). And compounds 62, 64–67, 71, and 72 were first reported for Alternaria sonchi (Dalinova et al., 2020). In addition, (2′S)-2-(2-acetoxypropyl)-7-hydroxy-5-methylchromone (73) was isolated from the Vitex rotundifolia endophytic fungus Alternaria brassicae JS959 (Kim et al., 2019). Compounds (74–75) with xanthone moiety were isolated from the marine Alternaria sp. R6 (Wang J. et al., 2015). 4-chloro-1,5-dihydroxy-3-hydroxymethyl-6-methoxycarbonyl-xanthen-9-one (74) bearing a chlorine atom was also named 4-chlorofischexanthone. Two new cephalochromin derivatives, prenylcephalochromin A (76) and prenylcephalochromin B (77), along with cephalochromin (78), were isolated from the Dasymaschalon rostratum fungus Alternaria sp. ZG22 (Song et al., 2021). Notably, 76 were elucidated by comprehensive spectroscopic methods, indicating that 76 bears a bis-naphtho-γ-pyrone skeleton. Polluxochrin (79) and dioscin (80), two new dimers of sulochrin linked by thioether bonds, as well as another five compounds (81–85), were purified from an Alternaria sp. isolate obtained from Hawaiian soil (Cai et al., 2014). Compounds 80–81 were produced by intramolecular cyclization of 82, and metabolites 82–85 were four secalonic acid analogs (Cai et al., 2014). Compound 83 is a symmetrical dimer. Overall, the planar structure of 83, especially the C-6–C-6′ linkage, was established by the HMBC correlation spectrum. Subsequently, 84 was determined to share the same planar structure as 83. However, the presence of two distinct sets of resonances representing the two monomeric portions of 84 denoted it was an asymmetric diastereomer of 83.
Other polyketides include aliphatic polyketone (86), aromatic polyketone dimer (87–89), and alternative acid B (90) (Ding et al., 2017; Xu et al., 2019; Yang C. L. et al., 2019). One new cyclohexanone derivative with unsaturated ketone groups was (±)-(4S*,5S*)-2,4,5-trihydroxy-3-methoxy-4-methoxycarbonyl-5-methyl-2-cyclopentene-1-one (91), which was characterized to originate from the mangrove Alternaria strain (Wang J. et al., 2015). Isobenzofuranone A (92) bearing isobenzofuranone moiety was isolated from the Morinda officinalis fungus Alternaria sp. A744 (Wang et al., 2017). Finally, four new pyrenochaetic acid derivatives (93–96) isolated from soil samples have the same carbon skeleton by analysis of the 1H and 13C NMR data (Cai et al., 2014).
2.2. Nitrogen-containing compounds
Nitrogen-containing compounds, isolated from Alternaria, include amides, peptides, and alkaloids. A total of 35 nitrogen-containing compounds, 16 amides (97–112) (Figure 6), 5 peptides (113–117) (Figure 7), and 14 alkaloids (118–131) (Figure 8) have been summarized and are described in detail as follows.
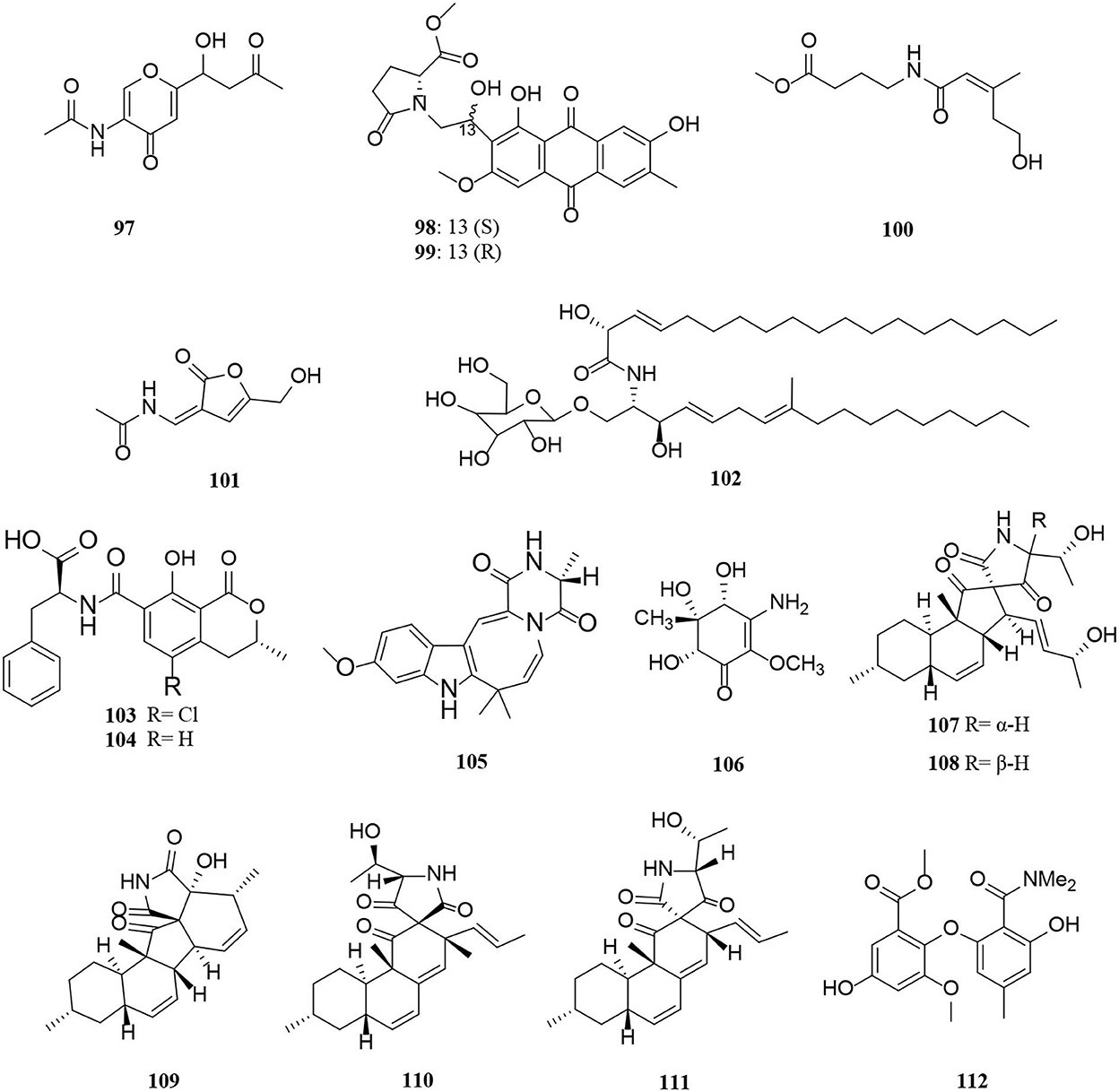
A pair of enantiomeric nitrogen-containing compounds, alternaritin A [(±)-97], is composed of the amide bond and γ-pyranone composition isolated from Alternaria sp. MG1 (Tian et al., 2021). Structurally, two new anthraquinones named anthrininones B-C (98–99) with a 4,5-disubstituted butylaminolate unit were obtained from the marine fungus Alternaria tenuissima DFFSCS013 (Pan et al., 2019). In addition, alteamide (100) bearing oxygenated prenyl group was obtained from Alternaria alternata (Wang et al., 2021). 2-(N-vinylacetamide)-4-hydroxymethyl-3-ene-butyrolactone (101) and chrysogeside F (102) were isolated from a marine-derived fungus Alternaria sp. NH-F6 bearing 3-ene-butyrolactone moiety and methyl D-glucopyranoside moiety structures, respectively (Ding et al., 2017). Compounds 103–106 were amide derivatives extracted from marine microorganisms (Li et al., 2015; Wang J. et al., 2015). Of these, (±)-(4R*,5S*,6S*)-3-amino-4,5,6-trihydroxy-2-methoxy-5-methyl-2-cyclohexen-1-one (106) is a new cyclohexenone derivative isolated from the marine Nerium indicum Alternaria sp. SPS-04 (Wang J. et al., 2015). Five new decalin derivatives, altercrasins A-E (107–111), contain lactam-ring structures from a sea-urchin-derived Alternaria sp. (Yamada et al., 2019). The absolute stereostructure of altercrasins A (107) was determined by NMR chemical shifts, NOESY correlations, and electronic circular dichroism (ECD) spectral analyses, and furthermore deduced by chemical transformation and the modified Mosher's method. As a result, the compound pairs of 107/108 and 110/111 were ascertained to be stereoisomers, deduced by the aforementioned methods respectively (Yamada et al., 2019). Dimethylamide asterrate (112), one new asterric acid analog with two new methyl groups, was obtained from an Alternaria sp. isolate (Cai et al., 2014).
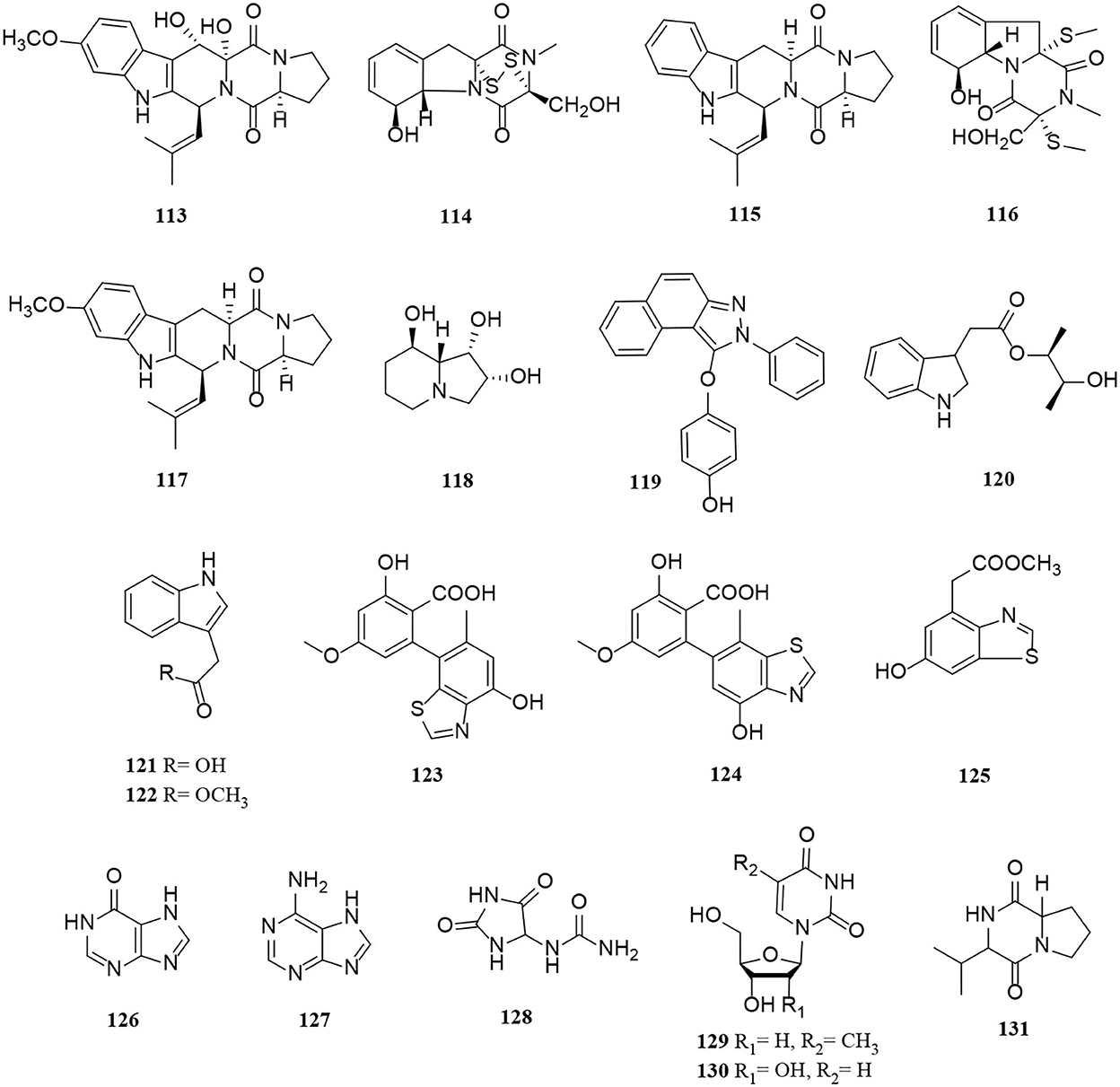
Diketopiperazines (DKPs) consisting of two α-amino acids and cyclic dipeptides are amino acid peptides (He et al., 2019). Five new diketopiperazine derivatives (113–117) were isolated from the marine Alternaria alternate HK-25 (He et al., 2019). In comparison with conventional column chromatography with either C18 or C8 columns, compounds 114 and 116 were successfully separated from crude samples by a new high-speed counter-current chromatography (HSCCC) elution method with high efficiency and recovery (He et al., 2019).
Most alkaloids have heterocyclic structures, such as swainsonine (118), 2H-benzindazole derivative (119), indole derivatives (120–122), and thiazoles (123–125) (Chen et al., 2018; Tan et al., 2019; Wu J. C. et al., 2019; Xu et al., 2019). Alterindazolin A (119) is a rare heterocyclic aromatic compound, containing indazole from Alternaria alternata Shm-1 (Wu X. et al., 2019). Similarly, altenusinoide A (123) and altenusinoide B (124) have an unusual altenusin-thiazole-fused skeleton core (6/6/5) (Chen et al., 2018). Moreover, compound 125 was identified as the first benzothiazole secondary metabolite from the marine sponge-derived fungus Alternaria sp. SCSIOS02F49 (Chen et al., 2018). Compounds (126–130) were purine and pyrimidine derivatives from different Alternaria strains (Miao et al., 2017). Compound (131) was a maculosin derivative isolated from Alternaria alternata (Hawas et al., 2015).
2.3. Quinones
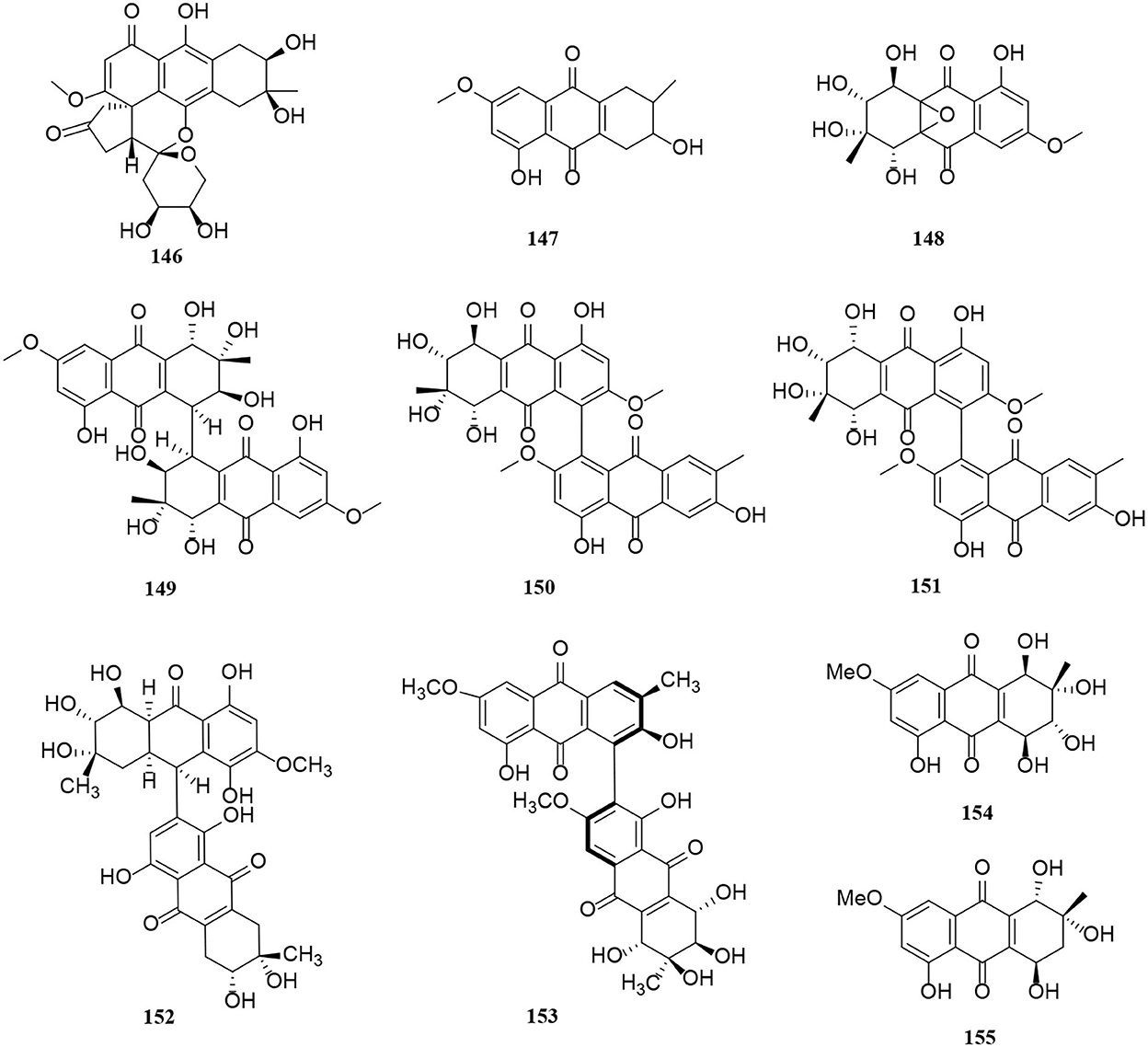
So far, there are two groups of quinones among Alternaria metabolites that have been isolated, perylenequinones and anthraquinones. In this part of the research, 14 perylenequinones (132–145) (Figure 8) and 10 anthraquinones (146–155) (Figure 9) were produced. Perylenequinones are a class of highly conjugated pentacyclic nuclear aromatic polyketones, which are described in detail as follows (Zhao et al., 2019).
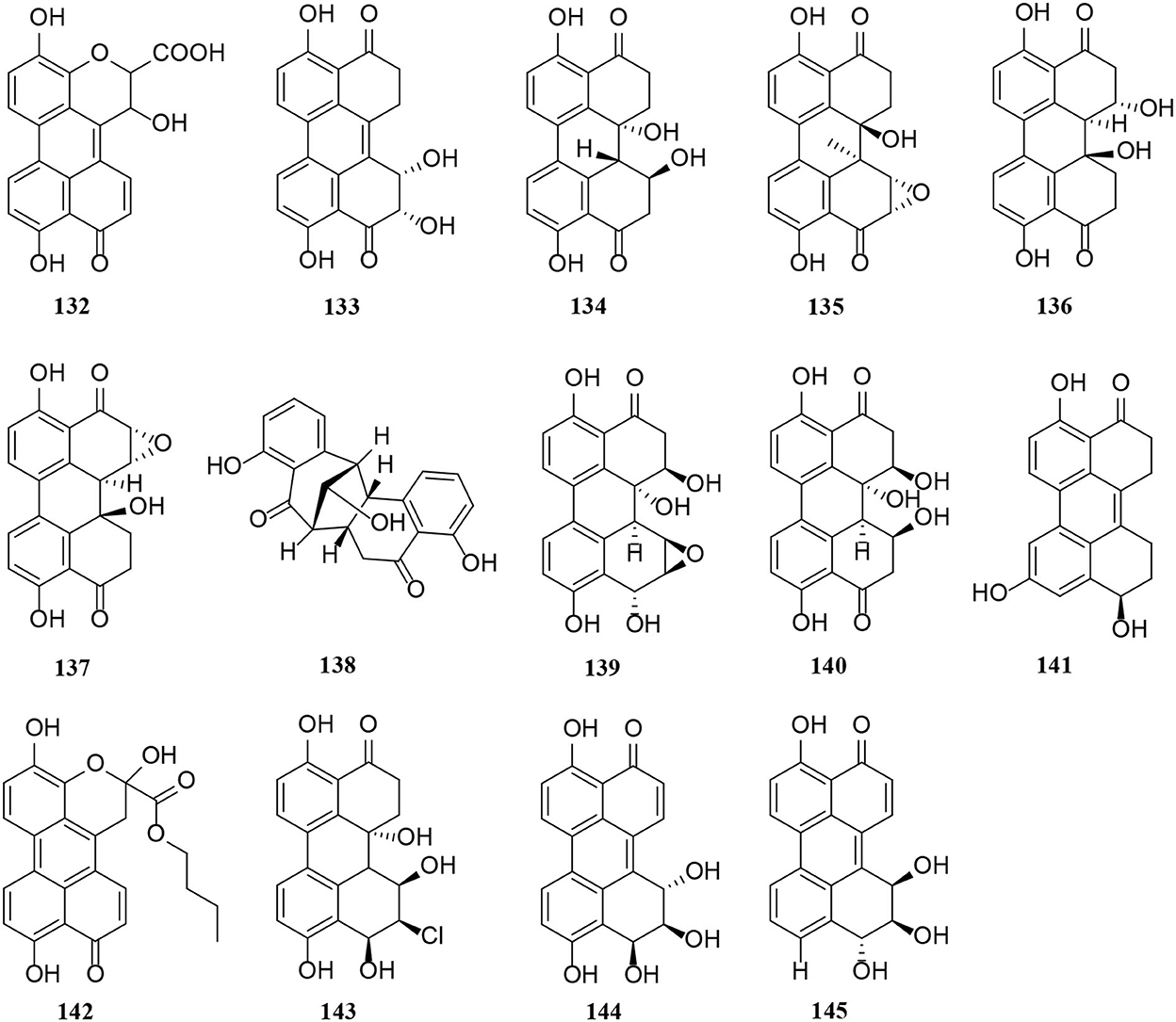
Perylenequinone is generally a dark-colored pigment characterized by an oxidized pentacyclic nuclear skeleton and has been widely used in traditional Chinese herbal medicine (Tantry et al., 2018). Four compounds (132–135) also have the structural skeleton of perylene quinone, namely isoxanalteric acid I (132), altertoxin VII (133), altertoxin I (134), and altertoxin II (135) (Kong et al., 2020; Mahmoud et al., 2021; Tian et al., 2021). In addition, altertoxin I (136) and altertoxin II (137) are two perylene quinone cytotoxins from Alternaria alternata (Hohenbichler et al., 2020). A novel perylenequinone-related derivative, known as alternatone A (138), was isolated from the marine Alternaria alternata L3111′, which possessed an unprecedented tricyclo [6.3.1.0] dodecane skeleton (Zhao et al., 2019). Furthermore, two new perylenequinones (139–140) were isolated from the Pinus ponderosa endophytic Alternaria sp. (Tantry et al., 2018). Compared with compound 140, compound 139 has a significantly epoxide ring. Notably, altertoxin VII (141) and butyl xanalterate (142) are two new polyketides from the sponge-derived fungus Alternaria sp. SCSIO41014. And 141 is the first example to bear a novel 4,8-dihydroxy-substituted perylenequinone structure, while the phenolic hydroxy groups be commonly substituted at C-4 and C-8 (Pang et al., 2018). Moreover, two new perylenequinones (143–144) have a similar structure to deep-sea sediment fungus Alternaria sp. NH-F6, which is characterized as a tetrahydroperylenone (Ding et al., 2017). Altertoxin IV (145) is also a new tetrahydroperylene ketone derivative from the Broussonetia papyrifera fungus Alternaria species G7 (Zhang et al., 2016).
A novel hydroanthraquinone, anthrininone A (146), possessing an unprecedented hexacyclic spiro-fused ring skeleton, was isolated from the marine fungus Alternaria tenuissima DFFSCS013 (Pan et al., 2019). In addition, macrosporin (147) is an anthraquinone from marine Alternaria species (Wang Y. N. et al., 2015). Four new anthraquinone derivatives, compounds (148–151), were isolated from the saline lake Alternaria sp. XZSBG-1 (Chen et al., 2014). In this study, altersolanol O (148) and alterporriol S (149) are relatively rare compounds, representing a novel tetrahydroanthraquinone bearing an epoxy ether bond between C-4a and C-9a and a tetrahydroanthraquinone dimer bearing a C-4-C-4' linkage, respectively (Chen et al., 2014). Alterporriol S (152) and (+)-aS-alterporriol C (153) were also obtained from the marine Alternaria sp. SK11 (Xia et al., 2014). A novel alterporriol-type anthranoid dimer, alterporriol S (152), was represented as the first member of the alterporriol family to possess a unique C-10–C-2′ linkage (Xia et al., 2014). In addition, two anthraquinones (154–155) were isolated from the endophyte Alternaria sp. in Erythrina variegata (Pompeng et al., 2013).
2.4. Terpenes
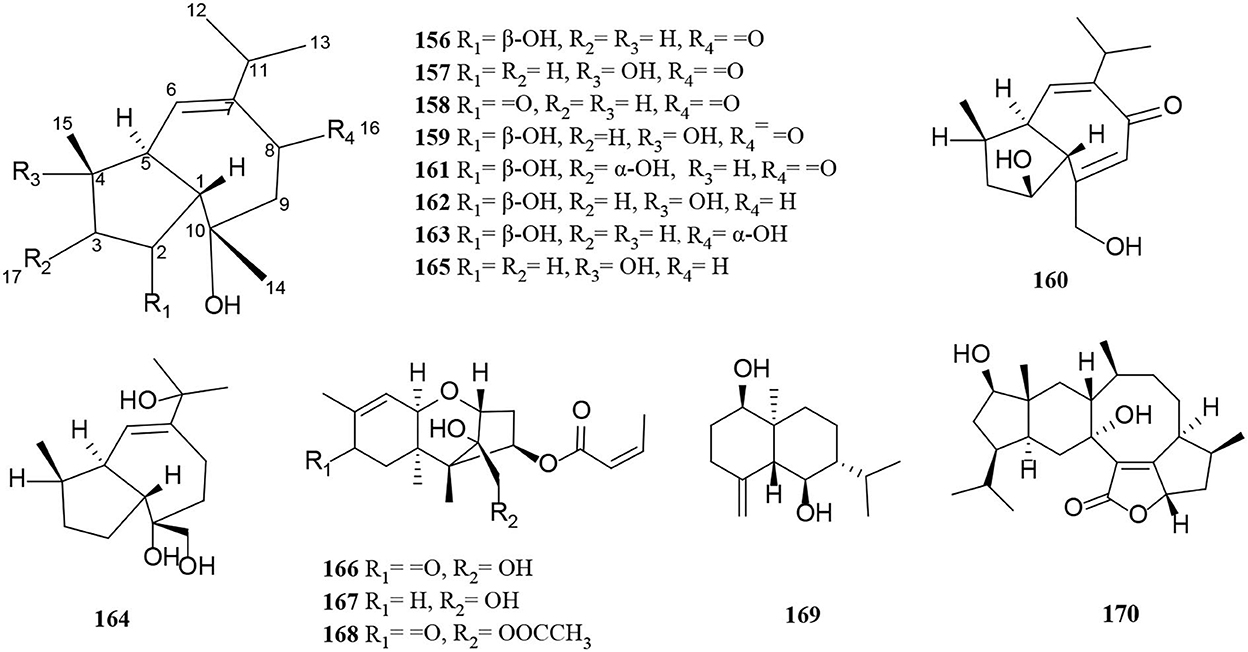
Terpenoids from Alternaria fungi include sesquiterpenes, diterpenes, and meroterpenoids. In this section, a total of 60 terpenoids, comprising 15 sesquiterpenes (156–170) (Figure 10), 16 diterpenes (171–186) (Figure 11), and 29 meroterpenoids (187–215) (Figure 12), are summarized. The specific description is as follows.
Oxytropiols A-J (156–165) were found in 10 undescribed guaiane-type sesquiterpenoids isolated from Alternaria oxytropis (Tan et al., 2019). Their typical structural feature is that they construct a seven-membered ring and fuse a five-membered ring, indicating a guaiacol-type sesquiterpene skeleton. New trichothecene derivatives with a 1, 2-diol moiety at C-12 and C-13, alterchothecenes A-C (166–168), were isolated from Alternaria sp. sb23 bearing a 12, 13-epoxytrichothec-9-ene ring moiety (Gao et al., 2020). Spectra data analysis of NMR, DEPT, and HSQC suggested that 167 is 8-dihydrogeneated derivatives and 168 is 13-acetylated derivatives of 166 respectively (Gao et al., 2020). Similarly, (1R,5R,6R,7R,10S)-1,6-Dihroxyeudesm-4(15)-ene (169) is a new sesquiterpenoid isolated from Alternaria alternate (Xu et al., 2019). In addition, sesteralterin (170) represents the first nitidasane sesterterpene obtained from the marine Alternaria alternata strain (k21-1) (Shi et al., 2017).
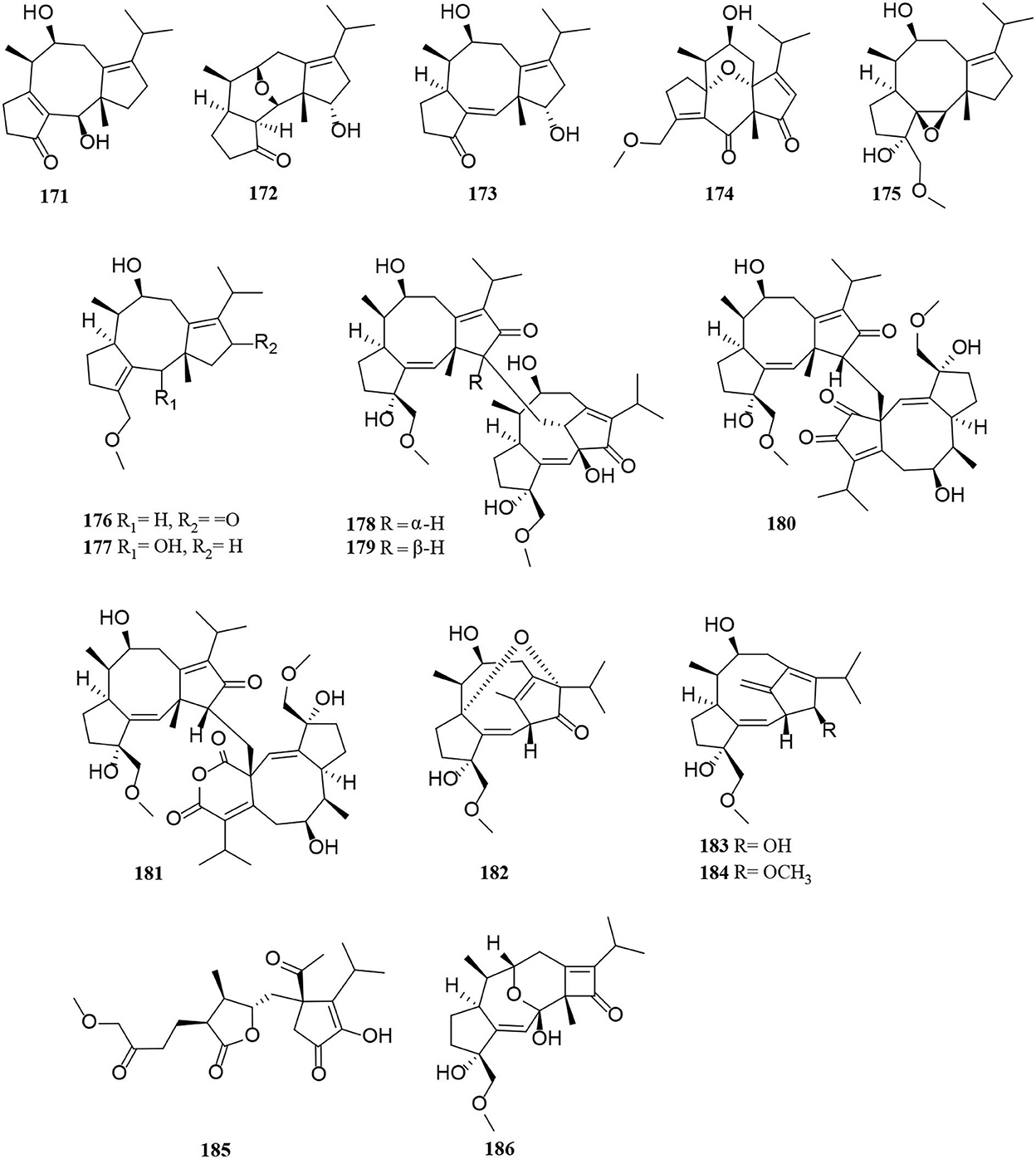
Compounds (171–177) were new fusicoccane-like diterpenoids isolated from modified rice cultures medium of Alternaria brassicicola, among which compounds (171–173) possess a rare 16-nor-dicyclopenta [a, d] cyclooctane structure, compounds 172 and 174 feature two previously new tetracyclic 5/6/6/5 ring systems that represent the typical examples of fusicoccane-type diterpenoids, and compound 175 features a new tetracyclic 5/8/5/3 ring system (Li et al., 2020a). Interestingly, four unprecedented diterpene dimers, alterbrassinoids A-D (178–181), were obtained in the same manner as above (Li et al., 2019a). Compounds (178–181) are the first examples of fusicoccane-derived diterpenoid dimers furnished by forming an undescribed C-12–C-18′ linkage, in which 178 and 179 represent unprecedented heterodimers, whereas 180 and 181 represent unprecedented homodimers (Li et al., 2019a). This suggests that the production of new compounds can be achieved by modifying the medium (Li et al., 2019a, 2020a). Three new rearranged fusicoccane diterpenoids, alterbrassicenes B–D (182–184) bearing a rare bridgehead double-bond-containing tricyclo [9.2.1.0] tetradecane core skeleton found from Alternaria brassicicola (Li et al., 2020b). A highly functionalized diterpenoid, alterbrassicicene A (185), with a new monocyclic carbon skeleton bearing unique dihydro-2(3H)-furanone and 2-cyclopenten1-one motifs, was obtained from Alternaria brassicicola (Li et al., 2018). Alterbrassicene A (186) was characterized as a fusicoccane-derived diterpenoid, possessing an undescribed 5/9/4-fused carbocyclic framework bearing a rare 2-cyclobuten-1-one motif, which were obtained from Alternaria brassicicola (Hu et al., 2018).
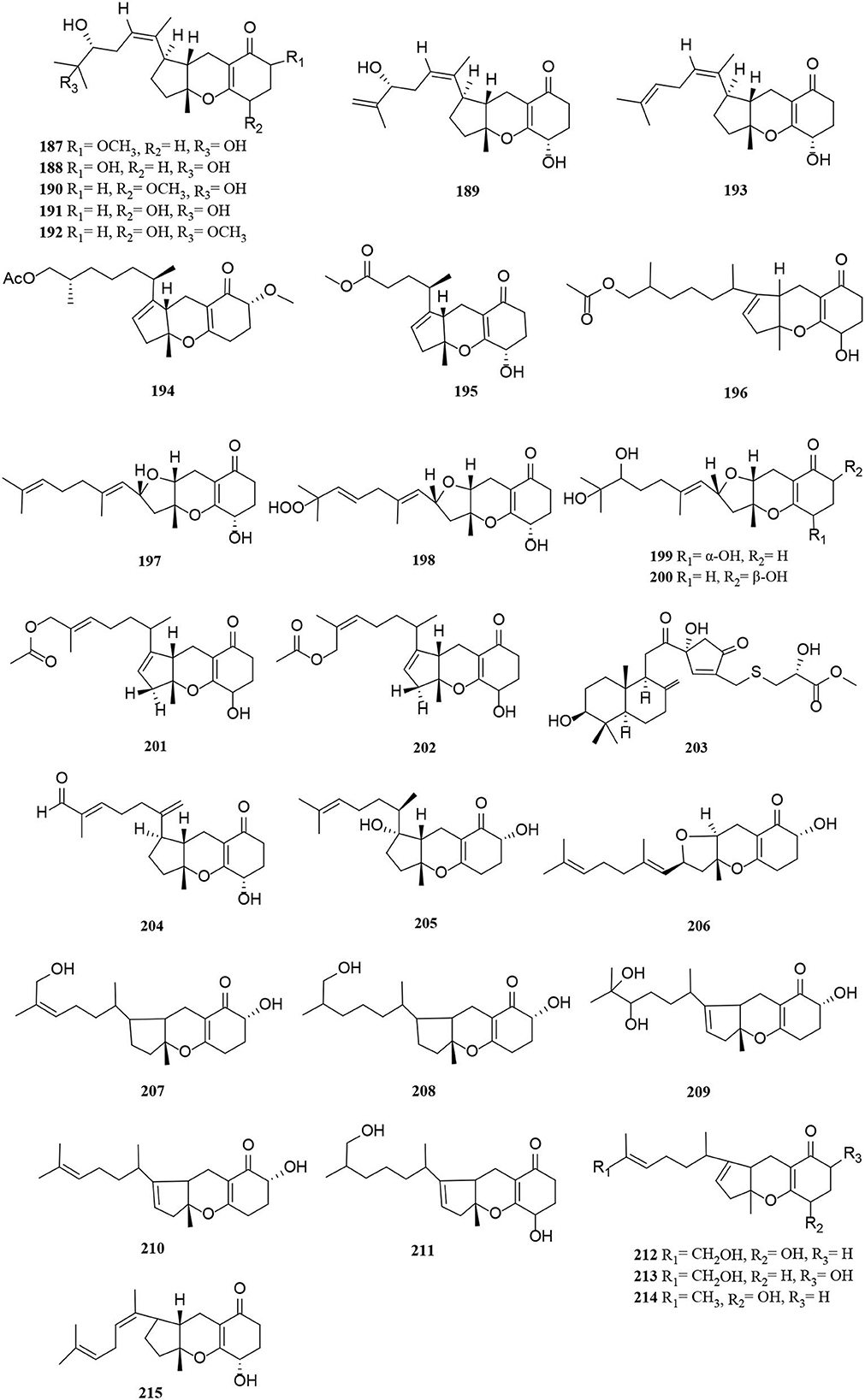
The new compounds (187–196) have a similar tricycloalternarene structure to each other (Shen et al., 2018; Shi et al., 2018a; Li et al., 2019b). Of these, tricycloalternarenes Q-W (187–193) were characterized as seven unprecedented metabolites from Alternaria brassicicola (Li et al., 2019b). Four new meroterpenes, tricycloalterfurenes A-D (197–200), rarely occur in tricycloalternarenes and bear a tetrahydrofuran unit obtained from an Alternaria alternata strain (k21-1). Compound 199 represents the first hydroperoxy-containing tricycloalternarene (Shi et al., 2017). Two new 15-hydroxytricycloalternarenes (201–202) represent a pair of E and Z isomers, possessing a double bond linked by an acetoxymethylene group (Shi et al., 2018a). A rearranged drimane meroterpenoid with a thioglycerate moiety, alternarin A (203), was obtained from the marine fungi Alternaria sp. ZH-15 (Wang H. L. et al., 2020). Tricycloalternarenes X-Y (204–205) and metabolites (206–211) were meroterpenoid compounds isolated similarly from the marine fungi (Pan et al., 2018; Wang L. et al., 2020). Compounds 212–215 were mixed terpenoids isolated from the endophyte Alternaria sp. Be-1 of the insect Pierisrapae Linne (Zhang et al., 2015).
2.5. Other classes
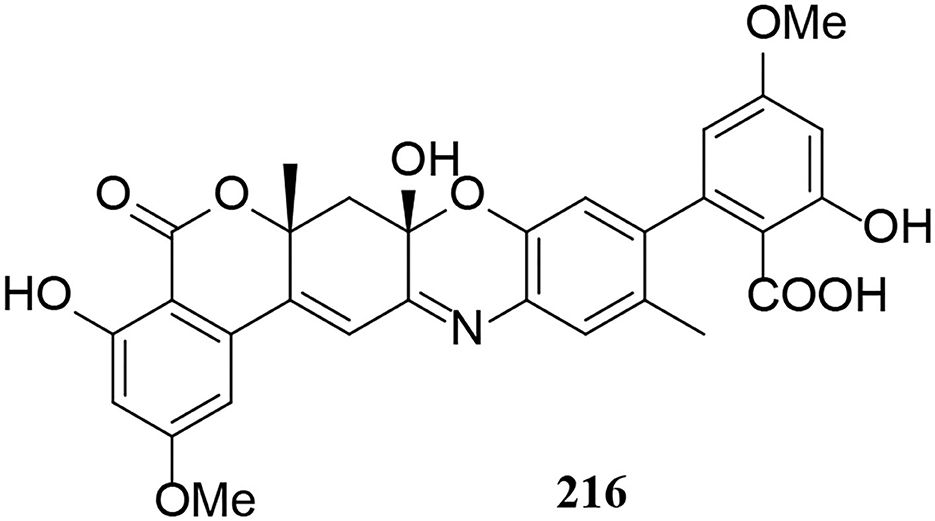
One miscellaneous metabolite 216 was isolated from Alternaria fungi (Figure 13). Notably, bialternacins A (216) is a racemic mixture of aromatic polyketone dimer with an unprecedented 6/6/6/6/6/6-hexacyclic scaffold (Yang C. L. et al., 2019).
3. Biological activity
The biological activities of secondary metabolites of Alternaria fungi are listed in Table 1. As shown in Table 1, antitumor, antibacterial, and antioxidant properties were characterized as the main indexes to assess the biological activity of these natural products (Zhang et al., 2021). Detailed descriptions of the compounds with excellent biological activities are provided as follows.
3.1. Antibacterial activity
Pyranones (37–39, 43, 47) can effectively inhibit fungal growth and have a great impact in the application of biofungicide. Of these, (+)-37 and (+)-38 showed a productive inhibitory effect on Candida albicans with IC50 of 19.5 ± 1.5 and 24.0 ± 1.0 μg/ml, while (–)-37 and (–)-38 were less active, suggesting different antifungal abilities between enantiomers (Wang et al., 2014). Notably, pyranone (43) showed significant activities toward the phytopathogenic bacteria Xoo, Xanthomonas oryzae pv. oryzicola (Xoc), and Rs with minimal inhibitory concentration (MIC) value of 0.5–64 μg/ml, indicating the potential of 43 for the development of novel bactericides (Zhao et al., 2021). Similarly, enantiomeric dibenzo-α-pyrone derivative (47) exhibited moderate antibacterial activities on phytopathogenic bacteria Xoo and Xoc with MIC value of 32–100 μg/ml (Zhao et al., 2020). Pyranone (63) exhibited antimicrobial activity toward Bacillus subtilis and Candida tropicalis with MIC of 0.5–5 μg/disk, which proved that they may be effective biological probes for antibacterial agents (Dalinova et al., 2020). γ-pyranones 79–81 inhibited methicillin-resistant Staphylococcus aureus (MRSA) with an MIC of 2.9, 3.2, and 2.0 μg/ml, respectively (Cai et al., 2014). The structure–activity relationship (SAR) of 79–81 indicated that the possible intramolecular cyclization caused by sulfur atom was necessary. Pyranones 74 and 91 showed antibacterial activity against Fusarium graminearum with MIC values of 107.14 and 215.52 μM, respectively (Wang J. et al., 2015). Compared with 91, 74 showed better activity, probably due to the presence of chlorine atoms in molecular. Moreover, perylenequinone (133) showed antibacterial activity against Streptococcus agalactiae, with an MIC of 17.3 μg/ml (Kong et al., 2020). Moreover, anthraquinone (147) had a strong inhibitory effect on Vibrio anguillarum with an MIC value of 17.6 μmol/L, which can destroy the cell wall and cell membrane, and its effect was equivalent to that of streptomycin at the same concentration (Wang Y. N. et al., 2015). In antimicrobial and antifungal activity tests, meroterpenoid (210) showed a significant inhibitory effect on E. coli and B. subtilis (Pan et al., 2018). Ethyl acetate (EA) fraction of endophytic A. tenuissima OE7 had an inhibitory effect on C. albicans (Chatterjee et al., 2020). Two fractions that could inhibit α-glucosidase activity were obtained from Alternaria destruens, which showed broad-spectrum antibacterial activity (Kaur et al., 2020). The Alternaria extracts with excellent antibacterial activity provide an important direction for future research on antibacterial drugs and will guide bioactivity isolation.
3.2. Antioxidant activity
Antioxidants acknowledged as “free-radical scavengers” have been widely connected to the treatment of aging, cancer, diabetes, etc., (Neha et al., 2019). Pyranone (59) showed scavenging activity of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, with an IC50 of 56.3 μg/ml (Liu et al., 2021). Compounds 91 and 106 showed strong free-radical scavenging efficiency for 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) with EC50 values of 8.19 ± 0.15 and 16.09 ± 0.01 μM, respectively, which were stronger than that of the positive control ascorbic acid (EC50, 17.14 ± 0.11 μM) (Wang J. et al., 2015). A free-radical scavenging test showed that pyranone (35) and nitrogenous metabolites (127–128) also had significant antioxidant activity (Miao et al., 2017; Tian et al., 2017). The discovery of antioxidant compounds is of great significance to various nutraceuticals and cosmetic medicine industries, which has been widely considered as a promising source of new therapeutics.
3.3. Enzyme-inhibitory metabolites
Inhibitory enzymes are often used as biocatalysts to participate in the catalysis of various metabolism activities in living organisms. They attach to the enzyme's active site and reduce the its activity, which can be used as medicine, pathogens, or insecticides in biotechnological applications. Pyranones (1–2) and perylene quinone (132) showed a moderate inhibitory effect on cyclooxygenase-2 (COX-2), with IC50 of 1.50, 7.00, and 7.00 μM. For comparison, celecoxib showed IC50 values of 0.06 μM as a positive control, demonstrating their potential for pharmaceutical uses in antipyretic analgesic and anti-inflammatory drugs (Tian et al., 2021). Pyranone (27) showed antiplatelet and anticoagulant effects after intracoronary tent implantation, with an IC50 of 57.6 ± 3.2 μM (Yang H. et al., 2019). Pyranone (30) showed an inhibitory effect on acetylcholinesterase with an IC50 of 15.5 μM (Yang C. L. et al., 2019). Huperzine can also inhibit acetylcholinesterase activity, which can be a prospective therapeutic drug candidate for Alzheimer's disease (Zaki et al., 2019). In addition, compounds 61 and 65 displayed selective carboxylesterase inhibition activity at a concentration of 100 μg/ml as a key serine hydrolase with potential applications in the treatment of hypertriglyceridemia, obesity, and type 2 diabetes (Zou et al., 2018; Dalinova et al., 2020). Pyranones (76–78) and anthraquinone (150) showed inhibitory activity on α-glucosidase activity with IC50 values of 2.9, 2.8, 3.1, and 7.2 μM, respectively, indicating that they have potential in the treatment of diabetes (Chen et al., 2014; Ruiz-Vargas et al., 2019; Song et al., 2021). Notably, anthrinones A-C (146, 98–99) showed significant inhibitory activity on indoleamine 2,3-dioxygenase 1 (IDO1), and amides (98–99) had selective inhibitory activity on different protein tyrosine phosphatases (Pan et al., 2019). Comparatively, anthraquinone (153) showed strong inhibitory activity against Mycobacterium tuberculosis protein tyrosine phosphatase B (MptpB), with IC50 of 8.70 μM (Xia et al., 2014). Similarly, meroterpenoids (214–215) showed strong inhibitory activity on three tyrosine kinase (EGFR, VEGFR-1, and c-Met) with an inhibition rate of 28.4–56.2%, indicating stronger activity than that of the positive control erlotinib, pazopanib, and bms-777607 (inhibition rate, 100.2, 98.5, and 99.1%, respectively) (Zhang et al., 2015). However, alternative monomer ether (AME) showed selective inhibitory activity on monoamine oxidase A (MAO-α), which may be related to dibenzo of α-pyranone (Lee et al., 2017). The cytotoxin produced by Alternaria can also inhibit topoisomerase (Jarolim et al., 2017).
3.4. Antitumor activity
Some Alternaria metabolites that have been identified as cytotoxic are considered potential sources of cancer chemo-preventive agents. Pyranone 19 and perylene quinone 135 on A549 (EC50, 0.73, 0.40 μg/ml) and PC3 (EC50, 0.17, 0.12 μg/ml) cells exhibited potential cytotoxicity in vitro (Mahmoud et al., 2021). Pyranones 22 and 29 exhibited moderate cytotoxicity against different tumor cells (MDA-MB-231, MCF-7, HeLa, and HepG2), where compound 22 was the most active in MDA-MB-231 and MCF-7 with IC50s of 20.1 and 32.2 μM, respectively (Wu J. C. et al., 2019; Wang et al., 2022). Notably, one pair of new cyclopentaisochromenone enantiomers, (+)-33a and (–)-33b from Alternaria sp. TNXY-P-1, showed distinct selective antitumor activities against HL-60 cell lines with IC50 values of >200 and 75.3 μM, respectively (Lu et al., 2018). Pyranone (56) exhibited cytotoxicity to human myeloma cancer U266, with an IC50 of 24.99 μg/ml (Zhong et al., 2022). However, γ-pyranones 79–81 exhibited weak cytotoxicity to pancreatic cancer cells (MIA PaCa-2), with IC50s of 50.8, 30.3, and 29.3 μM, respectively (Cai et al., 2014). Amide 103 has certain cytotoxicity, strong nephrotoxicity, neurotoxicity, immunotoxicity, carcinogenicity, teratogenicity, and mutagenicity (Li et al., 2015). In comparison, the cytotoxicity of amides (110–111) was equivalent to that of 5-fluorouracil (Yamada et al., 2019). Alkaloid (118) was merely cytotoxic to A549 and HeLa, with IC50s of 10.93 ± 0.80 and 66.69 ± 1.58 μM, respectively (Tan et al., 2019). Antitumor activity of 118 to A549 is equivalent to the positive control cis-platinum (IC50 values of 8.73 ± 1.77) (Tan et al., 2019). Two variants of an extract from cultured Alternaria alternata, quinones 136–137, displayed dose-dependent enhancements of cytochrome P450 (CYP) activity by testing singularly the 7-ethoxy-resorufin-O-deethylase (EROD) assay in MCF-7 breast cancer cells (Hohenbichler et al., 2020). In addition, perylenequinone (141) had cytotoxicity to K562, SGC-7901, and BEL-7402 with IC50s are 26.58 ± 0.80, 8.75 ± 0.13, and 13.11 ± 0.95 μg/ml, respectively (Pang et al., 2018). Diterpenes 172, 173, 175, and 177 were active against certain human tumor cell lines, with IC50 values ranging from 25.0 to 38.2 μM, but had no obvious toxicity to the normal LO2 cells (Li et al., 2020a). Interestingly, terpenoids 178–184, 187, 188, 191, and 193 all had antitumor activity, of which diterpenes 178–181 exhibited moderate cytotoxicity to OCvar, MDA-MB-231, HeLa, and HT-29, while being non-toxic to normal cells (Li et al., 2019a). Diterpenes 182–184 exhibited moderate cytotoxic activity against certain human tumor cell lines, with IC50 values in the range of 15.87–36.85 μM, but no obvious cytotoxicity to human normal cell LO2 (Li et al., 2020b). Meroterpenoids 187, 188, 191, and 193 exhibited selective cytotoxicity to some human cancer cells, with IC50s ranging from 12.83 to 32.87 μM; meanwhile, they had no obvious effect on normal human LO2 cells, indicating their significant potential as selective cancer chemo-preventive agents (Li et al., 2019b). Meroterpenoid 196 displayed inhibitory activity against the growth of SMMC-7721 cells with an IC50 of 49.7 ± 1.1, which is comparable with that of the positive control, cisplatin (IC50 = 6.5 ± 0.5 μg/ml) (Shen et al., 2018). Similarly, meroterpenoid 204 showed cytotoxicity to HL-60 and HO8910 cells, with IC50 of 7.54 and 20.32 μM (Wang L. et al., 2020). The emergence of a large number of metabolites with antitumor activities provides more opportunities for the development of cancer-treatment drugs.
3.5. Phytotoxicity
Partial metabolites of Alternaria fungi have exhibited pathogenicity that causes damage to plants and possess the potential to be as herbicides on account of excellent phytotoxicity (Meena and Samal, 2019; Leyte-Lugo et al., 2020). In phytotoxicity assays, pyranone 10a and 10b showed a significant inhibition rate on the germination of monocotyledonous weed seeds (E. crusgalli and S. viridis), with inhibitory ratios ranging from 68.6 ± 6.4 to 84.2 ± 5.1%, which was equivalent to that of the positive control, glyphosate, at a concentration of 100 μg/ml (Li et al., 2021). At 1 mg/ml, pyranone 69 showed contact insecticidal activity against wheat aphids (Schizaphis graminum), indicating its use as a potential agricultural insecticide (Dalinova et al., 2020). In addition, sesquiterpenoid 156 showed an inhibition of the root growth of Arabidopsis thaliana but no remarkable effect on leaf growth (Tan et al., 2019). Sesquiterpene (170) and meroterpenoids 194–195 and 197–202 showed weak or moderate inhibition of the growth of marine algae and plankton (Shi et al., 2017, 2018a). Among the three tested marine phytoplankton (Chattonella marina, Heterosigma akashiwo, and Prorocentrum donghaiense), compounds 170 and 197–200 appeared more sensitive to C. marina (Shi et al., 2017). Compounds 170 and 197 showed inhibition of these three phytoplanktons but were inactive to the zooplankton A. salina, indicating that the hydroxy group positions on ring C had almost no effect on their activities. Hydroxylation at C-2 and C-3 (199 and 200) slightly reduced the inhibition of the three phytoplankton (17–56% inhibition) (Shi et al., 2017). Taking structure into account, α-pyranones and terpenoids have great potential as biological control candidates in the application of herbicide, insecticide and marine protection.
3.6. Other activities
The various activity of Alternaria metabolites is of great significance for research. Pyranones 4, 6, and 9 exhibited inhibitory activities related to the SARS-CoV-2 virus (EC50 = 0.02, 0.3, 0.07 μM), which is conducive for the development of antiviral drugs (Lu et al., 2021). In addition, pyranones 50–51 exhibited a specific inhibitory effect on L. donovani and P. falciparum (Shi et al., 2019). Interestingly, compounds 139 and 140 have insect-resistant activity, and 139 showed antibacterial activity against Leishmania donovani with IC50 = 2.55 μg/ml (Tantry et al., 2018). In the study of biological mechanisms, 73 inhibited the oxidation of human plasma high-density lipoprotein (HDL) and low-density lipoprotein (LDL) induced by Cu2+, which is of great significance for Cardiovascular and cerebrovascular drugs development (Kim et al., 2019). Compound 144 exhibited a potent inhibition rate of 88.1% at a concentration of 10 μM, which provides new bromodomain protein 4 (BRD4) inhibitors possessing potential antitumoral, antiviral and anti-inflammatory pharmaceutical effects (Ding et al., 2017). In addition, anthraquinone (154) was further characterized to have good anti-angiogenic activity in vivo and in vitro by aortic-sprouting assay in rats, related to inhibited proliferation, tube formation, and migration in endothelial cells (Pompeng et al., 2013). Compounds 122 and 177 exhibited neuroprotective effects and moderate anti-inflammatory effects, respectively (Shi et al., 2017; Tian et al., 2021). Diterpene (185) was the first fusicoccane-derived diterpenoid to function as a potent peroxisome proliferator-activated receptor (PPAR-γ) agonist (EC50 = 744.1 nM) (Li et al., 2018). In addition, diterpenes (186) can inhibit IKKβ in the NF-κB signal pathway and have obvious anti-inflammatory activity (Hu et al., 2018). Meroterpenoid 203 can inhibit neuronal excitation due to its unique cyclopentanone structure, which will be applied in antiepileptic drugs development (Wang H. L. et al., 2020).
4. Possible biosynthesis mechanism of secondary metabolites
Biosynthesis is indispensable in the application of natural products. The diversity of endophytic biosynthesis often depends on the diversity of the host and the complexity of its metabolism, which provide a new way for the biosynthesis of various novel compounds (Lin et al., 2019; He et al., 2021). The study of biosynthetic pathways in pharmaceutical chemistry contributes to the discovery of novel drugs and provides new research opportunities for the sustainable development and utilization of natural drugs (Lin et al., 2019; He et al., 2021).
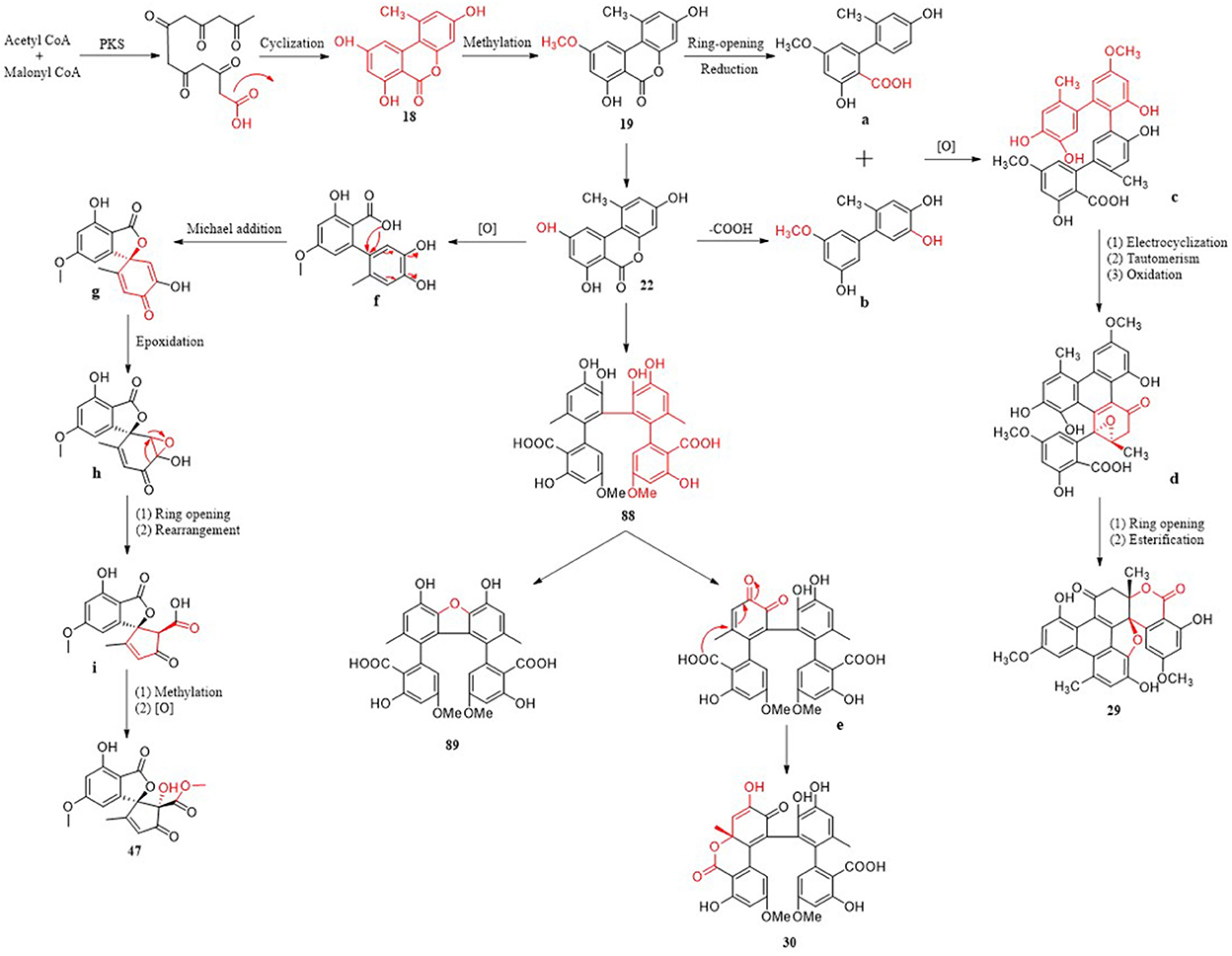
Polyketones have a variety of structural types and corresponding biosynthetic pathways. Three metabolic pathways of polyketones from Alternaria fungi are briefly described, and eight important metabolites are involved (Figure 14). The cinnamic acid–shikimic pathway, a familiar biosynthetic pathway, emerged as the basis of various biosynthetic pathways. Firstly, a heptapeptide intermediate can be produced by iterative condensation of acetyl-CoA(starter) with six molecules of malonyl-CoA (extenders) by polyketide synthase (PKS). Subsequently, the heptapeptide intermediate is cyclized to obtain compound 18, followed by methylation to obtain 19. The key intermediate molecule 22 is obtained from the loop-opened of 19, and then the carboxyl group is removed to form intermediate molecule b (Wu J. C. et al., 2019). Finally, compound 29 featuring an unprecedented seven-ring backbone, which was obtained from two molecular intermediates a and b through oxidative coupling, electrocyclization, tautomerism, oxidation, ring opening, and esterification (Wu J. C. et al., 2019). Complex compounds 30, 91, and 92 are also polymerized from two molecules with simple structures. Compound 22 can be dimerized via a C–C bond to form compound 88 through intermolecular oxidative phenol coupling, catalyzed most likely by a P450 monooxygenase or laccase. Dehydration of 88 gives compound 89 (Yang C. L. et al., 2019). Oxidation, regioselective intramolecular Michael additions, and Ketone–enol tautomerization of catechol in 88 afforded a new compound, 30, with a lactone ring (Yang C. L. et al., 2019). It is worth noting that a third possible biosynthetic pathway generates two five-membered rings, which are completely different from the first two pathways. f as an ortho-quinone intermediate is formed via oxidization of the catechol moiety in 22, followed by regioselective Michael additions that give intermediate g. Intermediate i was obtained after epoxidation and stereospecific acid-catalyzed rearrangement of intermediate g, indicating that the carbon skeleton of 47 was formed by the key epoxy-rearrangement step (Zhao et al., 2020). Then, compound 47 yielded the methylation and oxidization of i. As the starting materials of various metabolic pathways, compound 22 plays an important role in the biosynthesis and transformation of new compounds. This provides a new synthetic route for obtaining the novel structure of Alternaria fungi metabolites. In addition, the polyketide metabolites may also have a variety of metabolic pathways to be discovered, which is worthy of deep research.
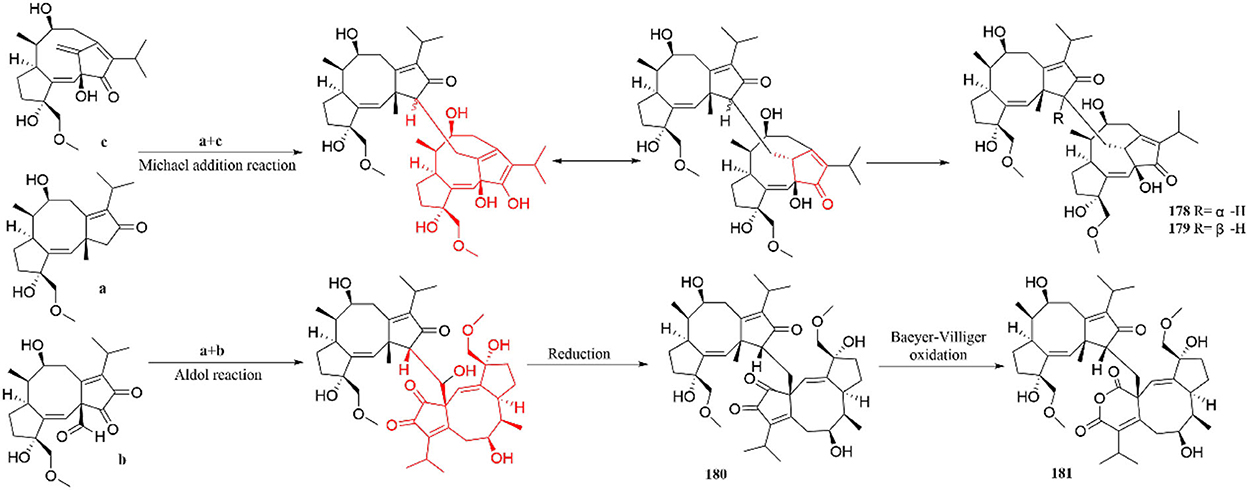
Furthermore, the possible biosynthetic pathways of terpenoid dimers are also described (Figure 15). Brassicicene A synthesizes three intermediates (a\b and c) through dehydrogenation, oxidation, and Wagner–Meerwein rearrangement. Intermediates a and c are formed through Michael addition reaction to produce 178 and 179. Interestingly, they are a pair of unprecedented heterodimers, bearing dicyclopentane [a, d], cyclooctane, and tricyclo [9.2.1.0] tetradecane diterpenoid subunits (Li et al., 2019a). In addition, compounds 180 and 181 are obtained by a series of aldol and reduction reactions, containing two dicyclopentadiene [a, d] cyclooctane diterpene subunits (Li et al., 2019a).
5. Conclusion and prospects
Fungi are ubiquitous in nature with their tenacious vitality and serve as a wealthy reservoir of structurally diverse metabolites. Alternaria fungi occupy a wide spectrum of habitats in diverse ecosystems worldwide. Remarkable progress has been made in the characterization of Alternaria fungi metabolites. Data showed that the number of articles published, the number of strains discovered, the number of new compounds, and the total compounds all increased dramatically from 2014 to 2019 (Figure 16). Numerous chemical studies suggest that Alternaria fungi are one of the prolific sources of functional biomolecules, including polyketides, terpenoids, quinones, and nitrogen-containing compounds. In this study, 216 metabolites from Alternaria species with diverse chemical structures and bioactivities were reviewed based on research from 2014 to 2022 (Figure 17). Polyketones, as the largest number of bio-metabolites, have immense potential in various fields of agriculture and the food and medical industries, considering their characteristics as being antibacterial and enzyme-inhibitory, as well as having antitumor, antioxidant, and phytotoxic properties, amongst others. Remarkably, terpenoids and quinones provided a higher proportion of active compounds. Additionally, the basic biosynthetic pathways of polyketones and terpenoid dimers have also been discussed, which would allow production for industrial purposes.
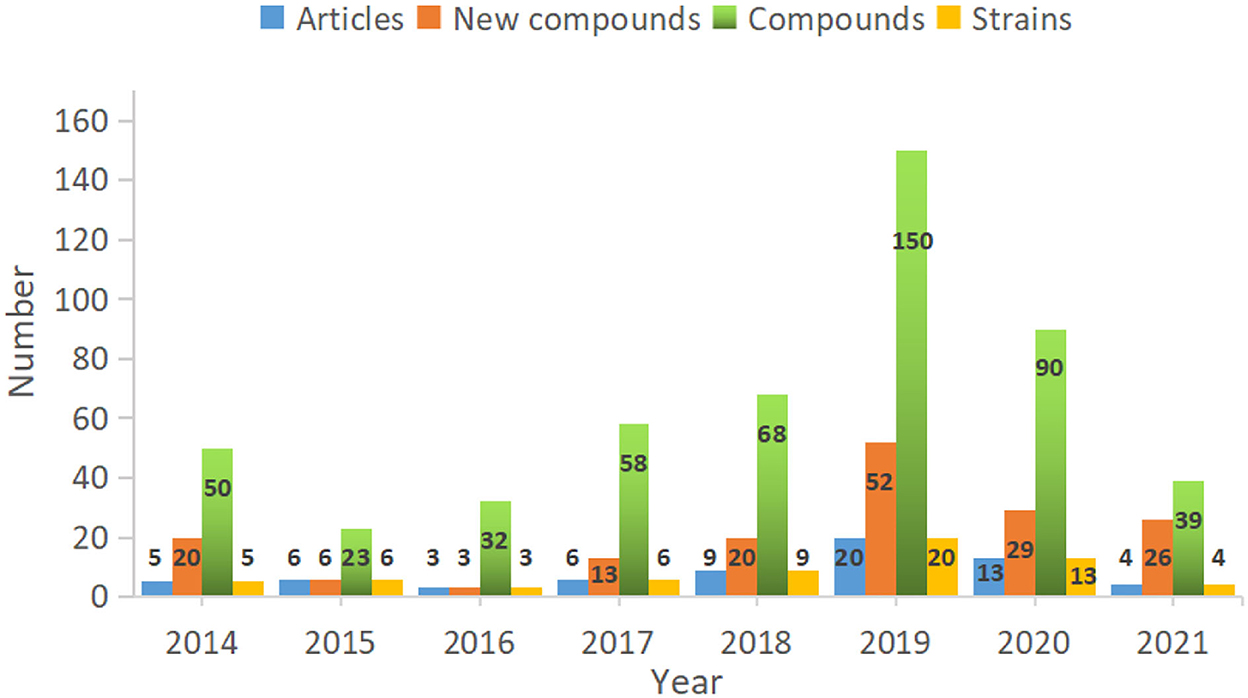
Figure 16. The number of articles, compounds, and strains reported from Alternaria fungi in recent years.
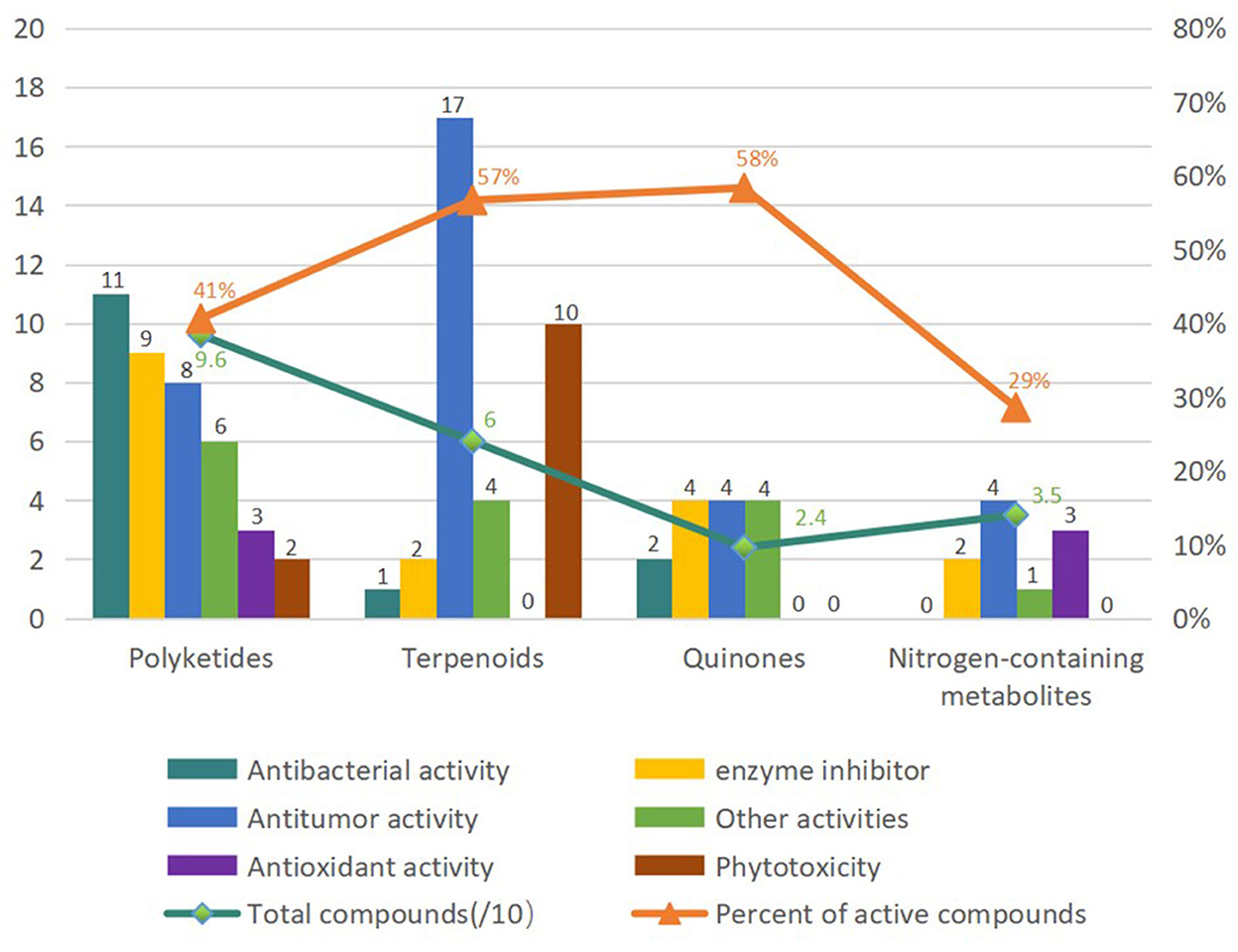
Figure 17. Classification of diverse chemicals of Alternaria fungi based on the pharmacological activities.
Unfortunately, the study of secondary metabolites has decreased in the past 2 years. Many metabolites remain to be discovered. Therefore, the construction and breeding of strains, as well as optimization of cultivation and fermentation processes, should be intensively conducted to accelerate the development of valuable products. In addition, a better understanding of the evaluation of bioactivities and pharmacological mechanisms would assist in ascertaining underlying therapeutic potential. Moreover, studying the molecular basis of biosynthetic pathways would be necessary for industrial production. More efforts should be made to explore further sources for the isolation of new Alternaria strains and to manufacture novel functional biomolecules using new strategies, such as the “one strain many compounds” (OSMAC) approach, genetic mining (phylogenomic analyses), combined with metabolic engineering.
Finally, we believe the therapeutic potential and chemical diversity of Alternaria fungi will provide new avenues for drug discovery with deep research.
Author contributions
JLi, SY, XY, and JM: conceptualization. SZ, SX, and MR: discussion of the contents. JM, MR, SW, and HZ: writing—original draft preparation. SZ, JLiu, SX, SY, JM, MR, and XY: writing—review and editing. All authors have read and approved the final manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, Grant Numbers 31900286 to XY and 81703380 to JLi and the Research and Innovation Fund of Wuhan Asia General Hospital, Grant Number 2022KYCX1-A02.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Obaidi, J. R., Jambari, N. N., and Ahmad-Kamil, E. I. (2021). Mycopharmaceuticals and nutraceuticals: promising agents to improve human well-being and life quality. J. Fungi. 7, 503. doi: 10.3390/jof7070503
Bemmann, W. (1986). Phytoeffective alternaria-metabolite phytoeffective metabolites of Alternaria. Zentralbl. Mikrobiol. 141, 49–66. doi: 10.1016/S0232-4393(86)80085-3
Brian, P. W., Curtis, P. J., Hemming, H. G., Jefferys, E. G., Unwin, C. H., and Wright, J. M. (1951). Alternaric acid; a biologically active metabolic product of Alternaria solani (Ell. and Mart.) Jones and Grout; its production, isolation and antifungal properties. J. Gen. Microbial. 5, 619–632. doi: 10.1099/00221287-5-4-619
Cai, S., King, J. B., Du, L., Powell, D. R., and Cichewicz, R. H. (2014). Bioactive sulfur-containing sulochrin dimers and other metabolites from an Alternaria sp. isolate from a Hawaiian soil sample. J. Nat. Prod. 77, 2280–2287. doi: 10.1021/np5005449
Chatterjee, S., Ghosh, R., and Mandal, N. C. (2020). Inhibition of biofilm- and hyphal- development, two virulent features of Candida albicans by secondary metabolites of an endophytic fungus Alternaria tenuissima having broad spectrum antifungal potential. Microbiol Res. 232, 126386. doi: 10.1016/j.micres.2019.126386
Chen, A., Mao, X., Sun, Q., Wei, Z., Li, J., You, Y., et al. (2021). Alternaria mycotoxins: an overview of toxicity, metabolism, and analysis in food. J. Agric. Food Chem. 69, 7817–7830. doi: 10.1021/acs.jafc.1c03007
Chen, B., Shen, Q., Zhu, X., and Lin, Y. (2014). The anthraquinone derivatives from the fungus Alternaria sp. XZSBG-1 from the saline lake in Bange, Tibet, China. Molecules 19, 16529–16542. doi: 10.3390/molecules191016529
Chen, Y., Chen, R., Xu, J., Tian, Y., Xu, J., and Liu, Y. (2018). Two new altenusin/thiazole hybrids and a new benzothiazole derivative from the marine sponge-derived fungus Alternaria sp. SCSIOS02F49. Molecules 23, 2844. doi: 10.3390/molecules23112844
Dalinova, A., Chisty, L., Kochura, D., Garnyuk, V., Petrova, M., Prokofieva, D., et al. (2020). Isolation and bioactivity of secondary metabolites from solid culture of the fungus, Alternaria sonchi. Biomolecules 10, 81. doi: 10.3390/biom10010081
Ding, H., Zhang, D., Zhou, B., and Ma, Z. (2017). Inhibitors of BRD4 protein from a marine-derived fungus Alternaria sp. NH-F6. Mar. Drugs 15, 76. doi: 10.3390/md15030076
Feng, Z. H., and Sun, G. Y. (2020). Advances in the classification of Alternaria and related genera. J. Fungal Res. 18, 294–303. doi: 10.13341/j.jfr.2020.8010
Gao, Y., Zhou, J., and Ruan, H. (2020). Trichothecenes from an Endophytic Fungus Alternaria sp. sb23. Planta Med. 86, 976–982. doi: 10.1055/a-1091-8831
Hawas, U. W., El-Desouky, S., Abou El-Kassem, L., and Elkhateeb, W. (2015). Alternariol derivatives from Alternaria alternata, an endophytic fungus residing in red sea soft coral, inhibit HCV NS3/4A protease. Appl. Biochem. Microbiol. 51, 579–584. doi: 10.1134/S0003683815050099
He, T., Bao, J., Leng, Y., Snow, D., Kong, S., Wang, T., et al. (2021). Biotransformation of doxycycline by Brevundimonas naejangsanensis and Sphingobacterium mizutaii strains. J. Hazard. Mater. 411, 125126. doi: 10.1016/j.jhazmat.2021.125126
He, X., Ding, L., Yi, M., Xu, J., Zhou, X., Zhang, W., et al. (2019). Separation of five diketopiperazines from the marine fungus Alternaria alternate HK-25 by high-speed counter-current chromatography. J. Sep. Sci. 42, 2510–2516. doi: 10.1002/jssc.201801284
Hohenbichler, J., Aichinger, G., Rychlik, M., Del Favero, G., and Marko, D. (2020). Alternaria alternata toxins synergistically activate the aryl hydrocarbon receptor pathway in vitro. Biomolecules 10, 1018. doi: 10.3390/biom10071018
Hu, Z., Sun, W., Li, F., Guan, J., Lu, Y., Liu, J., et al. (2018). Fusicoccane-derived diterpenoids from Alternaria brassicicola: investigation of the structure-stability relationship and discovery of an IKKβ inhibitor. Org. Lett. 20, 5198–5202. doi: 10.1021/acs.orglett.8b02137
Ibrahim, S., Choudhry, H., Asseri, A. H., Elfaky, M. A., Mohamed, S., and Mohamed, G. A. (2022). Stachybotrys chartarum-A hidden treasure: secondary metabolites, bioactivities, and biotechnological relevance. J. Fungi 8, 504. doi: 10.3390/jof8050504
Ibrahim, S., Sirwi, A., Eid, B. G., Mohamed, S., and Mohamed, G. A. (2021). Bright side of Fusarium oxysporum: secondary metabolites bioactivities and industrial relevance in biotechnology and nanotechnology. J. Fungi 7, 943. doi: 10.3390/jof7110943
Jarolim, K., Del Favero, G., Ellmer, D., Stark, T. D., Hofmann, T., Sulyok, M., et al. (2017). Dual effectiveness of Alternaria but not Fusarium mycotoxins against human topoisomerase II and bacterial gyrase. Arch. Toxicol. 91, 2007–2016. doi: 10.1007/s00204-016-1855-z
Kaur, J., Sharma, P., Kaur, R., Kaur, S., and Kaur, A. (2020). Assessment of alpha glucosidase inhibitors produced from endophytic fungus Alternaria destruens as antimicrobial and antibiofilm agents. Mol. Biol. Rep. 47, 423–432. doi: 10.1007/s11033-019-05145-3
Keller, N. P. (2019). Fungal secondary metabolism: regulation, function and drug discovery. Nat Rev Microbiol 17, 167–180. doi: 10.1038/s41579-018-0121-1
Kim, J. W., Kim, J. Y., Li, W., Ryu, J. Y., Kim, S., and Shim, S. H. (2019). Chromones with lipoprotein oxidation inhibitory activity from an endophytic fungus Alternaria brassicae JS959 derived from Vitex rotundifolia. J. Antibiot. 72, 709–713. doi: 10.1038/s41429-019-0198-4
Kong, F. D., Yi, T. F., Ma, Q. Y., Xie, Q. Y., Zhou, L. M., Chen, J. P., et al. (2020). Biphenyl metabolites from the patchouli endophytic fungus Alternaria sp. PfuH1. Fitoterapia 146, 104708. doi: 10.1016/j.fitote.2020.104708
Lee, C., Li, W., Bang, S., Lee, S. J., Kang, N. Y., Kim, S., et al. (2019). Secondary metabolites of the endophytic fungus Alternaria alternata JS0515 isolated from Vitex rotundifolia and their effects on pyruvate dehydrogenase activity. Molecules 24, 4450. doi: 10.3390/molecules24244450
Lee, H. W., Kim, Y. J., Nam, S. J., and Kim, H. (2017). Potent selective inhibition of monoamine oxidase a by alternariol monomethyl ether isolated fROM Alternaria brassicae. J. Microbiol. Biotechnol. 27, 316–320. doi: 10.4014/jmb.1610.10053
Leyte-Lugo, M., Richomme, P., Poupard, P., and Peña-Rodriguez, L. M. (2020). Identification and quantification of a phytotoxic metabolite from Alternaria dauci. Molecules 25, 4003. doi: 10.3390/molecules25174003
Li, F., Lin, S., Zhang, S., Hao, X., Li, X. N., Yang, B., et al. (2019a). Alterbrassinoids A–D: fusicoccane-derived diterpenoid dimers featuring different carbon skeletons from Alternaria brassicicola. Org. Lett. 21, 8353–8357. doi: 10.1021/acs.orglett.9b03133
Li, F., Lin, S., Zhang, S., Pan, L., Chai, C., Su, J. C., et al. (2020a). Modified fusicoccane-type diterpenoids from Alternaria brassicicola. J. Nat. Prod. 83, 1931–1938. doi: 10.1021/acs.jnatprod.0c00165
Li, F., Pan, L., Lin, S., Zhang, S., Li, H., Yang, B., et al. (2020b). Fusicoccane-derived diterpenoids with bridgehead double-bond-containing tricyclo[9.2.1.0(3,7)]tetradecane ring systems from Alternaria brassicicola. Bioorg. Chem. 100, 103887. doi: 10.1016/j.bioorg.2020.103887
Li, F., Sun, W., Guan, J., Lu, Y., Zhang, S., Lin, S., et al. (2018). Alterbrassicicene A, a highly transformed fusicoccane-derived diterpenoid with potent PPAR-γ agonistic activity from Alternaria brassicicola. Org. Lett. 20, 7982–7986. doi: 10.1021/acs.orglett.8b03553
Li, F., Tang, Y., Sun, W., Guan, J., Lu, Y., Zhang, S., et al. (2019b). New cytotoxic tricycloalternarenes from fungus Alternaria brassicicola. Bioorg. Chem. 92, 103279. doi: 10.1016/j.bioorg.2019.103279
Li, F., Ye, Z., Huang, Z., Chen, X., Sun, W., Gao, W., et al. (2021). New α-pyrone derivatives with herbicidal activity from the endophytic fungus Alternaria brassicicola. Bioorg. Chem. 117, 105452. doi: 10.1016/j.bioorg.2021.105452
Li, Y. F., Wang, H. W., Xu, J. Y., Li, J., and Liu, L. (2015). The secondary metabolites of the crinoid (Comanthina schlegeli) epipsymbiosis fungus Alternaria brassicae 93. Acta Scientiarum Naturalium Universitatis Sunyatseni 54, 75–78. doi: 10.13471/j.cnki.acta.snus.2015.04.014
Lin, H. C., Hewage, R. T., Lu, Y. C., and Chooi, Y. H. (2019). Biosynthesis of bioactive natural products from basidiomycota. Org. Biomol. Chem. 17, 1027–1036. doi: 10.1039/C8OB02774A
Liu, G., Niu, S., and Liu, L. (2021). Alterchromanone A, one new chromanone derivative from the mangrove endophytic fungus Alternaria longipes. J. Antibiot. 74, 152–155. doi: 10.1038/s41429-020-00364-4
Lou, J., Fu, L., Peng, Y., and Zhou, L. (2013). Metabolites from Alternaria fungi and their bioactivities. Molecules 18, 5891–5935. doi: 10.3390/molecules18055891
Lu, X., Tang, X. Y., Wang, H. X., Huang, W. J., Feng, W. X., and Feng, B. M. (2021). Polyketone metabolites isolated from Rhodiola tibetica endohytic fungus Alternaria sp. HJT-Y7 and their SARS-CoV-2 virus inhibitory activitives. Bioorg. Chem. 116, 105309. doi: 10.1016/j.bioorg.2021.105309
Lu, X. J., Chen, S. F., Xu, X. W., Zhao, D., Wang, H. F., Bai, J., et al. (2018). One pair of new cyclopentaisochromenone enantiomer from Alternaria sp. TNXY-P-1 and their cytotoxic activity. J. Asian Nat. Prod. Res. 20, 328–336. doi: 10.1080/10286020.2017.1336164
Mahmoud, M. M., Abdel-Razek, A. S., Soliman, H., Ponomareva, L. V., Thorson, J. S., Shaaban, K. A., et al. (2021). Diverse polyketides from the marine endophytic Alternaria sp. LV52: Structure determination and cytotoxic activities. Biotechnol. Rep. 33, e00628. doi: 10.1016/j.btre.2021.e00628
Meena, M., and Samal, S. (2019). Alternaria host-specific (HSTs) toxins: an overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol. Rep. 6, 745–758. doi: 10.1016/j.toxrep.2019.06.021
Miao, Z., Ma, Y. M., Kong, Y., Yan, M. R., and Wang, J. (2017). Study on alkaloid metabolites of a strain of Alternaria fungi. Jiangsu Agric. Sci. 45, 314–316. doi: 10.15889/j.issn.1002-1302.2017.22.081
Miyanaga, A. (2017). Structure and function of polyketide biosynthetic enzymes: various strategies for production of structurally diverse polyketides. Biosci. Biotechnol. Biochem. 81, 2227–2236. doi: 10.1080/09168451.2017.1391687
Neha, K., Haider, M. R., Pathak, A., and Yar, M. S. (2019). Medicinal prospects of antioxidants: a review. Eur. J. Med. Chem. 178, 687–704. doi: 10.1016/j.ejmech.2019.06.010
Noor, A. O., Almasri, D. M., Bagalagel, A. A., Abdallah, H. M., Mohamed, S., Mohamed, G. A., et al. (2020). Naturally occurring isocoumarins derivatives from endophytic fungi: sources, isolation, structural characterization, biosynthesis, and biological activities. Molecules 25, 395. doi: 10.3390/molecules25020395
Pan, D., Zhang, X., Zheng, H., Zheng, Z., Nong, X., Liang, X., et al. (2019). Novel anthraquinone derivatives as inhibitors of protein tyrosine phosphatases and indoleamine 2,3-dioxygenase 1 from the deep-sea derived fungus Alternaria tenuissima DFFSCS013. Org. Chem. Front. 6, 3252–3258. doi: 10.1039/C9QO00775J
Pan, D. Y., Huang, Z. H., Liang, X., Ma, X., and Qi, S. H. (2018). Study of tricycloalternarenes from the deep-sea derived fungus Alternaria tenuissma DFFSCS013 and their antimicrobial activity. Nat. Prod. Res. Dev. 30, 1166–1169. doi: 10.16333/j.1001-6880.2018.7.012
Pang, X., Lin, X., Wang, P., Zhou, X., Yang, B., Wang, J., et al. (2018). Perylenequione derivatives with anticancer activities isolated from the marine sponge-derived fungus, Alternaria sp. SCSIO41014. Mar. Drugs 16, 280. doi: 10.3390/md16080280
Pinto, V. E., and Patriarca, A. (2017). Alternaria species and their associated mycotoxins. Methods Mol. Biol. 1542, 13–32. doi: 10.1007/978-1-4939-6707-0_2
Pompeng, P., Sommit, D., Sriubolmas, N., Ngamrojanavanich, N., Matsubara, K., and Pudhom, K. (2013). Antiangiogenetic effects of anthranoids from Alternaria sp., an endophytic fungus in a Thai medicinal plant Erythrina variegata. Phytomed. Int. J. Phytotherapy Phytopharmacol. 20, 918–922. doi: 10.1016/j.phymed.2013.03.019
Ruiz-Vargas, J. A., Morales-Ferra, D. L., Ramírez-Ávila, G., Zamilpa, A., Negrete-León, E., Acevedo-Fernández, J. J., et al. (2019). α-Glucosidase inhibitory activity and in vivo antihyperglycemic effect of secondary metabolites from the leaf infusion of Ocimum campechianum mill. J. Ethnopharmacol. 243, 112081. doi: 10.1016/j.jep.2019.112081
Shen, L., Tian, S. J., Song, H. L., Chen, X., Guo, H., Wan, D., et al. (2018). Cytotoxic tricycloalternarene compounds from endophyte Alternaria sp. W-1 associated with Laminaria japonica. Mar. Drugs 16, 402. doi: 10.3390/md16110402
Sheng, S. Y., Zhou, Q., Qiu, D. W, and Yang, X. F. (2017). Effects and mechanism of disease resistance and yield improvement induced by plant immune protein preparation atailing in wheat. Chinese J. Biol. Control 33, 213–218. doi: 10.16409/j.cnki.2095-039x.2017.02.011
Shi, Y. N., Pusch, S., Shi, Y. M., Richter, C., Maciá-Vicente, J. G., Schwalbe, H., et al. (2019). (±)-Alternarlactones A and B, two antiparasitic alternariol-like dimers from the fungus Alternaria alternata P1210 isolated from the halophyte Salicornia sp. J. Org. Chem. 84, 11203–11209. doi: 10.1021/acs.joc.9b01229
Shi, Z. Z., Fang, S. T., Miao, F. P., and Ji, N. Y. (2018a). Two new tricycloalternarene esters from an alga-epiphytic isolate of Alternaria alternata. Nat. Prod. Res. 32, 2523–2528. doi: 10.1080/14786419.2017.1423312
Shi, Z. Z., Miao, F. P., Fang, S. T., Liu, X. H., Yin, X. L., and Ji, N. Y. (2017). Sesteralterin and tricycloalterfurenes A–D: terpenes with rarely occurring frameworks from the marine-alga-epiphytic fungus Alternaria alternata k21-1. J. Nat. Prod 80, 2524–2529. doi: 10.1021/acs.jnatprod.7b00478
Shi, Z. Z., Yin, X. L., Fang, S. T., Miao, F. P., and Ji, N. Y. (2018b). Two new isomeric tricycloalternarenes from the marine alga-epiphytic fungus Alternaria alternata k23-3. Magnet. Resonance Chem. MRC 56, 210–215. doi: 10.1002/mrc.4676
Song, X. M., Zhou, X. M., Li, Y. L., Huang, L. B., Chen, C. C., Gao, Y., et al. (2021). Two new cephalochromin derivative from the Alternaria sp. ZG22. Nat. Prod. Res. 35, 3370–3375. doi: 10.1080/14786419.2019.1700248
Tan, X., Zhang, X., Yu, M., Yu, Y., Guo, Z., Gong, T., et al. (2019). Sesquiterpenoids and mycotoxin swainsonine from the locoweed endophytic fungus Alternaria oxytropis. Phytochemistry 164, 154–161. doi: 10.1016/j.phytochem.2019.05.012
Tang, J. W., Xu, H. C., Wang, W. G., Hu, K., Zhou, Y. F., Chen, R., et al. (2019). (+)- and (–)-Alternarilactone A: enantiomers with a diepoxy-cage-like scaffold from an endophytic Alternaria sp. J. Nat. Prod. 82, 735–740. doi: 10.1021/acs.jnatprod.8b00571
Tantry, M. A., Idris, A. S., Williamson, J. S., Shafi, T., Dar, J. S., Malik, T. A., et al. (2018). Perylenequinones from an endophytic Alternaria sp. of Pinus ponderosa. Heliyon 4, e01046. doi: 10.1016/j.heliyon.2018.e01046
Tian, J., Fu, L., Zhang, Z., Dong, X., Xu, D., Mao, Z., et al. (2017). Dibenzo-α-pyrones from the endophytic fungus Alternaria sp. Samif01: isolation, structure elucidation, and their antibacterial and antioxidant activities. Nat. Prod. Res. 31, 387–396. doi: 10.1080/14786419.2016.1205052
Tian, L. L., Ren, H., Xi, J. M., Fang, J., Zhang, J. Z., and Wu, Q. X. (2021). Diverse anti-inflammation and anti-cancer polyketides isolated from the endophytic fungi Alternaria sp. MG1. Fitoterapia 153, 105000. doi: 10.1016/j.fitote.2021.105000
Wang, H., Guo, Y., Luo, Z., Gao, L., Li, R., Zhang, Y., et al. (2022). Recent advances in Alternaria phytotoxins: a review of their occurrence, structure, bioactivity, and biosynthesis. J. Fungi 8, 168. doi: 10.3390/jof8020168
Wang, H. L., Li, R., Li, J., He, J., Cao, Z. Y., Kurtán, T., et al. (2020). Alternarin A, a drimane meroterpenoid, suppresses neuronal excitability from the coral-associated fungi Alternaria sp. ZH-15. Org. Lett. 22, 2995–2998. doi: 10.1021/acs.orglett.0c00746
Wang, J., Ding, W., Wang, R., Du, Y., Liu, H., Kong, X., et al. (2015). Identification and bioactivity of compounds from the mangrove endophytic fungus Alternaria sp. Mar. Drugs 13, 4492–4504. doi: 10.3390/md13074492
Wang, J. T., Ma, Z. H., Wang, G. K., Xu, F. Q., Yu, Y., Wang, G., et al. (2021). Chemical constituents from plant endophytic fungus Alternaria alternata. Nat. Prod. Res. 35, 1199–1206. doi: 10.1080/14786419.2019.1639699
Wang, L., Jiao, J., Liu, D., Zhang, X., Li, J., Che, Q., et al. (2020). Cytotoxic meroterpenoids from the fungus Alternaria sp. JJY-32. Chem. Biodivers. 17, e2000226. doi: 10.1002/cbdv.202000226
Wang, Y., Liu, H. X., Chen, Y. C., Sun, Z. H., Li, H. H., Li, S. N., et al. (2017). Two new metabolites from the endophytic fungus Alternaria sp. A744 derived from morinda officinalis. Molecules 22, 765. doi: 10.3390/molecules22050765
Wang, Y., Yang, M. H., Wang, X. B., Li, T. X., and Kong, L. Y. (2014). Bioactive metabolites from the endophytic fungus Alternaria alternata. Fitoterapia 99, 153–158. doi: 10.1016/j.fitote.2014.09.015
Wang, Y. N., Zeng, C. J., Shao, C. L., and Wang, C. Y. (2015). Isolation of antibacterial macrosporin from gorgonian-derived fungus Alternaria sp. and preliminary study on its antimicrobial mechanism. Chinese J. Marine Drugs 34, 10–16. doi: 10.13400/j.cnki.cjmd.2015.02.002
Wu, J. C., Hou, Y., Xu, Q., Jin, X. J., Chen, Y., Fang, J., et al. (2019). (±)-Alternamgin, a pair of enantiomeric polyketides, from the endophytic fungi Alternaria sp. MG1. Org. Lett. 21, 1551–1554. doi: 10.1021/acs.orglett.9b00475
Wu, X., Wang, S., Liu, C., Zhang, C., Guo, J., and Shang, X. (2019). A new 2H-benzindazole compound from Alternaria alternata Shm-1, an endophytic fungus isolated from the fresh wild fruit of Phellinus igniarius. J. Nat. Med. 73, 620–626. doi: 10.1007/s11418-019-01291-x
Xia, G. Li, J., Li, H., Long, Y., Lin, S., Lu, Y., He, L., et al. (2014). Alterporriol-type dimers from the mangrove endophytic fungus, Alternaria sp. (SK11), and their MptpB inhibitions. Mar. Drugs 12, 2953–2969. doi: 10.3390/md12052953
Xu, G. B., Pu, X., Bai, H. H., Chen, X. Z., and Li, G. Y. (2015). A new alternariol glucoside from fungus Alternaria alternate cib-137. Nat. Prod. Res. 29, 848–852. doi: 10.1080/14786419.2014.990905
Xu, J., Hu, Y. W., Qu, W., Chen, M. H., Zhou, L. S., Bi, Q. R., et al. (2019). Cytotoxic and neuroprotective activities of constituents from Alternaria alternate, a fungal endophyte of Psidium littorale. Bioorg. Chem. 90, 103046. doi: 10.1016/j.bioorg.2019.103046
Yamada, T., Tanaka, A., Nehira, T., Nishii, T., and Kikuchi, T. (2019). Altercrasins A?E, decalin derivatives, from a sea-urchin-derived Alternaria sp.: isolation and structural analysis including stereochemistry. Mar. Drugs 17, 218. doi: 10.3390/md17040218
Yang, C. L., Wu, H. M., Liu, C. L., Zhang, X., Guo, Z. K., Chen, Y., et al. (2019). Bialternacins A–F, aromatic polyketide dimers from an endophytic Alternaria sp. J. Nat. Prod. 82, 792–797. doi: 10.1021/acs.jnatprod.8b00705
Yang, H., Qi, B., Ding, N., Jiang, F., Jia, F., Luo, Y., et al. (2019). Polyketides from Alternaria alternata MT-47, an endophytic fungus isolated from Huperzia serrata. Fitoterapia 137, 104282. doi: 10.1016/j.fitote.2019.104282
Zaki, A. G., El-Shatoury, E. H., Ahmed, A. S., and Al-Hagar, O. (2019). Production and enhancement of the acetylcholinesterase inhibitor, huperzine A, from an endophytic Alternaria brassicae AGF041. Appl. Microbiol. Biotechnol. 103, 5867–5878. doi: 10.1007/s00253-019-09897-7
Zhang, J. L., Tang, W. L., Huang, Q. R., Li, Y. Z., Wei, M. L., Jiang, L. L., et al. (2021). Trichoderma: a treasure house of structurally diverse secondary metabolites with medicinal importance. Front. Microbiol. 12, 723828. doi: 10.3389/fmicb.2021.723828
Zhang, N., Zhang, C., Xiao, X., Zhang, Q., and Huang, B. (2016). New cytotoxic compounds of endophytic fungus Alternaria sp. isolated from Broussonetia papyrifera (L.) Vent. Fitoterapia 110, 173–180. doi: 10.1016/j.fitote.2016.03.014
Zhang, Q. Q., Guo, L. Z., Chen, J. F., Zou, K., and Gou, Z. Y. (2015). Secondary metabolites from insect-derived endophytic fungus Alternaria sp. J. China Three Gorges Univ. 37, 110–112. doi: 10.13393/j.cnki.issn.1672-948X.2015.05.025
Zhao, D. L., Cao, F., Wang, C. Y., Yang, L. J., Shi, T., Wang, K. L., et al. (2019). Alternatone A, an unusual perylenequinone-related compound from a soft-coral-derived strain of the fungus Alternaria alternata. J. Nat. Prod. 82, 3201–3204. doi: 10.1021/acs.jnatprod.9b00905
Zhao, S., Tian, K., Li, Y., Ji, W., Liu, F., Khan, B., et al. (2020). Enantiomeric dibenzo-α-pyrone derivatives from Alternaria alternata ZHJG5 and their potential as agrochemicals. J. Agric. Food Chem. 68, 15115–15122. doi: 10.1021/acs.jafc.0c04106
Zhao, S., Wang, B., Tian, K., Ji, W., Zhang, T., Ping, C., et al. (2021). Novel metabolites from the Cercis chinensis derived endophytic fungus Alternaria alternata ZHJG5 and their antibacterial activities. Pest Manag. Sci. 77, 2264–2271. doi: 10.1002/ps.6251
Zhong, T. H., Zeng, X. M., Feng, S. B., Zhang, H. T., Zhang, Y. H., Luo, Z. H., et al. (2022). Three new phomalone derivatives from a deep-sea-derived fungus Alternaria sp. MCCC 3A00467. Nat. Prod. Res. 36, 414–418. doi: 10.1080/14786419.2020.1771706
Keywords: fungi, Alternaria, metabolites, bioactivity, biosynthesis, application
Citation: Zhao S, Li J, Liu J, Xiao S, Yang S, Mei J, Ren M, Wu S, Zhang H and Yang X (2023) Secondary metabolites of Alternaria: A comprehensive review of chemical diversity and pharmacological properties. Front. Microbiol. 13:1085666. doi: 10.3389/fmicb.2022.1085666
Received: 31 October 2022; Accepted: 17 November 2022;
Published: 06 January 2023.
Edited by:
Peng Zhang, Tobacco Research Institute (CAAS), ChinaReviewed by:
Xuefeng Zhou, South China Sea Institute of Oceanology (CAS), ChinaLixin Duan, Guangzhou University of Chinese Medicine, China
Copyright © 2023 Zhao, Li, Liu, Xiao, Yang, Mei, Ren, Wu, Zhang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiliang Yang, eXhseXhsMTE3QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Shiqin Zhao
Shiqin Zhao Juan Li2†
Juan Li2† Xiliang Yang
Xiliang Yang