
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 09 January 2023
Sec. Food Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1084500
This article is part of the Research TopicInteraction Between Food Homologous Plants and Intestinal MicrobiotaView all 22 articles
In recent years, with the harm caused by the abuse of antibiotics and the increasing demand for green and healthy food, people gradually began to look for antibiotic alternatives for aquaculture. As a Chinese herbal medicine, leaf extract chlorogenic acid (CGA) of Eucommia ulmoides Oliver can improve animal immunity and antioxidant capacity and can improve animal production performance. In this study, crucian carp (Carassius auratus) was fed with complete feed containing 200 mg/kg CGA for 60 days to evaluate the antioxidant, immuno-enhancement, and regulation of intestinal microbial activities of CGA. In comparison to the control, the growth performance indexes of CGA-added fish were significantly increased, including final body weight, weight gain rate, and specific growth rate (P < 0.01), while the feed conversion rate was significantly decreased (P < 0.01). Intestinal digestive enzyme activity significantly increased (P < 0.01); the contents of triglyceride in the liver (P < 0.01) and muscle (P > 0.05) decreased; and the expression of lipid metabolism-related genes in the liver was promoted. Additionally, the non-specific immune enzyme activities of intestinal and liver tissues were increased, but the expression level of the adenylate-activated protein kinase gene involved in energy metabolism was not affected. The antioxidant capacity of intestinal, muscle, and liver tissues was improved. Otherwise, CGA enhanced the relative abundance of intestinal microbes, Fusobacteria and Firmicutes and degraded the relative abundance of Proteobacteria. In general, our data showed that supplementation with CGA in dietary had a positive effect on Carassius auratus growth, immunity, and balance of the bacteria in the intestine. Our findings suggest that it is of great significance to develop and use CGA as a natural non-toxic compound in green and eco-friendly feed additives.
Crucian carp (Carassius auratus) is an important economical freshwater fish with good meat quality, strong disease resistance, and rich nutrition. Thus, it is deeply welcomed by farmers and consumers (Liu et al., 2022). With the continuous improvement of the aquaculture scale and intensification degree, fish diseases caused by microbial pathogens are increasingly aggravated. It is of great significance to the development and use of green and eco-friendly feed additives with effective preventive effects. Chinese herbal medicine, as a natural and innocuous compound, has been used for replacing antibiotics to prevent and control fish diseases (Santos and Ramos, 2016; Zhang et al., 2022).
Eucommia ulmoides Oliver is a unique deciduous tree in China, which is listed as top quality in Shennong Ben Cao Jing. Eucommia ulmoides’ bark is a valuable tonic and has been used in traditional Chinese herbal medicine formulations for 2,000 years (Luo et al., 2020). Since modern times, some studies have found that the chemical composition of Eucommia ulmoides’ leaves is basically of similar efficacy to that of Eucommia ulmoides’ bark, and the content of some active ingredients in leaves is much higher than that of bark, such as chlorogenic acid (CGA) (Nakamura et al., 1997). CGA has been shown to have a variety of physiological activities, including antibacterial, antiviral (Abaidullah et al., 2021; Chen et al., 2022), protecting the liver (Zhu et al., 2022), anti-tumor (Yagasaki et al., 2000; Belkaid et al., 2006), lowering blood pressure and blood lipids (Zhao et al., 2012), anti-inflammatory (Xu et al., 2020), antioxidant (Liang and Kitts, 2015), and stimulating the central nervous system (Kumar et al., 2019). Additionally, the role of gut microbiota in the protective effect of CGA on obesity and metabolic endotoxemia was identified in mice (Ye et al., 2021). However, the regulatory effect of CGA on immunity and intestinal microecology of freshwater fish in aquaculture remains to be studied.
In this study, we used crucian carp (Carassius auratus gibelio) as a model; physiological and biochemical detection technology, high throughput sequencing, real-time fluorescent quantitative PCR, and histological analysis were used to explore the effects of CGA on crucian carp. Our study showed that feeding CGA had a significant positive effect on the growth, immunity, and gut flora balance of Carassius auratus. Our findings provide a reference for the strategy of CGA replacing some chemical drugs and antibiotics to prevent and control fish diseases, which is of great significance to healthy aquaculture and ecological environmental protection.
The experiment was carried out in fish tanks with 1 m3 with identical management including water inputs, daily water exchange rate (∼5%), feed type, and rearing schedule. Carassius auratus was the Zhongke 3 Carassius auratus gibelio. The larval fish were cultured at a stocking density of ∼60 individuals per tank. All the fish were fed a commercial non-medicated feed (Tongwei Co., Ltd.) for 15 days, two times per day at 9 a.m. and 5 p.m. After a 15-day adaptation, the experimental group was fed a diet supplemented with 200 mg/kg CGA for 60 days (Gao et al., 2019), while the control group was fed an ordinary diet. The CGA extract from Eucommia ulmoides leaves came from Jiangsu Lvkee Biotechnology Co., Ltd., (Yangzhou, China). The experiments were conducted with three experimental replications. The experimental animal feed formulation is shown in Table 1.
After the experiment, fish were randomly selected from each tank separately at 4 h after the fish feeding in the morning. The fish dissection and sampling were performed as described previously (Li et al., 2020). The gut, liver, and muscle tissue samples were collected separately for subsequent experimental determination. Part of these tissue samples was directly stored at −80°C, and part of them was stored in an RNA protection solution. The foregut of gut was preserved in a formaldehyde fixation solution.
More than 10 fish were randomly selected from each tank for weighing before and after the experiment. The average body weight, weight gain rate, and specific growth rate of Carassius auratus in each group were calculated before and after the experiment, after a 12 h fast. The growth performance indexes were calculated according to the following formula:
In the formula:
W0—Carassius auratus initial average body mass (g/tail)
Wt—Average body weight of Carassius auratus (g/tail)
t—Feeding days (d)
F—Average total feed intake per fish (g).
The tissue of three fish was isolated on an ice tray and homogenized with cold saline with a weight-to-volume ratio of 1:9. The homogenate was centrifuged at 9,000 × g for 10 min at 4°C. The supernatant was collected for measuring the indices. The enzyme activity of lipase (LPS) and α-amylase (AMS) in gut tissue homogenate, the antioxidant indices including superoxide dismutase (SOD), peroxidase (POD), reduced glutathione (GSH), and malondialdehyde (MDA) concentration of gut, muscle, and liver tissues, the TG concentration in muscle and liver, and the non-specific immune enzyme activities including acid phosphatase (ACP), alkaline phosphatase (AKP) in gut and liver tissues were determined with commercially assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions (Matozzo et al., 2013; Wang et al., 2020; Zhao et al., 2021).
The total RNA was extracted from the liver and muscle by Biyuntian’s animal tissue RNA extraction kit (Shanghai, China), following the manufacturer’s instructions. After concentration determination (experimental group RNA concentration was 458.261 ng/μL and control group RNA concentration was 650.192 ng/μL), the RNA was reverse transcribed by the reverse transcription kit to obtain cDNA (Nanjing Novezan Biological Co., Ltd., Nanjing, China) (Sun et al., 2018; Tan et al., 2018).
Real-time quantitative PCR (RT-PCR) was performed to quantify the presence of the 12 genes: PPARα (peroxisome proliferator-activated receptor alpha), ACOX1 (acyl-coenzyme A oxidase 1), HSL (hormone-sensitive lipase), ACC (acetyl-CoA carboxylase), FAS (fatty acid synthase), SREBP-1c (sterol regulatory element-binding proteins-lc), DGAT2 (diacylglycerol acyltransferase 2), ATGL (adipose triglyceride lipase), MAGL (monoacylglycerol lipase), AMPK (AMP-activated protein kinase), and EP1α (translation elongation factor 1 alpha) gene which was a reference in the intestinal contents of fish.
The 24 pairs of specific primers were synthesized by Qingke Biotechnology Co., Ltd., (Wuhan, China) and verified by PCR and electrophoresis (Supplementary Table 1). The primer information is shown in Supplementary Table 1. A 10-μl reaction mixture contained 5 μl of the qPCR mix (without ROX) (Zhuangmeng International Biotechnology, Beijing, China), 0.2 μl each of forward and reverse primers (10 μM), 1 μL of template cDNA, and 3.6 μL of DEPC water. Real-time PCR reaction conditions were as follows: the cDNA unchained element was 95°C for 20 s, the denaturation condition was 95°C for 15 s, the annealing condition was 57°C for 20 s, and extension at 72°C for 20 s, for 40 cycles. Fluorescence was read during extension The conditions of the final melting section are 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s. The experiments were conducted with three experimental replications.
The morphology of the ileum was analyzed by PAS staining as reported by Wang et al. (2009). Sliced samples were viewed under an optical microscope (Wuhan Sevier Company, Wuhan, China). Five pictures and five fields in each picture were used to analyze villus height, villus number, and goblet cells on villous epithelium using image analysis software under 40 and 200 magnification microscopes (Wang et al., 2009; Yang et al., 2021).
The genomic DNA of the gut content was extracted using the Fast DNA Stool Mini kit (Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. The primer pair (forward: CCTAYGGGRBGCASCAG and reverse: GGACTACNNGGGTATCTAAT) was used to amplify the V3–V4 region of the 16S rRNA. The obtained amplicons were sequenced on the Illumina Hiseq 2500 platform to generate paired-end 250 bp reads. The resultant paired-end reads were merged using FLASH VI.2.7. The tool QIIME 2 was employed to perform raw read filtration, chimera removal, denoising (with plugin deblur), and taxonomic classification of OTUs (with q2-feature-classifier pre-trained with 99% OTU dataset of the SILVA Release 138). After 30,000 reads per sample (the smallest number of reads per sample) were normalized to generate OTU tables, based on which Chao 1, Observed specie, Shannon, Simpson, and phylogenetic diversity indices were estimated.
All the experimental data were presented as mean ± standard error of the mean (SEM) (Wu et al., 2022). The results were analyzed using a T-Test to compare the means of groups. A value of P < 0.05 was regarded to be statistically significantly different. Intestinal fecal samples were sequenced by ITS, and OTU clustering was performed according to 97% similarity after quality control, and then compared with UNITE database for annotation (Liang et al., 2020). The 2–ΔΔCT method was used to calculate the mRNA level of target genes accordingly (Livak and Schmittgen, 2001). After OUTs were normalized by the copy numbers, functional pathways were predicted by the Kyoto Encyclopedia of Genes and Genomes (KEGG) catalog at level 3 KEGG orthology groups (KOs) using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt).
The effects of oral CGA on the growth performance of crucian carp are shown in Table 2. The fish dietary CGA-added significantly increased the weight, weight gain rate (WGR, P = 0.000252) and specific growth rate (SGR, P = 0.000305) and tended to reduce the feed coefficient (FCR, P = 0.000323) significantly, indicating that the CGA in the diet helps to improve the feed utilization rate and promote the growth of Carassius auratus.
In our study, the intestinal lipase and amylase activities of the CGA-added group were significantly higher than those of the control group, P = 0.00007 and P = 0.0012, respectively (Figure 1), indicating that adding CGA to the feed could improve the ability of secretion of intestinal lipase and amylase, and enhance the digestion level of fat and starch in Carassius auratus.
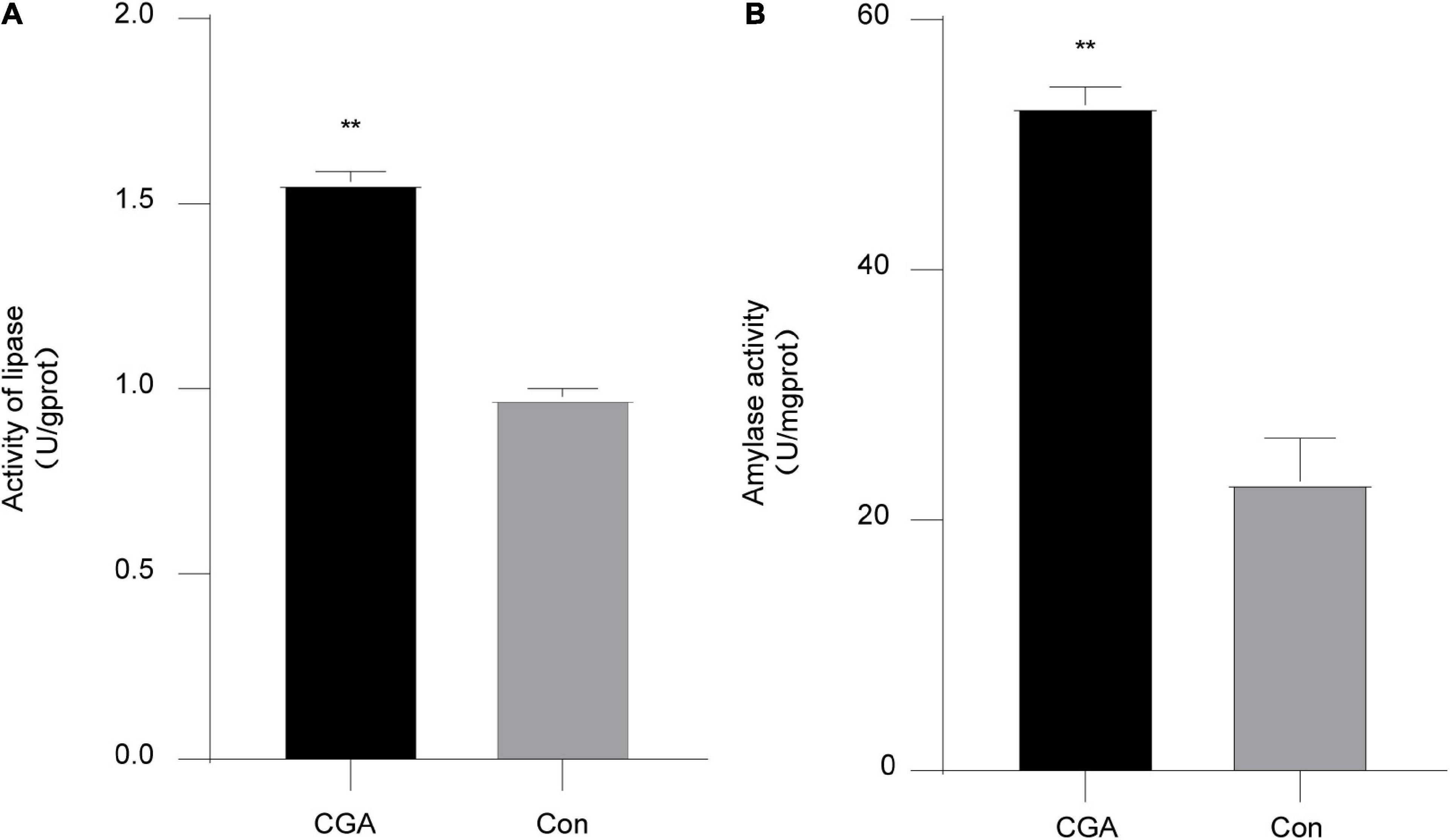
Figure 1. Intestinal digestive enzyme activities. (A) Effect of chlorogenic acid (CGA) on intestinal lipase. (B) Effect of CGA on intestinal amylase. **Indicates a significant difference compared with the control group (P< 0.01). The acronym for the CGA-added group is CGA and the acronym for the control group is Con.
To explore the effect of CGA on the lipid metabolism of Carassius auratus, the contents of triglycerides (TG) in the muscle and liver of Carassius auratus were determined. As shown in Figure 2A, the TG content in the liver of the CGA-added group was significantly lower than that of the control group (P = 0.003), while there had no significant effect on TG content in the muscle (P = 0.113) (Figure 2B). In general, CGA had a certain reduction effect on the TG content in the muscle and liver of Carassius auratus.
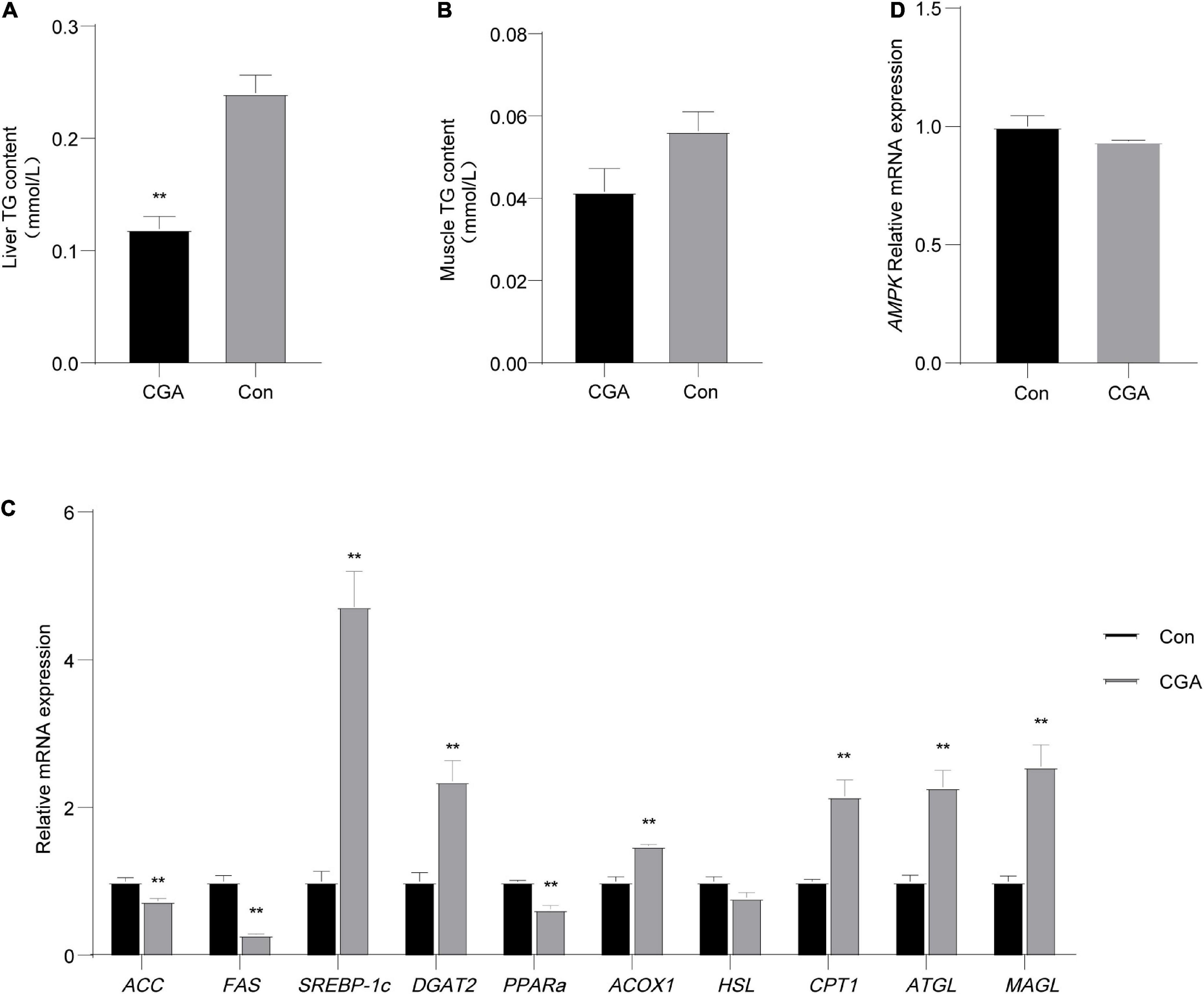
Figure 2. Effect of chlorogenic acid (CGA) on lipid and energy metabolism of Carassius auratus. (A) Effect of CGA on triglycerides (TG) in the liver. (B) Effect of CGA on TG in muscle. (C) Expression of lipid metabolism-related genes in liver tissue. (D) Expression of energy metabolism gene AMP-activated protein kinase (AMPK) in liver tissue. **Indicates a significant difference compared with the control group (P< 0.01). The acronym for the CGA-added group is CGA and the acronym for the control group is Con.
Then we measured the expression of 10 genes related to lipid metabolism in liver tissue. Among these genes, the acetyl-CoA carboxylase (ACC) gene, fatty acid synthase (FAS) gene, sterol regulatory element-binding proteins-lc (SREBP-1c) gene, and diacylglycerol acyltransferase 2 (DGAT2) gene were related to fat synthesis, and peroxisome proliferator-activated receptor alpha (PPARα) gene, acyl-coenzyme A oxidase 1 (ACOX1) gene, hormone-sensitive lipase (HSL) gene, carnitine palmitoyltransferase-1 (CPT1) gene, adipose triglyceride lipase (ATGL) gene, and monoacylglycerol lipase (MAGL) gene were related to lipolysis. As shown in Figure 2C, the expression levels of lipolysis genes (ATGL, CPT1, ACOX1, and MAGL) in the CGA-added group were significantly increased (P = 0.0065, 0.0064, 0.0016, and 0.0066 respectively), while fat synthesis gene ACC and FAS in the CGA-added group were significantly decreased (P = 0.013 and 0.00089), which may be due to the body self-regulation caused by the less fat content in the body. In general, CGA inhibited the expression of genes related to fat synthesis and promoted the expression of genes related to fat decomposition. Thereby, it is speculated that dietary supplementation of CGA could reduce the storage of fat in the liver, corresponding to the lower hepatic TG content in the CGA-added group than in the control group.
Meanwhile, there was no significant difference in the expression of the AMP-activated protein kinase (AMPK) gene between the CGA-added group and the control group in liver tissue (Figure 2D).
The non-specific immune responses of fish are related to the enzyme activities of acid phosphatase (ACP) and alkaline phosphatase (AKP). Compared with the control group, ACP (P = 0.000006) and AKP (P = 0.003627) activities in the CGA-added group were significantly increased in the intestinal tissue (P < 0.01). The enzyme activity also increased in the liver tissue, while there was no significant effect (Figure 3). In general, it showed that CGA could improve the liver and intestinal non-specific immunity of Carassius auratus.

Figure 3. Intestinal and liver non-specific immune enzyme activities. (A) Alkaline phosphatase (AKP) activity in intestinal tissue. (B) Acid phosphatase (ACP) activity in intestinal tissue. (C) AKP activity in liver tissue. (D) ACP activity in liver tissue. **Indicates a significant difference compared with the control group (P< 0.01). Acid phosphatase (ACP), alkaline phosphatase (AKP). The acronym for the chlorogenic acid (CGA)-added group is CGA and the acronym for the control group is Con.
The increase of intestinal villus height and density can cause the extension of the contact area with nutrients and promote nutrient absorption. The mucus layer formed by glycoproteins secreted by goblet cells and immune molecules (such as antimicrobial peptides and cytokines) is the first line of defense against the invasion of pathogenic substances (Alesci et al., 2022). As shown in Figures 4A,B, intestinal villi were leaf-like. Intestinal villi in the CGA-added group were arranged orderly and closely, while sparse and shorter in the control group. Meanwhile, there were slightly more goblet cells in the CGA-added group than in the control group (Figures 4C,D). According to Table 3, dietary supplementation of CGA increased intestinal villus height, villus density, and goblet cells per unit length.
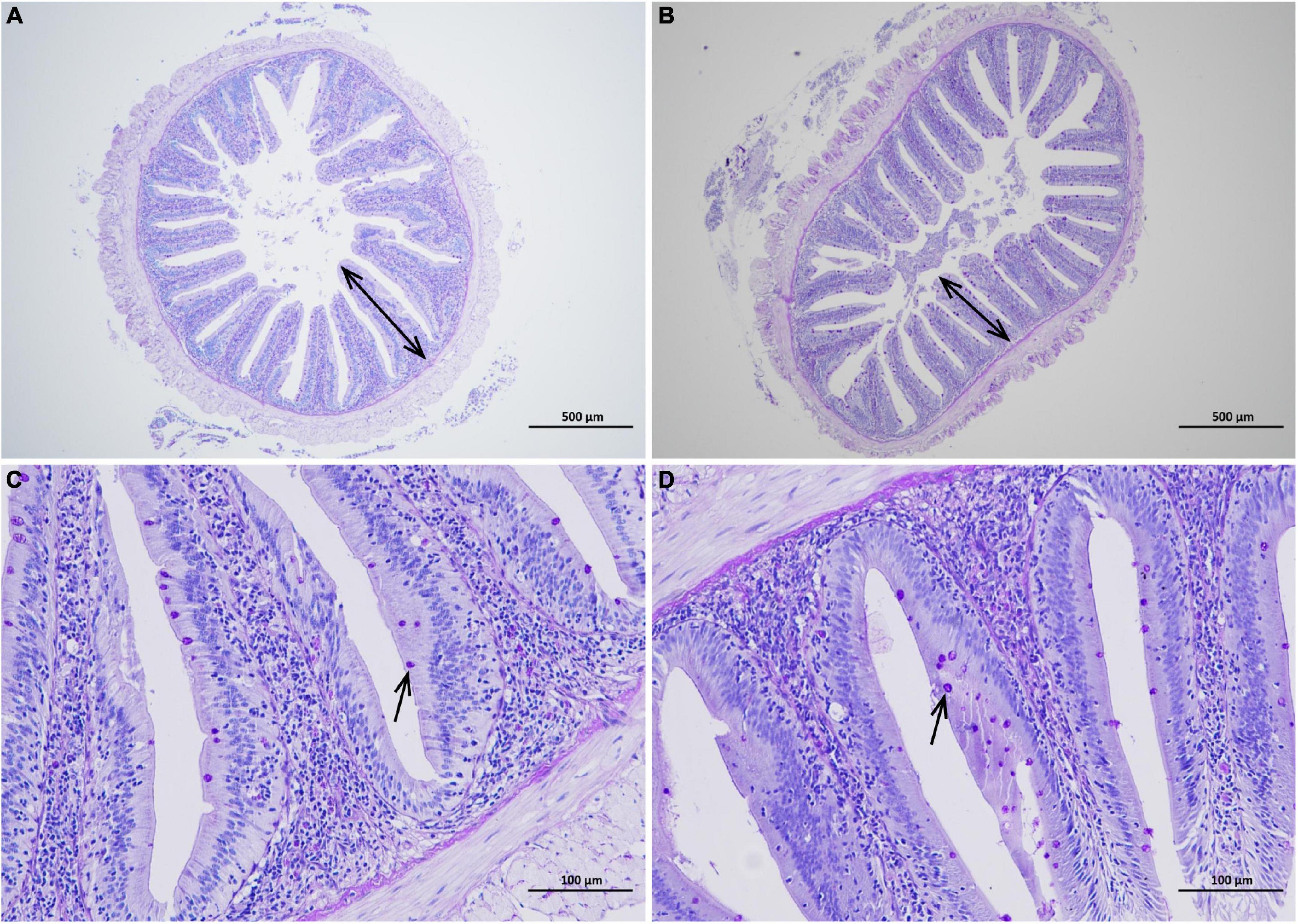
Figure 4. Effects of chlorogenic acid (CGA) on the intestinal morphology. (A) Observation of intestinal morphology in the control group under a 40 magnification microscope. (B) Observation of intestinal morphology in the experimental group under a 40 magnification microscope. (C) Observation of intestinal morphology in the control group under a 200 magnification microscope. (D) Observation of intestinal morphology in the experimental group under a 200 magnification microscope. Arrows in panels (A,B) indicate villus height. Arrows in panels (C,D) indicate goblet cells.
Lipid peroxidation can cause the formation of MDA, which is an indicator of the degree of oxygen-free radical damage in the human body. In our study, we found that when CGA was added to the fish diet, MDA content in multiple tissues was reduced (Figures 5A,E,I). Especially, the content of MDA in the liver tissue was significantly lower than that in the control group (P = 0.000012). Three antioxidant enzymes, namely GSH, POD, and SOD, are important participants in cellular anti-oxidation. Compared with the control group, the antioxidant GSH in intestinal, muscle, and liver tissue of the CGA-added group were all increased (Figures 5B,F,J). Additionally, the activities of POD and total SOD in the CGA-added group were higher than those in the control group (Figures 5C,D,G,H,K,L).
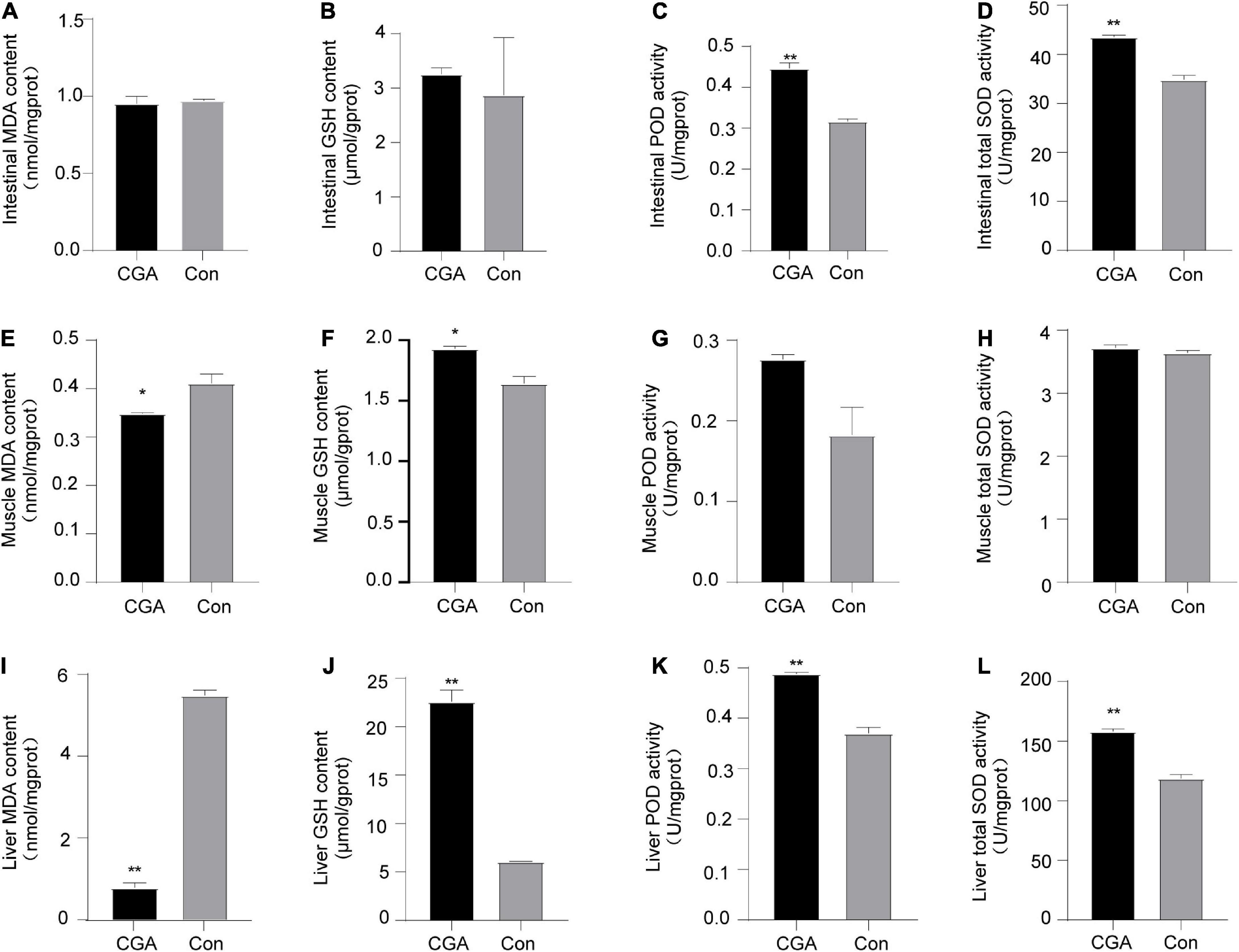
Figure 5. Effect of chlorogenic acid (CGA) on antioxidant capacity of intestinal, muscle, and liver of Carassius auratus. (A) Malondialdehyde (MDA) content in intestinal tissue. (B) Glutathione (GSH) activity in intestinal tissue. (C) Peroxidase (POD) content in intestinal tissue. (D) Superoxide dismutase (SOD) activity in intestinal tissue. (E) MDA content in muscle tissue. (F) GSH activity in muscle tissue. (G) POD content in muscle tissue. (H) SOD activity in muscle tissue. (I) MDA content in liver tissue. (J) GSH activity in liver tissue. (K) POD content in liver tissue. (L) SOD activity in liver tissue. Malondialdehyde (MDA), glutathione (GSH), peroxidase (POD), and superoxide dismutase (SOD). *Indicates a significant difference compared to the control group (P< 0.05); **Indicates a significant difference compared with the control group (P< 0.01). The acronym for the CGA-added group is CGA and the acronym for the control group is Con.
It is worth noting that the MDA, GSH, POD, and SOD in the CGA-administrated liver tissue of Carassius auratus were all significantly different from those in the control group. These results showed that supplementation of CGA in diet could improve the antioxidant capacity of Carassius auratus, especially in the liver.
An Illumina Hiseq 2500 sequencing platform was utilized to analyze the structure of the fish gut microbiota. A total of 499,848 microbial 16S rRNA genes raw reads were assembled using FLASH at quality settings, obtaining 492,686 clean reads. After applying the Usearch clustering algorithm, all 2,947 unique OTUs were identified and allotted.
Furthermore, the core microbial communities in each group were analyzed at the phylum and family levels (Figures 6A,B). Four predominant phyla accounted for more than 96% abundances of the total sequences at the phylum level, including Proteobacteria, Fusobacteriota, Firmicutes, and Bacteroidota in both two groups (Figure 6A). The relative abundance of Proteobacteria occupied the vast majority in the control group, and supplementation of CGA in the fish diet significantly increased the abundance of Firmicutes (from 2.00 to 31.27%, p = 0.027) and decreased the abundance of Proteobacteria (from 89.95 to 29.29%, p = 0.033) (Figure 6C).
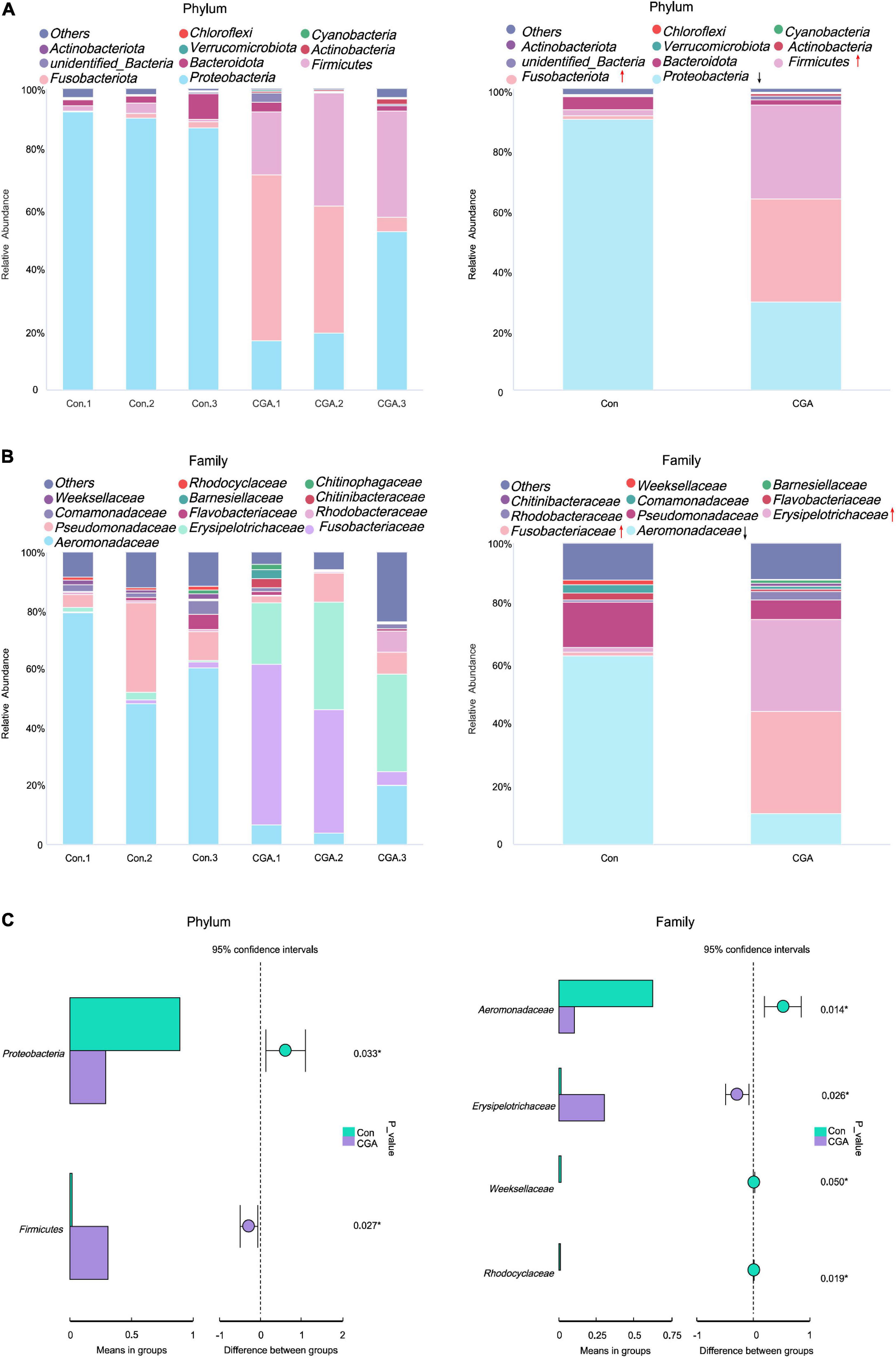
Figure 6. Bar chart of relative abundance of intestinal species. (A) Histogram of phyla level relative species abundance. (B) Histogram of relative abundance of species at the family level. (C) The changes of the main species between the two groups. con.1, con.2, and con.3 are the control group (Con), and chlorogenic acid (CGA).1, CGA.2, and CGA.3 are the CGA-added group (CGA). The green box represented Con; the purple box represents CGA. *Indicates a significant difference compared to the control group (P< 0.05).
The bacteria with higher abundance (> 0.01%) was shown in Figure 6B. The families Aeromonadaceae and Pseudomonadaceae dominated in the control group, and their relative abundances were 62.74 and 15.01%, respectively (Figure 6B). In the CGA-added group, The families Fusobacteriaceae and Erysipelotrichaceae were predominant, and their relative abundances were 34.10 and 30.40%, respectively. Otherwise, the relative abundance of the Aeromonadaceae significantly decreased from 62.74 to 10.27% as the CGA was added (Figure 6C). In summary, the composition of dominant bacterial communities was affected by dietary supplementation of CGA.
The dynamics of alpha diversity were further studied. As shown in Table 4, the Ace, Chao1, and observed species indices reduced from 148 to 142, 150 to 144, and 146 to 138, respectively, as CGA was added to the feed. Meanwhile, Shannon and Simpson’s indices increased from 3.05 to 3.22 and 0.72 to 0.79, respectively. Accordingly, the alpha diversity showed a slightly declined trend with the supplementation of CGA in the fish diet.
To analyze the functions of gut microbiota among the samples, sequences were conducted and submitted to the KEGG database for analysis. Metagenome potentials were predicted by PICRUSt. The top 40 relative abundance of KEGG pathways are revealed in Figure 7A, and the KEGG pathways with significant differences with supplementation of CGA in fish diet are shown in Figure 7B. Briefly, the largest relative abundance of KEGG pathways was found in transporters, general function prediction, and the DNA repair and recombination proteins both in the two groups. The relative abundance of amino acid-related enzymes, DNA repair and recombination proteins, and DNA replication was significantly increased with supplementation of CGA in fish diet.
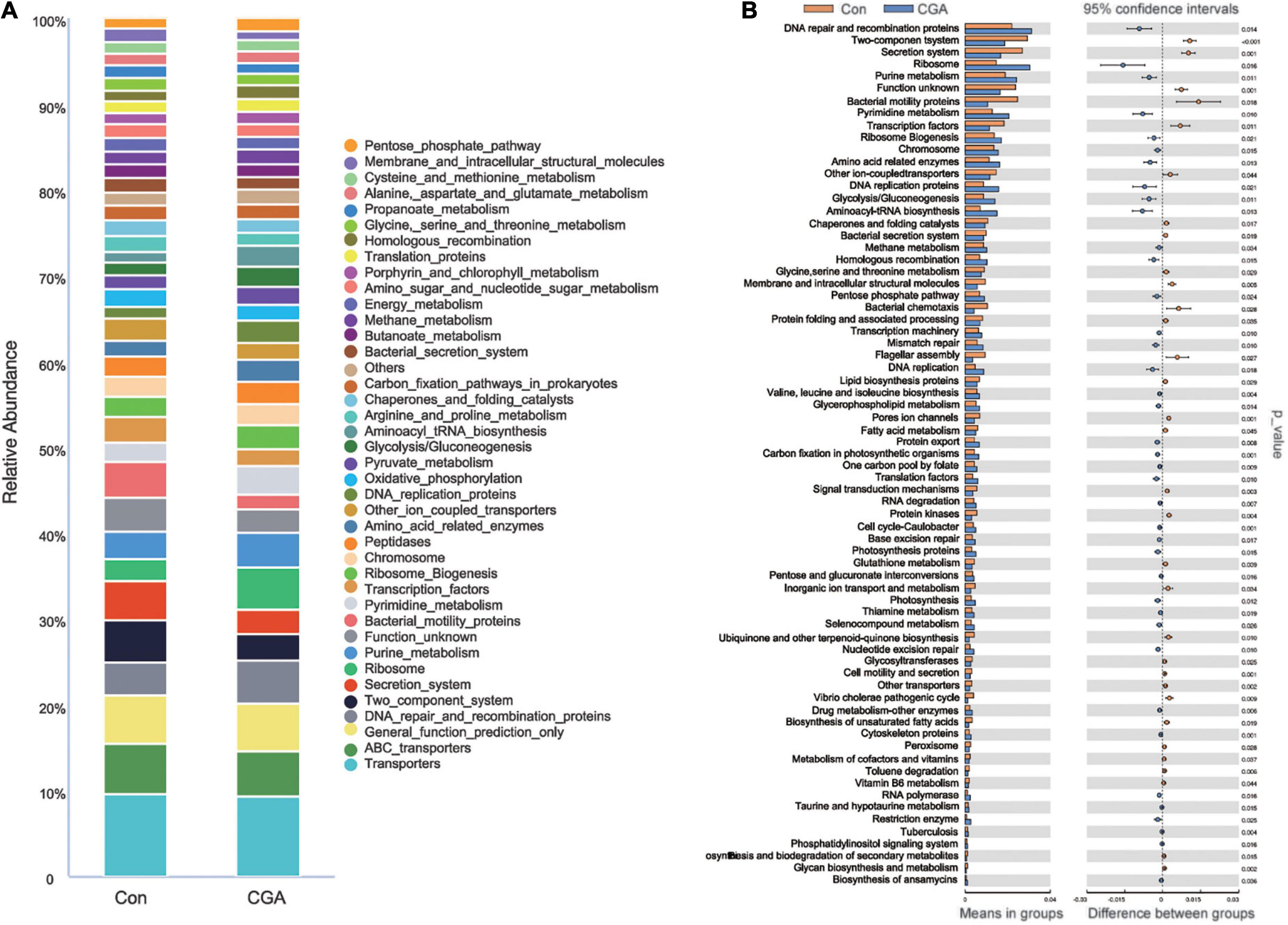
Figure 7. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways analysis by Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt). (A) Relative abundance of KEGG pathways for two groups. (B) KEGG pathways with significant differences between two groups (T-test). The acronym for the chlorogenic acid (CGA)-added group is CGA and the acronym for the control group is Con.
Our study indicated that the feeding supplemented with 200 mg/kg CGA had a positive effect on the growth performance of Carassius auratus. It has been reported that CGA can help to promote piglet growth and improve the feed conversion ratio (Chen et al., 2018). The mechanism of action may be related to the significant increase of the intestinal amylase and lipase activities of Carassius auratus, as the enhancement of the digestive function of the intestine will contribute to the full absorption of nutrients. Meanwhile, oral CGA also had a positive effect on the density and height of the intestinal villi of Carassius auratus. The density and height of villi in the intestine can increase the contact area between the intestine and intestinal contents, thus increasing the deposition of nutrients, which is of great significance for improving the health of animals (Fu et al., 2021).
Acid phosphatase and AKP are important hydrolases and play an important role in the body’s immune defenses (Faccioli et al., 2016). These two enzymes, collectively called phosphatase, are divided according to the optimal PH value of catalytic conditions. They can participate in the process of protein dephosphorylation, digestion, and absorption of nutrients and other functions, and are also an important detoxification system in the body. In our study, CGA significantly increased the activities of ACP and AKP in the intestinal tissue of Carassius auratus, that corresponded to the results of the increase in the number of goblet cells per unit length, which both improved intestinal immunity to a certain extent. Additionally, TG content in liver tissue decreased significantly as fed with CGA, which corresponded to the result of the expression of genes related to lipid metabolism in liver tissue. These results indicated that feeding supplemented with CGA could promote lipid metabolism in the liver of Carassius auratus, thus reducing the accumulation of lipids in liver tissue.
The increase of free radical content in organisms may lead to an increase in lipid peroxidation content and lipid peroxidation damage, and the decomposition of lipid peroxides will produce some harmful substances, such as MDA (Ayala et al., 2014). Therefore, the key to maintaining the health of the body is to eliminate the free radical content of the body, inhibit the appearance of lipid peroxidation damage, and protect the integrity and function of the cell membrane. The antioxidant enzymes total SOD, POD, and some non-enzymatic antioxidant substances such as GSH can eliminate the reactive oxygen free radicals of the body, thus improving the antioxidant capacity of the body (Liu et al., 2013). We found that feeding CGA could reduce the production of lipid peroxides in the Carassius auratus intestinal and muscle, and improve the total antioxidant enzyme SOD, POD, and the antioxidant substances GSH levels of the liver tissue. Thus, the enhancement of the metabolism index inflected the improvement body’s antioxidant and immune capacity. In our study, there was no significant difference in the expression of the AMP-activated protein kinase (AMPK) gene between the CGA-added group and the control group in liver tissue (Figure 2D). The results of Kong et al. show that under the action of CGA, the expression of AMPK was significantly increased, the expression of FAS was decreased, and the expression of HSL was increased (Kong et al., 2021), which was partially consistent with the results of this study, and it may be caused by the different growth cycles of the experimental crucian carp.
Intestinal microorganisms play an important role in the growth and development of fish as well as their immune ability. It was reported that CGA-induced changes in the gut microbiota played an important role in the inhibition of metabolic endotoxemia in mice (Ye et al., 2021). In this study, a change in Carassius auratus’ gut microbial composition was found as the application of CGA. According to previous studies, Proteobacteria and Firmicutes were the dominant phyla in the intestinal tract of fish (Zou et al., 2020). It was reported that the gut microbiota of Carassius auratus was initially dominated by Proteobacteria, and in adulthood, it was jointly dominated by Proteobacteria, Fusobacteriota, and Firmicutes (Li et al., 2017). Additionally, these three phyla play an important role in the growth and metabolism of fish. Proteobacteria have been reported to be involved in the metabolism and cycling of carbon, nitrogen, and sulfur in fish (Fjellheim et al., 2010; Han et al., 2010); Bacteroidota is involved in the fermentation process and degradation of oligosaccharides (Li et al., 2015). Firmicutes contribute to carbon metabolism (Corrigan et al., 2015). In this study, our results were similar to those reported. In the control group, Proteobacteria was dominated by intestinal bacteria and the relative abundance of Proteobacteria was 91.76%, Fusobacteriota was 1.33%, Firmicutes was 1.68%, and Bacteroidota was 4.21%. While in the CGA-added group, fish gut microbes mainly composed by Proteobacteria, Fusobacteriotas, and Firmicutes. Practically, Proteobacteria accounted for 30.13%, Fusobacteriota 34.52%, Firmicutes 31.50%, and Bacteroidota 1.48%. The relative abundance ratio of Firmicutes and Bacteroidota may also be related to the growth of the fish (Li et al., 2013).
Chlorogenic acid had various antimicrobial effects on CGA, while it was not sensitive to probiotic bacteria which made it even more appropriate to use in the food industry (Naveed et al., 2018). In our study, the relative abundance of Aeromonadaceae, Fusobacteriaceae, Erysipelotrichaceae, and Pseudomonadaceae was increased in the intestinal bacteria of Carassius auratus with the application of CGA. Aeromonadaceae and Pseudomonadaceae are essential colony members in the normal intestinal bacteria of fish and are potential probiotics that play an important role in the digestion and health of fish (Wu et al., 2012). Additionally, it was reported that the Erysipelotrichaceae family could help to digest high-fat and polysaccharide diets. Meanwhile, Erysipelotrichaceae may enhance fish immunity by stimulating the TLR4 pathway, which was involved in innate immune defense in teleost fish (Conterno et al., 2011; El Kasmi et al., 2013; Harris et al., 2014; Qi et al., 2017). Some strains of the Fusobacteriaceae, such as Cetobacterium somerae, played a catalytic role in the synthesis of vitamin B12 in fish (Tsuchiya et al., 2008). Therefore, the increased relative abundance of Aeromonadaceae, Fusobacteriaceae, Erysipelotrichaceae, and Pseudomonadaceae in the intestinal bacteria may promote the immunity and growth performance of Carassius auratus as the application of CGA. The composition and diversity of intestinal flora can predict different biological functions. In this study, KEGG analysis revealed that one of the most abundant KOs related to DNA repair, recombination, and replication was significantly increased with supplementation of CGA in a fish diet, which showed that CGA may have anti-inflammatory and antioxidant physiological activities.
This study revealed that the growth performance of Carassius auratus fed with CGA was significantly improved which may be related to the enhancement of the intestinal digestive enzyme activity and increased density and height of the intestinal villi. It was shown that CGA is of great significance for improving the growth of animals. In addition, our results showed that feeding supplemented with CGA could increase the expression of genes related to lipid metabolism in the liver, improve the activities of non-specific immune enzymes in intestinal and liver tissues, promote the antioxidant capacity of intestinal, muscle, and liver tissues. Meanwhile, our data also demonstrated the relative abundance of the most dominant bacterial communities interchangeably with dietary supplementation of CGA. At the phylum level, Proteobacteria decreased and Firmicutes increased in the CGA-added group. At the family level, Fusobacteriaceae and Erysipelotrichaceae were the dominant families in the CGA-added group, while the relative abundance of Aeromonadaceae decreased significantly.
Overall, our study showed that supplementation with CGA in diet had a significant positive impact on Carassius auratus. It is of great significance to develop and use CGA, a natural and innocuous compound, in green and eco-friendly feed additives with effective preventive effects. Our findings provided a reference for exploring the CGA for replacing some chemical drugs and antibiotics to prevent and control fish diseases.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA897832.
The animal study was reviewed and approved by the university’s Animal Care and Use Committee (SCXK2020-0084) and performed in accordance with internationally accepted guidelines and ethical principles.
XJ and MS: designed research ideas and content, wrote the manuscript, performed experiments, and analyzed data. YXL and YJL: project administration and funding acquisition. YJL: reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (32170096) project (2662022SKPY001), the Fundamental Research Funds for the Central Universities, and the Cooperation Fund of Huazhong Agricultural University—Agricultural Genomics Institute at Shenzhen (CAAS) (SZYJY2021002).
We thank laboratory members Tong Liu and Yurong Fan for their technical help in our experiments, and Jiangsu Lvkee Biotechnology Co., Ltd., for their help in the cultivation of Carassius auratus.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1084500/full#supplementary-material
Abaidullah, M., Peng, S., Song, X., Zou, Y., Li, L., Jia, R., et al. (2021). Chlorogenic acid is a positive regulator of MDA5, TLR7 and NF-κB signaling pathways mediated antiviral responses against Gammacoronavirus infection. Int. Immunopharmacol. 96:107671. doi: 10.1016/j.intimp.2021.107671
Alesci, A., Pergolizzi, S., Savoca, S., Fumia, A., Mangano, A., Albano, M., et al. (2022). Detecting intestinal goblet cells of the broadgilled hagfish Eptatretus cirrhatus (forster, 1801): A confocal microscopy evaluation. Biology 11:1366. doi: 10.3390/biology11091366
Ayala, A., Munoz, M. F., and Arguelles, S. (2014). Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014:360438. doi: 10.1155/2014/360438
Belkaid, A., Currie, J. C., Desgagnes, J., and Annabi, B. (2006). The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell Int. 6:7. doi: 10.1186/1475-2867-6-7
Chen, J. L., Li, Y., Yu, B., Chen, D. W., Mao, X. B., Zheng, P., et al. (2018). Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 96, 1108–1118. doi: 10.1093/jas/skx078
Chen, K., Peng, C., Chi, F., Yu, C., Yang, Q., and Li, Z. (2022). Antibacterial and antibiofilm activities of chlorogenic acid against Yersinia enterocolitica. Front. Microbiol. 13:885092. doi: 10.3389/fmicb.2022.885092
Conterno, L., Fava, F., Viola, R., and Tuohy, K. M. (2011). Obesity and the gut microbiota: Does up-regulating colonic fermentation protect against obesity and metabolic disease? Genes Nutr. 6, 241–260. doi: 10.1007/s12263-011-0230-1
Corrigan, A., de Leeuw, M., Penaud-Frezet, S., Dimova, D., and Murphy, R. A. (2015). Phylogenetic and functional alterations in bacterial community compositions in broiler ceca as a result of mannan oligosaccharide supplementation. Appl. Environ. Microbiol. 81, 3460–3470. doi: 10.1128/Aem.04194-14
El Kasmi, K. C., Anderson, A. L., Devereaux, M. W., Vue, P. M., Zhang, W., Setchell, K. D. R., et al. (2013). Phytosterols promote liver injury and kupffer cell activation in parenteral nutrition–associated liver disease. Sci. Transl. Med. 5:206ra137. doi: 10.1126/scitranslmed.3006898
Faccioli, C. K., Chedid, R. A., Mori, R. H., Amaral, A. C., Franceschini-Vicentini, I. B., and Vicentini, C. A. (2016). Acid and alkaline phosphatase localization in the digestive tract mucosa of the Hemisorubim platyrhynchos. Acta Histochem. 118, 722–728. doi: 10.1016/j.acthis.2016.08.001
Fjellheim, A. J., Klinkenberg, G., Skjermo, J., Aasen, I. M., and Vadstein, O. (2010). Selection of candidate probionts by two different screening strategies from Atlantic cod (Gadus morhua L.) larvae. Vet. Microbiol. 144, 153–159. doi: 10.1016/j.vetmic.2009.12.032
Fu, Y., Liang, X., Li, D., Gao, H., Wang, Y., Li, W., et al. (2021). Effect of dietary tryptophan on growth, intestinal microbiota, and intestinal gene expression in an improved triploid crucian carp. Front. Nutr. 8:676035. doi: 10.3389/fnut.2021.676035
Gao, W., Wang, C., Yu, L., Sheng, T., Wu, Z., Wang, X., et al. (2019). Chlorogenic acid attenuates dextran sodium sulfate-induced ulcerative colitis in mice through MAPK/ERK/JNK pathway. Biomed. Res. Int. 2019:6769789. doi: 10.1155/2019/6769789
Han, S. F., Liu, Y. C., Zhou, Z. G., He, S. X., Cao, Y. A., Shi, P. J., et al. (2010). Analysis of bacterial diversity in the intestine of grass carp (Ctenopharyngodon idellus) based on 16S rDNA gene sequences. Aquac. Res. 42, 47–56. doi: 10.1111/j.1365-2109.2010.02543.x
Harris, J. K., El Kasmi, K. C., Anderson, A. L., Devereaux, M. W., Fillon, S. A., Robertson, C. E., et al. (2014). Specific microbiome changes in a mouse model of parenteral nutrition associated liver injury and intestinal inflammation. PLoS One 9:e110396. doi: 10.1371/journal.pone.0110396
Kong, L., Xu, M., Qiu, Y., Liao, M., Zhang, Q., Yang, L., et al. (2021). Chlorogenic acid and caffeine combination attenuates adipogenesis by regulating fat metabolism and inhibiting adipocyte differentiation in 3T3-L1 cells. J. Food Biochem. 45:e13795. doi: 10.1111/jfbc.13795
Kumar, G., Mukherjee, S., Paliwal, P., Singh, S. S., Birla, H., Singh, S. P., et al. (2019). Neuroprotective effect of chlorogenic acid in global cerebral ischemia-reperfusion rat model. Naunyn Schmiedebergs Arch. Pharmacol. 392, 1293–1309. doi: 10.1007/s00210-019-01670-x
Li, H., Zhong, Q. P., Wirth, S., Wang, W. W., Hao, Y. T., Wu, S. G., et al. (2015). Diversity of autochthonous bacterial communities in the intestinal mucosa of grass carp (Ctenopharyngodon idellus) (Valenciennes) determined by culture-dependent and culture-independent techniques. Aquac. Res. 46, 2344–2359. doi: 10.1111/are.12391
Li, L., Zhu, R., Li, M., Yu, Z., Wang, H., Quan, Y., et al. (2020). Effects of β−conglycinin on growth performance, antioxidant capacity and immunity in Rhynchocypris lagowski Dybowski. Aquac. Nutr. 26, 2059–2073. doi: 10.1111/anu.13147
Li, X., Yan, Q., Xie, S., Hu, W., Yu, Y., and Hu, Z. (2013). Gut microbiota contributes to the growth of fast-growing transgenic common carp (Cyprinus carpio L.). PLoS One 8:e64577. doi: 10.1371/journal.pone.0064577
Li, X., Zhou, L., Yu, Y., Ni, J., Xu, W., and Yan, Q. (2017). Composition of gut microbiota in the gibel carp (Carassius auratus gibelio) varies with host development. Microb. Ecol. 74, 239–249. doi: 10.1007/s00248-016-0924-4
Liang, M., Johnson, D., Burslem, D., Yu, S., Fang, M., Taylor, J. D., et al. (2020). Soil fungal networks maintain local dominance of ectomycorrhizal trees. Nat. Commun. 11:2636. doi: 10.1038/s41467-020-16507-y
Liang, N., and Kitts, D. D. (2015). Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 8:16. doi: 10.3390/nu8010016
Liu, B., Xu, L., Ge, X., Xie, J., Xu, P., Zhou, Q., et al. (2013). Effects of mannan oligosaccharide on the physiological responses, HSP70 gene expression and disease resistance of Allogynogenetic crucian carp (Carassius auratus gibelio) under Aeromonas hydrophila infection. Fish Shellfish Immunol. 34, 1395–1403. doi: 10.1016/j.fsi.2013.02.028
Liu, J., Wang, B., Lai, Q., Lu, Y., Li, L., Li, Y., et al. (2022). Boosted growth performance, immunity, antioxidant capacity and disease resistance of crucian carp (Carassius auratus) by single or in combination dietary Bacillus subtilis and xylo-oligosaccharides. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 256:109296. doi: 10.1016/j.cbpc.2022.109296
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Luo, X., Wu, J., Li, Z., Jin, W., Zhang, F., Sun, H., et al. (2020). Safety evaluation of Eucommia ulmoides extract. Regul. Toxicol. Pharmacol. 118:104811. doi: 10.1016/j.yrtph.2020.104811
Matozzo, V., Chinellato, A., Munari, M., Bressan, M., and Marin, M. G. (2013). Can the combination of decreased pH and increased temperature values induce oxidative stress in the clam Chamelea gallina and the mussel Mytilus galloprovincialis? Mar. Pollut. Bull. 72, 34–40. doi: 10.1016/j.marpolbul.2013.05.004
Nakamura, T., Nakazawa, Y., Onizuka, S., Satoh, S., Chiba, A., Sekihashi, K., et al. (1997). Antimutagenicity of Tochu tea (an aqueous extract of Eucommia ulmoides leaves): 1. The clastogen-suppressing effects of Tochu tea in CHO cells and mice. Mutat. Res. 388, 7–20. doi: 10.1016/s1383-5718(96)00096-4
Naveed, M., Hejazi, V., Abbas, M., Kamboh, A. A., Khan, G. J., Shumzaid, M., et al. (2018). Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 97, 67–74. doi: 10.1016/j.biopha.2017.10.064
Qi, D. L., Xia, M. Z., Chao, Y., Zhao, Y. L., and Wu, R. R. (2017). Identification, molecular evolution of toll-like receptors in a Tibetan schizothoracine fish (Gymnocypris eckloni) and their expression profiles in response to acute hypoxia. Fish Shellfish Immunol. 68, 102–113. doi: 10.1016/j.fsi.2017.07.014
Santos, L., and Ramos, F. (2016). Analytical strategies for the detection and quantification of antibiotic residues in aquaculture fishes: A review. Trends Food Sci. Technol. 52, 16–30. doi: 10.1016/j.tifs.2016.03.015
Sun, Z., Tan, X., Ye, H., Zou, C., Ye, C., and Wang, A. (2018). Effects of dietary Panax notoginseng extract on growth performance, fish composition, immune responses, intestinal histology and immune related genes expression of hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) fed high lipid diets. Fish Shellfish Immunol. 73, 234–244. doi: 10.1016/j.fsi.2017.11.007
Tan, X., Sun, Z., Liu, Q., Ye, H., Zou, C., Ye, C., et al. (2018). Effects of dietary Ginkgo biloba leaf extract on growth performance, plasma biochemical parameters, fish composition, immune responses, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀) fed high lipid diets. Fish Shellfish Immunol. 72, 399–409. doi: 10.1016/j.fsi.2017.10.022
Tsuchiya, C., Sakata, T., and Sugita, H. (2008). Novel ecological niche of Cetobacterium somerae, an anaerobic bacterium in the intestinal tracts of freshwater fish. Lett. Appl. Microbiol. 46, 43–48. doi: 10.1111/j.1472-765X.2007.02258.x
Wang, J., Liang, D., Yang, Q., Tan, B., Dong, X., Chi, S., et al. (2020). The effect of partial replacement of fish meal by soy protein concentrate on growth performance, immune responses, gut morphology and intestinal inflammation for juvenile hybrid grouper (Epinephelus fuscoguttatus ♂ × Epinephelus lanceolatus ♀). Fish Shellfish Immunol. 98, 619–631. doi: 10.1016/j.fsi.2019.10.025
Wang, Y. H., Xu, M., Wang, F. N., Yu, Z. P., Yao, J. H., Zan, L. S., et al. (2009). Effect of dietary starch on rumen and small intestine morphology and digesta pH in goats. Livest. Sci. 122, 48–52. doi: 10.1016/j.livsci.2008.07.024
Wu, D., Zhang, Y., Li, J., Fan, Z., Xu, Q., and Wang, L. (2022). Assessment of chicken intestinal hydrolysates as a new protein source to replace fishmeal on the growth performance, antioxidant capacity and intestinal health of common carp (Cyprinus carpio). Fish Shellfish Immunol. 125, 161–170. doi: 10.1016/j.fsi.2022.05.011
Wu, S. G., Wang, G. T., Angert, E. R., Wang, W. W., Li, W. X., and Zou, H. (2012). Composition, diversity, and origin of the bacterial community in grass carp intestine. PLoS One 7:e30440. doi: 10.1371/journal.pone.0030440
Xu, X., Chang, J., Wang, P., Yin, Q., Liu, C., Li, M., et al. (2020). Effect of chlorogenic acid on alleviating inflammation and apoptosis of IPEC-J2 cells induced by deoxyniyalenol. Ecotoxicol. Environ. Saf. 205:111376. doi: 10.1016/j.ecoenv.2020.111376
Yagasaki, K., Miura, Y., Okauchi, R., and Furuse, T. (2000). Inhibitory effects of chlorogenic acid and its related compounds on the invasion of hepatoma cells in culture. Cytotechnology 33, 229–235. doi: 10.1023/A:1008141918852
Yang, Z., Wang, Y., He, T., Ziema Bumbie, G., Wu, L., Sun, Z., et al. (2021). Effects of dietary Yucca schidigera extract and oral candida utilis on growth performance and intestinal health of weaned piglets. Front. Nutr. 8:685540. doi: 10.3389/fnut.2021.685540
Ye, X., Liu, Y., Hu, J., Gao, Y., Ma, Y., and Wen, D. (2021). Chlorogenic acid-induced gut microbiota improves metabolic endotoxemia. Front. Endocrinol. 12:762691. doi: 10.3389/fendo.2021.762691
Zhang, W., Zhao, J., Ma, Y., Li, J., and Chen, X. (2022). The effective components of herbal medicines used for prevention and control of fish diseases. Fish Shellfish Immunol. 126, 73–83. doi: 10.1016/j.fsi.2022.05.036
Zhao, Y., Wang, J., Ballevre, O., Luo, H., and Zhang, W. (2012). Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 35, 370–374. doi: 10.1038/hr.2011.195
Zhao, Y., Yan, M.-Y., Jiang, Q., Yin, L., Zhou, X.-Q., Feng, L., et al. (2021). Isoleucine improved growth performance, and intestinal immunological and physical barrier function of hybrid catfish Pelteobagrus vachelli × Leiocassis longirostris. Fish Shellfish Immunol. 109, 20–33. doi: 10.1016/j.fsi.2020.09.035
Zhu, H., Jiang, W., Liu, C., Wang, C., Hu, B., Guo, Y., et al. (2022). Ameliorative effects of chlorogenic acid on alcoholic liver injury in mice via gut microbiota informatics. Eur. J. Pharmacol. 928:175096. doi: 10.1016/j.ejphar.2022.175096
Keywords: chlorogenic acid, Carassius auratus, metabolism, antioxidation, immunity, intestinal flora
Citation: Jin X, Su M, Liang Y and Li Y (2023) Effects of chlorogenic acid on growth, metabolism, antioxidation, immunity, and intestinal flora of crucian carp (Carassius auratus). Front. Microbiol. 13:1084500. doi: 10.3389/fmicb.2022.1084500
Received: 30 October 2022; Accepted: 02 December 2022;
Published: 09 January 2023.
Edited by:
Kun Li, Nanjing Agricultural University, ChinaReviewed by:
Xiaolin Meng, Henan Normal University, ChinaCopyright © 2023 Jin, Su, Liang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingjun Li,  eWluZ2p1bkBtYWlsLmh6YXUuZWR1LmNu; Yunxiang Liang,
eWluZ2p1bkBtYWlsLmh6YXUuZWR1LmNu; Yunxiang Liang,  ZmEtbHl4QDE2My5jb20=
ZmEtbHl4QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.