- 1Institute of Microbiology, School of Ecology and Nature Conservation, Beijing Forestry University, Beijing, China
- 2Administration of Yuyuantan Park, Beijing, China
- 3Retired, Orliénas, France
- 4Tasmanian Institute of Agriculture, Hobart, TAS, Australia
In the present study, fourteen Exidia-like specimens were collected from China, France, and Australia. Based on morphological characteristics and phylogenetic analyses using the internal transcribed spacer regions (ITS) and the large subunit of nuclear ribosomal RNA gene (nLSU), four species in Exidia sensu lato, including Exidia saccharina and Tremellochaete atlantica, and two new species, Exidia subsaccharina and Tremellochaete australiensis, were identified. The four species are described and illustrated in detail. E. saccharina and T. atlantica, two known species from China are reported for the first time. E. subsaccharina and T. australiensis, two new species from France and Australia, respectively are also described. E. subsaccharina is characterized by its reddish brown to vinaceous brown basidiomata, slightly papillate hymenial surface, and narrowly allantoid basidiospores without oil drop measuring 12.5–17.5 × 4.2–5.5 μm. It differs from the similar species, E. saccharina, by distinctly larger basidiospores (12.5–17.5 × 4.2–5.5 vs. 10–14.2 × 3.2–4.5 μm). Tremellochaete australiensis is characterized by its white to grayish blue basidiomata, obviously and densely papillate hymenial surface, and allantoid basidiospores with oil drop measuring 13.8–16.2 × 4.8–6.5 μm. It also can be distinguished from the similar species, T. atlantica and T. japonica, by its distinctly larger basidiospores (13.5–17.8 × 4–5.2 vs. 10–11.8 × 4–4.8 μm in T. atlantica; 9.4–11.8 × 3.5–4.2 μm in T. japonica).
Introduction
Exidia Fr. was proposed by Fries and typified by Exidia glandulosa (Bull.) Fr. The genus is characterized by gelatinous bassidiomata, ellipsoid to subglobose, longitudinally cruciate septate, 4-celled basidia, cylindrical to allantoid basidiospores, and the ability to cause white rot in woody plants (Lowy, 1971; Liu, 1992; Roberts, 2001; Spirin et al., 2018; Ye et al., 2020; Wu et al., 2022a). Because of their morphological similarities, Exidia sensu lato traditionally includes three genera Exidia, Myxarium Wallr., and Tremellochaete Raitv (Roberts, 1998; Weiß and Oberwinkler, 2001; Malysheva, 2012), as confirmed by phylogenetic analyses in recent studies (Malysheva and Spirin, 2017; Spirin et al., 2018, 2019a; Wu et al., 2020a). Some species in Hyaloria Möller, Stypella Möller, and Sebacina C. Tul and C. Tul. et al., have waxy, very small, and effused basidiomata different from the usually gelatinous, thick, orbicular basidiomata of Exidia, were recently transferred into Myxarium based on morphological and phylogenetic analyses (Spirin et al., 2018). Myxarium phylogenetically forms a monophyletic clade distantly related to Exidia and Tremellochaete (Spirin et al., 2019a; Stalpers et al., 2021). In addition, Myxarium belongs to Hyaloriaceae, whereas Exidia and Tremellochaete belong to Auriculariaceae, and Myxarium can be distinguished from the latter two genera by its distinctly stalked basidia (Spirin et al., 2018, 2019a). Therefore, Exidia sensu lato is defined here as a group of fungi that includes Exidia and Tremellochaete.
Exidia was less studied in the latter part of the 20th century but has received attention more recently (Weiß and Oberwinkler, 2001; Wells et al., 2004; Roberts, 2009) due to its edible species and medicinal values (Łopusiewicz, 2018; Wu et al., 2020a). One edible species from China, Exidia yadongensis described by F. Wu et al., contains rich amino acids and plays a key role in the balance of physiological functions (Chen et al., 2019; Wu et al., 2020a). Recently, E. reflexa F. Wu et al., E. subglandulosa F. Wu et al., and E. qinghaiensi S.R. Wang and Thorn, were described based on multigene phylogenies (Ye et al., 2020; Wang and Thorn, 2021). However, the genus is still polyphyletic in the phylogeny, and species of the genus are scattered in several genera of Auriculariaceae (Yuan et al., 2018; Spirin et al., 2019a,b; Ye et al., 2020). Tremellochaete was reinstated to accommodate T. japonica (Yasuda), Raitv. and T. nigerrima (Viégas), Spirin and Malysheva (Malysheva and Spirin, 2017), and T. atlantica Alvarenga and T. cerradensis Alvarenga, and one new combination species, T. ciliata (Möller) Spirin and Alvarenga, were described and proposed in this genus (Alvarenga et al., 2019; Phookamsak et al., 2019). In total, there are more than 70 species in Exidia and six species in Tremellochaete worldwide according to Index Fungorum (http://www.indexfungorum.org) and MycoBank (https://www.mycobank.org), but < 20 species have molecular data (Alvarenga et al., 2019; Wu et al., 2020a; Wang and Thorn, 2021). Tremellochaete was reinstated at the genus level, it is not accepted by some researchers (Wang and Thorn, 2021), and the generic demarcation of Exidia and Tremellochaete is unclear both in morphology and phylogeny (Alvarenga et al., 2019; Ye et al., 2020). Further studies are urgently needed based on more samples and taxa.
In the present study, fourteen Exidia-like specimens were collected from China, France, and Australia. After morphological examinations and phylogenetic analyses using the internal transcribed spacer regions (ITS) and the large subunit of the nuclear ribosomal RNA gene (nLSU), four species were identified in Exidia and Tremellochaete, among which two are new to science, and a detailed description of these species is given in the present study.
Materials and methods
Morphology
The studied specimens were deposited at the herbarium of the Institute of Microbiology, Beijing Forestry University (BJFC), with color terms following those outlined by Petersen (1996). Sections mounted in 5% KOH and 2% phloxine B (C20H2Br4Cl4Na2O5) were studied at a magnification of up to 1,000 × using a Nikon Eclipse 80i microscope and phase contrast illumination. A Nikon Digital Sight DS-L3 camera was used to photograph microscopic structures. We also used other reagents, including Cotton Blue and Melzer's reagent to observe micromorphology following Wu et al. (2022b). To show the variation in spore sizes, 5% of measurements were excluded from each end of the range and shown in parentheses. At least thirty basidiospores from each specimen were measured. Stalks were excluded from basidia measurements, and the hilar appendage was excluded from basidiospore measurements. The following abbreviations were used: KOH, potassium hydroxide (5%); L, mean length (arithmetic average of all basidiospores length); W, mean width (arithmetic average of all basidiospores width); Q, L/W ratio for each specimen studied; n (a/b), number of basidiospores (a) measured from a given number of specimens (b).
DNA extraction, PCR reaction, and sequencing
DNA was extracted from dried specimens using a rapid plant genome extraction kit (Aidlab Biotechnologies Co., Ltd., Beijing, China) and modified following Wu et al. (2021). The internal transcribed spacer regions (ITS) and the large subunit of the nuclear ribosomal RNA gene (nLSU) were amplified with primer pairs ITS 4 and ITS 5 (White et al., 1990) and LR0R and LR7 (Vilgalys and Hester, 1990), respectively. The PCR (polymerase chain reaction) procedure for ITS was initial denaturation at 95°C for 3 min, followed by 35 cycles at 94°C for 40 s, 58°C for 45 s, and 72°C for 1 min, and a final extension at 72°C for 10 min. The PCR procedure for nLSU was initial denaturation at 94°C for 1 min, followed by 35 cycles at 94°C for 1 min, 48°C for 1 min, and 72°C for 1.5 min, and a final extension at 72°C for 10 min (Wu et al., 2018). The PCR products were purified and sequenced at the BGI (Beijing Genomics Institute, China), with the same primers that are used in the PCR reactions. The nLSU sequences were obtained by splicing bidirectional sequences because LR0R-LR7 is >1,000 bp.
Phylogenetic analyses
The new sequences generated in this study and reference sequences retrieved from GenBank (Table 1) were aligned with MAFFT (version 7; Katoh and Standley, 2013) and then manually adjusted in BioEdit and Mesquite version 3.04 software (Hall, 1999; Maddison and Maddison, 2017). A dataset composed of concatenated ITS+nLSU sequences was used in the phylogenetic analyses using the maximum likelihood (ML), maximum parsimony (MP), and Bayesian inference (BI) methods. Bourdotia galzinii (Bres.) Trotter was selected as the outgroup in the phylogenetic analyses because the species was closer to species of Auriculariaceae than others but not closely related to species in Exidia sensu lato (Spirin et al., 2019a). Except for the outgroup, sequences from the other eleven Auriculariales genera were added to the phylogenetic analyses because Exidia was previously shown to be polyphyletic (Yuan et al., 2018; Spirin et al., 2019a).
Maximum likelihood (ML), Bayesian inference (BI), and maximum parsimony (MP) phylogenetic analyses were performed using RAxML (version 8; Stamatakis, 2014), MrBayes (version 3.2.7a; Ronquist et al., 2012), and PAUP (version 4.0b10; Swofford, 2002), respectively, following the study of Wu et al. (2020b). The optimal substitution models for the combined dataset are determined using the Akaike information criterion (AIC) implemented in MrModeltest 2.3 (Posada and Crandall, 1998; Nylander, 2004) after scoring 24 models of evolution by PAUP (version 4.0b10; Swofford, 2002). The GTR + I + G model was applied in the BI and ML analyses.
Branches that received bootstrap support for maximum likelihood (BS), Bayesian posterior probabilities (BPP), and maximum parsimony (BP) >50% (BS), 0.90 (BPP), and 50% (BP), respectively, are considered to be significantly supported. The phylograms were viewed using FigTree version 1.4.2 (Rambaut, 2012).
Results
Phylogenetic analyses
The combined ITS+nLSU dataset included 81 fungal specimens representing 44 species in the Auriculariales. The dataset had an aligned length of 1,898 characters, including 1,406 constants, 156 parsimony-uninformative characters, and 336 parsimony-informative characters. MP analysis yielded four equally parsimonious trees (tree length = 1,607, consistency index = 0.432, retention index = 0.725, rescaled consistency index = 0.313, and homoplasy index = 0.568). The average standard deviation of split frequencies in BI analysis was 0.005425. The topology of the ML tree with bootstrap values for BP, BS, and BPP was chosen to represent the phylogenetic relationship of species in the Auriculariales since ML, MP, and BI resulted in similar topologies (Figure 1). The phylogeny demonstrated our fourteen Exidia-like specimens were clustered into four different lineages with high support, including two new lineages that represented two new species, E. subsaccharina (100% BS, 1.00 BPP, and 100% BP; Figure 1) and Tremellochaete australiensis (100% BS, 1.00 BPP, and 100% BP; Figure 1). The four Chinese specimens and one German sample (Roki 88) identified as E. saccharina by Weiß and Oberwinkler (2001) were nested in the same lineage with high support in the phylogeny (Figure 1), so these specimens were treated as E. saccharina, and this represents the first record of the species in China.
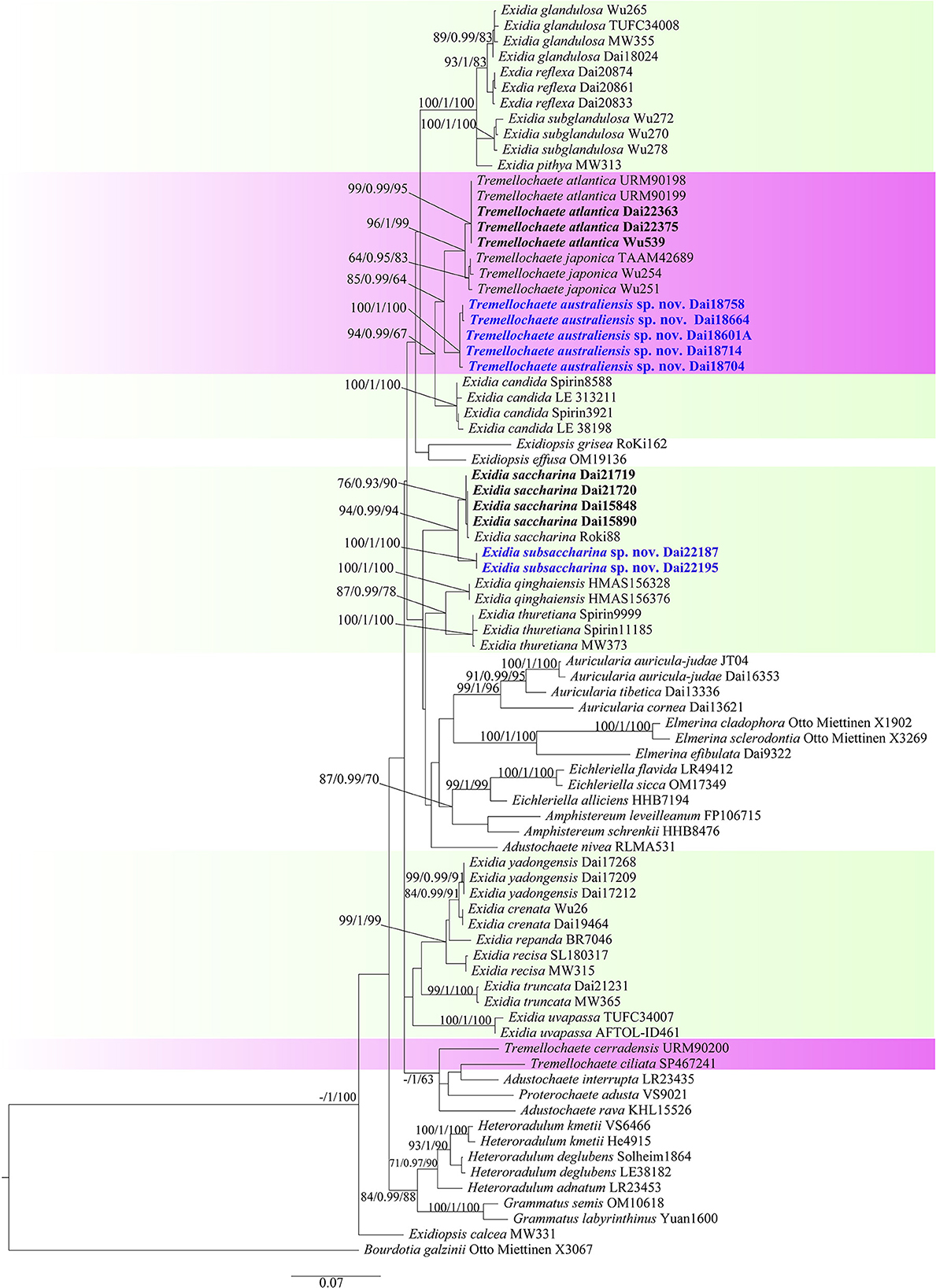
Figure 1. Maximum likelihood tree illustrating the phylogeny of Exidia sensu lato based on the combined ITS+nLSU dataset. Branches are labeled with maximum likelihood bootstrap >50%, Bayesian posterior probabilities >0.90, and maximum parsimony bootstrap >50%, respectively. New species are in blue.
Taxonomy
Exidia saccharina Fr., Syst. mycol. (Lundae) 2(1): 225 (1822), Figures 2A, 3.
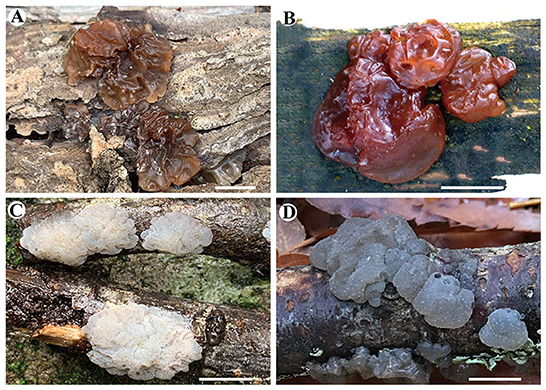
Figure 2. Basidiomata of species in Exidia and Tremellochaete. (A) Exidia saccharina (Dai 21719); (B) E. subsaccharina (Dai 22187 and LY BR 337, holotype); (C) Tremellochaete atlantica (Dai 22375); (D) T. australiensis (Dai 18664, holotype). Scale bars: 1 cm.
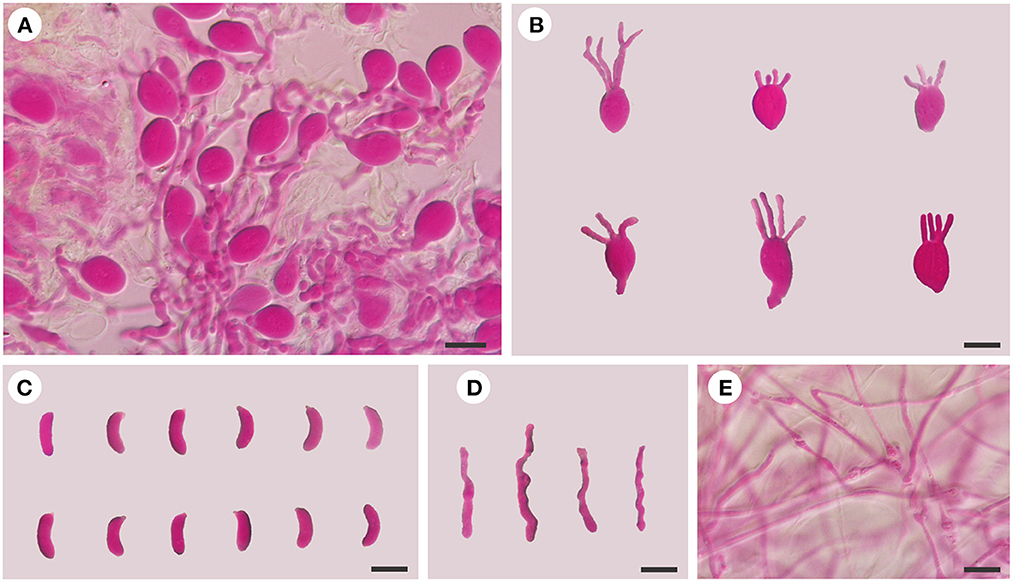
Figure 3. Microscopic structures of E. saccharina (Dai 21720). (A) A section of hymenium; (B) basidia; (C) basidiospores; (D) hyphidia; (E) hyphae. Scale bars: 10 μm.
Basidiomata: When fresh, the basidiomata are gelatinous, fawn to orange-brown, suborbicular to cerebriform, sessile, usually remaining separate, occasionally coalescing; are up to 10 cm wide and 1.5 cm thick; have free margins; have a hymenial surface that is clearly ridged, with sparse papillae, becoming vinaceous brown when dry; and are absent of mineral inclusions.
Internal features: Hyphal structure is monomitic; hyphae are clamped (clamps are usually open), usually branched, hyaline, thin-walled, 0.5–2.5 μm in diameter, and embedded in a gelatinous matrix. Basidia are longitudinally cruciate septate, 4-celled, subglobose to ovoid, and thin-walled, measuring 13–15.5 × 8.5–11.8 μm. Hyphidia are simple, thin-walled, and hyaline. Basidiospores are narrowly allantoid, slightly to distinctly curved, hyaline, thin-walled, smooth, usually without oil drop, neither amyloid, dextrinoid, nor cyanophilous, measuring (9.8–)10–14.2(−14.5) × (3–)3.2–4.5 μm, L = 11.71 μm, W = 3.82 μm, and Q = 2.98–3.12 (n = 60/2).
Specimens examined: CHINA. Hebei Province, Weichang County, Saihanba National Forest Park, on fallen trunk of Larix, 27.VIII.2020, Dai 21719 (BJFC 035621), and Dai 21720 (BJFC 035622); Xinjiang Autonomous Region, Burjin County, Karnas Nature Reserve, on fallen trunk of Larix, 11.IX.2015, Dai 15890 (BJFC 019991); Habahe County, Baihaba River Forest Park, on the fallen trunk of Larix, 10.IX.2015, Dai 15848 (BJFC 019949).
Exidia subsaccharina F. Wu, B. Rivoire, A. Tohtirjap, and Y.C. Dai, sp. nov. Figures 2B, 4.
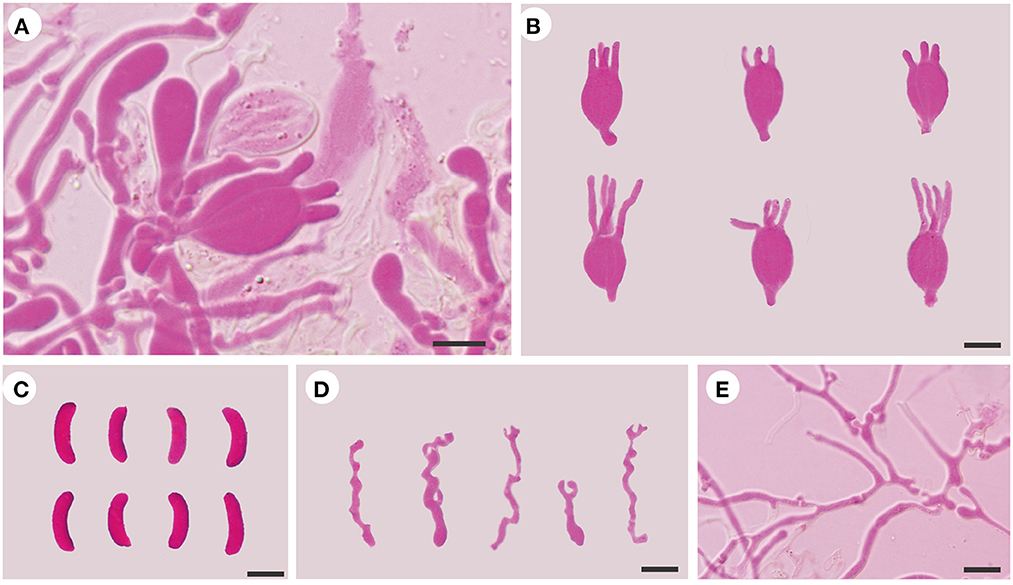
Figure 4. Microscopic structures of E. subsaccharina (Dai 22187 and LY BR 337, holotype). (A) A section of hymenium; (B) basidia; (C) basidiospores; (D) hyphidia; (E) hyphae. Scale bars: 10 μm.
MycoBank: MB846784.
Holotype: FRANCE. Chaussan, on dead tree of Pinus sylvestris, 10.VIII.2008, Dai 22187 and LY BR 337 (BJFC 036778).
Etymology: Subsaccharina (Latin) refers to the micromorphology being similar to that of E. saccharina.
Diagnosis: E. subsaccharina may be confused with E. saccharina when fresh, but E. saccharina differs from the species by its slightly smaller basidia (13–15.5 × 8.5–11.8 μm), usually simple hyphidia, and distinctly smaller basidiospores (10–14.2 × 3.2–4.5 μm).
Basidiomata: When fresh, the basidiomata are gelatinous, reddish brown to vinaceous brown, orbicular to suborbicular, sessile, usually remaining coalescing, occasionally separate; are fused together with up to 10 cm in width and 1 cm in thickness; have free margins; have a hymenial surface that is slightly ridged, with papillae, becoming fuscous when dry; and are absent of mineral inclusions.
Internal features: Hyphal structure is monomitic; hyphae are clamped (clamps are usually open), usually branched, hyaline, thin-walled, 0.5–3 μm in diameter, and embedded in a gelatinous matrix. Basidia are longitudinally cruciate septate, 4-celled, subglobose to ovoid, and thin-walled, measuring 13.5–19.2 × 9.2–14.2 μm. Hyphidia are usually branched, sometimes simple, thin-walled, and hyaline. Basidiospores are narrowly allantoid, slightly to distinctly curved, hyaline, thin-walled, smooth, usually without oil drop, neither amyloid, dextrinoid, nor cyanophilous, measuring (12–)12.5–17.5(−18.5) × (4–)4.2–5.5(−5.8) μm, L = 15.78 μm, W = 4.73 μm, and Q = 3.23–3.44 (n = 60/2).
Additional specimen examined (paratype): FRANCE. Orliénas, on dead tree of Pinus sylvestris, 22.XII.2011, Dai 22195 and LY BR 4290 (BJFC 036786).
Tremellochaete atlantica Alvarenga, in Phookamsak et al., Fungal Diversity 95: 242 (2019) Figures 2C, 5.
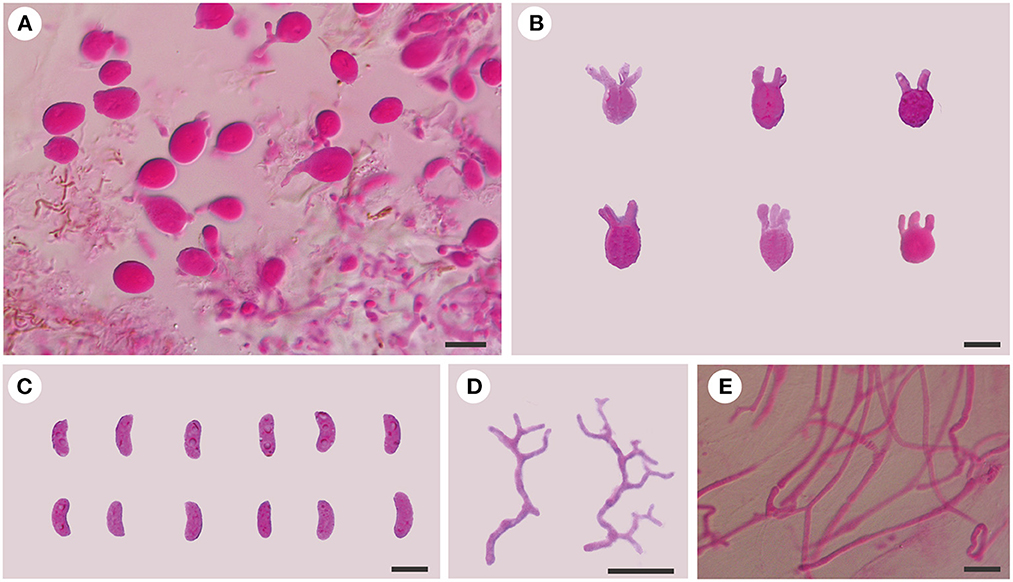
Figure 5. Microscopic structures of T. atlantica (Dai 23375). (A) A section of hymenium; (B) basidia; (C) basidiospores; (D) hyphidia; (E) hyphae. Scale bars: 10 μm.
Basidiomata: When fresh, the basidiomata are gelatinous, white to ash-gray or brownish, suborbicular to slightly cerebriform, sessile, usually remaining coalescing, occasionally separate; are fused, with up to 10 cm width and 1 cm thickness; have free margins; have occasionally ridged hymenial surface, are obviously and densely studded with irregular papillae, becoming dark gray or grayish brown when dry; and are absent of mineral inclusions.
Internal features: Hyphal structure is monomitic; hyphae are usually simple septate, rarely clamped, branched, hyaline, thin-walled, 0.5–2 μm in diameter, and embedded in a gelatinous matrix. Basidia are longitudinally cruciate septate, 4-celled, subglobose to globose, and thin-walled, measuring 10–12.8 × 9–10.8 μm. Hyphidia are distinctly branched, thin-walled, and hyaline. Basidiospores are allantoid, slightly to distinctly curved, hyaline, thin-walled, smooth, usually with oil drop, neither amyloid, dextrinoid, nor cyanophilous, measuring (9.8–)10–11.8(−12.8) × (3.8–)4–4.8(−5) μm, L = 10.95 μm, W = 4.24 μm, and Q = 2.58 (n = 30/1).
Specimens examined: CHINA. Fujian Province, Yongtai County, Tianmenshan National Forest Park, on fallen angiosperm branch, 5.VI.2021, Dai 22363 (BJFC 036947) and Dai 22375 (BJFC 036959); Yunnan Province, Xishuangbanna, Mengla County, Rainforest Valley Scenic Area, on fallen angiosperm branch, 3.VII.2021, Wu 539 (BJFC 036394).
Tremellochaete australiensis F. Wu, G.M. Gates, A. Tohtirjap, and Y.C. Dai, sp. nov. Figures 2D, 6.
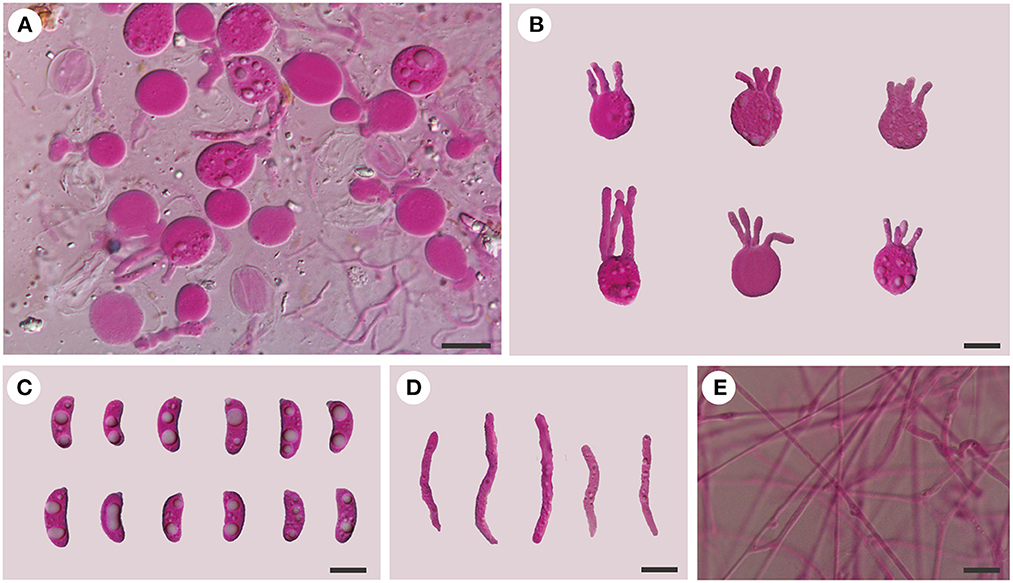
Figure 6. Microscopic structures of Tremellochaete australiensis (Dai 18664, holotype). (A) A section of hymenium; (B) basidia; (C) basidiospores; (D) hyphidia; (E) hyphae. Scale bars: 10 μm.
MycoBank: MB846785.
Holotype: AUSTRALIA. Melbourne, Dandenong Ranges Botanical Garden, on the dead tree of Rhododendron, 12.V.2018, Dai 18664 (BJFC 027132).
Etymology: Australiensis (Latin) refers to the species being found in Australia.
Diagnosis: Tremellochaete australiensis is morphologically similar to T. atlantica and T. japonica, but the latter two species have shorter basidia (< 13 μm in length) and basidiospores (< 12 μm in length) and branched hyphidia.
Basidiomata: When fresh, the basidiomata are gelatinous, white to grayish blue, suborbicular to slightly cerebriform, sessile, usually remaining coalescing, occasionally separate; are fused together with up to 20 cm in width and 0.5–1 cm in thickness; have free margins; have a hymenial surface occasionally ridged, is clearly and densely studded with irregular papillae, becoming dark gray to black when dry; and are absent of mineral inclusions.
Internal features: Hyphal structure is monomitic; hyphae are clamped (clamps are usually open), usually branched, hyaline, thin-walled, 0.5–2.5 μm in diameter, and embedded in a gelatinous matrix. Basidia are longitudinally cruciate septate, 4-celled, subglobose to globose, and thin-walled, measuring 13–15.8 × 11.5–15 μm. Hyphidia are simple, cylindrical, thin-walled, and hyaline. Basidiospores are allantoid, slightly to distinctly curved, hyaline, thin-walled, smooth, usually with oil drop, neither amyloid, dextrinoid, nor cyanophilous, measuring (12.8–)13.8–16.2(−18) × (4.5–)4.8–6.5 μm, L = 14.94 μm, W = 5.59 μm, and Q = 2.6 (n = 30/1).
Additional specimens examined (paratypes): AUSTRALIA. Tasmania, Hobart, Mt. Wellington, on rotten wood of Olearia, 13.V.2018, Dai 18704 (BJFC 027173) and Dai 18714 (BJFC 027183); Mount Field Forest, close to Mount National Park, on the fallen trunk of Nothofagus, 14.V.2018, Dai 18758 (BJFC 027226); Victoria, Yarra Ranges National Park, on rotten wood of Eucalyptus, 9.V.2018, Dai 18601A (BJFC 027070).
Discussion
Exidia sensu lato is a genus of wood-inhabiting fungi that grows on dead branches and logs and is best known in the temperate regions of Europe, America, and Asia (Malysheva, 2012; Spirin et al., 2018; Wu et al., 2020a; Ye et al., 2020; Wang and Thorn, 2021). Although nearly eighty taxa were recorded in Exidia sensu lato, most species were described in the 20th century (Fries, 1822; Lowy, 1964, 1971). In recent years, four species in Exidia and two species in Tremellochaete were described based on morphology and phylogenetic analyses (Alvarenga et al., 2019; Wu et al., 2020a; Ye et al., 2020; Wang and Thorn, 2021), which improved knowledge of Exidia sensu lato across the world. However, since the demarcation of Exidia and Tremellochaete is still ambiguous, multilocus analyses based on taxonomically and geographically broad sampling are needed.
Tremellochaete was accepted by most researchers (Malysheva and Spirin, 2017; Malysheva et al., 2018; Yuan et al., 2018; Alvarenga et al., 2019). However, Wang and Thorn (2021) rejected Tremellochaete because its type species T. japonica was closely related to E. candida Lloyd in their phylogeny. In our phylogeny (Figure 1), three Tremellochaete species, T. atlantica, T. australiensis, and T. japonica, are also closely related to E. candida, but they formed a separate clade with robust support; two other species placed in Tremellochaete, T. cerradensis and T. ciliata, are distantly related (Figure 1). Tremellochaete may be a polyphyletic genus like other genera, e.g., Exidia and Exidiopsis (Yuan et al., 2018; Alvarenga et al., 2019; Spirin et al., 2019b). In addition, Tremellochaete can be distinguished from Exidia by its clear and dense papillae on the hymenial surface (Malysheva and Spirin, 2017; Alvarenga et al., 2019; Figure 2).
Exidia subsaccharina is morphologically similar to E. saccharina by sharing gelatinous and brownish basidiomata, a slightly papillate hymenial surface, and narrowly allantoid basidiospores usually without oil drop and grows on rotten conifer wood (Spirin et al., 2018), and both species are closely related in the phylogeny (Figure 1). However, E. subsaccharina can be distinguished from E. saccharina by its slightly larger basidia (13.5–19.2 × 9.2–14.2 vs. 13–15.5 × 8.5–11.8 μm), usually branched hyphidia (usually simple in E. saccharina), and distinctly bigger basidiospores (12.5–17.5 × 4.2–5.5 vs. 10–14.2 × 3.2–4.5 μm), and they form two distinct lineages with robust support (Figure 1). Exidia pithya (Alb. and Schwein.) Fr. usually grows on conifer wood too, but it differs from E. subsaccharina by its resupinate and black basidiomata and distinctly smaller basidiospores (10–13 × 3–5 μm; Malysheva, 2012), and it is distantly related to E. subsaccharina in the phylogeny (Figure 1).
Tremellochaete australiensis may be confused with T. atlantica and T. japonica due to their gelatinous and white to gray basidiomata, densely papillated hymenial surface, and allantoid basidiospores usually with oil drop (Phookamsak et al., 2019; Ye et al., 2020), but it has longer basidia (13–15.8 × 11.5–15 μm in T. australiensis; 10–12.8 × 9–10.8 μm in T. atlantica; 9.4–12.4 × 9.1–14.2 μm in T. japonica) and basidiospores (13.8–16.2 × 4.8–6.5 μm in T. australiensis; 10–11.8 × 4–4.8 μm in T. atlantica; 9.4–11.8 × 3.5–4.2 μm in T. japonica). Furthermore, T. australiensis usually has cylindrical hyphidia, but they are distinctly branched in T. atlantica and T. japonica.
Tremellochaete atlantica was originally described from Brazil by Phookamsak et al. (2019), and our Chinese samples and the type of T. atlantica share almost the same ITS sequences, with < 2 base pair differences in the ITS region between the Chinese samples and the type of T. atlantica. The morphology of the Chinese samples fits the descriptions of T. atlantica except for slightly longer basidiospores (10–11.8 × 4–4.8 vs. 7.75–10 × 2–5 μm) and usually simple septate hyphae, with Brazillian specimens usually having clamped hyphae) (Phookamsak et al., 2019). These minor differences are considered intraspecific, so we consider this the first report of T. atlantica from China.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
FW and Y-CD coordinated the project, designed the experimental plan, and acquired funding. AT and FW analyzed the data and prepared the original draft. BR and Y-CD collected the samples from the field. S-XH, GG, BR, and Y-CD reviewed and edited the manuscript. All authors contributed to the study and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Project Nos. 32070006 and 32270011), the Tibet Autonomous Region Science and Technology Project (XZ202201ZY0006N), and the Fundamental Research Funds for the Central Universities (No. 2021ZY91).
Acknowledgments
We thank Long-Fei Fan, Zhan-Bo Liu, and Ya-Ping Lian for their guidance on DNA extraction, PCR reaction, sequencing, and illustration.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alvarenga, R. L. M., Spirin, V., Malysheva, V., Gibertoni, T. B., and Larsson, K. H. (2019). Two new genera and six other novelties in Heterochaete sensu lato (Auriculariales, Basidiomycota). Botany 97, 439–451. doi: 10.1139/cjb-2019-0046
Chen, H. Y., Bao, D. P., Yang, R. H., Wang, Y., Gao, Y. Y., Li, Y., et al. (2019). Amino acid profile and protein quality of Exidia sp. Acta Agric.Nucl. Sin. 33, 81–87.
Hall, T. A. (1999). Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98.
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Liu, B. (1992). Flora of Fungorum Sinicorum, vol. 2 (Tremellales and Dacrymycetales). Beijing: Science Press. p. 93.
Łopusiewicz, Ł. (2018). Isolation, characterisation and biological activity of melanin from Exidia nigricans. World Sci. News 91, 111–129.
Lowy, B. (1971). Flora Neotropica Monograph 6 (Tremellales). New York: Hafner Publishing Co., Inc. 153.
Maddison, W. P., and Maddison, D. R. (2017). Mesquite: a Modular System for Evolutionary Analysis, version 3.2. Available online at: http://mesquiteproject.org
Malysheva, V. (2012). A revision of the genus Exidia (Auriculariales, Basidiomycota) in Russia. Mikol. Fitopat. 46, 365–376.
Malysheva, V., and Spirin, V. (2017). Taxonomy and phylogeny of the Auriculariales (Agaricomycetes, Basidiomycota) with stereoid basidiocarps. Fungal Biol. 121, 689–715. doi: 10.1016/j.funbio.2017.05.001
Malysheva, V., Spirin, V., Miettinen, O., Motato-Vásquez, V., Hernawati, Seelan, J. S. S., et al. (2018). Revision of Protohydnum (Auriculariales, Basidiomycota). Mycol. Prog. 17, 805–814. doi: 10.1007/s11557-018-1393-6
Nylander, J. A. A. (2004). MrModeltest v2. Program Distributed by the Author. Uppsala: Uppsala University, Evolutionary Biology Centre.
Petersen, J. H. (1996). The Danish Mycological Society's colour-chart. Foreningen til Svampekundskabens Fremme (Greve), 1–6.
Phookamsak, R., Hyde, K. D., Jeewon, R., Bhat, J., Jones, E. B., Maharachchikumbura, S. S., et al. (2019). Fungal diversity notes 929–1035: taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers. 95, 1–273. doi: 10.1007/s13225-019-00421-w
Posada, D., and Crandall, K. A. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics. 14, 817–818. doi: 10.1093/bioinformatics/14.9.817
Rambaut, A. (2012). Molecular Evolution, Phylogenetics and Epidemiology. FigTree ver. 1.4 Software. Available online at: http://tree.bio.ed.ac.uk/software/figtree/ (accessed 7 August, 2022).
Roberts, P. (1998). A revision of the genera Heterochaetella, Myxarium, Protodontia and Stypella (Heterobasidiomycetes). Mycotaxon 69, 209–248.
Roberts, P. (2001). A key to British Exidia species. Field Mycol. 2, 134–135. doi: 10.1016/S1468-1641(10)60535-X
Roberts, P. (2009). Exidia nigricans: a new and legitimate name for Exidia plana. Mycotaxon 109, 219–220. doi: 10.5248/109.219
Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Spirin, V., Malysheva, V., and Larsson, K. H. (2018). On some forgotten of Exidia and Myxarium (Auriculariales, Basidiomycota). Nord. J. Bot. 36, e01601. doi: 10.1111/njb.01601
Spirin, V., Malysheva, V., Miettinen, O., Alvarenga, R. L. M., Gibertoni, T. B., Ryvarden, L., et al. (2019b). On Protomerulius and Heterochaetella (Auriculariales, Basidiomycota). Mycol. Prog. 18, 1079–1099. doi: 10.1007/s11557-019-01507-0
Spirin, V., Malysheva, V., Roberts, P., Trichies, G., Savchenko, A., and Larsson, K. H. (2019a). A convolute diversity of the Auriculariales (Agaricomycetes, Basidiomycota) with sphaeropedunculate basidia. Nord. J. Bot. 37, e02394. doi: 10.1111/njb.02394
Stalpers, J., Redhead, S., May, T. W., Rossman, A. Y., Crouch, J. A., Cubeta, M. A., et al. (2021). Competing sexual-asexual generic names in Agaricomycotina (Basidiomycota) with recommendations for use. IMA Fungus 12, 1–31. doi: 10.1186/s43008-021-00061-3
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Swofford, D. L. (2002). PAUP*: Phylogenetic Analysis Using Parsimony (* and Other Methods); Version 4.0b10; Sinauer Associates. Sunderland, MA, USA: Sinauer Associates.
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Wang, S. R., and Thorn, R. G. (2021). Exidia qinghaiensis, a new species from China. Mycoscience 62, 212–216. doi: 10.47371/mycosci.2021.03.002
Weiß, M., and Oberwinkler, F. (2001). Phylogenetic relationships in Auriculariales and related groups–hypotheses derived from nuclear ribosomal DNA sequences. Mycol. Res. 105, 103–415. doi: 10.1017/S095375620100363X
Wells, K., Bandoni, R. J., Lim, S. R., and Berbee, M. L. (2004). “Observations on some species of Myxarium and reconsideration of the Auriculariaceae and Hyaloriaceae (Auriculariales),” in Frontiers Basidiomycete Mycol. Eching: IHW-Verlag. eds, Agerer, R., Piepenbring, and M., Blanz, P. p. 237–248.
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR protocols: a guide to methods and applications. New York, NY: Academic Press. p. 315–322.
Wu, F., Chen, J. J., Ji, X. H., Vlasák, J., and Dai, Y. C. (2018). Phylogeny and diversity of morphologically similar polypore genera Rigidoporus, Physisporinus, Oxyporus and Leucophellinus. Mycologia 109, 749–765. doi: 10.1080/00275514.2017.1405215
Wu, F., Man, X. W., Tohtirjap, A., and Dai, Y. C. (2022a). A comparison of polypore funga and species composition in forest ecosystems of China, North America, and Europe. For. Ecosyst. 9, 100051. doi: 10.1016/j.fecs.2022.100051
Wu, F., Tohtirjap, A., Fan, L. F., Zhou, L. W., Alvarenga, R. L. M., Gibertoni, T. B., et al. (2021). Global diversity and updated phylogeny of Auricularia (Auriculariales, Basidiomycota). J. Fungi 7, 933. doi: 10.3390/jof7110933
Wu, F., Yuan, Y., Chen, J. J., Cui, B. K., Zhou, M., and Dai, Y. C. (2020b). Terrestriporiaceae fam. nov., a new family of Russulales (Basidiomycota). Mycosphere 11, 2755–2766. doi: 10.5943/mycosphere/11/1/21
Wu, F., Zhao, Q., Yang, Z. L., Ye, S. Y., Rivoire, B., and Dai, Y. C. (2020a). Exidia yadongensis, a new edible species from East Asia. Mycosystema 39, 1203–1214. doi: 10.13346/j.mycosystema.200205
Wu, F., Zhou, L. W., Vlasák, J., and Dai, Y. C. (2022b). Global diversity and systematics of Hymenochaetaceae with poroid hymenophore. Fungal Diver. 113, 1–192. doi: 10.1007/s13225-021-00496-4
Ye, S. Y., Zhang, Y. B., Wu, F., and Liu, H. X. (2020). Multi-locus phylogeny reveals two new species of Exidia (Auriculariales, Basidiomycota) from China. Mycol. Prog. 19, 859–868. doi: 10.1007/s11557-020-01601-8
Keywords: Auriculariaceae, phylogenetic analysis, taxonomy, wood-rotting fungi, diversity
Citation: Tohtirjap A, Hou S-X, Rivoire B, Gates G, Wu F and Dai Y-C (2023) Two new species of Exidia sensu lato (Auriculariales, Basidiomycota) based on morphology and DNA sequences. Front. Microbiol. 13:1080290. doi: 10.3389/fmicb.2022.1080290
Received: 26 October 2022; Accepted: 16 December 2022;
Published: 14 February 2023.
Edited by:
Jesús Navas-Castillo, Spanish National Research Council (CSIC), SpainReviewed by:
R. Greg Thorn, Western University, CanadaŁukasz Łopusiewicz, West Pomeranian University of Technology, Poland
Copyright © 2023 Tohtirjap, Hou, Rivoire, Gates, Wu and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Wu,  ZmFuZ3d1YmpmdTIwMTRAYmpmdS5lZHUuY24=; Yu-Cheng Dai,
ZmFuZ3d1YmpmdTIwMTRAYmpmdS5lZHUuY24=; Yu-Cheng Dai,  eXVjaGVuZ2RhaUBiamZ1LmVkdS5jbg==
eXVjaGVuZ2RhaUBiamZ1LmVkdS5jbg==
 Ablat Tohtirjap
Ablat Tohtirjap Shi-Xing Hou2
Shi-Xing Hou2 Genevieve Gates
Genevieve Gates Fang Wu
Fang Wu