- 1College of Pharmacy, Guangxi University of Chinese Medicine, Nanning, China
- 2Guangxi Key Laboratory of Medicinal Resources Conservation and Genetic Improvement, Guangxi Botanical Garden of Medicinal Plants, Nanning, China
- 3Guangxi Zhuang Yao Key Laboratory of Medicine, Guangxi University of Chinese Medicine, Nanning, China
Endophytic fungi from medicinal plants with specific pharmacological functions attract much attention to provide the possibility of discovering valuable natural drugs with novel structures and biological activities. Nervilia fordii is a rare and endangered karst endemic plant that is used as medicine and food homology in Guangxi, China. These plants have been reported to have antimicrobial, antitumor, antiviral, and anti-inflammatory activities. However, few studies have focused on the diversity and antibacterial activity of endophytic fungi from N. fordii. In the present study, 184 endophytic fungi were isolated from the healthy tissues of N. fordii, and their molecular diversity and antimicrobial activities were analyzed for the first time. These fungi were categorized into 85 different morphotypes based on the morphological characteristics and the similarity between the target sequence and the reference sequence in the GenBank database. With the exception of 18 unidentified fungi, the fungal isolates belonged to at least 2 phyla, 4 classes, 15 orders, 45 known genera, and 45 different species, which showed high abundance, rich diversity, and obvious tissue specificity. All isolates were employed to screen for their antimicrobial activities via the agar diffusion method against Escherichia coli, Staphylococcus aureus, and Candida tropicalis. Among these endophytes, eight strains (9.41%) displayed inhibitory activity against E. coli, 11 strains (12.94%) against S. aureus, and two strains (2.35%) against C. tropicalis, to some extent. In particular, our study showed for the first time that the fungal agar plugs of Penicillium macrosclerotiorum 1151# exhibited promising antibacterial activity against E. coli and S. aureus. Moreover, the ethyl acetate (EA) extract of P. macrosclerotiorum 1151# had antibacterial effects against E. coli and S. aureus with a minimum inhibitory concentration (MIC) of 0.5 mg ml–1. Further research also confirmed that one of the antimicrobial compounds of P. macrosclerotiorum 1151# was methyl chloroacetate and exhibited excellent antibacterial activity against E. coli and S. aureus up to 1.71-fold and 1.13-fold compared with tetracycline (TET) (5 mg ml–1), respectively. Taken together, the present data suggest that various endophytic fungi of N. fordii could be exploited as sources of novel natural antimicrobial agents.
Introduction
The emergence of novel coronavirus pathogens, monkeypox, and mucormycosis (Feldman and Anderson, 2021; Salem et al., 2022a), as well as the re-emergence of microbial diseases such as tuberculosis, whooping cough, and urinary tract infections, have posed an unprecedented threat to human lives and health (Rajivgandhi et al., 2016; Mahendra et al., 2022). In fact, antimicrobial resistance is the most serious obstacle to dealing with such threats. Unfortunately, no new antibiotics have been discovered in the last two decades, although the search for new antibiotics has never stopped (Rajivgandhi et al., 2020; Böttcher et al., 2021). Therefore, it is necessary to identify new antibacterial drugs to deal with emerging microbial diseases (Andryukov et al., 2019; Seukep et al., 2020).
Endophytic fungi are microorganisms that live within the cells or tissues of their host plants at a certain stage without causing obvious disease to plant tissues (Petrini et al., 1992). Studies have shown that endophytic fungi are prevalent in various ecosystems worldwide, with the exception of Antarctica (Guo, 2018). The number of endophytic fungi is conservatively estimated to be more than 1 million. However, about 95% of endophytic fungi have not been described, which means that many novel natural active ingredients may be discovered from endophytic fungi. In fact, some active substances, such as penicillin, cephalosporin, and ß-lactam antibiotics, produced by endophytic fungi have shown great economic value and application prospects in the development of antimicrobial drugs (Terfehr et al., 2017). In addition, biologists have also used fungi biological techniques to rapidly synthesize nanocrystals (Ag, Zn, Se, etc.) with antibacterial, antifungal, and anticancer activities (Hashem et al., 2022; Salem et al., 2022b; Shehabeldine et al., 2022a). Therefore, endophytic fungi provide the possibility of discovering valuable natural drugs with novel structures and biological activities (Elghaffar et al., 2022).
In recent years, many studies have found that endophytic fungi and their host plants have similar metabolite-synthesis pathways, which can produce the secondary metabolites of host plants. For example, Taxomyces andreanae isolated from Taxus brevifolia produces the anticancer component paclitaxel (Stierle et al., 1993), whereas Phialocephala fortinii isolated from Rhodiola angusta produces the antioxidant components salidroside and p-tyrosol (Cui et al., 2016). Therefore, endophytic fungi from medicinal plants with specific pharmacological functions have become a research hotspot.
Nervilia fordii (Hance) Schltr. is a small, terrestrial, and short-lived perennial plant (Orchidaceae) that is endemic to the karst limestone mountains (Figures 1A–D) in Guangxi Province, China. The whole plant or the aerial part of N. fordii (Figure 1E) are used as premium dishes, herbal tea, and folk medicine (Qiu et al., 2013) because of their excellent pharmacological capacities, such as antimicrobial (Li et al., 2017; Xu et al., 2017), anticancer (Yao et al., 2021), antiviral (Tian et al., 2009), and anti-inflammatory (Yin et al., 2021) effects. In particular, this plant was widely used as a main ingredient in traditional Chinese medicine formulations to treat patients with severe acute respiratory syndrome in 2013 (Qiu et al., 2013). Further studies have reported that flavonoids, terpenes, sterols, volatile oils, and amino acids are the bioactive ingredients of N. fordii (Wei et al., 2016). However, to date, few studies have reported the diversity and antimicrobial activities of endophytic fungi associated with N. fordii plants. The aims of this present study were: (1) to provide the first evidence of the diversity, phylogeny, and taxonomic composition of culturable endophytic fungi isolated from N. fordii in the karst region; (2) to evaluate the potential antimicrobial activities of these endophytic fungi against Gram-negative bacteria, Gram-positive bacteria, and unicellular fungi using the agar diffusion method; and (3) to investigate the antimicrobial compounds of selected endophytic fungi exhibiting excellent antimicrobial capacities.

Figure 1. Habitat of Nervilia fordii. (A) Nervilia fordii often located in the limestone mountains of the karst area in Guangxi Province, China. (B) The corm is the living state of N. fordii in every cold season. (C) The corm (↑) of N. fordii always sprout and break through the soil when the temperature rises in every March or April. (D) A community of N. fordii were found under bushes of the karst. (E) The whole plant of N. fordii is composed of the leaves, roots (*), and corms (↑).
Materials and methods
Pant material collection
Nervilia fordii samples were collected from Yongfu county (altitude, 270 m; E109°66′, N24°94′) (Figure 1A) in Guangxi province, China. They were identified by Li-Ying Yu and voucher specimens were preserved in the herbarium of the Guangxi Botanical Garden of Medicinal Plants (voucher ID: SHNF20200618).
Fungal isolation and cultivation
The procedures used for the surface sterilization of the N. fordii samples were according to the methods described by Tan et al. (2018). Briefly, the roots, corms, and leaves were separated from the plants and thoroughly washed under running tap water. Subsequently, surface sterilization was performed sequentially by soaking the plant materials in 70% ethanol (v/v) for 30 s, followed by soaking in 2.5% sodium hypochlorite (v/v) for 4–5 min; the materials were then rinsed with sterile distilled water three times. All sterilized materials were finally dried with sterile filter paper and then divided into segments using a sterilized scalpel. The segments of tissue were placed in Petri dishes containing Potato Dextrose Agar (PDA) medium with 50 μg ml–1 oxytetracycline and 50 μg ml–1 streptomycin (SM). The Petri dishes were then sealed with parafilm and incubated at 25 ± 2°C in the dark for up to 4 weeks to allow the isolation of slow growing endophytic fungi (Pecundo et al., 2021). The colonies that emerged from segments of tissue were periodically examined, and transferred in a timely manner to fresh antibiotic-free PDA medium by using the hyphal method to obtain the purified colonies of endophytic fungi. Then, all purified isolates were categorized and maintained at the Scientific Laboratory Center of the Guangxi University of Chinese Medicine.
Molecular analysis of endophytic fungi
Fresh mycelia were used to extract DNA according to the instructions provided by E.Z.N.A.TM Fungal DNA Mini Kits (Omega Bio-tek, Norcross, GA, USA). The rDNA region, including the internal transcribed spacer 1, internal transcribed spacer 2, and 5.8s gene, was amplified by PCR using the following primer pair: ITS1 (5′–TCCGTAGGTGAACCTGCGG–3′) and ITS4 (5′–TCCTCCGCTTATTGATATGC–3′) (White et al., 1990). The PCR mixture (50 μl) included 25 μl of 2 × SanTaq PCR Mix (Sangon Biotech, Shanghai), 2 μl of each primer (5 μM), 10 μl of genomic DNA (50 ng⋅μl–1), and 11 μl of autoclaved double-distilled water. PCR amplification was performed in a thermal cycler (BioRad) as follows: initial denaturation at 94°C for 3 min; followed by 35 cycles of 94°C for 30 s, 55°C for 25 s, and 72°C for 30 s; and a final extension at 72°C for 7 min. All PCR products were visualized by electrophoresis on a 1.5% (wt/v) agarose gel in 1 × TBE buffer (40 mmol L–1 Tris; 1 mmol L–1 EDTA, pH 8.0). Then, the certified products were sent to Shanghai Shengon Company Ltd. (Shanghai, China) for sequencing. The sequence data from 85 representative culturable endophytic fungi obtained in this study were submitted to GenBank. The phylogenetic analysis was performed by the Neighbor-joining (NJ) method using Molecular evolutionary genetics analysis (MEGA) software (Tejesvi et al., 2011; Rajivgandhi et al., 2018a).
Sequence accessions
The sequence data from the 85 fungal isolates were deposited in GenBank (the accession numbers are provided in Supplementary Table 1).
Antimicrobial screening of fungal agar plug
The agar well diffusion method described by Gauchan et al. (2020) was used to evaluate the antimicrobial activity of the 85 fungal isolates against Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 35401), and Candida tropicalis (ATCC 66029). First, the pre-cultured pathogenic indicator microorganism at approximately 107 CFU ml–1 was inoculated into 20 ml of molten nutrient agar. The solidified culture medium was used to punch wells with a sterile cork borer (φ = 6 mm). A sterile inoculating needle was employed to remove the agar plugs. Subsequently, the fungal endophyte agar plugs (φ = 6 mm) with different concentrations of the fungal extract were placed into separate wells, respectively. The PDA agar plugs was used as the negative control. The plates were incubated at 37°C (S. aureus and E. coli) or 28°C (C. tropicalis) for 24 h and the diameter of the inhibition zone was measured using a vernier caliper. The experiments were performed in triplicate. Streptomycin and tetracycline (Sigma, USA) were employed as the positive controls.
Bacterial species S. aureus and E. coli, were pre-incubated at 37°C on LB medium [yeast extract, 0.5% (w/v); tryptone, 1% (w/v); NaCl, 0.5% (w/v); agar, 2%(w/v); and pH 7.2] periodically. The fungal pathogenic C. tropicalis was pre-incubated at 28°C on Sabouraud medium [peptone, 1% (w/v); glucose, 4% (w/v); agar, 2% (w/v); and pH 5.8] periodically. All fungal isolates were maintained on PDA (Difco) at 25°C for 2 weeks before the antimicrobial assay.
Determination of antimicrobial composition from Penicillium macrosclerotiorum 1151#
The antimicrobial composition of the fungal isolate Penicillium macrosclerotiorum 1151#, which exhibited the highest antimicrobial activity, was determined by 1H Nuclear Magnetic Resonance (NMR) and 13C NMR. The specific experimental methods used were as follows.
Fermentation and extraction
Penicillium macrosclerotiorum 1151# was pre-cultured on PDA at 25 ± 2°C in the dark for 7 days. Four mycelial agar plugs (φ = 6 mm) collected from the edge of the fungal colony were inoculated into a 500-ml Erlenmeyer flask containing 120 g of sterile rice medium. The flasks were incubated at 25 ± 2°C in the dark for 2 weeks. The fungal fermentation product in the rice medium was extracted three times with 10-fold (w/v) petroleum ether (PET), ethyl acetate (EA), or n-butanol (n-BuOH) by ultrasonication for 1 h, to yield crude extraction solutions, respectively. The three crude extraction solutions were combined and filtered using Whatman No. 1 filter paper. The filtrate was further concentrated under reduced pressure (8 × 103 Pa) to remove the organic solvent, and the concentrates were then volatilized in a water bath at 60°C, to obtain the dried crude extract.
In vitro antibacterial activity and minimum inhibitory concentration determination of ethyl acetate crude extract
The antibacterial activity of EA crude extract of P. macrosclerotiorum 1151# was evaluated in vitro against Gram-negative bacteria (E. coli) and Gram-positive bacteria (S. aureus) using the agar well diffusion method as described by Shehabeldine et al. (2022b). In this study, each pre-cultured pathogenic bacteria at approximately 107 CFU ml–1 was inoculated into 20 ml of sterilized Luria-Bertani (LB) plate. Wells of 6-mm diameter was made on the LB plates using a sterile cork borer. The well was loaded with 20 μL of EA crude extracts diluted in 10% (v/v) dimethyl sulfoxide (DMSO) at various concentrations (0.1, 0.5, 1.0, 2.5, 5, and 10 mg⋅ml–1). DMSO was used as negative control, and TET dissolved in 10% (v/v) DMSO was employed as positive controls. The plates were incubated at 37°C for 24 h, and the radius of the inhibition zone was measured with a vernier caliper. Minimal inhibitory concentration (MIC) was recorded as the lowest concentration of extract that inhibited the growth of E. coli or S. aureus. All experiments were performed in triplicate and repeated twice.
Separated and purification of most active compound
In order to analyze the most active compound, the EA crude extract was chromatographed on a D101 macroporous resin column using a gradient of PET:acetone. The most active fractions were assessed and further purified by semi-preparative HPLC (MeOH:H2O, 1:1, v/v), according to the antibacterial assays.
Structure determination of the compound by 1H and 13C-NMR
To confirm the structure of the separated compound, NMR spectra were recorded on a Bruker AM-400 spectrometer (Bruker Corporation, Fallanden, Switzerland), which was operated at 400 MHz for 1H NMR and 100 MHz for 13C NMR. The chemical shifts are given in δ (ppm) using TMS as the internal standard. HR-ESI-MS was measured on a Q-TOF Ultima GLOBAL GAA076 LC mass spectrometer. Semi-preparative HPLC was performed on a Shimadzu SPD-20A Liquid Chromatograph with an SPD-20A detector using a C18 column (5 μm, ϕ 250 mm × 20 mm, YMC-pack ODS-A, Agilent, 2 ml min–1).
Statistical analyses
The Shannon–Wiener and Simpson indices were employed to compare the diversity of endophytic fungal communities among the leaves, roots, and corms of N. fordii. The two diversity indices were evaluated using the methods reported by Koukol et al. (2012). All antimicrobial experiments were performed in triplicate and the data are presented as the mean ± standard deviation. Statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Phylogenetic analyses of culturable endophytic fungi
A total of 184 culturable endophytic fungi were isolated from the leaves, roots, and corm of N. fordii. Among them, 85 different morphotypes (Supplementary Table 1) were recognizable according to their fungal morphological characteristics and used for phylogenetic reconstruction. The phylogenetic analysis was performed using the MEGA program, and the resulting NJ phylogenetic tree is shown in Figure 2; the tree was constructed from an ITS rDNA dataset comprising all morphotype sequences obtained in this study and the GenBank sequences of available close relatives. The resulting phylogenetic tree showed that the endophytic fungi of N. fordii belonged to a richly diverse group including Ascomycetes (95.29%) and Basidiomycetes (4.71%). Among the Ascomycetes groups, three classes (Dothideomycetes, Eurotiomycetes, and Sordariomycetes) and 13 orders (Diaporthales, Hypocreales, Magnaporthales, Sordariales, Glomerellales, Xylariales, Chaetothyriales, Eurotiales, Pleosporales, Cladosporiales, Muyocopronales, Botryosphaeriales, and Sordariomycetidae incertae sedis) were identified. Other groups involving Cantharellales, Corticiales, and Polyporales were placed within Agaricomycetes of Basidiomycetes.
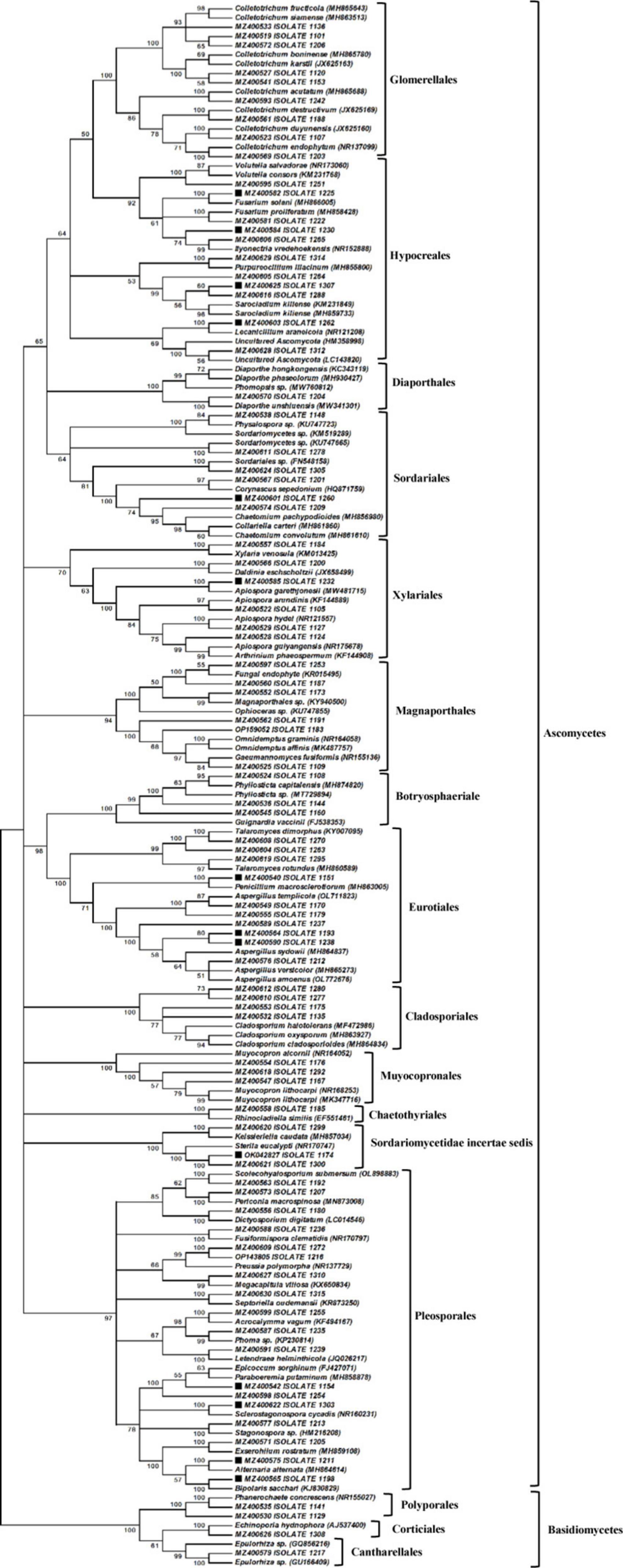
Figure 2. Neighbor-joining (NJ) analysis revealing the phylogenetic relationships of endophytic fungi associated with Nervilia fordii. The NJ phylogenetic tree without outside groups includes a total of 169 fungal sequences, in which 85 sequences from our study, 84 reference sequences of close relatives from the NCBI database. Bootstrap support values are indicated for major nodes having values ≥50%. The names of 16 different groups are shown in bold letter such as: Glomerellales, Hypocreales, Diaporthales, Sordariales, Magnaporthales, Xylariales, Botryosphaeriale, Eurotiales, Cladosporiales, Muyocopronales, Chaetothyriales, Sordariomycetidae incertae sedis, Pleosporales, Polyporales, Corticiales, and Cantharellales. The solid square (■) represents the isolates with antimicrobial activity.
Further analysis of the tree (Figure 2) showed that Pleosporales was the dominant fungal order of N. fordii. Among the Pleosporales, 18 isolates and 17 reference taxa formed a clade with 97% bootstrap support, and further formed six subclades. In the first subclade, three morphologically distinct isolates, i.e., 1192#, 1207#, and 1180#, clustered to Scolecohyalosporium submersum (OL898883), Periconia macrospinosa (MN873008), and Dictyosporium digitatum (LC014546) with 100% bootstrap support, respectively. The second subclade only had one isolate 1236#, which clustered with Fusiformispora clematidis (NR170797) with 100% bootstrap support; however, there were relatively low sequence similarities (86.64%) between the morphotype and F. clematidis. In another subclade, two isolates (1272# and 1216#) clustered with Preussia polymorpha (NR137729) with 99% bootstrap support. In the same subclade, isolate 1310# and the reference taxa Megacapitula villosa (KX650834) formed a group with 99% bootstrap support. In the fourth subclade, isolate 1315# and Septoriella oudemansii (KR873250) shared a subclade with 100% bootstrapping. In the sixth subclade, two isolates, i.e., 1255# and 1235#, clustered together with Acrocalymma vagum (KF494167) and Phoma sp. (KP230814), respectively, and formed a terminal clade with 98% bootstrapping. Isolate 1239# and the reference species Letendraea helminthicola (JQ026217) formed another terminal clade with 100% bootstrap support. Seven isolates clustered in the last subclade of the order Pleosporales, two isolates of which were closely related to Epicoccum sorghinum (FJ427071) and Paraboeremia putaminum (MH858878) with 99% sequence similarity, respectively, and formed a terminal clade with 100% bootstrap support. Another five isolates, i.e., 1303#, 1213#, 1205#, 1211#, and 1198#, clustered with Sclerostagonospora cycadis, Stagonospora sp., Exserohilum rostratum (MH859108), Alternaria alternata (MH864614), and Bipolaris sacchari (KJ830829) with 100% bootstrap values and relatively high nucleotide similarities (98.78–100%).
The clade representing the order Glomerellales had nine morphologically distinct isolates (Figure 2) and grouped with the genus Colletotrichum with 100% bootstrapping, four of which were closely related to C. acutatum (MH865688), C. destructivum (JX625169), C. duyunensis (JX625160), and C. endophytum (NR_137099) with 100% bootstrap support, respectively. In the same clade of Glomerellales, five isolates clustered with C. camelliae (MH864126), C. fructicola (MH865643), C. siamense (MH863513), C. boninense (MH865780), and C. karstii (JX625163) with 100% bootstrap support and with 99.02–99.64% nucleotide similarity. In another clade, 11 isolates formed the order Hypocreales, in which one isolate clustered to Volutella, two isolates to Fusarium, and two to Ilyonectria with high bootstrap support. In another subclade of Hypocreales, one isolate grouped with Purpureocillium lilacinum, three with Sarocladium kiliense, one with Lecanicillium araneicola, and one with two unidentified fungal species. Within the clade representing the order Diaporthales, isolate 1204# formed a group with Physalospora and three reference isolates of Diaporthe with 100% bootstrap support. The clade of the order Sordariales was composed of six isolates, one of which grouped with the genus of Physalospora, two of which showed similarities with 100% bootstrap support to two unidentified fungi of Sordariomycetes, and three to Corynascus sepedonium, Collariella carteri, and Chaetomium pachypodioides with 100% bootstrap support. In the clade of Xylariales belonging to Sordariomycetes, the six isolates identified in the current study showed high relatedness to four genera, i.e., Xylaria, Daldinia, Apiospora, and Arthrinium. Five isolates belonging to the order Magnaporthales formed a clade with 94% bootstrapping and had affinity with Omnidemptus, Gaeumannomyces, and two unidentified fungi.
In addition, 11 isolates belonged to the Dothideomycetes class and formed a clade composed of three subclades, i.e., Botryosphaeriale, Cladosporiales, and Muyocopronales. Within Botryosphaeriale, three isolates (1108#, 1144#, and 1160#) were identified as being closely related to four different species of the genus Phyllosticta and its teleomorph Guignardia. In Cladosporiales, four isolates clustered with three different species of the genus Cladosporium with 100% bootstrapping. In another subclade, three endophytic and two Muyocopron species formed an assembly of Muyocopronales with 100% bootstrap support.
Another 11 fungal isolates were placed in the Eurotiomycetes class within two orders, i.e., Eurotiales and Chaetothyriales, and formed four fungal assemblies with high bootstrap support. The first fungal assembly included three isolates (1270#, 1263#, and 1295#), that were closely related and formed a subclade with Talaromyces dimorphus (KY007095) and T. rotundus (MH860589). The 1151# isolate closely clustered with P. macrosclerotiorum (MH863005) and formed the second fungal assembly with 100% bootstrap support. Six isolates (1170#, 1179#, 1237#, 1193#, 1238#, and 1212#) were confirmed as being closely related to four different species of the genus Aspergillus. The clade representing Chaetothyriales was composed of a singleton isolate (1185#) grouped with Rhinocladiella similis (EF551461) with 100% bootstrap support. In addition, three isolates (1299#, 1174#, and 1300#) were well placed in a singleton clade with Keissleriella caudata (MH857034) and Sterila eucalypti (NR_170747) with 100% bootstrap support, albeit with low nucleotide similarity (85.52–89.39%).
Lastly, four isolates were classified as Basidiomycetes and formed a clade comprising three orders with 100% bootstrap support (Figure 2). In the order Polyporales subclade, two isolates grouped with Phanerochaete concrescens (NR_155027) with 100% bootstrap support, but only isolate (1141#) displayed high relatedness with P. concrescens with 98.19% nucleotide similarities. In the Corticiales order, isolate 1,308 was well placed in a singleton subclade with Echinoporia sp. (MH553213). In the Cantharellales order, isolate 1217# clustered with two unidentified species of the genus Epulorhiza and formed a subclade with 99% bootstrap support.
Diversity analysis of fungal endophytic species from Nervilia fordii
The results of the current study revealed that the fungal endophytic species isolated from N. fordii exhibited rich and diverse characteristics as showed in Table 1. The total fungal isolates and species diversity of the different tissues of N. fordii were comparatively researched using multiple analytical indexes, such as species richness (S), Camargo’s index (1/S), Simpson’s index (D), Simpson’s index of diversity (1−D), and the Shannon index of diversity (H′). First, the number of total fungal isolates collected from the different tissues of N. fordii was 103, 39, and 42 for leaves, roots, and corms, respectively. The species richness (S) of endophytic fungi from N. fordii was 85 species, of which 48 were collected from leaves, 30 from roots, and 22 from corms. The predominant fungal species collected from the leaves and corms of N. fordii was Apiospora hydei (Pi = 0.086 and 1/S = 0.012), followed by an undefined fungus of Magnaporthaceae (Pi = 0.054) from leaves; Fusarium sp. (Pi = 0.043) from leaves, roots, and corms; Aspergillus sydowii (Pi = 0.038) from corms; and C. karstii (Pi = 0.032) from leaves. Other isolates, i.e., E. sorghinum, Omnidemptus sp., Epulorhiza sp., A. arundinis, Arthrinium sp., C. fructicola, F. proliferatum, Muyocopron lithocarpi, and Phoma sp., were also common in the tissues of N. fordii. Our results showed that the highest endophytic population diversities were found in the leaves (1 − D = 0.953; H′ = 3.485), followed by the roots (1 − D = 0.953; H′ = 3.247), and the corms (1 − D = 0.926; H′ = 2.846). Moreover, the diversity index of the fungal community of N. fordii was confirmed by diversity values of H′ = 4.248 and 1 − D = 0.974.
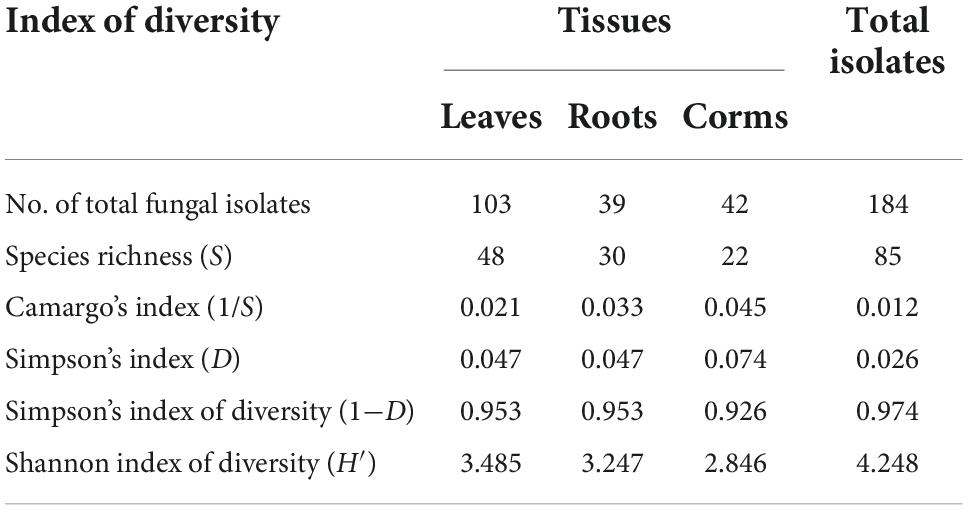
Table 1. The diversity of culturable endophytic fungi isolated from the health tissues of Nervilia fordii.
Antimicrobial activity of endophytic fungi
The other research aim of this study was to screen for the antimicrobial activity of all endophytic fungi from N. fordii using S. aureus, E. coli, and C. tropicalis as targets via the agar diffusion method. The results obtained are provided in Table 2. Eight fungal species (9.41%) displayed antibacterial activity against E. coli, whereas 11 (12.94%) had activity against S. aureus and two (2.35%) against C. tropicalis. Interestingly, eight isolates displayed not only antagonistic action toward S. aureus, but also antibacterial activity against E. coli. In particular, P. macrosclerotiorum 1151# showed efficient activity against E. coli, providing an inhibition of up to 1.32- and 1.53-fold (Figure 3A) compared with SM and TET, respectively. Moreover, this strain 1151# was also the most efficient in inhibiting S. aureus, up to 1.13- and 1.21-fold (Figure 3B) compared with SM and TET, respectively. Other fungal species, i.e., E. sorghinum 1154#, B. sacchari 1198#, and A. versicolor 1238#, were also effective in inhibiting the growth of Gram-negative (Figure 3C) and Gram-positive (Figure 3D) pathogens, although their antibacterial effects were weaker than those of the control. In addition, compared with SM and TET, Fusarium sp. 1225# and S. cycadis 1303# showed a wider antimicrobial spectrum to inhibit significantly the growth of both pathogenic bacteria and fungi.
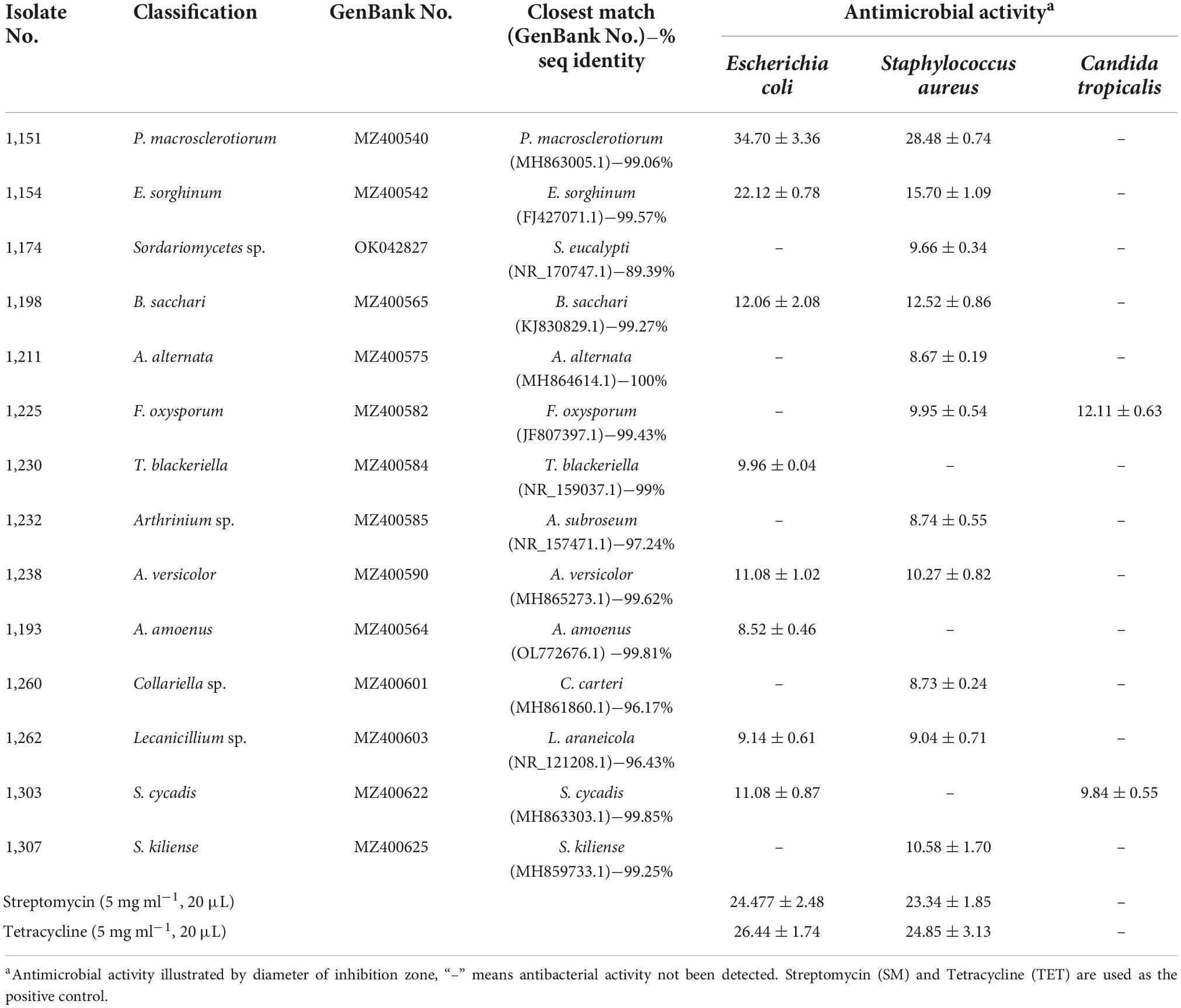
Table 2. Antimicrobial activity of 14 endophytic fungi isolated from Nervilia fordii against human pathogenic microorganisms by agar well diffusion method.
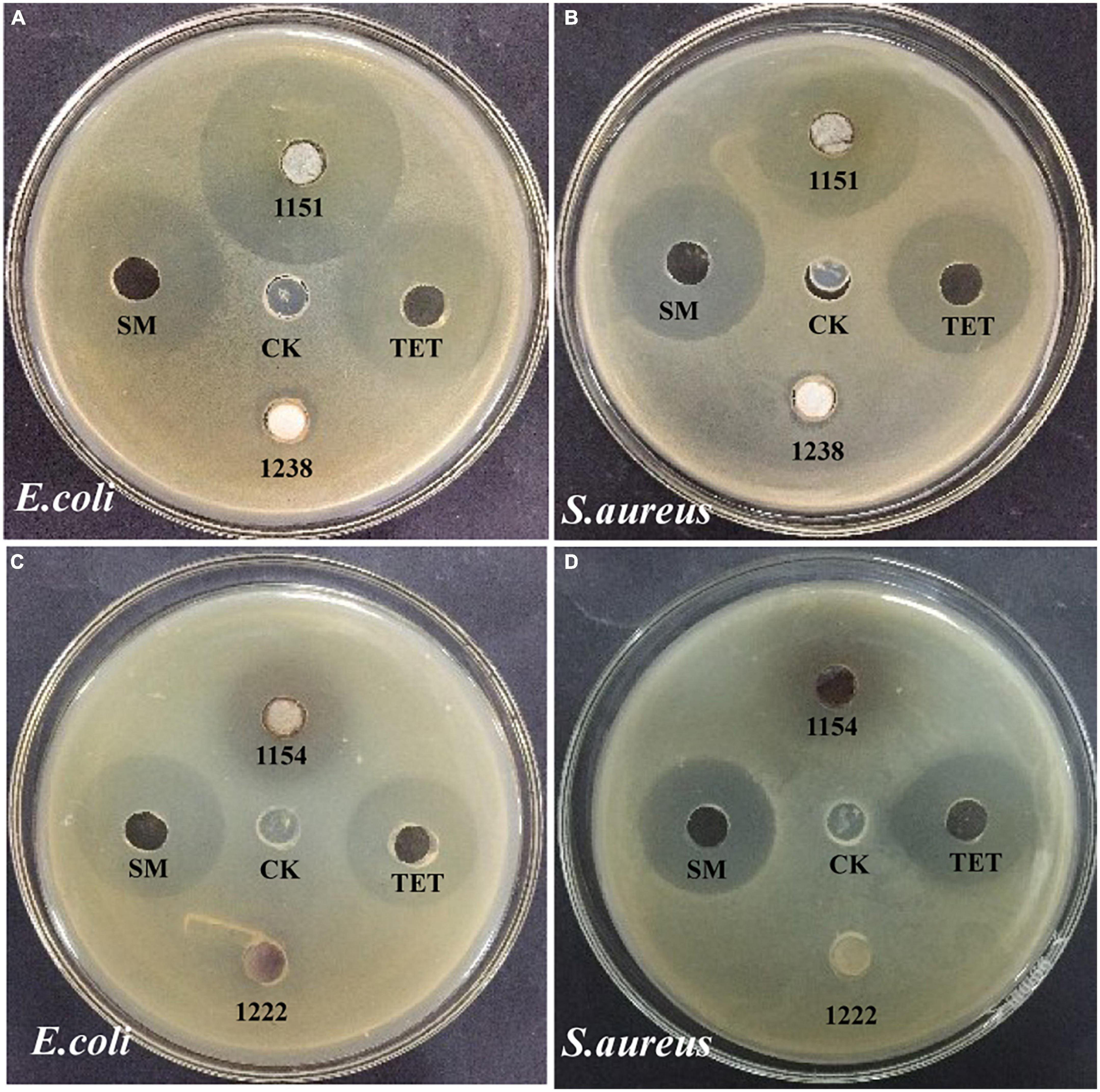
Figure 3. Antimicrobial activity of the fungal endophyte agar plugs of Penicillium macrosclerotiorum 1151# and Epicoccum sorghinum 1154# against Escherichia coli (A,C) and Staphylococcus aureus (B,D) by agar well diffusion method. Dimethyl sulfoxide (DMSO) was used as the blank control (CK); Tetracycline (TET), and Streptomycin (SM) were used as positive control.
These results also indicated that fungal species with antimicrobial activities were mainly collected from the roots of N. fordii, and were classified into six clades, i.e., Pleosporales, Hypocreales, Eurotiales, Xylariales, Sordariales, and Sordariomycetidae incertae sedis (Figure 2 and Supplementary Table 1).
Determination of minimum inhibitory concentration
During the antimicrobial assays of fungal agar plug, only P. macrosclerotiorum 1151# showed potent activity against Gram-negative and Gram-positive bacteria as having an inhibition zone of 34.70 ± 3.36 and 28.48 ± 0.74 mm (Table 2). This suggested that the P. macrosclerotiorum 1151# may be explored as a microbial factory of great potential antibacterial ingredients for industrial applicactions. Therefore, this endophytic fungi has been employed to determine the MIC values of crude extracts by using agar well diffusion method. Interestingly, only the EA crude extracts showed a strong inhibitory activity against tested Gram-negative and Gram-positive bacteria, whereas the PET crude extracts exhibited few growth inhibition against tested organisms as well as n-BuOH crude extracts. Moreover, the EA course extracts of P. macrosclerotiorum 1151# still had a clear zone of antibacterial activity against S. aureus and E. coli with MIC at 0.5 mg⋅ml–1. The diameters of inhibition zones are showed in Table 3 and illustrated in Figure 4. These results suggested that most antibacterial active fraction of P. macrosclerotiorum 1151# were extracted from the EA fraction, but not PET and n-BuOH fractions.
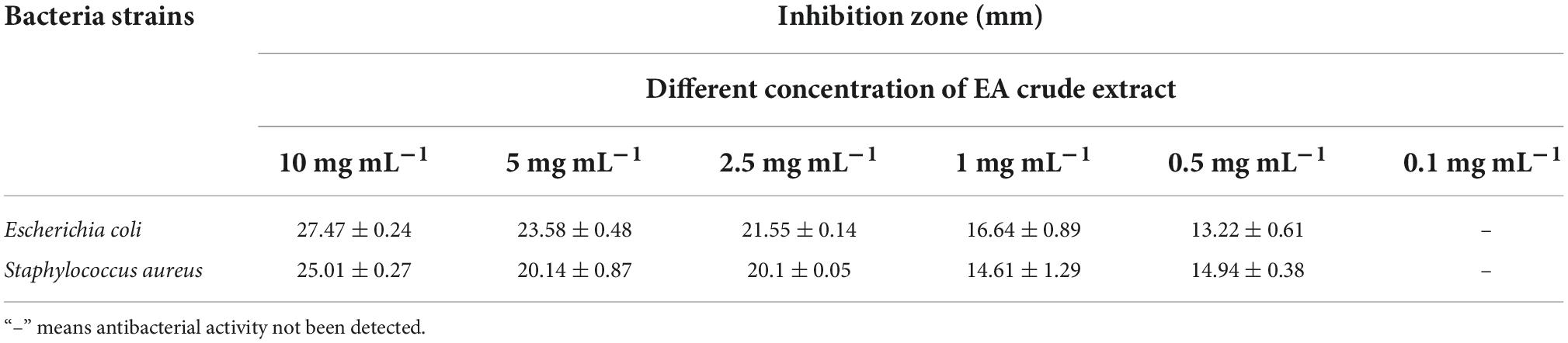
Table 3. Antimicrobial activity as indicated by growth-inhibition zone of different concentration of ethyl acetate (EA) crude extract of Penicillium macrosclerotiorum 1151# against gram-negative and gram-positive bacteria.
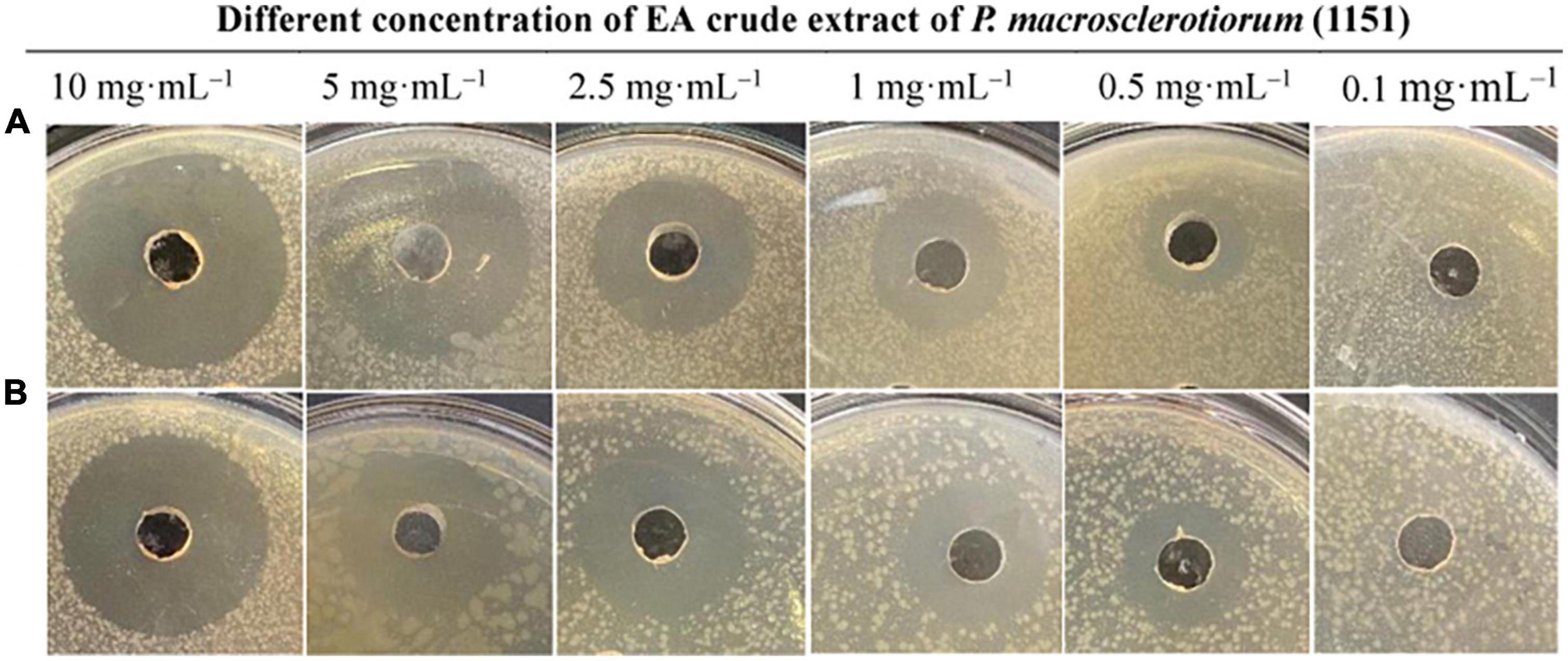
Figure 4. Antimicrobial activity of different concentration of the ethyl acetate (EA) crude extract of Penicillium macrosclerotiorum 1151# against Escherichia coli (A) and Staphylococcus aureus (B) by agar well diffusion method.
Characterization of the purified compound
In order to analyze the most antibacterial active components of P. macrosclerotiorum 1151#, 5.64 kg of the fungal rice medium was extracted with 10-fold (w/v) EA, and about 35.81 g of crude extract was obtained. The EA crude extract was chromatographed on a D101 macroporous resin column using a gradient of PET:acetone (30:1, 15:1, 3:1, 1:1, and 0:1), to yield 18 fractions (Fr1–Fr18). However, only the Fr13, Fr14, and Fr15 fractions displayed higher antibacterial activity against S. aureus and E. coli. Among them, the Fr15 fractions showed a strongest inhibitory activity against tested organisms, and consequently were separated into 12 subfractions (Fr15–1–Fr15–12) via silica gel CC, with elution using PET:EA (10:1–0:1). The results of the antimicrobial assays (Figure 5) suggested that the Fr15–6 subfraction was main active compound, which was recorded against E. coli and S. aureus as having an inhibition zone of 27.53 ± 1.65 and 23.33 ± 2.36 mm, up to 1.71- and 1.13-fold compared with tetracyclin, respectively. Finally, the Fr15–6 subfraction was further purified by semi-preparative HPLC (MeOH:H2O, 1:1, v/v) and obtain one compound. The chemical structure of this monomer component was confirmed as methyl chloroacetate by analyzing the ESI-MS (Figure 6) and NMR data (Supplementary Table 2 and Supplementary Figures 1–3). All data presented above were consistent with methyl chloroacetate reported in previous studies (Liu et al., 2015; Said et al., 2018).
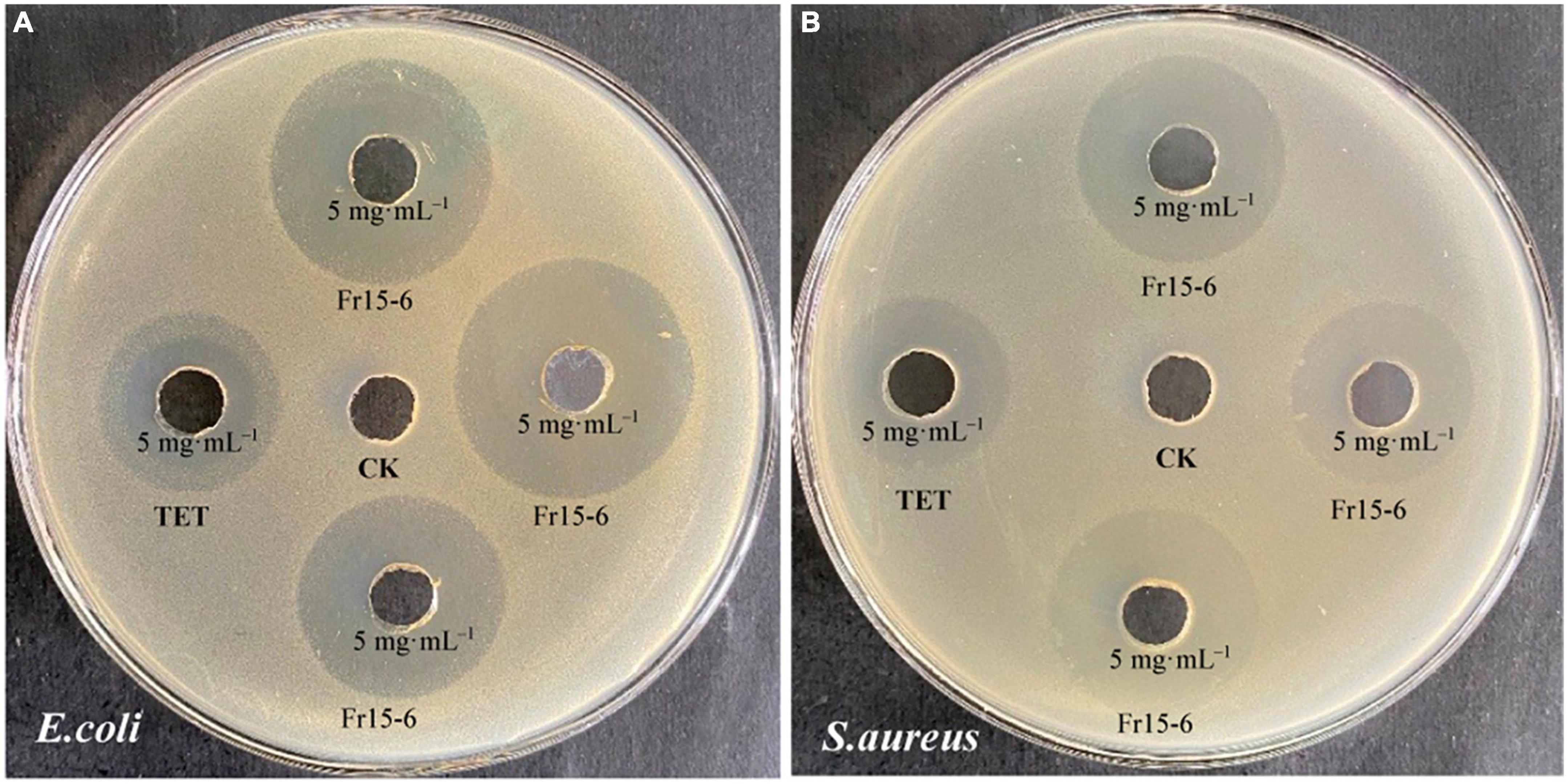
Figure 5. Antimicrobial activity of the monomer component (Fr15–6) isolated from the ethyl acetate (EA) crude extract of Penicillium macrosclerotiorum 1151# against Escherichia coli (A) and Staphylococcus aureus (B) by agar well diffusion method. Dimethyl sulfoxide (DMSO) was used as the blank control (CK), and the tetracycline (TET) as positive control. The concentration of all samples was 5 mg ml– 1. The sample of the monomer component (Fr15–6) was repeated for three wells in each Petri-dish plate.
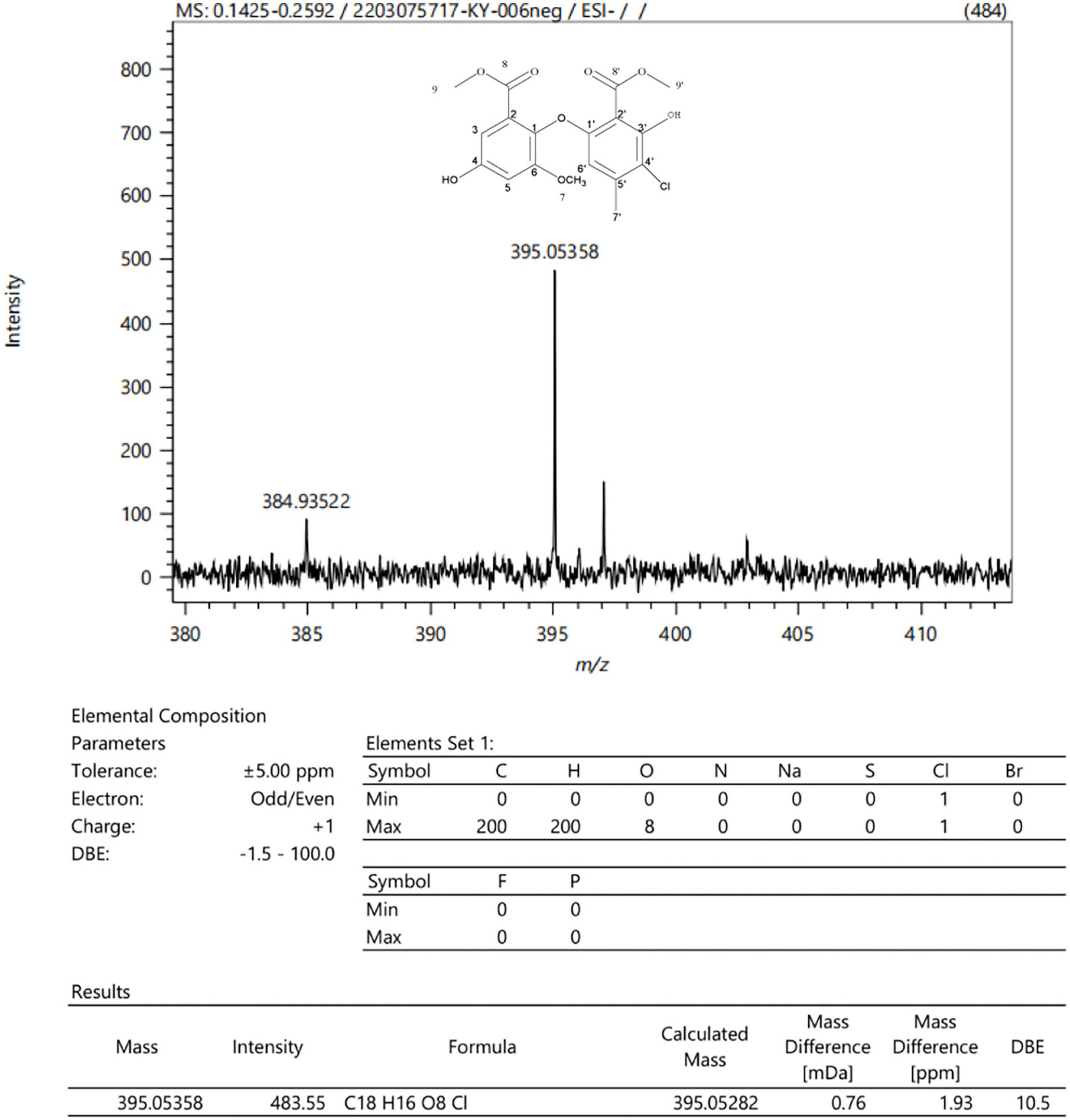
Figure 6. ESI-MS chromatogram and molecular formula of the monomer component isolated from the ethyl acetate (EA) crude extracts of Penicillium macrosclerotiorum 1151#.
Discussion
Antibiotic resistance has become a serious problem threating global public health (Malhadas et al., 2017; Wang et al., 2021), suggesting roles of new antimicrobial drugs in solving this increasingly serious problem in addition to the proper use of antibiotics. Unfortunately, it has become increasingly difficult to find new antibiotics from the conventional soil environment, resulting in nearly three decades without a new antibiotic (Böttcher et al., 2021). Endophytes are being used as an increased important resource for searching new natural antimicrobial compounds, due to their characteristics of wide distribution, species diversity, and the potential to yield novel metabolites (Rajivgandhi et al., 2018b). In this study, we report the rich diverse endophytic fungi have been isolated from the karst endemic plants N. fordii. Our in vitro antimicrobial assays using the well diffusion methods demonstrated that the fungal agar plugs of some strains can show potent activity against Gram-negative and Gram-positive bacteria. More importantly, for the first time, our study found that the EA crude extracts of P. macrosclerotiorum 1151# had stronger antibacterial effects against E. coli and S. aureus. Notably, a monomer component seperated from the EA fractions of this isolate 1151# was firstly identified as the methyl chloroacetate, which displaying a stronger inhibitory activity against all Gram-negative and Gram-positive bacteria, compared with tetracyclin. These findings provide evidence that various endophytic fungi of N. fordii could be exploited as potential sources of novel natural antimicrobial agents.
These endophytic fungi isolated from N. fordii were represented by mostly Ascomycetes and relatively small number of Basidiomycetes, in consistent with the results of previous studies on the endophytic fungi of plants (Macia-Vicente et al., 2008; Pecundo et al., 2021). In our study, we described the first time that Apiospora sp. was firstly reported as the dominant species of N. fordii, followed by Colletotrichum sp., Aspergillus sp., and Fusarium sp. Previously, it was reported that Colletotrichum (Huang et al., 2008), Aspergillus (Naik et al., 2021), and Fusarium (Tejesvi et al., 2011) are the dominant endophytic fungi of plants. In addition, widespread species, such as Chaetomium, Penicillium, Phoma, Xylaria, Phyllosticta, and Epicoccum, have been reported as being endophytic fungi of many plants and were mainly isolated from the leaves and roots (Arnold, 2007; Tejesvi et al., 2011); all were reported in N. fordii here for the first time. However, previous study reported a total of 23 strains belonging only a genus Colletotrichum isolated from N. fordii in the Daxing county of Guangxi Province (Song et al., 2019). Interestingly, most of endophytic fungal genera in the current study were not previously isolated from N. fordii, suggesting a unique fungal community with high abundance and rich diversity existed naturally in the roots, leaves, and corms of the karst endemic N. fordii plants, compared with the study reported by Song et al. (2019).
In the current study, 15 genera, i.e., Epulorhiza, Echinoporia, Thelonectria, Septoriella, Scolecohyalosporium, Sclerostagonospora, Sarocladium, Purpureocillium, Paraboeremia, Ilyonectria, Exserohilum, Daldinia, Corallomycetella, and Collariella, were only found in the roots of N. fordii, and were absent from its leaves and corms. Epulorhiza has been confirmed as an important mycorrhizal fungus isolated from the roots of many photosynthetic orchids (Kristiansen et al., 2004; McCormick et al., 2004; Tan et al., 2014); however, here, it was reported for the first time as the dominant fungal group of the roots of N. fordii. Moreover, 11 fungal genera (Arthrinium, Gaeumannomyces, Letendraea, Muyocopron, Omnidemptus, Penicillium, Physalospora, Rhinocladiella, Stagonospora, Xylaria, and Phanerochaete) and eight unidentified genera were only detected in the leaves of N. fordii, whereas six genera were detected exclusively in the corms (Table 1). In contrast, only five fugal communities, i.e., Apiospora, Epicoccum, Phoma, Phyllosticta, and Talaromyces) were found among the roots and leaves, and only two genera (Aspergillus and Chaetomium) were detected in all tissues. These results suggest that our reported endophytic fungal genera have obvious tissue specificity features to some extent, which similar to the previous studies (Xing and Guo, 2011; D’Souza and Hiremath, 2013; Cui et al., 2015; Ming et al., 2022).
Increasing evidence is showing that plants with antimicrobial activities may provide the most viable opportunity to screen out novel fungal endophytes with bioactive compounds (Strobel et al., 2004; Lv et al., 2010; Cui et al., 2015; Malhadas et al., 2017). For example, the endophytic A. alternata isolated from the leaves of the medicinal plant Ziziphus spina-christi displayed positive inhibition activity against several pathogenic bacteria and fungi (Elghaffar et al., 2022). Similarly, endophytic fungi isolated from many plants, i.e., Dysosma versipellis, Saussurea involucrate, and Rhododendron tomentosum (Lv et al., 2010; Tejesvi et al., 2011; Tan et al., 2018), with high antimicrobial efficiency also potentially represent new antibacterial compounds. In our study, 14 endophytic fungi with strong antimicrobial activities were identified in the healthy tissues of N. fordii plants and exhibited high antimicrobial capacities, as determined using the agar well diffusion method. These isolates showed an abundant diversity of species, including Penicillium, Epicoccum, Bipolaris, Alternaria, Fusarium, Arthrinium, Thelonectria, Lecanicillium, Sclerostagonospora, Sordariomycetes sp., and Corallomycetella (Table 2). For instance, the EA crude extracts of E. sorghinum 1154# exerted a strong inhibitory activity against E. coli and S. aureus. A previous study had reported that E. sorghinum L28 had a strong inhibitory effect against the growth of plant pathogenic fungi (Feng et al., 2022). In particular, the 1151# isolate obtained from the leaves of N. fordii had a high nucleotide similarity (99.06%) with P. macrosclerotiorum (CBS 116871; MH863005), and showed highest antimicrobial activities against E. coli and S. aureus with a MIC of 0.5 mg ml–1. In fact, previous studies had revealed that several fungal species of the genus Penicillium can produce various antimicrobial compounds (Nicoletti et al., 2014; Malhadas et al., 2017). The endophytes P. canescens and P. commune showed antimicrobial activities against to the human pathogens (Gao et al., 2011; Malhadas et al., 2017) and the phytopathogenic fungi (Bertinetti et al., 2009). Other species, such as P. cinnamopurpureum (Dinesh et al., 2022), P. chrysogenum (Orfali et al., 2022), and P. citrinum (Kumari et al., 2021), had also been reported as being prolific producers of antimicrobial compounds. However, no studies have reported the antibacterial activity of P. macrosclerotiorum, which was identified as a new species of the genus Penicillium in 2017 (Wang et al., 2007; Xu et al., 2021). Our study reported for the first time the strong antimicrobial potential of EA crude extracts of P. macrosclerotiorum against Gram-negative and Gram-positive pathogenic bacteria compared with two commercial antibiotics. Furthermore, our study also confirmed that an antimicrobial component, i.e., methyl chloroacetate, which had been previously isolated from two coastal saline soil fungi [A. iizukae (Liu et al., 2015) and Aspergillus sp. (Said et al., 2018)], was detected in P. macrosclerotiorum for the first time. However, due to insufficient sample, the antimicrobial compounds of other fractions in the current study needs to be clarified in the future, as well as the MIC values of methyl chloroacetate, etc.
Conclusion
We firstly concludes that an unique and diverse cultivable endophytic fungal assemblage inhabiting the roots, corms, and leaves of karst endemic N. fordii plants. We show that the fungal communities displayed obvious tissue specificity features and may be closely related to the different function of organizations as well as the external conditions. The endophytic fungus P. macrosclerotiorum 1151# isolated from the leaves of N. fordii exhibit the high ability to inhibit the growth of the tested Gram-positive and Gram-negative bacteria. In fact, the current study confirm, for the first time, one of the antibacterial active substances within the EA crude extracts of P. macrosclerotiorum 1151# was methyl chloroacetate through Semi-preparative HPLC and NMR analyses. Therefore, our studies provide a basis for the exploration of new natural antimicrobial agents from medicinal plants with antimicrobial function in the future, which may contribute to the solution of worldwide antimicrobial resistance in the long run.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
Y-QZ, X-MT, and RS-H designed the research. X-FY, X-YX, and Y-QZ performed the experiments. S-CY, YT, and JW analyzed the data. X-MT, L-YY, and PF collected the samples. X-MT, S-CY, and Y-QZ co-wrote the manuscript. All authors contributed to the manuscript and approved the submitted version.
Funding
This research work was financially supported by the National Natural Science Foundation of China (No. 31860128) and the Natural Science Foundation of Guangxi (No. 2019GXNSFDA245017).
Acknowledgments
We express our great thanks to Jinlong Cui (Institute of Applied Chemistry, Shanxi University) for native language polishing, and Hua Wei (College of Biological Resources and Environmental Sciences, Jishou University) for the identification of chemical composition.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1063897/full#supplementary-material
References
Andryukov, B., Mikhailov, V., and Besednova, N. (2019). The biotechnological potential of secondary metabolites from marine bacteria. J. Mar. Sci. Eng. 7:176. doi: 10.3390/jmse7060176
Arnold, A. E. (2007). Understanding the diversity of foliar endophytic fungi: Progress, challenges, and frontiers. Fungal Biol. Rev. 21, 51–66. doi: 10.1016/j.fbr.2007.05.003
Bertinetti, B. V., Peña, N. I., and Cabrera, G. M. (2009). An antifungal tetrapeptide from the culture of Penicillium canescens. Chem. Biodivers. 6, 1178–1184. doi: 10.1002/cbdv.200800336
Böttcher, L., Gersbach, H., and Wernli, D. (2021). Restoring the antibiotic R&D market to combat the resistance crisis. Sci. Public Policy 49, 127–131. doi: 10.1093/scipol/scab067
Cui, J. L., Guo, T. T., Chao, J. B., Wang, M. L., Wang, J. H., and Iriti, M. (2016). Potential of the endophytic fungus Phialocephala fortinii Rac56 found in Rhodiola plants to produce salidroside and p-tyrosol. Molecules 21:502. doi: 10.3390/molecules21040502
Cui, J. L., Guo, T. T., Ren, Z. X., Zhang, N. S., and Wang, M. L. (2015). Diversity and antioxidant activity of culturable endophytic fungi from alpine plants of Rhodiola crenulata, R. angusta, and R. sachalinensis. PLoS One 10:e0118204. doi: 10.1371/journal.pone.0118204
Dinesh, B., Monisha, N., Shalini, H. R., Prathap, G. K., Poyya, J., Shantaram, M., et al. (2022). Antibacterial activity of silver nanoparticles synthesized using endophytic fungus—; Penicillium cinnamopurpureum. Spectrosc. Lett. 55, 20–34. doi: 10.1080/00387010.2021.2010764
D’Souza, M. A., and Hiremath, K. G. (2013). Composition and tissue specificity of endophytic fungi associated with Nothapodytes nimmoniana (J. Graham) Mabberly from the Western Ghats of India. Med. Plants 5, 27–33. doi: 10.5958/j.0975-6892.5.1.006
Elghaffar, R. Y. A., Amin, B. H., Hashem, A. H., and Sehim, A. E. (2022). Promising endophytic Alternaria alternata from leaves of Ziziphus spina-christi: Phytochemical analyses, antimicrobial and antioxidant activities. Appl. Biochem. Biotechnol. 194, 3984–4001. doi: 10.1007/s12010-022-03959-9
Feldman, C., and Anderson, R. (2021). The role of co-infections and secondary infections in patients with COVID-19. Pneumonia 13:5. doi: 10.1186/s41479-021-00083-w
Feng, N. N., Li, Q. Q., Mu, Q., Lin, S. Y., Li, H. J., Li, C. Y., et al. (2022). Metabolites and antifungal activities of an endophytic fungus Epicoccum sorghinum from mangrove. J. S. China Agric. Univ. 43, 77–81.
Gao, S. S., Li, X. M., Zhang, Y., Li, C. S., Cui, C. M., and Wang, B. G. (2011). Comazaphilones A-F, azaphilone derivatives from the marine sediment-derived fungus Penicillium commune QSD-17. J. Nat. Prod. 74, 256–261. doi: 10.1021/np100788h
Gauchan, D. P., Kandel, P., Tuladhar, A., Acharya, A., Kadel, U., Baral, A., et al. (2020). Evaluation of antimicrobial, antioxidant and cytotoxic properties of bioactive compounds produced from endophytic fungi of Himalayan yew (Taxus wallichiana) in Nepal. F1000 Fac. Rev. 9:379. doi: 10.12688/f1000research.23250.1
Guo, S. X. (2018). The recent progress and prospects of research on endophytic fungi in medicinal plants. Mycosystema 37, 1–13. doi: 10.13346/j.mycosystema.170252
Hashem, A. H., Shehabeldine, A. M., Ali, O. M., and Salem, S. S. (2022). Synthesis of chitosan-based gold nanoparticles: Antimicrobial and wound-healing activities. Polymers 14:2293. doi: 10.3390/polym14112293
Huang, W. Y., Cai, Y. Z., Hyde, K. D., Corke, H., and Sun, M. (2008). Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants. Fungal Divers. 33, 61–75. doi: 10.1002/yea.1639
Koukol, O., Kolařík, M., Kolářová, Z., and Baldrian, P. (2012). Diversity of foliar endophytes in wind-fallen Picea abies trees. Fungal Divers. 54, 69–77. doi: 10.1007/s13225-011-0112-2
Kristiansen, K. A., Freudenstein, J. V., Rasmussen, F. N., and Rasmussen, H. N. (2004). Molecular identification of mycorrhizal fungi in Neuwiedia veratrifolia (Orchidaceae). Mol. Phylogenet. Evol. 33, 251–258. doi: 10.1016/j.ympev.2004.05.015
Kumari, P., Singh, A., Singh, D. K., Sharma, V. K., Kumar, J., Gupta, V. K., et al. (2021). Isolation and purification of bioactive metabolites from an endophytic fungus Penicillium citrinum of Azadirachta indica. S. Afr. J. Bot. 139, 449–457. doi: 10.1016/J.SAJB.2021.02.020
Li, D. M., Pan, H. M., Huang, P. L., Yang, L., and Liang, Y. B. (2017). Inhibition effect of Nerviliae fordii (Hance) Schltr on five kinds of fungi in vitro. J. Guangxi Med. Univ. 34, 1550–1552.
Liu, D., Yan, L., Ma, L., Huang, Y. L., Pan, X. H., Liu, W. Z., et al. (2015). Diphenyl derivatives from coastal saline soil fungus Aspergillus iizukae. Arch. Pharm. Res. 38, 1038–1043. doi: 10.1007/s12272-014-0371-z
Lv, Y. L., Zhang, F. S., Chen, J., Cui, J. L., Xing, Y. M., Li, X. D., et al. (2010). Diversity and antimicrobial activity of endophytic fungi associated with the alpine plant Saussurea involucrata. Biol. Pharm. Bull. 33, 1300–1306. doi: 10.1248/bpb.33.1300
Macia-Vicente, J. G., Jansson, H.-B., Abdullah, S. K., Descals, E., Salinas, J., and Lopez-Llorca, L. V. (2008). Fungal root endophytes from natural vegetation in Mediterranean environments with special reference to Fusarium spp. FEMS Microbiol. Ecol. 64, 90–105. doi: 10.1111/j.1574-6941.2007.00443.x
Mahendra, R., Beata, Z., Aniket, G., and Pramod, I. (2022). Promising antimicrobials from Phoma spp.: Progress and prospects. AMB Express 12:60. doi: 10.1186/S13568-022-01404-Y
Malhadas, C., Malheiro, R., Pereira, J. A., de Pinho, P. G., and Baptista, P. (2017). Antimicrobial activity of endophytic fungi from olive tree leaves. World J. Microbiol. Biotechnol. 33:46. doi: 10.1007/s11274-017-2216-7
McCormick, M. K., Whigham, D. F., and O’Neill, J. (2004). Mycorrhizal diversity in photosynthetic terrestrial orchids. New Phytol. 163, 425–438. doi: 10.1111/J.1469-8137.2004.01114.X
Ming, Q., Huang, X., Guo, L., Liu, D., Qin, L. Y., He, Y. M., et al. (2022). Diversity of endophytic fungi in Coptis chinensis Franch. and their activity against methicillin-resistant Staphylococcus aureus. Folia Microbiol. doi: 10.1007/s12223-022-00994-1 [Epub ahead of print].
Naik, B., Goyal, S. K., Tripathi, A. D., and Kumar, V. (2021). Exploring the diversity of endophytic fungi and screening for their pullulanase-producing capabilities. J. Genet. Eng. Biotechnol. 19:110. doi: 10.1186/s43141-021-00208-0
Nicoletti, R., Fiorentino, A., and Scognamiglio, M. (2014). Endophytism of Penicillium species in woody plants. Open Mycol. J. 8, 1–26. doi: 10.2174/1874437001408010001
Orfali, R., Perveen, S., AlAjmI, M. F., Ghaffar, S., Rehman, M. T., AlanzI, A. R., et al. (2022). Antimicrobial activity of dihydroisocoumarin isolated from Wadi Lajab sediment-derived fungus Penicillium chrysogenum: In vitro and in silico study. Molecules 27:3630. doi: 10.3390/MOLECULES27113630
Pecundo, M. H., dela Cruz, T. E. E., Chen, T., Notarte, K. I., Ren, H., and Li, N. (2021). Diversity, phylogeny and antagonistic activity of fungal endophytes associated with endemic species of Cycas (Cycadales) in China. J. Fungi 7:572. doi: 10.3390/jof7070572
Petrini, O., Sieber, T. N., Toti, L., and Viret, O. (1992). Ecology, metabolite production, and substrate utilization in endophytic fungi. Nat. Toxins 1, 185–196. doi: 10.1002/nt.2620010306
Qiu, L., Jiao, Y., Xie, J. Z., Huang, G. K., Qiu, S. L., Miao, J. H., et al. (2013). Five new flavonoid glycosides from Nervilia fordii. J. Asian Nat. Prod. Res. 15, 589–599. doi: 10.1080/10286020.2013.790377
Rajivgandhi, G., Muneeswaran, T., Maruthupandy, M., Saravanan, K., Ravikumar, V., and Manoharan, N. (2018a). Antibacterial and anticancer potential of marine endophytic actinomycetes Streptomyces coeruleorubidus GRG 4 (KY457708) compound against colistin resistant uropathogen. Microb. Pathog. 125, 325–335. doi: 10.1016/j.micpath.2018.09.025
Rajivgandhi, G., Ramachandran, G., Maruthupandy, M., Senthil, R., Vaseeharan, B., and Manoharan, N. (2018b). Molecular characterization and antibacterial investigation of marine endophytic actinomycetes Nocardiopsis sp. GRG 2 (KT 235641) compound against isolated ESBL producing bacteria. Microb. Pathog. 126, 138–148. doi: 10.1016/j.micpath.2018.10.014
Rajivgandhi, G., Vijayan, R., Kannan, M., Santhanakrishnan, M., and Manoharan, N. (2016). Molecular characterization and antibacterial effect of endophytic actinomycetes Nocardiopsis sp. GRG1 (KT235640) from brown algae against MDR strains of uropathogens. Bioact. Mater. 1, 140–150. doi: 10.1016/j.bioactmat.2016.11.002
Rajivgandhi, G. N., Ramachandran, G., Li, J. L., Yin, L. Z., Manoharan, N., Kannan, M. R., et al. (2020). Molecular identification and structural detection of anti-cancer compound from marine Streptomyces akiyoshiensis GRG 6 (KY457710) against MCF-7 breast cancer cells. J. King Saud Univ. Sci. 32, 3463–3469. doi: 10.1016/j.jksus.2020.10.008
Said, G., Mou, X. F., Fang, Y. W., Liang, T. M., Wei, M. Y., Chen, G. Y., et al. (2018). Secondary metabolites isolated from the soft coral-derived fungus Aspergillus sp. from the South China Sea. Chem. Nat. Compd. 54, 547–549. doi: 10.1007/s10600-018-2402-3
Salem, S. S., Ali, O. M., Reyad, A. M., Abd-Elsalam, K. A., and Hashem, A. H. (2022a). Pseudomonas indica-mediated silver nanoparticles: Antifungal and antioxidant biogenic tool for suppressing mucormycosis fungi. J. Fungi 8:126. doi: 10.3390/JOF8020126
Salem, S. S., Hashem, A. H., Sallam, A.-A. M., Doghish, A. S., Al-Askar, A. A., Arishi, A. A., et al. (2022b). Synthesis of silver nanocomposite based on carboxymethyl cellulose: Antibacterial, antifungal and anticancer activities. Polymers 14:3352. doi: 10.3390/polym14163352
Seukep, A. J., Kuete, V., Nahar, L., Sarker, S. D., and Guo, M. (2020). Plant-derived secondary metabolites as the main source of efflux pump inhibitors and methods for identification. J. Pharm. Anal. 10, 277–290. doi: 10.1016/j.jpha.2019.11.002
Shehabeldine, A. M., Amin, B. H., Hagras, F. A., Ramadan, A. A., Kamel, M. R., Ahmed, M. A., et al. (2022a). Potential antimicrobial and antibiofilm properties of copper oxide nanoparticles: Time-kill kinetic essay and ultrastructure of pathogenic bacterial cells. Biotechnol. Appl. Biochem. 1–19. doi: 10.1007/s12010-022-04120-2 [Epub ahead of print].
Shehabeldine, A. M., Salem, S. S., Ali, O. M., AbdElsalam, K. A., Elkady, F. M., and Hashem, A. H. (2022b). Multifunctional silver nanoparticles based on chitosan: Antibacterial, antibiofilm, antifungal, antioxidant, and wound-healing activities. J. Fungi 8:612. doi: 10.3390/JOF8060612
Song, L. S., Jiang, N., Lan, Z. Z., Guo, X. Y., and Zhang, Z. J. (2019). Identification and antibacterial activity of the endophytic fungus of the endangered medicinal plant, Nervilia fordii. Jiangsu Agric. Sci. 47, 175–178. doi: 10.15889/j.issn.1002-1302.2019.21.041
Stierle, A., Strobel, G., and Stierle, D. (1993). Taxol and taxane production by taxomyces-andreanae, an endophytic fungus of pacific yew. Science 260, 214–216. doi: 10.1126/science.8097061
Strobel, G., Daisy, B., Castillo, U., and Harper, J. (2004). Natural products from endophytic microorganisms. J. Nat. Prod. 67, 257–268. doi: 10.1021/np030397v
Tan, X. M., Wang, C. L., Chen, X. M., Zhou, Y. Q., Wang, Y. Q., Luo, A. X., et al. (2014). In vitro seed germination and seedling growth of an endangered epiphytic orchid, Dendrobium officinale, endemic to China using mycorrhizal fungi (Tulasnella sp.). Sci. Hortic. 165, 62–68. doi: 10.1016/j.scienta.2013.10.031
Tan, X. M., Zhou, Y. Q., Zhou, X. L., Xia, X. H., Wei, Y., He, L. L., et al. (2018). Diversity and bioactive potential of culturable fungal endophytes of Dysosma versipellis; a rare medicinal plant endemic to China. Sci. Rep. 8:e5929. doi: 10.1038/s41598-018-24313-2
Tejesvi, M. V., Kajula, M., Mattila, S., and Pirttilä, A. M. (2011). Bioactivity and genetic diversity of endophytic fungi in Rhododendron tomentosum Harmaja. Fungal Divers. 47, 97–107. doi: 10.1007/s13225-010-0087-4
Terfehr, D., Dahlmann, T. A., and Kück, U. (2017). Transcriptome analysis of the two unrelated fungal β-lactam producers Acremonium chrysogenum and Penicillium chrysogenum: Velvet-regulated genes are major targets during conventional strain improvement programs. BMC Genomics 18:272. doi: 10.1186/s12864-017-3663-0
Tian, L. W., Pei, Y., Zhang, Y. J., Wang, Y. F., and Yang, C. R. (2009). 7-O-methylkaempferol and-quercetin glycosides from the whole plant of Nervilia fordii. J. Nat. Prod. 72, 1057–1060. doi: 10.1021/np800760p
Wang, L., Zhang, X. M., and Zhuang, W. Y. (2007). Penicillium macrosclerotiorum, a new species producing large sclerotia discovered in south China. Mycol. Res. 111(Pt 10), 1242–1248. doi: 10.1016/j.mycres.2007.06.017
Wang, T., Yang, J., Lin, G., Li, M., Zhu, R., Zhang, Y., et al. (2021). Effects of dietary mannan oligosaccharides on non-specific immunity, intestinal health, and antibiotic resistance genes in pacific white shrimp Litopenaeus vannamei. Front. Immunol. 12:772570. doi: 10.3389/fimmu.2021.772570
Wei, Q., Xie, J. Z., Qiu, L., Jiao, Y., Zou, L. H., and Zhu, Q. L. (2016). Two new flavonoid glycosides from Nervilia fordii. Yao Xue Xue Bao 51, 961–964. doi: 10.16438/j.0513-4870.2015-0966
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR protocols, a guide to methods and applications, eds M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (New York, NY: Academic Press), 315–322.
Xing, X., and Guo, S. (2011). Fungal endophyte communities in four Rhizophoraceae mangrove species on the south coast of China. Ecol. Res. 26, 403–409. doi: 10.1007/s11284-010-0795-y
Xu, H. T., Li, H., Zhao, Y. Z., Zhou, J. Y., Yang, L. F., and Ma, S. M. (2017). Antioxidant and antibacterial activities of total flavonoids from Nervilia fordii. North. Hortic. 16, 161–165. doi: 10.11937/bfyy.20164715
Xu, Z. L., Cao, S. M., Qin, Y. Y., Mo, T. X., Li, B. C., Qin, X. Y., et al. (2021). Chemical constituents of the endophytic fungus Penicillium macrosclerotiorum from Sophora tonkinensis. Chem. Nat. Compd. 57, 542–544. doi: 10.1007/s10600-021-03409-8
Yao, Y. F., Yuan, Y., Lu, Z. H., Ma, Y. X., Xie, Y. Y., Wang, M. Q., et al. (2021). Effects of Nervilia fordii extract on pulmonary fibrosis through TGF-β/Smad signaling pathway. Front. Pharmacol. 12:659627.
Yin, S. M., Ding, M. Z., Fan, L., Yu, X. H., Liang, Z. Y., Wu, L., et al. (2021). Inhibition of inflammation and regulation of AQPs/ENaCs/Na+-K+-ATPase mediated alveolar fluid transport by total flavonoids extracted from Nervilia fordii in lipopolysaccharide-induced acute lung injury. Front. Pharmacol. 12:603863. doi: 10.3389/FPHAR.2021.603863
Keywords: endophytic fungi, antimicrobial activity, culturable fungal diversity, N. fordii, tissue specificity, P. macrosclerotiorum, methyl chloroacetate
Citation: Zhou Y-Q, Yao S-C, Wang J, Xie X-Y, Tan X-M, Huang R-S, Yang X-F, Tan Y, Yu L-Y and Fu P (2022) Cultivable endophytic fungal community associated with the karst endemic plant Nervilia fordii and their antimicrobial activity. Front. Microbiol. 13:1063897. doi: 10.3389/fmicb.2022.1063897
Received: 07 October 2022; Accepted: 07 November 2022;
Published: 24 November 2022.
Edited by:
Seok Hoon Jeong, Yonsei University, South KoreaReviewed by:
Gamal Mohamed El-Said El-Sherbiny, Al-Azhar University, EgyptAmr Shehabeldine, Al-Azhar University, Egypt
Copyright © 2022 Zhou, Yao, Wang, Xie, Tan, Huang, Yang, Tan, Yu and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Ming Tan, dGFueG1AZ3h0Y211LmVkdS5jbg==
†These authors have contributed equally to this work
 Ya-Qin Zhou
Ya-Qin Zhou Shao-Chang Yao1†
Shao-Chang Yao1† Jie Wang
Jie Wang