
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 09 November 2022
Sec. Evolutionary and Genomic Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1049007
This article is part of the Research Topic Insights on Fungal Diversity of Ascomycetes and Basidiomycetes: Taxonomy and Interaction with Their Host View all 24 articles
The genus Hydnellum is a kind of ectomycorrhizal fungi that can play a role in the material cycle by connecting the plant roots to the soil, and some species of Hydnellum are medicinal fungi with vital research value. The species diversity of Hydnellum is unclear in China. In this study, five new species of Hydnellum are described from China based on morphological characters and phylogenetic analyses inferred from two datasets of ITS + LSU and ITS + LSU + SSU + RPB2 sequences. H. chocolatum is characterized by its chocolate basidiomata with the fibrillose, spongy to tomentose pileal surface, and subglobose to globose basidiospores measuring (4.5–)5–6 × 4–5(–5.8) μm. H. concentricum is characterized by its zonate pileal surface, thin context, short stipe, presence of both simple septa and clamp connections in generative hyphae of spines, and subglobose to ellipsoidal basidiospores measuring (3.5–)4–5(–5.2) × (3.2–)3.5–5 μm. H. crassipileatum is characterized by its thick pileus with the reddish brown to grayish brown pileal surface, and subglobose to ellipsoidal basidiospores measuring 4–6(–6.5) × 4–5.5 μm. H. melanocarpum is characterized by its vinaceous brown to black pileus with spongy pileal surface, presence of both simple septa and clamp connections in generative hyphae of spines, and subglobose basidiospores measuring 4.5–5.5(–6) × (3.5–)3.8–5.1 μm. H. radiatum is characterized by its radially aligned stripes on pileal surface, grayish brown context, short stipe, and subglobose to ellipsoidal basidiospores measuring (3.5–)4–5 × 3–4.5(–5) μm. Full descriptions, illustrations, and phylogenetic trees to show the placement of the new species are provided.
Stipitate hydnoid fungi of the family Bankeraceae are ectomycorrhizal symbionts of trees in a broad spectrum of forests (Holec and Kučera, 2018), which can provide nutrients and water to roots by exchanging photosynthates from trees and improve the absorption capacity of trees to soil nutrients such as phosphorous (Erland and Taylor, 1999; Parfitt et al., 2007). In some forests, fewer ectomycorrhizal fungi have produced basidiomata (Arnolds, 1991, 2010) and become the emphasis of conservation in Europe (Parfitt et al., 2007). And some species are medicinal fungi, such as H. concrescens (Pers.) Banker which has an inhibitory action on syncytium formation, trafficking of glycoprotein and hemagglutinin-neuraminidase (HN) to the cell surface (Lee et al., 2012).
Hydnellum P. Karst., typed by H. suaveolens (Scop.) P. Karst., is a member of stipitate hydnoids. The genus Hydnellum together with Phellodon P. Karst. and Sarcodon Quél. ex P. Karst was affiliated to Bankeraceae Donk of Thelephorales Corner ex Oberw. The genus Hydnellum was established by Karsten (1879). At first, many species of the Bankeraceae including Hydnellum were originally classified into the Hydnaceae because of their dentate hymenium (Banker, 1906). Donk established the family Bankeraceae, which consisted of only two genera Bankera and Phellodon (Donk, 1961). Jülich (1981) revised the classification system of Basidiomycetes and classified Hydnellum into the Bankeraceae. The genus Hydnellum is characterized by annual basidiomata with a zonate or an azonate pileal surface, spinous and white to orange, gray blue, light brown, or dark brown spines, and centrically or eccentrically stipitate; a monomitic hyphal system with simple septa or clamped generative hyphae, and subglobose to globose and tuberculate basidiospores (Baird and Khan, 1986; Baird et al., 2013). Hydnellum is often confused with Phellodon and Sarcodon because of the similar basidiomata with a hymenium made up of spines (Baird et al., 2013). While the basidiospores in the Phellodon species are hyaline, the basidiospores in Hydnellum and Sarcodon are yellow to brown-tinted (Maas Geesteranus, 1975). Besides, Sarcodon differs from Hydnellum mainly by its brittle fleshy substance (Banker, 1906) and larger basidiospores (7.4-9 μm; Larsson et al., 2019).
Morphological features, including macroscopic morphological and microscopic morphological characteristics, were commonly used to identify Hydnellum species in the past (Banker, 1906; Maas Geesteranus, 1962, 1971; Harrison, 1964; Hrouda, 1999). Banker (1906) conducted a study of hydnaceous fungi of the Czech Republic and Slovakia, and 11 species of Hydnellum were newly described. Harrison (1964) conducted a systematic study of the stipitate hydnoids of the Bankeraceae from North America and described 10 species of Hydnellum. However, traditional morphology-based generic restrictions are ambiguous (Larsson et al., 2019). Mycologists have used morphological characters and phylogenetic analyses to study the taxonomy of Hydnellum in recent years (Ainsworth et al., 2010; Baird et al., 2013; Larsson et al., 2019; Mu et al., 2021). Baird et al. (2013) reevaluated the species of stipitate hydnoids from the southern United States, and 41 distinct taxa were determined including 19 species of Hydnellum. Larsson et al. (2019) reassessed the generic limits for Hydnellum and Sarcodon, and transferred 12 species from Sarcodon to Hydnellum based on ITS and nLSU sequences, which make the division of the genera clearer. Currently, about 70 species have been described and transferred to the genus according to the records in Index Fungorum (Accessed 7 May 2022)1. In recent years, the genus has been studied in China. Mu et al. (2021) described 11 new species from China based on morphological characters and multi-gene phylogenetic analysis.
Species in Bankeraceae are associated with coniferous trees in forest ecosystems and are widely distributed in the northern hemisphere. Stipitate hydnoids were often found in forests on mesic to dry, sandy to loamy soils with, at the most, a thin humus and litter layer (Arnolds, 2010). According to a survey conducted in the Netherlands about 22 species of hydnoid fungi, 12 are associated with deciduous trees older than 40 years, mainly Quercus robur, Quercus rubra, and Fagus sylvatica, and 10 are associated with coniferous trees, almost exclusively with Scots pine (Pinus sylvestris) (Arnolds, 2003). However, herb-rich spruce (Picea abies) forests on more or less calcareous soils rich in minerals constitute a third important habitat for hydnoid fungi (Arnolds, 2010). These three types of hosts corresponded well in our investigation (Table 2).
Macrofungi have important ecological and economical values. The species diversity, taxonomy, and phylogeny of macrofungi have been extensively investigated in recent years, and many new species have been discovered (Han et al., 2016; Cui et al., 2019; Mu et al., 2019, 2021; Shen et al., 2019; Sun et al., 2020; Cao et al., 2021; Deng et al., 2021, 2022; Liu et al., 2021a,b, 2022a,2022b; Song et al., 2021, 2022; Zhang et al., 2021; Ji et al., 2022; Wang et al., 2022). During our investigations on macrofungi from China, 90 specimens of Hydnellum were collected with different morphological characteristics. The morphological observation and phylogenetic analyses based on ITS + nLSU and ITS + LSU + nSSU + RPB2 combined matrixes were conducted to confirm the affinity of the undescribed species corresponding to Hydnellum. Five new species were described in detail and illustrated.
The specimens used in this study were deposited at the herbarium of the Institute of Microbiology, Beijing Forestry University (BJFC). Macro-morphological descriptions were based on field notes and laboratory measurements. The microscopic measures used in this study were followed by Sun et al. (2020, 2022) under a light microscope (Nikon Eclipse E 80i microscope, Nikon, Tokyo, Japan). Microscopic characteristics, measurements, and drawings were made from slide preparations stained with Cotton Blue and Melzer’s reagent, following Liu et al. (2021a). The following abbreviations were used: IKI = Melzer’s reagent, IKI– = neither amyloid or dextrinoid, KOH = 5% potassium hydroxide, CB = Cotton Blue, CB += cyanophilous, CB– = acyanophilous, L = mean spore length (arithmetic average of all spores), W = mean spore width (arithmetic average of all spores), Q = variation in the L/W rationes between the specimens studied, and n = number of spores measured from given number of specimens. A field Emission Scanning Electron Microscope (FESEM) Hitachi SU-8010 (Hitachi, Ltd., Tokyo, Japan) was used to photograph the ornamentation of the basidiospores, and the materials were studied at up to 2,200 times magnification, according to Song et al. (2021, 2022).
The CTAB rapid plant genome extraction kit DN14 (Aidlab Biotechnologies, Beijing, China) was used to acquire total genomic DNA from dried specimens according to the manufacturer’s instructions with some modifications (Sun et al., 2022). ITS4 and ITS5 were used as primers for the internal transcribed spacer (ITS), LR0R and LR7 were used for the large subunit of nuclear ribosomal RNA gene (nLSU), NS1/NS4 were used for the small subunit of nuclear ribosomal RNA gene (nSSU), and 5F/7Cr were used to the second largest subunit of RNA polymerase II (RPB2) gene. The Polymerase Chain Reaction (PCR) procedure for ITS was as follows: initial denaturation at 95°C for 3 min, followed by 35 cycles at 94°C for 40 s, 56°C for 45 s, and 72°C for 1 min, and a final extension of 72°C for 10 min. The PCR procedure for nLSU and nSSU was as follows: initial denaturation at 94°C for 1 min, followed by 35 cycles at 94°C for 30 s, 50°C for 1 min, and 72°C for 1.5 min, and a final extension of 72°C for 10 min. The PCR process for RPB2 was as follows: initial denaturation at 94°C for 2 min, 9 cycles at 94°C for 45 s, 60°C for 45 s, followed by 36 cycles at 94°C for 45 s, 53°C for 1 min, 72°C for 90 s, and a final extension of 72°C for 10 min. The PCR products were purified and sequenced at the Beijing Genomics Institute, China, with the same primers. The newly generated sequences were deposited at GenBank. All sequences analyzed in this study were deposited at GenBank and are listed in Table 1.
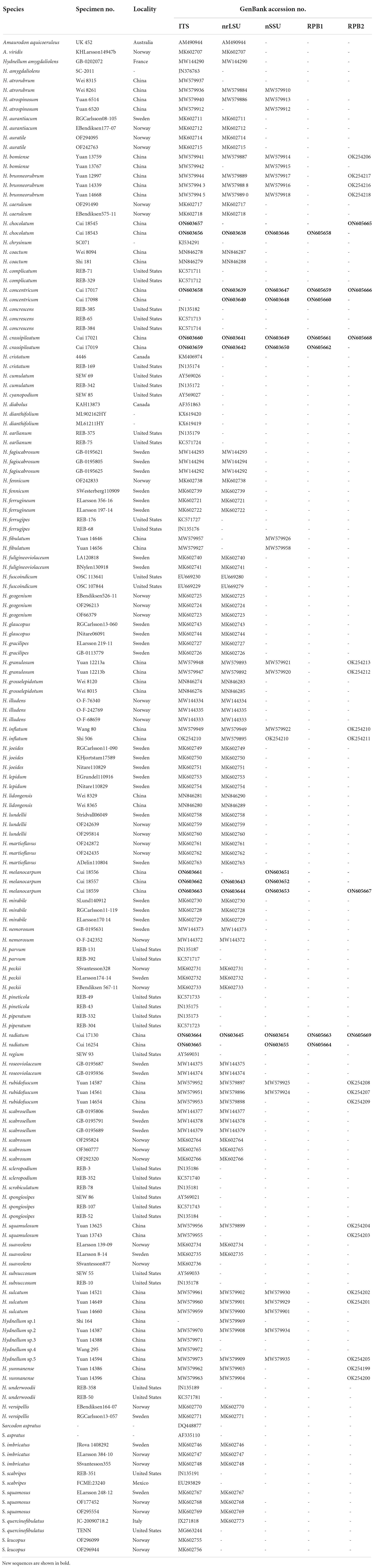
Table 1. A list of species, specimens, and GenBank accession numbers of sequences used in this study.
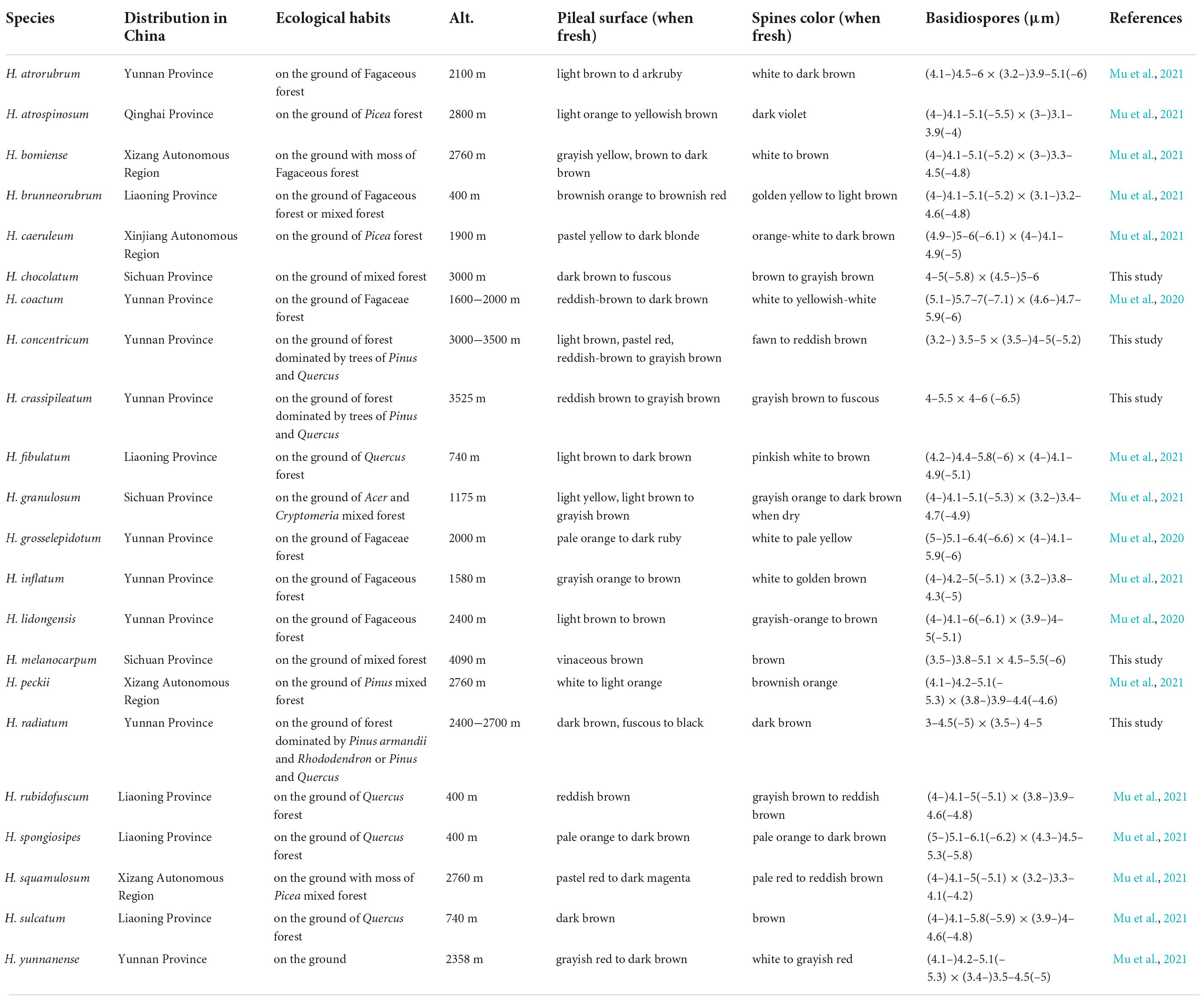
Table 2. The distribution areas, ecological habits, and main morphological characters of species in Hydnellum from China.
The new sequences generated in this study were combined with the sequences downloaded from GenBank and are listed in Table 1. Amaurodon aquicoeruleus Agerer and A. viridis (Alb. and Schwein.) J. Schröt were used as the outgroups, according to Mu et al. (2021). Sequences were aligned by MAFFT v.7 with the G-INI-I option (Katoh and Standley, 2013) and manually adjusted in BioEdit v. 7.0.9. (Hall, 1999). Alignments were spliced in Mesquite v. 3.2. (Maddison and Maddison, 2017). The partition homogeneity test (PHT) (Farris et al., 1994) of the four-gene dataset was tested by PAUP v. 4.0b10 (Swofford, 2002) under 1,000 homogeneity replicates. The best-fit evolutionary model was selected with AIC (Akaike Information Criterion) using ModelTest 2.3 (Guindon and Gascuel, 2003; Darriba et al., 2012). Phylogenetic analyses were carried out according to the previous studies (Cui et al., 2019; Liu et al., 2022a).
Maximum parsimony (MP) analyses were applied to the combined datasets. The construction was performed in PAUP* version 4.0b10 (Swofford, 2002). All characters were equally weighted and gaps were treated as missing data. Trees were inferred using the heuristic search option with TBR branch swapping and 1,000 random sequence additions. Max trees were set to 5,000, branches of zero length were collapsed, and all parsimonious trees were saved. Clade robustness was assessed using a bootstrap analysis with 1,000 replicates (Felsenstein, 1985). Descriptive tree statistics of tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC), and homoplasy index (HI) were calculated for each maximum parsimonious tree generated. RAxML-HPC2 was used to construct the maximum likelihood (ML) analyses with the GTRGAMMA model. All model parameters were estimated by the program, and only the best ML tree from all searches was kept. The ML bootstrap values were performed using rapid bootstrapping with 1,000 replicates.
MrModeltest 2.3 (Posada and Crandall, 1998; Nylander, 2004) was used to determine the best-fit evolution model for each dataset for Bayesian inference (BI). BI was calculated using MrBayes 3.1.2 with four Markov chains running for two runs from random starting trees for one million generations, and trees were sampled every 100 generations (Ronquist and Huelsenbeck, 2003). The first 25% of the sampled trees were discarded as burn-in and a majority rule consensus tree of all remaining trees was calculated. All trees were viewed in FigTree v. 1.4.2.
The combined ITS + nLSU dataset included 257 sequences from 156 specimens representing 76 taxa. The dataset had an aligned length of 2,605 characters, including gaps (1,202 characters for ITS, 1,403 characters for nLSU), of which 1,527 characters were constant, 122 were variable and parsimony-uninformative, and 956 were parsimony-informative. Maximum parsimony analysis yielded 12 equally parsimonious trees (TL = 5,489, CI = 0.355, RI = 0.798, RC = 0.283, HI = 0.645). The best model for the combined ITS + nLSU sequences dataset estimated and applied in the Bayesian analysis was the GTR + I + G model. Bayesian and ML analysis resulted in a topology similar to that of MP analysis. Bayesian analysis has an average standard deviation of split frequencies = 0.005071. Only the ML tree was provided in Figure 1, and the MP bootstrap values (≥75%), ML bootstrap values (≥75%), and BPP (≥0.95) were shown at the nodes.
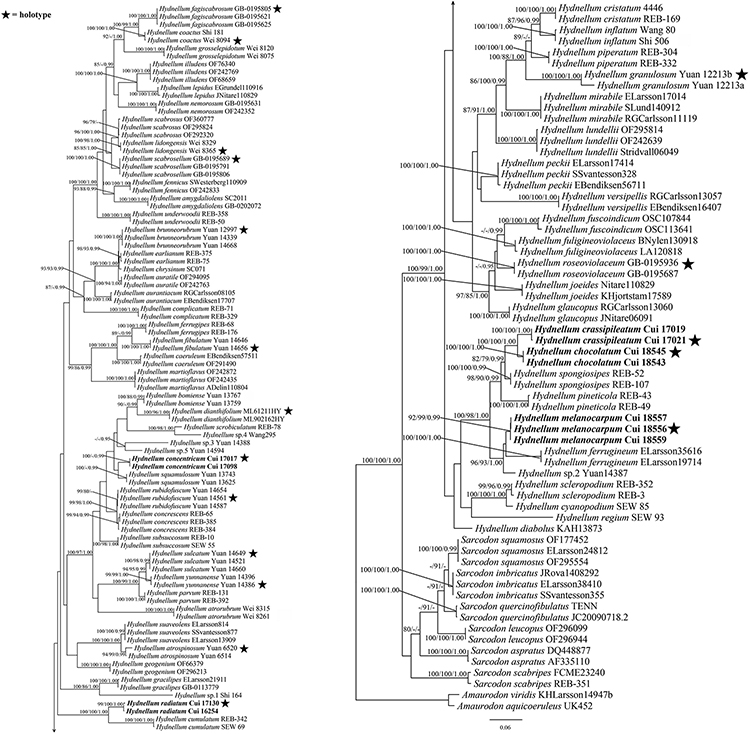
Figure 1. Maximum likelihood (ML) tree of the Hydnellum species based on ITS + nLSU sequences data. Branches are labeled with parsimony bootstrap values equal to or higher than 75%, maximum likelihood bootstrap values equal to or higher than 75%, and Bayesian posterior probabilities equal to or higher than 0.95. Bold names = New species.
The combined ITS + nLSU + nSSU + RPB2 dataset included 312 sequences from 156 specimens representing 76 taxa. The dataset had an aligned length of 4,639 characters, including gaps (1,181 characters for ITS, 1,399 characters for nLSU, 986 characters for nSSU, and 1,073 characters for RPB2), of which 3,168 characters were constant, 186 were variable and parsimony-uninformative, and 1,285 were parsimony-informative. Maximum parsimony analysis yielded 15 equally parsimonious trees (TL = 6,171, CI = 0.392, RI = 0.800, RC = 0.313, HI = 0.608). The best model for the combined ITS + nLSU + nSSU + RPB2 sequences dataset estimated and applied in the Bayesian analysis was the GTR + I + G model. Bayesian and ML analysis resulted in a topology similar to that of MP analysis. Bayesian analysis has an average standard deviation of split frequencies = 0.007966. Only the ML tree was provided in Figure 2, and the MP bootstrap values (≥75%), ML bootstrap values (≥75%), and BPP (≥0.95) were shown at the nodes.
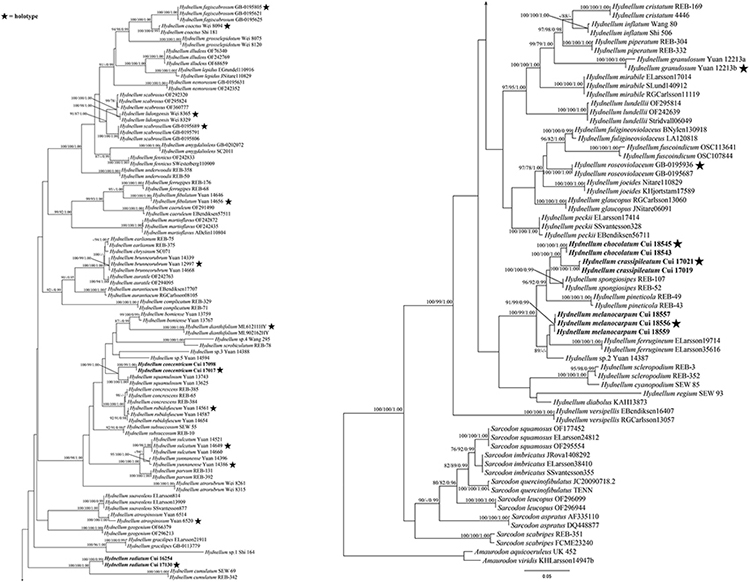
Figure 2. Maximum likelihood tree of the Hydnellum species based on the combined ITS + nLSU + nSSU + RPB2 sequences data. Branches are labeled with parsimony bootstrap values equal to or higher than 75%, ML bootstrap values equal to or higher than 75%, and Bayesian posterior probabilities equal to or higher than 0.95. Bold names = New species.
Hydnellum chocolatum B. K. cui and C. G. Song, sp. nov. (Figures 3A,B, 4A,B, 5).

Figure 3. Basidiomata of Hydnellum species. (A) H. chocolatum (paratype, Cui 18543), (B) H. chocolatum (holotype Cui 18545), (C,D) H. concentricum (holotype, Cui 17017), (E,F) H. crassipileatum (paratype, Cui 17019), (G) H. melanocarpum (paratype, Cui 18557), and (H) H. melanocarpum (paratype, Cui 18559), (I,J) H. radiatum (holotype, Cui 17130) Scale bars: 2 cm.
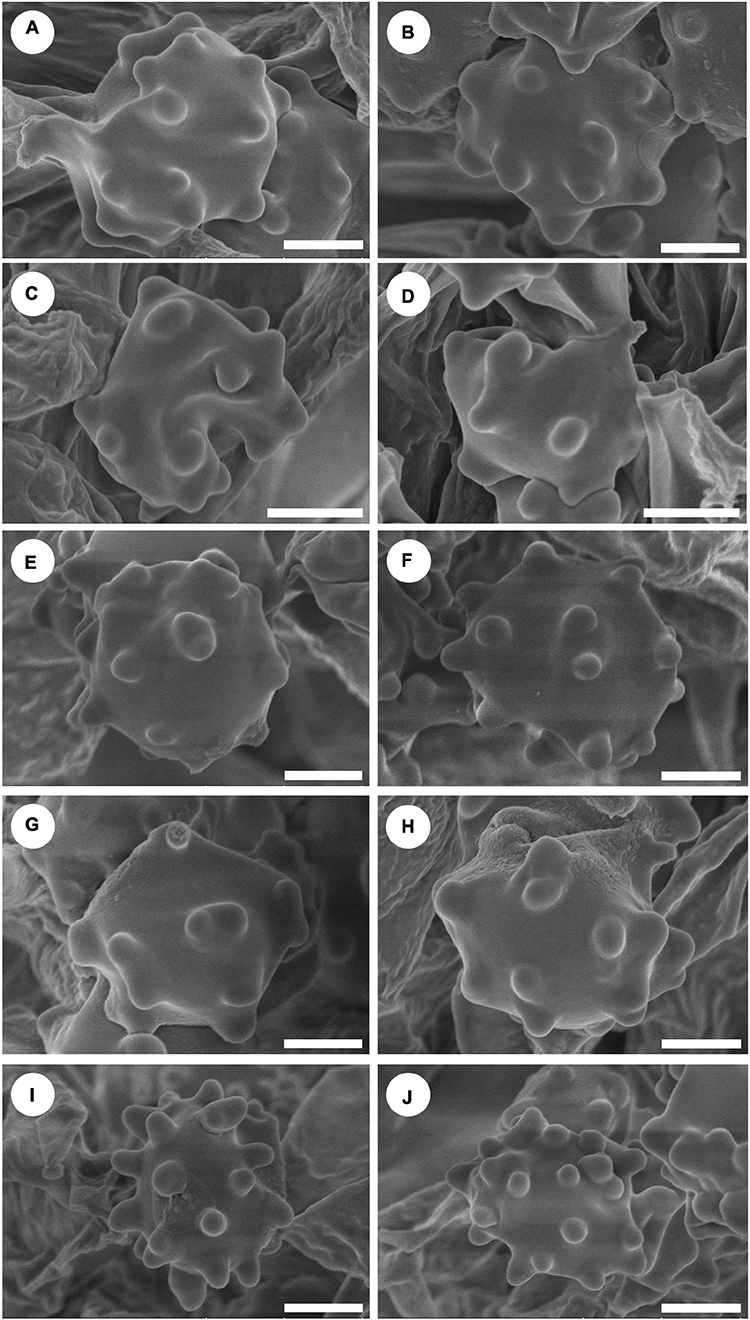
Figure 4. Scanning Electron Microscope (SEM) of basidiospores of Hydnellum species. (A,B) H. chocolatum, (C,D) H. concentricum, (E,F) H. crassipileatum, (G,H) H. melanocarpum, and (I,J) H. radiatum Scale bars: 1.5 μm.
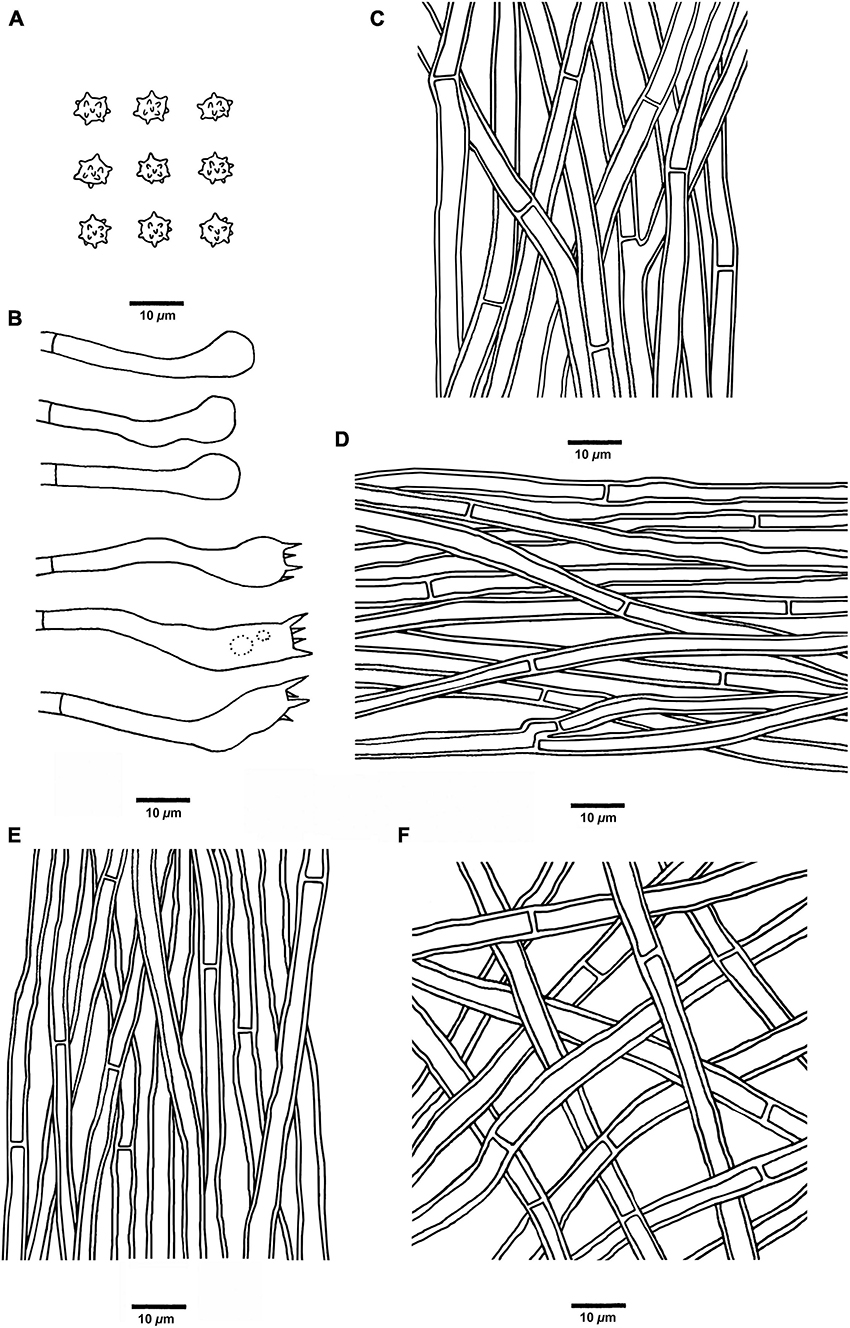
Figure 5. Microscopic structures of H. chocolatum (drawn from Cui 18545). (A) Basidiospores, (B) Basidia and basidioles, (C) Hyphae from context, (D) Hyphae from spines, (E) Hyphae from the inner layer of stipe, and (F) Hyphae from the surface layer of stipe.
MycoBank no.: 846115
Diagnosis: Differs from others by its fibrillose, spongy to tomentose pileal surface in chocolate color.
Type: CHINA. Sichuan Province, Jiuzhaigou County, on the ground of the mixed forest, elev. 2,600 m, 19 September 2020, Bao-Kai Cui, Cui 18545 (holotype, BJFC 035406).
Etymology: chocolatum (Lat.) refers to the chocolate-colored pileal surface.
Fruiting body: Basidiomata annual, eccentrically stipitate, single to concrescent, and odorless when fresh. The pileus is circular to irregular, with irregular folds in the middle, and up to 7.4 cm in diam and 0.7 cm thick at the center. Pileal surface is chocolate to fuscous when fresh, becoming grayish brown upon drying, azonate, fibrillose to spongy at the center, and tomentose near the margin, with radially aligned stripes toward the margin; margin white to light brown when fresh, becoming grayish brown upon drying, up to 7 mm wide. Context is brown to vinaceous gray upon drying, corky to fragile, and up to 3 mm thick. Spines are soft, brown to grayish brown when fresh, becoming grayish brown upon drying, fragile, and up to 5 mm long. Stipe is cylindrical and glabrous, surface layer is dark brown to vinaceous gray, and inner layer is grayish brown, and up to 3.8 cm long and 0.9 cm in diam.
Hyphal structure: Hyphal system monomitic; generative hyphae with simple septa; all the hyphae IKI–, CB–; tissues turned to olive-green or black in KOH.
Context: Generative hyphae grayish brown, thick-walled, branched, regular arranged, 2.5 to 6 μm in diam.
Spines: Generative hyphae clay-buff, slightly thick-walled, occasionally branched, regular arranged, 2 to 4.5 μm in diam. Cystidia and cystidioles are absent. Basidia clavate, bearing four sterigmata (2–4 μm long) and a basal simple septum, 32–45 × 5–7 μm; basidioles similar to basidia in shape, but slightly smaller.
Stipe: Generative hyphae clay-buff to grayish brown, slightly thick-walled, rarely branched, interwoven in the surface layer, regularly arranged in the inner layer, and 2 to 4 μm in diam.
Spores: Basidiospores subglobose to globose, hyaline, thin-walled, echinulate, IKI–, CB– (4.5–)5–6 × 4–5(–5.8) μm, L = 5.2 μm, W = 4.7 μm, Q = 1–1.25 (n = 60/2, without the ornamentation).
Additional specimen (paratype) examined: CHINA. Sichuan Province, Jiuzhaigou County, on the ground of the mixed forest, elev. 2,600 m, 19 September 2020, Bao-Kai Cui, Cui 18543 (BJFC 035404).
Ecological habits: Hydnellum chocolatum was collected in Southwest China under a plateau humid climate. It grows on the moist ground of the mixed forest, with well-watered bryophytes.
Hydnellum concentricum B. K. Cui and C. G. Song, sp. nov. (Figures 3C,D, 4C,D, 6).
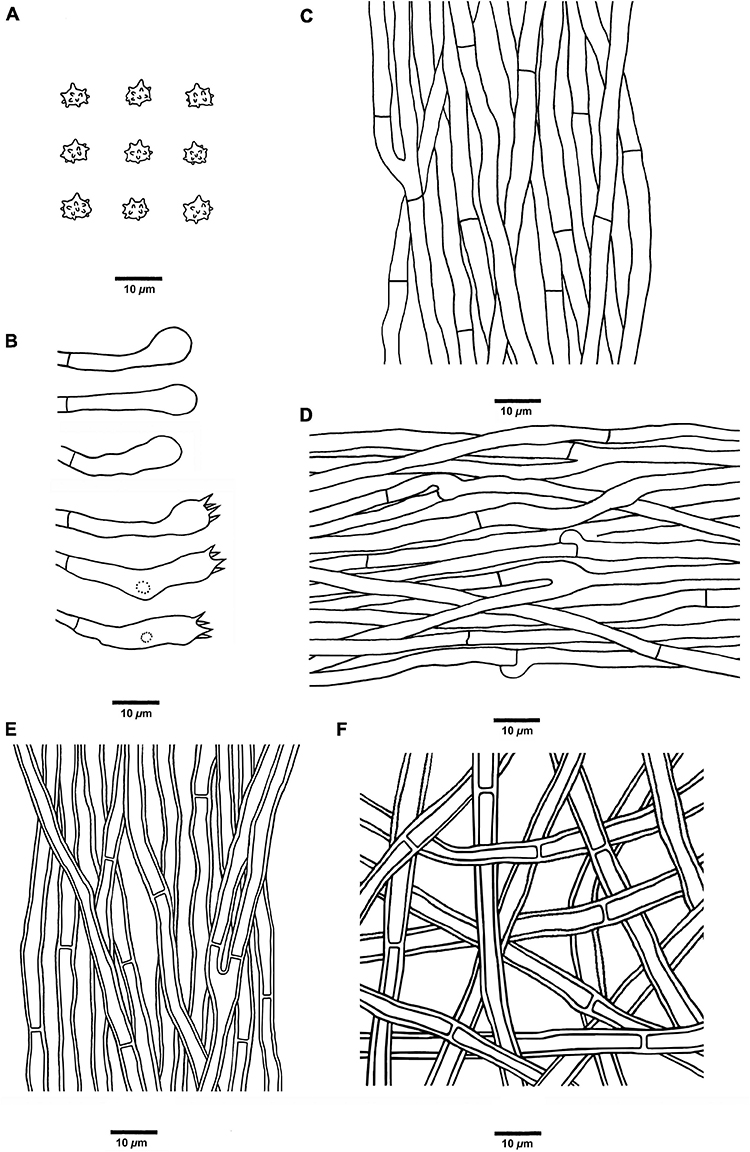
Figure 6. Microscopic structures of H. concentricum (drawn from Cui 17017). (A) Basidiospores, (B) Basidia and basidioles, (C) Hyphae from context, (D) Hyphae from spines, (E) Hyphae from the inner layer of stipe, and (F) Hyphae from the surface layer of stipe.
MycoBank no.: 846116
Diagnosis: Differs from other Hydnellum species by its zonate pileal surface, thin context, short stipe, and presence of both simple septa and clamp connections in generative hyphae of spines.
Type: CHINA. Yunnan Province, Lijiang City, Yulong County, Jiuhe, Laojun Mountain, Jiushijiulongtan, on the ground of forest dominated by trees of Pinus sp. and Quercus sp., elev. 2,800 m, 15 September 2018, Bao-Kai Cui, Cui 17017 (holotype, BJFC 030316).
Etymology: concentricum (Lat.) refers to the concentric bands on the pileal surface.
Fruiting body: Basidiomata annual, centrally stipitate, single, and odorless when fresh. Pileus infundibuliform, and up to 3.2 cm in diam and 0.4 cm thick at the center. Pileal surface is light brown, pastel red, reddish-brown to grayish brown when fresh, becoming brown to grayish brown upon drying, zonate, glabrous, with radially aligned stripes; margin fawn to orange-brown when fresh, becoming fawn upon drying, and up to 0.8 cm wide. Context is grayish brown upon drying, fragile, and up to 1 mm thick. Spines are soft, fawn to reddish-brown when fresh, grayish brown upon drying, fragile, and up to 3 mm long. Stipe cylindrical, glabrous, surface layer honey yellow to grayish brown upon drying, inner layer grayish brown upon drying; and up to 1.8 cm long and 0.5 cm in diam.
Hyphal structure: Hyphal system monomitic; generative hyphae in context and stipe with simple septa, generative hyphae in spines mostly with simple septa, occasionally with clamp connections; all the hyphae IKI–, CB–; tissues turned to olive-green or black in KOH.
Context: Generative hyphae clay-buff to grayish brown, slightly thick-walled, branched, regularly arranged, and 2 to 6 μm in diam.
Spines: Generative hyphae clay-buff, thin-walled, occasionally branched, occasionally with clamp connections, regularly arranged, and 2 to 4.5 μm in diam. Cystidia and cystidioles are absent. Basidia clavate, bearing four sterigmata (2–4 μm long) and a basal simple septum, 22–48 × 5–7 μm; basidioles are similar to basidia in shape but slightly smaller.
Stipe: Generative hyphae clay-buff, slightly thick-walled, rarely branched, interwoven in the surface layer, regularly arranged in the inner layer, and 2 to 4.5 μm in diam.
Spores: Basidiospores subglobose to ellipsoidal, hyaline, thin-walled, echinulate, IKI–, CB– (3.5–)4–5(–5.2) × (3.2–)3.5–5 μm, L = 4.6 μm, W = 3.9 μm, Q = 1.04–1.37 (n = 60/2, without the ornamentation).
Additional specimen (paratype) examined: CHINA. Yunnan Province, Shangri-La, on the ground of forest dominated by trees of Pinus yunnanensis, elev. 3,200 m, 17 September 2018, Bao-Kai Cui, Cui 17098 (BJFC 030398).
Ecological habits: Hydnellum concentricum was collected in Southwest China, under a plateau monsoon climate. It grows on the moist ground of a forest dominated by trees of Pinus yunnanensis.
Hydnellum crassipileatum B. K. Cui and C. G. Song, sp. nov. (Figures 3E,F, 4E,F, 7).
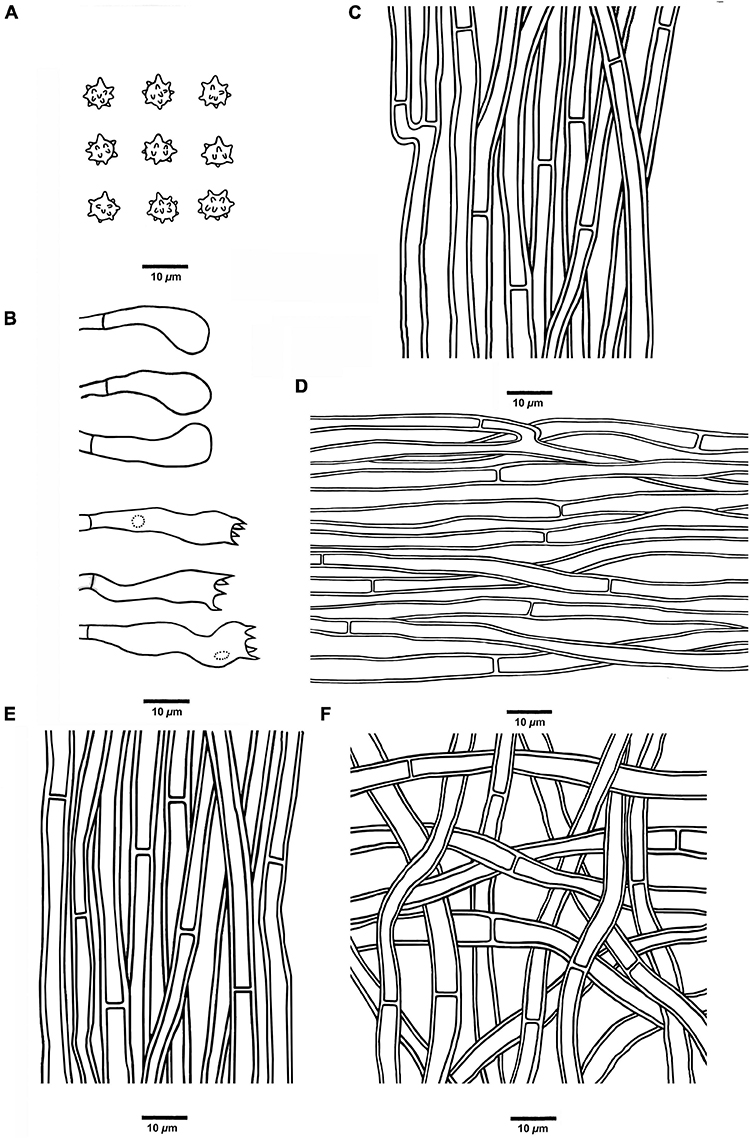
Figure 7. Microscopic structures of H. crassipileatum (drawn from Cui 17021). (A) Basidiospores, (B) Basidia and basidioles, (C) Hyphae from context, (D) Hyphae from spines, (E) Hyphae from the inner layer of stipe, and (F) Hyphae from the surface layer of stipe.
MycoBank no.: 846117
Diagnosis: Differs from other Hydnellum species by its thick pileus with reddish brown to grayish brown pileal surface.
Type: CHINA. Yunnan Province, Lijiang City, Yulong County, Jiuhe, Laojun Mountain, Jiushijiulongtan, on the ground of forest dominated by trees of Pinus sp. and Quercus sp., elev. 2,800 m, 15 September 2018, Bao-Kai Cui, Cui 17021 (holotype, BJFC 030320).
Etymology: crassipileatum (Lat.) refers to the thick pileus.
Fruiting body: Basidiomata annual, eccentrically stipitate, single, and odorless when fresh. Pileus circular to elliptical, up to 5.5 cm in diam, and 0.5 cm thick at the center. Pileal surface is reddish-brown to grayish brown when fresh, becoming grayish brown upon drying, azonate, fibrillose, spongy to tomentose when young, and glabrous with age; margin white to grayish brown when fresh, becoming olivaceous buff upon drying, up to 6 mm wide. Context light grayish-brown upon drying, fragile, up to 5 mm thick. Spines soft, grayish brown to fuscous when fresh, fuscous to black upon drying, fragile, up to 4 mm long. Stipe cylindrical, glabrous, surface layer grayish-brown, inner layer grayish brown to fuscous; up to 4.8 cm long, and 1.9 cm in diam.
Hyphal structure: Hyphal system monomitic; generative hyphae with simple septa; all the hyphae IKI–, CB–; tissues turned to olive-green or black in KOH.
Context: Generative hyphae clay-buff, thick-walled, occasionally branched, regularly arranged, and 2.5 to 5 μm in diam.
Spines: Generative hyphae clay-buff, thick-walled, occasionally branched, more or less regularly arranged, and 2.5 to 4 μm in diam. Cystidia and cystidioles are absent. Basidia clavate, bearing four sterigmata (2.5–3.5 μm long) and a basal simple septum, 14–31 × 5–7 μm; basidioles similar to basidia in shape, but slightly smaller.
Stipe: Generative hyphae clay-buff, slightly thick-walled, rarely branched, interwoven in the surface layer, regularly arranged in the inner layer, and 2.5 to 5 μm in diam.
Spores: Basidiospores subglobose to ellipsoidal, hyaline, thin-walled, echinulate, IKI–, CB–, 4–6(–6.5) × 4–5.5 μm, L = 5.6 μm, W = 4.5 μm, Q = 1–1.38 (n = 60/2, without the ornamentation).
Additional specimen (paratype) examined: CHINA. Yunnan Province, Lijiang City, Yulong County, Jiuhe, Laojun Mountain, Jiushijiulongtan, on the ground of forest dominated by trees of Pinus and Quercus, elev. 2,800 m, 15 September 2018, Bao-Kai Cui, Cui 17019 (BJFC 030318).
Ecological habits: Hydnellum crassipileatum was collected in Southwest China, under a plateau monsoon climate. It grows on the ground of a moist forest dominated by trees of Pinus and Quercus.
Hydnellum melanocarpum B. K. Cui and C. G. Song, sp. nov. (Figures 3G,H, 4G,H, 8).
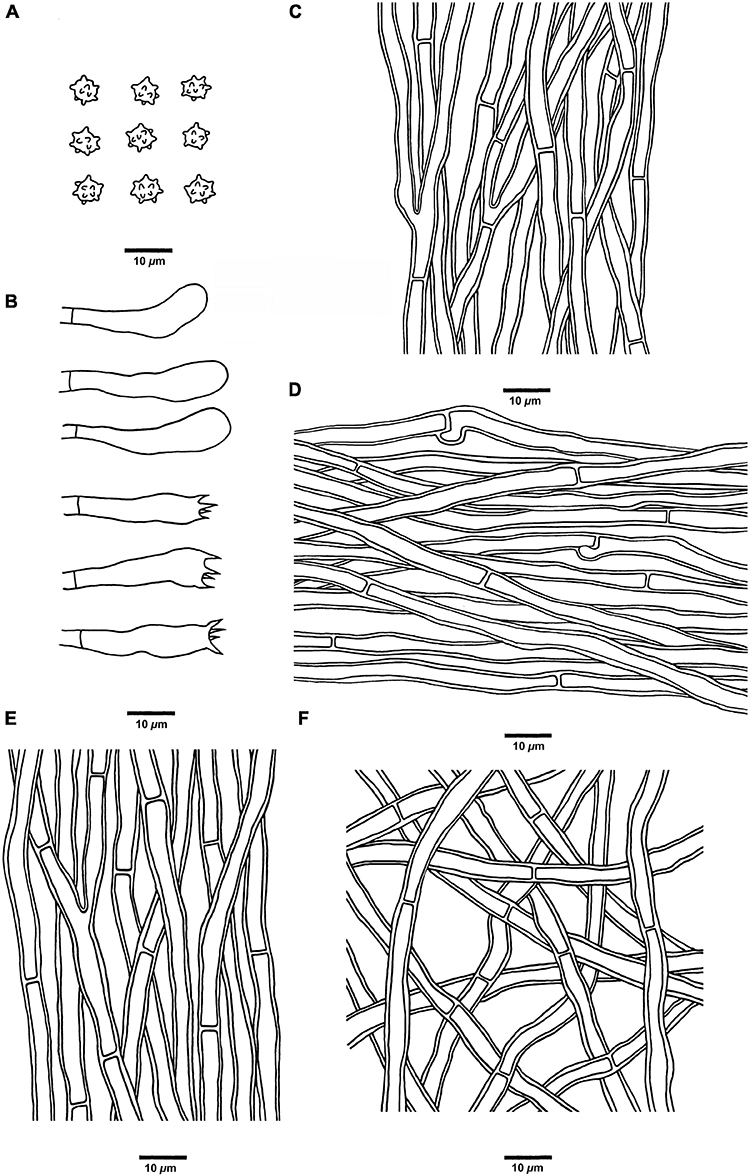
Figure 8. Microscopic structures of H. melanocarpum (drawn from Cui 18556). (A) Basidiospores, (B) Basidia and basidioles, (C) Hyphae from context, (D) Hyphae from spines, (E) Hyphae from the inner layer of stipe, and (F) Hyphae from the surface layer of stipe.
MycoBank no.: 846118
Diagnosis: Differs from other Hydnellum species by its vinaceous brown to black pileus with spongy pileal surface, and the presence of both simple septa and clamp connections in generative hyphae of spines.
Type: CHINA. Sichuan Province, Jiuzhaigou County, Jiuzhaigou Reverse, on the ground of the mixed forest, elev. 2,500 m, 20 September 2020, Bao-Kai Cui, Cui 18556 (holotype, BJFC 035417).
Etymology: melanocarpum (Lat.) refers to the vinaceous brown to black pileus.
Fruiting body: Basidiomata annual, centrally or eccentrically stipitate, single to concrescent, and odorless when fresh. Pileus is circular to irregular, up to 4.8 cm in diam, and 0.7 cm thick at the center. Pileal surface is vinaceous brown to black when fresh and becoming grayish brown upon drying, azonate, and glabrous to spongy at the center; margin cream, clay-buff, to orange-brown when fresh, light vinaceous gray at the lower tip, and becoming grayish brown to fuscous upon drying, and up to 0.6 cm wide. Spines are soft, brown when fresh, grayish brown to black upon drying, fragile, and up to 4 mm long. Context is grayish brown upon drying, fragile, and up to 3 mm thick. Stipe is cylindrical, glabrous, and grayish brown; and up to 2.6 cm long and 0.8 cm in diam.
Hyphal structure: Hyphal system monomitic; generative hyphae in context and stipe with simple septa, generative hyphae in spines mostly with simple septa, occasionally with clamp connections; all the hyphae IKI–, CB–; tissues turned to olive green in KOH.
Context: Generative hyphae clay-buff to grayish brown, thick-walled, branched, regularly arranged, and 2 to 4 μm in diam.
Spines: Generative hyphae clay-buff, thin-walled, occasionally branched, regularly arranged, and 2 to 3.5 μm in diam. Cystidia and cystidioles are absent. Basidia clavate, bearing four sterigmata (1.5–3 μm long) and a basal simple septum, 18–38 × 5–7 μm; basidioles similar to basidia in shape, but slightly smaller.
Stipe: Generative hyphae clay-buff, slightly thick-walled, rarely branched, interwoven in the surface layer, regularly arranged in the inner layer, and 2 to 4 μm in diam.
Spores: Basidiospores subglobose, hyaline, thin-walled, echinulate, IKI–, CB–, 4.5–5.5(–6) × (3.5–)3.8–5.1 μm, L = 5 μm, W = 4.6 μm, Q = 1–1.25 (n = 90/3, without the ornamentation).
Additional specimens (paratypes) examined: CHINA. Sichuan Province, Jiuzhaigou County, Jiuzhaigou Nature Reverse, on the ground of the mixed forest, elev. 2,500 m, 20 September 2020, Bao-Kai Cui, Cui 18557 (BJFC 035418) and Cui 18559 (BJFC 035420).
Ecological habits: Hydnellum melanocarpum was collected in Southwest China, under a plateau monsoon climate. It grows on the ground of the mixed forest, in well-watered bryophytes, and its roots are often interspersed with pine needles.
Hydnellum radiatum B. K. Cui and C. G. Song, sp. nov. (Figures 3I,J, 4I,J, 9).
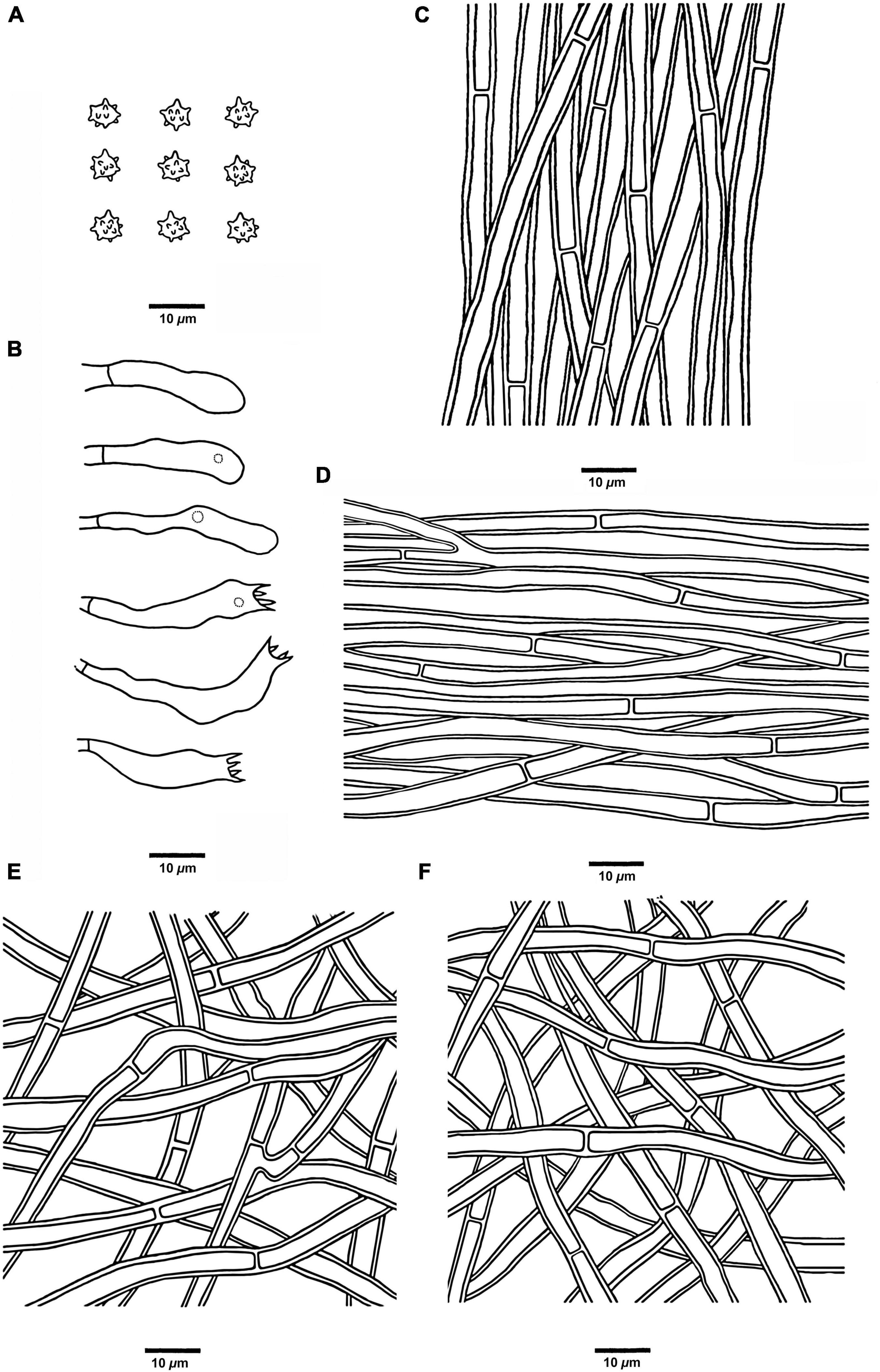
Figure 9. Microscopic structures of H. radiatum (drawn from Cui 17130). (A) Basidiospores, (B) Basidia and basidioles, (C) Hyphae from context, (D) Hyphae from spines, (E) Hyphae from the inner layer of stipe, and (F) Hyphae from the surface layer of stipe.
MycoBank no.: 846120
Diagnosis: Differs from other Hydnellum species by its radially aligned stripes on pileal surface, grayish brown context, and short stipe.
Type: CHINA. Yunnan Province, Lanping County, Tongdian, Jianganchang, on the ground of forest dominated by Pinus armandii and Rhododendron, elev. 2,480 m, 18 September 2018, Bao-Kai Cui, Cui 17130 (holotype, BJFC 030430).
Etymology: radiatum (Lat.) refers to the radially aligned stripes on the pileal surface.
Fruiting body: Basidiomata annual, eccentrically stipitate, single, and odorless when fresh. Pileus is subcircular, plicate, and up to 2.9 cm in diam and 0.5 cm thick at the center. Pileal surface is dark brown, fuscous to black when fresh and becoming fuscous to black upon drying, azonate, fibrillose, and with strong radially aligned stripes; margin white, cream to light brown when fresh, becoming grayish brown upon drying, and up to 0.3 cm wide. Context is grayish brown upon drying, tough, and up to 3 mm thick. Spines are soft, fuscous to black when fresh, grayish brown upon drying, fragile, and up to 3 mm long. Stipe is cylindrical, glabrous, surface layer fuscous to black upon drying, inner layer grayish brown upon drying, and up to 4 cm long and 1 cm in diam.
Hyphal structure: Hyphal system monomitic; generative hyphae with simple septa; all the hyphae IKI–, CB–; tissues of pileus and spines turned to olive green in KOH, tissues of stipe without reaction.
Context: Generative hyphae clay-buff, thick-walled, branched, regularly arranged, and 2 to 5.5 μm in diam.
Spines: Generative hyphae clay-buff, thick-walled, occasionally branched, regularly arranged, and 2 to 3.5 μm in diam. Cystidia and cystidioles are absent. Basidia clavate, bearing four sterigmata (1.5–4 μm long) and a basal simple septum, 14–21 × 3–4 μm; basidioles similar to basidia in shape, but slightly smaller.
Stipe: Generative hyphae clay-buff, thick-walled, rarely branched, interwoven in both the surface layer and the inner layer, and 2 to 5 μm in diam.
Spores: Basidiospores subglobose to ellipsoidal, hyaline, thin-walled, echinulate, IKI–, CB– (3.5–)4–5 × 3–4.5(–5) μm, L = 4.5 μm, W = 3.8 μm, Q = 1–1.35 (n = 60/2, without the ornamentation).
Additional specimen (paratype) examined: CHINA. Yunnan Province, Lanping County, Tongdian, Luoguqing, on the ground of forest dominated by Pinus and Quercus, elev. 2,630 m, 18 September 2017, Bao-Kai Cui, Cui 16254 (BJFC 029553).
Ecological habits: Hydnellum radiatum was collected in Southwest China, under a plateau monsoon climate. It grows on the ground of the forest dominated by trees of Pinus yunnanensis, in well-watered bryophytes, and its roots are often interspersed with pine needles.
Key to species of Hydnellum from China:
(1) Pileal surface scaled………………………………………………………… 2
(1) Pileal surface not scaled………………………………………………….. 3
(2) Pileal surface pale orange to dark ruby…………………………………………………………………………………….. H. grosselepidotum
(2) Pileal surface differently colored………………………………………………………………………………………………………….. H. lidongensis
(3) Pileus subinfundibuliform to infundibuliform………………. 4
(3) Pileus differently shaped……………………………………………….. 8
(4) Pileal surface glabrous……………………………. H. concentricum
(4) Pileal surface not glabrous……………………………………………… 5
(5) Pileal surface brownish orange to brownish red……………………………………………………………………….. H. brunneorubrum
(5) Pileus surface differently colored……………………………………. 6
(6) Pileal surface light brown to dark ruby………………………………………………………………………………………………. H. atrorubrum
(6) Pileus surface differently colored…………………………………… 7
(7) Pileus and spines grayish red…………………………………………………………………………………………………….. H. yunnanense
(7) Pileus and spines differently colored………………………………. …………………………………………………………………….. H. bomiense
(8) Context tissue becoming blue-green in KOH………………………………………………………………………………………….. H. peckii
(8) Context tissue differently colored in KOH……………………… 9
(9) Spines dark violet…………………………………… H. atrospinosum
(9) Spines differently colored…………………………………………….. 10
(10) Clamp connections present………………………………………….. 11
(10) Clamp connections absent…………………………………………… 13
(11) Pileal surface pastel yellow to dark blonde…………………………………………………………………………………….. H. caeruleum
(11) Pileal surface differently colored………………………………….. 12
(12) Clamp connections present in spines…………………………………………………………………………………………….. H. melanocarpum
(12) Clamp connections absent in spines…………………………………………………………………………………………………… H. fibulatum
(13) Pileal surface zonate……………………………………………………… 14
(13) Pileal surface azonate…………………………………………………… 16
(14) Pileal surface pastel red to dark magenta…………………… …………………………………………………………….. H. squamulosum
(14) Pileal surface differently colored…………………………………… 15
(15) Pileal surface glabrous to scrupose when fresh……. ………………………………………………………………. H. rubidofuscum
(15) Pileal surface scabrous to fibrous when fresh……………………………………………………………………………….. H. sulcatum
(16) Pileal surface with radially aligned stripes……………………. …………………………………………………………………………………… 17
(16) Pileal surface without radially aligned stripes…………………. ………………………………………………………………………………… 18
(17) Spines fuscous to black…………………………………………………… ……………………………………………………………………. H. radiatum
(17) Spines brown to grayish brown……………………………………………………………………………………………………. H. chocolatum
(18) Spines white to yellowish-white………………………. H. coatum
(18) Spines differently colored…………………………………………. 19
(19) Context grayish orange……………………………………………………… ……………………………………………………………… H. granulosum
(19) Context differently colored…………………………………………. 20
(20) Pileal surface reddish brown to grayish brown……………………………………………………………………………. H. crassipileatum
(20) Pileal surface differently colored…………………………………. 21
(21) Stipe light brown………………………………………….. H. inflatum
(21) Stipe orange white, pale orange, sunburn to cognac…………………………………………………………………. H. spongiosipes
The genus Hydnellum is easy to recognize in Bankeraceae by its corky to woody pileus with crowded spines, but identification among the species in Hydnellum is difficult due to the quite similar morphological features. The main morphological characters of each species in Hydnellum from China were summarized in Table 2. The morphological differences between five new species were emphasized here briefly.
Hydnellum chocolatum was clustered with H. crassipileatum and H. spongiosipes (Peck) Pouzar in our phylogenetic analyses (Figures 1, 2). Morphologically, H. chocolatum resembles H. crassipileatum and H. spongiosipes in having single to concrescent basidiomata. However, H. chocolatum can be distinguished by its tomentose and azonate pileal margin, and longer basidia (32–45 × 5–7 μm); H. crassipileatum differs by its thick pileus with the reddish brown to grayish brown pileal surface; Hydnellum spongiosipes can be distinguished by its orange white to pale orange pileus and longer basidiospores [6–7 × 5–6 μm in H. spongiosipes vs. (4.5–)5–6 × 4–5(–5.8) μm in H. chocolatum, Baird et al., 2013].
Our phylogenetic analyses showed that Hydnellum concentricum was sister to H. squamulosum Y. H. Mu and H. S. Yuan (Figures 1, 2). The two species were both described in Southwest China, and share the annual, solitary to gregarious basidiomata, pastel red pileus, and reddish-brown spines (Mu et al., 2021). However, H. squamulosum differs from H. concentricum by its floccose to woolly, squamulose pileal surface and smaller basidiospores [4.1–5 × 3.3–4.1 μm in H. squamulosum vs. (3.5–)4–5(–5.2) × (3.2–)3.5–5 μm in H. concentricum, Mu et al., 2021].
Hydnellum melanocarpum is closely related to Hydnellum ferrugineum (Fr.) P. Karst. In our phylogenetic analyses (Figures 1, 2). However, H. ferrugineum differs from H. melanocarpum by its pale orange to burnt umber pileus, and larger basidiospores [5–6(–7) × 5–6 μm in H. ferrugineum vs. 4.5–5.5(–6) × (3.5–)3.8–5.1 μm H. melanocarpum, Baird et al., 2013].
Hydnellum radiatum resembles H. cumulatum in having similar colored spines, which have close phylogenetic relationship (Figures 1, 2). However, H. cumulatum can be distinguished by its vinaceous buff, hessian brown to burnt umber pileus, wider basidia (20 × 5–7 μm in H. cumulatum vs. 14–21 × 3–4 μm), and larger basidiospores [4–5.5 × 4–5 μm in H. cumulatum vs. (3.5–)4–5 × 3–4.5(–5) μm in H. radiatum, Harrison, 1964].
Hydnellum species tend to grow in moist woodlands with thick mosses, under pine needles or oak leaves, which help to reduce water loss. Most of the specimens were collected from Pinaceae, Fagaceae forests, or mixed forests (Table 2). But the Fagaceae forests were regarded as the preference for Hydnellum by comparing the frequency of the collection sites (Table 2). It indicated that species in Hydnellum are host-biased, which can be used as an auxiliary basis for species discovery and identification.
The combination of the traditionally morphological observation and molecular systematics methods can objectively reveal the diversity and phylogenetic relationship of Hydnellum species. Few studies were using phylogenetic analyses of Hydnellum in the past, and most of them were only based on the ITS sequences of several species (Ainsworth et al., 2010; Baird et al., 2013; Loizides et al., 2016). Mu et al. (2021) conducted a phylogenetic analysis of Hydnellum and Sarcodon based on 4-gene sequences (ITS + nLSU + nSSU + RPB2), which undoubtedly filled in the blank of multiple gene fragments of Hydnellum. In this study, the phylogeny of Hydnellum was carried out based on four gene markers. In this study, both ITS + LSU and ITS + LSU + SSU + RPB2 datasets share a similar topology with Mu et al. (2021), but with discrepant bootstrap values.
Combining with the macro- and micro-morphological observation and scanning electron microscope shot, the number of Hydnellum species has been expanded to 22 in China, and it indicated that more potential species of Hydnellum could be discovered by combined evidence of morphological characters, molecular data, and ecological habits. Moreover, the sequences of the largest subunit of the RNA polymerase II (RPB1) gene of Hydnellum were also provided in this study, which might be useful for future phylogenetic studies. The primer pairs RPB1-Af and RPB1-Cr for the RPB1 gene used in this study are the same as in previous studies (White et al., 1990; Liu et al., 2022b). However, there are still many species of Hydnellum lacking molecular data, which limits the systematic study of this genus. For the time being, the common gene marker for the identification of most Hydnellum species is ITS, while more terminal nodes in phylogenetic trees are needed to investigate by using more gene markers, such as RPB1 and RPB2. There are only 22 RPB2 sequences and no RPB1 sequence of Hydnellum in NCBI (Accessed 7 May 2022)2. It is necessary to obtain diverse molecular sequences to build a more scientific system between the species and genera in hydnoid stipitate fungi.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, ON603638-ON603665 and https://www.ncbi.nlm.nih.gov/genbank/, ON605658-ON605669.
B-KC designed the research. B-KC, Y-FS, SL, T-MX, D-MW, NG, and C-GS prepared the samples. C-GS, SL, and T-MX conducted the molecular experiments and analyzed the data. C-GS, Y-FS, D-MW, NG, and B-KC drafted the manuscript. All authors read and agreed to the published version of the manuscript.
This research was supported by the National Natural Science Foundation of China (Nos. 31870008 and U2003211) and Beijing Forestry University Outstanding Young Talent Cultivation Project (No. 2019JQ03016).
We express our gratitude to Yu-Cheng Dai (Beijing Forestry University, China) for his help during field collections.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ainsworth, A., Parfitt, B., Rogers, H., and Boddy, L. (2010). Cryptic taxa within European species of Hydnellum and Phellodon revealed by combined molecular and morphological analysis. Fungal Ecol. 3, 65–80. doi: 10.1016/j.funeco.2009.07.001
Arnolds, E. (1991). Decline of ectomycorrhizal fungi in Europe. Agr. Ecosyst. Environ. 35, 209–244. doi: 10.1016/0167-8809(91)90052-Y
Arnolds, E. (2010). The fate of hydnoid fungi in the Netherlands and Northwestern Europe. Fungal Ecol. 3, 81–88. doi: 10.1016/j.funeco.2009.05.005
Baird, R. E., and Khan, S. R. (1986). The stipitate hydnums (Thelephoraceae) of Florida. Brittonia 38, 171–184. doi: 10.2307/2807273
Baird, R. E., Wallace, L. E., Baker, G., and Scruggs, M. (2013). Stipitate hydnoid fungi of the temperate southeastern United States. Fungal Divers. 62, 41–114. doi: 10.1007/s13225-013-0261-6
Banker, H. J. (1906). A contribution to a revision of the North American Hydnaceae. Mem. Torrey Bot. Club 12, 99–194. doi: 10.5962/bhl.title.97394
Cao, T., Hu, Y. P., Yu, J. R., Wei, T. Z., and Yuan, H. S. (2021). A phylogenetic overview of the Hydnaceae (Cantharellales, Basidiomycota) with new taxa from China. Stud. Mycol. 99, 100–121. doi: 10.1016/j.simyco.2021.100121
Cui, B. K., Li, H. J., Ji, X., Zhou, J. L., Song, J., Si, J., et al. (2019). Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Divers. 97, 137–392. doi: 10.1007/s13225-019-00427-4
Darriba, D., Taboada, G. L., Doallo, R., and Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9:772. doi: 10.1038/nmeth.2109
Deng, L. S., Kang, R., Zeng, N. K., Yu, W. J., Chang, Z., Xu, F., et al. (2021). Two new Inosperma (Inocybaceae) species with unexpected muscarine contents from tropical China. MycoKeys 85, 87–108. doi: 10.3897/mycokeys.85.71957
Deng, L. S., Yu, W. J., Zeng, N. K., Zhang, Y. Z., Wu, X. P., Li, H. J., et al. (2022). A new muscarine-containing Inosperma (Inocybaceae, Agaricales) species discovered from one poisoning incident occurring in tropical China. Front. Microbiol. 13:923435. doi: 10.3389/fmicb.2022.923435
Erland, S., and Taylor, A. F. S. (1999). “Resupinate ectomycorrhizal fungal genera,” in Ectomycorrhizal Fungi Key Genera in Profile, eds J. W. G. Cairney and S. M. Chambers (Berlin: Springer).
Farris, J. S., Kallersjo, M., Kluge, A. G., and Bult, C. (1994). Testing significance of incongruence. Cladistics 10, 315–319. doi: 10.1111/j.1096-0031.1994.tb00181.x
Felsenstein, J. (1985). Confidence intervals on phylogenetics: an approach using bootstrap. Evolution 39, 783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x
Guindon, S., and Gascuel, O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704. doi: 10.1080/10635150390235520
Hall, T. A. (1999). Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98.
Han, M. L., Chen, Y. Y., Shen, L. L., Song, J., Vlasák, J., Dai, Y. C., et al. (2016). Taxonomy and phylogeny of the brown-rot fungi: fomitopsis and its related genera. Fungal Divers. 80, 343–373. doi: 10.1007/s13225-016-0364-y
Harrison, K. A. (1964). New or little known north American stipitate hydnums. Can. J. Bot. 42, 1205–1233. doi: 10.1139/b64-116
Holec, J., and Kučera, T. (2018). Hydnoid fungi of the family Bankeraceae-their assemblages and vegetation ecology in Central Europe, Czech Republic. Fungal Ecol. 32, 40–48. doi: 10.1016/j.funeco.2017.11.007
Hrouda, P. (1999). Hydnaceous fungi of the Czech Republic and Slovakia. Czech Mycol. 51, 99–155. doi: 10.33585/cmy.51202
Ji, X., Zhou, J. L., Song, C. G., Xu, T. M., Wu, D. M., and Cui, B. K. (2022). Taxonomy, phylogeny and divergence times of Polyporus (Basidiomycota) and related genera. Mycosphere 13, 1–52. doi: 10.5943/mycosphere/13/1/1
Karsten, P. A. (1879). Symbolae ad mycologuam fennicam. Meddelanden Soc. Fauna Flora Fennica 5, 16–45.
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Larsson, K. H., Svantesson, S., Miscevic, D., Kljalg, U., and Larsson, E. (2019). Reassessment of the generic limits for Hydnellum and Sarcodon (Thelephorales, Basidiomycota). MycoKeys 54, 31–47. doi: 10.3897/mycokeys.54.35386
Lee, D., Boo, K. H., Lee, J. M., Viet, C. D., Quyen, N., Unno, T., et al. (2012). Anti-viral activity of Hydnellum concrescens, a medicinal mushroom. Afr. J. Biotechnol. 11, 15241–15245.
Liu, S., Han, M. L., Xu, T. M., Wang, Y., Wu, D. M., and Cui, B. K. (2021a). Taxonomy and phylogeny of the Fomitopsis pinicola complex with descriptions of six new species from east Asia. Front. Microbiol. 12:644979. doi: 10.3389/fmicb.2021.644979
Liu, S., Shen, L. L., Wang, Y., Xu, T. M., Gates, G., and Cui, B. K. (2021b). Species diversity and molecular phylogeny of Cyanosporus (Polyporales, Basidiomycota). Front. Microbiol. 12:631166. doi: 10.3389/fmicb.2021.631166
Liu, S., Song, C. G., Xu, T. M., Ji, X., Wu, D. M., and Cui, B. K. (2022a). Species diversity, molecular phylogeny and ecological habits of Fomitopsis (Polyporales, Basidiomycota). Front. Microbiol. 13:859411. doi: 10.3389/fmicb.2022.859411
Liu, S., Chen, Y. Y., Sun, Y. F., He, X. L., Song, C. G., Si, J., et al. (2022b). Systematic classification and phylogenetic relationships of the brown-rot fungi within the Polyporales. Fungal Divers. doi: 10.1007/s13225-022-00511-2 [Epub ahead of print].
Loizides, M., Alvarado, P., Assyov, B., Arnolds, E., and Moreau, P. A. (2016). Hydnellum dianthifolium sp. nov. Basidiomycota, Thelephorales, a new tooth-fungus from southern Europe with notes on H. concrescens and H. scrobiculatum. Phytotaxa 280, 23–35. doi: 10.11646/phytotaxa.280.1.2
Maas Geesteranus, R. A. (1971). Hydnaceous fungi of the eastern old world. Verh. Kon. Ned. Akad. Wetensch. Afd. Natuurk. 60:176.
Maas Geesteranus, R. A. (1975). The terrestrial hydnums of Europe. Verh. Kon. Ned. Akad. Wetensch. Afd. Natuurk. 65:127.
Maddison, W. P., and Maddison, D. R. (2017). Mesquite: a Modular System for Evolutionary Analysis. Version 3.2. Available online at: http://mesquiteproject.org (accessed August 15, 2022).
Mu, Y. H., Hu, Y. P., Wei, Y. L., and Yuan, H. S. (2020). Hydnaceous fungi of China 8. Morphological and molecular identification of three new species of Sarcodon and a new record from southwest China. MycoKeys 66, 83–103. doi: 10.3897/mycokeys.66.49910
Mu, Y. H., Wu, F., and Yuan, H. S. (2019). Hydnaceous fungi of China 7. morphological and molecular characterization of Phellodon subconfluens sp. nov. from temperate, deciduous forests. Phytotaxa 414, 280–288. doi: 10.11646/phytotaxa.414.6.2
Mu, Y. H., Yu, J. R., Cao, T., Wang, X. H., and Yuan, H. S. (2021). Multi-Gene phylogeny and taxonomy of Hydnellum (Bankeraceae, Basidiomycota) from China. J. Fungi 7:818. doi: 10.3390/jof7100818
Nylander, J. A. A. (2004). MrModeltest v2. Program. Distributed by the Author; Evolutionary Biology Center. Uppsala: Uppsala University.
Parfitt, D., Ainsworth, A. M., Simpson, D., Rogers, H. J., and Boddy, L. (2007). Molecular and morphological discrimination of stipitate hydnoids in the genera Hydnellum and Phellodon. Mycol. Res. 3, 761–777. doi: 10.1016/j.mycres.2007.05.003
Posada, D., and Crandall, K. A. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 14, 817–818. doi: 10.1093/bioinformatics/14.9.817
Ronquist, F., and Huelsenbeck, J. P. (2003). MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Shen, L. L., Wang, M., Zhou, J. L., Xing, J. H., Cui, B. K., and Dai, Y. C. (2019). Taxonomy and phylogeny of Postia. multi-gene phylogeny and taxonomy of the brown-rot fungi: postia (Polyporales, Basidiomycota) and related genera. Persoonia 42, 101–126. doi: 10.3767/persoonia.2019.42.05
Song, C. G., Chen, Y. Y., Liu, S., Xu, T. M., He, X. L., Wang, D., et al. (2022). A phylogenetic and taxonomic study on Phellodon (Bankeraceae, Thelephorales) from China. J. Fungi 8:429. doi: 10.3390/jof8050429
Song, C. G., Ji, X., Liu, S., He, X. L., and Cui, B. K. (2021). Taxonomy and molecular phylogeny of Phellodon (Thelephorales) with descriptions of four new species from Southwest China. Forests 12:932. doi: 10.3390/f12070932
Sun, Y. F., Costa-Rezende, D. H., Xing, J. H., Zhou, J. L., Zhang, B., Gibertoni, T. B., et al. (2020). Multi-gene phylogeny and taxonomy of Amauroderma s. lat. (Ganodermataceae). Persoonia 44, 206–239. doi: 10.3767/persoonia.2020.44.08
Sun, Y. F., Xing, J. H., He, X. L., Wu, D. M., Song, C. G., Liu, S., et al. (2022). Species diversity, systematic revision and molecular phylogeny of Ganodermataceae (Polyporales, Basidiomycota) with an emphasis on Chinese collections. Stud. Mycol. 101, 287–415. doi: 10.3114/sim.2022.101.05
Swofford, D. L. (2002). PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods); Version 4.0b10. Sunderland, MA: Sinauer Associates.
Wang, Y., Tuo, Y. L., Wu, D. M., Gao, N., Zhang, Z. H., Rao, G., et al. (2022). Exploring the relationships between four new species of boletoid fungi from Northern China and their related species. J. Fungi 8:218. doi: 10.3390/jof8030218
White, T., Bruns, T., Lee, S., Taylor, F., White, T., Lee, S. H., et al. (1990). Amplification and direct sequencing offungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 18, 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Keywords: Bankeraceae, ectomycorrhizal fungi, multi-gene phylogeny, stipitate hydnoids, taxonomy
Citation: Song C-G, Sun Y-F, Wu D-M, Gao N, Liu S, Xu T-M and Cui B-K (2022) Morphology and molecular phylogeny reveal five new species of Hydnellum (Bankeraceae, Thelephorales) from China. Front. Microbiol. 13:1049007. doi: 10.3389/fmicb.2022.1049007
Received: 20 September 2022; Accepted: 19 October 2022;
Published: 09 November 2022.
Edited by:
Ji-Chuan Kang, Guizhou University, ChinaReviewed by:
Samantha Chandranath Karunarathna, Qujing Normal University, ChinaCopyright © 2022 Song, Sun, Wu, Gao, Liu, Xu and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bao-Kai Cui, Y3VpYmFva2FpQGJqZnUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.