
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 08 December 2022
Sec. Evolutionary and Genomic Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1046777
This article is part of the Research Topic Insights on Fungal Diversity of Ascomycetes and Basidiomycetes: Taxonomy and Interaction with Their Host View all 24 articles
Megasporoporia sensu lato has recently been intensively studied in China and South America, and four independent clades representing four genera have been recognized phylogenetically. In this study, more samples, mostly from subtropical and tropical Asia, Oceania, and East Africa, are analyzed. A phylogeny based on a 4-gene dataset of sequences (ITS + nLSU + mtSSU + tef) has confirmed the presence of four genera in Megasporoporia sensu lato: Jorgewrightia, Mariorajchenbergia, Megasporia, and Megasporoporia sensu stricto. Six new species, Jorgewrightia austroasiana, Jorgewrightia irregularis, Jorgewrightia tenuis, Mariorajchenbergia subleucoplaca, Megasporia olivacea, and Megasporia sinuosa, are described based on morphology and phylogenetic analysis. Three new combinations are proposed, viz. Jorgewrightia kirkii, Mariorajchenbergia epitephra, and Mariorajchenbergia leucoplaca. To date, 36 species of Megasporoporia sensu lato are accepted and an identification key to these species is provided. In addition, the identification of Dichomitus amazonicus, Dichomitus cylindrosporus, and Megasporoporia hexagonoides is discussed.
Megasporoporia Ryvarden and J. E. Wright was established by Ryvarden et al. (1982). The genus is readily distinguished from other polypore genera by resupinate basidiocarps with large pores, a dimitic hyphal structure, usually the presence of hyphal pegs and dendrohyphidia, and hyaline, thin-walled, and big basidiospores. The species of the genus was found in tropical or subtropical Africa, America, and Asia (Ryvarden et al., 1982; Lira et al., 2021; Wang et al., 2021; Wu et al., 2022a). Li and Cui (2013) performed the first phylogenetic analysis of the genus based on ITS + nLSU sequences, demonstrating that Megasporoporia was a polyphyletic genus with three independent clades nested in Megasporoporia sensu lato. So, they proposed Megasporia B. K. Cui et al., Megasporoporia sensu stricto, and Megasporoporiella B. K. Cui et al. to represent these clades, although these three genera have similar morphology. Subsequently, Yuan et al. (2017) described three new species of Megasporia from China. Recently, Wang et al. (2021) made a comprehensive phylogenetic analysis based on a 4-gene dataset (ITS + nLSU + mtSSU + tef) from 21 species of Megasporoporia sensu lato, and they found a new clade, described four new species, and proposed two combinations. Lira et al. (2021) revised the definition of Megasporoporia sensu lato and proposed Jorgewrightia Gibertoni and C. R. S. Lira and Mariorajchenbergia Gibertoni and C. R. S. Lira. They also confirmed that the four clades nested in Megasporoporia sensu lato were Jorgewrightia, Mariorajchenbergia, Megasporia, and Megasporoporia sensu stricto. In addition, two new species were described and 20 combinations were proposed (Lira et al., 2021). Several new species have been confirmed recently in Megasporoporia sensu lato, especially from subtropical and tropical areas (Lira et al., 2021; Wang et al., 2021).
This study continues to research on the diversity and phylogeny of Megasporoporia sensu lato based on samples from subtropical and tropical Asia, Oceania, and East Africa. Six new species are described and three new combinations are proposed.
The voucher specimens are deposited at the herbaria of the Institute of Microbiology, Beijing Forestry University (BJFC); Universidade Federal de Pernambuco (URM); University of Oslo; the National Museum Prague of Czech Republic (PRM); and the private herbarium of Josef Vlasák (JV). Macro-morphological descriptions were based on field notes and herbarium specimens. Special color terms follow Anonymous (1969) and Petersen (1996). Microscopic analyses follow Wu et al. (2022b). The following abbreviations were used: CB = Cotton Blue, CB+ = cyanophilous in Cotton Blue, CB– = acyanophilous in Cotton Blue, IKI = Melzer's reagent, IKI– = neither amyloid nor dextrinoid, KOH = 2% potassium hydroxide, L = arithmetic average of all spore lengths, W = arithmetic average of all spore widths, Q = L/W ratios, and n = (a/b), where the number of spores (a) is measured from the given number of specimens (b).
The CTAB rapid plant genome extraction kit (Aidlab Biotechnologies Co., Ltd, Beijing) procedures were used to extract total genomic DNA from the fruiting bodies and for the polymerase chain reaction (PCR) according to the manufacturer's instructions with some modifications (Chen et al., 2016; Shen et al., 2019). The PCR primers for all genes are ITS5 (GGA AGT AAA AGT CGT AAC AAG G), ITS4 (TCC TCC GCT TAT TGA TAT GC), LR0R (ACC CGC TGA ACT TAA GC), LR7 (TAC TAC CAC CAA GAT CT), MS1 (CAG CAG TCA AGA ATA TTA GTC AAT G), MS2 (GCG GAT TAT CGA ATT AAA TAA C), 983F (GCY CCY GGH CAY CGT GAY TTY AT), and 1567R (ACH GTR CCR ATA CCA CCR ATC TT), according to White et al. (1990), Vilgalys and Hester (1990), and Rehner and Buckley (2005). The final PCR volume was 30 μl, which contained 1 μl of each primer, 1 μl extracted DNA, 12 μl ddH2O, and 15 μl 2 × EasyTaq PCR Supermix (TransGen Biotech Co., Ltd., Beijing, China). The PCR procedures for four genes followed Wang et al. (2021).
Nuclear ribosomal RNA genes of the known 36 species of Megasporoporia sensu lato were used to determine the phylogenetic position of the new species. Gene sequencing was performed at the Beijing Genomics Institute, and the newly-generated sequences were deposited in GenBank. Sequences generated for this study were aligned with additional sequences downloaded from GenBank. All newly generated sequences were deposited at GenBank (http://www.ncbi.nlm.nih.gov/) and are listed in Table 1. Clustal X (Thompson et al., 1997) and BioEdit (Hall, 1999) were used to align and collate these sequences. The data matrices were edited in Mesquite v3.04 software (Maddison and Maddison, 2021). The combined matrix was reconstructed for phylogenetic analysis as a 4-gene dataset (ITS + nLSU + mtSSU + tef). Sequences of Trametes hirsuta (Wulfen) Lloyd and T. ochracea (Pers.) Gilb. and Ryvarden were used as outgroups to root trees (Wang et al., 2021). The phylogenetic analysis used in this study followed the approach of Zhu et al. (2019) and Sun et al. (2020). Maximum parsimony (MP), maximum likelihood (ML), and Bayesian inference (BI) were employed to perform phylogenetic analysis.
Maximum Parsimony and bootstrap values (MP-BS) obtained from 1,000 replicates were performed using PAUP* version 4.0b10 (Swofford, 2002). All characters were equally weighted, and the gaps were treated as missing data. Trees were inferred using the heuristic search option with TBR branch swapping and 1,000 random sequence additions. Max trees were set to 5,000, branches of zero length were collapsed, and all parsimonious trees were saved. Clade robustness was assessed using bootstrap analysis with 1,000 replicates (Felsenstein, 1985). Descriptive tree statistics tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC), and homoplasy index (HI) were calculated for each maximum parsimonious tree generated (Farris, 1989; Farris et al., 1994).
Maximum likelihood (ML) was conducted with RAxML-HPC v. 8.2.3 (Stamatakis, 2014), involving 1,000 ML searches under the GTRGAMMA model, and only the maximum likelihood best tree from all searches was kept. In addition, 1,000 rapid bootstrap replicates were run with the GTRCAT model to assess the ML BS values (ML) of the nodes.
MrMODELTEST v. 2.3 (Posada and Crandall, 1998; Nylander, 2004) also was used to determine the best-fit evolution model for the combined datasets of ITS + nLSU and ITS + nLSU + mtSSU + tef sequences for estimating BI. BI was performed using MrBayes v. 3.2.6 (Ronquist and Huelsenbeck, 2003; Ronquist et al., 2012) with four simultaneous independent chains for two datasets, where 2 million generations were performed until the split deviation frequency value of <0.01 and were sampled every 100th generation. The first 25% of sampled trees were discarded as burn-in, while the remaining ones were used to calculate the Bayesian Posterior Probabilities (BPP) of the clades.
Branches that received bootstrap support for MP, ML, and BPP more than or equal to 50% (MP and ML) and 0.90 (BPP) were considered significantly supported (Figure 1). The phylogenetic tree was visualized with the program FigTree v. 1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/).
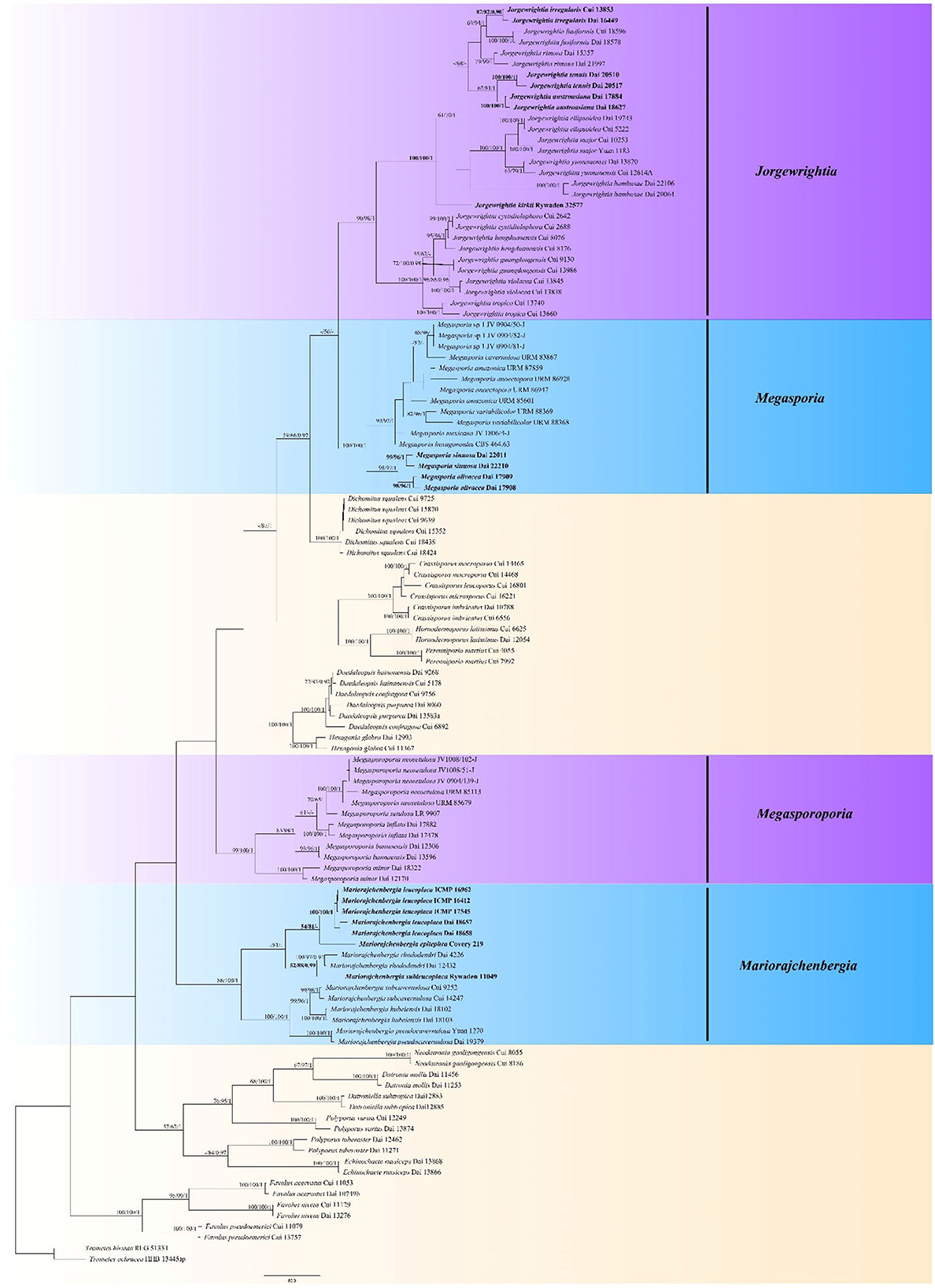
Figure 1. Phylogeny of Megasporoporia sensu lato and related species generated by Maximum Parsimony (MP) based on combined ITS + nLSU + mtSSU + tef sequences. Bootstrap supports for Maximum Parsimony (MP), Maximum Likelihood (ML), and Bayesian Posterior Probabilities (BPP) were not lower than 50% (MP and ML) and 0.90 (BPP) on the branches. The new species and new combinations are in bold.
Analyses were based on a combined dataset of 4-gene (ITS + nLSU + mtSSU + tef) sequences from 114 fungal collections representing 58 species. According to MrMODELTEST v.2.3, the most suitable model was GTR + I + G, lset nst = 6, rates = invgamma, and prset statefreqpr = Dirichlet (1,1,1,1). The alignment length of the dataset of each tree generated by MP analysis is 3,548 characters, of which 2,238 characters are constant, 1,146 characters are parsimony-informative, and other key data are TL = 5,018, CI = 0.392, RI = 0.772, RC = 0.302, and HI = 0.608. BI analysis generated a congruent topology with an average standard deviation of split frequencies = 0.007589 for MP and ML analyses. Thus, the topology from the MP tree is presented along with statistical values from the MP/ML/BPP algorithms (Figure 1).
The phylogeny shows that the samples of Megasporoporia sensu lato form four clades (Figure 1): Jorgewrightia (90% MP, 98% ML, 1.00 BPP), Mariorajchenbergia (86% MP, 100% ML, 1.00 BPP), Megasporia (100% MP, 100% ML, 1.00 BPP), and Megasporoporia sensu stricto (99% MP, 100% ML, 1.00 BPP).
Fifteen lineages are nested in the Jorgewrightia clade. Among them, three new lineages represent three new species: J. austroasiana sp. nov. (100% MP, 100% ML, 1.00 BPP), J. irregularis sp. nov. (87% MP, 82% ML, 0.90 BPP), and J. tenuis sp. nov. (100% MP, 100% ML, 1.00 BPP), and another lineage represents the taxon J. kirkii comb. nov. (100% MP, 100% ML, 1.00 BPP).
Seven lineages are nested in the Mariorajchenbergia clade. Among them, one lineage represents M. subleucoplaca sp. nov. (52% MP, 88% ML, 0.99 BPP), and two lineages represent M. epitephra comb. nov. (54% MP, 81% ML) and M. leucoplaca comb. nov. (100% MP, 100% ML, 1.00 BPP).
Nine lineages are nested in the Megasporia clade, and among them, two new lineages represent M. olivacea sp. nov. (98% MP, 96% ML, 1.00 BPP) and M. sinuosa sp. nov. (99% MP, 96% ML, 1.00 BPP).
Five lineages are nested in the Megasporoporia sensu stricto clade.
Jorgewrightia austroasiana Y. C. Dai, Yuan, Ya R. Wang and Y. D. Wu, sp. nov. Figures 2, 3
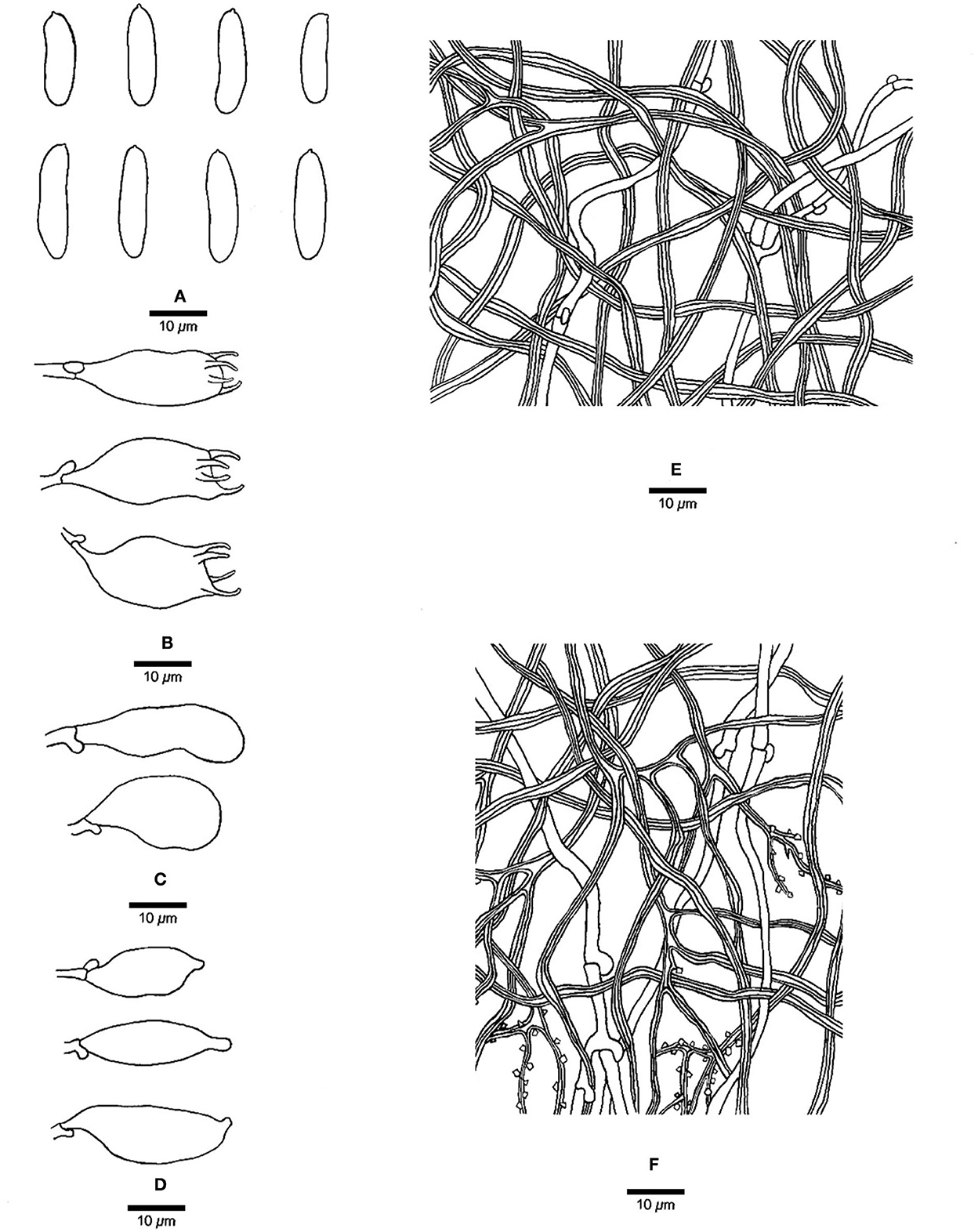
Figure 3. Microscopic structures of Jorgewrightia austroasiana (drawn from the holotype, Dai 17884). (A) basidiospores, (B) basidia, (C) basidioles, (D) cystidioles, (E) hyphae from subiculum, and (F) hyphae from tube trama.
MycoBank: 845309
Type: Singapore, Bukit Timah Nature Reserve, on a fallen angiosperm branch, 20 July 2017, Dai 17884 (holotype, BJFC025416!).
Etymology: austroasiana (Lat.) refers to the species being found in South Asia.
Basidiocarps annual, resupinate, adnate, corky, without odor or taste when fresh, becoming hard corky upon drying, up to 13 cm long, 2 cm wide, and 0.6 mm thick at the center; sterile margin thinning out, white when fresh, cream when dry, up to 1 mm wide. Pore surface white to cream when fresh, cream to buff when dry; pores round to angular, 3–3.5 per mm; dissepiments thick, entire; subiculum cream, corky, up to 0.2 mm thick; tubes cream, paler than subiculum, corky, up to 0.4 mm long. Hyphal system dimitic; generative hyphae with clamp connections; skeletal hyphae indextrinoid, CB+; tissues unchanged in KOH (not swollen). Subicular generative hyphae infrequent, hyaline, thin-walled, occasionally branched, 1.5–2 μm diameter; skeletal hyphae dominant, distinctly thick-walled with a narrow to medium lumen, occasionally branched, interwoven, 1.5–2.5 μm diameter. Tramal generative hyphae hyaline, thin-walled, occasionally branched, 1.5–3 μm diameter; skeletal hyphae dominant, thick-walled with a narrow lumen, frequently branched, mostly flexuous, interwoven, sometimes encrusted by crystals, 2–3.5 μm diameter. Dendrohyphidia absent. Hyphal pegs absent. Cystidia absent; cystidioles present, fusoid, thin-walled, smooth, 14–30 × 6.5–10 μm. Basidia more or less barrel-shaped, with four sterigmata and a basal clamp connection, 24–28 × 9–12 μm; basidioles in shape similar to basidia. Small tetrahedric or polyhedric crystals frequent among hymenium and trama. Basidiospores cylindrical, slightly curved, hyaline, thin-walled, smooth, IKI–, CB–, (14.5–) 15.0–19.5 (−20.0) × (3.2–) 3.5–6.0 (−6.5) μm, L = 16.27 μm, W = 4.50 μm, Q =3.54–3.74 (n = 60/2).
Additional materials (paratypes) examined: Malaysia, Selangor, Kota Damansara, Community Forest Reserve, on a fallen angiosperm branch, 17 April 2018, Dai 18627 (BJFC026915!).
Notes: Phylogenetically, Jorgewrightia austroasiana is related to J. rimosa, J, tenuis, J. fusiformis, and J. irregularis (Figure 1). However, J. rimosa differs from J. austroasiana by its dextrinoid skeletal hyphae and the presence of dendrohyphidia (Yuan et al., 2017). J. fusiformis and J. tenuis are readily distinguished from J. austroasiana by their fusiform basidiospores (Wang et al., 2021), J. irregularis differs from J. austroasiana by its bigger pores (0.5–1 per mm vs. 3–3.5 per mm) and the presence of dendrohyphidia and hyphal pegs.
Jorgewrightia irregularis Y. C. Dai, Yuan, Ya R. Wang and Y. D. Wu, sp. nov. Figures 4, 5
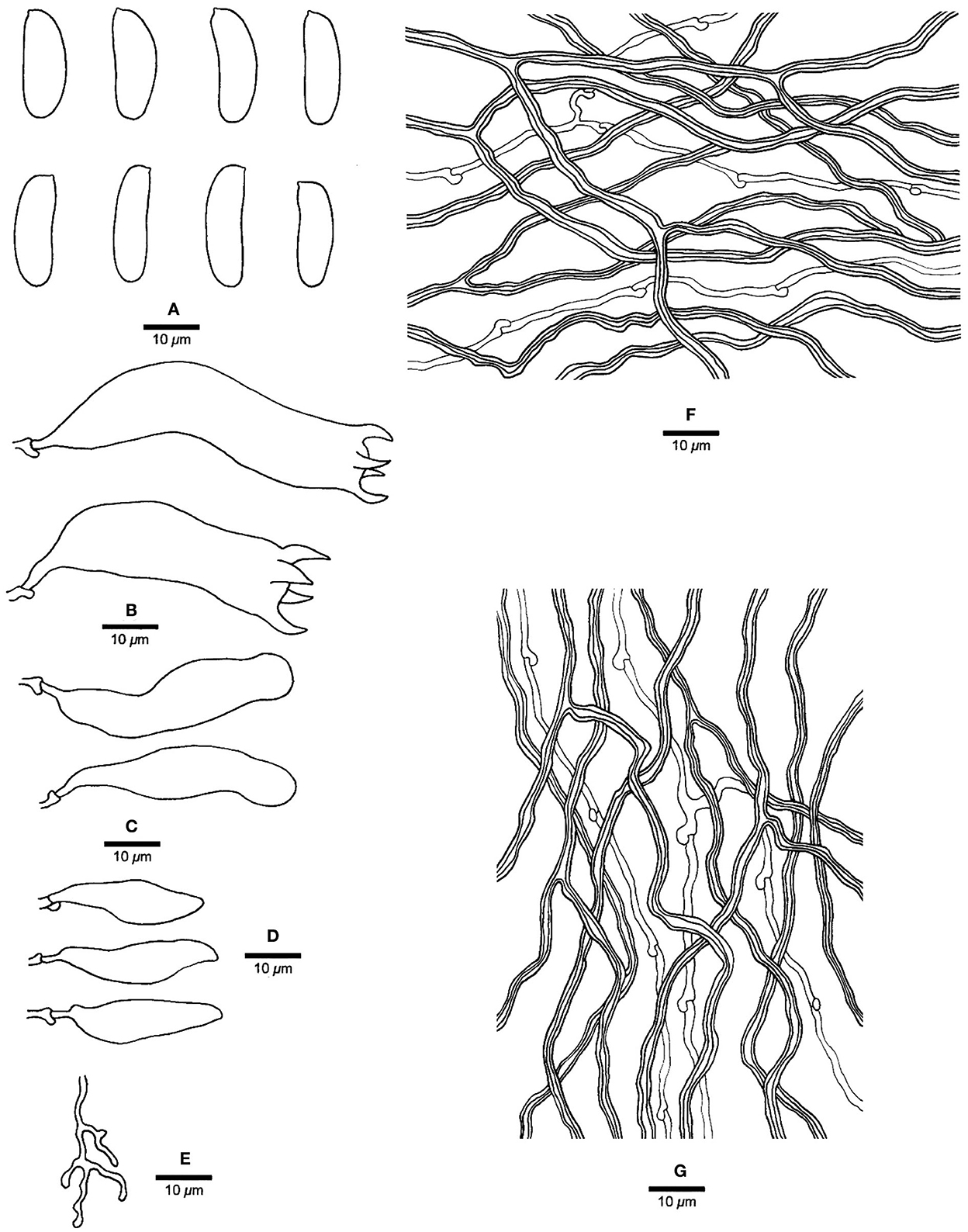
Figure 5. Microscopic structures of Jorgewrightia irregularis (drawn from holotype, Dai 13853). (A) basidiospores, (B) basidia, (C) basidioles, (D) cystidioles, (E) dendrohyphidia, (F) hyphae from subiculum, and (G) hyphae from tube trama.
MycoBank: 845312
Type: China, Hainan Prov., Baisha County, Yinggeling Nature Reserve, on a fallen angiosperm branch, 17 June 2016, Cui 13853 (holotype, BJFC028719!).
Etymology: irregularis (Lat.) refers to irregular pores of the basidiocarps.
Basidiocarps annual, resupinate, adnate, without odor or taste when fresh, becoming hard corky upon drying, up to 6 cm long, 1.5 cm wide, and 1.3 mm thick at the center; sterile margin cream when juvenile, brownish with age, up to 0.6 mm wide. Pore surface white to cream when fresh, buff to honey when dry; pores angular when juvenile, irregular with age, e.g., hexagonoid, sinuous or split, 0.5–1 per mm; dissepiments thick, entire to lacerate; subiculum pale cream, corky, up to 0.4 mm long; tubes buff, corky, up to 0.9 mm long. Hyphal system dimitic; generative hyphae with clamp connections; skeletal hyphae indextrinoid, CB+; tissues unchanged in KOH (not swollen). Subicular generative hyphae infrequent, hyaline, thin-walled, moderately branched, 1–1.2 μm diameter; skeletal hyphae dominant, thick-walled with a narrow to medium lumen, moderately branched, strongly flexuous, interwoven, 2.5–3 μm diameter. Tramal generative hyphae infrequent, hyaline, thin-walled, moderately branched, 1.2–1.5 μm diameter; skeletal hyphae dominant, thick-walled with a narrow lumen, moderately branched, strongly flexuous, interwoven, 2.2–2.5 μm diameter. Dendrohyphidia present. Hyphal pegs present. Cystidia absent; cystidioles present, subulate or ventricose, thin-walled, smooth, 25.5–32.5 × 6.5–8 μm. Basidia more or less clavate, usually constricted in middle, with four sterigmata and a basal clamp connection, 42–51 × 8–13 μm; basidioles in shape similar to basidia, but distinctly smaller. Small tetrahedric or polyhedric crystals present among hymenium and trama. Basidiospores cylindrical, slightly curved, hyaline, thin-walled, smooth, IKI–, CB–, (17–) 17.5–21.2 (−21.5) × (4.5–) 5–6.2 (−6.5) μm, L = 19.22 μm, W = 5.86 μm, Q = 3.22–3.34 (n = 60/2).
Additional materials (paratypes) examined: China, Hainan Prov., Ledong County, Jianfengling Nature Reserve, 11 May 2009, Cui 6592 (BJFC004445!); Qiongzhong County, Limushan Forest Park, on a fallen angiosperm branch, 8 June 2016, Dai 16449 (BJFC022566!).
Notes: Phylogenetically, Jorgewrightia irregularis is related to J. fusiformis (Figure 1), but the latter has fusiform basidiospores and lacks hyphal pegs (Wang et al., 2021).
Jorgewrightia tenuis Y. C. Dai, Yuan Yuan, Ya R. Wang and Y. D. Wu, sp. nov. Figures 6, 7
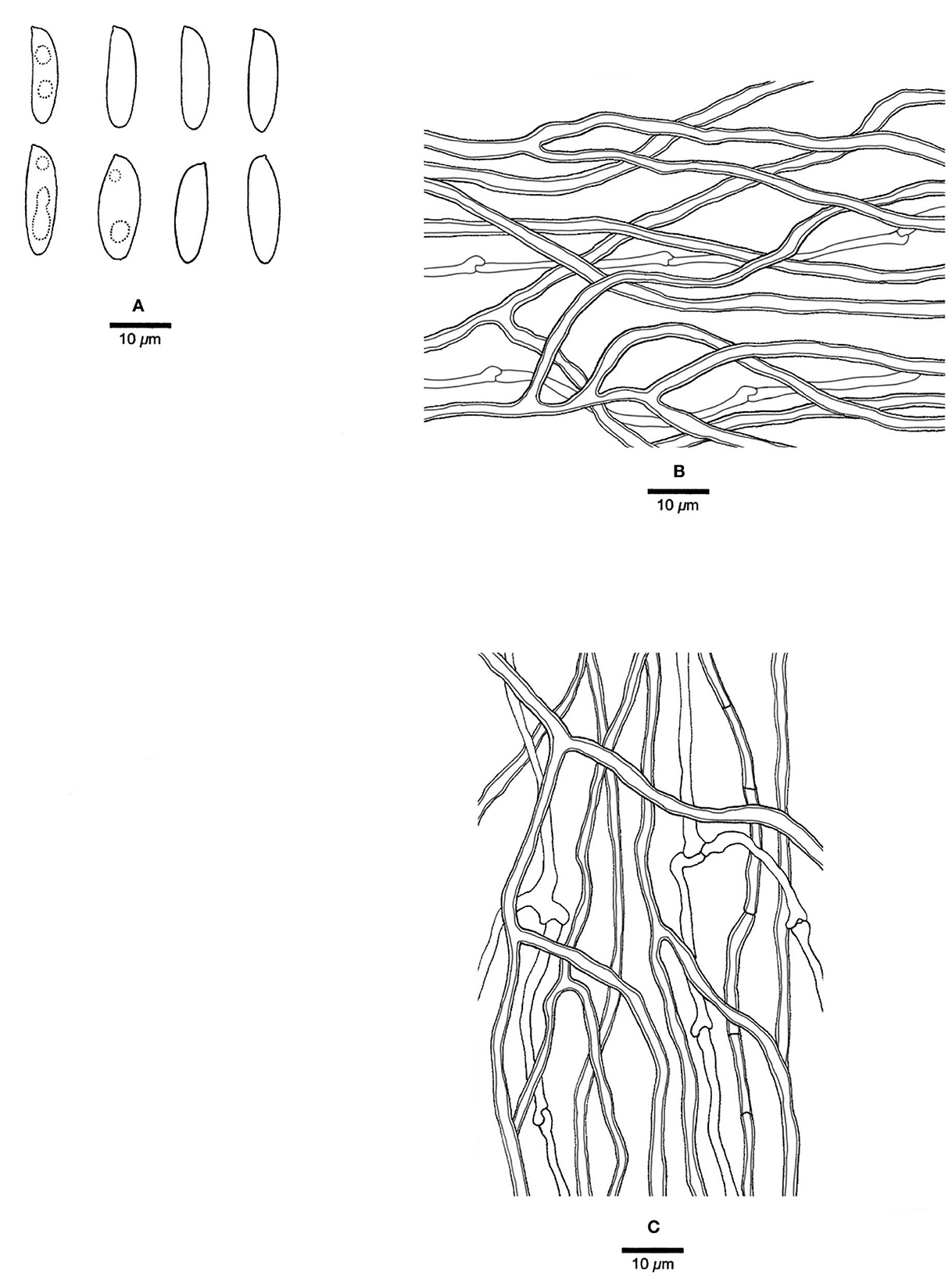
Figure 7. Microscopic structures of Jorgewrightia tenuis (drawn from holotype, Dai 20510). (A) basidiospores, (B) hyphae from subiculum, and (C) hyphae from tube trama.
MycoBank: 845313
Type: China, Yunnan Prov., Mengla County, Tropical Rain Forest Valley, on dead bamboo, 18 August 2019, Dai 20510 (holotype, BJFC032178!).
Etymology: tenuis (Lat.) refers to the extremely thin basidiocarps.
Basidiocarps annual, resupinate, adnate, corky, without odor or taste when fresh, becoming hard corky upon drying, up to 19 cm long, 3 cm wide, and 0.18 mm thick at the center; sterile margin very narrow to almost lacking. Pore surface cream when fresh, buff when dry; pores angular, 3–3.5 per mm; dissepiments thin, entire; subiculum pale cream, corky, extremely thin to almost absent; tubes cream, corky, up to 0.18 mm long. Hyphal system dimitic; generative hyphae with clamp connections; skeletal hyphae sometimes simple septate, weakly dextrinoid, CB+; tissues unchanged in KOH (not swollen). Subicular generative hyphae hyaline, thin-walled, unbranched, 1.5–1.8 μm diameter; skeletal hyphae dominant, thick-walled with a medium to wide lumen, moderately branched, flexuous, interwoven, 2–2.5 μm diameter. Tramal generative hyphae frequent, hyaline, thin-walled, moderately branched, 1.2–1.5 μm diameter; skeletal hyphae dominant, thick-walled with a medium to wide lumen, moderately branched, flexuous, interwoven, 1.5–2.5 μm diameter. Dendrohyphidia absent. Hyphal pegs absent. Cystidia absent; cystidioles absent. Basidia not seen. Small tetrahedric or polyhedric crystals frequent among hymenium and trama. Basidiospores fusiform, hyaline, thin-walled, smooth, sometimes with one or two small guttules, IKI–, CB–, (15–) 16.5–17 (−17.2) × (4.2–) 4.8–5.5 (−5.8) μm, L = 16.47 μm, W = 5.02 μm, Q = 3.28–3.42 (n = 60/2).
Additional materials (paratypes) examined: China, Yunnan Prov., Mengla County, Tropical Rain Forest Valley, on dead bamboo, 18 August 2019, Dai 20517 (BJFC032185!).
Notes: For the phylogenetic relationships of Jorgewrightia tenuis and other species, refer to the notes of J. austroasiana. Morphologically, J. tenuis resembles J. bambusae and J. rimosa by the adnate and extremely thin basidiocarps, but J. bambusae has thick-walled and ellipsoid basidiospores, and J. rimosa has dendrohyphidia (Yuan et al., 2017; Wang et al., 2021). In addition, J. tenuis is similar to J. fusiformis, both present with fusiform basidiospores, but J. fusiformis has indextrinoid skeletal hyphae and dendrohyphidia (Wang et al., 2021).
Jorgewrightia kirkii (Masuka and Ryvarden) Y. C. Dai, Yuan Yuan, Ya R. Wang and Y. D. Wu, comb. nov. Figure 8
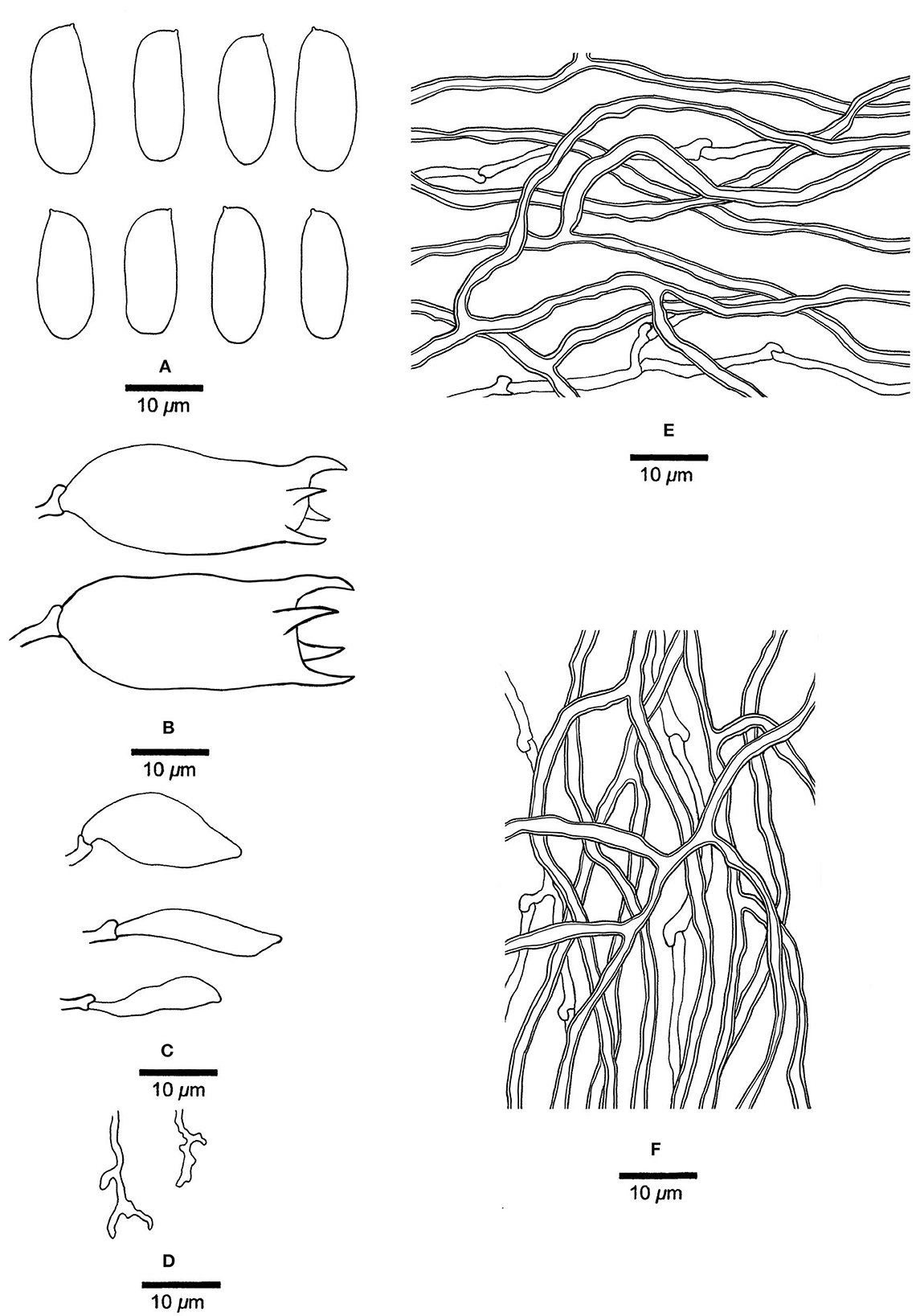
Figure 8. Microscopic structures of Jorgewrightia kirkii (drawn from Ryvarden 32577). (A) Basidiospores. (B) Basidia. (C) Cystidioles. (D) Dendrohyphidia. (E) Hyphae from subiculum. (F) Hyphae from tube trama.
MycoBank: 845318
Basionym: Dichomitus kirkii Masuka and Ryvarden, Mycological Research 103 (9): 1129 (1999).
Basidiocarps annual, resupinate, corky when dry, around 1 mm thick at the center; sterile margin very narrow to almost lacking. Pore surface clay buff to isabelline when dry; pores round, 1.5–2 per mm; dissepiments thin, entire; subiculum clay buff, corky, up to 0.2 mm thick; tubes concolorous with the pore surface, corky, up to 0.8 mm long. Hyphal system dimitic; generative hyphae with clamp connections; skeletal hyphae indextrinoid, CB+; tissues unchanged in KOH (not swollen). Subicular generative hyphae hyaline, thin-walled, occasionally branched, 1.5–1.8 μm diameter; skeletal hyphae dominant, thick-walled with a wide lumen, moderately branched, flexuous, interwoven, 2.5–3 μm diameter. Tramal generative hyphae, hyaline, thin-walled, moderately branched, 1.2–1.5 μm diameter; skeletal hyphae dominant, thick-walled with a wide lumen, frequently branched, flexuous, interwoven, 1.5–2.5 μm diameter. Dendrohyphidia present. Hyphal pegs absent. Cystidia absent; cystidioles present, fusoid to ventricose, thin-walled, smooth, 23–28 × 6.5–15 μm. Basidia barrel-shaped, with four sterigmata and a basal clamp connection, 30–40 × 16–18 μm; basidioles in shape similar to basidia. Small tetrahedric or polyhedric crystals frequent among hymenium and trama. Basidiospores cylindrical, hyaline, thin-walled, smooth, IKI–, CB–, (20.2–) 21–23.5 (−24) × (7–) 7.5–8.8 (−9) μm, L = 21.86 μm, W = 8.1 μm, Q = 2.70 (n = 30/1).
Materials examined: Zimbabwe, Mashonaland, Binga Forest East of Harare, on an angiosperm wood, 27 January 1993, Ryvarden 32577 (O, dupl. BJFC002897!); Ryvarden 33631 (holotype, O).
Notes: Jorgewrightia kirkii was originally described as Dichomitus kirkii Masuka and Ryvarden from Africa (Masuka and Ryvarden, 1999). It is extremely large basidiospores (21–23.5 × 7.5–8.8 μm) that are unique in Megasporoporia sensu lato. Our phylogeny (Figure 1) shows that the species is nested in Jorgewrightia clade with robust support (100% MP, 100% ML, 1.00 BPP). Hence, the above combination is proposed.
Mariorajchenbergia epitephra (Berk.) Y. C. Dai, Yuan Yuan, Ya R. Wang and Y. D. Wu, comb. nov. Figure 9
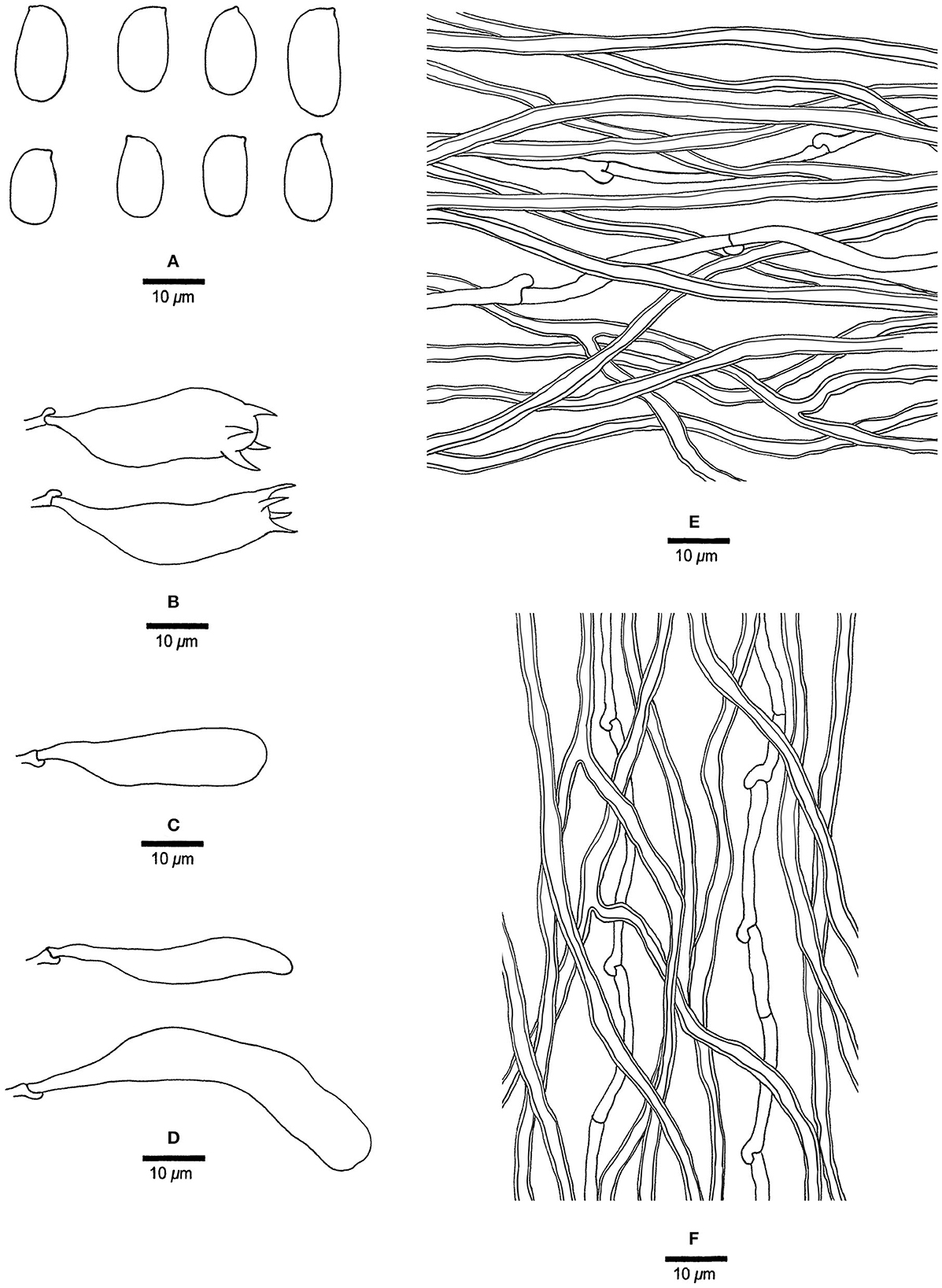
Figure 9. Microscopic structures of Mariorajchenbergia epitephra (drawn from Coveny 219). (A) Basidiospores. (B) Basidia. (C) A basidiole. (D) Cystidioles. (E) Hyphae from subiculum. (F) Hyphae from tube trama.
MycoBank: 845319
Basionym: Trametes epitephra Berk., J. Linn. Soc., Bot. 13: 165 (1872).
= Dichomitus epitephrus (Berk.) Ryvarden, Mycotaxon 20 (2): 339 (1984).
Basidiocarps biennial, pileate, solitary, attached by a broad lateral base. Pilei ungulate, hard corky when dry, projecting up to 5 mm, 7 mm wide, and 5.5 mm thick at the base. Pore surface cream to buff when dry; pores round to sinuous, 1–2 per mm; dissepiments thick, entire; subiculum pale buff, corky, up to 0.5 mm thick; tubes concolorous with the pore surface, corky, up to 5 mm long. Hyphal system dimitic; generative hyphae with clamp connections; skeletal hyphae indextrinoid, CB+; tissues become slightly swollen in KOH. Subicular generative hyphae hyaline, thin-walled, unbranched, 1.8–2 μm diameter; skeletal hyphae dominant, thick-walled with a wide lumen, infrequently branched, flexuous, interwoven, 2–2.8 μm diameter. Tramal generative hyphae hyaline, thin-walled, unbranched, 1.8–2.5 μm diameter; skeletal hyphae dominant, thick-walled with a medium to wide lumen, infrequently branched, flexuous, interwoven, 2.5–3 μm diameter. Dendrohyphidia absent. Hyphal pegs present. Cystidia absent; cystidioles present, fusoid to clavate, thin-walled, smooth, 26.5–50.5 × 6–13.2 μm. Basidia clavate, with four sterigmata and a basal clamp connection, 28.5–35.5 × 7.5–10.2 μm; basidioles in shape similar to basidia, but slightly smaller. Small tetrahedric crystals frequent among hymenium and trama. Basidiospores broadly ellipsoid, hyaline, thin-walled, smooth, IKI–, CB–, 9.5–16.5 × 7–9 μm, L = 13.28 μm, W =7.8 μm, Q = 1.7 (n = 15/1).
Material examined: Australia, New South Wales, Blackett, on Eucalyptus moluccana, 10 July 1983, Coveny 219 (H, JV, dupl. BJFC002895!).
Notes: Mariorajchenbergia epitephra was originally described as Trametes epitephra from South Australia. Our studied sample fits the original description of Trametes epitephra (Berkeley, 1872; Cunningham, 1965). Phylogenetically, the species is nested in the Mariorajchenbergia clade. Therefore, the above combination is proposed. The species has pileate basidiocarps that are unique to Megasporoporia sensu lato.
Mariorajchenbergia leucoplaca (Berk.) Y. C. Dai and P. K. Buchanan, comb. nov.
MycoBank: 845320
Basionym: Polyporus leucoplacus Berk., Fl. N. Zealand 2: 180 (1855).
= Dichomitus leucoplacus (Berk.) Ryvarden, Norweg. J. Bot. 24: 222 (1977).
= Megasporoporiella australiae Y. C. Dai, Yuan Yuan and Ya. R. Wang, in Wang, Wu, Vlasák, Yuan and Dai, Mycosphere 12 (1): 1027 (2021).
Megasporoporiella australiae was recently described in Australia (Wang et al., 2021). However, its vouchers and samples of Polyporus leucoplacus from New Zealand are nested together in a subclade with robust support in the Mariorajchenbergia clade. In addition, the morphology of these two taxa is similar (Wang et al., 2021), and the former becomes a synonym of the latter, with the above combination proposed.
Materials examined: Australia, Melbourne, Dandenong Ranges Botanic Garden, on a dead tree of Rhododendron, 12 May 2018, Y. C. Dai 18657 (BJFC027125!, holotype of Megasporoporiella australiae). New Zealand, Auckland, Waitakere Ranges, on a fallen wood, 11 April 1989, P. K. Buchanan 89/037 (ICMP 16412); Northland, William Hewett Reserve, on decaying wood, 2007, B. C. Paulus BCP3987 (ICMP 16962); Taupo, Kurua Reserve, Owhango, 3 Oct 2007, on a decaying branch (probably Dacrydium cupressinum), B. C. Paulus AOD348 (ICMP 17545).
Mariorajchenbergia subleucoplaca Y. C. Dai and P. K. Buchanan, sp. nov. Figure 10
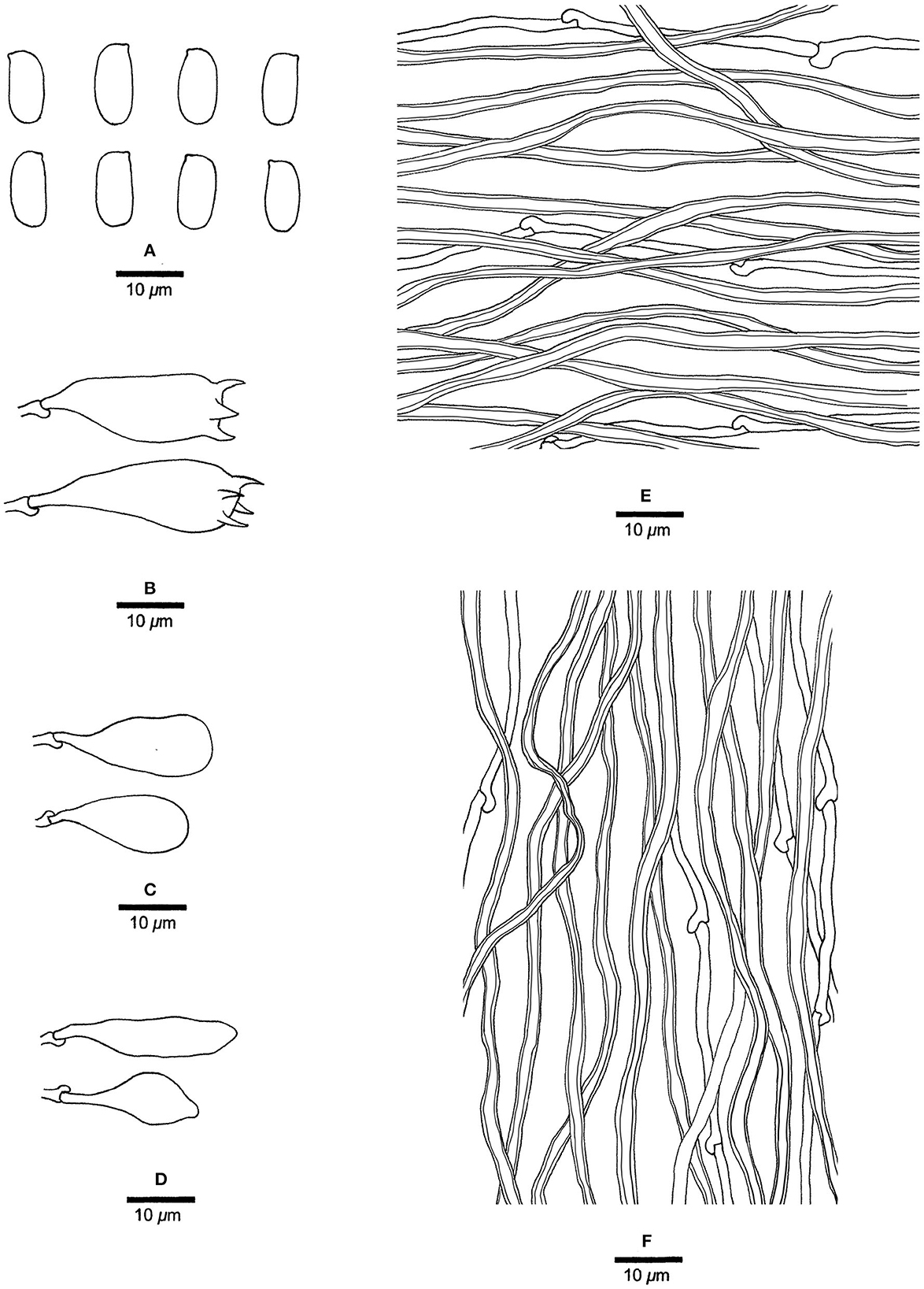
Figure 10. Microscopic structures of Mariorajchenbergia subleucoplaca (drawn from Ryvarden 11049). (A) Basidiospores. (B) Basidia. (C) Basidioles. (D) Cystidioles. (E) Hyphae from subiculum. (F) Hyphae from tube trama.
MycoBank: 845315
Type: Tanzania, Morogoro, Uluguri Mts., Morning Side Nature Reserve, 24 February 1973, Ryvarden 11049 (holotype, O; isotype, BJFC002898!).
Etymology: subleucoplaca (Lat.) refers to the species that somewhat resemble Mariorajchenbergia leucoplaca.
Basidiocarps annual, resupinate, corky when dry, around 0.4 mm thick at the center; sterile margin distinct, white, up to 0.2 mm wide. Pore surface cream to buff when dry; pores round, 4–5 per mm; dissepiments thick, entire; subiculum pale cream, corky, up to 0.3 mm thick; tubes concolorous with the pore surface, corky, up to 0.1 mm long. Hyphal system dimitic; generative hyphae with clamp connections; skeletal hyphae indextrinoid, CB+; tissues unchanged in KOH (not swollen). Subicular generative hyphae infrequent, hyaline, thin-walled, unbranched, 1.2–1.8 μm diameter; skeletal hyphae dominant, thick-walled with narrow to wide lumen, unbranched, flexuous, interwoven, 2.5–3 μm diameter. Tramal generative frequent, hyphae hyaline, thin-walled, unbranched, 1.2–1.5 μm diameter; skeletal hyphae dominant, thick-walled with a medium to wide lumen, unbranched, interwoven, 2.5–3.5 μm diameter. Dendrohyphidia absent. Hyphal pegs absent. Cystidia absent; cystidioles present, subulate or ventricose, thin-walled, smooth, 22.5–26.5 × 5.5–13.5 μm. Basidia more or less pyriform, with four sterigmata and a basal clamp connection, 23.2–26.5 × 9.5–11 μm; basidioles in shape similar to basidia, but distinctly smaller. Small tetrahedric or polyhedric crystals frequent among hymenium and trama. Basidiospores oblong ellipsoid, hyaline, thin-walled, smooth, IKI–, CB–, (10–) 11–12 × (4.5–) 5–6 μm, L = 10.94 μm, W = 5.2 μm, Q = 2.10 (n = 30/1).
Notes: Mariorajchenbergia leucoplaca was originally described as Polyporus leucoplacus from New Zealand [Berkeley, 1855; = Dichomitus leucoplacus (Berk.) Ryvarden (Ryvarden, 1977)]. Masuka and Ryvarden (1999) identified Tanzanian samples as D. leucoplacus. Our studied specimen (Ryvarden 11049) was also collected in Tanzania, and phylogenetically (Figure 1) is nested in the Mariorajchenbergia clade with robust support (53% MP, 91% ML, 0.98 BPP). Samples labeled as D. leucoplaca from Africa and New Zealand, therefore, nested into two independent lineages. Thus, we describe the African samples as a new species. It differs from M.leucoplaca by its smaller pores (4–5 per mm vs. 2–4 per mm) and shorter basidiospores (11–12 × 5–6 μm vs. 11.8–15 × 4–6 μm, Wang et al., 2021).
Megasporia sinuosa Y. C. Dai, Yuan Yuan, Ya R. Wang and Y. D. Wu, sp. nov. Figures 11, 12
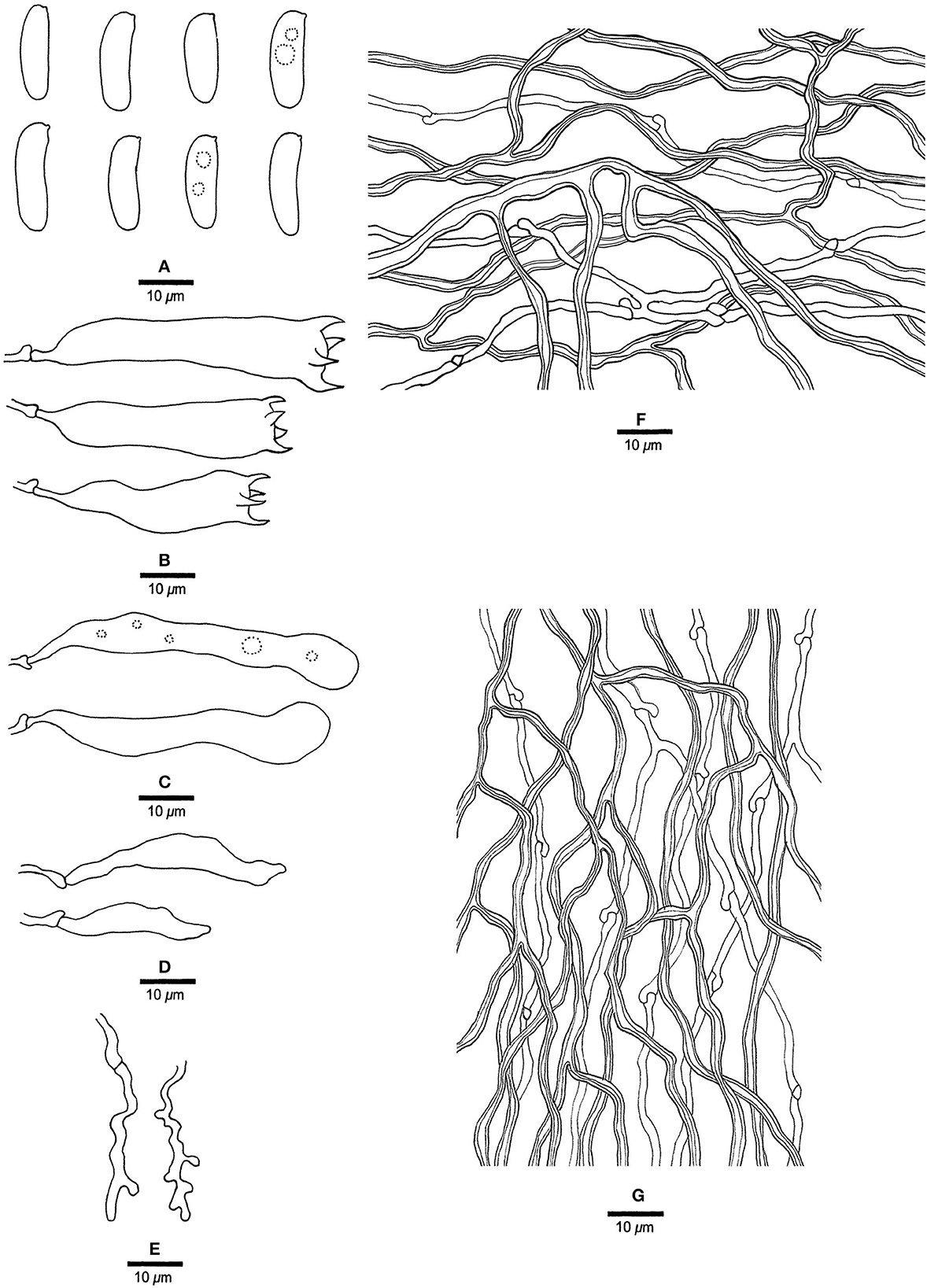
Figure 12. Microscopic structures of Megasporia sinuosa (drawn from holotype, Dai 22210). (A) Basidiospores. (B) Basidia. (C) Basidioles. (D) Cystidioles. (E) Dendrohyphidia. (F) Hyphae from subiculum. (G) Hyphae from tube trama.
MycoBank: 845316
Type: China, Hainan Prov., Qiongzhong County, Limushan Forest Park, on a fallen angiosperm branch, 31 March 2021, Dai 22210 (holotype, BJFC036801!).
Etymology: sinuosa (Lat.) refers to the species having sinuous pores.
Basidiocarps annual, resupinate, adnate, corky, without odor or taste when fresh, becoming hard corky upon drying, up to 3 cm long, 2 cm wide, and 0.6 mm thick at the center; sterile margin distinct, pale buff, up to 1.5 mm wide. Pore surface white to cream when fresh, cream to buff when dry; pores angular to sinuous, 1.5–2 per mm; dissepiments thick, entire to lacerate; subiculum pale buff, corky, up to 0.3 mm thick; tubes cream, corky, up to 0.3 mm long. Hyphal system dimitic; generative hyphae with clamp connections; skeletal hyphae strongly dextrinoid, CB+, slightly swollen in KOH. Subicular generative hyphae hyaline, thin-walled, occasionally branched, 1.5–2 μm diameter; skeletal hyphae dominant, thick-walled with a narrow to medium lumen, frequently branched, strongly flexuous, interwoven, 1.8–2.5 μm diameter. Tramal generative hyphae frequent, hyaline, thin-walled, moderately branched, 1.5–1.8 μm diameter; skeletal hyphae dominant, thick-walled with a narrow to medium lumen, frequently branched, strongly flexuous, interwoven, 1.5–2.8 μm diameter. Dendrohyphidia present. Hyphal pegs absent. Cystidia absent; cystidioles present, subulate or ventricose, thin-walled, smooth, 34.5–38.5 × 4.5–6.5 μm. Basidia clavate, usually constricted in middle, with four sterigmata and a basal clamp connection, 20–50.2 × 7–9.2 μm; basidioles similar to basidia, sometimes with a few guttules. Small tetrahedric or polyhedric crystals frequent among hymenium and trama. Basidiospores cylindrical to allantoid, hyaline, thin-walled, smooth, sometimes with one to two small guttules, IKI–, CB–, (15–) 15.2–16.8 (−17.2) × (4.5–) 4.8–5 μm, L = 16.3 μm, W = 4.87 μm, Q = 3.4 (n = 30/1).
Additional materials (paratypes) examined: China, Hainan Prov., Lingshui County, Diaoluoshan Forest Park, on a fallen angiosperm branch, 8 November 2020, Dai 22010 (BJFC035906!), Dai 22011 (BJFC035907!).
Notes: Phylogenetically, Megasporia sinuosa is closely related to M. olivacea (Figure 1), but the latter differs from the former by darker pore surface (deep olive vs. cream to buff), presence of hyphal pegs, and wider basidiospores (5.5–6.5 μm vs. 4.8–5 μm).
Megasporia olivacea Y. C. Dai, Yuan Yuan, Ya R. Wang and Y. D. Wu, sp. nov. Figures 13, 14
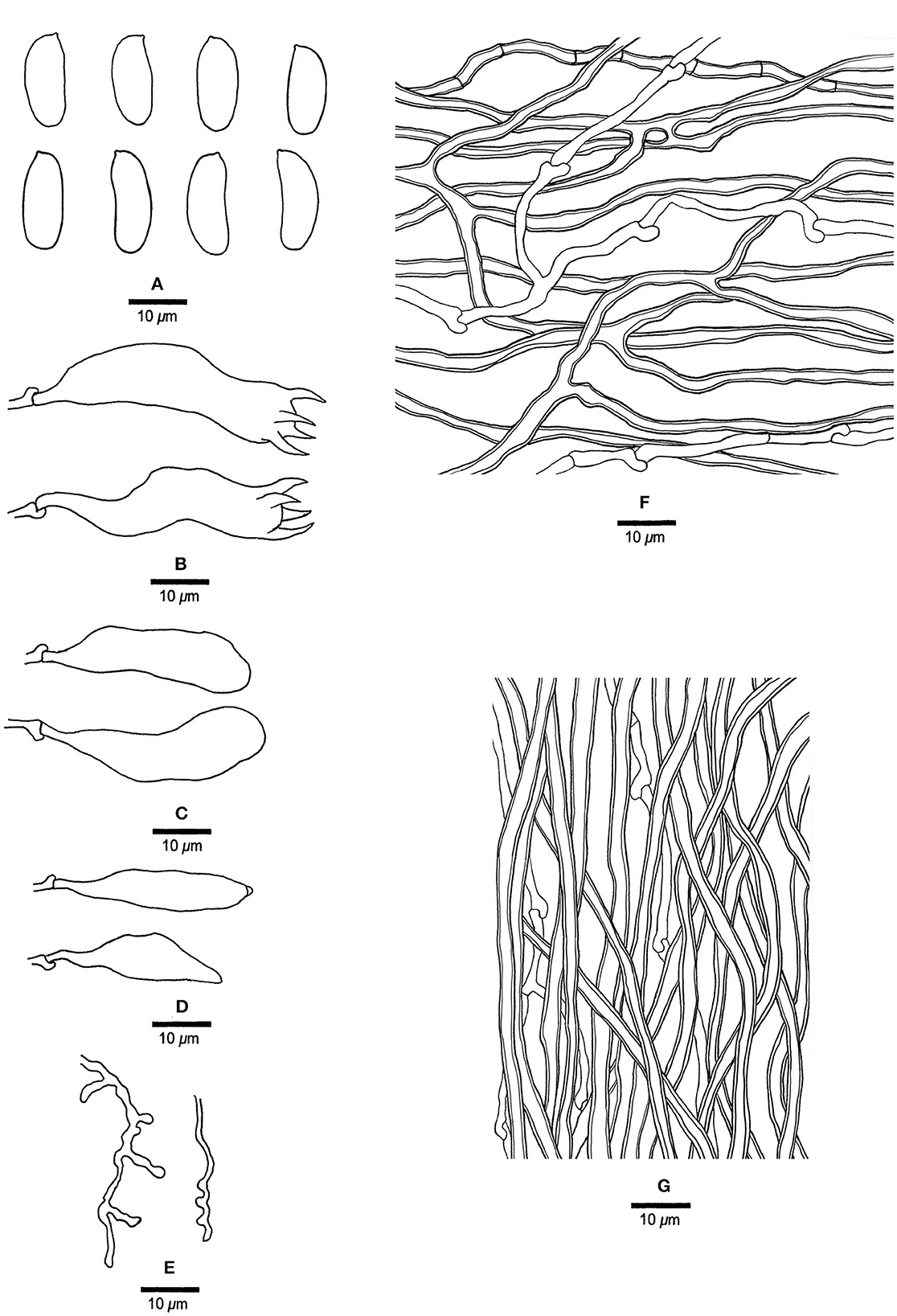
Figure 14. Microscopic structures of Megasporia olivacea (drawn from holotype, Dai 17908). (A) Basidiospores. (B) Basidia. (C) Basidioles. (D) Cystidioles. (E) Dendrohyphidia. (F) Hyphae from subiculum. (G) Hyphae from tube trama.
MycoBank: 845317
Type: China, Hubei Prov., Wufeng County, Chaibuxi Geopark, on the dead tree of Quercus, 14 August 2017, Dai 17908 (holotype, BJFC025437!).
Etymology: olivacea (Lat.), refers to the species with a deeply olivaceous pore surface when dry.
Basidiocarps annual, resupinate, adnate, hard corky, without odor or taste when fresh, becoming hard corky upon drying, up to 14 cm long, 4.5 cm wide, and 4 mm thick at the center; sterile margin distinct, cream, up to 1.5 mm wide. Pore surface white to cream when fresh, deep olive when dry; pores angular, 0.5–1 per mm; dissepiments thin, entire; subiculum grayish brown, hard corky, up to 0.2 mm thick; tubes grayish buff, hard corky, up to 3.8 mm long. Hyphal system dimitic; generative hyphae with clamp connections; skeletal hyphae sometimes simple septate, moderately dextrinoid, CB+, strongly swollen in KOH. Subicular generative hyphae hyaline, thin-walled, occasionally branched, 1.8–2.5 μm diameter; skeletal hyphae dominant, thick-walled with a narrow to medium lumen, moderately branched, flexuous, interwoven, 2–3.5 μm diameter. Tramal generative hyphae hyaline, thin-walled, occasionally branched, 2–2.5 μm diameter; skeletal hyphae dominant, thick-walled with a narrow to wide lumen, unbranched, flexuous, interwoven, 2–3 μm diameter. Dendrohyphidia present. Hyphal pegs present. Cystidia absent. Cystidioles present, subulate or ventricose, thin-walled, smooth, 26–31 × 5.5–6.5 μm. Basidia clavate, usually constricted in middle, with four sterigmata and a basal clamp connection, 38–44 × 9–10 μm; basidioles in shape similar to basidia, but distinctly smaller. Small tetrahedral crystals frequent among hymenium and trama. Basidiospores cylindrical, some slightly curved, hyaline, thin-walled, smooth, IKI–, CB–, (14.5–) 15.2–17 (−18.2) × (4.5–) 5.5–6.5 (−7) μm, L = 16.4 μm, W = 5.69 μm, Q = 2.73–2.95 (n = 60/2).
Additional materials (paratypes) examined: China, Hubei Prov., Wufeng County, Chaibuxi Geopark, on a dead tree of Quercus, 14 August 2017, Dai 17909 (BJFC025438!).
Notes: Morphologically, Megasporia olivacea has a deep olive pore surface when dry which is unique to Megasporoporia sensu lato.
Megasporia sp. 1 Figures 15, 16
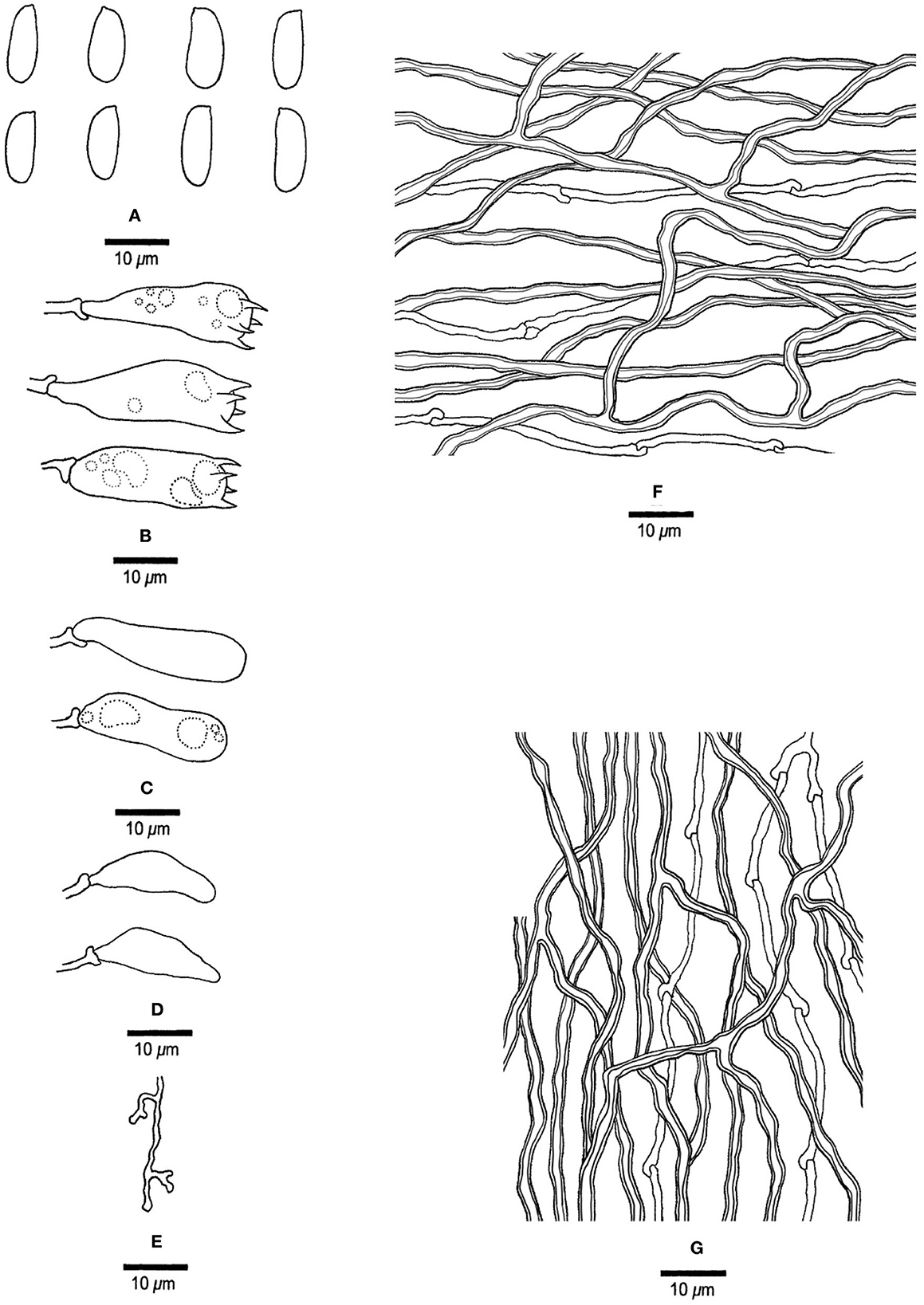
Figure 16. Microscopic structures of Megasporia sp. 1 (drawn from JV 0904/52-J). (A) basidiospores, (B) basidia, (C) basidioles, (D) cystidioles, (E) dendrohyphidia, (F) hyphae from subiculum, and (G) hyphae from tube trama.
Basidiocarps annual, resupinate, adnate, corky, without odor or taste when fresh, becoming hard corky upon drying, up to 8 cm long, 3 cm wide, and 0.6 mm thick at the center; sterile margin indistinct, white, very narrow to almost absent with age. Pore surface pale ochraceous when fresh and dry; pores mostly angular, 2–3 per mm; dissepiments thin, lacerate; subiculum cream, corky, up to 0.2 mm thick; tubes concolorous with the pore surface, corky, up to 0.4 mm long. Hyphal system dimitic; generative hyphae with clamp connections; skeletal hyphae strongly dextrinoid, CB+, strongly swollen in KOH. Subicular generative hyphae hyaline, thin-walled, unbranched, 1.8–2 μm diameter; skeletal hyphae dominant, thick-walled with a narrow to medium lumen, moderately branched, flexuous, interwoven, 2–2.5 μm diameter. Tramal generative hyphae hyaline, thin-walled, occasionally branched, 1.8–2 μm diameter; skeletal hyphae dominant, thick-walled with a narrow to medium lumen, moderately branched, strongly flexuous, interwoven, 2–3 μm diameter. Dendrohyphidia present. Hyphal pegs absent. Cystidia absent; cystidioles present, fusiform, thin-walled, smooth, 18–23 × 5–6 μm. Basidia pyriform to barrel-shaped, with four sterigmata and a basal clamp connection, usually with a few small guttules, 34.5–28.5 × 7.8–10.2 μm; basidioles in shape similar to basidia, but smaller. Small polyhedric crystals frequently present among hymenium and trama. Basidiospores cylindrical, hyaline, thin-walled, smooth, IKI–, CB–, (10.5–) 12.5–14.2 (−15) × (4.2–) 4.5–4.8 (−5) μm, L = 12.98 μm, W = 4.58 μm, Q = 2.76–3.09 (n = 30/2).
Materials examined: USA, Florida, Long Pine Key, April 2009, JV 0904/50-J (JV, BJFC038548!), JV 0904/52-J (JV, BJFC038549!).
Notes: Megasporoporia hexagonoides was originally described as Poria hexagonoides Speg. from Argentina (Spegazzini, 1898), and a detailed description was given by Ryvarden et al. (1982). Lira et al. (2021) analyzed the nLSU sequence of the Argentine specimen (CBS 464.63) and treated it as M. hexagonoides. Our studied samples from Florida, USA have the same nLSU sequence as CBS 464.63, but their morphological characteristics are very different from that of M. hexagonoides (the latter has pores 0.5–1 per mm, absence of dendrohyphidia, basidiospores 16.6–21.8 × 5.2–6.8 μm, Ryvarden et al., 1982).
Poria linearis Murrill, described from Panama (Murrill, 1920), was treated as a synonym of Megasporia cavernulosa (Berk.) C. R. S. Lira and T. B. Giberton (=Megasporoporia cavernulosa), described from Panuré, Brazil (Berkeley, 1856). The morphology of the taxon from Florida fits Poria linearis well, but so far no DNA data are available from the Panamanian sample, and for the time being, we treat the Florida samples as Megasporia sp. 1.
1. Pores < 1 per mm………………………………………. 2
1. Pores > 1 per mm………………………………………. 5
2. Dendrohyphidia present…………………………………3
2. Dendrohyphidia absent…………………………………. 4
3. Hyphal pegs absent…………………Jorgewrightia irregularis
3. Hyphal pegs present……………………Megasporia olivacea
4. Basidiospores > 20 μm long………….Megasporia mexicana
4. Basidiospores < 20 μm long…..Mariorajchenbergia epitephra
5. Pores 5–7 per mm………………………………………..6
5. Pores 1–5 per mm………………………………………..7
6. Pore surface violet to greyish violet……Jorgewrightia violacea
6. Pore surface cream to buff………….. Megasporoporia minor
7. Basidiospores ellipsoid……………………………………8
7. Basidiospores cylindrical, allantoid, or fusiform……….…13
8. Pores 4–5 per mm………………………………………..9
8. Pores 1–2 per mm………………………………………11
9. Basidiospores thick-walled…………Jorgewrightia bambusae
9. Basidiospores thin-walled……………….………………10
10. Skeletal hyphae dextrinoid……………….….….…………
…………………….….….…Mariorajchenbergia rhododendri
10. Skeletal hyphae indextrinoid………………………………
……………………………Mariorajchenbergia subleucoplaca
11. Basidiospores > 15 μm long; pore surface pale purplish brown………………………………Megasporia anoectopora
11. Basidiospores < 15 μm long; pore surface cream to yellow.12
12. Pores 1–1.5 per mm; hyphal pegs present……….…………
…..….………………………………Jorgewrightia ellipsoidea
12. Pores 2 per mm; hyphal pegs absent………………….……
………………………………………Megasporia amazonica
13. Basidiospores fusiform…………………………………14
13. Basidiospores cylindrical or allantoid….….….…………15
14. Dendrohyphidia present; skeletal hyphae indextrinoid.….…
…….….….….………………………Jorgewrightia fusiformis
14. Dendrohyphidia absent; skeletal hyphae weakly dextrinoid...
……………………………………….…Jorgewrightia tenuis
15. Hyphal pegs present……………………………………16
15. Hyphal pegs absent………………………….…………21
16. Cystidioles present………………………….….………17
16. Cystidioles absent…………………………….….….…19
17. Basidiospores > 15 μm long….….….…Jorgewrightia major
17. Basidiospores < 15 μm long……………………………18
18. Basidiospores cylindrical………Megasporoporia bannaensis
18. Basidiospores allantoid……………………………………
…………………….…Mariorajchenbergia pseudocavernulosa
19. Dendrohyphidia present…………………………….….…
…….….…………………Mariorajchenbergia subcavernulosa
19. Dendrohyphidia absent……………………….….….…20
20. Basidiospores 3–4 μm wide; American species……….….…
……………….…………………Megasporoporia neosetulosa
20. Basidiospores 4.2–5.7 μm wide; African species.….….….…
….….….……………………………Megasporoporia setulosa
21. Dendrohyphidia present………………….….…………22
21. Dendrohyphidia absent…………….….…….…………28
22. Skeletal hyphae indextrinoid……………………………23
22. Skeletal hyphae weakly to strongly dextrinoid….….….…24
23. Basidiocarps annual; basidiospores > 20 μm long…………
….….….…………………………………Jorgewrightia kirkii
23. Basidiocarps biennial; basidiospores < 20 μm long.….….…
….……………………………Mariorajchenbergia hubeiensis
24. Skeletal hyphae strongly dextrinoid….….………………25
24. Skeletal hyphae weakly dextrinoid….….….….…………27
25. Cystidioles absent…………………Megasporia cavernulosa
25. Cystidioles present…………………………….….….…26
26. Basidiospores > 15 μm long; Asian species….….….………
…….……………………………………Megasporia sinuosa
26. Basidiospores < 15 μm long; North American species.….…
…………………………….….……………Megasporia sp. 1
27. Basidiocarps cracked when dry…….…Jorgewrightia rimosa
27. Basidiocarps uncracked when dry……….….….….………
……………………………………Jorgewrightia yunnanensis
28. Skeletal hyphae indextrinoid……………………………29
28. Skeletal hyphae moderately to strongly dextrinoid………30
29. Basidiospores > 15 μm long……Jorgewrightia austroasiana
29. Basidiospores < 15 μm long………………….….….….…
………………………………Mariorajchenbergia leucoplaca
30. Cystidioles absent…………….….….…………………31
30. Cystidioles present….….………………………………32
31. Basidiospores 10–11.8 μm long; Asian species……….….…
………………………………………Megasporoporia inflata
31. Basidiospores 12–13 μm long; South American species……
……………………………………Megasporia variabilicolor
32. Skeletal hyphae strongly dextrinoid……………….….…33
32. Skeletal hyphae moderately dextrinoid…………………34
33. Pores 2–3 per mm……………………Jorgewrightia tropica
33. Pores 4–5 per mm…….….….Jorgewrightia guangdongensis
34. Basidiospores 16–21 μm long……Megasporia hexagonoides
34. Basidiospores < 15 μm long……………………………35
35. Pore surface cream to buff, pores 2–3 per mm….….………
…………………………………Jorgewrightia hengduanensis
35. Pore surface pale pinkish-brown to salmon, pores 3–5 per
mm ……………………………Jorgewrightia cystidiolophora.
Megasporoporia was established based on M. setulosa (the type from Tanzania), but the type has probably been lost (Ryvarden et al., 1982). Lira et al. (2021) analyzed sequences from an African specimen (LR 9907), providing the most reliable molecular data for the species, conforming to the Megasporoporia sensu stricto clade. An additional three species were included in Megasporoporia: M. cavernulosa (type from Brazil), M. hexagonoides (type from Argentina), and M. mexicana (type from Mexico). Phylogenetically these three are nested in another clade (Megasporia clade, Lira et al., 2021).
To date, four genera, Jorgewrightia, Mariorajchenbergia, Megasporia, and Megasporoporia sensu stricto, which include 36 species are accepted in Megasporoporia sensu lato.
The type of Megasporia cavernulosa was from Brazil, and the sequences KX584458 and KX619582 are from the Brazilian specimen URM 83867. Lira et al. (2021) considered this specimen to represent M. cavernulosa. This species was also reported from Florida, USA, and the morphology of our studied Florida samples (JV 0904/81-J, JV 0904/50-J, and JV 0904/52-J) is consistent with the description of M. cavernulosa. However, phylogenetically, these samples are distantly related to URM 83867. So, the occurrence of M. cavernulosa in the USA is uncertain.
Dichomitus amazonicus Gomes-Silva et al. was described from Amazonas (Gomes-Silva et al., 2012) and was later combined as Megasporia amazonica (Gomes-Silva et al.) C. R. S. Lira and Gibertoni (Lira et al., 2021). The molecular data of the type specimen of M. amazonica (Ryvarden 48295) are not available. Its phylogenetic analysis was based on specimens URM 85601 (Brazil-Pernambuco) and URM 87859 (Brazil-Bahia), but these two specimens did not cluster together (Lira et al., 2021). We studied a part of URM 87859 and found that it has a dimitic hyphal system, strongly dextrinoid and CB+ skeletal hyphae, and ellipsoid to subcylindrical basidiospores, (10–) 10.2–11.5 (−12.2) × (4.2–) 4.5–5 μm. These characteristics are consistent with the original description of M. amazonica. Therefore, we believe that URM 87859 represents M. amazonica, and URM 85601 is treated as “M. amazonica” in our phylogeny (Figure 1).
Lira et al. (2021) demonstrated that Dichomitus cylindrosporus (Ryvarden 45186) is nested in the Megasporia clade, and they combined it as Megasporia cylindrospora (Ryvarden) C. R. S. Lira and Gibertoni. However, we studied the type (Ryvarden 44248) of D. cylindrosporus and failed to extract the DNA. Morphologically, we found that Ryvarden 44248 has hyphal pegs and pores 2.5–3 per mm, while Ryvarden 45186 lacks hyphal pegs and has pores 1.5–2 per mm. So, Ryvarden 45186 most probably does not represent D. cylindrosporus, and the phylogenetic relationship between D. cylindrosporus and Megasporoporia sensu lato is uncertain.
Megasporoporia, Megasporia, Jorgewrightia, Mariorajchenbergia, and other genera (Dichomitus, Perenniporia, Crassisporus, Daedaleopsis, Datronia, Neodatronia, Polyporus, etc.) are nested together with robust support in our phylogeny based on our selected samples (Figure 1). Megasporia and Jorgewrightia are related to each other with moderate support. These two genera and Megasporoporia and Mariorajchenbergia seem to be strikingly unrelated to each other although the four genera share a very similar morphology. We currently cannot resolve this dilemma, which requires additional genomic data and further analyses.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Y-RW, YY, and Y-CD: design of the research. Y-RW: performance of the research. Y-RW, Y-CD, YY, and Y-DW: data analysis and interpretation. Y-RW, H-GL, JV, Y-CD, YY, Y-DW, and PB: the collection of materials. Y-RW, Y-CD, YY, Y-DW, and PB: writing and revising the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (Project Nos. 32000010 and U1802231).
We thank Prof. Leif Ryvarden (Norway), Prof. Bao-Kai Cui (China), and Dr. Slava Spirin (Finland) for allowing us to study their specimens.
PB was employed by Manaaki Whenua - Landcare Research.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anonymous (1969). Flora of British Fungi. Colour Identification Chart. London: Her Majesty's Stationery Office.
Berkeley, M. J. (1855). “Fungi,” in Flora Novae-Zelandiae. Part II. Flowerless Plants, ed J. D. Hooker (Lovell Reeve), 172–210, 338.
Berkeley, M. J. (1856). Decades of fungi. Decades LIX - LX. Rio Negro fungi. Hookers J. Bot. Kew Garden Miscellany 8, 233–241.
Berkeley, M. J. (1872). Australian Fungi, received principally from Baron F. von Mueller and Dr. R. Schomburgk. Bot. J. Linnean Soc. 13, 155–177. doi: 10.1111/j.1095-8339.1872.tb02397a.x
Chen, J. J., Cui, B. K., He, S. H., Cooper, J. A., Barrett, M. D., Chen, J. L., et al. (2016). Molecular phylogeny and global diversity of the remarkable genus Bondarzewia (Basidiomycota, Russulales). Mycologia 108, 697–708. doi: 10.3852/14-216
Cui, B. K., Li, H. J., Ji, X., Zhou, J. L., Song, J., Si, J., et al. (2019). Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Divers. 97, 137–392. doi: 10.1007/s13225-019-00427-4
Cunningham, G. H. (1965). Polyporaceae of New Zealand. Bull. New Zeal. Depart. Sci. Ind. Res. 164, 1–304.
Farris, J. S. (1989). The retention index and the rescaled consistency index. Cladistics 5, 417–419. doi: 10.1111/j.1096-0031.1989.tb00573.x
Farris, J. S., Källersjö, M., Kluge, A. G., and Bult, C. (1994). Testing significance of incongruence. Cladistics 10, 315–319. doi: 10.1111/j.1096-0031.1994.tb00181.x
Felsenstein, J. (1985). Confidence intervals on phylogenetics: an approach using bootstrap. Evolution 9, 783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x
Gomes-Silva, A. C., Ryvarden, L., and Gibertoni, T. B. (2012). Resupinate poroid fungi from tropical rain forests in Brazil: two new species and new records. Mycol. Prog. 11, 879–885. doi: 10.1007/s11557-011-0803-9
Hall, T. A. (1999). Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98.
Li, H. J., and Cui, B. K. (2013). Taxonomy and phylogeny of the genus Megasporoporia and its related genera. Mycologia 105, 368–383. doi: 10.3852/12-114
Li, H. J., Cui, B. K., and Dai, Y. C. (2014). Taxonomy and multi-gene phylogeny of Datronia (Polyporales, Basidiomycota). Persoonia 32, 170–182. doi: 10.3767/003158514X681828
Li, H. J., Si, J., and He, S. H. (2016). Daedaleopsis hainanensis sp. nov. (Polyporaceae, Basidiomycota) from tropical China based on morphological and molecular evidence. Phytotaxa 275, 294–300. doi: 10.11646/phytotaxa.275.3.7
Lira, C. R. S., Alvarenga, R. L. M., Soares, A. M. S., Ryvarden, L., and Gibertoni, T. B. (2021). Phylogeny of Megasporoporia s.lat. and related genera of Polyporaceae: new genera, new species and new combinations. Mycosphere 12, 1262–1289. doi: 10.5943/mycosphere/12/1/16
Maddison, W. P., and Maddison, D. R. (2021). Mesquite: A Modular System for Evolutionary analysis. Version 3.70. Available online at: http://www.mesquiteproject.org
Masuka, A., and Ryvarden, L. (1999). Dichomitus in Africa. Mycol. Res. 103, 1126–1130. doi: 10.1017/S0953756299008436
Murrill, W. A. (1920). Light-colored resupinate polypores 2. Mycologia 12, 299–308. doi: 10.1080/00275514.1920.12016844
Nylander, J. A. A. (2004). MrModeltest v2.2 Program Distributed by the Author. Uppsala: Evolutionary Biology Centre, Uppsala University.
Petersen, J. H. (1996). Farvekort. The Danish Mycological Society's Color-Chart. Greve: Foreningen til Svampekundskabens Fremme.
Posada, D., and Crandall, K. A. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 14, 817–818. doi: 10.1093/bioinformatics/14.9.817
Rehner, S. A., and Buckley, E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97, 84–98. doi: 10.3852/mycologia.97.1.84
Ronquist, F., and Huelsenbeck, J. P. (2003). MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D. L., Darling, A., and Höhna, S. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Ryvarden, L. (1977). Type-studies in the Polyporaceae 10. Species described by J.M. Berkeley, either alone or with other authors from 1844 to 1855. Norwegian J. Bot. 24, 213–230.
Ryvarden, L., Wright, J. E., and Rajchenberg, M. (1982). Megasporoporia, a new genus of resupinate polypores. Mycotaxon 16, 172–182.
Shen, L. L., Wang, M., Zhou, J. L., Xing, J. H., Cui, B. K., and Dai, Y. C. (2019). Taxonomy and phylogeny of Postia. Multi-gene phylogeny and taxonomy of the brown-rot fungi: Postia (Polyporales, Basidiomycota) and related genera. Persoonia 42, 101–126. doi: 10.3767/persoonia.2019.42.05
Spegazzini, C. (1898). Fungi Argentini novi vel critici. Anal Museo Nacl Hist Nat Buenos Aires 6, 81–288.
Stamatakis, A. (2014). RAxML Version 8: a tool for phylogenetic analyses and post analyses of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Sun, Y. F., Costa-Rezende, D. H., Xing, J. H., Zhou, J. L., Zhang, B., Gibertoni, T. B., et al. (2020). Multi-gene phylogeny and taxonomy of Amauroderma s. lat. (Ganodermataceae). Persoonia 44, 206–239. doi: 10.3767/persoonia.2020.44.08
Swofford, D. L. (2002). PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4.0 Beta. Sunderland, MA: Sinauer.
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., and Higgins, D. G. (1997). The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882. doi: 10.1093/nar/25.24.4876
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Wang, Y. R., Wu, Y. D., Vlasak, J., Yuan, Y., and Dai, Y. C. (2021). Phylogenetic analysis demonstrating four new species in Megasporoporia sensu lato (Polyporales, Basidiomycota). Mycosphere 12, 1012–1037. doi: 10.5943/mycosphere/12/1/11
White, T. J., Bruns, T. D., Lee, S., and Taylor, J. W. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR Protocols: A Guide to Methods and Applications, eds M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (New York, NY: Academic).
Wu, F., Man, X. W., Tohtirjap, A., and Dai, Y. C. (2022a). A comparison of polypore funga and species composition in forest ecosystems of China, North America, and Europe. Forest Ecosyst. 9, 540–546. doi: 10.1016/j.fecs.2022.100051
Wu, F., Zhou, L. W., Vlasák, J., and Dai, Y. C. (2022b). Global diversity and systematics of Hymenochaetaceae with poroid hymenophore. Fungal Divers. 113, 1–192. doi: 10.1007/s13225-021-00496-4
Yuan, Y., Ji, X. H., Chen, J. J., and Dai, Y. C. (2017). Three new species of Megasporia (Polyporales, Basidiomycota) from China. MycoKeys 20, 37–50. doi: 10.3897/mycokeys.20.11816
Zhao, C. L., and Cui, B. K. (2012). A new species of Perenniporia (Polyporales, Basidiomycota) described from southern China based on morphological and molecular characters. Mycol. Progress 11, 555–560. doi: 10.1007/s11557-011-0770-1
Zhou, J. L., and Cui, B. K. (2017). Phylogeny and taxonomy of Favolus (Basidiomycota). Mycologia 109, 766–779. doi: 10.1080/00275514.2017.1409023
Zhou, J. L., Zhu, L., Chen, H., and Cui, B. K. (2016). Taxonomy and phylogeny of polyporus group Melanopus (Polyporales, Basidiomycota) from China. PLoS One 11, e0159495. doi: 10.1371/journal.pone.0159495
Keywords: taxonomy, phylogeny, morphology, Polyporaceae, polymerase
Citation: Wang Y-R, Dai Y-C, Liu H-G, Vlasák J, Buchanan P, Yuan Y and Wu Y-D (2022) A new contribution to Megasporoporia sensu lato: Six new species and three new combinations. Front. Microbiol. 13:1046777. doi: 10.3389/fmicb.2022.1046777
Received: 17 September 2022; Accepted: 10 November 2022;
Published: 08 December 2022.
Edited by:
Ji-Chuan Kang, Guizhou University, ChinaReviewed by:
Hiran A. Ariyawansa, National Taiwan University, TaiwanCopyright © 2022 Wang, Dai, Liu, Vlasák, Buchanan, Yuan and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Yuan, eXVhbnl1YW4xMDE4QGJqZnUuZWR1LmNu; Ying-Da Wu, d3lkYmpmdUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.