
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 04 October 2022
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1038487
This article is part of the Research Topic Fungal Secondary Metabolites as Valuable Chemical Entities for Medicines and Agrochemicals View all 12 articles
Halometabolites, usually produced in marine environment, are an important group of natural halogenated compounds with rich biological functionality and drugability and thus play a crucial role in pharmaceutical and/or agricultural applications. In the exploration of novel halometabolites from marine microorganisms, the growing number of halogenated compounds makes it necessary to fully present these metabolites with diverse structures and considerable bioactivities. This review particularly focuses on the chemodiversity and bioactivities of halometabolites from marine-derived fungi. As a result, a total of 145 naturally halogenated compounds, including 118 chlorinated, 23 brominated, and four iodinated compounds, were isolated from 17 genera of marine-derived fungi. Interestingly, many of halometabolites, especially for the brominated and iodinated compounds, are generated by the substitution of bromide and iodide ions for the chloride ion in cultivation process. In addition, these compounds possess diverse structural types, which are classified into polyketides (62.7%), phenols (16.6%), alkaloids (14.5%), and terpenoids (6.2%). Their cytotoxic, antibacterial, and anti-inflammatory activities indicate the high potential of these halogenated compounds as lead compounds for drug discovery.
Halometabolites are a group of natural halogen-containing (Cl, Br, I, F) compounds which possess rich biological functionality and drugability. It is estimated that more than 5,000 halogenated compounds have been reported (Liao et al., 2016). Among them, chlorination is the predominant occurance, and then followed by bromination, while iodination and fluorination are extremely rare (Neumann et al., 2008). Halometabolites are generally produced from abiogenic and biogenic pathways. Biogenic halometabolites are formed by microorganisms (fungi and bacteria), plants, algae, and marine invertebrates (sponges and corals) (Kasanah and Triyanto, 2019). Biosynthetically, enzymatic halogenation through halogenases such as flavin adenine dinucleotide-dependent halogenases (FDHs) and non-heme FeII/α-ketoglutarate halogenases is the most common way to these compounds (Neumann et al., 2008; Liao et al., 2016). Halometabolites possess high diversity in structure, ranging in complexity from simple halogenated indoles, terpenes, and phenols to miscellaneous polypeptides and polyketides.
Apart from their novel structures, the presence of halogens in natural products significantly enhances their biological activities. The halogen substituents are responsible for the bioactivity, bioavailability, and stability of the compounds (Kasanah and Triyanto, 2019). Halometabolites also play an important role in pharmaceutical and agricultural applications. Many of them have been used for decades as pharmaceuticals and agrochemicals. It is worth mentioning that natural products have benefited significantly from the growth of the pharmaceutical industry, especially of pharmacologically attractive lead drugs and potential clinical therapeutic drugs. Among them, approximately 25% of clinically therapeutic drugs are halogenated, indicating halogen substituents as remarkable contributors to pharmacological applications. A large number of halogenated natural products-inspired pharmaceuticals are either FDA or EMEA approved. Representative examples of them include the antibiotics chloramphenicol and vancomycin, the anticancer drugs salinosporamide A, spongistatin, rebeccamycin, and calicheamicin (Supplementary Figure S1; Niu et al., 2021). Therefore, in this sense, halometabolites bioprospecting is a considerable approach to discover new innovative drugs.
Compared to those from terrestrial plants, halometabolites derived from marine environment are relatively unexplored. The marine environment is a crucial source of halotolerant microorganisms (Wang et al., 2011). Microorganisms living in marine extreme environment are suffered from low temperature, high pressure, high salinity, and low oxygen concentration, and have evolved extraordinary metabolic pathways to produce novel secondary metabolites (Xu et al., 2020). Marine-derived fungi have been largely explored due to their ability to generate structurally novel secondary metabolites with remarkable biological activities. Given the crucial role that halogen substituents can play in the bioactivity of these metabolites, high metabolic potential of halometabolites production can be expected from the marine-derived fungi. This present review illustrates the chemistry and biological activities of halometabolites produced by marine-derived fungi. A total of 145 naturally halogenated compounds, including 118 chlorinated, 23 brominated, and four iodinated compounds, were isolated in the past decades. Crucial insights into their chemical diversity and biological activities are provided herein. This review will reveal these halogenated compounds as lead compounds for the development of innovative drugs.
Thirty-nine halogenated azaphilones featured an oxabicyclic core were isolated from marine-derived fungi (Figures 1, 2; Table 1). Ten chlorinated azaphilones (1–10) including eight new nitrogenated azaphilones (1–8) were isolated from the deep-sea-derived fungus Chaetomium globosum MP4-S01-7 (Wang et al., 2020). Compounds 1–4 belong to N-(3,7-dimethyl-2,6-octadienyl) azaphilone polyketides, while compounds 5–8 are N-(3-methyl-2-butenyl) azaphilones. Most of them showed strong cytotoxic activity against the human gastric cancer MGC803 and AGS cell lines with IC50 values ranging from 0.12 to 10 μM. Importantly, compounds 1, 2, and 5, in particularly, demonstrated the strongest activity at a nanomole level. In-depth mechanism study revealed that 2 arrested gastric cancer MGC803 and AGS cells in the G1 phase, while 1 and 2 induced apoptosis of both cells in a concentration-dependent manner. Eight chlorinated azaphilones, including five new ones 11–15 as well as three known analogs 16–18 were isolated from the deep-sea-derived fungus Phomopsis tersa FS441 (Chen et al., 2021). It should be pointed out that, compound 12, which featured a cleaved tetrahydrofuranyl ring, possesses the novel 6/6–6 carbon framework. Moreover, compounds 14 and 15 are characterized as a pair of diastereomers with a characteristic epoxide ring, which are uncommon in azaphilones. In the cytotoxic assay, the new compounds 14 and 15 showed potent cytotoxicity against MCF-7, SF-268, and A549 cell lines with the IC50 values of 5.4–8.3 μM (compared with the positive control cisplatin, IC50 of 1.6–3.3 μM). Chemical investigations of Chaetomium sp. NA-S01-R1, which was isolated from the deep-sea seawater sample, yielded four new chlorinated azaphilone pigments (19–22) and two known ones (23–24; Wang et al., 2018). Compound 19 is a novel azaphilone bearing a fused tetrahydrofuran and δ-lactone moiety. The new azaphilones 20 and 21 exhibited antibacterial activities against aquatic pathogenic bacteria Vibrio rotiferianus and V. vulnificus, with MIC values of 7.3 and 7.4 μg/ml, respectively, while compounds 19, 21 and 22 were found to possess anti-methicillin resistant Staphylococcus aureus activity with MIC values ranging from 7.3 to 7.8 μg/ml (chloramphenicol as the positive control with an MIC value of 7.6 μg/ml). Moreover, compound 20 showed cytotoxic activity against the HepG2 cell line with an IC50 value of 3.9 μM. The marine-derived fungus Aspergillus falconensis, when cultured on solid rice medium containing 3.5% NaCl, yielded two new chlorinated azaphilones 25 and 26 as well as four known derivatives 27–30 (El-Kashef et al., 2020). Then, replacing NaCl with 3.5% NaBr induced accumulation of two additional brominated azaphilones 31 and 32 and a known analog 33. All of these compounds were examined for their nuclear factor kappa B (NF-κB) inhibitory activity in the triple negative breast cancer cell line MDA-MB-231. As a result, compounds 25 and 27–32 showed NF-κB inhibitory activity against the MDA-MB-231 cell line with IC50 values ranging from 11.9 to 72.0 μM. The mangrove rhizosphere soil-derived fungus Penicillium janthinellum HK1-6 was found to produce chlorinated azaphilones 36 and 37 (Chen et al., 2019). Cultivation of this fungal strain with NaBr instead of sea salt led to the isolation of two new brominated azaphilones 34 and 35. Structurally, compounds 34–37 have a 7-O-2′,4′-dimethyldec-2′-enoyl side chain. The NaBr-induced brominated azaphilones 34 and 35 possess the opposite configuration at C-7 to the chlorinated analogs 36 and 37. The brominated 35 exhibited antibacterial activity against the Gram-positive bacteria including both antibiotic-resistant (methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium) and antibiotic-susceptible (S. aureus and E. faecalis) strains with MIC values of 3.13–12.5 μg/ml. Fermentation of the fungus P. canescens 4.14.6a obtained from the Mediterranian sponge Agelas oroides with the addition of 5% NaBr yielded two new brominated azaphilones 38 and 39 (Frank et al., 2019). Compounds 38 and 39, which represent the first azaphilones with a benzene moiety and the pyranoquinone skeleton via a methylene group, were exclusively produced when the fungus was cultivated with NaBr. Compound 39 exerted mild cytotoxicity against the mouse lymphoma cell line L5178Y (IC50 = 8.9 μM) and the human ovarian cancer cell line A2780 (IC50 = 2.7 μM), while its epimer 38 was relatively less active.
As shown in Figure 3, 25 halogenated benzophenones (40–64) were isolated from marine-derived fungi. A chemical survey of the sponge-associated fungus Pestalotiopsis colombiensis yielded eight chlorinated benzophenone derivatives 40–47, which were isolated from this fungal species for the first time (Lei et al., 2020). These compounds, exclusively isolated from the genus Pestalotiopsis and never found in other genus, possess a great significance in the chemotaxonomic study of Pestalotiopsis. Therefore, they could be regarded as important chemotaxonomic markers for the genus of Pestalotiopsis. A new chlorinated benzophenone derivative 48 was isolated from the soft coral-derived fungus Pestalotiopsis sp. (Wei et al., 2013). Compound 48 demonstrated antibacterial activities against Escherichia coli, V. anguillarum, and V. parahaemolyticus with MIC values of 5.0, 10.0 and 20.0 μM, respectively. A new chlorinated xanthone 49 substituted with a tetrahydropyran ring was isolated from the marine-derived fungus Chaetomium sp. (Pontius et al., 2008). Compound 49 showed moderate antiprotozoal activity against Trypanosoma cruzi with an IC50 value of 1.5 μg/ml. Metabolomic investigations on the marine-derived fungus Aspergillus sp. SCSIO F063 unveiled seven new chlorinated anthraquinones 50–56 (Huang et al., 2012). Futhermore, when the fungus was fermented with 3% NaBr, two new brominated anthraquinones 57 and 58 were additionally isolated. Interestingly, no iodinated secondary metabolites were observed when the fungus was fermented with NaI. Among these metabolites, only compound 51 moderately inhibited the growth of three human tumor cell lines, SF-268, MCF-7, and NCI-H460, with IC50 values of 7.11, 6.64, and 7.42 μM, respectively. The above-mentioned fungal strain P. canescens 4.14.6a cultured in sea salt produced compounds 59 and 60 (Frank et al., 2019). Metabolic studies on two different developmental stages, the vegetative stage (asexual morph) and the sexual stage (sclerotial morph), of the marine algal-derived fungus A. alliaceus were performed (Mandelare et al., 2018). As a result, the asexual morph of A. alliaceus produced a chlorinated anthraquinone 61, whereas three chlorinated bianthrones 62–64 were generated by the coculture of the asexual and sclerotial morph of A. alliaceus. Compound 62 was active against the HCT-116 colon carcinoma and SK-Mel-5 skin cancer cell lines with IC50 values of 9.0 and 11.0 μM, respectively.
Diverse coumarin-/chromone/pyran-/furan-derived polyketides (65–85) isolated from marine-derived fungi are shown in Figure 4. Two chlorinated dihydro-isocoumarin derivatives 65 and 66 were isolated from the marine-derived fungus Phoma sp. 135 (Elsebai and Ghabbour, 2016). Two new chlorinated isocoumarins 67 and 68 with an exomethylene group at C-3 were isolated from a deep-sea-derived fungus Spiromastix sp. MCCC 3A00308 (Niu et al., 2021). The dichlorinated isocoumarin 68 showed higher antibacterial activity (Bacillus thuringiensis and B. subtilis, with an MIC value of 4 μg/ml) than the monochlorinated 67. The addition of metal bromides, NaBr and CaBr2, to the medium of marine-mudflat-derived fungus A. niger induced the production of a new brominated naphthopyranone 69 (Leutou et al., 2016), while the addition of NaBr to a marine-derived A. ochraceus led to the induced production of a new brominated isocoumarin 70 (Yun et al., 2013). Compounds 69 and 70 displayed strong radical scavenging activity against DPPH with IC50 values of 21 and 24 μM, respectively. Two new chlorinated benzofuran derivatives, 71 and 72, were isolated from the marine starfish-derived fungus Pseudallescheria boydii (Yan et al., 2015). A chlorinated griseofulvin-type spirocyclic polyketide 73 was isolated from P. canescens 4.14.6a (Frank et al., 2019). A new phenalenone 74, representing the first example of chlorinated acenaphthenquinone derivative, was characterized from the marine sediment-derived fungus Pleosporales sp. HDN1811400 (Han et al., 2021). Compound 74 displayed higher inhibitory activity against MRCNS (MIC = 25.0 μM) and MRSA (MIC = 12.5 μM) than the positive control ciprofloxacin (MICs of 25.0 and > 50 μM, respectively), suggesting the high potential of these heptaketide phenalenones as lead compounds for drug-resistant pathogens. A new naturally occurring 8–4′ linkage 1-tetralone dimeric derivative 75 was isolated from the deep-sea derived fungus Cladosporium cladosporioides HDN14-342 (Zhang et al., 2016). Compound 75, which represents the first halogenated cladosporol derivatives, showed cytotoxicity against HeLa, K562, and HCT-116 cell lines with IC50 values of 3.9, 8.8, and 19.4 μM. An unexpected iodinated dimeric naphtho-γ-pyrone 76 was obtained from the marine cold-seep fungus C. cladosporioides 8–1 (Li et al., 2022). Compared to chlorine- and bromine-containing compounds, iodine-bearing metabolites are rarely encountered. Compound 76 displayed potent antimicroalgal activity against the marine microalgae Prorocentrum minimum with an IC50 value being 0.61 μg/ml, compared with the positive control CuSO4 (IC50 = 2.4 μg/ml). Two new chlorinated cyclopentanoids 77 and 78 were isolated from A. sydowii, an endophyte associated with the marine alga Acanthophora spicifera (Teuscher et al., 2006). Both compounds are structurally related hydroxylated 2,5-diarylcyclopentenones, which have hitherto only been isolated from higher basidiomycetes. A novel dichlorinated compound 79 having an unprecedented polyketide skeleton was isolated from the marine-derived fungus Roussoella sp. DLM33 (Ferreira et al., 2015). Stable isotope feeding experiments revealed a complicated biosynthetic origin of 79 by Favorskii rearrangements in individual pentaketides before being linked via an intermolecular Diels−Alder reaction. Two new chlorinated quasi-precursors of sorbicillinoid-type polyketides, 80 and 81, were isolated from the marine sediment-derived fungus P. terrestre (Li et al., 2011). A furan lactone 82 was isolated from the soft coral-derived fungus Trichoderma harzianum (XS-20090075) cultured with rice medium (Yu et al., 2021). Chromatographic separation of the marine-derived fungus Phoma sp.135 resulted in the characterization of three new chlorinated cyclopentene derivatives 83–85 (Elsebai et al., 2018). Compounds 83–85 showed weak antimicrobial activity against E. coli, Bacillus subtilis, Mycobacterium phlei, and S. aureus, with MIC values ranging from 10 to 35 μM.
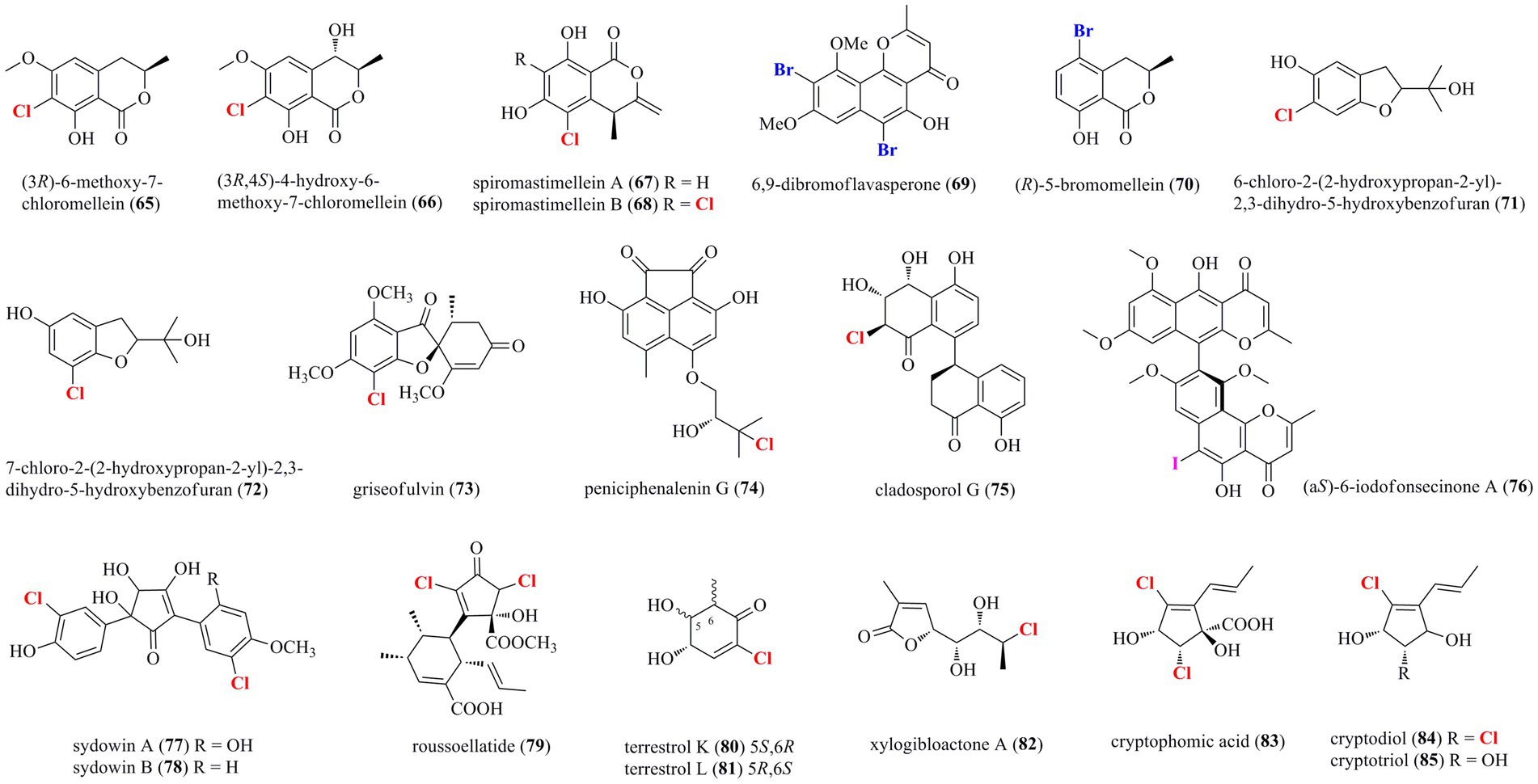
Figure 4. Halogenated coumarin−/chromone/pyran−/furan-derived polyketides from marine-derived fungi (65–85).
As shown in Figure 5, compounds 86 and 87, two novel chlorinated sorbicillinoids possessing an unprecedented bicyclo[2.2.2]octane-2-spiro cyclohexane skeleton, were isolated from P. terrestre (Li et al., 2011). Compounds 86 and 87 are identified as the first occurrence of spiro cyclohexane-containing and chlorinated sorbicillinoids. Interestingly, 86 was more active against HL-60 cell line with an IC50 value of 9.2 μM than 87 (IC50 = 37.8 μM), indicating that the stereochemistry may influence the cytotoxic activity. Chemical epigenetic modification, a promising approach to manipulate the silent fungal genes, was used to the marine-derived fungus Cochliobolus lunatus (TA26-46) with histone deacetylase inhibitors, led to the isolation and identification of two new brominated 14-membered resorcylic acid lactones 88 and 89 (Zhang W. et al., 2014). It should be noted that both compounds, which were identified as the first examples of brominated resorcylic acid lactones, were exclusively isolated via epigenetic modifying agents. Finally, two new dibrominated alkenoates 90 and 91 were isolated from an unidentified fungus (Li et al., 2004).
Diverse halogenated terpenoids isolated from marine-derived fungi, including one monoterpene 92, five sesquiterpenoids 93–97, one diterpenoid 98, and two meroterpenoids 99–100, are shown in Figure 6. A new chloro-monoterpene 92 was isolated from the mangrove-sourced endophytic fungal strain Tryblidiopycnis sp. 4,275 (Huang et al., 2006). A new chlorinated sesquiterpenoid 93 was obtained from the marine-derived Penicillium strain MMS351 (Vansteelandt et al., 2013). 93 is elucidated as an analog of fumagillin, a sesquiterpene esterified by a deca-2,4,6,8-tetraenedioic acid and functionalized by a spiro-epoxide fused with the cyclohexane ring. Compound 93 showed potent antiproliferative activity against the osteosarcoma cell line POS1 with an IC50 value of 117 nM. Four new chlorinated eremophilane-type sesquiterpenes 94–97 were obtained from the deep-sea derived fungus Penicillium sp. PR19N-1 (Wu et al., 2013). Compound 94, which is identified as a trinor-eremophilene core with an 8-oxo-1(2),9(10)-diene unit, was found to possess modest cytotoxic activity against HL-60 and A549 cell lines with IC50 values of 11.8 and 12.2 μM, respectively. A new chlorinated cleistanthane-type diterpenoid 98 was isolated from the soft coral-derived fungus T. harzianum (XS-20090075) cultured with 10 μM sodium butyrate (Shi et al., 2020). The cleistanthane-type diterpenoid, arisen owing to chemical epigenetic modification, was discovered from genus Trichoderma for the first time. Isolation of the marine worm (Sipunculus nudus)-derived fungus Penicillium sp. SCS-KFD09 afforded two new previously unreported chlorinated meroterpenoids 99 and 100 (Kong et al., 2017). Both meroterpenoids possess a drimane-type sesquiterpenoid substructure fused with an isochromanone moiety. Compound 99 showed strong antiviral activity against influenza A virus (H1N1) with an IC50 value of 74 μM (ribavirin as positive control with an IC50 of 103 μM).
A total of 21 halogenated alkaloids (101–121, Figure 7) were isolated from marine-derived fungi. A new chlorinated indole-diterpenoid 101 was isolated from the algal-endophytic fungus A. nidulans EN-330 (Zhang et al., 2015). Compound 101 inhibited the growth of brine shrimp (Artemia salina) with an LD50 value of 3.2 μM. Moreover, it also displayed antimicrobial activities against human- (E. coli and S. aureus) and aqua- (Edwardsiella tarda and V. anguillarum) pathogens with MIC values of 16–64 μg/ml. The chlorine-substitution may enhance bioactivities to some degree. Prenylated indole alkaloids possessing a characteristic bicyclo[2.2.2]diazaoctane or diketopiperazine ring are a diverse group of fungal secondary metabolites for biosynthetic investigations (Zhang et al., 2019). A systematic isolation of Malbranchea aurantiaca, obtained from an unidentified marine invertebrate, provided six new halogenated prenylated indole alkaloids 102–107 (Watts et al., 2011). Structurally, all of the isolated compounds are identified as prenylated indole alkaloids containing a halogenated indole ring and the bicyclo[2.2.2]diazaoctane skeleton. Compounds 102–105 were isolated in normal artificial seawater medium, while two brominated 106 and 107 were produced by modifying the solid growth medium with NaBr. Inspired by OSMAC approach, the mangrove-derived fungus Phomopsis sp. QYM-13 was cultured with the addition of NaBr or KI to afford halogen-substituted metabolites. As a result, a new brominated cytochalasin 108 and two new iodinated cytochalasins 109 and 110 were isolated from this strain treated with 3% NaBr and 3% KI, respectively (Chen et al., 2022). Compounds 109 and 110 represent the first iodinated cytochalasins. The brominated 108 displayed selective cytotoxicity to MDA-MB-435 cell line with an IC50 value of 7.4 μM. Research into the fungus Trichoderma sp. TPU199 derived from a red alga yielded a series of new epipolythiodiketopiperazines 111–115 with a sulfide bridge (–S–, –SS–, –SSS–, or –SSSS–) between the α- and β-positions of two amino acid residues (Yamazaki et al., 2020). This fungal strain afforded the halogenated 111, 113, and 114, when fermented with 3% NaCl, NaBr, and NaI, respectively. Moreover, compound 115, the first trisulfide derivative, was induced by cultivation of this strain with DMSO. A chlorinated mycotoxin 116 was isolated from sclerotial morph of A. alliaceus (Mandelare et al., 2018). Three unprecedented chlorinated PKS-NRPS hybrid metabolites 117–119 were isolated from the marine sponge symbiotic fungus A. flavipes 164,013 (Jiao et al., 2020). These compounds consisting of a chlorinated xanthone, an aminoethyl-modified pyrazol, and a methylated dipeptide represent a new structural family of PKS-NRPS hybrid metabolites. Compounds 117–119 showed significant inhibitory activity on pancreatic lipase with IC50 values of 0.23, 0.07, and 0.14 μM, respectively, which were 6–21 times more potent than that of the positive control kaempferol (IC50 = 1.50 μM). A new brominated chloroquinoline 120 was isolated from the fungus T. harzianum (Yu et al., 2021). 120 was isolated as the first halogenated quinoline derivative from the genus Trichoderma. A novel chlorinated alkaloid 121 featuring a rare oxazole moiety was isolated from the hydrothermal fungus Graphostroma sp. MCCC 3A00421 (Niu et al., 2018).
Figure 8 presents a total of 24 halogenated phenolic derivatives (122–145) isolated from marine-derived fungi. Two chlorinated diphenyl ethers, 122 and 123, were isolated from the sponge-associated fungus P. canescens 4.14.6a (Frank et al., 2019). Seven chlorinated phenolic derivatives, including two diphenyl ethers (124 and 125), four depsidones (126–129), and one depside (130), were isolated from the coral-derived fungus A. unguis GXIMD 02505 (Zhang et al., 2022). Compounds 124–128 and 130 were found to inhibit lipopolysaccharide (LPS)-induced NF-κB in RAW 264.7 macrophages at a concentration of 20 μM. Most importantly, compounds 125 and 130, acted as the most potent inhibitors, dose-dependently suppressed RANKL-induced osteoclast differentiation. In addition, compounds 124, 125, 127, 129, and 130 displayed moderate antibacterial activities against methicillin-resistant S. aureus, Microbulbifer variabilis, Marinobacterium jannaschii, and V. pelagius with the MIC values ranging from 2 to 64 μg/ml. Three new chlorinated depsidone-type compounds (131–133) were isolated from the deep-sea-derived Spiromastix fungus (Niu et al., 2021). Compound 133 was characterized as a tri-chlorinated derivative and possessed remarkable antibacterial activities against S. aureus, Bacillus thuringiensis, and B. subtilis, with MIC values of 0.5–1.0 μg/ml. Two tri-chlorinated depsidones 134 and 135 were isolated from a seaweed-derived A. unguis strain (Zhang Y. et al., 2014). Compound 135 strongly inhibited methicillin-resistant S. aureus (MIC = 4 μg/ml) and brine shrimp Artemia larva (LC50 = 2.8 μg/ml). A new dichlorinated compound 136 was isolated from the deep-sea sediment-derived fungus P. citreonigrum XT20-134 (Tang et al., 2019). Compound 136 possessed promising cytotoxicities against the human hepatoma tumor cell Bel7402 and the human fibrosarcoma tumor cell HT1080, with IC50 values of 13.14 and 16.53 μM, respectively. Seven halogenated phenolic derivatives, including three new chlorinated orsellinic aldehyde derivatives 137–139, two orsellinic acids (chlorinated 140 and brominated 141), and two phenols (chlorinated 142 and brominated 143), were isolated from the coral-associated fungus Acremonium sclerotigenum GXIMD 02501 (Lu et al., 2022). Compounds 137, 138, 140, and 143 showed certain inhibition of LPS-induced NF-κB activation in RAW 264.7 cells at 20 μM. Two new potent inhibitors (137 and 138) strongly suppressed RANKL-induced osteoclast differentiation. Finally, the addition of NaBr and CaBr2 in the fermentation of the marine-derived fungus Aspergillus sp. induced the production of two new brominated dihydroxyphenylacetic acid derivatives 144 and 145 (Leutou et al., 2013). Both compounds exerted strong DPPH scavenging activity with IC50 values of 14.2 and 12.1 μM.
In order to expand the structural diversity of the halometabolites from the marine-derived fungi, OSMAC (One Strain MAny Compounds) strategy was used to remodel the fungal metabolome and activate the cryptic biosynthetic pathways. Of all the isolated halometabolites from the marine-derived fungi, most of them are chlorinated (81.4%), then followed by brominated (15.9%), while iodinated compounds are rather rare (2.7%). It should be pointed out that the occurrence of halogenated metabolites depends on halogen salts in the fermentation of the producing fungi. It seems that most of the brominated and iodinated compounds are generated by the substitution of bromide and iodide ions for the chloride ion in cultivation (Figure 9). For example, fermentation of A. falconensis with 3.5% NaCl afforded chlorinated azaphilones 25–30, while replacing NaCl with 3.5% NaBr induced the production of additional brominated azaphilones 31 and 32 (El-Kashef et al., 2020). Cultivation of P. janthinellum HK1-6 with sea salt and NaBr yielded chlorinated azaphilones 36–37 and brominated 34–35, respectively (Chen et al., 2019). Interestingly, the NaBr-induced brominated 34–35 possess the opposite configuration at C-7 compared to the chlorinated analogs 36–37 cultured with normal sea salt condition. In addition to the chlorinated anthraquinones 50–56, two brominated anthraquinones 57 and 58 were obtained from Aspergillus sp. SCSIO F063 by the substitution of 3% NaBr for sea salt (Huang et al., 2012). The authors also fermented the fungus with NaI; however, no iodinated metabolites were observed. The fungus M. aurantiaca produced chlorinated prenylated indole alkaloids 102–105, when fermented in normal artificial seawater medium, while the brominated 106 and 107 were isolated from its culture broth in NaBr-containing medium (Watts et al., 2011). The fungus Phomopsis sp. QYM-13 cultured with the addition of 3% NaBr or 3% KI was found to produce a brominated cytochalasin 108 and two new iodinated cytochalasins 109 and 110, respectively (Chen et al., 2022). Finally, the fungus Trichoderma sp. TPU199 afforded the halogenated 111, 113, 114, and 115 when induced by cultivation of this fungal strain with 3% NaCl, 3% NaBr, 3% NaI, and DMSO, respectively (Yamazaki et al., 2020). These results indicated that the substitution of bromide or iodide ions for sea salt in the fermentation of the producing fungi may be an effective way to afford more intriguing halometabolites, expecially brominated and iodinated compounds, from the marine-derived fungi.
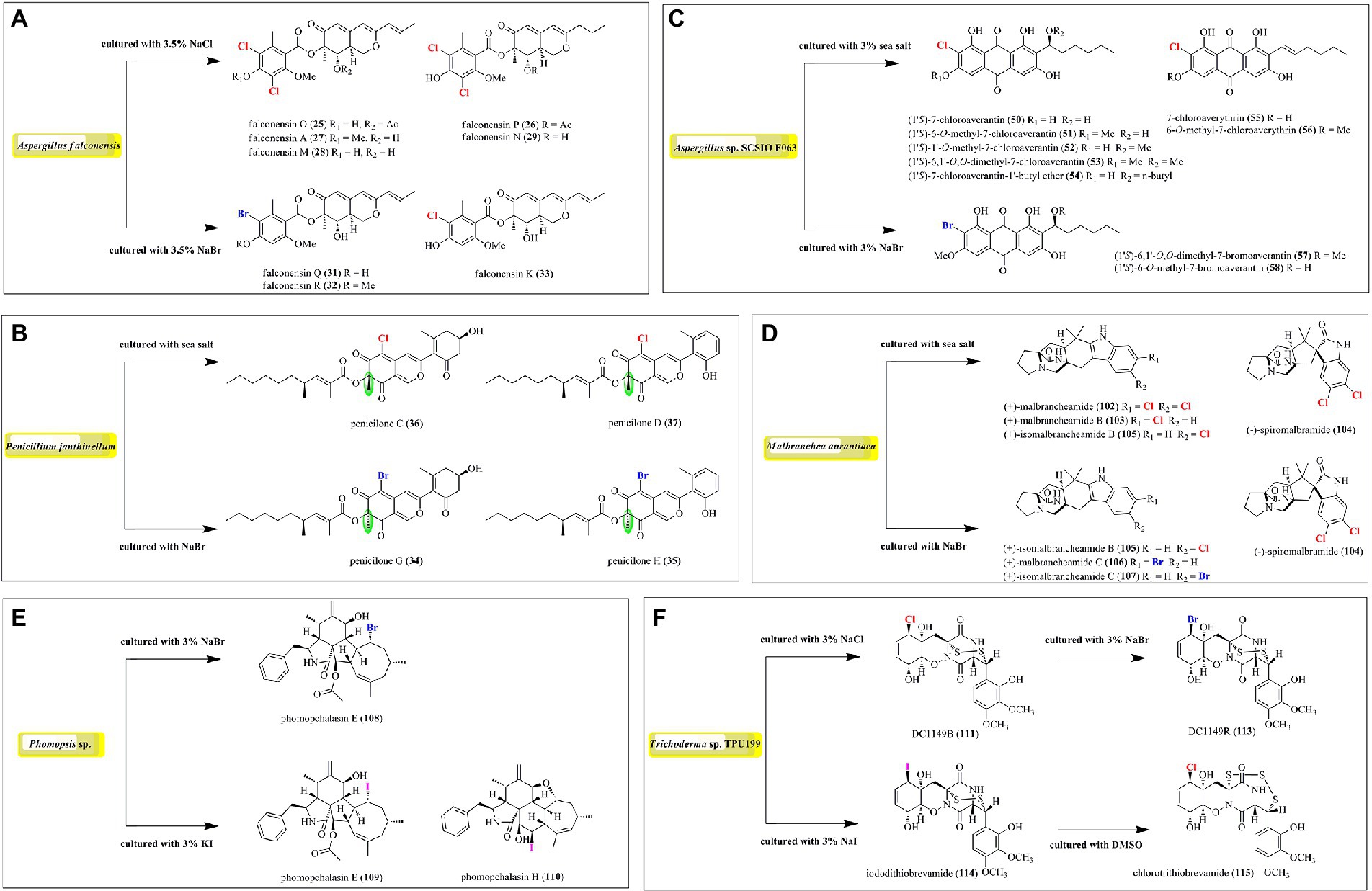
Figure 9. Induced production of halometabolites with different cultural conditions. (A) Azaphilones produced by Aspergillus falconensis; (B) Azaphilones produced by Penicillium janthinellum; (C) Anthraquinones produced by Aspergillus sp. SCSIO F063; (D) Prenylated indole alkaloids produced by Malbranchea aurantiaca; (E) Cytochalasin produced by Phomopsis sp. QYM-13; (F) Epipolythiodiketopiperazines produced by Trichoderma sp. TPU199.
Halometabolites are mainly produced by marine organisms due to the presence of chloride, bromine, and iodine ions in seawater. As previously discussed, among all of the halometabolites described herein, chlorination is the predominant modification, and then followed by bromination, while iodination is extremely rare. In this review, a total of 118 chlorinated (accounting for 81.4%), 23 brominated (15.9%), and four iodinated (2.7%) metabolites isolated from marine-derived fungi were summarized (Figure 10A). Marine fungi may possess the capability to oxidize chlorine more easily than bromide and iodine in the biosynthesis of these metabolites, thus the number of chlorinated compounds is quite higher than brominated and iodinated compounds. Moreover, these halometabolites possess a high structural diversity. The reported 145 halometabolites, shown in this review, are categorized into polyketides (1–91; including azaphilones 1–39, benzophenones 40–64, coumarin−/chromone/pyran−/furan-derived polyketides 65–85, and other types of polyketides 86–91), terpenoids (92–100), alkaloids (101–121), and phenolic derivatives (122–145). Structural classification of compounds based on biogenetic categories is unprecise, as many compounds are derived from mixed biosynthetic pathways. For example, compounds 1–6 are clearly classified as nitrogen-containing compounds. However, we categorize them as polyketides based on the biosynthetic origin of azaphilones. It is estimated that 62.8% of the reported halometabolites are polyketides (Figure 10B), especially azaphilones, which accounted for 42.9% of the reported halogenated polyketides. As for the halogenated alkaloids, a series of halogenated prenylated indole alkaloids 102–107 and epipolythiodiketopiperazines 111–115 were isolated and induced by the addition of additional halogen salts. Changing the cultural conditions will help to increase the chemical diversity of halometabolites produced by marine-derived fungi.
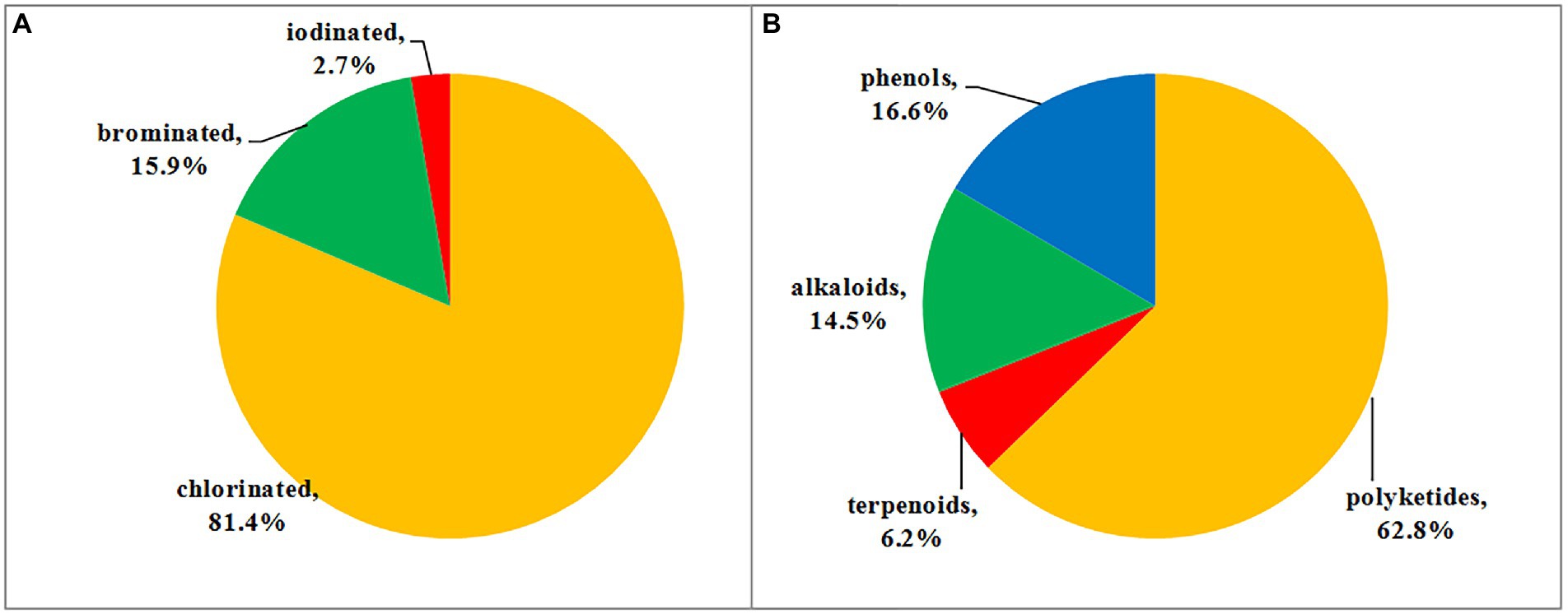
Figure 10. (A) Proportion of halometabolites from marine-derived fungi; (B) Structural classes of halometabolites.
Halometabolites isolated from marine microorganisms are relatively unexplored compared with those from marine macroorganisms, such as algae, sponges, and soft corals. Marine-derived fungi have proven to be a precious house of bioactive secondary metabolites with novel structures. Table 1 shows a total of 17 genera of marine-derived fungi as producers of these halometabolites. Among them, the species belonging to genera Aspergillus, Penicillium, Chaetomium, Phomopsis, Pestalotiopsis, Trichoderma, Acremonium, Malbranchea, Phoma, and Spiromastix are the Top 10 producers, with 42, 23, 17, 11, 9, 8, 7, 6, 5, and 5 halometabolites being isolated, respectively (Figure 11A). In addition, the distribution of these fungal producers is shown in Figure 11B. These fungal producers were obtained from a wide range of marine habitats, such as marine sediments (including mudflats and sludges), marine invertebrates (including sponges, soft corals, starfishes, and anemones), and marine plants (algae and mangroves). Marine sediments, marine sponges, marine algae, seawater, soft corals, and mangroves are dominating origins of these fungal strains, with 43, 23, 21, 19, 18, and 8 of the reported compounds characterized (Figure 11B).
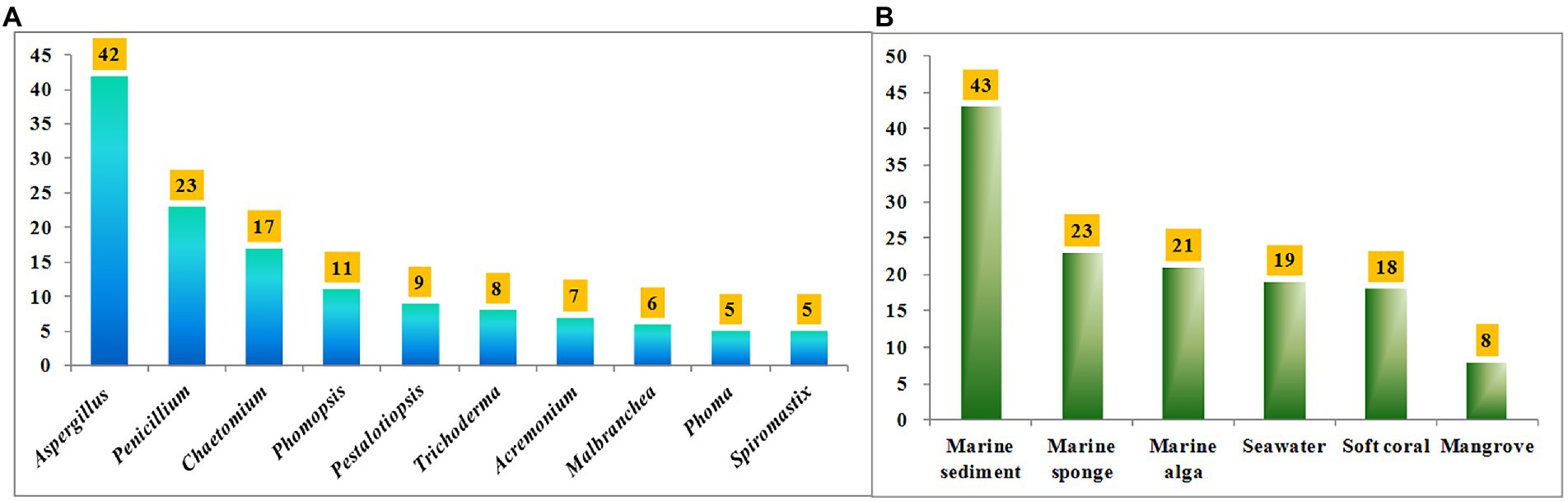
Figure 11. (A) Numbers of halometabolites from different marine-derived fungi; (B) Numbers of halometabolites from different sources of marine origins.
Halometabolites are vital sources for new drugs discovery given to their high diversity in structures and bioactivities. It is considered that the presence of halogen substituents profoundly enhances the bioactivity of natural compounds, as it is obvious that halometabolites often possess higher biological activity than that non-halogen substituted natural compounds. However, it lacks solid evidence that compounds with two or more halogen substituents, such as compounds 25–27 with two chlorine groups, 40–48 with two chlorine groups, and 134–135 with three chlorine groups, exhibit better activity than those with single substituent. The reported halometabolites derived from marine fungi demonstrated pronounced biological activities, including cytotoxic, antimicrobial, anti-inflammatory, antioxidant, and enzyme inhibitory properties (Figure 12). 31.3% of the isolated halometabolites were found to possess certain cytotoxicities. More importantly, some of them showed even higher activity than the positive controls. For example, the chlorinated azaphilones 1, 2, and 5 showed significant cytotoxic activity against the human gastric cancer MGC803 and AGS cell lines at a nanomole level (Wang et al., 2020), while compounds 19, 21 and 22 were found to possess anti-methicillin resistant S. aureus activity with MICs of 7.3–7.8 μg/ml (the positive control chloramphenicol, MIC = 7.6 μg/ml) (Wang et al., 2018). The phenalenone 74 displayed higher activity against MRCNS (MIC = 25.0 μM) and MRSA (MIC = 12.5 μM) than the positive control ciprofloxacin (MICs of 25.0 and > 50 μM, respectively), indicating the high potential of these heptaketide phenalenones as lead compounds for drug-resistant pathogens (Han et al., 2021). The iodinated dimeric naphtho-γ-pyrone 76 displayed potent antimicroalgal activity against the marine microalgae Prorocentrum minimum with an IC50 value of 0.61 μg/ml, compared with the positive control CuSO4 (IC50 = 2.4 μg/ml) (Li et al., 2022). It is well-known that some halometabolites have been on the market for decades as pharmaceuticals, as exemplified of antibiotic chloramphenicol and pyrrolnitrin and antitumor rebeccamycin. The promising bioactivities indicate that searching for new halometabolites is an important way to develop new drugs and agrochemicals.
In conclusion, in the exploration of bioactive natural compounds, we focus on the potential of marine-derived fungi as producers of halometabolites. This comprehensive review illustrates the chemistry and biological activities of halometabolites produced by marine-derived fungi. 145 halogenated compounds, including 118 chlorinated, 23 brominated, and 4 iodinated, which are classified into polyketides (62.7%), phenols (16.6%), alkaloids (14.5%), and terpenoids (6.2%), were isolated from 17 genera of marine-derived fungi. Their pronounced biological activities, such as cytotoxic, antimicrobial, anti-inflammatory, antioxidant, and enzyme inhibitory properties, revealed a high potential of these halogenated compounds as lead compounds for drug discovery. It should be pointed out that despite a large number of new halometabolites have been characterized; those halogenated compounds are relatively unexplored. Further OSMAC method by changing the cultural conditions will induce the production of more halometabolites.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
YC, L-CX, and SL: collected and reorganized the literature data. YC: wrote this manuscript. Z-XZ and G-YC: conceived the ideas and revised this manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1038487/full#supplementary-material
Chen, S., Liu, Z., Chen, Y., Tan, H., Liu, H., and Zhang, W. (2021). Tersaphilones A-E, cytotoxic chlorinated azaphilones from the deep-sea-derived fungus Phomopsis tersa FS441. Tetrahedron 78:131806. doi: 10.1016/j.tet.2020.131806
Chen, Y., Yang, W., Zou, G., Wang, G., Kang, W., Yuan, J., et al. (2022). Cytotoxic bromine- and iodine-containing cytochalasins produced by the mangrove endophytic fungus Phomopsis sp. QYM-13 using the OSMAC approach. J. Nat. Prod. 85, 1229–1238. doi: 10.1021/acs.jnatprod.1c01115
Chen, M., Zheng, Y. Y., Chen, Z. Q., Shen, N. X., Shen, L., Zhang, F. M., et al. (2019). NaBr-induced production of brominated azaphilones and related tricyclic polyketides by the marine-derived fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 82, 368–374. doi: 10.1021/acs.jnatprod.8b00930
El-Kashef, D. H., Youssef, F. S., Hartmann, R., Knedel, T. O., Janiak, C., Lin, W., et al. (2020). Azaphilones from the red sea fungus Aspergillus falconensis. Mar. Drugs 18:204. doi: 10.3390/md18040204
Elsebai, M. F., and Ghabbour, H. A. (2016). Isocoumarin derivatives from the marine-derived fungus Phoma sp. 135. Tetrahedron Lett. 57, 354–356. doi: 10.1016/j.tetlet.2015.12.024
Elsebai, M. F., Ghabbour, H. A., Legrave, N., Fontaine-Vive, F., and Mehiri, M. (2018). New bioactive chlorinated cyclopentene derivatives from the marine-derived fungus Phoma sp. Med. Chem. Res. 27, 1885–1892. doi: 10.1007/s00044-018-2201-1
Ferreira, E. L., Williams, D. E., Ióca, L. P., Morais-Urano, R. P., Santos, M. F., Patrick, B. O., et al. (2015). Structure and biogenesis of roussoellatide, a dichlorinated polyketide from the marine-derived fungus Roussoella sp. DLM33. Org. Lett. 17, 5152–5155. doi: 10.1021/acs.orglett.5b02060
Frank, M., Hartmann, R., Plenker, M., Mándi, A., Kurtán, T., Özkaya, F. C., et al. (2019). Brominated Azaphilones from the sponge-associated fungus Penicillium canescens strain 4.14.6a. J. Nat. Prod. 82, 2159–2166. doi: 10.1021/acs.jnatprod.9b00151
Han, Y., Sun, C., Li, C., Zhang, G., Zhu, T., Li, D., et al. (2021). Antibacterial phenalenone derivatives from marine-derived fungus Pleosporales sp. HDN1811400. Tetrahedron Lett. 68:152938. doi: 10.1016/j.tetlet.2021.152938
Huang, H., Wang, F., Luo, M., Chen, Y., Song, Y., Zhang, W., et al. (2012). Halogenated anthraquinones from the marine-derived fungus Aspergillus sp. SCSIO F063. J. Nat. Prod. 275, 1346–1352. doi: 10.1021/np3002699
Huang, H. R., Xia, X. K., She, Z. G., Lin, Y. C., Vrijmoed, L. L., and Gareth Jones, E. B. (2006). A new chloro-monoterpene from the mangrove endophytic fungus Tryblidiopycnis sp. (4275). J. Asian Nat. Prod. Res. 8, 609–612. doi: 10.1080/10286020500208493
Jiao, W. H., Xu, Q. H., Ge, G. B., Shang, R. Y., Zhu, H. R., Liu, H. Y., et al. (2020). Flavipesides A-C, PKS-NRPS hybrids as pancreatic lipase inhibitors from a marine sponge symbiotic fungus Aspergillus flavipes 164013. Org. Lett. 22, 1825–1829. doi: 10.1021/acs.orglett.0c00150
Kasanah, N., and Triyanto, T. (2019). Bioactivities of halometabolites from marine Actinobacteria. Biomol. Ther. 9:225. doi: 10.3390/biom9060225
Kong, F. D., Ma, Q. Y., Huang, S. Z., Wang, P., Wang, J. F., Zhou, L. M., et al. (2017). Chrodrimanins K-N and related meroterpenoids from the fungus Penicillium sp. SCS-KFD09 isolated from a marine worm, Sipunculus nudus. J. Nat. Prod. 80, 1039–1047. doi: 10.1021/acs.jnatprod.6b01061
Lei, H., Niu, H., Song, C., Fu, X., Luo, Y., Chen, S., et al. (2020). Chlorinated benzophenone derivatives as chemotaxonomic markers for the genus of pestalotiopsis. Biochem. Syst. Ecol. 91:104072. doi: 10.1016/j.bse.2020.104072
Leutou, A. S., Yun, K., Kang, J. S., and Son, B. W. (2013). Induced production of methyl bromodihydroxyphenyl acetates by the marine-derived fungus Aspergillus sp. Chem. Pharm. Bull. 61, 483–485. doi: 10.1248/cpb.c12-01048
Leutou, A. S., Yun, K., and Son, B. W. (2016). Induced production of 6,9-dibromoflavasperone, a new radical scavenging naphthopyranone in the marine-mudflat-derived fungus Aspergillus niger. Arch. Pharm. Res. 39, 806–810. doi: 10.1007/s12272-016-0764-2
Li, D., Chen, L., Zhu, T., Kurtán, T., Mándi, A., Zhao, Z., et al. (2011). Chloctanspirones A and B, novel chlorinated polyketides with an unprecedented skeleton, from marine sediment derived fungus Penicillium terrestre. Tetrahedron 67, 7913–7918. doi: 10.1016/j.tet.2011.08.037
Li, X., Kim, S. K., Kang, J. S., Choi, H. D., and Son, B. W. (2004). Polyketide and sesquiterpenediol metabolites from a marine-derived fungus. Bull. Kor. Chem. Soc. 25, 607–608.
Li, C. P., Song, Y. P., Wang, B. G., and Ji, N. Y. (2022). Sulfurated and iodinated metabolites from the cold-seep fungus Cladosporium cladosporioides 8-1. Tetrahedron Lett. 93:153689. doi: 10.1016/j.tetlet.2022.153689
Liao, L., Chen, R., Jiang, M., Tian, X., Liu, H., Yu, Y., et al. (2016). Bioprospecting potential of halogenases from arctic marine actinomycetes. BMC Microbiol. 16:34. doi: 10.1186/s12866-016-0662-2
Lu, H., Tan, Y., Zhang, Y., Li, Z., Chen, J., Gao, C., et al. (2022). Osteoclastogenesis inhibitory phenolic derivatives produced by the beibu gulf coral-associated fungus Acremonium sclerotigenum GXIMD 02501. Fitoterapia 159:105201. doi: 10.1016/j.fitote.2022.105201
Mandelare, P. E., Adpressa, D. A., Kaweesa, E. N., Zakharov, L. N., and Loesgen, S. (2018). Coculture of two developmental stages of a marine-derived Aspergillus alliaceus results in the production of the cytotoxic bianthrone allianthrone A. J. Nat. Prod. 81, 1014–1022. doi: 10.1021/acs.jnatprod.8b00024
Neumann, C. S., Fujimori, D. G., and Walsh, C. T. (2008). Halogenation strategies in natural product biosynthesis. Chem. Biol. 15, 99–109. doi: 10.1016/j.chembiol.2008.01.006
Niu, S., Liu, D., Shao, Z., Huang, J., Fan, A., and Lin, W. (2021). Chlorinated metabolites with antibacterial activities from a deep-sea-derived Spiromastix fungus. RSC Adv. 11, 29661–29667. doi: 10.1039/D1RA05736G
Niu, S., Liu, Q., Xia, J. M., Xie, C. L., Luo, Z. H., Shao, Z., et al. (2018). Polyketides from the deep-sea-derived fungus Graphostroma sp. MCCC 3A00421 showed potent antifood allergic activities. J. Agric. Food Chem. 66, 1369–1376. doi: 10.1021/acs.jafc.7b04383
Pontius, A., Krick, A., Kehraus, S., Brun, R., and König, G. M. (2008). Antiprotozoal activities of heterocyclic-substituted xanthones from the marine-derived fungus Chaetomium sp. J. Nat. Prod. 71, 1579–1584. doi: 10.1021/np800294q
Shi, T., Shao, C. L., Liu, Y., Zhao, D. L., Cao, F., Fu, X. M., et al. (2020). Terpenoids from the coral-derived fungus Trichoderma harzianum (XS-20090075) induced by chemical epigenetic manipulation. Front. Microbiol. 11:572. doi: 10.3389/fmicb.2020.00572
Tang, X. X., Liu, S. Z., Yan, X., Tang, B. W., Fang, M. J., Wang, X. M., et al. (2019). Two new cytotoxic compounds from a deep-sea Penicillum citreonigrum XT20-134. Mar. Drugs 17:509. doi: 10.3390/md17090509
Teuscher, F., Lin, W., Wray, V., Edrada, R., Padmakumar, K., Proksch, P., et al. (2006). Two new cyclopentanoids from the endophytic fungus Aspergillus sydowii associated with the marine alga Acanthophora spicifera. Nat. Prod. Comm. 1, 927–933. doi: 10.2174/138955706775197794
Vansteelandt, M., Blanchet, E., Egorov, M., Petit, F., Toupet, L., Bondon, A., et al. (2013). Ligerin, an antiproliferative chlorinated sesquiterpenoid from a marine-derived Penicillium strain. J. Nat. Prod. 76, 297–301. doi: 10.1021/np3007364
Wang, W., Liao, Y., Chen, R., Hou, Y., Ke, W., Zhang, B., et al. (2018). Chlorinated azaphilone pigments with antimicrobial and cytotoxic activities isolated from the deep sea derived fungus Chaetomium sp. NA-S01-R1. Mar. Drugs 16:61. doi: 10.3390/md16020061
Wang, Y., Lu, Z., Sun, K., and Zhu, W. (2011). Effects of high salt stress on secondary metabolite production in the marine-derived fungus Spicaria elegans. Mar. Drugs 9, 535–542. doi: 10.3390/md9040535
Wang, W., Yang, J., Liao, Y. Y., Cheng, G., Chen, J., Cheng, X. D., et al. (2020). Cytotoxic nitrogenated azaphilones from the deep-sea-derived fungus Chaetomium globosum MP4-S01-7. J. Nat. Prod. 83, 1157–1166. doi: 10.1021/acs.jnatprod.9b01165
Watts, K. R., Loveridge, S. T., Tenney, K., Media, J., Valeriote, F. A., and Crews, P. (2011). Utilizing DART mass spectrometry to pinpoint halogenated metabolites from a marine invertebrate-derived fungus. J. Org. Chem. 76, 6201–6208. doi: 10.1021/jo2009593
Wei, M. Y., Li, D., Shao, C. L., Deng, D. S., and Wang, C. Y. (2013). (±)-Pestalachloride D, an antibacterial racemate of chlorinated benzophenone derivative from a soft coral-derived fungus Pestalotiopsis sp. Mar. Drugs 11, 1050–1060. doi: 10.3390/md11041050
Wu, G., Lin, A., Gu, Q., Zhu, T., and Li, D. (2013). Four new chloro-eremophilane sesquiterpenes from an antarctic deep-sea derived fungus, Penicillium sp. PR19N-1. Mar. Drugs 11, 1399–1408. doi: 10.3390/md11041399
Xu, K., Wei, X. L., Xue, L., Zhang, Z. F., and Zhang, P. (2020). Antimicrobial meroterpenoids and erythritol derivatives isolated from the marine-algal-derived endophytic fungus Penicillium chrysogenum XNM-12. Mar. Drugs 18:578. doi: 10.3390/md18110578
Yamazaki, H., Takahashi, O., Kirikoshi, R., Yagi, A., Ogasawara, T., Bunya, Y., et al. (2020). Epipolythiodiketopiperazine and trichothecene derivatives from the NaI-containing fermentation of marine-derived Trichoderma cf. brevicompactum. J. Antibiot. 73, 559–567. doi: 10.1038/s41429-020-0314-5
Yan, D. F., Lan, W. J., Wang, K. T., Huang, L., Jiang, C. W., and Li, H. J. (2015). Two chlorinated benzofuran derivatives from the marine fungus Pseudallescheria boydii. Nat. Prod. Commun. 10, 621–622. doi: 10.1177/1934578X1501000421
Yu, J. Y., Shi, T., Zhou, Y., Xu, Y., Zhao, D. L., and Wang, C. Y. (2021). Naphthalene derivatives and halogenate quinoline from the coral-derived fungus Trichoderma harzianum (XS-20090075) through OSMAC approach. J. Asian Nat. Prod. Res. 23, 250–257. doi: 10.1080/10286020.2020.1729752
Yun, K., Feng, Z., Choi, H. D., Kang, J. S., and Son, B. W. (2013). New production of (R)-(−)-5-bromomellein, a dihydroisocoumarin derivative from the marine-derived fungus Aspergillus ochraceus. Chem. Nat. Compd. 49, 24–26. doi: 10.1007/s10600-013-0496-1
Zhang, Z., He, X., Liu, C., Che, Q., Zhu, T., Gu, Q., et al. (2016). Clindanones A and B and cladosporols F and G, polyketides from the deep-sea derived fungus Cladosporium cladosporioides HDN14-342. RSC Adv. 6, 76498–76504. doi: 10.1039/C6RA14640F
Zhang, Y., Li, Z., Huang, B., Liu, K., Peng, S., Liu, X., et al. (2022). Anti-osteoclastogenic and antibacterial effects of chlorinated polyketides from the beibu gulf coral-derived fungus Aspergillus unguis GXIMD 02505. Mar. Drugs 20:178. doi: 10.3390/md20030178
Zhang, P., Li, X. M., Li, X., and Wang, B. G. (2015). New indole-diterpenoids from the algal-associated fungus Aspergillus nidulans. Phytochem. Lett. 12, 182–185. doi: 10.1016/j.phytol.2015.03.017
Zhang, Y., Mu, J., Feng, Y., Wen, L., and Han, J. (2014). Four chlorinated depsidones from a seaweed-derived strain of Aspergillus unguis and their new biological activities. Nat. Prod. Res. 28, 503–506. doi: 10.1080/14786419.2013.879305
Zhang, W., Shao, C. L., Chen, M., Liu, Q. A., and Wang, C. Y. (2014). Brominated resorcylic acid lactones from the marine-derived fungus Cochliobolus lunatus induced by histone deacetylase inhibitors. Tetrahedron Lett. 55, 4888–4891. doi: 10.1016/j.tetlet.2014.06.096
Keywords: halometabolites, natural products, marine fungi, chemical diversity, biological activities
Citation: Chen Y, Xu L-C, Liu S, Zhang Z-X and Cao G-Y (2022) Halometabolites isolated from the marine-derived fungi with potent pharmacological activities. Front. Microbiol. 13:1038487. doi: 10.3389/fmicb.2022.1038487
Received: 07 September 2022; Accepted: 20 September 2022;
Published: 04 October 2022.
Edited by:
Peng Zhang, Tobacco Research Institute (CAAS), ChinaReviewed by:
Jianlong Zhang, Ludong University, ChinaCopyright © 2022 Chen, Xu, Liu, Zhang and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guan-Yi Cao, Y2hlZXJ5Y2h5QDE2My5jb20=; Zi-Xiang Zhang, emhhbmd6eHo2NkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.