- 1Yunnan Academy of Agricultural Sciences, Biotechnology and Germplasm Resources Institute, Kunming, China
- 2College of Agronomy and Biosciences, Dali University, Dali, Yunnan, China
Ganoderma is a globally distributed genus that encompasses species with forestry ecological, medicinal, economic, and cultural importance. Despite the importance of this fungus, the studies on the species diversity of Ganoderma in Yunnan Province, China (YPC) have poorly been carried out. During this study, opportunistic sampling was used to collect 21 specimens of Ganoderma from YPC. Morphology and multigene phylogeny of the internal transcribed spacer (ITS) regions, the large subunit of nuclear ribosomal RNA gene (nrLSU), the translation elongation factor 1-α gene (TEF1-α), and the second largest subunit of RNA polymerase II (RPB2) were used to identify them. Morphological and molecular characterization of the 21 specimens showed that they belong to 18 species of Ganoderma, of which three are novel viz. G. artocarpicola, G. obscuratum and G. yunnanense. Ganoderma artocarpicola is characterized by the sessile and concrescent basidiomata, reddish brown to yellowish brown pileus surface, heterogeneous context, wavy margin, and ovoid basidiospores. Ganoderma obscuratum is distinguished by small pores (6–9 per mm), dorsolaterally sub-stipitate basidiomata which become greyish-brown when dry, and narrow ellipsoid basidiospores. Ganoderma yunnanense is characterized by cream color pore surface and context, centrally to laterally stipitate basidiomata with reddish-brown to violet-brown strongly laccate pileus surface, and broadly ellipsoid basidiospores. With the help of an extensive literature survey and the results of this study, a checklist of 32 Ganoderma species from YPC was established, which accounts for 71.11% of the known species in China. In addition, a key to the Ganoderma in YPC is also provided.
Introduction
Ganoderma P. Karst. 1881 is a genus of white rot fungi in the Polyporales and Ganodermataceae containing species that were originally described in the United Kingdom (Moncalvo and Ryvarden, 1997). Ganoderma worldwide distribution from warm temperate to tropical, and is a facultative parasite on living, dead or rotting trees (Zhou et al., 2015). Ganoderma species cause white rot of hardwoods by decomposing lignin, cellulose, and related polysaccharides. Generally associated with the decay of roots and the lower trunk or stems flare, which can lead to hazardous tree conditions and tree failures, resulting in serious damage to property and life (Loyd et al., 2017). Previous studies have reported that some species of Ganoderma can cause diseases as pathogens of living trees such as Areca catechu (betel nut palm), Elaeis guineensis (oil palm), Hevea brasiliensis (rubber), and cause wood rot of forest trees and can contribute to tree mortality and failure by wind throw (Adaskaveg et al., 1991; Elliott and Broschat, 2001; Tonjock and Afui, 2015). Several species are responsible for stem and butt rots of commercially important crops such as stem rot of betel nut palm and oil palm caused by G. boninense or G. zonatum (Elliott and Broschat, 2001; Nur et al., 2019), and rubber root rot caused by G. philippi (Glen et al., 2009). Other species, such as G. australe, G. sessile and G. curtisii, seem to be opportunistic pathogens and typically only cause serious decay in old or stressed trees (Sinclair and Lyon, 2005). On the other hand, some of Ganoderma have been shown to selectively delignify wood and are recognized as a potentially important source of lignin degrading enzymes (Otjen et al., 1987). Obviously, Ganoderma are ecologically indispensable, but some of them are pathogenic and can cause diseases in forest trees.
Moreover, most Ganoderma species have biologically active components with nutritional and medicinal effects, which are economically important (Dai et al., 2009). Ganoderma has been used in Asian countries for over two millennia as a traditional medicine for maintaining vivacity and longevity, for its perceived health benefits, has gained wide popular use as a dietary supplement (Hapuarachchi et al., 2018a). Ganoderma lucidum (“lingzhi”) and G. sinense have been included in the Chinese Pharmacopoeia, and are used for anti-cancer treatment, lowering blood pressure, and improving immunity (Dai et al., 2009; Sun et al., 2022). Research of Ganoderma is a hot topic since its high potential to use in biotechnology.
As a consequence of several taxonomic and molecular phylogenetic studies on Ganoderma, an unexpectedly high level of species diversity has been uncovered worldwide, with the description of many new species (Cao et al., 2012; Cao and Yuan, 2013; Li et al., 2015; Xing et al., 2016, 2018; Hapuarachchi et al., 2018b, 2019; Liu et al., 2019; Wu et al., 2020; He et al., 2021). However, many taxonomy confusions have resulted from the great variability in the macroscopic characters of the Ganoderma basidiomata. As of 20 September 2022, there were 488 records of Ganoderma recorded in Index Fungorum,1 and 529 records in MycoBank.2 Nearly two-thirds of these records have been identified as synonyms. Up to now, 181 species are taxonomically accepted in Ganoderma, making it as one of the most species-rich genera in Ganodermataceae (Costa-Rezende et al., 2020). The genus is unique with characteristic double-walled basidiospores with a thin hyaline exosporium and ornamented endospore (Karsten, 1881; Moncalvo and Ryvarden, 1997).
China has a complex and diverse plant diversity, and a diversified three-dimensional climate environment that breeds abundant wild Ganoderma resources, thus, a total of 40 species of Ganoderma have been reported in China (Cao et al., 2012; Cao and Yuan, 2013; Li et al., 2015; Xing et al., 2018; Hapuarachchi et al., 2018b, 2019; Liu et al., 2019; Wu et al., 2020; He et al., 2021; Sun et al., 2022). Yunnan is an inland Province with low latitude and high altitudes in southwest China, which is a hotspot of global biodiversity and has abundant wildlife resources Nine type species of Ganoderma viz. Ganoderma alpinum, G. chuxiongense, G. dianzhongense, G. esculentum, G. mutabile, G. puerense, G. subangustisporum, G. weixiense and G. yunlingense have been reported in this region. In addition, several researchers have reported the diversity of Ganoderma in southwestern China, such as Luangharn et al. (2021), which reported 13 Ganoderma species viz. G. applanatum, G. australe, G. calidophilum, G. flexipes, G. gibbosum, G. leucocontextum, G. lucidum, G. multiplicatum, G. resinaceum, G. sanduense, G. sichuanense, G. sinense, and G. tsugae from YPC based on comprehensive morphological characteristics and molecular analyses. Apparently, there are many economically and medicinally important Ganoderma species in YPC (Figure 1; He et al., 2021; Luangharn et al., 2021; Sun et al., 2022). However, with the exception of the taxonomic and new species description studies, very little efforts have been made to identify the Ganoderma species diversity in YPC. Thus, the objectives of this research are, to identify and describe different species of Ganoderma including three new species in YPC based on morphology and multigene phylogeny, and to prepare a checklist of Ganoderma and a key to Ganoderma in YPC.

Figure 1. Basidiomata of the Ganoderma species collected in Yunnan Province, China. (A) Ganoderma applanatum found in Eriobotrya tree (HKAS123785); (B) Ganoderma dianzhongense in Cyclobalanopsis tree (HKAS 112719); (C) Ganoderma esculentum (HKAS123789); (D) Ganoderma gibbosum in Carya tree (HKAS123781); (E) Ganoderma lingzhi in Prunus tree (HKAS123768); (F) Ganoderma leucocontextum in Cyclobalanopsis tree (HKAS123767); (G) Ganoderma lucidum in Quercus tree (HKAS123773); (H) Ganoderma multipileum (HKAS123775); (I) Ganoderma sinense in Acer tree (HKAS123770). Photographs were taken by JH.
Materials and methods
Specimen collection
Twenty-one Ganoderma specimens were collected during the rainy season from July 2016 to September 2021 from jungle hill forests in Yunnan Province, China. They were photographed in the field, then collected and wrapped in aluminium foils or kept separately in a plastic collection box. Macro-morphology of fresh basidiomata was described, on the same day of collection. Specimens were then thoroughly dried at 40°C in a food drier, stored in sealed plastic bags with anhydrous silica gel, and deposited in the herbarium of Kunming Institute of Botany, Chinese Academy of Sciences Academia Sinica (HKAS section, KUN). MycoBank numbers were obtained as described in Jayasiri et al. (2015).
Morphological study
Macro-morphological studies were conducted following the protocols provided by Torres-Torres and Guzmán-Dávalos (2012). Key colors were obtained from Kornerup and Wanscher (1978). Micro-morphological data were obtained from the dried specimens and observed under a light microscope (Nikon). The temporary prepared microscope slides were placed under magnification up to 1,000 × using Nikon ECLIPSE80i (Nikon, Japan) compound stereomicroscope for observation and microscopic morphological photography. Microscopic observations were made from slide preparations stained with 10% potassium hydroxide (KOH), Melzer’s reagent, and Cotton Blue. Measurements were made using the Image Frame work v.0.9.7 To represent variation in the size of basidiospores, 5% of measurements were excluded from each end of the range and extreme values were given in parentheses (He et al., 2021).
The following abbreviations are used: IKI = Melzer’s reagent, IKI– = neither amyloid nor dextrinoid, KOH = 10% potassium hydroxide, CB = Cotton Blue, CB + = cyanophilous, L = mean spore length (arithmetic average of all spores), W = mean spore width (arithmetic average of all spores). The abbreviation for spore measurements (x/y/z) denote “x” spores measured from “y” basidiocarps of “z” specimens. Basidiospore dimensions (and “Q” values) are given as (a) b–av–c (d). Where “a” and “d” refer to the lower and upper extremes of all measurements, respectively, b-c the range of 95% of the measured values, and Q is the length/width ratio of basidiospores, is given as Qm ± standard deviation, where Qm is the average Q of all basidiospores. Where “a” and “d” refer to the lower and upper extremes of all measurements, “av” is the average “b,” respectively, b-c are the range of 95% of the measured values, and Q is the length/width ratio of basidiospores, which is given as Qm ± standard deviation, where Qm is the average Q of all basidiospores.
DNA extraction, PCR amplification, and sequencing
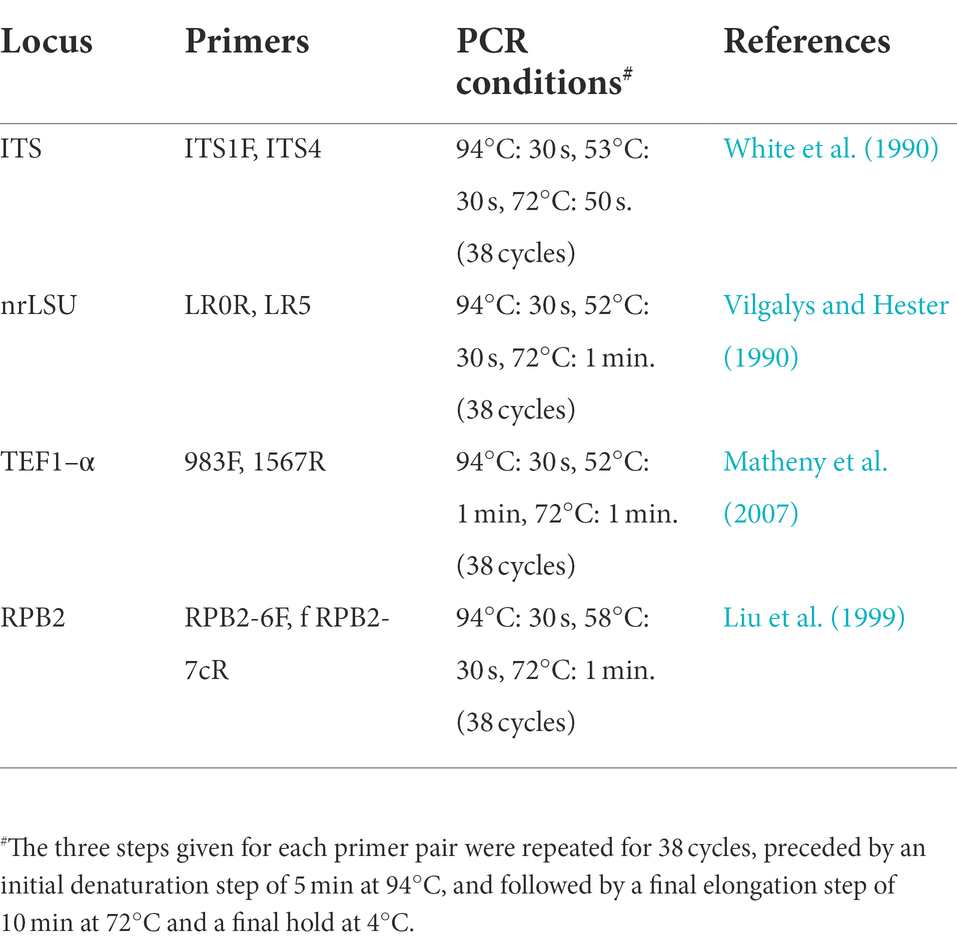
Genomic DNA isolation and PCR of the studied material were performed at the Yunnan Academy of Agricultural Sciences, China. Genomic DNA was extracted from dried specimens using Ezup Column Fungi Genomic DNA Purification Kit (Sangon Biotech Limited Company, Kunming, Yunnan, China) based on the manufacturer’s protocol. Primer pairs used for PCR were ITS1F/ITS5 (White et al., 1990) for ITS, LR5/LR0R (Vilgalys and Hester, 1990) for nrLSU, TEF1–983/TEF1–1567R (Matheny et al., 2007) for TEF1–α, and RPB2–6f/fRPB2–7cR (Liu et al., 1999) for RPB2. Primer sequences of the primers used in this study are available in the WASABI database of the AFTOL website (aftol.org). Gene regions were amplified in 30 μl reactions containing 15 μl 2 × Taq Plus Master Mix II (Sangon Biotechnology Co., Kunming, China), 13 μl ddH2O, 0.5 μl 10 μM of forward and reverse primers, 1 μl DNA. PCR conditions were used as in the Table 1, using a C1000 thermal cycler (Bio-Rad China). The PCR amplicons were sent to Sangon Biotech (China) for Sanger sequencing. Raw DNA sequences were assembled, and edited in Sequencher 4.1.4 and the assembled DNA sequences were deposited in GenBank (Table 2).
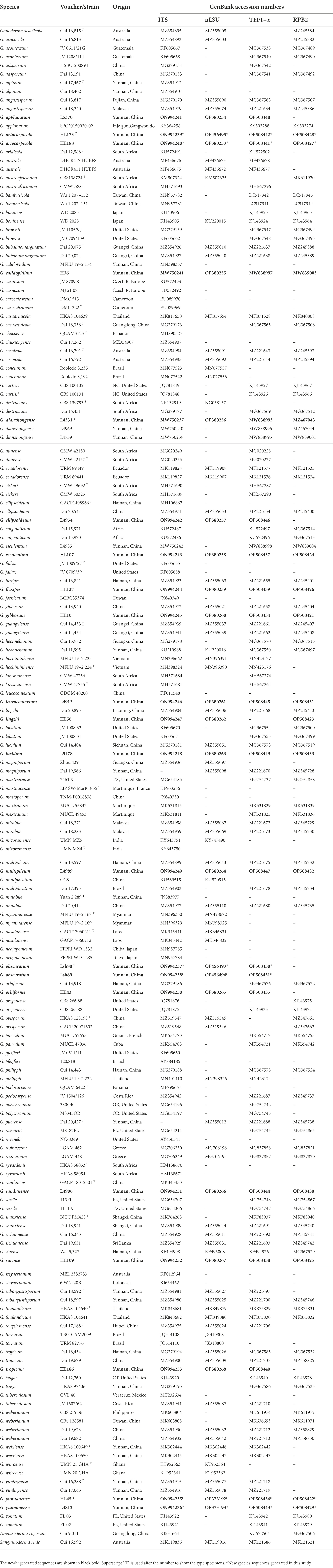
Table 2. Specimens used for phylogenetic analyses and their corresponding GenBank accession numbers.
Sequencing and sequence alignment
The sequences of the new species were subjected to standard BLAST searches in GenBank to find the most closely related sequences. All the sequences except those obtained from this study (Table 2), were retrieved from GenBank for phylogenetic analyses. Sequences were aligned using the online version of MAFFT v.7 (Katoh and Standley, 2013)3 and adjusted using BioEdit v.7.0.9 by hand (Hall, 1999) to minimize gaps and align properly. Ambiguous regions were excluded from the analyses and gaps were treated as missing data. The phylogeny website tool “ALTER” (Glez-Peña et al., 2010) was used to convert the Fasta alignment file to Phylip format for RAxML analysis and, AliView and PAUP 4.0b 10 were used to convert the Fasta alignment file to a Nexus file for Bayesian analysis (Swoford, 2003).
Phylogenetic analyses
Maximum likelihood (ML) analysis was performed for both gene regions separately using RAxML-HPC2 v. 8.2.12 (Stamatakis, 2014) as implemented on the CIPRES portal (Miller et al., 2010), with the GTR + G model for both genes and 1,000 rapid bootstrap (BS) replicates. Since no supported conflict (BS ≥ 60%) was detected among the topologies, the four single-gene alignments were concatenated using SequenceMatrix (Vaidya et al., 2011).
Bayesian analysis was performed in MrBayes 3.2 (Ronquist et al., 2012) and the best-fit model of sequences evolution was estimated via MrModeltest 2.3 (Guindon and Gascuel, 2003; Nylander, 2004; Darriba et al., 2012). The Markov Chain Monte Carlo (MCMC) sampling approach was used to calculate posterior probabilities (PP; Rannala and Yang, 1996). Bayesian analysis of six simultaneous Markov chains was run for 10,000,000 generations and trees were sampled every 1,000 generations. The first 5,000 trees, representing the burn-in phase of the analyses, were discarded, while the remaining 1,500 trees were used for calculating posterior probabilities in the majority rule consensus tree (the critical value for the topological convergence diagnostic is 0.01).
Phylogenetic trees were visualized using FigTree v1.4.0,4 editing and typesetting using Adobe Illustrator CS5 (Adobe Systems Inc., United States). Sequences derived in this study were deposited in GenBank.5 The final sequence alignments and the phylogenetic trees are available at TreeBase (http://www.treebase.org, accession number: 29691).
Results
Phylogenetic analyses
In this study, 71 Ganoderma sequences were newly generated from the specimens collected from YPC, and were deposited in GenBank (Table 2), i.e., 19 sequences of ITS, 21 sequences of nLSU, 18 sequences of tef1, and 13 sequences of rpb2. The combined two-gene dataset ITS + nrLSU (Figure 2) included sequences from 174 Ganodermataceae specimens representing 86 species. The dataset had an aligned length of 1,463 characters including gaps (ITS: 1–611; nrLSU: 612–1,463), of which Amauroderma rugosum Cui 9,011 and Sanguinoderma rude Cui 16,592 as the outgroup taxa (Figure 2; Sun et al., 2020, 2022). The Maximum likelihood analysis based on the concatenated ITS + nLSU dataset resulted in a similar topology as Bayesian Inference analysis. The RAxML analysis of the combined dataset yielded the best scoring tree with a final ML likelihood value of −8472.680716 (Figure 2). The matrix had 475 distinct alignment patterns, with 33.97% undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.230978, C = 0.222798, G = 0.276648, T = 0.269576; substitution rates AC = 1.230871, AG = 4.648437, AT = 1.401201, CG = 1.020212, CT = 9.538270, GT = 1.000000, α = 0.177171, Tree-Length: 1.586199. The best model for the ITS + nLSU dataset estimated and applied in the Bayesian analysis was HKY + I + G for ITS and GTR + I + G for nrLSU.
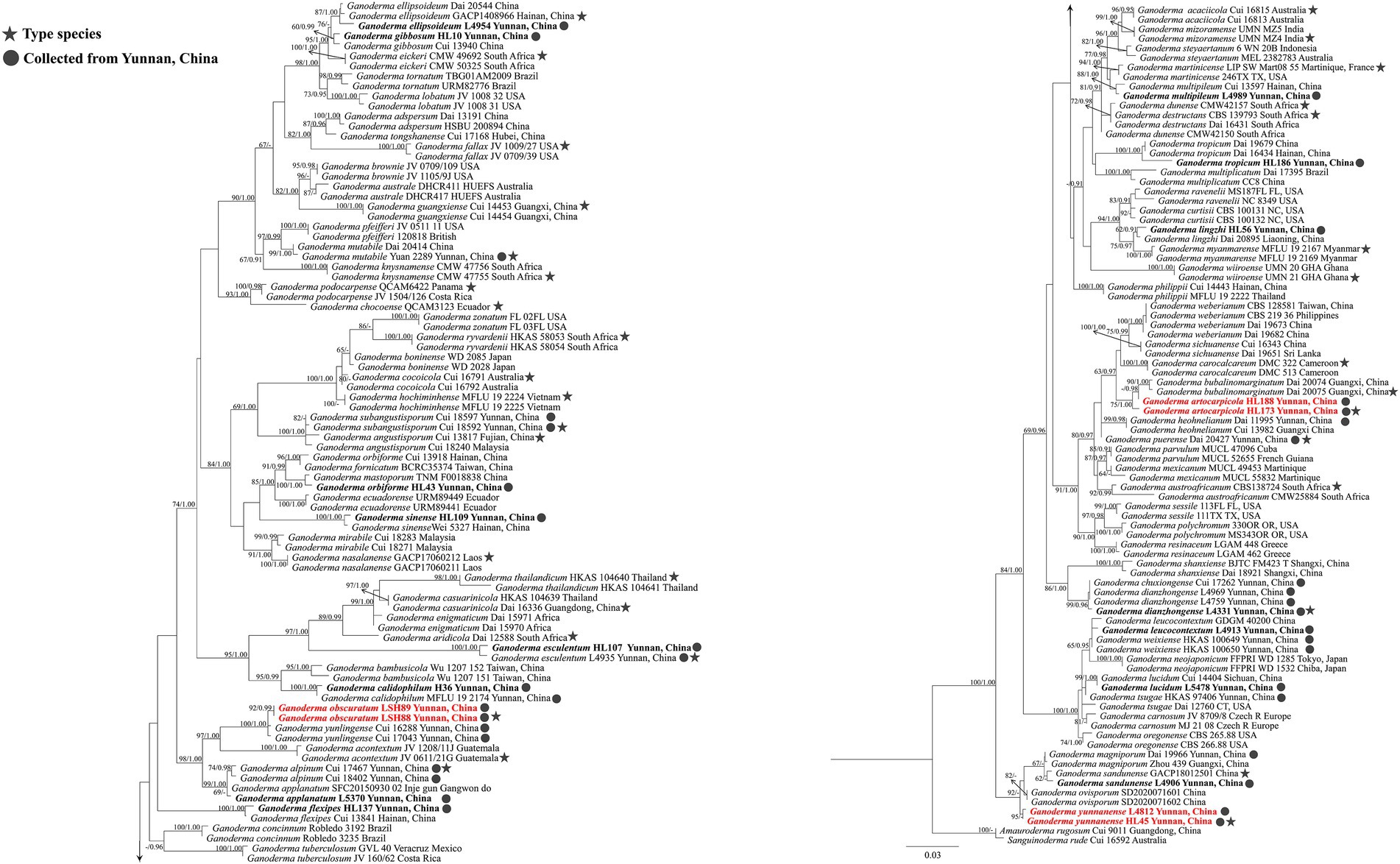
Figure 2. Maximum likelihood (ML) tree generated from a combined ITS + nrLSU sequence dataset. Bootstrap support values of maximum likelihood (ML) equal to or greater than 60% and Bayesian posterior probabilities (PP) equal to or greater than 0.90 are given above the nodes as “ML/PP.” New collections are indicated in black bold and new species are in red bold.
The dataset is composed of combined ITS + nrLSU + TEF1-α + RPB2 sequences data from 174 specimens, representing 86 taxa in Ganodermataceae. The aligned dataset comprised 2,995 characters including gaps (ITS: 1–611; nrLSU: 612–1,463; TEF1-α: 1,464–2002; RPB2: 2,003–2,663). Tree topology of the maximum likelihood analysis and Bayesian analysis is similar. The RAxML analysis of the combined dataset yielded the best scoring tree with a final ML likelihood value of −33599.741722 (Figure 3). The matrix had 1,087 distinct alignment patterns, with 36.13% undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.223924, C = 0.253042, G = 0.274308, T = 0.248726; substitution rates AC = 1.353439, AG = 6.944619, AT = 1.408316, CG = 1.653377, CT = 9.659772, GT = 1.000000, α = 0.194286, Tree-Length: 1.880697. Best model for the ITS + nLSU + TEF1-α + RPB2 dataset estimated and applied in the Bayesian analysis were HKY + I + G for ITS [Lset nst = 2, rates = invgamma; Prset statefreqpr = Dirichlet (1,1,1,1)] and GTR + I + G for nrLSU, TEF1-α and RPB2 [Lset nst = 6, rates = invgamma; Prset statefreqpr = Dirichlet (1,1,1,1)]. ML and BI analyses generated nearly identical tree topologies with minimal variations in statistical support values. Thus, only a ML tree is shown. Bootstrap support values in maximum likelihood (ML) equal to or greater than 60%, and Bayesian posterior probabilities (PP) equal to or greater than 0.90 are given above the nodes (Figures 2, 3).
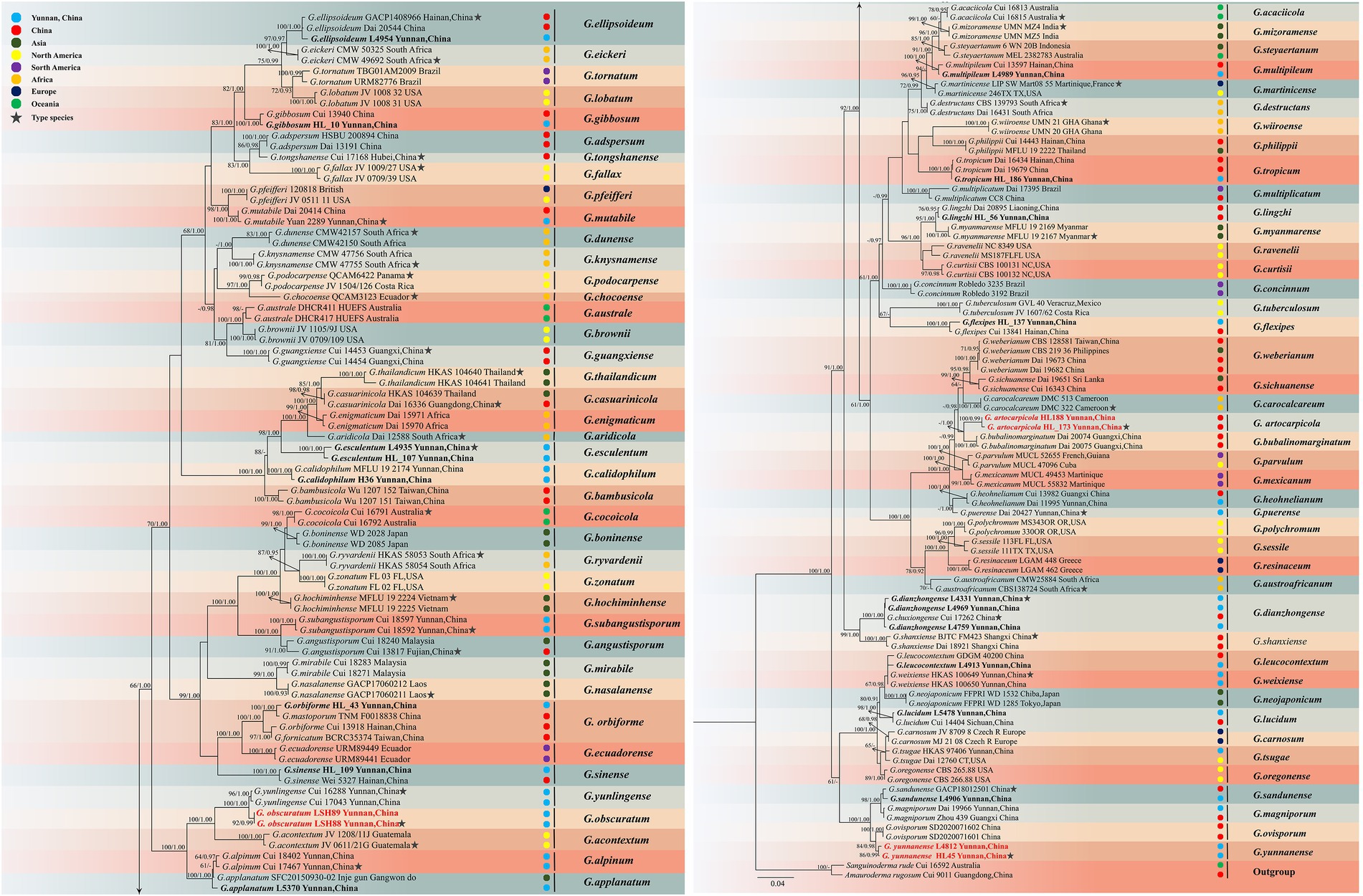
Figure 3. Maximum likelihood (ML) tree generated from a combined ITS + nrLSU + TEF1-α + RPB2 sequence dataset. Bootstrap support values with a maximum likelihood (ML) equal to or greater than 60% and Bayesian posterior probabilities (PP) equal to or greater than 0.90 given above the nodes as “ML/PP.” New collections are indicated in black bold while new species are in red bold.
The multigene phylogenetic analyses showed that 18 of our new specimens are nested in Ganoderma, of which three are described as new species. Ganoderma artocarpicola sp. nov. was sister to G. bubalinomarginatum B.K. Cui, J.H. Xing and Y.F. Sun with high statistical supports (−ML/1.00PP, Figure 3). Ganoderma obscuratum sp. nov. clustered as a sister clade with G. yunlingense B.K. Cui, J.H. Xing & Y.F. Sun and G. acontextum B.K. Cui, J.H. Xing & Vlasák with high statistical support (100%ML/1.00PP, Figure 3). The third species, G. yunnanense sp. nov. closely clustered with G. ovisporum H.D. Yang, T.C. Wen, G. magniporum J.D. Zhao & X.Q. Zhang and G. sandunense Hapuar., T.C. Wen and K.D. Hyde with high statistical support (100%ML/1.00PP), and a distinct lineage.
Taxonomy
Ganoderma artocarpicola J. He and S.H. Li, sp. nov. (Figure 4).

Figure 4. Ganoderma artocarpicola (HKAS 123782, holotype) (A–C) Basidiomata in situ on Artocarpus pithecogallus living tree. (D) Lower surface. (E) Transverse section of pileus. (F) Pore surface. (G) Sections of pileipellis. (H,I) Skeletal hyphae from context. (J) Binding hyphae from context. (K) Generative hyphae from tubes. (L) Basidia and basidioles. (M–P) Basidiospores. Scale bars: (G–J) = 20 μm, (K,L) = 10 μm, (M–P) = 5 μm. Photographs were taken by JH.
MycoBank number: MB845720
Diagnosis: Ganoderma artocarpicola is characterized by its sessile and concrescent basidiomata, reddish brown to yellowish brown pileus surface with shallow concentric furrows and radial rugose, heterogeneous context, wavy margin and ellipsoid to ovoid basidiospores (8.0–10.5 × 5.0–7.5 μm).
Etymology: The epithet ‘artocarpicola’ refers to the host tree genus Artocarpus.
Holotype: CHINA. Yunnan Province., Lincang City, Yongde County (24°54′51″N, 99°15′31″E), on living tree of Artocarpus pithecogallus, alt. 1,506 m, Jun He, 21 September 2021, HL188 (HKAS 123782).
Description: Basidiomata: annual, sessile and broadly attached, usually concrescent, woody hard. Pileus: imbricate, flabelliform to reniform, slightly convex to applanate, projecting up to 9 cm, 8 cm wide and 2 cm thick at the base. Pileus surface reddish brown (9E8) to yellowish brown (5C7), weakly to strongly laccate, with shallowly concentric furrows and radial rugose, concentrically zonate or azonate. Margin: buff (1A3) to grayish orange (6D8), entire, obtuse, irregularly wavy. Context: up to 1.8 cm thick, heterogeneous, the upper layer greyish white(2B1), the lower layer cinnamon brown (6D7) to chestnut brown (8E5), without black melanoid lines, hard corky and fibrous. Tubes: 0.2–0.5 cm long, dark brown (6E8), woody hard, unstratified. Pores: 5–7 per mm, circular to angular, dissepiments thick, entire; pores surface cream (2B2) to greyish white (2B1) when fresh, golden grey to greyish brown when bruising and drying.
Hyphal system trimitic: generative hyphae 2.0–3.5 μm in diameter, colorless, thin-walled, with clamp connections; skeletal hyphae 2.0–5.0 μm in diameter, thick-walled with a narrow lumen to sub-solid, arboriform and flexuous, pale yellow to yellowish brown; binding hyphae 1.5–3.0 μm in diameter, thick-walled, frequently branched, interwoven, colorless, scarce; all the hyphae IKI–, CB+; tissues darkening in KOH.
Pileipellis: a crustohymeniderm, cells 35–50 × 5–10 μm, thick-walled to sub-solid, apical cells clavate, inflated and flexuous, pale yellow to golden yellow, without granulations in the apex, moderately amyloid at maturity.
Basidiospores: ellipsoid to ovoid, not obviously truncated, with apical germ pore, yellowish to golden yellow, IKI–, CB+, inamyloid; double walled with slightly thick walls, exospore wall smooth, endospore wall with inconspicuous spinules; (60/3/2) 8.0 (8.5)–9.3–10.0 (10.5) × 5.0 (5.5)–6.2–7.0 (7.5) μm, L = 9.25 μm, W = 6.20 μm, Q = (1.23) 1.31–1.50–1.72 (1.78), Qm = 1.50 ± 0.14 (including myxosporium). Basidia: barrel-shaped to utriform, colorless, with a clamp connection and four sterigmata, thin-walled, 10–15 × 5–9 μm; basidioles pear-shaped to fusiform, colorless, thin-walled, 8–10 × 4–7 μm.
Additional specimen examined: China, Yunnan Province, Lincang City, Yongde County, Dedang Town (24°01′12″N, 99°15′34″E), on a living tree of Artocarpus pithecogallus, alt. 1,484 m, Qian-Qiu Luo, 22 August 2021, HL173 (HKAS 123783).
Notes: In the phylogenetic analyses, G. artocarpicola is sister to G. bubalinomarginatum, which was described from the southwest Guangxi Province in China (Figure 3; Sun et al., 2022). Morphologically, both species share similar characteristics of the connate and sessile basidiomata, reddish brown to yellowish brown pileus surface, and non-stratified tubes. However, G. bubalinomarginatum differs from G. artocarpicola in having buff and obtuse pileus margin, smaller basidiospores (7.0–8.8 × 4.3–5.8 μm), and larger basidia (15–22 × 7–11 μm, Sun et al., 2022).
Ganoderma weberianum and G. artocarpicola are similar in having imbricate, sessile and hard basidiomata. However, G. weberianum has a pale-yellow pore surface when dry, homogeneous greyish brown context, smaller basidiospores (6.0–7.0 × 4.0–6.0 μm), and longer pileipellis (60.0–90.0 × 6.0–12.0 μm, Steyaert, 1972; Pan and Dai, 2001). In addition, the pileus of G. weberianum is more laccate than G. artocarpicola. The comparison of the ITS sequences of G. weberianum and G. artocarpicola showed 2.12% (13/614 bp) nucleotide differences.
Ganoderma obscuratum J. He and S.H. Li, sp. nov. (Figure 5).
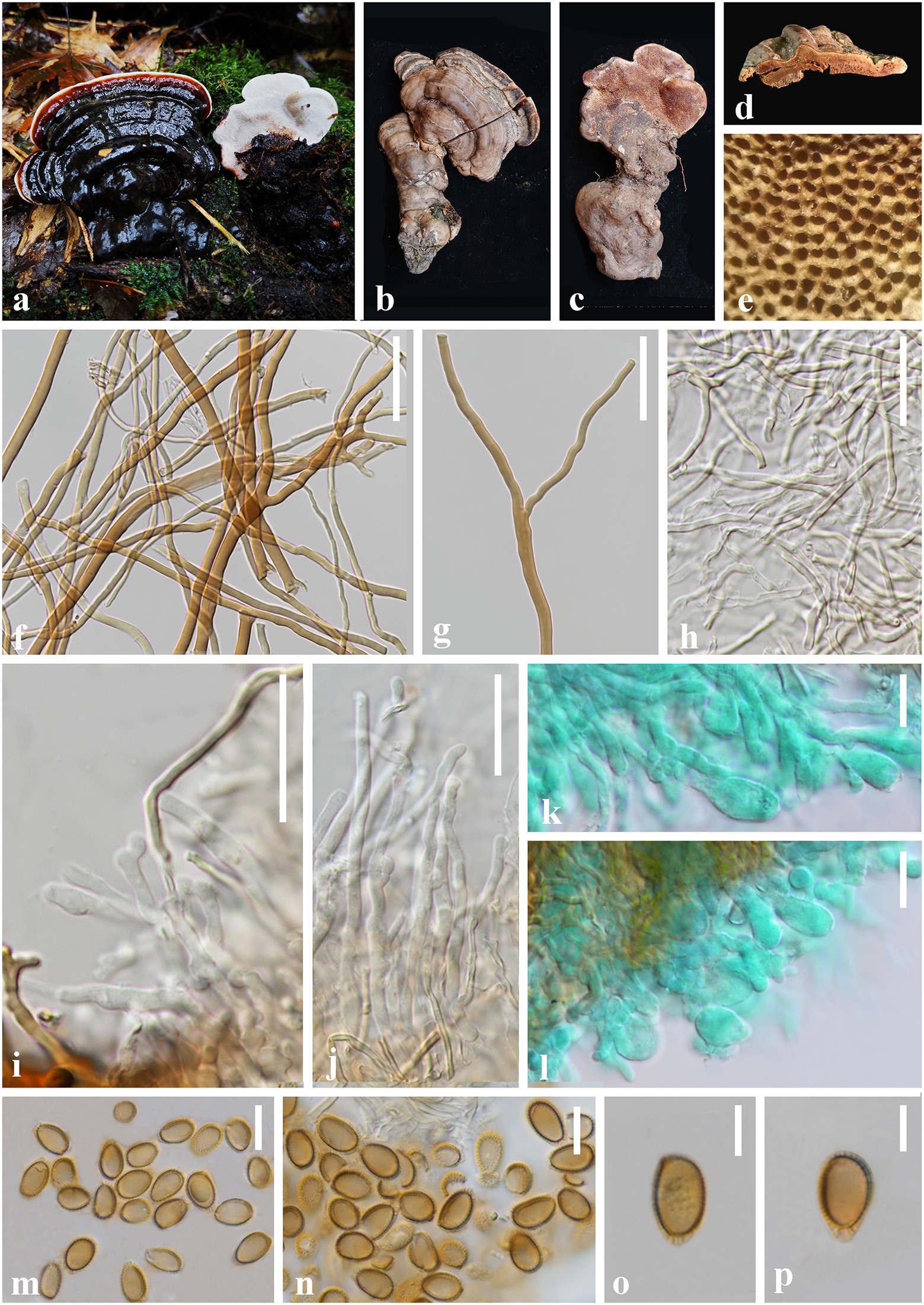
Figure 5. Ganoderma obscuratum (HKAS 123786, holotype) (A–C) Basidiomata. (D) Transverse section of pileus. (E) Pore surface. (F,G) Skeletal hyphae from context. (H) Binding hyphae from context. (I,J) Generative hyphae from tubes. (K,L) Basidia and basidioles. (M–P) Basidiospores. Scale bars: (F,G) = 30 μm, (H–N) = 10 μm, (O,P) = 5 μm. Photographs were taken by XH.
MycoBank number: MB845721
Diagnosis: Ganoderma obscuratum is characterized by its small and dorso-laterally stipitate basidiomata, dark brown to greyish brown and laccate pileus surface, small pores (6–9 per mm), corky context, and almond-shaped to narrow ellipsoid basidiospores (8.0–9.5 × 4.5–5.5 μm).
Etymology: The epithet ‘obscuratum’ refers to the obscure pileus surface when dry.
Holotype: CHINA. Yunnan Province., Zhaotong City, Yiliang County (104°14′55″E, 27°47′56″N), on a dead tree of Acer sp. alt. 1,859 m, Shu-Hong Li, 12 August 2019, Lsh88 (HKAS 123786).
Description: Basidiomata: annual, sessile to substipitate, coriaceous to woody hard, light in weight. Pileus: single, flabelliform to reniform or shell-shaped, applanate, projecting up to 6 cm, 4.5 cm wide and 1 cm thick at the base. Pileus surface dark brown (8E8) when fresh becoming greyish brown (7E8) when dry, and covered by a thin hard crust, laccate, glabrous and shiny, with dense concentric furrows. Margin: buff (8B2) to generally concolorous, entire, subacute to obtuse, slightly wavy, cracked when dry. Context: up to 0.7 cm thick, homogeneous, yellowish brown (5D5) to chestnut brown (6E8), with black melanoid lines, hard corky. Tubes: 0.2–0.4 cm long, concolorous with the base of the context, corky, unstratified. Pores: 6–9 per mm, circular, dissepiments slightly thick, entire; pores surface white to greyish white (2B1) when fresh, pale brown (6D6) to dark brown (7E7) when bruising and drying. Stipe: up to 6.5 cm long and 2.2 cm diam, flattened to cylindrical, fibrous to spongy, concolorous with pileus surface.
Hyphal system trimitic: generative hyphae 2.0–4.0 μm in diameter, colorless, thin-walled, with clamps connections; skeletal hyphae 2.0–8.0 μm in diameter, thick-walled with a wide to narrow lumen or sub-solid, arboriform with few branches, yellowish brown to golden yellow; binding hyphae 1.0–3.0 μm in diameter, thick-walled, branched and flexuous, colorless to pale yellow, scarce; all the hyphae IKI–, CB+; tissues darkening in KOH.
Basidiospores: almond-shaped to narrow ellipsoid, apex subacute, with apical germ pore, yellowish to yellowish brown, IKI–, CB+, inamyloid; double-walled with moderately thick walls, exospore wall smooth, endospore wall with inconspicuous spinules; (40/2/2; 8.0) 8.5–9.0–9.0 (9.5) × 4.5–5.2–5.0 (5.5) μm, L = 9.09 μm, W = 5.22 μm, Q = (1.58) 1.61–1.75–1.87 (2.08), Qm = 1.75 ± 0.11 (including myxosporium). Basidia: broadly clavate, colorless, with a clamp connection and four sterigmata, thin-walled, 15–25 × 5–9 μm; basidioles in shape like the basidia, colorless, thin-walled, 10–21 × 4–8 μm.
Additional specimens examined: China, Yunnan Province, Zhaotong City, Yiliang County, Xiaocaoba Town (104°14′18″E, 27°47′59″N), on a dead tree of Acer sp., alt. 1,905 m, Shu-Hong Li, 12 August 2019, Lsh89 (HKAS 123772).
Notes: Phylogenetic analyses showed that Ganoderma obscuratum clusters as a sister taxon to G. yunlingense with good statistical support (100% ML/1.00 PP, Figure 3). Morphologically, G. obscuratum differs from G. yunlingense by having thin basidiomata, dark brown and laccate pileus surface when fresh, homogeneous context and non-stratified tubes, smaller pores (6–9 per mm), and narrow ellipsoid basidiospores with spinules on the endospore wall (Sun et al., 2022).
Ganoderma alpinum described from Yunnan Province is morphologically similar to G. obscuratum by having the hard basidiomata with greyish brown pileus surface, homogeneous context and non-stratified tubes. However, G. alpinum differs by the larger pores (5–7per mm), and smaller basidiospores (6.2–7.8 × 4–5.5 μm, Sun et al., 2022). Ganoderma applanatum also has sessile basidiomata and homogeneous context, but it differs from G. obscuratum by having a perennial basidiomata with pale pileus surface and smaller basidiospores (5–8 × 4–6 μm, Moncalvo and Ryvarden, 1997; Hapuarachchi et al., 2019; Sun et al., 2022). Besides, G. applanatum and G. obscuratum were well separated in the phylogenetic analyses (Figure 3).
Ganoderma yunnanense J. He and S.H. Li, sp. nov. (Figure 6).
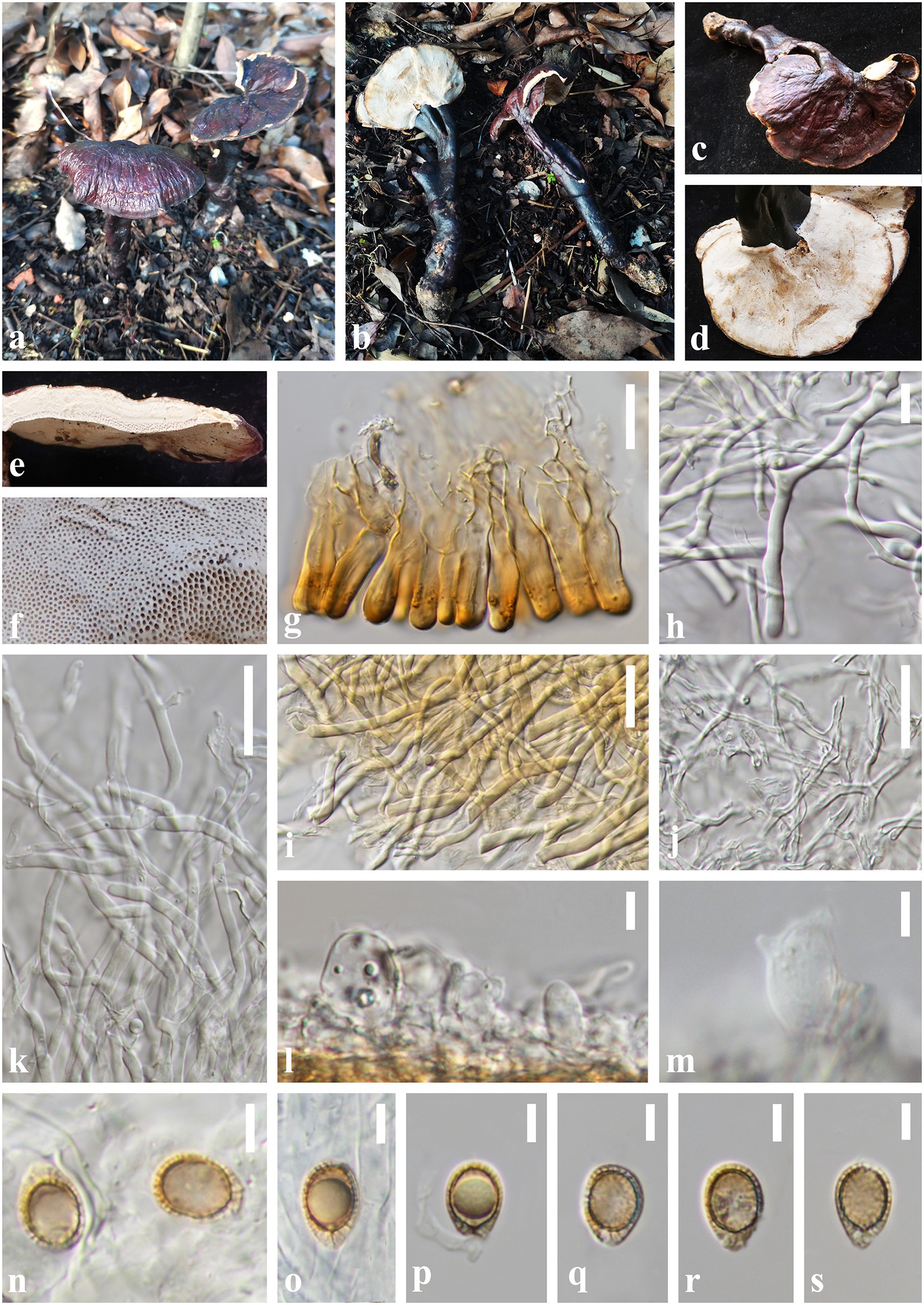
Figure 6. Ganoderma yunnanense (HKAS 123771, holotype) (A,B) Basidiomata. (C) Upper surface. (D) Lower surface. (E) Transverse section of pileus. (F) Pore surface. (G) Sections of pileipellis. (H,I) Skeletal hyphae from context. (J) Binding hyphae from context. (K) Generative hyphae from tubes. (L,M) Basidia and basidioles. (N–S) Basidiospores. Scale bars: (I–K) = 20 μm, (G,H) = 10 μm, (L–S) = 5 μm. Photographs were taken by JH.
MycoBank number: MB845722
Diagnosis: Ganoderma yunnanense is characterized by its centrally to laterally stipitate basidiomata with reddish brown to violet brown and strongly laccate pileus surface, cream color pore surface and context, and broadly ellipsoid basidiospores (8.0–12.5 × 7.0–9.0 μm).
Etymology: The epithet ‘yunnanense’ refers to Yunnan Province from where the holotype was collected.
Holotype: CHINA. Yunnan Province, Puer City, Jingdong County, Wuliang Mountains (100°48′48″E, 24°19′36″N), on a rotten broad-leaved tree, alt. 2,129 m, Song-Ming Tang, 8 August 2021, HL45 (HKAS 123771).
Description. Basidiomata: annual, centrally to laterally stipitate, hard corky. Pileus: single, flabelliform to reniform or suborbicular, projecting up to 9 cm, 6.5 cm wide and 0.5 cm thick at base. Pileus surface reddish brown (10F8) to violet brown (11F8), weakly to strongly laccate, glossy, with shallowly concentric furrows and radial rugose. Margin: pale yellow (3B2) to concolorous, entire, acute, incurved when dry. Context: up to 0.3 cm thick, homogeneous, white to cream (1B2), fibrous, corky, without black melanoid lines. Tubes: 0.1–0.2 cm long, concolorous with the base of the context, corky, unstratified. Pores: 4–6 per mm, round to angular, dissepiments thick, entire; pore surface white when fresh, lead grey (3B1) when bruising and drying. Stipe: 15.0–17.5 × 1.0–2.0 cm, dorsally lateral to nearly dorsal, cylindrical and solid, concolorous with pileus surface, strongly laccate, fibrous to woody.
Hyphal system trimitic: generative hyphae 2.0–3.0 μm in diameter, colorless, thin-walled, with clamps connections; skeletal hyphae 2.0–6.0 μm in diameter, subthick-walled to solid, non-septate, arboriform with few branches, colorless to pale yellow; binding hyphae 1.5–3.0 μm in diameter, thick-walled, frequently branched and flexuous, colorless, scarce; all the hyphae IKI–, CB+; tissues darkening in KOH.
Pileipellis: a crustohymeniderm, composed of a palisade of vertical, cells 23–40 × 6–9 μm, slightly thick-walled, clavate to cylindrical, slightly inflated, straw yellow to golden-yellow, granulations in the apex, moderately clavate to cylindrical amyloid at maturity.
Basidiospores: broadly ellipsoid to ellipsoid, apex not obviously truncated, with apical germ pore, yellowish to pale yellowish brown, IKI–, CB+, inamyloid; double-walled with distinctly thick walls, exospore wall smooth, endospore walls with inter-wall pillars; (40/2/2) (8.0) 9.0–10.7–12.0 (12.5) × 7.0–7.6–8.0 (8.5) μm, Q = (1.10) 1.25–1.41–1.55 (1.60), Qm = 1.41 ± 0.12 (including myxosporium). Basidia: widely clavate to barrel-shaped, colorless, with a clamp connection and four sterigmata, thin-walled, 15–18 × 8–11 μm; basidioles clavate, colorless, thin-walled, 10–14 × 6–9 μm.
Additional specimens examined: China, Yunnan Province., Puer City, Jingdong Co-unty, Ailao Mountains (101°01′29″E, 24°30′03 N), on a rotten broad-leaved tree, alt. 2,326 m, Jun He, 4 August 2019, L4812 (HKAS 123769).
Notes: Our multi-locus phylogenetic analyses show that Ganoderma yunnanense is sister to G. ovisporum with high statistical support (84% ML/0.98 PP, Figure 3), and together they group with G. sandunense and G. magniporum (Zhao et al., 1984; Hapuarachchi et al., 2019; Yang et al., 2022). Ganoderma yunnanense resembles G. ovisporum in having reddish-brown pileus and broadly ellipsoid basidiospores. However, G. ovisporum has heterogeneous context, shorter pileipellis cells (18–29 × 6–11 μm) and larger basidiospores (12.5–15.5 × 9.0–11.5 μm, Yang et al., 2022). Moreover, Ganoderma sandunens has a larger basidiospores (10.8–15.7 × 8.6–12.5 μm) and thicker context than those of G. yunnanense (Hapuarachchi et al., 2019; Yang et al., 2022). Ganoderma magniporum can be easily distinguished from G. yunnanense by the larger pores (2–2.5 per mm), black-brown to black pileus surface and ovoid basidiospores with truncated apex (8.7–10.4 × 5.2–7.0 μm, Zhao et al., 1984).
Morphologically, G. yunnanense resembles G. leucocontextum by white pore surface and context. However, G. leucocontextum has red to red brown pileus surface, white to yellowish margin, shorter stipe (5–10 cm) and broadly ellipsoid basidiospores with truncated apex (8.0–12.5 × 5.5–9.0 μm, Li et al., 2015). Among the species in the G. lucidum complex, G. yunnanense looks very similar to G. tsugae and G. weixiense morphologically, although they can be easily distinguished by phylogenetic analyses and ecological distribution (Murrill, 1902; Ye et al., 2019).
In addition, G. yunnanense also shares similarities with G. dianzhongense but the latter has dark-brown to putty context and wider pileipellis cells than those of G. yunnanense. The nucleotide comparison of ITS sequences of G. yunnanense and G. dianzhongense revealed 26 bp (26/614 bp, 4.23%) nucleotides differences (He et al., 2021).
Key to the species of Ganoderma in Yunnan Province, China
Discussion
Sun et al. (2022) revealed the species diversity, taxonomy and phylogeny of Ganodermataceae with emphasis on Chinese collections, which showed that 40 species of Ganoderma in China were confirmed by morphology and DNA sequence data. Among the 40 species, five new species of Ganoderma were discovered in YPC, namely G. alpinum, G. chuxiongense, G. puerense, G. subangustisporum, and G. yunlingense. Besides, Sun et al. (2022) summarized known species of Ganoderma in YPC viz. G. ellipsoideum、G. flexipes、G. hoehnelianum、G. lingzhi、and G. magniporum. However, results of our research showed that Ganoderma chuxiongense and G. dianzhongense are similar in morphology and phylogeny, and based on the time priority, G. chuxiongense is considered as a synonym of G. dianzhongense. In consideration of the authors’ contributions, it is suggested to use the sample Cui 17,262 (BJFC034120) as a paratype of Ganoderma dianzhongense (He et al., 2021; Sun et al., 2022).
To date, 25 species of Ganoderma have been recorded in YPC (Cao et al., 2012; Ye et al., 2019; He et al., 2021; Sun et al., 2022), however, the species diversity of Ganoderma is still not well known, especially in the subtropical and tropical areas. According to our survey of different sample sites in Yunnan Province from 2016 to 2021, a total of 268 samples of Ganoderma were collected. Based on comprehensive morphological characteristics and phylogenetic evidence, we report 15 known species of Ganoderma from YPC viz. Ganoderma applanatum, G. calidophilum, G. dianzhongense, G. ellipsoideum, G. esculentum, G. flexipes, G. gibbosum, G. leucocontextum, G. lingzhi, G. lucidum, G. multipileum, G. orbiforme, G. sandunense, G. sinense and G. tropicum. In addition, three new species viz. G. artocarpicola, G. obscuratum and G. yunnanense are proposed in this study. Up to now, 183 species of Ganoderma have been described all over the world, of which 42 species have been recorded in China (Wu et al., 2020; Sun et al., 2022; Yang et al., 2022). The discovery of three new species of Ganoderma in this study raises the known Ganoderma species in Yunnan Province to 32, accounting for 71.11% of the known Ganoderma species in China. Thus, Yunnan Province can be considered as one of the biodiversity center hot spots for Ganoderma.
A checklist of Ganoderma in YPC is given in Table 3. In addition, a key to Ganoderma in YPC is also provided. This paper enriches the knowledge of Ganoderma in YPC, and it is likely that more new taxa will be discovered in the future with extensive sampling in different areas and comprehensive molecular analyses.
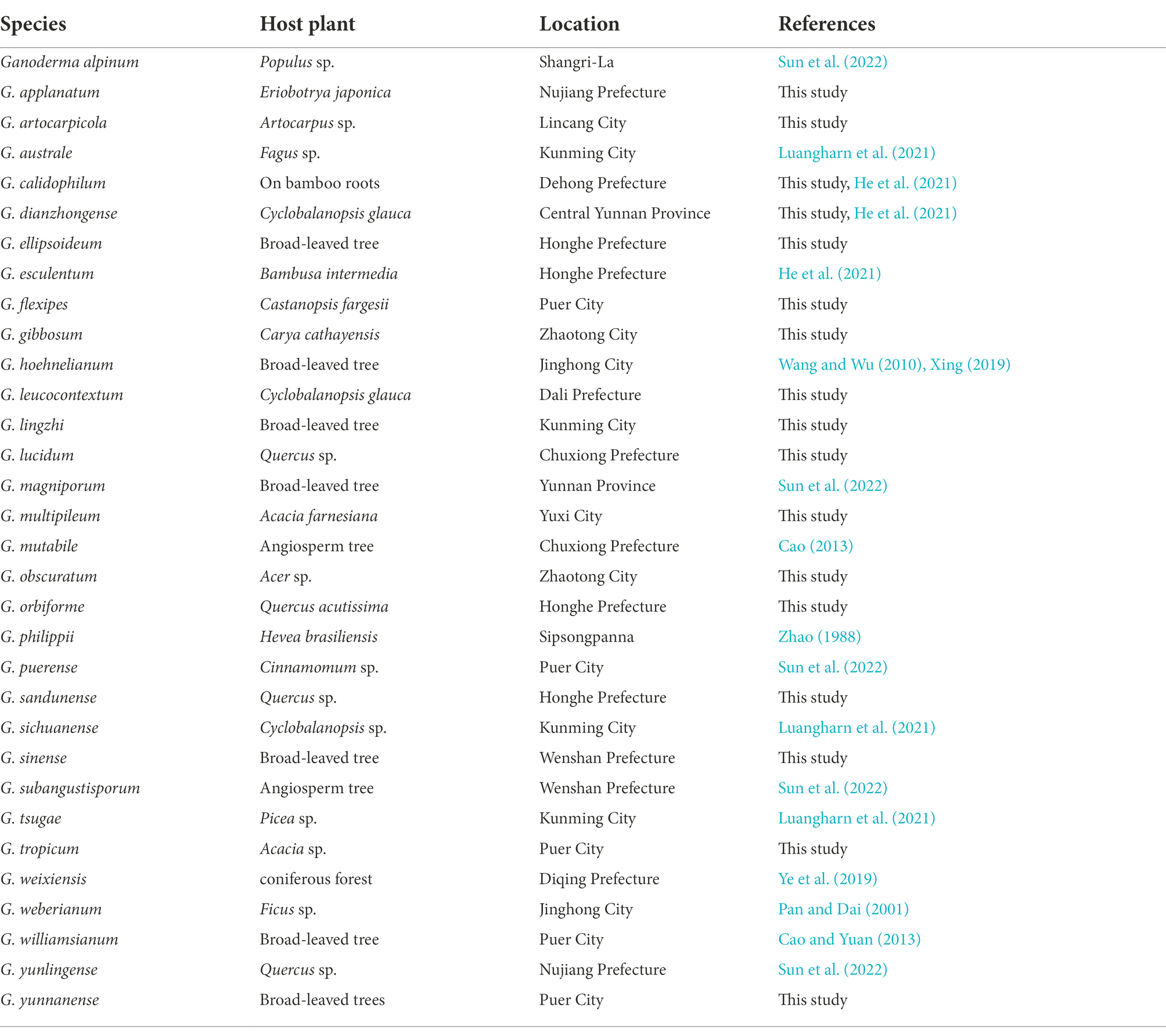
Table 3. Species, hosts, and geographical locations and corresponding references of Ganoderma in Yunnan Province, China.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
S-HL and Z-LL: conceptualization. JH: methodology, formal analysis, data curation, and writing—original draft preparation. JH and XH: investigation. S-HL and Z-ZL: resources. K-YN, S-MT, E-XL, H-ML, and S-HL: writing—review and editing. S-HL: funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the earmarked fund for CARS (Project ID: CARS-20) and the National Natural Science Foundation of China (Project ID: 32060006).
Acknowledgments
We would like to thanks Qian-Qiu Luo, Li Wang, Xin-Yu Ran, Cui-Qin Zhou, and Ying-Guo Shan for their help on sample collection, DNA extraction, and PCR amplification. Thanks to Shu-Qin Cao for her help in specimens’ preservation. We also thank Samantha C. Karunarathna at Qujing Normal University, China, and the reviewers for their helpful suggestions to improve this manuscript. At the same time, we would like to thank Hong-Yan Su for her support to our research work and the cultivation during our master’s degree before she passed away.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^http://www.indexfungorum.org/
3. ^http://mafft.cbrc.jp/alignment/server/
References
Adaskaveg, J. E., Blanchette, R. A., and Gilbertson, R. L. (1991). Decay of date palm wood by white–rot and brown–rot fungi. Can. J. Bot. 69, 615–629. doi: 10.1139/b91-083
Cao, Y. (2013). Taxonomy and phylogeny of Ganoderma in China. Ph.D. dissertation. China: The University of Chinese Academy of Sciences in Shenyang.
Cao, Y., Wu, S. H., and Dai, Y. C. (2012). Species clarification of the prize medicinal Ganoderma mushroom "Lingzhi". Fungal Divers. 56, 49–62. doi: 10.1007/s13225-012-0178-5
Cao, Y., and Yuan, H. S. (2013). Ganoderma mutabile sp. nov. from southwestern China based on morphological and molecular data. Mycol. Prog. 12, 121–126. doi: 10.1007/s11557-012-0819-9
Costa-Rezende, D. H., Robleo, G. L., Drechsler-Santos, E. R., Glen, M., Gates, G., de Madrignac, B. R., et al. (2020). Taxonomy and phylogeny of polypores with ganodermatoid basidiospores (Ganodermataceae). Mycol. Prog. 19, 725–741. doi: 10.1007/s11557-020-01589-1
Dai, Y. C., Yang, Z. L., Cui, B. K., Yu, C. J., and Zhou, L. W. (2009). Species diversity and utilization of medici-nal mushrooms and fungi in China. Int. J. Med. Mushrooms. 11, 287–302. doi: 10.1615/IntJMedMushr.v11.i3.80
Darriba, D., Taboada, G. L., Doallo, R., and Posada, D. J. (2012). ModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9:772. doi: 10.1038/nmeth.2109
Elliott, M., and Broschat, T. (2001). Observations and pathogenicity experiments on Ganoderma zonatum in Florida. Palms 45, 62–73.
Glen, M., Bougher, N. L., Francis, A. A., Nigg, S. Q., Lee, S. S., Irianto, R., et al. (2009). Ganoderma and Amauroderma species associated with root-rot disease of Acacia mangium plantation trees in Indonesia and Malaysia. Australas. Plant Pathol. 38, 345–356. doi: 10.1071/AP09008
Glez-Peña, D., Gómez-Blanco, D., Reboiro-Jato, M., Fdez-Riverola, F., and Posada, D. (2010). ALTER: program-oriented conversion of DNA and protein alignments. Nucleic Acids Res. 38, W14–W18. doi: 10.1093/nar/gkq321
Guindon, S., and Gascuel, O. A. (2003). Simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704. doi: 10.1080/10635150390235520
Hall, T. A. (1999). BioEdit: a user–friendly biological sequence alignment editor and analysis program for windows 95/98/NT. In Nucleic Acids Symposium Series. 95–98.
Hapuarachchi, K. K., Elkhateeb, W. A., Karunarathna, S. C., Cheng, C. R., Bandara, A. R., Kakumyan, P., et al. (2018a). Current status of global Ganoderma cultivation, products, industry and market. Mycosphere. 9, 1025–1052. doi: 10.5943/mycosphere/9/5/6
Hapuarachchi, K. K., Karunarathna, S. C., Phengsintham, P., Yang, H. D., Kakumyan, P., Hyde, K. D., et al. (2019). Ganodermataceae (Polyporales): diversity in greater Mekong subregion countries (China, Laos, Myanmar, Thailand and Vietnam). Mycosphere. 10, 221–309. doi: 10.5943/mycosphere/10/1/6
Hapuarachchi, K. K., Karunarathna, S. C., Raspé, O., De Silva, K. H. W. L., Thawthong, A., Wu, X. L., et al. (2018b). High diversity of Ganoderma and Amauroderma (Ganodermataceae, Polyporales) in Hainan Island, China. Mycosphere. 9, 931–982. doi: 10.5943/mycosphere/9/5/1
He, J., Luo, Z. L., Tang, S. M., Li, Y. J., Li, S. H., and Su, H. Y. (2021). Phylogenetic analyses and morphological characters reveal two new species of Ganoderma from Yunnan province. China. MycoKeys. 84, 141–162. doi: 10.3897/mycokeys.84.69449
Jayasiri, S. C., Hyde, K. D., Ariyawansa, H. A., Bhat, J., Buyck, B., Cai, L., et al. (2015). The faces of fungi database: fungal names linked with morphology, phylogeny and human impacts. Fungal Divers. 74, 3–18. doi: 10.1007/s13225-015-0351-8
Karsten, P. A. (1881). Enumeratio boletinearumet et polyproearum fennicarum, systemate novo dispositarum. Revue de Mycologie. 3, 16–19.
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Kornerup, A., and Wanscher, J. H. (1978). Methuen Handbook of Colour (3rd Edn.) London, England: Methuen.
Li, T. H., Hu, H. P., Deng, W. Q., Wu, S. H., Wang, D. M., and Tsering, T. (2015). Ganoderma leucocontextum, a new member of the G. lucidum complex from southwestern China. Mycoscience. 56, 81–85. doi: 10.1016/j.myc.2014.03.005
Liu, H., Guo, L. J., Li, S. L., and Fan, L. (2019). Ganoderma shanxiense, a new species from northern China based on morphological and molecular evidence. Phytotaxa. 406, 129–136. doi: 10.11646/phytotaxa.406.2.4
Liu, Y. j., Whelen, S., and Hall, B. D. (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 16, 1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092
Loyd, A. L., Smith, J. A., Richter, B. S., Blanchette, R. A., and Smith, M. E. (2017). The Laccate Ganoderma of the southeastern United States: a cosmopolitan and important genus of wood decay fungi: PP333, 1/2017. EDIS 2017:6. doi: 10.32473/edis-pp333-2017
Luangharn, T., Karunarathna, S. C., Dutta, A. K., Paloi, S., Lian, C. K., Huyen, L. T., et al. (2021). Ganoderma (Ganodermataceae, Basidiomycota) species from the greater Mekong subregion. J. Fungi. 7, 1–83. doi: 10.3390/jof7100819
Matheny, P. B., Wang, Z., Binder, M., Curtis, J. M., Lim, Y. W., Nilsson, R. H., et al. (2007). Hibbett DS contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, fungi). Mol. Phylogenet. Evol. 43, 430–451. doi: 10.1016/j.ympev.2006.08.024
Miller, M. A., Pfeiffer, W., and Schwartz, T. (2010). Creating the CIPRES science gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, United States, pp. 1–8.
Moncalvo, J. M., and Ryvarden, L. A. (1997). Nomenclatural study of the Ganodermataceae Donk. Syn-opsis Fungorum. 11, 1–114.
Murrill, W. A. (1902). The Polyporaceae of North America: I. the genus Ganoderma. Bull. Torrey. Bot. Club. 29, 599–608. doi: 10.2307/2478682
Nur, Q. O., Suhaila, S., Yang, P. L., and Joon, S. T. (2019). Transcriptomic data of mature oil palm basal trunk tissue infected with Ganoderma boninense. ELSEVIER. 25, 104–288. doi: 10.1016/j.dib.2019.104288
Nylander, J. A. A. (2004). MrModeltest 2.0; Program Distributed By the Author. Uppsala: Uppsala University.
Otjen, L., Blanchette, R., Effland, M., and Leatham, G. (1987). Assessment of 30 white rot basidiomycetes for selective lignin degradation. Holzforschung. 41, 343–349.
Pan, H. Y., and Dai, Y. C. (2001). Ganoderma weberianum newly recorded from Mainland of China. Fung. Sci., 16, 31–349.
Rannala, B., and Yang, Z. (1996). Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J. Mol. Evol. 43, 304–311. doi: 10.1007/BF02338839
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Sinclair, W. A., and Lyon, H. H. (2005). Diseases of Trees and Shrubs. New York, USA: Comstock Publishing Associates.
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Steyaert, R. L. (1972). Species of Ganoderma and related genera mainly of the Bogor and Leiden herbaria. Persoonia. 7, 55–118.
Sun, Y. F., Costa-Rezende, D. H., Xing, J. H., Zhou, J. L., Zhang, B., Gibertoni, T. B., et al. (2020). Multi-gene phylogeny and taxonomy of Amauroderma s. lat. (Ganodermataceae). Persoonia. 44, 206–239. doi: 10.3767/persoonia.2020.44.08
Sun, Y. F., Xing, J. H., He, X. L., Wu, D. M., Song, C. G., Liu, S., et al. (2022). Species diversity, systematic revision and molecular phylogeny of Ganodermataceae (Polyporales, Basidiomycota) with an emphasis on Chinese collections. Stud. Mycol. 101, 287–415. doi: 10.3114/sim.2022.101.05
Swoford, D. L. (2003). PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods). Sunderland, MA: Sinauer Associates.
Tonjock, R. K., and Afui, M. M. (2015). Diversity and distribution of species of Ganoderma in south western Cameroon. J. Yeast Fungal Res. 6, 17–24. doi: 10.5897/JYFR2014.0150
Torres-Torres, M. G., and Guzmán-Dávalos, L. (2012). The morphology of Ganoderma species with a laccate surface. Mycotaxon. 119, 201–216. doi: 10.5248/119.201
Vaidya, G., Lohman, D. J., and Meier, R. (2011). SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics. 27, 171–180. doi: 10.1111/j.1096-0031.2010.00329.x
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Wang, D., and Wu, S. H. (2010). Ganoderma hoehnelianum has priority over G. shangsiense, and G. williamsianum over G. meijiangense. Mycotaxon. 113, 343–349. doi: 10.5248/113.343
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). “Amplification and direct sequencing of fungal ri-bosomal RNA genes for phylogenetics,” in PCR Protocols: A Guide to Methods and Applications. eds. M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (San Diego, NY: Academic), 315–322.
Wu, S. H., Chern, C. L., Wei, C. L., Chen, Y. P., Akiba, M., and Hattori, T. (2020). Ganoderma bambusicola sp. nov. (Polyporales, Basidiomycota) from southern Asia. Phytotaxa. 456, 75–85. doi: 10.11646/phytotaxa.456.1.5
Xing, J. H. (2019). Species diversity, taxonomy and phylogeny of Ganoderma. Ph.D. dissertation. China: The University of Beijing Forestry in Beijing.
Xing, J. H., Song, J., Decock, C., and Cui, B. K. (2016). Morphological characters and phylogenetic analysis reveal a new species within the Ganoderma lucidum complex from South Africa. Phytotaxa. 266, 115–124. doi: 10.11646/phytotaxa.266.2.5
Xing, J. H., Sun, Y. F., Han, Y. L., Cui, B. K., and Dai, Y. C. (2018). Morphological and molecular identification of two new Ganoderma species on Casuarina equisetifolia from China. MycoKeys. 34, 93–108. doi: 10.3897/mycokeys.34.22593
Yang, H. D., Ding, Y., Wen, T. C., Hapuarachchi, K. K., and Wei, D. P. (2022). Ganoderma ovisporum sp. nov. (Polyporales, Polyporaceae) from Southwest China. Biodiv Data J. 10, 1–31. doi: 10.3897/BDJ.10.e80034
Ye, L., Karunarathna, S. C., Mortimer, P. E., Li, H. L., Qiu, M. H., Peng, X. R., et al. (2019). Ganoderma weixiensis (Polyporaceae, Basidiomycota), a new member of the G. lucidum complex from Yunnan Province, China. Phytotaxa. 423, 75–86. doi: 10.11646/phytotaxa.423.2.3
Zhao, J. D. (1988). Studies on the taxonomy of Ganodermataceae in China IX. Subgenus Elfvingia (Karst.) Imazeki. Acta Mycol. Sin. 13–22.
Zhao, J. D., Zhang, X. Q., and Xu, L. W. (1984). Studies on the taxonomy of Ganodermataceae in China III. Acta Mycol. Sin. 3, 15–23.
Keywords: 3 new taxa, basidiomycetes, Lingzhi, medicinal mushroom, multigene phylogeny, taxonomy
Citation: He J, Han X, Luo Z-L, Li E-X, Tang S-M, Luo H-M, Niu K-Y, Su X-j and Li S-H (2022) Species diversity of Ganoderma (Ganodermataceae, Polyporales) with three new species and a key to Ganoderma in Yunnan Province, China. Front. Microbiol. 13:1035434. doi: 10.3389/fmicb.2022.1035434
Edited by:
Nakarin Suwannarach, Chiang Mai University, ThailandReviewed by:
Kalani Hapuarachchi, Guizhou University, ChinaThatsanee Luangharn, Mae Fah Luang University, Thailand
Copyright © 2022 He, Han, Luo, Li, Tang, Luo, Niu, Su and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu-Hong Li, c2h1aG9uZ2Z1bmdpQDEyNi5jb20=
 Jun He
Jun He Xiao Han2
Xiao Han2 Zong-Long Luo
Zong-Long Luo Hong-Mei Luo
Hong-Mei Luo