
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 08 November 2022
Sec. Terrestrial Microbiology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1026102
This article is part of the Research Topic Exploration of Cold-Adapted Microorganisms For Sustainable Development View all 4 articles
Microorganisms including yeasts are responsible for mineralization of organic matter in cold regions, and their characterization is critical to elucidate the ecology of such environments on Earth. Strategies developed by yeasts to survive in cold environments have been increasingly studied in the last years and applied to different biotechnological applications, but their knowledge is still limited. Microbial adaptations to cold include the synthesis of cryoprotective compounds, as well as the presence of a high number of genes encoding the synthesis of proteins/enzymes characterized by a reduced proline content and highly flexible and large catalytic active sites. This study is a comparative genomic study on the adaptations of yeasts isolated from the Italian Alps, considering their growth kinetics. The optimal temperature for growth (OTG), growth rate (Gr), and draft genome sizes considerably varied (OTG, 10°C–20°C; Gr, 0.071–0.0726; genomes, 20.7–21.5 Mpb; %GC, 50.9–61.5). A direct relationship was observed between calculated protein flexibilities and OTG, but not for Gr. Putative genes encoding for cold stress response were found, as well as high numbers of genes encoding for general, oxidative, and osmotic stresses. The cold response genes found in the studied yeasts play roles in cell membrane adaptation, compatible solute accumulation, RNA structure changes, and protein folding, i.e., dihydrolipoamide dehydrogenase, glycogen synthase, omega-6 fatty acid, stearoyl-CoA desaturase, ATP-dependent RNA helicase, and elongation of very-long-chain fatty acids. A redundancy for several putative genes was found, higher for P-loop containing nucleoside triphosphate hydrolase, alpha/beta hydrolase, armadillo repeat-containing proteins, and the major facilitator superfamily protein. Hundreds of thousands of small open reading frames (SmORFs) were found in all studied yeasts, especially in Phenoliferia glacialis. Gene clusters encoding for the synthesis of secondary metabolites such as terpene, non-ribosomal peptide, and type III polyketide were predicted in four, three, and two studied yeasts, respectively.
Studies on the strategies exhibited by microbial communities inhabiting cold regions worldwide to thrive at low temperatures have increased in recent decades. Cold environments, characterized by temperatures below 5°C, predominate on Earth and represent a harsh habitat for microbial life leading to a strong reduction of growth rates (Feller and Gerday, 2003; D’Amico et al., 2006; Margesin and Feller, 2010; Margesin and Miteva, 2011). Cold-adapted (i.e., psychrophilic) microorganisms have evolved molecular and metabolic adaptations to overcome the adverse effects of low temperatures, including the synthesis of cryoprotectant molecules, cold-active enzymes, and polyunsaturated lipids regulating the degree of membrane fluidity (Margesin et al., 2007; Buzzini and Margesin, 2013; Alcaíno et al., 2015; Baeza et al., 2017). An increase in antioxidant enzymes activities was observed in Antarctic fungi after incubation at low temperatures, suggesting that the antioxidant defense could play a significant role in microbial survival when subjected to cold conditions (Gocheva et al., 2006, 2009; Kostadinova et al., 2012). When the Antarctic yeast Mrakia blollopis was submitted to a cold-shock an accumulation of TCA-cycle metabolites, lactic acid, and polyamines was observed (Tsuji, 2016). Polyamines are recognized to regulate important functions in cell growth, development, and cellular protection against stresses (Valdés-Santiago and Ruiz-Herrera, 2013). In the genome of the cold-adapted yeast Mrakia psychrophila there is a high numbers of genes encoding for Major Facilitator Superfamily (MFS) proteins (133), which play multiple roles including the incorporation of nutrients from the environment (Perlin et al., 2014; Su et al., 2016), and suggested as an adaptation to survive in its environment. Considering the protein 3D structural level, the highly flexible and larger active site exhibited by cold-active enzymes is considered responsible for their higher specific activity in comparison to their mesophilic counterparts (Collins et al., 2008; Jung et al., 2008; Feller, 2013). The reduced proline content found in bacterial cold-active proteins has been proposed as an adaptation strategy to attenuate the negative effect of proline isomerization on protein folding (Wedemeyer et al., 2002; Feller and Gerday, 2003; Feller, 2010).
OMICs technologies have become practical approaches for studying microorganisms, opening a window to find novel and holistic strategies of cold adaptation expressed by microbial cells (Casanueva et al., 2010). A higher number of rRNA and tRNA genes have been found in psychrophilic than mesophilic bacterial genomes suggesting an adaptation strategy to compensate the reduced translation rate under low-temperature conditions (Médigue et al., 2005; Methé et al., 2005; Riley et al., 2008; Choi et al., 2020). However, in the few studies about genomes of psychrophilic yeasts the number of rRNA and tRNA genes was variable and not consistently higher than mesophilic yeasts (Firdaus-Raih et al., 2018; Baeza et al., 2021; Yusof et al., 2021).
Genes associated with cold tolerance were found in genomes of the yeast Naganishia vishniacii isolated from McMurdo Dry Valleys, Antarctica; putative genes encoding for solute transfers, chaperones, associated with photoprotection, desaturases, trehalose synthesis, and lipid metabolism were found (Nizovoy et al., 2021). The yeast genus Glaciozyma includes psychrophilic species isolated from Antarctica and European glaciers, comprising some species formerly classified into different genera (e.g., Leucosporidium antarcticum, which has been re-classified as Glaciozyma antarctica; Turchetti et al., 2011). Genes encoding for proteins stimulating cold survival, e.g., antifreeze proteins (AFPs), were identified in the genome of G. antarctica PI12 (Wong et al., 2019). The comparisons with other genomes of two mesophilic yeasts, two thermophilic, and one psychrophilic fungi revealed that a third of the genes identified in G. antarctica were absent in the more closely related yeasts, suggesting that these genes, which are unique for G. antarctica, could be associated to its psychrophilic habitus (Firdaus-Raih et al., 2018). Furthermore, a 4% of the annotated open reading frames (ORFs) found in the G. antarctica genome corresponded to small ORFs (smORFs) not exceeding 100 amino acids, which may have a role in cold adaptation (Mat-Sharani et al., 2015). Thousands to millions of smORFs have been found in other organisms, including humans, insects (i.e., Drosophila melanogaster), metazoans, and yeasts (i.e., Saccharomyces cerevisiae), in which they fulfill key physiological functions (Couso and Patraquim, 2017).
The analysis of genomes and transcriptomes of a few Antarctic yeasts revealed the presence of a considerable number of putative genes involved in the synthesis of enzymes regulating the response to stress; most of them were classified as oxidative, general, and cold-associated responses, including desaturases, chaperones and chaperonins, glutathione S-transferase, catalase, and enzymes for the production and regulation of compatible osmolytes. Furthermore, putative genes involved in the synthesis of secondary metabolites, i.e., non-ribosomal peptide synthase (NRPS), terpenes, and type III polyketide synthases (PKSs) were found (Baeza et al., 2021, 2022). A global comparative analysis of in silico generated proteomes among Antarctic yeasts in comparison with their different optimal temperature for growth (OTG) and growth rates (Gr), revealed that a general trend of high content of flexible proteins associated with low OTG was found in yeasts characterized by fast growth. When subjected to cold stress, yeasts characterized by similar growth parameters exhibited similar transcriptomic level (Baeza et al., 2022). Interestingly, a significant upregulation of genes encoding for heat-shock proteins and chaperones, fatty acid desaturases, and proteasomal subunits, associated with the downregulation of ribosomal subunits were observed in yeasts exhibiting the higher OTG (e.g., Candida sake and Wickerhamomyces anomalus), but not in those with lower OTG (e.g., Mrakia gelida, and Cryptococcus sp.). In contrast, some yeasts characterized by lower OTG showed an upregulation of ribosomal subunits, suggesting the presence of differences strategies of adaptation to cold depending on their specific OTG (Baeza et al., 2022). In Rhodosporidium kratochvilovae strain YM25235 subjected to low-temperature stress, 1,300 differentially expressed genes (DEGs) were detected by next-generation deep sequencing technology (RNA-seq). From them, the pathways included the MAPK signaling pathway, metabolic pathways, and amino sugar and nucleotide sugar metabolism were overrepresented (Guo et al., 2021). The MAP-Kinase HOG1 would play a role in cold adaptation in R. kratochvilovae by promoting biosynthesis of polyunsaturated fatty acids and glycerol (Chen et al., 2022).
In recent years, the genomes of cold adapted yeasts from Antarctica were sequenced and characterized; their diverse responses to cold adaptations were studied, analyzing genomes and transcriptomes (Wong et al., 2019; Baeza et al., 2021, 2022; Nizovoy et al., 2021; Touchette et al., 2022). The extension of these studies to cold-adapted yeasts isolated from other geographical areas, which are characterized by chemical–physical and ecological conditions (including a different annual and seasonal temperature range) very different from those of the Antarctic habitats, is essential to advance toward a global understanding of yeasts’ adaptative strategies to live in cold habitat worldwide. In this work, the genomes of four strains of different cold-adapted yeast species isolated from the Italian Alps and Apennines were sequenced, analyzed, and compared in relation to their different optimal growth temperatures. In this framework, a comparative analysis among yeasts was performed considering their growth properties, genome size, protein flexibility, and putative genes encoding for cold stress response and secondary metabolites.
The yeast strains used in the present study were Goffeauzyma gilvescens DBVPG 4707, Glaciozyma martinii DBVPG 4841, Naganishia antarctica DBVPG 5271, and Phenoliferia glacialis DBVPG 5880, all preserved in the Industrial Yeast Collection DBVPG of the University of Perugia, Italy. They were all isolated from soil or sediments of cold habitats associated to high mountain glaciers of Italian Alps and Apennines. More in-depth information on strains used in this study are available on the website of DBVPG Collection (www.dbvpg.unipg.it). All the strains were routinely maintained in physiological inactive state (−80°C). Working cultures were grown on YEPG (yeast extract 10 g L−1, peptone 10 g L−1, glucose 20 g L−1, agar 15 g L−1) agar slants at 25°C (G. gilvescens) or 15°C (G. martinii, N. antarctica, and P. glacialis).
Calibrated (A600 = 0.8, which was considered an average cell concentration = 107 cells/mL) suspensions of 48 h-old cells of the 4 strains were used to inoculate 200 μl of YEPG into 96 wells microplate to obtain A600 = 0.1. Microplates were incubated at 4°C, 10°C, 15°C, 20°C, 25°C, and 30°C and A600 was checked every day for 10 days. Growth curves were determined in triplicate; the growth rates (Gr) of each strain were calculated from the exponential phase of the growth curves while, the temperature in which the highest A600 during exponential phase was observed was considered the optimum temperature for growth (OTG).
DNA Was extracted using standard methods based On The treatment of yeasts cells with CTAB (hexadecyltrimethylammonium bromide) and chloroform/isoamyl alcohol buffers. Briefly, 72 h yeast cells were suspended In pre-warmed CTAB extraction buffer containing CTAB, NaCl, EDTA, Tris HCl, PVP (Polyvinylpyrrolidone), β-mercaptoethanol, and proteinase K (sigma, Aldrich) and incubated at 60°C for 1 h. then chloroform/isoamylalcohol (24:1) Solution Was added and The Mix Was centrifuged To recover The aqueous phase. The DNA Was then purified using pectinase and RNAase (sigma, Aldrich) incubating The Mix at 37°C, and phenol:Chloroform:Isoamylalcohol (25:24:1; Sigma, Aldrich). After centrifugation, DNA contained In The aqueous phase Was additionally washed with sodium acetate (3 M), isopropanol and ethanol 70% (sigma, Aldrich). After an additional centrifugation, DNA Was suspended In ultrapure water and maintained at −20°C until quantification and sequencing The DNA Was quantified By qubit Fluorometric assay (ThermoFisher scientific) To obtain a concentration higher than 20 ng/μl.
NGS by the Omics2view company (Germany, http://www.omics2view.consulting) on MGI DNBSEQ-G400 in 2 × 150 bp mode, including sequence processing as follow. Quality of demultiplexed reads was checked with FastQC v0.11.7 (Andrews, 2018). A summary QC report created with MultiQC v1.10 (Ewels et al., 2016) is available for download. Read quality trimming was performed with the BBTools package v38.45 (Bushnell, 2019). This comprised the removal of optical duplicates, human sequences, adapter sequences, low entropy reads, and trimming of bases with quality scores <20. Reads with invalid or ambiguous bases and reads with a length < 50 base pairs (bp) were discarded. Only reads surviving quality trimming as pairs entered downstream analysis. Read quality recalibration and error correction was performed with the BBTools package v38.45 (Bushnell, 2019). Quality-trimmed reads were aligned to a preliminary de-novo assembly made with Tadpole from a subset of the quality-trimmed reads. Alignment information was used to recalibrate the base quality of all quality-trimmed reads. Sequencing errors were corrected by consecutively applying BBTools programs BBMerge, Clumpify, and Tadpole in error correction mode on the quality-recalibrated reads. Results are referred to as “filtered reads.” 31-bp kmers of filtered reads were normalized with BBNorm from the BBTools package v38.45 (Bushnell, 2019) to a target kmer depth of 100×, with a minimum kmer depth of 3×. Contiguous sequences (contigs) were assembled from normalized reads and further combined to Scaffolds ≥500 bp with SPAdes v3.15.2 (Bankevich et al., 2012) using k-mer lengths up to 127 bp. The genome completeness was evaluated by BUSCO v.5.0 (Nishimura et al., 2017, 2019) using the Fungi datasets.
Draft genomes have been deposited at DDBJ/ENA/GenBank under the following accession numbers and versions: G. gilvescens DBVPG 4707: JANGFI000000000; G. martinii DBVPG 4841: JANGFH000000000; N. antarctica DBVPG 5271: JANGFJ000000000; P. glacialis DBVPG 5880: JANHKM000000000.
The draft genomes were submitted to antiSMASH 6 fungal version to predict biosynthesis of secondary metabolites clusters in strict mode using default parameters (Blin et al., 2019). Each predicted core biosynthetic gene (CBG) and additional biosynthetic gene (ABG) of different gene clusters was compared to NCBI nr database by Blastp using default parameters, and the complete sequence of first 10 hits were retrieved. The sequences were analyzed using the maximum likelihood method and JTT matrix-based model (Jones et al., 1992) using the MEGA11 software (Kumar et al., 2018; Stecher et al., 2020). Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using a JTT model and then selecting the topology with a superior log-likelihood value.
Bioinformatic analysis was performed using Geneious Prime 2020.1.2 software (Kearse et al., 2012). The CDSs with lengths ≥210 nt were predicted in draft genomes using the generic training of the Augustus gene prediction program (Stanke and Morgenstern, 2005; Stanke and Tzvetkova, 2006), trough the Geneious Augustus plugin. The CDS were in silico extracted, translated, and compared by Blastp against a local curated fungal protein database (updated in June 2021). The hits having at least 30% similarity and E values ≤10−10 were considered for annotation. The annotated CDSs were classified according to cellular function predicted using KAAS – KEGG Automatic Annotation Server (Moriya et al., 2007)1 trough GHOSTX, and using default parameters and the datasets for Genes and Eukaryotes.
The translated and annotated CDS were used for comparative analysis of their flexibilities calculated based on their sequences of amino acids. For that, the percentage of amino acids classified according to their flexibility index (Li et al., 2006; Rao et al., 2011) either as very flexible (Vf: E, G, K, N, Q, and S) or moderately flexible (Mf: A, D, H, I, P, R, T, V) were calculated for each CDS. Furthermore, the global flexibility of each CDS was predicted using MEDUSA, a Deep-Learning based protein flexibility tool that uses as input the information from homologous protein sequences and amino acid physicochemical properties, using default parameters (Vander Meersche et al., 2021). From that, the percentage of residues grouped in three predicted flexibility classes was considered, M0, M1, and M2, from rigid to flexible. For each CDS the percentages of amino acids grouped as Vf, Vf plus Mf (VMf), M2, and M1 + 2, were calculated. The comparisons among yeasts were performed globally, considering the results for all CDS, and using the CDSs classified by cellular function by applying one-way ANOVA followed by a post hoc Tukey analysis. The data distribution was visualized using histograms and quantile-quantile (QQ) plots and did not find any indication that the data did not have a normal distribution. The CDS that displayed significant differences (significance threshold of 0.05) in their calculated flexibilities in the comparisons among yeasts at the global level or grouped by cellular function were selected to evaluate a probable correlation to yeast’s growth parameters. For that, the differences among yeasts in calculated flexibilities of CDS were plotted vs. their differences in the optimal temperature for growth or growth rate, and linear regressions were applied.
The ORFs between 30 and 300 nt were predicted in the draft genomes using the ORF finder included in Geneious software, annotated as SmORFs, and classified as those present in the CDS (CDSSmORFs), into 1,000 nt upstream (5’SmORFs) and into 1,000 nt downstream (3’SmORFs) from the CDS. SmORFs were translated and annotated by Blastp comparison to a local SmOrfs database constructed with the data downloaded from the web pages smORFunction2 (Ji et al., 2020) and SmProt3 (Hao et al., 2018; Li et al., 2021).
This analysis was conducted to compare the P. glacialis isolates from the Italian Alps and Antarctica and other members belonging to order Kriegeriales for which the genomes are available in the NCBI database were included. The corresponding genomes were downloaded (Supplementary Table S1), and the CDS were predicted and annotated, as mentioned above. The CDS encoding for putative proteins common to at least eight yeasts, including both P. glacialis isolates, were selected for more analysis. Furthermore, the analysis was performed for SmORFs. The translated SmORFs with at least 30% similarity in Blastp comparison against the local SmORF database mentioned above and common to three and all the studied yeasts were considered. The reference SmORF sequences were also included. The Evolutionary distances were computed for the individual proteins and concatenated CDS using the Jukes-Cantor method; the phylogenetic trees were obtained by neighbor-joining with 1,000 bootstrap replications. The analyses were conducted in MEGA11 software (Kumar et al., 2018; Stecher et al., 2020).
The complete growth curve was determined for each strain at temperature ranging from 4°C to 30°C, to obtain both OTG and Gr (Figure 1).
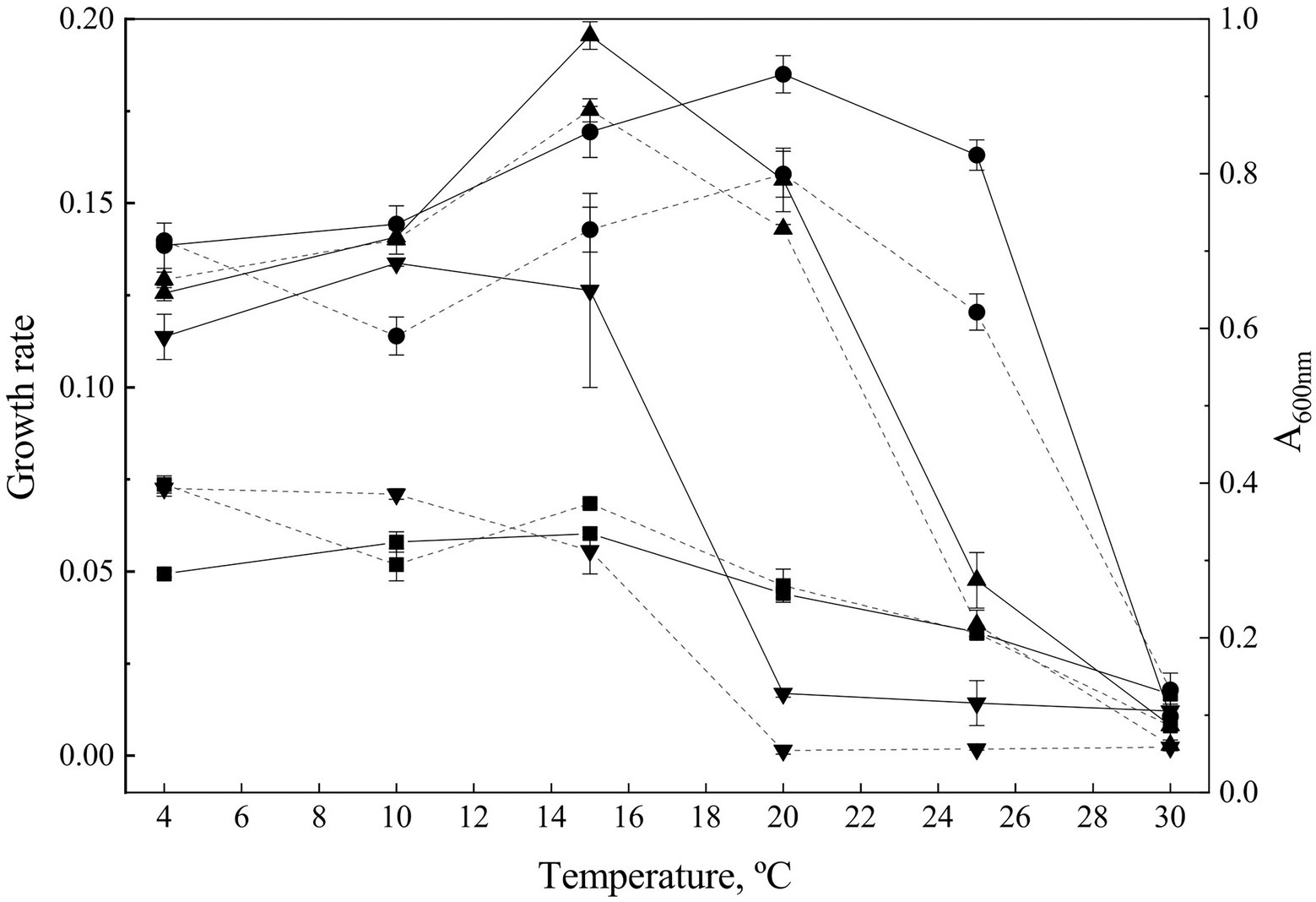
Figure 1. Growth parameters. Growth curves were determined at temperatures from 4°C to 30°C. The growth rate (discontinuous lanes) was calculated from the exponential phase of the growth curves. Continuous lanes show to the maximum A600 observed during exponential phase at different temperatures and express the optimal temperature for growth for each strain. Goffeauzyma gilvescens (circle), Glaciozyma martinii (down triangle), Naganishia antarctica (square), and Phenoliferia glacialis (up triangle).
G. martinii showed the lowest OTG (at 10°C), and the most limited temperature growth range (from 4°C to 15°C). N. antarctica and P. glacialis exhibited similar OTG at 15°C while G. gilvescens showed the highest OTG (20°C). All the strains exhibited growth at 4°C while only G. gilvescens and P. glacialis grew at 25°C. No growth was observed at 30°C (Figure 1).
The maximum Gr was showed by P. glacialis at 15°C (0.175 ± 0.003) while for G. gilvescens the maximum Gr was recorded at 20°C (0.1579 ± 0.0062).
The maximum Gr of G. martinii and N. antarctica corresponds to a value more than twice lower than the Gr of the other two species and were exhibited at 4°C–10°C with no significant differences for G. martinii (0.0726 ± 0.0022 and 0.0710 ± 0.0014, respectively) and at 4°C for N. antarctica (0.0736 ± 0.0023; Figure 1).
The contigs assembled in draft genomes ranged from 401 in N. antarctica to 1,908 in G. gilvescens, the size was from 20.7 Mpb in G. gilvescens to 33.8 Mpb in P. glacialis, and the GC content was from 50.9% in G. gilvescens to 61.2% in P. glacialis (Supplementary Table S2). The predicted CDS varied from 2,221 in G. gilvescens to 10,303 in P. glacialis, while the annotated ones ranged from 11% in P. glacialis to 20% in G. gilvescens. Secondary metabolite biosynthetic gene clusters were predicted in the draft genomes: terpene clusters in the four yeasts, type III polyketide synthase clusters (T3PKS) in G. gilvescens and N. antarctica, and non-ribosomal peptide synthetase clusters (NRSP) in G. gilvescens, G. martinii and P. glacialis (Table 1). The identification of protein encoded by core biosynthetic gene (CBG), additional biosynthetic gene (ABG), and transport related gene (TRG) in the clusters was inferred by a phylogenetic analysis that included the sequences of the best five Blastp hits for each sequence obtained from the NCBI database (Supplementary Figure S1). In terpene clusters, the CBG was closest to squalene synthase in G. martinii (seq 22,166) and P. glacialis (seq 24,553), to bifunctional farnesyl-diphosphate farnesyl transferase/squalene synthase in G. gilvescens (seq 14,972) and N. antarctica (seq 22,146), and to geranylgeranyl-diphosphate geranylgeranyl transferase, terpenoid cyclase, squalene/phytoene synthase and phytoene desaturase in G. gilvescens (seq 7,405), G. martinii (seq 17,625), N. antarctica (seq 20,744) and P. glacialis (seq 22,851), respectively. The ABG was close to amino acid transporter in N. antarctica and NAD-binding protein and NAD-dependent mannitol dehydrogenase in P. glacialis (seq 22,851 and 24,553, respectively). In T3PKS clusters the CBG found in G. gilvescens and N. antarctica apparently corresponded to chalcone synthase, and in N. antarctica the CBG and TRG were closest to anthranilate synthase and sugar transporter, respectively. The CBG of NRPS clusters predicted would correspond to nonribosomal peptide synthetase in G. martinii and P. glacialis, and to L-aminoadipate-semialdehyde dehydrogenase in G. gilvescens. The ABG of NRPS predicted in P. glacialis was close to an oxidoreductase (Supplementary Figure S1).
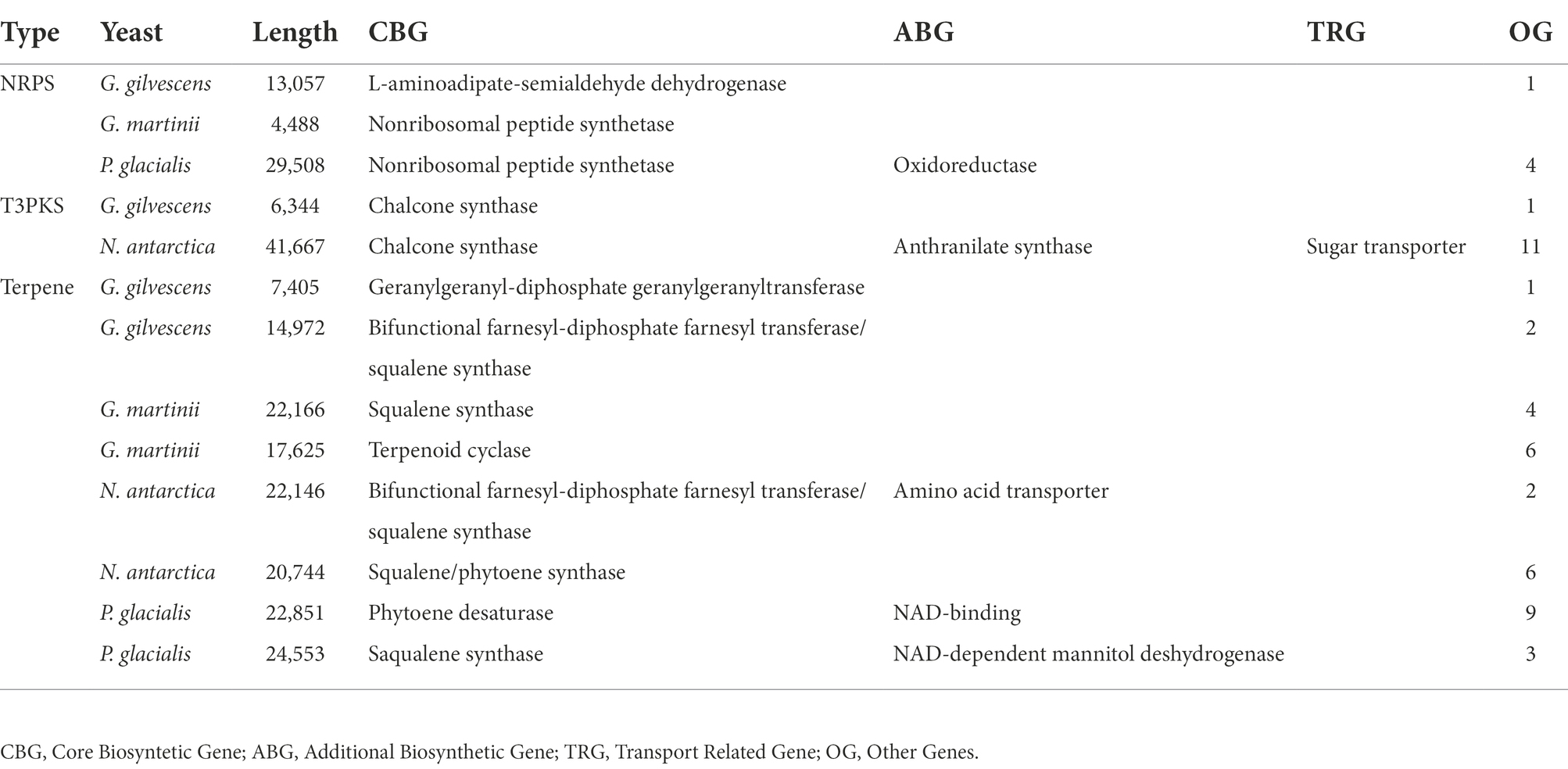
Table 1. Secondary metabolite biosynthesis clusters predicted on draft genomes: non-ribosomal peptide synthetase clusters (NRSP), type III polyketide synthase clusters (T3PKS), and terpene.
The annotated CDSs were classified according to general and specific completed modules and metabolic pathways. Forty-four modules were found, with the highest number in P. glacialis followed in decreasing order by N. antarctica, G. martinii, and G. gilvescens (Supplementary Table S3), 28 were found in the four studied yeasts and 9 in tree yeasts (i.e., Polyamine biosynthesis, arginine, assimilatory sulfate reduction and beta-oxidation acyl-CoA synthesis). The modules for which more putative genes were found were citrate cycle, beta-oxidation, leucine degradation, citrate cycle second carbon oxidation, and glycolysis. Seven modules associated to metabolism of cofactors and vitamins (i.e., lipoic acid biosynthesis, biotin biosynthesis and ubiquinone biosynthesis) were found only in two yeasts. Regarding metabolic pathways, more putative genes were found for P. glacialis and G. martinii, and the top5 at the middle level were signal transduction, carbohydrate metabolism, amino acid metabolism, transport and catabolism, and cell growth and death (Figure 2). At the specific level the top5 pathways were cell cycle, MAPK signaling pathway, spliceosome, ribosome, and autophagy (Supplementary Table S4). Of 201 specific pathways for which at least one putative gene was found, about the half were common to four yeasts, whiles 32 were found only in one yeast, most in G. martinii such as nitrogen metabolism, ether lipid metabolism, non-homologous end-joining, and atrazine degradation.
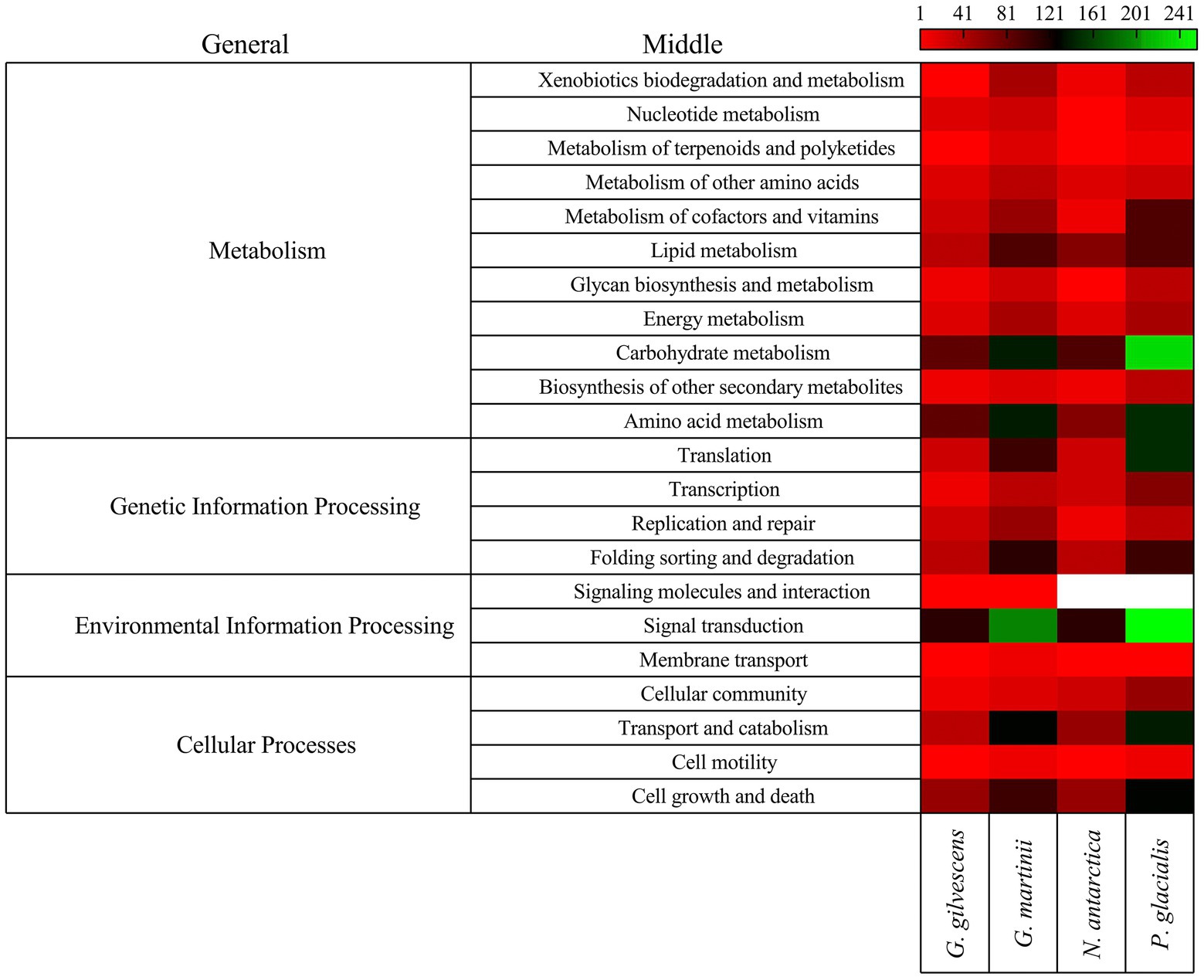
Figure 2. Classification of CDS by metabolic pathways. The CDSs were classified by comparison using GHOST X tools of the KAAS-KEGG Automatic Annotation Server. The number of CDSs grouped according to general and middle pathways are shown.
In all studied yeasts, two or more CDS encoding for the same putative protein was found, especially in G. martinii and P. glacialis. The 20 putative proteins encoded by a higher number of CDS found in all yeasts are shown in Figure 3. Among them, P-loop containing nucleoside triphosphate hydrolase protein, alpha/beta hydrolase protein, armadillo-type protein, major facilitator superfamily domain-containing protein, and general substrate transporter protein can be mentioned.
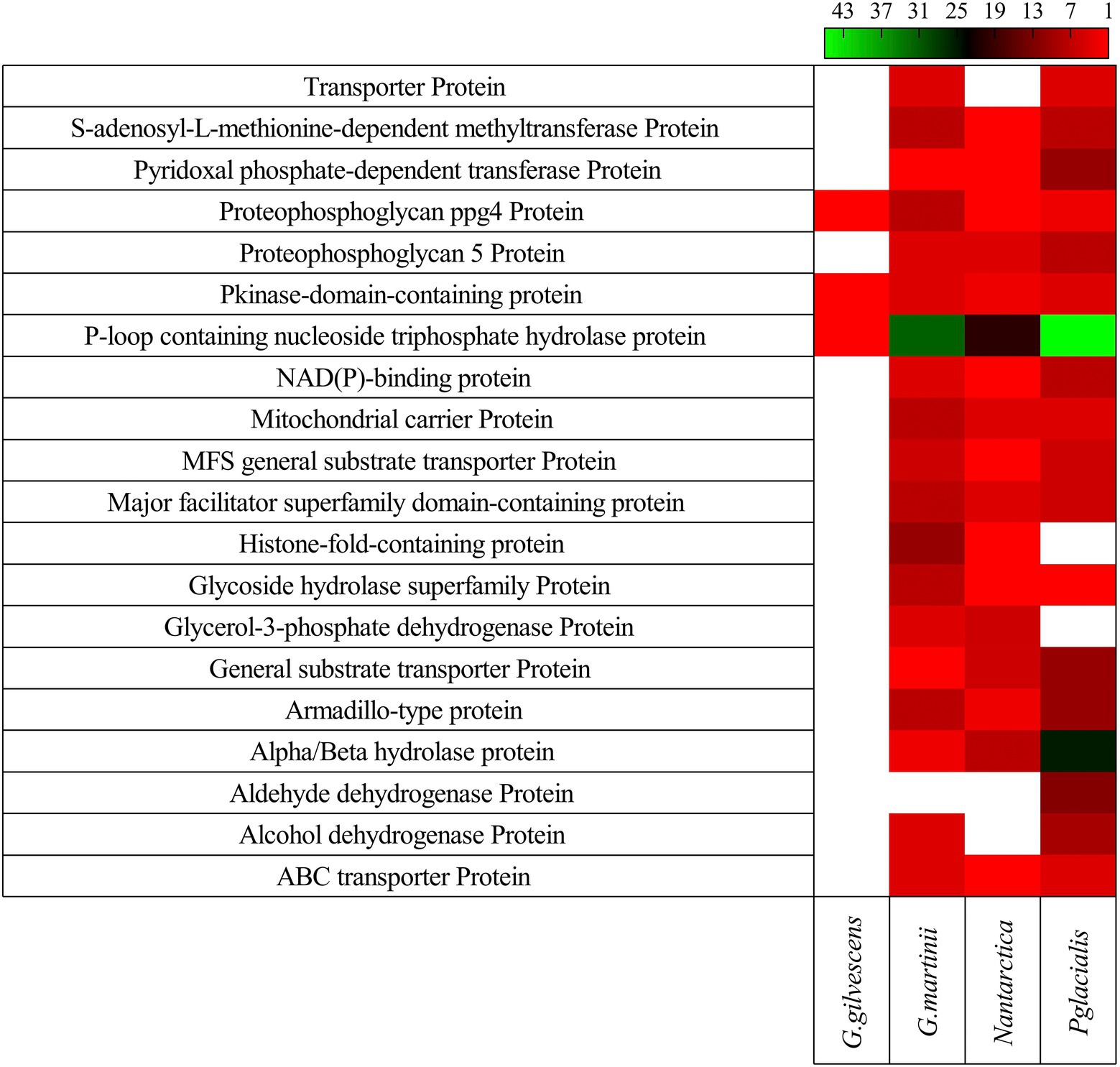
Figure 3. Gene redundancy for top 20 kinds of protein. The number of CDS predicted to encode for a protein in each yeast is shown.
A total of 423 orthologous genes associated to response to different kind of stresses were found, most of them related to oxidative and cold stress, followed by general, osmotic, and heat stress responses, especially in P. glacialis and G. martinii (Supplementary Table S5). The top5 predicted proteins related to stress were mitogen-activated protein kinase 1, catalase, dihydrolipoamide dehydrogenase, glyceraldehyde 3-phosphate dehydrogenase, glycogen synthase kinase 3 beta, ribulose-phosphate 3-epimerase.
A total of 13 putative stress related proteins were commonly found in the four studied yeasts, i.e., enoyl-[acyl-carrier protein] reductase II, mitogen-activated protein kinase 1, eukaryotic translation initiation factor 2-alpha kinase 4, formate dehydrogenase, UTP--glucose-1-phosphate uridylyl transferase, glycogen synthase kinase 3 beta, heat shock 70 kDa protein, and phosphoenolpyruvate carboxy kinase (ATP). A total of 73 orthologous genes related to cold stress were predicted, with enoyl-[acyl-carrier protein] reductase II, mitogen-activated protein kinase kinase 1, glycogen synthase, omega-6 fatty acid desaturase/acyl-lipid omega-6 desaturase (Delta-12 desaturase), and stearoyl-CoA desaturase (Delta-9 desaturase) found in at least three yeasts. Putative antifreeze glycopeptide AFGP polyprotein and antifreeze protein were identified in N. antarctica and P. glacialis, respectively.
Hundreds of thousands of SmORFs were found in all yeasts, in decreasing order in P. glacialis (772,037), G. martinii (462,525), N. antarctica (295,488), and G. gilvescens (199,985), and higher numbers of 3’SmOrfs were predicted (Figure 4A). No identical SmORFs nucleotide sequences were found among the studied yeasts, and very few considering translated sequences, being higher between P. glacialis and G. martinii in CDSSmOrfs and 3’SmOrfs (from 59 to 64 identities; Figure 4B). Compared to the SmORFs database, only 0.1%–0.5% of SmORFs yielded hits (considering at least 50% of coverage and similarity), most of them corresponding to the classifications Homo sapiens and mouse (Figure 4C). A phylogenetic analysis was performed considering the translated SmORFs with at least 30% similarity by Blastp to the SmORF database and common to three and all yeasts studied. The majority of common putative SmORFs corresponded to Literature mining classification. The yeasts were grouped close in either analysis of translated SmORFs common to three (Supplementary Figure S2A) and all yeasts (Supplementary Figure S2B), not observing a preference for hierarchical clustering among yeasts in any of the SmORF used for the analysis.
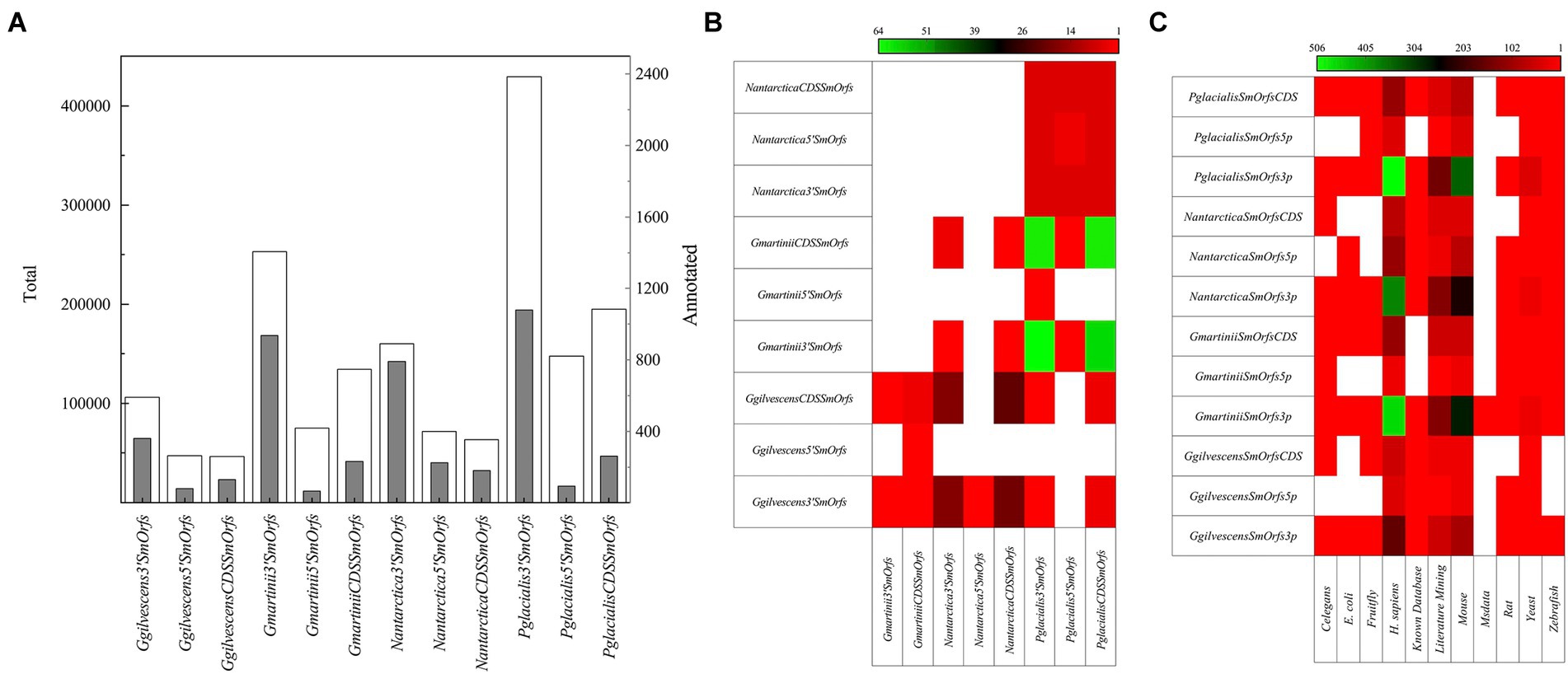
Figure 4. SmORFs prediction and comparison among yeasts. The total (white columns) SmORFs and those annotated (gray columns) by comparison SmOrfs database, are shown in (A) the number of translated SmORFs identical among yeasts is shown in (B) and those annotated by comparison to a SmORFs database in (C). CDSSmORFs, SmORF located into a CDS; 5’SmORFs, SmORF located until 1,000 nt upstream of a CDS; 3’SmORFs, SmORF located until 1,000 nt downstream of a CDS (3’SmORFs).
The analysis was performed considering the predicted flexibilities of translated CDSs annotated and classified in cellular pathways. The M1 + 2 percentage showed the highest degree of significant (p < 0.05) variations among almost all yeasts under study, being higher between G. martinii vs. G. gilvescens and N. antarctica, and P. glacialis vs. G. gilvescens and N. antarctica. The pathways exhibiting more significant differences were carbohydrate metabolism, cell growth and death, and transduction signal. The clustering of species according to their differences revealed that P. glacialis was close to G. martinii, while N. antarctica was close to G. gilvescens (Figure 5A). When the 4 yeast species were grouped according to their OTG (obtaining 3 groups: OTG = 10°C, OTG = 15°C, and OTG = 20°C), those with OTG equal to 10°C and 15°C clustered separately from yeast exhibiting an OTG of 20°C (Figure 5B). On the other hand, when Gr was considered (Gr = 0.07, Gr = 0.16, Gr = 0.18), the yeast species showing either highest or lowest Gr clustered together, differently from those with intermediate Gr, which clustered separately (Figure 5C). The significant differences found among studied yeasts in CDS groups and parameters were analyzed concerning their differences in growth parameters (Figure 5D). Overall, positive, and negative correlations were obtained between Vf and M1 + 2, respectively, and Gr, while a positive correlation was found between M1 + 2 and OTG. In the analysis by pathways, both positive and correlations between M1 + 2 and Gr in 2 and 3 pathways, respectively, and positive correlation between M1 + 2 and OTG in 6 pathways were observed. These correlations were generally found in curves with only two points, except for M1 + 2 vs. OTG in for global and general metabolism pathway (R2 ≥ 0.98).
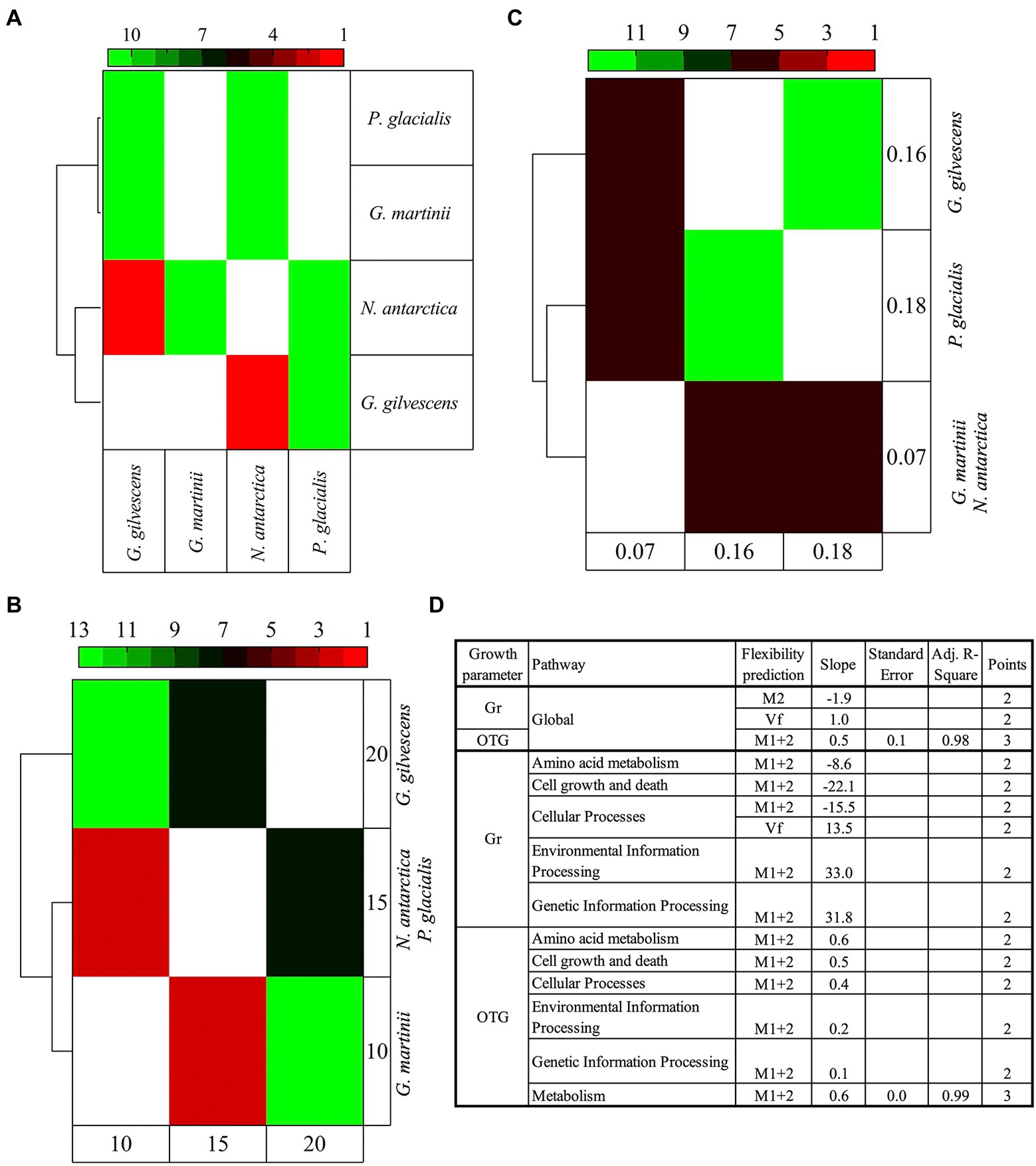
Figure 5. Comparative analysis of yeast species according to predicted protein flexibilities and growth parameters. The predicted flexibility of CDSs grouped by cellular functions were compared among species individually (A) and grouped according to their optimal temperature for growth (B) and growth rate (C). The heatmap shows the number of cellular pathways for which significant difference (Tukey post hoc tests, p < 0.05) were found between yeasts. In (D) the plot of the difference between yeasts for a pathway in flexibility parameters (P1-2) vs. difference in growth parameters (F1-2) is shown. OTG, optimal temperature for growth; Gr, growth rate; Vf, very flexible; M2, class2 flexibility predicted by MEDUSA; M1 + 2, class 1 + class 2 flexibility predicted by MEDUSA.
To highlight the differences between strains belonging to the same species but isolated from different geographical areas, the strain DBVPG 5880 of P. glacialis isolated from Italian Alps, was compared to an isolate from Antarctica, and to other members of the order Kriegeriales with available genomes at the NCBI database. The identified CDS encoding for proteins common to at least 8 yeasts (including both P. glacialis isolates) were considered for analysis at individual proteins and concatenated CDSs. In the trees generated from individual analysis of 65 proteins, both isolates of P. glacialis were generally closed related (Supplementary Figure S3). In some cases, alpine P. glacialis was closest to G. antarctica PI12, such as for proteins 2-isopropyl malate synthase, 3-hydroxybutyrate dehydrogenase, and acetyl-CoA carboxylase. Figure 6 summarized the results of trees classified for proteins common to 8–12 yeasts. The two strains of P. glacialis clustered in closed related positions in just 2 of the 6 trees obtained (Figures 6D,E); in the trees with higher number of analyzed proteins (Figures 6A–C,F) Antarctic P. glacialis was always positioned external to the entire group of strains, differently from Alpine P. glacialis. When the analysis was performed with concatenated CDS, both P. glacialis isolates clustered together and with Rhodotorula species (especially with R. diobovata, R. taiwanensis, and R. mucilaginosa) which vary in dependence of the number of CDS analyzed (Figures 7A–C), and with G. antarctica (Figures 7D,E). When the analysis was restricted to all CDS common exclusively to P. glacialis isolates and G. antarctica, these three yeasts clustered with R. taiwanensis MD1149, and R. mucilaginosa F6, with a close relation between alpine P. glacialis and G. antarctica PI12 (Figure 7F).
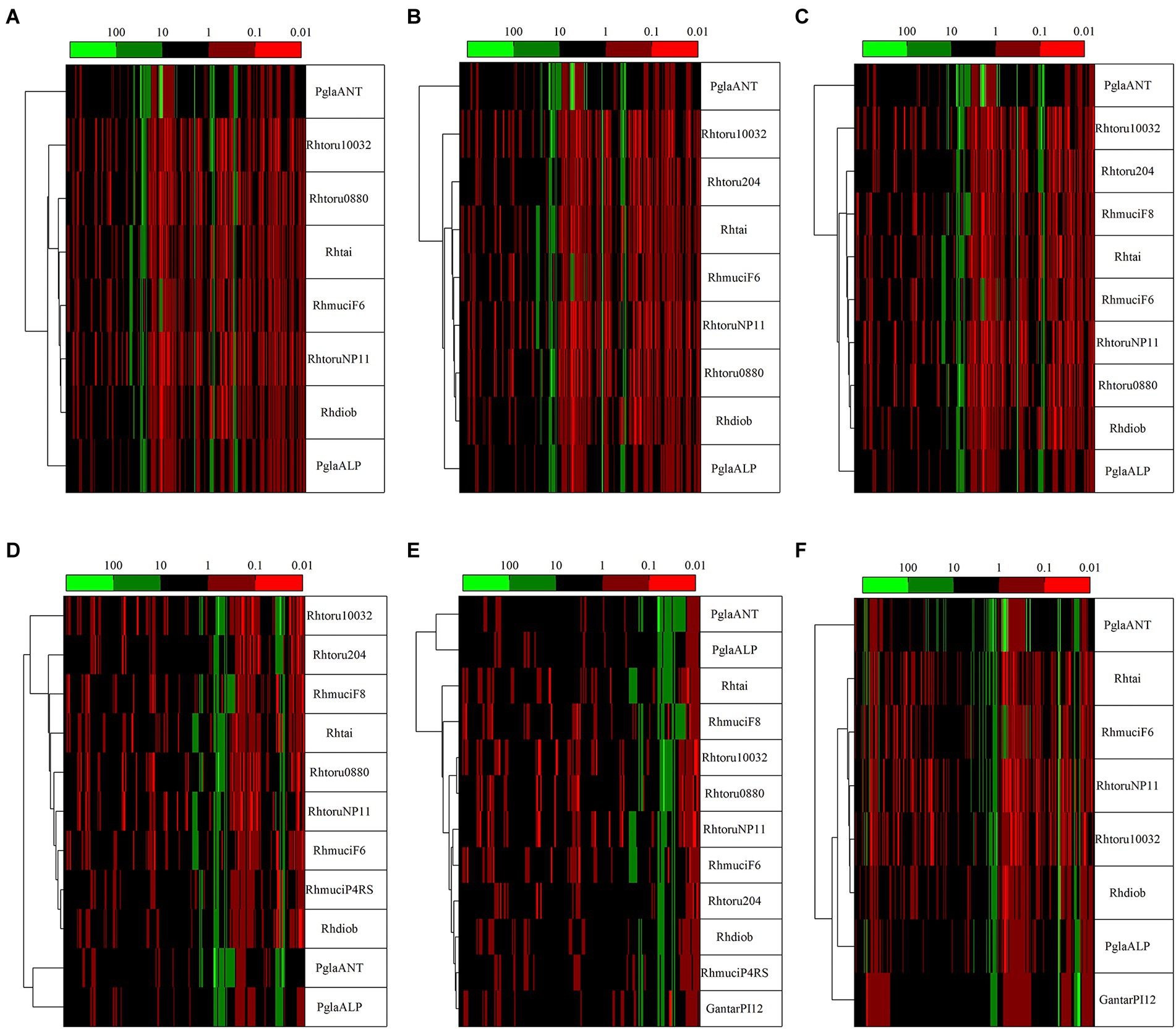
Figure 6. Comparison among yeasts based in phylogenetic analysis of individual proteins. Comparisons were made based on the patristic distances extracted from threes computed for proteins (Supplementary Figure S3) that were common to at least 8 yeasts, including both P. glacialis isolates. (A), 50 proteins common to 8 yeasts; (B), 41 proteins common to 9 yeasts; (C), 35 proteins common to 10 yeasts; (D), 23 proteins common to 11 yeasts; (E), 24 proteins common to 12 yeasts. In (F), was considered the proteins common at least for P. glacialis and G. antarctica (31 proteins common to 8 yeasts). PglaALP, P. glacialis isolate from Italian Alps; PglaANT, P. glacialis isolate from Antarctica; GantarPI12, Glaciozyma antarctica PI12; Rhdiob, Rhodotorula diobovata strain UCD-FST 08–225; RhmuciF6, Rhodotorula mucilaginosa strain F6; Rhmuci, Rhodotorula mucilaginosa strain F8; Rhhai, Rhodotorula taiwanensis strain MD1149; Rhtoru204, Rhodotorula toruloides ATCC 204091; RhtoruNP11C, Rhodotorula toruloides strain NP11C; Rhtoru10031, Rhodotorula toruloides strain NBRC10032; Rthoru0880, Rhodotorula toruloides strain NBRC 0880.
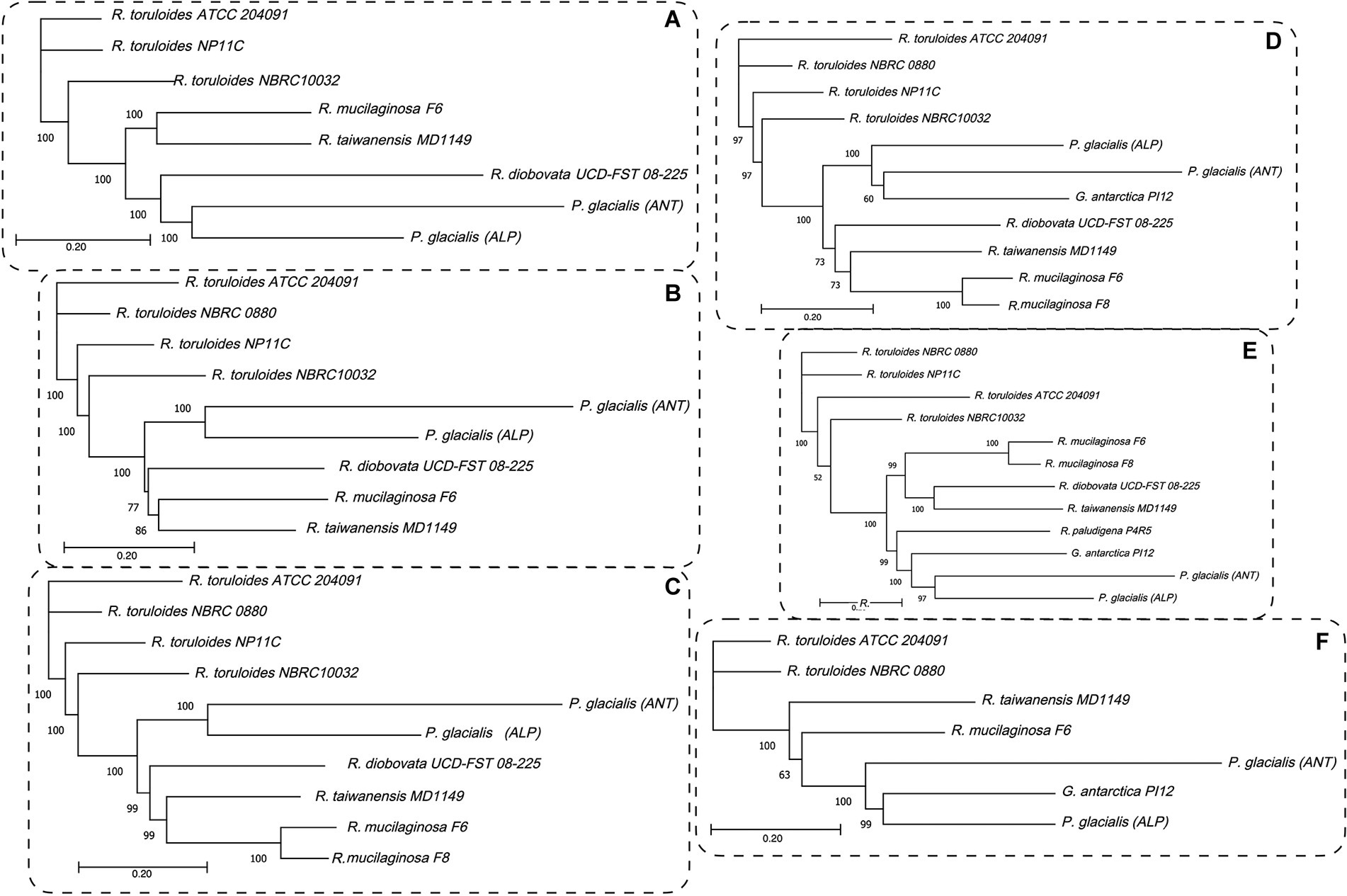
Figure 7. Maximum likelihood phylograms based on the concatenated CDSs. The coding sequences for proteins common to at least 8 yeasts, including both P. glacialis isolates, were concatenated and the evolutionary distances computed by Jukes-Cantor method. The phylogenetic trees obtained by neighbor-joining with 1,000 bootstrap replications and statistically supported bootstrap values (≥50%) are shown. (A), 50 proteins common to 8 yeasts; (B), 40 proteins common to 9 yeasts, (C), 34 proteins common to 10 yeasts; (D), 22 proteins common 11 yeasts; (E), 17 proteins common to 12 yeasts. In (F), was considered the proteins common at least for P. glacialis and G. antarctica (31 proteins, common to 7 yeasts). Abbreviation of yeasts as in Figure 6.
The four yeasts under study showed variable growth parameters (OTGs ranging from 10°C to 20°C). Interestingly, N. antarctica showed its max Gr at 4°C, but its max OTG (the highest A600 during the exponential phase) was found at 15°C. The long lag phase may explain the data observed at 4°C, in comparison with those obtained at 10°C and 15°C. The maximum A600 was reached by G. gilvescens and P. glacialis, which also showed the highest Gr and the ability to grow at 25°C, although the growth of P. glacialis was greatly reduced. All the strains were isolated from cold habitats of Italian Alps and Apennines and grow at 4°C, but only G. martinii and N. antarctica exhibited no growth at temperature equal or above 20°C. The Alpine P. glacialis strain herein studied differed from a previously studied Antarctic isolate of the same species exhibiting higher OTG and temperature range for growth (Baeza et al., 2021). This temperature range is narrower than that exhibited by isolates of the yeast-like fungus Aureobasidium subglaciale, which grew from 0°C to 30°C, and even tolerated incubation at 50°C for 2 h (Zajc et al., 2022). The sizes of draft genomes and GC contents ranged from 20.7 to 33.8 Mpb and 50.9% to 61.2%, respectively, in agreement with other yeasts isolated from cold habitats (Brejová et al., 2017; Coleine et al., 2019, 2020; Cunha et al., 2020; Baeza et al., 2021). Comparing the calculated protein flexibilities among yeasts, significant differences were found mainly for medium plus very flexible (M1 + 2, predicted by MEDUSA), which was higher for G. martinii than G. gilvescens and N. antarctica, and for P. glacialis than G. gilvescens and N. antarctica. Considering the growth parameters, the yeasts showing most significant differences in OTG were also discriminated according to their predicted protein flexibilities; similar clustering did not occur for Gr. Overall, a correlation between protein flexibilities calculated as M1 + 2 and yeast growth parameters was directly proportional when considering OTG and variable for Gr. Similarly, in previous genomic and transcriptomic studies of eight Antarctic yeasts, a correlation was observed between calculated protein flexibilities and OTGs, not Gr (Baeza et al., 2021, 2022). Interestingly, the correlation between OTG and calculated protein flexibilities was negative in Antarctic yeasts, while it was positive in the yeasts isolated from the Alps and Apennines. It could be an interesting point concerning potential differences in adaptation strategies in yeasts colonizing different cold environments, thus suggesting the need of a more in-depth study of cold-adapted yeasts at the genomic and biochemical levels.
A considerable number of putative genes involved in the regulation of response to different kinds of stress were found, including, in addition to cold, general, oxidative, and osmotic stress, thus highlighting the putative adaptation of yeasts to the challenging environmental conditions occurring in Alpine ecosystems. Some genes associated with response to cold were found, among them: dihydrolipoamide dehydrogenase, enoyl-[acyl-carrier protein] reductase II, glycogen synthase, omega-6 fatty acid, desaturase/acyl-lipid omega-6 desaturase, stearoyl-CoA desaturase (Delta-9 desaturase), ATP-dependent RNA helicase DeaD, elongation of very-long-chain fatty acids protein 6, glycogen debranching enzyme, and very-long-chain acyl-CoA dehydrogenase, involved in different cellular functions such as cell membrane adaptation, compatible solute accumulation, regulation of protein folding, and changes in RNA structure. Similar genes patterns were also found in other microorganism (including yeasts), as a common reply to cold stress (Aguilera et al., 2007; Inouye and Phadtare, 2014; Zhang and Gross, 2021). Among the more represented pathways MAPK signaling, spliceosome, ribosome, and autophagy were observed, especially in P. glacialis and G. martinii. In a genomic and comparative transcriptomic study of a cold-adapted oleaginous yeast R. kratochvilovae, cultivated at 15°C and 30°C, the significantly downregulated genes were primarily related to metabolic and cellular processes and cellular organelles, whereas the MAPK signaling pathway was among overrepresented pathways (Guo et al., 2021). A recent study of Chen et al. (2022) demonstrated that the overexpression and knockout of the MAP-Kinase HOG1 played an important role in cold adaptation of R. kratochvilovae, mainly by promoting the biosynthesis of polyunsaturated fatty acids, such as linoleic acid and linolenic acid, and glycerol (Chen et al., 2022). Autophagy denotes a variety of mechanisms for the metabolization via vacuole of aged and damaged proteins, lipids, and membrane-bound organelles and has also been associated play a critical role in aging (Tyler and Johnson, 2018). The role of autophagy in response to abiotic stress, including cold, has been studied mainly in plants and fishes (Chen et al., 2021; Ren et al., 2021). A considerable number of genes classified in the autophagy pathway were found in Antarctic yeasts, especially in Candida sake (Baeza et al., 2021). Among complete modules according to putative genes in the four studied yeasts, the pathways for polyamine biosynthesis and citrate cycle (TCA cycle Krebs cycle) were found. TCA-cycle metabolites, lactic acid, and polyamines were accumulated in the Antarctic yeast Mrakia blollopis after a cold shock exposure (Tsuji, 2016). Polyamines are aliphatic hydrocarbon chains containing one or more amine groups. They have recognized functions in cell growth, development, and cellular protection against stresses such as oxidative, osmotic and temperature (Valdés-Santiago and Ruiz-Herrera, 2013; Gevrekci, 2017; Murray Stewart et al., 2018). Putative antifreeze glycopeptide AFGP polyprotein and antifreeze protein were identified in N. antarctica and P. glacialis. Similarly, a putative gene encoding for ice-binding protein was found in the whole genome of the endemic Antarctic ascomycetous fungus Antarctomyces pellizariae (Batista et al., 2020). The antifreeze or Ice Binding Proteins (IBPs) can bind to the ice surface and be incorporated into the ice crystal matrix controlling the ice shape and inhibiting re-crystallization, potentially decreasing cell injury (DeVries, 1986; Bouvet and Ben, 2003; Garner and Harding, 2010). IBPs also stimulate microbial biofilm formation on ice and the formation of channels through the ice, helping interchange of nutrients and gasses (Davies et al., 2002; Bar Dolev et al., 2016; Cheung et al., 2017). Most studies revealed the production of IBPs by fish, bacteria, and plants, and in a more limited way by fungi. The IBP AFP1, produced by G. antarctica, was successfully expressed in E. coli (Hashim et al., 2013). Antifreeze proteins from the Antarctic yeast Goffeauzyma gastrica, maintained, after partial purification, a good antifreeze property after several freeze–thaw cycles (Villarreal et al., 2018).
The redundancy in putative CDS encoding for the same protein or proteins families was one additional aspect that can be putatively considered as an adaptation strategy of yeasts to their extreme environments. Gene duplication has been proposed as a mechanism of genomic adaptation (as a genetic robustness) to a changing environmental conditions, including transport of nutrients, and protection to biotic and abiotic stress (Levasseur and Pontarotti, 2011; Kondrashov, 2012). P-loop containing nucleoside triphosphate hydrolase (P-loop NTPase), Alpha/Beta hydrolase (α/β hydrolase), Armadillo repeat-containing proteins (ARMCs), and the major facilitator superfamily protein (MFS) were found in the studied genomes among the most redundant ones. P-loop NTPase is a superfamily of enzymes crucial for almost all aspects of life, and some members contribute to stress responses in plants (Leipe et al., 2002; Cheung et al., 2013, 2016). α/β Hydrolase is a superfamily of hydrolytic enzymes that share a common fold having widely catalytic functions and phylogenetic origin (Ollis et al., 1992). Some α/β hydrolases have been involved in salt and cold tolerance in plants and microorganisms, including yeasts (Brody et al., 2001; Takahashi et al., 2006; Liu et al., 2014; Wu et al., 2020). ARMCs form a large family of enzymes primarily characterized in multicellular organisms (e.g., in plants) and exhibiting very versatile and fundamental functions, including response to stress, such as osmotic (Kojima et al., 2013; Sharma and Pandey, 2015; Huang et al., 2021). MFS is a ubiquitous transporter superfamily whose members have a broad spectrum of substrates, such as inorganic and amino acids, nucleosides, lipids, and short peptides (Yan, 2015); some members of MFS are involved in resistance to oxidative stress and fungicides in fungal pathogen Alternaria alternata (Chen et al., 2017). An increase of MFS copy numbers led to an increase of the gene redundancy in Antarctic yeast Naganishia vishniacii (Nizovoy et al., 2021) and Mrakia psychrophila isolated from permafrost on the Qinghai-Tibet Plateau (Su et al., 2016) and suggested that it could be considered a further adaptation strategy contributing to the accumulation of nutrients under cold imposed conditions.
Gene clusters for the biosynthesis of secondary metabolites were predicted: terpene clusters were found in all four sequenced yeasts, NRSP in G. gilvescens, G. martinii, and P. glacialis, and T3PKS in G. gilvescens and N. antarctica. NRPS was mainly found in bacteria and fungi, especially Ascomycota, which assemble numerous peptides having functions in primary metabolism, cellular development and morphology, and stress responses such as oxidative in Aspergillus fumigatus (Stack et al., 2007; Süssmuth and Mainz, 2017; Sayari et al., 2019). Terpenoids are structurally the most diverse groups of natural compounds, widely distributed in nature, that play important physiological and metabolic functions, mainly antioxidant activities in microorganisms (Avalos and Carmen Limón, 2015; Jaeger and Cuny, 2016). Type III PKS has widely described in plants and bacteria and recently in Antarctic yeasts (Baeza et al., 2021), and produces a wide array of compounds such as chalcones, pyrones, acridones, phloroglucinols, and stilbenes, with several physiological roles such as pigmentation, salinity and dehydration resistance, stress adaptation, and cell wall remodeling (Yu et al., 2012; Parvez et al., 2018; Navarro-Muñoz and Collemare, 2019).
A class of genetic elements with increasing relevancy are putative functional small ORFs (SmORFs) of 10–100 codons (which millions can be found in eukaryotic genomes) generally considered non-coding sequences because of their short length that impair bioinformatic analysis (Albuquerque et al., 2015; Couso and Patraquim, 2017). In G. antarctica SmORFs represented 4% of annotated ORFs, validated as expressed transcripts via cross-referencing against available transcriptome data (Mat-Sharani et al., 2015). High numbers of SmORFs were found in all four Alpine yeasts studied, especially in P. glacialis, of which 19% were at 5′UTR. The SmORFs showed to be “exclusive” for each yeast as no identical SmORFs at nucleotide sequence were found and resulted infimum when translated sequences were compared; only 0.1%–0.5% of them showed similarity to SmORFs of other organisms, mainly from H. sapiens and mouse.
The function mainly described for SmORFs, especially those at 5′UTR, is related to the regulation of the expression of the main ORF by mechanisms that include the alteration of ribosome capacity to initiate, terminate and reinitiate translation (Wang and Rothnagel, 2004; Gunišová and Valášek, 2014; Renz et al., 2020). Although some examples of microproteins working independently have been found, such as repressors in S. cerevisiae, Neurospora crassa and H. sapiens (Renz et al., 2020), their appropriate functional study is still complicated. Anyway, it is possible to postulate a role for SmORFs as an additional adaptation strategy of yeasts to cold environments.
The results obtained by the comparison of the genomes showed that Alpine P. glacialis herein studied exhibited some differences when compared to another strain of the same species, but isolated from Antarctica (Baeza et al., 2021). The draft genome of Alpine P. glacialis was 33.8 Mbp with 61.2% GC (87% completeness), whiles Antarctic P. glacialis was 15.9 Mb with 50.4% GC (completeness 71%). Two terpene and one NRPS biosynthetic cluster were predicted in Alpine P. glacialis and three NRPS in Antarctic P. glacialis. The ratio sum of CDS / total of different putative proteins was 2.1 for Alpine strain and 1.8 for the Antarctic one. When comparing the top 20 redundant putative proteins, just 9 of them exhibited a coincidence between both yeasts: alcohol dehydrogenase, α/β hydrolase, general substrate transporter, MFS, NADP-dependent oxidoreductase domain-containing, P-loop NTPase, and pyridoxal phosphate-dependent transferase. In the comparisons by multigene phylogeny computed with protein sequences individually and concatenated CDS of both strains, including other yeasts of the order Kriegeriales, the two P. glacialis did not always cluster together and, in some trees, they showed a close relation with G. antarctica PI12.
The findings obtained from the genomic approach used in this study to analyze the cold adaptation of the four studied yeasts are consistent with those reported for other yeasts isolated from cold habitats regarding genomic structure. Furthermore, the high numbers of putative genes encoding for cold stress response and gene clusters encoding for the synthesis of secondary metabolites suggest the ability of such yeasts to successfully survive and adapt their physiology to these harsh ecological conditions. The different correlation between the OTG and calculated protein flexibilities found in yeasts isolated from the Italian Alps and Apennines and those from Antarctica suggest the existence of a certain degree of variability in adaptation strategies of yeasts thriving in different cold regions.
As expected, Alpine and Antarctic P. glacialis isolates were close in the multigene phylogenetic analysis, including other members of the order Kriegeriales. Interestingly, both strains were clustered close to G. antarctica PI12 suggesting more similitude among yeast from cold environments.
On the other hand, as already described for Antarctic yeasts, a considerable number of SmORFs and genetic redundancy were found. These two aspects are less studied and could be proposed to play roles in adaptation to cold, which are attractive to include in future comparative studies of adaptative yeast’ strategies from different cold environments worldwide.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: NCBI BioProject, accession no: PRJNA859078.
BT and PB carried out DNA purifications and determination of yeast growth parameters. MB performed bioinformatic and statistical analyses. MB, BT, and PB contributed to design of the experiments, discussion of the results, and manuscript writing. All authors have read and agreed to the published version of the manuscript.
This research was funded by Agencia Nacional de Investigación y Desarrollo, grant number Fondecyt 1180233.
Bioinformatic and statistical consulting was provided by omics2view.consulting GbR, Kiel (Germany).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1026102/full#supplementary-material
Supplementary FIGURE S1 | Prediction of function for genes in secondary metabolite clusters. The phylogenetic analysis included the best 10 Blastp hits for each sequence obtained from the NCBI database. The evolutionary history was inferred by using the Maximum Likelihood method and JTT matrix-based model. The percentage of trees in which the associated taxa clustered together is displayed next to the branches.
Supplementary FIGURE S2 | Phylogenetic analysis for translated SmORFs. The analyses were performed with SmORFs common to three (A), and all yeast (B) studied, including the reference Blastp hits SmORFs from Mouse and Literature mining classification. The evolutionary history was inferred by using the Maximum Likelihood method and JTT matrix-based model. The percentage of trees in which the associated taxa clustered together is displayed next to the branches.
Supplementary FIGURE S3 | Phylogenetic analysis for individual proteins used in multigene phylogeny analysis. The evolutionary history was inferred by using the Maximum Likelihood method and JTT matrix-based model. The percentage of trees in which the associated taxa clustered together is displayed next to the branches.
Aguilera, J., Randez-Gil, F., and Prieto, J. A. (2007). Cold response in Saccharomyces cerevisiae: new functions for old mechanisms. FEMS Microbiol. Rev. 31, 327–341. doi: 10.1111/j.1574-6976.2007.00066.x
Albuquerque, J. P., Tobias-Santos, V., Rodrigues, A. C., Mury, F. B., and Da Fonseca, R. N. (2015). Small ORFs: a new class of essential genes for development. Genet. Mol. Biol. 38, 278–283. doi: 10.1590/S1415-475738320150009
Alcaíno, J., Cifuentes, V., and Baeza, M. (2015). Physiological adaptations of yeasts living in cold environments and their potential applications. World J. Microbiol. Biotechnol. 31, 1467–1473. doi: 10.1007/s11274-015-1900-8
Andrews, S. (2018). Fast QC: a quality control tool for high throughput sequence data. Available at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (Accessed June, 2021).
Avalos, J., and Carmen Limón, M. (2015). Biological roles of fungal carotenoids. Curr. Genet. 61, 309–324. doi: 10.1007/s00294-014-0454-x
Baeza, M., Alcaíno, J., Cifuentes, V., Turchetti, B., and Buzzini, P. (2017). “Cold-active enzymes from cold-adapted yeasts” in Biotechnology of Yeasts and Filamentous Fungi. ed. A. A. Sibirny (Cham: Springer International Publishing), 297–324.
Baeza, M., Zúñiga, S., Peragallo, V., Barahona, S., Alcaino, J., and Cifuentes, V. (2021). Identification of stress-related genes and a comparative analysis of the amino acid compositions of translated coding sequences based on draft genome sequences of Antarctic yeasts. Front. Microbiol. 12:623171. doi: 10.3389/fmicb.2021.623171
Baeza, M., Zúñiga, S., Peragallo, V., Gutierrez, F., Barahona, S., Alcaino, J., et al. (2022). Response to cold: a comparative transcriptomic analysis in eight cold-adapted yeasts. Front. Microbiol. 13:828536. doi: 10.3389/fmicb.2022.828536
Bankevich, A., Nurk, S., Antipov, D., Gurevich, A. A., Dvorkin, M., Kulikov, A. S., et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477. doi: 10.1089/cmb.2012.0021
Bar Dolev, M., Braslavsky, I., and Davies, P. (2016). Ice-binding proteins and their function. Annu. Rev. Biochem. 2, 515–542. doi: 10.1146/annurev-biochem-060815-014546
Batista, T. M., Hilario, H. O., De Brito, G. A. M., Moreira, R. G., Furtado, C., de Menezes, G. C. A., et al. (2020). Whole-genome sequencing of the endemic Antarctic fungus Antarctomyces pellizariae reveals an ice-binding protein, a scarce set of secondary metabolites gene clusters and provides insights on Thelebolales phylogeny. Genomics 112, 2915–2921. doi: 10.1016/j.ygeno.2020.05.004
Blin, K., Shaw, S., Steinke, K., Villebro, R., Ziemert, N., Lee, S. Y., et al. (2019). antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 47, W81–W87. doi: 10.1093/nar/gkz310
Bouvet, V., and Ben, R. N. (2003). Antifreeze glycoproteins: structure, conformation, and biological applications. Cell Biochem. Biophys. 39, 133–144. doi: 10.1385/CBB:39:2:133
Brejová, B., Lichancová, H., Brázdovič, F., Cillingová, A., Neboháčová, M., Tomáška, Ľ., et al. (2017). Draft genome sequence of an obligate psychrophilic yeast, Candida psychrophila NRRL Y-17665T. Genome Announc. 5, e00851–e00817. doi: 10.1128/genomeA.00851-17
Brody, M. S., Vijay, K., and Price, C. W. (2001). Catalytic function of an alpha/beta hydrolase is required for energy stress activation of the sigma(B) transcription factor in Bacillus subtilis. J. Bacteriol. 183, 6422–6428. doi: 10.1128/JB.183.21.6422-6428.2001
Bushnell, B. (2019). BBTools. Available at: http://jgi.doe.gov/data-and-tools/bbtools/ (Accessed June, 2021).
Buzzini, P., and Margesin, R. (2013). Cold-Adapted Yeasts. Berlin, Germany: Springer Science & Business Media.
Casanueva, A., Tuffin, M., Cary, C., and Cowan, D. A. (2010). Molecular adaptations to psychrophily: the impact of ‘omic’ technologies. Trends Microbiol. 18, 374–381. doi: 10.1016/j.tim.2010.05.002
Chen, H., Dong, J., and Wang, T. (2021). Autophagy in plant abiotic stress management. Int. J. Mol. Sci. 22:4075. doi: 10.3390/ijms22084075
Chen, L.-H., Tsai, H.-C., Yu, P.-L., and Chung, K.-R. (2017). A major facilitator superfamily transporter-mediated resistance to oxidative stress and fungicides requires Yap1, Skn7, and MAP kinases in the citrus fungal pathogen Alternaria alternata. PLoS One 12:e0169103. doi: 10.1371/journal.pone.0169103
Chen, W., Zhang, X., Li, S., Cui, J., Yang, X., and Zhang, Q. (2022). The MAP-kinase HOG1 controls cold adaptation in Rhodosporidium kratochvilovae by promoting biosynthesis of polyunsaturated fatty acids and glycerol. Curr. Microbiol. 79:253. doi: 10.1007/s00284-022-02957-8
Cheung, M.-Y., Li, X., Miao, R., Fong, Y.-H., Li, K.-P., Yung, Y.-L., et al. (2016). ATP binding by the P-loop NTPase OsYchF1 (an unconventional G protein) contributes to biotic but not abiotic stress responses. Proc. Natl. Acad. Sci. U. S. A. 113, 2648–2653. doi: 10.1073/pnas.1522966113
Cheung, M. Y., Li, M. W., Yung, Y. L., Wen, C. Q., and Lam, H. M. (2013). The unconventional P-loop NTPase OsYchF1 and its regulator OsGAP1 play opposite roles in salinity stress tolerance. Plant Cell Environ. 36, 2008–2020. doi: 10.1111/pce.12108
Cheung, R. C. F., Ng, T. B., and Wong, J. H. (2017). Antifreeze proteins from diverse organisms and their applications: an overview. Curr. Protein Pept. Sci. 18, 262–283. doi: 10.2174/1389203717666161013095027
Choi, J. Y., Kim, S. C., and Lee, P. C. (2020). Comparative genome analysis of Psychrobacillus strain PB01, isolated from an iceberg. J. Microbiol. Biotechnol. 30, 237–243. doi: 10.4014/jmb.1909.09008
Coleine, C., Masonjones, S., Onofri, S., Selbmann, L., and Stajich, J. E. (2020). Draft genome sequence of the yeast Rhodotorula sp. strain CCFEE 5036, isolated from McMurdo dry valleys. Antarctica. Microbiol. Resour. Announce. 9:e00020. doi: 10.1128/MRA.00020-20
Coleine, C., Selbmann, L., Masonjones, S., Onofri, S., Zucconi, L., and Stajich, J. E. (2019). Draft genome sequence of an Antarctic isolate of the black yeast fungus Exophiala mesophila. Microbiol. Resour. Announc. 8:e00142–19. doi: 10.1128/MRA.00142-19
Collins, T., Roulling, F., Piette, F., Marx, J.-C., Feller, G., Gerday, C., et al. (2008). “Fundamentals of cold-adapted enzymes” in Psychrophiles: From Biodiversity to Biotechnology. eds. R. Margesin, F. Schinner, J-C. Marx, C. Gerday (Berlin: Springer), 211–227.
Couso, J. P., and Patraquim, P. (2017). Classification and function of small open reading frames. Nat. Rev. Mol. Cell Biol. 18, 575–589. doi: 10.1038/nrm.2017.58
Cunha, A. C., Santos, R. A. C. D., Riaño-Pachon, D. M., Squina, F. M., JVC, O., Goldman, G. H., et al. (2020). Draft genome sequence of Wickerhamomyces anomalus LBCM1105, isolated from cachaça fermentation. Genet. Mol. Biol. 43:e20190122. doi: 10.1590/1678-4685-gmb-2019-0122
D’Amico, S., Collins, T., Marx, J. C., Feller, G., and Gerday, C. (2006). Psychrophilic microorganisms: challenges for life. EMBO Rep. 7, 385–389. doi: 10.1038/sj.embor.7400662
Davies, P. L., Baardsnes, J., Kuiper, M. J., and Walker, V. K. (2002). Structure and function of antifreeze proteins. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 357, 927–935. doi: 10.1098/rstb.2002.1081
DeVries, A. L. (1986). Antifreeze glycopeptides and peptides: interactions with ice and water. Methods Enzymol. 127, 293–303. doi: 10.1016/0076-6879(86)27024-X
Ewels, P., Magnusson, M., Lundin, S., and Käller, M. (2016). MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048. doi: 10.1093/bioinformatics/btw354
Feller, G. (2010). Protein stability and enzyme activity at extreme biological temperatures. J. Phys. Condens. Matter 22:323101. doi: 10.1088/0953-8984/22/32/323101
Feller, G. (2013). Psychrophilic enzymes: from folding to function and biotechnology. Scientifica (Cairo) 2013:512840, 1–28. doi: 10.1155/2013/512840
Feller, G., and Gerday, C. (2003). “Catalysis and low temperature: molecular adaptations” in Extremophiles (Life Under Extreme Environmental Condition). ed. C. Gerday (Oxford, UK: Eolss Publishers)
Firdaus-Raih, M., Hashim, N. H. F., Bharudin, I., Abu Bakar, M. F., Huang, K. K., Alias, H., et al. (2018). The Glaciozyma Antarctica genome reveals an array of systems that provide sustained responses towards temperature variations in a persistently cold habitat. PLoS One 13:e0189947. doi: 10.1371/journal.pone.0189947
Garner, J., and Harding, M. M. (2010). Design and synthesis of antifreeze glycoproteins and mimics. Chembiochem 11, 2489–2498. doi: 10.1002/cbic.201000509
Gevrekci, A. Ö. (2017). The roles of polyamines in microorganisms. World J. Microbiol. Biotechnol. 33:204. doi: 10.1007/s11274-017-2370-y
Gocheva, Y. G., Krumova, E. T., Slokoska, L. S., Miteva, J. G., Vassilev, S. V., and Angelova, M. B. (2006). Cell response of Antarctic and temperate strains of Penicillium spp. to different growth temperature. Mycol. Res. 110, 1347–1354. doi: 10.1016/j.mycres.2006.08.007
Gocheva, Y. G., Tosi, S., Krumova, E. T., Slokoska, L. S., Miteva, J. G., Vassilev, S. V., et al. (2009). Temperature downshift induces antioxidant response in fungi isolated from Antarctica. Extremophiles 13, 273–281. doi: 10.1007/s00792-008-0215-1
Gunišová, S., and Valášek, L. S. (2014). Fail-safe mechanism of GCN4 translational control--uORF2 promotes reinitiation by analogous mechanism to uORF1 and thus secures its key role in GCN4 expression. Nucleic Acids Res. 42, 5880–5893. doi: 10.1093/nar/gku204
Guo, R., He, M., Zhang, X., Ji, X., Wei, Y., Zhang, Q. L., et al. (2021). Genome-wide transcriptional changes of Rhodosporidium kratochvilovae at low temperature. Front. Microbiol. 12:727105. doi: 10.3389/fmicb.2021.727105
Hao, Y., Zhang, L., Niu, Y., Cai, T., Luo, J., He, S., et al. (2018). SmProt: a database of small proteins encoded by annotated coding and non-coding RNA loci. Brief. Bioinform. 19, 636–643. doi: 10.1093/bib/bbx0005
Hashim, N. H., Bharudin, I., Nguong, D. L., Higa, S., Bakar, F. D., Nathan, S., et al. (2013). Characterization of Afp1, an antifreeze protein from the psychrophilic yeast Glaciozyma antarctica PI12. Extremophiles 17, 63–73. doi: 10.1007/s00792-012-0494-4
Huang, Y., Jiang, Z., Gao, X., Luo, P., and Jiang, X. (2021). ARMC subfamily: structures, functions, evolutions, interactions, and diseases. Front. Mol. Biosci. 8:791597. doi: 10.3389/fmolb.2021.791597
Inouye, M., and Phadtare, S. (2014). “Cold-shock response and adaptation to near-freezing temperature in cold-adapted yeasts” in Cold-Adapted Yeasts (Berlin: Springer), 243–257.
Jaeger, R., and Cuny, E. (2016). Terpenoids with special pharmacological significance: a review. Nat. Prod. Commun. 11:1934578X1601100. doi: 10.1177/1934578X1601100946
Ji, X., Cui, C., and Cui, Q. (2020). smORFunction: a tool for predicting functions of small open reading frames and microproteins. BMC Bioinformatics 21:455. doi: 10.1186/s12859-020-03805-x
Jones, D. T., Taylor, W. R., and Thornton, J. M. (1992). The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 8, 275–282.
Jung, S. K., Jeong, D. G., Lee, M. S., Lee, J. K., Kim, H. K., Ryu, S. E., et al. (2008). Structural basis for the cold adaptation of psychrophilic M37 lipase from Photobacterium lipolyticum. Proteins 71, 476–484. doi: 10.1002/prot.21884
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., et al. (2012). Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649. doi: 10.1093/bioinformatics/bts199
Kojima, T., Kinoshita, M., Yamada, T., Umezaki, S., Iwaizako, M., Saito, Y., et al. (2013). Molecular characterization of a novel armadillo repeat-like protein gene differentially induced by high-salt stress and dehydration from the model legume lotus Japonicus. Plant Mol. Biol. Report. 31, 698–706. doi: 10.1007/s11105-012-0542-3
Kondrashov, F. A. (2012). Gene duplication as a mechanism of genomic adaptation to a changing environment. Proc. Biol. Sci. 279, 5048–5057. doi: 10.1098/rspb.2012.1108
Kostadinova, N., Krumova, E., Angelova, M., and Stefanova, T. (2012). “Transient cold shock induces oxidative stress events in Antarctic fungi” in Oxidative Stress – Environmental Induction and Dietary Antioxidants. ed. V. Lushchak (Rijeka: Science, Technology and Medicine), 75–98.
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. doi: 10.1093/molbev/msy096
Leipe, D. D., Wolf, Y. I., Koonin, E. V., and Aravind, L. (2002). Classification and evolution of P-loop GTPases and related ATPases. J. Mol. Biol. 317, 41–72. doi: 10.1006/jmbi.2001.5378
Levasseur, A., and Pontarotti, P. (2011). The role of duplications in the evolution of genomes highlights the need for evolutionary-based approaches in comparative genomics. Biol. Direct 6:11. doi: 10.1186/1745-6150-6-11
Li, Z. R., Lin, H. H., Han, L. Y., Jiang, L., Chen, X., and Chen, Y. Z. (2006). PROFEAT: a web server for computing structural and physicochemical features of proteins and peptides from amino acid sequence. Nucleic Acids Res. 34, W32–W37. doi: 10.1093/nar/gkl305
Li, Y., Zhou, H., Chen, X., Zheng, Y., Kang, Q., Hao, D., et al. (2021). SmProt: a reliable repository with comprehensive annotation of small proteins identified from ribosome profiling. Genomics Proteomics Bioinformatics 19, 602–610. doi: 10.1016/j.gpb.2021.09.002
Liu, D., Wang, L., Zhai, H., Song, X., He, S., and Liu, Q. (2014). A novel α/β-hydrolase gene IbMas enhances salt tolerance in transgenic sweetpotato. PLoS One 9:e115128. doi: 10.1371/journal.pone.0115128
Margesin, R., and Feller, G. (2010). Biotechnological applications of psychrophiles. Environ. Technol. 31, 835–844. doi: 10.1080/09593331003663328
Margesin, R., and Miteva, V. (2011). Diversity and ecology of psychrophilic microorganisms. Res. Microbiol. 162, 346–361. doi: 10.1016/j.resmic.2010.12.004
Margesin, R., Neuner, G., and Storey, K. B. (2007). Cold-loving microbes, plants, and animals--fundamental and applied aspects. Naturwissenschaften 94, 77–99. doi: 10.1007/s00114-006-0162-6
Mat-Sharani, S., Bharudin, I., Zainuddin, N., Abdul-Murad, A.-M., Abu-Bakar, F.-D., Najimuddin, N., et al. (2015). Identification of small open reading frames in the Glaciozyma Antarctica genome. AIP Conf. Proc. 1678, 030016-1–030016-5. doi: 10.1063/1.4931237
Médigue, C., Krin, E., Pascal, G., Barbe, V., Bernsel, A., Bertin, P. N., et al. (2005). Coping with cold: the genome of the versatile marine Antarctica bacterium Pseudoalteromonas haloplanktis TAC125. Genome Res. 15, 1325–1335. doi: 10.1101/gr.4126905
Methé, B. A., Nelson, K. E., Deming, J. W., Momen, B., Melamud, E., Zhang, X., et al. (2005). The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc. Natl. Acad. Sci. U. S. A. 102, 10913–10918. doi: 10.1073/pnas.0504766102
Moriya, Y., Itoh, M., Okuda, S., Yoshizawa, A. C., and Kanehisa, M. (2007). KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 35, W182–W185. doi: 10.1093/nar/gkm321
Murray Stewart, T., Dunston, T. T., Woster, P. M., and Casero, R. A. (2018). Polyamine catabolism and oxidative damage. J. Biol. Chem. 293, 18736–18745. doi: 10.1074/jbc.TM118.003337
Navarro-Muñoz, J. C., and Collemare, J. (2019). Evolutionary histories of type III polyketide synthases in fungi. Front. Microbiol. 10:3018. doi: 10.3389/fmicb.2019.03018
Nishimura, O., Hara, Y., and Kuraku, S. (2017). gVolante for standardizing completeness assessment of genome and transcriptome assemblies. Bioinformatics 33, 3635–3637. doi: 10.1093/bioinformatics/btx445
Nishimura, O., Hara, Y., and Kuraku, S. (2019). Evaluating genome assemblies and gene models using gVolante. Methods Mol. Biol. 1962, 247–256. doi: 10.1007/978-1-4939-9173-0_15
Nizovoy, P., Bellora, N., Haridas, S., Sun, H., Daum, C., Barry, K., et al. (2021). Unique genomic traits for cold adaptation in Naganishia vishniacii, a polyextremophile yeast isolated from Antarctica. FEMS Yeast Res. 21:foaa056. doi: 10.1093/femsyr/foaa056
Ollis, D. L., Cheah, E., Cygler, M., Dijkstra, B., Frolow, F., Franken, S. M., et al. (1992). The α/β hydrolase fold. Protein Eng. Des. Sel. 5, 197–211. doi: 10.1093/protein/5.3.197
Parvez, A., Giri, S., Bisht, R., and Saxena, P. (2018). New insights on cyclization specificity of fungal type III polyketide synthase, PKSIIINc in Neurospora crassa. Indian J. Microbiol. 58, 268–277. doi: 10.1007/s12088-018-0738-9
Perlin, M. H., Andrews, J., and Toh, S. S. (2014). Essential letters in the fungal alphabet: ABC and MFS transporters and their roles in survival and pathogenicity. Adv. Genet. 85, 201–253. doi: 10.1016/B978-0-12-800271-1.00004-4
Rao, H. B., Zhu, F., Yang, G. B., Li, Z. R., and Chen, Y. Z. (2011). Update of PROFEAT: a web server for computing structural and physicochemical features of proteins and peptides from amino acid sequence. Nucleic Acids Res. 39, W385–W390. doi: 10.1093/nar/gkr284
Ren, J., Long, Y., Liu, R., Song, G., Li, Q., and Cui, Z. (2021). Characterization of biological pathways regulating acute cold resistance of zebrafish. Int. J. Mol. Sci. 22:3028. doi: 10.3390/ijms22063028
Renz, P. F., Valdivia-Francia, F., and Sendoel, A. (2020). Some like it translated: small ORFs in the 5’UTR. Exp. Cell Res. 396:112229. doi: 10.1016/j.yexcr.2020.112229
Riley, M., Staley, J. T., Danchin, A., Wang, T. Z., Brettin, T. S., Hauser, L. J., et al. (2008). Genomics of an extreme psychrophile, Psychromonas ingrahamii. BMC Genomics 9:210. doi: 10.1186/1471-2164-9-210
Sayari, M., Van Der Nest, M. A., Steenkamp, E. T., Soal, N. C., Wilken, P. M., and Wingfield, B. D. (2019). Distribution and evolution of nonribosomal peptide Synthetase gene clusters in the Ceratocystidaceae. Genes (Basel) 10, 1–17. doi: 10.3390/genes10050328
Sharma, M., and Pandey, G. K. (2015). Expansion and function of repeat domain proteins during stress and development in plants. Front. Plant Sci. 6:1218. doi: 10.3389/fpls.2015.01218
Stack, D., Neville, C., and Doyle, S. (2007). Nonribosomal peptide synthesis in aspergillus fumigatus and other fungi. Microbiology (Reading) 153, 1297–1306. doi: 10.1099/mic.0.2006/006908-0
Stanke, M., and Morgenstern, B. (2005). AUGUSTUS: a web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 33, W465–W467. doi: 10.1093/nar/gki458
Stanke, M., and Tzvetkova, A. (2006). AUGUSTUS at EGASP: using EST, protein and genomic alignments for improved gene prediction in the human genome. Genome Biol. 7, S11.1–S11.8. doi: 10.1186/gb-2006-7-s1-s11
Stecher, G., Tamura, K., and Kumar, S. (2020). Molecular evolutionary genetics analysis (MEGA) for macOS. Mol. Biol. Evol. 37, 1237–1239. doi: 10.1093/molbev/msz312
Su, Y., Jiang, X., Wu, W., Wang, M., Hamid, M. I., Xiang, M., et al. (2016). Genomic, transcriptomic and proteomic analysis provide insights into the cold adaptation mechanism of the obligate psychrophilic fungus Mrakia psychrophila. G3 (Bethesda) 6, 3603–3613. doi: 10.1534/g3.116.033308
Süssmuth, R. D., and Mainz, A. (2017). Nonribosomal peptide synthesis-principles and prospects. Angew. Chem. Int. Ed. Engl. 56, 3770–3821. doi: 10.1002/anie.201609079
Takahashi, M., Hikage, T., Yamashita, T., Saitoh, Y., Endou, M., and Tsutsumi, K.-I. (2006). Stress-related proteins are specifically expressed under non-stress conditions in the overwinter buds of the gentian plant Gentiana triflora. Breed. Sci. 56, 39–46. doi: 10.1270/jsbbs.56.39
Touchette, D., Ltshuler, I., Gostinčar, C., Zalar, P., Raymond-Bouchard, I., Zajc, J., et al. (2022). Novel Antarctic yeast adapts to cold by switching energy metabolism and increasing small RNA synthesis. ISME J. 16, 221–232. doi: 10.1038/s41396-021-01030-9
Tsuji, M. (2016). Cold-stress responses in the Antarctic basidiomycetous yeast Mrakia blollopis. R. Soc. Open Sci. 3:160106. doi: 10.1098/rsos.160106
Turchetti, B., Thomas Hall, S. R., Connell, L. B., Branda, E., Buzzini, P., Theelen, B., et al. (2011). Psychrophilic yeasts from Antarctica and European glaciers: description of Glaciozyma gen. Nov., Glaciozyma martinii sp. nov. and Glaciozyma watsonii sp. nov. Extremophiles 15, 573–586. doi: 10.1007/s00792-011-0388-x
Tyler, J. K., and Johnson, J. E. (2018). The role of autophagy in the regulation of yeast life span. Ann. N. Y. Acad. Sci. 1418, 31–43. doi: 10.1111/nyas.13549
Valdés-Santiago, L., and Ruiz-Herrera, J. (2013). Stress and polyamine metabolism in fungi. Front. Chem. 1:42. doi: 10.3389/fchem.2013.00042
Vander Meersche, Y., De Cretin, G., Brevern, A. G., Gelly, J. C., and Galochkina, T. (2021). MEDUSA: prediction of protein flexibility from sequence. J. Mol. Biol. 433:166882. doi: 10.1016/j.jmb.2021.166882
Villarreal, P., Carrasco, M., Barahona, S., Alcaíno, J., Cifuentes, V., and Baeza, M. (2018). Antarctic yeasts: analysis of their freeze-thaw tolerance and production of antifreeze proteins, fatty acids and ergosterol. BMC Microbiol. 18, 1–10. doi: 10.1186/s12866-018-1214-8
Wang, X. Q., and Rothnagel, J. A. (2004). 5′-untranslated regions with multiple upstream AUG codons can support low-level translation via leaky scanning and reinitiation. Nucleic Acids Res. 32, 1382–1391. doi: 10.1093/nar/gkh305
Wedemeyer, W. J., Welker, E., and Scheraga, H. A. (2002). Proline cis−trans isomerization and protein folding. Biochemistry 41, 14637–14644. doi: 10.1021/bi020574b
Wong, C. M. V. L., Boo, S. Y., Voo, C. L. Y., Zainuddin, N., and Najimudin, N. (2019). A comparative transcriptomic analysis provides insights into the cold-adaptation mechanisms of a psychrophilic yeast, Glaciozyma antarctica PI12. Polar Biol. 42, 541–553. doi: 10.1007/s00300-018-02443-7
Wu, D., Wang, D., and Hong, J. (2020). Effect of a novel alpha/Beta hydrolase domain protein on tolerance of K. marxianus to lignocellulosic biomass derived inhibitors. Front. Bioeng. Biotechnol. 8:844. doi: 10.3389/fbioe.2020.00844
Yan, N. (2015). Structural biology of the major facilitator superfamily transporters. Annu. Rev. Biophys. 44, 257–283. doi: 10.1146/annurev-biophys-060414-033901
Yu, D., Xu, F., Zeng, J., and Zhan, J. (2012). Type III polyketide synthases in natural product biosynthesis. IUBMB Life 64, 285–295. doi: 10.1002/iub.1005
Yusof, N. A., Hashim, N. H. F., and Bharudin, I. (2021). Cold adaptation strategies and the potential of psychrophilic enzymes from the Antarctic yeast, Glaciozyma antarctica PI12. J. Fungi 7: 528. doi: 10.3390/jof7070528
Zajc, J., Černoša, A., Sun, X., Fang, C., Gunde-Cimerman, N., Song, Z., et al. (2022). From glaciers to refrigerators: the population genomics and biocontrol potential of the black yeast Aureobasidium subglaciale. Microbiol. Spectr. 10:e0145522. doi: 10.1128/spectrum.01455-22
Keywords: cold-adapted yeasts, Alpine yeasts, draft genomes, stress genes, secondary metabolites
Citation: Turchetti B, Buzzini P and Baeza M (2022) A genomic approach to analyze the cold adaptation of yeasts isolated from Italian Alps. Front. Microbiol. 13:1026102. doi: 10.3389/fmicb.2022.1026102
Received: 23 August 2022; Accepted: 07 October 2022;
Published: 08 November 2022.
Edited by:
Ajar Nath Yadav, Eternal University, IndiaReviewed by:
Pankaj Bhatt, Purdue University, United StatesCopyright © 2022 Turchetti, Buzzini and Baeza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcelo Baeza, bWFiZXphQHVjaGlsZS5jbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.