
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Microbiol., 05 October 2022
Sec. Virology
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1011891
This article is part of the Research TopicInsights of Important Mammalian Viruses: Infection, Pathogenesis and DrugsView all 10 articles
 Julian Ruiz-Saenz1*†
Julian Ruiz-Saenz1*† Andres Diaz2
Andres Diaz2 D. Katterine Bonilla-Aldana3
D. Katterine Bonilla-Aldana3 Alfonso J. Rodríguez-Morales3,4
Alfonso J. Rodríguez-Morales3,4 Marlen Martinez-Gutierrez5
Marlen Martinez-Gutierrez5 Patricia V. Aguilar6,7
Patricia V. Aguilar6,7African swine fever (ASF) is a devastating disease for the swine industry, characterized by hemorrhagic fever with up to 100% mortality rate, and with a tremendous socioeconomic impact worldwide (Dixon et al., 2020). The disease was first reported in East Africa in the early 1920s as an acute hemorrhagic fever that caused the death of almost all infected domestic pigs (Montgomery, 1921; Plowright et al., 1969). Since then, the African swine fever virus (ASFV) has remained endemic in Africa affecting up to 35 African countries and has emerged in Europe, Asia, and now in the Americas.
Since the reemergence of ASFV in Europe through the Caucasus region in 2007, the virus has rapidly expanded and reached the Russian Federation, East Europe, and Asia (Dixon et al., 2020). In 2018, the detection of ASFV in China, killing at least half of the swine population of China (Zhou et al., 2018), and subsequent dissemination to Southeast Asia; threat one of the most significant swine industries of the world, which contains half the world's swine population (Dixon et al., 2020; Gaudreault et al., 2020). The high socioeconomic impact of ASFV infection results from direct death and culling of the animals and loss of business in the swine production chain, costs of disease control, and international trade blocks (Zhou et al., 2018). In addition, large epidemics can result in dramatic reductions in the size of national pig herds and inflation of prices of pig and pork products (Dixon et al., 2020; Gaudreault et al., 2020).
It is well-known that the highly virulent ASFV genotype II that emerged in the Caucasus region in 2007 is responsible for the contemporary European/Asiatic epidemic (Sánchez-Vizcaíno et al., 2013; Ge et al., 2018; Cwynar et al., 2019). The virus has spread to domestic pigs and wild boards across Eastern Europe and Asia (Gavier-Widén et al., 2015; Li et al., 2019). Multiple efforts are in place to avoid the dissemination of the ASFV throughout the European Union. However, the disease was first identified in Lithuania, Poland, Latvia, and Estonia in 2014 (de la Torre et al., 2015), and by 2019, it was in Belgium, Bulgaria, Slovakia, Estonia, Hungary, Latvia, Lithuania, Poland, and Romania. By the end of 2020, Germany reported their first case of ASF in domestic pigs (Sauter-Louis et al., 2021b). The current epidemiology of ASF in wild boars in East Europe plays a vital role in the risk of ASFV transmission to the domestic population (Sauter-Louis et al., 2021a; de la Torre et al., 2022).
In the Americas, ASFV emerged in late 1970s in Brazil, Cuba, and the Caribbean Island with a full eradication at early 1980s (De Paula Lyra et al., 1986). Nevertheless, ASFV re-emerged in the Americas in 2021. On 28 July 2021, the United States Department of Agriculture (USDA, 2021) confirmed the presence of ASFV in the Dominican Republic and it turned on the alarms for the swine industry in the Americas. According to the Department of Agriculture of the Dominican Republic, the virus has been detected in at least 22 out of the 31 provinces in the country (Agricultura, 2021). As stated by the World Organization of Animal Health (WOAH), the genotype II was detected in all positive samples (WOAH, 2021a,c).
On 20 September 2021, the Chief Veterinary Officer from Haiti reported a new case of ASF to the WOAH, becoming the second Country with ASFV positive samples in the Americas. The sample was collected from a backyard farm in a province bordering the Dominican Republic and was tested by the USDA Laboratories through a cooperative testing program. The report filed with the WOAH indicates the outbreak in Haiti began in late August and killed several swine (WOAH, 2021d) and by the end of October 2021, a total of seven outbreaks of ASF have been identified, affecting multiple provinces all over the country (WOAH, 2021b).
With a case fatality rate close to 87% in the Dominican Republic (WOAH, 2021a), lack of approved vaccines, the current ASF outbreaks highlight the devastating consequences for the swine industries (Busch et al., 2021). ASFV is now confirmed in at least 60 countries worldwide (in which ~80% of the swine population resides). Furthermore, global ASF outbreaks have increased 25% since 2018 (Gaudreault et al., 2020), changing the swine industry's global dynamics.
ASFV is a complex, enveloped virus that contains a large (170–190-kb) double-stranded DNA (dsDNA) genome (Tulman et al., 2009). ASFV is the only known member of the Asfarviridae family (Alonso et al., 2018) and it is currently grouped into 24 genotypes, all of them being associated with disease (Achenbach et al., 2017). Although most of the genotypes have been linked to ASF outbreaks in various parts of sub-Saharan Africa, genotype I dominates in Central and West Africa (Minoungou et al., 2021; Njau et al., 2021). The first description of AFSV outside Africa, was reported in 1958 and again in 1961 from Lisbon Portugal with subsequent spread to other regions of Europe and Latin America of the genotype I (Mur et al., 2016). During the 1970s and 1980s, ASF become endemic in the Iberian Peninsula and many other countries were affected by sporadic ASFV outbreaks due to the introduction and use of food waste from international planes or boats to feed pigs. Strong and long-lasting efforts were put in different places to achieve eradication in most of Europe, for a comprehensive review see Danzetta et al. (2020).
Virulence is not fully associated with the viral genotype, and infection can lead to a broad spectrum of disease severity, from highly lethal to subclinical or asymptomatic, depending on host characteristics and the specific viral strain (Boinas et al., 2004). While a highly virulent ASFV has been widely reported in Eurasia (90–100% mortality in domestic pigs and European wild boar), reduced virulence strains and attenuated strains of the ASFV genotype II were found in Estonia and Latvia (Zani et al., 2018; Gallardo et al., 2019; Vilem et al., 2020) suggesting a threat for control programs due to the risk of pigs with chronic and carriers behaviors besides of the difficulty of early detection of ASF epizootics due to a lack of clear specific clinical signs of infection, as well as the presence of non-viremic animals (Gallardo et al., 2018).
Based on genotyping, whole genome sequencing and phylogenetic analysis, the ASFV that spread through Europe (Georgia 2007/1) has been proved to originate in South East Africa, but the exact location of the origin of this genotype includes Mozambique, Malawi, Zambia, southern Tanzania, and Madagascar (Quembo et al., 2018; Njau et al., 2021). However, the recently confirmation of the ASFV genotype II in Lagos, Nigeria (West Africa), complicates the already constrained control measures against the disease in the region (Adedeji et al., 2021) and emphasize the risk of worldwide dissemination of this highly pathogenic genotype II.
ASFV can also be transmitted by soft ticks of the genus Ornithodoros in the family Argasidae, which act as biological vectors of ASFV (Pereira De Oliveira et al., 2019). To date, eight Ornithodoros taxa have been demonstrated as competent vectors of ASFV, including O. marocanus, O. puertoricensis, O. coriaceus, O. moubata porcinus, O. erraticus, O. moubata complex, O. turicata , and O. savignyi (Groocock et al., 1980; Mellor and Wilkinson, 1985; Hess et al., 1987; Endris et al., 1991, 1992; Kleiboeker et al., 1998; Rennie et al., 2000; Ribeiro et al., 2015; Golnar et al., 2019). Although O. erraticus ticks has been proven competent to replicate the ASFV genotype II, it failed to transmit the Eurasian ASFV strains to naive pigs (Diaz et al., 2012; Pereira De Oliveira et al., 2019) under experimental conditions, suggesting that other determinants beyond viral replication also influence ASFV vector competence (Pereira De Oliveira et al., 2020b). However, recent analysis had shown successful infection of domestic pigs by ingesting O. erraticus ticks that fed on ASFV-infected pigs suggesting that O. erraticus may act as a reservoir of ASFV and suggesting new transmission routes of ASFV (Pereira De Oliveira et al., 2020a).
In the Americas, multiple Ornithodoros species could be considered potential vectors of ASFV. At least three Ornithodoros species found in the US are considered high-risk competent vectors (O. coriaceus, O. turicata, and O. puertoricensis). However, it remains unknown if other soft ticks in the Americas can also transmit ASFV (Golnar et al., 2019). Critical role of certain Ornithodoros species in the Americas and the Caribbean need to be clarified since O. puertoricensis could be an efficient vector for ASFV (Butler and Gibbs, 1984; Butler et al., 1985). Nevertheless, the presence of these ticks in Haiti and the Dominican Republic did not appear to complicate the eradication of AFV from these countries in 1978 possibly due to a lack of contact between infected pigs and O. puertoricensis (Kleiboeker and Scoles, 2001) or perhaps due to a low stadial transmission of ASFV in the O. puertoricensis, which was found to decrease from nearly 100% to less than 35% from the nymphal to adult stage (Endris et al., 1991) that reduced the risk of transmission. This low transmission could be due to the presence of endogenous viral elements of the ASFV, which might have been integrated into soft tick genomes and serve as templates for siRNA and piRNA thereby possibly protecting the tick against viral infection as has been recently reported for O. moubata and O. porcinus field-collected ticks from Africa (Forth et al., 2020).
In regard to competent hosts, at least three susceptible species of swine are present in the Americas: domestic pigs (Sus scrofa domesticus), the invasive feral boars (Sus scrofa) in wildlife, and common warthogs (Phacochoerus africanus) in different zoos (Golnar et al., 2019). ASFV is easily transmitted from persistently infected host/reservoirs to uninfected animals (de Carvalho Ferreira et al., 2013; Gallardo et al., 2015). Therefore, the possibility that wild fauna in the Americas can become persistently infected and spread the virus to susceptible animals urge to be considered (Brown and Bevins, 2018). In fact, a recent analysis of the risk of ASFV establishment and spillover in the United States has been reported. Based on feral swine distribution, soft ticks, and the inventory of domestic swine in the US this report indicates that certain areas of California, Florida, and much of the southwestern United States are high risk zones for ASFV establishment and spillover (Wormington et al., 2019).
Boar hunting and human movements across borders with contaminated fomites or meat represent the major risk for naive populations. Hence, the presence of the ASFV genotype II in the Dominican Republic and Haiti highlights the urgent need for better transnational discussion regarding but not limited to (1) preventive measurements; (2) appropriate disposal of swine products potentially contaminated with ASFV; (3) early detection of the virus if introduced into a naive country; (4) prompt and coordinated response of producers, local authorities, and governments; (5) education; and (6) communication between countries. This discussion should include not only governments and decision-makers, but also large, medium, and small-sized pork producers.
Despite prevention and control efforts, ASF has led to an unprecedented crisis in the global pig sector representing a global risk to animal health and welfare, national and international economies, rural development, social/political behavior, national food security, and national and international markets (Gf-TADs, 2020). The Global Framework for the Progressive Control of Transboundary Animal Diseases (GF-TADs), funded by WOAH, and FAO has established a plan for preparation and early detection of a possible arrival of ASFV to the Americas (Komal, 2020). Once the virus was reported in China, countries of the Americas came together to discuss prevention and to establish preparedness plans. However, this was not enough to avoid the arrival and dissemination of the ASFV in the Dominican Republic and the subsequent transborder transmission to Haiti. The economic impact of ASFV in the pork industry of the Americas could be devastating. Five out of 15 of the major pork exporters of the world are in the Americas and the presence of ASF in a country will limit its trade capabilities of pork.
Given the current global situation and the detection of the virus in the Dominican Republic and Haiti, the swine industry recognizes a high risk of introducing ASFV into the continental part of the Americas (Figure 1). Hence, multiple preparedness efforts are being established in each country to avoid introducing and disseminating the disease. Responsible importation of supplies potentially contaminated with ASFV and appropriate downtime for hard-to-clean and disinfecting supplies is encouraged. Strict biosecurity practices and compliance at all swine production and management levels should be mandatory if producers want to keep the disease out of their system.
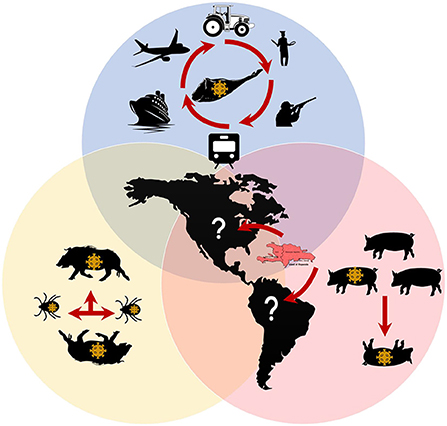
Figure 1. ASFV transmission known and gaps in the Americas. Red arrows represent potential routes for ASFV transmission. Blue shape: Human movement with contaminated fomites or contaminated pork represent the major risk for naive populations. Yellow shape: The potential role of wild Boars and Ornithodoros tick vectors still to be determined in the Americas. Orange shape: Virus transmission in pig farms has been confirmed in the Dominican Republic and Haiti. See text for references.
Since ASF is not a public health risk, people can regularly eat pork or pork-derived products. However, no pork or pork products should be transported from a country with ASF to another country. Additionally, no pork or pork products should be allowed into swine production facilities, given the risk of introduction through contaminated pork. ASFV DNA has been found in multiple pork products brought into South Korea and Taiwan by travelers (Kim et al., 2019; Wang et al., 2019), although no live virus has been isolated from such products. Furthermore, pigs should not be fed with food waste from restaurants or any kitchen. Clean and dirty areas must be clearly defined at feed mills, swine farms, and truck wash facilities (Li and Tian, 2018). There should be biosecurity protocols in place to avoid the contamination of feed or supplies for feed manufacturing. All supplies must be cleaned and disinfected before crossing into the clean area of swine facilities. Moreover, every swine production facility must have a response plan including management and disposal of mortality in case of an ASF outbreak.
Backyard production may possess different risks or perceptions regarding the disease than industrialized swine production. Hence, communication between authorities, producers, and local governments is key to reduce the risk of dissemination if the virus hits a given country. Active surveillance at different levels is required for early detection. Early detection of the virus is a crucial component minimize the impact of ASF introduction into a country. Unfortunately, in the Americas, PCR diagnosis can only be attempted until a suspect case is reported. Hence, if the clinical manifestation of the disease happens 2–3 weeks after the introduction into a farm, it might be too late to detect the virus (2–3 weeks after introduction into a commercial swine farm) and avoid dissemination within a region given the continuous flow and movement of pigs in the modern swine industry. Several European Union countries allow producers to monitor ASF status in the farms without needing to wait for clinical signs. It is also important to denote that recently based on expert perceived opinion, passive surveillance of wild boar and syndromic surveillance of pig mortality has been considered to be the most effective for controlling ASFV spread, whereas culling of all infected herds and movement bans for neighboring herds were considered as the most effective intervention strategies (Guinat et al., 2017).
As important measures, the USDA has recommended to the WOAH to establish the first foreign animal disease protection zone in United States, Puerto Rico, and the US Virgin Islands. Also, the Animal and Plant Health Inspection Service (APHIS) has suspended movement of live swine, swine germplasm, swine products, and swine byproducts from Puerto Rico and the US Virgin Island in an effort to keep ASF out of the US mainland. The establishment of an WOAH officially recognized protection zone would allow the US to maintain its ASF disease-free status, continue exporting pork and live swine, even if ASF is discovered within the protection zone (APHIS-USDA, 2021).
A new eradication of ASFV from the Haiti and the Dominican Republic can be achieved in spite we are facing a different epidemiological context. Previous eradication in the Americas showed that classical surveillance strategies, both at farm and slaughterhouse levels, conventional biosecurity and sanitary measures and well-structured collaboration among institutions in affected countries hand-in-hand to the international organizations (FAO and WOAH) is keystone for ASFV eradication (Danzetta et al., 2020). Countries should strengthen their surveillance systems based on previously known capabilities developed through the current “Continental Plan for the eradication of Classical Swine Fever (CSF) from the Americas,” that has achieved a CSF free status in most of the American continent in a step-by-step process of management, control, and elimination of the disease in infected countries (Ganges et al., 2020; WOAH, 2021e). The continuous support from USDA and other high-income countries will be crucial for the ASFV contention and eradication in the Hispaniola Island, which could be more economically profitable than assuming the losses of a possible arrival of the ASFV to continental soil.
The current economic crisis in Latin American countries like Venezuela (Suárez et al., 2018) highlights a possible risk of ASF introduction into South America, given the high probability of pork importation products to Venezuela from ASFV-positive countries. In addition, the current COVID-19 pandemic seems to be negative for the control of the ASFV. Low-income countries in Africa have reported increasing ASF incidence in association with the coronavirus emerging disease, mainly due to the redistribution of resources and attention toward mitigating the COVID-19 pandemic (Uwishema et al., 2021). It is essential to consider the socio-political conditions of the many nations of the Caribbean. The introduction of ASFV into Haiti and its dissemination throughout the country may increase the strong socio/economic disparities, and long-standing governance problems as well as ecological disturbance due to Natural disasters and uncontrolled COVID-19 infections (Oxford-Analytica, 2020; Hoffman, 2021). The same could apply for Cuba and other Caribbean nations in geographic proximity (Gorry, 2021); the close relationship, international trading and people movement between positive and naive nations could favor ASFV transmission.
Finally, no effective ASFV vaccine exists commercially. However, multiple vaccine developments are in progress due to the current situation of ASF worldwide (Bosch-Camós et al., 2020), Vaccine efficacy and vaccination strategies is a subject that needs to be taken into consideration to prevent a possible dissemination of ASFV in the Americas (Rivera-Benítez et al., 2021). Multiple vaccine development strategies have been employed, with varying levels of success (Gaudreault et al., 2020). The most promissory vaccine candidate has been achieved by deletion of the ASFV I177L gene (ASFV-G-ΔI177L) resulting in sterile immunity against the epidemic ASFV Genotype II (Borca et al., 2020). Besides, this attenuated ASFV strain has proven being effective to protect pig breeds against genotype II ASFV challenge (Tran et al., 2021) and completely protected against virulent ASFV challenge when it was administered by the oronasal route, even at large-scale experiments (Borca et al., 2021b; Tran et al., 2022). The stable adaptation of this live attenuated vaccine strain to cell culture (Borca et al., 2021a) and its oronasal distribution will allow to produce an ASF vaccine on a commercial scale and it possible use for wildlife. Moreover, an accompanying genetic test to discriminate between infected and vaccinated animals (DIVA) has been developed, and are a promising option to support the control and eradication of the ASFV genotype II during a potential vaccination program (Velazquez-Salinas et al., 2021).
Despite the multiple multilateral efforts to avoid ASFV emergence into the Americas, the virus has arrived and spread rapidly from one country to another. This situation highlights the need to establish local, regional, and transnational measures to avoid the spread of ASFV to other countries in the region (Kading et al., 2019). It is imperative to establish and follow national and international guidelines on preventive surveillance and diagnosis including enhancing communication among countries, strengthen vector surveillance, and increase public awareness of ASFV. Finally, we encourage to establish regional networks of cooperation, as has been implanted in the European Union (EFSA et al., 2021), that help to research/understand major gaps in knowledge in the Americas as much as to harmonize diagnostic techniques, share real-time surveillance data and help to avoid the possible dissemination of ASFV in the Region.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was financially supported CONADI-UCC Grant to JR-S and by the Fogarty International Center and the National Institute of Allergy and Infectious Diseases, of the National Institutes of Health under Award Number D43 TW010331 to PA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author AD is employed by Pig Improvement Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Achenbach, J., Gallardo, C., Nieto-Pelegrín, E., Rivera-Arroyo, B., Degefa-Negi, T., Arias, M., et al. (2017). Identification of a new genotype of African swine fever virus in domestic pigs from Ethiopia. Transbound. Emerg. Dis. 64, 1393–1404. doi: 10.1111/tbed.12511
Adedeji, A. J., Luka, P. D., Atai, R. B., Olubade, T. A., Hambolu, D. A., Ogunleye, M. A., et al. (2021). First-time presence of African swine fever virus genotype II in Nigeria. Microbiol. Resour. Announc. 10, e00350–e00321. doi: 10.1128/MRA.00350-21
Agricultura, M. D. (2021). “Boletin 2. ¡Juntos podemos vencer a la PPA!,” in Comisión Oficial Para El Control Y Erradicación De Brotes De La Peste Porcina Africana, ed M.D.A. (República Dominicana: Ministerio de Agricultura).
Alonso, C., Borca, M., Dixon, L., Revilla, Y., Rodriguez, F., Escribano, J. M., et al. (2018). ICTV virus taxonomy profile: Asfarviridae. J. Gen. Virol. 99, 613–614. doi: 10.1099/jgv.0.001049
APHIS-USDA (2021). USDA Submits Dossier to the World Organisation for Animal Health to Finalize African Swine Fever Protection Zone. Program Updates, USDA, USA.
Boinas, F., Hutchings, G., Dixon, L., and Wilkinson, P. (2004). Characterization of pathogenic and non-pathogenic African swine fever virus isolates from Ornithodoros erraticus inhabiting pig premises in Portugal. J. Gen. Virol. 85, 2177–2187. doi: 10.1099/vir.0.80058-0
Borca, M. V., Rai, A., Ramirez-Medina, E., Silva, E., Velazquez-Salinas, L., Vuono, E., et al. (2021a). A cell culture-adapted vaccine virus against the current African swine fever virus pandemic strain. J. Virol. 95, e00123–e00121. doi: 10.1128/JVI.00123-21
Borca, M. V., Ramirez-Medina, E., Silva, E., Vuono, E., Rai, A., Pruitt, S., et al. (2020). Development of a highly effective African swine fever virus vaccine by deletion of the I177L gene results in sterile immunity against the current epidemic Eurasia strain. J. Virol. 94, e02017–e02019. doi: 10.1128/JVI.02017-19
Borca, M. V., Ramirez-Medina, E., Silva, E., Vuono, E., Rai, A., Pruitt, S., et al. (2021b). ASFV-G-ΔI177L as an effective oral nasal vaccine against the Eurasia strain of Africa swine fever. Viruses 13:765. doi: 10.3390/v13050765
Bosch-Camós, L., López, E., and Rodriguez, F. (2020). African swine fever vaccines: a promising work still in progress. Porcine Health Manage. 6, 1–14. doi: 10.1186/s40813-020-00154-2
Brown, V. R., and Bevins, S. N. (2018). A review of African swine fever and the potential for introduction into the United States and the possibility of subsequent establishment in feral swine and native ticks. Front. Vet. Sci. 5:11. doi: 10.3389/fvets.2018.00011
Busch, F., Haumont, C., Penrith, M.-L., Laddomada, A., Dietze, K., Globig, A., et al. (2021). Evidence-based African swine fever policies: do we address virus and host adequately? Front. Vet. Sci. 8:637487. doi: 10.3389/fvets.2021.637487
Butler, J., and Gibbs, E. (1984). Distribution of potential soft tick vectors of African swine fever in the Caribbean region (Acari: Argasidae). Prev. Vet. Med. 2, 63–70. doi: 10.1016/0167-5877(84)90049-7
Butler, J., Wilson, D., Garris, G., Koch, H., Crum, J., and Castellanos, V. (1985). Survey for potential soft tick (Acari: Argasidae) vectors of African swine fever on the island of Hispaniola. Exp. Appl. Acarol. 1, 63–72. doi: 10.1007/BF01262200
Cwynar, P., Stojkov, J., and Wlazlak, K. (2019). African swine fever status in Europe. Viruses 11:310. doi: 10.3390/v11040310
Danzetta, M. L., Marenzoni, M. L., Iannetti, S., Tizzani, P., Calistri, P., and Feliziani, F. (2020). African swine fever: lessons to learn from past eradication experiences. A systematic review. Front. Vet. Sci. 7:296. doi: 10.3389/fvets.2020.00296
de Carvalho Ferreira, H., Weesendorp, E., Quak, S., Stegeman, J., and Loeffen, W. (2013). Quantification of airborne African swine fever virus after experimental infection. Vet. Microbiol. 165, 243–251. doi: 10.1016/j.vetmic.2013.03.007
de la Torre, A., Bosch, J., Iglesias, I., Muñoz, M. J., Mur, L., Martínez-López, B., et al. (2015). Assessing the risk of African swine fever introduction into the European Union by wild boar. Transbound. Emerg. Dis. 62, 272–279. doi: 10.1111/tbed.12129
de la Torre, A., Bosch, J., Sánchez-Vizcaíno, J. M., Ito, S., Muñoz, C., Iglesias, I., et al. (2022). African swine fever survey in a European context. Pathogens 11:137. doi: 10.3390/pathogens11020137
De Paula Lyra, T., Saraiva, V., Hermida Lage, G., and Samarcos, M. (1986). Eradication of African swine fever from Brazil. Rev. Sci. Tech. l'OIE (France) 5, 771–787. doi: 10.20506/rst.5.3.261
Diaz, A. V., Netherton, C. L., Dixon, L. K., and Wilson, A. J. (2012). African swine fever virus strain Georgia 2007/1 in Ornithodoros erraticus ticks. Emerg. Infect. Dis. 18, 1026–1028. doi: 10.3201/eid1806.111728
Dixon, L. K., Stahl, K., Jori, F., Vial, L., and Pfeiffer, D. U. (2020). African swine fever epidemiology and control. Annu. Rev. Anim. Biosci. 8, 221–246. doi: 10.1146/annurev-animal-021419-083741
EFSA European Food Safety Authority, Nielsen, S. S., Alvarez, J., Bicout, D. J., Calistri, P., Depner, K., et al. (2021). Research priorities to fill knowledge gaps in the control of African swine fever: possible transmission of African swine fever virus by vectors. EFSA J. 19:e06676. doi: 10.2903/j.efsa.2021.6676
Endris, R., Haslett, T., and Hess, W. (1991). Experimental transmission of African swine fever virus by the tick Ornithodoros (Alectorobius) puertoricensis (Acari: Argasidae). J. Med. Entomol. 28, 854–858. doi: 10.1093/jmedent/28.6.854
Endris, R., Hess, W., and Caiado, J. (1992). African swine fever virus infection in the Iberian soft tick, Ornithodoros (Pavlovskyella) marocanus (Acari: Argasidae). J. Med. Entomol. 29, 874–878. doi: 10.1093/jmedent/29.5.874
Forth, J. H., Forth, L. F., Lycett, S., Bell-Sakyi, L., Keil, G. M., Blome, S., et al. (2020). Identification of African swine fever virus-like elements in the soft tick genome provides insights into the virus' evolution. BMC Biol. 18:136. doi: 10.1186/s12915-020-00865-6
Gallardo, C., Nurmoja, I., Soler, A., Delicado, V., Simón, A., Martin, E., et al. (2018). Evolution in Europe of African swine fever genotype II viruses from highly to moderately virulent. Vet. Microbiol. 219, 70–79. doi: 10.1016/j.vetmic.2018.04.001
Gallardo, C., Soler, A., Nieto, R., Sánchez, M., Martins, C., Pelayo, V., et al. (2015). Experimental transmission of African swine fever (ASF) low virulent isolate NH/P68 by surviving pigs. Transbound. Emerg. Dis. 62, 612–622. doi: 10.1111/tbed.12431
Gallardo, C., Soler, A., Rodze, I., Nieto, R., Cano-Gómez, C., Fernandez-Pinero, J., et al. (2019). Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transbound. Emerg. Dis. 66, 1399–1404. doi: 10.1111/tbed.13132
Ganges, L., Crooke, H. R., Bohórquez, J. A., Postel, A., Sakoda, Y., Becher, P., et al. (2020). Classical swine fever virus: the past, present and future. Virus Res. 289:198151. doi: 10.1016/j.virusres.2020.198151
Gaudreault, N. N., Madden, D. W., Wilson, W. C., Trujillo, J. D., and Richt, J. A. (2020). African swine fever virus: an emerging DNA Arbovirus. Front. Vet. Sci. 7:215. doi: 10.3389/fvets.2020.00215
Gavier-Widén, D., Gortázar, C., Stahl, K., Neimanis, A. S., Rossi, S., Hard Av Segerstad, C., et al. (2015). African swine fever in wild boar in Europe: a notable challenge. Vet. Rec. 176, 199–200. doi: 10.1136/vr.h699
Ge, S., Li, J., Fan, X., Liu, F., Li, L., Wang, Q., et al. (2018). Molecular characterization of African swine fever virus, China, 2018. Emerg. Infect. Dis. 24, 2131–2133. doi: 10.3201/eid2411.181274
Gf-TADs (2020). African Swine Fever: An Unprecedented Global Threat—A Challenge to Livelihoods, Food Security and Biodiversity. Call for Action. Available online at: http://www.gf-tads.org/events/events-detail/fr/c/1152886/ (accessed 26 October, 2020).
Golnar, A. J., Martin, E., Wormington, J. D., Kading, R. C., Teel, P. D., Hamer, S. A., et al. (2019). Reviewing the potential vectors and hosts of African swine fever virus transmission in the United States. Vector Borne Zoonotic Dis. 19, 512–524. doi: 10.1089/vbz.2018.2387
Gorry, C. (2021). In Haiti, Cubans among first responders, again: Luis Orlando Oliveros-Serrano MD Coordinator, Cuban Medical Team in Haiti. MEDICC Rev. 24, 19–20. doi: 10.37757/MR2022.V24.N1.1
Groocock, C., Hess, W., and Gladney, W. (1980). Experimental transmission of African swine fever virus by Ornithodoros coriaceus, an argasid tick indigenous to the United States. Am. J. Vet. Res. 41, 591–594.
Guinat, C., Vergne, T., Jurado-Diaz, C., Sánchez-Vizcaíno, J. M., Dixon, L., and Pfeiffer, D. U. (2017). Effectiveness and practicality of control strategies for African swine fever: what do we really know? Vet. Rec. 180:97. doi: 10.1136/vr.103992
Hess, W. R., Endris, R. G., Haslett, T. M., Monahan, M. J., and Mccoy, J. P. (1987). Potential arthropod vectors of African swine fever virus in North America and the Caribbean basin. Vet. Parasitol. 26, 145–155. doi: 10.1016/0304-4017(87)90084-7
Hoffman, D. M. (2021). The Haitian orphanage crisis: exporting neoliberal family ideals in the debate on vulnerable childhoods in Haiti. Child. Soc. 35, 577–592. doi: 10.1111/chso.12442
Kading, R. C., Abworo, E. O., and Hamer, G. L. (2019). Rift valley fever virus, Japanese encephalitis virus, and African swine fever virus: three transboundary, vector-borne, veterinary biothreats with diverse surveillance, and response capacity needs. Front. Vet. Sci. 6:458. doi: 10.3389/fvets.2019.00458
Kim, H.-J., Lee, M.-J., Lee, S.-K., Kim, D.-Y., Seo, S.-J., Kang, H.-E., et al. (2019). African swine fever virus in pork brought into South Korea by travelers from China, August 2018. Emerg. Infect. Dis. 25, 1231–1233. doi: 10.3201/eid2506.181684
Kleiboeker, S. B., Burrage, T. G., Scoles, G. A., Fish, D., and Rock, D. L. (1998). African swine fever virus infection in the argasid host, Ornithodoros porcinus porcinus. J. Virol. 72, 1711–1724. doi: 10.1128/JVI.72.3.1711-1724.1998
Kleiboeker, S. B., and Scoles, G. A. (2001). Pathogenesis of African swine fever virus in Ornithodoros ticks. Anim. Health Res. Rev. 2, 121–128. doi: 10.1079/AHRR200133
Komal, J. (2020). Regional efforts in the Americas to prevent ASF introduction. African Swine Fever Unprecedented Global Threat: A Global Challenge to Livelihoods, Food Security, and Biodiversity. The Global Framework for the Progressive Control of Transboundary Animal Diseases (GF-TADs).
Li, L., Ren, Z., Wang, Q., Ge, S., Liu, Y., Liu, C., et al. (2019). Infection of African swine fever in wild boar, China, 2018. Transbound. Emerg. Dis. 66, 1395–1398. doi: 10.1111/tbed.13114
Li, X., and Tian, K. (2018). African swine fever in China. Vet. Rec. 183, 300–301. doi: 10.1136/vr.k3774
Mellor, P., and Wilkinson, P. (1985). Experimental transmission of African swine fever virus by Ornithodoros savignyi (Audouin). Res. Vet. Sci. 39, 353–361. doi: 10.1016/S0034-5288(18)31726-0
Minoungou, G. L., Diop, M., Dakouo, M., Ouattara, A. K., Settypalli, T. B. K., Lo, M. M., et al. (2021). Molecular characterization of African Swine fever viruses in Burkina Faso, Mali, and Senegal 1989–2016. Transbound. Emerg. Dis. 68, 2842–2852. doi: 10.1111/tbed.14240
Montgomery, R. E. (1921). On a form of swine fever occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 34, 159–191. doi: 10.1016/S0368-1742(21)80031-4
Mur, L., Atzeni, M., Martínez-López, B., Feliziani, F., Rolesu, S., and Sanchez-Vizcaino, J. M. (2016). Thirty-five-year presence of African swine fever in Sardinia: history, evolution and risk factors for disease maintenance. Transbound. Emerg. Dis. 63, e165–e177. doi: 10.1111/tbed.12264
Njau, E. P., Domelevo Entfellner, J.-B., Machuka, E. M., Bochere, E. N., Cleaveland, S., Shirima, G. M., et al. (2021). The first genotype II African swine fever virus isolated in Africa provides insight into the current Eurasian pandemic. Sci. Rep. 11:13081. doi: 10.1038/s41598-021-92593-2
Oxford-Analytica (2020). Pandemic to Worsen Recession and Instability in Haiti. Haiti: Emerald Expert Briefings.
Pereira De Oliveira, R., Hutet, E., Duhayon, M., Guionnet, J.-M., Paboeuf, F., Vial, L., et al. (2020a). Successful infection of domestic pigs by ingestion of the European soft tick O. Erraticus that fed on African swine fever virus infected pig. Viruses 12:300. doi: 10.3390/v12030300
Pereira De Oliveira, R., Hutet, E., Lancelot, R., Paboeuf, F., Duhayon, M., Boinas, F., et al. (2020b). Differential vector competence of Ornithodoros soft ticks for African swine fever virus: what if it involves more than just crossing organic barriers in ticks? Parasit. Vect. 13:618. doi: 10.1186/s13071-020-04497-1
Pereira De Oliveira, R., Hutet, E., Paboeuf, F., Duhayon, M., Boinas, F., Perez De Leon, A., et al. (2019). Comparative vector competence of the Afrotropical soft tick Ornithodoros moubata and Palearctic species, O. erraticus and O. verrucosus, for African swine fever virus strains circulating in Eurasia. PLoS ONE 14:e0225657. doi: 10.1371/journal.pone.0225657
Plowright, W., Parker, J., and Peirce, M. (1969). African swine fever virus in ticks (Ornithodoros moubata, Murray) collected from animal burrows in Tanzania. Nature 221, 1071–1073. doi: 10.1038/2211071a0
Quembo, C. J., Jori, F., Vosloo, W., and Heath, L. (2018). Genetic characterization of African swine fever virus isolates from soft ticks at the wildlife/domestic interface in Mozambique and identification of a novel genotype. Transbound. Emerg. Dis. 65, 420–431. doi: 10.1111/tbed.12700
Rennie, L., Wilkinson, P., and Mellor, P. (2000). Effects of infection of the tick Ornithodoros moubata with African swine fever virus. Med. Vet. Entomol. 14, 355–360. doi: 10.1046/j.1365-2915.2000.00251.x
Ribeiro, R., Otte, J., Madeira, S., Hutchings, G. H., and Boinas, F. (2015). Experimental infection of Ornithodoros erraticus sensu stricto with two portuguese African swine fever virus strains. Study of factors involved in the dynamics of infection in ticks. PLoS ONE 10:e0137718. doi: 10.1371/journal.pone.0137718
Rivera-Benítez, J. F., De La Luz-Armendáriz, J., Gómez-Núñez, L., Vargas, F. D., Escatell, G. S., Ramírez-Medina, E., et al. (2021). Swine health: history, challenges and prospects. Rev. Mex. Cienc. Pecu. 12, 149–185. doi: 10.22319/rmcp.v12s3.5879
Sánchez-Vizcaíno, J. M., Mur, L., and Martínez-López, B. (2013). African swine fever (ASF): five years around Europe. Vet. Microbiol. 165, 45–50. doi: 10.1016/j.vetmic.2012.11.030
Sauter-Louis, C., Conraths, F. J., Probst, C., Blohm, U., Schulz, K., Sehl, J., et al. (2021a). African swine fever in wild boar in Europe—a review. Viruses 13:1717. doi: 10.3390/v13091717
Sauter-Louis, C., Forth, J. H., Probst, C., Staubach, C., Hlinak, A., Rudovsky, A., et al. (2021b). Joining the club: first detection of African swine fever in wild boar in Germany. Transbound. Emerg. Dis. 68, 1744–1752. doi: 10.22541/au.160253806.62312023/v1
Suárez, J., Carreño, L., Paniz-Mondolfi, A., and Canosa, F. J. M. (2018). Infectious diseases, social, economic and political crises, anthropogenic disasters and beyond: Venezuela 2019—implications for public health and travel medicine. Rev. Panam. Enferm. Infecc. 1, 73–93. doi: 10.13140/RG.2.2.13082.90562/1
Tran, X. H., Le, T. T. P., Nguyen, Q. H., Do, T. T., Nguyen, V. D., Gay, C. G., et al. (2021). African swine fever virus vaccine candidate ASFV-G-DeltaI177L efficiently protects European and native pig breeds against circulating Vietnamese field strain. Transbound. Emerg. Dis.69, e497–e504. doi: 10.1111/tbed.14329
Tran, X. H., Phuong, L. T. T., Huy, N. Q., Thuy, D. T., Nguyen, V. D., Quang, P. H., et al. (2022). Evaluation of the safety profile of the ASFV vaccine candidate ASFV-G-andDelta;I177L. Viruses 14:896. doi: 10.3390/v14050896
Tulman, E. R., Delhon, G. A., Ku, B. K., and Rock, D. L. (2009). African swine fever virus. Curr. Top. Microbiol. Immunol. 328, 43–87. doi: 10.1007/978-3-540-68618-7_2
USDA (2021). USDA Statement on Confirmation of African Swine Fever in the Dominican Republic. USDOA Animal and Plant Health Inspection Service, USDA.
Uwishema, O., Chalhoub, E., Zahabioun, A., David, S. C., Khoury, C., Al-Saraireh, T. H., et al. (2021). The rising incidence of African swine fever during the COVID-19 pandemic in Africa: efforts, challenges and recommendations. Int. J. Health Plann. Manage. 37, 561–567. doi: 10.1002/hpm.3357
Velazquez-Salinas, L., Ramirez-Medina, E., Rai, A., Pruitt, S., Vuono, E. A., Espinoza, N., et al. (2021). Development real-time PCR assays to genetically differentiate vaccinated pigs from infected pigs with the Eurasian strain of African swine fever virus. Front. Vet. Sci. 8:768869. doi: 10.3389/fvets.2021.768869
Vilem, A., Nurmoja, I., Niine, T., Riit, T., Nieto, R., Viltrop, A., et al. (2020). Molecular characterization of African swine fever virus isolates in Estonia in 2014–2019. Pathogens 9:582. doi: 10.3390/pathogens9070582
Wang, W.-H., Lin, C.-Y., Chang Ishcol, M. R., Urbina, A. N., Assavalapsakul, W., Thitithanyanont, A., et al. (2019). Detection of African swine fever virus in pork products brought to Taiwan by travellers. Emerg. Microb. Infect. 8, 1000–1002. doi: 10.1080/22221751.2019.1636615
WOAH (2021a). Follow-up Report 1. African Swine Fever Virus (Inf. with), Dominican (Rep.)—REPORT ID FUR_151044. O. World Organization for Animal Health.
WOAH (2021b). Follow-up Report 1. African Swine Fever Virus (Inf. with), Haiti—REPORT ID FUR_151855. O. World Organization for Animal Health.
WOAH (2021c). Immediate Notification. African Swine Fever Virus (Inf. with), Dominican (Rep.)—REPORT ID IN_150921. O. World Organization for Animal Health.
WOAH (2021d). Immediate Notification. African Swine Fever Virus (Inf. with), Haiti—REPORT ID IN_151732. O. World Organization for Animal Health.
WOAH (2021e). RESOLUTION No. 20. Recognition of the Classical Swine Fever Status of Members. Oie: Paris, France.
Wormington, J. D., Golnar, A., Poh, K. C., Kading, R. C., Martin, E., Hamer, S. A., et al. (2019). Risk of African swine fever virus sylvatic establishment and spillover to domestic swine in the United States. Vector Borne Zoonotic Dis. 19, 506–511. doi: 10.1089/vbz.2018.2386
Zani, L., Forth, J. H., Forth, L., Nurmoja, I., Leidenberger, S., Henke, J., et al. (2018). Deletion at the 5'-end of Estonian ASFV strains associated with an attenuated phenotype. Sci. Rep. 8:6510. doi: 10.1038/s41598-018-24740-1
Keywords: African swine fever, reservoirs, Arbovirus, emerging disease, pigs
Citation: Ruiz-Saenz J, Diaz A, Bonilla-Aldana DK, Rodríguez-Morales AJ, Martinez-Gutierrez M and Aguilar PV (2022) African swine fever virus: A re-emerging threat to the swine industry and food security in the Americas. Front. Microbiol. 13:1011891. doi: 10.3389/fmicb.2022.1011891
Received: 04 August 2022; Accepted: 16 September 2022;
Published: 05 October 2022.
Edited by:
Chengming Wang, Auburn University, United StatesReviewed by:
Gerald Misinzo, SACIDS Foundation for One Health, Sokoine University of Agriculture, TanzaniaCopyright © 2022 Ruiz-Saenz, Diaz, Bonilla-Aldana, Rodríguez-Morales, Martinez-Gutierrez and Aguilar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julian Ruiz-Saenz, anVsaWFucnVpenNhZW56QGdtYWlsLmNvbQ==; anVsaWFuLnJ1aXpzQGNhbXB1c3VjYy5lZHUuY28=
†ORCID: Julian Ruiz-Saenz orcid.org/0000-0002-1447-1458
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.