- 1Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, ON, Canada
- 2Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, NM, United States
- 3Department of Mathematics, Trent University, Peterborough, ON, Canada
- 4School of Mathematics and Physics, University of Queensland, Saint Lucia, QLD, Australia
- 5Vector-borne Infectious Disease Group, Theoretical and Applied Biology, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
- 6Effective Basic Services (eBASE), Bamenda, Cameroon
- 7Black Creek Community Health Centre, Toronto, ON, Canada
- 8Department of Mathematics, Federal University of Technology, Owerri, Nigeria
- 9Abdus Salam School of Mathematical Sciences, Government College University, Lahore, Pakistan
- 10ASL Napoli 2 Nord, Naples, Italy
- 11School of Physics and Institute for Collider Particle Physics, University of the Witwatersrand, Johannesburg, South Africa
- 12Subatomic Physics, iThemba Laboratory for Accelerator Based Sciences, Somerset West, South Africa
Monkeypox is an emerging zoonotic disease caused by the monkeypox virus, which is an infectious agent belonging to the genus Orthopoxvirus. Currently, commencing from the end of April 2022, an outbreak of monkeypox is ongoing, with more than 43,000 cases reported as of 23 August 2022, involving 99 countries and territories across all the six World Health Organization (WHO) regions. On 23 July 2022, the Director-General of the WHO declared monkeypox a global public health emergency of international concern (PHEIC), since the outbreak represents an extraordinary, unusual, and unexpected event that poses a significant risk for international spread, requiring an immediate, coordinated international response. However, the real magnitude of the burden of disease could be masked by failures in ascertainment and under-detection. As such, underestimation affects the efficiency and reliability of surveillance and notification systems and compromises the possibility of making informed and evidence-based policy decisions in terms of the adoption and implementation of ad hoc adequate preventive measures. In this review, synthesizing 53 papers, we summarize the determinants of the underestimation of sexually transmitted diseases, in general, and, in particular, monkeypox, in terms of all their various components and dimensions (under-ascertainment, underreporting, under-detection, under-diagnosis, misdiagnosis/misclassification, and under-notification).
Introduction
Monkeypox is an emerging zoonotic disease caused by the monkeypox virus, which is an infectious agent belonging to the family of Poxviruses (Poxviridae), Chordopoxvirinae subfamily, and Orthopoxvirus genus (Hughes et al., 2010; Bunge et al., 2022). These viruses are large, brick-shaped, enveloped, double-stranded DNA viruses (Diven, 2001; Alakunle et al., 2020). Monkeypox virus is related to the Variola virus (VARV), the causative agent of smallpox, a life-threatening infectious disease fully eradicated in 1980, and another Orthopoxvirus (Barquet and Domingo, 1997; Riedel, 2005; Kmiec and Kirchhoff, 2022). Monkeypox has been endemic in some African countries, since 1970, when the first human case was reported in a 9-month-old child admitted to the Basankusu Hospital in the Democratic Republic of the Congo (DRC; Durski et al., 2018).
Currently, commencing from the end of April 2022, an outbreak of monkeypox is ongoing, with more than 43,000 cases reported as of 23 August 2022, involving 99 countries and territories across all the six World Health Organization (WHO) regions (Table 1). The most impacted WHO regions are the Region of the Americas (AMR; 52.0%) and the European Region (EUR; 47.5% of cases), followed by the Western Pacific Region (WPR; 0.3%), the African Region (AFR; 0.1%), the Eastern Mediterranean Region (EMR; 0.1%), and the South-East Asian Region (SEAR; 0.04%). On 23 July 2022, the Director-General of the WHO declared monkeypox a global public health emergency of international concern (PHEIC; Nuzzo et al., 2022), since the outbreak represents an extraordinary, unusual, and unexpected event that poses a significant risk for international spread, requiring an immediate, coordinated international response.
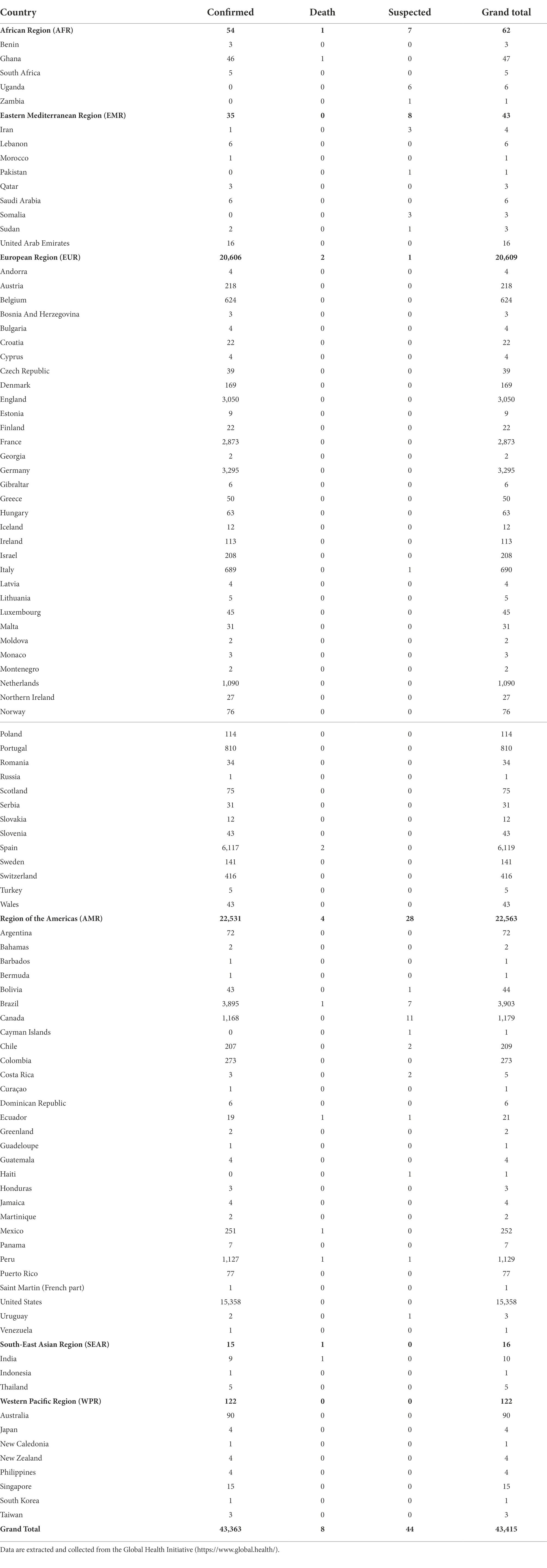
Table 1. Monkeypox cases (confirmed and suspected cases, deaths, and grand total) broken down according to the World Health Organization (WHO) region, and country.
The epidemiological and clinical features of the ongoing monkeypox outbreak are different from those established for monkeypox since its initial isolation and identification and during the previous outbreaks, with sexual transmission suspected as the major transmission route and with the community of men having sex with men (MSM) disproportionately impacted (Liu et al., 2022; Thornhill et al., 2022). According to a large-scale study, out of 528 monkeypox infections diagnosed and reported from 16 countries, between April 27 and June 24, 2022, the transmission was hypothesized to have occurred more likely via sexual intercourse in 95% of the cases during the current outbreak (Thornhill et al., 2022). Other transmission routes include contact with infected animals and travel to endemic countries, occupational exposure, and social and household contacts (Liu et al., 2022). As such, monkeypox is not an exclusively sexually transmitted disease (STD), but its transmission has been hypothesized to be associated with sexual contact. This is an important distinction because we are still not sure that transmission is occurring through body fluids exchanged during sex, but rather it could be via contact with mucosal surfaces, scarification, or even respiratory exposures.
The real magnitude of the burden of disease could be masked by failures in ascertainment and under-detection. As such, underestimation affects the efficiency and reliability of surveillance and notification systems and compromises the possibility of making informed and evidence-based policy decisions in terms of the adoption and implementation of ad hoc adequate preventive measures. For example, another infectious outbreak, the still ongoing “Coronavirus Disease 2019” (COVID-19) pandemic was initially underestimated and this, along with the high degree of contagiosity of the virus, contributed to its quick global spread (Wu et al., 2020; Nakamoto and Zhang, 2021).
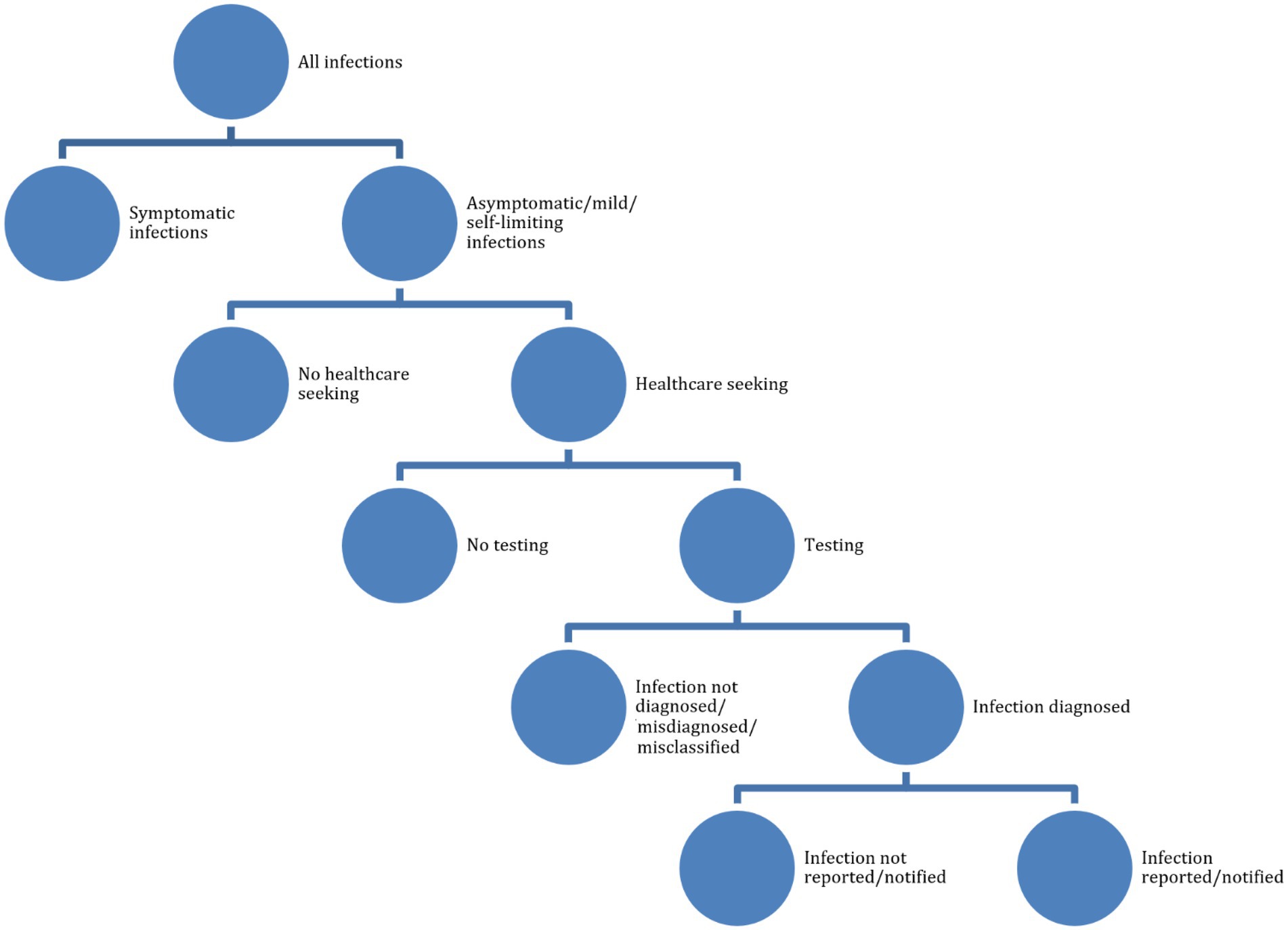
According to the working definitions of the “BCoDE-project” (Kretzschmar et al., 2012), underestimation can be due to various factors, including under-ascertainment. This can occur when infected subjects do not seek general practitioners or specialized health services, in that they perceive their illness as mild and/or self-limiting, do not have adequate health literacy and risk/disease perception, or they are asymptomatic and unaware of their disease status. Minority groups (including migrants, the lesbian/gay/bisexual/transgender/transsexual/queer/intersex, LGBTQI+, community, and other marginalized or difficult-to-reach communities) generally do not consult general practitioners or other healthcare workers (Kretzschmar et al., 2012). Cultural, religious, legal, administrative, economic, and financial factors can influence health-seeking behaviors. Underreporting, another component of underestimation, occurs when symptomatic cases in the community refer to health services but have their disease status not properly diagnosed or misclassified (under-diagnosis), or correctly diagnosed and classified but not effectively transmitted to the public health surveillance and monitoring bodies (under-notification). The reader is referred to Table 2 for further details.
The topic of underestimation of monkeypox cases is of crucial importance in the field of public and global health. However, to the best of our knowledge, there exists no comprehensive review addressing the determinants underlying the underestimation of STDs, in general, and, in particular, monkeypox. Therefore, the present study was undertaken to fill this gap in knowledge.
Materials and methods
An integrative review was conducted. Even though this technique dates back to the eighties, it is emerging as an innovative tool to synthesize and appraise the existing body of scholarly literature on the designated research problem/concept, enabling the combination of a heterogeneous array of sources, from empirical to conceptual/theoretical investigations, from quantitative to qualitative and mixed-method studies, and from observational to pilot, feasibility, and interventional studies (Broome, 2000). We employed this technique since we were able to identify and formulate a broad-scope research problem/concept/phenomenon of interest, particularly complex and articulated.
An integrative review enables to (i) overview and appraise theories and practices, (ii) to build bridges across diverse study fields, disciplines, and sectors, (iii) to generate and/or refine new knowledge and novel hypotheses, and (iv) to formulate and propose an actionable framework, being, as such, particularly suited for developing and informing healthcare policies and practices in an evidence-based fashion. More specifically, an integrative review study can be defined as “a review method that summarizes past empirical or theoretical literature to provide a more comprehensive understanding of a particular phenomenon or healthcare problem” (Broome, 2000).
To achieve the ambitious objectives of generating new knowledge and/or theories, an integrative review results in one or more of the following research synthesis forms: (i) a research agenda, (ii) a taxonomy or other conceptual classifications of constructs, (iii) alternative models or conceptual frameworks, and (iv) a metatheory/an array of metatheories.
Within the so-called “evidence synthesis ecosystem,” a systematic literature review and a meta-analysis have a highly focused, narrow research scope, whereas a scoping review has a broad research question and the objective of mapping, synthesizing, and combining the existing body of scholarly literature on the designated topic/research question.
We searched a major scholarly electronic database, PubMed/MEDLINE, for papers without language filters, using a search string consisting of several components. First, these components were related to (i) health-seeking behaviors (awareness, knowledge, attitudes, practices, health-literacy, and health-seeking behavior), (ii) underestimation (under-ascertainment, underreporting, under-diagnosis, misdiagnosis/misclassification, under-detection, or under-notification), and, (iii) STDs (sexual transmission, sexually transmitted disease, or sexually transmitted infection). We wanted, indeed, to study determinants of underestimation of STDs, including healthcare-seeking behaviors. During a second round of literature search, we added a fourth component related to the LGBTQI+ community, since it is being particularly impacted by the current monkeypox outbreak (see Figure 1 and Tables 2, 3 for further details). Google Scholar was searched too, looking for resources and items not indexed yet at the time of the literature search and for ensuring a broader relevant coverage of the literature.
Results
Underestimation of sexually transmitted diseases
Out of 230 items returned by searching PubMed/MEDLINE, 53 articles related to STDs (Franco, 1991; Koutsky et al., 1992; Lin et al., 1992; Schulte et al., 1992; Ashley et al., 1993; Webster et al., 1993; Brookmeyer et al., 1995; Maher and Hoffman, 1995; Petersen et al., 1995; Schachter and Chow, 1995; Wahdan, 1995; Schwebke et al., 1996; Agacfidan et al., 1997; Rompalo et al., 1997; Borisenko, 1998; Paget et al., 2002; Niccolai et al., 2005; Dhawan et al., 2006; Liu et al., 2006; Munson et al., 2008; Nguyen et al., 2008; Lusk et al., 2010; Andrea and Chapin, 2011; Hong et al., 2011; Roth et al., 2011; Wolfers et al., 2011; Koper et al., 2013; Krivochenitser et al., 2013; Oliffe et al., 2013; Gratzer et al., 2014; Mirzazadeh et al., 2014; Brown et al., 2015; Corbeto et al., 2015; Fakoya et al., 2015; Jenkins, 2015; Tomas et al., 2015; Johnson and Geffen, 2016; Kustec et al., 2016; Mlakar and Ramšak, 2016; Ni et al., 2016; Allard et al., 2017; Denison et al., 2017; Lee and Nishiura, 2017; Syme et al., 2017; Hall et al., 2018; Mangine et al., 2018; Shahesmaeili et al., 2018; Timsit et al., 2018; Steen et al., 2019; Knight et al., 2020; Moriña et al., 2021; Niekamp et al., 2021; Geba et al., 2022) were deemed eligible for inclusion in the present integrative review. More specifically, our comprehensive literature search enabled us to identify the following determinants of the underestimation of STDs: asymptomatic course (Wahdan, 1995; Shahesmaeili et al., 2018; Moriña et al., 2021); atypical clinical and epidemiological features (Ni et al., 2016), including atypical/unusual transmission routes (Allard et al., 2017; Lee and Nishiura, 2017; Timsit et al., 2018); differences in case definition (Schulte et al., 1992; Rompalo et al., 1997), and in regional/national testing rates (Koper et al., 2013; Kustec et al., 2016); underestimation among specific age groups, like the youth and the elderly, and populations, such as minority communities and visible racialized groups (Webster et al., 1993), migrant workers (Fakoya et al., 2015; Steen et al., 2019), sex workers (Agacfidan et al., 1997; Brown et al., 2015; Hong et al., 2011; Mirzazadeh et al., 2014; Shahesmaeili et al., 2018), or swingers (Niekamp et al., 2021); use of low-sensitivity and/or low-specificity diagnostic assays (Koutsky et al., 1992; Schulte et al., 1992; Ashley et al., 1993; Petersen et al., 1995; Schachter and Chow, 1995; Schwebke et al., 1996; Paget et al., 2002; Dhawan et al., 2006; Munson et al., 2008; Lusk et al., 2010; Andrea and Chapin, 2011; Gratzer et al., 2014), or inadequate clinical and microbe-isolation procedures (Koutsky et al., 1992; Lin et al., 1992); inadequate STD screening policies/protocols (Wahdan, 1995; Lusk et al., 2010; Roth et al., 2011; Corbeto et al., 2015; Geba et al., 2022); measurement error/misclassification (Franco, 1991; Krivochenitser et al., 2013; Tomas et al., 2015); barriers to accessing STD testing and management services (Mlakar and Ramšak, 2016; Denison et al., 2017), including psychological issues (Oliffe et al., 2013), or lack of available facilities and infrastructures in resource-limited contexts (Maher and Hoffman, 1995); self-treatment (Borisenko, 1998); disease perception/health literacy (Liu et al., 2006; Nguyen et al., 2008; Wolfers et al., 2011; Hall et al., 2018), including risk perception (Syme et al., 2017), that is to say, the subjective assessment about the characteristics and severity of a given risk; and limited/strained testing and diagnostic capacity (Schulte et al., 1992).
These studies concerned the following sexually transmitted pathogens/STDs: herpetic diseases (Koutsky et al., 1992; Ashley et al., 1993), human papillomavirus or HPV (Franco, 1991; Brown et al., 2015; Shahesmaeili et al., 2018; Moriña et al., 2021), chancroid (Schulte et al., 1992), Chlamydia trachomatis (Lin et al., 1992; Maher and Hoffman, 1995; Schachter and Chow, 1995; Agacfidan et al., 1997; Paget et al., 2002; Krivochenitser et al., 2013; Corbeto et al., 2015; Tomas et al., 2015; Kustec et al., 2016; Mlakar and Ramšak, 2016), syphilis (Webster et al., 1993; Gratzer et al., 2014; Shahesmaeili et al., 2018) and genital ulcer disease (GUD; Rompalo et al., 1997), gonorrhea (Webster et al., 1993; Maher and Hoffman, 1995; Borisenko, 1998; Krivochenitser et al., 2013; Tomas et al., 2015; Shahesmaeili et al., 2018), trichomoniasis (Maher and Hoffman, 1995; Petersen et al., 1995; Munson et al., 2008; Lusk et al., 2010; Andrea and Chapin, 2011; Roth et al., 2011; Tomas et al., 2015; Shahesmaeili et al., 2018), bacterial vaginosis (Schwebke et al., 1996), Ureaplasma urealyticum (Dhawan et al., 2006), Zika virus (Allard et al., 2017; Lee and Nishiura, 2017), amebiasis (Timsit et al., 2018), and human immunodeficiency virus, or HIV (Wahdan, 1995; Liu et al., 2006; Nguyen et al., 2008; Mirzazadeh et al., 2014; Fakoya et al., 2015; Ni et al., 2016; Hall et al., 2018; Steen et al., 2019).
Three articles (Niccolai et al., 2005; Jenkins, 2015; Mangine et al., 2018) contained recommendations to overcome these shortcomings: namely, (i) to use sensitive and specific assays, (ii) to accurately collect sexual history, including data related to sexual orientation, and identify high-risk sexual behaviors (Jenkins, 2015), (iii) to strengthen sentinel surveillance and establish further sites, to improve the quality of collected data, (iv) to deploy and link multiple data sources, such as self-reports, medical record reviews, and regional/state health department reports, harmonizing, when appropriate, the various and different reporting systems and case definitions (Niccolai et al., 2005), and, (v) to exploit the web, including social media and social networks to recruit high-risk populations, like the MSM community (Mangine et al., 2018).
Three other studies (Brookmeyer et al., 1995;Johnson and Geffen, 2016; Knight et al., 2020) focused on mathematical modeling, suggesting that the underestimation of STDs can occur when one fails to properly model high-risk sexual behaviors (such as unprotected, condomless sexual intercourse, use of recreational drugs or chemsex, sex with commercial partners, or with individuals the HIV status is unknown; Johnson and Geffen, 2016; Knight et al., 2020) or does not adjust for the follow-up bias (potential losses during the follow-up; Brookmeyer et al., 1995).
Specifically concerning behavioral determinants of STDs (i.e., healthcare-seeking behaviors), a series of qualitative in-depth interviews carried out among 24 university students, exhibiting risky sexual behaviors (Denison et al., 2017), identified three main types of barriers to STD testing: (i) personal (underestimation of risk, perception of STD as a not serious disease, fear of invasive procedures, self-consciousness in genital examination, and/or being too busy); (ii) structural (economic-financial cost of testing, environment–clinician attributes and attitudes); and, (iii) social (concern/fear of stigmatization).
Finally, seven of the 53 retrieved articles focused on the MSM community (Liu et al., 2006; Koper et al., 2013; Brown et al., 2015; Mlakar and Ramšak, 2016; Hall et al., 2018; Mangine et al., 2018; Knight et al., 2020).
Underestimation of monkeypox cases
So far, the only attempt to test the hypothesis of the impact of stigmatization on monkeypox case reporting in European countries has been done by Kenyon (Kenyon, 2022), employing Spearman’s correlation test to quantitatively explore whether the monkeypox national cumulative incidence was negatively associated with the intensity of screening for STIs and a composite indicator of LGBTQI+ rights (the “Rainbow Index”). The author found, instead, a positive correlation between the monkeypox epidemiological trend and the intensity of chlamydia/gonorrhea (rho 0.68, p < 0.0001), and syphilis (rho 0.62, p < 0.0001) screening, and the Rainbow Index (rho 0.65, p < 0.0001), suggesting that in several Eastern European countries, the real burden of monkeypox is underestimated.
Besides stigmatization and related issues, a few monkeypox infections are asymptomatic (Fleischauer et al., 2005; Karem et al., 2007; Guagliardo et al., 2020) and, when present, symptoms are atypical, in that this outbreak differs from previous outbreaks, in terms of a shift in mean age and the most affected age group, affected sex/gender, risk factors, clinical course, signs/symptoms, and, above all, the sexual transmission route (Bragazzi et al., 2022a). As such, physicians may not recognize the infection as monkeypox. A recent “knowledge, attitudes, and practices” (KAP) survey among Italian physicians showed unsatisfying monkeypox-related knowledge and attitude levels (Riccò et al., 2022). For example, systemic complications of monkeypox, especially among children, were generally largely overlooked. Of note, Italian physicians who took part in the survey showed substantial uncertainties and knowledge gaps related to monkeypox, in terms of clinical presentation and main features, risk factors, and preventative measures, with less than one-fifth of them confident in properly recognizing incident monkeypox cases during their clinical activities. Another survey conducted in Jordan (Sallam et al., 2022), among 615 university students in health schools/faculties (medicine, nursing, dentistry, pharmacy, medical laboratory sciences, and rehabilitation), identified serious gaps in knowledge, with only three out of 11 monkeypox-related knowledge items identified correctly by >70% of the respondents. Only 26.2% of the participants knew that monkeypox is a vaccine-preventable disease. However, information about knowledge of monkeypox among physicians and allied health professionals is scarce.
Also, the monkeypox case definition has only recently been revised to be adapted to the ongoing outbreak, in order to reflect the new findings and clinical and laboratory features (Bragazzi et al., 2022a; Centers for Disease Control and Prevention (CDC), 2022).
Another factor that could result in monkeypox underestimation is testing and diagnostic capacity, with a general lack of point-of-care tests currently available and, in some countries, overall testing (Nuzzo et al., 2022). Diagnostic/testing capacity for monkeypox varies substantially worldwide—some countries like the United States are able to process up to several thousand specimens per week (Cohen, 2022), while others have no diagnostic capacity at all; moreover, testing and diagnostic capacity are further strained by the still ongoing COVID-19 pandemic. Testing includes non-variola Orthopoxvirus (NVO) generic real-time polymerase chain reaction (PCR) test, monkeypox-specific PCR, and sequencing (Jiang et al., 2022).
Further, services and healthcare provisions offered by sexual health clinics in some countries, like the United Kingdom, are being significantly impacted and disrupted. This could result in a significant delay in the diagnosis, treatment, and reporting of cases.
Finally, in most cases, contact tracing (also known as partner notification) is unfeasible or presents particular challenges in the MSM community, given that contacts of infected individuals are casual sexual partners (Bell and Potterat, 2011; Bragazzi et al., 2022a).
Discussion
Sexually transmitted diseases are generally overlooked and underestimated (Sartorius, 2007; Bragazzi et al., 2022b). Based on our integrative review of the literature, monkeypox case underestimation could be significant. This has important implications for public and global health providers as well as policy- and decision-makers, epidemiologists, and mathematical modelers.
According to Andersen’s “Behavioral Model of Health Services Use,” health-seeking behaviors are complex and multidimensional, depending on an array of factors, including “predisposing factors” (such as age, sex/gender, ethnicity, or cultural and social variables), “enabling factors” (like financial variables—insurance coverage—or healthcare accessibility/availability), and “need factors” (health, risk, and disease perceptions, health literacy, medical conditions, or underlying co-morbidities; Babitsch et al., 2012). Symptoms of some STDs can be mild and individuals may not seek healthcare. Moreover, in the LGBTQI+ community, STDs are usually perceived as a “part of the way of life” and as inconvenient consequences of being sexually active. In the pre-HIV pre-exposure prophylaxis (PrEP) era, HIV was considered the most anxiety-provoking STD, followed by viral, recurring STDs, and bacterial STDs, which were conceived as trivial and treatable. On the other hand, while not generating particular concerns in terms of disease perception, a diagnosis of STDs was associated with feelings of being “dirty and ashamed” (Holt et al., 2010). Risk and disease perceptions regarding HIV have changed after PrEP introduction, but the general thought that STD is an untoward consequence of sexual activities has remained practically unchallenged. Intended and actual utilization of healthcare provisions has been found to be related to the endorsement of stigmatization of certain sexual practices, such as anal sexual intercourse (Kutner et al., 2022). Awareness and attitudes toward STDs are highly heterogenous among MSM, with some infections considered scarier and others less, depending on their transmission mechanisms, epidemiology (prevalence), visibility of symptoms, and impact on health, as well as the availability of vaccines and treatment options, based on both personal or friends’ experiences (Datta et al., 2019).
Sexual health clinics are usually the first point of access in the case of STDs. However, some sex and gender minorities (SGMs), despite being at higher risk for STDs, including monkeypox, could be underrepresented. Holmes and Beach (2020) found that individuals self-identifying as bisexuals were approximately one-quarter of sexual health clinic users, while they represent more than half of SGMs populations. The so-called “bisexual erasure” or “bisexual invisibility” may be one of the factors explaining the potential underestimation of monkeypox cases, with the number of cases reported among men having sex with men and women (MSMW) being tremendously underestimated.
All these behaviors can be explained utilizing the “minority stress theory” (MST), according to which some marginalized communities subjected to stigmatization and discrimination experience more stressors than the general population, resulting in increased stress-linked coping behaviors, substance use, encounters with random/casual sex partners, and poorer health outcomes and health-related inequalities. Health disparities could be due to lower access to healthcare services, including preventative and STD screening/testing ones (Holmes and Beach, 2020).
There are different interests and actors at stake and a holistic approach is required to address STDs, in general, and monkeypox, specifically. To really advance the field of STD- and sexual health-related research, institutional and governmental bodies should facilitate “sex-at-birth, sexual orientation, and gender identity” (SSOGI)-related data collection, dissemination, and utilization, to favor a more “inclusive STD reporting” (Baptiste-Roberts et al., 2017). Currently, SSOGI data collection is not routinely implemented, with the risk of invisibilizing individuals with bi/bi+ umbrella labels, such as bisexual, queer, and pansexual individuals (Baptiste-Roberts et al., 2017). Several LGBTQI+ organizations have been collecting SSOGI data, but current public health surveillance systems are not updated to incorporate such information (Baptiste-Roberts et al., 2017). Of note, a major shortcoming of the investigation by Kenyon (Bragazzi et al., 2022b) is that the incidence of monkeypox cases was computed utilizing the entire (general) population, rather than the MSM/SGM/LGBTQI+ population. The latter point reflects the challenges that can be encountered in measuring and collecting data related to the sexual orientation/gender identity of a patient, given that there exist several socio-cultural, historical, as well as political implications underlying these issues. Data collected by healthcare providers are affected by the patient’s willingness to disclose personal, sensitive information and their degree of openness, while self-report data suffer from selection/self-selection biases. As such, the real size of the MSM/SGM/LGBTQI+ population remains unknown and discrepancies among studies and differences among countries point to the influence of societal variables as well as the precise definition of what the MSM/SGM/LGBTQI+ population is (Marcus et al., 2013).
Specifically, concerning monkeypox cases, even though in a few cases, systemic prodromal symptoms (like fever, headache, lymphadenopathy, etc.) typical of the invasion period may be missing, with visible symptoms appearing during the skin eruption stage and a few asymptomatic individuals described in the current as in previous outbreaks, there are good reasons to suspect underestimation just by looking at data, since, as noted by Nuzzo et al. (2022), the United States, despite having a larger population size, have reported fewer cases than the United Kingdom.
The engagement of the LGBTQI+ community, and especially of bisexual/pansexual (bi/bi+) populations, with community-based sexual health providers is of paramount importance (Baptiste-Roberts et al., 2017) to offer LGBTQI+-tailored sexual health services. Scaling up community outreach and recruitment of LGBTQI+ members, including bi/bi+ people to engage in sexual health services represent challenges that need to be prioritized (Baptiste-Roberts et al., 2017). Adopting an intersectional lens, with a focus on populations reporting multiple stigmatization and discrimination, such as non-White communities, is crucial to address unmet needs. Educating staff to be culturally sensitive and competent, fighting against systemic and institutional stigmatization, and homo-bi-trans-phobia, and creating an inclusive environment represent another societal onus. Institutional bodies should conduct awareness campaigns to enhance health literacy, minimize structural or perceived barriers to STD testing, develop effective and innovative strategies aimed at addressing personal beliefs and improving STD testing rates, and favor the adoption of healthy sexual practices and behaviors (Pitts, 2020).
Social media, including news outlets, should also play their role in changing societal views of STDs (Pitts, 2020), combating disinformation and infodemic (Ennab et al., 2022), and creating awareness that monkeypox can infect all humans regardless of their age, sex/gender, sexual orientation, or gender identity. Moreover, there are various factors that may increase the potential risk for exposure, including close, sexual, and/or intimate contact with someone who has monkeypox and symptoms, such as rash, soreness, or scabs. Potentially, any sexually active individual could contract the infection, even if the focus is mainly on the MSM community. This could lead to a (further) underestimation of infectious transmission among other populations, as previously mentioned.
Conclusion and future prospects
Monkeypox is an emerging sexually transmitted infection, which is representing a global public health concern. Mathematical modeling of monkeypox should adjust for the underestimation of cases and public and global health policy- and decision-makers should consider the “hidden burden” of monkeypox when designing and implementing packages of interventions. Studies are urgently needed to quantify the degree of underestimation of monkeypox cases to better inform the responses to the outbreak.
Author contributions
JW and JDK conceived and drafted the paper. WAW, SAI, QH, XW, AS, KB, PO, CP, MW, AO, MC, and BM critically revised it. All authors contributed to the article and approved the submitted version.
Funding
This research is funded by Canada’s International Development Research Centre (IDRC) and the Swedish International Development Cooperation Agency (SIDA; Grant No. 109559–001). NLB and JDK acknowledge support from IDRC (Grant No. 109981), New Frontier in Research Fund-Exploratory (Grant No. NFRFE-2021-00879), and NSERC Discovery Grant (Grant No. RGPIN-2022-04559). AS would like to thank The University of Queensland’s AI4PAN research group. Portions of this work were performed at the Los Alamos National Laboratory under the auspices of the US Department of Energy contract 89233218CNA000001 and supported by NIH grant R01- OD011095 (SAI).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agacfidan, A., Chow, J. M., Pashazade, H., Ozarmagan, G., and Badur, S. (1997). Screening of sex workers in Turkey for chlamydia trachomatis. Sex. Transm. Dis. 24, 573–575. doi: 10.1097/00007435-199711000-00004
Alakunle, E., Moens, U., Nchinda, G., and Okeke, M. I. (2020). Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses 12:1257. doi: 10.3390/v12111257
Allard, A., Althouse, B. M., Hébert-Dufresne, L., and Scarpino, S. V. (2017). The risk of sustained sexual transmission of Zika is underestimated. PLoS Pathog. 13:e1006633. doi: 10.1371/journal.ppat.1006633
Andrea, S. B., and Chapin, K. C. (2011). Comparison of Aptima Trichomonas vaginalis transcription-mediated amplification assay and BD affirm VPIII for detection of T. vaginalis in symptomatic women: performance parameters and epidemiological implications. J. Clin. Microbiol. 49, 866–869. doi: 10.1128/JCM.02367-10
Ashley, R. L., Dalessio, J., Dragavon, J., Koutsky, L. A., Lee, F. K., Nahmias, A. J., et al. (1993). Underestimation of HSV-2 seroprevalence in a high-risk population by microneutralization assay. Sex. Transm. Dis. 20, 230–235. doi: 10.1097/00007435-199307000-00009
Babitsch, B., Gohl, D., and von Lengerke, T. (2012). Re-revisiting Andersen's behavioral model of health services use: a systematic review of studies from 1998-2011. Psychosoc. Med. 9. doi: 10.3205/psm000089
Baptiste-Roberts, K., Oranuba, E., Werts, N., and Edwards, L. V. (2017). Addressing health care disparities among sexual minorities. Obstet. Gynecol. Clin. N. Am. 44, 71–80. doi: 10.1016/j.ogc.2016.11.003
Barquet, N., and Domingo, P. (1997). Smallpox: the triumph over the most terrible of the ministers of death. Ann. Intern. Med. 127, 635–642. doi: 10.7326/0003-4819-127-8_part_1-199710150-00010
Bell, G., and Potterat, J. (2011). Partner notification for sexually transmitted infections in the modern world: a practitioner perspective on challenges and opportunities. Sex. Transm. Infect. 87, 34–36. doi: 10.1136/sextrans-2011-050229
Bragazzi, NL, Khamisy-Farah, R, Tsigalou, C, Mahroum, N, and Converti, M. (2022a). Attaching a stigma to the LGBTQI+ community should be avoided during the monkeypox epidemic. J. Med. Virol. doi: 10.1002/jmv.27913 [Epub ahead of print].
Bragazzi, NL, Kong, JD, Mahroum, N, Tsigalou, C, Khamisy-Farah, R, Converti, M., et al. (2022b). Epidemiological trends and clinical features of the ongoing monkeypox epidemic: a preliminary pooled data analysis and literature review. J. Med. Virol. doi: 10.1002/jmv.27931 [Epub ahead of print].
Brookmeyer, R., Quinn, T., Shepherd, M., Mehendale, S., Rodrigues, J., and Bollinger, R. (1995). The AIDS epidemic in India: a new method for estimating current human immunodeficiency virus (HIV) incidence rates. Am. J. Epidemiol. 142, 709–713. doi: 10.1093/oxfordjournals.aje.a117700
Broome, M. E. (2000). “Integrative literature reviews for the development of concepts,” in Concept Development in Nursing: Foundations, Techniques and Applications. eds. B. L. Rodgers and K. A. Knafl (Philadelphia: W. B. Saunders Company), 231–250.
Brown, B., Monsour, E., Klausner, J. D., and Galea, J. T. (2015). Sociodemographic and behavioral correlates of anogenital warts and human papillomavirus-related knowledge among men who have sex with men and transwomen in Lima. Peru. Sex Transm. Dis. 42, 198–201. doi: 10.1097/OLQ.0000000000000258
Bunge, E. M., Hoet, B., Chen, L., Lienert, F., Weidenthaler, H., Baer, L. R., et al. (2022). The changing epidemiology of human monkeypox-a potential threat? A systematic review. PLoS Negl. Trop. Dis. 16:e0010141. doi: 10.1371/journal.pntd.0010141
Centers for Disease Control and Prevention (CDC) (2022). National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), division of high-consequence pathogens and pathology (DHCPP). Case definitions for use in the 2022 Monkeypox Response. Available at https://www.cdc.gov/poxvirus/monkeypox/clinicians/case-definition.html
Cohen, E. (2022). Testing is crucial to getting monkeypox under control, but there's a 'shocking' lack of demand. CNN Health. Available at: https://edition.cnn.com/2022/07/28/health/monkeypox-testing-demand/index.html
Corbeto, E. L., Gonzalez, V., Lugo, R., Almirall, M. R., Espelt, R., Avecilla, A., et al. (2015). CT/NG study group. Discordant prevalence of chlamydia trachomatis in asymptomatic couples screened by two screening approaches. Int. J. STD AIDS 26, 27–32. doi: 10.1177/0956462414528686
Datta, J., Reid, D., Hughes, G., Mercer, C. H., Wayal, S., and Weatherburn, P. (2019). Awareness of and attitudes to sexually transmissible infections among gay men and other men who have sex with men in England: a qualitative study. Sex. Health 16, 18–24. doi: 10.1071/SH18025
Denison, H. J., Bromhead, C., Grainger, R., Dennison, E. M., and Jutel, A. (2017). Barriers to sexually transmitted infection testing in New Zealand: a qualitative study. Aust. N. Z. J. Public Health 41, 432–437. doi: 10.1111/1753-6405.12680
Dhawan, B., Gupta, V., Khanna, N., Singh, M., and Chaudhry, R. (2006). Evaluation of the diagnostic efficacy of PCR for Ureaplasma urealyticum infection in Indian adults with symptoms of genital discharge. Jpn. J. Infect. Dis. 59, 57–58.
Diven, D. G. (2001). An overview of poxviruses. J. Am. Acad. Dermatol. 44, 1–16. doi: 10.1067/mjd.2001.109302
Durski, K. N., McCollum, A. M., Nakazawa, Y., Petersen, B. W., Reynolds, M. G., Briand, S., et al. (2018). Emergence of Monkeypox-west and Central Africa, 1970-2017. MMWR Morb. Mortal. Wkly Rep. 67, 306–310. doi: 10.15585/mmwr.mm6710a5
Ennab, F., Nawaz, F. A., Narain, K., Nchasi, G., Essar, M. Y., Head, M. G., et al. (2022). Monkeypox outbreaks in 2022: battling another "pandemic" of misinformation. Int. J. Public Health 14:1605149. doi: 10.3389/ijph.2022.1605149
Fakoya, I., Álvarez-del Arco, D., Woode-Owusu, M., Monge, S., Rivero-Montesdeoca, Y., Delpech, V., et al. (2015). A systematic review of post-migration acquisition of HIV among migrants from countries with generalised HIV epidemics living in Europe: mplications for effectively managing HIV prevention programmes and policy. BMC Public Health 19:561. doi: 10.1186/s12889-015-1852-9
Fleischauer, A. T., Kile, J. C., Davidson, M., Fischer, M., Karem, K. L., Teclaw, R., et al. (2005). Kuehnert MJ. Evaluation of human-to-human transmission of monkeypox from infected patients to health care workers. Clin. Infect. Dis. 40, 689–694. doi: 10.1086/427805
Franco, E. L. (1991). The sexually transmitted disease model for cervical cancer: incoherent epidemiologic findings and the role of misclassification of human papillomavirus infection. Epidemiology 2, 98–106. doi: 10.1097/00001648-199103000-00003
Geba, M. C., Powers, S., Williams, B., Dort, K. R., Rogawski McQuade, E. T., and McManus, K. A. (2022). A missed opportunity: Extragenital screening for gonorrhea and chlamydia sexually transmitted infections in people with HIV in a southeastern Ryan white HIV/AIDS program clinic setting. Open Forum Infect. Dis. 9:ofac322. doi: 10.1093/ofid/ofac322
Gratzer, B., Pohl, D., and Hotton, A. L. (2014). Evaluation of diagnostic serological results in cases of suspected primary syphilis infection. Sex. Transm. Dis. 41, 285–289. doi: 10.1097/OLQ.0000000000000126
Guagliardo, S. A. J., Monroe, B., Moundjoa, C., Athanase, A., Okpu, G., Burgado, J., et al. (2020). Asymptomatic Orthopoxvirus circulation in humans in the wake of a Monkeypox outbreak among chimpanzees in Cameroon. Am. J. Trop. Med. Hyg. 102, 206–212. doi: 10.4269/ajtmh.19-0467
Hall, G. C., Koenig, L. J., Gray, S. C., Herbst, J. H., Matheson, T., Coffin, P., et al. (2018). Accuracy of HIV risk perceptions among episodic substance-using men who have sex with men. AIDS Behav. 22, 1932–1943. doi: 10.1007/s10461-017-1935-y
Holmes, N., and Beach, L. (2020). Bisexual people's utilization of sexual health services at an LGBTQ Community Center in Chicago. J. Bisex. 20, 342–359. doi: 10.1080/15299716.2020.1825270
Holt, M., Bernard, D., and Race, K. (2010). Gay men's perceptions of sexually transmissible infections and their experiences of diagnosis: 'part of the way of life' to feeling 'dirty and ashamed. Sex. Health 7, 411–416. doi: 10.1071/SH09117
Hong, Y., Fang, X., Zhou, Y., Zhao, R., and Li, X. (2011). Factors associated with sexually transmitted infection underreporting among female sex workers in China. J. Women's Health (Larchmt) 20, 129–136. doi: 10.1089/jwh.2010.2139
Hughes, A. L., Irausquin, S., and Friedman, R. (2010). The evolutionary biology of poxviruses. Infect. Genet. Evol. 10, 50–59. doi: 10.1016/j.meegid.2009.10.001
Jenkins, W. D. (2015). LeVault KR. Sexual history taking in the emergency department - more specificity required. J. Emerg. Med. 48, 143–151. doi: 10.1016/j.jemermed.2014.06.051
Jiang, Z., Sun, J., Zhang, L., Lai, A., and Shuo, S. (2022). Laboratory diagnostics for monkeypox: an overview of sensitivities from various published tests. Travel Med. Infect. Dis. 49:102425. doi: 10.1016/j.tmaid.2022.102425
Johnson, L. F., and Geffen, N. (2016). A comparison of two mathematical modeling frameworks for evaluating sexually transmitted infection epidemiology. Sex. Transm. Dis. 43, 139–146. doi: 10.1097/OLQ.0000000000000412
Karem, K. L., Reynolds, M., Hughes, C., Braden, Z., Nigam, P., Crotty, S., et al. (2007). Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin. Vaccine Immunol. 14, 1318–1327. doi: 10.1128/CVI.00148-07
Kenyon, C. (2022). Is Monkeypox being underdiagnosed in countries with more stigmatizing attitudes towards men who have sex with men? A simple ecological analysis. Epidemiologia 3, 363–368. doi: 10.3390/epidemiologia3030028
Kmiec, D., and Kirchhoff, F. (2022). Monkeypox: a new threat? Int. J. Mol. Sci. 23:7866. doi: 10.3390/ijms23147866
Knight, J., Baral, S. D., Schwartz, S., Wang, L., Ma, H., Young, K., et al. (2020). Contribution of high risk groups' unmet needs may be underestimated in epidemic models without risk turnover: a mechanistic modelling analysis. Infect. Dis. Model. 1, 549–562. doi: 10.1016/j.idm.2020.07.004
Koper, N. E., van der Sande, M. A., Gotz, H. M., and Koedijk, F. D. (2013). Dutch STI clinics. Lymphogranuloma venereum among men who have sex with men in the Netherlands: regional differences in testing rates lead to underestimation of the incidence, 2006-2012. Euro Surveill. 18:20561. doi: 10.2807/1560-7917.es2013.18.34.20561
Koutsky, L. A., Stevens, C. E., Holmes, K. K., Ashley, R. L., Kiviat, N. B., Critchlow, C. W., et al. (1992). Underdiagnosis of genital herpes by current clinical and viral-isolation procedures. N. Engl. J. Med. 326, 1533–1539. doi: 10.1056/NEJM199206043262305
Kretzschmar, M., Mangen, M. J., Pinheiro, P., Jahn, B., Fèvre, E. M., Longhi, S., et al. (2012). BCoDE consortium. New methodology for estimating the burden of infectious diseases in Europe. PLoS Med. 9:e1001205. doi: 10.1371/journal.pmed.1001205
Krivochenitser, R., Jones, J. S., Whalen, D., and Gardiner, C. (2013). Underrecognition of cervical Neisseria gonorrhoeae and chlamydia trachomatis infections in pregnant patients in the ED. Am. J. Emerg. Med. 31, 661–663. doi: 10.1016/j.ajem.2012.11.017
Kustec, T., Keše, D., and Klavs, I. (2016). Under-reporting of sexually transmitted infection with chlamydia trachomatis - a revision of surveillance system is required. Zdr. Varst. 55, 174–178. doi: 10.1515/sjph-2016-0022
Kutner, B. A., Simoni, J. M., DeWitt, W., Gaisa, M. M., and Sandfort, T. G. M. (2022). Gay and bisexual men who report anal sex stigma alongside discomfort discussing anal sex with health workers are less likely to have ever received an anal examination or anal swab. LGBT Health 9, 103–113. doi: 10.1089/lgbt.2021.0104
Lee, H., and Nishiura, H. (2017). Recrudescence of Ebola virus disease outbreak in West Africa, 2014-2016. Int. J. Infect. Dis. 64, 90–92. doi: 10.1016/j.ijid.2017.09.013
Lin, J. S., Jones, W. E., Yan, L., Wirthwein, K. A., Flaherty, E. E., Haivanis, R. M., et al. (1992). Underdiagnosis of chlamydia trachomatis infection. Diagnostic limitations in patients with low-level infection. Sex. Transm. Dis. 19, 259–265. doi: 10.1097/00007435-199209000-00004
Liu, D, Chi, Y, Song, P, Zeng, X, Du, L, Chen, Y, et al. (2022). Risk factors, clinical manifestation, precaution, and management of monkeypox. J. Evid. Med. doi: 10.1111/jebm.12490 [Epub ahead of print].
Liu, H., Yang, H., Li, X., Wang, N., Liu, H., Wang, B., et al. (2006). Men who have sex with men and human immunodeficiency virus/sexually transmitted disease control in China. Sex. Transm. Dis. 33, 68–76. doi: 10.1097/01.olq.0000187266.29927.11
Lusk, M. J., Naing, Z., Rayner, B., Rismanto, N., McIver, C. J., Cumming, R. G., et al. (2010). Konecny P. Trichomonas vaginalis: underdiagnosis in urban Australia could facilitate re-emergence. Sex. Transm. Infect. 86, 227–230. doi: 10.1136/sti.2009.039362
Maher, D., and Hoffman, I. (1995). Prevalence of genital infections in medical inpatients in Blantyre, Malawi. J. Inf. Secur. 31, 77–78. doi: 10.1016/s0163-4453(95)91674-1
Mangine, C., Kukk, A., Noormets, H., Jänes, J., and Rüütel, K. (2018). Internet recruitment for sexually transmitted infection screening among men who have sex with men in Eastern Europe. Int. J. STD AIDS 29, 237–243. doi: 10.1177/0956462417722477
Marcus, U., Hickson, F., Weatherburn, P., and Schmidt, A. J., EMIS Network (2013). Estimating the size of the MSM populations for 38 European countries by calculating the survey-surveillance discrepancies (SSD) between self-reported new HIV diagnoses from the European MSM internet survey (EMIS) and surveillance-reported HIV diagnoses among MSM in 2009. BMC Public Health 3:919. doi: 10.1186/1471-2458-13-919
Mirzazadeh, A., Nedjat, S., Navadeh, S., Haghdoost, A., Mansournia, M. A., McFarland, W., et al. (2014). HIV and related risk behaviors among female sex workers in Iran: bias-adjusted estimates from the 2010 National bio-Behavoral Survey. AIDS Behav. 18, S19–S24. doi: 10.1007/s10461-013-0548-3
Mlakar, B., and Ramšak, A. (2016). A suspected case of lymphogranuloma venereum (LGV) suggests underdiagnosed LGV infection among Slovenian men who have sex with men. Acta Dermatovenerol. Alp Pannonica Adriat. 25, 35–37. doi: 10.15570/actaapa.2016.10
Moriña, D., Fernández-Fontelo, A., Cabaña, A., Puig, P., Monfil, L., Brotons, M., et al. (2021). Quantifying the under-reporting of uncorrelated longitudal data: the genital warts example. BMC Med. Res. Methodol. 21:6. doi: 10.1186/s12874-020-01188-4
Munson, E., Napierala, M., Olson, R., Endes, T., Block, T., Hryciuk, J. E., et al. (2008). Impact of Trichomonas vaginalis transcription-mediated amplification-based analyte-specific-reagent testing in a metropolitan setting of high sexually transmitted disease prevalence. J. Clin. Microbiol. 46, 3368–3374. doi: 10.1128/JCM.00564-08
Nakamoto, I., and Zhang, J. (2021). Modeling the underestimation of COVID-19 infection. Results Phys. 25:104271. doi: 10.1016/j.rinp.2021.104271
Nguyen, T. Q., Gwynn, R. C., Kellerman, S. E., Begier, E., Garg, R. K., Pfeiffer, M. R., et al. (2008). Population prevalence of reported and unreported HIV and related behaviors among the household adult population in new York City, 2004. AIDS 22, 281–287. doi: 10.1097/QAD.0b013e3282f2ef58
Ni, M., Chen, X., Hu, X., and Ma, Y. (2016). Misreporting rate and influencing factors regarding the routes of transmission among reported HIV patients in Yili Kazakh autonomous prefecture of Xinjiang Uygur autonomous region of China. Zhonghua Liu Xing Bing Xue Za Zhi 37, 90–93. doi: 10.3760/cma.j.issn.0254-6450.2016.01.019
Niccolai, L. M., Kershaw, T. S., Lewis, J. B., Cicchetti, D. V., Ethier, K. A., and Ickovics, J. R. (2005). Data collection for sexually transmitted disease diagnoses: a comparison of self-report, medical record reviews, and state health department reports. Ann. Epidemiol. 15, 236–242. doi: 10.1016/j.annepidem.2004.07.093
Niekamp, A. M., Spauwen, L. W. L., Dukers-Muijrers, N. H. T. M., and Hoebe, C. J. P. A. (2021). How aware are swingers about their swing sex partners' risk behaviours, and sexually transmitted infection status? BMC Infect. Dis. 21:172. doi: 10.1186/s12879-021-05813-5
Nuzzo, J. B., Borio, L. L., and Gostin, L. O. (2022). The WHO Declaration of Monkeypox as a global public health emergency. JAMA 328, 615–617. doi: 10.1001/jama.2022.12513
Oliffe, J. L., Chabot, C., Knight, R., Davis, W., Bungay, V., and Shoveller, J. A. (2013). Women on men's sexual health and sexually transmitted infection testing: a gender relations analysis. Sociol. Health Illn. 35, 1–16. doi: 10.1111/j.1467-9566.2012.01470.x
Paget, W. J., Zbinden, R., Ritzler, E., Zwahlen, M., Lengeler, C., Stürchler, D., et al. (2002). Swiss sentinel surveillance Network of gynecologists. National laboratory reports of chlamydia trachomatis seriously underestimate the frequency of genital chlamydial infections among women in Switzerland. Sex. Transm. Dis. 29, 715–720. doi: 10.1097/00007435-200211000-00016
Petersen, C. S., Carl, L., Alnor, D., Thomsen, U., and Thomsen, H. K. (1995). Ignored trichomonal infestation diagnosed by Papanicolaou smear. Genitourin. Med. 71, 257–258. doi: 10.1136/sti.71.4.257
Pitts, C. J. (2020). Sexually transmitted infections: overlooked, underestimated, and undiagnosed. Nurs. Clin. North Am. 55, xiii–xiv. doi: 10.1016/j.cnur.2020.06.011.Epub
Riccò, M., Ferraro, P., Camisa, V., Satta, E., Zaniboni, A., Ranzieri, S., et al. (2022). When a neglected tropical disease goes global: knowledge, attitudes and practices of Italian physicians towards Monkeypox, preliminary results. Trop. Med. Infect. Dis. 7:135. doi: 10.3390/tropicalmed7070135
Riedel, S. (2005). Edward Jenner and the history of smallpox and vaccination. Proc. (Baylor Univ. Med. Cent.) 18, 21–25. doi: 10.1080/08998280.2005.11928028
Rompalo, A. M., Shepherd, M., Lawlor, J. P., Rand, S., Fox, R., Brookmeyer, R., et al. (1997). Definitions of genital ulcer disease and variation in risk for prevalent human immunodeficiency virus infection. Sex. Transm. Dis. 24, 436–442. doi: 10.1097/00007435-199708000-00009
Roth, A. M., Williams, J. A., Ly, R., Curd, K., Brooks, D., Arno, J., et al. (2011). Changing sexually transmitted infection screening protocol will result in improved case finding for trichomonas vaginalis among high-risk female populations. Sex. Transm. Dis. 38, 398–400. doi: 10.1097/OLQ.0b013e318203e3ce
Sallam, M., Al-Mahzoum, K., Dardas, L. A., Al-Tammemi, A. B., Al-Majali, L., Al-Naimat, H., et al. (2022). Knowledge of human Monkeypox and its relation to conspiracy beliefs among students in Jordanian health schools: filling the knowledge gap on emerging zoonotic viruses. Medicina 58:924. doi: 10.3390/medicina58070924
Schachter, J., and Chow, J. M. (1995). The fallibility of diagnostic tests for sexually transmitted diseases: the impact of behavioral and epidemiologic studies. Sex. Transm. Dis. 22, 191–196. doi: 10.1097/00007435-199505000-00010
Schulte, J. M., Martich, F. A., and Schmid, G. P. (1992). Chancroid in the United States, 1981-1990: evidence for underreporting of cases. MMWR CDC Surveill. Summ. 41, 57–61.
Schwebke, J. R., Hillier, S. L., Sobel, J. D., McGregor, J. A., and Sweet, R. L. (1996). Validity of the vaginal gram stain for the diagnosis of bacterial vaginosis. Obstet. Gynecol. 88, 573–576. doi: 10.1016/0029-7844(96)00233-5
Shahesmaeili, A., Karamouzian, M., Shokoohi, M., Kamali, K., Fahimfar, N., Nadji, S. A., et al. (2018). Symptom-based versus laboratory-based diagnosis of five sexually transmitted infections in female sex Workers in Iran. AIDS Behav. 22, 19–25. doi: 10.1007/s10461-018-2130-5
Steen, R., Hontelez, J. A. C., Mugurungi, O., Mpofu, A., Matthijsse, S. M., de Vlas, S. J., et al. (2019). Economy, migrant labour and sex work: interplay of HIV epidemic drivers in Zimbabwe over three decades. AIDS 33, 123–131. doi: 10.1097/QAD.0000000000002066
Syme, M. L., Cohn, T. J., and Barnack-Tavlaris, J. (2017). A comparison of actual and perceived sexual risk among older adults. J. Sex Res. 54, 149–160. doi: 10.1080/00224499.2015.1124379
Thornhill, J. P., Barkati, S., Walmsley, S., Rockstroh, J., Antinori, A., Harrison, L. B., et al. (2022). SHARE-net clinical group. Monkeypox virus infection in humans across 16 countries-April-June 2022. N. Engl. J. Med. 387, 679–691. doi: 10.1056/NEJMoa2207323
Timsit, B. L., Deroux, A., Lugosi, M., and Colombe, B. (2018). Bouillet L. Amibiase: au cours de rapports sexuels, un mode de transmission sous-estimé? [Amoebosis: May sexual transmission be an underestimated way of contamination?]. Rev. Med. Interne 39, 586–588. doi: 10.1016/j.revmed.2018.04.004
Tomas, M. E., Getman, D., Donskey, C. J., and Hecker, M. T. (2015). Overdiagnosis of urinary tract infection and Underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J. Clin. Microbiol. 53, 2686–2692. doi: 10.1128/JCM.00670-15
Webster, L. A., Berman, S. M., and Greenspan, J. R. (1993). Surveillance for gonorrhea and primary and secondary syphilis among adolescents, United States--1981-1991. MMWR CDC Surveill. Summ. 42, 1–11.
Wolfers, M., de Zwart, O., and Kok, G. (2011). Adolescents in the Netherlands underestimate risk for sexually transmitted infections and deny the need for sexually transmitted infection testing. AIDS Patient Care STDs 25, 311–319. doi: 10.1089/apc.2010.0186
Keywords: monkeypox, zoonotic disease, emerging and re-emerging infectious disease, underestimation, underreporting, under-detection, under-diagnosis, under-ascertainment
Citation: Bragazzi NL, Woldegerima WA, Iyaniwura SA, Han Q, Wang X, Shausan A, Badu K, Okwen P, Prescod C, Westin M, Omame A, Converti M, Mellado B, Wu J and Kong JD (2022) Knowing the unknown: The underestimation of monkeypox cases. Insights and implications from an integrative review of the literature. Front. Microbiol. 13:1011049. doi: 10.3389/fmicb.2022.1011049
Edited by:
Wenn-Chyau Lee, University of Malaya, MalaysiaReviewed by:
Ali Ahmed, Monash University Malaysia, MalaysiaSarah Guagliardo, Centers for Disease Control and Prevention (CDC), United States
Caleb McEntire, Department of Neurology, Massachusetts General Hospital, Harvard Medical School, United States
Copyright © 2022 Bragazzi, Woldegerima, Iyaniwura, Han, Wang, Shausan, Badu, Okwen, Prescod, Westin, Omame, Converti, Mellado, Wu and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Luigi Bragazzi, YnJhZ2F6emlAeW9ya3UuY2E=
 Nicola Luigi Bragazzi
Nicola Luigi Bragazzi Woldegebriel Assefa Woldegerima
Woldegebriel Assefa Woldegerima Sarafa Adewale Iyaniwura2
Sarafa Adewale Iyaniwura2 Qing Han
Qing Han Aminath Shausan
Aminath Shausan Kingsley Badu
Kingsley Badu Patrick Okwen
Patrick Okwen Cheryl Prescod
Cheryl Prescod Andrew Omame
Andrew Omame Manlio Converti
Manlio Converti Jude Dzevela Kong
Jude Dzevela Kong