- 1Nanchang Key Laboratory of Animal Health and Safety Production, Jiangxi Agricultural University, Nanchang, Jiangxi, China
- 2State Key Laboratory of Animal Nutrition, Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing, China
- 3Yangxin Yiliyuan Halal Meat Co., Ltd., Yangxin, Shandong, China
- 4School of Foreign Language, Jiangxi Agricultural University, Nanchang, Jiangxi, China
- 5Anyou Biotechnology Group Co., Ltd., Taicang, Jiangsu, China
Subacute ruminal acidosis (SARA) becomes the most common nutritional metabolic disease in high-yielding dairy cows and later fatting beef cattle because of the increasing consumption of high-concentrate diets in modern feeding patterns. Our previous research found a certain piece of evidence that adding 180 mg thiamine/kg DMI could increase the rumen pH and regulate the structure of the rumen microbial community in vivo. However, there is still limited experimental data on the effects of SARA on thiamine status, the damage to the structure of rumen epithelial cells, and the underlying mechanism of the epithelium alterations. For this purpose, a total of 18 Angus bulls (average 22.0-months-old) with an average live weight of 567.6 ± 27.4 kg were randomly allocated into a control treatment (CON), a high-concentrate diet treatment (HC), and a high-concentrate diet with the vitamin B1 supplement treatment (HCB). All bulls were conducted with a 7-day adjustment period followed by a 60-day-long main feeding procedure. Results indicated that ADFI and ADG significantly decreased in the HC treatment compared with CON (P < 0.05), while significantly increased after the VB1 supplement (P < 0.05). Besides, ruminal acetate content was significantly downregulated while propionate was significantly upregulated under the HC treatment compared with CON (P < 0.05); however, these alterations showed a completely inverse regulatory effect on the VB1 supplement compared with HC (P < 0.05). These changes causatively induced a significant decrease in the A/P ratio in the HC treatment compared with CON and HCB treatments (P < 0.05). Bacterial communities in the HC treatment could be separated from those in CON through PCoA axes 1 and 2. Meanwhile, the VB1 supplement significantly altered the bacterial communities compared with the HC treatment, except for HCB-3. Furthermore, the HC treatment significantly upregulated the expression of JNK, Bax, Caspase-8, Caspase-3, Caspase-9, and Cyt-C compared with CON, while significantly downregulated the expression of Bcl-2. The VB1 supplement showed a complete converse gene expression compared with HC. In conclusion, the VB1 supplement could effectively attenuate the alterations that occurred when exposed to high-concentrate diets, and help promote production performance through increased fermentability.
Introduction
Subacute ruminal acidosis (SARA) becomes the most common nutritional metabolic disease in high-yielding dairy cows and the later fattening beef cattle because of the increasing consumption of high-concentrate diets in the modern feeding patterns (Xue et al., 2018b). SARA leads to serious damage to dairy cows and the fattening period of beef cattle. It is necessary to find a suitable mitigation method to attenuate SARA.
It is well-established that thiamine (VB1) is an essential cofactor required for carbohydrate metabolism, and ruminants have no dietary Vitamin B1 requirements because of the ability of the rumen microbes to synthesize extensive ruminal Vitamin B1 (Breves et al., 1981; Miller et al., 1986). Several previous studies have indicated that increasing non-fiber carbohydrates decreased the daily apparent synthesis of Vitamin B1 in the rumen (Schwab et al., 2006), and increased the duodenal flow of Vitamin B1 (Zinn et al., 1987). However, SARA caused by high-fermentable carbohydrates significantly altered the ruminal pH and microbial activity (Mao et al., 2015), which is likely to affect the amount of Vitamin B1 production. Our previous research found that adding 180 mg of Vitamin B1/kg DMI could increase the rumen pH and regulate the structure of the rumen microbial community in vivo (Wang et al., 2015; Pan et al., 2018). However, there is still limited experimental data on the effects of SARA on Vitamin B1 status, the damage on the structure of rumen epithelial cells, and the underlying mechanism of the epithelium alterations.
Rumen epithelium is stratified squamous epithelium (SSE) composed of stratum basale, stratum spinosum, stratum granulosum, and stratum corneum, which pivotally acted on ruminal micro-ecosystem homeostasis through regulating nutrient transportation and absorption, the barrier function, and microbial diversity (Stumpff et al., 2011; Zhang et al., 2013). Continuous exposure to acidic pH, induced by long-term feeding high-concentrate diet fortified ruminal osmotic pressure, invasively acted on rumen epithelial cells, and further induced cell viability decline, and even apoptosis (Tao et al., 2014; Gui and Shen, 2016; Chen et al., 2018).
Previous studies indicated that continuously exposed to acidic pressure induced by both lactate and volatile fatty acids (VFAs) caused the occurrence of rumen epithelial apoptosis (Luo et al., 2019), and promoted apoptosis-related genes expression, such as Caspase-3, caspase-8, caspase-9, Bcl-2, Bax, and p53, of rumen epithelial cells in both bovine and sheep (Gui and Shen, 2016; Dai et al., 2020). However, the underlying mechanism of acidity-induced apoptosis is still unclear.
Generally considered, the accumulation of reactive oxygen species (ROS) induced by acidic pH first activated JNKs through apoptosis signal-regulating kinase 1 (ASK1) and IRE1 (Zhang et al., 2010; Němcová-Fürstová et al., 2013). Following, the activated JNKs improved the expression of downstream apoptosis-related protein Caspase 3, and further upregulated the poly-ADP-ribose polymerase (PARP), while downregulated the Bcl-2 (Miao et al., 2018; Ren et al., 2020). Moreover, C/EBP homologous protein (CHOP) may also be activated by the JNK signaling pathway (Huang et al., 2019) to intensify endoplasmic reticulum mediated apoptosis by upregulating the expression of caspase-12 (Su et al., 2016). All these genes may synergistically express under acidic conditions and causatively induced cell apoptosis.
Therefore, in the present study, rumen microbiota responses to vitamin B1 supplements combined with the apoptosis-related genes that participated in the JNK signaling pathway were investigated to determine the underlying mechanism of rumen epithelial apoptosis induced by acidity. We hypothesized that continuous exposure to an acidity environment triggered the JNKs signaling pathway, and further upregulated the apoptosis-related genes to induce cell apoptosis.
Materials and methods
Experimental design
The experiment was conducted on the cattle farm of Yangxin Yiliyuan Halal Meat Co., LTD, Yangxin, Shandong, China. A total of 18 Angus bulls (average 22.0-months-old) with an average live weight of 567.6 ± 27.4 kg were randomly allocated into three treatments, which included CON: a control treatment, HC: a high-concentrate diet treatment, and HCB: a high-concentrate diet with the vitamin B1 supplement treatment. Each treatment contained six repeats, with one bull in each repeat. All bulls received a 7-day-long adjustment period followed by a 60-day-long main feeding procedure. All animals were reared according to the Chinese Guidelines for Animal Welfare, which was approved by the Animal Care and Use Committee of Jiangxi Agricultural University, with the approval number JXAULL-20220121. All bulls were distributed in an individual barn, which was separated into 18 hurdles, with one bull in each hurdle. All bulls were free to walk and adapt to water ad libitum.
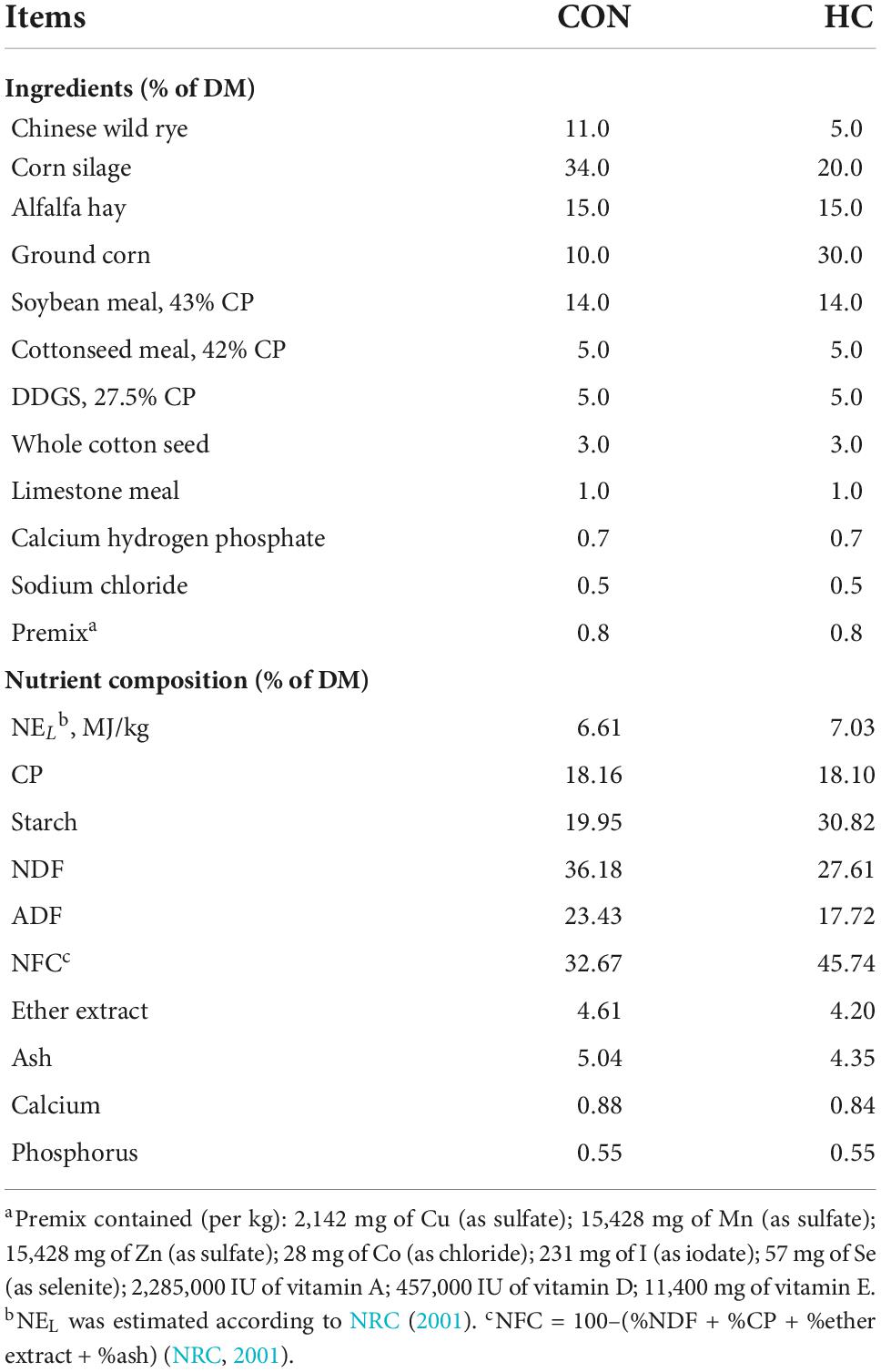
Diets were formulated according to the National Standard for Beef Cattle to meet the requirement of bulls with 550–600 kg body weight. All bulls were fed two times per day at 06:00 and 18:00. Details of ingredient analysis and chemical composition of diets are shown in Table 1. A total of 180 mg/kg DM of Vitamin B1 (Vitamin B1 hydrochloride, purity ≥ 99%; Wanrong Science and Technology Development Co., Ltd., Wuhan, China) was added into the premix first and further provided for the bulls that received the HCB treatment.
Determination of productive performances
Average daily intake was shown as the dry matter intake (DMI) of each treatment, which was calculated through the deviation between the weight of the supply and the residue. The DMI of each treatment group (CON, HC, and HCB) was calculated based on the average performance of the six cattle. The body weight gain (BWG) of each bull was measured at the beginning and end of the trail, with an empty stomach in the morning, and average daily gain (ADG) was calculated as the ratio of BWG/days. Feed conversion ratio (FCR) was then calculated through the following equation:
Ruminal fermentation parameters measurement
Rumen fluid samples of all 18 cows were collected on the last day through esophageal tubing for 3 h after morning feeding. The first tube of rumen fluid, which might be mixed with saliva, was discarded to ensure the purity of rumen microbiota. Each sample was divided into two portions. One was conducted to analyze ruminal pH [measured by Testo 206-pH1, Testo Instruments International (Shanghai) Co., Shanghai, China], rumen volatile fatty acids (VFAs, measured using a gas chromatograph, GC-2010, Shimadzu, Kyoto, Japan), and ammonia-N [NH3-N, determined by indophenol method and the absorbance value was measured through UV-2600 ultraviolet spectrophotometer (Shanghai Tianmei Scientific Instrument Co., LTD., Shanghai, China) at the 700 nm wavelength] (Xue et al., 2019). The other received part was first quick frozen by liquid nitrogen and then preserved at –80°C for further rumen microbial measurement.
Rumen microbial community measurement
Rumen microbial DNA of each sample was first extracted using the Bacterial Genome DNA Extraction kit [DP302, Tiangen, Tiangen Biotech (Beijing) Co., Ltd]. Following this, the 16S rRNA gene V4 region was amplified using the universal primers 520F and 802R (Xue et al., 2018a). The Qiagen Gel Extraction kit (Qiagen, Hilden, Germany) was used to purify the mixture of PCR products, followed by the generation of sequencing libraries using the TruSeq® DNA PCR-Free Sample Preparation kit (Illumina, USA). The Qubit@ 2.0 Fluorometer (Thermo Scientific) and the Agilent Bioanalyzer 2100 system were then applied for the assessment of the library quality. The Illumina HiSeq 4000 platform (Illumina Inc., San Diego, CA, USA) was used for the DNA sequencing process. High-quality clean tags were obtained according to the Quantitative Insights Into Microbial Ecology (QIIME, V1.7.0) quality controlling process, and sequences within similarity >97% were assigned to the same operational taxonomic unit (OTU). All following analyses included the α-diversity, β-diversity, and discrepancy bacterial community analyses were conducted based on the OTU results.
Rumen epithelium sampling and apoptosis-related genes expression
The rumen endodermis epithelial samples were isolated immediately after slaughtering and quickly placed into the ice-cold PBS. The epithelial tissues were washed five times with ice-cold PBS, which is added with 0.5 mg/ml amphotericin B and 100 μg/ml gentamicin, followed by being cut into small pieces and quickly frozen into the liquid nitrogen.
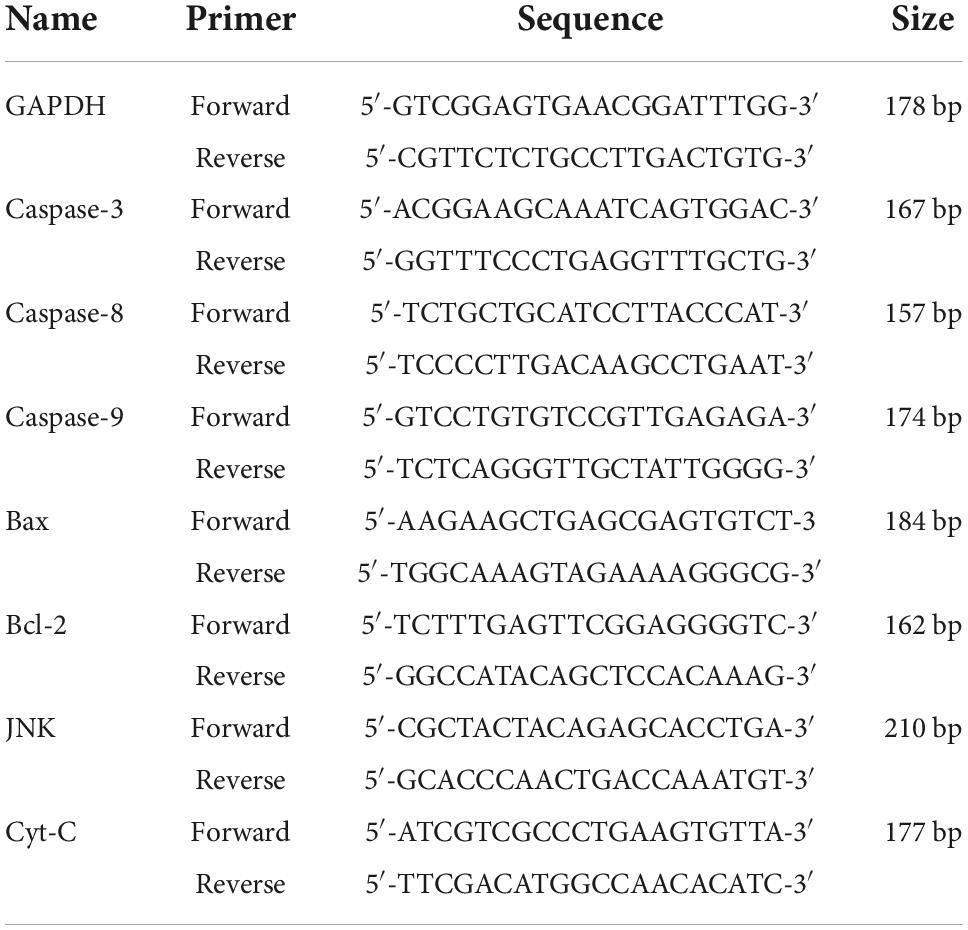
Total RNA was extracted from each rumen epithelial sample using the RNA kit (Takara) according to the manufacturer’s instructions. The amount and quality of RNA were determined by a spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA). Total RNA of all samples was first inverse-transcripted into cDNA according to the instructions of transcript reverse transcriptase (Bio-Rad Laboratories, Hercules, CA, USA), and then quantified by an RT-PCR amplifier (Roche, Applied Science, Mannheim, Germany) soon after the primers designation. All primers were designed using the Primer 5.0 software according to the gene sequence provided by GenBank in NCBI and displayed in Table 2.
Statistical analysis
Production performances and ruminal fermentation variables were first analyzed through a normal distribution test using the SAS procedure “proc univariate data = test normal,” and subsequently, the one-way ANOVA S-N-K test was received by SAS (SAS Institute, Inc., Cary, NC, USA). Significance would be considered when P < 0.05, while a tendency was considered when 0.05 ≤ P < 0.10. OTU abundances of each rumen bacteria were first conducted through a percentage transformation, and then a one-way ANOVA S-N-K test of SAS 9.2 was applied for the differential analysis. Alpha diversity and Beta diversity in our samples were calculated with QIIME 2 (Bolyen et al., 2018) and displayed with the R software (Version 3.3.1, R Core Team, Vienna, Austria). Principal coordinate analysis (PCoA) for different rumen methanogens was conducted using the R “vegan package.” Fold change of target gene expression was normalized by the housekeeping gene of GAPDH, and the discrepancies were determined by the 2–ΔΔCt method.
Results
Production performances and rumen fermentability parameters
The production performances, including dry matter intake (DMI), average daily body weight gain (ADG), and the feed conversion ratio (FCR) were first calculated. Then, rumen fermentable parameters, including ruminal pH, volatile fatty acids (acetate, propionate, and butyrate), and ammonia-N were measured. These results are shown in Table 3.
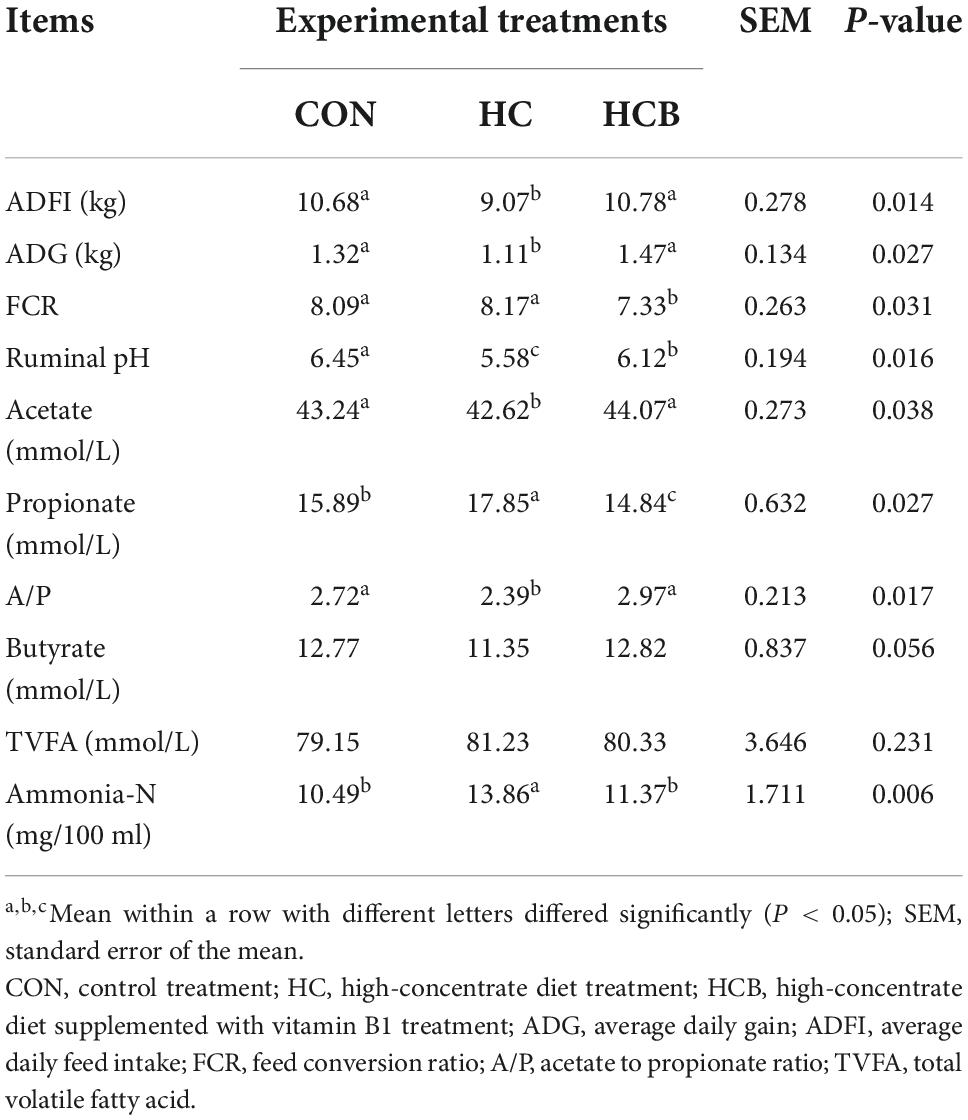
Table 3. Effects of high-concentrate diet and thiamine supplementation feeding treatments on production and ruminal fermentability parameters (n = 6).
Specifically, ADFI and ADG significantly decreased in the HC treatment compared with CON (P < 0.05), while significantly increased after the VB1 supplement (P < 0.05), which causatively induced the significant decrease of FCR in the HCB treatment compared with CON and HC treatments (P < 0.05). When referred to the fermentable parameters, ruminal pH significantly declined under the HC treatment, while significantly increased after the VB1 supplement (P < 0.05). Besides, ruminal acetate content was significantly downregulated while the propionate was significantly upregulated under the HC treatment compared with CON (P < 0.05), while these alterations showed a completely inverse regulatory effect after the VB1 supplement compared with HC (P < 0.05). All these changes caused a significant decrease in the A/P ratio in the HC treatment compared with CON and HCB treatments (P < 0.05). On the contrary, the ammonia-N content significantly increased in the HC treatment compared with CON and HCB (P < 0.05). No other significant alterations were observed among all three treatments.
Ruminal microbial communities measurement
In general, a total of 4,036 OTUs were obtained by performing OTU clustering on non-repetitive sequences according to 97% similarity. Taxonomic information indicated that a total of 18 phyla and 2,034 genera were identified after quality control, and it is displayed in Supplementary Table 1. All taxonomic bacteria were further applied for α-diversity, β-diversity, and differential communities’ investigation.
α-diversity
Alpha diversity was first investigated to determine the regulatory effects of HC and VB1 supplements on the complexity of rumen microbial diversity through Chao1, Shannon, Simpson, and ACE indexes. As shown in Table 4, the ACE index significantly decreased in the HC treatment compared with CON and HCB treatments (P < 0.05). No significant differences were found for the other indexes.
β-diversity
Differential analysis on rumen microbial communities among CON, HC, and HCB treatments was first proceeded through principal coordinates analysis (PCoA) to investigate the effects of HC and VB1 on the integrity of bacterial communities. As shown in Figure 1, PCoA axes 1 and 2 accounted for 39.91 and 23.38% of the total alteration, respectively. Bacterial communities in the HC treatment could be separated from those in CON through PCoA axes 1 and 2. VB1 supplement significantly altered the bacterial communities compared with the HC treatment, except for HCB-3.
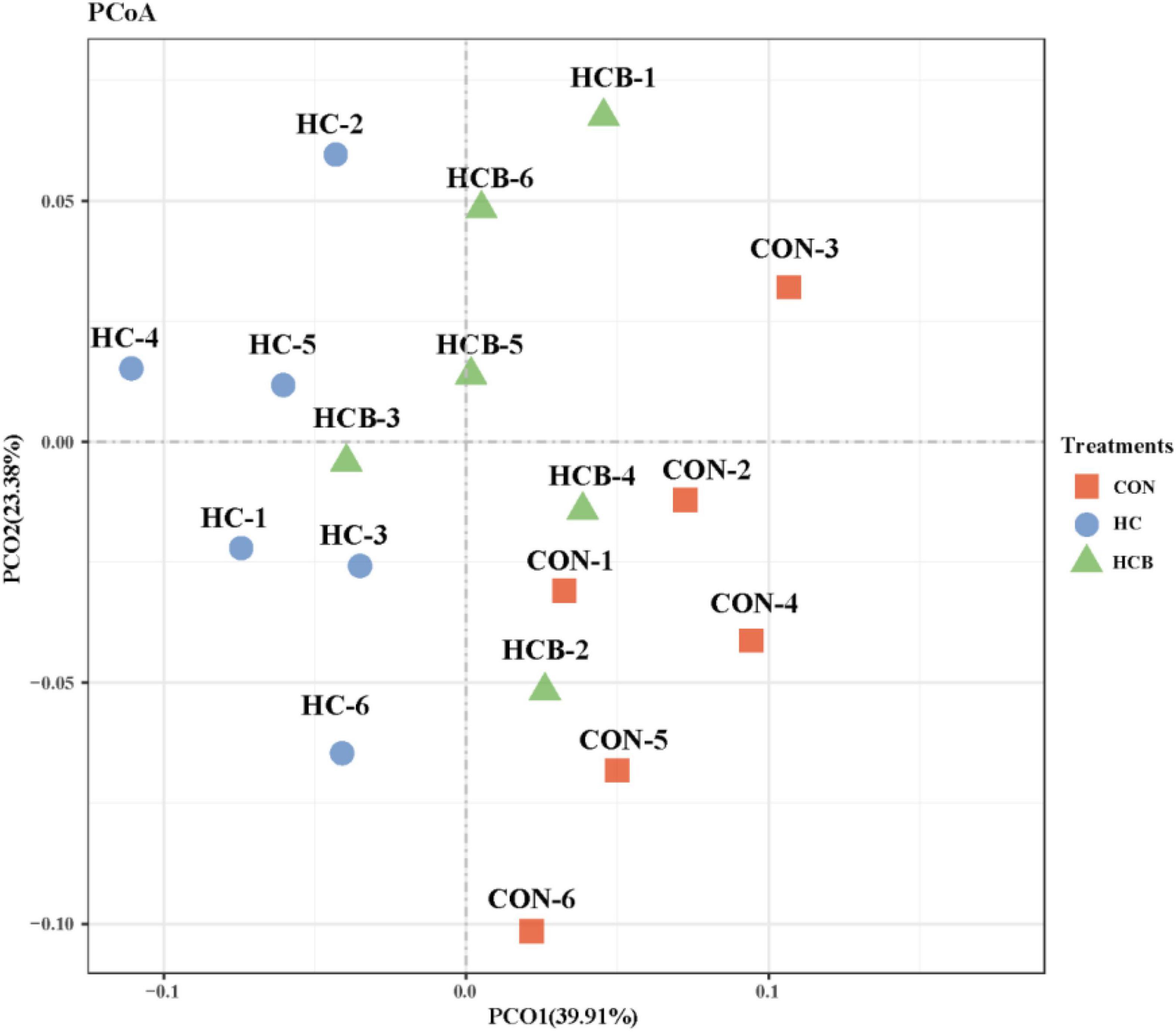
Figure 1. Principal coordinate analysis (PCoA) on community structures of the rumen microbiota between control treatment and small peptide supplement treatment. CON, control treatment; HC, high-concentrate diet treatment; HCB, high-concentrate diet supplemented with vitamin B1 treatment.
Then, discrepancies in the relative abundance of bacteria community proceeded, and all the results are shown in Tables 5, 6.
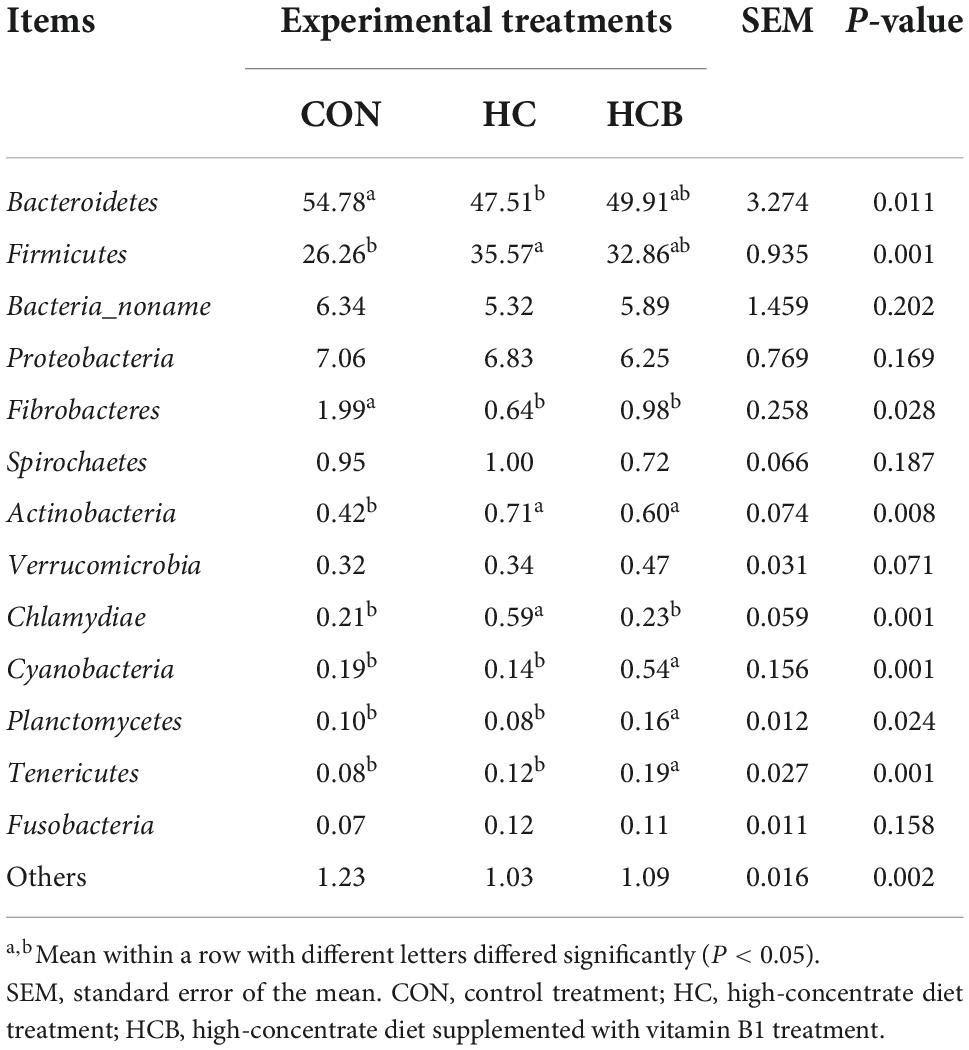
Table 5. Effect of high-concentrate diet feeding and thiamine supplementation on relative abundance of rumen bacteria (%, level of phyla, n = 6).
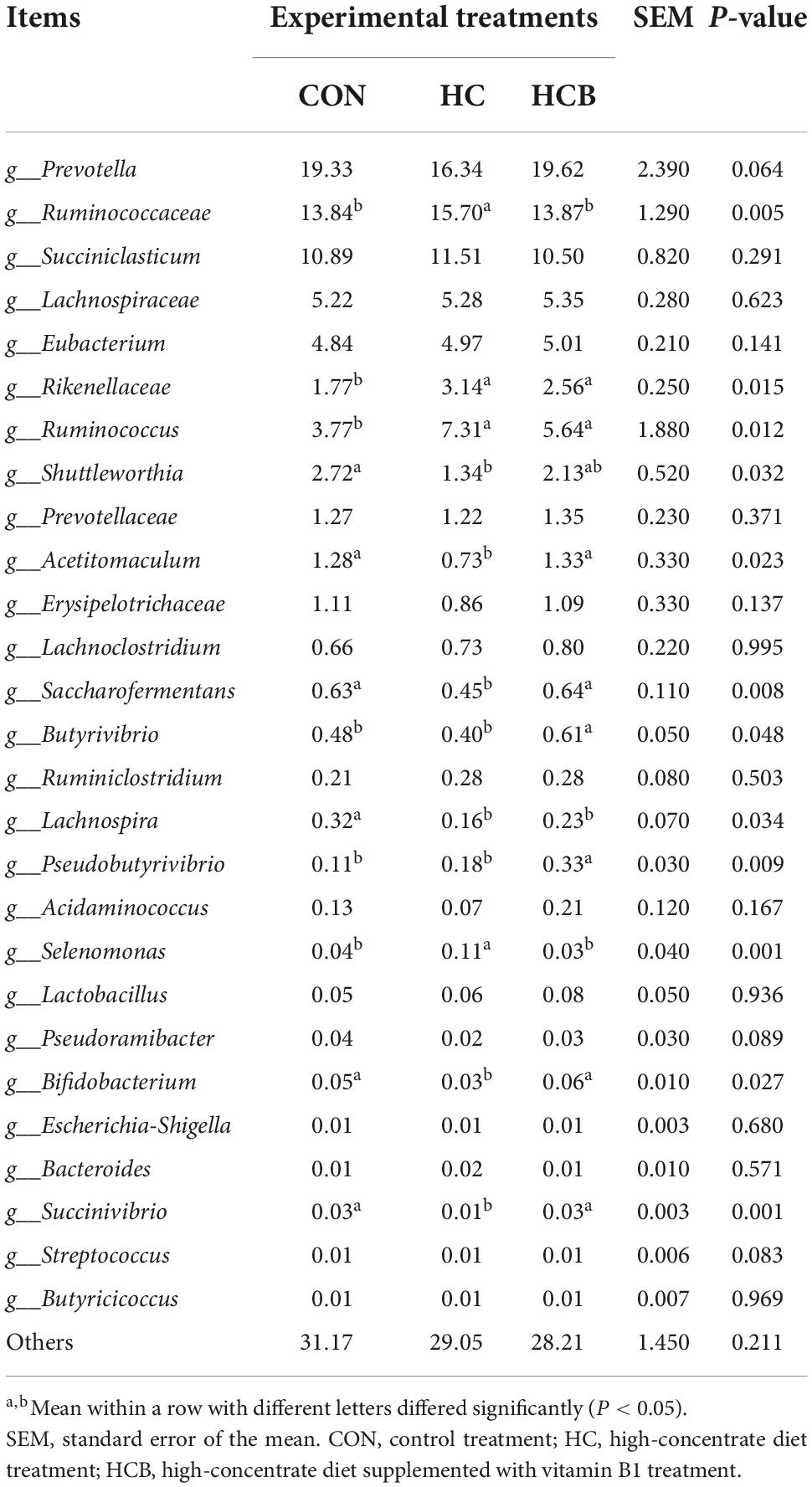
Table 6. Effect of high-concentrate diet feeding and thiamine supplementation on relative abundance of rumen bacteria (%, level of genera, n = 6).
Discrepancies at the phyla level are displayed in Table 5. Bacteroidetes and Firmicutes contributed as the most two abundant bacteria communities, which accounted for 90% of total microbial profiles. The relative abundance of Bacteroidetes significantly decreased, while of Firmicutes significantly increased in the HC treatment compared with the CON (P < 0.05). Compared with HC, VB1 supplement slightly increased the Bacteroidetes while decreased Firmicutes, however, not significantly. In addition, the HC treatment significantly decreased the relative abundances of Fibrobacteres, while significantly increased Actinobacteria and Chlamydiae (P < 0.05). The VB1 supplement significantly increased Cyanobacteria, Planctomycetes, and Tenericutes, while significantly decreased Chlamydiae compared with the HC treatment (P < 0.05). No other significant alterations were observed.
Then, differential analyses of the relative abundances at the genera level were conducted and the results are shown in Table 5. Prevotella, Ruminococcaceae, and Succiniclasticum contributed as the three most abundant genera and accounted for nearly half of all microbiota profiles. Microbial communities included Ruminococcaceae, Rikenellaceae, Ruminococcus, and Selenomonas significantly proliferated, while the abundances of Shuttleworthia, Saccharofermentans, Shuttleworthia, Acetitomaculum, Saccharofermentans, Lachnospira, Bifidobacterium, and Succinivibrio significantly suppressed in the HC treatment compared with CON. Besides, the VB1 supplement significantly increased Acetitomaculum, Saccharofermentans, Butyrivibrio, Pseudobutyrivibrio, Bifidobacterium, and Succinivibrio, while significantly declined abundances of Ruminococcaceae and Selenomonas compared with HC (P < 0.05). No significant changes were found for other bacterial genera.
Rumen epithelial apoptosis-related genes expression
The HC treatment significantly decreased the ruminal pH, which further caused epithelial cell apoptosis. Therefore, the apoptosis-related genes were detected and the results are shown in Figure 2. Compared with CON, HC treatment significantly upregulated the expression of JNK, Bax, Caspase-8, Caspase-3, Caspase-9, and Cyt-C, while significantly downregulated the expression of Bcl-2. The VB1 supplement remarkably decreased the expression of JNK, Caspase-9, Caspase-3, and Cyt-C, while significantly upregulated the expression of Bcl-2. Particularly, the ratio Bcl-2/Bax significantly decreased in HC compared with CON, while significantly increased after the VB1 supplement compared with the HC treatment.
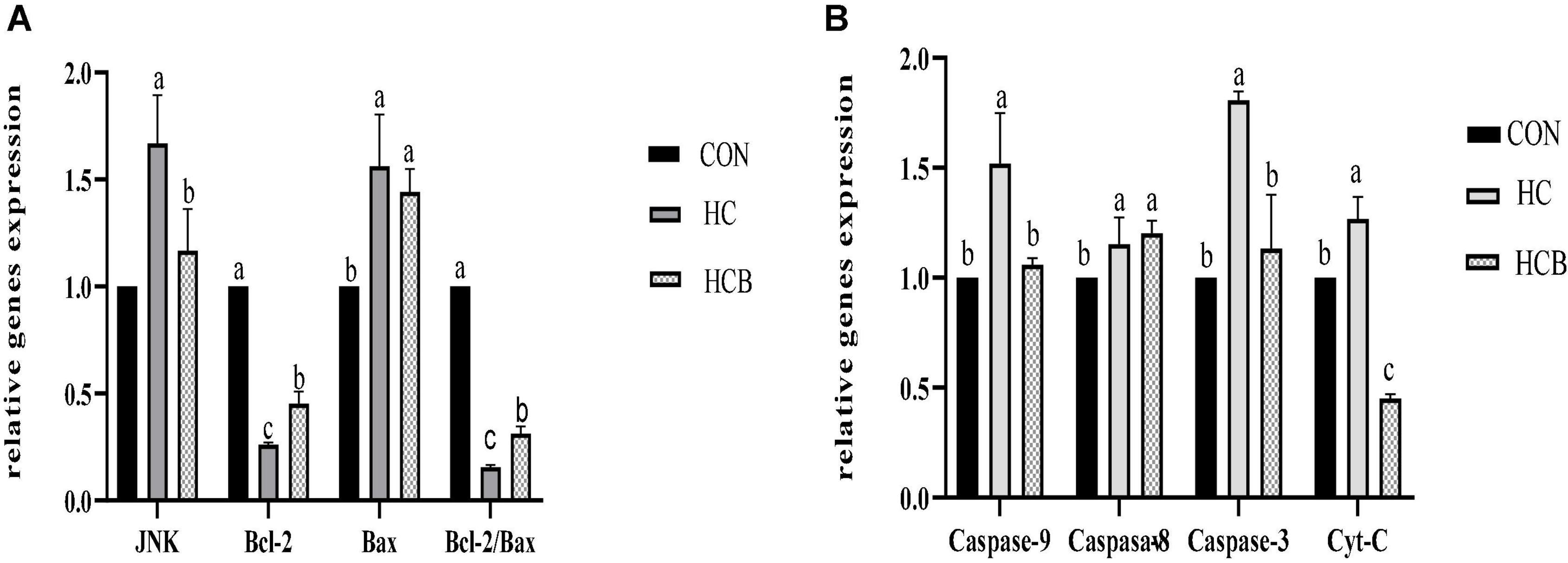
Figure 2. Effects of high-concentrate diets and vitamin B1 supplement treatments on the apoptosis-related genes expression of rumen epithelium. (A) Effects of high-concentrate diets and vitamin B1 supplement on the relative expression of JNK, Bcl-2, and Bax. (B) Effects of high-concentrate diets and vitamin B1 supplements on the relative expression of Caspase-9, Caspase-8, Caspase-3, and Cyt-C. CON, control treatment; HC, high-concentrate diet treatment; HCB, high-concentrate diet supplemented with vitamin B1 treatment. a,b,c means within different letters the gene expression differed significantly (P < 0.05).
Interactive analysis
Interactive analysis of the expression of apoptosis-related genes, productive performances, and the fermentability parameters are conducted and the result is shown in Figure 3.
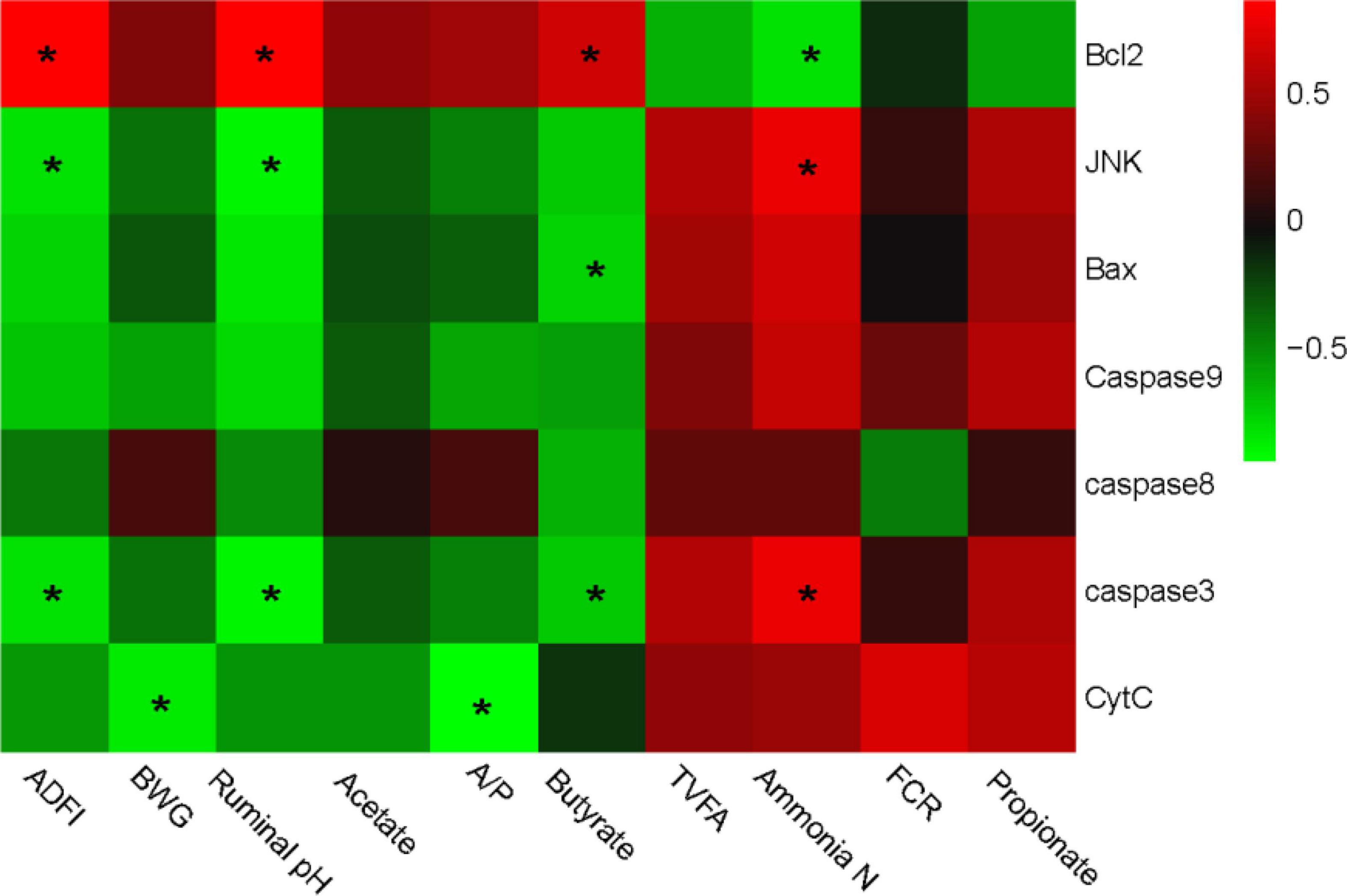
Figure 3. Correlation analysis between production performances, rumen fermentability parameters, and epithelial apoptosis-related genes expression. The red color represents a positive correlation while the green color represents a negative correlation. “*” means a significant correlation (| r| > 0.55, P < 0.05). ADFI, average daily feed intake, BWG, body weight gain; FCR, feed conversion ratio; TVFA, total volatile fatty acids.
Integrally, genotypic parameters could be separated into two clusters, based on the correlations with apoptosis-related genes. One consisted of ADFI, BWG, ruminal pH, acetate, and butyrate, which was positively correlated with Bcl-2, while negatively correlated with JNK, Bax, Caspase-8, Caspase-3, Caspase-9, and Cyt-C. The other cluster mainly consisted of TVFA, Ammonia-N, FCR, and propionate, which performed a completely contrary correlation with the apoptosis-related genes compared with the former one. Particularly, Bcl-2 gene expression was significantly positively correlated with ADFI, pH, and butyrate, while negatively correlated with ammonia-N content. JNK and caspase-3 showed a converse correlation compared with Bcl-2. Bax was negatively correlated with butyrate, while the cyt-C was negatively correlated with BWG and A/P. No other significant correlation was observed.
Furthermore, a correlation analysis between the bacterial communities and the genes expression was conducted and the relationship is displayed in Figure 4. Bacteria could also be separated into two big clusters based on their relationship with gene expressions. One mainly consisted of Bacteroidetes, Fibrobacteres, Cyanobacteria, and Planctomycetes, and shows a positive correlation with Bcl-2, while negatively correlated with JNK, Bax, Caspase-8, Caspase-3, Caspase-9, and Cyt-C. Meanwhile, the other cluster mainly consisted of Firmicutes, Actinobacteria, and Chlamydiae and showed a complete converse correlation with the apoptosis-related genes compared with the former cluster. Specifically, the expression of Bcl-2 was positively correlated with Bacteroidetes and Fibrobacteres, while negatively correlated with Firmicutes and Actinobacteria. Similar to the correlation with phenotypic parameters, the expressions of JNK and caspase-3 also showed a complete correlation with Bcl-2.
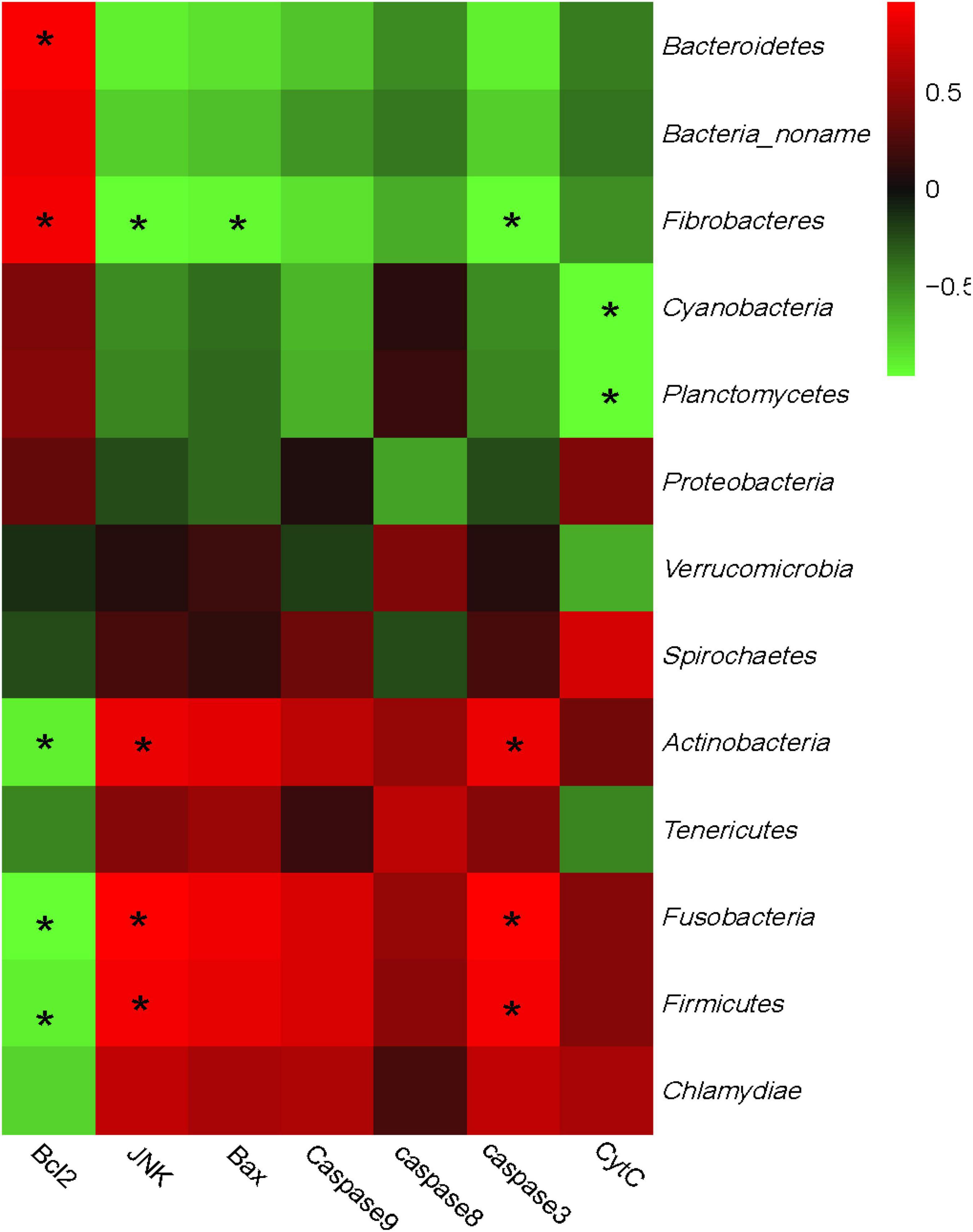
Figure 4. Correlation analysis between rumen bacteria community and epithelial apoptosis-related genes expression. The red color represents a positive correlation while the green color represents a negative correlation. “*” means a significant correlation (| r| > 0.55, P < 0.05).
Discussion
Subacute ruminal acidosis epidemically occurs among high-yielding dairy cows and later fattening beef cattle as a consequence of feeding higher grain diets (Plaizier et al., 2008). Vitamin B1 functionally participated in the physiological carbohydrate metabolism to regulate the potential metabolic disorders during SARA. However, the potential mechanism of VB1 on rumen epithelial function and cell transportation is still unclear. We systematically summarized the effects of VB1 on the rumen epithelial apoptosis process, and interpret the underlying attenuating mechanism of VB1 on SARA.
Effects of VB1 on rumen fermentability
Rumen fermentability determined the nutritional provision for body condition, while Vitamin B1 supplement significantly promoted the fermentability, including the enhancement content of acetate, propionate, and butyrate.
Ruminal infusion of Vitamin B1 is supposed to be an effective strategy to alleviate SARA in our study, as it helps in increasing the ruminal pH, reducing the ruminal lactate concentration, and improving the component of total VFA. The mechanism of Vitamin B1 on ruminal fermentation is related to its effects on microbes in the rumen. Wang et al. (2015) reported that Vitamin B1 supplementation significantly reduces the population of Streptococcus bovis (lactate-producing bacteria) and increases the population of Megasphaera elsdenii (lactate utilizers and convert ruminal lactate into propionate). Besides, a higher Vitamin B1 concentration in the rumen can support the protozoa population (Höltershinken et al., 2003); the increasing protozoa could store fermentable carbohydrates for a short time and consequently help to stabilize ruminal pH. Moreover, Vitamin B1 supplementation enhanced the activity of PDH and α-KGDHC, and consequently, increased the amount of pyruvate flow to the TCA cycle and reduced the conversion of pyruvate to lactate.
Specifically, the acetate concentration showed a high positive correlation to the Vitamin B1 concentration, this result was mainly attributed to the formation process of acetate. The major portion of the acetate is derived from the decarboxylation of pyruvate, which was degraded from structural carbohydrates (Baldwin et al., 1963). The decreased dietary structural carbohydrates and blocked pyruvate decarboxylation caused by Vitamin B1 deficiency in SARA cows inhibited the acetate generation and also lowered the ruminal acetate concentration.
Propionate is generally produced by succinate or from lactate via the acrylate pathway. The contribution of the acrylate pathway traditionally increased accompanied by higher carbohydrate availability (Baldwin et al., 1963). In our study, a high proportion of grain in the SARA diet could be rapidly fermented into large amounts of pyruvate, and then converted into propionate through the generating bacteria (Van Soest, 1994). Vitamin B1 deficiency under high-concentrate levels restricted the regular metabolism of pyruvate into acetyl-CoA, and may further lead to the accumulation of propionate. In the present study, vitamin B1 content significantly decreased, which interpreted the higher propionate concentration in the rumen.
Effects of VB1 on rumen epithelial functions
Rumen epithelial cell viability represented the underlying functions of nutrient absorption, the fundamental for bioactivities transportation, and the barrier functions. The viability was impacted by certain factors, including physical damages, such as pressure, and chemical injuries, such as the acidity treatment (Luo et al., 2019). When ruminants suffered SARA, the lower ruminal pH induced certain syndromes to threaten the homeostasis of the rumen micro-ecosystem (Mu et al., 2021). The increased hydrogen ions fortified ruminal osmotic pressure, which further aggressively impact the regular ion exchange process that occurred in epithelial cells and causatively induced decreased cell viability.
Simultaneously, alterations of the rumen microbiome generated unconventional metabolites, such as biogenic amines and lipopolysaccharide, which further induced inflammatory responses, suppressed cell viability, and disrupted the functions of epithelial cells (Wang et al., 2021). Apart from causing serious inflammatory responses, cellular anti-oxidative capacity is also a noticeable factor that impacts cell viability. The reactive oxygen scavenging ability ensured the normal cellular nutrients transportation. However, the previous study shows that the accumulation of reactive oxygen species (ROS) induced by acidic pH significantly restrained cell viability (Schmidt et al., 2021), which ultimately stimulated cell apoptosis.
Generally, apoptosis occurs when cells receive stress, invasive damage, and gene regulation. The rumen epithelial cell apoptosis process was stimulated when homeostasis was disturbed, as reported by Ma et al. (2021). The acidity treatment first promoted ROS-dependent intrinsic cell apoptosis by activating a JNK signaling pathway (Jiang et al., 2017). Indeed, in our present study, ROS levels, as well as the JNK gene and protein expression, significantly upregulated, which further confirmed the inductive effects of JNK signaling pathways. JNK signaling pathway executed an efficient function in cell apoptosis through c-Jun phosphorylation (Dhanasekaran and Reddy, 2008). The significant upregulation of JNK phosphorylation, as shown in the western blotting, further convincedly proves the inductive effects of JNK on cell apoptosis.
Underlying mechanism of the acidity treatment on apoptosis
Traditionally, cell apoptosis was primarily induced by the upregulation of the Caspase 3 gene expression, which could be stimulated through both mitochondrial and nucleonic pathways. JNK induced the cleavage of Bid, inhibited the anti-apoptotic proteins, such as Bcl2-family apoptotic proteins, and activated the expression of pro-apoptotic genes, such as Bax (Bcl2-associated protein). The activated Bax forms membrane channels through which apoptogenic proteins, such as cytochrome C, can be released (Ansari et al., 2021). In our research, the acidity treatment significantly up-regulated the expression of Bax and cyt-C, and down-regulated of the expression Bcl2. These findings showed a specific confirmation of the occurrence of mitochondrial apoptosis in an acidic environment.
The mitochondria-induced apoptosis is the most common process in the extrinsic pathway, of which the crucial event is the release of cytochrome C from the inner membrane space of mitochondria. Then, the released cytochrome C formed the apoptosomes combined with caspase-9, which activate the caspase-9 cascade process and finally triggered the epithelial cell apoptosis (Araya et al., 2021).
In conclusion, VB1 supplement could effectively attenuate metabolic disorders, and the potential attenuation mechanism scheme just as shown in Figure 5. VB1 supplement promote epithelial function by alleviating cell apoptosis when exposed to high-concentrate diets, and finally promoted production performance.
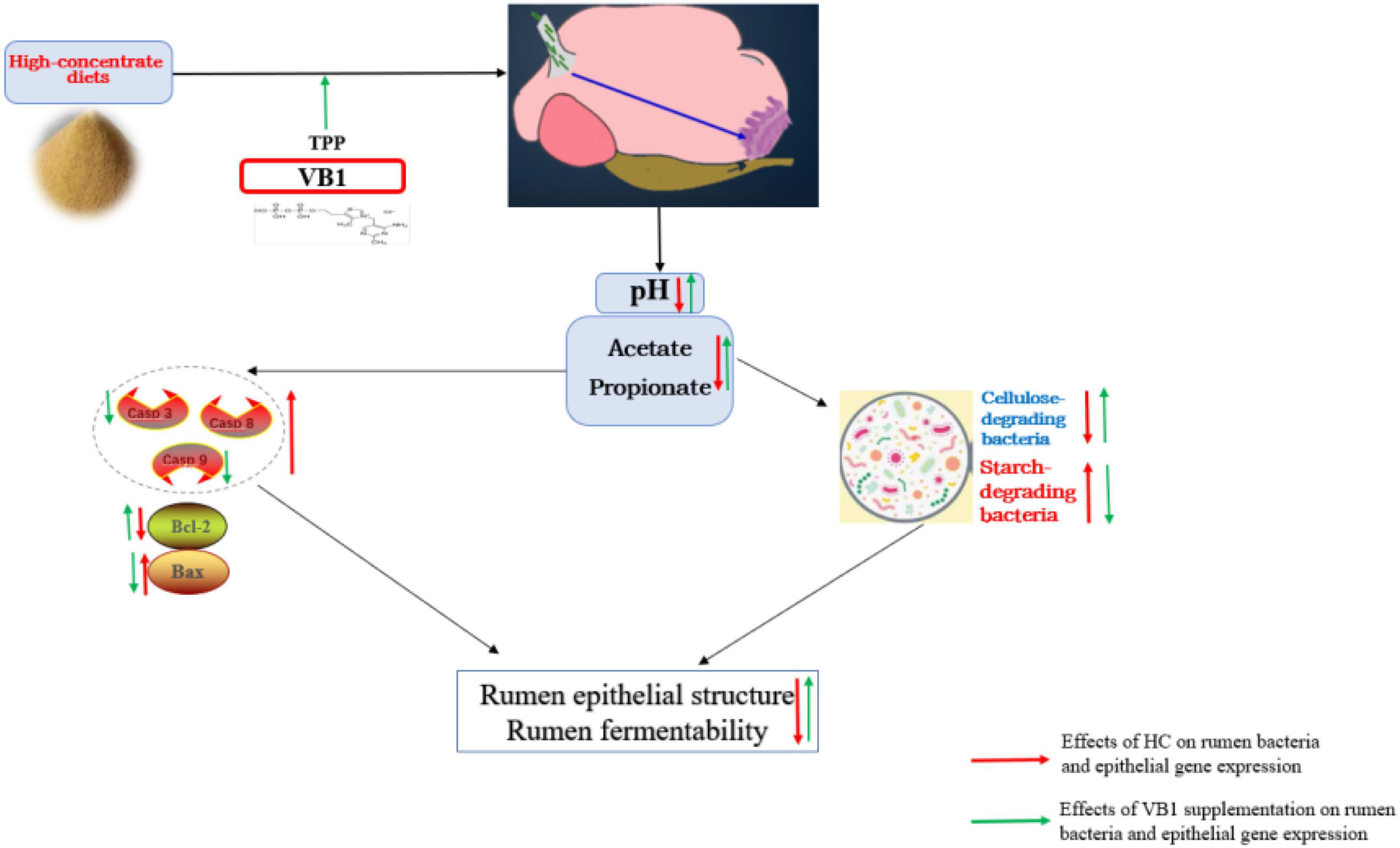
Figure 5. Potential attenuation mechanism scheme of Vitamin B1 on high-concentrate diets inducing rumen disorders.
Data availability statement
The original contributions presented in the study. are publicly available. This data can be found here: NCBI SRA database, accession number PRJNA644053.
Ethics statement
The animal study was reviewed and approved by JXAULL-20220121.
Author contributions
LY and FX designed the study. PM, CS, ML, YaS, and YK conducted the experiment. CS, HY, and PYM contributed to the manuscript writing and English editing. ZY and YuS contributed to parameter measurement and data analysis. All authors contributed to the article, approved the submitted version, carefully read the manuscript, and were accountable for all aspects of the work.
Funding
This study was supported by State Key Laboratory of Animal Nutrition of China (2004DA125184G2204), the China Agriculture Research System of MOF and MARA (CARS-37), the Science and Technology Planning Project of Jiangxi Educational Department (GJJ200414), and the Latitudinal Project of Jiangxi Agricultural University (2021JXAUHX021).
Acknowledgments
We thank the Nanchang Key Laboratory of Animal Health and Safety Production and the Jiangxi Province Key Laboratory of Animal Nutrition/Engineering Research Center of Feed Development.
Conflict of interest
ML, YuS, ZY, and FX were employed by Yangxin Yiliyuan Halal Meat Co., Ltd. PM was employed by Anyou Biotechnology Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1008373/full#supplementary-material
Supplementary Table 1 | Taxonomy results of each sample.
References
Ansari, M. Y., Ball, H. C., Wase, S. J., Novak, K., and Haqqi, T. M. (2021). Lysosomal dysfunction in osteoarthritis and aged cartilage triggers apoptosis in chondrocytes through BAX mediated release of Cytochrome c. Osteoarthr. Cartil. 29, 100–112. doi: 10.1016/j.joca.2020.08.014
Araya, L. E., Soni, I. V., Hardy, J. A., and Julien, O. (2021). Deorphanizing Caspase-3 and Caspase-9 substrates in and out of apoptosis with deep substrate profiling. ACS Chem. Biol. 16, 2280–2296. doi: 10.1021/acschembio.1c00456
Baldwin, R., Wood, W., and Emery, R. (1963). Conversion of glucose-C14 to propionate by the rumen microbiota. J. Bacteriol. 85, 1346–1349. doi: 10.1128/jb.85.6.1346-1349.1963
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., and Caporaso, J. G. (2018). QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science. PeerJ 6:e27295v2.
Breves, G., Brandt, M., Hoeller, H., and Rohr, K. (1981). Flow of thiamin to the duodenum in dairy cows fed different rations. J. Agric. Sci. 96, 587–591.
Chen, Y., Feng, X., Hu, X., Sha, J., Li, B., Zhang, H., et al. (2018). Dexmedetomidine ameliorates acute stress-induced kidney injury by attenuating oxidative stress and apoptosis through inhibition of the ROS/JNK signaling pathway. Oxid. Med. Cell. Longev. 2018, 1–12. doi: 10.1155/2018/4035310
Dai, H., Ma, N., Chang, G., Aabdin, Z. U., and Shen, X. (2020). Long-term high-concentrate diet feeding induces apoptosis of rumen epithelial cells and inflammation of rumen epithelium in dairy cows. Anim. Biotechnol. 33, 289–296. doi: 10.1080/10495398.2020.1806073
Gui, H., and Shen, Z. (2016). Concentrate diet modulation of ruminal genes involved in cell proliferation and apoptosis is related to combined effects of short-chain fatty acid and pH in rumen of goats. J. Dairy Sci. 99, 6627–6638. doi: 10.3168/jds.2015-10446
Höltershinken, M., Höhling, A., Wendelken, G., Elias, K., and Scholz, H. (2003). Einflüsse auf den ruminalen protozoenbesatz des rindes (in vitro) Teil 2: Wirkung einer subklinischen pansenazidose ohne und mit thiaminsubstitution. Tierärztliche Prax. Großtiere 31, 178–182.
Huang, C. C., Kuo, C. Y., Yang, C. Y., Liu, J. M., and Huang, C. F. (2019). Cadmium exposure induces pancreatic β-cell death via a Ca2+-triggered JNK/CHOP-related apoptotic signaling pathway. Toxicology 425:152252. doi: 10.1016/j.tox.2019.152252
Jiang, Z., Song, F., Li, Y., Xue, D., Zhao, N., Zhang, J., et al. (2017). Capsular polysaccharide of mycoplasma ovipneumoniae induces sheep airway epithelial cell apoptosis via ROS-dependent JNK/P38 MAPK pathways. Oxid. Med. Cell. Longev. 2017:6175841. doi: 10.1155/2017/6175841
Luo, D., Peng, Z., Yang, L., Qu, M., Xiong, X., Xu, L., et al. (2019). Niacin protects against butyrate-induced apoptosis in rumen epithelial cells. Oxid. Med. Cell. Longev. 2019:2179738. doi: 10.1155/2019/2179738
Ma, Y., Zhang, Y., Zhang, H., and Wang, H. (2021). Thiamine alleviates high-concentrate-diet-induced oxidative stress, apoptosis, and protects the rumen epithelial barrier function in goats. Front. Vet. Sci. 8:663698. doi: 10.3389/fvets.2021.663698
Mao, S. Y., Huo, W. J., and Zhu, W. Y. (2015). Microbiome–metabolome analysis reveals unhealthy alterations in the composition and metabolism of ruminal microbiota with increasing dietary grain in a goat model. Environ. Microbiol. 18, 525–541. doi: 10.1111/1462-2920.12724
Miao, L., Xinliang, C., Nan, W., Mingyang, W., Jiawen, P., Jingjing, T., et al. (2018). Proanthocyanidins protect epithelial cells from zearalenone-induced apoptosis via inhibition of endoplasmic reticulum stress-induced apoptosis pathways in mouse small intestines. Molecules 23:1508. doi: 10.3390/molecules23071508
Miller, B., Meiske, J., and Goodrich, R. (1986). Effects of grain source and concentrate level on B-vitamin production and absorption in steers. J. Anim. Sci. 62, 473–483.
Mu, Y. Y., Qi, W. P., Zhang, T., Zhang, J. Y., Mei, S. J., and Mao, S. Y. (2021). Changes in rumen fermentation and bacterial community in lactating dairy cows with subacute rumen acidosis following rumen content transplantation. J. Dairy Sci. 104, 10780–10795. doi: 10.3168/jds.2021-20490
Němcová-Fürstová, V., Balušíková, K., Srámek, J., James, R. F., and Kováø, J. (2013). Caspase-2 and JNK activated by saturated fatty acids are not involved in apoptosis induction but modulate ER stress in human pancreatic β-cells. Cell. Physiol. Biochem. 31, 277–289.
NRC (2001). Nutrient requirements of dairy cattle, 7th Rev. Edn. Washington, DC: National Academic Press.
Pan, X., Nan, X., Liang, Y., Jiang, L., and Xiong, B. (2018). Thiamine status, metabolism and application in dairy cows: A review. Br. J. Nutr. 120, 491–499. doi: 10.1017/S0007114518001666
Plaizier, J., Krause, D., Gozho, G., and McBride, B. (2008). Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 176, 21–31. doi: 10.1016/j.tvjl.2007.12.016
Ren, X., Wang, S., Zhang, C., Hu, X., Zhou, L., Li, Y., et al. (2020). Selenium ameliorates cadmium-induced mouse leydig TM3 cell apoptosis via inhibiting the ROS/JNK/c-jun signaling pathway. Ecotoxicol. Environ. Saf. 192:110266. doi: 10.1016/j.ecoenv.2020.110266
Schmidt, C., Schneble-Löhnert, N., Lajqi, T., Wetzker, R., Müller, J. P., and Bauer, R. (2021). PI3Kγ mediates microglial proliferation and cell viability via ROS. Cells 10:2534.
Schwab, E., Schwab, C., Shaver, R., Girard, C., Putnam, D., and Whitehouse, N. (2006). Dietary forage and nonfiber carbohydrate contents influence B-vitamin intake, duodenal flow, and apparent ruminal synthesis in lactating dairy cows. J. Dairy Sci. 89, 174–187. doi: 10.3168/jds.S0022-0302(06)72082-3
Stumpff, F., Georgi, M. I., Mundhenk, L., Rabbani, I., Fromm, M., Martens, H., et al. (2011). Sheep rumen and omasum primary cultures and source epithelia: Barrier function aligns with expression of tight junction proteins. J. Exp. Biol. 214, 2871–2882. doi: 10.1242/jeb.055582
Su, C. C., Kuan-I, L., Chen, M. K., Chun-Ying, K., Tang, C. H., Liu, S. H., et al. (2016). Cantharidin induced oral squamous cell carcinoma cell apoptosis via the JNK-regulated mitochondria and endoplasmic reticulum stress-related signaling pathways. PLoS One 11:e0168095. doi: 10.1371/journal.pone.0168095
Tao, S., Duanmu, Y., Dong, H., Tian, J., Ni, Y., and Zhao, R. (2014). A high-concentrate diet induced colonic epithelial barrier disruption is associated with the activating of cell apoptosis in lactating goats. BMC Vet. Res. 10:235. doi: 10.1186/s12917-014-0235-2
Van Soest, P. (1994). Nutritional ecology of the ruminant, 2nd Edn. Ithaca, NY: Cornell University Press.
Wang, H., Pan, X., Wang, C., Wang, M., and Yu, L. (2015). Effects of different dietary concentrate to forage ratio and thiamine supplementation on the rumen fermentation and ruminal bacterial community in dairy cows. Anim. Prod. Sci. 55, 189–193.
Wang, M. Y., Li, Y., Gao, M., Song, L. W., Xu, M., Zhao, X. L., et al. (2021). Effects of subacute ruminal acidosis on colon epithelial morphological structure, permeability, and expression of key tight junction proteins in dairy goats. J. Dairy Sci. 104, 4260–4270. doi: 10.3168/jds.2020-18738
Xue, F., Pan, X., Jiang, L., Guo, Y., and Xiong, B. (2018b). GC-MS analysis of the ruminal metabolome response to thiamine supplementation during high grain feeding in dairy cows. Metabolomics 14:67. doi: 10.1007/s11306-018-1362-8
Xue, F., Nan, X., Sun, F., Pan, X., Guo, Y., Jiang, L., et al. (2018a). Metagenome sequencing to analyze the impacts of thiamine supplementation on ruminal fungi in dairy cows fed high-concentrate diets. AMB Express 8:159. doi: 10.1186/s13568-018-0680-6
Xue, F., Sun, F., Jiang, L., Hua, D., Wang, Y., Nan, X., et al. (2019). Effects of partial replacement of dietary forage using kelp powder (Thallus laminariae) on ruminal fermentation and lactation performances of dairy cows. Animals 9:852. doi: 10.3390/ani9100852
Zhang, S., Aschenbach, J. R., Barreda, D. R., and Penner, G. B. (2013). Recovery of absorptive function of the reticulo-rumen and total tract barrier function in beef cattle after short-term feed restriction. J. Anim. Sci. 91, 1696–1706. doi: 10.2527/jas.2012-5774
Zhang, Y., Kong, C., Zeng, Y., Wang, L., Li, Z., Wang, H., et al. (2010). Ursolic acid induces PC-3 cell apoptosis via activation of JNK and inhibition of Akt pathways in vitro. Mol. Carcinog. 49, 374–385. doi: 10.1002/mc.20610
Keywords: vitamin B1, high-concentrate diets, rumen fermentability, rumen epithelium, rumen microbiome
Citation: Ma P, Sun C, Liu M, You H, Shen Y, Kang Y, Sun Y, Yang Z, Ma P, Yang L and Xue F (2022) Metagenomic insights into the rumen epithelial integrity responses to the vitamin B1 supplement under high-concentrate diets treatments. Front. Microbiol. 13:1008373. doi: 10.3389/fmicb.2022.1008373
Received: 31 July 2022; Accepted: 22 September 2022;
Published: 25 October 2022.
Edited by:
Dan Wan, Key Laboratory of Agro-ecological Processes in Subtropical Region, Institute of Subtropical Agriculture (CAS), ChinaReviewed by:
Yanghua He, University of Hawai’i at Mānoa,, United StatesZhenwei Zhang, Liaocheng University, China
Copyright © 2022 Ma, Sun, Liu, You, Shen, Kang, Sun, Yang, Ma, Yang and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuguang Xue, eHVlZnVndWFuZzEyM0AxNjMuY29t
†These authors have contributed equally to this work
 Peng Ma
Peng Ma Chaoqun Sun2†
Chaoqun Sun2† Liang Yang
Liang Yang Fuguang Xue
Fuguang Xue